Abstract
Advances in energy balance and cancer research to date have largely occurred in siloed work in rodents or patients. However, substantial benefit can be derived from parallel studies in which animal models inform the design of clinical and population studies or in which clinical observations become the basis for animal studies. The conference Translating Energy Balance from Bench to Communities: Application of Parallel Animal-Human Studies in Cancer, held in July 2021, convened investigators from basic, translational/clinical, and population science research to share knowledge, examples of successful parallel studies, and strong research to move the field of energy balance and cancer toward practice changes. This review summarizes key topics discussed to advance research on the role of energy balance, including physical activity, body composition, and dietary intake, on cancer development, cancer outcomes, and healthy survivorship.
Keywords: body composition, cancer, cancer survivorship, diet, exercise, nutrition, translational research
INTRODUCTION
The conference Translating Energy Balance from Bench to Communities: Application of Parallel Animal-Human Studies in Cancer, held in July 2021 at The University of Texas MD Anderson Cancer Center, highlighted the challenges and opportunities of parallel energy balance studies, in which mechanistic studies in animal models inform clinical or population studies, and vice versa. In this context, energy balance includes the study of diet, energy expenditure by physical activity or exercise, and body composition. The conference emphasized a transdisciplinary approach to translational research to overcome the challenges of moving bench research to clinical practice, and vice versa. Animal models are useful to inform clinical studies, and clinical studies likewise provide feedback for mechanistic animal models through real-world corroborating or contradictory evidence observed in patients. This feedback loop can produce more informative research to support clinical practice change, and it is clear that better integration between animal and clinical research is needed to obtain maximum benefit.
The virtual conference drew approximately 100 participants, including research leaders from clinical and basic science backgrounds. Presentations reviewed animal models and clinical trials, spanning the translational continuum from preclinical studies to community translation and the cancer control continuum from prevention to survivorship. The conference included open discussion among speakers and participants, potentially yielding new transdisciplinary collaborations. Proceedings of the conference are presented here, along with discussion and conclusions.
EXERCISE AND PHYSICAL ACTIVITY
Physical activity has been studied in all stages of cancer—prevention, treatment, and survival—for decades. There is evidence to support that exercise lowers the risk of cancer development, is safe during active therapy, and improves morbidity and mortality in survivors. In this section, we review the history of exercise and cancer, discuss possible mechanisms to explain associations between exercise and treatment outcomes, and innovative animal models helpful in studying cancer incidence and survival in humans.
Exercise and cancer overview (Melinda L. Irwin, PhD, MPH)
The relationship between physical activity (i.e., any movement engaging the muscles that requires energy) or exercise (i.e., purposeful physical activity designed to improve or maintain physical fitness, physical performance, or health) and cancer has emerged as a focus of study over the past several decades. Participation in physical activity increased during the 1960s through the 1980s but remained suboptimal,1 which brought attention to this public health crisis. Research in the 1990s merged exercise physiology with epidemiology, documenting the energy expenditure of many physical activities and studying disparities in activity across different races and ethnicities.2 Epidemiologic studies demonstrated an association of physical inactivity and breast cancer risk and mortality, and, together with randomized controlled trials (RCTs), showed that body fat and circulating levels of estrogens mediated this association.3–5 Although women stop ovarian production of estrogen after menopause, it continues to be produced in body fat, and a decrease in body fat through physical activity decreases cancer risk.6 These studies laid the groundwork for the study of the mechanisms of exercise-mediated effects on breast cancer.
More recently, through better understanding the effects of exercise on biologic mechanisms associated with breast cancer recurrence and survival (serum biomarkers, mammographic breast density, body fat, quality of life), collaborative oncology, surgery, and basic and population science teams have produced transdisciplinary research that can be translated from the bench to the clinic to the community. Exercise and diet intervention trials have shown improvements in fitness and body composition that translate into favorable decreases in insulin, metabolic biomarkers, and inflammation in people diagnosed with cancer.7,8
Exercise may improve outcomes by increasing treatment adherence through mitigating the toxic side effects of treatment. One trial reported a 30% improvement in joint pain, pain severity, and pain interference in women with breast cancer taking aromatase inhibitors and experiencing arthralgia.9 Observational data indicate an association of physical activity with chemotherapy completion rates.10 An RCT evaluating the effect of exercise on chemotherapy completion rates in 230 patients with breast cancer showed similar results.11 The National Cancer Institute has recently funded the Exercise and Nutrition Interventions to Improve Cancer Treatment-Related Outcomes (ENICTO) Consortium, which supports exercise and/or medical nutrition trials designed to improve cancer treatment-related outcomes and will generate additional evidence on exercise as supportive or adjuvant therapy to cancer treatment.
Despite the progress of research on physical activity and cancer, gaps in knowledge remain. For example, most clinical exercise trials in cancer have been performed in breast cancer, although low physical activity levels have been linked to the risk of 13 types of cancer.12 In survivorship research, certain outcomes (peripheral neuropathy, cognitive function, cardiotoxicity, etc.) are understudied, and there are research gaps in palliative care and pediatric and adolescent/young adult cancer.13 These areas require collaboration with preclinical and clinical researchers in oncology, aging, psychology, and social work to ensure proper validation and measurement of patient-related outcomes. The impact of exercise on disease-free survival warrants further study. Using animal models to understand the mechanisms of exercise’s effect on cancer treatment efficacy and survival will promote the development of individualized exercise interventions that can be translated to humans to provide evidence for implementation in cancer treatment and community interventions.
Clinical studies of exercise and cancer treatment response (Kerry S. Courneya, PhD)
Three main clinical pathways illustrate how exercise might improve cancer outcomes. First, as mentioned above, exercise can improve a patient’s ability to complete treatment by mitigating its side effects. Second, exercise may affect treatment efficacy by changing drug delivery or pharmacokinetics. Third, exercise may have a direct effect on a primary or metastatic tumor, which may manifest as an increase, a reduction, or no change in tumor burden.14,15 Understanding the direct effects of exercise on primary tumors and metastases is important to interpreting the effect of exercise on treatment efficacy (Figure 1).
FIGURE 1.
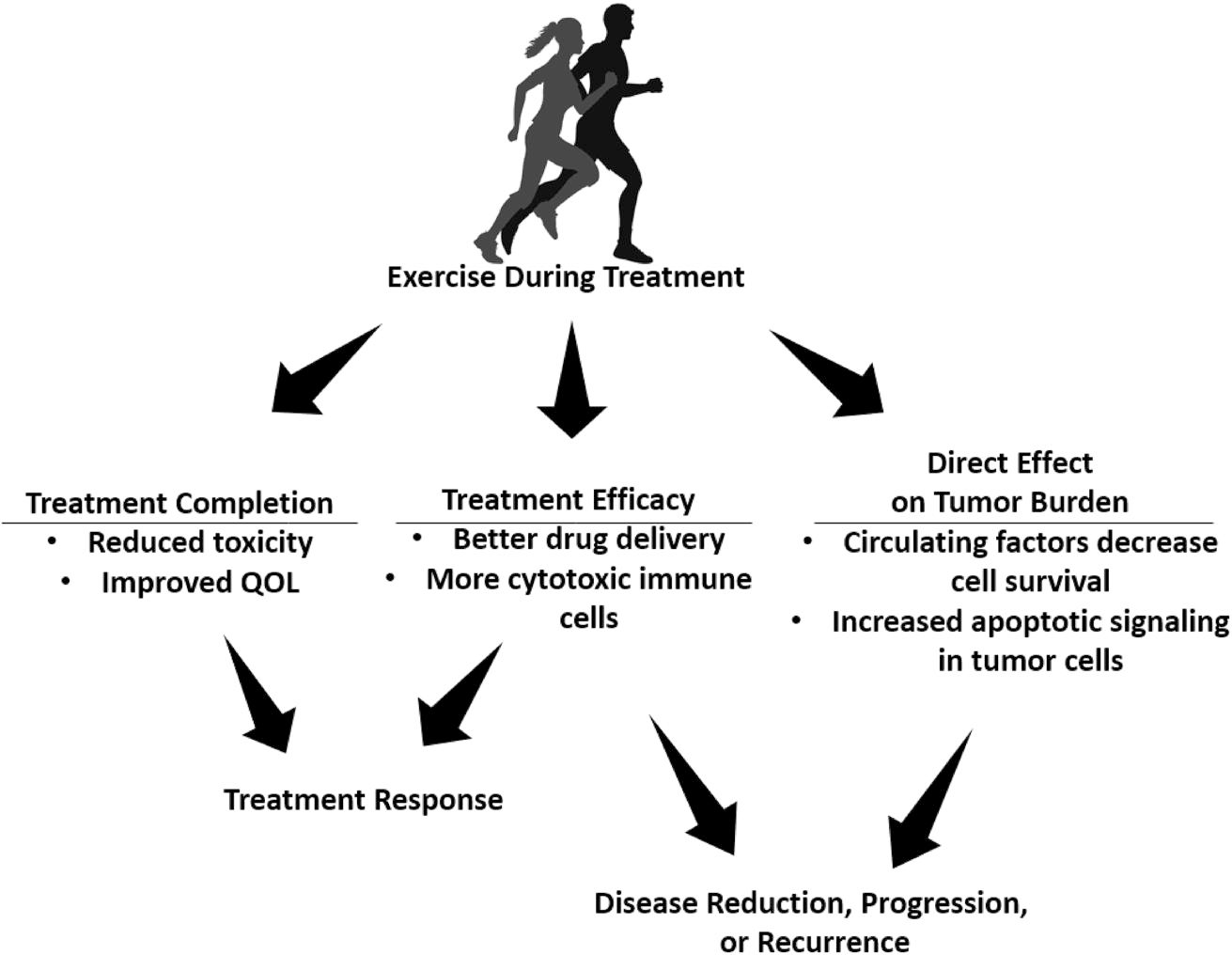
Clinical pathways from exercise to cancer outcomes. Shown are potential indirect and direct effects of exercise in the treatment course and disease outcomes. QOL indicates quality of life.
Preclinical and clinical studies of the effect of exercise on cancer treatment efficacy were recently reviewed.15 The review covered six preclinical studies with eight rodent models that included various cell lines (breast cancer, pancreatic cancer, melanoma, sarcoma) using single-agent chemotherapy or hormone therapy and tested effects using a factorial design (control, chemotherapy/hormone therapy, exercise, and exercise plus chemotherapy/hormone therapy). One study demonstrated an antagonistic effect of exercise against doxorubicin efficacy in a breast cancer model and made doxorubicin less effective16; however, seven demonstrated enhanced treatment effects that were both additive and synergistic. Defining the mechanism of the effects of exercise will better characterize its properties specific to treatment modality and cancer type and is critical for translating the findings to clinical care.
The review also covered seven clinical studies, five RCTs with a two-group controlled design and two single-arm studies. For exercise interventions in the neoadjuvant setting (three studies), one RCT in breast cancer showed a neutral effect,17 whereas two RCTs, in rectal cancer and lymphoma, showed higher rates of complete response.18,19 In the two studies of patients with breast cancer in the adjuvant setting, one RCT showed improvement20 and one nonrandomized trial showed no difference in disease-free survival compared with historic controls.21 In the two studies in the metastatic setting (no surgery), one nonrandomized study in colorectal cancer showed no difference in time to event,22 and one RCT of several cancer types showed improvements.23 Although early evidence is mixed, it suggests that exercise may improve clinical outcomes in patients with some types of cancer; limitations in study design and sample size leave room for further investigation. Heterogeneity in terms of cancer type, disease stage, treatment protocol, and cancer outcome precluded any calculation of an overall effect size. Potential mechanisms through which exercise may improve clinical outcomes are through increased chemotherapy completion rates,11 changes in tumor-infiltrating immune cells,24 changes in circulating factors that affect tumor cell survival,25 and improvement in tumor vascular function, as discussed in the next section.
