Abstract
Purpose
To investigate the molecular mechanism of pathological keratinization in the chronic phase of ocular surface (OS) diseases.
Methods
In this study, a comprehensive gene expression analysis was performed using oligonucleotide microarrays on OS epithelial cells obtained from three patients with pathological keratinization (Stevens-Johnson syndrome [n = 1 patient], ocular cicatricial pemphigoid [n = 1 patient], and anterior staphyloma [n = 1 patient]). The controls were three patients with conjunctivochalasis. The expression in some transcripts was confirmed using quantitative real-time PCR.
Results
Compared to the controls, 3118 genes were significantly upregulated by a factor of 2 or more than one-half in the pathological keratinized epithelial cells (analysis of variance P < 0.05). Genes involved in keratinization, lipid metabolism, and oxidoreductase were upregulated, while genes involved in cellular response, as well as known transcription factors (TFs), were downregulated. Those genes were further analyzed with respect to TFs and retinoic acid (RA) through gene ontology analysis and known reports. The expression of TFs MYBL2, FOXM1, and SREBF2, was upregulated, and the TF ELF3 was significantly downregulated. The expression of AKR1B15, RDH12, and CRABP2 (i.e., genes related to RA, which is known to suppress keratinization) was increased more than twentyfold, whereas the expression of genes RARB and RARRES3 was decreased by 1/50. CRABP2, RARB, and RARRES3 expression changes were also confirmed by qRT-PCR.
Conclusions
In pathological keratinized ocular surfaces, common transcript changes, including abnormalities in vitamin A metabolism, are involved in the mechanism of pathological keratinization.
Keywords: ocular surface disease, keratinization, retinoic acid receptor beta (RARB), vitamin A
Ocular surface (OS) diseases (OSDs) such as Stevens-Johnson syndrome (SJS) and ocular cicatricial pemphigoid (OCP), as well as thermal/chemical injuries, are very devastating ocular disorders, and the associated prolonged OS inflammation can cause further damage to the residual limbal stem cells, thus inducing limbal stem cell deficiency. In the most severe OSD cases, the conjunctival epithelium and goblet cells can also be compromised, thus resulting in severe dry eye disease with keratinization and symblepharon in addition to severe aqueous tear deficiency via the involvement of lacrimal gland ducts in the subconjunctival scarring.1–4
SJS is a systemic disease characterized by acute inflammatory vesiculobullous reaction of the skin, OS, and oral cavity, and is termed “toxic epidermal necrolysis” (i.e., the more severe variant of SJS) when the disease affects a larger surface area of the body.2,3,5 OCP is a type of pemphigoid of the mucosa characterized by chronic, recurrent, and progressive conjunctivitis. In the chronic phase of these OSDs, OS inflammation persists, as do OS complications such as severe dry eye, eyelash disorders, and conjunctival invasion.1,3,4,6
In cases of severe OSD, pathological keratinization develops on the OS, resulting in severe vision loss. Despite the variety of surgical procedures currently available and the advanced methods of postoperative management that have recently been developed, OS reconstruction in cases of severe OSD, including those with keratinization, remains one of the most challenging procedures in ophthalmology.7
The pathologic transformation from nonkeratinizing to keratinizing epithelium is termed 'squamous metaplasia'. In the OS, squamous metaplasia leads to loss of goblet cells, stratification of the epithelium, and pathological keratinization, and it has reportedly been found in various OSDs, such as SJS, OCP, thermal/chemical injury, and dry eye disease associated with Sjögren's syndrome, as well as in vitamin A deficiency cases.8,9
We hypothesized that although various pathological conditions are involved in OS keratinization, a common mechanism occurs for transformation to keratinizing epithelial cells. In this study, we clinically examined OSD cases with pathological keratinization of the OS to identify the expressed proteins via immunostaining and comprehensive microarray gene expression analysis to clarify the underlying process that leads to pathological keratinization.
Material and Methods
Patient and Public Involvement
The protocols of this study were approved by the Institutional Review Board of Kyoto Prefectural University of Medicine, Kyoto, Japan, and written informed consent was obtained from all participants before their involvement in the study. All study procedures were conducted in accordance with the tenets set forth in the Declaration of Helsinki.
This study involved three severe OSD patients (a 79-year-old woman with OCP, a 23-year-old man with SJS, and a 10-month-old male infant with anterior staphyloma who presented with severe keratinization) seen at the Department of Ophthalmology of Kyoto Prefectural University of Medicine. In all three cases, conjunctival invasion and subsequent pathological keratinization had occurred, so surgical OS reconstruction was performed to restore the conjunctival fornix and improve visual acuity,10 and a conjunctival tissue sample was obtained at the time of surgery. In addition, in the patient who had difficulty closing his eyelid because of anterior staphyloma with resultant gradual keratinization of the OS, a keratinized sample was obtained after exenterating the bulbar for the purpose of an ocular prosthesis stretched to enhance the development of the fornix and orbit (Fig. 1).11 The control subjects in this study were three female patients (age 45, 73, and 79 years ) with conjunctivochalasis. In all three patients, a conjunctival tissue sample was obtained during conjunctivochalasis surgery.
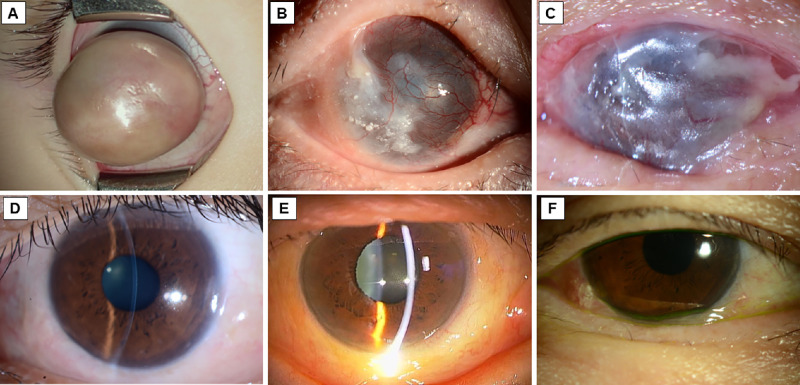
Figure 1.
Photographs of the eyes of three patients with OS keratinization (A–C) and the three control patients with conjunctivochalasis (D–F). (A) Intraoperative photo of the eye of a patient with anterior staphyloma that resulted in an eyelid closure disorder and the progressive development of skin-like keratinization. The patient underwent exenteration of the bulbar for the purpose of an ocular prosthesis stretched to enhance the development of the fornix and orbit. (B) The eye of an SJS patient with symblepharon, trichiasis, conjunctival invasion, and clinical keratinization. (C) The eye of a patient with OCP that resulted in conjunctival invasion because of limbal stem cell deficiency, symblepharon, and clinical keratinization. (D–F) Images of the eyes of the three control patients with inferior conjunctivochalasis.
Human Conjunctival Epithelium
Conjunctival epithelial tissue was immersed in 1.0 U/mL of Dispase II (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) overnight at 4°C to isolate epithelial cells.
Morphological Evaluation and Immunohistochemistry
For morphological evaluation and immunohistochemistry, tissue samples were obtained from the 23-year-old man with SJS and the 10-month-old male infant with anterior staphyloma. Briefly, the samples were first frozen-embedded with optimal cutting temperature compound and sectioned, and then fixed in methanol at 4°C for 10 minutes, washed with PBS, and permeabilized with 0.5% Triton X-100 (Thermo Fischer Scientific, Inc., Waltham, MA, USA)/0.01 M PBS for 15 minutes. Hematoxylin-eosin (H&E) staining was then used for morphological evaluation. All immunostaining was performed using mouse IgG antibodies as the primary antibodies, with the samples then being incubated overnight at 4°C under a moist condition. The primary antibodies used in this study were cytokeratin (CK)-1 (CK1), CK10 (both obtained from Santa Cruz Biotechnology, Santa Cruz, CA, USA), CK13 (obtained from Invitrogen Corporation, Carlsbad, CA, USA), and filaggrin (obtained from Biogenesis, Westminster, CO, USA). After the slides were washed, secondary antibodies were incubated with Alexa Fluor 488 donkey anti-mouse IgG (Life Technologies Corporation, San Diego, CA, USA) for one hour at room temperature for staining. The slides were once again washed and then encapsulated with an anti-fading encapsulant (Nacalai Tesque, Inc., Kyoto, Japan) containing DAPI (4′,6-diamidino-2-phenylindole).
