Abstract
Sex differences have complicated our understanding of the neurobiological basis of many behaviors that are key for survival. As such, continued elucidation of the similarities and differences between sexes is necessary to gain insight into brain function and vulnerability. The connection between the hippocampus (Hipp) and nucleus accumbens (NAc) is a crucial site where modulation of neuronal activity mediates reward-related behavior. Our previous work demonstrated that long-term potentiation (LTP) of Hipp→NAc synapses is rewarding, and mice can establish learned associations between LTP of these synapses and the contextual environment in which LTP occurred. Here, we investigated sex differences in the mechanisms underlying Hipp→NAc LTP using whole-cell electrophysiology and pharmacology. We observed similarities in basal synaptic strength between males and females and found that LTP occurs postsynaptically with similar magnitudes in both sexes. However, key sex differences emerged as LTP in males required NMDA receptors (NMDAR), whereas LTP in females utilized an NMDAR-independent mechanism involving L-type voltage-gated Ca2+ channels (VGCCs) and estrogen receptor α (ERα). We also uncovered sex-similar features as LTP in both sexes depended on CaMKII activity and occurred independently of dopamine-1 receptor (D1R) activation. Our results have elucidated sex-specific molecular mechanisms for LTP in an integral pathway that mediates reward-related behaviors, emphasizing the importance of considering sex as a variable in mechanistic studies. Continued characterization of sex-specific mechanisms underlying plasticity will offer novel insight into the neurophysiological basis of behavior, with significant implications for understanding how diverse processes mediate behavior and contribute to vulnerability to developing psychiatric disorders.
Keywords: hippocampus, long-term potentiation, nucleus accumbens, plasticity, sex differences
Significance Statement
Strengthening of hippocampus→nucleus accumbens (Hipp→NAc) synapses drives reward-related behaviors. Long-term potentiation (LTP) occurs with a similar magnitude in males and females, and both sexes have a predicted postsynaptic locus of plasticity. Despite these similarities, here we illustrate that sex-specific molecular mechanisms underlie LTP at Hipp→NAc synapses. Given the bidirectional relationship between Hipp→NAc synaptic strength in mediating reward-related behaviors, the use of distinct molecular mechanisms may explain sex differences observed in stress susceptibility or response to rewarding stimuli. Uncovering these latent sex differences offers a deeper understanding of the sex-specific function of this behaviorally relevant synapse with widespread implications for circuits that underlie learning and reward-related behavior.
Introduction
Sex differences in reward-related behaviors are prevalent across a variety of species. For instance, humans and rodents show clear sex differences in sensitivity to rewarding stimuli and reward value (Yararbas et al., 2010; Warthen et al., 2011; Holly et al., 2012; Becker, 2016; Alarcón et al., 2017; Sinclair et al., 2017; Legget et al., 2018; Westbrook et al., 2018; Cullity et al., 2021; Aubry et al., 2022). There are also well-documented sex differences in related disorders like major depressive disorder (Marcus et al., 2005; Brody et al., 2018; Huang et al., 2019) and depressive-like behaviors in rodents (Dalla et al., 2008; Trainor et al., 2011; Burke et al., 2016; Song et al., 2018; Baratta et al., 2019; Goodwill et al., 2019; L.-L. Liu et al., 2019; Williams et al., 2020; Pitzer et al., 2022), and males and females tend to respond differently to antidepressant treatment [reviewed in LeGates et al. (2019)]. This may be explained by clear sex differences in depression-related neuronal activity in humans and preclinical models (Bangasser and Cuarenta, 2021; X. Wang et al., 2023). However, the precise neuronal mechanisms underlying sex differences in behavior and circuit function remain unknown.
The nucleus accumbens (NAc) is a key node of the reward pathway that responds to rewarding stimuli (Richter et al., 2020), integrates information from various sources to mediate goal-directed behavior (Gruber et al., 2009; Francis and Lobo, 2017), and is altered in preclinical depression models (Wacker et al., 2009; Drysdale et al., 2017). The hippocampus (Hipp) provides crucial excitatory input to the NAc, which influences NAc activity and conveys spatial and contextual information to guide reward-related behavior (O’Donnell et al., 1999; Floresco et al., 2001; Belujon and Grace, 2008; Ito et al., 2008; Britt et al., 2012; Gill and Grace, 2013; Bagot et al., 2015; Okuyama et al., 2016; Oliva et al., 2016; Gauthier and Tank, 2018; LeGates et al., 2018; Sjulson et al., 2018; Trouche et al., 2019; Y. Zhou et al., 2019; Williams et al., 2020; Lind et al., 2023). Our previous work revealed that long-term potentiation (LTP) of Hipp→NAc synapses drives reward-related behaviors, while exposure to chronic stress reduced Hipp→NAc excitatory synaptic strength, abolished LTP, and produced a concomitant aberration in reward-related behaviors (LeGates et al., 2018). This is supported by data from human subjects showing that functional connectivity of Hipp→striatal pathways is correlated to fluctuations in positive affect due to experiential diversity (Heller et al., 2020). These findings demonstrate a key bidirectional relationship between the strength of Hipp→NAc synapses and reward-related behaviors, highlighting the Hipp→NAc pathway as a crucial component of reward circuitry.
Given numerous examples of sex differences in reward behaviors that may be impacted by the Hipp→NAc pathway, we were interested in characterizing the molecular mechanisms underlying Hipp→NAc LTP in male and female mice. We previously found that LTP at Hipp→NAc medium spiny neuron (MSN) synapses in the medial shell of male mice requires NMDARs, postsynaptic Ca2+ influx, and CaMKII activity but occurs independently of D1R activation (LeGates et al., 2018). Here, we performed whole-cell electrophysiology and pharmacology to characterize the mechanisms underlying Hipp→NAc MSN plasticity in females, comparing them to mechanisms used in males. We found that high-frequency stimulation (HFS) of hippocampal axons induced LTP of similar magnitude in males and females. While LTP was supported by postsynaptic mechanisms in both sexes, we observed several key sex differences: LTP in males was NMDAR dependent while LTP in females occurred through an NMDAR-independent mechanism involving L-type VGCCs and ERα activity. LTP at Hipp→NAc synapses in both sexes required CaMKII activation and occurred independent of D1R activity suggesting important sex similarities exist as well. Taken together, these data reveal latent sex differences produce similar LTP at Hipp→NAc synapses, which may be a key factor contributing to sex differences in behavior and disorder.
Materials and Methods
Animals
Adult (8–10-week-old) male and female D1dra-tdTomato or C57BL/6J mice were bred in-house or purchased directly from Jackson Laboratories. The use of D1dra-tdTomato mice allowed us to identify dopamine-1-receptor and putative dopamine-2-receptor-expressing MSNs (D1-MSN and pD2-MSN): D1-MSNs expressed tdTomato while pD2-MSNs were unlabeled. D1dra-tdTomato mice were used for the experiments described in Figures 1 and 6 while C57BL/6J mice were used for the remaining experiments. Mice were housed with same-sex cage mates in a temperature- and humidity-controlled environment under a 12 h light/dark cycle (lights on at 07:00). We did not track estrous cycle in females. All experiments were performed in accordance with the regulations set forth by the Institutional Animal Care and Use Committee at the University of Maryland, Baltimore County.
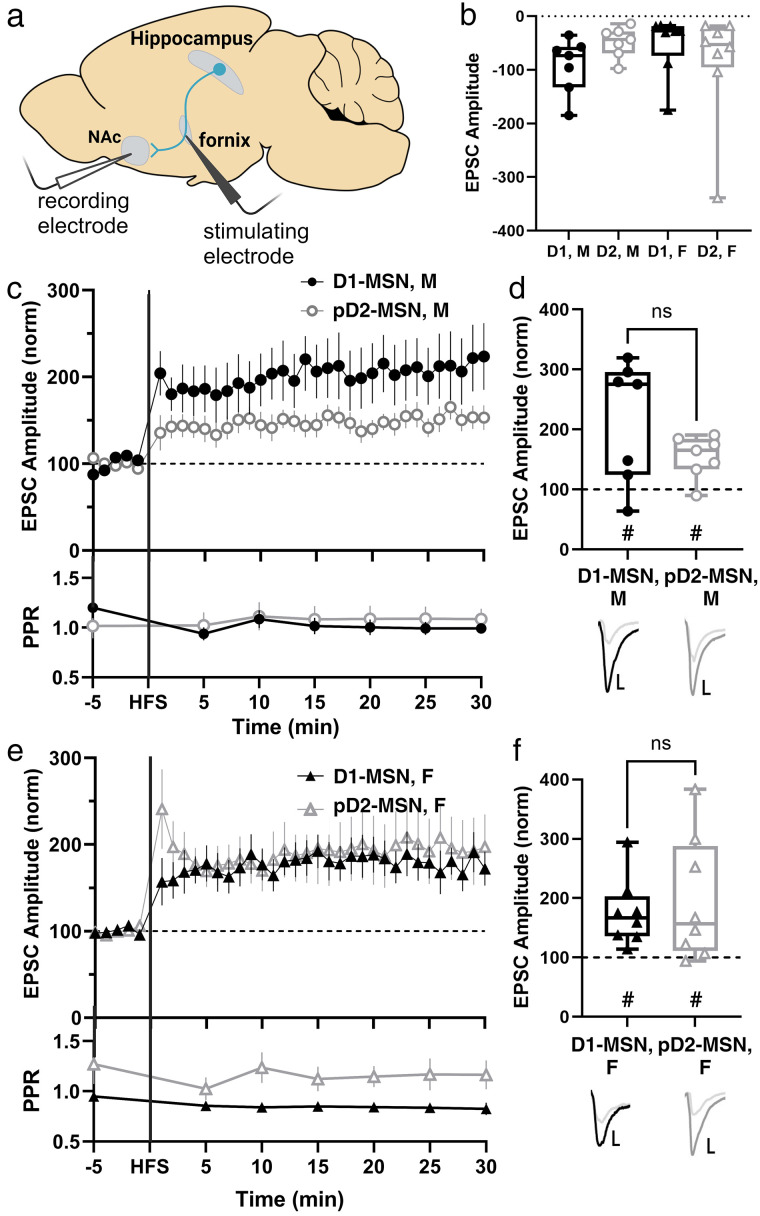
Figure 1.
Both sexes display a similar magnitude of LTP and a predicted postsynaptic locus of plasticity. a, Recording strategy with stimulating electrode placed in the fornix and recording electrode in the NAc medial shell to record Hipp-evoked EPSCs from MSNs. The shown parasagittal section represents a slice from lateral +0.48. b, Comparison of baseline, non-normalized EPSC amplitudes in male and female D1- and pD2-MSNs reveals no difference among sex or cell subtype (M D1-MSN n = 7 cells from 6 mice, M pD2-MSN n = 7 cells from 7 mice, F D1-MSN n = 8 cells from 7 mice, D2-MSN n = 8 cells from 8 mice; two-way ANOVA, ns p = 0.5191). c, Hippocampal-evoked EPSC amplitudes and PPR from D1- and pD2-MSNs in males. Data represent 1 min bins (means of all cells in each condition ± SEM; comparison of baseline PPR to 25–30 min PPR: M D1 n = 7 cells from 6 mice, p = 0.0781, two-tailed paired Wilcoxon test; M pD2 n = 7 cells from 7 mice, p > 0.9999, two-tailed paired Wilcoxon test). d, Summary EPSC data from 25 to 30 min post-HFS revealing similar magnitudes of LTP (M D1-MSN n = 7 cells from 6 mice, #p = 0.0469, two-tailed paired Wilcoxon test; M pD2-MSN n = 7 cells from 7 mice, #p = 0.0312, two-tailed paired Wilcoxon test; p = 0.3829, Mann–Whitney U test). Representative traces with scale bars, 40 pA/10 ms. e, Hippocampal-evoked EPSC amplitudes and PPR from D1- and pD2-MSNs in females. Data represent 1 min bins (means of all cells in each condition ± SEM; comparison of baseline PPR to 25–30 min PPR: F D1 n = 7 cells from 6 mice, p = 0.5781, two-tailed paired Wilcoxon test; M pD2 n = 8 cells from 8 mice, p = 0.3125, two-tailed paired Wilcoxon test). f, Summary EPSC data from 25 to 30 min post-HFS revealing similar magnitudes of LTP (F D1-MSN n = 8 cells from 7 mice, #p = 0.0078, two-tailed paired Wilcoxon test; F pD2-MSN n = 8 cells from 8 mice, #p = 0.0156, two-tailed paired Wilcoxon test; p = 0.9591, Mann–Whitney U test). Comparison of LTP magnitude between male and female D1- and pD2-MSNs reveal no significant difference (two-way ANOVA, ns p = 0.6067). Representative traces with scale bars, 40 pA/10 ms. *Differences between treatment and control by two-tailed Mann–Whitney U test. #Significant increase in EPSC amplitude above baseline revealed by two-tailed paired Wilcoxon test.
