Abstract
Irreversible damage to hair cells (HCs) in the cochlea leads to hearing loss. Cochlear supporting cells (SCs) in the murine cochlea have the potential to differentiate into HCs. Neuron membrane glycoprotein M6B (Gpm6b) as a four‐transmembrane protein is a potential regulator of HC regeneration according to our previous research. In this study, we found that AAV‐ie‐mediated Gpm6b overexpression promoted SC‐derived organoid expansion. Enhanced Gpm6b prevented the normal decrease in SC plasticity as the cochlea develops by supporting cells re‐entry cell cycle and facilitating the SC‐to‐HC transformation. Also, overexpression of Gpm6b in the organ of Corti through the round window membrane injection facilitated the trans‐differentiation of Lgr5+ SCs into HCs. In conclusion, our results suggest that Gpm6b overexpression promotes HC regeneration and highlights a promising target for hearing repair using the inner ear stem cells combined with AAV.
In this work, Sun et al. investigated the effect of up‐regulation of Gpm6b on the hair cell differentiation of progenitors using Adeno‐associated virus‐inner ear (AAV‐ie)–mediated gene overexpression. In this study, Sun et al. found that Gpm6b overexpression could promote supporting cell proliferation and hair cell differentiation through organoid culture experiments in vivo and viral injection in neonatal mice.
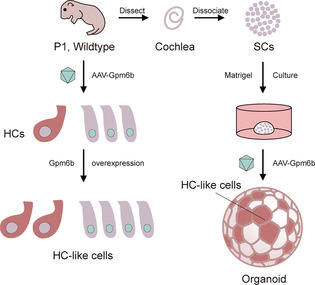
1. INTRODUCTION
Hearing loss is a common disease worldwide and has a profound impact on patients' communication and language acquisition. 1 There are many causes of deafness including ageing, ototoxic drugs, overstimulation, infection and genetic factors. 2 Hearing recovery is very difficult due to damaged HCs in the mature mammalian cochlea are unable to spontaneously regenerate. 3 Currently, the ideal treatment for sensorineural deafness would be to regenerate HCs from cochlear progenitor cells such as SCs to repair the structure and function recovery of the cochlea and restore hearing. Therefore, exploring effective methods for HC regeneration is the key question of current hearing research. 4
The sensory precursor cells proliferation and the HCs regeneration in the inner ear are regulated by various signalling pathways such as Wnt, Notch, Bmp/Smad, IGF (Insulin‐like growth factor) and FGF (fibroblast growth factor). 5 Simultaneously Wnt signalling pathway activation and Notch signalling pathway suppression can stimulate the hair cell differentiation of progenitors during the development of the cochlear epithelia, resulting in the production of ectopic HCs. 6 Inhibition of the Bmp signalling pathway increases HC regeneration after streptomycin injury, 7 and the Wnt signalling pathway can interact extensively with multiple signalling molecules such as TGF‐β and BMP to allow overlapping signalling pathways to specify cell fates, 8 thus playing a crucial role in regulating inner ear HC regeneration. However, it has not been possible to regenerate new HCs comparable to native HCs in mammals. Therefore, it is crucial to screen new factors that promote HC regeneration.
Glycoprotein GPM6B‐a four‐transmembrane protein belonging to the lipid‐protein family of integrated membrane proteins‐was originally defined as a structural protein of the myelin sheath of the central nervous system. 9 The GPM6B protein is generally expressed throughout the brain and expressed in neurons, oligodendrocytes and astrocytes, 10 , 11 and it is involved in the differentiation of terminally differentiated cells in various mammalian tissues. It also plays an important role in neuronal myelination and neuronal differentiation, which is essential for the correct extension and guidance of axons in the corpus callosum. 12 By stimulating the TGF‐β‐Smad2/3 signalling pathway, the GPM6B protein can induce the differentiation of vascular wall smooth muscle cells. 13 As a multifunctional cytokine, TGF‐β can regulate the morphogenesis of various cells as well as the processes of cell proliferation and differentiation. 14 TGF‐β1 is expressed in the inner ear and has been shown to enhance the protective effect of GDNF (Glial cell line‐derived neurotrophic factor) against ototoxicity caused by aminoglycoside‐induced HC loss. 15 In addition, Smad‐2 and Smad‐3 promote chondrogenesis in the developing middle ear capsule. 13 Therefore, we hypothesised that the GPM6B protein has a function in the regeneration of inner ear HCs.
The inner ear is structurally and anatomically independent from other organs, facilitating the direct delivery of exogenous genes, and the fluid‐filled environment allows the diffusion of therapeutic genes. AAV‐ie, screened by our team previously, as a new and safe AAV vector can efficiently infect mostly inner ear SCs in mice, and has been shown to effectively achieve the overexpression of Atoh1 in SCs and induce HC regeneration. 16 Moreover, SCs and HCs are derived from the same pool of progenitor cells. Therefore, in this research, we used the self‐designed AAV‐ie to deliver the Gpm6b into the cochlear progenitor cells through the round window membrane injection. We found that the overexpression of Gpm6b accelerated the SCs proliferation and HC differentiation in the cochlear organoids. RNA‐seq results showed that HC regeneration resulting from Gpm6b overexpression was related to Wnt, Hippo, and other signalling pathways. A similar phenomenon was observed in vivo, where Gpm6b overexpression slightly promoted SC division and HC differentiation, while lineage tracing indicated that regenerating HCs were partially derived from Lgr5+ SCs.
2. RESULTS
2.1. AAV‐Gpm6b promoted SCs proliferation in the cochlear organoids
It has been shown that SCs have the potential to differentiate into HCs. 17 , 18 SCs serve as HC progenitors in the postnatal mice cochlea, so we explored the effect of Gpm6b overexpression on SC proliferation and differentiation into HCs in vitro. The cochlear organoid is an excellent model for studying supporting cell plasticity. Control virus AAV‐mNeonGreen (a green fluorescent protein) and the experimental virus AAV‐Gpm6b‐mNeonGreen were added in the medium during the organoid culture (Figure 1A,B). We evaluated the diameter and number of organoids, which can be used as criteria to evaluate the proliferation ability of stem cells. 19 Our results showed that the organoids significantly increased in diameter but did not change in number after Gpm6b overexpression compared to the control group (Figure 1C,D). Before collecting the samples, EdU was added for 1 h in order to label the proliferating cells. The immunofluorescence images showed that the percentage of EdU+ SCs in each organoid and the proportion of EdU+ organoids increased significantly after Gpm6b overexpression compared to the control group (Figure 1E–G). Studies have shown that the plasticity of mouse SCs declines rapidly after birth. 19 In order to explore whether Gpm6b overexpression has a positive effect on the proliferation ability of cochlear SCs in the aged mice, a similar three‐dimensional organoid culture was performed on P4 WT mice and AAV‐Gpm6b was introduced in the organoid (Figure 1H,I). Similarly, after Gpm6b overexpression the diameter of the organoids increased significantly, without a changeable number of organoids (Figure 1J). Moreover, the immunofluorescence staining of the organoids showed that the percentage of EdU+ SCs also increased significantly in each organoid after Gpm6b overexpression (Figure 1K,L). In conclusion, Gpm6b overexpression can facilitate SC proliferation in the culture organoid.
