Abstract
Background
Early treatment of intracranial lesions in the emergency department is crucial, but it can be challenging to differentiate between them. This differentiation is essential because the treatment of each type of lesion is different. Cerebral computed tomography perfusion (CTP) imaging can help visualize the vascularity of brain lesions and provide absolute quantification of physiological parameters. Compared to magnetic resonance imaging, CTP has several advantages, such as simplicity, wide availability, and reproducibility.
Purpose
This study aimed to assess the effectiveness of Hounsfield units (HU) in measuring the density of hypercellular lesions and the ability of CTP to quantify hemodynamics in distinguishing intracranial space-occupying lesions.
Methods
A retrospective study was conducted from March 2016 to March 2022. All patients underwent CTP and CT scans, and relative cerebral blood volume (rCBV) and HU were obtained for intracranial lesions.
Results
We included a total of 244 patients in our study. This group consisted of 87 (35.7%) individuals with glioblastomas (GBs), 48 (19.7%) with primary central nervous system lymphoma (PCNSL), 45 (18.4%) with metastases (METs), and 64 (26.2) with abscesses. Our study showed that the HUs for METs were higher than those for GB (S 57.4% and E 88.5%). In addition, rCBV values for PCNSL and abscesses were lower than those for GB and METs. The HU in PCNSL was higher than those in abscesses (S 94.1% and E 96.6%).
Conclusion
PCT parameters provide valuable information for diagnosing brain lesions. A comprehensive assessment improves accuracy. Combining rCBV and HU enhances diagnostic accuracy, making it a valuable tool for distinguishing between lesions. PCT's widespread availability allows for the use of both anatomical and functional information with high spatial resolution for diagnosing and managing brain tumor patients.
Keywords: lymphoma, glioblastoma, metastasis, abscesses, perfusion parameters, intracranial brain tumors, ct perfusion
Introduction
It can be challenging to distinguish between intracranial lesions in an emergency department due to the urgency of early treatment. Oftentimes, the only available neuroimaging study is a cerebral computed tomography perfusion (CTP) scan, which sometimes cannot distinguish between the main types of lesions [1,2]. This is a problem because the treatment for each type of lesion is different, so it is essential to differentiate between them before surgery. Non-invasive in vivo methods are needed to assess and improve the accuracy of CTP diagnosis for intracranial lesions. Determining X-ray attenuation coefficients is one method that can help diagnose these lesions by differentiating abnormal tissues from normal ones using dose distribution and contrast differences [3-6]. Another method is perfusion imaging, which can be added to CTP to visualize the vascularity of brain lesions and quantify physiological parameters, such as blood flow, blood volume, mean transit time, and time to peak [7-9]. CTP has several advantages over magnetic resonance imaging, including simplicity, wide availability, and reproducibility. A study was conducted to evaluate the effectiveness of quantifying density by Hounsfield units (HU) in hypercellular lesions and quantifying hemodynamics by CTP in differentiating intracranial space-occupying lesions [10].
Materials and methods
We reviewed and analyzed the clinical data and medical files of patients who had a confirmed histologic diagnosis by a neuropathologist from March 2016 to March 2022 at the National Institute of Neurology and Neurosurgery in Mexico City. Before any neurosurgical treatment, all patients underwent CT and CTP. The institutional review board approved this study, with approval number INNN 43-22.
Image acquisition
CTP was performed with a Sensation 16 (Siemens, Germany) multislice helical tomography (16 slices). Before the CTP study, a non-contrast CT scan of the skull was performed to locate the region of interest. During the CTP study, a non-ionic contrast medium (350 mg/100 mL) of 40 mL (iohexol) was injected through a 20 G caliber antecubital intravenous line using an automated injector at a flow rate of 5 mL/s. A cine series was acquired using the following parameters: 80 kV, 80 mA, and 1 second per rotation.
