Abstract
The INSPIRE randomized clinical trial demonstrated that a high protein diet (HPRO) combined with neuromuscular electrical stimulation (NMES) attenuates muscle atrophy and may improve outcomes after aneurysmal subarachnoid hemorrhage We sought to identify specific metabolites mediating these effects. Blood samples were collected from subjects on admission prior to randomization to either standard of care (SOC; N = 12) or HPRO + NMES (N = 12) and at 7 days. Untargeted metabolomics were performed for each plasma sample. Sparse partial least squared discriminant analysis identified metabolites differentiating each group. Correlation coefficients were calculated between each metabolite and total protein per day and muscle volume. Multivariable models determined associations between metabolites and muscle volume. Unique metabolites (18) were identified differentiating SOC from HPRO + NMES. Of these, 9 had significant positive correlations with protein intake. In multivariable models, N-acetylleucine was significantly associated with preserved temporalis [OR 1.08 (95% CI 1.01, 1.16)] and quadricep [OR 1.08 (95% CI 1.02, 1.15)] muscle volume. Quinolinate was also significantly associated with preserved temporalis [OR 1.05 (95% CI 1.01, 1.09)] and quadricep [OR 1.04 (95% CI 1.00, 1.07)] muscle volume. N-acetylserine and β-hydroxyisovaleroylcarnitine were associated with preserved temporalis or quadricep volume. Metabolites defining HPRO + NMES had strong correlations with protein intake and were associated with preserved muscle volume.
Keywords: Aneurysmal subarachnoid hemorrhage, High protein diet, Neuromuscular electrical stimulation, Metabolomic, N-acetylleucine, Quinolinate
Subject terms: Stroke, Prognostic markers, Translational research
Introduction
Aneurysmal rupture causing subarachnoid hemorrhage (aSAH) accounts for 5–10% of all strokes in the United States but often affects younger patients contributing to significant morbidity and mortality1,2. Despite an improved understanding of the pathophysiology of aSAH effective treatments have remained elusive3.
Increased systemic breakdown of protein following injury has long been recognized to be associated with worse outcomes4–6. These findings have led to recommendations for increased protein (1.2–2.2 g/kg) to be delivered to patients following traumatic injury7. However, the benefits of a higher protein diet in critically ill patients remains to be proven and must be used cautiously in patients with acute kidney injury8.
Similar to patients with a traumatic injury, aSAH results in a systemic catabolic state due to increased catecholamine release and cytokine production9. Immune-mediated malnutrition characterized after SAH is characterized by a pro-inflammatory hypermetabolic state coupled with protein energy catabolism10–12. Specifically, acute reductions of glutamine, an amino acid essential in maintaining muscle mass, has been closely linked to the sequelae of malnutrition in critical illness13 and long-term recovery after SAH10. Muscle weakness and impaired neuromotor recovery can occur if nutrition delivered during the critical illness phase after aSAH is inadequate to compensate for increased catabolism10,14. The recent Impact of neuromuscular electrical stimulation (NMES) and high protein supplementation (HPRO) on Recovery After SAH (INSPIRE) trial demonstrated that a HPRO diet combined with NMES decreases muscle wasting after aSAH and contributes to improved functional outcomes15. However, the specific protein metabolites and molecular mechanisms subserving this benefit remain unclear.
Herein, we utilized an untargeted metabolomics approach to evaluate circulating metabolites in patients enrolled in the INSPIRE clinical trial who received either standard of care (SOC) or HPRO + NMES. We sought to evaluate whether specific metabolites of amino acid metabolism are elevated after HPRO intervention and to determine which metabolites may mitigate muscle wasting after aSAH.
Methods
Subjects
Subjects were selected among the 25 enrolled in the INSPIRE phase 2 randomized controlled trial (NCT03201094) who had available plasma samples15. Of those subjects, one in the SOC group had not consented to plasma collection, resulting in plasma samples available from 24 subjects. Detailed methods are available in the published trial results15. INSPIRE was approved by the institutional review board (IRB) at the University of Maryland School of Medicine (HP-00074174). All methods were performed in accordance with the Declaration of Helsinki. Prior to enrollment, informed consent was obtained from all patients or their legal guardians. All patients enrolled in INSPIRE had a diagnosis of aSAH, underwent aneurysm repair within 48 h of ictal hemorrhage, were at least 18 years of age, and had a Hunt Hess score (HHS) ≥ 2 and modified Fisher Scale (mFS) score > 1. Patients with subarachnoid hemorrhage for etiologies other than a ruptured aneurysm (trauma, arteriovenous malformation, neoplasm) were excluded. Patients were excluded if they were unlikely to survive one-week post hemorrhage or unlikely to remain in the intensive care unit (ICU) for more than 7 days. Patients with a body mass index < 15 or > 40 kg/m2, protein allergy, premorbid modified Rankin Scale (mRS) score > 1, or who were currently pregnant or diagnosed with a malignancy, inflammatory disorder, neuromuscular disorder, or renal failure were also excluded.
All subjects were randomized to either SOC or HPRO + NMES. Subjects in the HPRO + NMES group were administered a bolus of a whey protein power dissolved in water (8–10 oz) three times daily with a dose of 3 g leucine/feeding for a goal of 1.75 g/kg/d. SOC subjects received 1.2–1.4 g/kg/day of protein delivered via enteral nutritional formulas or specific oral diets with no additional protein supplementation permitted. The NMES device was a L300 Plus® system (Bioness, Inc, Valencia, CA) with thigh cuffs applied bilaterally to stimulate with stimulator pads across the quadricep muscles. The NMES intervention included two 30-min session per day. All subjects underwent study interventions until post-bleed day (PBD) 14. Each subject was followed up at 90-days in person for outcome measurements. Nitrogen balance was calculated as described 16,17.
Biosamples
Blood samples were collected from subjects within 24-h of admission (before randomization to SOC or HPRO + NMES) and at PBD 7 in ethylenediaminetetraacetic acid (EDTA) containing tubes according to the INSPIRE protocol. Samples were centrifuged at 4 °C and stored at − 80 °C until analysis. A total of 12 subjects from the SOC group and 12 subjects from the HPRO + NMES group had plasma samples available at both time points. The use of biosamples for metabolomics studies was approved by the University of Texas McGovern School of Medicine IRB (HSC-MS-12-0637).
