Abstract
Diabetic neuropathy (DN) represents a common and debilitating complication of diabetes, affecting a significant proportion of patients. Despite available treatments focusing on symptom management, there remains an unmet need for therapies that address the underlying pathophysiology. In pursuit of novel interventions, this study evaluated the therapeutic effects of caffeic acid—a natural phenolic compound prevalent in various foods—on diabetic neuropathy using a mouse model, particularly examining its interaction with the Insulin-like Growth Factor 1 (IGF-1) signaling pathway. Caffeic acid was administered orally at two dosages (5 mg/kg and 10 mg/kg), and a comprehensive set of outcomes including fasting blood glucose levels, body weight, sensory behavior, spinal cord oxidative stress markers, inflammatory cytokines, and components of the IGF-1 signaling cascade were assessed. Additionally, to determine the specific contribution of IGF-1 signaling to the observed benefits, IGF1R inhibitor Picropodophyllin (PPP) was co-administered with caffeic acid. Our results demonstrated that caffeic acid, at both dosages, effectively reduced hyperglycemia and alleviated sensory behavioral deficits in diabetic mice. This was accompanied by a marked decrease in oxidative stress markers and an increase in antioxidant enzyme activities within the spinal cord. Significantly lowered microglial activation and inflammatory cytokine expression highlighted the potent antioxidative and anti-inflammatory effects of caffeic acid. Moreover, increases in both serum and spinal levels of IGF-1, along with elevated phosphorylated IGF1R, implicated the IGF-1 signaling pathway as a mediator of caffeic acid's neuroprotective actions. The partial reversal of caffeic acid's benefits by PPP substantiated the pivotal engagement of IGF-1 signaling in mediating its effects. Our findings delineate the capability of caffeic acid to mitigate DN symptoms, particularly through reducing spinal oxidative stress and inflammation, and pinpoint the integral role of IGF-1 signaling in these protective mechanisms. The insights gleaned from this study not only position caffeic acid as a promising dietary adjunct for managing diabetic neuropathy but also highlight the therapeutic potential of targeting spinal IGF-1 signaling as part of a strategic treatment approach.
Keywords: Diabetic neuropathy, Caffeic acid, Insulin-like growth factor 1, Oxidative stress, Inflammation
1. Introduction
Diabetic neuropathy (DN) is a widespread and chronic complication that affects more than half of individuals with diabetes, manifesting through a spectrum of symptoms such as sensory disturbances, increased pain sensitivity, spontaneous discomfort, and numbness [1]. This condition not only deteriorates the quality of life but also predisposes patients to further morbidities, including skin ulcers and fractures [1]. Current analgesic treatments offer only temporary symptomatic relief and do not alter the underlying pathophysiology or the chronic nature of DN [2], highlighting an urgent need for more effective therapeutic strategies.
The relationship between DN and pathologies or functional anomalies in the peripheral [3,4] and central nervous systems (CNS) [5,6], notably the spinal cord, is well-established. Although initial damage frequently occurs in the PNS, dysfunction and sensitization within the CNS, especially among spinal neurons, are integral to the relentless progression and treatment resistance of DN [7,8]. Substantial evidence shows significant oxidative injuries to spinal sensory neurons in diabetic models, leading to mitochondrial dysfunctions and irreversible cellular disorders [9,10]. Additionally, diabetes stimulates the proliferation of inflammatory microglia within the spinal cord, exacerbating oxidative stress and neuronal damage [9]. Consequently, strategies to alleviate diabetes-induced spinal oxidative stress and inflammatory responses emerge as potentially effective interventions.
In this context, Insulin-like growth factor 1 (IGF-1), a hormone structurally akin to insulin and closely associated with diabetes [11,12], becomes particularly relevant. IGF-1 is instrumental in brain development, synaptic functions, and neuronal survival, mainly via activating the IGF1R receptor [13,14]. Brain IGF-1 deficits can precipitate oxidative injury and edema [15], while bolstering the IGF-1 signaling pathway may alleviate oxidative damage seen in traumatic brain injuries [16]. IGF-1 also influences inflammation related to microglial activation, with overexpression in aged rat brains leading to a shift toward an anti-inflammatory phenotype in microglia [17,18]. These discoveries point to the IGF-1 signaling pathway as a potential target for addressing the oxidative stress and inflammation characteristic of diabetes.
Given this scenario, dietary intervention plays a vital role in managing diabetes and its complications [19]. Caffeic acid, a natural phenolic compound abundant in common foods like vegetables, fruits, coffee, and tea, emerges as a compound of interest due to its antioxidant [[20], [21], [22]], anti-inflammatory [11,23], and neuroprotective properties [24]. It has been demonstrated to ameliorate oxidative stress and neural damage in animal models of various neurological conditions, such as stroke [25], Parkinson's disease [26], Alzheimer's disease [27] and traumatic brain injury [28]. With a high bioavailability, caffeic acid notably crosses the blood-brain barrier, allowing it to exert significant effects on the CNS when taken orally [29].
Despite these promising attributes, the specific impact of caffeic acid on DN, particularly its ability to modulate oxidative stress and inflammation by safeguarding the spinal IGF-1 signaling pathway, remains unexplored. Therefore, our study endeavors to investigate the effects of caffeic acid on DN and its underlying mechanisms, with an emphasis on how this compound influences spinal IGF-1 signaling, to uncover its potential neuroprotective benefits against diabetes-induced complications.
2. Materials and methods
2.1. Animals
Male C57BL/6J mice, within the age range of 8–12 weeks and exhibiting an average weight between 22 and 25 g, were procured from Xi'an Jiaotong University. These mice were housed under ethically sound laboratory conditions, with stringent control over temperature and humidity. The circadian rhythms of the animals were maintained with a fixed light/dark cycle of 12 h, initiating the light phase at 7:00. They were provided unrestricted access to both water and a standard diet. This study, including all animal-based experiments, was conducted in strict adherence to the ARRIVE guidelines and received due approval from the Ethics Committee of Shaanxi Provincial cancer Hospital (No. 2023016).
2.2. Establishment of diabetic neuropathy (DN) model
In order to develop a model of diabetic neuropathy (DN) within type 1 diabetes in mice, we embarked upon a regimen of daily intraperitoneal administration of streptozotocin (STZ; Sigma-Aldrich, St. Louis, MO, USA, #572201) at a dosage of 50 mg/kg over a period of five consecutive days. Each STZ injection was preceded by a phase of overnight fasting to ensure optimal conditions for the development of the disease model. The STZ solution was freshly prepared in a citrate buffer (pH 4.5) prior to each injection. A week following the last STZ injection, we proceeded with the measurement of fasting blood glucose levels. This was accomplished by extracting blood from the tail veins of the mice and conducting an analysis with a blood glucose meter (Roche). Mice that exhibited fasting blood glucose levels exceeding the threshold of 11.1 mmol/L were identified as diabetic and selected for further investigations. Apart from the periods of fasting required for the STZ injections, the mice were allowed unrestricted access to both water and a standard diet throughout the duration of the experiment.
2.3. Experimental design and drug administration
This study is bifurcated into two distinct segments as shown in Fig. 1. The primary objective of the initial segment is to scrutinize the impact of varied caffeic acid dosages on diabetic neuropathy, along with oxidative stress and inflammation in the spinal cord of murine subjects. Proceeding from these findings, the second segment aspires to validate the role of the IGF-1 signaling pathway in the protective attributes of caffeic acid against the aforementioned conditions.
Fig. 1.
Overview of experimental design. (A) Experiment 1 examines the effects of different caffeic acid dosages on diabetic neuropathy, oxidative stress, and inflammation in mouse models. The Control group was given vehicle (citrate buffer, 50 μL) via intraperitoneal injections for five days. The Diabetic Neuropathy (DN) group received STZ solution (50 mg/kg) intraperitoneally for five days. The CA1 group mirrored the DN protocol but also received oral caffeic acid (5 mg/kg) every other day for four weeks post-final STZ injection. The CA2 group followed the CA1 regime but with caffeic acid dosed at 10 mg/kg. (B) Experiment 2 seeks to establish the IGF-1 signaling pathway's role in caffeic acid's protective effects. The Control and DN groups replicated the Experiment 1 protocol. The CA group was given STZ solution (50 mg/kg) for five days and then caffeic acid (10 mg/kg) orally every other day for four weeks. The CA + PPP group mirrored the CA group, but with an added intraperitoneal dose of IGF-1R inhibitor, Picropodophyllin (PPP) (1 mg/kg), given 20 min before each caffeic acid dosage.
In the first segment of this investigation, mice were randomly allocated into four distinct cohorts (Fig. 1A). The first cohort, or the Control group, received solely the vehicle (citrate buffer, 50 μL) via intraperitoneal injections over a duration of five days. The second cohort, referred to as the Diabetic Neuropathy (DN) group, was administered continuous intraperitoneal injections of STZ solution at a dosage of 50 mg/kg for five consecutive days. The third cohort, termed the CA1 group, followed an identical protocol as the DN group for five days, after which they underwent an administration regime of orally intaking caffeic acid (Sigma-Aldrich, St. Louis, MO, #C0625) at a dosage of 5 mg/kg every other day for a period of four weeks, commencing after the final STZ injection. The fourth cohort, named the CA2 group, mirrored the CA1 protocol, differing only in the caffeic acid dosage which was escalated to 10 mg/kg.
The second segment of the experiment replicated the group division schema from the first part, albeit with modifications to the caffeic acid dosage and introduction of an IGF-1R inhibitor (Fig. 1B). The Control and DN groups followed the same protocol as in the first segment. The third group, designated as the CA group, received continuous intraperitoneal injections of the STZ solution at a dosage of 50 mg/kg for five days, followed by an administration regime of orally administrating caffeic acid at a dosage of 10 mg/kg every other day for four weeks, post the last STZ injection. The fourth cohort, referred to as the CA + PPP group, followed the same protocol as the CA group, but also received an additional intraperitoneal injection of the IGF-1R inhibitor, Picropodophyllin (PPP, Macklin, Shanghai, China, #P816528), at a dosage of 1 mg/kg, administered 20 min before each oral dose of caffeic acid.
2.4. Behavioral tests
The assessment of mechanical allodynia was executed utilizing the electronic von Frey device (Bioseb, France), adhering to a previously validated methodology [30,31]. The device is fitted with a flexible filament that delivers progressive force, ranging from 0 to 10 g, to the central region of the plantar surface of the mouse's hind paw. The protocol involved the monitoring of a nociceptive withdrawal reflex in response to the exerted mechanical stimulus. The stimulus was instantaneously discontinued upon the manifestation of the withdrawal response, and the corresponding level of mechanical pressure was noted. For the execution of the experiment, each mouse was situated in a specific testing chamber equipped with a wire mesh floor. The mice were granted an acclimation period of 1 h prior to the commencement of the testing process. To evaluate the mechanical withdrawal threshold of the paw at distinct time intervals, each mouse was subjected to a series of three tests, alternately conducted on each hind paw. In order to mitigate any potential bias introduced by prior stimuli, a mandatory interim period of 30 s was enforced between successive measurements. The mechanical withdrawal threshold for each mouse was then calculated by averaging the data gathered from these tests.
In accordance with the method proposed by Hervera et al. [32], we employed thermal radiation heat to evaluate the paw withdrawal latency (PWL) in mice [33,34]. This technique allows us to assess the sensitivity to temperature-related pain perception in the context of diabetic neuropathy. The experimental process involved positioning the mice on a glass platform integrated into the PL-200 Plantar Analgesia device (Chengdu Technology & Market CO., Ltd., Sichuan, China). Prior to data collection, the mice were given a minimum of 15 min to acclimate to the equipment. A radiant heat lamp, situated vertically beneath the right hind paw, was utilized to generate a focused light spot with a diameter of 5 mm. The PWL was determined through three independent trials, with the aim of calculating the average withdrawal latency for the hind paw subjected to the heat stimulus. To preclude thermal sensitization and behavioral interference, a recovery interval of 5 min was established between each trial. To ensure the welfare of the animal subjects and avoid potential tissue damage, the maximum duration of heat exposure was capped at 12 s.
