Abstract
Purpose
To compare the risk of immune-associated pneumonitis between PD-1 and PD-L1 inhibitors, the meta-analysis was designed.
Method
The difference in risk of immune-associated pneumonitis between PD-1 and PD-L1 inhibitors was assessed by two different meta-analysis methods, the Mirror-pairing and the PRISMA guidelines.
Results
A total of eighty-eight reports were used for meta-analysis, while thirty-two studies were used for the Mirror-pairing. Both PD-1 and PD-L1 inhibitors (used alone or combined with chemotherapy) increased the risk of developing immune-related pneumonitis (P < 0.00001; P < 0.00001). Based on indirect analyses results (subgroup analyses), the risk of PD-L1-induced pneumonitis was weaker than that of PD-1 inhibitors when the control group was chemotherapy (OR = 3.33 vs. 5.43) or placebo (OR = 2.53 vs. 3.19), while no obvious significant differences were found (P = 0.17; P = 0.53). For the Mirror-pairing-based meta-analysis, the risk of PD-1-induced pneumonitis was significantly higher than that of PD-L1 inhibitors (OR = 1.46, 95%CI [1.08, 1.98], I2 = 0%, Z = 2.47 (P = 0.01)). However, this difference was not significant, when they were combined with chemotherapy (OR = 1.05, 95%CI [0.68, 1.60], I2 = 38%, Z = 0.21 (P = 0.84)).
Conclusion
Both PD-1 and PD-L1 inhibitors increased the risk of immune-related pneumonitis, while the risk of PD-1-induced pneumonitis was significantly higher than that of PD-L1 inhibitors.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-024-03736-z.
Keywords: PD-1, PD-L1, Pneumonitis, Mirror-principle, Meta-analysis
Introduction
Many clinical trials have confirmed that programmed death-1 (PD-1) or Programmed cell death ligand 1 (PD-L1) inhibitors have excellent clinical efficacy for malignant tumors [1–87]. Due to their unique immune mechanism, many immune-related side effects have been reported as part of clinical trial results [1–87]. Of immunotoxic reactions, pneumonitis was mentioned and evaluated by clinicians for lung cancer patients [1–11, 13, 15, 16, 36–49, 67–71]. Due to the increasing diversity of drug combinations based on PD-1 or PD-L1 inhibitors, assessing the risk factors for pneumonitis have become much more difficult. There were no clinical trials involving a direct comparison between PD-1 and PD-L1 [1–87], which further increased the difficulty of directly comparing the differences in toxicity reactions between PD-1 and PD-L1. However, the report of the Mirror-pairing meta-analysis has made it possible for us to solve this dilemma [88, 89].
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline would also be followed [90]. The results of subgroup analyses were used to test whether the analysis results of the Mirror-pairing were consistent with the PRISMA meta-analysis results. In this study, the differences in the incidence risk of pneumonitis between PD-1 and PD-L1 inhibitors were evaluated by the above two analysis methods (PRISMA and Mirror-pairing). Furthermore, the applicability and reliability of the Mirror-pairing analysis method were further validated [88–90].
Method
The classic PRISMA analysis method was followed and prioritized for the subsequent analyses [90].
Search strategy and screening
The searching process for relevant literature in PubMed was carried out according to the PICOS (participants, interventions, comparisons, outcomes, and study design) references [90]. The searching keywords were not only limited to PD-1 or PD-L1, but also included specific product names and common names of related drugs. All clinical trials without a control group, meaning the single-arm clinical trial, would be excluded first. Randomized and controlled Phase III clinical trials would be prioritized, while other randomized controlled trials were considered as alternatives.
The time frame for all literature was just limited to the past ten years (August 2, 2013–August 2, 2023). The literature searching was completed by four participating authors, and the searching results would be checked by each other. In case of duplicated clinical trials, only one containing the most complete data could be used for the final analysis.
Mirror principle pairing
To increase the similarity and minimize heterogeneity and inconsistency between groups [88, 89], the Mirror-pairing criteria were listed as follows: (1) Tumor type: Due to the significant differences among different tumor types, this is the primary criteria for the Mirror-pairing; (2) Pathological type: Tumors occurring in the same organ need to be distinguished based on specific pathological types. (3) Treatment regimen: In the combination treatment regimens including PD-1 or PD-L1 inhibitors, it is necessary to keep consistent in drug composition of the Mirror-pairing groups; (4) Treatment line; (5) Phase stage; (6) Number of participants: The two paired groups have the same order of magnitude; (7) All clinical trials data could only be used once for the best Mirror-pairing; (8) Results choice: The analyses result involving single drug regimens will be prioritized, while the analyses results of combination therapy regimens are just considered as reference; (9)Other: While the above factors have been confirmed by subgroup analysis to be not the factor causing differences in subgroup analysis results, this factor can be moderately adjusted during pairing.
Evaluation of study quality and publication bias
Egger's test was used to test the symmetry of funnel plots [91, 92], while funnel plots and Harbor's test were used for publication bias evaluation [92, 93]. The Newcastle–Ottawa scale (NOS), recommended by Cochrane Collaboration, was used for quality assessments [94, 95]. The assessments contents were listed as follows: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and others. The evaluation method was to verify the original data of the reported clinical trials. P < 0.05 was considered to be statistically significant.
Exposure of interest, assessment of heterogeneity, and statistical analyses
The basic characteristics of clinical trials that met the inclusion criteria were collected and summarized in a separate table. This study focused on the incidence risk of pneumonitis in all grades.
Heterogeneity was assessed by Cochrane’s Q statistic test [94, 95], including the Mantel–Haenszel method and I2 values suggested by Higgins and colleagues [90, 95]. According to the different I2 values, heterogeneity was divided into three different levels: low (I2 < 25%), medium (I2 = 25–50%), and high (I2 > 50%) [90, 95]. The software Review Manager 5.3 was used for all the following analyses. Due to the inevitable existence of intergroup heterogeneity in the real world, random effects (RE) models were used for calculating odds ratio (OR) and 95% confidence interval (CI) [96]. The fixed effects (FE) model would just be used for funnel plot evaluations. All P values were calculated by two sides. P < 0.05 was deemed to be of statistical significance. Subgroup analysis was conducted based on PD-1 or PD-L1 types. When obvious heterogeneity was discovered, more detailed subgroup analyses would be conducted based on the specific situation. If heterogeneity was considered to be mainly caused by the data itself, further processing of the data would not be carried out, and the original data analysis results would still be adopted.
Results
Literature search results and characteristics of identified trials
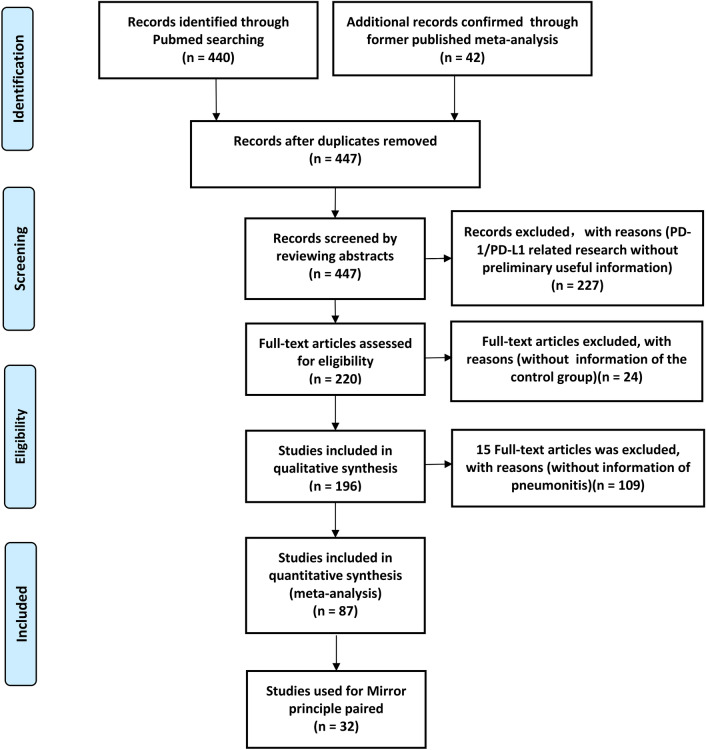
Through PubMed search, 440 studies met the basic criteria. After screening and verification, 87 studies, belonging to 77 clinical trials, were screened for the final comprehensive analyses (STable 1) [1–87], and 32 were selected for the Mirror-pairing (Fig. 1; Table 1) [1, 2, 4, 6–8, 10, 12, 14, 15, 17, 19, 21–24, 36–38, 42, 43, 45, 48, 49, 51, 53, 57, 63, 71, 78, 79]. Of these, 14 clinical trials were reported more than once (KEYNOTE-010 [4, 5], KEYNOTE-042 [12, 13], IMpower110 [14, 15], KEYNOTE‑061[17,18), KEYNOTE-177 [28, 29], KEYNOTE-189 [37, 41], IMpower133 [44, 47], KEYNOTE-355 [57, 62], KEYNOTE-522 [61, 63], IMpassion130 [51, 52], PACIFIC [68, 69], KEYNOTE-054 [72, 73], CheckMate227 [11, 82], CheckMate067 [83–86]). However, only one of the most completed data was used for the final comprehensive analysis or Mirror-pairing. Of all enrolled clinical trials, lung cancer (n = 36; STable 1) [1–16, 35–49, 65–71, 81, 82], including non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), accounted for the highest proportion, followed by esophageal squamous cell carcinoma (ESCC) (n = 7) [25–27, 55, 58–60]. Seventy-one were found to be phase III [1–3, 6–34, 36–73, 75–80, 82–87], two were phase II/III [4, 5], three were phase II [6, 35, 74], and one was phase I/II [81]. Previous treatments were found in 23 clinical trials [1–9, 17, 18, 20, 21, 25–27, 30–33, 68, 69, 77, 80, 81], while PD-1 or PD-L1 inhibitors were prescribed as the first line choice in the other reports [10–16, 19, 22–24, 28, 29, 34–67, 70–76, 78, 79, 82–87]. The quality assessments of all clinical trials were finished and provided in (SFigure 1). Data evaluated as high risk would be excluded (SFigure 1).
Fig. 1.
The flow diagram of the meta-analysis
Table 1.
