Abstract
Parent-of-origin-specific expression of imprinted genes is critical for successful mammalian growth and development. Insulin, coded by the INS gene, is an important growth factor expressed from the paternal allele in the yolk sac placenta of therian mammals. The tyrosine hydroxylase gene TH encodes an enzyme involved in dopamine synthesis. TH and INS are closely associated in most vertebrates, but the mouse orthologues, Th and Ins2, are separated by repeated DNA. In mice, Th is expressed from the maternal allele, but the parental origin of expression is not known for any other mammal so it is unclear whether the maternal expression observed in the mouse represents an evolutionary divergence or an ancestral condition. We compared the length of the DNA segment between TH and INS across species and show that separation of these genes occurred in the rodent lineage with an accumulation of repeated DNA. We found that the region containing TH and INS in the tammar wallaby produces at least five distinct RNA transcripts: TH, TH-INS1, TH-INS2, lncINS and INS. Using allele-specific expression analysis, we show that the TH/INS locus is expressed from the paternal allele in pre- and postnatal tammar wallaby tissues. Determining the imprinting pattern of TH/INS in other mammals might clarify if paternal expression is the ancestral condition which has been flipped to maternal expression in rodents by the accumulation of repeat sequences.
Subject terms: Imprinting, Evolutionary genetics
Introduction
In one model of the evolution of genomic imprinting, increased maternal provision of resources to offspring in the mammalian lineage created a conflict between the parental genomes (Haig 2004; Smits et al. 2008). The placenta contributes to offspring development through several functions, including delivery of maternal nutrients and growth factors, and has been a focal point for investigating allelic gene expression under genomic conflict (Coan et al. 2005; Renfree et al. 2008; Bartolomei and Ferguson-Smith 2011; Tucci et al. 2019). Imprinting can also influence maternal investment through its function in postnatal tissues, such as the mammary gland and brain (Stringer et al. 2014; Cleaton et al. 2014; Tucci et al. 2019; Hanin and Ferguson-Smith 2020; John 2023). Relative to eutherians, the provision of resources to marsupial offspring is more pronounced during postnatal development but fewer imprinting studies have been made on these stages (Stringer et al. 2014; Hanin and Ferguson-Smith 2020). Parental conflict is entrenched in the literature as the primary selection pressure for genomic imprinting but other models could explain the evolution of imprinting for at least some genes (Spencer and Clark 2014).
Genomic imprinting has traditionally been studied in the fetus and the chorioallantoic placenta of mice and humans. The yolk sac evolved in fish while amniotes additionally developed the amnion, allantois and chorion. Mammals then adapted these four fetal membranes to support their in utero development (Mossman 1937; Ross and Boroviak 2020). Mammalian pregnancy depends on the yolk sac as the early site of maternal-fetal exchange, hematopoeiesis, and biosynthesis (Gulbis et al. 1998; Burton and Jauniaux 2021). In most marsupials it is the yolk sac that forms the definitive choriovitelline placenta and consists of an avascular bilaminar region (“BOM”: bilaminar omphalopleure) and a vascular trilaminar region (“TOM”: trilaminar omphalopleure), that in most species are closely apposed to the maternal uterine epithelium until birth (Renfree 1973, 2010; Freyer et al. 2003; Guernsey et al. 2017). In humans and mice the yolk sac supports the early stages of development before the establishment of the definitive chorioallantoic placenta (Ross and Boroviak 2020; Burton and Jauniaux 2023).
Imprinted genes are special cases, comprising only a small proportion of all mammalian genes. More than 200 imprinted “genes” are known in humans, including distinct transcripts expressed from the same locus, with the majority paternally expressed (Morison et al. 2005; Tucci et al. 2019). Slightly more imprinted genes are known in mice, with a quarter of these genes imprinted in both human and mouse (Tucci et al. 2019). So far, 25 autosomal genes showing parent-of-origin-specific gene expression have been found in marsupials (Smits et al. 2008; Stringer et al. 2014; Douglas et al. 2014; Ishihara et al. 2022; Cao et al. 2023; Bond et al. 2023). In marsupials, and the early extraembryonic tissues of some eutherians, X-inactivation is non-random with the paternal X chromosome imprinted to undergo silencing (Sharman 1971; Cooper et al. 1971; Grant et al. 2012; Lee and Bartolomei 2013). Marsupial imprinted X-inactivation is known to involve paternal expression of an XIST-like (X-Inactive Specific Transcript) noncoding RNA, RSX (RNA-on-the-Silent X), and maternal expression of a TSIX (“XIST” backwards)-like RSX antisense transcript, XSR (“RSX” backwards) (Grant et al. 2012; Mahadevaiah et al. 2020).
Imprinting control regions (ICRs) regulate parent-of-origin-specific expression of a neighbouring cluster of genes (Jacob 2013; Juan and Bartolomei 2019; Chang and Bartolomei 2020). The most ancient ICR known is ICR1 which is paternally methylated in both eutherians and marsupials and associated with maternal expression of the noncoding RNA, H19, and paternal expression of the insulin-like growth factor 2, IGF2 (Sparago et al. 2004; Smits et al. 2008). A nearby ICR, ICR2, is located within the potassium voltage-gated channel gene, KCNQ1, and in eutherians has been associated with paternal expression of the KCNQ1 overlapping transcript (KCNQ1OT1) and maternal expression of several other genes (Chiesa et al. 2012). ICR2 may not be present in marsupials because, although a KCNQ1OT1 transcript is present in the TOM placenta of the tammar wallaby, the gene lacks a proximal CpG island (Ager et al. 2008). The genes that neighbour ICR2 in humans, mice and other eutherians, CDKN1C (cyclin dependent kinase inhibitor 1 C) and PHLDA2 (pleckstrin homology-like domain family A member 2), are biallelically-expressed in the tammar wallaby (Suzuki et al. 2005, 2011).
Aberrant imprinting in the human chromosome 11p15.5 region, containing ICR1 and ICR2, is associated with the Beckwith–Wiedemann (BWS) and Silver–Russell (SRS) syndromes. BWS and SRS are clinically opposite growth disorders, birth weights for BWS are >90th percentile while in SRS they are <3rd percentile (Wollman et al. 1995; Weksberg et al. 2010; Jacob et al. 2013; Eggermann et al. 2008). BWS/SRS have a range of molecular subtypes, ICR2 hypomethylation is the most frequent (50–60%) in BWS, with ICR1 hypermethylation (5–10%) also found; ICR1 hypomethylation is the most frequent (50–60%) for SRS (Eggermann et al. 2008; Wang et al. 2020). Loss of methylation at the maternal ICR2 allele in BWS reduces expression of the growth regulator CDKN1C while loss of methylation at the paternal ICR1 allele in SRS increases H19 which represses the growth promoting IGF2 (Nativio et al. 2011; Naveh et al. 2021). Expression of the genes in the 11p15.5 region is developmentally important but how parent-of-origin-specific gene expression is regulated at the interface of the two ICRs is unknown.
The tyrosine hydroxylase (TH) / insulin (INS) locus adjoining ICR1 and ICR2 has a dynamic evolutionary history of gene duplication and loss. The ancestral amino acid hydroxylase genes and insulin-like genes became linked in the genome of early chordates (Patton et al. 1998; Yamamoto et al. 2010). Duplication of the insulin-like gene early in the vertebrate lineage resulted in the neighbouring INS and IGF genes that encode structurally similar proteins (Chan and Steiner 2000). The duplication event that resulted in TH and phenylalanine hydroxylase (PAH) predates the divergence of invertebrates (Patton et al. 1998). A further duplication event in jawed vertebrates resulted in one paralogy group containing PAH, TH2 and IGF1 and another containing TH(1), INS and IGF2, with the TH2 gene subsequently lost in therians but not birds (Patton et al. 1998; Candy and Collet 2005; Yamamoto et al. 2010).
The insulin gene, INS, is an important regulator of carbohydrate metabolism that is paternally-expressed in the therian yolk sac (Ager et al. 2007). In humans, INS is monoallelically-expressed from the paternal allele in the yolk sac at weeks 9 and 10 of gestation (Moore et al. 2001) and there is circumstantial evidence for monoallelic expression in the thymus (Pugliese et al. 1997), neonatal pancreas and an adolescent spleen (Pugliese and Miceli 2002). Murine rodents, mice and rats, have two insulin-coding genes, Ins1 and Ins2, with Ins2 orthologous to the INS gene (Shiao et al. 2008). Ins1 and Ins2 are biallelically-expressed in mouse fetal pancreas and embryonic body, but only Ins2 is expressed in the yolk sac (Giddings et al. 1994; Deltour et al. 1995, 2004). Both parental alleles of Ins2 are expressed in the yolk sac at embryonic day E12.5, but by E14.5 the maternal allele is silenced so that Ins2 is paternally-expressed in the yolk sac (Giddings et al. 1994; Deltour et al. 1995, 2004). Deletion of the ICR1 and H19 region in mice results in activation of the Ins2 maternal allele in the E13.5 yolk sac, indicating that ICR1 can regulate imprinted expression of Ins2 in addition to Igf2 and H19 (Leighton et al. 1995).
The INS gene is paternally-expressed in the yolk sac and also in several postnatal tissues of the tammar wallaby (Stringer et al. 2012). INS has paternally-skewed expression in the BOM and TOM regions of the yolk sac placenta during the final third of the short, 26.5 day, tammar wallaby gestation after diapause (Ager et al. 2007). Marsupial mammals give birth to highly altricial young that are supported, usually in a pouch, by a sophisticated lactation process in which the composition of the milk changes dynamically throughout the whole of pouch life (Tyndale-Biscoe and Renfree 1987; Green et al. 1988; Trott et al. 2003; Stringer et al. 2014). Postnatally, INS is biallelically-expressed in the stomach and intestine, but monoallelically-expressed in the adult mammary gland and paternally-expressed in the tammar wallaby pouch young (PY) liver (Stringer et al. 2012).
