Abstract
Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic cancer in the United States. Additionally, the low survival rate makes PDAC the third-leading cause of cancer-related mortality in the United States, and it is projected that by 2030, it will become the second-leading cause of cancer mortality. Several biological factors contribute to PDAC aggressiveness, and their understanding will narrow the gap from biology to clinical care of PDAC, leading to earlier diagnoses and the development of better treatment options. In this review, we describe the origins of PDAC highlighting the role of cancer stem cells (CSC). CSC, also known as tumor initiating cells, which exhibit a unique metabolism that allows them to maintain a highly plastic, quiescent, immune- and therapy-evasive state. However, CSCs can exit quiescence during proliferation and differentiation, with the capacity to form tumors while constituting a small population in tumor tissues. Tumorigenesis depends on the interactions between CSCs and other cellular and non-cellular components in the microenvironment. These interactions are fundamental to support CSC stemness and are maintained throughout tumor development and metastasis. PDAC is characterized by a massive desmoplastic reaction, which result from the deposition of high amounts of extracellular matrix components by stromal cells. Here we review how this generates a favorable environment for tumor growth by protecting tumor cells from immune responses and chemotherapy and inducing tumor cell proliferation and migration, leading to metastasis formation ultimately leading to death. We emphasize the interactions between CSCs and the tumor microenvironment leading to metastasis formation and posit that better understanding and targeting of these interactions will improve patient outcomes.
1. Introduction
Cancer affects millions of people worldwide (National Cancer Institute, Accessed at 28th August 2022). In 2020, the United States (US) registered over 600,000 cancer related deaths, representing the second leading cause of cancer mortality. (American Cancer Research, Accessed at 28th August 2022). The incidence and mortality of cancer depend on multiple factors such as the organ of origin, the stage at the time of diagnosis, and the effectiveness of therapeutic options. Another important factor is the knowledge obtained from decades of research that allowed the discovery of new diagnostic tools and therapies, which facilitate early diagnosis and more effective treatment. In the last 20 years, the analysis of incidence and mortality trends from the top 5 deadliest types of cancer in the US shows that though high, the number of new cases and deaths for lung, breast, prostate, and colorectal cancers significantly decreased (National Cancer Institute, Accessed at 28th August 2022). This data translates the importance of the implementation of new preventive and diagnostic guidelines that allowed the identification of new cancer patients in earlier stages and the development of more effective therapeutic options. In contrast, pancreatic cancer cases and deaths have been increasing (National Cancer Institute, Accessed at 28th August 2022), reflecting the consequences of patients being diagnosed at later stages and the limited effectiveness of current therapies. More successful treatment of pancreatic cancer will require a better understanding of pancreas cancer biology. In this review, we analyze the clinical issues raised by this type of cancer and highlight the importance of developing new biomarkers that allow earlier diagnosis and new therapeutic strategies that improve the survival of patients. We will describe the importance of cancer stem cells (CSC) in the origin, progression, and metastasis of pancreatic cancer. Additionally, we will review how tumor microenvironment interactions are essential for pancreatic cancer progression. Finally, pancreatic tumors often disseminate to distant sites, greatly diminishing patient survival times. In this context, we highlight the importance of CSCs and the tumor microenvironment in facilitating the formation of metastasis. Altogether, the recent findings in pancreatic cancer biology may identify new biomarkers with an important impact on the earlier diagnosis of this disease and new therapeutic targets that will improve treatment.
2. Clinical issues in pancreatic ductal adenocarcinoma
Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic cancer with an estimated 62,000 new diagnoses and 50,0000 deaths predicted for 2022 in the US (American Cancer Research, Accessed at 28th August 2022). The 5-year survival rate for PDAC (11.5%) is the lowest of all major organ cancers for several reasons (American Cancer Research, Accessed at 28th August 2022; National Cancer Institute, Accessed at 28th August 2022,2022). First, symptoms of PDAC are often non-specific and are associated with many other conditions. For example, the three most common symptoms prior to PDAC diagnosis are abdominal pain, a change in stool habits, and jaundice (Deshwar et al., 2018). Second, at the time of diagnosis the vast majority of patients already have regional or distant metastasis, and only 9% of patients have localized disease (Chu et al., 2017). For patients with localized disease at the time of diagnosis, surgical resection combined with chemotherapy results in a 5-year survival rate of 31.5% (National Cancer Institute, Accessed at 28th August 2022, 2022). While this is a great improvement relative to patients with disseminated disease, who have a 5-year survival rate of only 3% this is the minority of patients and it is still dismal compared to the 5-year survival rates of localized disease for breast and prostate cancer (>99%) (National Cancer Institute, Accessed at 28th August 2022, 2022). PDAC is currently the third-leading cause of cancer-related mortality in the United States and is predicted to become the second-leading cause as early as 2030 due to increasing rates of obesity, which is a risk factor for PDAC, and the incredibly low survival rate for this disease (Kenner et al., 2016).
The lack of effective therapeutic options reflects a gap between understanding the biology of PDAC and its clinical management. To bridge this gap the disease must be diagnosed earlier, and more effective treatments must be available. Both goals require deeper understanding of PDAC biology. In this review we will outline the contributions of CSC, the tumor microenvironment, and the crosstalk between the two that contribute to the aggressiveness of PDAC. We posit that the poor clinical outcome in PDAC is due to a highly aggressive, plastic CSC population that is primed to metastasize early in disease progression and develop resistance to therapy. Advancements in the understanding of how PDAC arises and progresses to metastasis may lead to better treatments and better outcomes for patients with a disease that is most often fatal.
3. Cancer stem cells in pancreatic ductal adenocarcinoma
CSCs are defined as cancer cells with the ability to self-renew and generate a tumor from a single cell (Beck and Blanpain, 2013; D’Angelo and Wicha, 2010; Jordan et al., 2006; Nassar and Blanpain, 2016). In leukemia, these are the cells that can cause systemic leukemia from a single cell which generates leukemic cells that recapitulate the hierarchy of cells in the original patient or animal (Bonnet and Dick, 1997). In a carcinoma, these are the cells that can initiate a tumor in the organ of origin after injection of a single cell (Al-Hajj et al., 2003). There has been much debate about CSC since their discovery, however from a functional definition as tumor or disease-initiating cells, these cells undoubtably exist and are responsible for tumorigenesis, treatment resistance, and metastasis (Brooks et al., 2015). Much of the confusion over CSC has been a result of the overlap of terminology related to the cell of origin of the tumor as well as the idea that CSC states are highly plastic (Walcher et al., 2020). We describe CSC by the functional definition that is agnostic to the cell of origin of the tumor. In other words, the cell of origin does not need to be a tissue stem cell for the cells that are capable of tumor initiation to be CSCs. By describing the CSC state as highly plastic such that non-CSC may become CSC in an environment that supports their de-differentiation we focus on the functional definition of CSC—any tumor cell that can form a tumor (Fig. 1).
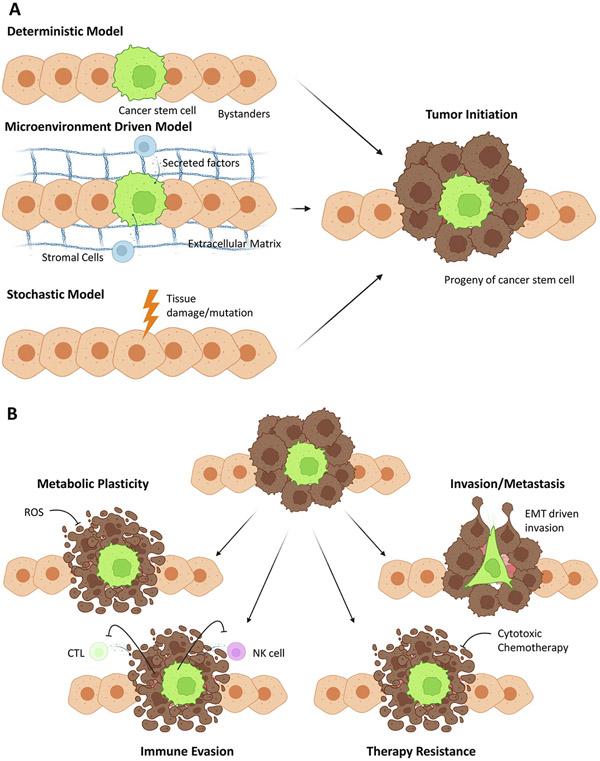
Fig. 1.
Cancer stem cell model and consequences. (A) Different models of tumor initiation that are compatible with the cancer stem cell hypothesis. In the deterministic model (top left), a tissue stem cell has intrinsic capabilities to form a tumor (right). In the microenvironment model (middle left), cancer stem cell phenotype is driven by the microenvironment. In the stochastic model (bottom left), cancer stem cell phenotype emergence is purely stochastic and driven by tissue damage or mutation. (B) Consequences of cancer stem cells include the fact that CSC (green) exhibit metabolic plasticity and survive under harsh conditions that kill differentiated cells (left). Furthermore, CSC are immune evasive and can escape T cell and NK cell control that kills differentiated cells (second from the left). The same concept holds true for therapy resistance (second from the right) where CSC have intrinsic therapy resistance and survive cytotoxic chemotherapy that kills bulk cells. Finally, CSCs can also drive invasion and metastasis (right).
The first description of CSC in PDAC was published in 2007 by Li et al. (2007) and a similar paper closely followed by Hermann et al. (2007). Li found that CD44 + CD24 + ESA+ cells made up <1% of the population of human PDAC cells and demonstrated more than 100-fold enrichment in orthotopic tumor formation relative to the unenriched population (Li, et al., 2007). Hermann et al. investigated CD133, a different maker population, and found that these cells make up 1–3% of the population of human PDAC cells, are exclusively tumorigenic and highly resistant to chemotherapy (Hermann, et al., 2007). In addition to CD44 + CD24 + ESA+ and CD133+, ALDH1 (Rasheed et al., 2010), c-Met (Li et al., 2011), CXCR4 (Hermann, et al., 2007; Van den Broeck et al., 2013), DCLK1 (Ito et al., 2016; Maruno et al., 2021; Westphalen et al., 2016) side-population (Ambrosini et al., 2020; Van den Broeck, et al., 2013; Wang et al., 2009), FoxO1-negative cells (Song et al., 2015), Nestin (Matsuda et al., 2014), and CD9 (Wang et al., 2019) have all been identified as markers of CSC in PDAC (Table 1). Additionally, CD44, CD24, CXCR4, and ESA expression were positively correlated with pancreatic carcinogenesis as they increase in frequency from normal ducts to low-grade PanINs to high-grade PanINs to PDAC (Kure et al., 2012).