2.5. Western blot
The mice (n = 6) were euthanized via an overdose of phenobarbital anesthetic (200 mg/kg, intraperitoneal). Following this, we extracted the lumbar enlargement (L4-L5) of the spinal cord. Upon extraction, the spinal cord tissue was promptly weighed and homogenized in a RIPA lysis buffer fortified with the proteinase inhibitor PMSF on ice. The homogenized mixture was subsequently centrifuged at 4 °C, with the supernatant collected for protein quantification via the BCA method. The resultant protein samples were partitioned into two equal segments. One segment was employed for Western blotting to determine specific protein concentrations in the spinal cord, while the other was analyzed for indicators related to oxidative stress.
In order to investigate the protein level of Nrf2 within the nuclear fraction of the spinal cord, we extracted nuclear proteins using the Nuclear Protein Extraction Kit (Solarbio Life Sciences, #R0050). The extraction process involved adding the kit's cytoplasmic and nuclear protein extraction reagents to the spinal cord tissue, mixing thoroughly for 15 min, and subsequent centrifugation at 4 °C at 12,000 to 16,000 g for 10 min. The resultant pellet signified the nuclear fraction. After removal of the remaining supernatant, 100 μl of nuclear protein extraction reagent was introduced, followed by a 10-min incubation on ice and a secondary centrifugation step. The supernatant was collected and represented the extracted nuclear protein.
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with equal quantities of protein (30 μg), followed by the transfer to a PVDF membrane. This membrane was subjected to a rinse with TBST, blocked with 5 % skim milk or 3 % BSA, and incubated overnight at 4 °C with Histone H3 (1:1000; Proteintech, Wuhan, China, #68345), IGF-1 (1:1000; Abcam, Cambridge, MA, USA, #ab9572), IGF1R (1:1000; Abcam, #ab182408), IL-1β (1:1000; Abclonal, Wuhan, China, #A16288), IL-6 (1:1000; Abclonal, #A0286), Nrf2 (1:1000; Cell Signaling Technology, Danvers, MA, USA, #12721), p-IGF1R (1:1000; Abclonal, #AP0367), β-actin (1:1000; Proteintech, #81115) primary antibodies. Subsequent to the primary antibody incubation, the membrane was cleansed with TBST, thrice for 10 min each. HRP-conjugated goat anti-rabbit IgG (diluted at 1:2000) was applied and the membrane incubated at room temperature for 2 h. The membranes were then incubated with chemifluorescent reagent ECL (Beyotime, Shanghai, China, #P0018FM) and then exposed to X-ray film in the dark room. The protein bands were quantitatively analyzed with ImageJ software.
2.6. Assessment of oxidative stress markers
To discern the extent of oxidative damage in the spinal cord, we quantified the levels of several oxidative stress markers in the spinal cord, including malondialdehyde (MDA; Yuanye Bio-Technology, Shanghai, China, #R21869), carbonyl protein content (Macklin, #P930080), and the enzymatic activity of superoxide dismutase (SOD; Macklin, #S926321), using commercially available assay kits.
In essence, the quantification of MDA, an indicator of lipid oxidation, involves its interaction with thiobarbituric acid, culminating in the formation of a red complex. This complex's absorbance is subsequently read at 532 nm using a spectrophotometer, which allows for the determination of MDA content. On the other hand, the evaluation of carbonyl protein content, a marker of protein oxidation, hinges on the interaction between protein carbonyls and 2,4-dinitrophenylhydrazine, forming a red 2,4-dinitrophenylhydrazone. Measurement of the absorbance of this complex at 370 nm with a spectrophotometer provides insights into the protein oxidation status within the spinal cord. Lastly, the detection of SOD activity, a critical component of the biological antioxidant system, involves tracking the transformation of superoxide anions, generated from the reaction between xanthine and xanthine oxidase, into a blue formazan via the reduction of nitroblue tetrazolium. As SOD counteracts superoxide anions, thereby inhibiting formazan formation, an inverse relationship is observed between the absorbance at 560 nm (which quantifies formazan levels) and SOD activity. Therefore, measuring this absorbance allows for the determination of SOD activity, yielding an overall picture of the spinal cord's oxidative stress status.
2.7. Immunofluorescence analysis
To execute an immunofluorescence analysis, the process commenced with a transcardial perfusion utilizing 50 mL of 4 % paraformaldehyde. Following this perfusion, key anatomical structures were meticulously harvested: the lumbar enlargement portion of the spinal cord, the lumbar dorsal root ganglia (DRG) spanning from L1 to L5, the sciatic nerves, and the plantar dermis. These tissues were subsequently embedded in paraffin for preservation. In preparation for examination, the paraffin-embedded tissues were sectioned into slices. The majority of the tissues were sectioned into coronal slices of 4 μm in thickness. However, to enhance the visualization of nerve fibers, the plantar dermis was sectioned into slices of 10 μm. These sections were subsequently deparaffinized and rehydrated. To expose the antigens for antibody binding, the spinal cord sections were subjected to a heat-induced antigen retrieval process using a citrate buffer (pH 6.0). Following this procedure, the sections were cooled down to room temperature and blocked with goat serum to prevent non-specific antibody binding.
Sections were incubated overnight at 4 °C with primary antibodies targeting NeuN (1:400; Abcam, #ab104224), PGP9.5 (1:200; Abcam, #ab8189), Iba-1 (1:400; Cell Signaling Technology, #17198), and GFAP (1:400; Cell Signaling Technology, #45946), aiming for specific antigen detection. Subsequently, these sections were washed with PBS and further incubated with Cy3-labeled goat anti-rabbit (1:400; Abcam, #ab6939), DyLight® 488-labeled goat anti-rabbit (1:400; Abcam, #ab96899), or DyLight® 488-labeled goat anti-mouse secondary antibodies (1:400; Abcam, #ab98794) for 2 h at ambient temperature, with the choice of secondary antibody being contingent upon the species origin of the primary antibody. Image capturing was conducted using immunofluorescence microscopy (Olympus BX53; Olympus, Tokyo, Japan) at magnifications of 200 × or 400 × . The resulting images were subjected to analysis using Image Pro™ Plus software.
2.8. Detection of hemoglobin A1c (HbA1c) levels
The measurement of HbA1c levels was performed utilizing the glycated hemoglobin assay kit supplied by the Nanjing Institute of Biotechnology (Nanjing, China, #A056-1-1). We initiated the procedure by extracting approximately 1 mL of blood from the retro-orbital plexus of the mice, following which the blood was deposited into an anticoagulant tube. After centrifuging the sample to separate the supernatant, we proceeded to wash the residual precipitate with physiological saline, thereby obtaining a solution of red blood cells. Subsequently, the absorbance of the resultant solution was determined at a wavelength of 540 nm using a spectrophotometer. By multiplying the absorbance value by 0.3677, we derived the total hemoglobin concentration (g/mL). Then, we combined the red blood cell solution with double distilled water, subsequently subjecting the mixture to stirring in a vortex mixer for a minute to ensure thorough dissolution of the blood. The blood solution was then subjected to acidification and hydrolysis. The absorbance was measured at 443 nm, allowing us to ascertain the concentration of HbA1c, which was denoted in terms of absorbance for every 10 g of Hemoglobin (OD/10gHb). Finally, we computed the percentage of HbA1c using the given formula: HbA1c (%) = (OD/10gHb) × 0.001 + 0.0154.
2.9. Serum IGF-1 and insulin detection by ELISA
We procured roughly 1 mL of blood from each mouse via the retro-orbital plexus. The collected samples were then left to stand at room temperature for 2 h, after which they were subjected to centrifugation at a speed of 1000 g/min for a duration of 15 min. This process allowed for the separation and collection of serum, which was subsequently stored at a temperature of −70 °C. The concentration of IGF-1 in the serum was measured using an ELISA kit from Solarbio Life Sciences (# SEKM-0096), and the insulin levels were assessed with an ELISA kit from Beyotime (# PI602). All the procedures were meticulously executed in line with the guidelines outlined in the documentation accompanying the ELISA kits.
2.10. Measurement of motor nerve conduction velocity (MCV)
To evaluate the function of sciatic nerve conduction in diabetic mice, motor conduction velocities (MCV) were quantified using an electromyograph evoked potential meter (NDI-097; Shanghai Haishen Medical Electronic Instrument Co., Ltd., Shanghai, China), eight weeks subsequent to STZ administration. This methodology was adapted from a previous study [35] with minor modifications. Initially, mice were sedated using a phenobarbital dose of 50 mg/kg and positioned prone. Subsequent to the depilation of hind limb skin, we ensured the maintenance of the animal's body temperature at 37 °C in a controlled environment with a room temperature of 22.0 ± 0.5 °C.
Specifically, the stimulating needle electrode was positioned between the femur and calcaneal tuberosity, while the recording needle electrode was located at the ankle adjacent to the sciatic nerve. The reference electrode was placed midway between the stimulating and recording electrodes, maintaining a distance of 1 cm from the latter. Stimulation parameters included a single pulse square wave with a pulse width of 0.1 ms and intensity set to 1.5 times the threshold required for stimulation, ensuring a minimum inter-stimulus interval of 6 s. To calculate MCV, we measured the distance between the stimulating and recording electrodes. The MCV (m/s) was then determined by dividing this distance by the latency period, defined as the interval from the onset of stimulation to the appearance of muscle evoked potentials.
2.11. Nissl staining
To determine if diabetic mice demonstrate neuronal damage within the spinal cord, we utilized Nissl staining to examine the spinal cord neurons. Following standard deparaffinization and rehydration procedures, spinal cord tissue sections were incubated with 0.1 % cresyl violet at 56 °C for a duration of 60 min. Subsequently, the sections underwent differentiation in 95 % ethanol for a brief period of 30 s, followed by a rapid dehydration process using 100 % ethanol for 2 min. The sections were then immersed in xylene for two 3-min periods. To finalize the preparation, neutral resin was applied to the tissue before the slides were coverslipped, facilitating microscopic analysis.
2.12. Primary spinal cord culture and in vitro experimental design
To elucidate the potential interaction between the glucose-lowering effect of caffeic acid and its impact on the spinal IGF-1 signaling pathway, primary spinal cord neuron cultures were established from mouse embryos on the 14th day of embryonic development (E14), adhering to the methodologies outlined in a previous study [36]. These neurons were cultured in NeuroBasal medium supplemented with 5 % fetal bovine serum and 2 % B-27 supplement (Gibco, Shanghai, China) until day 5. Neuronal purity was confirmed through immunocytochemistry for the neuronal marker microtubule-associated protein 2 (MAP2; 1:200, Cell Signaling Technology, #8707), visualized with fluorescence (Supplementary Fig. 2A).
To assess the effects under varying glucose conditions, the cultured neurons were randomly allocated into four experimental groups (Supplementary Fig. 2B): (1) the NGM-4d group, where neurons were maintained in a normal glucose medium (NGM, 5 mM glucose) for 96 h; (2) the HGM-4d group, with neurons cultured in a high glucose medium (HGM, 25 mM glucose) for 96 h; (3) the HGM-2d + NGM-2d group, where neurons experienced an initial 48 h in HGM followed by a switch to NGM for the remaining 48 h; (4) the HGM-4d + CA group, involving neurons cultured in HGM supplemented with 50 μM caffeic acid for 96 h.
