Abstract
Bladder cancer is a global health issue with sex differences in incidence and prognosis. Bladder cancer has distinct molecular subtypes with multiple pathogenic pathways depending on whether the disease is non-muscle invasive or muscle invasive. The mutational burden is higher in muscle-invasive than in non-muscle-invasive disease. Commonly mutated genes include TERT, FGFR3, TP53, PIK3CA, STAG2 and genes involved in chromatin modification. Subtyping of both forms of bladder cancer is likely to change considerably with the advent of single-cell analysis methods. Early detection signifies a better disease prognosis; thus, minimally invasive diagnostic options are needed to improve patient outcomes. Urine-based tests are available for disease diagnosis and surveillance, and analysis of blood-based cell-free DNA is a promising tool for the detection of minimal residual disease and metastatic relapse. Transurethral resection is the cornerstone treatment for non-muscle-invasive bladder cancer and intravesical therapy can further improve oncological outcomes. For muscle-invasive bladder cancer, radical cystectomy with neoadjuvant chemotherapy is the standard of care with evidence supporting trimodality therapy. Immune-checkpoint inhibitors have demonstrated benefit in non-muscle-invasive, muscle-invasive and metastatic bladder cancer. Effective management requires a multidisciplinary approach that considers patient characteristics and molecular disease characteristics.
Introduction
In 2020, 573,278 people were newly diagnosed with bladder cancer worldwide1,2, and this number is expected to double by 2040 based on World Health Organization predictions3. If detected early before muscle invasion, this disease is often treatable and can be managed with minimal effects on survival. Muscle-invasive disease can metastasize, predominantly to lymph nodes, bones, lungs and liver4, and is associated with a median survival of ~15 months5.
The bladder wall consists of 5–7 epithelial cell layers with surface umbrella cells (urothelium) with underlying layers of fibroconnective tissue and vessels (lamina propria), thick muscular bundles (muscularis propria or detrusor muscle) and perivesical fat (Fig. 1). Urothelial cells are the primary cells of origin of bladder cancer, and urothelial cancer is the most common form of bladder cancer, affecting ~95% of patients6,7. Tobacco use is the primary risk factor in ~50% of bladder cancer diagnoses8,9 as the urothelium is exposed to carcinogenic tobacco metabolites eliminated via the urine10. Other urothelial cell-derived bladder cancer types, occurring in <2% of patients, include small cell carcinoma, squamous cell carcinoma and adenocarcinoma7.
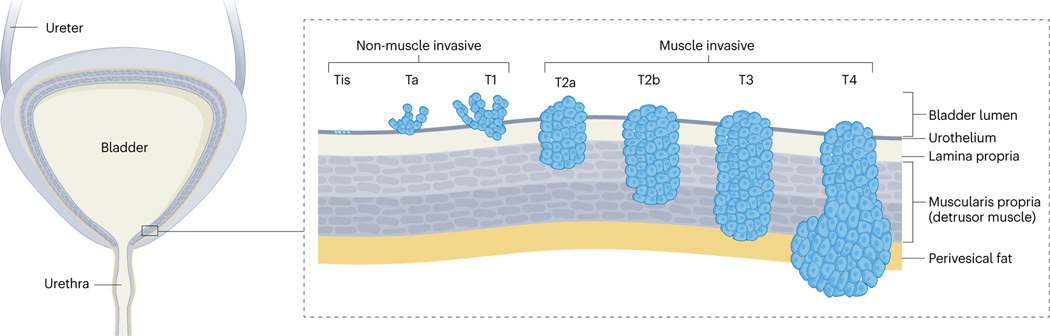
Fig. 1 |. Bladder cancer categories.
Bladder cancer can be categorized into grades, which is the cytological appearance of the urothelium, and stages, which are determined by the spread and depth of bladder wall invasion of the tumour. Non-invasive papillary carcinomas are classified as Ta disease, whereas urothelial carcinoma in situ is classified as Tis disease. All invasive urothelial cancers arise from either high-grade papillary carcinoma or urothelial carcinoma situ. Adapted from ref. 319, Springer Nature Limited.
At diagnosis, urothelial cancer is categorized as either non-muscle-invasive bladder cancer (NMIBC; stages Tis, Ta and T1) or muscle-invasive bladder cancer (MIBC; stages T2–T4) when the disease has grown into the muscularis propria. The overall categorization of the disease into NMIBC or MIBC is used frequently as treatment modalities differ substantially between these entities; however, within the NMIBC category, Ta tumours have a much more benign disease course than T1 and Tis tumours, and treatment of these subtypes is also markedly different7. The various tumour stages are associated with different genetic features, which can be used as markers for minimally invasive diagnostics and disease aggressiveness11,12. The importance of these markers in disease management will further increase as molecular pathology will become more predominant in diagnosis, treatment selection and follow-up planning. The most informative molecular markers to date are genetic variants of TP53, ERCC1 and FGFR3 as markers of disease progression, chemotherapy sensitivity and small-molecule therapeutic selection, respectively11,12.
Of note, bladder cancer incidence and aggressiveness differ considerably between men and women13. For instance, bladder cancer is the sixth most common cancer in biological males but only the seventeenth most common cancer in biological females14. However, women present clinically with more advanced disease and have a poorer prognosis15,16 and, perhaps, a lower survival than men (possibly confined to the first 2 years after diagnosis)17. In the past few years, efforts have also been made to understand the role of race in bladder cancer biology18 and further advances in this field are expected in the future.
This Primer focuses on urothelial cancer, the most common form of bladder cancer. We summarize the epidemiology of the disease with a focus on risk factors, discuss mechanisms of pathogenesis, including genetic alterations, and provide an overview of current diagnostic methods. In addition, we review current treatment modalities employed at different disease stages, discuss the quality of life (QoL) of patients with the disease, and discuss outstanding issues and research questions.
Epidemiology
Incidence and mortality
Bladder cancer incidence is highest in higher-income regions of the world, including Europe, North America and western Asia, and is also increased in regions affected by Schistosoma parasites such as Northern Africa19. By contrast, South America, eastern Asia, the Caribbean, and middle and southern Africa have much lower rates of bladder cancer. The differences in bladder cancer incidence between these regions have been linked to the prevalence of tobacco use, occupational exposure to aromatic amines in industry, arsenic in drinking water and other causes2,20. In 2020, nearly 600,000 people were diagnosed with bladder cancer globally, predominantly affecting individuals >55 years of age and men1,2 (Figs. 2 and 3). Bladder cancer is the tenth most common cause of cancer globally and the thirteenth most common cause of mortality from cancer19. Ongoing efforts to mitigate risk factors, improve timely diagnosis, better understand sex differences and expand therapy seem to have resulted in decreasing global rates of bladder cancer diagnoses and deaths21.
Fig. 2 |. Global incidence of bladder cancer.
Global estimated incidence of bladder cancer in 2020 in men and women of all ages. Data are expressed as age-standardized rates (ASRs; adjusted to World Standard Population) to account for differing age profiles among regions. Data were obtained from GLOBOCAN 2020. Map was produced by the World Health Organization/International Agency for Research on Cancer (https://gco.iarc.fr/today).
Fig. 3 |. Global mortality of bladder cancer.
Global estimated mortality due to bladder cancer in 2020 in men and women of all ages. Data are expressed as age-standardized rates (ASRs; adjusted to World Standard Population) to account for differing age profiles among regions. Data were obtained from GLOBOCAN 2020. Map was produced by the World Health Organization/International Agency for Research on Cancer (https://gco.iarc.fr/today).
Risk factors
Cigarette smoking.
Cigarette smoking is the most prominent contributor to bladder cancer development in most countries, with ~50% of all cases linked to this risk factor8,9. A global decline in smoking prevalence might have contributed to improving rates of bladder cancer diagnoses and deaths; however, trends vary considerably by country21. More than 1 billion people are estimated to smoke tobacco globally but smoking prevalence has decreased since 1990 by ~27% in men and 38% in women22,23. The highest reductions seem to have occurred in higher socioeconomic groups, which probably reflects higher health awareness and enhanced access to health care in this population22,23.
Parasitic infection and chronic inflammation.
Infection with Schistosoma haematobium, a parasite in the blood fluke family, is a relatively unique risk factor for bladder cancer in northern Africa24. Parasites infect individuals via the skin when swimming in water containing schistosome cercariae and, following maturation in the liver, can deposit eggs within the bladder and mesenteric plexus. Calcification of the eggs and resultant chronic inflammation of the bladder lining leads primarily to the development of squamous cell carcinoma25. Efforts to eradicate this parasite have resulted in a decrease in bladder cancer incidence26. In addition to parasitic infection, other conditions that can increase chronic inflammation may contribute to the development of bladder cancer, including the presence of diverticula, alterations in the gut or urinary tract microbiome, and dysfunction of the immune system27.
Sex and age.
Sex and age are two key epidemiological features associated with the development of bladder cancer. Men are more commonly affected by the disease, with the male-to-female ratio remaining relatively steady at approximately 4:1 (ref. 21). This discrepancy is reflected in the finding that bladder cancer is the sixth most common cancer in men worldwide and the fourth most common cancer in men in the USA1,21. Several explanations have been proposed, including differences in smoking rates and exposure to specific compounds in work environments, hormonal factors, and the effects of sex chromosomes13. Bladder cancer more commonly affects older individuals, with an average age at diagnosis of 73 years and >90% of cases occurring in persons >55 years of age. The discrepancy between sexes exists irrespective of age at diagnosis1,21.
Occupational exposure.
Occupational exposure to certain chemicals is another risk factor for bladder cancer. Exposure to aromatic amines, such as benzidine and β-naphthylamine in the dye industry, hair dyes, paint products, and other occupational exposures to organic compounds may increase the risk of bladder cancer28. Processing of rubber and textiles as well as exposure to diesel fumes may also be associated with an increased risk of bladder cancer29.
Genetic factors.
Risk factors in the development of bladder cancer include hereditary (germline) DNA alterations. For example, hereditary non-polyposis colon cancer (Lynch syndrome) is indicated in the development of urothelial carcinoma, accounting for ~5% of upper tract urothelial carcinomas and probably also cases of bladder cancer, although studies are ongoing30,31. In this hereditary disease, mutations in mismatch repair genes MLH1, MSH2, MSH6 and PMS2 result in microsatellite instability, with mutations in MSH2 and associated microsatellite instability posing a high risk for the development of urothelial carcinoma30.
Mechanisms/pathophysiology
Overall, NMIBC (stages Tis, Ta and T1) and MIBC (stages T2–T4) have distinct molecular profiles with considerable molecular heterogeneity within each disease category. T1 tumours often share molecular characteristics with MIBC and these tumours usually differ substantially from low-grade Ta tumours32–34 (Fig. 4). There is no obligate pathway from NMIBC to MIBC and it seems that these tumour categories have largely non-overlapping pathogenesis pathways. Histopathological and molecular data indicate that the flat lesion carcinoma in situ (CIS) is the major precursor of MIBC, whereas most papillary NMIBC arise from normal-appearing urothelium. Nevertheless, progression from initially non-invasive to invasive disease occurs in some patients with NMIBC, particularly those with tumours invading the lamina propria.
Fig. 4 |. Pathogenesis pathways.
Potential pathogenesis pathways to papillary non-muscle-invasive bladder cancer (NMIBC) and solid muscle-invasive bladder cancer (MIBC), including key genomic events, are shown (Tables 1 and 2). Solid arrows indicate pathways for which there is histopathological and/or molecular evidence. Dashed arrows indicate pathways for which there is uncertainty. Estimated time for tumour development is shown on the left. CIS, carcinoma in situ.
The normal urothelium
The urothelium is composed of basal, intermediate and superficial cell layers, the latter specialized to form a tight barrier that prevents urine absorption. This barrier function relies on the expression of uroplakins35 and claudin family members in tight junctions36. Keratin 20 is restricted to the umbrella cells37. This normally quiescent epithelium can proliferate rapidly in response to damage. Whether a definitive stem cell exists is unclear but evidence suggests that human basal cells have regenerative capacity38. In mouse models, both basal and intermediate cells are implicated as tumour cells of origin39. PPARγ, a member of the nuclear receptor superfamily, is a regulator of urothelial differentiation whose activation leads to expression of uroplakins, relevant keratins and claudins via transcription factors FOXA1, GATA2 and ELF3. In the absence of PPARγ activation, p63 maintains the undifferentiated (basal) phenotype40.
Field cancerization
Field cancerization, the acquisition of pro-tumorigenic mutations and genomic alterations in normal cell lineages, has been associated with the development of bladder cancer41. The origin of transformed cells among normal-appearing urothelial cells is unclear, with original speculation that cancer cells from tumours migrate in the urothelium or are shed from tumours and implanted between normal cells42. This is referred to as the ‘tumour-first-field-later’ theory. In the past decade, it has been suggested that field cancerization evolves from transformed stem cells in the urothelium that expand and drive tumour formation (‘field-first-tumour-later’ theory)43,44. Both theories may explain frequent recurrences of clonally related bladder tumours that develop years apart45. Whole-organ mapping studies demonstrated that genetic alterations can be divided into two categories: low-frequency mutations and high-frequency mutations increasing with disease progression. Based on this, it was estimated that bladder carcinogenesis spans 10–15 years, with a progressive phase of 1–2 years involving high-frequency mutations46. In another study, patients with a high level of field cancerization had poor survival, and tumours from these patients harboured a high mutational burden, high neoantigen load and high tumour-associated CD8+ T cell exhaustion47. Importantly, non-synonymous mutations in known bladder cancer driver genes, such as chromatin remodelling genes and TP53, STAG2 and PIK3CA, have been identified in non-diseased bladders48 as well as in histologically tumour-free urothelium from patients with bladder cancer47.
Common genetic alterations
Mutational signatures are similar regardless of tumour grade and stage despite largely non-overlapping pathogenesis pathways34,49. There is a major contribution from the activity of the APOBEC family of cytidine deaminases, accounting for more than 60% of all single-nucleotide mutations34,50,51 but only few known tobacco use-related signatures despite the association of tobacco use with risk. Compared with NMIBC, the overall mutational burden is much higher in MIBC (>7 mutations per Mb), surpassed only by lung cancer and melanoma52, and large structural alterations and aneuploidy are more common53.
Deletions of chromosome 9 are found in ~50% of both NMIBC and MIBC. These deletions include the CDKN2A locus (9p21), encoding p16 and p14ARF, which are regulators of the RB and p53 pathways, respectively. On 9q, loss of TSC1, which regulates mTOR signalling, has been found and 9q loss is associated with upregulated expression of mTOR targets54. Interestingly, mTOR has been implicated as a regulator of telomerase reverse transcriptase (TERT) gene transcription. In addition to the maintenance of telomere integrity, TERT has non-canonical functions, including upregulation of oncogenic signalling pathways55, is crucial in maintaining tumour immortality and contributes to tumour progression in bladder cancer56–59. Other copy number alterations in NMIBC (8–22%) include gains of 1q, 5p, 18q, 20p, and 20q and losses of 8p, 11p, 17p, and 18q, particularly in stage T1 tumours32. These regions are more commonly altered in MIBC, in which amplifications of 3p25 (PPARG), 6p22 (E2F3), 7p11 (EGFR), 17q12 (ERBB2) and 19q12 (CCNE1) are also found51. High-level DNA amplification is uncommon in NMIBC60.
Commonly mutated genes are shown in Tables 1 and 2. Extremely common in all tumour grades and stages (70–80%) are mutations in the promoter of the telomerase reverse transcriptase TERT57,61,62, which are associated with upregulated expression. Apart from TERT, mutated genes and mutation frequencies differ considerably between NMIBC and MIBC. The mutational profile of lamina propria-invasive tumours (stage T1) is more closely related to that of MIBC compared with stage Ta NMIBC. However, the mutational profile of stage T1 tumours does not indicate the presence of some tumours with MIBC-like features and of some with Ta-like features but rather that individual T1 tumours often contain both Ta-like and MIBC-like features34.
Table 1 |.
Oncogenes activated in bladder cancer
| Gene | Chromosome | Frequency (%) | Alteration | Functions affected | ||
|---|---|---|---|---|---|---|
| Ta | T1 | T2+ | ||||
| TERT | 5p15 | 70–80 | 70–80 | 70–80 | Point mutation | Senescence and other functions |
| FGFR3 | 4p16 | 80 | 30 | 10–15 | Point mutation | RAS–MAPK signalling |
| 70–80 | 50–60 | 40 | Upregulated expression | |||
| PIK3CA | 3q26 | 40 | 20 | 20 | Point mutation | PI3K signalling |
| HRAS and KRAS | 11p15 and 12p12 | 10–15 | 10–15 | 10–15 | Point mutation | RAS–MAPK and PI3K signalling |
| ERBB2 | 17q12 | ≤2 | 10–15 | 10–15 | Mutation or amplification | RAS–MAPK and PI3K signalling |
| ERBB3 | 12q13 | ≤2 | 10–15 | 10–15 | Mutation | RAS–MAPK and PI3K signalling |
| EGFR | 7p12 | ≤2 | ≥2 | 11 | Amplification | RAS–MAPK and PI3K signalling |
| PPARG | 3p25 | ≤2 | 10 | 15 | Amplification | PPARG signalling |
| ≤2 | 9 | 3 | Mutation | |||
| RXRA | 9q34 | 2 | 5 | 6 | Mutation | PPARG signalling |
| E2F3 | 6p22 | ≤2 | 5–10 | 10–15 | Amplification | Cell cycle regulation |
| MDM2 | 12q15 | 0 | 5–15 | 5–15 | Amplification | Cell cycle regulation |
| CCND1 | 11q13 | ≤2 | 10 | 10 | Amplification | Cell cycle regulation |
| CCNE1 | 19q12 | ≤2 | ≤2 | 10 | Amplification | Cell cycle regulation |
Genes with activating mutations or high-level DNA amplification in >10% of at least one bladder cancer stage are shown. If very low frequencies have been found in stage Ta tumours but samples were too few for accurate estimation, ≤2% is shown. Adapted from ref. 319, Springer Nature Limited.
Table 2 |.