The TH gene adjacent to INS encodes tyrosine hydroxylase: a key enzyme in the synthesis of catecholamines, particularly the neurotransmitter dopamine (Lelou et al. 2022). Th is maternally-expressed from E7.5 in the mouse placenta and embryo, but becomes biallelic within the embryo by E12.5 (Golding et al. 2011; Jones et al. 2011; Okae et al. 2012). In mice, the paternal Th allele is silenced in the E13.5 placenta but can be activated by deletion of ICR2 which prevents Kcnq1ot1 transcription (Jones et al. 2011), indicating that Th is imprinted by ICR2 in the mouse. Whether TH has parent-of-origin-specific expression is not known in humans (Lefebvre 2012; Cleaton et al. 2014), or any other species.
Synteny of TH and INS is well conserved in vertebrates, with the exception that INS was lost from the TH/IGF2 linkage group in some teleosts (Collet et al. 1998; Rotwein 2018). In chickens the distance between TH and INS is 15.5 kilobases (kb) while in humans the genes are 2.4 kb apart (Hernández-Sánchez et al. 2006). In contrast, the mouse orthologues, Th and Ins2 are separated by a 210 kb region of repetitive DNA rich in retro-elements (Shirohzu et al. 2004). Chimaeric TH-INS transcripts, which fuse exons from both TH and INS, have been observed in the developing chicken, quail (Hernández-Sánchez et al. 2006; De Pablo and de la Rosa 2011) and tammar wallaby (Stringer et al. 2012, 2014). The chimaeric TH-INS proteins have lower enzymatic activity than TH but any roles for the chimaeric transcripts in regulating the expression of two adjacent genes, and any physiological implications, are unknown (Hernández-Sánchez et al. 2006).
Since insulin is a key hormone for growth and lactation in all mammals, we asked whether the large distance between Th and Ins2 in the mouse was an ancestral mammalian trait or specific to mice. We examined a range of vertebrate species and found that the large distance between these two genes in mice is a feature specific to rodents. Despite the position of the TH and INS genes at the interface of ICR1 and ICR2, it is unknown if TH is expressed from the maternal allele in any other species, as in mice. This study investigated the orthologous region in the tammar wallaby to identify transcribed RNAs and analyse parent-of-origin-specific expression in tammar wallaby fetuses and PY. We identified five transcripts, TH, TH-INS1, TH-INS2, lncINS and INS, produced from the tammar wallaby region and show that each has paternal expression.
Materials and methods
Species comparison
The genomic location of TH and INS orthologues were taken from the 38 species listed in Supplementary Table 1. The distance between TH and INS was calculated as the end position of the TH gene minus the starting position of the INS gene, these values are provided in Supplementary Table 1. The species divergence times (mya) were taken from TimeTree 5 (Kumar et al. 2022), the divergence times relative to the house mouse are also noted in Supplementary Table 1. The phylogeny was visualised using the Environment for Tree Exploration: ETE v3 (Huerta-Cepas et al. 2016). Repeat sequences in the region between the rodent Th and Ins(2) genes were assessed using RepeatMasker v4.1.5 (Tarailo-Graovac and Chen 2009) with the “-species rodent” option.
The location of genes in the imprinted region was visualised for house mouse, human, cattle and tammar wallaby using the DNA Features Viewer library (Zulkower and Rosser 2020). ICR locations were from NCBI (Gene ID: 105317033, 105259599) or based on the position of published bisulfite primers (Smits et al. 2008; Oh et al. 2008; Robbins et al. 2012; Wang et al. 2015; Huang et al. 2021). The position of KCNQ1OT1 in cattle and the tammar wallaby is from primer positions used to generate a short amplicon of the transcript (Ager et al. 2007; Robbins et al. 2012). Parent-of-origin-specific expression of genes in the region was from online databases https://www.geneimprint.com and https://corpapp.otago.ac.nz/gene-catalogue or literature, in the case of cattle KCNQ1OT1 (Robbins et al. 2012; Chen et al. 2015).
Animal samples
Tammar wallabies of Kangaroo Island, South Australia origin, were held in open grassy yards in our breeding colony at the University of Melbourne. A postnatal sample set was prepared containing tissues from 11 pouch young (PY) aged between 37 and 41 days postpartum (pp) and from 12 PY aged between day 78 and 81 pp, matched to maternal tissues. A prenatal sample set was prepared from the BOM and TOM placental tissues and the maternal endometrial tissues from 16 pregnant females and their fetuses collected between day 18 and 25 of the 26.5 day gestation. Tissue samples were collected as described previously (Suzuki et al. 2005; Ishihara et al. 2021) snap frozen in liquid nitrogen and stored at -80°C. After retrieval of the snap frozen PY whole brain tissue samples, the anterior portion of the brain including the olfactory bulb and the front half of the cerebrum was taken (Renfree et al. 1982) for RNA extraction. All tammar animal handling, husbandry and experimental sampling were in accordance with the National Health and Medical Research Council of Australia (2013) guidelines and approved by the University of Melbourne Animal Experimentation Ethics committees.
lncRNA identification
To identify potential antisense lncRNA at the TH/INS locus, a publicly available tammar testis RNA-seq data set was analysed (NCBI, DRX012262). Reads were trimmed using TrimGalore! v0.6.10 (https://github.com/FelixKrueger/TrimGalore), aligned to the tammar wallaby genome v7 using HISAT2 v2.2.1 (Kim 2019) with the “--rna-strandness FR” option and mapped reads assigned to each strand with Samtools v1.16.1 (Li et al. 2009).
To determine the full-length of the antisense INS lncRNA transcript, RACE (rapid amplification of cDNA ends) was performed with the SMARTer RACE 5′/3′ kit (cat. no. 634923, Clontech, California, USA). The first round RACE reactions were performed with adult testis cDNA using SeqAmp DNA Polymerase (cat no. 638504, Clontech, California, USA) with gene specific primers (Supplementary Table 2). Nested RACE experiments were performed with GoTaq Master Mix (cat. no. M5123, Promega, Madison, WI, USA) and products cloned using the pGEM-T Easy Vector System (cat. no. A1360, Promega, Madison, WI, USA) and JM109 Competent Cells (cat. no. L2001, Promega, Madison, WI, USA). Plasmids were extracted using the Wizard Plus SV Minipreps DNA Purification System (cat. no. A1460, Promega, Wisconsin, USA) and sent for Sanger sequencing by the Australian Genome Research Facility (AGRF).
Genotyping
Maternal and PY gDNA was prepared from frozen tissue using the Wizard Genomic DNA Purification Kit (cat. no. A1120, Promega, Madison, WI, USA) with a T10 basic handheld homogenizer (IKA, Staufen, Germany). Genotyping was carried out by PCR using the genotyping primers listed in Supplementary Table 2. PCR products were extracted using the QIAquick Gel Extraction Kit (cat. no. 28706, Qiagen, Venlo, Netherlands) and sent for Sanger sequencing by AGRF. Informative animals were those in which the offspring was heterozygous at a site for which the corresponding maternal sample was homozygous.
RT-PCR
PY RNA was prepared from frozen tissue using the GenElute Mammalian Total RNA Miniprep Kit (cat. no. RTN70-1KT, Sigma-Aldrich, St. Louis, MO, USA). cDNA was prepared from 1 μg of RNA using the Superscript IV First-Strand Synthesis System (cat. no. 18091050, ThermoFisher Scientific, Waltham, MA, USA), primed with oligo(dT)20. Allele-specific expression analysis of each SNP was performed in multiple animals with a single technical replicate of RT-PCR using the expression primers listed (Supplementary Tables 2 and 3). PCR products were gel extracted using the QIAquick Gel Extraction Kit (cat. no. 28706, Qiagen, Venlo, Netherlands) and sent for Sanger sequencing by AGRF.
Quantification of allele use
Parent-of-origin-specific gene expression was quantified by extracting the signal data from the .ab1 trace file. The signal intensity value for the major and minor alleles at an offspring SNP site was compared with reference to the identity of the genotyped maternal allele. The parental expression of the allele in the offspring cDNA was presented as a “mat:pat ratio”, the maternal signal / (maternal signal + paternal signal), metric which ranged from 0 to 1, where paternal expression was 0, biallelic expression was 0.5, and a value of 1 would indicate maternal expression. A mat:pat ratio of 0.00–0.20 or 0.80–1.00 was interpreted as “monoallelic”, 0.20–0.35 or 0.65–0.80 was interpreted as “skewed”, and 0.35–0.65 was interpreted as “biallelic”. The mean of multiple SNPs was taken and the final value provided is the mean ± standard error of the mean (SEM), across n animals.
Results
Separation of the TH and INS genes in the rodent lineage
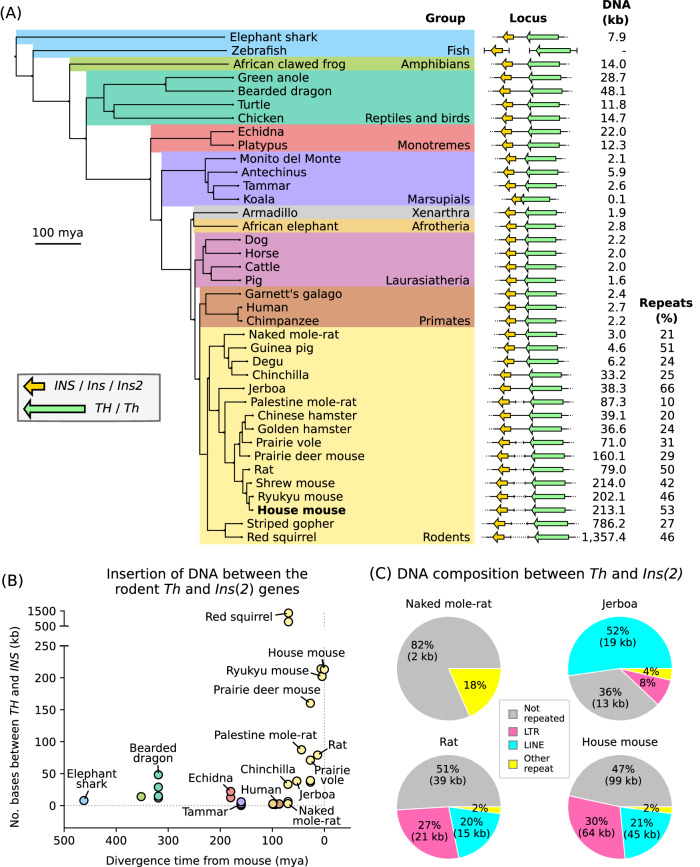
The DNA sequence between the orthologous TH and INS genes was assessed across 38 vertebrate species from diverse taxonomic groups (Fig. 1A). Across non-rodent therian species the length of the DNA segment between TH and INS was 2.4 kb with a standard deviation of 1.3 kb. In the fish, amphibians and reptiles that were assessed the DNA segment was 20 ± 13 kb, or linkage of the two genes was not maintained as was the case in the teleost zebrafish which had th on chr25 and ins on chr5. In the rodent lineage the DNA between Th and Ins(2) was highly variable in length ranging from 3 kb in the naked mole-rat (Heterocephalus glaber) to 1357 kb in the red squirrel (Sciurus vulgaris). The house mouse (Mus musculus) was similar to other species of mice and had a large 213.1 kb DNA segment between the Th and Ins2 genes.
