Abstract
Current models of retroviral entry hypothesize that interactions between the host cell receptor(s) and viral envelope protein induce structural changes in the envelope protein that convert it to an active conformation, allowing it to mediate fusion with the membrane. Recent evidence supporting this hypothesis is the demonstration that Tva, the receptor for subgroup A avian sarcoma and leukosis virus (ASLV-A), induces conformational changes in the viral envelope protein. These changes include conversion of the envelope protein to an active, membrane-binding state likely representing a fusogenic conformation. To determine whether binding of the soluble Tva (sTva) receptor was sufficient to activate fully the fusogenic potential of the ASLV-A envelope protein, we have evaluated the ability of ASLV-A to infect receptor-deficient cell lines in the presence of sTva. Soluble receptor efficiently mediated infection of cells devoid of endogenous Tva in a dose-dependent manner, and this infection was dependent absolutely on the addition of sTva. The infectivity of the virus was enhanced dramatically in the presence of the polycationic polymer Polybrene or when centrifugal forces were applied during inoculation, resulting in viral titers comparable to those achieved on cells expressing endogenous receptor. sTva functioned to mediate infection at low concentrations, approaching the estimated binding constant of the receptor and viral envelope protein. These results demonstrate that receptor binding can activate the ASLV-A envelope protein and convert it to a fusogenic conformation competent to mediate the fusion of the viral and cellular membranes.
Retroviral entry is determined by interactions between the host cell receptor and the envelope glycoproteins that spike the surface of the virus. Viral receptors play critical roles in entry and thus are important determinants of both host range and tissue tropism. The entry process is initiated by binding of the viral envelope protein to the receptor on the cell surface, thereby attaching the virus to the host cell. For retroviruses that enter cells at neutral pH such as subgroup A avian sarcoma and leukosis virus (ASLV-A) and the human and simian immunodeficiency viruses (HIV and SIV, respectively), it has been demonstrated that receptor binding triggers structural alterations in the viral glycoprotein. It is postulated that such conformational changes enable the envelope protein to catalyze the fusion of viral and host membranes necessary for delivery of the viral genome into the cell cytoplasm. Thus, retroviral receptors are believed to participate in both attachment of the virus to the cell and regulation of fusogenic properties of the viral envelope proteins.
Studies of both ASLV-A and its receptor, Tva, have provided evidence supporting this model of receptor-triggered activation during retroviral entry. In vitro analysis indicates that soluble receptor binding can induce temperature-dependent changes in the viral envelope protein EnvA (14, 30, 34). These changes include exposure of the hydrophobic fusion peptide located within the membrane-associated TM subunit of EnvA as well as changes in the receptor-binding SU subunit (30). In addition, binding of purified, soluble receptor converts a soluble form of EnvA into its membrane-binding form (14, 34). Conversion to a membrane-binding state appears to involve cooperative interactions within the oligomeric envelope protein that require binding of multiple receptor molecules (14). Similar molecular and biophysical changes have been detected in the pH-dependent envelope protein of influenza virus, hemagglutinin, under low-pH conditions known to convert this protein into one with a fusion-active conformation (19, 43). Thus, it has been postulated that Tva binding triggers the conversion of EnvA to a protein in a fusogenic state, which would mediate the membrane fusion-required viral entry.
To further substantiate the ability of receptor to activate the fusogenic potential of EnvA and bypass the requirements of a membrane-associated receptor, we evaluated the capacity of soluble Tva (sTva) to trigger ASLV-A infection of receptor-deficient cells. Here we report that Tva can act in solution to mediate infection of mammalian cells devoid of endogenous receptor, suggesting that sTva-induced changes in EnvA convert the envelope protein to a fusion-competent form of EnvA. Addition of the polycation Polybrene and/or application of centrifugal forces during inoculation enhanced dramatically sTva-triggered infection. This result suggests that while membrane association of the receptor is not an absolute requirement for infection, the proximity of the receptor-activated virus to the cell is likely an important determinant of infection efficiency. The titers achieved on receptor-deficient cells in the presence of sTva were comparable to those seen on lines expressing endogenous Tva, indicating that infection mediated by this means is highly effective. Soluble receptor-triggered infection was optimal over a broad range of concentrations and demonstrated a nonlinear response to receptor concentration, suggesting that binding of a critical number of sTva molecules may be required for EnvA-mediated membrane fusion. The ability of sTva to trigger ASLV-A infection will be discussed in terms of current models of pH-independent viral entry.
MATERIALS AND METHODS
Viruses and cells.
RCAS (A)-AP was produced by transfection of proviral vector DNA into QT6, a quail myosarcoma line (American Type Culture Collection). Virus-containing supernatants were clarified by centrifugation and filtration (pore size, 0.45 μm) and stored at −120°C. Murine leukemia virus (MLV) pseudotypes were produced as described previously (55). Avian cells were maintained in M199 supplemented with 10% tryptose phosphate broth, 5% fetal calf serum, and 1% chicken serum. Human 293T cells were maintained in Dulbecco modified Eagle medium (DMEM)–10% bovine calf serum (BCS). A stable Tva-expressing 293T cell line was generated by transfection of a plasmid bearing the gene encoding Tva using the CaPO4-DNA transfection method (54). Twenty-four hours posttransfection, cells were passed into media containing G418 and stable clones were selected. Clones were screened for expression of Tva by Western blotting.
Proteins.
sTva was produced and purified as described previously (3). Protein was quantified using a Coomassie protein reagent as per the instructions of the manufacturer (Pierce). sTva was stored at 4°C and diluted in DMEM–10% BCS to the concentrations indicated in the figures. Soluble HveA (sHveA) was generously provided by the laboratories of Gary Cohen and Ros Eisenberg of the University of Pennsylvania. The production and purification of sHVE-a were as previously described (51).
RCAS (A)-AP infection.
