Abstract
Background
Cancer patients with minor children but also their families suffer from significant psychological distress and comorbidity. Protective factors predicting successful coping are well known. Corresponding systematic interventions are rare and limited by access barriers. We developed a comprehensive family-centered intervention for cancer patients with at least one dependent minor.
Patients and methods
Family-SCOUT represents a multicentric, prospective, interventional, and controlled study for families with parental cancer and their minor children. In the intervention group (IG), all family members were addressed using a care and case management approach for nine months. Families in the control group (CG) received standard of care. Participating parents were asked to complete the Hospital-Anxiety-Depression-Scale (HADS) questionnaire at enrolment (T0) and after 9 months (T2). The primary outcome was a clinically relevant reduction of distress in at least one parent per family, measured as minimal important difference (MID) of ≥1.6 in the HADS total score. The percentage of families achieving MID is compared between the IG and CG by exact Fisher’s test, followed by multivariate confounder analyses.
Results
T0-questionnaire of at least one parent was available for 424 of 472 participating families, T2-questionnaire after 9 months was available for 331 families (IG n = 175, CG n = 156). At baseline, both parents showed high levels of distress (HADS total: sick parents IG: 18.7 ± 8.1; CG: 16.0 ± 7.2; healthy partners: IG: 19.1 ± 7.9; CG: 15.2 ± 7.7). The intervention was associated with a significant reduction in parental distress in the IG (MID 70.4% in at least one parent) compared with the CG (MID 55.8%; P = 0.008). Adjustment for group differences from specific confounders retained significance (P = 0.047). Bias from other confounders cannot be excluded.
Conclusions
Parental cancer leads to a high psychosocial burden in affected families. Significant distress reduction can be achieved through an optimized and structured care approach directed at the family level such as family-SCOUT.
Key words: parental cancer, minors, psychosocial burden, distress reduction, comprehensive support program, family-SCOUT
Highlights
-
•
Care and case management for families with parental cancer and underage children such as family-SCOUT is feasible.
-
•
Can be implemented in an outreach setting centered on a comprehensive cancer center.
-
•
Is significantly associated with a meaningful reduction of psychosocial distress at the parent level.
Introduction
More than half of all cancer patients experience an increased psychosocial burden and the 4-week prevalence of mental disorders amounts to about one-third (32%).1 Psychological sequelae of both cancer itself and/or cancer care occur more frequently in younger cancer patients.2
Overall, 18%-25% of cancer patients3,4 and more specifically, 35% of breast cancer patients have minor children.5 This group of patients has been demonstrated to experience higher psychosocial distress than cancer patients without children.6 This applies to both disease-specific distress but also fear about the future7 including concerns about the well-being of minor children and the maintenance of daily routines.8
Healthy partners of cancer patients are also at increased risk of acute psychosocial distress9 and the subsequent development of affective disorders.10 Their burden is multifold, as they are not only the most important source of support for their diseased partners11 but also in increasing responsibility for their children and the maintenance of everyday life both resulting in a measurable increase in fears and sorrows.12,13
Parental burden and changes in daily routines substantially impact children of parents with cancer.14,15 About half of the underage children living in families with parental cancer are considered to be substantially distressed16 and develop behavioral problems, with about a third developing psychological symptoms.15,17, 18, 19
Risk and protective factors known to influence the outcome of the children of patients suffering from cancer have recently been elucidated.17,20 Negative predictors are e.g. persistent parental psychological distress,21 maternal depression,22 lack of emotional availability,14 and financial worries.23 Protective factors are open communication in the family,23 adequate coping strategies, and the good functioning of the family system.24
Interventions aimed at the reduction of risk factors must involve all those affected, namely both parents, the healthy and the sick, the children, and the entire family system.25 Optimized structures should support parents in (i) maintaining everyday life and fostering financial security,4 (ii) dealing with their children, e.g. assisting in open family discussions,26 and (iii) developing emotional coping strategies.
To date, there is a substantial lack of structured support and psychosocial interventions for families with cancer in general.27 Existing interventions do not adequately address all key needs described.25,28 The few implemented structures are rarely used due to access barriers such as difficulty in scheduling appointments, fear of stigmatization, and lack of awareness.29 There are programs developed for children of parents with cancer23 or for mothers affected by cancer30 or for mourning parents31 to support them in their parenthood. There is, however, a lack of family-centered programs that support the family as a system according to an individual needs assessment and that offer continuous support to families during the illness and after a possible death. In a population-based sample in Germany, only 44% of parents with cancer use any psychosocial support at all and only 9% are known to receive specific family-centered support.32
Based on the existing evidence, the actively outreaching, family-centered, cross-sectoral intervention family-SCOUT was developed within the Comprehensive Cancer Center, Network Center of Integrated Oncology Aachen-Bonn-Cologne-Düsseldorf (CIOABCD) including four comprehensive cancer centers in the German Rhineland region, to address unmet needs of families suffering from parental cancer spanning all disease phases and transitions. It is based on dedicated multiprofessional comprehensive care and case management with a permanent contact person (the so-called family-scout).33 The intervention is described in detail in the intervention section of the following methods chapter.
Aim and hypotheses
The study aimed to evaluate the family-SCOUT intervention about its influence on the course of parental stress. We hypothesize that the family-SCOUT intervention is associated with a significant and clinically meaningful reduction of psychosocial parental distress in at least one parent per family when compared with a control group (CG). To test the hypothesis, a quasi-experimental two-armed, controlled, prospective interventional study was conducted in three German cancer centers.
Participants and methods
Participants
The study was conducted at three participating cancer centers of excellence certified by the German Cancer Aid (https://www.krebshilfe.de/), namely Aachen, Bonn, and Düsseldorf of the CIOABCD. Inclusion criteria were: (i) at least one parent had a confirmed ICD-10 C diagnosis, (ii) custody of at least one minor child (or child living in the household), (iii) sufficient German language skills, and (iv) sufficient cognitive abilities to consent to the study. The exclusion criterion for participation was the withdrawal of consent. The sample characteristics are described in detail in the results section.
Ethical approval
The study was approved by the ethics committees of the Medical Faculty of the RWTH Aachen University (EK 195/18), Bonn University (267/18), and Düsseldorf University (2018-215-ProspDEuA) and registered on ClinicalTrials.gov (identifier NCT04186923). All participants gave written informed consent.
