SUMMARY
Time-restricted eating (TRE) has become a popular strategy to treat obesity. TRE involves confining the eating window to 4–10 h per day and fasting for the remaining hours (14–20 h fast). During the eating window, individuals are not required to monitor food intake. The sudden rise in popularity of TRE is most likely due to its simplicity and the fact that it does not require individuals to count calories to lose weight. This feature of TRE may appeal to certain individuals with obesity, and this could help produce lasting metabolic health improvements. The purpose of this review is to summarize current evidence from randomized clinical trials of TRE (without calorie counting) on body weight and metabolic risk factors. The efficacy of TRE in various populations groups, including those with obesity, type 2 diabetes (T2DM), and polycystic ovary syndrome (PCOS), is also examined.
INTRODUCTION
Intermittent fasting has greatly increased in popularity over the past decade owing to its ability to produce clinically significant weight loss and confer protection against metabolic diseases.1–4 Currently, the most popular form of intermittent fasting in the United States is time-restricted eating (TRE).4,5 TRE typically involves confining the eating window to 4–10 h and fasting for the remaining hours of the day (14–20 h fast). During the eating window, individuals are not required to count calories or monitor food intake in any way. During the fasting window, individuals are encouraged to drink plenty of water and may also consume energy-free beverages.
The sudden rise in popularity of TRE is mostly likely due to its simplicity and the fact that it does not require individuals to count calories to lose weight. Indeed, evidence shows that when adults with obesity limit their eating window to 4–10 h per day, they naturally reduce energy intake by 200–550 kcal/day.6–9 These findings have important implications from a clinical standpoint. One of the barriers to traditional dieting approaches, such as daily calorie restriction (CR), is having to monitor calorie intake every day.10,11 TRE can bypass this requirement by allowing participants to simply “watch the clock” instead of monitoring energy intake while still producing weight loss.4,5 This feature of TRE may appeal to certain individuals with obesity, which could help produce lasting weight control and metabolic health improvements.
The purpose of this review is to summarize current evidence from randomized clinical trials of TRE (without calorie counting) on body weight and metabolic risk factors. The effects of TRE in various population groups, including those with obesity, type 2 diabetes (T2DM), and polycystic ovary syndrome (PCOS), are examined. We also look to the future of this field and comment on how TRE interventions may have a role in the treatment of obesity and metabolic diseases.
METHODS: HUMAN TRIAL SELECTION
A Medline search was conducted in July 2023 using the following keywords: “time-restricted eating,” “time limited eating,” “time-restricted feeding,” “intermittent fasting,” “intermittent energy restriction,” “fasting,” “meal timing,” “meal frequency,” “meal skipping,” “breakfast skipping,” “randomized trial,” “human,” “obesity,” “weight loss,” “diabetes,” “prediabetes,” “polycystic ovary syndrome,” “adolescents,” “teenagers,” “children,” “elderly,” and “older adults.” Inclusion criteria for research articles were as follows: (1) randomized controlled trials conducted in human subjects, (2) participants with overweight or obesity, and (3) endpoints that included changes in body weight and at least one relevant marker of metabolic disease. The following exclusion criteria were applied: (1) cohort and observational studies, (2) fasting performed as a religious practice (Ramadan or Seventh Day Adventist), (3) trial durations of less than 1 month, (4) trials that combined TRE with intentional energy restriction, and (5) trials that combined TRE with an exercise intervention. Our search retrieved 8 randomized controlled trials of TRE (without calorie counting) in individuals with obesity (Table 1). Since very few trials examined the effect of TRE in special population groups (i.e., adolescents, elderly, and individuals with type 2 diabetes [T2DM] or PCOS), we opted to include non-randomized controlled trials in this section. The TRE trials conducted in special population groups are summarized in Table 2. We also performed a risk-of-bias assessment using the Cochrane Collaboration tool for randomized trials.12 Random sequence generation, allocation concealment, blinding, and incomplete outcome data were graded as having a high, low, or unclear risk of bias.
Table 1.
Time-restricted eating (without calorie counting) on changes in body weight and metabolic risk factors in adults with overweight and obesity
| Reference |
Participants |
Length |
Design and intervention groups |
Body weight |
Body composition |
Blood pressure |
Plasma lipids |
Glucoregulatory factors |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Months | FM | FFM | VF | LDL | HDL | TG | Fasting glucose | Mean glucose | Fasting insulin | HOMA | HbA1c | |||||
|
| ||||||||||||||||
| 4-h time-restricted eating | ||||||||||||||||
|
| ||||||||||||||||
| Cienfuegos6 | n = 58, MF age: 47 years obese prediabetes |
2 | RCT: parallel-arm (1) 4-h TRE (3–7 p.m.) (2) control (no meal timing restrictions) | (1) ↓3%* (2) ∅ |
(1) ↓* (2) ∅ |
(1) ↓* (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
– | (1) ↓* (2) ∅ |
(1) ↓* (2) ∅ |
(1) ∅ (2) ∅ |
|
| ||||||||||||||||
| 6-h time-restricted eating | ||||||||||||||||
|
| ||||||||||||||||
| Sutton13 | n = 8, M age: 56 years obese prediabetes |
1 | RCT: cross-over (1) 6-h TRE (~8 a.m.– 2 p.m.) (2) control (12h eating window) | (1) ∅ (2) ∅ |
– | – | – | (1) ↓S*↓D* (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ↑* (2) ∅ |
(1) ∅ (2) ∅ |
– | (1) ↓* (2) ∅ |
(1) ↓* (2) ∅ |
– |
| Cienfuegos6 | n = 58, MF age: 47 years obese prediabetes |
2 | RCT: parallel-arm (1) 6-h TRE (1–7 p.m.) (2) control (no meal timing restrictions) | (1) ↓ 3%* (2) ∅ |
(1) ↓* (2) ∅ |
(1) ↓* (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
– | (1) ↓* (2) ∅ |
(1) ↓* (2) ∅ |
(1) ∅ (2) ∅ |
| Zhang14 | n = 63, MF age: 23 years overweight Ins resistant |
2 | RCT: parallel-arm (1) 6-h TRE (7 a.m.– 1 p.m.) (2) 6-h TRE (12 p.m.–6 p.m.) (3) control (no meal timing restrictions) | (1) ↓ 5%* (2) ↓ 4%* (3) ∅ |
(1) ↓* (2) ↓* (3) ∅ |
(1) ↓*† (2) ∅ (3) ∅ |
(1) ↓* (2) ↓* (3) ∅ |
(1) ↓S*∅ D (2) ∅ (3) ∅ |
(1) ↑* (2) ↑* (3) ∅ |
(1) ∅ (2) ∅ (3) ∅ |
(1) ∅ (2) ∅ (3) ∅ |
(1) ∅ (2) ∅ (3) ∅ |
(1) ↓*† (2) ∅ (3) ∅ |
(1) ↓* (2) ∅ (3) ∅ |
(1) ↓* (2) ∅ (3) ∅ |
(1) ∅ (2) ∅ (3) ∅ |
|
| ||||||||||||||||
| 8-h time-restricted eating | ||||||||||||||||
|
| ||||||||||||||||
| Chow15 | n = 21, MF age: 45 years overweight obese Ins resistant |
3 | RCT: parallel-arm (1) 8-h TRE (self-select) (2) control (no meal timing restrictions) | (1) ↓ 4%* (2) ∅ |
(1) ∅ (2) ∅ |
(1) ↓* (2) ∅ |
(1) ↓* (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
– | (1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
| Lowe16 | n = 141, MF age: 47 years obese normo-glycemic |
3 | RCT: parallel-arm (1) 8-h TRE (12–8 p.m.) (2) control (no meal timing restrictions) | (1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ↓* (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
– | (1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
| Gabel7 | n = 46, MF age: 49 years obese normo-glycemic |
3 | RCT: parallel-arm (1) 8-h TRE (10 a.m.–6 p.m.) (2) control | (1) ↓ 3%* (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ↓S*∅ D (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
– |
|
| ||||||||||||||||
| Reference |
Participants |
Diet length |
Design and intervention groups |
Body weight |
Body composition |
Blood pressure |
Plasma lipids |
Glucoregulatory factors |
||||||||
| Months | FM | FFM | VF | LDL | HDL | TG | Fasting glucose | Mean glucose | Fasting insulin | HOMA | HbA1c | |||||
|
| ||||||||||||||||
| 8-h time-restricted eating (continued) | ||||||||||||||||
|
| ||||||||||||||||
| Lin8 | n = 90, MF age: 44 years obese Ins resistant |
12 | RCT: parallel-arm (1) 8-h TRE (12–8 p.m.) (2) CR (25%) (3) control (no meal timing restrictions) | (1) ↓ 5%* (2) ↓ 5%* (3) ∅ |
(1) ↓* (2) ↓* (3) ∅ |
(1) ∅ (2) ∅ (3) ∅ |
(1) ∅ (2) ∅ (3) ∅ |
(1) ∅ (2) ∅ (3) ∅ |
(1) ∅ (2) ∅ (3) ∅ |
(1) ∅ (2) ∅ (3) ∅ |
(1) ∅ (2) ∅ (3) ∅ |
(1) ∅ (2) ∅ (3) ∅ |
– | (1) ∅ (2) ∅ (3) ∅ |
(1) ∅ (2) ∅ (3) ∅ |
(1) ∅ (2) ∅ (3) ∅ |
|
| ||||||||||||||||
| 10-h time-restricted eating | ||||||||||||||||
|
| ||||||||||||||||
| Manoogian17 | n = 150, MF age: 40 years overweight obese normo-glycemic |
3 | RCT: parallel-arm (1) 10-h TRE (self-select) (2) control (no meal timing restrictions) | (1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
– | – | (1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
– |
∅, non-significant change; –, not measured.
p < 0.05, significantly different from the control (between-group effect).
p< 0.05, significantly different from other intervention group (between-group effect).