Cells were plated for infection in six-well dishes (Costar) at a density of 3 × 105 to 5 × 105, 8 to 12 h before inoculation. Virus-receptor complexes were formed by incubating 100 μl of RCAS (A)-AP (generally 105 alkaline phosphatase-positive infectious units [AP+ IU]/ml) with or without filter-sterilized sTva or sHveA in DMEM–10% BCS (1.5-ml total volume) on ice for 30 min. Immediately before inoculation, 500 μl of DMEM–10% BCS containing 40 mM HEPES, pH 7.10, was added to the virus-receptor solution to buffer cells during the prolonged incubation period outside of the incubator. Cells were placed on ice, and medium was replaced with the HEPES-buffered virus-receptor solution. Where indicated in Fig. 3, Polybrene was added to a final concentration of 4 μg/ml. Plates were returned to a 37°C CO2 incubator or were centrifuged using a modified spinoculation protocol (27). Centrifugation was performed in an HB1000 low-speed, tabletop centrifuge (Sorvall) at 3,500 rpm for 2.5 h at 4°C. During an additional 30 min at 3,500 rpm, the temperature was gradually increased from 4 to between 36 and 38°C, typically in increments of 10°C per 10 min. Plates were immediately transferred to a 37°C CO2 incubator. Forty to 48 h postinoculation (p.i.), cells were rinsed with isotonic phosphate-buffered saline, formaldehyde fixed, and stained for AP activity as described previously (40). Viral titers were enumerated by counting AP-positive cells. Tva-expressing cell lines were infected as described above with the following modifications. Inhibition of RSCA A-(AP) infection was evaluated by incubating 100 μl of virus with sTva (5 nM) or sHveA (5 nM) at 37°C for 30 min, followed by inoculation of Tva-expressing 293T cells (Tva-293T) in the absence of Polybrene and centrifugation.
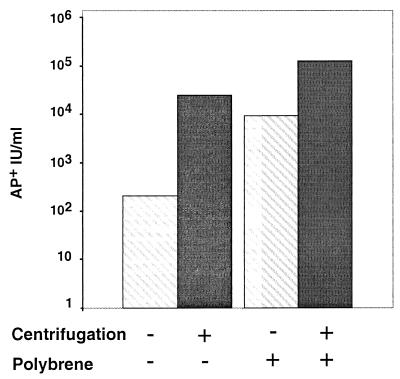
FIG. 3.
Polybrene and/or centrifugation enhances sTva-induced infection. RCAS (A)-AP was incubated with or without sTva. 293T cells were inoculated with virus-receptor complexes in the presence or absence of 4 μg of Polybrene per ml. Plates were immediately returned to a 37°C incubator or centrifuged as described in Materials and Methods. Forty to 48 h p.i., cells were stained and viral titers were determined. In the absence of sTva, no infected cells were detected. The data reflect results typical of multiple independent experiments employing approximately 104 AP+ IU.
RESULTS
ASLV-A can infect receptor-deficient cells in the presence of soluble receptor.
For pH-dependent viruses such as influenza virus and Semliki Forest virus, transiently lowering the pH of the medium triggers fusion of the virus and cell at the cell surface. Thus, it is possible to bypass the requirement of receptor-mediated endocytosis and the low-pH endosome and trigger the activation of the viral envelope protein extracellularly (52). Since in vitro studies indicate that Tva-induced changes in EnvA parallel acid-induced changes in hemagglutinin, we reasoned that by analogy it might be possible to bypass binding to a cell surface receptor and trigger viral entry. Thus, we evaluated the capacity of soluble receptor to trigger the fusion of ASLV-A with receptor-deficient cells to test the ability of receptor to activate fully the fusogenic potential of EnvA.
Mammalian cells are refractory to infection by ASLV-A but can be rendered susceptible by expression of the receptor Tva (6). Infection of mammalian cells results in a single round of infection, since mammalian cells do not produce infectious ASLV-A. The transformed human kidney cell line 293T was used as a source of receptor-deficient, mammalian cells.
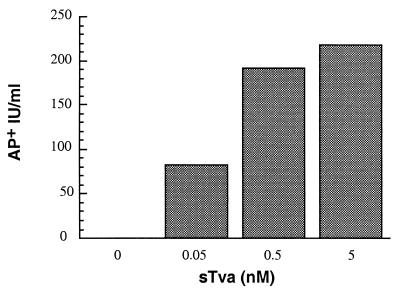
RSCA (A)-AP, a molecular clone of ALSV-A encoding the AP gene, was used in these studies to permit histochemical identification of infected cells (39). As anticipated, RCAS (A)-AP alone was unable to infect 293T cells. AP-positive cells were never detected in multiple, independent experiments even when the cells were challenged with 105 AP+ IU of virus. In contrast, when low concentrations of purified soluble receptor (0.05 to 5 nM) were incubated with 105 AP+ IU of virus at 4°C before inoculation, low levels of infectivity were detected, with titers approaching 102 AP+ IU/ml (Fig. 1). A receptor for herpes simplex virus (HSV), HveA, was used as a control of receptor specificity for activation. sHveA was expressed in insect cells and purified by affinity chromatography similarly to sTva. Preincubation of 105 IU of RCAS A-(AP) with this purified, noncognate viral receptor did not result in infection of the 293T cells, indicating that specific interactions between the virus and sTva are required for infection. To access whether sTva altered the inherent susceptibility of the target cells to retroviral infection, we evaluated the effects of sTva on the infectivity of MLV pseudotypes. The infectivity of MLV pseudotyped with vesicular stomatitis virus envelope protein was not altered in the presence of sTva (5 nM) (data not shown), suggesting that sTva does not act directly or indirectly on postentry steps in the retroviral life cycle. Thus, highly purified soluble receptor could specifically mediate low levels of ASLV-A infection of cells that expressed no functional receptor on their surfaces. This finding suggested that membrane association of the receptor is not a prerequisite for function and that Tva can act in solution to mediate viral entry. These results, in combination with those of previous in vitro studies (14, 30, 34), support strongly the hypothesis that Tva is sufficient to activate the viral glycoprotein, converting it to a fusogenic conformation, which can then catalyze the membrane fusion required for viral entry.
FIG. 1.
sTva-induced infection of receptor-deficient cells. Approximately 104 AP+ IU of RCAS (A)-AP was incubated with or without sTva at the indicated concentrations as described in Materials and Methods. Receptor-deficient 293T cells were inoculated with the virus-receptor complexes and stained for AP activity 40 to 48 h p.i. AP-positive cells were identified and enumerated microscopically. Results are representative of multiple independent experiments.
