Abstract
The use of ex vivo compound action potential (CAP) recordings from intact optic nerves is an ideal model to study white matter function without the influence of gray matter. Here, we describe how freshly dissected optic nerves are placed in a humidified recording chamber and how evoked CAPs are recorded and monitored in real time for up to 10 h. Evoked CAP recordings allow for white matter to be studied under acute challenges such as anoxia, hypoxia, aglycemia, and ischemia.
Keywords: Compound action potential, White matter, Axon, Ischemia, Aglycemia, Anoxia, Optic nerve
1. Introduction
The optic nerve is a purely myelinated central nervous system white matter tract that serves as a stable ex vivo tissue model to study axon function. The dissected intact optic nerve is devoid of neuronal cell bodies and synapses; nonetheless, it consists of all components of white matter, including axons and supporting glial cells, thus making it an optimal model to study white matter. Under controlled conditions ex vivo, the optic nerve can be maintained structurally and functionally for up to 18 h. The advantages of this model are that a) it requires relatively minimum dissection time due to the ease of access to the optic nerve (Fig. 1a, b), thus minimizing injury, and b) evoked compound action potentials (CAPs) can be monitored for several hours to study axon function (Fig. 2a).
Fig. 1.
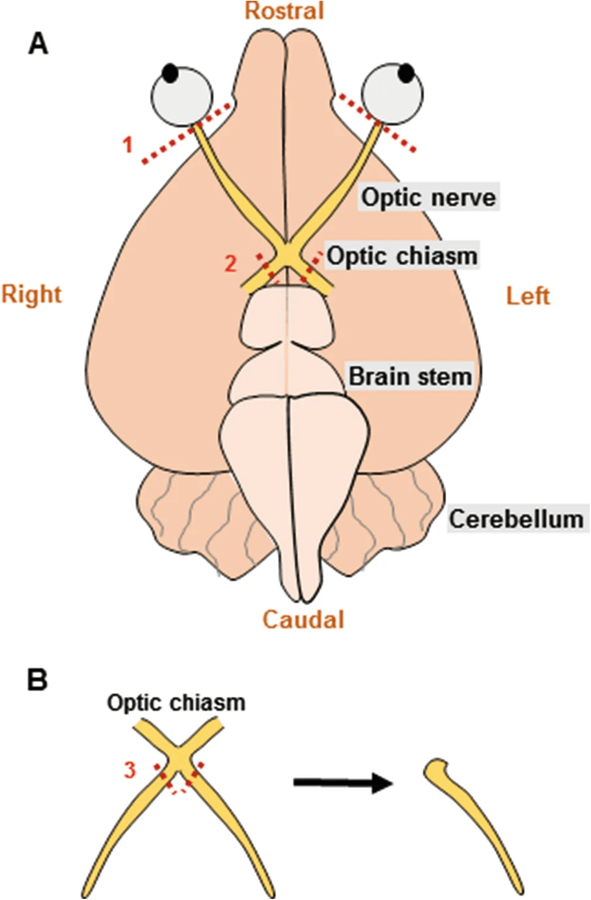
(a) Schematic showing the optic nerves attached at the optic chiasm located at the base of the brain. During dissection to isolate the nerves, the right and left optic nerves are cut right behind the retina (#1), following which the brain is lifted out of the skull and the optic chiasm is removed from the brain (#2). (b) Optic nerves are transferred to the recording chamber and cut twice under a dissecting microscope (#3) to separate the right and left optic nerves for recordings
Fig. 2.