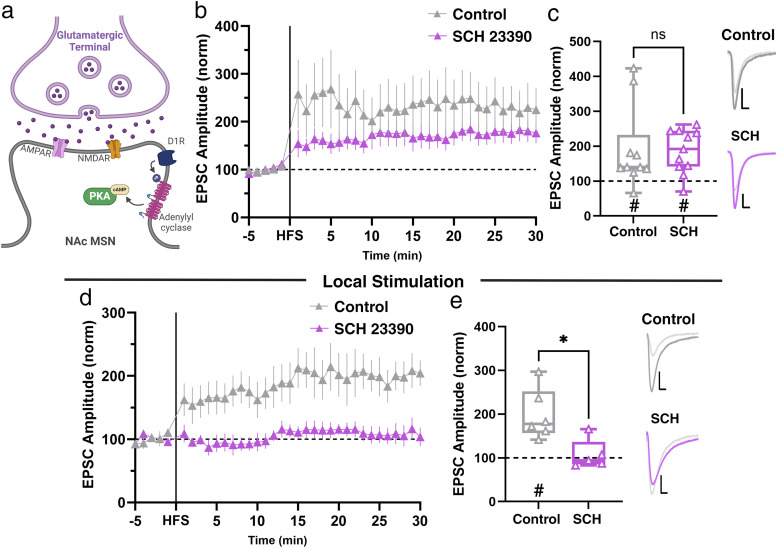
Figure 6.
Dopamine receptor activity is not required for Hipp→NAc LTP in females. a, Schematic of D1R downstream signaling that can contribute to LTP. b, Comparison of LTP in the presence and absence of D1R antagonist, SCH23390 (SCH). Data represent 1 min bins (means of all cells in each condition ± SEM). c, Summary EPSC data from 25 to 30 min post-HFS showing that SCH does not prevent LTP (control n = 10 cells from 10 mice, #p = 0.0020, two-tailed paired Wilcoxon test; SCH n = 11 cells from 9 mice, #p = 0.0020, two-tailed paired Wilcoxon test; p = 0.3867, Mann–Whitney U test). Representative trace with scale bars, 40 pA/10 ms. d, Comparison of LTP induced by local stimulation of NAc in the presence and absence of SCH. Data represent 1 min bins (means of all cells in each condition ± SEM). e, Summary EPSC data from 25 to 30 min post-HFS showing that LTP induced by local stimulation is prevented by application of SCH (control n = 6 cells from 5 mice, #p = 0.0312, two-tailed paired Wilcoxon test; SCH n = 5 cells from 4 mice, p = 0.8125, two-tailed paired Wilcoxon test; *p = 0.0173, Mann–Whitney U test). Representative trace with scale bars, 40 pA/10 ms. *Differences between treatment and control by two-tailed Mann–Whitney U test. #Significant increase in EPSC amplitude above baseline revealed by two-tailed paired Wilcoxon test.
Mouse brain slice preparation
Acute parasagittal slices (lateral 0.36–0.72) containing the fornix and nucleus accumbens were prepared for whole-cell patch-clamp electrophysiology. Animals were deeply anesthetized with isoflurane and decapitated, and brains were quickly dissected and submerged in ice-cold, bubbled (carbogen: 95% O2/5% CO2) N-methyl-D-glucamine (NMDG) recovery solution containing the following (in mM): 93 NMDG, 2.5 KCl, 1.2 NaH2PO4, 11 glucose, 25 NaHCO3, 1.2 MgCl2, and 2.4 CaCl2, pH 7.3–7.4, osmolarity = 300–310 mOsm. Using a vibratome (VT1000S, Leica Microsystems), parasagittal slices (400 µm) were cut in cold, oxygenated NMDG. Slices were transferred to 32–34°C NMDG for 7–12 min to recover and were then transferred to room temperature artificial cerebrospinal fluid (aCSF) containing the following (in mM): 120 NaCl, 3 KCl, 1.0 NaH2PO4, 20 glucose, 25 NaHCO3, 1.5 MgCl2·7H2O, and 2.5 CaCl2, pH 7.3–7.4. Slices were allowed to recover for 1 h at room temperature before beginning electrophysiological recordings.
Whole-cell recordings
We performed whole-cell patch-clamp recordings using an Axopatch 200B amplifier (Axon Instruments, Molecular Devices) and a Digidata 1550B digitizer (Axon Instruments). Slices were placed in a submersion-type recording chamber and superfused with room temperature aCSF (flow rate, 0.5–1 ml/min). Patch pipettes (4–8 MΩ) were made from borosilicate glass (World Precision Instruments) using a Sutter Instruments P-97 model puller. Cells were visualized using a 60× water immersion objective (Nikon Eclipse FN-1). D1R-MSNs were identified by the expression of tdTomato while putative D2R-MSNs were cells with a similar morphology that lacked expression of tdTomato.
All recordings were performed in voltage-clamp conditions from MSNs in the NAc medial shell. A bipolar stimulating electrode (FHC) was placed in the fornix to electrically stimulate hippocampal axons and record evoked excitatory postsynaptic currents (EPSCs). For local stimulation experiments (Fig. 6c,d), a bipolar stimulating electrode (FHC) was placed in the NAc to nonspecifically stimulate all inputs to NAc MSNs. For LTP experiments, patch pipettes were filled with a solution containing the following (in mM): 130 K-gluconate, 5 KCl, 2 MgCl6-H2O, 10 HEPES, 4 Mg-ATP, 0.3 Na2-GTP, 10 Na2-phosphocreatine, and 1 EGTA, pH 7.3–7.4; osmolarity = 285–295 mOsm. EPSCs were recorded from paired pulses (100 ms apart) performed every 10 s. Paired pulse ratio (PPR) was calculated by dividing EPSC2 by EPSC 1 (i.e. PPR = EPSC2/EPSC1). A 5 min baseline EPSC recording was obtained, then HFS (four trains of 100 Hz stimulation for 1 s with 15 s between trains while holding the cell at −40 mV) was used to induce LTP, followed by a 30 min recording of EPSCs. For experiments determining current–voltage (I–V) relationship, the patch pipette solution was composed of 135 mM CsCl, 2 mM MgCl6-H2O, 10 mM HEPES, 4 mM Mg-ATP, 0.3 mM Na2-GTP, 10 mM Na2-phosphocreatine, 1 mM EGTA, 5 mM QX-314, and 100 μM spermine, pH 7.3–7.4; osmolarity = 285–295 mOsm. EPSCs were collected from holding potentials ranging from −80 to +40 mV to create an I–V curve. For all pharmacological experiments, drugs [APV (Tocris, 50 µM), NASPM (Tocris, 20 µM), nimodipine (Tocris, 3 µM), KN-62 (Tocris, 3 µM), SCH23390 (Tocris, 3 µM), MPP dihydrochloride (Tocris, 3 µM)] were superfused over the slice for at least 15 min prior to recording. We used the following exclusion criteria to eliminate unhealthy cells and unreliable recordings: (1) We only proceeded with experiments on cells with series resistances <10 MΩ, (2) cells were excluded if their series resistance changed by >20% (comparing the resistance at the beginning and end of the experiment), (3) cells in poor health or poor recording status were excluded (i.e., cell partially or fully sealed up, a decrease in holding current >100 pA that is consistent with the cell dying, an increase in jitter post-HFS, and/or an increase in response failure rate to >50%).
Quantification, statistical analysis, and reproducibility
A total of 223 cells were recorded from 194 mice for these experiments. Males and females were kept separate in our analyses. We found no statistically significant difference between D1R- and pD2R-MSNs, so they are plotted together unless otherwise indicated. Both the number of cells and number of mice is reported for each experiment. The sample size (n) per condition represents the number of cells unless otherwise indicated in the figure caption. For LTP experiments, the 5 min baseline and last 5 min of recording were used for statistical comparisons. Two-tailed, paired Wilcoxon tests were used to determine whether a group of cells had a significant increase above baseline, indicating LTP. Pairwise comparisons using the Mann–Whitney U test were used to assess experimental condition differences due to non-normal distributions of data. Significant pairwise comparisons were reported. A p value of <0.05 was considered statistically significant, where exact p values can be found in the figure captions. For statistical tests considering both sex and cell type, a two-way ANOVA was used. All statistical analyses were performed using GraphPad Prism 9/10 software. For box plots, the line in the middle of the box is plotted at the median. The box extends from the 25th to 75th percentiles. Whiskers represent minimum and maximum. Figures were created with BioRender.com.
Results
HFS induces Hipp→NAc MSN LTP of similar magnitude in males and females
We performed whole-cell patch-clamp electrophysiology while electrically stimulating the fornix to record Hipp-evoked EPSCs in MSNs in the medial shell of the NAc (Fig. 1a). Slices were taken from D1dra-tdTomato mice, allowing us to distinguish between dopamine-1- and putative dopamine-2-receptor-expressing MSNs (D1-MSN and pD2-MSN) based on expression of tdTomato. In response to high frequency stimulation (HFS), we observed LTP of similar magnitude in male and female mice, with no difference between D1- and pD2-MSNs (Fig. 1). Comparison of paired-pulse ratio (PPR) baseline and post-HFS 25–30 min values suggests that LTP involves postsynaptic mechanisms in both male and female mice (Fig. 1c,e). These results indicate that LTP occurs at Hipp→NAc MSN synapses similarly in males and females, with no difference in baseline EPSC amplitude (Fig. 1b), LTP magnitude (Fig. 1c–f), or predicted locus of plasticity mechanisms between the sexes (Fig. 1c,e). Since there was no difference between D1- and pD2-MSNs, cells were pooled for the remainder of the data shown.