Genes commonly inactivated by mutation in bladder cancer
| Gene | Chromosome | Frequency (%) | Alteration | Functions affected | ||
|---|---|---|---|---|---|---|
| Ta | T1 | T2+ | ||||
| CDKN2A | 9p21 | 30 | 60 | 60 | Loss of heterozygosity, deletion | Cell cycle |
| ≤2 | 12 | 22 | Homozygous deletion | |||
| 1 | 7 | 7 | Mutation | |||
| RB1 | 13q14 | 0 | 14 | 17 | Inactivating mutation | Cell cycle |
| ATM | 11q22 | 12 | 16 | 14 | Inactivating mutation | Cell cycle |
| CDKN1A | 6p21 | 11 | 11 | 9 | Inactivating mutation | Cell cycle |
| TP53 | 17p13 | 4 | 24 | 48 | Inactivating mutation | Transcription |
| ELF3 | 1q32 | 8 | 22 | 12 | Inactivating mutation | Transcription |
| ZFP36L1 | 14q24 | 12 | 11 | 6 | Inactivating mutation | Transcription |
| KDM6A | Xp11 | 40 | 40 | 26 | Inactivating mutation | Chromatin regulation |
| KMT2D | 12q13 | 35 | 27 | 28 | Inactivating mutation | Chromatin regulation |
| CREBBP | 16p13 | 23 | 20 | 12 | Inactivating mutation | Chromatin regulation |
| KMT2C | 7q36 | 23 | 14 | 18 | Inactivating mutation | Chromatin regulation |
| STAG2 | Xq25 | 30 | 9 | 14 | Inactivating mutation | Chromatin regulation |
| ARID1A | 1p36 | 11 | 27 | 25 | Inactivating mutation | Chromatin regulation |
| KMT2A | 11q23 | 11 | 15 | 11 | Inactivating mutation | Chromatin regulation |
| EP300 | 22q13 | 15 | 11 | 15 | Inactivating mutation | Chromatin regulation |
| ASH1L | 1q22 | 10 | 12 | 7 | Inactivating mutation | Chromatin regulation |
| ARID2 | 12q12 | 7 | 11 | 8 | Inactivating mutation | Chromatin regulation |
| ERCC2 | 19q13 | 4 | 24 | 18 | Inactivating mutation | DNA repair |
| BRCA2 | 13q13 | 10 | 10 | 9 | Inactivating mutation | DNA repair |
| PTEN | 10q23 | 7–12 | 20–30 | 50 | Loss of heterozygosity, deletion, mutation | Regulator of AKT signalling |
| TSC1 | 9q34 | 12 | 15 | 8 | Inactivating mutation | Regulator of mTOR signalling |
| RBM10 | Xp11 | 7 | 13 | 5 | Inactivating mutation | RNA splicing |
Genes affected in >10% of at least one bladder cancer stage are shown. Large genes not formally identified as significantly mutated or with unknown function are not listed.
Non-muscle-invasive bladder cancer
NMIBC is characterized by FGFR3 point mutations (in ~60% of patients), which are associated with low tumour grade and stage54. The most common of these mutations (S249C) is predicted to result from APOBEC activity63. In cultured normal human urothelial cells, mutant FGFR3 drives cell overgrowth at confluence, suggesting a potential contribution to urothelial hyperplasia in vivo64. Mutation of RAS genes and FGFR3 are mutually exclusive, with mutation of one or the other in 90% of stage Ta tumours54. APOBEC target mutations in PIK3CA hotspot codons are found in ~30% of patients with NMIBC, often with mutations in FGFR3 or RAS genes34, indicating that most NMIBC have activation of both RAS–MAPK and PI3K signalling. Loss of 9q, including TSC1 in 50% of patients, provides activation of the PI3K pathway downstream of mTOR. In stage T1, gain-of-function mutations in ERBB2 and ERBB3 that provide PI3K activation52 are present in ~15% of tumours and often co-occur34.
Mutations of STAG2 and other chromatin regulators (KDM6A, KMT2D, KMD2C, CREBBP, EP300 and ARID1A) are common. Inactivation of one or more of these regulators is found in >65% of patients with NMIBC, with KDM6A mutations more common in stage Ta than in stage T1 and ARID1A mutations more common in stage T1 tumours34. The exact roles of these genes in bladder cancer are not well understood and some mutations can be found in normal urothelium of cancer-bearing bladders. Compatible with this is the role of KDM6A in the regulation of normal urothelial differentiation65,66 and its antagonistic effect on FGFR3 activation65. Mutation of STAG2, a subunit of the cohesin complex, is more common in bladder cancer than in other cancers and is implicated in negative regulation of basal cell identity67. Inactivating mutations and loss of expression are present in ~30% of low-grade Ta tumours, often with FGFR3, PIK3CA and/or KDM6A mutations, but in fewer T1 tumours34,68,69.
MIBC and metastatic disease
MIBC exhibits remarkable intratumour genetic heterogeneity70. Despite limited sampling, key players have been clearly identified51 (Tables 1 and 2). Almost all MIBC have loss of cell cycle checkpoints via TP53, RB1, and/or ATM mutations and/or alterations affecting their regulators, for example, E2F3 and MDM2 amplification, mutation of FBXW7 (8%), and deletion of CDKN2A. Response to DNA damage and DNA repair pathways (for example, through loss of function of ATM or ERCC2 mutation71) are also affected; ERCC2 is also implicated in 24% of T1 tumours34.
Overall involvement of chromatin modifiers in MIBC is similar to that in NMIBC except that the distribution of mutations differs. Activating point mutations in FGFR3 and PIK3CA are less common than in NMIBC, although upregulated expression of FGFR3 is frequent. Activating translocations involving FGFR3 are found in some tumours (2–5%)72. Upregulated expression and/or isoform switching of FGFR1, with a potential effect on epithelial–mesenchymal transition73,74, are also found in some tumours. FGFR3, PIK3CA, KDM6A and STAG2 mutations often co-occur and, in the tumours with this mutation profile and luminal phenotype, loss of 9p (p16 and p14ARF) may contribute to progression75. Activation of the RAS–MAPK and PI3K pathways is estimated to occur in ~70% of MIBC51, commonly via mutation or upregulation of upstream regulators, including gain-of-function mutations of ERBB2 and ERBB3, or amplification of ERBB2 and EGFR51. Loss of PTEN and TSC1 also contributes to AKT–mTOR activation76. Other pathways implicated in MIBC include upregulated MET signalling77 and the NOTCH pathway78.
Tumour microenvironment
The tumour microenvironment (TME) comprises both malignant and non-malignant cells. Cancer-associated fibroblasts (CAFs) are the most common non-malignant cells in bladder cancer, forming distinct regions within the tumour79, and these CAFs have been associated with tumour aggressiveness, chemoresistance and reduced response to immune-checkpoint inhibitor (ICI) therapy79–81. Tumour-associated macrophages are another important non-malignant population in bladder cancer82. Tumour-associated macrophages are recruited to sites of inflammation and hypoxia within the TME but, like CAFs, they are co-opted by cancerous cells to promote an immune suppressive environment, drug resistance and metastasis83–89. Resistance to inhibition of PD1 or PDL1 in urothelial cancer has also been linked to a pro-inflammatory cellular state of myeloid phagocytic cells detectable in tumour and blood90. Tumour-infiltrating lymphocytes (TILs) are immune cells that clear cancerous cells. Mostly composed of CD8+ T cells, TILs develop and expand to recognize foreign antigens present on cancer cells or antigen-presenting cells. Of note, bladder cancer, and MIBC in particular, has a high level of mutational burden91,92, providing neoantigens for immune cells to recognize. However, the beneficial effect in bladder cancer is lower than expected because of low numbers of TILs in the tumour and/or the inactivation of TILs that do reach malignant cells. In MIBC, the presence of TILs in or adjacent to the tumour is a predictor of patient response to ICIs and survival93. The degree of stromal cell infiltration, most notably CAFs, into tumours also determines patient response to immune therapies. Patients with high numbers of TILs and low stromal gene tumour signatures have improved survival and response to immune therapies94. The discoidin domain (DDR1 and DDR2) collagen receptors, which are commonly found on cancer cells and fibroblasts, have been implicated as biomarkers for ICI response in bladder cancer and other cancer types in both the experimental setting88 and in patients95. This important finding supports the link between collagen deposition, fibroblasts and resistance to ICIs. Future clinical trials of targeted therapies, such as DDR1 and/or DDR2 inhibition combined with ICIs, would be expected to enhance the effectiveness of ICIs.
Biological sex differences
Bladder cancer incidence and aggressiveness differ substantially between men and women13. Absence of X chromosome gene KDM6A leads to an increased incidence of bladder cancer in mouse models96 but, notably, only in female animals. KDM6A is mutated in 24% of patients with bladder cancer and its experimental depletion in human bladder cancer cells enhanced in vitro cell proliferation, migration and in vivo tumour growth; however, the limited number of cell lines investigated prevents a conclusion of whether this effect is dependent on sex59.
In addition to sex chromosome-mediated effects, androgen receptor (AR) signalling can lead to sexual dimorphism in bladder cancer incidence and therapeutic response. Two studies in 2022 demonstrated that T cell-intrinsic AR promotes CD8+ T cell exhaustion in the TME97,98. Furthermore, AR can suppress the expression of CD44 (ref. 99), a well-known driver of tumour progression and metastasis in bladder cancer100–102 and other cancer types103. In mouse studies, AR deletion reduces the incidence of bladder cancer induced by standard orally ingested chemical carcinogens that accumulate in urine and are analogues of those found in cigarette smoke104. However, the role of AR in humans is less clear105,106. Use of the 5α-reductase inhibitor finasteride was found to reduce bladder cancer incidence in white and Hispanic men but not in Black men107. Intriguingly, Black men have higher free testosterone levels than white men108, yet a lower incidence of bladder cancer109. By contrast, reduced AR expression in bladder cancer is associated with more advanced stage99,110 and aggressive tumour subtypes111. Inhibition of AR signalling has shown promise in men with reduced recurrence of NMIBC112–114.
In a systematic review of 18 studies, the incidence and clinical outcomes of bladder cancer were investigated in patients who received androgen suppression therapy108. 5α-Reductase inhibitors or androgen deprivation therapy were not significantly associated with a reduced risk of bladder cancer incidence or cancer-specific, overall or progression-free survival. In a subgroup analysis, only finasteride use was associated with reduced bladder cancer risk, and recurrence-free survival was improved in those receiving androgen suppression therapy compared with those who were not. Hence, finasteride use may represent a strategy for reducing bladder cancer incidence, and overall androgen suppression may reduce recurrence risk in patients with a history of bladder cancer. Only randomized trials with well-characterized study populations can definitively prove these observations.
The Y chromosome is essential for male sex determination and spermatogenesis115. In ageing men, loss of the Y chromosome (LOY) in haematopoietic cells has been associated with increased risk of several diseases, including cardiac fibrosis116 and multiple cancer types116–119. In bladder cancer, LOY has been found in 10–40% of tumours120–126. This is unsurprising as bladder cancer is commonly caused by environmental exposures, such as tobacco and industrial chemicals, that are known to result in DNA damage and LOY127–129. Recent studies have shown that LOY and the corresponding loss of Y genes KDM5D and UTY, which are chromatin modifiers, confer an aggressive phenotype to bladder cancer through acquisition of the ability to evade the adaptive immune system18. Fortunately, this also makes LOY tumours more vulnerable to ICIs. This landmark study is the first to show that LOY drives cancer biology and the host immune response to cancer130.
Diagnosis and screening
Clinical presentation
Around 75% of patients with bladder cancer present with painless, visible (gross) haematuria, which warrants early medical attention131. In a prospective observational study, 22.4% of patients presenting with visible haematuria were found to have bladder cancer, with the incidence increasing with age: only 4.7% in those <35 years of age compared with 35% in those >75 years of age132. Rates of urological referral of patients with haematuria are generally low133 and, therefore, the reported rates of bladder cancer can differ in the literature. Patients may also present with microscopic or non-visible haematuria commonly detected upon health checkup, and bladder cancer was found in 3.3–5.2% of that population132,134. Presentation with microscopic haematuria seems to correspond to a low disease stage135. In a multi-centre cohort study in patients with microscopic haematuria, 68.8% had Ta or Tis disease, 19.6% had T1 disease, and 11.6% had T2 disease, whereas in patients presenting with gross haematuria, 55.9% had Ta or Tis disease, 19.6% had T1 disease, and 17.9% had T2 disease135.
Bladder cancer is rare in children, with an incidence of only 0.1–0.4%136,137. In a systematic review including 243 paediatric patients with bladder cancer138, gross haematuria was the most common presentation (75.6%), followed by lower urinary tract symptoms (8.6%) and abdominal and/or flank pain (3.4%). Most of the patients presented with Ta (86.4%) and low-grade (93.4%) disease; T2 or above disease was uncommon (4.1%).
Diagnosis
Diagnostic evaluation of patients with haematuria should involve a physical examination including rectal and vaginal bimanual palpation to assess for pelvic masses suggesting a locally advanced tumour139, although the risk of both clinical under-staging and over-staging is well known140,141. Cystoscopy is considered the gold standard for diagnosing bladder cancer. White-light imaging cystoscopy is the conventional method to detect bladder cancer but may miss some lesions such as CIS. CIS usually presents as a velvet-like, reddish area that is difficult to detect and differentiate from inflammation142, which has led to advanced cystoscopy technologies, such as narrow-band imaging, photodynamic diagnosis and Image 1S, to enhance bladder cancer detection (Supplementary Table 1).
If a lesion is seen on cystoscopy, this is followed by examination under anaesthesia at the time of transurethral resection of bladder tumour (TURBT), although the risk of both clinical under-staging and over-staging with this assessment is well known141. Pathological work-up of patients includes the use of urine-based evaluation to detect malignant cells and/or analysis of biopsy or TURBT samples of visibly identifiable lesions.
Urine-based diagnosis of bladder cancer.
Urine cytology is the most cost-effective urine-based method to diagnose high-grade bladder cancer143. The sensitivity of this analysis is suboptimal but its specificity is high, especially for high-grade urothelial carcinoma; thus, urine cytology remains the gold standard in the diagnosis of bladder cancer compared with marker-based studies in urine144,145. Urine cytology specimens are classified according to the Paris System for Reporting Urinary Cytology published in 2016, which subdivides specimens into non-diagnostic, negative for high-grade urothelial carcinoma, atypical urothelial cells, suspicious for high-grade urothelial carcinoma, high-grade urothelial carcinoma, low-grade urothelial neoplasm, and other malignancies144. The risk of cancer with a diagnosis of high-grade urothelial carcinoma is >90% using this classification system144,145. Of note, any cytology classification approach to low-grade urothelial carcinomas yields lower sensitivity than those for high-grade carcinomas owing to the more cohesive nature of low-grade lesions and the much closer similarity of low-grade lesions to normal cellular morphology146.
Over the past few decades, extensive effort has gone into the development of protein-based and molecular-based urine tests to diagnose bladder cancer. These efforts have resulted in numerous FDA-approved tests, including cell-free DNA tests147–150. Methodologies of these tests include, for example, analysis of proteins elevated in dividing cells using antibody-based methods to detect chromosome aneuploidy by fluorescence in situ hybridization148,151. Although many of these tests show higher sensitivity in detection of bladder cancer than urine cytology, they are often limited by lower specificity, false positive results and better utility in high-grade lesions147–150. Efforts to identify new markers, including TERT and FGFR3 alterations, are ongoing, but hurdles remain to determine whether these will outperform existing approaches to urine-based diagnosis152.
ctDNA analysis.
In addition to tumour markers in urine, cell-free DNA with tumour-specific alterations is released into the blood circulation (circulating tumour DNA; ctDNA) mainly by cell death153. ctDNA is cleared through nuclease digestion, renal clearance, and uptake by the liver and spleen154–157. The half-life of ctDNA is ~2 hours158, which makes ctDNA useful for real-time tracking of tumour burden following surgery and during oncological treatment. Analysis of ctDNA in plasma has shown promising results for the detection of minimal residual disease and metastatic relapse in multiple cancer types, including bladder cancer159. In one prospective study, ctDNA measurements detected clinical relapse on average 3 months earlier than CT scans and better predicted the outcome following neoadjuvant chemotherapy compared to pathological response159,160. Furthermore, ctDNA levels have been shown to correlate to pathological complete response and outcome following neoadjuvant immunotherapy161. Of note, another study used ctDNA measurements to document a survival benefit with adjuvant immunotherapy in patients positive for ctDNA162,163. These results are promising overall, especially for the detection of minimal residual disease and for guiding adjuvant treatment, but further replication in large cohorts and development of optimal laboratory procedures for clinical use are needed. Furthermore, additional knowledge of ctDNA assay sensitivity and specificity is needed to address false positive and false negative rates in specific settings. ctDNA-guided clinical intervention trials are currently ongoing to determine the benefit of blood-based tests to guide adjuvant immunotherapy (for example, IMVIGOR011 and TOMBOLA)164,165. Importantly, ctDNA analysis can also identify genomic alterations associated with metastatic disease166,167, potentially serving as actionable therapeutic targets.
Tissue-based diagnosis of bladder cancer.
Analysis of samples from biopsy or TURBT at the time of cystoscopy is the most common method of initial diagnosis. Pathological analysis confirms the presence of cancer, histological type and stage. Bladder carcinoma is subdivided by grade into low-grade and high-grade categories, with low-grade carcinomas showing frequent recurrence but limited progression168. High-grade carcinomas can be either NMIBC or MIBC, of which NMIBC commonly shows recurrence and progression to MIBC, requiring more aggressive clinical management and follow-up.