FIGURE 1.
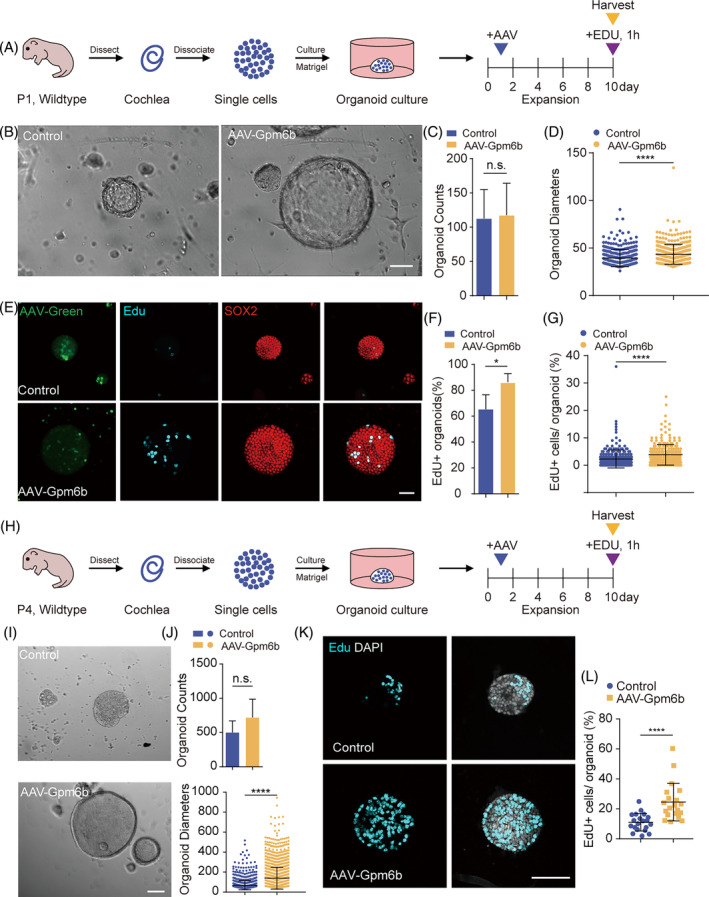
Gpm6b overexpression promoted the proliferation of cochlear organoids. (A) Experimental design of the expansion culture of SCs in three‐dimensional in vitro assay. Given a single EdU treatment for 1 h on day 10. (B) Bright‐field images of cochlear organoids after expansion with the administration of AAV‐mNeonGreen (Control) and AAV‐Gpm6b, respectively. Scale bar: 50 μm. (C and D) The number (C) and diameter (D) of the organoids in (B). (E) Immunofluorescence images of the organoids in (B). Sox2 (red) marks SCs. EdU (cyan) marks proliferating cells. AAV‐mNeonGreen (green) marks cells transduced by AAVs. Scale bar: 50 μm. (F and G) The ratio of EdU+ organoids (F) and the ratio of EdU+ cells per organoid (G) in (E). (H–L) Similar analyses to (A to G) of cochlear organoids from P4 mice. Scale bars: 100 μm. AAV dose: 2 × 1010 GCs per well. Data are displayed as the mean with SEM. The value of p was calculated by Student's t‐test. *p < 0.05; ****p < 0.0001; n.s., no significance.
2.2. AAV‐Gpm6b stimulated HC regeneration in cochlear organoids
Next, we explored the differentiation ability of organoids derived from P1 mouse cochlear SCs after Gpm6b overexpression (Figure 2A). On the first day of differentiation culture, the AAV‐mNeonGreen or AAV‐Gpm6b was added, and immunofluorescence staining indicated that the percentage of Myo7a + organoids remarkably increased after Gpm6b overexpression (Figure 2B–D), suggesting that Gpm6b overexpression can promote the transdifferentiation of SCs into HCs.
FIGURE 2.
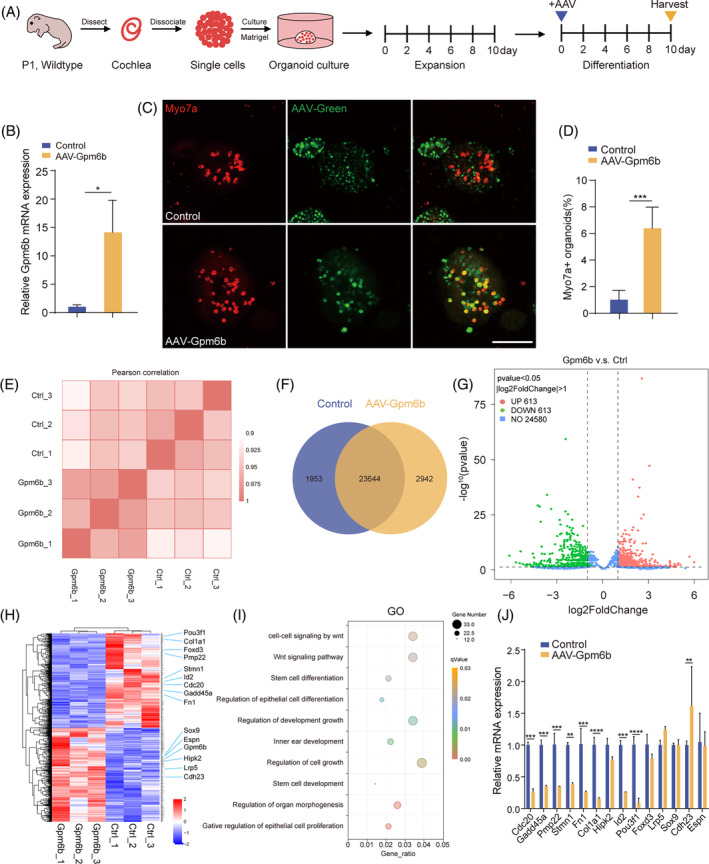
Gpm6b overexpression promoted HC regeneration in cochlear organoids. (A) Experimental design of the differentiation culture of cochlear organoids in the three‐dimensional in vitro assay. AAV dose: 2 × 1010 GCs per well. (B) Relative mRNA expression of Gpm6b in the cochlear organoids. (C) Immunofluorescence images of the organoids in (A). Scale bar: 100 μm. (D) The Myo7a+ organoids ratio was calculated in (C). (E–I) RNA sequencing data from cochlear organoids was added with control and AAV‐Gpm6b, respectively. (E) Correlation analysis between control and AAV‐Gpm6b–transduced organoids. (F) Venn diagram analysis of differentially expressed genes. (G) The volcano plot shows the overall profile of differentially expressed genes. Red/green dots indicate up/down‐regulated genes, respectively. Genes with no statistically significant expression difference are shown as blue dots. |log2FoldChange| > 1 and p‐value < 0.05 were the cutoffs for differentially expressed genes. (H) Heatmap of the differentially expressed genes. (I) GO terms significantly enriched in differentially expressed genes in Gpm6b‐overexpressing organoids. (J) The differentially expressed genes identified in (H) were verified by qPCR. Data are displayed as the mean with SEM. The value of p was calculated by Student's t‐test. **p < 0.01; ***p < 0.001; n.s. refers to no significance.