Centrifugal forces applied during inoculation dramatically enhance sTva-triggered ASLV-A infection.
During envelope virus entry, the cellular receptor plays a critical role in attaching the virus to the surface of a cell and thus brings the viral and host cell membranes in close proximity. The observation that sTva could mediate infection suggested that attachment via a membrane-associated receptor was not necessary for ASLV-A entry. However, we postulated that virus-cell fusion might be more efficient if the viral glycoproteins were in close proximity to the host cell membrane when they were activated by receptor binding. Thus, preformed virus-receptor complexes were added to cells and centrifugal forces were applied in an attempt to appose closely the viral and cellular membranes and bypass the role of receptor attachment in entry.
In addition, in vitro analysis indicates that receptor-induced changes in the viral glycoprotein are temperature dependent and are inefficient at temperatures below 16°C (14, 30, 32, 34). In contrast, conversion of the glycoprotein to a membrane-binding state occurs rapidly at physiological temperatures in the presence of sTva (14, 34). Therefore, by adding the sTva-virus complexes at 4°C and then shifting the temperature of the reaction mixture from 4 to 37°C, receptor-triggered activation of the viral envelope protein might be initiated.
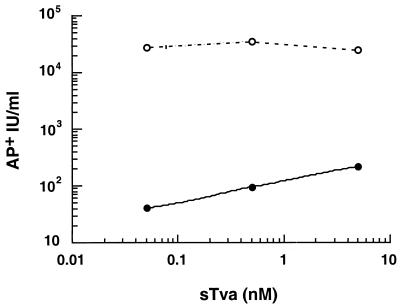
To address whether centrifugation would increase the efficiency of sTva-induced infection, we used a modified “spinoculation” protocol in which virus and sTva were incubated at 4°C to allow for formation of virus-receptor complexes (27). 293T cells were inoculated with these complexes followed by centrifugation. Under the conditions chosen, the approximately 500S retroviral particle would migrate from the supernatant onto the surfaces of the target cells. During the centrifugation, the temperature was gradually elevated from 4 to 37°C, allowing for temperature-dependent, receptor-triggered changes in the viral EnvA. The application of centrifugal force during inoculation increased the infectivities of the preformed virus-sTva complexes, giving a greater than 2-log-unit increase in viral titers compared to those obtained with inoculations without centrifugation (Fig. 2). Centrifugation did not alleviate the requirement for a cognate receptor since no infected cells were detected when they were challenged with 104 AP+ IU in the absence of sTva and they were not detected in the presence of 5 nM sHveA. Centrifugation of the target cells prior to inoculation with the soluble-receptor virus complexes did not increase the infectivities of the virus-receptor complexes, suggesting that centrifugal forces do not act by altering the inherent susceptibility of the cells to ASLV-A. Thus, centrifuging the sTva-bound ASLV-A to the cell surface increased the efficiency of viral entry into receptor-deficient cells, presumably by increasing the likelihood that the activated, viral glycoproteins came in contact with the target cellular membrane to mediate fusion.
FIG. 2.
Centrifugation of virus-receptor complexes increases viral infectivity. RCAS (A)-AP (approximately 104 AP+ IU) was incubated with or without sTva at the indicated concentrations as described in Materials and Methods. Preformed virus-receptor complexes were added to chilled 293T cells. Plates were returned to a 37°C incubator (filled circles) or centrifuged before being returned to the incubator (open circles) as described in Materials and Methods. Cells were stained for AP activity 40 to 48 h p.i., and viral titers were determined. In the absence of sTva, no infected cells were detected. The data reflect results typical of multiple independent experiments.
Polybrene dramatically enhances sTva-triggered ASLV-A infection.
The effects of centrifugation on sTva-triggered virus entry suggested that the proximity of the viral and target membranes was a critical parameter for infection of receptor-deficient cells. To determine whether centrifugation was necessary for efficient infection and whether other means of bringing the two membranes closer could substitute for centrifugation, we evaluated the effects of Polybrene on sTva-triggered infection. Polybrene is a cationic polymer which can increase the infectivities of many retroviruses such as MLV, HIV, and most ASLV subgroups with the exception of subgroup A. Polybrene acts during the attachment of the virus to the cell apparently by decreasing the repulsive surface charges of the viral and host cell membranes that would otherwise inhibit attachment. Polybrene has long been known to inhibit infection of ASLV-A (44). It has been suggested that this unique property may result from competition for Tva-binding sites between the polymer and critical basic residues within EnvA (41). With this in mind, virus-receptor complexes were formed by incubation of RCAS (A)-AP and sTva prior to the addition of Polybrene, thereby eliminating any effects of the polymer on receptor binding. The addition of Polybrene during inoculation with virus-receptor complexes increased the efficiency of infection 1 to 2 log units, giving titers approaching those obtained with centrifugation (Fig. 3). Centrifugation of virus-receptor complexes in the presence of Polybrene further increased titers approximately 10-fold to 105 AP+ IU/ml on receptor-deficient 293T cells. In the absence of purified Tva, no infection of 293T cells was detected when 104 AP+ IU of virus was centrifuged onto the target cells even in the presence of Polybrene. Thus, centrifugation is not required for efficient entry of virus-receptor complexes with receptor-deficient cells, yet this process remained absolutely dependent on sTva. The ability of Polybrene to substitute for, or enhance, centrifugal forces supported the hypothesis that centrifugation was acting to appose closely the fusion-competent virus and the target cell membrane.
Infectivity was nonlinear with respect to sTva concentration.