Following these treatments, the study proceeded to quantify the proportion of IGF-1 positive neurons via immunofluorescence (1:100, Abcam, #ab9572). Additionally, the concentration of IGF-1 in the culture medium was measured using ELISA (Solarbio Life Sciences, # SEKM-0096), and cell viability was assessed through the CCK-8 assay (Beyotime, #C0038).
2.13. Statistical analysis
The collected data are articulated as mean ± standard deviation (SD). Parameters such as fasting blood glucose concentration, body weight, mechanical withdrawal threshold, and thermal withdrawal latency were subjected to statistical analysis utilizing a two-way ANOVA, succeeded by Šídák's multiple comparisons test. These analyses were executed with the assistance of GraphPad Prism 5 software (GraphPad Software Inc., USA). Contrarily, for other group comparisons, the analytical approach involved a one-way ANOVA followed by Dunnett's multiple comparisons test. A p-value of less than 0.05 was set as the criterion to ascertain statistical significance.
3. Results
3.1. Caffeic acid counteracts diabetic neuropathy and rejuvenates spinal IGF-1 signaling
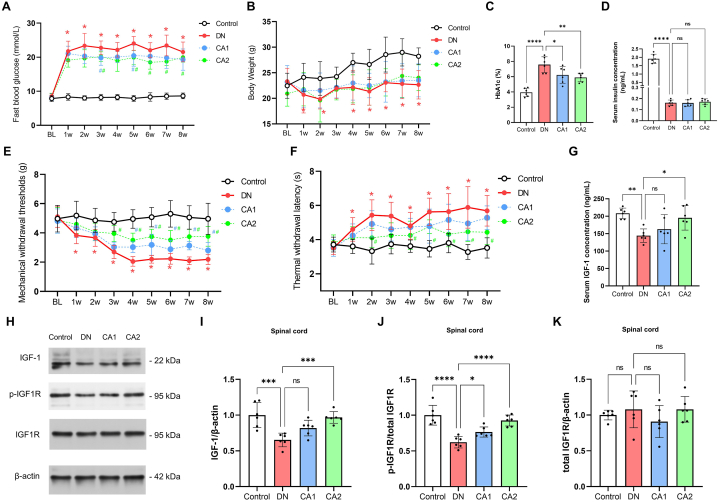
In an effort to develop a mouse model of diabetic neuropathy (DN), we administered streptozotocin (STZ) (50 mg/kg) intraperitoneally for five consecutive days. We first entailed measuring indicators such as fasting blood glucose, body weight, mechanical withdrawal thresholds, and thermal withdrawal latency, every week from the baseline (prior to STZ injection) until the eighth week post injection. By the first week following the STZ injection, a significant elevation in the DN group mice's fasting blood glucose levels was noticeable (Fig. 2A, n = 12, F (1, 22) = 2647, P < 0.0001, two-way ANOVA), in comparison to the control group. This trend continued throughout the observational period. Concurrently, the DN group mice experienced a marked decrease in body weight post STZ injection, with a modest rebound observable from the third week onwards. However, the body weight of the DN group remained discernibly lower than the control group (Fig. 2B, n = 12, F (1, 22) = 114.5, P < 0.0001, two-way ANOVA). In the eighth week following STZ injection, a significant elevation in the blood HbA1c ratio was observed in the DN group mice (Fig. 2C, n = 6, P < 0.0001), along with a notable reduction in serum insulin concentration (Fig. 2D, n = 6, P < 0.0001), compared to the control group. This is indicative of a persistent hyperglycemic state in the DN group mice.
Fig. 2.
Caffeic acid mitigates diabetic neuropathy (DN) and revives spinal IGF-1 signaling. (A) Longitudinal alterations in fasting blood glucose levels at Baseline (BL, immediately prior to STZ injection), and at weekly intervals for 8 weeks following STZ injection. Red asterisks (*) denote P < 0.05 when comparing DN group to control group at specified time points. Blue or green hashtags (#) represent P < 0.05 when comparing CA1 or CA2 groups, respectively, to the DN group at those time points. Statistical significance was determined using a multiple comparison test following a two-way ANOVA. (B) Time-course changes in body weight from BL for 8 weeks after STZ injection. Notations as explained in (A). (C) Comparative examination of blood HbA1c percentage among four experimental groups, 8 weeks after STZ administration. (D) Comparison of serum insulin levels among four experimental groups, 8 weeks after STZ administration. (E) Longitudinal variations in mechanical withdrawal thresholds from BL for 8 weeks following STZ injection. Notations as explained in (A). (F) Time-course changes in thermal withdrawal latency from BL for 8 weeks post-STZ injection. Notations as explained in (A). (G) Examination of serum IGF-1 concentration, 8 weeks after STZ administration. (H–K) Comparative assessment of spinal protein levels of IGF-1, phosphorylated IGF1R (p-IGF1R), and total IGF1R, 8 weeks post-STZ injection. Statistical significance is denoted as *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, while 'ns' denotes non-significant findings.
Turning our attention to mechanical withdrawal thresholds, the DN group mice showed a gradual decline when compared with the controls (Fig. 2E, n = 12, F (1, 22) = 517.9, P < 0.0001, two-way ANOVA, P < 0.05 at 1w, 2w, 3w, 4w, 5w, 6w, 7w, 8w after STZ injection by multiple comparisons test). This decrease stabilized four weeks post STZ injection, suggesting the onset of mechanical allodynia associated with diabetes. In contrast, the DN group mice displayed a significant increase in paw thermal withdrawal latency post STZ injection (Fig. 2F, n = 12, F (1, 22) = 289.2, P < 0.0001, two-way ANOVA; P < 0.05 at 1w, 2w, 3w, 4w, 5w, 6w, 7w, 8w after STZ injection by multiple comparisons test), compared to the control group. Taken together, these findings provide evidence that our method of STZ injection has been successful in establishing a mouse model of diabetic neuropathy.
Following the establishment of the DN mouse model, we proceeded to explore the effects of low-dose (5 mg/kg, CA1 group) and high-dose (10 mg/kg, CA2 group) oral caffeic acid administration on DN and associated diabetes indicators. Given that the most pronounced effects in the DN mice were observed within 4 weeks post STZ injection, we implemented a regimen of caffeic acid administration every other day for a duration of four weeks subsequent to the STZ injection. Our findings revealed that the CA1 and CA2 groups exhibited reduced fasting blood glucose levels when compared to the DN group (Fig. 2A, n = 12; for the CA1 group, F (1, 22) = 36.70, P < 0.0001, two-way ANOVA, P < 0.05 at 3w and 5w after STZ injection by multiple comparisons test; for the CA2 group, F (1, 22) = 108.4, P < 0.0001, two-way ANOVA, P < 0.05 at 3w, 5w, 6w, 7w after STZ injection by multiple comparisons test). Moreover, there was a notable decrease in the HbA1c levels eight weeks after STZ injection in both the CA1 and CA2 groups (Fig. 2B, n = 6, P = 0.0328 for CA1 and P = 0.0074 for CA2). However, their body weight did not show significant changes (Fig. 2C, n = 12; for CA1, F(1, 22) = 2.351, P = 0.1504; for CA2, F(1, 22) = 0.224, P = 0.6407, using two-way ANOVA). Similarly, serum insulin levels remained relatively stable in both groups (Fig. 2D, n = 6, with P > 0.05 for both CA1 and CA2). Notably, when compared to the DN group, both CA1 and CA2 groups demonstrated significant improvements in mechanical withdrawal thresholds (Fig. 2E, n = 12; for the CA1 group, F (1, 22) = 56.27, P < 0.0001, two-way ANOVA; for the CA2 group, F (1, 22) = 258.6, P < 0.0001, two-way ANOVA) and thermal withdrawal latency (Fig. 2F, n = 12; for the CA1 group, F (1, 22) = 21.34, P = 0.0001, two-way ANOVA; for the CA2 group, F (1, 22) = 76.81, P < 0.0001, two-way ANOVA). These results suggest that caffeic acid may possess the potential to alleviate the symptoms of diabetic neuropathy and counteract STZ-induced hyperglycemia in mice.
Previous research has underscored the pivotal role of decreased spinal IGF-1 in the development of DN [37]. Building on this knowledge, we sought to investigate the impact of caffeic acid administration on the IGF-1 signaling pathway within DN mice. We commenced our investigation by analyzing plasma IGF-1 concentrations at the eighth week post STZ injection via ELISA. The data revealed a substantial reduction in plasma IGF-1 concentration within DN mice when contrasted with the control group (Fig. 2G, n = 6, P = 0.0039). However, this downward trend was conspicuously mitigated in the high-dose caffeic acid (CA2) group (Fig. 2G, n = 6, P = 0.0209), while the low-dose caffeic acid (CA1) group did not exhibit significant alterations (Fig. 2G, n = 6, P = 0.5867). The primary receptor for IGF-1, IGF1R, and its activated form, phosphorylated IGF1R (p-IGF1R), play crucial roles in the IGF-1 signaling pathway. Our Western blot analysis demonstrated a significant decline in the spinal cord levels of both IGF-1 and p-IGF1R proteins in DN mice relative to the control group (Fig. 2 H–K, n = 6, P = 0.0002 for IGF-1, P < 0.0001 for p-IGF1R), indicating a palpable decrease in IGF-1 signaling pathway activity within the spinal cord of DN mice. Conversely, the CA2 group displayed a significant augmentation in spinal IGF-1 and p-IGF1R protein levels compared to the DN group (Fig. 2 H–K, n = 6, P = 0.0006 for IGF-1, P < 0.0001 for p-IGF1R). In the CA1 group, only the levels of p-IGF1R protein showed a significant increase (Fig. 2 H–K, n = 6, P = 0.0454), while the IGF-1 levels remained statistically unchanged (Fig. 2 H–K, n = 6, P = 0.0684). This pattern suggests a dose-dependent effect of caffeic acid in mitigating the inhibition of the IGF-1 signaling pathway in the spinal cord of DN mice. Additionally, no significant variations were observed in the total IGF1R protein levels across the different groups (Fig. 2 H–K, n = 6, F (3, 20) = 1.041, P = 0.3958).
Considering that IGF-1 is recognized as a neurotrophic factor, concerns arise about whether the reduced IGF-1 levels in the spinal cords of diabetic mice may suggest neuronal damage or pathological alterations. Consequently, we conducted Nissl staining on spinal cord samples from the four experimental groups. The outcomes of the Nissl staining revealed no significant pathological changes in the neurons located in either the dorsal or ventral horns of the spinal cord across all groups, as illustrated in Supplementary Fig. 1.
In light of our findings regarding the hypoglycemic activity of caffeic acid (CA), we further explored its potential to modulate the IGF-1 signaling pathway, specifically probing its direct influence on neurons within an in vitro setting (Supplementary Fig. 2). Initially, neurons were cultured in NeuroBasal medium for a period of five days, subsequently subjected to four distinct experimental conditions derived from variations in the culture medium's composition for 4 days. Mirroring the in vivo observations, analysis of the IGF-1 positive cell proportion and IGF-1 concentration in the culture medium disclosed a pronounced decrease in both the HGM-4d and HGM-2d + NGM-2d groups in comparison to the NGM-4d group, as evidenced in Supplementary Fig. 2 C-E (P < 0.05). In contrast, the HGM-4d + CA group demonstrated a significant uptick in both IGF-1 positive cells and IGF-1 concentration within the culture medium when juxtaposed with the HGM-4d group (P < 0.05), whereas the HGM-2d + NGM-2d group did not exhibit any noteworthy alteration (P > 0.05). This finding suggests that transitioning neurons from a high-glucose environment back to a normoglycemic condition does not necessarily rejuvenate neuron-derived IGF-1 production. Furthermore, aligning with in vivo Nissl staining data, an evaluation of cell viability through the CCK-8 assay revealed no significant disparities across the experimental groups (Supplementary Fig. 2F, P > 0.05).
