Abstract
Background
Prior systematic reviews addressing the impact of diet on cancer outcomes have focused on specific dietary interventions. In this systematic review, we assessed all randomized controlled trials (RCTs) investigating dietary interventions for cancer patients, examining the range of interventions, endpoints, patient populations, and results.
Methods
This systematic review identified all RCTs conducted before January 2023 testing dietary interventions in patients with cancer. Assessed outcomes included quality of life, functional outcomes, clinical cancer measurements (eg, progression-free survival, response rates), overall survival, and translational endpoints (eg, inflammatory markers).
Results
In total, 252 RCTs were identified involving 31 067 patients. The median sample size was 71 (interquartile range 41 to 118), and 80 (32%) studies had a sample size greater than 100. Most trials (n = 184, 73%) were conducted in the adjuvant setting. Weight or body composition and translational endpoints were the most common primary endpoints (n = 64, 25%; n = 52, 21%, respectively). Direct cancer measurements and overall survival were primary endpoints in 20 (8%) and 7 (3%) studies, respectively. Eight trials with a primary endpoint of cancer measurement (40%) met their endpoint. Large trials in colon (n = 1429), breast (n = 3088), and prostate cancer (n = 478) each showed no effect of dietary interventions on endpoints measuring cancer.
Conclusion
Most RCTs of dietary interventions in cancer are small and measure nonclinical endpoints. Although only a small number of large RCTs have been conducted to date, these trials have not shown an improvement in cancer outcomes. Currently, there is limited evidence to support dietary interventions as a therapeutic tool in cancer care.
Patients with cancer are often interested in exploring different dietary interventions before, during, or after standard cancer treatment. Although a balanced diet clearly contributes to overall health, whether specific dietary interventions can alter the trajectory of cancer or have anticancer activity remains uncertain (1).
Observational studies and nonrandomized trials have frequently reported improved cancer outcomes with adherence to various specific diets (2,3). However, these studies are biased by numerous confounders, both measured and unmeasured. Fundamentally, patients who can follow a specific diet may differ from those who are unable to do so, whether in terms of socioeconomic status, health literacy, pre-existing health, or other factors (4). Approximately 50% of patients with cancer use complementary or alternative medical products (5), and 37% of cancer survivors report using untested dietary interventions (6). There is, therefore, a need to appraise the evidence of the impact of dietary interventions on cancer outcomes.
To our knowledge, no systematic review has comprehensively examined all randomized trials of dietary interventions and supplements in cancer. This systematic review aims to assess all randomized controlled trials (RCTs) of dietary interventions in cancer, including the interventions studied, endpoints measured, patient populations enrolled, and the results of these interventions.
Methods
Protocol and registration
This systematic scoping review was performed according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Extension for Scoping Reviews guidelines (7). As no direct patient information was obtained, and the data were gathered from publicly available and deidentified sources, this study was considered exempt from institutional review board review. This was not registered on PROSPERO, as PROSPERO does not permit registration of broad scoping reviews. This study received no external funding.
Eligibility criteria
RCTs met our inclusion criteria if they focused on dietary intervention, including counseling or the use of dietary supplements in patients with cancer. We included trials on patients with an established history of cancer, including secondary prevention trials in such patients. Trials that focused on patients without cancer, including primary prevention studies, were excluded.
Publication type and study design
We included only RCTs published as papers in peer-reviewed scientific journals and those published as abstracts in conference proceedings. All other study types were excluded.
Information sources and search strategy
Our search strategy was restricted to RCTs assessing any dietary intervention published in paper or abstract form from October 1977 to January 2023. The search was last updated on January 20, 2023. A search strategy was made using keywords, and we searched across four electronic databases (MEDLINE, Embase, Web of Science Core Collection, and Cochrane Central Register of Controlled Trials) with the help of an experienced health sciences librarian (W.L.-S). We did not apply any restrictions on language. The Appendix highlights the Embase search strategy. All results were exported to EndNote (v. 20, Clarivate), and duplicate items were removed by successive iterations of EndNote’s duplicate detection features and manual inspection.
Study selection and screening
Three reviewers (N.P.I., A.H.S., C.S.) worked in pairs in the initial screening of abstracts and titles and assessed eligibility with the full text of selected articles when available. Abstracts from conference proceedings captured on these databases via our search strategy, such as those on Embase, were included. A fourth reviewer (G.R.M.) was consulted to resolve discrepancies and facilitate consensus.
Data charting and extraction
The independent data charting was done by three reviewers (N.P.I., A.H.S., C.S.). A data charting form was developed and independently piloted by a random sample of 10% of the included articles. It was modified as required based on feedback from within the team. The following characteristics of studies were identified: median age of participants, sample size, trial setting, primary endpoint and its outcome, country origin of trial, cancer types being studied, trial phase, blinding, and the dietary interventions employed. After initial charting, the reviewers validated the accuracy of data points and reached a consensus on each input. When discrepancies or uncertainties arose among reviewers, a fourth reviewer (G.R.M.) was consulted to facilitate consensus, and a dietician (E.H.) provided input.
Study outcomes
We defined standard quality-of-life primary endpoints as those using validated quality-of-life (QoL) measurements such as the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30) (8), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) (9), Functional Assessment of Chronic Illness Therapy—General (FACIT-G) (9), and Short Form 36 (SF-36) (10) health questionnaires. We defined nonstandard quality-of-life primary endpoints as endpoints that measured symptom burden without the use of standardized scales, such as chemotherapy or radiation-induced adverse events, infection rates, unspecified measurement scales of QoL, and functional outcomes (such as fatigue measured without the use of a standardized scale). Cancer measurement endpoints included response rates, clinical or biochemical recurrences, progression-free survival, relapse-free survival, time until cancer recurrence, and overall survival. Endpoints that focused on a related pre-cancer, such as polyps or actinic keratosis, were considered a cancer measurement endpoint. Perioperative endpoints included postoperative length of stay, complications (infective and noninfective), and functional outcomes (fatigue or pain) in the perioperative setting. Blinding categorization was defined as follows: single-blinded studies were studies in which participants were blinded to treatment assignment. Double-blinded were defined as studies in which both health-care providers and patients were masked to treatment assignment, and triple-blinded trials also blinded researchers to treatment assignment. Studies that did not report blinding for study participants or investigators were deemed open label. We analyzed countries where trials were conducted using the World Bank data to classify countries according to their Gross National Income (GNI) per capita, using it as a reference standard (11).
