Abstract
Background:
Changes in land use and climate change have been reported to reduce biodiversity of both the environment and human microbiota. These reductions in biodiversity may lead to inadequate and unbalanced stimulation of immunoregulatory circuits and, ultimately, to clinical diseases, such as asthma and allergies.
Objective:
We summarized available empirical evidence on the role of inner (gut, skin, and airways) and outer (air, soil, natural waters, plants, and animals) layers of biodiversity in the development of asthma, wheezing, and allergic sensitization.
Methods:
We conducted a systematic search in SciVerse Scopus, PubMed MEDLINE, and Web of Science up to 5 March 2024 to identify relevant human studies assessing the relationships between inner and outer layers of biodiversity and the risk of asthma, wheezing, or allergic sensitization. The protocol was registered in PROSPERO (CRD42022381725).
Results:
A total of 2,419 studies were screened and, after exclusions and a full-text review of 447 studies, 82 studies were included in the comprehensive, final review. Twenty-nine studies reported a protective effect of outer layer biodiversity in the development of asthma, wheezing, or allergic sensitization. There were also 16 studies suggesting an effect of outer layer biodiversity on increasing asthma, wheezing, or allergic sensitization. However, there was no clear evidence on the role of inner layer biodiversity in the development of asthma, wheezing, and allergic sensitization (13 studies reported a protective effect and 15 reported evidence of an increased risk).
Conclusions:
Based on the reviewed literature, a future systematic review could focus more specifically on outer layer biodiversity and asthma. It is unlikely that association with inner layer biodiversity would have enough evidence for systematic review. Based on this comprehensive review, there is a need for population-based longitudinal studies to identify critical periods of exposure in the life course into adulthood and to better understand mechanisms linking environmental exposures and changes in microbiome composition, diversity, and/or function to development of asthma and allergic sensitization. https://doi.org/10.1289/EHP13948
Introduction
Our planet is experiencing a massive decline in biodiversity, which is largely due to human activities and which could ultimately lead to the sixth extinction of animal and plant species on the Earth.1,2 This global change in the environments can affect ecosystem functioning by changing the time of natural events and cycles, reducing the ability of some species to survive, and/or by limiting the ability of ecosystems (e.g., forests) to absorb carbon dioxide, and lead to significant disruptions of ecosystems, which may threaten human livelihood and the current way of life. Loss of biodiversity is a global concern and may lead to a variety of possible adverse consequences for the human population, such as an increase in the occurrence of chronic inflammatory diseases, mental health disorders, and emerging of zoonotic diseases.3 The reasons underlying such loss of biodiversity are complex and have been suggested to be largely linked to the consequences of growing urbanization and industrialization, climate change, increasing pollution, and increasing utilization of chemicals, which have impact on the environment and microorganisms with which humans coevolve.4,5
Biodiversity was defined by von Hertzen et al.1 (from the Convention on Biological Diversity; https://www.cbd.int/convention/articles.shtml?a=cbd-02) as follows:
the variability among living organisms from all sources, including, inter alia, terrestrial, marine and other aquatic ecosystems and the ecological complexes of which they are part; this includes diversity within species, between species and of ecosystems.
Haahtela6 also suggested that microorganisms play a key role in the link between biodiversity-related environmental changes and human health. Loss of biodiversity and the disappearance of natural habitats may reduce the diversity of environmental microbiota, that is, the biodiversity of the outer layer.6 According to Haahtela,6 humans are protected by two nested layers of biodiversity, namely, microbiota of the outer and the inner layer (Figure 1). The outer layer is dependent on the environment we live in (including air, soil, natural waters, plants, and animals)6; the inner layer inhabits the human body (including gut, skin, and airways) and is dependent on colonization from the outer layer.6 Furthermore, environmental exposures (i.e., outer layer) may also influence the diversity and composition of the human microbiota (i.e., inner layer).7–9
Figure 1.
Layers of biodiversity.6
In 2011, von Hertzen et al.1 proposed that loss of biodiversity also leads to immune system dysfunction and increases the risk of chronic inflammatory diseases, including asthma and allergies, chronic obstructive pulmonary disease, type 1 diabetes, obesity, and inflammatory bowel diseases, and could therefore have important public health implications. This biodiversity hypothesis proposed by von Hertzen et al.1 is consistent with the observed declining trends of biodiversity indexes, such as the Waterbird Population Status Index and the Living Planet Index, and with increasing trends in the prevalence of asthma and allergic rhinitis since the 1970s.5 Rapidly declining biodiversity may be a contributing factor to another global megatrend, the rapidly increasing prevalence of allergies and other chronic inflammatory diseases among urban people.5 Increasing evidence suggests that the diversity of human microbiota influences the risk of asthma and allergies.10 Changes in the development of microbiota, evidenced by low gut and airways microbiota diversity in infancy, has been associated with the development of atopy and asthma later in life.10,11 The biodiversity hypothesis has stimulated substantial research on the role of biodiversity in the risk of developing asthma and allergic diseases, but the results have so far been inconsistent.10,11 This heterogeneity in results may be related to different definitions and measures of biodiversity, timing and duration of exposure, and differences in duration of the study periods, as well as differences in the phenotypes of asthma and allergic diseases. Therefore, this comprehensive review aimed to summarize the current knowledge on the relationship between exposure to inner and outer layers of biodiversity and the development of asthma, wheezing, and allergic sensitization.
Methods
Selection Criteria and Data Collection
We performed a systematic search of SciVerse Scopus, PubMed MEDLINE, and Web of Science databases from the inception of each database up to 5 March 2024, using the following Boolean search commands: [“biodiversity”] AND ([“allerg*”] OR [“asthma”]) AND [“environment*”] AND [“microbio*”]. The methodology used in this comprehensive review followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses literature search extension (PRISMA) reporting standards. The protocol was published in the International Register of Systematic Review Protocols (PROSPERO; registration no. CRD42022381725) in December 2022 (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022381725). Results were exported to Covidence Systematic Review Software for screening and to identify duplicates. A study was considered eligible if conducted in humans and published in English, and no restrictions were applied to the type of study or article format. We identified and screened a total of 2,419 articles in two phases: The first phase was initially based on the title and abstract to eliminate obviously irrelevant articles (i.e., not conducted in humans and published not in English), and the second phase was based on the full text. An individual search of the reference lists from all identified relevant original studies and review articles was also conducted to identify potential additional eligible studies (). Attempts were made to contact the corresponding author when the full-text article provided inadequate information for extracting relevant data.
A study was included in the comprehensive review if it a) was an original randomized controlled trial or an epidemiologic study (cohort, case–control, or cross-sectional study); b) provided data on the relationships between inner and/or outer layers of biodiversity and the risk of asthma, wheezing, and/or allergic sensitization; and c) included a definition and/or indexes of biodiversity and the main health outcomes (asthma, wheezing, and allergic sensitization). Studies reporting only asthma exacerbation as the outcome or assessing the effect of antibiotics, medication, or treatment were excluded. Studies assessing only the effect of endotoxins, fungi or virus, diet, breastfeeding, mode of delivery, use of probiotics/prebiotics/symbiotics, pet exposure, or exposure to farm, rural, or urban environments and to natural and green spaces were also excluded.
Exposure
The exposures of interest were the outer and/or inner layers biodiversity.6 The outer layer is dependent on the environment we live in (including air, soil, natural waters, plants, and animals). The inner layer inhabits human body (including gut, skin, and airways) and is influenced by colonization from the outer layer.6 The outer layer biodiversity was defined based on the environmental microbiota (including dust or soil) or on a score of environmental biodiversity (e.g., species richness index, land-use gradient, and plant diversity). The inner layer biodiversity was defined based on the diversity of human inner microbiota in the skin, stool, urine, and airway samples (including nasal, oropharyngeal, nasopharyngeal, and/or throat). The Shannon diversity index and the Simpson index, which weight the number of species by their relative evenness, and bacterial richness (that is, the total number of operational taxonomic units (OTUs) or species recorded, were considered as the diversity metrics for human and dust/soil samples.
Outcomes
The primary outcomes included asthma and the symptom wheezing. Different asthma definitions were considered for the inclusion of studies, such as the medical diagnosis, use of asthma medications, or the Global Initiative for Asthma (GINA)-based definition.12 The secondary outcome was allergic sensitization, which was defined based on a positive response to immunoglobulin E (IgE) antibodies for specific allergens, or a positive skin prick test.
Data Extraction
Three reviewers (I.P., N.S., B.H.) independently performed the initial screening by the title and the abstract. After this initial screening, full articles were reviewed by two independent reviewers (T.T.H., A.K.R.) and those fulfilling the inclusion criteria were selected for data extraction. When a conflict in assessments appeared at any stage of the review, a consensus between the original and two additional reviewers (T.T.H., A.K.R.) was required to achieve the resolution. For each study, the following information was collected: first author’s surname, publication year, country where the study was conducted, study design, population, definitions of exposure and outcome, methodology to assess or define the exposure parameters, mean/median (standard deviation/interquartile range) values or other measures of effect [95% confidence intervals (CIs)] and covariates.
Results
Studies
Our search returned 2,915 articles. After removal of duplicates, 2,419 studies were screened, 2,019 of which were excluded based on title and abstract review. Three hundred and ninety-six studies were retrieved for full-text review, and 365 were excluded (Figure 2). Reasons for exclusion included nonoriginal studies (244 were narrative reviews/meta-analysis), studies in which health outcomes were not relevant for the present objectives, and studies reporting results that were not suitable for inclusion in the present comprehensive review. The review of the reference lists of the identified articles resulted in the inclusion of an additional 51 articles. After a full-text review of the remaining 447 studies, 82 studies were included in the comprehensive review (Figure 2). Of the 82 included studies, 33 studies were related to the outer layer biodiversity [23 used dust samples and 10 used environmental biodiversity measures (e.g., species richness index, land-use gradient, and plant diversity)] and 49 studies were related to the inner layer biodiversity (2 studies characterized urine and skin microbiota diversity, 20 studies collected stool samples, and 27 characterized airway microbiota diversity) (Figure 3, Table S1).
Figure 2.
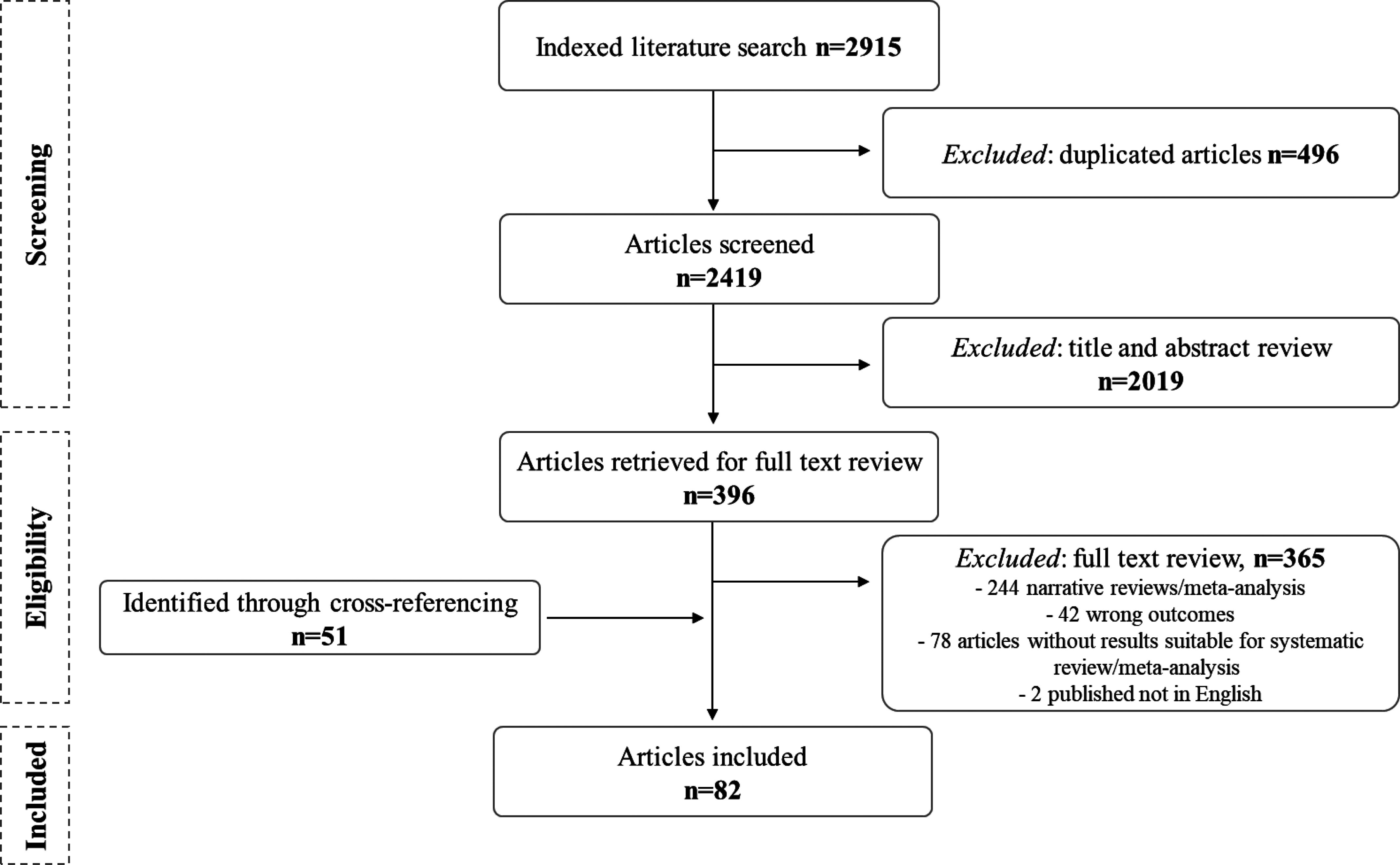
Selection of articles for the state-of-the-science review of the role of biodiversity in the development of asthma and allergic sensitization.
Figure 3.