Gene Expression Analysis
Gene expression analysis was performed using high-density oligonucleotide arrays (Clariom S Array, human ; Applied Biosystems, Waltham, MA, USA) for 21,448 genes. Total RNA of isolated epithelial cells was first isolated using the RNeasy Plus Mini Kit (Qiagen, Inc., Valencia, CA, USA) or TRIzol Reagent (Thermo Fisher Scientific, Waltham, MA, USA) and then purified using the NucleoSpin RNA Clean-up XS (Macherey-Nagel GmbH & Co. KG, Dueren, Germany) kit. Microarray experiments were then performed according to the protocols provided by Thermo Fisher Scientific, and scanned microarray images were obtained using the GeneChip Scanner 3000 7G (Thermo Fisher Scientific) microarray analysis system.
Quantitative Real-Time PCR
Isolated total RNA was reverse transcribed using the QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany). QRT-PCR was performed on a StepOnePlus (Applied Biosystems) RT-PCR system according to the manufacturer's instructions. The primers used in this study are listed in Supplementary Table S1. Quantitative comparisons were performed by normalizing for the expression of the housekeeping gene 18S.
Data Analysis
Microarray data was normalized using the Transcriptome Analysis Console v.4.0.3 (Thermo Fisher Scientific) to identify genes with statistically significant changes in gene expression (P value [analysis of variance {ANOVA}, false discovery rate {FDR}] < 0.05, |Fold Change| > 2). Principal component analysis plots and Volcano plots were plotted using the Transcriptome Analysis Console. Gene ontology (GO) analysis was performed using DAVID (Database for Annotation, Visualization and Integrated Discovery) (https://david.ncifcrf.gov) for genes with significant expression change (FDR < 0.05, P < 0.05), and charted using the QuickGO browser (https://www.ebi.ac.uk/QuickGO/) from the 10 GO terms with the lowest P values. Enrichment analysis was performed using ChIP-Atlas (https://chip-atlas.org) to analyze upstream transcription factors (TFs). QRT-PCR was analyzed using the t-test, and a P value < 0.05 was considered statistically significant. All statistical analyses were performed using EZR (Easy R) (Saitama Medical Center, Jichi Medical University, Saitama, Japan) statistics software (i.e., a graphical user interface for R ; The R Foundation for Statistical Computing, Vienna, Austria) that adds statistical functions frequently used in biostatistics.12
Results
Morphological Evaluation and Immunohistochemistry
In the two OSD patients with OCP, SJS, and anterior staphyloma, respectively, the conditions worsened over time, thus ultimately leading to pathological keratinization (Fig. 1). The three control patients with conjunctivochalasis who had pathologically nonkeratinized conjunctiva are included Figure 1 for comparison.
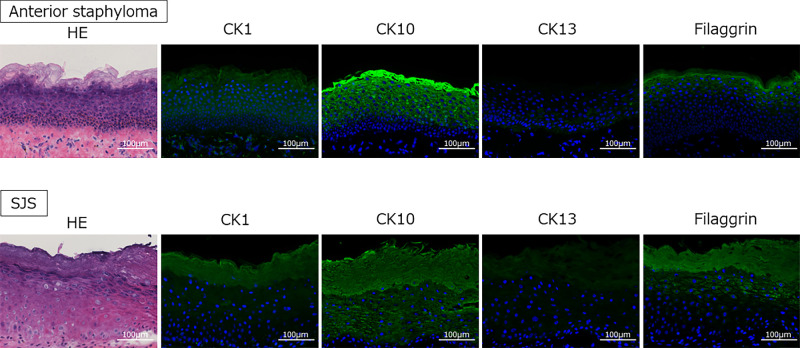
Morphological evaluation of the keratinized samples (SJS and anterior staphyloma) was performed using H&E staining, and expression of CK and filaggrin in the pathological keratinization was evaluated by immunohistochemistry (Fig. 2). The keratinized cases were highly stratified, forming a cornified envelope with some nucleus containing cells. The expression of CK1, CK10, and filaggrin (all three being keratinization markers) were elevated, whereas the expression of CK13 (a marker of nonkeratinization) was decreased.
Figure 2.
H&E and immunostaining images of representative cases of anterior staphyloma (top row) and SJS (bottom row) with OS keratinization. In the anterior staphyloma patient, thickened tissue over the cornea-like tissue was sectioned. In the SJS patient, sections were prepared from tissue from the peripheral cornea to the conjunctiva. In both cases, the more superficial tissues cornified, but the cornified layer also showed hyperkeratosis with some nucleus containing cells. CK-13, a marker of nonkeratinized mucosal epithelium, lost its staining, and the staining of keratinization markers CK1, CK10, and filaggrin was highly elevated. Scale bar: 100 µm.
Transcriptome Analysis
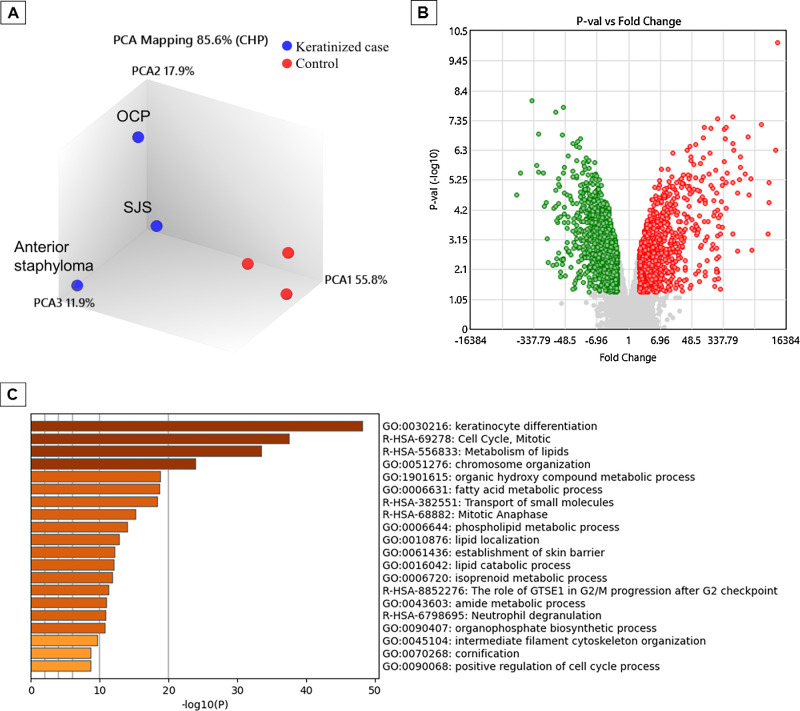
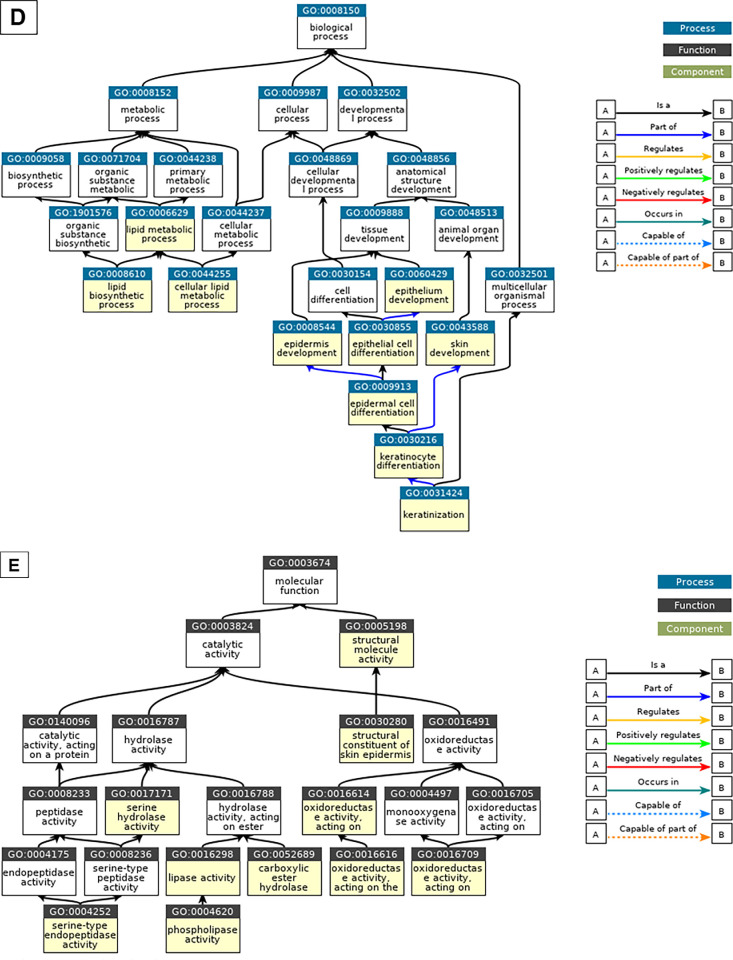
For comparison of the upregulated and downregulated transcripts in the three keratinized cases and the three control cases, comprehensive gene expression analysis using microarrays was performed in all six cases. Principal component analysis revealed distinct differences in disease characteristics between the keratinized cases and the nonkeratinized control cases, with the keratinized cases exhibiting a significant upregulation of 1272 genes and a significant downregulation of 1846 genes (Fig. 3). On the genes that were significantly upregulated, enrichment analysis was performed using Metascape (Fig. 3), a web-based portal used for cross-sectional analysis of enrichment in each GO term, KEGG (Kyoto Encyclopedia of Genes and Genomes), and canonical pathway classification. The GO term was assigned to classify genes based on their biological functions and other factors, and the same analysis was performed for genes with significantly decreased expression (Supplementary Fig. S1). In addition, on the genes that were significantly upregulated, GO analysis using DAVID allowed for identification of what kind of biological process or the molecular function of genes whose expression has changed by analyzing how many genes belong to a category with similar function based on the GO term. The 10 GO terms were charted with the lowest