Trial setting
To explore how dietary interventions are ascertained across the cancer treatment continuum, we categorized trials into four discrete groups to capture the setting in which they were studied. The “adjuvant” setting included interventions applied concurrently with curative-intent chemotherapy, radiotherapy, surgical resection, hematopoietic stem cell transplantation, or other procedures. All perioperative interventions were classified in this category. The “palliative” setting included interventions administered in patients who were being treated with palliative intent. This included patients with metastatic cancer, regardless of whether they were receiving chemotherapy. The third category was secondary prevention and/or survivorship among patients who have completed curative intent treatment and have no evidence of cancer. A fourth category was trials specifically done for patients with either cancer cachexia or malnutrition. Trials that fit none of these categories or included patients in multiple settings were classified as miscellaneous.
Results
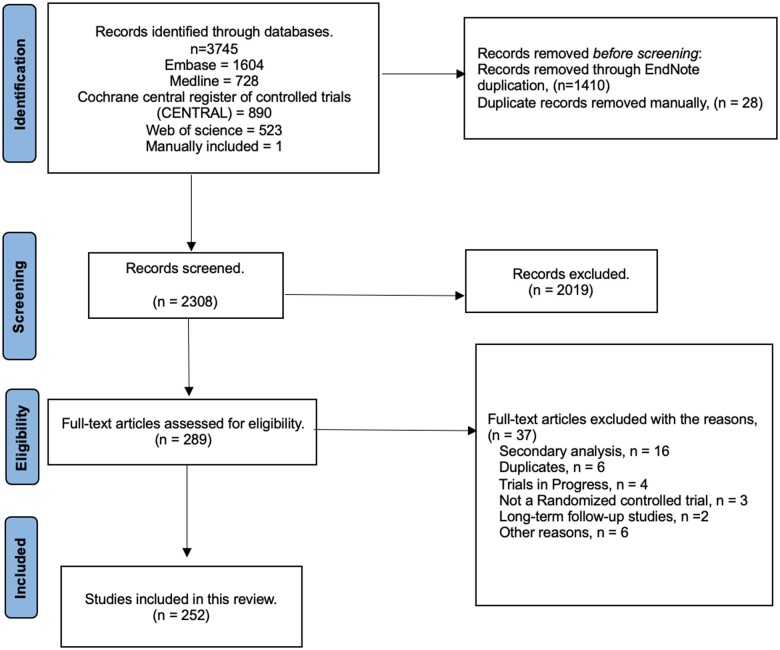
The initial search strategy identified 3745 studies, of which 252 were RCTs (Supplementary Table 1, available online; lists characteristics of all studies). Figure 1 highlights our screening process. The median sample size of eligible RCTs was 71 patients (Interquartile range: 41-118). Eighty studies (32%) had a sample size greater than 100. Characteristics of included studies are listed in Table 1.
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram.
Table 1.
Characteristics of included studies
| Median sample size | 71 (Interquartile range 41, 118) | |
|---|---|---|
| Median age | 61 (5,77) | |
| Studies by countries stratified by income (N = 252) | High-income Countries | 162 (64.3%) |
| Upper-middle-Income Countries | 77 (30.5%) | |
| Lower-middle-Income Countries | 13 (5.2%) | |
| Low-income Countries | None | |
| Studies grouped by primary endpoint | Weight, body composition, or muscle | 64 (25.4%) |
| Translational or preclinical endpoints | 52 (20.6%) | |
| Nonstandard measurements of QoL | 38 (15.1%) | |
| Postoperative endpoints | 35 (13.9%) | |
| Compliance or adherence or feasibility | 21 (8.3%) | |
| Direct measurements of cancer | 20 (7.9%) | |
| Quality-of-life | 15 (6.0%) | |
| Overall survival | 7 (2.8%) | |
| Cancers studied (N = 252), % | Gastrointestinal cancers | 97 (38.5%) |
| Mixed cancers | 36 (14.3%) | |
| Breast cancer | 28 (11.1%) | |
| Prostate cancer | 29 (11.5%) | |
| Head and neck cancer | 25 (9.9%) | |
| Hematologic malignancies | 12 (4.7%) | |
| Gynecologic malignancies | 9 (3.6%) | |
| Skin cancers | 4 (1.6%) | |
| Cancer type not specified | 3 (1.2%) | |
| Othera | 9 (3.6%) | |
| Trial setting (N = 252), % | Adjuvant | 184 (73.0%) |
| Miscellaneous or Mixed | 26 (10.3%) | |
| Secondary Prevention and/or Survivorship | 21 (8.3%) | |
| Palliative | 14 (5.5%) | |
| Cachexia or Malnutrition | 7 (2.8%) | |
Pediatric malignancies (n = 2, 0.8%), lung cancer (n = 2, 0.8%), malignant gliomas (n = 2, 0.8%), bladder (n = 1, 0.4%), renal (n = 1, 0.4%), thyroid (n = 1, 0.4%).
Cancer populations
The most common cancers studied were gastrointestinal (n = 97, 38%), followed by mixed cancer populations (n = 36, 14%), breast cancer (n = 28, 11%), prostate cancer (n = 29, 12%), head and neck cancer (n = 25, 10%), hematological malignancies (n = 12, 5%), and gynecological malignancies (n = 9, 4%).
