Abstract
Integrase (IN) is a key component of the preintegration nucleoprotein complex (PIC), which transports the retroviral genome from the cytoplasm to the nucleus of newly infected cells. Retroviral IN proteins have intrinsic karyophilic properties, which for human immunodeficiency virus type 1 (HIV-1) are currently attributed to regions that display sequence homology to previously characterized nuclear localization signals. We asked here whether the karyophilic properties of HIV-1 IN are involved in the nuclear import of PIC. We mutated three conserved basic regions in the C-terminal domain of IN and analyzed the effects of mutations on subcellular localization of the protein, viral particle composition, IN dimerization within virions, and infectivity. Alteration of two sequences caused the loss of nuclear accumulation of IN and drastically reduced the capacity of the protein to multimerize. Mutation of the most C-terminal sequence had no effect on the subcellular localization and dimerization of IN. Nevertheless, conservation of all three sequences was required for viral infectivity. Despite the perturbation of IN subcellular localization, all mutant viruses displayed normal reverse transcription and nuclear transport of PICs in newly infected cells. The replicative defect was instead at the level of integration, for which all mutants were markedly affected in vivo. Besides reinforcing the association between dimerization of IN and nuclear accumulation of the enzyme, our data demonstrate that subcellular localization of IN alone cannot predict the fate of the PICs.
The retroviral integrase (IN) protein catalyzes integration of the provirus into a chromosome of the infected cell, an essential step of the viral replication cycle (see references 1 and 4 for recent reviews on integration). IN is translated as part of the Gag-Pol precursor molecule, which is cleaved by the viral protease to allow viral particle maturation. Several in vitro studies have examined the biochemical properties of IN, and significant progress has been made in the understanding of its structure and of the mechanism of the integration reaction (1, 4, 15, 31). IN catalyzes the two steps of the integration process. The first step consists of the elimination of 2 nucleotides from each 3′ end of the proviral DNA. In the second step, the resulting 3′-OH ends of the viral DNA are covalently joined to newly created 5′ ends in the target DNA (11, 18, 39).
Retroviral IN proteins are composed of three functionally distinct domains (see Fig. 1A), all of which are required for a complete integration reaction. The N-terminal domain contains a zinc finger-like motif that stabilizes the folded structure of IN and enhances the catalytic activity of the enzyme (57). The core domain of retroviral IN contains the DDE motif to which the catalytic activity is attributed. This central domain is also involved in the recognition of the conserved nucleotide sequence at each end of the retroviral DNA. The carboxy-terminal domain is the least conserved among retroviruses, possesses intrinsic DNA binding activity, and is required for 3′-end processing and strand transfer (10, 51). The functional form of IN is multimeric, as was suggested by in vitro evidence of multimerization and demonstrated by trans-complementation of different IN mutants (16, 23, 34–36, 50). We recently demonstrated that the multimerization of human immunodeficiency virus type 1 (HIV-1) IN takes place in infectious viral particles and is dependent on disulfide bond formation (46).
FIG. 1.
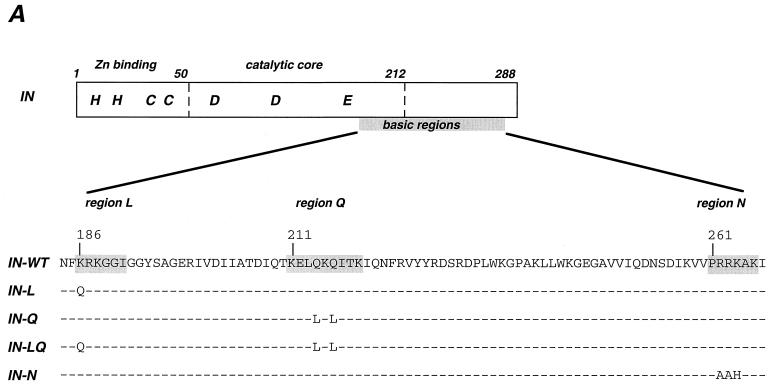
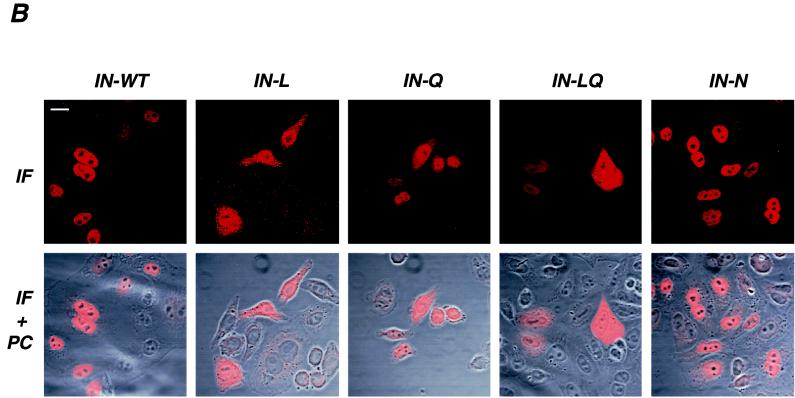
Schematic representation and intracellular localization of WT and mutant IN. (A) Schematic representation of HIV-1 IN domains and conserved basic regions that were altered by mutagenesis. Numbers correspond to amino acid position of the IN domain of the BRU viral clone. The L, Q, and N sequences were described previously as being conserved in lentiviruses (12). (B) Confocal microscopy analysis of WT and mutant (L, Q, LQ, and N) HIV-1 IN molecules. HeLa cells were transfected with the Flag-INT plasmid expressing the WT or mutant IN fused to the Flag epitope. Transfected cells were fixed, permeabilized, and stained with anti-Flag antibodies. Series of optical sections at 0.7-μm intervals were recorded. A representative medial section is shown in panels marked IF (immunofluorescence). Panels IF+PC represent a superimposition of IF staining and a phase-contrast image of the same field. Bar, 15 μm.
Besides the well characterized role in the integration process, IN participates in different steps of the virus cycle. Alterations of IN sequence were found to affect viral particle morphogenesis, reverse transcription, and nuclear import of the preintegration complex (PIC) (17, 28, 55), a nucleoprotein complex composed of viral and probably cellular proteins that carries the viral genome from the cytoplasm to the nucleus of the newly infected cell (9, 20, 21, 37, 38, 45).