To further analyze the requirements for soluble receptor-mediated infection of mammalian cells, we inoculated 293T cells with a constant amount of RCAS (A)-AP (104 AP+ IU) and increasing concentrations of sTva. Both Polybrene and centrifugation were used to maximize the infectivities of the preformed virus-receptor complexes. sTva induced efficient entry of ASLV over a broad range of concentrations, with the highest titers being observed at concentrations above 0.05 nM (Fig. 4). Concentrations of sTva between 0.05 and 500 nM resulted in maximal levels of infection with no apparent inhibition even at the highest concentration tested (500 nM; data not shown). Surprisingly, at extremely low concentrations of sTva (10−2 to 10−4 nM), we were able to detect residual infectivity of the virus-receptor complexes with titers of 20 to 60 AP+ IU/ml (Fig. 4). Further dilution of sTva to 10−7 nM still exhibited this low level of infection of 293T cells (data not shown). However, infection of receptor-deficient cells remained dependent absolutely on the addition of sTva since no infection was seen without added sTva. As above, in the absence of sTva or in the presence of 5 nM sHVE-a, no infected 293T cells were observed when the cells were challenged with up to 105 AP+ IU, suggesting that infectivity detected in the presence of extremely low concentrations of sTva was not merely background.
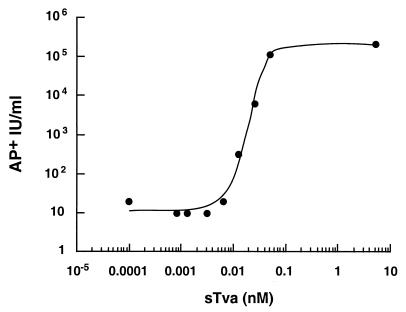
FIG. 4.
Infection of 293T cells was nonlinear in response to sTva concentration. RCAS (A)-AP was incubated without or with increasing concentrations of sTva from 5 × 10−7 to 10 nM, followed by inoculation of 293T cells in the presence of Polybrene (4 μg/ml) and centrifugation. AP-positive cells were enumerated 40 to 48 h p.i., and viral titers were calculated. No AP-positive cells were detected in the absence of sTva. The data reflect results typical of multiple independent experiments using 100 μl of RCAS (A)-AP (approximately 104 AP+ IU).
The viral titers achieved on the 293T cells were highly dependent on the concentration of sTva in the reaction mixture. A 4-log-unit increase in the infectivities of the virus-receptor complexes was observed with less than an eightfold increase in receptor concentration from 0.006 to 0.05 nM. This nonlinear and sharp dependence on receptor concentration suggests that a critical threshold of receptor molecules must bind the virus to trigger entry. Such nonlinearity in response to sTva may suggest that multiple interactions between or within EnvA trimers are required for efficient virus-cell fusion.
Soluble receptor-mediated infection is highly efficient.
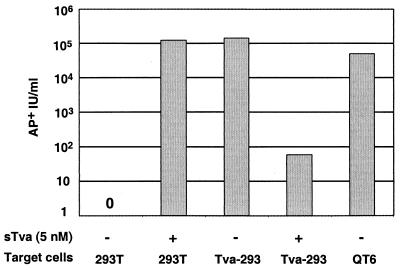
To evaluate the relative efficiencies of entry mediated by soluble and membrane-anchored receptors, we infected cells that express endogenous Tva in parallel with cells of the receptor-deficient line. At concentrations as low as 0.05 nM sTva, viral titers on 293T cells approached 105 AP+ IU/ml (Fig. 5). In the absence of sTva, similar viral titers were achieved on a 293T cell line which stably expresses superphysiological levels of Tva (Tva-293T) and on avian QT6 cells which expresses physiologic levels of Tva. Thus, the titers achieved by sTva-induced infection were greater than 95% of those obtained on 293T cells expressing Tva. Therefore, entry mediated by sTva was comparable to entry mediated by endogenous receptor and was, in relative terms, highly efficient.
FIG. 5.
sTva-induced infection is highly efficient. RCAP (A)-AP (104 AP+ IU in 100 μl) was incubated in the absence (−) or presence (+) of sTva (5 nM) as described in Materials and Methods. 293T cells were inoculated in the presence of Polybrene (4 μg/ml) and centrifuged. Tva-expressing 293T cells (Tva-293 or QT6 cells) were inoculated with virus alone (without sTva) or with virus preincubated with 5 nM sTva for 30 min at 37°C. Tva-expressing cells were inoculated in the absence of Polybrene and without centrifugation. Infected cells were enumerated 48 h p.i., and the viral titers were determined. The data reflect results typical of multiple independent experiments.
To confirm that infection of receptor-expressing lines remained Tva dependent, we evaluated the ability of soluble receptor to block infection. Preincubation of RCAS (A)-AP with 5 nM sTva for 30 min at 37°C inhibited infection of Tva-expressing 293T cells, reducing the AP titers from 1 × 105 to 2 × 105 down to less than 102 (Fig. 5). This result was consistent with a previous report of concentration of sTva required to inhibit RSCA (A)-AP (90% inhibitory concentration, 26 pM) (3). As predicted, preincubation of RCAS A-(AP) with the HSV type 1 (HSV-1) receptor sHveA (5 nM) had no effect on RCAS (A)-AP titers on Tva-293T cell lines and resulted in a titer of 105 AP+ IU/ml. The ability to specifically inhibit RCAS (A)-AP infection with sTva indicated that entry into this cell line was Tva dependent.
DISCUSSION
Entry of a virus into a cell is a critical initiating event in the infectious cycle. A greater understanding of this process is crucial both for exploitation of viruses as vehicles for targeted gene delivery and for development of therapeutic strategies to block the entry of viral pathogens. A critical parameter for viral entry is the availability of the host cell receptor to the virus, and thus receptor expression is an important determinant of both tropism and pathogenesis. Viral receptors are highly pleomorphic both in structure and in chemical composition. While viruses have evolved to use highly divergent receptors, there is ever growing evidence that many viral envelope proteins share architectural and structural similarities (53). This is particularly true of the membrane-associated subunits of divergent viruses such as members of the orthomyxovirus, paramyxovirus, filovirus, and retrovirus families (9, 10, 24, 25, 46–49). Conservation of structures and motifs within this subunit, believed to be responsible for the fusogenic properties of envelope proteins, suggests that these diverse viruses may use common molecular mechanisms to catalyze membrane fusion. Thus, our increased understanding of the molecular interactions between ASLV-A and its receptor may shed light on the entry of other viruses such as HIV.
