Abstract
Human T-cell lymphotropic virus type 1 (HTLV-1) is the causative agent of a chronic progressive myelopathy called tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP/HAM). In this disease, lesions of the central nervous system (CNS) are associated with perivascular infiltration by lymphocytes. We and others have hypothesized that these T lymphocytes infiltrating the CNS may play a prominent role in TSP/HAM. Here, we show that transient contact of human or rat astrocytes with T lymphocytes chronically infected by HTLV-1 impairs some of the major functions of brain astrocytes. Uptake of extracellular glutamate by astrocytes was significantly decreased after transient contact with infected T cells, while the expression of the glial transporters GLAST and GLT-1 was decreased. In two-compartment cultures avoiding direct cell-to-cell contact, similar results were obtained, suggesting possible involvement of soluble factors, such as cytokines and the viral protein Tax-1. Recombinant Tax-1 and tumor necrosis factor alpha (TNF-α) decreased glutamate uptake by astrocytes. Tax-1 probably acts by inducing TNF-α, as the effect of Tax-1 was abolished by anti-TNF-α antibody. The expression of glutamate-catabolizing enzymes in astrocytes was increased for glutamine synthetase and decreased for glutamate dehydrogenase, the magnitudes of these effects being correlated with the level of Tax-1 transcripts. In conclusion, Tax-1 and cytokines produced by HTLV-1-infected T cells impair the ability of astrocytes to manage the steady-state level of glutamate, which in turn may affect neuronal and oligodendrocytic functions and survival.
Human T-cell lymphotropic virus type 1 (HTLV-1) (49) is the etiological agent of an inflammatory demyelinating pathology of the central nervous system (CNS) known as tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP/HAM) (18, 47). This neurological syndrome is a chronic progressive encephalomyelopathy characterized by corticospinal attack (9, 36). To date, the precise mechanisms causing TSP/HAM remain largely undetermined. Nevertheless, several studies have emphasized the prime role of the high number of circulating HTLV-1-infected T lymphocytes (viral load) in the appearance of TSP/HAM (46, 63). Such high viral load has been considered a consequence of an inefficient immune response to HTLV-1 (26). In TSP/HAM patients, marked infiltration of the CNS by infected T cells is consistently observed (33, 59), particularly in demyelinating lesions. These T cells harboring provirus and expressing the viral protein Tax-1 (33, 42, 43) may cause bystander effects damaging neural cells or affecting their functions (25). Possible implication of direct infection of neural cells is not well documented in TSP/HAM, as viral products can hardly be detected in neural cells (34).
One important notion when considering the effects of the virus on the CNS is that certain impairments occurring in actually infected cells may be perpetuated via indirect effects of the virus on neural cells. Such impairment may persist and propagate via the secretion of soluble factors, such as cytokines, chemokines, or metalloproteinases (19, 20, 22, 57, 58, 60, 61), and eventually pervade the entire neuraxis. In the case of TSP/HAM, this view is consistent with (i) the presence, in the lesions, of cells expressing the viral product Tax-1 (43), which is known to transactivate many cellular genes including several inflammatory molecules (8), (ii) the expression of inflammatory cytokines in infiltrated T cells and astrocytes (57, 65), and (iii) the expression pattern of metalloproteinases and their inhibitors (20, 23, 60).
Our working hypothesis is that T lymphocytes persistently infected with HTLV-1 may initiate functional perturbations in astrocytes by expressing inflammatory molecules and viral proteins, in particular Tax-1. Previous studies in our laboratory have shown that astrocytes secrete inflammatory cytokines after transient contact with T cells persistently infected with HTLV-1, whether or not they produce virus (19). Such activated astrocytes may prolong and amplify the deleterious effects produced by invading T cells, given the crucial roles of astrocytes in brain homeostasis (production of energetic metabolites for neurons and oligodendrocytes, neurotransmitter catabolism, and ionic homeostasis [24]). One of the major roles of astrocytes is the control of the CNS excitability (13) by regulating the extracellular concentration of neurotransmitters, especially the major excitatory (glutamate) and inhibitory (γ-aminobutyric acid) amino acids. Astrocytes scavenge glutamate from the synaptic cleft and terminate its action via high-affinity sodium-dependent glutamate transporters specific to glia. These are the excitatory amino acid transporters 1 and 2 (EAAT1 and EAAT2 in humans, the rat counterparts being GLAST and GLT-1, respectively) (6, 29). Glutamate taken up by astrocytes is converted to glutamine by glutamine synthetase (GS; EC 6.3.1.2), and also passes into the astrocytic tricarboxylic (TCA) cycle (39) by conversion into α-ketoglutarate by glutamate dehydrogenase (GDH; EC.1.4.1.3). If glutamate management is impaired within astrocytes, this will compromise the functional integrity of the CNS in general and that of neurons and oligodendrocytes in particular, as these cells depend on metabolic precursors provided by astrocytes (48) and are highly sensitive to excessive concentrations of extracellular glutamate (7, 37, 54).
We have previously shown that transient exposure of human or rat astrocytes to cell lines of T lymphocytes chronically infected with HTLV-1 induces GS expression in these astrocytes (1). This altered catabolism of glutamate in astrocytes is mediated by the viral transactivator Tax-1 and has suggested that the deleterious effects of HTLV-1 infection may be caused by a compromised management of glutamate by astrocytes. In this study, we investigated glutamate uptake by astrocytes, since this step is crucial in the clearance of glutamate from the extracellular space and, subsequently, in the provision of metabolic precursors to neurons and oligodendrocytes. We show that glutamate accumulation and expression of mRNAs encoding glial glutamate transporters are significantly reduced in astrocyte culture after transient contact with HTLV-1-infected T lymphocytes. These effects result at least partly from paracrine effects of the viral protein Tax-1 via tumor necrosis factor alpha (TNF-α). Such bystander effects of Tax-1-producing cells emphasize the importance of the interaction between astrocytes and HTLV-1-infected T cells in the physiopathology of TSP/HAM.
MATERIALS AND METHODS
Unless otherwise noted, all reagents were obtained from Sigma (L'Isle d'Abeau, France).
Cell cultures.
Primary cultures of astrocytes were obtained by mechanical disaggregation of microdissected cortices from 1-day-old rat pups or of sensory motor cortices from the human fetus (embryonic day 116). The dissociated cells were diluted to a density of 2 × 105 cells/ml in Dulbecco's modified Eagle essential medium (DMEM) Glutamax (Life Technologies, Cergy Pontoise, France) containing 25 mM glucose, supplemented with 20% heat-inactivated fetal calf serum (FCS) and gentamicin (1 μg/ml). Cells were seeded in 35-mm-diameter culture dishes precoated with poly-l-lysine (3 μg/ml in 0.1 M borate buffer [pH 8.4]) as described by Yavin and Yavin (66). Cultures were incubated at 37°C in a moist 5% CO2–95% air atmosphere. The medium was changed 2 days after plating (DMEM Glutamax, 25 mM glucose, 10% FCS) and every 3 days thereafter until confluence. At that point, the FCS concentration was progressively decreased to 2% over 1 week. Using this procedure, we obtained 3-week-old cultures in which more than 95% of cells were of astrocytic phenotype. This was systematically determined by detection of glial fibrillary acidic protein (GFAP; a specific astrocytic marker) using immunocytochemistry (data not shown).
The human cell line Dev, established from a primitive neuroectodermic tumor (27), is similar to neural stem cells (38) and retains the capacity to differentiate toward astrocytes (21). We obtained various Dev cell lines after cloning and selected the one with the highest proportion (around 20%) of GFAP-positive cells. Culture conditions were the same as those described above.
The HTLV-1-producing T-cell line C91PL (50) and the HTLV-1-nonproducing T-cell line C8166/45 (53) were cultured in suspension in RPMI 1640 Glutamax medium (Life Technologies) supplemented with 20% heat-inactivated FCS and gentamicin (1 μg/ml). Cytokine and Tax-1 expression was checked before coculture (enzyme immunoassay [EIA], immunofluorescence). In some experiments, a mouse hybridoma cell line secreting antibodies against the viral protein Tax-1 (NIH 1314) was used to neutralize the effect of Tax-1 (106 B cells in culture inserts above astrocyte monolayer [see below]).
Transient coculture of astrocytes and T lymphocytes.