P values in each category of Biological Process and Molecular Function (Fig. 3). The same analysis was performed for the genes in which expression was significantly decreased (Supplementary Fig. S1). Genes involved in keratinization, lipid metabolism, and oxidoreductase were upregulated, while those involved in cellular response, TFs, and the immune system were downregulated (Fig. 3, Supplementary Fig. S1). In addition, 32 transcripts were upregulated more than 300-fold, and 12 transcripts were downregulated less than one-hundredth as genes with particularly large expression changes (Tables 1A and 1B). Among the 32 genes that were most highly upregulated, 21 of the 32 transcripts were genes that are clearly involved in keratinocyte differentiation and cornification. The genes that were highly downregulated included polymeric immunoglobulin receptor (PIGR), clusterin (CLU), and paired box 6 (PAX6).
Figure 3.
Image showing the results of the comprehensive gene expression analysis in the three patients with OS keratinization (i.e., anterior staphyloma, SJS, and OCP) and the three control patients. The results of the gene expression analysis using microarrays were normalized using the Transcriptome Analysis Console (QIAGEN) to identify genes with statistically significant changes in gene expression (P value [ANOVA], FDR < 0.05, |Fold Change| > 2). An analysis console was used for mapping the principal component analysis (PCA) plots (A) and Volcano plots (B). In the clinical keratinized cases, 1272 genes were significantly upregulated (red dots), and 1846 genes were significantly downregulated (green dots) (B). Using the Metascape web-based portal (https://www.metascape.org) to analyze genes significantly upregulated in clinical keratinization, we first identified all statistically enriched terms (i.e., GO/KEGG terms, canonical pathways, hallmark gene sets, etc., based on default selections in Express Analysis). Then, accumulative hypergeometric P values and enrichment factors were computed and used for filtering. The remaining significant terms were clustered hierarchically in a tree based on kappa statistical similarity between gene members. A kappa score of 0.3 was then applied as a threshold to classify the tree into term clusters (C). In the biological process and molecular function, enrichment analysis was performed using DAVID (https://david.ncifcrf.gov) on the significantly upregulated genes to obtain the top 10 P values of GO terms, and charts were created. The QuickGO browser (https://www.ebi.ac.uk/QuickGO/) was used for charting (D and E).
Table 1A.
Transcripts Upregulated in the Keratinized Conjunctival Epithelium
| Fold Change | P Value | Public Gene IDs | Gene Symbol | Description |
|---|---|---|---|---|
| 9613.76 | 7.69E-11 | NM_001244847 | KRTDAP | Keratinocyte differentiation-associated protein |
| 8579.93 | 5.02E-07 | NM_006121 | KRT1 | Keratin 1, type II |
| 5827.96 | 3.44E-05 | NM_002016 | FLG | Filaggrin |
| 5769.91 | 6.58E-06 | NM_001166034 | SBSN | Suprabasin |
| 5267.29 | 0.0005 | NM_001014342 | FLG2 | Filaggrin family member 2 |
| 3554.39 | 5.93E-08 | NM_000427 | LOR | Loricrin |
| 2019.41 | 0.0016 | NM_002963 | S100A7 | S100 calcium binding protein A7 |
| 1923.08 | 5.09E-06 | NM_019060 | CRCT1 | Cysteine rich C-terminal 1 |
| 1626.71 | 1.91E-05 | NM_012114 | CASP14 | Caspase 14 |
| 1609.66 | 1.62E-07 | NM_006919 | SERPINB3 | Serpin peptidase inhibitor, clade B (ovalbumin), member 3 |
| 1338.83 | 3.43E-06 | NM_174932 | BPIFC | BPI fold containing family C |
| 994.74 | 8.46E-07 | NM_001012964; NM_001012965; NM_002774 | KLK6 | Kallikrein related peptidase 6 |
| 991.9 | 2.04E-06 | NM_173483 | CYP4F22 | Cytochrome P450, family 4, subfamily F, polypeptide 22 |
| 941 | 5.46E-06 | NM_002638 | PI3 | Peptidase inhibitor 3, skin-derived |
| 813.1 | 1.64E-05 | NM_001127698; NM_001127699; NM_006846 | SPINK5 | Serine peptidase inhibitor, Kazal type 5 |
| 803.59 | 0.0018 | NM_016190 | CRNN | Cornulin |
| 664.75 | 4.92E-07 | NM_002974; NM_175041 | SERPINB4 | Serpin peptidase inhibitor, clade B (ovalbumin), member 4 |
| 627.28 | 2.82E-07 | NM_018004 | TMEM45A | Transmembrane protein 45A |
| 615.49 | 0.0004 | NM_004948; NM_024421 | DSC1 | Desmocollin 1 |
| 602.44 | 6.76E-06 | NM_001258333; NM_001258334; NM_002108 | HAL | Histidine ammonia-lyase |
| 596.94 | 3.22E-08 | NM_001307928; NM_080474 | SERPINB12 | Serpin peptidase inhibitor, clade B (ovalbumin), member 12 |
| 441.04 | 3.14E-06 | NM_001011709 | PNLIPRP3 | Pancreatic lipase-related protein 3 |
| 409.7 | 5.56E-05 | NM_016321 | RHCG | Rh family, C glycoprotein |
| 406.17 | 6.79E-06 | NM_025261 | LY6G6C | Lymphocyte antigen 6 complex, locus G6C |
| 400.81 | 7.60E-08 | NM_032488 | CNFN | Cornifelin |
| 385.9 | 2.00E-07 | NM_001077491; NM_001077492; NM_012427 | KLK5 | Kallikrein related peptidase 5 |
| 354.35 | 9.20E-08 | NM_001024209 | SPRR2E | Small proline-rich protein 2E |
| 352.9 | 1.22E-06 | NM_000067; NM_001293675 | CA2 | Carbonic anhydrase II |
| 323.31 | 5.03E-06 | NM_006945 | SPRR2D | Small proline-rich protein 2D |
| 316.16 | 8.30E-06 | NM_022726 | ELOVL4 | ELOVL fatty acid elongase 4 |
| 313.73 | 0.0003 | NM_014058 | TMPRSS11E | Transmembrane protease, serine 11E |
| 303.19 | 3.18E-05 | NM_001207053; NM_001243126; NM_005046; NM_139277 | KLK7 | Kallikrein related peptidase 7 |
The 32 transcripts upregulated more than 300 times and showed significant differences (ANOVA P < 0.05) in the keratinized conjunctival epithelium.