Fig. 1. Accumulation of DNA between Th and Ins(2) orthologues over evolutionary time in the rodent lineage.
A The number of bases between the TH/Th and INS/Ins/Ins2 genes is given for different species and the relationships of those species are placed in terms of either their phylogenetic grouping or (B) the species divergence relative to the house mouse. Divergence times, millions of years ago (mya), are those indicated by TimeTree. Illustration of the gene locus (A) is not to scale. C The percent of the rodent DNA segment between Th and Ins(2) comprised of different classes of repeated elements is shown for the naked mole-rat, jerboa, rat and house mouse.
There was a progressive extension of the DNA segment between the Th and Ins2 genes in rodents (Fig. 1B). The Hystricognathi clade, including the naked mole-rat and degu (Octodon degus), shared an ancestor with the murine rodents (Muroidea), including the house mouse, 70 mya and, with the exception of the chinchilla (Chinchilla lanigera), had a short DNA segment that was comparable to non-rodent therians. The jerboa (Jaculus jaculus) and the prairie vole (Microtus ochrogaster) diverged from mice 53 and 27 mya and had DNA segments that were 38 and 71 kb long respectively. The rat (Rattus norvegicus) shared an ancestor with the house mouse 13 mya and had 79 kb of DNA between Th and Ins2, reflecting a recent rapid extension in the length of the DNA segment in mice. The squirrel-related clade, including the red squirrel and striped gopher (Ictidomys tridecemlineatus), was an exception to this progression diverging from mice 69 mya yet having a DNA segment longer than 785 kb, suggesting a distinct evolutionary process in the squirrel lineage.
To gain insight into the DNA separating Th and Ins(2) in rodents we profiled the repeated element composition (Fig. 1A, C). Across the rodent species assessed, repeated elements comprised 35 ± 15% of the DNA segment. The amount of repeated DNA progressively increased between the Th and Ins(2) genes in the mouse-related clade. The jerboa which diverged before Muroidea had a distinct accumulation of long interspersed nuclear elements (LINEs) that made up 52% of the DNA between Th and Ins in this species. In Muroidea long terminal repeats (LTRs) were the largest subclass of repeated elements found. In the house mouse LTRs made up 30%, or 63.9 kb, of the DNA between the Th and Ins2 genes. The most common individual LTR was MYSERV-int: a murine-specific endogenous retroelement which alone made up 6%, or 7 kb, of the house mouse DNA segment, the MYSERV6-int element contributed an additional 6.1 kb. LTRs were also prominent in the squirrel-related clade, making up 450.7 kb of the DNA segment in the red squirrel.
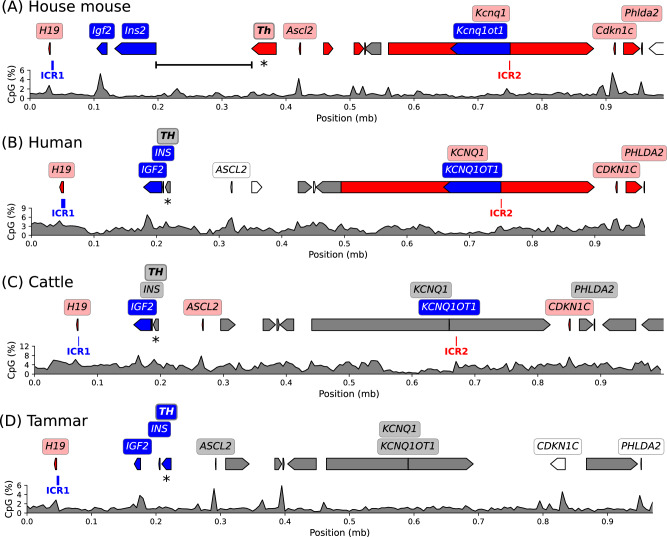
To put the mouse Th/Ins2 locus in the context of imprinting regulation, the gene positions were visualised to scale in the broader region (Fig. 2). In the house mouse the start site of the paternally-expressed Ins2 gene was closer to ICR1 while the start site of the maternally-expressed Th gene was positioned halfway (48.9% of the distance) between ICR1 and ICR2 (Fig. 2A). In humans and cattle, species for which ICR positions are known, the start site of TH gene was positioned closer to ICR1, less than a quarter of the way between ICR1 and ICR2 (Fig. 2B, C). Parent-of-origin-specific expression for genes within the ICR1/ICR2 region has been best characterised in the house mouse with the imprinting status of TH, and multiple other genes, unknown in other species. Here we find, below, that TH is paternally-expressed in the tammar wallaby; though no ICR2 has been found, the position of TH relative to ICR1 in tammar wallabies resembles humans and cattle (Fig. 2D).
Fig. 2. Parent-of-origin-specific expression in the broader ICR1/ICR2 imprinted region.
The genomic context of the orthologous TH/INS locus is shown for the (A) house mouse, (B) human, (C) cattle and (D) tammar wallaby. The colour of the gene indicates parental expression; blue is paternal, red is maternal, grey is unknown, white is biallelic. The paternally-methylated ICR1 position is indicated in blue, the maternally-methylated ICR2 position is noted in red. The TH gene is marked with an asterisk. The genomic distance between Th and Ins2 is indicated for the mouse (A). The KCNQ1OT1 position in cattle and tammar indicates 500 and 400 bp amplicons that have been detected for this transcript (Ager et al. 2007; Robbins et al. 2012). Plots below display CpG density as a percentage averaged over 5 kilobase (kb) windows, mb: million base pairs.
The tammar wallaby chimaeric TH-INS transcript is paternally-expressed
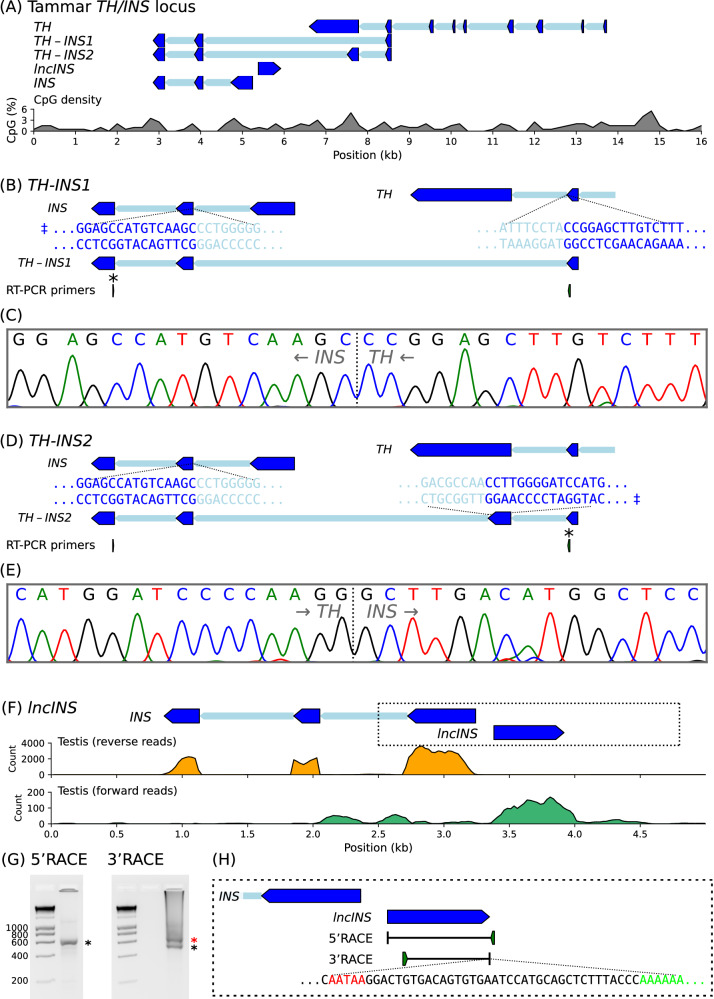
The close physical association between the TH and INS genes at the tammar wallaby locus (Fig. 3A) is exemplified by the production of a previously observed chimaeric TH-INS transcript (Stringer et al. 2012, 2014). RT-PCR, using an INS primer and a TH primer (Figs. 3B, D and 4A), resulted in two products that differed in size by 274 bp; these transcripts are referred to here as TH-INS1 (GenBank: PP646883) and TH-INS2 (GenBank: PP646884), where the latter includes part of the final exon of the TH gene. Analysis of the DNA sequence in the RT-PCR products showed the presence of a chimaeric junction between TH and INS sequences (Fig. 3C, E). When appropriate we refer to TH-INS1 and TH-INS2 collectively as TH-INS.
Fig. 3. Organisation of the tammar wallaby TH/INS locus.
A The tammar wallaby TH/INS gene locus showing exons as arrowheads indicating the direction of transcription, introns are thinner shaded regions. The blue colouring reflects the paternal expression pattern. Plot below displays CpG density as a percentage averaged over 200 bp windows. B, D Structure of the chimaeric TH-INS1 and TH-INS2 transcripts noting exonic (dark blue) and intronic (light blue) DNA sequences for the TH exon used and the second INS exon. An asterisk indicates the primer used for sequencing of the RT-PCR product and the double dagger indicates the sequence strand assessed. C, E Sanger sequencing chromatogram showing the junction, indicated by a dotted line, between the TH sequence and the INS sequence. Arrows indicate the direction of transcription. F Identification of an antisense transcript at the INS start site (dotted box) in testis transcriptome data with reads split into forward strand (green) and reverse strand (orange). Amplification of the antisense lncRNA was performed by (G) 5′ and 3′ RACE using adult testis cDNA. One of the two 3′RACE products (black asterisks) was isolated which encoded (H) a non-coding transcript with polyadenylation signal (red) and poly-A tail (green). The sequence of the larger 3′RACE product (red asterisks) was not confirmed.