Transient contact of astrocytes (previously FCS deprived) with HTLV-1-infected T lymphocytes was performed by replacing the medium in the astrocyte culture with C91PL or C8166/45 cell suspensions (astrocyte/T-cell ratio, 10:1), which had been gamma irradiated (136 Gy) to prevent further proliferation. After 20 h, the T cells were removed by several washes with fresh medium, and the astrocytes were cultured for up to 30 days postcontact in DMEM Glutamax–25 mM glucose–2% FCS, changed twice a week. Using rat astrocyte cultures, the complete elimination of T cells from astrocyte culture after transient contact (day 4) was verified by the absence of human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and TNF-α (by reverse transcription-PCR [RT-PCR]) and of CD4+ T lymphocytes (by fluorescence-activated cell sorting). Transient contact with the C91PL cell suspension led to astrocyte infection; the proportions of infected cells were around 2% for primary rat astrocytes, 20% for human Dev cells, and 90% for human fetal primary astrocytes. To test the effect of soluble factors produced by chronically infected T cells, such as Tax-1 and cytokines, the C91PL and C8166/45 T-cell lines were seeded in cell culture inserts (0.4-μm pore size; Becton Dickinson, La Pont de Claix, France) and placed for 96 h over astrocyte cultures, thus avoiding effects due to cell-cell contact. This procedure thus allows only soluble factors to diffuse from the inserts. The use of virus-producing T cells (C91PL) in the inserts never led to infection of astrocytes (no p24 and Tax-1 as assessed by immunofluorescence).
Glutamate uptake.
The uptake protocol was adapted from that used by Drejer and coworkers (12). The assay buffer was prepared from phenol-free DMEM containing 5 mM glucose, but without glutamate and glutamine (Life Technologies). l-[3H]glutamate (l-3,4-[3H]glutamic acid; 22,000 Ci/mol; NEN, DuPont) was added to the assay buffer (1 μCi/well, i.e., 46 nmol), and the final glutamate concentrations were obtained with unlabeled l-glutamate. The preassay buffer was prepared similarly but without [3H]glutamate. The assay and preassay buffers were prewarmed in a cell incubator (37°C, atmosphere with 5% CO2 and 95% air).
The astrocyte cultures were rinsed with 1 ml of the phenol-free DMEM without glutamate and glutamine and then preincubated at least for 30 min in a cell culture incubator. Uptake assays were performed at 37°C in a water bath in two steps of 5-min incubation (1 ml/well), first with preassay buffer then with assay buffer containing [3H]glutamate. Within these 10 min, the kinetics of glutamate uptake is linear, as reported by others (30). The uptake medium was rapidly removed, cell plates were kept on ice, and the wells were rinsed with 2 ml of ice-cold preassay buffer containing excess (1 mM) glutamate to avoid reverse transport. The cells were immediately lysed with radioimmunoprecipitation assay buffer (150 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 10 mM Tris-HCl [pH 7.2], 1 mM EDTA, 1% sodium deoxycholate, 1% aprotinin; 500 μl/well) and 100-μl aliquots were analyzed for incorporated radioactivity by scintillation spectrometry (2 ml of UltimaGold; Packard, Groningen, The Netherlands) at an efficiency of about 60% (Packard Tri-Carb TR1600 LS analyzer).
Glutamate uptake was expressed with respect to the DNA rather than protein content, as preliminary data showed that infection by HTLV-1 resulted in a significant increase in protein levels compared to control cells (13% ± 0.11%, mean ± standard error of the mean [SEM], n = 48, P < 0.01). As there was no cell loss, we examined the DNA amount which is related to the number of cells. The amount of DNA per culture well was not altered (35.8 ± 0.3 versus 37.4 ± 0.3 μg, mean ± SEM, n = 48, corresponding to 4.5% ± 0.8% increase; quantification using the DNA-binding fluorochrome Hoechst 33258). Briefly, 10-μl aliquots of the cell suspension were added to the Hoechst 33258 assay solution (100 mM Tris base, 2 M NaCl, 10 mM EDTA, 0.1 μg of H33258/ml [pH 7.4]) in 96-well black microplates (Black Cliniplate; Labsystems). The fluorescence was read using a Titertek Fluoroskan II fluorometer (360-nm excitation and 460-nm emission), and the DNA amount was expressed in nanograms per milliliter (DNA standard curve established with fish sperm DNA [Boehringer]). We checked the correlation between DNA contents and cell numbers using cell suspensions of various densities.
Glutamate concentrations ranging from 1 to 500 μM were used to determine the Km and Vmax for glutamate uptake using a hyperbolic model (Origin 5.0; Microcal, Northampton, Mass.). The specificity of glutamate transport was assessed using 1 mM competitive inhibitor, dl-threo-hydroxyaspartic acid (β-THA), an excitatory amino acid analogue known to have a high apparent affinity for all glial transporters (15). Nonspecific glutamate transport was evaluated at 4°C, conditions under which active transport of glutamate is abolished.
Effect of Tax-1 and TNF-α on glutamate transport.
The plasmids containing one sequence encoding Tax-1–glutathione S-transferase (GST) or GST alone (courtesy of P. Jalinot, Lyon, France) were used to synthesize the corresponding proteins (5). The specific binding of GST with glutathione is used to purify subsequently Tax-1–GST or GST. Recombinant Tax-1–GST was diluted in fresh medium (DMEM Glutamax, 5 mM glucose, 2% FCS) for treatment; an equal volume of sterile water was added to the same medium as a control. Treatments with the protein GST alone served as another control ensuring the specificity of the effect of the recombinant Tax-1–GST protein. Rat recombinant TNF-α (Diaclone Research) was used under the same conditions. Cotreatments, with or without specific antibodies, were made under the same conditions. In treated astrocyte cultures, supernatants were collected at different days posttreatment and assayed for TNF-α using human- or rat-specific kits (CytoScreen ultrasensitive enzyme-linked immunosorbent assay kit; BioSource International, Camarillo, Calif.). In Tax-1-treated cultures, the samples were collected at 24 h posttreatment.
DNA-RNA purification and RT-PCR analysis.
Total RNAs from astrocyte cultures (control or treated, at different days posttreatment) were prepared by solubilization and extraction with RNAzol (Bioprobe, Montreuil/Bois, France) using a protocol adapted from that of Chomczynski and Sacchi (10). The concentration and purity of the extracted RNA were determined spectrophotometrically (Beckman DU-640 instrument). RNA integrity was checked by electrophoresis on denaturating agarose gels, subsequently stained by ethidium bromide.
The primers were designed using the Wisconsin Package version 9.1 (Genetics Computer Group, Madison, Wis.) supporting SRS (Sequence Retrieval System) Indexes version 4.08 (EBI, Hinxton, United Kingdom). The specificity of the primers and internal probes was verified using the GenBank database (European Molecular Biology Laboratory, Heidelberg, Germany). Computer services were available at GIS INFOBIOGEN servers (MESR/AFM, Villejuif, France). The primers designed by Kinoshita and coworkers were used for Tax-1 amplification (31). Table 1 summarizes the selected PCR primers and specific internal probes for GS, GDH, GLAST, GLT-1, TNF-α, GFAP, cyclophilin A (CyP-A), and Tax-1.
TABLE 1.
PCR primer pairs and specific internal probes used for semiquantitative PCRa
| Target | Accession no. | Forward primer | Reverse primer | Internal probe (reverse) | Product size (bp) |
|---|---|---|---|---|---|
| Cyclophilin | M19533 | 135-GACAGCAGAAAACTTTCGTGC-155 | 410-TCCAGCCACTCAGTCTTGG-392 | 229-GGCACATGAATCCTGGAATAAT-208 | 276 |
| Tax-1b | J02029 | 4743-ATCCCGTGGAGACTCCTCAA-4762 | 7379-AACACGTAGACTGGGTATCC-7360 | 5197-AACACCATGGCCCACTTCCC-7334 | 145 |
| GS | M29579 | 157-TGAACAAAGGCATCAAGCAG-176 | 350-GGAGCCTTCAGACTGAAACG-331 | 266-ACGGGTCTTGCAGCGTAG-249 | 194 |
| GDH | X14223 | 1227-CAAGATCATTGCTGAAGGAGC-1247 | 1570-TGTAGGCCAAGCCAGAGTG-1552 | 1449-CTCTTGAACGGACATGAGCA-1430 | 343 |
| GLAST | X63744 | 1475-AAGTATCACAGCCACAGC-1492 | 1488-TCTCTGGTTCATTGTCCTGG-1769 | 1642-AGCACGTTGGTGGTGGTT-1625 | 295 |
| GLT-1 | X67857 | 1511-GCTGCTGGATAGAATGAGAACTTCGG-1536 | 1807-TTCCAAGGTTCTTCCTCAACACTGC-1783 | 1715-GGCATAGACACACTGATTAGAG-1594 | 297 |
| Rat TNF-α | X66539 | 259-TCGAGTGACAAGCCCGTAG-277 | 443-CAGCCTTGTCCCTTGAAGAG-424 | 366-GAGATCCATGCCATTGGC-349 | 166 |
| Human TNF-α | X01394 | 293-CTTCTGCCTGCTGCACTTTGGA-314 | 840-TCCCAAAGTAGACCTGCCCAGA-819 | 515-ATCTCTCAGCTCCACGCCATTGGCCAGGAG-486 | 527 |
Nucleotide numbers are given in the 5′→3′ sense and correspond to those used in the GenBank cDNA sequences.