Metabolomics
Plasma samples (200 μL) were sent to Metabolon (Durham, NC, USA) for untargeted metabolomics analysis in a single batch. Detailed descriptions of the metabolomics platform, which consists of four independent ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) instruments and methods have been published elsewhere18–20. Median and standard deviation of internal standards are used to assess instrument variability. Identification of each metabolite was accomplished by automated comparison of each ion to a standard library. Areas of under the curve (AUC) were calculated for each peak. Raw AUC values were normalized correcting for between day variation in instrument calibration using internal standards and median value for each run day. Missing values were imputed using k-nearest neighbors with 10 neighbors used for each imputation. All results were subsequently log transformed.
Image acquisition
Included patients were scanned using one of two scanners: a 64-section CT unit (Brilliance; Philips Healthcare, Andover, MA) or a 128-section dual-source CT unit (SOMATOM Force; Siemens, Erlangen Germany). Images were archived at 3 mm section thickness. Baseline and follow-up series also were saved as Neuroimaging Informatics Technology Initiative (NifTI) files for voxel-wise labeling and volumetric analysis using 3D slicer (version 4.11.20210226, slicer.org).
Image Analysis and muscle volume measurement
Temporal muscle volumes were measured from 120 kV images using label masks of the temporal muscle that was created using a 3D threshold paint tool initially in the axial plane and editing in coronal and sagittal planes. Thresholding was set to a range of 30–80 units to minimize noise and avoid neighboring hyperdense blood and calvarium. Labeling was performed with small (1–3 mm diameter) spherical ROI to carefully delineate the interface of the membrane with adjacent structures. Labeling was performed by a resident and all scans were subsequently reviewed and edited by a radiology attending with 14 years of experience. Both reviewers were blinded to clinical data. Muscle volume was measured from the origin to the coronoid process of mandible (first slice depicting the coronoid process). Quantitative values (cm3) were derived for each label class using the Segment Statistics slicer module. Quadricep muscle volumes were assessed by CT with scan conducted from the patella to the femoral head. Cross-sectional areas were calculated at the thigh by manually outlining the regions of interest using MIPAV (Medical Image Processing, Analysis, and Visualization v10) as described21. Cross-sectional areas were multiplied by the number of slices and distance between slices in order to obtain volumetric measurements in cm3.
Bioinformatics
All bioinformatics analyses were performed in R (R Foundation for Statistical Computing). Fold changes (FC) were calculated for each metabolite comparing either late (7-days) vs early (within 24 h of admission) or between HPRO + NMES and SOC at day 7. Changes in metabolites were considered to be significant increased at FC > 2 and decreased at FC < 0.5 with false discovered rate (FDR) corrected P-values < 0.05. Sparse partial least squared discriminant analysis (sPLS-DA) was conducted using the mixOmics library in R (http://mixomics.org). Adding discriminant analysis to the PLS algorithm allows for classification of large datasets22–24. We used sPLS-DA to select the most discriminative metabolites to classify groups. The perf function was used to determine the optimal number of components to use in order to maintain a classification error rate less than 0.1. The tune.splsda function was used to determine the number of metabolites in order to minimize the balanced error rate.
Statistics
All statistical analyses were performed in R (R Foundation for Statistical Computing). Distribution of each variable was assessed with a Shapiro–Wilk test25. Demographic variables were compared using a t-test (age, protein per day, nitrogen balance, muscle volume), Mann–Whitney U test (mRS), or χ-squared test (sex, race, HHS, DCI) based on the distribution of each. After confirming normal distributions for each metabolite, Pearson’s correlation coefficients were calculated between metabolites and protein per day as well as nitrogen balance. Multivariable linear regression models were developed evaluate the relationship between selected metabolites (change in levels from admission to 7 days) and muscle volume (temporalis and quadricep muscles). All models were adjusted for age, sex, and aSAH severity (HHS < 4 vs ≥ 4).
Results
Demographics
Demographics for all subjects are shown in Table 1. A total of 24 subjects were included with 12 in both the SOC and HPRO + NMES groups. The groups were well matched with no significant differences in age, sex, race, HHS, DCI, mRS at discharge, and mRS at 3-months between the groups. Protein per day, nitrogen balance, and muscle volumes are also displayed in Supplementary Fig. 1.
Table 1.
Demographics and intervention characteristics.
| All | SOC | HPRO + NMES | P value* | |
|---|---|---|---|---|
| N | 24 | 12 | 12 | |
| Age (mean ± SD) | 57.9 ± 11.1 | 55.6 ± 13.3 | 60.2 ± 8.1 | 0.323 |
| Sex [Female (%)] | 16 (66.7) | 9 (75) | 7 (58) | 0.665 |
| Race [N (%)] | 0.999 | |||
| Black | 9 (37.5) | 5 (41.7) | 4 (33.3) | |
| White | 15 (62.5) | 7 (58.3) | 8 (66.7) | |
| HHS [median (IQR)] | 3.5 (2.75, 4.0) | 3.5 (2.0, 4.0) | 3.5 (3.0, 4.0) | 0.738 |
| DCI [N (%)] | 6 (25) | 3 (25) | 3 (25) | 1.00 |
| mRS discharge [median (IQR)] | 4 (3, 4) | 4 (3, 4.25) | 4 (2.75, 4) | 0.647 |
| mRS 3-months [median (IQR)] | 1.0 (0.75, 2.0) | 1.5 (1.0, 2.0) | 1 (0.0, 2) | 0.028 |
| Protein per day (g/kg/d, mean ± SD) | 1.21 ± 0.52 | 0.9 ± 0.37 | 1.51 ± 0.47 | 0.0020 |
| Nitrogen balance (g/d, mean ± SD) | − 1.29 ± 5.24 | − 4.74 ± 4.35 | 1.88 ± 3.86 | 9.01 × 10−4 |
| Muscle volume† | ||||
| Quadricep [% ± SD] | − 7.23 ± 7.89 | − 12.1 ± 6.17 | − 3.17 ± 6.94 | 0.0049 |
| Temporalis [% ± SD] | − 11.5 ± 8.59 | − 16.1 ± 9.07 | − 6.96 ± 5.19 | 0.0089 |
SOC standard of care, HPRO + NMES high protein plus neuromuscular electrical stimulation, HHS Hunt Hess scale, DCI delayed cerebral ischemia, mRS modified Rankin scale, SBBP short performance physical battery, IQR interquartile range.
*P-values reflect comparisons between SOC and HPRO + NMES. †Muscle volumes are represented as percent changes, however original volumetric measurements were in cm3.