More than 90% of all bladder carcinoma histological subtypes are of urothelial histology, with the remainder comprising squamous cell carcinoma, adenocarcinoma and neuroendocrine carcinoma168,169 (Fig. 5). These broad categories describe ‘pure’ or non-mixed carcinomas representing a single histological type of carcinoma. Urothelial carcinoma itself can occur as a broad array of variants or subtypes such as micropapillary, plasmacytoid, nested and lymphoepithelioma-like carcinomas. These categories are defined by the WHO Classification of Tumours of the Urinary System and Male Genital Organs168. Several subtypes have been associated with unique molecular and/or therapeutic considerations. Micropapillary urothelial carcinoma, which shows clusters of inversely polarized nests of tumour cells within prominent retraction spaces, has a disproportionately higher rate of ERBB2 amplification than conventional urothelial carcinoma170–172. This amplification has been identified in up to 40% of micropapillary urothelial carcinomas, resulting in efforts to selectively target this pathway170. Plasmacytoid urothelial carcinoma, which is defined by distinct CDH1 mutations and a morphology that shows single, plasma cell-like cells that are highly infiltrative, is another research focus173. Micropapillary and plasmacytoid urothelial carcinomas are biologically aggressive subtypes and optimizing the approach to these diagnostic categories has resulted in some institutions advocating early cystectomy regardless of stage168. Furthermore, micropapillary urothelial carcinoma is often variably mixed with conventional urothelial carcinoma, with higher proportions of micropapillary urothelial carcinoma portending a more aggressive pathological behaviour174,175. Despite their urothelial carcinoma origin, these two examples of urothelial carcinoma subtypes highlight the dramatic differences in urothelial carcinoma evolution and differentiation, which complicates a unified approach to understanding and treating bladder cancer.
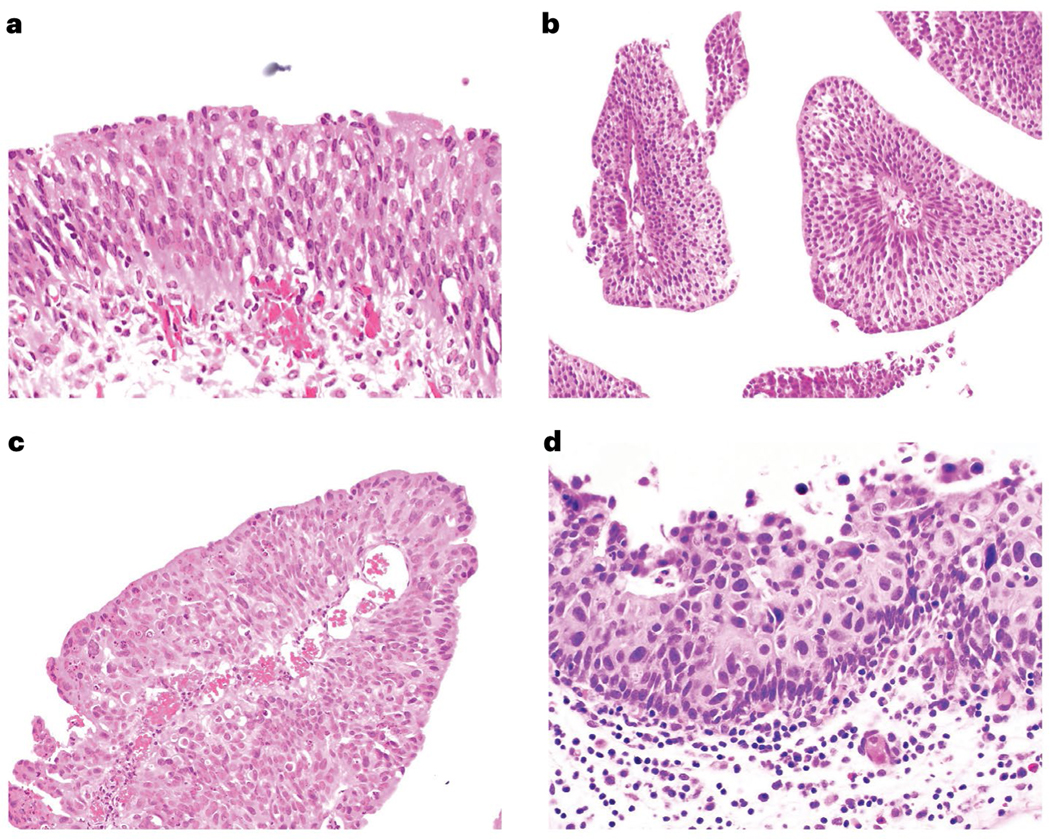
Fig. 5 |. Histopathology of bladder cancer.
Normal urothelium (part a) is defined by cellular polarization towards the luminal surface with individual cells relatively monotonous in appearance and containing open chromatin. Low-grade papillary urothelial carcinoma (part b) shows papillary cores, in this image cut in cross-section, lined by urothelium that remains relatively monotonous and polarized but with hyperchromasia of some nuclei. Non-invasive high-grade neoplasia in the bladder may be papillary (part c) or flat (part d) and demonstrates disorganization, nuclear enlargement, nuclear pleomorphism, and hyperchromasia. High-grade lesions have the potential to invade beyond the basement membrane and into the underlying bladder wall.
In addition to histological subtyping, pathological analysis determines the depth of invasion of the carcinoma at biopsy or TURBT and also following cystectomy. Pathological (after cystectomy) staging is defined by the American Joint Committee on Cancer (AJCC), currently in its eighth edition176. NMIBC occurs as either papillary (pTa) or flat urothelial CIS (pTis). Invasion of the lamina propria (pT1), invasion of the muscularis propria (pT2), perivesical fat (pT3) and involvement of adjacent organs (pT4) are associated with a progressive reduction in survival176. Determination of pathological stage on cystectomy specimens is straight-forward but diagnosis and staging on TURBT samples are challenging owing to the extent of sampling, interpretation artefact due to cautery or crush phenomenon, and lack of objective markers to conclusively determine if muscularis propria is present.
Use of tissue to predict progression from lamina propria-invasive (T1) disease to muscle-invasive carcinoma has been a subject of interest for some time. A recommendation was made in the AJCC manual to attempt substaging T1 disease based on numerous studies that showed that a larger amount of tumour in the lamina propria correlated with a higher rate of progression176. However, various approaches were used in the studies, including different cut-off criteria for substaging, surface orientation in some approaches that was impossible to perform on a considerable subset of specimens and diverse outcome end points. An additional confounder was the challenge of not knowing with certainty whether the lesion was fully resected. Comparison of these various approaches showed that an aggregate tumour measurement of ≥2.3 mm outperformed other histology-based approaches in predicting progression to muscle-invasive disease177. Since the endorsement of attempted substaging of T1 disease by the AJCC, numerous studies have evaluated additional approaches to predicting progression to MIBC, including histological, molecular and/or protein biomarkers178,179. Ultimately, these are challenging endeavours given the uncertainty regarding the presence of residual tumour, effects of precedent therapies on disease progression, and cellular heterogeneity associated with bladder cancer.
Staging
Diagnostic imaging is critical for both local and distant staging. During a work-up of haematuria, abdominopelvic imaging including imaging of the upper urinary tract (renal pelvis and ureters) should be performed to assess for a bladder mass (ideally prior to TURBT)180–182. Imaging informs both location and extent of disease (including potential upper tract involvement, extravesical extension, hydronephrosis, nodal involvement or distant metastatic disease). CT urography with and without an intravenous contrast agent is preferred and has largely replaced intravenous pyelogram183,184. In patients with poor renal function or allergy to iodinated contrast agents, MR urogram with a gadolinium-based contrast agent may be considered185. Renal ultrasonography or CT without a contrast agent combined with a retrograde ureteropyelography is conducted in patients who cannot receive iodinated or gadolinium-based contrast agents183,184.
In addition to CT urography, MRI of the pelvis with and without an intravenous contrast agent may be considered for further local staging, especially regarding depth of bladder wall invasion186. The best evidence supporting the use of MRI is in MIBC in the pre-TURBT setting to improve staging187. Multiparametric MRI has improved soft tissue resolution compared with CT, and the Vesical Imaging Reporting and Data System (VI-RADS) score has been developed to predict the likelihood of muscle invasion188. MRI may also have the potential to assess response after treatment, including TURBT, neoadjuvant chemotherapy and/or chemoradiation189.
For patients with NMIBC, chest and other metastatic imaging is not necessary, whereas for patients with MIBC, chest CT is recommended140. Bone scans and brain MRI have limited value and are typically reserved for symptomatic patients or those at very high risk (stage, tumour size, adverse pathology)190. 18F-fluorodeoxy glucose-PET (FDG PET)-CT is not as commonly used and does not have a clearly established role in patients with localized disease, although it may have more value in locally advanced disease and when metastatic disease is suspected191–194.
Prognostic and predictive biomarkers
In NMIBC, several prognostic biomarkers have been described; however, none have yet been implemented in clinical decision-making. For example, in one study, patients with NMIBC at high risk for progression were subdivided into groups with good, moderate and poor risk of progression based on mutations in FGFR3 and methylation of GATA2 (ref. 195). In addition, studies using measurements of genome-wide copy number alterations through array-based comparative genomic hybridization54 or SNP array analysis32 separated patients with Ta tumours or NMIBC, respectively, into different groups and found an association between a high level of copy number alterations and poor outcomes. Furthermore, tumour mutational burden (TMB) and APOBEC-associated mutations have been associated with increased NMIBC aggressiveness32. However, when analysing T1 tumours only, a high TMB was associated with better survival196. Earlier studies of gene expression subtypes in NMIBC identified two major molecular subtypes associated with disease aggressiveness197,198. Five subtypes of bladder cancer were identified when considering the whole spectrum of bladder cancer stages. The subtypes urothelial-like, genomically unstable, and a group of infiltrated cases were specifically associated with NMIBC199. Three expression-based subtypes were reported by the UROMOL consortium, which showed different clinical outcomes and molecular characteristics33. The work from the UROMOL consortium was later expanded and four subtypes were identified: the UROMOL2021 classification system showed overlap with previously reported subtypes but with increased granularity32. In another multi-omics approach, further molecular heterogeneity within disease stage categories was discovered, enabling further subclassification of Ta and T1 tumours34.
In MIBC, several classification systems based on gene expression subtypes have been reported, ranging from two major subtypes (luminal and basal)200 to six subtypes201. A consensus classification of six subtypes using previous classification systems has been reported202. The subtypes harbour different molecular alterations and immune cell characteristics and, overall, have been reported to be prognostic. In patients with MIBC, high TMB and neoantigen loads have been associated with particularly good survival and a high mutational contribution from APOBEC mutational processes was also associated with improved survival51, similar to observations in T1 tumours196.
Several studies sought to develop predictive biomarkers in both NMIBC and MIBC. In relation to Bacillus Calmette–Guérin (BCG) treatment in NMIBC, high PDL1 expression has been associated with BCG unresponsiveness, linking immune inhibitory pathways to BCG failure203. In another study, T cell exhaustion in the tumour was associated with outcome following BCG instillations204. In one study, molecular profiling of high-risk BCG-naive NMIBC and recurrent tumours after BCG treatment found three distinct BCG response subtypes (BRS1–3)205. Patients with BRS3 tumours had reduced recurrence-free and progression-free survival than patients with BRS1 and BRS2. BRS3 tumours expressed high epithelial–mesenchymal transition and basal markers and had an immunosuppressive profile. Tumours that recurred after BCG were enriched for BRS3. In a second cohort of BCG-naive patients with high-risk NMIBC, BRS molecular subtypes outperformed guideline-recommended risk stratification based on clinicopathological variables.
In MIBC, expression of and mutations in genes involved in DNA damage response are associated with a particularly good outcome following chemotherapy and chemoradiation206–210. Some of these genomic alterations have been tested in a clinical trial evaluating bladder-sparing approaches; however, the study did not reach the primary end point and further study refinements are needed211. In addition, a CD8+ T effector cell phenotype, high TMB and high neoantigen load have been demonstrated to be predictors of immunotherapy response in MIBC, whereas lack of response was associated with a signature of transforming growth factor-β (TGFβ) signalling in fibroblasts212. Other studies demonstrated that MIBC tumours of the luminal subtypes show an improved response to chemotherapy213,214 but contradicting results have also been reported215. Further gene expression profiling studies have shown that increased immune cell infiltration in MIBC is associated with improved outcomes after chemoradiation, whereas increased stromal infiltration is associated with worse outcomes after neoadjuvant chemotherapy and cystectomy216. Several seminal studies have shown substantial intratumour heterogeneity using single-cell and spatial transcriptomic analysis, which is likely complicating the utility of current subtype classifications for clinical outcome prediction79,217.
Management
The management of bladder cancer requires careful consideration of disease stage and tumour characteristics as well as patient demographics, comorbidities and preferences. Optimal treatment involves a multidisciplinary approach that may include surgery, chemotherapy, radiation therapy, immunotherapy and targeted therapy.
TURBT and en bloc resection of bladder tumour
TURBT is a diagnostic, staging and, for NMIBC, therapeutic tool, making it a cornerstone in management. The procedure starts with a comprehensive inspection of the bladder, followed by resection of the exophytic part of the tumour, and separate resection of the underlying bladder wall and edges of the resection area142. TURBT has two main goals: complete (possibly curative) resection in the case of NMIBC, and proper local staging and expediting subsequent definite treatment in the case of MIBC. To ensure complete tumour eradication in NMIBC, the quality of resection is extremely important, but the procedure is highly dependent on operator skills and experience218. Although TURBT aims to completely resect NMIBC, this is not always possible due to its technical difficulty and fear of bladder perforation. A second TURBT, 2–6 weeks later, is indicated if the tumour was not completely resected in the first TURBT, if the patient has T1 disease, or if detrusor muscle is absent in the first TURBT specimen with the exception of Ta low-grade tumours and primary CIS142. Second TURBT may be associated with improved progression-free survival in patients with T1 NMIBC219. A meta-analysis of 81 studies found that the pooled rates of any residual tumours and upstaging on second TURBT were 31.4% and 2.8%, respectively220, highlighting the limitations of the conventional TURBT procedure. In the case of MIBC, maximal TURBT is also important to optimize subsequent treatment such as radical cystectomy and trimodality therapy (TMT)221,222. Maximal resection of all visible bladder tumours down to the detrusor muscle layer should be pursued even when MIBC is suspected endoscopically221,222.
En bloc resection of bladder tumour (ERBT), that is, removal of the bladder tumour in one piece, has been proposed as a potentially more favourable surgical approach than conventional TURBT223,224. Results from three randomized trials comparing ERBT and TURBT have been reported225–227. In one trial225, the rate of detrusor muscle presence for ERBT was non-inferior to TURBT (94% versus 95%), and T1 substaging was more feasible in the ERBT group (100% versus 80%; P = 0.02). In a second trial226, the ERBT group had a higher rate of detrusor muscle presence (80.7% versus 71.1%; P = 0.01) and a lower rate of bladder perforation (5.6% versus 12%, difference –6.4%, 95% CI –12.2 to −0.6%) than the TURBT group. In a third trial227, ERBT resulted in a reduction in the 1-year recurrence rate from 38.1% to 28.5% (P = 0.007), and 30-day complications were similar between the two groups.
A single dose of intravesical chemotherapy (commonly mitomycin C or epirubicin) immediately after TURBT is associated with a decreased risk of recurrence228. A systematic review and individual patient data meta-analysis of a total of 2,278 patients found that a single dose of intravesical chemotherapy reduced the risk of recurrence by 35% (P < 0.001)228. However, this benefit was not observed in patients with a prior recurrence rate of >1 per year, or in patients with a European Organization for Research and Treatment of Cancer (EORTC) recurrence score of ≥5 (ref. 228). Single-dose intravesical chemotherapy should not be given when there is a concern for bladder perforation as chemotherapy extravasation can result in severe consequences229.
Although TURBT with or without single-dose intravesical chemotherapy is the standard of care for the treatment of NMIBC, it is a major surgery requiring formal anaesthesia, which could be a burden for patients with recurring diseases. As the risk of disease progression for recurrent Ta low-grade bladder tumours is low, fulguration or laser vaporization of small papillary recurrences on an outpatient basis has been proposed to reduce the therapeutic burden142,230,231. In particular for patients at advanced age, watchful waiting with urine cytology and regular cystoscopy without resection can also be considered232.
Intravesical therapy for NMIBC
Intravesical therapy with BCG vaccine was first proposed in 1976 as an immunotherapy to treat bladder cancer233 and became a standard of care for NMIBC. A randomized study to investigate the optimal BCG schedule for intermediate-risk and high-risk NMIBC with a primary outcome of disease-free interval, concluded that 1 year and 3 years of full-dose BCG should be given to patients with intermediate-risk and high-risk NMIBC, respectively234. Adverse effects of BCG include inflammation and/or infection of the bladder, prostate, epididymis, and testis as well as general malaise, fever and BCG sepsis142. Since 2013, an intermittent BCG shortage has been a global problem and alternative treatment options are urgently needed235,236. Intravesical maintenance chemotherapy can be an alternative in intermediate-risk NMIBC, but its efficacy in high-risk NMIBC is limited142. New intravesical therapies, such as intravesical gene therapy with nadofaragene firadenovec237 and systemic ICI therapy with pembrolizumab238 have been approved by the FDA for BCG-unresponsive NMIBC with CIS, with or without papillary tumours.
Intravesical maintenance chemotherapy, given repeatedly on a weekly or monthly basis239,240, has been investigated as an alternative to intravesical BCG therapy. A meta-analysis compared TURBT plus intravesical maintenance chemotherapy with TURBT only and found that the use of intravesical maintenance chemotherapy was associated with a 44% reduction in 1-year recurrence (P < 0.001)241. In an individual patient data meta-analysis comparing intravesical maintenance chemotherapy and intravesical BCG, the use of BCG was associated with a 32% reduction in the risk of recurrence (P < 0.001)240. In patients with intermediate-risk NMIBC who cannot tolerate intravesical BCG, intravesical maintenance chemotherapy can be considered noting its inferiority in oncological efficacy.