3.2. Caffeic acid mitigates oxidative damage in the spinal cord of DN mice
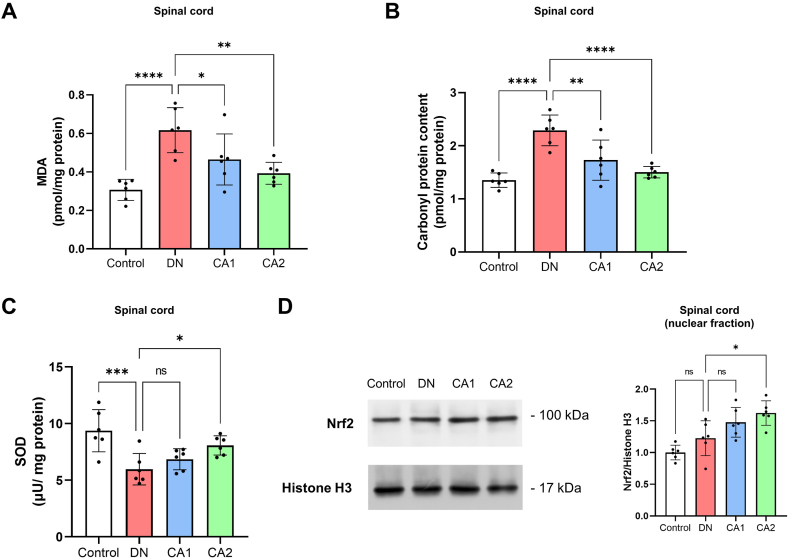
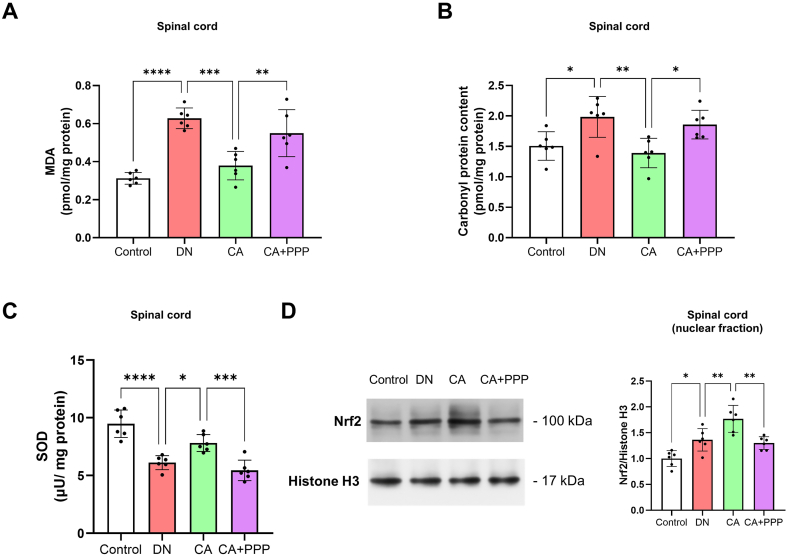
Numerous studies have affirmed that oxidative stress-triggered damage in the spinal cord plays a key role in the pathological mechanism of diabetic neuropathy [9,10]. Caffeic acid's neuroprotective effects are closely tied to its antioxidant properties [22,38]. In line with this, we assessed the levels of oxidative stress markers in the spinal cord, including MDA (a marker for lipid oxidation), carbonyl protein content (indicating protein oxidation), and the activity of the antioxidant enzyme SOD (superoxide dismutase). The findings revealed that MDA and carbonyl protein levels in the spinal cord were considerably elevated in DN mice when compared to the control group (Fig. 3 A and B, n = 6, P < 0.0001 for MDA, P < 0.0001 for carbonyl protein), whereas SOD concentration was markedly diminished (Fig. 3C, n = 6, P = 0.0007). As anticipated, when juxtaposed with the DN group, the CA1 group mice displayed a significant decrease in spinal MDA and carbonyl protein content (Fig. 3 A and B, n = 6, P = 0.0346 for MDA, P = 0.0029 for carbonyl protein), but SOD content showed no substantial difference (Fig. 3C, n = 6, P = 0.5338). Furthermore, mice in the CA2 group, in comparison to the DN group, demonstrated a significant decline in MDA and carbonyl protein levels (Fig. 3 A and B, n = 6, P = 0.0020 for MDA, P < 0.0001 for carbonyl protein), coupled with a significant increase in SOD concentration in the spinal cord (Fig. 3C, n = 6, P = 0.0320).
Fig. 3.
Caffeic acid protects against oxidative damage in the spinal cord of DN mice. (A) Comparison of spinal MDA levels between the control, DN, CA1, and CA2 groups, 8 weeks post-STZ injection. (B) The analysis of spinal carbonyl protein content in the control, DN, CA1, and CA2 groups, 8 weeks after STZ administration. (C) Comparison of spinal SOD levels among the control, DN, CA1, and CA2 groups, 8 weeks post-STZ injection. (D) The evaluation of spinal Nrf2 protein levels in the nuclear fraction among the control, DN, CA1, and CA2 groups, 8 weeks after STZ administration. Nrf2 protein levels were normalized to Histone H3. Statistical significance is indicated as *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, whereas 'ns' represents non-significant results.
Nrf2 is a vital antioxidant stress transcription factor that under conditions of oxidative stress [39], relocates from the cytoplasm to the cell nucleus, thereby activating downstream antioxidant stress signaling pathways. Consequently, we also quantified the protein levels of Nrf2 in the nuclear fraction of the spinal cord. The results suggest that, compared to the control group, nuclear Nrf2 levels were marginally elevated in DN mice, but the difference lacked statistical significance (Fig. 3D, n = 6, P = 0.1898). Notably, in the CA2 group mice, nuclear Nrf2 levels in the spinal cord were substantially higher than those in the DN group (Fig. 3D, n = 6, P = 0.0114). Collectively, these findings indicate that caffeic acid can attenuate oxidative damage in the spinal cord of DN mice.
3.3. Caffeic acid reduces spinal inflammation in DN mice
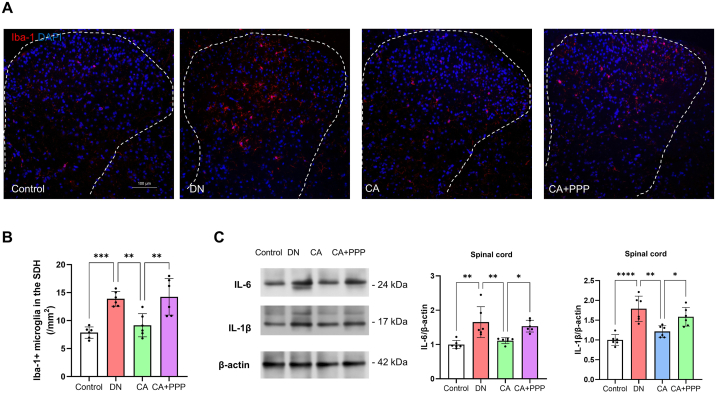
Alongside oxidative stress-induced damage, glial cell-driven spinal inflammation is a significant contributing factor in DN [40,41]. To delve into this, we evaluated the impact of caffeic acid on microglia and astrocytes in DN mice. Results highlighted a significant rise in the count of Iba-1+ microglia in the spinal dorsal horn (SDH) of DN mice relative to the control group (Fig. 4 A and C, n = 6, P < 0.0001). Intriguingly, this increase was curtailed by caffeic acid at both low (CA1) and high (CA2) doses (Fig. 4 A and C, n = 6, P = 0.0272 for CA1, P = 0.0017 for CA2). Contrarily, no noticeable differences were observed in the fluorescence intensity of GFAP, an astrocyte marker, across all groups in the mouse SDH (Fig. 4 B and D, n = 6, F (3, 20) = 1.024, P = 0.4031).
Fig. 4.
Caffeic acid moderates microglial activation and pro-inflammatory mediator levels in the spinal cord of DN mice. (A) Graphs showing Iba-1 immunostaining (red) in the SDH of the control, DN, CA1, and CA2 groups, 8 weeks post-STZ injection. The sections were counterstained with DAPI (blue) to highlight nuclei. Scale bars represent 100 μm. (B) Graphs displaying GFAP immunostaining (red) in the SDH across control, DN, CA1, and CA2 groups, 8 weeks post-STZ injection. Sections were counterstained with DAPI (blue). Scale bars denote 100 μm. (C) Quantification of Iba-1+ microglial cells in the SDH. (D) Evaluation of GFAP immunoreactivity in the SDH. (E) Assessment of spinal protein levels of pro-inflammatory cytokines IL-6 and IL-1β in control, DN, CA1, and CA2 groups, 8 weeks post-STZ injection. Statistical significance is indicated as *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, with 'ns' denoting non-significant results.
In a complementary line of investigation, we quantified the protein levels of pro-inflammatory cytokines IL-6 and IL-1β in the spinal cord. The data obtained signified that relative to the control group, the protein concentrations of IL-6 and IL-1β were significantly escalated in the spinal cord of DN mice (Fig. 4E, n = 6, P = 0.0015 for IL-6 and P < 0.0001 for IL-1β). Notwithstanding, a substantial reduction in the protein levels of IL-6 and IL-1β was noted in the CA2 group when compared to the DN group (Fig. 4E, n = 6, P = 0.0051 for IL-6 and P = 0.0011 for IL-1β), while the CA1 group demonstrated a decrease solely in IL-1β levels (Fig. 4E, n = 6, P = 0.0731 for IL-6 and P = 0.0038 for IL-1β). These findings suggest a dose-dependent anti-inflammatory effect of caffeic acid in the context of DN.
3.4. PPP inhibition of IGF-1 signaling partially counteracts the positive effects of caffeic acid on DN
To further elucidate the role of the IGF-1 signaling pathway in the ameliorative effects of caffeic acid on DN, we employed a strategy in which mice received an intraperitoneal injection of the IGF1R inhibitor Picropodophyllin (PPP) at a dosage of 1 mg/kg, 20 min prior to each oral dose of caffeic acid (10 mg/kg) - termed the CA + PPP group. We specifically chose the 10 mg/kg dose of caffeic acid (CA group) for these experiments, based on its demonstrated superior efficacy over the lower dose (5 mg/kg).
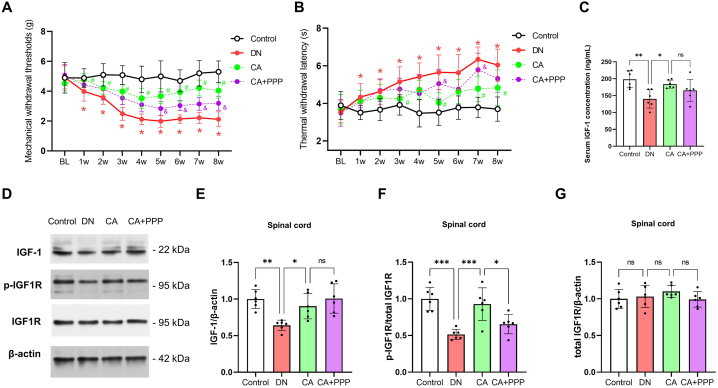
Behavioral evaluations indicated a partial reversal in the reduction of mechanical withdrawal thresholds (Fig. 5A, n = 12, F (1, 22) = 45.84, P < 0.0001, two-way ANOVA, P < 0.05 at 5w, 6w, 7w, 8w after STZ injection by multiple comparisons test) and a progressive elongation in thermal withdrawal latency (Fig. 5B, n = 12, F (1, 22) = 8.313, P = 0.0086, two-way ANOVA, P < 0.05 at 5w and 7w, after STZ injection by multiple comparisons test) in the CA + PPP group compared to the CA group. These findings suggest that inhibition of the IGF-1 signaling pathway via PPP partially negated the beneficial effects of caffeic acid on DN symptoms.