NMDAR activity is required for male, but not female, Hipp→NAc MSN LTP
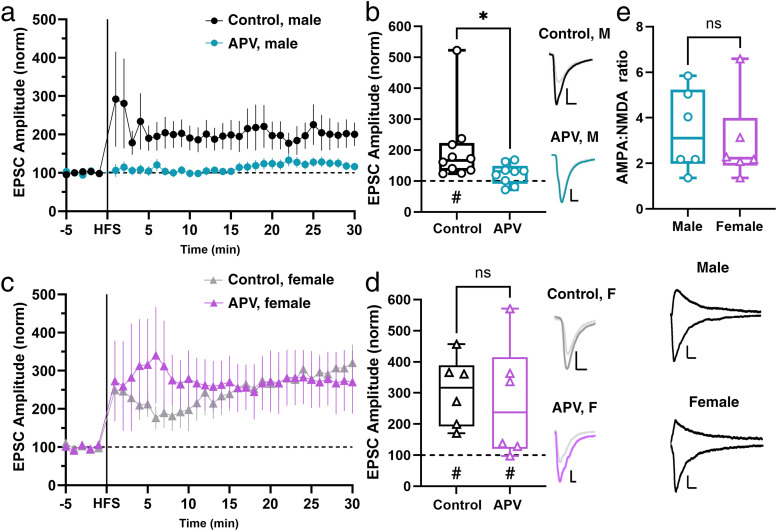
The prediction that both sexes use postsynaptic mechanisms for LTP suggests a rise in postsynaptic Ca2+ levels. At Hipp→NAc synapses in male mice, the proximate means of this Ca2+ is NMDARs (LeGates et al., 2018). We reproduced this result by repeating the experiment described above in the presence of 50 µM 2-aminophosphonovaleric acid (APV), an NMDAR antagonist (Fig. 2a,b). However, in slices taken from female mice, we found that APV was unable to block LTP (Fig. 2c,d), suggesting that while NMDARs are necessary for LTP at Hipp→NAc synapses in males, they are not required for LTP in females. We measured AMPA:NMDA ratio at Hipp→NAc synapses in male and female mice and found no sex difference in this ratio (Fig. 2e), suggesting that there is no difference in the relative strength of these synapses. The ability to collect NMDAR-mediated currents also shows that females have NMDARs present at Hipp→NAc synapses, suggesting that the lack of requirement for NMDARs in female LTP is not explained by a lack of NMDARs in the synapse. These experiments reveal the surprising sex-specific use of an NMDAR-independent pathway for LTP at Hipp→NAc synapses in females.
Figure 2.
LTP is NMDAR independent at Hipp→NAc synapses in females. a, Comparison of LTP in the presence and absence of NMDAR antagonist, APV, in males. Data represent 1 min bins (means of all cells in each condition ± SEM). b, Summary EPSC data from 25 to 30 min post-HFS showing abolishment of LTP in APV condition (Ctrl M n = 10 cells from 9 mice, #p = 0.0248, two-tailed paired Wilcoxon test; APV M n = 9 cells from 8 mice, p = 0.0750, two-tailed paired Wilcoxon test; *p = 0.0172, Mann–Whitney U test). Statistical difference is not driven by the control cell with large magnitude LTP (exclusion of this cell results in *p = 0.0315, Mann–Whitney U test). Representative trace with scale bars, 20 pA/10 ms. c, Comparison of LTP in the presence and absence of NMDAR antagonist, APV, in female mice. Data represent 1 min bins (means of all cells in each condition ± SEM). d, Summary EPSC data from 25 to 30 min post-HFS showing similar LTP magnitude in control and APV conditions (Ctrl F n = 6 cells from 6 mice, #p = 0.0312, two-tailed paired Wilcoxon test; APV F n = 7 cells from 5 mice, #p = 0.0312, two-tailed paired Wilcoxon test; p = 0.4248, Mann–Whitney U test). Representative trace with scale bars, 20 pA/10 ms. e, AMPA:NMDA ratio comparison in male and female mice reveals no sex differences in basal Hipp→NAc synaptic properties (male n = 6 cells from 4 mice; female n = 6 cells from 3 mice; p = 0.7338, Mann–Whitney U test). Representative trace with scale bars, 20 pA/50 ms. *Differences between treatment and control by two-tailed Mann–Whitney U test. #Significant increase in EPSC amplitude above baseline revealed by two-tailed paired Wilcoxon test.
L-type VGCC is required for Hipp→NAc MSN LTP in females
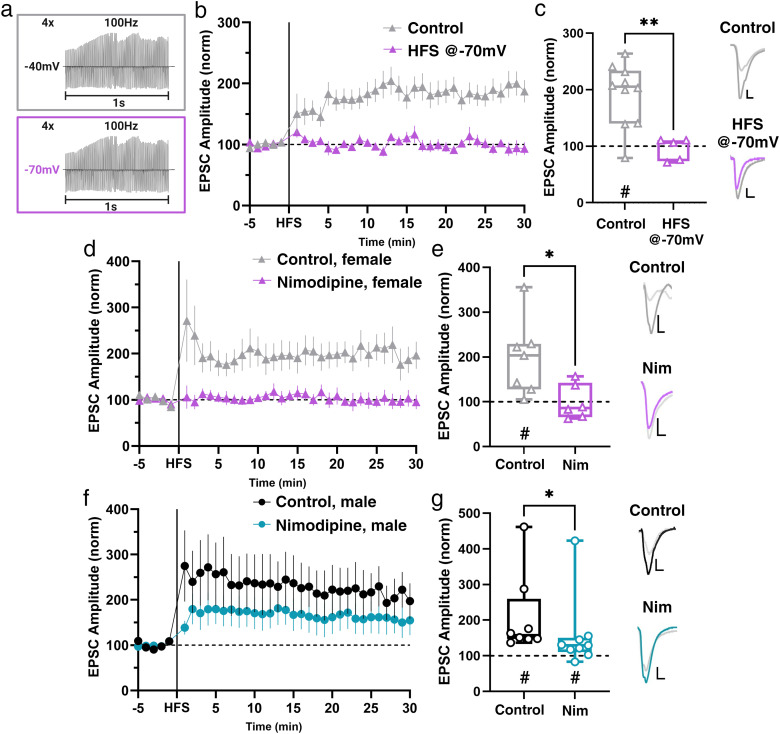
There are various NMDAR-independent mechanisms that have been shown to underlie postsynaptically expressed LTP which involve Ca2+-permeable AMPA receptors (CP-AMPAR), mobilization of intracellular Ca2+ stores, or VGCCs (Nanou and Catterall, 2018; Park et al., 2018; Padamsey et al., 2019; Alkadhi, 2021). To begin to understand which mechanisms may underlie LTP at Hipp→NAc synapses, we modified our LTP induction protocol to deliver HFS while holding the cell at −70 mV rather than the depolarized (−40 mV) potential we typically use. This modification effectively prevents the activation of any voltage-dependent processes during LTP induction. We found that delivering HFS in the absence of simultaneous depolarization prevented LTP induction (Fig. 3a–c), implicating the involvement of a voltage-dependent means of external Ca2+ in LTP at female Hipp→NAc synapses.
Figure 3.
L-type VGCCs are required for LTP at Hipp→NAc synapses in females. a, Control (depolarizing cell to −40 mV) and experimental (−70 mV) HFS protocols. b, Comparison of LTP with control HFS and HFS at −70 mV protocols. Data represent 1 min bins (means of all cells in each condition ± SEM). c, Summary EPSC data from 25 to 30 min post-HFS showing that HFS while holding the cell at −70 mV prevents LTP (control n = 10 cells from 9 mice, #p = 0.0006, two-tailed paired Wilcoxon test; HFS at −70 mV n = 5 cells from 5 mice, p = 0.5998, two-tailed paired Wilcoxon test; *p = 0.0047, Mann–Whitney U test). Representative trace with scale bars, 20 pA/10 ms. d, Comparison of LTP in the presence and absence of L-type VGCC antagonist, nimodipine (Nim), in female mice. Data represent 1 min bins (means of all cells in each condition ± SEM). e, Summary EPSC data from 25 to 30 min post-HFS reveals that Nim prevents LTP (control n = 7 cells from 6 mice, #p = 0.0223, two-tailed paired Wilcoxon test; Nim n = 6 cells from 5 mice, p = 0.9624, two-tailed paired Wilcoxon test; *p = 0.0221, Mann–Whitney U test). Representative trace with scale bars, 20 pA/10 ms. f, Comparison of LTP in the presence and absence of L-type VGCC antagonist, nimodipine (Nim), in male mice. Data represent 1 min bins (means of all cells in each condition ± SEM). g, Summary EPSC data from 25 to 30 min post-HFS reveals that Nim causes a decrease in the magnitude of LTP in males (control n = 8 cells from 7 mice, #p = 0.0078, two-tailed paired Wilcoxon test; Nim n = 9 cells from 8 mice, #p = 0.0195, two-tailed paired Wilcoxon test; *p = 0.0206, Mann–Whitney U test). Representative trace with scale bars, 20 pA/10 ms. *Differences between treatment and control by two-tailed Mann–Whitney U test. #Significant increase in EPSC amplitude above baseline revealed by two-tailed paired Wilcoxon test.
From here, we wanted to determine which type of voltage-gated channel was necessary for LTP at female Hipp→NAc synapses. L-type VGCCs have been implicated in postsynaptic forms of LTP in the amygdala and CA1 region of the hippocampus (Huber et al., 1995; Weisskopf et al., 1999) and are expressed postsynaptically within the NAc, allowing for voltage-dependent influx of Ca2+ into MSNs. Therefore, we hypothesized that L-type VGCCs mediate LTP at Hipp→NAc synapses in females. We tested this idea by pretreating slices with the L-type VGCC antagonist nimodipine (10 µM). Bath application of nimodipine was sufficient to block LTP in female mice (Fig. 3d,e), suggesting that L-type VGCCs are required for LTP at Hipp→NAc synapses in females. In contrast, inhibition of L-type VGCCs was not sufficient to block LTP in males, although LTP magnitude was reduced in the presence of nimodipine (Fig. 3f,g). Together, with our recordings in the presence of APV, this demonstrates that males and females utilize distinct sources of postsynaptic Ca2+ to mediate LTP at Hipp→NAc synapses, where males rely primarily on NMDARs with some contribution from L-type VGCCs while females utilize an NMDAR-independent mechanism that requires L-type VGCCs.
CP-AMPAR are not involved in Hipp→NAc MSN LTP
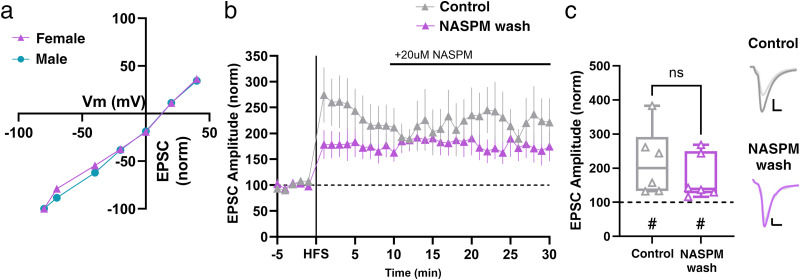
Given the role of CP-AMPARs in sex-specific mechanisms of synaptic potentiation in hippocampal CA1 neurons (Jain and Woolley, 2023), their contributions to multiple forms of NAc plasticity and behaviors that occur in response to drug exposure (Guire et al., 2008; Mameli et al., 2009; McCutcheon et al., 2011; Wolf and Tseng, 2012; Terrier et al., 2016; Carr, 2020; Park et al., 2021), and presence at ventral subiculum→NAc synapses (Boxer et al., 2023), we sought to investigate their potential role in LTP at Hipp→NAc synapses in females. CP-AMPARs have unique electrophysiological properties and are inwardly rectifying, whereas Ca2+-impermeable AMPARs display a linear current–voltage relationship (Cull-Candy et al., 2006; S. J. Liu and Zukin, 2007). This allows us to electrophysiologically determine the predominant population of AMPARs present at a particular synapse. We found that basal current–voltage relationships at Hipp→NAc MSN synapses were linear, demonstrating the absence of CP-AMPARs prior to HFS and precluding their involvement in LTP induction (Fig. 4a). Since CP-AMPARs can also be involved in LTP through preferential insertion following HFS (Whitehead et al., 2013), we aimed to determine whether they might instead play a role in LTP at Hipp→NAc synapses through this mechanism. We washed in 1-naphthylacetyl spermine (NASPM; 20 µM), a CP-AMPAR antagonist, 10 min after HFS, but found that NASPM wash-in had no effect on EPSC amplitude (Fig. 4b,c), suggesting that insertion of CP-AMPARs does not contribute to LTP at Hipp→NAc MSN synapses in female mice. Altogether, these results rule out the involvement of CP-AMPARs in LTP at Hipp→NAc MSN synapses in females which aligns with our previous observations in males (LeGates et al., 2018).