After administering the contrast medium, a five-second cine scanner was started five seconds later. The scanner then took one axial image every two seconds for the remaining 52 seconds, making the total acquisition time 57 seconds. Using CT Perfusion 3 software on the Advantage Windows workstation (version 4.2, GE Medical Systems, USA), perfusion maps of relative cerebral blood volume (rCBV) were generated for all patients. Regions of interest (ROIs) were manually placed in the tumor-enhancing area, peritumoral region, and contralateral normal-appearing white matter, as confirmed on both contrast-enhanced and non-contrast-enhanced CT images. The peritumoral region was defined as low density around the tumor, which was enhanced on the CT image. Parameters of CTP were normalized by those of the contralateral normal-appearing white matter to normalize parameters in the tumor (rCBV) and peritumoral region.
Statistical analysis
Demographics and different histopathological subgroups were analyzed using descriptive statistics. To determine the sensitivity and specificity parameters, an analysis was carried out, from which the positive predictive value and negative predictive value were obtained. This was done with a contingency table, which provided various probable results for the CT. Both CTP and CT were performed on all patients, and rCBV and HU were obtained for all intracranial lesions. These parameters were evaluated with the Mann-Whitney U test and receiver operating characteristics (ROC) analysis with an area under the curve (AUC) calculation to evaluate and compare prognostic performance. In addition, we compared these characteristics and obtained four groups: >30 HU/>2rCBV, >30 HU/<2rCBV, <30HU/>2 rCBV, and <30HU/<2rCBV. The results were analyzed using a binary logistic regression analysis model. The odds ratio (OR) and 95% confidence intervals (CIs) of each variable were estimated. Statistical significance was calculated using Pearson's chi-square and unpaired t-test with confidence levels of 95%. A P-value <0.05 was considered to be significant.
Results
This study was conducted retrospectively in a tertiary care hospital in Mexico City between March 2016 and March 2022. It included 244 patients, all of whom had a confirmed histologic diagnosis by a neuropathologist. The patients had a mean age of 54 years, with 99 (40.6%) women and 145 (59.4%) men. The study groups comprised 87 (35.7%) for glioblastomas (GBs), 48 (19.7%) for primary central nervous system CNS lymphoma, 45 (18.4) for metastases (METs), and 64 (26.2%) for abscess (Table 1, Figure 1).
Table 1. Demographics of the cases .
| Parameter | n(%) |
| Male | 145 (59.4) |
| Female | 99 (40.6) |
| Glioblastoma | 87 (35.7) |
| Metastases | 45 (18.4) |
| Lymphoma | 48 (19.7) |
| Abscess | 64 (26.2) |
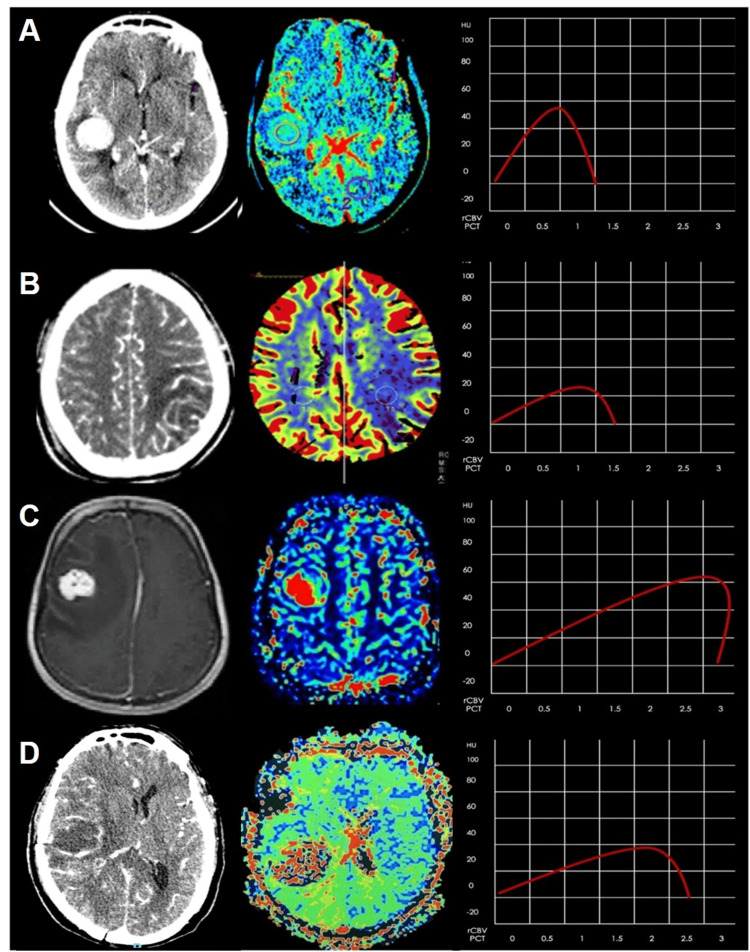
Table 1 shows the demographic and clinical details. Figure 1 presents the PCT/CT images, HU, and color maps of four histologically proven lesions: CNS lymphoma, abscess, and METs.
