Abstract
Natural killer (NK) cells are important immune cells in the organism and are the third major type of lymphocytes besides T cells and B cells, which play an important function in cancer therapy. In addition to retaining the tumor cell killing function of natural killer cells, natural killer cell-derived exosomes cells also have the characteristics of high safety, wide source, easy to preserve and transport. At the same time, natural killer cell-derived exosomes are easy to modify, and the engineered exosomes can be used in combination with a variety of current cancer therapies, which not only enhances the therapeutic efficacy, but also significantly reduces the side effects. Therefore, this review summarizes the source, isolation and modification strategies of natural killer cell-derived exosomes and the combined application of natural killer cell-derived engineered exosomes with other antitumor therapies, which is expected to accelerate the clinical translation process of natural killer cell-derived engineered exosomes in cancer therapy.
Keywords: Natural killer cells, Cancer therapy, Natural killer cell-derived exosomes, Modification, Clinical translation
Introduction
More than four decades ago, natural killer (NK) cells were identified as lymphocytes with the innate ability to lyse tumor cells, destroying target cells without prior sensitization, without MHC restriction, and with a fast response rate, acting early in the immune response, and being responsible for killing abnormal cells such as aging cells and tumor cells in the body [1–4].Natural killer cells are a specific immune effector cell type which is an important component of tumor immune surveillance, plays a key role in immune activation against aberrant cells, has innate virulence and immunomodulatory capacity, and accounts for 5–20% of circulating lymphocytes in the body [5].
With a deeper understanding of natural killer cells, researchers have come to realize that natural killer cell-derived exosomes have many unique advantages in addition to retaining most of their tumor-killing functions [6, 7]. For example, it is easy to modify, including physical modification, chemical modification, biological modification and immune modification, to enhance certain properties of natural killer cell-derived exosome [8, 9]. Second, Natural killer cell-derived exosomes are relatively safe in tumor therapy, as cell-based therapies, including NK cell-based therapies, carry the risk of triggering a “cytokine storm,” which may force patients to suspend treatment and, in some cases, may even be life-threatening; however, the use of natural killer cell-derived exosomes may not be accompanied by such serious side effects [10, 11]. Meanwhile, compared with natural killer cells, natural killer cell-derived exosomes are also widely available and easy to store and transport [12, 13]. These advantages make natural killer cell-derived exosomes show a strong potential for clinical application.
What is more, natural killer cell-derived exosomes can be used as a new drug delivery platform, which, in addition to their own anti-tumor efficacy, can also reduce the side effects of treatments such as radiotherapy [14]. Meanwhile, in the process of tumor treatment, we have already realized that each tumor treatment method has its own advantages and disadvantages, and a “one-size-fits-all” treatment method does not yet exist [15]. Therefore, researchers must choose to integrate multiple strategies to enhance the effectiveness of tumor therapy, and natural killer cell-derived exosomes have the ability to work in conjunction with other tumor treatments, which are often more effective and have fewer side effects [16]. In conclusion, this paper reviews the roles of natural killer cell-derived exosomes, working modes, and recent advances in engineered exosomes, as well as summarizes the clinical value and application of natural killer cell-derived exosomes based on natural killer cell-derived exosomes, which is expected to accelerate the clinical translation of natural killer cell-derived exosomes in targeted cancer therapies.
Origin of natural killer cell and cancer immunotherapy
Natural killer cells come from a wide range of sources and can currently be obtained from peripheral blood, cord blood, pluripotent stem cells and NK cell lines (Table 1) [17]. Based on their sources and efficacy in cancer treatment, several NK cell lines have been established internationally over the years, including HANK1, KHYG-1, NK92, NK92MI, NKL, NKT, NK-YS and YT, these cell lines have often contain different molecules that are capable and therapeutic potential, greatly expanding the scope of action of natural killer cells [18]. This review will summarize the origins as well as the role of natural killer cells in cancer immunotherapy.
Table 1.
Comparison of commonly used allogeneic NK cell sources
| NK cell source | Advantages | Limitations | Status of CAR NK cell immunotherapy programme |
|---|---|---|---|
| Cord blood | Easily collected and highly amplified in vivo | Heterogeneous with poor genetic modification | Reported [31] |
| Pluripotent stem cells | High proliferation capacity, homogeneous cell population, ability to cryopreserve UCBs | Immature phenotype, only modified to fulfill ADCC role | Reported [32] |
| Peripheral blood | Mature phenotype, highly cytotoxic | Requires in vivo amplification and is not readily obtained | Unreported |
| NK cell line | Homogeneous cell population, can give multiple doses, easy to expand, easy to genetically modify | Lack of certain receptors, no ADCC effect, limited number of generations amplified in vivo | Unreported |
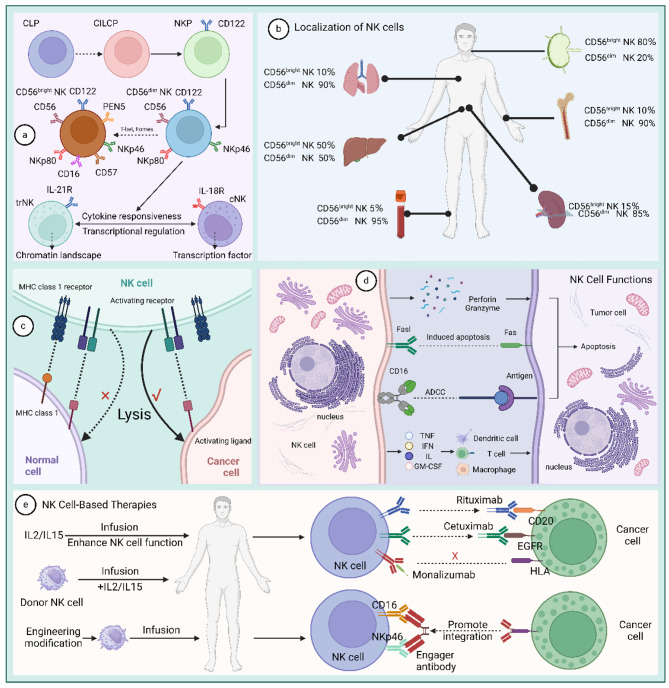
Natural killer cells originate from common lymphoid progenitors (CLP), pass through a common ILC precursor stage (CILCP), and eventually develop into NK progenitors (NKP) [19]. Natural killer cells are characterized by the expression of CD122, loss of CD34 and CD127, and its further differentiation is dependent on the expression of T-bet and Eomes. Based on CD56 expression, human NK cells are usually subdivided into two main subpopulations: the CD56brightCD16dim/− and the CD56dimCD16, the former traditionally considered to be more cytokine-producing and less mature, and the latter, more cytotoxic and maturer Higher. Both subpopulations express the activating surface receptors NKp4 and NKp80, and the transformation of CD56bright NK cells to CD56dim NK cells is achieved by expression of CD16, PEN5, and CD57 [5, 19–21]. The bone marrow is the primary site of natural killer cell development and may also develop and mature in stimulated lymphoid organs, including the tonsils, spleen, and lymph nodes [22]. Subsequently, based on their tissue-resident properties, natural killer cells can be classified into classical natural killer cells with cycling properties and tissue-resident natural killer cells [23].
Only activated natural killer cells can function [24]. The surface of natural killer cells contains a range of inhibitory and activating surface receptors, and its activation is dependent on ligand/receptor interactions, the dynamic balance between inhibitory and activating receptors on the cell surface ensures the regulation of NK cell effector functions (Table 2) [25]. Healthy cells express few ligands that activate natural killer cells, but express high levels of major histocompatibility complex class I molecules (MHC I), which bind to the killer immunoglobulin-like (KIR) family of inhibitory receptors on natural killer cells in order to protect them from natural killer cell attack [26]. In tumor cells, the expression level of MHC I is down-regulated, and the expression level of natural killer cell-activating ligands is up-regulated, thereby activating natural killer cells [27].