Mechanisms of exercise and cancer treatment response: Tumor vascular function (Keri L. Schadler, PhD)
One potential mechanism for the exercise-cancer treatment response relationship is related to tumor vasculature and drug delivery. Tumor vasculature is dysfunctional, and only 50% of vessels have blood-carrying capacity.26 Laminar shear stress increased by exercise is key to normalizing vessel function. Thus exercise can transform a tumor’s abnormal vasculature to functional vasculature, leading to better chemotherapy delivery and a decrease in tumor growth during treatment.27
Vascular function was examined in a mouse model of pancreatic ductal adenocarcinoma using a factorial study design involving treatment with exercise (treadmill running) and chemotherapy (gemcitabine). A dose of exercise intensity comparable to that of moderate-intensity exercise in humans was chosen. Gemcitabine alone reduced tumor size compared with untreated controls. Exercise enhanced its efficacy, yielding significantly better suppression of tumor growth than gemcitabine alone.27 Exercise alone had no effect compared with controls. Vascular function was observed using fluorescent-labeled lectin, showing that exercised mice had significantly increased functional vessels.
Similarly, mice bearing subcutaneous, patient-derived pancreatic ductal adenocarcinoma xenografts were treated in the same factorial design for 20 days after tumors were established. At the end of that period, mice in the gemcitabine-alone group and the gemcitabine/exercise combination group had undetectable tumors. Tumors grew back significantly faster in the gemcitabine-alone group than in the combination group.28 The tumor vessels of nonexercised mice were disordered, unlike the more elongated vessels of exercised mice, with a statistically significant contrast in the number of open lumens in the combination group compared with the gemcitabine-alone group.
It may eventually be possible to predict whether or how individual patients will respond to exercise if the mechanisms responsible for response are known. Ongoing work aims to identify blood biomarkers that indicate that tumor vascular remodeling has occurred in response to exercise. These biomarkers will be useful for measuring whether patients are exercising enough to get the maximum benefit within the tumor microenvironment.
Based on the preclinical studies described above, a single-arm pilot study tested an exercise intervention in combination with preoperative therapy for patients with pancreatic cancer. Patients received chemotherapy and/or chemoradiation and then had a period of rest. During this time, they exercised unsupervised at home (60 minutes per week of aerobic training and 60 minutes per week of strength training). Of 50 patients, 80% achieved at least 120 minutes per week of moderate-to-vigorous physical activity (MVPA), demonstrating feasibility.29 For patients who underwent surgery, evaluation of tumor specimens showed vascular changes similar to those observed in mice, including a significantly higher number of blood vessels than historical controls.28 Taken together, these studies illustrate the bidirectional information exchange between preclinical and clinical studies and accelerated knowledge generation that parallel studies can produce.
Innovative animal models: Use of companion dogs with cancer (Mark W. Dewhirst, DVM, PhD)
Animal models other than rodents are useful in studies of the role of energy balance in cancer. Companion dogs can develop naturally occurring cancers that are similar to human cancers in their growth and spread. Dogs and humans share some cancer predisposition genes and driver mutations that predict cancer risk and treatment success, and cancers in dogs have natural histories similar to those in humans.30 Importantly, human and canine populations are diverse in size, age, and other health risk factors. This diversity is relevant to clinical responses and represents a major advantage over the use of rodents, which lack significant diversity. Like humans, companion-animal models allow for the incorporation of aging and comorbidities in an immunocompetent host. Their complex tumor microenvironment similarly influences recurrence, metastasis, and treatment resistance.31,32 Because standards of care do not exist in many canine cancers, it is possible to initiate phase 0, 1, and 2 trials before other treatments.33 Cancer development and progression occur at faster rates in dogs, allowing for shorter, less costly, yet relevant clinical trials.32 One example of an ongoing canine cancer trial, The Golden Retriever Lifetime Study, is a prospective observational study to determine cancer risk factors over the life course of 3000 golden retrievers.34 Its primary outcomes are to determine the incidence of osteosarcoma, mast cell tumor, hemangiosarcoma, and lymphoma. Dog owners provide annual lifestyle and medical surveys and biosamples for their pets, and associations with outcomes through genetics, lifestyle, exercise, diet, reproductive history, and environmental exposures are evaluated. As of May 31, 2021, 45% of the projected 500 primary end points (dogs with cancer development) had been obtained. Hemangiosarcoma was the most common, and leukemia/lymphoma was the second most common, cancer type.35
To our knowledge, there are currently no interventional exercise and cancer studies being performed in dogs. However, an example of how canine studies may inform human studies in cancer is found in a randomized phase 3 trial performed in pets and then translated to a parallel study that included humans, assessing how hyperthermia affected outcomes of irradiated tumors.36 Two hundred thirty cats and dogs were randomized to receive either radiotherapy alone or radiotherapy with hyperthermia, where hyperthermia was administered before radiation. The addition of hyperthermia to radiotherapy resulted in higher complete response rates and longer lasting local control.37 Subsequently, prospective parallel phase 3 clinical trials involving human superficial tumors and canine sarcomas were designed, which randomized 109 humans and 122 dogs to high-heat or low-heat treatments with radiotherapy. In both dogs and humans, a higher thermal dose yielded better response and local control.38,39 The observation that higher thermal dose was more effective in both trials greatly strengthened the argument for careful control of thermal dose in thermoradiotherapy trials. Importantly, this type of embedded clinical trial design could be used in the context of exercise studies.
Studying dogs with cancer can provide preclinical data; in addition, canine studies can be conducted after phase 1 or 2 trials, or even in phase 4 trials, to provide guidance with dosing regimens or insight into efficacy.32,33 Translational studies among companion animals and humans are being supported through the Comparative Oncology Program of the National Cancer Institute to answer therapeutic intervention questions that cannot effectively be addressed in mouse models or safely examined in human studies.
OBESITY AND BODY COMPOSITION
Systematic reviews completed by the World Cancer Research Fund and the American Institute for Cancer Research have established a strong link between obesity and the development of at least 12 types of cancer.40,41 The urgent public health crisis of chronic obesity must be addressed by research to understand how to reduce obesity and its impact on cancer development. In this section, we summarize data on the mechanisms by which obesity increases the risk of various cancers and evidence in preclinical models and clinical studies for the usefulness of preventing obesity to prevent cancer.
Established and emerging mechanisms underlying the obesity-cancer link (Stephen D. Hursting, PhD)
Well-established mechanisms by which obesity and metabolic syndrome promote the development of many cancers include modulation of growth factor signaling, such as the interactive insulin receptor insulin-like growth factor and mechanistic target of rapamycin (mTOR) signaling pathways; adipokine signaling, including the adiponectin and leptin receptor pathways; inflammatory signaling involving the Janus kinase/signal transducer and activator of transcription, nuclear factor kappa B, and cyclooxygenase pathways; and vascular perturbations through vascular endothelial growth factor and its downstream mediators.42 For estrogen-responsive cancers, such as endometrial and estrogen receptor-positive breast cancer subtypes, increased estrogen receptor signaling associated with increased adiposity is a prominent contributor. Evidence is also accumulating for several emerging mechanisms, including obesity-driven epigenetic reprogramming, immunosuppression, and gut dysbiosis.43–45
In the context of these established and emerging mechanisms, four key questions have been raised. First, whereas it has become clear, primarily from bariatric surgery studies, that the pro-cancer effects of chronic obesity can be reversed by intentional weight loss.46 However, a key unanswered question is: what nonsurgical interventions can effectively break obesity-cancer links? Bariatric surgery is expensive and carries risks of adverse events, and lifestyle approaches to induce sufficient weight loss and/or metabolic reprogramming to overcome the cancer-enhancing effects of obesity remain challenging to sustain. Data in rodent and human studies suggest that several cancer-related effects of obesity can persist even after moderate weight loss, and sustained weight loss may be required for significant cancer risk reduction in chronically obese mice or humans.43,47,48 The impact of various forms of dietary restriction, including intermittent fasting and time-restricted feeding, on cancer risk or progression is an active area of research49 and is likely to benefit from studies in canines, as discussed. Moreover, recent advances in pharmacologic approaches to induce robust and sustainable weight loss, such as tirzepatide, a novel glucagon-like peptide-1 and glucose-dependent insulinotrophic polypeptide receptor agonist, hold great promise, but these agents have not been well characterized in relation to obesity and cancer.50
The second key gap in knowledge is the need to identify mechanistic targets and intervention strategies to offset the metastasis-promoting effects of obesity.51 Thus far, obesity-associated dysregulation of immune responses, as well as glucose and lipid metabolism, are emerging as key links between obesity and metastatic potential.52 The third key gap relates to the limitations of preclinical studies in studying the obesity-cancer relationship in the context of the abundant genetic diversity observed in humans. Most rodent studies of obesity and cancer have used inbred strains that do not approximate the genetic diversity of the human population. Progress toward closing this gap is being made by the mouse genetics community by generating mouse models of genetic heterogeneity. These include Collaborative Cross mice, a mosaic of eight founder mouse strains, and Diversity Outbred mice, derived from Collaborative Cross mice.53 These types of models will be important for assessing specific diet-gene interactions as the field moves toward a precision obesity research approach54 and will help address the fourth key unmet need–better integration of cell culture, animal model, and human research on obesity and cancer (Figure 2). To this end, preclinical study factors, such as age, sex, immune status, and genetic background of the animal model, choice of tumor model and mode of tumor induction, and dietary formulation, must all be considered because each variable will affect experimental outcomes.55 With these gaps in knowledge in mind, research on energy balance and cancer is evolving so that animal models are more reflective of human biology, and parallel human-animal co-trials (Figure 2) can generate transdisciplinary data in animals and patients.
FIGURE 2.
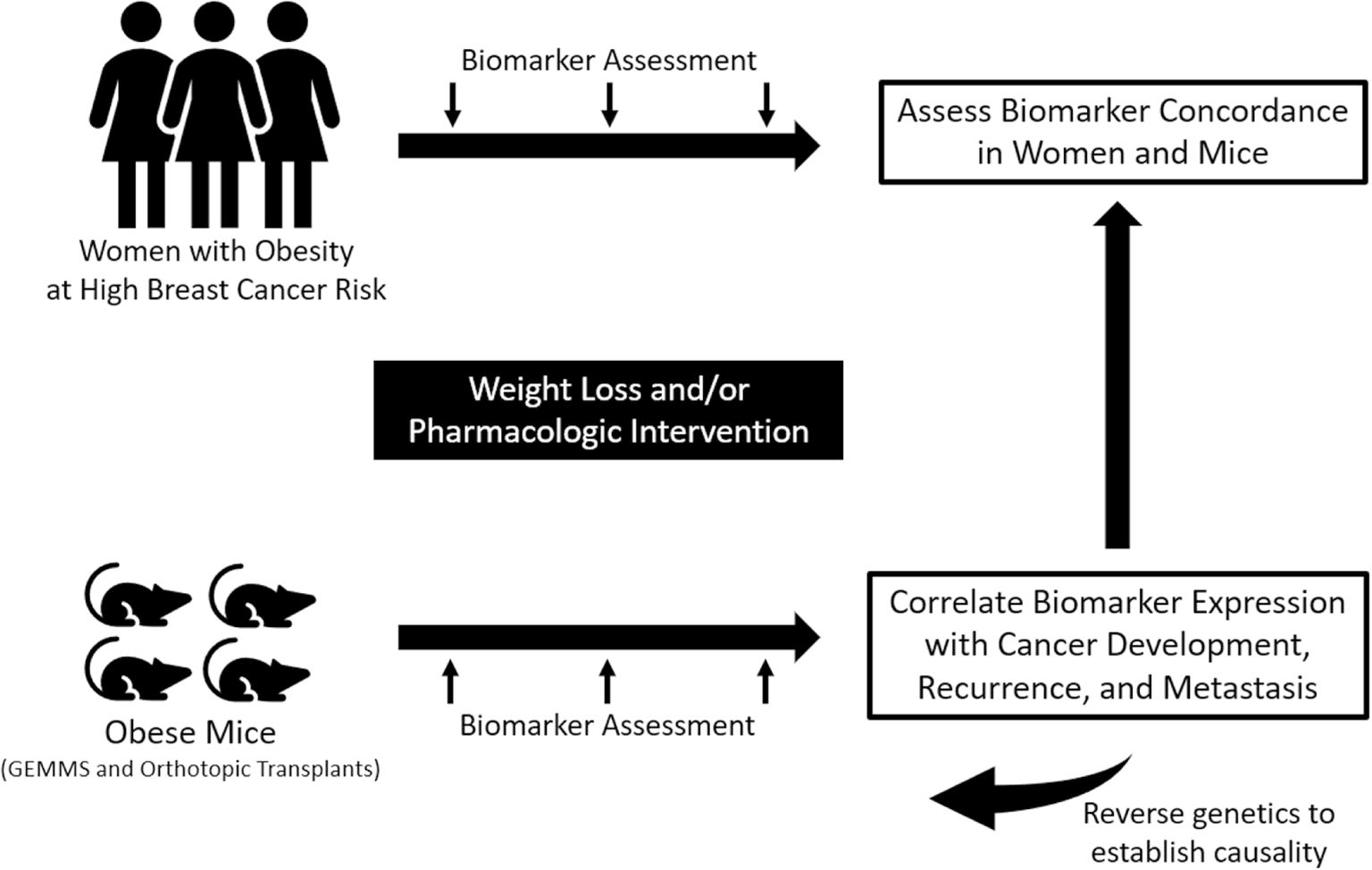
Example of integrated human-animal co-trials. Parallel trials in human and animal models integrate preclinical and clinical research allowing for concordant biomarker assessment and correlation with cancer development, recurrence, and metastases. GEMMs indicates genetically engineered mouse models.