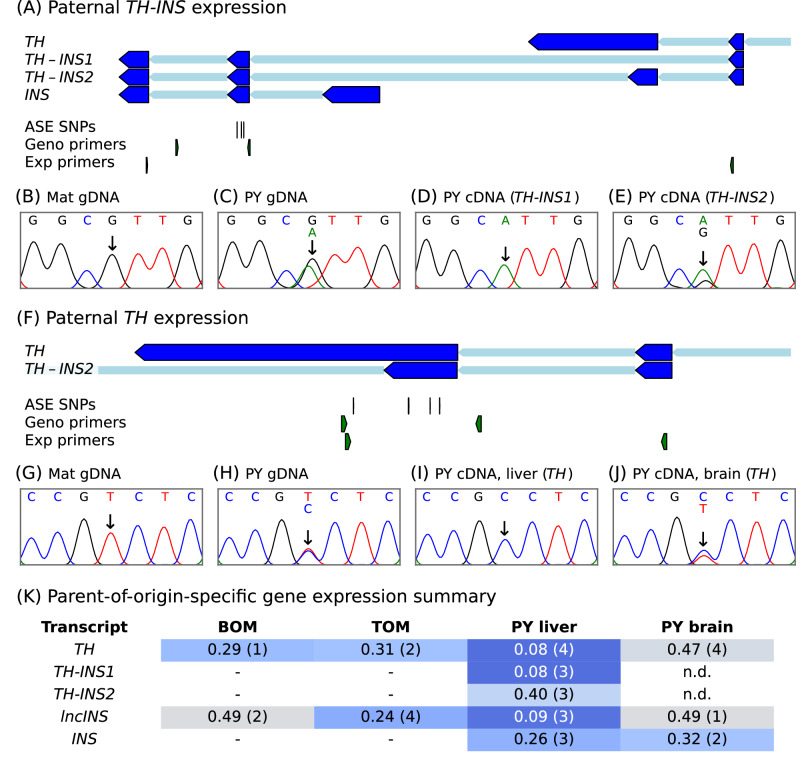
Fig. 4. Paternal transcription of TH/INS in the tammar wallaby.
A, F Primer and SNP positions for allele-specific expression (ASE) analysis of TH-INS and TH. The blue colour of the exons reflects the paternal expression pattern. The TH-INS genotyping primer set (Geno primers) had at least one intronic primer; the cDNA expression primer set (Exp primers) had an intron-spanning primer. B–E, G–J Parent-of-origin-specific transcription of TH-INS and TH. Sanger sequencing chromatograms showing the presence of (B, G) the homozygous maternal genotype, (C, H) the heterozygous SNP in PY gDNA, and (D, E, I, J) the allele present in PY cDNA. K Tabular summary of parent-of-origin-specific gene expression for the different RNA species detected from the TH/INS locus. A mean “mat:pat ratio” value closer to 0 indicates paternal expression (blue shading) while values closer to 0.5 indicate biallelic expression (grey shading). The number of animals is in brackets. “n.d.” indicates not detected. Placenta tissues taken from fetal stage samples, liver and brain taken from PY stage samples. BOM bilaminar omphalopleure, TOM trilaminar omphalopleure. See also, Supplementary Fig. 1 and Supplementary Table 3.
To test whether TH-INS showed allele-specific expression we genotyped 23 PY for which liver and brain tissue was available and identified three informative animals with SNPs in an INS exon (Fig. 4B). Transcription of TH-INS was tissue-specific, with the TH-INS1 and TH-INS2 transcripts detected in PY liver, but not brain tissue. Allele-specific expression analysis (Fig. 4A–E) showed monoallelic paternal transcription of TH-INS1 and skewed paternal transcription of TH-INS2 in the PY liver. TH-INS1 expression was from the paternal allele (Fig. 4K) in the three animals assessed, where the mat:pat ratio values were 0.01, 0.20 and 0.04. TH-INS2 expression was variable, in the three animals assessed the mat:pat ratios values were 0.18, 0.28 and 0.75. As we expected from previous studies (Agar et al. 2008, Stringer et al. 2012) the INS gene itself (Fig. 4L, Supplementary Fig. 1F–J) had paternally-skewed expression across three PY livers and had paternally-skewed expression in two PY brain samples (mat:pat ratio values 0.34 and 0.30).
lncINS: a new paternally-expressed long noncoding RNA antisense to INS
In eutherians, some imprinted genes are associated with long non-coding RNAs (lncRNAs): H19, Kcnq1ot1 and Airn (Wan and Bartolomei 2008). Since INS and the associated chimaeric transcript TH-INS1 showed paternal expression, it is possible that there is another lncRNA which regulates the coordinated imprinted expression, as seen in mouse Kcnq1ot1. In searching for additional elements relevant to the regulation of the INS locus, a new transcript was found (Fig. 3F–H). This 603 bp transcript (GenBank: PP646882) was discovered in a tammar wallaby adult testis transcriptome dataset (NCBI SRA: DRX012262). This RNA was transcribed from a position proximal to the INS start site in the antisense orientation (Fig. 3F). The Coding Potential Calculator 2 (Kang et al. 2017) gave this transcript a protein coding probability of less than 1%. LncRNAs are defined as RNAs longer than 200 nucleotides that are not translated into functional proteins (Statello et al. 2021), here this lncRNA is referred to as lncINS.
Genotyping for SNPs in lncINS identified six informative pre-natal animals of which four were assessed and three informative postnatal animals, out of 12 tested. Allele-specific expression analysis showed lncINS to be monoallelically expressed from the paternal allele in the PY liver (Fig. 4K, Supplementary Fig. 1K–S) with a mat:pat ratio of 0.09 ± 0.03 (n = 3 animals). In placenta lncINS expression was paternally-skewed in the TOM with a mat:pat ratio of 0.24 ± 0.04 (n = 4 animals) whilst in BOM expression was biallelic with a mat:pat ratio of 0.53 and 0.44 in n = 2 animals (Fig. 3K, Supplementary Fig. 1K–S). Parent-of-origin-specific expression of lncINS was particularly apparent in the TOM tissue of one fetus and the liver tissue of a PY, these animals both had five informative SNPs in lncINS that all were all preferentially expressed from the paternal allele (Supplementary Table 3). Further evidence for parent-of-origin-specific, but not allele-specific, expression of lncINS was illustrated by a C/T SNP site in which the T allele was maternally provided to a fetus that had skewed expression of the paternal C allele in the TOM and a PY that inherited a maternal C allele and expressed the paternal T allele in the liver (Supplementary Fig. 1L–S).
The tammar wallaby has paternal-expression of TH
Since INS, lncINS and TH-INS had paternal expression, it was possible that imprinting might extend to the marsupial TH gene. We examined the allele-specific expression of the TH gene which is maternally-expressed in mice (Golding et al. 2011; Jones et al. 2011; Okae et al. 2012; Bonthuis et al. 2015). Genotyping for SNPs in TH identified five informative fetuses of which two were assessed and four informative PYs. Allele-specific expression analysis showed TH was also paternally-expressed (Fig. 3F–K). Paternal transcription of TH was monoallelic in the PY liver (mat:pat ratio of 0.08 ± 0.08, n = 4 animals), and paternally-skewed in both BOM and TOM placental tissue (Supplementary Fig. 1A–E). Providing further evidence for parent-of-origin-specific expression of TH, one of the SNP sites (C/T) showed inheritance of a maternal C allele in one animal and inheritance of a maternal T allele in another animal with the paternal allele expressed in the PY liver in both cases (SNP loc. 11, Supplementary Table 3). In contrast, TH expression was biallelic in the PY brain, with a mat:pat ratio for the four animals being 0.39, 0.39, 0.47 and 0.62.
Discussion
The TH gene is expressed from the paternal allele in the tammar wallaby but from the maternal allele in the mouse. Changes in the parental origins of gene expression are rare. Of the 63 imprinted genes in common between human and mouse, differences in which allele is expressed have only been noted for Wilms’ tumour 1 (Wt1), bladder cancer-associated protein (Blcap), and zinc finger imprinted 2 (Zim2) (Tucci et al. 2019). Zim2 may be the best example of a change in the parental origins of transcription between species. In humans ZIM2 is paternally-expressed (Murphy et al. 2001), whereas in mice Zim2 is maternally-expressed (Kim et al. 2004) like TH. ZIM2 is located downstream of paternally expressed gene 3 (PEG3) at a similar distance in humans and in mice (Kim et al. 2004). So far no marsupial orthologue has been found for PEG3 or ZIM2 (Suzuki et al. 2011; Stringer et al. 2014). The change in the imprinting status of Zim2 in the mouse appears to have resulted from an insertional event that placed the Zim1 gene between Peg3 and Zim2 (Kim et al. 2004).
The large distance between mouse Th and Ins2 is not the ancestral state of mammals but instead reflects the unique evolutionary path of rodents. The mouse genome is 36.5% transposon-derived and has a high activity of transposable elements, particularly LINEs (Mouse Genome Sequencing Consortium 2002; Thybert et al. 2018). The activity of transposable elements has varied over time in the primate and rodent lineages, with Muridae showing a recent expansion of LINEs (Thybert et al. 2018). While LINEs are present in the expanded region between the rodent Th and Ins(2) genes, particularly in the jerboa, the majority of the DNA separating these genes in mice is made up of LTRs (Shirohzu et al. 2004; Lefebvre et al. 2009). Though the function of the prominent MYSERV6-int element in this region is unclear, this element is environmentally responsive, and is upregulated in fetal mouse testicular cells after exposure of pregnant mothers to the obesogen tributyltin (Shioda et al. 2022).