Metabolites detected
A total of 1370 metabolites were detected from plasma samples with 261 of these metabolites unable to be identified. These metabolites were excluded resulting in a total of 1109 metabolites available for analysis. Metabolites consisted of amino acids, carbohydrates, cofactors and vitamins, energy related pathways (glycolysis, gluconeogenesis, and TCD cycle), lipids, nucleotides, peptides, as well as partially characterized molecules (Supplementary Fig. 2).
Metabolite FC
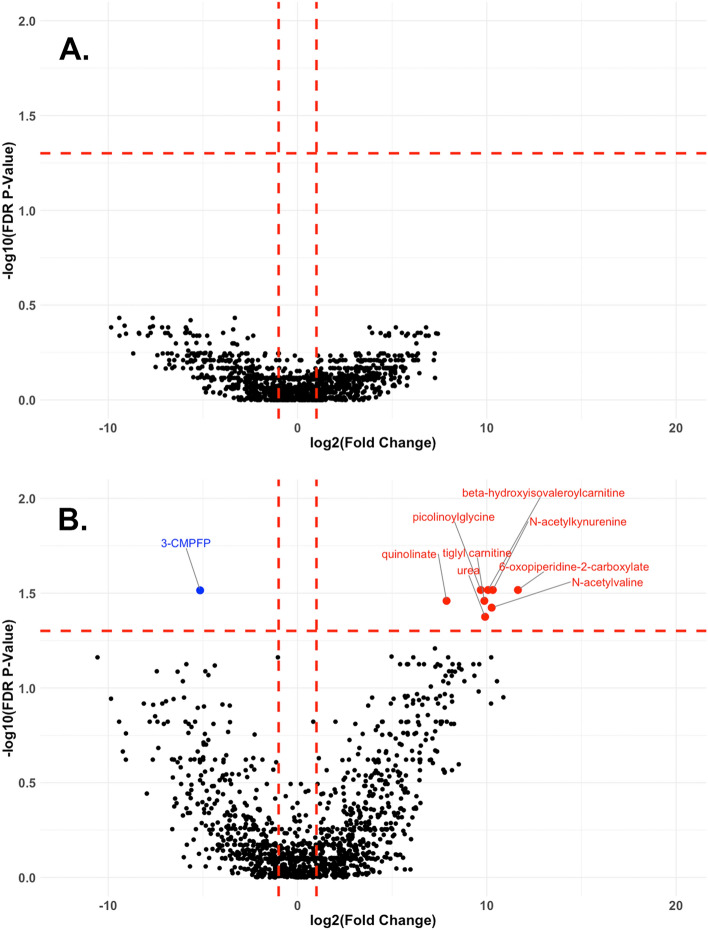
Paired FC and corresponding FDR corrected P-values were calculated for each metabolite. Figure 1 shows paired FC between metabolite levels measured at PBD 7 compared to metabolite levels measures within 24 h of ictus (before randomization) in the SOC group (Fig. 1A), as well as those in the HPRO + NMES group (Fig. 1B). Data are displayed as volcano plots with metabolites considered to be significantly different at a FC of > 2 or < 0.5 [represented as log2(fold change) of 1 or − 1 in each plot] and FDR P-value < 0.05. No metabolite fold changes were significantly different in the SOC group, while 8 metabolites were significantly increased and 1 significantly decreased in the HPRO + NMES group. All differentially expressed metabolites are shown in Supplementary Table 1.
Figure 1.
Volcano plots comparing paired metabolites at early and late time points. Paired fold changes (FC) were calculated for each subject in the SOC group (A) and HPRO + NEMS group (B) comparing the early and 7-day timepoints. Volcano plots were created using log2(FC) and − log10(FDR corrected P-values). Changes were considered to be significant if FC was greater than 2 or less than 0.5 and FDR corrected P-value was < 0.05. Metabolite that significantly increased from admission to 7-day are shown in red, and those that decreased are shown in blue. SOC (standard of care), HPRO + NMES (high protein plus neuromuscular electrical stimulation), FC (fold change), FDR (false discovery rate), 3-CMPFP (3-Carboxy-4-methyl-5-propyl-2-furanpropionate3-Carboxy-4-methyl-5-propyl-2-furanpropionate).
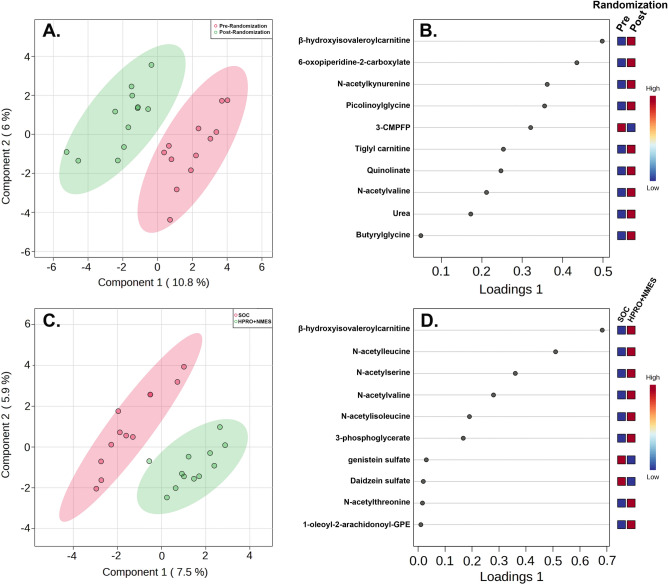
sPLS-DA analysis
sPLS-DA analysis was performed to determine metabolites contributing to differences between the HPRO + NMES group pre and post randomization (Fig. 2A). The top 10 metabolites that differentiated the HPRO + NMES group pre and post randomization are shown in Fig. 2B and are also listed in Supplementary Table 2. We also compared each metabolite between the SOC and HPRO + NMES groups. sPLS-DA was performed comparing SOC and HPRO + NMES groups using changes in each metabolite (Fig. 2C). The top 10 metabolites distinguishing SOC from HPRO + NMES are shown in Fig. 2D and also listed in Supplementary Table 2. Metabolites identified to have significant changes attributable to HPRO + NMES protocol were often amino acid metabolic intermediates. Considering both comparisons (1. HPRO + NMES pre and post randomization, and 2. SOC vs HPRO + NMES, Fig. 2B,D, respectively), a total of 18 unique metabolites were identified. Changes from baseline to 7 days in each of the 18 metabolites comparing SOC and HPRO + NMES are shown in Supplementary Fig. 3.
Figure 2.