Table 2.
Common human NK cell receptors and their ligands
| Active/ Inhibitory | Receptor | Ligands |
|---|---|---|
| Active | NCR1 | HS GAGs, Complement factor P, vimentin, viral HA |
| NCR2 | PCNA, Syndecan-4, Nidogen-1, viral HA, HS GAGs, PDGF-DD, 21spe-MLL5 | |
| NCR3 | B7-H6, galectin-3, GAGs, viral hemagglutinin, pp65 | |
| CD16 | Fc portion of IgG antibodies | |
| NKG2D | MICA, MICB and UL16-binding proteins | |
| DNAM1 | PVR, nectin-2 | |
| Inhibitory | KIR2DL1 | HLA-C, group 2 |
| KIR2DL2/3 | HLA-C, group 1 | |
| KIR3DL1 | HLA-Bw4 | |
| KIR3DL2 | HLA-A3, A11 | |
| NKG2A | HLA-E |
Due to these properties, natural killer cell-based immunotherapies are gradually being developed. The first NK cell-based therapies were discovered in hematopoietic stem cell transplants (HSCTs), in which NK cells have the ability to exert graft-versus-leukemia effects. A study by Ruggeri et al. found that KIR-mismatched allogeneic-reactive donor NK cells protected bone marrow transplanted AML patients from AML relapse while avoiding graft-versus-host disease (GVHD) [28, 29]. The study by Miller et al. pioneered the use of natural killer cells in a non-transplant setting, further demonstrating the impact of effective lymphocyte depletion preconditioning on NK cell expansion and persistence in vivo [30]. This was followed by the gradual emergence of Chimeric Antigen Receptor (CAR)-NK cells as an alternative to CAR-T therapy and the mainstream use of natural killer cells for immunotherapy [5]. With new tools for genetic engineering approaches and new understanding of NK cell biology, NK-based immunotherapies are bound to show greater potential in preclinical and clinical development. Also, natural killer cell-derived exosomes will be used for cancer immunotherapy (Fig. 1).
Fig. 1.
Origin of natural killer cell and cancer immunotherapy. (a) The origin of natural killer cells. (b) Localization of NK cells. (c) Natural killer cells function selectively. (d) Four ways natural killer cells fight tumors. (e) Natural killer cell-based therapies
Spatio-temporal specificity of natural killer cell-derived engineered exosomes
Isolation of natural killer cell-derived exosomes
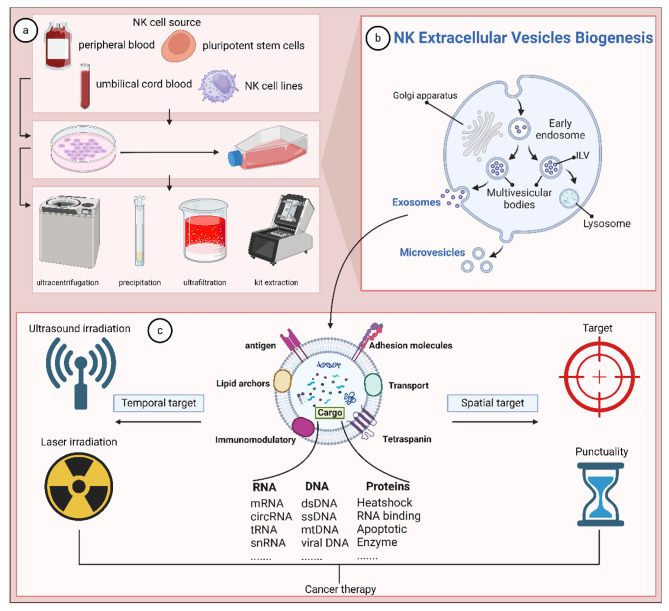
The isolation of exosomes is important for the study of mechanistic and clinical research. Currently, popular methods of exosome isolation include ultracentrifugation, kit extraction, ultrafiltration, precipitation, and so on. Among them, the immunosorbent method utilizes antigen-antibody interactions to extract exosomes, which is not only highly specific, but also yields a higher rate of exosomes than ultracentrifugation [33]. To further improve this method, Li et al. utilized sub-micron level magnetic particles for immunoaffinity, which resulted in exosome yields that were 10–15 times higher than the ultracentrifugation method [34]. The immunoaffinity method has no body level limitation and has potential for clinical application, but the high cost and complexity of the technique limit its use in the clinic [33].
Recently, a microfluidic technique based on fluid properties for exosome separation has gradually come into the limelight. Microfluidics enables a fusion of traditional and emerging technologies, including the usual separation determinants such as size, density, and immunoaffinity, as well as innovative sorting mechanisms such as electromagnetic manipulation, nanowire-based traps, acoustics, electrophoresis, nano-size-deterministic transverse displacement, and viscoelastic flow [35, 36]. For example, the micro- and nanofluidic device designed by Davies RT et al. uses mis-flow filtration to separate and capture liposarcoma-derived exosomes. Its combination of differently sized separation units and CD63-based antibody immunoaffinity resulted in a 5-fold increase in the number of key liposarcoma-associated extracellular vesicle cargoes within 30 min [36–38].
There is no absolute best isolation method for exosomes, and its isolation results are closely related to downstream applications and scientific issues, but high recovery and high specificity are two recognized basic requirements [39]. At the same time, we also have to consider purity, yield, cost and many other aspects. Therefore, conventional methods alone usually cannot meet the separation requirements, and the integration of basic methods such as microfluidics is essential for the separation of exosomes with high purity and high yield (Table 3) (Fig. 2).
Table 3.
Common Exosome Isolation Techniques
| Technique | Specificity/ Recovery rate | Advantages | Limitations | Reference |
|---|---|---|---|---|
| Asymmetric flow field-flow fractionation | High/Low | Time-saving, reproducible, fully automated assay, simulates physiological conditions | Small sample volume and low throughput | [40, 41] |
| Density gradient centrifugation | High/Low | Higher purity and biostructural integrity | Preliminary work is tedious, complicated, time-consuming, and difficult to remove high-density chemicals. | [42–44] |
| Hydrostatic filtration dialysis | Middle/ Middle | Low cost, no chemical contamination, high throughput | Extraction requires additional sterilization and is less efficient with larger sample sizes | [45] |
| Immunoaffinity-based isolation methods | High/Low | High purity, high specificity, good integrity of isolated exosomes | High cost, low throughput and separation efficiency, only for cell-free samples | [46] |
| Mass density-based ultracentrifugation | Low/ High | Simple operation, high throughput, no chemical contamination | Instruments are costly, time-consuming, and prone to mixing with other types of EVs to interfere with subsequent analyses. | [47–50] |
| Magneto-immunoprecipitation | High/Low | Simple operation and good reproducibility | High cost, low flux, low biological activity | [34] |
| Microfluidic | High/Low | Portability and purity with low reagent and sample consumption to combine exosome extraction and analysis | Lack of standardized methodology, high cost, complex equipment, low throughput | [51, 52] |
| Size-based isolation methods Ultrafiltration | Middle/ Middle | Low instrument requirements, no chemical reagent pollution, time-saving and efficient | Soluble proteins are difficult to remove, the purity, shape and charge of the sample can affect the separation, low biological activity | [53] |
| Size-exclusion chromatography | Middle/ Middle | High purity, sensitivity, integrity and bioactivity without chemical contamination | High instrument cost, low throughput, time-consuming, low purification yields | [54, 55] |
| Precipitation | Low/ High | High yield, high recovery, high integrity without special instruments or techniques | Complicated sample preparation procedures, difficult to remove isolated exosomal lipoproteins, non-uniform particle size, exosomes are easily damaged | [33, 56] |
Fig. 2.