Dietary interventions
The types of dietary interventions are listed in Supplementary Table 2 (available online). Diets were categorized into 7 categories, which included nutrient-modified diets (eg, supplemented vitamins, minerals, amino acids, or certain foods), nutritional counseling, energy-modified diets (these diets modified caloric intake or relative proportions of macronutrients), nutrition support (eg, parenteral and enteral nutrition), restrictive eating patterns (eg, ketogenic diet, neutropenic diet, vegan diet, plant-based diet, Chinese medicated diet), the Mediterranean diet, and fiber-modified diets. The most common intervention was nutrient-modified diets (n = 126, 50%), followed by nutrition counseling interventions (n = 44, 17%), energy-modified diets (n = 34, 13%), nutrition support (n = 27, 11%), restrictive eating patterns (n = 11, 4%), Mediterranean diets (n = 6, 2%), and fiber-modified diets (n = 4, 2%).
Settings and endpoints
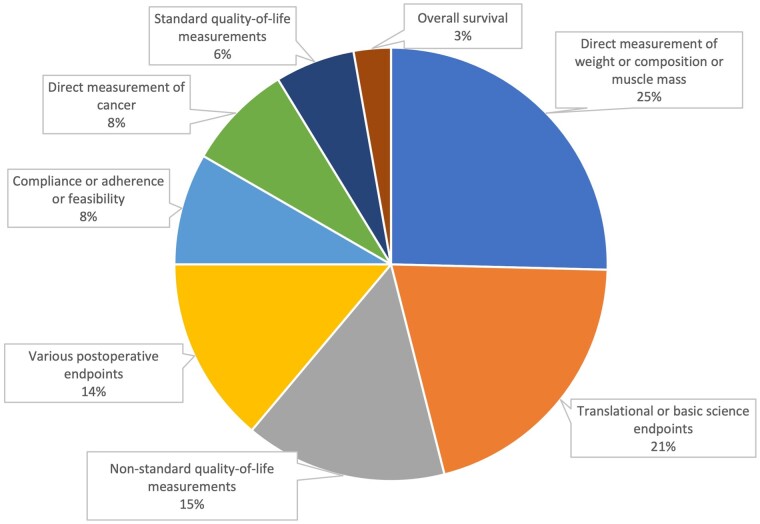
Endpoints used in included trials are summarized in Figures 2 and 3, and demonstrate the proportions of trials meeting their primary endpoint based on the type of endpoint used. Most trials (n = 184, 73%) were conducted in the adjuvant setting. Weight or body composition endpoints and translational endpoints were the most common primary endpoints (n = 64, 25%; n = 52, 21%, respectively). Direct cancer measurements were a primary endpoint in 20 (8%) studies, and overall survival was the primary endpoint in 7 (3%) studies.
Figure 2.
Primary endpoints of dietary intervention trials.
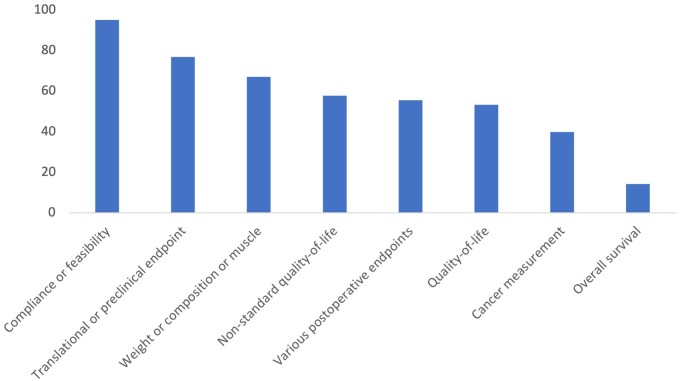
Figure 3.
Percentage of RCTs with achieved primary endpoint by endpoint categorization. Endpoints from RCTs testing dietary interventions in cancer patients were categorized into the following categories: Compliance or feasibility, Translational or preclinical, measurements of Weight or composition or muscle mass, Nonstandard quality-of-life, Various postoperative endpoints, Quality-of-life, Cancer measurements, and Overall survival. Quality-of-life endpoints were defined as validated measurements such as the EORTC QLQ-C30, FACIT-F, FACIT-G, and SF-36 health questionnaires. Nonstandard quality-of-life were measurements of symptom burden without the use of standardized scales, such as chemotherapy or radiation-induced adverse events, infection rates, unspecified measurement scales of QoL, and functional outcomes (such as fatigue). Cancer measurement endpoints were defined as response rates, clinical/biochemical recurrences, progression-free survival, relapse-free survival, and time until cancer recurrence.
Trials that measured cancer outcomes and overall survival
Among the 20 studies with a primary endpoint of cancer (or precancer) measurement, 8 (40%) studies achieved their endpoint (Table 2). Among the 20 studies for which direct measurement of cancer or associated precancer was a primary endpoint, the endpoints were biochemical recurrences of prostate-specific antigen in 8 (40%) studies, clinical recurrence of cancer in 3 (15%) trials, progression-free survival in 3 (15%) studies, response rates in 2 (10%) studies, relapse-free survival in 1 (5%), disease-free survival in 1 (5%), and occurrence of new skin cancers and precancerous keratosis in 1 (5%) trial.
Table 2.