Basic characteristics of Mirror-pairing clinical trials
| Mirror Group | References | NCT number | Phase | Drug name | Treatment regimen | Treatment lines | Tumor type | Involving patients |
|---|---|---|---|---|---|---|---|---|
| PD-1 VS. PD-L1 | ||||||||
| Group 1 | Reck M,et al. [10] | NCT02142738 (KEYNOTE-024) | III | Pembrolizumab (PD-1) | Pembrolizumab VS. Chemotherapy | 1 | Advanced NSCLC | 154 |
| Jassem J,et al. [15] | NCT02409342 ( IMpower110) | III | Atezolizumab (PD-L1) | Atezolizumab VS. Chemotherapy | Treatment-Naive PD-L1–Selected NSCLC | 286 | ||
| Group 2 | Borghaei H,et al. [1] | NCT01673867 (CheckMate 057) | III | Nivolumab (PD-1) | Nivolumab VS. Docetaxel | 2 | Advanced non-squamous NSCLC | 287 |
| Fehrenbacher L,et al. [6] | NCT01903993 (POPLAR) | II | Atezolizumab (PD-L1) | Atezolizumab VS. Docetaxel | Previously treated NSCLC | 142 | ||
| Group 3 | Wu YL,et al. [12] | NCT02613507 (CheckMate078) | III | Nivolumab (PD-1) | Nivolumab VS. Docetaxel | 2 | Advanced NSCLC | 337 |
| Barlesi F,et al. [8] | NCT02395172 (JAVELIN Lung 200) | III | Avelumab (PD-L1) | Avelumab VS. Docetaxel | platinum-treated advanced NSCLC | 393 | ||
| Group 4 | Brahmer J,et al. [2] | NCT01642004 (CheckMate017) | III | Nivolumab (PD-1) | Nivolumab VS. Docetaxel | 2 | Advanced Squamous Cell NSCLC | 131 |
| Hida T,et al. [7] | NCT02008227 (OAK) | III | Atezolizumab (PD-L1) | Atezolizumab VS. Docetaxel | Advanced NSCLC | 56 | ||
| Group 5 | Bajorin DF,et al. [78] | NCT02632409 (CheckMate 274) | III | Nivolumab (PD-1) | Nivolumab VS Placebo | 1 | muscle-invasive UC | 351 |
| Bellmunt J,et al. [79] | NCT02450331 (IMvigor010) | III | Atezolizumab (PD-L1) | Atezolizumab vs Observation | muscle-invasive UC | 390 | ||
| Group 6 | Powles T,et al. [21] | NCT02853305 (KEYNOTE-361) | III | Pembrolizumab (PD-1) | Pembrolizumab VS Chemotherapy | 1 | advanced UC | 302 |
| Powles T,et al. [23] | NCT02516241 (DANUBE) | III | Durvalumab (PD-L1) | Durvalumab VS Durvalumab + tremelimumab(CTLA-4) | unresectable, locally advanced or metastatic UC | 345 | ||
| Group 7 | Shitara K,et al. [17] | NCT02370498 (KEYNOTE-061) | III | Pembrolizumab (PD-1) | Pembrolizumab VS. Paclitaxel | 2 | Advanced GC or GEJC | 294 |
| Moehler M,et al. [19] | NCT02625610 (JAVELINGastric 100) | III | Avelumab (PD-L1) | Avelumab VS. Chemotherapy | 1 | GC or GEJC | 240 | |
| Group 8 | Herbst RS, [4] | NCT01905657 (KEYNOTE-010) | II/III | Pembrolizumab (PD-1) | Pembrolizumab 10 mg/kg VS. Docetaxel | 2 | PD-L1-positive, advanced NSCLC | 339 |
| Herbst RS, [14] | NCT02409342 (IMpower110) | III | Atezolizumab (PD-L1) | Atezolizumab VS. Chemotherapy | 1 | Metastatic non-squamous or squamous NSCLC | 286 | |
| Group 9 | Herbst RS,et al. [4] | NCT01905657 (KEYNOTE-010) | II/III | Pembrolizumab (PD-1) | Pembrolizumab 2 mg/kg VS Docetaxel | 2 | PD-L1-positive, advanced NSCLC | 339 |
| Felip E,et al. [71] | NCT02486718 (IMpower010) | III | Atezolizumab (PD-L1) | Atezolizumab VS BSC | 1 | Resected stage IB–IIIA NSCLC | 495 | |
| PD-1 + chemotherapy VS. PD-L1 + chemotherapy | ||||||||
| Group 1 + | Gandhi L,et al. [37] | NCT02578680 (KEYNOTE-189) | III | Pembrolizumab (PD-1) | Pembrolizumab + Chemotherapy VS. Chemotherapy | 1 | Metastatic NSCLC | 405 |
| West H,et al. [42] | NCT02367781 (IMpower130) | III | Atezolizumab (PD-L1) | Atezolizumab + Carboplatin + nab-paclitaxel VS. Carboplatin + nab-paclitaxel | 1 | Metastatic non-squamous NSCLC | 473 | |
| Group 2 + | Cheng Y,et al. [48] | NCT04063163 (ASTRUM-005) | III | Serplulimab (PD-1) | Serplulimab + EP VS. EP | 1 | ES -SCLC | 389 |
| Paz-Ares L,et al. [45] | NCT03043872 | III | Durvalumab (PD-L1) | Durvalumab + EP VS. EP | 1 | ES -SCLC | 265 | |
| Group 3 + | Paz-Ares L,et al. [36] | NCT02775435 (KEYNOTE-407) | III | Pembrolizumab (PD-1) | Pembrolizumab + Carboplatin + Paclitaxel VS. Carboplatin + Paclitaxel | 1 | untreated metastatic, squamous NSCLC | 278 |
| Jotte R,et al. [49] | NCT02367794 (IMpower131) | III | Atezolizumab (PD-L1) | Atezolizumab + Carboplatin + Paclitaxel VS. Carboplatin + nab-paclitaxel | 1 | Advanced Squamous NSCLC | 332 | |
| Group 4 + | Zhou C,et al. [38] | NCT03629925 (ORIENT-12) | III | Sintilimab (PD-1) | Sintilimab + GP VS. GP | 1 | Advanced or Metastatic Squamous NSCLC | 179 |
| Zhou C. [43] | NCT03789604 (GEMSTONE-302) | III | Sugemalimab (PD-L1) | Sugemalimab + GP VS. GP | 1 | metastatic NSCLC | 320 | |
| Group 5 + | Powles T,et al. [22] | NCT02853305 (KEYNOTE-361) | III | Pembrolizumab (PD-1) | Pembrolizumab + Chemotherapy VS. Chemotherapy | 1 | Advanced UC | 349 |
| Galsky MD,et al. [24] | NCT02807636 (IMvigor130) | III | Atezolizumab (PD-L1) | Atezolizumab + Chemotherapy VS. Chemotherapy | 1 | Locally advanced or metastatic UC | 453 | |
| Group 6 + | Cortes J,et al. [57] | NCT02819518 (KEYNOTE-355) | III | Pembrolizumab (PD-1) | Pembrolizumab + Chemotherapy VS. Chemotherapy | 1 | Untreated locally recurrent inoperable or metastatic TNBC | 562 |
| Emens LA,et al. [51] | NCT02425891 (IMpassion130) | III | Atezolizumab (PD-L1) | Atezolizumab + nab-paclitaxel VS. nab-paclitaxel | 1 | Unresectable locally advanced, or metastatic TNBC | 460 | |
| Group 7 + | Schmid P,et al. [63] | NCT03036488 (KEYNOTE-522) | III | Pembrolizumab (PD-1) | Pembrolizumab + Chemotherapy VS. Chemotherapy | 1 | Early TNBC | 784 |
| Mittendorf EA,et al. [53] | NCT03197935 (IMpassion031) | III | Atezolizumab (PD-L1) | Atezolizumab + Chemotherapy VS. Chemotherapy | 1 | Early Stage TNBC | 164 | |
PD-L1 = Programmed Cell Death-1; PD-L1 = Programmed Cell Death Ligand 1; CTLA-4 = Cytotoxic T lymphocyte associate protein-4; OSCC = Oesophageal Squamous Cell Carcinoma; UC = Urothelial Cancer; NSCLC = Non-Small Cell Lung Cancer, HNSCC = Head and Neck Squaous Cell Carcinoma, GC/GEJC = Gastric or Gastro-oesophageal Junction Cancer, TNBC = Triple-negative Breast Cancer, SCLC = Small Cell Lung Cancer, HCC = Hepatocellular Carcinoma, RCC = Renal Cell Carcinoma, CRC = Colorectal Cancer; MPM = malignant pleural mesothelioma
According to the composition of treatment regimens, all enrolled clinical trials were divided into five groups for the comprehensive analyses. The specific groupings were listed as follows: Group A (PD-1/PD-L1 VS. Chemotherapy) [1–34], Group B (PD-1/PD-L1 + Chemotherapy VS. Chemotherapy) [35–67], Group C (PD-1/PD-L1 VS. Placebo) [68–80], Group D (PD-1/PD-L1 VS. PD-1/PD-L1 + CTLA-4) [11, 23, 81–86], and Group E (PD-1/PD-L1 VS.PD-1/PD-L1 + Chemotherapy) [19, 21, 24, 34, 82].
Results of mirror pairing
After comprehensive analyses and comparison of all enrolled clinical trials, 32 clinical trials were paired according to the Mirror-pairing principle and divided into 16 groups [1, 2, 4, 6–8, 10, 12, 14, 15, 17, 19, 21–24, 36–38, 42, 43, 45, 48, 49, 51, 53, 57, 63, 71, 78, 79], including 9 pairs for the PD-1 versus PD-L1 group and 7 pairs for the chemotherapy combination group (Table 1). Among these Mirror-pairing clinical trials, NSCLC (n = 17) accounted for the highest proportion [1, 2, 4, 6–8, 10, 12, 14, 15, 36–38, 42, 43, 49, 71], followed by urothelial carcinoma (UC) (n = 6) [21–24, 78, 79].
Risk of pneumonitis
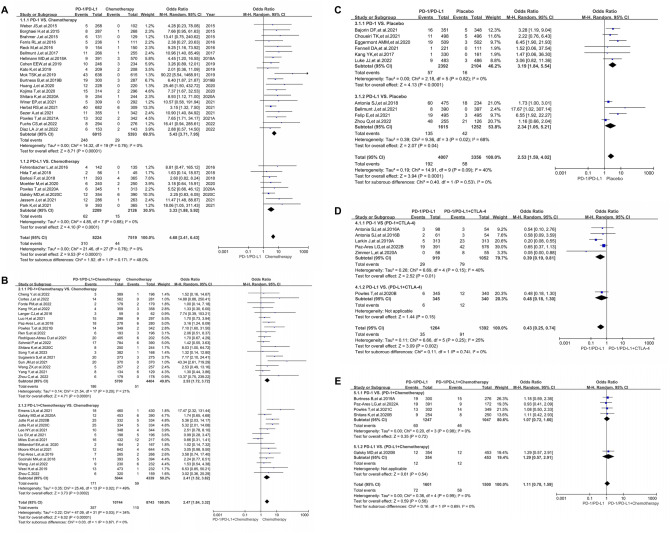
Compared with chemotherapy (PD-1/PD-L1 VS. Chemotherapy), PD-1/PD-L1 inhibitors significantly increased the risk of immune-related pneumonitis (OR = 4.68, 95%CI [3.41, 6.43], I2 = 0%, Z = 9.53 (P < 0.00001); Fig. 2A), and similar risk trend was also found in the subgroup analysis [1–3, 5–11, 13, 15–27, 29–34]. The PD-1 subgroup (OR = 5.43) had a higher risk of developing immune-related pneumonitis than the PD-L1 subgroup (OR = 3.33; Fig. 2A), while there was no statistical significance (P = 0.17). No heterogeneity was found in the above results (I2 = 0%). No significant publication bias was found through the corresponding funnel plots (SFigure 2A). Further subgroup analysis based on different tumor types revealed a higher risk of pneumonitis in the PD-1 subgroup of different tumor types (SFigure 4), especially for the Gastric or Gastro-oesophageal Junction Cancer (GC/GEJC) and UC subgroups.