Figure 1. PCT/CT images, HU, and color maps in four different histologically proven lesions: (A) PCNSL, (B) abscess, (C) METs, and (D) GB.
PCT/CT: perfusion computed tomography/computed tomography, PCNSL: primary central nervous system lymphoma, METs: metastases, GB: glioblastoma
The rCBV maximum values were 4 for metastasis and 3.4 for PCNSL; the minimum was 0.2 for abscess and 0.6 for PCNSL. In the HU values, the maximum value was 55 for PCNSL and 43 for metastasis; the minimum was 12 for GB and 14 for abscesses. According to Kruskal-Wallis H results presented in Table 2, rCBV shows a significant difference according to the four intracranial lesions (X2 = 162.10; p ≤ 0.05) and for HU (X2 = 129.46; p ≤ 0.05) (Table 2).
Table 2. Statical values of rCBV and HU in different intracerebral lesions.
rCBV: relative cerebral blood volume; HU: Hounsfield unit; Asymp. Sig (Kolmogorov-Smirnov test), mean rank (Kruskal-Wallis H test)
| Diagnosis | rCBV | HU | |
| Glioblastoma | Median (std. deviation) | 3.94 (+1.30) | 20.89 (+5.73) |
| Skew | 1.58 | 1.54 | |
| Kurtosis | 2.61 | 3.37 | |
| Asymp. sig | 0.000 | 0.000 | |
| Mean rank | 192.24 | 73.34 | |
| Metastases | Median (std. deviation) | 2.04 (+1.23) | 31.80 (+4.46) |
| Skew | 0.00 | -0.30 | |
| Kurtosis | -1.60 | 4.14 | |
| Asymp. sig | 0.000 | 0.000 | |
| Mean rank | 118.17 | 173.34 | |
| Lymphoma | Median (std. deviation) | 1.46 (+0.92) | 34.08 (+5.43) |
| Skew | 0.96 | 0.49 | |
| Kurtosis | -0.19 | 4.54 | |
| Asymp. sig | 0.001 | 0.000 | |
| Mean rank | 98.24 | 197.94 | |
| Abscess | Median (std. deviation) | 0.68 (+0.34) | 23.08 (+5.48) |
| Skew | 1.04 | 0.04 | |
| Kurtosis | 0.08 | -0.40 | |
| Asymp. sig | 0.000 | 0.200 | |
| Mean rank | 48.95 | 96.99 |
ROC analysis for differentiating PCNSL, abscess, METs, and GB with rCBV showed a higher discriminatory value (AUC of 0.909, 95% CI 0.874-0.943, p = 0.000). The mean cut-off value was 2.08, with a sensitivity and specificity of 81.8% and 88.2%, respectively. ROC analysis was performed for differentiating the same intracerebral lesions with HU, showing a discriminant value (AUC of 0.885, 95% CI 0.835-0.935, p = 0.000). The mean cut-off value was 30.05 with a sensitivity and specificity of 85.8% and 79.2%, respectively.
Comparison of GBs and METs
The rCBV values of GB were found to be greater than those of METs, but the HU values were low. On the other hand, we observed that the HU of METs was greater than that of GB. We could identify metastases with sensitivity at 57.4% and specificity at 88.5% (Table 3).
