Abstract
Objective
Clinicians treating patients with patellofemoral pain (PFP) rely on consensus statements to make the best practice recommendations in the absence of definitive evidence on how to manage PFP. However, the methods used to generate and assess agreement for these recommendations have not been examined. Our objective was to map the methods used to generate consensus-based recommendations for PFP and apply four novel questions to assess the rigour of consensus development.
Design
Scoping review.
Data sources
We searched Medline, SPORTDiscus, CINAHL and Embase from inception to May 2022 to identify consensus-derived statements or practice guidelines on PFP. The Joanna Briggs Institute Manual for Evidence Synthesis was followed to map the existing evidence. We measured the consensus methods based on four sets of questions addressing the panel composition, application of the consensus method chosen, agreement process and the use of evidence mapping.
Eligibility criteria
All consensus statements or clinical guidelines on PFP were considered.
Results
Twenty-two PFP consensus statements were identified. Panel composition: 3 of the 22 (14%) consensus groups reported the panellists’ experience, 2 (9%) defined a desired level of expertise, 10 (45%) reported panellist sex and only 2 (9%) included a patient. Consensus method: 7 of 22 (32%) reported using an established method of consensus measurement/development. Agreement process: 10 of 22 (45%) reported their consensus threshold and 2 (9%) acknowledged dissenting opinions among the panel. Evidence mapping: 6 of 22 (27%) reported using systematic methods to identify relevant evidence gaps.
Conclusions
PFP consensus panels have lacked diversity and excluded key partners including patients. Consensus statements on PFP frequently fail to use recognised consensus methods, rarely describe how ‘agreement’ was defined or measured and often neglect to use systematic methods to identify evidence gaps.
Keywords: Osteoarthritis, Consensus, Methods, Patellofemoral Pain Syndrome, Review
WHAT IS ALREADY KNOWN?
Consensus statements aim to provide direction when evidence is not available, or when conflicts or interpretations of the evidence diverge. Currently, there is no standard method to evaluate the rigour of consensus statements.
WHAT ARE THE NEW FINDINGS?
Published patellofemoral pain consensus statements have not used recognised methods to generate recommendations or assess agreement.
Patellofemoral consensus processes have used a narrow definition of ‘expert’, seldom including ‘expertise’ outside of professional clinical experience. This has left key stakeholders, such as patients, under-represented and with a limited voice.
Consensus panels have been male dominated and failed to include representatives from low or lower-middle income countries.
Patellofemoral consensus statements often did not synthesize the evidence to identify knowledge gaps.
Introduction
Consensus statements and their closely related cousins, position statements and clinical practice guidelines (herein referred to as ‘statements’), significantly influence clinical and research practices. Consensus methods are most often used by the scientific community to answer questions where scientific evidence is lacking, or when disagreements arise on the interpretation of the evidence.1 2 The employment of consensus methods and publication of their subsequent statements can direct large-scale research projects with significant implications for the future assessment and management of patients (for instance the Young Athlete’s Hip Research Collaborative or OPTIKNEE processes).3–5
Authors have criticised consensus processes for lacking methodological rigour, and neglecting to include all the key partners relevant to the problems they purport to address.6–9 This may call into question the authority of consensus statements and the utility of their recommendations.10
Expert agreement has often been sought on topics related to patellofemoral pain (PFP) due to evidence gaps, or a lack of knowledge/disagreement on how to apply what is known. For instance, the lack of definitive studies to inform the aetiology, prognosis and management of PFP, has necessitated the use of consensus methods to establish the best practice assessment and treatment, and to set research priorities.11–13 No previous study has mapped the methods used to gain consensus on topics related to PFP or patellofemoral osteoarthritis nor have the methods to generate recommendations and gain agreement been subject to scrutiny.8
Therefore, the objectives of this scoping review were to:
Map the consensus methods used to make practice recommendations on PFP or patellofemoral osteoarthritis.
Review the rigour of the methods using four novel questions related to: who was invited to participate; how consensus was generated; how subsequent agreement/dissent was reported; and whether scientific literature reviews were used to highlight gaps in the evidence, generate statements and/or inform panellist decisions.10
Methods
This scoping review was conducted according to the Joanna Briggs Institute Manual for Evidence Synthesis,14 15 and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) for reporting scoping reviews.16 The published protocol is available on the open science framework (https://osf.io/y2m3p/).
Definitions
The only taxonomy of consensus-based studies that exist in the medical literature is from the European Cystic Fibrosis Society (ECFS, 2014).17 Building on the ECFS taxonomy, the following definitions were used for the purposes of this scoping review:
Consensus statement: a statement that results from a consensus generation process involving interested partners, which explicitly includes a voting process to measure level of agreement.
Position statement: a statement from a specific group(s) or party that may or may not include methods to generate consensus, nor an explicit voting process.
Clinical practice guideline: a report that may or may not include a rigorous systematic review and synthesis of the published medical literature.18 These may also involve a consensus process and a formal rating of the evidence (eg, using The Grading of Recommendations Assessment, Development and Evaluation (GRADE)).19
Eligibility criteria
We included consensus statements, position statements or clinical practice guidelines (as described above) that provided recommendations on the assessment, diagnosis and/or management of PFP. Although there is some debate over whether PFP is a direct precursor to patellofemoral osteoarthritis (ie, that they exist on a continuum), we decided to include statements on patellofemoral osteoarthritis. Consensus was operationalised as a report that voting or another method of consensus generation among participants was used to arrive at a set of final reported recommendations. Examples of a clearly identified consensus methodology included the modified or unmodified Delphi, Nominal Group Technique, RAND-UCLA appropriateness method, or informal agreement among participants. Any report that identified as a ‘consensus statement’ was included for review, even in the absence of clear consensus methods.
We excluded reports of clinical practice guidelines that did not use a recognised consensus method—normally due to their reliance on evidence summaries such as GRADE—to reach their recommendations (eg, Willy et al, 2019—Patellofemoral Pain Clinical Practice Guidelines).19 Statements that focused on traumatic causes of PFP including patellofemoral instability post dislocation or PFP in the presence of hypermobility were also excluded.
Information sources
To identify appropriate statements, the following bibliographic databases were searched: Medline (via Ovid); SPORTDiscus; CINAHL (via EBSCO); and Embase (via Ovid). All databases were searched from database inception to 4 May 2022. A medical research librarian supported the development of a comprehensive search strategy (see acknowledgements). An example of the full search strategy is presented for Medline (via Ovid) in table 1. The search strategies for all databases can be found in online supplemental appendices A1-A4.
Table 1.