Study design
This was a quasi-experimental, non-randomized, unblinded, prospective superiority CG design with two study arms (intervention and control). Data collection was carried out at one time point for baseline and two time points for follow-up measurements.33
Procedures
Multiprofessional oncology care teams and outreach partners of the CIOABCD cancer centers identified affected parents by asking oncological patients between the ages of 20 and 55 years whether they live with or have custody of minors. If the patients consented, they forwarded the families’ contact details to the project manager at the study sites. To overcome known access barriers,29,34 project management actively contacted the families once they received the contact details from the oncology teams. For families in the intervention group (IG) only, a family-scout was informed, who contacted the family and arranged an initial meeting to start the intervention. Families were included in the study from October 2018 until December 2020.
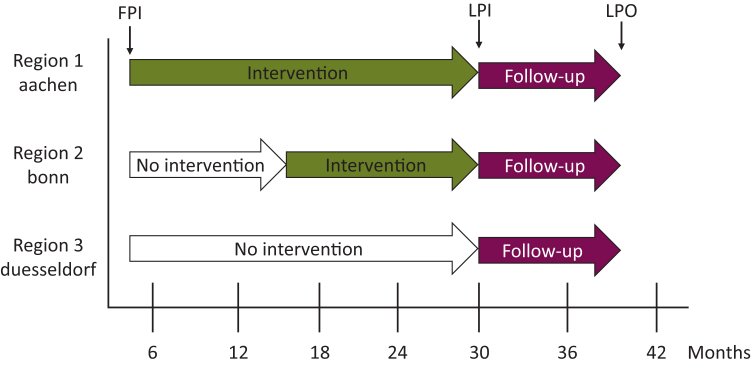
The allocation of affected families into CG or IG was executed in terms of an effectiveness-implementation hybrid study type 2.35 Region 1 (piloting before the study): all recruited families were assigned to the IG. Region 2 (first year of recruitment): all families were assigned to the CG, after implementation of family-SCOUT all following newly identified families were assigned to the IG. Region 3: all recruited families were assigned to the CG. Therewith additional knowledge for intervention roll-out to other locations was obtained (Figure 1).
Figure 1.
Study flow. FPI, first patient in; LPI, last patient in; LPO, last patient out.
Intervention
Family-SCOUT was developed in accordance with empirical findings on protective and risk factors for the development of secondary mental illnesses after parental cancer,20,36 on the basics of systemic family therapy, in regards to child developmental psychology and the children of somatically ill parents (COSIP) counseling concept.37 Empirical needs for support25 and clinical experience from the health system and youth welfare were taken into account.
The intervention addressed all family members, was provided by a permanent contact person, the family-scout (social worker), and was delivered via home visits, telephone support, text/email messages, or video calls. According to the psychosocial needs assessment and the expressed needs of the family, an individual intervention was planned. Components were organizational support (e.g. establishing household help or advising on securing finances), communicative support (e.g. providing age-appropriate information material on cancer for the children), and emotional support (e.g. developing functional coping strategies). Counseling took place on the level of the family, parental, couple, or on an individual basis. If necessary, forwarding information to or counseling additional professionals was initiated. The duration of the intervention depended on the families’ needs, planned to last ∼9 months (if reasonable beyond the death of the affected parent), and included on average 18.6 contacts with the family lasting in total ∼15 h. All intervention units were electronically documented (content and time spent).
Family-scouts received an 80-unit training course (60 h) that includes oncological, psycho-oncological, social-legal, systemic, and communication-promoting knowledge.38,39 Continuous external monitoring and regular case discussions ensured adherence to the manual and implementation quality.
Measures
Baseline variables and the primary outcome of the family-SCOUT project were defined as described previously.33
Primary outcome
Sick (SP) and healthy parents (HP) filled out the German version of the Hospital-Anxiety-Depression-Scale (HADS) at enrolment (T0) and 9 months after (T2).
The HADS is an established instrument for measuring psychosocial distress in somatically ill patients. The reliability and validity of the scale have been demonstrated in cancer patients.40 Patients rate 14 items on a four-point Likert scale. The total score ranges from 0 to 42. Higher scores indicate higher levels of anxiety and depression. For the overall scale, thresholds between 13 and 18 are reported as appropriate for various cancer samples.41 The best thresholds for screening for mental disorders were 10 or 11, respective thresholds for depression screening were 15 for the HADS total (sensitivity 0.87; specificity 0.88).42 For a conservative approach, we chose a cut-off ≥16 for post hoc stratified analysis.
The primary outcome was a reduction in the HADS total score at the family level, which means in at least one of the two parents after 9 months (time from T0 to T2). For the HADS, the value for a minimally important, i.e. clinically relevant, stress reduction (MID = minimal important difference) was assumed with a HADS total score reduction of ≥1.6.43,44 We planned this combined outcome because we were convinced that reducing the emotional stress of a parent—regardless of whether they are SP or HP—will benefit all family members and allow the greatest possible completeness of data even when an SP dies.
Independent variables (self-reported) in the adjusted multivariate analysis (Table 1)
Table 1.