Radical cystectomy
Radical cystectomy is a standard of care in localized MIBC182 and in patients with BCG-unresponsive NMIBC182. The surgery itself includes three major components: cystectomy, pelvic lymph node dissection (LND) and urinary diversion. In men, standard radical cystectomy includes removal of the bladder, prostate, seminal vesicles and distal ureters182. In women, standard radical cystectomy includes removal of the bladder, the entire urethra, anterior vaginal wall, uterus and distal ureters182. Standard LND includes removal of bilateral obturator, internal and external iliac lymph nodes. Two randomized trials investigated the role of extended LND (including the common iliac, presacral and up to, at least, the aortic bifurcation) and found that extended LND was associated with more grade ≥3 complications242,243 but no benefit in recurrence-free survival242, cancer-specific survival242, disease-free survival243 and overall survival242,243. For urinary diversion, ileal conduit and orthotopic neobladder are commonly performed. The choice of urinary diversion depends on patient factors (for example, age, renal function, ability to perform self-catheterization and patient preference) and disease factors (for example, urethral involvement, locally advanced disease and need for adjuvant therapy)244. Patients should be carefully counselled about the advantages and disadvantages of each option so that a shared decision can be made in the best interest of the patient. Radical cystectomy can be performed in an open, laparoscopic or robot-assisted approach. In a meta-analysis comparing robot-assisted radical cystectomy (RARC) with open radical cystectomy (ORC), no difference in terms of recurrence-free survival (HR 0.99, 95% CI 0.75–1.31) and overall survival (HR 0.98, 95% CI 0.73–1.30) was found245. RARC had a lower transfusion rate (OR 0.42, 95% CI 0.30–0.59) but a longer operative time (mean difference 78.54 min, 95% CI 45.87–111.21 min) than ORC245. Overall complications, major complications, positive margin rates and length of hospital stay did not differ245. High-quality data comparing RARC with intracorporeal versus extracorporeal urinary diversion are lacking, although non-randomized studies favoured the intracorporeal approach showing benefits in blood loss and hospital stay246,247. High-quality data on laparoscopic radical cystectomy is limited245.
Some patients with pT3/T4 pN0–2 bladder cancer (N0, no regional lymph node metastasis; N1, metastasis in a single regional lymph node; N2, metastasis in multiple regional lymph nodes) may be candidates for postoperative adjuvant pelvic radiotherapy to the pelvic lymph nodes with or without the cystectomy bed following radical cystectomy248,249. Addition of adjuvant radiotherapy to chemotherapy alone was associated with improved local relapse-free survival250.
Partial cystectomy may be considered in highly selected patients, including those with solitary tumours at favourable locations, such as the bladder dome, without concomitant CIS251. Special caution must be taken to avoid urine and tumour spillage during the procedure. To date, there are no randomized trials comparing partial with radical cystectomy, but previous retrospective studies showed comparable results251. Patient selection is key should partial cystectomy be contemplated.
Trimodality therapy
TMT is a bladder-preserving treatment of MIBC that includes a maximal, ideally visibly complete, TURBT followed by concurrent radiosensitizing chemotherapy and radiotherapy (chemoradiotherapy). TMT is an accepted alternative to radical cystectomy for selected patients with MIBC who have a desire to retain their native bladder or who are medically unfit for radical cystectomy181,182,252 and may be most effective in patients with specific characteristics (Box 1). Randomized controlled trials comparing TMT to radical cystectomy closed due to lack of accrual253, but best available data from prospective TMT trials (including from NRG/RTOG in the USA and from UK-based trials), meta-analyses and multi-institutional cohorts demonstrate comparable survival254–258. Chemoradiotherapy is considered standard in patients who can tolerate combined therapy, following a phase III randomized BC2001 trial that showed that concurrent chemoradiotherapy with 5-fluorouracil and mitomycin leads to improved locoregional disease control compared with external beam radiotherapy alone257. Other options for concurrent chemotherapy include cisplatin-based regimens or single-agent gemcitabine259. Ongoing randomized trials are investigating the addition of immunotherapy (for example, atezolizumab or pembrolizumab) to TMT260,261.
Box 1.
Optimal patient characteristics for trimodality bladder-sparing treatment for muscle-invasive bladder cancer
Predominant urothelial cancer histology
Unifocal tumour <7 cm in size
Visibly complete transurethral resection of bladder tumour
Clinical stage T2–T3a
Lack of extensive carcinoma in situ
Absence of hydronephrosis
Good bladder function
Lifelong post-treatment bladder surveillance is essential for the detection of in-bladder recurrences (10-year rates: NMIBC 20–26%, MIBC 13–18%) or second primary tumours, and 10–15% of patients may require a salvage cystectomy, which is associated with a higher risk of overall and major late complications than primary cystectomy and most often requires an incontinent urinary diversion262. Patients with MIBC and who are appropriate candidates should be offered the choice between radical cystectomy and TMT approaches. MIBC treatment, and in particular TMT, requires close multidisciplinary collaboration and environments that enable shared and informed decision-making263. A multi-institutional study in 722 patients (440 radical cystectomy, 282 TMT) used propensity score matching and logistic regression to show similar oncological outcomes between these two treatment modalities258. Although there are no conclusive randomized trials supporting the equivalence of TMT to radical cystectomy for selected patients in bladder cancer, the current evidence from other studies as summarized above supports that TMT, in the setting of multidisciplinary shared decision-making, should be offered to all suitable candidates with MIBC and not only to patients with considerable comorbidities for whom surgery is not an option258.
Bladder-preserving TMT has also been evaluated in a small phase II single-arm study in patients with recurrent high-grade NMIBC following intravesical therapy for whom the next step would be cystectomy, with chemoradiotherapy leading to favourable (88%) cystectomy-free survival results at 3 years264.
Radiotherapy of the primary tumour and possible sites of metastases may also have a role in oligometastatic bladder cancer. Studies suggest a possible survival benefit when adding local therapy to the bladder (including radiotherapy over chemotherapy alone) in metastatic disease265,266 and when using metastasis-directed therapy267,268. However, data are limited in the adjuvant, recurrent NMIBC and oligometastatic settings, and further prospective research is needed.
Perioperative systemic therapy
For patients with MIBC, the risk of metastatic recurrence despite curative-intent local therapy (that is, radical cystectomy or TMT) is high and systemic therapy has been explored to further improve outcomes. The BA06 30894 trial compared neoadjuvant cisplatin, methotrexate plus vinblastine followed by definitive local therapy versus definitive local therapy alone in patients with clinical stage T2–T4aN0M0 and is the largest neoadjuvant study reported to date269. This trial revealed that neoadjuvant cisplatin, methotrexate plus vinblastine improved survival (HR 0.84, 95% CI 0.72–0.99). The Southwest Oncology Group 8710 trial randomized patients with clinical stage T2–T4aN0M0 to neoadjuvant methotrexate, vinblastine, doxorubicin plus cisplatin (MVAC) followed by cystectomy versus cystectomy alone270. This trial reported an improvement in overall survival with neoadjuvant MVAC (HR 0.75, 95% CI 0.57–1.00). Importantly, these trials of neoadjuvant cisplatin-based chemotherapy have revealed an increased likelihood of achieving a pathological complete response at cystectomy with neoadjuvant chemotherapy followed by cystectomy versus cystectomy alone270. Meta-analyses of the neoadjuvant chemotherapy trials in MIBC have confirmed the survival benefit leading to this approach becoming standard care271. The optimal form of neoadjuvant chemo therapy, gemcitabine plus cisplatin or dose-dense MVAC remains controversial272–274.
Deferring decisions regarding the use of systemic therapy for MIBC to the postoperative setting is attractive given the ability to base treatment decisions on more precise pathological staging rather than clinical staging. Notwithstanding, clinical trials exploring adjuvant chemotherapy in patients with pT3–4 and/or pN+ urothelial cancer of the bladder have provided less robust evidence275 despite observational analyses and meta-analyses suggesting a benefit275,276.
There has historically been no standard perioperative systemic therapy to decrease the risk of recurrence after curative-intent surgery in cisplatin-ineligible patients with high-risk pathological features at cystectomy (pT3 and/or pN+) or patients who received prior neoadjuvant therapy with high-risk pathological features at cystectomy (pT3 and/or pN+). Two phase III trials with a similar design sought to define the role of adjuvant PD1 or PDL1 blockade in this population by randomly allocating patients to 1 year of adjuvant PD1 or PDL1 blockade versus observation or placebo. Checkmate 274 demonstrated a significant improvement in disease-free survival in the overall population (HR 0.70, 95% CI 0.55–0.90) and in the subset of patients with tumours with increased PDL1 expression (HR 0.55, 95% CI 0.35–0.85)277, leading to regulatory approval of adjuvant nivolumab for bladder cancer in several parts of the world. IMvigor 010 did not demonstrate an improvement in the primary end point of disease-free survival278. However, an exploratory analysis suggested a disease-free and overall survival benefit with adjuvant atezolizumab versus placebo in patients with detectable baseline ctDNA162, paving the way for ctDNA-based studies of adjuvant therapy in bladder cancer.
Systemic therapy for metastatic bladder cancer
Cisplatin-based combination chemotherapy became a standard treatment for metastatic bladder cancer in the early 1990s after a randomized clinical trial demonstrated a survival benefit with MVAC versus cisplatin alone279. A series of subsequent randomized trials found that administration of MVAC in a dose-dense fashion and/or with granulocyte colony-stimulating factor support was associated with less toxicity and possibly enhanced efficacy280,281 and that the combination of gemcitabine plus cisplatin yielded similar efficacy but less toxicity than MVAC282. Although cisplatin-based chemotherapy became a standard of care for patients with metastatic urothelial cancer, many patients with bladder cancer are of advanced age and many are ineligible for cisplatin283. For these patients, gemcitabine plus carboplatin is generally substituted284.
By 2015, PD1 and PDL1 ICIs had demonstrated durable responses in 20–25% of patients with metastatic urothelial cancer and received regulatory approval initially in patients progressing despite first-line platinum-based chemotherapy and, subsequently, as first-line treatment for cisplatin-ineligible patients285–289. Only the approval of pembrolizumab in patients with platinum-resistant metastatic urothelial cancer was based on a randomized phase III trial287 with the remainder based on single-arm phase II studies. Potential adverse events with PD1 and PDL1 ICIs include but are not limited to immune-related adverse events such as colitis, pneumonitis, dermatitis, hepatitis and endocrinopathies. Although requiring thorough validation in larger series, if the early data showing that LOY tumours are more vulnerable to ICIs holds, this would be a potentially valuable marker to stratify patients to this approach130.
Several phase III trials were launched to optimize the use of these therapies. IMvigor 130 (ref. 290) and Keynote 361 (ref. 291) compared platinum-based chemotherapy versus PD1 or PDL1 blockade versus platinum-based chemotherapy plus PD1 or PDL1 blockade as first-line treatment for metastatic urothelial cancer. These trials failed to demonstrate a benefit of concurrent platinum-based chemotherapy plus PD1 or PDL1 blockade versus platinum-based chemotherapy alone. A randomized phase II and III trial compared switch maintenance PD1 or PDL1 blockade (pembrolizumab and atezolizumab, respectively) versus placebo or observation in patients with at least stable disease after initial platinum-based chemotherapy292,293. These trials met their primary endpoints, with the phase III JAVELIN-Bladder 100 study demonstrating an overall survival benefit, resulting in switch maintenance ICI being adopted into standard treatment paradigms. After decades of investigation, platinum-based chemotherapy remains the standard-of-care first-line treatment for most patients with metastatic urothelial cancer with switch maintenance ICI employed for patients, with stable disease after ~4–6 cycles of chemotherapy. However, in some regions, the combination of an antibody–drug conjugate (enfortumab vedotin) plus pembrolizumab has received regulatory approval as first-line treatment for cisplatin-ineligible patients based on relatively high response rates and promising response durations294. Several new therapies with distinct mechanisms of action have subsequently been integrated into standard therapeutic strategies for metastatic bladder cancer (Table 3).
Table 3 |.
New systemic therapies for metastatic bladder cancer
| Drug | Mechanism of action | Evidence | Select adverse events |
|---|---|---|---|
| Erdafitinib | Small-molecule inhibitor of fibroblast growth factor receptor 3 | In a phase II study of patients with FGFR3-mutated metastatic urothelial cancer progressing despite prior platinum-based chemotherapy, erdafitinib demonstrated an objective response rate of 42%320 | Hyperphosphataemia, stomatitis, hand–foot syndrome as well as ocular disorders such as central serous retinopathy |
| Enfortumab vedotin | Antibody–drug conjugate comprised of a monoclonal antibody directed against nectin 4 linked to a monomethyl auristatin E payload | The phase III EV-301 trial321 randomized patients with metastatic urothelial cancer progressing despite prior platinum-based chemotherapy and PD1 or PDL1 blockade to treatment with enfortumab vedotin versus standard chemotherapy (docetaxel, paclitaxel or vinflunine); the trial demonstrated an improvement in overall survival with enfortumab vedotin versus chemotherapy (HR 0.70, 95% CI 0.56–0.89; P = 0.001); the combination of enfortumab vedotin plus pembrolizumab has been explored as first-line treatment in cisplatin-ineligible patients with metastatic urothelial cancer322, yielding a 73% response rate | Peripheral neuropathy, hyperglycaemia, rash |
| Sacituzumab govitecan | Antibody–drug conjugate comprising a monoclonal antibody directed against TROP2 linked to the topoisomerase I inhibitor SN-38 payload | A large phase II trial demonstrated an objective response rate of 27% with sacituzumab govitecan in patients with metastatic urothelial cancer progressing despite prior platinum-based chemotherapy and PD1 or PDL1 immune-checkpoint inhibition323 | Diarrhoea, neutropenia |
New systemic therapies that have received regulatory approval in at least one region of the world are shown.
Quality of life
A cross-sectional survey investigated the health-related QoL (HRQoL) of 1,796 patients with bladder cancer, of whom 868 (48%) had NMIBC, 893 (50%) received radical cystectomy or radiotherapy, and 35 (1.9%) had unknown treatment295. Most patients (69%) reported at least one problem in any EQ-5D dimension295. HRQoL outcomes adjusted for age and sex were similar across all stages and treatment groups. Sexual problems were common in male patients and increased with younger age and radical treatment295. A prospective study of 133 patients using the Short-Form 36-item survey (SF-36) found that physical functioning, social functioning and role-emotional of patients worsened with first, second and third TURBT, and finally improved when TURBT was performed ≥4 times296. Patient mental health was also impaired at first TURBT but gradually returned to normal with repeated TURBT.
A study investigated the QoL of 103 patients with NMIBC who received intravesical BCG or mitomycin C using the EORTC QLQ-C30 and QLQ-BLS24 questionnaires297. QoL seemed to drop after the induction course and returned to baseline at 3 months. QoL was more affected in patients aged >70 years, especially in those who received intravesical BCG therapy. In another study, QoL of 106 patients with NMIBC who underwent intravesical chemotherapy was evaluated using the EORTC QLQ-C30 and the Core Lower Urinary Tract Symptom Score questionnaire, finding that global health status and social functioning decreased and that Core Lower Urinary Tract Symptom Score also worsened significantly298.
A meta-analysis investigated the HRQoL following radical cystectomy and urinary diversion299. All included studies reported an initial deterioration in overall HRQoL but general health, functional and emotional domains at 12 months after surgery were similar to or better than baseline. Overall, there was no significant difference in HRQoL between continent and incontinent urinary diversion. Subgroup analysis showed greater improvement in physical health for patients undergoing incontinent urinary diversion but mental health and social health did not differ between diversion types299. Qualitative analysis showed that patients with neobladder had better emotional function and body image than those with cutaneous diversion299.
A meta-analysis comparing RARC and ORC showed no significant difference in QoL (standard mean difference –0.02, 95% CI –0.17 to 0.13; P = 0.78)300. In the RAZOR study comparing RARC plus extracorporeal urinary diversion and ORC, no significant difference in the Functional Assessment of Cancer (FACT)-Vanderbilt Cystectomy Index was found between the two groups at any time point. In the iROC study comparing RARC plus intracorporeal urinary diversion and ORC, patients undergoing ORC had worse QoL at 5 weeks and greater disability at 5 weeks and 12 weeks, but their QoL improved with time and QoL did not differ between RARC and ORC after 12 weeks301.
TMT experiences have shown favourable toxicity profiles and good long-term QoL. Late pelvic (genitourinary or gastrointestinal) grade ≥3 toxicity rates from the NRG/RTOG and BC2001 trials are acceptable and low (1–6%)257,302. Analysis of long-term survivors from four NRG/RTOG trials showed that TMT was associated with 5.7% genitourinary and 1.9% gastrointestinal late grade 3 toxic effects (that rarely persist) and no late grade 4 toxic effects or treatment-related deaths302. In TMT series, <1% of patients required cystectomy due to treatment-related toxicity222,258. Other studies from prospective trials and retrospective cohorts and using validated instruments as well as urodynamic studies in long-term survivors of TMT for MIBC made three QoL-related findings. First, the BC2001 trial showed short-term declines in HRQoL during treatment and immediately following chemoradiation, as would be expected, but these improved to baseline levels after 6 months with no impairment from the addition of chemotherapy303. Second, most patients have normally functioning bladders following therapy304. Third, TMT resulted in QoL gains compared with radical cystectomy, including modestly better general HRQoL, markedly better sexual function and QoL, better-informed decision-making, less concerns about appearance, and less life interference from cancer or cancer treatment305.
A Swedish bladder cancer data base investigated the natural history of patients unable or unwilling to receive therapy with curative intent306. Among patients with T2–3 M0 disease, a median of 2.4 hospitalizations per patient occurred during the first 12 months of diagnosis and half of these hospitalizations were due to cancer or genitourinary symptoms306. These patients experienced substantial disease-specific morbidity, which might have been avoided if they underwent treatment with curative intent306.
Several large phase III trials have evaluated QoL of patients with bladder cancer receiving systemic therapy. There are limited available instruments that have been designed and validated to assess both general and bladder cancer-specific QoL domains in these patients. The FACT-Bladder (FACT-BI) is a 39-item questionnaire that integrates questions regarding general QoL domains (FACT-General) as well as a cancer site-specific bladder subscale and has been assessed for validity in a cohort of patients with metastatic bladder cancer receiving ICIs307. This tool and the National Comprehensive Cancer Network/Functional Assessment of Cancer Therapy Bladder Symptom Index-18 (FBlSI-18), EORTC QLQ-C30 and EuroQol-4D (EQ-5D) have been most commonly employed in bladder cancer trials.