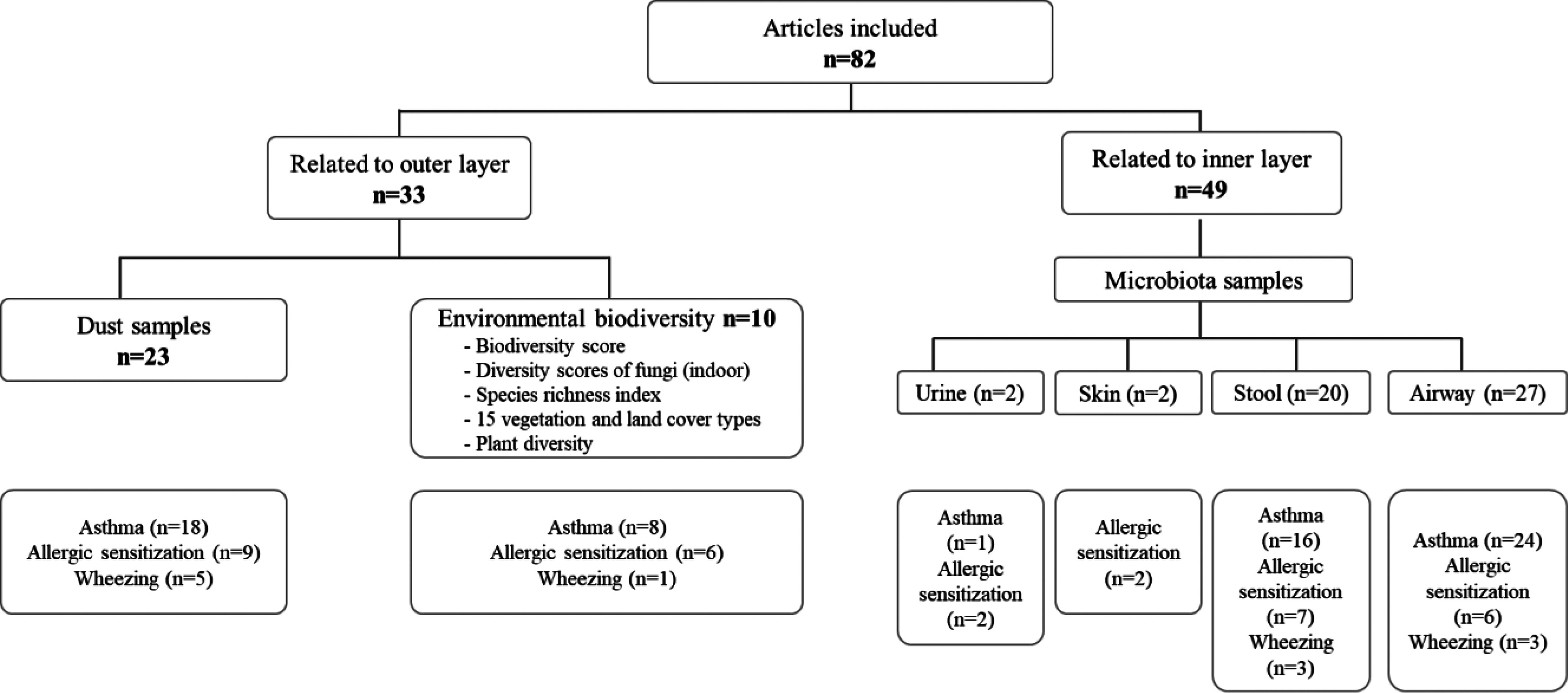
Characterization of the studies included () in the state-of-the-science review based on outer or inner layer biodiversity.
Characteristics of the 82 included studies are shown in Table S1. Among the 82 included studies, 40 studies () were conducted in Europe, 10 of these in more than one country; 22 in the Americas (17 in the United States); 17 in Asia; 2 in Oceania and only 1 in Africa. Health outcomes were ascertained based on a) parental reports of symptoms or doctor-diagnosis of asthma, wheezing, and/or allergic sensitization; b) a clinical diagnosis based on clinical examination or medical records; c) pharmacy and hospital discharge records (asthma, 1 study); d) census tract (asthma, 1 study); e) lung function or methacholine challenge test for asthma; and f) skin prick testing or assessment of specific IgE to characterize allergic sensitization (Table S1). Information on exposure was assessed by validated measurement tools: a) amplicon sequencing targeting the 16S rRNA gene; b) bacteria-derived extracellular vesicles; c) culture-based approaches [indoor (dust) and biological (urine, stool, skin, and airway) samples]; or d) diversity of land-use/cover types using satellite data, plant diversity, diversity scores of fungi, and species richness index. Nineteen of the 41 studies used similar approaches to assess biodiversity (including relative abundance of bacteria, bacterial richness, or the Shannon diversity index) (Table S1).
Among the 82 studies included in the comprehensive review, most of the studies focused on children [ (70%), age of participants ranged from 0 to 18 y], 5 studies focused on children and adults (age ranged from 0.5 to 52 y), whereas 20 studies focused on adults (age of participants ranged from 18 to 80 y) (Table S1). Among the studies focused on children, 35 studies reported a protective effect of increased biodiversity on asthma (), allergic sensitization (), or wheezing (); 25 studies presented evidence on increased biodiversity being related to higher risk of developing asthma (), allergic sensitization (), and wheezing (); 7 studies presented contradictory findings; and 24 studies reported no evidence on the effect of outer or inner layer biodiversity on respiratory outcomes (Table 1 and Table S2; some studies contributed more than one result). Nevertheless, the studies provided inconsistent estimates of the association between biodiversity and asthma, wheezing, or allergic sensitization among adults (7 studies reported a protective effect of increased biodiversity; 6 reported an adverse effect; 5 presented contradictory findings; and 8 reported no evidence on the effect of biodiversity on asthma, allergic sensitization, or wheezing) (Table 1 and Table S2; some studies contributed more than one result).
Table 1.
Main results of studies included in the comprehensive review ().
| Reference, study year (country) | Study population (age) | Covariates | Main results |
|---|---|---|---|
| Outer layer biodiversity | |||
| Ege et al., 2011 (Germany)102 | Children (6–13 y) | Living in a farm | Bacteria diversity score (number of detectable bands) PARSIFAL (95% CI: 0.45, 0.94) (95% CI: 0.65, 1.15) GABRIELA (95% CI: 0.73, 1.03) (95% CI: 0.79, 1.11) PCA 4 PARSIFAL (95% CI: 0.42, 0.91) PCA 5 PARSIFAL (95% CI: 0.42, 0.91) |
| Ege et al., 2012 (Germany)103 | Children ( y) | Farming, family history of atopy, parental education, and mutually adjusted for all associated bands (asthma: bands 248, 300, 318, 314, 427 and 506; allergic sensitization: bands 160, 265, 333 and 539) | Band 248 (95% CI: 0.24, 0.89) Band 394 (95% CI: 0.32, 0.97) Band 506 (95% CI: 0.38, 0.88) For a low cut-off: Band 394 (95% CI: 0.04, 0.72) Band 506 (95% CI: 0.20, 0.83) For a high cut-off: Band 300 (95% CI: 1.18, 4.95) Band 318 (95% CI: 1.07, 5.06) Band 427 (95% CI: 0.23, 0.89) |
| Hanski et al., 2012 (Finland)34 | Children (17–18 y) | — | PC1env (forested and agricultural land): , Flowering: , Gammaproteobacteria on the skin , |
| Lynch et al., 2014 (USA)7 | Children (3 y) | Race/ethnicity, gender, mean perceived stress of the mother in the year after birth, and number of smokers in the home | Children with the highest exposure to specific bacteria during their first year were least likely to develop recurrent wheeze and allergic sensitization |
| Ciaccio et al., 2015 (USA)104 | Children (2.4–4.8 y) | — | No significant difference in genus-level richness was found between the asthma homes and control homes |
| Ruokolainen et al., 2015 (Finland and Estonia)35 | Children and young adults (0.5–20 y) | Age and dataset (study cohort) | Land-use gradient () among children 0.5–1 years of age () among children 1.5–3 years of age () among children 6–12 years of age () among children/young adults 13–20 years of age () among children/young adults 0.5–20 years of age |
| Valkonen et al., 2015 (Germany, Austria, and Switzerland)105 | Children (6–12 y) | Sex and age | There was also a protective trend () of high bacterial diversity (Shannon index) on atopy. Individual bacterial groups and diversity were not clearly associated with asthma 7 and 2 bands: associated to a protective and adverse risk from developing allergic sensitization, respectively 5 and 1 bands: associated to be a protective and adverse risk from developing asthma, respectively Exposed nonfarm children: Shannon index (min, max: 1.93, 2.95) (min, max: 0.68, 3.01) (min, max: 2.30, 3.05) Nonexposed nonfarm children: Shannon index (min, max: 2.36, 2.88) (min, max: 2.01, 3.02) (min, max: 1.87, 3.04) |
| Tischer et al., 2016 (Germany)106 | Children (6 and 10 y) | Sex, maternal education, and season of dust sampling | Bacterial diversity: Sensitization to aero-allergens at 6 years of age 3rd tertile: (95% CI: 0.18, 1.11) Sensitization to aero-allergens at 10 years of age 3rd tertile: (95% CI: 0.18, 1.11) Wheezing at 10 years of age 3rd tertile: (95% CI: 0.45, 2.06) |
| Birzele et al., 2017 (Austria)20 | Children (6–12 y) | Farming and for the respective diversity measurement in nasal swabs or mattress dust | Mattress dust: Richness (95% CI: 0.22, 1.02) Shannon index (95% CI: 0.21, 0.83) Nasal samples: Richness (95% CI: 0.38, 1.06) Shannon index (95% CI: 0.39, 1.12) |
| Cavaleiro Rufo et al., 2017 (Portugal)107 | Children (8–10 y) | Age and height | No significant associations were observed between diversity score and asthma 3rd quartile: (95% CI: 0.40, 0.98) 4th quartile: (95% CI: 0.40, 0.92) |
| Campbell et al., 2017 (14 different countries)87 | Adults (26–54 y) | Age, sex, study center, smoking, and family history of allergic disease | Lived in an inner city: Microbial load score 2 (95% CI: 0.55, 1.09) (95% CI: 0.32, 2.00) (95% CI: 0.47, 2.88) Microbial load score 3 (95% CI: 0.49, 1.00) (95% CI: 0.17, 1.34) (95% CI: 0.50, 3.13) Microbial load score 4/5 (95% CI: 0.41, 0.90) (95% CI: 0.08, 1.11) (95% CI: 0.60, 4.10) |
| Dannemiller et al., 2016 (USA)108 | Children (5–10 y) | — | Bacterial richness (95% CI: 0.30, 0.99) among all children (95% CI: 0.16, 0.90) among atopic children Low bacterial richness: (95% CI: 0.34, 1.75) among atopic children Bacterial concentration (95% CI: 0.60, 1.82) among all children (95% CI: 0.51, 2.49) among atopic children (95% CI: 0.42, 2.11) among nonatopic children |
| Karvonen et al., 2017 (Finland)109 | Children (1 and 6 y) | Study cohort, farming, maternal history of allergic diseases, gender, number of older siblings, and smoking during pregnancy. Models of sensitization to inhalant allergens are additionally adjusted for floor type of dust sampling | No significant association were observed between microbial quantity or diversity scores and asthma or respiratory symptoms up to 6 years of age and current asthma and sensitization to inhalant allergen at 6 years of age Quantity score 2nd quintile: (95% CI: 0.67, 4.45) 3rd quintile: (95% CI: 0.87, 5.75) 4th quintile: (95% CI: 0.67, 4.69) 5th quintile: (95% CI: 0.09, 1.36) 2nd quintile: (95% CI: 0.38, 4.61) 3rd quintile: (95% CI: 0.56, 6.79) 4th quintile: (95% CI: 0.55, 6.32) 5th quintile: (95% CI: 0.07, 2.11) 2nd quintile: (95% CI: 0.35, 1.22) 3rd quintile: (95% CI: 0.49, 1.62) 4th quintile: (95% CI: 0.55, 1.75) 5th quintile: (95% CI: 0.43, 1.24) 2nd quintile: (95% CI: 0.92, 5.23) 3rd quintile: (95% CI: 0.55, 3.64) 4th quintile: (95% CI: 0.51, 3.40) 5th quintile: (95% CI: 0.61, 3.70) Diversity score 5 score: (95% CI: 0.27, 1.78) 6 score: (95% CI: 0.43, 2.49) 7–8 score: (95% CI: 0.15, 2.76) 5 score: (95% CI: 0.10, 0.89) 6 score: (95% CI: 0.12, 0.94) 7–8 score: (95% CI: 0.07, 2.29) 5 score: (95% CI: 0.38, 1.20) 6 score: (95% CI: 0.30, 0.97) 7–8 score: (95% CI: 0.06, 0.32) 5 score: (95% CI: 0.84, 5.27) 6 score: (95% CI: 0.86, 5.45) 7–8 score: (95% CI: 0.12, 1.50) |
| Donovan et al., 2018 (New Zealand)33 | Children (18 y) | Roads, air pollution, ethnicity, gender, birth outcomes, parents’ occupation, parents’ education, parents’ smoking status, antibiotic use, number of siblings, mesh block size, and birth order | Vegetation diversity (lifetime) (95% CI: 0.885, 0.985) |
| Lai et al., 2018 (USA)110 | Children ( y) | Age, gender, ethnicity, and season | Classroom microbial diversity (95% CI: 1.00, 1.14) Home microbial diversity (95% CI: 1.00, 1.00) |
| Loo et al., 2018 (Singapore)111 | Children (3–60 months) | — | No significant difference in Shannon or Simpson’s diversity indexes of bed, play area, and sofa dust samples of the participants who were allergic and those who were nonallergic |
| O’Connor et al., 2018 (USA)31 | Children (3 and 7 y) | Gender, race, maternal asthma, and maternal Perceived Stress Scale score | Bacterial and did not differ significantly between the homes of children that did or did not develop asthma, nor did these diversity measures differ between the homes of the children that did or did not develop atopy at 7 years of age |
| Pekkanen et al., 2018 (7 European countries)112 | Adults (29–55 y) | Age, sex, parental allergy, current smoking, and household density | Band L3B49_8 (2nd tertile): (95% CI: 1.455, 4.291) Band L3B53_7 (2nd tertile): (95% CI: 1.214, 4.007) Band L3B57_6 (2nd tertile): (95% CI: 1.378, 4.178) 3rd tertile: (95% CI: 1.299, 4.140) Band L3B71_9 (2nd tertile): (95% CI: 1.214, 3.974) |
| Valkonen et al., 2018 (7 European countries)113 | Adults (29–55 y) | Parental allergy, smoking status, household density, gender, age, and center | No significant associations were observed between microbial species and asthma and allergic sensitization |
| Karvonen, 2019 (Finland)19 | Children (10.5 y) | Follow-up time, study cohort, living on a farm, and well-known risk factors for asthma (maternal history of allergic diseases, sex, number of older siblings, and smoking during pregnancy) | Bacterial richness: (95% CI: 0.39, 0.95) (95% CI: 0.37, 1.12) Shannon diversity index: (95% CI: 0.55, 1.07) (95% CI: 0.45, 1.30) |
| Kirjavainen et al., 2019 (Finland)13 | Children (6 y) | Living on a farm, cohort, gender, maternal history of allergic diseases, number of older siblings, and smoking during pregnancy. For FaRMI (farm home-resembling microbiota index)—paternal history of atopic diseases and asthma, maternal and paternal education levels, birth weight, mode of delivery, indoor exposure to dog and/or cat at the age of 2 months, distance to farm, breastfeeding, consumption of farm milk, child care attendance, regular exposure to passive tobacco smoke at the age of 1 y, house type and age, season, type of vacuumed floor, and time from last vacuuming with reference to dust sampling | Farm-like relative abundance of bacteria/archaea: (95% CI: 0.19, 0.82) (95% CI: 0.27, 0.81) (95% CI: 0.23, 0.98) |
| Cavaleiro Rufo et al., 2020 (Portugal)114 | Children (8–10 y) | Age (in years), sex, school, classroom, and maternal education, allergic sensitization status, and diagnosis of asthma | School SRI (95% CI: 0.992, 1.005) (95% CI: 0.998, 1.011) (95% CI: 0.992, 1.007) |
| Fu et al., 2020 (Malaysia)115 | Children (14–16 y) | Gender, race, smoking, and parental asthma/allergy | Number of OTUs (95% CI: 0.99, 1.01) |
| Gangneux et al., 2020 (France)116 | Children and adults | — | Shannon index asthma group: (): ; control group: , |
| Adams et al., 2021 (Finland and Netherlands)117 | Children () | Gender, age, moisture damage in the home, and educational level | Bacterial richness and diversity Middle category: (95% CI: 1.36, 1.50) Highest category (95% CI: 1.09, 1.46) |
| Cavaleiro Rufo et al., 2021 (Portugal)118 | Children (4 and 7 y) | Distance to the nearest major road/motorway/highway, sex, household crowding, maternal education, neighborhood socioeconomic deprivation, and maternal history of diagnosed asthma |
(95% CI: 1.09, 11.79) (95% CI: 1.04, 3.86) (95% CI: 1.20, 4.63) Highest tertile of SRI: (95% CI: 0.67, 7.64) (95% CI: 1.08, 5.88) |
| Cox et al., 2021 (USA)119 | Children (7 and 12 y) | Race (Black or non-Black), pets, neighborhood socioeconomic status, cockroaches, dust mites, and rodents | Bacteria associated with the absence of the health outcomes (95% CI: 0.64, 0.91) (95% CI: 0.81, 1.01) (95% CI: 0.73, 0.96) (95% CI: 0.69, 1.03) (95% CI: 0.87, 1.00) (95% CI: 0.83, 1.00) Bacteria associated with the presence of the health outcomes (95% CI: 0.97, 1.15) (95% CI: 0.97, 1.15) (95% CI: 1.15, 1.49) (95% CI: 1.05, 1.31) (95% CI: 1.05, 1.22) (95% CI: 1.04, 1.34) |
| Donovan et al., 2021 (USA)120 | Adults | Race, socioeconomic status, air pollution, and proximity to roads | Taxonomic plant diversity |
| Fu et al., 2021 (China)121 | Young adults (IQR: 20–23 y) | Gender, smoking, and parental asthma | Bacterial richness settled air dust: (95% CI: 0.86, 1.1) floor dust: (95% CI: 0.63, 1.14) |
| Fu et al., 2021 (China)122 | Children (15–18 y) | Current smoking, gender, and parental asthma and allergies | Number of OTUs |
| Hyytiäinen et al., 2021 (Finland and Germany)123 | Children (10 y) | Gender, parental atopy, number of older siblings, and season of dust sampling in both cohorts; maternal education, living on a farm and cohort, and the number of different pet species indoors in LUKAS (Finnish rural-suburban birth cohort); and parental education, study center and age of the mother at delivery in LISA (German urban birth cohort). | Bacterial richness LISA cohort Middle tertile: (95% CI: 0.62, 1.58) Highest tertile: (95% CI: 0.44, 1.15) LUKAS cohort Middle tertile: (95% CI: 0.43, 1.65) Highest tertile: (95% CI: 0.66, 3.87) Shannon index LISA cohort Middle tertile: (95% CI: 0.48, 1.20) LUKAS cohort Middle tertile: (95% CI: 1.04, 3.78) Rural children: Bacterial richness Middle tertile: (95% CI: 0.57, 3.15) Highest tertile: (95% CI: 1.05, 12.56) Shannon index Middle tertile: (95% CI: 0.84, 3.98) Highest tertile: (95% CI: 1.06, 14.11) Suburban children: Bacterial richness Middle tertile: (95% CI: 0.01, 0.70) Highest tertile: (95% CI: 0.07, 7.92) Shannon index Middle tertile: (95% CI: 0.12, 2.90) Highest tertile: (95% CI: 0.24, 18.44) Farm children: Bacterial richness Middle tertile: (95% CI: 0.14, 35.54) Highest tertile: (95% CI: 0.11, 18.70) Shannon index Middle tertile: (95% CI: 0.4, 79.77) Highest tertile: (95% CI: 0.12, 17.5) |
