Abstract
Following a request from the European Commission, the EFSA Panel on Plant Health performed a quantitative risk assessment for the EU of Phlyctinus callosus (Coleoptera: Curculionidae), a polyphagous pest occurring in Australia, New Zealand and South Africa. The current risk assessment focused on potential pathways for entry, the climatic conditions allowing establishment, the expected spread capacity and the impact considering a time horizon of 10 years (2023–2032). The Panel identified the import of apples, cut flowers and table grapes as the most relevant entry pathways. Over the next 10 years, an annual median estimate of approximately 49.5 (90% certainty range, CR, ranging from 4.0 to 881.2) potential P. callosus founder populations are expected. When the probability of establishment is considered and climatic indicators are used to define the areas in the EU where establishment is possible, the model estimated a median of 1 founder population every 1.3 years (90% CR: 1 every 30.8 years to 23.3 per year) in the scenario where the areas are defined by the union of all the climatic indicators and 1 founder population every 11.9 years (90% CR: 1 every 256.6 years to 2.5 per year) in the scenario where establishment is possible only in the areas defined by the climatic indicator of minimum soil temperature. The estimated number of founder populations per year is mostly driven by the probability of establishment in the rural areas, infestation rate in table grapes and the probability of transfer to a suitable host in the rural area. The risk of entry for cut flowers and apples is substantially lower than the risk from the table grapes. If such founder populations were to establish, P. callosus is estimated to spread by natural dispersal and common agricultural practices at a rate of 15.5 m/year (90% CR 5.1–46.8 m/year) after a lag phase of 4.0 years (90% CR 1.3–8.7 years). The impact, expressed as percentage loss of the production directly attributable to P. callosus in the areas where establishment is possible and assuming farmers do not apply specific control measures was estimated at 0.5% (90% CR 0.01%–2.8%) for cut flowers/foliage, 5.2% (90% CR 2.2%–11.7%) for apples and 2% (90% CR 1.3%–5.2%) for table grapes. Options for risk reduction are discussed, but their effectiveness is not quantified.
Keywords: banded fruit weevil, pathway model, pest prevalence, phytosanitary measures, risk assessment, uncertainty
SUMMARY
Following a request from the European Commission, the EFSA Panel on Plant Health performed a quantitative risk assessment of Phlyctinus callosus (Coleoptera: Curculionidae), for the EU. The assessment focused on potential pathways for entry, climatic conditions allowing establishment, spread and subsequent impact considering a time horizon of 10 years (2023–2032). Options for risk reduction are discussed, but their effectiveness has not been quantified.
Phlyctinus callosus is a pest with a limited geographical distribution. It is native to South Africa, and is also known to occur in Australia, New Zealand, Norfolk Island (Australia), Reunion Island (overseas department of France) and Saint Helena (British overseas territory). The Panel identified the most relevant pathways for entry in the EU by considering interception data, the scientific evidence of association of P. callosus with the host plants and the possible presence on the plant products for which there is evidence of trade from the third countries where P. callosus is reported; as a result, the import of cut flowers, apples (Malus domestica) and table grapes (Vitis vinifera) were identified as the most relevant entry pathways.
Using expert knowledge elicitation (EKE) and pathway modelling, the Panel estimated a median number of potential founder populations of 49.5 (90% certainty range, CR, ranging from 4.0 to 881.2). However, when accounting for the actual probability of establishment, the number of founder populations drops to approximately one founder population every 1.3 years (90% CR: 1 every 30.8 years to 23.3 per year) when considering the scenario of the maximum area of establishment. Under this scenario, the areas suitable for pest establishment are identified by the overlay of three climatic indicators: (i) absolute minimum soil temperature, (ii) hardiness zones to annual minimum temperature and (iii) average maximum number of consecutive days below the lower development threshold. In the scenario where establishment is possible in the areas when only the climatic indicator of minimum soil temperature is considered (minimum area of establishment), the median number of founder population per year was 1 founder population every 11.9 years (90% CR: 1 every 256.6 years to 2.5 per year).
Should P. callosus establish in the climatically suitable areas of the EU, the Panel estimated a lag phase of 4.0 years (90% CR 1.3–8.7 years) before P. callosus populations reach a steady rate of spread estimated at 15.5 m/year (90% CR 5.1–46.8 m/year) within a production site.
In case P. callosus populations become a naturalised species within suitable areas of the EU, the average yield loss directly attributable to P. callosus was estimated at 5.2% (90% CR 2.2%–11.7%) for apples, 0.5% (90% CR 0.01%–2.8%) for cut flowers/foliage and 2% (90% CR 1.3%–5.2%) for table grapes of the total production. The estimations above do not consider that specific measures to control P. callosus are taken.
Potential risk reduction options applied either pre‐harvest or post‐harvest include the use of trunk barriers, application of biological control or chemical insecticides, inspections at export and at points of entry.
1. INTRODUCTION
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
The new Plant Health Regulation (EU) 2016/2031, on the protective measures against pests of plants, is applying from 14 December 2019. Conditions are laid down in this legislation in order for pests to qualify for listing as Union quarantine pests, protected zone quarantine pests or Union regulated non‐quarantine pests. The lists of the EU‐regulated pests together with the associated import or internal movement requirements of commodities are included in Commission Implementing Regulation (EU) 2019/2072. Additionally, as stipulated in the Commission Implementing Regulation 2018/2019, certain commodities are provisionally prohibited to enter in the EU (high‐risk plants, HRP). EFSA is performing the risk assessment of the dossiers submitted by exporting to the EU countries of the HRP commodities, as stipulated in Commission Implementing Regulation 2018/2018. Furthermore, EFSA has evaluated a number of requests from exporting to the EU countries for derogations from specific EU import requirements.
In line with the principles of the new plant health law, the European Commission with the Member States are discussing monthly the reports of the interceptions and the outbreaks of pests notified by the Member States. Notifications of an imminent danger from pests that may fulfil the conditions for inclusion in the list of the Union quarantine pest are included. Furthermore, EFSA has been performing horizon scanning of media and literature.
As a follow‐up of the abovementioned activities (reporting of interceptions and outbreaks, HRP, derogation requests and horizon scanning), a number of pests of concern have been identified. EFSA is requested to provide scientific opinions for these pests, in view of their potential inclusion in the lists of Commission Implementing Regulation (EU) 2019/2072 and the inclusion of specific import requirements for relevant host commodities, when deemed necessary.
1.1.2. Terms of Reference
EFSA is requested, pursuant to Article 29(1) of Regulation (EC) No 178/2002, to provide scientific opinions in the field of plant health.
EFSA is requested to deliver 50 pest categorisations for the pests listed in Annex 1A, 1B and 1D. Additionally, EFSA is requested to perform pest categorisations for the pests so far not regulated in the EU, identified as pests potentially associated with a commodity in the commodity risk assessments of the HRP dossiers (Annex 1C). Such pest categorisations are needed in the case where there are not available risk assessments for the EU.
When the pests of Annex 1A are qualifying as potential Union quarantine pests, EFSA should proceed to phase 2 risk assessment. The opinions should address entry pathways, spread, establishment, impact and include a risk reduction options analysis.
ANNEX 1 List of pests A
Amyelois transitella
Citripestis sagittiferella
Colletotrichum fructicola
Elasmopalpus lignosellus
Phlyctinus callosus
Resseliella citrifrugis
Retithrips syriacus
Xylella taiwanensis
1.2. Interpretation of the Terms of Reference
The EFSA Panel on Plant Health (hereafter Panel) published a pest categorisation on P. callosus (EFSA PLH Panel, 2021), which concluded that the pest met the criteria for consideration as Union quarantine pest.
The terms of reference relevant to P. callosus specify that the requested opinion should address entry pathways, spread, establishment, impact and include a risk reduction options analysis. The Panel therefore undertook a quantitative pest risk assessment according to the principles laid down in its guidance on quantitative pest risk assessment.
2. DATA AND METHODOLOGIES
A literature search on P. callosus was conducted at the beginning of the risk assessment (15th of March 2023) in the ISI Web of Science bibliographic database and Scopus using the scientific (Phlyctinus callosus, Ocynoma rhysa, Peritelus (Phlyctinus) callosus, Rhyncogonus germanus and Sciobius subnodosus), and common (garden weevil, vine calandra, banded fruit weevil, kalander, v‐back snoutbeetle, grapevine beetle and vine snout beetle) names of the pest as search terms. After removal of duplicates, 98 records were retained. All the documents were uploaded on DistillerSR (https://www.distillersr.com/products/distillersr‐systematic‐review‐software) and screened to extract data on: (i) geographical distribution, (ii) host(s), (iii) behaviours and biological parameters, (iv) spread, (v) symptoms & impact and (vi) control measures.
Data on interceptions and outbreaks of P. callosus within the risk assessment area were searched in Europhyt (1995‐until May 2020) and TRACES (June 2020‐ongoing database, last check 24th of April 2024) and no records of interceptions were found. However, considering that P. callosus could have been intercepted but simply reported as ‘Coleoptera’ or ‘Curculionidae’, the Panel repeated the search for each of these terms. It is anticipated that although not specific for P. callosus, this search was intended to provide a broad understanding of what could the interception magnitude could be in the unlikely scenario that all the interceptions recorded as ‘Coleoptera’ or ‘Curculionidae’ on P. callosus known hosts originating from the countries where P. callosus was reported, were indeed attributable to P. callosus.
Information on the pest distribution was used to inform the climate suitability analysis (Section 2.2.1) while literature data on hosts and interception data were used to inform identification of the priority entry pathways to be considered in the risk assessment when modelling the risk of entry (see Section 2.1.1).
Historical import data from the countries where the pest is known to occur and for the commodities identified as relevant entry pathways were retrieved from EUROSTAT.
The main features of the biology and life cycle of P. callosus are summarised in the pest categorisation prepared by EFSA PLH Panel (2021); however, the key biological aspects considered in this assessment are reported in Section 3.2 (review of pest biology).
Literature data on biology, spread, impact and control, integrated with information collected during interviews with hearing experts were used to prepare evidence dossiers in support of expert knowledge elicitation (EKE) sessions aimed to estimate, by means of expert judgements, quantities that could not be well characterised from the literature alone (EFSA, 2014). EKE sessions involved Panel members, members of the working group and EFSA staff.
The Panel performed this risk assessment following the Panel's guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018).
The probability of entry via imported plants for planting and plant products was assessed using pathway modelling in @Risk (https://www.palisade.com/risk/default.asp); the file is available as supplementary material in the online version of the scientific Opinion.
P. callosus is a polyphagous insect and the list of possible host plants is extensive. Therefore, in the assessment of entry, the Panel first identified the most relevant pathways for entry into the EU and identified three main pathways that should be considered (see Section 3.3.1). For each pathway, the volume of trade to the EU from the countries where P. callosus is reported was estimated together with the proportion of infested products (see Section 2.1.2) and the number of infested products delivered to each Member State and NUTS2 region according to a redistribution model (see Section 2.1.2.1). Finally, the number of founder populations was estimated after the identification of the areas in the EU that are suitable for establishment should entry take place (see Sections 2.2.2 and 3.3.3).
2.1. Entry
2.1.1. Identification of the relevant entry pathways
P. callosus is a polyphagous weevil and many different hosts could provide a pathway for entry into the EU (EFSA PLH Panel, 2021). The guidelines on quantitative pest risk assessment (EFSA PLH Panel, 2018) indicate that when multiple pathways are possible, the most relevant should be considered for estimating the probability of entry. In agreement, the Panel identified the most relevant entry pathways by considering:
Commodities for which there is evidence of interception, including non‐EU interception data, to establish evidence of pest association with a potential entry pathway.
Commodities for which P. callosus is a known pest in the country of origin.
EUROSTAT data regarding evidence of import into the EU of commodities associated with P. callosus from the countries where the pest is known to occur.
The volume of import (e.g. tons per year) for commodities with trade into the EU.
Following the above considerations, the Panel identified three main entry pathways: apples (CN 080810), table grapes (CN 08061010) and cut flowers (CN 06031970). These pathways would only allow the introduction of adult specimens. Conditions during the adults' lifetime remaining after harvest, packing, export, shipping and distribution are unfavourable to complete mating and oviposition (see Section 3.2). Therefore, for the potential arrivals in the EU, only mated females are considered.
For apples and table grapes, trade import data from countries where the pest is reported were retrieved from EUROSTAT. The Panel had sufficient commodity‐specific information for apples and table grapes to directly estimate the number of infested units in the countries of origin that enter the EU.
The entry pathway for cut flowers (coded as CN 06031970) includes a large variety of different product types of which: ‘Other living plants: cut flowers and branches with foliage’ was considered relevant for the possible presence of P. callosus from interception data (see Section 3.1.1). However, by querying the TRACES database, considering only the consignments from the third countries where the pest is known to occur (i.e. Australia, New Zealand and South Africa), from 2021 to 2023, it appears that an average of about 570 consignments per year were inspected across all the border control points. If considering that only few cut flower species are listed in Annex XI, Part A of Regulation (EU) 2019/2072 and the other species can be subjected to a reduced frequency of inspection regime as per Regulation (EU) 2022/2389, the number of inspected consignments reported on TRACES likely represents only a fraction of the total imported. Due to the lack of import data with adequate resolution (i.e. number of consignments and number of units per consignment, or number of units), the Panel used a different approach to approximate the number of P. callosus specimens entering the EU through this pathway. The methodology for the entry model, hence, is described in Section 2.1.2 separately for fruits and cut flowers.
2.1.2. Conceptual model
Apples and table grapes. The pathway model for the entry of P. callosus starts estimating the expected number of units infested with mated P. callosus females entering the EU (NInfEU). Then, the infested units are distributed to the NUTS2 regions of the Member States considering the proportion of population living in the rural and urban areas. Subsequently, the potential transfer to a host is evaluated to estimate the number of potential founder populations. Calculation uses the parameters listed in Table 1.
TABLE 1.
Description, unit and source of evidence of the parameters used to estimate the probability of entry of Phlyctinus callosus into the EU in terms of number of infested units (NInfEU) and number of potential founder populations (NPFPEU).
| Parameter | Description | Unit | Data source |
|---|---|---|---|
| Tv | Yearly trade volume in the time horizon of the risk assessment | kg/year | EUROSTAT |
| Uw | Typical weight of a single unit:
|
g | EFSA PPR Panel (2018) |
| P(Infested) | Prevalence of infested units before export at the point of departure in the country of origin | – a | EKE |
|
Pr(TrOC), Pr(TrAC), |
Portion of trade flow transported by ocean cargo Pr(TrOC), and air cargo Pr(TrAC):
|
– | |
|
Transport TimeAC Transport TimeOC |
Transport duration via air cargo (AC) and ocean cargo (OC) for apples and table grapes:
|
Days | |
| Transport temperature |
Recommended transport temperature for table grapes and apples: Apples = −0.5°C Table grapes = −0.5 ± 0.5°C |
°C | |
| P(SurvivalOC) | Probability of survival of the pest during transport via ocean cargo from the country of origin to the EU according to the commodity and transportation conditions (transport duration, temperature and treatments if any) | – | Interpolation from experimental data (Myburgh & Kriegler, 1968) |
| P(SurvivalAC) | Probability of survival of the pest during transport via air cargo from the country of origin to the EU according to the commodity and transportation conditions (transport duration, temperature and treatments if any) | – | Interpolation from experimental data (Myburgh & Kriegler, 1968) |
| P(TransferR) | Probability of successful transfer to a suitable host in the rural area | – | EKE |
| P(TransferU) | Probability of successful transfer to a suitable host in the urban area | – | EKE |
| ᵞ |
Conversion factor explaining by which order of magnitude the estimate for the probability of transfer for the rural area should be corrected to obtain the probability of transfer for the urban area P(TransferU) = P(TransferR)*10ᵞ |
– | EKE |
| Pr(Urban) | Proportion of population living in the urban areas of the EU | – | EUROSTAT |
| Pr(Rural) | Proportion of population living in the rural areas of the EU | – | EUROSTAT |
| PopEU | Population in the EU into which the commodity is transferred i.e. where infested units potentially may arrive | – | EUROSTAT |
These parameters are dimensionless.
The total number of units infested with mated P. callosus females imported in the EU (NInfEU) is estimated from the number of units being imported (Tv/Uw), the probability of the unit being infested P(Infested), the probability of P. callosus surviving the transport P(Survival_Transport) and the probability of being a (mated) female according to an assumed F:M sex ratio of 1:1 (i.e. females represent 50% of the total). For simplicity and following a conservative approach, it was further assumed that all female individuals are mated:
| (1) |
The probability of surviving transport comprises two alternative transport flows, via ocean cargo (proportion, Pr(TrOC)) and air cargo (Pr(TrAC)). The two flows come with a survival probability, i.e. P(SurvivalOC) and P(SurvivalAC), considering the commodity‐specific transport durations and storage conditions (e.g. use of SO2 with table grapes):
| (2) |
The survival probabilities, i.e. P(SurvivalOC) and P(SurvivalAC), are estimated from experimental data of P. callosus survival as function of duration of various low temperature treatments with or without SO2 (Myburgh & Kriegler, 1968) (see Table 6 in Section 3.3.2.3 for details). Today, transport at low temperature and use of SO2 is commonly applied to storage and transport of table grapes (de Aguiar et al., 2023).
TABLE 6.
| Days in pre‐cooling (T ≈ ‐0.5°C) | Days in storage (T ≈ +1.1°C) | Days in storage (T ≈ ‐0.5°C) | Total N# days in treatment | % survival (T° ≈ −0.5°C) | % survival (T° ≈ −0.5°C) + SO2 | % survival (T° ≈ +1.1°C) |
|---|---|---|---|---|---|---|
| 0 | ||||||
| 1 | 17 | 18 | 5 | 1 | ||
| 4 | 17 | 21 | 2 | 1 | ||
| 7 | 17 | 24 | 1 | 0 | ||
| 10 | 17 | 27 | 0 | 0 | ||
| 0 | 21 | 21 | 94 | |||
| 0 | 24 | 24 | 91 | |||
| 0 | 27 | 27 | 88 | |||
| 0 | 9* | 9 | 93 | 1 | ||
| 0 | 14* | 14 | 63 | 1 | ||
| 0 | 18* | 18 | 24 | 2 | ||
| 0 | 21* | 21 | 15 | 0 |
Only the results of the experiments conducted under storage conditions relevant for the assessment are reported. Results marked with × pertain to a small‐scale experiment with table grapes packed with polyethylene liners.
The number of units infested with mated females annually imported into the EU (NInfEU) is redistributed across NUTS2 regions of each MS according to a model presented in Section 2.1.2.1. Thereafter, the number of infested units delivered to a NUTS2 region (NInfNUTS2) is converted into the number of potential founder populations (NPFPNUTS2). A potential founder population is constituted by a mated P. callosus female that has found a suitable oviposition site and laid eggs on a host plant. The NPFPNUTS2 is calculated by multiplying NInfNUTS2 with the probability of transfer to a suitable host P(Transfer). However, the Panel assumed that the transfer to a suitable host is only possible after an infested unit has reached the consumer, who may reside in rural (R) or urban (U) areas. Considering that P. callosus cannot fly, the Panel deemed it important to explicitly consider whether the final destination of the infested unit is in rural or urban environment, because the factors facilitating or preventing transfer are different. The total number of potential founder populations in a NUTS2 region (NPFPNUTS2) is the sum of both environments:
| (3) |
Finally, the number of potential founder populations per year in the EU (NPFPEU) is obtained as the sum of the founder populations of all NUTS2 regions in the EU. Denoting the i‐th NUTS2 region of the j‐th EU Member State by NUTS2_ji the overall number of founder population per year is:
| (4) |
where Ʃi denotes the summation over all NUTS2 regions of a Member State and Ʃj those over all Member States.
Cut flowers. As described above (Section 2.1.1) for the cut flowers, the Panel could not implement a pathway model at the same level of details as for apples and table grapes. The number of potential founder population of P. callosus was estimated by using information from interceptions of P. callosus on cut flowers in Japan and data from TRACES on cut flowers.
According to the Japanese report of Genka and Yoshitake (2018) describing quarantine inspections on imported plants between 1978 and 2016 at Narita Airport, 1696 consignments were found infested with (one or more) weevils. Out of the total number of intercepted weevils (not the consignments), 82 were identified as P. callosus (Genka & Yoshitake, 2018). Hence, at maximum, 82 consignments could have been infested with P. callosus. Considering 82 consignments out of 1696 intercepted consignments from the Japanese border inspection data, this gives 1 consignment with P. callosus per maximum 20.7 consignments intercepted.
According to TRACES (accessed 26/3/2024), on average, 570 consignments/year (data from 2021 to 2023) are inspected for the commodity CN 06031970, product type: ‘Other living plants: cut flowers and branches with foliage’ from Australia, New Zealand and South Africa. In the worst case, in all the inspected consignments, a weevil would be intercepted. Under the assumption that: (i) the 570 inspections on TRACES represent in the worst case 1% of the total number of consignments inspected for a commodity under a reduced frequency checks regime (DTU31, 2023) and (ii) according to Japanese data, P. callosus is expected to be intercepted every 20.7th consignment, the 570 TRACES inspections translate into about 2754 P. callosus/year as a maximum number of P. callosus specimens introduced in the EU through this pathway ((570 × 100)/20.7 = 2754). For simplicity and following a conservative approach, the Panel considered the specimens introduced through this pathway as mated females.
This number i.e. the contribution of the cut flowers pathway, was added to the NInfEU in Equation 1. Further these introductions underwent the redistribution and transfer calculations together with those P. callosus specimens from the table grapes and apples pathway.
2.1.2.1. Redistribution model
The approach described in this section was used to convert the output of the entry pathway model (i.e. number of potential founder populations per year) to the regional resolution of NUTS2. Infested commodity units entering the EU are transported from the port of entry to EU MSs for consumption or further trading. To ascertain the destination of an individual consignment is not feasible. Therefore, the Panel implemented a redistribution model to estimate the share of the units imported into the EU for each MS (EFSA PLH Panel, 2024b). Briefly, the commodity‐specific inputs of the redistribution model are: (i) the weight of annually imported commodity from third countries with/without reported presence of the pest to the importing countries in the EU; (ii) the intra‐EU trade flows, (iv) the export of the commodity from the EU countries to third countries and (iii) the commodity‐specific production data of the MSs. With these data, the redistribution model returns the estimated median value of the share of the commodity imported from third countries where the pest is present that reaches each member state. Within each MS, the number of infested units is then redistributed to NUTS2 regions proportionate to the number of inhabitants. Due to a lack of data at a sufficient level of resolution for the cut flowers entry pathway, the Panel assumed that the Intra‐EU trade flows of this commodity are comparable to those of cut roses, as estimated in a recent EFSA Opinion (EFSA PLH Panel, 2024b).
Considering that the model explicitly separates between the urban and the rural environments, the redistribution of the infested units at NUTS2 level was performed considering the proportion of population living in rural and urban areas. This was achieved by integrating the urban–rural typology classification data at NUTS3 level (EUROSTAT database: NUTS‐2021, available at https://ec.europa.eu/eurostat/web/rural‐development/methodology) with the corresponding NUTS3 level population data (EUROSTAT database: demo_r_pjanaggr3). For each NUTS2, the number of inhabitants in the rural (PopNUTS2_R) and urban (PopNUTS2_U) areas is obtained as the sum of the inhabitants residing in the NUTS3 of the corresponding area type. It should be noted that the urban–rural typology data set distinguishes three population characteristics on the NUTS3 level: ‘predominantly urban’, ‘intermediate’ and ‘predominantly rural’. The ‘intermediate’ region is defined as a NUTS3 where more than 50% and up to 80% of the population live in urban clusters (EUROSTAT, 2018). For these NUTS3, the Panel adopted a conservative approach, assuming 50:50 partition between the population residing in areas resembling urban and rural environments. For example, the Austrian NUTS2 AT12 is divided into seven NUTS3, of which: four (AT121, AT123, AT124 and AT125) classified as ‘Predominantly Rural’, one (AT126) as ‘predominantly Urban’ and two (AT122 and AT127) as ‘Intermediate’. In this case, PopNUTS2i_R and PopNUTS2i_U (designated for Austria as PopNUTS2AT12_R and PopNUTS2AT12_U) are calculated as:
| (5a) |
| (5b) |
If NInfEU is the estimated number of infested units imported in the EU, then the number of infested units reaching a particular NUTS2 (NInfNUTS2) equals:
| (6) |
where NInfNUTS2_R and NInfNUTS2_U are the number of infested units delivered to the population living in rural (PopNUTS2_R) and urban (PopNUTS2_U) part of the NUTS2:
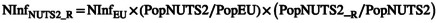 |
(7a) |
| (7b) |
with NinfEU being the number of infested units entering in the EU as estimated in Section 2.1.2, (PopNUTS2/PopEU) is the share of the NUTS2 population in the part of the EU to which the product is transferred, and PopNUTS2 = PopNUTS2_R + PopNUTS2_U.