Spatio-temporal specificity of natural killer cell-derived engineered exosomes. (a) Sources and methods of isolation of natural killer cell-derived exosomes. (b) Biogenesis of natural killer cell-derived exosomes. (c) Natural killer cell-derived exosomes are spatiotemporally specific in their action
Spatio-temporal specificity
Exosomes of natural killer cell origin containing specific targeting molecules modified to enable precise drug delivery. At the same time, the point in time at which the engineered exosomes release their cargo can be controlled by additional physical influences, such as laser and ultrasound radiation [16]. Whiteside et al. demonstrated that exosomes of all cell origins can cross the blood-brain barrier at different rates [57]. Liu et al. designed ExoCAR/T7@Micelle (where Micelle is PEG-TK-Ce6@RSL3) that allows them to precisely target to HER2-positive breast cancer cells. Compared to exosomes without targeting molecules, engineered exosomes trigger more intense tumor. Meanwhile, for internal micellar structures, the Photodynamic therapy (PDT) strategy was used to generate cytotoxic reactive oxygen species (ROS) and facilitate ROS-triggered drug release with the help of the photosensitizer chlorine e6 (Ce6) and the ROS-sensitive linkage of the chemical thioketone of bone (TK) [16]. The above engineering modifications enabled the treatment of HER2-positive breast cancer brain metastases.
Taken together, the targeted delivery of engineered exosomes of natural killer cell origin depends on surface targeting molecules that allow full access of the exosomes into tumor cells and lower concentrations in healthy organs. And by additional physical influences the engineered exosomes release biologically active therapeutic molecules at specific times. Thus, engineered exosomes of natural killer cell origin are able to prolong the survival time of tumor patients and are expected to open new frontiers for modern drug delivery (Fig. 2).
Natural killer cell-derived engineered exosomes can serve as drug delivery platforms
Natural killer cell-derived exosomes exert tumor-killing functions
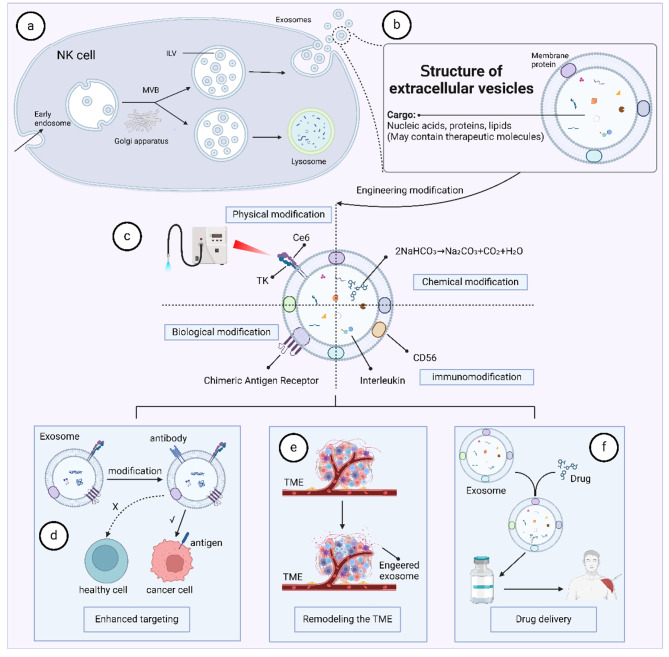
Natural killer cell-derived exosomes are able to kill tumors through several pathways. One, natural killer cell-derived exosomes contain a variety of cleavage particles (perforin, granzyme, granulocyte fusion) that induce target cell death [58]. Second, natural killer cell-derived exosomes contain a series of cytokines (TNF-α, IL-10, IFN-Y), chemokines (CCL3, CCL4, CCL5, XCL1), and growth factors (GM-CSF), which can interact with macrophages and dendritic cells to exert immune-response effects [59]. Third, natural killer cell-derived exosomes contain multiple members of the tumor necrosis factor superfamily, such as FASL, which can induce apoptosis in target cells by binding to the corresponding receptor (such as FAS) [60]. Fourth, natural killer cell-derived exosomes can also mediate antibody-dependent cell-mediated cytotoxicity (ADCC), which mediates the direct action of killer cells on target cells. Fifth, natural killer cell-derived exosomes contain a variety of therapeutic molecules such as miR-1249-3p [61]. Thus, natural killer cell-derived exosomes can themselves be used as a drug for tumor therapy.
Strategies for engineering modification of natural killer cell-derived exosomes
A variety of modifications can be added to natural killer cell-derived exosomes to enhance or confer multiple capabilities. For one, natural killer cell-derived exosomes containing physical modifications enable more precise tumor-targeting effects, such as laser irradiation. Liu et al. fused natural killer cell-derived exosomes with the photosensitizer chlorine e6 (Ce6) and ROS-sensitively linked chemical thioketone (TK), and in the case of laser irradiation the TK was destroyed by the ROS generated by Ce6. As a result, exosomes containing therapeutic molecules can be delivered and released with precision [16]. Second, exosomes can be chemically modified. For example, Thuy et al. encapsulated NaHCO3 (sodium bicarbonate, SBC) and paclitaxel into exosomes, which produce carbon monoxide and cleave when swallowed by tumor cells, thereby effectively releasing the drug [62]. Third, natural killer cell-derived exosomes can be biologically modified, and the most widely used strategy is membrane modification to enhance targeting. Liu et al. fused natural killer cell-derived exosome membranes with CAR modification and a T7 peptide (sequence HAIYPRH) to achieve precision delivery of engineered exosomes [16]. Fourth, natural killer cell-derived exosomes can be immunomodified to enhance their anti-tumor immune function.CD56 is not only a marker of natural killer cells, but also has powerful immunostimulatory effector functions, including the production of paracrine T-cell factor 1 and highly efficient cytotoxicity [63]. Fabbri et al. used CD56 antibody to modify natural killer cell-derived exosomes and enhanced the natural cytotoxicity of natural killer cell-derived exosomes and ultimately treated neuroblastoma by combination therapy [64]. In conclusion, natural killer cell-derived exosomes have the advantages of easy operation, high safety, and wide source, etc. In addition to their own tumor-killing function, they are also very suitable to be used as drug delivery carriers, which is expected to open up a new platform for future drug delivery (Fig. 3).
Fig. 3.
Natural killer cell-derived engineered exosomes can serve as drug delivery platforms. (a, b) Source and structure of natural killer cell-derived exosomes. (c) Engineering modification strategies for natural killer cell-derived exosomes. (d, e, f) Engineering modification strategies to confer or enhance novel properties of natural killer cell-derived exosomes
Synergies of engineered natural killer cell-derived exosomes with other tumor therapies
Radiotherapy
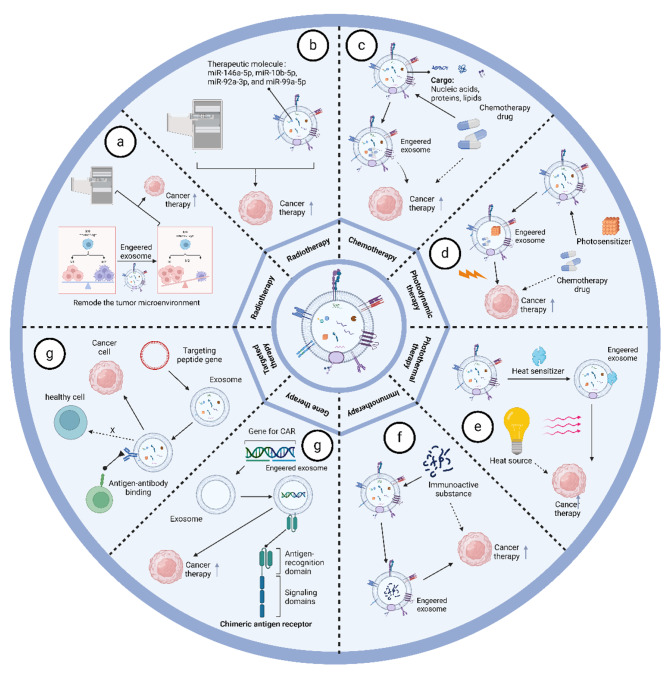
Radiation therapy plays an important role in the treatment of almost all solid cancers, with wide clinical application and satisfactory results, but there are still some obstacles limiting the efficacy of treatment, such as chemotherapy resistance. An example of the combination of radiation therapy with exosomes of natural killer cell origin is that they contain molecules that activate radio sensitization pathways [65]. Zhu et al. showed that exosomes of natural killer cell origin can enter tumor cells and improve the efficacy of antitumor radiation therapy, such as in melanoma [18]. Combined with microRNA sequencing results of natural killer cell-derived exosomes, we found that natural killer cell-derived exosomes contain molecules such as miR-146a-5p, miR-10b-5p, miR-92a-3p, and miR-99a-5p, which are capable of specifically attenuating tumor radio resistance involving multiple molecular pathways, such as the ATM/ ATR serine/threonine kinases, protein kinase B/mTOR and Janus kinase. Thus, natural killer cell-derived exosomes are able to increase the sensitivity of a wide range of tumors to radiotherapy by transducing miRNAs into tumor cells [66–70] (Fig. 4).