A preponderance of evidence indicates that Tva is the only host protein required for ASLV-A entry. Susceptibility to ASLV-A maps to a single locus encoding Tva (5). Expression of Tva confers susceptibility to ASLV-A entry in all cells evaluated from different tissues and divergent species (P. Bates, unpublished data), suggesting that Tva is sufficient or that any other factor is conserved among birds, fish, and mammals. Incorporation of Tva into retroviral pseudotypes lacking viral envelope proteins directs viral infection of cells expressing EnvA (2). Receptor pseudotypes appear to maintain the same receptor requirements for fusion as those of the parental virus, as demonstrated by the requirement of both CD4 and a cognate coreceptor for receptor-pseudotype targeting of cells expressing HIV envelope (23). Finally, sTva binds specifically and avidly to EnvA and antiserum against Tva blocks ASLV-A infection of avian cells (3, 6, 12, 29). Thus, genetic, cell biological, and biochemical evidence supports the hypothesis that Tva is necessary and sufficient for viral entry.
In vitro studies suggest that Tva binding induces a number of conformational changes in the viral envelope protein. These changes, which include exposure of the fusion peptide and acquisition of membrane-binding capacity (14, 30, 34), are reminiscent of the structural and biophysical changes that occur in pH-dependent viral envelope proteins under low-pH conditions. Collectively, these in vitro data strongly support the hypothesis that receptor binding triggers the structural changes in EnvA necessary for conversion to an active state. Here, we provide further evidence that Tva-induced changes in the viral envelope represent conversion of EnvA to a fusion-competent conformation capable of catalyzing membrane mixing and virus-cell fusion necessary for viral entry.
ASLV-A complexed with sTva can infect mammalian cells that lack cell surface receptors and are therefore resistant to virus alone. Since membrane fusion is a prerequisite for enveloped-virus entry, infection of these cells indicates that sTva can act in solution to activate the fusogenic potential of EnvA on the surface of the virus. Therefore, membrane association of the receptor is not required absolutely for membrane fusion and viral entry. The ability to increase the infectivities of the preformed complexes with treatments predicted to reduce the distance between the virus and cell surface suggests that the proximity of the viral and cellular membranes is likely an important determinant for efficient fusion triggered by sTva. Studies of the measles virus receptor CD46 also demonstrate that the proximity of the virus to the cell membrane is an important determinant for viral entry (8). Infection of these receptor-deficient cells was dependent absolutely on the presence of highly purified soluble receptor since infection was never detected in the absence of sTva or in the presence of another, unrelated viral receptor. Thus, soluble receptor can be used to bypass the absence of membrane-associated receptors on the cell surface and confer susceptibility to viral entry.
Soluble-receptor-triggered infection was highly dependent on receptor concentration and demonstrated a nonlinear response to concentration. Such a nonlinear response suggests that multiple receptor-binding events are required for viral infection and therefore virus-cell fusion. All indications suggest that Tva is a monomer (J. Balliet, personal communication). In contrast, EnvA is a trimer (21, 31) capable of binding multiple receptor molecules. Furthermore, the viral particle, containing many EnvA molecules, is multivalent. The nonlinearity of sTva-dependent infection suggests that multiple receptor-EnvA interactions are required for receptor-mediated entry of ASLV-A. At minimum, there are three events in the entry process that may be influenced by cooperative interactions: (i) binding of the receptor to the oligomeric EnvA, (ii) receptor-triggered conversion of EnvA to EnvA with a membrane-binding conformation, and (iii) fusion pore formation mediated by this membrane-bound EnvA. Binding studies indicate that a monomer of EnvA constitutes the minimal binding unit, while oligomers of EnvA can bind multiple receptor molecules (L. Rong, personal communication). There is no indication from binding studies to suggest that the Tva-EnvA interaction is cooperative (3, 12), suggesting that the nonlinearity of sTva-triggered infection is unlikely to be a consequence of EnvA-Tva binding directly. In contrast, a study using a water-soluble form of EnvA indicates sTva-induced conversion of the viral envelope protein to one in a membrane-binding state is highly dependent on receptor concentration (14). Thus, in a cell-free experimental system, there is evidence that sTva-induced changes in EnvA are cooperative in nature. From these results, we infer that the nonlinearity of sTva-triggered infection in part reflects that cooperative nature of receptor-induced conversion of EnvA to a protein in a membrane-binding conformation. Fusion pore formation and subsequent membrane fusion mediated by EnvA may require multiple, membrane-bound EnvA molecules and thus may also contribute to the nonlinear response described here. Future studies are required to determine the molecular requirements and stoichiometry of fusion pore formation during EnvA-mediated entry and the role, if any, of cooperative interactions. There is, however, evidence from other viral systems, such as influenza virus, that fusion pore formation requires multiple viral envelope proteins (15). By analogy, EnvA-mediated fusion is likely to require multiple, activated trimers. Thus, both cell-free and virus-based assays suggest that cooperativity within the EnvA oligomer, and perhaps between oligomers, is critical for efficient receptor-induced membrane fusion.
Classically, soluble receptors have been used to inhibit viral infection in vitro (17, 26, 35, 45). Such inhibition may reflect the ability of the soluble receptor to compete with the membrane-anchored receptor for virus binding. Alternatively, inhibition may involve receptor-induced changes in the virus that result in the inactivation of the virus in addition to a simple blockade. This is true of soluble-CD4-mediated inhibition of some HIV-1 isolates (7, 36, 38). ASLV-A infection of receptor-expressing cells is blocked in the presence of sTva, likely through competition, and also by receptor-induced structural changes possibly leading to virus inactivation (3). Interestingly, in line with the experiments described here, the inhibitory effect of sTva occurred at very low concentrations of sTva, with a 90% inhibitory concentration of 25 pM (3).