| Lehtimäki et al., 2021 (Denmark)124 | Children (6 y) | Pet ownership, child care attendance during the first year of life, length of the breastfeeding period, exposure to passive smoking, family income, parental education, number of older siblings, home type, mode of delivery, parental diagnosis of asthma/eczema/rhinitis, and use of antibiotics during the first year of life | Urbanized airway bacterial profile At 1 wk of age: (95% CI: 1.01, 1.55) At 1 month of age: (95% CI: 1.00, 1.48) Urbanized gut bacterial profile At 1 month of age: (95% CI: 1.05, 1.59) At 1 year of age: (95% CI: 1.02, 1.53); (95% CI: 1.03, 1.59); (95% CI: 0.98, 1.57) |
| Winnicki et al., 2022 (Denmark)125 | Individuals born 1995–2015 | Family history of asthma, income, education, age, and sex | Medium levels of the bioscore: (95% CI: 0.95, 1.08) (95% CI: 0.87, 1.09) High levels of the bioscore: (95% CI: 0.99, 1.28) (95% CI: 0.55, 0.94) |
| Inner layer biodiversity | |||
| Bisgaard et al., 2007 (Denmark)57 | Children (5 y) | Sex, gestational age at birth, maternal smoking during the third trimester, maternal use of antibiotics during the third trimester, breastfeeding, lung function, bronchial responsiveness, and the presence or absence of older children at home | The prevalence of asthma was 33% in colonized children and 10% in those not colonized [ (95% CI: 2.18, 9.57)]. No significant association was observed between colonization and allergic sensitization at 4 years of age [ (95% CI: 0.65, 2.54) |
| Hilty et al., 2010 (Ireland)126 | Children (1–17 y) and adults (– y) | — | Species Number of sequences of different phyla and genera were different among the groups (participants with asthma, COPD, and healthy) and considering the sample |
| Bisgaard et al., 2011 (Denmark)21 | Children (6 y) | Cesarean section, mother’s use of antibiotics in the third trimester; solely breastfeeding of the baby, or having a dog or cat at home at birth | Band richness At 1 month of age (95% CI: 0.83, 1.16) At 12 months of age (95% CI: 0.84, 1.12) |
| Cardenas et al., 2012 (Ecuador)127 | Children ( months) | — | No differences were found between individuals with wheeze and healthy individuals in species richness, taxa abundances, or evenness, as well as in microbial community cluster patterns ( diversity) |
| Marri et al., 2013 (USA)55 | Adults () | — | Samples from patients with asthma were associated with significantly greater bacterial diversity compared with samples from patients who were non-asthmatic |
| Abrahamsson et al., 2014 (Sweden)16 | Children (7 y) | — | Shannon diversity index At 1 wk of age (IQR: 0.95–1.64), (IQR: 1.42–1.75), (IQR: 1.38–1.75) (IQR: 1.42–1.79) (IQR: 1.42–1.80) At 1 month of age (IQR: 0.92–1.46), (IQR: 1.48–2.10), (IQR: 1.42–1.88) (IQR: 2.22–3.24) (IQR: 1.42–1.75) At 12 months of age (IQR: 2.26–3.24) (IQR: 2.25–3.24) (IQR: 2.32–3.21) (IQR: 2.22–3.24) (IQR: 2.32–3.25) Number of bacterial OTUs At 1 wk of age (IQR: 10–22) (IQR: 13–18) At 1 month of age (IQR: 12–17) (IQR: 14–22) At 12 months of age (IQR: 40–73) (IQR: 33–59) |
| Park et al., 2014 (Korea)128 | Adults ( to y) | — | Shannon index Number of OTUs |
| Arrieta et al., 2015 (Canada)70 | Children (3 y) | — | Gut community composition and diversity did not differ substantially among clinical phenotypes |
| Denner et al., 2016 (USA)129 | Adults ( and y) | — | Specific species Differential feature selection analysis revealed significant differences in microbial diversity between asthmatic and control brush and lavage samples |
| Hevia et al., 2016 (Spain)130 | Adults () | — | Specific species |
| Hua et al., 2016 (USA)131 | Adults ( y) | Sex, age, BMI, season, time since last antibiotic use, and probiotic and vitamin use | Shannon index Middle tertile (95% CI: 0.642, 1.64) Lowest tertile (95% CI: 0.85, 2.13) Richness Middle tertile (95% CI: 1.04, 2.71) Lowest tertile (95% CI: 0.94, 2.47) Chao1 Middle tertile (95% CI: 0.99, 2.52) Lowest tertile (95% CI: 0.82, 2.11) |
| Stiemsma et al., 2016 (Canada)71 | Children (4 y) | Antibiotic use, delivery mode, breastfeeding, sex, parental asthma, and atopic dermatitis | Gut microbial community composition at 3 months or 1 year of age did not differ between children with asthma and controls |
| Zhang et al., 2016 (UK)132 | Adults ( to y) | — | There were no significant differences in alpha diversity between healthy, non-severe, and severe asthma in adults |
| Chiu et al., 2017 (Taiwan)133 | Children (3–5 y) | Age, sex, maternal atopy, passive smoking, older siblings, and household income | Relatively lower Chao1 and Shannon indexes were found in children with asthma compared with the healthy controls, but these differences were not significant. However, the Chao1 and Shannon indexes in the mite-sensitized children with asthma were significantly lower than those in the healthy children without mite sensitization |
| Depner et al., 2017 (Germany, Austria, and Switzerland)59 | Children (12 y) | Farming | There was no association of bacterial load with asthma status Bacterial richness (95% CI: 0.25, 2.31) |
| Li et al., 2017 (China)134 | Adults ( to y) | — | Comparison between the three groups (asthmatics, severe, and non-severe asthma in adults) and healthy individuals showed that there was no significant difference in species richness and bacterial diversity as assessed by the Chao, Ace, Shannon, and Simpson indexes Number of OTUs |
| Ruokolainen et al., 2017 (Finnish and Russian Karelia)50 | Children (14–20 y) | — | Specific species The profile of Acinetobacter lwoffii in skin samples from sensitized Finns differed from all other samples (, ). No significant differences were found between sensitized and healthy participants for Acinetobacter johnsonii. For Micrococcus sp. profiles tended to differ between healthy and sensitized among the Russian participants (, ) |
| Arrieta et al., 2018 (Ecuador)135 | Children (5 y) | Antibiotic use during pregnancy or the first year of life, duration of antibiotic use during pregnancy or the first year of life, type of delivery, household potable water, number of respiratory tract infections during the first year of life, eosinophilia at 7 months of age, and number of diarrheal episodes during the first year of life | Atopic wheeze did not explain any significant changes in or bacterial diversity |
| Durack et al., 2018 (USA)136 | Adults (28–39 y) | — | Faith’s phylogenetic diversity trended to be higher in adults with allergic asthma compared those without asthma who were nonallergic in bronchial brushing samples. No such trend was observed in comparison of the other sample types from the same individuals No significant difference in the relative abundance of specific genera were observed in any of the four samples between participants with atopic asthma and healthy participants |
| Fazlollahi et al., 2018 (USA)51 | Adults () | Age, sex, allergic rhinitis, last upper respiratory infection, recent antibiotic use, antihistamine use, nasal steroid use, inhaled steroid use, and systemic steroid use | There was a positive trend between nasal bacterial alpha diversity and asthma activity. However, these differences in phylogenic diversity were not statistically significant Healthy controls, participants with nonexacerbated asthma, and participants with exacerbated asthma demonstrated distinct nasal microbiome compositions |
| Kim et al., 2018 (South Korea)137 | Children (6–10 y) | — | The number of observed OTUs and the Shannon diversity index in control group were lower than those in the asthma and remission groups, but the differences were not statistically significant |
| Okba et al., 2018 (Egypt)138 | Adults (18–45 y) | Sex, age, body mass index, time since last antibiotic use, and probiotic and vitamin use | Specific species Atopic asthma is significantly associated with gut microbiota Lactobacilli and E. coli |
| Stokholm et al., 2018 (Denmark)67 | Children (5 y) | Older siblings, duration of exclusive breastfeeding, hospitalization after birth, antibiotic use, and delivery mode | There were no significant associations between (Shannon diversity and Chao1 indexes) at any time point and asthma risk Microbial populations were significantly different at 1 year of age in children who had asthma at 5 years of age compared with those who were non-asthmatic (, |
| Wang et al., 2018 (UK)139 | Adults (36–80 y) | — | The at the three levels [genes, MGSs (meta-genomic species), and KEGG] show difference between asthma and control groups |
| Bannier et al., 2019 (Netherlands)140 | Children (6 y) | Sex, breastfeeding, birth season, atopy parents, siblings, parental smoking status, and child care attendance | At preschool age, microbial richness and Shannon index were not different between participants with wheeze and healthy controls |
| Espuela-Ortiz et al., 2019 (USA)53 | Children and young adults (6–21 y) | Age, sex, or genetic ancestry between cases and controls | Shannon index Pielou index |
| Lee et al., 2019 (South Korea)141 | Adults (18–45 y; ) | — | Young adults: Bacterial diversity was not significantly different between adults with asthma and those without Elderly: Bacterial diversity was not significantly different between adults with asthma and those without |
| Pang et al., 2019 (China)142 | Adults (37–41 y) | — | Eosinophilic asthma and non-eosinophilic asthma showed a significant difference on Chao1, observed species, and Shannon indexes among the three groups. Compared with healthy individuals, the asthmatics showed a significant decreased diversity (observed species index), richness (Chao1 and Shannon indexes), and evenness (Pielou evenness index). As for the participants with asthma, non-eosinophilic asthma showed a significant decreased diversity, richness, and evenness compared with eosinophilic asthma |
| Powell et al., 2019 (UK)44 | Children (24 months) | Ethnicity, family history of atopy (fixed), and presence of fever and the use of antibiotics in the 4 weeks prior to visit (time-varying) | Considering Bray–Curtis dissimilarities, no differences in microbiota composition were found between children who were wheezers and non-wheezers |
| Samra et al., 2019 (South Korea)143 | Children (5–12 y) | — | Dysbiosis among children with atopic asthma compared with the controls |
| Thorsen et al., 2019 (Denmark)15 | Children (6 y) | Paternal asthma, older siblings, and season of birth | Shannon index (at 1 month of age) (IQR: 1.11–1.90) Richness at 2,000 reads (IQR: 25–36) Richness at 10,000 reads (IQR: 40–57) , , Bacterial asthma score (abundance) (95% CI: 1.13, 1.63) (95% CI: 1.04, 1.72) (95% CI: 1.23, 3.11) (95% CI: 1.17, 1.79) (95% CI: 1.15, 2.30) (95% CI: 1.03, 2.69) |
| Al Bataineh et al., 2020 (United Arab Emirates)144 | Children (7 y) and adults (52 y) | — | Specific species A significant difference of bacterial composition (Bacteroidetes, Firmicutes, Fusobacteria, and Proteobacteria phyla) between participants with asthma and non-asthmatic controls was found among asthmatic groups compared with healthy groups |
| Chiu et al., 2020 (Taiwan)145 | Children (4–5 y) | — | In the airway microbiota, Chao1 and Shannon indexes were significantly reduced in children with mite sensitization and were significantly lower in children with mite-sensitized rhinitis but not asthma than those in the healthy children without mite sensitization In the stool microbiota, no difference was noted in the bacterial richness and diversity regarding the mite sensitization and its relevance to rhinitis and asthma |
| Patrick et al., 2020 (Canada)146 | Children (5 y) | Study center, sex, presence of older siblings, mode of delivery, birthweight, season of birth, breastfeeding, ethnicity, tobacco smoke exposure, parental atopy, and exposure to environmental nitrogen dioxide | Chao1 index (95% CI: 0.46, 0.99) |
| Ruokolainen et al., 2020 (Finland and Estonia)94 | Children (18 months) | — | In the skin samples from Estonia, diversity tended to be higher in sensitized children as compared with healthy children (). Significant differences between healthy and sensitized children were observed both in the nasal and stool samples, but only among Finnish children (MRM on Bray–Curtis: nasal: ; stool ) |
| Toivonen et al., 2020 (Finland)17 | Children (7 y) | Sex, household siblings, parental asthma, and child’s eczema at 13 months of age | Shannon index At 2 months of age (IQR: 0.71–1.38) (IQR: 0.50–1.40) At 13 months of age (IQR: 0.47–2.26) (IQR: 0.45–1.70) At 24 months of age (IQR: 0.29–1.03) (IQR: 0.32–1.31) |
| Ham et al., 2021 (South Korea)147 | Adults (49–58.44 y) | — | The patients with asthma did not differ from the heathy individuals in terms of the and diversity of the lung and gut microbiomes. Similarly, the two groups did not differ in terms of the relative abundance of microbiome genera or species in the sputum Stool samples: the patients with asthma and the healthy individuals did not differ significantly in terms of diversity, composition, or relative abundance of genera or species |
| Niemeier-Walsh et al., 2021 (USA)14 | Children (12 y) | Gender, asthma status, and mother’s education as a measure of socioeconomic status | Sputum: Shannon index (95% CI: 3.6, 3.8) (95% CI: 3.4, 3.7) Observed amplicon sequence variants (95% CI: 154, 204) (95% CI: 147, 185) Phylogenetic diversity (95% CI: 7.6, 9.6) (95% CI: 7.8, 9.0) |
| Samra et al., 2021 (South Korea)148 | Children () | — | Bacterial composition, Shannon diversity index, and Faith phylogenetic diversity were higher in the participants with allergies compared with the those who were healthy |
| Schei et al., 2021 (Norway)18 | Children (6 y) | — | Bacterial abundance (at 2 years of age) (95% CI: 0.53, 2.78) |
| Seppo et al., 2021 (USA)52 | Children (3 y) | Maternal atopy, delivery mode, cat and dog exposure, infant gender, and maternal and infant antibiotics | diversity in gut microbiome was not significantly enriched in infants with atopy compared with those who were nonatopic |
| Turek et al., 2021 (Australia)149 | Adults ( y) | — | 84 OTUs were in relatively low abundance among participants with asthma. Results shows differences in diversity between adults with asthma and those who were unaffected and nonsmoking |
| Bar et al., 2022 (Poland)150 | Children (6–17 y) | — | EBC samples: Asthmatic children had a higher abundance of bacterial species (Shannon diversity index, mean of vs. , ) |
