Abstract
Behavioral activation (BA) is a well-established method of evidence-based treatment for depression. There are clear links between the neural mechanisms underlying reward processing and BA treatment for depressive symptoms, including anhedonia; however, integrated interpretations of these two domains are lacking. Here we examine brain imaging studies involving BA treatments to investigate how changes in brain networks, including the reward networks, mediate the therapeutic effects of BA, and whether brain circuits are predictors of BA treatment responses. Increased activation of the prefrontal and subcortical regions associated with reward processing has been reported after BA treatment. Activation of these regions improves anhedonia. Conversely, some studies have found decreased activation of prefrontal regions after BA treatment in response to cognitive control stimuli in sad contexts, which indicates that the therapeutic mechanism of BA may involve disengagement from negative or sad contexts. Furthermore, the decrease in resting-state functional connectivity of the default-mode network after BA treatment appears to facilitate the ability to counteract depressive rumination, thereby promoting enjoyable and valuable activities. Conflicting results suggest that an intact neural response to rewards or defective reward functioning is predictive of the efficacy of BA treatments. Increasing the benefits of BA treatments requires identification of the unique individual characteristics determining which of these conflicting findings are relevant for the personalized treatment of each individual with depression.
Keywords: depression, cognitive behavioral therapy, psychotherapy, functional magnetic resonance imaging, neuroimaging
Graphical Abstract
INTRODUCTION
Behavioral activation (BA) is a structured and brief psychotherapeutic approach that aims to 1) increase engagement in adaptive activities (which are often associated with the experience of pleasure or mastery), 2) decrease engagement in activities that maintain depression or increase the risk of depression, and 3) solve problems that restrict access to rewards or maintain or increase aversive control.1 Several studies, which include large-scale randomized controlled trials (RCTs), have investigated the efficacy of BA treatments for depression,2,3,4,5,6 and meta-analyses of the cumulative findings have found moderate-to-large effect sizes for BA treatments.7,8,9,10,11 Several guidelines based on the accumulating research have given BA a high status in the treatment of depression; for example, the American Psychological Association (APA) guidelines designate BA as a “well-established, validated treatment for depression.”
The increasing amount of clinical evidence for the efficacy of several psychotherapy approaches and the advances in basic and translational neuroscientific research have recently led to a greater focus on neurobiological mechanisms with the aim of elucidating the mechanisms of action underlying both diseases and treatments. Research on the mechanisms underlying changes in brain networks is pivotal for the development and refinement of psychological treatments,12 because identifying these mechanisms will improve the understanding of which treatment components are causally associated with symptom improvement and will allow treatments to be refined. In particular, cognitive behavioral therapy (CBT) for depression has been the focus of various functional magnetic resonance imaging (fMRI) studies, which have identified the neurobiological mechanisms underlying treatment effects by examining changes in brain networks during CBT, and have aided in predicting the therapeutic response and prognosis of individual patients with depression by studying the activity in specific brain regions and networks.13,14,15,16,17,18,19 However, unlike for CBT, insufficient research has been conducted on the neurobiological mechanisms of BA at the brain network level. Although many fMRI studies have included BA as one of the therapeutic components, such comparative studies cannot replace investigations of the independent neurobiological effects of BA alone. Despite the strong empirical support for the efficacy of BA treatment, the understanding of the mechanisms of change that lead to symptom improvement remains inadequate.
Symptoms of major depressive disorder (MDD), such as decreases in motivation, activity levels, and ability to experience pleasure, have been linked to dysfunction of the systems governing the behavioral responsiveness to positive stimuli (i.e., reward function).20 Considering that the primary goal of BA is to increase the degree to which an individual engages in rewarding activities, BA treatment for depression is probably highly relevant to the activity of the brain’s reward system. Considering the etiology and treatment mechanisms of depression, there are clear links between BA treatments for depression and the neural mechanisms involved in reward function. Despite this strong connection, the body of research on the efficacy of BA treatments has mostly evolved independently of basic and translational neuroscience studies of the biobehavioral mechanisms underlying reward functioning.21
Therefore, this review comprehensively examines the clinical literature describing brain network changes following BA treatments, with a particular focus on changes in BA treatment mechanisms at the brain network level, especially those related to reward function/processing, as well as exploring whether functional neuroimaging markers can predict BA treatment outcomes. The aim in writing this review was to enhance our understanding of the therapeutic mechanisms of BA in the treatment of depression and thereby improve the ability to provide personalized treatments based on the underlying neural circuits.
BA IN DEPRESSION AND EMPIRICAL EVIDENCE
Brief history of BA
BA is rooted in behaviorism.22,23,24 The first wave of behaviorism involved the behavioral model of depression, which was based on the premise that depression occurs after losing a major source of positive reinforcement. However, further developments of the behavioral model were overshadowed during the 1960s by a second wave of psychotherapy, called the “cognitive revolution.” Beck and colleagues recognized the value of behavioral techniques and incorporated behavioral therapy strategies into cognitive therapy (CT),25 and interest in behavioral treatments for depression resurfaced during the 1990s and 2000s. This included Jacobson et al.26 comparing treatment effects in 150 adult patients with MDD randomized to receiving a BA component; to receiving a BA component plus strategies designed to restructure automatic thoughts; and to receiving the full CT package, including BA. They found that the outcomes in the BA-only group were equivalent to those under the other two conditions,26,27 which rekindled interest in behavior-based approaches to depression treatment and BA as a stand-alone intervention.28,29,30 Emphasis was placed on the functional analysis of contextual factors and behavior in depression assessments and interventions, or functional contextualism, as described by Hayes et al.31 Therefore, rather than focusing on changing or eliminating the form of a problem, psychotherapy searched for secondary changes by identifying the context in which problematic cognitions, emotions, and behaviors occur and intervening to change their function. This has led to BA becoming one of the modern third wave (“contemporary contextual”) approaches to psychotherapy.
Psychological mechanism underlying BA treatments
Lewinsohn and colleagues23,24,32 established a behavioral model of depression that was initially based on three assumptions: 1) response-contingent positive reinforcement at low levels eliciting stimuli for depressive behavior (mood and somatic feelings), 2) reduced response-contingent positive reinforcement being a sufficient explanation for depression, and 3) the total amount of response-contingent reinforcement being a function of the number of events that occur in the environment, potentially reinforcing the individual, the probability of the event occurring, and the frequency of the individual’s instrumental behavior triggering the event. Furthermore, depression was believed to be preceded by a decrease in response-contingent reinforcement and to act as a covariate with the level of this reinforcement. Low levels of reinforcement are key antecedents in depression development, with depression decreasing when positive reinforcement increases with treatment. Martell et al.29 proposed the TRAP/TRAC (Trigger, Response, Avoidance Pattern/Trigger, Response, Alternative Coping) model to describe the contexts in which individuals with depression exhibit avoidance behaviors and BA interventions for these avoidance patterns. “Avoidance Patterns” are exhibited when an event triggers a negative response (“Trigger”), causing a loss of opportunities to solve issues or access available reinforcers. Avoidance behavior creates a feedback loop of depressed moods by missing opportunities to cope with subsequent contextual triggers. However, engaging in constructive behaviors such as “Alternative Coping” breaks the avoidance cycle and depressed moods. Thus, a reduction in avoidance coping leads to increased opportunities for engagement with sources of reward in the environment. Another previous study33 extracted the following eight BA techniques from six BA treatment manuals: activity monitoring, contingency management, value and goal assessments, activity scheduling, procedures targeting verbal behavior, procedures targeting avoidance, skill training, and relaxation.25,29,34,35,36,37,38 The psychotherapeutic components of BA are discussed in more detail by Kanter et al.33
Efficacy of BA treatments
A large RCT involving 241 adults with MDD compared the efficacy of BA treatments for 16 weeks in reducing depressive symptoms with those of medication (paroxetine) and CT, and with a placebo control group. The results showed that the efficacy of BA was comparable to that in pharmacotherapy in the acute treatment of MDD, and better than CT in patients with more-severe depressive symptoms.2 A subsequent study followed up those individuals who had responded to treatment 1 year later, and found that the rate of depression relapse after medication discontinuation was lower in patients who received BA or CT, with no significant difference between BA and CT.3 These findings suggest that BA treatment is at least as effective in improving acute depressive symptoms and preventing relapse as the existing standard of care and well-established psychotherapy strategies for depression.
A meta-analysis of BA treatments that included 16 studies involving 780 participants observed that the pooled effect size for the difference between the treatment and control groups at posttest was significant at 0.87, while in 10 of the included studies that compared BA with CT found no significant difference between the two groups, even at follow-up.7 Another study extended that analysis7 by including 26 RCTs involving 1,524 participants,8 and found a large effect size of 0.74 standard mean differences (SMDs) between the BA and control groups in post-hoc analyses, and a small but significant superiority of 0.42 SMDs in the 4 studies in which BA was compared with pharmacotherapy, confirming the clinical efficacy of BA.
BA is highly regarded as an evidence-based psychotherapy strategy for the treatment of depression. The APA guidelines list BA as 1 of 12 empirically supported treatments for depression. The United Kingdom National Institute for Health and Care Excellence guideline39 for treating depression in adults recommends that the applied treatment should be based on the depression severity, and includes BA as a first-line treatment for both severe and mild depression. The clinical guidelines for MDD treatment in adults published by the Canadian Psychiatric Association and Canadian Network for Mood and Anxiety Treatments40 recommend BA as a first-line treatment for acute depressive symptoms, along with CBT and interpersonal therapy (IPT), and as a second-line maintenance treatment to prevent relapse. Guidelines produced by the Scottish Intercollegiate Guidelines Network41 for nondrug treatments of adult depression recommend BA as a first-line treatment along with CBT and IPT.