While soluble forms of viral receptors can have antiviral activity and inhibit infection, there is evidence that soluble receptors can also enhance viral infection. Studies have demonstrated the enhanced infection of some SIV and HIV-2 isolates in the presence of the soluble receptor sCD4 (1, 11, 50). It has long been recognized that CD4 is not sufficient to mediate HIV entry into some nonhuman cell lines (37). The observation that sCD4 could enhance infection, coupled with evidence that CD4 was insufficient, suggested that secondary host factors are required for HIV and SIV infection. Chemokine receptors have been identified as the coreceptors for these viruses (4, 18), and, in fact, some HIV-2 and SIV isolates enter and fuse in a CD4-independent manner through interactions with these chemokine receptors (20, 22). Binding of the viral envelope protein gp120 to CD4 triggers conformational changes in gp120 and appears to promote association between gp120 and the chemokine receptors (56). Thus, the apparent role of CD4 in facilitating interactions of gp120 with the chemokine receptors suggests that sCD4-enhanced infection is most likely mediated through the ability of CD4 to prime the virus to interact with the coreceptors in the target cell membrane. In this way, sCD4-enhanced infection appears to be distinct from sTva-induced infection. ASLV-A entry, unlike that of HIV and SIV, appears to require a single receptor, which is sufficient to convert the viral glycoprotein to one with a membrane-binding conformation (14, 34).
Analyses of poliovirus, a pH-independent nonenveloped virus, suggest that receptor binding triggers changes in virus that are functionally similar to the changes observed in EnvA following Tva binding. Receptor binding induces conformational changes in poliovirus, converting it from the native, 160S particle to a 135S particle at physiological temperatures (33). This conformationally altered particle gains the ability to bind target membranes (28). Similarly, sTva-triggered conversion of EnvA to a membrane-binding conformation is coincident with a decrease in receptor-binding activity, perhaps facilitating later events in the entry pathway. Thus, in both systems there is evidence that receptor binding induces temperature-dependent changes in viral proteins that lead to insertion of viral proteins into a target membrane and that are associated with the loss of receptor binding activity. In addition, there is indirect evidence that these receptor-induced changes in poliovirus prime the virus, allowing for infection of otherwise resistant cell lines. 135S virus particles can also be produced in vitro by heating the native virus to 50°C (13). Particles produced in this manner can infect cells resistant to the native virus, perhaps suggesting that receptor-induced changes in poliovirus may enable the virus to enter the cell. A recent report indicates that expression of the poliovirus receptor in the mouse gut is insufficient to confer susceptibility to poliovirus following oral inoculation of virus, suggesting that other host factors are essential for viral replication (16). Whether these still undefined host factors represent an entry cofactor or a coreceptor remains to be determined.
Snitkovsky and Young have recently described the use of a soluble chimeric Tva reagent to direct retroviral infection into specific cells (42). A fusion protein containing the extracellular domain of Tva and the mature form of human epidermal growth factor (EGF) was used to bind to the cell surface EGF receptor. Binding of the soluble chimera conferred sensitivity to ASLV-A as assessed by specific transduction of virally encoded resistance markers. In contrast with our results, those authors were unable to detect infection of cells lacking the EGF receptor and concluded from this that cell surface binding through the EGF receptor was necessary for viral infection and thus that it conferred specificity. While it is not possible to directly compare the absolute viral titers achieved in the two studies, it is clear that the relative efficiency of infection mediated by sTva was much higher than was achieved with the Tva-EGF chimera. sTva-induced infection resulted in viral titers essentially equivalent to the titers (>95%) obtained on the same line expressing cell surface Tva. In contrast, even when Tva-deficient cells were challenged with a larger viral inoculum than the Tva-expressing cells, the infectivity mediated by the Tva-EGF chimera represented only 10 to 23% of the level achieved on the Tva-positive line. The discrepancy between these two reports may be accounted for by a number of differences, including variations in the inoculation protocols, such as the use of centrifugation and the addition of polycations; the source, purity, and concentration of the soluble receptor; and differences in target cell specificities and the means of identifying infected cells. Although our results using sTva suggest that the specificity of targeting mediated by the Tva-EGF chimera may not be absolute, both reports demonstrate clearly that the Tva-EnvA interaction activates the fusogenic potential of this viral envelope protein.
ACKNOWLEDGMENTS
We thank Kristin Gendron for providing the Tva-expressing mammalian cell line used in this study, John Balliet for the sTva used in some of the experiments, and Warren Pear for suggesting the spinoculation procedure. We also thank the members of the Bates laboratory for useful discussions.
This work was supported by grants to P.B. from the National Institutes of Health (CA63531 and CA76256). R.D. was supported by grant T32 GM07229 from the NIH.
REFERENCES
- 1.Allan J S, Strauss J, Buck D W. Enhancement of SIV infection with soluble receptor molecules. Science. 1990;247:1084–1088. doi: 10.1126/science.2309120. [DOI] [PubMed] [Google Scholar]
- 2.Balliet J W, Bates P. Efficient infection mediated by viral receptors incorporated into retroviral particles. J Virol. 1998;72:671–676. doi: 10.1128/jvi.72.1.671-676.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balliet J W, Berson J, D'Cruz C M, Huang J, Crane J, Gilbert J M, Bates P. Production and characterization of a soluble, active form of Tva, the subgroup A avian sarcoma and leukosis virus receptor. J Virol. 1999;73:3054–3061. doi: 10.1128/jvi.73.4.3054-3061.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates P. Chemokine receptors and HIV-1: an attractive pair? Cell. 1996;86:1–3. doi: 10.1016/s0092-8674(00)80070-7. [DOI] [PubMed] [Google Scholar]