Abbreviations are as follows: Age, reported as mean years for all participants; CR, calorie-restriction (percent energy restriction shown in parentheses); D, diastolic blood pressure; FM, fat mass; FFM, fat-free mass; F, female; HbA1c, glycated hemoglobin, HDL, high-density lipoprotein; HOMA, homeostatic model assessment of insulin resistance; Ins resistant, insulin resistant, LDL, low-density lipoprotein, M, male; RCT, randomized controlled trial; S, systolic blood pressure; TG, triglycerides; TRE, time-restricted eating (prescribed eating window shown in parentheses); VF, visceral fat mass.
Table 2.
Time-restricted eating (without calorie counting) in special populations with obesity
| Reference |
Participants |
Diet length |
Design and intervention groups |
Body weight |
Body composition |
Blood pressure |
Plasma lipids |
Glucoregulatory factors |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Months | FM | FFM | VF | LDL | HDL | TG | Fasting glucose | Mean glucose | Fasting insulin | HOMA | HbA1c | |||||
|
| ||||||||||||||||
| Adolescents | ||||||||||||||||
|
| ||||||||||||||||
| Vidmar18 | n = 50, MF age: 16 years obese prediabetes |
3 | RCT: parallel-arm (1) 8-h TRE (self-select) + blinded CGM (2) 8-h TRE (self-select) + CGM not blinded (3) control (12-h eating window) |
(1) ∅ (2) ∅ (3) ∅ |
– | – | – | – | – | – | – | – | (1) ∅ (2) ∅ (3) ∅ |
– | – | – |
|
| ||||||||||||||||
| Elderly | ||||||||||||||||
|
| ||||||||||||||||
| Anton19 | n = 10, MF age: 77 years obese normo-glycemic |
1 | single-arm trial (1) 8-h TRE (self-select) | ↓2%** | – | – | – | (1) ∅ | – | – | – | (1) ∅ | – | – | – | – |
| Domaszew-ski20 | n = 46, M age: 69 years overweight normo-glycemic |
1.5 | RCT: parallel-arm (1) 8-h TRE (12–8 p.m.) (2) control (no meal timing restrictions) | (1) ↓ 2%* (2) ∅ |
(1) ↓* (2) ∅ |
(1) ∅ (2) ∅ |
(1) ↓* (2) ∅ |
– | – | – | – | – | – | – | – | – |
|
| ||||||||||||||||
| Type 2 diabetes | ||||||||||||||||
|
| ||||||||||||||||
| Andriessen21 | n = 14, MF age: 68 years A1c: 6.5% obese T2DM |
1 | RCT: cross-over (1) 10-h TRE (8 a.m.–6 p.m.) (2) control (no meal timing restrictions) | (1) ↓ 1%* (2) ∅ |
(1) ∅ (2) ∅ |
(1) ∅ (2) ∅ |
– | – | – | – | (1) ∅ (2) ∅ |
(1) ↓* (2) ∅ |
(1) ↓* (2) ∅ |
(1) ∅ (2) ∅ |
– | – |
| Che22 | n = 120, MF age: 48 years A1c: 8.4% overweight T2DM |
3 | RCT: parallel-arm (1) 10-h TRE (8 a.m.–6 p.m.) (2) control (no meal timing restrictions) | (1) ↓ 4%* (2) ∅ |
– | – | – | – | (1) ↓* (2) ∅ |
(1) ∅ (2) ∅ |
(1) ↓* (2) ∅ |
(1) ↓* (2) ∅ |
– | (1) ↓* (2) ∅ |
(1) ↓* (2) ∅ |
(1) ↓* (2) ∅ |
|
| ||||||||||||||||
| Polycystic ovary syndrome (PCOS) | ||||||||||||||||
|
| ||||||||||||||||
| Li23 | n = 18, F age: 18–31 years overweight PCOS |
1 | single-arm trial (1) 8-h TRE (8 a.m.–4 p.m.) | ↓2%** | ↓** | (1) ∅ | ↓** | – | (1) ∅ | (1) ∅ | (1) ∅ | (1) ∅ | – | (1) ∅ | ↓** | – |
∅, non-significant change; –, not measured.
p < 0.05, significantly different from the control (between-group effect).
p < 0.05, significantly different from baseline (within-group effect)—reported for single-arm studies only.
p < 0.05, significantly different from other intervention group (between-group effect).
Abbreviations are as follows: Age, reported as mean years or range of years for all participants; A1c, glycated hemoglobin; CGM, continuous glucose monitor; D, diastolic blood pressure; FM, fat mass; FFM, fat-free mass; F, female; HbA1c, glycated hemoglobin, HDL, high-density lipoprotein; HOMA, momeostatic model assessment of insulin resistance; LDL, low-density lipoprotein; M, male; PCOS, polycystic ovary syndrome; RCT, randomized controlled trial; S, systolic blood pressure; T2DM, type 2 diabetes; TG, triglycerides; TRE, time-restricted eating (prescribed eating window shown in parentheses); VF, visceral fat mass.
OVERVIEW OFTIME-RESTRICTED EATINGPROTOCOLS
Figure 1 provides a general overview of different forms of TRE. Participants randomized to the TRE group were instructed to eat ad libitum during a specified number of hours each day, ranging from 4 to 10 h. Subjects were not required to monitor food or caloric intake during the eating windows. Many studies specified where the eating window should be placed in the day.6–8,13,14,16,20–23 For example, if an 8-h eating window was tested, participants were asked to eat all food between 12 and 8 p.m. that day and fast from 8 p.m. that evening to 12 p.m. the following day. These pre-specified protocols helped to standardize the TRE intervention and alleviate the confounding effects of early versus late eating on certain outcomes (e.g., glycemic control). However, some trials allowed individuals to self-select their eating windows according to personal preference.15,18,19,17 During the fasting period, participants were encouraged to drink plenty of water and were permitted to consume energy-free drinks, such as coffee and tea without additives. Some trials also permitted the consumption of diet sodas during the fasting period.6–8 However, diet soda consumption was limited to two drinks per day since these beverages have been shown to increase sugar craving.24
Figure 1. Overview of time-restricted eating protocols.
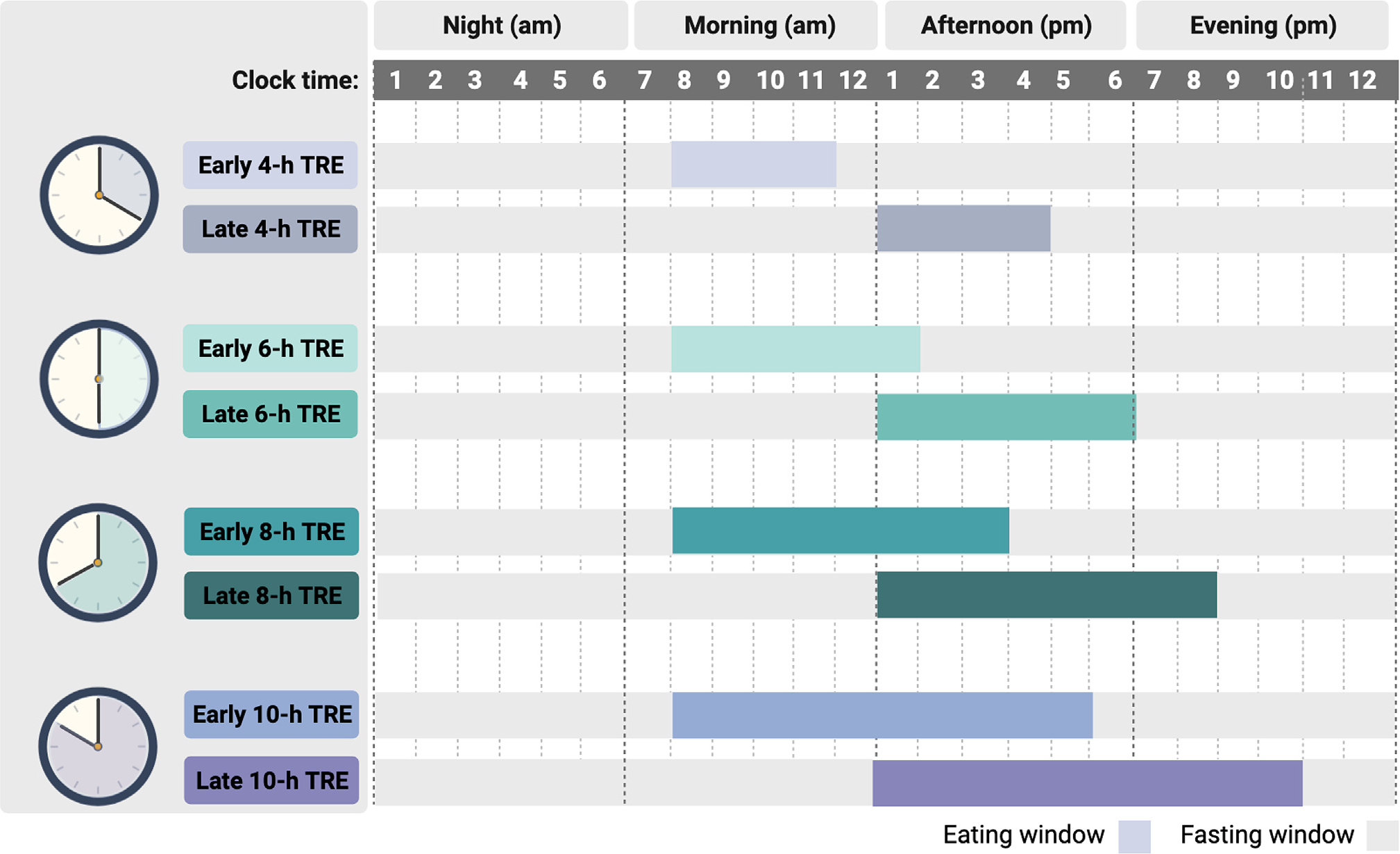
Time-restricted eating (TRE) typically involves confining the eating window to 4, 6, 8, or 10-h and fasting for the remaining hours of the day. During the eating window, individuals are not required to count calories or monitor food intake. During the fasting window, individuals are encouraged to drink plenty of water and may also consume energy-free beverages such as tea and coffee without additives. The eating window can be placed earlier in the day (early TRE) or later in the day (late TRE). Alternatively, participants can choose where they want to place their eating window (self-selected eating window).