Figure 4.
CP-AMPARs are not present at Hipp→NAc synapses and insertion of CP-AMPARs is not required for LTP in females. a, Linear I–V relationship demonstrates AMPARs at Hipp→NAc synapses are Ca2+-impermeable (male n = 7 cells from 4 mice; female n = 9 cells from 3 mice). b, CP-AMPAR antagonist, NASPM, wash-on 10 min after HFS. Data represent 1 min bins (means of all cells in each condition ± SEM). c, Summary EPSC data from 25 to 30 min post-HFS showing that NASPM wash has no effect on LTP (control n = 6 cells from 6 mice, #p = 0.0156, two-tailed paired Wilcoxon test; NASPM wash n = 6 cells from 6 mice, #p = 0.0312, two-tailed paired Wilcoxon test; p = 0.3939, Mann–Whitney U test). Representative trace with scale bars, 40 pA/10 ms. *Differences between treatment and control by two-tailed Mann–Whitney U test. #Significant increase in EPSC amplitude above baseline revealed by two-tailed paired Wilcoxon test.
CaMKII is required for LTP in female mice
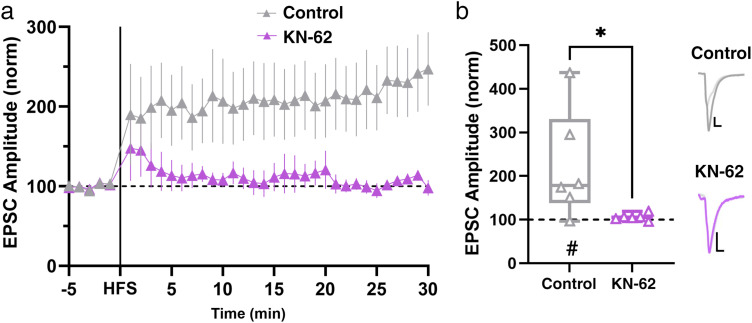
Despite males and females differing in their proximate means of postsynaptic Ca2+, we hypothesized that similar, Ca2+-dependent molecular players may be recruited downstream of this Ca2+ to mediate LTP. In male mice, the postsynaptic rise in Ca2+ initiates activation of CaMKII to cause LTP (LeGates et al., 2018). To determine whether this is consistent for LTP at Hipp→NAc synapses in females, we applied a CaMKII inhibitor (KN-62, 3 µM) before recording from MSNs. We found that blocking CaMKII prevented LTP in female mice (Fig. 5), suggesting that CAMKII activity is required for LTP in both sexes.
Figure 5.
Downstream of Ca2+ influx, CAMKII activity is required for LTP in females. a, Comparison of LTP in the presence and absence of CAMKII antagonist, KN-62. Data represent 1 min bins (means of all cells in each condition ± SEM). b, Summary EPSC data from 25 to 30 min post-HFS showing that KN-62 prevents LTP (control n = 6 cells from 6 mice, #p = 0.0312, two-tailed paired Wilcoxon test; KN-62 n = 6 cells from 6 mice, p = 0.1562, two-tailed paired Wilcoxon test; *p = 0.0411, Mann–Whitney U test). Representative trace with scale bars, 40 pA/10 ms. *Differences between treatment and control by two-tailed Mann–Whitney U test. #Significant increase in EPSC amplitude above baseline revealed by two-tailed paired Wilcoxon test.
Female Hipp→NAc MSN LTP occurs independently of dopamine
Dopamine is a well-known modulator of reward-related behaviors and plays a crucial role in regulating excitatory synapses within the NAc (Speranza et al., 2021). Many characterized forms of LTP at excitatory synapses within the NAc require D1R activity (Floresco et al., 2001; Goto and Grace, 2005; Hernandez et al., 2005; Mameli and Lüscher, 2011; Du Hoffmann and Nicola, 2014; Pignatelli and Bonci, 2015; Madadi Asl et al., 2018; Yu et al., 2022), but at Hipp→NAc synapses, HFS-induced LTP in males is unaffected by dopamine receptor blockade, demonstrating that LTP at these synapses in males occurs independent of dopamine receptor signaling (LeGates et al., 2018). To test whether this was also true at Hipp→NAc synapses in females, we blocked D1R activity with SCH 23390 (3 µM) and found that pretreatment of slices with SCH 23390 had no impact on Hipp→NAc MSN LTP in female mice (Fig. 6a–c). In separate slices, we used local stimulation to elicit EPSCs that were not pathway specific and found that HFS induces LTP that was blocked by pretreatment with SCH 23390 (Fig. 6d,e). Together, these results support previous findings on the importance of D1Rs in excitatory synaptic plasticity broadly in the NAc and highlight a key distinction at Hipp→NAc synapses where LTP occurs independent of dopamine receptor signaling in male and female mice.
Estrogen receptor activity is required for female Hipp→NAc MSN LTP
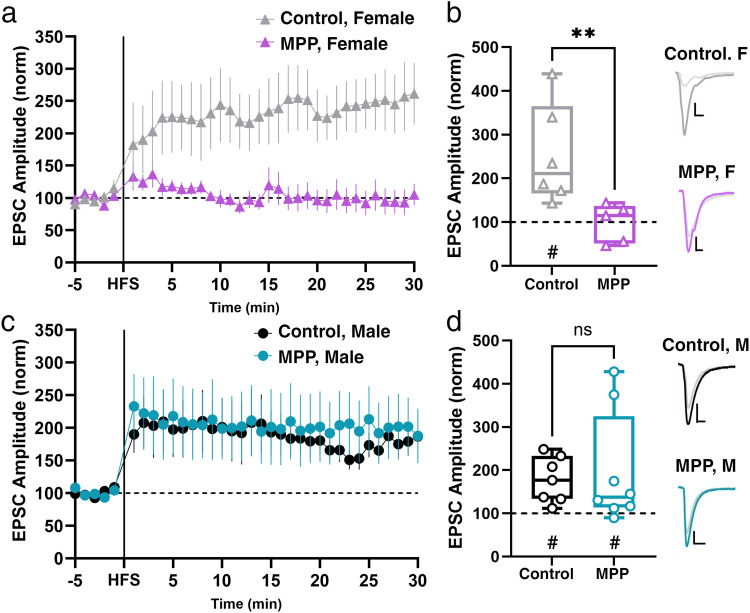
Estrogen can alter excitatory synapse function and plasticity (described in detail by Frick et al., 2015; Oberlander and Woolley, 2017; Jain and Woolley, 2023) and can regulate Ca2+ influx via L-type VGCCs in the striatum (Mermelstein et al., 1996; Sarkar et al., 2008). Additionally, while the mechanism underlying LTP at hippocampal CA1 is similar in male and females, females have an additional requirement of membrane-localized estrogen receptor-α (ERα) activation (X. Wang et al., 2018; Gall et al., 2023). Within the NAc, ERα is expressed primarily at the membrane in adult mice (Almey et al., 2022) and has been shown to interact with GPCRs to promote other forms of plasticity (Krentzel and Meitzen, 2018; Tonn Eisinger et al., 2018). Since ERα is moderately expressed in the NAc (Mitra et al., 2003) and has the potential to alter postsynaptic Ca2+ influx, we postulated that ERα is required for LTP at Hipp→NAc synapses in females. We used bath application of the ERα antagonist, MPP dihydrochloride (3 µM), to test the involvement of ERα in LTP at Hipp→NAc synapses. We found that pretreating slices with MPP prevented LTP in female mice but not male mice (Fig. 7), demonstrating the sex-specific requirement of ERα activation for LTP.
Figure 7.
Sex-specific requirement for ERα activity for Hipp→NAc LTP. a, Comparison of LTP in the presence and absence of an ERα antagonist, MPP dihydrochloride (MPP) in female mice. Data represent 1 min bins (means of all cells in each condition ± SEM). b, Summary EPSC data from 25 to 30 min post-HFS showing that ERα inhibition prevents LTP in female mice (Ctrl F n = 6 cells from 6 mice, #p = 0.0312, two-tailed paired Wilcoxon test; MPP F n = 5 cells from 5 mice, p = 0.8125, two-tailed paired Wilcoxon test; **p = 0.0087, Mann–Whitney U test). Representative trace with scale bars, 20 pA/10 ms. c, Comparison of LTP in the presence and absence of an ERα antagonist, MPP dihydrochloride (MPP) in male mice. Data represent 1 min bins (means of all cells in each condition ± SEM). d, Summary EPSC data from 25 to 30 min post-HFS showing that ERα inhibition has no effect on LTP in male mice (Ctrl M n = 7 cells from 7 mice, #p = 0.0156, two-tailed paired Wilcoxon test; MPP M n = 8 cells from 8 mice, #p = 0.0391, two-tailed paired Wilcoxon test; p = 0.6126, Mann–Whitney U test). Representative trace with scale bars, 20 pA/10 ms. *Differences between treatment and control by two-tailed Mann–Whitney U test. #Significant increase in EPSC amplitude above baseline revealed by two-tailed paired Wilcoxon test.
Discussion
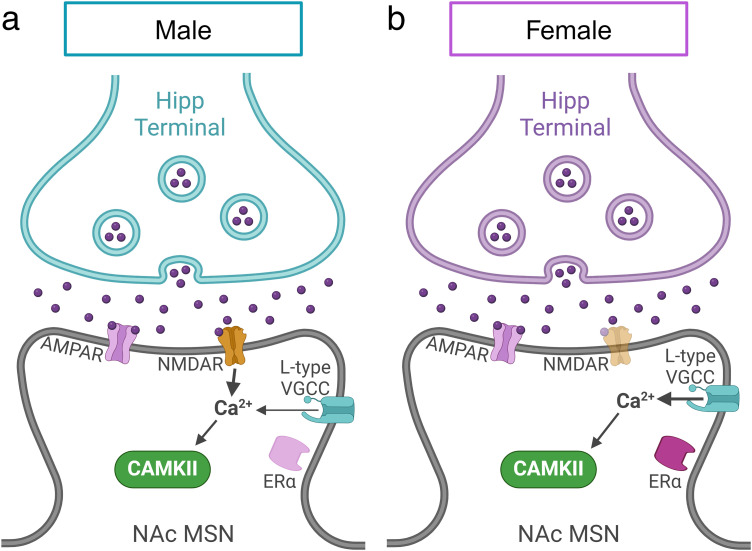
Our data reveal key sex-specific and sex-similar molecular mechanisms underlying LTP at Hipp→NAc synapses (Fig. 8). Males and females displayed LTP of similar magnitude that relies on common mechanisms like postsynaptic Ca2+ influx and CaMKII activity. However, key differences emerged when we investigated the proximate means of postsynaptic Ca2+; NMDARs are required for LTP at Hipp→NAc synapses in males, while L-type VGCCs are required in females. Furthermore, we identified a requirement for ERα in females that was not observed in males. Together, our results highlight the discovery of latent sex differences in the molecular mechanisms underlying LTP at Hipp→NAc synapses. Given the important role for these synapses in mediating reward-related behaviors, the identified sex differences have major implications for uncovering the neurobiological basis of sex variation in motivated behaviors and related psychiatric disorders.
