Summary
Background
Cyclin-dependent kinase 4/6 (CDK4/6) inhibitors in combination with traditional endocrine therapy (ET) are now the recommended first-line treatment for hormone receptor (HR)-positive and HER2-negative metastatic breast cancer (MBC). However, the benefits of adding CDK4/6 inhibitors to ET in HER2-low-positive and HER2-0 subgroups remain unclear. We aimed to assess the effectiveness of CDK4/6 inhibitors in combination with ET in patients with HR-positive, HER2-low-positive and HER2-0 MBC.
Methods
This secondary analysis assessed progression-free survival (PFS) among HER2-low-positive and HER2-0 patients enrolled in the double-blind, placebo-controlled randomised clinical trials PALOMA-2 and PALOMA-3. The study included 1186 HER2-negative, HR-positive female patients, with available immunohistochemistry (IHC) and/or in situ hybridization (ISH) results, across 17 countries enrolled between February 2013 and August 2014. HER2-low-positive status was defined by IHC 1+ or 2+ with negative ISH, and HER2-zero by IHC 0. Data analyses were conducted between March and May 2023. In the PALOMA-2 trial, patients were randomly assigned to receive either palbociclib or placebo, in combination with letrozole in the first-line treatment for HR-positive MBC. Patients in the PALOMA-3 study, who had progression or relapse during previous ET, were randomly allocated to receive either palbociclib plus fulvestrant or placebo plus fulvestrant. The primary endpoint was investigator-assessed PFS. Kaplan–Meier approach and Cox proportional hazards model were applied to estimate the association of treatment strategies with PFS among HER2-0 and HER2-low-positive populations. The two trials are registered with ClinicalTrials.gov, number NCT01740427 and NCT01942135.
Findings
Of the 666 patients with MBC from the PALOMA-2 study, there were 153 HER2-0 and 513 HER2-low-positive patients. In the HER2-0 population, no significant difference in PFS was observed between the palbociclib-letrozole and placebo-letrozole groups (hazard ratio = 0.79, 95% confidence interval [CI] 0.48–1.30, p = 0.34). In the HER2-low-positive population, palbociclib-letrozole demonstrated a significantly lower risk of PFS than placebo-letrozole group (hazard ratio = 0.52, 95% CI 0.41–0.66, p < 0.0001). The PALOMA-3 study analysed 520 patients with MBC. Within the 153 HER2-0 patients, the palbociclib-fulvestrant group showed a significantly longer PFS than the placebo-fulvestrant group (hazard ratio = 0.54, 95% CI 0.30–0.95, p = 0.034). Among the 367 HER2-low-positive patients, palbociclib-fulvestrant improved PFS (hazard ratio = 0.39, 95% CI 0.28–0.54, p < 0.0001).
Interpretation
The combination of a CDK4/6 inhibitor with ET significantly improved PFS in HER2-low-positive patients, while for HER2-0 patients, benefits were primarily observed in patients who had progressed on previous ET. Furthermore, HER2-0 patients may derive limited benefits from first-line CDK4/6 inhibitor treatment. Further work is needed to validate these findings and to delineate patient subsets that are most likely to benefit from the combination of CDK4/6 inhibitors and ET as first-line treatments.
Funding
None.
Keywords: HER2-low-positive, HER2-0, CDK4/6 inhibitor, Endocrine therapy, First-line treatment, Metastatic breast cancer
Research in context.
Evidence before this study
We conducted a comprehensive literature search on PubMed and major congress abstracts using the search terms “HER2-low”, “CDK4/6 inhibitors”, and “breast cancer”, without any publication date or language restrictions. Our search revealed only six retrospective studies that have explored the prognostic impact of HER2-low-positive status for HR-positive metastatic breast cancer (MBC) patients treated with CDK4/6 inhibitors, and these studies yielded conflicting findings. However, none have investigated the efficacy of CDK4/6 inhibitors in the context of endocrine-based therapy for HER2-low-positive and HER2-0 patients. Additionally, most of the existing publications were review articles, which indicated future therapeutic strategies might be contingent upon the identification of patient populations that are responsive to CDK4/6 inhibitors.
Added value of this study
In this secondary analysis of two phase 3 clinical trials, we found that among HER2-low-positive patients, CDK4/6 inhibitors combined with endocrine therapy (ET) led to a significant improvement in PFS compared to ET-alone. Conversely, the benefits of incorporating CDK4/6 inhibitors for HER2-0 tumours were primarily observed in patients who had progressed on previous ET, indicating that patients with HER2-0 expression were less likely to benefit from first-line CDK4/6 inhibitor treatment. As an exploratory study, this study provided insights into the role of CDK4/6 inhibitors in the context of ET for patients with HR-positive, HER2-low-positive and HER2-0 MBC, both in the first-line and endocrine-resistant settings.
Implications of all the available evidence
The current National Comprehensive Cancer Network and European Society for Medical Oncology guidelines recommend the use of CDK4/6 inhibitors in combination with ET as the first-line standard treatment for HR-positive, HER2-negative MBC. However, our study suggests that patients with HER2-0, HR-positive MBC may not significantly benefit from selected CDK4/6 inhibitors in first-line treatment. These findings potentially identify patient subsets that are most likely to benefit from the addition of CDK4/6 inhibitors, and may have an impact on future clinical guidelines for the first-line treatment of HR-positive, HER2-negative MBC.
Introduction
The PALOMA-2, Monaleesa-2, and Monarch-3 clinical trials have demonstrated the promising efficacy of cyclin-dependent kinase 4/6 (CDK4/6) inhibitors, such as palbociclib, ribociclib, and abemaciclib, in combination with endocrine therapy (ET), particularly in extending progression-free survival (PFS) and overall survival (OS) during first-line treatment.1, 2, 3 This compelling evidence has led to National Comprehensive Cancer Network (NCCN) and European Society for Medical Oncology (ESMO) guidelines endorsing the combined use of CDK4/6 inhibitors with ET as the first-line standard treatment for hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer (MBC).4,5
However, the 2023 SONIA clinical study presented at the American Society of Clinical Oncology (ASCO) conference showed contrasting results. While there was no marked enhancement in PFS, OS, or quality of life in patients receiving CDK4/6 inhibitors as a first-line treatment compared to second-line use, the risk of grade 3–4 adverse events saw a 42% increase.6 This discovery raises a critical question about the universality of the clinical benefits of first-line CDK4/6 inhibitors and encourages us to explore additional biomarkers for identifying patient subgroups who respond well to first-line ET alone, thereby potentially delaying the additional toxicity of CDK4/6 inhibitors.
Recently, a retrospective analysis of 463 patients with HR-positive, HER2-negative MBC treated with first-line ET plus CDK4/6 inhibitors found an inferior PFS in the HER2-low-positive cohort compared to HER2-0 patients.7 Conversely, Mouabbi et al. investigated 1649 patients with HR-positive MBC who received first-line ET plus CDK4/6 inhibitors and reported similar clinical outcomes between the HER2-low-positive and HER2-0 subgroups.8 Regardless, none have investigated the efficacy of the addition of CDK4/6 inhibitors in the context of endocrine-based therapy for the HER2-low-positive and HER2-0 subgroups, both in the first-line and endocrine-resistant settings. In the present study, we aim to conduct a secondary analysis of two phase 3 trials (PALOMA-2 and PALOMA-3) to assess the potential efficacy of a CDK4/6 inhibitor in combination with ET (versus ET alone) in the first-line or endocrine-resistant setting, stratified by HER2-low-positive and HER2-0 status. This study endeavours to address the gap in the existing literature and holds the potential to provide valuable insights for clinicians when selecting personalised treatment strategies for patients with HR-positive MBC based on HER2-low-positive versus HER2-0 status.
