Abstract
Background:
Neoadjuvant therapy prior to resection of adenocarcinoma of the pancreatic head increases time to surgery and thus the possibility of biliary complications. We hypothesized that biliary complications during neoadjuvant therapy negatively impact clinical outcomes.
Methods:
We completed a retrospective study of a cohort of borderline resectable patients consistently treated with neoadjuvant therapy from May 2014 through March 2019. Biliary complications were defined as new-onset biliary obstruction, existing stent failure, cholecystitis, and cholangitis.
Results:
Of 59 patients that met inclusion criteria, 34 (57.6%) went on to resection. Biliary complications affected 16 patients (27%); 8 (50%) of these patients went on to surgical resection. Of those 43 patients who did not have a biliary intervention, 26 went on to surgical resection (60.4%). There was no significant effect of a biliary complication on total number of chemotherapy cycles (p = 0.12), proceeding to surgical resection (p = 0.56) or on median survival (p = 0.23). Among patients who did proceed to surgery, there was a notable difference in median survival for patients who required a biliary intervention (17.9 vs 31.0 months) that did not reach significance (p=0.35).
Conclusion:
The need for further biliary interventions during neoadjuvant therapy for pancreatic adenocarcinoma is common, but does not appear to have a significant effect on number of cycles of neoadjuvant therapy or proceeding to surgical resection. Larger studies are necessary to determine if these events compromise overall survival.
Keywords: cholecystitis, cholecystostomy, cholecystectomy, cholangitis, chemotherapy
Introduction
The surgical treatment of pancreatic adenocarcinoma is evolving to favor neoadjuvant therapy, particularly for borderline resectable cancers, and even some select patients with locally advanced disease.1, 2 Most neoadjuvant regimens are based upon evidence from metastatic patients3 and data suggests these regimens are safe and effective in the neoadjuvant setting.4–13 Further, neoadjuvant therapy does not appear to negatively affect postoperative outcomes14 and is associated with a decrease in post-operative complications such as peripancreatic fistula15.
The premise of this study is that neoadjuvant therapy prior to resection of adenocarcinoma of the pancreatic head increases time to surgery and thus the possibility of biliary complications including new-onset biliary obstruction, existing stent failure, cholecystitis, and cholangitis. However, the frequency of biliary complications requiring interventions and the subsequent impact on outcomes in patients undergoing a neoadjuvant treatment strategy for borderline resectable cancer of the pancreatic head remains unknown. Most patients with adenocarcinoma of the pancreatic head present with jaundice and require up front endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic biliary drainage (PTHD) with stenting at time of diagnosis.16–20 These catheters can fail. Further, in a series of patients receiving neoadjuvant therapy for pancreatic cancer who had previously been stented, 6% were subsequently diagnosed with acute cholecystitis. For those who were managed with cholecystostomy drainage, this did not impact completion of neoadjuvant therapy and surgical resection whereas a very small minority who had cholecystectomies did not progress on to surgical resection of their pancreatic cancer.21
We aimed to quantify the incidence and clinical significance of biliary complications that occur during neoadjuvant therapy in a cohort of borderline resectable patients with adenocarcinoma of the pancreatic head. We hypothesized that biliary interventions interfere with neoadjuvant therapy and negatively impact overall survival. We specifically focused on the impact of biliary complications during neoadjuvant therapy on progressing to surgery, surgical outcome, and overall survival. This included the need for new biliary tree decompression, failure of existing biliary drainage, cholecystitis, or cholagitis occurring during neoadjuvant therapy for borderline resectable pancreatic cancers.
Methods
This protocol was approved by the University of Florida Institutional Review Board. A retrospective analysis of all patients from a single center diagnosed with pancreatic cancer between May 2014 and March 2019 was performed. Inclusion criteria were presence of a borderline resectable pancreatic cancer of the head (International Association of Pancreatology definition)22 and assignment to a course of neoadjuvant therapy with planned subsequent surgical extirpation. Patients with resectable disease that underwent neoadjuvant therapy were excluded, as that approach in this group was infrequently and inconsistently applied. All patients were discussed at an interdisciplinary tumor board, determined to be anatomically borderline resectable, and assigned to neoadjuvant therapy prior to attempt at surgical resection. The time frame of the study coincides with an institution-wide algorithm to insist on neoadjuvant therapy in all patients with borderline resectable pancreatic cancer prior to surgical resection. The goal of neoadjuvant, systemic therapy was completion a minimum of four and ideally eight cycles without biochemical or radiologic evidence of progression. The chemotherapy regimen was not prescribed and was determined by the treating medical oncologist. March 2019 was chosen as the closing date to assure at least 12 months of follow-up.
