Abstract
Background
The role of tyrosine kinase inhibitors (TKIs) in early-stage and metastatic oncogene-driven non–small cell lung cancer (NSCLC) is established, but it remains unknown how best to integrate TKIs with concurrent chemoradiotherapy (cCRT) in locally advanced disease. The phase 2 ASCENT trial assessed the efficacy and safety of afatinib and cCRT with or without surgery in locally advanced epidermal growth factor receptor (EGFR)-mutant NSCLC.
Patients and Methods
Adults ≥18 years with histologically confirmed stage III (AJCC 7th edition) NSCLC with activating EGFR mutations were enrolled at Mass General and Dana-Farber/Brigham Cancer Centers, Boston, Massachusetts. Patients received induction afatinib 40 mg daily for 2 months, then cisplatin 75 mg/m2 and pemetrexed 500 mg/m2 IV every 3 weeks during RT (definitive or neoadjuvant dosing). Patients with resectable disease underwent surgery. All patients were offered consolidation afatinib for 2 years. The primary endpoint was the objective response rate (ORR) to induction TKI. Secondary endpoints were safety, conversion to operability, progression-free survival (PFS), and overall survival (OS). Analyses were performed on the intention-to-treat population.
Results
Nineteen patients (median age 56 years; 74% female) were enrolled. ORR to induction afatinib was 63%. Seventeen patients received cCRT; 2/9 previously unresectable became resectable. Ten underwent surgery; 6 had a major or complete pathological response. Thirteen received consolidation afatinib. With a median follow-up of 5.0 years, median PFS and OS were 2.6 (95% CI, 1.4-3.1) and 5.8 years (2.9-NR), respectively. Sixteen recurred or died; 6 recurrences were isolated to CNS. The median time to progression after stopping consolidation TKI was 2.9 months (95% CI, 1.1-7.2). Four developed grade 2 pneumonitis. There were no treatment-related deaths.
Conclusion
We explored the efficacy of combining TKI with cCRT in oncogene-driven NSCLC. Induction TKI did not compromise subsequent receipt of multimodality therapy. PFS was promising, but the prevalence of CNS-only recurrences and rapid progression after TKI discontinuation speak to unmet needs in measuring and eradicating micrometastatic disease.
Keywords: lung cancer, radiation oncology, surgical oncology, EGFR-mutant, stage III, oncogenes
The phase 2 ASCENT trial assessed the efficacy and safety of afatinib and concurrent chemoradiotherapy with or without surgery in locally advanced epidermal growth factor receptor-mutant non–small cell lung cancer.
Implications for Practice.
It remains unclear how best to combine TKIs and local therapy in stage III EGFR-mutant NSCLC. ASCENT is a single-arm phase 2 study of afatinib and cCRT with/without surgery in stage III EGFR-mutant NSCLC. Though a small study, ASCENT shows that some patients can have long-term disease control and even cure with ≤2 years of afatinib. Of note, a phase 3 trial has been completed in which similar patients receive cCRT and indefinite consolidation osimertinib. Our findings could help guide shared decision-making between patients and providers about whether and when to stop EGFR TKIs after cCRT.
Introduction
Local and distant tumor recurrences have been a major challenge in the treatment of locally advanced, stage III non–small cell lung carcinoma (NSCLC), but the introduction of immune checkpoint inhibitors improved outcomes. In unresectable disease, immunotherapy is added after definitive concurrent chemoradiation therapy (cCRT) based on the PACIFIC study,1,2 and in resectable disease, immunotherapy is added with chemotherapy pre- or perioperatively.3-8 However, the benefits of immune checkpoint inhibition have not generally extended to patients with oncogene-driven NSCLC, such as those harboring epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) rearrangements.9,10 Indeed, patients with EGFR or ALK alterations were excluded from most perioperative immunotherapy trials by design, given the known lack of durable benefit in oncogene-driven metastatic cases. In particular, EGFR patients treated with the PACIFIC strategy do not appear to benefit.1,11,12
Tyrosine kinase inhibitors (TKIs) have improved outcomes, including overall survival, in both metastatic and early-stage oncogene-driven lung adenocarcinoma.13-20 However, it remains unclear how best to combine TKIs and local therapy (ie, cCRT and/or surgery) in locally advanced NSCLC. A large-scale trial (LAURA) is ongoing in which patients with stage III unresectable EGFR-mutant lung cancer receive cCRT followed by indefinite consolidation osimertinib.21 In early 2024, a press release announced that LAURA had shown a significant progression-free survival (PFS) benefit.22 A few more years will likely be needed for overall survival (OS) data to mature. In the interim, this is the final report of the ASCENT study, which was designed in the pre-osimertinib era to assess whether afatinib, a second-generation, irreversible, pan-ERBB TKI could be safely incorporated both before and after trimodality therapy or definitive cCRT in patients with locally advanced EGFR-mutant lung adenocarcinoma.
Patients and methods
Patient selection
This multi-institutional phase 2 trial (NCT01553942) enrolled patients aged ≥18 years with histologically confirmed locally advanced, stage III (AJCC 7th edition) NSCLC. Tumors were required to have an activating EGFR mutation confirmed by next-generation sequencing or PCR-based assay on tumor tissue. For further eligibility and details, see Study Protocol in Supplementary Data. The study protocol was approved by our local institutional review board, all patients provided written, informed consent, and the study was funded by Boehringer Ingelheim Pharmaceuticals, Inc.
Study design and treatment
Required staging included fluorodeoxyglucose-positron emission tomography, computed tomography (CT) of the chest, abdomen, and pelvis, and brain magnetic resonance imaging, and all participants were assessed for surgical resectability by a multidisciplinary team including a thoracic surgeon. Induction afatinib was administered at 40 mg orally per day for two 4-week cycles. In the absence of progression on post-afatinib CT scan, patients proceeded to cCRT either with preoperative or definitive dosing (45-54 Gy or up to 72 Gy, respectively), which was the standard practice at our institutions at the time. Cisplatin 75 mg/m2 and pemetrexed 500 mg/m2 were administered intravenously concurrent with RT for 2 cycles if RT was preoperative and up to 4 cycles if RT was given with definitive intent. Unless there was a lack of response to induction, afatinib was continued as consolidation for up to 2 years after cCRT with or without surgery.
