Abstract
There are significant sex-based differences in immune responses to pathogens and self-antigens, with human females exhibiting increased susceptibility to various autoimmune diseases, and human males displaying preferential susceptibility to some viral, bacterial, parasitic, and fungal infections. While sex hormones clearly contribute to sex differences in immune cell composition and function, the presence of two X chromosomes in human females suggests that differential gene expression of numerous X-linked immune-related genes may also influence sex-biased innate and adaptive immune cell function in health and disease. Here, we review the sex differences in immune system composition and function, examining how hormones and genetics influence the immune system. We focus on the genetic and epigenetic contributions responsible for altered X-linked gene expression, and how this impacts sex-biased immune responses in the context of pathogen infection and systemic autoimmunity.
Keywords: sex hormones, X chromosome inactivation, adaptive immune cells, innate immune cells, female biased autoimmune disease, systemic sclerosis, systemic lupus erythematosus, Sjögren’s syndrome, XCI escape
INTRODUCTION
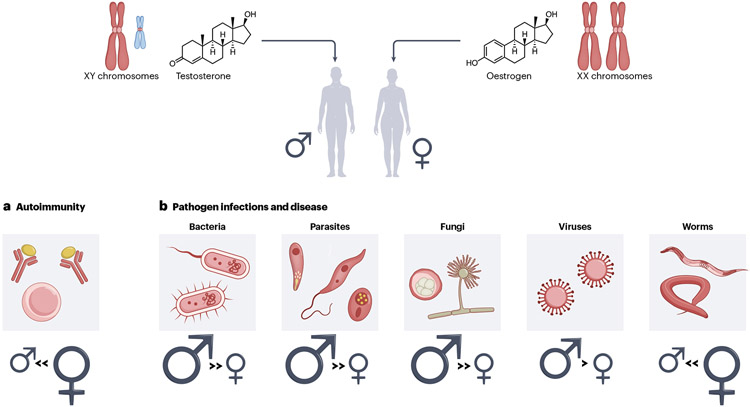
Biological sex contributes to physiological differences between human males and females that can influence pathogen exposure, recognition and clearance, and replication. Recent studies investigating how mammals respond to immunogenic challenges have revealed numerous differences between male and female susceptibility to pathogens such as bacteria, fungi, viruses, and parasites, likely via sex-biased differences in immune responses (Figure 1). In general, human females have greater innate and adaptive immune responses compared to males. Females usually clear infections faster than males, and males exhibit increased mortality rates across all ages, including in pre-term births and infants, after infection1. Females often exhibit stronger serologic responses compared to males following vaccination. Even in mice, females are more resistant to bacterial and viral infections and generate stronger and longer-lasting immune responses2. This seemingly advantageous female sex bias in the potency of immune responses is juxtaposed by the female-biased proclivity to autoimmunity. Human females are at increased risk for some autoimmune diseases, including systemic lupus erythematosus (SLE), Sjogren’s Syndrome, and systemic sclerosis3. However, the diverse molecular mechanism underlying these sex biased immune responses remain incompletely understood, and clarifying their origin may help provide new insights into disease pathogenesis.
Figure 1. Sex differences with pathogen infections and autoimmune disease.
The sex chromosomes and the sex hormones in biological males (XY) and females (XX) are responsible for the observed sex differences with responses to various pathogens (a) and in autoimmune disease (b). (a) The overall sex bias associated with a particular class of pathogen infection (and associated disease) is shown. Size of the symbol reflects amount of sex bias. (b) Autoimmune diseases are strongly female-biased.
The molecular origins for sex differences in immune responses originate from the X and Y chromosomes, the reproductive organs, and variations in levels of sex hormones over time. Sex hormones, specifically androgens and estrogens, can have either pro- or anti-inflammatory effects, and many genes with immune functions are regulated by the presence or absence of nuclear hormone receptor binding (BOX 1). The sex chromosomes provide the genetic and epigenetic foundations for altered gene expression in a heritable, sex-specific fashion in immune cells. The X chromosome contains about 50 immune-related genes, including some important for immune cell identity (FOXP3), cellular activation and intracellular signaling (CD40LG, TLR7, IRAK1, IL13RA1/2, NEMO, TASL, IL-9R), leukocyte trafficking (CD99, CXCR3), immune cell differentiation and proliferation (IL-2RG, BTK), and cellular metabolism (OGT, CYBB). Thus, the X chromosome is likely an important contributor to the molecular basis of female-biased antimicrobial responses and autoimmunity. In this Review, we review current literature on sex differences in immune system composition and function, examining how hormones and genetics of the X-chromosome influence the immune system. We focus on genetic and epigenetic contributions to altered X-linked gene expression and how this impacts female-biased autoimmune disorders and highlight examples of altered X-linked gene expression that influence pathogen susceptibility and disease progression.
Text Box 1: Sex hormones and inflammation.
Estrogens (estrone, 17β-estradiol, estriol), progesterone, and androgens (testosterone, dihydrotestosterone) are the predominant gonadal sex hormones. The sex hormone receptors – estrogen receptors (ER alpha, beta), progesterone receptor (PR), and androgen receptor (AR) – are hormone-activated transcription factors that bind to hormone response elements to regulate gene expression. ERs, PRs, and ARs are expressed by a variety of immune cells (T cells, B cells, dendritic cells, monocytes and macrophages, NK cells, type 2 innate lymphoid cells, and granulocytes) and cells at the interface of skin and mucosal barrier sites, such as thymic epithelial cells128. Sex-biased gene expression can result from sex-specific differences in steroid production. For example, numerous ER response elements in the IFN-gamma promoter allow for increased downstream gene expression when 17β-estradiol levels are increased, thereby contributing to female biased increased production of IFN-gamma129.
Estrogens can exhibit either anti-inflammatory (at high concentrations) or pro-inflammatory effects (at low concentrations)130. During pregnancy or periovulation when estrogen concentrations are high, estrogens exert inhibitory effects on T cells (specifically Th1 and Th17-polarized cells), M1 macrophages, dendritic cells, neutrophils, and microglia through NF-kB inhibition, and can also increase Treg function. ERalpha in T cells can promote T cell activation induced apoptosis and downregulates Foxp3 expression131, and can also suppress TFH cell development132. Estrogens can also signal through ER-beta in Treg cells in the intestine to regulate immune suppression133. Both ER-alpha and ER-beta in CD4+ T cells are important for ligand-mediated suppression of autoimmunity of the central nervous system (Multiple Sclerosis; MS), specially targeting pathogenic Th17 cells134,135. ER-beta is also required for Tregs to regulate macrophage proinflammatory responses for resolving lung inflammation resulting from pneumonia136.
The impact of estrogen on the immune response is also influenced by the immune microenvironment. In animal models of lupus-like disease, estrogens can be pro- or anti-inflammatory, depending on the proinflammatory milieu responsible for the observed lupus-like phenotypes. Deletion of ER-alpha in B cells reduced autoantibody production and nephritis in lupus-prone NZB/W F1 mice137. In the MRL/lpr model of spontaneous lupus-like disease, exogenous estrogen administration resulted in accelerated immune-complex glomerulonephritis, reflecting a proinflammatory effect via B-cell activation and autoantibody production; however, T-cell mediated disease, including periarticular inflammation, focal sialadenitis, and renal vasculitis, was significantly reduced130,138. Inflammatory cytokines including TNF-alpha, IL-1, and IL-6 can stimulate the activity of aromatase, an enzyme required for estrogen biosynthesis. Thus, the inflammatory milieu may confer contextual effects by altering the local concentrations of estrogen139. The concentration- and context-dependent effects of estrogen are also apparent in examples of systemic autoimmune disease. In rheumatoid arthritis and MS, disease activity often spontaneously decreases during pregnancy, a phenomenon which has been attributed to the anti-inflammatory effects of high concentrations of estrogen134,135,140. Nevertheless, the cumulative effects of local estrogen abundance are not so straightforward, as disease activity in systemic lupus erythematosus often spontaneously increases during pregnancy141. These observations from disease activity in pregnancy highlight the complex interactions between sex hormones, immune cells, immune tolerance, and the developing fetus.
Androgens have anti-inflammatory effects on immune responses in vivo and in vitro, through diverse mechanisms. In the context of their effects on the innate immune system, they have been shown to modify signal transduction through pattern recognition receptors, suppressing both NF-kB and Tlr4 expression142. Androgens also lower cytokine secretion of TNF-alpha, IL-1β, and IL-6 from monocytes and macrophages, and IL-33 production from mast cells41,143,144. Androgens impact adaptive immune responses, and can inhibit both humoral and cellular immune responses by reducing T and B cell proliferation and decreasing immunoglobulin and cytokine production35 41,143,144. In terms of B cells, androgens have inhibitory effects on B cell lymphopoiesis and specifically affect the production of B cell precursors, which express AR; conversely, mature B cells, which lack this receptor, are not directly impacted by androgens41. CD4+ T cells from female mice treated with dihydrotestosterone (DHT) produce more IL-10 compared to untreated female animals 36.
Sex differences in immune cell composition
The observed sex-biased differences in innate, humoral, and cellular immunity in humans are accompanied by differential immune composition in males and females. For example, flow cytometric immunophenotyping of healthy volunteers in Europe and Asia have consistently identified female-specific elevations of CD4+ T-cells, specifically naïve cells, which may reflect enhanced thymic function4,5. A recent whole blood transcriptional profiling of multiple international and intercontinental cohorts of healthy individuals similarly identified a higher proportion of CD4+ T cells in human females 6. In addition, human females have more circulating CD19+ B-cells, plasma cells, regulatory T cells (defined by CD25hiCD127lo), and both naïve CD8+ and mucosa-associated invariant T-cells relative to males4,7-10. Conversely, human females have lower proportions of both CD14+ and CD16+ monocytes, a generally lower proportion of myeloid cells 6,8 and fewer NK cells 4,7,8,11 relative to males. Differences in humoral immunity have also been observed between the sexes, with human females having higher quantities of IgM antibodies12.
Sex differences in immune cell composition have also been observed across mouse strains 13,14. Within resident immune populations of C57BL/6 mice and Wistar rats, there are greater total leukocyte quantities, and specifically more B and T cells and F4/80+ macrophages in female animals15. Consistent with these findings, mesenteric tissues from female animals express higher levels of both innate and adaptive immune cell chemokines and chemokine receptor genes that could enhance recruitment. A recent study found that male mice have higher numbers of splenic NK cells, yet have reduced effector function due to reduced expression of the X-linked gene Kdm6a (Utx), a histone lysine demethylase. Notably, Kdm6a (Utx) is expressed in higher levels in female NK cells16. Curiously, sex hormones do not significantly influence NK number or effector function, as a female-specific Utx deletion in NK cells increases NK cell frequency and reduces effector function through aberrant upregulation of Bcl2 expression, an anti-apoptosis factor, and downregulation of IFN-gamma16. The identification of additional X-linked genes that are capable of influencing both innate and adaptive immune cell frequencies may help further clarify the basis of the observed differences in immune cell composition between the sexes.
Sex hormones influence immune cell function
Sex differences in immune cell function arise from differential gene expression in immune cell subsets of males and females, and sex hormones can contribute towards these observed gene expression differences. Transcriptional profiling of innate and adaptive immune cells – including monocytes, naïve B cells, and various T cell subsets – identified about 1875 transcripts exhibiting sex biased expression, the majority of which were autosomal17. Most of these transcripts exhibited sex-biased expression in one type of immune cell, underscoring the cellular specificity of sex-specific gene expression and simultaneously highlighting the broad impact of sex hormonal and trans-acting sex chromosomal elements on the observed gene expression differences17. Estrogens can have either pro-inflammatory or anti-inflammatory functions (BOX 1), depending on both the local hormone concentration and the immune cell examined. For example, transcription of AICDA, which encodes the AID enzyme which is important for somatic hypermutation and class switch recombination in B cells, is regulated by binding of the estrogen-ER-alpha complex to its promoter region of AICDA. In autoimmune disease, AID contributes to the production of high-affinity IgG auto-antibodies18. Estrogens also increase the expression of endosomal Toll like Receptors (TLR) 3, 7, 9, which are important regulators of Type I interferons (IFNs)19. Co-culture of the SLE-derived estrogen-treated CD4+ T cells with B cells from healthy controls resulted increased antibody production in vitro20. Type I IFN production and antiviral responses downstream of TLRs 3, 7, and 9 are regulated by TRIM21, whose expression is increased by estrogen. TRIM21 expression also induces IL-23, which promotes Th17-differentiation, an adaptive immune pathway integral to the pathogenesis of several systemic autoimmune diseases, including SLE, SSc, SS, and RA21. The female-bias for enhanced type I IFN signaling has also been observed in human plasmacytoid dendritic cells (pDCs), which produce IFNα following TLR stimulation. Female pDCs treated with TLR7 and TLR8 agonists, but not TLR9 agonists, produce more IFNα compared to male pDCs, and more mRNA transcripts of the IFN-stimulated gene, IRF522-24. Although a sex-bias with type I IFN production was not originally observed for TLR9, this could be due to the type of CpG used in the original study22, as sex biased type I IFN production by pDCs stimulated through TLR9 has been recently reported25,26. Both sex chromosomal and sex hormonal mechanisms likely contribute towards this female bias because TLR7 and TLR8 are X-linked, and IRF5 transcription is regulated by estrogen receptor 1 (ESR1)27.
Sex differences in immune functions, particularly within the context of microbial infection, also reflect sex differences in the cellular expression of pattern recognition receptors. Peritoneal macrophages from female rodents express higher levels of Tlr2, Tlr3, and Tlr4, and exhibit enhanced phagocytosis and NADPH-mediated bacterial elimination compared to their male counterparts15. Accordingly, peritoneal-derived macrophages from female mice also exhibit increased expression of interferon-stimulated genes, and female mice exhibited blunted sepsis severity and fewer recoverable live bacteria in circulation28,29. These findings were abrogated by prior ovariectomy and support a role for sex hormones in shaping the tissue resident immunophenotype. The B1 cell-dependent neutralizing antibody response to LPS-O-antigens of enteropathogenic E. coli (EPEC) occurred in sexually mature females, resulting from estrogen-mediated upregulation of BAFF and APRIL cytokines secreted by peritoneal macrophages29. Bone marrow-derived macrophages from female mice have higher levels of Tlr8 compared to male mice30, and peripheral blood mononuclear cells from human females express more TLR7 compared to males31. Vaccination or viral challenge increases expression of TLR pathway and pro-inflammatory genes in female PBMCs from humans and rats, and it has been suggested that estrogen binding at sex hormone receptor elements in the promoter regions of these genes is responsible for increased gene expression 32. However, neutrophils from human males have higher expression of TLR4 and this expression increases following activation with LPS, which results in higher pro-inflammatory cytokine production and may contribute towards the male bias observed with endotoxic shock33. Notably, in line with its recognized generally anti-inflammatory role, androgen treatment of male mice decreases Tlr4 expression in macrophages, and it can also suppress the invasion and colonization of uropathogenic E.coli through inhibition of the JAK-STAT signaling pathway and decreased production of IL-1β, IL-6, and IL-8 34. Androgens have anti-inflammatory effects (BOX 1) on both humoral and cellular immune responses by reducing T and B cell proliferation and decreasing immunoglobulin and cytokine production35. Female mice treated with dihydrotestosterone (DHT) produce more IL-10 and less IL-12 compared to untreated female animals, resulting from increased AR signaling in CD4+ T cells36.
Androgens also play an important role in central tolerance through regulated expression of AIRE in the thymus, the expression of which exhibits a sex bias. While androgens recruit ARs to the AIRE promoter to increase transcription in male mice and humans, estrogens inhibit AIRE expression37,38. Androgens can also influence expression of FOXP3, a transcription factor important for CD4+ regulatory T cells (Tregs) that play an important role in tolerance. Treg-intrinsic AR signaling increases Treg suppression during an allergen challenge, and human Tregs treated with androgen reduced IL-33 induced ST2 expression and human bronchial epithelial cells produced less IL-3339. There is evidence that androgen treatment can increase FOXP3 expression in Tregs from human females during the ovulation phase of the menstrual cycle yet had no effect on Tregs from males40. Androgens have inhibitory effects on B cell lymphopoiesis and specifically impact B cell progenitors, which express AR while mature B cells lacking this receptor were not affected41. Unlike estrogen which upregulates expression of B cell activating factor (BAFF), BAFF expression is inhibited by androgens42. Thus androgens block BAFF-dependent B cell clonal expansion and class switch recombination, and downregulate GC responses, acting through B cell extrinsic mechanisms43. Androgens also increase expression of PTPN1, which functions in cell growth, differentiation, mitosis and immune cell function. AR-signaling prevents Th1 development by upregulating Ptpn1, which is an inhibitory phosphatase of Tyk2 and Jak2 kinases upstream of Sta4, thereby inhibiting IL12/Stat4 signaling 44.
Genetic and epigenetic contributions to sex differences in immune responses: X-Chromosome Inactivation (XCI)
Female mammals regulate X-linked gene expression using XCI (BOX 2), in which one X chromosome (the inactive X chromosome, or Xi) is transcriptionally silenced during development, and a memory of this silencing event is maintained with each cell division and persists into adulthood. Various epigenetic modifications, as well as the long noncoding RNA Xist, are enriched across the Xi, and function to maintain transcriptional repression of most, but not all, of the genes on the Xi. Xist expression is required to form the Xi and loss of Xist in early development is lethal due to the requirement for an appropriate dosage of X-linked genes in both embryo and placental development 45. Xist deletion or Xist gene silencing reduces, and often eliminates, enrichment of heterochromatic modifications from the Xi46 and results in reactivation of some X-linked genes on the Xi, depending on the cell type and timing relative to XCI initiation. Mouse models with conditional Xist deletion in post-XCI somatic cells yields variable phenotypes, including myeloproliferative neoplasia (deletion in hematopoietic stem cells), increased polyp formation (intestinal deletion, following azoxymethane/dextran sulfate treatment), or even the lack of a perceptible phenotype (deletion in neurons, B cells, epithelial cells, intestinal cells) where animals are viable and have normal lifespans47-49. Collectively, these studies highlight three fundamental observations: (1) that the identify and number of X-linked genes exhibiting reactivation upon Xist deletion varies by cell type; (2) X-linked dosage imbalances are, in fact, variably tolerated in a cell-type-specific manner; (3) inflammatory stress may exacerbate Xi reactivation.
