Abstract
Infections with herpes simplex virus type 1 (HSV-1) in humans and in animal models are accompanied by enhanced natural killer (NK) activity. In vitro, HSV-1 also enhances the NK activity of human peripheral blood mononuclear cells (PBMC). The molecular basis of this enhanced NK activity, however, is not well characterized. We investigated the role of human interleukin-15 (IL-15) in this phenomenon and report here that HSV-1-mediated enhanced NK activity was abrogated by neutralizing antibodies for IL-15 but not for other cytokines (i.e., IL-2, IL-12, gamma interferon [IFN-γ], tumor necrosis factor alpha, or IFN-α). Anti-CD122 antibodies which block signaling through IL-2 receptor β chain, and therefore neutralize the effects of IL-15 (and IL-2), also abrogated this enhancement. Furthermore, HSV-1 increased the levels of IL-15 mRNA and the production of IL-15 in HSV-1-infected PBMC cultures. The neutralization of IL-15 in cocultures of PBMC with HSV-1-infected cells significantly increased HSV-1 production. These results strongly suggest a role for IL-15 in the HSV-1-mediated in vitro enhancement of NK activity and in the PBMC-mediated suppression of HSV-1 replication.
Herpes simplex virus type 1 (HSV-1), a ubiquitously occurring human herpesvirus (reviewed in references 24 and 67), infects human beings early in childhood. Primary infections generally occur early in life and are usually mild or can be symptomless. These human infections are chronic and incurable, as the virus persists latently for the lifetime of the host in peripheral nervous system, mostly in trigeminal ganglia. In newborns and immunocompromised individuals, these infections may be severe and cause fatal encephalitis (67). Latent HSV-1 infections frequently become reactivated following physical or emotional stress, exposure to UV radiation, local tissue damage, and immunosuppression, and they usually manifest as herpes labialis (commonly called cold sores or fever blisters) (55, 67). These reactivations cause considerable discomfort and morbidity, and they represent a serious health problem. Studies from animal models of HSV-1 infection as well as from human beings have established that the sequelae and the control of primary and reactivated HSV-1 infections depend on the host immune response (24, 25, 67). Natural killer (NK) cells, which constitute an important cellular component of the innate immune system, play an important role in controlling these infections (6, 14–16, 60, 66). These cells can kill a wide variety of malignant and virus-infected cells without prior sensitization and constitute a first line of defense against viral infections and malignancy (5, 16). In fact, individuals with NK cell defects are known to be highly susceptible to progressive herpesvirus infections (10, 20, 25). The infected hosts, in general, respond to viral infections by increasing NK activity (5, 58). The enhanced NK activities after HSV-1 infection have been well documented both in vitro and in vivo (28, 31, 34, 41, 47, 48); however, the molecular mechanism(s) responsible for this innate immune response has not been fully investigated.
Interleukin-15 (IL-15) is a cytokine that was discovered independently by two groups in 1994 as an IL-2-like activity in the culture supernatants (SN) of two transformed cell lines. Its gene has been cloned and sequenced (17, 39; reviewed in references 61 and 64). Although it has no sequence homology at the amino acid level with IL-2, the two cytokines have similar tertiary structures and belong to the four-α-helix bundle family of cytokines. They also share the same receptor components for signal transduction (i.e., β and γ chains of the IL-2 receptor [IL-2R] complex [4, 18, 20, 22, 35]). It is not surprising, therefore, that many of their biological effects are similar. IL-15 markedly enhances the cytolytic potential of NK cells and induces the secretion of gamma interferon (IFN-γ) from these cells, alone and in synergism with IL-12 (4, 17, 18, 25, 59, 65). It has been shown to be essential for the development and differentiation of NK cells from their precursors (19, 27, 29, 50, 57). More importantly, IL-15 has been proposed as an immediate response gene which becomes activated and serves as a signal for the recruitment of immunocytes when body cells or tissues undergo stress. The stress may be physical or may occur due to an infectious agent or adverse environmental conditions (64). This characteristic makes IL-15 an appropriate candidate molecule that may be involved in the innate host response of enhanced NK activity. Because of our long-term interest in the innate responses to herpesviruses (1, 3, 33), we investigated whether in vitro infection of human peripheral blood mononuclear cells (PBMC) with HSV-1 increases their NK activity and whether IL-15 plays any role in this enhancement. The results of these studies are reported here.
MATERIALS AND METHODS
Virus preparation.
Cell-free HSV-1 (McIntyre strain) was prepared from the SN of HSV-1-infected Vero cells as described earlier (37, 38). The viral preparations were titrated by plaque-forming assay as described elsewhere (49). Briefly, 1 million Vero cells were incubated with 100 μl of logarithmically diluted virus preparations for 1 h at 37°C with intermittent shaking. After the addition of 5 ml of 1% methylcellulose, the cells were incubated in 100-mm-diameter culture dishes at 37°C in humidified (85%) 5% CO2 atmosphere. After 3 days, methylcellulose was removed by gentle suction, and the cell monolayers were washed with phosphate-buffered saline (PBS; pH 7.2) and fixed with formalin (diluted 1:5 with PBS). The fixed cells were stained with 0.1% crystal violet, and the plaques were counted under an inverted microscope. The average number of plaques per culture dish was multiplied by 10 and the dilution factor to determine the number of PFU per milliliter. The viral stock used contained 108 PFU per ml. In some experiments, concentrated HSV-1 was used. For this purpose, the virus was pelleted from the culture SN of HSV-1-infected Vero cells by ultracentrifugation, washed with PBS, and redissolved in PBS to 1/10 of the original volume as described elsewhere (1). To prepare noninfectious HSV-1, this virus preparation was either heated in a water bath at 56°C for 1 h or irradiated by exposure to a UV source that delivered 400 μJ/s for 30 min as described previously (1). Heat-inactivated and irradiated virus preparations lost infectivity, as tested by their plaque-forming ability on Vero monolayers. The viral stocks were aliquoted and kept at −80°C.
PBMC and virus treatment.
Peripheral venous blood was obtained from normal healthy donors in heparinized tubes (Vacutainer; Becton Dickinson, Oakville, Ontario, Canada). Two of these donors were seronegative for anti-HSV-1 antibodies, as determined by indirect immunofluorescence assays performed as described elsewhere (38). PBMC were obtained by centrifugation over Ficoll-Hypaque (Pharmacia, Montréal, Québec, Canada) and washed with culture medium. In some experiments, we used PBMC after depleting CD3+, CD16+, or CD56+ cells. For this purpose, PBMC were successively incubated with anti-CD16, anti-CD56, or anti-CD3 monoclonal antibodies (from Ortho Diagnostics, Raritan, N.J.) and fresh rabbit serum as detailed in our earlier publications (2, 33). For virus treatment, the cell pellets were incubated with the virus preparation (to provide 10 PFU per cell unless indicated otherwise) at 37°C for 1 h, washed thrice, and resuspended in the culture medium at a concentration of 4 × 106 cells per ml. The cells were incubated at 37°C in a humidified 5% CO2 atmosphere for 24 h, after which SN were collected and cells were used for NK assays unless indicated otherwise. The SN were concentrated 10-fold for proteins by using Microconcentrator 10 filters (Amicon Inc., Beverly, Mass.). In some experiments, the direct effect of purified HSV-1 on the NK activity of PBMC was observed. For this purpose, the virus preparation (5 to 100 μl) was added directly to the NK assay wells (see below).