Demographic data included age, sex, BMI and race. Other key clinical data included ASA class, ultimate stage of disease (either clinical for non-surgical patients or pathological for surgical patients) and neoadjuvant regimen. Primary outcomes included completion of surgical resection, decision to not pursue surgical resection or aborted resection, and overall survival. Secondary outcomes for those undergoing surgical resection included Clavien-Dindo Class 3 or greater complications, and 30- and 90-day mortality.
Continuous variables are expressed as the median with interquartile range (IQR). Categorical variables are presented as the raw number and percent of the total number from the cohort. Continuous numerical variables were assessed for statistical significance using Wilcoxon-Mann-Whitney Test. Categorical variables were assessed for significance by Fisher’s exact test. A p value of < 0.05 was considered statistically significant. A Kaplan-Meier estimator and log-rank test were used to determine significant differences in survival. Kaplan-Meier reverse method was used to determine median follow up time. All statistical analyses were completed using R Studio Version 1.1.383.
Results
Demographics and Neoadjuvant Therapy
Fifty-nine patients met inclusion criteria (of 279 pancreatoduodenectomy candidates during the timeframe of the study). Median follow up time was 36.5 months. Table 1 presents the patient demographics. Specific neoadjuvant treatment regimens were not prescribed and subsequently varied based upon patient- and provider-dependent variables. For completeness, the majority of patients (29, 49%) received a course of gemcitabine and nab-paclitaxel followed by chemo-radiation (Table 1). Another 14 (24%) received a course of modified leucovorin, fluoruracil, irinotecan, oxaliplatin (mFOLFIRINOX) followed by chemo-radiation. Eight (14%) patients received a combination of mFOLFIRINOX which was changed to gemcitabine/nab-paclitaxel (or vice versa) based on evidence of progression of disease or intolerance. Eight patients (14%) received other treatment.
Table 1.
Demographic information, neoadjuvant therapy and biliary interventions.
| Age | 67 (61.3–76.0) |
|
| |
| Male sex | 30 (51%) |
|
| |
| BMI | 27.2 (24.1–31.1) |
|
| |
| Race | |
| Black | 6 (10%) |
| White | 51 (86%) |
| Hispanic | 1 (2%) |
| Asian | 1 (2%) |
|
| |
| ASA | |
| II # (%) | 6 (10%) |
| III # (%) | 52 (88%) |
| IV # (%) | 1 (2%) |
|
| |
| Ultimate Stage | |
| IA # (%) | 8 (14%) |
| IB # (%) | 12 (20%) |
| IIA # (%) | 10 (17%) |
| IIB # (%) | 20 (34%) |
| III # (%) | 6 (10%) |
| IV # (%) | 3 (5%) |
|
| |
| Neoadjuvant Regimen | |
|
| |
| Gemcitabine/nab-paclitaxel ± chemoradiation | 29 (49%) |
| FOLFIRINOX ± chemoradiation | 14 (24%) |
| Combo FOLFIRINOX ± gemcitabine/nab-paclitaxel | 8 (14%) |
| Other | 8 (14%) |
|
| |
| # cycles | 4.0 (3.0–6.0) |
|
| |
| # patients with cycles unknown | 7 |
|
| |
| ERCP with stent | |
| Metal ➜ Metal | 1 |
| Plastic ➜ Metal | 2 |
| New metal | 2 |
| PTHD | 4 |
| Percutaneous cholecystostomy | 3 |
| Cholecystectomy | 2 |
| Medical treatment of cholangitis | 2 |
The median number of cycles of neoadjuvant therapy was 4 (IQR 3–6), although cycle number data for seven (12%) of these patients treated outside the institution was incomplete. Of the sixteen patients who had a biliary intervention, three experienced a delay in neoadjuvant treatment due specifically to the complication, but there was no difference in number of total cycles based on the need for a biliary intervention (W = 408, p = 0.12).