BOX 2: X-Chromosome Inactivation (XCI) & ‘Escape’ from XCI.
Female eutherian mammals use XCI for dosage compensation of X-linked genes between the sexes145. XCI is initiated during early embryonic development, during which one X chromosome is randomly selected for transcriptional silencing. The process begins with upregulation of the long non-coding RNA Xist, which spreads across the future inactive X chromosome (Xi) in cis together with Polycomb Repressive Complexes (PRCs)146-151. X-linked gene promoters and enhancers rapidly lose active histone acetylation marks concurrently with transcriptional repression148-150. The repressive histone modifications H2AK119Ub and H3K27me3 are deposited on the Xi by PRC1 and PRC2, respectively, and the histone variant macroH2A becomes enriched152-155, together creating a heterochromatic environment across the Xi. As a final layer of regulation to maintain transcriptional repression of the Xi, DNA methylation becomes enriched at promoters and enhancers156-160. Thus, in most somatic cells, transcriptional silencing of the Xi is maintained with each cell division through Xi enrichment of Xist RNA, heterochromatic histone marks, and DNA methylation, some of which can be visualized cytologically using RNA FISH and immunofluorescence.
Although most genes on the Xi are silenced, about 15-23% of X-linked genes in humans and ~3-7% of X-linked genes in mice are transcribed from the Xi and ‘escape’ XCI, either constitutively or in a cell type-specific manner161-164. The promoter regions of XCI escape genes lack Xist RNA enrichment148,149,151, heterochromatic histone marks148,150, and DNA methylation159,160, and are enriched for RNA Polymerase II, DNAse I hypersensitivity sites, and CTCF occupancy, which collectively allow for gene expression from a highly heterochromatic environment. X-linked genes with a Y-linked counterpart (XY gene pairs) commonly escape XCI silencing and are expressed from the active (Xa) and inactive X (Xi) chromosomes, although transcript levels from the Xi are typically less than levels from the Xa165. Importantly, X-linked genes lacking a Y-linked homolog can also escape XCI. Both the magnitude and extent of XCI gene escape vary considerably by cell type, as well as between individual cells, and among individuals. Additionally, some escape genes display sex-biased expression 162,166-168. Of the approximately 54 immunity-related genes located on the mouse and human X chromosomes, 20.4% are expressed more highly in females and 16.7% are expressed more highly in males169. However, these values are likely underestimates due to the methodological challenges associated with measuring transcription specifically from the Xi. Because XCI is random and because expression from the Xi is typically less than that of the Xa, the identification of XCI escape genes requires single-cell approaches with high sequencing depth, or bulk cell analyses using F1 hybrid mice with skewed XCI70,170,171. Nevertheless, ongoing efforts to identify additional escape genes are likely to provide important insights into sex-biased immune responses—to date, several proinflammatory genes have been found to aberrantly escape XCI in SLE and other autoimmune diseases, suggesting a key role for the transcription of X-linked genes from the Xi in the context of female-biased immunological processes (see Table 1).
Female mammals are mosaic for the X chromosome, as each individual female cell will contain an inactive X (Xi) that is either maternal or paternal in origin. Most somatic cells examined to date have similar epigenetic features enriched on the Xi, including Xist RNA, heterochromatic histone modifications, and histone variant macroH2A, which can be visualized cytologically50-53 . However, immune cells lack some hallmark epigenetic features of canonical XCI maintenance. Specifically, splenic B and T cells from mice and circulating lymphocytes from humans lack cytologically visible Xist RNA and heterochromatic mark enrichment at the Xi, despite any significant changes with Xist transcription54-57. Thus, Xist transcription and its localization to the Xi are genetically independent processes. In vitro activation of B and T cells stimulates the return of Xist RNA and heterochromatic modifications to the Xi, prior to the first cell division54,56,58,59. This dynamic localization of Xist RNA and heterochromatic marks to the Xi is also observed during lymphocyte development, as Xist RNA is lost from the Xi after the common lymphoid progenitor stage, at the pro-B cell and DN1 thymocyte stages, and Xist transcription remains constant 56,58.
Surprisingly, non-canonical XCI maintenance is a feature of both adaptive and innate immune cells. Xist RNA exhibits substantial diversity in the robustness of its relocalization to the Xi depending on the type of immune cell. Neutrophils and pDCs lack detectable Xist RNA transcripts at the Xi; in contrast, NK cells exhibit clusters of Xist RNA pinpoints within the Xi territory60. Bone marrow-derived macrophages stimulated with either LPS or CpG have dispersed Xist RNA patterns and about half of the nuclei have a H3K27me3 focus that co-localizes with Xist signals. In vitro stimulation of mouse pDCs with either CpG or a Tlr7 agonist did not result in detectable Xist RNA localization, despite persistent Xist transcription, and both resting and in vitro activated pDCs have few H3K27me3 foci60.
The paucity of epigenetic features on the Xi in many immune cells, including lymphocyte progenitors, supports the hypothesis that the chromatin of the Xi in immune cells is more euchromatic than in fibroblasts, and therefore prone to aberrant reactivation at certain loci. The facultative nature of the chromatin of the Xi may facilitate the increased expression of proinflammatory X-linked genes in response to pathogen infections, which would provide a favorable advantage to females, and yet simultaneously disrupt the balance of self-tolerance resulting in the development of female-biased autoimmune disease (Figure 1).
Female-biased systemic autoimmune disease and the X chromosome
Autoimmune diseases predominantly affect human females, and 80% of all individuals with autoimmune disease are female61,62,63. For some autoimmune diseases, such as multiple sclerosis (MS), this sex bias is modest (~65% female), while for other autoimmune diseases such as primary biliary cirrhosis, the sex bias is strongly skewed (~90% female)64. The female sex bias with some autoimmune diseases has increased over time. For example, in the mid-twentieth century, MS originally exhibited equal prevalence between the sexes; by the 1980s the sex ratio was 2:1 (female:male); currently, the sex ratio is 3:165. The observed increase in the female-bias of disease likely reflects advancements in the medical classification and diagnosis of these diseases but could also reflect environmental changes. While the female bias for many autoimmune diseases is quite high, the incidence and prevalence across rheumatic autoimmune diseases can vary. For example, the sex bias with SLE is typically ~66-93% female, and the prevalence ranges from 5 - 241 per 100,000 people in the United States; for dermatomyositis (DM) and polymyositis (PM), the sex bias is ~60-75% female yet the prevalence is only about 6 per 100,000 (Table 1). Importantly, beyond disease prevalence, there are also sex differences in disease severity, specifically with respect to the degree of organ involvement, and symptom burden/disability. Male patients with MS, SLE, and SSc typically exhibit greater disease severity66,67, and male patients with SLE are more likely to exhibit renal and cardiovascular co-morbidities68. However, this male-biased increase in autoimmune disease severity is not universal; in RA for example, biological sex does not correlate with articular disease severity69. Additional translational studies systematically examining the relationship between biological sex and both disease manifestations and disease severity are necessary to better understand how biological sex may impact the phenotype, pathogenesis, and natural history of disease.
Table 1. Prevalence, incidence, and sex bias for the female-biased autoimmune diseases SLE, SS, scleroderma, inflammatory myopathies, and RA.
| Autoimmune Disease | Prevalence (per 100,000 people in United States) |
Incidence (per 100,000 person- years in United States) |
Sex Bias (% of Affected Individuals who are Women) |
|---|---|---|---|
| Systemic lupus erythematosus (SLE) | 5-2411 | 1.0 – 23.21 | 66%-93%1 83.71%2 |
| Sjogren’s syndrome (SS) | 22-1033 | 3.94 | 90.54%2 96.2%4 |
| Systemic sclerosis/scleroderma (SSc) | 27.65 | 1.935 | 83.7%5 75%-93.5%6 83.80%2 |
| Inflammatory myopathies: dermatomyositis and polymyositis (DM/PM) | 6.37 | 0.116-0.67 | 65.08%2 60%-75%7 |
| Rheumatoid arthritis (RA) | 10708 10009 |
75.38 | 73.4%8 |
Dosage imbalances of some X-linked genes are detrimental for cell function and can result in features of systemic autoimmune disease64 . Because XCI ‘escape’ (BOX 2) is variable across cell types, it is likely that some of these X-linked genes are biallelically expressed in specific cells (or escape silencing in a portion of the total cell population) in healthy individuals, where they accordingly exhibit sex-biased expression70. The X chromosome contains many genes that function, either directly or indirectly, in immune processes1. It is therefore likely that these genes are dosage sensitive and therefore subject to XCI silencing in immune cells59,71, although direct evidence from specific cell types in mice and human samples is lacking. Also unclear is whether any of these genes can become reactivated from the Xi in response to either antigen-mediated stimulation or pathogen infection, or as a result of chronic inflammation during autoimmunity. Aberrant XCI maintenance, where XIST RNA and some heterochromatic histone modifications (H3K27me3, H2AK119-ubiquitin) are missing from the Xi in circulating lymphocytes from female SLE patients, both pediatric and adults, and also in various mouse models of spontaneous lupus-like disease which exhibit a female bias 56,59,72,73. We have proposed the hypothesis that autoimmune disease, such as SLE, results in reduced enrichment of epigenetic features across the Xi which permits reactivation of immunity-related genes, resulting in the observed abnormal increased expression in lymphocytes (Figure 3). Additional work is necessary to determine whether perturbed XCI maintenance is a feature of other autoimmune diseases that predominantly affect human females.
Figure 3. Impairments with dynamic XCI maintenance result in aberrant overexpression of X-linked genes in female-biased autoimmune disease.
(a) The Xi in naïve lymphocytes lack cytological enrichment of Xist RNA (pink curvey lines) and heterochromatic marks (colored circles), and these modifications return to the Xi in activated cells. (b) Activated lymphocytes from patients with autoimmune disease have dispersed Xist RNA and heterochromatic marks from the Xi, and increased expression of some X-linked genes (denoted with red arrows). Prevention of Xist RNA tethering to the Xi reduces enrichment of histone heterochromatic marks on this chromosome, and persistent absence of these epigenetic modifications across multiple cell divisions may increase abnormal overexpression across the Xi.
Individuals with more than one X chromosome, including XX females, patients with Klinefelter Syndrome (XXY), and individuals with polysomy X (XXX), have increased susceptibility to some female biased autoimmune diseases (Figure 2), including SLE, Sjogren’s syndrome (SS), SSc, and PM/DM74,75. The association between female-biased autoimmunity and X chromosome dosage may also apply more broadly to other autoimmune diseases pending additional karyotype-stratified case-control studies. The contribution of multiple X chromosomes to systemic autoimmune disease risk (independent of sex hormones) is supported by mouse models using the ‘four core genotypes’: (1) ovary-bearing XX mice , (2) ovary-bearing XY mice deficient in the Y-linked Sry gene required for sex determination and male gonad formation (XYSry mice), (3) testes-bearing XYSry− and (4) testes-bearing XXSry transgenic mice76. Pristane-induced SLE-like disease, with elevated type I interferon production, and induced experimental autoimmune encephalomyelitis (EAE) results in increased onset and disease severity in XX and XXSry animals77, reflecting genetic and epigenetic contributions from the X, although the causal genes responsible are unknown. Bone marrow chimera experiments in which hematopoietic stem cells or fetal liver cells from ovariectomized female NZB/W F1 mice, a classic spontaneous mouse model of SLE-like disease exhibiting a female bias (see BOX 3), are injected into hormonally intact lethally irradiated NZB/W F1 males results in the development of lupus-like disease with increased numbers of germinal center B cells, memory B cells, and plasma cells in all male recipients, similar to NZB/W F1 females78. Thus X-linked genes expressed in female (XX) immune cells, independent of prior exposure to female sex hormones, accelerate SLE-like disease onset in male animals predisposed to develop autoimmune disease, underscoring the significance of genetic contributions from the X chromosome in autoimmunity.
Figure 2. Number of X chromosomes and risk for autoimmune disease.
Increased numbers of X chromosomes is associated with higher risk for the female-biased autoimmune diseases SLE, SS, scleroderma, polymyositis, and dermatomyositis.
Box 3: Mouse models of autoimmune disease exhibiting a sex bias.
Spontaneous and induced mouse models of lupus-like disease recapitulate some features of human autoimmune disease (primarily autoantibody production, lymphoid activation and hyperplasia, and lupus nephritis), and some of these models exhibit a sex bias. Male BXSB/Yaa bear a translocation of the telomeric end of the X chromosome containing Tlr7 region and 15 neighboring genes onto the Y-chromosome (Yaa mutation)172,173 and acquire a lupus-like phenotype over time. Notably, Tlr7 knockdown in BXSB/Yaa mice abrogates disease development174, and transgenic overexpression of Tlr7 on a non-lupus prone background results in lupus-like disease96. The BXSB strain also contains mutations in the MHC locus, and loci on chromosomes 1, 3, and 13 (Bsx1-6) that contribute to disease activity175. The F1 hybrid NZB/W mouse model develops spontaneous lupus-like disease that exhibits a female bias (100% females and < 40% males develop disease phenotypes by 1 year)176,177. NZB/W mice also develop features of SS and female mice exhibit more extensive salivary gland lesions compared to male mice178. Both X chromosome number and hormones appear to influence disease onset and severity in NZM/W F1 mice, as estrogen accelerates disease and testosterone provides anti-inflammatory protection in some studies, but not others 179,180. In vitro activated lymphocytes from female NZB/W F1 mice with lupus-like disease exhibit mislocalized Xist RNA and reduced enrichment of H3K27me3 at the Xi, and sex biased gene expression differences56,73. Backcrosses between NZB/W F1 and NZW generated various recombinant inbred strains of New Zealand Mixed (NZM) mice, among which NZM2328 and NZM2410 have earlier disease onset, and NZM2328 displaying a stronger female bias than NZM2410181. Lupus-like disease can be induced in various mouse strains (with variable efficiency) using intraperitoneal injections of pristane, during which females develop more severe disease phenotypes including anti-Sm, anti-dsDNA, anti-ribosomal P, anti-Su autoantibodies, arthritis, immune complex-mediated glomerulonephritis, and pulmonary capillaritis182,183. Finally, there is a female bias with the experimental autoimmune encephalomyelitis model of MS 184.
X-linked immunity genes that are dosage sensitive in autoimmune disease
There are a number of X-linked immunodeficiencies, thus underscoring the importance of appropriate X-linked gene dosage for immune function and health.79 Accordingly, X-linked genes that are aberrantly expressed in immune cells of patients with female-biased autoimmune disease suggest perturbations in the regulation of XCI contributes to this bias. Abnormal X-linked gene expression has been observed in both adaptive and innate immune cells from patients with autoimmune rheumatic diseases, including SLE, SS, and SSc (Table 2). Interestingly, transgenic mouse models overexpressing some X-linked immune related genes have altered immune function and result in features of autoimmune disease (see Table 2). The X-linked gene CD40LG is primarily expressed by activated CD4+ T cells, and encodes a receptor that binds to CD40, which is expressed by dendritic cells, B cells, and endothelial cells. CD40LG is aberrantly overexpressed in T and B cells from female SLE80,81 and SSc82 patients relative to healthy female controls. While the biological significance of this overexpression in these patients is not well-established, transgenic overexpression of Cd40lg in mice results in autoimmune disease, with increased IgG antibodies, chronic inflammation, glomerulonephritis, thymic atrophy, and increased lethality83. CXCR3 is an inducible chemokine receptor that functions in adaptive immune responses by regulating Th1 cells and T cell trafficking. CXCR3 is overexpressed in circulating CD4+ T cells of female patients with SLE and the proportion of CXCR3+ CD4+ T cells is increased in the urine and kidneys from patients with lupus nephritis81,84. In SS patients, there are also elevated numbers of infiltrating CXCR3+ CD3+ T cells in salivary gland tissue, and the CXCR3 ligands CXCL9 and CXCL10 are overexpressed85. BTK is a kinase associated with the B cell receptor and its activation induces a signaling cascade required for B cell proliferation, activation, and survival. SLE patients (samples were not distinguished by sex) with active lupus nephritis overexpress BTK in peripheral blood mononuclear cells86, and Btk overexpression in mice increases germinal center B cells, positive ANA staining, and immune complex deposition in kidneys 87. Decreasing the activity of Btk through small molecule inhibition in both a spontaneous (NZB/W F1 mice) and an inducible mouse model of lupus reduces lupus-like disease phenotypes88. FOXP3 is a transcription factor important for differentiation and maintenance of regulatory T cells (Tregs). SLE and SSc patient Tregs express less FOXP3, which likely compromises Treg function, and while there are conflicting reports regarding the abundance of Tregs in the peripheral blood of patients with SLE and SSc, the available evidence suggests that Tregs may be dysfunctional in these diseases 89-91. Tregs in the skin of SSc patients exhibit abnormal production of Th2 cytokines, indicative of tissue specific Treg cellular effects that may influence localized dysfunction and contribute towards fibrosis92. Overexpression of Foxp3 is protective against renal dysfunction in a mouse model of accelerated crescentic glomerulonephritis due to increased Treg number and function, which blocks Th1, Th2, and Th17 responses systemically93.
Table 2. Immunity-related X-linked genes that are dosage-sensitive for autoimmune diseases.