Relationships between BA treatments and anhedonia in depression
It has recently been suggested that popular depression treatments such as CBT and antidepressants are less effective in treating anhedonia and low positive affect,42 which underscores the need for a deeper understanding of the mechanisms of change underlying reward-focused treatments. New strategies are needed that target low positive affect, decreased motivation and pleasure, and anhedonia, which are primary symptoms of MDD.20,43 Accordingly, Nagy et al.44 proposed the transdiagnostic behavioral activation therapy for anhedonia (BATA) for restoring reward motivation and responsiveness. Clinical studies have shown that BA is associated with improvements in anhedonia. The administration of BA treatment to 33 outpatients diagnosed with MDD resulted in a significant reduction in their anhedonia symptoms.45 Another study involving 440 patients with MDD randomized to BA or CBT demonstrated an improvement in anhedonia symptoms in both treatment groups during acute treatment, with no significant intergroup difference.46 The efficacy of BA treatment for anhedonia has also been validated in adolescent populations.47,48
DYSFUNCTION OF THE REWARD-PROCESSING NETWORK AND DEPRESSION
What is the reward-processing network?
Deficits in reward processing have been proposed as a candidate mechanism underlying anhedonia, which involves a consistent lack of pleasure and interest in nearly all daily life activities.49 fMRI studies have made significant progress over the past decade in improving the understanding of brain mechanisms underlying anhedonia and reward-processing dysfunction in MDD. Although numerous brain regions react to rewards, the cortical-basal ganglia circuit is the cardinal component of the reward system.50 The key structures in this network are the anterior cingulate cortex, orbital prefrontal cortex (OFC), ventral striatum (i.e., the nucleus accumbens [NAcc]), ventral pallidum, and midbrain dopamine neurons. Other structures are core components in regulating the reward circuit, including the dorsal prefrontal cortex (PFC), amygdala, hippocampus, thalamus, lateral habenular nucleus, and specific brainstem structures such as the pedunculopontine and raphe nuclei.50,51,52,53
Relationship between dysfunction of the reward-processing network and anhedonia in depression
Altered functional connectivity within reward-processing network observed in patients with MDD and anhedonia provides evidence that reward processing is dysfunctional in anhedonia.49,51,54,55,56,57,58,59,60,61,62 Functional neuroimaging data primarily underscore the changes in neural activation and functional connectivity during reward processing. Regarding altered activation, anhedonia was found to be significantly negatively correlated with medial OFC activation in adolescents with MDD.63 Anhedonia in patients with MDD has been associated with decreased activity in limbic regions such as the NAcc and caudate nucleus, and with hyperactivity in cortical regions such as the ventromedial prefrontal cortex (vmPFC) and dorsolateral prefrontal cortex (dlPFC).64,65 A review of anhedonic subtypes associated striatal hypoactivation—along with hypoactivation and hyperactivation of several prefrontal regions—with reward-liking and desire, and the blunted sensitivity of the prefrontal striatum to positive feedback with reward learning.52 Additionally, increased low-frequency fluctuations in the left dorsal anterior cingulate cortex (dACC) were correlated with anticipatory anhedonia in patients with MDD.66 Regarding changes in functional connectivity, anhedonia was negatively correlated with connectivity to the vmPFC and various reward regions in patients with MDD, including the NAcc, ventral tegmental area, OFC, and insula.67 Changes in the functional connectivity of frontostriatolimbic circuits and cortical networks may be associated with MDD combined with anhedonia.68 However, conflicting findings have been reported regarding the relationship between anhedonia and connectivity between the NAcc and the default-mode network (DMN). Reduced reward sensitivity (e.g., reward-liking, motivation, and pleasure) as measured using the Behavioral Inhibition System and Behavioral Activation System (BIS/BAS) scale69 was associated with a decreased resting-state NAcc connectivity to the DMN and increased connectivity to the salience network across psychiatric conditions, including patients with MDD.70 Conversely, enhanced functional connectivity between the DMN and NAcc in subthreshold depression was positively correlated with loss of interest, as measured by the two anhedonia items on the Center for Epidemiological Studies-Depression scale.71 However, it should be remembered that these discrepant findings regarding NAcc–DMN connectivity may be due to differences in anhedonia measures applied in different studies or in the severity of depressive symptoms between the included subjects.
With respect to structural brain changes, several structural magnetic resonance imaging studies have revealed correlations between anhedonia and the reward network. Brain volume reductions in the reward networks (e.g., striatum, NAcc, fornix, and right basal forebrain), decreased white-matter integrity in reward-related brain regions, and reduced cortical thickness (e.g., in the left rostral anterior cingulate cortex and lateral OFC) have been associated with MDD combined with anhedonia.66,72,73,74,75,76,77,78,79 Additionally, trait anhedonia has been linked to decreased volumes of the caudate nucleus80 and NAcc81 in nonclinical cohorts, and the bilateral volume reduction of the putamen has been utilized in predicting anhedonia severity.81
BRAIN NETWORK CHANGES AFTER BA AND PREDICTORS OF BA TREATMENT RESPONSES IN FMRI STUDIES ON DEPRESSION
Brain network changes after BA treatments in depression
Previous fMRI studies have examined the neural responses following BA treatments to elucidate their underlying neural mechanisms. Dichter et al.82 using a probabilistic monetary decision-making task comprising selection, anticipation, and feedback phases, and noted that patients with MDD showed activation changes in the frontal, temporal, and occipital regions associated with reward processing after receiving brief behavioral activation therapy for depression (BATD).83 The low activation of the putamen neural circuit observed in anhedonia58 normalized (i.e., increased) during the reward-selection phase. During the reward-anticipation phase there was increased activation of the left caudate nucleus (i.e., the dorsal striatum), which is strongly associated with reward processing (Table 1).50 Dichter et al.84 applied fMRI to the same subjects as in their earlier study82 and observed that patients with MDD who received BATD showed reduced activation of the prefrontal regions in response to cognitive control stimuli presented in sad contexts (Table 1). The finding of decreased prefrontal activation following psychotherapy contrasts with studies of the effects of psychopharmacological interventions in MDD typically showing increased prefrontal activation.85,86,87,88 These results are consistent with those of previous studies showing decreased prefrontal activity in response to cognitive control stimuli presented in sad contexts to patients with MDD following antidepressant treatment.89,90 Additionally, the medial prefrontal cortex (mPFC), dlPFC,91 and occipital cortex92 have been shown to be hyperactivated in response to negative versus neutral pictures in depression, and overrecruitment of the mPFC during transient sadness may reflect disturbed mood regulation.93 Thus, the therapeutic effects of BA may be attributable to normalization of the hyperactivity in prefrontal regions within negative or sad contexts.
Table 1. Research on changes in brain networks following behavioral activation treatment.
| Study | Participants | Intervention | Imaging paradigm/scan time points | Task | BA treatment outcomes | |
|---|---|---|---|---|---|---|
| Dichter et al.,82 2009 | 12 Adults with MDD and 15 adults without MDD | 8–14 weekly BATD sessions | fMRI/pre- to posttreatment | Wheel of Fortune task: a computerized two-choice decision-making task with probabilistic monetary outcomes, comprising selection, anticipation, and feedback phases | (1) Selection phase | |
| - Increased activation of the paracingulate gyrus, left putamen, right supramarginal gyrus, and left posterior temporal fusiform cortex | ||||||
| - Decreased activation of the left amygdala, left superior frontal gyrus, left superior occipital cortex, left occipital pole, left postcentral gyrus, left precentral gyrus, left supramarginal gyrus, and right inferior temporal gyrus | ||||||
| (2) Anticipation phase | ||||||
| - Increased activation of the left caudate, anterior cingulate gyrus, left middle frontal gyrus, left superior frontal gyrus, left lingual gyrus, left lateral and superior-lateral occipital cortex, left posterior parahippocampal gyrus, right insular cortex, right precuneus, right subcallosal cortex, right posterior temporal fusiform cortex, and bilateral precentral gyrus and temporal poles | ||||||
| - Decreased activation of the anterior inferior temporal gyrus | ||||||
| (3) Feedback phase | ||||||
| - Increased activation of the left planum temporale, right superior lateral occipital cortex, and right posterior temporal fusiform cortex | ||||||
| - Decreased activation of the left posterior cingulate, left caudate, left postcentral gyrus, and left paracingulate gyrus | ||||||
| Dichter et al.,84 2010 | 12 Adults with MDD and 15 adults without MDD | 8–14 weekly BATD sessions | fMRI/pre- to posttreatment | Event-related target-detection task | Decreased activation in response to cognitive control stimuli presented in sad contexts in prefrontal structures, including the paracingulate gyrus, right orbital frontal cortex, right frontal pole, left Heschl’s gyrus, left occipital pole, bilateral postcentral gyrus, bilateral precentral gyrus, left superior temporal gyrus, left middle temporal gyrus, right inferior posterior temporal gyrus, and right anterior supramarginal gyrus | |
| Mori et al.,94 2016 | 15 Adults with subthreshold depression and 15 controls | 5 weekly BA sessions | fMRI/pre- to posttreatment | Monetary incentive delay task involving gain, loss, and neutral trials | Significant activity increases in the left vlPFC and left angular gyrus for loss anticipation | |
| Shiota et al.,99 2017 | 56 Adults with subthreshold depression randomized to 27 and 29 in intervention and nonintervention groups, respectively | 5 weekly BA sessions | fMRI/pre- to posttreatment | Self-referential task: with two viewpoints (self/other) and two emotional valences (positive/negative) | Significant increase in dmPFC activation during other-perspective self-judgement on positive words | |
| Yokoyama et al.,102 2018 | 40 Adults with subthreshold depression randomized to 19 and 21 in intervention and nonintervention groups, respectively | 5 weekly BA sessions | rs-fMRI/pre- to posttreatment | Task-free condition: measurements from resting-state echo planar imaging | Reductions in connectivity of the subnetwork of the anterior DMN that connects to the dACC | |
| Cernasov et al.,112 2021 | 73 Participants with anhedonia: 38 and 35 underwent BATA and MBCT, respectively | 15 treatment sessions each | 7-T MRI/pre- to posttreatment | Task-free condition: measurements of rsFC | Significant reductions in anhedonia symptoms and in the average rsFC within the DMN and FPN over time; however, there is no association between the reduction in rsFC within the DMN and improvements in anhedonia | |
BA, behavioral activation; BATA, behavioral activation therapy for anhedonia; BATD, brief behavioral activation therapy for depression; dACC, dorsal anterior cingulate cortex; DMN, default-mode network; dmPFC, dorsal medial prefrontal cortex; fMRI, functional magnetic resonance imaging; FPN, frontoparietal network; MBCT, mindfulness-based cognitive therapy; MDD, major depressive disorder; MRI, magnetic resonance imaging; rsFC, resting-state functional connectivity; rs-fMRI, resting-state functional magnetic resonance imaging; vlPFC, ventrolateral prefrontal cortex.