Fig. 5.
Partial nullification of caffeic acid's beneficial effects on DN through IGF-1 signaling inhibition via PPP. (A) Longitudinal changes in mechanical withdrawal thresholds from Baseline (BL) up to 8 weeks post-STZ injection. An asterisk (*) represents P < 0.05 when comparing the DN group with the control group at specific time points. Blue hashtags (#) denote P < 0.05 when comparing the CA group with the DN group at the indicated time points. Purple symbols (&) signify P < 0.05 when comparing the CA + PPP group with the CA group at these time points. Statistical significance was evaluated via a multiple comparison test following a two-way ANOVA. (B) Sequential alterations in thermal withdrawal latency from BL to 8 weeks post-STZ injection. The notations are as explained in (A). (C) Assessment of serum IGF-1 concentration, 8 weeks post-STZ administration. (D–F) Comparative analysis of spinal protein levels of IGF-1, phosphorylated IGF1R (p-IGF1R), and total IGF1R, 8 weeks after STZ injection. Statistical significance is indicated as *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, with 'ns' denoting non-significant results.
Notably, plasma IGF-1 concentration analyses showed that the upregulation induced by caffeic acid in DN mice was not significantly affected by PPP (Fig. 5C, n = 6, P = 0.5898). In addition, Western blot analysis revealed a marked decrease in the protein level of p-IGF1R in the CA + PPP group compared to the CA group (Fig. 5 D-G, n = 6, P = 0.0298), while there were no significant alterations in the protein levels of IGF-1 and total IGF1R (Fig. 5 D-G, n = 6, P = 0.6400 for IGF-1 and P = 0.4198 for total IGF1R). These findings further substantiate the involvement of the IGF-1 signaling pathway in the protective effects of caffeic acid on DN.
3.5. Co-administration of PPP mitigates the antioxidative effects of caffeic acid in the spinal cord of DN mice
Building upon the evidence that suggests the IGF-1 signaling pathway can exert antioxidant properties in the CNS, and that its deficiency may induce oxidative damage and edema in brain nerve cells [15], we aimed to understand if PPP-induced inhibition of the IGF-1 signaling pathway could impact the antioxidative effects of caffeic acid in the spinal cords of DN mice.
Our investigation revealed that the co-administration of PPP with caffeic acid (in the CA + PPP group) led to a significant increase in MDA levels and carbonyl protein content in the spinal cord (Fig. 6 A and B, n = 6, P = 0.0062 for MDA and P = 0.0297 for carbonyl protein content), alongside a pronounced decrease in SOD content compared to the group that received caffeic acid alone (CA group) (Fig. 6C, n = 6, P = 0.0008). Moreover, our assessment of the protein expression levels of nuclear Nrf2 in the spinal cord suggested that the co-administration of PPP significantly diminished the upregulation of nuclear Nrf2 mediated by caffeic acid (Fig. 6D, n = 6, P = 0.0025). This evidence underscores the possible interplay between the IGF-1 signaling pathway and the antioxidative mechanisms of caffeic acid in managing diabetic neuropathy.
Fig. 6.
The inhibitory effect of PPP on IGF-1 signaling counteracts the antioxidative actions of caffeic acid in the spinal cord of DN mice. (A) Depicts the comparative analysis of spinal MDA levels among the control, DN, CA, and CA + PPP groups, 8 weeks after STZ injection. (B) Illustrates the assessment of spinal carbonyl protein content across the control, DN, CA, and CA + PPP groups, 8 weeks post-STZ administration. (C) Represents the comparison of spinal SOD levels between the control, DN, CA, and CA + PPP groups, 8 weeks following STZ injection. (D) Portrays the examination of nuclear Nrf2 protein levels in the spinal cord across the control, DN, CA, and CA + PPP groups, 8 weeks subsequent to STZ administration, with Nrf2 protein levels normalized to Histone H3. Statistical significance is denoted as *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, with 'ns' indicating non-significant findings.
3.6. The anti-inflammatory effects of caffeic acid in the spinal cord of DN mice are attenuated by the co-administration of PPP
The IGF-1 signaling pathway is known to play a pivotal role in modulating inflammation triggered by microglia [17,42]. To better understand its role and interaction with the anti-inflammatory effects of Caffeic acid, we targeted this pathway with the IGF1R inhibitor, PPP, in our DN mouse model. Our findings indicate a notable upregulation in the number of Iba-1+ microglia in the SDH of the CA + PPP group, relative to the CA group (Fig. 7 A and B, n = 6, P = 0.0024). Similarly, protein level analysis using Western blot revealed a significant rise in the inflammatory cytokines IL-6 and IL-1β in the spinal cord of the CA + PPP group when compared to the CA group (Fig. 7C, n = 6, P = 0.0396 for IL-6 and P = 0.0414 for IL-1β). These findings emphasize the probable role of the IGF-1 signaling pathway in mediating the anti-inflammatory effects of caffeic acid in DN mice.
Fig. 7.
The anti-inflammatory effects of caffeic acid in the spinal cord of DN mice are mitigated by the co-administration of PPP. (A) Depictions of Iba-1 immunostaining (red) in the SDH of the control, DN, CA, and CA + PPP groups, 8 weeks after STZ injection. DAPI (blue) counterstaining was employed to highlight nuclei. Scale bars correspond to 100 μm. (B) Quantitative evaluation of Iba-1+ microglial cells in the SDH. (C) Comparative analysis of spinal protein levels of the pro-inflammatory cytokines IL-6 and IL-1β among the control, DN, CA, and CA + PPP groups, 8 weeks following STZ injection. Levels of statistical significance are designated as *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, while 'ns' signifies non-significant outcomes.
3.7. Caffeic acid partially diminishes neuropathic changes in PNS of diabetic mice
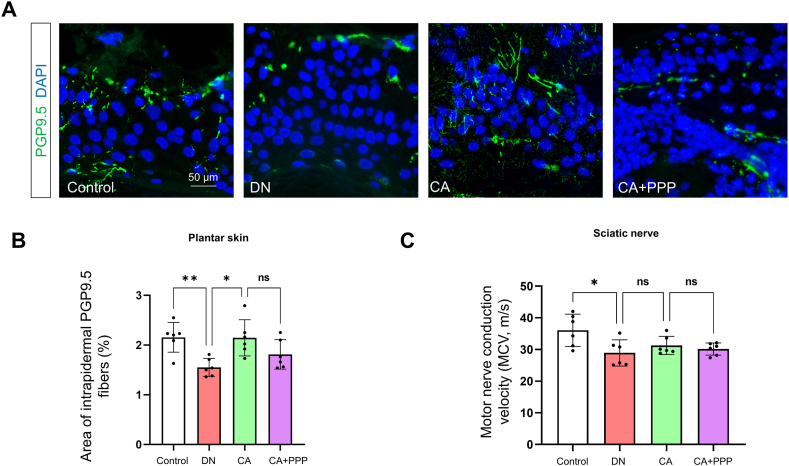
We expanded our study on the effects of caffeic acid on the peripheral nervous system (PNS) of diabetic mice, specifically targeting the intraepidermal nerve fiber density (IENFD) and motor conduction velocities (MCV) of the sciatic nerve. Immunofluorescence assays were employed to measure the coverage of plantar intradermal PGP9.5-positive nerve fibers. Our findings indicate a significant reduction in PGP9.5-positive fibers in the diabetic group (Fig. 8 A and B, n = 6, P = 0.0093). In contrast, administration of caffeic acid (CA group) significantly increased these fibers (Fig. 8 A and B, n = 6, P = 0.0106). However, the CA + PPP group, which also received caffeic acid, did not show a statistically significant difference in the area of PGP9.5-positive fibers compared to controls (Fig. 8 A and B, n = 6, P = 0.2313).
Fig. 8.
Caffeic acid partially mitigates neuropathic alterations in PNS of diabetic mice. (A) Visualization of PGP9.5-positive intrapidermal nerve fibers (green) in plantar skin from control, DN, CA, and CA + PPP groups, 8 weeks post STZ administration. Nuclei are counterstained in blue (DAPI). Scale bars: 50 μm. (B) Measurement of the percentage area of PGP9.5-positive nerve fibers. (C) Measurement of motor nerve conduction velocity (MCV) in the sciatic nerve across control, DN, CA, and CA + PPP groups, 8 weeks post STZ administration. Statistical significance levels are indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. 'ns' denotes non-significant differences.
Furthermore, MCV assessments conducted 8 weeks post-STZ injection served as indicators of neural functional integrity. The analysis revealed a significant decline in MCV for the DN group compared to controls (Fig. 8C, n = 6, P = 0.0163). Notably, the caffeic acid-treated mice (CA group) exhibited no significant differences in MCV compared to the DN group (Fig. 8C, n = 6, P = 0.6962). This suggests that the potential of caffeic acid to enhance nerve conduction functionality in diabetic mice may be limited.
4. Discussion
The underlying cause of diabetic neuropathy (DN) remains a significant challenge, underscoring the need for innovative therapeutic approaches. Dietary regulation stands out as a pivotal strategy in the management of diabetes and its complications, with our study exploring the protective impact of caffeic acid, a naturally occurring dietary component, on DN in a mouse model. We have comprehensively demonstrated that caffeic acid not only markedly attenuate sensory behavioral changes in diabetic mice but also sheds light on the mechanisms involved, specifically through decreasing oxidative stress and inflammation in the spinal cord. Additionally, our research offers insights into the essential role of the spinal IGF-1 signaling pathway in the positive effects of caffeic acid on DN. By using an IGF1R inhibitor PPP, our findings further verify the pathway's contribution, as evidenced by the inhibitor's ability to diminish caffeic acid's protective, antioxidant, and anti-inflammatory effects in the diabetic mice's spinal cord.
Clinical evidence has revealed that coffee consumption can induce comprehensive modifications in human blood glucose and lipid metabolism, implying the potential anti-diabetic advantages of its components and metabolites [43]. Caffeic acid, a phenolic acid predominantly found in coffee beans and other foodstuffs, is one such component of interest. Prior investigations have illustrated the potential of caffeic acid in significantly diminishing hyperglycemia in type 2 diabetic mice (db/db mice) and attenuating oxidative damage in erythrocytes and the liver [44]. Echoing these results, our study has shown that an oral administration of caffeic acid at a dosage of 10 mg/kg could potentially lower fasting blood glucose in STZ-induced diabetic mice. However, the hypoglycemic effect observed was less pronounced than in previous studies involving type 2 diabetic mice [44]. Additionally, the treatment with caffeic acid did not achieve significant glycemic control within the normal range, nor substantially ameliorate alterations in body weight.