Viral tax/rex mRNA is double spliced. The second splice occurs between nucleotides 5207 and 7224. The internal probe was designed to cover this second splice junction site, thus increasing the specificity (see reference 31).
mRNAs (1 μg of total denatured RNA, 10 min at 70°C) were reverse transcribed into sense-strand cDNAs using 100 ng of oligo(dT)12–18 (Pharmacia) and 800 U of murine leukemia virus reverse transcriptase (Life Technologies). The reaction proceeded at 42°C for 1.75 h in a 20-μl reaction mix (1× buffer from Life Technologies, 0.5 mM each deoxynucleoside triphosphate, 10 mM dithiothreitol, 40 U of RNAsin from Promega). The RT products (1/20 of total volume for each sample, corresponding to 50 ng of extracted total RNA) were subjected to PCR with final volume of 50 μl (1× buffer, 3 mM MgCl2, 0.2 mM each deoxynucleoside triphosphate, 0.4 mM each specific couple of primers, 2 U of Taq DNA polymerase [Promega]) according to the criteria for semiquantification of mRNAs as defined by Mohler and Butler (41). The PCR was run on a PTC-200 thermocycler (MJ Research, Watertown, Mass.) and consisted of a denaturation step (10 min at 95°C) followed by 25 to 35 cycles of 45-s denaturation (95°C), 45-s annealing (62°C), and 1.75-min elongation (72°C). The optimal number of cycles for each cDNA was 25 for GFAP, 27 for CyP-A, and 28 for GS, GDH, GLAST, GLT-1, and TNF-α. The PCR products were quantified by Southern blotting (separation by 1.8% agarose gel electrophoresis and electroblotting on positively charged nylon membranes; ICN) and hybridization with specific [γ-32P]ATP-5′-end-labeled internal oligonucleotide probes (Table 1). After exposure to an NEN-Reflection autoradiographic film, the specific labeled bands were cut out and counted on a liquid scintillation analyzer. Slot blot (Bio-Rad) analysis confirmed the reliability of our Southern blot procedure. Tax-1 mRNA was detected using the same RT-PCR procedure (35 cycles). Genomic DNA contamination was assessed by the same PCR procedure but without RT. In rat astrocyte cultures, the absence of residual C91PL T lymphocytes was checked by PCR using human-specific GAPDH primers (day 4 postcontact). RT-PCR on HTLV-1-infected human cells were run on DNase-treated total RNA as previously described (1). For correlation analyses between transcripts encoding Tax-1, GS, and GDH, the samples were amplified in one PCR session, and the amounts of mRNAs were evaluated using the same three radiolabeled probes for all experiments.
Immunocytochemistry and TNF-α assays.
HTLV-1-specific proteins were detected by indirect immunofluorescence using a polyclonal rabbit antiserum raised against the viral transactivator Tax-1 (NIH 467). Human and rat astrocytes were cultured in Labtek chambers (Nalge Nunc, Naperville, Ill.) and then fixed with acetone (10 min at −20°C) on various days postcontact. The primary antibody incubation was in phosphate-buffered saline for 45 min at 37°C. Fluorescein isothiocyanate-labeled anti-rabbit immunoglobulin antibodies (Biosys) were used as second antibodies (30 min at room temperature). TNF-α was assayed in the collected culture supernatants (stored at −20°C) using EIA kits specific for rat TNF-α (CytoScreen ultrasensitive, 0.7 pg/ml; BioSource International) or human TNF-α (CytoScreen).
Data analysis.
Results are expressed as the mean ± SEM. Student's t test was used to compare the results obtained by treated versus naive cultures.
RESULTS
Experimental design.
The effects on astrocyte cultures of transient coculture with HTLV-1-infected T cells were investigated using persistently infected producer (C91PL) or nonproducer (C8166/45) T-cell lines. In most of the study, rat astrocytes were used because of the difficulty of obtaining normal human material; nevertheless, the relevance of the data obtained in rat cells was confirmed using a human cell line, Dev, exhibiting the astrocytic phenotype and human primary astrocytes obtained from fetal cortex (one experiment). Infected T cells and astrocytes were cocultured for 20 h. These T cells were carefully eliminated, as verified by the absence of human-specific GAPDH and TNF-α by RT-PCR and of CD4+ T cells by fluorescence-activated cell sorting. No cell loss occurred in the cultures tested, as shown by cell counting and quantitation of protein or DNA. At various intervals postcontact, we studied glutamate uptake and expression of mRNAs encoding glutamate transporters (GLT-1 and GLAST), GS, and GDH. The influence of soluble factors was evaluated using two-compartment culture in which infected T cells were placed in inserts, thereby avoiding cell-to-cell contact. To identify the signaling molecules mediating the observed effects, astrocyte cultures were treated by recombinant Tax-1 and TNF-α, with or without their specific antibodies.
Glutamate transport after transient contact with HTLV-1-infected T cells.
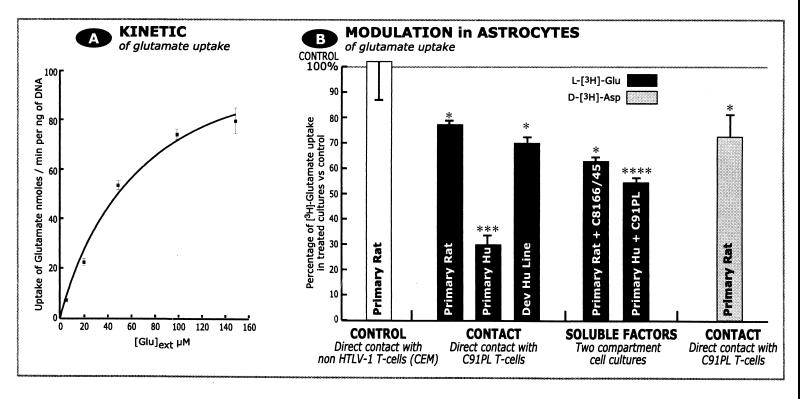
Glutamate uptake was estimated by the concentration of radiolabeled glutamate accumulating within astrocytes, once transported from culture medium. We first determined the optimal conditions for glutamate uptake in naive astrocyte cultures obtained from rat cortex (n = 4). The uptake velocity was linear for at least 20 min for extracellular glutamate concentrations of 100 to 200 μM. The glutamate concentration in the culture medium under investigation can be considered constant, as less than 3% of radiolabeled glutamate was cleared from the extracellular space. Thereafter, we determined the kinetic properties of tritiated l-glutamate uptake with respect to the extracellular concentration of glutamate at 10 min by measuring the rate of radiolabeled glutamate accumulation in astrocytes. Curve fitting and subsequent statistical analyses showed that glutamate transport was compatible with a hyperbolic model characterized by a Km of 72 ± 16 μM and a Vmax of 121 ± 12 nmol of glutamate/min/ng of DNA (mean ± SEM, n = 3, P < 0.05) (Fig. 1A). Hill plot analysis using linear regression gave Hill coefficients of 0.9 to 1, which is consistent with a single-site transport system. The specificity of glutamate transport was verified by evaluating (i) the passive diffusion of glutamate (accumulation at 4°C) into the cellular compartment (1.8% ± 0.1% of the mean value obtained at 37°C, n = 3, extracellular glutamate concentration of 100 μM) and (ii) the degree of inhibition of glutamate transport by the competitive inhibitor, β-THA (1 mM at 37°C; 97.1% ± 0.1% inhibition, n = 6, P < 0.005). These results are in agreement with those published for cultured astrocytes obtained from rat cortices (see review by Robinson and Dowd [51]). Thereafter, glutamate uptake was measured with extracellular concentration of 100 μM glutamate unless otherwise specified. In addition, in situ autoradiography of tritiated d-aspartate uptake indicated that all cultured astrocytes had the capacity to uptake glutamate (not shown). We also determined the affinity and maximal velocity of glutamate uptake in human Dev cell cultures containing around 20% of cells of astrocytic phenotype (Km = 14.6 ± 2.7 μM and Vmax = 5.1 ± 0.2 nmol glutamate/min/ng of DNA; n = 3).
FIG. 1.