| Lee-Sarwar et al., 2022 (USA)151 | Children (6 y) | Sex, race/ethnicity, VDAART (Vitamin D Antenatal Asthma Reduction Trial) study site, and analyses of stool samples collected at age 3–6 months were additionally adjusted for exact age at stool sample collection | Age at stool sample: 3–6 months of age Shannon index Transient asthma (vs. no asthma): Active asthma (vs. no active asthma): Early asthma (vs. no early asthma): Faith’s phylogenetic diversity Transient asthma (vs. no asthma): Active asthma (vs. no active asthma): Early asthma (vs. no early asthma): 1 year of age Shannon index Transient asthma (vs. no asthma): Active asthma (vs. no active asthma): Early asthma (vs. no early asthma): Faith’s phylogenetic diversity Transient asthma (vs. no asthma): Active asthma (vs. no active asthma): Early asthma (vs. no early asthma): 3 years of age Shannon index Transient asthma (vs. no asthma): Active asthma (vs. no active asthma): Early asthma (vs. no early asthma): Faith’s phylogenetic diversity Transient asthma (vs. no asthma): Active asthma (vs. no active asthma): Early asthma (vs. no early asthma): |
| Lee-Sarwar et al., 2022 (USA)152 | Children (6 y) | VDAART study site | Fecal alpha diversity was not associated with wheeze proportion (Shannon index: Pearson , ; Simpson index: Pearson , ) |
| Tsai et al., 2022 (Taiwan)153 | Children (36 months) | — | Children with atopy alone had a significantly lower Chao1 index than healthy controls (). Higher Shannon index was present in children with atopy alone than in the healthy controls () |
| Zheng et al., 2022 (China)154 | Children (5–14 y) | Age, gender, and body mass index | Chao1 () and Simpson () indexes showed significant differences among the three groups (nonallergic asthma, allergic asthma, and healthy controls). A higher bacterial richness and a lower diversity in asthma group was observed |
| Mubanga et al., 2023 (Sweden)155 | Children (9–14 y) | Twin pairs | No statistically significant evidence for differences in within-sample alpha diversity between exposure groups (Shannon: , Simpson: ) |
| Thorsen et al., 2023 (Denmark)156 | Children (7 y) | Log(library size) and sequencing run | Shannon diversity index: (95% CI: 0.42, 1.22) Faith’s phylogenetic diversity: (95% CI: 0.88, 1.18) |
Note: aHR; adjusted hazard ratio; aOR, adjusted odds ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; IQR, interquartile range; KEGG, Kyoto Encyclopedia of Genes and Genomes; MRM, multiple regression on distance matrixes; OR, odds ratio; OTUs, operational taxonomic units; PC, principal component; PCA, principal component analysis; SD, standard deviation; SRI; species richness index.
Outer Layer (Environmental) Biodiversity
The summary results on the association between outer layer biodiversity and asthma, wheezing, and allergic sensitization are shown in Table 1 and Table S2 (significant evidence of lower risk: includes statistically significant results, ; suggestive evidence of lower risk: includes results with a tendency toward lower risk, ; contradictory findings: evidence for both a lower and higher risk; suggestive evidence of higher risk: includes results with a tendency toward higher risk, ; significant evidence of higher risk: includes statistically significant results, ; no evidence of effect: includes the qualitative results). Eleven studies showed significant evidence of a protective effect of exposure to biodiversity on asthma, whereas 7 studies reported significant evidence of an increased risk of asthma among individuals exposed to greater biodiversity. Six studies provided inconclusive evidence on the role of exposure to biodiversity in the development of asthma. The results for wheezing were also inconsistent, 4 studies reported a protective effect (2 reported significant evidence and 2 reported suggestive evidence of lower risk), whereas 2 reported significant evidence of an increased risk, and one study suggested inconclusive evidence on the effect of exposure to biodiversity on wheezing (Tables S2 and S4). Consistent with the findings on asthma, most of the studies suggested a protective effect of biodiversity on the development of allergic sensitization (6 reported significant evidence and 5 reported suggestive evidence of lower risk). There were 5 studies suggesting an effect of outer layer biodiversity on increasing allergic sensitization, and 4 studies provided inconclusive evidence on the role of outer layer biodiversity on the development of allergic sensitization (Tables S2 and S4).
Considering the studies that reported a significant or suggestive protective effect of exposure to biodiversity on asthma, wheezing, and allergic sensitization, seven defined biodiversity based on land-use types, number of different fungal species, exposure to cats or to dogs, type of child care, bedroom sharing and on having older siblings, vegetation diversity, and plant diversity. Among those studies that reported evidence on increased risk for asthma, wheezing, and allergic sensitization, four studies were based on the assessment of species richness index (SRI) and on diversity of land-use types (Table 1).
Inner Layer (Human Microbiota) Biodiversity
Table 1 summarizes the evidence on the associations between inner layer biodiversity and asthma, wheezing, and allergic sensitization. Most of the studies () provided no clear evidence on the role of inner layer biodiversity on asthma development, and 6 studies reported controversial results. Nine studies provided significant () or suggestive () evidence on the protective effect of high biodiversity on asthma. However, 12 studies reported significant () or suggestive () evidence on the higher risk of developing asthma among individuals with higher inner layer biodiversity (Tables S2 and S5). The results for wheezing were also inconsistent, 2 studies reported opposite results (1 reported suggestive evidence of lower risk and 1 reported significant evidence of an increased risk), and 3 studies suggested inconclusive evidence on the effect of exposure to biodiversity on wheezing (Tables S2 and S5). Among studies on allergic sensitization, 4 studies provided no clear evidence on the role of inner layer biodiversity, whereas 3 studies reported a protective effect of inner layer biodiversity on allergic sensitization (Tables S2 and S5).
Among the studies that included airway samples, no evidence was reported on the role of diversity of microbiota on asthma () or wheezing (). Further, 8 studies presented significant evidence on increased biodiversity being related to higher risk of developing asthma () and allergic sensitization (), and 5 studies reported a protective effect of increased biodiversity on asthma (, 1 reporting significant evidence and 2 reporting suggestive evidence) and allergic sensitization (, significant evidence). Of the studies that characterized human microbiota using stool samples, 12 reported no evidence on the role of inner layer biodiversity on asthma (), wheezing (), or allergic sensitization (), whereas 6 studies showed evidence on a protective effect of biodiversity on asthma () and allergic sensitization () (Table 1).
Relative Abundance of Taxa on Different Taxonomic Levels
Table S3 summarizes the main or significant difference in relative abundance of taxa analyzed in the studies included in the comprehensive review between cases (individuals with asthma, wheezing, or allergic sensitization) and controls (healthy individuals). Some of the studies presented in Table S3 reported a difference in the abundance or presence of specific bacterial taxa between individuals with asthma, wheezing, or allergic sensitization and healthy individuals, suggesting that the composition of the human microbiota may also modulate allergic sensitization and asthma risk.
Discussion
Based on a systematic search, to our knowledge ours is the first comprehensive review aiming to summarize current knowledge on the role of outer (environmental) and inner layer (human microbiota) biodiversity in the development of asthma, wheezing, and allergic sensitization in humans. This comprehensive review revealed that the study-specific associations between outer layer biodiversity and asthma, wheezing, and allergic sensitization were heterogenous across studies. A number of studies showed a protective trend of exposure to high environmental biodiversity on the development of asthma, wheezing, and allergic sensitization. Although our results showed that in more studies bacterial diversity was slightly higher among individuals with asthma, there was no clear evidence of a significant association between inner layer biodiversity and the risk of asthma, wheezing, or allergic sensitization, that is, the observed differences were within chance variation (Table S5).
Validity of Results
In addition to the four databases, we also searched the reference lists of all the relevant articles identified. Our comprehensive review included also evidence from longitudinal studies on biodiversity and respiratory outcomes,13–21 which allowed assessment of the time-dependent effects related to outer and inner layer biodiversity on the development of asthma, wheezing, and allergic sensitization. The different exposure assessment, sampling methods applied, and different types of samples (including stool and airway samples) may also complicate the comparison of different studies For example, the multitude of locations (nasal cavity; naso-, oro-, and hypopharynx; trachea; and/or bronchi) and diverse sampling techniques (brush, swab, nasal wash, induced sputum, or bronchial alveolar lavage) used to assess the airway microbiome may introduce differences in the characterization of the microbiome composition22 and limit comparison between different studies. Another challenge in comparing studies may be the temporal variation in biodiversity, namely, the human microbiome.22 Previous studies indicate that human microbiome exhibits high variability in early life,22 throughout seasons, and in response to respiratory tract infections (i.e., the airway microbiome)15,17,23 and is also influenced by interactions between the environment24 and host- and microbiome-associated factors.25–28 Moreover, the different metrics used for diversity throughout different studies (e.g., Shannon diversity index, Simpson index, phylogenetic diversity, Chao1) may also complicate comparison between studies.29 However, the aim of this comprehensive review was to summarize current knowledge on the role of biodiversity, regardless the definition of biodiversity used in each study, namely, in the outer layer (i.e., diversity of land-use types, number of different fungal species, or SRI), or the type of samples, which represents different body sites, and/or the composition of the human microbiota (i.e., the inner layer). Study design-related limitations were largely due to potential unadjusted confounding, which varied from study to study, as well as to self-reported outcomes, which may limit the conclusions about the role of biodiversity in the development of asthma, wheezing, and allergic sensitization. In addition, heterogeneity and inaccuracy of self-reported outcomes may lead to information bias. The variation in the outcome assessment methods may have had an impact on the ability to reach consistent summary results. Furthermore, this comprehensive review did not consider the different routes of exposure to outer layer biodiversity (i.e., air, soil, natural waters, plants, and animals), limiting conclusions about the different effects of exposure to biodiversity in soil, air, and/or water on asthma, wheezing, and/or allergic sensitization.
Synthesis with Previous Knowledge
Although the evidence on the role of biodiversity in the development of respiratory outcomes was inconsistent, the findings of the present systematic search suggest that the effects related to the inner and outer layer biodiversity on asthma, wheezing, and allergic sensitization are diverse. However, caution is needed when interpreting the results, given the heterogeneity in definitions of exposure and outcomes, and confounders that were adjusted for.
Outer Layer (Environmental) Biodiversity
Previous studies have reported an association between early life environmental exposures and airway inflammation.11,30 These studies provided evidence that contact with animals and allergens during early life reduces the risk to developing asthma.7,31,32 Furthermore, exposure to higher environmental biodiversity, assessed based on land-use or vegetation types, has been associated with a lower risk of respiratory outcomes, such as asthma and allergic sensitization.33–35 Moreover, according to the biodiversity hypothesis, contact with natural environments, including environmental microbiota, enriches the human microbiome, promotes immune balance, and protects from developing allergies and/or inflammatory diseases.6 Although exposure to beneficial microbiota seems to play an important role, the complexity of different routes of exposure to microbiota and their timing, duration, intensity, and frequency make studying the role of outer and inner biodiversity on respiratory health challenging.36 Several previous studies have investigated the association between the composition of the immediate living environment and health and found that the composition and diversity of environmental microbiota seem to differ among different land-use types.34,37,38 Environments, such as traditional farms39,40 and green spaces,35,41 which contain enriched and specific microbial exposures, may be protective against asthma and allergies. More recently a systematic review and meta-analysis reported that the associations between exposure to green spaces and asthma (current and ever) and allergic rhinitis were inconsistent.42 The authors suggested that their result may be explained by a variable balance between the positive and negative effects related to biodiversity exposure.42 According to Hanski et al.34 environmental biodiversity, human microbiota, and the function of the immune system are dynamic and complex systems that include different components that interact with each other. They hypothesized that the association between environmental biodiversity and atopy reflects the immunologic responses that have been developed by individuals with long-term exposure to specific environmental microbiota and allergens.34 Ruff et al.43 have also suggested that the drastic changes in modern environments and lifestyles may have reduced microbial biodiversity and led to an imbalance of the evolutionarily processes, which in turn may have led to more unstable and less resilient microbiota. This change in the microbiota—dysbiosis—may, consequently, alter the balance maintained in the gut, skin, and airway microbiomes, impair immune homeostasis, and increase the risk of many chronic inflammatory diseases, such as asthma and allergic diseases.