The specific mechanism by which caffeic acid exerts its hypoglycemic effects remains unclear. We observed that there were no significant differences in serum insulin levels between groups treated with CA and the DN group. This finding implies that the role of CA in reducing blood glucose may not primarily involve the direct stimulation of insulin secretion. Instead, it may act through alternative pathways, such as enhancing glucose uptake and utilization in the liver. This hypothesis receives partial support from a study conducted on db/db mice [44], which demonstrated that caffeic acid significantly diminished the activities of hepatic PEPCK and G6Pase—critical enzymes involved in gluconeogenesis and the hepatic output of glucose—as well as reduced the expression of GLUT2, which is associated with decreased hepatic glucose output. Furthermore, an increase in plasma IGF-1 levels was observed in the CA-treated group. It is well-documented that IGF-1 possesses notable structural resemblances to insulin and is capable of binding to insulin receptors, thus facilitating glucose transport in adipose and muscle tissues [45], enhancing insulin sensitivity [46], diminishing hepatic glucose production [47], and ultimately contributing to the reduction of blood glucose levels. Although the impact of IGF-1 on blood glucose is relatively modest compared to that of insulin, the induction of IGF-1 production by CA could further contribute to the normalization of blood glucose levels.
Despite the relatively modest hypoglycemic effects observed, we noted a significant alleviation of sensory behavioral changes in diabetic mice following treatment with caffeic acid. This improvement was evidenced by an increased mechanical withdrawal threshold and a partial restoration of thermal withdrawal latency. Hence, our findings imply that the beneficial effects of caffeic acid on diabetes extend beyond its ability to lower blood sugar levels, potentially offering relief from the neural functional changes induced by diabetes. Although the hypoglycemic impact appears modest, we cannot dismiss the possibility that caffeic acid's ability to lower blood sugar may contribute to its neuroprotective effects in diabetic mice. Consequently, we carried out further in vitro experiments with neuronal cell cultures (Supplementary Fig. 2). These experiments showed that while high glucose conditions can inhibit IGF-1 signaling, merely normalizing glucose levels does not suffice to revive this pathway. However, in vitro experiments are often oversimplified and may not fully represent the actual situation in animals. Thus, we acknowledge that the hypoglycemic effect of caffeic acid might also contribute to its neuroprotective action in diabetic mice.
Diabetes, while causing significant damage to the PNS including the DRG [3] and sciatic nerve [48], can also incite various alterations within the CNS [7,8,49]. These changes, particularly those impacting spinal cord neurons, are integral to the persistent progression and refractory nature of DN. In light of this, our study pivots towards examining pathological alterations within the spinal cords of diabetic mice. In concordance with previous research findings [9,10,49], we observed a marked elevation in oxidative stress products, specifically MDA and carbonyl protein content, accompanied by a notable reduction in the antioxidant enzyme SOD, within the spinal cords of diabetic mice. These findings underscore the potential contribution of spinal oxidative damage to DN. In this context, caffeic acid emerges as a compound of interest due to its well-known antioxidant capacities [50]. Research evidence also suggests that the concentrations of caffeic acid in blood plasma and cerebrospinal fluid are comparable [29], hinting at its promising protective potential for disorders pertaining to the CNS. On this basis, we hypothesized that caffeic acid may serve as a beneficial intervention in attenuating oxidative stress within the spinal cords of diabetic mice. Our research lends credence to this hypothesis, illustrating that both low (5 mg/kg) and high (10 mg/kg) doses of caffeic acid can appreciably curtail the levels of MDA and carbonyl protein content within the spinal cords of diabetic mice. Furthermore, caffeic acid bolstered the activity of SOD, implying it may counteract neurological dysfunction in the spinal cord of DN mice via its antioxidant properties.
In addition to its antioxidant attributes, caffeic acid also demonstrates a protective role against neuroinflammation [51,52]. Consistent with previous research [37], we noted a marked increase in the number of microglia and inflammatory markers IL-6 and IL-1β in the SDH in diabetic mice. However, no significant changes were observed in the immunofluorescence intensity of the astrocytic marker GFAP. This observation emphasizes the critical role of microglial-mediated neuroinflammation in the spinal cord of DN mice, though the role of astrocytes cannot be completely ruled out, given that diabetes/hyperglycemia could lead to functional alterations in astrocytes [53,54] without necessarily affecting their proliferation. Our data further substantiate that oral administration of caffeic acid significantly reduces the number of microglia and the levels of inflammatory markers IL-6 and IL-1β. These findings are in line with previous research [18] exploring the beneficial influence of caffeic acid on microglial phenotypes and associated inflammatory responses. Consequently, it can be posited that the direct anti-inflammatory action of caffeic acid in the spinal cord of diabetic mice may be one of the mechanisms through which it provides protective benefit in DN.
While caffeic acid has established beneficial impact for our mouse DN model, the precise mechanism by remains elusive. Our study proposes that the protective effects of caffeic acid on DN might be intrinsically linked with the activation of the IGF-1 signaling pathway. Predominantly synthesized and secreted by the liver [55], IGF-1 plays a crucial role across various organs and metabolic processes within the body [56,57]. Within the spinal cord, IGF-1 expression is seen across multiple cell types, including neurons, microglia, and astrocytes [56,58]. Earlier investigations have demonstrated that caffeic acid phenethyl ester, a derivative of caffeic acid, can effectively attenuate the downregulation of hepatic and renal IGF-1 mRNA in STZ-induced diabetic rats [59]. Our research mirrors these findings, identifying that high-dose oral administration of caffeic acid can enhance serum IGF-1 concentrations in diabetic mice. This suggests that caffeic acid might directly interact with hepatocytes to stimulate the synthesis and release of IGF-1. More intriguingly, we observed that the influence of caffeic acid transcended serum IGF-1 levels, effectively countering the downregulation of IGF-1 and p-IGF1R protein levels in diabetic mice. IGF-1 exerts its effects primarily by binding to IGF1R [55], triggering receptor phosphorylation and consequent activation of downstream intracellular signaling pathways. Therefore, our findings suggest that caffeic acid plays a crucial role in preserving and activating the IGF-1 signaling pathway within the spinal cord of diabetic mice.
Our investigation into the role of the IGF-1 signaling pathway in the beneficial effects of caffeic acid on DN was further substantiated using the IGF1R inhibitor PPP. We noted that the co-administration of PPP did not markedly alter the serum IGF-1 concentration or the protein expression of spinal IGF-1. However, it significantly attenuated the upregulation of phosphorylated IGF1R (the activated form of IGF1R) induced by caffeic acid. Alongside this, the co-administration of PPP also dampened the improvement in the pain behavior of diabetic mice brought about by caffeic acid, as demonstrated by a reduction in mechanical withdrawal threshold and an elevation in thermal withdrawal latency. These observations suggest that caffeic acid may mitigate DN by preserving the IGF-1 signaling pathway within the spinal cord of diabetic mice.
IGF-1 signaling pathway signaling pathway is integral to crucial neurological processes, including brain development, the formation of neuronal synapses, and cell viability [56]. Within the CNS, specifically in the spinal cord, IGF-1 is primarily produced by neurons [60], while in the PNS, it is produced by Schwann cells, playing a vital role in their viability [61]. Moreover, the IGF-1 pathway plays a vital role in regulating oxidative stress and neuroinflammation [[15], [16], [17], [18]]. Our study not only corroborates these established findings but also provides new insights. We found that that the co-administration of the IGF-1 receptor inhibitor, PPP, notably reduces the antioxidative effects of caffeic acid in the spinal cord of diabetic mice. Prior investigations have connected IGF-1's antioxidative mechanisms with its capacity to activate the Nrf2/HO-1 antioxidative signaling pathway [62,63]. Consistently, our observations show that PPP co-administration diminishes the upregulation of Nrf2 and HO-1 in the spinal cord, which was otherwise induced by caffeic acid. Furthermore, activation of the IGF-1 signaling pathway is known to suppress the inflammatory HMGB-1/NF-κB signaling pathway [62,64], a correlation that our findings reaffirm. Specifically, PPP co-administration decreased the inhibitory effect of caffeic acid on pro-inflammatory cytokines IL-6 and IL-1β in the spinal cord. Although the direct impact of IGF-1 on microglial cell phenotype towards the anti-inflammatory M2 phenotype [18] was not the focus of our study, we did observe that PPP co-administration reduced the suppressive effect of caffeic acid on the microglial population in the spinal dorsal horn. Collectively, these results suggest that the antioxidative and anti-inflammatory effects of caffeic acid in diabetic mice may, in part, be facilitated through the activation of the IGF-1 signaling pathway.
Our research initially focused on the modifications occurring within the spinal cord of diabetic mice; however, during the course of our investigation, we observed that caffeic acid may exert a protective effect on the PNS. This was particularly evident in the preservation of intraepidermal nerve fiber density, as evidenced by the maintenance of PGP9.5-positive fibers in caffeic acid-treated groups. Despite this finding, caffeic acid's potential to improve motor conduction velocities in the context of diabetes was found to be modest, suggesting that its protective impact on the PNS is selective and not uniformly applicable across different neural functions.
The present study is subject to several limitations that merit attention. Firstly, the ambiguity in distinguishing between the direct actions of caffeic acid and its hypoglycemic effects presents a significant challenge. This is due to the compound's potential hypoglycemic properties, which complicates the isolation of its protective effects solely to its direct intervention. Secondly, technical difficulties, particularly those associated with our antibodies, impeded our capacity to conclusively ascertain the cellular origin of IGF-1 using immunofluorescence techniques. This limitation underscores the need for improved methodologies in identifying specific cellular contributions. Lastly, while our investigation predominantly centered on the spinal cord, preliminary observations from the PNS indicate a possible action of caffeic acid within this domain as well. Such findings suggest the necessity for future research to undertake exhaustive experimental approaches, employing diverse techniques and samples. These efforts are crucial for a more comprehensive understanding of caffeic acid's protective mechanisms against DN.
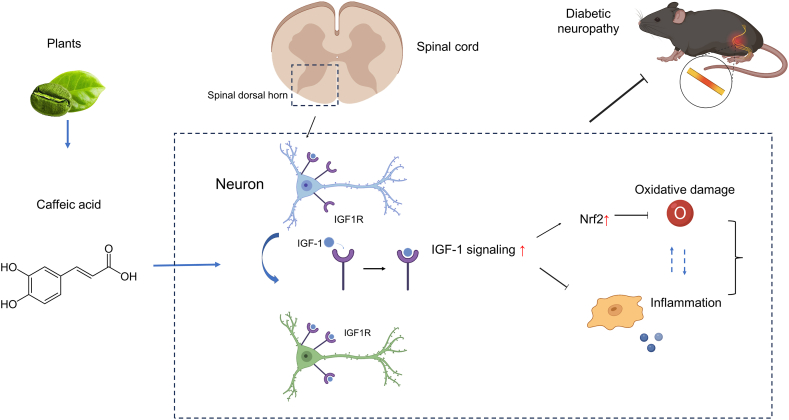
In conclusion, our study delineates the beneficial effects of caffeic acid in attenuating symptoms of diabetic neuropathy, specifically by reducing spinal cord oxidative damage and inflammation, as depicted in Fig. 9. Caffeic acid was found to stimulate the IGF-1 signaling pathway, which emerged as a crucial component in mediating these protective effects. Furthermore, co-administration of an IGF1R inhibitor curtailed the beneficial impacts of caffeic acid, underscoring the significance of the IGF-1 signaling pathway. These findings suggest that caffeic acid holds promise as a dietary intervention for DN. Future research should explore the detailed molecular interactions between caffeic acid and the IGF-1 signaling pathway and their implications for DN treatment.
Fig. 9.
Schematic illustration of the possible protective mechanism of caffeic acid for diabetic neuropathy. This figure presents a schematic overview of how caffeic acid may confer protective effects against diabetic neuropathy. According to our findings, caffeic acid contributes to the mitigation of diabetic neuropathy symptoms through the activation of the IGF-1 signaling pathway in the spinal cord. This activation is associated with a decrease in spinal cord oxidative damage and inflammation, illustrating the compound's potential protective benefits.