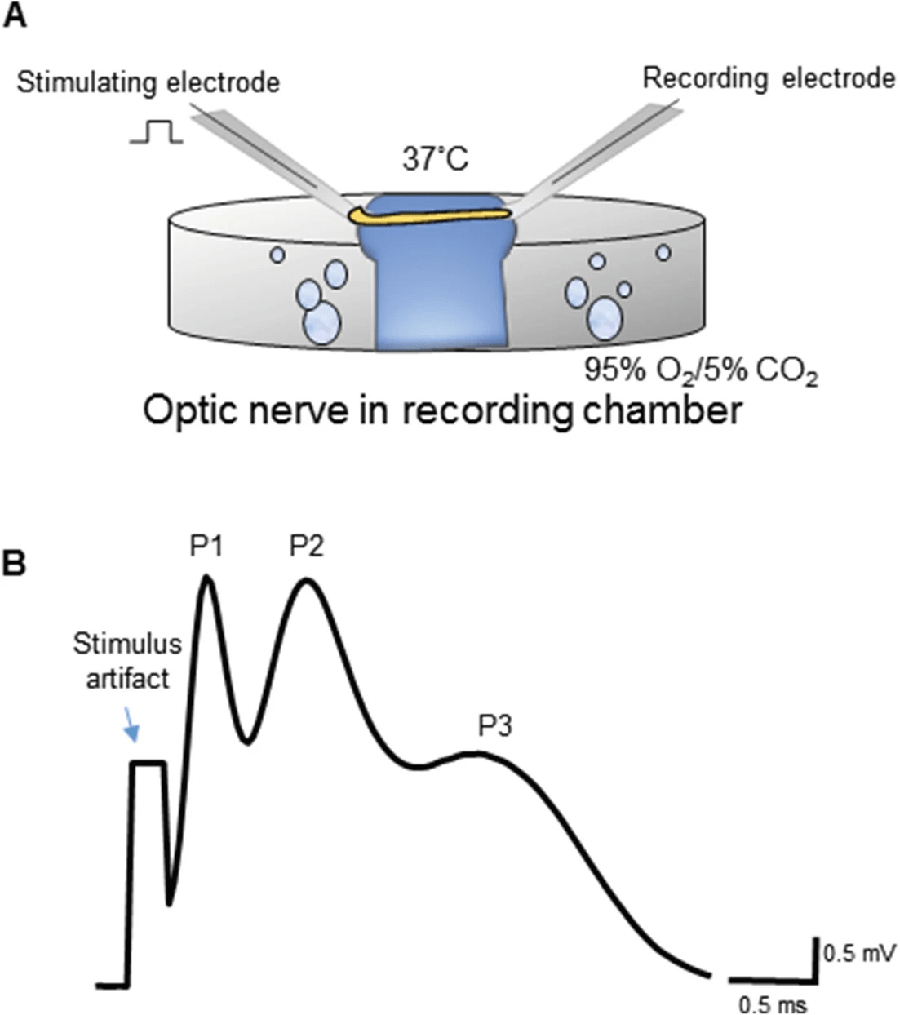
(a) A diagram representing a typical interface-typed chamber to record from optic nerves bubbled with 95% O2/5% CO2 and kept at 37 °C. The optic nerve is placed between two suction electrodes that are back-filled with ACSF. (b) A representative evoked CAP from optic nerve. Note the three characteristic peaks (P1, P2, and P3), where P1 closest to the stimulus artifact represents the fastest axons and P3 represents the slowest axons
Both rat optic nerve and mouse optic nerve have been widely used to study white matterinjury to determine the effects of anoxia [1, 2], aglycemia [3], and ischemia [4–11]. Mouse optic nerve is advantageous over rat optic nerve because of its thinner diameter. The thicker rat optic nerve is limited by a lack of glucose diffusion into the core of the optic nerve, especially under conditions that increase white matter glucose demand [12]. Mouse optic nerves are also highly desirable compared to rat because of the availability of various mouse transgenic lines to take advantage of studying white matter function under specific conditions [5]. Evoked CAPs recorded from white matter tracts are quantified typically by measuring the area under the curve [2, 13].Optic nerve recordings typically show three peaks (Fig. 2b), which represents the fast (P1), medium (P2), and slow (P3) myelinated axons, respectively. CAP area measures the summation of action potentials in functional axons residing in the optic nerve. Furthermore, for the duration of each experiment, CAPs overlap, thus providing visual confirmation of any changes in conduction properties and allowing additional CAP characteristics such as duration, latency, and amplitude to be quantified.
2. Materials
Prepare solutions for optic nerveelectrophysiology experiments using double-distilled deionized water (ddH2O) that is ultrapurified to achieve a resistivity of at least 18 MΩ.cm at room temperature. Purchase analytical grade reagents to prepare all solutions. Follow all Institutional Animal Care and Use Committee regulations on animal use for experiments. Observe all institutional policies for proper waste disposal.
2.1. Materials Required
Dissected optic nerves from the preferred mouse strain, gender, and/or age (see Note 1) for optic nerveelectrophysiology.
Graduated glass cylinders (see Note 2) to prepare solutions.