EFFICACY OF TIME-RESTRICTED EATING IN HEALTHY INDIVIDUALS WITH OBESITY
Risk-of-bias assessment
Results of the risk-of-bias assessment using the Cochrane Collaboration tool12 are displayed in Figure 2. We found that 5 of 12 randomized trials were at high risk of bias because of missing participant outcome data. Moreover, none of the studies blinded study personnel or participants, as this was not possible due to the nature of the dietary interventions. In addition, only 4 of 12 trials blinded personnel performing the outcomes assessment. These findings highlight the need for more high-quality randomized controlled trials that clearly and accurately report outcome data to be performed.
Figure 2. Risk of bias assessment for randomized trials.
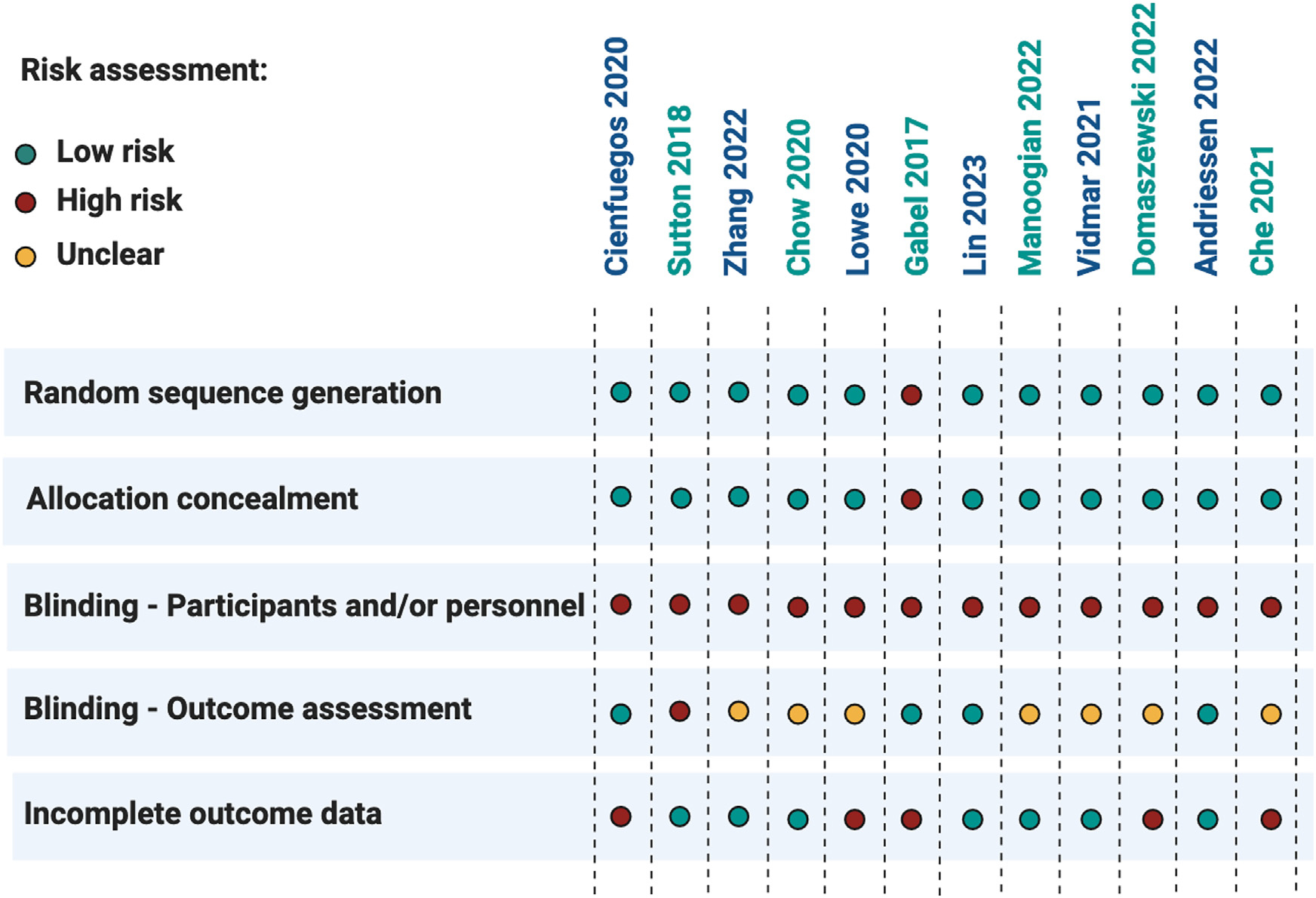
Risk of bias was assessed using the Cochrane Collaboration tool for the randomized controlled trials presented in Tables 1 and 2. Random sequence generation, allocation concealment, blinding, and incomplete outcome data were graded for each trial as having a high, low, or unclear risk of bias. Single-arm, non-randomized trials were not included in the risk-of-bias assessment.
Changes in body weight and body composition
Body weight
Weight loss ranging from 5% to 10% is associated with improvements in several metabolic disease parameters, including blood pressure, triglycerides, and low-density lipoprotein (LDL) cholesterol levels.25–27 Whether TRE can produce clinically significant weight loss (>5% from baseline28,29) compared with a non-intervention control group, has been tested in several trials to date (Table 1; Figure 3). After 1–3 months of TRE ignoring calories, body weight decreased by 3% to 5% versus controls,6,7,14,15 but not always.16,17 Only one study evaluated the longer-term effects of TRE.8 After 12 months of 8-h TRE (12–8 p.m.), Lin et al.8 demonstrated 5% reductions in body weight in a racially diverse group of men and women with obesity. Since this longer-term study (12 months)8 did not produce greater body weight reductions than short-term trials (1–3 months),6,7,14,15 it is possible that the weight loss efficacy of TRE may peak around 3 months. However, more long-term studies will be required to confirm this.
Figure 3. Summary of time-restricted eating effects on metabolic outcomes in various populations.
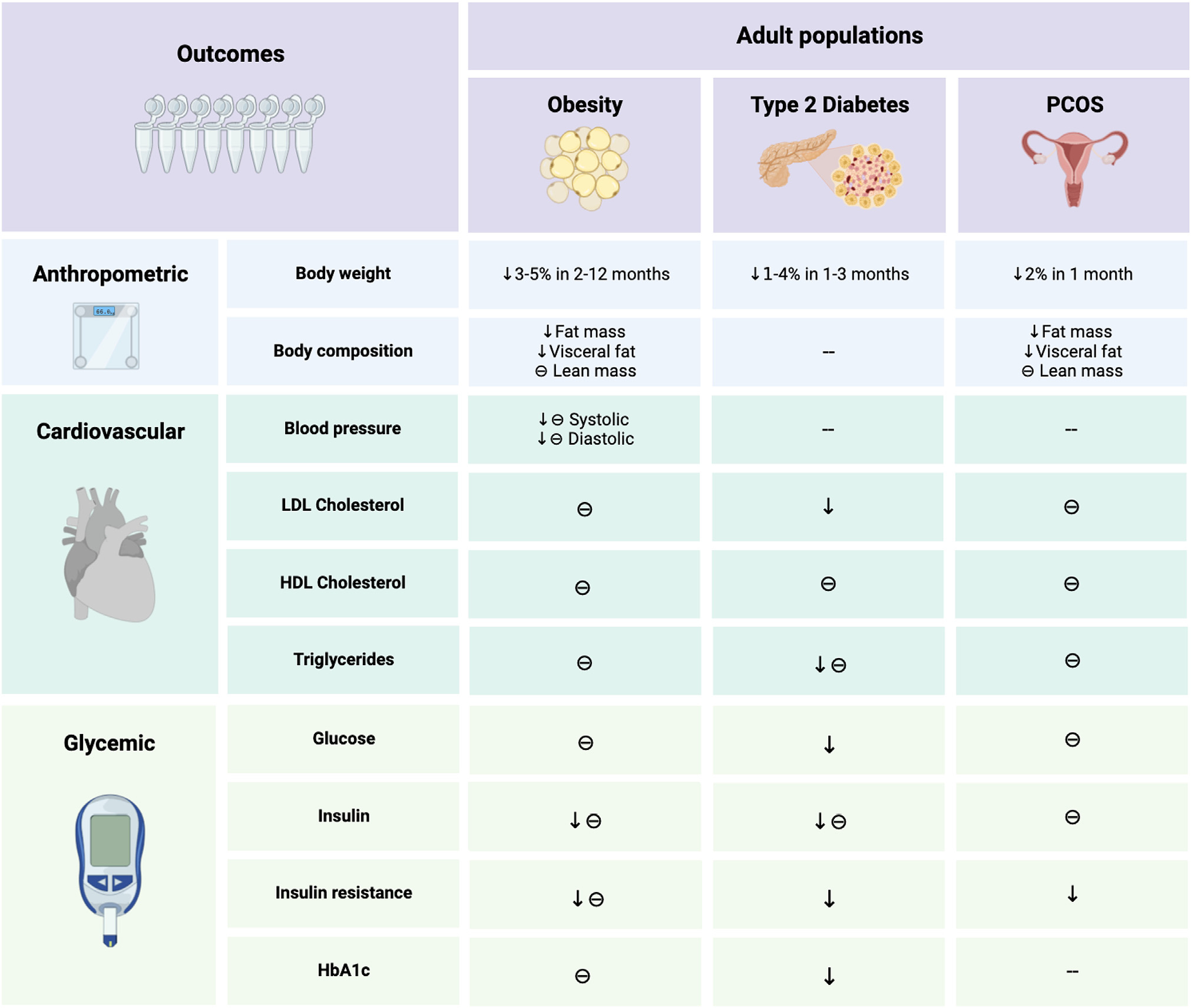
In participants with obesity, time-restricted eating results in 3%–5% weight loss over 2–12 months, versus controls. Reductions in body weight by TRE generally result from decreases in fat mass rather than lean mass. In terms of cardiovascular effects, TRE may improve blood pressure but has little effect on plasma lipids. In terms of glycemic control, TRE lowers fasting insulin and insulin resistance in adults with obesity and prediabetes but has little effect on fasting glucose. In adults with type 2 diabetes, TRE lowers body weight and reduces HbA1c. In women with polycystic ovary syndrome, TRE produces mild weight loss and improves androgen markers.