Examples of X-linked genes that function in the adaptive and innate immune systems are shown.
| Dosage sensitive X-linked gene |
Function | Evidence for XCI escape |
Gain/loss of function mouse models & phenotypes |
Aberrant expression in autoimmune patients |
|---|---|---|---|---|
| Adaptive Immune System | ||||
| CD40LG |
|
|
||
| CXCR3 | ||||
| BTK |
|
|
|
|
| FOXP3 |
|
|
|
|
| OGT |
|
|
||
| Innate Immune System | ||||
| TASL (Cxorf21) | Variable XCI escape in human lymphoblastoid cells2 | |||
| TLR7 |
|
|
|
|
X-linked immune genes also exhibit dosage sensitivity and are overexpressed in female-biased autoimmune diseases. TLR7 encodes an endosomal pattern recognition receptor that induces Type I IFN production and activates IFN signature genes. Increased TLR7 expression was observed among female pediatric SLE patients94, perhaps in part due to its ability to escape XCI and exhibit biallelic expression in immune cells, such as B cells31. Indeed, TLR7 exhibits variable escape from XCI in human immune cells and B cells from female NZB/W F1 mice73, suggesting that aberrant overexpression resulting from XCI escape is a mechanism that may contribute to the pathogenesis of female-biased autoimmune disease. Biallelic expression of TLR7 in human female B cells increases cell responsiveness to TLR7 ligands and increased class switching compared to monoallelic TLR7 expressing B cells31. The resulting increase in TLR7 signaling is likely biologically significant. Recently, a gain-of-function mutation in TLR7 was identified in a young female with SLE. Introduction of this mutation into BL6 mice results in the spontaneous development of lupus-like disease. 95. Mouse models with two or more copies of Tlr7, including the BXSB-Yaa strain (BOX 3), have increased autoantibody production, autoreactive lymphocytes, and glomerulonephritis96,97. TRL7 exhibits variable escape from XCI in human immune cells and B cells from female NZB/W F1 mice73, suggesting that aberrant overexpression resulting from XCI escape is a mechanism that may contribute towards female-biased autoimmune disease. TASL (CXorf21), an X-linked adaptor protein critical for Tlr7 signaling is aberrantly overexpressed in SLE patient lymphoblastoid cell lines compared to healthy controls and may escape XCI in immune cells 98. Thus, abnormal overexpression of TLR7 and TASL due to potential perturbations with XCI escape could represent a mechanism which contributes towards increased IFN signaling in female individuals with autoimmune disease. Retrospective identification of additional disease-related genes abnormally expressed from the X chromosome is challenged by the omission of reads from the sex chromosomes in microarray and transcriptional profiling datasets when normalizing samples between the sexes in many publications. However, many patient datasets for autoimmune diseases with a strong female bias include a female-majority of patient samples and therefore retain X-linked transcript information, permitting future re-analyses of the data to identify additional differentially expressed X-linked genes in patient samples56,59. Future translational investigations in well clinically characterized patient cohorts will be instrumental in further defining the spectrum of X-linked genes exhibiting dysregulated expression in a sex-specific and disease-specific manner.
Sex differences with immune responses to pathogens
Sex differences in the incidence or severity of infectious diseases have also been observed across a variety of distinct pathogens (Table 3). Biological sex impacts pathogen replication and transmission, as well as the host immune response to the pathogen. The sex bias of infectious disease is typically explained by the stronger female immune response, which results in higher levels of inflammation. While sexual dimorphism among infectious diseases is likely explained in part by behavioral differences (lifestyle choices, exposures, access to healthcare, etc), underlying physiological differences, both hormonal and genetic, may have an even greater impact. The hormonal and genetic contributions responsible for the sex-biased responses to a variety of pathogens are summarized in Table 3 but have not been well characterized (particularly the genetic component). Mouse and rodent models often recapitulate the observed sex bias in humans (Table 3) and have been used to identify sex-specific molecular pathways resulting from infection of some pathogens. Pathogen type also influences infection incidence and disease severity, often exhibiting a sex bias in both humans and rodent models. Males typically exhibit greater incidence for most bacterial, parasitic, some viral, and fungal infections (Table 3); females have greater incidence of parasitic worm infections99-101. Some pathogens, including HIV, Ebola, M. tuberculosis, Cryptosporidium, and Sars-CoV-2, exhibit altered rates of replication, transmission, or incidence of multiple infections of different pathogens between the sexes, which likely influence incidence rates 102-107. Continued use of relevant mouse models, when they recapitulate some features of human disease, with these different pathogens is likely to reveal genetic and epigenetic mechanisms that contribute to the sex differences following infection by these specific pathogens.
Table 3. Sex differences across various classes of pathogens (virus, bacteria, worms, parasites, fungi) for humans and mouse/rodent models.
Pathogen prevalence and incidence, pathogen loads and disease severity, and mouse models exhibiting sex bias with infection or disease are shown in each column. Information regarding hormonal or genetic influences on the sex bias is provided (when available) for each pathogen.
| Pathogen | Pathogen Prevalence and Incidence |
Pathogen Intensity/Load; Resulting Disease Severity |
Mouse Models & Sex Biased Mechanisms/Pathways |
|
|---|---|---|---|---|
| VIRUS | Coronaviruses; SARS-CoV; SARS-CoV-2 | Males have higher incidence1-10 | Adult males (45-79yrs) have higher mortality5,6
9 Females more likely to be diagnosed with Long Covid syndrome11,12 |
Male mice are more susceptible to SARS-CoV infection13-15 Male mice have increased accumulation of inflammatory macrophages and neutrophils in lung (SARS-CoV)3,13 Estrogen receptor signaling is protective after SARS-CoV-infection13,16 SARS-CoV-2 entry receptor Ace2 is biallelically expressed in females, which might reflect its dual role in mediating viral replication vs renin-angiotensin-aldosterone system 4,17 |
| Influenza | Infant males and older adult males have increased incidence18-23 | Males (pre-pubescent, elderly) have high mortality.19,23 Females (pre-menopausal; pregnancy) also have high mortality18,24-26 |
Male mice have greater disease severity and this increases with age27 Female mice have greater mortality28 Female mice are more protected following influenza vaccination29,30 Low levels of testosterone in male mice correlate with poorer protection27,31-33 but estriol protects female mice from severe disease and decreases influenza replication34,35 |
|
| Hepatitis A | Males have more hospitalizations36 | Males have higher mortality36 | n/a | |
| Hepatitis C | Similar incidence rates between males and females37 | Males have greater disease severity (HCV-associated cirrhosis) 37 Females more likely to clear virus37 |
n/a | |
| West Nile Virus | Higher percentage of affected males in this case study38 | Similar initial viremia38 Females have more symptoms38 Males have longer lived cytokine response38 Males have increased mortality38 Males more likely to develop neuroinvasive disease38 |
n/a | |
| Human Immunodeficiency Virus (HIV) | Females have higher incidence 39 Females have higher levels of immune activation and interferon signature gene expression40 |
Females have lower viral loads in early stages of infection (but comparable in advanced stage)40,41 No sex difference with disease progression or clinical outcomes |
Male-to-female transmission appears more efficient than female-to-male transmission42 | |
| HCMV | Females (post-puberty pre-menopausal) have higher incidence of HCMV seroprevalence43 | n/a | ||
| Herpes Simplex Virus (HSV) | Females have higher prevalence44,45 | Female mice more susceptible to infection28 Female mice have higher HSV titers in brain tissue28 Higher mortality in male mice46 Ovariectomy of female mice or estrogen treatment of male mice eliminated sex differences after infection28 Sex-biased survival differences depend on type I IFN signaling and DAP12 signaling28 |
||
| Coxsackievirus | Male mice have increased mortality28,47 Males develop more severe cardiac inflammation due to Th1 response47 Females are more resistant; exhibit predominantly Th2 responses47 |
|||
| Ebola | Males have higher mortality48 | n/a | ||
| Measles | Females (age 45-64) have higher incidence49; Males (age 0-45) have higher incidence50 | Females (ages 0-49) have higher mortality, particularly post-puberty pre-menopausal49. | n/a | |
| Respiratory Syncytial Virus (RSV) | Males have higher incidence51,52, but a metanalysis of acute respiratory infections in Africa did not identify sex as a factor in RSV prevalence53 | Males have higher rates of hospitalizations51 | Male neonatal mice have higher viral gene expression after RSV infection, and delayed viral resolution54 After early-life RSV infection, male mice exposed to allergen have severe allergic exacerbation (female mice are protected). TSLP pathway (which impacts IFN-beta production) alters male immune environment after neonatal infection54 |
|
| BACTERIA | Heliobactor pylori | Males have greater H. Pylori sero-prevalence55,56 Infection has a male bias55 |
Males have more severe inflammation, atrophy, and intestinal metaplasia57 | Male mice are more susceptible Males have higher colonization levels for babA virualence factor of H. pylori58 Male mice treated with estradiol produce less IFN-gamma and IL-1-beta, and increased IL-10 and Th2 associated IgG1 levels58 Estrogen is protective against gastric lesions; ovarectomy increases severity of gastritis and gastric cancer58 |
| Pseudomonas aeruginosa | Males have higher prevalence | Female CF patients have worse disease prognosis59 | Female mice more susceptible to infection60 Females mount strong inflammatory response in lungs60 Estradiol upregulates expression of secretory leucoprotease which inhibits Tlr-dependent IL-8 release in bronchial epithelial cells during P.aeruginosa infection |
|
| Salmonella | Higher incidence rates in male children for salmonellosis (up to age 15) 61 Females have higher incidence rates (ages 15–44 and 45–64) |
n/a | ||
| Chlamydia trachomatis; Chlamydia pneumoniae | Males have greater prevalence (C. pneumoniae)62 Females have higher prevalence (C. trachomatis)63 |
Males have higher levels of C. pneumoniae64 Females have higher infection rates because they are more likely to be screened (C.trachomatis)65 Estrogen levels correlate with chlamydial load64 Chlamydia-induced arthritis more common in men66 |
n/a | |
| Brucella spp. | Males have higher incidence67 No sex bias with prevalence |
Males more likely to develop Brucellosis68 | n/a | |
| Borrelia burgdorferi (Lyme disease) | Males have higher incidence (USA 1992-1998)69 Females >45 greater incidence (Sweden 1992-1993)70,71 Females more likely to be re-infected after 5 years. |
Males have more hospitalizations and likelihood for disseminated disease72 Lyme neuroborreliosis is more common in female patients73 Females have increased production of IFN-gamma, IL-4, IL6, IL-10, TNF-alpha70 |
Male mice have more infected tissues and higher spirochete loads74 | |
| Mycobacterium tuberculosis | Males have higher incidence (male/female 1.7)75 | Males exhibit higher mortality rates (global)76 Pregnancy increases risk of disease complications77 Females usually have less symptoms64 |
Male mice have accelerated disease progression, increased morbidity and mortality78 Males have higher M. tuberculosis loads78 Testosterone treatment increases susceptibility to infection |
|
| Mycoplasma pulmonis | Male mice more susceptible79 Male mice develop more severe disease in lung parenchyma79 Removal of reproductive organs reduced disease severity79 |
|||
| Coxiella burnettii | Males have higher incidence80 | Human males more likely to become sympotomatic with Q fever (symptoms include fever, granulomatous hepatitis, myocarditis, pericarditis, pneumonia)64,80 Pregnancy increases risk for persistent infections, and impaired immunity negatively impacts pregnancy64 |
Male mice have higher bacterial loads64 Estrogen treatment of ovariectomized mice reduces bacterial loads and granulomas81 C. burnetti infection results in sex-specific gene expression profiles: males upregulate IL-10 and interferon-gamma production; females exhibt altered expression of circadian rhythm genes.82 |
|
| Campylobacter spp. | Males have higher incidence64 | Males are more susceptible to infection and colonization83 Males have higher shedding rates |
||
| Clostridiodes difficile | Females have higher incidence84 Females have increased risk of recurrent infection57 |
Increased disease severity in pregnant and peripartum females84 | Progesterone and estrogen intermediates can inhibit spore germination in mice57 | |
| Listeria monocytogenes | Females have higher incidence rates of invasive listeriosis85 Pregnant females have higher incidence64 Among older individuals, males have 2-4 higher incidence rates85 |
Pregnant females and older males have greater incidences of invasive disease85 Older males have increased fatality rates85 |
Female mice more susceptible to infection and exhibit greater lethality86 Females have higher bacterial load; Infected females have increased IL-10, which inhibits Th1 differentiation and Th1-derived cytokines86 Estrogen treatment reduced IL-12, IFN-gamma, TNF-alpha; increased IL-4 and IL-10; reduced monocytes and lymphocyte accumulation at infection87 |
|
| Legionella pneumophila | Males have higher incidence | Males more likely to develop legionellosis and males more likely to have poor prognosis64 | n/a | |
| Leptospira spp. | Males have higher incidence64 | n/a | ||
| Francisella tularensis | Males have higher incidence65 | No sex difference with susceptibility Vaccinated female mice are more resistant to infection, with lower bacterial burdens, less tissue inflammation, and less proinflammatory cytokine production, and more Ft-specific antibodies in serum and lung85 |
||
| Escherichia coli | Females have higher incidence64 | No sex difference with enterohemorrhagic E.coli disease in mice | ||
| Treponema pallidum (syphilis) | Males have higher incidence88,89 | n/a | ||
| Neisseria gonorrhea | Males have higher incidence64 Infected males may also have increased expression of gonococcal antimicrobial resistance genes90 |
Most females lack symptoms64 Complications in males include epididymitis, infertility, prostatitis, seminal vesiculitis91 Elevated progesterone promotes gonococcal infection (human cervical epithelial cells) |
Estrogen treated mice have increased susceptibility to gonococcal infection92 | |
| Streptococcus pneumoniae | Males have higher incidence for all types of pneumonia64 Males (pre-puberty) have higher incidence |
Males have greater hospitalization rates and increased mortality64 Males more frequently diagnosed with Legionellosis (1.7:5 male to female)93 |
Male mice are more susceptible & have more severe disease94 Males exhibit increased pro-inflammatory cytokines (IL-6, IL-17A, IFN-gamma)94 Estrogen is protective, regulating macrophage activity (for pneumococcal pneumonia)95 |
|
| Yersinia enterocolitica | Males have higher incidence for Yersiniosis96 | Males have higher levels of IgG4 antibodies for Yersinia outer membrane proteins, which is associated with anti-inflammatory response that is resistant to treatment57 | n/a | |
| Sepsis: Staphylococcus, Escherichia coli, Pseduomonas, etc | Males have higher rates of sepsis and septic shock65 Males more likely to develop sepsis after trauma or surgery65 |
Conflicting results for a sex bias with mortality97 | Male mice develop greater inflammatory response, producing more pro-inflammatory cytokines64 Males have more severe sepsis-induced cardiac dysfunction85 Estrogen is protective, and female mice produced protective antibodies in response to estrogen; estrogen-driven antibodies were maternally transferrable to offspring98 |
|
| WORMS | Pork tapeworm (Taenia solium) Neurocysticercosis |
Females have higher incidence in some countries (Nigeria, Tanzania, Guatemala)99 Females have more transitional cysts in brain (Ecuador)100 No sex difference with incidence in Vietnam99 |
Female patients have greater number of transitional cysts100 | Estrogen increases parasite loads and androgens decrease loads in mice, either acting directly on the worm’s reproduction or by altering host’s immune response to favor Th2 or Th1 pathways (Taenia crassiceps)101 |
| A. Lumbricoides | Females have higher incidence102 | n/a | ||
| Schistosoma masoni | Males have higher prevalence for infection103 | Female and castrated male mice have greater morbidity after Schistosoma infection104 Female mice have higher worm loads104 Testosterone is protective for Schistosoma mansoni infections; female mice treated with testosterone had reduced worm burdens (if treated before infection)104 |
||
| PARASITES | Plasmodium falciparum (malaria) | Male patients have greater disease severity105 | n/a | |
| Cryptosporidium | Males have higher incidence106 | Male patients have greater incidence of hospitalizations107 | n/a | |
| Entamoeba histolytica (amoebiasis) | Asymtopmatic infection rates are the same across sexes108 | Invasive amebiasis predominantly affects males; males have higher rates of invasive disease108 Males have higher incidence of hepatic amebiasis109 |
Testosterone treatment induces proinflammatory responses in mouse (& human) classical monocytes, with increased production of CXCL1 and TNF109 | |
| Leishmania | Males have higher incidence even when accounting for exposure110 Adult males have higher incidence of cutaneous leishmaniasis111 Childhood cutaneous leishmaniasis does not exhibit a sex bias112 Males have higher incidence and greater risk ratio of visceral leishmaniasis113 No sex bias for childhood cutaneous leishmaniasis114 |
Male patients exhibit higher rates of treatment failure and adverse effects110 | Male mice have higher parasite burdens following infection (L.infantum)115 Male mice express higher levels of IL-10 and TNF after infection and exhibit greater disease severity115 Male mice (BALB/c congenic strains) are more susceptible to subcutanteous L.major, and exhibit more severe disease110,116 Female mice heal small lesions following L. Mexicana infection, yet male mice exhibit persistent lesions, dependent on IL-4 levels117 Male hamsters have increased disease severity and parasite burden with L. viannia infection. Testosterone-treated female animals had larger lesions than untreated females. Disease severity correlated with increased expression of IL-4, IL-10, and TGF-beta118 X-linked Cxcr3 is biallelically expressed in T cells of female mice and contributes to increased cytokine production119 |
|
| Toxoplasma gondii | Sex differences with infection-induced behavioral changes and personality shifts120 | Female mice are more susceptible to infection and have higher cyst burdens121 Female mice exhibit higher mortality after acute infection121 Male mice produce higher TNF-alpha after day 10 of infection; female mice mortality did not correlate with lower TNF-alpha levels. Male mice produce higher IFN-gamma and IL-10 early during infection121 |
||
| FUNGI | Aspergillus fumigatus | Males have higher incidence (invasive pulmonary aspergillosis)122 Male bias with prevalence, incidence and severity123 Males more susceptible to infection123 |
Female mice have higher levels of immune components (antibody titers, neutrophil eosinophil, and lymphocyte cells) after infection124 | |
| Cryptococcus neoformans | Males have higher incidence125 Males more affected than females125 |
Female mice express more cytokines in plasma and increased expression of TNF-alpha, interferon-gamma in spleen126 Increased lethality for young male mice126 Survival and fungal loads are similar between male and female mice126 |
||
| Paracoccidioides brasiliensis | Males have greater incidence (10:1 male to female, Latin America)127 | Male patients have faster disease progression128 | Male mice are more susceptible129 Macrophages from infected female mice exhibit greater fungicidal activity, with higher nitric oxide production129 Estrogen is protective following P. brasiliensis infection, as castrated male treated with estradiol have higher levels of IFN-gamma and lower levels of IL-10 compared to normal males. Ovariectomized female mice treated with testosterone produce less IFN-gamma and more IL-10 compared to normal female mice after infection129 |
|
| Microsporum, Trichophyton, epigermophyton (Tinea or Dermatophytosi) | Males have higher incidence 130 | n/a | ||
| Candida albicans | Females have higher incidence (oral candidiasis)131 Females have higher incidence (candida onychomycosis), with 3/4 females (childbearing age) infected at least once in their life; and 1/10 females having a recurring event131 |
Male patients with seropositivity for C.albicans have increased odds for schizopherenia132 Female patients with seropositivity for C.albicans have increased odds for lower cognitive scores132 |
n/a |
Sex differences in response to vaccination
Females often have greater antibody responses, and typically experience more adverse reactions to vaccinations108. Activation of the innate immune system immediately following vaccination often results in localized inflammation of the injection site. Subsequent activation of the adaptive immune system is also critical for generating an effective memory response to inactivated viruses or viral particles. Females often develop more inflammation around the vaccine injection site, which may result from sex differences with innate immune activation and produce stronger class-switched antibody profiles for vaccines against influenza, smallpox, measles mumps and rubella, yellow fever, hepatitis A and B, and herpes simplex (Table 3). Female mice injected with inactivated virus produce higher antibody titers and higher numbers of germinal center B cells and CD8+ and CD4+ T cells in lymph nodes compared to male mice109. Because females often have stronger responses to viral and bacterial vaccines and have more severe reactions, it has been suggested that female-specific reductions with vaccine doses should be considered32.