Figure 3.
Continued.
Table 1B.
Transcripts Downregulated in the Keratinized Conjunctival Epithelium
| Fold Change | P Value | Public Gene IDs | Gene Symbol | Description |
|---|---|---|---|---|
| −975.17 | 1.83E-05 | NM_002644 | PIGR | Polymeric immunoglobulin receptor |
| −797.06 | 3.11E-06 | NM_001177998; NM_001177999; NM_006424 | SLC34A2 | Solute carrier family 34 (type II sodium/phosphate cotransporter), member 2 |
| −393.2 | 8.80E-09 | NM_000096 | CP | Ceruloplasmin (ferroxidase) |
| −291.15 | 1.70E-06 | NM_001201546; NM_001201547; NM_001201548; NM_001201549; NM_004696 | SLC16A4 | Solute carrier family 16, member 4 |
| −254.75 | 1.28E-07 | NM_001177355; NM_005823; NM_013404 | MSLN | Mesothelin |
| −254.62 | 3.00E-06 | NM_005727 | TSPAN1 | Tetraspanin 1 |
| −193.1 | 3.11E-06 | NM_181644 | MFSD4 | Major facilitator superfamily domain containing 4 |
| −174.79 | 4.81E-05 | NM_001775 | CD38 | CD38 molecule |
| −163.27 | 3.36E-05 | NM_001831 | CLU; MIR6843 | Clusterin; microRNA 6843 |
| −147.98 | 0.0007 | NM_001112706; NM_033128 | SCIN | Scinderin |
| −141.56 | 0.0043 | NM_000280; NM_001127612; NM_001258462; NM_001258463; NM_001258464; NM_001258465; NM_001310158; NM_001310159; NM_001310160; NM_001310161; NM_001604 | PAX6 | Paired box 6 |
| −104.59 | 0.0056 | NM_006186 | NR4A2 | Nuclear receptor subfamily 4, group A, member 2 |
The 12 transcripts downregulated less than 1/100 and showed significant differences (ANOVA P < 0.05).
DNA-Templated Transcription
To clarify the pathogenesis of pathological keratinization, we focused on the trends of TFs that regulate the expression of many genes. We extracted genes related to DNA-templated genes from GO term as TF-related genes, and found 61 genes significantly upregulated and 216 genes significantly downregulated in the microarray. Enrichment analysis was performed on the significantly upregulated and downregulated genes using ChIP-Atlas to identify the upstream TFs. Refining our microarray data with the results of the enrichment analysis identified 4 transcripts for upregulated TFs and 56 transcripts for downregulated TFs. The upregulated TFs in this study included MYB proto-oncogene-like 2 (MYBL2) and forkhead box M1 (FOXM1), which are involved in cell-cycle progression, and sterol regulatory element binding TF 2 (SREBF2), which is considered a master TF for adipocyte differentiation, while E74 like ETS TF 3 (ELF3), which is involved in maintaining normal epidermal cells, was highly downregulated (Tables 2A and 2B).
Table 2A.
Transcripts Associated with DNA-Binding Transcription Factor Activity, and Representative Transcripts that are Upregulated in Keratinizing Conjunctival Epithelium
| Fold Change | P Value | Public Gene IDs | Gene Symbol | Description |
|---|---|---|---|---|
| 7 | 0.0013 | NM_001278610; NM_002466 | MYBL2 | Sterol regulatory element binding transcription factor 2 |
| 3.68 | 0.0008 | NM_004599 | SREBF2 | V-myb avian myeloblastosis viral oncogene homolog-like 2 |
| 3.46 | 0.0054 | NM_001243088; NM_001243089; NM_021953; NM_202002; NM_202003 | FOXM1 | Histone deacetylase 1 |
| 2.18 | 0.0022 | NM_004964 | HDAC1 | Forkhead box M1 |
DNA-templated genes (GO0006351-5) were extracted from the GO term as those related to transcription factors, and among them, four genes that were significantly upregulated in the microarray and were included in the transcription factors that were speculated to be upregulated were identified by enrichment analysis using ChIP-Atlas from differentially expressed genes in the microarray. ANOVA P < 0.05 was considered a significant difference.
Table 2B.
Transcripts Associated with DNA-Binding Transcription Factor Activity, and Representative Transcripts that are Downregulated in Keratinizing Conjunctival Epithelium
| Fold Change | P Value | Public Gene IDs | Gene Symbol | Description |
|---|---|---|---|---|
| −52.24 | 1.54E-05 | NM_001114309; NM_004433 | ELF3 | E74-like factor 3 (ets domain transcription factor, epithelial-specific) |
| −24.07 | 4.55E-06 | NM_001105539; NM_001277145; NM_023929 | ZBTB10 | Zinc finger and BTB domain containing 10 |
| −23.05 | 0.015 | NM_001114171; NM_006732 | FOSB | FBJ murine osteosarcoma viral oncogene homolog B |
| −22 | 0.0042 | NM_001030287; NM_001040619; NM_001206484; NM_001206486; NM_001206488; NM_001674 | ATF3 | Activating transcription factor 3 |
| −15.41 | 0.0007 | NM_001105077; NM_001105078; NM_001163999; NM_001164000; NM_001205194; NM_004991; NM_005241 | MECOM | MDS1 and EVI1 complex locus |
| −15.34 | 0.0008 | NM_004496 | FOXA1 | Forkhead box A1 |
| −15.01 | 1.82E-06 | NM_001145155; NM_001145156; NM_001145157; NM_021005 | NR2F2 | Nuclear receptor subfamily 2, group F, member 2 |
| −9.34 | 4.62E-05 | NM_014323; NM_032050; NM_032051; NM_032052 | PATZ1 | POZ (BTB) and AT hook containing zinc finger 1 |
| −8.13 | 0.0064 | NM_001164342; NM_001164343; NM_001164344; NM_001164345; NM_001164346; NM_001164347; NM_015642 | ZBTB20; MIR568 | Zinc finger and BTB domain containing 20; microRNA 568 |
| −7.49 | 7.42E-05 | NM_002198 | IRF1 | Interferon regulatory factor 1 |
| −7.22 | 0.0004 | NM_001206 | KLF9 | Kruppel-like factor 9 |
| −6.96 | 0.0081 | NM_001964 | EGR1 | Early growth response 1 |
| −6.57 | 0.0001 | NM_001430 | EPAS1 | Endothelial PAS domain protein 1 |
| −6.18 | 3.26E-05 | NM_001145102; NM_001145103; NM_001145104; NM_005902 | SMAD3 | SMAD family member 3 |