Sparse partial least squared discriminant analysis (sPLS-DA). sPLS-DA was performed to determine metabolites driving differences between groups. Groups consisted of paired changes in metabolites for subjects in the HPRO + NMES group from baseline to 7-days (A, B) and SOC versus HPRO + NMES (C, D). Loadings plots detail the top 10 metabolites accounting for differences between groups.
Correlations between metabolites and protein intake
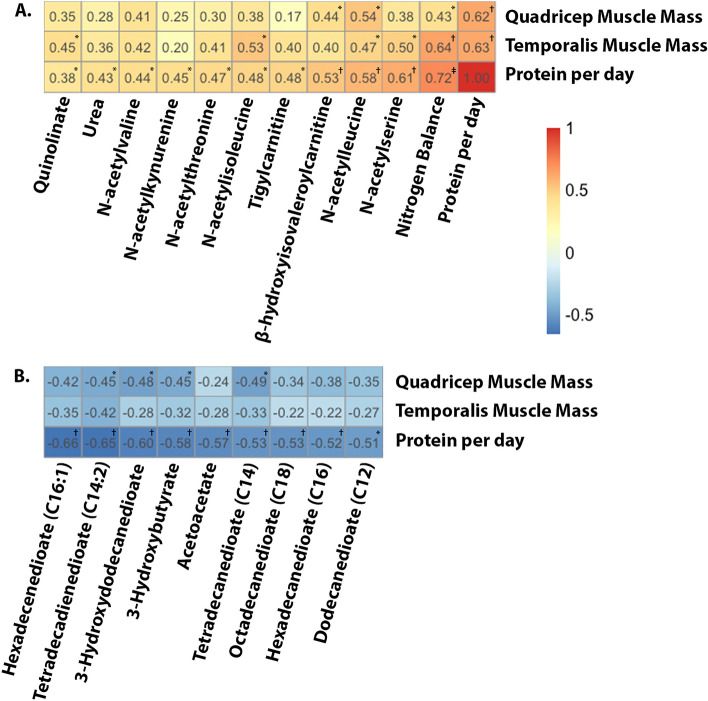
In order to determine the effect of treatment on each of the 18 metabolites above, correlation coefficients were determined between changes in each metabolite from baseline to 7-days and protein intake per day considering all subjects (SOC and HPRO + NMES). As expected, there was a significant correlation between protein per day and daily nitrogen balance (ρ = 0.72, P = 9.57 × 10−5). Significant correlations were also detected between protein intake and 9 of the 18 metabolites detected from the sPLS-DA analysis from Fig. 2: N-acetylserine (ρ = 0.61, P = 1.56 × 10−3), N-acetylleucine (ρ = 0.58, P = 2.97 × 10−3), β-hydroxyisovaleroylcarnitine (ρ = 0.53, P = 8.35 × 10−3), tiglyl carnitine (ρ = 0.48, P = 0.0168), N-acetylisoleucine (ρ = 0.48, P = 0.0183), N-acetylthreonine (ρ = 0.47, P = 0.0218), N-acetylkynurenine (ρ = 0.45, P = 0.0263), N-acetylvaline (ρ = 0.44, P = 0.0306), and urea (ρ = 0.43, P = 0.0381). Correlations between protein per day intake as well as nitrogen balance and all 18 metabolites are shown in Supplementary Table 3.
Metabolites most negatively correlated with protein intake were long chain fatty acid derivates and ketones. Metabolites with correlation coefficients < − 0.50 included: hexadecenedioate (C16:1) (ρ = − 0.66, P = 4.71 × 10–4), tetradecadienedioate (C14:2) (ρ = -0.65, P = 6.67 × 10–4), 3-hydroxydodecanedioate (ρ = − 0.60, P = 0.0021), 3-hydroxybutyrate (ρ = − 0.58, P = 0.0032), acetoacetate (ρ = − 0.57, P = 0.0038), tetradecanedioate (C14) (ρ = − 0.53, P = 0.0071), octadecanedioate (C18) (ρ = − 0.53, P = 0.0082), hexadecanedioate (C16) (ρ = − 0.52, P = 0.0089), and dodecanedioate (C12) (ρ = − 0.51, P = 0.011).
Metabolites and muscle volume
Muscle volumes decreased from baseline to 7 days, however this decrease was significantly less in the HPRO + NEMS compared with SOC (Supplementary Fig. 1). Total daily protein intake was strongly correlated with muscle volume (less muscle loss) in both the quadricep and temporalis muscles (ρ = 0.63, P = 0.0015 and ρ = 0.62, P = 0.002, respectively). Similarly, nitrogen balance was significantly positively correlated with quadricep and temporalis muscle volume (ρ = 0.43, P = 0.044 and ρ = 0.64, P = 0.0012, respectively). Preserved temporalis muscle volume was correlated with increased levels of N-acetylisoleucine (ρ = 0.53, P = 0.012), N-acetylserine (ρ = 0.50, P = 0.017), N-acetylleucine (ρ = 0.47, P = 0.027), and quinolinate (ρ = 0.45, P = 0.037). Preserved quadricep muscle volume was correlated with increased levels of N-acetylleucine (ρ = 0.54, P = 0.010) and β-hydroxyisovaleroylcarnitine (ρ = 0.44, P = 0.038). Heatmaps depicting significant correlations between metabolites and muscle volumes are shown in Fig. 3A. Metabolites included in Fig. 3A all have significant positive correlations with protein intake per day. Figure 3B includes those metabolites with the most negative correlations with protein intake per day and depicts their correlations with quadricep and temporalis muscle volume. Correlations between each of the 18 metabolites and temporalis and quadricep muscle preservation as well as those with significant negative correlations are shown in Supplementary Table 4.
Figure 3.
Heatmaps demonstrating correlations between metabolites and muscle volume. Pearson’s correlations coefficients were calculated between each metabolite and quadricep muscle, temporalis muscle, and protein per day (g/kg/d). Correlations were determined using changes in metabolites from baseline to 7-days. Metabolites are arranged by those having the most positive (A) and most negative (B) correlations with protein per day. Significance: *P < 0.05, †P < 0.01, ‡P < 0.0001.