Table 1.
Markers of cancer stem cells in pancreatic adenocarcinoma. References that demonstrate enrichment in tumor initiation via limiting dilution assay are reported here, those that only investigate sphere formation as a proxy for CSC activity are not included since this is not the gold-standard.
| CSC Marker | Cell line/model(s) | References |
|---|---|---|
| CD44+CD24+ESA+ | Human primary PDAC tumors | Lodestijn, et al. (2021) |
| CD133+CXCR4+ | L3.6pl (metastatic variant of COLO 357) | Hermann, et al. (2007) |
| ALDH1 + | CAPAN-1, PANC-1, DAN-G, L3.6pl | |
| Human primary PDAC tumors | Rasheed, et al. (2010) | |
| c-Met+ | Human primary PDAC tumors | Li, et al. (2011) |
| DCLK1 + | KLM1, BxPC3 | |
| LSL-KrasG12D, Dclk1-CreERT mice | Ito, et al. (2016); Westphalen, et al. (2016) | |
| FoxO1-negative | Human primary PDAC tumors, | |
| PANC-1 | Song, et al. (2015) | |
| Side-population | Human primary PDAC tumors, PANC-1, BxPC3 | Van den Broeck, et al. (2013); Wang, et al. (2009) |
| CD9+ | LSL-KRasG12D; Fbw7F/F;Ck19-CreER;R26-LSL-YFP(KFCkY) | Wang, et al. (2019) |
Another key distinction of the CSC state is its unique metabolic phenotype (Perusina Lanfranca et al., 2019). For example, CD9 serves as a marker for PDAC CSC by mechanistically enhancing glutamine uptake (Wang, et al., 2019). Valle et al. found that PDAC cells could be pushed to a CSC phenotype in vitro simply by increasing OXPHOS (Valle et al., 2020). Similarly, Ambrosini et al. found that de-differentiated PDAC cells shift from glycolytic to oxidative metabolism and during this process enter a quiescent state with high clonogenicity and stemness (Ambrosini, et al., 2020). Blocking OXPHOS impairs mitophagy, mitochondrial metabolism and reduces PDAC CSC metabolic plasticity and stemness (Alcala et al., 2020). PDAC CSC also have altered fatty acid biosynthesis relative to non-CSCs (Di Carlo et al., 2021). Nimmakayala et al. found that in KPC mice liver metastasis showed a more glycolytic metabolism while lung metastasis displayed more oxidative metabolism, with different CSC subsets enriched in each (Nimmakayala et al., 2021). This indicates that there may be unique metabolic states of CSCs that are selected in an organotropic fashion during the metastatic process. In another publication the authors demonstrated that PGC1α drove metabolic reprogramming to OXPHOS associated with increased stemness (Nimmakayala et al., 2021). These findings suggest a unique metabolic phenotype of CSCs that is crucial to their function providing a possible therapeutic target.
Recently, work from Lodestijn et al. has investigated the role of the microenvironment in tumor initiation via marker-free lineage tracing in xenograft models of PDAC (Lodestijn et al., 2021). Importantly, they found that clonogenicity of PDAC cells is determined by the microenvironment. Though the authors describe their findings as contrasting with the CSC model, their findings are consistent with the description of CSCs as a state and not as a fixed entity (Hermann and Sainz, 2018). Additionally, it is likely an oversimplification to view clonogenicity and CSC status as equivalent. While linked to clonogenicity, the CSC model also allows for the possibility that the most stem-like cells are only transiently proliferative and primarily quiescent, while more differentiated progenitor cells that are derived from the most stem-like cells are the most proliferative. This finding that the niche is crucial for PDAC CSC function is not new. In fact, in 2012, Lonardo et al. and Hamada et al. both independently reported that pancreatic stellate cells promote stemness (Hamada et al., 2012; Lonardo et al., 2012), a finding that is also supported by Lodestijn et al. Furthermore Askan et al. recently found that PDAC CSC frequency was strongly associated with both loose stroma and the highest cumulative rate of local recurrence in patient samples (Askan et al., 2021). Various immune signals have also been demonstrated to support stemness including IL-22 (He et al., 2018) and tumor-infiltrating macrophages (Mitchem et al., 2013). Finally, Ball et al. also reported that the long-term progression of PDAC during serial xenograft transplants is determined by transiently active CSCs (Ball et al., 2017). Taken together these findings demonstrate that CSC states have both tumor-intrinsic and microenvironmentally driven characteristics and that are both important to consider for CSC function in PDAC.
The CSC state in PDAC is also intricately linked to an immune evasive phenotype (Tsuchiya and Shiota, 2021). The initial identification of PDAC CSC was performed largely in patient derived xenograft models and with human PDAC tumor cell lines in nude (Foxn1nu/Foxn1nu), NOD scid (NOD.Cg-Prkdcscid), or NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl) mice which lack a full immune system to prevent rejection of human tumor cell xenografts (Li, et al., 2007). In these models, the role of the immune system in regulation of CSC cannot be reliably studied. However, both in patient samples and in transgenic mouse models of spontaneous PDAC development, the interplay of CSC and the immune system is becoming clearer. Due to limitations of tumor xenograft models, the first area of study was macrophages, which have a defective function in NOD scid and NSG mice (Serreze et al., 1993; Shultz et al., 1995). In patients, CD204+ tumor associated macrophages and CD44 + CD133+ CSC are associated with poor prognosis, suggesting an interplay between macrophages and CSC (Hou et al., 2014). Hou et al. found that CD44 + CD133+ CSC are associated with low CD8+ T cell infiltration and high PD-L1 expression in human PDAC samples (Hou et al., 2019). This association with an immune evasive phenotype and CSC was also linked to unfavorable prognosis and reduced survival times in patients. Recently, in a spontaneous murine model of pancreatic cancer Lytle et al. reported a multiscale map of the CSC state in PDAC (Lytle et al., 2019). Using this unbiased approach, they found a major genetic program active in PDAC CSC’s to be cytokine signaling and immune pathways. Inhibition of these immune signals had a tumor-intrinsic effect characterized by the loss of sphere formation and tumor-extrinsic effects such as increased immune infiltration. Similarly, Valle et al. found that PDAC CSCs are enriched for expression of ligands and receptors related to immune suppression and immune evasion such as PD-L1, CD47, CD155, CD206, and ULPB2/5/6 (Valle, et al., 2020). The authors also found that PDAC CSC were less sensitive to macrophage phagocytosis and T cell killing than non-CSC. Taken together, these findings indicate that a key characteristic of PDAC CSC is their immune evasive phenotype.
Cancer stem cell characteristics and the epithelial to mesenchymal transition (EMT) are inextricably linked for carcinomas (Mani et al., 2008; Zhang and Weinberg, 2018) and this phenomenon holds for PDAC (Rodriguez-Aznar et al., 2019). Even from the early papers on CSC in PDAC, Hermann et al. found that CXCR4 + CD133+ CSC were more mesenchymal, migratory and invasive than their differentiated counterparts (Hermann, et al., 2007). Furthermore, ALDH1 expression in PDAC CSC was also associated with a migratory, invasive, and mesenchymal phenotype (Rasheed, et al., 2010). Both early and more recent investigations have found that Dclk1+ PDAC cells are more migratory/invasive (Ito, et al., 2016) and show significantly higher expression levels of EMT transcription factors such as Snai1, Snai2, Twist1, and Twist2 (Maruno, et al., 2021). Rhimet al. also demonstrated that PDAC cells which have progressed through a partial EMT exhibit CSC properties and are capable of metastasis (Rhim et al., 2012). Interestingly, hyperglycemia and type 2 diabetes mellitus are significant risk factors for PDAC development in patients (Rahn et al., 2018). Sascha et al. found that hyperglycemia promotes EMT and CSC-features via a TGF-beta1 dependent mechanism (Rahn, et al., 2018). In fact, it is becoming clearer that many of the microenvironmental and metabolic factors that support and define stemness also support EMT (Rodriguez-Aznar, et al., 2019).
Taken together, these findings point to the idea that poor clinical outcomes in PDAC are due to a highly aggressive, plastic CSC population primed to metastasize early in disease progression, evade the immune system, and develop resistance to therapy. Greater understanding of the cells that drive disease initiation, progression, and metastasis will lead to better therapies and better outcomes for patients.
4. Tumor microenvironment in pancreatic cancer
One common characteristic shared by many solid cancers is the presence of desmoplasia (Thomas and Radhakrishnan, 2019) (Fig. 2). The formation of fibrotic tissue characterizes desmoplastic reaction (DR), which occurs as a consequence of the abnormal deposition of extracellular matrix (ECM) proteins, growth factors, and cytokines produced by an accumulation of stromal cells surrounding the tumor tissue (Suklabaidya et al., 2018) (Fig. 2A). Additionally, fibroblasts and immune cells largely contribute to this reaction, as they are responsible for the deposition of most of the proteins that constitute the ECM and represent most of the stroma from the tumor microenvironment (TME) (Apte et al., 2012; Ene-Obong et al., 2013; Ino et al., 2013) (Fig. 2A). Moreover, stromal tissues represent a large part of tumor volume, conferring structural support and promoting tumor cell growth, metastasis, and chemoresistance (Erkan et al., 2012).