X-linked genes involved in sex-biased responses to pathogen infections and vaccination
Accumulating evidence suggests that XCI escape of some X-linked immunity genes in innate and adaptive immune cells contributes towards increased female protection from bacteria and parasites. Transcription of ACE2, one of the receptors utilized by coronaviruses for cellular entry, is influenced by both genetic and hormonal factors106,107,110,111, and ACE2 escapes XCI in human cells and mouse AT2 cells inside alveoli of the lung71,112. One study using Cxcr3 dual reporter mice found that Cxcr3 escapes XCI in activated T cells, and that following Leishmania infection, biallelic Cxcr3-expressing cells produce more IFN-gamma, IL-2, and CD69 than do monoallelic Cxcr3 expressing cells113. In general, males have less potent antiviral responses (Table 3), which may be influenced by sex-dependent differences in UTX/Utx expression levels in NK cells that result in increased numbers of NK cells with reduced functionality in males compared to females16. NK cells are necessary for antiviral responses to various viruses including cytomegalovirus (CMV), and Utx deletion increases lethality following CMV infection16. It is likely that in addition to Utx and Cxcr3, other X-linked genes that escape XCI in immune cells impact the response to pathogens that exhibit sex biases in infection (Table 3). The identification of these genes may reveal one pathway which underlies the mechanistic basis for sex-biased immune protection in response to a variety of pathogens.
The importance of X-linked immunity related genes for sex-biased responses to pathogens is underscored by observations of sex-specific phenotypes in genetic deletion models or in patients with specific mutations following infections. For example, the X-linked gene Ddx3x, an RNA helicase that impacts RNA processing and transcription and regulates type I IFN production following viral and bacterial infections114, escapes XCI in female cells. Although male cells contain a Y-linked functional homolog (Ddx3y), conditional deletion of Ddx3x using VAV-Cre impairs the ability of male mice (female-specific deletion is embryonic lethal) to respond to listeria monocytogenes115. Consistent with these findings, female bone marrow derived macrophages (BMDMs) with homozygous Ddx3x deletion are unable to restrict listeria growth115. Ddx3x deletion in BMDMs exhibits sex-specific gene expression patterns, and female BMDMs lacking Ddx3x have greater reductions in cytokine (IL-1, IL-6, Il-12, and TNF-alpha) and chemokine expression compared to male Ddx3x mutants115. Additional research investigating other XCI escape genes, especially those that encode for chromatin modifying enzymes, through the use of gain and loss of function mutations in specific immune cell types will reveal other potential genetic contributors to the immune responses to pathogens that exhibit a sex bias (Table 3).
Tuberculosis exhibits a male bias in humans and mouse models, and TB is a leading cause globally of a disease caused by a bacterial pathogen (Table 3). Mutations in two X-linked genes, CYBB and IKBKG (NEMO), increase susceptibility to mycobacterial disease. Missense mutations in CYBB, which encodes for the gp91 subunit of the NADPH oxidase complex, results in a hypomorphic protein that impairs respiratory burst activity in macrophages necessary for protection from mycobacteria116. IKBKG/NEMO is the regulatory subunit of the inhibitor of kappa B kinase (IKK) complex, which regulates canonical NF-kappa B activation of genes involved in inflammation and immunity. While IKBKG/NEMO mutations result in incontinentia pigmenti, ectodermal dysplasia, and various immunodeficiencies, there are some NEMO mutations that increase predisposition of male patients to mycobacterial infection through reduction of IL-12 and IFN-gamma production117.
Despite ample evidence for sex biased responses to vaccinations, few X-linked genes that contribute to this finding have been identified. B cells from female immunized mice do express higher levels of Tlr7109, which may result from increased levels of XCI escape and expression from the Xi in response to flu vaccines. Tlr7 can recognize single-stranded viruses including SARS-CoV-2, and may contribute to Type I IFN production118,119. Loss of function TLR7 mutations in human males reduce Type I IFN levels and prevent interferon-stimulated gene (ISG) induction, resulting in severe COVID-19 disease118,120-122. RNA vaccines for SARS-CoV-2 induce adverse which exhibit sex biases 123, as human females are more susceptible to thrombosis thrombocytopaenia syndrome (from adenoviral vectors)124, yet young human males appear to be more susceptible to myocarditis and pericarditis from mRNA vaccines125.
CONCLUSIONS AND PERSPECTIVES
Biological sex is an important factor for immune responses and immune health, and the importance of understanding hormonal and genetic contributions to sex differences in immunity is increasing. In 2009, about >60% of immunology-related research publications using animal models lacked information about biological sex, and over half of human immunology publications included male and female samples, yet >90% of these publications lacked sex-specific analyses126. Inclusion rates for both sexes in immunological research was 16% in 2009, and increased to 46% by 2019, but suggests that more work is needed to ensure that sex as a biological variable is addressed in future immunologic research. While the influence of sex hormones on sex-specific immune cell function and cytokine production following infection has been investigated for some of the pathogens in Table 3, the contribution from the X chromosome in biased immune responses is not well understood. Future experiments examining XCI escape genes in specific immune cell populations, and use of genetic gain and loss of function experiments of immunity-related X-linked genes will reveal important mechanisms of sex differences in infection susceptibility and resulting disease severity. While the sensitivity of X-linked gene dosage for some female-biased autoimmune diseases has been examined, whether X-linked gene dosage influences immune responses to pathogens is not well known. Understanding the origins of the sex biases using rodent models that recapitulate sex differences observed in clinical studies of infectious and autoimmune disease are likely to inform sex-specific treatment strategies that could improve patient outcomes.
The interplay of the sex chromosomes and how they influence sex hormones is likely to influence sex differences in immune responses and sex-biased autoimmune disease. In addition to the Y-linked Sry gene, which functions in primary sex determination and the formation of the male gonads responsible for testosterone production, the influence of other X- and Y-linked genes on sex hormones in the context of sex-biased immune responses has not been carefully investigated. Given the large number of steroid receptor binding sites across the genome, including on the X chromosome, it is likely that inflammatory pathways resulting from infections and autoimmunity will promote sex hormone receptor binding to promoters of X-linked immunity-related genes, perhaps promoting increased XCI escape in female immune cells. Moreover, AR and ER expression levels change with cellular activation in immune cells127, potentially impacting X-linked gene expression and contributing to sex-biased gene expression. Hormonally-induced expression changes of X-linked genes are likely to occur on the active X, yet it is possible that XCI escape genes on the Xi could be differentially regulated by AR and ER in the context of immune activation. Investigation of allele-specific transcriptional changes in response to cellular activation and inflammation are necessary to determine the complex interplay of sex hormones and X-linked gene expression for sex differences in immune responses. Understanding the genetic and hormonal contributions for sex differences with immune health will undoubtedly result in novel therapeutic approaches for effective precision medicine.
Acknowledgements
We thank L. King and members of the Anguera lab for their input and feedback on the manuscript and figures. This work was supported by grants from the National Institutes of Health (R01-AI134834 to M.C.A; T32-AR076951-01 to N.J.), the Lupus Research Alliance TIL grant (to M.C.A); T32 DK-07780 to K.S.F., the Rheumatology Research Foundation Future Physician Scientist Award (to C.D.L.), the Lupus Foundation of America, Philadelphia Tri-State Chapter Goldie Simon Preceptorship Award (to C.D.L. and N.E.T.), the Lupus Foundation of America, Philadelphia Tri-State Chapter Gina M. Finzi Memorial Student Summer Awards (to C.D.L. and N.E.T.), the H. Ralph Schumacher Rheumatology Research Fund, salary support from the Division of Rheumatology at the University of Pennsylvania, the Penn Skin Biology and Diseases Resource-based Center Pilot grant, and a scholarship (for NJ) from the Institute for Translational Medicine and Therapeutics of the Perelman School of Medicine at the University of Pennsylvania. All figures were created using BioRender.
Footnotes
Competing Interests
The authors declare no competing interests.
REFERENCES
- 1.Libert C, Dejager L & Pinheiro I The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol 10, 594–604 (2010). 10.1038/nri2815 [DOI] [PubMed] [Google Scholar]
- 2.Pasche B. et al. Sex-dependent susceptibility to Listeria monocytogenes infection is mediated by differential interleukin-10 production. Infect Immun 73, 5952–5960 (2005). 10.1128/IAI.73.9.5952-5960.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Natri H, Garcia AR, Buetow KH, Trumble BC & Wilson MA The Pregnancy Pickle: Evolved Immune Compensation Due to Pregnancy Underlies Sex Differences in Human Diseases. Trends Genet 35, 478–488 (2019). 10.1016/j.tig.2019.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patin E. et al. Natural variation in the parameters of innate immune cells is preferentially driven by genetic factors. Nat Immunol 19, 302–314 (2018). 10.1038/s41590-018-0049-7 [DOI] [PubMed] [Google Scholar]
- 5.Clave E. et al. Human thymopoiesis is influenced by a common genetic variant within the TCRA-TCRD locus. Sci Transl Med 10 (2018). 10.1126/scitranslmed.aao2966 [DOI] [PubMed] [Google Scholar]
- 6.Bongen E. et al. Sex Differences in the Blood Transcriptome Identify Robust Changes in Immune Cell Proportions with Aging and Influenza Infection. Cell Rep 29, 1961–1973 e1964 (2019). 10.1016/j.celrep.2019.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdullah M. et al. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell Immunol 272, 214–219 (2012). 10.1016/j.cellimm.2011.10.009 [DOI] [PubMed] [Google Scholar]
- 8.Melzer S. et al. Reference intervals for leukocyte subsets in adults: Results from a population-based study using 10-color flow cytometry. Cytometry B Clin Cytom 88, 270–281 (2015). 10.1002/cyto.b.21234 [DOI] [PubMed] [Google Scholar]
- 9.Huang Z. et al. Effects of sex and aging on the immune cell landscape as assessed by single-cell transcriptomic analysis. Proc Natl Acad Sci U S A 118 (2021). 10.1073/pnas.2023216118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wikby A, Mansson IA, Johansson B, Strindhall J & Nilsson SE The immune risk profile is associated with age and gender: findings from three Swedish population studies of individuals 20-100 years of age. Biogerontology 9, 299–308 (2008). 10.1007/s10522-008-9138-6 [DOI] [PubMed] [Google Scholar]
- 11.Carr EJ et al. The cellular composition of the human immune system is shaped by age and cohabitation. Nat Immunol 17, 461–468 (2016). 10.1038/ni.3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan SR et al. Determinants of Serum Immunoglobulin Levels: A Systematic Review and Meta-Analysis. Front Immunol 12, 664526 (2021). 10.3389/fimmu.2021.664526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hensel JA, Khattar V, Ashton R & Ponnazhagan S Characterization of immune cell subtypes in three commonly used mouse strains reveals gender and strain-specific variations. Lab Invest 99, 93–106 (2019). 10.1038/s41374-018-0137-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breznik JA, Schulz C, Ma J, Sloboda DM & Bowdish DME Biological sex, not reproductive cycle, influences peripheral blood immune cell prevalence in mice. J Physiol 599, 2169–2195 (2021). 10.1113/JP280637 [DOI] [PubMed] [Google Scholar]
- 15.Scotland RS, Stables MJ, Madalli S, Watson P & Gilroy DW Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood 118, 5918–5927 (2011). 10.1182/blood-2011-03-340281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng MI et al. The X-linked epigenetic regulator UTX controls NK cell-intrinsic sex differences. Nat Immunol 24, 780–791 (2023). 10.1038/s41590-023-01463-8 [DOI] [PubMed] [Google Scholar]
- 17.Schmiedel BJ et al. Impact of Genetic Polymorphisms on Human Immune Cell Gene Expression. Cell 175, 1701–1715 e1716 (2018). 10.1016/j.cell.2018.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pauklin S, Sernandez IV, Bachmann G, Ramiro AR & Petersen-Mahrt SK Estrogen directly activates AID transcription and function. J Exp Med 206, 99–111 (2009). 10.1084/jem.20080521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young NA et al. Estrogen modulation of endosome-associated toll-like receptor 8: an IFNalpha-independent mechanism of sex-bias in systemic lupus erythematosus. Clin Immunol 151, 66–77 (2014). 10.1016/j.clim.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J. et al. Oestrogen up-regulates interleukin-21 production by CD4(+) T lymphocytes in patients with systemic lupus erythematosus. Immunology 142, 573–580 (2014). 10.1111/imm.12263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S. et al. Interleukin-23 drives expansion of Thelper 17 cells through epigenetic regulation by signal transducer and activators of transcription 3 in lupus patients. Rheumatology (Oxford) 59, 3058–3069 (2020). 10.1093/rheumatology/keaa176 [DOI] [PubMed] [Google Scholar]
- 22.Berghofer B. et al. TLR7 ligands induce higher IFN-alpha production in females. J Immunol 177, 2088–2096 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Meier A. et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med 15, 955–959 (2009). 10.1038/nm.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seillet C. et al. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor alpha signaling. Blood 119, 454–464 (2012). 10.1182/blood-2011-08-371831 [DOI] [PubMed] [Google Scholar]
- 25.Regis E. et al. Sex differences in innate anti-viral immune responses to respiratory viruses and in their clinical outcomes in a birth cohort study. Sci Rep 11, 23741 (2021). 10.1038/s41598-021-03044-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Congy-Jolivet N. et al. Monocytes are the main source of STING-mediated IFN-alpha production. EBioMedicine 80, 104047 (2022). 10.1016/j.ebiom.2022.104047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griesbeck M. et al. Sex Differences in Plasmacytoid Dendritic Cell Levels of IRF5 Drive Higher IFN-alpha Production in Women. J Immunol 195, 5327–5336 (2015). 10.4049/jimmunol.1501684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gal-Oz ST et al. ImmGen report: sexual dimorphism in the immune system transcriptome. Nat Commun 10, 4295 (2019). 10.1038/s41467-019-12348-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng Z. et al. Sex-hormone-driven innate antibodies protect females and infants against EPEC infection. Nat Immunol 19, 1100–1111 (2018). 10.1038/s41590-018-0211-2 [DOI] [PubMed] [Google Scholar]
- 30.McDonald G. et al. Female Bias in Systemic Lupus Erythematosus is Associated with the Differential Expression of X-Linked Toll-Like Receptor 8. Front Immunol 6, 457 (2015). 10.3389/fimmu.2015.00457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Souyris M. et al. TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol 3 (2018). 10.1126/sciimmunol.aap8855 [DOI] [PubMed] [Google Scholar]
- 32.Klein SL & Flanagan KL Sex differences in immune responses. Nat Rev Immunol 16, 626–638 (2016). 10.1038/nri.2016.90 [DOI] [PubMed] [Google Scholar]
- 33.Aomatsu M, Kato T, Kasahara E & Kitagawa S Gender difference in tumor necrosis factor-alpha production in human neutrophils stimulated by lipopolysaccharide and interferon-gamma. Biochem Biophys Res Commun 441, 220–225 (2013). 10.1016/j.bbrc.2013.10.042 [DOI] [PubMed] [Google Scholar]
- 34.Ho CH et al. Testosterone suppresses uropathogenic Escherichia coli invasion and colonization within prostate cells and inhibits inflammatory responses through JAK/STAT-1 signaling pathway. PLoS One 12, e0180244 (2017). 10.1371/journal.pone.0180244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fish EN The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol 8, 737–744 (2008). 10.1038/nri2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liva SM & Voskuhl RR Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J Immunol 167, 2060–2067 (2001). 10.4049/jimmunol.167.4.2060 [DOI] [PubMed] [Google Scholar]
- 37.Dragin N. et al. Estrogen-mediated downregulation of AIRE influences sexual dimorphism in autoimmune diseases. J Clin Invest 126, 1525–1537 (2016). 10.1172/JCI81894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu ML et al. Sex bias in CNS autoimmune disease mediated by androgen control of autoimmune regulator. Nat Commun 7, 11350 (2016). 10.1038/ncomms11350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gandhi VD et al. Androgen receptor signaling promotes Treg suppressive function during allergic airway inflammation. J Clin Invest 132 (2022). 10.1172/JCI153397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walecki M. et al. Androgen receptor modulates Foxp3 expression in CD4+CD25+Foxp3+ regulatory T-cells. Mol Biol Cell 26, 2845–2857 (2015). 10.1091/mbc.E14-08-1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trigunaite A, Dimo J & Jorgensen TN Suppressive effects of androgens on the immune system. Cell Immunol 294, 87–94 (2015). 10.1016/j.cellimm.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 42.Wilhelmson AS et al. Testosterone is an endogenous regulator of BAFF and splenic B cell number. Nat Commun 9, 2067 (2018). 10.1038/s41467-018-04408-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao R. et al. A GPR174-CCL21 module imparts sexual dimorphism to humoral immunity. Nature 577, 416–420 (2020). 10.1038/s41586-019-1873-0 [DOI] [PubMed] [Google Scholar]