| −5.54 | 4.00E-05 | NM_000246; NM_001286402; NM_001286403 | CIITA | Class II, major histocompatibility complex, transactivator |
| −5.26 | 0.0093 | NM_001015051; NM_001024630; NM_001278478 | RUNX2 | Runt-related transcription factor 2 |
| −4.95 | 6.41E-05 | NM_002199 | IRF2 | Interferon regulatory factor 2 |
| −4.7 | 0.0196 | NM_032575 | GLIS2 | GLIS family zinc finger 2 |
| −3.98 | 0.0009 | NM_001204961; NM_001204963; NM_002585 | PBX1 | Pre-B-cell leukemia homeobox 1 |
| −3.85 | 0.0046 | NM_001453 | FOXC1 | Forkhead box C1 |
| −3.77 | 0.0001 | NM_007146 | VEZF1 | Vascular endothelial zinc finger 1 |
| −3.6 | 0.0118 | NM_001079526; NM_016260 | IKZF2 | IKAROS family zinc finger 2 |
| −3.59 | 0.001 | NM_000449; NM_001025603 | RFX5 | Regulatory factor X, 5 (influences HLA class II expression) |
| −3.37 | 0.0006 | NM_001130845; NM_001134738; NM_001706 | BCL6 | B-cell CLL/lymphoma 6 |
| −3.34 | 0.0007 | NM_005253 | FOSL2 | FOS-like antigen 2 |
| −3.24 | 0.0015 | NM_003670 | BHLHE40 | Basic helix-loop-helix family, member e40 |
| −2.96 | 0.0018 | NM_006084 | IRF9 | Interferon regulatory factor 9 |
| −2.7 | 0.004 | NM_005349; NM_015874; NM_203283; NM_203284OTTHUMT00000215047 | RBPJ | Recombination signal binding protein for immunoglobulin kappa J region |
| −2.67 | 0.0112 | NM_001101802; NM_016621 | PHF21A | PHD finger protein 21A |
| −2.56 | 0.0055 | NM_024671 | ZNF768 | Zinc finger protein 768 |
| −2.53 | 0.0052 | NM_001166693; NM_005935 | AFF1 | AF4/FMR2 family, member 1 |
| −2.39 | 0.0153 | NM_001184772; NM_021946 | BCORL1 | BCL6 corepressor-like 1 |
| −2.34 | 0.0025 | NM_001245002; NM_001245004; NM_001245005; NM_005597; NM_205843 | NFIC | Nuclear factor I/C (CCAAT-binding transcription factor) |
| −2.32 | 0.0313 | NM_002467 | MYC | V-myc avian myelocytomatosis viral oncogene homolog |
| −2.3 | 0.0099 | NM_001206794; NM_016374; NM_031371 | ARID4B | AT rich interactive domain 4B (RBP1-like) |
| −2.25 | 0.0094 | NM_017617 | NOTCH1 | Notch 1 |
| −2.24 | 0.0024 | NM_002892; NM_023000; NM_023001 | ARID4A | AT rich interactive domain 4A (RBP1-like) |
| −2.22 | 0.0052 | NM_001286818; NM_001730 | KLF5 | Kruppel-like factor 5 (intestinal) |
| −2.19 | 0.0036 | NM_001271068; NM_001271069; NM_002382; NM_145112; NM_145113; NM_145114; NM_197957 | MAX | MYC associated factor X |
| −2.18 | 0.0014 | NM_001184896; NM_001184897; NM_001184898; NM_015107 | PHF8 | PHD finger protein 8 |
| −2.17 | 0.0402 | NM_001039920; NM_001135734; NM_133476 | ZNF384 | Zinc finger protein 384 |
| −2.15 | 0.0046 | NM_014663 | KDM4A | Lysine (K)-specific demethylase 4A |
| −2.1 | 0.014 | NM_001197104; NM_005933 | KMT2A | Lysine (K)-specific methyltransferase 2A |
| −2.1 | 0.0177 | NM_001987 | ETV6 | Ets variant 6 |
| −2.07 | 0.0134 | NM_001303425; NM_001303426; NM_016331 | ZNF639 | Zinc finger protein 639 |
| −2.06 | 0.0172 | NM_001012505; NM_001244808; NM_001244810; NM_001244812; NM_001244813; NM_001244814; NM_001244815; NM_001244816; NM_032682 | FOXP1 | Forkhead box P1 |
| −2.06 | 0.0059 | NM_001003688; NM_005900 | SMAD1 | SMAD family member 1 |
| −2.05 | 0.0069 | NM_001267039; NM_015454; NM_016648 | LARP7 | La ribonucleoprotein domain family, member 7 |
DNA-templated genes (GO0006351-5) were extracted from the GO term as those related to transcription factors, and among them, 56 genes that were significantly downregulated in the microarray and were included in the transcription factors that were speculated to be downregulated were identified by enrichment analysis using ChIP-Atlas from differentially expressed genes in the microarray. ANOVA P < 0.05 was considered a significant difference.
Vitamin A–Related Transcriptions
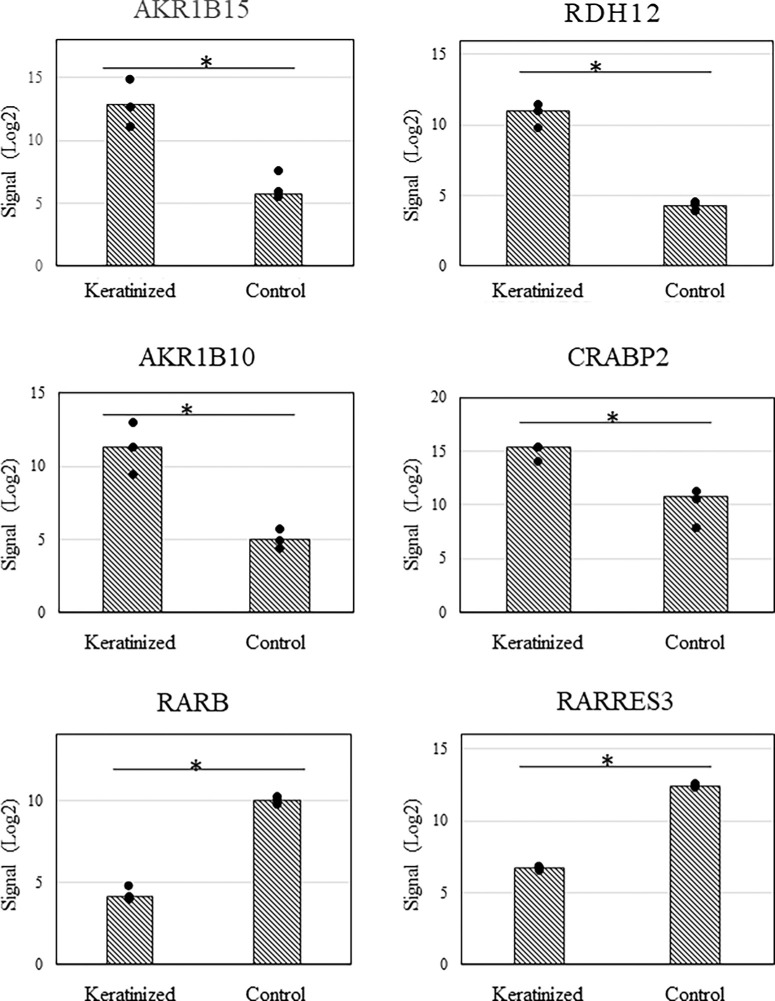
We investigated the metabolism and receptors of vitamin A, which is known to suppress cell keratinization. We analyzed the expression of 65 genes in this comprehensive gene expression analysis using the GO term as a reference for genes involved in vitamin A metabolism and receptors. As a result, significant expression changes were observed in 18 genes, of which 12 genes were upregulated and 6 genes were downregulated (Tables 3A and 3B). Binding of vitamin A to nuclear receptors occurs when vitamin A is taken up by epithelial cells as retinol, which is then oxidized to retinal and then retinoic acid (RA), which binds to cellular retinoic acid-binding protein 2 (CRABP2) and acts as a ligand for nuclear receptors. The differentially expressed genes were found to have increased mRNA for enzymes that reduce RA, the active form of vitamin A, and convert it to its inactive forms, retinol and then retinyl esters. On the other hand, the expression of CRABP2, which allows RA to be taken up by nuclear RA receptors (RARs), was significantly upregulated, while the expression of fatty acid binding protein 5, which competes with CRABP2 to bind RA to peroxisome proliferator-activated receptors, was significantly downregulated. Interestingly, the expression of RAR-beta (RARB) was selectively downregulated, while the expression of RAR-alpha (RARA) and RAR-gamma (RARG) was not significantly altered. In addition, when representative genes such as aldo-keto reductase family 1 member (AKR1)-B15 (AKR1B15), AKR1-B10 (AKR1B10), retinol dehydrogenase-12 (RDH12), CRABP2, RAR responder (RARRES) protein 3 (RARRES3), and RARB, whose expression varied more than 10-fold, were examined, there was little difference in expression of the transcripts because of disease (Fig. 4).
Table 3A.
