Highlights
-
•
STAT3 plays a pivotal role in tumor growth and progression.
-
•
Targeted inhibition of STAT3 can overcome resistance to chemotherapeutic drugs and enhance their effectiveness.
-
•
STAT3 inhibition as a promising therapeutic approach for treating medulloblastoma.
-
•
Combining STAT3 inhibitors with radiotherapy, chemotherapy, or immunotherapy can improve treatment outcomes and prognosis.
Keywords: STAT3 inhibitors, Medulloblastoma, Chemotherapy resistance, Cancer, Targeted therapy
Abstract
Medulloblastoma is a type of brain cancer that primarily affects children. While chemotherapy has been shown to be effective in treating medulloblastoma, the development of chemotherapy resistance remains a challenge. One potential therapeutic approach is to selectively inhibit the inducible transcription factor called STAT3, which is known to play a crucial role in the survival and growth of tumor cells. The activation of STAT3 has been linked to the growth and progression of various cancers, including medulloblastoma. Inhibition of STAT3 has been shown to sensitize medulloblastoma cells to chemotherapy, leading to improved treatment outcomes. Different approaches to STAT3 inhibition have been developed, including small-molecule inhibitors and RNA interference. Preclinical studies have shown the efficacy of STAT3 inhibitors in medulloblastoma, and clinical trials are currently ongoing to evaluate their safety and effectiveness in patients with various solid tumors, including medulloblastoma. In addition, researchers are also exploring ways to optimize the use of STAT3 inhibitors in combination with chemotherapy and identify biomarkers that can predict treatment that will help to develop personalized treatment strategies. This review highlights the potential of selective inhibition of STAT3 as a novel approach for the treatment of medulloblastoma and suggests that further research into the development of STAT3 inhibitors could lead to improved outcomes for patients with aggressive cancer.
Graphical abstract
Introduction
Medulloblastoma is a type of malignant brain tumor that mainly affects children. It arises from the cerebellum, which is the part of the brain responsible for coordinating movement and maintaining balance [1]. Medulloblastoma is a fast-growing cancer that can spread to other parts of the brain and spinal cord, making it a life-threatening condition if not treated promptly.[2] Medulloblastoma accounts for approximately 15% to 20% of all childhood brain tumors, and it is the most common malignant brain tumor in children [3]. The incidence of medulloblastoma varies by geographic region, with higher incidence rates reported in North America and Western Europe than in other parts of the world [4]. In the United States, medulloblastoma accounts for approximately 7% of all childhood cancers, with an estimated 500-600 new cases diagnosed each year [5]. The incidence rate of medulloblastoma in the United States is approximately 0.5–1.0 cases per 100,000 children under the age of 15 [6]. The cause of medulloblastoma is still not fully understood, but there are some genetic and environmental factors that may increase the risk of developing this type of cancer [7]. Symptoms of medulloblastoma can vary depending on the location and size of the tumor, but they often include headaches, vomiting, dizziness, and problems with balance and coordination [8].
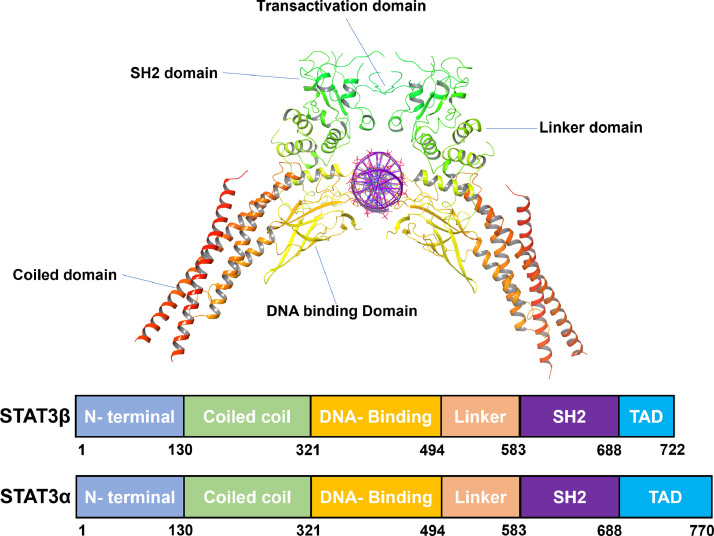
The signal transducer and activator of transcription (STAT3) is a transcription factor comprising several distinct domains that orchestrate its activation and function [9]. STAT3 is a 770-amino acid-long protein with six domains shown in Fig. 1. The structural organization of STAT3 consists of an N-terminal domain (1–130 amino acids) responsible for protein–protein interactions and nuclear localization, followed by a coiled-coil domain (131–320 amino acids) that mediates dimerization. Adjacent to this, a DNA-binding domain (321–465 amino acids) is essential for sequence-specific DNA recognition, followed by a linker region (466–585 amino acids). The linker domain connects the DNA-binding domain to the Src homology 2 (SH2) domain (586–688 amino acids), which plays a crucial role in receptor tyrosine kinase recognition and phosphorylation. Finally, a C-terminal transactivation domain (689–770 amino acids) regulates target gene transcription [[10], [11], [12]].
Fig. 1.
Structural organization of STAT3 [161].
STAT3 activation involves phosphorylation at a tyrosine residue in the SH2 domain, leading to dimerization, nuclear translocation, and subsequent gene regulation, making it a pivotal component in mediating cellular responses to various cytokines and growth factors [13]. The SH2 domain of STAT3 is essential for the activation and dimerization of the protein; the majority of STAT3 inhibitors target this domain [14]. A variety of amino acid residues, such as Arg609, Ser613, Glu614, Asp618, and Leu662, are implicated in inhibitor binding [15] as shown in Fig. 2. To avoid STAT3 activation, small compounds are designed to bind to the SH2 domain containing the tyrosine residue to compete with the natural ligands. To avoid STAT3 activation, small compounds that are made to resemble the phosphorylated tyrosine residue may compete with natural ligands for binding to the SH2 domain [16]. Numerous peptides and small compounds have been researched as possible STAT3 inhibitors. These substances frequently act to obstruct STAT3′s capacity to bind to the DNA and activate target genes, as well as to interfere with the protein–protein interactions necessary for STAT3 dimerization [17,18].
Fig. 2.
(A) This diagram illustrates the mechanism of STAT3. Upon cytokine stimulation, JAK activates STAT3 through phosphorylation. Phosphorylated STAT3 forms dimers and translocates to the nucleus, initiating specific gene expression. (B) Binding site. (C) Interaction with ligand.
There are two isoforms of STAT3: the full-length STAT3α and the truncated STAT3β [19]. STAT3α is the main mediator of interleukin 6 (IL-6)-type cytokine signaling [20]. It has non-redundant roles, such as modulation of cellular responses to IL-6 and mediation of interleukin 10 (IL-10) function in macrophages. STAT3β is a distinct isoform of STAT3 that differs from STAT3α by the replacement of the C-terminal transactivation domain with a unique 23-amino acid sequence [21]. STAT3β is generally thought to act as a dominant negative factor. It is a truncated isoform that lacks the 55-residue C-terminal transactivation domain of STAT3α [22]. STAT3β can rescue the embryonic lethality of a STAT3-null mutation and can by itself induce the expression of specific STAT3 target genes. STAT3β has unique and specific functions, such as the regulation of cell migration and invasion [23]. STAT3β demonstrates distinct intracellular dynamics with prolonged nuclear retention, mapping to its unique C-terminal end [24].
The activation of the STAT3 pathway is initiated by the binding of extracellular ligands, such as cytokines, to their respective cell surface receptors [25]. These ligand–receptor interactions trigger a cascade of intracellular signaling events that ultimately result in the activation of STAT3 [26]. One of the key signaling pathways that activate STAT3 is the Janus kinase (JAK)-signal transducer and activator of the transcription (STAT) pathway. JAKs are cytoplasmic tyrosine kinases that are associated with cytokine receptors. Ligand binding activates JAKs and leaves phosphorylated tyrosine residues on the cytoplasmic tail of the receptor which [26] in turn, creates docking sites for STAT proteins, which are then phosphorylated by JAKs. Phosphorylated STAT proteins form dimers, translocate to the nucleus, and bind to specific DNA sequences, thereby regulating gene expression to promote cell proliferation, survival, and migration in cancer cells, making them an attractive target for cancer therapy [27]. Another signaling pathway that activates STAT3 is the phosphatidylinositol 3-kinase (PI3K)-AKT pathway. PI3K is a lipid kinase that is activated by growth factors and cytokines [28]. Activated PI3K generates phosphatidylinositol-3,4,5-trisphosphate (PIP3), to which Akt binds, bringing it into active conformation, which is then phosphorylated by 3-phosphoinositide-dependent kinase 1 (PDK1) and is a mechanistic target of rapamycin (mTOR) complex 2 (mTORC2) to activate AKT [29]. AKT, in turn, phosphorylates and activates various downstream targets, including STAT3. The inactivation of the STAT3 pathway is mediated by several negative regulators, including protein tyrosine phosphatases (PTPs), suppressors of cytokine signaling (SOCS), and protein inhibitors of activated STAT (PIAS) [30]. PTPs are enzymes that dephosphorylate tyrosine residues on proteins. They play a crucial role in terminating signaling pathways by removing phosphate groups from phosphorylated proteins.[31] Several PTPs have been shown to regulate the STAT3 pathway, including SHP1, SHP2, and PTPN11 [32].
Role of STAT3 in medulloblastoma pathogenesis
The STAT3 pathway is activated in medulloblastoma and has been implicated in the development and progression of this tumor. Several studies have shown that STAT3 activation is associated with increased proliferation and survival of medulloblastoma cells [33]. STAT3 was highly activated in medulloblastoma samples, and its expression was significantly associated with poor overall survival [34]. The researchers also found that inhibition of STAT3 using small-molecule inhibitors or short hairpin RNA (shRNA)-mediated knockdown led to decreased proliferation and increased apoptosis in medulloblastoma cells [35]. Another study found that cytokine IL-6, a known activator of the STAT3 pathway, was highly expressed in medulloblastoma samples [36]. The researcher showed that IL-6 promoted medulloblastoma cell proliferation and survival through activation of the STAT3 pathway [37]. In addition, STAT3 has been shown to interact with other signaling pathways that are dysregulated in medulloblastoma. For example, STAT3 has been shown to interact with the sonic hedgehog (SHH) pathway, which is commonly dysregulated in medulloblastoma [38]. Another study found that STAT3 was required for the proliferation of SHH-driven medulloblastoma cells and that inhibition of STAT3 led to decreased tumor growth in a mouse model of the medulloblastoma [39] mechanism of STAT3, shown in Fig. 3.