Endpoints and statistics
The primary endpoint was the objective response rate (ORR) to induction afatinib, assessed using RECIST.23 Notably, no RECIST-defined confirmatory scans were possible with our study design because patients with partial or complete response proceeded directly to cCRT without waiting 4 weeks to obtain a second scan to confirm the response. A sample size of 30 patients was predicted to provide 87% power to detect the hypothesized ORR of 65%, which was chosen based on afatinib’s activity in the metastatic setting.24-26 Secondary endpoints included safety assessments, graded using the Common Terminology Criteria for Adverse Events version 4.0,27 conversion to operability following induction afatinib, PFS, and overall survival (OS). Pathologic responses were evaluated in resected specimens. The study closed early for slow accrual. All analyses were performed on the intention-to-treat population unless specifically noted. Time-to-event analyses were performed using the Kaplan-Meier method.
Results
Study population
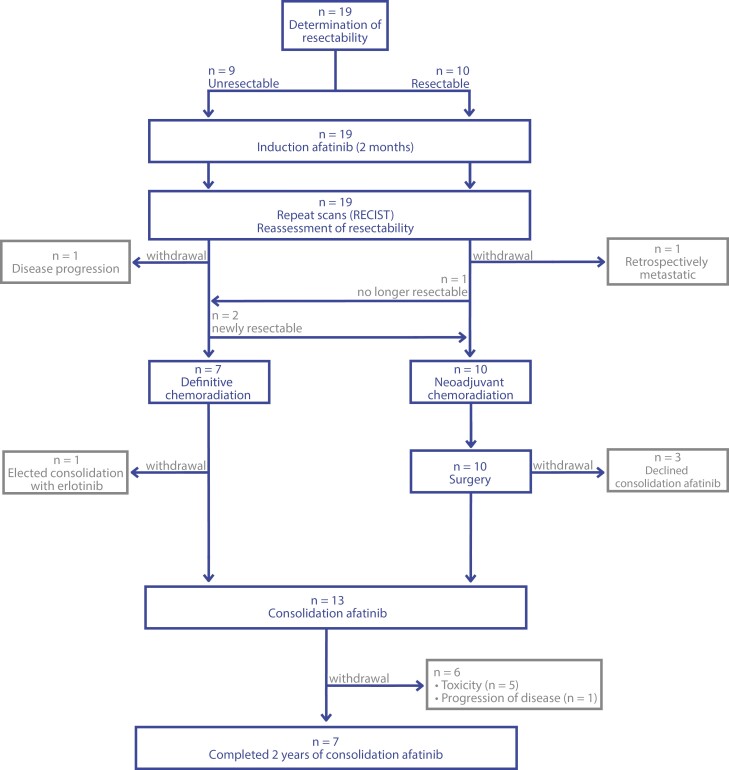
Nineteen patients were enrolled and initiated on protocol therapy from September 2012 to January 2020 (Figure 1). Patient and tumor characteristics are summarized in Table 1. Of note, eligibility criteria were based on 7th edition AJCC staging, but conversion to 8th edition staging is reported here to better inform current clinical decision-making.28,29 There were 14 females, and the median age was 56 years. Activating EGFR mutations were exon 19 deletions in 12 cases and L858R mutations in 6 cases. One patient had a NSCLC with an EGFR exon 18 deletion delE709_T710insD. At the time of diagnosis, the cancer in 10 patients was classified as potentially resectable, while in 9 it was classified as unresectable.
Figure 1.
Modified CONSORT diagram.
Table 1.
Patient and tumor characteristics in the ITT population.
| Characteristic | n = 19, No. (% or range) |
|---|---|
| Age | 56 (34-75) |
| Gender | |
| Female | 14 (74) |
| Male | 5 (26) |
| Race | |
| White | 13 (68) |
| Asian | 6 (32) |
| Tobacco use history | |
| Never | 9 (47) |
| Former | 10 (53) |
| Histology | |
| Adenocarcinoma | 19 (100) |
| Other | 0 (0) |
| EGFR mutation | |
| Exon 19 deletion | 11 (58) |
| L858R | 7 (37) |
| Other | 1 (5) |
| Clinical AJCC stage (8th edition) | |
| IIIA (T1-4 N0-2) | 4 (21) |
| T1N2 | 1 (5) |
| T2N2 | 3 (16) |
| IIIB (T1-4 N2-3) | 11 (58) |
| T2N3 | 3 (16) |
| T3N2 | 7 (37) |
| T4N2 | 1 (5) |
| IIIC (T3-4 N3) | 4 (21) |
| T3N3 | 2 (10) |
| T4N3 | 2 (10) |
| Deemed resectable at diagnosis | |
| Yes | 10 (53) |
| No | 9 (47) |
Abbreviations: ITT, intention to treat; AJCC, American Joint Committee on Cancer.
Treatments
All 19 patients completed 2 months of induction afatinib (Figure 1). Upon restaging, 2 patients initially characterized as having potentially resectable tumor developed disease progression or exhibited findings that clarified their initial presentation as metastatic and did not continue to cCRT. Ten patients underwent neoadjuvant cCRT followed by surgery (including 2 with disease initially deemed unresectable who exhibited dramatic responses to afatinib), and 7 patients underwent definitive cCRT. For patients proceeding to surgery, the median radiation dose was 54 Gy (range, 45-66 Gy) and the median number of chemotherapy cycles was 2 (range 1-2; Supplementary Figure S1). For patients receiving definitive cCRT, the median radiation dose was 66.6 Gy (range, 63-72 Gy) and the median number of chemotherapy cycles was 3 (range 2-4).
Among the 17 patients who received cCRT, 13 proceeded to consolidation afatinib, 3 opted for surveillance off of TKI, and 1 patient came off-protocol to receive consolidation erlotinib instead of afatinib. Seven out of 13 patients completed the planned 2-year course of consolidation afatinib, while 5 discontinued early for toxicity and one discontinued for progressive disease (median time on afatinib was 1.73 years, 95% CI 0.76-1.65; Supplementary Figure S1). In total, 3 patients received EGFR TKIs off-protocol prior to any evidence of recurrence: 2 were treated with consolidation erlotinib (one transitioned to erlotinib after discontinuing afatinib due to toxicity, and the other started immediately after cCRT as mentioned above), and 1 extended consolidation afatinib for an additional 4.5 years beyond the planned 2-year course. Post-progression therapies are summarized in Supplementary Table S1.