ACSF with glucose (ACSFG): 126 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 2 mM MgSO4·7H2O, 26 mM NaHCO3, and 10 mM glucose (Table 1). Prepare each salt solution separately, mix into a graduated cylinder, and bring to final volume using ddH2O. Dissolve glucose and NaHCO3 in deionized distilled H2O separately at 60 °C on a heating plate (see Note 3, Table 1). Aerate the solution with 95% O2/5% CO2 for 15–20 min to attain a pH of 7.4. Add 2 M CaCl2 after bubbling to achieve a final working concentration of 2 mM CaCl2 (see Note 4).
ACSF without glucose (ACSF0): 126 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 2 mM MgSO4, 26 mM NaHCO3, and 10 mM sucrose (see Note 5). Prepare each salt solution separately, mix into a graduated cylinder and bring to final volume using ddH2O. Aerate the solution with 95% O2/5% CO2 for 15–20 min to attain a pH of 7.4. Add 2 M CaCl2 after bubbling to achieve a final working concentration of 2 mM CaCl2 (see Note 4).
Table 1. Table lists the chemical constituents of ACSF (1 L).
From: Ex Vivo Studies of Optic Nerve Axon Electrophysiology
| ACSF (1 L) | Weight | Concentration |
|---|---|---|
| NaCl | 7.3625 g | 126 mM |
| KCl | 0.2238 g | 3 mM |
| NaH2PO4·H2O | 0.1725 g | 1.25 mM |
| MgSO4·7H2O | 0.493 g | 2 mM |
| NaHCO3 | 2.184 g | 26 mM |
| Glucosea | 1.8 g | 10 mM |
| CaCl2·2H2O | 2 mM |
For ACSF0, 10 mM glucose is replaced with 10 mM sucrose
2.2. Electrophysiology Setup
Gas tanks: 95% N2/5% CO2 and 95% O2/5% CO2 for optic nerve recordings, 100% N2 for vibration-isolating floating table, and 100% CO2 for mouse anesthesia.
Floating table.
Faraday cage, aspirator glass bottles (500 mL or 250 mL) for ACSF, and shelves on the faraday cage to set the aspirator bottles to hold ACSF solutions.
Tungsten–halogen lamps.
Micromanipulators.
Temperature controller.
Teflon tubing and assembly, clips and flow regulators to control ACSF flow and flow for gases to the interphase chamber.
To make suction electrodes: capillary tubes, Bunsen burner, forceps, and silver wire.
5 mL syringes.
Amplifier, preamplifier, low-noise data acquisition system, and stimulus isolator.
2.3. Optic Nerve Dissection
Scalpel with #10 scalpel blade.
Fine forceps.
Microtapered stainless steel spatula.
Large blunt dissecting scissors.
Sharp pointed dissecting scissors.
Microdissecting scissors.
5 mL syringe filled with ACSFG.
3. Methods
3.1. Electrophysiology Setup for Optic Nerve Recordings
Set up an interface chamber on a vibration-isolating floating table connected to a 100% N2 tank.
Build a Faraday cage open at the front to isolate electrical noise (see Note 6).
Use a tungsten or halogen lamp inside the faraday cage in order to provide adequate lighting to observe the optic nerves under the dissecting microscope and to aid in guiding the nerves to the suction electrodes.
Utilize micromanipulators to hold the electrodes in place during the recordings.
Make stimulating and recording electrodes using glass capillary tubes of appropriate diameter and a Bunsen burner to polish the ends. Attach Teflon tubing to one end of the capillary tube and attach a 5-mL syringe to the other end (see Note 1).
Fill two glass bottles, one each with ACSFG and ACSF0. Aerate the ACSF with 95% N2/5% CO2 and the interface chamber with 95% O2/5% CO2.
Turn on the gas tanks (100% N2) for the floating tables.
Let the ACSF flow through the interface chamber aided by gravity and adjust the flow rate of ACSF through the chamber to 2 mL/min (see Note 7).
Fill the inner part of the interphase chamber with ddH2O.
Turn on the temperature controller attached to the interphase chamber. Wait until the temperature in the chamber equilibrates to 37 °C (see Note 8).
Back-fill the suction electrodes with ACSFG.
Switch on the amplifier, preamplifier, and the data acquisition system.
3.2. Optic Nerve Dissection
Anesthetize animals deeply using CO2 asphyxiation, and using large scissors isolate the head by decapitation.
Make a midline incision dorsally extending from the snout to the base of the skull, and retract the skin flaps to expose the skull and eyeballs.
Carefully loosen the muscle behind the eyeball using a sharp scalpel, and cut behind the eye ball to detach the right and left optic nerves from retina (Fig. 1a).
Starting from the occipital bone, remove the skull to expose the brain.