2.2. Establishment
2.2.1. Climate suitability analysis
To perform the assessment of establishment, information on the global distribution of P. callosus was collected together with information on the climate requirements of the pest. In total 99 confirmed P. callosus occurrences were georeferenced locations. However, the Panel also searched for additional occurrences on GBIF (Global Biodiversity Information Facility) platform. The search resulted in an additional 520 points.
The climate suitability methodology description is available (Golic et al., 2024). In summary, three climate indicators were considered for the analysis: (CI1) absolute minimum soil temperature, (CI2) the hardiness zones to annual minimum temperature and (CI3) the average maximum number of days below the lower development threshold (LDT).
Considering the areas identified by the different climate indicators, and taking into account the biology of the pest, two scenarios for establishment were considered:
-
–
Scenario 1 (SC1): Any area in the EU where climate coincides with at least one of the climate indicators CI1‐3 is considered climatically suitable for P. callosus; i.e. union of CI1‐3. The Köppen–Geiger map was not considered, because this classification is too broad to identify regional climate characterising observed pest occurrences.
-
–
Scenario 2 (SC2): Areas in the EU are considered climatically suitable if the absolute minimum soil temperature is equal or above the minimum value in locations where P. callosus was observed (CI2). The Panel deemed it informative to evaluate this restrictive scenario because large part of the life cycle of P. callosus is completed in the soil and thus, the indicator affects the capacity of the pest to develop and overwinter (see Section 3.2).
2.2.2. Identification of the areas suitable for pest establishment
The high‐resolution gridded climate‐suitability maps obtained in Section 2.2.1 were upscaled to higher administrative levels. To do so, for each NUTS3, the actual number of grid cells of the map valued as climatically suitable was counted and put into percentage of the NUTS3's area. The percentage data were aggregated at NUTS2 level, separately for the rural and urban part. Parts of NUTS2, climatically suitable or not suitable under SC1 and SC2, were considered as the area suitable or not suitable for the pest establishment.
2.2.3. Estimation of the number of founder populations for the EU
The number of founder populations in each NUTS2 (NFPNUTS2) is calculated from those potential founder populations (NPFPNUTS2; Equation 3) that are located in parts of the NUTS2 that are suitable for establishment. Therefore, taking into account the suitable (S) and non‐suitable (NS) shares of the urban and rural parts, the area of a NUTS2 is divided into the four parts (PR) according to (8):
| (8) |
Now for the suitable part of a NUTS2, probabilities of pest establishment – estimated separately for the suitable urban and the suitable rural areas (Table 2) i.e. P(EstablishmentU) and P(EstablishmentR), − are integrated with the number of potential founder populations to calculate the number of founder populations per NUTS2 (NFPNUTS2):
| (9) |
TABLE 2.
Description and source of the evidence of the parameters used to estimate the number of founder population of Phlyctinus callosus into the EU.
| Parameter | Description | Source |
|---|---|---|
| P(EstablishmentR) | Probability of establishment in the climatically suitable RURAL areas of the EU | EKE |
| P(EstablishmentU) | Probability of establishment in the climatically suitable URBAN areas of the EU | EKE |
The total number of founder populations for the EU (NFPEU) can be obtained as the sum of the founder populations in each NUTS2 region (from all the entry pathways).
| (10) |
where ƩNUTS2_MS denotes the summation over all NUTS2 regions of a MS and ƩMS those over all MSs.
When confronted with modelling decisions in the development of the entry model, the Panel adopted a precautionary principle by assuming the most pessimistic (i.e. worst case) scenarios. The uncertainties affecting the entry model are listed in Section 3.5.
2.3. Lag phase and spread
To assess the potential spread after establishment, the Panel assumed that each founder population of P. callosus occupies at the beginning a limited proportion of available habitat due to small population number (i.e. a fraction of the habitat's carrying capacity). Similarly, it is considered that the population growth of P. callosus might be below maximum due to the lack of fitness of the species in a new environment (i.e. Allee effects). Therefore, as the Panel previously proposed (EFSA PLH Panel, 2024a), a lag phase parameter is considered to account for the average duration of the time from establishment to subsequent spread.
At the end of this lag phase, the pest is expected to reach population numbers large enough to enhance expansive spread. Both natural spread (i.e. walking) and human assisted spread by common agricultural practices within a production site (i.e. movement of machinery and equipment) were considered. The average spread rate of the pest was elicited.
In the absence of specific data, the uncertainty distributions characterising the lag phase and spread rate of P. callosus within the suitable regions of the EU, were reflecting consideration of the expected life span, number of generations and the survival rate of the different life stages. In addition, since no quantitative information on P. callosus dispersal capacity could be retrieved, the uncertainty distribution of the spread was based on the behaviour of P. callosus as reported by the consulted experts (Dr. S. Hansen, Dr. E. Allsopp) and the spread capacity of curculionid species with a similar biology and behaviour (i.e. Otiorhynchus spp.).
The uncertainty distributions of the lag phase and the spread rate were fitted to five consensus points obtained by means of EKE.
2.4. Impact
The scientific literature on P. callosus was screened for information on impact of the pest on host plants and its potential role as vector of plant viruses.
Phlyctinus callosus is known as a highly polyphagous species: adults damage the aboveground parts – leaves, shoots, fruit stalks and fruits – of a wide variety of deciduous fruit trees, various berries and a large number of ornamental plants, whereas larvae feed on the underground parts, roots, bulbs and corms (Oberprieler & Zimmerman, 2020; Whittle, 1986). Infestations have been recorded in various cultivated crops, including blueberry (Vaccinium corymbosum), carrots (Daucus carota) and asparagus (Asparagus officinalis) (see Table A.1, Appendix A). P. callosus, however, is regarded as a key pest in apple orchards (Malus domestica) and vineyards (Vitis vinifera) in South Africa, Australia and New Zealand. In fact, information on the fruit‐feeding behaviour and extent of fruit damage by P. callosus is scarce, quantitative data are limited and only available for apples and grapes.
In its current area of distribution, P. callosus has been recorded from various garden plants in South Africa (Haran et al., 2020; Hevin et al., 2022), and various ornamental plants in New Zealand (Scott & Mason, 1984), and Australia (Miller, 1979; Oberprieler & Zimmerman, 2020; Walker, 1981). P. callosus is known to feed on leaves of ornamental flowers and shrubs (Miller, 1979), bulbs and corms (May, 1966; Scott & Mason, 1984) of e.g. Iris xiphium and Cyclamen persicum. The pest is particularly damaging to succulents, such as Sedum spp. Echevaria spp., Faucaria spp., Crassula spp. and cacti, but the host list also includes Callistephus cinensis, Chrysanthemum spp., Dahlia sp., Gerbera jamesonii, Kalanchoe tubiflora, Narcissus pseudonarcissus and Pelargonium sp. ((Whittle, 1986) and references therein) however, quantitative data are missing. In addition, there are various records of the presence of P. callosus on native flowers, made during pre‐export inspections in South Africa (Huysamer, 2018) as well as during import inspections in Japan (Genka & Yoshitake, 2018) and USA (APHIS, 1984). To what extent cut flowers serve as food resources for adults, actual hosts or merely places to hide is uncertain, and quantitative data of the impact of P. callosus on cut flowers are lacking. Therefore, the Panel elicited the uncertainty distribution of the impact of P. callosus on ornamentals, i.e. cut flowers/cut foliage, using the evidence (gathered from literature and opinions from consulted experts) available on the impact on ornamentals by a similar pest, Otiorhynchus sulcatus (F.), known to occur in the EU, as a reasonable proxy. Due to the scarcity of information, informed opinions of experts from the countries where the pest is known to occur and professionals from the ornamental plants production sector in the EU were considered.
2.5. Temporal and spatial scales of the risk assessment
The risk assessment area was the EU territory. The temporal horizon considered for the risk assessment was 10 years (2023–2032). This temporal horizon delimits the scope of the parameter elicitations done by the Panel. Entry was considered as a separate process for each year. No time‐cumulative processes were accounted for in the entry model. The risk assessment was performed considering the current ecological factors and conditions for the host plants growing areas of the EU (risk assessment area) and countries of origin.
3. ASSESSMENT
3.1. Identity and taxonomy
Following recent taxonomic revisions, P. callosus, previously classified under the monotypic P. callosus sensu lato taxon, was proposed as a species complex comprising 8–10 species (Hansen, Haran, et al., 2024). Within this species complex, P. callosus and P. xerophilus seem to be the only two species found associated with commercial agriculture (Haran et al., 2020; Hevin et al., 2022). P. callosus naturally occurs in the coastal areas and valleys of the Western Cape, while P. xerophilus appears restricted to inland valleys and lower mountain slopes. Only some lineages within both P. callosus and P. xerophilus appear to be highly polyphagous and associated with agricultural crops. However, no major differences were observed in behaviour and ecology between the two species, leading to the conclusion that similar control methods have the same efficacy on both species (Hansen, Haran, et al., 2024).
According to a recent taxonomical revision (Hansen, Haran, et al., 2024), no other Phlyctinus species was found associated with agricultural hosts, neither among museum records nor in recent studies. It remains still unclear why only P. callosus and P. xerophilus successfully shifted onto agricultural hosts to the extent of being economically significant pests. Hansen and colleagues suggest to considering all pre‐2020 records of ‘Phlyctinus callosus’ in South Africa inclusive of both P. callosus and P. xerophilus (Hansen, Haran, et al., 2024). Considering the similarity in upper and lower critical thermal thresholds that have been obtained under laboratory acclimation regimes for field‐collected P. callosus and P. xerophilus adults, as well as the similar efficacy of control methods (mainly physical), the naming of P. xerophilus as a pest can be regarded principally as a taxonomic clarification within a species complex. For the scope of this scientific opinion, the name P. callosus will be used, referring to both P. callosus sensu stricto and P. xerophilus.
3.2. Review of pest biology
A description of the biology of the pest is provided in the EFSA pest categorisation (EFSA PLH Panel, 2021), in Barnes (Barnes, 1989b) and the review by Dlamini and colleagues (Dlamini, Addison, & Malan, 2019). Here, we provide a summary overview of the key aspects relevant for the risk assessment.
Host range and number of generations. P. callosus is a polyphagous pest that has been shown to be able to shift to diverse ornamental plants, crops and orchards, in addition to its native asteraceous hosts (Hevin et al., 2022). According to Dlamini and colleagues, P. callosus can have one or two generations per year, depending on the ground cover and the irrigation system used during dry summer (Dlamini, Addison, & Malan, 2019). However, in its natural habitat, away from the irrigated orchards or vineyards, the pest tends to have only one generation per year. Similar information is reported by Barnes (Barnes, 1989a), who indicates the occurrence of two generations per year in orchard fully irrigated during summer. Similarly, in laboratory conditions, eggs were laid at distinctly different periods, suggesting two generations may occur during summer (Giliomee, 1961).
Egg stage. P. callosus adults start egg‐laying approximately 3 weeks after emerging from the soil in summer and continue for 3–6 months. Female adults produce less than 5 eggs/week in the first 4 weeks, subsequently, eggs are usually laid in batches of 20 (Dlamini, Addison, & Malan, 2019) (although up to 70 eggs/week have been observed [CABI, 2020]) in loose organic litter, in small cavities and cracks in bits of bark (Giliomee, 1961), or near the surface of the soil.
In general, eggs viability is reported to be strictly related to high humidity and moisture levels, both in air and soil (Barnes & Swart, 1977). From experimental studies on egg storage and survival, when placed at a constant temperature of 11°C and 14°C, the eggs were found to start hatching, prior to their relocation at 25°C; when stored at 4°C for 70 days, a mean percentage hatch of 45.7% was observed (Ferreira, 2010). In an earlier study, the results showed that freshly laid eggs of P. callosus can be stored for as long as 12 and 10 weeks at 5°C and 8°C without loss of viability (Walker, 1981). In the same study, the author conducted a population growth experiment with eggs placed in sterilised and fertilised soil at constant temperatures of 30, 25, 20, 15 and 10.5°C. Results of the experiment showed that eggs survival ranged from 76% to 86% when stored at 10.5°C and 25°C, when stored at 30°C, egg survival dropped to 1.7%. From these data, the theoretical minimum threshold temperature for development was calculated to be 6.0°C (Walker, 1981).
Larval and pupal stages. Within 1–2 weeks after hatching, the first‐instar larvae burrow into the soil and start feeding on roots or tubers (Ferreira & Malan, 2010; Swart, Barnes, & Greeff, 1976; Swart, Barnes, & Myburgh, 1976). Feeding has been reported on roots of weeds and grasses (Barnes & Swart, 1977), but also on roots of cultivated plants like asparagus (Prestidge & Willoughby, 1989) and roots, bulbs and corms of ornamentals (Scott & Mason, 1984). Most larvae are found in the top 10 cm of soil where they over‐winter progressing through a variable number (6–8) of instars (CABI, 2020; Walker, 1981). In the case of the first generation, pupation occurs in the soil and is reported to last from 1 to 3 weeks; adult emergence from the soil occurs from October to December in South Africa (Dlamini, Addison, & Malan, 2019).
Adult stage. Adults are ~ 7 mm long, greyish‐brown with a bulbous abdomen (CABI, 2020). They cannot fly, but are described as highly mobile and are able to climb on trees (Ferreira & Malan, 2010). Emerged adults feed on fruits, shoots and leaves during the night, while hiding during the day (Barnes & Swart, 1977). Nocturnal feeding is also reported on asparagus (Prestidge & Willoughby, 1989). Adult feeding causes superficial scars on fruits and leaves. During the day adults hide in the leaf litter on the ground in the vicinity of the host plant, or under the bark or in plant material. They climb onto the aerial plant parts during the night to feed on leaves, stems and fruits and to mate. They show thigmotactic behaviour when they are inactive during the day (Barnes, 1989a) and feign death when disturbed (Magagula, 2019). Adults may be found all year round if the habitat remains wet. Longevity decreases with temperature with 15% mortality in the first 100 days after emergence at 15°C compared to 70% mortality at 20°C observed in laboratory trials (Walker, 1981). Expected lifespan of adults in natural environment is 3–4 months on average (Dr S. P. Hansen, personal communication, May 2024). Phlyctinus callosus overwinters at larval stage, although it is reported that mated females can survive the winter (Barnes, 1989a).
Within orchards/vineyards hotspots are reported, where the most damage and weevil numbers occur. This aggregation behaviour is potentially facilitated by a pheromone in adult frass (Barnes & Capatos, 1989).
Influence of temperature and humidity. In areas in their native range where ambient temperatures drop below 0°C during winter, adults of P. callosus can be found in sheltered habitats (Dr S. Hansen & Dr J. M. Haran personal communication, January 2024). Experimental data showed that cooling at temperatures of 1°C is not causing significant mortality to adults, unless combined with prolonged precooling periods or controlled atmosphere (Myburgh & Kriegler, 1968). For example, in one experiment, the survival of 400 specimens stored for 17 days at 1.1°C was 74% after a precooling period of 1 day at −0.5°C; the percentage of survival dropped to 13% when 400 specimens were exposed to a prolonged precooling period of 7 days at −0.5°C and further reduced to 5% when in addition to the pre‐cooling period, the cold storage at 1.1°C was accompanied by SO2 treatment.
Barnes (1987) reports that the numbers of weevils and damage inflicted on a crop can fluctuate drastically from one season to the next, possibly due to different prevailing weather conditions and/or biotic factors like natural enemies (Barnes, 1987). Natural mortality of larvae and pupae seems to be high (Barnes, 1987; Walker, 1981) and some may physically drown or potentially get attacked by soil‐based fungi during very wet winters in poorly drained soils (Dr S. Hansen, personal communication January 2024 (Barnes, 1987)). Under laboratory rearing conditions, the insect was able to increase in numbers over a restricted temperature range and only temperatures above 30°C were observed to be lethal for eggs, and those above 25°C for larvae (Walker, 1981). In field conditions, adults prefer to hide at the base of vines and trunk collars at temperatures between 28 and 32°C, and occur mostly inside grape bunches with cooler temperatures (25–27°C) (Pryke & Samways, 2007). P. callosus adults have shown to be sensitive to heat treatments (Johnson & Neven, 2011). Post‐harvest heat treatments demonstrated mortality rates between 97.5% and 100% after 120 min under 45°C.
In terms of humidity, the presence of Phlyctinus species within the Cape Floristic Region in South Africa seems to be restricted to areas receiving 300–500 mm/year of rainfall (Hevin et al., 2022). Soil moisture and relative humidity is also hypothesised to play a major role in egg‐hatching, larval penetration into soil and therefore larval survival (Barnes, 1989a). In the absence of adequate relative humidity and soil moisture during the growing season (summer in a Mediterranean region), egg‐eclosion is delayed until the first rains, subsequently resulting in larger larval and adult populations later during the year (Barnes, 1989a; Barnes & Swart, 1977).
3.3. Entry
3.3.1. Identification of the relevant entry pathways
Interception data. The Panel consulted the TRACES/EUROPHYT database for the period 1995–2023 and no evidence of official reporting of P. callosus OR Coleoptera OR Curculionidae from the countries where the pest is known to occur (i.e. Australia, New Zealand and South Africa) was found (last check on March 6th, 2024). However, the following additional evidence should be noted:
-
–
The presence of live adults of P. callosus has been reported in table grapes from South Africa imported into the USA as early as 1939 (USDA, 1940), 77 times between 1948 and 1963 (USDA, 1948–1963), 40 times between 1964 and 1970 (USDA, 1964–1970), and more repeatedly since at least the late 1970s (APHIS, 1974, 1979, 1982, 1986).
-
–
One male P. callosus was found in Davis, California USA in 1976 on imported olives (Haran et al., 2020).
-
–
An adult P. callosus was intercepted in cargo with Protea flowers from South Africa in New York USA in 1965 (USDA, 1964–1970), on Protea barbigera (flower) in Illinois USA in 1984 (APHIS, 1984), on Protea sp. (flower) and Protea cynaroides (flower) in Florida USA in 1986 (APHIS, 1987).
-
–
Adult P. callosus have been intercepted on apples imported into the USA from South Africa, e.g. in 1981 (APHIS, 1982) in 1984 in Illinois (APHIS, 1984) in 1986 in Florida (APHIS, 1987).
-
–
Single adults of P. callosus have been intercepted on propagation material from South Africa, in 1972 on Haworthia sp. (succulent) in New Jersey, USA (APHIS, 1974) and between 1973 and 1975 on Villarsia sp. (aquatic plant) in California, USA (APHIS, 1979), as well as in 1981 on consumer material of Crassula sp. (succulent) in the USA (APHIS, 1982).
-
–
P. callosus ranked first in the list of most common quarantine pests intercepted (32 times between October 2000 and September 2003) on table grapes intended for export from South Africa to USA (https://slideplayer.com/slide/4456437/; accessed: 10 December 2023).
-
–
P. callosus has been intercepted once from table grapes imported into New Zealand, between 1987 and 2008 (MAF, 2009).
-
–
P. callosus was intercepted in the UK on apple fruits (Malus) and on peach fruit (Prunus) both from South Africa in 2014 and 2015 respectively (Defra unpublished data).
-
–
P. callosus was intercepted in Ireland on apple fruit for consumption from South Africa during a phytosanitary inspection at the Irish border in May 2020 (Bourke, 2020).
-
–
A single P. callosus adult was found on a potted azalea plant at a public market in Wolverhampton, England, UK, in March 2004 (Smith, 2004).
-
–
Adults of P. callosus intercepted at Paris Charles de Gaulle Airport (France) in March 2021 on a consignment of table grapes from South Africa (Dr J. M. Haran pers. comm. January 2024).
-
–
A paper describing the chronological change of taxonomic composition of exotic weevils (Coleoptera: Curculionidae) found in imported plants at Narita International Airport (Japan) reports 82 interceptions of P. callosus in consignments of various cut flowers (Genka & Yoshitake, 2018).
In 1984, one adult P. callosus was intercepted on a Protea barbigera flower from South Africa in Illinois, USA (APHIS, 1984). In a more recent post‐harvest survey on export Proteaceae cut flowers in South Africa, Huysamer (2018) made 82 insect interceptions, which consisted of 8 orders and 26 families (Huysamer, 2018). The large inflorescence of the Proteaceae and high nectar production contribute to high insect populations, leading to either consignment rejection or mandatory fumigation. From the Coleoptera intercepted, most were single individuals. However, in one case of P. callosus, approximately 21 individuals were found within a single box of Leucospermum ‘Tango’.
P. callosus as a known pest in the country of origin. P. callosus is indigenous to South Africa where it is described as a major pest of grapes, apples and nectarines (Barnes & Pringle, 1989), and sporadic fruit damage on a smaller scale is also reported for pears, plums and peaches (Barnes, 1989a). From South Africa, P. callosus has been introduced onto several islands in the southern hemisphere, including New Zealand and Australia (Kuschel, 1972) where it is reported to attack a range of commercially valuable plants including grapevine, apples, carrots and potatoes (Horne & Stacpoole, 1989) P. callosus is also recognised as a pest of ornamental and garden plants (Haran et al., 2020; Whittle, 1986), feeding on roots, bulbs and corms (Scott & Mason, 1984). P. callosus is known to occur on native flowers in South Africa as well (Huysamer, 2018), but it has not been recorded as a pest.
Entry pathways for P. callosus. Although one specimen has been found on an ornamental Azalea plant in a UK market (Smith, 2004), the actual origin of the pest remains uncertain. The life cycle of P. callosus occurs to a large extent in the soil where eggs are laid, and where the larvae feed on the roots and over‐winter. Taking into account that the current regulatory framework in the EU prohibits the import of soil, the Panel considered the plants for planting entry pathway, including ornamentals, as not relevant. Other interception data strongly suggest table grapes, deciduous fruit and cut flowers, as most relevant entry pathways.
Trade volume. The trade volume of the products associated with P. callosus aggregated by the countries where the pest is known to occur is shown in Figure 1.
FIGURE 1.
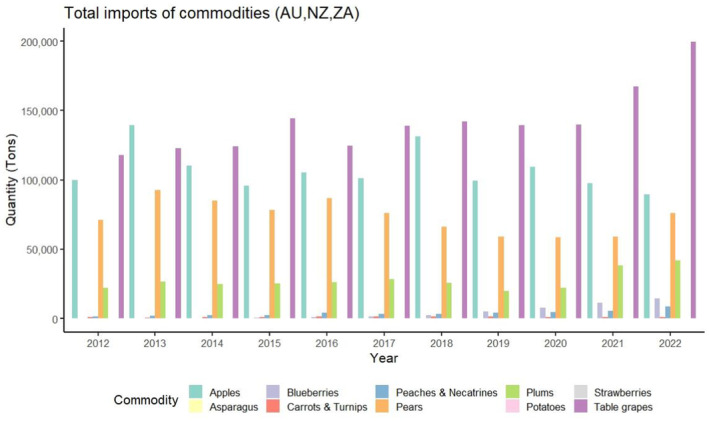
Aggregated import data (2012–2023) for the plant products for which there is evidence of association with Phlyctinus callosus in the countries of origin.
When considering the import data and the plausibility for the host to act as a vehicle for entry, the Panel identified the import of apples, cut flowers and table grapes as relevant entry pathways. A summary of the justification for inclusion or exclusion of the considered pathways is outlined in Table 3.
TABLE 3.