Representative Transcripts Related to Vitamin A and Upregulated in Keratinizing Conjunctival Epithelium
| Fold Change | P-Value | Public Gene IDs | Gene Symbol | Description |
|---|---|---|---|---|
| 136.09 | 0.0002 | NM_001080538 | AKR1B15 | Aldo-keto reductase family 1, member B15 |
| 102.35 | 8.82E-07 | NM_152443 | RDH12 | Retinol dehydrogenase 12 (all-trans/9-cis/11-cis) |
| 80.07 | 0.0001 | NM_020299 | AKR1B10 | Aldo-keto reductase family 1, member B10 (aldose reductase) |
| 24.88 | 0.0005 | NM_001199723; NM_001878 | CRABP2 | Cellular retinoic acid binding protein 2 |
| 8.17 | 0.0071 | NM_001253908; NM_001253909; NM_003739 | AKR1C3 | Aldo-keto reductase family 1, member C3 |
| 7.22 | 0.0114 | NM_001301645; NM_004744 | LRAT | Lecithin retinol acyltransferase (phosphatidylcholine–retinol O-acyltransferase) |
| 7.03 | 0.0182 | NM_001135241; NM_001354; NM_205845 | AKR1C2 | Aldo-keto reductase family 1, member C2 |
| 6.02 | 0.0132 | NM_001243325; NM_001243327; NM_001243328; NM_139165 | RAET1E | Retinoic acid early transcript 1E |
| 4.42 | 0.0053 | NM_130900 | RAET1L | Retinoic acid early transcript 1L |
| 3.88 | 0.0002 | NM_052960 | RBP7 | Retinol binding protein 7, cellular |
| 3.01 | 0.0031 | NM_001252650; NM_016026 | RDH11 | Retinol dehydrogenase 11 (all-trans/9-cis/11-cis) |
| 2.06 | 0.0321 | NM_001818 | AKR1C4 | Aldo-keto reductase family 1, member C4 |
We analyzed the expression of 65 genes in this comprehensive gene expression analysis, using the GO term as a reference for genes involved in vitamin A metabolism and receptors. Twelve genes that were significantly upregulated and showed significantly differences (ANOVA P < 0.05).
Figure 4.
Graphs showing the results of the representative transcripts of significantly differentially expressed genes related to vitamin A metabolism in microarrays. Representative genes AKR1B15, RDH12, AKR1B10, CRABP2, RARRES3, and RARB up- or downregulated more than tenfold in normalized signal among 65 genes related to vitamin A metabolism and receptors based on GO term. Little difference was found in these transcripts for each disease.
Table 3B.
Representative Transcripts Related to Vitamin A and Downregulated in Keratinizing Conjunctival Epithelium
| Fold Change | P-Value | Public Gene IDs | Gene Symbol | Description |
|---|---|---|---|---|
| −59.12 | 1.41E-07 | NM_000965; NM_001290216; NM_001290217; NM_001290266; NM_001290276; NM_001290277; NM_001290300; NM_016152 | RARB | Retinoic acid receptor, beta |
| −54.18 | 1.55E-08 | NM_004585 | RARRES3 | Retinoic acid receptor responder (tazarotene induced) 3 |
| −5.1 | 0.0158 | NM_001145520; NM_001145521; NM_001145522; NM_001145523; NM_001145525; NM_015577 | RAI14 | Retinoic acid induced 14 |
| −4.11 | 0.009 | NM_000689 | ALDH1A1 | Aldehyde dehydrogenase 1 family, member A1 |
| −2.9 | 0.0002 | NM_001202413; NM_001202414; NM_006066; NM_153326 | AKR1A1 | Aldo-keto reductase family 1, member A1 (aldehyde reductase) |
| −2.29 | 0.0099 | NM_030665 | RAI1 | Retinoic acid induced 1 |
We analyzed the expression of 65 genes in this comprehensive gene expression analysis, using the GO term as a reference for genes involved in vitamin A metabolism and receptors. Six genes that were significantly downregulated and showed significantly differences (ANOVA P < 0.05).
QRT-PCR Analysis
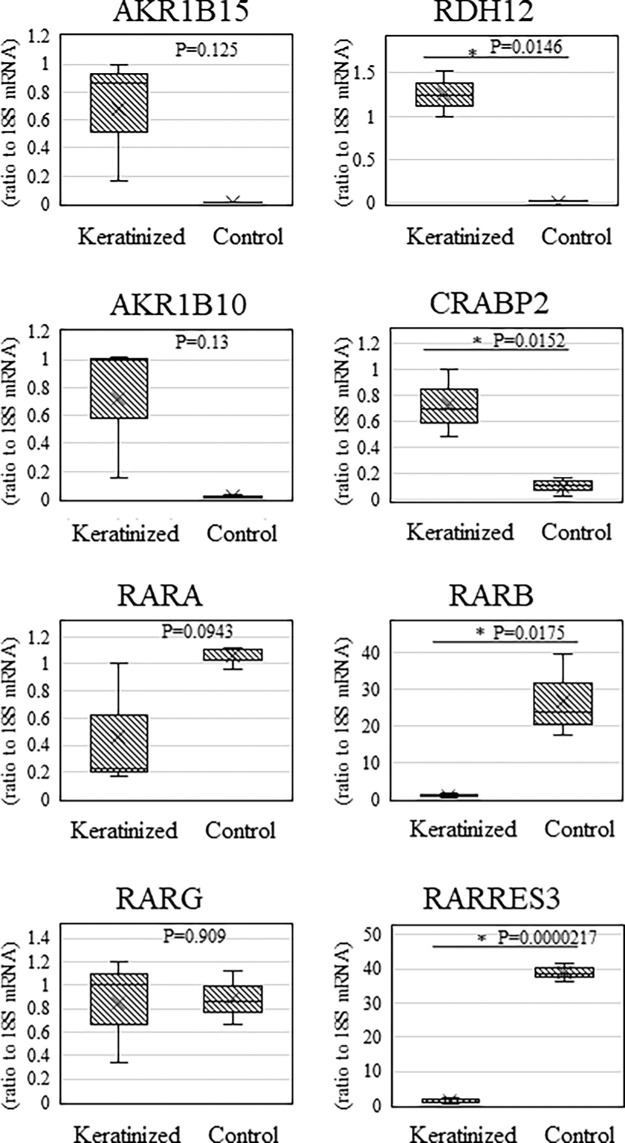
For comparison of the upregulated and downregulated transcripts in the epithelium from patients with keratinization and the controls, we performed qRT-PCR on the three keratinization cases and the three nonkeratinization controls in this study to confirm the expression of vitamin A-related transcripts whose expression was significantly changed in the comprehensive gene expression analysis. In this study, qRT-PCR was also performed for AKR1B15, RDH12, AKR1B10, and CRABP2, which are upregulated more than twentyfold among the significantly-upregulated genes and promote binding to RAR, two genes (RARB and RARRES3) whose expression were downregulated less than 1/50, and RARA and RARG, which are other RAR types. The results of the qRT-PCR were similar to the results of the comprehensive gene expression analysis.
Similar to the results of the comprehensive gene expression analysis, AKR1B15, RDH12, AKR1B10, and CRABP2 were significantly upregulated in the keratinized cases. On the other hand, RARB and RARRES3 mRNAs were significantly downregulated, while RARA and RARG mRNAs showed no significant changes in expression (Fig. 5).
Figure 5.
Graphs showing the representative transcripts of genes related to vitamin A metabolism in qRT-PCR. QRT-PCR was also performed for the genes AKR1B15, RDH12, AKR1B10, and CRABP2, which are upregulated more than twentyfold among the significantly upregulated genes and promote binding to RAR, two genes (RARB and RARRES3) whose expression was downregulated more than 1/50, and RARA and RARG, which are other types of RAR. By normalizing the expression of the gene of interest to the expression of 18S rRNA, we were able to obtain a relative measure of the expression level of each gene (*P < 0.05, **P < 0.005, ***P < 0.0005). Similar to the microarray results, RARB, CRABP2, and RARRES3 showed significant differences, whereas RARA and RARG showed no significant differences.
Discussion
To the best of our knowledge, this is the first study to clarify the relationship between pathological keratinization. The expression of 3118 genes in keratinized epithelial cells was significantly upregulated compared to controls. The upregulated transcripts were enriched with genes involving keratinization and lipid metabolism, while the downregulated transcripts were enriched with genes involving immune response and TFs. The most predominantly differentially expressed genes were associated with keratinization. PAX6 and CLU, which are known to be downregulated in OSDs, were also significantly downregulated. Interestingly, the expression of genes related to vitamin A metabolism and signaling pathways were also significantly differentiated.13 This may play an important role in the cause of cases of OS pathological keratinization.