HTLV-1-infected T lymphocytes reduce glutamate uptake in rat and human astrocytes via released soluble factors. (A) Kinetic parameters of tritiated l-glutamate uptake by astrocytes. Uptake fits to a hyperbolic model. Each point represents a mean obtained from three experiments. (B) Glutamate uptake was assayed using tritiated l-glutamate (black bars) or its nonmetabolizable analogue tritiated d-aspartate (grey bars). Glutamate uptake by astrocytes was decreased by all treatments (direct contact with infected T cells or use of inserts containing infected T cells, allowing only soluble factors to diffuse) except contact with the noninfected CD4 T lymphocytes, CEM (left). Human primary astrocytes were more sensitive than rat astrocytes or a human cell line. Results are expressed as percentage of glutamate uptake in treated versus naive astrocytes. Numbers of experiments, from left to right: 3, 9, 3, 3, 3, 3, 9. Student's t test was used to evaluate statistical significance. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.005; ∗∗∗∗, P < 0.001.
Transient contact of rat primary astrocytes with HTLV-1-infected T cells (C91PL) reduced astrocytic accumulation of tritiated l-glutamate in all cases examined (68.5% ± 1.8% of control value, n = 9, P < 0.05) (Fig. 1B). This reduction was consistently observed within 1 week after contact with C91PL T cells. Contact with noninfected T lymphocytes (CEM cell line) did not significantly affect glutamate accumulation (97.7% ± 17.2% of control value, n = 3) (Fig. 1B). A reduction in the intracellular accumulation of glutamate generally reflects decreased uptake, rather than increased catabolism of glutamate by GS or GDH, the resulting glutamate metabolites being released into the extracellular space. To discount the latter possibility, tritiated d-aspartate was used instead of tritiated l-glutamate; this structural analogue of l-glutamate is transported in the same way but is not catabolized by GS and GDH. Using d-aspartate, we observed a reduction similar to that seen for l-glutamate (61.1% ± 9.9% of control value, n = 9, P < 0.05) (Fig. 1B), showing that the reduced glutamate accumulation is not due to increased intracellular catabolism of l-glutamate by GS or GDH. The relevance of the decrease in glutamate accumulation in rat astrocytes after contact with infected T cells was confirmed by the decrease observed in human astrocytic cultures established from fetal brain and Dev cell line (30% ± 4% and 70% ± 3% of control values, respectively, n = 3 for both) (Fig. 1B). In control experiments, we used a noninfected CD4+ T-cell line (CEM) with or without prior activation (phorbol myristate acetate and ionomycin). Such contact had no effect on glutamate uptake in human Dev cells (104% ± 2% of control value with inactivated CEM cells, n = 3; 97% ± 6% with CEM cells activated by phorbol myristate acetate and ionomycin, n = 3). Inconsistent results were obtained in similar experiments conducted with primary T lymphocytes obtained from healthy humans (not shown).
In rat astrocytes transiently exposed to HTLV-1-infected T cells, the decrease in glutamate uptake was always associated with downregulation of the mRNAs coding for glial glutamate transporters as evaluated by RT-PCR. At 1 week postcontact, GLT-1 and GLAST mRNA levels were reduced by 31 to 76% (four experiments) and by 20 to 45% (three experiments), respectively.
Involvement of soluble factors released by HTLV-1-infected T lymphocytes.
As HTLV-1-infected T lymphocytes are known to continuously express the viral protein Tax-1 and cytokines (35, 56), we examined the paracrine effect of soluble factors released by HTLV-1-infected T cells. Primary astrocyte cultures established from rat or human fetal cortex were cocultivated in two separate compartments with the C8166/45 T-cell line. We have previously verified the high level of Tax-1 expression by immunocytochemistry and RT-PCR in this cell line. These T lymphocytes were seeded in inserts and placed over the astrocyte monolayer for 96 h without direct T-cell astrocyte contact. Such transient exposure decreased glutamate uptake by 37.4% ± 2.3% (n = 3, P < 0.05) in rat astrocyte cultures (Fig. 1B). The relevance of this result obtained in rat was verified in human astrocyte cultures with insert containing C91PL T lymphocytes: glutamate uptake was decreased by 44.1% ± 2.0% (n = 3, P < 0.001) (Fig. 1B). These data indicate that HTLV-1-infected T lymphocytes, whether or not they produce virus, are able to alter glutamate uptake in astrocytes via soluble factors.
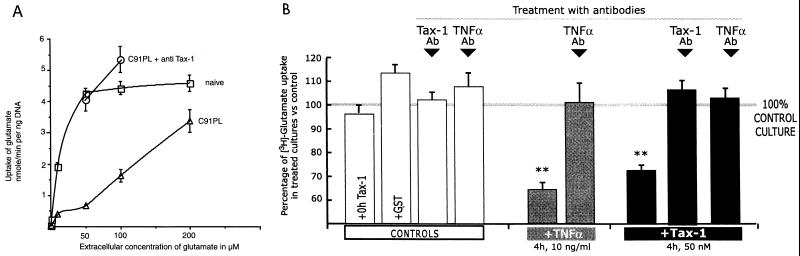
The possible implication of Tax-1 was assessed using hybridoma B cells secreting antibodies against Tax-1. In these experiments, human Dev cells were directly exposed to HTLV-1-infected T cells (C91PL), while hybridoma cells were seeded in culture inserts (106 cells) above the Dev cell monolayer. As shown in Fig. 2A, coculture with anti-Tax-1 hybridoma completely reversed the effect of infected T cells on glutamate uptake in Dev cells. Note that glutamate uptake in human Dev cells was more dramatically decreased for extracellular concentrations of glutamate smaller than 100 μM.
FIG. 2.
(A) Implication of Tax-1 in the decrease in glutamate uptake induced by HTLV-1-infected T cells. Glutamate uptake was consistently decreased in human Dev cells after transient contact with infected T cells (C91PL) compared to naive cells. C91PL + anti Tax-1, Dev cells cocultured with inserts containing hybridoma B cells secreting anti-Tax-1 antibodies. (B) Tax-1 reduces glutamate uptake by rat astrocytes via TNF-α. Treatment by recombinant rat TNF-α (grey bars) or Tax-1–GST (black bars) significantly reduced glutamate uptake. The effects of recombinant proteins were specific, as they were abolished by pretreatment with their corresponding antibodies (Ab). Moreover, the reduction in glutamate uptake induced by Tax-1 was suppressed by pretreatment with anti-TNF-α antibody (rightmost column). Controls show the lack of effect of GST protein, anti-TNF-α antibody, or anti-Tax-1 antibody when tested alone on glutamate uptake. Tritiated l-glutamate uptake by treated astrocytes was expressed as percentage of tritiated l-glutamate uptake by naive astrocytes. ∗∗, P < 0.01.
Tax-1 protein decreases glutamate uptake by astrocytes via TNF-α.
The potent transactivator protein Tax-1 may be one of the soluble factors (3, 5, 11) responsible for the effect of HTLV-1-infected T lymphocytes on glutamate uptake by astrocytes. To determine the effect of extracellular Tax-1 on glutamate transport, rat astrocytes were treated with the recombinant protein Tax-1–GST (5). The control was the immediate rinsing with the medium containing recombinant Tax-1 (25 nM, i.e., 1.5 μg of Tax-1–GST/ml, 0-h treatment), which showed no difference in glutamate uptake compared to naive astrocytes (96% ± 7% of control value, n = 3) (Fig. 2B). Glutamate uptake was decreased by treatment with 25 and 50 nM Tax-1 (2, 4, 8, 16, and 24 h) but not with the protein GST alone (113% ± 7% of control value, n = 4). The maximal reduction (63% ± 4% of control value, n = 9, P < 0.01) (Fig. 2B) in glutamate transport was obtained after 4 h of treatment with 50 nM Tax-1 (3 μg of Tax-1–GST/ml). The specificity of this effect was confirmed using anti-Tax-1 antibodies (NIH 467, diluted 1/100), which completely neutralized the effect of Tax-1 protein (106% ± 6% of control value, n = 3) (Fig. 2B) at its optimal concentration (Tax-1–GST at 3 μg/ml, 4 h). The specificity of the blockade by anti-Tax-1 antibody was checked using irrelevant antibodies (63% ± 6% of control value, n = 3, P < 0.01).
We then examined whether the effect of Tax-1 may be mediated by TNF-α secreted by astrocytes, as Tax-1 has been shown to induce cultured astrocytes to secrete inflammatory cytokines, including TNF-α (11, 40). Treatment of rat astrocytes with 50 nM Tax-1 (3 μg of Tax-1–GST/ml, 4 h) induced a secretion of TNF-α in these astrocytes (2.6 ± 0.2 pg/ml, n = 3), whereas TNF-α was not secreted with the protein GST alone (n = 6). Treatment (for 4 h) of rat astrocytes with exogenous rat recombinant TNF-α (10 ng/ml; Diaclone Research) reduced glutamate uptake by 35% ± 5% (n = 3, P < 0.01; range, 20 to 55%) (Fig. 2B). The specificity of the effect was shown using anti-TNF-α antibodies, which prevented the decrease (101% ± 8% of control value, n = 3). Finally, Tax-1 seems to decrease glutamate uptake in astrocytes via TNF-α, as anti-TNF-α antibodies virtually suppressed (103% ± 5% of control value, n = 3) the effect of Tax-1 on glutamate transport (Tax-1–GST [3 μg/ml] and anti-TNF-α antibody [1/100], 4 h) (Fig. 2B).
