Abstract
Objective
Imaging parameters of Chiari malformation type I (CMI) development are not well established. This study aimed to collect evidence of general or specific imaging measurements in patients with CMI, analyze indicators that may assist in determining the severity of CMI, and guide its diagnosis and treatment.
Methods
A comprehensive search was conducted across various databases including the Cochrane Library, PubMed, MEDLINE, Scopus, and Embase, covering the period from January 2002 to October 2023, following predefined inclusion criteria. Meta-analyses were performed using RevMan (ver. 5.4). We performed a quantitative summary and systematic analysis of the included studies. This study was registered in the PROSPERO (International Prospective Register of Systematic Reviews) prior to initiation (CRD42023415454).
Results
Thirty-three studies met our inclusion criteria. The findings indicated that out of the 14 parameters examined, 6 (clivus length, basal angle, Boogard’s angle, supraocciput lengths, posterior cranial fossa [PCF] height, and volume) exhibited significant differences between the CMI group and the control group. Furthermore, apart from certain anatomical parameters that hold prognostic value for CMI, functional parameters like tonsillar movement, obex displacement, and cerebrospinal fluid dynamics serve as valuable indicators for guiding the clinical management of the disease.
Conclusion
We collated and established a set of linear, angular, and area measurements deemed essential for diagnosing CMI. However, more indicators can only be analyzed descriptively for various reasons, particularly in prognostic prediction. We posit that the systematic assessment of patients’ PCF morphology, volume, and other parameters at a 3-dimensional level holds promising clinical application prospects.
Keywords: Chiari malformation, MRI-related parameters, Posterior cranial fossa, Meta-analysis, Systematic review
INTRODUCTION
Chiari malformation type I (CMI) is a neuroanatomical abnormality, in which the cerebellar tonsils extend up to 5 mm below the foramen magnum (FM) [1]. Patients with CMI suffer from various pain, sensory or motor deficits, and cognitive dysfunction. The primary diagnostic tool for this disorder is magnetic resonance imaging (MRI). With the development of medical imaging techniques, a wealth of anatomical data are available for diagnostic purposes. Many asymptomatic patients and those who are symptomatic but have minimal tonsillar descent have been identified with MRI [2]. With the development of research, many MRI-related structural and functional parameters have been identified; some of them have been proven to be relevant to postoperative recovery, which can be used for the selection of surgery. However, because of the structural complexity of the posterior cranial fossa (PCF) and occipital bone and the different parameters chosen to be measured in each study, there is a need for a systematic collation and analysis of these parameters. Therefore, this study aimed to conduct a comprehensive literature review, collect evidence of general or specific imaging measurements in patients with CMI, analyze indicators that may assist in determining the severity of CMI and guide its diagnosis and treatment, and identify gaps in previous studies and priorities for future research.
MATERIALS AND METHODS
The meta-analysis and systematic review were registered on the PROSPERO (International Prospective Register of Systematic Reviews) with the registration number of CRD42023415454.
1. Data Sources and Search Strategy
We systematically searched published and unpublished literature between 2002 (January) and 2023 (October) from Cochrane Library, PubMed, MEDLINE, Scopus, and Embase databases. We concatenated terms and phrases using appropriate Boolean operators. Retrieval included (“CM” OR “Chiari malformation” OR “Arnold-Chiari Malformation”) and (“MRI” OR “Magnetic Resonance Imaging” OR “Magnetic Resonance”). Language restrictions were not applied. We manually searched reference lists of all articles and gray literature to identify potentially eligible studies.
2. Inclusion and Exclusion Criteria
Studies considered for meta-analysis and systematic evaluation met the following inclusion criteria: (1) simple CMI without congenital craniocervical junction malformations; (2) studies that examined children or adults who had a clinical diagnosis of CMI; (3) complete data records through prospective or retrospective studies; and (4) studies published in peer-reviewed journals. Adult participants were those aged ≥ 18 years. The exclusion criteria were studies without effect sizes, unpublished duplicate publications and conference abstracts, and studies with incomplete data.
3. Risk of Bias and Analysis Plan
Since most of the included studies were nonrandom comparative studies, the risk of study bias was assessed using the Newcastle-Ottawa Scale [3], and the preferred Cochrane tool for nonrandomized studies (Risk Of Bias In Non-randomized Studies - of Interventions, ROBINS-I) [4]. Each study was rated based on the scores of the Newcastle-Ottawa Scale obtained. A maximum score of 9 was set for 8 items. If a study scored > 6, it was considered to have a good quality. Using the ROBINS-I tool, a study’s risk of bias is evaluated by considering confounding, participants’ selection, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of reported results. The risk assessment for each bias category can be classified as low, moderate, serious, or critical. The overall bias risk of a study was determined by the highest risk identified within each category. Two reviewers separately evaluated study quality; any differences will be resolved by discussion or consensus with the third reviewer.
4. Study Selection and Data Collection Process
All titles and abstracts identified from the search process were independently assessed by 2 reviewers (ZW and SH). Instances of disagreement were resolved by discussion between the 2 reviewers or by introduction of a third reviewer (ZL). All articles were summarized in detail, and data from full-text articles that met the study benchmarks were incorporated, including subjects’ demographics and narrative summaries of outcomes and methods. Summaries were performed using predesigned standardized tables. If there was a lack of relevant data in the included studies, we request data by contacting the corresponding author of the paper.
5. Quality Assessment
This study included reports with a control group or pre- and postoperative comparisons; there were no systematic differences between the groups. All studies were approved by the ethics committee and conducted with patients’ informed consent. Only studies that met the quality criteria were included.
6. Quality of the Evidence
A modified Recommendations Assessment, Development, and Evaluation grading (GRADE) method was used to categorized the quality of evidence as high, moderate, low, or very low. Because publication bias is difficult to assess in observational studies [5], only 4 factors were assessed in our meta-analysis: risk of bias, inconsistency, indirectness, and imprecision. When there was a risk for a factor results, the quality of evidence for the related factor correspondingly decreased by 1 or 2 grades [6]. The level of evidence and the strength of the recommendation were determined through discussion by all members of the research group.
7. Data Analysis and Systematic Review
Meta-analyses were performed using RevMan (ver. 5.4, Cochrane Collaboration, Oxford, UK) and IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA). The primary outcome was the mean (M) and standard deviation of the objective assessment measures for subjects. Meta-analyses of mean differences (MDs) were expressed as 95% confidence intervals (CIs). The heterogeneity of results was estimated using I2, Z, and chi-square tests (p < 0.05). Risk of publication bias was assessed by examining funnel plots for symmetry and summarization and analyzing data that could not be combined. A leave-one-out sensitivity analysis was used to test the robustness of the results. Studies in which data could not be combined adopted a systematic narrative approach.
RESULTS
1. Search Results
The initial search identified 3,411 studies. A total of 1,832 studies remained after removing duplicate studies. After selecting titles and abstracts and browsing the complete text, a total of 33 studies which met the requirements and had complete data were finally determined [7-38]. A total of 17 studies [7-23] (2,097 patients with CMI and 1,055 controls) and 16 studies [21,24-38] (involving 1,270 patients with CMI) were included to evaluate the role of imaging parameters in disease diagnosis and explore the correlation between imaging parameters and postoperative prognosis, respectively. Two of these studies [8,13] were by the same author. Therefore, in order to reduce the possibility of duplication of data, only their most recent study [8] was included in the meta-analysis to reduce bias. Among 1,055 individuals in the control group, 377 were recruited healthy volunteers and 678 were patients with no obvious posterior fossa pathology or medical problems; therefore, they were inferred to reflect PCF morphology of the normal population. A flow chart of the search is shown in Fig. 1.
Fig. 1.