Target cell line.
The cell line used in this study was K562, an erythroleukemic cell line which does not express major histocompatibility complex class I antigens and has been used extensively as a target cell line for NK assays (2, 56). These cells were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum and antibiotics as described earlier (2).
Determination of NK activity.
A standard 51Cr release assay was used to determine the NK activity of PBMC as described elsewhere (2). Briefly, K562 target cells were radiolabeled by incubation of cell pellets with 100 μCi of [51Cr]sodium chromate (NEN/Dupont, Boston, Mass.) at 37°C for 1 h with intermittent shaking. After four washings, 10,000 radiolabeled target cells were incubated in triplicate with 2 × 105 PMBC in the wells of a V-bottomed microculture plate (target/effector cell ratio of 1:20) in a total volume of 200 μl. The plates were incubated at 37°C in a humidified incubator in 5% CO2 atmosphere for 16 h. After this incubation, 100 μl of the SN was aspirated from each well, and the radioactivity released in the SN was measured in a gamma counter (LKB/Wallac, Turku, Finland). NK activity was determined as percent lysis, calculated as [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100; data are presented as average ± standard error (SE). Spontaneous release was determined by incubating 10,000 labeled K562 cells in a 200-μl volume without effector cells (PBMC), whereas maximum release was determined by lysing 10,000 labeled K562 cells by 1% Triton X-100 in a 200-μl volume.
Antibodies.
Sources of neutralizing monoclonal antibodies (MAbs) were as follows: to IFN-γ, Genzyme (Boston, Mass.); to IL-2, IL-12, and tumor necrosis factor alpha (TNF-α), R & D Systems (Minneapolis, Minn.); to IFN-α, Sigma; to CD122, Becton Dickinson. The IL-15-neutralizing MAb (M112) was a kind gift from Immunex (Seattle, Wash.). The concentrations of these and the control antibodies are detailed elsewhere (3, 33).
RT-PCR for IL-15 mRNA.
To determine whether HSV-1 treatment of PBMC had any effect on their level of expression of IL-15 gene transcripts, we used a quantitative reverse transcription-PCR (RT-PCR) assay as described earlier (3, 33). Briefly, 4 × 106 PBMC were exposed for up to 12 h to the viral preparation, and total RNA was isolated from these cells at different time points using a modified guanidinium thiocyanate method as described elsewhere (22). These time points were chosen since earlier studies had shown that herpesvirus-induced transcriptional activation of cytokine genes (including IL-15) peaks at 6 to 12 h postinfection (33, 37, 38). The total RNA was reverse transcribed in a 25-μl volume using 100 U of Moloney murine leukemia virus reverse transcriptase (Gibco/BRL, Burlington, Ontario, Canada) and first-strand synthesis buffer (Gibco/BRL). The reaction mixture contained 10 mM dithiothreitol, 1 μl of RNA Guard (Pharmacia, Baie d'Urfé, Québec, Canada), 10 pmol of the gene-specific reverse primer, and 0.2 mM each of the four deoxynucleoside triphosphates. The reaction was carried out at 30°C for 1 h. All of the RT product was used for amplification of a segment of IL-15 cDNA, whereas for the housekeeping β-actin gene, 1/10 of the product was used. PCR was carried out with 2 U of Taq polymerase (Gibco/BRL) in a 50-μl volume with the accompanying PCR buffer to which 0.2 mM deoxynucleoside triphosphates, 1.5 mM MgCl2, and 0.5 μM gene-specific reverse and forward primers were added. The reaction mixture was heated at 95°C for 1 min and chilled on ice before adding the Taq polymerase. Amplification was carried out for 25 cycles for β-actin and 35 cycles for IL-15; each cycle comprised denaturation at 94°C for 45 s, annealing at 55°C for 45 s, and extension at 72°C for 2 min, with a final 10-min extension at 72°C.
The PCR products were run on ∼2.0% agarose gels and validated by Southern blotting using 32P-end-labeled oligonucleotide probes. The primers and probes have been described previously (3, 33). The concentration of mRNA for IL-15 was normalized with respect to the β-actin mRNA, using a Pharmacia LKB Bromma laser densitometer.
Determination of IL-15.
IL-15 was determined in the concentrated SN of PBMC cultures by using a commercial enzyme-linked immunosorbent assay (ELISA) kit (detection limit, 10 pg/ml; Immunocorp, Montréal, Québec, Canada) according to the manufacturer's recommendations.
Effect of IL-15 neutralization and rhIL-15 on HSV-1 production.
To determine whether HSV-induced IL-15 production and recombinant human IL-15 (rhIL-15) have any effect on viral replication, we stimulated PBMC with UV-inactivated HSV-1 and cocultured them with HSV-1-infected K562 cells in the presence or absence of rhIL-15- or IL-15-neutralizing antibodies. For this purpose, 105 K562 cells were infected with HSV-1 (10 PFU/cell) for 1 h at 37°C. After extensive washings, the cells were mixed with 5 × 106 PBMC that had been either mock treated or treated with UV-inactivated HSV-1 for 1 h at 37°C and washed. The reason for treating PBMC with UV-inactivated viral preparation was to avoid interference with the titration of HSV-1 in the infected K562 target cells. In pilot experiments, we determined that the minimum number of PBMC added to the HSV-1-infected K562 cells that caused a persistent significant decrease in HSV-1 titers in 3-day cultures was 5 × 106 (i.e., at a target/effector cell ratio of 1:500). The cell mixtures were incubated at 37°C in 5% CO2 atmosphere with or without rhIL-15 (100 ng/ml)- or IL-15 (5 μg/ml)-neutralizing antibodies. These and control antibodies were again added on the second day after the start of the cultures. The cultures were terminated on day 3, and HSV-1 titers were determined in the cultures as described above.
RESULTS
HSV-1 enhances the NK activity of PBMC.