The effects of neoadjuvant cisplatin-based chemotherapy on QoL are not well studied. In a randomized trial comparing two cycles of neoadjuvant MVAC followed by cystectomy versus cystectomy alone in 99 patients, QoL was assessed using the FACT-Bl instrument308. QoL after completion of chemotherapy was lower than baseline scores in domains including physical and functional well-being as well as for total FACT-Bl scores; however, there was no difference in these domains between study arms on follow-up after radical cystectomy. In the Checkmate 274 trial comparing adjuvant nivolumab versus placebo in 709 patients, QoL was assessed using the EORTC QLQ-C30 and the EQ-5D-3L309: adjuvant nivolumab was non-inferior to placebo on changes from baseline across all major domains. In the JAVELIN-Bladder 100 trial of switch maintenance avelumab versus observation in 700 patients with at least stable disease after first-line platinum-based chemotherapy, the FBlSI-18 and EQ-5D-5L instruments were explored310. Switch maintenance avelumab was demonstrated to have minimal effects on QoL. QoL with ICIs was also assessed in the Keynote-045 trial comparing pembrolizumab versus chemotherapy in 519 patients with platinum-resistant metastatic bladder cancer311. Pembrolizumab prolonged the time to deterioration in global QoL compared with chemotherapy (median 3.5 months versus 2.3 months, hazard ratio 0.72; nominal one-sided P = .004). QoL with systemic therapy in patients with bladder cancer is complex to measure and interpret given the variability of instruments, time points and heterogeneity in clinical disease states with differential effects of disease-related and treatment-related burden.
Because of its unique biology, such as high recurrence rates, procedural requirements related to surveillance and expensive treatments, bladder cancer management contributes considerably to medical costs. In the USA, the overall annual costs of cancer were US$183 billion in 2015 and are projected to increase to US$246 billion by 2030 (ref. 312). Bladder cancer contributed US$7.93 billion in 2015, with an anticipated increase of US$11.6 billion by 2030. Similarly, among European Union members, cancer costs totalled € 152.8 billion in 2012, of which bladder cancer contributed € 5.24 billion (adjusted to 2019 values)313. Multiple cost-effectiveness analyses and reviews have been published and provide perspectives on the cost, efficacy and effects on QoL of interventions in patients with bladder cancer314–316.
Outlook
Bladder cancer is a considerable and growing global health issue and its prevalence is expected to increase by 2040. However, with advances in molecular biology and therapy culminating progress over the past 100 years (Fig. 6), there is hope for the development of more effective diagnostic and treatment options that can improve patient outcomes.
Fig. 6 |. Landmarks in understanding, diagnosis and treatment of bladder cancer.
This timeline shows seminal developments in bladder cancer, highlighting clinical, scientific and technical advances that have changed or will change clinical practice or scientific thinking in the field51,130,233,271,277,285,287,289,293,324–332. BCG, Bacillus Calmette–Guérin; MVAC, methotrexate, vinblastine, doxorubicin plus cisplatin; NAC, neoadjuvant chemotherapy; TCGA, The Cancer Genome Atlas; UC, urothelial carcinoma.
One promising area of research is the development of minimally invasive diagnostic tools, such as urine-based or blood-based tests, that can detect disease recurrences and minimal residual disease. These tests could provide a less invasive and more convenient alternative to current diagnostic methods. Furthermore, the tests could ultimately lead to new ways of guiding oncological decisions and follow-up programmes. Further research in this area should focus on validation of clinical applicability of the tests in clinical trials to demonstrate clinical utility. Furthermore, development of robust multi-cancer early detection tests may ultimately lead to better screening for bladder cancer and in this way detect the disease at earlier stages.
The development of precision medicine approaches is also critical for improving bladder cancer management. The distinct molecular profiles of NMIBC and MIBC suggest that personalized treatment approaches based on the specific genetic mutations of a tumour could lead to more effective outcomes. Similarly, understanding the sex and race differences in bladder cancer incidence and prognosis can help tailor treatment approaches to individual patients and improve outcomes. In addition, there is an urgent need to delineate tumour heterogeneity using single-cell and spatial transcriptomic analysis, which is likely compromising the utility of current subtype classifications for clinical outcome prediction79,217. These approaches will likely provide much-needed clues to clinically tractable approaches that can be used to determine the primary driver populations in each specific tumour and use that driver as a prognosticator, predictor or therapeutic target. Finally, it is essential to continue to prioritize research into the causes and risk factors for bladder cancer. With a better understanding of the underlying biology of the disease, more effective prevention strategies can be developed to identify patients who are at increased risk for developing bladder cancer. This could include lifestyle interventions, such as smoking cessation and dietary changes as well as targeted screening for populations at high risk.
Machine learning, a subdiscipline of artificial intelligence that focuses on data analytics, has played a prominent role in cancer research and care because of the complexity of the disease and the availability of big data from technologies such as genomics and imaging. Applications include predicting regulatory elements in DNA sequences, predicting disease risk in populations, and diagnosing cancer from pathology and radiology images as well as modelling and predicting physiological and biological behaviours or systems biology317,318.
In conclusion, the outlook for bladder cancer is promising, with multiple advances in the understanding of the biological context of bladder cancer, development of novel non-invasive test methods to potentially guide treatment and, finally, the development of multiple novel oncological treatments. A multidisciplinary approach that considers sex and race differences as well as the genetic and molecular characteristics of the disease, will be critical for improving patient outcomes and reducing the global burden of bladder cancer.
Supplementary Material
Footnotes
Competing interests
L.D. has sponsored research agreements with Natera, C2i Genomics, AstraZeneca, Photocure and Ferring, has an advisory/consulting role at Ferring, MSD and UroGen, has received speaker honoraria from AstraZeneca, Pfizer and Roche, and is a board member for BioXpedia. D.E.H. is an advisory board member for AstraZeneca. M.D.G. receives or has received research funding from Bristol Myers Squibb, Novartis, Dendreon, AstraZeneca, Merck and Genentech. M.D.G. is or was a consultant for Bristol Myers Squibb, Merck, Genentech, AstraZeneca, Pfizer, EMD Serono, SeaGen, Janssen, Numab, Dragonfly, GlaxoSmithKline, Basilea, UroGen, Rappta Therapeutics, Alligator, Silverback, Fujifilm, Curis, Gilead, Bicycle, Asieris, Abbvie, Analogue Devices and Veracyte. J.A.E. is or was a consultant/advisory board member and receives or has received honoraria from Blue Earth Diagnostics, Boston Scientific, AstraZeneca, Lantheus, IBA, Astellas, Pfizer, Merck, Roivant Pharma, Myovant Sciences, Janssen, Bayer Healthcare, Progenics Pharmaceuticals, Genentech, Gilead, Angiodynamics and UptoDate. The other authors declare no competing interests.
Additional information
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41572-023-00468-9.
References
- 1.Sung H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Antoni S. et al. Bladder cancer incidence and mortality: a global overview and recent trends. Eur. Urol. 71, 96–108 (2017). [DOI] [PubMed] [Google Scholar]
- 3.IARC. Global Cancer Observatory: Cancer Tomorrow. WHO; https://gco.iarc.fr/tomorrow/en (2023). [Google Scholar]
- 4.Tran L, Xiao J-F, Agarwal N, Duex JE & Theodorescu D. Advances in bladder cancer biology and therapy. Nat. Rev. Cancer 21, 104–121 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Facchini G. et al. Advanced/metastatic bladder cancer: current status and future directions. Eur. Rev. Med. Pharmacol. Sci. 24, 11536–11552 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Dancik GM, Owens CR, Iczkowski KA & Theodorescu D. A cell of origin gene signature indicates human bladder cancer has distinct cellular progenitors. Stem Cell 32, 974–982 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Partin AW, Peters CA, Kavoussi LR, Dmochowski RR & Wein AJ Campbell-Walsh-Wein Urology Twelfth Edition Review (Elsevier Health Sciences, 2020). [Google Scholar]
- 8.Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A. & Abnet CC Association between smoking and risk of bladder cancer among men and women. JAMA 306, 737–745 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilhelm-Benartzi CS et al. Association of secondhand smoke exposures with DNA methylation in bladder carcinomas. Cancer Causes Control 22, 1205–1213 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellamri M. et al. DNA damage and oxidative stress of tobacco smoke condensate in human bladder epithelial cells. Chem. Res. Toxicol. 35, 1863–1880 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matuszczak M. & Salagierski M. Diagnostic and prognostic potential of biomarkers CYFRA 21.1, ERCC1, p53, FGFR3 and TATI in bladder cancers. Int. J. Mol. Sci. 21, 3360 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mertens LS et al. Prognostic markers in invasive bladder cancer: FGFR3 mutation status versus P53 and KI-67 expression: a multi-center, multi-laboratory analysis in 1058 radical cystectomy patients. Urol. Oncol. 40, 110.e1–110.e9 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Theodorescu D, Li Z. & Li X. Sex differences in bladder cancer: emerging data and call to action. Nat. Rev. Urol. 19, 447–449 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bladder Cancer Statistics. WCRF International https://www.wcrf.org/cancer-trends/bladder-cancer-statistics/ (2022). [Google Scholar]
- 15.Dobruch J. et al. Gender and bladder cancer: a collaborative review of etiology, biology, and outcomes. Eur. Urol. 69, 300–310 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Hyldgaard JM & Jensen JB The inequality of females in bladder cancer. APMIS 129, 694–699 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Radkiewicz C. et al. Sex differences in urothelial bladder cancer survival. Clin. Genitourin. Cancer 18, 26–34.e6 (2020). [DOI] [PubMed] [Google Scholar]
- 18.You S. et al. Characterizing molecular subtypes of high-risk non-muscle-invasive bladder cancer in African American patients. Urol. Oncol. 40, 410.e19–410.e27 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saginala K. et al. Epidemiology of bladder cancer. Med. Sci. 8, 15 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richters A, Aben KKH & Kiemeney LALM The global burden of urinary bladder cancer: an update. World J. Urol. 38, 1895–1904 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Safiri S, Kolahi A-A & Naghavi M, Global Burden of Disease Bladder Cancer Collaborators. Global, regional and national burden of bladder cancer and its attributable risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease study 2019. BMJ Glob. Health 6, e004128 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai X, Gakidou E. & Lopez AD Evolution of the global smoking epidemic over the past half century: strengthening the evidence base for policy action. Tob. Control 31, 129–137 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Flor LS, Reitsma MB, Gupta V, Ng M. & Gakidou E. The effects of tobacco control policies on global smoking prevalence. Nat. Med. 27, 239–243 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishida K. & Hsieh MH Understanding urogenital schistosomiasis-related bladder cancer: an update. Front. Med. 5, 223 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaghloul MS, Zaghloul TM, Bishr MK & Baumann BC Urinary schistosomiasis and the associated bladder cancer: update. J. Egypt. Natl Canc. Inst. 32, 44 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Salem S, Mitchell RE, El-Alim El-Dorey A, Smith JA & Barocas DA Successful control of schistosomiasis and the changing epidemiology of bladder cancer in Egypt. BJU Int. 107, 206–211 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Martin A, Woolbright BL, Umar S, Ingersoll MA & Taylor JA 3rd Bladder cancer, inflammageing and microbiomes. Nat. Rev. Urol. 19, 495–509 (2022). [DOI] [PubMed] [Google Scholar]
- 28.Lobo N. et al. Epidemiology, screening, and prevention of bladder cancer. Eur. Urol. Oncol. 5, 628–639 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Letašiová S. et al. Bladder cancer, a review of the environmental risk factors. Environ. Health 11, S11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Post RS et al. Risk of urothelial bladder cancer in Lynch syndrome is increased, in particular among MSH2 mutation carriers. J. Med. Genet. 47, 464–470 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindner AK et al. Lynch syndrome: its impact on urothelial carcinoma. Int. J. Mol. Sci. 22, 531 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lindskrog SV et al. An integrated multi-omics analysis identifies prognostic molecular subtypes of non-muscle-invasive bladder cancer. Nat. Commun. 12, 2301 (2021). An update to the 2016 UROMOL consortium study, representing the largest multi-omics analysis to characterize the molecular landscape in early-stage bladder cancer.
- 33.Hedegaard J. et al. Comprehensive transcriptional analysis of early-stage urothelial carcinoma. Cancer Cell 30, 27–42 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Hurst CD et al. Stage-stratified molecular profiling of non-muscle-invasive bladder cancer enhances biological, clinical, and therapeutic insight. Cell Rep. Med. 2, 100472 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun TT, Zhao H, Provet J, Aebi U. & Wu XR Formation of asymmetric unit membrane during urothelial differentiation. Mol. Biol. Rep. 23, 3–11 (1996). [DOI] [PubMed] [Google Scholar]
- 36.Varley CL et al. PPARgamma-regulated tight junction development during human urothelial cytodifferentiation. J. Cell. Physiol. 208, 407–417 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Southgate J, Harnden P. & Trejdosiewicz LK Cytokeratin expression patterns in normal and malignant urothelium: a review of the biological and diagnostic implications. Histol. Histopathol. 14, 657–664 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Wezel F, Pearson J. & Southgate J. Plasticity of in vitro-generated urothelial cells for functional tissue formation. Tissue Eng. Part. A 20, 1358–1368 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Wiessner GB, Plumber SA, Xiang T. & Mendelsohn CL Development, regeneration and tumorigenesis of the urothelium. Development 149, dev198184 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fishwick C. et al. Heterarchy of transcription factors driving basal and luminal cell phenotypes in human urothelium. Cell Death Differ. 24, 809–818 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curtius K, Wright NA & Graham TA An evolutionary perspective on field cancerization. Nat. Rev. Cancer 18, 19–32 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Sidransky D. et al. Clonal origin of bladder cancer. N. Engl. J. Med. 326, 737–740 (1992). [DOI] [PubMed] [Google Scholar]
- 43.Höglund M. On the origin of syn- and metachronous urothelial carcinomas. Eur. Urol. 51, 1185–1193 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Höglund M. Bladder cancer, a two phased disease? Semin. Cancer Biol. 17, 225–232 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Lamy P. et al. Paired exome analysis reveals clonal evolution and potential therapeutic targets in urothelial carcinoma. Cancer Res. 76, 5894–5906 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Bondaruk J. et al. The origin of bladder cancer from mucosal field effects. iScience 25, 104551 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strandgaard T. et al. Field cancerization is associated with tumor development, T-cell exhaustion, and clinical outcomes in bladder cancer. Eur. Urol. 10.1016/j.eururo.2023.07.014 (2023). [DOI] [PubMed] [Google Scholar]
- 48.Lawson ARJ et al. Extensive heterogeneity in somatic mutation and selection in the human bladder. Science 370, 75–82 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Alexandrov LB et al. The repertoire of mutational signatures in human cancer. Nature 578, 94–101 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nordentoft I. et al. Mutational context and diverse clonal development in early and late bladder cancer. Cell Rep. 7, 1649–1663 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Robertson AG et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 171, 540–556 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alexandrov LB et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sandberg AA Chromosome changes in bladder cancer: clinical and other correlations. Cancer Genet. Cytogenet. 19, 163–175 (1986). [DOI] [PubMed] [Google Scholar]
- 54. Hurst CD et al. Genomic subtypes of non-invasive bladder cancer with distinct metabolic profile and female gender bias in KDM6A mutation frequency. Cancer Cell 32, 701–715.e7 (2017). The authors defined two major genomic subtypes of primary-stage Ta tumors and found that more mutations in the histone lysine demethylase KDM6A were present in non-invasive tumours from women than in those from men, supporting the hypothesis that male and female bladder cancers have both common and different biological drivers.