Using fine forceps or a thin spatula, lift the brain from the rostral end out of the calvaria and let the optic nerves slip out of the optic canal with ease. Note that at this step, optic nerves are still attached at the optic chiasm.
With the base of the brain facing upward, make two cuts on either side of the optic chiasm using fine microscissors to free the optic nerves (Fig. 1a).
Without delay, using fine forceps gently transfer the dissected optic nerves to the interface chamber superfused with ACSFG and bubbled continuously with 95% O2/5% CO2 (Fig. 2a) throughout the experiment while recording from the optic nerve.
While looking through a dissecting microscope, cut the right and left nerves from the optic chiasm using fine microscissors (Fig. 1b).
Trim ½ mm off the retinal and chiasmal ends of the optic nerves.
The nerves are ready to be placed into suction electrodes filled with ACSF for CAP recordings.
3.3. Compound Action Potential Recordings
Use the syringe attached to the suction electrodes to suction the retinal and the chiasmal ends of the optic nerve into the stimulating and the recording electrodes, respectively, taking care not to stretch the nerves (Fig. 2a).
Connect the recording electrode to the amplifier and amplify the signals 20 or 50 times, filter at 3 kHz, and acquire at 20–30 kHz.
Turn on the stimulus isolator and stimulate the retinal end of the nerve using a stimulus pulse strength (30 μs duration) to obtain a CAP with the typical three peaks (Fig. 2b).
Turn up the intensity of stimulation to achieve the maximal CAP amplitude and set the isolator to evoke a CAP every 30 s and start the recording using the digital data acquisition system.
Anoxia: For these experiments, switch the gases from 95% O2/5% CO2 to 95% N2/5% CO2 for the desired duration.
Glucose deprivation or aglycemia: For these experiments, switch ACSFG flowing through the interface chamber to ACSF0 for the desired duration.
Oxygen glucose deprivation (OGD): For these experiments, superfuse the interphase chamber with ACSF0 and aerate the inner chamber with 95% N2/5% CO2 for the desired duration of OGD.
It is possible to add pharmacological agents or drugs to the ACSF during the experiment to test for white matter function under different conditions (see Note 9).
3.4. Compound Action Potential Analysis
Using electrophysiology analysis software, analyze the CAP parameters over the time course of the experiment.
CAP area and time course: To monitor axon function over time, measure the area under the three peaks (Fig. 2b) of the CAP over the course of the experiment.
CAP duration and CAP amplitude: To determine the efficiency of summation of axon potentials in the optic nerve, measure the duration of the three peaks of the CAP (Fig. 2b) and the highest point of each peaks (amplitude).
Time to peak: To determine the conduction velocity of action potentials in the optic nerve, measure the time from the stimulus artifact to the first peak (P1; Fig. 2b) and the distance between the tips of the stimulating and recording electrodes (Fig. 2a) and calculate the conduction velocity (distance/time).
Acknowledgments
This work was supported by grants from National Institute on Aging (NIA) to Selva Baltan and NINDS to Sylvain Brunet and Selva Baltan, as well as a gift from Rose Mary Kubik. Selva Baltan has previously published as Selva Tekkök. The authors thank Dr. Chris Nelson, medical writer, for his help in editing this chapter. Materials and data were provided by the Cleveland Clinic Foundation (CCF). All rights, title, and interest in the materials and data are owned by the CCF.
Footnotes
Note that optic nerves from aging mice are thicker when compared to young mice [14]. Furthermore, rat optic nerves are twice as thick as mouse optic nerves. Accordingly, it is important to create suction electrodes with thicker ends to accommodate thicker nerves.
Rinse all glassware with deionized double-distilled water before use. Any impurities remaining on glassware can significantly impact electrophysiology experiments.
Glucose and NaHCO3 do not easily dissolve. It is important that they completely dissolve. Heating ddH2O to 60 °C helps complete dissolution of these solutes.
CaCl2·2H2O can easily precipitate. Wait until all other ingredients are mixed in the ACSF solution and ensure that pH is adjusted to 7.4 by bubbling with 95% O2/5% CO2 before adding CaCl2·2H2O.
For experiments where optic nerves are exposed to glucose deprivation or OGD, during the period of exposure, nerves are superfused with ACSF with no glucose (ACSF0). This is achieved by replacing glucose with isomolar sucrose (10 mM), which is a disaccharide and thus cannot be utilized by optic nerves for energy metabolism.