HIV-1 IN has karyophilic properties which were demonstrated by the nuclear accumulation of this protein both after transient expression of a Flag- or green fluorescent protein-tagged IN (46, 47) and after microinjection of HIV-1 IN fused to glutathione S-transferase (GST) (28). The GST-IN fusion protein was additionally shown to bind in vitro to karyopherin-α (28), a cellular mediator of nuclear transport which is specific for nuclear localization signal-bearing proteins. The interaction between IN and karyopherin-α was suggested to be functionally relevant in vivo, providing one additional mechanism for the nuclear import of HIV-1 PIC (28). Participation of IN in this process would be partially redundant with the functions proposed for the viral proteins matrix and Vpr, which were suggested to be implicated in the active transport of the PICs to the nuclei of resting cells (8, 24, 28, 29, 32, 52), although some of these reports remain disputed (5, 25, 27, 40). Besides viral proteins, a cis-acting viral DNA structure, the DNA flap, generated during lentivirus-specific reverse transcription, was recently described as also playing an important role in the nuclear import of the HIV-1 genome (56). Several sequences within HIV-1 IN were pointed out as possible nuclear localization signals for their high content of basic residues (28). Three such motifs (186KRK, 211KELQKQITK, and 261PRRKAK) mapped in the C-terminal domain of HIV-1 IN, deletion of which resulted in the loss of interaction with karyopherin-α (28). These sequences were previously highlighted as being conserved in lentiviruses, leading to the suggestion that they may also be responsible for the ability of these viruses to infect interphasic cells (12). The 186KRK tripeptide is part of a lentivirus-specific exposed loop, called sequence L, which is absent in other retroviruses (12, 15). The 211KELQKQITK motif falls within the so-called glutamine-rich basic region of lentiviruses (sequence Q), corresponding to the proline-rich region of oncoviruses (12). Finally, the 261PRRKAK sequence is part of the N region, for which the lentivirus consensus sequence clearly differs from the oncovirus motif (12). It is important to note, however that avian sarcoma virus IN was shown to readily accumulate in the nucleus of transfected cells (41), although this virus cannot infect nondividing cells.
In the present study we asked whether the karyophilic properties of HIV-1 IN are required for nuclear import of PICs. We analyzed the effect of mutations in the three above-described sequences both on the karyophilic properties of HIV-1 IN expressed in the absence of other viral proteins and on viral replication, with particular interest in the nuclear import of viral PICs. Alteration of the sequences L and Q separately or in combination resulted in the loss of nuclear accumulation of HIV-1 IN, while wild-type and mutant IN molecules carrying substitutions in the IN motif concentrated in the nucleus of transfected cells. Nevertheless, alteration of each of the three sequences resulted in the loss of viral replication. The inability of the L- and Q-mutated IN proteins to accumulate in the nucleus was associated with a markedly reduced capacity to dimerize in viral particles. Most interestingly, for all four virus mutants the loss of replicative capacity was due not to a deficient nuclear import of viral DNA but to a defect in the integration process in vivo.
MATERIALS AND METHODS
Plasmid construction.
The Flag-INT vector, which allows the expression of HIV-1 integrase fused to the Flag epitope at its N terminus, has been described previously (46). Mutagenesis was performed using the Quick-Change mutagenesis kit (Stratagene). BRU-HA and BRU-Flag infectious molecular clones of HIV-1, which carry an epitope-tagged IN (HA and Flag, respectively), have been described previously (46). To obtain the BRU, BRU-HA, and BRU-Flag full-length clones carrying mutated IN, the BspMI fragment from the mutated Flag-INT vectors was used to replace the corresponding sequence of the three clones. Constructions were confirmed by DNA sequencing of the entire PCR-amplified fragment.
Cells, virus infection, and reagents.
Human epithelial HeLa, P4 (HeLa-CD4+, LTR-LacZ) (13), and P4p56 (HeLa CD4+, p56lck+, LTR-LacZ) cells were grown in Dulbecco's modified Eagle's medium (Gibco) supplemented with glutamine, antibiotics, and 10% fetal calf serum. P4 cells were grown in the presence of G418 (1 mg/ml), and P4p56 cell medium was supplemented with G418 and hygromycin (100 μg/ml). P4p56 cells were used for measuring viral DNA synthesis and integration, since they express higher levels of surface CD4 than do P4 cells and are more susceptible to HIV infection. MT4 lymphoid cells were grown in RPMI medium supplemented with glutamine, antibiotics, and 10% fetal calf serum. Viruses were produced by transfection with the plasmids as described previously (49). Supernatants were analyzed for HIV-1 p24 antigen content by an enzyme-linked immunosorbent assay (Dupont). P4 cells, plated in 96-well plates, were infected with different viral doses. Infectivity was measured as previously described (43). The anti-gag monoclonal antibodies (MAb) 25A (anti-CA) and 18A (anti-MA) were a kind gift from François Traincart (Institut Pasteur, Paris, France). The rabbit polyclonal anti-Flag antibodies were from Zymed Laboratories (San Francisco, Calif.), and the mouse anti-Flag MAb M2 was from Sigma. Cy3-conjugated anti-mouse immunoglobulin G (heavy plus light chains) and peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin G were from Amersham Life Science.
Indirect immunofluorescence staining.
HeLa cells (2 × 105) were spread on glass coverslips in 24-well plates, transfected with the indicated plasmids, and stained for immunofluorescence 24 to 40 h later. The cells were fixed in 3.7% formaldehyde–phosphate-buffered saline (PBS) for 20 min, washed three times in PBS, and incubated for 10 min in 50 mM NH4Cl to quench free aldehydes. After one wash in PBS and a 15-min incubation in permeabilization buffer (0.05% saponin, 0.01% Triton X-100, and 2% bovine serum albumin in PBS), cells were incubated for 1 h with the first Ab (M2 anti-Flag MAb) at 7.5 μg/ml in permeabilization buffer. The cells were then washed three times in permeabilization buffer and incubated with Cy3-conjugated anti-mouse Abs (Amersham) at a final dilution of 1:200. The cells were washed three times in permeabilization buffer and once in PBS and were mounted in 133 mg of Mowiol (Hoechst) per ml–33% glycerol, 133 mM Tris HCl (pH 8.5). Confocal microscopy was performed on a Leica TCS4D microscope. Series of optical sections at 0.7-μm intervals were recorded. One representative medial section was mounted using Adobe Photoshop software.
Western blot analysis.
Viral supernatants were collected from transfected HeLa cells. Viruses were concentrated by ultracentrifugation (15 min at 60,000 rpm in a Beckman TL100 centrifuge), and pellets were resuspended in lysis buffer (20 mM HEPES, 150 mM NaCl, 0.5% Triton). Proteins corresponding to 100 and 200 ng of p24 were used for Gag and Flag analysis, respectively. Samples were diluted in loading buffer in the absence or presence of dithiothreitol (60 mM final concentration), boiled for 5 min, and analyzed by Western blotting as previously described (46). Final concentrations for each antibody were as follows: anti-gag 25A plus 18A, 0.5 μg/ml each; rabbit anti-Flag, 2 μg/ml.