Biliary Interventions
Table 1 shows the biliary interventions performed in the entire cohort. Fifteen patients were not stented prior to initiation of therapy; two of these patients developed biliary obstruction after initiation of chemotherapy and required ERCP with biliary stenting. Other biliary complications during neoadjuvant therapy impacted an additional 14 patients (total of 16 of 59 patients, 27%). Emergent/urgent interventions among these patients included percutaneous cholecystostomy (3 patients, 5.1%), laparoscopic cholecystectomy (2 patients, 3.3%), ERCP with stent exchange (3 patients, 5.1%), PTHD catheter exchange (4 patients, 6.8%) and hospital admission for cholangitis with medical treatment only (2 patients, 3.3%). Of those requiring repeat stenting, one patient had a partially occluded metal stent replaced with a second metal stent. Two patients had plastic stents exchanged for metal stents when they presented with stent obstruction. One patient had a scheduled plastic to plastic stent exchange; this was not considered a biliary complication. All patients who received a PTHD had them placed after failed attempts at ERCP stenting.
Procession to Surgical Resection and Survival
Among our entire cohort, six (10%) patients died during neoadjuvant therapy prior to any reassessment for surgery. Of the remaining patients that completed neoadjuvant therapy, 34 (58%) of the original 59 patients went on to resection and four of these included a vein resection, while 18 (31%) did not go on to resection due to disease progression. Of the 16 patients in the bilary complications cohort, eight went on to surgical resection (50%) compared to 26 of the 43 (60.4%) patients in the cohort who did not have biliary complications (p = 0.56), thus there was not a significant difference in proceeding to surgical resection based on the need for biliary intervention (Table 2). Of note, one patient did not undergo surgical resection as a direct result of a biliary complication that led to severe sepsis with multi-organ dysfunction. Similarly, there was no significant difference in post-operative length of stay (W = 83.5, p = 0.41) or Clavien-Dindo class 3+ complications (p = 1) based upon need for a biliary intervention. Thirty-day mortality was zero for all surgical patients (p = 1) and there was no difference in 90-day mortality (p = 0.23, Table 2) between the biliary intervention and non-intervention cohorts.
Table 2.
Primary and Secondary Outcomes
| Table 4 Outcomes | Total | Intervention | No Intervention | |
|---|---|---|---|---|
| Resection | 34 (58%) | 8 (50%) | 26 (60%) | p = 0.56 |
| CD>3 complication | 4 (7%) | 1 (12.5%) | 3 (7%) | p = 1 |
| post-op LOS | 10.1 (7.4) | 12.4 (6.7) | 9.4 (7.6) | p = 0.41 |
| 30 day mortality | 0 (0%) | 0 (0%) | 0 (0%) | p = 1 |
| 90 day mortality | 1 (12.5%) | 1 (12.5%) | 0 (0%) | p = 0.23 |
| No Resection | 25 (42%) | 8 (50%) | 17 (40%) | |
| # cycles chemo | 4(3–6) | 4(3–6) | 4 (3–6.5) | p = 0.12 |
Rate of resection is compared to no resection by intervention. Complications, LOS (mean (standard deviation)) and mortality are compared in intervention vs. no intervention group.
CD 3+: Clavien-Dindo Class 3 or greater complication. LOS: length of stay.
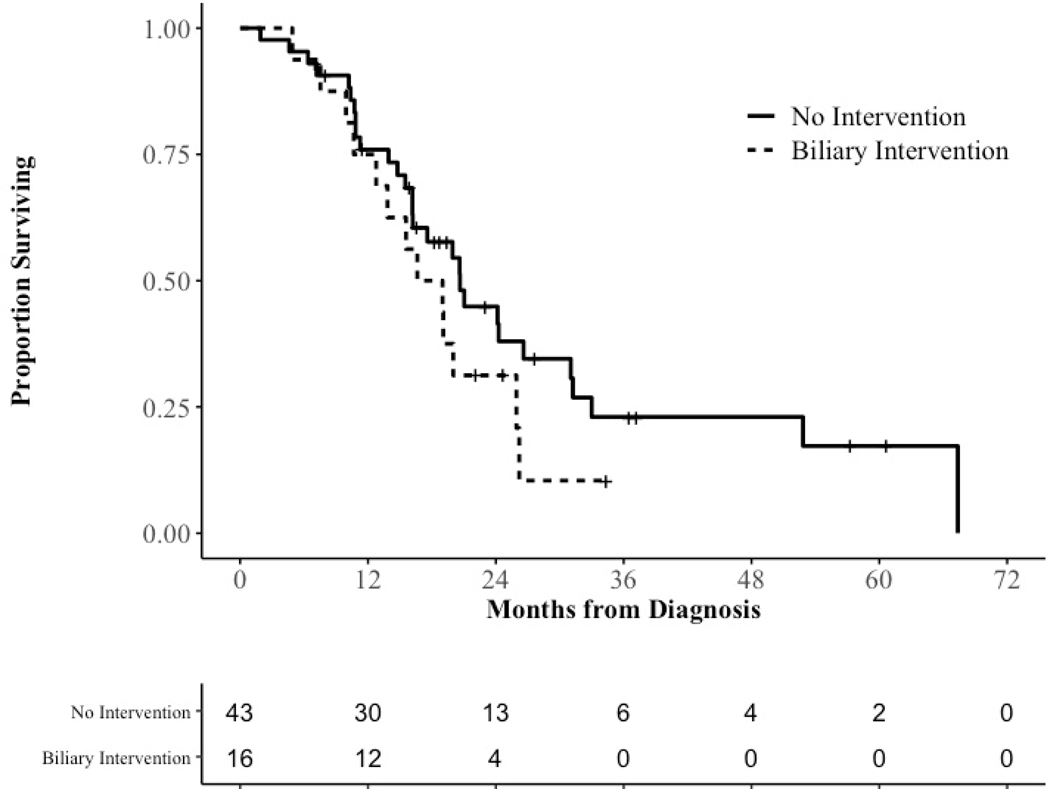