Table 3. Sensitivity and specificity of the mean rCBV and HU to identify different intracerebral lesions and the positive and negative predictive values between them.
rCBV: relative cerebral blood volume; HU: Hounsfield unit; PPV: positive predictive value; NPV: negative predictive value
| Abscess (n = 64) | Glioblastoma (n = 87) | Lymphoma (n = 48) | Metastases (n = 45) | |
| Sensitivity | 84.4 | 81.4 | 94.1 | 57.4 |
| Specificity | 96.2 | 87.9 | 96.9 | 88.5 |
| PPV | 88.5 | 76.1 | 88.9 | 54 |
| NPV | 94.6 | 89 | 98.4 | 89.8 |
Comparison of CNS lymphoma and abscess
The study found that both PCNSL and abscess had lower rCBV values compared to GB and METs. In addition, the HU in PCNSL was higher than that of abscess, with sensitivity at 94.1% and specificity at 96.6% (Table 3).
Table 3 presents the positive and negative predictive values between different subgroups.
Variables that were independently associated have been studied with the four study groups: >30 HU/>2rCBV, >30 HU/<2rCBV, <30HU/>2 rCBV, and <30HU/<2rCBV (Table 4).
Table 4. Comparison and analysis of the HU and rCBV groups.
rCBV: relative cerebral blood volume; HU: Hounsfield unit; rCBV: relative cerebral blood volume, P: p-value
| rCBV | |||
| <2 n(%) | >2 n(%) | P-value | |
| HU <30 | |||
| Glioblastoma | 2(3) | 80(95.2) | 0.000 |
| Metastases | 4(6.1) | 2(2.4) | 0.406 |
| CNS lymphoma | 5(7.6) | 2(2.4) | 0.241 |
| Abscess | 55(83.3) | 0(0.0) | 0.000 |
| HU >30 | |||
| Glioblastoma | 0(0.0) | 5(13.5) | 0.017 |
| Metastases | 15(26.3) | 24(64.9) | 0.000 |
| CNS lymphoma | 33(57.9) | 8(21.6) | 0.001 |
| Abscess | 9(15.8) | 0(0.0) | 0.011 |
Those with significant differences (p < 0.05) were abscess on <30HU/<2rCBV (OR = 2.64; 95%CI = 1.46-14.90), CNS lymphoma on >30 HU/<2rCBV (OR = 4.91; 95%CI = 1.94-12.79), GB on >30 HU/>2rCBV (OR = 6.42; 95%CI = 1.13-16.58), and metastases on <30HU/>2 rCBV (OR = 5.16; 95%CI = 2.11-12.66) (Table 5).
Table 5. Result of the multivariable analysis.
rCBV: relative cerebral blood volume; HU: Hounsfield unit; P: p-value; OR: odds ratio; CI: confidence interval
| rCBV <2 | P | OR | CI | rCBV >2 | P | OR | CI | |
| HU <30 | Abscess | 0.000 | 2.64 | 1.46-14.90 | Glioblastoma | 0.000 | 6.42 | 1.13-16.58 |
| HU >30 | CNS lymphoma | 0.001 | 4.91 | 1.94-12.79 | Metastases | 0.000 | 5.16 | 2.11-12.66 |
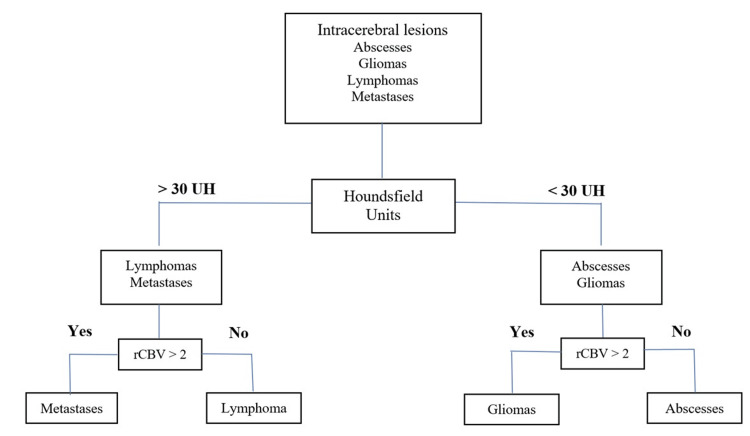
Figure 2 presents a flow diagram that can help improve diagnostic accuracy.