Details of studies with a primary endpoint of cancer measurement or overall survivala
| Study | Sample size |
Dietary intervention | Trial setting | Primary endpoint | Endpoint met? |
|---|---|---|---|---|---|
| Dequadros Camargo et al. (14) | 30 | Fish oil supplements | Adjuvant | Time to disease progression (days) | Yes |
| Khodabakhshi et al. (15) | 80 | Ketogenic diet | Adjuvant | Response rate (reduction in tumor size) | Yes |
| Nojiri, S. et al. (16) | 51 | Branched-chain amino acids supplements | Adjuvant | Time until intrahepatic recurrence of HCC | Yes |
| Rangel Huerta et al. (18) | 74 | Lycopene supplements | Adjuvant | PSA levels | Yes |
| Thomas et al. (47) | 203 | Supplements with pomegranate seeds, green tea, broccoli, and turmeric | Adjuvant | Median rise in PSA | Yes |
| De Waele et al. (32) | 60 | Intensive personalized dietary counseling | Adjuvant | Overall Survival | Yes |
| Black et al. (12) | 115 | Low-fat diet | Secondary prevention or survivorship | Cumulative incidence of new AK | Yes |
| Chen et al. (13) | 386 | 500 mg of nicotinamide twice daily | Secondary prevention or survivorship | Number of new histologically confirmed nonmelanoma skin cancers | Yes |
| Kranse et al. (17) | 37 | Supplements with plant estrogens, carotenoids, and selenium | Misc or mixed | Slope of the rise in PSA | Yes |
| Bougnoux et al. (23) | 65 | Long-chain | Adjuvant | Progression-free survival | No |
| PUFA | |||||
| supplements | |||||
| Voss et al. (31) | 50 | Ketogenic diet | Adjuvant | Progression-free survival rate at six months | No |
| Bourdel-Marchasson et al. (35) | 341 | Dietary counseling aiming to increase energy and protein intake | Adjuvant | One-year survival | No |
| Lin et al. (35) | 97 | Zinc supplements | Adjuvant | Overall survival | No |
| Sykorova et al. (37) | 44 | Glutamine rich parenteral nutrition | Adjuvant | Overall survival | No |
| Toma et al. (38) | 214 | Beta-carotene supplements | Adjuvant | Overall survival | No |
| Freedland et al. (25) | 57 | Low-carbohydrate diet | Palliative | PSA doubling time | No |
| Van Zweeden et al. (30) | 82 | Vitamin B12 and B9 supplements | Palliative | Response rate | No |
| Antunac et al. (33) | 71 | Vitamin D supplements | Palliative | Overall survival | No |
| Baldwin et al. (34) | 358 | Nutritional supplements with high-calorie diet | Palliative | One-year survival | No |
| Alberts et al. (21) | 1429 | High-fiber diet | Secondary prevention or survivorship | Presence or absence of new adenomas at the time of follow-up colonoscopy | No |
| Bosland et al. (22) | 177 | Soy protein supplements | Secondary prevention or survivorship | Biochemical recurrence of prostate cancer (PSA levels) | No |
| DeVere White et al. (24) | 66 | High-dose isoflavone supplements | Secondary prevention or survivorship | Serum PSA | No |
| Chlebowski et al. (20) | 2437 | Low-fat diet | Secondary prevention or survivorship | Relapse-free survival | No |
| Johansson et al. (28) | 104 | Vitamin D supplements | Secondary prevention or survivorship | Disease-free survival | No |
| Parsons et al. (26) | 478 | Telephone-based counseling | Secondary prevention or survivorship | Time to progression (PSA) | No |
| Pierce et al. (29) | 3088 | Diet rich in vegetables, fruits, and fiber, but with low fat | Secondary prevention or survivorship | Invasive breast cancer event (recurrence or new primary) and all-cause mortality | No |
| Schröder et al. (27) | 49 | Soy-based supplements | Misc or mixed | PSA slope and doubling time | No |
AK = actinic keratosis; HCC = hepatocellular cancer; PSA = prostate specific antigen.
The 8 studies that met their endpoint evaluating cancer-related outcomes consisted of a low-fat diet study at reducing actinic keratosis in patients with skin cancer (n = 115) (12), a trial supplementing nicotinamide aimed at reducing new histologically confirmed nonmelanoma skin cancers in patients with a history of nonmelanoma skin cancer (n = 386) (13), a fish oil study that aimed at reducing time to progression for gastrointestinal (GI) cancers (n = 30) (14), a study of a ketogenic diet that aimed at increasing breast cancer response rates (n = 80) (15), a branched-chain amino-acid supplementation study measuring the recurrence of hepatocellular cancer (n = 51) (16), and three trials of various supplements or diets that aimed at reducing prostate specific antigen (PSA) values (n = 37, 74, and 203, respectively) (17-19).
The 12 studies that did not meet their endpoint when evaluating cancer-related outcomes included a low-fat diet in breast cancer (n = 2437) on relapse-free survival (20), a high-fiber diet study on adenoma rates among patients with colorectal cancer (n = 1429) (21), a soy-protein supplement study on prostate cancer recurrence rates (n = 177) (22), a long-chain fatty acid study on breast cancer progression-free survival (n = 65) (23), high-dose isoflavone supplementation, low-carbohydrate diet, telephone-based dietary counseling on PSA rates (n = 66 and 57, respectively) (24,25), a telephone-based dietary counseling study measuring time to progression (n = 478) (26), a soy-based dietary supplementation study on PSA doubling time (n = 49) (27), a vitamin D supplementation study on skin cancer (n = 104) (28), a diet high in vegetables, fruits, and fiber study on breast cancer survival (n = 3088) (29), a vitamin B12 and folic acid study on response rates in GI cancers (n = 82) (30), and an intermittent fasting and ketogenic diet study on progression-free survival of gliomas (n = 50) (31).
One trial with a primary endpoint of overall survival met its endpoint (n = 60) (32). This trial tested counseling patients to increase caloric intake among a mixed cancer population and showed that at 12 months follow-up, the control group had a median survival of 45.5 weeks vs not reached in the intervention group (P = .0378) (32).
Trials that did not meet their overall survival endpoint included a vitamin D supplement study in colorectal cancer (n = 71) (33), two studies that focused on increasing caloric intake with one among patients with GI cancers, non-small-cell lung cancers, and mesothelioma (n = 358) (34) and the other in a mixed cancer population (n = 341) (35), a zinc supplementation trial for patients with head and neck cancer (n = 97) (36), a trial that tested a glutamine-enriched parental supplementation in patients with hematological malignancies (n = 44) (37), and a study that used beta-carotene supplements for head and neck cancer patients (n = 214) (38).
Likelihood of meeting endpoint based on trial design
The majority of trials reported either an open-label design or did not report any blinding procedures, 184 (73%). A total of 19 studies (8%) incorporated a single-blind design, and 49 (19%) studies reported a double- or triple-blind design. Trials that were open label were more likely to meet their primary endpoint (68%) vs those that were blinded (38%), χ2 (1, n = 252) equals 18.9, P less than .05.