The repeat-rich region between Th and Ins2 in the mice may serve as a boundary between imprinted domains (Shirohzu et al. 2004). But, despite its size, this region does not pose a barrier to imprinting. In the mouse, the silencing effect of the Kcnq1ot1 lncRNA extends from ICR2 some 470 kb, across the genetic region separating Th and Ins2 (Jones and Lefebvre 2009). An isoform of Ins2, Ins2-006, is regulated by Kcnq1ot1 and maternally-expressed in the E13.5 embryonic mouse head from an alternative promoter 20 kb upstream of the main Ins2 gene (Jones et al. 2011). It is not known if Th-Ins2 chimaeric transcripts are produced in the mouse. If a barrier is present between ICR1 and ICR2 then in mice that barrier should be positioned close to Ins2, between the alternative promoter that drives expression from the maternal isoform and the main promoter from which the paternal Ins2 gene is transcribed (Golding et al. 2011).
Variation in the size of the DNA segment separating the INS and TH genes can have phenotypic consequences in humans. A polymorphic region comprising of a variable number of tandem repeats (VNTR) of a 14–15 nucleotide sequence is positioned upstream of the INS gene start site (Bennett and Todd 1996). The VNTR alleles occur in three size classes; class III (141–209 repeats) alleles are the largest and have a DNA segment at least 1 kb larger than the class I (26-63 repeats) alleles (Bennett and Todd 1996). In addition, a tetranucleotide microsatellite, TH01, is located within the TH gene and can affect gene expression (Berumen 2023). The class III allele is associated with protection against type 1 diabetes but susceptibility to type 2 diabetes when paternally derived (Bennett and Todd 1996; Huxtable et al. 2000). In the presence of the autoimmune regulator, AIRE, the class III VNTR locus drives expression of insulin at levels three-fold higher than the class I locus in thymic endothelial cells (Cai et al. 2011).
There are multiple modes of monoallelic expression (Reinius and Sandberg 2015) and we conclude that the tammar wallaby TH/INS gene locus has parent-of-origin-specific gene expression from the paternal allele. Paternal expression of the TH/INS region may result from differential methylation at the distal ICR1 site, the imprint associated with H19 and IGF2. The imprinting effect of ICR1 could extend to the TH/INS region. Binding of the insulator CTCF to the H19 DMR on the maternal allele changes the chromatin architecture such that IGF2, the gene adjacent to INS, is maternally-silenced (Smits et al. 2008; Hore et al. 2010; Llères et al. 2019). In mice, H19 and Igf2 are imprinted in both embryonic and extraembryonic tissues while paternal expression of Ins2 is restricted to the yolk sac (Deltour et al. 1995, 2004; Hudson et al. 2010).
We expanded the list of transcripts from the marsupial TH/INS locus to include TH, TH-INS1, TH-INS2, lncINS, and INS. Imprints are often associated with lncRNAs that can regulate gene expression through various mechanisms (Autuoro et al. 2014; Statello et al. 2021), the antisense lncRNA is typically expressed from a different parental origin but lncINS is expressed from the same parental origin as INS. The novel marsupial lncINS is not as long as other imprinted lncRNAs in eutherians, this was similar to the marsupial antisense lncRNA in the IGF2R DMR, ALID (Suzuki et al. 2018). The degree of paternal transcription was stronger in the PY liver, implying the locus had undergone broader maternal silencing in this tissue. In placental tissue and the PY brain, transcripts from the TH/INS locus showed a mix of biallelic and paternally skewed expression suggesting that regions of the domain are individually regulated.
The levels of transcript expression from the TH/INS locus were not assessed. The abundance of a transcript does not provide any indication of its functional importance, for example lncRNAs are important cellular regulators that are often found at low levels (Seiler et al. 2017; Grammatikakis 2022). The promiscuity of RNA polymerase can result in “spurious transcription”: a low background level of genome-wide transcription, even of the non-coding DNA (Jensen et al. 2013; Wade and Grainger 2018). The structure of the transcripts assessed from the TH/INS locus is much more organised than would be expected of spurious transcripts. Detection of known tissue-specific transcripts at low abundance in non-specific cells, without any likely role, has been referred to as “illegitimate transcription” (Chelly et al. 1989). Low transcript abundance could make allele-specific expression analysis more subject to gene-intrinsic noise (Kærn et al. 2005), but the transcripts from the TH/INS locus had consistent paternal monoallelic expression.
It is unlikely that the TH-INS transcripts are products of transcription termination failure. The chimaeric tammar TH-INS transcripts have a structure consistent with the most frequent form of cis-SAGe (cis-splicing of adjacent gene) chimaeras which are formed by the uninterrupted transcription of two nearby genes in the same orientation joining the second-to-last exon of the 5′ gene to the second exon of the 3′ gene (Chwalenia et al. 2017). Several mechanistic models have been proposed for how the transcriptional termination signal might be omitted from the 5′ gene, including; mutation of the polyA signal, torsional stress and “transcriptional slippage” (Chwalenia et al. 2017). The two parental genes that generate a fusion transcript typically have a small intergenic distance (Varley et al. 2014). The association of the TH and INS genes in many lineages may have been conserved to allow transcriptional read-through from TH to INS (Hernández-Sánchez et al. 2006).
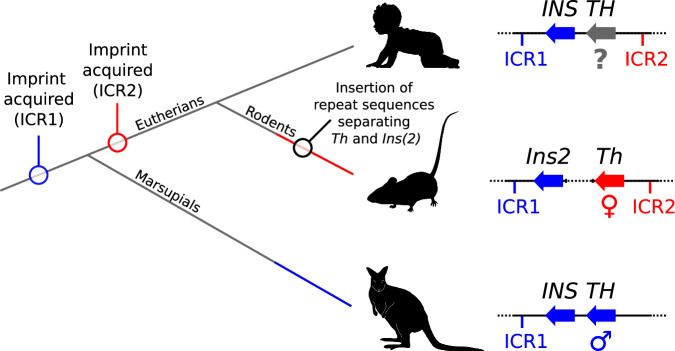
As the TH gene can vary between species in its parental origin of expression (Fig. 5), TH could be an “innocent bystander” to the effects of local gene regulation (Duvillié et al. 1998; Barlow and Bartolomei 2014). If the parental origin of TH expression correlated with the length of the DNA region between the TH and INS genes then all rodents that have inserted repeat sequences separating Th and Ins2 would have maternal expression of Th and all non-rodent therians that have a close physical association of the TH and INS genes would have paternal expression of TH. However, the imprinting status of the KCNQ1OT1 lncRNA is another important variable.
Fig. 5. The possible evolutionary path leading to a change in the parental origins of TH/Th expression.
(Left) Cladogram indicating the acquisition of ICR1 in the therian ancestor, acquisition of ICR2 in the eutherian ancestor and the separation of Th and Ins(2) in the rodent lineage. Maternal Th expression in the rodent lineage is indicated by the branch having a red colour. Paternal TH expression in the marsupial lineage is indicated by the branch having a blue colour. The unknown parent-of-origin-specific expression of TH in non-rodent eutherians and ancestral therians is indicated by the grey line colour. (Right) Simplified diagram of the TH/INS region highlighting the unknown parental origin of expression (grey) of TH in non-rodent eutherians, maternal expression (red) of Th in the mouse and paternal expression (blue) of TH in the wallaby. Also indicated is the acquisition of the paternally-methylated (blue) ICR1 and maternally-methylated (red) ICR2. The larger distance between Th and Ins2 in the mouse is indicated by a dotted line between the genes. Species silhouettes include Homo sapiens sapiens by Andrew A. Farke (CC BY 3.0), Murinae by Katy Lawler (CC BY 4.0) and Notamacropus eugenii by Geoff Shaw (CC BY 4.0), courtesy of https://www.phylopic.org.
Maternal expression of Th could be rodent-specific. Placenta-specific imprinted Th isoforms in the mouse are promoted from a rodent LTR located between Th and the adjacent achaete-scute complex homologue 2 gene, Ascl2 (Jones et al. 2011). Ascl2 is also maternally-expressed in the mouse placenta (Guillemot et al. 1995), but ASCL2 is biallelically-expressed in the human placenta from 12 to 39 weeks gestation (Miyamoto et al. 2002). A lack of imprinting at ASCL2 and other neighbouring genes, such as CD81, in humans could mean that the domain of paternal-silencing by KCNQ1OT1 is shorter in humans, relative to mice, potentially allowing for paternal expression of TH. However, mouse extraembryonic stem cells have maternal expression of Th and biallelic Ascl2, so it is possible for ASCL2 to be either included or excluded from repression of the paternal locus (Golding et al. 2011).
The TH and INS genes lie at the interface of two imprints implicated in developmental pathology. In humans, and other non-rodent therians, whether TH is imprinted is unknown and few studies of INS imprinting have been performed. Mice are commonly used to model the Beckwith–Wiedemann and Silver–Russell syndromes, but some genomic features in this region are specific to rodents. As the mouse and tammar wallaby express TH from different parental alleles, it would be informative to assess parent-of-origin-specific expression in non-rodent therians. Our findings raise the possibility that paternal expression of TH was the ancestral condition in therians (Fig. 5) with the parental origins of expression changing in either the eutherian lineage with acquisition of the maternally methylated ICR2 or more recently in the rodent lineage following the extensive insertion of repeat elements between TH and INS.
Supplementary information
Acknowledgements
The authors thank all members of the wallaby research group for helpful discussions and assistance with sampling. We also thank the Hore Lab (The University of Otago, New Zealand) for helpful discussions. The authors acknowledge the use of the services and facilities of the Australian Genome Research Facility. This study was supported by a Melbourne Research Scholarship and the Albert Shimmins postgraduate writing up award to TI and grants from the Australian Research Council to MBR, GS and Tim Hore.
Author contributions
TN, TI, GS, and MBR designed the research; TN, TI, GS, and MBR collected the samples; TN and TI performed experiments; TN and MBR wrote the paper with critical feedback from TI and GS.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Data availability
The analysed datasets are available, further information provided in Supplementary Table 1. Sequences for lncINS (PP646882), TH-INS1 (PP646883), TH-INS2 (PP646884) are available at GenBank (https://www.ncbi.nlm.nih.gov/genbank/).