Figure 2. Flow diagram proposed in the present study (the patient on the top undergos several nodes to discriminate intracerebral lesions).
rCBV: relative cerebral blood volume; HU: Hounsfield unit
Discussion
This study aimed to examine CT and CTP parameters to distinguish between different conditions based on the cellularity and neovascularity of present lesions. The combined use of several parameters was found to be beneficial. We measured HU in all images by creating an ROI to establish the correlation between tumor cellularity and regional CTP values, and we found a strong correlation between the two. Advanced imaging techniques such as perfusion and spectroscopy are helpful in distinguishing between different types of lesions in the brain [11,12]. There have been few comprehensive studies using CTP to differentiate the rCBV of ring-enhancing intracranial lesions, although a few studies have been conducted to evaluate vascular permeability and perfusion in brain tumors and gliomas. CTP techniques have been validated and can be compared with xenon CT and MR perfusion techniques, showing consistent results [13-16].
Previously, the clinical efficacy of CTP in histologic grading of glioma has already been reported [16]. The differentiation between low-grade and high-grade gliomas was successfully achieved through perfusion studies. Low-grade tumors exhibited lower perfusion compared to high-grade tumors. Kamble RB et al. [17] found a statistically significant difference between the rCBV of low-grade glioma and high-grade glioma, with an rCBV cut-off value of 1.64 to differentiate between them. This value showed a sensitivity of 86.36% and a specificity of 100%. Similarly, Aronen et al. found that none of the low-grade gliomas had an rCBV greater than 1.5 [18].
However, there was no statistical difference in rCBV between GB and METs. Ellika SK et al. [19] demonstrated that the mean rCBV of high-grade gliomas is significantly higher, with a cut point for rCBV of >1.92, yielding 85.7% sensitivity and 100% specificity. Our study used CTP parameters to differentiate between GB, METs, PCNSL, and infective lesions. According to reports, the rCBV of GB obtained by CTP is more significant than those of PCNSL and abscess [19-21].
Infective lesions and primary CNS lymphoma show a reduced rCBV, as observed by Kamble RB et al. [17] in tuberculomas. To differentiate these lesions, we measured HU to determine cellularity. Our study shows a good correlation between cellularity and density with respect to HU, and we used a cut point for HU of 30, yielding 84.4% sensitivity, 96.2% specificity, and 94.1% and 96.9%, respectively. We found higher values of HU in PCNSL than infective lesions, and we concluded that a HU >30 and low rCBV values correlate with the lack of vascular proliferation and the higher cellularity of histopathological properties of PCNSL [10-13,22,23].
It should be noted that there is no standard threshold for rCBV to distinguish between different entities. However, we have adopted a cutoff value of 2 for our study. Our findings align with previous studies, such as those conducted by Okaii AL et al. [1], which also reported a good correlation with rCBV values >1.79.
Post-contrast acute kidney injury (AKI) and contrast-induced AKI can vary significantly depending on factors such as the presence or absence of risk factors, the volume and method of contrast material administration, and the specific patient population under examination [2]. In the absence of risk factors, the occurrence of contrast-induced AKI is very low. A major risk factor for kidney injury is a decline in kidney function, which may be due to chronic kidney disease or a current episode of AKI [23-26]. Other potential risk factors may include diabetes mellitus, heart failure, hypovolemia, proteinuria, and the use of intra-arterial (rather than intravenous) contrast material during medical procedures [27].
Limitations in the diagnosis accuracy of a single CTP parameter can be overcome by combining the analysis of rCBV and HU, which can be a useful tool in differentiating intracranial lesions. CTP techniques have been thoroughly validated and found to be comparable with xenon CT and MR perfusion techniques. These findings demonstrate a high level of agreement with quantitative results, highlighting the reliability and accuracy of CTP in assessing perfusion [24,27-32].
Conclusions
The PCT parameters offer valuable information for preoperative diagnosis of PCNSL, abscesses, METs, and GB. We found that a comprehensive PCT parameter assessment improved diagnostic accuracy for intracranial lesions. This study showed that combining rCBV and HU can enhance diagnostic accuracy, making it a valuable tool for distinguishing between intracranial lesions. The widespread availability of PCT allows for the use of both anatomical and functional information with high spatial resolution for the initial diagnosis and subsequent management of brain tumor patients.