RA is produced from vitamin A through two sequential oxidation steps,14 and vitamin A is an essential nutrient required for embryogenesis, determining cell lineage, and fate commitment.15–17 RA is a ligand transduced by two types of nuclear receptors, RAR and RXR (retinoid X receptor), both of which composed of three isotypes, alpha, beta, and gamma,16 and it is attributed to the trans-repression of keratinization by RAR stimulation in OS cells.18 In this study, the expression of RARB was significantly decreased, yet there was no significant change in the expression of RARA and RARG, the receptors for vitamin A. The uptake of RA into the nuclear receptors of RARs was likely enhanced by the increase in CRABP2 and the decrease in FABP5.19,20 However, RA seemed to be decreased because the expression of enzymes that convert RA, an active vitamin A, to inactive forms, such as retinal, retinol, and retinyl esters, was increased, and decreased expression of transcripts was induced by RA such as RARB, RARRES, and PAX6.19,21–24 In addition, the expression of genes related to vitamin A was significantly different, and although the number of cases was limited, there were few differences in the expression of transcripts because of disease (i.e., OCP, SJS, anterior staphyloma).
RARB is reportedly upregulated by RA, and plays a very important role in ocular development.15–17 Although the role of RARB on the OS after development has yet to be fully elucidated in detail, its expression is decreased in some malignant tumors. It has been reported that decreased RARB expression correlates with keratinization in premalignant oral lesions and squamous cell carcinoma of the head and neck, and that the expression of RARB or stimulation with pharmacological doses of vitamin A converts the keratinized cell phenotype into nonkeratinized cells.25–27 In this study, we found that significantly lower RARB expression correlated with keratinization in pathological keratinized compared to nonkeratinized conjunctival epithelium.
It is known that vitamin A inhibits squamous metaplasia of corneal limbal stem cells in late cultures in vitro.18,28 The efficacy of topically administered vitamin A as a therapeutic agent to reduce OS keratinization has previously been reported, and there have been attempts to use it as a novel therapeutic agent in SJS cases. However, even with topical treatment, side effects such as meibomian gland inflammation and keratitis have been reported, and it has not yet reached any practical use in the clinical setting.29–31 Recently, a report regarding the safety and efficacy of retinol palmitate ophthalmic solution for SJS with OS keratinization is of great interest.29 We consider it necessary to use more specific ligands such as RARB as therapeutic agents and to find new therapeutic targets by clarifying the causes of decreased expression of only RARB.
The TF differentially expressed genes in this study may reflect the pathogenesis of pathological keratinization. SREBF2, whose expression was upregulated, is a TF related to cholesterol synthesis that is known to contribute to cornification in the epidermis.32,33 In pathological keratinization, the OS is significantly keratinized, which may reflect the formation of cornified layer. Furthermore, it is involved in keratinocyte differentiation through lipid synthesis, and its expression is suppressed with RA inhibition of keratinization.34 It is known that MYBL2, a member of the MYN TF family, is overexpressed in malignant tumors, and its expression is involved in cell proliferation and cell-cycle progression.35 FOXM1 is a TF known to regulate mainly the G2/M phase. In ophthalmology, it reportedly plays an important role in the pathogenesis of pterygium, and it has functions such as cell proliferation and maintenance of stemness.36,37 The histone deacetylase HDAC1 is considered a fibrogenesis acceleration and a proinflammatory molecule on the OS. Therefore it is considered as a therapeutic target for the prevention of symblepharon.38 These TFs may be highly relevant to the regulation of OS cell turnover in pathological keratinization.
ELF3 is one of the epithelium-specific ETS TFs defined by their highly conserved ETS DNA binding domain and predominant epithelial-specific expression profile.39,40 On the OS, ELF3 is reportedly expressed in the corneal epithelium and goblet cells. FOSB (FBJ murine osteosarcoma viral, oncogene homolog B), ATF3 (activating transcription factor 3), and EGR1 (early growth response protein 1), which are reportedly downregulated in pterygium, were also downregulated in this analysis.41 These may be involved in normal tissue homeostasis. To the best of our knowledge, there are no previous reports of these upregulated TFs being involved in disease. Thus these TFs may provide novel targets for understanding the pathogenesis and treatment of keratinization.
We previously reported that CLU and PAX6 are downregulated in pathological keratinization and that the expression of genes related to epidermis development is upregulated and PIGR expression is downregulated in SJS.13,42 Reportedly, the expression of aldehyde dehydrogenase 1 family member 1 (ALDH1) is elevated in OCP cases and is associated with fibrosis.43 In this study, ALDH1 upregulation was common in OCP, and these findings are consistent with the results of the present comprehensive gene expression analysis.
We found that genes involved in keratinization were significantly upregulated in conjunctival epithelium, while expression of genes involved in OS immune system, including PIGR, was significantly downregulated.42 In addition, we found that TFs thought to be involved in differentially expressed genes were downregulated.
Limitations of the present study were the low number of cases analyzed and the significant difference in the age of keratinized cases due to the age at onset of disease characteristics. The diseases recruited for this study are rare, and it is difficult to perform OS reconstruction surgery for an OSD with pathological keratinization. However, we were fortunately able to apply cultivated oral mucosal epithelial transplantation in challenging cases. In addition, the samples in this study were of different diseases and different patient ages and it can be assumed that the pathophysiology leading to keratinization is also different from the pattern of gene expression, but they have a common pathology of keratinization. In the future, further investigation involving a larger number of cases is needed for a broader clarification of the pathogenesis of the keratinization. In conclusion, the findings in this study suggest that the pathological keratinization of the OS involves cell proliferation, keratinization, and increased lipid synthesis and that decreased expression of RARB may be involved in this pathology.
Supplementary Material
Acknowledgments
The authors thank John Bush for the critical reading of this manuscript, and Hiromi Nishigaki for technical assistance with the experiments.
Supported by grants from the Japan Agency for Medical Research and Development (AMED) (Grant No. JP23ek0109572h0003) and grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology Scientific Research (B) (Grant No. JP22h03244).