Other studies have also found changes in regions primarily related to emotion regulation and executive control networks, with these changes differing from those reported by Dichter et al.84 Mori et al.94 found that BA treatment increased attenuated neural responses in the left ventrolateral prefrontal cortex (vlPFC) and angular gyrus during loss anticipation in a cohort with subthreshold depression. Earlier research had found that vlPFC activation during distracting sad emotions was lower in patients with MDD than in controls.95 Additionally, left-lateralized dysfunction is correlated with avoidance and decreased motivation, especially in patients with MDD.96 BA exerts the strongest and most-varied effects on avoidance modification since it directly addresses the nature of avoidance and specific mechanisms for managing it.97 Therefore, the increased activations of the left vlPFC and angular gyrus during anticipation of loss after BA treatment may be due to a reduced tendency to avoid loss; that is, an enhanced ability to engage in rewarding behaviors even in aversive situations. In contrast to previous studies,82,98 Mori et al.94 found no changes in the reward-related striatum were observed after BA treatment. This discrepancy may be due to differences in depression severity, with participants with subthreshold depression experiencing smaller neural changes than those in the clinical population, and these changes not being reflected in subcortical structures.94 Similarly, Shiota et al.99 observed that BA treatment increased activation of the dorsal medial prefrontal cortex (dmPFC) during other-perspective self-referential processing of positive words in participants with subthreshold depression. This increased activation was associated with improved depressive symptoms. Previous research has shown increased mPFC activation in individuals with depression that is associated with increased positive self-reference after CBT,100 and Shiota et al.99 replicated these findings using BA treatment. The making of judgements regarding one’s future when faced with undesirable information that requires adjusting beliefs about unfavorable future prospects requires the activation of networks that prevent harmful updating, which include the ventral striatum, thalamus, hippocampus, and dmPFC.101 Thus, dmPFC activation following BA treatment may contribute to preventing unfavorable self-updates and improving objective monitoring, which will alleviate depressive symptoms.
Changes in resting-state functional connectivity (rsFC) have been reported following BA treatments. Yokoyama et al.102 noted that connectivity of the subnetwork of the anterior DMN that connects to the dACC decreased after BA intervention in participants with subthreshold depression. These decreases were correlated with an increase in the health-related quality of life. The dACC is the core region of the salience circuit103 that can detect salience changes in internal and external environments, signal the need for further processing, and initiate appropriate cognitive control.103,104 In contrast, DMN connectivity has been deeply implicated in depressive self-referential processing, such as rumination,105,106,107,108,109 and hyperfunctioning of DMN circuits has been associated with higher levels of depressive rumination in MDD.110 BA treatment programs increase access to positive activities accompanied by reinforcement from the environment111 and provide specific methods to deal with rumination.30 Therefore, the findings of Yokoyama and colleagues suggest that BA treatments improve the quality of life by activating the dACC, which aids in detecting rewards and choosing positive behaviors, and by controlling the DMN, which is associated with rumination. The BA mechanism may be related to an enhanced ability to use the dACC and DMN independently, which can exert attentional control to favor positive stimuli in the external environment.102
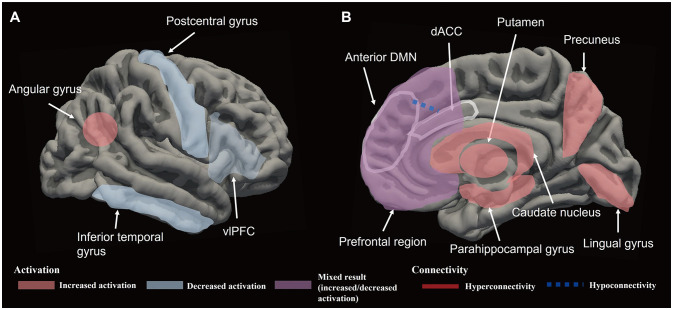
Cernasov et al.112 randomly assigned participants with anhedonic symptoms to BATA113 and mindfulness-based CT,114 and estimated changes in anhedonia and rsFC over time using the connectivity between the average regions of interest within the DMN, frontoparietal network (FPN), salience network, and reward network. The results showed significant reductions in anhedonia symptoms and average rsFC within the DMN and FPN over time in the groups receiving the different interventions. BATA-induced reductions in rsFC within the DMN were consistent with the findings of Yokoyama et al.102 However, no relationship was observed between within-person reductions in rsFC within the DMN and improvements in anhedonia. Previous studies have produced inconsistent results for the changes in rsFC within the DMN and symptom reduction after depression treatment (e.g., antidepressants and transcranial magnetic stimulation).115,116,117,118 These unexpected results may be due to DMN modulation having an indirect effect on anhedonia and more directly involving changes in related domains such as rumination.112 In addition, attenuation within the FPN was unexpected considering that hypoconnectivity in the FPN is an MDD hallmark.119,120,121 However, reductions in rsFC within the FPN have been reported in patients with MDD who were treated with mindfulness-based interventions122 and electroconvulsive therapy.123 Moreover, studies not involving rsFC have produced evidence of PFC hyperactivation during working-memory tasks in MDD.124,125 Thus, FPN hypoactivation following BA treatment may be related to the alleviation of compensatory forms of dysfunction associated with cognitive overload. An overall schematic of brain network changes after BA treatment in depression can be seen in Fig. 1.
Fig. 1. Schematic representations of the brain network changes following behavioral activation (BA) treatment in lateral (A) and medial (B) views. The brain network changes after BA treatments shown in the figure above are thought to contribute to the reduction in anhedonia and depresive symptoms. dACC, dorsal anterior cingulate cortex; DMN, default-mode network; vlPFC, ventrolateral prefrontal cortex.
Pretreatment brain network patterns and BA treatment responses
Independent of changes in brain activity patterns following BA treatment, studies have shown that the pretreatment brain activity status is predictive of the treatment effects of BA. Walsh et al.126 compared the neural connectivity in patients with MDD and healthy controls prior to treatment to investigate the role of global connectivity and connectivity attenuation as predictors of BATD responses. Their results indicated that patients exhibiting more-diverse patterns of brain connectivity (greater global connectivity or connectivity attenuation) in frontostriatal regions relative to controls responded better to BATD (Table 2). Furthermore, the significant clusters were primarily localized in regions within or bordering the dACC and mPFC, which play crucial roles in reward-based decision-making and behavior.127,128 A subsequent study by Walsh et al.129 found that reduced connectivity between the left middle frontal gyrus and right temporoparietal regions (which are involved in emotion regulation and selective attention)130 prior to treatment in MDD patients predicted a greater improvement in anhedonic symptoms following BATD. This is consistent with the earlier work of Walsh et al.126 suggesting that BATD is more effective in individuals who exhibit greater impairments in reward network function. Overall, patients with deficits in the connectivity of reward-processing-related regions and in the ability to sustain connectivity may especially benefit from the BA treatment model of increasing engagement in goal-directed activities and value-driven behaviors as well as increasing the focus on rewarding activities.1 Whereas most of these results suggest that individuals with greater disturbances in reward network function benefit more from BATD, normal connectivity patterns predicted better treatment outcomes in the single study of Walsh et al.126
Table 2. Research on pretreatment brain network activation patterns as predictors of BA treatment responses.
| Study | Participants | Intervention | Imagining paradigm/scan time points | Task | Pretreatment predictor | |
|---|---|---|---|---|---|---|
| Walsh et al.,126 2017 | 33 Outpatients with MDD and 20 controls | 15 weekly BATD sessions | fMRI/pretreatment | Monetary incentive delay task: anticipation and outcome phases | (1) Diverse patterns in patients compared with controls: | |
| - Greater connectivity between the left caudate seed and right paracingulate gyrus during reward anticipation | ||||||
| - Greater connectivity attenuation between the right frontal medial cortex seed and paracingulate gyrus during reward anticipation | ||||||
| - Greater connectivity attenuation between the left NAcc seed and paracingulate gyrus when receiving a reward outcome | ||||||
| (2) Similar patterns in patients compared with controls: | ||||||
| - Less connectivity attenuation between the right putamen seed and right OFC/temporal pole during reward anticipation | ||||||
| Walsh et al.,129 2019 | 33 Outpatients with MDD and 20 nondepressed controls | 15 weekly BATD sessions | fMRI/pretreatment | Positive-emotions regulation task: passive viewing and positive-upregulation condition | Decreased connectivity between the left middle frontal gyrus and right temporoparietal regions during upregulation of positive emotions | |
| Dichter et al.,84 2010 | 12 Adults with MDD and 15 adults without MDD | 8–14 weekly BATD sessions | fMRI/pre- to posttreatment | Event-related target-detection task | Lower activation of the paracingulate gyrus in sad contexts | |
| Webb et al.,137 2023 | 39 Anhedonic adolescents and 41 typically developing adolescents | 12 weekly BA sessions | fMRI/pre- to posttreatment | Event-related card-guessing task: win, loss, and neutral trial types | Larger right striatal response to reward | |
BA, behavioral activation; BATD, behavioral activation therapy for depression; fMRI, functional magnetic resonance imaging; MDD, major depressive disorder; NAcc, nucleus accumbens; OFC, orbital prefrontal cortex.