Competing interests
The authors declare no competing interests.
Research Ethics
All tammar animal handling, husbandry and experimental sampling were in accordance with the National Health and Medical Research Council of Australia (2013) guidelines and approved by the University of Melbourne Animal Experimentation Ethics committees.
Footnotes
Associate editor: Louise Johnson.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41437-024-00689-y.
References
- Ager EI, Pask AJ, Gehring HM, Shaw G, Renfree MB. Evolution of the CDKN1C-KCNQ1 imprinted domain. BMC Evolut Biol. 2008;8:1–14. doi: 10.1186/1471-2148-8-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ager E, Suzuki S, Pask A, Shaw G, Ishino F, Renfree MB. Insulin is imprinted in the placenta of the marsupial, Macropus eugenii. Develop Biol. 2007;309:317–328. doi: 10.1016/j.ydbio.2007.07.025. [DOI] [PubMed] [Google Scholar]
- Autuoro JM, Pirnie SP, Carmichael GG. Long noncoding RNAs in imprinting and X chromosome inactivation. Biomol. 2014;4:76–100. doi: 10.3390/biom4010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow DP, Bartolomei MS. Genomic imprinting in mammals. Cold Spring Harb Perspect Biol. 2014;6:a018382–a018382. doi: 10.1101/cshperspect.a018382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomei MS, Ferguson-Smith AC. Mammalian genomic imprinting. Cold Spring Harb Perspect Biol. 2011;3:a002592. doi: 10.1101/cshperspect.a002592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett ST, Todd JA. Human type 1 diabetes and the insulin gene: principles of mapping polygenes. Annu Rev Genet. 1996;30:343–370. doi: 10.1146/annurev.genet.30.1.343. [DOI] [PubMed] [Google Scholar]
- Berumen J. Association of tyrosine hydroxylase 01 (TH01) microsatellite and insulin gene (INS) variable number of tandem repeat (VNTR) with type 2 diabetes and fasting insulin secretion in Mexican population. J Endocrinol Investig. 2023;1:13. doi: 10.1007/s40618-023-02175-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond DM, Ortega-Recalde O, Laird MK, Hayakawa T, Richardson KS, Reese FCB, et al. The admixed brushtail possum genome reveals invasion history in New Zealand and novel imprinted genes. Nat Commun. 2023;14:6364. doi: 10.1038/s41467-023-41784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthuis PJ, Huang W-C, Stacher Hörndli CN, Ferris E, Cheng T, Gregg C. Noncanonical genomic imprinting effects in offspring. Cell Rep. 2015;12:979–991. doi: 10.1016/j.celrep.2015.07.017. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E (2021) Placentation in the human and higher primates. In: Geisert RD, Spencer T (eds) Placentation in Mammals, Advances in Anatomy, Embryology and Cell Biology. Springer International Publishing Vol 234, pp 223–254. [DOI] [PubMed]
- Burton GJ, Jauniaux E. The human placenta: new perspectives on its formation and function during early pregnancy. Proc R Soc B Biol Sci. 2023;290:20230191. doi: 10.1098/rspb.2023.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai CQ, Zhang T, Breslin MB, Giraud M, Lan MS. Both Polymorphic Variable Number of Tandem Repeats and Autoimmune Regulator Modulate Differential Expression of Insulin in Human Thymic Epithelial Cells. Diabetes. 2011;60:336–344. doi: 10.2337/db10-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candy J, Collet C. Two tyrosine hydroxylase genes in teleosts. Biochimica et Biophysica Acta. 2005;1727:35–44. doi: 10.1016/j.bbaexp.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Cao W, Douglas KC, Samollow PB, VandeBerg JL, Wang X, Clark AG. Origin and evolution of marsupial-specific imprinting clusters through lineage-specific gene duplications and acquisition of promoter differential methylation (M Wilson, Ed.) Mol Biol Evol. 2023;40:msad022. doi: 10.1093/molbev/msad022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SJ, Steiner DF. Insulin through the ages: phylogeny of a growth promoting and metabolic regulatory hormone. Am Zool. 2000;40:213–222. [Google Scholar]
- Chang S, Bartolomei MS (2020) Modeling human epigenetic disorders in mice: Beckwith–Wiedemann syndrome and Silver–Russell syndrome. Dis Model Mech dmm.044123. [DOI] [PMC free article] [PubMed]
- Chelly J, Concordet J-P, Kaplan J-C, Kahn A. Illegitimate transcription: transcription of any gene in any cell type. Proc Natl Acad Sci. 1989;86:2617–2621. doi: 10.1073/pnas.86.8.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Hagen DE, Elsik CG, Ji T, Morris CJ, Moon LE, et al. Characterization of global loss of imprinting in fetal overgrowth syndrome induced by assisted reproduction. Proc Natl Acad Sci. 2015;112:4618–4623. doi: 10.1073/pnas.1422088112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa N, De Crescenzo A, Mishra K, Perone L, Carella M, Palumbo O, et al. The KCNQ1OT1 imprinting control region and non-coding RNA: new properties derived from the study of Beckwith–Wiedemann syndrome and Silver–Russell syndrome cases. Hum Mol Genet. 2012;21:10–25. doi: 10.1093/hmg/ddr419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwalenia K, Facemire L, Li H. Chimeric RNAs in cancer and normal physiology. WIREs RNA. 2017;8:e1427. doi: 10.1002/wrna.1427. [DOI] [PubMed] [Google Scholar]
- Cleaton MAM, Edwards CA, Ferguson-Smith AC. Phenotypic outcomes of imprinted gene models in mice: elucidation of pre- and postnatal functions of imprinted genes. Annu Rev Genom Hum Genet. 2014;15:93–126. doi: 10.1146/annurev-genom-091212-153441. [DOI] [PubMed] [Google Scholar]
- Coan PM, Burton GJ, Ferguson-Smith AC. Imprinted genes in the placenta—a review. Placenta. 2005;26:S10–S20. doi: 10.1016/j.placenta.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Collet C, Candy J, Sara V. Tyrosine hydroxylase and insulin‐like growth factor II but not insulin are adjacent in the teleost species barramundi, Lates calcarifer. Animal Genetics. 1998;29:30–33. doi: 10.1046/j.1365-2052.1998.00227.x. [DOI] [PubMed] [Google Scholar]
- Cooper DW, Vandeberg JL, Sharman GB, Poole WE. Phosphoglycerate kinase polymorphism in kangaroos provides further evidence for paternal X inactivation. Nat New Biol. 1971;230:155–157. doi: 10.1038/newbio230155a0. [DOI] [PubMed] [Google Scholar]
- De Pablo F, de la Rosa E. Proinsulin: from hormonal precursor to neuroprotective factor. Front Mol Neurosci. 2011;4:1–7. doi: 10.3389/fnmol.2011.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deltour L, Montagutelli X, Guenet J-L, Jami J, Páldi A. Tissue-and developmental stage-specific imprinting of the mouse proinsulin gene, Ins2. Dev Biol. 1995;168:686–688. doi: 10.1006/dbio.1995.1114. [DOI] [PubMed] [Google Scholar]
- Deltour L, Vandamme J, Jouvenot Y, Duvillié B, Kelemen K, Schaerly P, et al. Differential expression and imprinting status of Ins1 and Ins2 genes in extraembryonic tissues of laboratory mice. Gene Expr Patterns. 2004;5:297–300. doi: 10.1016/j.modgep.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Douglas KC, Wang X, Jasti M, Wolff A, VandeBerg JL, Clark AG, et al. Genome-wide histone state profiling of fibroblasts from the opossum, Monodelphis domestica, identifies the first marsupial-specific imprinted gene. BMC Genom. 2014;15:89. doi: 10.1186/1471-2164-15-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvillié B, Bucchini D, Tang T, Jami J, Pàldi A. Imprinting at the mouse Ins2 locus: evidence for cis- and trans-allelic interactions. Genomics. 1998;47:52–57. doi: 10.1006/geno.1997.5070. [DOI] [PubMed] [Google Scholar]
- Eggermann T, Eggermann K, Schönherr N. Growth retardation versus overgrowth: Silver-Russell syndrome is genetically opposite to Beckwith-Wiedemann syndrome. Trends Genet. 2008;24:195–204. doi: 10.1016/j.tig.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Freyer C, Zeller U, Renfree MB. The marsupial placenta: a phylogenetic analysis. J Exp Zool. 2003;299A:59–77. doi: 10.1002/jez.a.10291. [DOI] [PubMed] [Google Scholar]
- Giddings SJ, King CD, Harman KW, Flood JF, Carnaghi LR. Allele specific inactivation of insulin 1 and 2, in the mouse yolk sac, indicates imprinting. Nat Genet. 1994;6:310–313. doi: 10.1038/ng0394-310. [DOI] [PubMed] [Google Scholar]
- Golding MC, Magri LS, Zhang L, Lalone SA, Higgins MJ, Mann MRW. Depletion of Kcnq1ot1 non-coding RNA does not affect imprinting maintenance in stem cells. Development. 2011;138:3667–3678. doi: 10.1242/dev.057778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammatikakis I. Significance of lncRNA abundance to function. Mamm Genome. 2022;33:271–280. doi: 10.1007/s00335-021-09901-4. [DOI] [PubMed] [Google Scholar]
- Grant J, Mahadevaiah SK, Khil P, Sangrithi MN, Royo H, Duckworth J, et al. Rsx is a metatherian RNA with Xist-like properties in X-chromosome inactivation. Nature. 2012;487:254–258. doi: 10.1038/nature11171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B, Merchant J, Newgrain K. Milk consumption and energetics of growth in pouch young of the tammar wallaby, Macropus eugenii. Aust J Zool. 1988;36:217. doi: 10.1071/ZO9880217. [DOI] [Google Scholar]
- Guernsey MW, Chuong EB, Cornelis G, Renfree MB, Baker JC. Molecular conservation of marsupial and eutherian placentation and lactation. eLife. 2017;6:e27450. doi: 10.7554/eLife.27450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot F, Caspary T, Tilghman SM, Copeland NG, Gilbert DJ, Jenkins NA, et al. Genomic imprinting of Mash2, a mouse gene required for trophoblast development. Nat Genet. 1995;9:235–242. doi: 10.1038/ng0395-235. [DOI] [PubMed] [Google Scholar]