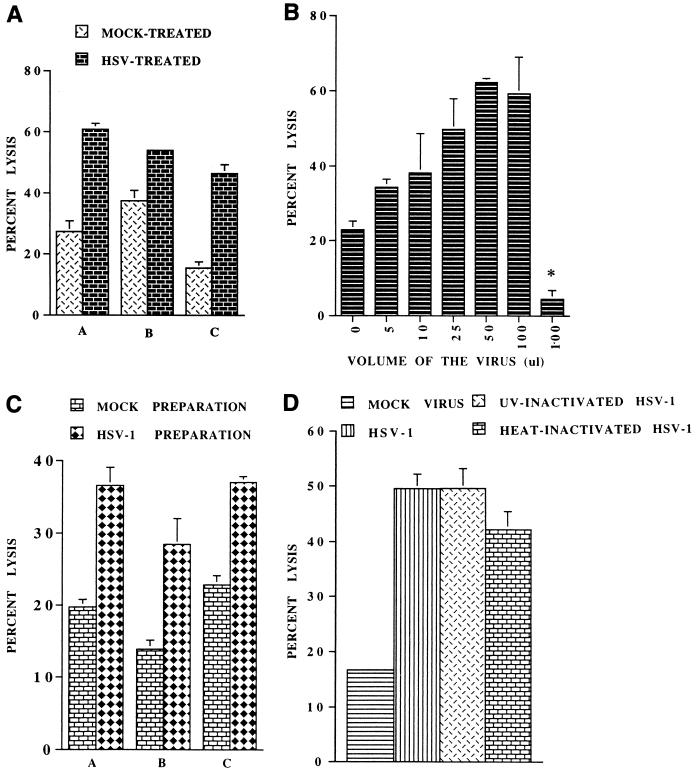
To determine the effect of HSV-1 on the NK activity of PBMC, 4 × 106 PBMC from a donor were incubated with 5 × 106 PFU of HSV-1 at 37°C for 1 h, washed and incubated overnight in 1 ml of culture medium, and then used as effectors in NK assay. The PBMC of all donors tested showed a significant increase in lysis of K562 targets. The results of a typical experiment using three donors are shown in Fig. 1A, in which donor C is seronegative for HSV-1. It is evident from these data that the extent of the increase depended on the baseline NK activity of the donor. Donors with low NK activity showed an up to threefold increase in NK activity following infection with HSV-1. Furthermore, essentially similar results were obtained for individuals that were seronegative or seropositive for anti-HSV-1 antibodies (Fig. 1A). We further determined whether direct addition of the virus preparation to NK assays (in which fresh PBMC were used as effector cells without prior treatment with the virus) also increased the NK activity. After adding different doses of the virus to the assays, we found that addition of the virus preparation resulted in enhanced NK activity and that 50 μl (containing 5 × 106 PFU) was the optimum dose to induce maximal increase in the NK assay (Fig. 1B). It is noteworthy that direct addition of 100 μl of the viral preparation per se had no cytolytic effect on the target cells in the 16-h 51Cr release assay. The effects of addition of 50 μl of the viral preparation on the NK activity of PBMC of three donors are shown in Fig. 1C. In additional experiments, we determined whether concentrated HSV-1 (without culture SN from infected Vero cells) could also increase the NK activity of PBMC and whether infection of PBMC by HSV-1 was necessary for this increase. For this purpose, we treated PBMC with purified infectious HSV-1 and with noninfectious heat-inactivated and UV-irradiated HSV-1 separately for 1 h at 37°C, washed the cells, and determined their NK activities. The results of a typical experiment are shown in Fig. 1D. Both concentrated infectious and noninfectious viruses were able to induce this enhancement, indicating that the interaction of HSV-1 with PBMC, and not the infection process per se or a soluble factor present in the culture SN of the infected Vero cells, was responsible for this effect.
FIG. 1.
HSV-1 exposure induces enhancement of NK activity of PBMC. (A) Four million PBMC were incubated with HSV-1 or with mock virus preparation at 37°C for 1 h, washed, and incubated at 37°C in 5% CO2. After 24 h, their NK activity was determined at a target/effector cell ratio of 1:20 against 104 51Cr-labeled K562 cells. Data represent the average NK activity (percent lysis) ± SE of PBMC from three different donors (A, B, and C) with and without (mock) treatment with HSV-1. (B) NK assays were set up in triplicate in 96-well microculture plates using PBMC without treatment with HSV-1. Different doses of HSV-1 (0 to 100 μl) were added to these assays, and NK activity was determined 16 h later. The data show that the optimum dose of virus to induce maximum lysis was 50 μl. Note that 100 μl of the virus itself (without PBMC) did not cause significant lysis of the target cells (bar with asterisk). (C) Average lysis ± SE from three different donors (A, B, and C) after the addition of 50 μl of HSV-1 or mock virus preparation to NK assays (as described for panel B). (D) PBMC were incubated with purified HSV-1 or with similar noninfectious UV- or heat-treated viral preparations, and NK activities were determined 24 h later as described for panel A. Both UV- and heat-inactivated viruses also enhanced the NK activities of PBMC as did the concentrated infectious HSV-1.
To determine whether the lytic activity observed in our microcytotoxicity assays was authentic NK activity effected by NK cells, we depleted PBMC of NK cells or of CD3+ T cells and determined their ability to kill NK-sensitive target cells with and without infection with HSV-1. The results of a typical experiment are shown in Table 1. It is evident from these data that the enhanced cytotoxicity due to HSV-1 exposure is mediated mainly by CD16+ and/or CD56+ NK cells. CD3+ T cells (which also include NK T cells) do not seem to play a significant role in this cytotoxicity (Table 1).
TABLE 1.
Effect of depletion of NK and CD3+ cells on the HSV-induced enhancement in NK activitya
| Depletion | NK activity (% lysis)
|
|
|---|---|---|
| Mock-treated PBMC | HSV-treated PBMC | |
| None | 23.31 ± 5.69 | 59.56 ± 1.95 |
| CD56 | 3.66 ± 1.54 | 4.05 ± 1.61 |
| CD16 | 7.33 ± 1.07 | 11.68 ± 1.60 |
| CD3 | 16.42 ± 3.54 | 42.48 ± 4.00 |
| Control | 17.15 ± 0.68 | 49.04 ± 5.72 |
PBMC were treated with HSV-1 or mock infected, depleted for cells positive for the indicated markers as described in Materials and Methods, and used as effectors in NK assays. Shown is the average percent lysis ± standard deviation from three replicate NK assay wells.
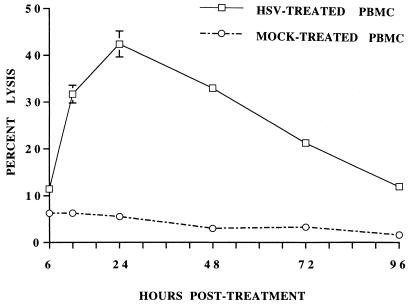
The kinetics of the enhancement of NK activity by HSV-1 treatment was studied by incubating PBMC with HSV-1 for 1 h at 37°C and then testing their NK activity at different time points postincubation (p.i.) as shown in Fig. 2. The NK activity of HSV-1-treated cells enhanced as early as 6 h p.i., reached its peak at 24 h p.i., and then gradually declined to the baseline level by day 4.
FIG. 2.
PBMC were incubated with HSV-1, washed, and incubated at 37°C in 5% CO2 as described for Fig. 1A. NK activity against 51Cr-labeled K562 targets was determined at different time points postincubation. Note the peak increase in NK activity in HSV-treated PBMC at 24 h after the treatment.
Presence of NK-enhancing factor in SN.