Analysis of viral DNA by Southern blotting.
P4p56 target cells (12 × 106 per sample) were infected with wild-type (WT) and mutated BRU-HA viruses adjusted for equivalent p24 concentration (2,500 ng in a final volume of 5 ml), in the presence of 20 μg of DEAE-dextran per ml. At 24 h after infection, low-molecular-weight DNA was prepared by Hirt extraction (33). Samples were digested with DpnI to remove residual plasmids that may contaminate viral supernatants and then with EcoRI and subjected to Southern blot analysis. DNA from approximately 4 × 106 target cells was analyzed in each lane. The 32P-labeled DNA probe used for hybridization was the 1.9-kb MscI fragment from the pol region of pBRU. Total amounts of low-molecular-weight DNA were detected by using a probe specific for the mitochondrial cytochrome b gene. Gels were visualized by autoradiography and with a PhosphorImager.
Analysis of integrated viral DNA by PCR.
For the analysis of integrated viral DNA, P4p56 cells were infected as described above and total DNA was extracted, digested with DpnI, and subjected to PCR using the method described by Chun et al. (14). Briefly, 50 ng of total DNA was subjected to nested PCR, using for the first round a 5′ primer from the conserved human Alu sequence and a 3′ primer from the conserved HIV-1 long terminal repeat (LTR) sequence (14). This round amplifies both cellular DNA upstream of the integration site and integrated HIV-1 LTR. An aliquot (1/400) of the first PCR product was subjected to the second round of PCR using nested HIV-1 LTR-specific primers (14). In parallel, the presence of total viral DNA was verified by performing the second round of PCR directly on 50 ng of DNA sample. One-fifth of each PCR product was analyzed by gel electrophoresis.
RESULTS
Subcellular localization of HIV-1 IN mutated in C-terminal basic sequences.
Our first aim was to generate HIV-1 IN mutants with altered karyophilic properties. The lentivirus-specific L, Q, and N sequences were mutated in the Flag-INT expression vector. This vector encoding HIV-1 IN fused to the Flag epitope allows the expression of IN in the absence of other viral proteins and its detection by immunofluorescence staining (46). Two sets of mutations shown to reduce the interaction of IN with karyopherin-α (28) were inserted: K186-Q in the lentivirus exposed loop L (mutant IN-L) and the double glutamine substitution Q214-L Q216-L in the lentivirus glutamine-rich sequence (mutant IN-Q) (Fig. 1A). Additionally, we reconstructed the double mutation (IN-LQ) (Fig. 1A), which was previously reported to abrogate the IN–karyopherin-α interaction (28). We also asked whether the basic region N was involved in the karyophilic properties of HIV-1 IN. To test this hypothesis, three basic residues in the lentivirus consensus sequence N (262RRK) were replaced by the corresponding residues of Moloney murine leukemia virus (AAH, mutant IN-N).
As we previously demonstrated (46), expression of WT HIV-1 IN in HeLa cells resulted in the almost exclusive nuclear localization of IN (Fig. 1B), indicating active transport of IN. Alteration of the lentivirus L and Q sequence, either alone or in combination, resulted in the loss of nuclear accumulation of IN and a homogeneous staining, both in the nucleus and in the cytoplasm of the transfected cells (Fig. 1B). This pattern suggests that IN molecules mutated in the L and/or Q sequence can freely diffuse through the nuclear pores, as expected for a protein of the size of monomeric IN (22). In contrast, substitution of the charged HIV-1 tripeptide in the N sequence with the corresponding Moloney murine leukemia virus residues resulted in a staining profile indistinguishable from that of WT IN (Fig. 1B). Thus, the charged lentivirus consensus of the N sequence is not required for the nuclear accumulation of HIV-1 IN and can be replaced by an oncovirus sequence. Taken together, our data show that the subcellular localization of HIV-1 IN is markedly perturbed by alteration of the conserved L and Q sequences but not of the N sequence.
Impact of mutations on virus infectivity and integrase multimerization.
Given the proposed implication of IN in the nuclear import of the PICs, we evaluated the impact of the above-described mutations on the different steps of the viral cycle. We inserted the IN mutations in the replication-competent molecular clone pBRU, yielding L, Q, LQ, and N viruses. We then measured virus particle production and infectivity of WT and mutant viruses. Gag p24 antigen production, measured in the supernatant of HeLa cells transfected with the different molecular clones, was similar for wild-type and mutant viruses (data not shown). Virus infectivity was monitored both by a colorimetric test in a single-cycle infectivity assay using P4 (HeLa CD4 LTR-LacZ) reporter cells and in a multiple-cycle assay using T-lymphoid MT4 cells. As shown in Fig. 2A, the four mutant viruses were noninfectious in both assays. Therefore, mutations in the L, Q, and N sequences do not affect viral production, measured by p24 release in the supernatant of virus-producing cells, but do abrogate viral infectivity.
FIG. 2.
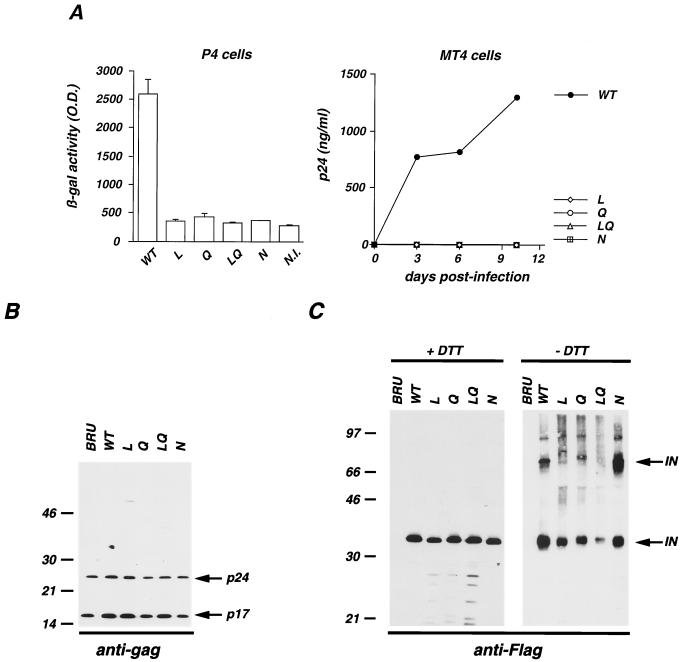
Infectivity and protein content of viral particles carrying WT and mutated IN. (A) WT and IN mutated pBRU clones were transfected in HeLa cells. Viral particles released in the supernatant of transfected cells were normalized for p24 content and used to infect P4 (left) and MT4 (right) cells. Infectivity in P4 cells was tested in a single-cycle assay, as previously described (43). N.I., noninfected cells. Replication in MT4 cells was assessed by p24 accumulation in the culture supernatant over a 10-day period. β-gal, β-galactosidase; O.D., optical density. (B and C) HeLa cells were transfected with pBRU molecular clone (lanes BRU) and with the isogenic construct (pBRU-Flag molecular clone) in which IN was fused to the Flag epitope and contains either WT (lanes WT) or mutated IN (lanes L, Q, LQ, and N) sequences. Viral particles were harvested, concentrated by ultracentrifugation, and analyzed by Western blotting with antibodies specific for the HIV-1 gag matrix (p17) and capsid (p24) (B) or specific for the Flag epitope (C) under reducing (+DTT) or nonreducing (−DTT) conditions. Proteins corresponding to 100 and 200 ng of p24 were used for Gag and Flag analysis, respectively. Molecular mass markers are indicated on the left in kilodaltons.