Breaking the obesity, inflammation, and breast cancer connection (Andrew J. Dannenberg, MD)
Obesity is a risk factor for hormone receptor-positive postmenopausal breast cancer. Potential mediators of the obesity-breast cancer relationship include obesity’s effects on estrogen synthesis, insulin resistance, and altered adipokine and cytokine production. Breast white adipose tissue inflammation (BWATi) is associated with each of these potential mediators.56,57 Obesity causes an inflammatory state characterized by adipocyte hypertrophy, in which the adipocyte eventually dies. Cytokines recruit monocytes from the bloodstream into adipose tissue, and the monocytes differentiate into macrophages that envelop the dying adipocyte, forming a crown-like structure. These structures are found in the breast adipose tissue of approximately 90% of women with obesity and in one half of those with overweight.56,58 Importantly, BWATi, as indicated by crown-like structures, is also seen in approximately one third of women with normal body mass index (BMI).59
BWATi is associated with an increased risk of breast cancer in women with a history of benign breast disease60 and increased levels of aromatase, the rate-limiting enzyme for converting androgens to estrogens.56,61 An increased ratio of select estrogens to androgens has been observed in association with crown-like structures in breast adipose tissue and serum in patients with breast cancer.62 Elevation of estrogens is believed to have a paracrine effect on breast epithelial cells, resulting in an increased risk of hormone receptor-positive breast cancer. Taken together, these results indicate that the obesity-inflammation-aromatase axis may contribute to the increased risk of hormone receptor-positive breast cancer in postmenopausal women.60
In addition to the link between obesity and elevated risk of postmenopausal breast cancer, women with normal BMI do develop hormone receptor-positive breast cancer. It is possible to have normal BMI but increased adiposity (a high percentage of total body fat even with normal weight). The potential importance of excess body fat and BWATi in the development of breast cancer in postmenopausal women with normal BMI was evaluated. An RNA-sequencing analysis showed that BWATi was associated with numerous transcriptome changes, including elevated levels of aromatase.61 Moreover, the relationship between excess body fat in postmenopausal women with normal BMI and breast cancer risk was determined using data from the Women’s Health Initiative.63 In 3460 postmenopausal women with normal BMI, the risk of breast cancer was compared in those with high versus low body fat (measured by dual-energy absorptiometry scan). Among women with normal BMI and high body fat, the risk of postmenopausal breast cancer was doubled. This high-risk group also had increased levels of insulin, C-reactive protein, and leptin and a decrease in sex hormone-binding globulin, which contributes to increased levels of free estrogen in blood. Given the link between elevated body fat, BWATi, and the risk of breast cancer, a separate RNA-sequencing study was performed in women with normal BMI. In these women, excess body fat was associated with numerous changes in gene expression in the nontumorous breast that may contribute to an increased risk of breast cancer.64 Taken together, in women with normal BMI, excess body fat is associated with BWATi, changes in gene expression in the nontumorous breast tissue, and systemic changes, which collectively appear to increase the risk of breast cancer in postmenopausal women.
Potential nonsurgical interventions for patients with obesity include diet-induced weight loss, exercise, medication, and combination regimens. One study of caloric restriction in a mouse model showed regression of inflammation,48 indicating that mouse models could be useful in testing interventions. In addition, a clinical trial has been initiated to determine whether an exercise and diet intervention improves body composition and the metaboinflammatory profile in women with normal BMI who have excess trunk fat.
Focus on visceral adipose with weight-loss interventions in breast cancer survivors with obesity (Carol J. Fabian, MD)
The complexities of the relationship between body fatness and breast cancer risk and outcomes are not fully understood. Weight loss through lifestyle intervention change has not yet been established to improve disease-free survival for breast cancer survivors, in part because of the complex relationships between obesity, adiposity, and cancer risk. Interventions for weight loss to improve disease-free survival can be confounded by inclusion of metabolically healthy patients in obesity studies. Approximately one third of individuals with a BMI ≥30 kg/m2 (the current definition of obesity) are considered metabolically healthy, with normal lipids, insulin sensitivity measures, and blood pressure. These individuals tend to be fit and have low visceral adiposity. Including these individuals, who likely do not share the same increased health risks as individuals with high BMI and high visceral adipose, may mask the importance of weight loss for cancer-mitigating breast cancer risk.65–67
Many trials of behavioral interventions may not have produced sufficient initial or sustained weight loss likely to reduce the risk of breast cancer recurrence. Although the minimum loss remains unclear, an 8%–10% weight loss, when maintained, has been shown to reduce the risk of breast cancer.68 Studies suggest that a ≥10% weight loss is reliably associated with significant improvement in multiple metabolic and proinflammatory markers identified in preclinical and clinical studies of the obesity-breast cancer link.47,69–72 These include insulin, adiponectin, leptin, the leptin-to-adiponectin ratio, and C-reactive protein. Adiponectin not only plays a key role in insulin sensitivity but is also thought to counter the oncogenic effects of leptin.72 A 25% decrease in visceral adipose, which generally can be achieved with a 10% weight loss, has been shown to reduce the proportion of individuals with metabolic syndrome, which is associated with a worse breast cancer prognosis.73,74
Physical activity or exercise may confound the relationships of weight loss or visceral adipose loss and cancer risk. A high-volume (>200 minutes per week) of MVPA reduces visceral adipose even without weight loss and promotes maintenance of adipose loss with caloric restriction.75 A pilot trial to determine the feasibility of rapid ramp-up of MVPA to ≥200 minutes in 9 weeks was begun concomitantly with modest caloric restriction in sedentary, obese, postmenopausal breast cancer survivors with <60 minutes per week of exercise.71 Assessments were also made of weight loss, body composition, and biomarkers of metabolic health. Two cohorts both received personal training twice per week, YMCA memberships, monitoring devices for weight and exercise, individual feedback, and weekly group sessions conducted by phone. The first cohort completed the intervention after 12 weeks; the second cohort continued another 12 weeks but without the personal trainer. The median MVPA for weeks 9–12 (postescalation period) was 219 minutes per week for both cohorts. By week 12, participants from both cohorts had substantial loss in total mass (8%), lean mass (3%), fat mass (13%), and visceral adipose (19%) as well as improvements in multiple biomarkers, including insulin, C-reactive protein, sex hormone-binding globulin, omentin, and the leptin-to-adiponectin ratio.71 Minutes of MVPA were correlated with visceral fat loss, which, in turn was associated with improvement in the leptin-to-adiponectin ratio. This ratio has been shown both in preclinical and clinical studies to be related to breast cancer risk.76–78 Participants in the second cohort that continued another 12 weeks without a personal trainer continued at an increased MVPA level from baseline, with only a moderate reduction from week 12, and continued to show improved body composition.
This work suggests that MVPA with modest caloric restriction leads to reduction of visceral adipose and improvement of metabolic biomarkers, such as the leptin-to-adiponectin ratio. The use of a personal trainer in sedentary women during a behavioral weight loss is associated with a marked increase in MVPA, which can be sustained post-trainer provided the woman has a place to exercise.
Effect of physical activity on inflammation and obesity-associated pancreatic ductal adenocarcinoma (Zobeida Cruz-Monserrate, PhD)
An upward trajectory of pancreatic cancer incidence correlates with the increasing prevalence of obesity in the United States.79 Although much is unknown regarding the mechanisms linking obesity and pancreatic cancer, some are related to the activation of proinflammatory molecules and their related pathways, such as cyclooxygenase-2 and lipocalin 2; blocking these pathways has proven beneficial in preclinical models of pancreatic cancer.80,81
Substantial weight loss after bariatric surgery reduces the risk of several cancer types, including pancreatic cancer,46 but it remains unclear whether lifestyle interventions for weight loss can reduce the risk, development, and/or progression of pancreatic cancer. Many clinical studies that incorporate physical activity interventions have not focused on prevention of the disease but rather on its effects after diagnosis of pancreatic cancer.82
To address these gaps in knowledge, the effects of weight loss have been tested through increased physical activity (wheel-running) and/or diet using a diet-induced obesity, genetically engineered mouse model of pancreatic cancer. Physically active mice gained significantly less weight, had decreased systemic inflammatory markers, developed fewer precancerous lesions, and experienced a slower rate of tumor development relative to controls on a locked wheel.83 The mechanisms of these findings, mostly as they relate to the activation of anti-inflammatory pathways, circulating cytokines, and adipokines and how they affect the adipose-tumor tissue crosstalk, are still under investigation and will provide a foundation for developing interventions that could be translated into obese individuals at high risk of developing pancreatic cancer. Important support for the translational relevance of this work was provided by an exercise intervention in individuals with overweight or obesity who participated in a 10-week running program. In these individuals, exercise caused a decrease in proinflammatory circulating cytokines and decreased body weight, similar to the results observed in mice.83 Taken together, these ongoing preliminary studies support the use of physical activity and/or exercise and diet as a weight loss and cancer prevention tool in individuals at a high-risk of cancer.
DIET AND NUTRITION
Nutrition plays a critical role in energy balance, obesity, and cancer. Dietary factors are difficult to mimic with drugs or supplements as well as to measure and characterize, calling attention to the need for further research in the field of nutrition and cancer. Addressing cancer through dietary interventions is a dynamic and challenging field requiring multidisciplinary teams and creative approaches.
Impacting energy balance in human studies: Intensity, personalization, and maintenance (Steven K. Clinton, MD, PhD)
Experimental studies of the impact of energy balance, nutrients, and bioactives in food on the cancer cascade began in earnest nearly a century ago when scientists developed a host of new rodent models of chemical-induced and radiation-induced carcinogenesis and strains of mice genetically disposed to specific cancers. Historically, research in diet/nutrition, energy, and cancer has taken a reductionist, mechanistic approach to determine how specific dietary components affect cancer. For example, the association between diets rich in fruits and vegetables and lower cancer risk led to the reductionist hypothesis that β-carotene was a major mediating factor,84 but clinical trials were profoundly disappointing.85 Indeed, it is unlikely that energy balance, or many nutrients, foods, and bioactives, act through a single mechanism when so many metabolic, neuroendocrine, immunologic, and other highly integrated host and tissue processes are affected.
It is imperative to go beyond the reductionist strategy alone and focus on complex and integrative interventions of dietary patterns and physical fitness, particularly as we transition to the future of personalized nutrition and begin to tailor interventions to individuals’ unique health concerns. It is clear that optimizing guidance for individuals at increased risk of cancer because of family history/genetics, exposure to carcinogens, and the presence of premalignant lesions, as well as for those who are undergoing therapy or are in posttherapy survivorship, requires evidence from epidemiologic research to identify relationships in different populations, preclinical research to understand underlying mechanisms and identify biomarkers, and high-quality RCTs.
Scientists often use epidemiology and laboratory models to establish hypotheses that warrant testing in well designed and appropriately powered phase 1 and 2 clinical trials to demonstrate safety and compliance, validate relevant biomarkers, and generate evidence for efficacy. Such studies provide the foundation for large phase 3 RCTs to establish a true estimate of beneficial impact and for translation into standards of care. A critical step is that the guidelines for evidence-based dietary interventions/counseling in the clinical setting must be supported by health care insurance, as are pharmaceutical agents, to ensure that impactful diet and fitness interventions are effectively translated into community practice for each individual (Figure 3).
