Relapsed or refractory multiple myeloma (RRMM) is associated with severe symptoms, some of which have been strongly linked to impairments in health-related quality of life (HRQoL), notably pain, fatigue, and a decline in physical and emotional functioning.1 Furthermore, HRQoL deteriorates with each subsequent line of therapy in RRMM.2 Hence, treatment goals, particularly in later lines of therapy, should include preserving HRQoL. Melphalan flufenamide (melflufen) is a first-in-class peptide-drug conjugate that utilizes increased peptidase expression to selectively release potent alkylating agents inside tumor cells. Melflufen is approved in Europe for the treatment of patients with triple-class refractory RRMM with ≥3 prior lines of therapy and time to progression (TTP) >36 months after prior autologous stem cell transplant (ASCT), if received. Approval was based on the results of the phase II HORIZON study and further supported by those of the phase III OCEAN study.3-5 OCEAN met its primary endpoint with melflufen plus dexamethasone demonstrating superior progression-free survival compared with pomalidomide plus dexamethasone in RRMM.4 Across trials, the safety profile of melflufen plus dexamethasone has been characterized primarily by hematologic adverse events that are clinically manageable, with infrequent grade 3/4 non-hematologic adverse events.3,4,6 HRQoL over time was preserved with melflufen plus dexamethasone in patients with advanced RRMM in the HORIZON trial..7 Pomalidomide plus dexamethasone has also been shown to be safe and effective without negatively affecting HRQoL, including in later lines of therapy.8 In this letter, we report HRQoL based on patient-reported outcomes (PRO) in a subset of patients from OCEAN receiving either melflufen plus dexamethasone or pomalidomide plus dexamethasone. Overall, melflufen plus dexamethasone treatment resulted in HRQoL comparable to that of pomalidomide plus dexamethasone, further supporting the use of melflufen plus dexamethasone in heavily pretreated patients with RRMM.
The OCEAN study design has been previously published.4 The study was conducted in compliance with the ethical principles set forth in the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice Guidelines and was approved by national regulatory authorities and independent ethics committees/ review boards. All patients provided written consent to participate. PRO assessments were added as an exploratory endpoint on May 24, 2019 (protocol v4.1), approximately 2 years after the study started.4 Only patients enrolled on/after protocol v4.1 and who completed ≥1 PRO questionnaires were included in this analysis.. PRO questionnaires were administered before dosing at baseline and day 1 of each treatment cycle, at the end-of-treatment visit, disease progression, and the start of a new treatment. Three PRO assessments were used: the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30) version 3, scored from 0-100, with higher scores indicating better functional status/ QoL and functional scales, and an increase in severity of symptoms for the symptom scales;9,10 the EORTC Quality of Life Questionnaire-Multiple Myeloma module (EORTC MY20), scored from 0-100 with higher scores indicating greater symptom severity;10 and the European Quality of Life 5 Dimensions 3-Level (EQ-5D-3L) questionnaire that evaluates generic health status, also with scores ranging from 0-100 (death=0; perfect health=100) with higher scores indicating a better health state.11 PRO results were compared between the treatment groups (melflufen and pomalidomide), and also within the melflufen group - between patients with TTP >36 months after prior ASCT or without prior ASCT (target population) and those with TTP <36 months after ASCT (non-target population). Mean scores at baseline, at each treatment cycle up to cycle 6, and mean change from baseline through cycle 6 were analyzed. Responder analysis was conducted, defined as the proportion of patients with improved, stable, or worsening EORTC QLQ-C30 and MY20 scores at cycle 6. We used a cutoff of a 10-point change for improvement or worsening scores, since this is the minimal important difference resulting in clinical benefit.12
Of the 495 patients enrolled and randomized in OCEAN, 158 with PRO assessments were included in the pre-specified PRO analysis (melflufen group, n=77; pomalidomide group, n=81). Forty-four patients in the melflufen group had not received previous ASCT or had TTP >36 months after previous ASCT, and 33 had TTP <36 months after previous ASCT.