Study population—sick parents
| Sick parent n = 424 |
||||
|---|---|---|---|---|
| Total | Intervention groupX1 (n = 239) | Control group (n = 185) | P | |
| Sociodemographic parameters | ||||
| AgeX11, years | n = 389 m = 35 | 219 m = 20 | 170 m = 15 | 0.0138 |
| Mean ± SD | 44.6 ± 7.7 | 42.7 ± 6.9 | ||
| GenderX10 | n = 424 m = 0 | 239 | 185 | 0.4315 |
| Male/female | 107/317 | 64/175 (27/73%) | 43/142 (23/77%) | |
| Marital status | n = 397 m = 27 | 223 | 174 | 0.6618 |
| Married | 322 | 176 (79%) | 146 (84%) | |
| Widowed, single, divorced | 6/39/30 | 4/25/18 (2/11/8%) | 2/14/12 (1/8/7%) | |
| Partner | n = 395 m = 29 | 221 | 174 | 0.1173 |
| Yes/no | 336/59 | 182/39 (82/18%) | 154/20 (89%/11%) | |
| Highest educational degree (classified) | n = 398 m = 26 | 223 | 175 | 0.0406 |
| Without, basic school attendanceX6 | 53 | 37 (17%) | 16 (9%) | |
| Middle maturityX7 | 84 | 45 (20%) | 39 (22%) | |
| College degreeX8 | 55 | 37 (17%) | 18 (10%) | |
| University entrance qualification, high school diplomaX9 | 197 | 100 (45%) | 97 (55%) | |
| Another | 9 | 4 (2%) | 5 (3%) | |
| Child <4 yearsX5 | n = 402 m = 22 | 226 | 176 | 0.8209 |
| Yes/no | 108/294 | 62/164 (27/73%) | 46/130 (26/74%) | |
| Employment | n = 373 m = 51 | 207 | 166 | 0.7558 |
| Full-time, part-time >50%, self-employed | 111/66/16 | 56/39/6 (27/19/3%) | 55/27/10 (33/16/6%) | |
| Part-time up to 50%, minor employment | 77/14 | 44/7 (21/3%) | 33/7 (20/4%) | |
| Occupational rehabilitation, unemployed | 4/20 | 2/13 (1/6%) | 2/7 (1/4%) | |
| Completely disabled, pensioner | 17/7 | 10/5 (5/2%) | 7/2 (4/1%) | |
| House(-wo)man, pupil, student | 39/2 | 23/2 (11/1%) | 16/0 (10/0%) | |
| Disease-specific parameters | ||||
| Diagnosis | ||||
| Multiple selections due to primary, secondary, and tertiary cancer | n = 403 m = 21 | n = 228 m = 11 | n = 175 m = 10 | |
| MammaCa | 158 | 91 | 67 | 0.7584 |
| Leukemia and lymphoma | 57 | 27 | 30 | 0.1497 |
| GI (except pancreatic) cancer | 37 | 25 | 12 | 0.1683 |
| Pancreatic cancer | 8 | 8 | 0 | 0.0111 |
| Brain | 34 | 17 | 17 | 0.4714 |
| Gyn. | 24 | 17 | 7 | 0.2022 |
| Skin | 18 | 11 | 7 | 0.8098 |
| Lung | 15 | 10 | 5 | 0.5970 |
| Urological | 10 | 6 | 4 | 1.0000 |
| Other | 19 | 14 | 5 | 0.1565 |
| Time since diagnosisX2 | n = 374 m = 50 | 210 m = 29 | 164 m = 21 | |
| Mean ± SD (median) | 23.2 ± 46.7 (3.0) | 26.5 ± 44.5 (9.0) | <0.0001a | |
| <3 months | 136 (36%) | 103 (49%) | 33 (20%) | <0.0001 |
| Secondary diagnosisX3 | n = 365 m = 59 | 200 | 165 | |
| Yes/no/unknown | 121/200/44 | 77/93/30 (39/46/15%) | 44/107/14 (27/65/8%) | 0.0020 |
| Death within 12 months after T0X4 | n = 424 m = 0 | 239 | 185 | 0.0002 |
| Yes/no | 71/353 | 54/185 (23/77%) | 17/168 (9/91%) | |
| Comorbidities | n = 395 m = 29 | 222 | 173 | |
| Physically (yes/no) | 165/230 | 94/128 (42/58%) | 71/102 (41/59%) | 0.8373 |
| Mentally (yes/no) | 52/343 | 25/197 (11/89%) | 27/146 (16/84%) | 0.2313 |
| Sick leave | n = 375 m = 49 | 211 | 164 | 0.0458 |
| Yes/no | 254/121 | 152/59 (72/28%) | 102/62 (62/38%) | |
Bold indicates P < 0.05.
GI, Gastrointestinal; Gyn., gynecological cancer; m, missing; ns, non-significant; SD, standard deviation; xnVariable in multivariate analysis.
Wilcoxon test.
Before modeling, the following confounders were selected after discussion of primarily significant differences in baseline variables. The adjusted multivariate analysis included sociodemographic variables: the age of the SP (at study enrolment, in years), gender of the SP (male/female), children younger than 4 years, and school certification of the SP, and disease-specific variables: the duration since the first diagnosis (in months), recurrence (yes/no), and unclear recurrence (I do not know). Additionally, the model included the death of the SP 12 months after T0 (family reports and information from the local population registers).
Sample size and statistical power
A sample size of families (IG: 330; CG: 230) was planned to detect a difference in the estimated rates of MID in HADS total score on a family level between 28% (IG) and 16% (CG) at an α of 0.05 and a power of 0.9.33
Statistical methods
The observation units were families, each family including maximal one SP and one HP. Analyzing variables of SP or HP or of the whole family correspond uniquely to the family, such that cluster adjustment is guaranteed.
Treatment groups were described by their baseline variables. To investigate potential bias from non-randomization, statistical tests corresponding to their distribution (Fisher’s test, t-test, Wilcoxon test) were carried out to compare both groups. Baseline variables with significant differences were considered in the above-mentioned selection of potential confounders for the following multiple regression analyses.
The primary analysis was carried out using the intention-to-treat principle. The HADS response rates between IG and CG were compared by Fisher’s exact test. As a measure of association, the raw odds ratio was calculated. To adjust for potential confounders multiple logistic regression analyses were carried out. Furthermore, the primary analysis (including regression) was carried out after the imputation of missing values of the primary outcome and the covariables from above by multiple (100) imputations assuming missing at random (MAR) and using the fully conditional specification.
Post hoc subgroup analysis for families with baseline HADS ≥16 (at least one parent) was carried out using the corresponding multiple logistic regression model to investigate the intervention effect in a more distressed subpopulation in both groups. For statistical calculations, SAS version 9.4 was used. The significance level was 5%.
Results
Sample characteristics and descriptive statistics
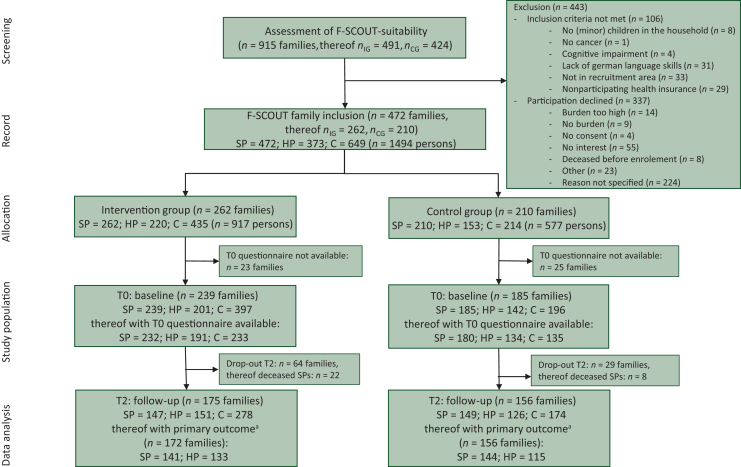
Of n = 915 screened families, n = 472 families were recruited in the study (n = 262 families in the IG and n = 210 families in the CG). Reasons for non-inclusion and non-participation are shown within the CONSORT flowchart45 (Figure 2).