Search strategy for Ovid Medline
| Search number | Query | Results |
| 1 | Patellofemoral Pain Syndrome/ | 1082 |
| 2 | (patellofemoral adj3 (pain or syndrome)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | 2290 |
| 3 | (patellar femoral adj3 (pain or syndrome)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | 3 |
| 4 | PFPS.mp. | 533 |
| 5 | anterior knee pain.mp. | 2044 |
| 6 | Chondromalacia Patellae/ | 96 |
| 7 | Sinding larsen johansson.mp. | 43 |
| 8 | runner* knee.mp. | 29 |
| 9 | plica syndrome.mp. | 119 |
| 10 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 | 4228 |
| 11 | Consensus/ | 18 440 |
| 12 | Consensus Development Conference/ | 12 306 |
| 13 | Consensus Development Conference, NIH/ | 801 |
| 14 | (Consensus adj3 (statement or paper)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | 7476 |
| 15 | ((position or policy) adj3 (statement or paper)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | 11 880 |
| 16 | Practice Guideline/ | 29 792 |
| 17 | practice guideline.mp. | 35 320 |
| 18 | Declaration.mp. | 9682 |
| 19 | 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 | 84 269 |
| 20 | 10 and 19 | 14 |
bjsports-2023-107552supp001.pdf (411.7KB, pdf)
All articles that met the inclusion criteria for full-text review underwent bibliometric indexing (backward citation tracking) of their references to search for references to previous consensus or position statements, or clinical practice guidelines on PFP. Where articles were not published in English, they were translated using Google Translate. No article was excluded due to language restrictions.
A comprehensive grey literature search was also developed in collaboration with the medical librarian, based on search guidelines from Godin et al.20 Briefly, this strategy involves four key themes: targeted website searching and browsing; grey literature database searches using sites such as Proquest Dissertations and Theses Global; search engine searches conducted in line with the best practice guidance offer by Haddaway et al 21; and contacting knowledge experts. Detailed explanation of all grey literature searches can be found in online supplemental appendix A5.
All searches were transferred into Covidence (Veritas Health Innovation). All titles and abstracts were screened by two reviewers (PB and JML). Articles that passed title and abstract screening were retrieved in full text to further gauge eligibility against the eligibility criteria. A pilot was conducted with three studies to ensure consistency between reviewers. Once calibration had taken place, all texts were read in full by both reviewers. Where disagreements occurred over inclusion in the final review, these were resolved via discussion and if necessary the vote of a third team member (KMK).
Data charting
A data charting template was created to extract data from included studies. This was piloted with five studies (PB and JML) to ensure consistency in reporting or ranking items, as recommended best practice data extraction techniques for scoping reviews.22 Where information was not available, the contact authors for each source were contacted via email on at least two separate occasions to request further information.
Data extraction (see online supplemental appendix B for the full data charting template) included the following categories, divided into research metadata, and the primary and secondary aims.
Metadata
Title.
First author.
Year published.
Years since previous iteration (if applicable).
Stated aim of the consensus process (examples include to derive treatment recommendations, or set priorities for future research).
Data extracted on consensus development process
Number of panellists/experts.
Experience of panellists (years).
Definition of expertise (if present).
Inclusion criteria for panellists (if present).
Sex balance of the panel.
Countries represented on the panel.
Low/lower-middle income countries represented on the panel.
Mix of partners (professions, patients, policy-makers) included.
Whether a Stakeholder Analysis was completed.
Whether questions were explicitly systematic or scoping review informed.
Whether the questions asked of panelists were presented (either in the text or online supplemental material).
What consensus method was reported (examples include Delphi, RAND-UCLA, Nominal Group Technique).
Which method of consensus was used (if different from that reported in the methods or if no method stated then listed as ‘unclear’).
Was the consensus level of agreement decided a priori (before the process began).
What was the method used to represent agreement of the panel.
Were dissenting opinions acknowledged and reported.
Were funding/conflicts of interest reported.
Box 1 provides definitions to explain how we operationalised some of the criteria listed in the methods of consensus development.
Box 1. Glossary of definitions.
Definition of expertise: would include any rationale supplied by the authors to explain why their panel qualified as ‘experts’ to answer the questions their process aimed to address.
Sex-balance among panels: the sex split of panels was estimated from given names reported in the final manuscript, or where unclear from web searches.
Low or lower-middle income countries: the involvement of representatives from low or lower-middle income countries was defined by noting the inclusion of at least one panel member from a country listed in either category by the World Bank (https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups).
Stakeholder analysis: the use of a formal method to identify potential parties or partners that would be either interested or impacted by the statement, and therefore invited to participate in the process (including but not limited to: gaining consensus; approving the statement; implementation of the recommendations).
Questions informed by systematic or scoping review: is there a clear process for how the scientific review of available literature led to the questions presented to the panel either in the main statement or online supplemental material?
Acknowledgement of dissent: did the statement include any information on items that proved contentious among the panel? Simply saying an item was removed from agreement was not enough, there needed to be a clear discussion of what items may have been included despite a large number of votes against inclusion. Ideally with additional explanation as to why.
Critical appraisal: using a novel tool to assess methodological rigour
Our original protocol outlined data charting, but no process of appraisal. Scoping reviews have been criticised for not including a quality assessment, which makes interpretation of the data challenging.23 In a deviation from our protocol (https://osf.io/y2m3p/), we decided to perform a qualitative content analysis.22
There is currently no known quality-rating system with which to design or judge consensus-based methods, and the reporting guideline for consensus-based methods in biomedical research was published following the completion of our work.24 25 Therefore, in the absence of a reporting or quality guideline with which to describe or assess a consensus development process and its subsequent statement, we used four sets of questions as a lens through which to view existing statements.10 These four sets of questions were previously described as supporting an evidence-informed appraisal of the conduct of consensus development in sport and exercise medicine.10 Critically, the four sets of questions were based on both the Conducting and Reporting Delphi Studies guideline and critiques from the literature on consensus development processes.26
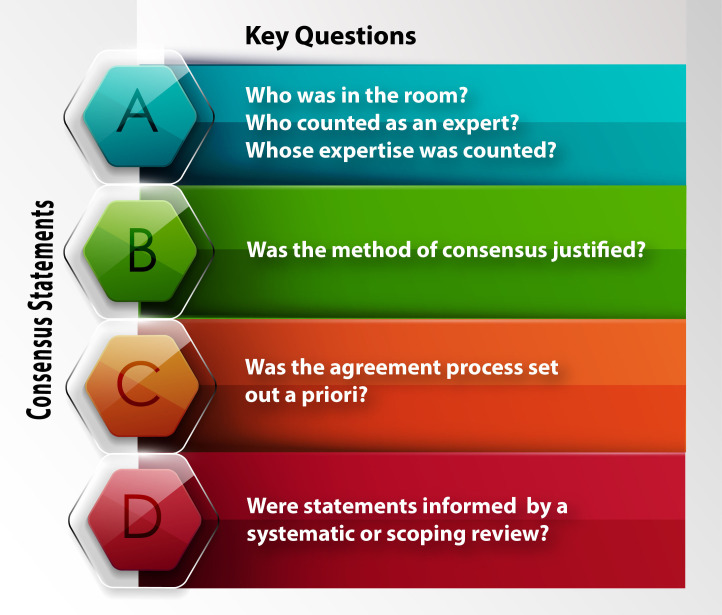
The four sets of questions that were used to frame existing consensus development processes are outlined as A–D in figure 1.