Methods
Study design and participants
The present study utilised data from the PALOMA-2 and PALOMA-3 clinical trials, registered as NCT01740427 and NCT01942135 with ClinicalTrials.gov, both being prospective multicentre randomised phase 3 trials. The PALOMA-2 trial enrolled 666 postmenopausal women with previously untreated oestrogen receptor (ER)-positive/HER2-negative MBC. Patients were randomised in a 2:1 ratio to receive either palbociclib (125 mg/day for 3 weeks, followed by 1 week off in a 28-day cycle) or a placebo, in combination with letrozole (2.5 mg/day). In the PALOMA-3 trial, 521 women with HR-positive/HER2-negative MBC who had previously received ET were randomised 2:1 to receive either palbociclib (125 mg/day for 3 weeks, followed by 1 week off in a 28-day cycle) plus fulvestrant (500 mg) or placebo plus fulvestrant. Further study design details and eligibility criteria for these studies have been published elsewhere (Appendix p 2).1,9, 10, 11 Our analysis included 666 patients from the PALOMA-2 trial and 520 patients from the PALOMA-3 trial, who had available data regarding HER2 immunohistochemistry (IHC) score and/or in situ hybridization (ISH) status (Appendix p 12). Baseline characteristics, including age, race, Eastern Cooperative Oncology Group (ECOG) performance status, HR status, HER2 sample site, site of metastatic disease, disease-free interval, prior treatment, etc., were collected for patients enrolled in PALOMA-2 and PALOMA-3, as listed in Tables 1 and 2. HR and HER2 status were assessed locally via the Food and Drug Administration (FDA)-approved assays. Patients were then stratified into two subgroups based on HER2 expression, i.e., HER2-0 for tumours scored IHC 0 and HER2-low-positive for tumours scored IHC 1+, or 2+ with ISH-negative.
Table 1.
Baseline characteristics within HER2-0 and HER2-low-positive populations in PALOMA-2 trial.
| HER2-0b |
HER2-low-positiveb |
|||||||
|---|---|---|---|---|---|---|---|---|
| Total (N = 153) | Palbociclib + Letrozole (N = 104) | Placebo + Letrozole (N = 49) | p valuea | Total (N = 513) | Palbociclib + Letrozole (N = 340) | Placebo + Letrozole (N = 173) | p valuea | |
| Age | ||||||||
| Mean (SD)—yr | 62.4 (10.5) | 62.2 (10.1) | 63.0 (11.2) | 0.58 | 60.9 (10.9) | 61.5 (10.8) | 59.9 (11.2) | 0.13 |
| <65 yr—no. (%) | 83 (54.2) | 58 (55.8) | 25 (51.0) | 0.58 | 321 (62.6) | 205 (60.3) | 116 (67.1) | 0.13 |
| ≥65 yr—no. (%) | 70 (45.8) | 46 (44.2) | 24 (49.0) | 192 (37.4) | 135 (39.7) | 57 (32.9) | ||
| Race—no. (%) | 0.89 | 0.98 | ||||||
| White | 108 (77.1) | 75 (75.0) | 33 (82.5) | 408 (81.6) | 269 (81.5) | 139 (81.8) | ||
| Asian | 26 (18.6) | 20 (20.0) | 6 (15.0) | 69 (13.8) | 45 (13.6) | 24 (14.1) | ||
| Black | 2 (1.4) | 2 (2.0) | 0 (0.0) | 9 (1.8) | 6 (1.8) | 3 (1.8) | ||
| Other | 4 (2.9) | 3 (3.0) | 1 (2.5) | 14 (2.8) | 10 (3.0) | 4 (2.4) | ||
| ECOG score at baseline—no. (%) | 0.60 | 0.010 | ||||||
| 0 | 84 (54.9) | 60 (57.7) | 24 (49.0) | 275 (53.6) | 197 (57.9) | 78 (45.1) | ||
| 1 | 66 (43.1) | 42 (40.4) | 24 (49.0) | 229 (44.6) | 136 (40.0) | 93 (53.8) | ||
| 2 | 3 (2.0) | 2 (1.9) | 1 (2.0) | 9 (1.8) | 7 (2.1) | 2 (1.2) | ||
| Site of metastatic disease—no. (%) | 0.35 | 0.38 | ||||||
| Visceral | 74 (48.4) | 53 (51.0) | 21 (42.9) | 250 (48.7) | 161 (47.4) | 89 (51.4) | ||
| Nonvisceral | 79 (51.6) | 51 (49.0) | 28 (57.1) | 263 (51.3) | 179 (52.6) | 84 (48.6) | ||
| Bone only, yes—no. (%) | 32 (20.9) | 20 (19.2) | 12 (24.5) | 0.46 | 119 (23.2) | 83 (24.4) | 36 (20.8) | 0.36 |
| No. of disease site—no. (%) | 0.18 | 0.15 | ||||||
| 1–3 | 125 (81.7) | 82 (78.8) | 43 (87.8) | 421 (82.1) | 285 (83.8) | 136 (78.6) | ||
| ≥4 | 28 (18.3) | 22 (21.2) | 6 (12.2) | 92 (17.9) | 55 (16.2) | 37 (21.4) | ||
| Disease-free interval—no. (%) | 0.52 | 0.83 | ||||||
| ≤12 Months | 30 (19.6) | 23 (22.1) | 7 (14.3) | 117 (22.8) | 76 (22.4) | 41 (23.7) | ||
| >12 Months | 68 (44.4) | 45 (43.3) | 23 (46.9) | 203 (39.6) | 133 (39.1) | 70 (40.5) | ||
| de novo metastatic | 55 (35.9) | 36 (34.6) | 19 (38.8) | 193 (37.6) | 131 (38.5) | 62 (35.8) | ||
| Measurable disease, yes—no. (%) | 119 (77.8) | 83 (79.8) | 36 (73.5) | 0.38 | 390 (76.0) | 255 (75.0) | 135 (78.0) | 0.45 |
| Most recent therapy—no. (%) | 0.10 | 0.18 | ||||||
| Anti-Oestrogens | 51 (58.6) | 34 (54.8) | 17 (68.0) | 178 (61.8) | 120 (64.2) | 58 (57.4) | ||
| Aromatase inhibitors | 35 (40.2) | 28 (45.2) | 7 (28.0) | 100 (34.7) | 63 (33.7) | 37 (36.6) | ||
| Other | 1 (1.1) | 0 (0.0) | 1 (4.0) | 10 (3.5) | 4 (2.1) | 6 (5.9) | ||
| Histopathological classification—no. (%)c | 0.65 | 0.88 | ||||||
| Ductal | 115 (75.2) | 76 (73.1) | 39 (79.6) | 424 (82.7) | 279 (82.1) | 145 (83.8) | ||
| Lobular | 28 (18.3) | 20 (19.2) | 8 (16.3) | 70 (13.6) | 48 (14.1) | 22 (12.7) | ||
| Other or unknown or data missing | 10 (6.5) | 8 (7.7) | 2 (4.1) | 19 (3.7) | 13 (3.8) | 6 (3.5) | ||
| Recurrence type—no. (%) | 0.35 | 0.65 | ||||||
| Distant | 100 (65.4) | 69 (66.3) | 31 (63.3) | 339 (66.1) | 225 (66.2) | 114 (65.9) | ||
| LFd | 1 (0.7) | 0 (0.0) | 1 (2.0) | 3 (0.6) | 3 (0.9) | 0 (0.0) | ||
| Local | 3 (2.0) | 3 (2.9) | 0 (0.0) | 6 (1.2) | 3 (0.9) | 3 (1.7) | ||
| Locoregional | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (0.8) | 2 (0.6) | 2 (1.2) | ||
| Newly diagnosed | 49 (32.0) | 32 (30.8) | 17 (34.7) | 161 (31.4) | 107 (31.5) | 54 (31.2) | ||
| Prior hormonal therapy, yes—no. (%) | 87 (56.9) | 62 (59.6) | 25 (51.0) | 0.32 | 288 (56.1) | 187 (55.0) | 101 (58.4) | 0.47 |
| Prior chemotherapy, yes—no. (%) | 73 (47.7) | 49 (47.1) | 24 (49.0) | 0.83 | 249 (48.5) | 164 (48.2) | 85 (49.1) | 0.85 |
| HER2 sample site—no. (%) | 0.46 | 0.53 | ||||||
| Primary tumour | 100 (65.4) | 70 (67.3) | 30 (61.2) | 321 (62.6) | 216 (63.5) | 105 (60.7) | ||
| Metastasis | 53 (34.6) | 34 (32.7) | 19 (38.8) | 192 (37.4) | 124 (36.5) | 68 (39.3) | ||
Wilcoxon rank sum test; Pearson's Chi-squared test; Fisher's exact test.