Figure 2.
CONSORT flowchart. C, children; HADS, Hospital-Anxiety-Depression-Scale; HP, healthy parent; SP, sick parent. aDifference in HADS total score available for at least one parent per family.
The age range of children in the participating families was between 0 and 36 years, and the average number of children was 1.7. HADS-questionnaire data at T0 were available for n = 424/472 families so these families (IG = 239/CG = 185) were defined as the final study population. Evaluable data sets of the HADS score (T0 and T2) for the calculation of the MID were available in the IG on family level for n = 172 (either SP n = 141 and/or HP n = 133) and in the CG for n = 156 (either SP n = 144 and/or HP n = 115).
Sociodemographic and disease-specific properties of the study sample were compared for the IG and CG, divided into SP and HP. The SPs of the IG and CG differed significantly in age (IG > CG), classified school leaving certificate (IG < CG), duration since the initial cancer diagnosis (IG < CG, continuous or categorized), follow-up diagnosis, sick leaves, and death within 12 months (all IG > CG, Table 1). HP of the IG and CG differed significantly in age (IG > CG), and sick leaves (IG > CG, Table 2). Mean HADS baseline values (T0) differed significantly in IG and CG, with means (±standard deviation) of 18.7 (± 8.1) for SP (IG) versus 16.0 (± 7.2) in the CG and for HP (IG) of 19.1 (± 7.9) and in the CG 15.2 (± 7.7) (Table 3).
Table 2.
Study population—healthy parents
| HP n = 343 (no HP for n = 81 families, 38 in IG and 43 in CG) |
||||
|---|---|---|---|---|
| Total | IG (n = 201) | CG (n = 142) | P | |
| Sociodemographic parameters | ||||
| Age, years | n = 313 m = 30 | 184 | 129 | 0.0008 |
| Mean ± SD | 46.0 ± 7.8 | 43.0 ± 7.3 | ||
| Gender | n = 343 m = 0 | 201 | 142 | 0.9053 |
| Male/female | 240/103 | 140/61 (70/30%) | 100/42 (70/30%) | |
| Relationship of HP to SP | n = 320 m = 23 | 187 | 133 | 0.9179 |
| Spouse/partner | 288/26 | 167/16 (89/9%) | 121/10 (91/8%) | |
| Relative/other | 2/4 | 1/3 (1/2%) | 1/1 (1/1%) | |
| Highest educational degree (classified) | n = 305 m = 38 | 177 | 128 | 0.0694 |
| Without, basic school attendance | 45 | 31 (18%) | 14 (11%) | |
| Middle maturity7 | 64 | 41 (23%) | 23 (18%) | |
| College degree | 45 | 28 (16%) | 17 (13%) | |
| University entrance qualification, high school diploma | 140 | 69 (39%) | 71 (56%) | |
| Another | 11 | 8 (5%) | 3 (2%) | |
| Child <4 years | n = 304 m = 39 | 177 | 127 | 0.2968 |
| Yes/no | 83/221 | 44/133 (25/75%) | 39/88 (31/69%) | |
| Employment | n = 295 m = 48 | 170 | 125 | 0.6870 |
| Full-time, part-time >50%, self-employed | 195/33/16 | 109/18/7 (64/11/4%) | 86/15/9 (69/12/7%) | |
| Part-time up to 50%, minor employment | 18/11 | 11/8 (6/5%) | 7/3 (6/2%) | |
| Occupational rehabilitation, unemployed | -/5 | -/4 (-/2%) | -/1 (-/1%) | |
| Completely disabled, pensioner | 1/2 | 1/2 (1/1%) | 0/0 (0/0%) | |
| House(-wo)man, pupil, student | 12/2 | 8/2 (5/1%) | 4/0 (3/0%) | |
| Disease-specific parameters | ||||
| Comorbidities | n = 311 m = 32 | 182 | 129 | |
| Physically (yes/no) | 153/158 | 89/93 (49/51%) | 64/65 (50/50%) | 0.9089 |
| Mentally (yes/no) | 49/262 | 28/154 (15/85%) | 21/108 (16/84%) | 0.8751 |
| Sick leave | n = 283 m = 60 | 162 | 121 | 0.0017 |
| Yes/no | 31/252 | 26/136 (16/84%) | 5/116 (4/96%) | |
Bold indicates P < 0.05.
CG, control group; HP, healthy parent; IG, intervention group; m, missing; ns, non-significant; SD, standard deviation; SP, sick parent.
Table 3.
HADS values and primary analysis (n = 424)—individual and family-level analysis
| IG |
CG |
|||
|---|---|---|---|---|
| SP |
HP |
SP |
HP |
|
| Primary analysis | ||||
| Individual level | ||||
| n = 141 m = 98 | n = 133 m = 106 | n = 144 m = 41 | n = 115 m = 70 | |
| Response/non-response | 80/61 (56.7/43.3%) | 73/60 (54.9/45.1%) | 61/83 (42.4/57.6%) | 46/69 (40.0/60.0%) |
| Response | Combination parents Families with/without values in the primary outcome: n = 328 m = 96 (IG n = 172 m = 67, CG n = 156 m = 29) |
|||
| Both parents | 32 | 20 | ||
| Only SPa | 48 | 41 | ||
| Only HPa | 41 | 26 | ||
| None | 51 | 69 | ||
| Family-levelb | ||||
| Responsec/non-response | 121/51 (70.4/29.7%) | 87/69 (55.8/44.2%) | 0.0082 | |
| HADS values | ||||
| Baseline – T0 | ||||
| Mean ± SD | 18.7 ± 8.1 n = 227 m = 12 P = 0.0006d |
19.1 ± 7.9 n = 190 m = 49 P< 0.0001d |
16.0 ± 7.2 n = 177 m = 8 P = 0.0006d |
15.2 ± 7.7 n = 133 m = 52 P< 0.0001d |
| After intervention – T2 | ||||
| Mean ± SD | 15.9 ± 8.3 n = 141 m = 98 | 16.2 ± 8.1 n = 134 m = 105 | 14.5 ± 7.6 n = 147 m = 38 | 14.8 ± 7.1 n = 120 m = 65 |
Bold indicates P < 0.05.
CG, control group; HADS, Hospital-Anxiety-Depression-Scale; HP, healthy parent; IG, intervention group; m, missing; MID, minimal important difference; SD, standard deviation; SP, sick parent.
Non-response or missing value for spouse.
Family-level includes SP and HP.
Response at family-level if at least one parent had an MID of ≥1.6.
t-test comparing IG and CG.