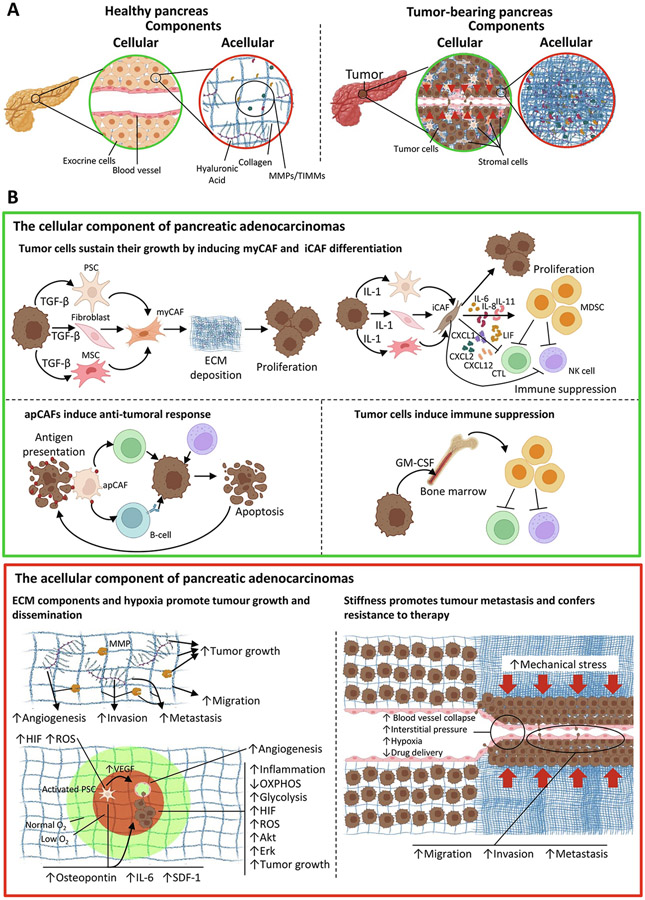
Fig. 2.
Tumor microenvironment interactions in PDAC. (A) Model comparing the cellular (green circles) and acellular components (red circles) of the healthy pancreas (left) and tumor-bearing pancreas with desmoplastic reaction (right). In the healthy pancreas, we observe a tissue region where exocrine cells grow in a structured manner with a blood vessel of normal size (left green circle) and an organized ECM composed of collagen, hyaluronic acid, MMPs, and TIMMs (left red circle). In the tumor-bearing pancreas, we observe that the uncontrolled growth of tumor and stromal cells changed the cellular composition of pancreas, causing the invasion and collapsing of blood vessels (right green circle). Additionally, the ECM in PDAC has an abnormal accumulation of proteins such as collagen and hyaluronic acid, which support tumor growth (right red circle). (B) In-depth observation of the interactions established in the cellular (green box) and acellular (red box) components of PDAC. At the cellular level (green box), we observed that tumor cells induce the differentiation of PSCs, fibroblasts, and MSCs into myCAFs by secreting TGF-β. Consequently, myCAFs support tumor growth by increasing the deposition of ECM molecules (top left panel). Additionally, tumor cells also secrete IL-1 to induce the differentiation of PSCs, fibroblasts, and MSCs into iCAFs. These cells secrete different cytokines responsible to promote a pro-inflammatory environment that supports tumor growth and inhibits anti-tumoral immune responses (top right panel). Contrarily, apCAFs can boost the immune system to eliminate tumor cells by presenting tumor antigens to CTLs and B-cells. Furthermore, innate immune cells such as NK cells also contribute to this anti-tumoral response (bottom left panel). However, tumor cells can evade the immune system by secreting growth factors such as GM-CSF, which recruits MDSCs from the bone marrow to inhibit the activity of immune cells such as CTLs and NK-cells (bottom right panel). On the acellular level (red box), we observe that ECM components such as collagen, hyaluronic acid, and MMPs promote tumor growth and dissemination by increasing angiogenesis and tumor cell invasion, migration, and metastasis (top left panel). Additionally, hypoxia has a similar effect on tumor growth since low levels of oxygen increase the levels of HIF and ROS in PSCs leading to their activation. Consequently, activated PSCs increase the expression of VEGF, which leads to angiogenesis, thus favoring tumor growth and dissemination by conferring new oxygen supplies. Moreover, activated PSCs under hypoxic conditions increase the expression of osteopontin, IL-6, and SDF-1, which forms an inflammatory environment that promotes tumor cell migration and proliferation. Similar to PSCs, hypoxia increases the levels of HIF and ROS in tumor cells resulting in a metabolic shift from oxidative phosphorylation to glycolysis and activating signaling pathways such as Erk and Akt, which are responsible to increase tumor cell proliferation (bottom left panel). Finally, the abnormal deposition of ECM molecules increases the stiffness of PDAC tissue resulting in mechanical stress. Consequently, blood and lymphatic vessels collapse causing hypoxia, increasing interstitial pressure, and compromising PDAC treatment due to inefficient drug delivery. Additionally, mechanical stress increases tumor cell migration and invasion, thus facilitating metastasis (right panel).
Notably, the DR in PDAC is considered a histopathological hallmark of this type of cancer. For example, DR constitutes around 80% of the tumor volume (Erkan, et al., 2012), which changes the normal parenchyma of the pancreas. Consequently, pancreatic endocrine and exocrine functions are drastically compromised (Erkan, et al., 2012).
Hence, PDAC malignancy is not solely a consequence of the existence of cancer cells but also a result of the exacerbated accumulation of stromal cells in TME (Apte, et al., 2012) and the interactions between stromal and cancer cells. In this context, pancreatic stellate cells (PSCs) are pivotal players in PDAC development since they establish pro-tumoral interactions with tumor and immune cells and produce most of the proteins from the ECM (Apte, et al., 2012).
PSCs locate near pancreatic acinar cells and around pancreatic ducts and blood vessels (Apte, et al., 2012). In healthy individuals, PSCs are present in a quiescent state characterized by their content of lipid droplets rich in Vitamin A (Park et al., 2012). They also maintain the homeostasis of the pancreatic matrix by producing ECM components such as collagen III and fibronectin, but also matrix-degrading metalloproteinases (MMP) and their inhibitors (TIMP) (Apte et al., 1999; Phillips et al., 2003). However, under pathological conditions such as pancreatitis, PSCs become activated, leading to the loss of lipid droplets, followed by an increased expression of α smooth muscle actin (α-SMA) that leads to their conversion into a myofibroblast-like phenotype (Yen et al., 2002). Additionally, in such conditions, PSCs destabilize the matrix synthesis and degradation balance towards the excessive production of ECM, thus leading to fibrosis (Apte, et al., 2012). PSCs show similar behavior to PDAC cancer cells since they are the principal producers of fibrosis due to the synthesis of collagen, fibronectin, and laminin (Apte et al., 2004). Tumor cells interact with PSCs promoting their activation and leading to increased proliferation, ECM production, and migration (Vonlaufen et al., 2008). Activated PSCs sustain tumor growth by inhibiting tumor cell apoptosis and promoting proliferation and migration (Kikuta et al., 2010). Several studies reported the existence of different PSC subtypes, which upon activation, associate with tumor cells and become cancer-associated fibroblasts (CAFs) (Elyada et al., 2019; Ohlund et al., 2017). Other sources of CAFs include tissue resident fibroblasts and tissue-infiltrating mesenchymal stem cells (Manoukian et al., 2021). Ohlund et al. identified two distinct CAFs populations, namely, myofibroblastic CAFs (myCAFs) and inflammatory CAFs (iCAFs) (Ohlund, et al., 2017). Additionally, Elyada et al. confirmed the existence of these subtypes by scRNA-seq and observed a third CAF subtype named antigen-presenting CAFs (apCAFs) (Elyada, et al., 2019).
The expression of fibroblast activation protein (FAP), high expression of α-SMA, and low expression of interleukin 6 (IL6) characterize the myCAF subtype, which localizes in the periglandular regions (Ohlund, et al., 2017). Signaling through C-X-C motif ligand 3 (CXCL3)-C-X-C motif chemokine receptor 2 (CXCR2) activates myCAFs and increases the metastatic potential of cancer cells (Sun et al., 2022).
Tumor cells express transforming growth factor-β (TGF-β), which determines fibroblast transformation fate including transition to both myCAFs and iCAFs (Biffi et al., 2019; Elyada, et al., 2019). For example, TGF-β promotes myCAFs activation through SMAD2/3 pathways and consequent expression of connective tissue growth factor (Ctgf) and collagen type I alpha 1 (Col1α1) (Biffi, et al., 2019) (Fig. 2B, green box top left panel). Simultaneously, tumor-derived TGF-β inhibits fibroblast transformation into iCAFs by decreasing the expression of interleukin-1 receptor-1 (IL1R1) (Elyada, et al., 2019).
Ohlund et al. also characterized iCAFs by their low α-SMA and high IL-6 expression (Ohlund, et al., 2017). These cells locate in distant sites from tumor cells. However, tumor cells secrete factors that lead to iCAF activation. Another characteristic of iCAFs is the upregulation of cytokines and chemokines such as IL-6, IL-8, IL-11, CXCL1, CXCL2, and CXCL12 (Ohlund, et al., 2017), conferring a chronic inflammatory environment that favors tumor cell proliferation. For example, the expression of CXCL12 by iCAFs has an immunosuppressive effect and promotes tumor growth (Garg et al., 2018). Interestingly, the IL-1 secreted by tumor cells seems to have an opposing role to that observed by TGF- β since IL-1 inhibits the expression of Ctgf in myCAFs and induces the expression of leukemia inhibitory factor (LIF) (Biffi, et al., 2019) (Fig. 2B, green box top right panel). In turn, LIF suppresses the immune system and decreases the deposition of ECM, which leads to tumor progression (Biffi, et al., 2019). Moreover, iCAFs synthesize hyaluronic acid (HA), which may also facilitate PDAC progression by reducing the efficiency of PDAC therapy (Provenzano et al., 2012).
Both myCAFs and iCAFs show characteristics that support tumor growth, and their localization suggests that CAFs can change their subtype accordingly to their proximity to tumor cells (Biffi, et al., 2019). Tumor cells and PSCs interact in a vicious cycle that perpetuates tumor growth. Tumor cells contact with PSCs activating and transforming those in their neighborhoods into myCAFs. Simultaneously, tumor cells secrete factors that will transform distant PSCs into iCAFs. Together, myCAFs and iCAFs secrete factors important to sustain tumor growth by producing ECMs (myCAFs) and inhibiting the anti-tumoral immune activity (iCAFs). While the tumor grows, iCAFs become closer to tumor cells and change their phenotype to myCAF, thus supporting local tumor growth as tumor cells continue to secrete the factors needed to transform distant PSCs into iCAFs.