- 5.Bates P, Rong L, Varmus H E, Young J A T, Crittenden L B. Genetic mapping of the cloned subgroup A avian sarcoma and leukosis virus receptor gene to the TVA locus. J Virol. 1998;72:2505–2508. doi: 10.1128/jvi.72.3.2505-2508.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bates P, Young J A, Varmus H E. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell. 1993;74:1043–1051. doi: 10.1016/0092-8674(93)90726-7. [DOI] [PubMed] [Google Scholar]
- 7.Berger E A, Lifson J D, Eiden L E. Stimulation of glycoprotein gp120 dissociation from the envelope glycoprotein complex of human immunodeficiency virus type 1 by soluble CD4 and CD4 peptide derivatives: implications for the role of the complementarity-determining region 3-like region in membrane fusion. Proc Natl Acad Sci USA. 1991;88:8082–8086. doi: 10.1073/pnas.88.18.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchholz C J, Schneider U, Devaux P, Gerlier D, Cattaneo R. Cell entry by measles virus: long hybrid receptors uncouple binding from membrane fusion. J Virol. 1996;70:3716–3723. doi: 10.1128/jvi.70.6.3716-3723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bullough P A, Hughson F M, Skehel J J, Wiley D C. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- 10.Chan D C, Fass D, Berger J M, Kim P S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 11.Clapham P R, McKnight A, Weiss R A. Human immunodeficiency virus type 2 infection and fusion of CD4-negative human cell lines: induction and enhancement by soluble CD4. J Virol. 1992;66:3531–3537. doi: 10.1128/jvi.66.6.3531-3537.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connolly L, Zingler K, Young J A. A soluble form of a receptor for subgroup A avian leukosis and sarcoma viruses (ALSV-A) blocks infection and binds directly to ALSV-A. J Virol. 1994;68:2760–2764. doi: 10.1128/jvi.68.4.2760-2764.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curry S, Chow M, Hogle J M. The poliovirus 135S particle is infectious. J Virol. 1996;70:7125–7131. doi: 10.1128/jvi.70.10.7125-7131.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damico R L, Crane J, Bates P. Receptor-triggered membrane association of a model retroviral glycoprotein. Proc Natl Acad Sci USA. 1998;95:2580–2585. doi: 10.1073/pnas.95.5.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danieli T, Pelletier S L, Henis Y I, White J M. Membrane fusion mediated by the influenza virus hemagglutinin requires the concerted action of at least three hemagglutinin trimers. J Cell Biol. 1996;133:559–569. doi: 10.1083/jcb.133.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deatly A M, Taffs R E, McAuliffe J M, Nawoschik S P, Coleman J W, McMullen G, Weeks-Levy C, Johnson A J, Racaniello V R. Characterization of mouse lines transgenic with the human poliovirus receptor gene. Microb Pathog. 1998;25:43–54. doi: 10.1006/mpat.1998.0212. [DOI] [PubMed] [Google Scholar]
- 17.Deen K C, McDougal J S, Inacker R, Folena-Wasserman G, Arthos J, Rosenberg J, Maddon P J, Axel R, Sweet R W. A soluble form of CD4 (T4) protein inhibits AIDS virus infection. Nature. 1988;331:82–84. doi: 10.1038/331082a0. [DOI] [PubMed] [Google Scholar]
- 18.Dimitrov D S. How do viruses enter cells? The HIV coreceptors teach us a lesson of complexity. Cell. 1997;91:721–730. doi: 10.1016/s0092-8674(00)80460-2. [DOI] [PubMed] [Google Scholar]
- 19.Doms R W, Helenius A, White J. Membrane fusion activity of the influenza virus hemagglutinin. The low pH-induced conformational change. J Biol Chem. 1985;260:2973–2981. [PubMed] [Google Scholar]
- 20.Edinger A L, Mankowski J L, Doranz B J, Margulies B J, Lee B, Rucker J, Sharron M, Hoffman T L, Berson J F, Zink M C, Hirsch V M, Clements J E, Doms R W. CD4-independent, CCR5-dependent infection of brain capillary endothelial cells by a neurovirulent simian immunodeficiency virus strain. Proc Natl Acad Sci USA. 1997;94:14742–14747. doi: 10.1073/pnas.94.26.14742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Einfeld D, Hunter E. Oligomeric structure of a prototype retrovirus glycoprotein. Proc Natl Acad Sci USA. 1988;85:8688–8692. doi: 10.1073/pnas.85.22.8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Endres M J, Clapham P R, Marsh M, Ahuja M, Turner J D, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N, Power C A, Sutterwala S S, Doms R W, Landau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 23.Endres M J, Jaffer S, Haggarty B, Turner J D, Doranz B J, O'Brien P J, Kolson D L, Hoxie J A. Targeting of HIV- and SIV-infected cells by CD4-chemokine receptor pseudotypes. Science. 1997;278:1462–1464. doi: 10.1126/science.278.5342.1462. [DOI] [PubMed] [Google Scholar]
- 24.Fass D, Harrison S C, Kim P S. Retrovirus envelope domain at 1.7 angstrom resolution. Nat Struct Biol. 1996;3:465–469. doi: 10.1038/nsb0596-465. [DOI] [PubMed] [Google Scholar]
- 25.Fass D, Kim P S. Dissection of a retrovirus envelope protein reveals structural similarity to influenza hemagglutinin. Curr Biol. 1995;5:1377–1383. doi: 10.1016/s0960-9822(95)00275-2. [DOI] [PubMed] [Google Scholar]
- 26.Fisher R A, Bertonis J M, Meier W, Johnson V A, Costopoulos D S, Liu T, Tizard R, Walker B D, Hirsch M S, Schooley R T, et al. HIV infection is blocked in vitro by recombinant soluble CD4. Nature. 1988;331:76–78. doi: 10.1038/331076a0. [DOI] [PubMed] [Google Scholar]
- 27.Forestell S P, Dando J S, Bohnlein E, Rigg R J. Improved detection of replication-competent retrovirus. J Virol Methods. 1996;60:171–178. doi: 10.1016/0166-0934(96)02052-6. [DOI] [PubMed] [Google Scholar]