Abbreviations are as follows: θ, non-significant change; ↓, decrease; −, not measured; HbA1c, glycated hemoglobin; PCOS, polycystic ovary syndrome; TRE, time-restricted eating.
Several different population groups were evaluated. Evidence to date shows that TRE is an effective weight loss strategy in individuals with either overweight (BMI 25–29.9 kg/m2)14,15 or obesity (BMI > 30 kg/m2).6–8,15 Individuals with insulin resistance8,14,15 or prediabetes6 also benefit from these eating plans and may lose similar amounts of weight as those who do not have these conditions.7 In addition, the weight loss efficacy of TRE does not appear to vary according to sex30 or menopausal status.31 However, it should be noted that no study to date has directly compared these different population groups in one study, so it is difficult to draw comparisons between studies due to the heterogeneity of trial designs and study durations.
Whether or not TRE produces comparable weight loss as traditional dieting (i.e., daily CR) has also been examined. After 12 months, it was shown that 8-h TRE (12–8 p.m., without calorie counting) produced similar degrees of energy restriction and weight loss (425 kcal/day; 4.9%) as daily CR (405 kcal/day; 5.3%), versus controls.8 These findings suggest that TRE may be just as effective as traditional dieting approaches for weight control in adults with obesity, but more studies are warranted.
Body composition
Based on the trials reviewed here (Table 1), the weight loss produced by TRE is primarily attributable to reductions in fat mass rather than lean mass. The only trial that observed drastic decreases in lean mass with TRE was the study by Lowe et al.16 After 3 months of 8-h TRE (12–8 p.m.) adults with obesity lost ~1% of body weight.16 Surprisingly, approximately 65% of the weight loss was fat-free mass.16 Though the precise reason for this remains unknown, it is possible reductions in lean mass occurred because participants reported reduced protein intake.32 Alternatively, these effects may have happened because hydration status may not have been controlled before body composition was measured by dual-energy X-ray absorptiometry (DXA). Acute ingestion of water before the scan can result in overestimations of fat-free mass and underestimations of fat mass.33 Changes in abdominal fat by TRE were also assessed in several studies reviewed here.6–8,14–16 Visceral fat mass only decreased in the trials that observed 4%–5% weight loss, versus controls,14,15 with one exception.8 Thus, TRE may only reduce visceral fat mass when overall weight loss approaches clinically significant levels.
Changes in coronary heart disease risk factors
Blood pressure
The impact of TRE on blood pressure levels is highly variable (Table 1). Although some studies report decreases in systolic blood pressure (4%–8%)7,13,14 and diastolic blood pressure (13%),13 versus controls, others show no benefit.6,8,15,16,17 The studies that observed improvements in blood pressure included participants with borderline hypertension (i.e., systolic blood pressure: >120 mm Hg and/or diastolic blood pressure: >80 mm Hg).7,13,14 Thus, TRE may only be effective at reducing blood pressure in those with elevated levels at the onset of treatment. Interestingly, the studies that implemented early eating windows (all food consumed before ~2 p.m.) observed consistent reductions in blood pressure.13,14 The reason for this remains unclear but may involve early TRE facilitating natriuresis by shifting salt consumption to earlier in the day when sodium excretion is upregulated by the circadian system.34
Plasma lipids
TRE appears to have little to no effect on plasma lipid levels (Table 1). For instance, high-density lipoprotein (HDL) cholesterol levels remained unaffected by TRE, relative to controls, in all studies that examined this parameter.6,7,13–16,17 Likewise, LDL cholesterol concentrations remained unchanged, relative to controls, in the majority of studies.6,7,13,15,16,17 The only study that observed changes in LDL cholesterol was conducted by Zhang et al.14 After 2 months of either early 6-h TRE (7 a.m.–1 p.m.) or late 6-h TRE (12–6 p.m.), LDL cholesterol levels increased by 20% and 21%, respectively, versus controls, in overweight adults with normal LDL cholesterol values at baseline.14 Similar increases in LDL cholesterol concentrations have been reported in a study testing the acute (4-day) effects of early 6-h TRE (8 a.m.–2 p.m.).35 It is possible that LDL cholesterol levels increased because of the prolonged fasting period and greater reliance on fat oxidation.36 Whether early TRE produces mild increases in LDL cholesterol concentrations warrants further investigation.
Triglycerides levels remained unchanged in all studies6,7,14–16,17 but one.13 However, participants in all of these trials had triglyceride levels within the normal range at baseline.6,7,14–16,17 Since the participants already had healthy concentrations of this lipid parameter at the onset of the intervention, this could explain why no additional improvements were observed. The only trial that observed changes in triglycerides was performed by Sutton et al.13 After 1 month of early 6-h TRE (8 a.m.–2 p.m.), triglyceride levels increased by 48% in men with normal triglyceride levels at baseline.13 It is possible that the elevation in triglycerides resulted from an extended acute fast prior to the blood draw. For instance, the participants in the Sutton et al.13 trial fasted for 18-h prior to blood collection, whereas participants in the other studies generally fasted for shorter durations (8–12 h) before testing.6,7,14–16,17 Acute fasting has been shown to augment lipolysis and produce sharp increases in fatty acids and triglycerides.37–40 Therefore, the elevation in fasting triglycerides may be a transient byproduct of extended daily fasting with early TRE. More research will be required to confirm that this phenomenon is not pathogenic.
Changes in diabetes risk factors
Fasting glucose
Fasting glucose was assessed in all the trials reviewed here (Table 1). Results reveal that fasting glucose levels did not change after 2–12 months of TRE in adults with obesity,6–8,13–16,17 even when participants achieved 5% weight loss.8,14 Although it should be noted that participants in these trials were primarily normo-glycemic (fasting glucose < 100 mg/dL), fasting glucose is not likely to change when baseline values are in the healthy range.41
Mean glucose
Mean glucose levels were assessed in one trial14 using continuous glucose monitoring (CGM) (Table 1). CGMs are a powerful research tool that tracks glucose concentrations in the body’s interstitial fluid every 10 s of the day.42 These data are then used to calculate mean 24-h glucose levels, which is a more accurate reflection of an individual’s glycemic control than fasting glucose alone.42 The only trial that used CGMs to assess glucoregulation was the study by Zhang et al.14 In this trial, adults with overweight and insulin resistance were randomized to early 6-h TRE (7 a.m.–1 p.m.), late 6-h TRE (12–6 p.m.), or a no-intervention control group.14 After 2 months of diet, mean glucose levels significantly decreased in the early TRE group, relative to the late TRE group, and controls.14 It was also noted that interstitial glucose levels in the evening (i.e., between 6:30 and 11:00 p.m.) were approximately 10% lower in the early TRE group compared with the late TRE group and controls.14 These findings are not surprising as glucose tolerance exhibits circadian variation, with better glycemic control in the morning and poorer control in the evening.43,44 Specifically, glucose tolerance is typically 20%–30% lower in the evening compared with the morning.45 As such, early TRE interventions that require participants to consume all their food in the morning/early afternoon may be more effective at improving glucoregulation versus later TRE eating patterns.