Fig. 3.
Mechanisms by which STAT3 inhibitors act on medulloblastoma [162].
Medulloblastoma stem cells (MBSCs) are a subpopulation of cells within the tumor responsible for tumor initiation, progression, and recurrence. The STAT3 pathway is critical in the regulation of MBSCs. One study found that STAT3 was highly expressed in MBSCs and that its inhibition led to decreased self-renewal and tumor-initiating capacity of MBSCs [40]. The researchers also showed that STAT3 inhibition led to increased differentiation of MBSCs into non-tumorigenic cells [41]. Another study found that the cytokine leukemia inhibitory factor (LIF), which is known to activate the STAT3 pathway, was highly expressed in MBSCs and showed that LIF promoted the self-renewal and survival of MBSCs through activation of the STAT3 pathway [42].
Targeting STAT3 is a new direction for treating medulloblastoma
Targeting STAT3 has emerged as a promising therapeutic strategy for the treatment of medulloblastoma. The activation of the STAT3 pathway in medulloblastoma is associated with increased proliferation, survival, and stemness of tumor cells, as well as resistance to chemotherapy and radiation therapy [43]. Therefore, targeting STAT3 can potentially overcome these limitations and improve the efficacy of current treatment modalities [44].
One of the key advantages of targeting STAT3 is its specificity for tumor cells. While the STAT3 pathway is activated in medulloblastoma, it is not active in normal brain tissue [42]. This differential activation provides a therapeutic window for selectively targeting tumor cells while sparing normal tissue. This specificity can minimize the side effects associated with conventional chemotherapy and radiation therapy, which often result in significant damage to healthy tissue. Several preclinical studies have demonstrated the potential of targeting STAT3 in medulloblastoma [45], and of targeting STAT3-sensitized medulloblastoma cells for chemotherapy and radiation therapy. Stattic, a small-molecule inhibitor of STAT3, has shown promising results in preclinical studies. Treatment with Stattic led to decreased tumor growth and increased survival in a mouse model of medulloblastoma [46]. Furthermore, combination therapy with Stattic and cisplatin was found to be more effective than monotherapy [47]. Another approach to targeting STAT3 is through the use of monoclonal antibodies. The use of monoclonal antibodies targeting IL-6 could be a potential strategy to suppress STAT3 signaling pathway [48]. Siltuximab, an IL-6 neutralizing monoclonal antibody, is undergoing clinical trials for treatment of ovarian, colorectal, pancreatic, lung, and head and neck cancer for its potential to target IL-6R/JAK/STAT3 signaling pathway [49]. In addition to small-molecule inhibitors, other approaches to targeting STAT3 like targeting upstream signaling molecules that activate STAT3, as well as developing novel delivery systems to improve the efficacy and specificity of STAT3 inhibitors are being explored [12]. Overall, targeting STAT3 has emerged as a promising therapeutic strategy for the treatment of medulloblastoma. Its specificity for tumor cells, ability to sensitize tumor cells to chemotherapy and radiation therapy, and potential for combination therapy make it an attractive target for further development. However, more research is needed to optimize the delivery and dosing of STAT3 inhibitors and to better understand the potential side effects associated with targeting this pathway [50].
STAT3 inhibitor in combination with radiotherapy
Radiation therapy has been used in cancer treatment for decades. In medulloblastoma, radiation therapy is typically performed after surgery to remove the remaining cancer cells and it is administered alone or combined with chemotherapy for older children [51]. The combination of different therapeutic strategies may enhance efficacy and reduce nonspecific toxicity due to what otherwise would be high-dose monotherapy [52]. Inhibition of STAT3 in combination with radiotherapy reduces the expression of STAT3 downstream targets, such as Cyclin D1 and Survivin, and induces apoptosis in cancer cells [53]. Combination therapy with STAT3 inhibitors and radiotherapy has shown promise for the treatment of medulloblastoma in preclinical studies. Studies have shown that the combination of the STAT3 inhibitor LLY17 and radiotherapy was more effective at inhibiting tumor growth in a mouse model of medulloblastoma than either treatment alone [54]. While radiotherapy is an effective treatment for medulloblastoma, it can be limited by resistance and toxicity. Targeting the STAT3 pathway can potentially overcome these limitations and enhance the efficacy of radiotherapy [55]. Inhibition of STAT3 leads to decreased DNA repair, increased apoptosis, and decreased survival of medulloblastoma cells exposed to radiation therapy [56]. This suggests that targeting STAT3 can enhance the effects of radiation therapy by reducing the ability of tumor cells to repair DNA damage and increasing their sensitivity to radiation-induced cell death [57]. In one study, treatment with the STAT3 inhibitor Stattic in combination with radiation therapy led to increased survival and decreased tumor growth in a mouse model of medulloblastoma compared with those for either treatment alone [58]. Another study found that treatment with the STAT3 inhibitor S3I-201 in combination with radiation therapy resulted in increased apoptosis and decreased proliferation of medulloblastoma cells [59]. The combination of STAT3 inhibitors and radiotherapy has also been investigated in other types of cancer. In a preclinical study of head and neck cancer, treatment with the STAT3 inhibitor JSI-124 (cucurbitacin I) in combination with radiation therapy led to increased tumor cell death and decreased tumor growth compared with those for either treatment alone [60]. Similarly, a study of breast cancer cells found that treatment with the STAT3 inhibitor OPB-31121 in combination with radiation therapy resulted in increased apoptosis and decreased cell viability [61]. While these preclinical studies are promising, more research is needed to optimize the combination of STAT3 inhibitors and radiotherapy for the treatment of medulloblastoma [62]. This includes determining the optimal dosing and timing of both treatments and evaluating potential side effects.
STAT3 inhibitor in combination with chemotherapy
Several studies have shown that STAT3 inhibitors can synergize with chemotherapy to kill cancer cells in a variety of cancer types, including lung cancer, breast cancer, and pancreatic cancer [63]. Directly targeting STAT3 and/or inhibiting its functions may be a promising strategy for developing safe and effective anticancer therapeutics [64]. Several STAT3 inhibitors have entered clinical trials, and some of them have been combined with chemotherapy to exert synergistic effects in treating triple-negative breast cancer [53]. Combining STAT3 inhibitors with Chimeric Antigen Receptor T (CAR-T) cells can reduce excessive expansion of CAR-T cells and alleviate the cytokine release syndrome (CRS) [65]. In addition, STAT3 inhibition enhances the therapeutic efficacy of immunogenic chemotherapy by stimulating type 1 interferon production by cancer cells. Therefore, combining STAT3 inhibitors with chemotherapy may be a promising strategy for cancer treatment [66].
Combination therapy with STAT3 inhibitors and chemotherapy has also shown promise for the treatment of medulloblastoma in preclinical studies. The effectiveness of chemotherapy in medulloblastoma is limited by toxicity and development of resistance. Targeting the STAT3 pathway can potentially overcome these limitations and enhance the efficacy of chemotherapy. Preclinical studies have shown that inhibition of STAT3 can sensitize medulloblastoma cells to chemotherapy [67]. Inhibition of STAT3 leads to decreased expression of anti-apoptotic proteins, increased apoptosis, and decreased survival of medulloblastoma cells exposed to chemotherapy. This suggests that targeting STAT3 can enhance the effects of chemotherapy by reducing the ability of tumor cells to survive and promoting their death [56].
STAT3 inhibitor in combination with immunotherapy
Currently, one of the most promising methods for treating cancer is immunotherapy. Immune checkpoint blockade (ICB) and CAR-T cells are the major components of this treatment approach, which has produced substantially better outcomes in patients with otherwise incurable malignancies [68,69]. A variety of cancers exploit immune checkpoint malfunction as a defense strategy to evade immune monitoring, enabling the progression of cancer. The notion of enhancing the host immune system as a potential anti-cancer treatment evolved from this belief [70]. The basic processes of cell division, differentiation, angiogenesis, and survival are all impacted by STAT3 [71,72]. In normally functioning cells, brief activation of STAT3 through phosphorylation allows cytokines and growth factor receptors to convey transcriptional signals to the nucleus [73]. On the other hand, STAT3 attains hyperactivation in most of the cancer malignancies, which results in poor clinical outcome of various cancer therapies [74]. The cancer-associated fibroblasts (CAFs), endothelial cells, tumor cells, and smooth muscle cells make up an extremely multifaceted and varied ecosystem known as the tumor microenvironment (TME) [75,76]. TME is able to accelerate the development of cancer and cause resistance to therapy, especially to cancer immunotherapy [77,78]. Studies indicate that STAT3 is overactive in the TME, including immune cells, CAFs, and cancer cells themselves [[79], [80], [81], [82], [83]]. The increased activity of STAT3 in the TME may have a considerable effect on the immune response against tumors through several pathways. Hyperactivated STAT3 exerts significant immune effects on tumor cells by decreasing the production of immune-stimulating molecules such as chemokines, pro-inflammatory cytokines, and interferons, and increasing the levels of several cytokines and growth factors that include Transforming growth factor-β (TGFβ) and IL-6 [84]. To provide resilience for growth of tumor cells, STAT3 frequently interacts with other signaling pathways, such as nuclear factor kappa B (NF-κB), which is responsible for inflammation-induced carcinogenesis, as well as immune responses against tumors. Cyclooxygenase 2 (COX-2), interleukin 1 (IL-1), IL-6, and interleukin 23 (IL-23) are a few of the molecules that can be stimulated by NF-κB, particularly v-rel avian reticuloendotheliosis viral oncogene homolog A (RELA); they are also implicated in chronic inflammation and cancer development [[85], [86], [87]].