Our proposed imaging strategy has been highly accurate in differentiating intracranial lesions. When combined with the clinical context, our strategy is the best predictor for early and adequate treatment. This can be particularly beneficial for hospitals that perform neurological surgeries but lack access to reference MRI, which is a common issue in developing countries. Our treatment algorithm provides a standardized CTP approach, which is an effective alternative for diagnosing intracranial space-occupying lesions.
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study. National Institute of Neurology and Neurosurgery issued approval 43-22.
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Héctor A. Rodríguez-Rubio, Eliezer Villanueva-Castro, Rodrigo Uribe-Pacheco, Edgardo de Jesus Mateo-Nouel, Alberto Gonzalez-Aguilar, Sergio Moreno-Jiménez, Juan Antonio Alvaro-Heredia, Luis A. Rodríguez-Hernández, Elvira Castro-Martinez, Guillermo A. Gutierrez-Aceves, Ignacio Reyes-Moreno, Michel G. Mondragon-Soto, Isidro Alvaro-Heredia, Ivan A. Rodríguez-Hernández
Acquisition, analysis, or interpretation of data: Héctor A. Rodríguez-Rubio, Eliezer Villanueva-Castro, Rodrigo Uribe-Pacheco, Edgardo de Jesus Mateo-Nouel, Alberto Gonzalez-Aguilar, Sergio Moreno-Jiménez, Juan Antonio Alvaro-Heredia, Luis A. Rodríguez-Hernández, Elvira Castro-Martinez, Guillermo A. Gutierrez-Aceves, Ignacio Reyes-Moreno, Michel G. Mondragon-Soto, Isidro Alvaro-Heredia, Ivan A. Rodríguez-Hernández
Drafting of the manuscript: Héctor A. Rodríguez-Rubio, Eliezer Villanueva-Castro, Rodrigo Uribe-Pacheco, Edgardo de Jesus Mateo-Nouel, Alberto Gonzalez-Aguilar, Sergio Moreno-Jiménez, Juan Antonio Alvaro-Heredia, Luis A. Rodríguez-Hernández, Elvira Castro-Martinez, Guillermo A. Gutierrez-Aceves, Ignacio Reyes-Moreno, Michel G. Mondragon-Soto, Isidro Alvaro-Heredia, Ivan A. Rodríguez-Hernández
Critical review of the manuscript for important intellectual content: Héctor A. Rodríguez-Rubio, Eliezer Villanueva-Castro, Rodrigo Uribe-Pacheco, Edgardo de Jesus Mateo-Nouel, Alberto Gonzalez-Aguilar, Sergio Moreno-Jiménez, Juan Antonio Alvaro-Heredia, Luis A. Rodríguez-Hernández, Elvira Castro-Martinez, Guillermo A. Gutierrez-Aceves, Ignacio Reyes-Moreno, Michel G. Mondragon-Soto, Isidro Alvaro-Heredia, Ivan A. Rodríguez-Hernández
Supervision: Héctor A. Rodríguez-Rubio, Eliezer Villanueva-Castro, Rodrigo Uribe-Pacheco, Edgardo de Jesus Mateo-Nouel, Alberto Gonzalez-Aguilar, Sergio Moreno-Jiménez, Juan Antonio Alvaro-Heredia, Luis A. Rodríguez-Hernández, Elvira Castro-Martinez, Guillermo A. Gutierrez-Aceves, Ignacio Reyes-Moreno, Michel G. Mondragon-Soto, Isidro Alvaro-Heredia, Ivan A. Rodríguez-Hernández
References
- 1.Advanced MR imaging techniques in the diagnosis of intraaxial brain tumors in adults. Al-Okaili RN, Krejza J, Wang S, Woo JH, Melhem ER. Radiographics. 2006;26 Suppl 1:0–89. doi: 10.1148/rg.26si065513. [DOI] [PubMed] [Google Scholar]
- 2.Contrast nephrotoxicity. Barrett BJ. J Am Soc Nephrol. 1994;5:125–137. doi: 10.1681/ASN.V52125. [DOI] [PubMed] [Google Scholar]
- 3.Prediction of malignancy grading using computed tomography perfusion imaging in nonenhancing supratentorial gliomas. Beppu T, Sasaki M, Kudo K, et al. J Neurooncol. 2011;103:619–627. doi: 10.1007/s11060-010-0433-0. [DOI] [PubMed] [Google Scholar]