However, a third subtype of CAFs seems to have an opposing role in tumor progression due to its capacity to present tumor antigens. As previously mentioned, Elyada et al. identified apCAFs, which are described as a subtype of CAFs expressing major histocompatibility (MHC) class II genes, thus resembling the immune functions of antigen-presenting cells (Elyada, et al., 2019). Among the MHC II genes expressed by apCAFs are H2-aa, H2-Ab1, CD74, serum amyloid A3 (Saa3), and secretory leukocyte peptidase inhibitor (SLPI). Additionally, apCAFs show enrichment of pathways for antigen presentation and processing and express costimulatory factors similarly to APCs, though at lower levels (Elyada, et al., 2019) (Fig. 2B, green box bottom left panel). Despite its presumable immune activity, the mechanisms leading to apCAFs activation and their involvement in the induction of immune responses require further investigation.
Like other solid tumors, PDAC tissue shows infiltration of different types of immune cells such as T-cells, B-cells, NK cells, neutrophils, macrophages, and myeloid-derived suppressor cells (MDSCs) (Ene-Obong, et al., 2013; Ino, et al., 2013). Immune cell infiltration results in different outcomes, depending on the relationships between these cells and tumor cells. For example, immune cells such as cytotoxic T-cells (CTLs) and NK cells show a high cytotoxic activity towards tumor cells, which associates with better survival rates (Ene-Obong, et al., 2013; Ino, et al., 2013) (Fig. 2B, green box bottom left panel). However, tumor tissues infiltrated with macrophages, neutrophils, and MDSC show an opposite tendency (Ene-Obong, et al., 2013; Ino, et al., 2013). The fact that these immune cells confer tumor cells’ tolerance to antigen-specific T-cell cytotoxic activity may explain the observation of poor survival rates (Ene-Obong, et al., 2013; Ino, et al., 2013). Indeed, the evasion of the immune system is a well-established hallmark of cancer (Hanahan and Weinberg, 2011). For example, tumor cells archive immune evasion by producing granulocyte-macrophage colony-stimulating factor (GM-CSF), which induces MDSCs to suppress CD8 T-cell activity (Marigo et al., 2010) (Fig. 2B, green box bottom right panel).
PSCs also seem to contribute to immune evasion. For example, PSCs reduce CD8 T-cell migration towards tumor cells (Ene-Obong, et al., 2013). Additionally, PSCs secrete tryptase and IL13, which activate mast cells (Ma et al., 2013). Consequently, these cells can activate other PSCs through IL1 secretion, and this loop promotes tumor cell proliferation (Ma, et al., 2013).
So far, we discussed how the interactions established by the cellular components of TMEs either promote tumor growth or suppression in PDAC. Yet, TMEs also contain acellular elements that participate in tumor fate, from which we highlight the ECMs mainly produced by PSCs.
The ECM consists of a network of non-cellular components that confers structural support and participates in cellular communications, which result in different cellular processes such as cell growth, migration, and death (Frantz et al., 2010). The dynamic deposition of structural molecules such as collagens and their degradation by enzymes such as MMPs control ECM homeostasis, leading to unique chemical and mechanical properties (Frantz, et al., 2010). Additionally, the interaction of cell-surface receptors with ECM and the bound growth factors to it leads to the activation of several signaling cues, which ultimately result in the regulation of gene transcription (Frantz, et al., 2010).
As mentioned before, PSCs are responsible for synthesizing and depositing high amounts of ECM molecules such as collagen, fibronectin, and laminin, which sustain tumor growth (Fig. 2B, red box top left panel). For example, Grzesiak et al. show that type I collagen promotes cell adhesion, proliferation, and migration of different PDAC cell lines, thus facilitating EMT and the consequent development of metastasis (Grzesiak and Bouvet, 2006). Moreover, the expression of such fibrous proteins leads to the acquisition of tumor cell resistance to therapies such as gemcitabine, 5-fluorouracil, cisplatin, and doxorubicin (Miyamoto et al., 2004).
Additionally, matrix glycosaminoglycans such as HA are present in the ECM of PDAC. The expression of these molecules by PDAC tumor and stromal cells enhances tumor progression by promoting angiogenesis, tumor growth, and metastasis (Sato et al., 2016) (Fig. 2B, red box top left panel). Conversely, the degradation of HA increased the survival of mice with PDAC, suggesting that this molecule is a potential target for PDAC therapy (Provenzano, et al., 2012). However, clinical studies in metastatic pancreatic cancer patients combining hyaluronidase with standard of care treatments failed to improve patient progression free survival and overall survival rates (Ramanathan et al., 2019; Van Cutsem et al., 2020).
Tumor cells depend on the degradation of the ECM and the release of ECM-bonded growth factors to grow and invade neighboring tissues, thus laying on the expression of MMPs (Cabral-Pacheco et al., 2020). For example, the expression of MMP-7 leads to tumor invasiveness and adhesion through the activation of EGFR signaling (Tan et al., 2005). Other MMPs, such as MMP-2 and MMP-9, promote tumor invasion by degrading type IV collagen (Liotta et al., 1980). Interestingly, MMP-9 releases vascular epithelial growth factor (VEGF) from the ECM, thus inducing endothelial cell sprouting and consequently promoting angiogenesis in response to hypoxia (Hawinkels et al., 2008) (Fig. 2B, red box top left panel). This mechanism has particular importance since most PDACs exhibit hypoxic regions (Tao et al., 2021).
In fact, the combination of DR with the proliferation of tumor cells, and poor vascularization of PDAC results in high consumption of the oxygen supplies and consequent formation of hypoxic regions (Tao, et al., 2021). Therefore, tumors need to adapt to this condition to survive. They do so by hijacking the mechanisms used by healthy cells to counter the stress caused by hypoxia, by increasing the expression of hypoxia-inducible factors (HIF) (Tao, et al., 2021) (Fig. 2B, red box bottom left panel). These transcription factors allow tumor cells to change glucose metabolism from oxidative phosphorylation to glycolysis through the Warburg effect (Chiche et al., 2010) (Fig. 2B, red box bottom left panel).
Under hypoxic conditions, tumor and stromal cells increase the levels of reactive oxygen species (ROS) in a HIF-independent manner (Sendoel and Hengartner, 2014). Yet, increased levels of ROS stabilize HIF transcription activity, which prolongs the response to hypoxia (Calvani et al., 2012). Interestingly, moderate levels of ROS favor tumor growth by increasing cell survival and proliferation through the activation of signaling pathways such as ERK and PI3K/AKT (Fruehauf and Meyskens, 2007) (Fig. 2B, red box bottom left panel). However, increased levels of ROS lead to tumor cell death as a consequence of oxidative stress (Nogueira et al., 2008). Hence, tumor cells need to control ROS levels in a favorable manner, which allows them to survive. In this context, hypoxic stromal cells contribute to the survival of hypoxic PDAC cells (Tao, et al., 2021). Hypoxia activates PSCs, which in turn also increase the levels of ROS (Cao et al., 2019). Contrarily to PDAC cells, PSCs endure hypoxia-driven oxidative stress caused by high levels of ROS and consequently express different factors that support PDAC cell viability (Cao, et al., 2019) (Fig. 2B, red box bottom left panel). For example, PSCs secrete osteopontin in a ROS-dependent manner, which promotes EMT and stemness in PDAC cells (Cao, et al., 2019). Another consequence of PSCs activation under hypoxia is the expression of angiogenic and inflammatory factors such as IL-6, stromal cell-derived factor-1 (SDF-1), and VEGF, which contribute to tumor cell invasion and confer a new supply of oxygen through the formation of blood vessels (Lei et al., 2014) (Fig. 2B, red box bottom left panel). The ECM also generates cytotoxic levels of ROS, resulting in PDAC cell death through fibronectin-mediated stimulation of α5β1 integrin (Topalovski et al., 2016). However, PSCs express fibulin-5, which blocks fibronectin signaling, thus protecting tumor cells from oxidative stress (Topalovski, et al., 2016). Moreover, PDAC cells can reduce the levels of ROS by upregulating the expression of heme oxygenase-1 (HO-1) (Abdalla et al., 2019; Topalovski, et al., 2016) or by increasing the activity of HIF-1α (Lang et al., 2016). Furthermore, HIF-1α leads to chemoresistance by reducing the sensitivity of PDAC cells drugs such as gemcitabine (Yokoi and Fidler, 2004).
Finally, the vicious loop established by continuous tumor cell proliferation and PSCs deposition of ECM proteins stiffens tumor tissues, which increases the tension and interstitial pressure in PDACs (Piersma et al., 2020) (Fig. 2B, red box right panel). As stiffness increases in tumor tissues due to tumor growth and ECM deposition, blood and lymphatic vessels collapse, thus increasing the interstitial tissue pressure, developing hypoxic regions, and compromising drug delivery (Piersma, et al., 2020) (Fig. 2B, red box right panel). Consequently, tumor and stromal cells are under extreme mechanical forces, translated in mechanotransduction, a process by which cells activate different signaling pathways in response to mechanical stress (Friedland et al., 2009). As an outcome, mechanical stress further induces stromal cells to support tumor growth and potentiates the migratory capacity of tumor cells, which leads to metastasis (DuChez et al., 2019; Lo et al., 2000) (Fig. 2B, red box right panel). Even in this condition, migratory tumor cells take advantage of ECM proteins to form clusters and travel across tracks of collagen, fibronectin, and tenascin C secreted by PSCs (Sunyer et al., 2016).
The aggressiveness of PDAC is broader than the growth of tumor cells and results from the interactions established with different types of non-cancerous cells and non-cellular components in the TME. Such interactions are fundamental to support tumor development by inducing tumor cell proliferation, protecting tumor cells from immune surveillance and therapy, supplying tumor cells with growth factors, and adapting tumor cells to stress conditions such as hypoxia and mechanical pressures. Additionally, the TME can perpetuate tumor development by inducing tumor cells to adopt stem-like states and boost tumor cell dissemination by increasing their metastatic potential. Therefore, targeting the tumor microenvironment is a promising therapeutic strategy to improve PDAC patient survival.