- Gulbis B. Protein and enzyme patterns in the fluid cavities of the first trimester gestational sac: relevance to the absorptive role of secondary yolk sac. Mol Hum Reprod. 1998;4:857–862. doi: 10.1093/molehr/4.9.857. [DOI] [PubMed] [Google Scholar]
- Haig D. Genomic imprinting and kinship: how good is the evidence? Annu Rev Genet. 2004;38:553–585. doi: 10.1146/annurev.genet.37.110801.142741. [DOI] [PubMed] [Google Scholar]
- Hanin G, Ferguson-Smith AC. The evolution of genomic imprinting: epigenetic control of mammary gland development and postnatal resource control. WIREs Syst Biol Med. 2020;12:e1476. doi: 10.1002/wsbm.1476. [DOI] [PubMed] [Google Scholar]
- Hernández-Sánchez C, Bártulos Ó, Valenciano AI, Mansilla A, de Pablo F. The regulated expression of chimeric tyrosine hydroxylase–insulin transcripts during early development. Nucleic Acids Res. 2006;34:3455–3464. doi: 10.1093/nar/gkl436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickford D, Frankenberg S, Renfree MB. The tammar wallaby, Macropus eugenii: a model kangaroo for the study of developmental and reproductive biology. Cold Spring Harb Protoc. 2009;2009:pdb-emo137. doi: 10.1101/pdb.emo137. [DOI] [PubMed] [Google Scholar]
- Hore TA, Renfree MB, Pask AJ, Graves JAM. The evolution of genomic imprinting—a marsupial perspective. In: Deakin JE, Waters PD, Marshall Graves JA, editors. Marsupial Genetics and Genomics. Dordrecht: Springer Netherlands; 2010. pp. 233–257. [Google Scholar]
- Huang T-C, Chang K-C, Chang J-Y, Tsai Y-S, Yang Y-J, Chang W-C, et al. Variants in maternal effect genes and relaxed imprinting control in a special placental mesenchymal dysplasia case with mild trophoblast hyperplasia. Biomedicines. 2021;9:544. doi: 10.3390/biomedicines9050544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson QJ, Kulinski TM, Huetter SP, Barlow DP. Genomic imprinting mechanisms in embryonic and extraembryonic mouse tissues. Heredity. 2010;105:45–56. doi: 10.1038/hdy.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta-Cepas J, Serra F, Bork P. ETE 3: reconstruction, analysis, and visualization of phylogenomic data. Mol Biol Evolution. 2016;33:1635–1638. doi: 10.1093/molbev/msw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable SJ, Saker PJ, Haddad L, Walker M, Frayling TM, Levy JC, et al. Analysis of parent-offspring trios provides evidence for linkage and association between the insulin gene and type 2 diabetes mediated exclusively through paternally transmitted class III variable number tandem repeat alleles. Diabetes. 2000;49:126–130. doi: 10.2337/diabetes.49.1.126. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Griffith OW, Suzuki S, Renfree MB. Placental imprinting of SLC22A3 in the IGF2R imprinted domain is conserved in therian mammals. Epigenetics Chromatin. 2022;15:32. doi: 10.1186/s13072-022-00465-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara T, Griffith OW, Tarulli GA, Renfree MB. Male germline development in the tammar wallaby, Macropus eugenii. Reproduction. 2021;161:333–341. doi: 10.1530/REP-20-0634. [DOI] [PubMed] [Google Scholar]
- Jacob K, Robinson W, Lefebvre L. Beckwith–Wiedemann and Silver–Russell syndromes: opposite developmental imbalances in imprinted regulators of placental function and embryonic growth: Beckwith-Wiedemann and Silver-Russell syndromes. Clin Genet. 2013;84:326–334. doi: 10.1111/cge.12143. [DOI] [PubMed] [Google Scholar]
- Jensen TH, Jacquier A, Libri D (2013) Dealing with pervasive transcription. Mol Cell 473–484 [DOI] [PubMed]
- John RM. Imprinted genes and the manipulation of parenting in mammals. Nat Rev Genet. 2023;24:783–796. doi: 10.1038/s41576-023-00644-3. [DOI] [PubMed] [Google Scholar]
- Jones MJ, Bogutz AB, Lefebvre L. An extended domain of Kcnq1ot1 silencing revealed by an imprinted fluorescent reporter. Mol Cell Biol. 2011;31:2827–2837. doi: 10.1128/MCB.01435-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MJ, Lefebvre L. An imprinted GFP insertion reveals long-range epigenetic regulation in embryonic lineages. Dev Biol. 2009;336:42–52. doi: 10.1016/j.ydbio.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan AM, Bartolomei MS. Evolving imprinting control regions: KRAB zinc fingers hold the key. Genes Dev. 2019;33:1–3. doi: 10.1101/gad.322990.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kærn M, Elston TC, Blake WJ, Collins JJ. Stochasticity in gene expression: from theories to phenotypes. Nat Rev Genet. 2005;6:451–464. doi: 10.1038/nrg1615. [DOI] [PubMed] [Google Scholar]
- Kang Y-J, Yang D-C, Kong L, Hou M, Meng Y-Q, Wei L, et al. CPC2: a fast and accurate coding potential calculator based on sequence intrinsic features. Nucleic Acids Res. 2017;45:W12–W16. doi: 10.1093/nar/gkx428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D (2019) Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol 37. [DOI] [PMC free article] [PubMed]
- Kim J, Bergmann A, Lucas S, Stone R, Stubbs L. Lineage-specific imprinting and evolution of the zinc-finger gene ZIM2. Genomics. 2004;84:47–58. doi: 10.1016/j.ygeno.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Kumar S, Suleski M, Craig JM, Kasprowicz AE, Sanderford M, Li M, et al. TimeTree 5: an expanded resource for species divergence times. Mol Biol Evol. 2022;39:msac174. doi: 10.1093/molbev/msac174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JT, Bartolomei MS. X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell. 2013;152:1308–1323. doi: 10.1016/j.cell.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Lefebvre L. The placental imprintome and imprinted gene function in the trophoblast glycogen cell lineage. Reprod BioMed Online. 2012;25:44–57. doi: 10.1016/j.rbmo.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre L, Mar L, Bogutz A, Oh-McGinnis R, Mandegar MA, Paderova J, et al. The interval between Ins2 and Ascl2 is dispensable for imprinting centre function in the murine Beckwith–Wiedemann region. Hum Mol Genet. 2009;18:4255–4267. doi: 10.1093/hmg/ddp379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton PA, Ingram RS, Eggenschwiler J, Efstratiadis A, Tilghman SM. Disruption of imprinting caused by deletion of the H19 gene region in mice. Nature. 1995;375:34–39. doi: 10.1038/375034a0. [DOI] [PubMed] [Google Scholar]
- Lelou E, Corlu A, Nesseler N, Rauch C, Mallédant Y, Seguin P, et al. The role of catecholamines in pathophysiological liver processes. Cells. 2022;11:1021. doi: 10.3390/cells11061021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llères D, Moindrot B, Pathak R, Piras V, Matelot M, Pignard B, et al. CTCF modulates allele-specific sub-TAD organization and imprinted gene activity at the mouse Dlk1-Dio3 and Igf2-H19 domains. Genome Biol. 2019;20:272. doi: 10.1186/s13059-019-1896-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevaiah SK, Sangrithi MN, Hirota T, Turner JMA. A single-cell transcriptome atlas of marsupial embryogenesis and X inactivation. Nature. 2020;586:612–617. doi: 10.1038/s41586-020-2629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Hasuike S, Jinno Y, Soejima H, Yun K, Miura K, et al. The human ASCL2 gene escaping genomic imprinting and its expression pattern. J Assist Reprod Genet. 2002;19:240–244. doi: 10.1023/A:1015362903486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GE, Abu-Amero SN, Bell G, Wakeling EL, Kingsnorth A, Stanier P, et al. Evidence that insulin is imprinted in the human yolk sac. Diabetes. 2001;50:199–203. doi: 10.2337/diabetes.50.1.199. [DOI] [PubMed] [Google Scholar]
- Morison IM, Ramsay JP, Spencer HG. A census of mammalian imprinting. Trends Genet. 2005;21:457–465. doi: 10.1016/j.tig.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Mossman HW. Comparative morphogenesis of the fetal membranes and accessory uterine structures. Contrib Embryol Carne Instn. 1937;26:129–246. doi: 10.1016/0143-4004(91)90504-9. [DOI] [PubMed] [Google Scholar]
- Mouse Genome Sequencing Consortium Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- Murphy SK, Wylie AA, Jirtle RL. Imprinting of PEG3, the human homologue of a mouse gene involved in nurturing behavior. Genomics. 2001;71:110–117. doi: 10.1006/geno.2000.6419. [DOI] [PubMed] [Google Scholar]
- National Health and Medical Research Council (Australia) (2013) Australian code for the care and use of animals for scientific purposes, 8th edn. Government Printer, Canberra, ACT Australia
- Nativio R, Sparago A, Ito Y, Weksberg R, Riccio A, Murrell A. Disruption of genomic neighbourhood at the imprinted IGF2-H19 locus in Beckwith–Wiedemann syndrome and Silver–Russell syndrome. Hum Mol Genet. 2011;20:1363–1374. doi: 10.1093/hmg/ddr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveh NSS, Deegan DF, Huhn J, Traxler E, Lan Y, Weksberg R, et al. The role of CTCF in the organization of the centromeric 11p15 imprinted domain interactome. Nucleic Acids Res. 2021;49:6315–6330. doi: 10.1093/nar/gkab475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh R, Ho R, Mar L, Gertsenstein M, Paderova J, Hsien J, et al. Epigenetic and phenotypic consequences of a truncation disrupting the imprinted domain on distal mouse chromosome 7. Mol Cell Biol. 2008;28:1092–1103. doi: 10.1128/MCB.01019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okae H, Hiura H, Nishida Y, Funayama R, Tanaka S, Chiba H, et al. Re-investigation and RNA sequencing-based identification of genes with placenta-specific imprinted expression. Hum Mol Genet. 2012;21:548–558. doi: 10.1093/hmg/ddr488. [DOI] [PubMed] [Google Scholar]