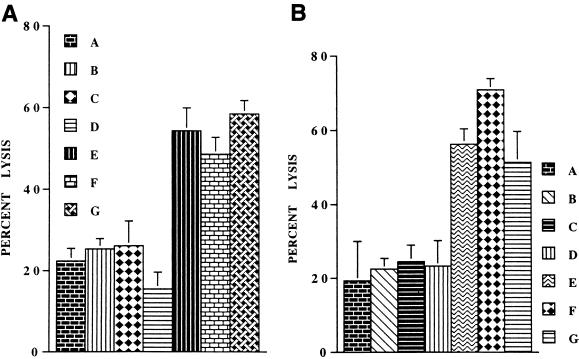
To determine whether any soluble factor(s) released from the interaction of HSV-1 with PBMC mediated the enhancement of NK activity, we tested the effect of culture SN from HSV-treated and mock-treated PBMC on the NK activity of uninfected freshly isolated human PBMC. These culture SN were collected 24 h p.i. and added to the cultures of fresh human PBMC, which were then incubated for 24 h at 37°C in 5% CO2 atmosphere. As shown in Fig. 3A, the SN from HSV-treated, and not from mock-treated, PBMC cultures markedly enhanced the NK activity of untreated PBMC, indicating that the interaction of HSV-1 with PBMC caused the release of one or more soluble factors that were responsible for the observed increase in the NK activity. We also added 50 μl of the culture SN to the NK assay wells to determine their effect on the NK activity of freshly isolated human PBMC. In these assays, these SN did not significantly increase NK activity (P ≥ 0.05 [data not shown]). However, when 10-fold-concentrated SN were added to these NK assay wells, the SN from HSV-treated PBMC, but not from mock-treated PBMC, significantly increased the NK activity (Fig. 3B). These SN alone (without effector cells) had no cytolytic effect on the target cells in these assays.
FIG. 3.
The culture SN from HSV-1-treated PBMC increases NK activity of untreated PBMC. (A) PBMC (4 × 106) were treated with HSV-1, washed, and incubated at 37°C for 24 h as described in the legend to Fig. 1A. Their SN were collected and filtered through 0.1-μm-pore-size filters. Freshly isolated PBMC were incubated at 37°C in this SN or with SN from the mock-treated PBMC for 24 h and then used as effectors in NK assays. The effects of these SN from three different donors on the NK activity of the untreated PBMC are shown. A, NK activity of PBMC incubated for 24 h in culture medium; B to D, NK activity of the same PBMC after incubation with SN from mock-treated PBMC of three donors; E to G, NK activity of the PBMC after incubation with SN from HSV-treated PBMC of the same three donors, respectively. (B) The addition of 50 μl of 10-fold-concentrated culture SN from HSV-treated, but not from mock-treated, PBMC to NK assays enhances the NK activity of freshly isolated untreated PBMC. A, NK activity of PBMC without SN; B to D, NK activity of PBMC with the addition of SN from the mock-treated PBMC of three different donors; E to G, NK activity of PBMC with the addition of SN from HSV-treated PBMC of the same three donors, respectively.
Identification of the NK activity-enhancing factor.
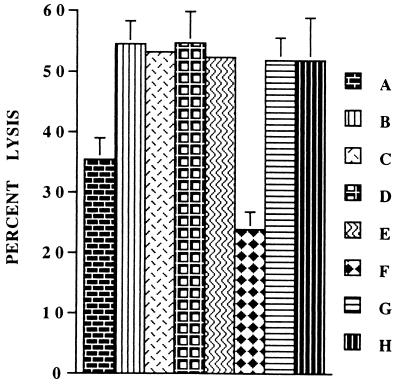
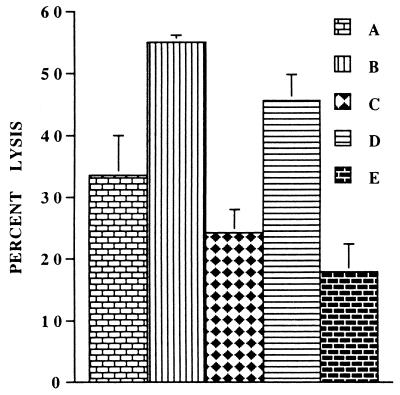
To identify the NK activity-enhancing factor(s) induced by the HSV-1 treatment of PBMC, we added saturating concentrations of neutralizing antibodies for different cytokines to the PBMC cultures after incubation with HSV-1 and then tested their NK activity after 24 h. As shown in Fig. 4, only anti-IL-15 antibodies abrogated the enhanced NK activity due to HSV-1 infection; the effect of other cytokines was nonsignificant (P ≥ 0.05). In separate experiments, we also determined that IL-2- and IL-18-neutralizing antibodies had no effect on the HSV-induced enhancement of the NK activity. Essentially similar results were obtained when these experiments were repeated with noninfectious UV- or heat-inactivated HSV-1 (data not shown). Furthermore, as we reported previously, none of these antibodies except for IL-15 decreased NK activity of untreated, freshly isolated PBMC (Fig. 4). These data show that the NK activity enhanced by exposure of PBMC to HSV-1 is induced by IL-15.
FIG. 4.
IL-15-neutralizing antibodies inhibit the HSV-1-mediated increase in the NK activity of PBMC. PBMC were treated with HSV-1 and incubated in the presence of different cytokine neutralizing antibodies. Their NK activities were determined 24 h later. The effects of neutralization of different cytokines on the HSV-1-mediated increase in NK activity of PBMC are shown. A, mock-treated PBMC; B, HSV-treated PBMC; C to H, HSV-infected PBMC incubated in the presence of neutralizing antibodies for cytokines IL-12, TNF-α, IFN-γ, IL-15, control, and IFN-α, respectively. Note that only significant (P < 0.05) inhibition of the HSV-mediated enhancement of NK activity was caused by IL-15-neutralizing antibodies (compare F with B).
Anti-CD122 MAb abrogates the NK-enhancing effect of HSV-1.
The foregoing experiments identified IL-15 as the soluble factor induced after interaction of HSV-1 with human PBMC that was responsible for the enhancement of NK activity in human PBMC. Since IL-15 uses β and γ chains of the IL-2R system for signal transduction and anti-IL-2Rβ (i.e., anti-CD122) antibodies are shown to block the NK cell-stimulatory activity of IL-15 (4, 18, 39), we investigated whether blocking signal transduction by IL-2Rβ would also abolish the NK activity enhancement by HSV-1. For this purpose, we added anti-CD122 antibodies to the PBMC immediately before incubating them with HSV-1. As shown in Fig. 5, the addition of anti-CD122 antibodies to the PBMC at this time resulted in complete loss of the NK activity enhancement by HSV-1. Interestingly, this treatment also resulted in NK activity of PBMC even lower than that of untreated PBMC, further supporting the results obtained with IL-15-neutralizing antibodies that IL-15 and signaling via IL-2Rβ play a role in the NK function of PBMC under physiological conditions.
FIG. 5.