In contrast to the above studies, other studies have found that intact neuronal responses are predictive of better BA treatment outcomes. The study of Dichter et al.84 involving patients with MDD found that hypoactivation of the paracingulate gyrus cluster in sad contexts before BATD predicted improvement in depressive symptoms posttreatment. The PFC mediates affect regulation in emotional contexts131 and several goal-directed behaviors.132,133,134 When the task demand increases, PFC activity typically increases in a compensatory manner to ensure that the behavioral performance remains constant.135 Increased activation of multiple prefrontal regions has been reported in the MDD group in sad contexts,136 which suggests that a greater cognitive effort is needed from patients with MDD to disengage from sad pictures and respond to a positive stimuli.125 Thus, hypersensitivity to sad contexts may compromise treatment efficacy by increasing the difficulty in identifying and select rewarding positive activities, because greater cognitive effort is required to respond appropriately to the applied cognitive control stimuli. This suggests that individuals with a lower degree of hypersensitivity would benefit more from BA treatments. Additionally, Webb et al.137 found that in adolescents with anhedonia, a larger right striatal response pre-BA was associated with a greater improvement in anhedonia posttreatment. In order words, adolescents exhibiting relatively large striatal responses to rewards exhibited better BA treatment outcomes. This is similar to findings obtained when applying CBT for anxiety disorders, where greater striatal responsivity to rewards was associated with symptom improvement.138 The striatum is involved in reward processing, specifically in learning and updating behaviors that lead to rewards.139,140 Accordingly, it is believed that individuals with more-responsive reward circuits, such as the striatum, are more likely to engage in reward-focused activities that are addressed in BA (e.g., enjoyable and valuable activities), and that increased reward activity leads to increased pleasure and positive reinforcement, which in turn improves anhedonia.
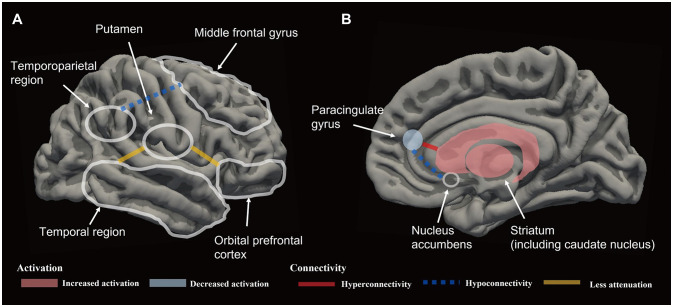
The association between brain network changes and BA treatment has been primarily investigated using fMRI, with few studies employing other modalities. However, some electroencephalography (EEG) studies have attempted to identify biomarkers for evaluating the effects of BA treatment. Gollan et al.141 examined alpha EEG asymmetry before and after BA treatment in patients with depression, and investigated how this was associated with emotions. They found that BA treatment did not change frontal alpha EEG asymmetry, which is known to be associated with motivation and emotional affect.142 However, the pretreatment level of frontal alpha asymmetry was predictive of treatment responses. Specifically, the presence of middle frontal and middle lateral alpha asymmetry prior to treatment did not predict posttreatment reductions in depressive symptoms, whereas pretreatment middle frontal alpha asymmetry did predict a posttreatment negative mood. These results suggest that BA treatment is less beneficial when greater prefrontal alpha asymmetry is present prior to treatment. Therefore, the pretreatment level of prefrontal alpha asymmetry may serve as a predictor of treatment response and relapse. Additionally, an analysis of resting EEG data by Anderson and Perone143 identified functional networks associated with the BAS and BIS,69 which are brain systems that regulate motivation, approach behavior, reward-seeking, and positive affect.144 That study revealed that the BAS and BIS are associated with different brain networks, with differences in the strengths of these networks potentially accounting for individual differences, particularly in the specific frequency bands (theta and beta) associated with the BAS subscales. Applying this to BA treatment would involve developing a personalized treatment plan by measuring the resting EEG signal to assess the strengths of the networks related to the BAS and BIS. Furthermore, the efficacy of the treatment can be objectively assessed by monitoring the changes in network strength between before and after treatment. In other words, the motivational BAS/BIS has potential as a biomarker for personalized interventions and objective assessments in BA treatment. An overall schematic of the pretreatment brain network patterns indicative of BA treatment response in depression can be seen in Fig. 2.
Fig. 2. Schematic representations of the pretreatment brain network patterns that are predictors of larger behavioral activation treatment responses in lateral (A) and medial (B) views. The pretreatment brain activation and connectivity shown in the figure above appears to be assoicated with improved treatment response.
Limitations of previous fMRI studies on BA and suggestions for future research
There have been few fMRI studies of BA and they have produced inconsistent results. These discrepancies can be attributed to several factors. One possible factor is the different treatment protocols used for BA. For example, Martell et al.29 emphasized the importance of avoidance in depression and so included techniques to recognize and overcome avoidance in their treatment, whereas BATD38 does not offer specific techniques for addressing avoidance. Additionally, BATA44 includes several new components specific to anhedonia, such as removing depression-specific content, adding psychoeducation about anhedonia and responses to rewards, and simplifying activity monitoring for patients with low motivation. Other possible explanations for the differences in study results include sample smallness and different traits being measured in each fMRI study task, such as reward sensitivity, cognitive control, and self-evaluation. Future research should address some of these issues in larger samples and by comparing the findings across multiple fMRI tasks that measure similar properties. Accumulating fMRI data from meta-analytical studies of BA treatment may also lead to the acquisition of consistent findings. For example, a group of researchers specializing in large-scale neuroimaging studies formed the so-called ENIGMA (Enhancing Neuroimaging Genetics through Meta-Analysis) network145 that has produced reliable and consistent neuroimaging findings by comparing healthy populations with various cohorts having psychiatric disorders including MDD,146 obsessive-compulsive disorder,147 and anxiety disorders.148 We propose that an fMRI consortium similar to the ENIGMA one could facilitate the identification of reliable neural correlates of BA treatment effects and treatment predictors.
CONCLUSION
The accumulating evidence for the efficacy of BA treatment on depression is leading it to becoming one of the main evidence-based treatments. Technological and conceptual advances in brain imaging have provided new insights into the brain circuits underlying the reward-processing network associated with depression and anhedonia. However, there is a gap between the advances in brain imaging and its application in real-world clinical practice. A literature review addressing neuroimaging endophenotypes can aid in bridging this gap by strengthening the understanding of therapeutic mechanisms for psychiatric disorders and suggesting predictors of treatment responses.
This review has provided insights into the mechanisms underlying BA treatment by revealing how it affects reward-related neural circuits. BA treatment induces increased activation of the prefrontal and subcortical regions (e.g., vlPFC, mPFC, anterior cingulate gyrus, posterior parahippocampal gyrus, insular cortex, and caudate putamen), which are known to be involved in reward processing.50,51,52,53,55,149 The functioning of brain circuits associated with sensitivity to rewards improved posttreatment, reflecting the therapeutic goal of BA of enabling individuals to increase the rewards that they experience in their daily lives.30 Deficits in regions involved in reward processing are strongly associated with anhedonia and other reward-related deficits.52,63,64,65,66,67 Together the results suggest that BA treatment induces a series of changes in the brain circuitry that processes rewards so as to increase the responsiveness to positive rewarding behaviors that are meaningful to the individual. Increases in positive rewarding behaviors improve anhedonia, which in turn leads to engagement in behaviors that can lead to greater rewards in life. Furthermore, the prefrontal and subcortical regions that exhibited increased activation after BA have been shown to be involved in reward processing and higher-order cognitive functions, such as problem-solving, decision-making,150,151,152,153 and emotion regulation.154,155,156,157 Thus, the beneficial effects of BA treatment may be due to improvements in reward circuits and the brain circuits that regulate cognitive and emotional responses to problems that are encountered. BA therapy addresses problem-solving, overcoming avoidance, and counteracting rumination that otherwise further entrench depression. These strategies may contribute to improved cognition and emotion regulation, adding to the therapeutic effects of BA.
However, some studies have found decreased activation of the prefrontal regions during cognitive control tasks in sad contexts after BA treatment. This contradictory activation direction may be attributable to studies that show increased activation posttreatment measuring reward selection or expectancy, while studies showing decreased activation posttreatment measure cognitive control in a negative context; that is, these two tasks are measuring different aspects. Prefrontal activation has been associated with exaggerated threat processing in depression and anxiety disorders,158 and hyperactivation of the mPFC in negative or sad contexts has additionally been reported in depression.91,93 Therefore, decreased activation of the prefrontal regions posttreatment suggests that BA aids patients with depression to reduce their engagement with negative or sad contexts. Hypersensitivity to sad contexts results in greater cognitive effort being needed to respond appropriately to cognitive control stimuli,125 which increases the difficulty of identifying and engaging in positive activities. Also, hypoactivation in negative contexts may cause cognitive attention to be diverted elsewhere. Thus, the therapeutic component of BA helps individuals with depression to counteract rumination by refocusing their attention on sensory experiences and positive stimuli.30
Studies have also found reductions in rsFC within the DMN after BA treatment. Resting-state analyses of depression have revealed a consistent profile of functional overactivation and hyperconnectivity of the default-mode circuits, with the hyperactivation of default-mode circuits in patients with MDD being strongly associated with maladaptive rumination about depressive thoughts.110,159 Rumination disconnects an individual from their surrounding real-world environment, causing the individual to become lost in their internal thoughts, ultimately hindering effective problem-solving.30 Therefore, one therapeutic mechanism of BA involves helping patients to escape from their own internal world, to stop ruminating and to re-engage in activities that are enjoyable and valuable to them.
This review has also provided insights into whether pretreatment reward reactivity or specific neural patterns are predictors of treatment outcomes. Analyses of neurocircuit biotypes can help to differentiate between individuals who respond well to BA treatment and those who do not, and to guide interventions tailored to each individual.160 Neuroimaging data have been used to perform brain-network-based biotyping to identify patients who may benefit from BA, ultimately enabling precision psychiatry. However, there are two contradictory findings: First, compared with a healthy cohort, improvements in depression or anhedonia were associated with deviations in connectivity between the paracingulate gyrus and left caudate seed, left NAcc seed, and right mPFC seed, and with reduced connectivity between the middle frontal gyrus and right temporoparietal regions, suggesting that BA treatment will be more effective in individuals with functional deficits in their reward network. Second, improvements in depression or anhedonia have been linked to larger right striatal responses, which are associated with reward processing, and lower activation of the paracingulate gyrus, which frees up cognition-regulation resources, indicating that intact neuronal responses to rewards may predict better BA treatment outcomes. These discrepancies can be respectively attributed to the capitalization model, which suggests that individuals with higher pretreatment reward functioning will benefit more from treatment that leverages their existing interest in and motivation to receive rewards, and to the compensatory model, which suggests that individuals with greater deficits in reward functioning will benefit more from treatment.21 Mixed results supporting the capitalization and compensation models have been obtained for CBT and BA.161,162,163 Considering the insufficient and contradictory evidence yielded by previous studies, increasing the benefit of BA treatments will require further research to elucidate the unique characteristics of individuals that determine whether a capitalization or compensation model is appropriate to apply to a specific individual.