- 28.Fricks C E, Hogle J M. Cell-induced conformational change in poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J Virol. 1990;64:1934–1945. doi: 10.1128/jvi.64.5.1934-1945.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilbert J M, Bates P, Varmus H E, White J M. The receptor for the subgroup A avian leukosis-sarcoma viruses binds to subgroup A but not to subgroup C envelope glycoprotein. J Virol. 1994;68:5623–5628. doi: 10.1128/jvi.68.9.5623-5628.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilbert J M, Hernandez L D, Balliet J W, Bates P, White J M. Receptor-induced conformational changes in the subgroup A avian leukosis and sarcoma virus envelope glycoprotein. J Virol. 1995;69:7410–7415. doi: 10.1128/jvi.69.12.7410-7415.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbert J M, Hernandez L D, Chernov-Rogan T, White J M. Generation of a water-soluble oligomeric ectodomain of the Rous sarcoma virus envelope glycoprotein. J Virol. 1993;67:6889–6892. doi: 10.1128/jvi.67.11.6889-6892.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilbert J M, Mason D, White J M. Fusion of Rous sarcoma virus with host cells does not require exposure to low pH. J Virol. 1990;64:5106–5113. doi: 10.1128/jvi.64.10.5106-5113.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez Yafal A, Kaplan G, Racaniello V R, Hogle J M. Characterization of poliovirus conformational alteration mediated by soluble cell receptors. Virology. 1993;197:501–505. doi: 10.1006/viro.1993.1621. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez L D, Peters R J, Delos S E, Young J A, Agard D A, White J M. Activation of a retroviral membrane fusion protein: soluble receptor-induced liposome binding of the ALSV envelope glycoprotein. J Cell Biol. 1997;139:1455–1464. doi: 10.1083/jcb.139.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hussey R E, Richardson N E, Kowalski M, Brown N R, Chang H C, Siliciano R F, Dorfman T, Walker B, Sodroski J, Reinherz E L. A soluble CD4 protein selectively inhibits HIV replication and syncytium formation. Nature. 1988;331:78–81. doi: 10.1038/331078a0. [DOI] [PubMed] [Google Scholar]
- 36.Layne S P, Merges M J, Dembo M, Spouge J L, Nara P L. HIV requires multiple gp120 molecules for CD4-mediated infection. Nature. 1990;346:277–279. doi: 10.1038/346277a0. [DOI] [PubMed] [Google Scholar]
- 37.Maddon P J, Dalgleish A G, McDougal J S, Clapham P R, Weiss R A, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 38.Moore J P, McKeating J A, Norton W A, Sattentau Q J. Direct measurement of soluble CD4 binding to human immunodeficiency virus type 1 virions: gp120 dissociation and its implications for virus-cell binding and fusion reactions and their neutralization by soluble CD4. J Virol. 1991;65:1133–1140. doi: 10.1128/jvi.65.3.1133-1140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rong L, Bates P. Analysis of the subgroup A avian sarcoma and leukosis virus receptor: the 40-residue, cysteine-rich, low-density lipoprotein receptor repeat motif of Tva is sufficient to mediate viral entry. J Virol. 1995;69:4847–4853. doi: 10.1128/jvi.69.8.4847-4853.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rong L, Edinger A, Bates P. Role of basic residues in the subgroup-determining region of the subgroup A avian sarcoma and leukosis virus envelope in receptor binding and infection. J Virol. 1997;71:3458–3465. doi: 10.1128/jvi.71.5.3458-3465.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rong L J, Gendron K, Strohl B, Shenoy R, Wool-Lewis R, Bates P. Characterization of determinants for envelope binding and infection in Tva, the subgroup a avian sarcoma and leukosis virus receptor. J Virol. 1998;72:4552–4559. doi: 10.1128/jvi.72.6.4552-4559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snitkovsky S, Young J A. Cell-specific viral targeting mediated by a soluble retroviral receptor-ligand fusion protein. Proc Natl Acad Sci USA. 1998;95:7063–7068. doi: 10.1073/pnas.95.12.7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stegmann T, Delfino J M, Richards F M, Helenius A. The HA2 subunit of influenza hemagglutinin inserts into the target membrane prior to fusion. J Biol Chem. 1991;266:18404–18410. [PubMed] [Google Scholar]
- 44.Toyoshima K, Vogt P K. Enhancement and inhibition of avian sarcoma viruses by polycations and polyanions. Virology. 1969;38:414–426. doi: 10.1016/0042-6822(69)90154-8. [DOI] [PubMed] [Google Scholar]
- 45.Traunecker A, Luke W, Karjalainen K. Soluble CD4 molecules neutralize human immunodeficiency virus type 1. Nature. 1988;331:84–86. doi: 10.1038/331084a0. [DOI] [PubMed] [Google Scholar]
- 46.Weissenhorn W, Calder L J, Dessen A, Laue T, Skehel J J, Wiley D C. Assembly of a rod-shaped chimera of a trimeric GCN4 zipper and the HIV-1 gp41 ectodomain expressed in Escherichia coli. Proc Natl Acad Sci USA. 1997;94:6065–6069. doi: 10.1073/pnas.94.12.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weissenhorn W, Carfr A, Lee K, Skehel J, Wiley D. Crystal structure of the Ebola virus membrane fusion subunit, GP2, from the envelope glycoprotein ectodomain. Mol Cell. 1998;2:605–616. doi: 10.1016/s1097-2765(00)80159-8. [DOI] [PubMed] [Google Scholar]
- 48.Weissenhorn W, Dessen A, Harrison S C, Skehel J J, Wiley D C. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 49.Weissenhorn W, Wharton S A, Calder L J, Earl P L, Moss B, Aliprandis E, Skehel J J, Wiley D C. The ectodomain of HIV-1 env subunit gp41 forms a soluble, alpha-helical, rod-like oligomer in the absence of gp120 and the N-terminal fusion peptide. EMBO J. 1996;15:1507–1514. [PMC free article] [PubMed] [Google Scholar]
- 50.Werner A, Winskowsky G, Kurth R. Soluble CD4 enhances simian immunodeficiency virus SIVagm infection. J Virol. 1990;64:6252–6256. doi: 10.1128/jvi.64.12.6252-6256.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitbeck J C, Peng C, Lou H, Xu R, Willis S H, Ponce de Leon M, Peng T, Nicola A V, Montgomery R I, Warner M S, Soulika A M, Spruce L A, Moore W T, Lambris J D, Spear P G, Cohen G H, Eisenberg R J. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J Virol. 1997;71:6083–6093. doi: 10.1128/jvi.71.8.6083-6093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.White J, Matlin K, Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981;89:674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White J M. Viral and cellular membrane fusion proteins. Annu Rev Physiol. 1990;52:675–697. doi: 10.1146/annurev.ph.52.030190.003331. [DOI] [PubMed] [Google Scholar]
- 54.Wigler M, Silverstein S, Lee L S, Pellicer A, Cheng Y, Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977;11:223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- 55.Wool-Lewis R J, Bates P. Characterization of Ebola virus entry by using pseudotyped viruses: identification of receptor-deficient cell lines. J Virol. 1998;72:3155–3160. doi: 10.1128/jvi.72.4.3155-3160.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]