All the HER2 testing results were based on local laboratory test using FDA-approved assay. Among HER2-low-positive group, 182 (35.5%) patients had immunohistochemistry (IHC) 1+ and 331 (64.5%) patients had IHC 2+/in situ hybridization (ISH)-negative. Specifically, in Palbociclib + Letrozole arm, 121 (35.6%) patients had IHC 1+ and 219 (64.4%) patients had IHC 2+/ISH-negative. While in Placebo + Letrozole arm, 61 (35.3%) patients had IHC 1+ and 112 (64.7%) patients had IHC 2+/ISH-negative.
Ductal carcinoma includes mixed adenocarcinoma, adenocarcinoma and ductal carcinoma. “Other” and “Unknown” were options for the site to select on the clinical report form; “data missing” means that the site did not complete that field because the information was not available; one patient with “LF” was reclassified as “Other or unknown or data missing” per suggestion by Pfizer's study team, which indicates that some redaction was done here for a certain value in order to reduce the risk of patient re-identification.
The “LF” was presented as a separate category per discussion with Pfizer's study team, which indicates that some redaction was done here for certain values in order to reduce the risk of patient re-identification.
Table 2.
Baseline characteristics within HER2-0 and HER2-low-positive populations in PALOMA-3 trial.
| HER2-0b |
HER2-low-positiveb |
|||||||
|---|---|---|---|---|---|---|---|---|
| Overall (N = 153) | Palbociclib + Fulvestrant (N = 107) | Placebo + Fulvestrant (N = 46) | p valuea | Overall (N = 367) | Palbociclib + Fulvestrant (N = 239) | Placebo + Fulvestrant (N = 128) | p valuea | |
| Age | ||||||||
| Mean (SD)—yr | 57.8 (10.4) | 57.9 (11.1) | 57.7 (8.7) | 0.93 | 56.6 (11.6) | 56.6 (11.9) | 56.5 (10.9) | 0.99 |
| <65 yr—no. (%) | 117 (76.5) | 79 (73.8) | 38 (82.6) | 0.24 | 274 (74.7) | 181 (75.7) | 93 (72.7) | 0.52 |
| ≥65 yr—no. (%) | 36 (23.5) | 28 (26.2) | 8 (17.4) | 93 (25.3) | 58 (24.3) | 35 (27.3) | ||
| Race | 0.20 | 0.95 | ||||||
| White | 116 (75.8) | 77 (72.0) | 39 (84.8) | 268 (73.4) | 174 (73.1) | 94 (74.0) | ||
| Asian | 33 (21.6) | 26 (24.3) | 7 (15.2) | 72 (19.7) | 48 (20.2) | 24 (18.9) | ||
| Black or other | 4 (2.6) | 4 (3.7) | 0 (0.0) | 25 (6.8) | 16 (6.7) | 9 (7.1) | ||
| Menopausal status at study entry | 0.56 | 0.75 | ||||||
| Post-Menopausal | 124 (81.0) | 88 (82.2) | 36 (78.3) | 289 (78.7) | 187 (78.2) | 102 (79.7) | ||
| Pre/Peri-Menopausal | 29 (19.0) | 19 (17.8) | 10 (21.7) | 78 (21.3) | 52 (21.8) | 26 (20.3) | ||
| Sensitivity to previous hormonal therapy, yes—no. (%) | 114 (74.5) | 83 (77.6) | 31 (67.4) | 0.19 | 296 (80.7) | 191 (79.9) | 105 (82.0) | 0.62 |
| ECOG score at baseline—no. (%) | 0.33 | 0.22 | ||||||
| 0 | 84 (54.9) | 56 (52.3) | 28 (60.9) | 237 (64.6) | 149 (62.3) | 88 (68.8) | ||
| 1 | 69 (45.1) | 51 (47.7) | 18 (39.1) | 130 (35.4) | 90 (37.7) | 40 (31.2) | ||
| Hormone receptor status – no. (%) | 0.76 | 0.53 | ||||||
| ER-positive and PR-positive | 111 (75.5) | 80 (76.2) | 31 (73.8) | 240 (69.4) | 160 (70.5) | 80 (67.2) | ||
| ER-positive and PR-negative | 36 (24.5) | 25 (23.8) | 11 (26.2) | 106 (30.6) | 67 (29.5) | 39 (32.8) | ||
| Previous lines of therapies for metastatic disease—no. (%) | 0.18 | 0.49 | ||||||
| 0 | 30 (19.6) | 19 (17.8) | 11 (23.9) | 84 (22.9) | 55 (23.0) | 29 (22.7) | ||
| 1 | 73 (47.7) | 48 (44.9) | 25 (54.3) | 151 (41.1) | 92 (38.5) | 59 (46.1) | ||
| 2 | 37 (24.2) | 28 (26.2) | 9 (19.6) | 94 (25.6) | 66 (27.6) | 28 (21.9) | ||
| ≥3 | 13 (8.5) | 12 (11.2) | 1 (2.2) | 38 (10.4) | 26 (10.9) | 12 (9.4) | ||
| Site of metastatic disease—no. (%) | 0.44 | 0.52 | ||||||
| Visceral | 87 (56.9) | 63 (58.9) | 24 (52.2) | 224 (61.0) | 143 (59.8) | 81 (63.3) | ||
| Nonvisceral | 66 (43.1) | 44 (41.1) | 22 (47.8) | 143 (39.0) | 96 (40.2) | 47 (36.7) | ||
| Bone only, yes—no. (%) | 40 (26.1) | 28 (26.2) | 12 (26.1) | 0.99 | 83 (22.6) | 57 (23.8) | 26 (20.3) | 0.44 |
| Number of disease sites—no. (%) | 0.81 | 0.51 | ||||||
| 1–3 | 128 (83.7) | 89 (83.2) | 39 (84.8) | 295 (80.4) | 190 (79.8) | 105 (82.7) | ||
| ≥4 | 25 (16.3) | 18 (16.8) | 7 (15.2) | 70 (19.2) | 48 (20.2) | 22 (17.3) | ||
| Disease-free interval—no. (%) | 0.81 | 0.70 | ||||||
| ≤24 Months | 25 (25.0) | 17 (25.8) | 8 (23.5) | 37 (14.6) | 23 (13.9) | 14 (15.7) | ||
| >24 Months | 75 (75.0) | 49 (74.2) | 26 (76.5) | 217 (85.4) | 142 (86.1) | 75 (84.3) | ||
| Measurable disease, yes—no. (%) | 117 (76.5) | 83 (77.6) | 34 (73.9) | 0.62 | 289 (78.7) | 185 (77.4) | 104 (81.2) | 0.39 |
| Histopathological classification—no. (%)c | 0.31 | 0.053 | ||||||
| Ductal | 113 (73.9) | 82 (76.6) | 31 (67.4) | 276 (75.2) | 188 (78.7) | 88 (68.8) | ||
| Lobular | 17 (11.1) | 12 (11.2) | 5 (10.9) | 45 (12.3) | 28 (11.7) | 17 (13.3) | ||
| Other or unknown | 23 (15.0) | 13 (12.1) | 10 (21.7) | 46 (12.5) | 23 (9.6) | 23 (18.0) | ||
| Type of previous chemotherapy—no. (%) | 0.31 | 0.42 | ||||||
| Neoadjuvant or adjuvant treatment only | 65 (42.5) | 42 (39.3) | 23 (50.0) | 149 (40.6) | 98 (41.0) | 51 (39.8) | ||
| Treatment for metastatic disease | 50 (32.7) | 35 (32.7) | 15 (32.6) | 126 (34.3) | 77 (32.2) | 49 (38.3) | ||
| None | 38 (24.8) | 30 (28.0) | 8 (17.4) | 92 (25.1) | 64 (26.8) | 28 (21.9) | ||
| Disease stage at study entry—no. (%)d | 0.65 | 0.71 | ||||||
| Metastatic | 130 (86.7) | 91 (85.8) | 39 (88.6) | 311 (85.2) | 204 (85.7) | 107 (84.3) | ||
| Recurrent locally advanced | 20 (13.3) | 15 (14.2) | 5 (11.4) | 54 (14.8) | 34 (14.3) | 20 (15.7) | ||
| Type of most recent therapy—no. (%) | 0.34 | 0.94 | ||||||
| Neoadjuvant or adjuvant | 30 (19.7) | 19 (17.8) | 11 (24.4) | 84 (22.9) | 55 (23.0) | 29 (22.7) | ||
| Advanced disease | 122 (80.3) | 88 (82.2) | 34 (75.6) | 283 (77.1) | 184 (77.0) | 99 (77.3) | ||
| Prior endocrine therapy—no. (%) | 0.42 | 0.68 | ||||||
| Aromatase inhibitors only | 69 (45.1) | 51 (47.7) | 18 (39.1) | 138 (37.6) | 86 (36.0) | 52 (40.6) | ||
| Tamoxifen only | 23 (15.0) | 17 (15.9) | 6 (13.0) | 50 (13.6) | 33 (13.8) | 17 (13.3) | ||
| Aromatase inhibitors and tamoxifen | 61 (39.9) | 39 (36.4) | 22 (47.8) | 179 (48.8) | 120 (50.2) | 59 (46.1) | ||
| HER2 sample site—no. (%) | 0.75 | 0.22 | ||||||
| Primary tumour | 73 (48.0) | 50 (47.2) | 23 (50.0) | 139 (38.1) | 96 (40.3) | 43 (33.9) | ||
| Metastasis | 79 (52.0) | 56 (52.8) | 23 (50.0) | 226 (61.9) | 142 (59.7) | 84 (66.1) | ||
Abbreviations: ER, oestrogen receptor; PR, progesterone receptor.