The metabolic fate of glutamate taken up by astrocytes largely depends on the catabolic enzymes, GS and GDH (see the introduction). Therefore, the effect of HTLV-1-infected T cells (C91PL) on the expression of GS and GDH was investigated by RT-PCR in rat astrocytes transiently exposed to HTLV-1-infected T cells at a period when glutamate uptake was reduced (n = 3). GS and GDH mRNAs were upregulated and downregulated, respectively (Table 2). The magnitudes of these opposite changes were inversely correlated. These changes were also correlated with the level of Tax-1 transcripts expressed by cultured cells (note that 2 to 5% of astrocytes were expressing Tax-1 immunoreactivity, as shown below).
TABLE 2.
Expression of GS, GDH, and Tax-1 transcripts in astrocytes after contact with infected T-cells (C91PL)
| Expt | % Change with respect to naive cultures
|
Tax mRNAa | |
|---|---|---|---|
| GS mRNA | GDH mRNA | ||
| 1 | +20 | −10 | 0.09 |
| 2 | +22 | −33 | 0.2 |
| 3 | +37 | −52 | 1.64 |
Expressed in arbitrary units after normalization with CyP-A mRNA expression.
Prolonged expression of Tax-1 and TNF-α.
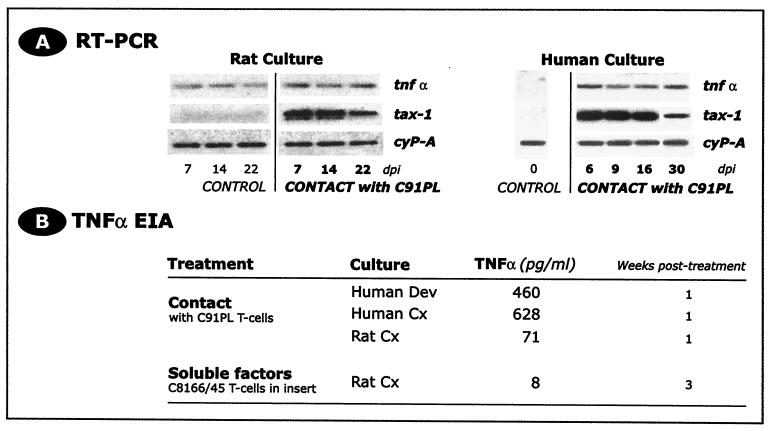
As we observed a long-term decrease in glutamate uptake induced by infected T cells (79.4% ± 4.1% of control value, n = 4, day 22, P < 0.05), we examined the expression of Tax-1 and TNF-α in primary astrocytes and the Dev cell line over the month following contact with the T-cell line C91PL. mRNA encoding Tax-1 (Fig. 3A) and Tax-1 protein were detected as early as 2 days postcontact and were persistently expressed throughout the period of observation, but without production of viral particles (55a). We found that 2 to 5% of rat astrocytes and about 20% of human Dev cells showed Tax-1 immunoreactivity during long-term cultures. Such persistent expression of Tax-1 was closely associated with a sustained secretion of the proinflammatory cytokine TNF-α. Within the first week following transient contact with the HTLV-1-infected T-cell line C91PL, TNF-α (Fig. 3B) was secreted in the culture supernatants from the human Dev cell line (460 pg/ml) and human primary astrocytes (628 pg/ml), and TNF-α mRNA was detected in Dev cells until 30 days postcontact (Fig. 3A). In rat primary astrocytes, TNF-α secretion was observed within the first week postcontact (71 pg/ml), and TNF-α transcripts were detected at least until 22 days postcontact. Stimulation of rat astrocyte cultures by HTLV-1-infected T lymphocytes also expressing Tax-1 (C8166/45, placed for 96 h in cell culture inserts over the astrocyte monolayer) induced sustained secretion of TNF-α by astrocytes at least for 3 weeks postcontact (8 pg/ml) (Fig. 3B). Control cultures of rat and human astrocytes with noninfected CD4+ T lymphocytes (CEM cell line) did not result in TNF-α secretion. These data indicate that TNF-α secretion is associated with the presence of Tax-1-expressing cells, regardless of their lymphocytic or astrocytic phenotype.
FIG. 3.
(A) Tax-1 is persistently expressed in HTLV-1-infected cultures of astrocytes, at least until 22 and 30 days postinfection (dpi) in rat and human cells, respectively. Tax-1 mRNA was detected in astrocyte cultures after transient contact with HTLV-1-infected T lymphocytes (Southern blotting of RT-PCR products hybridized with a specific internal radiolabeled probe). CyP-A was used as the quality control for RT-PCR. (B) The proinflammatory cytokine TNF-α was secreted following transient treatment of rat and human astrocyte cultures with infected T cells (direct contact or use of insert allowing only soluble factors to diffuse). Note that TNF-α was not detected in control cultures (naive astrocytes and astrocytes transiently exposed to noninfected CD4+ T lymphocytes [CEM cell line]. TNF-α was assayed using rat- or human-specific EIA kits.
DISCUSSION
Examination of TSP/HAM patients has consistently revealed invasion of the CNS by T cells harboring provirus and expressing the viral protein Tax-1 (42, 43). These data have led to a consensus on the importance of CNS infiltration by HTLV-1-infected T cells in the physiopathology of TSP/HAM, although many subsequent events remain unaddressed. In this study, we used transient coculture of astrocytes with HTLV-1-infected T lymphocytes to mimic CNS invasion by infected T lymphocytes and examined the functional consequence on astrocytes. Indeed, an effective operation of astrocytes is critical in brain homeostasis and neural cell survival. These glial cells are essential managers of a variety of metabolic pathways involved in energy storage, ionic equilibrium, and the control of extracellular concentrations of various neurotransmitters. In particular, the main excitatory amino acid glutamate is specifically taken up and then catabolized by astrocytes. Our data show that glutamate management is significantly impaired in astrocytes following transient contact with infected T cells, whether or not they produce virus. Such astrocyte impairments triggered by HTLV-1 may have deleterious effects on neighboring cells by affecting their electrical activity and energy metabolism (55). In human astrocytes, the effects were even greater than in rat cells, confirming the clinical relevance of our model.
Activation of astrocytes by infected T lymphocytes.
Transient contact of cultured astrocytes with infected T cells did not affect the amount of DNA or the number of cells. There was even an increase in the expression of astrocyte-specific proteins, such as GFAP and GS, indicating that the presence of HTLV-1-infected T cells did not promote a general shutoff, but rather induced factors targeting specific astrocytic functions. Marked activation of astrocytes was observed after transient contact with HTLV-1-infected T lymphocytes (19). This activation was characterized by the sustained expression of the proinflammatory cytokine TNF-α and upregulation of the gliofilament protein GFAP. These changes in astrocytes produced by infected T lymphocytes are typical of astrocytes engaging a variety of regulatory processes in response to several types of insults (4).
Glutamate uptake by astrocytes.
Glutamate uptake by astrocytes is crucial in the regulation of its extracellular concentration and intracellular metabolism (glutamine synthesis, ammonia detoxification, and energy/cell respiration). Glutamate accumulation was consistently reduced in astrocyte cultures treated with HTLV-1-infected T lymphocytes. This decrease was due to a change in the net transport activity rather than to an accelerated turnover of glutamate catabolism (e.g., by increased GS activity), as a similar decrease was observed using tritiated d-aspartate, which is not metabolized by GS and GDH.
Glutamate is mainly transported into astrocytes via two high-affinity, sodium-dependent transporters (6, 28), GLAST/EAAT1 and GLT-1/EAAT2. Analysis of the expression of their messengers shows a significant decrease in both, occurring as early as 3 days after contact with HTLV-1-producing T lymphocytes. The decreased glutamate transport probably results from the downregulation of genomic expression of glial glutamate transporters, as glutamate uptake was found to correlate with the expression of the transporters' messengers (16, 52). At a more functional level, such decreased glutamate uptake by astrocytes should increase the extracellular level of glutamate, which in turn may perturb neuronal transmission (e.g., a decreased signal-to-noise ratio or temporal detuning) or even exert an excitotoxic effect. Another retrovirus, feline immunodeficiency virus, has also been shown to decrease glutamate uptake in cultured astrocytes. In this model, the effect was interpreted as a result of direct infection of astrocytes (67), whereas with HTLV-1, the decreased glutamate uptake was observed even after contact with a noninfectious HTLV-1-infected T-cell line.