Fasting insulin and insulin resistance (HOMA-IR)
Fasting insulin decreased in a few TRE trials,6,13,14 but most studies showed no change in this parameter versus controls (Table 1).7,8,15,16,17 Interestingly, all the studies that reported reductions in fasting insulin involved TRE interventions with shorter eating windows (ranging from 4 to 6 h).6,13,14 In the trial by Cienfuegos et al.,6 fasting insulin significantly decreased in both the 4-h TRE group (20%) and 6-h TRE group (12%), versus controls, after 2 months of intervention in adults with obesity and prediabetes. Sutton et al.13 also reported reductions in fasting insulin levels by 14% after 1 month of early 6-h TRE (8 a.m.–2 p.m.), relative to controls, in men with obesity and prediabetes. Complementary to these findings, Zhang et al.14 demonstrated 33% decreases in fasting insulin by early 6-h TRE (7 a.m.–1 p.m.), but not late 6-h TRE (12–6 p.m.), relative to controls, in adults with overweight and insulin resistance. By contrast, none of the studies that implemented longer eating windows (ranging from 8 to 10 h) reported changes in fasting insulin, relative to controls.7,8,15,16,17 Thus, it would appear as though TRE interventions with shorter eating windows (4–6h), placed earlier in the day (before 2 p.m.),may produce the most consistent reductions in fasting insulin levels in adults with insulin resistance or prediabetes.
Changes in insulin resistance were quantified by the homeostatic model assessment of insulin resistance (HOMA-IR) (Table 1). HOMA-IR calculates insulin resistance based on fasting glucose and fasting insulin values.46 It serves as a simple and minimally invasive surrogate marker of insulin resistance and is commonly used in dietary intervention trials. Changes in HOMA-IR were assessed in all the studies reviewed here.6–8,13–16,17 Insulin resistance was reduced only in the studies that also reported reductions in fasting insulin.6,13,14 Since the HOMA-IR calculation is based on fasting insulin and glucose levels, these findings are not surprising. Overall, insulin resistance was reduced by 12%–29%, versus controls, in the TRE studies, which implemented shorter eating windows of 4–6 h (i.e., longer fasting durations of 18–20 h).6,13,14 The studies that demonstrated no change in insulin resistance employed either late TRE (10 a.m.–6 p.m. or 12–8 p.m.)7,8,16 or self-selected eating windows,15,17 where participants chose their preferred eating times. We speculate that the self-selected eating windows resulted in food consumed late into the evening, as individuals tend to prioritize eating dinner with their families.47 However, this is simply as assumption, as the timing of the eating window was not reported in these trials of self-selected TRE.15,17 Overall, these data suggest that short eating windows placed earlier in the day may be the most effective for lowering insulin resistance in adults at risk for developing diabetes.
HbA1c
The impact of TRE on glycated hemoglobin (HbA1c) levels in adults without diabetes was assessed in most of the trials reviewed here (Table 1).6,8,14–16 Circulating levels of HbA1c remained unchanged with 2–12 months of 4, 6, or 8-h TRE in individuals with obesity, insulin resistance, or prediabetes.6,8,14–16 However, changes in HbA1c are typically only demonstrated with larger amounts of weight loss (5%–10%),48–50 so the body weight reductions in these studies (3%–5%)6,8,14–16 were probably not sufficient to change this parameter. Moreover, most of these studies ran for less than 3 months.6,14–16 Since HbA1c levels generally take at least 3 months to change,51,52 it is not surprising that these short-term studies showed no effect.
EFFICACY OF TIME-RESTRICTED EATING IN SPECIAL POPULATIONS WITH OBESITY
Adolescents
In the United States, one in five adolescents has obesity, and 30%–50% of those children will go on to develop T2DM as adults.53,54 Due to its simplicity, TRE may serve as a feasible intervention to treat childhood obesity. Only one randomized controlled trial18 to date has examined the effects of TRE in adolescents (Table 2; Figure 3). In this study by Vidmar et al.,18 50 adolescents (aged 14–18 years) with BMI >95th percentile were randomized to one of three groups: 8-h TRE (with real-time CGM feedback), 8-h TRE (with a blinded CGM), or control (standard care) for 3 months. Participants self-selected the placement of the eating window during the day. Adolescents were excluded from the study if they were previously diagnosed with type 2 diabetes or had a history of eating disorders (anorexia, bulimia, or binge eating disorder). CGMs were used to capture glycemic excursions, monitor adherence, and detect episodes of hypoglycemia. Participants were adherent to the 8-h TRE window on 5–6 days per week, and no serious adverse events or hypoglycemia were reported.18 Most participants chose to place their eating windows in the afternoons and evenings. Shortening the eating window to 8 h per day resulted in energy intake reductions of approximately 375 kcal/day (~25% reduction).18 Body weight was reduced by ~2% from baseline in the TRE groups; however, this was not significant when compared with controls.18 Mean glucose levels and time in euglycemic range (i.e., glucose levels between 70 and 180 mg/dL) did not differ between the TRE groups with and without real-time CGM biofeedback or versus controls.18 These preliminary findings suggest that TRE may be a safe and well-tolerated diet intervention in adolescents with obesity. However, since there were no differences between the TRE and control groups in terms of weight loss, mean glucose levels, and time in euglycemic range, the efficacy of this eating pattern in this population remains uncertain. Notably, TRE did not result in unhealthy eating behaviors.18 Since disordered eating is prevalent among adolescents with obesity, the potential for unhealthy eating behaviors should be continuously monitored in future trials of TRE.55,56
Elderly
Aging is associated with many biological changes that can lead to a progressive decline in cognitive and physical functioning.57,58 These changes are accelerated by obesity, which can result from low levels of physical activity accompanied by excessive energy intake.59,60 As such, the ability of TRE to aid with weight control and slow the rate of functional decline in elderly populations is of great interest. Two studies19,20 to date have assessed the efficacy and safety of TRE in older adults with overweight or obesity (Table 2; Figure 3). In the study by Anton et al.,19 10 sedentary elderly participants with obesity (mean age 77 years) participated in an 8-h TRE (self-selected window) for 1 month. Participants were adherent with the 8-h eating window on 85% of days, and body weight decreased by approximately 2% from baseline.19 Clinically meaningful improvements in walking speed and quality of life were reported, with few adverse events.19 Domaszewski et al.20 also evaluated the impact of TRE in older adults. In this trial, older men with overweight (mean age 69 years) were randomized to 8-h TRE (12–8 p.m.) or control.20 After 1.5 months, weight loss was 2%, relative to controls.20 Reductions in body weight primarily resulted from decreases in fat mass and visceral fat mass.20 Skeletal muscle mass did not change significantly versus controls.20 These preliminary data suggest that TRE may be a well-tolerated eating plan for weight control in older adults, at least in the short term (1–2 months). TRE does not appear to lead to deleterious reductions in skeletal muscle mass, though more studies with larger sample sizes will be needed to confirm this. Future studies in this area should also comprehensively examine how metabolic disease risk factors and bone metrics change during TRE in this population. Moreover, the optimal level of protein intake to maintain lean mass in older adults during TRE should also be investigated.
Type 2 diabetes (T2DM)
Approximately 1 in 10 adults in the United States have T2DM.61 If current trends continue, one in three adults in the United States will have T2DM by 2050. TRE may serve as an effective diet strategy for weight loss and glycemic control in patients with T2DM. However, clinical trial evidence in this area is very limited. Only two trials21,22 to date have examined the effect of TRE in patients with T2DM (Table 2; Figure 3). In a 1-month cross-over trial by Andriessen et al.,21 10-h TRE (8 a.m.–6 p.m.) decreased body weight by 1%, lowered mean glucose levels, and increased time spent in the euglycemic range (i.e., glucose levels between 70 and 180 mg/dL) in adults with obesity and T2DM versus controls. Complementary to these findings, Che et al.22 showed that 3 months of 10-h TRE (8 a.m.–6 p.m.) lowered body weight by 4%, reduced fasting glucose levels, and decreased HbA1c by 1.5% in adults with obesity and T2DM, versus controls. LDL cholesterol and triglycerides concentrations were also reduced in the trial by Che et al.22 Occurrences of hypoglycemia and hyperglycemia were rare in both trials,21,22 but patients were monitored closely by their physicians throughout the fasting protocol. These preliminary findings suggest that TRE may be safe and effective in patients with T2DM, though much more evidence from randomized controlled trials will be needed before TRE can be incorporated into clinical guidelines.
PCOS
PCOS is the most common cause of anovulatory infertility,62 and it affects up to 18% of reproductive-aged females.63 This complex disorder is characterized by hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphologic features.63 Many women with PCOS have insulin resistance and compensatory hyperinsulinemia, which augments ovarian androgen production.62 Additionally, 50%–70% of women with PCOS have obesity.64 Weight loss of as little as 5% can improve hyperandrogenism by reducing testosterone, androstenedione, and dehydroepiandrosterone-sulfate (DHEA-S) and increasing sex hormone-binding globulin (SHBG).63 This degree of weight loss can also improve insulin resistance and menstrual function in women with PCOS.63 Whether TRE is an effective weight loss strategy in individuals with this condition has been evaluated in two trials23,65 (Table 2; Figure 3). Li et al.23 conducted an 8-h TRE trial, where young women with PCOS and obesity ate all their energy needs early in the day (8 a.m.–4 p.m.) and fasted with water for the rest of the day. After 1 month, body weight significantly decreased by 2%, total testosterone levels were reduced, and SHBG was increased relative to baseline.23 Another study65 examined the effect of meal timing on androgens in women with PCOS. In this trial by Jakubowicz et al.,65 women with PCOS were randomized to one of two isoenergetic diets where they ate >50% of calories at dinner or >50% of calories at breakfast. After 3 months, circulating levels of DHEA-S and androstenedione decreased, whereas levels of SHBG increased, in the breakfast group relative to the dinner group.65 Inflammation and insulin resistance were also reduced in the breakfast group, further illustrating the link between hyperandrogenism and metabolic disturbances.65 Altogether, these preliminary findings23,65 suggest that TRE and early meal timing may improve androgen markers in females with PCOS, but more research is warranted.