5. Metastasis in pancreatic ductal adenocarcinoma
Early dissemination is a key feature of PDAC, as evidenced both by the course of disease in patients and in genetically engineered mouse models (Das and Batra, 2015). Rhim et al. found that in the KPCY model dissemination of pancreatic cells precedes tumor formation (Rhim, et al., 2012), a phenomenon that has also been found for other cancers such as breast (Hosseini et al., 2016). These early circulating tumor cells can be detected in the blood (Rhim, et al., 2012), liver (Rhim, et al., 2012), and even in implanted biomaterial scaffolds that recruit metastatic tumor cells in murine models of spontaneous PDAC development (Bushnell et al., 2021). More recent results from Ray et al. suggest that the ECM plays a key role in PDAC early dissemination (Ray et al., 2022). Specifically, they investigate the role of tumor-associated collagen signatures (TACS) and find that TACS regulate cell extrusion and invasion from pre-malignant and malignant lesions. Lymphovascular invasion is also commonly associated with PDAC and poor prognosis (Takahashi et al., 2020). Recently, an HDAC inhibitor was demonstrated to reduce lymphangiogensis and as a result, reduced dissemination, and metastasis (Wang et al., 2021). The concept of early dissemination is further complicated by the fact that tumor cells disseminate and lie dormant in target organs. Specifically, Pommier et al. found livers of PDAC patients contain single disseminated cancer cells (DCC) that lack CK19 and MHCI expression (Pommier et al., 2018). The authors generated a mouse model that recapitulated this finding by immunizing mice against PDAC cells prior to injection in the portal vein. Recently, Luksza et al. report a model wherein neoantigen quality determines the degree of immunoediting that indicates whether patients will survive long term or not (Luksza et al., 2022). Interestingly, this model demonstrates that long-term survivors of PDAC develop recurrent tumors with fewer high-quality neoantigens due to editing over time. These findings suggest that even if PDAC can be diagnosed earlier, there may be additional complications from tumor cells that have already disseminated and sit dormant in distant organs and may or may not be visible to the immune system. However, at present the interaction between dormant DCC and the primary tumor is unknown. These are crucial questions to answer in the quest for better outcomes for patients.
As described above, CSC play a crucial role in metastasis. As cells that can form tumors, CSC are responsible for metastasis initiation. There is some debate in the field whether the CSC that initiate tumors in the pancreas are the same phenotype as those that initiate tumors in metastatic organs such as the lungs and liver. As previously mentioned, Nimmakayala et al. found liver metastases were enriched in glycolytic SP cells while lung metastases were enriched in oxidative CD133+ALDH+ PDAC cells (Nimmakayala, et al., 2021). The CSC model side-steps this debate by recognizing that the CSC state is plastic and not a single entity. Hermann et al., in one of the first reports on PDAC CSC identified CXCR4 + CD133+ CSC as those that determine the metastatic phenotype of the tumor (Hermann, et al., 2007). Maeda et al. also found that CD133 expression correlates with lymph node metastasis (Maeda et al., 2008). Side population (SP) cells are also enriched in a CSC phenotype (Van den Broeck, et al., 2013; Wang, et al., 2009) and are enriched in metastases relative to the primary tumor.
The pre-metastatic niche also plays a crucial role in pancreatic cancer metastasis (Gumberger et al., 2022). The term pre-metastatic niche was coined by a landmark paper by Kaplan et al. in which they found that VEGFR1+ bone marrow cells prepare the metastatic microenvironment to receive and support tumor cells (Kaplan et al., 2005). Though first validated in Lewis lung carcinoma, this concept has been extended to PDAC via Costa-Silva et al. who found that exosomes from PDAC cells initiate the pre-metastatic niche in the liver (Costa-Silva et al., 2015). This field is rapidly growing; thus, we refer readers to the following review of the hepatic pre-metastatic niche for further information (Houg and Bijlsma, 2018). The concept of the pre-metastatic niche is key to the idea that metastasis formation may not be purely stochastic but at least determined, in part, by the preparation of the microenvironment enabling it to attract and receive DCCs. For patients, this indicates that there is an additional step that can be targeted to abrogate metastasis (Aguado et al., 2017). CSC are crucial to the concept of the pre-metastatic niche in two ways. First, cells that initiate metastases are CSC and this model is agnostic to whether they are CSC when they arrive at the primed niche or whether they are turned into CSC by the microenvironment. Second, many studies have demonstrated that CSC contribute strongly to the microenvironment of the tumor as well as the tumor secretome, including exosomes. For an excellent review on this concept please see Chang et al. (Chang and Pauklin, 2021). Taken together, the contribution of CSC to the pre-metastatic niche is a promising avenue for targeting metastasis at the earliest stages.
The tumor microenvironment (TME) also plays a crucial role in promoting metastasis. Beyond the direct role of CSC on priming the microenvironment for dissemination and metastasis as described above, there are many cell types present in the TME that contribute to dissemination, including cancer associated fibroblasts (von Ahrens et al., 2017), macrophages (Kemp et al., 2021; Mitchem, et al., 2013; Nielsen et al., 2016), monocytes (Kemp, et al., 2021; Nedjadi et al., 2018), endothelial cells (Choi et al., 2021), pancreatic stellate cells (Hamada, et al., 2012; Lonardo, et al., 2012), and endocrine cells of the pancreas (Mutgan et al., 2018). For an excellent, in depth review of the TME contribution to metastasis in PDAC we recommend Truong et al. (Truong and Pauklin, 2021). In brief, each of these TME components has been shown to contribute to metastasis that fall into three possible categories with some cells demonstrating contributions to metastasis in all three. These categories include (i) enhancing stemness which in turn facilitates metastasis, (ii) contributing to the tumor secretome which alters the pre-metastatic niche in myriad ways discussed above, (iii) directly contributing to invasion, extravasation and therefore, subsequent metastasis. Better understanding of how each of these cell types contribute to metastasis at varying levels is crucial for finding new therapeutic targets to prevent or treat metastasis in PDAC (Fig. 3).
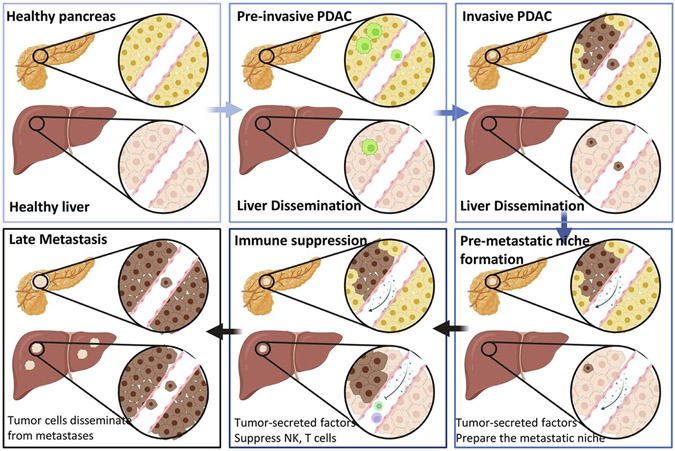
Fig. 3.
Metastasis in PDAC. Schematic illustration of the progression from healthy pancreas (top left) to pre-invasive PDAC (top middle). In pre-invasive disease, even prior to true tumor formation it is possible for PDAC cells to disseminate to the liver (top middle). In invasive PDAC, tumor cells can intravasate into the blood stream arrive in the liver, and extravasate into liver tissue (top right). Once there is an invasive tumor, tumor secreted factors contribute to the formation of the pre-metastatic niche in the liver that attracts and supports tumor cell growth in this organ (bottom right). Furthermore, tumor secreted factors also suppress the immune system and prevent NK and T cell surveillance and elimination of tumor cells at metastatic sites (bottom middle). Finally, in the late metastasis stage, tumor cells not only disseminate from the original tumor, but also from metastases (bottom left). During this phase the number and size of metastases can increase exponentially, ultimately resulting in patient death.
6. Conclusion
Despite basic and clinical research efforts, the prognosis of patients with pancreatic ductal adenocarcinoma is worse than most other cancers. The vast majority of patients already have distant metastasis at the time of diagnosis (Chu, et al., 2017) and current therapies do not offer significant increases in survival times. Clinical trials in PDAC are successful if patients survive 1 month longer in the experimental arm compared to the standard of care (Adamska et al., 2017). We have argued in this review that one of the reasons for poor patient outcomes in PDAC is the existence of highly aggressive, phenotypically plastic CSC populations that are highly metastatic and therapy resistant. Without eliminating the CSC populations that can recreate the entire heterogeneous structure of the original tumor, therapies will not be successful. Most current PDAC clinical trials utilize an immunotherapy, targeted therapy, or metabolic targeting (Hosein et al., 2022; Katayama et al., 2020). Given the importance of the immune system and metabolism in regulating CSC, as described in this review, these therapies are promising.
PDAC CSCs interact with the tumor microenvironment to support the growth of both primary and metastatic tumors. For example, PDAC characterizes by a marked desmoplastic reaction, which result from the recruitment of stromal cells such as PSCs, MSCs, and fibroblasts that differentiate into CAFs responsible to produce high amounts of ECM components such as collagens, MMPs, and hyaluronic acid (Xu et al., 2016). The continuous growth of tumor cells, the recruitment of stromal cells and the accumulation of ECM proteins stiffens PDAC tissues and leads to the collapse of blood and lymphatic vessels (Nieskoski et al., 2017). Consequently, this generates mechanical stress, high interstitial pressure, and hypoxia, which compromise the penetration of chemotherapeutic drugs such as gemcitabine, 5-fluorouracil, cisplatin, and doxorubicin (Lei et al., 2019). Simultaneously, tumor cells adapt to these stress conditions and acquire resistance to mechanical stress, interstitial pressure, hypoxia, and chemotherapy further potentiating their aggressiveness (Yamasaki et al., 2020). Hence, the use of therapies targeting the microenvironment may represent a promising approach to inhibit PDAC growth. Targeting the stroma has been an active area of investigation, however these approaches have had limited success in patients as the desmoplastic reaction is partly tumor inhibitory (Jiang et al., 2020). These findings highlight the need to better understand the interplay between the tumor microenvironment and PDAC cells to target true vulnerabilities and not exacerbate tumor progression. Tumor microenvironment targets currently under clinical investigation have recently been reviewed by Tsang et al. and include CXCR4 antagonist, CXCL12 antagonist, IL-6 inhibitors, IL-1beta inhibition, and Vitamin D analogues (Tsang and Tempero, 2021).