Fig. 4.
Synergies of engineered natural killer cell-derived exosomes with other tumor therapies. (a, b) Combined potentiation of natural killer cell-derived engineered exosomes with radiotherapy. (c) Natural killer cell-derived engineered exosomes facilitate chemotherapeutic drug delivery. (d) Natural killer cell-derived engineered exosomes are used in combination with photodynamic therapy to promote precise drug release. (e) Natural killer cell-derived engineered exosomes are used in combination with photothermal therapy treatment to control the release of tumor drugs. (f) Natural killer cell-derived engineered exosomes deliver immune substances into tumor cells. (g) Natural killer cell-derived engineered exosomes enter tumor cells and regulate gene expression. (h) Natural killer cell-derived engineered exosomes for precision therapy
Chemotherapy
Chemotherapy is a common form of tumor treatment and is often administered systemically due to the lack of targeting of chemotherapeutic agents. Systemic administration of chemotherapeutic agents leads to various TRAEs, including cardiotoxicity and frontal peripheral sensory neuropathy, and reduced anti-tumor efficiency [71–74]. Therefore, combination with engineered exosomes of natural killer cell origin would be a solution to these challenges. Cisplatin is a broad-spectrum chemotherapeutic agent that can be encapsulated into natural killer cell-derived exosomes. Zhao et al. showed that exosomes derived from natural killer cells express protein markers typical of natural killer cells, can be preferentially taken up by SKOV3 cells, and show cytotoxicity to OC cells. Meanwhile, the combined use of the chemotherapeutic drug cisplatin can sensitize drug-resistant ovarian cancer cells to the frontal proliferative effect of cisplatin and achieve significant anti-tumor effects with less damage to major organs [75]. Paclitaxel is another commonly used chemotherapeutic agent, and the results of Gao et al. demonstrated that the use of natural killer cell-derived exosomes in combination with paclitaxel effectively inhibited tumor cell proliferation induced apoptosis and reduced damage to normal cells [76]. In conclusion, combination therapy is able to significantly enhance its tumor targeting and delivery capabilities compared to chemotherapeutic agents alone. At the same time, it can trigger stronger anti-tumor activity and less systemic toxicity (Fig. 4).
Photodynamic therapy
Photodynamic therapy is a minimally invasive cancer treatment that uses ROS produced by photosensitizers to treat tumors [77]. A distinguishing feature of photodynamic therapy is its ability to sensitize cancer immunotherapy with the controlled release of therapeutic molecules encapsulated in exosomes [78]. An example of photodynamic therapy based on natural killer cell-derived exosomes is ExoCar/T7@Micelle designed by Liu et al. ExoCar/T7@Micelle is a bionic nano-bomb that facilitates ROS-triggered drug release with the help of the photosensitizer chlorine e6 (Ce6) and ROS-sensitively linked chemical thioketone of bone (TK) to achieve precision strikes against breast cancer tumors through double targeting by to achieve a precise strike against breast cancer tumor cells [16]. Loading photosensitizers into engineered exosomes is the key to achieving precision therapy, and photodynamic therapy, which releases therapeutic molecules from exosomes at a specific time and space compared to conventional administration, is the key to alleviating TRAE. At the same time, we can also use photodynamic therapy to selectively kill tumor cells. Another example is the LASNEO system developed by Huang et al. The LASNEO system is prepared by loading hydrophilic siRNA into natural killer cell-derived exosomes, which are then incubated with a hydrophobic photosensitizer of chlorine e6. Under 660 nm laser irradiation, an effective PDT effect is produced. In addition, ROS disrupts the endolysosomal membrane and promotes the release of siRNA from exosomes, which in turn silences the PD-L1 gene [79]. In conclusion, photodynamic therapy used in combination with exosomes can enhance its anti-tumor effects (Fig. 4).
Photothermal therapy
Nanomaterial-based photothermal therapies (PTT) have entered clinical studies for some solid tumor types compared to traditional tumor treatments [80–82]. Photothermal therapy does not rely on oxygen and eliminates solid tumors by overcoming the hypoxic tumor microenvironment [83]. A range of photothermal agents have been developed such as carbon nanotubes, and double-based nanoparticles [84, 85]. However, these materials have significant drawbacks, including complex synthesis processes and low biodegradability. Meanwhile, the tumor-killing efficacy of photothermal therapy requires high temperatures in order to ablate tumor tissues and overcome heat shock protein-induced thermotolerance, which is highly susceptible to thermal damage to normal organs near tumors [86]. To address these problems, Liu et al. designed an integrated photothermal therapy based on natural killer cells. A novel PTA was constructed through the coordination of tetrahydroxyanthraquinone and Mn, and by further adsorption of polyetherimide/DNAzymes on the surface, DNAzymes@Mn-CONASHs exhibited excellent photothermal conversion, enhanced tumor microenvironmental T1-MRI-directed ability, and thermal durability resistance. At the same time, the researchers used artificially engineered NK cells with HCC-specific targeting of TLS11a-adaptor modifications to obtain engineered exosomes for specific elimination of any possible residual tumor cells after PTT [87]. This combination therapy has achieved excellent and significantly improved anti-tumor efficiency in vivo. Another example of the use of photothermal therapy in conjunction with natural killer cell-derived exosomes is the study by Li et al. Granzyme B is a serine protease enriched in natural killer cells and their exosomes. Granzyme B is able to enter target cells in the presence of perforin and induce apoptosis in target cells [88–91]. Li et al. designed exosome-based and photothermal-sensitive liposome fusion nanoplatforms with excellent cascade tumor-targeting and cytotoxicity properties under laser irradiation, showing excellent therapeutic efficacy [92]. In conclusion, the combination of exosomes and photothermal therapy significantly increases the penetration capacity of photothermal therapy and reduces the use of laser dose compared with conventional photothermal therapy, thus enhancing the tumor treatment outcome (Fig. 4).