UNRESOLVED QUESTIONS IN THE FIELD
Is TRE easier to adhere to than other diet interventions?
Since TRE does not require complicated carbohydrate or calorie counting, it has been postulated that TRE might be easier to adhere to than other diet interventions. In the trials reviewed here, adherence to TRE was measured using an app (e.g., myCircadianClock or RedCap)8,15,16,17 or a paper log6,7,14 which documented the times that participants started and stopped eating each day. On average, subjects reported adhering to their prescribed eating windows on 5–6 days per week (70%–85% of days) for up to 12 months.6–8,14–16,17 Compliance did not appear to wane over longer durations of TRE, but long-term data are very limited.8 Adherence was lowest on Saturdays and Sundays, due to increased engagement in social eating events on the weekends.8
Only one study8 compared TRE adherence with that of another diet. In this study by Lin et al.,8 adults with obesity followed an 8-h TRE diet (eating only 12–8 p.m., without calorie counting) or daily CR (25% energy restriction) for 12 months. Adherence to TRE was high, with subjects complying with the 8-h eating window on average 6.1 out of 7 days per week (87%) over 12 months. In comparison, CR subjects showed moderately high compliance, with 61% of participants adhering to their prescribed calorie goals over 1 year. The adherence data for TRE and CR are difficult to compare as different metrics were used to assess compliance. However, since the average degree of energy reduction achieved with TRE and CR were comparable (~400 kcal/day), it is likely that overall adherence was also similar. Based on the limited data available, it is impossible to say if TRE is easier to comply with compared with other diets. Much more research will be needed before any solid conclusions can be reached.
Are there any weight-loss-independent effects of TRE?
Some evidence suggests that TRE may improve metabolic health even in the absence of weight loss. For instance, Sutton et al.13 evaluated the effects of early 6-h TRE (8 a.m.–2 p.m.) versus controls in men with obesity and prediabetes. By the end of the study, body weight remained stable, but several metabolic benefits were still observed, such as improved insulin sensitivity, pancreatic beta-cell function, and blood pressure.13 Complementary to these findings, Jamshed et al.35 observed reductions in mean glucose levels and glycemic excursions (measured by CGM) with early 6-h TRE (8 a.m.–2 p.m.), versus controls, in the absence of weight loss. Although these short-term studies offer promise for the weight-loss-independent effects of TRE, they need to be confirmed by larger and longer randomized controlled trials.
What are the effects of TRE on diet quality?
There is some concern among clinicians that limiting the eating window to 4–10 h per day may lead to the increased consumption of energy-dense foods. There is also concern that individuals may consume more caffeinated beverages during the fasting window to boost their energy levels, which could lead to sleep disturbances. The effects of TRE on diet quality have only been assessed in a few clinical trials to date.6,8,22 Cienfuegos et al.6 reported no changes in diet quality or caffeinated beverage consumption with shorter TRE eating windows (4 or 6 h) in adults with obesity after 2 months, relative to controls. In the 8-h TRE study by Lin et al.,8 key diet quality indicators, such as sugar, saturated fat, cholesterol, fiber, sodium, and caffeine intake, did not change over 12 months in the TRE group versus CR or controls. Complementary to these findings, Che et al.22 showed no changes in percent energy from macronutrients during 3 months of 10-h TRE in adults with T2DM. These preliminary studies show that limiting the eating window to 4–10 h per day does not negatively impact diet quality, but more research is warranted.
How does TRE impact physical activity?
Changes in habitual physical activity were measured by pedometers or actigraphers in most of the studies reviewed here.6–8,14–16,22 At baseline, subjects were taking approximately 5,500–7,500 steps/day, which is classified as “low activity.”66 This level of physical activity remained stable during the TRE intervention in all studies,6–8,14,15,22 but one,16 suggesting that TRE probably does not impact regular physical activity habits. Whether TRE impacts spontaneous physical activity has also been investigated. Spontaneous physical activity is defined as an activity behavior that emanates from an unconscious drive for movement and includes fidgeting, gesticulating, and more time spent standing.67 In a trial by Ravussin et al.,36 spontaneous physical activity was measured using a metabolic chamber over 4 days in adults with overweight following an early 6-h TRE (8 a.m.–2 p.m.) intervention. By the end of the study, there were no changes in spontaneous physical activity by the TRE group, relative to controls. Taken together, these preliminary findings suggest that TRE does not affect habitual physical activity or spontaneous physical activity in individuals with obesity.
What is the optimal eating window length?
TRE eating windows lengths vary from short (4–6 h) to long (8–10 h). When choosing the eating window length, it is important to consider both feasibility (adherence) and efficacy (weight reduction). In terms of feasibility, data from randomized controlled trials show that adherence to shorter eating windows (4–6 h)6,13,14 is similar to that of longer windows (8–10 h).7,8,15,17 In general, adults with obesity comply with their eating window on 5–6 days per week, regardless of eating window length. Though it should be noted that only one trial evaluated compliance with very short windows (4 h), so data are lacking.6 As for efficacy, shorter eating windows tend to produce greater energy restriction by simply limiting the total time available to eat, which in turn produces greater weight loss. For instance, in the study by Cienfuegos et al.,6 it was demonstrated that TRE with shorter (4–6 h) eating windows reduced energy intake by ~500–550 kcal/day in adults with obesity, which produced ~3% weight loss over 2 months. In comparison, in the studies by Gabel et al.7 and Lin et al.8 it was shown that eating within an 8-h window reduced calorie intake by ~350–400 kcal/day which produced ~2.5% weight loss over 2 months. Whereas, Haganes et al.9 showed that eating within a 10-h window reduced energy intake by ~200 kcal/day and decreased body weight by ~1.5% over 2 months. Taken together, shorter eating windows (4–6 h) may produce superior weight loss, but the same level of adherence, as longer windows (8–10 h). Thus, shorter eating windows may serve as better weight loss strategies, though more research that directly compares various eating window lengths will be needed to confirm this.
Are early eating windows better than late windows for glycemic control?
Glucose tolerance is highest a few hours after waking and steadily decreases over the course of the day.45,68 As such, concerns have been raised about later eating windows during TRE promoting insulin resistance. Several recent studies14,69–71 have directly compared the effects of early versus late TRE on glycemic control. Queiroz et al.69 found that early 8-h TRE (8 a.m.–4 p.m.) produced similar improvements in fasting insulin, insulin sensitivity, and functional beta-cell capacity when compared with late 8-h TRE (12–8 p.m.) in adults with obesity. Ruddick-Collins et al.70 tested the effects of isoenergetic weight loss diets with morning-loaded or evening-loaded calories and found that both protocols produced similar improvements in glucose tolerance. By contrast, Zhang et al.14 showed that mean glucose levels (assessed by CGM) were improved only in the early 6-h TRE group (7 a.m.–1 p.m.) when compared with late 6-h TRE (12–6 p.m.) and controls, after 2 months in adults with overweight and insulin resistance. Similarly, Xie et al.71 demonstrated that early 8-h TRE (7 a.m.–3 p.m.), but not late 8-h TRE (12–8 p.m.), decreased insulin resistance and fasting glucose levels, versus controls, in healthy adults who were normal weight. In view of these equivocal findings, it remains unclear if earlier eating windows produce greater improvements in glycemic control versus later eating windows. On one hand, it is possible that the weight loss benefits of TRE outweigh any differences arising from calorie distribution. TRE regimens that produce energetic deficits, regardless of meal timing, may improve insulin sensitivity. On the other hand, human metabolism is optimized for food intake in the morning. As such, early eating windows, which properly align with our circadian rhythms, may prove to be better for glycemic control than eating later in the day.45,68 More randomized controlled studies with larger sample sizes that implement robust techniques (i.e., hyperinsulinemic euglycemic clamps) will be needed before solid conclusions can be reached.
LIMITATIONS
This review has some limitations. First and foremost, the body of literature in this area is still quite small. Only eight randomized controlled trials6–8,13–16,17 have examined the effects of TRE (without calorie counting) on body weight and metabolic risk parameters in adults with obesity. Second, most studies were short (1–3 months)6,7,13–16,17 and only one8 examined the long-term (12 months) effects of this diet. Third, the sample size in each study was small (n = 8–150). As such, it is unlikely that most of the studies reviewed here were adequately powered to detect statistically significant differences in primary and secondary outcomes, such as blood pressure, plasma lipids, and glycemic endpoints. Fourth, only one randomized controlled trial8 to date has compared the weight loss efficacy of TRE (without calorie counting) to a traditional dieting approach, such as daily CR. In view of these limitations, future randomized controlled trials that run for longer durations (>6 months) with larger sample sizes, which directly compare TRE to other weight loss approaches, will be needed before solid conclusions can be reached.