Inner Layer (Human Microbiota) Biodiversity
This comprehensive review suggests that the direction of associations between the inner layer biodiversity in different samples (i.e., stool and/or airway samples), representing different body sites, and the development of asthma, wheezing, and/or allergic sensitization is not consistent. Among the 27 studies that included airway samples (e.g., hypopharyngeal and oropharyngeal, nose, oropharynx samples, sputum, bronchial brushings, and oral swabs), 5 studies reported a protective effect of inner layer biodiversity on the development of such outcomes, 10 reported a risk factor, and 12 reported nonsignificant associations between inner layer biodiversity and asthma, wheezing, or allergic sensitization. Among the studies that characterized human microbiota using stool samples, 12 studies reported no evidence on the role of inner layer biodiversity on asthma, wheezing, or allergic sensitization, 4 studies reported an adverse effect of inner layer biodiversity, whereas 6 studies showed evidence on a protective effect of biodiversity on asthma and allergic sensitization.
As suggested in the previous studies,15,17,44,45 different sampling time points and different anatomical sites may affect the outcome and, consequently, the consistency of the results. However, the concept of the gut–lung axis suggests that gut microbiota dysbiosis affects the immune responses in the lungs, and vice versa, and the development of asthma and allergic disease.46–48 In addition, Marsland et al.49 reported that there is an important “crosstalk” between the gut and lungs, suggesting an association between asthma and allergic diseases and a dysbiosis in not only the airway microbiota but also in the gut microbiota. Furthermore, several environmental exposures influence the diversity and composition of human microbiota.7–9 A study conducted in the Russian and the Finnish Karelia showed significant differences in the skin and nasal microbiota composition between the countries.50 The microbial diversity was higher in the Russian samples than in the Finnish samples. However, no significant associations were observed between nasal and skin microbiota diversity and asthma among the Finnish individuals.50 Consistently, a study including 72 adult participants (20 with asthma exacerbation, 31 with nonexacerbated asthma, and 21 healthy individuals) found no statistically significant difference in nasal bacterial diversity among these three study groups, using Faith’s phylogenetic diversity.51 A recent cohort of mother–infant pairs from the United States also showed that alpha diversity in gut microbiome was not significantly enriched in atopic compared with nonatopic infants.52 On the other hand, Espuela-Ortiz et al.53 reported significant differences in salivary microbiome, using both the Shannon diversity index and the Pielou index, between individuals with asthma and those without asthma.
These results are consistent with the studies conducted by Huang et al.54 and Marri et al.55 regarding alpha diversity in airway samples. Huang et al.54 reported a significantly higher bronchial bacterial diversity among individuals having asthma compared with control individuals. The authors suggested that bacterial diversity (variation in composition and relative abundance of specific phylotypes) is associated with the degree of bronchial hyperresponsiveness in individuals having asthma treated with inhaled corticosteroids.54 Although lower gut bacterial biodiversity is usually associated with disease status,56 in airways, some diseases, including asthma, have been associated with greater bacterial biodiversity.54,55 Furthermore, several studies highlight that an increase in the abundance of specific taxa, including Moraxellaceae and Pasteurellaceae in asthmatic airway samples (to which Moraxella catarrhalis and Haemophilus influenza belong, respectively), supports the role of specific bacteria in the development of asthma.55,57,58 According to Følsgaard et al.58 the colonization with Moraxella catarrhalis and Haemophilus influenzae induced a mixed Th1/Th2/Th17 response of the airway mucosa, which may result in chronic inflammation and, consequently, increase the risk of asthma in early childhood.57 In addition, Depner et al.59 reported that the detrimental effect of Moraxella colonization may be related to environmental exposures. The effect of Moraxella was not observed among farm children [ (95% CI: 0.34, 2.63)] compared with nonfarm children [ (95% CI: 2.27, 20.86)], suggesting that this natural environment may neutralize the effect of Moraxella colonization. These findings are consistent with the interpretation of a role for pathogenic bacteria, which may disturb the local bacterial ecology, supporting the hypothesis of an association between inner layer biodiversity and composition and the development of allergic diseases. Moreover, Niemeier-Walsh et al.14 highlight that given that there were fewer individuals with asthma than those without asthma, it may be difficult to make a clear determination of differences in relative abundance between the asthma status groups.
Although our results showed that the majority of studies found bacterial diversity was slightly higher among individuals with asthma, the evidence on the effect of inner layer biodiversity on asthma, wheezing, or allergic sensitization remains unclear. This might align with the different genetics of asthma and allergic sensitization, as recently demonstrated by genomic analyses.21 Consistently with our comprehensive review, the results of these previous studies on microbiota diversity and respiratory outcomes are heterogeneous.
The small number of studies focusing on adults limits the interpretation of the effects of outer and inner layer biodiversity on respiratory outcomes at all ages. The relationship between environmental biodiversity, microbial composition, and the onset of allergic disease has been extensively studied in pediatric populations.60–63 Recent studies also suggest that events and exposures during early life can influence the development of immune regulation, contributing to the epidemic increase in allergic diseases.16,64,65 In addition, the neonatal period of life is one of remarkable developmental changes, including the maturation of the microbiota and the establishment of functional immune system.66 Stokholm et al.67 found robust associations between gut microbiota maturity at 1 year of age and asthma at 5 years of age. However, the timeframe during which maturation of regulatory immune mechanisms occurs is not well known,68 and thus it is uncertain at what age outer and inner layer biodiversity would be of most importance. Although early childhood is an important period, the interaction between the outer and inner microbial layers never stops.6 Rook69 and Abrahamsson et al.16 also reported that innate immunity needs constant and lifelong exposure to new bacterial and/or repeated exposure to create and maintain tolerance. Longitudinal studies that capture the early outer and inner layer biodiversity and the exposure to biodiversity throughout childhood to adulthood are needed to elucidate the role of biodiversity over time.
In addition to diversity, several studies have suggested that the composition of human and environmental microbiota may contribute to the development of asthma and allergies.11,15,70,71 The microbiota composition may be related to a decrease in diversity, promoting less resilient microbiota. This would alter the ecosystem provided by the microbiota and the balance of the immune system response.72 Moreover, there are several factors that can modify the effect of inner and outer layers of biodiversity on the risk of asthma, wheezing, and/or allergic sensitization. The increase in the prevalence of asthma and allergic diseases is strongly related to urbanization trends.73 In urban areas, the exposure to higher levels of air pollution may also affect the composition of airway microbiota, cause bacterial dysbiosis and promote the development of asthma and/or allergies. In China, a study including 114 healthy participants (18–21 y old), suggested that exposure to higher levels of particulate matter in aerodynamic diameter () was associated with a decrease in the relative abundance of Bacteroidetes and Fusobacteria and with an increase of Firmicutes, Proteobacteria, and Actinobacteria in the oropharyngeal mucosa.74 The authors also highlighted that exposure to high levels of sulfur dioxide, nitrogen dioxide, and ozone may also contribute to changes in microbial composition.74 A Finnish study also supported the hypothesis that people living in urban areas are less exposed to outer layer biodiversity compared with those living in rural areas. The diversity and richness of total bacterial, Proteobacteria, Actinobacteria, and Bacteroidetes decreased as the percentage of built area increased. In addition, an opposite trend was observed for potentially pathogenic bacterial communities, which relative abundance increased as the percentage of built area increased.75 Contrasting with urban areas, those who are living in rural areas possibly carry more soil and plant material,75 which can be expected to cause a higher exposure to environmental diverse microbiota and might have a positive effect on the immune system and respiratory health. Rural exposures, including cattle farms and consumption of raw milk, may also promote the contact (i.e., dermal contact, inhalation, and/or consumption/ingestion) with diverse environmental microbial products (e.g., endotoxin, muramic acid, higher content of whey proteins and omega-3 fatty acids), which may exert their effects directly on the immune system, reducing the risk of asthma and allergic diseases.20,76–78
In addition, there are other factors, including the socioeconomic status, use of antibiotics and functional biodiversity, that can modify the association between inner and outer layers biodiversity and respiratory outcomes. The use of antibiotics has been associated with temporary changes in microbiota, that is, with a reduction in the amount and diversity of bacteria and loss of functional diversity that can affect the microbiota–host interaction and long-term immunological functions.79 In addition, McAleer et al.80 reported that changes in intestinal microbiota, and of their metabolites, may increase the susceptibility to asthma, especially during early life.67,81,82 Accordingly, it has been suggested that airway microbiota may modify the association between the use of antibiotics and the risk of asthma among children.83,84 Furthermore, the influence of inner layer biodiversity on the development of asthma and/or allergic diseases may be related to the presence of specific bacterial species capable of fulfilling certain functions, such as the production of short-chain fatty acids or production of vitamins— the functional microbiome.78 According to Hufnagl et al.,78 the upper and lower airway microbiota may produce metabolites that interact with the host, thus changing the development and progression of asthma. Moreover, environmental factors, including pollens and air pollutants, can also interact with these compounds and change the growth conditions of microbiota and/or the production of compounds and metabolites. The social environment may also influence the association between exposure to biodiversity and asthma. A small study of healthy volunteers () conducted in Chicago found that lower socioeconomic status was associated with a lower alpha diversity (in sigmoid biopsies and stool samples).85 The authors suggested that highly processed foods, physical inactivity, visceral adiposity, and frequency of antibiotic use (especially in childhood), may mediate the association between social environment and gut alpha diversity.78 Similar results were observed in TwinsUK cohort study, where lower individual income was associated with a lower gut biodiversity even after adjustment for individual health status.86
The effect of exposure to inner and outer layers of biodiversity may be time dependent. Several studies included in this comprehensive review (14 related to outer layer and 12 related to inner layer of biodiversity) assessed the effect of early exposure to biodiversity on asthma, asthma-like symptoms, and allergic sensitization, highlighting that exposure during early life (prenatal and early childhood) may have a strong effect on asthma and allergic diseases pathogenesis. Of the 82 included studies, only one cohort study87 assessed the effect of exposure to outer layer biodiversity during childhood on asthma and allergic sensitization among adults. Among those studies assessing the effect of exposure to inner layer of biodiversity, only 12 investigated a possible longitudinal association between microbiota diversity in early infancy and development of asthma, asthma-like symptoms, and/or allergic sensitization later in life. However, the direction of the association and the estimate effects were inconsistent. The time period between exposure (environmental exposure and collection of human samples) and outcomes assessment may also limit the causal inference of the results. The findings that early dysbiosis may be associated with asthma and allergic diseases later in life suggests that exposure to the microbiome may be associated with the development of asthma and allergic diseases.88 However, this does not exclude the possibility that the association is bidirectional.89,90 Therefore, interpretation of these associations requires consideration of temporality and underlying biology.89 This comprehensive review underlines exposure to biodiversity as a key modulator of immune system, which is associated with susceptibility to develop asthma. This comprehensive review suggests that the heterogeneity of exposure and outcomes assessment or definition may induce variability in the results. It should also be noted that other factors can play a role in variability and/or inconsistence of the results. For instance, the geographic distribution of the included studies, and inclusion of individuals from different populations, may contribute to the variability in results due to exposure to different environments and social and behavioral risk factors (e.g., medications,91 dietary habits,92,93 exposure to natural/built-up areas,8,36,75,94 socioeconomic status95).89,96 In addition, the results suggest that further efforts are needed for causal inference and elaboration of the exact underlying mechanisms by which biodiversity exposure influences the development of asthma and allergic diseases.
Furthermore, most studies addressing the role of biodiversity in the development of respiratory outcomes have analyzed only a single point in time in cross-sectional studies, which has not allowed assessment of responses of the immune system to changes in human microbiome caused by exposure to environmental and biological factors.45 The results of this comprehensive review may not fully represent the general population given that in some of the included studies participants may have had a genetic predisposition to develop asthma. Genetic predisposition increases the susceptibility to immune dysfunction, which may be triggered or further amplified by specific immunomodulatory exposures involving inner and outer layers of biodiversity.89 Recent studies suggested that host genetics is also associated the composition of human microbiota.97–99 Neonates with a family history of allergic diseases have been reported to have a higher abundance of Enterobacteriaceae and a lower abundance of Lactobacillaceae and Bifidobacteriaceae.98,99 Another important limitation in the previous studies has been that the link between biodiversity and pathophysiological mechanisms underlying asthma may have been confounded by the asthma subtype and the inflammatory process.45 Based on this comprehensive review, there is a need for population-based longitudinal studies, including a) cohort studies, especially in previously underrepresented populations (e.g., in Asian and African regions); b) standardized outcome definitions; and c) studies with recruitment at an early developmental phase (e.g., from preconception) and having a longitudinal follow-up to identify critical periods of exposure in the life course into adulthood and to better understand mechanisms linking environmental exposures and changes in microbiome composition, diversity and/or function to development of asthma and allergic sensitization. Furthermore, climate change is affecting the biodiversity of both outer and inner layers.100 Climate change is responsible for environmental degradation and loss of biodiversity in plants, animals, and microorganisms, thus affecting the distribution, composition, and interactions between microorganisms.101 Climate change can also disrupt the relation between environmental microorganisms and humans, resulting in loss of inner layer biodiversity.100,101 Therefore, understanding how the interactions between outer and inner layer, biodiversity, human being, and immune system respond to climate change is also needed for assessing the role of biodiversity in the development of asthma, wheezing, and allergic sensitization.
In conclusion, the present comprehensive review revealed that the associations between the exposure to environmental biodiversity and asthma, wheezing, and allergic sensitization were heterogenous across studies. Although more studies showed that exposure to higher environmental biodiversity may have a protective effect on the development of asthma, wheezing, and allergic sensitization, there was no consistent evidence of an association between inner layer biodiversity and asthma, wheezing, or allergic sensitization.
Supplementary Material
Acknowledgments
J.J.K.J., I.P., T.T.H., and M.S.J. identified the need for this comprehensive review. All authors contributed to the design of the review protocol. I.P., N.S., and B.H. conducted the initial screening and selected the studies for inclusion. I.P. extracted the data from the included studies. T.T.H. and A.K.R. reviewed the initial screening and checked the data from the included studies. J.J.K.J. checked the data from the included studies. I.P. and N.S. performed the study quality assessment. J.J.K.J. supervised all the steps. All steps from screening to quality assessment were done in consultation with the wider review team. I.P. analyzed the data and drafted the manuscript. All authors contributed to the critical revision and approved the final version of the manuscript.
We gratefully acknowledge funding from the University of Oulu and the Academy of Finland Profi Biodiverse Anthropocenes no. 336449 (J.J.K.J.), Academy of Finland grant no. 310371, and no. 310372 [GLORIA consortium (J.J.K.J.)], as well as Horizon 2020 Framework Programme [Project 101056883—INCHILDHEALTH HORIZON-HLTH-2021-ENVHLTH-02 (J.J.K.J.)].