Effects of anti-CD122 antibodies on HSV-induced enhancement of NK activity. PBMC were mock treated or treated with HSV-1, washed and incubated in the presence of anti-CD122 or control antibodies for 24 h, and used as effectors in NK assays. The data show the average ± SE NK activities of these PBMC. A, mock-treated PBMC; B, HSV-treated PBMC; C, HSV-treated PBMC incubated in the presence of anti-CD122 antibodies; D, HSV-treated PBMC incubated in the presence of control antibodies; E, HSV-treated PBMC incubated in the presence of anti-IL-15 antibodies.
Production of IL-15 by HSV-treated PBMC.
Having determined that the NK-enhancing factor in the SN of HSV-treated PBMC was indeed IL-15, we titrated the SN of the HSV-infected and mock-infected PBMC from healthy individuals for IL-15, using a commercial ELISA kit with a detection limit of 10 pg/ml. Since no signal could be detected in the SN from HSV-infected and mock-infected PBMC from these individuals, these SN were concentrated as described in Materials and Methods and used in the ELISA. IL-15 was detected in the culture SN from HSV-1-infected PBMC (mean, 72.38 pg/ml; standard deviation, 15.83), whereas it remained below the detection limit (10 pg/ml) in the culture SN of the mock-infected PBMC.
HSV-1 increases IL-15 mRNA expression.
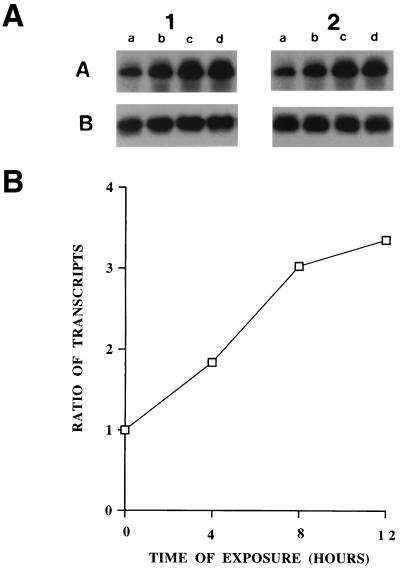
Increased synthesis of cytokines is generally accompanied by increased gene transcription and/or increased stability of their transcripts, causing increased steady-state levels of their transcripts. A quantitative RT-PCR was used to compare the expression of IL-15 mRNA in HSV-1-treated and mock-treated PBMC at different time points after treatment. The results for two different donors are shown in Fig. 6A; densitometric analysis of a donor showing the ratio of IL-15 to β-actin transcripts is shown in Fig. 6B. A threefold increase in the IL-15 mRNA is evident in the HSV-1-treated PBMC 12 h after the treatment. These data demonstrate that HSV-1 increases IL-15 gene expression in HSV-1-treated PBMC.
FIG. 6.
HSV-1 increases IL-15 mRNA levels in PBMC. (A) Four million PBMC were incubated with HSV-1. Total RNA was extracted from the PBMC at the indicated time points, reverse transcribed, and used in PCR for amplification of the IL-15 or β-actin cDNA segments as described in Materials and Methods. The PCR products were validated on Southern blots using gene-specific 32P-labeled probes. The amplified transcripts from the PBMC of two different donors (1 and 2) are shown. The bands in rows A and B show RT-PCR products of IL-15 and β-actin, respectively. Lanes a to d represent 0, 4, 8, and 12 h of incubation with the virus. (B) Densitometric analysis of the IL-15 transcripts expressed as a ratio with β-actin transcripts at different time points after incubation of the PBMC with HSV-1.
Effect of HSV-induced IL-15 on viral replication.
We determined the effect of HSV-1-induced IL-15 in PBMC on the virus replication in K562 cells as described in Materials and Methods. As shown in Table 2, the addition of PBMC to HSV-infected K562 cells caused a 2-log decrease in HSV-1 titers. The addition of IL-15-neutralizing antibodies inhibited this decrease. There was no difference between the viral titers when HSV-1-treated or mock-treated PBMC were added to these cultures. This may be due to activation of the uninfected PBMC upon contact with HSV-1-infected target cells. Furthermore, the addition of rhIL-15 also caused a marked decrease in HSV-1 titers in the PBMC-K562 coculture but not in K562 cells to which no PBMC were added. Thus, IL-15 had no direct effect on HSV-1 replication in K562 cells, and its antiviral effects in the PBMC-K562 cocultures might be mediated indirectly via PBMC due to their IL-15-induced enhanced NK activity and/or release of some other IL-15-induced antiviral mediator(s). These data suggest a role for IL-15 in the PBMC-mediated repression of HSV-1 replication in these cell cultures.
TABLE 2.
Effect of PBMC and IL-15 neutralization on HSV-1 replicationa
| Treatment | HSV-1 titer
|
||
|---|---|---|---|
| K562 | K562 + PBMC I | K562 + PBMC II | |
| Anti-IL-15 antibodies | 4.7 × 105 | 3.43 × 104 | 4.83 × 104 |
| Control antibodies | 3.50 × 105 | 2.17 × 103 | 2.46 × 103 |
| rhIL-15 | 6.10 × 105 | 6.2 × 102 | 9.5 × 102 |
K562 cells (105) were infected with HSV-1 (106 PFU), washed, and cocultured with mock-treated (PBMC I) or UV-inactivated HSV-1-treated (PBMC II) cells (5 × 106) with or without the presence of IL-15-neutralizing antibodies or of rhIL-15 (100 ng/ml). On day 3 postinfection, HSV-1 titers in these cultures were determined; the results of a typical experiment are shown.
DISCUSSION
We have demonstrated here that HSV-1 induces the production of IL-15 from human PBMC and that this induced IL-15 subsequently enhances the NK activity of these cells. Increased NK activity in acute viral infections particularly with herpesviruses has been well documented both in infected human beings and in animal models of these infections (11, 62; reviewed in reference 14). In the case of HSV-1, activation and blastogenesis of NK cells occur in mice within 2 to 3 days after an experimental infection (6, 66). In vitro, HSV-1 also activates NK cells and increases their cytolytic activity. HSV-1-infected cells of several lineages not only become more susceptible to NK cell-mediated killing but also activate NK cells (6, 66). The molecular mechanisms responsible for this enhanced NK cell activity are not fully understood. Our studies clearly show that virus-induced IL-15 plays a role in this phenomenon. It is generally assumed that virus-induced IFN-α and -β are responsible for NK cell activation. However, we could not find any study in which the in vitro virus-mediated NK cell activation was shown to be caused by IFN-α/β or by any other soluble factor. In fact, on the contrary, several workers observed that this NK cell activation either was not correlated to the production of IFN-α/β or could not be abrogated by neutralizing these cytokines (9, 12, 28, 31, 32). In vivo studies in mice with HSV-1 and murine cytomegalovirus, however, have shown that administration of IFN-α-neutralizing antibodies can abrogate virus-mediated NK cell activation and the antiviral effects of this cytokine (41, 53). In view of the facts that (i) antiviral effects of IFNs are mediated by IFN response factor 1 (46) and (ii) this factor has been shown to be necessary and sufficient for inducing IL-15 (52), it would not be surprising if the ultimate effector molecule of these IFN-mediated effects was found to be IL-15 or some IL-15-induced factor(s). This observation is further supported by the reports that (i) IFN-α and -β can activate NK cells, but their in vitro effects are antiproliferative for NK and T cells (7, 63, 69), and (ii) the in vivo effects of inducing proliferation of CD8+ memory T cells by these IFNs have been clearly shown to be mediated via IL-15 (69).