A total of 79 (31%) studies used counseling techniques, whereas the remaining trials (n = 173, 68.6%) directly administered dietary meals or nutritional supplements. In total, 56 (70.9%) of the studies using counseling and 105 (60.7%) of studies providing meals or supplementation met their primary endpoint, χ2 (1, n = 252) equals 2.44, P equals .12.
Discussion
This is the first comprehensive systematic review to assess all RCTs evaluating dietary interventions in patients with cancer. We find that dietary intervention trials for cancer patients are typically small and most often assess translational and body composition endpoints. These trials often met their primary endpoints, showing improvements in translational parameters and body composition with dietary interventions. In addition, most trials were done in the adjuvant setting in conjunction with other approaches such as chemotherapy, radiation, and surgery. However, direct measurements of cancer were a primary endpoint in only 8% of studies.
Only a small number of trials both had adequate statistical power and assessed an endpoint of cancer measurement; these generally did not meet their endpoint, such as large trials in colon cancer (n = 1429) (21), and breast cancer (n = 3088) (29). Our results cumulatively indicate that it has been difficult to demonstrate the clinical benefit of specific dietary interventions as a therapeutic anticancer strategy in RCTs in patients with cancer. Given the lack of adequately powered RCTs with clinical endpoints, the benefits of dietary interventions remain largely untested. However, based on the existing evidence from the small number of conducted large RCTs, there is currently very limited evidence to support dietary interventions as a therapeutic tool in cancer care. These results serve as an important call for further high-quality large RCTs in this space, powered to show improvements in clinically meaningful endpoints.
Our study sought to systematically evaluate the collective RCT evidence of diverse dietary interventions in cancer. Prior reports have focused on specific dietary patterns across multiple cancer populations, some comprising RCTs and observational studies (39-44). These systematic reviews have observed that dietary interventions for cancer patients or survivors are feasible with positive outcomes for translational endpoints (41,44,45). Our work corroborates this finding, indicating that many feasibility and translational trials meet their primary endpoints; it seems, however, that many of these interventions are not subsequently tested in larger trials.
Some RCTs testing dietary interventions and measuring cancer outcomes met their primary endpoint, but these trials have been limited by small sample sizes and lack of reproducibility. In prostate cancer, although some trials of dietary interventions powered for rise of PSA met their endpoint (17,18,19), other trials of dietary interventions or counseling with longer follow-up did not, including a large study of 478 patients in which dietary interventions led to an increase in vegetable consumption but did not change time to progression (26). In other tumor types, trials that met their endpoints had small sample sizes necessitating confirmation with larger trials. For example, a fish oil study (n = 30) reduced time to progression for GI cancers (593 days vs 330 days) (14), and a branched-chain amino-acid supplementation trial (n = 51) also reduced recurrence (44% vs 68% at 3 years) of hepatocellular cancer (16). Additionally, one trial that showed improved overall survival with counseling to increase caloric intake had a sample size of 60 patients and a variety of cancers were included, limiting its applicability to routine clinical practice in terms of recommending a specific diet for specific patients with cancer (32). An exception to this is an oral nicotinamide trial with a sample size of 386 participants, which was effective in reducing new nonmelanoma skin cancers in those who already had skin cancers, with a distinct biological mechanism explaining this effect (13). Overall, although these trials have demonstrated that some dietary interventions may have an effect on cancer, due to the limitations of these studies, there is insufficient evidence to recommend specific diets (beyond general lifestyle changes for better health) for patients with cancer. Future work should look to replicate positive findings seen in small studies in trials with larger sample sizes.
Several RCTs with adequate statistical power have been conducted to evaluate the impact of interventions on cancer outcomes, particularly in breast and prostate cancers. Unfortunately, the results of these trials have often been negative. For example, a large RCT that used a telephone-based counseling method to increase vegetable intake in patients with prostate cancer showed no difference in PSA between the control and intervention arms (n = 478) (26). Additionally, a well-designed trial to increase fruit and vegetable intake among breast cancer patients showed no change in breast cancer recurrence (n = 3088) (29). Although an RCT showed a potential effect of a low-fat diet in breast cancer (n = 2437) with improved relapse-free survival (relapse events in 9.8% of the intervention group vs 12.4% in the control group) (20), this trial did not have a statistically significant P value for the primary endpoint when analyzed via the prespecified stratified log-rank test (P = .077), indicating that the results could be due to chance alone. When considering these results, RCTs cumulatively indicate that dietary interventions studied to date generally have offered limited benefits in reducing the burden of cancer. In these RCTs, the compliance to the intervention was frequently sufficient to lead to a change in the diet as measured by surveys and nutritional indices (such as plasma carotenoids), but this change did not translate to a difference in cancer outcomes such as progression-free survival. This challenges the previously suggested causal connections between adherence to specific diets seen in observational data and cancer outcomes, highlighting the role confounding may play in observational studies that suggest a large effect size of diet in cancer (2,3).
We found that smaller trials assessing translational endpoints or endpoints directly measuring weight or body composition often achieved their primary endpoint (77% and 67%, respectively), whereas larger RCTs are often negative. This shows intuitively that dietary interventions may help maintain or restore an ideal body weight or improve certain laboratory values, as they are known to do in patients without cancer. Nevertheless, these studies provide limited information about the potential clinical benefit of dietary interventions as therapeutic measures to reduce cancer burden.
Certain diets such as the ketogenic diet, or other diets that aim to starve cancer cells of sugar, attract considerable media attention as a mechanism to cure cancer (46). Our review finds no convincing evidence that any diet “cures” cancer, with no large robust randomized study demonstrating this, and smaller studies producing conflicting results (15,46).
The strength of this systematic review lies in its focus on RCTs that have investigated the role of diet in patients with cancer. The review also encompasses studies from diverse geographical regions and various cancer types, lending weight to the generalizability of our conclusions. A limitation is that some of the included abstracts did not clearly describe the study’s primary endpoint, requiring us to infer the primary endpoint. In addition, because no RCTs were performed in low-income countries and only 5% of RCTs were performed in lower-middle-income countries (Table 1), these results may not be generalizable to patients in countries where nutritional deficiencies may be more prevalent.