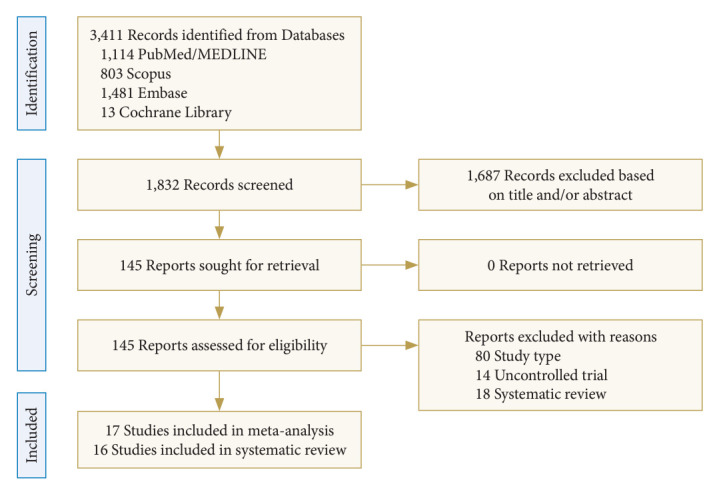
Flow chart of study through different phases of the meta-analysis and systematic review.
2. Quality Assessment of the Studies
The authors used meta-analysis for the role of imaging parameters in diagnosing disease. According to the Newcastle-Ottawa Scale, 7 studies scored 8 points, while 10 studies scored 7 points, indicating relatively high quality of each study. Table 1 showed the characteristics and quality scores of the included meta-analysis studies. Supplementary Table 1 showed the risk of bias assessments of the included studies using the ROBINS-I tool. One study had a critical risk of bias due to missing data. Seven studies scored moderately biased mainly due to bias in confounding or measurement of outcomes. The quality of evidence based on GRADE was downgraded to moderate (imprecision or inconsistency) or low (imprecision and inconsistency) for these findings of the basal angle, Boogard’s angle, supraocciput lengths, PCF height, and PCF volume, respectively. The detailed data for GRADE scales were presented in Table 2. In contrast, discussing the correlation between imaging parameters and postoperative prognosis used a systematic narrative approach rather than meta-analysis. The reasons for the systematic narrative approach were (1) methodological differences between studies were too great to allow for straightforward comparisons, (2) inconsistencies in the data used to calculate effect sizes, (3) inconsistencies in the data for potential confounders, and (4) heterogeneity of the data used in previous studies, which did not allow for the calculation of moderating effects.
Table 1.
Key characteristics and quality scores of the included studies regarding the value of MRI-related parameters to diagnose CMI
| Study | Country | No. patients with CMI/controls | Mean age (yr) | Included quantitative parameters | Quality score (NOS) |
|---|---|---|---|---|---|
| Karagöz et al. [7] 2002 | Turkey | 22/21 | 32.9 ± 12.0 | Basal angle, Boogard’s angle, tentorium angle, the slope of the tentorium, PCF area | 7 |
| Milhorat et al. [8] 2010 | USA | 388/80 | 33.6 ± 10.1 | Tentorium angle, clivus length, FMAP, PCFV and PCFBV | 7 |
| Alperin et al. [9] 2014 | USA | 36/37 | 37.0 ± 11.0 | Clivus length, supraocciput lengths, PCFV | 8 |
| Aydin et al. [10] 2005 | Turkey | 60/30 | 35.1 ± 12.7 | Clivus length, supraocciput lengths, FMAP, AP diameter of PCF, PCF height | 7 |
| Urbizu et al. [11] 2014 | Spain | 100/50 | 45.5 ± 12.2 | Basal angle, Wackenheim angle, the slope of the tentorium, clivus length, supraocciput lengths, FMAP, AP diameter of PCF, PCF height, PCF area | 7 |
| Krishna et al. [12] 2016 | Canada | 8/16 | 42.6 ± 10.4 | Tentorium angle, PCFV | 7 |
| Milhorat et al. [13] 2009 | USA | 280/75 | 33.7 ± 10.4 | Tentorium angle, clivus length, FMAP, PCFV, and PCFBV | 8 |
| Dufton et al. [14] 2011 | Canada | 81/107 | 42.6 ± 13.0 | Boogard’s angle, clivus length | 7 |
| Heiss et al. [15] 2012 | USA | 48/18 | 36.8 ± 11.2 | Clivus length, supraocciput lengths | 7 |
| Houston et al. [16] 2018 | USA | 162/140 | 38.3 ± 10.0 | Basal angle, Wackenheim angle, Boogard’s angle, odontoid angle, clivus length, supraocciput lengths, AP diameter of PCF, PCF height, PCF area | 7 |
| Nair and Rajshekhar [17] 2022 | India | 27/10 | < 18 | Boogard’s angle, tentorium angle, the slope of the tentorium, clivus length, supraocciput lengths, FMAP, AP diameter of PCF, PCF height | 7 |
| 94/20 | > 18 | ||||
| Nishikawa et al. [18] 2022 | Japan | 50/20 | 4–7† | Clivus length, supraocciput lengths, PCFV, and PCFBV | 8 |
| 65/24 | 8–11† | ||||
| 70/23 | 12–15† | ||||
| 32/25 | 16–19† | ||||
| 230/58 | 20–49† | ||||
| Wang et al. [19] 2014 | China | 52/17 | 37.0 (22–59)‡ | Tentorium angle, clivus length | 8 |
| Yan et al. [20] 2016 | China | 67/40 | 15.3 ± 3.9 | Boogard’s angle, clivus length, supraocciput lengths, FMAP, AP diameter of PCF | 8 |
| Yuksel et al. [21] 2022 | Turkey | 70/69 | 38.5 (17–70)‡ | PCF area | 8 |
| Tubbs et al. [22] 2003 | USA | 100/50 | 9.0 (< 18) | Odontoid angle | 8 |
| Besachio et al. [23] 2015 | USA | 55/125 | 34.0 ± 10.2 | Wackenheim angle, odontoid angle | 7 |
MRI, magnetic resonance imaging; CMI, Chiari malformation type I; NOS, Newcastle-Ottawa Scale; PCF, posterior cranial fossa; FMAP, anteroposterior diameter of the foramen magnum; PCFV, posterior cranial fossa volume; PCFBV, posterior cranial fossa brain volume; AP, anteroposterior.
Range.
Mean (range).
Table 2.
The certainty assessment of evidences based on GRADE
| No. of studies | Certainty assessment |
Effect |
Certainty | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of CMIs | No. of controls | Incidence (95% CI) | ||
| Clivus length | |||||||||
| 7 (ref. #10, 11, 15-19) | Observational studies | Not serious | Not serious | Not serious | Not serious | 990 | 435 | -1.02 to -0.78 | ⊕⊕⊕⊕ HIGH |
| Basal angle | |||||||||
| 3 (ref. #7, 11, 16) | Observational studies | Not serious | Not serious | Not serious | Serious† | 283 | 211 | 3.81–6.21 | ⊕⊕⊕◯ MODERATE |
| Boogard's angle | |||||||||
| 4 (ref. #7, 14, 16, 17) | Observational studies | Not serious | Not serious | Not serious | Serious† | 385 | 298 | 0.45–0.77 | ⊕⊕⊕◯ MODERATE |
| Supraocciput lengths | |||||||||
| 6 (ref. #10, 11, 15-18) | Observational studies | Not serious | Serious‡ | Not serious | Not serious | 938 | 418 | -0.45 to -0.21 | ⊕⊕⊕◯ MODERATE |
| Height of the posterior cranial fossa | |||||||||
| 3 (ref. #10, 11, 17) | Observational studies | Not serious | Not serious | Not serious | Serious† | 281 | 110 | -1.49 to -1.01 | ⊕⊕⊕◯ MODERATE |
| Volume of the posterior cranial fossa | |||||||||
| 4 (ref. #8, 9, 12, 18) | Observational studies | Not serious | Serious‡ | Not serious | Serious§ | 617 | 200 | -21.99 to -18.88 | ⊕⊕◯◯ LOW |
GRADE, Grading of Recommendations, Assessment, Development, and Evaluations; CMI, Chiari malformation type I; CI, confidence interval.