FIGURE 3.
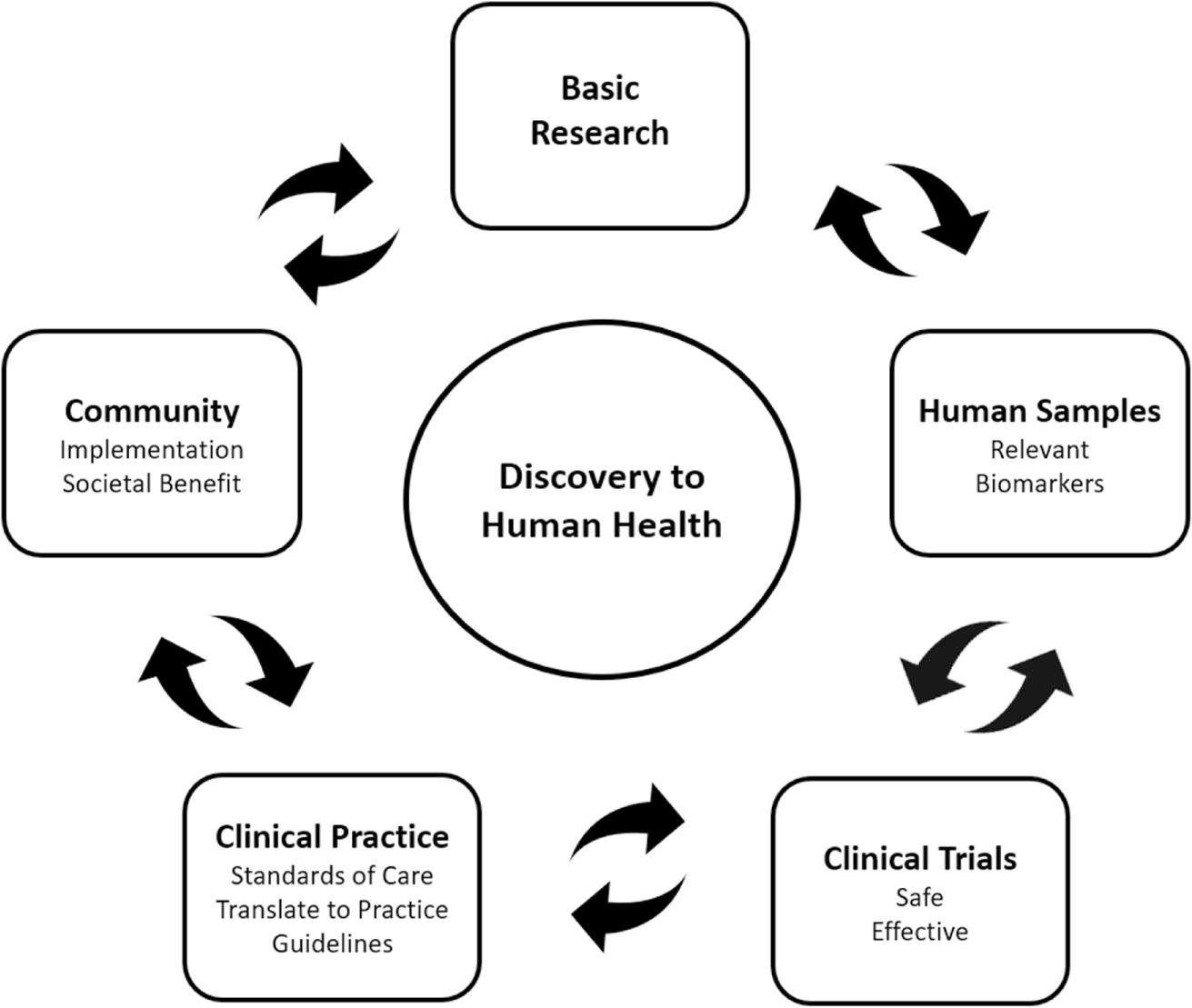
Discovery to human health. Basic research informs clinical research to be translated to clinical practice and implemented in the community. Community implementation can go on to further inform basic research and continue the cycle of discovery, practice, and implementation. To move through the cycle, research must first be relevant in humans with measurable biomarkers. Second, treatments must be safe and effective to be translated to standard practice guidelines to provide society benefit when implemented in the community.
Public health diet guidelines for populations are mature,86 although implementation remains a challenge. One approach to personalized nutrition is to integrate healthy dietary patterns with exercise and fitness programs for cancer survivors who are at high risk of cardiometabolic disease as a consequence of their cancer therapy compounded by unhealthy lifestyles. Two examples include the Individualized Diet and Exercise Adherence Trial–Prostate (IDEA-P) study (ClinicalTrials.gov identifier NCT02050906)87 and the Garden of Hope study.88 IDEA-P was a pilot project for men with prostate cancer that involved a personalized exercise program combined with modest dietary goals provided by an integrated team of exercise physiologists and dietitians. The individualized approach enhanced compliance and improved function and quality of life for men with prostate cancer on hormone therapy.87,89 The Garden of Hope pilot study for cancer survivors also integrated diet and fitness but with greater focus on diet using more intense combinations of group and individual guidance, motivational interviewing and coaching, along with digital health strategies (Table 1). These studies led to the larger phase 2 clinical trial, the Nutrition and Exercise Trial (NExT; ClinicalTrials.gov identifier NCT03489213), for cancer survivors with overweight and obesity that is now undergoing analysis. The intervention consisted of biobehavioral lifestyle changes based on the Dietary Guidelines for Americans/World Cancer Research Fund guidelines, followed by a 6-month monitoring and maintenance phase. The study goals are to improve overall adherence to evidence-based diet and exercise guidelines over a 6-month intervention and to maintain clinically meaningful, sustainable weight loss, healthy dietary patterns, and fitness during a 6-month maintenance phase. Components of the study are conducted at an urban garden; participants work in the garden and harvest fruits and vegetables weekly, and the program integrates expert chefs to provide education regarding food selection, using in-season crops, and cooking skills. Real-time feedback is provided by self-monitoring through the use of technology and by counselors. Preliminary results suggest a remarkable and statistically significant change in dietary patterns along with improved biomarkers of health: decreased weight/BMI with reductions in fat mass measured by dual-energy absorptiometry scan, improved systolic blood pressure, increased skin and total plasma carotenoids, improved fitness, and improved quality-of-life scores in multiple domains.
TABLE 1.
Key phases and factors for development of clinical trials in energy balance.
| Intervention phase | Maintenance phase |
|---|---|
|
| |
| • Group education with individualized support | • Skills reinforcement |
| • Web-based training accessible 24/7 | |
| • Physical setting that promotes group sharing | • Continued health education |
| • Hands-on skills development | |
| • Motivational interviewing and coaching | |
| • Real-time feedback from experts | • Group support |
| • Wearable device for performance tracking and self-monitoring | |
More phase 2 trials are needed that test specific and highly defined intervention strategies using a portfolio of tools that help individuals overcome their unique barriers (see Table 2 for intervention considerations). Once the key components crucial for high adherence and efficacy are demonstrated in phase 2 trials, support from the National Institutes of Health for large phase 3 trials will be imperative. Investment in personalized nutrition research for cancer prevention, optimizing cancer therapy, and promoting healthy survivorship will improve health and productivity while reducing health care costs. The evidence generated will ensure future guideline development and support by insurers so that individuals have access to the expertise, resources, and monitoring for success.
TABLE 2.
Considerations for developing impactful diet and exercise interventions that can be moved into standard of care.
| Considerations |
|---|
|
|
| • Combine diet and exercise |
| • Ground in behavioral theory and incorporate tested strategies |
| • Optimize intensity, duration, and frequency |
| • Include a maintenance phase |
| • Integrate the individual and the group |
| • Involve multidisciplinary experts |
| • Utilize available and acceptable technologies |
| • Provide access to tools/equipment |
| • Consider service reimbursement |
| • Include unique populations |
| • Maximize patient benefit |
Using parallel studies to define nutritional and circadian parameters for enhancing leukemia therapy (Joya Chandra, PhD)
Acute leukemia is the most common malignancy in children worldwide but affects only a small population of adults. Survival rates for patients with acute lymphocytic leukemia are heralded as a success because of decades of improvements in combination chemotherapy and supportive care. Acute myelogenous leukemia (AML) is molecularly heterogeneous across children, adolescents and young adults, and adults; however, across all ages, the most common genetic alteration is mutation in FLT3. This mutation confers a worse prognosis in all three populations and has been a focus of energy balance research, particularly in nutrition.
Many patients with leukemia consume diets that are high in sugar, salt, and fat, and previous studies have shown that high-fat diets promote leukemia progression.90 Studies have also shown that circadian-clock genes regulate stem cells in AML through the disruption of canonical circadian-pathway components.91 However, whether delivering diet interventions and modulating timing of diet can augment therapy efficacy has not been studied in AML. By using an orthotopic xenograft FLT3-mutant AML mouse model, mice were fed a chow-based diet with a macronutrient composition relevant to that of human diets. Researchers examined whether fat and sugar modulation within the diet can augment chemotherapy efficacy. The mice were treated with chemotherapy (doxorubicin) or targeted therapy (the tyrosine kinase inhibitor quizartinib, which targets the FLT3 mutation) and/or a special diet or circadian modulation of diet. The diet treatments were either low-fat/low-sugar, low-sugar, high-sugar, low-fat, or high-fat. The low-fat/low-sugar diet enhanced doxorubicin and quizartinib efficacy in FLT3-mutant AML. Combination therapy with the low-fat/low-sugar diet and doxorubicin was more effective than doxorubicin alone or the diet alone, and combination of quizartinib with a low-fat/low-sugar diet showed enhanced survival compared with quizartinib and a regular chow diet. Low-fat and low-sucrose diets were also evaluated in separate mouse survival experiments. The longest surviving mice were those receiving the low-sugar and doxorubicin combination or the low-fat and doxorubicin combination.
Separate experiments looked at circadian effects by restricting food consumption to the active phase and for 12 hours. In these experiments, mice not receiving doxorubicin had a lower leukemia burden if they received restricted feeding, but time-restricted feeding did not influence disease burden in mice receiving doxorubicin, likely because of the high effectiveness of the drug. Western blot analysis demonstrated that levels of the circadian regulator Bmal1 were higher in peripheral blood mononuclear cells of mice receiving the low-sucrose diet and higher in mice on the time-restricted feeding diet, demonstrating a circadian component to both the diet and its timing.
To translate these findings to clinical trials, diets should consider cultural aspects and needs of families, particularly for pediatric patients. In a small institutional trial, children with leukemia were enrolled in a tasting study of low-fat and/or low-sugar foods. The children and their caregivers participated in tasting sessions in which the macronutrients in the foods matched those tested in the mouse studies described above. Most patients liked the foods tested, demonstrating the feasibility of delivering a low-fat and/or low-sugar diet as an intervention in future studies.
Reverse translation in nutrition: From observation to mechanism to intervention (Jennifer L. McQuade, MD)
The development and approval of immune checkpoint inhibitors has dramatically improved the outcomes of many cancers. For example, historically, metastatic melanoma was deadly, with <10% of patients surviving 3 years after diagnosis,92 but, since the advent of immune checkpoint inhibitors, the 5-year survival rate has improved to >50%.93 However, responses are heterogeneous; nearly 50% of patients do not derive any benefit.
Factors that determine tumor response versus resistance can be cancer cell intrinsic, such as oncogenic signaling pathways; extrinsic, such as immune infiltration into the tumor; or both. One dimension that has not been well studied is the role of the host’s modifiable factors, such as obesity,94 diet, exercise, stress, sleep, and the gut microbiome. The microbiome has more gene content than the human genome and is inherently modifiable because it is largely environmentally determined. Genetic inheritability only contributes to 10% of the variation in the human microbiome, with the remaining 90% determined by exposures such as diet, medications, geography, or stress.