Summary table outlining, for each of plant product whether the Panel considers the import of that commodity as a relevant entry pathway for Phlyctinus callosus in the EU.
| Commodity | Comment | Relevant entry pathway? |
|---|---|---|
| Allium, Asparagus, Carrots, Pastinaca, potatoes, Blackberries and strawberries | No evidence of interception, import data show substantially lower amounts of pears and plums being imported from the countries where the pest is reported, compared to apples or table grapes | No |
| Peaches and nectarines |
While there is one record of interception involving P. callosus on one peach and the pest is reported to damage peaches and nectarines, it is also reported to affect these fruits to a lower extent than apples. In addition, import data show much lower amounts of peaches and nectarines being imported as compared to apples from countries where the pest is reported The Panel considered the import of peaches and nectarines as a less relevant entry pathway when compared to the import of apples or table grapes |
No |
| Pears and plums |
No evidence of interception involving pears or plums from countries where the pest is known to occur; although P. callosus is reported to damage pears and plums, it is also reported to affect these commodities to a lower extent than apples. In addition, import data show substantially lower amounts of pears and plums being imported from the countries where the pest is reported, compared to apples or table grapes The Panel considered the import of pears and plums as less relevant entry pathways when compared to the import of apples |
No |
| Table grapes, apples | Evidence of interception and significant trade volume from countries where P. callosus is known to occur | Yes |
| Cut flowers | Evidence of interception and significant annual trade volume from countries where P. callosus is known to occur, in particular Cape flora cut flowers, with foliage, from South Africa | Yes |
3.3.2. Parameters of the entry model
3.3.2.1. Trade volumes ( T V )
Annual trade data (2012–2022) for the plant products identified as relevant entry pathways for P. callosus (apples and table grapes) from the countries where the pest is reported, were retrieved from EUROSTAT (disaggregated country data are reported in detail in Appendix B, Tables B.1, B.2). The Panel assumed that the yearly average volume of trade in the time horizon would be similar to the trade in the recent past (2012–2022) and can be adequately described as a normal distribution fitted to trade data (Table 4). The Panel acknowledges that in reality, trade may increase or decrease quickly in response to markets and consumer preferences. This uncertainty was not accounted for but was considered small compared to uncertainty in other parameters of the pathway model. It is reminded to the readers that the trade volume for cut flowers is not reported because of the different approach being used for this commodity (see Section 2.1.2).
TABLE 4.
Percentiles of the normal distribution assumed to describe the parameter trade volume (T V ) for the table grapes and apples.
| Parameter | Percentile (%) | ||||
|---|---|---|---|---|---|
| 1 | 25 | 50 | 75 | 99 | |
| T V – Table grapes (tons) | 86,990.0 | 125,941.7 | 141,846.4 | 157,751.2 | 196,702.8 |
| T V – Apples (tons) | 71,633.7 | 96,812.1 | 107,093 | 117,373.9 | 142,552.3 |
3.3.2.2. Infestation rate
The infestation rate of the fruit at the point of departure in the country of origin was elicited considering: (i) the biology of the pest, (ii) the harvest time in relation to the pest life cycle, (iii) the pre‐harvest management (monitoring and control) and (iv) the expected pest behaviour during harvesting and post‐harvest processing. Results and justifications are reported in detail in the Appendix C.1, the results of the fitting of the consensus values are summarised in Table 5.
TABLE 5.
Percentiles of the distributions of uncertainty of the parameter infestation rate for apples and table grapes bunches.
| Parameter | Percentile (%) | ||||
|---|---|---|---|---|---|
| 1 | 25 | 50 | 75 | 99 | |
| Infestation rate of P. callosus on apples (per 10,000 apples) | 0.000002 | 0.0003 | 0.001 | 0.008 | 1.5 |
| Infestation rate of P. callosus on table grapes (per 10,000 bunches) | 3 | 22 | 52 | 122 | 973 |
3.3.2.3. Probability of survival
As a starting point, the Panel considered that should P. callosus be present in the exported units, the probability for the pest to survive the transport from the country of origin is a function of the transport time, temperature and treatments (if any). Experimental data by Myburgh and Kriegler (1968), reporting the survival of P. callosus in packed table grapes under: (i) continuous exposure to T° of ≈ −0.5°C (31°F) and ≈1.1°C (34°F) and (ii) continuous exposure to T° of ≈ − 0.5°C (31°F) + treatment with SO2 (Table 6) were used to infer the probability of P. callosus survival during transport of apples and table grapes (Myburgh & Kriegler, 1968).
From the experimental data presented in Table 6, the Panel established P(Survival) for apples and table grapes under the following reasonings:
Apples are transported at 0.5 ± 0.5°C for 18–23 days (average of 20.5 days considered); for this temperature a precise probability of P. callosus survival cannot be inferred from the experimental data in Table 6 because of the unknown survival rates at temperatures of 0 and 0.5°C. The Panel therefore referred to the worst‐case scenario with experimental data for apples during transport i.e. P(Survival) = 0.94 for P. callosus exposed to ≈ 1.1°C for 21 days in Table 6.
Table grapes are currently transported at −0.5°C for 18–23 days (average of 20.5 days considered), and with SO2 generating pads inserted in the boxes as a standard operation procedure to protect table grapes intended for export from infection by the fungus Botrytis cinerea (de Aguiar et al., 2023).
Table 6 shows a percentage of survival ranging between 5% and 2% when cold storage is preceded by pre‐cooling and between 24% and 15% after 18 and 21 days of storage in the small‐scale experiment with table grapes packed with polyethylene liners under continuous exposure to −0.5°C. Both the experiments after 18 and 21 days at T ≈ −0.5°C + SO2 resulted in a percentage of P. callosus survival of 1%. From these experimental data, the Panel described the probability of P. callosus survival in table grapes during transport as a uniform distribution from 0.01 to 0.24.
Recently, the effect of SO2 and cooling (+1°C) during storage of table grapes was tested on various insects (Tomkins, 2018). In that report, the authors recorded 100% mortality of the long‐tailed mealybug (Pseudococcus longispinus (Tagioni Tozzetti)), a ladybird beetle (Chilocorus sp.) and a the dried‐fruit beetle (Carpophilus hemipterus (L.)) in table grapes stored for 14–17 days at 1°C with or without an SO2 generating pad placed inside the cartons. However, 100% mortality was achieved only after 28 days of cold storage (with or without a SO2) for the Argentine ant (Linepithema humile (Mayr)) and storage for 28 days (with or without an SO2) resulted in 0 and 91.7% mortality for the European earwig (Forficula auricularia (L.)) and 96.7% and 94.4% for the two‐spotted spider mite (Tetranychus urticae (Koch)).
These results indicate that insect genera can exhibit diverse tolerances. While it cannot be excluded that the survival of P. callosus at temperatures close to 1°C could be in fact lower than the assumed 94% for apples, the Panel could not find specific evidence for P. callosus to justify assuming a lower value.
3.3.2.4. Redistribution model
The results of the redistribution model for apples, cut flowers and table grapes are reported in Figure 2. The output of the redistribution model for each MS is the percentage of product imported into the EU from the countries where the pest is known to occur that arrives in the MS. As explained in Section 2.1.2.1, cut flowers are assumed to follow the redistribution of the cut roses as estimated in (EFSA PLH Panel, 2024b).
FIGURE 2.
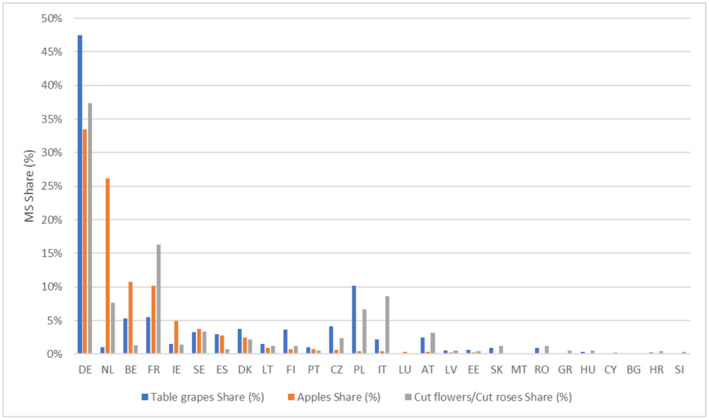
Percentage of product (apples, table grapes and cut flowers) imported into the EU that arrives in the Member States. The median values of the redistribution model are reported, cut flowers are assumed to follow the redistribution of cut roses as estimated in a recent EFSA Opinion (EFSA PLH Panel, 2024b).
3.3.2.5. Probability of transfer
The probability of transfer for a mated female of P. callosus was elicited considering the situation that apples, cut flowers and table grapes are purchased by consumers living either in predominantly rural or urban areas of the EU. The Panel proceeded with the elicitation of the uncertainty distribution describing the probability of transfer for infested units in the rural area and continued with the elicitation of a conversion factor ‘γ’ describing the uncertainty in the orders of magnitude the estimate of the rural area should be corrected for the urban area: P(TransferU) = P(TransferR) × 10γ.
Factors considered during elicitation of both parameters were: (i) temperature at the time of arrival in the EU (trade windows), (ii) transport time and temperature from the countries of origin (fitness of the pest), (iii) expected maximum walking distance (walking ability), (iv) expected presence of suitable hosts at the time of arrival within the walking distance, (v) factors such as physical barriers and predators along the way to the suitable host (survival of P. callosus along the way to the suitable host). Results and justifications are reported in detail in Appendix C.2, the results of the fitting of the consensus values are summarised in Table 7.
TABLE 7.
Percentiles of the distributions of uncertainty of the parameter probability of transfer for pest individuals in the rural and the conversion factor γ in the urban area.
| Percentile | Percentile (%) | ||||
|---|---|---|---|---|---|
| 1 | 25 | 50 | 75 | 99 | |
| Successful transfer rate in the rural area (per 10,000 mated females) | 4.4 | 5 | 10 | 29 | 114 |
| Conversion factor (order of magnitude, γ) | −4 | −3.1 | −2.4 | −1.3 | −1.1 |
3.3.3. Entry assessment results
Key results from the entry pathway model are the number of potential P. callosus founder populations per year in the EU shown in Table 8. A potential founder population is represented by a mated P. callosus female that has reached a suitable oviposition site and laid eggs on a host plant (see Section 2.1.1) The potential founder populations are distributed across MSs according to (i) the redistribution model (see Section 2.1.2.1); and (ii) within each MS, proportional to the population size in the rural and urban areas of each NUTS2 region as an indicator of potential demand.
TABLE 8.
Percentiles of the output distribution for the total number of potential Phlyctinus callosus founder populations per year in the EU (NPFPEU).
| Parameter | Percentile (%) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 5 | 25 | 50 | 75 | 95 | 99 | |
| NPFPEU | 1.8 | 4.0 | 16.5 | 49.5 | 159.2 | 881.2 | 2752.7 |
From the contribution of all the considered entry pathways, the model estimated a median number of 49.5 potential founder populations of P. callosus (90% CR: 4.0–881.2) per year in the EU. From the partial results of all pathways, it can be appreciated how the risk of entry is driven by the table grapes (Table 9).
TABLE 9.
Percentiles of the output distribution for the total number of potential Phlyctinus callosus founder populations per year in the EU (NPFPEU) for the considered entry pathways.
| Entry pathway | Percentile (%) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 5 | 25 | 50 | 75 | 95 | 99 | |
| Apple | 1.4 × 10−5 | 1.7 × 10−4 | 2.9 × 10−3 | 0.02 | 0.11 | 2.01 | 22.91 |
| Cut flowers | 0.48 | 0.49 | 0.56 | 1.13 | 3.21 | 8.87 | 12.72 |
| Table grapes | 1.11 | 3.13 | 14.96 | 46.72 | 153.01 | 861.97 | 2653.50 |
The median value of the output distribution for the table grapes entry pathway was estimated at ~ 47 potential founder populations per year. In contrast, the median values of the output distributions for the other pathways indicate the entry of 1 potential founder population every ~ 57 years for apples and ~ 1 potential founder population/year for the cut flowers. The values at the 99th percentile of the output distributions for these pathways are about 23 and 13 potential founder populations per year. Both these values are lower than the median value of the output distribution of table grapes, providing some reassurance about the worst‐case conditions assumed for the cut flower entry pathway. The predicted median number of potential founder populations per MS is shown in Figure 3 where the median value and the 90% CR are reported. Results by NUTS2 are made available in the Excel file as supplementary material.
FIGURE 3.
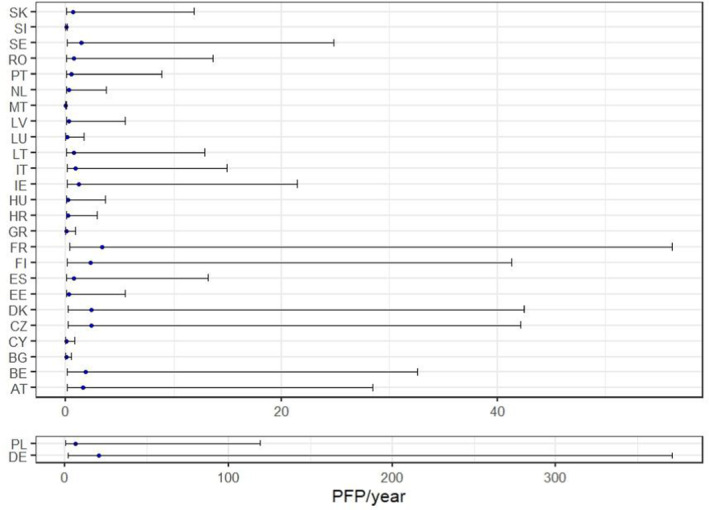
Graphical representation of the median number (blue dot) and 90% credible range of the potential founder populations per Member State as a result of the redistribution model and probability of successful transfer to a suitable host. Results for Germany (DE) and Poland (PL) are shown separately due to the different scale of the results.
From the predicted distribution of potential founder populations, it can be appreciated how many of the infested units originating a potential founder populations of P. callosus are predicted in areas of the EU where the establishment is considered negligible under SC2 but not under SC1 (see maps in Section 3.4.3).
3.4. Establishment
3.4.1. Background information and host distribution
The extensive literature search and the inclusion of GBIF distribution data yielded 619 specific geographic coordinates (directly reported or reporting enough information to obtain coordinates from Google Earth) (Golic et al., 2024). The obtained distribution is displayed in the map shown in Figure 4 and shows that all the data points consistently indicated the presence of P. callosus as limited to Australia, New Zealand, South Africa and several islands in the southern hemisphere.
FIGURE 4.
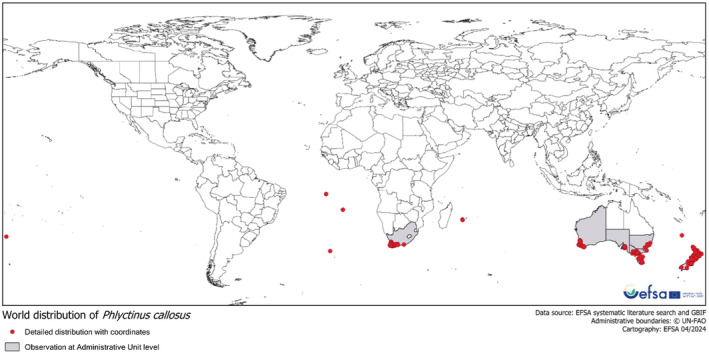
Map showing the location points (red) where precise coordinates for the presence of Phlyctinus callosus could be obtained overlayed to the areas (grey background) where the presence of the pest was only reported at a FAO.GAUL Administrative 0, 1 or 2.
3.4.2. Climate suitability analysis
With the obtained distribution, the Panel identified the areas suitable for the establishment of P. callosus in the EU by combining different climate indicators.
Köppen–Geiger climate comparison. The climate types present in the observed locations of P. callosus were identified and mapped. For the climate matching, the Panel decided to use only the locations for which a point observation (indicated with red dots in Figure 4) was available. This is because the use of larger administrative units (i.e. FAO GAUL 2 or 1) was judged of too low resolution and poor specificity for the purpose of climate matching. P. callosus has been observed in the following Köppen–Geiger climate types that also occur in the EU: hot semiarid (BSh), cold semiarid (BSk), humid subtropical (Cfa), oceanic (Cfb), Mediterranean hot summer (Csa) and a Mediterranean warm summer climate (Csb) (Appendix D.2, Figure D.2).
Absolute minimum soil temperature. The map of absolute minimum soil temperature (used to identify the areas suitable for establishment in scenario 2, Figure 6) shows the areas where the absolute minimum soil temperature is equal or higher than the minimum observed based on the pest occurrence (0.53°C in Te Anau, New Zealand). The areas potentially suitable for P. callosus in the EU are Republic of Ireland, Portugal, Spain, Western and Southern France, Coastal Northern, Central and Southern Italy, coast of Croatia, Malta, Cyprus and Greece.
FIGURE 6.
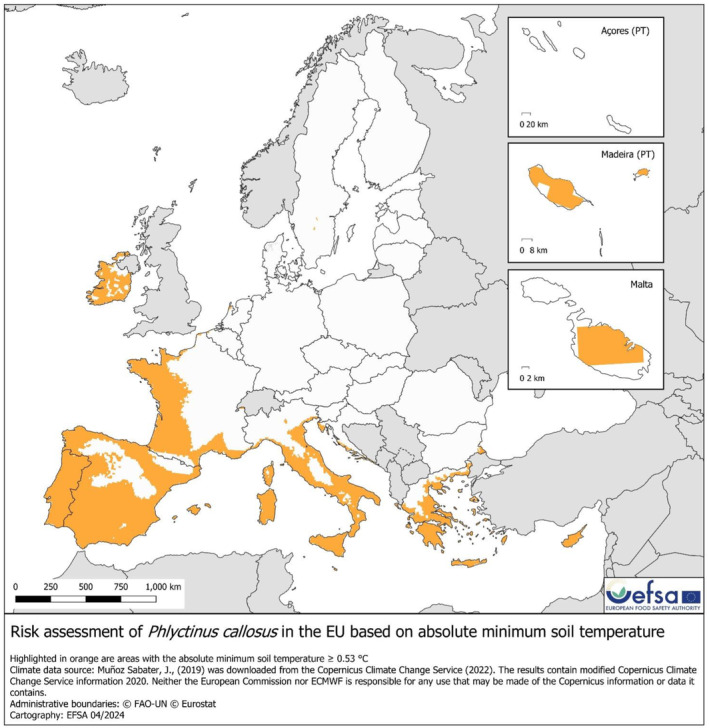
Map of the EU showing the areas where the absolute minimum soil temperature is equal or higher the observed minimum (0.53°C in Te Anau, New Zealand). In orange are the areas where the soil temperature is above the threshold, and in the Scenario2 (SC2), this corresponds to the area where establishment is assumed to be possible.
Hardiness zone. The map represents the area where the 30‐year average absolute minimum air temperature per year is equal or higher than the minimum observed temperature, based on pest occurrence (−7.51°C in Geraldine, New Zealand). This value is categorised in 26 classes. The threshold recovered from the worldwide distribution data showed that P. callosus occurs in areas included in the 8b:13b cold‐hardiness zones (Appendix D, Figure D.3).
Maximum number of consecutive days below the LDT. The map of the maximum number of consecutive days below the LDT (6°C, (Walker, 1981)) shows the area where the average maximum number of days below the LDT is equal or less than the observed maximum number of consecutive days (84.53 days in Geraldine, New Zealand) (Appendix D, Figure D.4).
3.4.3. Identification of the regions suitable for establishment
As explained in Section 2.2.1, the Panel identified two scenarios:
-
–
Scenario 1 (SC1): Where the area in the EU suitable for pest establishment is identified by a ‘Union’ map that overlays the hardiness map, the absolute minimum soil temperature map and average maximum number of days below the LDT map (Figure 5).
-
–
Scenario 2 (SC2): Where the area in the EU suitable for pest establishment is identified based on absolute minimum soil temperature map (Figure 6).
FIGURE 5.
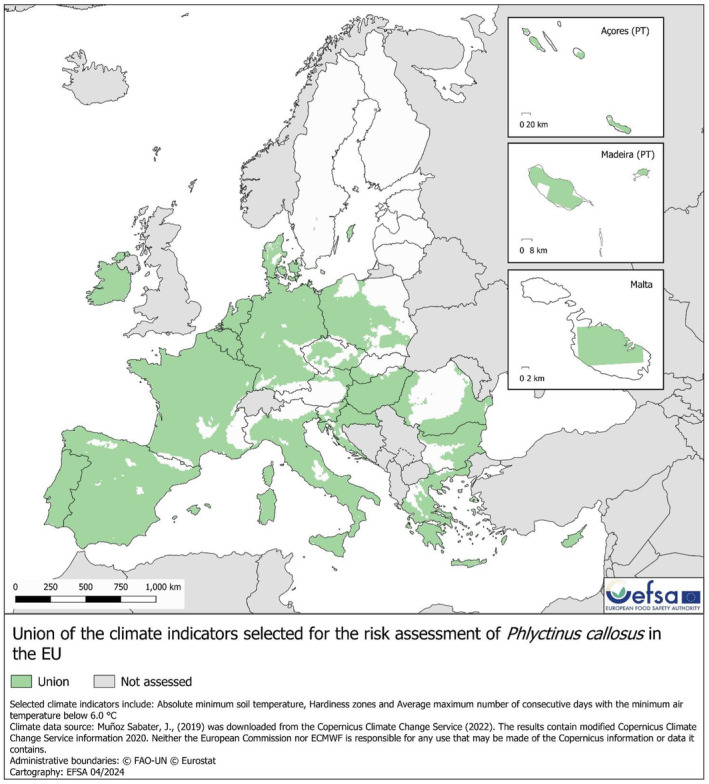
Map of the EU showing the result of overlapping absolute minimum soil temperature, hardiness zones, and the average maximum number of days below the LDT. In light green (‘Union’), the areas of the EU where at least one of the considered climate indicators coincides with those where Phlyctinus callosus was observed in the countries of its current distribution, and in the Scenario 1 (SC1), this corresponds to the area where establishment is assumed to be possible.
For both the scenarios, information at the grid level were summarised at NUTS3 level to identify the administrative units potentially suitable for establishment and extract the percentage of area at risk for each NUTS2 as explained in Sections 2.2.2 and 2.2.3.
The probability of establishment for the rural and the urban areas of the EU was elicited as an establishment rate per 10,000 potential founder populations and considered factors such as the Allee effect, the cold stress experienced by the P. callosus specimens due to the transport conditions and how these might have had an impact on the reproductive performance and the expected survival of adults and immature stages in the two environments. Results and justifications are reported in detail in Appendix D.3; the results of the fitting of the consensus values are summarised in Table 10.
TABLE 10.
Percentiles of the distributions of uncertainty of the parameter establishment rate (out of 10,000 potential founder populations) for the rural and urban areas of the EU with climatically favourable conditions for Phlyctinus callosus.
| Percentile | Percentile (%) | ||||
|---|---|---|---|---|---|
| 1 | 25 | 50 | 75 | 99 | |
| Establishment rate (Rural) | 14 | 96 | 212 | 468 | 3253 |
| Establishment rate (Urban) | 2 | 22 | 58 | 155 | 1714 |
3.4.4. Number of founder populations
From the contribution of all the considered entry pathways, the median number of P. callosus founder population for the EU (NFPEU) was estimated for both the scenarios (Table 11) as described in Section 2.2.1.
TABLE 11.
Percentiles of the output distribution for the total number of Phlyctinus callosus founder populations per year in the EU (NFPEU) in the areas suitable for pest establishment under scenario 1 (SC1) and scenario 2 (SC2).
| Parameter | Percentile (%) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 5 | 25 | 50 | 75 | 95 | 99 | |
| NFPEU – SC1 | 0.01 | 0.03 | 0.20 | 0.75 | 3.05 | 23.35 | 92.77 |
| NFPEU – SC2 | 0.00 | 0.004 | 0.02 | 0.08 | 0.33 | 2.50 | 9.79 |
In the scenario where the areas in the EU that are suitable for the pest establishment are identified by combination of different climatic indicators (SC1), the model predicted a median of 1 founder population every 1.3 years (90% CR: 1 every 30.8 years to 23.3 per year). The location of the founder populations under the conditions of SC1 are shown in Figure 7 where the median value and the 90% CR for each MS are reported.
FIGURE 7.