Previous studies from this laboratory have shown that HSV-1 induces TNF-α but little IL-1β in human PBMC cultures (37, 38). TNF-α, however, was not induced in HSV-infected PBMC cultures until 4 to 5 days after infection. The virus-mediated enhancement of NK cell activity, on the other hand, peaks within 24 h postinfection. Furthermore, the target K562 cells used in our study are resistant to TNF-α-mediated killing (51), and we also demonstrated that neutralization of TNF-α did not affect the virus-mediated enhancement of NK cell activity reported here. All of these observations strongly argue against a role of TNF-α in this enhanced NK cell activity. Some viruses (e.g., Epstein-Barr virus [38]) induce the production of IL-6 in human PBMC cultures, and this cytokine can also increase their NK activity. However, we can rule out a role of this cytokine in the HSV-mediated increase in NK activity of PBMC, since previous studies from this laboratory have demonstrated that HSV-1 does not induce the production of IL-6 in human PBMC cultures (37, 38). HSV-1 was recently reported to induce IL-12 in PBMC cultures, and this cytokine was shown to be important in in vivo virus-induced IFN-γ production (54; reviewed in reference 8). Both IL-15 and IL-12 are produced from monocytes/macrophages in PBMC. Compared to IL-12, IL-15 is a weaker stimulus for inducing IFN-γ from human NK cells. The effects of both cytokines are, however, synergistic (59, 61). Therefore, virus-induced IL-15 may also contribute to IFN-γ production from NK cells. IFN-γ production is important for activating macrophages and for inducing a strong pathogen-specific immune response. IL-12 and IFN-γ, however, are not involved in the virus-mediated enhancement of NK cell activity reported here, as the saturation concentrations of neutralizing antibodies specific for these cytokines did not significantly reduce the virus-mediated increase in NK cell cytotoxicity. Cousens et al. (26) have recently shown that IFN-α and -β produced by PBMC in response to a viral infection strongly inhibit IL-12 production from monocytes. Monocytes are the cell type which also produce IL-15. Its production from monocytes is, however, much more resistant to the downregulatory effects of inhibitory cytokines (e.g., transforming growth factor β, IL-4, IL-10, and IFN-α [29]). Thus, the inhibition of IL-12 production in virus-infected PBMC by IFNs may be responsible for a lack of its role in the enhancement of HSV-mediated NK activity.
HSV-1 infects a wide variety of target cells and shuts off the synthesis of macromolecules in these cells within 24 h after infection. The viral protein Vhs mediates this shutoff. Freshly isolated monocytes and T, B, and NK cells are resistant to infection with this virus (13, 42). Previous results from this and other laboratories have shown that monocytes are the main cell type in PBMC that produces IL-15. This may explain why HSV-1 does not shut off the synthesis of this (and other monokines) from human PBMC.
With respect to inducing IL-15 in human PBMC cultures, HSV-1 behaves like human herpesviruses 6 and 7 (3, 33). In fact, the induction of IL-15 by viral infections seems to be a general phenomenon, as several different viruses including HSV-1 have recently been reported to activate this cytokine gene in human PBMC cultures (30, 36, 45). Interestingly, extremely variable levels of this cytokine have been found in biological fluids and PBMC cultures (21, 29, 30, 44, 45). Nevertheless, it seems to be a defensive mechanism on the part of host to ensure enhanced NK cell activity and a strong adaptive immune response, as IL-15 has been shown to be an excellent adjuvant (21, 40, 43, 44, 63, 68). The NK cell activation may be particularly effective in controlling the so-called NK cell-sensitive viruses like HSV-1. This virus, like many others, downregulates major histocompatibility complex class I antigen expression on the surface of infected cells (to evade host cytotoxic T-cell responses) and thus renders these cells more susceptible to NK cell-mediated killing. However, it seems that the virus has also evolved strategies to escape this host response. Virus-infected cells have been reported to become resistant to NK cell-mediated killing in the later phase of infection, probably by expressing certain viral glycoproteins on the surface (23).
In this in vitro study, we have demonstrated a role of IL-15 in HSV-1-mediated activation of NK cells. It is highly likely that this cytokine also plays an important role in controlling viral infections in vivo. Further studies to address this issue should be forthcoming. The availability of IL-15 knockout mice will facilitate these studies.
ACKNOWLEDGMENTS
We thank the Medical Research Council of Canada (MRCC) and J-L Lévesque Foundation for support. A.A. is an MRCC scholar.
We thank Immunex Corporation (Seattle, Wash.) for IL-15-related reagents and Micheline Patenaude and Sylvie Julien for secretarial assistance.