Figure 1.
Four sets of questions that support the assessment of rigour during consensus development.
Synthesis of the results
Data are grouped into both narrative summaries and summary tables of the extracted data. Part A presents the data on participants on the consensus panel or steering committee including:
panel number;
panel expertise/experience;
inclusion criteria for panellists;
sex split of panels;
and participant groups represented.
Part B focuses on the method and justification for reaching consensus.
Part C focuses on the individual procedures identified for observing when consensus was achieved including:
was consensus operationally defined a priori;
what was the level of agreement (expressed either as a percentage or categorical measure);
and were dissenting opinions acknowledged in the final report.
Finally, part D looks at the methods for generating questions or providing information to the panel. This included description of whether a systematic or scoping review was performed prior to the consensus process, and whether the questions asked were explicitly reported.
All items were tabulated using Microsoft Excel.
Patient and public involvement
No patients were involved in the development of this review.
Equity, diversity and inclusion statement
The authorship group consists of early, mid and late-career researchers and clinician scientists inclusive of a Master’s student, PhD candidate, assistant, associate and full professor. The researchers or clinician–researchers originate from the UK, Canada, the USA and Australia. Five are registered physiotherapists, one sport and exercise medicine specialist, and one professor of health economics. The authors are 43% female, and 86% identify as white.
This is a synthesis of existing research but the results focus on sex balance, patient and professional representation and the representation on consensus panels of those from low or lower-middle income countries (with crossover between income status as defined by the World Bank, and nations considered part of the ‘Global South’). Our study considered diversity as a marker of rigourous and representative consensus development. It is possible (hopeful) that the results of this work will inform future consensus processes and encourage the inclusion of members from more diverse and representative backgrounds.
Results
Selection of sources of evidence
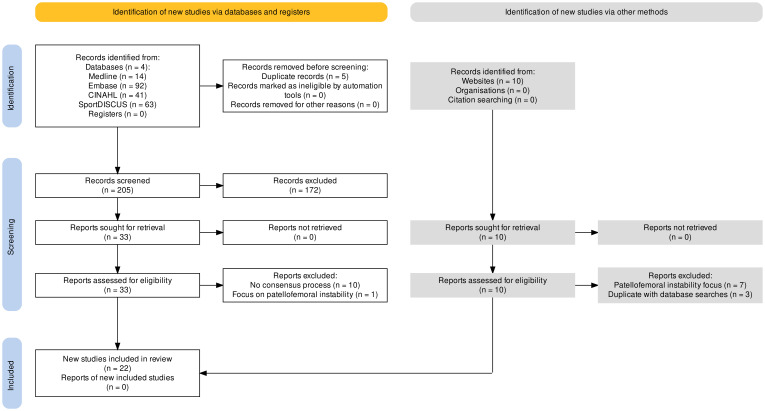
Figure 2 shows the PRISMA flow chart of evidence management. We identified 225 records. After title/abstract screening, 33 records were screened at full-text and 22 articles were included. Online supplemental appendix A contains the database and grey literature search results.
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart of returned searches.78
Characteristics of sources of evidence
Table 2 provides an overview of the included statement’s characteristics. Of the 22 statements included, 15 focused directly on PFP, and 7 included at least 1 statement on PFP (or patellofemoral osteoarthritis). Consensus statements on PFP have become more popular with four published in each of 2018 and 2021. The aims of the consensus processes have been heterogenous. The majority (13%–59%) have looked to establish agreement on treatments or interventions related to PFP. Other aims have included: definitions—1 (5%); diagnosis—5 (23%); natural history of PFP—5 (23%); agree on patient-reported outcome measures—2 (9%); a reporting checklist for PFP studies—1 (5%); and priority setting for research related to PFP—2 (9%).
Table 2.
Characteristics of the included statements (see online supplemental material for detailed version of table 2)
| First author | Year published | Stated aim (eg, treatment recommendation, develop definitions, priority setting exercise, etc) |
| Herring27 | 2008 | Help the team physician improve the care of the adolescent athlete by understanding the medical, musculoskeletal (shoulder/knee—including patellofemoral pain (PFP)/elbow/spine), and psychological issues (sport specialisation) common in this age group |
| Davis11 | 2010 | Unclear—presentation of research and some future research recommendations |
| Powers79 | 2012 | To understand the factors that contribute to the development and, consequently the treatment of PFP |
| Witvrouw80 | 2014 | Gain consensus for three specific areas: (1) the natural history of PFP and local (knee region) factors that influence PFP; (2) trunk and distal factors that influence PFP; and (3) innovations in rehabilitation for PFP |
| McAlindon31 | 2014 | To develop concise, up-to-date, patient-focused, evidence-based, expert consensus guidelines for the management of knee osteoarthritis (OA) *including patellofemoral osteoarthritis* |
| Crossley71 | 2016a | To agree on: terminology, definitions, clinical examination, natural history and PROMs (patient-reported outcome measures) |
| Crossley39 | 2016b | To agree on expert-recommended physical interventions for PFP |
| Herring28 | 2016 | To help the team physician improve the care of the athlete by understanding and practising methods of injury and illness prevention in specific sports medicine problems. |
| Powers81 | 2017 | To place known associated factors within the context of a pathomechanical model of PFP |
| Herring29 | 2018 | To improve the care of the female athletes by understanding select injuries and illnesses |
| Van Middlekoop36 | 2018 | Mixed consensus on: diagnosis; burden; PROMs; prognosis; risk factors; and treatment. Adds in a priority-setting process. |
| Collins37 | 2018 | To agree on expert-recommended physical interventions for PFP |
| Huang82 | 2018 | To formulate Chinese Pain Specialist Consensus on the diagnosis and treatment of degenerative knee osteoarthritis (DKOA) |
| Fox30 | 2018 | To provide clinicians with the best practices for ordering imaging examinations |
| Guanghua83 | 2020 | Guidelines on the diagnosis and treatment of patellofemoral OA |
| Chahla32 | 2020 | Consensus on the functional anatomy, indications, donor graft considerations, surgical treatment and rehabilitation of large chondral and osteochondral defects in the patellofemoral joint |
| Kolasinski38 | 2020 | To develop an evidence-based guideline for the comprehensive management of osteoarthritis (OA) as a collaboration between the American College of Rheumatology and the Arthritis Foundation, updating the 2012 ACR recommendations for the management of hand, hip and knee OA. |
| Keshmiri84 | 2021 | Consensus on therapy for different patellofemoral abnormalities in patients suffering from isolated patellofemoral arthritis. |
| Kunene33 | 2021 | To develop a community-based rehabilitation implementation framework for PFP in runners from under-resourced communities |
| Barton34 | 2021 | Consensus statement and associated checklist provide standards for REPORTing of quantitative PatelloFemoral Pain (REPORT-PFP) research to enhance clinical translation and evidence synthesis, and support clinician engagement with research and data collection |
| Guanghua85 | 2021 | To summarise the latest research progress in the surgical treatment of patellofemoral osteoarthritis, and refer to the latest domestic and foreign guidelines and consensus |
| Vicenzino35 | 2022 | The objective of this consensus development process was to decide clinical and research priorities for pain features and psychological factors in persons with PFP. |
ACR, American College of Rheumatology.