- Patton SJ, Luke GN, Holland P. Complex history of a chromosomal paralogy region: insights from amphioxus aromatic amino acid hydroxylase genes and insulin-related genes. Mol Biol Evol. 1998;15:1373–1380. doi: 10.1093/oxfordjournals.molbev.a025865. [DOI] [PubMed] [Google Scholar]
- Pugliese A, Miceli D. The insulin gene in diabetes. Diabetes/Metab Res Rev. 2002;18:13–25. doi: 10.1002/dmrr.261. [DOI] [PubMed] [Google Scholar]
- Pugliese A, Zeller M, Fernandez A, Jr, Ricordi C, Pietropaolo M, Eisenbarth G, et al. The insulin gene is transcribed in the human thymus and transcription levels correlate with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet. 1997;15:293–297. doi: 10.1038/ng0397-293. [DOI] [PubMed] [Google Scholar]
- Reinius B, Sandberg R. Random monoallelic expression of autosomal genes: stochastic transcription and allele-level regulation. Nat Rev Genet. 2015;16:653–664. doi: 10.1038/nrg3888. [DOI] [PubMed] [Google Scholar]
- Renfree MB. The composition of fetal fluids of the marsupial Macropus eugenii. Dev Biol. 1973;33:62–79. doi: 10.1016/0012-1606(73)90165-6. [DOI] [PubMed] [Google Scholar]
- Renfree MB. Marsupials: placental mammals with a difference. Placenta. 2010;31:S21–S26. doi: 10.1016/j.placenta.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Renfree MB, Ager EI, Shaw G, Pask AJ. Genomic imprinting in marsupial placentation. Reproduction. 2008;136:523–531. doi: 10.1530/REP-08-0264. [DOI] [PubMed] [Google Scholar]
- Renfree MB, Holt AB, Green SW, Carr JP, Cheek DB. Ontogeny of the brain in a marsupial (Macropus eugenii) throughout pouch life. Brain Behav Evol. 1982;20:57–71. doi: 10.1159/000121581. [DOI] [PubMed] [Google Scholar]
- Robbins KM, Chen Z, Wells KD, Rivera RM. Expression of KCNQ1OT1, CDKN1C, H19, and PLAGL1 and the methylation patterns at the KvDMR1 and H19/IGF2 imprinting control regions is conserved between human and bovine. J Biomed Sci. 2012;19:1–10. doi: 10.1186/1423-0127-19-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C, Boroviak TE. Origin and function of the yolk sac in primate embryogenesis. Nat Commun. 2020;11:3760. doi: 10.1038/s41467-020-17575-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotwein P. The complex genetics of human insulin-like growth factor 2 are not reflected in public databases. J Biol Chem. 2018;293:4324–4333. doi: 10.1074/jbc.RA117.001573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler J, Breinig M, Caudron-Herger M, Polycarpou-Schwarz M, Boutros M, Diederichs S. The lncRNA VELUCT strongly regulates viability of lung cancer cells despite its extremely low abundance. Nucleic Acids Res. 2017;45:5458–5469. doi: 10.1093/nar/gkx076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharman GB. Late DNA replication in the paternally derived X chromosome of female kangaroos. Nature. 1971;230:231–232. doi: 10.1038/230231a0. [DOI] [PubMed] [Google Scholar]
- Shiao M-S, Liao B-Y, Long M, Yu H-T. Adaptive evolution of the insulin two-gene system in mouse. Genetics. 2008;178:1683–1691. doi: 10.1534/genetics.108.087023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda K, Odajima J, Blumberg B, Shioda T. Transgenerational transcriptomic and DNA methylome profiling of mouse fetal testicular germline and somatic cells after exposure of pregnant mothers to tributyltin, a potent obesogen. Metabolites. 2022;12:95. doi: 10.3390/metabo12020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirohzu H, Yokomine T, Sato C, Kato R, Toyoda A, Purbowasito W, et al. A 210-kb segment of tandem repeats and retroelements located between imprinted subdomains of mouse distal chromosome 7. DNA Res. 2004;11:325–334. doi: 10.1093/dnares/11.5.325. [DOI] [PubMed] [Google Scholar]
- Smits G, Mungall AJ, Griffiths-Jones S, Smith P, Beury D, Matthews L, et al. Conservation of the H19 noncoding RNA and H19-IGF2 imprinting mechanism in therians. Nat Genet. 2008;40:971–976. doi: 10.1038/ng.168. [DOI] [PubMed] [Google Scholar]
- Sparago A, Cerrato F, Vernucci M, Ferrero GB, Silengo MC, Riccio A. Microdeletions in the human H19 DMR result in loss of IGF2 imprinting and Beckwith-Wiedemann syndrome. Nat Genet. 2004;36:958–960. doi: 10.1038/ng1410. [DOI] [PubMed] [Google Scholar]
- Spencer HG, Clark AG. Non-conflict theories for the evolution of genomic imprinting. Heredity. 2014;113:112–118. doi: 10.1038/hdy.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statello L, Guo C-J, Chen L-L, Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22:96–118. doi: 10.1038/s41580-020-00315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer JM, Pask AJ, Shaw G, Renfree MB. Postnatal imprinting: evidence from marsupials. Heredity. 2014;113:145–155. doi: 10.1038/hdy.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer JM, Suzuki S, Pask AJ, Shaw G, Renfree MB. Selected imprinting of INS in the marsupial. Epigenet Chromatin. 2012;5:14. doi: 10.1186/1756-8935-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Ono R, Narita T, Pask AJ, Shaw G, Wang C, et al. Retrotransposon silencing by DNA methylation can drive mammalian genomic imprinting. PLOS Genet. 2007;3:e55. doi: 10.1371/journal.pgen.0030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Renfree MB, Pask AJ, Shaw G, Kobayashi S, Kohda T, et al. Genomic imprinting of IGF2, p57KIP2 and PEG1/MEST in a marsupial, the tammar wallaby. Mech Dev. 2005;122:213–222. doi: 10.1016/j.mod.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Shaw G, Kaneko-Ishino T, Ishino F, Renfree MB. Characterisation of marsupial PHLDA2 reveals eutherian specific acquisition of imprinting. BMC Evolut Biol. 2011;11:244. doi: 10.1186/1471-2148-11-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Shaw G, Renfree MB. Identification of a novel antisense noncoding RNA, ALID, transcribed from the putative imprinting control region of marsupial IGF2R. Epigenet Chromatin. 2018;11:55. doi: 10.1186/s13072-018-0227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarailo‐Graovac M, Chen N (2009) Using RepeatMasker to identify repetitive elements in genomic sequences. Curr Protoc Bioinform 25 [DOI] [PubMed]
- Thybert D, Roller M, Navarro FCP, Fiddes I, Streeter I, Feig C, et al. Repeat associated mechanisms of genome evolution and function revealed by the Mus caroli and Mus pahari genomes. Genome Res. 2018;28:448–459. doi: 10.1101/gr.234096.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott JF, Simpson KJ, Moyle RLC, Hearn CM, Shaw G, Nicholas KR, et al. Maternal regulation of milk composition, milk production, and pouch young development during lactation in the tammar wallaby (Macropus eugenii) Biol Reprod. 2003;68:929–936. doi: 10.1095/biolreprod.102.005934. [DOI] [PubMed] [Google Scholar]
- Tucci V, Isles AR, Kelsey G, Ferguson-Smith AC, Bartolomei MS, Benvenisty N, et al. Genomic imprinting and physiological processes in mammals. Cell. 2019;176:952–965. doi: 10.1016/j.cell.2019.01.043. [DOI] [PubMed] [Google Scholar]
- Tyndale-Biscoe H, Renfree M (1987) Reproductive physiology of marsupials. Cambridge University Press
- Varley KE, Gertz J, Roberts BS, Davis NS, Bowling KM, Kirby MK, et al. Recurrent read-through fusion transcripts in breast cancer. Breast Cancer Res Treatment. 2014;146:287–297. doi: 10.1007/s10549-014-3019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade JT, Grainger DC. Spurious transcription and its impact on cell function. Transcription. 2018;9:182–189. doi: 10.1080/21541264.2017.1381794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Bartolomei MS. Regulation of imprinting in clusters: noncoding RNAs versus insulators. Adv Genet. 2008;61:207–223. doi: 10.1016/S0065-2660(07)00007-7. [DOI] [PubMed] [Google Scholar]
- Wang KH, Kupa J, Duffy KA, Kalish JM. Diagnosis and management of Beckwith-Wiedemann syndrome. Front Pediatr. 2020;7:562. doi: 10.3389/fped.2019.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Li D, Zhang M, Yang W, Cui Y, Li S. Methylation of KvDMR1 involved in regulating the imprinting of CDKN1C gene in cattle. Anim Genet. 2015;46:354–360. doi: 10.1111/age.12297. [DOI] [PubMed] [Google Scholar]
- Weksberg R, Shuman C, Beckwith JB. Beckwith–Wiedemann syndrome. Eur J Hum Genet. 2010;18:8–14. doi: 10.1038/ejhg.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmann HA, Kirchner T, Ranke MB, Enders H, Preece MA. Growth and symptoms in Silver-Russell syndrome: review on the basis of 386 patients. Eur J Pediatr. 1995;154:958–968. doi: 10.1007/BF01958638. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Ruuskanen JO, Wullimann MF, Vernier P. Two tyrosine hydroxylase genes in vertebrates: new dopaminergic territories revealed in the zebrafish brain. Mol Cell Neurosci. 2010;43:394–402. doi: 10.1016/j.mcn.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Zulkower V, Rosser S. DNA Features Viewer: a sequence annotation formatting and plotting library for Python. Bioinformatics. 2020;36:4350–4352. doi: 10.1093/bioinformatics/btaa213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The analysed datasets are available, further information provided in Supplementary Table 1. Sequences for lncINS (PP646882), TH-INS1 (PP646883), TH-INS2 (PP646884) are available at GenBank (https://www.ncbi.nlm.nih.gov/genbank/).