Analysis of primary outcome
Fisher’s test
A distress reduction at the family level according to the defined MID of ≥1.6 was achieved significantly more frequently in the IG. In the IG, 121 of 172 families (70.4%) showed a clinically relevant reduction in distress for at least one parent, and in the CG, 87 of 156 families (55.8%). The proportion of HADS responders in the IG was significantly greater (P = 0.008) (Table 3). The 95% confidence intervals for the HADS responder probabilities were 70.4% (62.9% to 77.1%) and 55.8% (47.6% to 63.7%). The unadjusted odds ratio was 1.88 (1.19-2.97) to be compared with the adjusted odds ratio below.
The response rates for HP and SP are similar on a descriptive level in both groups and higher in the IG compared with CG; IG ∼55%, CG ∼41% (Table 3).
Multivariate model
After model-based confounder adjustment, the odds ratio remained similar at 1.85 (1.01-3.41) (P = 0.047). There was only a minor change of the odds ratio by adjustment for these confounders, and the confidence interval was a bit enlarged but is still located completely above 1. The baseline HADS variable was significantly associated with the HADS response. After multiple imputations, the corresponding model yielded very similar results, the age of SP at enrolment was additionally significantly associated with the HADS response (Table 4).
Table 4.
Multivariate analysis before and after imputation
| Dependent variable | Multivariate model |
I (before imputation) n = 269 |
II (after multiple imputation, n = 424) |
||
|---|---|---|---|---|---|
| Independent variable | OR covariable (95% CI) | P | OR covariable (95% CI) | P | |
| Primary outcome | Intervention versus control | 1.853 (1.007-3.409) | 0.047 | 1.787 (1.036-3.084) | 0.037 |
| (HADS response of ≥MID 1.6) | Maximum parental HADS sum score at baseline | 1.104 (1.060-1.151) | <0.001 | 1.108 (1.068-1.151) | <0.001 |
| Time since diagnosis <3 months: yes versus no | 0.904 (0.457-1.790) | 0.772 | 1.063 (0.554-2.039) | 0.855 | |
| Secondary diagnosis yes versus no | 0.708 (0.333-1.503) | 0.367 | 0.928 (0.451-1.907) | 0.838 | |
| Unsure secondary diagnosis (‘don’t know’) versus no | 1.035 (0.401-2.671) | 0.943 | 0.911 (0.374-2.218) | 0.838 | |
| Death of SP within 12 months after T0 yes versus no | 0.573 (0.184-1.784) | 0.335 | 0.465 (0.193-1.122) | 0.088 | |
| Child <4 years yes versus no | 0.680 (0.314-1.471) | 0.325 | 0.757 (0.371-1.543) | 0.443 | |
| School degree of SP 1 versus 0 (ref) | 1.394 (0.454-4.283) | 0.561 | 1.217 (0.409-3.620) | 0.724 | |
| School degree of SP 2 versus 0 (ref) | 1.194 (0.364-3.915) | 0.769 | 1.047 (0.339-3.232) | 0.936 | |
| School degree of SP 3 versus 0 (ref) | 1.829 (0.639-5.232) | 0.259 | 1.503 (0.551-4.100) | 0.426 | |
| School degree of SP 4 versus 0 (ref) | 0.548 (0.091-3.306) | 0.510 | 0.517 (0.083-3.222) | 0.480 | |
| Male gender versus female gender of SP | 1.798 (0.865-3.739) | 0.116 | 1.338 (0.702-2.548) | 0.376 | |
| Age of SP at enrolment (years) | 0.969 (0.921-1.020) | 0.230 | 0.953 (0.912-0.997) | 0.035 | |
0, without school attendance; 1, middle maturity; 2, college degree; 3, university entrance qualification, high school diploma; 4, other.
Bold indicates P < 0.05.
CI, confidence interval; HADS, Hospital-Anxiety-Depression-Scale; OR, odds ratio; SP, sick parent.
Stratified analysis
As a post hoc planned subgroup analysis, we compared the families with at least one parent scoring an initial distress level over cut-off ≥16 in IG and CG. In n = 310/424 of the families (73.1%, 2 missing), the maximum HADS value at T0 was found to be ≥16. HADS score difference was available for n = 131 in IG, 54 missing, and for n = 105 in CG, 20 missing.
Assuming HADS baseline values at T0 ≥16, a distress reduction at the family level (MID of ≥1.6 for at least one parent) was achieved significantly more frequently in the IG for 79.4% of the families versus for 62.9%, P = 0.006 in the CG (Table 5). The confounder-adjusted odds ratio concerning distress reduction was 2.32 (1.05-5.13), P = 0.038.
Table 5.
Post hoc stratified analysis for HADS baseline ≥16 (after imputation). Primary outcome, HADS-response of MID ≥1.6
| Family level | IG (n = 131, m = 54) | CG (n = 105, m = 20) | P |
|---|---|---|---|
| Response/non-response | 104/27 (79.4/20.6%) | 66/39 (62.9/37.1%) | 0.0057 |
| Dependent variable | Independent variable | OR covariable (CI) | P |
| Univariate model n = 236 | |||
| Intervention versus control | 2.276 (1.271-4.076) | 0.006 | |
| Bivariate model n = 236 | |||
| Intervention versus control | 2.050 (1.127-3.728) | 0.019 | |
| Maximum parental HADS sum score | 1.040 (0.985-1.099) | 0.156 | |
| Multivariate modelan = 191 | |||
| Primary outcome | Intervention versus control | 2.320 (1.050-5.129) | 0.038 |
CG, control group; CI, confidence interval; HADS, Hospital-Anxiety-Depression-Scale; IG, intervention group; m, missing; MID, minimal important difference; OR, odds ratio.
Bold indicates P < 0.05.
Other independent variables as in Table 4.
Discussion
To our knowledge, the family-SCOUT intervention represents the first structured comprehensive care and case management intervention for families with minors suffering from parental cancer. The aim of this quasi-experimental two-armed, controlled, prospective interventional study was to analyze the impact of family-SCOUT on the reduction of psychosocial parental distress.
The family-SCOUT intervention is outreaching, cross-sectoral, along disease trajectories, and addresses the needs of all family members in the areas of support in maintaining everyday life, open disease-related communication, and emotional coping with the disease. In our study, the intervention was significantly associated with a clinically meaningful reduction of distress in at least one parent per family assessed after 9 months. This might be attributable to different parts of the intervention. The structured needs assessment at the beginning ensured that the individual needs were being addressed. The combination of organizational and emotional support could be particularly effective because it is only through organizational support that the individual space is being created in which emotional support can become effective. Financial security counseling could also be a positive factor, as financial concerns contribute to increased parental distress.23 Support for all family members instead of an index member could potentially make a crucial difference since family communication is best guaranteed when all family members are involved. In this way, the protective factors known for the best child outcome, such as open family communication23 and good functioning of the family system,24 can be particularly promoted.