bjsports-2023-107552supp002.pdf (117.5KB, pdf)
Synthesis and appraisal of results
Representativeness of PFP statement panels (part A)
Table 3 provides detail on the representativeness of panels. The number of panellists included ranged from 10 to 71. Only 3 (14%) of the 22 reports detailed the experience of their respective panels, and only 2 (9%) of these 3 gave further details as to how they defined expertise prior to recruiting their panellists. Eight (36%) studies provided inclusion criteria for the selection of their panellists. Four (18%) reports had existing criteria for panellist selection detailed on linked websites.27–30 Five (23%) studies outlined their own individual methods for highlighting experts.31–35 Five (23%) were classed as ‘unclear’ because they reported panellists had to have been part of a recent meeting related to the topic under discussions without providing qualifying criteria as to why presence at the meeting made the panellists suitable.
Table 3.
Representativeness of patellofemoral pain (PFP) statement panels (see online supplemental material for detailed version of table 3)
| First author | No. panellists | Definition of expertise | Inclusion criteria for panellists | Sex split | No countries represented |
| Herring (2008) | 11 | Not applicable | Yes* | 7 male: 4 female | 1—USA |
| Davis (2010) | Unclear | Not applicable | Unclear | Unclear | 10 |
| Powers (2012) | Unclear | Not applicable | Unclear | Unclear | 9 |
| Witvrouw (2014) | Not reported | Not applicable | Unclear | Unclear | Not reported |
| McAlindon (2014) | 13 | Not applicable | Yes | 10 male: 3 female | 10 |
| Crossley (2016a) | Not reported | Not applicable | No | Unclear | Not reported |
| Crossley (2016b) | 35 | Not applicable | Unclear | Unclear | Not reported |
| Herring (2016) | 12 | Not applicable | Yes* | 10 male: 2 female | 1—USA |
| Powers (2017) | Not reported | Not applicable | Unclear | Unclear | Not reported |
| Herring (2018) | 10 | Not applicable | Yes* | 4 male: 6 female | 2—USA and Canada |
| Van Middlekoop (2018) | Unclear | Not applicable | Unclear |
Authorship
8 male : 9 female |
Unclear |
| Collins (2018) | 41 | ‘active researchers in the field’ | Unclear |
Authorship
6 male: 6 female |
Unclear |
| Huang (2018) | 15 | Not applicable | No | Unclear | 1—China |
| Fox (2018) | 17 | Not applicable | Yes† | 8 male: 9 female | 1—USA |
| Guanghua (2020) | 30 | Not applicable | No | Unclear | 1—China |
| Chahla (2020) | 28 | Not applicable | Yes | 26 male: 2 female | 2—USA and Canada |
| Kolasinski (2020) | 15 | Not applicable | No. | 8 male: 7 female | USA and Canada |
| Keshmiri (2021) | 13 | Not applicable | No. | 12 male: 1 female | 3—Germany, Austria and Switzerland |
| Kunene (2021) | 19 | > 5 years post qualification | Yes. | 10 female: 9 male | 2—South Africa and UK |
| Barton (2021) | 24 (2015); 51 (2019) |
Measured in clinical experience | Yes. | Unclear* | 10 countries |
| Guanghua (2021) | 35 | Not applicable | No | Unclear | 1—China |
| Vicenzino (2022) | Survey: 35 healthcare workers and 36 patients In-person: 20 healthcare workers |
Years of experience or exposure to patients with PFP | Yes | Survey: healthcare workers (20 male:15 female); and patients (12 male:24 female) In-person: (11 male: 9 female) |
9—health professions survey 4—patient survey 8—in-person process |
*Panel is made up from two nominated representatives from each of American Academy of Family Physicians, American Academy of Orthopaedic Surgeons, American College of Sports Medicine, American Medical Society for Sports Medicine, American Orthopaedic Society for Sports Medicine and American Osteopathic Academy of Sports Medicine. Representatives are chosen by their organisation based on their experience as team physicians with expertise in the topic area.
† Available on American College of Radiology website—‘Following regulatory requirements, we survey panel members on their skills and expertise to ensure that panels include expertise in the clinical topic, primary care medicine, medical imaging, statistics and clinical trial design. Panel members’ expertise is determined using self-attestation and calculated by the amount of education, training and experience the member reports for that skill area’.
bjsports-2023-107552supp003.pdf (177.6KB, pdf)
One statement explicitly reported participant sex,35 and one reported panellists preferred gender identity.33 Ten (45%) studies included enough information on panellists or authors for us to estimate their sex on the basis of names and/or internet profile data. Of the 10 articles, 8 (80%) had greater male representation than female, with the greatest difference being a 26:2 male:female panel.32 There were two further studies where the panel size had a large discrepancy from the authorship; in these instances, we collected the estimated sex of the authors. One authorship team had greater female representation than male (9:8),36 and one authorship was balanced (6:6).37
Countries represented on the panels ranged from 1 to 10, with 16 (73%) statements appearing to be based on the opinions of multicountry panels. The USA was the most commonly represented country with clear indications that panellists or authors originated from the USA in 16 (73%) of the statements. Only one consensus statement—Barton et al (2021)—included a panellist where a member was considered to be from a low or lower-middle income country (India).
Thirteen of the 22 (59%) articles detailed the professional designations of their panellists. The most commonly represented professions invited to provide statements on topics related to PFP were medical doctors of no known specialty (n=11%–50%), orthopaedic surgeons or specialists (n=11%–50%) and physiotherapists (n=8%–36%). Patients were part of the panel in two studies (9%).31 38 Vicenzino et al. (2022) did include patients at the survey stage of their development process to support clinical decisions, but patients were not invited to be part of the final decision-making process.