- 44.Kissick HT et al. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc Natl Acad Sci U S A 111, 9887–9892 (2014). 10.1073/pnas.1402468111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marahrens Y, Panning B, Dausman J, Strauss W & Jaenisch R Xist-deficient mice are defective in dosage compensation but not spermatogenesis. Genes Dev 11, 156–166 (1997). [DOI] [PubMed] [Google Scholar]
- 46.Anguera MC et al. Molecular signatures of human induced pluripotent stem cells highlight sex differences and cancer genes. Cell Stem Cell 11, 75–90 (2012). https://doi.org:S1934-5909(12)00125-7 [pii] 10.1016/j.stem.2012.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adrianse RL et al. Perturbed maintenance of transcriptional repression on the inactive X-chromosome in the mouse brain after Xist deletion. Epigenetics Chromatin 11, 50 (2018). 10.1186/s13072-018-0219-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang L, Yildirim E, Kirby JE, Press W & Lee JT Widespread organ tolerance to Xist loss and X reactivation except under chronic stress in the gut. Proc Natl Acad Sci U S A 117, 4262–4272 (2020). 10.1073/pnas.1917203117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yildirim E. et al. Xist RNA is a potent suppressor of hematologic cancer in mice. Cell 152, 727–742 (2013). 10.1016/j.cell.2013.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boggs BA et al. Differentially methylated forms of histone H3 show unique association patterns with inactive human X chromosomes. Nat Genet 30, 73–76 (2002). 10.1038/ng787ng787 [pii] [DOI] [PubMed] [Google Scholar]
- 51.Plath K. et al. Role of histone H3 lysine 27 methylation in X inactivation. Science 300, 131–135 (2003). 10.1126/science.10842741084274 [pii] [DOI] [PubMed] [Google Scholar]
- 52.Costanzi C, Stein P, Worrad DM, Schultz RM & Pehrson JR Histone macroH2A1 is concentrated in the inactive X chromosome of female preimplantation mouse embryos. Development 127, 2283–2289 (2000). [DOI] [PubMed] [Google Scholar]
- 53.Clemson CM, McNeil JA, Willard HF & Lawrence JB XIST RNA paints the inactive X chromosome at interphase: evidence for a novel RNA involved in nuclear/chromosome structure. J Cell Biol 132, 259–275 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J et al. Unusual maintenance of X chromosome inactivation predisposes female lymphocytes for increased expression from the inactive X. Proceedings of the National Academy of Sciences 113, E2029–E2038 (2016). 10.1073/pnas.1520113113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Syrett CM et al. Loss of Xist RNA from the inactive X during B cell development is restored in a dynamic YY1-dependent two-step process in activated B cells. PLOS Genetics 13, e1007050–e1007050 (2017). 10.1371/journal.pgen.1007050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Syrett CM et al. Altered X-chromosome inactivation in T cells may promote sex-biased autoimmune diseases. JCI Insight 4 (2019). 10.1172/jci.insight.126751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Syrett CM et al. Diversity of epigenetic features of the inactive X-chromosome in NK cells, dendritic cells, and macrophages. Frontiers in Immunology (2019). 10.3389/fimmu.2018.03087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Syrett CM et al. Loss of Xist RNA from the inactive X during B cell development is restored in a dynamic YY1-dependent two-step process in activated B cells. PLoS Genet 13, e1007050 (2017). 10.1371/journal.pgen.1007050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pyfrom S. et al. The dynamic epigenetic regulation of the inactive X chromosome in healthy human B cells is dysregulated in lupus patients. Proc Natl Acad Sci U S A 118 (2021). 10.1073/pnas.2024624118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Syrett CM et al. Diversity of Epigenetic Features of the Inactive X-Chromosome in NK Cells, Dendritic Cells, and Macrophages. Front Immunol 9, 3087 (2018). 10.3389/fimmu.2018.03087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramos-Casals M et al. Google-driven search for big data in autoimmune geoepidemiology: analysis of 394,827 patients with systemic autoimmune diseases. Autoimmun Rev 14, 670–679 (2015). 10.1016/j.autrev.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 62.Jacobson DL, Gange SJ, Rose NR & Graham NM Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol 84, 223–243 (1997). 10.1006/clin.1997.4412 [DOI] [PubMed] [Google Scholar]
- 63.Beeson PB Age and sex associations of 40 autoimmune diseases. Am J Med 96, 457–462 (1994). 10.1016/0002-9343(94)90173-2 [DOI] [PubMed] [Google Scholar]
- 64.Syrett CM & Anguera MC When the balance is broken: X-linked gene dosage from two X chromosomes and female-biased autoimmunity. J Leukoc Biol 106, 919–932 (2019). 10.1002/JLB.6RI0319-094R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ngo ST, Steyn FJ & McCombe PA Gender differences in autoimmune disease. Front Neuroendocrinol 35, 347–369 (2014). 10.1016/j.yfrne.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 66.Runmarker B & Andersen O Prognostic factors in a multiple sclerosis incidence cohort with twenty-five years of follow-up. Brain 116 (Pt 1), 117–134 (1993). 10.1093/brain/116.1.117 [DOI] [PubMed] [Google Scholar]
- 67.Peoples C, Medsger TA Jr., Lucas M, Rosario BL & Feghali-Bostwick CA Gender differences in systemic sclerosis: relationship to clinical features, serologic status and outcomes. J Scleroderma Relat Disord 1, 177–240 (2016). 10.5301/jsrd.5000209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crosslin KL & Wiginton KL Sex differences in disease severity among patients with systemic lupus erythematosus. Gend Med 8, 365–371 (2011). 10.1016/j.genm.2011.10.003 [DOI] [PubMed] [Google Scholar]
- 69.Courvoisier N. et al. Prognostic factors of 10-year radiographic outcome in early rheumatoid arthritis: a prospective study. Arthritis Res Ther 10, R106 (2008). 10.1186/ar2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sierra I. et al. Unusual X chromosome inactivation maintenance in female alveolar type 2 cells is correlated with increased numbers of X-linked escape genes and sex-biased gene expression. Stem Cell Reports (2022). 10.1016/j.stemcr.2022.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tukiainen T. et al. Landscape of X chromosome inactivation across human tissues. Nature 550, 244–248 (2017). 10.1038/nature24265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiwrajka N. et al. Impaired dynamic X-chromosome inactivation maintenance in T cells is a feature of spontaneous murine SLE that is exacerbated in female-biased models. J Autoimmun 139, 103084 (2023). 10.1016/j.jaut.2023.103084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Syrett CM, Sierra I, Beethem ZT, Dubin AH & Anguera MC Loss of epigenetic modifications on the inactive X chromosome and sex-biased gene expression profiles in B cells from NZB/W F1 mice with lupus-like disease. J Autoimmun 107, 102357 (2020). 10.1016/j.jaut.2019.102357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scofield RH et al. Klinefelter’s syndrome (47,XXY) in male systemic lupus erythematosus patients: Support for the notion of a gene-dose effect from the X chromosome. Arthritis and Rheumatism 58, 2511–2517 (2008). 10.1002/art.23701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scofield RH et al. 47XXY and 47XXX in Scleroderma and Myositis. ACR Open Rheumatology (2022). 10.1002/acr2.11413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Vries GJ et al. A Model System for Study of Sex Chromosome Effects on Sexually Dimorphic Neural and Behavioral Traits. The Journal of Neuroscience 22, 9005–9014 (2002). 10.1523/jneurosci.22-20-09005.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith-Bouvier DL et al. A role for sex chromosome complement in the female bias in autoimmune disease. Journal of Experimental Medicine 205, 1099–1108 (2008). 10.1084/jem.20070850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.David A. et al. Intrinsic autoimmune capacities of hematopoietic cells from female New Zealand hybrid mice. Genes Immun 15, 153–161 (2014). 10.1038/gene.2014.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Geha RS et al. Primary immunodeficiency diseases: an update from the International Union of Immunological Societies Primary Immunodeficiency Diseases Classification Committee. J Allergy Clin Immunol 120, 776–794 (2007). 10.1016/j.jaci.2007.08.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Desai-Mehta A, Lu L, Ramsey-Goldman R & Datta SK Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J Clin Invest 97, 2063–2073 (1996). 10.1172/JCI118643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hewagama A. et al. Overexpression of X-linked genes in T cells from women with lupus. J Autoimmun 41, 60–71 (2013). 10.1016/j.jaut.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lian X. et al. DNA demethylation of CD40l in CD4+ T cells from women with systemic sclerosis: a possible explanation for female susceptibility. Arthritis Rheum 64, 2338–2345 (2012). 10.1002/art.34376 [DOI] [PubMed] [Google Scholar]
- 83.Perez-Melgosa M, Hollenbaugh D & Wilson CB Cutting edge: CD40 ligand is a limiting factor in the humoral response to T cell-dependent antigens. J Immunol 163, 1123–1127 (1999). [PubMed] [Google Scholar]
- 84.Enghard P. et al. CXCR3+CD4+ T cells are enriched in inflamed kidneys and urine and provide a new biomarker for acute nephritis flares in systemic lupus erythematosus patients. Arthritis Rheum 60, 199–206 (2009). 10.1002/art.24136 [DOI] [PubMed] [Google Scholar]
- 85.Ogawa N, Ping L, Zhenjun L, Takada Y & Sugai S Involvement of the interferon-gamma-induced T cell-attracting chemokines, interferon-gamma-inducible 10-kd protein (CXCL10) and monokine induced by interferon-gamma (CXCL9), in the salivary gland lesions of patients with Sjogren’s syndrome. Arthritis Rheum 46, 2730–2741 (2002). 10.1002/art.10577 [DOI] [PubMed] [Google Scholar]
- 86.Kong W. et al. Increased expression of Bruton’s tyrosine kinase in peripheral blood is associated with lupus nephritis. Clin Rheumatol 37, 43–49 (2018). 10.1007/s10067-017-3717-3 [DOI] [PubMed] [Google Scholar]
- 87.Kil LP et al. Btk levels set the threshold for B-cell activation and negative selection of autoreactive B cells in mice. Blood 119, 3744–3756 (2012). 10.1182/blood-2011-12-397919 [DOI] [PubMed] [Google Scholar]
- 88.Katewa A. et al. Btk-specific inhibition blocks pathogenic plasma cell signatures and myeloid cell-associated damage in IFNalpha-driven lupus nephritis. JCI Insight 2, e90111 (2017). 10.1172/jci.insight.90111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Valencia X & Lipsky PE CD4+CD25+FoxP3+ regulatory T cells in autoimmune diseases. Nat Clin Pract Rheumatol 3, 619–626 (2007). 10.1038/ncprheum0624 [DOI] [PubMed] [Google Scholar]
- 90.Wang YY et al. DNA hypermethylation of the forkhead box protein 3 (FOXP3) promoter in CD4+ T cells of patients with systemic sclerosis. Br J Dermatol 171, 39–47 (2014). 10.1111/bjd.12913 [DOI] [PubMed] [Google Scholar]
- 91.Bonelli M, von Dalwigk K, Savitskaya A, Smolen JS & Scheinecker C Foxp3 expression in CD4+ T cells of patients with systemic lupus erythematosus: a comparative phenotypic analysis. Ann Rheum Dis 67, 664–671 (2008). 10.1136/ard.2007.074690 [DOI] [PubMed] [Google Scholar]
- 92.MacDonald KG et al. Regulatory T cells produce profibrotic cytokines in the skin of patients with systemic sclerosis. J Allergy Clin Immunol 135, 946–955 e949 (2015). 10.1016/j.jaci.2014.12.1932 [DOI] [PubMed] [Google Scholar]
- 93.Yang C, Huang XR, Fung E, Liu HF & Lan HY The Regulatory T-cell Transcription Factor Foxp3 Protects against Crescentic Glomerulonephritis. Sci Rep 7, 1481 (2017). 10.1038/s41598-017-01515-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garcia-Ortiz H et al. Association of TLR7 copy number variation with susceptibility to childhood-onset systemic lupus erythematosus in Mexican population. Ann Rheum Dis 69, 1861–1865 (2010). 10.1136/ard.2009.124313 [DOI] [PubMed] [Google Scholar]
- 95.Brown GJ et al. TLR7 gain-of-function genetic variation causes human lupus. Nature 605, 349–356 (2022). 10.1038/s41586-022-04642-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deane JA et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity 27, 801–810 (2007). 10.1016/j.immuni.2007.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pisitkun P. et al. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science 312, 1669–1672 (2006). 10.1126/science.1124978 [DOI] [PubMed] [Google Scholar]
- 98.Harris VM et al. Characterization of cxorf21 Provides Molecular Insight Into Female-Bias Immune Response in SLE Pathogenesis. Front Immunol 10, 2160 (2019). 10.3389/fimmu.2019.02160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kelvin EA et al. The association of host age and gender with inflammation around neurocysticercosis cysts. Annals of tropical medicine and parasitology. 103, 487–499 (2009). 10.1179/000349809x12459740922291 [DOI] [PubMed] [Google Scholar]
- 100.Wright JE, Werkman M, Dunn JC & Anderson RM Current epidemiological evidence for predisposition to high or low intensity human helminth infection: a systematic review. Parasites & Vectors 11 (2018). 10.1186/s13071-018-2656-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tchuem Tchuenté LA, Behnke JM, Gilbert FS, Southgate VR & Vercruysse J Polyparasitism with Schistosoma haematobium and soil-transmitted helminth infections among school children in Loum, Cameroon. Tropical medicine & international health : TM & IH. 8, 975–986 (2003). 10.1046/j.1360-2276.2003.01120.x [DOI] [PubMed] [Google Scholar]
- 102.Nicolosi A. et al. The Efficiency of Male-to Female and Female-to-Male Sexual Transmission of the Human Immunodeficiency Virus. Epidemiology. 5, 570–575 (1994). 10.1097/00001648-199411000-00003 [DOI] [PubMed] [Google Scholar]
- 103.WHO Ebola Response Team, J. A-A, , Archchun Ariyarajah, Isobel M. Blake, Anne Cori, Christl A. Donnelly, Ilaria Dorigatti, Christopher Dye, Tim Eckmanns, Neil M. Ferguson, Christophe Fraser, Tini Garske, Wes Hinsley, Thibaut Jombart, Harriet L. Mills, Gemma Nedjati-Gilani, Emily Newton, Pierre Nouvellet, Devin Perkins, Steven Riley, Dirk Schumacher, Anita Shah, Lisa J. Thomas, Maria D. Van Kerkhove. Ebola Virus Disease among Male and Female Persons in West Africa. New England Journal of Medicine 374, 96–98 (2016). 10.1056/nejmc1510305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hertz D & Schneider B Sex differences in tuberculosis. Seminars in Immunopathology 41, 225–237 (2019). 10.1007/s00281-018-0725-6 [DOI] [PubMed] [Google Scholar]
- 105.Shaheen AAM, Somayaji R, Myers R & Mody CH Epidemiology and trends of cryptococcosis in the United States from 2000 to 2007: A population-based study. International Journal of STD & AIDS 29, 453–460 (2018). 10.1177/0956462417732649 [DOI] [PubMed] [Google Scholar]
- 106.Gemmati D. et al. COVID-19 and Individual Genetic Susceptibility/Receptivity: Role of ACE1/ACE2 Genes, Immunity, Inflammation and Coagulation. Might the Double X-Chromosome in Females Be Protective against SARS-CoV-2 Compared to the Single X-Chromosome in Males? International Journal of Molecular Sciences 21, 3474 (2020). 10.3390/ijms21103474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Devaux CA, Rolain J-M & Raoult D ACE2 receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. Journal of microbiology, immunology and infection = Wei mian yu gan ran za zhi. 53, 425–435 (2020). 10.1016/j.jmii.2020.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Klein SL, Marriott I & Fish EN Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg 109, 9–15 (2015). 10.1093/trstmh/tru167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fink AL, Engle K, Ursin RL, Tang WY & Klein SL Biological sex affects vaccine efficacy and protection against influenza in mice. Proc Natl Acad Sci U S A 115, 12477–12482 (2018). 10.1073/pnas.1805268115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Channappanavar R. et al. Sex-Based Differences in Susceptibility to Severe Acute Respiratory Syndrome Coronavirus Infection. The Journal of Immunology 198, 4046 (2017). 10.4049/jimmunol.1601896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stelzig KE et al. Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells. American Journal of Physiology-Lung Cellular and Molecular Physiology 318, L1280–L1281 (2020). 10.1152/ajplung.00153.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sierra I. et al. Unusual X chromosome inactivation maintenance in female alveolar type 2 cells is correlated with increased numbers of X-linked escape genes and sex-biased gene expression. Stem Cell Reports 18, 489–502 (2023). 10.1016/j.stemcr.2022.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oghumu S. et al. Cutting Edge: CXCR3 Escapes X Chromosome Inactivation in T Cells during Infection: Potential Implications for Sex Differences in Immune Responses. J Immunol 203, 789–794 (2019). 10.4049/jimmunol.1800931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Soulat D. et al. The DEAD-box helicase DDX3X is a critical component of the TANK-binding kinase 1-dependent innate immune response. EMBO J 27, 2135–2146 (2008). 10.1038/emboj.2008.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Szappanos D. et al. The RNA helicase DDX3X is an essential mediator of innate antimicrobial immunity. PLoS Pathog 14, e1007397 (2018). 10.1371/journal.ppat.1007397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bustamante J. et al. Germline CYBB mutations that selectively affect macrophages in kindreds with X-linked predisposition to tuberculous mycobacterial disease. Nat Immunol 12, 213–221 (2011). 10.1038/ni.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Filipe-Santos O. et al. Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Semin Immunol 18, 347–361 (2006). 10.1016/j.smim.2006.07.010 [DOI] [PubMed] [Google Scholar]