Wilcoxon rank sum test; Pearson's Chi-squared test; Fisher's exact test.
All the HER2 testing results were based on local laboratory test using FDA-approved assays. Among HER2-low-positive group, 148 (40.3%) patients had immunohistochemistry (IHC) 1+ and 219 (59.7%) patients had IHC 2+/in situ hybridization (ISH)-negative. Specifically, in Palbociclib + Fulvestrant arm, 100 (41.8%) patients had IHC 1+ and 139 (58.2%) patients had IHC 2+/ISH-negative. While in Placebo + Fulvestrant arm, 48 (37.5%) patients had IHC 1+ and 80 (62.5%) patients had IHC 2+/ISH-negative.
Ductal carcinoma includes diffuse adenocarcinoma, mixed adenocarcinoma, adenocarcinoma and ductal carcinoma. “Other” and “Unknown” were options for the site to select on the clinical report form.
Recurrent locally advanced disease included local and regional involvement and data on disease stage at study entry were missing or unknown for five patients.
The present study was deemed exempt by the Duke University Institutional Review Board (IRB) as no identifiers of the patients, physicians, or hospitals were included (IRB number: Pro00113712).
Outcomes
To evaluate the effectiveness of palbociclib in combination with ET (i.e., letrozole or fulvestrant) in patients with HR-positive, HER2-low-positive and HER2-0 MBC, the primary endpoint in the present secondary analysis for both the PALOMA-2 and PALOMA-3 trials was investigator-assessed PFS, defined as the time from randomization to the first documentation of progressive disease (PD) defined by Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 or death due to any cause, whichever occurred first. The secondary endpoints in the present study were comprised of OS, objective response rate (ORR), and clinical benefit rate (CBR). OS was defined as the time from randomization to death from any cause. ORR was defined as the proportion of patients who had a confirmed complete response or partial response, and CBR was defined as the proportion of patients who had a confirmed complete response, a partial response, or stable disease for 24 weeks. Additionally, a blinded independent central review (BICR) of PFS was performed as a supportive analysis.
Statistical analysis
Baseline characteristics between study subgroups, HER2-0 or HER2-low-positive, for both the PALOMA-2 and PALOMA-3 studies, were compared using the Wilcoxon rank sum test for continuous variables, and Fisher's exact or Pearson test for categorical variables. Median PFS or OS with survival curves were estimated by the Kaplan–Meier method, and two-sided log-rank tests were used to compare survival differences between groups. After evaluating the assumptions underlying Cox proportional hazards regression, including proportional hazards and linearity for quantitative predictors if needed, the univariable Cox proportional hazards models would be constructed to present the hazard ratios with 95% confidence interval (CI). The ORR and the CBR between the study groups within the HER2-0 and HER2-low-positive subgroups were examined using odds ratio and Pearson test (or Fisher's exact test).
To account for heterogeneity, sensitivity analyses were performed to estimate the adjusted hazard ratios using two different multivariable Cox models. The first model included the study group and the most imbalanced baseline variable identified from Tables 1 and 2 (i.e., a baseline covariate with the smallest p value between the two treatment groups within the HER2-0 or HER2-low-positive subgroups). The second model was adjusted by two empirical confounders as suggested by physicians with clinical expertise, i.e., age (≤65 or >65 years) and bone-only disease (yes or no).
All statistical analyses were performed using R software version 4.1.1 (Vivli research environment). A two-sided p < 0.05 was considered statistically significant. No adjustments were made for multiple comparisons.
Role of the funding source
There was no funding source for this study.
Results
PALOMA-2
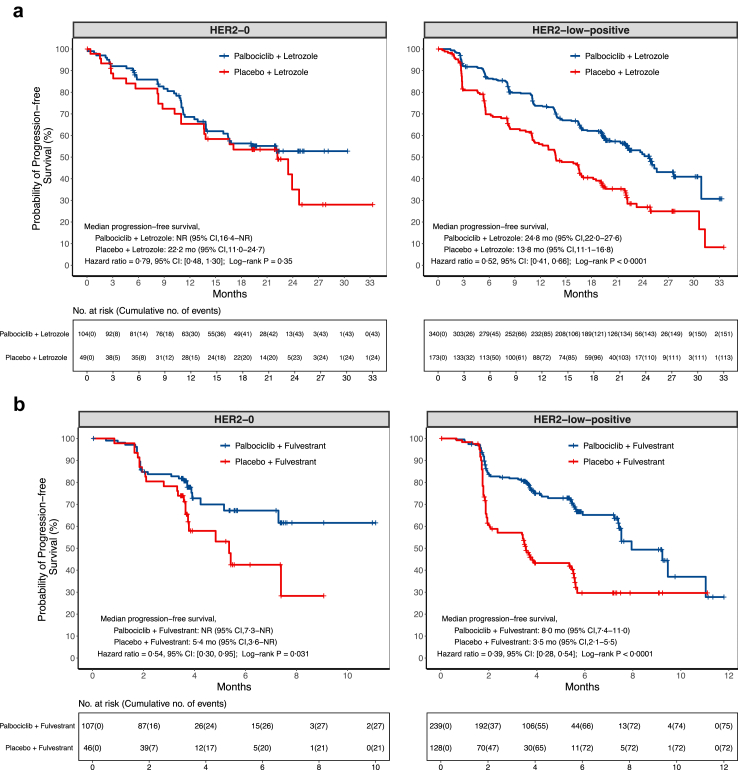
The PALOMA-2 primary endpoint analyses included 666 patients (data cutoff date, February 26, 2016), with 153 (23.0%) classified as HER2-0 and 513 (77.0%) as HER2-low-positive. Among the HER2-0 patients (n = 153), 104 patients (68.0%) were assigned to the palbociclib plus letrozole group, and 49 patients (32.0%) to the placebo plus letrozole group. Whereas, in the HER2-low-positive population (n = 513), 340 patients (66.3%) were included in the palbociclib group, and 173 patients (33.7%) were in the placebo group (Table 1). Among the HER2-0 patients, all the baseline characteristics listed in Table 1 were well-balanced across the two treatment groups. In the HER2-low-positive subgroup, most of the baseline characteristics were balanced across the two treatment groups, except ECOG score at baseline (p = 0.010; Table 1). By the data cutoff date, the median follow-up was 23.0 months.1
Within the HER2-0 population, the proportions of censoring for PFS were 58.7% and 51.0% in the palbociclib and placebo groups respectively, and the reasons of censoring could be found in the Appendix (p 3). The median PFS in the palbociclib group was not reached (NR, 95% confidence interval [CI] 16.4 months–NR), compared with 22.2 months (95% CI 11.0–24.7) in the placebo group (Fig. 1; Appendix pp 4, 13). The PFS rates by each landmark time were provided in the Appendix (p 5). There was no significant difference in PFS, assessed by the investigator, between the two treatment groups (hazard ratio = 0.79, 95% CI 0.48–1.30, p = 0.34; log-rank p = 0.35; Fig. 1; Appendix pp 6, 13). Additionally, the confirmed ORRs were not significantly different at 36.5% (95% CI 27.3–46.6) among the patients in the palbociclib group, and 30.6% (95% CI 18.3–45.4) among the patients in the placebo group (p = 0.47; Table 3). The CBRs were also similar at 85.6% (95% CI 77.3–91.7) within the palbociclib group, and 73.5% (95% CI 58.9–85.1) within the placebo group (p = 0.071; Table 3).