Fig. 2.
Forest blots of the analysis results for different groups. A The OR of pneumonitis for all-grade checked using the random effect (RE) model in Group A (PD-1/PD-L1 VS. Chemotherapy): Subgroup analyses were carried out according to the types of immune checkpoint inhibitors (PD-1 or PD-L1). B The OR of pneumonitis for all-grade checked using the random effect (RE) model in Group B (PD-1/PD-L1 + Chemotherapy VS. Chemotherapy): Subgroup analyses were carried out according to the types of immune checkpoint inhibitors (PD-1 or PD-L1). C The OR of pneumonitis for all-grade checked using the random effect (RE) model in Group C (PD-1/PD-L1 VS. Placebo): Subgroup analyses were carried out according to the types of immune checkpoint inhibitors (PD-1 or PD-L1). D The OR of pneumonitis for all-grade checked using the random effect (RE) model in Group D (PD-1/PD-L1 VS. PD-1/PD-L1 + CTLA-4): Subgroup analyses were carried out according to the types of immune checkpoint inhibitors (PD-1 or PD-L1). E The OR of pneumonitis for all-grade checked using the random effect (RE) model in Group E (PD-1/PD-L1 VS. PD-1/PD-L1 + Chemotherapy): Subgroup analyses were carried out according to the types of immune checkpoint inhibitors (PD-1 or PD-L1)
When PD-1 or PD-L1 inhibitors combined with chemotherapy were compared with chemotherapy alone (PD-1/PD-L1 + Chemotherapy VS. Chemotherapy), the risk of immune-related pneumonitis was also significantly increased (OR = 2.47, 95%CI [1.84, 3.32], I2 = 34%, Z = 6.02 (P < 0.00001); Fig. 2B) [20, 21, 23, 35, 36, 38–43, 45–51, 53–56, 58–60, 62–67]. A slightly higher risk of developing pneumonitis could be found in the PD-1 subgroup (2.53 vs. 2.41; Fig. 2B). For moderate heterogeneity (I2 = 34%), further subgroup analysis indicated that it might be caused by 3 Triple-negative Breast Cancer (TNBC) clinical trials (IMpassion130, IMpassion031, IMpassion131; SFigure 5) [51, 53, 56]. No significant publication bias was found through the corresponding funnel plots (SFigure 2B).
When the control group was placebo but chemotherapy (PD-1/PD-L1 VS. Placebo), the incidence risk of pneumonitis was also increased by PD-1/PD-L1 inhibitors (OR = 2.53, 95%CI [1.59, 4.02], I2 = 40%, Z = 3.94(P < 0.0001); Fig. 2C) [69–73, 75–80]. Similar to the above, subgroup analysis indicated that the PD-1 subgroup presented a higher risk of developing pneumonitis (3.19 VS. 2.34; Fig. 2C). For moderate heterogeneity (I2 = 40%), further subgroup analysis indicated that it might be caused by three NSCLC clinical trials (PACIFIC, GEMSTONE-301, IMpower010; SFigure 6) [69–71]. No significant publication bias was found through the corresponding funnel plots (SFigure 2C).
Compared with the combination of PD-1/PD-L1 and CTLA-4 (PD-1/PD-L1 VS. PD-1/PD-L1 + CTLA-4), the impact of PD-1/PD-L1 on the risk of pneumonitis was weaker than that of the control group (OR = 0.43, 95%CI [0.25, 0.74], I2 = 25%, Z = 3.09 (P = 0.002); Fig. 2D) [23, 74, 81, 82, 86]. For moderate heterogeneity (I2 = 25%), subgroup analysis suggested that heterogeneity might originate from the data themselves (SFigure 7) [23, 74, 81, 82, 86]. No significant publication bias was found through the corresponding funnel plots (SFigure 2D).
Compared with PD-1/PD-L1 in combination with chemotherapy (PD-1/PD-L1 VS. PD-1/PD-L1 + Chemotherapy), the risk of pneumonitis was not significantly increased (OR = 1.11, 95%CI [0.78, 1.59], I2 = 0%, Z = 0.59 (P = 0.56); Fig. 2E) [19, 21, 24, 34, 82]. No significant publication bias was found through the corresponding funnel plot (SFigure 2E).
Risk of pneumonitis for mirror-pairing
The basic characteristics of 16 Mirror pairings were provided in (Table 1) [1, 2, 4, 6–8, 10, 12, 14, 15, 17, 19, 21–24, 36–38, 42, 43, 45, 48, 49, 51, 53, 57, 63, 71, 78, 79]. Through the Mirror-pairing (n = 9) analysis of PD-1 versus PD-L1, it indicated that PD-1 had a much more significant impact on the risk of pneumonitis (OR = 1.46, 95%CI [1.08, 1.98], I2 = 0%, Z = 2.47 (P = 0.01); Fig. S3A) [1, 2, 4, 6–8, 10, 12, 14, 15, 17, 19, 21, 23, 71, 78, 79]. This risk trend was obviously evident in the UC subgroup (OR = 2.39, 95%CI [1.25, 4.57], I2 = 0%, Z = 2.64 (P = 0.008); Fig. S3A) [21, 23, 78, 79]. No heterogeneity was found. No significant publication bias was found through the corresponding funnel plots (SFigure 3A).
When chemotherapy was added to both experimental and control groups (n = 7), the difference became no longer significant (OR = 1.05, 95%CI [0.68, 1.60], I2 = 38%, Z = 0.21 (P = 0.84); Fig. Fig. S3B) [22, 24, 36–38, 42, 43, 45, 48, 49, 51, 53, 57, 63]. For moderate heterogeneity (I2 = 38%), subgroup analysis suggested that heterogeneity might originate from the NSCLC subgroup (Fig. Fig. S3B) [36–38, 42, 43, 45, 48, 49]. No significant publication bias was found through the corresponding funnel plot (SFigure 3B).
Discussion
With the increasing use of PD-1/PD-L1 inhibitors in clinical practice, the complex and diverse forms of immune-related toxic side effects are increasingly reported and valued by clinical doctors [1–87]. Pneumonitis, as an important clinical event of pulmonary toxicities, requires rapid identification and management. Once suspected, the scope of differential diagnosis between infectious and vegetative processes might make the physician's diagnostic process challenging [97]. A comprehensive assessment of the incidence risk of immune-related pneumonitis would have important guiding significance for physicians. However, due to the lack of clinical trials comparing PD-1 and PD-L1 head to head, it was difficult to determine the differences in risk of pneumonitis occurrence between the two. To address this dilemma, this study was designed [88, 89].
A literature searching was conducted according to PRISMA guidelines and PICOS principles [90], and a total of 77 clinical trial data were collected (Fig. 1; STable 1) [1–87]. Seventy-seven clinical trials were taken into account for a more comprehensive and detailed analysis by grouping in more ways, which increased the possibility of obtaining more Mirror pairings and reduced the possibility of bias due to insufficient data [88, 89]. We carefully reviewed the data of all enrolled clinical trials and conducted a comprehensive systematic evaluation of random sequence generation (selection bias), allocation consideration (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and others. After the comprehensive evaluation, data with any kind of high risk biases would be excluded, and only the high-quality and complete clinical trial data were retained, ensuring the reliability and authenticity of our analyses results (SFigure 1; STable 1). The previous Mirror-pairing principles had been improved[88.89], which would make the pairing much more accurate. After a detailed analysis of clinical trials using the Mirror-pairing principle, 16 Mirror pairings were obtained, which was the largest number of PD-1/PD-L1 related Mirror pairings first reported so far (Table 1; SFigure 3) [1, 2, 4, 6–8, 10, 12, 14, 15, 17, 19, 21–24, 36–38, 42, 43, 45, 48, 49, 51, 53, 57, 63, 71, 78, 79]. These further strengthened the innovation of our research.
Through analysis, regardless of whether the control group was chemotherapy or placebo (Fig. 2A and C), PD-1/PD-L1 inhibitors increased the risk of immune-related pneumonitis [1–3, 5–11, 13, 15–27, 29–34, 69–73, 75–80]. Subgroup analysis indicated that the PD-1 subgroup presented a higher risk of developing pneumonitis (Fig. 2A and C) [1–3, 5–11, 13, 15–27, 29–34, 69–73, 75–80]. Although subgroup analyses could not draw statistically significant conclusions, PD-1 might cause a higher risk trend for pneumonitis (Fig. 2A and C) [1–3, 5–11, 13, 15–27, 29–34, 69–73, 75–80], which laid the foundation for the following Mirror-pairing meta-analysis.
Compared with chemotherapy, the PD-1/PD-L1 inhibitors played a much more important role in increasing the risk of immune-related pneumonitis (Fig. 2B and E) [19–24, 34–36, 38–43, 45–51, 53–56, 58–60, 62–67, 82]. When PD-1/PD-L1 inhibitors were combined with CTLA-4, this effect was obviously evident (Fig. 2D) [23, 74, 81, 82, 86]. Based on the above analyses, we concluded that PD-1/PD-L1 inhibitors increased the risk of pneumonitis; Furthermore, it seemed that PD-1 inhibitors had a higher risk of causing pneumonitis (Fig. 2) [1–87], which further enhanced the necessity of conducting Mirror-pairing analysis.
When using the Mirror-pairing for comparing PD-1 with PD-L1, the risk of pneumonitis caused by PD-1 was significantly higher than that of the PD-L1 group (OR = 1.46, 95%CI [1.08, 1.98], I2 = 0%, Z = 2.47 (P = 0.01); Fig. S3A) [1, 2, 4, 6–8, 10, 12, 14, 15, 17, 19, 21, 23, 71, 78, 79], which the difference was statistically significant. When chemotherapy was incorporated into the Mirror-pairing, this difference became no longer statistically significant (OR = 1.05, 95%CI [0.68, 1.60], I2 = 38%, Z = 0.21 (P = 0.84); Fig. S3B) [22, 24, 36–38, 42, 43, 45, 48, 49, 51, 53, 57, 63]. In the previous subgroup analyses (Fig. 2A and B), similar results could also be found after the addition of chemotherapy. Therefore, we concluded that chemotherapy might induce excessive heterogeneity and inconsistency and desalinate the true differences between PD-1 and PD-L1 (Figs. 2A, B, and S3A). When there were fewer interfering factors, whether it was indirect subgroup analysis (Fig. 2A) or the Mirror-pairing analysis (Fig. S3A), the conclusions drawn were consistent, which further confirmed the practicality and feasibility of this improved Mirror-pairing analysis method [88, 89]. The difference in the risk of pneumonitis between PD-1 and PD-L1 was evaluated using the Mirror-pairing meta-analysis, accompanied by improvements in the Mirror-pairing method, which indicated a better innovation. This comparative method solves the dilemma of lacking head-to-head clinical trials of PD-1 versus PD-L1.
