Abstract
Despite clinical and scientific advancements, heart failure is the major cause of morbidity and mortality worldwide. Both mitochondrial dysfunction and inflammation contribute to the development and progression of heart failure. Although inflammation is crucial to reparative healing following acute cardiomyocyte injury, chronic inflammation damages the heart, impairs function, and decreases cardiac output. Mitochondria, which comprise one third of cardiomyocyte volume, may prove a potential therapeutic target for heart failure. Known primarily for energy production, mitochondria are also involved in other processes including calcium homeostasis and the regulation of cellular apoptosis. Mitochondrial function is closely related to morphology, which alters through mitochondrial dynamics, thus ensuring that the energy needs of the cell are met. However, in heart failure, changes in substrate use lead to mitochondrial dysfunction and impaired myocyte function. This review discusses mitochondrial and cristae dynamics, including the role of the mitochondria contact site and cristae organizing system complex in mitochondrial ultrastructure changes. Additionally, this review covers the role of mitochondria-endoplasmic reticulum contact sites, mitochondrial communication via nanotunnels, and altered metabolite production during heart failure. We highlight these often-neglected factors and promising clinical mitochondrial targets for heart failure.
Keywords: cardiovascular diseases, heart failure, hypertension, mitochondria, myocardium
Cardiovascular diseases (CVDs), which affect blood vessels or the heart, are the leading cause of death globally,1 accounting for 25% of US deaths.2 Although CVD generally affects the left ventricle, the right ventricle plays a crucial role in cardiovascular diseases, including pulmonary hypertension.3 Other forms of heart disease include valvular heart disease, cardiomyopathy, and arrhythmias. The most common form of heart disease is coronary artery disease, which results from the buildup of plaque in the arteries that supply blood to the heart. Coronary artery disease leads to chest pain, heart attacks, and heart failure (HF),4–6 which is defined by the American Heart Association as a condition in which the heart pumps insufficient blood to meet the body’s needs.7 HF, which presents clinically with or without preserved ejection fraction8 and is the result of cardiomyocyte injury, is characterized by the inability of the heart to fill and expel blood from the left ventricle effectively.7,9 Injury to the myocardium can result from hypertension, diabetes, and coronary artery disease.9 Various types of HF present with different symptoms that can include shortness of breath, fatigue, congestion, and peripheral edema (Figure 1).9,10
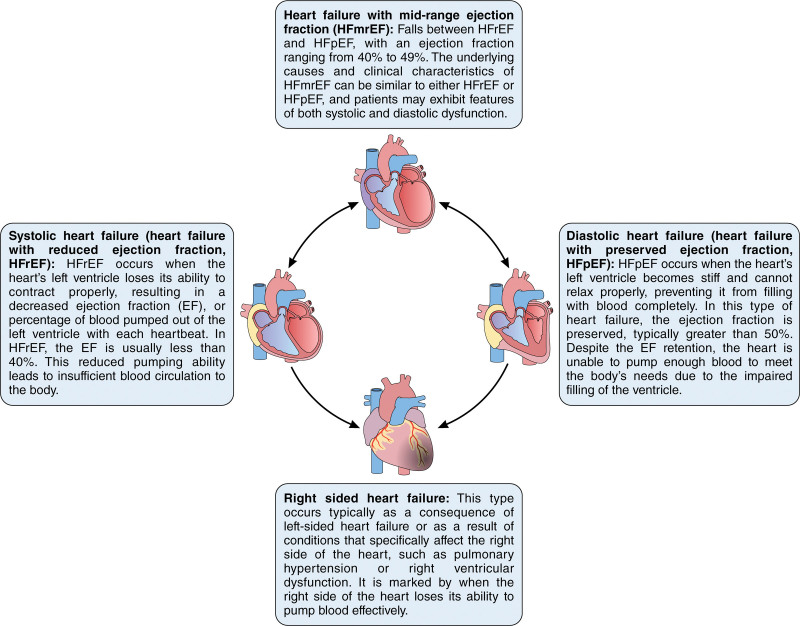
Figure 1.
Overview of the main types of heart failure (HF). This schematic illustration describes HF with reduced ejection fraction (HFrEF), HF with preserved ejection fraction (HFpEF), HF with mid-range ejection fraction (HFmrEF), right-sided HF, and congestive HF. It describes the affected side of the heart, the ejection fraction range, and key symptoms for each type. Understanding the various types of HF is critical for accurate diagnosis and treatment. Illustration credit: Sceyence Studios.
It is important to understand the molecular bases of the various types of HF. CVD increases with age,11 as does mitochondrial dysfunction.12 Many lines of evidence from animal models and human studies implicate mitochondrial DNA (mtDNA) heteroplasmy13 and mitochondrial biogenesis impairment14 occurring before and during early HF,15 suggesting mitochondrial dysfunction is an early hallmark of HF and a potential therapeutic target in CVD.16,17 Reviews that consider mitochondria for the treatment of HF16–22 have focused on mitochondrial dynamics or function. The multifaceted regulation of mitochondrial structure and function23 is poorly understood, especially in the context of age-dependent HF. Tools that allow for this understanding have only been developed and become more widely available within the last 10 years. For example, focus ion-beam scanning electron microscopy (SEM),24 serial block face SEM,25 and correlative light electron microscopy,26 have all undergone significant improvements, which have allowed for 3-dimensional (3D) structures of organelles including mitochondria to be better interrogated in heart development27 and failure.28 3D tomography has also been used to perform morphological analysis of cardiac mitochondria to provide greater insights into ultrastructural changes.29 Concurrent advances in cryo-electron microscopy have enabled the resolution of the mitochondrial oxidative phosphorylation machinery, such as the mitochondrial supercomplex I1III2IV1.30 Outside of microscopy, proteome, and acetylome analyses have accelerated, which have allowed researchers to glean the functional role of specific acetylation sites.31 The study of in vivo freshly excised hearts has used high-resolution respirometry to study respiratory function,32 while mechanistic studies have continued to be guided by broader advancements such as those in CRISPR/Cas9.33 Together, these technologies have continued to bolster our understanding of mitochondria.
Mitochondria are central to many of the signaling cascades involved in HF (reviewed by He et al34). Here, we highlight recent developments and important future directions in the field, including the potential role of mitochondrial dynamics and interactions in HF. We discuss the dynamic nature of mitochondria and cristae and the role of the mitochondria contact site and cristae organizing system (MICOS) complex in these dynamics. We also discuss mitochondrial communication via both mitochondria-endoplasmic reticulum contacts (MERCs) and nanotunnels, as well as HF-associated changes in metabolite production. By taking an interconnected view of mitochondria, we consider areas of further study that may offer greater insights into mitochondrial-mediated roles in HF.
RISK FACTORS OF HF
It is pertinent to study mitochondria in the context of HF as the burden of HF continues to grow and new therapies are necessary. Within the United States, the burden of HF continues to grow, with roughly 6.2 million American adults (ie, 20+ years old) having HF in the span of time from 2013 to 2016, representing approximately a 10% increase over the preceding 3-year period.35 In 2012, this cumulative prevalence represents an estimated cost of over $30 billion.35 Current projections expect this prevalence to only continue growing, nearly by 50%, to reach over 8 million American adults with HF by 2030.35 The probability of developing HF during one’s lifetime varies significantly by race and gender, with estimates ranging from 20% to 42% in men and 24% to 46% in women, highlighting higher risks in Black women, but overall exuberated risk in men.35
HF disproportionately affects racial minorities within the United States: Black people, who are affected by socioeconomic and social factors, such as John Henryism,36,37 have higher rates of hypertension compared with other racial and ethnic groups. Moreover, as a result of disparities in health care access and socioeconomic factors, Black and Hispanic individuals are more likely to have both poor prognoses and outcomes in the context HF.38 Race is a social construct; however, the cumulative effects of intergenerational stressors, hereditary factors, and exposome (ie, the totality of external environmental exposures one experiences throughout their life) factors may change mitochondrial function, leading to an increased risk of HF. Other risk factors for CVD include obesity, diabetes, smoking, a sedentary lifestyle, and poor diet, all of which can be correlated with lower socioeconomic status.35 As a result, while non-Hispanic White women have an ≈1.9% prevalence of HF, this is more than double, at 3.9%, in Black women.35 Although exposome factors have been implicated in affecting mitochondria, it remains unclear the full extent which these factors are involved in mitochondrial regulation.
Generally, men develop heart disease at younger ages than women.11 Risk for the development of CVD in women increases significantly after menopause when they experience a decline in estrogen, a hormone with beneficial effects on blood vessels and lipid metabolism.39–43 In addition to the protective effect of estrogens on blood pressure,44 estrogens may also alter mitochondrial membrane permeability and microviscosity,45,46 concurrently improving mitochondrial 3D shape.47 In general, women with diabetes are at a higher risk for heart disease than men,39–41 suggesting that the protective effects of estrogens premenopause are unable to overcome this risk factor. Yet, while the loss of estrogen in postmenopausal women blunts the sex-dependent difference, unlike many other CVDs, men typically are still at a higher risk of HF in old age.35 This suggests that even in the absence of active estrogen, women have long-lasting cardioprotective effects against HF. However, other factors could conceivably be at play beyond estrogen, and it is still too early to clearly explicate the impact of gender-affirming hormonal treatments on the risks associated with HF.
Age is the greatest risk factor for HF.48 According to the 2013 to 2016 National Health and Nutrition Examination Survey, a small percent of the population from 20 to 30 years old have HF (ie, 0.3% for men and 0.2% for women).35 However, this progressively increases with a more pronounced sex-dependent difference, especially at 60 to 79 years of age (ie, HF prevalence is 6.9% for men and 4.8% for women) and over 80 years old (ie, HF prevalence is 12.8% for men and 12.0% for women).35 Aging may have a further confluence with race, as HF before 50 years of age is more common among Blacks, with Black men of 55 to 64 years old having an incidence of HF of 11.2 per 1000 person-years, which is much higher than White men of the same age (3.9 per 1000 person-years) and even among White men of 65 to 74 years old (11.0 per 1000 person-years).35 Generally, however, with age these differences begin to narrow, while sex-dependent differences remain more pronounced. Hypertension, another risk factor for the development of HF, increases with age.49 Thus, mechanisms associated with aging may provide a key to understanding and treating CVD, particularly the progressive loss of mitochondrial function.50,51 Although there is some dispute concerning the mechanisms associated with aging and mitochondrial dysfunction,52 the escalating decline of mitochondria with age and its potential role in HF has led to greater interest in mitochondria as a therapeutic target for HF.52
MITOCHONDRIAL FUNCTION IN THE HEART
In the 1960s, Mitchell introduced the chemiosmotic hypothesis, proposing a mechanism for ATP synthesis in biological systems, with energy transfer and ATP synthesis in mitochondria, coupled by a transmembrane electrochemical potential gradient.53 We have now come to understand this mechanism as oxidative phosphorylation (OxPhos), the process through which mitochondria respond to the energetic needs of the body, with reactive oxygen species (ROS) as a deleterious byproduct. The heart needs large amounts of energy in the form of ATP to produce contractions strong enough to fulfill systemic demands,54 but ATP storage is low. To overcome storage limitations, mitochondria, the primary producers of ATP,54 comprise ≈30% to 40% of the cardiomyocyte volume. Every day, this large volume of mitochondria produces ≈90% of the ATP that the heart uses, generating 30 kg a day and nearly 880 000 kg across one’s lifespan.55 The other 60% volume of cardiomyocytes is typically occupied by myofibrils,56 which can undergo changes in orientation and size in HF.28
ATP is generated by OxPhos machinery, which primarily consists of 5 complexes. Deficiency of Complex I—a 45 subunit complex that serves as an entry point for electrons through oxidization of NADH, —through inhibition of Ndufs4 decreases respiration and accelerates HF.57 Right-ventricular failure, conversely, is associated with increased levels of Complex II—a direct link between the TCA cycle and the electron transport chain that couples the oxidation of succinate to the reduction of coenzyme Q without pumping protons—which in turn increases oxidative stress, a byproduct of the electron transport chain.58 In interfibrillar mitochondrial, aging decreases complex III activity—transfers electrons from reduced coenzyme Q to cytochrome c via a mechanism that is coupled to proton pumping—leading to ischemia-reperfusion injury due to tandem defects in electron flow and increased oxidant production.59 In HF, unchanged cardiolipin levels in cardiac mitochondria concomitant with increased phosphorylation of complex IV—the terminal electron acceptor that moves electrons from cytochrome c to molecular oxygen while vectorially transporting protons into the intermembrane space—alters oxidative phosphorylation by either hindering the incorporation of complex IV into supercomplexes or reducing their stability.60 Although the extent of OxPhos changes in HF goes beyond these specific examples, a key regulator of mitochondrial cardiomyopathies are mitochondrial mutations which can cause deficiencies through both mutations in the nuclear and mitochondrial genome (reviewed by El-Hattab and Scaglia61).
Mitochondrial DNA (mtDNA) plays a central role in the pathogenesis of human HF. A study from left ventricular tissue from end-stage HF has shown that in a failing heart, mtDNA content is decreased by >40%, with significantly reduced replication, which in turn impairs mitochondrial biogenesis.15 Additionally, mitochondrial DNA depletion is an early sign in right-ventricular hypertrophy during the transition from hypertrophy to failure in patients with congenital heart disease.14 The Atherosclerosis Risk in Communities, a broad study conducted over ≈30 years, showed an inverse relationship between the mtDNA copy number and the risk of incident HF.62 This loss in mtDNA content is preceded by oxidative stress and results in decreased expression of mtDNA-encoded genes and respiratory chain complex enzymes.63 Notably, this oxidative stress only affects mtDNA, not the nuclear genome,63 potentially due to the lack of protective histones in mtDNA, which generally has less clearly defined epigenetic modification.64 Still, this oxidative stress affecting mtDNA has been targeted through MitoQ antioxidants, which can in turn reduce oxidative stress.65
Mutations in mtDNA similarly may contribute to the pathogenesis of HF through the release of cytochrome c.66 However, the cardiac involvement of mutations varies across cohorts and specific mutations (eg, mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes, myoclonus epilepsy with ragged-red fibers, and Kearns-Sayre syndrome),67 so it remains unclear if certain polymorphisms across certain genetic backgrounds may be responsible for the increased risk of HF in certain populations. Notably, Cao et al68 showed that Acsl6 is a genetic determinant of diastolic function, which may confer sex-dependent differences in HF. This involvement of mtDNA mutations in HF has raised mtDNA repair mechanisms as potential therapeutic targets (reviewed by Marín-García69). Nuclear genomics are equally important, as recent results have demonstrated that IFIT3, XAF1, RSAD2, and MX1 are all mitochondrial-related genes that serve as biomarkers for HF.70
HETEROGENEITY IN HF
In addition to ATP production, mitochondria play a vital role in cellular metabolism, calcium (Ca2+) homeostasis, lipid synthesis, and redox regulation in the myocardium.54 Altered metabolism, a principal pathomechanism of human HF, and its relevance to the treatment of HF have been reviewed extensively.22 This altered metabolism can confer heterogeneity in HF across regions and types of HF.
There are several types of HF (Figure 1), including HF with preserved ejection fraction (ie, diastolic HF) and HF with reduced ejection fraction (ie, systolic HF). Although over 50% of patients have diastolic HF, in both of these types of HF, mitochondrial function is central to the pathogenesis, with abnormal myocardial stiffness in diastolic HF and impaired energy production in systolic HF.18,71 In HF with preserved ejection fraction, oxidative stress is a key regulator with increased proinflammatory and pro-fibrotic signaling, myocardial fibrosis, and altered Ca2+ handling.72 Furthermore, in diastolic HF, impaired mitochondrial function and altered TCA cycle metabolites are significantly associated with a protein hyperacetylation pattern, which was effectively ameliorated by nicotinamide riboside supplementation.73 In a mouse model of cardiac hypertrophy, reduced mitochondrial oxidative metabolism led to an energy deficit, potentially driving the progression from hypertrophy to systolic HF.74 Yet, it remains unclear if mitochondrial function is directly correlated to ejection fraction. Knez et al75 found a correlation between mtDNA content and ejection fraction in ion-ischemic HF. Another study found that cardiac phosphocreatine/β-ATP ratios, while inversely related to age, was not significantly linked to ejection fraction.76 Thus, it remains an open area of research to understand whether distinct hallmarks of mitochondrial dysfunction are associated with specific types of HF and can serve as a biomarker of relative ejection fraction.
Cardiomyocytes have heterogeneous populations of mitochondria with discrete roles.77,78 The organization of mitochondria within the heart include intermyofibrillar, subsarcolemmal, or perinuclear locations (Figure 2). Generally, HF may cause mitochondrial structure to change on the basis of these mitochondrial subpopulations.79 Heterogeneity also arises on the basis of cardiac region. Interestingly, differences in the rates of aging of various tissues affect the function of the heart and other organs79; thus, mitochondria may exhibit different characteristics in HF tissues compared with other organs. Based on 3D analysis, murine cardiac tissue mitochondria display different phenotypes than their murine skeletal muscle counterparts during aging.80 An earlier review focused on the role of mitochondria in regulating neonatal cardiomyocyte maturation, which may be due to the unique mitochondrial network that forms in cardiac tissue.81 Because energy changes may differ by heart tissue subtype and by region-specific mitochondria, mitochondrial fusion, and fission proteins could be expressed differently in each region. For example, the endocardium (ie, the inner lining of the heart chambers), the myocardium (ie, the thick, muscular middle layer responsible for the heart’s contraction), and the pericardium (ie, protective double-layered sac encasing the heart)82 may functionally express different dynamics components that impact mitochondrial function and distribution. In particular, the elastic pericardium plays important roles in systolic function, hemodynamics, and ventricular function.83 Conversely, endocardial smooth muscle cells may play a role in reducing left ventricular wall stress and systolic dysfunction.84 Because we do not know mitochondrial structures across the entire geography of the heart and whether there exist correlations between a given structure and how that area pumps blood, we do not understand how the structural and functional relationships depend on unique signatures of aging in the various cardiac regions. Understanding these differences is crucial to developing targeted therapies for HF.
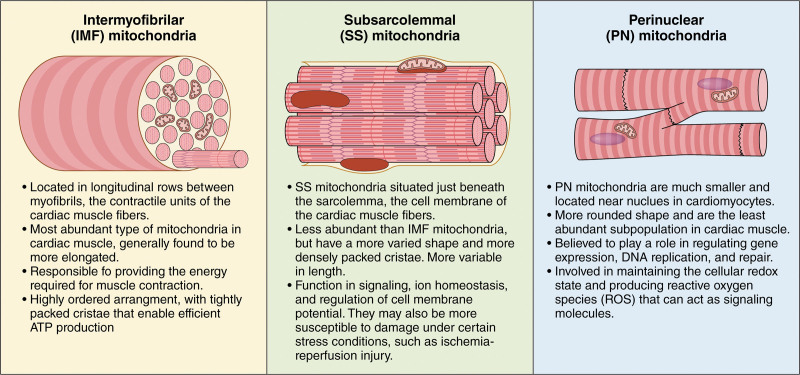
Figure 2.
Distribution and characteristics of mitochondrial subpopulations in cardiac muscle fibers. Mitochondria in cardiac muscle fibers include intermyofibrillar (IMF), subsarcolemmal (SSL), and perinuclear (PN) mitochondria. Each has a distinct relative abundance, location within the cardiac muscle cell, and structural features. Characterizing the differences among these mitochondrial subpopulations is crucial to understanding the impact of mitochondrial dysfunction in cardiac diseases and developing targeted therapies to improve cardiac function. Illustration credit: Sceyence Studios.
DYNAMIC NATURE OF MITOCHONDRIA
Mitochondrial dynamics refers to the coordinated, continuous cycles of fusion and fission85–87 as mitochondria respond to the changing energy demands of the cell. Mitochondrial morphology is intricately linked to function88; thus, mitochondrial dynamics affect HF. Mitochondrial fusion pools mtDNA, preserving the mitochondrial genome, and contributes to the maintenance of mitochondrial OxPhos machinery.88,89 Conversely, mitochondrial fission facilitates the elimination of damaged mitochondrial components through autophagy, also called mitophagy, regulates mitochondrial size and energy distribution, and modulates mitochondrial quality control.85,90 A healthy mitochondrial network balances fusion and fission cycles,88 and identifying the regulators of these cycles may provide therapeutic targets for HF.