To determine how Gpm6b overexpression improves the capacity of SCs to transdifferentiate into HCs, we analysed the transcriptomes of the control and Gpm6b‐overexpressing organoids using the RNA sequencing (Figure 2A). Pearson correlation between samples indicated that the samples between control and AAV‐Gpm6b transduced organoids had high similarity (Figure 2E). A Venn diagram showed that 1953 and 2942 genes were enriched in the control and Gpm6b group, respectively, and 23,644 genes were co‐expressed in both groups (Figure 2F). The gene expression changes induced by Gpm6b overexpression are shown in the volcano plot and heat map (|log2FoldChange| > 2.0, p‐value < 0.05) (Figure 2G,H). After Gpm6b overexpression, 613 genes were down‐regulated (blue) and 613 genes were up‐regulated (red) compared with the control group. To identify the signalling pathways and biological processes that might be altered by Gpm6b overexpression, gene ontology (GO) enrichment analysis was performed. Differentially expressed genes were highly enriched in functional categories such as the Wnt signalling pathway, stem cell differentiation and inner ear development (Figure 2I). We performed qPCR verification of the differentially expressed genes obtained by the RNA sequencing, and it showed that genes related to the cell cycle (Cdc20, Gadd45a, Pmp22 and Stmn1), transcription factors (Pou3f1 and Foxd3), the EGF signalling pathway (Fn1 and Col1a1) and the TGF‐β signalling pathway (Id2) were down‐regulated, while genes associated with the Wnt signalling pathway (Lrp5), the Hippo signalling pathway (Hipk2) and the cochlear hair cell (Cdh23 and Espn) were all up‐regulated (Figure 2J). Taken together, the above results suggested that Gpm6b overexpression can facilitate the transdifferentiation of SCs into HCs via multiple signalling pathways.
2.3. AAV‐Gpm6b promoted HC regeneration in the murine cochlea
We further investigated the influence of Gpm6b overexpression on SC proliferation and HC regeneration in vivo. AAV‐mNeonGreen and AAV‐Gpm6b viruses were injected into P1 WT neonatal mice through the round window membrane, respectively. And, EdU was injected subcutaneously into P2–P4 mice to label the proliferative SCs. The cochleae were dissected at P7 and immunofluorescence staining was performed (Figure 3A). No EdU+ SCs (Sox2+/EdU+ cells) was detected in the control or Gpm6b group (Figure 3C). It was known that Lgr5 is a receptor of the Wnt signalling pathway and is considered as a marker of SCs in cochlea. 20 , 21 Lgr5 expression decreases gradually during SC development. Therefore, we obtained the cochleae of P15 Lgr5‐EGFP mice injected with AAV‐mNeonGreen and AAV‐Gpm6b viruses respectively, and our qPCR and immunofluorescence results showed that Gpm6b overexpression had no effect on the expression of Lgr5 in cochlea (Figure 3B,D,E). These results indicated that Gpm6b overexpression had no effect on the proliferation of SCs in vivo. We then explored the influence of Gpm6b on the SC‐to‐HC trans‐differentiation process in vivo. In P1 WT mice, the control and Gpm6b viruses were injected through the round window membrane, and the cochleae were dissected at P7 for immunofluorescence staining (Figure 3F,G). The data indicated the number of ectopic inner HCs (IHCs) in the cochlea increased after Gpm6b overexpression. The number of ectopic IHCs in the middle turn was remarkably different from the control group, while in the apical turn and basal turn were not significantly different. In addition, the number of ectopic outer HCs (OHCs) increased remarkably. The number of total OHCs and HCs were also increased significantly (Figure 3H). These results suggested that Gpm6b overexpression may promote the transdifferentiation of SCs into HCs in the cochlea.
FIGURE 3.
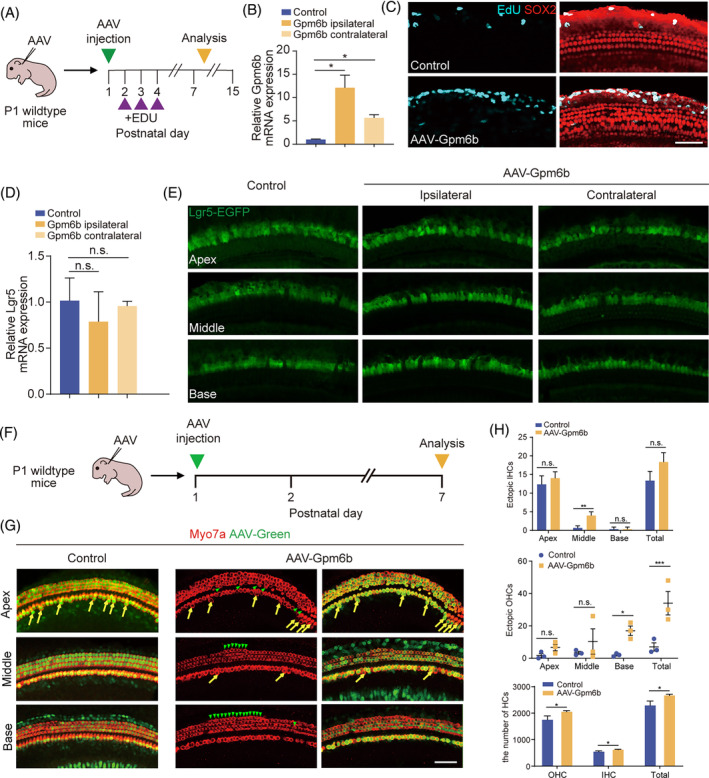
Gpm6b overexpression promoted hair cell regeneration in vivo. (A) Experimental design. (B) mRNA expression level of Gpm6b in P15 cochleae after the injection of AAV‐Gpm6b. (C) EdU immunostaining after Gpm6b overexpression in cochlear. EdU (cyan) marks proliferating cells. Sox2 (red) marks the SCs. Scale bar: 50 μm. (D) mRNA expression level of Lgr5 in P15 cochleae after the injection of AAV‐Gpm6b. (E) Immunofluorescence images of the AAV‐transduced cochleae of Lgr5‐EGFP mice. Lgr5‐EGFP (green) marks the Lgr5+ cells. (F) Experimental design. Tamoxifen was injected intraperitoneally into P1 wildtype mice. The AAVs were injected through the round window membrane, and the cochleae were collected at P7. (G) Immunofluorescence images of cochlear epithelia infected by control and AAV‐Gpm6b, respectively. Ectopic HCs are marked by yellow arrows (IHC area) and green arrows (OHC area). Scale bar: 50 μm. (H) The number of ectopic IHCs, ectopic OHCs, and total HCs in (B). AAV dose: 2.8 × 1010 GCs per cochlea. Data are displayed as the mean with SEM. The value of p was calculated by Student's t‐test. *p < 0.05; **p < 0.01; ***p < 0.001; n.s. refers to no significance.