Disclosure: H. Yoshioka, None; M. Ueta, None; H. Fukuoka, None; N. Yokoi, None; K. Mizushima, None; Y. Naito, None; S. Kinoshita, None; C. Sotozono, None
References
- 1. Deng SX, Borderie V, Chan CC, et al.. Global consensus on definition, classification, diagnosis, and staging of limbal stem cell deficiency. Cornea . 2019; 38: 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kohanim S, Palioura S, Saeed HN, et al.. Acute and chronic ophthalmic involvement in Stevens-Johnson syndrome/toxic epidermal necrolysis—a comprehensive review and guide to therapy. II. Ophthalmic disease. Ocul Surf . 2016; 14: 168–188. [DOI] [PubMed] [Google Scholar]
- 3. Dart JK. The 2016 Bowman Lecture Conjunctival curses: scarring conjunctivitis 30 years on. Eye (Lond) . 2017; 31: 301–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yoshikawa Y, Ueta M, Fukuoka H, et al.. Long-term progression of ocular surface disease in Stevens-Johnson syndrome and toxic epidermal necrolysis. Cornea . 2020; 39: 745–753. [DOI] [PubMed] [Google Scholar]
- 5. Jain R, Sharma N, Basu S, et al.. Stevens-Johnson syndrome: the role of an ophthalmologist. Surv Ophthalmol . 2016; 61: 369–399. [DOI] [PubMed] [Google Scholar]
- 6. Elder MJ, Bernauer W, Leonard J, Dart JK.. Progression of disease in ocular cicatricial pemphigoid. Br J Ophthalmol . 1996; 80: 292–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Santos MS, Gomes JA, Hofling-Lima AL, Rizzo LV, Romano AC, Belfort R Jr. Survival analysis of conjunctival limbal grafts and amniotic membrane transplantation in eyes with total limbal stem cell deficiency. Am J Ophthalmol . 2005; 140: 223–230. [DOI] [PubMed] [Google Scholar]
- 8. Di Girolamo N, Park M.. Cell identity changes in ocular surface Epithelia. Prog Retin Eye Res . 2022; 95: 101148. [DOI] [PubMed] [Google Scholar]
- 9. Fogagnolo P, De Cilla S, Alkabes M, Sabella P, Rossetti L.. A review of topical and systemic vitamin supplementation in ocular surface diseases. Nutrients . 2021; 13: 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Komai S, Inatomi T, Nakamura T, et al.. Long-term outcome of cultivated oral mucosal epithelial transplantation for fornix reconstruction in chronic cicatrising diseases. Br J Ophthalmol . 2021; 106: 1355–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wan Y, Xiao G, Yu T, Zhang P, Hong J.. Histopathological examination of congenital corneal staphyloma and prognosis after penetrating keratoplasty. Medicine (Baltimore) . 2020; 99: e21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kanda Y. Investigation of the freely available easy-to-use software `EZR' for medical statistics. Bone Marrow Transplant . 2013; 48: 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakamura T, Nishida K, Dota A, Kinoshita S.. Changes in conjunctival clusterin expression in severe ocular surface disease. Invest Ophthalmol Vis Sci . 2002; 43: 1702–1707. [PubMed] [Google Scholar]
- 14. Carazo A, Macakova K, Matousova K, Krcmova LK, Protti M, Mladenka P.. Vitamin A update: forms, sources, kinetics, detection, function, deficiency, therapeutic use and toxicity. Nutrients . 2021; 13: 1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clagett-Dame M, Knutson D.. Vitamin A in reproduction and development. Nutrients . 2011; 3: 385–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lohnes D, Mark M, Mendelsohn C, et al.. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development . 1994; 120: 2723–2748. [DOI] [PubMed] [Google Scholar]
- 17. Matt N, Ghyselinck NB, Pellerin I, Dupe V.. Impairing retinoic acid signalling in the neural crest cells is sufficient to alter entire eye morphogenesis. Dev Biol . 2008; 320: 140–148. [DOI] [PubMed] [Google Scholar]
- 18. Samarawickrama C, Chew S, Watson S.. Retinoic acid and the ocular surface. Surv Ophthalmol . 2015; 60: 183–195. [DOI] [PubMed] [Google Scholar]
- 19. Napoli JL. Cellular retinoid binding-proteins, CRBP, CRABP, FABP5: effects on retinoid metabolism, function and related diseases. Pharmacol Ther . 2017; 173: 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Celik SD, Ates O.. Analysis of CRABP2 and FABP5 genes in primary and recurrent pterygium tissues. Mol Biol Rep . 2020; 47: 6105–6110. [DOI] [PubMed] [Google Scholar]
- 21. Lee SA, Belyaeva OV, Popov IK, Kedishvili NY.. Overproduction of bioactive retinoic acid in cells expressing disease-associated mutants of retinol dehydrogenase 12. J Biol Chem . 2007; 282: 35621–35628. [DOI] [PubMed] [Google Scholar]
- 22. Chung YT, Matkowskyj KA, Li H, et al.. Overexpression and oncogenic function of aldo-keto reductase family 1B10 (AKR1B10) in pancreatic carcinoma. Mod Pathol . 2012; 25: 758–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Endo S, Morikawa Y, Matsunaga T, Hara A, Nishinaka T.. Porcine aldo-keto reductase 1C subfamily members AKR1C1 and AKR1C4: substrate specificity, inhibitor sensitivity and activators. J Steroid Biochem Mol Biol . 2022; 221: 106113. [DOI] [PubMed] [Google Scholar]
- 24. Berenguer M, Meyer KF, Yin J, Duester G.. Discovery of genes required for body axis and limb formation by global identification of retinoic acid-regulated epigenetic marks. PLoS Biol . 2020; 18: e3000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rotondo JC, Borghi A, Selvatici R, et al.. Association of retinoic acid receptor beta gene with onset and progression of lichen sclerosus-associated vulvar squamous cell carcinoma. JAMA Dermatol . 2018; 154: 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Youssef EM, Chen XQ, Higuchi E, et al.. Hypermethylation and silencing of the putative tumor suppressor Tazarotene-induced gene 1 in human cancers. Cancer Res . 2004; 64: 2411–2417. [DOI] [PubMed] [Google Scholar]
- 27. Wan H, Oridate N, Lotan D, Hong WK, Lotan R.. Overexpression of retinoic acid receptor beta in head and neck squamous cell carcinoma cells increases their sensitivity to retinoid-induced suppression of squamous differentiation by retinoids. Cancer Res . 1999; 59: 3518–3526. [PubMed] [Google Scholar]
- 28. Hori Y, Spurr-Michaud SJ, Russo CL, Argueso P, Gipson IK.. Effect of retinoic acid on gene expression in human conjunctival epithelium: secretory phospholipase A2 mediates retinoic acid induction of MUC16. Invest Ophthalmol Vis Sci . 2005; 46: 4050–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ravindra AP, Sinha R, Bari A, et al.. Retinol palmitate in management of chronic Steven-Johnson Syndrome with ocular surface keratinization. Ocul Surf . 2023; 30: 160–167. [DOI] [PubMed] [Google Scholar]
- 30. Srividya G, Angayarkanni N, Iyer G, Srinivasan B, Agarwal S.. Altered retinoid metabolism gene expression in chronic Stevens-Johnson syndrome. Br J Ophthalmol . 2019; 103: 1015–1023. [DOI] [PubMed] [Google Scholar]
- 31. Ubels JL. A retrospective on topical retinoids occasioned by observation of unexpected interactions of retinoic acid with androgens and glucocorticoids in immortalized lacrimal acinar cells. Exp Eye Res . 2005; 80: 281–284. [DOI] [PubMed] [Google Scholar]
- 32. Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol . 2005; 125: 183–200. [DOI] [PubMed] [Google Scholar]
- 33. Harris IR, Farrell AM, Holleran WM, et al.. Parallel regulation of sterol regulatory element binding protein-2 and the enzymes of cholesterol and fatty acid synthesis but not ceramide synthesis in cultured human keratinocytes and murine epidermis. J Lipid Res . 1998; 39: 412–422. [PubMed] [Google Scholar]
- 34. Lee DD, Stojadinovic O, Krzyzanowska A, Vouthounis C, Blumenberg M, Tomic-Canic M.. Retinoid-responsive transcriptional changes in epidermal keratinocytes. J Cell Physiol . 2009; 220: 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Musa J, Aynaud MM, Mirabeau O, Delattre O, Grunewald TG.. MYBL2 (B-Myb): a central regulator of cell proliferation, cell survival and differentiation involved in tumorigenesis. Cell Death Dis . 2017; 8: e2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu Y, Qiao C, He S, et al.. Identification of functional genes in pterygium based on bioinformatics analysis. Biomed Res Int . 2020; 2020: 2383516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Enzo E, Secone Seconetti A, Forcato M, et al.. Single-keratinocyte transcriptomic analyses identify different clonal types and proliferative potential mediated by FOXM1 in human epidermal stem cells. Nat Commun . 2021; 12: 2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Swarup A, Ta CN, Wu AY.. Molecular mechanisms and treatments for ocular symblephara. Surv Ophthalmol . 2022; 67: 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Luk IY, Reehorst CM, Mariadason JM.. ELF3, ELF5, EHF and SPDEF transcription factors in tissue homeostasis and cancer. Molecules . 2018; 23: 2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Swamynathan SK, Swamynathan S.. Corneal epithelial development and homeostasis. Differentiation . 2023; 132: 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li J, Tao T, Yu Y, et al.. Expression profiling suggests the involvement of hormone-related, metabolic, and Wnt signaling pathways in pterygium progression. Front Endocrinol (Lausanne) . 2022; 13: 943275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ueta M, Sotozono C, Nishigaki H, et al.. Gene expression analysis of conjunctival epithelium of patients with Stevens-Johnson syndrome in the chronic stage. BMJ Open Ophthalmol . 2019; 4: e000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ahadome SD, Abraham DJ, Rayapureddi S, et al.. Aldehyde dehydrogenase inhibition blocks mucosal fibrosis in human and mouse ocular scarring. JCI Insight . 2016; 1: e87001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.