Limited sample size.
High inconsistency (I2>50%).
The data for the subgroup (child group) analysis came from the same study.
3. Meta-Analysis Results of MRI-Related Parameters in Patients With CMI
Numerous MRI-related parameters are used to assess patients with CMI. After pooling, a total of 14 indicators were included in the meta-analysis (Fig. 2, Supplementary Fig. 1).
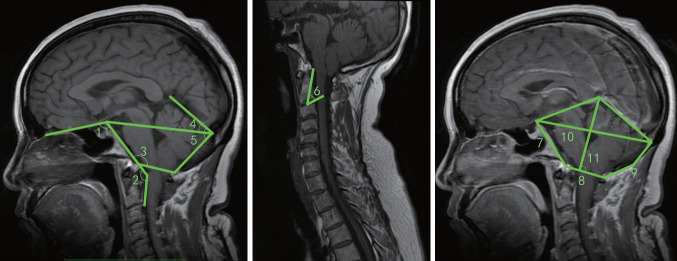
Fig. 2.
Photographs demonstrate the measurement techniques of magnetic resonance imaging-related parameters. 1, basal angle; 2, Wackenheim angle; 3, Boogard’s angle; 4, tentorium angle; 5, the slope of the tentorium; 6, odontoid angle; 7, clivus length; 8, anteroposterior diameter of the foramen magnum; 9, supraocciput lengths; 10, anteroposterior diameter of posterior cranial fossa; 11, height of posterior cranial fossa.
Two studies [17,18] grouped different age populations, and we included each age group as a separate sample for analysis. One study 18 classified CMI into 3 subtypes (type A, normal PCF volume [PCFV] and occipital bone size; type B, normal PCFV and small volume of the area surrounding the FM [VAFM] and occipital bone size; and type C, small VAFM, PCFV, and occipital bone size). We combined these 3 datasets to exclude bias caused by screening data from this study. The results showed that 6 (clivus length, basal angle, Boogard’s angle, supraocciput lengths, PCF height, and PCFV) of the 14 parameters were significantly different in the CMI group compared with the control group.
1) Clivus length
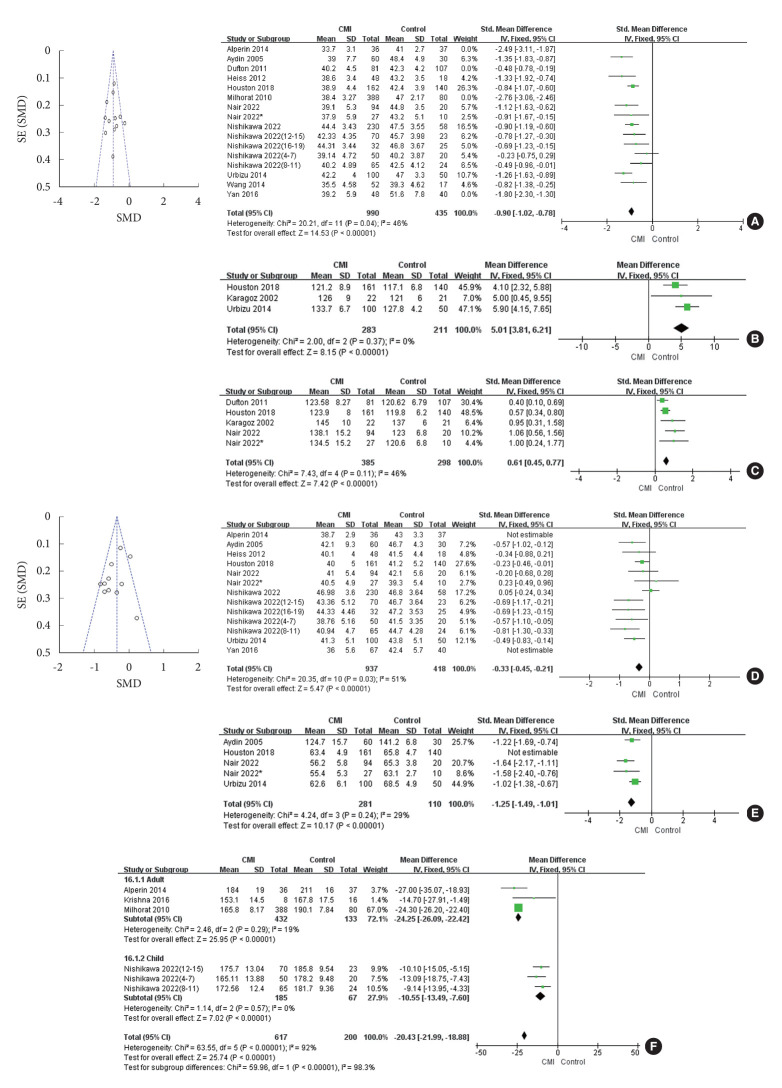
A combined analysis of this parameter included data from 16 groups [8-11,14-20], 1,543 patients with CMI and 699 controls, and showed a significant difference in clivus length between the groups (MD = -1.14; 95% CI, -1.51 to -0.77; I2= 92%; Z = 5.97; p < 0.00001). Clivus lengths were smaller in patients with CMI than in the controls. However, we found that the p-value after the Q-test was too small. Sensitivity analyses and regression studies on the results did not show significant bias factors. By looking at the funnel plot, we found that 4 datasets [8,9,14,20] of the included datasets were heavily biased. Exclusion of these 4 datasets resulted in significantly lower heterogeneity (χ2 = 20.21, p = 0.04; I2= 46%), thus suggesting them to be the primary source of higher heterogeneity. After excluding these 4 datasets, the results still showed a significant difference in the CMI group compared with the control group (MD = -0.90; 95% CI, -1.02 to -0.78; Z = 14.53; p < 0.00001) (Fig. 3A).
Fig. 3.
Comparison of magnetic resonance imaging-related parameters in patients with Chiari malformation type I (CMI) and controls. Effect sizes are presented as mean difference with 95% confidence intervals (CIs). The heterogeneity of results was estimated using I2, Z, and chi-square tests (p < 0.05). Risk of publication bias was assessed by examining funnel plots for symmetry. (A) The change in clivus length. (B) The change in basal angle. (C) The change in Boogard’s angle. (D) The change in supraocciput lengths. (E) The change in the height of posterior cranial fossa. (F) The change in the volume of posterior cranial fossa. SE, standard error; SMD, standard mean difference; SD, standard deviation; df, degrees of freedom.
2) Basal angle
The basal angle (cranial base flexion angle) is the inclination angle measured from the nasion, top of the dorsum sellae, and the basal slopes of the occipital and sphenoid bones. The combined analysis of this parameter included 3 studies [7,11,16] with 283 patients with CMI and 211 controls. The studies showed that patients with CMI had a wider basal angle than controls (MD = 5.01; 95% CI, 3.81–6.21; heterogeneity: χ2 = 2.00, p = 0.37; I2= 0%; Z = 8.15; p < 0.00001) (Fig. 3B).
3) Boogard’s angle
Boogard’s angle is defined by the angle measured between the top of the dorsum sellae, basion, and opisthion. The 5 datasets for this parameter were from 4 studies [7,14,16,17], including 385 patients with CMI and 298 controls. One study [17] included data of children and adults and did not produce significant bias in the combined analysis. The results showed a greater Boogard’s angle in patients with CMI than in the controls (MD = 0.61; 95% CI, 0.45–0.77; heterogeneity: χ2 = 7.43; p = 0.11; I2= 46%; Z = 7.42; p < 0.00001) (Fig. 3C).