2.4. AAV‐Gpm6b facilitated Lgr5+ SC‐to‐HC transformation in murine cochlea
Lgr5+ SCs are usually considered as the HC progenitors, which can differentiate into HCs. 22 , 23 Therefore, we used Lgr5‐EGFPCreER/+/Rosa26‐tdTomatoloxp/+ mice to explore the source of regenerated HCs. AAV‐ie can infect Lgr5+ SCs. 16 Via round window membrane, we injected AAV‐mNeonGreen and AAV‐Gpm6b viruses in P1.5 mice, in which the Cre enzyme was activated by tamoxifen at P1. At P7, the cochleae were dissected for immunofluorescence staining (Figure 4A,B). Myo7a+/Tomato+ IHCs, Myo7a+/Tomato+ OHCs and Myo7a+/Tomato+ HCs were found in the control group and Gpm6b overexpression group. After Gpm6b overexpression, Tomato‐labelled OHC, not IHC, was increased significantly (Figure 4C). These results indicated that Gpm6b overexpression promoted the regeneration of cochlear HCs and that these regenerated HCs were derived from Lgr5+ SCs.
FIGURE 4.
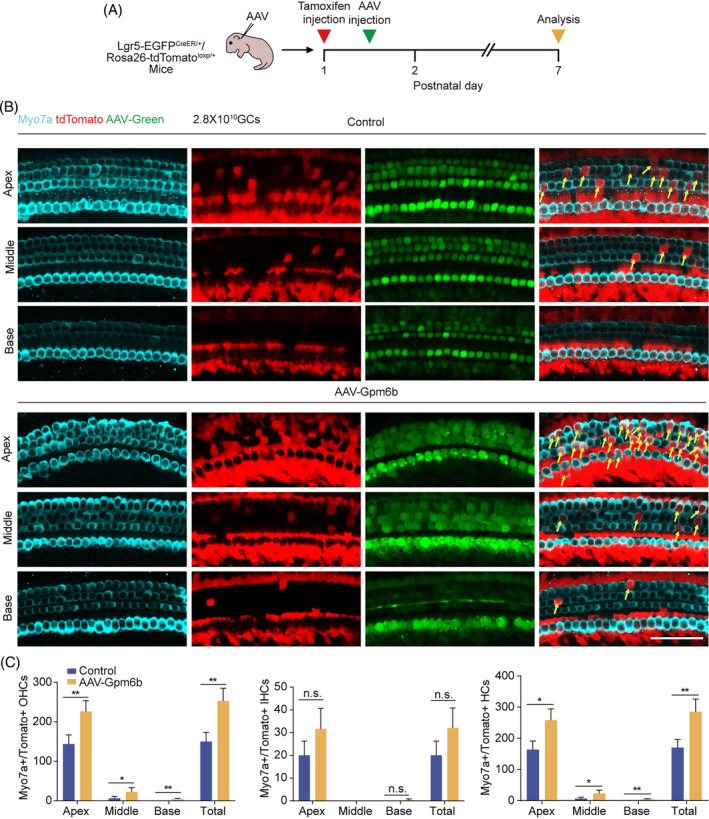
Gpm6b overexpression facilitated the trans‐differentiation of Lgr5+ progenitors into HCs. (A) Experimental design. P1 Lgr5‐EGFPCreER/+/Rosa26‐tdTomatoloxp/+ mice were injected with tamoxifen intraperitoneally. AAVs were injected 0.5 days later through the round window membrane, and the cochleae were collected at P7. (B) Immunofluorescence of AAV‐transduced cochleae. Scale bar: 50 μm. (C) The number of Myo7a+/Tomato+ OHCs, IHCs and HCs per cochlea. AAV dose: 2.8 × 1010 GCs per cochlea. Data are displayed as the mean with SEM. The value of p was calculated by Student's t‐test. *p < 0.05; **p < 0.01; n.s. refers to no significance.
3. DISCUSSION
In previous studies, our team compared differentially expressed genes of different transcriptomes of SCs including Lgr5+ SCs in the apical/basal turn, the Lgr5+/Lgr5− SCs, and Lgr5+ SCs from neomycin‐treated and non‐treated cochleae, and intersection analysis showed that Gpm6b expression changed significantly, 24 , 25 , 26 suggesting that Gpm6b might be play a role in the regulation of HC regeneration. In this study, we used inner ear organoid culture in vitro and lineage tracing of neonatal inner ear HCs to show that the overexpression of Gpm6b improved the monopotency of SCs and promoted the transdifferentiation of SCs into HCs. This study identified the new gene that directs HC regeneration via AAV‐mediated gene delivery, thus providing new therapeutic targets for the prevention and recovery of auditory dysfunction caused by HC degeneration.
Due to the lack of spontaneous regeneration ability of inner ear HCs in mammals, there is currently no effective means to completely recover the loss of inner ear HCs. 27 Studies have shown that SCs can be used as progenitor cells of the inner ear, which can divide or transdifferentiate to regenerate HCs, 28 and some signalling pathways or transcription factors are associated with HC regeneration, for example, Atoh1, Wnt and Notch signalling pathways. 29 Many other genes and related pathways have also been shown to play important roles in HC regeneration. These pathways can communicate with each other to influence the SC proliferation or HC regeneration. 30 Gpm6b was originally identified as a structural protein in the nervous system of vertebrate embryos. 10 And it is also expressed in peripheral tissues such as the smooth muscle, heart, skeletal muscle, liver, spleen and kidney. 31 Gpm6b is a pleiotropic molecule with different function. It is involved in cellular housekeeping functions such as membrane transport and intercellular communication in the central nervous system. 32 While, there are no studies of Gpm6b in the auditory system. In this study, we found that AAV‐Gpm6b could promote more SCs differentiation into HCs under physiological conditions compared with the control in mice, and perhaps our study will provide a theoretical basis for future human auditory system research about Gpm6b.