At this point, many levels of STAT3-NF-κB crosstalk have been discovered: (i) With many shared targets involved in cell angiogenesis, proliferation, survival, and metastasis, NF-κB and STAT3 are commonly stimulated in tumor cells and TME-bound immune cells [86]. (ii) By p300-mediated acetylation, STAT3 can extend the retention time of RELA in the nucleus, resulting in continuous activation of NF- κB. (iii) Numerous cytokines, such IL-6, can activate STAT3 and NF-κB at the same time [88]. (iv) Recent research has shown that NF-κB functioning in pancreatic CAFs enhanced CXCL12 expression, protecting malignant cells from immune onslaught [89]. Considering the widely recognized relationship between CXCL12 and STAT3, it is likely that STAT3 is involved in this NF-κB -associated immune escape [90,91]. Additionally, STAT3 is essential for a variety of immune cells that mostly make up the TME. Immunosuppression is brought on by the increased activity of STAT3 in tumor-bound immune cells, which prevents immune responses that are innate and adaptive. Overall, increased STAT3 activity in the innate immune cell subgroup may suppress antigen presentation, reduce the synthesis of pro-inflammatory mediators such as Interferon‐gamma (IFNγ), and prevent effector cells from destroying malignancies [[92], [93], [94]].
It has been demonstrated that increased expression of immune checkpoint molecules such as Cytotoxic T-lymphocyte associated protein 4 (CTLA-4), Programmed cell death 1 (PD-1), and Programmed death-ligand 1 (PD-L1) helps tumor immune escape. There is a lot of evidence that STAT3 can control these immune checkpoint molecules directly or indirectly. By interacting directly with the promoters of PD-1, PD-L1, and PD-L2 genes, STAT3 functions as a transcription factor that can boost gene expression [[95], [96], [97], [98]]. In addition, it has been discovered that STAT3 can indirectly stimulate the production of immune checkpoint molecules by altering several signaling mechanisms. According to reports, PD-1 overexpression in Clusters of differentiation 4 (CD4+) T cells increases the expression of STAT3 mRNA by an unidentified process, and this is necessary for the synthesis of Interleukin 17 (IL-17) and TGFβ1 [99]. In CD4+ T cells, PD-1 can reduce the TCR's ability to activate the PI3K/Akt pathway [100]. Given that PI3K/Akt is a transcriptional regulator of STAT3, it is likely that PD-1 indirectly increases STAT3 activity via PI3K inhibition. In addition to indicating that STAT3 is involved in anti-tumor immunity, the inverse relationship of immune checkpoint molecules and STAT3 offers a possible method for enhancing the effectiveness of the existing immune checkpoint inhibitors [101]. The inclusion of STAT3 inhibitors can increase curative effectiveness and simultaneously decrease resistance to ICB immunotherapy, which is an optimistic finding of paired STAT3 and immune checkpoint blocking. Hematologic malignancies' anti-cancer treatments have been transformed by CAR-T cell therapy, an immunotherapeutic strategy that is quickly gaining popularity. The primary tumor stromal component CAFs influence the extracellular matrix, secrete soluble molecules, promote angiogenesis and metastasis, and suppress anti-tumor immune responses, which all lead to the development of cancer and treatment failure [102]. Recent research has shown that Leukemia inhibitory factor (LIF), among other cytokines, can activate STAT3 in CAFs [103]. Due to the overstimulation of STAT3, CAFs produce different immunosuppressive agents such as C-C Motif Chemokine Ligand 2 (CCL2), Vascular endothelial growth factor (VEGF), TGFβ, IL-6, and EGF, which is responsible for their pro-oncogenic activity [104,105]. It is becoming clearer that STAT3 signaling has a role in CAR-T treatment. Chronic lymphocytic leukemia patients that responded to anti-CD19 CAR-T cells exhibited higher IL-6/STAT3 levels, which encouraged the growth of CAR-T cell therapy, according to transcriptomic profiling [106]. Accordingly, a new anti-CD19 CAR-T cell with STAT3 stimulation demonstrated higher CAR-T cell proliferation and decreased terminal differentiation, and provided superior anti-cancer effects [106]. These results imply that stimulation of STAT3 in CAR-T cells has a positive effect. As discussed earlier, STAT3 excessive stimulation in tumor stroma suppresses the immune system and may result in a rise in the production of specific cytokines and growth factors. In light of this, constitutive production of a variety of cytokines, including IL-6 and IL-10, may raise the likelihood of severe side effects of CAR-T treatment [107]. Therefore, there have been some efforts to integrate STAT3 inhibitors with CAR-T treatment to increase its stability and anti-tumor effects while reducing CAR-T cell toxicity in vivo. Several human malignancies cause increased STAT3 activation, which serves as a key signaling link for tumor cells and TME constituent cells, particularly tumor-infiltrating immune cells. Addressing STAT3 thus seems to have various advantages, including decreased intrinsic tumor cell growth, greater immunosuppressive interaction within the TME, and increased anti-tumor effects of immune cells invading the tumor. Due to these outcomes, STAT3 has emerged as an intriguing prospective approach to the treatment of cancer [84].
The combination of a STAT3 inhibitor with immunotherapy could yield several benefits:
-
•
Enhanced immune response: STAT3 inhibition may restore immune cell function and improve their ability to recognize and attack tumor cells.
-
•
Reduced tumor growth: Blocking STAT3 could hinder tumor cell proliferation and survival, making them more susceptible to immune attack.
-
•
Improved immune infiltration: STAT3 inhibition might increase immune cell infiltration into the tumor, bolstering the effectiveness of immunotherapy.
-
•
Reduced immunosuppression: STAT3 inhibition can modulate the tumor microenvironment, reducing factors that suppress the immune response.
Various STAT3 inhibitors under development
Several STAT3 inhibitors are currently under clinical trial. Some of the small-molecule inhibitors that have entered clinical trials include STAT3 Inhibitor C188-9 (TTI-101) and STAT3 Inhibitor WP1066. These inhibitors are being tested for their safety and efficacy in treating various types of cancer, including head and neck cancer and other solid tumors. The development of STAT3 inhibitors is a promising area of research, and various novel approaches are being explored to overcome the challenges associated with targeting STAT3. List of various STAT3 inhibitors under development is shown in Table 1.
Table 1.
Various STAT3 inhibitors under development.
| Inhibitor Name | Cancer Type | Brief Description | Phase of Development | Ref |
|---|---|---|---|---|
 |
Advanced solid tumor, and hepatocellular carcinoma |
|
Status Phase II (Ongoing) |
[1,2] |
|
NCT number NCT00955812 NCT01406574 | ||||
 |
Recurrent glioblastoma, Metastatic malignant neoplasm in the brain |
|
Status Phase II (Recruiting) |
[3] |
|
NCT number NCT05879250 NCT04334863 | ||||
 |
Breast cancer, Hepatocellular carcinoma, head and neck squamous cell carcinoma |
|
Status Phase II (Recruiting) |
[4,5] |
|
NCT number NCT05440708, NCT03195699 | ||||
 |
Glioblastoma, Advanced Malignancies, Colorectal Cancer |
|
Status Phase II (Ongoing) |
[10,11] |
|
NCT number NCT02315534 NCT01775423 NCT02753127 | ||||
 |
Brain metastasis (BM) from non-small-cell lung cancer (NSCLC), Breast Cancer |
|
Preclinical | [6,7] |
 |
Chronic myeloid leukemia (CML) |
|
Preclinical | [8] |
 |
Head and neck squamous cell carcinoma |
|
Preclinical | [9] |
 |
Colorectal cancer |
|
Preclinical | [12] |
 |
Gastric cancer |
|
Preclinical | [13,14] |
 |
Breast cancer, Pancreatic cancer |
|
Preclinical | [15,16] |
STAT3 in polarization of macrophages in medulloblastoma
The complicated process of macrophage polarization, which gives rise to different activation states, is generally believed to be controlled by a number of intracellular signaling molecules and their associated pathways [108,109]. Furthermore, STAT3 controls macrophage polarization to carry out a variety of critical functions in both healthy and malignant human tissues, including angiogenesis, proliferation, differentiation, and survival, as well as immune system regulation [64]. There is a crosstalk between different signaling pathways like PI3K/Akt/mTOR, MAPK, and AMPK during the STAT3-dependent macrophage polarization process [110].
The JAK/STAT pathway is activated by cytokines and growth factors, which are secreted glycoproteins acting as intercellular messengers. These factors bind specialized cell surface receptors on target cells which have intracellular domains that are constitutively coupled to members of the JAK family, which includes JAK1, JAK2, JAK3, and TYK2. The cytokine-receptor engagement activates JAK which phosphorylates the tyrosine residues on the cytoplasmic tail, which in turn causes auto/transphosphorylation. This phosphorylation forms binding sites for latent cytoplasmic STATs, which are then drawn to the receptor complex and phosphorylate themselves by the action of tyrosine-phosphate-binding SH2 domains. Finally, the phosphorylated STATs separate from the receptors, form a dimer in the cytoplasm, and travel to the nucleus, where they bind to the target gene's promoter region to start the transcription of that gene [111]. Through JAK/STAT signaling, the multifunctional protein STAT3 affects human metabolism, immunological inflammation, and damage repair. It is controlled by numerous cytokines and growth factors [112]. Research on the function of JAK/STAT3 in macrophage polarization has demonstrated that this system can either be stimulated or inhibited to improve macrophage M2 polarization, depending on the disease. However, STAT3 activation often promotes macrophage M2 polarization [110].