Overall survival was the primary outcome of interest in this study. Median overall survival for all 59 patients was 19.9 months. There was no difference in median survival for patients with no biliary intervention (20.6 months) compared to those who had biliary interventions (17.8 months, log-rank, p = 0.23, Figure 1). Median overall survival for patients who went on to surgical resection was 24.3 months. A difference in median survival for surgical patients without biliary intervention (31.0 months) compared to those who required biliary interventions (17.9 months) was noted, but failed to reach significance (log-rank, p = 0.35, Supplementary Figure 1).
Figure 1.
Kaplan-Meier estimator for all patients by need for biliary intervention.
Discussion
This study identifies the rate and measures impact on surgical outcomes and overall survival of biliary interventions required during neoadjuvant therapy for borderline resectable pancreatic head adenocarcinoma. Based on our findings, biliary complications during neoadjuvant therapy for pancreatic head cancer are relatively common, do not appear to impact number of chemotherapy cycles or progression to surgery, but may have an effect on survival outcomes as our findings demonstrate a trend towards an effect of these interventions on survival, suggesting the present sample size may be underpowered to detect this effect.
Our study builds upon recent reports of other specific biliary complications during neoadjuvant therapy for pancreatic cancer. In particular, the incidence of acute cholecystitis after stenting has been reported to be 6%.21 Similarly, the percentage of patients undergoing ERCP with stenting for biliary obstruction during neoadjuvant therapy ranges from 6% to 10%.23, 24 These interventions did not disrupt neoadjuvant therapy or proceeding to surgery. This suggests that interventions to manage biliary complications do not compromise continuation of chemotherapy or proceeding to surgery.
While the sample size limits analysis, our findings suggest metal stents may prove more robust during neoadjuvant therapy. In our cohort, five patients required urgent/emergent ERCP with stenting during their neoadjuvant therapy. Among these, one patient with a metal stent compared to two patients with plastic stents became obstructed. All stents placed during neoadjuvant therapy were replaced with metal stents (other than one scheduled plastic to plastic exchange outside the institution) and did not require further intervention. Similarly, the two patients that developed obstructive jaundice after the initiation of chemotherapy received metal stents. Neither went on to require further intervention. These findings are consistent with previous reports of the superiority of metal over plastic stents.16–18, 20
Finally, our resection rate and survival are comparable to other series in the literature. The proportion of patients who went onto resection in this study (57.6%) is slightly lower than previous studies that report 62% to 74%.25, 26 Median survival (19.9 months) was likewise comparable to previous reports of 20–25 months.25, 26
This study has a number of limitations including all the biases intrinsic to a retrospective, single-center study. Further, this study is from a tertiary care center with a large referral catchment area. Many patients at our institution receive neoadjuvant therapy in their local community and this impacts influence of the selected chemotherapy regimen, the availability of medical records, and the possibility of failing to identify necessary biliary interventions. Importantly, the limited sample size opens the study to the possibility of a type II error. This is particularly notable given the reduced survival rates following surgical resection in those patients requiring biliary interventions compared to the control cohort that did not reach significance (17.9 versus 31.0 months, p = 0.35).