Fig. 1.
Kaplan–Meier curves for progression-free survival assessed by investigator. Panel a shows progression-free survival assessed by the investigator in HER2-0 or HER2-low-positive population from PALOMA-2 trial, the relative excess risk due to interaction (RERI) = −0.68 (95% CI −1.43 to 0.08, p = 0.078 based on null hypothesis RERI = 0) with HER2-low-positive and Palbociclib + Letrozole as the reference groups. Panel b shows progression-free survival assessed by the investigator in HER2-0 or HER2-low-positive population from PALOMA-3 trial, the RERI = 0.86 (95% CI −0.23 to 1.95, p = 0.12 based on null hypothesis RERI = 0) with HER2-0 and Palbociclib + Fulvestrant as the reference groups.
Table 3.
Best overall response in the HER2-0 or HER2-low-positive population.
| Study | Variable | Palbociclib + Letrozole (or Fulvestrant) | Placebo + Letrozole (or Fulvestrant) | Odds ratio (95% CI) | p value |
|---|---|---|---|---|---|
| PALOMA-2 | All HER2-0 patients–no. | 104 | 49 | ||
| Rate of objective response–% (95% CI)a | 36.5 (27.3,46.6) | 30.6 (18.3,45.4) | 1.30 (0.60,2.92) | 0.47 | |
| Rate of clinical benefit response–% (95% CI)b | 85.6 (77.3,91.7) | 73.5 (58.9,85.1) | 2.13 (0.84,5.36) | 0.071 | |
| All HER2-low-positive patients–no. | 340 | 173 | |||
| Rate of objective response–% (95% CI)a | 43.8 (38.5,49.3) | 35.8 (28.7,43.5) | 1.40 (0.94,2.08) | 0.082 | |
| Rate of clinical benefit response–% (95% CI)b | 84.7 (80.4,88.4) | 69.4 (61.9,76.1) | 2.44 (1.54,3.88) | <0.0001 | |
| PALOMA-3 | All HER2-0 patients–no. | 107 | 46 | ||
| Rate of objective response–% (95% CI)a,c | 8.4 (3.9,15.4) | 8.7 (2.4,20.8) | 0.96 (0.25,4.53) | 1.00 | |
| Rate of clinical benefit response–% (95% CI)b | 24.3 (16.5,33.5) | 21.7 (10.9,36.4) | 1.15 (0.48,2.97) | 0.73 | |
| All HER2-low-positive patients–no. | 239 | 128 | |||
| Rate of objective response–% (95% CI)a | 11.3 (7.6,16.0) | 5.5 (2.2,10.9) | 2.20 (0.90,6.16) | 0.066 | |
| Rate of clinical benefit response–% (95% CI)b | 38.5 (32.3,45.0) | 18.0 (11.7,25.7) | 2.85 (1.66,5.04) | <0.0001 |
Abbreviations: CI, confidence interval.
Rate of objective response was defined as the percentage of patients who had a confirmed complete response or a partial response. And the exact 95% CI was obtained by the Clopper-Pearson method.
Rate of clinical benefit response was defined as the percentage of patients who had a confirmed complete response, a partial response, or stable disease for 24 weeks or more. And the exact 95% CI was obtained by the Clopper-Pearson method.
There were only four individuals, with HER2-0 and receiving Placebo + Fulvestrant, achieved objective response in PALOMA-3 study, so the corresponding p value was obtained by the Fisher's exact test. Other p values were obtained by the Pearson test.
In the HER2-low-positive population, the proportions of censoring for PFS were 55.6% and 34.7% in the palbociclib and placebo groups respectively, and the reasons of censoring could be found in the Appendix (p 3). The median PFS in the palbociclib group was 24.8 months (95% CI 22.0–27.6), as compared with 13.8 months (95% CI 11.1–16.8) in the placebo group (Fig. 1; Appendix pp 4, 13). The HER2-low-positive patients in the palbociclib group had a significantly lower risk of disease progression or death than those in the placebo group (hazard ratio = 0.52, 95% CI 0.41–0.66, p < 0.0001; log-rank p < 0.0001; Fig. 1; Appendix pp 6, 13). Confirmed ORRs were higher among the patients in the palbociclib group (43.8%, 95% CI 38.5–49.3) compared to those in the placebo group (35.8%, 95% CI 28.7–43.5; p = 0.082; Table 3). The CBRs were also higher within the palbociclib group (84.7%, 95% CI 80.4–88.4) compared to the placebo group (69.4%, 95% CI 61.9–76.1; p < 0.0001; Table 3). The relevant relative risks (RRs) were also reported in Table S6 (Appendix p 7).
Sensitivity analyses using the two different multivariable Cox models yielded consistent results with those from the univariable Cox regression model (Appendix p 8). Furthermore, results from the analysis of BICR-assessed PFS also supported the findings from the investigator-assessed PFS analysis showing that the palbociclib group had a better PFS than the placebo group among patients with HER2-low-positive BC (hazard ratio = 0.57, 95% CI 0.43–0.76; log-rank p = 0.0001), while no significant differences in PFS between treatment groups were observed among the HER2-0 patients (hazard ratio = 1.10, 95% CI 0.62–1.97; log-rank p = 0.74; Appendix p 14). Notably, all Cox regression applied in the present study met the assumption (Appendix p 9). By the data cutoff date, OS data were immature, and as such, they were not included in the present study.
PALOMA-3
In the PALOMA-3 study, 520 of the intention-to-treat patients, who had available HER2-IHC and/or -ISH status, were included in these outcome analyses. The data cutoff date for PFS, ORR, and CBR was December 5, 2014. OS data were analysed based on the study cutoff date of April 13, 2018, with a median follow-up of 44.8 (interquartile range [IQR], 43.9–46.8) months. Among the study population, 153 (29.4%) were classified as HER2-0 and 367 (70.6%) as HER2-low-positive. Of the HER2-0 patients, 107 (69.9%) patients were included in the palbociclib plus fulvestrant group, and 46 (30.1%) patients were in the placebo plus fulvestrant group. Among the HER2-low-positive patients, 239 (65.1%) patients were assigned to the palbociclib group, and 128 (34.9%) patients were in the placebo group (Table 2). All of the baseline characteristics were balanced across the treatment groups in the HER2-0 and HER2-low-positive populations (Table 2).