Funding information
This work was supported by Natural Science Foundation of Shaanxi Province (Grant No. 2020JQ-953)
Funding
This work was supported by Natural Science Foundation of Shaanxi Province (Grant No. 2020JQ-953).
Ethical approval
Our study strictly adhered to the ARRIVE guidelines for all animal-based experiments and was duly approved by the Ethics Committee of Shaanxi Provincial cancer Hospital (No. 2023016).
Informed consent
N/A.
Data availability statement
All datasets produced or analyzed during this study can be obtained from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Leina Hou: Writing – original draft, Methodology, Investigation, Conceptualization. Jiaqi Ma: Writing – review & editing, Funding acquisition, Conceptualization. Xugang Feng: Writing – review & editing, Methodology, Investigation. Jing Chen: Writing – review & editing, Investigation. Bu-huai Dong: Writing – review & editing, Supervision. Li Xiao: Writing – review & editing, Investigation, Formal analysis. Xi Zhang: Writing – review & editing, Formal analysis. Bin Guo: Writing – review & editing, Supervision, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Jiaqi Ma reports administrative support, article publishing charges, equipment, drugs, or supplies, statistical analysis, and writing assistance were provided by Natural Science Foundation of Shaanxi Province. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e32623.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Feldman E.L., Callaghan B.C., Pop-Busui R., Zochodne D.W., Wright D.E., Bennett D.L., Bril V., Russell J.W., Viswanathan V. Diabetic neuropathy. Nat. Rev. Dis. Prim. 2019;5:42. doi: 10.1038/s41572-019-0097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boulton A.J., Kempler P., Ametov A., Ziegler D. Whither pathogenetic treatments for diabetic polyneuropathy? Diabetes Metab Res Rev. 2013;29:327–333. doi: 10.1002/dmrr.2397. [DOI] [PubMed] [Google Scholar]
- 3.Bhandari R., Sharma A., Kuhad A. Novel nanotechnological approaches for targeting dorsal root ganglion (DRG) in mitigating diabetic neuropathic pain (DNP) Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.790747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie Y.K., Luo H., Zhang S.X., Chen X.Y., Guo R., Qiu X.Y., Liu S., Wu H., Chen W.B., Zhen X.H., Ma Q., Tian J.L., Li S., Chen X., Han Q., Duan S., Shen C., Yang F., Xu Z.Z. GPR177 in A-fiber sensory neurons drives diabetic neuropathic pain via WNT-mediated TRPV1 activation. Sci. Transl. Med. 2022;14 doi: 10.1126/scitranslmed.abh2557. [DOI] [PubMed] [Google Scholar]
- 5.Newton V.L., Guck J.D., Cotter M.A., Cameron N.E., Gardiner N.J. Neutrophils infiltrate the spinal cord parenchyma of rats with experimental diabetic neuropathy. J. Diabetes Res. 2017;2017 doi: 10.1155/2017/4729284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva M., Costa-Pereira J.T., Martins D., Tavares I. Pain modulation from the brain during diabetic neuropathy: uncovering the role of the rostroventromedial medulla. Neurobiol. Dis. 2016;96:346–356. doi: 10.1016/j.nbd.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Fan T., Yu Y., Chen Y.L., Gu P., Wong S., Xia Z.Y., Liu J.A., Cheung C.W. Histone deacetylase 5-induced deficiency of signal transducer and activator of transcription-3 acetylation contributes to spinal astrocytes degeneration in painful diabetic neuropathy. Glia. 2023;71:1099–1119. doi: 10.1002/glia.24328. [DOI] [PubMed] [Google Scholar]
- 8.Zhu D., Fan T., Huo X., Cui J., Cheung C.W., Xia Z. Progressive increase of inflammatory CXCR4 and TNF-alpha in the dorsal root ganglia and spinal cord maintains peripheral and central sensitization to diabetic neuropathic pain in rats. Mediat. Inflamm. 2019;2019 doi: 10.1155/2019/4856156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng Y.C., Chiu Y.M., Dai Z.K., Wu B.N. Loganin ameliorates painful diabetic neuropathy by modulating oxidative stress, inflammation and insulin sensitivity in streptozotocin-nicotinamide-induced diabetic rats. Cells. 2021;10 doi: 10.3390/cells10102688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raposo D., Morgado C., Pereira-Terra P., Tavares I. Nociceptive spinal cord neurons of laminae I-III exhibit oxidative stress damage during diabetic neuropathy which is prevented by early antioxidant treatment with epigallocatechin-gallate (EGCG) Brain Res. Bull. 2015;110:68–75. doi: 10.1016/j.brainresbull.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Taïlé J., Bringart M., Planesse C., Patché J., Rondeau P., Veeren B., Clerc P., Gauvin-Bialecki A., Bourane S., Meilhac O., Couret D., Gonthier M.P. Antioxidant polyphenols of antirhea borbonica medicinal plant and caffeic acid reduce cerebrovascular, inflammatory and metabolic disorders aggravated by high-fat diet-induced obesity in a mouse model of stroke. Antioxidants. 2022;11 doi: 10.3390/antiox11050858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teppala S., Shankar A. Association between serum IGF-1 and diabetes among U.S. adults. Diabetes Care. 2010;33:2257–2259. doi: 10.2337/dc10-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashpole N.M., Sanders J.E., Hodges E.L., Yan H., Sonntag W.E. Growth hormone, insulin-like growth factor-1 and the aging brain. Exp. Gerontol. 2015;68:76–81. doi: 10.1016/j.exger.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhalla S., Mehan S., Khan A., Rehman M.U. Protective role of IGF-1 and GLP-1 signaling activation in neurological dysfunctions. Neurosci. Biobehav. Rev. 2022;142 doi: 10.1016/j.neubiorev.2022.104896. [DOI] [PubMed] [Google Scholar]
- 15.Puche J.E., Muñoz Ú., García-Magariño M., Sádaba M.C., Castilla-Cortázar I. Partial IGF-1 deficiency induces brain oxidative damage and edema, which are ameliorated by replacement therapy. Biofactors. 2016;42:60–79. doi: 10.1002/biof.1255. [DOI] [PubMed] [Google Scholar]
- 16.Montivero A.J., Ghersi M.S., Silvero C.M., Artur de la Villarmois E., Catalan-Figueroa J., Herrera M., Becerra M.C., Hereñú C.B., Pérez M.F. Early IGF-1 gene therapy prevented oxidative stress and cognitive deficits induced by traumatic brain injury. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.672392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arroba A.I., Campos-Caro A., Aguilar-Diosdado M., Valverde Á M. IGF-1, inflammation and retinal degeneration: a close network. Front. Aging Neurosci. 2018;10:203. doi: 10.3389/fnagi.2018.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falomir-Lockhart E., Dolcetti F.J.C., Herrera M.L., Pennini J., Zappa Villar M.F., Salinas G., Portiansky E., Spittau B., Lacunza E., Hereñú C.B., Bellini M.J. IGF-1 gene transfer modifies inflammatory environment and gene expression in the caudate-putamen of aged female rat brain. Mol. Neurobiol. 2022;59:3337–3352. doi: 10.1007/s12035-022-02791-w. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds A.N., Akerman A.P., Mann J. Dietary fibre and whole grains in diabetes management: systematic review and meta-analyses. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan F., Bamunuarachchi N.I., Tabassum N., Kim Y.M. Caffeic acid and its derivatives: antimicrobial drugs toward microbial pathogens. J. Agric. Food Chem. 2021;69:2979–3004. doi: 10.1021/acs.jafc.0c07579. [DOI] [PubMed] [Google Scholar]
- 21.Lu L., Luo K., Luan Y., Zhao M., Wang R., Zhao X., Wu S. Effect of caffeic acid esters on antioxidant activity and oxidative stability of sunflower oil: molecular simulation and experiments. Food Res. Int. 2022;160 doi: 10.1016/j.foodres.2022.111760. [DOI] [PubMed] [Google Scholar]
- 22.Mude H., Maroju P.A., Balapure A., Ganesan R., Ray Dutta J. Water-soluble caffeic acid-dopamine acid-base complex exhibits enhanced bactericidal, antioxidant, and anticancer properties. Food Chem. 2022;374 doi: 10.1016/j.foodchem.2021.131830. [DOI] [PubMed] [Google Scholar]
- 23.Paciello F., Di Pino A., Rolesi R., Troiani D., Paludetti G., Grassi C., Fetoni A.R. Anti-oxidant and anti-inflammatory effects of caffeic acid: in vivo evidences in a model of noise-induced hearing loss. Food Chem. Toxicol. 2020;143 doi: 10.1016/j.fct.2020.111555. [DOI] [PubMed] [Google Scholar]
- 24.Muhammad Abdul Kadar N.N., Ahmad F., Teoh S.L., Yahaya M.F. Comparable benefits of stingless bee honey and caffeic acid in mitigating the negative effects of metabolic syndrome on the brain. Antioxidants. 2022;11 doi: 10.3390/antiox11112154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y., Fang S.H., Ye Y.L., Chu L.S., Zhang W.P., Wang M.L., Wei E.Q. Caffeic acid ameliorates early and delayed brain injuries after focal cerebral ischemia in rats. Acta Pharmacol. Sin. 2006;27:1103–1110. doi: 10.1111/j.1745-7254.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y., Wu Q., Zhang L., Wang Q., Yang Z., Liu J., Feng L. Caffeic acid reduces A53T α-synuclein by activating JNK/Bcl-2-mediated autophagy in vitro and improves behaviour and protects dopaminergic neurons in a mouse model of Parkinson's disease. Pharmacol. Res. 2019;150 doi: 10.1016/j.phrs.2019.104538. [DOI] [PubMed] [Google Scholar]
- 27.Khan A., Park J.S., Kang M.H., Lee H.J., Ali J., Tahir M., Choe K., Kim M.O. Caffeic acid, a polyphenolic micronutrient rescues mice brains against aβ-induced neurodegeneration and memory impairment. Antioxidants. 2023;12 doi: 10.3390/antiox12061284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L., Zhang W.P., Chen K.D., Qian X.D., Fang S.H., Wei E.Q. Caffeic acid attenuates neuronal damage, astrogliosis and glial scar formation in mouse brain with cryoinjury. Life Sci. 2007;80:530–537. doi: 10.1016/j.lfs.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 29.Grabska-Kobylecka I., Kaczmarek-Bak J., Figlus M., Prymont-Przyminska A., Zwolinska A., Sarniak A., Wlodarczyk A., Glabinski A., Nowak D. The presence of caffeic acid in cerebrospinal fluid: evidence that dietary polyphenols can cross the blood-brain barrier in humans. Nutrients. 2020;12 doi: 10.3390/nu12051531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Latacz G., Sałat K., Furgała-Wojas A., Martyniak A., Olejarz-Maciej A., Honkisz-Orzechowska E., Szymańska E. Phenylalanine-based AMPA receptor antagonist as the anticonvulsant agent with neuroprotective activity-in vitro and in vivo studies. Molecules. 2022;27 doi: 10.3390/molecules27030875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thibault K., Calvino B., Dubacq S., Roualle-de-Rouville M., Sordoillet V., Rivals I., Pezet S. Cortical effect of oxaliplatin associated with sustained neuropathic pain: exacerbation of cortical activity and down-regulation of potassium channel expression in somatosensory cortex. Pain. 2012;153:1636–1647. doi: 10.1016/j.pain.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Hervera A., Negrete R., Leánez S., Martín-Campos J., Pol O. The role of nitric oxide in the local antiallodynic and antihyperalgesic effects and expression of delta-opioid and cannabinoid-2 receptors during neuropathic pain in mice. J. Pharmacol. Exp. Therapeut. 2010;334:887–896. doi: 10.1124/jpet.110.167585. [DOI] [PubMed] [Google Scholar]