REFERENCES
- 1.Ahmad A, Menezes J. The binding of Epstein-Barr virus to human platelets causes the release of transforming growth factor-β. J Immunol. 1997;159:3984–3988. [PubMed] [Google Scholar]
- 2.Ahmad A, Menezes J. Defective killing activity against gp120/41-expressing human erythroleukaemic K562 cell line by monocytes and natural killer cells from HIV-infected individuals. AIDS. 1996;10:143–149. doi: 10.1097/00002030-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Atedzoé B N, Ahmad A, Menezes J. Enhancement of natural killer cell cytotoxicity by the human herpesvirus-7 via IL-15 induction. J Immunol. 1997;159:4966–4972. [PubMed] [Google Scholar]
- 4.Bamford R N, Grant A J, Burton J D, Peters C, Kuzys G, Goldman C D, Brennan J, Roessler E, Waldman T A. The interleukin (IL) 2 receptor β chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T cell proliferation and the induction of lymphokine activated killer cells. Proc Natl Acad Sci USA. 1994;91:4940–4944. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bancroft G J. The role of natural killer cells in innate resistance to infection. Curr Opin Immunol. 1993;5:503–510. doi: 10.1016/0952-7915(93)90030-v. [DOI] [PubMed] [Google Scholar]
- 6.Biron C A. Activation and function of natural killer cell responses during viral infections. Curr Opin Immunol. 1997;9:24–34. doi: 10.1016/s0952-7915(97)80155-0. [DOI] [PubMed] [Google Scholar]
- 7.Biron C A, Sonnenfield G, Welsh R M. Interferon induces NK cell blastogenesis in vivo. J Leukoc Biol. 1984;35:31–37. doi: 10.1002/jlb.35.1.31. [DOI] [PubMed] [Google Scholar]
- 8.Biron C A, Orange J S. IL-12 in acute viral infectious diseases. Res Immunol. 1995;146:590–600. doi: 10.1016/0923-2494(96)83036-7. [DOI] [PubMed] [Google Scholar]
- 9.Biron C A, Nguyen K B, Pien G C, Cousens L P, Solazar-Mather T P. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 10.Biron C A, Byron K S, Sullivan J L. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 11.Biron C A, Turgiss L R, Welsh R M. Increase in NK cell number and turnover rate during acute viral infection. J Immunol. 1983;131:1539–1545. [PubMed] [Google Scholar]
- 12.Borysiewicz L K, Rodgers B, Morris S, Graham S, Sissons J G P. Lysis of human cytomegalovirus infected fibroblasts by natural killer cells: demonstration of an interferon-independent component requiring expression of early viral proteins and characterization of effector cells. J Immunol. 1985;134:2695–2701. [PubMed] [Google Scholar]
- 13.Braun R W, Taute H K, Kirchner H, Munk K. Replication of herpes simplex virus in human T lymphocytes: characterization of the viral target cell. J Immunol. 1984;132:914–919. [PubMed] [Google Scholar]
- 14.Brutkiewicz R R, Welsh R M. Major histocompatibility complex class I antigens and the control of viral infections by natural killer cells. J Virol. 1995;69:3967–3971. doi: 10.1128/jvi.69.7.3967-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bukowski J F, Woda B A, Habu S, Okumura K, Welsh R M. Natural killer cell depletion enhances virus synthesis and virus induced hepatitis in vivo. J Immunol. 1983;131:1531–1538. [PubMed] [Google Scholar]
- 16.Bukowski J F, Warner J F, Dennert G, Welsh R M. Adoptive transfer studies demonstrating the antiviral effect of natural killer cells in vivo. J Exp Med. 1985;161:40–52. doi: 10.1084/jem.161.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burton J D, Bamford R N, Peters C, Grant A J, Kurys G, Goldman C K, Brennan J, Roessler E, Waldman T A. A lymphokine, provisionally designated interleukin T and produced by a human adult T cell leukemia line, stimulates T-cell proliferation and induction of lymphokine-activated killer cells. Proc Natl Acad Sci USA. 1994;91:4935–4939. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carson W E, Giri J G, Lindemann M J, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carson W E, Fehniger T A, Haldar S, et al. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Investig. 1997;99:937–943. doi: 10.1172/JCI119258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chae D-W, Nosaka Y, Stromand T B, Maslinski W. Distribution of IL-15 receptor α-chain on human peripheral blood mononuclear cells and effect of immunosuppressive drugs on receptor expression. J Immunol. 1996;157:2813–2819. [PubMed] [Google Scholar]
- 21.Chehimi J, Marshall J D, Salvucci O, Frank I, Chehimi S, Kawecki S, Bacheller D, Rifat S, Chouaib S. IL-15 enhances immune functions during HIV infection. J Immunol. 1997;158:5978–5987. [PubMed] [Google Scholar]
- 22.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Ann Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 23.Confer D L, Vercellotti G M, Kotasek D, Goodman J L, Ochoa A, Jacob H S. Herpes simplex virus-infected cells disarm killer lymphocytes. Proc Natl Acad Sci USA. 1990;87:3609–3613. doi: 10.1073/pnas.87.9.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corey L, Spear P G. Infections with herpes simplex viruses. N Engl J Med. 1986;314:686–691. doi: 10.1056/NEJM198603133141105. [DOI] [PubMed] [Google Scholar]
- 25.Corey L, Spear P G. Infections with herpes simplex viruses. N Engl J Med. 1986;314:749–757. doi: 10.1056/NEJM198603203141205. [DOI] [PubMed] [Google Scholar]
- 26.Cousens L P, Orange J S, Su H C, Biron C A. INFα/β inhibition of IL-12 and INF-γ production in vitro and endogenously during viral infection. Proc Natl Acad Sci USA. 1997;94:634–639. doi: 10.1073/pnas.94.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daniel W W. Biostatistics: a foundation of analysis in health sciences. 6th ed. Toronto, Ontario, Canada: John Wiley & Sons; 1991. pp. 166–170. [Google Scholar]
- 28.Djeu J Y, Stocks N, Zoon K, Stanton G J, Timonen T, Herberman R B. Self regulation of cytotoxicity in human natural killer cells by production of interferon upon exposure to influenza and herpes simplex viruses. J Exp Med. 1982;156:1222–1236. doi: 10.1084/jem.156.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doherty T M, Seder R A, Sher A. Induction and regulation of IL-15 expression in murine macrophages. J Immunol. 1996;156:735–741. [PubMed] [Google Scholar]
- 30.Fawaz L M, Sharif-Askari E, Menezes J. Up-regulation of NK cytotoxic activity via IL-15 induction by different viruses: a comparative study. J Immunol. 1999;163:4473–4480. [PubMed] [Google Scholar]
- 31.Fitzgerald P A, Mendelsohn M, Lopez C. Human natural killer cells limit replication of herpes simplex virus type I in vitro. J Immunol. 1985;134:2666–2672. [PubMed] [Google Scholar]
- 32.Fitzgerald P A, vonWussow P, Lopez C. Role of interferon in natural kill of HSV-1-infected fibroblasts. J Immunol. 1982;129:819–825. [PubMed] [Google Scholar]
- 33.Flamand L, Stefanescu I, Menezes J. Human herpes virus-6 enhances natural killer cytotoxicity via IL-15. J Clin Investig. 1996;97:1373–1381. doi: 10.1172/JCI118557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fleisher G, Starr S, Koven N, Kamiya H, Douglas S D, Henle W. A non-x-linked syndrome with susceptibility to severe Epstein-Barr virus infections. J Pediatr. 1982;100:727–730. doi: 10.1016/s0022-3476(82)80572-6. [DOI] [PubMed] [Google Scholar]
- 35.Giri J G, Anderson D M, Kumaki S, Park L S, Grabstein K H, Cosman D. IL-15, a novel T cell growth factor that shares activities and receptor components with IL-2. J Leukoc Biol. 1995;57:763–766. doi: 10.1002/jlb.57.5.763. [DOI] [PubMed] [Google Scholar]