According to research on tumor conditions including glioma and ovarian cancer, JAK/STAT3 signaling axis activation promotes M2 macrophage polarization and influences the course of the disease by either activating or suppressing associated cytokines [[113], [114], [115], [116], [117]]. The two distinct sets of data indicate that, in addition to the diversity of STAT3 and macrophages, other factors that may influence macrophage polarization include immune cells, tumor cells, the JAK/STAT3 pathway, multiple cytokines, chemokines, immune cells, and interaction between diverse signaling pathways in macrophages [118,119]. The pattern of macrophage polarization may depend on how these parameters are balanced. IL-6 and IL-10 are the two most prominent regulatory cytokines of the JAK/STAT3 signaling pathway. IL-6 is found abundantly in the TME and is a vital cytokine that promotes tumor cell cycle development and inhibits apoptosis [120]. IL-6 promotes the JAK/STAT3 signaling pathway by interacting with host cell receptor complex glycoprotein 130/IL-6 receptor (IL-6R) [121]. According to recent studies, IL-6/JAK/STAT3 may be expressed directly in tumor cells and promote tumor cell proliferation, differentiation, and metastasis. It may also be present in macrophages and affect the beginning and development of disease indirectly through macrophage M2 polarization [122]. As a result, by blocking this signaling pathway, macrophage polarization to the M2 sub-type can be blocked, which in turn suppresses pro-tumor related cytokines including IL-6, IL-10, and VEGF production, which will eventually restrict the advancement of the tumor condition [123].
The transcriptional regulator, also known as the "master switch" that controls the production of many pro-inflammatory mediator genes, is called NF-κB. The NF-κB's p65 subunit controls the polarization of macrophage M1. Whenever the Toll-like receptor 4 (TLR4) on the surface of macrophages attaches to lipopolysaccharide (LPS) via a route reliant on the myeloid differentiation factor 88 (MyD88) or the interferon regulatory factor (IRF) 3, the conventional NF-κB pathway is activated [124]. Typically, STAT3 activation is essential for the anti-inflammatory M2 phenotype, whereas NF-κB activation results in an inflammatory macrophage M1 phenotype. Together, these processes regulate responses to diverse microenvironments and preserve M1/M2 homeostasis [125]. (MicroRNA) let7Wb, one of the most significant factors associated with immune system regulation and inflammation, has been shown to affect the TLR4 pathway adversely [126]. The TLR4/NF-κB/STAT3/AKT signaling pathway was found to be responsible for the significant increase in p-STAT3 and p-AKT expression levels in macrophages. In the presence of let-7b inhibitor, STAT3 stimulation completely stopped, suggesting that the TLR4/NF-κB/STAT3/AKT regulatory circuit can regulate the modification of macrophage polarization, a process that causes inflammation [127].
Studies have shown that migration, proliferation, and survival of macrophages depend on the PI3K/AKT signaling system [128]. Numerous human diseases such as heart-related disease, diabetes, cancer, and neurological problems have been related to dysregulation of this signaling system [[129], [130], [131], [132]]. Additionally, research has shown that PI3K/AKT activation enhances STAT3 phosphorylation and nuclear translocation, which helps macrophages polarize toward M2 and has pro-cancer and immunosuppressive effects through the release of many mediators [133,134].
In conclusion, a variety of signaling pathways can control macrophage polarization by influencing STAT3, which then influences the progression of the illness. Consequently, activating or inhibiting these signaling pathways may be able to control the M1/M2 balance and offer fresh treatment options for disorders that are associated with it [110].
Exosomes-based therapies targeting STAT3
Material exchange between cells is required for good communication and cell viability [135]. Recently, extracellular vehicles (EVs), notably exosomes, have emerged as new cell–cell communication mediators in healthy and pathological situations [136]. Exosomes are distinct from other forms of EVs in terms of biogenesis, release mechanisms, size, content, and function; see Fig. 4. Exosomes are generated by the inward budding of early endosome membranes, which later develop into multivesicular structures [137]. Microvesicles (MVs), conversely, are formed by direct outward pinching or budding of the cell's plasma membrane, whereas apoptotic bodies are discharged into the extracellular space by dying cells [137]. Recent research has revealed that tumor-cell-derived exosomes play an important role in communication by transporting numerous biomolecules such as proteins, lipids, DNA, and RNA [138]. The cargo of exosomes closely resembles the intracellular components of their parent cells. Therefore, real-time detection of these exosomal components could provide critical insights for diagnosis, prognosis, and disease monitoring. In a clinical setting, exosomes can serve as diagnostic biomarkers and even as carriers for anticancer drugs. Their clathrin-coated membranes confer exceptional stability and resistance against degradation enzymes, such as RNases, making them an attractive tool for diagnosis and therapy [139]. Exosomes and other extracellular vesicles are essential in regulating a wide range of physiological and pathological cellular processes, which can be utilized for therapeutic purposes [136]. Recently, mesenchymal stem cells (MSCs) derived from various sources, such as bone marrow, adipose tissue, and cord blood, have gained significant attention as potential therapeutic agents with regenerative properties [140]. Studies have shown that in pig and mouse models, MSC-derived exosomes have significant cardio-protective paracrine effects against myocardial ischemia/reperfusion injury [141]. Furthermore, MSC-derived exosomes have been shown to treat pulmonary hypertension by suppressing early inflammation and vascular remodeling. These act by inhibiting hyper-proliferative pathways, including STAT3 mediated signaling [142]. Another study explored the potential of exosome-based strategies targeting STAT3 for treating neurovascular injuries. Microglia-secreted miR-424-5p is crucial in exacerbating endothelial cell damage and vascular integrity loss during oxygen–glucose deprivation (OGD). MiR-424-5p inhibition mitigated these effects by targeting the FGF2-mediated STAT3 signaling pathway. In vivo, mouse experiments confirmed that blocking miR-424-5p reduced neurological dysfunction and endothelial cell injury caused by middle cerebral artery occlusion (MCAO) [143].
Fig. 4.
Workflow for exosome-based strategy used for STAT3 inhibition.
Mesenchymal stromal cell-derived exosomes (MEX) have shown potential in treating pulmonary hypertension. They inhibit early lung inflammation while promoting vascular remodeling. By inhibiting the activation of the STAT3 pathway, MEX significantly reduces hypoxia, and lowers miR-17 levels while raising miR-204, which is typically reduced in pulmonary hypertension. MEX also inhibits STAT3 signaling in pulmonary artery endothelial cells, directly influencing hypoxic vascular cells [144].
A study proposed a unique way to improve glioblastoma (GBM) therapy by employing Angiopep-2 (An2)-functionalized exosomes loaded with small interfering RNA (siRNA) targeting STAT3. GBM treatment with siRNA has several problems, including low absorption, immunogenicity, instability, short circulation time, and limited blood–brain barrier penetration. An Exo-An2-siRNA formulation demonstrated exceptional properties such as increased blood stability, effective cellular uptake, and significant BBB penetration. Exo-An2-siRNA displayed potent in vitro anti-GBM activities, protected siRNA, and successfully targeted GBM cells, enhancing tumor inhibition and increased survival in mice with GBM [145]. Formulations such as exosomal curcumin (Exo-cur) and exosomal cucurbitacin I (Exo-JSI124) have been developed by independently encapsulating curcumin and a STAT3 inhibitor named JSI124 into exosomes. When administered intranasally to brain microglial cells, these exosomes caused apoptosis in microglial cells, substantially decreasing LPS-driven brain inflammation. Similarly, Exo-JSI124 slowed tumor growth and increased subject survival in a glioblastoma tumor model [146]. Therefore, exosome-based formulations are used for targeting STAT3 in various applications, including inflammation control and cancer therapy, and offer a promising approach.
STAT3: a potential player in personalized medicine
STAT3 is emerging as a key component of personalized medicine for medulloblastoma patients, offering exciting new therapeutic opportunities [147]. Complete comprehension pertaining to STAT3 activation pathways is still lacking and there is a need for refining STAT3 inhibitors for therapeutic application [148]. Repurposing drugs is another effective way to get STAT3 inhibitors into the clinic quickly [149]. Recently FDA approved compounds like Pyrimethamine and Celecoxib as STAT3 inhibitors, offering new avenues for cancer therapy. Use of STAT3 inhibitors in combination with other targeted therapies or conventional procedures like radiation therapy or chemotherapy could increase treatment efficacy [150]. Certain natural substances, such as salidroside and isoharringtonine, have been found in multiple preclinical investigations to increase the efficacy of STAT3 inhibitors. These compounds have also been shown to exert anti-cancer activity against triple negative breast cancer (TNBC) by preventing STAT3 from binding to DNA. Pectolinarigenin inhibited the migration and invasion of breast cancer cells in vitro and suppressed growth and metastasis of osteosarcoma by inhibiting the STAT3 signaling pathway [53,151]. Numerous other natural products have demonstrated strong anticancer effects by blocking the STAT3 signaling pathway in different types of cancer, including resveratrol, curcumin, alantolactone, curvularin, osthole, piperlongumine, withaferin A, trichothecin, angoline, norcantharidin, 2-O-methylmagnolol, and cosmomycin C. These organic products will reduce STAT3 inhibitor toxicities and side effects, and dramatically maximize their tolerability. In addition, targeted therapy is becoming equally promising in order to ensure better therapeutic approaches and overcome blood-brain barrier, chemoresistance, tumor microenvironment, and cancer stem cells [[152], [153], [154], [155], [156]]. These therapies include drugs aimed at certain pathways, oncoviruses, and modified T-cells. Cancer-specific therapy is based on innovative tailored therapy techniques. Genetic testing is a method to determine the specific abnormalities in a patient's cell of their cancer [157,158]. However, in this situation, it is necessary to use a different procedure to identify the STAT3 expression in the tumor cells before beginning the treatment. This alternative solution must encompass genomic profiling of your tumor to discover some essential mutations or other differences that may influence your response to STAT3 inhibitors and molecular testing to determine the existence of STAT3 and how hard it is working. Also, new ways of targeting STAT3, like aptamers and oligonucleotides, have shown promise in reducing tumor growth. While causing minimal damage, STAT3 also shows much promise as a personalized target, given its involvement in immune infiltration and drug response in cancer [159,160].