Additionally, interpreting RCTs testing dietary interventions in cancer may be subject to limitations. These include insufficient blinding leading to an increased probability of a false negative finding due to contamination of the control arm, and self-selected enrollment of comparatively more motivated patients, which may decrease generalizability to a broader population. Furthermore, dietary interventions can be very heterogenous (as can cancer), and results of dietary interventions in one cancer may not extrapolate to others. A final limitation of our analysis is that no formal meta-analytic method was employed due to the heterogeneity of assessed interventions and outcomes.
Our review confirms that dietary interventions in these studies often positively impact translational endpoints, symptom burden, and/or body composition. However, despite smaller RCTs often meeting their primary outcome, when larger RCTs of dietary interventions directly measuring cancer are conducted, they have not yet consistently shown any effect on cancer progression. Given immense interest from patients in this field, it is paramount to generate high-quality, randomized data with clinical endpoints, as the results of observational studies are subject to confounding and cannot be relied on. Although there are challenges in running RCTs in this space, they remain essential to further our understanding of diet as an anticancer modality. Our results suggest that larger studies directly measuring cancer should be prioritized for funding rather than further small studies with nonclinical endpoints. An ideal randomized trial in this setting would be a trial of a dietary intervention that is scalable and easy to adhere to, with adequate power to capture a meaningful difference in a clinically relevant endpoint (eg, cancer response rate, progression-free survival, or validated quality of life measurement). Such a trial may be done in conjunction with effective cancer pharmacological therapy and may be designed to isolate the effect of the dietary intervention. A holistic, multidisciplinary approach to optimizing the health of patients with cancer remains paramount; however, there is currently limited evidence to support specific dietary interventions as a therapeutic tool to target cancer.
Supplementary Material
Acknowledgments
Not applicable.
Contributor Information
Nosakhare Paul Ilerhunmwuwa, One Brooklyn Health/Brookdale University Hospital and Medical Center, Brooklyn, NY, USA.
Abul Hasan Shadali Abdul Khader, Government Kilpauk Medical College, Chennai, Tamil Nadu, India.
Calvin Smith, Frank H. Netter MD School of Medicine, Quinnipiac University, North Haven, CT, USA.
Edward R Scheffer Cliff, Program on Regulation, Therapeutics and Law, Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Christopher M Booth, Division of Cancer Care and Epidemiology, Queen’s Cancer Research Institute, Kingston, ON, Canada.
Evevanne Hottel, Division of Hematology, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
Muhammad Aziz, Division of Gastroenterology and Hepatology, University of Toledo, Toledo, OH, USA.
Wade Lee-Smith, Mulford Health Science Library, University of Toledo, Toledo, OH, USA.
Aaron Goodman, Division of Blood and Marrow Transplantation, University of California San Diego, San Diego, CA, USA.
Rajshekhar Chakraborty, Division of Hematology, Columbia University Cancer Center, New York, NY, USA.
Ghulam Rehman Mohyuddin, Division of Hematology, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
Data availability
All data used to inform this paper are publicly available.
Author contributions
Nosakhare Paul Ilerhunmwuwa, MD (Data curation; Formal analysis; Project administration; Writing—original draft; Writing—review & editing), Abul Hasan Shadali Abdul Khader, MBBS (Data curation; Formal analysis; Writing—original draft; Writing—review & editing), Calvin Smith, B.Sc. (Data curation; Formal analysis; Project administration; Writing—original draft), Edward R. Scheffer Cliff, MBBS, MPH (Project administration; Writing—review & editing), Christopher M. Booth, MD (Writing—review & editing), Evevanne Hottel, RD (Data curation; Project administration), Muhammad Aziz, MD (Project administration; Writing—review & editing), Wade Lee-Smith, MLS, BS (Data curation; Project administration; Resources), Aaron Goodman, MD (Project administration; Writing—review & editing), Rajshekhar Chakraborty, MD (Project administration; Writing—review & editing), Ghulam Rehman Mohyuddin, MBBS (Conceptualization; Data curation; Formal analysis; Methodology; Project administration; Resources; Supervision; Writing—original draft; Writing—review & editing).
Funding
No specific funding.
Conflicts of interest
Dr Rajshekhar Chakraborty: Consults for Janssen, Sanofi, and Adaptive.
Dr Edward R. Scheffer Cliff: Research funding from Arnold Ventures.
Dr Ghulam Rehman Mohyuddin: Royalties for writing from MashupMD.
Dr Nosakhare Paul Ilerhunmwuwa: Research funding from American Society of Hematology as part of the Minority Hematology Resident Program Initiative.