Immunotherapy
Immunotherapy refers to the method of causing the body to produce an immune response through active or passive means, specifically and efficiently exerting the function of suppressing and killing tumor cells. Unlike traditional treatment methods, immunotherapy does not kill the cells directly, but mobilizes the immune cells in the interior of the body. Given the properties of natural killer cell-derived exosomes, the combined use of engineered exosomes with immunotherapy is of increasing interest. For example, Shoae et al. showed that natural killer cell-derived exosomes were able to modify the expression of the natural cytotoxicity receptor of NK cells in vivo, making them more toxic to neuroblastoma cells, which led to much more effective immunotherapy [93]. Natural killer cell-derived exosomes also stimulate T cells, monocytes, and substances that act on the TGF-β pathway, thereby attenuating immunosuppression and enhancing immunotherapy [94–96]. Natural killer cell-derived exosomes can carry specific targeting molecules into tumor cells to perform their killing function, in addition to their own tumor-killing function. Neviani et al. showed that exosomes released by NK cells contain miR-186, a tumor growth inhibitor. miR-186 interferes with TGF-β-mediated inhibition of NK function by decreasing NB cell migration and proliferation through the targeting and down-regulation of TGFβR1, TGFβR2, and SMAD3. In addition, Neviani et al. blocked TGF-β-mediated inhibition by specifically delivering miR-186 mimics to NK cells using nanoparticles loaded with CD56 antibody. From these results, it is clear that natural killer cell-derived exosomes maintain their properties even under immunosuppressive conditions, suggesting that they can be used in combination with immunotherapy to improve the prognosis of patients with high-risk neuroblastoma [64]. The tumor immunosuppressive microenvironment hampers the effectiveness of immunotherapy, and the use of natural killer cell-derived exosomes to remodel the tumor immune microenvironment has received increasing attention. The tumor immunosuppressive microenvironment hinders the effectiveness of immunotherapy, and the use of natural killer cell-derived exosomes to remodel the tumor immune microenvironment has received increasing attention.Huang et al. developed a light-activated silencing NK-derived exocytosis (LASNEO) system. Not only achieved effective PDT, but also promoted macrophage reprogramming to M1 phenotype and mature dendritic cells, and activated CD4 + T cells and CD8 + T cells in TME through PD-L1 inhibition [79]. In summary, our understanding of the tumor microenvironment has advanced considerably over the past few decades, which provides a wealth of potential targets for engineering exosomes. In the future, the use of natural killer cell-derived exosomes in combination with immunotherapy will be a great addition to the field of cancer treatment efficacy (Fig. 4).
Gene therapy
Gene therapy is a biological treatment method in which exogenous normal genes are introduced into target cells through gene transfer technology to correct or compensate for diseases caused by genetic defects and abnormalities, and ultimately achieve therapeutic goals [97, 98]. Exosomal non-coding RNAs (e.g. miRNAs) have shown great therapeutic potential in a variety of cancers and can be used as gene expression vectors for tumor therapy [99–101]. However, the poor controllability of the expression level and location of expression is a major obstacle to the use of non-coding RNAs for gene therapy at present. For example, Wang et al. delivered miR-1249-3p using natural killer cell-derived exosomes to regulate insulin resistance by directly targeting SKOR1 to regulate the formation of the ternary complex SMAD6/MYD88/SMURF1 and inhibit the TLR4/NF-κB signaling pathway to mediate glucose homeostasis [61]. In another study, Hu et al. delivered miR-207 using natural killer cells. miR-207 interacted with TLR4 and inhibited NF-κB signaling in astrocytes, reducing the release of pro-inflammatory cytokines and alleviating depressive symptoms [102]. Thus, the therapeutic efficacy of non-coding RNAs in disease has been demonstrated, and the combination of gene therapy with exosomes of natural killer cell origin has improved therapeutic efficacy and accelerated the use of gene therapy in clinical trials (Fig. 4).
Targeted therapy
The targeted therapy of cancer refers to the design of drugs at the cellular or molecular level against known cancer-causing sites, which specifically enter the receptor cells and act to cause the specific death of tumor cells without affecting the normal cells surrounding the tumor. In recent years, CAR-T cell-derived exosomes have gained a great deal of attention due to their more potent anti-tumor effects [103, 104]. Recently, the study by Liu et al. successfully applied chimeric antigen receptors to natural killer cell-derived exosomes, aiming to enhance the effectiveness of antitumor therapy by disrupting the iron death defense mechanism. The modification of transferrin receptor-binding peptide and the expression of CAR on the exosome surface successfully crossed the blood-brain barrier and facilitated the release of therapeutic molecules at specific sites and times [16]. Compared to CAR-T cell-derived exosomes, CAR-NK cell-derived exosomes offer several advantages: safer, multiple tumor-killing mechanisms, more widely available, and relatively homogeneous and efficient quality [105] (Fig. 4).
Conclusion
Currently, many studies on exosomes of natural killer cell origin have demonstrated their potential as drug delivery, as well as their ability to be used in combination with other tumor therapeutics and to improve therapeutic efficacy. However, exosomes of natural killer cell origin also have certain limitations, such as the differentiation potential of natural killer cells, and the exosomes secreted by them may be heterogeneous, affecting the therapeutic effect of the disease. The emergence of exosome engineering technology provides new methods and ideas to solve this problem, and how to choose the appropriate exosome modification strategy will be a new direction for future tumor therapy. In conclusion, in view of the current maturity of engineered exosome technology, natural killer cell-derived exosomes containing engineered modifications will certainly provide a new method and idea for tumor therapy.
Acknowledgements
Not applicable.
Abbreviations
- NK
Natural killer
- CLP
lymphoid progenitors
- CILCP
ILC precursor stage
- NKP
NK progenitors
- PDT
Photodynamic therapy
- Ce6
Chlorine e6
- GVHD
Graft-versus-host disease
- ROS
Reactive oxygen species
- CAR
Chimeric Antigen Receptor
- PTT
Photothermal therapies
Author contributions
CS wrote the manuscript. XM and JG revised the manuscript. All authors have read and approved the final draft.
Funding
Not applicable.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval
Not applicable.
Consent for publication
All authors consent to publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jianen Gao, Email: gaojianen@nrifp.org.cn.
Xu Ma, Email: maxu@nrifp.org.cn.
References