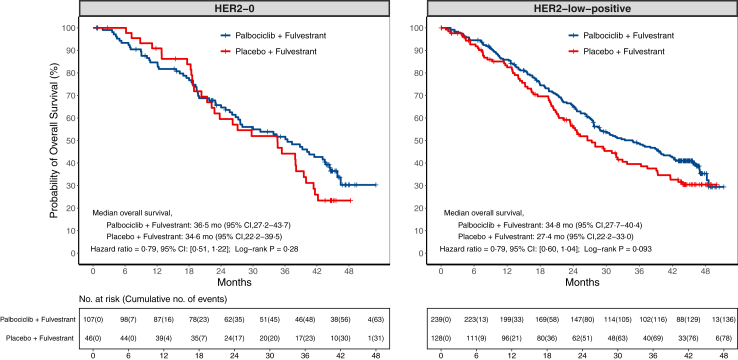
Within the HER2-0 population, the proportions of censoring for PFS were 74.8% and 54.3% in the palbociclib and placebo groups respectively, and the reasons of censoring could be found in the Appendix (p 3). The median PFS in the palbociclib group was NR (95% CI 7.3 months–NR), compared with 5.4 months (95% CI 3.6–NR) in the placebo group (Fig. 1; Appendix pp 4, 15). The PFS rates by each landmark time are listed in the Appendix (p 10). The palbociclib group showed a significantly longer PFS than the placebo group (hazard ratio = 0.54, 95% CI 0.30–0.95, p = 0.034; log-rank p = 0.031; Fig. 1; Appendix pp 6, 15). The overall ORRs were similar at 8.4% (95% CI 3.9–15.4) in the palbociclib group and 8.7% (95% CI 2.4–20.8) in the placebo group (p = 1.00; Table 3). The CBRs at the time of best overall response analysis were also similar at 24.3% (95% CI 16.5–33.5) within the palbociclib group and 21.7% (95% CI 10.9–36.4) within the placebo group (p = 0.73; Table 3). Furthermore, the proportions of censoring for OS were 41.1% and 32.6% in the palbociclib and placebo groups respectively (Appendix p 3), and there was no statistically significant difference in OS between the two groups, with a median OS of 36.5 months (95% CI 27.2–43.7) in the palbociclib group, and 34.6 months (95% CI 22.2–39.5) in the placebo group (hazard ratio = 0.79, 95% CI 0.51–1.22, p = 0.29; log-rank p = 0.28; Fig. 2; Appendix pp 4, 6). Using the Kaplan–Meier method, the estimated OS rate at 3 years was 52% (95% CI 43–63) in the palbociclib group and 44% (95% CI 31–63) in the placebo group (Appendix p 11).
Fig. 2.
Kaplan–Meier curves for overall survival in PALOMA-3 trial. This figure shows overall survival in HER2-0 or HER2-low-positive population from PALOMA-3 trial, the relative excess risk due to interaction (RERI) = −0.04 (95% CI −0.66 to 0.58, p = 0.90 based on null hypothesis RERI = 0) with HER2-low-positive and Palbociclib + Fulvestrant as the reference groups.
In the HER2-low-positive population, the proportions of censoring for PFS were 68.6% and 43.8% in the palbociclib and placebo groups respectively, and the reasons of censoring could be found in the Appendix (p 3). The median PFS in the palbociclib group was 8.0 months (95% CI 7.4–11.0), as compared with 3.5 months (95% CI 2.1–5.5) in the placebo group (Fig. 1; Appendix pp 4, 15). The addition of palbociclib to fulvestrant resulted in a significantly improved PFS (hazard ratio = 0.39, 95% CI 0.28–0.54, p < 0.0001; log-rank p < 0.0001; Fig. 1; Appendix pp 6, 15). The overall ORRs were similar at 11.3% (95% CI 7.6–16.0) in the palbociclib group and 5.5% (95% CI 2.2–10.9) in the placebo group (p = 0.066; Table 3). While the CBRs were higher among the patients in the palbociclib group (38.5%, 95% CI 32.3–45.0), compared to those in the placebo group (18.0%, 95% CI 11.7–25.7; p < 0.0001; Table 3). And the corresponding RRs were also reported in Table S6 (Appendix p 7). Additionally, the proportions of censoring for OS were 42.3% and 39.1% in the palbociclib and placebo groups respectively, and the reasons of censoring could be found in the Appendix (p 3). The median OS at the time of the final analysis was 34.8 months (95% CI 27.7–40.4) in the palbociclib group and 27.4 months (95% CI 22.2–33.0) in the placebo (Fig. 2; Appendix p 4). Although the addition of palbociclib to fulvestrant led to an absolute OS prolongation of 7.4 months, this difference did not reach statistical significance within the follow-up period (hazard ratio = 0.79, 95% CI 0.60–1.04, p = 0.093; log-rank p = 0.093; Fig. 2; Appendix p 6). Using the Kaplan–Meier method, the estimated OS rate at 3 years was 49% (95% CI 43–56) in the palbociclib group and 40% (95% CI 31–50) in the placebo group (Appendix p 11).
Sensitivity analyses using the two different multivariable Cox models yielded consistent findings in the HER2-low-positive group, indicating a significant difference in PFS between the treatment groups. However, no significant differences in PFS between the two treatment groups were observed in the HER2-0 population from the sensitivity analyses (Appendix p 8). Approximately 40% of the patients (211 patients with 58 events) were included in the PFS analysis assessed by BICR. The results of the BICR-assessed PFS were consistent only with the investigator-assessed PFS analysis in the HER2-low-positive population (hazard ratio = 0.20, 95% CI 0.11–0.37; log-rank p < 0.0001), while no significant differences in BICR-assessed PFS between treatment groups were observed in HER2-0 patients (hazard ratio = 0.45, 95% CI 0.18–1.13; log-rank p = 0.081; Appendix p 14). No Cox regression applied in the present study violated the proportional hazards assumption (Appendix p 9).
Discussion
To our knowledge, this secondary analysis of the PALOMA-2 and PALOMA-3 trials represents the primary exploratory investigation into the role of a CDK4/6 inhibitor with endocrine-based treatment for patients with HR-positive, HER2-low-positive and HER2-0 MBC, both in the first-line and endocrine-resistant settings. Our study demonstrated that in the first-line treatment, no significant difference was observed in PFS for HER2-0 patients treated with palbociclib in combination with ET versus ET alone. Conversely, HER2-low-positive patients experienced an improvement in PFS with the addition of palbociclib. However, in the context of endocrine resistance, adding palbociclib to ET improved PFS compared with ET monotherapy in both the HER2-low-positive and HER2-0 subgroups. As such, it may be time to re-evaluate the prevailing recommendation of using CDK4/6 inhibitors combined with ET as the default first-line treatment for patients with HER2-0 MBC.
CDK4/6 inhibitors exert their action by blocking the activity of the CDK-D-type cyclin complex (CCND), which subsequently leads to the upregulation of the retinoblastoma protein (pRb). This increase in pRb levels negatively regulates E2F transcription factors, thereby hindering cell cycle progression and promoting apoptosis in tumour cells.12 The use of CDK4/6 inhibitors in combination with ET has been a breakthrough in the treatment of advanced HR-positive BC.
In the HER2-low-positive subgroup, the addition of CDK4/6 inhibitors to ET in the first-line setting improved PFS, while no such benefit was observed in the HER2-0 subgroup. It is noteworthy that HER2 expression induces significantly higher levels of CDK4/6 activity and cell proliferation.13,14 This finding is consistent with previous studies,1,10 and supports the notion that CDK4/6 inhibitors can effectively target the cell cycle that was enhanced by HER2 signalling, thereby improving the efficacy of ET in HER2-low-positive patients. Furthermore, the benefits of CDK4/6 inhibitors were also observed in endocrine-resistant settings in the HER2-low-positive subgroup, indicating their potential as a possible treatment option across different stages of disease progression for patients with HER2-low expression.