- 55.Ségal-Bendirdjian E. & Geli V. Non-canonical roles of telomerase: unraveling the imbroglio. Front. Cell Dev. Biol. 7, 332 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agarwal N. et al. TRIM28 is a transcriptional activator of the mutant TERT promoter in human bladder cancer. Proc. Natl Acad. Sci. USA 118, e2102423118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borah S. et al. Cancer. TERT promoter mutations and telomerase reactivation in urothelial cancer. Science 347, 1006–1010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nickerson ML et al. Molecular analysis of urothelial cancer cell lines for modeling tumor biology and drug response. Oncogene 36, 35–46 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nickerson ML et al. Concurrent alterations in TERT, KDM6A, and the BRCA pathway in bladder cancer. Clin. Cancer Res. 20, 4935–4948 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hurst CD, Platt FM, Taylor CF & Knowles MA Novel tumor subgroups of urothelial carcinoma of the bladder defined by integrated genomic analysis. Clin. Cancer Res. 18, 5865–5877 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allory Y. et al. Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome. Eur. Urol. 65, 360–366 (2014). [DOI] [PubMed] [Google Scholar]
- 62.Stern JL, Theodorescu D, Vogelstein B, Papadopoulos N. & Cech TR Mutation of the TERT promoter, switch to active chromatin, and monoallelic TERT expression in multiple cancers. Genes Dev. 29, 2219–2224 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi M-J et al. APOBEC-mediated mutagenesis as a likely cause of FGFR3 S249C mutation over-representation in bladder cancer. Eur. Urol. 76, 9–13 (2019). [DOI] [PubMed] [Google Scholar]
- 64.di Martino E, L’Hôte CG, Kennedy W, Tomlinson DC & Knowles MA Mutant fibroblast growth factor receptor 3 induces intracellular signaling and cellular transformation in a cell type- and mutation-specific manner. Oncogene 28, 4306–4316 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barrows D, Feng L, Carroll TS & Allis CD Loss of UTX/KDM6A and the activation of FGFR3 converge to regulate differentiation gene-expression programs in bladder cancer. Proc. Natl Acad. Sci. USA 117, 25732–25741 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qiu H. et al. KDM6A loss triggers an epigenetic switch that disrupts urothelial differentiation and drives cell proliferation in bladder cancer. Cancer Res. 83, 814–829 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Richart L. et al. STAG2 loss-of-function affects short-range genomic contacts and modulates the basal-luminal transcriptional program of bladder cancer cells. Nucleic Acids Res. 49, 11005–11021 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taylor CF, Platt FM, Hurst CD, Thygesen HH & Knowles MA Frequent inactivating mutations of STAG2 in bladder cancer are associated with low tumour grade and stage and inversely related to chromosomal copy number changes. Hum. Mol. Genet. 23, 1964–1974 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gordon NS et al. STAG2 protein expression in non-muscle-invasive bladder cancer: associations with sex, genomic and transcriptomic changes, and clinical outcomes. Eur. Urol. Open Sci. 38, 88–95 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meeks JJ et al. Genomic heterogeneity in bladder cancer: challenges and possible solutions to improve outcomes. Nat. Rev. Urol. 17, 259–270 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Q. et al. ERCC2 helicase domain mutations confer nucleotide excision repair deficiency and drive cisplatin sensitivity in muscle-invasive bladder cancer. Clin. Cancer Res. 25, 977–988 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Williams SV, Hurst CD & Knowles MA Oncogenic FGFR3 gene fusions in bladder cancer. Hum. Mol. Genet. 22, 795–803 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tomlinson DC, Baxter EW, Loadman PM, Hull MA & Knowles MA FGFR1induced epithelial to mesenchymal transition through MAPK/PLCγ/COX-2-mediated mechanisms. PLoS ONE 7, e38972 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tomlinson DC & Knowles MA Altered splicing of FGFR1 is associated with high tumor grade and stage and leads to increased sensitivity to FGF1 in bladder cancer. Am. J. Pathol. 177, 2379–2386 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rebouissou S. et al. CDKN2A homozygous deletion is associated with muscle invasion in FGFR3-mutated urothelial bladder carcinoma. J. Pathol. 227, 315–324 (2012). [DOI] [PubMed] [Google Scholar]
- 76.Huan J, Grivas P, Birch J. & Hansel DE Emerging roles for mammalian target of rapamycin (mTOR) complexes in bladder cancer progression and therapy. Cancers 14, 1555 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miyata Y, Sagara Y, Kanda S, Hayashi T. & Kanetake H. Phosphorylated hepatocyte growth factor receptor/c-Met is associated with tumor growth and prognosis in patients with bladder cancer: correlation with matrix metalloproteinase-2 and −7 and E-cadherin. Hum. Pathol. 40, 496–504 (2009). [DOI] [PubMed] [Google Scholar]
- 78.Goriki A. et al. Unravelling disparate roles of NOTCH in bladder cancer. Nat. Rev. Urol. 15, 345–357 (2018). [DOI] [PubMed] [Google Scholar]
- 79. Gouin KH III et al. An N-Cadherin 2 expressing epithelial cell subpopulation predicts response to surgery, chemotherapy and immunotherapy in bladder cancer. Nat. Commun. 12, 4906 (2021). To our knowledge, this is the first publication evaluating, through single-cell and spatial transcriptomics and proteomics, tumor heterogeneity in muscle-invasive bladder cancer and defines a new subtype architecture and specific tumor cell population whose presence predicts clinical outcomes after surgery and immunotherapy.
- 80.Su S. et al. CD10+GPR77+ cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell 172, 841–856.e16 (2018). [DOI] [PubMed] [Google Scholar]
- 81.Lee Y-C et al. The dynamic roles of the bladder tumour microenvironment. Nat. Rev. Urol. 19, 515–533 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qiu S. et al. Tumor-associated macrophages promote bladder tumor growth through PI3K/AKT signal induced by collagen. Cancer Sci. 110, 2110–2118 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mezheyeuski A. et al. Fibroblasts in urothelial bladder cancer define stroma phenotypes that are associated with clinical outcome. Sci. Rep. 10, 281 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Long X. et al. Cancer-associated fibroblasts promote cisplatin resistance in bladder cancer cells by increasing IGF-1/ERβ/Bcl-2 signalling. Cell Death Dis. 10, 375 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tran L. & Theodorescu D. Determinants of resistance to checkpoint inhibitors. Int. J. Mol. Sci. 21, 1594 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen Y. et al. Tumor-associated macrophages: an accomplice in solid tumor progression. J. Biomed. Sci. 26, 78 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tu MM et al. Inhibition of the CCL2 receptor, CCR2, enhances tumor response to immune checkpoint therapy. Commun. Biol. 3, 720 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tu MM et al. Targeting DDR2 enhances tumor response to anti-PD-1 immunotherapy. Sci. Adv. 5, eaav2437 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Said N, Sanchez-Carbayo M, Smith SC & Theodorescu D. RhoGDI2 suppresses lung metastasis in mice by reducing tumor versican expression and macrophage infiltration. J. Clin. Invest. 122, 1503–1518 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang L. et al. Myeloid cell-associated resistance to PD-1/PD-L1 blockade in urothelial cancer revealed through bulk and single-cell RNA sequencing. Clin. Cancer Res. 27, 4287–4300 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Samstein RM et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 51, 202–206 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cristescu R. et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 362, eaar3593 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pfannstiel C. et al. The tumor immune microenvironment drives a prognostic relevance that correlates with bladder cancer subtypes. Cancer Immunol. Res. 7, 923–938 (2019). [DOI] [PubMed] [Google Scholar]
- 94.Wang L. et al. EMT- and stroma-related gene expression and resistance to PD-1 blockade in urothelial cancer. Nat. Commun. 9, 3503 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.You S. et al. Discoidin domain receptor-driven gene signatures as markers of patient response to anti-PD-L1 immune checkpoint therapy. J. Natl Cancer Inst. 114, 1380–1391 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaneko S. & Li X. X chromosome protects against bladder cancer in females via a KDM6A-dependent epigenetic mechanism. Sci. Adv. 4, eaar5598 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Z, Azar JH & Rubinstein MP Converting tumoral PD-L1 into a 4–1BB agonist for safer and more effective cancer immunotherapy. Cancer Discov. 12, 1184–1186 (2022). [DOI] [PubMed] [Google Scholar]
- 98.Kwon H. et al. Androgen conspires with the CD8+ T cell exhaustion program and contributes to sex bias in cancer. Sci. Immunol. 7, eabq2630 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sottnik JL et al. Androgen receptor regulates CD44 expression in bladder cancer. Cancer Res. 81, 2833–2846 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Calvete J. et al. The coexpression of fibroblast activation protein (FAP) and basal-type markers (CK 5/6 and CD44) predicts prognosis in high-grade invasive urothelial carcinoma of the bladder. Hum. Pathol. 91, 61–68 (2019). [DOI] [PubMed] [Google Scholar]
- 101.Bellmunt J. Stem-like signature predicting disease progression in early stage bladder cancer. the role of E2F3 and SOX4. Biomedicines 6, 85 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sottnik JL & Theodorescu D. CD44: a metastasis driver and therapeutic target. Oncoscience 3, 320–321 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Senbanjo LT & Chellaiah MA CD44: a multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front. Cell Dev. Biol. 5, 18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miyamoto H. et al. Promotion of bladder cancer development and progression by androgen receptor signals. J. Natl Cancer Inst. 99, 558–568 (2007). [DOI] [PubMed] [Google Scholar]
- 105.Morales EE et al. Finasteride reduces risk of bladder cancer in a large prospective screening study. Eur. Urol. 69, 407–410 (2016). [DOI] [PubMed] [Google Scholar]
- 106.Sathianathen NJ, Fan Y, Jarosek SL, Lawrentschuk NL & Konety BR Finasteride does not prevent bladder cancer: a secondary analysis of the Medical Therapy for Prostatic Symptoms Study. Urol. Oncol. 36, 338.e13–338.e17 (2018). [DOI] [PubMed] [Google Scholar]
- 107.Zhu D. et al. Finasteride use and risk of bladder cancer in a multiethnic population. J. Urol. 206, 15–21 (2021). [DOI] [PubMed] [Google Scholar]
- 108.Richard A. et al. Racial variation in sex steroid hormone concentration in black and white men: a meta-analysis. Andrology 2, 428–435 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Giaquinto AN et al. Cancer statistics for African American/Black People 2022. CA Cancer J. Clin. 72, 202–229 (2022). [DOI] [PubMed] [Google Scholar]
- 110.Miyamoto H. et al. Expression of androgen and oestrogen receptors and its prognostic significance in urothelial neoplasm of the urinary bladder. BJU Int. 109, 1716–1726 (2012). [DOI] [PubMed] [Google Scholar]
- 111.Tripathi A. & Gupta S. Androgen receptor in bladder cancer: a promising therapeutic target. Asian J. Urol. 7, 284–290 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xiang P. et al. Impact of androgen suppression therapy on the risk and prognosis of bladder cancer: a systematic review and meta-analysis. Front. Oncol. 11, 784627 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Creta M. et al. Inhibition of androgen signalling improves the outcomes of therapies for bladder cancer: results from a systematic review of preclinical and clinical evidence and meta-analysis of clinical studies. Diagnostics 11, 351 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu S-C et al. Androgen suppression therapy is associated with lower recurrence of non-muscle-invasive bladder cancer. Eur. Urol. Focus 7, 142–147 (2021). [DOI] [PubMed] [Google Scholar]
- 115.Maan AA et al. The Y chromosome: a blueprint for men’s health? Eur. J. Hum. Genet. 25, 1181–1188 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sano S. et al. Hematopoietic loss of Y chromosome leads to cardiac fibrosis and heart failure mortality. Science 377, 292–297 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Forsberg LA et al. Mosaic loss of chromosome Y in peripheral blood is associated with shorter survival and higher risk of cancer. Nat. Genet. 46, 624–628 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kido T. & Lau Y-FC Roles of the Y chromosome genes in human cancers. Asian J. Androl. 17, 373–380 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brown DW & Machiela MJ Why Y? Downregulation of chromosome Y genes potentially contributes to elevated cancer risk. J. Natl Cancer Inst. 112, 871–872 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Panani AD & Roussos C. Sex chromosome abnormalities in bladder cancer: Y polysomies are linked to PT1-grade III transitional cell carcinoma. Anticancer. Res. 26, 319–323 (2006). [PubMed] [Google Scholar]
- 121.Fadl-Elmula I. et al. Karyotypic characterization of urinary bladder transitional cell carcinomas. Genes Chromosomes Cancer 29, 256–265 (2000). [PubMed] [Google Scholar]
- 122.Sauter G, Moch H, Mihatsch MJ & Gasser TC Molecular cytogenetics of bladder cancer progression. Eur. Urol. 33, 9–10 (1998). [DOI] [PubMed] [Google Scholar]
- 123.Smeets W, Pauwels R, Laarakkers L, Debruyne F. & Geraedts J. Chromosomal analysis of bladder cancer. III. Nonrandom alterations. Cancer Genet. Cytogenet. 29, 29–41 (1987). [DOI] [PubMed] [Google Scholar]
- 124.Sauter G. et al. Y chromosome loss detected by FISH in bladder cancer. Cancer Genet. Cytogenet. 82, 163–169 (1995). [DOI] [PubMed] [Google Scholar]
- 125.Neuhaus M. et al. Polysomies but not Y chromosome losses have prognostic significance in pTa/pT1 urinary bladder cancer. Hum. Pathol. 30, 81–86 (1999). [DOI] [PubMed] [Google Scholar]
- 126.Powell I, Tyrkus M. & Kleer E. Apparent correlation of sex chromosome loss and disease course in urothelial cancer. Cancer Genet. Cytogenet. 50, 97–101 (1990). [DOI] [PubMed] [Google Scholar]
- 127.Siegel RL, Miller KD, Fuchs HE & Jemal A. Cancer statistics, 2022. CA Cancer J. Clin. 72, 7–33 (2022). [DOI] [PubMed] [Google Scholar]
- 128.Johansson SL & Cohen SM Epidemiology and etiology of bladder cancer. Semin. Surg. Oncol. 13, 291–298 (1997). [DOI] [PubMed] [Google Scholar]
- 129.Dumanski JP et al. Mutagenesis. Smoking is associated with mosaic loss of chromosome Y. Science 347, 81–83 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Abdel-Hafiz HA et al. Y chromosome loss in cancer drives growth by evasion of adaptive immunity. Nature 619, 624–631 (2023). To our knowledge, this is the first publication that mechanistically links cancer aggressiveness with LOY and shows that this is due to the cancer cell evading T cell-mediated immunity, opening up possibilities for biomarker and therapeutic development in cancer.
- 131.Cummings KB, Barone JG & Ward WS Diagnosis and staging of bladder cancer. Urol. Clin. North Am. 19, 455–465 (1992). [PubMed] [Google Scholar]
- 132.Khadhouri S. et al. The IDENTIFY study: the investigation and detection of urological neoplasia in patients referred with suspected urinary tract cancer — a multicentre observational study. BJU Int. 128, 440–450 (2021). [DOI] [PubMed] [Google Scholar]
- 133.Ghandour R, Freifeld Y, Singla N. & Lotan Y. Evaluation of hematuria in a large public health care system. Bladder Cancer 5, 119–129 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rai BP et al. Systematic review of the incidence of and risk factors for urothelial cancers and renal cell carcinoma among patients with haematuria. Eur. Urol. 82, 182–192 (2022). [DOI] [PubMed] [Google Scholar]
- 135.Ramirez D. et al. Microscopic haematuria at time of diagnosis is associated with lower disease stage in patients with newly diagnosed bladder cancer. BJU Int. 117, 783–786 (2016). [DOI] [PubMed] [Google Scholar]
- 136.Alanee S. & Shukla AR Bladder malignancies in children aged <18 years: results from the surveillance, epidemiology and end results database. BJU Int. 106, 557–560 (2010). [DOI] [PubMed] [Google Scholar]
- 137.Kutarski PW & Padwell A. Transitional cell carcinoma of the bladder in young adults. Br. J. Urol. 72, 749–755 (1993). [DOI] [PubMed] [Google Scholar]
- 138.Rezaee ME, Dunaway CM, Baker ML, Penna FJ & Chavez DR Urothelial cell carcinoma of the bladder in pediatric patients: a systematic review and data analysis of the world literature. J. Pediatr. Urol. 15, 309–314 (2019). [DOI] [PubMed] [Google Scholar]
- 139.Czech AK et al. Diagnostic accuracy of bimanual palpation in bladder cancer patients undergoing cystectomy: a prospective study. Urol. Oncol. 41, 390.e27–390.e33 (2023). [DOI] [PubMed] [Google Scholar]
- 140.Flaig TW et al. Bladder cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J. Natl Compr. Canc. Netw. 18, 329–354 (2020). [DOI] [PubMed] [Google Scholar]
- 141.Ploeg M. et al. Discrepancy between clinical staging through bimanual palpation and pathological staging after cystectomy. Urol. Oncol. 30, 247–251 (2012). [DOI] [PubMed] [Google Scholar]
- 142.Babjuk M. et al. European Association of Urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 81, 75–94 (2022). [DOI] [PubMed] [Google Scholar]
- 143.Xing J. & Reynolds JP Diagnostic advances in urine cytology. Surg. Pathol. Clin. 11, 601–610 (2018). [DOI] [PubMed] [Google Scholar]
- 144.Barkan GA et al. The Paris system for reporting urinary cytology: the quest to develop a standardized terminology. Adv. Anat. Pathol. 23, 193–201 (2016). [DOI] [PubMed] [Google Scholar]
- 145.Nikas IP et al. The Paris system for reporting urinary cytology: a meta-analysis. J. Pers. Med. 12, 170 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Saprykina EV & Sal’nik BI The role of lipid metabolism disorders in the mechanism of the hepatotoxic effects of rubomycin [Russian]. Antibiot. Khimioter. 33, 452–455 (1988). [PubMed] [Google Scholar]
- 147.Guo A. et al. Bladder tumour antigen (BTA stat) test compared to the urine cytology in the diagnosis of bladder cancer: a meta-analysis. Can. Urol. Assoc. J. 8, E347–E352 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dimashkieh H. et al. Evaluation of urovysion and cytology for bladder cancer detection: a study of 1835 paired urine samples with clinical and histologic correlation. Cancer Cytopathol. 121, 591–597 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zippe C, Pandrangi L. & Agarwal A. NMP22 is a sensitive, cost-effective test in patients at risk for bladder cancer. J. Urol. 161, 62–65 (1999). [PubMed] [Google Scholar]
- 150.He H, Han C, Hao L. & Zang G. ImmunoCyt test compared to cytology in the diagnosis of bladder cancer: a meta-analysis. Oncol. Lett. 12, 83–88 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang Z. et al. Evaluation of the NMP22 bladderchek test for detecting bladder cancer: a systematic review and meta-analysis. Oncotarget 8, 100648–100656 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jeong S-H & Ku JH Urinary markers for bladder cancer diagnosis and monitoring. Front. Cell Dev. Biol. 10, 892067 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Heitzer E, Auinger L. & Speicher MR Cell-free DNA and apoptosis: how dead cells inform about the living. Trends Mol. Med. 26, 519–528 (2020). [DOI] [PubMed] [Google Scholar]
- 154.Cherepanova AV, Tamkovich SN, Bryzgunova OE, Vlassov VV & Laktionov PP Deoxyribonuclease activity and circulating DNA concentration in blood plasma of patients with prostate tumors. Ann. N. Y. Acad. Sci. 1137, 218–221 (2008). [DOI] [PubMed] [Google Scholar]
- 155.Tamkovich SN et al. Circulating DNA and DNase activity in human blood. Ann. N. Y. Acad. Sci. 1075, 191–196 (2006). [DOI] [PubMed] [Google Scholar]
- 156.Yu SCY et al. High-resolution profiling of fetal DNA clearance from maternal plasma by massively parallel sequencing. Clin. Chem. 59, 1228–1237 (2013). [DOI] [PubMed] [Google Scholar]
- 157.Khier S. & Gahan PB Hepatic clearance of cell-free DNA: possible impact on early metastasis diagnosis. Mol. Diagn. Ther. 25, 677–682 (2021). [DOI] [PubMed] [Google Scholar]
- 158.Khier S. & Lohan L. Kinetics of circulating cell-free DNA for biomedical applications: critical appraisal of the literature. Future Sci. OA 4, FSO295 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Christensen E. et al. Early detection of metastatic relapse and monitoring of therapeutic efficacy by ultra-deep sequencing of plasma cell-free DNA in patients with urothelial bladder carcinoma. J. Clin. Oncol. 37, 1547–1557 (2019). To our knowledge, this is the first larger prospective study showing that ctDNA measurements during chemotherapy and after cystectomy may be a very powerful biomarker for guiding treatment.