All datasets generated and analyzed, including the study protocol, search strategy, list of the included and excluded studies, data extracted, and quality assessment are available in the article and upon request from the corresponding author.
Conclusions and opinions are those of the individual authors and do not necessarily reflect the policies or views of EHP Publishing or the National Institute of Environmental Health Sciences.
References
- 1.von Hertzen L, Hanski I, Haahtela T. 2011. Natural immunity. Biodiversity loss and inflammatory diseases are two global megatrends that might be related. EMBO Rep 12(11):1089–1093, PMID: 21979814, 10.1038/embor.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ceballos G, Ehrlich PR, Dirzo R. 2017. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc Natl Acad Sci USA 114(30):E6089–E6096, PMID: 28696295, 10.1073/pnas.1704949114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Díaz S, Fargione J, Chapin FS III, Tilman D. 2006. Biodiversity loss threatens human well-being. PLoS Biol 4(8):e277, PMID: 16895442, 10.1371/journal.pbio.0040277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marselle MR, Lindley SJ, Cook PA, Bonn A. 2021. Biodiversity and health in the urban environment. Curr Environ Health Rep 8(2):146–156, PMID: 33982150, 10.1007/s40572-021-00313-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haahtela T, Holgate S, Pawankar R, Akdis CA, Benjaponpitak S, Caraballo L, et al. 2013. The biodiversity hypothesis and allergic disease: world allergy organization position statement. World Allergy Organ J 6(1):3, PMID: 23663440, 10.1186/1939-4551-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haahtela T. 2019. A biodiversity hypothesis. Allergy 74(8):1445–1456, PMID: 30835837, 10.1111/all.13763. [DOI] [PubMed] [Google Scholar]
- 7.Lynch SV, Wood RA, Boushey H, Bacharier LB, Bloomberg GR, Kattan M, et al. 2014. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol 134(3):593–601.e512, PMID: 24908147, 10.1016/j.jaci.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haahtela T, Laatikainen T, Alenius H, Auvinen P, Fyhrquist N, Hanski I, et al. 2015. Hunt for the origin of allergy—comparing the Finnish and Russian Karelia. Clin Exp Allergy 45(5):891–901, PMID: 25772429, 10.1111/cea.12527. [DOI] [PubMed] [Google Scholar]
- 9.Lehtimäki J, Karkman A, Laatikainen T, Paalanen L, von Hertzen L, Haahtela T, et al. 2017. Patterns in the skin microbiota differ in children and teenagers between rural and urban environments. Sci Rep 7:45651, PMID: 28361981, 10.1038/srep45651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riiser A. 2015. The human microbiome, asthma, and allergy. Allergy Asthma Clin Immunol 11(1):35, PMID: 26664362, 10.1186/s13223-015-0102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ver Heul A, Planer J, Kau AL. 2019. The human microbiota and asthma. Clin Rev Allergy Immunol 57(3):350–363, PMID: 30426401, 10.1007/s12016-018-8719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Global Initiative for Asthma. 2021. A Pocket Guide for Asthma Management and Prevention (for Adults and Children Older than 5 years). A Pocket Guide for Health Professionals Updated 2021. Fontana, WI: Global Initiation for Asthma. [Google Scholar]
- 13.Kirjavainen PV, Karvonen AM, Adams RI, Täubel M, Roponen M, Tuoresmäki P, et al. 2019. Farm-like indoor microbiota in non-farm homes protects children from asthma development. Nat Med 25(7):1089–1095, PMID: 31209334, 10.1038/s41591-019-0469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niemeier-Walsh C, Ryan PH, Meller J, Ollberding NJ, Adhikari A, Reponen T. 2021. Exposure to traffic-related air pollution and bacterial diversity in the lower respiratory tract of children. PLoS One 16(6):e0244341, PMID: 34166366, 10.1371/journal.pone.0244341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorsen J, Rasmussen MA, Waage J, Mortensen M, Brejnrod A, Bønnelykke K, et al. 2019. Infant airway microbiota and topical immune perturbations in the origins of childhood asthma. Nat Commun 10(1):5001, PMID: 31676759, 10.1038/s41467-019-12989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. 2014. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy 44(6):842–850, PMID: 24330256, 10.1111/cea.12253. [DOI] [PubMed] [Google Scholar]
- 17.Toivonen L, Karppinen S, Schuez-Havupalo L, Waris M, He Q, Hoffman KL, et al. 2020. Longitudinal changes in early nasal microbiota and the risk of childhood asthma. Pediatrics 146(4):e20200421, PMID: 32934151, 10.1542/peds.2020-0421. [DOI] [PubMed] [Google Scholar]
- 18.Schei K, Simpson MR, Øien T, Salamati S, Rudi K, Ødegård RA. 2021. Allergy-related diseases and early gut fungal and bacterial microbiota abundances in children. Clin Transl Allergy 11(5):e12041, PMID: 34194728, 10.1002/clt2.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karvonen AM, Kirjavainen PV, Täubel M, Jayaprakash B, Adams RI, Sordillo JE, et al. 2019. Indoor bacterial microbiota and development of asthma by 10.5 years of age. J Allergy Clin Immunol 144(5):1402–1410, PMID: 31415782, 10.1016/j.jaci.2019.07.035. [DOI] [PubMed] [Google Scholar]
- 20.Birzele LT, Depner M, Ege MJ, Engel M, Kublik S, Bernau C, et al. 2017. Environmental and mucosal microbiota and their role in childhood asthma. Allergy 72(1):109–119, PMID: 27503830, 10.1111/all.13002. [DOI] [PubMed] [Google Scholar]
- 21.Bisgaard H, Li N, Bonnelykke K, Chawes BLK, Skov T, Paludan-Müller G, et al. 2011. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol 128(3):646–652.e5, PMID: 21782228, 10.1016/j.jaci.2011.04.060. [DOI] [PubMed] [Google Scholar]
- 22.Smulders T, Van Der Schee MP, Maitland-Van Der Zee AH, Dikkers FG, Van Drunen CM. 2024. Influence of the gut and airway microbiome on asthma development and disease. Pediatr Allergy Immunol 35(3):e14095, PMID: 38451070, 10.1111/pai.14095. [DOI] [PubMed] [Google Scholar]
- 23.Bogaert D, Keijser B, Huse S, Rossen J, Veenhoven R, van Gils E, et al. 2011. Variability and diversity of nasopharyngeal microbiota in children: a metagenomic analysis. PLoS One 6(2):e17035, PMID: 21386965, 10.1371/journal.pone.0017035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lunjani N, Walsh LJ, Venter C, Power M, MacSharry J, Murphy DM, et al. 2022. Environmental influences on childhood asthma—the effect of diet and microbiome on asthma. Pediatr Allergy Immunol 33(12):e13892, PMID: 36564884, 10.1111/pai.13892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galazzo G, van Best N, Bervoets L, Dapaah IO, Savelkoul PH, Hornef MW, et al. 2020. Development of the microbiota and associations with birth mode, diet, and atopic disorders in a longitudinal analysis of stool samples, collected from infancy through early childhood. Gastroenterology 158(6):1584–1596, PMID: 31958431, 10.1053/j.gastro.2020.01.024. [DOI] [PubMed] [Google Scholar]
- 26.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. 2010. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA 107(26):11971–11975, PMID: 20566857, 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, et al. 2000. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr 30(1):61–67, PMID: 10630441, 10.1002/j.1536-4801.2000.tb02655.x. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka S, Kobayashi T, Songjinda P, Tateyama A, Tsubouchi M, Kiyohara C, et al. 2009. Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota. FEMS Immunol Med Microbiol 56(1):80–87, PMID: 19385995, 10.1111/j.1574-695X.2009.00553.x. [DOI] [PubMed] [Google Scholar]
- 29.Kers JG, Saccenti E. 2021. The power of microbiome studies: some considerations on which alpha and beta metrics to use and how to report results. Front Microbiol 12:796025, PMID: 35310396, 10.3389/fmicb.2021.796025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dick S, Friend A, Dynes K, AlKandari F, Doust E, Cowie H, et al. 2014. A systematic review of associations between environmental exposures and development of asthma in children aged up to 9 years. BMJ Open 4(11):e006554, PMID: 25421340, 10.1136/bmjopen-2014-006554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Connor GT, Lynch SV, Bloomberg GR, Kattan M, Wood RA, Gergen PJ, et al. 2018. Early-life home environment and risk of asthma among inner-city children. J Allergy Clin Immunol 141(4):1468–1475, PMID: 28939248, 10.1016/j.jaci.2017.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ownby DR, Johnson CC, Peterson EL. 2002. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA 288(8):963–972, PMID: 12190366, 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 33.Donovan GH, Gatziolis D, Longley I, Douwes J. 2018. Vegetation diversity protects against childhood asthma: results from a large New Zealand birth cohort. Nat Plants 4(6):358–364, PMID: 29735984, 10.1038/s41477-018-0151-8. [DOI] [PubMed] [Google Scholar]
- 34.Hanski I, von Hertzen L, Fyhrquist N, Koskinen K, Torppa K, Laatikainen T, et al. 2012. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc Natl Acad Sci USA 109(21):8334–8339, PMID: 22566627, 10.1073/pnas.1205624109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruokolainen L, von Hertzen L, Fyhrquist N, Laatikainen T, Lehtomäki J, Auvinen P, et al. 2015. Green areas around homes reduce atopic sensitization in children. Allergy 70(2):195–202, PMID: 25388016, 10.1111/all.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruokolainen L, Fyhrquist N, Haahtela T. 2016. The rich and the poor: environmental biodiversity protecting from allergy. Curr Opin Allergy Clin Immunol 16(5):421–426, PMID: 27490122, 10.1097/ACI.0000000000000304. [DOI] [PubMed] [Google Scholar]
- 37.Mhuireach G, Johnson BR, Altrichter AE, Ladau J, Meadow JF, Pollard KS, et al. 2016. Urban greenness influences airborne bacterial community composition. Sci Total Environ 571:680–687, PMID: 27418518, 10.1016/j.scitotenv.2016.07.037. [DOI] [PubMed] [Google Scholar]
- 38.Tischer C, Kirjavainen P, Matterne U, Tempes J, Willeke K, Keil T, et al. 2022. Interplay between natural environment, human microbiota and immune system: a scoping review of interventions and future perspectives towards allergy prevention. Sci Total Environ 821:153422, PMID: 35090907, 10.1016/j.scitotenv.2022.153422. [DOI] [PubMed] [Google Scholar]
- 39.von Mutius E, Vercelli D. 2010. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol 10(12):861–868, PMID: 21060319, 10.1038/nri2871. [DOI] [PubMed] [Google Scholar]
- 40.Müller-Rompa SEK, Markevych I, Hose AJ, Loss G, Wouters IM, Genuneit J, et al. 2018. An approach to the asthma-protective farm effect by geocoding: good farms and better farms. Pediatr Allergy Immunol 29(3):275–282, PMID: 29314275, 10.1111/pai.12861. [DOI] [PubMed] [Google Scholar]
- 41.Ferrante G, Asta F, Cilluffo G, De Sario M, Michelozzi P, La Grutta S. 2020. The effect of residential urban greenness on allergic respiratory diseases in youth: a narrative review. World Allergy Organ J 13(1):100096, PMID: 32071664, 10.1016/j.waojou.2019.100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu B, Guo X, Liang M, Sun C, Gao J, Xie P, et al. 2022. Association of individual green space exposure with the incidence of asthma and allergic rhinitis: a systematic review and meta-analysis. Environ Sci Pollut Res Int 29(59):88461–88487, PMID: 36329245, 10.1007/s11356-022-23718-x. [DOI] [PubMed] [Google Scholar]
- 43.Ruff WE, Greiling TM, Kriegel MA. 2020. Host–microbiota interactions in immune-mediated diseases. Nat Rev Microbiol 18(9):521–538, PMID: 32457482, 10.1038/s41579-020-0367-2. [DOI] [PubMed] [Google Scholar]
- 44.Powell EA, Fontanella S, Boakes E, Belgrave D, Shaw AG, Cornwell E, et al. 2019. Temporal association of the development of oropharyngeal microbiota with early life wheeze in a population-based birth cohort. EBioMedicine 46:486–498, PMID: 31353293, 10.1016/j.ebiom.2019.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alamri A. 2021. Diversity of microbial signatures in asthmatic airways. Int J Gen Med 14:1367–1378, PMID: 33889017, 10.2147/IJGM.S304339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Budden KF, Gellatly SL, Wood DLA, Cooper MA, Morrison M, Hugenholtz P, et al. 2017. Emerging pathogenic links between microbiota and the gut–lung axis. Nat Rev Microbiol 15(1):55–63, PMID: 27694885, 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 47.Barcik W, Boutin RCT, Sokolowska M, Finlay BB. 2020. The role of lung and gut microbiota in the pathology of asthma. Immunity 52(2):241–255, PMID: 32075727, 10.1016/j.immuni.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frati F, Salvatori C, Incorvaia C, Bellucci A, Di Cara G, Marcucci F, et al. 2018. The role of the microbiome in asthma: the gut–lung axis. Int J Mol Sci 20(1):123, PMID: 30598019, 10.3390/ijms20010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marsland BJ, Trompette A, Gollwitzer ES. 2015. The gut-lung axis in respiratory disease. Ann Am Thorac Soc 12(suppl 2):S150–S156, PMID: 26595731, 10.1513/AnnalsATS.201503-133AW. [DOI] [PubMed] [Google Scholar]
- 50.Ruokolainen L, Paalanen L, Karkman A, Laatikainen T, von Hertzen L, Vlasoff T, et al. 2017. Significant disparities in allergy prevalence and microbiota between the young people in Finnish and Russian Karelia. Clin Exp Allergy 47(5):665–674, PMID: 28165640, 10.1111/cea.12895. [DOI] [PubMed] [Google Scholar]