Four statements (18%) clearly reported any conflicts of interest among invited panellists. Four further studies (18%) included either a statement declaring authors had no conflicts of interest or where funding had been given to generate the statement. This left 14 articles (64%) without either a conflict of interest statement, or a disclosure of any funding received.
Method of assessing/achieving consensus and definition of consensus (parts B and C)
Table 4 details the methods used for measuring and/or facilitating consensus on PFP. Seven (32%) articles reported an identified method of consensus to elucidate their panellists’ views (five Delphi, and two RAND-UCLA technique). A further three studies reported their own methods (two scale-based and one survey plus in-person). Nine had no identifiable method, and three were unclear.
Table 4.
Methods used for measuring, and/or facilitating consensus among panel member (see online supplemental material for detailed version of table 4)
| First author | What consensus method was reported? | Which method of consensus was used? | Was consensus level decided a priori? | What was the method or level of agreement set at? | Were dissenting opinions acknowledged and reported? |
| Herring (2008) | None | Informal—iterative development over written rounds and in-person meeting | No | Unanimous agreement among invited panel—assumed not measured | No |
| Davis (2010) | None | Consensus conference—informal | No | Unclear | No |
| Powers (2012) | None | Consensus conference—informal | No | Unclear | No |
| Witvrouw (2014) | None | Consensus conference—informal | No | Unclear | No |
| McAlindon (2014) | RAND-UCLA and Delphi | RAND-UCLA | Yes | RAND method | Yes |
| Crossley (2016a) | None | Consensus conference—informal | No | Unclear | No |
| Crossley (2016b) | Unclear | Modified version of RAND/UCLA | Yes | Had to be rated 'appropriate' (7–9 on a 10-point Likert) on average (median) AND consistent with evidence | No |
| Herring (2016) | None | Informal—iterative development over written rounds and in-person meeting | No | Unanimous agreement among invited panel—assumed not measured | No |
| Powers (2017) | None | Informal—not reported fully | No | Unclear | No |
| Herring (2018) | None | Informal—iterative development over written rounds and in-person meeting | No | Unanimous agreement among invited panel - assumed not measured | No |
| Van Middlekoop (2018) | Numerical Rating Scale 0–10 | Unclear | Yes | For priority setting part only, consensus was >7.5 out of 10 on numerical rating scale | No |
| Collins (2018) | 10 point Likert scale—median score must be between 7 and 9 | Unclear | Yes | Median agreement between 7 and 9 | No |
| Huang (2018) | None | Unclear | Unclear | Unclear | No |
| Fox (2018) | RAND-UCLA | RAND-UCLA | Yes | median agreement between 7 and 9 | Yes |
| Guanghua (2020) | Delphi | Delphi | Unclear | Unclear | No |
| Chahla (2020) | Delphi | Modified Delphi | Yes | Over 75% of respondents agreed and fewer than 20% disagreed in the final voting round | No |
| Kolasinski (2020) | Unclear | Unclear | Yes | >70% agreement | No |
| Keshmiri (2021) | Delphi | Modified Delphi | No | Unclear | No |
| Kunene (2021) | Delphi | Modified—Delphi | Yes | >70% agreement | No |
| Barton (2021) | Delphi | Modified Delphi mixed with priority setting process | Yes | >70% agreement | No |
| Guanghua (2021) | Unclear | Unclear | Unclear | Unclear | No |
| Vicenzino (2022) | Survey plus in person meeting | Survey plus in-person meeting | Yes | >70% agreement on survey and paper-based votes (single round) | No |
bjsports-2023-107552supp004.pdf (139.9KB, pdf)
Qualitative assessment revealed substantial deviations from the reported method in all but two studies.30 35 Many of the articles that did not report a method used either an informal process of developing a written document over successive editing rounds without a formal voting structure (authors’ signing off at the end of the process)—sometimes called ‘Glaser’s State-of-the-Art Approach’,6 or used a form of consensus conference to generate statements which were taken away by a small group to be written up. Many of those who reported using a Delphi method used a modified Delphi with an in-person element to decide on final statements.
Ten (45%) articles reported deciding on what was considered consensus among panellists a priori. Of these, four studies fixed consensus as meaning 70% of panellists agreed with the statements. Three used a derivation of the RAND-UCLA criteria with the mean among panellists falling within the 7–9 range on a 9-point Likert scale when 9 was full agreement (one used a 10-point). One article39 reported that the median rank of ‘appropriate’ (using a 10-point Likert where agreement was a median score between 7–9 on a 0–9 scale) but final statements had to be in agreement with objective evidence from literature searches.39 It was unclear how (or who) this was decided by. One study32 set criteria that 75% had to agree with a statement while no more than 20% could disagree on a 5-point Likert scale where 4 and 5 were agree/strongly agree.32 One study36 did not explain how statements were voted on or agreed on among panellists, but did report the results of consensus on subsequent research priorities (numerical scale 0–10, with consensus set at>7.5).36
Two of the 22 (9%) articles reported on dissenting opinions. Both consensus processes used the RAND-UCLA technique where dissent is expressed as part of the traditional quantitative assessment. No report explored the meaning of any expressed dissent among panellists.
Use of scientific literature searches to support question formation or delegate decision-making and conflicts of interest (part D)
Six of the 22 (27%) articles reported using systematic methods to inform the statements used in their consensus development.30 31 35 37–39 Four of the six30 31 35 38 provided links to their systematic searches and/or summaries of the evidence which were given to panel members to support decisions made during the consensus process. One further article reported a partial literature review, and three reported informal literature reviews, with no supporting information provided.
Eight of the 22 (36%) articles explicitly recorded the questions that panellists were asked to vote on. Table 5 summarises which consensus processes used literature searches, whether they reported the search results, and whether or not they made the questions that were produced by said searches explicit in their reports or the supplementary material.
Table 5.