However, in the HER2-0 subgroup, our analysis demonstrated that first-line treatment with palbociclib combined with ET did not lead to a significant improvement in PFS compared to ET alone. This finding is consistent with the notion that HER2 signalling through CDKs impacts the response to CDK4/6 inhibitors in the first-line setting. In contrast, the CDK4/6 inhibitors combined with ET did result in an improvement in PFS among patients who had progressed on previous ET in the HER2-0 subgroup. This could be attributed to potential CDK activation in patients exhibiting endocrine resistance in the pretreated group of patients. Various mechanisms of endocrine resistance including ESR1 mutations, transcription factor alterations, and cell cycle-related mechanisms, such as CCND1 amplification, EGFR/HER2 amplification, and the bypass activation mechanism involving the activation of Ras/MAPK and PI3K/AKT/mTOR pathways, which may potentially enhance the efficacy of CDK4/6 inhibitors for HER2-0 patients who progressed on previous ET.15, 16, 17, 18
In summary, our analysis revealed distinct responses between the PALOMA-2 and PALOMA-3 trials regarding the efficacy of CDK4/6 inhibitors in combination with different ET agents across the HER2 subgroups. In the PALOMA-2 trial, the combination of CDK4/6 inhibitors with aromatase inhibitors (AIs) showed limited additional benefit in the HER2-0 subgroup in the first-line setting. Low HER2 expression may contribute to ET resistance due to the cross-talk between ER and HER2 signalling pathways,19 and this limited cross-talk in HER2-0 patients could result in similar efficacy between AIs and CDK4/6 inhibitors. Conversely, in the PALOMA-3 trial, combining fulvestrant with CDK4/6 inhibitors revealed an additional benefit among HER2-low-positive and HER2-0 patients with previous ET. This discrepancy could be attributed to variations in the mechanisms of endocrine resistance. HER2-0 patients who experienced progression on prior ET may harbour distinct molecular alterations, such as amplifications in cell cycle-related genes, which could enhance the efficacy of CDK4/6 inhibitors in overcoming endocrine resistance.17,18
These findings, if validated, could potentially have clinical implications for the treatment of patients with HER2-low-positive and HER2-0 MBC. For HER2-low-positive patients, the combination of CDK4/6 inhibitors with ET may be considered a treatment option, regardless of the treatment line. This approach may significantly improve PFS and potentially prolong OS. On the other hand, for HER2-0 patients, HER2-0 expression may indicate minimal benefit from CDK4/6 inhibitors in the first-line setting. This finding should prompt a re-evaluation of the current paradigm advocating “CDK4/6 inhibitors as first-line treatment for all patients”.20 A head-to-head prospective study with and without CDK4/6 inhibitors should be explored for HER2-0 patients. Additionally, our study revealed an 8.4-month PFS advantage in HER2-0 patients treated with letrozole alone in the first-line setting compared to HER2-low-positive patients. This suggests that, for HER2-0 patients, ET monotherapy as the first-line treatment might represent a more effective therapeutic approach. However, among the HER2-0 patients who had progressed or relapsed during previous ET, CDK4/6 inhibitors combined with ET appeared to provide a substantial clinical benefit. In contrast, ET monotherapy in the first-line setting for patients with HER2-low-positive BC exhibited less efficacy compared to the HER2-0 subgroup, suggesting a need for consideration of combination therapies with CDK4/6 inhibitors to enhance efficacy.
Notably, HER2-low-positive expression exhibits variability between primary and recurrent tumours due to prior treatments. Miglietta et al.,21 reported a 38% overall rate of HER2-low-positive discordance between primary tumour and relapse. Similarly, Tarantino et al.,22 observed changes in HER2 expression between primary tumours and metastatic biopsies, with 22% of HER2-low-positive primary tumours transitioning to HER2-0. In the DB-04 study, patients were included based on the most recent available tumour tissue, encompassing both primary (35%) and metastatic (65%) samples.23 Notably, T-DXd's efficacy remained consistent across different tumour sample characteristics.24 However, further investigation is warranted to understand the potential impact of HER2 status changes on treatment response, particularly in the context of endocrine therapy. Utilizing data from the PALOMA-2 and PALOMA-3 trials, our study encompassed both primary and recurrent tumours, with balanced baseline characteristics across treatment arms. However, further subgroup analyses yielded small sample sizes, compromising treatment effect estimates due to insufficient statistical power. Subsequent studies should aim to separately analyse populations based on primary versus recurrent tumour, which may ultimately inform clinical decisions.
As an exploratory study focused on the role of CDK4/6 inhibitors along with the endocrine-based treatment of HR-positive, HER2-low-positive and HER2-0 MBC across different prior-treatment scenarios, the present study has several noteworthy strengths, such as its relatively large sample size and extended follow-up period based on RCT data. However, this present study using data from PALOMA-2 and PALOMA-3 also has potential limitations, including the post hoc nature of our exploratory study, and residual bias from unmeasured confounders. We acknowledge the inherent selection bias in hazard ratios in our study, attributable to differential survival or dropout rates among HER2-low-positive and HER2-0 patient subgroups receiving palbociclib in combination with ET versus ET alone, as a limitation when interpreting progression-free survival outcomes.25 Moreover, it is noteworthy that all HER2 testing results were derived from local laboratory assessments utilizing FDA-approved assays. However, other studies have demonstrated disparities between local assessments and central laboratory evaluations of HER2-0 and HER2-low-positive (with consistency rates ranging from 78.0% to 81.3%) due to differences in antibodies and immunostaining protocols at each facility.24,26 Also, the sample size of the HER2-0 subgroup was relatively small; thereby, these findings should be interpreted with caution. Nevertheless, our analysis has revealed noteworthy differences in responsiveness among the HER2-0 subgroup to CDK4/6 inhibitors between PALOMA-2 and PALOMA-3 trials, despite the consistently small sample size within both HER2-0 subgroups. Additionally, we refrained from conducting an OS analysis for PALOMA-2 due to the immaturity of the OS data. Furthermore, the definition of HER2-low-positive expression used in this analysis was not based on the latest guidelines for HER2 testing,27 which may affect the interpretation of the results. And it is important to note that statistical significance does not always mean that there is or will be a clinical significance.28,29 While our results section is meant to convey our study findings without subjective commentary, we have demonstrated the meaningful significance in clinical practice of the present study in this discussion section. Regardless, these findings emphasise the necessity for future head-to-head clinical studies based on central laboratory testing results to determine patient subsets that may be most likely to benefit from CDK4/6 inhibitors and ETs combination in first-line treatments, and ultimately have the potential to influence clinical guidelines.
In conclusion, our secondary analysis of the PALOMA-2 and PALOMA-3 trials highlights the significance of CDK4/6 inhibitors in the treatment of HER2-low-positive and HER2-0 MBC. The combination of CDK4/6 inhibitors with ET significantly improved PFS in HER2-low-positive patients in both first-line and endocrine-resistant setting, while the benefit in HER2-0 tumours was primarily observed among patients who had progressed on previous ET. Thus, patients with HER2-0 expression appear to be less likely to benefit from first-line CDK4/6 inhibitor treatment. Overall, our study emphasises the need to reconsider HER2-low-positive and HER2-0 status in first-line treatment for HR-positive MBC, and suggests that future studies may demonstrate that HER2-0 patients could be exempted from CDK4/6 inhibitors in first-line treatment. Further targeted clinical studies are needed to identify patient subsets that are most likely to benefit from the combination of CDK4/6 inhibitors and ET in first-line treatment.
Contributors
HL, YW, HZ, and SK are co-first authors and contributed equally to the work, SL and FM are co-corresponding authors. HL, YW, JKP, JZ, YH, LT, XC, KL, FM, and SL contributed to conceptualisation of the study. HL and SL contributed to data acquisition. HL, HZ, and SK did the data analysis and interpreted the data. HL, YW, and FM wrote the original draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version for submission. SL, HZ, and SK have accessed and verified the underlying study data. All authors had access to the underlying study data, and had final responsibility for the decision to submit for publication.