4) Supraocciput lengths
Supraocciput lengths were defined as the length of the occipital bone medial to the PCF in the midsagittal plane. Thirteen datasets from 8 studies [9-11,15-18,20] were included in evaluation of this parameter. Supraocciput lengths were shorter in patients with CMI than in the controls. However, the heterogeneity was slightly higher after the combined study (χ2 = 46.25, I2= 74%). We analyzed the included datasets and found that 2 datasets [9,20] of them were derived from subgroup studies of patients with CMI. Exclusion of these 2 datasets resulted in significantly lower heterogeneity (χ2 = 20.35, p = 0.03, I2= 51%), thus suggesting them to be the primary source of higher heterogeneity. After excluding these 2 groups, the results still showed a significant difference between the 2 groups (MD = -0.33; 95% CI, -0.45 to -0.21; Z = 5.47; p < 0.00001) (Fig. 3D).
5) Height of the PCF
The height of the PCF was measured with a line drawn from the most anterior portion of the tentorium, perpendicular to the McRae line. Five datasets from 4 studies [10,11,16,17] were included in assessment of this parameter, and the results showed significant heterogeneity (χ2 = 23.82, p < 0.0001, I2= 83%). We performed a sensitivity analysis using a leave‐one‐out method. The analysis results (Supplementary Fig. 2) showed the source of heterogeneity was the study of Houston et al. [16], which had only female subjects; sex may have been a factor in determination of the height of the PCF. Because of a lack of studies on sexual differences in the included literature, a subgroup analysis could not be performed. After removing the data from this group, the heterogeneity was significantly reduced (χ2 = 4.24, p = 0.24, I2= 29%). The height of the PCF was significantly reduced in patients with CMI (n = 281) compared with that in controls (n = 110) (MD = -1.25; 95% CI, -1.49 to -1.01; Z = 10.17; p < 0.00001) (Fig. 3E).
6) Volume of the PCF
A total of 8 datasets (879 patients with CMI and 283 controls) from 4 studies [8,9,12,18] were included in the analysis of PCFV. The results showed a significant difference in PCFV between the 2 groups, but with high heterogeneity (χ2 = 242.48, p < 0.00001, I2= 97%). We analyzed the data across the groups and found that age could be the reason for the high heterogeneity. We divided the data into adult and child groups, while excluding the study of Nishikawa et al. [18], which, unlike other studies, analyzed 16- to 19-year-olds as a subgroup. In most studies, 18 years of age was the threshold to distinguish between adults and children. When data from this age group were removed and tested for subgroups, heterogeneity was significantly reduced (adults: χ2 = 2.46, p = 0.29, I2= 19%; children: χ2 = 1.14, p = 0.57, I2= 0%). PCFV was significantly reduced in patients with CMI compared with controls, in both adults (MD = -24.25; 95% CI, -26.09 to -22.42; Z = 25.95; p < 0.00001) and children (MD = -10.55; 95% CI, -13.49 to -7.60; Z = 7.02; p < 0.00001) (Fig. 3F).
4. MRI-Related Parameters in Predicting Prognosis of Patients With CMI
MRI-related anatomical parameters such as McRae line, pB-C2 line, clivoaxial angle, FM-C2 cistern, condylar-C2 sagittal vertical alignment may be associated with the prognosis of CMI. The introduction of functional parameters, such as tonsillar movement, obex displacement, and cerebrospinal fluid dynamics provide adequate guidance for clinical management of the disease. Table 3 summarizes the main characteristics and outcomes of the collected studies conducted on MRI-related imaging parameters for prediction of prognosis in patients with CMI (Supplementary Fig. 1).
Table 3.
The main characteristics and outcomes of the included studies on the value of MRI-related parameters for the prognosis of patients with CMI
| Study | Country | No. of cases (M/F) | Mean age (yr) | Intervening method | Parameters | Effect evaluation | Follow-up period | MRI sequences | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Collins et al. [34] 2022 | USA | 44 (20/24) | 9.73 ± 4.56 | PFD/nonsurgery | CSF flow, tonsillar motion | Clinical outcomes | > 2 yr | PC-MRI and 2D fast imaging employing steady-state acquisition | Tonsillar motion was positively correlated with the exacerbation of clinical symptoms in patients with CMI. Tonsillar motion was associated with reduced CSF flow, manifested as aggravated clinical symptoms. |
| 11.36 ± 4.71 | |||||||||
| Furtado et al. [26] 2011 | India | 20 (9/11) | 13.08 ± 4.74 | FMD, shrinkage of tonsils, and duraplasty | Dimensions of the syrinx and cord, FM morphometry, dimensions of the PCF, ICV, PCFV | Modified Asgari scoring system | 24.6 (3–84) mo | Craniocervical MRI | The age at presentation, duration, type, morphological measurements of the cranial and FM, and syrinx-related changes were not associated with outcomes at short-term postoperative follow-up. |
| Ladner et al. [28] 2015 | USA | 119 (64/55) | 8.5 ± 4.2 | PFDD | pB–C2 line | Clinical outcomes | 2.4 ± 2.9 yr | Brain/cervical spine MRI | Better outcomes after PFDD treatment in patients with grade I pB-C2 lines with increased ventral canal obstruction than in those with grade 0 pB-C2 lines. |
| Liu et al. [27] 2019 | China | 39 (10/29) | 48.3 ± 9.7 | PFD | Morphological indicators of PCF (13 linear, 8 angular, 4 areal, and 4 ratios) | CCOS | 27 (2–82) mo | Craniocervical MRI | The morphometric measurements of the PCF did not predict the response to PFD in patients with CMI. |
| Mantha et al. [32] 2021 | Australia | 59 (20/39) | 3.9/12.7 | Surgical decompression with suboccipital craniectomy, C1 laminectomy, Y-shaped durotomy and expansile duraplasty | FM-C2 cistern, pB-C2 distance, CXA, clival length, clival angle and BoA | CCOS | 4–12 mo | Brain/cervical spine MRI | In younger patients with CMI, the preoperative volume of the FM-C2 cistern is a particular indicator that may play a good role in predicting postoperative outcomes. |
| Marianayagam et al. [29] 2021 | USA | 35 (20/15) | 11.7 ± 5.1 | OCF with or without endoscopic endonasal odontoidectomy | CXA, pB-C2, canal diameter, atlantodental, basion-dens, and basion-axial interval | Clinical outcomes | 21.3 ± 13.5 mo | CT/MRI | CXA may be the most critical morphological index for predicting clinical outcomes in pediatric patients with CMI after OCF. |
| McGirt et al. [24] 2006 | USA | 130 (59/71) | 16 ± 13 | PFD | Hindbrain CSF flow | Clinical outcomes | 19 ± 17 mo | Cine PC-MRI | Preoperatively normal hindbrain CSF flow was an independent risk factor for intervention failure after decompression of patients with CMI. |
| McGirt et al. [25] 2008 | USA | 44 (24/20) | 8± 6 | PFD | Ventral or dorsal CSF flow | Clinical outcomes | 27 ± 16 mo | Cine PC-MRI | Combined ventral and dorsal CSF flow analysis of the hindbrain could better predict patient response to PFD. |
| Ravindra et al. [31] 2021 | USA | 206 (77/129) | 11.5 ± 4.96 | PFD, VBD/OCF | C–C2SVA, pB–C2 line, CXA | Sensitivity and specificity | 2.28 ± 1.36/2.62 ± 1.43 yr | Brain/cervical spine MRI | A C-C2SVA ≥ 5 mm was a high predictor of requiring OCF/VBD surgery in patients with CMI. |
| 11.4 ± 5 | |||||||||
| Thakar et al. [33] 2018 | India | 57 (30/27) | 38.29 ± 14.32 | FMD, C-1 laminectomy, and duraplasty | M-line–FVV distance, caudal displacement of the obex | CCOS | 40.29 ± 10.36 mo | Craniocervical MRI | Obex caudal displacement and shorter distances of the M-line–FVV were associated with good CCOS scores, suggesting that patients with higher hindbrain pathology responded better to surgery. |
| Yuksel et al. [21] 2022 | Turkey | 70 (17/53) | 38.5 (17–70) | Conservative treatment and/or follow-up, surgery | Tonsillar herniation, Chamberlain line, McRae line, and odontoid process-McRae line angle | Annual MRI and periodic examinations | NA | Craniocervical MRI | McRae line value and symptom severity can be used as predictors of surgical intervention decisions. A new CHIASURG scale can effectively and reliably predict the risk of surgical intervention. |
MRI, magnetic resonance imaging; CMI, Chiari malformation type I; PFD, posterior fossa decompression; CSF, cerebrospinal fluid; PC, phase contrast; 2D, 2-dimensional; FM, foramen magnum; PCF, posterior cranial fossa; ICV, intracranial volume; PCFV, posterior cranial fossa volume; PFDD, posterior fossa decompression with duraplasty; CCOS, Chicago Chiari Outcome Scale; FM-C2, foramen magnum-C2; pB-C2, perpendicular to the basion to C2 line; CXA, clivo-axial angle; BoA, Boogard’s angle; OCF, occipitocervical fusion; CT, computed tomography; VBD, ventral brainstem decompression; C-C2SVA, condylar-C2 sagittal vertical alignment; FMD, foramen magnum decompression; FVV, fourth ventricle vertex; NA, not applicable.