Methods informed by appropriate systematic or scoping review
| First author | Were questions informed by a systematic or scoping review? | Searches and information summary in the report or online? | Were the questions asked of panellists made explicit? |
| Herring (2008) | No | N/A | No |
| Davis (2010) | No | N/A | No |
| Powers (2012) | No | N/A | No |
| Witvrouw (2014) | No | N/A | No |
| McAlindon (2014) | Yes | Yes | Yes |
| Crossley (2016a) | Partial literature review of natural history (PFP and patellofemoral osteoarthritis) and patient reported outcome measures for PFP | No | No |
| Crossley (2016b) | Yes | No | Yes |
| Herring (2016) | No | N/A | No |
| Powers (2017) | No | N/A | No |
| Herring (2018) | No | N/A | No |
| Van Middlekoop (2018) | No | N/A | No |
| Collins (2018) | Yes | No | Yes |
| Huang (2018) | No | N/A | No |
| Fox (2018) | Yes | Yes | Yes |
| Guanghua (2020) | No | N/A | No |
| Chahla (2020) | No | N/A | Yes |
| Kolasinski (2020) | Yes | Yes | No |
| Keshmiri (2021) | No | N/A | Yes |
| Kunene (2021) | No | N/A | No |
| Barton (2021) | No | N/A | Yes |
| Guanghua (2021) | No | N/A | No |
| Vicenzino (2022) | Yes | Yes | Yes |
PFP, patellofemoral pain.
Discussion
Consensus methods have evolved over the past 70 years. The most common methods include Delphi outlined in the 1950s40 41; Nominal Group Technique originating in the 1970s42 43 and the RAND-UCLA method developed in the early 1990s.44 Choosing to bypass these recognised methods of consensus development is not necessarily a weakness when there is a clear rationale for that decision.25 Authors should pick the methods that best suit their aims and fit with the resources available to them. It is logical that there is heterogeneity among the approaches groups choose to generate consensus. We found that consensus seekers in PFP or patellofemoral osteoarthritis chose recognised methods of consensus development (eg, the Delphi method or RAND-UCLA appropriateness method) less often (32% of statements) than consensus statements in some other areas of medicine. For instance, Delphi or modified Delphi was used in 196 out of 257 (76%) of consensus approaches to medical education topics between 2009 and 2016.8
Our review found that many consensus statements on PFP (or patellofemoral osteoarthritis) published between 2008 and 2022 missed steps that support the rigorous development of consensus recommendations.10 45 46 However, we acknowledge that the framework we used to evaluate rigour was published in 2021 and has not been validated. Our use of the four questions outlined in figure 1 to interrogate the rigour of past consensus processes will, we hope, increase researchers’ awareness of key questions to consider.
Bearing in mind the historical context in which some of the existing consensus statements were conducted, we used four sets of questions to evaluate the rigour of existing PFP consensus development. We found that most consensus statements failed to address at least one of the four key areas. These four areas constitute: panel representation and diversity; using recognised methods of consensus development; defining what constituted ‘agreement’; and/or appraising literature to identify knowledge gaps.
Panel representation and diversity (part A—who was in the room? Who was counted as an ‘expert’? Whose ‘expertise’ counted?)
To obtain a clear and useful answer from a consensus panel, it is important that invited panellists are both knowledgeable, and representative of the population the answers will serve.47 The panellists recruited to develop consensus on topics related to PFP have been: male dominated (80%); largely from high income countries (especially North America—USA or Canada represented in 73% of panels, Western Europe—52% and Australia—43%); and, without justification, focus on medical doctors, allied health professionals and researchers. Low or lower-middle income countries were represented in only one consensus panel (5%). Patients have largely been absent—only two statements included a patient on their panel. Questions on diagnosis and treatment (ie, those most concerning patients) were the most commonly asked in the PFP/patellofemoral osteoarthritis consensus-based research, and therefore it might have been expected that patients would be more involved.
In some cases, it may be appropriate for consensus panels to focus on ‘experts-only’.48 49 The recently developed reporting guideline for consensus exercises recommends detailed reporting of the criteria for panellist inclusion.2 25 We note that most consensus developers did not provide definitions of expertise other than ‘experience’. Expertise and experience are conceptually different and we encourage deeper consideration of the use of ‘expertise’ to justify the make-up of consensus panels. Too much group homogeneity may lead to a lack of critical questioning among the panel, or panellists not being able to recognise potential conflicts of interest.47 50–52 The narrow definition of ‘expert’ and exclusion of patients also ignores the ethical consideration of patients being integral to decisions made about their care.53 No PFP statements thus far have used stakeholder analysis or engagement theories to select their panels.54–58 We propose that a lack of key group involvement in decision-making processes could harm subsequent implementation of recommendations.
Using recognised methods and defining consensus (parts B ‘was the method of consensus justified?’ and C ‘was the agreement process set out a priori?’)
Fewer than half (32%) of the statements on PFP used identifiable methods of consensus development. Failing to use a formal method runs the risk that consensus seekers will miss the steps associated with rigorous scientific research.59 Although consensus is iterative, it should also be guided by a framework, without which there is a risk that decisions are made based on individual (potentially biased) opinions.6 60 61
Two (9%)30 31 of the included studies did have rigorous methodology underpinning their statements, having identified and used the RAND-UCLA appropriateness method which has an extensive open-access guide available at https://www.rand.org/pubs/monograph_reports/MR1269.html.44 The organisations (Osteoarthritis Research Society International, and the American College of Radiology) supporting statements that used the RAND-UCLA appropriateness method also had extensive supporting literature detailing their processes for arriving at their statements, how they selected panellists, and consistently applied these criteria across several other consensus statements on topics not eligible within this review.
Fewer than half (45%) of the consensus statements developers used a predefined threshold to establish when agreement existed among their panel. Failing to define agreement can lead to prolonged processes or premature declarations of agreement among panellists in the absence of unanimity.62 However, it has to be acknowledged that there is no gold standard for measuring when agreement exists among a group. There were several statements where no apparent vote was used. Implicit agreement among a panel is potentially misleading, and may be a result of people feeling they have not been given a platform to voice opinions. This runs the risk that those with the greatest power (loudest voice) will dominate such proceedings.42 60 63 64
Only two studies reported the presence of dissent among their panel.30 31 Both consensus-based studies that acknowledged dissent used the RAND-UCLA method. However, neither study formally explored the reasons for the dissenting opinions among their respective panels. Not acknowledging disagreement (and the reasons behind disagreement or dissent) may seem normal in statements that report on agreement, but risks suppressing relevant counteropinions.9 10 Groups that are forced to agree run the risk of agreeing to watered-down statements.65 Suppression of minority opinions is just one of the reasons the Concussion in Sport Group was criticised for their statements on concussion in sport.7
Appraising literature and identifying knowledge gaps (part D—‘Were statements informed by a systematic or scoping review?’)
Consensus is often used to arrive at statements (or guidance) on topics when evidence is lacking, or to help integrate the available evidence into clinical practice.2 10 66 67 If there is no review of the existing evidence, it is hard to judge what consensus judgements should be focused on. Around a third of guidelines (34%) have been criticised for lacking systematic methods to synthesise information, and underpin their recommendations.68 Scoping reviews can generate valuable evidence ‘gap maps’.15 69 Previous critiques have already recommended that systematic literature synthesis be integrated into consensus methods.66 70
Only six (27%)30 31 35 37 38 71 of the statements on PFP or patellofemoral osteoarthritis explicitly reported using a formal review of the evidence to either guide statement formation, or to inform panellist decisions in the subsequent consensus process. Five out of the six statements using a formal review reported the questions their panellists were asked to generate recommendations explicitly, either within their manuscript or as online supplemental material.