We then analyzed the protein composition of viral particles and in particular the multimeric status of IN. To this aim, the IN mutants were reconstructed in the replication-competent BRU-HA and BRU-Flag clones, in which IN is fused to a tagging epitope (hemagglutinin and the Flag peptide, respectively), allowing a high sensitivity of detection of IN by Western blotting (46). The protein content of virions was analyzed by Western blotting for the WT and IN mutated BRU-Flag viral clones (Fig. 2B and C). As previously described (46), IN epitope tagging did not alter viral particle production or Gag protein composition; accordingly, BRU-Flag (Fig. 2B, lane WT) displayed a Gag profile identical to that of the original BRU clone (Fig. 2B, lane BRU). Disruption of the lentivirus-specific sequences in mutants L, Q, LQ, and N did not alter the Gag protein profile (Fig. 2B). More interestingly, we analyzed IN incorporation and multimerization in viral particles produced by WT and the corresponding IN-mutated BRU-Flag viral clones (Fig. 2C). We recently showed that Western blot analysis conducted under nonreducing conditions, preserving disulfide bonds, allowed the detection of IN multimers in viral particles (46). Here we used the same approach to detect and characterize mutated IN molecules in viral particles. As shown in Fig. 2C (left panel), under reducing conditions WT and mutant IN molecules were readily detectable at similar levels. Mutants L and Q displayed minor IN specific degradation products (left panel), indicating a reduced stability of the mutated proteins. This phenotype was enhanced when the two mutations were present in the same IN molecules (mutant LQ, left panel). Mutant N, on the other hand, displayed a WT protein profile.
As we previously reported (46), under nonreducing conditions an additional IN-specific, strongly reactive signal with an apparent molecular mass of 70 kDa was observed in WT virions, confirming that disulfide bridges are required to form IN multimers. The dimeric IN signal was also observed with mutant N, indicating that this mutant is not affected in its ability to multimerize. A slight reduction of the signal corresponding to monomeric IN was detected for mutants L and Q, and the reduction was marked for the double mutant LQ (Fig. 2C, right panel). Since similar amounts of WT and mutated IN are present in the analyzed virions (Fig. 2C, left panel), we concluded that the exposure of the tagging epitope was decreased under nonreducing conditions, indicating that IN mutants L, Q, and particularly LQ display structural perturbation. Moreover, the ratio of dimer to monomer signals was strongly reduced for mutants L and Q, while no signal corresponding to IN multimers was observed for the double mutant LQ (Fig. 2C, right panel). This result suggests that alteration of sequences L and Q is detrimental for IN dimerization. Of note, analysis of the particle content of mutant viral clones constructed in the BRU-HA context gave similar results (data not shown), demonstrating that these observations are independent of the tagging epitope. Also, it was previously reported that IN mutants L and Q displayed reduced dimerization in a yeast two-hybrid system (28), an observation that supports our findings with viral particles.
Taken together, these results strongly suggest that modification of lentivirus-specific sequences L and Q induces conformational changes of IN molecules, which impair their ability to multimerize and increase their degradation in viral particles. The substitution within the N sequence did not appreciably alter IN conformation or reduce the dimerization potential of IN despite the sharp change in the local charge.
Mutant viruses display normal entry, DNA synthesis and nuclear import of viral DNA.
The inability of IN-mutated viruses to induce the expression of the reporter gene in P4 cells (Fig. 2A) indicates that these mutants were affected in the early phases of the virus life cycle. To determine precisely which step was implicated, we analyzed viral entry, synthesis and nuclear import of viral DNA, and integration into target cell DNA. We first examined whether mutations in the IN sequence could influence viral entry by measuring the cytosolic HIV-1 p24 antigen content of newly infected P4 cells. Cytosolic p24 is a reliable index of infectious events eventually leading to productive infection (44). We did not observe a significant difference between WT and mutant viruses (data not shown), indicating that the entry step of the viral cycle is not affected by the mutations in IN.
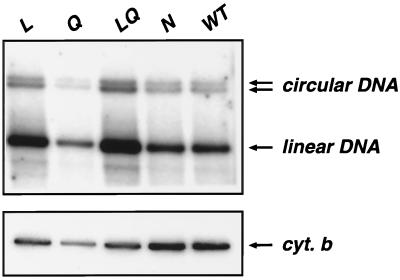
We examined the reverse transcription and the nuclear import of preintegrative viral DNA in newly infected P4p56 cells exposed to WT or mutant viruses adjusted to contain equivalent amounts of p24. Low-molecular-weight DNA was extracted by the Hirt procedure 24 h after exposure of target cells to virion preparations and was analyzed by Southern blotting with a probe specific for the pol gene. Samples were digested by EcoRI to produce diagnostic fragments which discriminate the linear and circular forms of viral DNA (2). As expected, after infection with wild-type BRU virus, two forms of circular viral DNA (containing one or two LTRs) were observed in addition to the linear form of the genome (Fig. 3, top panel). No signal was detected when target cells were treated with azidothymidine or exposed to an env-deleted virus (data not shown), indicating that the DNA detected by the pol probe was the product of de novo synthesis. Circular and linear forms of viral DNA were instead observed after infection of target cells with IN mutant viruses (top panel). To compare the reverse transcription products of WT and mutant viruses, we first needed to measure the efficiency of DNA extraction. The total amount of cellular low-molecular-weight DNA present in cell extracts was measured by hybridization of the same blot with a probe specific for the mitochondrial cytochrome b gene (Fig. 3, bottom panel). After correlation with the cytochrome b signal, the total (i.e., linear plus circular) amounts of viral DNA synthesized during the reverse transcription process were comparable for WT and IN mutant viruses (Fig. 3, top panel). Therefore, all IN mutant viruses analyzed here were competent for the synthesis of DNA genome of the expected size. Although this analysis does not provide precise quantitative evaluation of the viral DNA content, it nevertheless shows that viral mutants L, Q, LQ, and N were competent for reverse transcription.