FUTURE DIRECTIONS
Although the number of published trials in this area is still limited, there are many trials currently underway. We searched Clinicaltrials.gov and retrieved approximately 50 records of ongoing TRE studies. Most trials aim to examine the efficacy of TRE in promoting weight loss in various patient populations, such as people with T2DM (NCT05290246; NCT05365529; NCT03940482; NCT04762251), type 1 diabetes (NCT05031429), non-alcoholic fatty liver disease (NCT03848390; NCT05579158; NCT05220956), PCOS (NCT06031753; NCT05629858), Alzheimer’s disease (NCT05732935), and certain obesity-related cancers, such as breast cancer (NCT05639829; NCT05038137; NCT05259410), and colorectal cancer (NCT05114798). Two studies are examining the safety and efficacy of TRE in adolescents with obesity (aged 13–18 years) (NCT05740254; NCT05107726), whereas four other studies are assessing how TRE impacts healthy aging in older adults (aged > 65 years) (NCT06019195; NCT05997316; NCT05482711; NCT05732935). In addition, there are some mechanistic studies currently underway that aim to differentiate the effects of early TRE (all food eating before 3 p.m.) versus late TRE (all food eating after 3 p.m.) on body weight and glycemic control (NCT05486702; NCT04618133; NCT03504683). Most of these trials employ an 8–10 h eating windows, placed in the afternoon or evening. The durations of these studies are generally quite short (3–6 months); however, we were able to identify two studies that are running for 12 months (NCT05453617; NCT04762251). There are also several trials examining the effects of TRE combined with either resistance training or endurance training on body composition and metabolic endpoints (NCT05486702; NCT05908201; NCT05897073; NCT05908201; NCT05167903). Based on the large number of ongoing studies, it is clear that this is a very exciting time for TRE research. These data will undoubtedly help to fill in critical knowledge gaps and bolster our understanding of the role of TRE in improving human health.
Conclusions
In summary, data from randomized controlled trials suggest individuals can adhere to TRE interventions, at least in the short term. TRE is a unique diet therapy in that it produces a daily energy deficit of 200–550 kcal, without calorie counting. These reductions in energy intake result in mild to moderate weight loss of 3%–5% over the course of 2–12 months versus no-intervention controls. TRE may produce metabolic benefits, such as improved blood pressure and glycemic control. However, improvements in these variables generally result from reductions in body weight and do not generally change with insufficient weight loss. The optimal timing of the eating window, weight-independent effects, and clinical utility of TRE in special populations are still uncertain due to limited evidence to date. Although these preliminary results offer promise for the use of TRE as a weight loss intervention, larger and longer-term human trials with suitable comparison to other diet interventions will be needed to confirm these findings.
ACKNOWLEDGMENTS
This study was funded by the National Institutes of Health, NIDDK (R01DK119783), NIDDK (R01DK128180), NCI (R01CA257807), and NCI (K12HD101373).
Footnotes
DECLARATION OF INTERESTS
K.A.V. received author fees from Hachette Book Group for the book The Every Other Day Diet and from Pan MacMillan Publishing for the book The Fasted Diet. She also serves on two Data Safety Monitoring Boards (DSMBs) for the NIH HALLO-P study and the DiAL Health study.
REFERENCES
- 1.Chaix A, Manoogian ENC, Melkani GC, and Panda S (2019). Timerestricted eating to prevent and manage chronic metabolic diseases. Annu. Rev. Nutr. 39, 291–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longo VD, and Panda S (2016). Fasting, circadian rhythms, and timerestricted feeding in healthy lifespan. Cell Metab. 23, 1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mattson MP, and de Cabo R (2020). Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med. 382, 1771–1774. [DOI] [PubMed] [Google Scholar]
- 4.Varady KA, Cienfuegos S, Ezpeleta M, and Gabel K (2022). Clinical application of intermittent fasting for weight loss: progress and future directions. Nat. Rev. Endocrinol. 18, 309–321. [DOI] [PubMed] [Google Scholar]
- 5.Manoogian ENC, Chow LS, Taub PR, Laferrère B, and Panda S (2022). Time-restricted eating for the prevention and management of metabolic diseases. Endocr. Rev. 43, 405–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Wiseman E, Pavlou V, Lin S, Oliveira ML, and Varady KA (2020). Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab. 32, 366–378.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabel K, Hoddy KK, Haggerty N, Song J, Kroeger CM, Trepanowski JF, Panda S, and Varady KA (2018). Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr. Healthy Aging 4, 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin S, Cienfuegos S, Ezpeleta M, Gabel K, Pavlou V, Mulas A, Chakos K, McStay M, Wu J, Tussing-Humphreys L, et al. (2023). Timerestricted eating without calorie counting for weight loss in a racially diverse population: a randomized controlled trial. Ann. Intern. Med. 176, 885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haganes KL, Silva CP, Eyjólfsdóttir SK, Steen S, Grindberg M, Lydersen S, Hawley JA, and Moholdt T (2022). Time-restricted eating and exercise training improve HbA1c and body composition in women with overweight/obesity: a randomized controlled trial. Cell Metab. 34, 1457–1471.e4. [DOI] [PubMed] [Google Scholar]
- 10.Dorling JL, Das SK, Racette SB, Apolzan JW, Zhang D, Pieper CF, Martin CK, and K C. (2020). Changes in body weight, adherence, and appetite during 2 years of calorie restriction: the CALERIE 2 randomized clinical trial. Eur. J. Clin. Nutr. 74, 1210–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall KD, and Kahan S (2018). Maintenance of lost weight and long-term management of obesity. Med. Clin. North Am. 102, 183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et al. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, and Peterson CM (2018). Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 27, 1212–1221.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang LM, Liu Z, Wang JQ, Li RQ, Ren JY, Gao X, Lv SS, Liang LY, Zhang F, Yin BW, et al. (2022). Randomized controlled trial for time-restricted eating in overweight and obese young adults. iScience 25, 104870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow LS, Manoogian ENC, Alvear A, Fleischer JG, Thor H, Dietsche K, Wang Q, Hodges JS, Esch N, Malaeb S, et al. (2020). Timerestricted eating effects on body composition and metabolic measures in humans who are overweight: a feasibility study. Obesity (Silver Spring) 28, 860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, Philip E, Vittinghoff E, Heymsfield SB, Olgin JE, et al. (2020). Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern. Med. 180, 1491–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manoogian ENC, Zadourian A, Lo HC, Gutierrez NR, Shoghi A, Rosander A, Pazargadi A, Ormiston CK, Wang X, Sui J, et al. (2022). Feasibility of time-restricted eating and impacts on cardiometabolic health in 24-h shift workers: the Healthy Heroes randomized control trial. Cell Metab. 34, 1442–1456.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vidmar AP, Naguib M, Raymond JK, Salvy SJ, Hegedus E, Wee CP, and Goran MI (2021). Time-limited eating and continuous glucose monitoring in adolescents with obesity: a pilot study. Nutrients 13, 3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anton SD, Lee SA, Donahoo WT, McLaren C, Manini T, Leeuwenburgh C, and Pahor M (2019). The effects of time restricted feeding on overweight, older adults: a pilot study. Nutrients 11, 1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Domaszewski P, Konieczny M, Pakosz P, Łukaniszyn-Domaszewska K, Mikuláková W, Sadowska-Krępa E, and Anton S (2022). Effect of a six-week times restricted eating intervention on the body composition in early elderly men with overweight. Sci. Rep. 12, 9816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andriessen C, Fealy CE, Veelen A, van Beek SMM, Roumans KHM, Connell NJ, Mevenkamp J, Moonen-Kornips E, Havekes B, Schrauwen-Hinderling VB, et al. (2022). Three weeks of timerestricted eating improves glucose homeostasis in adults with type 2 diabetes but does not improve insulin sensitivity: a randomised crossover trial. Diabetologia 65, 1710–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Che T, Yan C, Tian D, Zhang X, Liu X, and Wu Z (2021). Timerestricted feeding improves blood glucose and insulin sensitivity in overweight patients with type 2 diabetes: a randomised controlled trial. Nutr. Metab. (Lond.) 18, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Xing C, Zhang J, Zhao H, Shi W, and He B (2021). Eight-hour time-restricted feeding improves endocrine and metabolic profiles in women with anovulatory polycystic ovary syndrome. J. Transl. Med. 19, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swithers SE (2013). Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol. Metab. 24, 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beavers DP, Beavers KM, Lyles MF, and Nicklas BJ (2013). Cardiometabolic risk after weight loss and subsequent weight regain in overweight and obese postmenopausal women. J. Gerontol. A Biol. Sci. Med. Sci. 68, 691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown JD, Buscemi J, Milsom V, Malcolm R, and O’Neil PM (2016). Effects on cardiovascular risk factors of weight losses limited to 5–10. Transl. Behav. Med. 6, 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dow CA, Thomson CA, Flatt SW, Sherwood NE, Pakiz B, and Rock CL (2013). Predictors of improvement in cardiometabolic risk factors with weight loss in women. J. Am. Heart Assoc. 2, e000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williamson DA, Bray GA, and Ryan DH (2015). Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity (Silver Spring) 23, 2319–2320. [DOI] [PubMed] [Google Scholar]