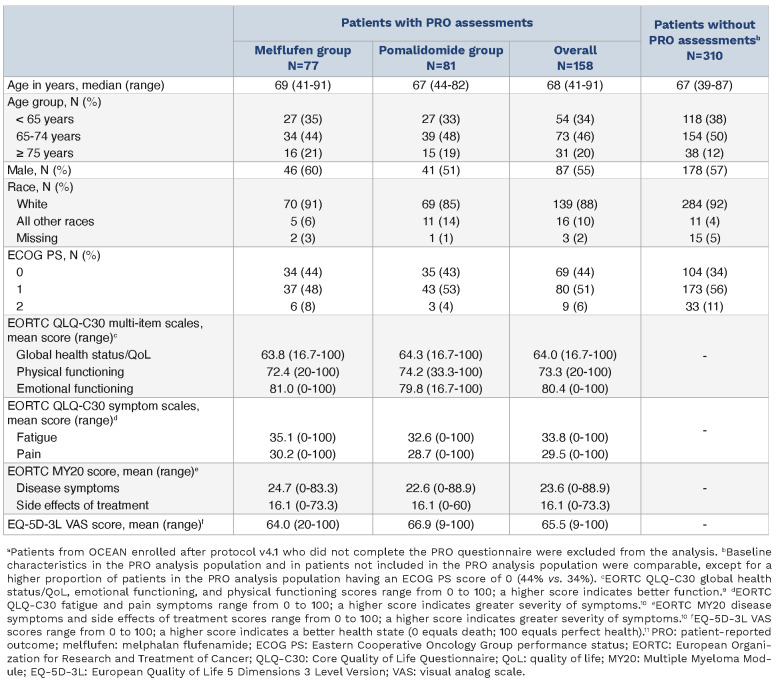
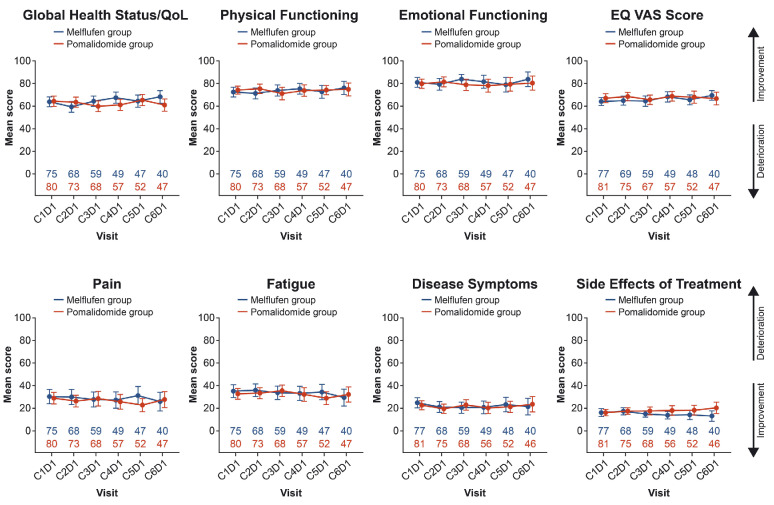
Baseline characteristics were generally well matched between treatment groups for patients with PRO data. Both treatment groups showed similar PRO scores at baseline with respect to general health and well-being, as measured by the EORTC QLQ-C30 multi-item scales, including global health status/QoL and physical and emotional functioning; symptom burden, as measured by the EORTC QLQ-C30 fatigue and pain scales; EORTC MY20 disease symptom scale and side effects of treatment score; and “health today,” as measured by the EQ-5D-3L visual analog scale (VAS) score (Table 1). Importantly, mean PRO scores remained generally consistent with baseline ones through cycle 6 in both treatment groups (Figure 1). Consistent with this, mean change from baseline in PRO scores through cycle 6 was also largely unchanged (Online Supplementary Figure S1).
Table 1.
Baseline characteristics of patients with and without patient-reported outcomes assessments.a
Figure 1.
Mean scores at baseline through cycle 6 of treatment with melflufen plus dexamethasone or pomalidomide plus dexamethasone. C: cycle; D: day; dex: dexamethasone; melflufen: melphalan flufenamide; QoL: quality of life; VAS: visual analog scale.