Figure 8.
Comparison of sex-specific mechanisms involved in Hipp→NAc LTP. a, LTP at Hipp→NAc synapses in males requires NMDAR-mediated Ca2+ influx and CAMKII activation but does not require ERα or D1R activity. b, In females, LTP at Hipp→NAc synapses occurs with a mechanism involving L-type VGCCs instead of NMDARs for Ca2+ influx, CAMKII, and ERα activity but does not require D1R activity.
Similarities in synaptic strength and LTP magnitude across sex and cell subtype
A wealth of evidence has established clear sex differences in excitatory circuitry throughout the brain (McLaughlin et al., 2009; McEwen, 2010; Duarte-Guterman et al., 2015; Cao et al., 2018; Bangasser and Cuarenta, 2021; Johnson et al., 2023). This includes the NAc core where it is related to sex differences in cocaine-induced behaviors and synaptic plasticity (Forlano and Woolley, 2010; Wissman et al., 2011, 2012; Catalfio et al., 2023; Knouse et al., 2023; Lewitus and Blackwell, 2023). Our investigation of Hipp→NAc shell synapses revealed no differences in AMPA:NMDA ratio, AMPA subunit composition (CP-AMPA vs AMPA), or LTP magnitude across sex or MSN subtype demonstrating similarities between sexes and subtypes in basal synaptic strength and activity-dependent plasticity. We also noted that a small subset of cells across our study displayed HFS-induced long-term depression (LTD). Given its infrequency, we did not investigate this further, but HFS-induced LTD has been observed within the nucleus accumbens (Kombian and Malenka, 1994; Chergui, 2011) and at Hipp→NAc synapses in a rodent model of schizophrenia (Belujon et al., 2014) making it an interesting avenue of future research.
Sex differences in excitatory synaptic plasticity mechanisms
Despite similarities in LTP magnitude and the locus of plasticity, our experiments demonstrate latent sex differences underlie LTP at Hipp→NAc synapses. Hipp→NAc LTP in males requires NMDARs while L-type VGCCs facilitate typical LTP magnitude (Figs. 2, 3). In contrast, LTP in females occurs independent of NMDARs and instead relies on L-type VGCC (Fig. 3). This key difference in the type of calcium channel involved is a particularly unique finding as numerous studies have shown that other excitatory synapses onto MSNs primarily use NMDAR-dependent forms of plasticity (Floresco et al., 2001; Thomas and Malenka, 2003; Popescu et al., 2007; Vega-Villar et al., 2019), and to our knowledge, this is the first description of latent sex differences composed of NMDAR-dependent and NMDAR-independent mechanisms.
L-type VGCCs and NMDARs, which are both critical in long-lasting synaptic plasticity, are both voltage-dependent channels that facilitate Ca2+ influx to bind calmodulin, leading to Ca2+-dependent activation of CaMKII that is required for Hipp→NAc LTP in both sexes (Ataman et al., 2007; Berger and Bartsch, 2014; LeGates et al., 2018; Fig. 5). Despite these similarities, NMDARs and L-type VGCCs are differentially regulated, and their dysregulation is implicated in different behaviors and diseases (Q. Zhou and Sheng, 2013; Ortner and Striessnig, 2015; Myers et al., 2019; Laryushkin et al., 2021; Mielnik et al., 2021; Sanderson et al., 2022). For example, deletion of L-type VGCCs impairs learning and memory in females (Zanos et al., 2015; Klomp et al., 2022). Moreover, Cacna1c, which encodes the Cav1.2 subunit of the L-type VGCC, is associated with genetic risk for multiple mood disorders (Sklar et al., 2008; Bigos et al., 2010; Dedic et al., 2018; Moon et al., 2018; Jiang et al., 2023) and shows sex-specific interactions influencing depression (Dao et al., 2010). Since Hipp→NAc communication is a key mediator of reward-related behaviors, elucidation of sex-specific mechanisms underlying LTP may offer essential insight into sex differences in behavior and pathophysiology.
Hormones regulate Hipp→NAc synapses
Estrogen and testosterone are important regulators of synaptic transmission and plasticity in both sexes (Barth et al., 2015; W. Wang et al., 2016; Lu et al., 2019; Williams et al., 2020; Chen et al., 2022). We did not track estrous cycle in our experiments but observed variability in LTP magnitude in females, which may suggest a role for the estrous cycle in modulating plasticity at Hipp→NAc synapses. Additionally, the bimodal distribution of our data obtained in the presence of NMDAR antagonism may indicate estrous cycle-dependent differences in LTP mechanisms. To our knowledge, there are no descriptions of shifts in LTP mechanisms across the estrous cycle (e.g., NMDA-dependent to -independent), though there is evidence indicating estrous cycle-dependent changes in hippocampal LTP magnitude as well as expression and posttranslational modifications of key synaptic proteins including NMDARs (Warren et al., 1995; Good et al., 1999; Bi et al., 2001; Diao et al., 2007; Smith et al., 2009; Waters et al., 2009; Tada et al., 2015; Iqbal et al., 2020). Given the behavioral role for Hipp→NAc synapses in mediating learning and motivation, determining how the estrogen impacts plasticity of these synapses will provide key insight into the synaptic basis for sex-different and estrous cycle-dependent alterations in behavior.
A key distinction we observed was in the sex-specific requirement of ERα in LTP in females (Fig. 7). This is congruent with observations in the hippocampus demonstrating sex differences in the requirement of estrogen receptors for LTP in CA1 (Vierk et al., 2012; W. Wang et al., 2018) and may stem from differences in expression or function. Membrane-localized ERα (mERα) is prevalent in the hippocampus and NAc where nuclear ERα is less abundant (Mitra et al., 2003; Vasudevan and Pfaff, 2007; Schultz et al., 2009; Grove-Strawser et al., 2010; Stanić et al., 2014; Krentzel and Meitzen, 2018; Krentzel et al., 2020; Almey et al., 2022), and females express higher levels of synapse-localized ERα (W. Wang et al., 2018). mERα influences dendritic structure and synaptic function (P. Micevych and Christensen, 2012; W. Wang et al., 2018; Mazid et al., 2023), and in the NAc, where dendritic spine density is modulated by estradiol, mERα can rapidly modulate mEPSCs (Staffend et al., 2011; Peterson et al., 2015; Proaño et al., 2018, 2020; Krentzel et al., 2019; Beeson and Meitzen, 2023; Miller et al., 2023). mERα can functionally couple with mGluRs (P. E. Micevych and Mermelstein, 2008; Grove-Strawser et al., 2010; Tonn Eisinger et al., 2018) to influence L-type VGCCs (Subbamanda and Bhargava, 2022), supporting the idea that estradiol can have rapid, transcriptionally independent effects on excitatory synapses. Alternatively, our observed sex-specific effect of ERα antagonism could be due to sex-specific functions of ERα activation, which has been reported in brain regions with no sex differences in ERα expression (Oberlander and Woolley, 2017; Krentzel and Meitzen, 2018). Further studies are necessary to identify the source of the sex-specific requirement for ERα in LTP at Hipp→NAc synapses.
D1R is not required for LTP at Hipp→NAc synapses
Dopamine is a critical regulator of the reward system and typically an important factor in LTP within the NAc (Floresco et al., 2001; Jay et al., 2004; Goto and Grace, 2005; Mameli and Lüscher, 2011; Pignatelli and Bonci, 2015; Madadi Asl et al., 2018; Speranza et al., 2021; Yu et al., 2022). While D1R is required for LTP at many excitatory synapses in the NAc, our results (Fig. 6) and previous work (LeGates et al., 2018) show that D1R activation is not required for LTP at Hipp→NAc synapses, a finding observed elsewhere in the striatum and in hedonic reward learning behaviors (Pennartz et al., 1993; Berridge and Robinson, 1998; Cannon and Palmiter, 2003). Our findings do not preclude the possibility that dopamine can influence plasticity in this pathway. In fact, others have shown dopamine-dependent modulation of LTP magnitude even when not required for induction (Otani et al., 2003; FitzGerald et al., 2015; Palacios-Filardo and Mellor, 2019). Further examination is required to fully understand dopamine-dependent effects on Hipp→NAc synaptic plasticity.
Implications of latent sex differences in LTP at Hipp→NAc synapses
The modulation of Hipp→NAc synaptic transmission is a critical contributor to reward-related behaviors, and these synapses are altered in response to stress and cocaine (LeGates et al., 2018; Sjulson et al., 2018; Williams et al., 2020). As such, the sex differences in LTP mechanisms at Hipp→NAc synapses holds significant implications for stress- and reward-related behavior and physiology. Recent studies support the idea of sex-specific LTP mechanisms underlying sex differences in spatial learning and memory (Monfort et al., 2015; Sneider et al., 2015; Safari et al., 2021; Gall et al., 2023), so the use of distinct mechanisms for Hipp→NAc LTP may explain some sex-dependent behavioral changes that occur in response to stress and mood disorders (Seney and Sibille, 2014; Wei et al., 2014; Hodes et al., 2015; Brancato et al., 2017; Salk et al., 2017; Huang et al., 2019; Williams et al., 2020). Together, our findings highlight sex-specific mechanisms underlying plasticity in a reward pathway that redefines our knowledge about LTP and offers potential molecular targets for therapeutics to treat conditions linked to aberrant reward processing and stress.
References
- Alarcón G, Cservenka A, Nagel BJ (2017) Adolescent neural response to reward is related to participant sex and task motivation. Brain Cogn 111:51–62. 10.1016/j.bandc.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkadhi KA (2021) NMDA receptor-independent LTP in mammalian nervous system. Prog Neurobiol 200:101986. 10.1016/j.pneurobio.2020.101986 [DOI] [PubMed] [Google Scholar]
- Almey A, Milner TA, Brake WG (2022) Estrogen receptors observed at extranuclear neuronal sites and in glia in the nucleus accumbens core and shell of the female rat: evidence for localization to catecholaminergic and GABAergic neurons. J Comp Neurol 530:2056–2072. 10.1002/cne.25320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataman ZA, Gakhar L, Sorensen BR, Hell JW, Shea MA (2007) The NMDA receptor NR1 C1 region bound to calmodulin: structural insights into functional differences between homologous domains. Structure 15:1603–1617. 10.1016/j.str.2007.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry AV, Joseph Burnett C, Goodwin NL, Li L, Navarrete J, Zhang Y, Tsai V, Durand-de Cuttoli R, Golden SA, Russo SJ (2022) Sex differences in appetitive and reactive aggression. Neuropsychopharmacology 47:1746–1754. 10.1038/s41386-022-01375-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, et al. (2015) Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun 6:7062. 10.1038/ncomms8062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Cuarenta A (2021) Sex differences in anxiety and depression: circuits and mechanisms. Nat Rev Neurosci 22:674–684. 10.1038/s41583-021-00513-0 [DOI] [PubMed] [Google Scholar]
- Baratta MV, Gruene TM, Dolzani SD, Chun LE, Maier SF, Shansky RM (2019) Controllable stress elicits circuit-specific patterns of prefrontal plasticity in males, but not females. Brain Struct Funct 224:1831–1843. 10.1007/s00429-019-01875-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth C, Villringer A, Sacher J (2015) Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci 9:37. 10.3389/fnins.2015.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB (2016) Sex differences in addiction. Dialogues Clin Neurosci 18:395–402. 10.31887/DCNS.2016.18.4/jbecker [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson ALS, Meitzen J (2023) Estrous cycle impacts on dendritic spine plasticity in rat nucleus accumbens core and shell and caudate–putamen. J Comp Neurol 531:759–774. 10.1002/cne.25460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Grace AA (2008) Critical role of the prefrontal cortex in the regulation of hippocampus–accumbens information flow. J Neurosci 28:9797–9805. 10.1523/JNEUROSCI.2200-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Patton MH, Grace AA (2014) Role of the prefrontal cortex in altered hippocampal-accumbens synaptic plasticity in a developmental animal model of schizophrenia. Cereb Cortex 24:968–977. 10.1093/cercor/bhs380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger SM, Bartsch D (2014) The role of L-type voltage-gated calcium channels Cav1.2 and Cav1.3 in normal and pathological brain function. Cell Tissue Res 357:463–476. 10.1007/s00441-014-1936-3 [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev 28:309–369. 10.1016/S0165-0173(98)00019-8 [DOI] [PubMed] [Google Scholar]
- Bi R, Foy MR, Vouimba R-M, Thompson RF, Baudry M (2001) Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci U S A 98:13391–13395. 10.1073/pnas.241507698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigos KL, Mattay VS, Callicott JH, Straub RE, Vakkalanka R, Kolachana B, Hyde TM, Lipska BK, Kleinman JE, Weinberger DR (2010) Genetic variation in CACNA1C affects brain circuitries related to mental illness. Arch Gen Psychiatry 67:939–945. 10.1001/archgenpsychiatry.2010.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer EE, Kim J, Dunn B, Aoto J (2023) Ventral subiculum inputs to nucleus accumbens medial shell preferentially innervate D2R medium spiny neurons and contain calcium permeable AMPARs. J Neurosci 43:1166–1177. 10.1523/JNEUROSCI.1907-22.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancato A, Bregman D, Ahn HF, Pfau ML, Menard C, Cannizzaro C, Russo SJ, Hodes GE (2017) Sub-chronic variable stress induces sex-specific effects on glutamatergic synapses in the nucleus accumbens. Neuroscience 350:180–189. 10.1016/j.neuroscience.2017.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A (2012) Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron 76:790–803. 10.1016/j.neuron.2012.09.040.Synaptic [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody DJ, Pratt LA, Hughes JP (2018) Prevalence of depression among adults aged 20 and over: United States, 2013-2016. NCHS Data Brief, 303, 1–8.