- 29.Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, Hill JO, Brancati FL, Peters A, Wagenknecht L, et al. (2011). Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 34, 1481–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu D, Huang Y, Huang C, Yang S, Wei X, Zhang P, Guo D, Lin J, Xu B, Li C, et al. (2022). Calorie restriction with or without time-restricted eating in weight loss. N. Engl. J. Med. 386, 1495–1504. [DOI] [PubMed] [Google Scholar]
- 31.Kalam F, Akasheh RT, Cienfuegos S, Ankireddy A, Gabel K, Ezpeleta M, Lin S, Tamatam CM, Reddy SP, Spring B, et al. (2023). Effect of time-restricted eating on sex hormone levels in premenopausal and postmenopausal females. Obesity (Silver Spring) 31 (Suppl 1), 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bopp MJ, Houston DK, Lenchik L, Easter L, Kritchevsky SB, and Nicklas BJ (2008). Lean mass loss is associated with low protein intake during dietary-induced weight loss in postmenopausal women. J. Am. Diet. Assoc. 108, 1216–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barreira TV, and Tseh W (2020). The effects of acute water ingestion on body composition analyses via dual-Energy X-ray absorptiometry. Clin. Nutr. 39, 3836–3838. [DOI] [PubMed] [Google Scholar]
- 34.Johnston JG, Speed JS, Jin C, and Pollock DM (2016). Loss of endothelin B receptor function impairs sodium excretion in a time- and sex-dependent manner. Am. J. Physiol. Renal Physiol. 311, F991–F998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, and Peterson CM (2019). Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients 11, 1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, and Peterson CM (2019). Early time-restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity (Silver Spring) 27, 1244–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antoni R, Johnston KL, Collins AL, and Robertson MD (2016). Investigation into the acute effects of total and partial energy restriction on postprandial metabolism among overweight/obese participants. Br. J. Nutr. 115, 951–959. [DOI] [PubMed] [Google Scholar]
- 38.Browning JD, Baxter J, Satapati S, and Burgess SC (2012). The effect of short-term fasting on liver and skeletal muscle lipid, glucose, and energy metabolism in healthy women and men. J. Lipid Res. 53, 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halberg N, Henriksen M, Söderhamn N, Stallknecht B, Ploug T, Schjerling P, and Dela F (2005). Effect of intermittent fasting and refeeding on insulin action in healthy men. J. Appl. Physiol. (1985) 99, 2128–2136. [DOI] [PubMed] [Google Scholar]
- 40.Salgin B, Marcovecchio ML, Humphreys SM, Hill N, Chassin LJ, Lunn DJ, Hovorka R, and Dunger DB (2009). Effects of prolonged fasting and sustained lipolysis on insulin secretion and insulin sensitivity in normal subjects. Am. J. Physiol. Endocrinol. Metab. 296, E454–E461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freckmann G, Hagenlocher S, Baumstark A, Jendrike N, Gillen RC, Rössner K, and Haug C (2007). Continuous glucose profiles in healthy subjects under everyday life conditions and after different meals. J. Diabetes Sci. Technol. 1, 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janapala RN, Jayaraj JS, Fathima N, Kashif T, Usman N, Dasari A, Jahan N, and Sachmechi I (2019). Continuous glucose monitoring versus self-monitoring of blood glucose in type 2 diabetes mellitus: a systematic review with meta-analysis. Cureus 11, e5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stenvers DJ, Scheer FAJL, Schrauwen P, la Fleur SE, and Kalsbeek A (2019). Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 15, 75–89. [DOI] [PubMed] [Google Scholar]
- 44.Poggiogalle E, Jamshed H, and Peterson CM (2018). Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism 84, 11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, Buxton OM, Shea SA, and Scheer FA (2015). Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl. Acad. Sci. USA 112, E2225–E2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gutch M, Kumar S, Razi SM, Gupta KK, and Gupta A (2015). Assessment of insulin sensitivity/resistance. Indian J. Endocrinol. Metab. 19, 160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torres L, Lee JL, Park S, Di Lorenzo RC, Branam JP, Fraser SA, and Salisbury BA (2022). Retention, fasting patterns, and weight loss with an intermittent fasting app: large-scale, 52-week observational study. JMIR Mhealth Uhealth 10, e35896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bauman V, Ariel-Donges AH, Gordon EL, Daniels MJ, Xu D, Ross KM, Limacher MC, and Perri MG (2019). Effect of dose of behavioral weight loss treatment on glycemic control in adults with prediabetes. BMJ Open Diabetes Res. Care 7, e000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choy S, Kjellsson MC, Karlsson MO, and de Winter W (2016). Weight-HbA1c-insulin-glucose model for describing disease progression of type 2 diabetes. CPT Pharmacometrics Syst. Pharmacol. 5, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gummesson A, Nyman E, Knutsson M, and Karpefors M (2017). Effect of weight reduction on glycated haemoglobin in weight loss trials in patients with type 2 diabetes. Diabetes Obes. Metab. 19, 1295–1305. [DOI] [PubMed] [Google Scholar]
- 51.Bunn HF, Haney DN, Kamin S, Gabbay KH, and Gallop PM (1976). The biosynthesis of human hemoglobin A1c. Slow glycosylation of hemoglobin in vivo. J. Clin. Invest. 57, 1652–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim H, Jung DY, Lee SH, Cho JH, Yim HW, and Kim HS (2022). Long-term changes in HbA1c according to blood glucose control status during the first 3 months after visiting a tertiary University Hospital. J. Korean Med. Sci. 37, e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar S, and Kelly AS (2017). Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin. Proc. 92, 251–265. [DOI] [PubMed] [Google Scholar]
- 54.Marcus MD, Wilfley DE, El Ghormli L, Zeitler P, Linder B, Hirst K, Ievers-Landis CE, van Buren DJ, and Walders-Abramson N; TODAY Study Group (2017). Weight change in the management of youth-onset type 2 diabetes: the TODAY clinical trial experience. Pediatr. Obes. 12, 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jebeile H, Gow ML, Baur LA, Garnett SP, Paxton SJ, and Lister NB (2019). Treatment of obesity, with a dietary component, and eating disorder risk in children and adolescents: a systematic review with meta-analysis. Obes. Rev. 20, 1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jebeile H, Lister NB, Baur LA, Garnett SP, and Paxton SJ (2021). Eating disorder risk in adolescents with obesity. Obes. Rev. 22, e13173. [DOI] [PubMed] [Google Scholar]
- 57.Anton SD, Karabetian C, Naugle K, and Buford TW (2013). Obesity and diabetes as accelerators of functional decline: can lifestyle interventions maintain functional status in high risk older adults? Exp. Gerontol. 48, 888–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dye L, Boyle NB, Champ C, and Lawton C (2017). The relationship between obesity and cognitive health and decline. Proc. Nutr. Soc. 76, 443–454. [DOI] [PubMed] [Google Scholar]
- 59.Vásquez E, Batsis JA, Germain CM, and Shaw BA (2014). Impact of obesity and physical activity on functional outcomes in the elderly: data from NHANES 2005–2010. J. Aging Health 26, 1032–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Long T, Zhang K, Chen Y, and Wu C (2022). Trends in diet quality among older US adults from 2001 to 2018. JAMA Netw. Open 5, e221880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mendola ND, Chen TC, Gu Q, Eberhardt MS, and Saydah S (2018). Prevalence of total, diagnosed, and undiagnosed diabetes among adults: United States, 2013–2016. NCHS Data Brief, 1–8. [PubMed] [Google Scholar]
- 62.Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, and Legro RS (2015). Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr. Rev. 36, 487–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCartney Ch.R., and Marshall JC. (2016). Polycystic ovary syndrome. N. Engl. J. Med. 375, 1398–1399. [DOI] [PubMed] [Google Scholar]
- 64.Barber TM, Hanson P, Weickert MO, and Franks S (2019). Obesity and polycystic ovary syndrome: implications for pathogenesis and novel management strategies. Clin. Med. Insights Reprod. Health 13. 1179558119874042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jakubowicz D, Barnea M, Wainstein J, and Froy O (2013). Effects of caloric intake timing on insulin resistance and hyperandrogenism in lean women with polycystic ovary syndrome. Clin. Sci. (Lond.) 125, 423–432. [DOI] [PubMed] [Google Scholar]
- 66.Tudor-Locke C, Craig CL, Brown WJ, Clemes SA, De Cocker K, Giles-Corti B, Hatano Y, Inoue S, Matsudo SM, Mutrie N, et al. (2011). How many steps/day are enough? For adults. Int. J. Behav. Nutr. Phys. Act. 8, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kotz CM, Perez-Leighton CE, Teske JA, and Billington CJ (2017). Spontaneous physical activity defends against obesity. Curr. Obes. Rep. 6, 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qian J, and Scheer FAJL (2016). Circadian system and glucose metabolism: implications for physiology and disease. Trends Endocrinol. Metab. 27, 282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Queiroz JDN, Macedo RCO, Dos Santos GC, Munhoz SV, Machado CLF, de Menezes RL, Menzem EN, Moritz CEJ, Pinto RS, Tinsley GM, et al. (2022). Cardiometabolic effects of early v. delayed time-restricted eating plus energetic restriction in adults with overweight and obesity: an exploratory randomised clinical trial. Br. J. Nutr. 129, 637–649. [DOI] [PubMed] [Google Scholar]
- 70.Ruddick-Collins LC, Morgan PJ, Fyfe CL, Filipe JAN, Horgan GW, Westerterp KR, Johnston JD, and Johnstone AM (2022). Timing of daily calorie loading affects appetite and hunger responses without changes in energy metabolism in healthy subjects with obesity. Cell Metab. 34, 1472–1485.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xie Z, Sun Y, Ye Y, Hu D, Zhang H, He Z, Zhao H, Yang H, and Mao Y (2022). Randomized controlled trial for time-restricted eating in healthy volunteers without obesity. Nat. Commun. 13, 1003. [DOI] [PMC free article] [PubMed] [Google Scholar]