Systematic searches can be used to form statements (which the consensus panel vote on), and/or to produce evidence summaries for panellists before they vote in a consensus process. No formal guidance exists on how to translate systematic literature searches into unbiased statements. Transparent and well-reported consensus statements should include all the material that was used to inform decisions made in the consensus process (often as online supplemental material).25
Limitations
As yet no quality framework exists to judge consensus statements, and the reporting guideline (Accurate Consensus Reporting Document—ACCORD) was published in January 2024.25 The four sets of questions used to frame the consensus development processes in this study were derived from previous critiques of the consensus literature.8 10 61 62 72 73 These questions provide a means to view the data in this scoping review, but are not designed as a comprehensive quality assessment tool. Scoping reviews should not be used to evaluate the quality of existing evidence.22 The four sets of questions we used to frame our report on the rigour of consensus development here have not been validated. It is possible we missed questions that may have enhanced our understanding of the rigour of consensus development in statements reporting on PFP or patellofemoral osteoarthritis.
To assess the number of countries represented, we used panellists’ self-reported affiliations. This ignores the regular movement of people between countries. Panellists do not ‘lose’ their experiences or ‘knowledge’ of their countries of origin. It is possible that some of those counted among high-income countries originated from low-income or middle-income countries. Panellists who originated from low-income or middle-income countries may have brought valuable additional insights to their consensus processes that were not captured.
There are flaws in using conventional names to estimate the balance of sex or gender on panels. We consciously decided to report our data using sex and not gender, as sex provides a binary model (male vs female), as opposed to the spectrum of gender diversity. We do not wish to inadvertently misgender the panellists. We believed that we were less likely to mistake sex based on naming conventions and tried to coordinate our data using publicly available information on panellists or statement authors. We acknowledge that there may be errors where we have made assumptions. Automated tools have been used to assess gender balance in research reports but these suffer from only being able to produce binary reports, for example, https://genderize.io/ or https://namsor.app/about-us. The 2020 Elsevier report on gender in science which used the NamSor application to assert gender balance in research reported precision rates of 93% for men and 98% for women.74 For consensus panels to meet diversity and inclusion criteria, it would be useful for all future consensus projects to ask panellists their gender to facilitate clear and accurate reporting of the genders represented.
This review highlights the lack of key representative groups being included in consensus processes. However, and with regret, we—the authors—recognise our own failure to include a patient partner in this research project. While stating the need for diversity in consensus processes, we also recognise the lack of diversity among the authors. In hindsight, we feel adding patients and a more diverse steering committee would have added richness to our appraisal, especially with regards to our assessment of diversity, representation and expertise.
Future directions
Future consensus statements on PFP should focus on developing representative panels to enhance creativity, and avoid the problems associated with ‘groupthink’. Sex and gender diversity among panels improves group decision-making, and thus this analysis, although crude, may still help to increase awareness among consensus seekers that panel memberships need to be diverse.50 52 75 76 Stakeholder analysis might form an innovative and objective way to develop future panels who represent all of those who might be impacted by the aims of a consensus exercise in PFP, or other topics in sports and exercise medicine. Consensus organisers could consider adopting the ‘7Ps Framework to Identify Stakeholders in Patient-Centered Outcomes Research’ where stakeholders are broken down into seven key groups: Patients and the public; Providers; Purchasers; Payers; Policy-makers; Product makers; and Principle investigators.55
Statements often reported involving clinician–researchers; if these panellists were predominantly research based, it could have affected the adoption of recommendations in clinical practice.77 Therefore, future statements should consider involving those actively practising with patients. Systematic or scoping reviews should be used to analyse gaps in existing literature, and guide consensus development panels on where their efforts should be directed.
This review framed existing consensus statements against questions on the rigour of consensus development. We did not assess whether consensus developers had begun to answer these four questions more often in more recently published work (ie, whether there was a time trend among published consensus statements). Future studies could assess whether consensus development methods are improving to inform what future actions may be needed to enhance the rigour of future consensus-based approaches.
Future assessments of quality should focus on the quality of consensus development methods (eg, effective use of Delphi, RAND-UCLA) and not the subsequent statements or recommendations of the consensus panel. The quality (accuracy) of the statement recommendations only becomes apparent over time and should evolve as new evidence and clinical solutions emerge. As a result, trust in consensus statements relies on the rigour of methods used to develop recommendations and agreement, and from the inclusion of diverse and representative panel members.
Conclusion
Clinicians and researchers have sought consensus with increasing frequency on topics related to PFP. However, consensus statements on PFP have often failed to rigorously develop consensus recommendations with respect to the four questions we outlined in this review. The lack of systematic searching to identify potential evidence gaps may have resulted in statements focusing on areas with well-established research evidence, or missing important topics where no information exists. Given the potential for consensus to direct whole bodies of research, it is perhaps most concerning that the patient voice has been almost totally absent.
Future consensus statements that are rigorous, representative (of all interested or impacted parties) and clearly report their development processes could be seen as more credible.
Acknowledgments
The search strategy or this review was developed in collaboration with Charlotte Beck librarian at the University of British Columbia Woodward Library.
Footnotes
@blazey85, @clare_ardern, @DrJenniferCDav1, @jwhittak_physio, @JayLos18, @KarimKhan_IMHA
Contributors: PB and KMK proposed the review. PB, AS, CLA and KMK identified the method and the framework for the review. PB developed and executed the search strategy. PB and JML undertook data charting. PB produced all data summaries and produced each draft of the research manuscript. All authors edited, subsequently reviewed and approved the final manuscript.