DISCUSSION
This study showed that patients with CMI had significant changes in clivus and supraoccipital length and PCFV compared with controls, except for tonsillar herniation. For postoperative predictors of CMI, traditional anatomical parameters are not satisfactory predictors, whereas cerebrospinal fluid dynamics and tonsillar motion parameters may be more convincing.
1. The Value of MRI-Related Parameters in the Diagnosis of CMI
1) Measurement and assessment of PCF structures
Some studies [11,16,17] did not reveal a difference in the anteroposterior (AP) diameter of the PCF compared with normal controls with CMI, whereas others have found AP diameters of the PCF in patients with CMI to be shorter [10,20]. Herein, the AP diameter of the PCF in patients with CMI was not significantly different from that of the controls. In contrast, the height of the PCF showed some differences. The study of Houston et al. [16] had only female subjects; application of the meta-analysis to evaluate the change in PCF height in patients with CMI showed significant heterogeneity. However, the other PCF parameters in that study did not show substantial heterogeneity when subjected to the meta-analysis, suggesting that the variation in PCF height may be related to sex. The reduction in PCF height may account for the smaller PCF in women. However, very few studies have focused on the effect of sex differences on the structure of the PCF to allow further analysis.
Some studies have concluded that patients with symptomatic CMI have a normal PCF area [2,12,39], whereas other studies revealed that patients with CMI have a smaller PCF area [11,40,41]. We found no significant difference in the PCF area and a high degree of heterogeneity between the data of patients with CMI compared with that of the controls. This can be attributed to variation in the measurement method and selection range. However, the measurement of area, which is usually manually delineated, is highly biased.
Volumetric measurements give a more realistic picture of the size of the PCF than linear and area measurements. We showed a significant difference in PCFV between patients with CMI and normal subjects, thus confirming that a smaller PCF is a characteristic feature of patients with CMI. It is also clear from the subgroup analysis that PCFV is associated with demographic characteristics. Age, race, sex, and body mass index have statistically significant effects on intracranial measurements and must be considered [39]. Herein, age was found to be a significant factor affecting PCFV. Subgroup analysis showed that grouping by age significantly reduced statistical heterogeneity and demonstrated that adult and pediatric patients with CMI had a substantially smaller PCFV than the corresponding control group. The PCFV is significantly smaller or the PCF is underdeveloped in patients with CMI [8,12,42], which may be the leading cause of the caudal downward protrusion of the normally developing hindbrain [10]. One study has explored the extent of the association between linear and volumetric measures. In addition to occipital bone length being mildly correlated with PCF and fourth ventricle volume in the CMI cohort, the other expected linear measures were not correlated in each cohort [9]. These findings suggest that linear measures only complement volumetric measures.
Despite the potential diagnostic and prognostic value of PCF morphology, volumetric assessment of PCF size is not commonly used in clinical practice. It is very time-consuming to manually outline the PCF size on multiple images. In contrast, manual measurement of the length of different markers of the PCF is more time efficient and more commonly used as an alternative measurement of PCFV. With the development of 3-dimensional (3D) imaging technology and artificial intelligence, the results of the 3D evaluation are likely to be more accurate and representative, which is a good direction for future research.
2) Measurement and assessment of clivus lengths and associated angles
We found a significant difference in clivus length between patients with CMI and controls. Although heterogeneity of the data included in the meta-analysis was high, the overall effect showed that the clivus was shorter in patients with CMI. We also found that patients with CMI exhibit a wider basal angle and Boogard’s angle than those without. In the author’s opinion, angle combined with clivus length is strong evidence for diagnosing CMI, and separate indices often do not provide a comprehensive characterization.
3) Measurement and evaluation of the occipital structure
Shorter occipital bone length [9-11,15,18,20,42] and wider tentorium angulation [2,17,19] are typical features of CMI. This study used a meta-analysis to reveal that the patients with CMI and controls showed significant differences between occipital bone length measurements; however, it did not show significant differences in either the tentorium angle or slope of the tentorium. Shorter occipital bone significantly affects the overall layout of the PCF. There is an abnormality in the length of the occipital bone in patients with adult Chiari malformation due to hypoplasia of the occipital body originating from the paraxial mesoderm.
Further analysis showed that the brain tissue volume in the PCF did not differ in patients with CMI compared to controls [8,18]. This allows for overcrowding of the PCF in patients with CMI, which affects the flow of cerebrospinal fluid and alters the form of cerebrospinal fluid flow in the posterior part of the PCF. As symptoms worsen, they will eventually affect the neural structures of the PCF. We believe that the rear aspect of the PCF is a better indicator of the degree of crowding than the structures on the anterior part of the PCF. Shorter occipital bone length makes the PCF morphology shallower and more likely to cause crowding of the PCF structures. Changes in tentorium angle are strong evidence to explain the narrowing of the PCF but may not be a characteristic change in CMI. To compensate for the small PCF, the cerebellar tentorium shifts upwards, resulting in an abnormal tentorium angle, which may be an alteration secondary to CMI [43,44]. In the future, exploring the correlation between changes in tentorium angle and CMI symptoms may be done, using the angle change as an adjunct to CMI staging rather than a basis for diagnosis. Considering the PCF to be a container, shorter clivus and occipital lengths make the area of the mouth of the container much larger than the area of the bottom. The area of PCF in the midsagittal plane showed no difference, but the height had decreased. If the PCF of an ordinary person is like a bowl, then the PCF of a patient with CMI is more like a plate. Thus, it is important to measure the shape, volume, and other parameters of PCF in patients with CMI at the 3D level.
Many studies have been conducted on measuring FM in patients with CMI; however, the conclusions differ. We found no significant difference in the diameter of the FM in the midsagittal plane between patients with CMI and controls. We speculate one of the 2 possibilities: firstly, because of the small sample size of the study, the measurement of the FM area was not perfect; secondly, there may have been a lack of relationship between the FM and tonsillar herniation and obstruction of cerebrospinal fluid flow. One study found that cerebrospinal fluid reflux at FM may not be due to bone structure [45].