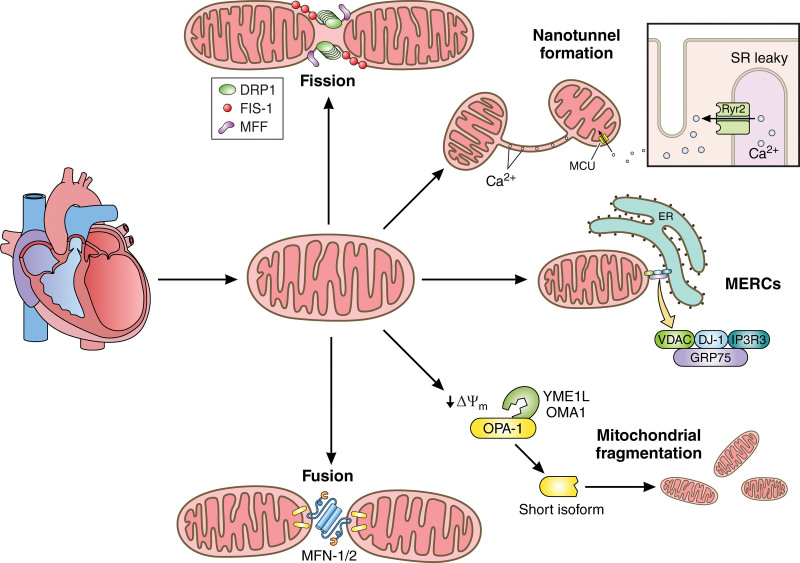
A key hallmark of the failing heart is the dysregulation of mitochondrial dynamics. There is generally an increase in levels of fission-associated proteins (eg, DRP1 [dynamin-related protein 1]) and a decrease in levels of fusion-associated proteins (eg, MFN2 (mitofusin 2) and OPA1 [optic atrophy 1]).91 The fusion of outer versus inner membranes of 2 mitochondria is mediated by different proteins. Fusion of the outer mitochondrial membrane (OMM) depends on conformational changes and GTP-dependent dimerization of 2 dynamin-like proteins, mitofusin 1 (MFN1) and MFN2, which are reduced across aging.88,92,93 This is counterbalanced by fission, which is typically mediated by DRP1 . The absence of MFN1 or MFN2 leads to distinct types of fragmented mitochondria.94 Cells lacking both MFN1 and MFN2 have severe cellular defects, including impaired growth, mitochondrial membrane potential heterogeneity, and reduced cellular respiration.95 In HF, there is decreased expression of the mitochondrial dynamics regulator MFN2 (eg, in diabetic mice), leading to excessive mitochondrial fission that is linked to the development of HF in rats and humans with pulmonary arterial hypertension.96 As previously reviewed, this results in a shift in the MFN2:DRP1 ratio toward pro-fission states in HF.97 As discussed, beyond shaping mitochondria, these dynamics-related dysfunctions also alter the structure of cristae, in turn disrupting ATP production.98 Although excessive fission drives decreased respiration, inhibition of this fission may in turn restore ATP production, improve cardiac fractional output, and reduce mitochondrial dysfunction, cumulatively protecting against HF.99 Yet, the complete ablation of DRP1 causes dilated cardiomyopathy, since fission is necessary for the distribution of mtDNA nucleoids, protein-bound structures that contain active mtDNA.100 Thus, there must be a careful balance of fusion and fission in the heart.
Fusion of the inner mitochondrial membrane (IMM) is facilitated by OPA1, a membrane-bound GTPase.101 OPA1 is highly vulnerable to mutations, which can in turn increase ROS, reduce mtDNA content, and increase the risk of cardiomyopathy.102 OPA1 is decreased in HF102 likely by a posttranscriptional mechanism.21 Fusion requires partial proteolytic cleavage of OPA1, yielding a soluble short form and a long transmembrane form.103,104 Both forms of OPA1 also contribute to the formation of the MICOS complex that regulates the width of cristae.105 An imbalance of mitochondrial fusion versus fission can lead to devastating alterations in mtDNA and trigger cellular apoptosis.102 Proteolytic processing of OPA1 by 2 ATP-dependent metalloproteases, YME1L (YME1 Like 1 ATPase) and OMA1 (OMA1 zinc metallopeptidase), results in the inhibition of IMM fusion.103,106,107 OPA1 also regulates cristae morphology and mitochondrial fragmentation.80,102,108–111 Overexpression of OPA1 decreases mitochondrial fission, inhibiting autophagy.108 Conversely, low OPA1 caused by its excessive processing by the overlapping stress-activated proteolytic activity of OMA1,110 inhibits fusion, resulting in mitochondrial fragmentation102,109 that leads to cell death and eventually heart disease.110 Similarly, samples from human patients with HF show reduced OPA1 expression, which correlates with mitochondrial fragmentation,21,88 and a reduction of OPA1 increases the risk for cardiomyopathy due to decreased ATP production.102
Importantly, imbalanced OPA1 processing, leading to mitochondrial fragmentation, contributes to dilated cardiomyopathy and HF, while preventing mitochondrial fragmentation by deleting OMA1 protects against these cardiac issues.112 OMA1 also protects against cardiomyopathy through the mitochondrial stress response, which protects against ferroptosis and associated lipid peroxidation–dependent impairments of OxPhos.113 When activated by mitochondrial stress, OMA1 also cleaves DELE1 (DAP3 binding cell death enhancer 1), leading to heme-regulated inhibitor (HRI)-mediated eIF2α (eukaryotic initiation factor 2 alpha) phosphorylation and ATF4 (activating transcription factor 4) translation.114 We have recently found that OPA1 action in forming contact sites occurs in an ATF4-dependent manner,115 showing that OMA1 may further affect OPA1 downstream of DELE1 cleavage. Similarly, this also involves OMA1 as a novel regulator of the integrated stress response,116 which may in turn effect dynamics through mitochondrial contact site formation with endoplasmic reticulum. Finally, OMA1 may also provide an alternative mode of fragmentation: in the context of a stress-inducing CHCHD10 mutation, OMA1 facilitates mitochondrial fragmentation alongside alterations in the electron transport chain (ETC).117 However, there is little information on the differential expression of the isoforms of OPA1 in HF or whether changes in YME1L and OMA1 proteases contribute to alterations in mitochondrial structure and function in HF. The development of an immunoblotting technique that differentiates OPA1 isoforms118 may help to determine the role of isoforms in heart disease, as certain isoforms, such as 1 and 7, may confer a protective phenotype.107
Mitochondrial fission occurs via 2 mechanisms. The first mechanism involves the recruitment of DRP1 to the OMM, where it binds to adaptor proteins and oligomerizes around mitochondria, driving membrane constriction.119,120 However, as the diameter of helical DRP1 is insufficient to drive the initial constriction,121 actin filament nucleators at mitochondria-ER Contact sites (MERCs) facilitate preconstriction of the mitochondrial membrane, which is required for DRP1 localization to the site of scission.121 The second pathway involves the phosphorylation of DRP1, which occurs at serine (S)40, S44, S579, S585, S616, S637, S656, and S693.122–124 Phosphorylation at S616, mediated by mitogen-activated protein kinases, Cdk1 (cyclin-dependent kinase 1), and Ca2+/CaMKII (calmodulin-dependent protein kinase II),125,126 is associated with increased DRP1 activity and mitochondrial fission. Similarly, phosphorylation at S693 is mediated by GSK-3β (glycogen synthase kinase 3β) and promotes DRP1 activation and mitochondrial fission. Interestingly, β-amyloid plaques increase GSK-3β activation, thus, promoting mitochondrial fission, a hallmark of Alzheimer disease.127 DRPl, in response to pressure overload-induced cardiac hypertrophy, is translocated to mitochondria which coincides with the activation of mitochondrial autophagy; DRP1 haploinsufficiency exacerbates mitochondrial dysfunction and HF, presumably due to impaired turnover of dysfunctional mitochondria.128
Beyond these proteolytic processing units, a multitude of other proteins are emerging as regulators of fusion and fission. Stress-induced mitochondrial hyperfusion, which represents a compensatory mechanism to increase ATP, requires concurrent expression of SLP-2, MFN1, and long-isoforms of OPA1.129 Mitochondrial elongation factors 1 and 2 (also known as MiD51 [mitochondrial elongation factor 1] and MiD49 [mitochondrial elongation factor 2]) recruit DRP1, with slightly distinct effects due to Mid49 having a stronger affinity toward binding Drp1 (dynamin-related protein 1/dynamin-1-like protein).130 How the recruitment of DRP1 by MiD51 or MiD49 impacts mitochondrial dynamics is controversial. It was reported that MiD49- and MiD51-mediated recruitment of DRP1 to the mitochondrial surface inhibited DRP1’s ability to initiate fission and caused mitochondrial elongation.130 Contrarily, MiD51 and MiD49 were shown to recruit DRP1 to initiate fission in the absence of other fission receptors.124 Although not studied in HF, in pulmonary arterial hypertension, MiD49 and MiD51 are upregulated leading to increased fission.131 Another promoter of DRP1-recruitment, mitochondrial FIS1 (fission 1 protein), has been shown to drive to septic cardiomyopathy upon engagement with DRP1.132 Notably, FIS1 displays a different type of fission than MFF (mitochondria fission factor)-mediated fission, which govern only midzone fission.133 Thus, the roles of MFF, FIS1, MiD49, and MiD51 in HF must be clarified. Additionally, posttranslational modifications are important. Phosphoglycerate mutase family member 5 (PGAM5) is an emerging posttranslational modifier that dephosphorylates MFN2 to drive mitochondrial network formation.134 In the context of cardiac ischemia-reperfusion injury, MFN2 and OPA1 levels are increased in cardiac-specific PGAM5 knockout mice alongside the retained phosphorylation of DRP1 at Ser-637.135
Activation of DRP1 by phosphorylation and the accompanying fission-induced mitochondrial fragmentation are linked to cardiac dysfunction and HF.136 DRP1 can be phosphorylated at sites that include Ser-40, Ser-44, Ser-579, Ser-585, Ser-592, Ser-616, Ser-637, Ser-656, Ser-693, with particular attention paid to S637 and S656.122 Phosphorylation at S637, mediated by a cAMP-dependent protein kinase, inhibits DRP1 activity and promotes mitochondrial fusion.136 Dephosphorylation of S637 by calcineurin, a Ca2+-dependent phosphatase, enhances DRP1 activity and mitochondrial fission.136 In ischemia-reperfusion, dephosphorylation at S637 contributes to left ventricular dysfunction137; thus, calcineurin may play a role in the pathophysiology of HF. PGAM5 deletion can inhibit dephosphorylation at S637 additionally, despite inverse increases in fusion proteins, showing a slightly paradoxical role.135 Similarly, phosphorylation at S656 by AMPK (AMP-activated protein kinase) activation inhibits DRP1 activity and mitochondrial fission,138 which serves as a potential mediator for endoplasmic reticulum (ER) stress. Interestingly, during HF, a shift from AMPKα2 to AMPKα1 expression correlates with the prevention of mitochondrial fragmentation.139 Although different functions are associated with the various phosphorylation sites, HF-associated differences in phosphorylation sites are not well described. Additionally, as previously reviewed,140 DRP1 can be SUMOylated, which provides a protective role in ischemia-reperfusion injury,141 and S-palmitoylated, which is necessary for DRP1 mitochondrial translocation.142 Thus, diverse DRP1 posttranslational modifications regulate mitochondrial dynamics in myriad ways. Further research is needed to understand the roles of site-specific DRP1 phosphorylation and dephosphorylation in the heart and whether pathways that promote or inhibit fission run in tandem during HF, modulating the fission responses in cardioprotective environments.
Although OPA1 and DRP1 are the main focus of studies on mitochondrial dynamics, modulating factors may be equally important. For example, it is not known whether the rates of FIS1 and MFF-mediated fission change in HF, which could explain how disruptions in the fusion-fission balance in HF143 lead to bioenergetic dysfunctions (Figure 3). Equally, there are other, often-neglected regulators in mitochondrial dynamics which have important roles in HF pathology. Although not required for fission, deletion of Mitochondrial Fission Process 1 in the heart leads to dilated cardiomyopathy, mitochondrial defects, and increased sensitivity to cell death.144 Additionally BNIP3, through its multifaceted molecular interactions including MICOS, alters metabolic pathways in HF with reduced ejection fraction.145 In brain aging, BNIP3 has unresolved roles in healthy aging by modulating mitochondrial dynamics.146 Additionally deficiency in Prohibitin 2, an important modulator of mitochondrial dynamics, causes impairment of fatty acid oxidation concomitant with HF progression.147 Finally, overexpressing transmembrane protein 135 causes increased mitochondrial fragmentation and ER stress antecedent to cardiac abnormalities,148 suggesting an alternative mechanism through which fission may be modulated in the absence of DRP1 (reviewed by Beasley et al149). Many dynamics proteins are thus involved in the pathogenesis of HF, potentially modulating the relationship between HF progression, mitochondrial dynamics, and altered HF metabolism.
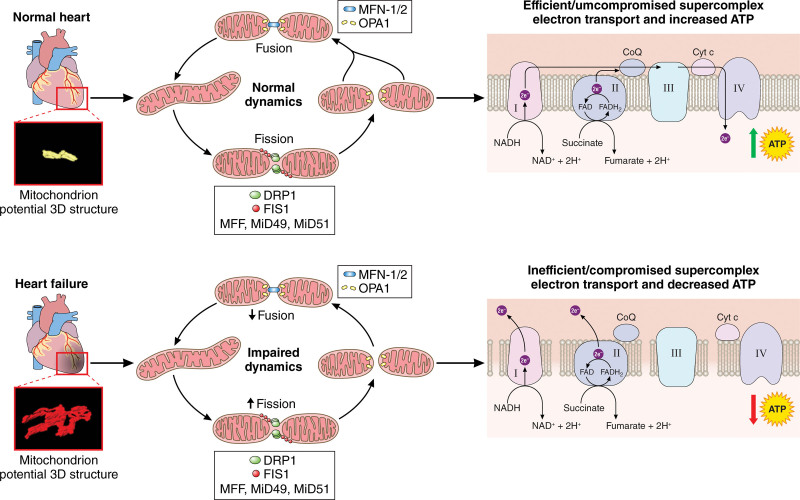
Figure 3.
Biopsies from normal vs failing heart tissue. The schematic illustrates the dysfunction in mitochondrial dynamics that disrupts ATP production. Although results have shown varied changes in structure and dynamics in heart failure (HF), the schema demonstrates commonly reported changes. From our previous 3-dimensional (3D) reconstruction, we found normal mitochondrial structure in healthy cardiac tissue, indicating proper fusion and fission dynamics; however, in HF, there are complex, altered mitochondrial structures, while other studies have also reported fragmentation. The dysregulation of key fusion and fission mitochondrial proteins, such as OPA1 (optic atrophy 1) and DRP1 (dynamin-related protein 1), in failing cardiac tissue results in disruption of the electron transport chain. Commonly this is an uptick in fission and decrease in fusion, but previous results show varied dynamic changes. Restoring phenotypically normal mitochondria may also restore ATP production and reduce cardiomyopathy. MiD4 indicates mitochondrial elongation factor 2; and MiD51, mitochondrial elongation factor 1. Illustration credit: Sceyence Studios.
TARGETING MITOCHONDRIAL DYNAMICS
As extensively reviewed, targeting mitochondrial dynamics is highly promising across numerous pathologies (reviewed in Refs.18,150–152). To briefly summarize some therapies that modulate dynamics in the context of HF, long-term therapy with elamipretide (ie, Szeto-Schiller peptide SS-31 or Bendavia) has been shown to reverse HF-dependent changes in dynamics and biogenesis.91 Elamipretide treatment significantly increases the long Opa1:short Opa1 isoform ratio compared with controls, resulting in a reduction of mitochondrial fragmentation in fibroblasts from patients with dilated cardiomyopathy with ataxia syndrome.153 Although not directly modifying mitochondrial structure, pyrroloquinoline quinone can protect against HF by altering Ca2+ overload (see Disrupted Calcium Homeostasis and ROS Production in HF section) through increased biogenesis.154 Mitochondrial-mimetic therapy, represented by the bioactive conjugate TPT encapsulated in nanomicelles, plays roles in effectively regulating mitochondrial dynamics.155 In a rat model of HF, Neuregulin-1 was suggested to protect against mitochondrial dysfunction by maintaining its structural integrity.156 Finally, while mdivi-1 is a standard DRP1 inhibitor, Drpitor1, and Drpitor1a inhibit fission with higher potency and exert cardioprotective properties in the context of cardiac ischemia-reperfusion injury.87 As treatments continue to develop, there are several additional promising avenues to pursue.
As ROS accumulate during aging,157 antioxidants may protect against HF. Antioxidants such as MitoQ158 mitigate murine age-related aortic stiffness, a predictor of CVD. ROS pathways may be intrinsically linked to mitochondrial dynamics, as mutations in Opa1 can lead to an increase in ROS.159,160 HF is associated with increases in the cardiomyocyte oxidative stress state,161 and an increase in ROS is accompanied by decreased antioxidant capacity in HF mitochondria.162,163 Loss of DRP1 or downregulation of Fis1 results in abnormally elongated mitochondria as fission is blunted,124 but overexpression of Fis1 induces cellular apoptosis, demonstrating the importance of mitochondrial dynamic homeostasis.20 Maintenance of normal amounts of mitochondrial dynamic proteins and their associated machinery may inhibit ROS,159,160 but FIS1 can also be modulated by ROS,160 indicating their complex but poorly understood interdependence. Although FIS1 and MFF are independently predictive of fission pathways,133 it is not known whether these pathways are involved in alternative ROS generation. Thus, it will be important to determine whether an imbalance in mitochondrial dynamics is a cause or effect of the damaged myocardium.90 This may allow us to target ROS to mitigate HF through modulation of mitochondrial dynamics.
Potential therapeutic candidates that directly target mitochondrial dynamics include LARP7 (La ribonucleoprotein domain family member 7), a master regulator of the RNA polymerase II pausing pathway and the DNA damage response.164 LARP7 is decreased in patients with HF and mitochondrial dynamics are impaired in LARP7 deficient cardiomyocytes.164 Notably, the reintroduction of LARP7 may restore bioenergetics and associated dynamics. Other possible targets to improve mitochondrial dynamics include linoleic acid,165 voltage-dependent anion channels,166 and the transcription factor PRDM16 (PR-domain containing 16).167 However, many pathways can alter mitochondrial dynamics. In noncardiac tissue, estrogen receptor-α has been linked to increases in blood pressure,43 and treatment with 17β-estradiol restores mitochondrial dynamics.46 To reveal the full complement of possible therapeutic targets to mitigate HF damage, it is essential to explicate mitochondrial dynamics changes in gene knockouts across a variety of model organisms.168
CRISTAE DYNAMICS AND THE MICOS COMPLEX IN HF
Mitochondria have 4 subcompartments separated into the OMM, the intermembrane space, the IMM, and the matrix.121 The IMM is further separated into the inner boundary membrane that runs parallel to the OMM, and the IMM invaginations, called cristae,121 that house the oxidative phosphorylation machinery.86 High-resolution electron microscopy has shown that cristae, like mitochondria, undergo dynamic morphological cycles to accommodate the changing needs of the cell.169,170 Cristae dynamics refers to the morphological changes of cristae in response to energetic, developmental, or physiological cues.171 Altered cristae morphology can modulate oxidative phosphorylation efficiency by changing the spatial arrangement of respiratory chain components. The sites where the OMM, IMM, and cristae coalesce are cristae junctions, which are formed and maintained by the components of the MICOS complex.121 The mammalian MICOS complex comprises 7 subunits,MIC60, MIC27, MIC26, MIC25, MIC19, MIC13, and MIC10 (reviewed by Kozjak-Pavlovic172)—distributed in the MIC10 and MIC60 subcomplexes, named for their core components.173,174 In addition to maintaining cristae morphology, cristae junctions serve as diffusion barriers for metabolites and proteins,171 which suggests that they might also maintain the IMM pH gradient and limit the diffusion of ADP/ATP.171
The MIC10 and MIC60 subunits, which mediate the membrane bending that is needed to form cristae junctions, are the best-characterized MICOS complex components (reviewed by Anand et al175). The loss of MIC60 results in a loss of cristae architecture, resulting in the formation of membrane stacks.176 This is accompanied by a decrease in the expression and destabilization of all components of the MICOS complex. The loss of MIC10 results in aberrant cristae morphology and the components of the MIC10 subcomplex are reduced, while the MIC60 subcomplex remains largely unaffected.171–174,177 MIC13, which bridges the MIC10 and MIC60 subcomplexes, is also a key MICOS subunit. Although MIC13 knockdown results in destabilization of the MIC10 subcomplex,175,178 MIC60 subcomplex is largely stable in the absence of MIC13.173,179,180 In addition, the MICOS complex interplays with cardiolipin, a signature phospholipid residing in the IMM (reviewed in Refs. 181, 182). Specifically, MIC27 binds to cardiolipin.183 Double knockout of MIC26 and MIC27 reduces cardiolipin levels and destabilizes respiratory complexes.180 These studies further highlight the crucial role of the MICOS complex in maintaining optimal mitochondrial respiratory function. The other MICOS subunits may also have roles unrelated to the cristae organization. For example, in addition to maintaining cristae morphology, MIC19 and MIC25 regulate key mitochondrial dynamic proteins, such as DRP1 and OPA1.172,184,185 However, OPA1 also has nonfusion mitochondrial roles; not only do endogenous OPA1 and MIC60 interact, OPA1 deletion results in increased amounts of MIC60 in mouse embryonic fibroblasts.186 OPA1 is epistatic to MIC60;187 however, MIC60 also has a unique role in the nucleoid (ie, active mtDNA) distribution.173,188 Future studies should determine the functional importance of the OPA1-MIC60 association and whether this interaction is affected by HF.