The strength of the intervention could also be that it is on the one hand structured (in terms of access, assessment, regular contact, duration, and structured information on existing support offers) and on the other hand, at the same time needs-oriented (especially in terms of the focus in the individual family). Concurrently, the comparability and evaluation within the study may of course be somewhat limited.
Even in families in which the SP dies, the family-scout stays for a total of 9 months and is available to the surviving partner and children as a permanent contact person for the grieving process and for the establishment of new everyday structures. Not feeling left alone and having expert advice available at all times could also have reduced the stress on the HP at T2 in this special situation.
HADS baseline values for the affected parents in our study were higher than typically expected in cancer patients.46,47 Compared with earlier studies in parental cancer,48 both the participants in the IG and the CG showed a higher initial burden. In >73% of the families, at least one parent scored HADS values above the cut-off ≥16. Only one former study6 described similarly high values. One explanation might be that a wide variety of cancer scenarios were included in this study, i.e. not only families early in the course of their disease, but also families in later-line and/or palliative condition who elsewhere might have been excluded from participation were allowed to take part.49,50
It has to be discussed whether the more frequent reduction of HADS values in the IG (70.4% versus 56% in CG) occurred even though the patients were more distressed at baseline or could be explained by a regression to mean. Interestingly, the significance persisted after adjustment for confounders, and families with HADS baseline values ≥16 also showed a significant difference in the outcome between IG and CG. Altogether, this suggests that HADS baseline values and other covariables in the model did not heavily confound the primary results. The assumed MID rate of 28% in the IG and 16% in the CG was in line with our expectation of lower baseline values and lower size of possible intervention effects according to the literature.30,51
The natural course of psychosocial distress in most cancer patients shows a decline over time,46,47 but in some patients, the burden remains at high levels, sometimes anxiety increases again after years,52 and distress after 8 months seems to be a predictor for long-term persisting stress.53 Risk factors for persistent depression and anxiety are female gender, age under 50 years, and having responsibility for the care of minors.46,52,54 The natural course of psychosocial distress in the context of parental cancer has not been extensively studied.
Family-SCOUT was implemented to finally achieve a more rapid reduction of psychosocial burden for all family members, a quicker return to everyday life, and to support children to sooner resume their own development. In this study, parental burden was defined as a proxy indicator of family burden. This is mainly because it is impossible to objectively survey young children’s distress. Data on the quality of life of children over the age of 8 years were collected, however, and are currently being analyzed about their relationship to parental stress. In addition, a follow-up study to investigate the usefulness of the intervention retrospectively from the perspective of the participating children who are now older is being planned.
Conduction of this large family study with nearly 1500 participants, evaluation of the complex data structure as well as the implementation of the intervention family-SCOUT in a new region of our consortium during the study period were successful. As families with underage children and parental cancer inevitably reach their organizational limits rather quickly55 and become unable to organize support for themselves, the outreach study and intervention design led to sufficient recruitment and use of family-SCOUT. Hereby, the initiating support from the CIOABCD oncology care team including outreach partners seems to be of key importance.29 Compared with other studies in this field, our study design is of improved quality due to the CG and its quasi-experimental approach.29
As the study was not formally randomized and not blinded, the families knew from inclusion whether they would receive support or standard of care. Families with higher levels of parental stress and more severe illnesses were thus more likely to be found in the IG. The high mortality rate of SP in the IG (22.6% within 12 months after T0) suggests a higher prevalence of terminally ill patients in IG than in CG (9.2% died within 12 months). Higher HADS values at baseline in the IG seem to reflect this difference.
The motivation of the families to participate and the recruitment or selection of the families by the project managers from different sites could have differed between the two study arms. Furthermore, there are indications that the pre-selection of the referrers was also different at the three sites. Accordingly, a selection bias between the two groups with different baseline variables cannot be excluded. Further important limitations are biases caused by unknown or not documented confounders, missing values, dropouts, and deaths (more frequently in IG).
Conclusions
Care and case management for families with parental cancer and underage children such as family-SCOUT is feasible, can be implemented in an outreach setting centered by a comprehensive cancer center network such as CIOABCD, and is significantly associated with a meaningful reduction of psychosocial distress at the parent level. Follow-up studies of the family-SCOUT cohort are planned to evaluate the long-term effects of the intervention on both the parental and children’s levels.
Acknowledgments
Funding
This work was supported by the German Innovation Fund of the Federal Joint Committee [grant number 01NVF17043]. The CIOABCD network is supported through a grant by the German Cancer Aid Foundation, DKH [grant numbers 70113470, 70114996].
Disclosure
The authors have declared no conflicts of interest.