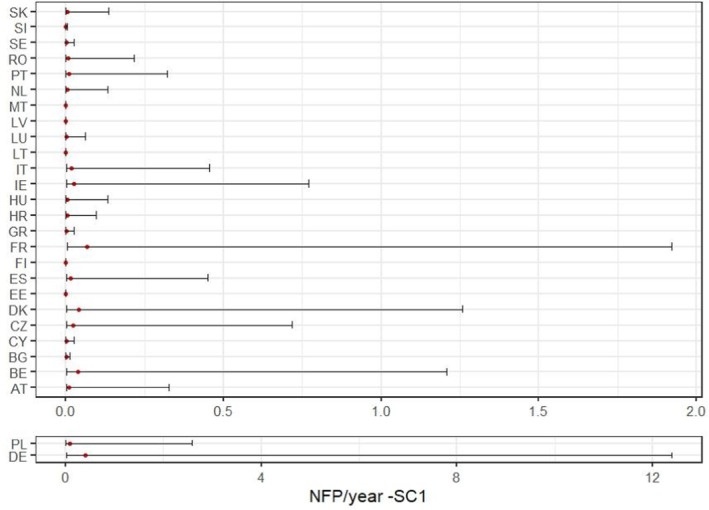
Graphical representation of the median number (red dot) and 90% credible range of the founder populations per Member State as a result of the redistribution model and the probability of establishment under the ‘Union’ conditions of Scenario 1 (SC1). Results for Germany (DE) and Poland (PL) are shown separately due to the different scale of the results.
When considering the scenario where the areas suitable for establishment in the EU are identified on the basis of the minimum soil temperature (SC2), the model predicted a median of 1 founder population every 11.9 years (90% CR: 1 every 256.6 years to 2.5 per year). The location of the founder populations under the conditions of SC2 is graphically shown in Figure 8 where the median value and the 90% CR for each MS are reported.
FIGURE 8.
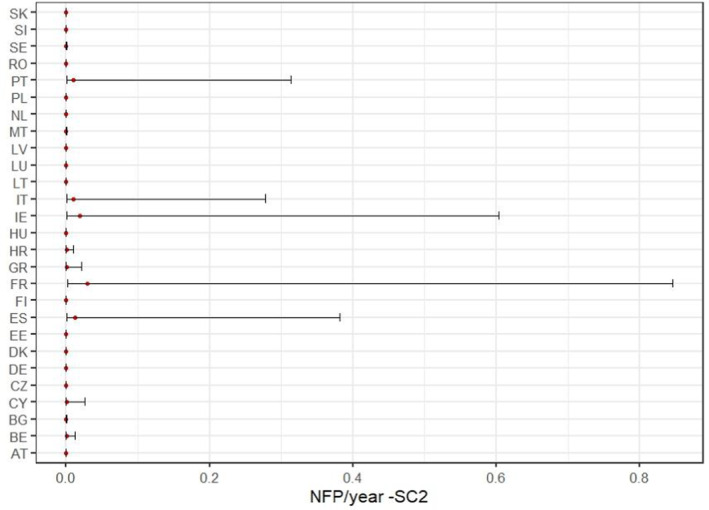
Graphical representation of the median number (red dot) and 90% credible range of the founder populations per Member State as a result of the redistribution model and the probability of establishment under the ‘soil temperature’ conditions of Scenario 2 (SC2).
3.4.5. Sensitivity analysis
A sensitivity analysis was conducted to estimate the correlations between the output variable (NFPEU) and the parameters of the entry pathway model. Correlations were computed using the Spearman rank coefficient which is nonparametric and able to compute both linear and nonlinear relationships between parameters and outputs. Results (Figure 9) show that the highest correlations were obtained for the following parameters:
-
–
The infestation rate in table grapes.
-
–
The probability of establishment in the rural areas,
-
–
The probability of transfer to a suitable host in the rural area.
FIGURE 9.
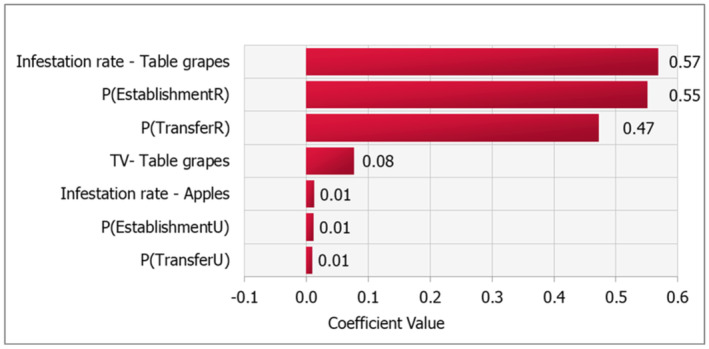
Correlations between the output variable (NFPEU) and the parameters of the entry pathway model.
Therefore, to reduce uncertainty regarding entry risk, it would be a priority to collect more information about these parameters.
3.5. Uncertainties affecting entry
-
–
For consignments arriving in the EU, there is uncertainty on the chance of P. callosus detection in case of an infestation; in addition, there is no obligation to notify interceptions of non‐quarantine pests.
-
–
The identification of the most relevant entry pathways was based on the available interception data and the fulfilment of a set of logical criteria. This led the Panel to consider the import of cut flowers, apples and table grapes as relevant entry pathways for P. callosus in the EU. Nonetheless, for commodities not considered as a relevant entry pathway, trade volume might increase in the future.
-
–
Trying to model accurately the destination of goods imported from third countries is challenging due to lack of the intra‐EU trade at a sufficient level of resolution. For the commodities where data allow (apples and table grapes), the Panel elaborated a specific redistribution model that tries to capture the general trends emerging from the combination of the import, export and production data of each MS. While representing a source of uncertainty itself, the Panel considered that for these commodities, this modelling approach would provide a more representative figure of the real flows as compared to the distribution based on population size of the MSs.
-
–
Due to the lack of sufficient data, the flows of the cut flowers across the MSs and NUTS2 were assumed as comparable to that of the cut roses estimated as part of a recent Opinion (EFSA PLH Panel, 2024b).
-
–
The urban–rural typology classification at NUTS3 level is based upon a preliminary classification of raster cells of 1 km2 followed by agglomeration and adjustment procedures; the shares of population living in urban clusters within predominantly rural NUTS3 and thinly populated areas within predominantly urban NUTS3 represent sources of uncertainty that the Panel did not quantify.
-
–
A full entry pathway model for the cut flowers was not possible due to the absence of specific quantitative data at a sufficient level of resolution (e.g. the total number of consignments and number of pieces per consignment). Furthermore, the number of imported species of cut flowers is considerable. However, obtaining detailed data on production location and method of all imported species of cut flowers and how these might impact the probability of P. callosus being present on these commodities was not feasible.
-
–
The proportion of infested units discarded before reaching the consumers for various reasons (e.g. wasted) was not quantified.
-
–
Adults of P. callosus have been repeatedly intercepted on table grapes and other fruit; however, it remains unclear whether the pest was ON the fruit/IN the grape bunches or on the crates/packaging material. The Panel considered the most pessimistic scenario (higher risk) assuming all the specimens are either within a bunch of table grapes or on an apple fruit and reach the consumer.
-
–
Storage and transport conditions (e.g. modified atmosphere at different levels of O2 and CO2, gaseous ozone (O3), ozone in water, hot water or vapour heat treatments), could be used (de Aguiar et al., 2023) and affect P. callosus survival; however, no specific data could be retrieved.
4. SPREAD
4.1. Assessment of lag phase and spread
For the duration of the lag phase, it was considered that in its native area P. callosus has one generation per year in natural ecosystems and up to two generations per year in agricultural areas where irrigated orchards provide food and moisture during the summer. The pest is polyphagous, so larvae can develop feeding on the roots of various broadleaved plants and a mated female can produce 20–70 eggs. Egg survival rates range between 76% and 86% at 10–25°C, but above 30°C survival drops to 1.7% (Ferreira, 2010; Walker, 1981). The cryptic behaviour and nocturnal activity of the pest may allow it to remain undetected in agricultural systems that are not monitored for similar pests. In addition, the similarity of symptoms on above‐ground plant parts with those caused by other weevils (e.g. Otiorhynchus spp.) may delay the detection of P. callosus.
For the spread rate, it was considered that P. callosus is a slow‐moving, flightless insect, which tends to aggregate in certain spots where it mates, feeds and shelters. Closely related curculionid species were used as proxy organisms to estimate the spread rate of P. callosus adults by their natural capacity. Possible human‐assisted spread within the same vineyard/orchard by machinery use (assuming good agricultural practices) during harvest was also considered as a possible means of spreading.
The median duration of the lag period in the regions where P. callosus could potentially establish in the EU was estimated to be approximately 4 years (90% CR 1.3–8.7 years). After the lag period, P. callosus populations were estimated to spread in those areas at a rate of 15.5 m/year (90% CR 5.1–46.8 m/year). Justifications are reported in detail in Appendix C.4; the results of the fitting of the consensus values are summarised in Table 12.
TABLE 12.
Percentiles of the distributions of uncertainty of the parameters lag phase and spread rate of Phlyctinus callosus.
| Parameter | Percentile (%) | ||||
|---|---|---|---|---|---|
| 1 | 25 | 50 | 75 | 99 | |
| Lag phase (years) | 1.0 | 2.5 | 4.0 | 6.0 | 10.0 |
| Spread rate (m/year) | 3.2 | 9.8 | 15.5 | 24.4 | 74.1 |
4.2. Uncertainties affecting lag phase and spread
Uncertainties affecting the lag phase and spread of P. callosus in suitable areas of the EU include:
-
–
To what extent data on closely related weevil species, of which the larvae are also root feeders and adults are flightless foliage feeders, can be used as a proxy for P. callosus.
-
–
To what extent the pest‐specific dispersal capacity, which remains poorly understood, and the impact of host species communities, including species composition, patch distribution, distance between suitable patches and availability within EU environments can be related to observations from the insect's native area. Detailed assessment of these factors is hindered by insufficient information.
-
–
The actual number of generations of P. callosus per year, which is related to summer precipitation/crop irrigation.
5. IMPACT
The potential impact of P. callosus in terms of crop yield loss was assessed for an area where establishment has occurred and under the following defining characteristics:
-
–
The pest has spread throughout the suitable regions of the EU to its maximum extent. In each location where the pest is found, its abundance is balanced with available resources and environmental conditions.
-
–
P. callosus has established itself in a climatically suitable area for a prolonged period, reaching its carrying capacity and exerting maximum impact.
-
–
Data on percentage of fruit yield from control plots of field trials, extracted from literature, are assumed to represent a good approximation of the yield losses that can be expected when no specific control measures are in place.
-
–
The agricultural practices and management options mirror those currently implemented within the potential pest distribution areas of the EU.
-
–
The efficacy of existing monitoring and control measures against other pests is taken into consideration. This involves assessing the additional burden P. callosus would impose on top of existing pest pressures in the EU.
The average yield reduction directly attributable to P. callosus in apples, cut flowers/foliage and table grapes in the regions where P. callosus could potentially establish in the EU was assessed in separate EKEs. Results of the elicitations are summarised in Table 13. The evidence and justifications which support the elicited uncertainty distributions are reported in detail in Appendix C.5.
TABLE 13.
Percentiles of the distributions of uncertainty of the impact of Phlyctinus callosus on yield reduction (%).
| Parameter | Percentile (%) | ||||
|---|---|---|---|---|---|
| 1 | 25 | 50 | 75 | 99 | |
| % Apple yield reduction | 2 | 3.4 | 5.2 | 7.8 | 13.9 |
| % Cut flowers/foliage production loss | 0.01 | 0.08 | 0.5 | 1.5 | 3.0 |
| % Table grape yield reduction | 1.3 | 1.5 | 2 | 3 | 7 |
5.1. Uncertainties affecting impact
The main uncertainties affecting the assessment of impact include:
-
–
Quantitative information on yield losses directly attributable to P. callosus is scarce, mostly available from a collection of studies conducted in South Africa from the ‘70s to mid ‘90s (for apples) and a project report of 2011 from Australia (for grapes). There is uncertainty on the transferability of the evidence from these countries to the situations in the EU, particularly in relation to fruit varieties and management practices.
-
–
The cosmetic damage, caused by feeding activity of P. callosus, that could render fruits unmarketable, depends on the tolerance of each fruit variety. Tolerance of cosmetic damage of fruits also depends on the market and the actual level of production (higher cosmetic standards when production is high). This tolerance may change in the future. There is uncertainty about the actual cosmetic damage P. callosus could cause to commercial cultivation of ornamental plants.
-
–
Crop management practices, such as insecticide applications targeting other insect pests (e.g. Anthonomus pomorum or mandatory control of Scaphoideus titanus [Regulation 2022/1630]), type of pruning of the trees and weed control affecting the level of damage by P. callosus.
-
–
Phlyctinus callosus is mentioned as a pest of ornamental plants and has been intercepted on cut flowers, but no quantitative evidence of impact (either as economic or production loss) emerged from the scientific literature. The assessment of the impact on cut flowers/foliage was based on the assumption that Otiorhynchus sulcatus would provide a reasonable proxy for the expected impact of P. callosus on this commodity in the EU. However, the extent to which this is a valid assumption remains uncertain.
-
–
Uncertainty remains regarding to what extent there will be impact on crops not considered as relevant entry pathways.
6. POTENTIAL RISK REDUCTION OPTIONS
Specific import requirements for relevant host commodities can be identified as risk reduction options (RROs) for countries or areas where P. callosus is present. This may be stand‐alone RROs or may be combined in a system approach, which according to ISPM 14 requires two or more measures that are independent of each other and may include any number of measures that are dependent on each other.
Pre‐harvest RROs
Cultural control is important for decreasing adult emergence from the soil. Destruction of weeds and grasses reduces the larvae population that feeds on their roots and eventually the abundance of adults in the fields. In addition, restricting the routes for reaching the fruits on a plant by pruning host plant branches hanging down to the undergrowth or soil, is considered as a useful management tactic. Application of kaolin particles is also considered for discouraging adult crossing and feeding.
The use of trunk barriers is considered as highly effective in reducing the number of adults reaching the plant canopy where feeding adults cause damage to host plants. The barriers are installed at a lower part of the trunk and it is usually a strip of fibre (batting), most times soaked in a synthetic pyrethroid, or a sticky plate (Dlamini, Addison, & Malan, 2019). The use of trunk barriers is a requirement for the export of table grapes to some countries (Opatowski, 2006).
As biological control options, in the last decades, efforts are orientated to control the pest using products based on entomopathogenic nematodes (Heterorhabditis sp.) which seem to achieve high mortality for all life stages of P. callosus (Dlamini, Addison, & Malan, 2019; Ferreira & Malan, 2010). Entomopathogenic fungi (Beauveria bassiana and Metarhizium anisopliae) are also currently evaluated (Dlamini et al., 2020a; Dlamini, Malan, & Addison, 2019; Hansen, Malan, et al., 2024). However, the efficacy of commercial available products in the EU on P. callosus is unknown. Recently, Cleruchus depressus (Annecke) (Hymenoptera: Mymaridae) was found parasitising eggs of P. callosus in South Africa (Barnes, 2014); however, what impact it may have on populations of P. callosus, needs further study. To what extent natural enemies affect the population dynamics of P. callosus is largely unknown. Generalist predators, such as helmeted guineafowl or chickens, which feed on the adults, larvae and pupae in the soil, have been reported to reduce the weevil's population below the economic threshold on some occasions (Pringle et al., 2015), but overall have a negligible impact on large weevil populations (Witt et al., 1995).
Several pesticides are also used for chemical control of P. callosus in orchards and vineyards. The most common insecticide group currently used is synthetic pyrethroids targeting adults with variable efficacy. The pest, however, is also known to be capable of developing resistance to pyrethroids. In South Africa currently two to three insecticide applications per seasons are targeting P. callosus (Dlamini, Addison, & Malan, 2019).
A pest‐free place of production can be identified following ISPM 10 particularly for crops growing in greenhouse conditions where entry of adults is restricted. Pest freedom in places of production should be substantiated by surveys and/or growing‐season inspections. All the operations need to be supported by appropriate documentation.
Post‐harvest RROs
Post‐harvest control could include several RROs.
Inspections of commodities prior to export to detect and exclude infested consignments. Official inspections of consignments for the presence of P. callosus should be in accordance with International Standards for Phytosanitary Measures (ISPM 1).
The thermal biology of the species has not yet been studied thoroughly. However, experiments have shown that at cold storage conditions of −0.5°C and with simultaneous application of SO2 the mortality observed could be substantial. Table grapes for instance are currently transported under conditions of low temperature and SO2 emission to achieve significant mortality of pests and diseases, incl. P. callosus adults (de Aguiar et al., 2023). For Cape flora cut flowers (Proteaceae) this does not apply; there the effect of controlled atmosphere and (high) temperature treatment systems (CATTS) and ethyl formate (EF) fumigation are currently under investigation to establish the efficacy on problematic phytosanitary pests, incl. P. callosus, while maintaining post‐treatment flower quality (Huysamer, 2016; Huysamer, 2018; Huysamer et al., 2017; Ngwenya, 2021). Gamma irradiation is considered as an efficient post‐harvest control with no side effects on fruit quality (Duvenhage & Johnson, 2014).
7. CONCLUSIONS OF THE PRA
Following the previous pest categorisation conducted on P. callosus (EFSA PLH Panel, 2021), this quantitative PRA confirms the potential of this pest for entry, establishment, spread and impact in the EU. Following a prioritisation approach based on interception data and the biology of the pest, the Panel considered three entry pathways for P. callosus in the EU as relevant: import of cut flowers, apples (Malus domestica) and table grapes (Vitis vinifera) from Australia, New Zealand and South Africa. The identification of the most relevant entry pathways was based on the available interception data and the fulfilment of a set of logical criteria. While P. callosus has been observed on a range of other host plants, at time of writing the Opinion, the set of criteria considered for the identification of the entry pathways (including the trade volume and the strength of association of the pest with the commodity from the literature) did not suggest that other entry pathways would affect the outcome.
The risk of entry for cut flowers and apples is substantially lower than the risk from the table grapes. From the contribution of all the considered entry pathways, the model estimated a median number of 49.5 potential founder populations of P. callosus (90% CR: 4.0–881.2) per year in the EU.
By combining different climate indicators, the Panel was able to identify the areas suitable for the establishment of P. callosus. Two specific scenarios were considered. In scenario 1, three climatic indicators were taken together and any area in the EU where climate coincides with at least one of the climate indicators is considered climatically suitable for P. callosus. In scenario 2, only areas in the EU are considered climatically suitable if the absolute minimum soil temperature is equal or above the minimum value in locations where P. callosus was observed. The Panel deemed it informative to evaluate this restrictive scenario because large part of the life cycle of P. callosus is completed in the soil and thus the indicator affects the capacity of the pest to overwinter.
From the contribution of all the considered entry pathways and the probabilities of establishment in the rural and urban areas of the EU, the model estimated a median number of 1 founder populations of P. callosus every 1.3 years in the EU (90% CR: 1 every 30.8 years to 23.3 per year) for SC1 and 1 every 11.9 years (90% CR: 1 every 256.6 years to 2.5 per year) for SC2. The main knowledge gaps affecting the assessment of entry and establishment are whether other commodities are possible pathways insufficient data to develop a full pathway model for cut flowers, as well as the precise final destinations of the imported commodities.
After establishment of P. callosus in the risk assessment area, the median duration of the lag period between establishment and spread, defined as the time needed for a founder population to build up to a population size enabling the colonisation of neighbouring hosts, was estimated to be approximately 4 years (90% CR: 1.3 and 8.7 years). After the lag period, the median spread rate of P. callosus by natural means and human‐assisted spread in the areas where the pest could potentially establish in the EU is estimated at a rate of 15.5 m/year (90% CR 5.1–46.8 m/year). The main uncertainties affecting the assessment of the lag phase are the extent to which natural enemies, agroclimatic characteristics and crop management practices could hamper the build‐up of the population. For the spread rate, the main uncertainties are whether the closely related species used as proxy for P. callosus is a valid assumption and the actual environment in relation to its patchy distribution that is affecting the spread ability.
Assuming P. callosus has reached its maximum geographical range in the EU, the pest is regarded as a naturalised species and no specific control measures are adopted, the average yield loss directly attributable to P. callosus was estimated 5.2% as a median value (90% CR 2.2–11.7%) for apples, 0.5% (90% CR 0.01–2.8%) for cut flowers/foliage and 2% (90% CR 1.3–5.2%) for table grapes of the total production throughout the suitable regions of the EU. Other crops (e.g. pears, peaches) might also be affected by P. callosus, but there was not sufficient information to estimate the impact.
Potential risk reduction options for P. callosus were described and consisted of pre‐harvest and post‐harvest options. Main RROs during pre‐harvest in its native area is the application of chemical pesticides and biopesticides (entomopathogens) and the installation of trunk barriers to reduce the number of individuals climbing on the host plants. Several post‐harvest RROs are under consideration including transportation at controlled atmosphere and appropriate treatments during transport.
ABBREVIATIONS
- AC
Air Cargo
- CATTS
Controlled Atmosphere/Temperature Treatment System
- CI
Climatic Indicator
- CR
Certainty Range
- EF
Ethyl Formate
- EKE
Expert Knowledge Elicitation
- GBIF
Global Biodiversity Information Facility
- HRP
High Risk Plant
- IQR
Inter Quartile Range
- ISI
Institute for Scientific Information
- ISPM
International Standards for Phytosanitary Measures
- LDT
Lower Development Threshold
- MS
Member State
- OC
Ocean Cargo
- PLH
PLant Health
- PRA
Pest Risk Assessment
- RRO
Risk Reduction Option
- SC
Scenario
CONFLICT OF INTEREST
If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
REQUESTOR
European Commission
QUESTION NUMBER
EFSA‐Q‐2021‐00754
COPYRIGHT FOR NON‐EFSA CONTENT
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
PANEL MEMBERS
Claude Bragard, Paola Baptista, Elisavet Chatzivassiliou, Francesco Di Serio, Paolo Gonthier, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A. Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L. Reignault, Emilio Stefani, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent Civera, Jonathan Yuen, and Lucia Zappalà.
MAP DISCLAIMER
The designations employed and the presentation of material on any maps included in this scientific output do not imply the expression of any opinion whatsoever on the part of the European Food Safety Authority concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.
Supporting information
Supplementary material to the ‘Pest risk assessment of Phlyctinus callosus for the European Union‘ ‐ Model
ACKNOWLEDGEMENTS
The Panel thanks for the information provided to this scientific output: Irene Pilar Munoz Guajardo and Marika De Santis from EFSA. EFSA wishes to thank the ISA experts: Caterina Campese (Lincoln University, New Zealand) and Dr. Francesco Paoli (Council for Agricultural Research and Economics); the Hearing Experts: Dr. Steffan Hansen (Department of Conservation Ecology and Entomology, Stellenbosch University), Dr. Julien Haran (UMR CBGP, CIRAD, INRAE, IRD, InstitutAgro, Univ. Montpellier, Montpellier, France) and Dr. Samuel Brown (The New Zealand Institute for Plant and Food Research Limited). The Panel also thanks Dr. Elleunorah Allsopp (Agricultural Research Council of South Africa, Stellenbosch) and Dr. Pasquale Restuccia (consultant for Florcoop Sanremo) for sharing technical information. The Panel acknowledges all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
APPENDIX A. P. callosus hosts potentially posing a risk for pest introduction into EU
A.1.
For a plant species to be considered a host, the insect must be able to complete its development by feeding only on that plant species and adults that emerge are able to reproduce. The Panel considered the observation of immature stages of P. callosus as a sufficient indication that the pest can complete immature development on that host. Evidence of adult feeding on a plant species was also considered. The list of plants supporting immature development and/or adult feeding of P. callosus together with the supporting reference is shown in Table A.1. The Panel acknowledges that when specific species are reported (e.g. V. corymbosum), given the polyphagy of P. callosus, it cannot be excluded that the pest may develop on and/or feed on other species belonging to the genus (e.g. Vaccinium spp.). However, in Table A.1, the names of the host plants are shown as mentioned in the original reference.
TABLE A.1.