- 1.Kiessling R, Klein E, Wigzell H. Natural killer cells in the mouse. I. cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–7. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 2.Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. distribution of reactivity and specificity. Int J Cancer. 1975;16:216–29. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- 3.Bald T, Krummel MF, Smyth MJ, Barry KC. The NK cell-cancer cycle: advances and new challenges in NK cell-based immunotherapies. Nat Immunol. 2020;21:835–47. doi: 10.1038/s41590-020-0728-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melaiu O, Lucarini V, Cifaldi L, Fruci D. Influence of the Tumor Microenvironment on NK cell function in solid tumors. Front Immunol. 2019;10:3038. doi: 10.3389/fimmu.2019.03038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu S, Galat V, Galat Y, Lee YKA, Wainwright D. Wu. NK cell-based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol. 2021;14:7. doi: 10.1186/s13045-020-01014-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zafarani A, Taghavi-Farahabadi M, Razizadeh MH, Amirzargar MR, Mansouri M, Mahmoudi M. The role of NK Cells and their exosomes in Graft Versus host Disease and Graft Versus Leukemia. Stem Cell Rev Rep. 2023;19:26–45. doi: 10.1007/s12015-022-10449-2. [DOI] [PubMed] [Google Scholar]
- 7.Fiore PF, Di Pace AL, Conti LA, Tumino N, Besi F, Scaglione S, et al. Different effects of NK cells and NK-derived soluble factors on cell lines derived from primary or metastatic pancreatic cancers. Cancer Immunol Immunother. 2023;72:1417–28. doi: 10.1007/s00262-022-03340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong Y, Klein Wolterink RGJ, Wang J, Bos GMJ, Germeraad WTV. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J Hematol Oncol. 2021;14:73. doi: 10.1186/s13045-021-01083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batista IA, Quintas ST, Melo SA. The interplay of exosomes and NK cells in Cancer Biology. Cancers (Basel). 2021; 13. [DOI] [PMC free article] [PubMed]
- 10.Dagher OK, Posey AD. Jr. Forks in the road for CAR T and CAR NK cell cancer therapies. Nat Immunol. 2023;24:1994–2007. doi: 10.1038/s41590-023-01659-y. [DOI] [PubMed] [Google Scholar]
- 11.Sadowski K, Olejarz W, Basak G. Modern advances in CARs therapy and creating a New Approach to Future Treatment. Int J Mol Sci. 2022; 23. [DOI] [PMC free article] [PubMed]
- 12.Cai Y, Li J, Jia C, He Y, Deng C. Therapeutic applications of adipose cell-free derivatives: a review. Stem Cell Res Ther. 2020;11:312. doi: 10.1186/s13287-020-01831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 2021;19:47. doi: 10.1186/s12964-021-00730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang PF, Gao C, Huang XY, Lu JC, Guo XJ, Shi GM, et al. Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma. Mol Cancer. 2020;19:110. doi: 10.1186/s12943-020-01222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18:75. doi: 10.1186/s12943-019-0991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tao B, Du R, Zhang X, Jia B, Gao Y, Zhao Y, et al. Engineering CAR-NK cell derived exosome disguised nano-bombs for enhanced HER2 positive breast cancer brain metastasis therapy. J Control Release. 2023;363:692–706. doi: 10.1016/j.jconrel.2023.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Fang F, Xie S, Chen M, Li Y, Yue J, Ma J, et al. Advances in NK cell production. Cell Mol Immunol. 2022;19:460–81. doi: 10.1038/s41423-021-00808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu L, Kalimuthu S, Gangadaran P, Oh JM, Lee HW, Baek SH, et al. Exosomes Derived from Natural Killer cells exert therapeutic effect in Melanoma. Theranostics. 2017;7:2732–45. doi: 10.7150/thno.18752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crinier A, Narni-Mancinelli E, Ugolini S, Vivier E. SnapShot: natural killer cells. Cell. 2020;180:1280–1280. doi: 10.1016/j.cell.2020.02.029. [DOI] [PubMed] [Google Scholar]
- 20.Freud AG, Mundy-Bosse BL, Yu J. Caligiuri. The Broad Spectrum of Human Natural Killer Cell Diversity. Immunity. 2017;47:820–33. doi: 10.1016/j.immuni.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abel AM, Yang C, Thakar MS, Malarkannan S. Natural killer cells: Development, Maturation, and clinical utilization. Front Immunol. 2018;9:1869. doi: 10.3389/fimmu.2018.01869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bjorkstrom NK, Ljunggren HG, Michaelsson J. Emerging insights into natural killer cells in human peripheral tissues. Nat Rev Immunol. 2016;16:310–20. doi: 10.1038/nri.2016.34. [DOI] [PubMed] [Google Scholar]
- 23.Hu L, Han M, Deng Y, Gong J, Hou Z, Zeng Y, et al. Genetic distinction between functional tissue-resident and conventional natural killer cells. iScience. 2023;26:107187. doi: 10.1016/j.isci.2023.107187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnon TI, Markel G, Mandelboim O. Tumor and viral recognition by natural killer cells receptors. Semin Cancer Biol. 2006;16:348–58. doi: 10.1016/j.semcancer.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Tomaipitinca L, Russo E, Bernardini G. NK cell surveillance of hematological malignancies. Therapeutic implications and regulation by chemokine receptors. Mol Aspects Med. 2021;80:100968. doi: 10.1016/j.mam.2021.100968. [DOI] [PubMed] [Google Scholar]
- 26.Handgretinger R, Lang P, Andre MC. Exploitation of natural killer cells for the treatment of acute leukemia. Blood. 2016;127:3341–9. doi: 10.1182/blood-2015-12-629055. [DOI] [PubMed] [Google Scholar]
- 27.Smyth MJ, Cretney E, Kelly JM, Westwood JA, Street SE, Yagita H, et al. Activation of NK cell cytotoxicity. Mol Immunol. 2005;42:501–10. doi: 10.1016/j.molimm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 28.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 29.Ruggeri L, Capanni M, Casucci M, Volpi I, Tosti A, Perruccio K, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94:333–9. doi: 10.1182/blood.V94.1.333.413a31_333_339. [DOI] [PubMed] [Google Scholar]
- 30.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 31.Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR-Transduced Natural Killer cells in CD19-Positive lymphoid tumors. N Engl J Med. 2020;382:545–53. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Hermanson DL, Moriarity BS. Kaufman. Human iPSC-Derived natural killer cells Engineered with chimeric Antigen receptors enhance anti-tumor activity. Cell Stem Cell. 2018;23:181–92. doi: 10.1016/j.stem.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zarovni N, Corrado A, Guazzi P, Zocco D, Lari E, Radano G, et al. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods. 2015;87:46–58. doi: 10.1016/j.ymeth.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 34.Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress Exosome Isolation Techniques Theranostics. 2017;7:789–804. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woo HK, Sunkara V, Park J, Kim TH, Han JR, Kim CJ, et al. Exodisc for Rapid, Size-Selective, and efficient isolation and analysis of Nanoscale Extracellular vesicles from Biological samples. ACS Nano. 2017;11:1360–70. doi: 10.1021/acsnano.6b06131. [DOI] [PubMed] [Google Scholar]
- 36.Contreras-Naranjo JC, Wu HJ. Ugaz. Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine. Lab Chip. 2017;17:3558–77. doi: 10.1039/C7LC00592J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies RT, Kim J, Jang SC, Choi EJ, Gho YS. Park. Microfluidic filtration system to isolate extracellular vesicles from blood. Lab Chip. 2012;12:5202–10. doi: 10.1039/c2lc41006k. [DOI] [PubMed] [Google Scholar]