In addition to better treatments, it is possible that earlier diagnosis of PDAC will have a significant positive impact on patient survival. The cells that initiate a PDAC tumor or metastasis are CSCs. Thus, the detection problem can be understood that it is crucial to detect PDAC CSCs themselves or biomarkers that are associated with the presence of CSCs. Liquid biopsy is a promising avenue to detect the presence of circulating tumor cells, ctDNA, or exosomes (Nagai et al., 2020). Another approach is detection of alterations in the immune system associated with PDAC progression (van der Sijde et al., 2021). Interestingly, this approach can also be used to detect both immune alterations and tumor cells in a biomaterial implant (Bushnell, et al., 2021). Though PDAC CSCs may disseminate at the earliest stages of disease progression, earlier diagnosis in patients leads to better outcomes (Kanno et al., 2019). Better understanding of the biology of PDAC CSCs—their biomarkers and vulnerabilities—will lead to earlier diagnosis, more effective treatments, and better patient outcomes.
Acknowledgments
This study was supported by Fundação para a Ciência e a Tecnologia Grant 2020.05319.BD to A.M.P., SNSF, Swiss Cancer League, LB692, FCT LB506, the VCU Massey Cancer Center core grant NIH-NCI Cancer Center Support Grant P30 CA016059 (R.G.), NIH-NCI R35 CA197585 (M.W.), Breast Cancer Research Foundation BCRF-18-173 (M.W.), and NIH NCI K99 CA267261 (G.B.). All figures are original and were prepared using BioRender.
Footnotes
All authors declare that they have no conflict of interests.
References
- Abdalla MY, Ahmad IM, Rachagani S, Banerjee K, Thompson CM, Maurer HC, et al. (2019). Enhancing responsiveness of pancreatic cancer cells to gemcitabine treatment under hypoxia by heme oxygenase-1 inhibition. Translational Research, 207, 56–69. [DOI] [PubMed] [Google Scholar]
- Adamska A, Domenichini A, & Falasca M (2017). Pancreatic ductal adenocarcinoma: Current and evolving therapies. International Journal of Molecular Sciences, 18, 1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguado BA, Bushnell GG, Rao SS, Jeruss JS, & Shea LD (2017). Engineering the pre-metastatic niche. Nat. Biomedical Engineering, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, & Clarke MF (2003). Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America, 100, 3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcala S, Sancho P, Martinelli P, Navarro D, Pedrero C, Martin-Hijano L, et al. (2020). ISG15 and ISGylation is required for pancreatic cancer stem cell mitophagy and metabolic plasticity. Nature Communications, 11, 2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosini G, Dalla Pozza E, Fanelli G, Di Carlo C, Vettori A, Cannino G, et al. (2020). Progressively De-differentiated pancreatic Cancer cells shift from glycolysis to oxidative metabolism and gain a quiescent stem state. Cell, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Cancer Research. Cancer Facts & Figures. (2022). American Cancer Society.
- Apte MV, Haber PS, Darby SJ, Rodgers SC, McCaughan GW, Korsten MA, et al. (1999). Pancreatic stellate cells are activated by proinflammatory cytokines: Implications for pancreatic fibrogenesis. Gut, 44, 534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, Kumar RK, et al. (2004). Desmoplastic reaction in pancreatic cancer: Role of pancreatic stellate cells. Pancreas, 29, 179–187. [DOI] [PubMed] [Google Scholar]
- Apte MV, Pirola RC, & Wilson JS (2012). Pancreatic stellate cells: A starring role in normal and diseased pancreas. Frontiers in Physiology, 3, 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askan G, Sahin IH, Chou JF, Yavas A, Capanu M, Iacobuzio-Donahue CA, et al. (2021). Pancreatic cancer stem cells may define tumor stroma characteristics and recurrence patterns in pancreatic ductal adenocarcinoma. BMC Cancer, 21, 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball CR, Oppel F, Ehrenberg KR, Dubash TD, Dieter SM, Hoffmann CM, et al. (2017). Succession of transiently active tumor-initiating cell clones in human pancreatic cancer xenografts. EMBO Molecular Medicine, 9, 918–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck B, & Blanpain C (2013). Unravelling cancer stem cell potential. Nature Reviews. Cancer, 13, 727–738. [DOI] [PubMed] [Google Scholar]
- Biffi G, Oni TE, Spielman B, Hao Y, Elyada E, Park Y, et al. (2019). IL1-induced JAK/STAT signaling is antagonized by TGFbeta to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discovery, 9, 282–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet D, & Dick JE (1997). Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Medicine, 3, 730–737. [DOI] [PubMed] [Google Scholar]
- Brooks MD, Burness ML, & Wicha MS (2015). Therapeutic implications of cellular heterogeneity and plasticity in breast cancer. Cell Stem Cell, 17, 260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell GG, Orbach SM, Ma JA, Crawford HC, Wicha MS, Jeruss JS, et al. (2021). Disease-induced immunomodulation at biomaterial scaffolds detects early pancreatic cancer in a spontaneous model. Biomaterials, 269, 120632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral-Pacheco GA, Garza-Veloz I, Castruita-De la Rosa C, Ramirez-Acuna JM, Perez-Romero BA, Guerrero-Rodriguez JF, et al. (2020). The roles of matrix metalloproteinases and their inhibitors in human diseases. International Journal of Molecular Sciences, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvani M, Comito G, Giannoni E, & Chiarugi P (2012). Time-dependent stabilization of hypoxia inducible factor-1alpha by different intracellular sources of reactive oxygen species. PLoS One, 7, e38388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Li J, Sun L, Qin T, Xiao Y, Chen K, et al. (2019). Hypoxia-driven paracrine osteopontin/integrin alphavbeta3 signaling promotes pancreatic cancer cell epithelial-mesenchymal transition and cancer stem cell-like properties by modulating forkhead box protein M1. Molecular Oncology, 13, 228–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, & Pauklin S (2021). Extracellular vesicles in pancreatic cancer progression and therapies. Cell Death & Disease, 12, 973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiche J, Brahimi-Horn MC, & Pouyssegur J (2010). Tumour hypoxia induces a metabolic shift causing acidosis: A common feature in cancer. Journal of Cellular and Molecular Medicine, 14, 771–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JI, Jang SI, Hong J, Kim CH, Kwon SS, Park JS, et al. (2021). Cancer-initiating cells in human pancreatic cancer organoids are maintained by interactions with endothelial cells. Cancer Letters, 498, 42–53. [DOI] [PubMed] [Google Scholar]
- Chu LC, Goggins MG, & Fishman EK (2017). Diagnosis and detection of pancreatic Cancer. Cancer Journal, 23, 333–342. [DOI] [PubMed] [Google Scholar]
- Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. (2015). Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nature Cell Biology, 17, 816–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo RC, & Wicha MS (2010). Stem cells in normal development and cancer. Progress in Molecular Biology and Translational Science, 95, 113–158. [DOI] [PubMed] [Google Scholar]
- Das S, & Batra SK (2015). Pancreatic cancer metastasis: Are we being pre-EMTed? Current Pharmaceutical Design, 21, 1249–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshwar AB, Sugar E, Torto D, De Jesus-Acosta A, Weiss MJ, Wolfgang CL, et al. (2018). Diagnostic intervals and pancreatic ductal adenocarcinoma (PDAC) resectability: A single-center retrospective analysis. Annals of Pancreatic Cancer, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carlo C, Sousa BC, Manfredi M, Brandi J, Dalla Pozza E, Marengo E, et al. (2021). Integrated lipidomics and proteomics reveal cardiolipin alterations, upregulation of HADHA and long chain fatty acids in pancreatic cancer stem cells. Scientific Reports, 11, 13297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuChez BJ, Doyle AD, Dimitriadis EK, & Yamada KM (2019). Durotaxis by human cancer cells. Biophysical Journal, 116, 670–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, et al. (2019). Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting Cancer-associated fibroblasts. Cancer Discovery, 9, 1102–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ene-Obong A, Clear AJ, Watt J, Wang J, Fatah R, Riches JC, et al. (2013). Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology, 145, 1121–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, et al. (2012). The role of stroma in pancreatic cancer: Diagnostic and therapeutic implications. Nature Reviews. Gastroenterology & Hepatology, 9, 454–467. [DOI] [PubMed] [Google Scholar]
- Frantz C, Stewart KM, & Weaver VM (2010). The extracellular matrix at a glance. Journal of Cell Science, 123, 4195–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland JC, Lee MH, & Boettiger D (2009). Mechanically activated integrin switch controls alpha5beta1 function. Science, 323, 642–644. [DOI] [PubMed] [Google Scholar]
- Fruehauf JP, & Meyskens FL Jr. (2007). Reactive oxygen species: A breath of life or death? Clinical Cancer Research, 13, 789–794. [DOI] [PubMed] [Google Scholar]
- Garg B, Giri B, Modi S, Sethi V, Castro I, Umland O, et al. (2018). NFkappaB in pancreatic stellate cells reduces infiltration of tumors by cytotoxic T cells and killing of Cancer cells, via up-regulation of CXCL12. Gastroenterology, 155(880–891), e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzesiak JJ, & Bouvet M (2006). The alpha2beta1 integrin mediates the malignant phenotype on type I collagen in pancreatic cancer cell lines. British Journal of Cancer, 94, 1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumberger P, Bjornsson B, Sandstrom P, Bojmar L, & Zambirinis CP (2022). The liver pre-metastatic niche in pancreatic Cancer: A potential opportunity for intervention. Cancers (Basel), 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S, Masamune A, Takikawa T, Suzuki N, Kikuta K, Hirota M, et al. (2012). Pancreatic stellate cells enhance stem cell-like phenotypes in pancreatic cancer cells. Biochemical and Biophysical Research Communications, 421, 349–354. [DOI] [PubMed] [Google Scholar]