List of plants used by Phlyctinus callosus for immature development and/or adult feeding.
| Scientific name | Reference supporting the plant as a P. callosus host for immature development and/or adult feeding |
|---|---|
| Adromischus phillipsiae | Whittle (1986) |
| Aeonium holochrysum, A. urbicum | Whittle (1986) |
| Agapanthus praecox * | Martin (2011) |
| Allium cepa |
Whittle (1986) Plant Pest Information Network Database. Ministry for Primary Industries New Zealand (Accessed 22 September 2023) |
| Asparagus officinalis | Prestidge and Willoughby (1989, 1990), Haran et al. (2020) |
| Athanasia sp. | Hevin et al. (2022) |
| Beta vulgaris * | Plant Pest Information Network Database. Ministry for Primary Industries New Zealand (Accessed 22 September 2023) |
| Capiscum spp.* | Plant Pest Information Network Database. Ministry for Primary Industries New Zealand (Accessed 22 September 2023) |
| Bergeranthus multiceps, B. scapiger | Whittle (1986) |
| Cacti | Whittle (1986) |
| Callistephus chinensis | Whittle (1986) |
| Carpobrotus edulis | Hevin et al. (2022) |
| Carpobrotus spp. | Dlamini, Addison, and Malan (2019) |
| Chlorophytum comosum | Dlamini, Addison, and Malan (2019) |
| Chrysanthemoides monilifera |
Scurr et al. (2008) Plant Pest Information Network Database. Ministry for Primary Industries New Zealand (Accessed 22 September 2023) |
| Chrysanthemum spp. | Whittle (1986) |
| Citrus limon * | Plant Pest Information Network Database. Ministry for Primary Industries New Zealand (Accessed 22 September 2023) |
| Citrus sinensis * | Plant Pest Information Network Database. Ministry for Primary Industries New Zealand (Accessed 22 September 2023) |
| Citrus spp. |
Whittle (1986) Plant Pest Information Network Database. Ministry for Primary Industries New Zealand (Accessed 22 September 2023) |
| Coprosoma sp. | Dlamini, Addison, and Malan (2019) |
| Cotyledon sp. | Dlamini, Addison, and Malan (2019) |
| Crassula sp. | Dlamini, Addison, and Malan (2019) |
| Crassula corymbulosa, C. cultrata, C. multicava, C. rosularis | Whittle (1986) |
| Crocus sp. | Whittle (1986) |
| Cyclamen persicum | May (1966) |
| Dahlia pinnata | Haran, Hansen et al. (2020) |
| Daucus carota |
Anonymous (1970), Horne & Stacpoole (1989), Miller (1979), Whittle (1986) Plant Pest Information Network Database. Ministry for Primary Industries New Zealand (Accessed 22 September 2023) |
| Dianthus caryophyllus | Whittle (1986) |
| Echeveria agavoides, E. fulgens, E. gibbiflora, E. × gilva, E. multicaulis, E. nodulosa, E. pubescens, E. × scaphophylla | Whittle (1986) |
| Epiphyllum spp. | Whittle (1986) |
| Erepsia inclaudens | Whittle (1986) |
| Eucalyptus viminalis ** | Plant Pest Information Network Database. Ministry for Primary Industries New Zealand (Accessed 22 September 2023) |
| Euryops sp. | Hevin, Hansen et al. (2022) |
| Fragaria × ananassa | Curran & Patel (1988), Miller (1979) |
| Gazania spp. | Haran, Hansen et al. (2020), Hevin, Hansen et al. (2022) |
| Gerbera jamesonii | Whittle (1986) |
| Hypochaeris radicata | Barnes & Pringle (1989) |
| Iris xiphium | May (1966) |
| Kalanchoe tubiflora | Whittle (1986) |
| Lolium multiflorum | Barnes & Pringle (1989) |
| Malva parviflora | Dlamini, Addison, & Malan (2019) |
| Malus domestica * |
Myburgh, Bosman et al. (1975), Myburgh, Rust et al. (1975), Barnes & Swart (1977), Barnes (1989b), Nel & Addison (1993), Ferreira & Malan (2010) Plant Pest Information Network Database. Ministry for Primary Industries New Zealand (accessed 22 September 2023) |
| Medicago sp. | Dlamini, Addison, & Malan (2019) |
| Narcissus pseudonarcissus | Whittle (1986) |
| Osteospermum sp. | Haran, Hansen et al. (2020), Hevin, Hansen et al. (2022) |
| Paspalum dilatatum | Barnes & Pringle (1989) |
| Pastinaca sativa | Miller (1979), Whittle (1986) |
| Pelargonium sp. | Hevin, Hansen et al. (2022) |
| Pennisetum clandestinum | Barnes & Pringle (1989) |
| Phaseolus sp.** | Plant Pest Information Network Database. Ministry for Primary Industries New Zealand (Accessed 22 September 2023) |
| Plantago lanceolata | Barnes & Pringle (1989), Dlamini, Addison, & Malan (2019), Haran, Hansen et al. (2020), Hevin, Hansen et al. (2022) |
| Plumbago auriculata | Hevin, Hansen et al. (2022) |
| Portulaca oleracea | Pringle, Barnes et al. (2015) |
| Prunus domestica * | Barnes (1989a), Barnes & Giliomee (1992), Ferreira & Malan (2010) |
| Prunus persica * | Barnes (1989a), Barnes & Giliomee (1992), Ferreira & Malan (2010) |
| Prunus persica var. nucipersica * | Barnes (1989a), Barnes & Giliomee (1992), Ferreira & Malan (2010) Plant Pest Information Network Database. Ministry for Primary Industries New Zealand (Accessed 22 September 2023) |
| Pyrus communis * | Myburgh, Rust et al. (1975), Barnes (1989a), Barnes & Giliomee (1992), Ferreira & Malan (2010) |
| Rheum rhabarbarum * | Plant Pest Information Network Database. Ministry for Primary Industries New Zealand (Accessed 22 September 2023) |
| Rhododendron (as Azalea)* | Brown (2004) |
| Rosa sp.* | Plant Pest Information Network Database. Ministry for Primary Industries New Zealand (Accessed 22 September 2023) |
| Sedum sp.* | Anonymous (1970) |
| Sonchus oleraceus * | Pringle, Barnes et al. (2015), Dlamini, Addison, & Malan (2019) |
| Solanum tuberosum |
Horne & Stacpoole (1989), Walker (1981) Plant Pest Information Network Database. Ministry for Primary Industries New Zealand (Accessed 22 September 2023)** |
| Tulbaghia spp. | Haran, Hansen et al. (2020) |
| Trifolium repens | Barnes & Pringle (1989) |
| Vaccinium corymbosum | Bredenhand, van Hoorn et al. (2010), Ferreira (2010) |
| Vitis vinifera | Giliomee (1961), Van Den Berg (1971), Swart, Barnes, & Greeff (1976), Swart, Barnes, & Myburgh (1976), Whittle (1986), Schwartz (1988), Barnes (1989b), Lo, Blank et al. (1990), Pryke & Samways (2007), de Villiers & Pringle (2008), Dlamini, Malan, & Addison (2019), Dlamini et al. (2020a, 2020b, 2020c) |
| Zantedeschia sp.* | Plant Pest Information Network Database. Ministry for Primary Industries New Zealand (Accessed 22 September 2023) |
| X. gerbera* | Haran, Hansen et al. (2020) |
Adult feeding only, no evidence found for immature development.
Unrecorded pest life stage.
APPENDIX B. Trade data
B.1. Import of plant products from countries with reported presence of Phlyctinus callosus
The EUROSTAT import data for the years from 2012 to 2022 used to estimate the amounts of table grapes and apples imported into the EU from countries where P. callosus was reported are presented in Tables B.1 and B.2.
TABLE B.1.
EU imports of table grapes from South Africa, New Zealand and Australia, 2012–2022 [×100 kg] (Eurostat, EU trade since 1988 by HS2‐4‐6 and CN8, CN 08061010, online, accessed 16th August 2023).
| Country | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Australia | 0 | 0 | 151 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| New Zealand | 0 | 0 | 0 | 827 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| South Africa | 1,180,229 | 1,226,597 | 1,240,844 | 1,441,706 | 1,244,196 | 1,388,339 | 1,418,506 | 1,395,776 | 1,397,163 | 1,672,887 | 1,995,888 |
TABLE B.2.
Imports of apples from South Africa, New Zealand and Australia, 2012–2022 [×100 kg] (Eurostat, EU trade since 1988 by HS2‐4‐6 and CN8, CN 080810, online, accessed 16 August 2023).
| Country | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Australia | 0 | 0 | 0 | 0 | 1049 | 4926 | 9159 | 8311 | 3639 | 6971 | 5526 |
| New Zealand | 712,776 | 878,668 | 872,855 | 700,287 | 751,628 | 754,737 | 966,921 | 728,052 | 759,371 | 564,387 | 470,950 |
| South Africa | 283,830 | 516,151 | 228,359 | 257,699 | 298,163 | 252,069 | 334,616 | 258,077 | 329,086 | 405,555 | 416,417 |
B.2. Trade windows
Of these three players, the European market for table grapes is dominated by South Africa, most of the table grapes is imported in the European winter–spring, between December and May with peak in February (Figure B.1).
FIGURE B.1.
Total imports (tons) of table grapes by country, monthly average (2012–2022 data).
Apples are mostly imported from New Zealand and South Africa in the European spring–summer, between April and September with peak in July (Figure B.2).
FIGURE B.2.
Total imports (tons) of table grapes by country, monthly average (2012–2022 data).
APPENDIX C. Model Input parameters
C.1. Infestation rate
The elicited mean annual rates of infestation per 10,000 table grapes bunches and apples (in infested vineyards and infested orchards) are reported in Tables C.1 and C.2 together with the fitted probability distributions in Figures C.1 and C.2. EKE estimates are values proposed by the expert working group as consensus estimates.
TABLE C.1.
Estimated mean number of table grapes infested with Phlyctinus callosus when leaving the packing house in the country of origin (per 10,000 units).
| Question: | How many out of 10,000 table grapes (grapes bunches) coming from vineyards producing table grapes for export to the EU, are on average infested with living individuals of P. callosus after leaving the packing house? | ||||||
| Results | Infestation rate of table grapes after leaving the packing house (per 10,000 units) | ||||||
| Percentiles: | 1% | 5% | 25% | 50% | 75% | 95% | 99% |
| EKE estimates: | 1 | – | 23 | 50 | 125 | – | 1000 |
| Fitted values: | 3 | 7 | 22 | 52 | 122 | 412 | 973 |
| Fitted distribution | Lognorm (114.86, 226.29) | ||||||
TABLE C.2.
Estimated mean number of apples infested with Phlyctinus callosus when leaving the packing house in the country of origin (per 10,000 units).
| Question: | How many out of 10,000 apples coming from orchards producing apples for export to the EU, are on average infested with living individuals of P. callosus after leaving the packing house? | ||||||
| Results | Infestation rate of table grapes after leaving the packing house (per 10,000 units) | ||||||
| Percentiles: | 1% | 5% | 25% | 50% | 75% | 95% | 99% |
| EKE estimates: | 1 × 10−8 | – | 0.0005 | 0.001 | 0.01 | – | 1 |
| Fitted values: | 1 × 10−6 | 2 × 10−5 | 0.0003 | 0.001 | 0.008 | 0.1 | 1.5 |
| Fitted distribution | Loglogistic (0, 0.0015809, 0.67108) | ||||||
FIGURE C.1.
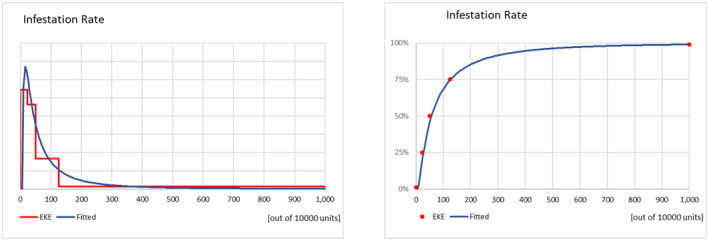
Distribution of the estimated infestation rate of Phlyctinus callosus in table grapes (per 10,000 units) when leaving the packing house fitted to EKE estimates.
FIGURE C.2.
Distribution of the estimated infestation rate of Phlyctinus callosus in apples (per 10,000 units) when leaving the packing house fitted to EKE estimates.
Table grapes: V. vinifera is one of the plants most often associated with P. callosus, and table grapes is a commodity already identified by an EU project as one of the most relevant entry pathways for this insect (DROPSA, 2016b). For the estimation of the infestation rate, the Panel considered (i) the quality standards normally required for the EU market, (ii) the pre‐ and post‐harvest processing agricultural practices – expected either to favour or to prevent the occurrence of the pest on table grapes intended for export (CBI, 2023; OECD, 2007; PPECB, 2023b) and (iii) the biology of P. callosus.
Of the countries where P. callosus is known to occur, South Africa is the leading exporter of table grapes in the EU (Figure B.1); the pest is a known pest problem for the grape industry and monitoring is expected to be in place, particularly for the vineyards producing table grapes intended for export in the EU. Nonetheless, since P. callosus is widespread in the table grapes producing regions, and control is often difficult, the pest can be assumed to be present. However, in infested vineyards, the infestation is normally described as localised (Dr. S. Hansen & Dr. E. Allsopp, personal communication, January 2024).
Harvesting time for grapes is assumed to coincide with the trade (i.e. no storage of grapes at place of origin) and the peak presence of P. callosus in table grapes bunches was observed to overlay only partially with that of the trade; in addition, the mean number of P. callosus in table grapes bunches was observed to be lower in the morning (harvest time) as compared to later in the day (Pryke & Samways, 2007).
Table grapes for fresh consumption are harvested by hand and carefully treated to ensure high product quality; in fact, also the post‐harvest processing is limited to sorting and shaping always by hand to avoid damaging the berries, P. callosus is relatively easy to detect, some specimens are expected to be detected and removed either pre‐ or post‐harvest.
The results of the elicitation for table grapes are reported in Table C.1 and Figure C.1.
The reasoning in support of the elicited values for the table grapes is listed below.
1st percentile
-
–
At the time of harvest, the population is unlikely to be at its maximum extent.
-
–
Table grapes intended for export is of high quality and buyers normally have strict quality requirements.
-
–
The pest is relatively easy to detect at inspection, although it is not a quarantine pest, if the infestation rate was high more reports of interceptions would be expected.
99th percentile
-
–
Infestation rate in infested vineyards can be high but some grape bunches might come from less affected areas, in addition, too high infestation rates in vineyards for table grapes for export to the EU is unlikely to remain unnoticed and not managed.
-
–
The structure of the grape bunches represents an ideal place for the pest to hide and remain undetected during harvesting.
-
–
The structure of the grape bunches reduces the chances for the pest to simply drop on the soil during manual fruit picking.
-
–
Table grapes is minimally handled, not washed nor polished, this minimal post‐harvest processing reduces the chances for the pest to be mechanically removed.
Median
-
–
P. callosus is a known pest issue in table grapes especially for exporting to the USA and infested vineyards will most likely be treated; however, the pest is known to be difficult to control and the treatment might not be 100% effective.
-
–
P. callosus is active during the night, at day it will hide; grape bunches are one of the options, but it might move to hide in other places (e.g. soil, weed).
-
–
Applications of insecticides to control other pests might have a collateral effect on the weevil, even by spraying only might remove the weevil from the vine.
Interquartile range (IQR): the interquartile range was set to reflect the consensus of more uncertainty for the higher values and more certainty for the lower values.
Apples: For the estimation of the infestation rate, the Panel considered (i) the quality standards normally required for the EU market, (ii) the pre‐ and post‐harvest processing agricultural practices – expected either to favour or to prevent the occurrence of the pest on apples intended for export and (iii) the biology of P. callosus.
Of the countries where P. callosus is known to occur, New Zealand is the leading exporter of apples followed by South Africa (Figure B.2); however, although it is present in the apple growing regions, P. callosus is not reported as a pest of major concern for the New Zealand apple industry (Dr S. Brown, personal communication, January 2024).
Although the presence of P. callosus in apple orchards cannot be excluded, the harvest‐time for apples does not necessarily coincide with that of the trade (i.e. apples can be stored for being exported when needed) and therefore the peak presence of P. callosus might not overlay with that of the harvest, the elicitation of the infestation rate was largely driven by the post‐harvest events. In fact, as opposite to a table grapes bunch, the structure of the apple only offers the peduncle (stem) or the calyx as possible places for the pest to (partially) hide. In addition, apples are at least washed and polished; these mechanical operations are expected to result in an effective removal of the pest from the fruit before packaging.
Reasoning in support of the elicited values for the apples are listed below:
1st percentile
-
–
The structure of the apple does not represent an ideal place for the pest to hide; the weevil can only stay in the peduncle (stem) or the calyx (flower) of the apple.
-
–
The structure of the apples makes likely the mechanical removal of the pest during manual fruit picking, movement of boxes or the rolling on conveyor belts at the packing house.
-
–
At the packing house, apples are washed and polished, weevils cannot hold easily on the fruit and mechanical removal is facilitated.
-
–
At the time of harvest the population is unlikely to be at its maximum extent.
-
–
Apples intended for export is of high quality and buyers normally have strict quality requirements.
-
–
P. callosus has never been reported from apples from New Zealand, the major exporting country for apple.
-
–
The pest is relatively easy to detect at inspection, although it is not a quarantine pest, if the infestation rate was high more reports of interceptions would be expected.
99th percentile
-
–
Although apples can be stored for being exported when needed, the harvest period (January until June with peak from February to April) is well aligned with that of the presence of P. callosus adults.
-
–
Infestation rate in infested orchards can be high but apples might come from less affected areas, in addition, too high infestation rates in orchards of apples for export to the EU is unlikely to remain unnoticed and not managed.
-
–
Washing and shaking are expected to be highly effective in removing the pest from the fruit even under the most pessimistic scenarios.
Median
-
–
P. callosus is a known pest issue in apples and especially for those for export and infested orchards will most likely be treated; however, the pest is known to be difficult to control and the treatment might not be 100% effective.
-
–
P. callosus is active during the night, at day it will hide; the fruit itself should not be the first choice as there are better places for hiding (e.g. soil, weeds, trunk)
-
–
Applications of insecticides to control for other pests might have a collateral effect on the beetle, even by spraying only might remove the beetle from the apples.
-
–
Post‐harvest processing is expected to be effective in the mechanical removal of the pest.
Interquartile range (IQR): the interquartile range was set to reflect the consensus of more uncertainty for the higher values and more certainty for the lower values.
C.2. Probability of transfer
The elicited probability of P. callosus being in the conditions of moving from the consumers' house to a suitable host and surviving along the way to the host until egg laying was informed by the expected time of pest arrival in the EU (see trade windows in Figures B.1, B.2) and the corresponding temperature (see Figure D.1 in Appendix D) to evaluate whether, at the time of pest arrival, the environmental conditions are expected to be favourable for movement and egg laying.
FIGURE C.3.
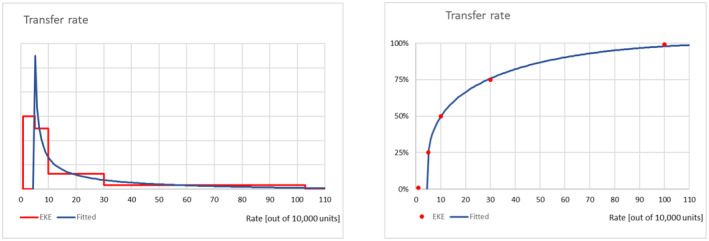
Distribution of the estimated transfer rate of Phlyctinus callosus for the rural area fitted to EKE estimates.
FIGURE C.4.
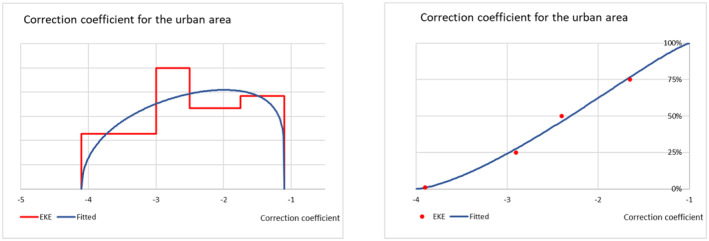
Distribution of the estimated correction coefficient for the urban area fitted to EKE estimates.
The Panel also considered the expected fitness of the arriving specimens in relation to their travel history. For example, P. callosus specimens surviving a journey of 2 weeks at a T° of about 0°C (transport time and temperature for table grapes via ocean cargo) are expected to be in overall worse fit if compared to P. callosus specimens arriving in the EU through consignments delivered via air cargo. The final destination of the apples, cut flowers and table grapes is the consumers' house, which could be located within a predominantly rural or urban area. In addition of the limited walking capacity of P. callosus, these different locations are expected to pose different challenges (physical barriers, predators) and opportunities (presence of suitable hosts) for the pest transfer.
The Panel therefore proceeded with the elicitation of two different probabilities of establishment, one to be used for the infested units delivered in predominantly rural areas and one for those units delivered in predominantly urban areas.
C.2.1. Probability of transfer (rural area)
The elicited probabilities of P. callosus being in the conditions of translocate from the consumers house to a suitable host and survive along the way to the host until egg laying for the rural area is reported in Table C.3, the resulting probability distribution characterising the uncertainty in the parameter is showed in Figure C.3.
TABLE C.3.
Estimated transfer rate for the rural area.
| Question: | 10,000 transfer units are purchased by consumers in the rural area and all units are infested with 1 mated Phlyctinus callosus female. How many of the 10,000 mated P. callosus females will be in the condition of translocating from the consumers house to a suitable host and survive along the way to the host until egg laying? | ||||||
| Results | Successful transfer rate (per 10,000 units) | ||||||
| Percentiles: | 1% | 5% | 25% | 50% | 75% | 95% | 99% |
| EKE estimates: | 1 | – | 5 | 10 | 30 | – | 100 |
| Fitted values: | 4.4 | 4.4 | 5 | 10 | 29 | 79 | 114 |
| Fitted distribution | BetaGeneral (0.30748, 2.3112, 4.4097, 154.39) | ||||||
Reasoning in support of the elicited values for the transfer in the rural area are listed below:
1st percentile
-
–
Most of the mated P. callosus specimens would arrive in the EU via ocean cargo, which takes about 2 weeks. Fruits are stored at a very low temperature and these conditions are expected to affect the fitness of the pest.
-
–
P. callosus adults are flightless and would need to overcome several physical barriers by walking to reach the nearest suitable host.
-
–
If detected on the fruit by the consumers, P. callosus would probably be eliminated.
-
–
Considering the trade windows, P. callosus is more likely to arrive during the winter when most host plants are hibernating/not present.
-
–
In the rural area, a variety of predators are expected to be present.
-
–
Events for the transfer to take place (remaining unnoticed by the consumer, safely walking to the host plant making successful contact with suitable host) are all characterised by a low probability of occurrence.
99th percentile
-
–
A small proportion of specimens might arrive via air cargo. Although the storage temperature is still very low the journey is considerably shorter and the adult P. callosus females arriving via this route are expected to be more fit.
-
–
P. callosus is polyphagous pest and although most of the import takes place in winter months, some suitable hosts in the rural area might be present.
-
–
Some import still happens in autumn and spring when more hosts are available.
-
–
If detected on the fruit by the consumers, the pest might be thrown to the external environment, facilitating its transfer to the host plant.
Median
-
–
P. callosus is more likely to arrive in winter when less hosts are expected to be available.
-
–
Most of the import is travelling via ocean cargo.
-
–
P. callosus does not fly, it must reach to the nearest suitable host and survive along the way in a rural area where predators are expected to be present.
Inter‐Quartile‐Range (IQR): the Inter‐Quartile‐Range was set to reflect the consensus of more uncertainty for the higher values and more certainty for the lower values.
C.2.2. Probabilty of transfer (urban area)
The probability of transfer for the urban area was computed by means of a conversion factor explaining by which order of magnitude the estimate of the rural area should be corrected. EKE results for the conversion factor are presented in Table C.4, the resulting probability distribution characterising the uncertainty in the parameter is showed in Figure C.4.
TABLE C.4.