Because the MICOS complex is vital to the maintenance and integrity of cristae, it is important to understand how this complex changes in disease. The MICOS complex facilitates the folding of cristae, which increases their surface area to maximize cellular respiration.185 The density and morphology of cristae depends on the energy demands of the cell.189 Cardiomyocytes and skeletal muscle cells have more densely packed stacks of cristae than less energetically demanding tissues.185 Mitochondrial and cristae degeneration correlate with impaired heart function in patients with mitochondrial cardiomyopathy arising due to an mtDNA mutation.190 Furthermore, Hypertrophic Cardiomyopathy multi-omics profiling showed decompensation concomitantly with reduced cristae density.191 Aberrant cristae morphology can affect the heart pluralistically in cardiomyocytes, dysregulated cristae led to declines in the mitochondrial network concomitant with the overproduction of mitochondrial ROS, resulting in increased myocardial size.192 Thus, it is possible that cristae abnormalities in muscle cells, like in cardiomyocytes, increase disease severity and the risk of complications compared with similar abnormalities in less energetically demanding environments. However, it is unclear whether cristae density and morphology differ in different regions of cardiac tissue or whether cristae density can serve as a diagnostic predictor of cardiac dysfunction.
Cristae remodeling also controls other critical cellular processes; for example, during apoptosis, cristae junctions widen for the release of cytochrome c from the intercristal space.171 In addition, human diseases, including diabetes and cardiomyopathy, are associated with altered expression of MICOS complex components.171,185 Rare variants in MIC19 and MIC25 are associated with hypoplastic left heart syndrome.193 Consistent with a causal role, depletion of Mic19 in the Drosophila heart compromises heart function, decreases ATP, and results in the accumulation of mitochondrial aggregates, suggestive of altered mitochondrial dynamics.193 In mammals, knockout of either MIC10 or MIC60 results in a reduction in maximal mitochondrial respiration.171,173,185 However, it is not known whether there are differences in MICOS complex expression across different types of HF (Figure 1) or whether only certain MICOS complex subunits are essential for stability in HF. However, mitochondrial dysfunction and decreases in ATP production are both hallmarks of the pathophysiology of HF20; thus, understanding the maintenance of cristae integrity will be vital in developing potential therapeutics for HF.90
A critical player in apoptosis is the Bcl-2 family member, Bid, which also regulates metabolism and DNA damage.194 Following cleavage by caspase-8 during apoptosis, tBID facilitates the rearrangement of cristae and the permeability of the OMM.194 The loss of BID leads to the loss of most cristae, with some aberrant cristae that remain. In Bid−/− mice, the induction of acute cardiac stress amplifies the disordered cristae phenotype and results in cardiac dysfunction.194 Stressed Bid−/− mice have a lower threshold for developing HF from unmet energetic demands. Moreover, Bid−/− acute cardiac stress mice also have a reduced ejection fraction and an increased diameter of left ventricles.194 Although tBID is not MICOS specific, there is a link between cristae and HF. Therefore, understanding the proteins involved in cristae dynamics may identify potential therapeutic targets for HF.
To better understand how HF affects bioenergetics, we need highly resolved cristae structures. There are 2-dimensional 195 and 3D reconstructions of cristae for quantitative evaluation, including volume. We have characterized cristae and mitochondrial 3D structural changes using a knockout of the MICOS complex subunits MIC19, MIC25, and MIC60 in fibroblasts,80 which parallels age-related changes;80,111,196 however, it is not clear whether these changes also exist in HF. We have also developed a method for quantifying and scoring 3D cristae structures.196 This technique can provide measurements of cristae that can be correlated with biochemical and bioenergetic changes in the MICOS complex and mitochondria, respectively, that may occur in HF.172 Currently, volume microscopy techniques, including serial block-face SEM, focused ion-beam SEM, and automated tape-collecting ultramicrotome SEM are commonly used to measure 3D structures, as described and compared previously.24,25,197–199 Emerging techniques such as correlative light electron microscopy26 and cryo-soft x-ray tomography, which have been used for 3D analysis of cristae,200 will continue to expand our understanding of cristae structure. However, there are few studies using these modern tools on HF samples.
UNDERSTANDING MITOCHONDRIAL SHAPES DURING HF
In HF, mitochondria become fragmented or swollen, are heterogenous, and lose cristae (reviewed by Daghistani et al201). We found that in aging mice, cardiac mitochondria adopt a more compact phenotype,111 suggesting that mitochondrial changes in HF are independent of their age-related changes. Therefore, it is important to understand changes in mitochondrial structure and the functional implications of these changes in HF. In sheep HF, 3D rearrangement of the sarcoplasmic reticulum (SR) alters mitochondrial organization.202 In our 3D structure studies of human HF mitochondria, we found that mitochondrial volume increases, as does mitochondrial complexity, with diverse phenotypes that include increased branching, mitochondrial donuts, and nanotunnels28 with concomitant shifts in myofibril orientation and quantity.28 Similarly, the 3D structure of intercalated discs, involved in communication across cardiac myocytes, is altered in HF.203 However, the small sample size and the heterogeneity across and within patient samples in our study28 underscores the need for further analysis of how HF across various models affects the frequency of mitochondrial phenotypes. Although this study analyzed intermyofibrillar mitochondria in scarred tissue, it is not clear whether HF affects mitochondrial structure equally across the heart and regions of cardiac tissue.
Mitochondria can adopt 8 distinct phenotypes that may be expressed concomitantly, including small or large, indicating relative size for ATP synthesis, and elongated or compact, indicating relative surface area for organelle interactions (reviewed Glancy et al88). Thus, it is important to view mitochondria holistically, considering the interplay between mtDNA mutations, structure, and bioenergetics that all play a role in HF.
While typically arising in liver, megamitochondria are adaptive responses to reduce intracellular ROS, yet if megamitochondria are overwhelmed by an excess of free radicals, an apoptotic signaling cascade occurs.204 Unusually large megamitochondria may affect cellular energy metabolism by reducing ETC activity.204 In a cardiomyopathy case study of a patient at risk for HF, SEM revealed abnormal mtDNA that resulted in pleomorphic megamitochondria in the myocardium;205 however, this contrasts with several studies that found fragmented mitochondria in HF across several regions.201 While some of this variation may be due to interregional variability, it also suggests that megamitochondria may represent a stress-adaptive mechanism in only certain circumstances. Ang II‐induced cardiac damage has similarly been demonstrated to cause the formation of megamitochondria in vivo.206 Similarly, knockout of Klf15, which plays an important cardioprotective role in myocardial growth and remodeling, causes an increase in megamitochondria formation in cardiomyocytes.207 Cumulatively, while discussed more in depth in past reviews,208 it is clear that the pluralistic formation mechanisms and roles of megamitochondria in HF remain poorly explicated.
Mitochondria undergo structural changes associated with aging,88 such as donut-shaped mitochondria in monkey brain tissue, which are a hallmark of cognitive decline.47 In our study of mitochondria in human HF, we observed donut-shaped mitochondria28 but no megamitochondria. DRP1-associated fission factors, including MFF and FIS1, may be differentially expressed in various cellular conditions, such as in neurodegeneration where mitochondrial dynamics are dysregulated,133,209 providing a possible path to selecting mitochondrial phenotypes. For example, it is possible that spatial distribution of these proteins is a determinant in phenotype formation. Similarly, alterations in fission associated with HF may alter mitochondrial phenotypes. HF modulation of brain function via changes in blood flow due to reduced cardiac efficiency has been studied,210 but the brain-heart interface may also influence mitochondrial structure,210 which remains understudied. The donut-shaped mitochondria that result from hypoxia-reoxygenation stress and related alterations in OPA1 levels211 suggest at least 1 mechanism by which HF affects mitochondrial structure in distinct tissue regions.
Immobilized mitochondria develop nanotunnels that allow the transfer of materials between 2 mitochondria212,213 and serve as a means of communication through thin double-membrane protrusions that involve both the IMM and OMM. Nanotunnels, which are associated with Ca2+ transfer, form in response to impaired homeostasis,213–215 and nanotunnels increase in cells with genetic mitochondrial defects and perturbed Ca2+ signaling.214 As nanotunnel formation depends on MICOS, OPA1, and F1/F0 ATP synthase,212 and OPA1 prevents ROS formation, nanotunnels may help to maintain a balance between ROS production and elimination.216 Nanotunnels may also be affected by fission dynamics, as it has previously been hypothesized that nanotunnels form as a result of incomplete fission.213,217 For example, whereas both forms of fission depend on DRP1, peripheral fission and midzone fission depend on FIS1 and MFF, respectively.133 However, it is unknown whether mitochondrial nanotunnels are a precursor or end result of either of these dual fates of mitochondrial fission. Finally, the abundance of nanotunnels may increase with the mitochondrial stress that occurs in HF. However, neither nanotunnels nor other mitochondrial phenotypes have been rigorously characterized in HF.
FUTURE STUDY OF HF THROUGH IMAGING
Although biochemical and functional techniques have elucidated much of the pathophysiology of HF, imaging techniques such as transmission electron microscopy provide 2-dimensional micrographs of mitochondria195,218 that reveal the structural causes or consequences of mitochondrial dysfunction. However, characterizing specialized mitochondrial structures, like MERCs and nanotunnels, requires newer techniques, such as 3D reconstruction, which provides quantitation of volume, branching of mitochondria, nanotunnels, and other features.24,25,197,214 Confocal imaging can be used to assess the intracellular real-time movement of proteins that are associated with mitochondrial dynamics, such as estrogen.219 Furthermore, single particle analysis and cryo-electron tomography can be performed together, providing a visualization of cristae alongside protein-protein binding sites.201 These advanced imaging technologies can be used to characterize features of mitochondria that are not easily captured through traditional 2-dimensional histological analyses. Furthermore, these new methods use machine learning for faster analysis of various organelles in HF under many conditions. In particular, focused ion-beam SEM has high contrast, which makes it ideal for machine learning applications,197 but new techniques must be developed for serial block-face SEM to expedite workflows. CDeep3M is a promising technology for isolating mitochondria from cardiac myocytes for serial block-face SEM.220 Machine learning may be used in future studies to characterize other alterations in cardiac mitochondria.
Further characterization of mitochondrial 3D structure in human HF is needed to determine how aging and pathology affect mitochondrial morphology and function in a tissue-specific manner. Such studies would determine the frequency and distribution of various mitochondrial structures in different tissues, contributing to a mitochondrial connectome for human HF. For example, MitoCarta provides a systematic catalog of mitochondrial proteins and their functions.221 However, it does not address the interplay between mitochondria and other cellular components, highlighting the need for a more integrated approach to studying mitochondria in HF. An effective connectome would consider not only mitochondria but also their relationships with other organelles, as well as the relative frequency of their morphology and spatial distribution, both across subregions (eg, intermyofibrillar and SS) as well as cardiac regions (eg, pericardium and myocardium). Additionally, such studies may further categorize other factors affecting mitochondria including sex, ethnicity, hormonal regulation, and aging (Figure 4), which may also contribute to tissue-dependent structure. For example, although recent studies have investigated the role of sex-based mitochondrial differences in sex-dependent HF development68 and estrogen as a regulator of HF through mitochondria,90 little is known of their effects on mitochondrial structure; however, past reviews have summarized pathologies related to mitochondria.222,223 Despite earlier studies on the interplay between age-related pathologies, including HF, mitochondrial alterations, and epigenetic changes64 or ethnicity,224 more in-depth research is needed. Thus, to better understand the mechanisms that underlie the formation of these 3D structural phenotypes, we need better information on the localization of mitochondrial fusion and fission proteins by techniques such as mass spectroscopy imaging,225 as well as changes in the distribution of active mtDNA (ie, nucleoids), which may be determined by ER contact sites,226 across a diverse (both in sampling site and population) data set.
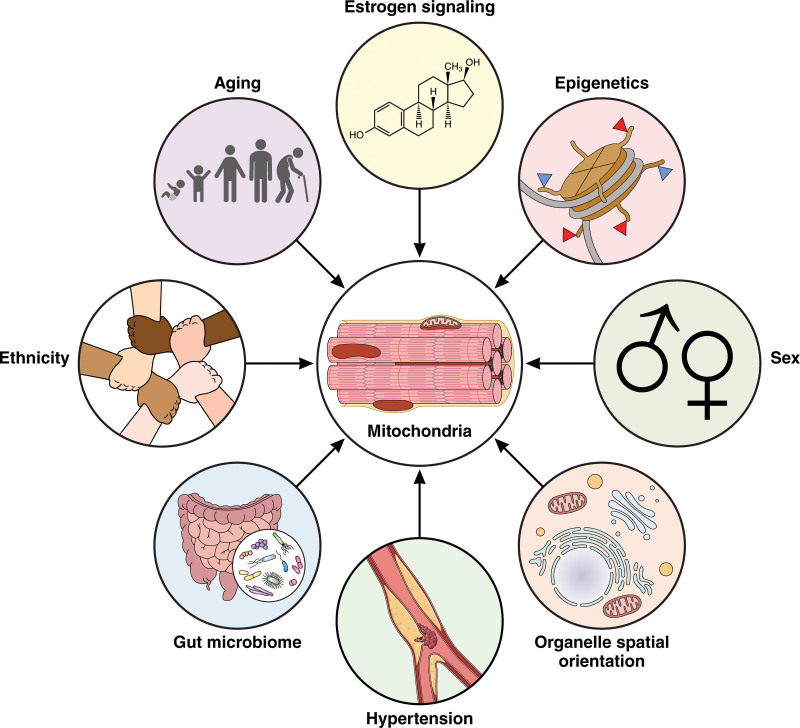
Figure 4.
Diverse effectors of mitochondria in cardiac tissue. Mitochondria in cardiac tissue sarcomeres are affected by aging, estrogen signaling, epigenetics, sex, relative organelle rearrangement (eg, mitochondria-endoplasmic reticulum contact sites), changes in pulse wave velocity and arterial stiffness, the gut microbiome, and potentially ethnicity. These are all avenues of research as they may confer risks for heart failure and should be researched for future studies. Illustration credit: Sceyence Studios.
DISRUPTED CALCIUM HOMEOSTASIS AND ROS PRODUCTION IN HF
Abnormal Ca2+ signaling is a hallmark of HF initiation,227 which is in turn linked to mitochondrial dysfunction.228 In healthy hearts, Ca2+ enters cardiomyocytes primarily through L-type Ca2+ channels during each heartbeat, triggering a larger release of Ca2+ from the SR, yet, in HF this positive feedback loop is inhibited by a reduction in Ca2+ influx.228 As reviewed by Gorski et al,229 the cardiac sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA2a), which pumps Ca2+ back into the SR and is regulated by phospholamban, has reduced activity, resulting in a duality of insufficient systolic Ca2+ release and diastolic uptake.229 Resultingly, SERCA2a and phospholamban have become key targets aimed at modulating HF Ca2+, with gene-therapies increasing SERCA2a offering cardioprotective effects.230 Impaired Ca2+ signaling may be further exacerbated by a dysregulated Ca2+-calcineurin-Nuclear Factor of Activated T-cells (NFAT) pathway.231 Notably, as previously reviewed, mitochondria, well-known to be intertwined with Ca2+ signaling, may contribute to the decreased Ca2+ availability for each contraction and impaired relaxation characteristic of HF, thus representing a valuable potential therapeutic target.232
Mitochondria form organelle-to-organelle contacts and engage in crosstalk with the cytoplasm for macromolecular synthesis, and dysregulation of this communication is linked to pathology.233 Communication between the mitochondria and the ER depends on MERCs,234 which facilitate the transfer of lipids, Ca2+, and ROS between the ER and mitochondria.234 MERCs are formed by interconnecting membrane protein complexes that mediate interactions between the mitochondria and ER without fusion.92 The isolation of MERCs via biophysical protocols yields mitochondrial-associated membranes (reviewed by Giacomello and Pellegrini235). Although MERCs can vary in structure and composition, we are using the term MERCs, analogous to their biochemical fractional counterpart mitochondrial-associated membranes, as the most common term to refer to these exchange sites. MERCs are essential for transferring lipids and maintaining Ca2+ homeostasis,236 and disturbances to MERCs result in perturbed lipid transport, the loss of Ca2+ homeostasis, and mitochondrial destruction.237 MERCs regulate Ca2+ flow from the ER to the mitochondrion; when normal Ca2+ flow is altered, the increased mitochondrial Ca2+ leads to changes in mitochondrial function and morphology.237 Moreover, an excessive mitochondrial Ca2+ load is a key contributor to myocardial hypertrophy and subsequent HF.238 The accumulation of mitochondrial Ca2+ triggers a vicious cycle of self-destruction in cardiomyocytes. The increased Ca2+ load leads to the accumulation of ROS,239 which damages the components of the respiratory chain and reduces ATP production (Figure 3). ROS accumulation also damages mtDNA and polyunsaturated membrane lipids.240,241 This positive feedback loop triggers apoptosis, leading to cardiomyocyte death.
Several mitochondrial-associated proteins are involved in Ca2+ signaling. Overexpression of mitochondrial mCa2+ uniporter (MCU) decreases oxidative stress and alleviates HF.242 Conversely, chronic inhibition of the MCU in the myocardium results in increased oxygen consumption rates and impaired physiological intracellular Ca2+ homeostasis, yet, paradoxically, does not protect against ischemia-reperfusion injury despite reduced ROS and preserved mitochondrial membrane potential.243 Specific study of this uniporter has further explicated that MICU1 (mitochondrial calcium uptake 1), a regulatory subunit of MCU, restricts cation flux in the absence of Ca2+, suggesting that the Ca2+ binding status of MICU1’s EF-hands may serve to influence MCU activity.244 Although the role of MCU in the development of HF is promising, and it has been reviewed extensively,245 scant research has been reported on the roles of other potential effectors of Ca2+ homeostasis in HF.
Oxidative metabolism that generates ATP requires Ca2+ signaling,246 and Ca2+ transfer is hypothesized to occur across MERCs.247 Mitofusin 2, a protein essential for MERC formation,92 is reduced in HF in mice.248 MERCs are composed of several proteins involved in the regulation of Ca2+ homeostasis: GRP75 (glucose-regulated protein 75), a chaperone protein, acts as a crucial linker between the IP3Rs (inositol trisphosphate receptors) on the ER and the voltage-dependent anion channel (VDAC) 1 on the outer mitochondrial membrane.234,235 In vitro studies have shown that IP3R3 is responsible for Orai-mediated Ca2+ entry and store-operated Ca2+ entry through the formation of a complex with STIM1 (stromal interaction molecule 1) following an inositol trisphosphate signaling cascade in low Ca2+ conditions.249 The role of IP3R (inositol 1,4,5-trisphosphate receptors) is further intriguing as it facilitates direct Ca2+ transfer to mitochondria, stimulating oxidative metabolism in states of limited MERC coupling.250 However, VDACs’ role in mitochondrial Ca2+ exchange, beyond forming Ca2+ permeable pores in the OMM, remains controversial (reviewed by Sander et al251). Furthermore, a tertiary and necessary component of this IP3R3-GRP75-VDAC1 complex is DJ-1 (protein deglycase DJ-1; Parkinsonism-associated deglycase), which is deficient in Parkinson disease.252 Conversely, in neurological disorders, increased DJ-1 activity is associated with deficits in L-type voltage-gated Ca2+ channel activity,253 showing that DJ-1 has poorly defined pluralistic roles in Ca2+ homeostasis. Translocase of the mitochondrial outer membrane 70, a subunit of the OM translocase that mediates the import of nuclear-encoded mitochondrial precursors, is downregulated in hypertrophic hearts.254 Depletion of translocase of mitochondrial outer membrane 70 in neonatal rat ventricular myocytes results in decreased OPA1 import and elevated ROS; its forced expression is protective against pro-hypertrophic perturbations.254 Furthermore, translocase of mitochondrial outer membrane 70 has emerged as a modulator of IP3R3-GRP75-VDAC1 through reinforcing ER-embedded IP3R3.255 Despite, this defined multicomponent tether system, few studies have examined it in depth in HF. One study in cardiomyocytes showed that the mitochondrial chaperone, CypD (cyclophilin D), inhibits MERC Ca2+ transfer by interacting with the VDAC1/GRP75/IP3R1 complex, thereby protecting the cells from mitochondrial Ca2+ overload and subsequent death during hypoxia-reoxygenation injury.256 Since CypD is a matrix chaperone, further investigation is necessary to see if this is only a functional interaction, or a structural interaction as well. Since Ca2+ overload is a key determinant in HF,238 this suggests that, while a lack of tethering may contribute to Ca2+ deficiency, an overabundance of MERC tethering is involved in HF pathogenesis. Therefore, future analyses should focus on MERCs, which may establish a strategy to restore Ca2+ homeostasis through structural alterations that optimize these inter-organelle gap widths to modify their crosstalk.257,258
In the myocardium, the 2 main types of intracellular Ca2+ release channels are the RyR2 (type 2 ryanodine receptor)/Ca2+ release channel and the IPCR2 (type 2 inositol 1,4,5-triphosphate receptor).238 In mouse models mimicking HF, RyR2 Ca2+ leak contributes to increased Ca2+ load and excessive ROS generation in cardiomyocytes.238 Mitochondria from the HF mouse model are dysfunctional and have distorted morphologies, with a marked decrease in form factor, size, and aspect ratio, indicating that the fusion-to-fission ratio is low.238 In addition, the oxidation and phosphorylation of RyR2 channels by protein kinase A results in Ca2+ leak from the SR, the ER of muscle cells.238 In Alzheimer disease, Ca2+ release via RyR2 channels and ROS generation, leads to mitochondrial Ca2+ overload and fragmentation.259 The RyR2 receptor modulation Ca2+ leak is also believed to contribute to the progression of HF,238 but the full role of RyR2 in this mechanism has not yet been established. Distinctly, inhibition of DRP1 with a short-term inhibitor known as Drpitor1a, independent from its roles in fission, decreases RyR2 concomitantly with reduced ROS production.260 Yet, this same study found that prolonged DRP1 deletion conversely leads to cardiomyopathy.260 Another recent study has had similar findings, showing that in hyperglycemic conditions DRP1 inhibition, normalizes Ca2+ homeostasis through reducing RyR2-dependent Ca2+ leak.261 Together, this suggests that beneficial targeting RyR2 may be achieved through short-term DRP1 inhibition.