- Burke NN, Coppinger J, Deaver DR, Roche M, Finn DP, Kelly J (2016) Sex differences and similarities in depressive- and anxiety-like behaviour in the Wistar-Kyoto rat. Physiol Behav 167:28–34. 10.1016/j.physbeh.2016.08.031 [DOI] [PubMed] [Google Scholar]
- Cannon CM, Palmiter RD (2003) Reward without dopamine. J Neurosci 23:10827–10831. 10.1523/JNEUROSCI.23-34-10827.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Willett JA, Dorris DM, Meitzen J (2018) Sex differences in medium spiny neuron excitability and glutamatergic synaptic input: heterogeneity across striatal regions and evidence for estradiol-dependent sexual differentiation. Front Endocrinol 9:173. 10.3389/fendo.2018.00173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD (2020) Homeostatic regulation of reward via synaptic insertion of calcium-permeable AMPA receptors in nucleus accumbens. Physiol Behav 219:112850. 10.1016/j.physbeh.2020.112850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalfio AM, Fetterly TL, Nieto AM, Robinson TE, Ferrario CR (2023) Cocaine-induced sensitization and glutamate plasticity in the nucleus accumbens core: effects of sex. Biol Sex Differ 14:41. 10.1186/s13293-023-00525-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, et al. (2022) Effects of membrane androgen receptor binding on synaptic plasticity in primary hippocampal neurons. Mol Cell Endocrinol 554:111711. 10.1016/j.mce.2022.111711 [DOI] [PubMed] [Google Scholar]
- Chergui K (2011) Dopamine induces a GluN2A-dependent form of long-term depression of NMDA synaptic responses in the nucleus accumbens. Neuropharmacology 60:975–981. 10.1016/j.neuropharm.2011.01.047 [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Kelly L, Farrant M (2006) Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr Opin Neurobiol 16:288–297. 10.1016/j.conb.2006.05.012 [DOI] [PubMed] [Google Scholar]
- Cullity ER, Guerin AA, Perry CJ, Kim JH (2021) Examining sex differences in conditioned place preference or aversion to methamphetamine in adolescent and adult mice. Front Pharmacol 12:770614. 10.3389/fphar.2021.770614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Edgecomb C, Whetstone AS, Shors TJ (2008) Females do not express learned helplessness like males do. Neuropsychopharmacology 33:1559–1569. 10.1038/sj.npp.1301533 [DOI] [PubMed] [Google Scholar]
- Dao DT, et al. (2010) Mood disorder susceptibility gene CACNA1C modifies mood-related behaviors in mice and interacts with sex to influence behavior in mice and diagnosis in humans. Biol Psychiatry 68:801–810. 10.1016/j.biopsych.2010.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedic N, et al. (2018) Cross-disorder risk gene CACNA1C differentially modulates susceptibility to psychiatric disorders during development and adulthood. Mol Psychiatry 23:533–543. 10.1038/mp.2017.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao W-F, Höger H, Chen W-Q, Pollak A, Lubec G (2007) Estrous-cycle-dependent hippocampal levels of signaling proteins. Hippocampus 17:563–576. 10.1002/hipo.20293 [DOI] [PubMed] [Google Scholar]
- Drysdale AT, et al. (2017) Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med 23:28–38. 10.1038/nm.4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte-Guterman P, Yagi S, Chow C, Galea LAM (2015) Hippocampal learning, memory, and neurogenesis: effects of sex and estrogens across the lifespan in adults. Horm Behav 74:37–52. 10.1016/j.yhbeh.2015.05.024 [DOI] [PubMed] [Google Scholar]
- Du Hoffmann J, Nicola SM (2014) Dopamine invigorates reward seeking by promoting cue-evoked excitation in the nucleus accumbens. J Neurosci 34:14349–14364. 10.1523/JNEUROSCI.3492-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald THB, Dolan RJ, Friston K (2015) Dopamine, reward learning, and active inference. Front Comput Neurosci 9:136. 10.3389/fncom.2015.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG (2001) Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection. J Neurosci 21:2851–2860. 10.1523/JNEUROSCI.21-08-02851.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano PM, Woolley CS (2010) Quantitative analysis of pre- and postsynaptic sex differences in the nucleus accumbens. J Comp Neurol 518:1330–1348. 10.1002/cne.22279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis TC, Lobo MK (2017) Emerging role for nucleus accumbens medium spiny neuron subtypes in depression. Biol Psychiatry 81:645–653. 10.1016/j.biopsych.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Kim J, Tuscher JJ, Fortress AM (2015) Sex steroid hormones matter for learning and memory: estrogenic regulation of hippocampal function in male and female rodents. Learn Mem 22:472–493. 10.1101/lm.037267.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall CM, Le AA, Lynch G (2023) Sex differences in synaptic plasticity underlying learning. J Neurosci Res 101:764–782. 10.1002/jnr.24844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier JL, Tank DW (2018) A dedicated population for reward coding in the hippocampus. Neuron 99:179–193.e7. 10.1016/j.neuron.2018.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill KM, Grace AA (2013) Differential effects of acute and repeated stress on hippocampus and amygdala inputs to the nucleus accumbens shell. Int J Neuropsychopharmacol 16:2013–2025. 10.1017/S1461145713000618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M, Day M, Muir JL (1999) Cyclical changes in endogenous levels of oestrogen modulate the induction of LTD and LTP in the hippocampal CA1 region. Eur J Neurosci 11:4476–4480. 10.1046/j.1460-9568.1999.00920.x [DOI] [PubMed] [Google Scholar]
- Goodwill HL, Manzano-Nieves G, Gallo M, Lee H-I, Oyerinde E, Serre T, Bath KG (2019) Early life stress leads to sex differences in development of depressive-like outcomes in a mouse model. Neuropsychopharmacology 44:711–720. 10.1038/s41386-018-0195-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA (2005) Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron 47:255–266. 10.1016/j.neuron.2005.06.017 [DOI] [PubMed] [Google Scholar]
- Grove-Strawser D, Boulware MI, Mermelstein PG (2010) Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience 170:1045–1055. 10.1016/j.neuroscience.2010.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber AJ, Hussain RJ, O’Donnell P (2009) The nucleus accumbens: a switchboard for goal-directed behaviors. PLoS One 4:e5062. 10.1371/journal.pone.0005062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guire ES, Oh MC, Soderling TR, Derkach VA (2008) Recruitment of calcium-permeable AMPA receptors during synaptic potentiation is regulated by CaM-kinase I. J Neurosci 28:6000–6009. 10.1523/JNEUROSCI.0384-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Shi TC, Ezie CEC, Reneau TR, Baez LM, Gibbons CJ, Hartley CA (2020) Association between real-world experiential diversity and positive affect relates to hippocampal-striatal functional connectivity. Nat Neurosci 23:800–804. 10.1038/s41593-020-0636-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez PJ, Andrzejewski ME, Sadeghian K, Panksepp JB, Kelley AE (2005) AMPA/kainate, NMDA, and dopamine D1 receptor function in the nucleus accumbens core: a context-limited role in the encoding and consolidation of instrumental memory. Learn Mem 12:285–295. 10.1101/lm.93105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, et al. (2015) Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J Neurosci 35:16362–16376. 10.1523/JNEUROSCI.1392-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly EN, Shimamoto A, DeBold JF, Miczek KA (2012) Sex differences in behavioral and neural cross-sensitization and escalated cocaine taking as a result of episodic social defeat stress in rats. Psychopharmacology 224:179–188. 10.1007/s00213-012-2846-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, et al. (2019) Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry 6:211–224. 10.1016/S2215-0366(18)30511-X [DOI] [PubMed] [Google Scholar]
- Huber KM, Mauk MD, Kelly PT (1995) Distinct LTP induction mechanisms: contribution of NMDA receptors and voltage-dependent calcium channels. J Neurophysiol 73:270–279. 10.1152/jn.1995.73.1.270 [DOI] [PubMed] [Google Scholar]
- Iqbal J, Tan Z-N, Li M-X, Chen H-B, Ma B, Zhou X, Ma X-M (2020) Estradiol alters hippocampal gene expression during the estrous cycle. Endocr Res 45:84–101. 10.1080/07435800.2019.1674868 [DOI] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Pennartz CM, Everitt BJ (2008) Functional interaction between the hippocampus and nucleus accumbens shell is necessary for the acquisition of appetitive spatial context conditioning. J Neurosci 28:6950–6959. 10.1523/JNEUROSCI.1615-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Woolley CS (2023) Mechanisms that underlie expression of estradiol-induced excitatory synaptic potentiation in the hippocampus differ between males and females. J Neurosci 43:1298–1309. 10.1523/JNEUROSCI.2080-19.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM, Rocher C, Hotte M, Naudon L, Gurden H, Spedding M (2004) Plasticity at hippocampal to prefrontal cortex synapses is impaired by loss of dopamine and stress: importance for psychiatric diseases. Neurotox Res 6:233–244. 10.1007/BF03033225 [DOI] [PubMed] [Google Scholar]
- Jiang X, Sultan AA, Dimick MK, Zai CC, Kennedy JL, MacIntosh BJ, Goldstein BI (2023) The association of genetic variation in CACNA1C with resting-state functional connectivity in youth bipolar disorder. Int J Bipolar Disord 11:3. 10.1186/s40345-022-00281-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CS, Chapp AD, Lind EB, Thomas MJ, Mermelstein PG (2023) Sex differences in mouse infralimbic cortex projections to the nucleus accumbens shell. Biol Sex Differ 14:87. 10.1186/s13293-023-00570-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klomp AJ, Plumb A, Mehr JB, Madencioglu DA, Wen H, Williams AJ (2022) Neuronal deletion of CaV1.2 is associated with sex-specific behavioral phenotypes in mice. Sci Rep 12:22152. 10.1038/s41598-022-26504-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knouse MC, Deutschmann AU, Nenov MN, Wimmer ME, Briand LA (2023) Sex differences in pre- and post-synaptic glutamate signaling in the nucleus accumbens core. Biol Sex Differ 14:52. 10.1186/s13293-023-00537-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombian SB, Malenka RC (1994) Simultaneous LTP of non-NMDA- and LTD of NMDA-receptor-mediated responses in the nucleus accumbens. Nature 368:242–246. 10.1038/368242a0 [DOI] [PubMed] [Google Scholar]