- 51.Fazlollahi M, Lee TD, Andrade J, Oguntuyo K, Chun Y, Grishina G, et al. 2018. The nasal microbiome in asthma. J Allergy Clin Immunol 142(3):834–843.e2, PMID: 29518419, 10.1016/j.jaci.2018.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seppo AE, Bu K, Jumabaeva M, Thakar J, Choudhury RA, Yonemitsu C, et al. 2021. Infant gut microbiome is enriched with Bifidobacterium longum ssp. infantis in Old Order Mennonites with traditional farming lifestyle. Allergy 76(11):3489–3503, PMID: 33905556, 10.1111/all.14877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Espuela-Ortiz A, Lorenzo-Diaz F, Baez-Ortega A, Eng C, Hernandez-Pacheco N, Oh SS, et al. 2019. Bacterial salivary microbiome associates with asthma among African American children and young adults. Pediatr Pulmonol 54(12):1948–1956, PMID: 31496123, 10.1002/ppul.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang YJ, Nelson CE, Brodie EL, DeSantis TZ, Baek MS, Liu J, et al. 2011. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol 127(2):372–381.e3, PMID: 21194740, 10.1016/j.jaci.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marri PR, Stern DA, Wright AL, Billheimer D, Martinez FD. 2013. Asthma-associated differences in microbial composition of induced sputum. J Allergy Clin Immunol 131(2):346–352.e3, PMID: 23265859, 10.1016/j.jaci.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cho I, Blaser MJ. 2012. The human microbiome: at the interface of health and disease. Nat Rev Genet 13(4):260–270, PMID: 22411464, 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bønnelykke K, et al. 2007. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med 357(15):1487–1495, PMID: 17928596, 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 58.Følsgaard NV, Schjørring S, Chawes BL, Rasmussen MA, Krogfelt KA, Brix S, et al. 2013. Pathogenic bacteria colonizing the airways in asymptomatic neonates stimulates topical inflammatory mediator release. Am J Respir Crit Care Med 187(6):589–595, PMID: 23370914, 10.1164/rccm.201207-1297OC. [DOI] [PubMed] [Google Scholar]
- 59.Depner M, Ege MJ, Cox MJ, Dwyer S, Walker AW, Birzele LT, et al. 2017. Bacterial microbiota of the upper respiratory tract and childhood asthma. J Allergy Clin Immunol 139(3):826–834.e13, PMID: 27576124, 10.1016/j.jaci.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 60.Melli LCFL, do Carmo-Rodrigues MS, Araújo-Filho HB, Solé D, de Morais MB. 2016. Intestinal microbiota and allergic diseases: a systematic review. Allergol Immunopathol (Madr) 44(2):177–188, PMID: 25985709, 10.1016/j.aller.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 61.Shah R, Bunyavanich S. 2021. The airway microbiome and pediatric asthma. Curr Opin Pediatr 33(6):639–647, PMID: 34412069, 10.1097/MOP.0000000000001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haahtela T. 2022. Biodiversity for resilience—what is needed for allergic children. Pediatr Allergy Immunol 33(5):e13779, PMID: 35616890, 10.1111/pai.13779. [DOI] [PubMed] [Google Scholar]
- 63.Lucey M. 2017. Urban biodiversity affects children’s respiratory health. Lancet Respir Med 5(8):613, PMID: 28748807, 10.1016/S2213-2600(17)30272-2. [DOI] [PubMed] [Google Scholar]
- 64.Prescott SL. 2003. Early origins of allergic disease: a review of processes and influences during early immune development. Curr Opin Allergy Clin Immunol 3(2):125–132, PMID: 12750609, 10.1097/00130832-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 65.Christensen ED, Hjelmsø MH, Thorsen J, Shah S, Redgwell T, Poulsen CE, et al. 2022. The developing airway and gut microbiota in early life is influenced by age of older siblings. Microbiome 10(1):106, PMID: 35831879, 10.1186/s40168-022-01305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gollwitzer ES, Marsland BJ. 2015. Impact of early-life exposures on immune maturation and susceptibility to disease. Trends Immunol 36(11):684–696, PMID: 26497259, 10.1016/j.it.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 67.Stokholm J, Blaser MJ, Thorsen J, Rasmussen MA, Waage J, Vinding RK, et al. 2018. Maturation of the gut microbiome and risk of asthma in childhood. Nat Commun 9(1):141, PMID: 29321519, 10.1038/s41467-017-02573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang M, Karlsson C, Olsson C, Adlerberth I, Wold AE, Strachan DP, et al. 2008. Reduced diversity in the early fecal microbiota of infants with atopic eczema. J Allergy Clin Immunol 121(1):129–134, PMID: 18028995, 10.1016/j.jaci.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 69.Rook GA. 2013. Regulation of the immune system by biodiversity from the natural environment: an ecosystem service essential to health. Proc Natl Acad Sci USA 110(46):18360–18367, PMID: 24154724, 10.1073/pnas.1313731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, et al. 2015. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med 7(307):307ra152, PMID: 26424567, 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 71.Stiemsma LT, Arrieta MC, Dimitriu PA, Cheng J, Thorson L, Lefebvre DL, et al. 2016. Shifts in Lachnospira and Clostridium sp. in the 3-month stool microbiome are associated with preschool age asthma. Clin Sci (Lond) 130(23):2199–2207, PMID: 27634868, 10.1042/CS20160349. [DOI] [PubMed] [Google Scholar]
- 72.Fiuza BSD, Fonseca HF, Meirelles PM, Marques CR, da Silva TM, Figueiredo CA. 2021. Understanding asthma and allergies by the lens of biodiversity and epigenetic changes. Front Immunol 12:623737, PMID: 33732246, 10.3389/fimmu.2021.623737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pearce N, Aït-Khaled N, Beasley R, Mallol J, Keil U, Mitchell E, et al. 2007. Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 62(9):758–766, PMID: 17504817, 10.1136/thx.2006.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li X, Sun Y, An Y, Wang R, Lin H, Liu M, et al. 2019. Air pollution during the winter period and respiratory tract microbial imbalance in a healthy young population in Northeastern China. Environ Pollut 246:972–979, PMID: 31126003, 10.1016/j.envpol.2018.12.083. [DOI] [PubMed] [Google Scholar]
- 75.Parajuli A, Grönroos M, Siter N, Puhakka R, Vari HK, Roslund MI, et al. 2018. Urbanization reduces transfer of diverse environmental microbiota indoors. Front Microbiol 9:84, PMID: 29467728, 10.3389/fmicb.2018.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Depner M, Taft DH, Kirjavainen PV, Kalanetra KM, Karvonen AM, Peschel S, et al. 2020. Maturation of the gut microbiome during the first year of life contributes to the protective farm effect on childhood asthma. Nat Med 26(11):1766–1775, PMID: 33139948, 10.1038/s41591-020-1095-x. [DOI] [PubMed] [Google Scholar]
- 77.Loss G, Apprich S, Waser M, Kneifel W, Genuneit J, Büchele G, et al. 2011. The protective effect of farm milk consumption on childhood asthma and atopy: the GABRIELA study. J Allergy Clin Immunol 128(4):766–773.e4, PMID: 21875744, 10.1016/j.jaci.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 78.Hufnagl K, Pali-Schöll I, Roth-Walter F, Jensen-Jarolim E. 2020. Dysbiosis of the gut and lung microbiome has a role in asthma. Semin Immunopathol 42(1):75–93, PMID: 32072252, 10.1007/s00281-019-00775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wypych TP, Wickramasinghe LC, Marsland BJ. 2019. The influence of the microbiome on respiratory health. Nat Immunol 20(10):1279–1290, PMID: 31501577, 10.1038/s41590-019-0451-9. [DOI] [PubMed] [Google Scholar]
- 80.McAleer JP, Kolls JK. 2018. Contributions of the intestinal microbiome in lung immunity. Eur J Immunol 48(1):39–49, PMID: 28776643, 10.1002/eji.201646721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chernikova D, Yuan I, Shaker M. 2019. Prevention of allergy with diverse and healthy microbiota: an update. Curr Opin Pediatr 31(3):418–425, PMID: 31090586, 10.1097/MOP.0000000000000766. [DOI] [PubMed] [Google Scholar]
- 82.Obiakor CV, Tun HM, Bridgman SL, Arrieta MC, Kozyrskyj AL. 2018. The association between early life antibiotic use and allergic disease in young children: recent insights and their implications. Expert Rev Clin Immunol 14(10):841–855, PMID: 30198345, 10.1080/1744666X.2018.1521271. [DOI] [PubMed] [Google Scholar]
- 83.Toivonen L, Schuez-Havupalo L, Karppinen S, Waris M, Hoffman KL, Camargo CA Jr, et al. 2021. Antibiotic treatments during infancy, changes in nasal microbiota, and asthma development: population-based cohort study. Clin Infect Dis 72(9):1546–1554, PMID: 32170305, 10.1093/cid/ciaa262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, et al. 2015. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 17(5):704–715, PMID: 25865368, 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller GE, Engen PA, Gillevet PM, Shaikh M, Sikaroodi M, Forsyth CB, et al. 2016. Lower neighborhood socioeconomic status associated with reduced diversity of the colonic microbiota in healthy adults. PLoS One 11(2):e0148952, PMID: 26859894, 10.1371/journal.pone.0148952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bowyer RCE, Jackson MA, Le Roy CI, Ni Lochlainn M, Spector TD, Dowd JB, et al. 2019. Socioeconomic status and the gut microbiome: a TwinsUK cohort study. Microorganisms 7(1):17, PMID: 30641975, 10.3390/microorganisms7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Campbell B, Raherison C, Lodge CJ, Lowe AJ, Gislason T, Heinrich J, et al. 2017. The effects of growing up on a farm on adult lung function and allergic phenotypes: an international population-based study. Thorax 72(3):236–244, PMID: 27672121, 10.1136/thoraxjnl-2015-208154. [DOI] [PubMed] [Google Scholar]
- 88.Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. 2014. The intestinal microbiome in early life: health and disease. Front Immunol 5:427, PMID: 25250028, 10.3389/fimmu.2014.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang HHF, Teo SM, Sly PD, Holt PG, Inouye M. 2021. The intersect of genetics, environment, and microbiota in asthma—perspectives and challenges. J Allergy Clin Immunol 147(3):781–793, PMID: 33678251, 10.1016/j.jaci.2020.08.026. [DOI] [PubMed] [Google Scholar]
- 90.von Mutius E. 2016. The microbial environment and its influence on asthma prevention in early life. J Allergy Clin Immunol 137(3):680–689, PMID: 26806048, 10.1016/j.jaci.2015.12.1301. [DOI] [PubMed] [Google Scholar]
- 91.Abdel-Aziz MI, Vijverberg SJH, Neerincx AH, Kraneveld AD, Maitland-van der Zee AH. 2019. The crosstalk between microbiome and asthma: exploring associations and challenges. Clin Exp Allergy 49(8):1067–1086, PMID: 31148278, 10.1111/cea.13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Frei R, Lauener RP, Crameri R, O’Mahony L. 2012. Microbiota and dietary interactions: an update to the hygiene hypothesis? Allergy 67(4):451–461, PMID: 22257145, 10.1111/j.1398-9995.2011.02783.x. [DOI] [PubMed] [Google Scholar]
- 93.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. 2014. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20(2):159–166, PMID: 24390308, 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 94.Ruokolainen L, Parkkola A, Karkman A, Sinkko H, Peet A, Hämäläinen AM, et al. 2020. Contrasting microbiotas between Finnish and Estonian infants: exposure to Acinetobacter may contribute to the allergy gap. Allergy 75(9):2342–2351, PMID: 32108360, 10.1111/all.14250. [DOI] [PubMed] [Google Scholar]
- 95.Lotfata A, Moosazadeh M, Helbich M, Hoseini B. 2023. Socioeconomic and environmental determinants of asthma prevalence: a cross-sectional study at the U.S. county level using geographically weighted random forests. Int J Health Geogr 22(1):18, PMID: 37563691, 10.1186/s12942-023-00343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Valverde-Molina J, García-Marcos L. 2023. Microbiome and asthma: microbial dysbiosis and the origins, phenotypes, persistence, and severity of asthma. Nutrients 15(3):486, PMID: 36771193, 10.3390/nu15030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Benson AK, Kelly SA, Legge R, Ma F, Low SJ, Kim J, et al. 2010. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc Natl Acad Sci USA 107(44):18933–18938, PMID: 20937875, 10.1073/pnas.1007028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Johansson MA, Sjögren YM, Persson JO, Nilsson C, Sverremark-Ekström E. 2011. Early colonization with a group of Lactobacilli decreases the risk for allergy at five years of age despite allergic heredity. PLoS One 6(8):e23031, PMID: 21829685, 10.1371/journal.pone.0023031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gosalbes MJ, Llop S, Vallès Y, Moya A, Ballester F, Francino MP. 2013. Meconium microbiota types dominated by lactic acid or enteric bacteria are differentially associated with maternal eczema and respiratory problems in infants. Clin Exp Allergy 43(2):198–211, PMID: 23331561, 10.1111/cea.12063. [DOI] [PubMed] [Google Scholar]
- 100.Jain PK, Purkayastha SD, De Mandal S, Passari AK, Govindarajan RK. 2020. Chapter 13 – effect of climate change on microbial diversity and its functional attributes. In: Recent Advancements in Microbial Diversity. De Mandal S, Bhatt P, eds. New York, NY: Academic Press, 315–331. [Google Scholar]
- 101.Cavicchioli R, Ripple WJ, Timmis KN, Azam F, Bakken LR, Baylis M, et al. 2019. Scientists’ warning to humanity: microorganisms and climate change. Nat Rev Microbiol 17(9):569–586, PMID: 31213707, 10.1038/s41579-019-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WOM, Braun-Fahrländer C, et al. 2011. Exposure to environmental microorganisms and childhood asthma. N Engl J Med 364(8):701–709, PMID: 21345099, 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 103.Ege MJ, Mayer M, Schwaiger K, Mattes J, Pershagen G, van Hage M, et al. 2012. Environmental bacteria and childhood asthma. Allergy 67(12):1565–1571, PMID: 22994424, 10.1111/all.12028. [DOI] [PubMed] [Google Scholar]
- 104.Ciaccio CE, Barnes C, Kennedy K, Chan M, Portnoy J, Rosenwasser L. 2015. Home dust microbiota is disordered in homes of low-income asthmatic children. J Asthma 52(9):873–880, PMID: 26512904, 10.3109/02770903.2015.1028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Valkonen M, Wouters IM, Täubel M, Rintala H, Lenters V, Vasara R, et al. 2015. Bacterial exposures and associations with atopy and asthma in children. PLoS One 10(6):e0131594, PMID: 26121165, 10.1371/journal.pone.0131594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tischer C, Weikl F, Probst AJ, Standl M, Heinrich J, Pritsch K. 2016. Urban dust microbiome: impact on later atopy and wheezing. Environ Health Perspect 124(12):1919–1923, PMID: 27232328, 10.1289/EHP158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cavaleiro Rufo J, Madureira J, Paciência I, Aguiar L, Pereira C, Silva D, et al. 2017. Indoor fungal diversity in primary schools may differently influence allergic sensitization and asthma in children. Pediatr Allergy Immunol 28(4):332–339, PMID: 28208225, 10.1111/pai.12704. [DOI] [PubMed] [Google Scholar]