The connection between MERCs and nanotunnels is unclear. MERCs, as a critical site of Ca2+ transfer,262 may affect nanotunnel formation, which arises in conditions of impaired Ca2+ homeostasis.213 Notably, nanotunnels can form in cardiomyocytes with leaky RyR2 Ca2+ release channels.166,215 The role of Ca2+ signaling in HF is a critical avenue to explore, as reestablishing the balance of Ca2+ to ADP is important in restoring cardiac contractile function in HF.263 It may be that when VDACs and other MERC proteins are unable to perform mitochondrial Ca2+ uptake in overloaded conditions, such as those caused by RyR2 mutations, nanotunnels exist as a final compensatory mechanism. The involvement of DRP1 in modulating RyR2260 further underscores the importance of further explicating how DRP1 and its associated fission factors may be related to the formation of nanotunnels. Nanotunnels may also be important in HF because they facilitate the exchange of metabolites and other essential molecules to optimize ATP production.213,214 Thus, compromised nanotunnels may negatively affect energy metabolism in cardiomyocytes, thereby contributing to HF.
It is known that the ER can have different conformations, which should be better characterized in general,264 as different types of ER influence MERC functionality. Yet, it is not known whether different types of MERCs are associated with HF; however, certain cellular conditions give rise to new mitochondrial contacts. For example, a wrappER is a rough ER that wraps around mitochondria and functions in lipid flux.265 However, there may be additional MERC phenotypes in HF in response to changes in mitochondrial structure. It is possible that ER-associated protein degradation mechanisms result in the formation of certain MERC phenotypes, as opposed to general ER stress states. Furthermore, mitochondrial-ER synthetic linkers, previously reviewed by Csordás et al 234 may, as therapeutics, prevent the formation of nanotunnels or impairment of Ca2+ homeostasis. Synthetic linkers that tighten individual mitochondria-junctional SR contacts mitigate myocyte death and attenuate mitochondrial Ca2+ overload in ex vivo ischemia/reperfusion injury.266 However, alterations in tethering may also negatively affect cardiac function,266 so further research is needed on artificial tethers that modulate Ca2+ overload and increase mitochondrial connectivity.
ALTERED METABOLITE PRODUCTION
Mitochondrial metabolism responds to changing environments. For example, embryonic development is partially moderated by mitochondria, which serve as a pacemaker that slows overall metabolism.267 Changes in cardiomyocyte metabolism underlie the progression of HF. At rest, the heart primarily uses fatty acid oxidation to produce energy,268 with glucose providing the remaining ≈20%. As the heart fails, mitochondrial oxidation declines, resulting in an energy deficit.268 The failing myocardium compensates for the energy loss by switching to glycolysis, and mitochondrial ATP production decreases with the oxidation of amino acids and glucose.268 However, fatty acid oxidation changes are complex and depend on the type (with or without reduced ejection fraction) and severity of HF. The importance of mitochondria in maintaining redox homeostasis is evident as the loss of the manganese antioxidant superoxide dismutase in HF leads to the disruption of cristae and mitochondrial gross architecture.269 The combined metabolic alterations in HF cause the already failing heart to become even less efficient.268,270 In advanced stages of HF, circulating ketone bodies, which may provide fuel, reflect the severity of cardiac dysfunction270,271 and are neurohormonal triggers for the activation of HF.270 Thus, impaired mitochondrial function contributes to the development and progression of HF by affecting energy metabolism and the redox balance in cardiomyocytes. As in embryonic species-specific development, mitochondria may be the governing pacemakers for HF through their roles in balancing the redox state and metabolism. Thus, HF must be viewed through the dual lenses of metabolic changes concomitant with mitochondrial structural changes.
Central to metabolic dysregulation is NAD(+):NADH ratios. NAD(+):NADH regulates ATP production, but also pluralistically influences anaerobic and aerobic metabolism, posttranslational protein modification, and energy substrate preference, conferring alterations in cardiac efficiency in HF.272 Loss of NAD+ in aging or stress leads to altered metabolic status and increased disease susceptibility, which are intertwined with HF pathology.272 Additionally, NAD+ homeostasis is modulated through autophagic flux in cardiomyocytes, with autophagy impairment causing loss of NAD+ due to the induction of nicotinamide N-methyltransferase and a process regulated by the SQSTM1-NF-κB (nuclear factor-κB)-NNMT signaling pathway.273 This study further shows that inhibition of nicotinamide N-methyltransferase restores NAD+.273 Notably, mitochondrial ETC complex I deficiency leads to an decreased NAD(+):NADH ratio, accelerating HF progression, which may also be reversed by NAD+ redox balance.31 Finally, another promising avenue to restore NAD+:NADH is through manipulation using an engineered enzyme or tool, as performed by Patgiri et al,274 Sharma et al,275 and Titov et al. 276 For example, LOXCAT, an engineered enzyme that converts lactate to pyruvate, rectifies intracellular NAD(+):NADH imbalance, suggesting a novel approach for treating intracellular redox imbalances by targeting circulating metabolites.274
In addition to understanding how changes in metabolism affect the heart, we need a better understanding of the effects of these changes on organismal systems. Although ketones are important, we need to understand the interplay between amino acids and mitochondria,277 in particular, whether amino acids modulate mitochondrial structure and mediate region-dependent mitochondrial changes. The integrated stress response is linked to amino acid metabolism via ATF4 regulation, which ameliorates amino acid starvation.278 Defects in branched-chain amino acid catabolism increase the risk of HF279; thus, ATF4 may serve as a therapeutic target to limit oxidative stress and the associated ketogenesis caused by amino acid starvation.
Mitokines, communicable markers of mitochondrial stress, which include FGF21 and GDF15, are best known in skeletal muscle, where they provide inter-tissue crosstalk in healthy aging (reviewed by Burtscher et al280) and are beneficial for preventing HF in aging (reviewed by Duan et al281). However, mitokines show differences in expression in type 2 diabetes, and GDF15 plasma levels are associated with a higher risk of HF.282 One question concerning mitokines is whether HF sends mitokine signals to other tissues, leading to cachexia and other skeletal muscle diseases, which are common in HF.283 If so, then mitochondria could be targeted to prevent the cross-organ dysregulation caused by HF. Similarly, more information on the role of mitokines in mitochondrial structure could improve our understanding of HF. For example, mitokine FGF21, which is associated with healthy aging in type 2 diabetes,282 is secreted following Opa1 deletion in skeletal muscle.284 Although this effect is not known for HF, it suggests that changes in mitochondrial dynamics may affect mitokine signaling, which may participate in a bidirectional relationship.
Recent findings have demonstrated that there is altered myocardial metabolism in HF with preserved ejection fraction, particularly through reduced utilization of fatty acids and other alternative fuels like glucose, ketones, and branched-chain amino acids.285 Additionally, expression of genes associated with lipolysis and oxidation are reduced in HFpEF myocardium, suggesting wider lipidomic changes that remain unclear.285 This differential catabolism in HFpEF is evidenced by lower glucose, ketone, and branched-chain amino acid utilization compared with HF with reduced ejection fraction.285 Yet it is unclear if mitochondrial changes are driving this altered energetic utilization and differential mitochondrial regulation may be responsible for differences in metabolism between HF types. Notably, recent studies in brown adipose tissue have shown that SLC25 (soluble carrier family 25) A44 determines active branched-chain amino acids catabolism.286 More broadly, other mitochondrial SLC25 proteins may drive altered metabolism. SLC25A37 and SLC25A39, which are essential for mitochondrial glutathione import and iron uptake, respectively, have recently appreciated roles in supporting OxPhos.287 In HF, SLC11A1, and SLC2A3 have been suggested as biomarkers for acute myocardial infarction.288 In septic cardiomyopathy, HIF1A and SLC25A37 are differentially expressed iron metabolism-related genes in both the myocardium and blood monocytes of patients with sepsis.289 Although the role of transporters in HF has been more comprehensively reviewed by Kumar et al,290 together these findings suggest that soluble carrier transport family members may contribute to differences between HF types.
Although 70% of cardiac fuel sources are derived from fatty acids, HF can cause an uptick in nonoxidized fatty acid derivatives, leading to lipotoxicity.291 In HF, the HIF1alpha-PPARgamma axis mediates metabolic changes, leading to increased glycolytic flux and decreased fatty acid utilization accompanied by lipid accumulation.292 This lipid accumulation, often caused by increased ROS generation, leads to lipotoxicity and apoptosis, ultimately exacerbating HF.291 In the context of mitochondria, mitochondrial very-long-chain acyl-CoA dehydrogenase, which is involved in the β-oxidation of very-long-chain fatty acids, is required for normal mitochondrial bioenergetics and associated cardiac heart rate regulation.293 Additionally, lipotoxicity causes ROS generation, which in turn posttranslationally modifies mitochondrial fusion and fission proteins, causing dysfunctional dynamics.160 This suggests that the switch from fatty acid utilization, and associated lipid accumulation, pluralistically targets mitochondria. Yet, advanced HF shows decreased myocardial lipid content and increased myocardial ketone utilization.294 This suggests that as HF continues, the body has natural mechanisms to abrogate lipid accumulation. It is possible that treatments, such as sulforaphane, may accelerate this response by promoting lipid utilization through increasing mitochondrial biogenesis.295 Additionally, a subpopulation of cells effectively collects and stores lipids in nonalcoholic fatty liver disease to reduce lipotoxicity,296 yet it is unclear if this same cellular heterogeneity can be exploited in HF.
More broadly, we do not understand whether the formation of mitochondrial structural phenotypes leads to or is the result of changes in metabolite utilization and production. Do changes in ketone levels regulate mitochondrial shape and organization in different regions? For example, lipid flux may alter as failing hearts shift to ketone utilization,294 leading to changes in MERCs (see previous section). Thus, mitochondrial structure and dynamics are interconnected with factors critical to HF pathology. Furthermore, we must target therapeutics instigated to explicate if mitochondria can reverse lipid accumulation. The utilization of new tools, such as MitoCarta 3.0, which offers a comprehensive catalog of mitochondrial proteins,221 has provided increased insight into the specific proteins involved in mitochondrial structural changes and their relationship to altered metabolite utilization, thereby informing the development of targeted therapeutics for conditions like lipid accumulation reversal.
Although mitochondrial energetics are reduced across all types of HF, fatty acid oxidation is different in HFpEF versus in HF with reduced ejection fraction (reviewed by De Jong et al297). If different types of HF lead to different mitochondrial dynamics and energetic capacities, could mitochondria regulate the various forms of HF? If so, what are the implications for the treatments needed to restore a normal mitochondrial structural phenotype if metabolism is not changed consistently across all forms of HF? This suggests that mitochondria could be a target for individualized medicine that considers the specific type of HF. Furthermore, if mitochondria serve as determinants of ejection fraction, biopsies from patients with HF may provide new information on the factors that lead to phenotypic differences.
CONCLUSIONS AND FUTURE PERSPECTIVES
Here, we have presented the current knowledge on the roles of mitochondrial and cristae dynamics, including the role of the MICOS complex in mitochondrial ultrastructure changes in the context of HF. Furthermore, we analyzed the roles of MERC sites, mitochondrial communication via nanotunnels, altered metabolite production, and external factors that affect mitochondrial structure in the failing heart. Each component provides a potential mitochondrial therapeutic target for mitigating cardiomyocyte death in HF. Given the complexity of the development and progression of human HF, effective measures will likely require a combination of treatments with distinct targets that require a better understanding of mitochondrial structure and dynamics and modulatory factors in HF. New insights into mitochondrial structural changes in HF and other factors of HF pathology that affect mitochondria (Figure 4) should provide a better understanding of the development of mitochondria in HF, allowing for individualized treatment regimens.
Mitochondria are indispensable for energy production, Ca2+ homeostasis, and other cellular processes. Aging is a major risk factor for HF and other cardiovascular diseases,71 and aging mitochondria produce ROS, leading to mutations in mtDNA.13 Because decreasing mutations in mtDNA can reduce mitochondrial dysfunction,13 it is important to understand how mitochondria change during aging to reverse these changes. In advanced HF cases in which damaged mitochondria are beyond repair, autogenous mitochondrial transplantation might restore function.298,299 Although autogenous mitochondrial transplantation reduces cardiomyocyte loss, it is not known whether these transplanted mitochondria can establish MERCs and other important structures needed for long-term health.
In the future, as we examine age-related pathologies and their relation to HF, it is important to consider the mitochondrial role, both within the heart and outside of the heart. In particular, recent studies have illustrated the organ-dependent aging rates, with accelerated aging in the heart conferring increased risk of HF.300 In practice, as reviewed by Xie et al,301 this comes about, in part, via alterations in circulating metabolic signatures. However, equally important is understanding the role of mitochondria in these age-related signature alterations. Notably, different types of HF may come about, in part, due to these altered metabolic signatures. Additionally, recent findings suggest that HFpEF can cause skeletal muscle wasting through mitochondrial dysfunction, suggesting that accelerated aging of the heart may have systemic effects.302 Yet, it is unclear if skeletal muscle mitochondrial structure and dynamics differ on the basis of ejection fraction, which would offer a potential biomarker for the different types of HF (Figure 1). This may offer insight into how mitochondria change in accelerated aging and pathology states, and the pluralistic roles mitochondria play in HF pathology (Figure 5).303
Figure 5.
Normal vs pathological alterations of mitochondrial morphology. Mitochondria are not static but change in response to stimuli to survive under stress. Fusion and fission result in changes in mitochondrial size; however, unregulated fission may result in fragmentation, whereas unregulated fusion may result in hyperbranching. Mitochondria may burst or be recycled via mitophagy. In addition to the typical spheres, mitochondria can assume many shapes and sizes, including megamitochondria. For transport of ions, including Ca2+, mitochondria may form nanotunnels and mitochondria-endoplasmic reticulum contacts (MERCs). Understanding these mitochondrial states and developing therapies that induce or prevent them is an important avenue in heart failure (HF) research. DJ-1 indicates protein deglycase DJ-1; DRP1, dynamin-related protein 1; FIS1, fission 1 protein; GRP75, glucose-regulated protein 75; IP3R3, inositol trisphosphate receptor 3; MFF, mitochondria fission factor; Mfn1/2, mitofusin 1 or 2; OMA1, OMA1 zinc metallopeptidase; OPA1, optic atrophy 1; Ryr2, type 2 ryanodine receptor/Ca2+ release channel; SR, sarcoplasmic reticulum; S616, serine 616; S637, serine 637; S656, serine 656; S693, serine 693; VDAC, voltage-dependent anion channel; and YME1L, YME1 Like 1 ATPase. Illustration credit: Sceyence Studios.
ARTICLE INFORMATION
Sources of Funding
The study was funded by United Negro College Fund (UNCF)/Bristol Myers Squibb E.E. Just Faculty Fund, Career Award at the Scientific Interface (Career Awards at the Scientific Interface [CASI] award) from Burroughs Welcome Fund (BWF) ID number 1021868.01, BWF ad hoc award, National Institutes of Health (NIH) Small Research Pilot Subaward to 5R25HL106365-12 from the NIH Programs to Increase Diversity among Individuals Engaged in Health-Related Research (PRIDE) Program, DK020593, Vanderbilt Diabetes and Research Training Center (DRTC) Alzheimer’s Disease Pilot and Feasibility Program. The Chan Zuckerberg Initiative (CZI) Science Diversity Leadership grant number 2022-253529 from the Chan Zuckerberg Initiative DAF, an advised fund of the Silicon Valley Community Foundation (A. Hinton). A postdoctoral fellowship from the American Heart Association and the Barth Syndrome Foundation (award ID: 828058 to N. Senoo). NIH Grants R01HL147818 and R01HL144941 (A. Kirabo) and R01HL165729 and R01GM151746 (S.M. Claypool). Doris Duke CSDA 2021193 (CNW), K23 HL156759 (CNW), Burroughs Wellcome Fund 1021480 (CNW). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Disclosures
None.
Nonstandard Abbreviations and Acronyms
- 3D
- 3 dimensional
- AMPK
- AMP-activated protein kinase
- CaMKII
- Ca2+/calmodulin-dependent protein kinase II
- Cdk1
- cyclin-dependent kinase 1
- CVD
- cardiovascular disease
- CypD
- cyclophilin D
- DRP1
- dynamin-related protein 1
- ER
- endoplasmic reticulum
- FIS1
- fission 1 protein
- GRP75
- glucose-regulated protein 75
- GSK-3β
- glycogen synthase kinase 3β
- HF
- heart failure
- HFpEF
- heart failure with preserved ejection fraction
- IMM
- inner mitochondrial membrane
- IP3R
- inositol trisphosphate receptor
- IPCR2
- type 2 inositol 1,4,5-triphosphate receptor
- MCU
- Mitochondrial mCa2+ uniporter
- MERC
- mitochondria-endoplasmic reticulum contact
- MFF
- mitochondria fission factor
- MFN1
- mitofusin 1
- MFN2
- mitofusin 2
- MICOS
- mitochondria contact site and cristae organizing system
- mtDNA
- mitochondrial DNA
- NF-κB
- nuclear factor-κB
- OMM
- outer mitochondrial membrane
- OPA1
- optic atrophy 1
- OxPhos
- oxidative phosphorylation
- PGAM5
- phosphoglycerate mutase family member 5
- ROS
- reactive oxygen species
- RyR2
- type 2 ryanodine receptor/Ca2+ release channel
- SEM
- scanning electron microscopy
- SERCA2a
- sarco(endo)plasmic reticulum Ca2+ ATPase
- SLC25
- soluble carrier family 25
- SR
- sarcoplasmic reticulum
- VDAC
- voltage-dependent anion channel
A. Hinton and S.M. Claypool contributed equally as co-first and co-senior authors.
For Sources of Funding and Disclosures, see page 389.