Conclusion
Medulloblastoma is an aggressive brain tumor in children which requires prompt attention to avoid fatality. The complex molecular profile is characterized by the activation of STAT3 pathway facilitated by JAK-STAT and PI3K-AKT signaling. Targeting these pathways presents the best hope for curing this devastating disease, and thus more studies are needed to develop new therapies that can ameliorate the prognosis in these severely impacted patients. The process of preclinical testing on Stattic and other STAT3 inhibitors has proven to be encouraging and demonstrates potential for combination therapy. STAT3 inhibitors combined with other modalities of treatment like radiation, chemotherapy, immunotherapy, and exosome-based methods could lead to synergistic effects that would help overcome resistance as well as enhance therapeutic outcomes. There is effective therapy for targeting the STAT3 pathway through an exosome-based method for various applications, including neurovascular injuries and glioblastoma therapy. Nevertheless, there are certain limitations. For example, most of the evidence is based on preclinical research, thus failing to comprehensively capture the mechanistic and physiological consequences on actual pathologies. Therefore, any treatment decisions based on these findings should be made with caution. Additionally, there is a lack of sufficient long-term usage data on safety, efficacy, and patient outcomes, and the majority of reviewed clinical studies are in the developing stages. Future research on STAT3 inhibition in the therapy of medulloblastoma should focus on several aspects. More research should be performed on the molecular processes underlying STAT3 activation and its interaction with other signaling pathways. This will allow for the development of predictable biomarkers and combination therapies. Identifying novel and better STAT3 inhibitors with higher bioavailability and specificity and research on drug delivery systems for selective inhibition of STAT3 in the central nervous system will also be needed for clinical applications.
Availability of data and materials
All the data were from publicly available search engines, namely PubMed, Scopus, and Web of Science. These articles are available online.
CRediT authorship contribution statement
Sachindra Kumar: Writing – review & editing, Writing – original draft, Conceptualization. Dube Aakash Arwind: Writing – original draft. Harish Kumar B: Writing – review & editing. Samyak Pandey: Writing – review & editing. Raksha Nayak: Writing – original draft. Megh Pravin Vithalkar: Writing – original draft. Nitesh Kumar: Writing – review & editing. K Sreedhara Ranganath Pai: Writing – review & editing, Conceptualization.
Declaration of competing interest
The authors declare no conflicts of interest.
References
- 1.Doger de Spéville E, Kieffer V, Dufour C, et al. Neuropsychological consequences of childhood medulloblastoma and possible interventions: a review. Neurochirurgie. 2021;67(1):90–98. doi: 10.1016/j.neuchi.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Flepisi BT, Balmith M. An overview of central nervous system tumours. SciMedicine J. 2021;3(4):360–386. doi: 10.28991/scimedj-2021-0304-8. [DOI] [Google Scholar]
- 3.K. K., Kabitha; Rajan, M.S.; Hegde, K.; Koshy, S.; Shenoy A. EBSCOhost | 115876553 | A COMPREHENSIVE REVIEW ON BRAIN TUMOR. doi:https://web.p.ebscohost.com.
- 4.Sorajja N, Moore KJ, Sample JM, Hubbard AK, Williams LA. Global variation in young adult central nervous system tumor incidence by region, age, and sex from 1988 to 2012. Cancer Epidemiol. 2022;78 doi: 10.1016/j.canep.2022.102151. [DOI] [PubMed] [Google Scholar]
- 5.Parkes J, Hendricks M, Ssenyonga P, et al. SIOP PODC adapted treatment recommendations for standard-risk medulloblastoma in low and middle income settings. Pediatr. Blood Cancer. 2015;62(4):553–564. doi: 10.1002/pbc.25313. [DOI] [PubMed] [Google Scholar]
- 6.Wu J, Lin F, Zhuang Q, et al. Trends in the incidence and survival of adult-onset medulloblastoma. SSRN Electron. J. 2020 doi: 10.2139/ssrn.3556649. Published online. [DOI] [Google Scholar]
- 7.Northcott PA, Robinson GW, Kratz CP, et al. Medulloblastoma. Nat. Rev. Dis. Prim. 2019;5(1):11. doi: 10.1038/s41572-019-0063-6. [DOI] [PubMed] [Google Scholar]
- 8.Jacques G, Cormac O. Central nervous system tumors: 2013:931-958. doi:10.1016/B978-0-444-52910-7.00015-5. [DOI] [PubMed]
- 9.Bharadwaj U, Kasembeli MM, Robinson P, Tweardy DJ. Targeting Janus kinases and signal transducer and activator of transcription 3 to treat inflammation, fibrosis, and cancer: rationale, progress, and caution. Ye RD, ed. Pharmacol. Rev. 2020;72(2):486–526. doi: 10.1124/pr.119.018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sgrignani J, Garofalo M, Matkovic M, Merulla J, Catapano CV., Cavalli A. Structural biology of STAT3 and its implications for anticancer therapies development. Int. J. Mol. Sci. 2018;19(6):1591. doi: 10.3390/ijms19061591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang R, Song Y, Shakoor K, Yi W, Peng C, Liu S. Insights into the role of STAT3 in intrahepatic cholangiocarcinoma (Review) Mol. Med. Rep. 2022;25(5):171. doi: 10.3892/mmr.2022.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Jiang W, Dong S, Li W, Zhu W, Zhou W. STAT3 Inhibitors: a novel insight for anticancer therapy of pancreatic cancer. Biomolecules. 2022;12(10):1450. doi: 10.3390/biom12101450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris R, Kershaw NJ, Babon JJ. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018;27(12):1984–2009. doi: 10.1002/pro.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang T, Kee WH, Seow KT, Fung W, Cao X. The coiled-coil domain of Stat3 is essential for its SH2 domain-mediated receptor binding and subsequent activation induced by epidermal growth factor and interleukin-6. Mol. Cell Biol. 2000;20(19):7132–7139. doi: 10.1128/MCB.20.19.7132-7139.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar A, Bora U. Molecular docking studies on inhibition of Stat3 dimerization by curcumin natural derivatives and its conjugates with amino acids. Bioinformation. 2012;8(20):988–993. doi: 10.6026/97320630008988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Resetca D, Haftchenary S, Gunning PT, Wilson DJ. Changes in signal transducer and activator of transcription 3 (STAT3) dynamics induced by complexation with pharmacological inhibitors of Src Homology 2 (SH2) domain dimerization. J. Biol. Chem. 2014;289(47):32538–32547. doi: 10.1074/jbc.M114.595454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pranada AL, Metz S, Herrmann A, Heinrich PC, Müller-Newen G. Real time analysis of STAT3 nucleocytoplasmic shuttling. J. Biol. Chem. 2004;279(15):15114–15123. doi: 10.1074/jbc.M312530200. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Yin Z, Huang B, Xu K, Su J. Stat3 signaling pathway: a future therapeutic target for bone-related diseases. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.897539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maritano D, Sugrue ML, Tininini S, et al. The STAT3 isoforms α and β have unique and specific functions. Nat. Immunol. 2004;5(4):401–409. doi: 10.1038/ni1052. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Sun Q, Wu J, Tian W, Wang H, Liu H. Identification of four STAT3 isoforms and functional investigation of IL-6/JAK2/STAT3 pathway in blunt snout bream (Megalobrama amblycephala) Dev. Comp. Immunol. 2022;135 doi: 10.1016/j.dci.2022.104484. [DOI] [PubMed] [Google Scholar]
- 21.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003;374(1):1–20. doi: 10.1042/bj20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tolomeo M, Cascio A. The multifaced role of STAT3 in cancer and its implication for anticancer therapy. Int. J. Mol. Sci. 2021;22(2) doi: 10.3390/ijms22020603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy DE, kuo Lee C. What does Stat3 do? J. Clin. Invest. 2002;109(9):1143–1148. doi: 10.1172/JCI15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y, Qiu J, Dong S, et al. Stat3 isoforms, α and β, demonstrate distinct intracellular dynamics with prolonged nuclear retention of Stat3β mapping to its unique C-terminal end. J. Biol. Chem. 2007;282(48):34958–34967. doi: 10.1074/jbc.M704548200. [DOI] [PubMed] [Google Scholar]
- 25.Kotenko SV, Pestka S. Jak-Stat signal transduction pathway through the eyes of cytokine class II receptor complexes. Oncogene. 2000;19(21):2557–2565. doi: 10.1038/sj.onc.1203524. [DOI] [PubMed] [Google Scholar]
- 26.Subramaniam A, Shanmugam MK, Perumal E, et al. Potential role of signal transducer and activator of transcription (STAT)3 signaling pathway in inflammation, survival, proliferation and invasion of hepatocellular carcinoma. Biochim. Biophys. Acta Rev. Cancer. 2013;1835(1):46–60. doi: 10.1016/j.bbcan.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal. Transduct. Target. Ther. 2021;6(1):402. doi: 10.1038/s41392-021-00791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogt PK, Hart JR. PI3K and STAT3: A new alliance. Cancer Discov. 2011;1(6):481–486. doi: 10.1158/2159-8290.CD-11-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franke TF. PI3K/Akt: getting it right matters. Oncogene. 2008;27(50):6473–6488. doi: 10.1038/onc.2008.313. [DOI] [PubMed] [Google Scholar]