Due to the inevitable existence of intergroup heterogeneity in the real world, RE models were used for OR and 95% CI calculations [96]. Although no highly heterogeneous results were found, we conducted sufficient subgroup analyses and speculated on the source of the corresponding heterogeneity (SFigure 4, SFigure 5, SFigure 6, and SFigure 7) [1–87]. There were no data found that affected the analysis results. Furthermore, no significant bias was found through the corresponding funnel plot (SFigure 2 and SFigure 3), which confirmed the authenticity and reliability of the above analysis results.
Based on the subgroup analysis results (Fig. S3A, SFigure 4, SFigure 5, and SFigure 6), we found that the risk of pneumonitis in UC patients receiving PD-1 inhibitors was the highest among all tumor types. This meant that special attention should be paid to the risk of immune-related pneumonitis for PD-1 inhibitor use in UC patients.
By comparing the subgroup analysis results of the PRISMA meta-analysis with the results of the Mirror-pairing analysis, we found that the risk trend of the analysis results was basically consistent, while the analysis results of the Mirror-pairing seemed to be much more sensitive (Figs. 2A,2C; S3A). It indicated that when mild differences in subgroup analysis was found, the Mirror-pairing analysis could be conducted to clarify the significance of these differences. Furthermore, this would be beneficial for clinicians to determine the choice of drugs (PD-1 or PD-L1) based on the degree of toxicities, as well as whether PD-1 was needed to be replaced by PD-L1.
Conclusions
Both PD-1 and PD-L1 inhibitors increased the risk of immune-related pneumonitis, while the risk of PD-1-induced pneumonitis was significantly higher than that of PD-L1 inhibitors.
The limitations of the study
The Mirror-pairing analysis is an indirect paired comparison of existing clinical trials while minimizing heterogeneity. Its reliability still needs to be validated with more head-to-head clinical trial data in the real world.
Supplementary Information
Below is the link to the electronic supplementary material.
S Table 1: Basic characteristics of all enrolled clinical trials. (DOCX 72 KB)
S Figure 1: Risk of bias summary: review authors' judgements about each risk of bias item for each included study. (TIF 1698 KB)
S Figure 2: Funnel plots of the analysis results for different groups. A: The OR of pneumonitis for all-grade checked using the fixed effect (FE) model in Group A (PD-1/PD-L1 VS. Chemotherapy): Subgroup analyses were carried out according to the types of immune checkpoint inhibitors (PD-1 or PD-L1). B: The OR of pneumonitis for all-grade checked using the fixed effect (FE) model in Group B (PD-1/PD-L1+Chemotherapy VS. Chemotherapy): Subgroup analyses were carried out according to the types of immune checkpoint inhibitors (PD-1 or PD-L1). C: The OR of pneumonitis for all-grade checked using the fixed effect (FE) model in Group C (PD-1/PD-L1 VS. Placebo): Subgroup analyses were carried out according to the types of immune checkpoint inhibitors (PD-1 or PD-L1). D: The OR of pneumonitis for all-grade checked using the fixed effect (FE) model in Group D (PD-1/PD-L1 VS. PD-1/PD-L1+CTLA-4): Subgroup analyses were carried out according to the types of immune checkpoint inhibitors (PD-1 or PD-L1). E: The OR of pneumonitis for all-grade checked using the fixed effect (FE) model in Group E (PD-1/PD-L1 VS. PD-1/PD-L1+Chemotherapy): Subgroup analyses were carried out according to the types of immune checkpoint inhibitors (PD-1 or PD-L1). (TIF 2042 KB)
S Figure 3: Funnel plots of comparison in Mirror-pairing clinical trials. A: The OR of pneumonitis for all grades was checked using the fixed effect (FE) model (PD-1 VS. PD-L1). Subgroup analyses were carried out according to the tumor types. B: The OR of pneumonitis for all grades was checked using the fixed effect (FE) model (PD-1+Chemotherapy VS. PD-L1+Chemotherapy). Subgroup analyses were carried out according to the tumor types. (TIF 2023 KB)
S Figure 4: Forest blots of the subgroup analysis in Group A (PD-1/PD-L1 VS. Chemotherapy): The OR of pneumonitis for all-grade checked using the random effect (RE) model: Subgroup analyses were carried out according to the tumor types and PD-1/PD-L1. (TIF 2856 KB)
S Figure 5: Forest blots of the subgroup analysis in Group B (PD-1/PD-L1+Chemotherapy VS. Chemotherapy): The OR of pneumonitis for all-grade checked using the random effect (RE) model: Subgroup analyses were carried out according to the tumor types and PD-1/PD-L1. (TIF 2913 KB)
S Figure 6: Forest blots of the subgroup analysis in Group C (PD-1/PD-L1 VS. Placebo): The OR of pneumonitis for all-grade checked using the random effect (RE) model: Subgroup analyses were carried out according to the tumor types and PD-1/PD-L1. (TIF 2504 KB)
S Figure 7: Forest blots of the subgroup analysis in Group D (PD-1/PD-L1 VS. PD-1/PD-L1+CTLA-4): The OR of pneumonitis for all-grade checked using the random effect (RE) model: Subgroup analyses were carried out according to the tumor types and PD-1/PD-L1. (TIF 1597 KB)
Acknowledgements
This study was funded by the Clinical Research Special Support Fund of Wu Jieping Medical Foundation (320.6570.2023-16-12; Yuan Tian), Shandong Medical Association Clinical Special Fund—Qilu Special Fund (YXH2022ZX02016; Yuan Tian), and Shandong Second People's Hospital Research Fund (2023MS01; Yuan Tian).
Abbreviations
- CI
Confidence interval
- CnP
Carboplatin + Nab-paclitaxel
- CP
Carboplatin + Paclitaxel
- CRC
Colorectal cancer
- CTLA-4
Cytotoxic T lymphocyte associate protein-4
- EC
Etoposide + Carboplatin
- EP
Etoposide + Platinum
- ESCC
Oesophageal squamous cell carcinoma
- GC/GEJC
Gastric or gastro-oesophageal junction cancer
- GC/GP
Carboplatin/Cisplatin + Gemcitabine
- HCC
Hepatocellular carcinoma
- HNSCC
Head and neck squamous cell carcinoma
- HR
Hazard ratios
- NOS
Newcastle–Ottawa scale
- NSCLC
Non-small cell lung cancer
- OR
Odds ratio
- PD-1
Programmed cell death-1
- PD-L1
Programmed cell death ligand 1
- PF
Cisplatin + Fluorouracil
- PICOS
Participants, interventions, comparisons, outcomes, and study design
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- RCC
Renal cell carcinoma
- RE
Random effect
- SCLC
Small cell lung cancer
- TNBC
Triple-negative breast cancer
- UC
Urothelial cancer
- UC
Urothelial cancer
Author contributions
Qi Dang designed the meta-analysis. Yuan Tian, Zongxiu Yin, Chi Zhang, Zhuoqi Li, Yuanyuan Wang, Kai Zhang, and Feng Chen had the full data of the manuscript. Yuan Tian, Zongxu Yin, Chi Zhang, and Zhuoqi Li conducted literature search and quality evaluation. Yuan Tian drafted the manuscript.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
No ethical issues were involved, and the need for ethical approval was waived.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuan Tian, Zongxiu Yin, Chi Zhang, and Zhuoqi Li contributed equally to this work.
References
- 1.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373(17):1627–1639. 10.1056/NEJMoa1507643 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373(2):123–135. 10.1056/NEJMoa1504627 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu YL, Lu S, Cheng Y, Zhou C, Wang J, Mok T, Zhang L, Tu HY, Wu L, Feng J, Zhang Y, Luft AV, Zhou J, Ma Z, Lu Y, Hu C, Shi Y, Baudelet C, Cai J, Chang J (2019) Nivolumab versus docetaxel in a predominantly chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol 14(5):867–875. 10.1016/j.jtho.2019.01.006. (Epub 2019 Jan 17 PMID: 30659987) 10.1016/j.jtho.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 4.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro GM Jr, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027):1540–1550. 10.1016/S0140-6736(15)01281-7. (Epub 2015 Dec 19 PMID: 26712084) 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 5.Herbst RS, Garon EB, Kim DW, Cho BC, Gervais R, Perez-Gracia JL, Han JY, Majem M, Forster MD, Monnet I, Novello S, Gubens MA, Boyer M, Su WC, Samkari A, Jensen EH, Kobie J, Piperdi B, Baas P (2021) Five year survival update from KEYNOTE-010: pembrolizumab versus docetaxel for previously treated, programmed death-ligand 1-positive advanced NSCLC. J Thorac Oncol 16(10):1718–1732. 10.1016/j.jtho.2021.05.001. (Epub 2021 May 26 PMID: 34048946) 10.1016/j.jtho.2021.05.001 [DOI] [PubMed] [Google Scholar]
- 6.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, Braiteh F, Waterkamp D, He P, Zou W, Chen DS, Yi J, Sandler A, Rittmeyer A (2016) Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387(10030):1837–1846 10.1016/S0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- 7.Hida T, Kaji R, Satouchi M, Ikeda N, Horiike A, Nokihara H, Seto T, Kawakami T, Nakagawa S, Kubo T (2018) Atezolizumab in Japanese patients with previously treated advanced non-small-cell lung cancer: a subgroup analysis of the phase 3 OAK study. Clin Lung Cancer 19(4):e405–e415. 10.1016/j.cllc.2018.01.004. (Epub 2018 Feb 1 PMID: 29525239) 10.1016/j.cllc.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 8.Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, Özgüroğlu M, Szczesna A, Polychronis A, Uslu R, Krzakowski M, Lee JS, Calabrò L, Arén Frontera O, Ellers-Lenz B, Bajars M, Ruisi M, Park K (2018) Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol 19(11):1468–1479. 10.1016/S1470-2045(18)30673-9 10.1016/S1470-2045(18)30673-9 [DOI] [PubMed] [Google Scholar]