REFERENCES
- 1.Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol. 2022;80:2361–2371. doi: 10.1016/j.jacc.2022.11.005 [DOI] [PubMed] [Google Scholar]
- 2.Office of the Associate Director for Policy and Strategy. Health Topics: Heart Disease and Heart Attack. Centers for Disease Control and Prevention; 2021 [Google Scholar]
- 3.Haddad F, Hunt S, Rosenthal D, Murphy D. Right ventricular function in cardiovascular disease, Part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117:1436–1448. doi: 10.1161/CIRCULATIONAHA.107.653576 [DOI] [PubMed] [Google Scholar]
- 4.Fox KF, Cowie MR, Wood DA, Coats AJS, Gibbs JSR, Underwood SR, Turner RM, Poole-Wilson PA, Davies SW, Sutton GC. Coronary artery disease as the cause of incident heart failure in the population. Eur Heart J. 2001;22:228–236. doi: 10.1053/euhj.2000.2289 [DOI] [PubMed] [Google Scholar]
- 5.Bauersachs R, Zeymer U, Brière J-B, Marre C, Bowrin K, Huelsebeck M. Burden of coronary artery disease and peripheral artery disease: a literature review. Cardiovasc Ther. 2019;2019:8295054. doi: 10.1155/2019/8295054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown JC, Gerhardt TE, Kwon E. Risk Factors for Coronary Artery Disease. StatPearls: StatPearls Publishing; 2024. [PubMed] [Google Scholar]
- 7.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 8.Golla MSG, Shams P. Heart Failure With Preserved Ejection Fraction (HFpEF). StatPearls, Treasure Island (FL): StatPearls Publishing; 2024. [PubMed] [Google Scholar]
- 9.Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol. 2012;21:365–371. doi: 10.1016/j.carpath.2011.11.007 [DOI] [PubMed] [Google Scholar]
- 10.Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. 2020;22:1342–1356. doi: 10.1002/ejhf.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodgers JL, Jones J, Bolleddu SI, Vanthenapalli S, Rodgers LE, Shah K, Karia K, Panguluri SK. Cardiovascular risks associated with gender and aging. J Cardiovasc Dev Dis. 2019;6:19. doi: 10.3390/jcdd6020019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amorim JA, Coppotelli G, Rolo AP, Palmeira CM, Ross JM, Sinclair DA. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat Rev Endocrinol. 2022;18:243–258. doi: 10.1038/s41574-021-00626-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elorza AA, Soffia JP. mtDNA heteroplasmy at the core of aging-associated heart failure. An integrative view of OXPHOS and mitochondrial life cycle in cardiac mitochondrial physiology. Front Cell Dev Biol. 2021;9:625020. doi: 10.3389/fcell.2021.625020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karamanlidis G, Bautista-Hernández V, Fynn-Thompson F, Nido PD, Tian R. Impaired mitochondrial biogenesis precedes heart failure in right ventricular hypertrophy in congenital heart disease. Circ Heart Fail. 2011;4:707–713. doi: 10.1161/CIRCHEARTFAILURE.111.961474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karamanlidis G, Nascimben L, Couper G, Shekar P, Monte F, Tian R. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ Res. 2010;106:1541–1548. doi: 10.1161/CIRCRESAHA.109.212753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu M, Lv J, Pan Z, Wang D, Zhao L, Guo X. Mitochondrial dysfunction in heart failure and its therapeutic implications. Front Cardiovasc Med. 2022;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu C, Zhang Z, Zhang W, Liu X. Mitochondrial dysfunction and mitochondrial therapies in heart failure. Pharmacol Res. 2022;175:106038. doi: 10.1016/j.phrs.2021.106038 [DOI] [PubMed] [Google Scholar]
- 18.Bayeva M, Gheorghiade M, Ardehali H. Mitochondria as a therapeutic target in heart failure. J Am Coll Cardiol. 2013;61:599–610. doi: 10.1016/j.jacc.2012.08.1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown DA, Perry JB, Allen ME, Sabbah HN, Stauffer BL, Shaikh SR, Cleland JGF, Colucci WS, Butler J, Voors AA, et al. Mitochondrial function as a therapeutic target in heart failure. Nat Rev Cardiol. 2017;14:238–250. doi: 10.1038/nrcardio.2016.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L, Knowlton AA. Mitochondrial dynamics in heart failure. Congest Heart Fail (Greenwich, Conn.). 2011;17:257–261. doi: 10.1111/j.1751-7133.2011.00255.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Gong Q, Stice JP, Knowlton AA. Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res. 2009;84:91–99. doi: 10.1093/cvr/cvp181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou B, Tian R. Mitochondrial dysfunction in pathophysiology of heart failure. J Clin Invest. 2018;128:3716–3726. doi: 10.1172/JCI120849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monzel AS, Enríquez JA, Picard M. Multifaceted mitochondria: moving mitochondrial science beyond function and dysfunction. Nat Metab. 2023;5:546–562. doi: 10.1038/s42255-023-00783-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall AG, Damo SM, Hinton A. Revisiting focused ion beam scanning electron microcopy. Trends Biochem Sci. 2023;48:585–586. doi: 10.1016/j.tibs.2023.02.005 [DOI] [PubMed] [Google Scholar]
- 25.Marshall AG, Neikirk K, Stephens DC, Vang L, Vue Z, Beasley HK, Crabtree A, Scudese E, Lopez EG, Shao B, et al. Serial block face-scanning electron microscopy as a burgeoning technology. Adv Biol (Weinh). 2023;7:e2300139. doi: 10.1002/adbi.202300139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall AG, Krystofiak E, Damo SM, Hinton A. Correlative light-electron microscopy: integrating dynamics to structure. Trends Biochem Sci. 2023;48:826–827. doi: 10.1016/j.tibs.2023.05.003 [DOI] [PubMed] [Google Scholar]
- 27.Kim Y, Ajayi PT, Bleck CKE, Glancy B. Three-dimensional remodelling of the cellular energy distribution system during postnatal heart development. Philos Trans R Soc Lond B Biol Sci. 2022;377:20210322. doi: 10.1098/rstb.2021.0322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vue Z, Ajayi PT, Neikirk K, Murphy AC, Prasad P, Jenkins BC, Vang L, Garza-Lopez E, Mungai M, Marshall AG, et al. Human heart failure alters mitochondria and fiber 3D structure triggering metabolic shifts. 2023;2023.11.28.569095: doi: 10.1101/2023.11.28.569095
- 29.Kalkhoran S, Hall A, Cole A, White I, Yellon D, Hausenloy D. 3D Electron microscopy tomography to assess mitochondrial morphology in the adult heart. Heart. 2014;100:A10.1–A1A10. doi: 10.1136/heartjnl-2014-306916.29 [Google Scholar]
- 30.Althoff T, Mills D, Popot J, Kühlbrandt W. Arrangement of electron transport chain components in bovine mitochondrial supercomplex I1III2IV1. EMBO J. 2011;30:4652–4664. doi: 10.1038/emboj.2011.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee CF, Chavez J, Garcia-Menendez L, Choi Y, Roe N, Chiao Y, Edgar JS, Goo YA, Goodlett DR, Bruce JE, et al. Normalization of NAD+ redox balance as a therapy for heart failure. Circulation. 2016;134:883–894. doi: 10.1161/CIRCULATIONAHA.116.022495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cordero-Reyes AM, Gupte AA, Youker KA, Loebe M, Hsueh WA, Torre-Amione G, Taegtmeyer H, Hamilton DJ. Freshly isolated mitochondria from failing human hearts exhibit preserved respiratory function. J Mol Cell Cardiol. 2014;68:98–105. doi: 10.1016/j.yjmcc.2013.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li T, Yang Y, Qi H, Cui W, Zhang L, Fu X, He X, Liu M, Li P, Yu T. CRISPR/Cas9 therapeutics: progress and prospects. Sig Transduct Target Ther. 2023;8:1–23. doi: 10.1038/s41392-023-01309-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He X, Du T, Long T, Liao X, Dong Y, Huang ZP. Signaling cascades in the failing heart and emerging therapeutic strategies. Sig Transduct Target Ther. 2022;7:1–36. doi: 10.1038/s41392-022-00972-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 36.Fernander AF, Durán REF, Saab PG, Schneiderman N. John henry active coping, education, and blood pressure among urban blacks. J Natl Med Assoc. 2004;96:246–255. [PMC free article] [PubMed] [Google Scholar]
- 37.Rolle T, Vue Z, Murray S, Shareef SA, Shuler H, Beasley HK, Marshall AG, Hinton A. Toxic stress and burnout: John henryism and social dominance in the laboratory and STEM workforce. Pathog Dis. 2021;79:ftab041. doi: 10.1093/femspd/ftab041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piña IL, Jimenez S, Lewis EF, Morris AA, Onwuanyi A, Tam E, Ventura HO. Race and ethnicity in heart failure. J Am Coll Cardiol. 2021;78:2589–2598. doi: 10.1016/j.jacc.2021.06.058 [DOI] [PubMed] [Google Scholar]
- 39.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 40.Khatibzadeh S, Farzadfar F, Oliver J, Ezzati M, Moran A. Worldwide risk factors for heart failure: a systematic review and pooled analysis. Int J Cardiol. 2013;168:1186–1194. doi: 10.1016/j.ijcard.2012.11.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dunlay SM, Weston SA, Jacobsen SJ, Roger VL. Risk factors for heart failure: a population-based case-control study. Am J Med. 2009;122:1023–1028. doi: 10.1016/j.amjmed.2009.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knowlton AA, Korzick DH. Estrogen and the female heart. Mol Cell Endocrinol. 2014;389:31–39. doi: 10.1016/j.mce.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hinton AO, He Y, Xia Y, Xu P, Yang Y, Saito K, Wang C, Yan X, Shu G, Henderson A, et al. Estrogen receptor-α in the medial amygdala prevents stress-induced elevations in blood pressure in females. Hypertension. 2016;67:1321–1330. doi: 10.1161/HYPERTENSIONAHA.116.07175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ashraf MS, Vongpatanasin W. Estrogen and hypertension. Curr Hypertens Rep. 2006;8:368–376. doi: 10.1007/s11906-006-0080-1 [DOI] [PubMed] [Google Scholar]
- 45.Burstein SR, Kim HJ, Fels JA, Qian L, Zhang S, Zhou P, Starkov AA, Iadecola C, Manfredi G. Estrogen receptor beta modulates permeability transition in brain mitochondria. Biochim Biophys Acta Bioenerg. 2018;1859:423–433. doi: 10.1016/j.bbabio.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torres MJ, Kew KA, Ryan TE, Pennington ER, Lin CT, Buddo KA, Fix AM, Smith CA, Gilliam LA, Karvinen S, et al. 17β-Estradiol directly lowers mitochondrial membrane microviscosity and improves bioenergetic function in skeletal muscle. Cell Metab. 2018;27:167–179.e7. doi: 10.1016/j.cmet.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hara Y, Yuk F, Puri R, Janssen WGM, Rapp PR, Morrison JH. Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proc Natl Acad Sci USA. 2014;111:486–491. doi: 10.1073/pnas.1311310110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christiansen MN, Køber L, Weeke P, Vasan RS, Jeppesen JL, Smith JG, Gislason GH, Torp-Pedersen C, Andersson C. Age-specific trends in incidence, mortality, and comorbidities of Heart Failure in Denmark, 1995 to 2012. Circulation. 2017;135:1214–1223. doi: 10.1161/CIRCULATIONAHA.116.025941 [DOI] [PubMed] [Google Scholar]
- 49.Buford TW. Hypertension and aging. Ageing Res Rev. 2016;26:96–111. doi: 10.1016/j.arr.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akbari M, Kirkwood TBL, Bohr VA. Mitochondria in the signaling pathways that control longevity and health span. Ageing Res Rev. 2019;54:100940. doi: 10.1016/j.arr.2019.100940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campo A, Contreras-Hernández I, Castro-Sepúlveda M, Campos CA, Figueroa R, Tevy MF, Eisner V, Casas M, Jaimovich E. Muscle function decline and mitochondria changes in middle age precede sarcopenia in mice. Aging (Milano). 2018;10:34–55. doi: 10.18632/aging.101358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57:660–671. doi: 10.1007/s00125-014-3171-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0 [DOI] [PubMed] [Google Scholar]
- 54.Nguyen BY, Ruiz‐Velasco A, Bui T, Collins L, Wang X, Liu W. Mitochondrial function in the heart: the insight into mechanisms and therapeutic potentials. Br J Pharmacol. 2019;176:4302–4318. doi: 10.1111/bph.14431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrari R, Censi S, Mastrorilli F, Boraso A. Prognostic benefits of heart rate reduction in cardiovascular disease. Eur Heart J Suppl. 2003;5:G10–G14. [Google Scholar]
- 56.Lyra-Leite DM, Petersen AP, Ariyasinghe NR, Cho N, McCain ML. Mitochondrial architecture in cardiac myocytes depends on cell shape and matrix rigidity. J Mol Cell Cardiol. 2021;150:32–43. doi: 10.1016/j.yjmcc.2020.10.004 [DOI] [PubMed] [Google Scholar]
- 57.Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz SC, Suthammarak W, Gong G, Sedensky MM, Morgan PG, Wang W, Tian R. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab. 2013;18:239–250. doi: 10.1016/j.cmet.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Redout EM, Wagner MJ, Zuidwijk MJ, Boer C, Musters RJP, van Hardeveld C, Paulus WJ, Simonides WS. Right-ventricular failure is associated with increased mitochondrial complex II activity and production of reactive oxygen species. Cardiovasc Res. 2007;75:770–781. doi: 10.1016/j.cardiores.2007.05.012 [DOI] [PubMed] [Google Scholar]
- 59.Lesnefsky EJ, Chen Q, Hoppel CL. Mitochondrial metabolism in aging heart. Circ Res. 2016;118:1593–1611. doi: 10.1161/CIRCRESAHA.116.307505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosca M, Minkler P, Hoppel CL. Cardiac mitochondria in heart failure: Normal cardiolipin profile and increased threonine phosphorylation of complex IV. Biochim Biophys Acta. 2011;1807:1373–1382. doi: 10.1016/j.bbabio.2011.02.003 [DOI] [PubMed] [Google Scholar]
- 61.El-Hattab AW, Scaglia F. Mitochondrial cardiomyopathies. Front Cardiovasc Med. 2016;3:25. doi: 10.3389/fcvm.2016.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hong YS, Longchamps RJ, Zhao D, Castellani CA, Loehr LR, Chang PP, Matsushita K, Grove ML, Boerwinkle E, Arking DE, et al. Mitochondrial DNA copy number and incident heart failure. Circulation. 2020;141:1823–1825. doi: 10.1161/CIRCULATIONAHA.120.046001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ide T, Tsutsui H, Hayashidani S, Kang D, Suematsu N, Nakamura K, Utsumi H, Hamasaki N, Takeshita A. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res. 2001;88:529–535. doi: 10.1161/01.res.88.5.529 [DOI] [PubMed] [Google Scholar]
- 64.Mongelli A, Mengozzi A, Geiger M, Gorica E, Mohammed SA, Paneni F, Ruschitzka F, Costantino S. Mitochondrial epigenetics in aging and cardiovascular diseases. Front Cardiovasc Med. 2023;10:1204483. doi: 10.3389/fcvm.2023.1204483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ribeiro Junior RF, Dabkowski ER, Shekar KC, O Connell KA, Hecker PA, Murphy MP. MitoQ improves mitochondrial dysfunction in heart failure induced by pressure overload. Free Radic Biol Med. 2018;117:18–29. doi: 10.1016/j.freeradbiomed.2018.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang D, Mott JL, Farrar P, Ryerse JS, Chang S-W, Stevens M, Denniger G, Zassenhaus HP. Mitochondrial DNA mutations activate the mitochondrial apoptotic pathway and cause dilated cardiomyopathy. Cardiovasc Res. 2003;57:147–157. doi: 10.1016/s0008-6363(02)00695-8 [DOI] [PubMed] [Google Scholar]
- 67.Anan R, Nakagawa M, Miyata M, Higuchi I, Nakao S, Suehara M, Osame M, Tanaka H. Cardiac involvement in mitochondrial diseases. Circulation. 1995;91:955–961. doi: 10.1161/01.cir.91.4.955 [DOI] [PubMed] [Google Scholar]
- 68.Cao Y, Vergnes L, Wang YC, Pan C, Chella Krishnan K, Moore TM, Rosa-Garrido M, Kimball TH, Zhou Z, Charugundla S, et al. Sex differences in heart mitochondria regulate diastolic dysfunction. Nat Commun. 2022;13:3850. doi: 10.1038/s41467-022-31544-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marín-García J. Mitochondrial DNA repair: a novel therapeutic target for heart failure. Heart Fail Rev. 2016;21:475–487. doi: 10.1007/s10741-016-9543-x [DOI] [PubMed] [Google Scholar]
- 70.Yu H, Yu M, Li Z, Zhang E, Ma H. Identification and analysis of mitochondria-related key genes of heart failure. J Transl Med. 2022;20:410. doi: 10.1186/s12967-022-03605-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kenny HC, Abel ED. Heart failure in type 2 diabetes mellitus. Circ Res. 2019;124:121–141. doi: 10.1161/CIRCRESAHA.118.311371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lozhkin A, Vendrov AE, Ramos-Mondragón R, Canugovi C, Stevenson MD, Herron TJ, Hummel SL, Figueroa CA, Bowles DE, Isom LL, et al. Mitochondrial oxidative stress contributes to diastolic dysfunction through impaired mitochondrial dynamics. Redox Biol. 2022;57:102474. doi: 10.1016/j.redox.2022.102474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu X, Zhang Y, Deng Y, Yang L, Ou W, Xie M, Ding L, Jiang C, Yu H, Li Q, et al. Mitochondrial protein hyperacetylation underpins heart failure with preserved ejection fraction in mice. J Mol Cell Cardiol. 2022;165:76–85. doi: 10.1016/j.yjmcc.2021.12.015 [DOI] [PubMed] [Google Scholar]
- 74.Zhang L, Jaswal JS, Ussher JR, Sankaralingam S, Wagg C, Zaugg M, Lopaschuk GD. Cardiac insulin-resistance and decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy. Circ Heart Fail. 2013;6:1039–1048. doi: 10.1161/CIRCHEARTFAILURE.112.000228 [DOI] [PubMed] [Google Scholar]
- 75.Knez J, Lakota K, Božič N, Okrajšek R, Cauwenberghs N, Thijs L, Kneževič I, Vrtovec B, Tomšič M, Čučnik S, et al. Correlation between mitochondrial DNA content measured in myocardium and peripheral blood of patients with non-ischemic heart failure. Genet Test Mol Biomarkers. 2017;21:736–741. doi: 10.1089/gtmb.2017.0099 [DOI] [PubMed] [Google Scholar]
- 76.Esterhammer R, Klug G, Wolf C, Mayr A, Reinstadler S, Feistritzer H, Metzler B, Schocke MFH. Cardiac high-energy phosphate metabolism alters with age as studied in 196 healthy males with the help of 31-phosphorus 2-dimensional chemical shift imaging. PLoS One. 2014;9:e97368. doi: 10.1371/journal.pone.0097368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mietsch M, Hinkel R. “Empowering” cardiac cells via stem cell derived mitochondrial transplantation-does age matter? Int J Mol Sci . 2021;22:1824–1824. doi: 10.3390/ijms22041824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hollander JM, Thapa D, Shepherd DL. Physiological and structural differences in spatially distinct subpopulations of cardiac mitochondria: influence of cardiac pathologies. American Journal of Physiology-Heart and Circulatory Physiology. Am J Physiol Heart Circ Physiol. 2014;307:H1–14. doi: 10.1152/ajpheart.00747.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schaum N, Lehallier B, Hahn O, Pálovics R, Hosseinzadeh S, Lee SE, Sit R, Lee DP, Losada PM, Zardeneta ME, et al. ; Tabula Muris Consortium. Ageing hallmarks exhibit organ-specific temporal signatures. Nature. 2020;583:596–602. doi: 10.1038/s41586-020-2499-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vue Z, Garza-Lopez E, Neikirk K, Katti P, Vang L, Beasley H, Shao J, Marshall AG, Crabtree A, Murphy AC, et al. 3D reconstruction of murine mitochondria reveals changes in structure during aging linked to the MICOS complex. Aging Cell. 2023;22:e14009. doi: 10.1111/acel.14009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao Q, Sun Q, Zhou L, Liu K, Jiao K. Complex regulation of mitochondrial function during cardiac development. J Am Heart Assoc. 2019;8:e012731. doi: 10.1161/JAHA.119.012731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oakley CM. Diseases of the Myocardium, Pericardium, and Endocardium. In: Messerli FH, editor. Cardiovascular Disease in the Elderly, Springer US; 1987, p. 197–224. doi:10.1007/978-1-4684-9925-4_12. [Google Scholar]
- 83.Janicki JS. Influence of the pericardium and ventricular interdependence on left ventricular diastolic and systolic function in patients with heart failure. Circulation. 1990;81:15–20. [PubMed] [Google Scholar]
- 84.Okada H, Takemura G, Kanamori H, Tsujimoto A, Goto K, Kawamura I, Watanabe T, Morishita K, Miyazaki N, Tanaka T, et al. Phenotype and physiological significance of the endocardial smooth muscle cells in human failing hearts. Circ Heart Fail. 2015;8:149–155. doi: 10.1161/CIRCHEARTFAILURE.114.001746 [DOI] [PubMed] [Google Scholar]