- 36.Gosselin J, Tomolu A, Gallo R C, Flamand L. Interleukin-15 as an activator of natural killer cell-mediated antiviral response. Blood. 1999;94:4210–4219. [PubMed] [Google Scholar]
- 37.Gosselin J, Flamand L, D'Addario M, Hiscott J, Stefanescu I, Ablashi D V, Gallo R C, Menezes J. Modulatory effects of Epstein-Barr, herpes simplex, and human herpes-6 viral infections and co-infections on cytokine synthesis: a comparative study. J Immunol. 1992;149:181–187. [PubMed] [Google Scholar]
- 38.Gosselin J, Flamand L, D'Addario M, Hiscott J, Menezes J. Infection of peripheral blood mononuclear cells by herpes simplex and Epstein-Barr viruses: differential induction of interleukin 6 and tumor necrosis factor-α. J Clin Investig. 1992;89:1849–1856. doi: 10.1172/JCI115789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grabstein K H, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with β chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 40.Gursel M, Gregoriadis G. Interleukin-15 acts as an immunological co-adjuvant for liposomal antigen in vivo. Immunol Lett. 1997;55:161–165. doi: 10.1016/s0165-2478(97)02699-0. [DOI] [PubMed] [Google Scholar]
- 41.Habu S, Akamatsu K, Tamaoki N, Okumura K. In vivo significance of NK cells on resistance against virus (HSV-1) infections in mice. J Immunol. 1984;133:2743–2447. [PubMed] [Google Scholar]
- 42.Hayward A R, Read G S, Cosysn M. Herpes simplex virus interferes with monocyte accessory cell function. J Immunol. 1993;150:190–196. [PubMed] [Google Scholar]
- 43.Hunter C A, Ellis-Neyer L, Gabriel K E, Kennedy M K, Grabstein K H, Linsley P S, Remington J S. The role of the CD28/B7 interaction in the regulation of NK cell responses during infection with Toxoplasma gondii. J Immunol. 1997;158:2285–2293. [PubMed] [Google Scholar]
- 44.Jullien D, Sieling P A, Uyemura K, Mar N D, Rea T H, Modlin R L. IL-15, an immunomodulator of T cell responses in intracellular infection. J Immunol. 1997;158:800–806. [PubMed] [Google Scholar]
- 45.Kacani L, Stroiber H, Dierich M P. Role of IL-15 in HIV-associated hypergammaglobulinaemia. Clin Exp Immunol. 1997;108:14–18. doi: 10.1046/j.1365-2249.1997.d01-972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kimura T, Katsutoshi N, Penninger J, et al. Involvement of IRF-1 transcription factor in antiviral responses to interferons. Science. 1994;264:1921–1924. doi: 10.1126/science.8009222. [DOI] [PubMed] [Google Scholar]
- 47.Lopez C, Kirkpatrick D, Read S E, Fitzgerald P A, Pitt J, Pahwa S, Ching C Y, Smithwick E M. Correlation between low natural killing of fibroblasts infected with herpes simplex virus type 1 and susceptibility to herpesvirus infections. J Infect Dis. 1983;147:1030–1035. doi: 10.1093/infdis/147.6.1030. [DOI] [PubMed] [Google Scholar]
- 48.Lopez C, Ryshke R, Bennett M. Marrow dependent cells depleted by 98Sr mediate genetic resistance to herpes simplex virus 1 infection. Infect Immun. 1980;28:1028–1032. doi: 10.1128/iai.28.3.1028-1032.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Menezes J, Bourkas A E. Herpesvirus-lymphoid cell interactions: comparative studies on the biology of herpes simplex virus-induced Fc receptors in B, T and “null” lymphoid cell lines. J Virol. 1980;33:115–122. doi: 10.1128/jvi.33.1.115-122.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morzek E, Anderson P, Caligiuri M A. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87:2632–2639. [PubMed] [Google Scholar]
- 51.Munker R, Koeffler P. In vitro action of tumor necrosis factor on myeloid leukemic cells. Blood. 1987;69:1102–1108. [PubMed] [Google Scholar]
- 52.Ohteki T, Yoshida H, Matsuyama T, Duncan G S, Mak T W, Ohashi P S. The transcription factor interferon regulatory factor-1 (IRF-1) is important during the maturation of natural killer 1-1+ T cell receptor-α/β (NK1+T) cells, natural killer cells, and intestinal intraepithelial T cells. J Exp Med. 1998;187:967–972. doi: 10.1084/jem.187.6.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orange J S, Biron C A. Characterization of early IL-12, INF-αβ, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J Immunol. 1996;156:4746–4756. [PubMed] [Google Scholar]
- 54.Orange J S, Biron C A. An absolute and restricted requirement for IL-12 in natural killer cell INF-gamma production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J Immunol. 1996;156:1138–1142. [PubMed] [Google Scholar]
- 55.Posavad C M, Koelle D M, Corey L. Tipping the scales of herpes simplex virus reactivation: the important responses are local. Nat Med. 1998;4:381–382. doi: 10.1038/nm0498-381. [DOI] [PubMed] [Google Scholar]
- 56.Pross H F, Callewart D, Rubin P. Assays for NK cell cytotoxicity-their values and pitfalls. In: Lotzova E, editor. Immunobiology of natural killer cells. Boca Raton, Fla: CRC Press, Inc.; 1986. pp. 87–90. [Google Scholar]
- 57.Puzanov I J, Bennett M, Kumar V. IL-15 can substitute for marrow microenvironment in the differentiation of natural killer cells. J Immunol. 1996;157:4282–4285. [PubMed] [Google Scholar]
- 58.Robertson M J, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76:2421–2438. [PubMed] [Google Scholar]
- 59.Seder R A. High-dose IL-2 and IL-15 enhance the in vitro priming of naive CD4+ T cells for INF-γ but have differential effects on priming for IL-4. J Immunol. 1996;156:2413–2422. [PubMed] [Google Scholar]
- 60.Stohlman S A, Brayton P R, Harmon R C, Stevenson D, Ganges R G, Matsushima G M. Natural killer cell activity during mouse hepatitis virus infection: response in the absence of interferon. Int J Cancer. 1983;31:309–314. doi: 10.1002/ijc.2910310310. [DOI] [PubMed] [Google Scholar]
- 61.Tagaya Y, Bamford R N, de Filipis A P, Waldmann T A. IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity. 1996;4:329–336. doi: 10.1016/s1074-7613(00)80246-0. [DOI] [PubMed] [Google Scholar]
- 62.Takahashi K, Segal E, Kondo T, Mukai T, Moriyama M, Takahashi M, Yamanishi K. Interferon and natural killer cell activity in patients with xanthem subitum. Paedr Infect Dis J. 1992;11:369–373. doi: 10.1097/00006454-199205000-00006. [DOI] [PubMed] [Google Scholar]
- 63.Trinchieri G, Santoli D, Dee R R, Knowles B B. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Identification of the anti-viral activity as interferon and characterization of the human effector lymphocyte population. J Exp Med. 1978;147:1299–1313. doi: 10.1084/jem.147.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waldmann T A, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in the NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 65.Warren H S, Kinnear B F, Kastelein R L, Lanier L L. Analysis of the costimulatory role of IL-2 and IL-15 in initiating proliferation of resting (CD56dim) human NK cells. J Immunol. 1996;156:3254–3259. [PubMed] [Google Scholar]
- 66.Welsh R M. Regulation of virus infections by natural killer cells. Nat Immun Cell Growth Regul. 1986;5:169–199. [PubMed] [Google Scholar]
- 67.Whitley R J. Herpes simplex viruses. In: Fields B N, et al., editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. [Google Scholar]
- 68.Wilkinson P C, Liew F Y. Chemoattraction of human blood T lymphocytes by interleukin-15. J Exp Med. 1995;181:1255–1259. doi: 10.1084/jem.181.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang X, Sun S, Hwang I, Tough D F, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]