- 30.Wu M, Song D, Li H, et al. Negative regulators of STAT3 signaling pathway in cancers. Cancer Manage. Res. 2019;11:4957–4969. doi: 10.2147/CMAR.S206175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burke TR, Zhang ZY. Protein-tyrosine phosphatases: structure, mechanism, and inhibitor discovery. Biopolymers. 1998;47(3):225–241. doi: 10.1002/(SICI)1097-0282(1998)47:3<225::AID-BIP3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 32.Kim M, Morales L, Jang IS, Cho YY, Kim D. Protein tyrosine phosphatases as potential regulators of STAT3 signaling. Int. J. Mol. Sci. 2018;19(9):2708. doi: 10.3390/ijms19092708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang F, Jove V, Xin H, Hedvat M, Van Meter TE, Yu H. Sunitinib induces apoptosis and growth arrest of medulloblastoma tumor cells by inhibiting STAT3 and AKT signaling pathways. Mol. Cancer Res. 2010;8(1):35–45. doi: 10.1158/1541-7786.MCR-09-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C, Li H, Zhang P, et al. SHP2, SOCS3 and PIAS3 expression patterns in medulloblastomas: relevance to STAT3 activation and resveratrol-suppressed STAT3 signaling. Nutrients. 2016;9(1):3. doi: 10.3390/nu9010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Lathia JD, Wu Q, et al. Targeting interleukin 6 signaling suppresses glioma stem cell survival and tumor growth. Stem Cells (1981) 2009;27(10):2393–2404. doi: 10.1002/stem.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sreenivasan L, Wang H, Yap SQ, Leclair P, Tam A, Lim CJ. Autocrine IL-6/STAT3 signaling aids development of acquired drug resistance in Group 3 medulloblastoma. Cell Death. Dis. 2020;11(12):1035. doi: 10.1038/s41419-020-03241-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X, Wei J, Li C, Pierson C, Finlay J, Lin J. Blocking interleukin-6 signaling inhibits cell viability/proliferation, glycolysis, and colony forming activity of human medulloblastoma cells. Int. J. Oncol. 2017 doi: 10.3892/ijo.2017.4211. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon RE, Zhang L, Peri S, et al. Statins synergize with hedgehog pathway inhibitors for treatment of medulloblastoma. Clin. Cancer Res. 2018;24(6):1375–1388. doi: 10.1158/1078-0432.CCR-17-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan L, Zhang H, Liu J, et al. STAT3 is required for Smo-dependent signaling and mediates Smo-targeted treatment resistance and tumorigenesis in Shh medulloblastoma. Mol. Oncol. 2022;16(4):1009–1025. doi: 10.1002/1878-0261.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang G, Xu Q, Cui Y, Li N, Bian X, Lv S. Medulloblastoma stem cells: promising targets in medulloblastoma therapy. Cancer Sci. 2016;107(5):583–589. doi: 10.1111/cas.12925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehdipour P. Cancer Genetics and Psychotherapy. Springer International Publishing; 2017. Metastatic breast cancer at a glance: scenarios of BC brain- and BC bone-metastasis by illustrations; pp. 1029–1070. [DOI] [Google Scholar]
- 42.Zaiter A, Audi ZF, Shawraba F, et al. STAT3 in medulloblastoma: a key transcriptional regulator and potential therapeutic target. Mol. Biol. Rep. 2022;49(11):10635–10652. doi: 10.1007/s11033-022-07694-6. [DOI] [PubMed] [Google Scholar]
- 43.Chang CJ, Chiang CH, Song WS, et al. Inhibition of phosphorylated STAT3 by cucurbitacin I enhances chemoradiosensitivity in medulloblastoma-derived cancer stem cells. Child's Nerv. Syst. 2012;28(3):363–373. doi: 10.1007/s00381-011-1672-x. [DOI] [PubMed] [Google Scholar]
- 44.Yue P, Turkson J. Targeting STAT3 in cancer: how successful are we? Expert. Opin. Investig. Drugs. 2009;18(1):45–56. doi: 10.1517/13543780802565791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Ruysscher D, Niedermann G, Burnet NG, Siva S, Lee AWM, Hegi-Johnson F. Radiotherapy toxicity. Nat. Rev. Dis. Prim. 2019;5(1):13. doi: 10.1038/s41572-019-0064-5. [DOI] [PubMed] [Google Scholar]
- 46.Page BDG, Croucher DC, Li ZH, et al. Inhibiting aberrant signal transducer and activator of transcription protein activation with tetrapodal, small molecule Src Homology 2 domain binders: promising agents against multiple myeloma. J. Med. Chem. 2013;56(18):7190–7200. doi: 10.1021/jm3017255. [DOI] [PubMed] [Google Scholar]
- 47.Tilija Pun N, Jeong CH. Statin as a potential chemotherapeutic agent: current updates as a monotherapy, combination therapy, and treatment for anti-cancer drug resistance. Pharmaceuticals. 2021;14(5):470. doi: 10.3390/ph14050470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furtek SL, Backos DS, Matheson CJ, Reigan P. Strategies and approaches of targeting STAT3 for cancer treatment. ACS. Chem. Biol. 2016;11(2):308–318. doi: 10.1021/acschembio.5b00945. [DOI] [PubMed] [Google Scholar]
- 49.Siveen KS, Sikka S, Surana R, et al. Targeting the STAT3 signaling pathway in cancer: Role of synthetic and natural inhibitors. Biochim. Biophys. Acta Rev. Cancer. 2014;1845(2):136–154. doi: 10.1016/j.bbcan.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Lee H, Pal SK, Reckamp K, Figlin RA, Yu H. STAT3: A Target to Enhance Antitumor Immune Response; 2010:41-59. doi:10.1007/82_2010_51. [DOI] [PMC free article] [PubMed]
- 51.Seidel C, Heider S, Hau P, Glasow A, Dietzsch S, Kortmann RD. Radiotherapy in medulloblastoma—evolution of treatment, current concepts and future perspectives. Cancers. (Basel) 2021;13(23):5945. doi: 10.3390/cancers13235945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar K, Rani V, Mishra M, Chawla R. New paradigm in combination therapy of siRNA with chemotherapeutic drugs for effective cancer therapy. Curr. Res. Pharmacol. Drug Discov. 2022;3 doi: 10.1016/j.crphar.2022.100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qin JJ, Yan L, Zhang J, Zhang WD. STAT3 as a potential therapeutic target in triple negative breast cancer: a systematic review. J. Exp. Clin. Cancer Res. 2019;38(1):195. doi: 10.1186/s13046-019-1206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buck J, Dyer PJC, Hii H, et al. Veliparib is an effective radiosensitizing agent in a preclinical model of medulloblastoma. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.633344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Y, Zheng C, Huang Y, He M, Xu WW, Li B. Molecular mechanisms of chemo- and radiotherapy resistance and the potential implications for cancer treatment. MedComm (2020) 2021;2(3):315–340. doi: 10.1002/mco2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ray S, Coulter DW, Gray SD, et al. Suppression of STAT3 NH 2 -terminal domain chemosensitizes medulloblastoma cells by activation of protein inhibitor of activated STAT3 via de-repression by microRNA-21. Mol. Carcinog. 2018;57(4):536–548. doi: 10.1002/mc.22778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, Zhang X, Qiu C, Yang N. STAT3 contributes to radioresistance in cancer. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.01120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rohrer KA, Song H, Akbar A, et al. STAT3 inhibition attenuates MYC expression by modulating Co-activator recruitment and suppresses medulloblastoma tumor growth by augmenting cisplatin efficacy in vivo. Cancers. (Basel) 2023;15(8):2239. doi: 10.3390/cancers15082239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao H, Bid HK, Jou D, et al. A novel small molecular STAT3 inhibitor, LY5, inhibits cell viability, cell migration, and angiogenesis in medulloblastoma cells. J. Biol. Chem. 2015;290(6):3418–3429. doi: 10.1074/jbc.M114.616748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spitzner M, Ebner R, Wolff H, Ghadimi B, Wienands J, Grade M. STAT3: a novel molecular mediator of resistance to chemoradiotherapy. Cancers. (Basel) 2014;6(4):1986–2011. doi: 10.3390/cancers6041986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang PL, Liu LX, Li EM, Xu LY. STAT3, the challenge for chemotherapeutic and radiotherapeutic efficacy. Cancers. (Basel) 2020;12(9):2459. doi: 10.3390/cancers12092459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zagozewski J, Borlase S, Guppy BJ, et al. Combined MEK and JAK/STAT3 pathway inhibition effectively decreases SHH medulloblastoma tumor progression. Commun. Biol. 2022;5(1):697. doi: 10.1038/s42003-022-03654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao M, Jiang B, Gao FH. Small molecule inhibitors of STAT3 for cancer therapy. Curr. Med. Chem. 2011;18(26):4012–4018. doi: 10.2174/092986711796957284. [DOI] [PubMed] [Google Scholar]
- 64.Wang X, Crowe PJ, Goldstein D, Yang JL. STAT3 inhibition, a novel approach to enhancing targeted therapy in human cancers. Int. J. Oncol. 2012;41(4):1181–1191. doi: 10.3892/ijo.2012.1568. [DOI] [PubMed] [Google Scholar]
- 65.Li X, Shao M, Zeng X, Qian P, Huang H. Signaling pathways in the regulation of cytokine release syndrome in human diseases and intervention therapy. Signal. Transduct. Target. Ther. 2021;6(1):367. doi: 10.1038/s41392-021-00764-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang H, Yamazaki T, Pietrocola F, et al. STAT3 inhibition enhances the therapeutic efficacy of immunogenic chemotherapy by stimulating type 1 interferon production by cancer cells. Cancer Res. 2015;75(18):3812–3822. doi: 10.1158/0008-5472.CAN-15-1122. [DOI] [PubMed] [Google Scholar]
- 67.Qureshy Z, Johnson DE, Grandis JR. Targeting the JAK/STAT pathway in solid tumors. J. Cancer Metastasis Treat. 2020;6 http://www.ncbi.nlm.nih.gov/pubmed/33521321 [PMC free article] [PubMed] [Google Scholar]
- 68.Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 70.Christofi T, Baritaki S, Falzone L, Libra M, Zaravinos A. Current perspectives in cancer immunotherapy. Cancers. (Basel) 2019;11(10):1472. doi: 10.3390/cancers11101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hanlon MM, Rakovich T, Cunningham CC, et al. STAT3 mediates the differential effects of oncostatin M and TNFα on RA synovial fibroblast and endothelial cell function. Front. Immunol. 2019;10:2056. doi: 10.3389/fimmu.2019.02056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J. Cell Sci. 2004;117(8):1281–1283. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- 73.Darnell JE, Jr., Kerr lan M, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 74.Johnson DE, O'Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018;15(4):234–248. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pearce OMT, Delaine-Smith RM, Maniati E, et al. Deconstruction of a metastatic tumor microenvironment reveals a common matrix response in human cancers. Cancer Discov. 2018;8(3):304–319. doi: 10.1158/2159-8290.CD-17-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Azizi E, Carr AJ, Plitas G, et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell. 2018;174(5):1293–1308. doi: 10.1016/j.cell.2018.05.060. e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martin JD, Cabral H, Stylianopoulos T, Jain RK. Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat. Rev. Clin. Oncol. 2020;17(4):251–266. doi: 10.1038/s41571-019-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Phuengkham H, Ren L, Shin IW, Lim YT. Nanoengineered immune niches for reprogramming the immunosuppressive tumor microenvironment and enhancing cancer immunotherapy. Adv. Mater. 2019;31(34) doi: 10.1002/adma.201803322. [DOI] [PubMed] [Google Scholar]