Estimated correction factor for the urban area.
| Question: | By which order of magnitude the probability of transfer estimated for the rural area would be reduced if the 10,000 mated Phlyctinus callosus females were introduced in the urban environment? | ||||||
| Results | Order of magnitude | ||||||
| Percentiles: | 1% | 5% | 25% | 50% | 75% | 95% | 99% |
| EKE estimates: | –4 | – | –3 | −2.5 | −1.75 | – | −1 |
| Fitted values: | −4 | −3.8 | −3.1 | −2.4 | −1.8 | −1.3 | −1.1 |
| Fitted distribution | BetaGeneral (1.4563, 1.193, –4.1, –1.1) | ||||||
Reasoning in support of the elicited values for the transfer in the urban area.
1st percentile
-
–
Similar considerations made for the rural area regarding arrival time, and the effect of the transport conditions on the fitness of the mated P. callosus adults.
-
–
In the urban area fewer predators are found compared to the rural area, but still some mortality is expected due to hemerophiles species (species dwelling or thriving in habitats influenced by the activities of man or under cultivation such as rats, mice, pigeons) that are adjusted to human‐altered habitat.
-
–
More physical barriers than the rural area would have to be overcome by walking P. callosus adults to reach to a suitable host.
-
–
Overall, less suitable hosts are expected to be present in the urban environment.
99th percentile
-
–
Natural enemies and entomopathogenic organisms most probably will be absent in the urban environment.
-
–
Some hosts may be available (urban forests, community gardens, parks).
Median
The conditions favouring the probability of transfer towards the lower limit are more likely in the urban area as the majority of consumers live in apartments. The distance and physical barriers from the consumers' house to a suitable host are expected to be greater compared to the rural areas.
Interquartile range (IQR): The interquartile range set to leave the median slightly closer to the upper limit and leave more uncertainty towards the lower values.
C.3. Probability of establishment
C.3.1. Probability of establishment (rural area)
Consistently with the approach used for the probability of transfer, also for the establishment the Panel proceeded with the elicitation of two probabilities one to be used for the potential founder populations in the rural areas and one for those founder populations in the urban areas. The elicited probabilities of P. callosus females initiating a founder population that persists in the rural area are reported in Table C.5, the resulting probability distribution, characterising the uncertainty in the parameter, is showed in Figure C.5.
TABLE C.5.
Estimated establishment rate for the rural area.
| Question: | How many out of 10,000 mated females that successfully completed the transfer (and laid eggs) on a suitable host in the RURAL areas of the EU where climatic conditions are suitable for the establishment will be able to initiate a founder population that persists? | ||||||
| Results | Successful establishment rate (out of 10,000 females) | ||||||
| Percentiles: | 1% | 5% | 25% | 50% | 75% | 95% | 99% |
| EKE estimates: | 10 | – | 100 | 200 | 500 | – | 2000 |
| Fitted values: | 14 | 31 | 96 | 212 | 468 | 1461 | 3253 |
| Fitted distribution | Lognorm (422.11, 727.21) | ||||||
FIGURE C.5.
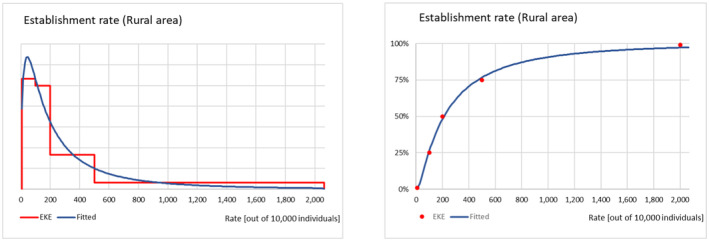
Distribution of the estimated correction coefficient for the urban area fitted to EKE estimates.
Reasoning in support of the elicited values for the establishment in the rural area are listed below.
1st percentile
-
–
There is a very narrow window of abiotic conditions for the eggs and larvae of P. callosus to hatch and survive.
-
–
Survival rate of eggs is low even at optimal conditions.
-
–
All immature stages of the insect will be subjected to mortality factors and few of them will develop to adults.
-
–
Strong Allee effects.
-
–
There is uncertainty about the lower temperature developmental threshold of 6°C.
-
–
At least some of the P. callosus females that were transferred by air cargo and suffered lower levels of cold stress will be successful in establishing a founder population.
99th percentile
-
–
P. callosus females have already managed to reach a host and lay eggs at a climate suitable environment; the probability of establishment will be limited by random factors affecting the development of the offspring generation.
-
–
Many of the P. callosus females that arrived by air cargo and did not suffer cold stress will manage to reach a suitable host and start reproducing.
-
–
Natural enemies will not manage to control the population.
-
–
Egg survival will be around the highest ratio of 54% observed at optimal conditions, but larval survival expected to be lower.
-
–
Successful cases of invasion and establishment of the species have been documented in New Zealand, although there is uncertainty whether these established populations were initiated by transferred adults or by immature stages of the insect.
Median
-
–
P. callosus females will most probably be from an agricultural area of the origin country, so their lineage will be a polyphagous one and not be restricted by host availability.
-
–
Some uncertainty exists on the polyphagous ability of the species since other non‐polyphagous lineages exist in the origin country. There is a need for specific environmental conditions for polyphagy to be expressed, so it is not assumed as certain.
-
–
Even if optimal conditions exist, low rate of survival is expected for the immature stages and Allee effects will restrict establishment.
-
–
Tiny niche for establishment: a need for dry climate, but soil humidity is also important during the first stages of larval development.
-
–
At the rural environment, pest control measures will be applied and P. callosus presence will be noticed, especially due the damage caused by the second‐generation adults.
Inter‐Quartile‐Range (IQR): the Inter‐Quartile‐Range was set to represent the consensus of high uncertainty for Q1 and more certainty for the lower values, so Q3 closer to the median.
C.3.2. Probability of establishment (urban area)
The elicited probabilities of P. callosus females initiating a founder population that persists in the urban area are reported in Table C.6, the resulting probability distribution characterising the uncertainty in the parameter is showed in Figure C.6.
TABLE C.6.
Estimated establishment rate for the urban area.
| Question: | How many out of 10,000 mated females that successfully completed the transfer (and laid eggs) on a suitable host in the URBAN areas of the EU where climatic conditions are suitable for the establishment will be able to initiate a founder population that persists? | ||||||
| Results | Successful establishment rate (out of 10,000 females) | ||||||
| Percentiles: | 1% | 5% | 25% | 50% | 75% | 95% | 99% |
| EKE estimates: | 1 | – | 25 | 50 | 175 | – | 1000 |
| Fitted values: | 2 | 5 | 22 | 58 | 155 | 637 | 1714 |
| Fitted distribution | Lognorm (167.64, 451.95) | ||||||
FIGURE C.6.
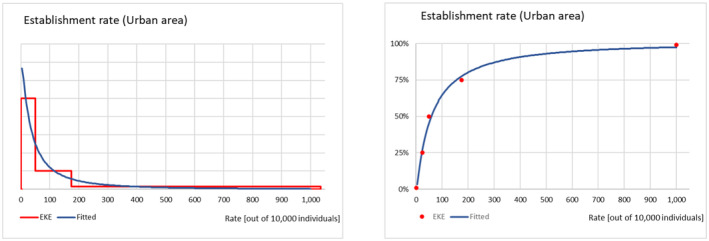
Distribution of the estimated correction coefficient for the urban area fitted to EKE estimates.
Reasoning in support of the elicited values for the establishment in the urban area.
1st percentile
-
–
In an urban environment, the presence of soil borne enemies is less likely, but on the other hand there is no continuous host presence, so egg and larvae survival are affected by counteracting factors.
-
–
Lower food resources compared to the rural environment.
-
–
More fluctuations of temperature and humidity compared to the rural environment. High temperatures exceeding the higher developmental threshold of the insect are more probable in the urban environment.
-
–
P. callosus females will most probably land in a private garden and their presence will be noticed and managed.
99th percentile
-
–
The two limiting factors are mortality due to natural enemies and available food resources. Restricted host availability in the urban environment is compensated by the lower abundance of natural enemies.
-
–
Presence of generalist predators (rats and pigeons) in the urban environment, but their populations are controlled, so there is uncertainty of their ability to restrict P. callosus.
Median
-
–
In the urban environment, more human interactions are expected to have a negative effect on the survival of the offspring generation.
Inter‐Quartile‐Range (IQR): the Inter‐Quartile‐Range was set to represent the consensus of more uncertainty for the higher values.
C.4. Probability of lag phase and spread rate
For the elicitation of the spread parameters, the Panel considered the lag phase as the period where the pest is reproducing and increasing in numbers until it reaches a population level to be able to spread to non‐infested areas. The spread as the spatial expansion of a population either due to the natural spread capacity of the pest or human assisted spread (human‐assisted spread is limited to the spread of the pest facilitated by common agricultural practices).
C.4.1. Lag phase
For the elicitation of the lag phase, it was considered that P. callosus is a polyphagous species and females lay 20–70 eggs (May, 1966, Pringle, Barnes et al. 2015, Dlamini, Addison, & Malan, 2019). Normally, one generation is completed per year, but two generations per year can be observed in orchards/vineyards depending on the ground cover and irrigation (Barnes, 1989a). High mortality is observed at temperatures above 30°C (Walker, 1981). The elicited values for the lag phase (in years) are reported in Table C.7, the resulting probability distribution, characterising the uncertainty in the parameter, is showed in Figure C.7.
TABLE C.7.
Estimated Phlyctinus callosus lag phase in years.
| Question: | What is the average duration of the lag phase, defined as the time between first establishment and the continuous expansion of the infested area (linear phase) in the climatic suitable areas of the EU? | ||||||
| Results | Lag phase duration in years | ||||||
| Percentiles: | 1% | 5% | 25% | 50% | 75% | 95% | 99% |
| EKE estimates: | 1.00 | – | 2.50 | 4.00 | 6.00 | – | 10.00 |
| EKE fitted: | 1.01 | 1.29 | 2.49 | 4.03 | 5.97 | 8.70 | 10.09 |
| Fitted distribution | BetaGeneral (1.2151, 2.5227, 0.90468, 11.615) | ||||||
FIGURE C.7.
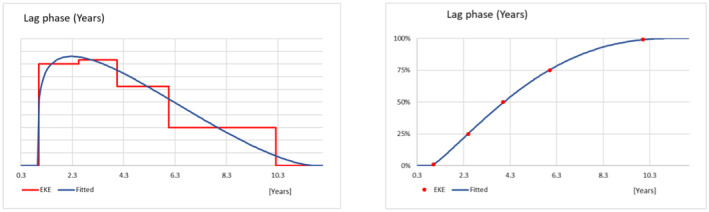
Distribution of the estimated Phlyctinus callosus lag phase (in years) fitted to EKE estimates.
The reasoning in support of the elicited values for the lag phase of P. callosus are listed below.
1st percentile
-
–
One or two generations would be enough to produce sufficient offspring of P. callosus before it could subsequently spread to the neighbouring tree/vine or a secondary host. Most orchards and vineyards would be irrigated, allowing a second generation during the same cropping season to develop, which boosts the number of offspring per year.
-
–
Food availability and weather conditions would not be a limiting factor.
-
–
The pest management practices currently applied in the EU would not affect P. callosus either by not being applied at the appropriate time or active ingredients would not be lethal to this specific pest.
-
–
Weed control and soil cultivation would only remove weeds from the inter‐rows, not from the base of the plants.
99th percentile
-
–
At least three to four generations would be needed to produce sufficient offspring of P. callosus before it could subsequently spread to the neighbouring tree/vine or a secondary host. In addition, the pest tends to aggregate in certain hotspots to mate and feed and can only move further during its adult life stage.
-
–
Compared to other insects, this species is univoltine and does not produce many offspring/year.
-
–
The survival of P. callosus is limited to a strict temperature/humidity window and is not tolerant to humidity fluctuations especially during the early larval developmental stages.
-
–
Weed control and soil cultivation practices would sufficiently remove weeds from a field to hamper population build‐up.
-
–
The pest management practices currently applied against other pests in the EU, would affect P. callosus significantly, as they are taken at the right time and include active ingredients which are also lethal to this specific pest, e.g. in apple orchards that already monitor and treat for apple blossom weevil (Anthonomus pomorum) in spring and in vineyards that monitor and treat for black vine weevil (Otiorhynchus spp.) and Scaphoideus titanus (vector of Flavescence dorée).
-
–
There is the possibility that P. callosus populations would be knocked down each year due to various other reasons (low winter temperatures, dry summers, lack of food, etc.) and the lag phase duration would be prolonged.
Median
-
–
Three to four generations would be needed to produce enough offspring so that the carrying capacity of the host crop would be exceeded and the pest would start spreading.
-
–
Natural enemies and mortality rate would restrict the reproduction rate of the population.
Interquartile range (IQR): the interquartile range was set to represent the consensus of more uncertainty for the higher values.
C.4.2. Spread rate
For the elicitation of the spread rate, it was considered that since P. callosus adults cannot fly, they can spread only by walking short distances on its own or assisted by human activities during crop management practices (thinning, harvest, post‐harvest activities involving use of machinery moving on a single day between the borders of the orchard/vineyard).
The average size of a vineyard in Europe is 1.4 ha, although in certain countries (e.g. France), a single vineyard could be 10.5 ha (EUROSTAT, 2022). The diagonal distance between the corners of a single vineyard holding of an average size of 1.4 ha, would be approximately 100 m and it was assumed as a maximum distance P. callosus adults could be moved by human‐assisted means.
As no data on the spread rate or dispersal capacity were found for P. callosus, data on dispersal distance reported in Mark‐Release‐Recapture studies of some closely related flightless, polyphagous, nocturnal curculionid species, that deposit eggs at the root collar of host plants were used as a proxy. For Otiorhynchus sulcatus (Fabricius) most adults were recovered 10 m from their release site, up to maximum 100 m in an urban setting (Maier, 1978); in studies with the same species in strawberry fields by Pope and colleagues, adults moved a distance of between 2.65 and 17.30 m (average 7.50 m) during 5 weeks (Pope, Gundalai et al. 2015); Otiorhynchus rugosostriatus Goeze and Otiorhynchus raucus Fabricius dispersed 2–12 m in ornamental plantations (Reineke, Hirsch et al. 2011); and Hylobius warreni Wood moved between 5 and 9 m in conifer forests, with a maximum of 50–100 m a year or more, especially when suitable hosts were scarce (Klingenberg, Björklund et al. 2010). About 50% of the monophagous weevil, Rhyssomatus lineaticollis, even moved less than 1 m (St Pierre & Hendrix, 2003). Within most of these studies there were always odd weevils that moved much larger distances than the average or median. In the assessment of the spread, host availability was not considered as a limiting factor, as peaches, pears, apples, grapes, asparagus, cut flowers and soft fruits are possible hosts of P. callosus.
The elicited values for the spread rate (in m/year) are reported in Table C.8, the resulting probability distribution, characterising the uncertainty in the parameter, is showed in Figure C.8.
TABLE C.8.
Estimated Phlyctinus callosus spread rate in metres per year.
| Question: | After the lag period the pest population spreads continuously (linear spread) until the saturation phase. What is the average rate of expansion in metres during this linear phase under all conditions in the climatic suitable area of the EU? [m/year] | ||||||
| Results | Spread rate in m/year | ||||||
| Percentiles: | 1% | 5% | 25% | 50% | 75% | 95% | 99% |
| EKE estimates: | 4.00 | – | 10.00 | 15.00 | 25.00 | – | 80.00 |
| EKE fitted: | 3.23 | 5.11 | 9.82 | 15.47 | 24.36 | 46.83 | 74.10 |
| Fitted distribution | Lognorm (19.404, 14.699) | ||||||
FIGURE C.8.
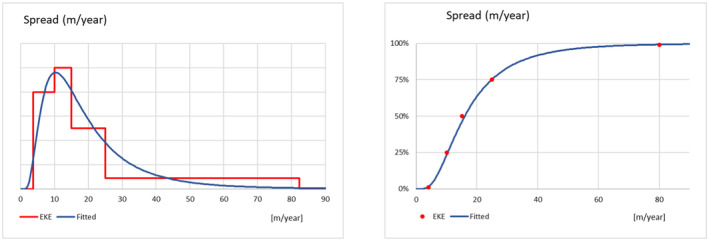
Distribution of the estimated Phlyctinus callosus spread rate (in m/years) fitted to EKE estimates.
The reasoning in support of the elicited values for the spread rate of P. callosus are listed below.
1st percentile
-
–
The pest's population would move to its neighbouring tree and the average distance between two host plants would be about 4 m (apple trees are planted at 3 × 4 m distance).
99th percentile
-
–
Human assisted spread during normal agricultural practices would have the predominant effect. However, the longest distance a P. callosus individual would be translocated this way would be the diagonal of a vineyard holding (average size of EU vineyard is 1.4 ha and the diagonal of a single vineyard holding would be ~ 100 m).
Median
-
–
P. callosus does not fly and only moves during adult life. With no specific P. callosus data, it was assumed that the average spread distance would be similar to that of O. sulcatus (~ 10 m).
-
–
Unanimous opinion of the consulted experts is that, from field experience, the spreading capacity of P. callosus is very limited when not aided by human intervention (e.g. human assisted long‐distance spread due to movement of plants).
Inter‐Quartile‐Range (IQR): the Inter‐Quartile‐Range was set to represent the consensus of more uncertainty for the higher values.
C.5. Impact assessment
C.5.1. Apples
Quantitative data on the yield loss directly attributable to P. callosus is reported or can be extrapolated from surveys or experimental studies conducted in South Africa from the early ‘70 to ‘90s. When considering orchard‐level estimates, Myburgh and colleagues observed that about 90% of the 90 untreated apple orchards were severely infested with a degree of fruit damage from 1% to 32% but most often ranging from 4% to 8% (Myburgh, Rust et al. 1975). These results are consistent with a previous survey in an apple orchard (Myburgh, Bosman et al. 1975) resulting in an average crop loss of 4.0%.
Other sources reporting P. callosus yield loss data are available from experimental trials intended to assess the effectiveness of specific control measures. The results of these experiments, all conducted in South Africa between 1979 and 1996 (Barnes & Swart, 1980, Barnes & Giliomee, 1992, Barnes, Knipe et al. 1994, 1995, 1996), are summarised in Figure C.9 where the percentage of fruit damaged in untreated experimental units is plotted. The original dataset behind the figure is made available as supplementary material in the online version of the Opinion.
FIGURE C.9.
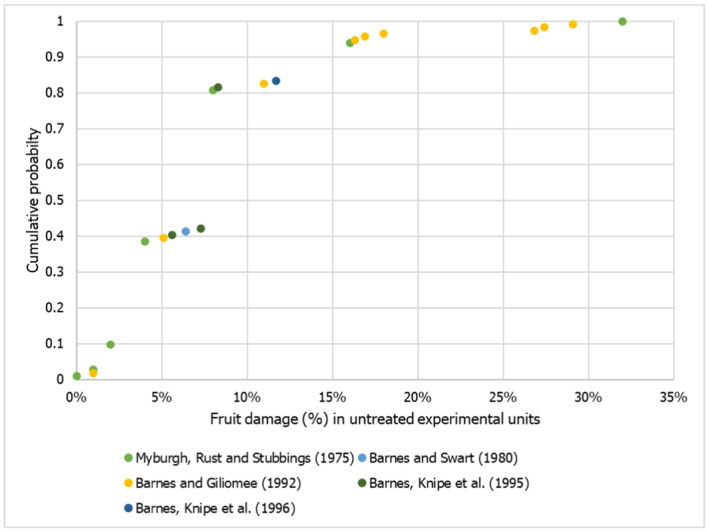
Distribution of the observed percentage of fruit damage (apples) in untreated experimental units.
It should be noted that the Figure C.9 reporting the data for the untreated experimental units, includes the results of experiments conducted in different growing seasons and under different experimental design. In some cases the trials were conducted under high pest pressure (Barnes, Knipe et al. 1996). Additional information from individual experiments were considered in the weight of the evidence considering the fact that between trees noticeable variation was observed, with crop loss due to P. callosus on individual trees varying from less than 1% up to 66% (Barnes & Giliomee, 1992). By observing the dispersion pattern on the tree of fruit damaged by P. callosus, it appears that adults did not feed randomly on the trees but aggregated in certain areas to feed and possibly also to mate (Barnes & Swart, 1980). Myburgh and colleagues observed that numerous cases of fruit damage occurred on lateral branches weighted down by fruit and touching weeds near the rootzone of apple trees (Myburgh, Rust et al. 1975). The elicited values for the impact on apples (in % yield reduction) are reported in Table C.9, the resulting probability distribution, characterising the uncertainty in the parameter, is showed in Figure C.10.
TABLE C.9.
Estimated yield loss for apples by Phlyctinus callosus.
| Question: | What is the average percentage of yield loss for apples attributed to P. callosus in climatically suitable areas of the EU, when the pest is present to its maximum extent? | ||||||
| Results | % yield reduction | ||||||
| Percentiles: | 1% | 5% | 25% | 50% | 75% | 95% | 99% |
| EKE estimates: | 2.00% | – | 3.50% | 5.00% | 8.00% | – | 13.00% |
| EKE fitted: | 1.96% | 2.19% | 3.40% | 5.22% | 7.85% | 11.68% | 13.88% |
| Fitted distribution | BetaGeneral (1.0222, 2.8111, 1.9, 16.728) | ||||||
FIGURE C.10.
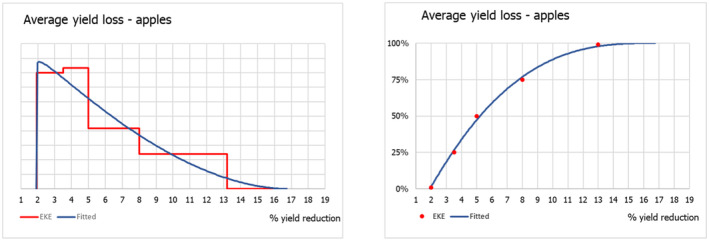
Distribution of the estimated % apple yield reduction directly attributable to Phlyctinus callosus fitted to EKE estimates.
Reasoning for the impact on apple yield reduction.
1st percentile
-
–
Conventional apple orchards having a high input of synthetic insecticides to treat other major pests and at least pyrethroids are expected to have contingent effect on P. callosus adults.
-
–
Growers may be aware of the presence of apple blossom weevil (A. pomorum) in their orchards and monitor for it or apply control measures.
-
–
Levels of fruit damage might be more than 1% if the pest becomes widespread.
99th percentile
-
–
According to evidence data, high levels of fruit damage are possible when no treatment is applied.
-
–
There is low tolerance for cosmetic injury in apples and a single feeding scar could render the apple fruit unmarketable or of lower quality class.
-
–
Floor management in apple orchards, may allow weeds near the irrigated rootzone of trees.
Median
-
–
It is unlikely that the whole orchard will be infested, as the pest is typically observed in aggregations at localised areas.
-
–
According to evidence data, median values of fruit damage are in the range of 4%–8%.
Interquartile range: Highest uncertainty for Q1 and more certainty for Q3.
C.5.2. Table Grapes
Quantitative data of yield losses caused by P. callosus on grape are also very scarce. In general, P. callosus adults tend to aggregate in specific spots, so the damage is not expected to involve the entire vineyard. In fact, under the assumption that the infestation rate of P. callosus on table grapes can be inferred from the extent of the area where management methods are normally applied, this typically involves less than 10% of the vineyard and most often around 2%–3% (Dr E. Allsopp, personal communication, March 2024). While it is important to note that this remains only an approximation, it is in accordance with information from New Zealand, where the field incidence of P. callosus appears to be localised within subregions and ‘hot spots’ within blocks (https://www.nzwine.com/media/20546/bri‐research‐fact‐sheet_weevilmonitoring.pdf, Accessed: April 2024).
Information reported on a specific webpage of the Government of Western Australia are also on the same line in stating that damage by P. callosus may be local, and therefore inspecting a representative sample of vines and host weed species across the vineyard is necessary and areas where garden weevils were a problem the previous season should be included (https://www.agric.wa.gov.au/pome‐fruit/garden‐weevil‐vineyards?page=0%2C2, Accessed: April 2024).
As mentioned above, quantitative data are very limited but some indication of the impact of P. callosus in vineyard can be inferred from the results of a project investigating a number of aspects in relation to the sustainable management of garden weevil in grapevines in Australia (Learmonth, Gibberd et al. 2011). In that report, the authors reported useful quantitative data also in relation to weevil damage.