- 9.Park K, Özgüroğlu M, Vansteenkiste J, Spigel D, Yang JCH, Ishii H, Garassino M, de Marinis F, Szczesna A, Polychronis A, Uslu R, Krzakowski M, Lee JS, Calabrò L, Arén Frontera O, Xiong H, Bajars M, Ruisi M, Barlesi F (2021) Avelumab versus docetaxel in patients with platinum-treated advanced NSCLC: 2-year follow-up from the JAVELIN lung 200 phase 3 trial. J Thorac Oncol 16(8):1369–1378. 10.1016/j.jtho.2021.03.009. (Epub 2021 Apr 9 PMID: 33845211) 10.1016/j.jtho.2021.03.009 [DOI] [PubMed] [Google Scholar]
- 10.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19):1823–1833. 10.1056/NEJMoa1606774 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 11.Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers S, Salman P, Borghaei H, Ramalingam SS, Brahmer J, Reck M, O’Byrne KJ, Geese WJ, Green G, Chang H, Szustakowski J, Bhagavatheeswaran P, Healey D, Fu Y, Nathan F, Paz-Ares L (2018) Nivolumab plus Ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 378(22):2093–2104. 10.1056/NEJMoa1801946 10.1056/NEJMoa1801946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu YL, Zhang L, Fan Y, Zhou J, Zhang L, Zhou Q, Li W, Hu C, Chen G, Zhang X, Zhou C, Dang T, Sadowski S, Kush DA, Zhou Y, Li B, Mok T (2021) Randomized clinical trial of pembrolizumab vs chemotherapy for previously untreated Chinese patients with PD-L1-positive locally advanced or metastatic non-small-cell lung cancer: KEYNOTE-042 China Study. Int J Cancer 148(9):2313–2320. 10.1002/ijc.33399 10.1002/ijc.33399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK, Bondarenko I, Kubota K, Lubiniecki GM, Zhang J, Kush D, Lopes G (2019) Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 393(10183):1819–1830. 10.1016/S0140-6736(18)32409-7 10.1016/S0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- 14.Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, Morise M, Felip E, Andric Z, Geater S, Özgüroğlu M, Zou W, Sandler A, Enquist I, Komatsubara K, Deng Y, Kuriki H, Wen X, McCleland M, Mocci S, Jassem J, Spigel DR (2020) Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med 383(14):1328–1339. 10.1056/NEJMoa1917346. (PMID: 32997907) 10.1056/NEJMoa1917346 [DOI] [PubMed] [Google Scholar]
- 15.Jassem J, de Marinis F, Giaccone G, Vergnenegre A, Barrios CH, Morise M, Felip E, Oprean C, Kim YC, Andric Z, Mocci S, Enquist I, Komatsubara K, McCleland M, Kuriki H, Villalobos M, Phan S, Spigel DR, Herbst RS (2021) Updated overall survival analysis from IMpower110: atezolizumab versus platinum-based chemotherapy in treatment-naive programmed death-ligand 1-selected NSCLC. J Thorac Oncol 16(11):1872–1882. 10.1016/j.jtho.2021.06.019. (Epub 2021 Jul 12 PMID: 34265434) 10.1016/j.jtho.2021.06.019 [DOI] [PubMed] [Google Scholar]
- 16.Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, Turk HM, Cicin I, Bentsion D, Gladkov O, Clingan P, Sriuranpong V, Rizvi N, Gao B, Li S, Lee S, McGuire K, Chen CI, Makharadze T, Paydas S, Nechaeva M, Seebach F, Weinreich DM, Yancopoulos GD, Gullo G, Lowy I, Rietschel P (2021) Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 397(10274):592–604. 10.1016/S0140-6736(21)00228-2. (PMID: 33581821) 10.1016/S0140-6736(21)00228-2 [DOI] [PubMed] [Google Scholar]
- 17.Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu MH, Fornaro L, Olesiński T, Caglevic C, Chung HC, Muro K, Goekkurt E, Mansoor W, McDermott RS, Shacham-Shmueli E, Chen X, Mayo C, Kang SP, Ohtsu A, Fuchs CS (2018) Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 392(10142):123–133. 10.1016/S0140-6736(18)31257-1. (Epub 2018 Jun 4 PMID: 29880231) 10.1016/S0140-6736(18)31257-1 [DOI] [PubMed] [Google Scholar]
- 18.Fuchs CS, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandala M, Ryu MH, Fornaro L, Olesinski T, Caglevic C, Chung HC, Muro K, Van Cutsem E, Elme A, Thuss-Patience P, Chau I, Ohtsu A, Bhagia P, Wang A, Shih CS, Shitara K (2022) Pembrolizumab versus paclitaxel for previously treated PD-L1-positive advanced gastric or gastroesophageal junction cancer: 2-year update of the randomized phase 3 KEYNOTE-061 trial. Gastric Cancer 25(1):197–206. 10.1007/s10120-021-01227-z 10.1007/s10120-021-01227-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moehler M, Dvorkin M, Boku N, Özgüroğlu M, Ryu MH, Muntean AS, Lonardi S, Nechaeva M, Bragagnoli AC, Coşkun HS, Cubillo Gracian A, Takano T, Wong R, Safran H, Vaccaro GM, Wainberg ZA, Silver MR, Xiong H, Hong J, Taieb J, Bang YJ (2021) Phase III trial of avelumab maintenance after first-line induction chemotherapy versus continuation of chemotherapy in patients with gastric cancers: results from JAVELIN gastric 100. J Clin Oncol 39(9):966–977. 10.1200/JCO.20.00892 10.1200/JCO.20.00892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Lee J, Castro HR, Mansoor W, Braghiroli MI, Karaseva N, Caglevic C, Villanueva L, Goekkurt E, Satake H, Enzinger P, Alsina M, Benson A, Chao J, Ko AH, Wainberg ZA, Kher U, Shah S, Kang SP, Tabernero J (2020) Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 Phase 3 randomized clinical trial. JAMA Oncol 6(10):1571–1580. 10.1001/jamaoncol.2020.3370.PMID:32880601;PMCID:PMC7489405 10.1001/jamaoncol.2020.3370.PMID:32880601;PMCID:PMC7489405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi A, Gerritsen W, Gurney H, Quinn DI, Culine S, Sternberg CN, Mai Y, Poehlein CH, Perini RF, Bajorin DF (2017) Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 376(11):1015–1026. 10.1056/NEJMoa1613683 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powles T, Csőszi T, Özgüroğlu M, Matsubara N, Géczi L, Cheng SY, Fradet Y, Oudard S, Vulsteke C, Morales Barrera R, Fléchon A, Gunduz S, Loriot Y, Rodriguez-Vida A, Mamtani R, Yu EY, Nam K, Imai K, Homet Moreno B, Alva A (2021) Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol 22(7):931–945. 10.1016/S1470-2045(21)00152-2 10.1016/S1470-2045(21)00152-2 [DOI] [PubMed] [Google Scholar]
- 23.Powles T, van der Heijden MS, Castellano D, Galsky MD, Loriot Y, Petrylak DP, Ogawa O, Park SH, Lee JL, De Giorgi U, Bögemann M, Bamias A, Eigl BJ, Gurney H, Mukherjee SD, Fradet Y, Skoneczna I, Tsiatas M, Novikov A, Suárez C, Fay AP, Duran I, Necchi A, Wildsmith S, He P, Angra N, Gupta AK, Levin W, Bellmunt J (2020) Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 21(12):1574–1588. 10.1016/S1470-2045(20)30541-6 10.1016/S1470-2045(20)30541-6 [DOI] [PubMed] [Google Scholar]
- 24.Galsky MD, Arija JÁA, Bamias A, Davis ID, De Santis M, Kikuchi E, Garcia-Del-Muro X, De Giorgi U, Mencinger M, Izumi K, Panni S, Gumus M, Özgüroğlu M, Kalebasty AR, Park SH, Alekseev B, Schutz FA, Li JR, Ye D, Vogelzang NJ, Bernhard S, Tayama D, Mariathasan S, Mecke A, Thåström A, Grande E (2020) Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 395(10236):1547–1557. 10.1016/S0140-6736(20)30230-0. (PMID: 32416780) 10.1016/S0140-6736(20)30230-0 [DOI] [PubMed] [Google Scholar]
- 25.Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, Doi T, Moriwaki T, Kim SB, Lee SH, Bennouna J, Kato K, Shen L, Enzinger P, Qin SK, Ferreira P, Chen J, Girotto G, de la Fouchardiere C, Senellart H, Al-Rajabi R, Lordick F, Wang R, Suryawanshi S, Bhagia P, Kang SP, Metges JP (2020) Randomized Phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol 38(35):4138–4148. 10.1200/JCO.20.01888 10.1200/JCO.20.01888 [DOI] [PubMed] [Google Scholar]
- 26.Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, Yen CC, Kubota Y, Kim SB, Hsu CH, Holtved E, Xynos I, Kodani M, Kitagawa Y (2019) Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20(11):1506–1517. 10.1016/S1470-2045(19)30626-6 10.1016/S1470-2045(19)30626-6 [DOI] [PubMed] [Google Scholar]
- 27.Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, Chen J, Zhang H, Niu Z, Fan Q, Lin L, Gu K, Liu Y, Ba Y, Miao Z, Jiang X, Zeng M, Chen J, Fu Z, Gan L, Wang J, Zhan X, Liu T, Li Z, Shen L, Shu Y, Zhang T, Yang Q, Zou J (2020) Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol 21(6):832–842. 10.1016/S1470-2045(20)30110-8 10.1016/S1470-2045(20)30110-8 [DOI] [PubMed] [Google Scholar]
- 28.André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Bendell J, Le DT, Yoshino T, Van Cutsem E, Yang P, Farooqui MZH, Marinello P, Diaz LA Jr (2020) Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med 383(23):2207–2218. 10.1056/NEJMoa2017699 10.1056/NEJMoa2017699 [DOI] [PubMed] [Google Scholar]
- 29.Diaz LA Jr, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fourchardiere C, Rivera F, Elez E, Le DT, Yoshino T, Zhong WY, Fogelman D, Marinello P, Andre T (2022) Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol 23(5):659–670. 10.1016/S1470-2045(22)00197-8 10.1016/S1470-2045(22)00197-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winer EP, Lipatov O, Im SA, Goncalves A, Muñoz-Couselo E, Lee KS, Schmid P, Tamura K, Testa L, Witzel I, Ohtani S, Turner N, Zambelli S, Harbeck N, Andre F, Dent R, Zhou X, Karantza V, Mejia J, Cortes J (2021) Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol 22(4):499–511. 10.1016/S1470-2045(20)30754-3 10.1016/S1470-2045(20)30754-3 [DOI] [PubMed] [Google Scholar]
- 31.Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD, Linette GP, Thomas L, Lorigan P, Grossmann KF, Hassel JC, Maio M, Sznol M, Ascierto PA, Mohr P, Chmielowski B, Bryce A, Svane IM, Grob JJ, Krackhardt AM, Horak C, Lambert A, Yang AS, Larkin J (2015) Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 16(4):375–384. 10.1016/S1470-2045(15)70076-8. (Epub 2015 Mar 18 PMID: 25795410) 10.1016/S1470-2045(15)70076-8 [DOI] [PubMed] [Google Scholar]