References
- 1.Mehnert A., Braehler E., Faller H., et al. Four-week prevalence of mental disorders in patients with cancer across major tumor entities. J Clin Oncol. 2014;32:3540–3546. doi: 10.1200/JCO.2014.56.0086. [DOI] [PubMed] [Google Scholar]
- 2.Singer S., Szalei C., Briest S., et al. Co-morbid mental health conditions in cancer patients at working age–prevalence, risk profiles, and care uptake. Psycho Oncol. 2013;22(10):2291–2297. doi: 10.1002/pon.3282. [DOI] [PubMed] [Google Scholar]
- 3.Weaver K.E., Rowland J.H., Alfano C.M., et al. Parental cancer and the family: a population-based estimate of the number of US cancer survivors residing with their minor children. Cancer. 2010;116(18):4395–4401. doi: 10.1002/cncr.25368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inhestern L., Johannsen L.M., Bergelt C. Families affected by parental cancer: quality of life, impact on children and psychosocial care needs. Front Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.765327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erdmann F., Spix C., Katalinic A., et al. Krebs in Deutschland für 2017/2018. 2021, 13. Ausgabe. Robert Koch-Institut (Hrsg) und die Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V. (Hrsg). Berlin. [DOI]
- 6.Akter J., Khan J.G., Khan M.H., et al. Psychological distress in cancer patients with underage children. J Dhaka Med Coll. 2016;24(2):146–151. [Google Scholar]
- 7.Mehnert A., Hartung T.J., Friedrich M., et al. One in two cancer patients is significantly distressed: prevalence and indicators of distress. Psychooncology. 2018;27:75–82. doi: 10.1002/pon.4464. [DOI] [PubMed] [Google Scholar]
- 8.Inhestern L., Bergelt C. When a mother has cancer: strains and resources of affected families from the mother’s and father’s perspective - a qualitative study. BMC Womens Health. 2018;18:72. doi: 10.1186/s12905-018-0562-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Northouse L.L., Katapodi M.C., Song L., et al. Interventions with family caregivers of cancer patients: meta-analysis of randomized trials. CA Cancer J Clin. 2010;60(5):317–339. doi: 10.3322/caac.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakaya N., Saiko-Nakaya K., Bidstruo P.E., et al. Increased risk of severe depression in male partners of women with breast cancer. Cancer. 2010;116(23):5527–5534. doi: 10.1002/cncr.25534. [DOI] [PubMed] [Google Scholar]
- 11.Rhee Y.S., Yun Y.H., Park S., et al. Depression in family caregivers of cancer patients: the feeling of burden as a predictor of depression. J Clin Oncol. 2008;26(36):5890–5895. doi: 10.1200/JCO.2007.15.3957. [DOI] [PubMed] [Google Scholar]
- 12.Götze H., Weißflog G., Braehler E., et al. Partner von krebspatientinnen mit minderjährigen kindern – psychische belastung im vergleich zur allgemeinbevölkerung und zur krebskranken partnerin. Psychother Psychosom Med Psychol. 2012;62(02):73–79. doi: 10.1055/s-0032-1301899. [DOI] [PubMed] [Google Scholar]
- 13.Götze H., Braehler E., Romer G., et al. Partnerinnen von krebspatienten mit minderjährigen kindern – psychische belastung im vergleich zur allgemeinbevölkerung und zum krebskranken partner. Psychother Psychosom Med Psychol. 2012;62:170–176. doi: 10.1055/s-0032-1306303. [DOI] [PubMed] [Google Scholar]
- 14.Stefanou K., Zografos E., Zografos G.C., et al. Emotional and behavioural problems in children dealing with maternal breast cancer: a literature review. Br J Guid Couns. 2020;48(3):394–405. [Google Scholar]
- 15.Visser A., Huizinga G., van der Graaf W.T. The impact of parental cancer on children and the family: a review of the literature. Cancer Treat Rev. 2004;30(8):683–694. doi: 10.1016/j.ctrv.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Bergelt C., Ernst J.C., Beierlein V. Reaktive veränderungen in befinden und verhalten von kindern bei elterlicher krebserkrankung - ergebnisse einer epidemiologischen patientenbefragung. Prax Kinderpsychol Kinderpsychiatr. 2012;61(6):378–395. doi: 10.13109/prkk.2012.61.6.378. [DOI] [PubMed] [Google Scholar]
- 17.Watson M, St, James-Roberts I., Ashley S., et al. Factors associated with emotional and behavioural problems among school age children of breast cancer patients. Br J Cancer. 2006;94(1):43–50. doi: 10.1038/sj.bjc.6602887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romer G., Barkmann C., Sculte-Markwort M., et al. Children of somatically ill parents. Clin Child Psychol Psychiatry. 2002;7(1):17–38. [Google Scholar]
- 19.Birenbaum L.K., Yancey D.Z., Phillips D.S., et al. School-age children's and adolescents' adjustment when a parent has cancer. Oncol Nurs Forum. 1999;26(10):1639–1645. [PubMed] [Google Scholar]
- 20.Möller B., Barkmann C., Krattenmacher T., et al. Children of cancer patients: prevalence and predictors of emotional and behavioral problems. Cancer. 2014;120(15):2361–2370. doi: 10.1002/cncr.28644. [DOI] [PubMed] [Google Scholar]
- 21.Thastum M., Watson M., Kienbacher C., et al. Prevalence and predictors of emotional and behavioural functioning of children where a parent has cancer: a multinational study. Cancer. 2009;115(17):4030–4039. doi: 10.1002/cncr.24449. [DOI] [PubMed] [Google Scholar]
- 22.Kliem A. Universitätsklinikum Hamburg-Eppendorf. University of Hamburg; 2018. Psychosoziale Symptombelastung und Familienfunktion bei Kindern krebskranker Mütter in Einelternfamilien.https://ediss.sub.uni-hamburg.de/handle/ediss/8208 [Google Scholar]
- 23.Phillips F., Prezio E.A., Panisch L.S., et al. Factors affecting outcomes following a psychosocial intervention for children when a parent has cancer. J Child Life Psychosocial Theory Pract. 2021;2(2):1–13. [Google Scholar]
- 24.Faccio F., Ferrari F., Pravettoni G. When a parent has cancer: how does it impact on children’s psychosocial functioning? A systematic review. Eur J Cancer Care. 2018;27(6) doi: 10.1111/ecc.12895. [DOI] [PubMed] [Google Scholar]
- 25.Ellis S., Wakefield C.E., Antill G., et al. Supporting children facing a parent's cancer diagnosis: a systematic review of children's psychosocial needs and existing interventions. Eur J Cancer Care. 2017;26(1) doi: 10.1111/ecc.12432. [DOI] [PubMed] [Google Scholar]
- 26.Park E.M., Jensen C., Song M.K., et al. Talking with children about prognosis: the decisions and experiences of mothers with metastatic cancer. JCO Oncol Pract. 2021;17(6):e840–e847. doi: 10.1200/OP.21.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ernst J., Beierlein V., Romer G., et al. Psychosoziale versorgung von kindern mit einem an krebs erkrankten elternteil - eine bestandsaufnahme spezifischer versorgungsangebote in Deutschland. Psychother Psychosom Med Psychol. 2011;61(09/10):426–434. doi: 10.1055/s-0031-1286303. [DOI] [PubMed] [Google Scholar]