RNA‐seq data showed cycle genes (Cdc20, Gadd45a, Pmp22 and Stmn1), transcription factors (Pou3f1 and Foxd3), signalling pathway genes, including Wnt (Lrp5), Hippo (Hipk2), TGF‐β (Id2) and EGF (Fn1 and Col1a1), and cochlea‐related genes (Cdh23, and Espn) were differentially expressed in the two groups of organoids. Several genes above have been confirmed to be associated with inner ear development and HC regeneration in mice. For example, the downstream target Id2 is downregulated in AAV‐Gpm6b‐transduced TGF‐β signalling, which engages in cross‐talk with the classical Notch, Wnt and Hippo signalling pathways regulating the differentiation of HC progenitors, and this negatively regulates HC generation during the development of inner ear. 8 , 33 Gpm6b can also stimulate TGF‐β‐Smad2/3‐related signalling pathways that participate in the differentiation of osteoblasts and smooth muscle cells. 13 The TGF‐β signalling pathway is also associated with the regulation of inner ear sensory epithelial cell status. 34 Moreover, it has been shown that TGF‐β1 is expressed in the inner ear and enhance the protective effect of GDNF (Glial cell line‐derived neurotrophic factor) against ototoxicity caused by aminoglycoside‐induced HC loss. 15 RNA‐seq results showed that TGF‐β1 was up‐regulated after Gpm6b overexpression, which might have a protective effect on HCs. Wnt signalling pathway activation can enhance the expansion of Lgr5+ cochlear progenitor cells, 22 and Smad protein, a key mediator of the TGF‐β signalling pathway, can also respond to Wnt signalling. Notch signalling inhibition can cause Lgr5+ progenitor cells to re‐enter the cell cycle and transdifferentiate into HCs, 35 and the TGF‐β‐Smad and Notch signals can affect the regenerative ability of stem cells through antagonistic interactions. 36 Furthermore, the Hippo signalling pathway as well as YAP‐mediated overexpression of Lin28a can activate the Wnt pathway to facilitate HC regeneration, 37 and YAP has been shown to be associated with Smad2/3/4 protein complexes and to determine their intracellular localisation. 38 Thus, there are complex interactions between the Notch, Wnt and Hippo signalling pathways, indicating that regulation of multiple pathways may be more effective in promoting HC regeneration than regulating individual pathways. TGF‐β signalling plays an important role in these signalling networks, so it might be possible to use TGF‐β as a connector to further explore the signalling pathway crosstalk including Gpm6b to promote inner ear HC regeneration. Therefore, multiple signals upon Gpm6b overexpression may synergistically promote inner ear organoid proliferation and differentiation into HCs.
Cadherin 23 (Cdh23) is an important constituent of the HC tip link in the organ of Corti. Mutations in Cdh23 are associated with age‐related hearing loss (AHL). 39 Similarly, Espn has a crucial role in the structural maintenance and development of HC stereocilium. 40 RNA seq. results showed that Cdh23 and Espn were upregulated when Gpm6b overexpression. Therefore, Gpm6b overexpression can promote the relative HC genes high expression and HC differentiation. HCs act as specialised neurons, so we speculate that Gpm6b may also have a role in neuron regeneration and protection.
We also found reduced expression of cell cycle factor Cdc20 in the AAV‐Gpm6b‐induced differentiation of inner ear organoids. Cdc20 is involved in proteolysis mediated by ubiquitin and is highly expressed in proliferating cells and mediates the co‐regulation of MAD mitotic spindle checkpoint proteins, with the anaphase promoting complex (APC). 41 Cdc20 is expressed in the inner ear. At embryonic day 12.5, Cdc20 is expressed throughout the cochlear duct epithelium, and it is significantly decreased in the prosensory region at embryonic day 14.5, which corresponds with the exit of prosensory cells from the cell cycle. Conditional knockdown of Cdc20 in the anterior sensory region induces four or more rows of OHCs at the apex of the cochlea and leads to a reduction in the length of the cochlear canal. 42 It is unclear whether Cdc20 can control the generation of SCs and HCs by regulating the proliferation and differentiation of progenitor cells, but it is known that Cdc20‐APC is needed in presynaptic axonal differentiation of cerebellum postmitotic neurons. 43 The relationship between Cdc20 and HCs requires further investigation. In addition, many other differentially expressed genes, such as Gadd45a, Pmp22, Stmn1, Fn1, Col1a1 and so forth, may be potential targets for HC regeneration.
Our organoid expansion experiments showed that Gpm6b could promote the proliferation of SCs in vitro. However, EdU+/SOX2+ proliferative SCs were not observed in vivo. At the stage of organoid proliferation culture, a variety of cytokines promoting cell growth were added to the medium, including EGF, IGF, FGF and CHIR. CHIR is a small molecule agonist of Wnt, 44 and the presence of these cytokines makes it easier for cells to enter the cell cycle. The synergistic effect of these factors may be involved in the promotion of organoid formation by Gpm6b. However, the in vivo environment lacks the cofactors mentioned above. This explains, to a certain extent, the failure of Gpm6b to promote SC proliferation in vivo and suggests that Gpm6b might have a stronger effect on SC proliferation in the presence of other signals, such as Wnt. Thus, multiple signalling pathways and multiple genes may be required for cooperative regulation if proliferation is also desired in vivo.
In summary, we used AAV‐ie to up‐regulate Gpm6b in the cochlear SCs of newborn mice and discovered that Gpm6b overexpression led to a remarkable increase in HCs. Our study demonstrated for the first time the role of the Gpm6b gene in HC regeneration and its potential to participate in multiple signalling pathways to promote inner ear HC regeneration. At the same time we demonstrated that AAV was a powerful tool in hair cell regeneration applications. It is valuable for the future utilise of multiple genes and signalling pathways to jointly regulate inner ear HC regeneration via the recombined AAV.