- 32.Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R, Burtness B, Zhang P, Cheng J, Swaby RF, Harrington KJ (2019) Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 393(10167):156–167. 10.1016/S0140-6736(18)31999-8 10.1016/S0140-6736(18)31999-8 [DOI] [PubMed] [Google Scholar]
- 33.Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML (2016) Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 375(19):1856–1867. 10.1056/NEJMoa1602252 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro GM Jr, Psyrri A, Basté N, Neupane P, Bratland Å, Fuereder T, Hughes BGM, Mesía R, Ngamphaiboon N, Rordorf T, Wan Ishak WZ, Hong RL, González Mendoza R, Roy A, Zhang Y, Gumuscu B, Cheng JD, Jin F, Rischin D (2019) Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394(10212):1915–1928. 10.1016/S0140-6736(19)32591-7 10.1016/S0140-6736(19)32591-7 [DOI] [PubMed] [Google Scholar]
- 35.Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI, Panwalkar A, Yang JC, Gubens M, Sequist LV, Awad MM, Fiore J, Ge Y, Raftopoulos H, Gandhi L (2016) Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 17(11):1497–1508. 10.1016/S1470-2045(16)30498-3 10.1016/S1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, Rodríguez-Cid J, Wilson J, Sugawara S, Kato T, Lee KH, Cheng Y, Novello S, Halmos B, Li X, Lubiniecki GM, Piperdi B, Kowalski DM (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379(21):2040–2051. 10.1056/NEJMoa1810865 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- 37.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092. 10.1056/NEJMoa1801005 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 38.Zhou C, Wu L, Fan Y, Wang Z, Liu L, Chen G, Zhang L, Huang D, Cang S, Yang Z, Zhou J, Zhou C, Li B, Li J, Fan M, Cui J, Li Y, Zhao H, Fang J, Xue J, Hu C, Sun P, Du Y, Zhou H, Wang S, Zhang W (2021) Sintilimab plus platinum and gemcitabine as first-line treatment for advanced or metastatic squamous nsclc: results from a randomized, double-blind, phase 3 trial (ORIENT-12). J Thorac Oncol 16(9):1501–1511. 10.1016/j.jtho.2021.04.011. (Epub 2021 May 25 PMID: 34048947) 10.1016/j.jtho.2021.04.011 [DOI] [PubMed] [Google Scholar]
- 39.Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson SJ, Kerr K, Wang C, Ciuleanu TE, Saylors GB, Tanaka F, Ito H, Chen KN, Liberman M, Vokes EE, Taube JM, Dorange C, Cai J, Fiore J, Jarkowski A, Balli D, Sausen M, Pandya D, Calvet CY, Girard N (2022) Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med 386(21):1973–1985. 10.1056/NEJMoa2202170 10.1056/NEJMoa2202170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ren S, Chen J, Xu X, Jiang T, Cheng Y, Chen G, Pan Y, Fang Y, Wang Q, Huang Y, Yao W, Wang R, Li X, Zhang W, Zhang Y, Hu S, Guo R, Shi J, Wang Z, Cao P, Wang D, Fang J, Luo H, Geng Y, Xing C, Lv D, Zhang Y, Yu J, Cang S, Yang Z, Shi W, Zou J, Zhou C (2022) Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC (CameL-Sq): A Phase 3 Trial. J Thorac Oncol 17(4):544–557. 10.1016/j.jtho.2021.11.018 10.1016/j.jtho.2021.11.018 [DOI] [PubMed] [Google Scholar]
- 41.Rodríguez-Abreu D, Powell SF, Hochmair MJ, Gadgeel S, Esteban E, Felip E, Speranza G, De Angelis F, Dómine M, Cheng SY, Bischoff HG, Peled N, Reck M, Hui R, Garon EB, Boyer M, Kurata T, Yang J, Pietanza MC, Souza F, Garassino MC (2021) Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol 32(7):881–895. 10.1016/j.annonc.2021.04.008. (Epub 2021 Apr 22 PMID: 33894335) 10.1016/j.annonc.2021.04.008 [DOI] [PubMed] [Google Scholar]
- 42.West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Kopp HG, Daniel D, McCune S, Mekhail T, Zer A, Reinmuth N, Sadiq A, Sandler A, Lin W, Ochi Lohmann T, Archer V, Wang L, Kowanetz M, Cappuzzo F (2019) Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20(7):924–937. 10.1016/S1470-2045(19)30167-6. (Epub 2019 May 20 PMID: 31122901) 10.1016/S1470-2045(19)30167-6 [DOI] [PubMed] [Google Scholar]
- 43.Zhou C, Wang Z, Sun Y, Cao L, Ma Z, Wu R, Yu Y, Yao W, Chang J, Chen J, Zhuang W, Cui J, Chen X, Lu Y, Shen H, Wang J, Li P, Qin M, Lu D, Yang J (2022) Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol 23(2):220–233. 10.1016/S1470-2045(21)00650-1. (Epub 2022 Jan 14 PMID: 35038432) 10.1016/S1470-2045(21)00650-1 [DOI] [PubMed] [Google Scholar]
- 44.Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio M, Reck M, Mok T, Lam S, Shames DS, Liu J, Ding B, Lopez-Chavez A, Kabbinavar F, Lin W, Sandler A, Liu SV (2018) First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379(23):2220–2229. 10.1056/NEJMoa1809064 10.1056/NEJMoa1809064 [DOI] [PubMed] [Google Scholar]
- 45.Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH, Voitko O, Poltoratskiy A, Ponce S, Verderame F, Havel L, Bondarenko I, Kazarnowicz A, Losonczy G, Conev NV, Armstrong J, Byrne N, Shire N, Jiang H, Goldman JW (2019) Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 394(10212):1929–1939. 10.1016/S0140-6736(19)32222-6 10.1016/S0140-6736(19)32222-6 [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Zhou C, Yao W, Wang Q, Min X, Chen G, Xu X, Li X, Xu F, Fang Y, Yang R, Yu G, Gong Y, Zhao J, Fan Y, Liu Q, Cao L, Yao Y, Liu Y, Li X, Wu J, He Z, Lu K, Jiang L, Hu C, Zhao W, Zhang B, Shi W, Zhang X, Cheng Y (2022) Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 23(6):739–747. 10.1016/S1470-2045(22)00224-8 10.1016/S1470-2045(22)00224-8 [DOI] [PubMed] [Google Scholar]
- 47.Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, Garassino MC, De Castro CJ, Califano R, Nishio M, Orlandi F, Alatorre-Alexander J, Leal T, Cheng Y, Lee JS, Lam S, McCleland M, Deng Y, Phan S, Horn L (2021) Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol 39(6):619–630. 10.1200/JCO.20.01055 10.1200/JCO.20.01055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng Y, Han L, Wu L, Chen J, Sun H, Wen G, Ji Y, Dvorkin M, Shi J, Pan Z, Shi J, Wang X, Bai Y, Melkadze T, Pan Y, Min X, Viguro M, Li X, Zhao Y, Yang J, Makharadze T, Arkania E, Kang W, Wang Q, Zhu J (2022) Effect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer: The ASTRUM-005 randomized clinical trial. JAMA 328(12):1223–1232. 10.1001/jama.2022.16464 10.1001/jama.2022.16464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodríguez-Abreu D, Hussein M, Soo R, Conter HJ, Kozuki T, Huang KC, Graupner V, Sun SW, Hoang T, Jessop H, McCleland M, Ballinger M, Sandler A, Socinski MA (2020) Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III Trial. J Thorac Oncol 15(8):1351–1360. 10.1016/j.jtho.2020.03.028. (Epub 2020 Apr 14 PMID: 32302702) 10.1016/j.jtho.2020.03.028 [DOI] [PubMed] [Google Scholar]
- 50.Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, Chung IJ, Yamaguchi K, Kato K, Sym SJ, Kadowaki S, Tsuji K, Chen JS, Bai LY, Oh SY, Choda Y, Yasui H, Takeuchi K, Hirashima Y, Hagihara S, Boku N (2022) Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 23(2):234–247. 10.1016/S1470-2045(21)00692-6. (Epub 2022 Jan 11 PMID: 35030335) 10.1016/S1470-2045(21)00692-6 [DOI] [PubMed] [Google Scholar]
- 51.Emens LA, Adams S, Barrios CH, Diéras V, Iwata H, Loi S, Rugo HS, Schneeweiss A, Winer EP, Patel S, Henschel V, Swat A, Kaul M, Molinero L, Patel S, Chui SY, Schmid P (2021) First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann Oncol 32(8):983–993. 10.1016/j.annonc.2021.05.355 10.1016/j.annonc.2021.05.355 [DOI] [PubMed] [Google Scholar]
- 52.Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Henschel V, Molinero L, Chui SY, Maiya V, Husain A, Winer EP, Loi S, Emens LA (2020) Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 21(1):44–59. 10.1016/S1470-2045(19)30689-8 10.1016/S1470-2045(19)30689-8 [DOI] [PubMed] [Google Scholar]
- 53.Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, Koehler A, Sohn J, Iwata H, Telli ML, Ferrario C, Punie K, Penault-Llorca F, Patel S, Duc AN, Liste-Hermoso M, Maiya V, Molinero L, Chui SY, Harbeck N (2020) Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet 396(10257):1090–1100. 10.1016/S0140-6736(20)31953-X. (Epub 2020 Sep 20 PMID: 32966830) 10.1016/S0140-6736(20)31953-X [DOI] [PubMed] [Google Scholar]
- 54.Yang Y, Qu S, Li J, Hu C, Xu M, Li W, Zhou T, Shen L, Wu H, Lang J, Hu G, Luo Z, Fu Z, Qu S, Feng W, Chen X, Lin S, Zhang W, Li X, Sun Y, Lin Z, Lin Q, Lei F, Long J, Hong J, Huang X, Zeng L, Wang P, He X, Zhang B, Yang Q, Zhang X, Zou J, Fang W, Zhang L (2021) Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 22(8):1162–1174. 10.1016/S1470-2045(21)00302-8. (Epub 2021 Jun 23 PMID: 34174189) 10.1016/S1470-2045(21)00302-8 [DOI] [PubMed] [Google Scholar]
- 55.Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, Zhang Y, Zhao K, Chen Z, Gao S, Li J, Fu Z, Gu K, Liu Z, Wu L, Zhang X, Feng J, Niu Z, Ba Y, Zhang H, Liu Y, Zhang L, Min X, Huang J, Cheng Y, Wang D, Shen Y, Yang Q, Zou J, Xu RH (2021) Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA 326(10):916–925. 10.1001/jama.2021.12836 10.1001/jama.2021.12836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miles D, Gligorov J, André F, Cameron D, Schneeweiss A, Barrios C, Xu B, Wardley A, Kaen D, Andrade L, Semiglazov V, Reinisch M, Patel S, Patre M, Morales L, Patel SL, Kaul M, Barata T, O’Shaughnessy J (2021) Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol 32(8):994–1004. 10.1016/j.annonc.2021.05.801. (Epub 2021 Jul 1 PMID: 34219000) 10.1016/j.annonc.2021.05.801 [DOI] [PubMed] [Google Scholar]
- 57.Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Holgado E, Iwata H, Masuda N, Otero MT, Gokmen E, Loi S, Guo Z, Zhao J, Aktan G, Karantza V, Schmid P (2020) Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396(10265):1817–1828. 10.1016/S0140-6736(20)32531-9 10.1016/S0140-6736(20)32531-9 [DOI] [PubMed] [Google Scholar]