Multivariable models for muscle volume
Separate multivariable models were developed to assess the association between individual metabolites and temporalis and quadricep muscle volume (Table 2). Models were developed for each of the 18 metabolites, with those models discussed below having significant associations with either temporalis or quadricep volume. Each model was adjusted for age, sex, and aSAH severity (HHS 1–3 vs 4–5). In the first model, the amount of protein intake per day was associated with increased volume of both temporalis [OR 1.13 (95% CI 1.07, 1.20), P = 5.69 × 10–4] and quadricep [OR 1.13 (1.07, 1.19), P = 2.21 × 10−4] muscles. Similarly, in the second model, higher nitrogen balance was associated with increased volume of both temporalis [OR 1.01 (1.00, 1.02), P = 0.0019] and quadricep [OR 1.01(1.00, 1.01), P = 0.045] muscles. In the third model, larger increases in N-acetylleucine were associated with increased volume of both temporalis [1.08 (1.01, 1.16), P = 0.038] and quadricep [1.08 (1.02, 1.15), P = 0.012] muscles. In the fourth model, larger increases in quinolinate were associated with increased volume of both temporalis [OR 1.05 (95% CI 1.01, 1.09), P = 0.018] and quadricep [OR 1.05 (95% CI 1.01, 1.09), P = 0.040] muscles. Larger increases in N-acetylserine [model 5: OR 1.18 (95% CI 1.04, 1.34), P = 0.017] and N-acetylisoleucine [model 6: OR 1.11 (1.01, 1.22), P = 0.048] were associated with increased temporalis muscle volume. Larger increases in β-hydroxyisovaleroylcarnitine [model 7: OR 1.07 (95% CI 1.01, 1.13), P = 0.026] were associated with increased quadricep muscle volume.
Table 2.
Multivariable regression models for muscle volume.
| Multivariable model | Temporalis volume | Quadricep volume | ||
|---|---|---|---|---|
| AOR (CI) | P | AOR (CI) | P | |
| 1. Protein per day | 1.13 (1.07, 1.20) | 5.69 × 10−4 | 1.13 (1.07, 1.19) | 2.21 × 10−4 |
| 2. Nitrogen balance | 1.01 (1.00, 1.02) | 0.0019 | 1.01 (1.00, 1.01) | 0.045 |
| 3. N-acetylleucine | 1.08 (1.01, 1.16) | 0.038 | 1.08 (1.02, 1.15) | 0.012 |
| 4. Quinolinate | 1.05 (1.01, 1.09) | 0.018 | 1.04 (1.00, 1.07) | 0.040 |
| 5. N-acetylserine | 1.18 (1.04, 1.34) | 0.017 | 1.13 (1.01, 1.27) | 0.055 |
| 6. N-acetylisoleucine | 1.11 (1.01, 1.22) | 0.048 | 1.08 (0.99, 1.18) | 0.12 |
| 7. β-hydroxyisovaleroylcarnitine | 1.07 (1.00, 1.15) | 0.059 | 1.07 (1.01, 1.13) | 0.026 |
Bold numbers represent statistically significant p-values
All models are adjusted for age, sex, and aSAH severity (HHS 1–3 vs 4–5).
AOR adjusted odds ratio, CI 95% confidence interval.
Discussion
The INSPIRE trial demonstrated that a high protein diet delivered enterally combined with neuromuscular electrical stimulation reduces muscle atrophy in the quadricep muscle after aSAH15. Herein, we identify the effects of HPRO + NMES on circulating plasma metabolites and examine the associations between changes in metabolite levels and muscle volume in both quadricep and temporalis muscles.
Using untargeted metabolomics, we identified the effects of a combined treatment (HPRO + NMES) on metabolite concentrations. Specifically, 18 metabolites that were prominently affected by the treatment. Our findings support the concept that nutritional and muscle stimulation interventions change the metabolome and these changes can contribute to overall muscle preservation in the acute stages of aSAH. Not surprisingly, among HPRO + NMES patients, there were notable increases in amino acid derivatives in plasma. Many of these amino acid derivatives, especially as related to glutamine metabolism are established important building blocks to preserving muscle mass. We also found carnitine esters (β-hydroxyisovaleroylcarnitine, tigylcarnitine), a tryptophan catabolite (quinolinate), a monocarboxylic acid (6-oxopiperidine-2-carboxylate), a phosphatidylethanolamine (1-oleoyl-2-arachidonoyl-GPE (18:1/20:4), a monophosphoglycerate involved in glycolysis and the calvin cycle (3-phosphoglycerate), and urea to be higher after HPRO + NMES treatment. Other metabolites including the isoflavones genistein and daidzein were lower in the HPRO + NEMS group. These metabolites are present in their sulfonic acid conjugates, which is their most common circulating form after undergoing hepatic metabolism by sulfotransferase enzyme26. The furoic acid 3-carboxy-4-methyl-5-pentyl-2-furanpropionate (3-CMPFP) was also decreased in the HPRO + NMES group. Classification and suspected biological function of each metabolite are shown in 5.
Our analysis showed that as expected, half of the 18 that were affected by the treatment were significantly positively correlated with protein intake per day (Supplementary Table 3), namely the acetylated amino acids as well as other metabolites, such as the carnitine ester β-hydroxyisovalerocylcarnitine, which plays a role in leucine catabolism. Urea, the end product of the urea cycle that plays a pivotal role in metabolizing excess nitrogen was also correlated with increased protein intake per day27. Additionally, an acylcarnitine (tiglylcarnitine) was correlated with protein intake per day. Although not a direct amino acid derivative, tiglyl containing compounds play a role in the metabolism of isoleucine28. Consistently, metabolites that were most negatively correlated with protein intake per day consisted of fatty acids and ketones (Supplementary Table 3) suggesting a shift away from fatty acid and ketone metabolism in those subjects receiving more dietary protein. While a shift away from fatty acid metabolism may attenuate inflammation29, some fatty acids such as omega-3 polyunsaturated fatty acids as well as ketones are thought to play a beneficial role30. Future studies will be necessary to understand the functional significance of decreased fatty acids and ketones on functional recovery.
In our previous study, atrophy of the quadricep muscle correlated with protein intake. In this analysis, we add to our findings by confirming in a multivariable model adjusting for age, sex and aSAH severity that higher protein intake per day as well as higher nitrogen balance were associated with not only perseveration of the quadricep muscle but temporalis muscle as well. Recent reports indicate that differences in temporalis muscle volume may be a marker of disease severity and prognosis after aSAH31. Our findings would suggest that this muscle may also be a sensitive marker of nutritionally-driven metabolomic changes after aSAH.