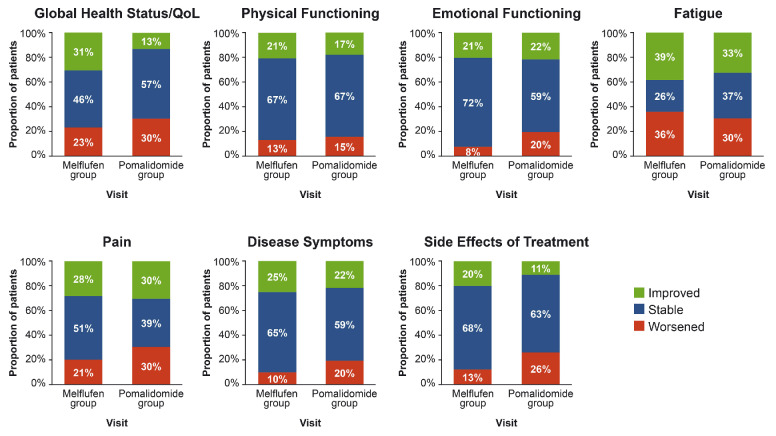
Overall, there was no difference in the proportion of patients with improvements or stable or worsening PRO measures over time between treatment groups, as exemplified by a snapshot at cycle 6 (Figure 2). Most patients had improved or stable PRO measures at cycle 6. The proportions of patients with improved PRO measures at cycle 6 ranged from 20% to 39% in the melflufen group and 11% to 33% in the pomalidomide group, and the proportions with stable PRO measures ranged from 26% to 72% for melflufen and from 37% to 67% for pomalidomide. In contrast, the proportion of patients with worsening PRO measures was generally low, particularly for physical functioning (melflufen: 13% and pomalidomide: 15%), emotional functioning (8% and 20%), disease symptoms (10% and 20%), and side effects of treatment (13% and 26%). Because a TTP <36 months after previous ASCT has been shown to be associated with worse outcomes in patients treated with melflufen and dexamethasone,5 PRO assessments were compared between the target and non-target populations within the melflufen group.
Figure 2.
Proportion of patients with improved, stable, or worsened patient-reported outcome measures at cycle 6. An improvement or worsening of an outcome was defined as a change of ≥10 points. QoL: quality of life; melflufen: melphalan flufenamide.
Mean PRO scores at baseline with melflufen and dexamethasone were similar between the target and non-target populations and trended similar to those in the overall population (Online Supplementary Table S1). Mean baseline global health status/QoL scores were 65.3 in the target population and 61.9 in the non-target population, physical functioning scores were 73.2 and 71.5, and emotional functioning scores were 83.3 and 78.0. Similarly, mean baseline scores for fatigue (30.7 and 40.7), pain (26.2 and 35.4), MM-specific disease symptoms (23.0 and 27.1), side effects of treatment (15.9 and 16.3), and EQ-5D-3L VAS scores (64.8 and 62.8) were similar between the target and non-target populations. Likewise, mean PRO scores remained generally consistent through cycle 6 of treatment with melflufen and dexamethasone between the target and non-target populations (Online Supplementary Figure S2).
These results are consistent with the preserved HRQoL seen with melflufen plus dexamethasone in the HORIZON study.7 Although progression in <36 months after prior ASCT is a negative prognostic factor for overall survival,5 PRO measures of the target and non-target populations within the melflufen group were similar at baseline and remained consistent through cycle 6 of therapy. In patients with RRMM, HRQoL tends to decrease with each subsequent line of therapy.2 Currently available therapeutic options for RRMM mostly maintain HRQoL but do not achieve clinically meaningful improvements.13 Comparisons across trials, however, are limited by differences in reporting of clinically meaningful improvements, and differences in patient populations and trial design. In the phase III APOLLO study of pomalidomide plus dexamethasone with or without daratumumab, HRQoL measured using the EORTC QLQ-C30 and EORTC QLQ-MY20 questionnaires remained generally consistent with baseline in both arms, although between-group differences did not favor the pomalidomide-dexamethasone arm for most QoL scales.14 In the phase II DREAMM-2 study, HRQoL measured by the EORTC QLQ-MY20 scale was maintained with long-term follow-up with belantamab mafodotin, with only up to 38% of evaluable patients (n=45) experiencing improvements in pain in different locations at week 7.15
Results from our analysis are limited by the fact that PRO assessments were collected only from patients who were randomized after protocol amendment 4.1.