Considering neurocircuit biotypes in treatment is consistent with a transdiagnostic and dimensional view of mental disorders, which attempts to understand mental disorders in terms of multiple factors or dimensions rather than categorizing them according to diagnostic criteria. Interventions need to be targeted at the specific neural circuitry of an individual, rather than one-size-fits-all interventions based on the diagnosis only. BA treatment has been found to be effective for depression, anxiety disorders,164 posttraumatic stress disorder,165 schizophrenia,166 substance use disorders,167 and other conditions, supporting the existence of a transdiagnostic component to the efficacy of this treatment modality. BATA is a transdiagnostic treatment for anhedonia that restores reward motivation and responsiveness in patients with various mental disorders and subthreshold disorders associated with high levels of anhedonia.
Finally, the neuroimaging findings for BA have implications for the development of preventive psychiatry. In a study168 of adolescents at a high sociodemographic risk of psychopathologies, researchers observed that adolescents with a higher BAS sensitivity69 exhibited weaker DMN connectivity with the subgenual anterior cingulate cortex (sgACC). Strong DMN–sgACC connectivity is associated with depressive disorders and negative affect,110,169 suggesting that the sensitivity of the approach-related positive-motivation system is a potential protective factor in the stress vulnerability circuitry of the brain. BA activates and engages individuals in specific ways, allowing them to experience more rewards in their daily lives.97 BA treatments may therefore aid in preventing the development of stress-related psychopathologies by increasing the BAS sensitivity to achieving goals. Adolescence, in particular, is a developmental period of increased sensitivity in reward-related neural circuits,170,171 and using an appropriate intervention such as BA during this period may be especially beneficial in preventing future depressive symptoms.
Footnotes
- Conceptualization: Kyu-Man Han.
- Funding acquisition: Kyu-Man Han.
- Investigation: Minjee Jung.
- Resources: Kyu-Man Han.
- Supervision: Kyu-Man Han.
- Writing—original draft: Minjee Jung, Kyu-Man Han.
- Writing—review & editing: Minjee Jung, Kyu-Man Han.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding Statement: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT) (No. 2022R1A2C4001313) and by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (No. HI23C0035).
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
References
- 1.Dimidjian S, Barrera M, Jr, Martell C, Muñoz RF, Lewinsohn PM. The origins and current status of behavioral activation treatments for depression. Annu Rev Clin Psychol. 2011;7:1–38. doi: 10.1146/annurev-clinpsy-032210-104535. [DOI] [PubMed] [Google Scholar]
- 2.Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol. 2006;74:658–670. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- 3.Dobson KS, Hollon SD, Dimidjian S, Schmaling KB, Kohlenberg RJ, Gallop RJ, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the prevention of relapse and recurrence in major depression. J Consult Clin Psychol. 2008;76:468–477. doi: 10.1037/0022-006X.76.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gawrysiak M, Nicholas C, Hopko DR. Behavioral activation for moderately depressed university students: randomized controlled trial. J Couns Psychol. 2009;56:468–475. [Google Scholar]
- 5.Jacobson NS, Gortner ET. Can depression be de-medicalized in the 21st century: scientific revolutions, counter-revolutions and the magnetic field of normal science. Behav Res Ther. 2000;38:103–117. doi: 10.1016/s0005-7967(99)00029-7. [DOI] [PubMed] [Google Scholar]
- 6.McCauley E, Gudmundsen G, Schloredt K, Martell C, Rhew I, Hubley S, et al. The adolescent behavioral activation program: adapting behavioral activation as a treatment for depression in adolescence. J Clin Child Adolesc Psychol. 2016;45:291–304. doi: 10.1080/15374416.2014.979933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuijpers P, van Straten A, Warmerdam L. Behavioral activation treatments of depression: a meta-analysis. Clin Psychol Rev. 2007;27:318–326. doi: 10.1016/j.cpr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Ekers D, Webster L, Van Straten A, Cuijpers P, Richards D, Gilbody S. Behavioural activation for depression; an update of meta-analysis of effectiveness and sub group analysis. PLoS One. 2014;9:e100100. doi: 10.1371/journal.pone.0100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazzucchelli T, Kane R, Rees C. Behavioral activation treatments for depression in adults: a meta-analysis and review. Clin Psychol Sci Pract. 2009;16:383–411. [Google Scholar]
- 10.Tindall L, Mikocka-Walus A, McMillan D, Wright B, Hewitt C, Gascoyne S. Is behavioural activation effective in the treatment of depression in young people? A systematic review and meta-analysis. Psychol Psychother. 2017;90:770–796. doi: 10.1111/papt.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin F, Oliver T. Behavioral activation for children and adolescents: a systematic review of progress and promise. Eur Child Adolesc Psychiatry. 2019;28:427–441. doi: 10.1007/s00787-018-1126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazdin AE. Treatment outcomes, common factors, and continued neglect of mechanisms of change. Clin Psychol Sci Pract. 2005;12:184–188. [Google Scholar]
- 13.Sankar A, Melin A, Lorenzetti V, Horton P, Costafreda SG, Fu CHY. A systematic review and meta-analysis of the neural correlates of psychological therapies in major depression. Psychiatry Res Neuroimaging. 2018;279:31–39. doi: 10.1016/j.pscychresns.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Chalah MA, Ayache SS. Disentangling the neural basis of cognitive behavioral therapy in psychiatric disorders: a focus on depression. Brain Sci. 2018;8:150. doi: 10.3390/brainsci8080150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franklin G, Carson AJ, Welch KA. Cognitive behavioural therapy for depression: systematic review of imaging studies. Acta Neuropsychiatr. 2016;28:61–74. doi: 10.1017/neu.2015.41. [DOI] [PubMed] [Google Scholar]
- 16.Ball TM, Stein MB, Paulus MP. Toward the application of functional neuroimaging to individualized treatment for anxiety and depression. Depress Anxiety. 2014;31:920–933. doi: 10.1002/da.22299. [DOI] [PubMed] [Google Scholar]
- 17.Tanguay-Sela M, Rollins C, Perez T, Qiang V, Golden G, Tunteng JF, et al. A systematic meta-review of patient-level predictors of psychological therapy outcome in major depressive disorder. J Affect Disord. 2022;317:307–318. doi: 10.1016/j.jad.2022.08.041. [DOI] [PubMed] [Google Scholar]
- 18.Chakrabarty T, Ogrodniczuk J, Hadjipavlou G. Predictive neuroimaging markers of psychotherapy response: a systematic review. Harv Rev Psychiatry. 2016;24:396–405. doi: 10.1097/HRP.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 19.Seeberg I, Kjaerstad HL, Miskowiak KW. Neural and behavioral predictors of treatment efficacy on mood symptoms and cognition in mood disorders: a systematic review. Front Psychiatry. 2018;9:337. doi: 10.3389/fpsyt.2018.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forbes CN. New directions in behavioral activation: using findings from basic science and translational neuroscience to inform the exploration of potential mechanisms of change. Clin Psychol Rev. 2020;79:101860. doi: 10.1016/j.cpr.2020.101860. [DOI] [PubMed] [Google Scholar]
- 22.Ferster CB. A functional anlysis of depression. Am Psychol. 1973;28:857–870. doi: 10.1037/h0035605. [DOI] [PubMed] [Google Scholar]
- 23.Lewinsohn PM. In: The psychology of depression. Friedman RJ, Katz MM, editors. Oxford: John Wiley & Sons; 1974. A behavioral approach to depression; pp. 157–174. [Google Scholar]
- 24.Lewinsohn PM, Shaffer M. Use of home observations as an integral part of the treatment of depression; preliminary report and case studies. J Consult Clin Psychol. 1971;37:87–94. doi: 10.1037/h0031297. [DOI] [PubMed] [Google Scholar]
- 25.Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York: Guilford Press; 1979. [Google Scholar]
- 26.Jacobson NS, Dobson KS, Truax PA, Addis ME, Koerner K, Gollan JK, et al. A component analysis of cognitive-behavioral treatment for depression. J Consult Clin Psychol. 1996;64:295–304. doi: 10.1037//0022-006x.64.2.295. [DOI] [PubMed] [Google Scholar]
- 27.Gortner ET, Gollan JK, Dobson KS, Jacobson NS. Cognitive-behavioral treatment for depression: relapse prevention. J Consult Clin Psychol. 1998;66:377–384. doi: 10.1037//0022-006x.66.2.377. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson NS, Martell CR, Dimidjian S. Behavioral activation treatment for depression: returning to contextual roots. Clin Psychol Sci Pract. 2001;8:255–270. [Google Scholar]
- 29.Martell CR, Addis ME, Jacobson NS. Depression in context: strategies for guided action. New York: W. W. Norton & Company; 2001. [Google Scholar]
- 30.Martell CR, Dimidjian S, Herman-Dunn R. Behavioral activation for depression: a clinician’s guide. New York: Guilford Press; 2010. [Google Scholar]
- 31.Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy. New York: Guilford Press; 1999. [Google Scholar]
- 32.Lewinsohn PM, Youngren MA, Grosscup SJ. In: The psychobiology of the depressive disorders: implications for the effects of stress. Depue RA, editor. New York: Academic Press; 1979. Reinforcement and depression; pp. 291–316. [Google Scholar]
- 33.Kanter JW, Manos RC, Bowe WM, Baruch DE, Busch AM, Rusch LC. What is behavioral activation? A review of the empirical literature. Clin Psychol Rev. 2010;30:608–620. doi: 10.1016/j.cpr.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Lewinsohn PM, Biglan A, Zeiss AM. In: Behavioral management of anxiety, depression and pain. Davidson PO, editor. New York: Brunner/Mazel; 1976. Behavioral treatment of depression; pp. 91–146. [Google Scholar]