FIG. 3.
Synthesis and circularization of viral DNA. P4p56 cells were exposed to WT or IN-mutated (L, Q, LQ, and N) virions in amounts adjusted to ensure equivalent levels of p24. At 24 h following infection, low-molecular-weight DNA was prepared by Hirt extraction, EcoRI digested and analyzed by Southern blotting with a 32P-labeled probe specific for the pol gene of HIV-1 (top). Arrows indicate the circular and linear forms of viral DNA. Hybridization of the same blot with a probe specific for the mitochondrial cytochrome b gene (cyt. b) is shown (bottom).
The linear form of viral DNA can be found in both the cytoplasm and the nucleus of newly infected cells, while the circular forms are generated in the nucleus, probably by nuclear host enzymes as an alternative (dead end) to correct integration, and are commonly used as markers for nuclear import of the PICs (3, 6, 19, 20, 38). The ratios of circular to total (circular and linear) forms of viral DNA were equivalent for WT and mutant viruses (Fig. 3, top panel), indicating that mutant viral DNA could access the nuclear compartment with an efficiency comparable to that of the WT. Similar results were obtained with BRU, BRU-HA, and BRU-Flag viruses and with MT4 target cells or when the analysis was performed 15 h after infection (data not shown). Taken together, these results demonstrated that viral DNA molecules synthesized by WT and IN mutant viruses gain access to the nucleus in similar proportions. Our findings are in agreement with previous reports showing by a different technique that mutants L and Q were not affected in the nuclear import of proviral DNA in dividing or in nondividing cells (12, 28). Therefore, we conclude that the nuclear import of PICs was not affected in L, Q, LQ, and N mutant viruses.
Mutant viruses are impaired in the integration process.
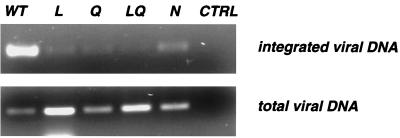
Once we determined that virions carrying mutations in the L, Q, and N sequences performed the nuclear import of PICs efficiently, we analyzed the capacity of these mutants to integrate proviral DNA into the host genome. P4p56 cells were exposed for 24 h to WT and IN mutant viruses adjusted to contain equivalent amounts of p24, and DNA was extracted to determine the amount of total and integrated viral DNA. The total viral DNA was amplified with primers specific for the HIV-1 LTR sequence and was found to be at least as abundant in cells exposed to the four mutant viruses as in cells infected by the WT virus (Fig. 4, bottom panel). This finding is in agreement with our observation of normal synthesis of viral DNA in the IN mutant viruses, obtained by Southern blotting analysis of viral DNA without a PCR step (Fig. 3). We then analyzed integrated proviral DNA by the Alu-LTR PCR technique (14). The boundary of host and viral DNA was amplified in a first PCR using a primer specific for the human Alu sequence and a primer complementary to the viral 5′ LTR. An aliquot of this reaction product was further amplified using nested HIV LTR-specific primers. A strong signal was observed with WT virus (Fig. 4, top panel), confirming that integrated DNA can be efficiently detected in this experimental setting. Under the same conditions, cells exposed to mutants L, Q, LQ, and N produced barely detectable signals (top panel), indicating that the four replication-defective IN mutants were markedly affected at the integration step. The weak signal observed with IN mutants could represent residual integration activity but could also be due to aberrant or HIV-1 IN-independent integration products previously observed with IN mutants (30). Such drastic reduction of integrated viral DNA observed for the IN mutated viruses in vivo, combined with our data on normal nuclear import of viral DNA, supports the interpretation that the lack of infectivity is due to a direct effect on IN enzymatic properties including viral DNA binding, processing, and insertion rather than to defective nuclear import of viral PICs.
FIG. 4.
Integration of viral DNA. P4p56 cells were exposed to WT or IN-mutated (L, Q, LQ, and N) virions in amounts adjusted to ensure equivalent levels of p24. At 24 h following infection, DNA was prepared and analyzed by PCR to visualize integrated and total viral DNA. Integrated viral DNA (top) was amplified by nested PCR. In the first round of PCR, a 5′ primer from the conserved human Alu sequence and a 3′ primer from the conserved HIV-1 LTR sequence were used. This round amplifies both cellular DNA upstream of the integration site and integrated HIV-1 LTR. An aliquot (1/400) of the first PCR product was further subjected to the second round of PCR by using nested HIV-1 LTR-specific primers. To verify that only integrated viral DNA was amplified by the two-round procedure, control reactions (including the 1/400 dilution step) were performed in which the primers and the enzyme were omitted in the first PCR (not shown). Total viral DNA (bottom) was amplified using primers from the HIV-1 LTR sequence. Mock-infected cells were similarly analyzed as a negative control (lane CTRL).
DISCUSSION
We and others have previously shown that HIV-1 IN has intrinsic karyophilic properties, since it accumulates in the nuclear compartment when expressed in the absence of other viral proteins (28, 46, 47). To alter the subcellular localization of IN, we mutated the three C-terminal basic sequences, L, Q, and N. We first examined the subcellular distribution of IN mutated in basic motifs (L and Q) that were proposed to act as NLS (28). We observed that in contrast to the WT protein, which accumulated in the nucleus, IN proteins mutated in the L or Q sequence and the double LQ mutant displayed a homogenous distribution, both in the nucleus and in the cytosol, indicating that these mutations affected the karyophilic properties of IN. Similarly, IN fused to GST and microinjected into the cytoplasm of Cos cells was found to display an almost exclusive nuclear localization while IN mutants L and Q in this context failed to localize to the nucleus (28). The finding that these mutations prevented the nuclear import of IN in the context of a large fusion protein (IN-GST) suggests that the residual nuclear staining we observed when IN was fused to a small Flag epitope was the consequence of passive diffusion through nuclear pores.