2. Value of MRI-Related Parameters in Predicting Prognosis of Patients With CMI
1) Anatomical structure parameters
The degree of cerebellar tonsillar ectopia is the basis of CMI diagnosis; however, is not a prognostic factor [24,25]. An earlier study collected the following data from pediatric patients with CMI (with or without improvement): age at presentation, duration, type, morphological measurements of the cranial and FM, and presence of syringomyelia and found that these variables were not associated with outcomes at short-term postoperative follow-up [26]. CMI is associated with smaller PCFV [46-49], which is also an effective parameter for diagnosis. However, a study based on characteristic PCF parameters found that morphometric measurements of the PCF did not predict the response to posterior fossa decompression in patients with CMI [27]. This study encompassed 13 linear, 8 angular, and 4 area parameters associated with PCF features and 4 ratios associated with these linear and area parameters to assess.
McRae line value and symptom severity can be used as predictors of surgical intervention decisions [21]. Authors of this study also developed a CHIASURG scale including depth of tonsillar herniation, Chamberlain line, and McRae line to predict surgical interventions in patients with CMI [21]. The scale can effectively and reliably predict the risk of surgical intervention.
The pB-C2 line is drawn perpendicular to the line from the C-2 body and the basion at the posterior extent of the odontoid process. The results of a large retrospective study showed better outcomes after posterior fossa decompression with duraplasty treatment in patients with grade I pB-C2 lines (pB-C2 line ≥ 3 mm) with increased ventral canal obstruction than those with grade 0 pB-C2 lines (pB-C2 line < 3 mm) [28]. A pB-C2 line of > 9 mm may indicate brainstem compression and is an indication for anterior decompression surgery [50]. Another study found that the clivoaxial angle increased significantly only in the postoperative improvement group by analyzing clivoaxial angle, pB-C2 line, atlantodental interval, basion-dens interval, basion-axial interval, and canal diameter at the level of C1 [29]. Additionally, other studies suggested that traditional anatomical parameters do not predict prognosis [40-42], which may be related to the age and sex of the patients involved in these studies.
The preoperative volume of the FM-C2 cistern is a prevalent indicator. It is a novel cerebrospinal fluid space of the upper cervical canal extending from the FM to the inferior cortex of the C2 body. In younger patients with CMI, the preoperative volume of the FM-C2 cistern is an indicator that may play a good role in predicting postoperative outcomes [32]. The condylar-C2 sagittal vertical alignment provides a more accurate description of the anatomical loading relationship between the atlantooccipital joint and the cervical segment of the upper spine. A single-center study found that children with CMI had a higher condylar-C2 sagittal vertical alignment than controls [30]. A multicenter cohort study further validated the predictive value of condylar-C2 sagittal vertical alignment with a sensitivity of 100%, specificity of 86%, and misclassification rate of 12.6% in identifying high-risk patients [31]. A value of condylar-C2 sagittal vertical alignment > 5 mm was a major predictor of the requirement of occipitocervical fusion or ventral brainstem decompression surgery in patients with CMI [30,31]. The obex caudal displacement and shorter distances of the M-line–fourth ventricle vertex were associated with good Chicago Chiari Outcome Scale scores, suggesting that patients with higher hindbrain pathology responded better to surgery [33].
Although these studies have yielded optimistic results, further research is needed to demonstrate the predictive value of these indicators.
2) Cerebrospinal fluid dynamic parameters
Qualitative analysis of phase contrast-MRI can provide additional information to help clinicians decide whether to operate [24,25,34,51]. The results of Fan et al. [35] revealed that the subarachnoid manipulation procedure was more feasible for CMI patients with type III cerebrospinal fluid kinetic abnormalities (cerebrospinal fluid flow blockage found in the posterior fossa of the cerebellum and tonsil, the IV ventricle and the central aqueduct, and the ventral space between the clivus and the brainstem). However, the subdural decompression procedure was more suitable for patients with CMI with type I cerebrospinal fluid kinetic abnormalities (cerebrospinal fluid flow blockage found in the posterior fossa space behind the cerebellum and tonsils) [35]. Moreover, preoperative normal hindbrain cerebrospinal fluid flow is an independent risk factor for intervention failure after decompression of patients with CMI [24]. In other words, abnormal cerebrospinal fluid flow in the hindbrain region preoperatively may predict a better surgical outcome. One study found that combined ventral and dorsal cerebrospinal fluid flow analysis of the hindbrain could better predict patient response to PCF decompression [25]. Aqueductal stroke volume was crucial for determining the need of surgical treatment [36]. An aqueductal stroke volume of ≤ 12 µL is an essential factor for considering surgical intervention. Conservative treatment is indicated for adult patients with CMI who are symptomatic and have an aqueductal stroke volume > 15 µL. This study also noted that clinical improvement was positively correlated with an increase in aqueductal stroke volume after treatment.
3) Tonsillar motion
Slight movement of the mesencephalon and brainstem that occurs with the arterial pulsations in the cardiac cycle due to the descending tonsils is a nonnegligible factor in causing abnormal cerebrospinal fluid flow as well as altered neuromotor function. The tonsillar motion was positively correlated with the exacerbation of clinical symptoms in patients with CMI [34]. Tonsillar motion is associated with reduced cerebrospinal fluid flow that manifests as aggravated clinical symptoms [34]. Similar results were obtained in another study, which compared morphological and physiological parameters in patients with CMI with excellent and poor prognosis after decompression surgery and found that maximal spinal cord displacement during the cardiac cycle was a better predictor of prognosis than morphological indicators [38]. One study even found normalization of the cerebellar tonsils and brainstem in patients, 6 months after craniocervical decompression by follow-up observation. Thus, the authors inferred that CMI is not a congenital disorder but an acquired malformation caused by cerebellar tonsillar pulsation embedded into the greater occipital foramen [14].
3. Limitations
There were some limitations to this study. Primarily, we collected and established some MRI-related morphological and functional parameters in patients with CMI, and obtained some good results. However, most of these parameters are based on traditional measurement methods and are too affected by human factors. Second, most studies did not clearly differentiate between symptomatic and asymptomatic CMI subjects, therefore, imaging-based features specific to the onset and nononset of symptoms are not available. Third, there is very little data in the literature on female subjects. Due to the lack of studies on gender differences, subgroup analysis was not possible. Finally, due to the lack of studies on the prognostic value of MRI-related parameters in patients with conservative follow-up, this aspect has not been evaluated.
CONCLUSION
With the development of MRI technology, diagnosing CMI should become more accurate and comprehensive. The traditional measurement method is affected by human factors and has too many parameters. The complexity of the cranial structure also makes it more challenging to select valid parameters. We collated and established a set of linear, angular, and area measurements deemed essential for diagnosing CMI. However, more indicators can only be analyzed descriptively for various reasons, particularly in prognostic prediction. It has become a major clinical challenge to determine and manage the disease faster and more accurately through multi-dimensional analysis. We posit that the systematic assessment of patients’ PCF morphology, volume, and other parameters at a 3D level holds promising clinical application prospects. Alternatively, additional criteria could be introduced to evaluate the occipital bone, slope, and basal angle as references for confirming the disease diagnosis. Multimodal MRI can be used for determination of disease and prediction of prognosis by introducing parameters to provide adequate guidance for clinical management.
Footnotes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This work was supported by the National High Level Hospital Clinical Research Funding (2022-PUMCH-B-112); the CAMS Innovation Fund for Medical Sciences (CIFMS) (2021-I2M-C&T-B-016); and the Yinhua Public Welfare Foundation.
Author Contribution
Conceptualization: ZW, YL, JG; Formal analysis: ZW, ZL, SH, SP; Investigation: ZW, ZL, SH, XH, SP; Methodology: ZW, ZL, XH; Project administration: YL, JG; Writing – original draft: ZW, ZL, SH; Writing – review & editing: ZW, ZL, SH, XH, SP, YL, JG.
Supplementary Materials
Supplementary Table 1 and Figs. 1-2 can be found via https://doi.org/10.14245/ns.2347150.575.