Increased levels the acylated amino acid N-acetylleucine were associated with both temporalis and quadricep muscles preservation, while other acetylated amino acids were only significantly associated with temporalis muscle preservation. Although the quadricep muscle was exposed to facilitated exercise with NMES during the study period, the smaller size of the temporalis may have made smaller changes in muscle mass easier to detect. The branched chain amino acid leucine and its metabolic derivatives are increased in paradigms of amino acid supplementation combined with exercise. Leucine in particular impacts the mTORC1 pathway and plays an integral role in energy homeostasis32. N-acetylleucine (NAL) has therapeutic potential, with studies showing that it may improve motor function in cerebral ataxia33 as well as lysosomal storage disease including Niemann Pick34,35 and GM2 Gangliosidosis36,37. More recently, NAL treatment has been shown to improve motor and cognitive outcomes after TBI in a mouse model38. NAL therefore may be a promising treatment for functional recovery after aSAH.
Increased levels of quinolinate were also shown to be associated with muscle preservation. Quinolinate plays an important role in tryptophan metabolism via the kynurenine pathway39,40. While upstream metabolites such as kynurenine have been associated with muscle wasting during critical illness41,42, quinolinate does not have this effect43. Quinolinate can be used to synthesize nicotinamide adenine dinucleotide (NAD+), which is depleted in response to proinflammatory stimuli44. Numerous reactions rely on NAD+ such as DNA repair via poly-ADP ribosylation and sirtuins, which have regulatory roles in cellular metabolism45,46. Although it is unclear whether quinolinate has a benefit on functional outcomes after aSAH, it is possible that the higher protein intake provides more tryptophan as a substrate to produce quinolinate resulting in the ability to restore NAD+ depleted after aSAH and the subsequent robust inflammatory response. Although we measured circulating quinolinate in plasma, quinolinate is also well known to have neurotoxic effects in the CNS as it is an agonist of the N-methyl-D-aspartate (NMDA) receptor and acts as an excitotoxin47. Therefore, additional studies will be needed to determine whether circulating quinolinate plays a beneficial role or is primarily a bioproduct of higher protein intake.
Although we have identified circulating metabolites that are increased after the HPRO + NMES intervention, our results are primarily correlative regarding which metabolites are associated with preserved muscle volume. It is well known that dietary protein intake stimulates muscle synthesis48. While we cannot exclude the possibility that some metabolites identified herein are byproducts of protein metabolism rather than being directly responsible for muscle synthesis, we believe that a number of these metabolites likely have beneficial roles. For instance, acetylated amino acids may play a role in muscle structure and function49. Furthermore, the acetylated amino acid NAL modulates metabolism broadly through its effects on mammalian target of rapamycin (mTOR) and activation of autophagy38,50,51. Dietary protein intake acts in concert with exercise to increase muscle synthesis52, and NMES acts as a surrogate for exercise53. Although many of the 18 metabolites that we have identified are strongly correlated with protein intake, NMES also contributes to their levels. For example, muscle contraction induced by NMES likely increases levels of acetylcarnitines54. We therefore suspect preserved muscle volume as well as increases in the metabolites identified in this manuscript are due to a combination of both HPRO and NMES.
High protein diets have been extensively evaluated in critically ill patients. Data from patients with traumatic brain injury (TBI) have suggested that a higher protein intake (1.5–2.0 g/kg/day) may be beneficial for recovery55. However, the recent EFFORT Protein trial found no significant survival benefit of a high protein diet alone with an increased risk of acute kidney injury (AKI) in at risk patients8. This large, randomized trial was conducted in mechanically ventilated patients without acquired brain injury, limiting its applicability to our study. In this study, we have identified specific metabolites that are increased by HPRO + NMES treatment which in turn were also associated with intermediaries of energy homeostasis and muscle preservation. This suggests that a more tailored intervention including a combination of metabolites such as acetylated amino acids like N-acetylleucine along with facilitated exercise with NMES may be able to preserve muscle mass while avoiding the potentially deleterious effects of a high protein diet.
This study has several important limitations. First, the small sample size (12 subjects in each group) as well as numerous comparisons being made for a broad panel of metabolites may have resulted in some associations been found by random chance. We attempted to mitigate this by utilizing false discovery rate corrected P-values. This small samples size also precluded being able to make associations with functional outcomes. Second, patients in the intervention arm received a combination of HPRO and NMES making it difficult to deconvolute the effects of these two interventions, as discussed above. Although we determined associations between metabolites and protein per day, overlapping effects of NMES likely contributed the changes in metabolites observed. Third, this study was conducted at a single tertiary care facility, therefore given variability in practices and patient populations, these results may not be broadly generalizable. Finally, although we assessed changes in metabolites from admission to 7 days after aneurysm rupture, we are not able to determine the exact mechanisms by which certain metabolites may mitigate muscle atrophy. Nevertheless, this is one of the first studies to better understand the systemic metabolic effects of HPRO and NMES and provides an important foundation for additional studies. Further, the study groups were randomized, there was careful implementation of the study intervention, and metabolites were analyzed with statistical rigor.
Conclusions
The HPRO + NMES intervention results in a distinction profile of circulating metabolites including a variety of amino acid derivatives. Further larger studies are required to elucidate the mechanisms by which amino acid metabolic intermediates such as N-acetylleucine prevent muscle atrophy and evaluate their potential therapeutic use after aSAH.
Supplementary Information
Acknowledgements
The authors acknowledge all patients who participated in the INSPIRE study.
Abbreviations
- aSAH
Aneurysmal subarachnoid hemorrhage
- HPRO
High protein diet
- NMES
Neuromuscular electrical stimulation
- sPLS-DA
Squared discriminant analysis
- SOC
Standard of care
- HHS
Hunt Hess score
- mFS
Modified Fisher scale
- mRS
Modified Rankin scale
- PBD
Post-bleed day
- EDTA
Ethylenediaminetetraacetic acid
- UPLC-MS/MS
Ultra-high-performance liquid chromatography-tandem mass spectrometry
- AUC
Area of under the curve
- NifTI
Neuroimaging informatics technology initiative
- FC
Fold change
- FDR
False discovered rate
- NAL
N-Acetylleucine
- NAD +
Nicotinamide adenine dinucleotide
- NMDA
N-Methyl-D-aspartate
- TBI
Traumatic brain injury
- AKI
Acute kidney injury
Author contributions
A.M.G. designed the study, analyzed data, and wrote the manuscript. J.P.J.S. analyzed data and edited the manuscript. D.F. analyzed data and edited the manuscript. AS collected and curated data and edited the manuscript. G.L. collected and curated data and edited the manuscript. U.B. analyzed radiographic data pertaining to muscle volume and edited the manuscript. W.Z. collected and curated data and edited the manuscript. A.S.R. was involved in study design and helped to write and revise the manuscript. H.A.C. was involved in study design and helped to write and revise the manuscript. NB designed the study, analyzed data, wrote and revised the manuscript.