- 79.Kortylewski M, Kujawski M, Wang T, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat. Med. 2005;11(12):1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 80.Herrmann A, Kortylewski M, Kujawski M, et al. Targeting Stat3 in the myeloid compartment drastically improves the in vivo antitumor functions of adoptively transferred T cells. Cancer Res. 2010;70(19):7455–7464. doi: 10.1158/0008-5472.CAN-10-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iwata-Kajihara T, Sumimoto H, Kawamura N, et al. Enhanced cancer immunotherapy using STAT3-depleted dendritic cells with high Th1-inducing ability and resistance to cancer cell-derived inhibitory factors. J. Immunol. 2011;187(1):27–36. doi: 10.4049/jimmunol.1002067. [DOI] [PubMed] [Google Scholar]
- 82.Siegel AM, Heimall J, Freeman AF, et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity. 2011;35(5):806–818. doi: 10.1016/j.immuni.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gotthardt D, Putz EM, Straka E, et al. Loss of STAT3 in murine NK cells enhances NK cell–dependent tumor surveillance. Blood. 2014;124(15):2370–2379. doi: 10.1182/blood-2014-03-564450. [DOI] [PubMed] [Google Scholar]
- 84.Zou S, Tong Q, Liu B, Huang W, Tian Y, Fu X. Targeting STAT3 in cancer immunotherapy. Mol. Cancer. 2020;19(1):1–19. doi: 10.1186/s12943-020-01258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer. 2009;9(11):798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fan Y, Mao R, Yang J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell. 2013;4:176–185. doi: 10.1007/s13238-013-2084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat. Rev. Immunol. 2018;18(5):309–324. doi: 10.1038/nri.2017.142. [DOI] [PubMed] [Google Scholar]
- 88.Lee H, Herrmann A, Deng JH, et al. Persistently activated Stat3 maintains constitutive NF-κB activity in tumors. Cancer Cell. 2009;15(4):283–293. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garg B, Giri B, Modi S, et al. NFκB in pancreatic stellate cells reduces infiltration of tumors by cytotoxic T cells and killing of cancer cells, via up-regulation of CXCL12. Gastroenterology. 2018;155(3):880–891. doi: 10.1053/j.gastro.2018.05.051. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pramanik S, Saha D. The genetic influence in fluorosis. Environ. Toxicol. Pharmacol. 2017;56:157–162. doi: 10.1016/j.etap.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 91.Shaim H, Estrov Z, Harris D, et al. The cXcr4–sTaT3–il-10 Pathway controls the immunoregulatory Function of chronic lymphocytic leukemia and is Modulated by lenalidomide. Front. Immunol. 2018;8:1773. doi: 10.3389/fimmu.2017.01773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rébé C, Ghiringhelli F. STAT3, a master regulator of anti-tumor immune response. Cancers. (Basel) 2019;11(9):1280. doi: 10.3390/cancers11091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huynh J, Chand A, Gough D, Ernst M. Therapeutically exploiting STAT3 activity in cancer—using tissue repair as a road map. Nat. Rev. Cancer. 2019;19(2):82–96. doi: 10.1038/s41568-018-0090-8. [DOI] [PubMed] [Google Scholar]
- 94.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 2007;7(1):41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 95.Atsaves V, Tsesmetzis N, Chioureas D, et al. PD-L1 is commonly expressed and transcriptionally regulated by STAT3 and MYC in ALK-negative anaplastic large-cell lymphoma. Leukemia. 2017;31(7):1633–1637. doi: 10.1038/leu.2017.103. [DOI] [PubMed] [Google Scholar]
- 96.Song TL, Nairismägi ML, Laurensia Y, et al. Oncogenic activation of the STAT3 pathway drives PD-L1 expression in natural killer/T-cell lymphoma. Blood. 2018;132(11):1146–1158. doi: 10.1182/blood-2018-01-829424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Garcia-Diaz A, Shin DS, Moreno BH, et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017;19(6):1189–1201. doi: 10.1016/j.celrep.2017.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yoyen-Ermis D, Tunali G, Tavukcuoglu E, et al. Myeloid maturation potentiates STAT3-mediated atypical IFN-γ signaling and upregulation of PD-1 ligands in AML and MDS. Sci. Rep. 2019;9(1):11697. doi: 10.1038/s41598-019-48256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Celada LJ, Kropski JA, Herazo-Maya JD, et al. PD-1 up-regulation on CD4+ T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β1 production. Sci. Transl. Med. 2018;10(460):eaar8356. doi: 10.1126/scitranslmed.aar8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Celada LJ, Rotsinger JE, Young A, et al. Programmed death-1 inhibition of phosphatidylinositol 3-kinase/AKT/mechanistic target of rapamycin signaling impairs sarcoidosis CD4+ T cell proliferation. Am. J. Respir. Cell Mol. Biol. 2017;56(1):74–82. doi: 10.1165/rcmb.2016-0037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Krasilnikov M, Ivanov VN, Dong J, Ronai Z. ERK and PI3K negatively regulate STAT-transcriptional activities in human melanoma cells: implications towards sensitization to apoptosis. Oncogene. 2003;22(26):4092–4101. doi: 10.1038/sj.onc.1206598. [DOI] [PubMed] [Google Scholar]
- 102.Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019;18(2):99–115. doi: 10.1038/s41573-018-0004-1. [DOI] [PubMed] [Google Scholar]
- 103.Albrengues J, Bertero T, Grasset E, et al. Epigenetic switch drives the conversion of fibroblasts into proinvasive cancer-associated fibroblasts. Nat. Commun. 2015;6(1):10204. doi: 10.1038/ncomms10204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang X, Lin Y, Shi Y, et al. FAP promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3–CCL2 signaling. Cancer Res. 2016;76(14):4124–4135. doi: 10.1158/0008-5472.CAN-15-2973. [DOI] [PubMed] [Google Scholar]
- 105.Li X, Xu Q, Wu Y, et al. A CCL2/ROS autoregulation loop is critical for cancer-associated fibroblasts-enhanced tumor growth of oral squamous cell carcinoma. Carcinogenesis. 2014;35(6):1362–1370. doi: 10.1093/carcin/bgu046. [DOI] [PubMed] [Google Scholar]
- 106.Fraietta JA, Lacey SF, Orlando EJ, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 2018;24(5):563–571. doi: 10.1038/s41591-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol. 2019;16(6):372–385. doi: 10.1038/s41571-019-0184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kolliniati O, Ieronymaki E, Vergadi E, Tsatsanis C. Metabolic regulation of macrophage activation. J. Innate Immun. 2022;14(1):51–68. doi: 10.1159/000516780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Natoli G, Pileri F, Gualdrini F, Ghisletti S. Integration of transcriptional and metabolic control in macrophage activation. EMBo Rep. 2021;22(9):e53251. doi: 10.15252/embr.202153251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xia T, Zhang M, Lei W, et al. Advances in the role of STAT3 in macrophage polarization. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1160719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK–STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77:521–546. doi: 10.1007/s40265-017-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huynh J, Etemadi N, Hollande F, Ernst M, Buchert M. The JAK/STAT3 axis: a comprehensive drug target for solid malignancies. Semin. Cancer Biol. 2017;45:13–22. doi: 10.1016/j.semcancer.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 113.Yang J, Chen G, Guo TW, Qin WY, Jia P. Simiao Wan attenuates monosodium urate crystal-induced arthritis in rats through contributing to macrophage M2 polarization. J. Ethnopharmacol. 2021;275 doi: 10.1016/j.jep.2021.114123. [DOI] [PubMed] [Google Scholar]
- 114.Jiang B, Zhu SJ, Xiao SS, Xue M. MiR-217 inhibits M2-like macrophage polarization by suppressing secretion of interleukin-6 in ovarian cancer. Inflammation. 2019;42:1517–1529. doi: 10.1007/s10753-019-01004-2. [DOI] [PubMed] [Google Scholar]
- 115.Liu YJ, hong Zeng S, hua Qian W, xian Tao M, ying Zhu Y, pin Li J. DNTTIP2 expression is associated with macrophage infiltration and malignant characteristics in low-grade glioma. Pharmgenomics. Pers. Med. 2022:261–275. doi: 10.2147/PGPM.S356326. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang H, Wang SQ, Hang L, et al. GRP78 facilitates M2 macrophage polarization and tumour progression. Cell Mol. Life Sci. 2021;78:7709–7732. doi: 10.1007/s00018-021-03997-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li M, Xu H, Qi Y, et al. Tumor-derived exosomes deliver the tumor suppressor miR-3591-3p to induce M2 macrophage polarization and promote glioma progression. Oncogene. 2022;41(41):4618–4632. doi: 10.1038/s41388-022-02457-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Malyshev IY. Phenomena and signaling mechanisms of macrophage reprogramming. Patol. Fiziol. Eksp. Ter. 2015;59(2):99–111. [PubMed] [Google Scholar]