The emerging role of the gut microbiome in both shaping the immune response and as an integrator of modifiable host factors has brought new attention to energy balance in the setting of immune checkpoint inhibitors. Early work in mice showed that the gut microbiome influenced responses to immunotherapy and that fecal microbiome transplants between mice could convey responsiveness to immunotherapy through microbiome modulation.95,96 To determine relevance to humans, researchers compared the gut microbiome of patients who responded to immunotherapy to those who did not. The microbiome of responders to immunotherapy was distinct from that of nonresponders.97–100
Recent studies have examined the interaction between modifiable lifestyle factors, the microbiome, and immunotherapy response. Many of the bacteria associated with immunotherapy response have roles in the fermentation and digestion of fiber. Fiber is broken down by bacteria to produce short-chain fatty acids that serve as the primary nutrient source for gut enterocytes and formation of a gut mucosal barrier. In one study, 128 patients with melanoma being treated with immune checkpoint blockade responded to surveys about their diet, supplements, depression and anxiety symptoms, and physical activity. Patients consuming sufficient fiber had higher BMI than those with insufficient intake, but the groups were otherwise balanced. Patients with sufficient fiber consumption had a higher 2-year progression-free survival than those with insufficient fiber consumption.101 These findings should be interpreted as correlative rather than causative because of the possibility of residual confounding by lifestyle and demographic factors.
To control for potential confounders, mouse models have been used to inform human studies related to fiber consumption. Mice treated with either a standard adequate-fiber chow diet or a fiber-deprived diet were injected with melanoma cells and treated with immunotherapy. Only mice receiving a high-fiber chow diet mounted a significant response to the therapy, whereas a much-diminished response to checkpoint inhibition was observed in the mice receiving low-fiber chow. Flow cytometry analysis of tumor-infiltrating lymphocytes demonstrated activation of immune pathways only in mice that received the high-fiber chow. Furthermore, there was a distinct difference in the microbiome profile between the two groups. When this experiment was repeated in germ-free mice, there was no difference in the tumor burden between the two groups, suggesting that treatment response was indeed microbiome-mediated.101
Based on these results from human observational and experimental mouse studies, a human study at The University of Texas MD Anderson Cancer Center will deliver a fully controlled dietary intervention to evaluate whether diet enhances approved therapies by changing the microbiome. In this prospective study in patients with melanoma, all calorie-containing food and beverages are provided in a controlled feeding study. This feeding study approach removes the behavioral component of diet choice and evaluates whether the microbiome and immune response can be changed by diet. A phase 1 study was performed in melanoma survivors to assess the feasibility of diet delivery and diet adherence as well as changes in the microbiome (ClinicalTrials.gov identifier NCT03950635). The current phase 2 study (ClinicalTrials.gov identifier NCT04645680) will evaluate biomarkers of changes in immunity and changes in the microbiome in patients being treated with checkpoint inhibitors. If phase 2 is successful, phase 3 will consist of a large-scale behavioral intervention diet study to assess disease outcomes.
Translational diet studies are important to inform the public health recommendations regarding behavioral change in the population and support practice change by clinical providers. Nutritional aspects of care are often overlooked because of a focus on immune and tumor targets and the lack of current evidence supporting nutrition’s role in cancer therapy.102 Demonstrating that nutrition or the microbiome is critical in the progression or regression of tumor burden will eventually lead to the incorporation of nutrition into standard-of-care clinical practices.
MOVING ENERGY BALANCE RESEARCH TOWARD CLINICAL PRACTICE CHANGE
This conference session summarized what is known and what remains to be elucidated related to physical activity/exercise, weight loss, dietary intake, and cancer. Multiple studies have focused on evidence that exercise, weight loss, and diet provide benefit to cancer survivors and patients with cancer.13,103,104 Multidisciplinary teams are developing diet and exercise trials with a clinical phase system similar to that of pharmaceutical trials to begin establishing these measures and to configure individualized plans that can be more easily prescribed to patients by providers. Concurrently, these teams are also examining how to best approach patients with evidence to promote change and ensure all stakeholders are informed, regardless of background or means.
Translation to practice: Exercise and physical activity (Neil M. Iyengar, MD)
Exercise, among several lifestyle-management strategies, has been shown to improve quality-of-life parameters and patient-reported outcomes in survivors of cancer.13 Consequently, clinicians often view exercise as a supportive intervention. Yet there is a rapidly growing body of preclinical and clinical data indicating that exercise may also reduce the risk of cancer recurrence through the modulation of multiple local and systemic pathways.105 To harness the potential anticancer effect of exercise, rigorous clinical testing using metrics adapted from traditional oncology therapeutic development is needed to identify the optimal dose and timing of exercise and to select populations most likely to respond.106 Further research on these mechanisms is important for selecting the populations most likely to respond to exercise or physical activity.
A survey conducted among 971 oncologists in the United States and internationally queried whether lifestyle management, including nutrition and exercise, should be included as part of standard-of-care treatment.107 These physicians supported the inclusion of nutrition and physical activity as a standard part of cancer treatment, with 79% reporting that the treating physician is responsible for recommending these lifestyle interventions. However, <40% refer patients for physical activity or nutrition interventions, and nearly 90% agree that clinicians need more knowledge about the resources available to help patients with weight and exercise. These findings emphasize a key reality in modern cancer care: oncology practitioners want to incorporate nutrition and exercise into the standard of care, but they are not equipped to do so. Dissemination of data regarding energy balance and cancer would likely provide oncologists with support to expand their treatment strategies to include diet and physical activity recommendations.
As described in previous sections, interventions that favorably modulate metabolic health, body fat, and metabolic or inflammatory markers with strategies such as exercise can serve an important purpose in oncology practice to which clinicians are receptive. However, effects may be variable. Although epidemiologic evidence shows that physical activity is associated with reduced cancer-specific mortality,108 differential effects of exercise may exist and may be mediated by tumor molecular subtype or microenvironment, type of chemotherapy or immunotherapy, underlying inflammatory state, and other intrinsic host factors.106
The American Institute for Cancer Research provides readily accessible lifestyle guidelines for reducing cancer risk that can be easily incorporated into clinical practice.104 However, greater specificity is needed, including the optimal dosing and timing of exercise during and after cancer treatment. Oncologists are generally reluctant to recommend treatments without established dosing or modification guidelines specific to tumor subtypes and/or treatment setting. Dose-finding studies and studies that interrogate exercise modality are needed. The next generation of exercise oncology trials could leverage the precision oncology paradigm by testing for a differential effect of exercise by tumor subtype using classical oncologic outcomes, such as objective response rate and progression-free survival.
Using a drug-development paradigm that is adapted for exercise as an anti-cancer strategy may be useful in developing dosing and modification guidelines necessary for practice change,106 as described above in the Diet and Nutrition section. To translate exercise research to practice, phase 1 trials could allocate participants to multiple exercise doses, test feasibility, and identify maximum feasible doses (the highest dose with acceptable toxicity or side effects). Subsequent phase 2 testing could provide preliminary clinical data using the maximum feasible dose while identifying predictors of response by measuring biomarkers and investigating tumor tissue biology and the tumor microenvironment response. Identification of populations most likely to benefit could guide phase 3 trials that test the effects of predetermined exercise doses versus the standard of care (i.e., no specific exercise guidelines) for improving cancer-specific and overall survival.
An example of a phase 1a/1b trial exercise trial is currently open for patients with estrogen receptor-positive, metastatic breast cancer (ClinicalTrials.gov identifier NCT03988595). Forty eligible patients are being allocated to four different dosing groups ranging from 90 minutes per week to 300 minutes per week for a 6-month intervention period. The trial was recently amended to add a fifth, higher dose of exercise (375 minutes per week), which will be tested in a separate dosing cohort. The biologic activity seen with the different doses of exercise will also inform the selection of the dose carried forward to phase 2 testing.
Phase 2 trials in the adjuvant setting can build on prior diet and exercise studies. For example, a current phase 2 study in postmenopausal women on adjuvant aromatase-inhibitor therapy for breast cancer is testing the combination of aerobic exercise with a plant-based diet deployed by digitally supervised, home-based exercise and preprepared meal delivery (ClinicalTrials.gov identifier NCT04298086). This study combines exercise and groundwork laid in support of a plant-based diet for favorably modifying the biologic effects of obesity that promote tumor growth.109,110
Future directions for the incorporation of exercise into standard-of-care therapy for cancer should include preclinical studies with parallel clinical testing to determine when and how to optimally harness a potential anticancer effect of exercise. Certainly, exercise prescription is recommended throughout the cancer continuum when used for symptom control and/or management of treatment-related adverse effects.13,102
Translation to practice: Weight loss and dietary intake (Wendy Demark-Wahnefried, PhD, RD)
Obesity is a well established risk factor for cancer40; body size and weight gain over the course of time into adulthood can also affect cancer risk. Purposeful weight loss is known to improve survival in the general population. A meta-analysis of 54 RCTs involving 30,200 adults with obesity found that the risk ratio for all-cause mortality was reduced by 18% when adults intentionally lost weight.111 There is evidence of reduced risk for several obesity-related cancers with intentional weight loss among women. In the Iowa Women’s Health Study, a 20% reduction in breast cancer was demonstrated among adult women with sustainable purposeful weight loss.112 Intentional weight loss was also shown to protect against development of colon and endometrial cancers.113
However, the relationship between weight change and cancer risk is not always clear. Unlike intentional weight loss, unintentional caloric restriction can be associated with increased cancer risk. In a meta-analysis of seven prospective cohorts, caloric restriction during childhood and adolescence was associated with an increased risk of prostate cancer and breast cancer.114 These analyses suggest that there may be critical periods in the lifecycle during which energy balance may have long-term effects on energy dysregulation and subsequent cancer risk.
Obesity is a poor prognostic factor for several, but not all, cancers.115 Weight gain has been shown to adversely affect cancer-related outcomes for breast and prostate cancer.116 A meta-analysis of 82 studies of >200,000 women with breast cancer found that every 5-kg/m2 increase in BMI from prediagnosis to postdiagnosis was associated with an increase in breast cancer-specific mortality of almost 30% and in total mortality of 8%. Similarly, a prostate cancer cohort of >18,000 men found that every 5-kg/m2 increase in BMI from prediagnosis to postdiagnosis was associated with increases of 20% in both biochemical recurrence and prostate cancer-specific mortality. However, in patients who had metastatic melanoma being treated with immune and targeted therapies, obesity was linked with improved progression-free survival and overall survival compared with those who had normal BMI.94 Clearly, diagnosis-specific differences will indicate when weight loss is a necessary goal.
To date, published weight-loss trials in cancer survivors have focused on weight/body composition, quality-of-life, and biomarker outcomes. A Cochrane review of 20 weight management RCTs in 2028 breast cancer survivors found positive changes in weight loss, quality-of-life outcomes, and biomarkers; however, evidence quality was low, in part because of inability to blind participants and assessors.117 A similar review of three RCTs that pooled data from 161 endometrial cancer survivors who participated in diet and exercise behavioral modifications found little evidence of benefit related to quality of life or body composition and actually observed an increase in musculoskeletal symptoms.118 Whether purposeful weight loss improves cancer-related outcomes and survival in populations with cancer requires further study. More intervention trials investigating survival end points in cancer survivors are necessary. Examples of recent trials in this area are the Lifestyle Intervention for Ovarian Cancer Enhanced Survival (LIVES) study (ClinicalTrials.gov identifier NCT00719303)119 and the Breast Cancer Weight Loss (BWEL) study (ClinicalTrials.gov identifier NCT02750826),120 which should produce findings within the next few years.
The optimal diet composition and rate of weight loss to produce benefits are unknown for patients with cancer and cancer survivors. Rapid weight loss with suboptimal physical activity has been associated with a higher tumor proliferation index in two presurgical RCTs in men with prostate cancer.121,122 Weight loss in patients with cancer is difficult to assess because it may be secondary to the cachexia of cancer progression.