Subgroup analyses for the target and non-target populations within the melflufen group were further limited by small patient numbers. Nevertheless, in both groups, more than half of the patients for whom PRO assessments were available were still on study at cycle 6. Although not a statistically powered comparison, 31% of patients receiving melflufen and 13% receiving pomalidomide showed improvement (≥10 points’ change) in global health status/QoL scores at cycle 6, whereas 46% of patients receiving melflufen and 57% receiving pomalidomide showed stable global health status/ QoL scores at cycle 6. These results from later timepoints likely suggest a continuous selection of patients benefiting from therapy and being able to tolerate it.
Pomalidomide plus dexamethasone was the standard-of-care for patients with RRMM when the OCEAN study was initiated, and the combination does not have a negative impact on HRQoL. Despite the higher frequency of hematologic adverse events in the melflufen group than in the pomalidomide group in OCEAN,4 these adverse effects had limited impact on patients’ HRQoL in the melflufen group. A greater proportion of patients were stable or even experienced improvements than those with worsening PRO measures with both treatment regimens, suggesting that, in terms of impact on HRQoL, melflufen plus dexamethasone is comparable to pomalidomide plus dexamethasone, despite the different routes of administration (intravenous for melflufen and oral for pomalidomide). These findings aid in meaningful translation of melflufen plus dexamethasone treatment to real-world practice.
Supplementary Material
Acknowledgments
The authors would like to thank the patients and their families for participating in this trial, and the trial investigators and coordinators for their contributions to the trial. We thank Jayasri Srinivasan, MD, MBA, and Swati Ghatpande, PhD, of Team 9 Science for providing medical writing assistance under the guidance of the authors, which was funded by Oncopeptides AB in accordance with Good Publications Practice (GPP) 2022 guidelines.
Funding Statement
Funding: Funding for the study (ClinicalTrials.gov identifier: NCT03151811) and for editorial assistance was provided by Oncopeptides AB.
Data-sharing statement
Oncopeptides is committed to sharing clinical study data with qualified researchers to enable enhancement of public health. As such, Oncopeptides will share anonymized patient-level data on request or if required by law or regulation. Qualified scientific and medical researchers can request patient-level data for studies of Oncopeptides’ pharmaceutical substances listed on ClinicalTrials. gov and approved by health authorities in the USA and the European Union. Patient-level data for studies of newly approved pharmaceutical substances or indications can be requested 9 months after approval by the US Food and Drug Administration and European Medicines Agency. Such requests are assessed at Oncopeptides’ discretion, and the decisions depend on the scientific merit of the proposed request, data availability, and the purpose of the proposal. The applicants should be willing to submit both positive and negative findings to a scientific journal. If Oncopeptides agrees to share clinical data for research purposes, the applicant is required to sign an agreement for data sharing before data release to ensure that the patients’ data are deidentified. In case of any risk of re-identification on anonymized data despite measures to protect patients’ confidentiality, the data will not be shared. The patients’ informed consent will always be respected. If the anonymization process will provide futile data, Oncopeptides has the right to refuse the request. Oncopeptides will provide access to patient-level clinical trial analysis datasets in a secured environment upon execution of the data-sharing agreement. Oncopeptides will also provide the protocol, statistical analysis plan, and the clinical study report synopsis if needed. For additional information or requests for access to Oncopeptides’ clinical trial data for research purposes, please contact us at: medinfoglobal@oncopeptides.com.