References
- 1. Burden S, Jones DJ, Sremanakova J, et al. Dietary interventions for adult cancer survivors. Cochrane Database Syst Rev. 2019;2019(11):CD011287. doi: 10.1002/14651858.CD011287.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Romanos-Nanclares A, Willett WC, Rosner BA, et al. Healthful and unhealthful plant-based diets and risk of breast cancer in U.S. women: results from the nurses’ health studies. Cancer Epidemiol Biomarkers Prev. 2021;30(10):1921-1931. doi: 10.1158/1055-9965.EPI-21-0352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ricci C, Freisling H, Leitzmann MF, et al. Diet and sedentary behaviour in relation to cancer survival: a report from the national health and nutrition examination survey linked to the U.S. mortality registry. Clin Nutr. 2020;39(11):3489-3496. doi: 10.1016/j.clnu.2020.03.013 [DOI] [PubMed] [Google Scholar]
- 4. Gong CL, Zawadzki NK, Hay JW.. The assessment of different diets and mortality fails to address unmeasured confounding. JAMA Intern Med. 2021;181(1):137-138. doi: 10.1001/jamainternmed.2020.1391 [DOI] [PubMed] [Google Scholar]
- 5. Horneber M, Bueschel G, Dennert G, Less D, Ritter E, Zwahlen M.. How many cancer patients use complementary and alternative medicine: a systematic review and metaanalysis. Integr Cancer Ther. 2012;11(3):187-203. doi: 10.1177/1534735411423920 [DOI] [PubMed] [Google Scholar]
- 6. Sullivan ES, Rice N, Kingston E, et al. A national survey of oncology survivors examining nutrition attitudes, problems and behaviours, and access to dietetic care throughout the cancer journey. Clin Nutr ESPEN. 2021;41:331-339. doi: 10.1016/j.clnesp.2020.10.023 [DOI] [PubMed] [Google Scholar]
- 7. Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467-473. doi: 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 8. Fayers P, Aaronson NK, Bjordal K, Sullivan M.. EORTC QLQ–C30 Scoring Manual. Brussels, Belgium: European Organisation for Research and Treatment of Cancer; 1995. [Google Scholar]
- 9. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E.. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63-74. [DOI] [PubMed] [Google Scholar]
- 10. Ware JE, Sherbourne CD.. The MOS 36-Item Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483. [PubMed] [Google Scholar]
- 11. World Health Organization. World Bank Country and Lending Groups. World Health Organization. 2017. https://datahelpdesk worldbank org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed August 15, 2023.
- 12. Black HS. Influence of dietary factors on actinically-induced skin cancer. Mutat Res. 1998;422(1):185-190. doi: 10.1016/S0027-5107(98)00191-2 [DOI] [PubMed] [Google Scholar]
- 13. Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373(17):1618-1626. doi: 10.1056/NEJMoa1506197 [DOI] [PubMed] [Google Scholar]
- 14. De Quadros Camargo C, Mocellin MC, De Aguiar Pastore Silva J, De Souza Fabre ME, Nunes EA, De Moraes Trindade EBS.. Fish oil supplementation during chemotherapy increases posterior time to tumor progression in colorectal cancer. Nutr Cancer. 2016;68(1):70-76. doi: 10.1080/01635581.2016.1115097 [DOI] [PubMed] [Google Scholar]
- 15. Khodabakhshi A, Akbari ME, Mirzaei HR, Seyfried TN, Kalamian M, Davoodi SH.. Effects of Ketogenic metabolic therapy on patients with breast cancer: a randomized controlled clinical trial. Clin Nutr.2021;40(3):751-758. doi: 10.1016/j.clnu.2020.06.028 [DOI] [PubMed] [Google Scholar]
- 16. Nojiri S, Fujiwara K, Shinkai N, Iio E, Joh T.. Effects of branched-chain amino acid supplementation after radiofrequency ablation for hepatocellular carcinoma: a randomized trial. Nutrition. 2017;33:20-27. doi: 10.1016/j.nut.2016.07.013 [DOI] [PubMed] [Google Scholar]
- 17. Kranse R, Dagnelie PC, Van Kemenade MC, et al. Dietary intervention in prostate cancer patients: PSA response in a randomized double-blind placebo-controlled study. Int J Cancer. 2005;113(5):835-840. doi: 10.1002/ijc.20653 [DOI] [PubMed] [Google Scholar]
- 18. Rangel Huerta OD, Paur I, Bastani N, et al. Association between amino acids, biomarkers of prostate cancer and inflammation in Norwegian prostate cancer patients. Ann Nutr Metabol. 2017;71:1174-1175. doi: 10.1159/000480486 [DOI] [Google Scholar]
- 19. Thomas RJ, Williams MMA, Sharma H, Chaudry A, Bellamy P.. A double-blind, placebo RCT evaluating the effect of a polyphenol-rich whole food supplement on PSA progression in men with prostate cancer: the U.K. National Cancer Research Network (NCRN) Pomi-T study. J Clin Oncol. 2013;31(suppl 15):5008. [Google Scholar]
- 20. Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the women’s intervention nutrition study. J Natl Cancer Inst. 2006;98(24):1767-1776. [DOI] [PubMed] [Google Scholar]
- 21. Alberts DS, Martínez ME, Roe DJ, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. New Engl J Med.2000;342(16):1156-1162. doi: 10.1056/NEJM200004203421602 [DOI] [PubMed] [Google Scholar]
- 22. Bosland MC, Kato I, Zeleniuch-Jacquotte A, et al. Effect of soy protein isolate supplementation on biochemical recurrence of prostate cancer after radical prostatectomy: a randomized trial. JAMA. 2013;310(2):170-178. doi: 10.1001/jama.2013.7842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bougnoux P, Bonneterre J, Mercier-Blas A, et al. Modification of the response to chemotherapy of HER2 negative metastatic breast cancer by lipids of marine origin: a controlled, randomized, double blind dietary supplementation trial. Cancer Res. 2015;75(suppl 9):P3-13-08. doi: 10.1158/1538-7445.SABCS14-P3-13-08 [DOI] [Google Scholar]
- 24. DeVere White RW, Tsodikov A, Stapp EC, Soares SE, Fujii H, Hackman RM.. Effects of a high dose, aglycone-rich soy extract on prostate-specific antigen and serum isoflavone concentrations in men with localized prostate cancer. Nutr Cancer. 2010;62(8):1036-1043. doi: 10.1080/01635581.2010.492085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Freedland SJ, Allen J, Jarman A, et al. A randomized controlled trial of a 6-month low-carbohydrate intervention on disease progression in men with recurrent prostate cancer: Carbohydrate and Prostate Study 2 (CAPS2). Clin Cancer Res/ 2020;26(12):3035-3043. doi: 10.1158/1078-0432.Ccr-19-3873 [DOI] [PubMed] [Google Scholar]