- 38.Casadei L, Choudhury A, Sarchet P, Mohana Sundaram P, Lopez G, Braggio D, et al. Cross-flow microfiltration for isolation, selective capture and release of liposarcoma extracellular vesicles. J Extracell Vesicles. 2021;10:e12062. doi: 10.1002/jev2.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H, Lyden D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat Protoc. 2019;14:1027–53. doi: 10.1038/s41596-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018;20:332–43. doi: 10.1038/s41556-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113:E968–77. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeppesen DK, Hvam ML, Primdahl-Bengtson B, Boysen AT, Whitehead B, Dyrskjot L, et al. Comparative analysis of discrete exosome fractions obtained by differential centrifugation. J Extracell Vesicles. 2014;3:25011. doi: 10.3402/jev.v3.25011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JO, Kwon EJ, Song DW, Lee JS, Kim DH. miR-185 inhibits endoplasmic reticulum stress-induced apoptosis by targeting Na+/H + exchanger-1 in the heart. BMB Rep. 2016;49:208–13. doi: 10.5483/BMBRep.2016.49.4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Musante L, Tataruch D, Gu D, Benito-Martin A, Calzaferri G, Aherne S, et al. A simplified method to recover urinary vesicles for clinical applications, and sample banking. Sci Rep. 2014;4:7532. doi: 10.1038/srep07532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kukreja RC, Yin C, Salloum FN. MicroRNAs: new players in cardiac injury and protection. Mol Pharmacol. 2011;80:558–64. doi: 10.1124/mol.111.073528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu N, Zhang D, Xie H, Zhou Z, Chen H, Hu T, et al. Endothelial-specific intron-derived miR-126 is down-regulated in human breast cancer and targets both VEGFA and PIK3R2. Mol Cell Biochem. 2011;351:157–64. doi: 10.1007/s11010-011-0723-7. [DOI] [PubMed] [Google Scholar]
- 49.Momen-Heravi F, Balaj L, Alian S, Trachtenberg AJ, Hochberg FH, Skog J, et al. Impact of biofluid viscosity on size and sedimentation efficiency of the isolated microvesicles. Front Physiol. 2012;3:162. doi: 10.3389/fphys.2012.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Linares R, Tan S, Gounou C, Arraud N, Brisson AR. High-speed centrifugation induces aggregation of extracellular vesicles. J Extracell Vesicles. 2015;4:29509. doi: 10.3402/jev.v4.29509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin S, Yu Z, Chen D, Wang Z, Miao J, Li Q, et al. Progress in Microfluidics-based exosome separation and Detection technologies for diagnostic applications. Small. 2020;16:e1903916. doi: 10.1002/smll.201903916. [DOI] [PubMed] [Google Scholar]
- 52.Ingato D, Lee JU, Sim SJ, Kwon YJ. Good things come in small packages: overcoming challenges to harness extracellular vesicles for therapeutic delivery. J Control Release. 2016;241:174–85. doi: 10.1016/j.jconrel.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 53.Peterson MF, Otoc N, Sethi JK, Gupta A. Antes. Integrated systems for exosome investigation. Methods. 2015;87:31–45. doi: 10.1016/j.ymeth.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 54.Wagner J, Riwanto M, Besler C, Knau A, Fichtlscherer S, Roxe T, et al. Characterization of levels and cellular transfer of circulating lipoprotein-bound microRNAs. Arterioscler Thromb Vasc Biol. 2013;33:1392–400. doi: 10.1161/ATVBAHA.112.300741. [DOI] [PubMed] [Google Scholar]
- 55.Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, El-Andaloussi S, et al. Methodological guidelines to study Extracellular vesicles. Circ Res. 2017;120:1632–48. doi: 10.1161/CIRCRESAHA.117.309417. [DOI] [PubMed] [Google Scholar]
- 56.Kim J, Shin H, Kim J, Kim J, Park J. Isolation of high-purity Extracellular vesicles by extracting proteins using aqueous two-phase system. PLoS ONE. 2015;10:e0129760. doi: 10.1371/journal.pone.0129760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yue L, Zheng M, Wang M, Khan IM, Ding X, Zhang Y, et al. Water-soluble chlorin e6-hydroxypropyl chitosan as a high-efficiency photoantimicrobial agent against Staphylococcus aureus. Int J Biol Macromol. 2022;208:669–77. doi: 10.1016/j.ijbiomac.2022.03.140. [DOI] [PubMed] [Google Scholar]
- 58.Prager I, Liesche C, van Ooijen H, Urlaub D, Verron Q, Sandstrom N, et al. NK cells switch from granzyme B to death receptor-mediated cytotoxicity during serial killing. J Exp Med. 2019;216:2113–27. doi: 10.1084/jem.20181454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kramer B, Knoll R, Bonaguro L, ToVinh M, Raabe J, Astaburuaga-Garcia R, et al. Early IFN-alpha signatures and persistent dysfunction are distinguishing features of NK cells in severe COVID-19. Immunity. 2021;54:2650–e266914. doi: 10.1016/j.immuni.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meggyes M, Nagy DU, Balassa T, Godony K, Peterfalvi A, Szereday L et al. Influence of Galectin-9 Treatment on the Phenotype and Function of NK-92MI Cells in the Presence of Different Serum Supplements. Biomolecules. 2021; 11. [DOI] [PMC free article] [PubMed]
- 61.Wang Y, Li M, Chen L, Bian H, Chen X, Zheng H, et al. Natural killer cell-derived exosomal mir-1249-3p attenuates insulin resistance and inflammation in mouse models of type 2 diabetes. Signal Transduct Target Ther. 2021;6:409. doi: 10.1038/s41392-021-00805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nguyen Cao TG, Kang JH, Kim W, Lim J, Kang SJ, You JY, et al. Engineered extracellular vesicle-based sonotheranostics for dual stimuli-sensitive drug release and photoacoustic imaging-guided chemo-sonodynamic cancer therapy. Theranostics. 2022;12:1247–66. doi: 10.7150/thno.65516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Acker HH, Capsomidis A, Smits EL, Van Tendeloo VF. CD56 in the Immune System: more than a marker for cytotoxicity? Front Immunol. 2017;8:892. doi: 10.3389/fimmu.2017.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neviani P, Wise PM, Murtadha M, Liu CW, Wu CH, Jong AY, et al. Natural killer-derived exosomal miR-186 inhibits Neuroblastoma Growth and Immune escape mechanisms. Cancer Res. 2019;79:1151–64. doi: 10.1158/0008-5472.CAN-18-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng W, Ling S, Cao Y, Shao C, Sun X. Combined use of NK cells and radiotherapy in the treatment of solid tumors. Front Immunol. 2023;14:1306534. doi: 10.3389/fimmu.2023.1306534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dosil SG, Lopez-Cobo S, Rodriguez-Galan A, Fernandez-Delgado I, Ramirez-Huesca M, Milan-Rois P et al. Natural killer (NK) cell-derived extracellular-vesicle shuttled microRNAs control T cell responses. Elife. 2022; 11. [DOI] [PMC free article] [PubMed]
- 67.Penha RCC, Pellecchia S, Pacelli R, Pinto LFR. Fusco. Ionizing Radiation deregulates the MicroRNA expression Profile in differentiated thyroid cells. Thyroid. 2018;28:407–21. doi: 10.1089/thy.2017.0458. [DOI] [PubMed] [Google Scholar]
- 68.Wang Z, Liu L, Du Y, Mi Y, Wang L. The HNF1A-AS1/miR-92a-3p axis affects the radiosensitivity of non-small cell lung cancer by competitively regulating the JNK pathway. Cell Biol Toxicol. 2021;37:715–29. doi: 10.1007/s10565-021-09595-z. [DOI] [PubMed] [Google Scholar]
- 69.Luo J, Si ZZ, Li T, Li JQ, Zhang ZQ, Chen GS, et al. MicroRNA-146a-5p enhances radiosensitivity in hepatocellular carcinoma through replication protein A3-induced activation of the DNA repair pathway. Am J Physiol Cell Physiol. 2019;316:C299–311. doi: 10.1152/ajpcell.00189.2018. [DOI] [PubMed] [Google Scholar]
- 70.Liu Z, Wu K, Gu S, Wang W, Xie S, Lu T, et al. A methyltransferase-like 14/miR-99a-5p/tribble 2 positive feedback circuit promotes cancer stem cell persistence and radioresistance via histone deacetylase 2-mediated epigenetic modulation in esophageal squamous cell carcinoma. Clin Transl Med. 2021;11:e545. doi: 10.1002/ctm2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu RH, Zhang Y, Pan H, Feng J, Zhang T, Liu T, et al. Efficacy and safety of weekly paclitaxel with or without ramucirumab as second-line therapy for the treatment of advanced gastric or gastroesophageal junction adenocarcinoma (RAINBOW-Asia): a randomised, multicentre, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2021;6:1015–24. doi: 10.1016/S2468-1253(21)00313-7. [DOI] [PubMed] [Google Scholar]
- 72.Conroy T, Bosset JF, Etienne PL, Rio E, Francois E, Mesgouez-Nebout N, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:702–15. doi: 10.1016/S1470-2045(21)00079-6. [DOI] [PubMed] [Google Scholar]
- 73.Marupudi NI, Han JE, Li KW, Renard VM, Tyler BM. Brem. Paclitaxel: a review of adverse toxicities and novel delivery strategies. Expert Opin Drug Saf. 2007;6:609–21. doi: 10.1517/14740338.6.5.609. [DOI] [PubMed] [Google Scholar]