- 4.Diagnostic imaging for the emergency physician. Broder J. https://books.google.com.ph/books?hl=en&lr=&id=q3_EthzU3dcC&oi=fnd&pg=PP1&dq=Diagnostic+imaging+for+the+emergency+physician&ots=mFlfXs7oqz&sig=74qc2w2jWppacZewDprSYZdNo68&redir_esc=y#v=onepage&q=Diagnostic%20imaging%20for%20the%20emergency%20physician&f=false Elsevier Health Sciences. 2011 [Google Scholar]
- 5.A quantitative theory of the Hounsfield unit and its application to dual energy scanning. Brooks RA. J Comput Assist Tomogr. 1977;1:487–493. doi: 10.1097/00004728-197710000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Brain perfusion: computed tomography and magnetic resonance techniques. Copen WA, Lev MH, Rapalino O. Handb Clin Neurol. 2016;135:117–135. doi: 10.1016/B978-0-444-53485-9.00006-4. [DOI] [PubMed] [Google Scholar]
- 7.Radiographic findings in 37 cases of primary CNS lymphoma in immunocompetent patients. Coulon A, Lafitte F, Hoang-Xuan K, Martin-Duverneuil N, Mokhtari K, Blustajn J, Chiras J. Eur Radiol. 2002;12:329–340. doi: 10.1007/s003300101037. [DOI] [PubMed] [Google Scholar]
- 8.DenOtter TD, Schubert J. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2022. Hounsfield unit. [PubMed] [Google Scholar]
- 9.Quantitative analysis of CT-perfusion parameters in the evaluation of brain gliomas and metastases. Di Nallo AM, Vidiri A, Marzi S, et al. J Exp Clin Cancer Res. 2009;28:38. doi: 10.1186/1756-9966-28-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comparison of cerebral blood volume and permeability in preoperative grading of intracranial glioma using CT perfusion imaging. Ding B, Ling HW, Chen KM, Jiang H, Zhu YB. Neuroradiology. 2006;48:773–781. doi: 10.1007/s00234-006-0120-1. [DOI] [PubMed] [Google Scholar]
- 11.Cerebral blood flow, blood volume, and vascular permeability of cerebral glioma assessed with dynamic CT perfusion imaging. Eastwood JD, Provenzale JM. Neuroradiology. 2003;45:373–376. doi: 10.1007/s00234-003-0996-y. [DOI] [PubMed] [Google Scholar]
- 12.Potential role of CT perfusion parameters in the identification of solitary intra-axial brain tumor grading. Fainardi E, Di Biase F, Borrelli M, et al. Acta Neurochir Suppl. 2010;106:283–287. doi: 10.1007/978-3-211-98811-4_53. [DOI] [PubMed] [Google Scholar]
- 13.Differentiating glioblastomas from solitary brain metastases: an update on the current literature of advanced imaging modalities. Fordham AJ, Hacherl CC, Patel N, Jones K, Myers B, Abraham M, Gendreau J. Cancers (Basel) 2021;13 doi: 10.3390/cancers13122960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perfusion imaging in brain disease. Grand S, Tahon F, Attye A, Lefournier V, Le Bas JF, Krainik A. Diagn Interv Imaging. 2013;94:1241–1257. doi: 10.1016/j.diii.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Medical imaging physics. Hendee W, Ritenour E, Hoffmann K, et al. Med Phys. 2003;4:730. [Google Scholar]
- 16.Perfusion CT imaging of brain tumors: an overview. Jain R. AJNR Am J Neuroradiol. 2011;32:1570–1577. doi: 10.3174/ajnr.A2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Role of dynamic CT perfusion study in evaluating various intracranial space-occupying lesions. Kamble RB, Jayakumar PN, Shivashankar R. Indian J Radiol Imaging. 2015;25:162–166. doi: 10.4103/0971-3026.155866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Aronen HJ, Gazit IE, Louis DN, et al. Radiology. 1994;191:41–51. doi: 10.1148/radiology.191.1.8134596. [DOI] [PubMed] [Google Scholar]