Besides the loss of nuclear accumulation of IN, alteration of the L and Q sequences induced other phenotypes. We observed a drastic reduction of IN multimers in viral particles, associated with an increased degradation of the protein. Moreover, under nonreducing conditions, IN mutants were less well recognized by anti-Tag antibodies. Altogether, these results strongly suggest that the overall conformation of IN mutants L, Q, and LQ was markedly affected. It should be pointed out that perturbation of the subcellular localization of IN by mutations does not imply that the targeted sequences are NLS (25). A structural perturbation imposed by the mutations could in fact impair the exposure of a distal NLS. In this respect, we previously reported the loss of nuclear accumulation associated with the IN mutation C130-G, a position that is not part of any NLS-like sequence (46). The loss of karyophilic properties was attributed for this mutant to the significant alteration of IN structure. A similar distal effect could also be responsible for the previously reported loss of the karyopherin-α in vitro binding activity of IN mutant LQ (28).
It is interesting to note that the loss of nuclear accumulation in mutants L, Q, and LQ was associated with a drastic reduction of the multimerization of IN in viral particles. We previously observed that the two phenotypes were also associated for the mutation C130-G (46). Accordingly, alteration of the N sequence, although lethal for the virus, did not impair nuclear localization or dimerization of IN. One can therefore speculate that dimerization of IN favors nuclear accumulation of the protein, possibly by allowing interaction with host proteins or DNA. After penetration in the cytoplasm of a target cell, specific viral components need to gain access to the nucleus. How retroviruses manage to reach this compartment is not fully understood. Lentiviruses in particular are even more complex than other retroviruses, being able to infect both dividing and nondividing cells (8, 42, 54). Whether they use different mechanisms to gain access to the nucleus depending on the replicative status of target cells is unknown. The requirement for specific sequences in the matrix, Vpr, and IN proteins to infect differentiated or growth-arrested cells in tissue culture was proposed by some authors but discounted by others (7, 8, 24–29, 32). In particular, HIV-1 IN has been proposed to participate in the nuclear import of PICs in nonproliferating cells by recruiting phosphorylated matrix proteins to the core of the PICs and by interacting with karyopherin-α (28, 29). The relevance of karyophilic properties of lentivirus IN for infection of interphasic cells can, however, be challenged by the finding that oncovirus IN proteins have comparable karyophilic characteristics (41).
We have analyzed the different steps of virus replication for HIV-1 mutants carrying karyophilic IN proteins (viruses WT and N) and nonkaryophilic ones (L, Q, and LQ), with particular interest in the nuclear import of PICs. Our results demonstrate that conservation of the L, Q, and N sequences is required for viral infectivity. Alteration of these motifs was, however, compatible with viral particle formation, target cell entry, and viral DNA synthesis and did not impair nuclear transport of viral DNA. It is clear from these results that one cannot determine the fate of the PICs on the basis of the localization of IN protein expressed alone. Besides, nuclear import of viral DNA does not appear to require the karyophilic potential of IN, since it takes place with normal efficiency in the presence of IN molecules that are unable to accumulate in the nuclear compartment. Instead, we observed a drastic reduction of integrated viral DNA for the IN-mutated viruses in vivo, indicating that the lack of infectivity was due to a direct effect on IN enzymatic properties. Accordingly, normal nuclear import of viral PICs for mutants L and Q was reported independently of the replicative status of target cells (12, 28). Gallay et al. proposed a complex interpretation of their results to take into account their observation of reduced in vitro binding to karyopherins observed with mutated IN (28). These authors acknowledged that mutants L and Q were unable to replicate due to a block in integration, independently of the replicative status of target cells, but in addition suggested that in nondividing cells the recruitment of PICs by karyopherins might be inefficient due to mutations in IN (28). Our results support the simpler view that a defective integration process is the cause of the lack of replication of these mutant viruses in both dividing and nondividing cells.
It would be interesting to know whether the mutated IN proteins studied here could still associate with viral DNA and enter the nucleus as part of the PICs. Although the association between viral DNA and IN takes place early after reverse transcription, probably while the DNA is still in the cytoplasm (20, 45, 48, 53), we cannot exclude the possibility that viral DNA of IN mutants entered the nucleus independently of IN, as part of abortive PICs. An attempt to detect IN molecules in the nuclei of newly infected cells by immunofluorescence staining did not give definitive results (data not shown).
Impaired enzymatic activity of IN mutants L, Q, and LQ was not totally unexpected, given the structural perturbation of the proteins, which also had major repercussion on their dimerization and karyophilic properties. Mutant N instead appears to be specifically affected at the integration step, since IN structure, dimerization, and karyophilic properties were preserved and all the analyzed steps of the viral cycle were performed with WT efficiency. This mutant provides a useful tool for studies of the integration process in a cellular context, since other mutations in IN were shown to have pleiotropic effects (17, 55).
In conclusion, our data demonstrate that alteration of the L and Q sequences affects the karyophilic properties of HIV-1 IN but not those of viral PICs. Rather, conservation of the L, Q, and N sequences is required for efficient integration in vivo. The diverse effects of mutations on IN properties are most probably due to structural perturbation of this enzyme. In the virus context, a possible role of IN for nuclear import of PICs may be indirect, such as a structural determinant allowing the correct architecture of the complex, and should not depend on IN karyophilic properties.
ACKNOWLEDGMENTS
We thank Jean Michel Heard and François Clavel for support and critical suggestions. We thank Guillermina Bolcini and Elisabeth Menu for helpful advice on the Alu-LTR PCR technique. We thank Emmanuelle Perret for confocal microscopy analysis, Virginie Trouplin for help with cloning, and François Traincart for the kind gift of reagents.
C.P. is a fellow of Agence Nationale de Recherche sur le SIDA (ANRS). This work was supported by grants from the ANRS, SIDACTION, and the Pasteur Institute.