Efficacy and characteristics of recurrent cancers
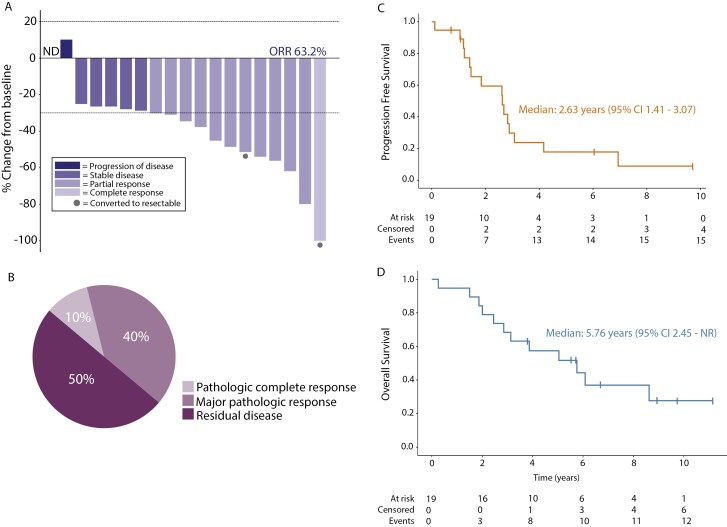
By ITT analysis, the ORR to afatinib induction was 63% (12/19 patients; 95% CI 38%-84%; Figure 2A). Among 10 patients who underwent lobectomy and nodal dissection after induction TKI and cCRT, a major pathologic response (defined as <10% viable tumor cells in resected lung mass) was noted in 5 patients, and a pathologic complete response (pCR) was seen in 1 patient (Figure 2B). Of note, the single pCR was observed in one of the patients who was initially deemed to have unresectable disease. The other patient with previously unresectable disease who underwent surgery had a major pathological response. Mediastinal nodal sterilization (ypN0) was seen in 50% (5/10) of operable patients.
Figure 2.
Efficacy. (A) Individual patient RECIST response after 2 months of induction afatinib (n = 19). ND denotes lack of measurable disease by RECIST. Circles denote patients who were thought to be unresectable at study entry, but had dramatic responses to therapy and underwent surgery. Note: RECIST responses were not confirmed with a second scan as patients proceeded directly to chemoradiotherapy. ORR, objective response rate. (B) Distribution of pathologic response at time of surgery (n = 10 patients). Major pathologic response was defined as <10% residual tumor cells. (C) Progression-free survival of cohort. (D) Overall survival of cohort.
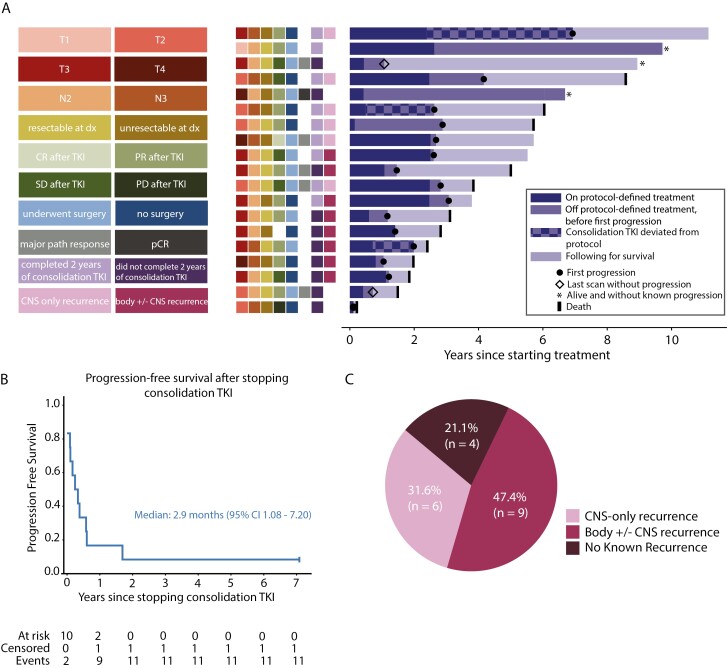
At the time of analysis, 8/19 patients are alive, and 3 of these 8 remain progression-free on their most recent scans (Figure 3A). With a median follow-up of 5.04 years (range, 3 months-11.15 years), the median PFS and OS were 2.63 years (95% CI, 1.41-3.07) and 5.76 years (95% CI, 2.45-NR), respectively (Figure 2C, 2D). Three patients remained alive and without evidence of recurrent disease now more than 7 years since trial enrollment, but one of these patients (third bar down in the swimmer’s plot, Figure 3A) elected to no longer undergo scans soon after coming off-protocol.
Figure 3.
Patterns of disease progression. (A) Right: the longitudinal course of all 19 patients is depicted by horizontal bars of varying length. Periods of deviation from protocol-defined therapy are noted with a checkered bar; these deviations included continuation of consolidation afatinib beyond the predefined 2-year period or off-protocol consolidation erlotinib. Left: colored boxes denote T-stage, N-stage, resectability at diagnosis, RECIST response category after induction afatinib, surgical disposition, pathologic response at time of surgery (major pathological response is <10% residual tumor cells, pCR is pathological complete response), completion of 2 years of consolidation afatinib, and recurrence isolated to the central nervous system (CNS). (B) Progression-free survival after stopping consolidation afatinib (n = 12, includes only those patients who received consolidation afatinib and no other consolidation TKI off-protocol). (C) Distribution of site of first recurrence (n = 19, ITT group).