For each experiment intended to test the effectiveness of control measures against P. callosus, the yields of the controls and the higher yields of treated units were extrapolated from the figures using WebPlotDigitizer (https://apps.automeris.io/wpd/) and used to calculate the yield loss fraction as: (YieldTREATED – YieldUNTREATED)/YieldTREATED. Results of this exercise are presented in Table C.10, where the reference to the experiment is reported together with the unit and whether the difference between treated and controls was statistically significant. Readers are invited to read the original report (Learmonth, Gibberd et al. 2011) for the details of the experiments.
TABLE C.10.
Summary of the yield loss fraction calculated from the extrapolated yields of the controls and the higher yields of treated units from the experiments intended to evaluate the effectiveness of control measures against Phlyctinus callosus. The reference to the experiment in the original report is reported together with the unit and whether the difference between treated and controls was statistically significant.
| Yield (untreated) | Yield (treated) | Experiment reference (objective/experiment) | Unit | Significant | Yield loss fraction |
|---|---|---|---|---|---|
| 8.90 | 10.30 | Objective3/Experiment(a) | kg/plot | Yes | 0.136 |
| 9.70 | 12.00 | Objective3/Experiment(a) | kg/plot | Yes | 0.192 |
| 6.05 | 7.5 | Objective3/Experiment(a) | kg/panel | No | 0.193 |
| 5.67 | 7.5 | Objective3/Experiment(a) | kg/panel | No | 0.244 |
| 11 | 15.1 | Objective3/Experiment(a) | kg/panel | No | 0.272 |
| 10 | 15.1 | Objective3/Experiment(a) | kg/panel | No | 0.338 |
| 100% | 80% | Objective3/Experiment(b) | % Of untreated plots | N/A | −0.250 |
| 100% | 92% | Objective3/Experiment(b) | % Of untreated plots | N/A | −0.087 |
| 100% | 93% | Objective3/Experiment(b) | % Of untreated plots | N/A | −0.075 |
| 100% | 98% | Objective3/Experiment(b) | % Of untreated plots | N/A | −0.020 |
| 100% | 100% | Objective3/Experiment(b) | % Of untreated plots | N/A | 0.000 |
| 100% | 105% | Objective3/Experiment(b) | % Of untreated plots | N/A | 0.048 |
| 100% | 111% | Objective3/Experiment(b) | % Of untreated plots | N/A | 0.099 |
| 100% | 114% | Objective3/Experiment(b) | % Of untreated plots | N/A | 0.123 |
| 100% | 117% | Objective3/Experiment(b) | % Of untreated plots | N/A | 0.145 |
| 100% | 119% | Objective3/Experiment(b) | % Of untreated plots | N/A | 0.160 |
| 100% | 120% | Objective3/Experiment(b) | % Of untreated plots | N/A | 0.167 |
| 100% | 120% | Objective3/Experiment(b) | % Of untreated plots | N/A | 0.167 |
| 100% | 125% | Objective3/Experiment(b) | % Of untreated plots | N/A | 0.200 |
| 100% | 127% | Objective3/Experiment(b) | % Of untreated plots | N/A | 0.213 |
| 100% | 135% | Objective3/Experiment(b) | % Of untreated plots | N/A | 0.259 |
| 100% | 145% | Objective3/Experiment(b) | % Of untreated plots | N/A | 0.310 |
| 100% | 154% | Objective3/Experiment(b) | % Of untreated plots | N/A | 0.351 |
| 100% | 178% | Objective3/Experiment(b) | % Of untreated plots | N/A | 0.438 |
| 10.30 | 12.40 | Objective5/Experiment(a) | kg/panel | No | 0.169 |
| 10.50 | 14.60 | Objective5/Experiment(b) | kg/panel | Yes | 0.281 |
| 3.80 | 7.60 | Objective5/Experiment(b) | kg/plot | Yes | 0.500 |
| 14.10 | 16.50 | Objective5/Experiment(c) | kg/panel | No | 0.145 |
| 5.70 | 10.10 | Objective5/Experiment(c) | kg/panel | Yes | 0.436 |
| 8.90 | 10.90 | Objective5/Experiment(d) | kg/panel | No | 0.183 |
| 6.80 | 7.60 | Objective5/Experiment(e) | kg/panel | No | 0.105 |
| 9.80 | 18.20 | Objective5/Experiment(e) | kg/panel | Yes | 0.462 |
While these experiments provide useful data particularly in highlighting that occasionally the severity of pest damage in infested areas may be significant (e.g. for objective 5 in experiment (e) it was reported that ‘The trial undertaken in the Pemberton region was subjected to a heavy infestation of garden weevil that caused a high level of damage to untreated vines and some of kaolin treated vines’) these results should be evaluated considering that the experiments were carried out in different locations, seasons, grape varieties and experimental design. In addition, it should be noted that results of many trials were not significant and others (i.e. Objective3/Experiment(b)) lacking sufficient detail. This indicates uncertainty regarding whether the calculated yield loss fraction can be attributed to P. callosus or is merely a result of variability in plot yields.
The elicited values for the impact on table grapes (in % yield reduction) are reported in Table C.11, the resulting probability distribution, characterising the uncertainty in the parameter, is showed in Figure C.11.
TABLE C.11.
Estimated yield loss for table grapes by Phlyctinus callosus.
| Question: | What is the average percentage of yield loss for table grapes attributed to P. callosus in climatically suitable areas of the EU, when the pest is present to its maximum extent? | ||||||
| Results | % yield reduction | ||||||
| Percentiles: | 1% | 5% | 25% | 50% | 75% | 95% | 99% |
| EKE estimates: | 1.00% | – | 1.5% | 2% | 3% | – | 7% |
| EKE fitted: | 1.30% | 1.31% | 1.5% | 2% | 3% | 5.2% | 7% |
| Fitted distribution | BetaGeneral (0.60703, 4.9758, 1.3007, 12.046) | ||||||
FIGURE C.11.
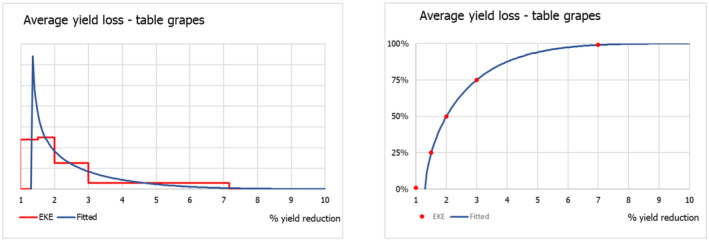
Distribution of the estimated % table grapes yield reduction directly attributable to Phlyctinus callosus fitted to EKE estimates.
Reasoning for the impact on table grapes yield reduction:
1st percentile
-
–
Pest management practices in the countries where the pest is present (weed control, insecticide applications, trunk barriers) can successfully control the impact of P. callosus at almost zero level. Similar practices (except the trunk barriers) are applied in EU countries. Typically, vineyard soil is cultivated in the inter‐ rows and herbicides are applied near the rootzone. Insecticide applications against various table grape pests (Scaphoideus titanus, thrips, Popillia japonica) would also control P. callosus adults.
-
–
Trunk exclusion barriers are not widely used in table grape vineyards in the EU, except for some areas where Otiorynchus spp. are present. This may allow for a damage level a little higher than zero.
-
–
Compared to apple crops, where a single scar would render the fruit unmarketable, there is higher tolerance for cosmetic damage in table grapes. Phlyctinus callosus adults would feed on the young berries and possible damage the stem of the bunch but would not feed on the mature grapes.
99th percentile
-
–
If P. callosus was to be introduced in the EU, there is uncertainty about how the pest will behave in the new environment. Damage levels are kept low where the pest is present, but the cropping system is designed accordingly, e.g. vines are trained on a trellis that does not let long shoots hangi near the ground.
-
–
The incidence rate of the pest in the vineyard is low, as it tends to aggregate, but infestation rate can be high at the spots where it is present.
-
–
Impact could vary depending on the area, even at the origin country. Higher impact is expected at coastal areas with moderate temperatures compared to inland areas with colder winters or semi‐arid regions with high summer temperatures.
Median
-
–
Yield losses would be expected only at parts of the crop due to localised infestations of P. callosus. The average infestation rate is 2%–3% of the vineyard area.
-
–
Feeding damage of adults would be easily spotted by visual inspection early in season.
-
–
Similar species already exist in certain table grape producing regions where monitoring and treatments are applied e.g. against Otiorynchus spp.
Interquartile range: Highest uncertainty for Q1, but more certainty on the median.
C.5.3. Cut flowers/foliage
With no reports on the impact of P. callosus in cut flowers/foliage production in the origin countries, related Curculionidae species (Otiorhynchus spp. etc.) were used as proxy organisms. Particularly, the Panel considered the following evidence in relation to Otiorhyncus sulcatus:
-
–
Growers of cut flowers/foliage in the EU are generally aware of the possible high economic impact of O. sulcatus infestation (Dr. P. Restuccia, personal communication, March 2024).
-
–
Despite control efforts, in case of O. sulcatus infestation, a residual loss is expected. This is quantified in the order of 3%–5% by some growers in the UK (Bennison, Chandler et al. 2014)
-
–
From a survey involving 217 nurseries across diverse range of production scale and location in the US (Hort, 2001), more than 65% of growers who responded to economic queries (N = 57) reported 0%–2% losses in saleable materials to root weevils. Only 13% of nurseries described their losses to weevils > 5%. From the same survey, a 2% crop loss emerged as a benchmark for grower's satisfaction with their weevil management strategies.
-
–
A commercial formulation of the entomopathogenicc nematode Heterorhabditis bacteriophora was tested for the control of Otiorhynchus sulcatus in a nursery of cyclamen stock in New Zealand during 1990. More than 1400 plants were treated and the infestation rate of pots was reduced from 27% to 3.8% (Popay, 1990).
-
–
During consultation with a subject matter expert (Dr. P. Restuccia personal communication March 2024) it emerged that: ‘If left untreated, an infestation of O. sulcatus can lead to a yield reduction of 15%–20% in the first year and complete production loss within three years. However, O. sulcatus is a known issue, producers are aware and actively monitor for it. Control methods consist in applying nematodes against the larvae and insecticides (pyrethroids) against the adults. Despite these efforts, control is not entirely effective, and a residual yield loss can be expected’.
The elicited values for the impact on cut flowers/foliage (in % production reduction) are reported in Table C.12, the resulting probability distribution, characterising the uncertainty in the parameter, is showed in Figure C.12.
TABLE C.12.
Estimated yield loss for cut flowers and cut foliage by Phlyctinus callosus.
| Question: | What is the average percentage of yield loss for cut flowers and cut foliage attributed to P. Callosus in climatically suitable areas of the EU, when the pest is present to its maximum extent? | ||||||
| Results | % yield reduction | ||||||
| Percentiles: | 1% | 5% | 25% | 50% | 75% | 95% | 99% |
| EKE estimates: | 0.01% | – | 0.15% | 0.30% | 1.65% | – | 3.00% |
| EKE fitted: | 0.010% | 0.011% | 0.08% | 0.50% | 1.54% | 2.78% | 3.01 |
| Fitted distribution | BetaGeneral (0.34362, 0.85554, 0.01, 3.0605) | ||||||
FIGURE C.12.
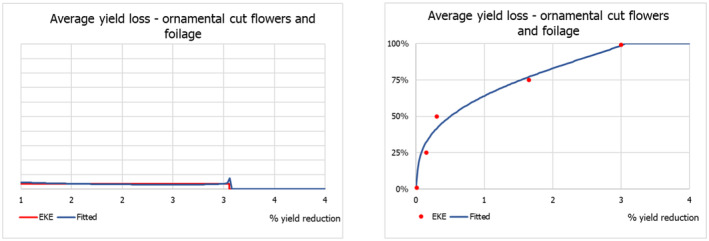
Distribution of the estimated % of cut flowers and cut foliage yield reduction directly attributable to Phlyctinus callosus fitted to EKE estimates.
Reasoning for the impact on ornamental plants yield reduction.
1st percentile
-
–
Cut flowers and cut foliage plants are grown in greenhouses where strict measures are used to prevent entry of pests. However, it is possible that pests enter via infested plant material from the nursery.
-
–
No reports exist on the impact of P. callosus on cut flowers and cut foliage crops in the countries where the pest is present.
-
–
Interceptions of P. callosus on (Cape) cut flowers (APHIS, 1984; Genka & Yoshitake, 2018) and ornamentals exist (Smith, 2004), but on plant species only that are not reported as hosts (Haran, Hansen et al. 2020). Therefore, these plants could be merely a hiding place for the weevil.
99th percentile
-
–
Similar species cause damage even though growers are aware and apply measures.
-
–
There is high uncertainty about how the pest would behave in a new environment and it is possible that P. callosus would shift to new hosts.
Median
-
–
Otiorhynchus spp. have similar biology and control measures currently applied for it would be effective also for P. callosus. Entomopathogenic nematodes currently used have shown to have lethal effect for P. callosus during experimental trials.
-
–
Phlyctinus callosus adults have been observed on plant species used as ornamentals (e.g. Agapanthus spp., Crassula spp.) in their original habitat, but in home gardens or in the wild, and not on cultivated crops.
Interquartile range: Maximum uncertainty considered.
APPENDIX D. Climate suitability maps
D.1. Monthly average temperature for the EU
The monthly average temperature for the EU is shown in Figure D.1. These maps were used in combination with the trade windows to evaluate the likely temperature encountered by P. callosus at arrival in the EU.
Figure D.1.
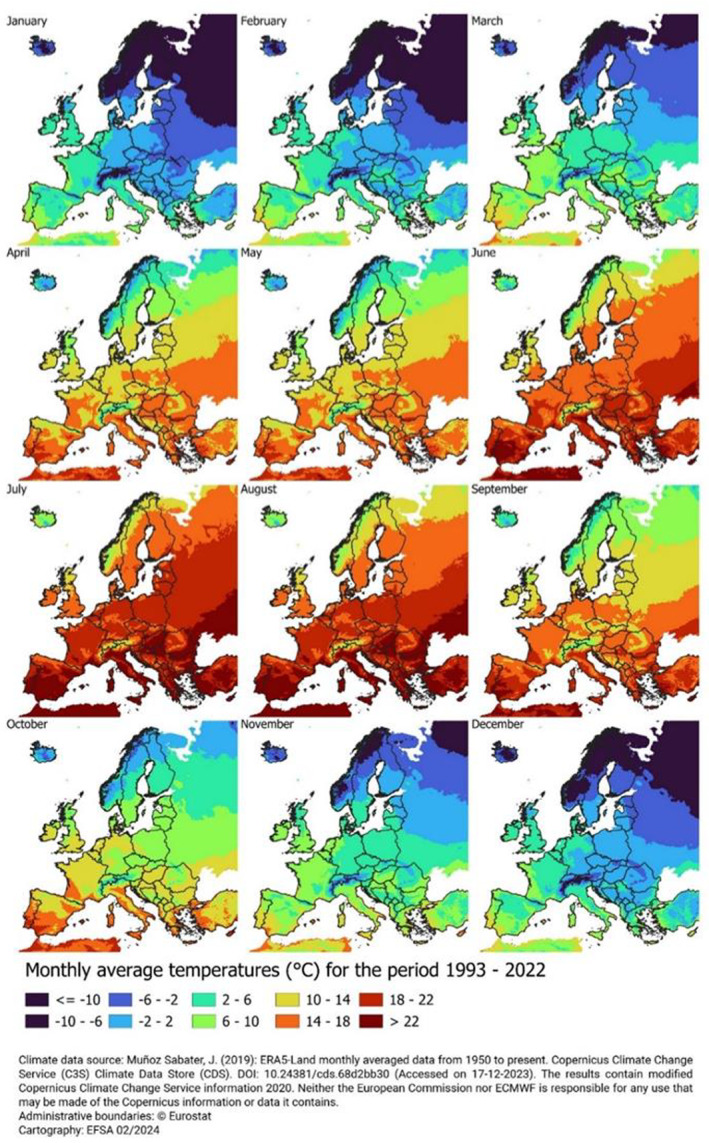
Monthly average temperature for the EU
D.2. Köppen–Geiger
Since the area of the assessment is the EU, the Köppen–Geiger climate matching map (Figure D.2) shows only climate types that are present in this area. If P. callosus was reported occurring in a climate type that does not occur in the EU, this climate is not mapped as a relevant climate for the assessment.
Figure D.2.
Köppen–Geiger climate types occurring in places where Phlyctinus callosus has been reported and in the EU.
D.3. Hardiness zone
The observed pest distribution was used to sample the average annual minimum temperature raster layer, corresponding to a specific hardiness zone, where the pest was observed. All the areas in EU having an equal or higher hardiness zone corresponding to the sampled minimum temperature were mapped. The hardiness zones map implemented for this analysis was based on the updated version of the USDA Plant Hardiness Zones (2023) which identifies 13 main zones, each divided into two subzones, for a total of 26 hardiness zones.
Figure D.3.
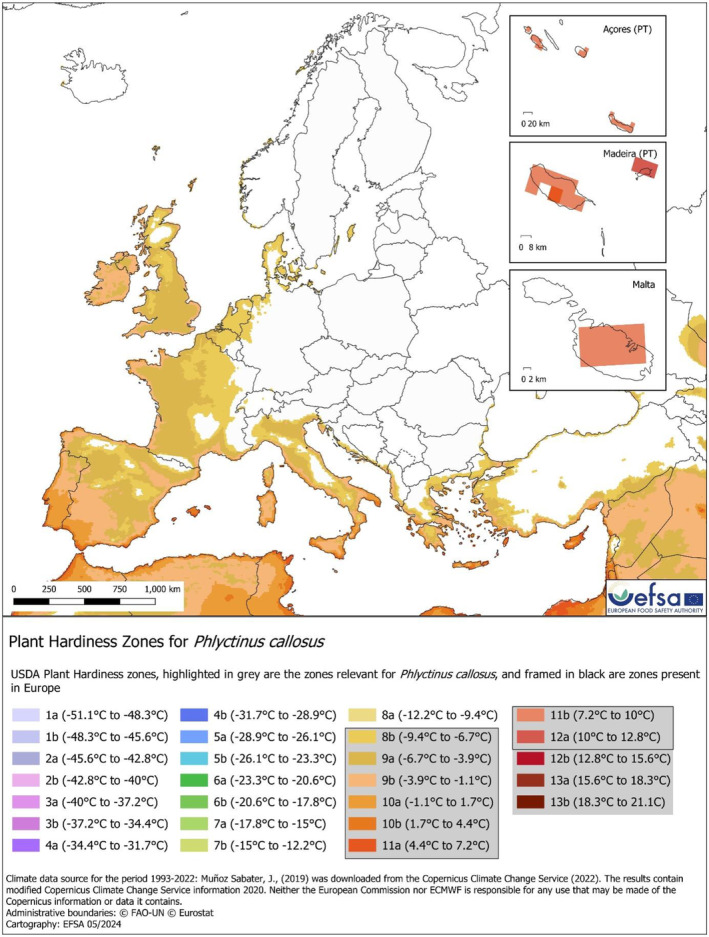
Hardiness zone map based on the average annual minimum temperature for the period 1993–2022. The map highlights the hardiness zones (highlighted in grey in the legend) in the EU where the average minimum temperature is higher or equal to the minimum value sampled using Phlyctinus callosus occurrences. The Hardiness zone map is based on the recent implementation of the USDA Plant Hardiness Zones (2023).
D.4. Maximum number of consecutive days below the LDT
The average maximum number of consecutive days below the lower development threshold was used as an indicator of climate conditions particularly unfavourable to the organism. The average maximum number of consecutive days below LDT for P. callosus was obtained by extracting the average maximum number of consecutive days (period 1993–2022) below the lower development threshold (6°C) for each pest observation point. The highest value obtained (84.53 days in Geraldine, New Zealand) was used as a threshold assuming longer period would not be suitable for the pest. The map in Figure D.4 shows in yellow the areas in EU having an equal or lower average maximum number of consecutive days below the LDT.
FIGURE D.4.
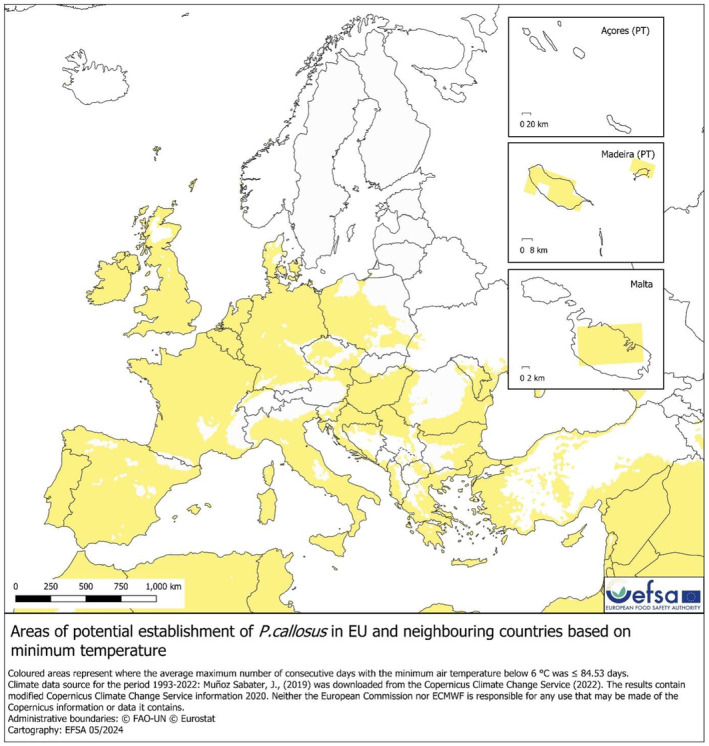
Areas in EU where the average maximum number of consecutive days with temperature below lower development threshold (6°C) is equal or below the maximum value (84.53 days in Geraldine, New Zealand) derived from the Phlyctinus callosus distribution occurrences.
EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard, C. , Baptista, P. , Chatzivassiliou, E. , Di Serio, F. , Gonthier, P. , Jaques Miret, J. A. , Justesen, A. F. , MacLeod, A. , Magnusson, C. S. , Navas‐Cortes, J. A. , Parnell, S. , Potting, R. , Reignault, P. L. , Stefani, E. , Civera, A. V. , van der Werf, W. , Yuen, J. , Zappalà, L. , … Milonas, P. (2024). Risk assessment of Phlyctinus callosus for the EU . EFSA Journal, 22(7), e8832. 10.2903/j.efsa.2024.8832
Adopted: 23 May 2024
Annex A is available under the Supporting Information section
This article was originally published on the EFSA website http://www.efsa.europa.eu on 03 July 2024 as part of EFSA’s publication procedures.