- 85.Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet. 2012;46:265–287. doi: 10.1146/annurev-genet-110410-132529 [DOI] [PubMed] [Google Scholar]
- 86.Abel ED. Mitochondrial dynamics and metabolic regulation in cardiac and skeletal muscle. Trans Am Clin Climatol Assoc. 2018;129:266–278. [PMC free article] [PubMed] [Google Scholar]
- 87.Wu D, Dasgupta A, Chen KH, Neuber-Hess M, Patel J, Hurst TE, Mewburn JD, Lima PDA, Alizadeh E, Martin A, et al. Identification of novel dynamin-related protein 1 (Drp1) GTPase inhibitors: therapeutic potential of Drpitor1 and Drpitor1a in cancer and cardiac ischemia-reperfusion injury. FASEB J. 2020;34:1447–1464. doi: 10.1096/fj.201901467R [DOI] [PubMed] [Google Scholar]
- 88.Glancy B, Kim Y, Katti P, Willingham TB. The functional impact of mitochondrial structure across subcellular scales. Front Physiol. 2020;11:541040. doi: 10.3389/fphys.2020.541040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ni HM, Williams JA, Ding WX. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015;4:6–13. doi: 10.1016/j.redox.2014.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Knowlton AA, Chen L, Malik ZA. Heart failure and mitochondrial dysfunction: the role of mitochondrial fission/fusion abnormalities and new therapeutic strategies. J Cardiovasc Pharmacol. 2014;63:196–206. doi: 10.1097/01.fjc.0000432861.55968.a6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sabbah HN, Gupta RC, Singh-Gupta V, Zhang K, Lanfear DE. Abnormalities of mitochondrial dynamics in the failing heart: normalization following long-term therapy with elamipretide. Cardiovasc Drugs Ther. 2018;32:319–328. doi: 10.1007/s10557-018-6805-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534 [DOI] [PubMed] [Google Scholar]
- 93.Son JM, Sarsour EH, Kakkerla Balaraju A, Fussell J, Kalen AL, Wagner BA, Buettner GR, Goswami PC. Mitofusin 1 and optic atrophy 1 shift metabolism to mitochondrial respiration during aging. Aging Cell. 2017;16:1136–1145. doi: 10.1111/acel.12649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200 [DOI] [PubMed] [Google Scholar]
- 96.Hu H, Tian M, Ding C, Yu S. The C/EBP Homologous Protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front Immunol. 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Givvimani S, Pushpakumar S, Veeranki S, Tyagi SC. Dysregulation of Mfn2 and Drp-1 proteins in heart failure. Can J Physiol Pharmacol. 2014;92:583–591. doi: 10.1139/cjpp-2014-0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Caron C, Bertolin G. Cristae shaping and dynamics in mitochondrial function. J Cell Sci. 2024;137:jcs260986. doi: 10.1242/jcs.260986 [DOI] [PubMed] [Google Scholar]
- 99.Disatnik M, Ferreira JCB, Campos JC, Gomes KS, Dourado PMM, Qi X, Mochly-Rosen D. Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long‐term cardiac dysfunction. J Am Heart Assoc.2013;2:e000461. doi: 10.1161/JAHA.113.000461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ishihara T, Ban-Ishihara R, Maeda M, Matsunaga Y, Ichimura A, Kyogoku S, Aoki H, Katada S, Nakada K, Nomura M, et al. Dynamics of mitochondrial DNA nucleoids regulated by mitochondrial fission is essential for maintenance of homogeneously active mitochondria during neonatal heart development. Mol Cell Biol. 2015;35:211–223. doi: 10.1128/MCB.01054-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alexander C, Votruba M, Pesch UEA, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–215. doi: 10.1038/79944 [DOI] [PubMed] [Google Scholar]
- 102.Chen L, Liu T, Tran A, Lu X, Tomilov AA, Davies V, Cortopassi G, Chiamvimonvat N, Bers DM, Votruba M, et al. OPA 1 mutation and late‐onset cardiomyopathy: mitochondrial dysfunction and mtDNA instability. J Am Heart Assoc. 2012;1:e003012–e003012. doi: 10.1161/JAHA.112.003012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Del Dotto V, Mishra P, Vidoni S, Fogazza M, Maresca A, Caporali L, McCaffery JM, Cappelletti M, Baruffini E, Lenaers G, et al. OPA1 Isoforms in the hierarchical organization of mitochondrial functions. Cell Rep. 2017;19:2557–2571. doi: 10.1016/j.celrep.2017.05.073 [DOI] [PubMed] [Google Scholar]
- 104.Wang R, Mishra P, Garbis SD, Moradian A, Sweredoski MJ, Chan DC. Identification of new OPA1 cleavage site reveals that short isoforms regulate mitochondrial fusion. Mol Biol Cell. 2021;32:157–168. doi: 10.1091/mbc.E20-09-0605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simula L, Campello S. Monitoring the mitochondrial dynamics in mammalian cells. Mitochondrial Bioenergetics. 2018;1782:267–285. doi: 10.1007/978-1-4939-7831-1_15 [DOI] [PubMed] [Google Scholar]
- 106.Anand R, Wai T, Baker MJ, Kladt N, Schauss AC, Rugarli E, Langer T. The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J Cell Biol. 2014;204:919–929. doi: 10.1083/jcb.201308006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maloney DM, Chadderton N, Millington-Ward S, Palfi A, Shortall C, O’Byrne JJ, Cassidy L, Keegan D, Humphries P, Kenna P, et al. Optimized OPA1 isoforms 1 and 7 provide therapeutic benefit in models of mitochondrial dysfunction. Front Neurosci. 2020;14:571479. doi: 10.3389/fnins.2020.571479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xie L, Shi F, Tan Z, Li Y, Bode AM, Cao Y. Mitochondrial network structure homeostasis and cell death. Cancer Sci. 2018;109:3686–3694. doi: 10.1111/cas.13830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arnoult D, Grodet A, Lee Y-J, Estaquier J, Blackstone C. Release of OPA1 during apoptosis participates in the rapid and complete release of cytochrome c and subsequent mitochondrial fragmentation. J Biol Chem. 2005;280:35742–35750. doi: 10.1074/jbc.M505970200 [DOI] [PubMed] [Google Scholar]
- 110.MacVicar T, Langer T. OPA1 processing in cell death and disease–the long and short of it. J Cell Sci. 2016;129:2297–2306. doi: 10.1242/jcs.159186 [DOI] [PubMed] [Google Scholar]
- 111.Vue Z, Neikirk K, Vang L, Garza-Lopez E, Christensen TA, Shao J, Lam J, Beasley HK, Marshall AG, Crabtree A, et al. Three-Dimensional mitochondria reconstructions of murine cardiac muscle changes in size across aging. Am J Physiol Heart Circ Physiol. 2023;325:H965–H982. doi: 10.1152/ajpheart.00202.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wai T, García-Prieto J, Baker MJ, Merkwirth C, Benit P, Rustin P, Rupérez FJ, Barbas C, Ibañez B, Langer T. Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science. 2015;350:aad0116. doi: 10.1126/science.aad0116 [DOI] [PubMed] [Google Scholar]
- 113.Ahola S, Rivera Mejías P, Hermans S, Chandragiri S, Giavalisco P, Nolte H, Langer T. OMA1-mediated integrated stress response protects against ferroptosis in mitochondrial cardiomyopathy. Cell Metab. 2022;34:1875–1891.e7. doi: 10.1016/j.cmet.2022.08.017 [DOI] [PubMed] [Google Scholar]
- 114.Guo X, Aviles G, Liu Y, Tian R, Unger BA, Lin Y-HT, Wiita AP, Xu K, Correia MA, Kampmann M. Mitochondrial stress is relayed to the cytosol by an OMA1-DELE1-HRI pathway. Nature. 2020;579:427–432. doi: 10.1038/s41586-020-2078-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hinton A, Jr, Katti P, Mungai M, Hall DD, Koval O, Shao J, Vue Z, Lopez EG, Rostami R, Neikirk K, et al. ATF4-dependent increase in mitochondrial-endoplasmic reticulum tethering following OPA1 deletion in skeletal muscle. J Cell Physiol. 2024;239:e31204. doi: 10.1002/jcp.31204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fessler E, Eckl E-M, Schmitt S, Mancilla IA, Meyer-Bender MF, Hanf M, Philippou-Massier J, Krebs S, Zischka H, Jae LT. A pathway coordinated by DELE1 relays mitochondrial stress to the cytosol. Nature. 2020;579:433–437. doi: 10.1038/s41586-020-2076-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shammas MK, Huang X, Wu BP, Fessler E, Song IY, Randolph NP, Li Y, Bleck CK, Springer DA, Fratter C, et al. OMA1 mediates local and global stress responses against protein misfolding in CHCHD10 mitochondrial myopathy. J Clin Invest. n.d;132:e157504. doi: 10.1172/JCI157504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stephens DC, Mungai M, Crabtree A, Beasley HK, Garza-Lopez E, Neikirk K, Bacevac S, Vang L, Vue Z, Vue N, et al. Creating optimal conditions for OPA1 isoforms by western blot in skeletal muscle cells and tissue. bioRxiv. 2023;2023.05.20.541601: doi: 10.1101/2023.05.20.541601 [Google Scholar]
- 119.Ong S-B, Hausenloy DJ. Mitochondrial morphology and cardiovascular disease. Cardiovasc Res. 2010;88:16–29. doi: 10.1093/cvr/cvq237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Favaro G, Romanello V, Varanita T, Desbats MA, Morbidoni V, Tezze C, Albiero M, Canato M, Gherardi G, Stefani DE, et al. DRP1-mediated mitochondrial shape controls calcium homeostasis and muscle mass. Nat Commun. 2019;10:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang Z, Wang L, Yang C, Pu S, Guo Z, Wu Q, Zhou Z, Zhao H. Mitochondrial membrane remodeling. Front Bioeng Biotechnol. 2022;9:786806–786806. doi: 10.3389/fbioe.2021.786806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Qi Z, Huang Z, Xie F, Chen L. Dynamin‐related protein 1: a critical protein in the pathogenesis of neural system dysfunctions and neurodegenerative diseases. J Cell Physiol. 2019;234:10032–10046. doi: 10.1002/jcp.27866 [DOI] [PubMed] [Google Scholar]
- 123.Chen C, Huang J, Shen J, Bai Q. Quercetin improves endothelial insulin sensitivity in obese mice by inhibiting Drp1 phosphorylation at serine 616 and mitochondrial fragmentation. Acta Biochim Biophys Sin. 2019;51:1250–1257. doi: 10.1093/abbs/gmz127 [DOI] [PubMed] [Google Scholar]
- 124.Losón OC, Song Z, Chen H, Chan DC. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. MBoC. 2013;24:659–667. doi: 10.1091/mbc.e12-10-0721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zaja I, Bai X, Liu Y, Kikuchi C, Dosenovic S, Yan Y, Canfield SG, Bosnjak ZJ. Cdk1, PKCδ and calcineurin-mediated Drp1 pathway contributes to mitochondrial fission-induced cardiomyocyte death. Biochem Biophys Res Commun. 2014;453:710–721. doi: 10.1016/j.bbrc.2014.09.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200 [DOI] [PubMed] [Google Scholar]
- 127.DaRocha-Souto B, Coma M, Perez-Nievas BG, Scotton TC, Siao M, Sánchez-Ferrer P, Hashimoto T, Fan Z, Hudry E, Barroeta I, et al. Activation of glycogen synthase kinase-3 beta mediates β-amyloid induced neuritic damage in Alzheimer’s disease. Neurobiol Dis. 2012;45:425–437. doi: 10.1016/j.nbd.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shirakabe A, Zhai P, Ikeda Y, Saito T, Maejima Y, Hsu CP, Nomura M, Egashira K, Levine B, Sadoshima J. Drp1-Dependent mitochondrial autophagy plays a protective role against pressure overload–induced mitochondrial dysfunction and heart failure. Circulation. 2016;133:1249–1263. doi: 10.1161/CIRCULATIONAHA.115.020502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, Herzig S, Da Cruz S, Clerc P, Raschke I, Merkwirth C, et al. SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J. 2009;28:1589–1600. doi: 10.1038/emboj.2009.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu T, Yu R, Jin SB, Han L, Lendahl U, Zhao J, Nistér M. The mitochondrial elongation factors MIEF1 and MIEF2 exert partially distinct functions in mitochondrial dynamics. Exp Cell Res. 2013;319:2893–2904. doi: 10.1016/j.yexcr.2013.07.010 [DOI] [PubMed] [Google Scholar]
- 131.Chen KH, Dasgupta A, Lin J, Potus F, Bonnet S, Iremonger J, Fu J, Mewburn J, Wu D, Dunham-Snary K, et al. Epigenetic dysregulation of the dynamin-related protein 1 binding partners MiD49 and MiD51 increases mitotic mitochondrial fission and promotes pulmonary arterial hypertension. Circulation. 2018;138:287–304. doi: 10.1161/CIRCULATIONAHA.117.031258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Haileselassie B, Mukherjee R, Joshi AU, Napier BA, Massis LM, Ostberg NP, Queliconi BB, Monack D, Bernstein D, Mochly-Rosen D. Drp1/Fis1 interaction mediates mitochondrial dysfunction in septic cardiomyopathy. J Mol Cell Cardiol. 2019;130:160–169. doi: 10.1016/j.yjmcc.2019.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kleele T, Rey T, Winter J, Zaganelli S, Mahecic D, Perreten Lambert H, Ruberto FP, Nemir M, Wai T, Pedrazzini T, et al. Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature. 2021;593:435–439. doi: 10.1038/s41586-021-03510-6 [DOI] [PubMed] [Google Scholar]
- 134.Nag S, Szederkenyi K, Gorbenko O, Tyrrell H, Yip CM, McQuibban GA. PGAM5 is an MFN2 phosphatase that plays an essential role in the regulation of mitochondrial dynamics. Cell Rep. 2023;42:112895. doi: 10.1016/j.celrep.2023.112895 [DOI] [PubMed] [Google Scholar]
- 135.Zhu H, Tan Y, Du W, Li Y, Toan S, Mui D, Tian F, Zhou H. Phosphoglycerate mutase 5 exacerbates cardiac ischemia-reperfusion injury through disrupting mitochondrial quality control. Redox Biol. 2021;38:101777. doi: 10.1016/j.redox.2020.101777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tong M, Zablocki D, Sadoshima J. The role of Drp1 in mitophagy and cell death in the heart. J Mol Cell Cardiol. 2020;142:138–145. doi: 10.1016/j.yjmcc.2020.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sharp WW, Fang YH, Han M, Zhang HJ, Hong Z, Banathy A, Morrow E, Ryan JJ, Archer SL. Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J. 2014;28:316–326. doi: 10.1096/fj.12-226225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li J, Wang Y, Wang Y, Wen X, Ma XN, Chen W, Huang F, Kou J, Qi LW, Liu B, et al. Pharmacological activation of AMPK prevents Drp1-mediated mitochondrial fission and alleviates endoplasmic reticulum stress-associated endothelial dysfunction. J Mol Cell Cardiol. 2015;86:62–74. doi: 10.1016/j.yjmcc.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 139.Wang B, Nie J, Wu L, Hu Y, Wen Z, Dong L, Zou MH, Chen C, Wang DW. AMPKα2 Protects against the development of heart failure by enhancing mitophagy via PINK1 phosphorylation. Circ Res. 2018;122:712–729. doi: 10.1161/CIRCRESAHA.117.312317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jin J, Wei X, Zhi X, Wang X, Meng D. Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacol Sin. 2021;42:655–664. doi: 10.1038/s41401-020-00518-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bian X, Xu J, Zhao H, Zheng Q, Xiao X, Ma X, Li Y, Du X, Liu X. Zinc-Induced SUMOylation of dynamin-related protein 1 protects the heart against ischemia-reperfusion injury. Oxid Med Cell Longev. 2019;2019:1232146. doi: 10.1155/2019/1232146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Napoli E, Song G, Liu S, Espejo A, Perez CJ, Benavides F, Giulivi C. Zdhhc13-dependent Drp1 S-palmitoylation impacts brain bioenergetics, anxiety, coordination and motor skills. Sci Rep. 2017;7:12796. doi: 10.1038/s41598-017-12889-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Marín-García J, Akhmedov AT. Mitochondrial dynamics and cell death in heart failure. Heart Fail Rev. 2016;21:123–136. doi: 10.1007/s10741-016-9530-2 [DOI] [PubMed] [Google Scholar]
- 144.Donnarumma E, Kohlhaas M, Vimont E, Kornobis E, Chaze T, Gianetto QG, Matondo M, Moya-Nilges M, Maack C, Wai T. Mitochondrial Fission Process 1 controls inner membrane integrity and protects against heart failure. Nat Commun. 2022;13:6634. doi: 10.1038/s41467-022-34316-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chaanine AH, Higgins L, Lauterboeck L, Markowski T, Yang Q, Delafontaine P. Multiomics approach reveals an important role of BNIP3 in myocardial remodeling and the pathogenesis of heart failure with reduced ejection fraction. Cells. 2022;11:1572. doi: 10.3390/cells11091572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Neikirk K, Marshall AG, Santisteban MM, Hinton A, Jr. BNIP3 as a new tool to promote healthy brain aging. Aging Cell. n.d.;n/a;23:e14042. doi: 10.1111/acel.14042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu D, Jian C, Peng Q, Hou T, Wu K, Shang B, Zhao M, Wang Y, Zheng W, Ma Q, et al. Prohibitin 2 deficiency impairs cardiac fatty acid oxidation and causes heart failure. Cell Death Dis. 2020;11:1–14. doi: 10.1038/s41419-020-2374-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lewis SA, Takimoto T, Mehrvar S, Higuchi H, Doebley A-L, Stokes G, Sheibani N, Ikeda S, Ranji M, Ikeda A. The effect of Tmem135 overexpression on the mouse heart. PLoS One. 2018;13:e0201986. doi: 10.1371/journal.pone.0201986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Beasley HK, Rodman TA, Collins GV, Hinton A, Exil V. TMEM135 is a novel regulator of mitochondrial dynamics and physiology with implications for human health conditions. Cells. 2021;10:1750. doi: 10.3390/cells10071750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Murphy MP, Hartley RC. Mitochondria as a therapeutic target for common pathologies. Nat Rev Drug Discov. 2018;17:865–886. doi: 10.1038/nrd.2018.174 [DOI] [PubMed] [Google Scholar]
- 151.Kopecka J. Mitochondria-targeted drug delivery. Pharmaceutics. 2022;14:178. doi: 10.3390/pharmaceutics14010178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zacharioudakis E, Gavathiotis E. Mitochondrial dynamics proteins as emerging drug targets. Trends Pharmacol Sci. 2023;44:112–127. doi: 10.1016/j.tips.2022.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Machiraju P, Wang X, Sabouny R, Huang J, Zhao T, Iqbal F, King M, Prasher D, Lodha A, Jimenez-Tellez N, et al. SS-31 peptide reverses the mitochondrial fragmentation present in fibroblasts from patients with DCMA, a mitochondrial cardiomyopathy. Front Cardiovasc Med. 2019;6:167. doi: 10.3389/fcvm.2019.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xu X, Chen C, Lu WJ, Su YL, Shi JY, Liu Y-C, Wang L, Xiao CX, Wu X, Lu Q. Pyrroloquinoline quinone can prevent chronic heart failure by regulating mitochondrial function. Cardiovasc Diagn Ther. 2020;10:453–469. doi: 10.21037/cdt-20-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen H, Pu W, Hu X, Yang C, Zhao G, Hu H, Zhang J. Rational engineering of a mitochondrial-mimetic therapy for targeted treatment of dilated cardiomyopathy by precisely regulating mitochondrial homeostasis. Adv Funct Mater. 2023;33:2301918. doi: 10.1002/adfm.202301918 [Google Scholar]
- 156.Guo Y, Zhang X, Liu Y, Duan H, Jie B, Wu X. Neuregulin-1 attenuates mitochondrial dysfunction in a rat model of heart failure. Chin Med J (Engl). 2012;125:807–814. doi: 10.3760/cma.j.issn.0366-6999.2012.05.015 [PubMed] [Google Scholar]
- 157.Dikalov SI, Harrison DG. Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid Redox Signal. 2014;20:372–382. doi: 10.1089/ars.2012.4886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gioscia-Ryan RA, Battson ML, Cuevas LM, Eng JS, Murphy MP, Seals DR. Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. APSselect. 2017;4:1194–1202. doi: 10.1152/japplphysiol.00670.2017@apsselect.2017.4.issue-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Quintana-Cabrera R, Manjarrés-Raza I, Vicente-Gutiérrez C, Corrado M, Bolaños JP, Scorrano L. Opa1 relies on cristae preservation and ATP synthase to curtail reactive oxygen species accumulation in mitochondria. Redox Biol. 2021;41:101944. doi: 10.1016/j.redox.2021.101944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tsushima K, Bugger H, Wende AR, Soto J, Jenson GA, Tor AR, McGlauflin R, Kenny HC, Zhang Y, Souvenir R, et al. Mitochondrial reactive oxygen species in lipotoxic hearts induces post-translational modifications of AKAP121, DRP1 and OPA1 that promote mitochondrial fission. Circ Res. 2018;122:58–73. doi: 10.1161/CIRCRESAHA.117.311307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Moris D, Spartalis M, Spartalis E, Karachaliou G-S, Karaolanis GI, Tsourouflis G, Tsilimigras DI, Tzatzaki E, Theocharis S. The role of reactive oxygen species in the pathophysiology of cardiovascular diseases and the clinical significance of myocardial redox. Ann Transl Med. 2017;5:326–326. doi: 10.21037/atm.2017.06.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ashrafian H, Czibik G, Bellahcene M, Aksentijević D, Smith AC, Mitchell SJ, Dodd MS, Kirwan J, Byrne JJ, Ludwig C, et al. Fumarate is cardioprotective via activation of the Nrf2 antioxidant pathway. Cell Metab. 2012;15:361–371. doi: 10.1016/j.cmet.2012.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hartwick Bjorkman S, Oliveira Pereira R. The interplay between mitochondrial reactive oxygen species, endoplasmic reticulum stress, and Nrf2 signaling in cardiometabolic health. Antioxid Redox Signal. 2021;35:252–269. doi: 10.1089/ars.2020.8220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yu H, Zhang F, Yan P, Zhang S, Lou Y, Geng Z, Li Z, Zhang Y, Xu Y, Lu Y, et al. LARP7 Protects against heart failure by enhancing mitochondrial biogenesis. Circulation. 2021;143:2007–2022. doi: 10.1161/CIRCULATIONAHA.120.050812 [DOI] [PubMed] [Google Scholar]