One patient had disease progression during consolidation afatinib therapy, after 12 months of treatment. The recurrent disease was biopsied and was negative for acquired T790M. Among the 12 patients who received only consolidation afatinib and no other consolidation TKI, all but one had progression after stopping afatinib, with a median PFS from the time of afatinib discontinuation of 2.9 months (95% CI 1.08-7.20 months; Figure 3B). Reasons for afatinib discontinuation included (a) toxicity (n = 5 total, note: one of these is excluded from the post-afatinib PFS analysis due to switching immediately to erlotinib consolidation) and (b) completion of the planned 2-year course (n = 7). One patient continued afatinib off-protocol for an additional 4.5 years beyond the protocol-planned 2 years before ultimately experiencing disease progression. Importantly, the trend of progression after stopping TKI was also seen among the 2 patients who elected to take consolidation erlotinib; one patient had progression 1 month after stopping erlotinib, and the other while still on erlotinib after ~14 months. Among the 15 total patients who recurred, 6 (40%) experienced isolated CNS recurrences (Figure 3C), 4 of which occurred within 8 months from stopping consolidation TKI. Of the patients who received cCRT, only one had an isolated locoregional recurrence at the time of first recurrence (Supplementary Figure S1). Eleven of 19 received osimertinib after recurrence (Supplementary Table S1).
Safety
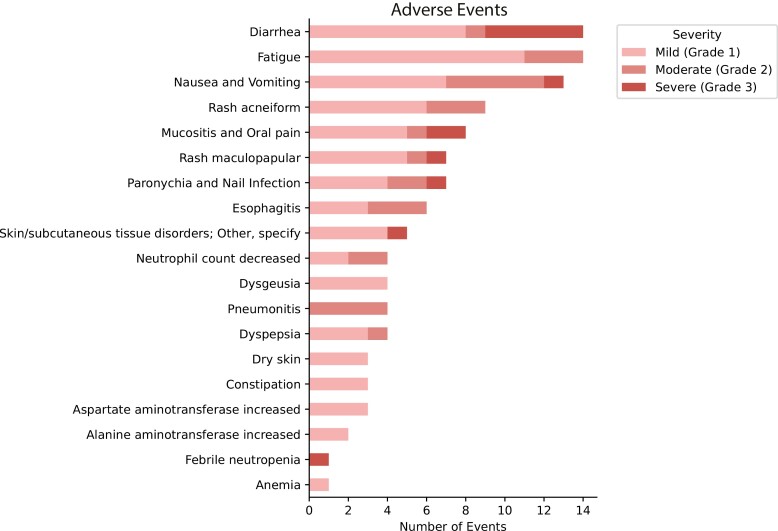
During the induction afatinib course, 7/19 patients required afatinib dose reduction from 40 to 30 mg once daily (Supplementary Figure S1). Overall, 5 patients had to discontinue consolidation TKI due to toxicity. Adverse events were consistent with prior observed side effects of afatinib and cCRT; no obvious synergistic toxicity was seen (Figure 4). The grade 3 side effects observed were GI symptoms (diarrhea, nausea, and vomiting; n = 6), mucositis (n = 2), febrile neutropenia (n = 1), and skin symptoms (rash and paronychia; n = 3). One patient had mediastinoscopy wound dehiscence during induction afatinib. CCRT was well tolerated, with 4 patients experiencing grade 2 pneumonitis (22.2%) and 3 grade 2 esophagitis (16.7%) events. One patient developed hypotension and hypoxemia requiring a brief ICU stay in the perioperative setting, but there were no other serious perioperative adverse events. There were no grade 4-5 events.
Figure 4.
Adverse events per CTCAE version 4.0.
Discussion
Over time, a series of clinical advances have iteratively prolonged the median survival for stage III NSCLC, from 16 months in the late 1990s,30,31 to 25 months in the 2010s with more modern chemotherapy and radiation techniques,32 to 47 months (after cCRT) with contemporaneous immune checkpoint inhibitor consolidation.2 Meanwhile, targeted TKIs have become the standard of care in metastatic oncogene-driven NSCLCs,13-16,19 and they have begun to demonstrate PFS and OS benefits in the adjuvant setting,17,18,33 but there are still no randomized trials to guide their use in locally advanced (stage III) disease. Here, we share the final results of the ASCENT trial, a nonrandomized phase 2 clinical study testing a strategy to introduce EGFR TKI induction and consolidation into the treatment paradigm for stage III NSCLC harboring an activating EGFR mutation. Patients received 2 months of induction afatinib, followed by definitive cCRT (with or without surgery) and then up to 2 years of consolidation afatinib.
Patients with EGFR-mutant NSCLC tend not to benefit from immunotherapy. In the PACIFIC trial, the subset of patients with EGFR mutations (n = 43) derived no benefit from consolidation durvalumab; in that study, the control arm showed a median PFS of 5.6 months and median OS of 29.1 months after completion of cCRT.1,2 While the control arm of PACIFIC may not generalize to EGFR-positive cancers overall given the small size of the EGFR cohort, a recent retrospective study of 136 stage III unresectable EGFR-mutant lung cancer showed similar PFS outcomes in patients receiving cCRT with and without consolidation durvalumab (12.7 months vs 9.7 months, respectively, from the time of cCRT initiation; P = .083), whereas they derived a significant PFS benefit with consolidation osimertinib (median PFS not reached).12 In ASCENT, the median PFS was 2.63 years (95% CI, 1.41-3.07) and the median OS was 5.76 years (95% CI 2.45-NR) from the start of induction afatinib. Though ASCENT is a much smaller and nonrandomized study and was designed in the pre-osimertinib and pre-immunotherapy era, the improvement in median PFS and OS relative to these other studies is noteworthy.
In the modern era, the benefit of neoadjuvant cCRT compared to chemotherapy alone is not defined in oncogene-driven cancers. Unfortunately, in the ASCENT trial, this aggressive multimodal treatment regimen with cCRT was not curative for most patients despite excellent local outcomes. Fourteen of 17 patients who underwent induction afatinib and cCRT with or without surgery ultimately recurred or died, with only 3 demonstrating long-term clinical benefit (these patients remain alive and without evidence of disease at 7-10 years since enrolling on trial). In light of these observations, the median PFS of 2.63 years appears to reflect that patients spent approximately 2 years on some form of treatment per our study design, and that many patients (n = 8) progressed shortly after stopping consolidation TKI (median ~3 months). In the ADAURA trial, the PFS curves similarly show a clear decrement after patients discontinued adjuvant osimertinib at 36 months. Taken together, these findings suggest that adjuvant or consolidation TKI prolongs disease control but does not reliably eradicate micrometastatic disease. This is consistent with the low pCR rate observed here (1/10) and in a recent study of neoadjuvant osimertinib (0/27).34
If targeted TKIs are rarely curative, how can they be most effectively combined with other treatment modalities to achieve cure? Are TKIs most effective in the neoadjuvant/induction setting or the adjuvant/consolidation setting? ASCENT was not designed to test the independent contributions of induction versus consolidation TKI, but its findings nonetheless establish important starting points for future trial design.