References
- 1.LeBlanc MR, Hirschey R, Leak Bryant A, LeBlanc TW, Smith SK. How are patient-reported outcomes and symptoms being measured in adults with relapsed/refractory multiple myeloma? A systematic review. Qual Life Res. 2020;29(6):1419-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engelhardt M, Ihorst G, Singh M, et al. Real-world evaluation of health-related quality of life in patients with multiple myeloma from Germany. Clin Lymphoma Myeloma Leuk. 2021;21(2):e160-e175. [DOI] [PubMed] [Google Scholar]
- 3.Richardson PG, Oriol A, Larocca A, et al. Melflufen and dexamethasone in heavily pretreated relapsed and refractory multiple myeloma. J Clin Oncol. 2021;39(7):757-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schjesvold FH, Dimopoulos MA, Delimpasi S, et al. Melflufen or pomalidomide plus dexamethasone for patients with multiple myeloma refractory to lenalidomide (OCEAN): a randomised, head-to-head, open-label, phase 3 study. Lancet Haematol. 2022;9(2):e98-e110. [DOI] [PubMed] [Google Scholar]
- 5.Sonneveld P, Richardson PG, Ludwig H, et al. Benefit versus risk assessment of melflufen and dexamethasone in relapsed/ refractory multiple myeloma: analyses from longer follow-up of the OCEAN and HORIZON studies. Clin Lymphoma Myeloma Leuk. 2023;23(9):P687-696. [DOI] [PubMed] [Google Scholar]
- 6.Richardson PG, Bringhen S, Voorhees P, et al. Melflufen plus dexamethasone in relapsed and refractory multiple myeloma (O-12-M1): a multicentre, international, open-label, phase 1-2 study. Lancet Haematol. 2020;7(5):e395-e407. [DOI] [PubMed] [Google Scholar]
- 7.Larocca A, Leleu X, Touzeau C, et al. Patient-reported outcomes in relapsed/refractory multiple myeloma treated with melflufen plus dexamethasone: analyses from the phase II HORIZON study. Br J Haematol. 2022;196(3):639-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song KW, Dimopoulos MA, Weisel KC, et al. Health-related quality of life from the MM-003 trial of pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone in relapsed and/or refractory multiple myeloma. Haematologica. 2015;100(2):e63-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A. on behalf of the EORTC Quality of Life Group. The EORTC QLQ-C30 Scoring Manual. 3rd ed. Brussels: European Organisation for Research and Treatment of Cancer, 2001. https://www.eortc.org/app/uploads/sites/2/2018/02/SCmanual.pdf. Accessed October 20, 2023. [Google Scholar]
- 10.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365-376. [DOI] [PubMed] [Google Scholar]
- 11.EuroQol G. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. [DOI] [PubMed] [Google Scholar]
- 12.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139-144. [DOI] [PubMed] [Google Scholar]
- 13.Ojo AS, Araoye MO, Ali A, Sarma R. The impact of current therapeutic options on the health-related quality of life of patients with relapse/refractory multiple myeloma: a systematic review of clinical studies. J Cancer Surviv. 2024; 18: 673-697 [DOI] [PubMed] [Google Scholar]
- 14.Terpos E, Dimopoulos MA, Boccadoro M, et al. Health-related quality of life in patients with relapsed/refractory multiple myeloma treated with pomalidomide and dexamethasone +/-subcutaneous daratumumab: patient-reported outcomes from the APOLLO trial. Am J Hematol. 2022;97(4):481-490. [DOI] [PubMed] [Google Scholar]
- 15.Popat R, Lonial S, Lee HC, et al. DREAMM-2: belantamab mafodotin effect on disease symptoms and health-related quality of life in patients with relapsed/refractory multiple myeloma (RRMM). Presented at the European Hematology Association (EHA) Virtual Congress, 2020; Abstract EP1746. https://library.ehaweb.org/eha/2020/eha25th/294226/rakesh.popat.dreamm-2.belantamab.mafodotin.effect.on.disease.symptoms.and.html. Accessed October 18, 2023. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Oncopeptides is committed to sharing clinical study data with qualified researchers to enable enhancement of public health. As such, Oncopeptides will share anonymized patient-level data on request or if required by law or regulation. Qualified scientific and medical researchers can request patient-level data for studies of Oncopeptides’ pharmaceutical substances listed on ClinicalTrials. gov and approved by health authorities in the USA and the European Union. Patient-level data for studies of newly approved pharmaceutical substances or indications can be requested 9 months after approval by the US Food and Drug Administration and European Medicines Agency. Such requests are assessed at Oncopeptides’ discretion, and the decisions depend on the scientific merit of the proposed request, data availability, and the purpose of the proposal. The applicants should be willing to submit both positive and negative findings to a scientific journal. If Oncopeptides agrees to share clinical data for research purposes, the applicant is required to sign an agreement for data sharing before data release to ensure that the patients’ data are deidentified. In case of any risk of re-identification on anonymized data despite measures to protect patients’ confidentiality, the data will not be shared. The patients’ informed consent will always be respected. If the anonymization process will provide futile data, Oncopeptides has the right to refuse the request. Oncopeptides will provide access to patient-level clinical trial analysis datasets in a secured environment upon execution of the data-sharing agreement. Oncopeptides will also provide the protocol, statistical analysis plan, and the clinical study report synopsis if needed. For additional information or requests for access to Oncopeptides’ clinical trial data for research purposes, please contact us at: medinfoglobal@oncopeptides.com.