Funding
This study was supported by funding from the NINDS awarded to AMG (5K23NS121628). Support for the INSPIRE clinical was awarded to NB from the Neurocritical Care Society (INCLINE grant).
Data availability
All data utilized for analyses in this study are available upon reasonable request made to the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-64666-5.
References
- 1.Lawton MT, Vates GE. Subarachnoid Hemorrhage. N. Engl. J .Med. 2017;377:257–266. doi: 10.1056/NEJMcp1605827. [DOI] [PubMed] [Google Scholar]
- 2.Rincon F, Rossenwasser RH, Dumont A. The epidemiology of admissions of nontraumatic subarachnoid hemorrhage in the United States. Neurosurgery. 2013;73:217–22. doi: 10.1227/01.neu.0000430290.93304.33. [DOI] [PubMed] [Google Scholar]
- 3.Connolly ES, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43:1711–1737. doi: 10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- 4.Davies JW. Protein metabolism following injury. J. Clin. Pathol. Suppl. (R. Coll. Pathol.) 1970;4:56–64. doi: 10.1136/jcp.s3-4.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuthbertson DP. Observations on the disturbance of metabolism produced by injury to the limbs. QJM: Int. J. Med. 1932;1:233–246. [Google Scholar]
- 6.Cuthbertson DP. The disturbance of metabolism produced by bony and non-bony injury, with notes on certain abnormal conditions of bone. Biochem. J. 1930;24:1244–1263. doi: 10.1042/bj0241244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor BE, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically Ill patient. Crit. Care Med. 2016;44:390–438. doi: 10.1097/CCM.0000000000001525. [DOI] [PubMed] [Google Scholar]
- 8.Heyland DK, et al. The effect of higher protein dosing in critically ill patients with high nutritional risk (EFFORT protein): An international, multicentre, pragmatic, registry-based randomised trial. Lancet. 2023;401:568–576. doi: 10.1016/S0140-6736(22)02469-2. [DOI] [PubMed] [Google Scholar]
- 9.Ahn SH, et al. Inflammation in delayed ischemia and functional outcomes after subarachnoid hemorrhage. J. Neuroinflamm. 2019;16:1–10. doi: 10.1186/s12974-019-1578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badjatia N, et al. Inflammation, negative nitrogen balance, and outcome after aneurysmal subarachnoid hemorrhage. Neurology. 2015;84:680–687. doi: 10.1212/WNL.0000000000001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Galea I, Macdonald RL, Wong GKC, Zhang JH. Rethinking the initial changes in subarachnoid haemorrhage: Focusing on real-time metabolism during early brain injury. EBioMedicine. 2022;83:104223. doi: 10.1016/j.ebiom.2022.104223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Touho H, et al. Hypermetabolism in the acute stage of hemorrhagic cerebrovascular disease. J. Neurosur.g. 1990;72:710–714. doi: 10.3171/jns.1990.72.5.0710. [DOI] [PubMed] [Google Scholar]
- 13.Tao K-M, et al. Glutamine supplementation for critically ill adults. Cochrane Database Syst. Rev. 2014;2014:CD010050. doi: 10.1002/14651858.CD010050.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puthucheary ZA, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 15.Badjatia N, et al. Neuromuscular electrical stimulation and high-protein supplementation after subarachnoid hemorrhage: A single-center phase 2 randomized clinical trial. Neurocrit. Care. 2021;35:46–55. doi: 10.1007/s12028-020-01138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickerson RN, Tidwell AC, Minard G, Croce MA, Brown RO. Predicting total urinary nitrogen excretion from urinary urea nitrogen excretion in multiple-trauma patients receiving specialized nutritional support. Nutrition. 2005;21:332–338. doi: 10.1016/j.nut.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Guralnik JM, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald KC, et al. Multi-omic evaluation of metabolic alterations in multiple sclerosis identifies shifts in aromatic amino acid metabolism. Cell. Rep. Med. 2021;2:100424. doi: 10.1016/j.xcrm.2021.100424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhargava P, Fitzgerald KC, Calabresi PA, Mowry EM. Metabolic alterations in multiple sclerosis and the impact of vitamin D supplementation. JCI Insight. 2017 doi: 10.1172/jci.insight.9530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun D, et al. A prospective study of serum metabolites and risk of ischemic stroke. Neurology. 2019;92:e1890–e1898. doi: 10.1212/WNL.0000000000007279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan AS, Buscemi A, Forrester L, Hafer-Macko CE, Ivey FM. Atrophy and intramuscular fat in specific muscles of the thigh: Associated weakness and hyperinsulinemia in stroke survivors. Neurorehabil. Neural Repair. 2011;25:865–872. doi: 10.1177/1545968311408920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lê Cao K-A, Boitard S, Besse P. Sparse PLS discriminant analysis: biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinf. 2011;12:253. doi: 10.1186/1471-2105-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lê Cao K-A, Boitard S, Besse P. Sparse PLS discriminant analysis: biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinf. 2011;12:253. doi: 10.1186/1471-2105-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pérez-Enciso M, Tenenhaus M. Prediction of clinical outcome with microarray data: A partial least squares discriminant analysis (PLS-DA) approach. Hum. Genet. 2003;112:581–592. doi: 10.1007/s00439-003-0921-9. [DOI] [PubMed] [Google Scholar]
- 25.Mishra P, et al. Descriptive statistics and normality tests for statistical data. Ann. Card. Anaesth. 2019;22:67–72. doi: 10.4103/aca.ACA_157_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karakaya S. Bioavailability of phenolic compounds. Crit. Rev. Food Sci. Nutr. 2004;44:453–464. doi: 10.1080/10408690490886683. [DOI] [PubMed] [Google Scholar]
- 27.Kumari A. Sweet Biochemistry. Netherlands: Elsevier; 2018. Urea Cycle Disorders. [Google Scholar]
- 28.Robinson WG, Bachhawat BK, Coon MJ. Tiglyl coenzyme A and alpha-methylacetoacetyl coenzyme A, intermediates in the enzymatic degradation of isoleucine. J. Biol. Chem. 1956;218:391–400. doi: 10.1016/S0021-9258(18)65902-2. [DOI] [PubMed] [Google Scholar]
- 29.Calder PC. Fatty acids and inflammation: the cutting edge between food and pharma. Eur. J. Pharmacol. 2011;668(Suppl 1):S50–S58. doi: 10.1016/j.ejphar.2011.05.085. [DOI] [PubMed] [Google Scholar]