First, we observed that induction TKI can sometimes transform unresectable stage III disease to being amenable to resection, typically when a centrally located tumor exhibited a strong response. In our cohort, disease in 2 of 9 patients was converted from unresectable to resectable, and one of these (cT4N2 at diagnosis, pCR following induction, and declined consolidation) still has no evidence of disease more than 6 years after completing treatment. This trial is too small to measure how often induction TKI can convert unresectable disease to resectable, but it provides proof-of-concept to motivate a future trial of induction TKI and confirms other similar case series.35-37 Subsequent work could also assess whether these “converted” patients have better outcomes with surgery or with definitive cCRT alone. A phase 2 trial (NEOLA, NCT06194448) is planned to open in 2024 that will assess outcomes with 8 weeks of induction osimertinib followed by cCRT and indefinite consolidation osimertinib in patients with unresectable stage III EGFR-mutant NSCLC. The NEOLA trial—along with the forthcoming LAURA trial (discussed in more detail below) of consolidation osimertinib after cCRT and the ongoing NeoADAURA trial of neoadjuvant osimertinib prior to surgery—will also provide insights into the role of induction TKI before definitive local therapy21,38 Additional work needs to be done to determine whether short-course induction TKI improves radiation outcomes through reducing target volumes, thereby decreasing dose to normal organs at risk such as lungs.36,39,40
Second, induction TKI could inform adjuvant or consolidation treatment. For example, 2 of the 3 patients who remain without evidence of disease recurrence had strong responses to induction therapy (one pCR and one near-pCR, with <1% residual tumor) and elected to forego consolidation TKI. Determining how to tailor adjuvant/consolidation treatment is even more important now that preliminary findings from LAURA reportedly show an improvement in PFS with indefinite osimertinib after definitive cCRT.22 We need to see the results from LAURA to judge if indefinite TKI is feasible and affordable, but the results from ASCENT confirm that not all patients require indefinite TKI as some patients can be cured without indefinite therapy. Identifying which patients can safely stop or even forego consolidation TKI will be a challenge, as both ASCENT and ADAURA show substantial rates of recurrence shortly after stopping TKI, but this personalization is critical for quality of life.41,42 Circulating tumor DNA is a potential tool for risk stratifying patients—for example, assessing residual disease after induction TKI and/or after cCRT.36,43 A similar approach could be used for guiding adjuvant treatment for patients with earlier-stage resectable disease.44 Other novel biomarkers should also be studied.
Of note, many patients who recurred in the ASCENT trial did so only in the CNS (6/15), and most of these (4/6) occurred after stopping consolidation TKI. Interestingly, this phenomenon was also observed in ADAURA, with many post-TKI recurrences occurring in the CNS.45,46 Our study is too small to offer meaningful insights into the relative CNS efficacy of afatinib and osimertinib, although both are known to have excellent CNS activity.45-47 Together, these observations are consistent with the known predilection of EGFR-mutant NSCLC for the brain and/or the blood-brain barrier reducing CNS penetrance of anti-cancer therapy and point to a need for increased CNS surveillance in patients with locally advanced EGFR-mutant lung cancer.48
Sequential afatinib and cCRT did not produce unexpected toxicities. Most notably, treatment-related pneumonitis was not a pervasive side effect, and the 4 patients who developed it had only moderate (grade 2) cases. The most severe side effects were consistent with previous afatinib reports, including diarrhea, mucositis, and rash.25,26,49,50 But importantly, treatment with induction afatinib did not prevent any patients from proceeding to definitive local therapy. These findings are similar to those in 2 other small prospective trials of induction EGFR TKI prior to thoracic radiotherapy36,51 as well as a meta-analysis of 16 prospective clinical trials using both thoracic radiotherapy and anti-EGFR TKI for non-biomarker selected advanced NSCLC, which showed that that the addition of TKI does not significantly increase toxicity.52
Limitations of the study include the small sample size as well as the use of afatinib, an older TKI that has since been supplanted by the third-generation EGFR TKI osimertinib. However, despite enrolling only 19 patients, to our knowledge, ASCENT represents the largest prospective clinical trial combining TKI with definitive cCRT in a cohort of patients with locally advanced, oncogene-driven cancer. One smaller study of erlotinib combined with cCRT in locally advanced EGFR-mutant NSCLC suggested this combination was safe, but with only 7 patients receiving erlotinib throughout treatment, the findings must be interpreted with caution.53
The observations described here have important implications not only for EGFR-mutant lung cancer but also for all oncogene-driven lung cancer. We await the results of the LAURA trial, which will guide our use of osimertinib as consolidation after cCRT. In the meantime, however, we can use the results of the ASCENT trial to begin thinking about how best to tailor induction and consolidation TKI for each of our patients.
Supplementary Material
Acknowledgments
The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors. This was an independent, investigator-initiated study supported by Boehringer Ingelheim Pharmaceuticals, Inc (BIPI). BIPI had no role in the design, analysis, or interpretation of the results in this study. BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as it relates to BIPI substances, as well as intellectual property considerations.
Contributor Information
Allison E B Chang, Department of Medicine, Division of Hematology/Oncology, Massachusetts General Hospital, Boston, MA 02114, United States.
Andrew J Piper-Vallillo, Department of Medicine, Division of Hematology/Oncology, Lahey Hospital and Medical Center, Burlington, MA 01805, United States.
Raymond H Mak, Department of Radiation Oncology, Dana Farber Cancer Institute, Boston, MA 02215, United States.
Michael Lanuti, Department of Surgery, Division of Thoracic Surgery, Massachusetts General Hospital, Boston, MA 02114, United States.
Alona Muzikansky, Massachusetts General Hospital Biostatistics Center, Boston, MA 02114, United States.
Julia Rotow, Lowe Center for Thoracic Medical Oncology, Dana Farber Cancer Institute, Boston, MA 02115, United States.