REFERENCES
- 1.Asante-Appiah E, Skalka A M. Molecular mechanisms in retrovirus DNA integration. Antiviral Res. 1997;36:139–156. doi: 10.1016/s0166-3542(97)00046-6. [DOI] [PubMed] [Google Scholar]
- 2.Barbosa P, Charneau P, Dumey N, Clavel F. Kinetic analysis of HIV-1 early replicative steps in a coculture system. AIDS Res Hum Retroviruses. 1994;10:53–59. doi: 10.1089/aid.1994.10.53. [DOI] [PubMed] [Google Scholar]
- 3.Bowerman B, Brown P O, Bishop J M, Varmus H E. A nucleoprotein complex mediates the integration of retroviral DNA. Genes Dev. 1989;3:469–478. doi: 10.1101/gad.3.4.469. [DOI] [PubMed] [Google Scholar]
- 4.Brown P O. Integration. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 161–204. [PubMed] [Google Scholar]
- 5.Bukrinskaya A G, Ghorpade A, Heinzinger N K, Smithgall T E, Lewis R E, Stevenson M. Phosphorylation-dependent human immunodeficiency virus type 1 infection and nuclear targeting of viral DNA. Proc Natl Acad Sci USA. 1996;93:367–371. doi: 10.1073/pnas.93.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukrinsky M, Sharova N, Stevenson M. Human immunodeficiency virus type 1 2-LTR circles reside in a nucleoprotein complex which is different from the preintegration complex. J Virol. 1993;67:6863–6865. doi: 10.1128/jvi.67.11.6863-6865.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bukrinsky M I, Haffar O K. HIV-1 nuclear import: matrix protein is back on center stage, this time together with Vpr. Mol Med. 1998;4:138–143. [PMC free article] [PubMed] [Google Scholar]
- 8.Bukrinsky M I, Haggerty S, Dempsey M P, Sharova N, Adzhubei A, Spitz L, Lewis P, Goldfarb D, Emerman M, Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukrinsky M I, Sharova N, McDonald T L, Pushkarskaya T, Tarpley W G, Stevenson M. Association of integrase, matrix, and reverse transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc Natl Acad Sci USA. 1993;90:6125–6129. doi: 10.1073/pnas.90.13.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bushman F D, Engelman A, Palmer I, Wingfield P, Craigie R. Domains of the integrase protein of human immunodeficiency virus type 1 responsible for polynucleotidyl transfer and zinc binding. Proc Natl Acad Sci USA. 1993;90:3428–3432. doi: 10.1073/pnas.90.8.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bushman F D, Fujiwara T, Craigie R. Retroviral DNA integration directed by HIV integration protein in vitro. Science. 1990;249:1555–1558. doi: 10.1126/science.2171144. [DOI] [PubMed] [Google Scholar]
- 12.Cannon P M, Byles E D, Kingsman S M, Kingsman A J. Conserved sequences in the carboxyl terminus of integrase that are essential for human immunodeficiency virus type 1 replication. J Virol. 1996;70:651–657. doi: 10.1128/jvi.70.1.651-657.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charneau P, Mirabeau G, Roux P, Paulous S, Buc H, Clavel F. HIV-1 reverse transcription: a termination step at the center of the genome. J Mol Biol. 1994;241:651–662. doi: 10.1006/jmbi.1994.1542. [DOI] [PubMed] [Google Scholar]
- 14.Chun T W, Stuyver L, Mizell S B, Ehler L A, Mican J A, Baseler M, Lloyd A L, Nowak M A, Fauci A S. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyda F, Hickman A B, Jenkins T M, Engelman A, Craigie R, Davies D R. Crystal structure of the catalytic domain of HIV-1 integrase: similarity to other polynucleotidyl transferases. Science. 1994;266:1981–1986. doi: 10.1126/science.7801124. [DOI] [PubMed] [Google Scholar]
- 16.Engelman A, Bushman F D, Craigie R. Identification of discrete functional domains of HIV-1 integrase and their organization within an active multimeric complex. EMBO J. 1993;12:3269–3275. doi: 10.1002/j.1460-2075.1993.tb05996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engelman A, Englund G, Orenstein J M, Martin M A, Craigie R. Multiple effects of mutations in human immunodeficiency virus type 1 integrase on viral replication. J Virol. 1995;69:2729–2736. doi: 10.1128/jvi.69.5.2729-2736.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engelman A, Mizuuchi K, Craigie R. HIV-1 DNA integration: mechanism of viral DNA cleavage and DNA strand transfer. Cell. 1991;67:1211–1221. doi: 10.1016/0092-8674(91)90297-c. [DOI] [PubMed] [Google Scholar]
- 19.Farnet C, Haseltine W A. Circularization of human immunodeficiency virus type 1 DNA in vitro. J Virol. 1991;65:6942–6952. doi: 10.1128/jvi.65.12.6942-6952.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farnet C, Haseltine W A. Determination of viral proteins present in the Human Immunodeficiency Virus type 1 preintegration complex. J Virol. 1991;65:1910–1915. doi: 10.1128/jvi.65.4.1910-1915.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farnet C M, Bushman F D. HIV-1 cDNA integration—requirement of HMG I(Y) protein for function of preintegration complexes in vitro. Cell. 1997;88:483–492. doi: 10.1016/s0092-8674(00)81888-7. [DOI] [PubMed] [Google Scholar]
- 22.Finlay D R, Forbes D J. Reconstitution of biochemically altered nuclear pores: transport can be eliminated and restored. Cell. 1990;60:17–29. doi: 10.1016/0092-8674(90)90712-n. [DOI] [PubMed] [Google Scholar]
- 23.Fletcher T M, Soares M A, McPhearson S, Hui H X, Wiskerchen M A, Muesing M A, Shaw G M, Leavitt A D, Boeke J D, Hahn B H. Complementation of integrase function in HIV-1 virions. EMBO J. 1997;16:5123–5138. doi: 10.1093/emboj/16.16.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fouchier R A, Meyer B E, Simon J H, Fischer U, Albright A V, Gonzalez-Scarano F, Malim M H. Interaction of the human immunodeficiency virus type 1 Vpr protein with the nuclear pore complex. J Virol. 1998;72:6004–6013. doi: 10.1128/jvi.72.7.6004-6013.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fouchier R A, Meyer B E, Simon J H, Fischer U, Malim M H. HIV-1 infection of non-dividing cells: evidence that the amino-terminal basic region of the viral matrix protein is important for Gag processing but not for post-entry nuclear import. EMBO J. 1997;16:4531–4539. doi: 10.1093/emboj/16.15.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freed E, Englund G, Maldarelli F, Martin M A. Phosphorylation of residue 131 of HIV-1 matrix is not required for macrophage infection. Cell. 1997;88:171–174. doi: 10.1016/s0092-8674(00)81836-x. [DOI] [PubMed] [Google Scholar]
- 27.Freed E O, Martin M. HIV-1 infection of non-dividing cells. Nature. 1994;369:107–108. doi: 10.1038/369107b0. [DOI] [PubMed] [Google Scholar]