- 58.Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, Cho BC, Mansoor W, Li SH, Sunpaweravong P, Maqueda MA, Goekkurt E, Hara H, Antunes L, Fountzilas C, Tsuji A, Oliden VC, Liu Q, Shah S, Bhagia P, Kato K (2021) Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 398(10302):759–771. 10.1016/S0140-6736(21)01234-4 10.1016/S0140-6736(21)01234-4 [DOI] [PubMed] [Google Scholar]
- 59.Song Y, Zhang B, Xin D, Kou X, Tan Z, Zhang S, Sun M, Zhou J, Fan M, Zhang M, Song Y, Li S, Yuan Y, Zhuang W, Zhang J, Zhang L, Jiang H, Gu K, Ye H, Ke Y, Li J, Wang Q, Zhu J, Huang J (2023) First-line serplulimab or placebo plus chemotherapy in PD-L1-positive esophageal squamous cell carcinoma: a randomized, double-blind phase 3 trial. Nat Med 29(2):473–482. 10.1038/s41591-022-02179-2 10.1038/s41591-022-02179-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng J, Yang S, Fan Y, Shi J, Zhang X, Shen L, Shu Y, Wang C, Dai T, Mao T, Chen L, Guo Z, Liu B, Pan H, Cang S, Jiang Y, Wang J, Ye M, Chen Z, Jiang D, Lin Q, Ren W, Wang J, Wu L, Xu Y, Miao Z, Sun M, Xie C, Liu Y, Wang Q, Zhao L, Li Q, Huang C, Jiang K, Yang K, Li D, Liu Y, Zhu Z, Chen R, Jia L, Li W, Liao W, Liu HX, Ma D, Ma J, Qin Y, Shi Z, Wei Q, Xiao K, Zhang Y, Zhang Y, Chen X, Dai G, He J, Li J, Li G, Liu Y, Liu Z, Yuan X, Zhang J, Fu Z, He Y, Ju F, Liu Z, Tang P, Wang T, Wang W, Zhang J, Luo X, Tang X, May R, Feng H, Yao S, Keegan P, Xu RH, Wang F (2022) Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell 40(3):277-288.e3. 10.1016/j.ccell.2022.02.007. (Epub 2022 Mar 3 PMID: 35245446) 10.1016/j.ccell.2022.02.007 [DOI] [PubMed] [Google Scholar]
- 61.Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, Takahashi M, Foukakis T, Fasching PA, Cardoso F, Untch M, Jia L, Karantza V, Zhao J, Aktan G, Dent R, O’Shaughnessy J (2020) Pembrolizumab for early triple-negative breast cancer. N Engl J Med 382(9):810–821. 10.1056/NEJMoa1910549 10.1056/NEJMoa1910549 [DOI] [PubMed] [Google Scholar]
- 62.Cortes J, Rugo HS, Cescon DW, Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Perez-Garcia J, Iwata H, Masuda N, Torregroza Otero M, Gokmen E, Loi S, Guo Z, Zhou X, Karantza V, Pan W, Schmid P (2022) Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med 387(3):217–226. 10.1056/NEJMoa2202809 10.1056/NEJMoa2202809 [DOI] [PubMed] [Google Scholar]
- 63.Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, Takahashi M, Untch M, Fasching PA, Cardoso F, Andersen J, Patt D, Danso M, Ferreira M, Mouret-Reynier MA, Im SA, Ahn JH, Gion M, Baron-Hay S, Boileau JF, Ding Y, Tryfonidis K, Aktan G, Karantza V, O’Shaughnessy J (2022) Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med 386(6):556–567. 10.1056/NEJMoa2112651 10.1056/NEJMoa2112651 [DOI] [PubMed] [Google Scholar]
- 64.Lee NY, Ferris RL, Psyrri A, Haddad RI, Tahara M, Bourhis J, Harrington K, Chang PM, Lin JC, Razaq MA, Teixeira MM, Lövey J, Chamois J, Rueda A, Hu C, Dunn LA, Dvorkin MV, De Beukelaer S, Pavlov D, Thurm H, Cohen E (2021) Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol 22(4):450–462. 10.1016/S1470-2045(20)30737-3. (PMID: 33794205) 10.1016/S1470-2045(20)30737-3 [DOI] [PubMed] [Google Scholar]
- 65.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Kelsch C, Lee A, Coleman S, Deng Y, Shen Y, Kowanetz M, Lopez-Chavez A, Sandler A, Reck M (2018) Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378(24):2288–2301. 10.1056/NEJMoa1716948. (Epub 2018 Jun 4 PMID: 29863955) 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 66.Moore KN, Bookman M, Sehouli J, Miller A, Anderson C, Scambia G, Myers T, Taskiran C, Robison K, Mäenpää J, Willmott L, Colombo N, Thomes-Pepin J, Liontos M, Gold MA, Garcia Y, Sharma SK, Darus CJ, Aghajanian C, Okamoto A, Wu X, Safin R, Wu F, Molinero L, Maiya V, Khor VK, Lin YG, Pignata S (2021) Atezolizumab, bevacizumab, and chemotherapy for newly diagnosed stage iii or iv ovarian cancer: placebo-controlled randomized Phase III Trial (IMagyn050/GOG 3015/ENGOT-OV39). J Clin Oncol 39(17):1842–1855. 10.1200/JCO.21.00306 10.1200/JCO.21.00306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sugawara S, Lee JS, Kang JH, Kim HR, Inui N, Hida T, Lee KH, Yoshida T, Tanaka H, Yang CT, Nishio M, Ohe Y, Tamura T, Yamamoto N, Yu CJ, Akamatsu H, Namba Y, Sumiyoshi N, Nakagawa K (2021) Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann Oncol 32(9):1137–1147. 10.1016/j.annonc.2021.06.004. (Epub 2021 Jun 15 PMID: 34139272) 10.1016/j.annonc.2021.06.004 [DOI] [PubMed] [Google Scholar]
- 68.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro CJ, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M (2017) Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 377(20):1919–1929. 10.1056/NEJMoa1709937 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 69.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro CJ, Faivre-Finn C, Reck M, Vansteenkiste J, Spigel DR, Wadsworth C, Melillo G, Taboada M, Dennis PA, Özgüroğlu M (2018) Overall Survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 379(24):2342–2350. 10.1056/NEJMoa1809697 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
- 70.Zhou Q, Chen M, Jiang O, Pan Y, Hu D, Lin Q, Wu G, Cui J, Chang J, Cheng Y, Huang C, Liu A, Yang N, Gong Y, Zhu C, Ma Z, Fang J, Chen G, Zhao J, Shi A, Lin Y, Li G, Liu Y, Wang D, Wu R, Xu X, Shi J, Liu Z, Cui N, Wang J, Wang Q, Zhang R, Yang J, Wu YL (2022) Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): interim results of a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 23(2):209–219. 10.1016/S1470-2045(21)00630-6. (Epub 2022 Jan 14 PMID: 35038429) 10.1016/S1470-2045(21)00630-6 [DOI] [PubMed] [Google Scholar]
- 71.Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, Luft A, Akopov A, Martinez-Marti A, Kenmotsu H, Chen YM, Chella A, Sugawara S, Voong D, Wu F, Yi J, Deng Y, McCleland M, Bennett E, Gitlitz B, Wakelee H (2021) Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 398(10308):1344–1357. 10.1016/S0140-6736(21)02098-5 10.1016/S0140-6736(21)02098-5 [DOI] [PubMed] [Google Scholar]
- 72.Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, Haydon A, Lichinitser M, Khattak A, Carlino MS, Sandhu S, Larkin J, Puig S, Ascierto PA, Rutkowski P, Schadendorf D, Koornstra R, Hernandez-Aya L, Maio M, van den Eertwegh AJM, Grob JJ, Gutzmer R, Jamal R, Lorigan P, Ibrahim N, Marreaud S, van Akkooi ACJ, Suciu S, Robert C (2018) Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 378(19):1789–1801. 10.1056/NEJMoa1802357. (Epub 2018 Apr 15 PMID: 29658430) 10.1056/NEJMoa1802357 [DOI] [PubMed] [Google Scholar]
- 73.Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson VG, Dalle S, Haydon AM, Meshcheryakov A, Khattak A, Carlino MS, Sandhu S, Larkin J, Puig S, Ascierto PA, Rutkowski P, Schadendorf D, Koornstra R, Hernandez-Aya L, Di Giacomo AM, van den Eertwegh AJM, Grob JJ, Gutzmer R, Jamal R, Lorigan PC, van Akkooi ACJ, Krepler C, Ibrahim N, Marreaud S, Kicinski M, Suciu S, Robert C (2020) Longer follow-up confirms recurrence-free survival benefit of adjuvant pembrolizumab in high-risk stage iii melanoma: updated results from the EORTC 1325-MG/KEYNOTE-054 Trial. J Clin Oncol 38(33):3925–3936. 10.1200/JCO.20.02110 10.1200/JCO.20.02110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zimmer L, Livingstone E, Hassel JC, Fluck M, Eigentler T, Loquai C, Haferkamp S, Gutzmer R, Meier F, Mohr P, Hauschild A, Schilling B, Menzer C, Kieker F, Dippel E, Rösch A, Simon JC, Conrad B, Körner S, Windemuth-Kieselbach C, Schwarz L, Garbe C, Becker JC, Schadendorf D (2020) Adjuvant nivolumab plus ipilimumab or nivolumab monotherapy versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 395(10236):1558–1568. 10.1016/S0140-6736(20)30417-7 10.1016/S0140-6736(20)30417-7 [DOI] [PubMed] [Google Scholar]
- 75.Luke JJ, Rutkowski P, Queirolo P, Del Vecchio M, Mackiewicz J, Chiarion-Sileni V, de la Cruz ML, Khattak MA, Schadendorf D, Long GV, Ascierto PA, Mandala M, De Galitiis F, Haydon A, Dummer R, Grob JJ, Robert C, Carlino MS, Mohr P, Poklepovic A, Sondak VK, Scolyer RA, Kirkwood JM, Chen K, Diede SJ, Ahsan S, Ibrahim N, Eggermont AMM (2022) Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): a randomised, double-blind, phase 3 trial. Lancet 399(10336):1718–1729. 10.1016/S0140-6736(22)00562-1 10.1016/S0140-6736(22)00562-1 [DOI] [PubMed] [Google Scholar]
- 76.Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, Chang YH, Hajek J, Symeonides SN, Lee JL, Sarwar N, Thiery-Vuillemin A, Gross-Goupil M, Mahave M, Haas NB, Sawrycki P, Gurney H, Chevreau C, Melichar B, Kopyltsov E, Alva A, Burke JM, Doshi G, Topart D, Oudard S, Hammers H, Kitamura H, Bedke J, Perini RF, Zhang P, Imai K, Willemann-Rogerio J, Quinn DI, Powles T (2021) Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med 385(8):683–694. 10.1056/NEJMoa2106391 10.1056/NEJMoa2106391 [DOI] [PubMed] [Google Scholar]
- 77.Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT (2017) Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390(10111):2461–2471. 10.1016/S0140-6736(17)31827-5. (Epub 2017 Oct 6 PMID: 28993052) 10.1016/S0140-6736(17)31827-5 [DOI] [PubMed] [Google Scholar]