- 118.Asano T. et al. X-linked recessive TLR7 deficiency in ~1% of men under 60 years old with life-threatening COVID-19. Sci Immunol 6 (2021). 10.1126/sciimmunol.abl4348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Onodi F. et al. SARS-CoV-2 induces human plasmacytoid predendritic cell diversification via UNC93B and IRAK4. J Exp Med 218 (2021). 10.1084/jem.20201387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fallerini C. et al. Association of Toll-like receptor 7 variants with life-threatening COVID-19 disease in males: findings from a nested case-control study. Elife 10 (2021). 10.7554/eLife.67569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Solanich X. et al. Genetic Screening for TLR7 Variants in Young and Previously Healthy Men With Severe COVID-19. Front Immunol 12, 719115 (2021). 10.3389/fimmu.2021.719115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van der Made CI et al. Presence of Genetic Variants Among Young Men With Severe COVID-19. JAMA 324, 663–673 (2020). 10.1001/jama.2020.13719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Green MS et al. Gender Differences in Adverse Events Following the Pfizer-BioNTech COVID-19 Vaccine. Vaccines (Basel) 10 (2022). 10.3390/vaccines10020233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tsilingiris D, Vallianou NG, Karampela I & Dalamaga M Vaccine induced thrombotic thrombocytopenia: The shady chapter of a success story. Metabol Open 11, 100101 (2021). 10.1016/j.metop.2021.100101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ling RR et al. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: a systematic review and meta-analysis. Lancet Respir Med 10, 679–688 (2022). 10.1016/S2213-2600(22)00059-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Beery AK & Zucker I Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev 35, 565–572 (2011). 10.1016/j.neubiorev.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guan X. et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature 606, 791–796 (2022). 10.1038/s41586-022-04522-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brown MA & Su MA An Inconvenient Variable: Sex Hormones and Their Impact on T Cell Responses. J Immunol 202, 1927–1933 (2019). 10.4049/jimmunol.1801403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Singh RP, Hahn BH & Bischoff DS Interferon Genes Are Influenced by 17beta-Estradiol in SLE. Front Immunol 12, 725325 (2021). 10.3389/fimmu.2021.725325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Straub RH The complex role of estrogens in inflammation. Endocr Rev 28, 521–574 (2007). 10.1210/er.2007-0001 [DOI] [PubMed] [Google Scholar]
- 131.Mohammad I. et al. Estrogen receptor alpha contributes to T cell-mediated autoimmune inflammation by promoting T cell activation and proliferation. Sci Signal 11 (2018). 10.1126/scisignal.aap9415 [DOI] [PubMed] [Google Scholar]
- 132.Kim DH et al. Estrogen receptor alpha in T cells suppresses follicular helper T cell responses and prevents autoimmunity. Exp Mol Med 51, 1–9 (2019). 10.1038/s12276-019-0237-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Goodman WA et al. Impaired estrogen signaling underlies regulatory T cell loss-of-function in the chronically inflamed intestine. Proc Natl Acad Sci U S A 117, 17166–17176 (2020). 10.1073/pnas.2002266117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Aggelakopoulou M, Kourepini E, Paschalidis N & Panoutsakopoulou V ERbeta in CD4+ T Cells Is Crucial for Ligand-Mediated Suppression of Central Nervous System Autoimmunity. J Immunol 196, 4947–4956 (2016). 10.4049/jimmunol.1600246 [DOI] [PubMed] [Google Scholar]
- 135.Garnier L. et al. Estrogen Signaling in Bystander Foxp3(neg) CD4(+) T Cells Suppresses Cognate Th17 Differentiation in Trans and Protects from Central Nervous System Autoimmunity. J Immunol 201, 3218–3228 (2018). 10.4049/jimmunol.1800417 [DOI] [PubMed] [Google Scholar]
- 136.Xiong Y. et al. Estradiol resolves pneumonia via ERbeta in regulatory T cells. JCI Insight 6 (2021). 10.1172/jci.insight.133251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tabor DE & Gould KA Estrogen receptor alpha promotes lupus in (NZBxNZW)F1 mice in a B cell intrinsic manner. Clin Immunol 174, 41–52 (2017). 10.1016/j.clim.2016.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Carlsten H. et al. Estrogen accelerates immune complex glomerulonephritis but ameliorates T cell-mediated vasculitis and sialadenitis in autoimmune MRL lpr/lpr mice. Cell Immunol 144, 190–202 (1992). 10.1016/0008-8749(92)90236-i [DOI] [PubMed] [Google Scholar]
- 139.Herrmann M, Scholmerich J & Straub RH Influence of cytokines and growth factors on distinct steroidogenic enzymes in vitro: a short tabular data collection. Ann N Y Acad Sci 966, 166–186 (2002). 10.1111/j.1749-6632.2002.tb04213.x [DOI] [PubMed] [Google Scholar]
- 140.de Man YA, Dolhain RJ, van de Geijn FE, Willemsen SP & Hazes JM Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis Rheum 59, 1241–1248 (2008). 10.1002/art.24003 [DOI] [PubMed] [Google Scholar]
- 141.Baer AN, Witter FR & Petri M Lupus and pregnancy. Obstet Gynecol Surv 66, 639–653 (2011). 10.1097/OGX.0b013e318239e1ee [DOI] [PubMed] [Google Scholar]
- 142.Cutolo M & Straub RH Sex steroids and autoimmune rheumatic diseases: state of the art. Nat Rev Rheumatol 16, 628–644 (2020). 10.1038/s41584-020-0503-4 [DOI] [PubMed] [Google Scholar]
- 143.Gubbels Bupp MR & Jorgensen TN Androgen-Induced Immunosuppression. Front Immunol 9, 794 (2018). 10.3389/fimmu.2018.00794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Traish A, Bolanos J, Nair S, Saad F & Morgentaler A Do Androgens Modulate the Pathophysiological Pathways of Inflammation? Appraising the Contemporary Evidence. J Clin Med 7 (2018). 10.3390/jcm7120549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lyon MF Gene Action in the X-chromosome of the Mouse (Mus musculus L.). Nature 190, 372–373 (1961). 10.1038/190372a0 [DOI] [PubMed] [Google Scholar]
- 146.Brown CJ et al. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature 349, 38–44 (1991). 10.1038/349038a0 [DOI] [PubMed] [Google Scholar]
- 147.Brown CJ et al. The human XIST gene: Analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell 71, 527–542 (1992). 10.1016/0092-8674(92)90520-M [DOI] [PubMed] [Google Scholar]
- 148.Colognori D, Sunwoo H, Kriz AJ, Wang CY & Lee JT Xist Deletional Analysis Reveals an Interdependency between Xist RNA and Polycomb Complexes for Spreading along the Inactive X. Molecular Cell 74, 101–117.e110 (2019). 10.1016/j.molcel.2019.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Simon MD et al. High-resolution Xist binding maps reveal two-step spreading during X-chromosome inactivation. Nature 504, 465–469 (2013). 10.1038/nature12719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Żylicz JJ et al. The Implication of Early Chromatin Changes in X Chromosome Inactivation. Cell 176, 182–197.e123 (2019). 10.1016/j.cell.2018.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Engreitz JM et al. The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science 341, 1–9 (2013). 10.1126/science.1237973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Colognori D, Sunwoo H, Kriz AJ, Wang CY & Lee JT in Molecular Cell Vol. 74 101–117.e110 (Elsevier Inc., 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Żylicz JJ et al. in Cell Vol. 176 182–197.e123 (2019).30595450 [Google Scholar]
- 154.Simon MD et al. in Nature Vol. 504 465–469 (Nature Publishing Group, 2013).24162848 [Google Scholar]
- 155.Costanzi C & Pehrson JR Histone macroH2A1 is concentrated in the inactive X chromosome of female mammals. Nature 393, 599–601 (1998). 10.1038/31275 [DOI] [PubMed] [Google Scholar]
- 156.Gendrel A-V et al. Smchd1-Dependent and -Independent Pathways Determine Developmental Dynamics of CpG Island Methylation on the Inactive X Chromosome. Developmental Cell 23, 265–279 (2012). 10.1016/j.devcel.2012.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lock LF, Takagi N & Martin GR Methylation of the Hprt gene on the inactive X occurs after chromosome inactivation. Cell 48, 39–46 (1987). 10.1016/0092-8674(87)90353-9 [DOI] [PubMed] [Google Scholar]
- 158.Norris DP, Brockdorff N & Rastan S Methylation status of CpG-rich islands on active and inactive mouse X chromosomes. Mammalian Genome 1, 78–83 (1991). 10.1007/BF02443782 [DOI] [PubMed] [Google Scholar]
- 159.Sharp AJ et al. DNA methylation profiles of human active and inactive X chromosomes. Genome Research 21, 1592–1600 (2011). 10.1101/gr.112680.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cotton AM et al. Landscape of DNA methylation on the X chromosome reflects CpG density, functional chromatin state and X-chromosome inactivation. Human Molecular Genetics 24, 1528–1539 (2015). 10.1093/hmg/ddu564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Carrel L & Willard HF X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 434, 400–404 (2005). 10.1038/nature03479 [DOI] [PubMed] [Google Scholar]
- 162.Tukiainen T. et al. Landscape of X chromosome inactivation across human tissues. Nature 550, 244–248 (2017). 10.1038/nature24265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Berletch JB et al. Escape from X Inactivation Varies in Mouse Tissues. PLoS Genetics 11, 1–26 (2015). 10.1371/journal.pgen.1005079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yang F, Babak T, Shendure J & Disteche CM Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Research 20, 614–622 (2010). 10.1101/gr.103200.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Berletch JB, Yang F, Xu J, Carrel L & Disteche CM Genes that escape from X inactivation. Hum Genet 130, 237–245 (2011). 10.1007/s00439-011-1011-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Aguet F et al. The impact of sex on gene expression across human tissues. Science 369 (2020). 10.1126/SCIENCE.ABA3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Souyris M. et al. TLR7 escapes X chromosome inactivation in immune cells. Science Immunology 3, 1–11 (2018). 10.1126/sciimmunol.aap8855 [DOI] [PubMed] [Google Scholar]
- 168.Zhang Y. et al. Genes that escape X-inactivation in humans have high intraspecific variability in expression, are associated with mental impairment but are not slow evolving. Mol Biol Evol 30, 2588–2601 (2013). 10.1093/molbev/mst148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Meester I. et al. SeXY chromosomes and the immune system: Reflections after a comparative study. Biology of Sex Differences 11, 1–13 (2020). 10.1186/s13293-019-0278-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Berletch JB et al. Escape from X inactivation varies in mouse tissues. PLoS Genet 11, e1005079 (2015). 10.1371/journal.pgen.1005079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Berletch JB et al. Identification of genes escaping X inactivation by allelic expression analysis in a novel hybrid mouse model. Data Brief 5, 761–769 (2015). 10.1016/j.dib.2015.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Andrews BS et al. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med 148, 1198–1215 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Subramanian S. et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A 103, 9970–9975 (2006). 10.1073/pnas.0603912103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fairhurst AM et al. Yaa autoimmune phenotypes are conferred by overexpression of TLR7. Eur J Immunol 38, 1971–1978 (2008). 10.1002/eji.200838138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Haywood ME et al. Identification of intervals on chromosomes 1, 3, and 13 linked to the development of lupus in BXSB mice. Arthritis Rheum 43, 349–355 (2000). [DOI] [PubMed] [Google Scholar]
- 176.Burnet FM & Holmes MC The natural history of the NZB/NZW F1 hybrid mouse: a laboratory model of systemic lupus erythematosus. Australas Ann Med 14, 185–191 (1965). 10.1111/imj.1965.14.3.185 [DOI] [PubMed] [Google Scholar]
- 177.Helyer BJ & Howie JB Renal disease associated with positive lupus erythematosus tests in a cross-bred strain of mice. Nature 197, 197 (1963). 10.1038/197197a0 [DOI] [PubMed] [Google Scholar]
- 178.Kessler HS A laboratory model for Sjogren’s syndrome. Am J Pathol 52, 671–685 (1968). [PMC free article] [PubMed] [Google Scholar]
- 179.Roubinian JR, Talal N, Greenspan JS, Goodman JR & Siiteri PK Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J Exp Med 147, 1568–1583 (1978). 10.1084/jem.147.6.1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Verheul HA, Verveld M, Hoefakker S & Schuurs AH Effects of ethinylestradiol on the course of spontaneous autoimmune disease in NZB/W and NOD mice. Immunopharmacol Immunotoxicol 17, 163–180 (1995). 10.3109/08923979509052727 [DOI] [PubMed] [Google Scholar]
- 181.Waters ST et al. NZM2328: a new mouse model of systemic lupus erythematosus with unique genetic susceptibility loci. Clin Immunol 100, 372–383 (2001). 10.1006/clim.2001.5079 [DOI] [PubMed] [Google Scholar]
- 182.Reeves WH, Lee PY, Weinstein JS, Satoh M & Lu L Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol 30, 455–464 (2009). 10.1016/j.it.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Satoh M. et al. Widespread susceptibility among inbred mouse strains to the induction of lupus autoantibodies by pristane. Clin Exp Immunol 121, 399–405 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Du S. et al. XY sex chromosome complement, compared with XX, in the CNS confers greater neurodegeneration during experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A 111, 2806–2811 (2014). 10.1073/pnas.1307091111 [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Rees F, Doherty M, Grainge MJ, Lanyon P & Zhang W The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies. Rheumatology (Oxford) 56, 1945–1961, doi: 10.1093/rheumatology/kex260 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Ramos-Casals M. et al. Google-driven search for big data in autoimmune geoepidemiology: analysis of 394,827 patients with systemic autoimmune diseases. Autoimmun Rev 14, 670–679, doi: 10.1016/j.autrev.2015.03.008 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Maciel G, Crowson CS, Matteson EL & Cornec D Prevalence of Primary Sjogren's Syndrome in a US Population-Based Cohort. Arthritis Care Res (Hoboken) 69, 1612–1616, doi: 10.1002/acr.23173 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pillemer SR et al. Incidence of physician-diagnosed primary Sjogren syndrome in residents of Olmsted County, Minnesota. Mayo Clin Proc 76, 593–599, doi: 10.4065/76.6.593 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Mayes MD et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum 48, 2246–2255, doi: 10.1002/art.11073 (2003). [DOI] [PubMed] [Google Scholar]
- 6.Hughes M. et al. Gender-related differences in systemic sclerosis. Autoimmun Rev 19, 102494, doi: 10.1016/j.autrev.2020.102494 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Meyer A. et al. Incidence and prevalence of inflammatory myopathies: a systematic review. Rheumatology (Oxford) 54, 50–63, doi: 10.1093/rheumatology/keu289 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Gabriel SE, Crowson CS & O'Fallon WM The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955-1985. Arthritis Rheum 42, 415–420, doi: (1999). [DOI] [PubMed] [Google Scholar]
- 9.Lawrence RC et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum 41, 778–799, doi: (1998). [DOI] [PubMed] [Google Scholar]
References
- 1.Zhou Y. et al. T cell CD40LG gene expression and the production of IgG by autologous B cells in systemic lupus erythematosus. Clinical Immunology 132, 362–370 (2009). 10.1016/j.clim.2009.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balaton BP, Cotton AM & Brown CJ Derivation of consensus inactivation status for X-linked genes from genome-wide studies. Biol Sex Differ 6, 35 (2015). 10.1186/s13293-015-0053-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu Q. et al. in The Journal of Immunology Vol. 179 6352–6358 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Clegg CH et al. Thymus dysfunction and chronic inflammatory disease in gp39 transgenic mice. Int Immunol 9, 1111–1122 (1997). 10.1093/intimm/9.8.1111 [DOI] [PubMed] [Google Scholar]
- 5.Perez-Melgosa M, Hollenbaugh D & Wilson CB Cutting edge: CD40 ligand is a limiting factor in the humoral response to T cell-dependent antigens. J Immunol 163, 1123–1127 (1999). [PubMed] [Google Scholar]
- 6.Desai-Mehta A, Lu L, Ramsey-Goldman R & Datta SK Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. Journal of Clinical Investigation 97, 2063–2073 (1996). 10.1172/JCI118643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Coz C. et al. CD40LG duplication-associated autoimmune disease is silenced by nonrandom X-chromosome inactivation. Journal of Allergy and Clinical Immunology 141, 2308–2311.e2307 (2018). 10.1016/j.jaci.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groom JR & Luster AD CXCR3 in T cell function. Experimental Cell Research 317, 620–631 (2011). 10.1016/j.yexcr.2010.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flier J. et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol 194, 398–405 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Hewagama A. et al. Overexpression of X-Linked genes in T cells from women with lupus. Journal of Autoimmunity 41, 60–71 (2013). 10.1016/j.jaut.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oghumu S. et al. Cutting Edge: CXCR3 Escapes X Chromosome Inactivation in T Cells during Infection: Potential Implications for Sex Differences in Immune Responses. The Journal of Immunology 203, 789–794 (2019). 10.4049/jimmunol.1800931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eriksson C, Eneslätt K, Ivanoff J, Rantapää-Dahlqvist S & Sundqvist KG Abnormal expression of chemokine receptors on T-cells from patients with systemic lupus erythematosus. Lupus 12, 766–774 (2003). 10.1191/0961203303lu467oa [DOI] [PubMed] [Google Scholar]
- 13.Hewagama A. et al. Overexpression of X-linked genes in T cells from women with lupus. J Autoimmun 41, 60–71 (2013). 10.1016/j.jaut.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogawa N, Ping L, Zhenjun L, Takada Y & Sugai S Involvement of the interferon-gamma-induced T cell-attracting chemokines, interferon-gamma-inducible 10-kd protein (CXCL10) and monokine induced by interferon-gamma (CXCL9), in the salivary gland lesions of patients with Sjogren's syndrome. Arthritis Rheum 46, 2730–2741 (2002). 10.1002/art.10577 [DOI] [PubMed] [Google Scholar]
- 15.Rankin AL et al. Selective inhibition of BTK prevents murine lupus and antibody-mediated glomerulonephritis. J Immunol 191, 4540–4550 (2013). 10.4049/jimmunol.1301553 [DOI] [PubMed] [Google Scholar]