- 165.Maekawa S, Takada S, Nambu H, Furihata T, Kakutani N, Setoyama D, Ueyanagi Y, Kang D, Sabe H, Kinugawa S. Linoleic acid improves assembly of the CII subunit and CIII2/CIV complex of the mitochondrial oxidative phosphorylation system in heart failure. Cell Commun Signal. 2019;17:128. doi: 10.1186/s12964-019-0445-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rosenberg P. VDAC2 as a novel target for heart failure: Ca2+ at the sarcomere, mitochondria and SR. Cell Calcium. 2022;104:102586. doi: 10.1016/j.ceca.2022.102586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cibi DM, Bi-Lin KW, Shekeran SG, Sandireddy R, Tee N, Singh A, Wu Y, Srinivasan DK, Kovalik J-P, Ghosh S, et al. Prdm16 deficiency leads to age-dependent cardiac hypertrophy, adverse remodeling, mitochondrial dysfunction, and heart failure. Cell Rep. 2020;33:108288. doi: 10.1016/j.celrep.2020.108288 [DOI] [PubMed] [Google Scholar]
- 168.Quintana-Cabrera R, Scorrano L. Determinants and outcomes of mitochondrial dynamics. Mol Cell. 2023;83:857–876. doi: 10.1016/j.molcel.2023.02.012 [DOI] [PubMed] [Google Scholar]
- 169.McMillan JD, Eisenback MA. Transmission electron microscopy for analysis of mitochondria in mouse skeletal muscle. Bio-Protocol. 2018;8:e2455–e2455. doi: 10.21769/BioProtoc.2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cogliati S, Enriquez JA, Scorrano L. Mitochondrial cristae: where beauty meets functionality. Trends Biochem Sci. 2016;41:261–273. doi: 10.1016/j.tibs.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 171.Kondadi AK, Anand R, Hänsch S, Urbach J, Zobel T, Wolf DM, Segawa M, Liesa M, Shirihai OS, Weidtkamp-Peters S, et al. Cristae undergo continuous cycles of membrane remodelling in a MICOS‐dependent manner. EMBO Rep. 2020;21:e49776. doi: 10.15252/embr.201949776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kozjak-Pavlovic V. The MICOS complex of human mitochondria. Cell Tissue Res. 2017;367:83–93. doi: 10.1007/s00441-016-2433-7 [DOI] [PubMed] [Google Scholar]
- 173.Li H, Ruan Y, Zhang K, Jian F, Hu C, Miao L, Gong L, Sun L, Zhang X, Chen S, et al. Mic60/Mitofilin determines MICOS assembly essential for mitochondrial dynamics and mtDNA nucleoid organization. Cell Death Differ. 2016;23:380–392. doi: 10.1038/cdd.2015.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rampelt H, Wollweber F, Licheva M, de Boer R, Perschil I, Steidle L, Becker T, Bohnert M, van der Klei I, Kraft C, et al. Dual role of Mic10 in mitochondrial cristae organization and ATP synthase-linked metabolic adaptation and respiratory growth. Cell Rep. 2022;38:110290. doi: 10.1016/j.celrep.2021.110290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Anand R, Reichert AS, Kondadi AK. Emerging roles of the MICOS complex in cristae dynamics and biogenesis. Biology (Basel). 2021;10:600. doi: 10.3390/biology10070600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.John GB, Shang Y, Li L, Renken C, Mannella CA, Selker JM, Rangell L, Bennett MJ, Zha J. The mitochondrial inner membrane protein mitofilin controls cristae morphology. Mol Biol Cell. 2005;16:1543–1554. doi: 10.1091/mbc.e04-08-0697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Stephan T, Brüser C, Deckers M, Steyer AM, Balzarotti F, Barbot M, Behr TS, Heim G, Hübner W, Ilgen P, et al. MICOS assembly controls mitochondrial inner membrane remodeling and crista junction redistribution to mediate cristae formation. EMBO J. 2020;39:e104105. doi: 10.15252/embj.2019104105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zeharia A, Friedman JR, Tobar A, Saada A, Konen O, Fellig Y, Shaag A, Nunnari J, Elpeleg O. Mitochondrial hepato-encephalopathy due to deficiency of QIL1/MIC13 (C19orf70), a MICOS complex subunit. Eur J Hum Genet. 2016;24:1778–1782. doi: 10.1038/ejhg.2016.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Guarani V, Jardel C, Chrétien D, Lombès A, Bénit P, Labasse C, Lacène E, Bourillon A, Imbard A, Benoist J-F, et al. QIL1 mutation causes MICOS disassembly and early onset fatal mitochondrial encephalopathy with liver disease. Elife. 2016;5:e17163. doi: 10.7554/eLife.17163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Anand R, Kondadi AK, Meisterknecht J, Golombek M, Nortmann O, Riedel J, Peifer-Weiß L, Brocke-Ahmadinejad N, Schlütermann D, Stork B, et al. MIC26 and MIC27 cooperate to regulate cardiolipin levels and the landscape of OXPHOS complexes. Life Sci Alliance. 2020;3:e202000711. doi: 10.26508/lsa.202000711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Acoba MG, Senoo N, Claypool SM. Phospholipid ebb and flow makes mitochondria go. J Cell Biol. 2020;219:e202003131. doi: 10.1083/jcb.202003131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dong J, Ye F, Lin J, He H, Song Z. The metabolism and function of phospholipids in Mitochondria. Mit Commun. 2023;1:2–12. doi: 10.1016/j.mitoco.2022.10.002 [Google Scholar]
- 183.Weber TA, Koob S, Heide H, Wittig I, Head B, van der Bliek A, Brandt U, Mittelbronn M, Reichert AS. APOOL is a cardiolipin-binding constituent of the Mitofilin/MINOS protein complex determining cristae morphology in mammalian mitochondria. PLoS One. 2013;8:e63683. doi: 10.1371/journal.pone.0063683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mukherjee I, Ghosh M, Meinecke M. MICOS and the mitochondrial inner membrane morphology–when things get out of shape. FEBS Lett. 2021;595:1159–1183. doi: 10.1002/1873-3468.14089 [DOI] [PubMed] [Google Scholar]
- 185.Eramo MJ, Lisnyak V, Formosa LE, Ryan MT. The “mitochondrial contact site and cristae organising system” (MICOS) in health and human disease. J Biochem. 2020;167:243–255. doi: 10.1093/jb/mvz111 [DOI] [PubMed] [Google Scholar]
- 186.Barrera M, Koob S, Dikov D, Vogel F, Reichert AS. OPA1 functionally interacts with MIC60 but is dispensable for crista junction formation. FEBS Lett. 2016;590:3309–3322. doi: 10.1002/1873-3468.12384 [DOI] [PubMed] [Google Scholar]
- 187.Glytsou C, Calvo E, Cogliati S, Mehrotra A, Anastasia I, Rigoni G, Raimondi A, Shintani N, Loureiro M, Vazquez J, et al. Optic atrophy 1 is epistatic to the core MICOS component MIC60 in mitochondrial cristae shape control. Cell Rep. 2016;17:3024–3034. doi: 10.1016/j.celrep.2016.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.He B, Yu H, Liu S, Wan H, Fu S, Liu S, Yang J, Zhang Z, Huang H, Li Q, et al. Mitochondrial cristae architecture protects against mtDNA release and inflammation. Cell Rep. 2022;41:111774. doi: 10.1016/j.celrep.2022.111774 [DOI] [PubMed] [Google Scholar]
- 189.Leveille CF, Mikhaeil JS, Turner KD, Silvera S, Wilkinson J, Fajardo VA. Mitochondrial cristae density: a dynamic entity that is critical for energy production and metabolic power in skeletal muscle. J Physiol. 2017;595:2779–2780. doi: 10.1113/JP274158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Saku T, Takashio S, Tsuruta Y, Otsuka Y, Takae M, Kiyama T, Yamamoto E, Kaikita K, Hotta T, Matsumoto S, et al. Comparison of electron microscopic findings and clinical presentation in three patients with mitochondrial cardiomyopathy caused by the mitochondrial DNA mutation m.3243A > G. Med Mol Morphol. 2021;54:181–186. doi: 10.1007/s00795-020-00268-0 [DOI] [PubMed] [Google Scholar]
- 191.Ranjbarvaziri S, Kooiker KB, Ellenberger M, Fajardo G, Zhao M, Roest ASV, Woldeyes RA, Koyano TT, Fong R, Ma N, et al. Altered cardiac energetics and mitochondrial dysfunction in hypertrophic cardiomyopathy. Circulation. 2021;144:1714–1731. doi: 10.1161/CIRCULATIONAHA.121.053575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xue R, Zhao M-Y, Wu Q, Yang S-R, Cui Y, Yu X-J, Liu J, Zang WJ. Regulation of mitochondrial cristae remodelling by acetylcholine alleviates palmitate-induced cardiomyocyte hypertrophy. Free Radic Biol Med. 2019;145:103–117. doi: 10.1016/j.freeradbiomed.2019.09.025 [DOI] [PubMed] [Google Scholar]
- 193.Birker K, Kirkland NJ, Theis JL, Fogarty ZC, Missinato MA, Kalvakuri S, et al. Mitochondrial MICOS complex genes, implicated in hypoplastic left heart syndrome, maintain cardiac contractility and actomyosin integrity. eLife. 2022;12:2022.06.13.22276366: doi: 10.1101/2022.06.13.22276366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Salisbury-Ruf CT, Bertram CC, Vergeade A, Lark DS, Shi Q, Heberling ML, Fortune NL, Okoye GD, Jerome WG, Wells QS, et al. Bid maintains mitochondrial cristae structure and function and protects against cardiac disease in an integrative genomics study. eLife. 2018;7:e40907. doi: 10.7554/eLife.40907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lam J, Katti P, Biete M, Mungai M, AshShareef S, Neikirk K, Garza Lopez E, Vue Z, Christensen TA, Beasley HK, et al. A universal approach to analyzing transmission electron microscopy with ImageJ. Cells. 2021;10:2177. doi: 10.3390/cells10092177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Crabtree A, Neikirk K, Marshall AG, Vang L, Whiteside AJ, Williams Q, Altamura CT, Owens TC, Stephens D, Shao B, et al. Defining mitochondrial cristae morphology changes induced by aging in brown adipose tissue. Adv Biol (Weinh). 2023;8:e2300186. doi: 10.1002/adbi.202300186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Garza-Lopez E, Vue Z, Katti P, Neikirk K, Biete M, Lam J, Beasley H, Marshall A, Rodman T, Christensen T, et al. Protocols for generating surfaces and measuring 3D organelle morphology using amira. Cells. 2022;11:65. doi: 10.3390/cells11010065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Baena V, Schalek RL, Lichtman JW, Terasaki M. Serial-section electron microscopy using automated tape-collecting ultramicrotome (ATUM). Methods Cell Biol. 2019;152:41–67. doi: 10.1016/bs.mcb.2019.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Titze B, Genoud C. Volume scanning electron microscopy for imaging biological ultrastructure. Biol Cell. 2016;108:307–323. doi: 10.1111/boc.201600024 [DOI] [PubMed] [Google Scholar]
- 200.Polo CC, Fonseca-Alaniz MH, Chen JH, Ekman A, McDermott G, Meneau F, Krieger JE, Miyakawa AA. Three-dimensional imaging of mitochondrial cristae complexity using cryo-soft X-ray tomography. Sci Rep. 2020;10:21045. doi: 10.1038/s41598-020-78150-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Daghistani HM, Rajab BS, Kitmitto A. Three-dimensional electron microscopy techniques for unravelling mitochondrial dysfunction in heart failure and identification of new pharmacological targets. Br J Pharmacol. 2019;176:4340–4359. doi: 10.1111/bph.14499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pinali C, Bennett H, Davenport JB, Trafford AW, Kitmitto A. Three-Dimensional reconstruction of cardiac sarcoplasmic reticulum reveals a continuous network linking transverse-tubules. Circ Res. 2013;113:1219–1230. doi: 10.1161/CIRCRESAHA.113.301348 [DOI] [PubMed] [Google Scholar]
- 203.Pinali C, Bennett HJ, Davenport JB, Caldwell JL, Starborg T, Trafford AW, Kitmitto A. Three-Dimensional structure of the intercalated disc reveals plicate domain and gap junction remodeling in heart failure. Biophys J. 2015;108:498–507. doi: 10.1016/j.bpj.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wakabayashi T. Megamitochondria formation ‐ physiology and pathology. J Cell Mol Med. 2002;6:497–538. doi: 10.1111/j.1582-4934.2002.tb00452.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kanzaki Y, Terasaki F, Okabe M, Otsuka K, Katashima T, Fujita S, Ito T, Kitaura Y. Giant Mitochondria in the myocardium of a patient with mitochondrial cardiomyopathy. Circulation. 2010;121:831–832. doi: 10.1161/CIR.0b013e3181d22e2d [DOI] [PubMed] [Google Scholar]
- 206.Sonwani H, Sahu P, Bandey R, Chandrawanshi Y, Verma P. Simvastatin’s mitochondrial defenses against angiotensin II-induced heart failure. Heart Fail. 2022;3:1030. [Google Scholar]
- 207.Tandler B, Fujioka H, Hoppel CL, Haldar SM, Jain MK. Megamitochondria in cardiomyocytes of a knockout (Klf15−/−) mouse. Ultrastruct Pathol. 2015;39:336–339. doi: 10.3109/01913123.2015.1042610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li A, Shami GJ, Griffiths L, Lal S, Irving H, Braet F. Giant mitochondria in cardiomyocytes: cellular architecture in health and disease. Basic Res Cardiol. 2023;118:39. doi: 10.1007/s00395-023-01011-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Seo AY, Joseph AM, Dutta D, Hwang JCY, Aris JP, Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci. 2010;123:2533–2542. doi: 10.1242/jcs.070490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mueller K, Thiel F, Beutner F, Teren A, Frisch S, Ballarini T, Möller HE, Ihle K, Thiery J, Schuler G, et al. Brain damage with heart failure. Circ Res. 2020;126:750–764. doi: 10.1161/CIRCRESAHA.119.315813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Liu X, Hajnóczky G. Altered fusion dynamics underlie unique morphological changes in mitochondria during hypoxia–reoxygenation stress. Cell Death Differ. 2011;18:1561–1572. doi: 10.1038/cdd.2011.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Eisner V, Picard M, Hajnóczky G. Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat Cell Biol. 2018;20:755–765. doi: 10.1038/s41556-018-0133-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Vincent AE, Turnbull DM, Eisner V, Hajnóczky G, Picard M. Mitochondrial nanotunnels. Trends Cell Biol. 2017;27:787–799. doi: 10.1016/j.tcb.2017.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Vincent AE, White K, Davey T, Philips J, Ogden RT, Lawless C, Warren C, Hall MG, Ng YS, Falkous G, et al. Quantitative 3D mapping of the human skeletal muscle mitochondrial network. Cell Rep. 2019;26:996–1009.e4. doi: 10.1016/j.celrep.2019.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lavorato M, Iyer VR, Dewight W, Cupo RR, Debattisti V, Gomez L, De la Fuente S, Zhao Y-T, Valdivia HH, Hajnóczky G, et al. Increased mitochondrial nanotunneling activity, induced by calcium imbalance, affects intermitochondrial matrix exchanges. Proc Natl Acad Sci USA. 2017;114:E849–E858. doi: 10.1073/pnas.1617788113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Robert P, Nguyen PMC, Richard A, Grenier C, Chevrollier A, Munier M, Grimaud L, Proux C, Champin T, Lelièvre E, et al. Protective role of the mitochondrial fusion protein OPA1 in hypertension. FASEB J. 2021;35:e21678. doi: 10.1096/fj.202000238RRR [DOI] [PubMed] [Google Scholar]
- 217.Zhang L, Trushin S, Christensen TA, Bachmeier BV, Gateno B, Schroeder A, Yao J, Itoh K, Sesaki H, Poon WW, et al. Altered brain energetics induces mitochondrial fission arrest in Alzheimer’s Disease. Sci Rep. 2016;6:18725. doi: 10.1038/srep18725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Neikirk K, Lopez EG, Marshall AG, Alghanem A, Krystofiak E, Kula B, Smith N, Shao J, Katti P, Hinton A. Call to action to properly utilize electron microscopy to measure organelles to monitor disease. Eur J Cell Biol. 2023;102:151365. doi: 10.1016/j.ejcb.2023.151365 [DOI] [PubMed] [Google Scholar]
- 219.Saito K, He Y, Yan X, Yang Y, Wang C, Xu P, Hinton AO, Shu G, Yu L, Tong Q, et al. Visualizing estrogen receptor-α-expressing neurons using a new ERα-ZsGreen reporter mouse line. Metabolism. 2016;65:522–532. doi: 10.1016/j.metabol.2015.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hatano A, Someya M, Tanaka H, Sakakima H, Izumi S, Hoshijima M, Ellisman M, McCulloch AD. Isolation and reconstruction of cardiac mitochondria from SBEM images using a deep learning-based method. J Struct Biol. 2022;214:107806. doi: 10.1016/j.jsb.2021.107806 [DOI] [PubMed] [Google Scholar]
- 221.Rath S, Sharma R, Gupta R, Ast T, Chan C, Durham TJ, Goodman RP, Grabarek Z, Haas ME, Hung WHW, et al. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 2021;49:D1541–D1547. doi: 10.1093/nar/gkaa1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Harper ME, Seifert EL. Thyroid hormone effects on mitochondrial energetics. Thyroid®. 2008;18:145–156. doi: 10.1089/thy.2007.0250 [DOI] [PubMed] [Google Scholar]
- 223.Ventura-Clapier R, Moulin M, Piquereau J, Lemaire C, Mericskay M, Veksler V, Garnier A. Mitochondria: a central target for sex differences in pathologies. Clin Sci (London, England : 1979). 2017;131:803–822. doi: 10.1042/CS20160485 [DOI] [PubMed] [Google Scholar]
- 224.Kenney MC, Chwa M, Atilano SR, Falatoonzadeh P, Ramirez C, Malik D, Tarek M, Del Carpio JC, Nesburn AB, Boyer DS, et al. Molecular and bioenergetic differences between cells with African versus European inherited mitochondrial DNA haplogroups: Implications for population susceptibility to diseases. Biochim Biophys Acta. 2014;1842:208–219. doi: 10.1016/j.bbadis.2013.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hogan KA, Zeidler JD, Beasley HK, Alsaadi AI, Alshaheeb AA, Chang Y-C, Tian H, Hinton AO, McReynolds MR. Using mass spectrometry imaging to visualize age-related subcellular disruption. Front Mol Biosci. 2023;10:906606. doi: 10.3389/fmolb.2023.906606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lewis SC, Uchiyama LF, Nunnari J. ER-mitochondria contacts couple mtDNA synthesis with mitochondrial division in human cells. Science. 2016;353:aaf5549. doi: 10.1126/science.aaf5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Davlouros AP, Gkizas V, Vogiatzi C, Giannopoulos G, Alexopoulos D, Deftereos S. Calcium homeostasis and kinetics in heart failure. Med Chem. 2016;12:151–161. [DOI] [PubMed] [Google Scholar]
- 228.Ho P, Fan J, Hayes NL, Saada NI, Palade P, Glembotski C, McDonough PM. Ras reduces L-type calcium channel current in cardiac myocytes: corrective effects of L-channels and SERCA2 on [Ca2+]i regulation and cell morphology. Circ Res. 2001;88:63–69. doi: 10.1161/01.RES.88.1.63 [DOI] [PubMed] [Google Scholar]
- 229.Gorski PA, Ceholski D, Hajjar R. Altered myocardial calcium cycling and energetics in heart failure--a rational approach for disease treatment. Cell Metab. 2015;21:183–194. doi: 10.1016/j.cmet.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zhihao L, Jingyu N, Lan L, Michael S, Rui G, Xiyun B, Xiaozhi L, Guanwei F. SERCA2a: a key protein in the Ca2+ cycle of the heart failure. Heart Fail Rev. 2020;25:523–535. doi: 10.1007/s10741-019-09873-3 [DOI] [PubMed] [Google Scholar]
- 231.Wilkins B, Molkentin J. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem Biophys Res Commun. 2004;322:1178–1191. doi: 10.1016/J.BBRC.2004.07.121 [DOI] [PubMed] [Google Scholar]
- 232.Xu H, Cui S, Zhang Y, Ren J. Mitochondrial Ca2+ regulation in the etiology of heart failure: physiological and pathophysiological implications. Acta Pharmacol Sin. 2020;41:1301–1309. doi: 10.1038/s41401-020-0476-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Poor TA, Chandel NS. SnapShot: mitochondrial signaling. Mol Cell. 2023;83:1012–1012.e1. doi: 10.1016/j.molcel.2023.01.008 [DOI] [PubMed] [Google Scholar]