Implication of soluble factors.
The possible involvement of soluble factors in the effects of HTLV-1-infected T cells was verified using transient cocultures with astrocytes and HTLV-1-infected T lymphocytes in two distinct compartments, which resulted in a reduction of glutamate uptake similar to that observed with single compartment coculture (direct cell-to-cell contact). This raised the possibility that infected T lymphocytes may impair glutamate uptake and catabolism by bystander effects via soluble factors, such as the viral protein Tax-1 and cytokines. In our model, we clearly showed that human as well as rat astrocytes secrete TNF-α after transient contact with infectious or noninfectious HTLV-1-infected T-cell lines (C91PL or C8166/45), both expressing Tax-1. The addition of recombinant Tax-1 protein to the culture medium was also able to induce TNF-α secretion. Involvement of Tax-1 and TNF-α in the effects of HTLV-1 infection on glutamate uptake was further substantiated by the decreased glutamate uptake after application of recombinant Tax-1 or TNF-α. Coincubation with both Tax-1 and anti-TNF-α antibody abolished the effect of Tax-1 on glutamate transport, providing evidence that TNF-α acts as the mediator of the Tax-1-induced decrease in glutamate uptake. These findings are in agreement with the induction of TNF-α expression by Tax-1 (11) and the decreased glutamate uptake induced by TNF-α in astrocytes (14). Thus, we can assume that the signaling cascade leading to the decreased uptake of glutamate in astrocytes successively involves two paracrine mediators, Tax-1 and TNF-α.
The critical role of TNF-α in the physiopathology of TSP/HAM is also suggested by the presence of this cytokine in astrocytes and T lymphocytes within CNS lesions of TSP/HAM patients (57). The molecular events downstream of TNF-α are not precisely known for its effects on glutamate uptake. But TNF-α may affect other molecular or cellular processes, such as migration and activation of lymphocytes, be toxic for oligodendrocytes, and alter the expression of other cytokines (2, 45). The present work underscores the importance of examining nonclassical effects of TNF-α (17), other than its well-documented ability to enhance inflammatory processes.
Glutamate catabolism and energy metabolism.
Effective inactivation of glutamate uptaken by astrocytes is achieved by the glial enzymes GS and GDH. Therefore, the functional outcome of the decreased glutamate accumulation induced by HTLV-1-infected T lymphocytes must be considered by taking into account the ability of astrocytes to metabolize glutamate via GS and GDH. In this regard, we show that transient contact with HTLV-1-infected T cells expressing Tax-1 (C91PL and C8166/45) led to imbalanced expression of glutamate-glutamine cycle enzymes in rat (this study) and human (1) astrocytes (increase for GS and decrease for GDH). We have previously shown that Tax-1 protein transactivates the GS gene promoter (1). In the present study, we further show that the magnitude of these opposite changes correlates with the level of viral Tax-1 mRNA, demonstrating the critical role of Tax-1-expressing T lymphocytes and astrocytes in the effects observed. The Tax-1-induced imbalance between GS and GDH is expected to preferentially drive glutamate catabolism toward the formation of glutamine, rather than toward the TCA cycle. Decreased GDH expression may result in insufficient energetic stores in astrocytes, as the mitochondrial enzyme GDH primarily catabolizes the metabolic pool of glutamate to generate α-ketoglutarate, which passes into the TCA cycle (48). Such effect on the energy store may be enhanced by the decreased intracellular pool of glutamate following its impaired uptake. Thus, astrocytes represent an important target for HTLV-1-infected T lymphocytes infiltrating the CNS, possibly depleting energy precursors for the surrounding cells, in particular neurons and oligodendrocytes, which need to be continuously fed with essential metabolites (lactate and α-ketoglutarate) released by astrocytes (24).
Functional significance.
The present study demonstrates that HTLV-1-infected T cells impair the uptake and metabolism of glutamate by astrocytes. Although the in vivo significance of our results must be evaluated cautiously, these data suggest that Tax-1 initiates a divergent and high-gain transduction cascade which may pervade the entire CNS. Indeed, at each step of this cascade, the input signal can affect several targets which, in turn, can amplify the output signal. The magnitude of the resulting bystander effects is probably enhanced by factors secreted by infected T cells, but also by astrocytes (cytokines, chemokines, integrins, and metalloproteinases and their endogenous inhibitors). These factors probably affect the number of infected/activated T cells infiltrating the CNS and the extent of migration of these cells through the CNS parenchyma (61, 62, 64). Once in the CNS, Tax-expressing T lymphocytes may persist and expand within the CNS, as Tax is able to prevent T cells from undergoing apoptosis (44) that may be stimulated by neighboring astrocytes (32). In conclusion, our results demonstrate a great susceptibility of astrocytes vis-à-vis HTLV-1-infected T cells, which significantly alters the metabolism of an important excitatory amino acid, glutamate. Clinically, current knowledge on glutamate neurotransmission and metabolism suggests that astrocytic glutamate metabolism may be an effective therapeutic target in TSP/HAM.
ACKNOWLEDGMENTS
This work was financially supported by French research associations for multiple sclerosis (ARSEP and LFSEP), ANRS, Sidaction, and INSERM. R.S. was a recipient of fellowships from ARSEP and LFSEP.
REFERENCES
- 1.Akaoka H, Hardin-Pouzet H, Bernard A, Verrier B, Belin M F, Giraudon P. Imbalanced expression of glutamate-glutamine cycle enzymes induced by human T-cell lymphotropic virus type 1 Tax protein in cultivated astrocytes. J Virol. 1996;70:8727–8736. doi: 10.1128/jvi.70.12.8727-8736.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barone F C, Feuerstein G Z. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Beimling P, Moelling K. Isolation and characterization of the tax protein of HTLV-I. Oncogene. 1989;4:511–516. [PubMed] [Google Scholar]
- 4.Belin M F, Didier-Bazes M, Akaoka H, Hardin-Pouzet H, Bernard A, Giraudon P. Changes in astrocytic glutamate catabolism enzymes following neuronal degeneration or viral infection. Glia. 1997;21:154–161. doi: 10.1002/(sici)1098-1136(199709)21:1<154::aid-glia17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 5.Beraud C, Lombard-Platet G, Michal Y, Jalinot P. Binding of the HTLV-I Tax1 transactivator to the inducible 21 bp enhancer is mediated by the cellular factor HEB1. EMBO J. 1991;10:3795–3803. doi: 10.1002/j.1460-2075.1991.tb04949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billups B, Rossi D, Oshima T, Warr O, Takahashi M, Sarantis M, Szatkowski M, Attwell D. Physiological and pathological operation of glutamate transporters. Prog Brain Res. 1998;116:45–57. doi: 10.1016/s0079-6123(08)60429-x. [DOI] [PubMed] [Google Scholar]
- 7.Bittigau P, Ikonomidou C. Glutamate in neurologic diseases. J Child Neurol. 1997;12:471–485. doi: 10.1177/088307389701200802. [DOI] [PubMed] [Google Scholar]
- 8.Buckle G J, Hafler D A, Hollsberg P. HTLV-I-induced T-cell activation. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13(Suppl. 1):S107–S113. doi: 10.1097/00042560-199600001-00018. [DOI] [PubMed] [Google Scholar]