4. MATERIALS AND METHODS
4.1. Animals
Lgr5‐EGFPCreERT2 and Rosa26‐tdTomatoloxP/+ (The Jackson Laboratory, Stock No. 007914 and No. 008875, respectively) mice were bred in the animal room. Tamoxifen (Sigma, #T5648) was dissolved in corn oil and injected into P1 mice intraperitoneally (dose: 0.075 mg/g body weight). EdU (Beyotime, ST067‐50 mg) was dissolved in PBS and injected subcutaneously into P2–4 mice (dose: 0.05 mg/g body weight). All animal experiments were approved by the Institutional Animal Care and Use Committee of Southeast University.
4.2. HEK 293T cell culture
HEK 293T cells were taken out from liquid nitrogen and rapidly resuscitated in a 37°C water bath. After centrifuged at 1000 × g for 3 min, the cells were seeded in cell culture dishes (Greiner, #664160). The culture medium were DMEM (Gibco, #C11995500BT‐500 mL), 10% FBS (Vivacell, #C04001‐500), and 1% penicillin/streptomycin (Gibco, #15140122). When the cells grew to about 90% confluence, they were passaged according to the appropriate ratio (e.g., 1:3). For cell passage, 0.05% trypsin–EDTA (Gibco, #25200072) was added to lysis the cells after PBS washed. The cell morphology was observed under the microscope, when the cell boundary became significantly brighter, the 0.05% trypsin–EDTA was removed, then the digestion was terminated with culture medium, and the cells were gently blown off the bottom of the dish. The cells were collected into tubes and centrifuged at 1000 × g for 3 min. Next the cells were inoculated in cell culture dishes at an appropriate proportion for passage.
4.3. Organoid culture
The cochleae basal membranes of P1 WT mice were taken out and then they were digested into single cells with 0.25% trypsin. The digestion was terminated by adding trypsin inhibitor (Worthington, #59S11627), centrifuging at 2500 rpm, discarding the supernatant, and resuspending in DMEM/F12 (ThermoFisher, #11320033). Then the cells were resuspended in DMEM/F12 mixed with 35% Matrigel (Corning, #354230), and the cell resuspension was seeded in 24‐well dishes at 30 μL/well. The plates were then placed in a 37°C incubator for 30 min. When the Matrigel had solidified, proliferation medium was added (500 μL/well). The next day the AAV‐ie‐CAG‐mNeonGreen and AAV‐ie‐CAG‐Gpm6b viruses were added. The proliferation medium consisted of DMEM/F12, 2% B27 (ThermoFisher, #12587010), 1% N2 (ThermoFisher, #17502001), β‐FGF (10 ng/mL; #Peprotech, 100‐18C), EGF (20 ng/mL; life, #PHG0311), CHIR99021 (3 μM; Sigma‐Aldrich, #SML1046), valproic acid (1 mM; Sigma‐Aldrich, #P4543), 616452 (2 μm; Sigma‐Aldrich, #446859‐33‐2) and 0.1% ampicillin (Sangon Biotech, #A610028‐0025). After proliferation culture, the culture medium was replaced with differentiation medium, and the AAV‐ie‐CAG‐mNeonGreen and AAV‐ie‐CAG‐Gpm6b viruses were added. After 10 days of differentiation culture, the samples were collected. The components of the differentiation medium included DMEM/F12, 1% N2, 2% B27, CHIR99021 (3 μM), LY411575 (5 μM; Sigma‐Aldrich, #SML0506), and 0.1% ampicillin.
4.4. Virus packaging and purification
In the three‐plasmid packaging system, the target gene, viral capsid, and auxiliary plasmid were mixed with the transfection reagent PEI (yeasen; #40816ES02/03) according to the appropriate proportions, incubated at room temperature for 20 min, and then dropped into the culture dish of HEK 293 T cells at a density of about 90%. The culture medium was changed to 1% medium at 12 h, the supernatant was collected at 48 h and the cells were collected at 96 h. The components of the 1% medium were DMEM, 1% FBS and 1% penicillin/streptomycin. For virus purification, the method in the previous literature was used. 16 The SYBR (Vazyme, #Q311) method was used for titre determination, and primers were designed from the WPRE region to determine the genomic titre of AAVs.
4.5. AAV injection through the round window membrane
Newborn mice were anaesthetised in ice. Under the microscope, a small incision was made between the ears and the neck of the mice with scissors. The fat and muscle were gently removed with tweezers to expose the round window of the cochlea. The AAV virus was injected into the mice cochlea through round window membrane via a glass micropipettes (Drummond, #5‐000‐1001‐X10). The incisions were sealed using tissue adhesive (3 M Vetbond, #1469SB), and the mice were placed on a 37°C heating pad to revive them before returning them to their mother's cage.
4.6. Immunofluorescence staining
Mouse cochlea and cells were fixed in 4% PFA (Beyotime, #P0099), and cochleae were decalcified by 0.5 M EDTA (Solarbio, #E1170). After the cochlea was cut under the microscope, it was blocked with 10% donkey serum (Solarbio, #017‐000‐121) for 1 h at room temperature. Primary antibodies against Myo7a (Proteus Biosciences, #25–6790, 1:1000 dilution) and Sox2 (Santa, #SC‐17320, 1:400 dilution) were incubated at 4°C overnight. Next day, they were washed with PBS and the corresponding secondary antibody was incubated at room temperature for 1 h. Finally, DAKO fluorescence mounting medium (DAKO, #S3023) was used to seal the samples. Images were taken under a laser confocal microscope.
4.7. Quantitative real‐time PCR and RNA sequencing
Total RNA from the tissue/organoids was extracted with Trizol (ThermoFisher, #15596018). Then the RNA was reverse‐transcribed into cDNA by a Reverse Transcription Kit (Vazyme, #R223‐01). The quantitative real‐time PCR (qPCR) was conducted with the SYBR (Vazyme, #Q712) on a Real‐Time PCR System (Bio‐Rad). The primer sequences are listed in Table 1. For RNA sequencing, all libraries were analysed through the bioanalyzer from Novogene for quality and concentration. Sequencing data was analysed using the NovoMagic Platform.
TABLE 1.