- 28.Ohan J.L., Jackson H.M., Bay S., et al. How psychosocial interventions meet the needs of children of parents with cancer: A review and critical evaluation. Eur J Cancer Care. 2020;29(5) doi: 10.1111/ecc.13237. [DOI] [PubMed] [Google Scholar]
- 29.Inhestern L, Haller AC, Wlodarczyk O, et al. Psychosocial interventions for families with parental cancer and barriers and facilitators to implementation and use – a systematic review. PLoS One. 11(6):e0156967. [DOI] [PMC free article] [PubMed]
- 30.Lewis F.M., Brandt P.A., Cochrane B.B., et al. The Enhancing Connections Program: a six-state randomized clinical trial of a cancer parenting program. J Consult Clin Psychol. 2015;83(1):12–23. doi: 10.1037/a0038219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandler I.N., Wolchik S.A., Ayers T.S., Tein J.Y., Luecken L. Family bereavement program (FBP) approach to promoting resilience following the death of a parent. Fam Sci. 2013;4(1):10. doi: 10.1080/19424620.2013.821763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ernst J.C., Beierlein V., Romer G., et al. Use and need for psychosocial support in cancer patients: a population-based sample of patients with minor children. Cancer. 2013;119(12):2333–2341. doi: 10.1002/cncr.28021. [DOI] [PubMed] [Google Scholar]
- 33.Dohmen M., Petermann-Meyer A., Blei D., et al. Comprehensive support for families with parental cancer (Family-SCOUT), evaluation of a complex intervention: study protocol for a non-randomized controlled trial. Trials. 2021;22:622. doi: 10.1186/s13063-021-05577-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romer G., Saha R., Haagen M., et al. Lessons learned in the implementation of an innovative consultation and liaison service for children of cancer patients in various hospital settings. Psychooncology. 2007;16:138–148. doi: 10.1002/pon.1105. [DOI] [PubMed] [Google Scholar]
- 35.Curran G.M., Bauer M., Mittmann B., et al. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50(3):217. doi: 10.1097/MLR.0b013e3182408812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krattenmacher T., Kuehne F., Ernst J., et al. Parental cancer: factors associated with children's psychosocial adjustment—a systematic review. J Psychosom Res. 2012;72(5):344–356. doi: 10.1016/j.jpsychores.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Paschen B., Saha R., Baldus C., et al. Evaluation eines präventiven beratungskonzeptes für kinder körperlich kranker eltern. Psychotherapeut. 2007;52(4):265–272. [Google Scholar]
- 38.Krüger L., Panse J.P., Hugot J., et al. Implementierung eines modellprojektes zur phasenübergreifenden unterstützung von krebskranken eltern mit minderjährigen kindern. Gesundheitswesen. 2023;85(6):529–536. doi: 10.1055/a-1775-8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petermann-Meyer A., Dohmen M., Ernstmann N., et al. Krebskranke Eltern mit minderjährigen Kindern. Onkologie. 2022;28:997–1004. [Google Scholar]
- 40.Hermann-Lingen C., Buss U., Snaith R. Hans Huber; Bern: 2011. Hospital Anxiety and Depression Scale–German Version (HADS-D) [Google Scholar]
- 41.Herschbach P., Weis J. (Hrsg.), editors. Vol. 2. Deutsche Krebsgesellschaft; Berlin: 2010. (Screeningverfahren in der Psychoonkologie. Testinstrumente zur Identifikation betreuungsbedürftiger Krebspatienten. Eine Empfehlung der PSO für die psychoonkologische Behandlungspraxis). [Google Scholar]
- 42.Vodermaier A., MIllmann R.D. Accuracy of the Hospital Anxiety and Depression Scale as a screening tool in cancer patients: a systematic review and meta-analysis. Support Care Cancer. 2011;19:1899–1908. doi: 10.1007/s00520-011-1251-4. [DOI] [PubMed] [Google Scholar]
- 43.Puhan M.A., Frey M., Buechi S., et al. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2008;6(1):1–6. doi: 10.1186/1477-7525-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guyatt G.H., Oscoba D., Wu A.W., et al. Methods to explain the clinical significance of health status measures. Mayo Clin Proc. 2002;77(4):371–383. doi: 10.4065/77.4.371. [DOI] [PubMed] [Google Scholar]
- 45.Moher D., Hopewell S., Schulz K.F., et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28–55. doi: 10.1016/j.ijsu.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Bidstrup P.E., Christensen J., Goldschmidt Mertz B., et al. Trajectories of distress, anxiety, and depression among women with breast cancer: looking beyond the mean. Acta Oncol. 2015;54(5):789–796. doi: 10.3109/0284186X.2014.1002571. [DOI] [PubMed] [Google Scholar]
- 47.Beesley V.L., Smith D.D., Nagle C.M., et al. Coping strategies, trajectories, and their associations with patient-reported outcomes among women with ovarian cancer. Support Care Cancer. 2018;26:4133–4142. doi: 10.1007/s00520-018-4284-0. [DOI] [PubMed] [Google Scholar]
- 48.Park E.M., Deal A.M., Check D.K., et al. Parenting concerns, quality of life, and psychological distress in patients with advanced cancer. Psycho Oncol. 2016;25(8):942–948. doi: 10.1002/pon.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Götze H., Friedrich M., Brähler E., et al. Psychological distress of cancer patients with children under 18 years and their partners—a longitudinal study of family relationships using dyadic data analysis. Support Care Cancer. 2017;25:255–264. doi: 10.1007/s00520-016-3411-z. [DOI] [PubMed] [Google Scholar]
- 50.Bultmann J.C., Beierlein V., Romer G., et al. Parental cancer: Health-related quality of life and current psychosocial support needs of cancer survivors and their children. Int J Cancer. 2014;135:2669–2677. doi: 10.1002/ijc.28905. [DOI] [PubMed] [Google Scholar]
- 51.Ferrell B., Wittenberg E. A review of family caregiving intervention trials in oncology. CA Cancer J Clin. 2017;67(4):318–325. doi: 10.3322/caac.21396. [DOI] [PubMed] [Google Scholar]
- 52.Breidenbach C., Heidkamp P., Hiltrop H. Prevalence and determinants of anxiety and depression in long-term breast cancer survivors. BMC Psychiatry. 2022;22:101. doi: 10.1186/s12888-022-03735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lam W.W., Shing Y.T., Bonanno G.A., et al. Distress trajectories at the first year diagnosis of breast cancer in relation to 6 years survivorship. Psychooncology. 2012;21:90–99. doi: 10.1002/pon.1876. [DOI] [PubMed] [Google Scholar]
- 54.Van Beek F.E., Jansen F., Mak L., et al. The course of symptoms of anxiety and depression from time of diagnosis up to 2 years follow-up in head and neck cancer patients treated with primary (chemo) radiation. Oral Oncol. 2020;102 doi: 10.1016/j.oraloncology.2020.104576. [DOI] [PubMed] [Google Scholar]
- 55.Kuswanto C.N., Stafford L., Sharp J., et al. Psychological distress, role, and identity changes in mothers following a diagnosis of cancer: a systematic review. Psycho Oncol. 2018;27(12):2700–2708. doi: 10.1002/pon.4904. [DOI] [PubMed] [Google Scholar]