Pasi A Jänne, Lowe Center for Thoracic Medical Oncology, Dana Farber Cancer Institute, Boston, MA 02115, United States.
Mari Mino-Kenudson, Department of Pathology, Massachusetts General Hospital, Boston, MA 02114, United States.
Scott Swanson, Department of Surgery, Division of Thoracic Surgery, Brigham and Women’s Hospital, Boston, MA 02115, United States.
Cameron D Wright, Department of Surgery, Division of Thoracic Surgery, Massachusetts General Hospital, Boston, MA 02114, United States.
David Kozono, Department of Radiation Oncology, Dana Farber Cancer Institute, Boston, MA 02215, United States.
Paul Marcoux, Lowe Center for Thoracic Medical Oncology, Dana Farber Cancer Institute, Boston, MA 02115, United States.
Zofia Piotrowska, Department of Medicine, Division of Hematology/Oncology, Massachusetts General Hospital, Boston, MA 02114, United States.
Lecia V Sequist, Department of Medicine, Division of Hematology/Oncology, Massachusetts General Hospital, Boston, MA 02114, United States.
Henning Willers, Department of Radiation Oncology, Massachusetts General Hospital, Boston, MA 02114, United States.
Author contributions
Allison E.B. Chang (Data curation, Formal Analysis, Visualization, Writing—original draft, Writing—review & editing), Andrew J. Piper-Vallillo (Data curation, Formal Analysis, Visualization, Writing—review & editing), Raymond H. Mak (Conceptualization, Investigation, Methodology, Writing—review & editing), Michael Lanuti (Conceptualization, Investigation, Methodology, Writing—review & editing), Alona Muzikansky (Conceptualization, Formal analysis, Methodology, Writing—review & editing), Julia Rotow (Investigation, Writing—review & editing), Pasi A. Jänne (Conceptualization, Investigation, Writing—review & editing), Mari Mino-Kenudson (Data curation, Investigation, Writing—review & editing), Scott Swanson (Investigation, Writing—review & editing), Cameron D. Wright (Investigation, Writing—review & editing), David Kozono (Investigation, Writing—review & editing), Paul Marcoux (Investigation, Writing—review & editing), Zofia Piotrowska (Data curation, Investigation, Writing—review & editing), Lecia V. Sequist (Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing—original draft, Writing—review & editing), and Henning Willers (Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing). Allison E. B. Chang and Andrew J. Piper-Vallillo are co-first authors. Lecia V. Sequist and Henning Willers are co-senior authors.
Conflicts of interest
A.C.: none. A.P.V.: Sanofi-Genzyme and Takeda (consulting). R.M.: ViewRay (Advisory Board, consulting, research funding), AstraZeneca (Advisory Board), Varian Medical Systems (Consulting) Sio Capital Management (Consulting), Novartis (Honorarium). M.L.: none. A.M.: none. J.R.: consulting fees or honoraria from Amgen, AstraZeneca, BioAtla, BMS, Daiichi Sankyo, Genentech, G1 Therapeutics, Guardant Health, Janssen, Jazz Pharmaceuticals, Sanofi-Genzyme, Summit Therapeutics and Takeda; contracted for research (institutional) with: AstraZeneca, BioAtla, Blueprint Medicines, Enliven Therapeutics, EpimAb Biotherapeutics, LOXO Oncology, ORIC. P.A.J.: grants: AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Puma Technology, Revolution Medicines, and Takeda Oncology; royalties and patents: LabCorp; consulting fees: AbbVie, Accutar Biotech, Allorion Therapeutics, AstraZeneca, Bayer, Biocartis, Boehringer Ingelheim, Chugai Pharmaceutical Co., Daiichi Sankyo, Duality, Eisai, Eli Lilly, Frontier Medicines, Hongyun Biotechnology, Merus, Mirati Therapeutics, Monte Rosa, Novartis, Nuvalent, Pfizer, Roche/Genentech, Scorpion Therapeutics, SFJ Pharmaceuticals, Silicon Therapeutics, Syndax, Takeda Oncology, Transcenta, and Voronoi. Stock or stock options: Gatekeeper Pharmaceuticals. M.M.K.: AstraZeneca, Pfizer, Repare, Boehringer Ingelheim, Sanofi, AbbVie, Daiichi Sankyo (consultancy), Elsevier (royalties). S.S.: none. C.W.: none. D.K.: Genentech/Roche (consultancy), RefleXion (honorarium). P.M.: none. Z.P.: served as a consultant and/or received honoraria from Eli Lilly, Boehringer Ingelheim, Bayer, Sanofi/Genzyme, C4 Therapeutics, Janssen, Takeda, Cullinan Oncology, Daiichi Sankyo Europe GmbH, Lilly, AstraZeneca, Taiho Pharmaceutical, Blueprint Medicines; received institutional research funding from Novartis, ARIAD/Takeda, Spectrum Pharmaceuticals, AstraZeneca, Cullinan Oncology, Daiichi Sankyo Europe GmbH, AbbVie, Janssen Oncology, Blueprint Medicines, GlaxoSmithKline/Tesaro; serves on a data safety monitoring committee for Genentech/Roche; and has received travel support from Janssen and AstraZeneca. L.V.S.: received institutional research funding from AstraZeneca, Novartis, and Delfi Diagnostics, and is involved in a clinical demonstration project funded by GRAIL and Point 32 Health. H.W.: none.