Risk of bias assessments of the included studies using the ROBINS-I tool
MRI-related anatomic and functional parameters for evaluating the diagnosis and prognosis of Chiari malformation type I. MRI, magnetic resonance imaging; PCF, posterior cranial fossa; CSF, cerebrospinal fluid; FM, foramen magnum; C-C2SVA, condylar-C2 sagittal vertical alignment; pB-C2, perpendicular to the basion to C2 line; FM-C2, foramen magnum-C2; FVV, fourth ventricle vertex.
Sensitivity analysis of heterogeneity about the result of posterior cranial fossa height. CI, confidence interval.
REFERENCES
- 1.Massimi L, Peppucci E, Peraio S, et al. History of Chiari type I malformation. Neurol Sci. 2011;32 Suppl 3:S263–5. doi: 10.1007/s10072-011-0700-7. [DOI] [PubMed] [Google Scholar]
- 2.Sekula RF, Jannetta PJ, Casey KF, et al. Dimensions of the posterior fossa in patients symptomatic for Chiari I malformation but without cerebellar tonsillar descent. Cerebrospinal Fluid Res. 2005;2:11. doi: 10.1186/1743-8454-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 4.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence--publication bias. J Clin Epidemiol. 2011;64:1277–82. doi: 10.1016/j.jclinepi.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–6. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Karagöz F, Izgi N, Kapíjcíjoğlu Sencer S. Morphometric measurements of the cranium in patients with Chiari type I malformation and comparison with the normal population. Acta Neurochir (Wien) 2002;144:165–71. doi: 10.1007/s007010200020. discussion 171. [DOI] [PubMed] [Google Scholar]
- 8.Milhorat TH, Nishikawa M, Kula RW, et al. Mechanisms of cerebellar tonsil herniation in patients with Chiari malformations as guide to clinical management. Acta Neurochir (Wien) 2010;152:1117–27. doi: 10.1007/s00701-010-0636-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alperin N, Loftus JR, Oliu CJ, et al. Magnetic resonance imaging measures of posterior cranial fossa morphology and cerebrospinal fluid physiology in Chiari malformation type I. Neurosurgery. 2014;75:515–22. doi: 10.1227/NEU.0000000000000507. discussion 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aydin S, Hanimoglu H, Tanriverdi T, et al. Chiari type I malformations in adults: a morphometric analysis of the posterior cranial fossa. Surg Neurol. 2005;64:237–41. doi: 10.1016/j.surneu.2005.02.021. discussion241. [DOI] [PubMed] [Google Scholar]
- 11.Urbizu A, Poca MA, Vidal X, et al. MRI-based morphometric analysis of posterior cranial fossa in the diagnosis of chiari malformation type I. J Neuroimaging. 2014;24:250–6. doi: 10.1111/jon.12007. [DOI] [PubMed] [Google Scholar]
- 12.Krishna V, Sammartino F, Yee P, et al. Diffusion tensor imaging assessment of microstructural brainstem integrity in Chiari malformation type I. J Neurosurg. 2016;125:1112–9. doi: 10.3171/2015.9.JNS151196. [DOI] [PubMed] [Google Scholar]
- 13.Milhorat TH, Bolognese PA, Nishikawa M, et al. Association of Chiari malformation type I and tethered cord syndrome: preliminary results of sectioning filum terminale. Surg Neurol. 2009;72:20–35. doi: 10.1016/j.surneu.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dufton JA, Habeeb SY, Heran MK, et al. Posterior fossa measurements in patients with and without Chiari I malformation. Can J Neurol Sci. 2011;38:452–5. doi: 10.1017/s0317167100011860. [DOI] [PubMed] [Google Scholar]
- 15.Heiss JD, Suffredini G, Bakhtian KD, et al. Normalization of hindbrain morphology after decompression of Chiari malformation type I. J Neurosurg. 2012;117:942–6. doi: 10.3171/2012.8.JNS111476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houston JR, Eppelheimer MS, Pahlavian SH, et al. A morphometric assessment of type I Chiari malformation above the McRae line: a retrospective case-control study in 302 adult female subjects. J Neuroradiol. 2018;45:23–31. doi: 10.1016/j.neurad.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Nair BR, Rajshekhar V. Posterior fossa morphometry in 170 South Asian children and adults with Chiari malformation and its correlation with tonsillar descent. Br J Neurosurg. 2022;36:762–9. doi: 10.1080/02688697.2021.2008873. [DOI] [PubMed] [Google Scholar]
- 18.Nishikawa M, Bolognese PA, Yamagata T, et al. Surgical management of Chiari malformation type I and instability of the craniocervical junction based on its pathogenesis and classification. Neurol Med Chir (Tokyo) 2022;62:400–15. doi: 10.2176/jns-nmc.2022-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang CS, Wang X, Fu CH, et al. Analysis of cerebrospinal fluid flow dynamics and morphology in Chiari I malformation with cine phase-contrast magnetic resonance imaging. Acta Neurochir (Wien) 2014;156:707–13. doi: 10.1007/s00701-013-1958-8. [DOI] [PubMed] [Google Scholar]
- 20.Yan H, Han X, Jin M, et al. Morphometric features of posterior cranial fossa are different between Chiari I malformation with and without syringomyelia. Eur Spine J. 2016;25:2202–9. doi: 10.1007/s00586-016-4410-y. [DOI] [PubMed] [Google Scholar]
- 21.Yuksel U, Burulday V, Akkaya S, et al. Possible predictive clinical and radiological markers in decision making for surgical intervention in patients with Chiari malformation type 1. Neurol Res. 2022;44:975–88. doi: 10.1080/01616412.2022.2089402. [DOI] [PubMed] [Google Scholar]
- 22.Tubbs RS, Wellons JC, 3rd, Blount JP, et al. Inclination of the odontoid process in the pediatric Chiari I malformation. J Neurosurg. 2003;98(1 Suppl):43–9. doi: 10.3171/spi.2003.98.1.0043. [DOI] [PubMed] [Google Scholar]
- 23.Besachio DA, Khaleel Z, Shah LM. Odontoid process inclination in normal adults and in an adult population with Chiari malformation type I. J Neurosurg Spine. 2015;23:701–6. doi: 10.3171/2015.3.SPINE14926. [DOI] [PubMed] [Google Scholar]
- 24.McGirt MJ, Nimjee SM, Fuchs HE, et al. Relationship of cine phase-contrast magnetic resonance imaging with outcome after decompression for Chiari I malformations. Neurosurgery. 2006;59:140–6. doi: 10.1227/01.NEU.0000219841.73999.B3. discussion 140-6. [DOI] [PubMed] [Google Scholar]
- 25.McGirt MJ, Atiba A, Attenello FJ, et al. Correlation of hindbrain CSF flow and outcome after surgical decompression for Chiari I malformation. Childs Nerv Syst. 2008;24:833–40. doi: 10.1007/s00381-007-0569-1. [DOI] [PubMed] [Google Scholar]
- 26.Furtado SV, Thakar S, Hegde AS. Correlation of functional outcome and natural history with clinicoradiological factors in surgically managed pediatric Chiari I malformation. Neurosurgery. 2011;68:319–27. doi: 10.1227/NEU.0b013e31820206e5. discussion 328. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Hao Z, Hu S, et al. Predictive value of posterior cranial fossa morphology in the decompression of Chiari malformation type I: a retrospective observational study. Medicine (Baltimore) 2019;98:e15533. doi: 10.1097/MD.0000000000015533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ladner TR, Dewan MC, Day MA, et al. Evaluating the relationship of the pB-C2 line to clinical outcomes in a 15-year single-center cohort of pediatric Chiari I malformation. J Neurosurg Pediatr. 2015;15:178–88. doi: 10.3171/2014.9.PEDS14176. [DOI] [PubMed] [Google Scholar]