- Hanahan D, & Weinberg RA (2011). Hallmarks of cancer: The next generation. Cell, 144, 646–674. [DOI] [PubMed] [Google Scholar]
- Hawinkels LJ, Zuidwijk K, Verspaget HW, de Jonge-Muller ES, van Duijn W, Ferreira V, et al. (2008). VEGF release by MMP-9 mediated heparan sulphate cleavage induces colorectal cancer angiogenesis. European Journal of Cancer, 44, 1904–1913. [DOI] [PubMed] [Google Scholar]
- He W, Wu J, Shi J, Huo YM, Dai W, Geng J, et al. (2018). IL22RA1/STAT3 signaling promotes stemness and tumorigenicity in pancreatic cancer. Cancer Research, 78, 3293–3305. [DOI] [PubMed] [Google Scholar]
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. (2007). Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell, 1, 313–323. [DOI] [PubMed] [Google Scholar]
- Hermann PC, & Sainz B Jr. (2018). Pancreatic cancer stem cells: A state or an entity? Seminars in Cancer Biology, 53, 223–231. [DOI] [PubMed] [Google Scholar]
- Hosein AN, Dougan SK, Aguirre AJ, & Maitra A (2022). Translational advances in pancreatic ductal adenocarcinoma therapy. Nature cancer, 3, 272–286. [DOI] [PubMed] [Google Scholar]
- Hosseini H, Obradovic MMS, Hoffmann M, Harper KL, Sosa MS, Werner-Klein M, et al. (2016). Early dissemination seeds metastasis in breast cancer. Nature, 540, 552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou YC, Chao YJ, Hsieh MH, Tung HL, Wang HC, & Shan YS (2019). Low CD8(+) T cell infiltration and high PD-L1 expression are associated with level of CD44(+)/CD133(+) cancer stem cells and predict an unfavorable prognosis in pancreatic cancer. Cancers (Basel), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou YC, Chao YJ, Tung HL, Wang HC, & Shan YS (2014). Coexpression of CD44-positive/CD133-positive cancer stem cells and CD204-positive tumor-associated macrophages is a predictor of survival in pancreatic ductal adenocarcinoma. Cancer, 120, 2766–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houg DS, & Bijlsma MF (2018). The hepatic pre-metastatic niche in pancreatic ductal adenocarcinoma. Molecular Cancer, 17, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, et al. (2013). Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. British Journal of Cancer, 108, 914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Tanaka S, Akiyama Y, Shimada S, Adikrisna R, Matsumura S, et al. (2016). Dominant expression of DCLK1 in human pancreatic cancer stem cells accelerates tumor invasion and metastasis. PLoS One, 11, e0146564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Zhou L, Lu J, Wang Y, Liu C, You L, et al. (2020). Stroma-targeting therapy in pancreatic cancer: One coin with two sides? Frontiers in Oncology, 10, 576399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CT, Guzman ML, & Noble M (2006). Cancer stem cells. The New England Journal of Medicine, 355, 1253–1261. [DOI] [PubMed] [Google Scholar]
- Kanno A, Masamune A, Hanada K, Kikuyama M, & Kitano M (2019). Advances in early detection of pancreatic cancer. Diagnostics, 9, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. (2005). VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature, 438, 820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama ES, Hue JJ, Bajor DL, Ocuin LM, Ammori JB, Hardacre JM, et al. (2020). A comprehensive analysis of clinical trials in pancreatic cancer: What is coming down the pike? Oncotarget, 11, 3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp SB, Steele NG, Carpenter ES, Donahue KL, Bushnell GG, Morris AH, et al. (2021). Pancreatic cancer is marked by complement-high blood monocytes and tumor-associated macrophages. Life Science Alliance, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenner BJ, Chari ST, Maitra A, Srivastava S, Cleeter DF, Go VL, et al. (2016). Early detection of pancreatic cancer-a defined future using lessons from other cancers: A white paper. Pancreas, 45, 1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuta K, Masamune A, Watanabe T, Ariga H, Itoh H, Hamada S, et al. (2010). Pancreatic stellate cells promote epithelial-mesenchymal transition in pancreatic cancer cells. Biochemical and Biophysical Research Communications, 403, 380–384. [DOI] [PubMed] [Google Scholar]
- Kure S, Matsuda Y, Hagio M, Ueda J, Naito Z, & Ishiwata T (2012). Expression of cancer stem cell markers in pancreatic intraepithelial neoplasias and pancreatic ductal adenocarcinomas. International Journal of Oncology, 41, 1314–1324. [DOI] [PubMed] [Google Scholar]
- Lang M, Wang X, Wang H, Dong J, Lan C, Hao J, et al. (2016). Arsenic trioxide plus PX-478 achieves effective treatment in pancreatic ductal adenocarcinoma. Cancer Letters, 378, 87–96. [DOI] [PubMed] [Google Scholar]
- Lei F, Xi X, Batra SK, & Bronich TK (2019). Combination therapies and drug delivery platforms in combating pancreatic cancer. Journal of Pharmacology and Experimental Therapeutics, 370, 682–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei J, Huo X, Duan W, Xu Q, Li R, Ma J, et al. (2014). Alpha-Mangostin inhibits hypoxia-driven ROS-induced PSC activation and pancreatic cancer cell invasion. Cancer Letters, 347, 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. (2007). Identification of pancreatic cancer stem cells. Cancer Research, 67, 1030–1037. [DOI] [PubMed] [Google Scholar]
- Li C, Wu JJ, Hynes M, Dosch J, Sarkar B, Welling TH, et al. (2011). C-met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology, 141(2218–2227), e5. [DOI] [PubMed] [Google Scholar]
- Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, & Shafie S (1980). Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature, 284, 67–68. [DOI] [PubMed] [Google Scholar]
- Lo CM, Wang HB, Dembo M, & Wang YL (2000). Cell movement is guided by the rigidity of the substrate. Biophysical Journal, 79, 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodestijn SC, Miedema DM, Lenos KJ, Nijman LE, Belt SC, El Makrini K, et al. (2021). Marker-free lineage tracing reveals an environment-instructed clonogenic hierarchy in pancreatic cancer. Cell Reports, 37, 109852. [DOI] [PubMed] [Google Scholar]
- Lonardo E, Frias-Aldeguer J, Hermann PC, & Heeschen C (2012). Pancreatic stellate cells form a niche for cancer stem cells and promote their self-renewal and invasiveness. Cell Cycle, 11, 1282–1290. [DOI] [PubMed] [Google Scholar]
- Luksza M, Sethna ZM, Rojas LA, Lihm J, Bravi B, Elhanati Y, et al. (2022). Neoantigen quality predicts immunoediting in survivors of pancreatic cancer. Nature, 606, 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle NK, Ferguson LP, Rajbhandari N, Gilroy K, Fox RG, Deshpande A, et al. (2019). A multiscale map of the stem cell state in pancreatic adenocarcinoma. Cell, 177(572–586), e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hwang RF, Logsdon CD, & Ullrich SE (2013). Dynamic mast cell-stromal cell interactions promote growth of pancreatic cancer. Cancer Research, 73, 3927–3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Shinchi H, Kurahara H, Mataki Y, Maemura K, Sato M, et al. (2008). CD133 expression is correlated with lymph node metastasis and vascular endothelial growth factor-C expression in pancreatic cancer. British Journal of Cancer, 98, 1389–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. (2008). The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell, 133, 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoukian P, Bijlsma M, & Van Laarhoven H (2021). The cellular origins of cancer-associated fibroblasts and their opposing contributions to pancreatic cancer growth. Frontiers in Cell and Development Biology, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, et al. (2010). Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity, 32, 790–802. [DOI] [PubMed] [Google Scholar]
- Maruno T, Fukuda A, Goto N, Tsuda M, Ikuta K, Hiramatsu Y, et al. (2021). Visualization of stem cell activity in pancreatic cancer expansion by direct lineage tracing with live imaging. eLife, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Yoshimura H, Ueda J, Naito Z, Korc M, & Ishiwata T (2014). Nestin delineates pancreatic cancer stem cells in metastatic foci of NOD/Shi-scid IL2Rgamma(null) (NOG) mice. The American Journal of Pathology, 184, 674–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, et al. (2013). Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Research, 73, 1128–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto H, Murakami T, Tsuchida K, Sugino H, Miyake H, & Tashiro S (2004). Tumor-stroma interaction of human pancreatic cancer: Acquired resistance to anticancer drugs and proliferation regulation is dependent on extracellular matrix proteins. Pancreas, 28, 38–44. [DOI] [PubMed] [Google Scholar]
- Mutgan AC, Besikcioglu HE, Wang S, Friess H, Ceyhan GO, & Demir IE (2018). Insulin/IGF-driven cancer cell-stroma crosstalk as a novel therapeutic target in pancreatic cancer. Molecular Cancer, 17, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Sho M, Akahori T, Nakagawa K, & Nakamura K (2020). Application of liquid biopsy for surgical management of pancreatic cancer. Annals of Gastroenterological Surgery, 4, 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar D, & Blanpain C (2016). Cancer stem cells: Basic concepts and therapeutic implications. Annual Review of Pathology, 11, 47–76. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. 2022. SEER*Explorer Application.