Data availability
The data underlying this article cannot be shared publicly in order to preserve the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
References
- 1. Antonia SJ, Villegas A, Daniel D, et al. ; PACIFIC Investigators. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342-2350. 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
- 2. Spigel DR, Faivre-Finn C, Gray JE, et al. Five-year survival outcomes from the PACIFIC Trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 2022;40(12):1301-1311. 10.1200/JCO.21.01308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Forde PM, Spicer J, Lu S, et al. ; CheckMate 816 Investigators. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973-1985. 10.1056/NEJMoa2202170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O’Brien M, Paz-Ares L, Marreaud S, et al. ; EORTC-1416-LCG/ETOP 8-15 – PEARLS/KEYNOTE-091 Investigators. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022;23(10):1274-1286. 10.1016/S1470-2045(22)00518-6 [DOI] [PubMed] [Google Scholar]
- 5. Felip E, Altorki N, Zhou C, et al. Overall survival with adjuvant atezolizumab after chemotherapy in resected stage II-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase III trial. Ann Oncol. 2023;34(10):907-919. 10.1016/j.annonc.2023.07.001 [DOI] [PubMed] [Google Scholar]
- 6. Heymach JV, Harpole D, Mitsudomi T, et al. ; AEGEAN Investigators. Perioperative durvalumab for resectable non-small-cell lung cancer. N Engl J Med. 2023;389(18):1672-1684. 10.1056/NEJMoa2304875 [DOI] [PubMed] [Google Scholar]
- 7. Cascone T, Awad MM, Spicer JD, et al. LBA1 CheckMate 77T: Phase III study comparing neoadjuvant nivolumab (NIVO) plus chemotherapy (chemo) vs neoadjuvant placebo plus chemo followed by surgery and adjuvant NIVO or placebo for previously untreated, resectable stage II–IIIb NSCLC. European Society for Medical Oncology Congress 2023; 2023. [Google Scholar]
- 8. Wakelee H, Liberman M, Kato T, et al. ; KEYNOTE-671 Investigators. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med. 2023;389(6):491-503. 10.1056/NEJMoa2302983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gainor JF, Shaw AT, Sequist LV, et al. EGFR Mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22(18):4585-4593. 10.1158/1078-0432.CCR-15-3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee CK, Man J, Lord S, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-a meta-analysis. J Thorac Oncol. 2017;12(2):403-407. 10.1016/j.jtho.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 11. Aredo JV, Mambetsariev I, Hellyer JA, et al. Durvalumab for stage III EGFR-mutated NSCLC after definitive chemoradiotherapy. J Thorac Oncol. 2021;16(6):1030-1041. 10.1016/j.jtho.2021.01.1628 [DOI] [PubMed] [Google Scholar]
- 12. Nassar AH, Kim SY, Aredo JV. et al. Consolidation osimertinib versus durvalumab versus observation after concurrent chemoradiation in unresectable EGFR-Mutant NSCLC: a multicenter retrospective cohort study. J Thorac Oncol. 2024. [DOI] [PubMed] [Google Scholar]
- 13. Peters S, Camidge DR, Shaw AT, et al. ; ALEX Trial Investigators. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377(9):829-838. 10.1056/NEJMoa1704795 [DOI] [PubMed] [Google Scholar]
- 14. Soria JC, Ohe Y, Vansteenkiste J, et al. ; FLAURA Investigators. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. 10.1056/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- 15. Ramalingam SS, Vansteenkiste J, Planchard D, et al. ; FLAURA Investigators. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41-50. 10.1056/NEJMoa1913662 [DOI] [PubMed] [Google Scholar]
- 16. Shaw AT, Bauer TM, de Marinis F, et al. ; CROWN Trial Investigators. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383(21):2018-2029. 10.1056/NEJMoa2027187 [DOI] [PubMed] [Google Scholar]
- 17. Wu YL, Tsuboi M, He J, et al. Osimertinib in resected EGFR-mutated non–small-cell lung cancer. N Engl J Med. 2020;383(18):1711-1723. 10.1056/nejmoa2027071 [DOI] [PubMed] [Google Scholar]
- 18. Tsuboi M, Herbst RS, John T, et al. ; ADAURA Investigators. Overall survival with osimertinib in resected EGFR-mutated NSCLC. N Engl J Med. 2023;389(2):137-147. 10.1056/NEJMoa2304594 [DOI] [PubMed] [Google Scholar]
- 19. Drilon A, Camidge DR, Lin JJ, et al. ; TRIDENT-1 Investigators. Repotrectinib in ROS1 fusion-positive non-small-cell lung cancer. N Engl J Med. 2024;390(2):118-131. 10.1056/NEJMoa2302299 [DOI] [PubMed] [Google Scholar]
- 20. Wu YL, Dziadziuszko R, Ahn JS, et al. ; ALINA Investigators. Alectinib in resected ALK-positive non-small-cell lung cancer. N Engl J Med. 2024;390(14):1265-1276. 10.1056/NEJMoa2310532 [DOI] [PubMed] [Google Scholar]
- 21. Lu S, Casarini I, Kato T, et al. Osimertinib maintenance after definitive chemoradiation in patients with unresectable EGFR mutation positive stage III non-small-cell lung cancer: LAURA trial in progress. Clin Lung Cancer. 2021;22(4):371-375. 10.1016/j.cllc.2020.11.004 [DOI] [PubMed] [Google Scholar]
- 22. Kemp, A. Tagrisso Demonstrated Overwhelming Efficacy Benefit for Patients With Unresectable, Stage III EGFR-Mutated Lung Cancer in LAURA Phase III Trial. AstraZeneca; 2024. [Google Scholar]
- 23. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 24. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327-3334. 10.1200/JCO.2012.44.2806 [DOI] [PubMed] [Google Scholar]
- 25. Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16(2):141-151. 10.1016/S1470-2045(14)71173-8 [DOI] [PubMed] [Google Scholar]
- 26. Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17(5):577-589. 10.1016/S1470-2045(16)30033-X [DOI] [PubMed] [Google Scholar]
- 27. USDOHAH Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2009. [Google Scholar]
- 28. Goldstraw P, Crowley J, Chansky K, et al. ; International Association for the Study of Lung Cancer International Staging Committee. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2(8):706-714. 10.1097/JTO.0b013e31812f3c1a [DOI] [PubMed] [Google Scholar]
- 29. Goldstraw P, Chansky K, Crowley J, et al. ; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39-51. 10.1016/j.jtho.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 30. Albain KS, Crowley JJ, Turrisi AT, et al. Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non-small-cell lung cancer: a Southwest Oncology Group phase II study, SWOG 9019. J Clin Oncol. 2002;20(16):3454-3460. 10.1200/JCO.2002.03.055 [DOI] [PubMed] [Google Scholar]