- 16.Katewa A. et al. Btk-specific inhibition blocks pathogenic plasma cell signatures and myeloid cell-associated damage in IFNalpha-driven lupus nephritis. JCI Insight 2, e90111 (2017). 10.1172/jci.insight.90111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kil LP et al. Btk levels set the threshold for B-cell activation and negative selection of autoreactive B cells in mice. Blood 119, 3744–3756 (2012). 10.1182/blood-2011-12-397919 [DOI] [PubMed] [Google Scholar]
- 18.Cornacchia E. et al. Hydralazine and procainamide inhibit T cell DNA methylation and induce autoreactivity. J Immunol 140, 2197–2200 (1988). [PubMed] [Google Scholar]
- 19.Kong W. et al. Increased expression of Bruton's tyrosine kinase in peripheral blood is associated with lupus nephritis. Clin Rheumatol 37, 43–49 (2018). 10.1007/s10067-017-3717-3 [DOI] [PubMed] [Google Scholar]
- 20.Yang C, Huang XR, Fung E, Liu HF & Lan HY The Regulatory T-cell Transcription Factor Foxp3 Protects against Crescentic Glomerulonephritis. Sci Rep 7, 1481 (2017). 10.1038/s41598-017-01515-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonelli M, von Dalwigk K, Savitskaya A, Smolen JS & Scheinecker C Foxp3 expression in CD4+ T cells of patients with systemic lupus erythematosus: a comparative phenotypic analysis. Ann Rheum Dis 67, 664–671 (2008). 10.1136/ard.2007.074690 [DOI] [PubMed] [Google Scholar]
- 22.Valencia X & Lipsky PE CD4+CD25+FoxP3+ regulatory T cells in autoimmune diseases. Nat Clin Pract Rheumatol 3, 619–626 (2007). 10.1038/ncprheum0624 [DOI] [PubMed] [Google Scholar]
- 23.Wang YY et al. DNA hypermethylation of the forkhead box protein 3 (FOXP3) promoter in CD4+ T cells of patients with systemic sclerosis. Br J Dermatol 171, 39–47 (2014). 10.1111/bjd.12913 [DOI] [PubMed] [Google Scholar]
- 24.Golks A, Tran TT, Goetschy JF & Guerini D Requirement for O-linked N-acetylglucosaminyltransferase in lymphocytes activation. EMBO J 26, 4368–4379 (2007). 10.1038/sj.emboj.7601845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu JL et al. O-GlcNAcylation is required for B cell homeostasis and antibody responses. Nat Commun 8, 1854 (2017). 10.1038/s41467-017-01677-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odhams CA et al. Interferon inducible X-linked gene CXorf21 may contribute to sexual dimorphism in Systemic Lupus Erythematosus. Nature Communications 10, 1–15 (2019). 10.1038/s41467-019-10106-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinz LX et al. TASL is the SLC15A4-associated adaptor for IRF5 activation by TLR7–9. Nature 581, 316–322 (2020). 10.1038/s41586-020-2282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bentham J. et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nature Genetics 47, 1457–1464 (2015). 10.1038/ng.3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heil F. et al. Species-Specific Recognition of Single-Stranded RNA via Till-like Receptor 7 and 8. Science 303, 1526–1529 (2004). 10.1126/science.1093620 [DOI] [PubMed] [Google Scholar]
- 30.Diebold SS, Kaisho T, Hemmi H, Akira S & Reis E Sousa C Innate Antiviral Responses by Means of TLR7-Mediated Recognition of Single-Stranded RNA. Science 303, 1529–1531 (2004). 10.1126/science.1093616 [DOI] [PubMed] [Google Scholar]
- 31.Souyris M. et al. TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol 3 (2018). 10.1126/sciimmunol.aap8855 [DOI] [PubMed] [Google Scholar]
- 32.Wang J. et al. Unusual maintenance of X chromosome inactivation predisposes female lymphocytes for increased expression from the inactive X. Proc Natl Acad Sci U S A (2016). 10.1073/pnas.1520113113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbas F. et al. HIV-1 infection enhances innate function and TLR7 expression in female plasmacytoid dendritic cells. Life Sci Alliance 5 (2022). 10.26508/lsa.202201452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hagen SH et al. Heterogeneous Escape from X Chromosome Inactivation Results in Sex Differences in Type I IFN Responses at the Single Human pDC Level. Cell Rep 33, 108485 (2020). 10.1016/j.celrep.2020.108485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Syrett CM et al. Diversity of Epigenetic Features of the Inactive X-Chromosome in NK Cells, Dendritic Cells, and Macrophages. Front Immunol 9, 3087 (2018). 10.3389/fimmu.2018.03087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deane JA et al. Control of Toll-like Receptor 7 Expression Is Essential to Restrict Autoimmunity and Dendritic Cell Proliferation. Immunity 27, 801–810 (2007). 10.1016/j.immuni.2007.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pisitkun P. et al. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science 312, 1669–1672 (2006). 10.1126/science.1124978 [DOI] [PubMed] [Google Scholar]
- 38.Deane JA et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity 27, 801–810 (2007). 10.1016/j.immuni.2007.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown GJ et al. TLR7 gain-of-function genetic variation causes human lupus. (2022). 10.1038/s41586-022-04642-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia-Ortiz H. et al. Association of TLR7 copy number variation with susceptibility to childhood-onset systemic lupus erythematosus in Mexican population. Annals of the Rheumatic Diseases 69, 1861–1865 (2010). 10.1136/ard.2009.124313 [DOI] [PubMed] [Google Scholar]
- 41.Brown GJ et al. TLR7 gain-of-function genetic variation causes human lupus. Nature 605, 349–356 (2022). 10.1038/s41586-022-04642-z [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Alwani M. et al. Sex-based differences in severity and mortality in COVID-19. Reviews in Medical Virology 31 (2021). 10.1002/rmv.2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haitao T. et al. COVID-19 and Sex Differences. Mayo Clinic Proceedings 95, 2189–2203 (2020). 10.1016/j.mayocp.2020.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin S et al. Sex- and age-specific clinical and immunological features of coronavirus disease 2019. PLOS Pathogens 17, e1009420 (2021). 10.1371/journal.ppat.1009420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gemmati D. et al. COVID-19 and Individual Genetic Susceptibility/Receptivity: Role of ACE1/ACE2 Genes, Immunity, Inflammation and Coagulation. Might the Double X-Chromosome in Females Be Protective against SARS-CoV-2 Compared to the Single X-Chromosome in Males? International Journal of Molecular Sciences 21, 3474 (2020). 10.3390/ijms21103474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R & Klein SL Impact of sex and gender on COVID-19 outcomes in Europe. Biology of Sex Differences 11, 29 (2020). 10.1186/s13293-020-00304-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scully EP, Haverfield J, Ursin RL, Tannenbaum C & Klein SL Considering how biological sex impacts immune responses and COVID-19 outcomes. Nature Reviews Immunology 20, 442–447 (2020). 10.1038/s41577-020-0348-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi T. et al. Sex differences in immune responses to SARS-CoV-2 that underlie disease outcomes. medRxiv, 2020.2006.2006.20123414 (2020). 10.1101/2020.06.06.20123414 [DOI] [Google Scholar]
- 8.Silva MVR et al. Men are the main COVID-19 transmitters: behavior or biology? Discover Mental Health 2 (2022). 10.1007/s44192-022-00004-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Driscoll M. et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 590, 140–145 (2021). 10.1038/s41586-020-2918-0 [DOI] [PubMed] [Google Scholar]
- 10.Harwood R. et al. Which children and young people are at higher risk of severe disease and death after hospitalisation with SARS-CoV-2 infection in children and young people: A systematic review and individual patient meta-analysis. eClinicalMedicine 44, 101287 (2022). 10.1016/j.eclinm.2022.101287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sylvester SV et al. Sex differences in sequelae from COVID-19 infection and in long COVID syndrome: a review. Current Medical Research and Opinion, 1–9 (2022). 10.1080/03007995.2022.2081454 [DOI] [PubMed] [Google Scholar]
- 12.Taquet M. et al. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med 18, e1003773 (2021). 10.1371/journal.pmed.1003773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Channappanavar R. et al. Sex-Based Differences in Susceptibility to Severe Acute Respiratory Syndrome Coronavirus Infection. The Journal of Immunology 198, 4046 (2017). 10.4049/jimmunol.1601896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sohn S-Y et al. Interferon-Lambda Intranasal Protection and Differential Sex Pathology in a Murine Model of SARS-CoV-2 Infection. mBio 12 (2021). 10.1128/mbio.02756-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis ME et al. Sex and age bias viral burden and interferon responses during SARS-CoV-2 infection in ferrets. Scientific Reports 11 (2021). 10.1038/s41598-021-93855-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stelzig KE et al. Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells. American Journal of Physiology-Lung Cellular and Molecular Physiology 318, L1280–L1281 (2020). 10.1152/ajplung.00153.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devaux CA, Rolain J-M & Raoult D ACE2 receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. Journal of microbiology, immunology and infection = Wei mian yu gan ran za zhi. 53, 425–435 (2020). 10.1016/j.jmii.2020.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein SL, Pekosz A, Passaretti C, Anker M & Olukoya P. (World Health Organization, 2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noymer A & Garenne M The 1918 influenza epidemic's effects on sex differentials in mortality in the United States. Popul Dev Rev 26, 565–581 (2000). 10.1111/j.1728-4457.2000.00565.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eshima N. et al. Sex- and age-related differences in morbidity rates of 2009 pandemic influenza A H1N1 virus of swine origin in Japan. PLoS One 6, e19409 (2011). 10.1371/journal.pone.0019409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs JH et al. Searching for sharp drops in the incidence of pandemic A/H1N1 influenza by single year of age. PLoS One 7, e42328 (2012). 10.1371/journal.pone.0042328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen-Fangel S. et al. Gender differences in hospitalization rates for respiratory tract infections in Danish youth. Scand J Infect Dis 36, 31–36 (2004). 10.1080/00365540310017618 [DOI] [PubMed] [Google Scholar]
- 23.Vom Steeg LG & Klein SL Sex and sex steroids impact influenza pathogenesis across the life course. Semin Immunopathol 41, 189–194 (2019). 10.1007/s00281-018-0718-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cervantes O. et al. Role of hormones in the pregnancy and sex-specific outcomes to infections with respiratory viruses*. Immunological Reviews 308, 123–148 (2022). 10.1111/imr.13078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herold S, Becker C, Ridge KM & Budinger GR Influenza virus-induced lung injury: pathogenesis and implications for treatment. Eur Respir J 45, 1463–1478 (2015). 10.1183/09031936.00186214 [DOI] [PubMed] [Google Scholar]
- 26.Kumar A. et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 302, 1872–1879 (2009). 10.1001/jama.2009.1496 [DOI] [PubMed] [Google Scholar]
- 27.Vom Steeg LG et al. Age and testosterone mediate influenza pathogenesis in male mice. Am J Physiol Lung Cell Mol Physiol 311, L1234–L1244 (2016). 10.1152/ajplung.00352.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geurs TL, Hill EB, Lippold DM & French AR Sex differences in murine susceptibility to systemic viral infections. J Autoimmun 38, J245–253 (2012). 10.1016/j.jaut.2011.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fink AL, Engle K, Ursin RL, Tang W-Y & Klein SL Biological sex affects vaccine efficacy and protection against influenza in mice. Proceedings of the National Academy of Sciences 115, 12477 (2018). 10.1073/pnas.1805268115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorenzo ME et al. Antibody responses and cross protection against lethal influenza A viruses differ between the sexes in C57BL/6 mice. Vaccine. 29, 9246–9255 (2011). 10.1016/j.vaccine.2011.09.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vermillion MS et al. Production of amphiregulin and recovery from influenza is greater in males than females. Biol Sex Differ 9, 24 (2018). 10.1186/s13293-018-0184-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.vom Steeg LG, Attreed SE, Zirkin B & Klein SL Testosterone treatment of aged male mice improves some but not all aspects of age-associated increases in influenza severity. Cellular Immunology 345, 103988 (2019). https://doi.org: 10.1016/j.cellimm.2019.103988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vom Steeg LG et al. Androgen receptor signaling in the lungs mitigates inflammation and improves the outcome of influenza in mice. PLoS Pathog 16, e1008506 (2020). 10.1371/journal.ppat.1008506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vermillion MS, Ursin RL, Attreed SE & Klein SL Estriol Reduces Pulmonary Immune Cell Recruitment and Inflammation to Protect Female Mice From Severe Influenza. Endocrinology 159, 3306–3320 (2018). 10.1210/en.2018-00486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peretz J, Pekosz A, Lane AP & Klein SL Estrogenic compounds reduce influenza A virus replication in primary human nasal epithelial cells derived from female, but not male, donors. American Journal of Physiology-Lung Cellular and Molecular Physiology 310, L415–L425 (2015). 10.1152/ajplung.00398.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen CM, Chen SCC, Yang HY, Yang ST & Wang CM Hospitalization and mortality due to hepatitis A in Taiwan: a 15-year nationwide cohort study. Journal of Viral Hepatitis 23, 940–945 (2016). 10.1111/jvh.12564 [DOI] [PubMed] [Google Scholar]
- 37.Baden R, Rockstroh JK & Buti M Natural history and management of hepatitis C: does sex play a role? J Infect Dis 209 Suppl 3, S81–85 (2014). 10.1093/infdis/jiu057 [DOI] [PubMed] [Google Scholar]
- 38.Jean CM, Honarmand S, Louie JK & Glaser CA Risk factors for West Nile virus neuroinvasive disease, California, 2005. Emerg Infect Dis 13, 1918–1920 (2007). 10.3201/eid1312.061265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hegdahl HK, Fylkesnes KM, Sandøy IF & Faragher EB Sex Differences in HIV Prevalence Persist over Time: Evidence from 18 Countries in Sub-Saharan Africa. PloS one. 11, e0148502 (2016). 10.1371/journal.pone.0148502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griesbeck M, Scully E & Altfeld M Sex and gender differences in HIV-1 infection. Clinical science. 130, 1435–1451 (2016). 10.1042/cs20160112 [DOI] [PubMed] [Google Scholar]
- 41.Meier A. et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med 15, 955–959 (2009). 10.1038/nm.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicolosi A. et al. The Efficiency of Male-to Female and Female-to-Male Sexual Transmission of the Human Immunodeficiency Virus. Epidemiology. 5, 570–575 (1994). 10.1097/00001648-199411000-00003 [DOI] [PubMed] [Google Scholar]
- 43.Cobelens F, Nagelkerke N & Fletcher H The convergent epidemiology of tuberculosis and human cytomegalovirus infection. F1000Research 7, 280 (2018). 10.12688/f1000research.14184.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yousuf W, Ibrahim H, Harfouche M, Abu Hijleh F & Abu-Raddad L Herpes simplex virus type 1 in Europe: systematic review, meta-analyses and meta-regressions. BMJ global health. 5, e002388 (2020). 10.1136/bmjgh-2020-002388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McQuillan G, Kruszon-Moran D, Flagg EW & Paulose-Ram R Prevalence of Herpes Simplex Virus Type 1 and Type 2 in Persons Aged 14-49: United States, 2015-2016. NCHS Data Brief, 1–8 (2018). [PubMed] [Google Scholar]
- 46.Han X. et al. Gender influences herpes simplex virus type 1 infection in normal and gamma interferon-mutant mice. J Virol 75, 3048–3052 (2001). 10.1128/JVI.75.6.3048-3052.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huber SA & Pfaeffle B Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J Virol 68, 5126–5132 (1994). 10.1128/JVI.68.8.5126-5132.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.WHO Ebola Response Team, J. A-A, , Archchun Ariyarajah, Isobel M. Blake, Anne Cori, Christl A. Donnelly, Ilaria Dorigatti, Christopher Dye, Tim Eckmanns, Neil M. Ferguson, Christophe Fraser, Tini Garske, Wes Hinsley, Thibaut Jombart, Harriet L. Mills, Gemma Nedjati-Gilani, Emily Newton, Pierre Nouvellet, Devin Perkins, Steven Riley, Dirk Schumacher, Anita Shah, Lisa J. Thomas, Maria D. Van Kerkhove. Ebola Virus Disease among Male and Female Persons in West Africa. New England Journal of Medicine 374, 96–98 (2016). 10.1056/nejmc1510305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garenne M. Sex Differences in Measles Mortality: A World Review. International journal of epidemiology. 23, 632–642 (1994). 10.1093/ije/23.3.632 [DOI] [PubMed] [Google Scholar]
- 50.Green MS, Schwartz N & Peer V Gender differences in measles incidence rates in a multi-year, pooled analysis, based on national data from seven high income countries. BMC Infectious Diseases 22 (2022). 10.1186/s12879-022-07340-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schuurhof A. et al. Interleukin-9 polymorphism in infants with respiratory syncytial virus infection: An opposite effect in boys and girls. Pediatric pulmonology. 45, 608–613 (2010). 10.1002/ppul.21229 [DOI] [PubMed] [Google Scholar]
- 52.Pisesky A. et al. Incidence of Hospitalization for Respiratory Syncytial Virus Infection amongst Children in Ontario, Canada: A Population-Based Study Using Validated Health Administrative Data. PLOS ONE 11, e0150416 (2016). 10.1371/journal.pone.0150416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kenmoe S. et al. Prevalence of human respiratory syncytial virus infection in people with acute respiratory tract infections in Africa: A systematic review and meta-analysis. Influenza and Other Respiratory Viruses 12, 793–803 (2018). 10.1111/irv.12584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malinczak CA et al. Sex-associated TSLP-induced immune alterations following early-life RSV infection leads to enhanced allergic disease. Mucosal Immunol 12, 969–979 (2019). 10.1038/s41385-019-0171-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferro A. et al. Sex differences in the prevalence of Helicobacter pylori infection: an individual participant data pooled analysis (StoP Project). Eur J Gastroenterol Hepatol 31, 593–598 (2019). 10.1097/MEG.0000000000001389 [DOI] [PubMed] [Google Scholar]