The primer sequences were used in this study.
| Gene | Forward sequence (5′–3′) | Reverse sequence (5′–3′) |
|---|---|---|
| Gpm6b | TGGGCTTACTTAAAGGATGCAAG | TTGAGTTGTTCTTTTGAGCGAGA |
| Lgr5 | TCTTCACCTCCTACCTGGACCT | GGCGTAGTCTGCTATGTGGTGT |
| Cdc20 | TTCGTGTTCGAGAGCGATTTG | ACCTTGGAACTAGATTTGCCAG |
| Gadd45a | CCGAAAGGATGGACACGGTG | TTATCGGGGTCTACGTTGAGC |
| Pmp22 | CATCGCGGTGCTAGTGTTG | AAGGCGGATGTGGTACAGTTC |
| Stmn1 | TCTGTCCCCGATTTCCCCC | AGCTGCTTCAAGACTTCCGC |
| Pou3f1 | GCGAGCACTCGGACGAGG | CGCAGACGGCTTGGGACACT |
| Foxd3 | CCCATCACGGACAGCCTCAG | TAGGCTGTTCTTGGGCTTGC |
| Fn1 | ATGTGGACCCCTCCTGATAGT | GCCCAGTGATTTCAGCAAAGG |
| Col1a1 | GCTCCTCTTAGGGGCCACT | CCACGTCTCACCATTGGGG |
| Id2 | ATGAAAGCCTTCAGTCCGGTG | AGCAGACTCATCGGGTCGT |
| Lrp5 | AAGGGTGCTGTGTACTGGAC | AGAAGAGAACCTTACGGGACG |
| Hipk2 | TTTCTCCCCTCACACCCTTCA | CCAGTTGGAACTTGGCTCTACT |
| Sox9 | AGTACCCGCATCTGCACAAC | ACGAAGGGTCTCTTCTCGCT |
| Cdh23 | GGAGGATTACCTACGGCTCAA | GTGTGGATCAGCTCGGAGA |
| Espn | CCACAGGCTACCTCTCTTGC | AGCAGCCACTTCACCACATC |
| Gapdh | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
| WPRE | GTCAGGCAACGTGGCGTGGTGTG | GGCGATGAGTTCCGCCGTGGC |
4.8. Statistical analysis
All of the data were displayed as the mean with SEM, and statistical analyses were performed using GraphPad Prism9 software. For all experiments, n represents the number of replicates, and at least three individual experiments were conducted. Two‐tailed, unpaired Student's t‐tests were used to determine statistical significance when comparing two groups. In all cases, p < 0.05 was considered significant.
AUTHOR CONTRIBUTIONS
R.C., F.Y. and J.Q. conceived and designed the experiments. Q.S., L.Z., T.C. and N.L. performed most of the experiments. X.G., Z.Z., Y.Z., J.L., X.Q. and Y.L. performed genotyping and cell culture. R.C., J.Q., F.T, B.G. and Q.S. discussed the data analysis, interpretation and presentation and wrote the manuscript with contributions from all authors.
FUNDING INFORMATION
This work was supported by the National Key Research and Development Program of China (2021YFA1101300, 2021YFA1101800, 2020YFA0113600 and 2020YFA0112503), the STI2030‐Major Projects (2022ZD0205400), the National Natural Science Foundation of China (82030029, 81970882, 93149304, 82000984, 82071046 and 82371156), the China National Postdoctoral Program for Innovative Talents (BX20200082), the China Postdoctoral Science Foundation (2020M681468), the Science and Technology Department of Sichuan Province (2021YFS0371), the Natural Science Foundation from Jiangsu Province (BK20211168), the Shenzhen Science and Technology Program (JCYJ20210324125608022), the Open Research Fund of State Key Laboratory of Genetic Engineering, Fudan University (SKLGE‐2104), the Jiangsu Postdoctoral Research Funding Program (2021K156B) and the Fundamental Research Funds for the Central Universities.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
Sun Q, Zhang L, Chen T, et al. AAV‐mediated Gpm6b expression supports hair cell reprogramming. Cell Prolif. 2024;57(7):e13620. doi: 10.1111/cpr.13620
Qiuhan Sun, Liyan Zhang, Tian Chen, Nianci Li and Fangzhi Tan contributed equally.
Contributor Information
Fangzhi Tan, Email: tanfangzhi@163.com.
Bing Guan, Email: aliceguan0685@sina.com.
Jieyu Qi, Email: jieyuqi@seu.edu.cn.
Fanglei Ye, Email: yefanglei000@sina.com.
Renjie Chai, Email: renjiec@seu.edu.cn.
DATA AVAILABILITY STATEMENT
All data associated with this study are present in the paper.
REFERENCES
- 1. Fukui H, Raphael Y. Gene therapy for the inner ear. Hear Res. 2013;297:99‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shibata SB, West MB, du X, Iwasa Y, Raphael Y, Kopke RD. Gene therapy for hair cell regeneration: review and new data. Hear Res. 2020;394:107981. [DOI] [PubMed] [Google Scholar]
- 3. Takeda H, Dondzillo A, Randall JA, Gubbels SP. Challenges in cell‐based therapies for the treatment of hearing loss. Trends Neurosci. 2018;41(11):823‐837. [DOI] [PubMed] [Google Scholar]
- 4. Chen Y, Zhang S, Chai R, Li H. Hair cell regeneration. Adv Exp Med Biol. 2019;1130:1‐16. [DOI] [PubMed] [Google Scholar]
- 5. Koehler KR, Nie J, Longworth‐Mills E, et al. Generation of inner ear organoids containing functional hair cells from human pluripotent stem cells. Nat Biotechnol. 2017;35(6):583‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu J, Li W, Lin C, et al. Co‐regulation of the notch and Wnt signaling pathways promotes supporting cell proliferation and hair cell regeneration in mouse utricles. Sci Rep. 2016;6:29418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bai H, Yang S, Xi C, et al. Signaling pathways (notch, Wnt, Bmp and Fgf) have additive effects on hair cell regeneration in the chick basilar papilla after streptomycin injury in vitro: additive effects of signaling pathways on hair cell regeneration. Hear Res. 2021;401:108161. [DOI] [PubMed] [Google Scholar]
- 8. Luo K. Signaling cross talk between TGF‐beta/Smad and other signaling pathways. Cold Spring Harb Perspect Biol. 2017;9(1):a022137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mikoshiba K, Okano H, Tamura TA, Ikenaka K. Structure and function of myelin protein genes. Annu Rev Neurosci. 1991;14:201‐217. [DOI] [PubMed] [Google Scholar]
- 10. Sanchez‐Roige S, Barnes SA, Mallari J, Wood R, Polesskaya O, Palmer AA. A mutant allele of glycoprotein M6‐B (Gpm6b) facilitates behavioral flexibility but increases delay discounting. Genes Brain Behav. 2022;21(4):e12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yan Y, Narayanan V, Lagenaur C. Expression of members of the proteolipid protein gene family in the developing murine central nervous system. J Comp Neurol. 1996;370(4):465‐478. [DOI] [PubMed] [Google Scholar]
- 12. Mita S, De Monasterio‐Schrader P, Fünfschilling U, et al. Transcallosal projections require glycoprotein M6‐dependent neurite growth and guidance. Cereb Cortex. 2015;25(11):4111‐4125. [DOI] [PubMed] [Google Scholar]
- 13. Zhang X, Xie H, Chang P, et al. Glycoprotein M6B interacts with TbetaRI to activate TGF‐beta‐Smad2/3 signaling and promote smooth muscle cell differentiation. Stem Cells. 2019;37(2):190‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roberts AB, Flanders KC, Heine UI, et al. Transforming growth factor‐beta: multifunctional regulator of differentiation and development. Philos Trans R Soc Lond Ser B Biol Sci. 1990;327(1239):145‐154. [DOI] [PubMed] [Google Scholar]