- 74.Ransom D, Wilson K, Fournier M, Simes RJ, Gebski V, Yip D, et al. Final results of Australasian gastrointestinal trials Group ARCTIC study: an audit of raltitrexed for patients with cardiac toxicity induced by fluoropyrimidines. Ann Oncol. 2014;25:117–21. doi: 10.1093/annonc/mdt479. [DOI] [PubMed] [Google Scholar]
- 75.Luo H, Zhou Y, Zhang J, Zhang Y, Long S, Lin X, et al. NK cell-derived exosomes enhance the anti-tumor effects against ovarian cancer by delivering cisplatin and reactivating NK cell functions. Front Immunol. 2022;13:1087689. doi: 10.3389/fimmu.2022.1087689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Han D, Wang K, Zhang T, Gao GC, Xu H. Natural killer cell-derived exosome-entrapped paclitaxel can enhance its anti-tumor effect. Eur Rev Med Pharmacol Sci. 2020;24:5703–13. doi: 10.26355/eurrev_202005_21362. [DOI] [PubMed] [Google Scholar]
- 77.Xie J, Wang Y, Choi W, Jangili P, Ge Y, Xu Y, et al. Overcoming barriers in photodynamic therapy harnessing nano-formulation strategies. Chem Soc Rev. 2021;50:9152–201. doi: 10.1039/D0CS01370F. [DOI] [PubMed] [Google Scholar]
- 78.Lu M, Xing H, Shao W, Zhang T, Zhang M, Wang Y, et al. Photoactivatable silencing Extracellular Vesicle (PASEV) sensitizes Cancer Immunotherapy. Adv Mater. 2022;34:e2204765. doi: 10.1002/adma.202204765. [DOI] [PubMed] [Google Scholar]
- 79.Zhang M, Shao W, Yang T, Liu H, Guo S, Zhao D, et al. Conscription of Immune cells by light-activatable silencing NK-Derived Exosome (LASNEO) for synergetic tumor eradication. Adv Sci (Weinh) 2022;9:e2201135. doi: 10.1002/advs.202201135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miao ZH, Lv LX, Li K, Liu PY, Li Z, Yang H, et al. Liquid Exfoliation of Colloidal Rhenium Disulfide nanosheets as a multifunctional Theranostic Agent for in vivo Photoacoustic/CT Imaging and Photothermal Therapy. Small. 2018;14:e1703789. doi: 10.1002/smll.201703789. [DOI] [PubMed] [Google Scholar]
- 81.Chen W, Ouyang J, Yi X, Xu Y, Niu C, Zhang W et al. Black phosphorus nanosheets as a neuroprotective nanomedicine for neurodegenerative disorder therapy. Adv Mater. 2018; 30. [DOI] [PubMed]
- 82.Lyu Y, Zeng J, Jiang Y, Zhen X, Wang T, Qiu S, et al. Enhancing both biodegradability and efficacy of Semiconducting Polymer nanoparticles for Photoacoustic Imaging and Photothermal Therapy. ACS Nano. 2018;12:1801–10. doi: 10.1021/acsnano.7b08616. [DOI] [PubMed] [Google Scholar]
- 83.Liu Z, Lin H, Zhao M, Dai C, Zhang S, Peng W, et al. 2D superparamagnetic Tantalum Carbide Composite MXenes for efficient breast-Cancer theranostics. Theranostics. 2018;8:1648–64. doi: 10.7150/thno.23369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Toshimitsu F, Nakashima N. Semiconducting single-walled carbon nanotubes sorting with a removable solubilizer based on dynamic supramolecular coordination chemistry. Nat Commun. 2014;5:5041. doi: 10.1038/ncomms6041. [DOI] [PubMed] [Google Scholar]
- 85.Yu X, Li A, Zhao C, Yang K, Chen X, Li W. Ultrasmall Semimetal nanoparticles of Bismuth for Dual-Modal Computed Tomography/Photoacoustic Imaging and synergistic thermoradiotherapy. ACS Nano. 2017;11:3990–4001. doi: 10.1021/acsnano.7b00476. [DOI] [PubMed] [Google Scholar]
- 86.Zhu X, Feng W, Chang J, Tan YW, Li J, Chen M, et al. Temperature-feedback upconversion nanocomposite for accurate photothermal therapy at facile temperature. Nat Commun. 2016;7:10437. doi: 10.1038/ncomms10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang D, Zheng Y, Lin Z, Lan S, Zhang X, Zheng A, et al. Artificial Engineered Natural Killer cells combined with Antiheat Endurance as a powerful strategy for enhancing photothermal-immunotherapy efficiency of solid tumors. Small. 2019;15:e1902636. doi: 10.1002/smll.201902636. [DOI] [PubMed] [Google Scholar]
- 88.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–46. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 89.Mangan MS, Bird CH, Kaiserman D, Matthews AY, Hitchen C, Steer DL, et al. A Novel Serpin Regulatory mechanism: SerpinB9 IS REVERSIBLY INHIBITED BY VICINAL DISULFIDE BOND FORMATION IN THE REACTIVE CENTER LOOP. J Biol Chem. 2016;291:3626–38. doi: 10.1074/jbc.M115.699298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bots M, Kolfschoten IG, Bres SA, Rademaker MT, de Roo GM, Kruse M, et al. SPI-CI and SPI-6 cooperate in the protection from effector cell-mediated cytotoxicity. Blood. 2005;105:1153–61. doi: 10.1182/blood-2004-03-0791. [DOI] [PubMed] [Google Scholar]
- 91.Bladergroen BA, Meijer CJ, ten Berge RL, Hack CE, Muris JJ, Dukers DF, et al. Expression of the granzyme B inhibitor, protease inhibitor 9, by tumor cells in patients with Non-hodgkin and Hodgkin lymphoma: a novel protective mechanism for tumor cells to circumvent the immune system? Blood. 2002;99:232–7. doi: 10.1182/blood.V99.1.232. [DOI] [PubMed] [Google Scholar]
- 92.Han R, Yu L, Zhao C, Li Y, Ma Y, Zhai Y, et al. Inhibition of SerpinB9 to enhance granzyme B-based tumor therapy by using a modified biomimetic nanoplatform with a cascade strategy. Biomaterials. 2022;288:121723. doi: 10.1016/j.biomaterials.2022.121723. [DOI] [PubMed] [Google Scholar]
- 93.Shoae-Hassani A, Hamidieh AA, Behfar M, Mohseni R, Mortazavi-Tabatabaei SA, Asgharzadeh S. NK Cell-derived Exosomes from NK cells previously exposed to Neuroblastoma cells augment the Antitumor activity of cytokine-activated NK cells. J Immunother. 2017;40:265–76. doi: 10.1097/CJI.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang L, Wang Y, Quan J. Exosomes derived from natural killer cells inhibit hepatic stellate cell activation and liver fibrosis. Hum Cell. 2020;33:582–9. doi: 10.1007/s13577-020-00371-5. [DOI] [PubMed] [Google Scholar]
- 95.Choucair K, Duff JR, Cassidy CS, Albrethsen MT, Kelso JD, Lenhard A, et al. Natural killer cells: a review of biology, therapeutic potential and challenges in treatment of solid tumors. Future Oncol. 2019;15:3053–69. doi: 10.2217/fon-2019-0116. [DOI] [PubMed] [Google Scholar]
- 96.O’Sullivan TE, Sun JC. L L Lanier Nat Killer Cell Memory Immun. 2015;43:634–45. doi: 10.1016/j.immuni.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Anguela XM. High. Entering the modern era of Gene Therapy. Annu Rev Med. 2019;70:273–88. doi: 10.1146/annurev-med-012017-043332. [DOI] [PubMed] [Google Scholar]
- 98.High KA. Roncarolo. Gene Therapy. N Engl J Med. 2019;381:455–64. doi: 10.1056/NEJMra1706910. [DOI] [PubMed] [Google Scholar]
- 99.Paunovska K, Loughrey D. Dahlman. Drug delivery systems for RNA therapeutics. Nat Rev Genet. 2022;23:265–80. doi: 10.1038/s41576-021-00439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–22. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 101.Winkle M, El-Daly SM, Fabbri M. Calin. Noncoding RNA therapeutics - challenges and potential solutions. Nat Rev Drug Discov. 2021;20:629–51. doi: 10.1038/s41573-021-00219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li D, Wang Y, Jin X, Hu D, Xia C, Xu H, et al. NK cell-derived exosomes carry miR-207 and alleviate depression-like symptoms in mice. J Neuroinflammation. 2020;17:126. doi: 10.1186/s12974-020-01787-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fu W, Lei C, Liu S, Cui Y, Wang C, Qian K, et al. CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat Commun. 2019;10:4355. doi: 10.1038/s41467-019-12321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zheng W, Zhu T, Tang L, Li Z, Jiang G, Huang X. Inhalable CAR-T cell-derived exosomes as paclitaxel carriers for treating lung cancer. J Transl Med. 2023;21:383. doi: 10.1186/s12967-023-04206-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. CAR-NK cells: a promising cellular immunotherapy for cancer. EBioMedicine. 2020;59:102975. doi: 10.1016/j.ebiom.2020.102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.