In summary, we build on recent reports of biliary complications impacting clinical care during neoadjuvant therapy for pancreatic cancer, presenting data that suggests these complications are common but do not lead to adverse clinical outcomes. Despite the incidence of such complications, biliary interventions do not appear to preclude surgical resection or decrease overall survival. Our results do suggest further studies of larger sample sets across multiple institutions are warranted.
Supplementary Material
Supplementary Figure 1. Kaplan-Meier estimator for surgical patients by need for biliary intervention.
Abbreviations:
- mFOLFIRINOX
leucovorin, fluoruracil, irinotecan, oxaliplatin
- ERCP
endoscopic retrograde cholangio-pancreatography
- PTHD
percutaneous transhepatic drain
- IQR
interquartile range
- CD 3+
Clavien-Dindo Class 3 or greater complication
- LOS
length of stay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Assifi MM, Lu X, Eibl G, Reber HA, Li G, and Hines OJ. “Neoadjuvant Therapy in Pancreatic Adenocarcinoma: A Meta-Analysis of Phase Ii Trials.” Surgery 150, no. 3 (Sep 2011): 466–73. 10.1016/j.surg.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee AJ, Simoneau E, Chiang YJ, et al. “Is Early-Stage Pancreatic Adenocarcinoma Truly Early: Stage Migration on Final Pathology with Surgery-First Versus Neoadjuvant Therapy Sequencing.” HPB (Oxford) 21, no. 9 (Sep 2019): 1203–10. 10.1016/j.hpb.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Conroy T, Desseigne F, Ychou M, et al. “Folfirinox Versus Gemcitabine for Metastatic Pancreatic Cancer.” N Engl J Med 364, no. 19 (May 12 2011): 1817–25. 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 4.Boone BA, Steve J, Krasinskas AM, et al. “Outcomes with Folfirinox for Borderline Resectable and Locally Unresectable Pancreatic Cancer.” JSurg Oncol 108, no. 4 (Sep : 236–41. 10.1002/jso.23392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christians KK, Tsai S, Mahmoud A, et al. “Neoadjuvant Folfirinox for Borderline Resectable Pancreas Cancer: A New Treatment Paradigm?”, Oncologist 19, no. 3 (Mar: 266–74. 10.1634/theoncologist.2013-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrone CR, Marchegiani G, Hong TS, et al. “Radiological and Surgical Implications of Neoadjuvant Treatment with Folfirinox for Locally Advanced and Borderline Resectable Pancreatic Cancer.” Ann Surg 261, no. 1 (Jan 2015): 12–7. 10.1097/sla.0000000000000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hackert T, Sachsenmaier M, Hinz U, et al. “Locally Advanced Pancreatic Cancer: Neoadjuvant Therapy with Folfirinox Results in Resectability in 60% of the Patients.” Ann Surg 264, no. 3 (Sep 2016): 457–63. 10.1097/sla.0000000000001850. [DOI] [PubMed] [Google Scholar]
- 8.Klaiber U, and Hackert T. “Conversion Surgery for Pancreatic Cancer-the Impact of Neoadjuvant Treatment.” Front Oncol 9 (2019): 1501. 10.3389/fonc.2019.01501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neyaz A, Tabb E, Shih A, et al. “Pancreatic Ductal Adenocarcinoma: Tumor Regression Grading Following Neoadjuvant Folfirinox and Radiation.” Histopathology (Feb 7 2020). 10.1111/his.14086. [DOI] [PubMed] [Google Scholar]
- 10.Suker M, Beumer BR, Sadot E, et al. “Folfirinox for Locally Advanced Pancreatic Cancer: A Systematic Review and Patient-Level Meta-Analysis.” Lancet Oncol 17, no. 6 (Jun 2016): 801–10. 10.1016/s1470-2045(16)00172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nitsche U, Wenzel P, Siveke JT, et al. “Resectability after First-Line Folfirinox in Initially Unresectable Locally Advanced Pancreatic Cancer: A Single-Center Experience.” Ann Surg Oncol 22 Suppl 3 (Dec 2015): S1212–20. 10.1245/s10434-015-4851-2. [DOI] [PubMed] [Google Scholar]