- 31. Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103(19):1452-1460. 10.1093/jnci/djr325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Senan S, Brade A, Wang LH, et al. PROCLAIM: randomized phase III Trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2016;34(9):953-962. 10.1200/JCO.2015.64.8824 [DOI] [PubMed] [Google Scholar]
- 33. Solomon BJ, et al. LBA2 ALINA: Efficacy and Safety of Adjuvant Alectinib Versus Chemotherapy in Patients With Early-Stage ALK+ Non-Small Cell Lung Cancer (NSCLC). European Society for Medical Oncology Congress 2023; 2023. [Google Scholar]
- 34. Aredo JV, Urisman A, Gubens MA, et al. Phase II Trial of Neoadjuvant Osimertinib for Surgically Resectable EGFR-Mutated Non-Small Cell Lung Cancer. American Society of Clinical Oncology 2023 Meeting; 2023. [Google Scholar]
- 35. Aredo JV, Wakelee HA, Hui AB, et al. Induction EGFR tyrosine kinase inhibitors prior to definitive chemoradiotherapy in unresectable stage III EGFR-mutated non-small cell lung cancer. Cancer Treat Res Commun. 2022;33:100659. 10.1016/j.ctarc.2022.100659 [DOI] [PubMed] [Google Scholar]
- 36. Peled N, Roisman LC, Levison E, et al. Neoadjuvant osimertinib followed by sequential definitive radiation therapy and/or surgery in stage III epidermal growth factor receptor-mutant non-small cell lung cancer: an open-label, single-arm, phase 2 study. Int J Radiat Oncol Biol Phys. 2023;117(1):105-114. 10.1016/j.ijrobp.2023.03.042 [DOI] [PubMed] [Google Scholar]
- 37. Kato T, Casarini I, Cobo M, et al. Targeted treatment for unresectable EGFR mutation-positive stage III non-small cell lung cancer: emerging evidence and future perspectives. Lung Cancer. 2024;187:107414. 10.1016/j.lungcan.2023.107414 [DOI] [PubMed] [Google Scholar]
- 38. Tsuboi M, Weder W, Escriu C, et al. Neoadjuvant osimertinib with/without chemotherapy versus chemotherapy alone for EGFR-mutated resectable non-small-cell lung cancer: NeoADAURA. Future Oncol. 2021;17(31):4045-4055. 10.2217/fon-2021-0549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lim YJ, Chang JH, Kim HJ, et al. Superior treatment response and in-field tumor control in epidermal growth factor receptor-mutant genotype of stage III nonsquamous non-small cell lung cancer undergoing definitive concurrent chemoradiotherapy. Clin Lung Cancer. 2017;18(3):e169-e178. 10.1016/j.cllc.2016.12.013 [DOI] [PubMed] [Google Scholar]
- 40. McClatchy DM, Willers H, Hata AN, et al. Modeling resistance and recurrence patterns of combined targeted-chemoradiotherapy predicts benefit of shorter induction period. Cancer Res. 2020;80(22):5121-5133. 10.1158/0008-5472.CAN-19-3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hechtner M, Eichler M, Wehler B, et al. Quality of life in NSCLC survivors - a multicenter cross-sectional study. J Thorac Oncol. 2019;14(3):420-435. 10.1016/j.jtho.2018.11.019 [DOI] [PubMed] [Google Scholar]
- 42. Chen Y, Xu N, Yang Y, et al. Quality-of-life, mental health, and perspective on TKI dose reduction as a prelude to discontinuation in chronic phase chronic myeloid leukemia. Cancer Med. 2023;12(16):17239-17252. 10.1002/cam4.6296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lebow ES, Murciano-Goroff YR, Jee J, et al. Minimal residual disease (MRD) detection by ctDNA in relation to radiographic disease progression in patients with stage I-III non–small cell lung cancer (NSCLC) treated with definitive radiation therapy. American Society of Clinical Oncology Meeting 2022; 2022. [Google Scholar]
- 44. Xia L, Mei J, Kang R, et al. Perioperative ctDNA-based molecular residual disease detection for non-small cell lung cancer: a prospective multicenter cohort study (LUNGCA-1). Clin Cancer Res. 2022;28(15):3308-3317. 10.1158/1078-0432.CCR-21-3044 [DOI] [PubMed] [Google Scholar]
- 45. Ballard P, Yates JWT, Yang Z, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-Mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22(20):5130-5140. 10.1158/1078-0432.CCR-16-0399 [DOI] [PubMed] [Google Scholar]
- 46. Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS Response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. 2018;36(33):JCO2018783118. 10.1200/JCO.2018.78.3118 [DOI] [PubMed] [Google Scholar]
- 47. Kim J, Jang TW, Choi CM, et al. Real-world analysis of first-line afatinib in patients with EGFR-mutant non-small cell lung cancer and brain metastasis: survival and prognostic factors. Transl Lung Cancer Res. 2023;12(6):1197-1209. 10.21037/tlcr-22-832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Khasraw M, Yalamanchili P, Santhanagopal A, et al. Clinical management of patients with non-small cell lung cancer, brain metastases, and actionable genomic alterations: a systematic literature review. Adv Ther. 2024;41(5):1815-1842. 10.1007/s12325-024-02799-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang JC, Sequist LV, Zhou C, et al. Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation-positive lung adenocarcinoma: post hoc analyses of the randomized LUX-Lung 3 and 6 trials. Ann Oncol. 2016;27(11):2103-2110. 10.1093/annonc/mdw322 [DOI] [PubMed] [Google Scholar]
- 50. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2023;41(16):2869-2876. 10.1200/JCO.22.02547 [DOI] [PubMed] [Google Scholar]
- 51. Hotta K, Saeki S, Yamaguchi M, et al. Gefitinib induction followed by chemoradiotherapy in EGFR-mutant, locally advanced non-small-cell lung cancer: LOGIK0902/OLCSG0905 phase II study. ESMO Open. 2021;6(4):100191. 10.1016/j.esmoop.2021.100191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu R, Wei S, Zhang Q, et al. Epidermal growth factor receptor tyrosine kinase inhibitors combined with thoracic radiotherapy or chemoradiotherapy for advanced or metastatic non-small cell lung cancer: a systematic review and meta-analysis of single-arm trials. Medicine (Baltimore). 2019;98(29):e16427. 10.1097/MD.0000000000016427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee Y, Han JY, Moon SH, et al. Incorporating erlotinib or irinotecan plus cisplatin into chemoradiotherapy for stage III non-small cell lung cancer according to EGFR mutation status. Cancer Res Treat. 2017;49(4):981-989. 10.4143/crt.2016.522 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly in order to preserve the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.