- 26. Parsons JK, Zahrieh D, Mohler JL, et al. Effect of a behavioral intervention to increase vegetable consumption on cancer progression among men with early-stage prostate cancer: the MEAL randomized clinical trial. JAMA. 2020;323(2):140-148. doi: 10.1001/jama.2019.20207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schröder FH, Roobol MJ, Boevé ER, et al. Randomized, double-blind, placebo-controlled crossover study in men with prostate cancer and rising PSA: effectiveness of a dietary supplement. Eur Urol. 2005;48(6):922-930. doi: 10.1016/j.eururo.2005.08.005 [DOI] [PubMed] [Google Scholar]
- 28. Johansson H, Spadola G, Tosti G,. et al. Vitamin D supplementation and disease-free survival in stage II melanoma: a randomized placebo controlled trial. Nutrients. 2021;13(6):1931.doi: 10.3390/nu13061931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pierce JP, Natarajan L, Caan BJ, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298(3):289-298. doi: 10.1001/jama.298.3.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Zweeden AA, van Groeningen CJ, Honeywell RJ, et al. Randomized phase 2 study of gemcitabine and cisplatin with or without vitamin supplementation in patients with advanced esophagogastric cancer. Cancer Chemother Pharmacol. 2018;82(1):39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Voss M, Wagner M, Von Mettenheim N, et al. ERGO2: A prospective randomized trial of a 9-day schedule of calorically restricted ketogenic diet and fasting or standard diet in addition to re-irradiation for malignant glioma. Neuro-Oncol.2019;21(suppl 3):iii13. [Google Scholar]
- 32. De Waele E, Pen J, Demol J, Huysentruyt K, Buyl R, Laviano A.. Nutrition therapy promotes overall survival in cachectic cancer patients through a biophysical pathway: the Ticaconco trial. Clin Nutr ESPEN. 2021;46:S715. doi: 10.1016/j.clnesp.2021.09.484 [DOI] [Google Scholar]
- 33. Antunac Golubić Z, Baršić I, Librenjak N, Pleština S.. Vitamin D supplementation and survival in metastatic colorectal cancer. Nutr Cancer. 2018;70(3):413-417. [DOI] [PubMed] [Google Scholar]
- 34. Baldwin C, Spiro A, McGough C, et al. Simple nutritional intervention in patients with advanced cancers of the gastrointestinal tract, non-small cell lung cancers or mesothelioma and weight loss receiving chemotherapy: a randomised controlled trial. J Hum Nutr Diet. 2011;24(5):431-440. [DOI] [PubMed] [Google Scholar]
- 35. Bourdel-Marchasson I, Blanc-Bisson C, Doussau A, et al. Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: a two-year randomized controlled trial. PLoS One. 2014;9(9):e108687. doi: 10.1371/journal.pone.0108687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin LC, Que J, Lin KL, Leung HWC, Lu CL, Chang CH.. Effects of zinc supplementation on clinical outcomes in patients receiving radiotherapy for head and neck cancers: a double-blinded randomized study. Int J Radiat Oncol Biol Phys. 2008;70(2):368-373. [DOI] [PubMed] [Google Scholar]
- 37. Sykorova A, Horacek J, Zak P, Kmonicek M, Bukac J, Maly J.. A randomized, double blind comparative study of prophylactic parenteral nutritional support with or without glutamine in autologous stem cell transplantation for hematological malignancies—three years’ follow-up. Neoplasma. 2005;52(6):476-482. [PubMed] [Google Scholar]
- 38. Toma S, Bonelli L, Sartoris A, et al. beta-carotene supplementation in patients radically treated for stage I-II head and neck cancer: results of a randomized trial. Oncol Rep. 2003;10(6):1895-1901. [PubMed] [Google Scholar]
- 39. Sargaço B, Oliveira PA, Antunes ML, Moreira AC.. Effects of the ketogenic diet in the treatment of gliomas: a systematic review. Nutrients. 2022;14(5):1007.doi:10.3390/nu14051007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de van der Schueren MAE, Laviano A, Blanchard H, Jourdan M, Arends J, Baracos VE.. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: current evidence and guidance for design of future trials. Ann Oncol. 2018;29(5):1141-1153. doi: 10.1093/annonc/mdy114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Allenby TH, Crenshaw ML, Mathis K, et al. A systematic review of home-based dietary interventions during radiation therapy for cancer. Tech Innov Patient Support Radiat Oncol. 2020;16:10-16. doi: 10.1016/j.tipsro.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Oliveira LC, Calixto-Lima L, Cunha GDC, et al. Effects of specialised nutritional interventions in patients with incurable cancer: a systematic review. BMJ Support Palliat Care. 2022;12(4):388-402. doi: 10.1136/spcare-2022-003893 [DOI] [PubMed] [Google Scholar]
- 43. Sonbol MB, Jain T, Firwana B, et al. Neutropenic diets to prevent cancer infections: updated systematic review and meta-analysis. BMJ Support Palliat Care. 2019;9(4):425-433. doi: 10.1136/bmjspcare-2018-001742 [DOI] [PubMed] [Google Scholar]
- 44. Cheng Y, Zhang J, Zhang L, Wu J, Zhan Z.. Enteral immunonutrition versus enteral nutrition for gastric cancer patients undergoing a total gastrectomy: a systematic review and meta-analysis. BMC Gastroenterol. 2018;18(1):11. doi: 10.1186/s12876-018-0741-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Saxe GA, Rock CL, Wicha MS, Schottenfeld D.. Diet and risk for breast cancer recurrence and survival. Breast Cancer Res Treat. 1999;53(3):241-253. doi: 10.1023/a:1006190820231 [DOI] [PubMed] [Google Scholar]
- 46. Grimes DR, O’Riordan E.. Starving cancer and other dangerous dietary misconceptions. Lancet Oncol. 2023;24(11):1177-1178. doi: 10.1016/S1470-2045(23)00483-7 [DOI] [PubMed] [Google Scholar]
- 47. Thomas R, Williams M, Sharma H, Chaudry A, Bellamy P.. A double-blind, placebo-controlled randomised trial evaluating the effect of a polyphenol-rich whole food supplement on PSA progression in men with prostate cancer—the UK NCRN Pomi-T study. Prostate Cancer Prostatic Dis.2014 2014;17(2):180-186. doi: 10.1038/pcan.2014.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used to inform this paper are publicly available.