- 19.Quantitative estimation of permeability surface-area product in astroglial brain tumors using perfusion CT and correlation with histopathologic grade. Jain R, Ellika SK, Scarpace L, et al. AJNR Am J Neuroradiol. 2008;29:694–700. doi: 10.3174/ajnr.A0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prediction of tumor cellularity in resectable PDAC from preoperative computed tomography imaging. Jungmann F, Kaissis GA, Ziegelmayer S, et al. Cancers (Basel) 2021;13 doi: 10.3390/cancers13092069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Improved differentiation between high- and low-grade gliomas by combining dual-energy CT analysis and perfusion CT. Kaichi Y, Tatsugami F, Nakamura Y, et al. Medicine (Baltimore) 2018;97:0. doi: 10.1097/MD.0000000000011670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Efficacy of perfusion computed tomography (PCT) in differentiating high-grade gliomas from low grade gliomas, lymphomas, metastases and abscess. Karegowda LH, Kadavigere R, Shenoy PM, Paruthikunnan SM. J Clin Diagn Res. 2017;11:0–33. doi: 10.7860/JCDR/2017/24835.9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Application of CT in the investigation of angiogenesis in oncology. Miles KA, Charnsangavej C, Lee FT, Fishman E, Horton K, Lee TY. Acad Radiol. 2000;10:840–850. doi: 10.1016/s1076-6332(00)80632-7. [DOI] [PubMed] [Google Scholar]
- 24.Perfusion computed tomography parameters are useful for differentiating glioblastoma, lymphoma, and metastasis. Onishi S, Kajiwara Y, Takayasu T, et al. World Neurosurg. 2018;119:0–7. doi: 10.1016/j.wneu.2018.07.291. [DOI] [PubMed] [Google Scholar]
- 25.Dynamic, contrast-enhanced CT of human brain tumors: quantitative assessment of blood volume, blood flow, and microvascular permeability: report of two cases. Roberts HC, Roberts TPL, Lee T-Y, et al. https://www.ajnr.org/content/23/5/828.short. AJNR Am J Neuroradiol. 2002;23:828–832. [PMC free article] [PubMed] [Google Scholar]
- 26.Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: a randomized trial. The Iohexol Cooperative Study. Rudnick MR, Goldfarb S, Wexler L, et al. Kidney Int. 1995;47:254–261. doi: 10.1038/ki.1995.32. [DOI] [PubMed] [Google Scholar]
- 27.Determination of linear x-ray attenuation coefficients of pathological brain tissues and use of filters in tissue contrast enhancement in computed tomography. Sagsoz ME, Erdogan F, Erzeneoglu SZ, Yuce İ. Eurasian J Med. 2010;42:53–56. doi: 10.5152/eajm.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dynamic CT perfusion imaging of intra-axial brain tumours: differentiation of high-grade gliomas from primary CNS lymphomas. Schramm P, Xyda A, Klotz E, Tronnier V, Knauth M, Hartmann M. Eur Radiol. 2010;20:2482–2490. doi: 10.1007/s00330-010-1817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evaluation of perfusion CT in grading and prognostication of high-grade gliomas at diagnosis: a pilot study. Shankar JJ, Woulfe J, Silva VD, Nguyen TB. AJR Am J Roentgenol. 2013;200:0–9. doi: 10.2214/AJR.12.8967. [DOI] [PubMed] [Google Scholar]
- 30.Using voxel count measurements based on X-ray attenuation coefficient principle to estimate organ volume during CT scan. Shirazu I, Mensah YB, Schandorf C, et al. Int J Sci Res Sci Eng Technol. 2017:653–659. [Google Scholar]
- 31.Dynamic perfusion CT in brain tumors. Yeung TP, Bauman G, Yartsev S, Fainardi E, Macdonald D, Lee TY. Eur J Radiol. 2015;84:2386–2392. doi: 10.1016/j.ejrad.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Hounsfield units variations: impact on CT-density based conversion tables and their effects on dose distribution. Zurl B, Tiefling R, Winkler P, Kindl P, Kapp KS. Strahlenther Onkol. 2014;190:88–93. doi: 10.1007/s00066-013-0464-5. [DOI] [PubMed] [Google Scholar]