- 9.Cartier L M, Cea J G, Vergara C, Araya F, Born P. Clinical and neuropathological study of six patients with spastic paraparesis associated with HTLV-I: an axomyelinic degeneration of the central nervous system. J Neuropathol Exp Neurol. 1997;56:403–413. doi: 10.1097/00005072-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 11.Cowan E P, Alexander R K, Daniel S, Kashanchi F, Brady J N. Induction of tumor necrosis factor alpha in human neuronal cells by extracellular human T-cell lymphotropic virus type 1 Tax. J Virol. 1997;71:6982–6989. doi: 10.1128/jvi.71.9.6982-6989.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drejer J, Larsson O M, Schousboe A. Characterization of l-glutamate uptake into and release from astrocytes and neurons cultured from different brain regions. Exp Brain Res. 1982;47:259–269. doi: 10.1007/BF00239385. [DOI] [PubMed] [Google Scholar]
- 13.Erecinska M, Silver I A. Metabolism and role of glutamate in mammalian brain. Prog Neurobiol. 1990;35:245–296. doi: 10.1016/0301-0082(90)90013-7. [DOI] [PubMed] [Google Scholar]
- 14.Fine S M, Angel R A, Perry S W, Epstein L G, Rothstein J D, Dewhurst S, Gelbard H A. Tumor necrosis factor alpha inhibits glutamate uptake by primary human astrocytes. Implications for pathogenesis of HIV-1 dementia. J Biol Chem. 1996;271:15303–15306. doi: 10.1074/jbc.271.26.15303. [DOI] [PubMed] [Google Scholar]
- 15.Garlin A B, Sinor A D, Sinor J D, Jee S H, Grinspan J B, Robinson M B. Pharmacology of sodium-dependent high-affinity l-[3H]glutamate transport in glial cultures. J Neurochem. 1995;64:2572–2580. doi: 10.1046/j.1471-4159.1995.64062572.x. [DOI] [PubMed] [Google Scholar]
- 16.Gegelashvili G, Schousboe A. High affinity glutamate transporters: regulation of expression and activity. Mol Pharmacol. 1997;52:6–15. doi: 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- 17.Gelbard H A, Dzenko K A, DiLoreto D, del Cerro C, del Cerro M, Epstein L G. Neurotoxic effects of tumor necrosis factor alpha in primary human neuronal cultures are mediated by activation of the glutamate AMPA receptor subtype: implications for AIDS neuropathogenesis. Dev Neurosci. 1993;15:417–422. doi: 10.1159/000111367. [DOI] [PubMed] [Google Scholar]
- 18.Gessain A, Barin F, Vernant J C, Gout O, Maurs L, Calender A, de The G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;ii:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 19.Giraudon P, Bernard A, Malcus C, Dufay N, Desgranges C, Belin M F. Retroviral infection (HTLV-I) induces cytokine-regulated immunomodulation and cytotoxicity of medulloblastoma cells. J Neuropathol Exp Neurol. 1995;54:165–174. doi: 10.1097/00005072-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Giraudon P, Buart S, Bernard A, Thomasset N, Belin M F. Extracellular matrix-remodeling metalloproteinases and infection of the central nervous system with retrovirus human T-lymphotropic virus type I (HTLV-I) Prog Neurobiol. 1996;49:169–184. doi: 10.1016/0301-0082(96)00017-2. [DOI] [PubMed] [Google Scholar]
- 21.Giraudon P, Dufay N, Hardin H, Reboul A, Tardy M, Belin M F. Differentiation of a medulloblastoma cell line towards an astrocytic lineage using the human T lymphotropic retrovirus-1. Neuroscience. 1993;52:1069–1079. doi: 10.1016/0306-4522(93)90553-r. [DOI] [PubMed] [Google Scholar]
- 22.Giraudon P, Thomasset N, Bernard A, Verrier B, Belin M F. Induction of MMP9 (92 kDa gelatinase) activity and expression of tissue inhibitor of metalloproteinase-2 mRNA (TIMP-2) in primitive neuroectodermal cells infected with retrovirus HTLV-I. Eur J Neurosci. 1995;7:841–848. doi: 10.1111/j.1460-9568.1995.tb01071.x. [DOI] [PubMed] [Google Scholar]
- 23.Giraudon P, Vernant J C, Confavreux C, Belin M F, Desgranges C. Matrix metalloproteinase 9 (gelatinase B) in cerebrospinal fluid of HTLV-1 infected patients with tropical spastic paraparesis. Neurology. 1998;50:1920. doi: 10.1212/wnl.50.6.1920. [DOI] [PubMed] [Google Scholar]
- 24.Hamprecht B, Dringen R. Energy metabolism. In: Kettenmann H, Ransom B R, editors. Neuroglia. New York, N.Y: Oxford University Press; 1995. pp. 473–487. [Google Scholar]
- 25.Ijichi S, Izumo S, Eiraku N, Machigashira K, Kubota R, Nagai M, Ikegami N, Kashio N, Umehara F, Maruyama I, et al. An autoaggressive process against bystander tissues in HTLV-I-infected individuals: a possible pathomechanism of HAM/TSP. Med Hypotheses. 1993;41:542–547. doi: 10.1016/0306-9877(93)90111-3. [DOI] [PubMed] [Google Scholar]
- 26.Jeffery K J, Usuku K, Hall S E, Matsumoto W, Taylor G P, Procter J, Bunce M, Ogg G S, Welsh K I, Weber J N, Lloyd A L, Nowak M A, Nagai M, Kodama D, Izumo S, Osame M, Bangham C R. HLA alleles determine human T-lymphotropic virus-I (HTLV-I) proviral load and the risk of HTLV-I-associated myelopathy. Proc Natl Acad Sci USA. 1999;96:3848–3853. doi: 10.1073/pnas.96.7.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jennings M T, Jennings D L, Ebrahim S A, Johnson M D, Turc-Carel C, Philip T, Philip I, Lapras C, Shapiro J R. In vitro karyotypic and immunophenotypic characterisation of primitive neuroectodermal tumours: similarities to malignant gliomas. Eur J Cancer. 1992;28A:762–766. doi: 10.1016/0959-8049(92)90111-e. [DOI] [PubMed] [Google Scholar]
- 28.Kanai Y. Family of neutral and acidic amino acid transporters: molecular biology, physiology and medical implications. Curr Opin Cell Biol. 1997;9:565–572. doi: 10.1016/s0955-0674(97)80035-x. [DOI] [PubMed] [Google Scholar]
- 29.Kanai Y, Trotti D, Nussberger S, Hediger M A. The high affinity glutamate transporter family: structure, function and physiological relevance. In: Reith M E A, editor. Neurotransmitter transporters. Totowa, N.J: Humana Press; 1997. pp. 151–170. [Google Scholar]
- 30.Kimelberg H K, Goderie S K, Conley P A, Higman S, Goldschmidt R, Amundson R H. Uptake of [3H]serotonin and [3H]glutamate by primary astrocyte cultures. I. Effects of different sera and time in culture. Glia. 1992;6:1–8. doi: 10.1002/glia.440060102. [DOI] [PubMed] [Google Scholar]
- 31.Kinoshita T, Shimoyama M, Tobinai K, Ito M, Ito S, Ikeda S, Tajima K, Shimotohno K, Sugimura T. Detection of mRNA for the tax1/rex1 gene of human T-cell leukemia virus type I in fresh peripheral blood mononuclear cells of adult T-cell leukemia patients and viral carriers by using the polymerase chain reaction. Proc Natl Acad Sci USA. 1989;86:5620–5624. doi: 10.1073/pnas.86.14.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohji T, Tanuma N, Aikawa Y, Kawazoe Y, Suzuki Y, Kohyama K, Matsumoto Y. Interaction between apoptotic cells and reactive brain cells in the central nervous system of rats with autoimmune encephalomyelitis. J Neuroimmunol. 1998;82:168–174. doi: 10.1016/s0165-5728(97)00198-7. [DOI] [PubMed] [Google Scholar]
- 33.Kubota R, Umehara F, Izumo S, Ijichi S, Matsumuro K, Yashiki S, Fujiyoshi T, Sonoda S, Osame M. HTLV-I proviral DNA amount correlates with infiltrating CD4+ lymphocytes in the spinal cord from patients with HTLV-I-associated myelopathy. J Neuroimmunol. 1994;53:23–29. doi: 10.1016/0165-5728(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 34.Lehky T J, Fox C H, Koenig S, Levin M C, Flerlage N, Izumo S, Sato E, Raine C S, Osame M, Jacobson S. Detection of human T-lymphotropic virus type I (HTLV-I) tax RNA in the central nervous system of HTLV-I-associated myelopathy/tropical spastic paraparesis patients by in situ hybridization. Ann Neurol. 1995;37:167–175. doi: 10.1002/ana.410370206. [DOI] [PubMed] [Google Scholar]
- 35.Lindholm P F, Reid R L, Brady J N. Extracellular Tax1 protein stimulates tumor necrosis factor beta and immunoglobulin kappa light chain expression in lymphoid cells. J Virol. 1992;66:1294–1302. doi: 10.1128/jvi.66.3.1294-1302.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattson D H, McFarlin D E, Mora C, Zaninovic V. Central-nervous-system lesions detected by magnetic resonance imaging in an HTLV-1 antibody positive symptomless individual. Lancet. 1987;ii:49. doi: 10.1016/s0140-6736(87)93091-1. [DOI] [PubMed] [Google Scholar]
- 37.McDonald J W, Althomsons S P, Hyrc K L, Choi D W, Goldberg M P. Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nat Med. 1998;4:291–297. doi: 10.1038/nm0398-291. [DOI] [PubMed] [Google Scholar]
- 38.McKay R, Renfranz P, Cunningham M. Immortalized stem cells from the central nervous system. C R Acad Sci Ser III. 1993;316:1452–1457. [PubMed] [Google Scholar]