- 234.Csordás G, Weaver D, Hajnóczky G. Endoplasmic Reticulum–Mitochondrial contactology: structure and signaling functions. Trends Cell Biol. 2018;28:523–540. doi: 10.1016/j.tcb.2018.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Giacomello M, Pellegrini L. The coming of age of the mitochondria–ER contact: a matter of thickness. Cell Death Differ. 2016;23:1417–1427. doi: 10.1038/cdd.2016.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Carreras-Sureda A, Jaña F, Urra H, Durand S, Mortenson DE, Sagredo A, Bustos G, Hazari Y, Ramos-Fernández E, Sassano ML, et al. Non-canonical function of IRE1α determines mitochondria-associated endoplasmic reticulum composition to control calcium transfer and bioenergetics. Nat Cell Biol. 2019;21:755–767. doi: 10.1038/s41556-019-0329-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Chen Q, Samidurai A, Thompson J, Hu Y, Das A, Willard B, Lesnefsky EJ. Endoplasmic reticulum stress-mediated mitochondrial dysfunction in aged hearts. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165899. doi: 10.1016/j.bbadis.2020.165899 [DOI] [PubMed] [Google Scholar]
- 238.Santulli G, Xie W, Reiken SR, Marks AR. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci USA. 2015;112:11389–11394. doi: 10.1073/pnas.1513047112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Seidlmayer LK, Kuhn J, Berbner A, Arias-Loza PA, Williams T, Kaspar M, Czolbe M, Kwong JQ, Molkentin JD, Heinze KG, et al. Inositol 1,4,5-trisphosphate-mediated sarcoplasmic reticulum–mitochondrial crosstalk influences adenosine triphosphate production via mitochondrial Ca 2+ uptake through the mitochondrial ryanodine receptor in cardiac myocytes. Cardiovasc Res. 2016;112:491–501. doi: 10.1093/cvr/cvw185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bhatti JS, Bhatti GK, Reddy PH. Mitochondrial dysfunction and oxidative stress in metabolic disorders: a step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1066–1077. doi: 10.1016/j.bbadis.2016.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Su LJ, Zhang JH, Gomez H, Murugan R, Hong X, Xu D, Jiang F, Peng ZY. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med Cell Longev. 2019;2019:5080843. doi: 10.1155/2019/5080843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Liu T, Yang N, Sidor A, O’Rourke B. MCU Overexpression rescues inotropy and reverses heart failure by reducing SR Ca2+ leak. Circ Res. 2021;128:1191–1204. doi: 10.1161/CIRCRESAHA.120.318562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Rasmussen TP, Wu Y, Joiner MA, Koval OM, Wilson NR, Luczak ED, Wang Q, Chen B, Gao Z, Zhu Z, et al. Inhibition of MCU forces extramitochondrial adaptations governing physiological and pathological stress responses in heart. Proc Natl Acad Sci USA. 2015;112:9129–9134. doi: 10.1073/pnas.1504705112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Rodríguez-Prados M, Berezhnaya E, Castromonte MT, Menezes-Filho SL, Paillard M, Hajnóczky G. MICU1 occludes the mitochondrial calcium uniporter in divalent-free conditions. Proc Natl Acad Sci USA. 2023;120:e2218999120. doi: 10.1073/pnas.2218999120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Garbincius JF, Luongo TS, Elrod JW. The debate continues – What is the role of MCU and mitochondrial calcium uptake in the heart? J Mol Cell Cardiol. 2020;143:163–174. doi: 10.1016/j.yjmcc.2020.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Schwemmlein J, Maack C, Bertero E. Mitochondria as therapeutic targets in heart failure. Curr Heart Fail Rep. 2022;19:27–37. doi: 10.1007/s11897-022-00539-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ziegler DV, Vindrieux D, Goehrig D, Jaber S, Collin G, Griveau A, Wiel C, Bendridi N, Djebali S, Farfariello V, et al. Calcium channel ITPR2 and mitochondria–ER contacts promote cellular senescence and aging. Nat Commun. 2021;12:720. doi: 10.1038/s41467-021-20993-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ren L, Gopireddy RR, Perkins G, Zhang H, Timofeyev V, Lyu Y, Diloretto DA, Trinh P, Sirish P, Overton JL, et al. Disruption of mitochondria–sarcoplasmic reticulum microdomain connectomics contributes to sinus node dysfunction in heart failure. Proc Natl Acad Sci USA. 2022;119:e2206708119. doi: 10.1073/pnas.2206708119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Sampieri A, Santoyo K, Asanov A, Vaca L. Association of the IP3R to STIM1 provides a reduced intraluminal calcium microenvironment, resulting in enhanced store-operated calcium entry. Sci Rep. 2018;8:13252. doi: 10.1038/s41598-018-31621-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Katona M, Bartók A, Nichtova Z, Csordás G, Berezhnaya E, Weaver D, Ghosh A, Várnai P, Yule DI, Hajnóczky G. Capture at the ER-mitochondrial contacts licenses IP3 receptors to stimulate local Ca2+ transfer and oxidative metabolism. Nat Commun. 2022;13:6779. doi: 10.1038/s41467-022-34365-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Sander P, Gudermann T, Schredelseker J. A calcium guard in the outer membrane: Is VDAC a regulated Gatekeeper of mitochondrial calcium uptake? Int J Mol Sci . 2021;22:946. doi: 10.3390/ijms22020946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Liu Y, Ma X, Fujioka H, Liu J, Chen S, Zhu X. DJ-1 regulates the integrity and function of ER-mitochondria association through interaction with IP3R3-Grp75-VDAC1. Proc Natl Acad Sci USA. 2019;116:25322–25328. doi: 10.1073/pnas.1906565116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Niere F, Uneri A, McArdle CJ, Deng Z, Egido-Betancourt HX, Cacheaux LP, Namjoshi SV, Taylor WC, Wang X, Barth SH, et al. Aberrant DJ-1 expression underlies L-type calcium channel hypoactivity in dendrites in tuberous sclerosis complex and Alzheimer’s disease. Proc Natl Acad Sci USA. 2023;120:e2301534120. doi: 10.1073/pnas.2301534120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Li J, Qi M, Li C, Shi D, Zhang D, Xie D, Yuan T, Feng J, Liu Y, Liang D, et al. Tom70 serves as a molecular switch to determine pathological cardiac hypertrophy. Cell Res. 2014;24:977–993. doi: 10.1038/cr.2014.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Filadi R, Leal NS, Schreiner B, Rossi A, Dentoni G, Pinho CM, Wiehager B, Cieri D, Calì T, Pizzo P, et al. TOM70 sustains cell bioenergetics by promoting IP3R3-mediated ER to mitochondria Ca2+ transfer. Curr Biol. 2018;28:369–382.e6. doi: 10.1016/j.cub.2017.12.047 [DOI] [PubMed] [Google Scholar]
- 256.Paillard M, Tubbs E, Thiebaut PA, Gomez L, Fauconnier J, Crola Da Silva C, Teixeira G, Mewton N, Belaidi E, Durand A, et al. Depressing mitochondria-reticulum interactions protects cardiomyocytes from lethal hypoxia-reoxygenation injury. Circulation. 2013;128:1555–1565. doi: 10.1161/circulationaha.113.001225 [DOI] [PubMed] [Google Scholar]
- 257.Golic I, Velickovic K, Markelic M, Stancic A, Jankovic A, Vucetic M, Otasevic V, Buzadzic B, Korac B, Korac A. Calcium-induced alteration of mitochondrial morphology and mitochondrial-endoplasmic reticulum contacts in rat brown adipocytes. Eur J Histochem. 2014;58:2377. doi: 10.4081/ejh.2014.2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Csordás G, Várnai P, Golenár T, Roy S, Purkins G, Schneider TG, Balla T, Hajnóczky G. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell. 2010;39:121–132. doi: 10.1016/j.molcel.2010.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.SanMartín CD, Veloso P, Adasme T, Lobos P, Bruna B, Galaz J, García A, Hartel S, Hidalgo C, Paula-Lima AC. RyR2-Mediated Ca2+ release and mitochondrial ROS generation partake in the synaptic dysfunction caused by amyloid β peptide oligomers. Front Mol Neurosci. 2017;10:115. doi: 10.3389/fnmol.2017.00115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Piao L, Fang Y-H, Fisher M, Hamanaka RB, Ousta A, Wu R, Mutlu GM, Garcia AJ, Archer SL, Sharp WW. Dynamin-related protein 1 is a critical regulator of mitochondrial calcium homeostasis during myocardial ischemia/reperfusion injury. FASEB J. 2024;38:e23379. doi: 10.1096/fj.202301040RR [DOI] [PubMed] [Google Scholar]
- 261.Dubois M, Boulghobra D, Rochebloine G, Pallot F, Yehya M, Bornard I, Gayrard S, Coste F, Walther G, Meyer G, et al. Hyperglycemia triggers RyR2-dependent alterations of mitochondrial calcium homeostasis in response to cardiac ischemia-reperfusion: Key role of DRP1 activation. Redox Biol. 2024;70:103044. doi: 10.1016/j.redox.2024.103044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Peruzzo R, Costa R, Bachmann M, Leanza L, Szabò I. Mitochondrial metabolism, contact sites and cellular calcium signaling: implications for tumorigenesis. Cancers. 2020;12:2574. doi: 10.3390/cancers12092574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kohlhaas M, Nickel AG, Maack C. Mitochondrial energetics and calcium coupling in the heart. J Physiol. 2017;595:3753–3763. doi: 10.1113/JP273609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Schwarz DS, Blower MD. The endoplasmic reticulum: structure, function and response to cellular signaling. Cell Mol Life Sci. 2016;73:79–94. doi: 10.1007/s00018-015-2052-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ilacqua N, Anastasia I, Raimondi A, Lemieux P, de Aguiar Vallim TQ, Toth K, Koonin EV, Pellegrini L. A three-organelle complex made by wrappER contacts with peroxisomes and mitochondria responds to liver lipid flux changes. J Cell Sci. 2021;135:jcs259091. doi: 10.1242/jcs.259091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Nichtová Z, Fernandez-Sanz C, De La Fuente S, Yuan Y, Hurst S, Lanvermann S, Tsai HY, Weaver D, Baggett A, Thompson C, et al. Enhanced Mitochondria-SR tethering triggers adaptive cardiac muscle remodeling. Circ Res. 2023;132:e171–e187. doi: 10.1161/CIRCRESAHA.122.321833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Liu YJ, Auwerx J. Mitochondria: A “pacemaker” for species-specific development. Mol Cell. 2023;83:824–826. doi: 10.1016/j.molcel.2023.02.025 [DOI] [PubMed] [Google Scholar]
- 268.Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED. Cardiac energy metabolism in heart failure. Circ Res. 2021;128:1487–1513. doi: 10.1161/CIRCRESAHA.121.318241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sharma S, Bhattarai S, Ara H, Sun G, St Clair DK, Bhuiyan MS, Kevil C, Watts MN, Dominic P, Shimizu T, et al. SOD2 deficiency in cardiomyocytes defines defective mitochondrial bioenergetics as a cause of lethal dilated cardiomyopathy. Redox Biol. 2020;37:101740–101740. doi: 10.1016/j.redox.2020.101740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Lommi J, Kupari M, Koskinen P, Näveri H, Leinonen H, Pulkki K, Härkönen M. Blood ketone bodies in congestive heart failure. J Am Coll Cardiol. 1996;28:665–672. doi: 10.1016/0735-1097(96)00214-8 [DOI] [PubMed] [Google Scholar]
- 271.Ritterhoff J, Tian R. Metabolism in cardiomyopathy: every substrate matters. Cardiovasc Res. 2017;113:411–421. doi: 10.1093/cvr/cvx017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ussher J, Jaswal J, Lopaschuk G. Pyridine nucleotide regulation of cardiac intermediary metabolism. Circ Res. 2012;111:628–641. doi: 10.1161/CIRCRESAHA.111.246371 [DOI] [PubMed] [Google Scholar]
- 273.Zhang Q, Li Z, Li Q, Trammell SA, Schmidt MS, Pires KM, Cai J, Zhang Y, Kenny H, Boudina S, et al. Control of NAD+ homeostasis by autophagic flux modulates mitochondrial and cardiac function. EMBO J. 2024;43:362–390. doi: 10.1038/s44318-023-00009-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Patgiri A, Skinner OS, Miyazaki Y, Schleifer G, Marutani E, Shah H, Sharma R, Goodman RP, To TL, Robert Bao X, et al. An engineered enzyme that targets circulating lactate to alleviate intracellular NADH:NAD+ imbalance. Nat Biotechnol. 2020;38:309–313. doi: 10.1038/s41587-019-0377-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sharma R, Reinstadler B, Engelstad K, Skinner OS, Stackowitz E, Haller RG, Clish CB, Pierce K, Walker MA, Fryer R, et al. Circulating markers of NADH-reductive stress correlate with mitochondrial disease severity. J Clin Invest. 2021;131:e136055. doi: 10.1172/JCI136055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Titov DV, Cracan V, Goodman RP, Peng J, Grabarek Z, Mootha VK. Complementation of mitochondrial electron transport chain by manipulation of the NAD+/NADH ratio. Science. 2016;352:231–235. doi: 10.1126/science.aad4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.King N. Amino Acids and the Mitochondria. In: Schaffer SW, Suleiman M-S, editors. Mitochondria: The Dynamic Organelle: Springer; 2007, p. 151–166. doi:10.1007/978-0-387-69945-5_6. [Google Scholar]
- 278.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9 [DOI] [PubMed] [Google Scholar]
- 279.Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, Wang Z, Jeyaraj D, Youn JY, Ren S, Liu Y, et al. Catabolic defect of branched-chain amino acids promotes heart failure. Circulation. 2016;133:2038–2049. doi: 10.1161/CIRCULATIONAHA.115.020226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Burtscher J, Soltany A, Visavadiya NP, Burtscher M, Millet GP, Khoramipour K, Khamoui AV. Mitochondrial stress and mitokines in aging. Aging Cell. 2023;22:e13770. doi: 10.1111/acel.13770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Duan J, Chen Z, Wu Y, Zhu B, Yang L, Yang C. Metabolic remodeling induced by mitokines in heart failure. Aging (Albany NY). 2019;11:7307–7327. doi: 10.18632/aging.102247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Conte M, Sabbatinelli J, Chiariello A, Martucci M, Santoro A, Monti D, Arcaro M, Galimberti D, Scarpini E, Bonfigli AR, et al. Disease-specific plasma levels of mitokines FGF21, GDF15, and Humanin in type II diabetes and Alzheimer’s disease in comparison with healthy aging. GeroScience. 2021;43:985–1001. doi: 10.1007/s11357-020-00287-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Suzuki T, Palus S, Springer J. Skeletal muscle wasting in chronic heart failure. ESC Heart Fail. 2018;5:1099–1107. doi: 10.1002/ehf2.12387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Pereira RO, Tadinada SM, Zasadny FM, Oliveira KJ, Pires KMP, Olvera A, Jeffers J, Souvenir R, Mcglauflin R, Seei A, et al. OPA 1 deficiency promotes secretion of FGF 21 from muscle that prevents obesity and insulin resistance. EMBO J. 2017;36:2126–2145. doi: 10.15252/embj.201696179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Hahn VS, Petucci C, Kim M-S, Bedi KC, Wang H, Mishra S, Koleini N, Yoo EJ, Margulies KB, Arany Z, et al. Myocardial metabolomics of human heart failure with preserved ejection fraction. Circulation. 2023;147:1147–1161. doi: 10.1161/CIRCULATIONAHA.122.061846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Yoneshiro T, Wang Q, Tajima K, Matsushita M, Maki H, Igarashi K, Dai Z, White PJ, McGarrah RW, Ilkayeva OR, et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature. 2019;572:614–619. doi: 10.1038/s41586-019-1503-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Shi X, Reinstadler B, Shah H, To TL, Byrne K, Summer L, Calvo SE, Goldberger O, Doench JG, Mootha VK, et al. Combinatorial GxGxE CRISPR screen identifies SLC25A39 in mitochondrial glutathione transport linking iron homeostasis to OXPHOS. Nat Commun. 2022;13:2483. doi: 10.1038/s41467-022-30126-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Qi Z, Pu Y, Guo H, Tang W, Xiong Y, Ran B. Identification and subtype analysis of biomarkers associated with the solute carrier family in acute myocardial infarction. Medicine (Baltimore). 2023;102:e36515. doi: 10.1097/MD.0000000000036515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Zhang R, Di C, Gao H, Zhu Y, Li C, Zhu Z, Wang Q, Wang J, Zhou F, Wang S. Identification of iron metabolism-related genes in the circulation and myocardium of patients with sepsis via applied bioinformatics analysis. Front Cardiovasc Med. 2023;10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Kumar V, Santhosh Kumar TR, Kartha CC. Mitochondrial membrane transporters and metabolic switch in heart failure. Heart Fail Rev. 2019;24:255–267. doi: 10.1007/s10741-018-9756-2 [DOI] [PubMed] [Google Scholar]
- 291.Schulze PC, Drosatos K, Goldberg IJ. Lipid use and misuse by the heart. Circ Res. 2016;118:1736–1751. doi: 10.1161/CIRCRESAHA.116.306842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Krishnan J, Suter M, Windak R, Krebs T, Felley A, Montessuit C, Tokarska-Schlattner M, Aasum E, Bogdanova A, Perriard E, et al. Activation of a HIF1alpha-PPARgamma axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab. 2009;9:512–524. doi: 10.1016/j.cmet.2009.05.005 [DOI] [PubMed] [Google Scholar]
- 293.Exil VJ, Gardner CD, Rottman JN, Sims H, Bartelds B, Khuchua Z, Sindhal R, Ni G, Strauss AW. Abnormal mitochondrial bioenergetics and heart rate dysfunction in mice lacking very-long-chain acyl-CoA dehydrogenase. Am J Physiol Heart Circ Physiol. 2006;290:H1289–H1297. doi: 10.1152/ajpheart.00811.2005 [DOI] [PubMed] [Google Scholar]
- 294.Bedi KC, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, et al. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation. 2016;133:706–716. doi: 10.1161/CIRCULATIONAHA.115.017545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Lei P, Tian S, Teng C, Huang L, Liu X, Wang J, Zhang Y, Li B, Shan Y. Sulforaphane improves lipid metabolism by enhancing mitochondrial function and biogenesis in vivo and in vitro. Mol Nutr Food Res. 2019;65: doi: 10.1002/mnfr.201800795 [DOI] [PubMed] [Google Scholar]
- 296.Herms A, Bosch M, Ariotti N, Reddy BJN, Fajardo A, Fernández-Vidal A, Alvarez-Guaita A, Fernández-Rojo MA, Rentero C, Tebar F, et al. Cell-to-cell heterogeneity in lipid droplets suggests a mechanism to reduce lipotoxicity. Curr Biol. 2013;23:1489–1496. doi: 10.1016/j.cub.2013.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.De Jong KA, Lopaschuk GD. Complex energy metabolic changes in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. Can J Cardiol. 2017;33:860–871. doi: 10.1016/j.cjca.2017.03.009 [DOI] [PubMed] [Google Scholar]
- 298.Weixler V, Lapusca R, Grangl G, Guariento A, Saeed MY, Cowan DB, Del Nido PJ, McCully JD, Friehs I. Autogenous mitochondria transplantation for treatment of right heart failure. J Thorac Cardiovasc Surg. 2021;162:e111–e121. doi: 10.1016/j.jtcvs.2020.08.011 [DOI] [PubMed] [Google Scholar]
- 299.Liu Y, Wu M, Zhong C, Xu B, Kang L. M2-like macrophages transplantation protects against the doxorubicin-induced heart failure via mitochondrial transfer. Biomater Res. 2022;26:14. doi: 10.1186/s40824-022-00260-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Oh HSH, Rutledge J, Nachun D, Pálovics R, Abiose O, Moran-Losada P, Channappa D, Urey DY, Kim K, Sung YJ, et al. Organ aging signatures in the plasma proteome track health and disease. Nature. 2023;624:164–172. doi: 10.1038/s41586-023-06802-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Xie H, Zhang B, Xie M, Li T. Circulating metabolic signatures of heart failure in precision cardiology. Precis Clin Med. 2023;6:pbad005. doi: 10.1093/pcmedi/pbad005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Scandalis L, Kitzman DW, Nicklas BJ, Lyles M, Brubaker P, Nelson MB, Gordon M, Stone J, Bergstrom J, Neufer PD, et al. Skeletal muscle mitochondrial respiration and exercise intolerance in patients with heart failure with preserved ejection fraction. JAMA Cardiol. 2023;8:575–584. doi: 10.1001/jamacardio.2023.0957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Vega-Vásquez T, Langgartner D, Wang JY, Reber SO, Picard M, Basualto-Alarcón C. Mitochondrial morphology in the mouse adrenal cortex: Influence of chronic psychosocial stress. Psychoneuroendocrinology. 2024;160:106683. doi: 10.1016/j.psyneuen.2023.106683 [DOI] [PMC free article] [PubMed] [Google Scholar]