Weight-loss guidelines123 note that loss of as little as 3% of body weight in individuals with overweight or obesity is associated with health benefits, including glycemic and lipid control. Recommendations include energy restriction of 1200–1800 kcal daily, an increase in physical activity, and behavior modifications. Effective strategies identified in 30 weight-loss trials for cancer survivors included goal setting, social support, and self-monitoring, which ranked highly in techniques used in effective interventions.124 These techniques can be applied in person, over the phone, or on the web. Web-based interventions are efficient, but participant engagement wanes over time; success depends on incorporating user input into intervention design. The National Cancer Institute-sponsored Aim Plan and Act on Lifestyles (AMPLIFY) Survivor Health study is currently enrolling survivors to test the impact of three web-based diet and/or exercise interventions on changes in health behaviors and outcomes (https://amplifymyhealth.org; ClinicalTrials.gov identifier NCT04000880).
Weight-control considerations differ between cancer survivors and individuals without cancer and may require personalized guidelines. Weight gain can be a common side effect of therapies, especially chemotherapy, as well as hormonal therapies used for some cancers, including breast cancer, prostate cancer, and leukemia.125–127 Up to 35% of cancer survivors have obesity, and obesity has been found to increase the risk of death for all cancers combined, with patients who have severe obesity experiencing a >50% increased risk of death than patients with cancer who have a healthy weight.128 Cancer survivors may have poorer quality diets that may reflect long-term dietary patterns before diagnosis or because changes in taste and other physical and quality-of-life factors affect dietary intake postdiagnosis.129 Survivors also may receive more negative feedback for their weight loss and may have more comorbidities or late effects of their therapies that interfere with weight loss.102 Despite these challenges, weight-loss trial retention rates are significantly higher in cancer survivors (71%–100%) compared with the general population (53%–65%),130 which suggests that cancer survivors are motivated to participate in weight-loss interventions with potential for improved long-term outcomes.
Expansion of research is needed in multiple areas within the context of cancer, diet, and weight-loss interventions. The best measures for the effect of weight-related changes on cancer have not yet been established, although several are being evaluated: weight, body composition, BMI, metabolic disruption, diet, and biomarkers. The best dietary approaches (composition and delivery; e.g., intermittent fasting vs. continuous caloric restriction) to achieve weight loss or improved cancer outcomes are unclear. The timing of these interventions should also be studied. Timing may also influence comorbidities, quality of life, and cost. The mechanisms underlying changes at the molecular level provide ample opportunity for preclinical and parallel studies. Distribution of knowledge to stakeholders will be critical as knowledge expands, with attention to ensuring access to underserved groups, including racial, ethnic, and sexual/gender minorities; those in rural areas; and patients with understudied cancers.
CONCLUSION
A goal of this conference was to explore the bidirectional relationship between preclinical and clinical research in energy balance and cancer and to explore translation to clinical and community practice. Several themes emerged from the work and ideas presented. First, designing preclinical studies to parallel clinical research is complex. Factors such as differences in genetic heterogeneity between animal models and humans and comparability of diet and exercise interventions between animal and human studies illustrate the complexity of trying to model the effects of energy balance on human cancer.14
Second, numerous examples of preclinical work being translated to clinical trials, or of clinical observations being modeled preclinically, were presented. However, there are very few truly parallel studies in which the preclinical and patient data inform each other within the context of a single study. This indicates the difficulty in getting such studies funded and the need for more of this type of parallel research. Although rare, truly parallel research was presented and discussed above in the example of the relationship between fiber, the microbiome, and the response of patients with melanoma to immune checkpoint blockade. The relationship between the microbiome and response to immune checkpoint blockade was first discovered in mice, then validated in patients. Next, in patients, the relationship between sufficient fiber intake and better outcomes was observed. This observation was taken to mouse models, in which confounding variables can be controlled, and validated. The mouse models were used to demonstrate that improved outcomes of melanoma in response to immune checkpoint blockade by fiber intake is mediated by the microbiome. In this example, observations in mice were validated in humans and, vice versa, through a series of parallel studies.
Finally, whereas silos between preclinical, clinical, and population researchers have lessened over the last several decades, there is still much work to be done. Some points for consideration in the building of successful parallel research include: the importance of a transdisciplinary team with equal input from individuals of varied expertise, careful consideration of the applicability of the chosen animal to the human disease being studied, and the utility of a flexible translational research continuum, akin to drug development and testing but incorporating bidirectional influences as new information is learned in humans or in mice. With the growing body of evidence pointing to positive effects of diet, exercise, and weight loss for patients with cancer and survivors, implementation research is sorely needed. As presented at this conference, the building blocks of preclinical and clinical research, which lay the foundation for implementation research and adoption of best practice, suggest a growing role for energy balance interventions as a mainstay of cancer care.
ACKNOWLEDGMENTS
The conference was sponsored by The University of Texas MD Anderson Cancer Center Divisions of Cancer Prevention and Population Sciences and Pediatrics. The authors thank the Conference Planning Committee and its organizers, including MD Anderson’s Center for Energy Balance (Miranda Baum, Thuan Le) and conference services (Dr James Cavalier Jr, Melissa Andrus, Sharlene Lockett, Dayna Gordon, Francisco Ostolaza, Coni Tierney). Conference grant support was provided by the National Cancer Institute (1R13CA254014-01).
Footnotes
CONFLICTS OF INTEREST
Carrie R. Daniel reports grants from the Melanoma Research Alliance and travel support from the American Association for Cancer Research, the American Gastroenterological Association, and the American Society for Radiation Oncology outside the submitted work. Andrew J. Dannenberg reports personal fees from SynDevRx outside the submitted work. Neil M. Iyengar reports grants from the American Cancer Society, the Conquer Cancer Foundation, Novartis, and SynDevRx and personal fees from Pfizer and Seattle Genetics outside the submitted work. Jennifer L. McQuade reports personal fees for service on advisory boards for Roche, Bristol Myers Squibb, Merck, and BioTax outside the submitted work. Kathryn H. Schmitz reports grants from the American Cancer Society outside the submitted work. Karen Basen-Engquist reports grants from the Cancer Prevention and Research Institute of Texas outside the submitted work. The remaining authors made no disclosures.
Andrew J. Dannenberg was retired at the time of this report.
REFERENCES
- 1.Stephens T Secular trends in adult physical activity: exercise boom or bust. Res Q Exerc Sport. 1987;58(2):94–105. doi: 10.1080/02701367.1987.10605432 [DOI] [Google Scholar]
- 2.Irwin ML, Ainsworth BE, Mayer-Davis EJ, Addy CL, Pate RR, Durstine JL. Physical activity and the metabolic syndrome in a tri-ethnic sample of women. Obes Res. 2002;10(10):1030–1037. doi: 10.1038/oby.2002.140 [DOI] [PubMed] [Google Scholar]
- 3.Bernstein L, Henderson BE, Hanisch R, Sullivan-Halley J, Ross RK. Physical exercise and reduced risk of breast cancer in young women. J Natl Cancer Inst. 1994;86(18):1403–1408. doi: 10.1093/jnci/86.18.1403 [DOI] [PubMed] [Google Scholar]
- 4.Bernstein L, Ross R, Lobo R, Hanisch R, Krailo M, Henderson B. The effects of moderate physical activity on menstrual cycle patterns in adolescence: implications for breast cancer prevention. Br J Cancer. 1987;55(6):681–685. doi: 10.1038/bjc.1987.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McTiernan A, Tworoger SS, Ulrich CM, et al. Effect of exercise on serum estrogens in postmenopausal women: a 12-month randomized clinical trial. Cancer Res. 2004;64(8):2923–2928. doi: 10.1158/0008-5472.can-03-3393 [DOI] [PubMed] [Google Scholar]
- 6.Irwin ML, Yasui Y, Ulrich CM, et al. Effect of exercise on total and intra-abdominal body fat in postmenopausal women: a randomized controlled trial. JAMA. 2003;289(3):323–330. doi: 10.1001/jama.289.3.323 [DOI] [PubMed] [Google Scholar]
- 7.Hu C, Tang J, Gao Y, Cao R. Effects of physical exercise on body fat and laboratory biomarkers in cancer patients: a meta-analysis of 35 randomized controlled trials. Support Care Cancer. 2022;30(9):1–12. doi: 10.1007/s00520-022-07013-6 [DOI] [PubMed] [Google Scholar]
- 8.Ligibel JA, Dillon D, Giobbie-Hurder A, et al. Impact of a preoperative exercise intervention on breast cancer proliferation and gene expression: results from the Pre-Operative Health and Body (PreHAB) Study. Clin Cancer Res. 2019;25(17):5398–5406. doi: 10.1158/1078-0432.ccr-18-3143 [DOI] [PubMed] [Google Scholar]
- 9.Harrigan M, Cartmel B, Loftfield E, et al. Randomized trial comparing telephone versus in-person weight loss counseling on body composition and circulating biomarkers in women treated for breast cancer: the Lifestyle, Exercise, and Nutrition (LEAN) Study. J Clin Oncol. 2016;34(7):669–676. doi: 10.1200/jco.2015.61.6375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Usiskin I, Li F, Irwin ML, Cartmel B, Sanft T. Association between pre-diagnosis BMI, physical activity, pathologic complete response, and chemotherapy completion in women treated with neoadjuvant chemotherapy for breast cancer. Breast Cancer. 2019;26(6):719–728. doi: 10.1007/s12282-019-00974-3 [DOI] [PubMed] [Google Scholar]
- 11.van Waart H, Stuiver MM, van Harten WH, et al. Effect of low-intensity physical activity and moderate-to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol. 2015;33(17):1918–1927. doi: 10.1200/jco.2014.59.1081 [DOI] [PubMed] [Google Scholar]
- 12.Moore SC, Lee IM, Weiderpass E, et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. 2016;176(6):816–825. doi: 10.1001/jamainternmed.2016.1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc. 2019;51(11):2375–2390. doi: 10.1249/mss.0000000000002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Courneya KS, Booth CM. Exercise as cancer treatment: a clinical oncology framework for exercise oncology research. Front Oncol. 2022;12:957135. doi: 10.3389/fonc.2022.957135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L, Morielli AR, Heer E, et al. Effects of exercise on cancer treatment efficacy: a systematic review of preclinical and clinical studies. Cancer Res. 2021;81(19):4889–4895. doi: 10.1158/0008-5472.can-21-1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones LW, Eves ND, Courneya KS, et al. Effects of exercise training on antitumor efficacy of doxorubicin in MDA-MB-231 breast cancer xenografts. Clin Cancer Res. 2005;11(18):6695–6698. doi: 10.1158/1078-0432.ccr-05-0844 [DOI] [PubMed] [Google Scholar]
- 17.Rao R, Cruz V, Peng Y, et al. Bootcamp during neoadjuvant chemotherapy for breast cancer: a randomized pilot trial. Breast Cancer (Auckl). 2012;6:39–46. doi: 10.4137/bcbcr.s9221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morielli AR, Usmani N, Boule NG, et al. Feasibility, safety, and preliminary efficacy of exercise during and after neoadjuvant rectal cancer treatment: a phase II randomized controlled trial. Clin Colorectal Cancer. 2021;20(3):216–226. doi: 10.1016/j.clcc.2021.05.004 [DOI] [PubMed] [Google Scholar]
- 19.Courneya KS, Sellar CM, Stevinson C, et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol. 2009;27(27):4605–4612. doi: 10.1200/jco.2008.20.0634 [DOI] [PubMed] [Google Scholar]
- 20.Courneya KS, Segal RJ, Mckenzie DC, et al. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med Sci Sports Exerc. 2014;46(9):1744–1751. doi: 10.1249/mss.0000000000000297 [DOI] [PubMed] [Google Scholar]