- 39.McKenna M C, Tildon J T, Stevenson J H, Huang X. New insights into the compartmentation of glutamate and glutamine in cultured rat brain astrocytes. Dev Neurosci. 1996;18:380–390. doi: 10.1159/000111431. [DOI] [PubMed] [Google Scholar]
- 40.Mendez E, Kawanishi T, Clemens K, Siomi H, Soldan S S, Calabresi P, Brady J, Jacobson S. Astrocyte-specific expression of human T-cell lymphotropic virus type 1 (HTLV-1) Tax: induction of tumor necrosis factor alpha and susceptibility to lysis by CD8+ HTLV-1-specific cytotoxic T cells. J Virol. 1997;71:9143–9149. doi: 10.1128/jvi.71.12.9143-9149.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohler K M, Butler L D. Quantitation of cytokine mRNA levels utilizing the reverse transcriptase-polymerase chain reaction following primary antigen-specific sensitization in vivo. I. Verification of linearity, reproducibility and specificity. Mol Immunol. 1991;28:437–447. doi: 10.1016/0161-5890(91)90157-f. [DOI] [PubMed] [Google Scholar]
- 42.Moritoyo T, Izumo S, Moritoyo H, Tanaka Y, Kiyomatsu Y, Nagai M, Usuku K, Sorimachi M, Osame M. Detection of human T-lymphotropic virus type I p40tax protein in cerebrospinal fluid cells from patients with human T-lymphotropic virus type I-associated myelopathy/tropical spastic paraparesis. J Neurovirol. 1999;5:241–248. doi: 10.3109/13550289909015810. [DOI] [PubMed] [Google Scholar]
- 43.Moritoyo T, Reinhart T A, Moritoyo H, Sato E, Izumo S, Osame M, Haase A T. Human T-lymphotropic virus type I-associated myelopathy and tax gene expression in CD4+ T lymphocytes. Ann Neurol. 1996;40:84–90. doi: 10.1002/ana.410400114. [DOI] [PubMed] [Google Scholar]
- 44.Mulloy J C, Kislyakova T, Cereseto A, Casareto L, LoMonico A, Fullen J, Lorenzi M V, Cara A, Nicot C, Giam C, Franchini G. Human T-cell lymphotropic/leukemia virus type 1 Tax abrogates p53-induced cell cycle arrest and apoptosis through its CREB/ATF functional domain. J Virol. 1998;72:8852–8860. doi: 10.1128/jvi.72.11.8852-8860.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munoz-Fernandez M A, Fresno M. The role of tumour necrosis factor, interleukin 6, interferon-gamma and inducible nitric oxide synthase in the development and pathology of the nervous system. Prog Neurobiol. 1998;56:307–340. doi: 10.1016/s0301-0082(98)00045-8. [DOI] [PubMed] [Google Scholar]
- 46.Nagai M, Usuku K, Matsumoto W, Kodama D, Takenouchi N, Moritoyo T, Hashiguchi S, Ichinose M, Bangham C R, Izumo S, Osame M. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J Neurovirol. 1998;4:586–593. doi: 10.3109/13550289809114225. [DOI] [PubMed] [Google Scholar]
- 47.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;i:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 48.Plaitakis A, Berl S, Yahr M D. Neurological disorders associated with deficiency of glutamate dehydrogenase. Ann Neurol. 1984;15:144–153. doi: 10.1002/ana.410150206. [DOI] [PubMed] [Google Scholar]
- 49.Poiesz B J, Ruscetti F W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Popovic M, Lange-Wantzin G, Sarin P S, Mann D, Gallo R C. Transformation of human umbilical cord blood T cells by human T-cell leukemia/lymphoma virus. Proc Natl Acad Sci USA. 1983;80:5402–5406. doi: 10.1073/pnas.80.17.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson M B, Dowd L A. Heterogeneity and functional properties of subtypes of sodium-dependent glutamate transporters in the mammalian central nervous system. Adv Pharmacol. 1997;37:69–115. doi: 10.1016/s1054-3589(08)60948-5. [DOI] [PubMed] [Google Scholar]
- 52.Rothstein J D, Dykes-Hoberg M, Pardo C A, Bristol L A, Jin L, Kuncl R W, Kanai Y, Hediger M A, Wang Y, Schielke J P, Welty D F. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 53.Salahuddin S Z, Markham P D, Wong-Staal F, Franchini G, Kalyanaraman V S, Gallo R C. Restricted expression of human T-cell leukemia-lymphoma virus (HTLV) in transformed human umbilical cord blood lymphocytes. Virology. 1983;129:51–64. doi: 10.1016/0042-6822(83)90395-1. [DOI] [PubMed] [Google Scholar]
- 54.Schousboe A, Sonnewald U, Civenni G, Gegelashvili G. Role of astrocytes in glutamate homeostasis. Implications for excitotoxicity. Adv Exp Med Biol. 1997;429:195–206. doi: 10.1007/978-1-4757-9551-6_14. [DOI] [PubMed] [Google Scholar]
- 55.Sibson N R, Dhankhar A, Mason G F, Rothman D L, Behar K L, Shulman R G. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci USA. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55a.Szymocha, R., et al. J. Neurovirol., in press. [DOI] [PubMed]
- 56.Tendler C, Greenberg S, Burton J, Danielpour D, Kim S, Blattner W, Manns A, Waldmann T. Cytokine induction in HTLV-I associated myelopathy and adult T-cell leukemia: alternate molecular mechanisms underlying retroviral pathogenesis. J Cell Biochem. 1991;46:302–311. doi: 10.1002/jcb.240460405. [DOI] [PubMed] [Google Scholar]
- 57.Umehara F, Izumo S, Ronquillo A T, Matsumuro K, Sato E, Osame M. Cytokine expression in the spinal cord lesions in HTLV-I-associated myelopathy. J Neuropathol Exp Neurol. 1994;53:72–77. doi: 10.1097/00005072-199401000-00009. [DOI] [PubMed] [Google Scholar]
- 58.Umehara F, Izumo S, Takeya M, Takahashi K, Sato E, Osame M. Expression of adhesion molecules and monocyte chemoattractant protein-1 (MCP-1) in the spinal cord lesions in HTLV-I-associated myelopathy. Acta Neuropathol (Berlin) 1996;91:343–350. doi: 10.1007/s004010050435. [DOI] [PubMed] [Google Scholar]
- 59.Umehara F, Nakamura A, Izumo S, Kubota R, Ijichi S, Kashio N, Hashimoto K, Usuku K, Sato E, Osame M. Apoptosis of T lymphocytes in the spinal cord lesions in HTLV-I-associated myelopathy: a possible mechanism to control viral infection in the central nervous system. J Neuropathol Exp Neurol. 1994;53:617–624. doi: 10.1097/00005072-199411000-00009. [DOI] [PubMed] [Google Scholar]
- 60.Umehara F, Okada Y, Fujimoto N, Abe M, Izumo S, Osame M. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in HTLV-I-associated myelopathy. J Neuropathol Exp Neurol. 1998;57:839–849. doi: 10.1097/00005072-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Van Der Voorn P, Tekstra J, Beelen R H, Tensen C P, Van Der Valk P, De Groot C J. Expression of MCP-1 by reactive astrocytes in demyelinating multiple sclerosis lesions. Am J Pathol. 1999;154:45–51. doi: 10.1016/S0002-9440(10)65249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weiss J M, Downie S A, Lyman W D, Berman J W. Astrocyte-derived monocyte-chemoattractant protein-1 directs the transmigration of leukocytes across a model of the human blood-brain barrier. J Immunol. 1998;161:6896–6903. [PubMed] [Google Scholar]
- 63.Wodarz D, Nowak M A, Bangham C R. The dynamics of HTLV-I and the CTL response. Immunol Today. 1999;20:220–227. doi: 10.1016/s0167-5699(99)01446-2. [DOI] [PubMed] [Google Scholar]
- 64.Wong D, Prameya R, Dorovini-Zis K. In vitro adhesion and migration of T lymphocytes across monolayers of human brain microvessel endothelial cells: regulation by ICAM-1, VCAM-1, E-selectin and PECAM-1. J Neuropathol Exp Neurol. 1999;58:138–152. doi: 10.1097/00005072-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 65.Wu E, Dickson D W, Jacobson S, Raine C S. Neuroaxonal dystrophy in HTLV-1-associated myelopathy/tropical spastic paraparesis: neuropathologic and neuroimmunologic correlations. Acta Neuropathol (Berlin) 1993;86:224–235. doi: 10.1007/BF00304136. [DOI] [PubMed] [Google Scholar]
- 66.Yavin E, Yavin Z. Attachment and culture of dissociated cells from rat embryo cerebral hemispheres on polylysine-coated surface. J Cell Biol. 1974;62:540–546. doi: 10.1083/jcb.62.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu N, Billaud J N, Phillips T R. Effects of feline immunodeficiency virus on astrocyte glutamate uptake: implications for lentivirus-induced central nervous system diseases. Proc Natl Acad Sci USA. 1998;95:2624–2629. doi: 10.1073/pnas.95.5.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]