- 119.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fu XL, Duan W, Su CY, et al. Interleukin 6 induces M2 macrophage differentiation by STAT3 activation that correlates with gastric cancer progression. Cancer Immunol. Immunther. 2017;66:1597–1608. doi: 10.1007/s00262-017-2052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Garbers C, Aparicio-Siegmund S, Rose-John S. The IL-6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Curr. Opin. Immunol. 2015;34:75–82. doi: 10.1016/j.coi.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 122.Lieblein JC, Ball S, Hutzen B, et al. STAT3 can be activated through paracrine signaling in breast epithelial cells. BMC Cancer. 2008;8:1–14. doi: 10.1186/1471-2407-8-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li W, Zhang X, Wu F, et al. Gastric cancer-derived mesenchymal stromal cells trigger M2 macrophage polarization that promotes metastasis and EMT in gastric cancer. Cell Death. Dis. 2019;10(12):918. doi: 10.1038/s41419-019-2131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen XX, Tang L, Fu YM, Wang Y, Han ZH, Meng JG. Paralemmin-3 contributes to lipopolysaccharide-induced inflammatory response and is involved in lipopolysaccharide-Toll-like receptor-4 signaling in alveolar macrophages. Int. J. Mol. Med. 2017;40(6):1921–1931. doi: 10.3892/ijmm.2017.3161. [DOI] [PubMed] [Google Scholar]
- 125.Gao S, Mao F, Zhang B, et al. Mouse bone marrow-derived mesenchymal stem cells induce macrophage M2 polarization through the nuclear factor-κB and signal transducer and activator of transcription 3 pathways. Exp. Biol. Med. 2014;239(3):366–375. doi: 10.1177/1535370213518169. [DOI] [PubMed] [Google Scholar]
- 126.He X, Jing Z, Cheng G. MicroRNAs: new regulators of Toll-like receptor signalling pathways. Biomed. Res. Int. 2014;2014 doi: 10.1155/2014/945169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ti D, Hao H, Tong C, et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015;13:1–14. doi: 10.1186/s12967-015-0642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Linton MF, Moslehi JJ, Babaev VR. Akt signaling in macrophage polarization, survival, and atherosclerosis. Int. J. Mol. Sci. 2019;20(11):2703. doi: 10.3390/ijms20112703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ghafouri-Fard S, Khanbabapour Sasi A, Hussen BM, et al. Interplay between PI3K/AKT pathway and heart disorders. Mol. Biol. Rep. 2022;49(10):9767–9781. doi: 10.1007/s11033-022-07468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kumar M, Bansal N. Implications of phosphoinositide 3-kinase-Akt (PI3K-Akt) pathway in the pathogenesis of Alzheimer's disease. Mol. Neurobiol. 2022;59(1):354–385. doi: 10.1007/s12035-021-02611-7. [DOI] [PubMed] [Google Scholar]
- 131.Xiao H, Sun X, Lin Z, et al. Gentiopicroside targets PAQR3 to activate the PI3K/AKT signaling pathway and ameliorate disordered glucose and lipid metabolism. Acta Pharm. Sin. B. 2022;12(6):2887–2904. doi: 10.1016/j.apsb.2021.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yu L, Wei J, Liu P. Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Semin. Cancer Biol. 2022;85:69–94. doi: 10.1016/j.semcancer.2021.06.019. [DOI] [PubMed] [Google Scholar]
- 133.Jiang Z, Zhang Y, Zhang Y, Jia Z, Zhang Z, Yang J. Cancer derived exosomes induce macrophages immunosuppressive polarization to promote bladder cancer progression. Cell Commun. Signal. 2021;19:1–13. doi: 10.1186/s12964-021-00768-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jeon H, Oh S, Kum E, Seo S, Park Y, Kim G. Immunomodulatory effects of an aqueous extract of black radish on mouse macrophages via the TLR2/4-mediated signaling pathway. Pharmaceuticals. 2022;15(11):1376. doi: 10.3390/ph15111376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Berumen Sánchez G, Bunn KE, Pua HH, Rafat M. Extracellular vesicles: mediators of intercellular communication in tissue injury and disease. Cell Commun. Signal. 2021;19(1):104. doi: 10.1186/s12964-021-00787-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yokoi A, Ochiya T. Exosomes and extracellular vesicles: rethinking the essential values in cancer biology. Semin. Cancer Biol. 2021;74:79–91. doi: 10.1016/j.semcancer.2021.03.032. [DOI] [PubMed] [Google Scholar]
- 137.Hur YH, Cerione RA, Antonyak MA. Extracellular vesicles and their roles in stem cell biology. Stem Cells (1981) 2020;38(4):469–476. doi: 10.1002/stem.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang N, Pei B, Yuan X, et al. Emerging roles of mesenchymal stem cell-derived exosomes in gastrointestinal cancers. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.1019459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dutta A. Exosomes-based cell-free cancer therapy: a novel strategy for targeted therapy. Immunol. Med. 2021;44(2):116–123. doi: 10.1080/25785826.2020.1818482. [DOI] [PubMed] [Google Scholar]
- 140.Prakash N, Kim J, Jeon J, et al. Progress and emerging techniques for biomaterial-based derivation of mesenchymal stem cells (MSCs) from pluripotent stem cells (PSCs) Biomater. Res. 2023;27(1):31. doi: 10.1186/s40824-023-00371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Janockova J, Slovinska L, Harvanova D, Spakova T, Rosocha J. New therapeutic approaches of mesenchymal stem cells-derived exosomes. J. Biomed. Sci. 2021;28(1):39. doi: 10.1186/s12929-021-00736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nikfarjam S, Rezaie J, Zolbanin NM, Jafari R. Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. J. Transl. Med. 2020;18(1):449. doi: 10.1186/s12967-020-02622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xie L, Zhao H, Wang Y, Chen Z. Exosomal shuttled miR-424-5p from ischemic preconditioned microglia mediates cerebral endothelial cell injury through negatively regulation of FGF2/STAT3 pathway. Exp. Neurol. 2020;333 doi: 10.1016/j.expneurol.2020.113411. [DOI] [PubMed] [Google Scholar]
- 144.Shahabipour F, Barati N, Johnston TP, Derosa G, Maffioli P, Sahebkar A. Exosomes: nanoparticulate tools for RNA interference and drug delivery. J. Cell Physiol. 2017;232(7):1660–1668. doi: 10.1002/jcp.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liang SF, Zuo FF, Yin BC, Ye BC. Delivery of siRNA based on engineered exosomes for glioblastoma therapy by targeting STAT3. Biomater. Sci. 2022;10(6):1582–1590. doi: 10.1039/D1BM01723C. [DOI] [PubMed] [Google Scholar]
- 146.Zhuang X, Xiang X, Grizzle W, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 2011;19(10):1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zou H, Poore B, Broniscer A, Pollack IF, Hu B. Molecular heterogeneity and cellular diversity: implications for precision treatment in medulloblastoma. Cancers. (Basel) 2020;12(3):643. doi: 10.3390/cancers12030643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fasinu P, Pillay V, Ndesendo VMK, du Toit LC, Choonara YE. Diverse approaches for the enhancement of oral drug bioavailability. Biopharm. Drug Dispos. 2011;32(4):185–209. doi: 10.1002/bdd.750. [DOI] [PubMed] [Google Scholar]
- 149.Polak K.L., Chernosky N.M., Smigiel J.M., Tamagno I., Jackson M.W. Balancing STAT activity as a therapeutic strategy. Cancers. (Basel) 2019;11(11):1716. doi: 10.3390/cancers11111716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Thilakasiri PS, Dmello RS, Nero TL, Parker MW, Ernst M, Chand AL. Repurposing of drugs as STAT3 inhibitors for cancer therapy. Semin. Cancer Biol. 2021;68:31–46. doi: 10.1016/j.semcancer.2019.09.022. [DOI] [PubMed] [Google Scholar]
- 151.Santoni M, Miccini F, Cimadamore A, et al. An update on investigational therapies that target STAT3 for the treatment of cancer. Expert. Opin. Investig. Drugs. 2021;30(3):245–251. doi: 10.1080/13543784.2021.1891222. [DOI] [PubMed] [Google Scholar]
- 152.Gan C, Li Y, Yu Y, et al. Natural product pectolinarigenin exhibits potent anti-metastatic activity in colorectal carcinoma cells in vitro and in vivo. Bioorg. Med. Chem. 2019;27(21) doi: 10.1016/j.bmc.2019.115089. [DOI] [PubMed] [Google Scholar]
- 153.Talib WH, Awajan D, Hamed RA, Azzam AO, Mahmod AI, AL-Yasari IH. Combination anticancer therapies using selected phytochemicals. Molecules. 2022;27(17):5452. doi: 10.3390/molecules27175452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang M, Jiang S, Zhou L, et al. Potential mechanisms of action of curcumin for cancer prevention: focus on cellular signaling pathways and miRNAs. Int. J. Biol. Sci. 2019;15(6):1200–1214. doi: 10.7150/ijbs.33710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang C, Mai Z, Liu C, Yin S, Cai Y, Xia C. Natural products in preventing tumor drug resistance and related signaling pathways. Molecules. 2022;27(11):3513. doi: 10.3390/molecules27113513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Costantino M, Corno C, Colombo D, Perego P. Curcumin and related compounds in cancer cells: new avenues for old molecules. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.889816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nayak A, Warrier NM, Kumar P. Cancer stem cells and the tumor microenvironment: targeting the critical crosstalk through nanocarrier systems. Stem Cell Rev. Rep. 2022;18(7):2209–2233. doi: 10.1007/s12015-022-10426-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fang B. Genetic interactions of STAT3 and anticancer drug development. Cancers. (Basel) 2014;6(1):494–525. doi: 10.3390/cancers6010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Amero P, Khatua S, Rodriguez-Aguayo C, Lopez-Berestein G. Aptamers: novel therapeutics and potential role in neuro-oncology. Cancers. (Basel) 2020;12(10):2889. doi: 10.3390/cancers12102889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Venkatesan S, Chanda K, Balamurali MM. Recent advancements of aptamers in cancer therapy. ACS. Omega. 2023;8(36):32231–32243. doi: 10.1021/acsomega.3c04345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bharadwaj U, Kasembeli M, Eckols T, et al. Monoclonal antibodies specific for STAT3β reveal its contribution to constitutive STAT3 phosphorylation in breast cancer. Cancers. (Basel) 2014;6(4):2012–2034. doi: 10.3390/cancers6042012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zou S, Tong Q, Liu B, Huang W, Tian Y, Fu X. Targeting stat3 in cancer immunotherapy. Mol. Cancer. 2020;19(1):1–19. doi: 10.1186/s12943-020-01258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data were from publicly available search engines, namely PubMed, Scopus, and Web of Science. These articles are available online.