- 29.Marianayagam NJ, Chae JK, Hussain I, et al. Increase in clivo-axial angle is associated with clinical improvement in children undergoing occipitocervical fusion for complex Chiari malformation: patient series. J Neurosurg Case Lessons. 2021;2:CASE21433. doi: 10.3171/CASE21433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravindra VM, Iyer RR, Awad AW, et al. Defining the role of the condylar-C2 sagittal vertical alignment in Chiari malformation type I. J Neurosurg Pediatr. 2020;26:439–44. doi: 10.3171/2020.4.PEDS20113. [DOI] [PubMed] [Google Scholar]
- 31.Ravindra VM, Iyer RR, Yahanda AT, et al. A multicenter validation of the condylar-C2 sagittal vertical alignment in Chiari malformation type I: a study using the Park-Reeves Syringomyelia Research Consortium. J Neurosurg Pediatr. 2021 Jun 4;:1–7. doi: 10.3171/2020.12.PEDS20809. doi: . [Epub] [DOI] [PubMed] [Google Scholar]
- 32.Mantha S, Coulthard LG, Campbell R. CSF-space volumetric change following posterior fossa decompression in paediatric Chiari type-I malformation: a correlation with outcome. Childs Nerv Syst. 2021;37:3861–9. doi: 10.1007/s00381-021-05307-4. [DOI] [PubMed] [Google Scholar]
- 33.Thakar S, Sivaraju L, Jacob KS, et al. A points-based algorithm for prognosticating clinical outcome of Chiari malformation type I with syringomyelia: results from a predictive model analysis of 82 surgically managed adult patients. J Neurosurg Spine. 2018;28:23–32. doi: 10.3171/2017.5.SPINE17264. [DOI] [PubMed] [Google Scholar]
- 34.Collins RA, John A, Daniel H, et al. Association of cerebellar tonsil dynamic motion and outcomes in pediatric Chiari I malformation. World Neurosurg. 2022;168:e518–29. doi: 10.1016/j.wneu.2022.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Fan T, Zhao H, Zhao X, et al. Surgical management of Chiari I malformation based on different cerebrospinal fluid flow patterns at the cranial-vertebral junction. Neurosurg Rev. 2017;40:663–70. doi: 10.1007/s10143-017-0824-1. [DOI] [PubMed] [Google Scholar]
- 36.Abdallah A, Çınar İ, Gündağ Papaker M, et al. Management of adult Chiari I patients based on CSF flow magnetic resonance imaging: experience of two neurosurgical centers. J Neurol Surg A Cent Eur Neurosurg. 2023;84:128–43. doi: 10.1055/s-0042-1745845. [DOI] [PubMed] [Google Scholar]
- 37.Chae JK, Haghdel A, Kelly A, et al. Ventral tonsillar herniation predicts headaches in adults with Chiari malformation. World Neurosurg. 2021;155:e453–9. doi: 10.1016/j.wneu.2021.08.085. [DOI] [PubMed] [Google Scholar]
- 38.Alperin N, Loftus JR, Bagci AM, et al. Magnetic resonance imaging-based measures predictive of short-term surgical outcome in patients with Chiari malformation type I: a pilot study. J Neurosurg Spine. 2017;26:28–38. doi: 10.3171/2016.5.SPINE1621. [DOI] [PubMed] [Google Scholar]
- 39.Roller LA, Bruce BB, Saindane AM. Demographic confounders in volumetric MRI analysis: is the posterior fossa really small in the adult Chiari 1 malformation. AJR Am J Roentgenol. 2015;204:835–41. doi: 10.2214/AJR.14.13384. [DOI] [PubMed] [Google Scholar]
- 40.George TM, Higginbotham NH. Defining the signs and symptoms of Chiari malformation type I with and without syringomyelia. Neurol Res. 2011;33:240–6. doi: 10.1179/016164111X12962202723760. [DOI] [PubMed] [Google Scholar]
- 41.Bagci AM, Lee SH, Nagornaya N, et al. Automated posterior cranial fossa volumetry by MRI: applications to Chiari malformation type I. AJNR Am J Neuroradiol. 2013;34:1758–63. doi: 10.3174/ajnr.A3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noudel R, Jovenin N, Eap C, et al. Incidence of basioccipital hypoplasia in Chiari malformation type I: comparative morphometric study of the posterior cranial fossa. Clinical article. J Neurosurg. 2009;111:1046–52. doi: 10.3171/2009.2.JNS08284. [DOI] [PubMed] [Google Scholar]
- 43.Milhorat TH, Chou MW, Trinidad EM, et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44:1005–17. doi: 10.1097/00006123-199905000-00042. [DOI] [PubMed] [Google Scholar]
- 44.Nishikawa M, Sakamoto H, Hakuba A, et al. Pathogenesis of Chiari malformation: a morphometric study of the posterior cranial fossa. J Neurosurg. 1997;86:40–7. doi: 10.3171/jns.1997.86.1.0040. [DOI] [PubMed] [Google Scholar]
- 45.Iskandar BJ, Quigley M, Haughton VM. Foramen magnum cerebrospinal fluid flow characteristics in children with Chiari I malformation before and after craniocervical decompression. J Neurosurg. 2004;101(2 Suppl):169–78. doi: 10.3171/ped.2004.101.2.0169. [DOI] [PubMed] [Google Scholar]
- 46.Eppelheimer MS, Biswas D, Braun AM, et al. Quantification of changes in brain morphology following posterior fossa decompression surgery in women treated for Chiari malformation type 1. Neuroradiology. 2019;61:1011–22. doi: 10.1007/s00234-019-02206-z. [DOI] [PubMed] [Google Scholar]
- 47.Khalsa SSS, Siu A, DeFreitas TA, et al. Comparison of posterior fossa volumes and clinical outcomes after decompression of Chiari malformation type I. J Neurosurg Pediatr. 2017;19:511–7. doi: 10.3171/2016.11.PEDS16263. [DOI] [PubMed] [Google Scholar]
- 48.Noudel R, Gomis P, Sotoares G, et al. Posterior fossa volume increase after surgery for Chiari malformation type I: a quantitative assessment using magnetic resonance imaging and correlations with the treatment response. J Neurosurg. 2011;115:647–58. doi: 10.3171/2010.11.JNS102148. [DOI] [PubMed] [Google Scholar]
- 49.Bollo RJ, Riva-Cambrin J, Brockmeyer MM, et al. Complex Chiari malformations in children: an analysis of preoperative risk factors for occipitocervical fusion. J Neurosurg Pediatr. 2012;10:134–41. doi: 10.3171/2012.3.PEDS11340. [DOI] [PubMed] [Google Scholar]
- 50.Grabb PA, Mapstone TB, Oakes WJ. Ventral brain stem compression in pediatric and young adult patients with Chiari I malformations. Neurosurgery. 1999;44:520–7. doi: 10.1097/00006123-199903000-00050. discussion527-8. [DOI] [PubMed] [Google Scholar]
- 51.Mauer UM, Gottschalk A, Mueller C, et al. Standard and cardiac-gated phase-contrast magnetic resonance imaging in the clinical course of patients with Chiari malformation type I. Neurosurg Focus. 2011;31:E5. doi: 10.3171/2011.7.FOCUS11105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Risk of bias assessments of the included studies using the ROBINS-I tool
MRI-related anatomic and functional parameters for evaluating the diagnosis and prognosis of Chiari malformation type I. MRI, magnetic resonance imaging; PCF, posterior cranial fossa; CSF, cerebrospinal fluid; FM, foramen magnum; C-C2SVA, condylar-C2 sagittal vertical alignment; pB-C2, perpendicular to the basion to C2 line; FM-C2, foramen magnum-C2; FVV, fourth ventricle vertex.
Sensitivity analysis of heterogeneity about the result of posterior cranial fossa height. CI, confidence interval.