- 28.Gallay P, Hope T, Chin D, Trono D. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc Natl Acad Sci USA. 1997;94:9825–9830. doi: 10.1073/pnas.94.18.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallay P, Swingler S, Song J, Bushman F, Trono D. HIV nuclear import is governed by the phosphotyrosine-mediated binding of matrix to the core domain of integrase. Cell. 1995;83:569–576. doi: 10.1016/0092-8674(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 30.Gaur M, Leavitt A D. Mutations in the human immunodeficiency virus type 1 integrase D,D(35)E motif do not eliminate provirus formation. J Virol. 1998;72:4678–4685. doi: 10.1128/jvi.72.6.4678-4685.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodarzi G, Im G J, Brackmann K, Grandgenett D. Concerted integration of retrovirus-like DNA by human immunodeficiency virus type 1 integrase. J Virol. 1995;69:6090–6097. doi: 10.1128/jvi.69.10.6090-6097.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinzinger N K, Bukrinsky M I, Haggerty S A, Ragland A M, Kewalramani V, Lee M A, Gendelman H E, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 34.Jenkins T M, Engelman A, Ghirlando R, Craigie R. A soluble active mutant of HIV-1 integrase: involvement of both the core and carboxyl-terminal domains in multimerization. J Biol Chem. 1996;271:7712–7718. doi: 10.1074/jbc.271.13.7712. [DOI] [PubMed] [Google Scholar]
- 35.Jones K S, Coleman J, Merkel G W, Laue T M, Skalka A M. Retroviral integrase functions as a multimer and can turn over catalytically. J Biol Chem. 1992;267:16037–16040. [PubMed] [Google Scholar]
- 36.Kalpana G V, Goff S P. Genetic analysis of homomeric interactions of human immunodeficiency virus type 1 integrase using the yeast two-hybrid system. Proc Natl Acad Sci USA. 1993;90:10593–10597. doi: 10.1073/pnas.90.22.10593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalpana G V, Marmon S, Wang W, Crabtree G R, Goff S P. Binding and stimulation of HIV-1 integrase by a human homolog of yeast transcription factor SNF5. Science. 1994;266:2002–2006. doi: 10.1126/science.7801128. [DOI] [PubMed] [Google Scholar]
- 38.Karageorgos L, Li P, Burrell C. Characterization of HIV replication complexes early after cell-to-cell infection. AIDS Res Hum Retroviruses. 1993;9:817–823. doi: 10.1089/aid.1993.9.817. [DOI] [PubMed] [Google Scholar]
- 39.Katzman M, Mack J P, Skalka A M, Leis J. A covalent complex between retroviral integrase and nicked substrate DNA. Proc Natl Acad Sci USA. 1991;88:4695–4699. doi: 10.1073/pnas.88.11.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kootstra N A, Schuitemaker H. Phenotype of HIV-1 lacking a functional nuclear localization signal in matrix protein of gag and Vpr is comparable to wild-type HIV-1 in primary macrophages. Virology. 1999;253:170–180. doi: 10.1006/viro.1998.9482. [DOI] [PubMed] [Google Scholar]
- 41.Kukolj G, Jones K S, Skalka A M. Subcellular localization of avian sarcoma virus and human immunodeficiency virus type 1 integrases. J Virol. 1997;71:843–847. doi: 10.1128/jvi.71.1.843-847.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis P, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mammano F, Petit C, Clavel F. Resistance-associated loss of viral fitness in human immunodeficiency virus: phenotypic analysis of protease and gag coevolution in protease inhibitor-treated patients. J Virol. 1998;72:7632–7637. doi: 10.1128/jvi.72.9.7632-7637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maréchal V, Clavel F, Heard J M, Schwartz O. Cytosolic Gag p24 as an index of HIV-1 productive entry. J Virol. 1998;72:2208–2212. doi: 10.1128/jvi.72.3.2208-2212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller M D, Farnet C M, Bushman F D. Human immunodeficiency virus type 1 preintegration complexes: studies of organization and composition. J Virol. 1997;71:5382–5390. doi: 10.1128/jvi.71.7.5382-5390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petit C, Schwartz O, Mammano F. Oligomerization within virions and subcellular localization of human immunodeficiency virus type 1 integrase. J Virol. 1999;73:5079–5088. doi: 10.1128/jvi.73.6.5079-5088.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pluymers W, Cherepanov P, Schols D, De Clercq E, Debyser Z. Nuclear localization of human immunodeficiency virus type 1 integrase expressed as a fusion protein with green fluorescent protein. Virology. 1999;258:327–332. doi: 10.1006/viro.1999.9727. [DOI] [PubMed] [Google Scholar]
- 48.Roth M, Schwartzberg P, Goff S P. Structure of the termini of DNA intermediates in the integration of retroviral DNA. Cell. 1989;58:47–54. doi: 10.1016/0092-8674(89)90401-7. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz O, Maréchal V, Danos O, Heard J M. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J Virol. 1995b;69:4053–4059. doi: 10.1128/jvi.69.7.4053-4059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Gent D C, Vink C, Groeneger A A, Plasterk R H. Complementation between HIV integrase proteins mutated in different domains. EMBO J. 1993;12:3261–3267. doi: 10.1002/j.1460-2075.1993.tb05995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vink C, Oude Groeneger A M, Plasterk R H. Identification of the catalytic and DNA-binding region of the human immunodeficiency virus type 1 integrase protein. Nucleic Acids Res. 1993;21:1419–1425. doi: 10.1093/nar/21.6.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.von Schwedler U, Kornblut R S, Trono D. The nuclear localization signal of the matrix protein of human immunodeficiency virus type 1 allows the establishment of infection in macrophages and quiescent T lymphocytes. Proc Natl Acad Sci USA. 1994;91:6992–6996. doi: 10.1073/pnas.91.15.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei S Q, Mizuuchi K, Craigie R. A large nucleoprotein assembly at the ends of the viral DNA mediates retroviral DNA integration. EMBO J. 1997;16:7511–7520. doi: 10.1093/emboj/16.24.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weinberg J B, Matthews T J, Cullen B R, Malim M H. Productive human immunodeficiency virus type 1 (HIV-1) infection of non proliferative human monocytes. J Exp Med. 1991;88:1477–1482. doi: 10.1084/jem.174.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu X, Liu H, Xiao H, Conway J A, Hehl E, Kalpana G V, Prasad V, Kappes J C. Human Immunodeficiency Virus type 1 Integrase protein promotes reverse transcription through specific interactions with the nucleoprotein reverse transcription complex. J Virol. 1999;73:2126–2135. doi: 10.1128/jvi.73.3.2126-2135.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zennou V, Petit C, Guetard D, Nerhbass U, Montagnier L, Charneau P. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell. 2000;101:173–185. doi: 10.1016/S0092-8674(00)80828-4. [DOI] [PubMed] [Google Scholar]
- 57.Zheng R L, Jenkins T M, Craigie R. Zinc folds the N-terminal domain of HIV-1 integrase, promotes multimerization, and enhances catalytic activity. Proc Natl Acad Sci USA. 1996;93:13659–13664. doi: 10.1073/pnas.93.24.13659. [DOI] [PMC free article] [PubMed] [Google Scholar]