- 160.Christensen E. et al. Cell-free urine and plasma DNA mutational analysis predicts neoadjuvant chemotherapy response and outcome in patients with muscle-invasive bladder cancer. Clin. Cancer Res. 14, 1582–1591 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.van Dorp J. et al. High- or low-dose preoperative ipilimumab plus nivolumab in stage III urothelial cancer: the phase 1B NABUCCO trial. Nat. Med. 29, 588–592 (2023). [DOI] [PubMed] [Google Scholar]
- 162.Powles T. et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature 595, 432–437 (2021). [DOI] [PubMed] [Google Scholar]
- 163.Powles T. et al. Updated overall survival by circulating tumor DNA status from the phase 3 IMvigor010 trial: adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma. Eur. Urol. 10.1016/j.eururo.2023.06.007 (2023). [DOI] [PubMed] [Google Scholar]
- 164.US National Library of Medicine. ClinicalTrials.gov; https://clinicaltrials.gov/ct2/show/NCT04660344 (2023). [Google Scholar]
- 165.US National Library of Medicine. ClinicalTrials.gov; https://clinicaltrials.gov/ct2/show/NCT04138628 (2022). [Google Scholar]
- 166.Vandekerkhove G. et al. Plasma ctDNA is a tumor tissue surrogate and enables clinical-genomic stratification of metastatic bladder cancer. Nat. Commun. 12, 184 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vandekerkhove G. et al. Circulating tumor DNA reveals clinically actionable somatic genome of metastatic bladder cancer. Clin. Cancer Res. 23, 6487–6497 (2017). [DOI] [PubMed] [Google Scholar]
- 168.Moch H. Urinary and Male Genital Tumours: WHO Classification of Tumours, 5th Edition, Volume 8. 576 (IARC Publications, 2022). [Google Scholar]
- 169.Pasin E, Josephson DY, Mitra AP, Cote RJ & Stein JP Superficial bladder cancer: an update on etiology, molecular development, classification, and natural history. Rev. Urol. 10, 31–43 (2008). [PMC free article] [PubMed] [Google Scholar]
- 170.Ching CB et al. HER2 gene amplification occurs frequently in the micropapillary variant of urothelial carcinoma: analysis by dual-color in situ hybridization. Mod. Pathol. 24, 1111–1119 (2011). [DOI] [PubMed] [Google Scholar]
- 171.Willis DL et al. Micropapillary bladder cancer: current treatment patterns and review of the literature. Urol. Oncol. 32, 826–832 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Isharwal S. et al. Intratumoral heterogeneity of ERBB2 amplification and HER2 expression in micropapillary urothelial carcinoma. Hum. Pathol. 77, 63–69 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Teo MY et al. Natural history, response to systemic therapy, and genomic landscape of plasmacytoid urothelial carcinoma. Br. J. Cancer 124, 1214–1221 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Edgerton N, Sirintrapun SJ, Munoz M, Chen Z. & Osunkoya AO Micropapillary urothelial carcinoma of the urinary bladder: a clinicopathological analysis of 24 cases. Int. J. Urol. 18, 49–54 (2011). [DOI] [PubMed] [Google Scholar]
- 175.Fernández MI et al. Clinical risk stratification in patients with surgically resectable micropapillary bladder cancer. BJU Int. 119, 684–691 (2017). [DOI] [PubMed] [Google Scholar]
- 176.Amin MB et al. AJCC Cancer Staging Manual (Springer International Publishing, 2017). [Google Scholar]
- 177.Leivo MZ et al. Analysis of T1 bladder cancer on biopsy and transurethral resection specimens: comparison and ranking of T1 quantification approaches to predict progression to muscularis propria invasion. Am. J. Surg. Pathol. 42, e1–e10 (2018). [DOI] [PubMed] [Google Scholar]
- 178.Soria F, Dutto D. & Gontero P. Clinical and biological markers for risk-stratification of T1 high-grade non-muscle invasive bladder cancer. Curr. Opin. Urol. 32, 517–522 (2022). [DOI] [PubMed] [Google Scholar]
- 179.Castaneda PR, Theodorescu D, Rosser CJ & Ahdoot M. Identifying novel biomarkers associated with bladder cancer treatment outcomes. Front. Oncol. 13, 1114203 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chang SS et al. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO guideline. J. Urol. 198, 552–559 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Flaig TW et al. NCCN guidelines® insights: bladder cancer, version 2.2022. J. Natl Compr. Canc. Netw. 20, 866–878 (2022). [DOI] [PubMed] [Google Scholar]
- 182.Witjes JA et al. European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur. Urol. 79, 82–104 (2021). [DOI] [PubMed] [Google Scholar]
- 183.Hensley PJ et al. Contemporary staging for muscle-invasive bladder cancer: accuracy and limitations. Eur. Urol. Oncol. 5, 403–411 (2022). [DOI] [PubMed] [Google Scholar]
- 184.Mirmomen SM, Shinagare AB, Williams KE, Silverman SG & Malayeri AA Preoperative imaging for locoregional staging of bladder cancer. Abdom. Radiol. 44, 3843–3857 (2019). [DOI] [PubMed] [Google Scholar]
- 185.Tekes A. et al. Dynamic MRI of bladder cancer: evaluation of staging accuracy. AJR Am. J. Roentgenol. 184, 121–127 (2005). [DOI] [PubMed] [Google Scholar]
- 186.Cornelissen SWE, Veenboer PW, Wessels FJ & Meijer RP Diagnostic accuracy of multiparametric MRI for local staging of bladder cancer: a systematic review and meta-analysis. Urology 145, 22–29 (2020). [DOI] [PubMed] [Google Scholar]
- 187.Gurram S, Muthigi A, Egan J. & Stamatakis L. Imaging in localized bladder cancer: can current diagnostic modalities provide accurate local tumor staging? Curr. Urol. Rep. 20, 82 (2019). [DOI] [PubMed] [Google Scholar]
- 188.Panebianco V. et al. Multiparametric magnetic resonance imaging for bladder cancer: development of VI-RADS (Vesical Imaging-Reporting And Data System). Eur. Urol. 74, 294–306 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bandini M. et al. The value of multiparametric magnetic resonance imaging sequences to assist in the decision making of muscle-invasive bladder cancer. Eur. Urol. Oncol. 4, 829–833 (2021). [DOI] [PubMed] [Google Scholar]
- 190.Furrer MA et al. Routine preoperative bone scintigraphy has limited impact on the management of patients with invasive bladder cancer. Eur. Urol. Focus 7, 1052–1060 (2021). [DOI] [PubMed] [Google Scholar]
- 191.Ha HK, Koo PJ & Kim S-J Diagnostic accuracy of F-18 FDG PET/CT for preoperative lymph node staging in newly diagnosed bladder cancer patients: a systematic review and meta-analysis. Oncology 95, 31–38 (2018). [DOI] [PubMed] [Google Scholar]
- 192.Mertens LS, Meijer RP & Alfred Witjes J. Positron emission tomography/computed tomography for staging of bladder cancer: a continuing clinical controversy. Eur. Urol. 83, 95–96 (2023). [DOI] [PubMed] [Google Scholar]
- 193.Apolo AB et al. Clinical value of fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in bladder cancer. J. Clin. Oncol. 28, 3973–3978 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kibel AS et al. Prospective study of [18F]fluorodeoxyglucose positron emission tomography/computed tomography for staging of muscle-invasive bladder carcinoma. J. Clin. Oncol. 27, 4314–4320 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.van Kessel KEM et al. Molecular markers increase precision of the european association of urology non-muscle-invasive bladder cancer progression risk groups. Clin. Cancer Res. 24, 1586–1593 (2018). [DOI] [PubMed] [Google Scholar]
- 196.Bellmunt J. et al. Genomic predictors of good outcome, recurrence, or progression in high-grade T1 non-muscle-invasive bladder cancer. Cancer Res. 80, 4476–4486 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dyrskjøt L. et al. A molecular signature in superficial bladder carcinoma predicts clinical outcome. Clin. Cancer Res. 11, 4029–4036 (2005). [DOI] [PubMed] [Google Scholar]
- 198.Dyrskjøt L. et al. Prognostic impact of a 12-gene progression score in non-muscle-invasive bladder cancer: a prospective multicentre validation study. Eur. Urol. 72, 461–469 (2017). [DOI] [PubMed] [Google Scholar]
- 199.Sjödahl G. et al. A molecular taxonomy for urothelial carcinoma. Clin. Cancer Res. 18, 3377–3386 (2012). [DOI] [PubMed] [Google Scholar]
- 200.Damrauer JS et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc. Natl Acad. Sci. USA 111, 3110–3115 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sjödahl G, Eriksson P, Liedberg F. & Höglund M. Molecular classification of urothelial carcinoma: global mRNA classification versus tumour-cell phenotype classification. J. Pathol. 242, 113–125 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Kamoun A. et al. A consensus molecular classification of muscle-invasive bladder cancer. Eur. Urol. 77, 420–433 (2019). A consensus classification system for MIBC that includes six subtype classes, demonstrating differences in underlying oncogenic mechanisms, infiltration by immune and stromal cells, and histological and clinical characteristics, including outcomes.
- 203.Kates M. et al. Adaptive immune resistance to intravesical BCG in non-muscle invasive bladder cancer: implications for prospective BCG-unresponsive trials. Clin. Cancer Res. 26, 882–891 (2020). [DOI] [PubMed] [Google Scholar]
- 204.Strandgaard T. et al. Elevated T-cell exhaustion and urinary tumor DNA levels are associated with Bacillus Calmette-Guérin failure in patients with non-muscle-invasive bladder cancer. Eur. Urol. 82, 646–656 (2022). [DOI] [PubMed] [Google Scholar]
- 205.de Jong FC et al. Non-muscle-invasive bladder cancer molecular subtypes predict differential response to intravesical Bacillus Calmette-Guérin. Sci. Transl. Med. 15, eabn4118 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Van Allen EM et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 4, 1140–1153 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Liu D. et al. Clinical validation of chemotherapy response biomarker ERCC2 in muscle-invasive urothelial bladder carcinoma. JAMA Oncol. 2, 1094–1096 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Magliocco AM, Moughan J. & Miyamoto DT Analysis of MRE11 and mortality among adults with muscle-invasive bladder cancer managed with trimodality therapy. JAMA Netw. Open 5, e2242378 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Miyamoto DT, Mouw KW, Feng FY, Shipley WU & Efstathiou JA Molecular biomarkers in bladder preservation therapy for muscle-invasive bladder cancer. Lancet Oncol. 19, e683–e695 (2018). [DOI] [PubMed] [Google Scholar]
- 210.Kamran SC et al. Genomic tumor correlates of clinical outcomes following organ-sparing chemoradiation therapy for bladder cancer. Clin. Cancer Res. 10.1158/1078-0432.CCR-23-0792 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Geynisman DM et al. A phase II trial of risk-enabled therapy after initiating neoadjuvant chemotherapy for bladder cancer (RETAIN). J. Clin. Orthod. 41, 438–438 (2023). [Google Scholar]
- 212.Mariathasan S. et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Taber A. et al. Molecular correlates of cisplatin-based chemotherapy response in muscle invasive bladder cancer by integrated multi-omics analysis. Nat. Commun. 11, 4858 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sjödahl G. et al. Different responses to neoadjuvant chemotherapy in urothelial carcinoma molecular subtypes. Eur. Urol. 81, 523–532 (2022). [DOI] [PubMed] [Google Scholar]
- 215.Seiler R. et al. Impact of molecular subtypes in muscle-invasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. Eur. Urol. 72, 544–554 (2017). [DOI] [PubMed] [Google Scholar]
- 216.Efstathiou JA et al. Impact of immune and stromal infiltration on outcomes following bladder-sparing trimodality therapy for muscle-invasive bladder cancer. Eur. Urol. 76, 59–68 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lindskrog SV et al. Single-nucleus and spatially resolved intratumor subtype heterogeneity in bladder cancer. Eur. Urol. Open Sci. 51, 78–88 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Poletajew S. et al. The learning curve for transurethral resection of bladder tumour: how many is enough to be independent, safe and effective surgeon? J. Surg. Educ. 77, 978–985 (2020). [DOI] [PubMed] [Google Scholar]
- 219.Divrik RT, Sahin AF, Yildirim U, Altok M. & Zorlu F. Impact of routine second transurethral resection on the long-term outcome of patients with newly diagnosed pT1 urothelial carcinoma with respect to recurrence, progression rate, and disease-specific survival: a prospective randomised clinical trial. Eur. Urol. 58, 185–190 (2010). [DOI] [PubMed] [Google Scholar]
- 220.Yanagisawa T. et al. Repeat transurethral resection for non-muscle-invasive bladder cancer: an updated systematic review and meta-analysis in the contemporary era. Eur. Urol. Focus 10.1016/j.euf.2023.07.002 (2023). [DOI] [PubMed] [Google Scholar]
- 221.Kirk PS et al. Impact of maximal transurethral resection on pathological outcomes at cystectomy in a large, multi-institutional cohort. J. Urol. 209, 882–889 (2023). [DOI] [PubMed] [Google Scholar]
- 222.Giacalone NJ et al. Long-term outcomes after bladder-preserving tri-modality therapy for patients with muscle-invasive bladder cancer: an updated analysis of the massachusetts general hospital experience. Eur. Urol. 71, 952–960 (2017). [DOI] [PubMed] [Google Scholar]
- 223.Kitamura K, Kataoka K, Fujioka H. & Kashiwai K. Transurethral resection of a bladder tumor by the use of a polypectomy snare. J. Urol. 124, 808–809 (1980). [PubMed] [Google Scholar]
- 224.Teoh JY-C et al. En-bloc resection of bladder tumour as primary treatment for patients with non-muscle-invasive bladder cancer: routine implementation in a multi-centre setting. World J. Urol. 39, 3353–3358 (2021). [DOI] [PubMed] [Google Scholar]
- 225.Gallioli A. et al. En bloc versus conventional transurethral resection of bladder tumors: a single-center prospective randomized noninferiority trial. Eur. Urol. Oncol. 5, 440–448 (2022). [DOI] [PubMed] [Google Scholar]
- 226.D’Andrea D. et al. En bloc versus conventional resection of primary bladder tumor (eBLOC): a prospective, multicenter, open-label, phase 3 randomized controlled trial. Eur. Urol. Oncol. 10.1016/j.euo.2023.07.010 (2023). [DOI] [PubMed] [Google Scholar]
- 227.Teoh YCJ et al. A0707 — Transurethral en bloc resection versus standard resection of bladder tumour: a multi-center randomized trial (EB-StaR Study). Eur. Urol. 83, S997–S998 (2023). [Google Scholar]
- 228.Sylvester RJ et al. Systematic review and individual patient data meta-analysis of randomized trials comparing a single immediate instillation of chemotherapy after transurethral resection with transurethral resection alone in patients with stage pTa-PT1 urothelial carcinoma of the bladder: which patients benefit from the instillation? Eur. Urol. 69, 231–244 (2016). [DOI] [PubMed] [Google Scholar]
- 229.Mertens LS, Meinhardt W, Rier WB, Nooter RI & Horenblas S. Extravasation of intravesical chemotherapy for non-muscle-invasive bladder cancer. Urol. Int. 89, 332–336 (2012). [DOI] [PubMed] [Google Scholar]
- 230.Xu Y. et al. Comparing the treatment outcomes of potassium-titanyl-phosphate laser vaporization and transurethral electroresection for primary nonmuscle-invasive bladder cancer: a prospective, randomized study. Lasers Surg. Med. 47, 306–311 (2015). [DOI] [PubMed] [Google Scholar]
- 231.Planelles Gómez J. et al. Holmium YAG photocoagulation: safe and economical alternative to transurethral resection in small nonmuscle-invasive bladder tumors. J. Endourol. 31, 674–678 (2017). [DOI] [PubMed] [Google Scholar]
- 232.Gofrit ON, Pode D, Lazar A, Katz R. & Shapiro A. Watchful waiting policy in recurrent Ta G1 bladder tumors. Eur. Urol. 49, 303–306 (2006). [DOI] [PubMed] [Google Scholar]
- 233.Morales A, Eidinger D. & Bruce AW Intracavitary bacillus calmette-guerin in the treatment of superficial bladder tumors. J. Urol. 116, 180–183 (1976). [DOI] [PubMed] [Google Scholar]
- 234.Oddens J. et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur. Urol. 63, 462–472 (2013). [DOI] [PubMed] [Google Scholar]
- 235.Balasubramanian A. et al. Adjuvant therapies for non-muscle-invasive bladder cancer: advances during BCG shortage. World J. Urol. 40, 1111–1124 (2022). [DOI] [PubMed] [Google Scholar]
- 236.Ourfali S. et al. Recurrence rate and cost consequence of the shortage of Bacillus Calmette-Guérin Connaught strain for bladder cancer patients. Eur. Urol. Focus 7, 111–116 (2021). [DOI] [PubMed] [Google Scholar]
- 237.Boorjian SA et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 22, 107–117 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Balar AV et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 22, 919–930 (2021). [DOI] [PubMed] [Google Scholar]
- 239.Shang PF et al. Intravesical Bacillus Calmette-Guérin versus epirubicin for Ta and T1 bladder cancer. Cochrane Database Syst. Rev. 5, CD006885 (2011). [DOI] [PubMed] [Google Scholar]
- 240.Malmström P-U et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur. Urol. 56, 247–256 (2009). [DOI] [PubMed] [Google Scholar]
- 241.Huncharek M, Geschwind JF, Witherspoon B, McGarry R. & Adcock D. Intravesical chemotherapy prophylaxis in primary superficial bladder cancer: a meta-analysis of 3703 patients from 11 randomized trials. J. Clin. Epidemiol. 53, 676–680 (2000). [DOI] [PubMed] [Google Scholar]
- 242.Gschwend JE et al. Extended versus limited lymph node dissection in bladder cancer patients undergoing radical cystectomy: survival results from a prospective, randomized trial. Eur. Urol. 75, 604–611 (2019). [DOI] [PubMed] [Google Scholar]
- 243.Lerner SP et al. SWOG S1011: a phase III surgical trial to evaluate the benefit of a standard versus an extended lymphadenectomy performed at time of radical cystectomy for muscle invasive urothelial cancer. J. Clin. Orthod. 41, 4508–4508 (2023). [Google Scholar]
- 244.Lee RK et al. Urinary diversion after radical cystectomy for bladder cancer: options, patient selection, and outcomes. BJU Int. 113, 11–23 (2014). [DOI] [PubMed] [Google Scholar]
- 245.Kowalewski K-F et al. Robotic-assisted versus laparoscopic versus open radical cystectomy-a systematic review and network meta-analysis of randomized controlled trials. Eur. Urol. Focus 9, 480–490 (2023). [DOI] [PubMed] [Google Scholar]
- 246.Zhang JH et al. Large single institution comparison of perioperative outcomes and complications of open radical cystectomy, intracorporeal robot-assisted radical cystectomy and robotic extracorporeal approach. J. Urol. 203, 512–521 (2020). [DOI] [PubMed] [Google Scholar]
- 247.Teoh JY-C et al. Perioperative outcomes of robot-assisted radical cystectomy with intracorporeal versus extracorporeal urinary diversion. Ann. Surg. Oncol. 28, 9209–9215 (2021). [DOI] [PubMed] [Google Scholar]
- 248.Baumann BC et al. Validating a local failure risk stratification for use in prospective studies of adjuvant radiation therapy for bladder cancer. Int. J. Radiat. Oncol. Biol. Phys. 95, 703–706 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Baumann BC et al. Development and validation of consensus contouring guidelines for adjuvant radiation therapy for bladder cancer after radical cystectomy. Int. J. Radiat. Oncol. Biol. Phys. 96, 78–86 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zaghloul MS et al. Adjuvant sandwich chemotherapy plus radiotherapy vs adjuvant chemotherapy alone for locally advanced bladder cancer after radical cystectomy: a randomized phase 2 trial. JAMA Surg. 153, e174591 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Peak TC & Hemal A. Partial cystectomy for muscle-invasive bladder cancer: a review of the literature. Transl. Androl. Urol. 9, 2938–2945 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Compérat E. et al. Current best practice for bladder cancer: a narrative review of diagnostics and treatments. Lancet 400, 1712–1721 (2022). [DOI] [PubMed] [Google Scholar]
- 253.Huddart RA, Hall E, Lewis R. & Birtle A, SPARE Trial Management Group. Life and death of spare (selective bladder preservation against radical excision): reflections on why the spare trial closed. BJU Int. 106, 753–755 (2010). [DOI] [PubMed] [Google Scholar]
- 254.Vashistha V. et al. Radical cystectomy compared to combined modality treatment for muscle-invasive bladder cancer: a systematic review and meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 97, 1002–1020 (2017). [DOI] [PubMed] [Google Scholar]
- 255.Mak RH et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J. Clin. Oncol. 32, 3801–3809 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kamran SC & Efstathiou JA The legacy of RTOG/NRG protocols in shaping current bladder preservation therapy in North America. Semin. Radiat. Oncol. 33, 26–34 (2023). [DOI] [PubMed] [Google Scholar]
- 257.James ND et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N. Engl. J. Med. 366, 1477–1488 (2012). [DOI] [PubMed] [Google Scholar]
- 258. Zlotta AR et al. Radical cystectomy versus trimodality therapy for muscle-invasive bladder cancer: a multi-institutional propensity score matched and weighted analysis. Lancet Oncol. 24, 669–681 (2023). In the absence of randomized trials, this is the most definitive work suggesting that trimodality therapy for muscle-invasive bladder cancer is of value and should be considered in patient management.