- 78.Bajorin DF, Witjes JA, Gschwend JE, Schenker M, Valderrama BP, Tomita Y, Bamias A, Lebret T, Shariat SF, Park SH, Ye D, Agerbaek M, Enting D, McDermott R, Gajate P, Peer A, Milowsky MI, Nosov A, Neif Antonio J Jr, Tupikowski K, Toms L, Fischer BS, Qureshi A, Collette S, Unsal-Kacmaz K, Broughton E, Zardavas D, Koon HB, Galsky MD (2021) Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med 384(22):2102–2114. 10.1056/NEJMoa2034442.Erratum.In:NEnglJMed.2021Aug26;385(9):864.PMID:34077643;PMCID:PMC8215888 10.1056/NEJMoa2034442.Erratum.In:NEnglJMed.2021Aug26;385(9):864.PMID:34077643;PMCID:PMC8215888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bellmunt J, Hussain M, Gschwend JE, Albers P, Oudard S, Castellano D, Daneshmand S, Nishiyama H, Majchrowicz M, Degaonkar V, Shi Y, Mariathasan S, Grivas P, Drakaki A, O’Donnell PH, Rosenberg JE, Geynisman DM, Petrylak DP, Hoffman-Censits J, Bedke J, Kalebasty AR, Zakharia Y, van der Heijden MS, Sternberg CN, Davarpanah NN, Powles T (2021) Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 22(4):525–537. 10.1016/S1470-2045(21)00004-8 10.1016/S1470-2045(21)00004-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fennell DA, Ewings S, Ottensmeier C, Califano R, Hanna GG, Hill K, Danson S, Steele N, Nye M, Johnson L, Lord J, Middleton C, Szlosarek P, Chan S, Gaba A, Darlison L, Wells-Jordan P, Richards C, Poile C, Lester JF, Griffiths G (2021) Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol 22(11):1530–1540. 10.1016/S1470-2045(21)00471-X 10.1016/S1470-2045(21)00471-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, Jäger D, Pietanza MC, Le DT, de Braud F, Morse MA, Ascierto PA, Horn L, Amin A, Pillai RN, Evans J, Chau I, Bono P, Atmaca A, Sharma P, Harbison CT, Lin CS, Christensen O, Calvo E (2016) Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 17(7):883–895. 10.1016/S1470-2045(16)30098-5 10.1016/S1470-2045(16)30098-5 [DOI] [PubMed] [Google Scholar]
- 82.Paz-Ares LG, Ramalingam SS, Ciuleanu TE, Lee JS, Urban L, Caro RB, Park K, Sakai H, Ohe Y, Nishio M, Audigier-Valette C, Burgers JA, Pluzanski A, Sangha R, Gallardo C, Takeda M, Linardou H, Lupinacci L, Lee KH, Caserta C, Provencio M, Carcereny E, Otterson GA, Schenker M, Zurawski B, Alexandru A, Vergnenegre A, Raimbourg J, Feeney K, Kim SW, Borghaei H, O’Byrne KJ, Hellmann MD, Memaj A, Nathan FE, Bushong J, Tran P, Brahmer JR, Reck M (2022) First-line nivolumab plus ipilimumab in advanced NSCLC: 4-year outcomes from the randomized, open-label, Phase 3 checkmate 227 Part 1 trial. J Thorac Oncol 17(2):289–308. 10.1016/j.jtho.2021.09.010. (Epub 2021 Oct 12 PMID: 34648948) 10.1016/j.jtho.2021.09.010 [DOI] [PubMed] [Google Scholar]
- 83.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373(1):23–34. 10.1056/NEJMoa1504030 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino MS, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbé C, Ascierto PA, Long GV, Cebon J, Sosman J, Postow MA, Callahan MK, Walker D, Rollin L, Bhore R, Hodi FS, Larkin J (2017) Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 377(14):1345–1356. 10.1056/NEJMoa1709684 10.1056/NEJMoa1709684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, Lao CD, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hill A, Hogg D, Marquez-Rodas I, Jiang J, Rizzo J, Larkin J, Wolchok JD (2018) Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 19(11):1480–1492. 10.1016/S1470-2045(18)30700-9 10.1016/S1470-2045(18)30700-9 [DOI] [PubMed] [Google Scholar]
- 86.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hogg D, Hill A, Márquez-Rodas I, Haanen J, Guidoboni M, Maio M, Schöffski P, Carlino MS, Lebbé C, McArthur G, Ascierto PA, Daniels GA, Long GV, Bastholt L, Rizzo JI, Balogh A, Moshyk A, Hodi FS, Wolchok JD (2019) Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381(16):1535–1546. 10.1056/NEJMoa1910836. (Epub 2019 Sep 28 PMID: 31562797) 10.1056/NEJMoa1910836 [DOI] [PubMed] [Google Scholar]
- 87.Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, Kalofonos H, Radulović S, Demey W, Ullén A, Loriot Y, Sridhar SS, Tsuchiya N, Kopyltsov E, Sternberg CN, Bellmunt J, Aragon-Ching JB, Petrylak DP, Laliberte R, Wang J, Huang B, Davis C, Fowst C, Costa N, Blake-Haskins JA, di Pietro A, Grivas P (2020) Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med 383(13):1218–1230. 10.1056/NEJMoa2002788. (Epub 2020 Sep 18 PMID: 32945632) 10.1056/NEJMoa2002788 [DOI] [PubMed] [Google Scholar]
- 88.Duan J, Cui L, Zhao X, Bai H, Cai S, Wang G, Zhao Z, Zhao J, Chen S, Song J, Qi C, Wang Q, Huang M, Zhang Y, Huang D, Bai Y, Sun F, Lee JJ, Wang Z, Wang J (2020) Use of immunotherapy with programmed cell death 1 vs programmed cell death ligand 1 inhibitors in patients with cancer: a systematic review and meta-analysis. JAMA Oncol 6(3):375–384. 10.1001/jamaoncol.2019.5367.PMID:31876895;PMCID:PMC6990765 10.1001/jamaoncol.2019.5367.PMID:31876895;PMCID:PMC6990765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Song F, Altman DG, Glenny AM, Deeks JJ (2003) Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ 326(7387):472. 10.1136/bmj.326.7387.472 10.1136/bmj.326.7387.472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moher D, Liberati A, Tetzlaff J, Altman DG (2009) PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(264–69):W64 [PMC free article] [PubMed] [Google Scholar]
- 91.Pustejovsky JE, Rodgers MA (2019) Testing for funnel plot asymmetry of standardized mean differences. Res Synth Methods 10(1):57–71. 10.1002/jrsm.1332. (Epub 2019 Jan 8 PMID: 30506832) 10.1002/jrsm.1332 [DOI] [PubMed] [Google Scholar]
- 92.Harbord RM, Egger M, Sterne JA (2006) A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 25(20):3443–3457. 10.1002/sim.2380. (PMID: 16345038) 10.1002/sim.2380 [DOI] [PubMed] [Google Scholar]
- 93.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JP (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 22(343):d4002. 10.1136/bmj.d4002. (PMID: 21784880) 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 94.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 18(343):d5928. 10.1136/bmj.d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Basagaña X, Pedersen M, Barrera-Gómez J, Gehring U, Giorgis-Allemand L, Hoek G, Stafoggia M, Nieuwenhuijsen MJ, Brunekreef B, Slama R (2018) Analysis of multicentre epidemiological studies: contrasting fixed or random effects modelling and meta-analysis. Int J Epidemiol 47(4):1343–1354. 10.1093/ije/dyy117 10.1093/ije/dyy117 [DOI] [PubMed] [Google Scholar]
- 97.Rashdan S, Minna JD, Gerber DE (2018) Diagnosis and management of pulmonary toxicity associated with cancer immunotherapy. Lancet Respir Med 6(6):472–478. 10.1016/S2213-2600(18)30172-3.PMID:29856320;PMCID:PMC7341891 10.1016/S2213-2600(18)30172-3.PMID:29856320;PMCID:PMC7341891 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S Table 1: Basic characteristics of all enrolled clinical trials. (DOCX 72 KB)
S Figure 1: Risk of bias summary: review authors' judgements about each risk of bias item for each included study. (TIF 1698 KB)
S Figure 2: Funnel plots of the analysis results for different groups. A: The OR of pneumonitis for all-grade checked using the fixed effect (FE) model in Group A (PD-1/PD-L1 VS. Chemotherapy): Subgroup analyses were carried out according to the types of immune checkpoint inhibitors (PD-1 or PD-L1). B: The OR of pneumonitis for all-grade checked using the fixed effect (FE) model in Group B (PD-1/PD-L1+Chemotherapy VS. Chemotherapy): Subgroup analyses were carried out according to the types of immune checkpoint inhibitors (PD-1 or PD-L1). C: The OR of pneumonitis for all-grade checked using the fixed effect (FE) model in Group C (PD-1/PD-L1 VS. Placebo): Subgroup analyses were carried out according to the types of immune checkpoint inhibitors (PD-1 or PD-L1). D: The OR of pneumonitis for all-grade checked using the fixed effect (FE) model in Group D (PD-1/PD-L1 VS. PD-1/PD-L1+CTLA-4): Subgroup analyses were carried out according to the types of immune checkpoint inhibitors (PD-1 or PD-L1). E: The OR of pneumonitis for all-grade checked using the fixed effect (FE) model in Group E (PD-1/PD-L1 VS. PD-1/PD-L1+Chemotherapy): Subgroup analyses were carried out according to the types of immune checkpoint inhibitors (PD-1 or PD-L1). (TIF 2042 KB)
S Figure 3: Funnel plots of comparison in Mirror-pairing clinical trials. A: The OR of pneumonitis for all grades was checked using the fixed effect (FE) model (PD-1 VS. PD-L1). Subgroup analyses were carried out according to the tumor types. B: The OR of pneumonitis for all grades was checked using the fixed effect (FE) model (PD-1+Chemotherapy VS. PD-L1+Chemotherapy). Subgroup analyses were carried out according to the tumor types. (TIF 2023 KB)
S Figure 4: Forest blots of the subgroup analysis in Group A (PD-1/PD-L1 VS. Chemotherapy): The OR of pneumonitis for all-grade checked using the random effect (RE) model: Subgroup analyses were carried out according to the tumor types and PD-1/PD-L1. (TIF 2856 KB)
S Figure 5: Forest blots of the subgroup analysis in Group B (PD-1/PD-L1+Chemotherapy VS. Chemotherapy): The OR of pneumonitis for all-grade checked using the random effect (RE) model: Subgroup analyses were carried out according to the tumor types and PD-1/PD-L1. (TIF 2913 KB)
S Figure 6: Forest blots of the subgroup analysis in Group C (PD-1/PD-L1 VS. Placebo): The OR of pneumonitis for all-grade checked using the random effect (RE) model: Subgroup analyses were carried out according to the tumor types and PD-1/PD-L1. (TIF 2504 KB)
S Figure 7: Forest blots of the subgroup analysis in Group D (PD-1/PD-L1 VS. PD-1/PD-L1+CTLA-4): The OR of pneumonitis for all-grade checked using the random effect (RE) model: Subgroup analyses were carried out according to the tumor types and PD-1/PD-L1. (TIF 1597 KB)