- 21.Kirkham AA, Gelmon KA, Van Patten CL, et al. Impact of exercise on chemotherapy tolerance and survival in early-stage breast cancer: a nonrandomized controlled trial. J Natl Compr Canc Netw. 2020;18(12):1670–1677. doi: 10.6004/jnccn.2020.7603 [DOI] [PubMed] [Google Scholar]
- 22.Chiarotto JA, Akbarali R, Bellotti L, Dranitsaris G. A structured group exercise program for patients with metastatic cancer receiving chemotherapy and CTNNB1 (β-catenin) as a biomarker of exercise efficacy. Cancer Manag Res. 2017;9:495–501. doi: 10.2147/cmar.s147054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rief H, Bruckner T, Schlampp I, et al. Resistance training concomitant to radiotherapy of spinal bone metastases—survival and prognostic factors of a randomized trial. Radiat Oncol. 2016;11(1):97. doi: 10.1186/s13014-016-0675-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedersen L, Idorn M, Olofsson G, et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016;23(3):554–562. doi: 10.1016/j.cmet.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 25.Metcalfe RS, Kemp R, Heffernan SM, et al. Anti-carcinogenic effects of exercise-conditioned human serum: evidence, relevance and opportunities. Eur J Appl Physiol. 2021;121(8):2107–2124. doi: 10.1007/s00421-021-04680-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2003;163(5):1801–1815. doi: 10.1016/s0002-9440(10)63540-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schadler KL, Thomas NJ, Galie PA, et al. Tumor vessel normalization after aerobic exercise enhances chemotherapeutic efficacy. Oncotarget. 2016;7(40):65429–65440. doi: 10.18632/oncotarget.11748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Florez Bedoya CA, Cardoso ACF, Parker N, et al. Exercise during preoperative therapy increases tumor vascularity in pancreatic tumor patients. Sci Rep. 2019;9(1):13966. doi: 10.1038/s41598-019-49582-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ngo-Huang A, Parker NH, Bruera E, et al. Home-based exercise prehabilitation during preoperative treatment for pancreatic cancer is associated with improvement in physical function and quality of life. Integr Cancer Ther. 2019;18:1534735419894061. doi: 10.1177/1534735419894061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LeBlanc AK, Breen M, Choyke P, et al. Perspectives from man’s best friend: National Academy of Medicine’s Workshop on Comparative Oncology. Sci Transl Med. 2016;8(324):324ps5. doi: 10.1126/scitranslmed.aaf0746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langsten KL, Kim JH, Sarver AL, Dewhirst M, Modiano JF. Comparative approach to the temporo-spatial organization of the tumor microenvironment. Front Oncol. 2019;9:185. doi: 10.3389/fonc.2019.01185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer. 2008;8(2):147–156. doi: 10.1038/nrc2273 [DOI] [PubMed] [Google Scholar]
- 33.Page R, Baneux P, Vail D, et al. Conduct, oversight, and ethical considerations of clinical trials in companion animals with cancer: report of a workshop on best practice recommendations. J Vet Intern Med. 2016;30(2):527–535. doi: 10.1111/jvim.13916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guy MK, Page RL, Jensen WA, et al. The Golden Retriever Lifetime Study: establishing an observational cohort study with translational relevance for human health. Philos Trans R Soc Lond B Biol Sci. 2015;370(1673):20140230. doi: 10.1098/rstb.2014.0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Labadie J, Swafford B, DePena M, Tietje K, Page R, Patterson-Kane J. Cohort profile: the Golden Retriever Lifetime Study (GRLS). PLoS One. 2022;17(6):e0269425. doi: 10.1371/journal.pone.0269425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oleson JR, Samulski TV, Leopold KA, et al. Sensitivity of hyperthermia trial outcomes to temperature and time: implications for thermal goals of treatment. Int J Radiat Oncol Biol Phys. 1993;25(2):289–297. doi: 10.1016/0360-3016(93)90351-u [DOI] [PubMed] [Google Scholar]
- 37.Dewhirst MW, Sim DA. The utility of thermal dose as a predictor of tumor and normal tissue responses to combined radiation and hyperthermia. Cancer Res. 1984;44(10 suppl):4772s–4780s. [PubMed] [Google Scholar]
- 38.Jones EL, Oleson JR, Prosnitz LR, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol. 2005;23(13):3079–3085. doi: 10.1200/jco.2005.05.520 [DOI] [PubMed] [Google Scholar]
- 39.Thrall DE, LaRue SM, Yu D, et al. Thermal dose is related to duration of local control in canine sarcomas treated with thermoradiotherapy. Clin Cancer Res. 2005;11(14):5206–5214. doi: 10.1158/1078-0432.ccr-05-0091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clinton SK, Giovannucci EL, Hursting SD. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: impact and future directions. J Nutr. 2020;150(4):663–671. doi: 10.1093/jn/nxz268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–798. doi: 10.1056/nejmsr1606602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ulrich CM, Himbert C, Holowatyj AN, Hursting SD. Energy balance and gastrointestinal cancer: risk, interventions, outcomes and mechanisms. Nat Rev Gastroenterol Hepatol. 2018;15(11):683–698. doi: 10.1038/s41575-018-0053-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bowers LW, Doerstling SS, Shamsunder MG, et al. Reversing the genomic, epigenetic and triple negative breast cancer-enhancing effects of obesity. Cancer Prev Res (Phila). 2022;15(9):581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karra P, Winn M, Pauleck S, et al. Metabolic dysfunction and obesity-related cancer: beyond obesity and metabolic syndrome. Obesity. 2022;30(7):1323–1334. doi: 10.1002/oby.23444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Habanjar O, Diab-Assaf M, Caldefie-Chezet F, Delort L. The impact of obesity, adipose tissue, and tumor microenvironment on macrophage polarization and metastasis. Biology. 2022;11(2):339. doi: 10.3390/biology11020339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schauer DP, Feigelson HS, Koebnick C, et al. Bariatric surgery and the risk of cancer in a large multisite cohort. Ann Surg. 2019;269(1):95–101. doi: 10.1097/sla.0000000000002525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fabian CJ, Kimler BF, Donnelly JE, et al. Favorable modulation of benign breast tissue and serum risk biomarkers is associated with >10% weight loss in postmenopausal women. Breast Cancer Res Treat. 2013;142(1):119–132. doi: 10.1007/s10549-013-2730-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rossi EL, de Angel RE, Bowers LW, et al. Obesity-associated alterations in inflammation, epigenetics, and mammary tumor growth persist in formerly obese mice. Cancer Prev Res (Phila). 2016;9(5):339–348. doi: 10.1158/1940-6207.capr-15-0348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clifton KK, Ma CX, Fontana L, Peterson LL. Intermittent fasting in the prevention and treatment of cancer. CA Cancer J Clin. 2021;71(6):527–546. doi: 10.3322/caac.21694 [DOI] [PubMed] [Google Scholar]
- 50.Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–216. doi: 10.1056/nejmoa2206038 [DOI] [PubMed] [Google Scholar]
- 51.Devericks EN, Carson MS, McCullough EH, Coleman MF, Hursting SD. The obesity-breast cancer link: a multidisciplinary perspective. Cancer Metastasis Rev. 2022;41(3):607–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quail DF, Olson OC, Bhardwaj P, et al. Obesity alters the lung myeloid cell landscape to enhance breast cancer metastasis through IL5 and GM-CSF. Nat Cell Biol. 2017;19(8):974–987. doi: 10.1038/ncb3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yam P, Albright J, VerHague M, Gertz ER, Pardo-Manuel de Villena F, Bennett BJ. Genetic background shapes phenotypic response to diet for adiposity in the collaborative cross. Front Genet. 2021;11:615012. doi: 10.3389/fgene.2020.615012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gordon-Larsen P, French JE, Moustaid-Moussa N, et al. Synergizing mouse and human studies to understand the heterogeneity of obesity. Adv Nutr. 2021;12(5):2023–2034. doi: 10.1093/advances/nmab040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glenny EM, Coleman MF, Giles ED, Wellberg EA, Hursting SD. Designing relevant preclinical rodent models for studying links between nutrition, obesity, metabolism, and cancer. Annu Rev Nutr. 2021;41(1):253–282. doi: 10.1146/annurev-nutr-120420-032437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morris PG, Hudis CA, Giri D, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila). 2011;4(7):1021–1029. doi: 10.1158/1940-6207.capr-11-0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iyengar NM, Zhou XK, Gucalp A, et al. Systemic correlates of white adipose tissue inflammation in early-stage breast cancer. Clin Cancer Res. 2016;22(9):2283–2289. doi: 10.1158/1078-0432.ccr-15-2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iyengar NM, Morris PG, Zhou XK, et al. Menopause is a determinant of breast adipose inflammation. Cancer Prev Res (Phila). 2015;8(5):349–358. doi: 10.1158/1940-6207.capr-14-0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iyengar NM, Brown KA, Zhou XK, et al. Metabolic obesity, adipose inflammation and elevated breast aromatase in women with normal body mass index. Cancer Prev Res (Phila). 2017;10(4):235–243. doi: 10.1158/1940-6207.capr-16-0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carter JM, Hoskin TL, Pena MA, et al. Macrophagic “crown-like structures” are associated with an increased risk of breast cancer in benign breast disease. Cancer Prev Res (Phila). 2018;11(2):113–119. doi: 10.1158/1940-6207.capr-17-0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cho BA, Iyengar NM, Zhou XK, et al. Blood biomarkers reflect the effects of obesity and inflammation on the human breast transcriptome. Carcinogenesis. 2021;42(10):1281–1292. doi: 10.1093/carcin/bgab066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mullooly M, Yang HP, Falk RT, et al. Relationship between crown-like structures and sex-steroid hormones in breast adipose tissue and serum among postmenopausal breast cancer patients. Breast Cancer Res. 2017;19(1):8. doi: 10.1186/s13058-016-0791-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iyengar NM, Arthur R, Manson JE, et al. Association of body fat and risk of breast cancer in postmenopausal women with normal body mass index: a secondary analysis of a randomized clinical trial and observational study. JAMA Oncol. 2019;5(2):155–163. doi: 10.1001/jamaoncol.2018.5327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cho BA, Iyengar NM, Zhou XK, et al. Increased trunk fat is associated with altered gene expression in breast tissue of normal weight women. NPJ Breast Cancer. 2022;8(1):15. doi: 10.1038/s41523-021-00369-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62(10):921–925. doi: 10.1016/j.jacc.2013.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bi X, Seabolt L, Shibao C, et al. DXA-measured visceral adipose tissue predicts impaired glucose tolerance and metabolic syndrome in obese Caucasian and African-American women. Eur J Clin Nutr. 2015;69(3):329–336. doi: 10.1038/ejcn.2014.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ortega FB, Cadenas-Sanchez C, Sui X, Blair SN, Lavie CJ. Role of fitness in the metabolically healthy but obese phenotype: a review and update. Prog Cardiovasc Dis. 2015;58(1):76–86. doi: 10.1016/j.pcad.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 68.Teras LR, Patel AV, Wang M, et al. Sustained weight loss and risk of breast cancer in women 50 years and older: a pooled analysis of prospective data. J Natl Cancer Inst. 2020;112(9):929–937. doi: 10.1093/jnci/djz226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bustamante-Marin XM, Merlino JL, Devericks E, Carson MS, Hursting SD, Stewart DA. Mechanistic targets and nutritionally relevant intervention strategies to break obesity–breast cancer links. Front Endocrinol (Lausanne). 2021;12:632284. doi: 10.3389/fendo.2021.632284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Befort CA, Klemp JR, Austin HL, et al. Outcomes of a weight loss intervention among rural breast cancer survivors. Breast Cancer Res Treat. 2012;132(2):631–639. doi: 10.1007/s10549-011-1922-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fabian CJ, Klemp JR, Marchello NJ, et al. Rapid escalation of high-volume exercise during caloric restriction; change in visceral adipose tissue and adipocytokines in obese sedentary breast cancer survivors. Cancers (Basel). 2021;13(19):4871. doi: 10.3390/cancers13194871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Esper RM, Dame M, McClintock S, et al. Leptin and adiponectin modulate the self-renewal of normal human breast epithelial stem cells. Cancer Prev Res (Phila). 2015;8(12):1174–1183. doi: 10.1158/1940-6207.capr-14-0334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dong S, Wang Z, Shen K, Chen X. Metabolic syndrome and breast cancer: prevalence, treatment response, and prognosis. Front Oncol. 2021;11:629666. doi: 10.3389/fonc.2021.629666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oh SW, Park CY, Lee ES, et al. Adipokines, insulin resistance, metabolic syndrome, and breast cancer recurrence: a cohort study. Breast Cancer Res. 2011;13(2):R34. doi: 10.1186/bcr2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Verheggen RJ, Maessen MFH, Green DJ, Hermus ARMM, Hopman MTE, Thijssen DHT. A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: distinct effects on body weight and visceral adipose tissue. Obes Rev. 2016;17(8):664–690. doi: 10.1111/obr.12406 [DOI] [PubMed] [Google Scholar]