- 259.Coen JJ et al. Bladder preservation with twice-a-day radiation plus fluorouracil/cisplatin or once daily radiation plus gemcitabine for muscle-invasive bladder cancer: NRG/RTOG 0712 — a randomized phase II trial. J. Clin. Oncol. 37, 44–51 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.US National Library of Medicine. ClinicalTrials.gov; https://clinicaltrials.gov/ct2/show/NCT03775265 (2023). [Google Scholar]
- 261.US National Library of Medicine. ClinicalTrials.gov; https://clinicaltrials.gov/ct2/show/NCT04241185 (2023). [Google Scholar]
- 262.Pieretti A. et al. Complications and outcomes of salvage cystectomy after trimodality therapy. J. Urol. 206, 29–36 (2021). [DOI] [PubMed] [Google Scholar]
- 263.Yerramilli D, Moghanaki DM & Efstathiou JA Safeguarding autonomy of patients with bladder cancer. Int. J. Radiat. Oncol. Biol. Phys. 103, 81–83 (2019). [DOI] [PubMed] [Google Scholar]
- 264.Dahl DM et al. NRG oncology/RTOG 0926: phase II protocol for patients with stage T1 bladder cancer to evaluate selective bladder preserving treatment by radiation therapy concurrent with radiosensitizing chemotherapy following a thorough transurethral surgical re-staging. Int. J. Radiat. Oncol. Biol. Phys. 111, S133–S134 (2021). [Google Scholar]
- 265.Seisen T. et al. Efficacy of high-intensity local treatment for metastatic urothelial carcinoma of the bladder: a propensity score-weighted analysis from the national cancer data base. J. Clin. Oncol. 34, 3529–3536 (2016). [DOI] [PubMed] [Google Scholar]
- 266.Fischer-Valuck BW et al. Association between local radiation therapy to the primary bladder tumor and overall survival for patients with metastatic urothelial cancer receiving systemic chemotherapy. Eur. Urol. Oncol. 5, 246–250 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Lehmann J. et al. Surgery for metastatic urothelial carcinoma with curative intent: the German experience (AUO AB 30/05). Eur. Urol. 55, 1293–1299 (2009). [DOI] [PubMed] [Google Scholar]
- 268.Palma DA et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J. Clin. Oncol. 38, 2830–2838 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.International Collaboration of Trialists et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J. Clin. Oncol. 29, 2171–2177 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Grossman HB et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 349, 859–866 (2003). [DOI] [PubMed] [Google Scholar]
- 271.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur. Urol. 48, 202–205 (2005). [DOI] [PubMed] [Google Scholar]
- 272.Galsky MD et al. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer 121, 2586–2593 (2015). [DOI] [PubMed] [Google Scholar]
- 273.Flaig TW et al. A randomized phase II study of coexpression extrapolation (COXEN) with neoadjuvant chemotherapy for bladder cancer (SWOG S1314; NCT02177695). Clin. Cancer Res. 27, 2435–2441 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Pfister C. et al. Dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin or gemcitabine and cisplatin as perioperative chemotherapy for patients with nonmetastatic muscle-invasive bladder cancer: results of the GETUG-AFU V05 VESPER trial. J. Clin. Oncol. 40, 2013–2022 (2022). [DOI] [PubMed] [Google Scholar]
- 275.Sternberg CN et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol. 16, 76–86 (2015). [DOI] [PubMed] [Google Scholar]
- 276.Galsky MD et al. Effectiveness of adjuvant chemotherapy for locally advanced bladder cancer. J. Clin. Oncol. 34, 825–832 (2016). [DOI] [PubMed] [Google Scholar]
- 277. Bajorin DF et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N. Engl. J. Med. 384, 2102–2114 (2021). This trial demonstrated an improvement in disease-free survival with adjuvant PD1 inhibition versus placebo as adjuvant therapy for patients with high-risk muscle-invasive urothelial cancer after radical resection.
- 278.Bellmunt J. et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 22, 525–537 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Loehrer PJ Sr et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J. Clin. Oncol. 10, 1066–1073 (1992). [DOI] [PubMed] [Google Scholar]
- 280.Gabrilove JL et al. Effect of granulocyte colony-stimulating factor on neutropenia and associated morbidity due to chemotherapy for transitional-cell carcinoma of the urothelium. N. Engl. J. Med. 318, 1414–1422 (1988). [DOI] [PubMed] [Google Scholar]
- 281.Sternberg CN et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur. J. Cancer 42, 50–54 (2006). [DOI] [PubMed] [Google Scholar]
- 282.von der Maase H. et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J. Clin. Oncol. 23, 4602–4608 (2005). [DOI] [PubMed] [Google Scholar]
- 283.Galsky MD et al. A consensus definition of patients with metastatic urothelial carcinoma who are unfit for cisplatin-based chemotherapy. Lancet Oncol. 12, 211–214 (2011). [DOI] [PubMed] [Google Scholar]
- 284.De Santis M. et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J. Clin. Oncol. 30, 191–199 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Sharma P. et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 18, 312–322 (2017). [DOI] [PubMed] [Google Scholar]
- 286.Patel MR et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 19, 51–64 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Bellmunt J. et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 376, 1015–1026 (2017). This trial demonstrated an improvement in survival with PD1 inhibition versus chemotherapy in patients with metastatic urothelial cancer progressing despite prior platinum-based chemotherapy.
- 288.Balar AV et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): multicentre, single-arm, phase 2 study. Lancet Oncol. 18, 1483–1492 (2017). [DOI] [PubMed] [Google Scholar]
- 289.Balar AV et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 389, 67–76 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Galsky MD et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 395, 1547–1557 (2020). [DOI] [PubMed] [Google Scholar]
- 291.Powles T. et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): randomised, open-label, phase 3 trial. Lancet Oncol. 22, 931–945 (2021). [DOI] [PubMed] [Google Scholar]
- 292.Galsky MD et al. Randomized double-blind phase II study of maintenance pembrolizumab versus placebo after first-line chemotherapy in patients with metastatic urothelial cancer. J. Clin. Oncol. 38, 1797–1806 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Powles T. et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 383, 1218–1230 (2020). This trial demonstrated an improvement in survival with switch maintenance PDL1 inhibition versus surveillance after first-line chemotherapy in patients with metastatic urothelial cancer.
- 294.Rugo HS et al. LBA76 Overall survival (OS) results from the phase III TROPiCS-02 study of sacituzumab govitecan (SG) vs treatment of physician’s choice (TPC) in patients (pts) with HR+/HER2-metastatic breast cancer (mBC). Ann. Oncol. 33, S1386 (2022). [Google Scholar]
- 295.Catto JWF et al. Quality of life after bladder cancer: a cross-sectional survey of patient-reported outcomes. Eur. Urol. 79, 621–632 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Yoshimura K. et al. Impact of superficial bladder cancer and transurethral resection on general health-related quality of life: an SF-36 survey. Urology 65, 290–294 (2005). [DOI] [PubMed] [Google Scholar]
- 297.Siracusano S. et al. Health-related quality of life after BCG or MMC induction for non-muscle invasive bladder cancer. Can. J. Urol. 25, 9480–9485 (2018). [PubMed] [Google Scholar]
- 298.Wei L, Li Q, Liang H. & Jianbo L. The quality of life in patients during intravesical treatment and correlation with local symptoms. J. Chemother. 26, 165–168 (2014). [DOI] [PubMed] [Google Scholar]
- 299.Yang LS et al. A systematic review and meta-analysis of quality of life outcomes after radical cystectomy for bladder cancer. Surg. Oncol. 25, 281–297 (2016). [DOI] [PubMed] [Google Scholar]
- 300.Khetrapal P. et al. Robot-assisted radical cystectomy versus open radical cystectomy: a systematic review and meta-analysis of perioperative oncological and quality of life outcomes using randomized controlled trials. Eur. Urol. 84, 393–405 (2023). [DOI] [PubMed] [Google Scholar]
- 301.Catto JWF et al. Effect of robot-assisted radical cystectomy with intracorporeal urinary diversion vs open radical cystectomy on 90-day morbidity and mortality among patients with bladder cancer: a randomized clinical trial. JAMA 327, 2092–2103 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Efstathiou JA et al. Late pelvic toxicity after bladder-sparing therapy in patients with invasive bladder cancer: RTOG 89–03, 95–06, 97–06, 99–06. J. Clin. Oncol. 27, 4055–4061 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Huddart RA et al. Patient-reported quality of life outcomes in patients treated for muscle-invasive bladder cancer with radiotherapy ± chemotherapy in the BC2001 phase III randomised controlled trial. Eur. Urol. 77, 260–268 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Zietman AL et al. Organ conservation in invasive bladder cancer by transurethral resection, chemotherapy and radiation: results of a urodynamic and quality of life study on long-term survivors. J. Urol. 170, 1772–1776 (2003). [DOI] [PubMed] [Google Scholar]
- 305.Mak KS et al. Quality of life in long-term survivors of muscle-invasive bladder cancer. Int. J. Radiat. Oncol. Biol. Phys. 96, 1028–1036 (2016). [DOI] [PubMed] [Google Scholar]
- 306.Westergren D-O, Gårdmark T, Lindhagen L, Chau A. & Malmström P-U A nationwide, population based analysis of patients with organ confined, muscle invasive bladder cancer not receiving curative intent therapy in Sweden from 1997 to 2014. J. Urol. 202, 905–912 (2019). [DOI] [PubMed] [Google Scholar]
- 307.Degboe A, Ivanescu C, Rohay JM, Turner RR & Cella D. Validity and performance of the Functional Assessment of Cancer Therapy-Bladder (FACT-Bl) among advanced urothelial cancer patients. Support. Care Cancer 27, 4189–4198 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Kitamura H. et al. Effect of neoadjuvant chemotherapy on health-related quality of life in patients with muscle-invasive bladder cancer: results from JCOG0209, a randomized phase III study. Jpn J. Clin. Oncol. 50, 1464–1469 (2020). [DOI] [PubMed] [Google Scholar]
- 309.Witjes JA et al. Health-related quality of life with adjuvant nivolumab after radical resection for high-risk muscle-invasive urothelial carcinoma: results from the phase 3 checkmate 274 trial. Eur. Urol. Oncol. 5, 553–563 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Grivas P. et al. Patient-reported outcomes from JAVELIN bladder 100: avelumab first-line maintenance plus best supportive care versus best supportive care alone for advanced urothelial carcinoma. Eur. Urol. 83, 320–328 (2023). [DOI] [PubMed] [Google Scholar]
- 311.Vaughn DJ et al. Health-related quality-of-life analysis from KEYNOTE-045: a phase III study of pembrolizumab versus chemotherapy for previously treated advanced urothelial cancer. J. Clin. Oncol. 36, 1579–1587 (2018). [DOI] [PubMed] [Google Scholar]
- 312.Mariotto AB, Enewold L, Zhao J, Zeruto CA & Yabroff KR Medical care costs associated with cancer survivorship in the United States. Cancer Epidemiol. Biomark. Prev. 29, 1304–1312 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Leal J, Luengo-Fernandez R, Sullivan R. & Witjes JA Economic burden of bladder cancer across the European Union. Eur. Urol. 69, 438–447 (2016). [DOI] [PubMed] [Google Scholar]
- 314.Botteman MF, Pashos CL, Redaelli A, Laskin B. & Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics 21, 1315–1330 (2003). [DOI] [PubMed] [Google Scholar]
- 315.Yeung C, Dinh T. & Lee J. The health economics of bladder cancer: an updated review of the published literature. Pharmacoeconomics 32, 1093–1104 (2014). [DOI] [PubMed] [Google Scholar]
- 316.Joyce DD, Sharma V. & Williams SB Cost-effectiveness and economic impact of bladder cancer management: an updated review of the literature. Pharmacoeconomics 41, 751–769 (2023). [DOI] [PubMed] [Google Scholar]
- 317.Kandoi G, Acencio ML & Lemke N. Prediction of druggable proteins using machine learning and systems biology: a mini-review. Front. Physiol. 6, 366 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Koprowski R. & Foster KR Machine learning and medicine: book review and commentary. Biomed. Eng. Online 17, 17 (2018). [Google Scholar]
- 319.Sanli O. et al. Bladder cancer. Nat. Rev. Dis. Primers 3, 17022 (2017). [DOI] [PubMed] [Google Scholar]
- 320.Loriot Y. et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 381, 338–348 (2019). [DOI] [PubMed] [Google Scholar]
- 321.Powles T. et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N. Engl. J. Med. 384, 1125–1135 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Hoimes CJ et al. Enfortumab vedotin plus pembrolizumab in previously untreated advanced urothelial cancer. J. Clin. Oncol. 41, 22–31 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Tagawa ST et al. TROPHY-U-01: a phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J. Clin. Oncol. 39, 2474–2485 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Berglund RK & Herr HW in Campbell-Walsh Urology 10th edn (eds McDougal W. et al. ) Ch. 83, 2375 (Elsevier Health Sciences, 2011). [Google Scholar]
- 325.Beer N. Removal of neoplasms of the urinary bladder. A new method, employing high-frequency (oudin) currents through a catheterizing cystoscope. JAMA LIV, 1768–1769 (1910). [DOI] [PubMed] [Google Scholar]
- 326.Sternberg CN et al. Preliminary results of M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for transitional cell carcinoma of the urothelium. J. Urol. 133, 403–407 (1985). [DOI] [PubMed] [Google Scholar]
- 327.Sánchez de Badajoz E. et al. Radical cystectomy and laparoscopic ileal conduit [Spanish]. Arch. Esp. Urol. 46, 621–624 (1993). [PubMed] [Google Scholar]
- 328.Housset M. et al. Combined radiation and chemotherapy for invasive transitional-cell carcinoma of the bladder: a prospective study. J. Clin. Oncol. 11, 2150–2157 (1993). [DOI] [PubMed] [Google Scholar]
- 329.von der Maase H. et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J. Clin. Oncol. 18, 3068–3077 (2000). [DOI] [PubMed] [Google Scholar]
- 330.Menon M. et al. Nerve-sparing robot-assisted radical cystoprostatectomy and urinary diversion. BJU Int. 92, 232–236 (2003). [DOI] [PubMed] [Google Scholar]
- 331.Bellmunt J. et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J. Clin. Oncol. 27, 4454–4461 (2009). [DOI] [PubMed] [Google Scholar]
- 332.Humphrey P. et al. The 2016 WHO classification of tumours of the urinary system and male genital organs-part B: prostate and bladder tumours. Eur. Urol. 70, 106–119 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.