- 35.Rehm LP. A self-control model of depression. Behav Ther. 1977;8:787–804. [Google Scholar]
- 36.McLean P. In: The behavioral management of anxiety, depression and pain. Davidson PO, editor. New York: Brunner/Mazel; 1976. Therapeutic decision-making in the behavioral treatment of depression; pp. 54–83. [Google Scholar]
- 37.Gallagher-Thompson D. Depression in the elderly: a behavioral treatment manual. Los Angeles: University of Southern California Press; 1981. [Google Scholar]
- 38.Lejuez CW, Hopko DR, Hopko SD. A brief behavioral activation treatment for depression. Treatment manual. Behav Modif. 2001;25:255–286. doi: 10.1177/0145445501252005. [DOI] [PubMed] [Google Scholar]
- 39.National Institute for Health and Care Excellence. Depression in adults: treatment and management. London: National Institute for Health and Care Excellence; 2022. [PubMed] [Google Scholar]
- 40.Parikh SV, Quilty LC, Ravitz P, Rosenbluth M, Pavlova B, Grigoriadis S, et al. Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 2. Psychological treatments. Can J Psychiatry. 2016;61:524–539. doi: 10.1177/0706743716659418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scottish Intercollegiate Guidelines Network. Non-pharmaceutical management of depression in adults: a national clinical guideline. Edinburgh: Scottish Intercollegiate Guidelines Network; 2010. [Google Scholar]
- 42.Dunn BD, German RE, Khazanov G, Xu C, Hollon SD, DeRubeis RJ. Changes in positive and negative affect during pharmacological treatment and cognitive therapy for major depressive disorder: a secondary analysis of two randomized controlled trials. Clin Psychol Sci. 2020;8:36–51. [Google Scholar]
- 43.Argyropoulos SV, Nutt DJ. Anhedonia revisited: is there a role for dopamine-targeting drugs for depression? J Psychopharmacol. 2013;27:869–877. doi: 10.1177/0269881113494104. [DOI] [PubMed] [Google Scholar]
- 44.Nagy GA, Cernasov P, Pisoni A, Walsh E, Dichter GS, Smoski MJ. Reward network modulation as a mechanism of change in behavioral activation. Behav Modif. 2020;44:186–213. doi: 10.1177/0145445518805682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carl H, Walsh E, Eisenlohr-Moul T, Minkel J, Crowther A, Moore T et al. Sustained anterior cingulate cortex activation during reward processing predicts response to psychotherapy in major depressive disorder. J Affect Disord. 2016;203:204–212. doi: 10.1016/j.jad.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alsayednasser B, Widnall E, O’Mahen H, Wright K, Warren F, Ladwa A, et al. How well do cognitive behavioural therapy and behavioural activation for depression repair anhedonia? A secondary analysis of the COBRA randomized controlled trial. Behav Res Ther. 2022;159:104185. doi: 10.1016/j.brat.2022.104185. [DOI] [PubMed] [Google Scholar]
- 47.Webb CA, Murray L, Tierney AO, Gates KM. Dynamic processes in behavioral activation therapy for anhedonic adolescents: modeling common and patient-specific relations. J Consult Clin Psychol. 2023 Jun 05; doi: 10.1037/ccp0000830. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watson R, Harvey K, Pass L, McCabe C, Reynolds S. A qualitative study exploring adolescents’ experience of brief behavioural activation for depression and its impact on the symptom of anhedonia. Psychol Psychother. 2021;94:266–288. doi: 10.1111/papt.12307. [DOI] [PubMed] [Google Scholar]
- 49.Halahakoon DC, Kieslich K, O’Driscoll C, Nair A, Lewis G, Roiser JP. Reward-processing behavior in depressed participants relative to healthy volunteers: a systematic review and meta-analysis. JAMA Psychiatry. 2020;77:1286–1295. doi: 10.1001/jamapsychiatry.2020.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Höflich A, Michenthaler P, Kasper S, Lanzenberger R. Circuit mechanisms of reward, anhedonia, and depression. Int J Neuropsychopharmacol. 2019;22:105–118. doi: 10.1093/ijnp/pyy081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borsini A, Wallis ASJ, Zunszain P, Pariante CM, Kempton MJ. Characterizing anhedonia: a systematic review of neuroimaging across the subtypes of reward processing deficits in depression. Cogn Affect Behav Neurosci. 2020;20:816–841. doi: 10.3758/s13415-020-00804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heshmati M, Russo SJ. Anhedonia and the brain reward circuitry in depression. Curr Behav Neurosci Rep. 2015;2:146–153. doi: 10.1007/s40473-015-0044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ng TH, Alloy LB, Smith DV. Meta-analysis of reward processing in major depressive disorder reveals distinct abnormalities within the reward circuit. Transl Psychiatry. 2019;9:293. doi: 10.1038/s41398-019-0644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Treadway MT, Zald DH. Parsing anhedonia: translational models of reward-processing deficits in psychopathology. Curr Dir Psychol Sci. 2013;22:244–249. doi: 10.1177/0963721412474460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Treadway MT. The neurobiology of motivational deficits in depression—an update on candidate pathomechanisms. Curr Top Behav Neurosci. 2016;27:337–355. doi: 10.1007/7854_2015_400. [DOI] [PubMed] [Google Scholar]
- 58.Wang S, Leri F, Rizvi SJ. Anhedonia as a central factor in depression: neural mechanisms revealed from preclinical to clinical evidence. Prog Neuropsychopharmacol Biol Psychiatry. 2021;110:110289. doi: 10.1016/j.pnpbp.2021.110289. [DOI] [PubMed] [Google Scholar]
- 59.Husain M, Roiser JP. Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nat Rev Neurosci. 2018;19:470–484. doi: 10.1038/s41583-018-0029-9. [DOI] [PubMed] [Google Scholar]
- 60.Schultz W. Neuronal reward and decision signals: from theories to data. Physiol Rev. 2015;95:853–951. doi: 10.1152/physrev.00023.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, et al. Reward processing in depression: a conceptual and meta-analytic review across fMRI and EEG studies. Am J Psychiatry. 2018;175:1111–1120. doi: 10.1176/appi.ajp.2018.17101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang X, Su Y, Yang F, Song Y, Yan J, Luo Y, et al. Neurofunctional mapping of reward anticipation and outcome for major depressive disorder: a voxel-based meta-analysis. Psychol Med. 2022;52:3309–3322. doi: 10.1017/S0033291722002707. [DOI] [PubMed] [Google Scholar]
- 63.Xie C, Jia T, Rolls ET, Robbins TW, Sahakian BJ, Zhang J, et al. Reward versus nonreward sensitivity of the medial versus lateral orbitofrontal cortex relates to the severity of depressive symptoms. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:259–269. doi: 10.1016/j.bpsc.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 64.Young CB, Chen T, Nusslock R, Keller J, Schatzberg AF, Menon V. Anhedonia and general distress show dissociable ventromedial prefrontal cortex connectivity in major depressive disorder. Transl Psychiatry. 2016;6:e810. doi: 10.1038/tp.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Faramarzi A, Sharini H, Shanbehzadeh M, Pour MY, Fooladi M, Jalalvandi M, et al. Anhedonia symptoms: the assessment of brain functional mechanism following music stimuli using functional magnetic resonance imaging. Psychiatry Res Neuroimaging. 2022;326:111532. doi: 10.1016/j.pscychresns.2022.111532. [DOI] [PubMed] [Google Scholar]
- 66.Liu X, Li L, Li M, Ren Z, Ma P. Characterizing the subtype of anhedonia in major depressive disorder: a symptom-specific multimodal MRI study. Psychiatry Res Neuroimaging. 2021;308:111239. doi: 10.1016/j.pscychresns.2020.111239. [DOI] [PubMed] [Google Scholar]
- 67.Yang XH, Huang J, Lan Y, Zhu CY, Liu XQ, Wang YF, et al. Diminished caudate and superior temporal gyrus responses to effort-based decision making in patients with first-episode major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:52–59. doi: 10.1016/j.pnpbp.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 68.Hu Y, Zhao C, Zhao H, Qiao J. Abnormal functional connectivity of the nucleus accumbens subregions mediates the association between anhedonia and major depressive disorder. BMC Psychiatry. 2023;23:282. doi: 10.1186/s12888-023-04693-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Pers Soc Psychol. 1994;67:319–333. [Google Scholar]
- 70.Sharma A, Wolf DH, Ciric R, Kable JW, Moore TM, Vandekar SN, et al. Common dimensional reward deficits across mood and psychotic disorders: a connectome-wide association study. Am J Psychiatry. 2017;174:657–666. doi: 10.1176/appi.ajp.2016.16070774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hwang JW, Xin SC, Ou YM, Zhang WY, Liang YL, Chen J, et al. Enhanced default mode network connectivity with ventral striatum in subthreshold depression individuals. J Psychiatr Res. 2016;76:111–120. doi: 10.1016/j.jpsychires.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Enneking V, Krüssel P, Zaremba D, Dohm K, Grotegerd D, Förster K, et al. Social anhedonia in major depressive disorder: a symptom-specific neuroimaging approach. Neuropsychopharmacology. 2019;44:883–889. doi: 10.1038/s41386-018-0283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu S, Wu C, Jia L, Fang Z, Lu J, Mou T, et al. Increased plasma levels of IL-6 are associated with striatal structural atrophy in major depressive disorder patients with anhedonia. Front Psychiatry. 2022;13:1016735. doi: 10.3389/fpsyt.2022.1016735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang XH, Wang Y, Wang DF, Tian K, Cheung EFC, Xie GR, et al. White matter microstructural abnormalities and their association with anticipatory anhedonia in depression. Psychiatry Res Neuroimaging. 2017;264:29–34. doi: 10.1016/j.pscychresns.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 76.Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage. 2009;46:327–337. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mu Q, Cui D, Zhang K, Ru Y, Wu C, Fang Z, et al. Volume changes of the subcortical limbic structures in major depressive disorder patients with and without anhedonia. Psychiatry Res Neuroimaging. 2023;336:111747. doi: 10.1016/j.pscychresns.2023.111747. [DOI] [PubMed] [Google Scholar]