- 76.Grossmann ME, Ray A, Nkhata KJ, et al. Obesity and breast cancer: status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev. 2010;29(4):641–653. doi: 10.1007/s10555-010-9252-1 [DOI] [PubMed] [Google Scholar]
- 77.Rogozina OP, Bonorden MJ, Seppanen CN, Grande JP, Cleary MP. Effect of chronic and intermittent calorie restriction on serum adiponectin and leptin and mammary tumorigenesis. Cancer Prev Res (Phila). 2011;4(4):568–581. doi: 10.1158/1940-6207.capr-10-0140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ollberding NJ, Kim Y, Shvetsov YB, et al. Prediagnostic leptin, adiponectin, C-reactive protein, and the risk of postmenopausal breast cancer. Cancer Prev Res (Phila). 2013;6(3):188–195. doi: 10.1158/1940-6207.capr-12-0374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi: 10.1158/0008-5472.can-14-0155 [DOI] [PubMed] [Google Scholar]
- 80.Philip B, Roland CL, Daniluk J, et al. A high-fat diet activates oncogenic Kras and COX2 to induce development of pancreatic ductal adenocarcinoma in mice. Gastroenterology. 2013;145(6):1449–1458. doi: 10.1053/j.gastro.2013.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gomez-Chou SB, Swidnicka-Siergiejko AK, Badi N, et al. Lipocalin-2 promotes pancreatic ductal adenocarcinoma by regulating inflammation in the tumor microenvironment. Cancer Res. 2017;77(10):647–2660. doi: 10.1158/0008-5472.can-16-1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hsueh HY, Pita-Grisanti V, Gumpper-Fedus K, et al. A review of physical activity in pancreatic ductal adenocarcinoma: epidemiology, intervention, animal models, and clinical trials. Pancreatology. 2022;22(1):98–111. doi: 10.1016/j.pan.2021.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pita-Grisanti V, Dubay K, Lahooti A, et al. Abstract PO-034: Increased physical activity delays development of obesity-induced pancreatic ductal adenocarcinoma in mice and modulates inflammation. Cancer Res. 2020;80(22 suppl):PO–034. doi: 10.1158/1538-7445.panca20-po-034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peto R, Doll R, Buckley JD, Sporn MB. Can dietary beta-carotene materially reduce human cancer rates? Nature. 1981;290(5803):201–208. doi: 10.1038/290201a0 [DOI] [PubMed] [Google Scholar]
- 85.Mayne ST, Ferrucci LM, Cartmel B. Lessons learned from randomized clinical trials of micronutrient supplementation for cancer prevention. Annu Rev Nutr. 2012;32(1):369–390. doi: 10.1146/annurev-nutr-071811-150659 [DOI] [PubMed] [Google Scholar]
- 86.Shams-White MM, Brockton NT, Mitrou P, et al. Operationalizing the 2018 World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) cancer prevention recommendations: a standardized scoring system. Nutrients. 2019;11(7):1572. doi: 10.3390/nu11071572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Focht BC, Lucas AR, Grainger E, et al. Effects of a group-mediated exercise and dietary intervention in the treatment of prostate cancer patients undergoing androgen deprivation therapy: results from the IDEA-P trial. Ann Behav Med. 2018;52(5):412–428. doi: 10.1093/abm/kax002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Spees CK, Braun AC, Hill EB, et al. Impact of a tailored nutrition and lifestyle intervention for overweight cancer survivors on dietary patterns, physical activity, quality of life, and cardiometabolic profiles. J Oncol. 2019;2019:1503195. doi: 10.1155/2019/1503195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chaplow ZL, Focht BC, Lucas AR, et al. Effects of a lifestyle intervention on body composition in prostate cancer patients on androgen deprivation therapy. JCSM Clin Rep. 2020;5(2):52–60. doi: 10.1002/crt2.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hermetet F, Mshaik R, Simonet J, Callier P, Delva L, Quere R. High-fat diet intensifies MLL-AF9-induced acute myeloid leukemia through activation of the FLT3 signaling in mouse primitive hematopoietic cells. Sci Rep. 2020;10(1):16187. doi: 10.1038/s41598-020-73020-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Puram RV, Kowalczyk M, de Boer C, et al. Core circadian clock genes regulate leukemia stem cells in AML. Cell. 2016;165(2):303–316. doi: 10.1016/j.cell.2016.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26(4):527–534. doi: 10.1200/jco.2007.12.7837 [DOI] [PubMed] [Google Scholar]
- 93.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–1546. doi: 10.1056/nejmoa1910836 [DOI] [PubMed] [Google Scholar]
- 94.McQuade JL, Daniel CR, Hess KR, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19(3):310–322. doi: 10.1016/s1470-2045(18)30078-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vétizou M, Pitt JM, Daillere R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–1089. doi: 10.1126/science.aac4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Frankel AE, Coughlin LA, Kim J, et al. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia. 2017;19(10):848–855. doi: 10.1016/j.neo.2017.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104–108. doi: 10.1126/science.aao3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. [DOI] [PubMed] [Google Scholar]
- 101.Spencer CN, McQuade JL, Gopalakrishnan V, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374(6575):1632–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ligibel JA, Bohlke K, May AM, et al. Exercise, diet, and weight management during cancer treatment: ASCO guideline. J Clin Oncol. 2022;40(22):2491–2507. doi: 10.1200/jco.22.00687 [DOI] [PubMed] [Google Scholar]
- 103.Parra-Soto S, Ahumada D, Petermann-Rocha F, et al. Association of meat, vegetarian, pescatarian and fish-poultry diets with risk of 19 cancer sites and all cancer: findings from the UK Biobank prospective cohort study and meta-analysis. BMC Med. 2022;20(1):79. doi: 10.1186/s12916-022-02257-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shams-White MM, Brockton NT, Mitrou P, Kahle LL, Reedy J. The 2018 World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) score and all-cause, cancer, and cardiovascular disease mortality risk: a longitudinal analysis in the NIH-AARP Diet and Health Study. Curr Dev Nutr. 2022;6(6):nzac096. doi: 10.1093/cdn/nzac096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. 2016;34(35):4270–4276. doi: 10.1200/jco.2016.67.4283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iyengar NM, Jones LW. Development of exercise as interception therapy for cancer: a review. JAMA Oncol. 2019;5(11):1620–1627. doi: 10.1001/jamaoncol.2019.2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ligibel JA, Jones LW, Brewster AM, et al. Oncologists’ attitudes and practice of addressing diet, physical activity, and weight management with patients with cancer: findings of an ASCO survey of the oncology workforce. J Oncol Pract. 2019;15(6):e520–e528. doi: 10.1200/jop.19.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Friedenreich CM, Neilson HK, Farris MS, Courneya KS. Physical activity and cancer outcomes: a precision medicine approach. Clin Cancer Res. 2016;22(19):4766–4775. doi: 10.1158/1078-0432.ccr-16-0067 [DOI] [PubMed] [Google Scholar]
- 109.Baden MY, Satija A, Hu FB, Huang T. Change in plant-based diet quality is associated with changes in plasma adiposity-associated biomarker concentrations in women. J Nutr. 2019;149(4):676–686. doi: 10.1093/jn/nxy301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shah UA, Iyengar NM. Plant-based and ketogenic diets as diverging paths to address cancer: a review. JAMA Oncol. 2022;8(8):1201–1208. doi: 10.1001/jamaoncol.2022.1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ma C, Avenell A, Bolland M, et al. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ. 2017;359:j4849. doi: 10.1136/bmj.j4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Parker ED, Folsom AR. Intentional weight loss and incidence of obesity-related cancers: the Iowa Women’s Health Study. Int J Obes Relat Metab Disord. 2003;27(12):1447–1452. doi: 10.1038/sj.ijo.0802437 [DOI] [PubMed] [Google Scholar]
- 113.Kawai M, Minami Y, Kuriyama S, et al. Adiposity, adult weight change and breast cancer risk in postmenopausal Japanese women: the Miyagi Cohort Study. Br J Cancer. 2010;103(9):1443–1447. doi: 10.1038/sj.bjc.6605885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Elands RJ, Simons CCJM, Dongen M, et al. A systematic literature review and meta-regression analysis on early-life energy restriction and cancer risk in humans. PLoS One. 2016;11(9):e0158003. doi: 10.1371/journal.pone.0158003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/nejmoa021423 [DOI] [PubMed] [Google Scholar]
- 116.Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila). 2011;4(4):486–501. doi: 10.1158/1940-6207.capr-10-0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shaikh H, Bradhurst P, Ma LX, Tan SYC, Egger SJ, Vardy JL. Body weight management in overweight and obese breast cancer survivors. Cochrane Database Syst Rev. 2020;12(12):CD012110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kitson S, Ryan N, MacKintosh ML, Edmondson R, Duffy JM, Crosbie EJ. Interventions for weight reduction in obesity to improve survival in women with endometrial cancer. Cochrane Database Syst Rev. 2018;2(2):CD012513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thomson CA, Crane TE, Miller A, Garcia DO, Basen-Engquist K, Alberts DS. A randomized trial of diet and physical activity in women treated for stage II-IV ovarian cancer: rationale and design of the Lifestyle Intervention for Ovarian Cancer Enhanced Survival (LIVES): an NRG Oncology/Gynecologic Oncology Group (GOG-225) study. Contemp Clin Trials. 2016;49:181–189. doi: 10.1016/j.cct.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ligibel JA, Barry WT, Alfano C, et al. Randomized phase III trial evaluating the role of weight loss in adjuvant treatment of overweight and obese women with early breast cancer (Alliance A011401): study design. NPJ Breast Cancer. 2017;3(1):37. doi: 10.1038/s41523-017-0040-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Demark-Wahnefried W, Nix JW, Hunter GR, et al. Feasibility outcomes of a presurgical randomized controlled trial exploring the impact of caloric restriction and increased physical activity versus a wait-list control on tumor characteristics and circulating biomarkers in men electing prostatectomy for prostate cancer. BMC Cancer. 2016;16(1):61. doi: 10.1186/s12885-016-2075-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Henning SM, Galet C, Gollapudi K, et al. Phase II prospective randomized trial of weight loss prior to radical prostatectomy. Prostate Cancer Prostatic Dis. 2018;21(2):212–220. doi: 10.1038/s41391-017-0001-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 suppl 2):S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hoedjes M, van Stralen MM, Joe STA, et al. Toward the optimal strategy for sustained weight loss in overweight cancer survivors: a systematic review of the literature. J Cancer Surviv. 2017;11(3):360–385. doi: 10.1007/s11764-016-0594-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.van den Berg MM, Winkels R, de Kruif J, et al. Weight change during chemotherapy in breast cancer patients: a meta-analysis. BMC Cancer. 2017;17(1):259. doi: 10.1186/s12885-017-3242-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Egnell C, Ranta S, Banerjee J, et al. Changes in body mass index during treatment of childhood acute lymphoblastic leukemia with the Nordic ALL2008 protocol. Eur J Haematol. 2022;105(6):797–807. doi: 10.1111/ejh.13517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schumacher O, Galvao DA, Taaffe DR, et al. Nationwide industry-led community exercise program for men with locally advanced, relapsed, or metastatic prostate cancer on androgen-deprivation therapy. JCO Oncol Pract. 2022;18(8):e1334–e1341. doi: 10.1200/op.21.00745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.National Cancer Institute (NCI). Annual Plan & Budget Proposal for Fiscal Year 2022. NCI; 2022. Accessed January 11, 2023. https://www.cancer.gov/research/annual-plan/2022-annual-plan-budget-proposal-aag.pdf [Google Scholar]
- 129.Zhang FF, Liu S, John EM, Must A, Demark-Wahnefried W. Diet quality of cancer survivors and noncancer individuals: results from a national survey. Cancer. 2015;121(23):4212–4221. doi: 10.1002/cncr.29488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Reeves MM, Terranova CO, Eakin EG, Demark-Wahnefried W. Weight loss intervention trials in women with breast cancer: a systematic review. Obes Rev. 2014;15(9):749–768. doi: 10.1111/obr.12190 [DOI] [PubMed] [Google Scholar]