- Krentzel AA, Barrett LR, Meitzen J (2019) Estradiol rapidly modulates excitatory synapse properties in a sex- and region-specific manner in rat nucleus accumbens core and caudate-putamen. J Neurophysiol 122:1213–1225. 10.1152/jn.00264.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentzel AA, Meitzen J (2018) Biological sex, estradiol and striatal medium spiny neuron physiology: a mini-review. Front Cell Neurosci 12:492. 10.3389/fncel.2018.00492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentzel AA, Willett JA, Johnson AG, Meitzen J (2020) Estrogen receptor alpha, g-protein coupled estrogen receptor 1, and aromatase: developmental, sex, and region-specific differences across the rat caudate-putamen, nucleus accumbens core and shell. J Comp Neurol 529:786–801. 10.1002/cne.24978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laryushkin DP, Maiorov SA, Zinchenko VP, Gaidin SG, Kosenkov AM (2021) Role of L-type voltage-gated calcium channels in epileptiform activity of neurons. Int J Mol Sci 22:10342. 10.3390/ijms221910342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates TA, Kvarta MD, Thompson SM (2019) Sex differences in antidepressant efficacy. Neuropsychopharmacology 44:140–154. 10.1038/s41386-018-0156-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates TA, Kvarta MD, Tooley JR, Francis TC, Lobo MK, Creed MC, Thompson SM (2018) Reward behaviour is regulated by the strength of hippocampus–nucleus accumbens synapses. Nature 564:258–262. 10.1038/s41586-018-0740-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legget KT, Cornier M-A, Bessesen DH, Mohl B, Thomas EA, Tregellas JR (2018) Greater reward-related neuronal response to hedonic foods in women compared to men. Obesity 26:362–367. 10.1002/oby.22082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewitus VJ, Blackwell KT (2023) Estradiol receptors inhibit long-term potentiation in the dorsomedial striatum. eNeuro 10:ENEURO.0071-23.2023. 10.1523/ENEURO.0071-23.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind EB, Sweis BM, Asp AJ, Esguerra M, Silvis KA, David Redish A, Thomas MJ (2023) A quadruple dissociation of reward-related behaviour in mice across excitatory inputs to the nucleus accumbens shell. Commun Biol 6:119. 10.1038/s42003-023-04429-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L-L, Li J-M, Su W-J, Wang B, Jiang C-L (2019) Sex differences in depressive-like behaviour may relate to imbalance of microglia activation in the hippocampus. Brain Behav Immun 81:188–197. 10.1016/j.bbi.2019.06.012 [DOI] [PubMed] [Google Scholar]
- Liu SJ, Zukin RS (2007) Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci 30:126–134. 10.1016/j.tins.2007.01.006 [DOI] [PubMed] [Google Scholar]
- Lu Y, et al. (2019) Neuron-derived estrogen regulates synaptic plasticity and memory. J Neurosci 39:2792–2809. 10.1523/JNEUROSCI.1970-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madadi Asl M, Vahabie AH, Valizadeh A (2018) Dopaminergic modulation of synaptic plasticity, its role in neuropsychiatric disorders, and its computational modeling. Basic Clin Neurosci 10:1–12. 10.32598/bcn.9.10.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Lüscher C (2009) Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci 12:1036–1041. 10.1038/nn.2367 [DOI] [PubMed] [Google Scholar]
- Mameli M, Lüscher C (2011) Synaptic plasticity and addiction: learning mechanisms gone awry. Neuropharmacology 61:1052–1059. 10.1016/j.neuropharm.2011.01.036 [DOI] [PubMed] [Google Scholar]
- Marcus SM, Young EA, Kerber KB, Kornstein S, Farabaugh AH, Mitchell J, Wisniewski SR, Balasubramani GK, Trivedi MH, Rush AJ (2005) Gender differences in depression: findings from the STAR*D study. J Affect Disord 87:141–150. 10.1016/j.jad.2004.09.008 [DOI] [PubMed] [Google Scholar]
- Mazid S, Waters EM, Lopez-Lee C, Poultan Kamakura R, Rubin BR, Levin ER, McEwen BS, Milner TA (2023) Both nuclear and membrane estrogen receptor alpha impact the expression of estrogen receptors and plasticity markers in the mouse hypothalamus and hippocampus. Biology 12:632. 10.3390/biology12040632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M (2011) Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci 31:5737–5743. 10.1523/JNEUROSCI.0350-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2010) Stress, sex and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann N Y Acad Sci 1204:E38–E59. 10.1111/j.1749-6632.2010.05568.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Conrad CD (2009) Chronic stress- and sex-specific neuromorphological and functional changes in limbic structures. Mol Neurobiol 40:166–182. 10.1007/s12035-009-8079-7 [DOI] [PubMed] [Google Scholar]
- Mermelstein P, Becker J, Surmeier D (1996) Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci 16:595–604. 10.1523/JNEUROSCI.16-02-00595.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Christensen A (2012) Membrane-initiated estradiol actions mediate structural plasticity and reproduction. Front Neuroendocrinol 33:331–341. 10.1016/j.yfrne.2012.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Mermelstein PG (2008) Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Mol Neurobiol 38:66–77. 10.1007/s12035-008-8034-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielnik CA, et al. (2021) Consequences of NMDA receptor deficiency can be rescued in the adult brain. Mol Psychiatry 26:2929–2942. 10.1038/s41380-020-00859-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CK, Krentzel AA, Meitzen J (2023) ERα stimulation rapidly modulates excitatory synapse properties in female rat nucleus accumbens core. Neuroendocrinology 113:1140–1153. 10.1159/000529571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SW, et al. (2003) Immunolocalization of estrogen receptor β in the mouse brain: comparison with estrogen receptor α. Endocrinology 144:2055–2067. 10.1210/en.2002-221069 [DOI] [PubMed] [Google Scholar]
- Monfort P, Gomez-Gimenez B, Llansola M, Felipo V (2015) Gender differences in spatial learning, synaptic activity, and long-term potentiation in the hippocampus in rats: molecular mechanisms. ACS Chem Neurosci 6:1420–1427. 10.1021/acschemneuro.5b00096 [DOI] [PubMed] [Google Scholar]
- Moon AL, Haan N, Wilkinson LS, Thomas KL, Hall J (2018) CACNA1C: association with psychiatric disorders, behavior, and neurogenesis. Schizophr Bull 44:958–965. 10.1093/schbul/sby096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers SJ, Yuan H, Kang J-Q, Tan FCK, Traynelis SF, Low C-M (2019) Distinct roles of GRIN2A and GRIN2B variants in neurological conditions. F1000Res 8, F1000 Faculty Rev-1940. 10.12688/f1000research.18949.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanou E, Catterall WA (2018) Calcium channels, synaptic plasticity, and neuropsychiatric disease. Neuron 98:466–481. 10.1016/j.neuron.2018.03.017 [DOI] [PubMed] [Google Scholar]
- Oberlander JG, Woolley CS (2017) 17β-Estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. J Neurosci 37:12314–12327. 10.1523/JNEUROSCI.3011-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P, Greene J, Pabello N, Lewis BL, Grace AA (1999) Modulation of cell firing in the nucleus accumbens. Ann N Y Acad Sci 877:157–175. 10.1111/j.1749-6632.1999.tb09267.x [DOI] [PubMed] [Google Scholar]
- Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S (2016) Ventral CA1 neurons store social memory. Science 353:1536–1541. 10.1126/science.aaf7003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva A, Fernández-Ruiz A, Buzsáki G, Berényi A (2016) Spatial coding and physiological properties of hippocampal neurons in the cornu ammonis subregions. Hippocampus 26:1593–1607. 10.1002/hipo.22659 [DOI] [PubMed] [Google Scholar]
- Ortner NJ, Striessnig J (2015) L-type calcium channels as drug targets in CNS disorders. Channels 10:7–13. 10.1080/19336950.2015.1048936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani S, Daniel H, Roisin M-P, Crepel F (2003) Dopaminergic modulation of long-term synaptic plasticity in rat prefrontal neurons. Cereb Cortex 13:1251–1256. 10.1093/cercor/bhg092 [DOI] [PubMed] [Google Scholar]
- Padamsey Z, Foster WJ, Emptage NJ (2019) Intracellular Ca2+ release and synaptic plasticity: a tale of many stores. Neuroscientist 25:208–226. 10.1177/1073858418785334 [DOI] [PubMed] [Google Scholar]
- Palacios-Filardo J, Mellor JR (2019) Neuromodulation of hippocampal long-term synaptic plasticity. Curr Opin Neurobiol 54:37–43. 10.1016/j.conb.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park P, Kang H, Georgiou J, Zhuo M, Kaang B-K, Collingridge GL (2021) Further evidence that CP-AMPARs are critically involved in synaptic tag and capture at hippocampal CA1 synapses. Mol Brain 14:26. 10.1186/s13041-021-00737-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park P, Kang H, Sanderson TM, Bortolotto ZA, Georgiou J, Zhuo M, Kaang B-K, Collingridge GL (2018) The role of calcium-permeable AMPARs in long-term potentiation at principal neurons in the rodent hippocampus. Front Synaptic Neurosci 10:42. 10.3389/fnsyn.2018.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CMA, Ameerun RF, Groenewegen HJ, Lopes da Silva FH (1993) Synaptic plasticity in an in vitro slice preparation of the rat nucleus accumbens. Eur J Neurosci 5:107–117. 10.1111/j.1460-9568.1993.tb00475.x [DOI] [PubMed] [Google Scholar]
- Peterson BM, Mermelstein PG, Meisel RL (2015) Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Struct Funct 220:2415–2422. 10.1007/s00429-014-0794-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli M, Bonci A (2015) Role of dopamine neurons in reward and aversion: a synaptic plasticity perspective. Neuron 86:1145–1157. 10.1016/j.neuron.2015.04.015 [DOI] [PubMed] [Google Scholar]
- Pitzer C, Kurpiers B, Eltokhi A (2022) Sex differences in depression-like behaviors in adult mice depend on endophenotype and strain. Front Behav Neurosci 16:838122. 10.3389/fnbeh.2022.838122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu AT, Saghyan AA, Paré D (2007) NMDA-dependent facilitation of corticostriatal plasticity by the amygdala. Proc Natl Acad Sci U S A 104:341–346. 10.1073/pnas.0609831104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proaño SB, Krentzel AA, Meitzen J (2020) Differential and synergistic roles of 17β-estradiol and progesterone in modulating adult female rat nucleus accumbens core medium spiny neuron electrophysiology. J Neurophysiol 123:2390–2405. 10.1152/jn.00157.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proaño SB, Morris HJ, Kunz LM, Dorris DM, Meitzen J (2018) Estrous cycle-induced sex differences in medium spiny neuron excitatory synaptic transmission and intrinsic excitability in adult rat nucleus accumbens core. J Neurophysiol 120:1356–1373. 10.1152/jn.00263.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A, Reinhard F, Kraemer B, Gruber O (2020) A high-resolution fMRI approach to characterize functionally distinct neural pathways within dopaminergic midbrain and nucleus accumbens during reward and salience processing. Eur Neuropsychopharmacol 36:137–150. 10.1016/j.euroneuro.2020.05.005 [DOI] [PubMed] [Google Scholar]