- 30.Giacobbe J, Benoiton B, Zunszain P, Pariante CM, Borsini A. The anti-inflammatory role of omega-3 polyunsaturated fatty acids metabolites in pre-clinical models of psychiatric, neurodegenerative, and neurological disorders. Front. Psychiatry. 2020 doi: 10.3389/fpsyt.2020.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katsuki M, et al. Temporal muscle and stroke—a narrative review on current meaning and clinical applications of temporal muscle thickness, area, and volume. Nutrients. 2022;14:687. doi: 10.3390/nu14030687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vellai T. How the amino acid leucine activates the key cell-growth regulator mTOR. Nature. 2021;596:192–194. doi: 10.1038/d41586-021-01943-7. [DOI] [PubMed] [Google Scholar]
- 33.Schniepp R, et al. Acetyl-DL-leucine improves gait variability in patients with cerebellar ataxia-a case series. Cerebellum Ataxias. 2016;3:8. doi: 10.1186/s40673-016-0046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bremova T, et al. Acetyl-dl-leucine in Niemann-Pick type C: A case series. Neurology. 2015;85:1368–1375. doi: 10.1212/WNL.0000000000002041. [DOI] [PubMed] [Google Scholar]
- 35.Bremova-Ertl T, et al. Efficacy and safety of N-acetyl-L-leucine in Niemann-Pick disease type C. J Neurol. 2022;269:1651–1662. doi: 10.1007/s00415-021-10717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bremova-Ertl T, Platt F, Strupp M. Sandhoff disease: Improvement of gait by acetyl-DL-leucine: A case report. Neuropediatrics. 2020;51:450–452. doi: 10.1055/s-0040-1715486. [DOI] [PubMed] [Google Scholar]
- 37.Kaya E, et al. Beneficial effects of acetyl-DL-leucine (ADLL) in a Mouse model of sandhoff disease. J. Clin. Med. 2020;9:1050. doi: 10.3390/jcm9041050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hegdekar N, Lipinski MM, Sarkar C. N-Acetyl-l-leucine improves functional recovery and attenuates cortical cell death and neuroinflammation after traumatic brain injury in mice. Sci. Rep. 2021;11:9249. doi: 10.1038/s41598-021-88693-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis I, Liu A. What is the tryptophan kynurenine pathway and why is it important to neurotherapeutics? Expert Rev. Neurother. 2015;15:719–721. doi: 10.1586/14737175.2015.1049999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramírez Ortega D, et al. On the antioxidant properties of L-kynurenine: An efficient ROS scavenger and enhancer of rat brain antioxidant defense. Antioxidants. 2021;11:31. doi: 10.3390/antiox11010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaiser H, et al. Kynurenine, a tryptophan metabolite that increases with age, induces muscle atrophy and lipid peroxidation. Oxid. Med. Cell. Longev. 2019;2019:1–9. doi: 10.1155/2019/9894238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie T, et al. Interleukin-6 promotes skeletal muscle catabolism by activating tryptophan–indoleamine 2,3-dioxygenase 1–kynurenine pathway during intra-abdominal sepsis. J. Cachexia Sarcopenia Muscle. 2023;14:1046–1059. doi: 10.1002/jcsm.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaiser H, Ding K-H, Isales C, Hamrick M. The effects of kynurenine metabolites on skeletal muscle in vivo and in vitro. FASEB J. 2020;34:1–1. [Google Scholar]
- 44.Moffett JR, et al. Quinolinate as a marker for kynurenine metabolite formation and the unresolved question of NAD+ synthesis during inflammation and infection. Front. Immunol. 2020 doi: 10.3389/fimmu.2020.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kane AE, Sinclair DA. Sirtuins and NAD+ in the development and treatment of metabolic and cardiovascular diseases. Circ. Res. 2018;123:868–885. doi: 10.1161/CIRCRESAHA.118.312498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim MY, Zhang T, Kraus WL. Poly(ADP-ribosyl)ation by PARP-1: `PAR-laying’ NAD+ into a nuclear signal. Genes Dev. 2005;19:1951–1967. doi: 10.1101/gad.1331805. [DOI] [PubMed] [Google Scholar]
- 47.Lugo-Huitrón R, et al. Quinolinic acid: An endogenous neurotoxin with multiple targets. Oxid. Med. Cell. Longev. 2013;2013:1–14. doi: 10.1155/2013/104024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mamerow MM, et al. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J. Nutr. 2014;144:876–880. doi: 10.3945/jn.113.185280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang D, et al. Alterations of lysine acetylation profile in murine skeletal muscles upon exercise. Front. Aging Neurosci. 2022;14:859313. doi: 10.3389/fnagi.2022.859313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ananieva EA, Powell JD, Hutson SM. Leucine metabolism in T cell activation: mTOR signaling and beyond. Adv. Nutr. 2016;7:798S–805S. doi: 10.3945/an.115.011221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarkar C, Lipinski MM. N-acetyl-L-leucine: A promising treatment option for traumatic brain injury. Neural Regen. Res. 2022;17:1957–1958. doi: 10.4103/1673-5374.335146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hartono FA, Martin-Arrowsmith PW, Peeters WM, Churchward-Venne TA. The effects of dietary protein supplementation on acute changes in muscle protein synthesis and longer-term changes in muscle mass, strength, and aerobic capacity in response to concurrent resistance and endurance exercise in healthy adults: a systematic review. Sports Med. 2022;52:1295–1328. doi: 10.1007/s40279-021-01620-9. [DOI] [PubMed] [Google Scholar]
- 53.Blazevich AJ, Collins DF, Millet GY, Vaz MA, Maffiuletti NA. Enhancing adaptations to neuromuscular electrical stimulation training interventions. Exerc. Sport Sci. Rev. 2021;49:244–252. doi: 10.1249/JES.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Furuichi Y, et al. Imaging mass spectrometry reveals fiber-specific distribution of acetylcarnitine and contraction-induced carnitine dynamics in rat skeletal muscles. Biochim. Biophys. Acta. 2014;1837:1699–1706. doi: 10.1016/j.bbabio.2014.05.356. [DOI] [PubMed] [Google Scholar]
- 55.Kurtz P, Rocha EEM. Nutrition therapy, glucose control, and brain metabolism in traumatic brain injury: a multimodal monitoring approach. Front. Neurosci. 2020;14:452910. doi: 10.3389/fnins.2020.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data utilized for analyses in this study are available upon reasonable request made to the corresponding author.