Data sharing statement
The data collected for this study, including individual patient data and a data dictionary defining each field in the data set, will not be available to others. This study is using data from data contributor, Pfizer that has been available through Vivli, Inc (https://vivli.org/). Summary statistical data are, however, available from the corresponding authors upon reasonable request with the permission of Vivli and/or Pfizer (https://vivli.org/resources/requestdata/). If interested in more details of requesting data, please contact corresponding authors.
Declaration of interests
JKP was the recipient of research funding by the Color Foundation (PI: Plichta). She serves on the National Comprehensive Cancer Network (NCCN) Breast Cancer Screening Committee. Both are unrelated to this work. She also receives funding from the National Institutes of Health (PI: Amundsen; K12HD043446).
SMT holds the consulting or advisory role for Novartis, Pfizer, Merck, Eli Lilly, AstraZeneca, Genentech/Roche, Eisai, Sanofi, Bristol Myers Squibb, Seattle Genetics, CytomX Therapeutics, Daiichi Sankyo, Gilead, Ellipses Pharma, 4D Pharma, OncoSec Medical Inc., BeyondSpring Pharmaceuticals, OncXerna, Zymeworks, Zentalis, Blueprint Medicines, Reveal Genomics, ARC Therapeutics, Infinity Therapeutics, Myovant, Zetagen, Umoja Biopharma, Menarini/Stemline, Aadi Biopharma, Bayer, which are unrelated to this work.
Acknowledgements
This “Clinical efficacy of CDK4/6 inhibitor plus endocrine therapy in HR-positive/HER2-0 and HER2-low-positive metastatic breast cancer: a secondary analysis of PALOMA-2 and PALOMA-3 trials” is based on research using data from data contributor, Pfizer that has been made available through Vivli, Inc. Vivli has not contributed to or approved, and Vivli and Pfizer are not in any way responsible for, the contents of this publication.
The authors thank Ms. Li Kong, President of Academy of Clinical Research and Study, for coordinating this research work and communication.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.105186.
Contributor Information
Fei Ma, Email: drmafei@126.com.
Sheng Luo, Email: sheng.luo@duke.edu.
Appendix A. Supplementary data
References
- 1.Finn R.S., Martin M., Rugo H.S., et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 2.Hortobagyi G.N., Stemmer S.M., Burris H.A., et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29(7):1541–1547. doi: 10.1093/annonc/mdy155. [DOI] [PubMed] [Google Scholar]
- 3.Goetz M.P., Toi M., Campone M., et al. Monarch 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638–3646. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 4.Gradishar W.J., Moran M.S., Abraham J., et al. Breast cancer, version 4.2022, NCCN clinical practice guidelines in Oncology. J Natl Compr Cancer Netw. 2022;20(6):691–722. doi: 10.6004/jnccn.2022.0030. [DOI] [PubMed] [Google Scholar]
- 5.Gennari A., André F., Barrios C.H., et al. ESMO clinical practice guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475–1495. doi: 10.1016/j.annonc.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Sonke G.S., Van Ommen-Nijhof A., Wortelboer N., et al. Primary outcome analysis of the phase 3 SONIA trial (BOOG 2017-03) on selecting the optimal position of cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors for patients with hormone receptor-positive (HR+), HER2-negative (HER2-) advanced breast cancer (ABC) J Clin Oncol. 2023;41(17_suppl) [Google Scholar]
- 7.Zattarin E., Sposetti C., Leporati R., et al. HER2-low status is associated with worse clinical outcomes in hormone receptor-positive, HER2-negative advanced breast cancer patients treated with first-line cyclin-dependent kinase 4/6 inhibitors plus endocrine therapy. San Antonio Breast Cancer Symposium; San Antonio, Texas: 2022. [Google Scholar]
- 8.Mouabbi J., Raghavendra A.S., Bassett R., Hassan A., Tripathy D., Layman R.M.J.C.R. Abstract P5-02-30: histology-based survival outcomes in HR+/HER2-metastatic breast cancer treated with targeted therapies plus endocrine therapy based on HER2 expression. Cancer Res. 2023;83(5_Supplement):P5. P2. [Google Scholar]
- 9.Turner N.C., Ro J., André F., et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373(3):209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 10.Cristofanilli M., Turner N.C., Bondarenko I., et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 11.Turner N.C., Slamon D.J., Ro J., et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379(20):1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 12.Klein M.E., Kovatcheva M., Davis L.E., Tap W.D., Koff A. CDK4/6 inhibitors: the mechanism of action may not be as simple as once thought. Cancer Cell. 2018;34(1):9–20. doi: 10.1016/j.ccell.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moasser M.M. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26(45):6469–6487. doi: 10.1038/sj.onc.1210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinclair W.D., Cui X. The effects of HER2 on CDK4/6 activity in breast cancer. Clin Breast Cancer. 2022;22(3):e278–e285. doi: 10.1016/j.clbc.2021.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Hanker A.B., Sudhan D.R., Arteaga C.L. Overcoming endocrine resistance in breast cancer. Cancer Cell. 2020;37(4):496–513. doi: 10.1016/j.ccell.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papadimitriou M.C., Pazaiti A., Iliakopoulos K., Markouli M., Michalaki V., Papadimitriou C.A. Resistance to CDK4/6 inhibition: mechanisms and strategies to overcome a therapeutic problem in the treatment of hormone receptor-positive metastatic breast cancer. Biochim Biophys Acta Mol Cell Res. 2022;1869(12) doi: 10.1016/j.bbamcr.2022.119346. [DOI] [PubMed] [Google Scholar]
- 17.Wander S.A., Cohen O., Gong X., et al. The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor-positive metastatic breast cancer. Cancer Discov. 2020;10(8):1174–1193. doi: 10.1158/2159-8290.CD-19-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saatci O., Huynh-Dam K.T., Sahin O. Endocrine resistance in breast cancer: from molecular mechanisms to therapeutic strategies. J Mol Med (Berl. 2021;99(12):1691–1710. doi: 10.1007/s00109-021-02136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osborne C.K., Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–247. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gradishar W.J., Moran M.S., Abraham J., et al. NCCN Guidelines® insights: breast cancer, version 4.2023. J Natl Compr Canc Netw. 2023;21(6):594–608. doi: 10.6004/jnccn.2023.0031. [DOI] [PubMed] [Google Scholar]
- 21.Miglietta F., Griguolo G., Bottosso M., et al. Evolution of HER2-low expression from primary to recurrent breast cancer. NPJ Breast Cancer. 2021;7(1):137. doi: 10.1038/s41523-021-00343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarantino P., Gandini S., Nicolò E., et al. Evolution of low HER2 expression between early and advanced-stage breast cancer. Eur J Cancer. 2022;163:35–43. doi: 10.1016/j.ejca.2021.12.022. [DOI] [PubMed] [Google Scholar]
- 23.Modi S., Jacot W., Yamashita T., et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cance. N Engl J Med. 2022;387(1):9–20. doi: 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prat A., Modi S., Tsurutani J., et al. San antonio breast cancer symposium annual meeting. 2022. Determination of HER2-low status in tumors of patients with unresectable and/or metastatic breast cancer in DESTINY-Breast04. [Google Scholar]
- 25.Hernán M.A. The hazards of hazard ratios. Epidemiology. 2010;21(1):13. doi: 10.1097/EDE.0b013e3181c1ea43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viale G., Basik M., Niikura N., et al. Retrospective study to estimate the prevalence and describe the clinicopathological characteristics, treatments received, and outcomes of HER2-low breast cancer. ESMO Open. 2023;8(4) doi: 10.1016/j.esmoop.2023.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolff A.C., Hammond M.E.H., Allison K.H., et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36(20):2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 28.Mansournia M.A., Nazemipour M., Etminan M. P-value, compatibility, and S-value. Glob Epidemiol. 2022;4 doi: 10.1016/j.gloepi.2022.100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenland S., Mansournia M.A., Joffe M. To curb research misreporting, replace significance and confidence by compatibility: a preventive medicine golden jubilee article. Prev Med. 2022;164 doi: 10.1016/j.ypmed.2022.107127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.