REFERENCES
- Anonymous . (1970). Weevil pests of plants in Victoria. Journal of Agricultural Victoria Department of Agriculture, 68, 244–249. [Google Scholar]
- APHIS . (1974). List of intercepted plant pests, 1972 (pests recorded from July 1, 1971, through June 30, 1972). APHIS 82 US Animal and Plant Health Inspection Service. [Google Scholar]
- APHIS . (1979). List of intercepted plant pests, (pests recorded from July 1, 1973, through September 30, 1977). APHIS 82 US Animal Plant Health Inspection Service. [Google Scholar]
- APHIS . (1982). List of intercepted plant pests, fiscal years 1980 and 1981. APHIS 82–8 US Animal Plant Health Inspection Service. [Google Scholar]
- APHIS . (1984). List of intercepted plant pests, fiscal years 1984. APHIS 82‐11 US Animal Plant Health Inspection Service. [Google Scholar]
- APHIS . (1986). List of intercepted plant pests, fiscal year 1985. APHIS 82‐12 US Animal Plant Health Inspection Service. [Google Scholar]
- APHIS . (1987). List of intercepted plant pests. Fiscal year 1986. APHIS 82‐13 US Animal Plant Health Inspection Service. [Google Scholar]
- Barnes, B. (2014). First record of a fairyfly, Cleruchus depressus (Annecke)(Hymenoptera: Mymaridae), parasitizing eggs of banded fruit weevil, Phlyctinus callosus Schönherr (coleoptera: Curculionidae), in South Africa. African Entomology, 22(4), 900–905. [Google Scholar]
- Barnes, B. N. (1987). Bionomics, behaviour and monitoring of the vine snoutbeetle, Phlyctinus callosus Boh., in deciduous fruit orchards, with proposals for an improved control strategy. Stellenbosch University. [Google Scholar]
- Barnes, B. N. (1989a). Different life and seasonal cycles of banded fruit weevil, Phlyctinus callosus (Coleoptera: Curculionidae), in apple orchards in the south‐western cape. Phytophylactica, 21(2), 147–158. [Google Scholar]
- Barnes, B. N. (1989b). Embryonic and immature stages of Phlyctinus callosus Boh. (coleoptera: Curculionidae): Aspects of biology and behaviour with respect to control in deciduous fruit orchardsl. Journal of the Entomological Society of Southern Africa, 52(1), 165–178. [Google Scholar]
- Barnes, B. N. , & Capatos, D. (1989). Evidence for an aggregation pheromone in adult frass of banded fruit weevil, Phlyctinus callosus (Schoenherr) (Col., Curculionidae). Journal of Applied Entomology, 108(1–5), 512–518. [Google Scholar]
- Barnes, B. N. , & Giliomee, J. H. (1992). Fruit‐feeding behaviour of banded fruit weevil, Phlyctinus callosus (Schönherr) (Col., Curculionidae), in apple orchards. Journal of Applied Entomology, 113(1–5), 407–415. [Google Scholar]
- Barnes, B. N. , Knipe, M. C. , & Calitz, F. J. (1994). Trunk barriers provide effective control of banded fruit‐weevil on apples and nectarines. Deciduous Fruit Grower, 44(9), 322–327. [Google Scholar]
- Barnes, B. N. , Knipe, M. C. , & Calitz, F. J. (1995). Effective weevil control on apple trees with batting trunk barriers. Deciduous Fruit Grower, 45(9), 376–378. [Google Scholar]
- Barnes, B. N. , Knipe, M. C. , & Calitz, F. J. (1996). Latest results with trunk exclusion barriers for weevil control on apples. Deciduous Fruit Grower, 46(8), 284–287. [Google Scholar]
- Barnes, B. N. , & Pringle, K. L. (1989). Oviposition by the banded fruit weevil, Phlyctinus callosus (Schoenherr) (coleoptera: Curculionidae), in deciduous fruit orchards in South Africa. Bulletin of Entomological Research, 79(1), 31–40. [Google Scholar]
- Barnes, B. N. , & Swart, P. L. (1977). A new look at snoutbeetles on apples. Deciduous Fruit Grower, 27(8), 258–263. [Google Scholar]
- Barnes, B. N. , & Swart, P. L. (1980). A setback for snoutbeetles in apple orchards. Deciduous Fruit Grower, 30(8), 280–284. [Google Scholar]
- Bennison, J. , Chandler, D. , Prince, G. , Pope, T. , Atwood, J. , Talbot, D. , Roberts, H. , & Creed, C. (2014). A review of vine weevil knowledge in order to design best‐practice IPM protocols suitable for implementation in UK horticulture. HDC Project CP111 Final Report, 5. [Google Scholar]
- Bourke, A. (2020). Rapid Pest Risk Analysis (PRA) for Phlyctinus callosus Ireland Department of Agriculture Foodand the Marine. 53 pp. https://pra.eppo.int/getfile/9c71c4b6‐fabc‐4e68‐a9ae‐e4a27f863fd9
- Bredenhand, E. , van Hoorn, A. , May, F. , Ferreira, T. , & Johnson, S. (2010). Evaluation of techniques for monitoring banded fruit weevil, Phlyctinus callosus (Schoenherr) (coleoptera: Curculionidae), infestation in blueberry orchards. African Entomology, 18(1), 205–209. [Google Scholar]
- Brown, E. B. (2004). Phlyctinus callosus Boheman 1834 (Curculionidae, Otiorhynchini) ‐ recorded in Great Britain. Coleopterist, 13(4), 160–161. [Google Scholar]
- CABI . (2020). Phlyctinus callosus (vine calandra). [Accessed: January 2024]. 10.1079/cabicompendium.40299 [DOI]
- CBI . (2023). The European market potential for table grapes. [Accessed: February 2024]. https://www.cbi.eu/market‐information/fresh‐fruit‐vegetables/table‐grapes/market‐potential
- Curran, J. , & Patel, V. (1988). Use of a trickle irrigation system to distribute entomopathogenic nematodes (Nematoda: Heterorhabditidae) for the control of weevil pests (coleoptera: Curculionidae) of strawberries. Australian Journal of Experimental Agriculture, 28(5), 639–643. [Google Scholar]
- de Aguiar, A. C. , Higuchi, M. T. , Yamashita, F. , & Roberto, S. R. (2023). SO2‐generating pads and packaging materials for postharvest conservation of table grapes: A review. Horticulturae, 9(6), 724. [Google Scholar]
- de Villiers, M. , & Pringle, K. L. (2008). Developing a generic sampling system for monitoring the key arthropod pests of table grapes, Vitis vinifera L. International Journal of Pest Management, 54(3), 207–217. [Google Scholar]
- Dlamini, B. E. , Addison, P. , & Malan, A. P. (2019). A review of the biology and control of Phlyctinus callosus (Schonherr) (coleoptera: Curculionidae), with special reference to biological control using entomopathogenic nematodes and fungi. African Entomology, 27(2), 279–288. [Google Scholar]
- Dlamini, B. E. , Malan, A. P. , & Addison, P. (2019). Control of the banded fruit weevil Phlyctinus callosus (coleoptera: Curculionidae) using entomopathogenic nematodes. Austral Entomology, 58(3), 687–695. [Google Scholar]
- Dlamini, B. E. , Malan, A. P. , & Addison, P. (2020a). Combined effect of entomopathogenic fungi and Steinernema yirgalemense against the banded fruit weevil, Phlyctinus callosus (coleoptera: Curculionidae). Biocontrol Science and Technology, 30(11), 1169–1179. [Google Scholar]
- Dlamini, B. E. , Malan, A. P. , & Addison, P. (2020b). Control of the banded fruit weevil, Phlyctinus callosus (Schonherr) (coleoptera: Curculionidae), using entomopathogenic fungi. African Entomology, 28(1), 106–114. [Google Scholar]
- Dlamini, B. E. , Malan, A. P. , & Addison, P. (2020c). Entomopathogens from agricultural soil and their potential to control the banded fruit weevil, Phlyctinus callosus (Schonherr) (coleoptera: Curculionidae). African Entomology, 28(2), 374–384. [Google Scholar]
- DROPSA . (2016a). DROPSA Deliverable 1.3 Report for Apples – Fruit pathway and Alert List. [Accessed: January 2024]. https://www.eppo.int/media/uploaded_images/RESOURCES/special_projects/dropsa/4_apple_report.pdf
- DROPSA . (2016b). DROPSA Deliverable 1.3 Report for Table grapes – Fruit pathway and Alert List. [Accessed: January 2024]. https://www.eppo.int/media/uploaded_images/RESOURCES/special_projects/dropsa/4_vitis_report.pdf
- DTU31 . (2023). Procedure operative per l'esecuzione dei controlli fitosanitari sulle merci in importazione. [Accessed: May 2024]. https://www.ilpuntocoldiretti.it/wp‐content/uploads/2024/01/dtu‐n.‐31‐procedure‐controlli‐allimport‐rev‐1_signed.pdf
- Duvenhage, A. J. , & Johnson, S. A. (2014). The potential of irradiation as a postharvest disinfestation treatment against Phlyctinus callosus (coleoptera: Curculionidae). Journal of Economic Entomology, 107(1), 154–160. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Saftey Authority) . (2014). Guidance on expert knowledge elicitation in food and feed safety risk assessment. EFSA Journal, 12(6), 3734, 278 pp. 10.2903/j.efsa.2014.3734 [DOI] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) . (2018). Guidance on quantitative pest risk assessment. EFSA Journal, 16(8), e05350. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) . Bragard, C. , Di Serio, F. , Gonthier, P. , Jaques Miret, J. A. , Justesen, A. F. , Magnusson, C. S. , Milonas, P. , Navas‐Cortes, J. A. , Parnell, S. , Potting, R. , Reignault, P. L. , Thulke, H.‐H. , Van der Werf, W. , Vicent Civera, A. , Yuen, J. , Zappalà, L. , Gregoire, J.‐C. , Malumphy, C. , … MacLeod, A. (2021). Pest categorisation of Phlyctinus callosus . EFSA Journal, 19(8), 6800. 10.2903/j.efsa.2021.6800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) . Bragard, C. , Baptista, P. , Chatzivassiliou, E. , DiSerio, F. , Gonthier, P. , Jaques Miret, J. A. , Justesen, A. F. , MacLeod, A. , Magnusson, C. S. , Milonas, P. , Navas‐ Cortes, J. A. , Parnell, S. , Potting, R. , Reignault, P. L. , Stefani, E. , Thulke, H.‐H. , Civera, A. V. , Yuen, J. , … Van der Werf, W. (2024a). Pest risk assessment of Leucinodes orbonalis for the European Union. EFSA Journal, 22(3), e8498. 10.2903/j.efsa.2024.8498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) . Bragard, C. , Baptista, P. , Chatzivassiliou, E. , DiSerio, F. , Gonthier, P. , Jaques Miret, J. A. , Justesen, A. F. , MacLeod, A. , Magnusson, C. S. , Milonas, P. , Navas‐Cortes, J. A. , Parnell, S. , Potting, R. , Reignault, P. L. , Stefani, E. , Thulke, H.‐H. , van der Werf, W. , Yuen, J. , … Vicent Civera, A. (2024b). Pest risk assessment of Retithrips syriacus for the EU. EFSA Journal, 22(4), e8741. 10.2903/j.efsa.2024.8741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PPR Panel (EFSA Plant Protection Products and their Residues) . Brancato, A. , Brocca, D. , Ferreira, L. , Greco, L. , Jarrah, S. , Leuschner, R. , Medina, P. , Miron, I. , Nougadere, A. , Pedersen, R. , Reich, H. , Santos, M. , Stanek, A. , Tarazona, J. , Theobald, A. , & Villamar‐Bouza, L. (2018). Use of EFSA pesticide residue intake model (EFSA PRIMo revision 3). EFSA Journal, 16(1), e05147. 10.2903/j.efsa.2018.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUROSTAT . (2018). Methodological manual on territorial typologies.
- EUROSTAT . (2022). Vineyards in the EU ‐ statistics. [Accessed: May 2024]. https://ec.europa.eu/eurostat/statistics‐explained/index.php?title=Vineyards_in_the_EU_‐_statistics
- Ferreira, T. (2010). Rearing of the banded fruit weevil, Phlyctinus callosus (Schonherr) (Coleoptera: Curculionidae) and control with entomopathogenic nematodes (Doctoral dissertation. University of Stellenbosch. [DOI] [PubMed] [Google Scholar]
- Ferreira, T. , & Malan, A. P. (2010). Controlling the banded fruit weevil using entomopathogenic nematodes. SA Fruit Journal, 9(4), 31–33. [Google Scholar]
- Genka, M. , & Yoshitake, H. (2018). Chronological change of taxonomic composition of exotic weevils (coleoptera: Curculionoidea) intercepted in import plant quarantine at Narita international airport. 植物防疫所調査研究報告(植防研報), 54, 1–47. [Google Scholar]
- Giliomee, J. H. (1961). Egg‐laying habits in the laboratory of six species of vine snout beetles (Coleoptera: Curculionidae). South African Journal of Agricultural Science, 4(2), 261–262. [Google Scholar]
- Golic, D. , Gobbi, A. , Campese, C. , Rossi, E. , Terzidou, A. , Crotta, M. , & Maiorano, A. (2024). EFSA climate suitability analysis of Phlyctinus callosus . Zenodo. 10.5281/zenodo.10693980 [DOI] [Google Scholar]
- Hansen, S. , Haran, J. M. , Johnson, S. A. , Hévin, N. M.‐C. , & Addison, P. (2024). New data on an old pest complex: The status of Phlyctinus callosus Schönherr and Phlyctinus xerophilus haran (coleoptera: Curculionidae) in South Africa. African Entomology, 32, 5 pp. [Google Scholar]
- Hansen, S. P. , Malan, A. P. , Haran, J. M. , & Addison, P. (2024). Susceptibility of adult Phlyctinus (coleoptera: Curculionidae) to entomopathogens: A first look at potential differences in a newly revised species complex. Journal of Applied Entomology, 148(2), 129–139. [Google Scholar]
- Haran, J. M. , Hansen, S. , Benoit, L. , & Addison, P. (2020). Description of five new species in the genus Phlyctinus Schoenherr (coleoptera, Curculionidae): A first step in deciphering the P. Callosus complex. European Journal of Taxonomy, 669, 1–29. [Google Scholar]
- Hevin, N. M. C. , Hansen, S. , Addison, P. , Benoit, L. , Kergoat, G. J. , & Haran, J. (2022). Late Cenozoic environmental changes drove the diversification of a weevil genus endemic to the cape floristic region. Zoologica Scripta, 51(6), 724–740. [Google Scholar]
- Horne, P. A. , & Stacpoole, C. A. (1989). An efficient technique for rearing Phlyctinus callosus Boheman (coleoptera: Curculionidae). Australian Journal of Entomology, 28(2), 152. [Google Scholar]
- Hort, F. (2001). Speak No weevil: What rhododendron growers said about their root weevil management. North American Root Weevil Workshop. [Google Scholar]
- Huysamer, A. , Hoffman, E. , & Johnson, S. (2017). Novel technologies for the postharvest treatment of Cape Flora to control phytosanitary insect pests. VII International Conference on Managing Quality in Chains (MQUIC2017) and II International Symposium on Ornamentals in 1201.
- Huysamer, A. J. (2016). New tools to bash bugs. Innovate Magazine, 6, 62–65. [Google Scholar]
- Huysamer, A. J. (2018). An assessment of alternative postharvest technologies for the disinfestation of fresh cape Flora cut flowers for export from South Africa. Stellenbosch University. [Google Scholar]
- Johnson, S. A. , & Neven, L. G. (2011). Heated‐controlled atmosphere postharvest treatments for Macchiademus diplopterus (Hemiptera: Lygaeidae) and Phlyctinus callosus (coleoptera: Curculionidae). Journal of Economic Entomology, 104(2), 398–404. [DOI] [PubMed] [Google Scholar]
- Klingenberg, M. D. , Björklund, N. , & Aukema, B. H. (2010). Seeing the forest through the trees: Differential dispersal of Hylobius warreni within modified forest habitats. Environmental Entomology, 39(3), 898–906. [DOI] [PubMed] [Google Scholar]
- Kuschel, G. (1972). The foreign Curculionidea established in New Zealand (lnsecta: Coleoptera). New Zealand Journal of Science, 15(3), 272–289. [Google Scholar]
- Learmonth, S. , Gibberd, M. , & Stanaway, M. (2011). Sustainable protection of grapevines from garden weevil. https://www.wineaustralia.com/getmedia/8ff7e0eb‐0184‐4a39‐9b24‐aadde05b5099/RD‐05‐01‐3
- Lo, P. L. , Blank, R. H. , & Parker, R. E. (1990). Insecticides for adult garden weevil and problems with control on glasshouse grapes. 43rd New Zealand weed and pest control conf, dunedin, New Zealand .
- MAF . (2009). Import risk analysis: Table grapes (Vitis vinifera) from China (p. 322). MAF Biosecurity New Zealand. [Google Scholar]
- Magagula, M. M. (2019). Diversity and ecology of phytophagous weevils in the deciduous fruit industry. Stellenbosch University. [Google Scholar]
- Maier, C. T. (1978). Dispersal of adults of the black vine weevil, Otiorhynchus sulcatus (coleoptera: Curculionidae), in an urban area. Environmental Entomology, 7(6), 854–857. [Google Scholar]
- Martin, N. (2011). Further observations on host plants of adult weevil (coleoptera: Curculionidae). Weta, 42, 14–20. [Google Scholar]
- May, B. M. (1966). Identification of the immature forms of some common soil‐inhabiting weevils, with notes on their biology. New Zealand Journal of Agricultural Research, 9(2), 286–316. [Google Scholar]
- Miller, L. A. (1979). Weevil pests of horticultural crops. Journal of Agriculture, Tasmania, 50(2), 52–53. [Google Scholar]
- Myburgh, A. C. , Bosman, I. P. , & Holtzhausen, H. P. (1975). Pest management: Methods of assessing results on apples and pears. The D Eciduous Fruit Grower, 25, 135–141. [Google Scholar]
- Myburgh, A. C. , & Kriegler, P. J. (1968). Experiments on sterilization of the snout‐beetles, Philyctinus callosus Boh. and Eremnus setulosus Boh., on export grapes in cold storage. Journal of the Entomological Society of Southern Africa, 29, 96–101. [Google Scholar]
- Myburgh, A. C. , Rust, D. J. , & Stubbings, D. (1975). Snoutbeetles (kalanders) on apples. Deciduous Fruit Grower, 25(8), 208–211. [Google Scholar]
- Nel, P. J. , & Addison, M. F. (1993). The development of an integrated pest management programme in apple orchards in Elgin, South Africa and the implications for integrated fruit production. Acta Horticulturae, 347, 323–326. [Google Scholar]
- Ngwenya, M. S. (2021). Postharvest insect pest disinfestation in export Proteaceae cut flowers‐the potential of new disinfestation strategies. Stellenbosch University. [Google Scholar]
- Oberprieler, R. , & Zimmerman, E. (2020). Australian weevils (coleoptera: Curculionoidea) IV: Curculionidae: Entiminae part I. CSIRO PUBLISHING. [Google Scholar]
- OECD . (2007). Table grapes, international standards for fruit and vegetables. OECD Publishing. [Google Scholar]
- Opatowski, D. (2006). Bilateral quarantine arrangement between the Department of Agriculture of the Republic of South Africa (DoA SA) and the Plant Protection and Inspection Services of Israel (PPIS) regarding the Conditions for the Importation of fresh grapes (Vitis vinifera) from the Republic of South Africa into Israel. Prepared by David Opatowski, PRA, PPIS, Israel. http://www.daff.gov.za/images/Branches/AgricProducHealthFoodSafety/PlantProductionHealth/PlantHealth/export‐from‐sa/specialexportprotocols‐prog‐dir/israel/plantex.pdf
- Popay, A. (1990). Proceedings of the forty‐third New Zealand weed and Pest control conference.
- Pope, T. , Gundalai, E. , Elliott, L. , Blackshaw, R. , Hough, G. , Wood, A. , Bennison, J. , Prince, G. , & Chandler, D. (2015). Recording the movement of adult vine weevil within strawberry crops using radio frequency identification tags. Journal of Berry Research, 5(4), 197–206. [Google Scholar]
- PPECB . (2023a). Intransit Handling Protocol ‐ HP04 ‐ USA (T107‐a) apples and pears. [Accessed: January 2024]. https://ppecb.com/wp‐content/uploads/2023/05/Intransit‐Handling‐Protocol‐HP04‐USA‐T107‐a‐apples‐and‐pears‐ID‐1819.pdf
- PPECB . (2023b). Ordinary Handling Protocol ‐ HP27 ‐ Handling Procedure for Table Grapes. [Accessed: January 2024]. https://ppecb.com/wp‐content/uploads/2023/05/Ordinary‐Handling‐Protocol‐HP27‐Handling‐Procedure‐for‐Table‐Grapes‐ID‐1856.pdf
- Prestidge, R. A. , & Willoughby, B. (1989). Garden weevil life cycle and insecticides for its control in asparagus. 42nd New Zealand weed and Pest control conf, New Plymouth, New Zealand.
- Prestidge, R. A. , & Willoughby, B. (1990). Control of garden weevil (Phlyctinus callosus) larvae and pupae with a parasitic nematode and a fungal pathogen. 43rd New Zealand weed and Pest control conference.
- Pringle, K. L. , Barnes, B. N. , & Blomfield, T. L. (2015). Deciduous fruit and nut trees and olive – Apple. In Prinsloo G. H. U. (Ed.), Insects of cultivated plants and natural pastures in southern Africa (pp. 350–365). Entomological Society of Southern Africa. [Google Scholar]
- Pryke, J. S. , & Samways, M. J. (2007). Current control of phytosanitary insect pests in table grape vineyards of the Hex River valley, South Africa. African Entomology, 15(1), 25–36. [Google Scholar]
- Reineke, A. , Hirsch, J. , & Kubach, G. (2011). Aggregation, abundance and dispersal capabilities of Otiorhynchus rugosostriatus Goeze and Otiorhynchus raucus Fabricius (coleoptera: Curculionidae) in plantations of ornamental plants. Journal of Pest Science, 84(3), 297–302. [Google Scholar]
- Saunders, C. M. , & Hayes, P. (2007). Air freight transport of fresh fruit and vegetables.
- Schwartz, A. (1988). Efficacy of trunk barriers for the control of key pests on trellised grapevines. South African Journal for Enology and Viticulture, 9(1), 16–18. [Google Scholar]
- Scott, R. , & Mason, K. (1984). New Zealand pest and beneficial insects. Lincoln University College of Agriculture. [Google Scholar]
- Scurr, G. , Kirkpatrick, J. B. , Daniels, G. D. , & McQuillan, P. B. (2008). Biotic resistance to Chrysanthemoides monilifera ssp monilifera in Tasmania. Austral Ecology, 33(8), 941–950. [Google Scholar]
- Smith, R. (2004). Pest risk Analysisfor Phlyctinus callosus. Central Science Laboratory, Sand Hutton, York YO411LZ UK. [Available on request from Defra].
- St Pierre, M. J. , & Hendrix, S. D. (2003). Movement patterns of Rhyssomatus lineaticollis say (coleoptera: Curculionidae) within and among Asclepias syriaca (Asclepiadaceae) patches in a fragmented landscape. Ecological Entomology, 28(5), 579–586. [Google Scholar]
- Swart, P. L. , Barnes, B. N. , & Greeff, H. J. (1976). Snoutbeetles (kalanders) on apple trees: Preliminary evaluation of candidate insecticides for their control. Deciduous Fruit Grower, 26(8), 308–312. [Google Scholar]
- Swart, P. L. , Barnes, B. N. , & Myburgh, A. C. (1976). Pests of table grapes in the Western cape. Deciduous Fruit Grower, 26(5), 169–172. 174–179, 181–183, 187–195. [Google Scholar]
- Tomkins, B. (2018). Effect of Sulphur Dioxide and Cold on Survival of Insects During Storage of Table Grapes.
- USDA . (1940). List of intercepted plant pests, 1939 (List of Pests Recorded During the Period July 1, 1938, to June 30, 1939, Inclusive, as Intercepted in, on, or with Plants and Plant Products Entering United States Territory.).
- USDA . (1948. –1963). List of intercepted plants pests, 1948–1963. List of pests recorded during the period July 1, 1947 to June 30, 1963, inclusive, as intercepted in, on, or with plants and plant products entering United States territory.
- USDA . (1964. –1970). List of intercepted plant pests, 1964–1970 (Pests recorded from July 1, 1963, through June 30, 1970).
- Van Den Berg, H. C. (1971). The biology and control of vine snout beetles. Deciduous Fruit Grower, 21, 83–85. [Google Scholar]
- Walker, P. L. (1981). Laboratory rearing of the garden weevil, Phlyctinus callosus boheman (coleoptera: Curculionidae), and the effect of temperature on its growth and survival. Australian Journal of Zoology, 29(1), 1–5. [Google Scholar]
- Whittle, K. (1986). Pests not known to occur in the United States or of limited distribution no. 72: Garden weevil. Phlyctinus callosus Boheman. U.S. Department of Agriculture APHIS‐PPQ APHIS 81‐49, 1–11.
- Witt, A. B. R. , Little, R. M. , & Crowe, T. M. (1995). The effectiveness of helmeted guineafowl Numida meleagris (Linnaeus 1766) in controlling the banded fruit weevil Phlyctinus callosus (Schonherr 1826), and their impact on other invertebrates in apple orchards in the Western Cape Province, South Africa. Agriculture Ecosystems & Environment, 55(3), 169–179. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material to the ‘Pest risk assessment of Phlyctinus callosus for the European Union‘ ‐ Model