- 12.Varadhachary GR, Tamm EP, Abbruzzese JL, et al. “Borderline Resectable Pancreatic Cancer: Definitions, Management, and Role of Preoperative Therapy.” Ann Surg Oncol 13, no. 8 (Aug 2006): 1035–46. 10.1245/aso.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Von Hoff DD, Ervin T, Arena FP, et al. “Increased Survival in Pancreatic Cancer with Nab-Paclitaxel Plus Gemcitabine.” N Engl J Med 369, no. 18 (Oct 31 2013): 1691–703. 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pecorelli N, Pagnanelli M, Cinelli L, et al. “Postoperative Outcomes and Functional Recovery after Preoperative Combination Chemotherapy for Pancreatic Cancer: A Propensity Score-Matched Study.” Front Oncol 9 (2019): 1299. 10.3389/fonc.2019.01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hank T, Sandini M, Ferrone CR, et al. “Association between Pancreatic Fistula and Long-Term Survival in the Era of Neoadjuvant Chemotherapy.” JAMA Surg (Aug 14 2019). 10.1001/jamasurg.2019.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boulay BR, Gardner TB, and Gordon SR. “Occlusion Rate and Complications of Plastic Biliary Stent Placement in Patients Undergoing Neoadjuvant Chemoradiotherapy for Pancreatic Cancer with Malignant Biliary Obstruction.” J Clin Gastroenterol 44, no. 6 (Jul 2010): 452–5. 10.1097/MCG.0b013e3181d2ef06. [DOI] [PubMed] [Google Scholar]
- 17.Crippa S, Cirocchi R, Partelli S, et al. “Systematic Review and Meta-Analysis of Metal Versus Plastic Stents for Preoperative Biliary Drainage in Resectable Periampullary or Pancreatic Head Tumors.” Eur J Surg Oncol 42, no. 9 (Sep 2016): 1278–85. 10.1016/j.ejso.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Decker C, Christein JD, Phadnis MA, Wilcox CM, and Varadarajulu S. “Biliary Metal Stents Are Superior to Plastic Stents for Preoperative Biliary Decompression in Pancreatic Cancer.” Surg Endosc 25, no. 7 (Jul 2011): 2364–7. 10.1007/s00464-010-1552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheufele F, Schorn S, Demir IE, et al. “Preoperative Biliary Stenting Versus Operation First in Jaundiced Patients Due to Malignant Lesions in the Pancreatic Head: A Meta-Analysis of Current Literature.” Surgery 161, no. 4 (Apr 2017): 939–50. 10.1016/j.surg.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Tol JA, van Hooft JE, Timmer R, et al. “Metal or Plastic Stents for Preoperative Biliary Drainage in Resectable Pancreatic Cancer.” Gut 65, no. 12 (Dec 2016): 1981–87. 10.1136/gutjnl-2014-308762. [DOI] [PubMed] [Google Scholar]
- 21.Jariwalla NR, Khan AH, Dua K, et al. “Management of Acute Cholecystitis During Neoadjuvant Therapy in Patients with Pancreatic Adenocarcinoma.” Ann Surg Oncol 26, no. 13 (Dec 2019): 4515–21. 10.1245/s10434-019-07906-7. [DOI] [PubMed] [Google Scholar]
- 22.Isaji S, Mizuno S, Windsor JA, et al. “International Consensus on Definition and Criteria of Borderline Resectable Pancreatic Ductal Adenocarcinoma 2017.” Pancreatology 18, no. 1 (Jan 2018): 2–11. 10.1016/j.pan.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu S, Naitoh I, Nakazawa T, et al. “Predictive Factors for Pancreatitis and Cholecystitis in Endoscopic Covered Metal Stenting for Distal Malignant Biliary Obstruction.” J Gastroenterol Hepatol 28, no. 1 (Jan 2013): 68–72. 10.1111/j.1440-1746.2012.07283.x. [DOI] [PubMed] [Google Scholar]
- 24.Suk KT, Kim HS, Kim JW, et al. “Risk Factors for Cholecystitis after Metal Stent Placement in Malignant Biliary Obstruction.” Gastrointest Endosc 64, no. 4 (Oct 2006): 522–9. 10.1016/j.gie.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 25.Barnes CA, Chavez MI, Tsai S, et al. “Survival of Patients with Borderline Resectable Pancreatic Cancer Who Received Neoadjuvant Therapy and Surgery.” Surgery 166, no. 3 (Sep 2019): 277–85. 10.1016/j.surg.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Tsai S, Christians KK, George B, et al. “A Phase Ii Clinical Trial of Molecular Profiled Neoadjuvant Therapy for Localized Pancreatic Ductal Adenocarcinoma.” Ann Surg 268, no. 4 (Oct 2018): 610–19. 10.1097/sla.0000000000002957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Kaplan-Meier estimator for surgical patients by need for biliary intervention.