- 33.Wang H., Li Y., Dun L., Xu Y., Jin S., Du J., Ma L., Li J., Zhou R., He X., Sun T., Yu J. Antinociceptive effects of oxymatrine from Sophora flavescens, through regulation of NR2B-containing NMDA receptor-ERK/CREB signaling in a mice model of neuropathic pain. Phytomedicine. 2013;20:1039–1045. doi: 10.1016/j.phymed.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Zhang M.T., Wang B., Jia Y.N., Liu N., Ma P.S., Gong S.S., Niu Y., Sun T., Li Y.X., Yu J.Q. Neuroprotective effect of liquiritin against neuropathic pain induced by chronic constriction injury of the sciatic nerve in mice. Biomed. Pharmacother. 2017;95:186–198. doi: 10.1016/j.biopha.2017.07.167. [DOI] [PubMed] [Google Scholar]
- 35.Wang H., Zhang H., Cao F., Lu J., Tang J., Li H., Zhang Y., Feng B., Tang Z. Protection of insulin-like growth factor 1 on experimental peripheral neuropathy in diabetic mice. Mol. Med. Rep. 2018;18:4577–4586. doi: 10.3892/mmr.2018.9435. [DOI] [PubMed] [Google Scholar]
- 36.Pollari E., Savchenko E., Jaronen M., Kanninen K., Malm T., Wojciechowski S., Ahtoniemi T., Goldsteins G., Giniatullina R., Giniatullin R., Koistinaho J., Magga J. Granulocyte colony stimulating factor attenuates inflammation in a mouse model of amyotrophic lateral sclerosis. J. Neuroinflammation. 2011;8:74. doi: 10.1186/1742-2094-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X., Le Y., Tang S.Q., He W.Y., He J., Wang Y.H., Wang H.B. Painful diabetic neuropathy is associated with compromised microglial IGF-1 signaling which can Be rescued by green tea polyphenol EGCG in mice. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/6773662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng X., Wu G., Zhao A., Huang K., Chai L., Natarajan B., Yang S., Chen H., Lin C. Synthesis of novel caffeic acid derivatives and their protective effect against hydrogen peroxide induced oxidative stress via Nrf2 pathway. Life Sci. 2020;247 doi: 10.1016/j.lfs.2020.117439. [DOI] [PubMed] [Google Scholar]
- 39.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qureshi S., Ali G., Muhammad T., Idrees M., Ullah S., Ali Khan S., Ullah R., Khan R., Ul-Haq Z., Haseeb Mohsin A., Kong I.K. Thiadiazine-thione derivatives ameliorate STZ-induced diabetic neuropathy by regulating insulin and neuroinflammatory signaling. Int. Immunopharm. 2022;113 doi: 10.1016/j.intimp.2022.109421. [DOI] [PubMed] [Google Scholar]
- 41.Wang D., Couture R., Hong Y. Activated microglia in the spinal cord underlies diabetic neuropathic pain. Eur. J. Pharmacol. 2014;728:59–66. doi: 10.1016/j.ejphar.2014.01.057. [DOI] [PubMed] [Google Scholar]
- 42.Myhre C.L., Thygesen C., Villadsen B., Vollerup J., Ilkjær L., Krohn K.T., Grebing M., Zhao S., Khan A.M., Dissing-Olesen L., Jensen M.S., Babcock A.A., Finsen B. Microglia express insulin-like growth factor-1 in the Hippocampus of aged APP(swe)/PS1(ΔE9) transgenic mice. Front. Cell. Neurosci. 2019;13:308. doi: 10.3389/fncel.2019.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hang D., Zeleznik O.A., He X., Guasch-Ferre M., Jiang X., Li J., Liang L., Eliassen A.H., Clish C.B., Chan A.T., Hu Z., Shen H., Wilson K.M., Mucci L.A., Sun Q., Hu F.B., Willett W.C., Giovannucci E.L., Song M. Metabolomic signatures of long-term coffee consumption and risk of type 2 diabetes in women. Diabetes Care. 2020;43:2588–2596. doi: 10.2337/dc20-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jung U.J., Lee M.K., Park Y.B., Jeon S.M., Choi M.S. Antihyperglycemic and antioxidant properties of caffeic acid in db/db mice. J. Pharmacol. Exp. Therapeut. 2006;318:476–483. doi: 10.1124/jpet.106.105163. [DOI] [PubMed] [Google Scholar]
- 45.Dimitriadis G., Parry-Billings M., Bevan S., Dunger D., Piva T., Krause U., Wegener G., Newsholme E.A. Effects of insulin-like growth factor I on the rates of glucose transport and utilization in rat skeletal muscle in vitro. Biochem. J. 1992;285(Pt 1):269–274. doi: 10.1042/bj2850269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woods K.A., Camacho-Hübner C., Bergman R.N., Barter D., Clark A.J., Savage M.O. Effects of insulin-like growth factor I (IGF-I) therapy on body composition and insulin resistance in IGF-I gene deletion. J. Clin. Endocrinol. Metab. 2000;85:1407–1411. doi: 10.1210/jcem.85.4.6495. [DOI] [PubMed] [Google Scholar]
- 47.Haluzik M., Yakar S., Gavrilova O., Setser J., Boisclair Y., LeRoith D. Insulin resistance in the liver-specific IGF-1 gene-deleted mouse is abrogated by deletion of the acid-labile subunit of the IGF-binding protein-3 complex: relative roles of growth hormone and IGF-1 in insulin resistance. Diabetes. 2003;52:2483–2489. doi: 10.2337/diabetes.52.10.2483. [DOI] [PubMed] [Google Scholar]
- 48.Yuan Q., Zhang X., Wei W., Zhao J., Wu Y., Zhao S., Zhu L., Wang P., Hao J. Lycorine improves peripheral nerve function by promoting Schwann cell autophagy via AMPK pathway activation and MMP9 downregulation in diabetic peripheral neuropathy. Pharmacol. Res. 2022;175 doi: 10.1016/j.phrs.2021.105985. [DOI] [PubMed] [Google Scholar]
- 49.Medras Z.J.H., Mostafa Y.M., Ahmed A.A.M., El-Sayed N.M. Arctigenin improves neuropathy via ameliorating apoptosis and modulating autophagy in streptozotocin-induced diabetic mice. CNS Neurosci. Ther. 2023;29(10):3068–3080. doi: 10.1111/cns.14249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gülçin I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid) Toxicology. 2006;217:213–220. doi: 10.1016/j.tox.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 51.Ruggiero M., Calvello R., Porro C., Messina G., Cianciulli A., Panaro M.A. Neurodegenerative diseases: can caffeine Be a powerful ally to weaken neuroinflammation? Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms232112958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsai C.F., Kuo Y.H., Yeh W.L., Wu C.Y., Lin H.Y., Lai S.W., Liu Y.S., Wu L.H., Lu J.K., Lu D.Y. Regulatory effects of caffeic acid phenethyl ester on neuroinflammation in microglial cells. Int. J. Mol. Sci. 2015;16:5572–5589. doi: 10.3390/ijms16035572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.García-Cáceres C., Quarta C., Varela L., Gao Y., Gruber T., Legutko B., Jastroch M., Johansson P., Ninkovic J., Yi C.X., Le Thuc O., Szigeti-Buck K., Cai W., Meyer C.W., Pfluger P.T., Fernandez A.M., Luquet S., Woods S.C., Torres-Alemán I., Kahn C.R., Götz M., Horvath T.L., Tschöp M.H. Astrocytic insulin signaling couples brain glucose uptake with nutrient availability. Cell. 2016;166:867–880. doi: 10.1016/j.cell.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herrera Moro Chao D., Kirchner M.K., Pham C., Foppen E., Denis R.G.P., Castel J., Morel C., Montalban E., Hassouna R., Bui L.C., Renault J., Mouffle C., García-Cáceres C., Tschöp M.H., Li D., Martin C., Stern J.E., Luquet S.H. Hypothalamic astrocytes control systemic glucose metabolism and energy balance. Cell Metabol. 2022;34:1532–1547.e6. doi: 10.1016/j.cmet.2022.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Higashi Y., Sukhanov S., Anwar A., Shai S.Y., Delafontaine P. IGF-1, oxidative stress and atheroprotection. Trends Endocrinol. Metabol. 2010;21:245–254. doi: 10.1016/j.tem.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dyer A.H., Vahdatpour C., Sanfeliu A., Tropea D. The role of Insulin-Like Growth Factor 1 (IGF-1) in brain development, maturation and neuroplasticity. Neuroscience. 2016;325:89–99. doi: 10.1016/j.neuroscience.2016.03.056. [DOI] [PubMed] [Google Scholar]
- 57.Papadimitriou A., Marakaki C., Papadimitriou D.T. Growth variations with opposite clinical outcomes and the emerging role of IGF-1. Trends Endocrinol. Metabol. 2022;33:359–370. doi: 10.1016/j.tem.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 58.Le Y., Chen X., Wang L., He W.Y., He J., Xiong Q.M., Wang Y.H., Zhang L., Zheng X.Q., Wang H.B. Chemotherapy-induced peripheral neuropathy is promoted by enhanced spinal insulin-like growth factor-1 levels via astrocyte-dependent mechanisms. Brain Res. Bull. 2021;175:205–212. doi: 10.1016/j.brainresbull.2021.07.026. [DOI] [PubMed] [Google Scholar]
- 59.Park S.H., Min T.S. Caffeic acid phenethyl ester ameliorates changes in IGFs secretion and gene expression in streptozotocin-induced diabetic rats. Life Sci. 2006;78:1741–1747. doi: 10.1016/j.lfs.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 60.Chen X., Le Y., He W.Y., He J., Wang Y.H., Zhang L., Xiong Q.M., Zheng X.Q., Liu K.X., Wang H.B. Abnormal insulin-like growth factor 1 signaling regulates neuropathic pain by mediating the mechanistic target of rapamycin-related autophagy and neuroinflammation in mice. ACS Chem. Neurosci. 2021;12:2917–2928. doi: 10.1021/acschemneuro.1c00271. [DOI] [PubMed] [Google Scholar]
- 61.Syroid D.E., Zorick T.S., Arbet-Engels C., Kilpatrick T.J., Eckhart W., Lemke G. A role for insulin-like growth factor-I in the regulation of Schwann cell survival. J. Neurosci. 1999;19:2059–2068. doi: 10.1523/JNEUROSCI.19-06-02059.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mahran Y.F. New insights into the protection of growth hormone in cisplatin-induced nephrotoxicity: the impact of IGF-1 on the Keap1-Nrf2/HO-1 signaling. Life Sci. 2020;253 doi: 10.1016/j.lfs.2020.117581. [DOI] [PubMed] [Google Scholar]
- 63.Wang Z., Xiong L., Wang G., Wan W., Zhong C., Zu H. Insulin-like growth factor-1 protects SH-SY5Y cells against β-amyloid-induced apoptosis via the PI3K/Akt-Nrf2 pathway. Exp. Gerontol. 2017;87:23–32. doi: 10.1016/j.exger.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 64.Hossain M.A., Adithan A., Alam M.J., Kopalli S.R., Kim B., Kang C.W., Hwang K.C., Kim J.H. IGF-1 facilitates cartilage reconstruction by regulating PI3K/AKT, MAPK, and NF-kB signaling in rabbit osteoarthritis. J. Inflamm. Res. 2021;14:3555–3568. doi: 10.2147/JIR.S316756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets produced or analyzed during this study can be obtained from the corresponding author upon reasonable request.