- 78.Dillon DG, Gonenc A, Belleau E, Pizzagalli DA. Depression is associated with dimensional and categorical effects on white matter pathways. Depress Anxiety. 2018;35:440–447. doi: 10.1002/da.22734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim MJ, Hamilton JP, Gotlib IH. Reduced caudate gray matter volume in women with major depressive disorder. Psychiatry Res. 2008;164:114–122. doi: 10.1016/j.pscychresns.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harvey PO, Pruessner J, Czechowska Y, Lepage M. Individual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in non-clinical subjects. Mol Psychiatry. 2007;12:767–775. doi: 10.1038/sj.mp.4002021. [DOI] [PubMed] [Google Scholar]
- 81.Auerbach RP, Pisoni A, Bondy E, Kumar P, Stewart JG, Yendiki A, et al. Neuroanatomical prediction of anhedonia in adolescents. Neuropsychopharmacology. 2017;42:2087–2095. doi: 10.1038/npp.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, Smoski MJ. The effects of psychotherapy on neural responses to rewards in major depression. Biol Psychiatry. 2009;66:886–897. doi: 10.1016/j.biopsych.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hopko DR, Lejuez CW, Ruggiero KJ, Eifert GH. Contemporary behavioral activation treatments for depression: procedures, principles, and progress. Clin Psychol Rev. 2003;23:699–717. doi: 10.1016/s0272-7358(03)00070-9. [DOI] [PubMed] [Google Scholar]
- 84.Dichter GS, Felder JN, Smoski MJ. The effects of brief behavioral activation therapy for depression on cognitive control in affective contexts: an fMRI investigation. J Affect Disord. 2010;126:236–244. doi: 10.1016/j.jad.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Norbury R, Mackay CE, Cowen PJ, Goodwin GM, Harmer CJ. Short-term antidepressant treatment and facial processing. Functional magnetic resonance imaging study. Br J Psychiatry. 2007;190:531–532. doi: 10.1192/bjp.bp.106.031393. [DOI] [PubMed] [Google Scholar]
- 86.Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 87.Fales CL, Barch DM, Rundle MM, Mintun MA, Mathews J, Snyder AZ, et al. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J Affect Disord. 2009;112:206–211. doi: 10.1016/j.jad.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Langenecker SA, Kennedy SE, Guidotti LM, Briceno EM, Own LS, Hooven T, et al. Frontal and limbic activation during inhibitory control predicts treatment response in major depressive disorder. Biol Psychiatry. 2007;62:1272–1280. doi: 10.1016/j.biopsych.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miskowiak KW, Favaron E, Hafizi S, Inkster B, Goodwin GM, Cowen PJ, et al. Effects of erythropoietin on emotional processing biases in patients with major depression: an exploratory fMRI study. Psychopharmacology (Berl) 2009;207:133–142. doi: 10.1007/s00213-009-1641-1. [DOI] [PubMed] [Google Scholar]
- 90.Benedetti F, Radaelli D, Bernasconi A, Dallaspezia S, Colombo C, Smeraldi E. Changes in medial prefrontal cortex neural responses parallel successful antidepressant combination of venlafaxine and light therapy. Arch Ital Biol. 2009;147(Suppl 1):83–93. [PubMed] [Google Scholar]
- 91.Wagner V, Müller JL, Sommer M, Klein HE, Hajak G. [Changes in the emotional processing in depressive patients: a study with functional magnetoresonance tomography under the employment of pictures with affective contents] Psychiatr Prax. 2004;31(Suppl 1):S70–S72. doi: 10.1055/s-2004-828410. German. [DOI] [PubMed] [Google Scholar]
- 92.Davidson RJ. Affective neuroscience and psychophysiology: toward a synthesis. Psychophysiology. 2003;40:655–665. doi: 10.1111/1469-8986.00067. [DOI] [PubMed] [Google Scholar]
- 93.Beauregard M, Leroux JM, Bergman S, Arzoumanian Y, Beaudoin G, Bourgouin P, et al. The functional neuroanatomy of major depression: an fMRI study using an emotional activation paradigm. Neuroreport. 1998;9:3253–3258. doi: 10.1097/00001756-199810050-00022. [DOI] [PubMed] [Google Scholar]
- 94.Mori A, Okamoto Y, Okada G, Takagaki K, Jinnin R, Takamura M, et al. Behavioral activation can normalize neural hypoactivation in subthreshold depression during a monetary incentive delay task. J Affect Disord. 2016;189:254–262. doi: 10.1016/j.jad.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 95.Wang L, LaBar KS, Smoski M, Rosenthal MZ, Dolcos F, Lynch TR, et al. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Res. 2008;163:143–155. doi: 10.1016/j.pscychresns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jacobs BL. Depression: the brain finally gets into the act. Curr Dir Psychol Sci. 2004;13:103–106. [Google Scholar]
- 97.Dimidjian S, Martell CR, Addis ME, Herman-Dunn R, Barlow DH. In: Clinical handbook of psychological disorders: a step-by-step treatment manual. 4th ed. Barlow DH, editor. New York: Guildford Press; 2008. Behavioral activation for depression; pp. 328–364. [Google Scholar]
- 98.Stoy M, Schlagenhauf F, Sterzer P, Bermpohl F, Hägele C, Suchotzki K, et al. Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. J Psychopharmacol. 2012;26:677–688. doi: 10.1177/0269881111416686. [DOI] [PubMed] [Google Scholar]
- 99.Shiota S, Okamoto Y, Okada G, Takagaki K, Takamura M, Mori A, et al. Effects of behavioural activation on the neural basis of other perspective self-referential processing in subthreshold depression: a functional magnetic resonance imaging study. Psychol Med. 2017;47:877–888. doi: 10.1017/S0033291716002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yoshimura S, Okamoto Y, Onoda K, Matsunaga M, Okada G, Kunisato Y, et al. Cognitive behavioral therapy for depression changes medial prefrontal and ventral anterior cingulate cortex activity associated with self-referential processing. Soc Cogn Affect Neurosci. 2014;9:487–493. doi: 10.1093/scan/nst009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kuzmanovic B, Jefferson A, Vogeley K. The role of the neural reward circuitry in self-referential optimistic belief updates. Neuroimage. 2016;133:151–162. doi: 10.1016/j.neuroimage.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 102.Yokoyama S, Okamoto Y, Takagaki K, Okada G, Takamura M, Mori A, et al. Effects of behavioral activation on default mode network connectivity in subthreshold depression: a preliminary resting-state fMRI study. J Affect Disord. 2018;227:156–163. doi: 10.1016/j.jad.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 103.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li B, Liu L, Friston KJ, Shen H, Wang L, Zeng LL, et al. A treatment-resistant default mode subnetwork in major depression. Biol Psychiatry. 2013;74:48–54. doi: 10.1016/j.biopsych.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 106.Sundermann B, Olde Lütke Beverborg M, Pfleiderer B. Toward literature-based feature selection for diagnostic classification: a meta-analysis of resting-state fMRI in depression. Front Hum Neurosci. 2014;8:692. doi: 10.3389/fnhum.2014.00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zeng LL, Wang D, Fox MD, Sabuncu M, Hu D, Ge M, et al. Neurobiological basis of head motion in brain imaging. Proc Natl Acad Sci U S A. 2014;111:6058–6062. doi: 10.1073/pnas.1317424111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zeng LL, Shen H, Liu L, Hu D. Unsupervised classification of major depression using functional connectivity MRI. Hum Brain Mapp. 2014;35:1630–1641. doi: 10.1002/hbm.22278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhou HX, Chen X, Shen YQ, Li L, Chen NX, Zhu ZC, et al. Rumination and the default mode network: meta-analysis of brain imaging studies and implications for depression. Neuroimage. 2020;206:116287. doi: 10.1016/j.neuroimage.2019.116287. [DOI] [PubMed] [Google Scholar]
- 110.Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol Psychiatry. 2015;78(BADS):224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Takagaki K, Okajima I, Kunisato Y, Nakajima S, Kanai Y, Ishikawa S, et al. Development and validation of the Japanese version of the behavioral activation for depression scale (BADS) Arch Psychiatr Diagn Clin Eval. 2013;6:76–85. [Google Scholar]
- 112.Cernasov P, Walsh EC, Kinard JL, Kelley L, Phillips R, Pisoni A, et al. Multilevel growth curve analyses of behavioral activation for anhedonia (BATA) and mindfulness-based cognitive therapy effects on anhedonia and resting-state functional connectivity: interim results of a randomized trial. J Affect Disord. 2021;292:161–171. doi: 10.1016/j.jad.2021.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lejuez CW, Hopko DR, Acierno R, Daughters SB, Pagoto SL. Ten year revision of the brief behavioral activation treatment for depression: revised treatment manual. Behav Modif. 2011;35:111–161. doi: 10.1177/0145445510390929. [DOI] [PubMed] [Google Scholar]
- 114.Teasdale JD. In: Buddhist thought and applied psychological research. Nauriyal DK, Drummond MS, Lal YB, editors. London: Routledge; 2006. Mindfulness-based cognitive therapy for depression; pp. 450–466. [Google Scholar]
- 115.Posner J, Hellerstein DJ, Gat I, Mechling A, Klahr K, Wang Z, et al. Antidepressants normalize the default mode network in patients with dysthymia. JAMA Psychiatry. 2013;70:373–382. doi: 10.1001/jamapsychiatry.2013.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.van de Ven V, Wingen M, Kuypers KP, Ramaekers JG, Formisano E. Escitalopram decreases cross-regional functional connectivity within the default-mode network. PLoS One. 2013;8:e68355. doi: 10.1371/journal.pone.0068355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry. 2014;76:517–526. doi: 10.1016/j.biopsych.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Philip NS, Barredo J, van’t Wout-Frank M, Tyrka AR, Price LH, Carpenter LL. Network mechanisms of clinical response to transcranial magnetic stimulation in posttraumatic stress disorder and major depressive disorder. Biol Psychiatry. 2018;83:263–272. doi: 10.1016/j.biopsych.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I. Resting-state functional connectivity in major depressive disorder: a review. Neurosci Biobehav Rev. 2015;56:330–344. doi: 10.1016/j.neubiorev.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 121.Soares JC, Mann JJ. The functional neuroanatomy of mood disorders. J Psychiatr Res. 1997;31:393–432. doi: 10.1016/s0022-3956(97)00016-2. [DOI] [PubMed] [Google Scholar]