- 108.Dannemiller KC, Gent JF, Leaderer BP, Peccia J. 2016. Indoor microbial communities: influence on asthma severity in atopic and nonatopic children. J Allergy Clin Immunol 138(1):76–83.e1, PMID: 26851966, 10.1016/j.jaci.2015.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Karvonen AM, Hyvärinen A, Rintala H, Korppi M, Täubel M, Doekes G, et al. 2014. Quantity and diversity of environmental microbial exposure and development of asthma: a birth cohort study. Allergy 69(8):1092–1101, PMID: 24931137, 10.1111/all.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lai PS, Kolde R, Franzosa EA, Gaffin JM, Baxi SN, Sheehan WJ, et al. 2018. The classroom microbiome and asthma morbidity in children attending 3 inner-city schools. J Allergy Clin Immunol 141(6):2311–2313, PMID: 29518428, 10.1016/j.jaci.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Loo EXL, Chew LJM, Zulkifli AB, Ta LDH, Kuo IC, Goh A, et al. 2018. Comparison of microbiota and allergen profile in house dust from homes of allergic and non-allergic subjects-results from the GUSTO study. World Allergy Organ J 11(1):37, PMID: 30534340, 10.1186/s40413-018-0212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pekkanen J, Valkonen M, Täubel M, Tischer C, Leppänen H, Kärkkäinen PM, et al. 2018. Indoor bacteria and asthma in adults: a multicentre case–control study within ECRHS II. Eur Respir J 51(2):1701241, PMID: 29437937, 10.1183/13993003.01241-2017. [DOI] [PubMed] [Google Scholar]
- 113.Valkonen M, Täubel M, Pekkanen J, Tischer C, Rintala H, Zock JP, et al. 2018. Microbial characteristics in homes of asthmatic and non-asthmatic adults in the ECRHS cohort. Indoor Air 28(1):16–27, PMID: 28960492, 10.1111/ina.12427. [DOI] [PubMed] [Google Scholar]
- 114.Cavaleiro Rufo J, Ribeiro AI, Paciência I, Delgado L, Moreira A. 2020. The influence of species richness in primary school surroundings on children lung function and allergic disease development. Pediatr Allergy Immunol 31(4):358–363, PMID: 31943397, 10.1111/pai.13213. [DOI] [PubMed] [Google Scholar]
- 115.Fu X, Norbäck D, Yuan Q, Li Y, Zhu X, Hashim JH, et al. 2020. Indoor microbiome, environmental characteristics and asthma among junior high school students in Johor Bahru, Malaysia. Environ Int 138:105664, PMID: 32200316, 10.1016/j.envint.2020.105664. [DOI] [PubMed] [Google Scholar]
- 116.Gangneux JP, Sassi M, Lemire P, Le Cann P. 2020. Metagenomic characterization of indoor dust bacterial and fungal microbiota in homes of asthma and non-asthma patients using next generation sequencing. Front Microbiol 11:1671, PMID: 32849345, 10.3389/fmicb.2020.01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Adams RI, Leppänen H, Karvonen AM, Jacobs J, Borràs-Santos A, Valkonen M, et al. 2021. Microbial exposures in moisture-damaged schools and associations with respiratory symptoms in students: a multi-country environmental exposure study. Indoor Air 31(6):1952–1966, PMID: 34151461, 10.1111/ina.12865. [DOI] [PubMed] [Google Scholar]
- 118.Cavaleiro Rufo J, Paciência I, Hoffimann E, Moreira A, Barros H, Ribeiro AI. 2021. The neighbourhood natural environment is associated with asthma in children: a birth cohort study. Allergy 76(1):348–358, PMID: 32654186, 10.1111/all.14493. [DOI] [PubMed] [Google Scholar]
- 119.Cox J, Stone T, Ryan P, Burkle J, Jandarov R, Mendell MJ, et al. 2022. Residential bacteria and fungi identified by high-throughput sequencing and childhood respiratory health. Environ Res 204(pt D):112377, PMID: 34800538, 10.1016/j.envres.2021.112377. [DOI] [PubMed] [Google Scholar]
- 120.Donovan GH, Landry SM, Gatziolis D. 2021. The natural environment, plant diversity, and adult asthma: a retrospective observational study using the CDC’s 500 Cities Project Data. Health Place 67:102494, PMID: 33321458, 10.1016/j.healthplace.2020.102494. [DOI] [PubMed] [Google Scholar]
- 121.Fu X, Li Y, Meng Y, Yuan Q, Zhang Z, Wen H, et al. 2021. Derived habitats of indoor microbes are associated with asthma symptoms in Chinese university dormitories. Environ Res 194:110501, PMID: 33221308, 10.1016/j.envres.2020.110501. [DOI] [PubMed] [Google Scholar]
- 122.Fu X, Ou Z, Zhang M, Meng Y, Li Y, Wen J, et al. 2021. Indoor bacterial, fungal and viral species and functional genes in urban and rural schools in Shanxi Province, China—association with asthma, rhinitis and rhinoconjunctivitis in high school students. Microbiome 9(1):138, PMID: 34118964, 10.1186/s40168-021-01091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hyytiäinen H, Kirjavainen PV, Täubel M, Tuoresmäki P, Casas L, Heinrich J, et al. 2021. Microbial diversity in homes and the risk of allergic rhinitis and inhalant atopy in two European birth cohorts. Environ Res 196:110835, PMID: 33582132, 10.1016/j.envres.2021.110835. [DOI] [PubMed] [Google Scholar]
- 124.Lehtimäki J, Thorsen J, Rasmussen MA, Hjelmsø M, Shah S, Mortensen MS, et al. 2021. Urbanized microbiota in infants, immune constitution, and later risk of atopic diseases. J Allergy Clin Immunol 148(1):234–243, PMID: 33338536, 10.1016/j.jaci.2020.12.621. [DOI] [PubMed] [Google Scholar]
- 125.Winnicki MH, Dunn RR, Winther-Jensen M, Jess T, Allin KH, Bruun HH. 2022. Does childhood exposure to biodiverse greenspace reduce the risk of developing asthma? Sci Total Environ 850:157853, PMID: 35940273, 10.1016/j.scitotenv.2022.157853. [DOI] [PubMed] [Google Scholar]
- 126.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. 2010. Disordered microbial communities in asthmatic airways. PLoS One 5(1):e8578, PMID: 20052417, 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cardenas PA, Cooper PJ, Cox MJ, Chico M, Arias C, Moffatt MF, et al. 2012. Upper airways microbiota in antibiotic-naïve wheezing and healthy infants from the tropics of rural Ecuador. PLoS One 7(10):e46803, PMID: 23071640, 10.1371/journal.pone.0046803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Park H, Shin JW, Park SG, Kim W. 2014. Microbial communities in the upper respiratory tract of patients with asthma and chronic obstructive pulmonary disease. PLoS One 9(10):e109710, PMID: 25329665, 10.1371/journal.pone.0109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Denner DR, Sangwan N, Becker JB, Hogarth DK, Oldham J, Castillo J, et al. 2016. Corticosteroid therapy and airflow obstruction influence the bronchial microbiome, which is distinct from that of bronchoalveolar lavage in asthmatic airways. J Allergy Clin Immunol 137(5):1398–1405.e3, PMID: 26627545, 10.1016/j.jaci.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hevia A, Milani C, López P, Donado CD, Cuervo A, González S, et al. 2016. Allergic patients with long-term asthma display low levels of Bifidobacterium adolescentis. PLoS One 11(2):e0147809, PMID: 26840903, 10.1371/journal.pone.0147809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hua X, Goedert JJ, Pu A, Yu G, Shi J. 2016. Allergy associations with the adult fecal microbiota: analysis of the American Gut Project. EBioMedicine 3:172–179, PMID: 26870828, 10.1016/j.ebiom.2015.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang Q, Cox M, Liang Z, Brinkmann F, Cardenas PA, Duff R, et al. 2016. Airway microbiota in severe asthma and relationship to asthma severity and phenotypes. PLoS One 11(4):e0152724, PMID: 27078029, 10.1371/journal.pone.0152724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chiu CY, Chan YL, Tsai YS, Chen SA, Wang CJ, Chen KF, et al. 2017. Airway microbial diversity is inversely associated with mite-sensitized rhinitis and asthma in early childhood. Sci Rep 7(1):1820, PMID: 28500319, 10.1038/s41598-017-02067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li N, Qiu R, Yang Z, Li J, Chung KF, Zhong N, et al. 2017. Sputum microbiota in severe asthma patients: relationship to eosinophilic inflammation. Respir Med 131:192–198, PMID: 28947029, 10.1016/j.rmed.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 135.Arrieta MC, Arévalo A, Stiemsma L, Dimitriu P, Chico ME, Loor S, et al. 2018. Associations between infant fungal and bacterial dysbiosis and childhood atopic wheeze in a nonindustrialized setting. J Allergy Clin Immunol 142(2):424–434.e10, PMID: 29241587, 10.1016/j.jaci.2017.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Durack J, Huang YJ, Nariya S, Christian LS, Ansel KM, Beigelman A, et al. 2018. Bacterial biogeography of adult airways in atopic asthma. Microbiome 6(1):104, PMID: 29885665, 10.1186/s40168-018-0487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kim BS, Lee E, Lee MJ, Kang MJ, Yoon J, Cho HJ, et al. 2018. Different functional genes of upper airway microbiome associated with natural course of childhood asthma. Allergy 73(3):644–652, PMID: 29052232, 10.1111/all.13331. [DOI] [PubMed] [Google Scholar]
- 138.Okba AM, Saber SM, Abdel-Rehim AS, Amin MM, Mohamed DA. 2018. Fecal microbiota profile in atopic asthmatic adult patients. Eur Ann Allergy Clin Immunol 50(3):117–124, PMID: 29479926, 10.23822/EurAnnACI.1764-1489.48. [DOI] [PubMed] [Google Scholar]
- 139.Wang Q, Li F, Liang B, Liang Y, Chen S, Mo X, et al. 2018. A metagenome-wide association study of gut microbiota in asthma in UK adults. BMC Microbiol 18(1):114, PMID: 30208875, 10.1186/s12866-018-1257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bannier MAGE, van Best N, Bervoets L, Savelkoul PHM, Hornef MW, van de Kant KDG, et al. 2020. Gut microbiota in wheezing preschool children and the association with childhood asthma. Allergy 75(6):1473–1476, PMID: 31838753, 10.1111/all.14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee JJ, Kim SH, Lee MJ, Kim BK, Song WJ, Park HW, et al. 2019. Different upper airway microbiome and their functional genes associated with asthma in young adults and elderly individuals. Allergy 74(4):709–719, PMID: 30242844, 10.1111/all.13608. [DOI] [PubMed] [Google Scholar]
- 142.Pang Z, Wang G, Gibson P, Guan X, Zhang W, Zheng R, et al. 2019. Airway microbiome in different inflammatory phenotypes of asthma: a cross-sectional study in Northeast China. Int J Med Sci 16(3):477–485, PMID: 30911282, 10.7150/ijms.29433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Samra M, Nam SK, Lim DH, Kim DH, Yang J, Kim YK, et al. 2019. Urine bacteria-derived extracellular vesicles and allergic airway diseases in children. Int Arch Allergy Immunol 178(2):150–158, PMID: 30415264, 10.1159/000492677. [DOI] [PubMed] [Google Scholar]
- 144.Al Bataineh MT, Hamoudi RA, Dash NR, Ramakrishnan RK, Almasalmeh MA, Sharif HA, et al. 2020. Altered respiratory microbiota composition and functionality associated with asthma early in life. BMC Infect Dis 20(1):697, PMID: 32962658, 10.1186/s12879-020-05427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chiu CY, Chan YL, Tsai MH, Wang CJ, Chiang MH, Chiu CC, et al. 2020. Cross-talk between airway and gut microbiome links to IgE responses to house dust mites in childhood airway allergies. Sci Rep 10(1):13449, PMID: 32778700, 10.1038/s41598-020-70528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Patrick DM, Sbihi H, Dai DLY, Al Mamun A, Rasali D, Rose C, et al. 2020. Decreasing antibiotic use, the gut microbiota, and asthma incidence in children: evidence from population-based and prospective cohort studies. Lancet Respir Med 8(11):1094–1105, PMID: 32220282, 10.1016/S2213-2600(20)30052-7. [DOI] [PubMed] [Google Scholar]
- 147.Ham J, Kim J, Choi S, Park J, Baek MG, Kim YC, et al. 2021. Interactions between NCR+ILC3s and the microbiome in the airways shape asthma severity. Immune Netw 21(4):e25, PMID: 34522438, 10.4110/in.2021.21.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Samra MS, Lim DH, Han MY, Jee HM, Kim YK, Kim JH. 2021. Bacterial microbiota-derived extracellular vesicles in children with allergic airway diseases: compositional and functional features. Allergy Asthma Immunol Res 13(1):56–74, PMID: 33191677, 10.4168/aair.2021.13.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Turek EM, Cox MJ, Hunter M, Hui J, James P, Willis-Owen SAG, et al. 2021. Airway microbial communities, smoking and asthma in a general population sample. EBioMedicine 71:103538, PMID: 34425308, 10.1016/j.ebiom.2021.103538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bar K, Żebrowska P, Łaczmański Ł, Sozańska B. 2022. Airway bacterial biodiversity in exhaled breath condensates of asthmatic children—does it differ from the healthy ones? J Clin Med 11(22):6774, PMID: 36431251, 10.3390/jcm11226774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee-Sarwar KA, Chen YC, Chen YY, Kozyrskyj AL, Mandhane PJ, Turvey SE, et al. 2023. The maternal prenatal and offspring early-life gut microbiome of childhood asthma phenotypes. Allergy 78(2):418–428, PMID: 36107703, 10.1111/all.15516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lee-Sarwar K, Dedrick S, Momeni B, Kelly RS, Zeiger RS, O’Connor GT, et al. 2022. Association of the gut microbiome and metabolome with wheeze frequency in childhood asthma. J Allergy Clin Immunol 150(2):325–336, PMID: 35196534, 10.1016/j.jaci.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tsai MH, Shih HJ, Su KW, Liao SL, Hua MC, Yao TC, et al. 2022. Nasopharyngeal microbial profiles associated with the risk of airway allergies in early childhood. J Microbiol Immunol Infect 55(4):777–785, PMID: 35288032, 10.1016/j.jmii.2022.01.006. [DOI] [PubMed] [Google Scholar]
- 154.Zheng P, Zhang K, Lv X, Liu C, Wang Q, Bai X. 2022. Gut microbiome and metabolomics profiles of allergic and non-allergic childhood asthma. J Asthma Allergy 15:419–435, PMID: 35418758, 10.2147/JAA.S354870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mubanga M, Ploner A, Schuppe-Koistinen I, Magnusson PKE, Boulund F, Debelius JW, et al. 2023. The gut microbiome and asthma in a Swedish twin study. Clin Exp Allergy 53(11):1212–1215, PMID: 37549655, 10.1111/cea.14379. [DOI] [PubMed] [Google Scholar]
- 156.Thorsen J, Li XJ, Peng S, Sunde RB, Shah SA, Bhattacharyya M, et al. 2023. The airway microbiota of neonates colonized with asthma-associated pathogenic bacteria. Nat Commun 14(1):6668, PMID: 37863895, 10.1038/s41467-023-42309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



![Figure 1 is an illustration that indicates that the outer layer is related to the inner layer and features a symbol of Earth and a stick figure of a human. The following information is given: Two nested layers of biodiversity: Outer layer dependent on the environment we live in (including air, soil, natural waters, plants, and animals). Inner layer inhabits the human body [internal (airways, gut) and external microbiota (skin)], dependent on colonization from the outer layer.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/08b6/11218706/5074740c48cf/ehp13948_f1.jpg)