- Nedjadi T, Evans A, Sheikh A, Barerra L, Al-Ghamdi S, Oldfield L, et al. (2018). S100A8 and S100A9 proteins form part of a paracrine feedback loop between pancreatic cancer cells and monocytes. BMC Cancer, 18, 1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SR, Quaranta V, Linford A, Emeagi P, Rainer C, Santos A, et al. (2016). Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nature Cell Biology, 18, 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieskoski MD, Marra K, Gunn JR, Hoopes PJ, Doyley MM, Hasan T, et al. (2017). Collagen complexity spatially defines microregions of total tissue pressure in pancreatic cancer. Scientific Reports, 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmakayala RK, Leon F, Rachagani S, Rauth S, Nallasamy P, Marimuthu S, et al. (2021). Metabolic programming of distinct cancer stem cells promotes metastasis of pancreatic ductal adenocarcinoma. Oncogene, 40, 215–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmakayala RK, Rauth S, Chirravuri Venkata R, Marimuthu S, Nallasamy P, Vengoji R, et al. (2021). PGC1alpha-mediated metabolic reprogramming drives the stemness of pancreatic precursor lesions. Clinical Cancer Research, 27, 5415–5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, et al. (2008). Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell, 14, 458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, et al. (2017). Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. The Journal of Experimental Medicine, 214, 579–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Choi S, Lee MG, Lim C, & Oh J (2012). Retinol binding protein-albumin domain III fusion protein deactivates hepatic stellate cells. Molecules and Cells, 34, 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perusina Lanfranca M, Thompson JK, Bednar F, Halbrook C, Lyssiotis C, Levi B, et al. (2019). Metabolism and epigenetics of pancreatic cancer stem cells. Seminars in Cancer Biology, 57, 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PA, McCarroll JA, Park S, Wu MJ, Pirola R, Korsten M, et al. (2003). Rat pancreatic stellate cells secrete matrix metalloproteinases: Implications for extracellular matrix turnover. Gut, 52, 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piersma B, Hayward MK, & Weaver VM (2020). Fibrosis and cancer: A strained relationship. Biochimica Et Biophysica Acta. Reviews on Cancer, 1873, 188356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier A, Anaparthy N, Memos N, Kelley ZL, Gouronnec A, Yan R, et al. (2018). Unresolved endoplasmic reticulum stress engenders immune-resistant, latent pancreatic cancer metastases. Science (New York, N.Y.), 360. eaao4908). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, & Hingorani SR (2012). Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell, 21, 418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn S, Zimmermann V, Viol F, Knaack H, Stemmer K, Peters L, et al. (2018). Diabetes as risk factor for pancreatic cancer: Hyperglycemia promotes epithelial-mesenchymal-transition and stem cell properties in pancreatic ductal epithelial cells. Cancer Letters, 415, 129–150. [DOI] [PubMed] [Google Scholar]
- Ramanathan RK, McDonough SL, Philip PA, Hingorani SR, Lacy J, Kortmansky JS, et al. (2019). Phase IB/II randomized study of FOLFIRINOX plus pegylated recombinant human hyaluronidase versus FOLFIRINOX alone in patients with metastatic pancreatic adenocarcinoma: SWOG S1313. Journal of Clinical Oncology, 37, 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed ZA, Yang J, Wang Q, Kowalski J, Freed I, Murter C, et al. (2010). Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. Journal of the National Cancer Institute, 102, 340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Callaway MK, Rodriguez-Merced NJ, Crampton AL, Carlson M, Emme KB, et al. (2022). Stromal architecture directs early dissemination in pancreatic ductal adenocarcinoma. JCI Insight, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. (2012). EMT and dissemination precede pancreatic tumor formation. Cell, 148, 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Aznar E, Wiesmuller L, Sainz B Jr., & Hermann PC (2019). EMT and stemness-key players in pancreatic Cancer stem cells. Cancers (Basel), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Kohi S, Hirata K, & Goggins M (2016). Role of hyaluronan in pancreatic cancer biology and therapy: Once again in the spotlight. Cancer Science, 107, 569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendoel A, & Hengartner MO (2014). Apoptotic cell death under hypoxia. Physiology, 29, 168–176. [DOI] [PubMed] [Google Scholar]
- Serreze DV, Gaedeke JW, & Leiter EH (1993). Hematopoietic stem-cell defects underlying abnormal macrophage development and maturation in NOD/Lt mice: Defective regulation of cytokine receptors and protein kinase C. Proceedings of the National Academy of Sciences of the United States of America, 90, 9625–9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, et al. (1995). Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. Journal of Immunology, 154, 180–191. [PubMed] [Google Scholar]
- Song W, Li Q, Wang L, Huang W, & Wang L (2015). FoxO1-negative cells are cancer stem-like cells in pancreatic ductal adenocarcinoma. Scientific Reports, 5, 10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suklabaidya S, Dash P, Das B, Suresh V, Sasmal PK, & Senapati S (2018). Experimental models of pancreatic cancer desmoplasia. Laboratory Investigation, 98, 27–40. [DOI] [PubMed] [Google Scholar]
- Sun X, He X, Zhang Y, Hosaka K, Andersson P, Wu J, et al. (2022). Inflammatory cell-derived CXCL3 promotes pancreatic cancer metastasis through a novel myofibroblast-hijacked cancer escape mechanism. Gut, 71, 129–147. [DOI] [PubMed] [Google Scholar]
- Sunyer R, Conte V, Escribano J, Elosegui-Artola A, Labernadie A, Valon L, et al. (2016). Collective cell durotaxis emerges from long-range intercellular force transmission. Science, 353, 1157–1161. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Katsuta E, Yan L, Tokumaru Y, Katz MHG, & Takabe K (2020). Transcriptomic profile of Lymphovascular invasion, a known risk factor of pancreatic ductal adenocarcinoma metastasis. Cancers, 12, 2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Egami H, Abe M, Nozawa F, Hirota M, & Ogawa M (2005). Involvement of MMP-7 in invasion of pancreatic cancer cells through activation of the EGFR mediated MEK-ERK signal transduction pathway. Journal of Clinical Pathology, 58, 1242–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Yang G, Zhou W, Qiu J, Chen G, Luo W, et al. (2021). Targeting hypoxic tumor microenvironment in pancreatic cancer. Journal of Hematology & Oncology, 14, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, & Radhakrishnan P (2019). Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis. Molecular Cancer, 18, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalovski M, Hagopian M, Wang M, & Brekken RA (2016). Hypoxia and transforming growth factor beta cooperate to induce Fibulin-5 expression in pancreatic cancer. The Journal of Biological Chemistry, 291, 22244–22252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong LH, & Pauklin S (2021). Pancreatic Cancer microenvironment and cellular composition: Current understandings and therapeutic approaches. Cancers (Basel), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang ES, & Tempero MA (2021). Therapeutic targets in the pancreatic adenocarcinoma microenvironment: Past challenges and opportunities for the future. Journal of Cancer Metastasis and Treatment, 7, 33. [Google Scholar]
- Tsuchiya H, & Shiota G (2021). Immune evasion by cancer stem cells. Regenerative Therapy, 17, 20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle S, Alcala S, Martin-Hijano L, Cabezas-Sainz P, Navarro D, Munoz ER, et al. (2020). Exploiting oxidative phosphorylation to promote the stem and immunoevasive properties of pancreatic cancer stem cells. Nature Communications, 11, 5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cutsem E, Tempero MA, Sigal D, Oh DY, Fazio N, Macarulla T, et al. (2020). Randomized phase III trial of Pegvorhyaluronidase alfa with nab-paclitaxel plus gemcitabine for patients with hyaluronan-high metastatic pancreatic adenocarcinoma. Journal of Clinical Oncology, 38, 3185–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Broeck A, Vankelecom H, Van Delm W, Gremeaux L, Wouters J, Allemeersch J, et al. (2013). Human pancreatic cancer contains a side population expressing cancer stem cell-associated and prognostic genes. PLoS One, 8, e73968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sijde F, Mustafa DA, Vietsch EE, Katsikis PD, & van Eijck CH (2021). Circulating immunological biomarkers: Prognosis of pancreatic cancer patients reflected by the immune system. Pancreas, 50, 933–941. [DOI] [PubMed] [Google Scholar]
- von Ahrens D, Bhagat TD, Nagrath D, Maitra A, & Verma A (2017). The role of stromal cancer-associated fibroblasts in pancreatic cancer. Journal of Hematology & Oncology, 10, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonlaufen A, Joshi S, Qu C, Phillips PA, Xu Z, Parker NR, et al. (2008). Pancreatic stellate cells: Partners in crime with pancreatic cancer cells. Cancer Research, 68, 2085–2093. [DOI] [PubMed] [Google Scholar]
- Walcher L, Kistenmacher AK, Suo H, Kitte R, Dluczek S, Strauss A, et al. (2020). Cancer stem cells-origins and biomarkers: Perspectives for targeted personalized therapies. Frontiers in Immunology, 11, 1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CA, Li CF, Huang RC, Li YH, Liou JP, & Tsai SJ (2021). Suppression of extracellular vesicle VEGF-C-mediated Lymphangiogenesis and pancreatic Cancer early dissemination by a selective HDAC1/2 inhibitor. Molecular Cancer Therapeutics, 20, 1550–1560. [DOI] [PubMed] [Google Scholar]
- Wang VM, Ferreira RMM, Almagro J, Evan T, Legrave N, Zaw Thin M, et al. (2019). CD9 identifies pancreatic cancer stem cells and modulates glutamine metabolism to fuel tumour growth. Nature Cell Biology, 21, 1425–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Li F, Luo B, Wang XH, Sun HC, Liu S, et al. (2009). A side population of cells from a human pancreatic carcinoma cell line harbors cancer stem cell characteristics. Neoplasma, 56, 371–378. [DOI] [PubMed] [Google Scholar]
- Westphalen CB, Takemoto Y, Tanaka T, Macchini M, Jiang Z, Renz BW, et al. (2016). Dclk1 defines quiescent pancreatic progenitors that promote injury-induced regeneration and tumorigenesis. Cell Stem Cell, 18, 441–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, P Zhou B, Tao M, Liu J, & Li W (2016). The role of stromal components in pancreatic cancer progression. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents), 16, 1117–1124. [DOI] [PubMed] [Google Scholar]
- Yamasaki A, Yanai K, & Onishi H (2020). Hypoxia and pancreatic ductal adenocarcinoma. Cancer Letters, 484, 9–15. [DOI] [PubMed] [Google Scholar]
- Yen TW, Aardal NP, Bronner MP, Thorning DR, Savard CE, Lee SP, et al. (2002). Myofibroblasts are responsible for the desmoplastic reaction surrounding human pancreatic carcinomas. Surgery, 131, 129–134. [DOI] [PubMed] [Google Scholar]
- Yokoi K, & Fidler IJ (2004). Hypoxia increases resistance of human pancreatic cancer cells to apoptosis induced by gemcitabine. Clinical Cancer Research, 10, 2299–2306. [DOI] [PubMed] [Google Scholar]
- Zhang Y, & Weinberg RA (2018). Epithelial-to-mesenchymal transition in cancer: Complexity and opportunities. Frontiers in Medicine, 12, 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]