- 122.Lifshitz M, Sacchet MD, Huntenburg JM, Thiery T, Fan Y, Gärtner M, et al. Mindfulness-based therapy regulates brain connectivity in major depression. Psychother Psychosom. 2019;88:375–377. doi: 10.1159/000501170. [DOI] [PubMed] [Google Scholar]
- 123.Perrin JS, Merz S, Bennett DM, Currie J, Steele DJ, Reid IC, et al. Electroconvulsive therapy reduces frontal cortical connectivity in severe depressive disorder. Proc Natl Acad Sci U S A. 2012;109:5464–5468. doi: 10.1073/pnas.1117206109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Harvey PO, Fossati P, Pochon JB, Levy R, Lebastard G, Lehéricy S, et al. Cognitive control and brain resources in major depression: an fMRI study using the n-back task. Neuroimage. 2005;26:860–869. doi: 10.1016/j.neuroimage.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 125.Matsuo K, Glahn DC, Peluso MA, Hatch JP, Monkul ES, Najt P, et al. Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol Psychiatry. 2007;12:158–166. doi: 10.1038/sj.mp.4001894. [DOI] [PubMed] [Google Scholar]
- 126.Walsh E, Carl H, Eisenlohr-Moul T, Minkel J, Crowther A, Moore T, et al. Attenuation of frontostriatal connectivity during reward processing predicts response to psychotherapy in major depressive disorder. Neuropsychopharmacology. 2017;42:831–843. doi: 10.1038/npp.2016.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, et al. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry. 2004;55:594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 128.Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, et al. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Walsh EC, Eisenlohr-Moul TA, Minkel J, Bizzell J, Petty C, Crowther A, et al. Pretreatment brain connectivity during positive emotion upregulation predicts decreased anhedonia following behavioral activation therapy for depression. J Affect Disord. 2019;243:188–192. doi: 10.1016/j.jad.2018.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Smoski MJ, Keng SL, Schiller CE, Minkel J, Dichter GS. Neural mechanisms of cognitive reappraisal in remitted major depressive disorder. J Affect Disord. 2013;151:171–177. doi: 10.1016/j.jad.2013.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 132.Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124(Pt 10):2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- 133.Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 134.Fichtenholtz HM, Dean HL, Dillon DG, Yamasaki H, McCarthy G, LaBar KS. Emotion-attention network interactions during a visual oddball task. Brain Res Cogn Brain Res. 2004;20:67–80. doi: 10.1016/j.cogbrainres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 135.Adler CM, Sax KW, Holland SK, Schmithorst V, Rosenberg L, Strakowski SM. Changes in neuronal activation with increasing attention demand in healthy volunteers: an fMRI study. Synapse. 2001;42:266–272. doi: 10.1002/syn.1112. [DOI] [PubMed] [Google Scholar]
- 136.Dichter GS, Felder JN, Smoski MJ. Affective context interferes with cognitive control in unipolar depression: an fMRI investigation. J Affect Disord. 2009;114:131–142. doi: 10.1016/j.jad.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Webb CA, Murray L, Tierney AO, Forbes EE, Pizzagalli DA. Reward-related predictors of symptom change in behavioral activation therapy for anhedonic adolescents: a multimodal approach. Neuropsychopharmacology. 2023;48:623–632. doi: 10.1038/s41386-022-01481-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sequeira SL, Silk JS, Ladouceur CD, Hanson JL, Ryan ND, Morgan JK, et al. Association of neural reward circuitry function with response to psychotherapy in youths with anxiety disorders. Am J Psychiatry. 2021;178:343–351. doi: 10.1176/appi.ajp.2020.20010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 140.Delgado MR, Miller MM, Inati S, Phelps EA. An fMRI study of reward-related probability learning. Neuroimage. 2005;24:862–873. doi: 10.1016/j.neuroimage.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 141.Gollan JK, Hoxha D, Chihade D, Pflieger ME, Rosebrock L, Cacioppo J. Frontal alpha EEG asymmetry before and after behavioral activation treatment for depression. Biol Psychol. 2014;99:198–208. doi: 10.1016/j.biopsycho.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: a meta-analytic review. J Abnorm Psychol. 2006;115:715–729. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- 143.Anderson AJ, Perone S. Predicting individual differences in behavioral activation and behavioral inhibition from functional networks in the resting EEG. Biol Psychol. 2023;177:108483. doi: 10.1016/j.biopsycho.2022.108483. [DOI] [PubMed] [Google Scholar]
- 144.Gray JA. The psychology of fear and stress. 2nd ed. Cambridge: Cambridge University Press; 1987. [Google Scholar]
- 145.Stein JL, Medland SE, Vasquez AA, Hibar DP, Senstad RE, Winkler AM, et al. Identification of common variants associated with human hippocampal and intracranial volumes. Nat Genet. 2012;44:552–561. doi: 10.1038/ng.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schmaal L, Pozzi E, C Ho T, van Velzen LS, Veer IM, Opel N, et al. ENIGMA MDD: seven years of global neuroimaging studies of major depression through worldwide data sharing. Transl Psychiatry. 2020;10:172. doi: 10.1038/s41398-020-0842-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.van den Heuvel OA, Boedhoe PSW, Bertolin S, Bruin WB, Francks C, Ivanov I, et al. An overview of the first 5-years of the ENIGMA obsessive-compulsive disorder working group: the power of worldwide collaboration. Hum Brain Mapp. 2022;43:23–36. doi: 10.1002/hbm.24972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bas-Hoogendam JM, Groenewold NA, Aghajani M, Freitag GF, Harrewijn A, Hilbert K, et al. ENIGMA-anxiety working group: rationale for and organization of large-scale neuroimaging studies of anxiety disorders. Hum Brain Mapp. 2022;43:83–112. doi: 10.1002/hbm.25100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sescousse G, Redouté J, Dreher JC. The architecture of reward value coding in the human orbitofrontal cortex. J Neurosci. 2010;30:13095–13104. doi: 10.1523/JNEUROSCI.3501-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cole MW, Schneider W. The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 152.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Friedman NP, Robbins TW. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology. 2022;47:72–89. doi: 10.1038/s41386-021-01132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167:545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Alexandra Kredlow M, Fenster RJ, Laurent ES, Ressler KJ, Phelps EA. Prefrontal cortex, amygdala, and threat processing: implications for PTSD. Neuropsychopharmacology. 2022;47:247–259. doi: 10.1038/s41386-021-01155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Suzuki Y, Tanaka SC. Functions of the ventromedial prefrontal cortex in emotion regulation under stress. Sci Rep. 2021;11:18225. doi: 10.1038/s41598-021-97751-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Williams JMG, Watts FN, MacLeod C, Mathews A. Cognitive psychology and emotional disorders. Chichester: John Wiley & Sons; 1988. [Google Scholar]
- 159.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Williams LM. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry. 2016;3:472–480. doi: 10.1016/S2215-0366(15)00579-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cheavens JS, Strunk DR, Lazarus SA, Goldstein LA. The compensation and capitalization models: a test of two approaches to individualizing the treatment of depression. Behav Res Ther. 2012;50:699–706. doi: 10.1016/j.brat.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 162.Burkhouse KL, Kujawa A, Kennedy AE, Shankman SA, Langenecker SA, Phan KL, et al. Neural reactivity to reward as a predictor of cognitive behavioral therapy response in anxiety and depression. Depress Anxiety. 2016;33:281–288. doi: 10.1002/da.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Burkhouse KL, Gorka SM, Klumpp H, Kennedy AE, Karich S, Francis J, et al. Neural responsiveness to reward as an index of depressive symptom change following cognitive-behavioral therapy and SSRI treatment. J Clin Psychiatry. 2018;79:17m11836. doi: 10.4088/JCP.17m11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hopko DR, Robertson SMC, Lejuez CW. Behavioral activation for anxiety disorders. Behav Anal Today. 2006;7:212–232. [Google Scholar]
- 165.Etherton JL, Farley R. Behavioral activation for PTSD: a meta-analysis. Psychol Trauma. 2022;14:894–901. doi: 10.1037/tra0000566. [DOI] [PubMed] [Google Scholar]
- 166.Choi KH, Jaekal E, Lee GY. Motivational and behavioral activation as an adjunct to psychiatric rehabilitation for mild to moderate negative symptoms in individuals with schizophrenia: a proof-of-concept pilot study. Front Psychol. 2016;7:1759. doi: 10.3389/fpsyg.2016.01759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pott SL, Delgadillo J, Kellett S. Is behavioral activation an effective and acceptable treatment for co-occurring depression and substance use disorders? A meta-analysis of randomized controlled trials. J Subst Abuse Treat. 2022;132:108478. doi: 10.1016/j.jsat.2021.108478. [DOI] [PubMed] [Google Scholar]
- 168.Iadipaolo AS, Marusak HA, Sala-Hamrick K, Crespo LM, Thomason ME, Rabinak CA. Behavioral activation sensitivity and default mode network-subgenual cingulate cortex connectivity in youth. Behav Brain Res. 2017;333:135–141. doi: 10.1016/j.bbr.2017.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol Psychiatry. 2013;74:898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Curr Opin Neurobiol. 2010;20:236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cereb Cortex. 2010;20:61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.