- 15. Kawamoto K, Yagi M, Stöver T, Kanzaki S, Raphael Y. Hearing and hair cells are protected by adenoviral gene therapy with TGF‐beta1 and GDNF. Mol Ther. 2003;7(4):484‐492. [DOI] [PubMed] [Google Scholar]
- 16. Tan F, Chu C, Qi J, et al. AAV‐ie enables safe and efficient gene transfer to inner ear cells. Nat Commun. 2019;10(1):3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kolla L, Kelly MC, Mann ZF, et al. Characterization of the development of the mouse cochlear epithelium at the single cell level. Nat Commun. 2020;11(1):2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu J, Ueno H, Xu CY, Chen B, Weissman IL, Xu PX. Identification of mouse cochlear progenitors that develop hair and supporting cells in the organ of Corti. Nat Commun. 2017;8:15046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li XJ, Doetzlhofer A. LIN28B/let‐7 control the ability of neonatal murine auditory supporting cells to generate hair cells through mTOR signaling. Proc Natl Acad Sci USA. 2020;117(36):22225‐22236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shi F, Kempfle JS, Edge ASB. Wnt‐responsive Lgr5‐expressing stem cells are hair cell progenitors in the cochlea. J Neurosci. 2012;32(28):9639‐9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lenz DR, Gunewardene N, Abdul‐Aziz DE, et al. Applications of Lgr5‐positive Cochlear progenitors (LCPs) to the study of hair cell differentiation. Front Cell Dev Biol. 2019;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chai R, Kuo B, Wang T, et al. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci USA. 2012;109(21):8167‐8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McLean WJ, McLean DT, Eatock RA, Edge ASB. Distinct capacity for differentiation to inner ear cell types by progenitor cells of the cochlea and vestibular organs. Development. 2016;143(23):4381‐4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cheng C, Guo L, Lu L, et al. Characterization of the transcriptomes of Lgr5+ hair cell progenitors and Lgr5‐ supporting cells in the mouse cochlea. Front Mol Neurosci. 2017;10:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Waqas M, Guo L, Zhang S, et al. Characterization of Lgr5+ progenitor cell transcriptomes in the apical and basal turns of the mouse cochlea. Oncotarget. 2016;7(27):41123‐41141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang S, Zhang Y, Yu P, et al. Characterization of Lgr5+ progenitor cell transcriptomes after neomycin injury in the neonatal mouse cochlea. Front Mol Neurosci. 2017;10:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shu Y, Li W, Huang M, et al. Renewed proliferation in adult mouse cochlea and regeneration of hair cells. Nat Commun. 2019;10(1):5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cox BC, Chai R, Lenoir A, et al. Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development. 2014;141(4):816‐829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Waqas M, Zhang S, He Z, Tang M, Chai R. Role of Wnt and notch signaling in regulating hair cell regeneration in the cochlea. Front Med. 2016;10(3):237‐249. [DOI] [PubMed] [Google Scholar]
- 30. Tao Y, Liu X, Yang L, et al. AAV‐ie‐K558R mediated cochlear gene therapy and hair cell regeneration. Signal Transduct Target Ther. 2022;7(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Choi KM, Kim JY, Kim Y. Distribution of the Immunoreactivity for glycoprotein M6B in the neurogenic niche and reactive glia in the injury penumbra following traumatic brain injury in mice. Exp Neurobiol. 2013;22(4):277‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Drabek K, Van De Peppel J, Eijken M, Van Leeuwen JPTM. GPM6B regulates osteoblast function and induction of mineralization by controlling cytoskeleton and matrix vesicle release. J Bone Miner Res. 2011;26(9):2045‐2051. [DOI] [PubMed] [Google Scholar]
- 33. Jones JM, Montcouquiol M, Dabdoub A, Woods C, Kelley MW. Inhibitors of differentiation and DNA binding (ids) regulate Math1 and hair cell formation during the development of the organ of Corti. J Neurosci. 2006;26(2):550‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCullar JS, Oesterle EC. Cellular targets of estrogen signaling in regeneration of inner ear sensory epithelia. Hear Res. 2009;252(1–2):61‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li W, Wu J, Yang J, et al. Notch inhibition induces mitotically generated hair cells in mammalian cochleae via activating the Wnt pathway. Proc Natl Acad Sci USA. 2015;112(1):166‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carlson ME, Hsu M, Conboy IM. Imbalance between pSmad3 and notch induces CDK inhibitors in old muscle stem cells. Nature. 2008;454(7203):528‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ye Z, Su Z, Xie S, et al. Yap‐lin28a axis targets let7‐Wnt pathway to restore progenitors for initiating regeneration. elife. 2020;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Varelas X, Sakuma R, Samavarchi‐Tehrani P, et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem‐cell self‐renewal. Nat Cell Biol. 2008;10(7):837‐848. [DOI] [PubMed] [Google Scholar]
- 39. Liu S, Li S, Zhu H, Cheng S, Zheng QY. A mutation in the cdh23 gene causes age‐related hearing loss in Cdh23nmf308/nmf308 mice. Gene. 2012;499(2):309‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang X, Qi J, Zhang L, et al. The role of Espin in the stereocilia regeneration and protection in Atoh1‐overexpressed cochlear epithelium. Cell Prolif. 2023;56(11):e13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hwang LH, Lau LF, Smith DL, et al. Budding yeast Cdc20: a target of the spindle checkpoint. Science. 1998;279(5353):1041‐1044. [DOI] [PubMed] [Google Scholar]
- 42. Yang LM, Stout L, Rauchman M, Ornitz DM. Analysis of FGF20‐regulated genes in organ of Corti progenitors by translating ribosome affinity purification. Dev Dyn. 2020;249(10):1217‐1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang Y, Kim AH, Yamada T, et al. A Cdc20‐APC ubiquitin signaling pathway regulates presynaptic differentiation. Science. 2009;326(5952):575‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Feng Z, Mabrouk I, Msuthwana P, et al. In ovo injection of CHIR‐99021 promotes feather follicles development via activating Wnt/beta‐catenin signaling pathway during chick embryonic period. Poult Sci. 2022;101(6):101825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data associated with this study are present in the paper.