- Safari S, Ahmadi N, Mohammadkhani R, Ghahremani R, Khajvand-Abedeni M, Shahidi S, Komaki A, Salehi I, Karimi SA (2021) Sex differences in spatial learning and memory and hippocampal long-term potentiation at perforant pathway-dentate gyrus (PP-DG) synapses in Wistar rats. Behav Brain Funct 17:9. 10.1186/s12993-021-00184-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salk RH, Hyde JS, Abramson LY (2017) Gender differences in depression in representative national samples: meta-analyses of diagnoses and symptoms. Psychol Bull 143:783–822. 10.1037/bul0000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson JL, Freund RK, Castano AM, Benke TA, DellAcqua ML (2022) The CaV1.2 G406R mutation decreases synaptic inhibition and alters L-type Ca2+ channel-dependent LTP at hippocampal synapses in a mouse model of Timothy syndrome. Neuropharmacology 220:109271. 10.1016/j.neuropharm.2022.109271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar SN, Huang R-Q, Logan SM, Yi KD, Dillon GH, Simpkins JW (2008) Estrogens directly potentiate neuronal L-type Ca 2+ channels. Proc Natl Acad Sci U S A 105:15148–15153. 10.1073/pnas.0802379105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz KN, Von Esenwein SA, Hu M, Bennett AL, Kennedy RT, Musatov S, Toran-Allerand CD, Kaplitt MG, Young LJ, Becker JB (2009) Viral vector-mediated overexpression of estrogen receptor-α in striatum enhances the estradiol-induced motor activity in female rats and estradiol-modulated GABA release. J Neurosci 29:1897–1903. 10.1523/JNEUROSCI.4647-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seney ML, Sibille E (2014) Sex differences in mood disorders: perspectives from humans and rodent models. Biol Sex Differ 5:17. 10.1186/s13293-014-0017-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair EB, Hildebrandt BA, Culbert KM, Klump KL, Sisk CL (2017) Preliminary evidence of sex differences in behavioral and neural responses to palatable food reward in rats. Physiol Behav 176:165–173. 10.1016/j.physbeh.2017.03.042 [DOI] [PubMed] [Google Scholar]
- Sjulson L, Peyrache A, Cumpelik A, Cassataro D, Buzsáki G (2018) Cocaine place conditioning strengthens location-specific hippocampal coupling to the nucleus accumbens. Neuron 98:926–934.e5. 10.1016/j.neuron.2018.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar P, et al. (2008) Whole-genome association study of bipolar disorder. Mol Psychiatry 13:558–569. 10.1038/sj.mp.4002151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Vedder LC, McMahon LL (2009) Estradiol and the relationship between dendritic spines, NR2B containing NMDA receptors, and the magnitude of long-term potentiation at hippocampal CA3–CA1 synapses. Psychoneuroendocrinology 34:S130–S142. 10.1016/j.psyneuen.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneider JT, Hamilton DA, Cohen-Gilbert JE, Crowley DJ, Rosso IM, Silveri MM (2015) Sex differences in spatial navigation and perception in human adolescents and emerging adults. Behav Processes 111:42–50. 10.1016/j.beproc.2014.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Kalyani M, Becker JB (2018) Sex differences in motivated behaviors in animal models. Curr Opin Behav Sci 23:98–102. 10.1016/j.cobeha.2018.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speranza L, di Porzio U, Viggiano D, de Donato A, Volpicelli F (2021) Dopamine: the neuromodulator of long-term synaptic plasticity, reward and movement control. Cells 10:735. 10.3390/cells10040735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffend NA, Loftus CM, Meisel RL (2011) Estradiol reduces dendritic spine density in the ventral striatum of female Syrian hamsters. Brain Struct Funct 215:187–194. 10.1007/s00429-010-0284-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanić D, Dubois S, Chua HK, Tonge B, Rinehart N, Horne MK, Boon WC (2014) Characterization of aromatase expression in the adult male and female mouse brain. I. Coexistence with oestrogen receptors α and β, and androgen receptors. PLoS One 9:e90451. 10.1371/journal.pone.0090451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbamanda YD, Bhargava A (2022) Intercommunication between voltage-gated calcium channels and estrogen receptor/estrogen signaling: insights into physiological and pathological conditions. Cells 11:3850. 10.3390/cells11233850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada H, Koide M, Ara W, Shibata Y, Funabashi T, Suyama K, Goto T, Takahashi T (2015) Estrous cycle-dependent phasic changes in the stoichiometry of hippocampal synaptic AMPA receptors in rats. PLoS One 10:e0131359. 10.1371/journal.pone.0131359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrier J, Lüscher C, Pascoli V (2016) Cell-type specific insertion of GluA2-lacking AMPARs with cocaine exposure leading to sensitization, cue-induced seeking, and incubation of craving. Neuropsychopharmacology 41:1779–1789. 10.1038/npp.2015.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Malenka RC (2003) Synaptic plasticity in the mesolimbic dopamine system. Philos Trans R Soc Lond B Biol Sci 358:815–819. 10.1098/rstb.2002.1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonn Eisinger KR, Gross KS, Head BP, Mermelstein PG (2018) Interactions between estrogen receptors and metabotropic glutamate receptors and their impact on drug addiction in females. Horm Behav 104:130–137. 10.1016/j.yhbeh.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Pride MC, Villalon Landeros R, Knoblauch NW, Takahashi EY, Silva AL, Crean KK (2011) Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus). PLoS One 6:e17405. 10.1371/journal.pone.0017405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouche S, et al. (2019) A hippocampus-accumbens tripartite neuronal motif guides appetitive memory in space. Cell 176:1393–1406.e16. 10.1016/j.cell.2018.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW (2007) Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr Rev 28:1–19. 10.1210/er.2005-0021 [DOI] [PubMed] [Google Scholar]
- Vega-Villar M, Horvitz JC, Nicola SM (2019) NMDA receptor-dependent plasticity in the nucleus accumbens connects reward-predictive cues to approach responses. Nat Commun 10:4429. 10.1038/s41467-019-12387-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierk R, et al. (2012) Aromatase inhibition abolishes LTP generation in female but not in male mice. J Neurosci 32:8116–8126. 10.1523/JNEUROSCI.5319-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker J, Dillon DG, Pizzagalli DA (2009) The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage 46:327–337. 10.1016/j.neuroimage.2009.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. (2023) The sex differences in anhedonia in major depressive disorder: a resting-state fMRI study. J Affect Disord 340:555–566. 10.1016/j.jad.2023.08.083 [DOI] [PubMed] [Google Scholar]
- Wang X, Christian KM, Song H, Ming G (2018) Synaptic dysfunction in complex psychiatric disorders: from genetics to mechanisms. Genome Med 10:9. 10.1186/s13073-018-0518-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Kantorovich S, Babayan AH, Hou B, Gall CM, Lynch G (2016) Estrogen’s effects on excitatory synaptic transmission entail integrin and TrkB transactivation and depend upon β1-integrin function. Neuropsychopharmacology 41:2723–2732. 10.1038/npp.2016.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Le AA, Hou B, Lauterborn JC, Cox CD, Levin ER, Lynch G, Gall CM (2018) Memory-related synaptic plasticity is sexually dimorphic in rodent hippocampus. J Neurosci 38:7935–7951. 10.1523/JNEUROSCI.0801-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SG, Humphreys AG, Juraska JM, Greenough WT (1995) LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res 703:26–30. 10.1016/0006-8993(95)01059-9 [DOI] [PubMed] [Google Scholar]
- Warthen DM, Wiltgen BJ, Provencio I (2011) Light enhances learned fear. Proc Natl Acad Sci U S A 108:13788–13793. 10.1073/pnas.1103214108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EM, Mitterling K, Spencer JL, Mazid S, McEwen BS, Milner TA (2009) Estrogen receptor alpha and beta specific agonists regulate expression of synaptic proteins in rat hippocampus. Brain Res 1290:1–11. 10.1016/j.brainres.2009.06.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Yuen EY, Liu W, Li X, Zhong P, Karatsoreos IN, McEwen BS, Yan Z (2014) Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Mol Psychiatry 19:588–598. 10.1038/mp.2013.83 [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Bauer EP, LeDoux JE (1999) L-type voltage-gated calcium channels mediate NMDA-independent associative long-term potentiation at thalamic input synapses to the amygdala. J Neurosci 19:10512–10519. 10.1523/JNEUROSCI.19-23-10512.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook SR, Hankosky ER, Dwyer MR, Gulley JM (2018) Age and sex differences in behavioral flexibility, sensitivity to reward value, and risky decision-making. Behav Neurosci 132:75–87. 10.1037/bne0000235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead G, et al. (2013) Acute stress causes rapid synaptic insertion of Ca2+-permeable AMPA receptors to facilitate long-term potentiation in the hippocampus. Brain 136:3753–3765. 10.1093/brain/awt293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ES, Manning CE, Eagle AL, Swift-Gallant A, Duque-Wilckens N, Chinnusamy S, Moeser A, Jordan C, Leinninger G, Robison AJ (2020) Androgen-dependent excitability of mouse ventral hippocampal afferents to nucleus accumbens underlies sex-specific susceptibility to stress. Biol Psychiatry 87:492–501. 10.1016/j.biopsych.2019.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissman AM, May RM, Woolley CS (2012) Ultrastructural analysis of sex differences in nucleus accumbens synaptic connectivity. Brain Struct Funct 217:181–190. 10.1007/s00429-011-0353-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissman AM, McCollum AF, Huang G-Z, Nikrodhanond AA, Woolley CS (2011) Sex differences and effects of cocaine on excitatory synapses in the nucleus accumbens. Neuropharmacology 61:217–227. 10.1016/j.neuropharm.2011.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Tseng KY (2012) Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front Mol Neurosci 5:72. 10.3389/fnmol.2012.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yararbas G, Keser A, Kanit L, Pogun S (2010) Nicotine-induced conditioned place preference in rats: sex differences and the role of mGluR5 receptors. Neuropharmacology 58:374–382. 10.1016/j.neuropharm.2009.10.001 [DOI] [PubMed] [Google Scholar]
- Yu J, Sesack SR, Huang Y, Schlüter OM, Grace AA, Dong Y (2022) Contingent amygdala inputs trigger heterosynaptic LTP at hippocampus-to-accumbens synapses. J Neurosci 42:6581–6592. 10.1523/JNEUROSCI.0838-22.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Bhat S, Terrillion CE, Smith RJ, Tonelli LH, Gould TD (2015) Sex-dependent modulation of age-related cognitive decline by the L-type calcium channel gene Cacna1c (Ca v 1.2). Eur J Neurosci 42:2499–2507. 10.1111/ejn.12952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, et al. (2019) A ventral CA1 to nucleus accumbens core engram circuit mediates conditioned place preference for cocaine. Nat Neurosci 22:1986–1999. 10.1038/s41593-019-0524-y [DOI] [PubMed] [Google Scholar]
- Zhou Q, Sheng M (2013) NMDA receptors in nervous system diseases. Neuropharmacology 74:69–75. 10.1016/j.neuropharm.2013.03.030 [DOI] [PubMed] [Google Scholar]