- 56.Murray LJ, McCrum EE, Evans AE & Bamford KB Epidemiology of Helicobacter pylori infection among 4742 randomly selected subjects from Northern Ireland. Int J Epidemiol 26, 880–887 (1997). 10.1093/ije/26.4.880 [DOI] [PubMed] [Google Scholar]
- 57.Vazquez-Martinez ER, Garcia-Gomez E, Camacho-Arroyo I & Gonzalez-Pedrajo B Sexual dimorphism in bacterial infections. Biol Sex Differ 9, 27 (2018). 10.1186/s13293-018-0187-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohtani M. et al. 17 beta-estradiol suppresses Helicobacter pylori-induced gastric pathology in male hypergastrinemic INS-GAS mice. Carcinogenesis 32, 1244–1250 (2011). 10.1093/carcin/bgr072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.FitzSimmons SC The changing epidemiology of cystic fibrosis. J Pediatr 122, 1–9 (1993). 10.1016/s0022-3476(05)83478-x [DOI] [PubMed] [Google Scholar]
- 60.Guilbault C. et al. Influence of gender and interleukin-10 deficiency on the inflammatory response during lung infection with Pseudomonas aeruginosa in mice. Immunology 107, 297–305 (2002). 10.1046/j.1365-2567.2002.01508.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peer V, Schwartz N & Green MS Sex Differences in Salmonellosis Incidence Rates-An Eight-Country National Data-Pooled Analysis. J Clin Med 10 (2021). 10.3390/jcm10245767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blasi F, Tarsia P, Arosio C, Fagetti L & Allegra L Epidemiology of Chlamydia pneumoniae. Clin Microbiol Infect 4 Suppl 4, S1–S6 (1998). [PubMed] [Google Scholar]
- 63.Matteelli A. et al. Prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae infection in adolescents in Northern Italy: an observational school-based study. BMC Public Health 16, 200 (2016). 10.1186/s12889-016-2839-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dias SP, Brouwer MC & van de Beek D Sex and Gender Differences in Bacterial Infections. Infect Immun 90, e0028322 (2022). 10.1128/iai.00283-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dielissen PW, Teunissen DA & Lagro-Janssen AL Chlamydia prevalence in the general population: is there a sex difference? a systematic review. BMC Infect Dis 13, 534 (2013). 10.1186/1471-2334-13-534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hannu T. Reactive arthritis. Best Pract Res Clin Rheumatol 25, 347–357 (2011). 10.1016/j.berh.2011.01.018 [DOI] [PubMed] [Google Scholar]
- 67.Zheng R. et al. A Systematic Review and Meta-Analysis of Epidemiology and Clinical Manifestations of Human Brucellosis in China. Biomed Res Int 2018, 5712920 (2018). 10.1155/2018/5712920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alkahtani AM, Assiry MM, Chandramoorthy HC, Al-Hakami AM & Hamid ME Sero-prevalence and risk factors of brucellosis among suspected febrile patients attending a referral hospital in southern Saudi Arabia (2014-2018). BMC Infect Dis 20, 26 (2020). 10.1186/s12879-020-4763-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Orloski KA, Hayes EB, Campbell GL & Dennis DT Surveillance for Lyme disease--United States, 1992-1998. MMWR CDC Surveill Summ 49, 1–11 (2000). [PubMed] [Google Scholar]
- 70.Jarefors S. et al. Lyme borreliosis reinfection: might it be explained by a gender difference in immune response? Immunology 118, 224–232 (2006). 10.1111/j.1365-2567.2006.02360.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berglund J. et al. An epidemiologic study of Lyme disease in southern Sweden. N Engl J Med 333, 1319–1327 (1995). 10.1056/NEJM199511163332004 [DOI] [PubMed] [Google Scholar]
- 72.Schwartz AM et al. Epidemiology and cost of Lyme disease-related hospitalizations among patients with employer-sponsored health insurance-United States, 2005-2014. Zoonoses Public Health 67, 407–415 (2020). 10.1111/zph.12699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kwit NA, Nelson CA, Max R & Mead PS Risk Factors for Clinician-Diagnosed Lyme Arthritis, Facial Palsy, Carditis, and Meningitis in Patients From High-Incidence States. Open Forum Infect Dis 5, ofx254 (2018). 10.1093/ofid/ofx254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zinck CB et al. Borrelia burgdorferi strain and host sex influence pathogen prevalence and abundance in the tissues of a laboratory rodent host. Mol Ecol 31, 5872–5888 (2022). 10.1111/mec.16694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hertz D & Schneider B Sex differences in tuberculosis. Semin Immunopathol 41, 225–237 (2019). 10.1007/s00281-018-0725-6 [DOI] [PubMed] [Google Scholar]
- 76.Tan W. et al. Sex influences the association between haemostasis and the extent of lung lesions in tuberculosis. Biol Sex Differ 9, 44 (2018). 10.1186/s13293-018-0203-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miele K, Bamrah Morris S & Tepper NK Tuberculosis in Pregnancy. Obstet Gynecol 135, 1444–1453 (2020). 10.1097/AOG.0000000000003890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dibbern J, Eggers L & Schneider BE Sex differences in the C57BL/6 model of Mycobacterium tuberculosis infection. Sci Rep 7, 10957 (2017). 10.1038/s41598-017-11438-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yancey AL, Watson HL, Cartner SC & Simecka JW Gender is a major factor in determining the severity of mycoplasma respiratory disease in mice. Infect Immun 69, 2865–2871 (2001). 10.1128/IAI.69.5.2865-2871.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raoult D, Marrie T & Mege J Natural history and pathophysiology of Q fever. Lancet Infect Dis 5, 219–226 (2005). 10.1016/S1473-3099(05)70052-9 [DOI] [PubMed] [Google Scholar]
- 81.Leone M. et al. Effect of sex on Coxiella burnetii infection: protective role of 17beta-estradiol. J Infect Dis 189, 339–345 (2004). 10.1086/380798 [DOI] [PubMed] [Google Scholar]
- 82.Textoris J. et al. Sex-related differences in gene expression following Coxiella burnetii infection in mice: potential role of circadian rhythm. PLoS One 5, e12190 (2010). 10.1371/journal.pone.0012190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Strachan NJ et al. Sexual dimorphism in campylobacteriosis. Epidemiol Infect 136, 1492–1495 (2008). 10.1017/S0950268807009934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Turner NA et al. Epidemiologic Trends in Clostridioides difficile Infections in a Regional Community Hospital Network. JAMA Netw Open 2, e1914149 (2019). 10.1001/jamanetworkopen.2019.14149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pohl AM et al. Differences Among Incidence Rates of Invasive Listeriosis in the U.S. FoodNet Population by Age, Sex, Race/Ethnicity, and Pregnancy Status, 2008-2016. Foodborne Pathog Dis 16, 290–297 (2019). 10.1089/fpd.2018.2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pasche B. et al. Sex-dependent susceptibility to Listeria monocytogenes infection is mediated by differential interleukin-10 production. Infect Immun 73, 5952–5960 (2005). 10.1128/IAI.73.9.5952-5960.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salem ML, Matsuzaki G, Madkour GA & Nomoto K Beta-estradiol-induced decrease in IL-12 and TNF-alpha expression suppresses macrophage functions in the course of Listeria monocytogenes infection in mice. Int J Immunopharmacol 21, 481–497 (1999). 10.1016/s0192-0561(99)00027-2 [DOI] [PubMed] [Google Scholar]
- 88.Bremer V, Marcus U & Hamouda O Syphilis on the rise again in Germany – results from surveillance data for 2011. Eurosurveillance 17 (2012). 10.2807/ese.17.29.20222-en [DOI] [PubMed] [Google Scholar]
- 89.Peeling RW et al. Syphilis. Nature Reviews Disease Primers 3 (2017). 10.1038/nrdp.2017.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nudel K. et al. Transcriptome Analysis of Neisseria gonorrhoeae during Natural Infection Reveals Differential Expression of Antibiotic Resistance Determinants between Men and Women. mSphere 3 (2018). 10.1128/mSphereDirect.00312-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xiong M. et al. Analysis of the sex ratio of reported gonorrhoea incidence in Shenzhen, China. BMJ Open 6, e009629 (2016). 10.1136/bmjopen-2015-009629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jerse AE et al. Estradiol-Treated Female Mice as Surrogate Hosts for Neisseria gonorrhoeae Genital Tract Infections. Front Microbiol 2, 107 (2011). 10.3389/fmicb.2011.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Farnham A, Alleyne L, Cimini D & Balter S Legionnaires' disease incidence and risk factors, New York, New York, USA, 2002-2011. Emerg Infect Dis 20, 1795–1802 (2014). 10.3201/eid2011.131872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kadioglu A. et al. Sex-based differences in susceptibility to respiratory and systemic pneumococcal disease in mice. J Infect Dis 204, 1971–1979 (2011). 10.1093/infdis/jir657 [DOI] [PubMed] [Google Scholar]
- 95.Yang Z. et al. Female resistance to pneumonia identifies lung macrophage nitric oxide synthase-3 as a therapeutic target. Elife 3 (2014). 10.7554/eLife.03711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rosner BM, Stark K & Werber D Epidemiology of reported Yersinia enterocolitica infections in Germany, 2001-2008. BMC Public Health 10, 337 (2010). 10.1186/1471-2458-10-337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pietropaoli AP, Glance LG, Oakes D & Fisher SG Gender differences in mortality in patients with severe sepsis or septic shock. Gend Med 7, 422–437 (2010). 10.1016/j.genm.2010.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zeng Z. et al. Sex-hormone-driven innate antibodies protect females and infants against EPEC infection. Nat Immunol 19, 1100–1111 (2018). 10.1038/s41590-018-0211-2 [DOI] [PubMed] [Google Scholar]
- 99.Ng-Nguyen D. et al. The epidemiology of Taenia spp. infection and Taenia solium cysticerci exposure in humans in the Central Highlands of Vietnam. BMC Infect Dis 18, 527 (2018). 10.1186/s12879-018-3434-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kelvin EA et al. The association of host age and gender with inflammation around neurocysticercosis cysts. Ann Trop Med Parasitol 103, 487–499 (2009). 10.1179/000349809X12459740922291 [DOI] [PubMed] [Google Scholar]
- 101.Morales-Montor J & Larralde C The role of sex steroids in the complex physiology of the host-parasite relationship: the case of the larval cestode of Taenia crassiceps. Parasitology 131, 287–294 (2005). 10.1017/s0031182005007894 [DOI] [PubMed] [Google Scholar]
- 102.Wright JE, Werkman M, Dunn JC & Anderson RM Current epidemiological evidence for predisposition to high or low intensity human helminth infection: a systematic review. Parasit Vectors 11, 65 (2018). 10.1186/s13071-018-2656-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ayabina DV et al. Gender-related differences in prevalence, intensity and associated risk factors of Schistosoma infections in Africa: A systematic review and meta-analysis. PLoS Negl Trop Dis 15, e0009083 (2021). 10.1371/journal.pntd.0009083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nakazawa M. et al. Schistosoma mansoni: susceptibility differences between male and female mice can be mediated by testosterone during early infection. Exp Parasitol 85, 233–240 (1997). 10.1006/expr.1997.4148 [DOI] [PubMed] [Google Scholar]
- 105.Bernin H & Lotter H Sex Bias in the Outcome of Human Tropical Infectious Diseases: Influence of Steroid Hormones. Journal of Infectious Diseases 209, S107–S113 (2014). 10.1093/infdis/jit610 [DOI] [PubMed] [Google Scholar]
- 106.Hajjeh RA, Brandt ME & Pinner RW Emergence of cryptococcal disease: epidemiologic perspectives 100 years after its discovery. Epidemiol Rev 17, 303–320 (1995). 10.1093/oxfordjournals.epirev.a036195 [DOI] [PubMed] [Google Scholar]
- 107.Shaheen AA, Somayaji R, Myers R & Mody CH Epidemiology and trends of cryptococcosis in the United States from 2000 to 2007: A population-based study. Int J STD AIDS 29, 453–460 (2018). 10.1177/0956462417732649 [DOI] [PubMed] [Google Scholar]
- 108.Acuna-Soto R, Maguire JH & Wirth DF Gender distribution in asymptomatic and invasive amebiasis. Am J Gastroenterol 95, 1277–1283 (2000). 10.1111/j.1572-0241.2000.01525.x [DOI] [PubMed] [Google Scholar]
- 109.Sellau J. et al. Androgens predispose males to monocyte-mediated immunopathology by inducing the expression of leukocyte recruitment factor CXCL1. Nat Commun 11, 3459 (2020). 10.1038/s41467-020-17260-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lockard RD, Wilson ME & Rodriguez NE Sex-Related Differences in Immune Response and Symptomatic Manifestations to Infection with Leishmania Species. J Immunol Res 2019, 4103819 (2019). 10.1155/2019/4103819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Soares L, Abad-Franch F & Ferraz G Epidemiology of cutaneous leishmaniasis in central Amazonia: a comparison of sex-biased incidence among rural settlers and field biologists. Trop Med Int Health 19, 988–995 (2014). 10.1111/tmi.12337 [DOI] [PubMed] [Google Scholar]
- 112.Layegh P, Moghiman T & Ahmadian Hoseini SA Children and cutaneous leishmaniasis: a clinical report and review. J Infect Dev Ctries 7, 614–617 (2013). 10.3855/jidc.2939 [DOI] [PubMed] [Google Scholar]
- 113.Cloots K. et al. Male predominance in reported Visceral Leishmaniasis cases: Nature or nurture? A comparison of population-based with health facility-reported data. PLoS Negl Trop Dis 14, e0007995 (2020). 10.1371/journal.pntd.0007995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Layegh P, Moghiman T & Ahmadian Hoseini SA Children and cutaneous leishmaniasis: a clinical report and review. The Journal of Infection in Developing Countries 7, 614–617 (2013). 10.3855/jidc.2939 [DOI] [PubMed] [Google Scholar]
- 115.Rodriguez NE et al. Epidemiological and Experimental Evidence for Sex-Dependent Differences in the Outcome of Leishmania infantum Infection. Am J Trop Med Hyg 98, 142–145 (2018). 10.4269/ajtmh.17-0563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kobets T. et al. Genetics of host response to Leishmania tropica in mice - different control of skin pathology, chemokine reaction, and invasion into spleen and liver. PLoS Negl Trop Dis 6, e1667 (2012). 10.1371/journal.pntd.0001667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bryson KJ et al. BALB/c mice deficient in CD4 T cell IL-4Ralpha expression control Leishmania mexicana Load although female but not male mice develop a healer phenotype. PLoS Negl Trop Dis 5, e930 (2011). 10.1371/journal.pntd.0000930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Travi BL et al. Gender is a major determinant of the clinical evolution and immune response in hamsters infected with Leishmania spp. Infect Immun 70, 2288–2296 (2002). 10.1128/IAI.70.5.2288-2296.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Oghumu S. et al. Transgenic expression of CXCR3 on T cells enhances susceptibility to cutaneous Leishmania major infection by inhibiting monocyte maturation and promoting a Th2 response. Infect Immun 83, 67–76 (2015). 10.1128/IAI.02540-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lindova J. et al. Gender differences in behavioural changes induced by latent toxoplasmosis. Int J Parasitol 36, 1485–1492 (2006). 10.1016/j.ijpara.2006.07.008 [DOI] [PubMed] [Google Scholar]
- 121.Roberts CW, Cruickshank SM & Alexander J Sex-determined resistance to Toxoplasma gondii is associated with temporal differences in cytokine production. Infect Immun 63, 2549–2555 (1995). 10.1128/iai.63.7.2549-2555.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sun KS, Tsai CF, Chen SC, Chen YY & Huang WC Galactomannan Testing and the Incidence of Invasive Pulmonary Aspergillosis: A 10-Year Nationwide Population-Based Study in Taiwan. PLoS One 11, e0149964 (2016). 10.1371/journal.pone.0149964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.vom Steeg LG & Klein SL SeXX Matters in Infectious Disease Pathogenesis. PLoS Pathog 12, e1005374 (2016). 10.1371/journal.ppat.1005374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schaefer AL et al. Factors Contributing to Sex Differences in Mice Inhaling Aspergillus fumigatus. Int J Environ Res Public Health 17 (2020). 10.3390/ijerph17238851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guess TE, Rosen J, Castro-Lopez N, Wormley FL Jr. & McClelland EE An inherent T cell deficit in healthy males to C. neoformans infection may begin to explain the sex susceptibility in incidence of cryptococcosis. Biol Sex Differ 10, 44 (2019). 10.1186/s13293-019-0258-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lortholary O, Improvisi L, Fitting C, Cavaillon JM & Dromer F Influence of gender and age on course of infection and cytokine responses in mice with disseminated Cryptococcus neoformans infection. Clin Microbiol Infect 8, 31–37 (2002). 10.1046/j.1469-0691.2002.00375.x [DOI] [PubMed] [Google Scholar]
- 127.Bellissimo-Rodrigues F, Bollela VR, Da Fonseca BA & Martinez R Endemic paracoccidioidomycosis: relationship between clinical presentation and patients' demographic features. Med Mycol 51, 313–318 (2013). 10.3109/13693786.2012.714529 [DOI] [PubMed] [Google Scholar]
- 128.Shankar J, Restrepo A, Clemons KV & Stevens DA Hormones and the resistance of women to paracoccidioidomycosis. Clin Microbiol Rev 24, 296–313 (2011). 10.1128/CMR.00062-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pinzan CF, Ruas LP, Casabona-Fortunato AS, Carvalho FC & Roque-Barreira MC Immunological basis for the gender differences in murine Paracoccidioides brasiliensis infection. PLoS One 5, e10757 (2010). 10.1371/journal.pone.0010757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shemer Avner and Meir B (2018). [Google Scholar]
- 131.d'Enfert C. et al. The impact of the Fungus-Host-Microbiota interplay upon Candida albicans infections: current knowledge and new perspectives. FEMS Microbiol Rev 45 (2021). 10.1093/femsre/fuaa060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Severance EG et al. Candida albicans exposures, sex specificity and cognitive deficits in schizophrenia and bipolar disorder. NPJ Schizophr 2, 16018 (2016). 10.1038/npjschz.2016.18 [DOI] [PMC free article] [PubMed] [Google Scholar]