To diminish electrical noise during recordings, dip the ground and silver wires used in electrodes into a bleach solution for 10 min, following which they are rinsed in ddH2O.
Using Teflon tubing, connectors, and clamps, build a gravity-driven course for solution from glass bottles to the interphase chamber. The solution will flow over the optic nerve and can be collected in a tray to be discarded.
This step not only ensures the right body temperature for the nerves but also humidifies the air inside the interface chamber such that the optic nerve does not dry out during the experiment.
Pharmacological agents can be added to the ACSFG or ACSF0 during the experiments to test for potential targets causing white matterinjury. At the end of electrophysiology experiments, optic nerves can be immersion-fixed in 4% paraformaldehyde and 0.025% glutaraldehyde solution for 24 h, followed by serial incubation in 10%, 20%, and 30% sucrose solutions in phosphate-buffered saline (pH 7.4), then cryosectioned and used for immunohistochemistry. However, optic nerves can also be pooled at the end of experiments, frozen in liquid nitrogen, and used for protein, DNA, or RNA analysis to study molecular targets in white matter.
References
- 1.Stys PK, Ransom BR, Waxman SG, Davis PK (1990) Role of extracellular calcium in anoxic injury of mammalian central white matter. Proc Natl Acad Sci U S A 87:4212–4216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stys PK, Waxman SG, Ransom BR (1992) Effects of temperature on evoked electrical activity and anoxic injury in CNS white matter. J Cereb Blood Flow Metab 12:977–986. 10.1038/jcbfm.1992.135 [DOI] [PubMed] [Google Scholar]
- 3.Brown AM, Wender R, Ransom BR (2001) Ionic mechanisms of aglycemic axon injury in mammalian central white matter. J Cereb Blood Flow Metab 21:385–395. 10.1097/00004647-200104000-00007 [DOI] [PubMed] [Google Scholar]
- 4.Bastian C, Quinn J, Tripathi A et al. (2018) CK2 inhibition confers functional protection to young and aging axons against ischemia by differentially regulating the CDK5 and AKT signaling pathways. Neurobiol Dis 126:47–61. 10.1016/j.nbd.2018.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastian C, Zaleski J, Stahon K et al. (2018) NOS3 inhibition confers post-ischemic protection to young and aging white matter integrity by conserving mitochondrial dynamics and miro-2 levels. J Neurosci 38:6247–6266. 10.1523/JNEUROSCI.3017-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bastian C, Politano S, Day J et al. (2018) Mitochondrial dynamics and preconditioning in white matter. Cond Med 1:64–72 [PMC free article] [PubMed] [Google Scholar]
- 7.Baltan S, Besancon EF, Mbow B et al. (2008) White matter vulnerability to ischemic injury increases with age because of enhanced excitotoxicity. J Neurosci 28:1479–1489. 10.1523/JNEUROSCI.5137-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baltan S, Murphy SP, Danilov CA et al. (2011) Histone deacetylase inhibitors preserve white matter structure and function during ischemia by conserving ATP and reducing excitotoxicity. J Neurosci 31:3990–3999. 10.1523/JNEUROSCI.5379-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy SP, Lee RJ, McClean ME et al. (2014) MS-275, a class I histone deacetylase inhibitor, protects the p53-deficient mouse against ischemic injury. J Neurochem 129:509–515. 10.1111/jnc.12498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baltan S (2012) Histone deacetylase inhibitors preserve function in aging axons. J Neurochem 123:108–115. 10.1111/j.1471-4159.2012.07949.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garthwaite G, Goodwin DA, Garthwaite J (1999) Nitric oxide stimulates cGMP formation in rat optic nerve axons, providing a specific marker of axon viability. Eur J Neurosci 11:4367–4372 [DOI] [PubMed] [Google Scholar]
- 12.Tekkök SB, Brown AM, Ransom BR (2003) Axon function persists during anoxia in mammalian white matter. J Cereb Blood Flow Metab 23:1340–1347. 10.1097/01.WCB.0000091763.61714.B7 [DOI] [PubMed] [Google Scholar]
- 13.Tekkök SB, Goldberg MP (2001) Ampa/kainate receptor activation mediates hypoxic oligodendrocyte death and axonal injury in cerebral white matter. J Neurosci 21:4237–4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stahon KE, Bastian C, Griffith S et al. (2016) Age-related changes in axonal and mitochondrial ultrastructure and function in white matter. J Neurosci 36:9990–10001. 10.1523/JNEUROSCI.1316-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]