Funding: Professor Khan holds a Canadian Institute of Health Research (CIHR) Scientific Director research grant (SOP-154942) which provided the main source of support to undertake this work. All other authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Rennie D. Consensus statements. N Engl J Med 1981;304:665–6. 10.1056/NEJM198103123041110 [DOI] [PubMed] [Google Scholar]
- 2. van Zuuren EJ, Logullo P, Price A, et al. Existing guidance on reporting of consensus methodology: a systematic review to inform ACCORD guideline development. BMJ Open 2022;12:e065154. 10.1136/bmjopen-2022-065154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dijkstra HP, Mc Auliffe S, Ardern CL, et al. Oxford consensus on primary cam morphology and femoroacetabular Impingement syndrome: part 2—research priorities on conditions affecting the young person’s hip. Br J Sports Med 2022;57:342–58. 10.1136/bjsports-2022-106092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dijkstra HP, Mc Auliffe S, Ardern CL, et al. Oxford consensus on primary cam morphology and femoroacetabular impingement syndrome: part 1—definitions, terminology, taxonomy and imaging outcomes. Br J Sports Med 2022;57:325–41. 10.1136/bjsports-2022-106085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Whittaker JL, Culvenor AG, Roos EM, et al. OPTIKNEE – Optimising knee health after injury. Br J Sports Med 2022;56:1391–2. 10.1136/bjsports-2022-106510 [DOI] [Google Scholar]
- 6. Fink A, Kosecoff J, Chassin M, et al. Consensus methods: characteristics and guidelines for use. Am J Public Health 1984;74:979–83. 10.2105/ajph.74.9.979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Casper ST, Bachynski KE, Buckland ME, et al. Toward complete, candid, and unbiased International consensus statements on concussion in sport. J Law Med Ethics 2021;49:372–7. 10.1017/jme.2021.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Humphrey-Murto S, Varpio L, Wood TJ, et al. The use of the Delphi and other consensus group methods in medical education research: a review. Acad Med 2017;92:1491–8. 10.1097/ACM.0000000000001812 [DOI] [PubMed] [Google Scholar]
- 9. Shrier I. Consensus statements that fail to recognise dissent are flawed by design: a narrative review with 10 suggested improvements. Br J Sports Med 2020. 10.1136/bjsports-2020-102545 [DOI] [PubMed] [Google Scholar]
- 10. Blazey P, Crossley KM, Ardern CL, et al. It is time for consensus on ‘consensus statements Br J Sports Med 2022;56:306–7. 10.1136/bjsports-2021-104578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis IS, Powers C. Patellofemoral pain syndrome: proximal, distal, and local factors—an international research retreat: April 30–May 2, 2009, Fells Point, Baltimore, MD. J Orthop Sports Phys Ther 2010;40:A1–48. 10.2519/jospt.2010.0302 [DOI] [PubMed] [Google Scholar]
- 12. Juhn MS. Patellofemoral pain syndrome: a review and guidelines for treatment. Am Fam Physician 1999;60:2012–22. [PubMed] [Google Scholar]
- 13. iPFRN . About us: International Patellofemoral pain research network. 2023. Available: https://ipfrn.org/about/
- 14. Peters MD, Godfrey CM. Chapter 11: Scoping reviews. In: JBI Manual for Evidence Synthesis. JBI, 2020. Available: https://reviewersmanual.joannabriggs.org/ [Google Scholar]
- 15. Peters MDJ, Marnie C, Tricco AC, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth 2020;18:2119–26. 10.11124/JBIES-20-00167 [DOI] [PubMed] [Google Scholar]
- 16. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-SCR): checklist and explanation. Ann Intern Med 2018;169:467–73. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 17. De Boeck K, Castellani C, Elborn JS, et al. Medical consensus, guidelines, and position papers: a policy for the ECFS. J Cyst Fibros 2014;13:495–8. 10.1016/j.jcf.2014.06.012 [DOI] [PubMed] [Google Scholar]
- 18. (NIH) NIoH . Clinical practice guidelines. national center for complementary and integrative health; 2023. Available: https://www.nccih.nih.gov/health/providers/clinicalpractice [Google Scholar]
- 19. Cruz JE, Fahim G, Moore K. Practice guideline development, grading, and assessment. P T 2015;40:854–7. [PMC free article] [PubMed] [Google Scholar]
- 20. Godin K, Stapleton J, Kirkpatrick SI, et al. Applying systematic review search methods to the grey literature: a case study examining guidelines for school-based breakfast programs in Canada. Syst Rev 2015;4:138. 10.1186/s13643-015-0125-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haddaway NR, Collins AM, Coughlin D, et al. The role of google scholar in evidence reviews and its applicability to grey literature searching. PLOS ONE 2015;10:e0138237. 10.1371/journal.pone.0138237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pollock D, Peters MDJ, Khalil H, et al. Recommendations for the extraction, analysis, and presentation of results in scoping reviews. JBI Evid Synth 2023;21:520–32. 10.11124/JBIES-22-00123 [DOI] [PubMed] [Google Scholar]
- 23. Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010;5:69. 10.1186/1748-5908-5-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gattrell WT, Hungin AP, Price A, et al. ACCORD guideline for reporting consensus-based methods in BIOMEDICAL research and clinical practice: a study protocol. Res Integr Peer Rev 2022;7:3. 10.1186/s41073-022-00122-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gattrell WT, Logullo P, van Zuuren EJ, et al. ACCORD (accurate consensus reporting document): a reporting guideline for consensus methods in biomedicine developed via a modified Delphi. PLOS Med 2024;21:e1004326. 10.1371/journal.pmed.1004326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jünger S, Payne SA, Brine J, et al. Guidance on conducting and reporting Delphi studies (CREDES) in palliative care: recommendations based on a methodological systematic review. Palliat Med 2017;31:684–706. 10.1177/0269216317690685 [DOI] [PubMed] [Google Scholar]
- 27. Herring SB, Bernhardt JA, Boyajian-O’Neill DT, et al. Selected issues for the adolescent athlete and the team physician: a consensus statement. Med Sci Sports Exerc 2008;40:1997–2012. 10.1249/MSS.0b013e31818acdcb [DOI] [PubMed] [Google Scholar]
- 28. Herring SW, Putukian M, et al. Selected issues in injury and illness prevention and the team physician: a consensus statement. Curr Sports Med Rep 2016;15:48–59. 10.1249/JSR.0000000000000231 [DOI] [PubMed] [Google Scholar]
- 29. Herring SK. Female athlete issues for the team physician: a consensus statement—2017 update. Med Sci Sports Exerc 2018;50:1113–22. 10.1249/MSS.0000000000001603 [DOI] [PubMed] [Google Scholar]
- 30. Fox MG, Chang EY, Amini B, et al. ACR appropriateness criteria: chronic knee pain. J Am Coll Radiol 2018;15:S302–12. 10.1016/j.jacr.2018.09.016 [DOI] [PubMed] [Google Scholar]
- 31. McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis and Cartilage 2014;22:363–88. 10.1016/j.joca.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 32. Chahla J, Hinckel BB, Yanke AB, et al. An expert consensus statement on the management of large chondral and osteochondral defects in the patellofemoral joint. Orthop J Sports Med 2020;8. 10.1177/2325967120907343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kunene S, Taukobong N, Ramklass S. Community-based rehabilitation implementation framework to address patellofemoral pain amongst runners in under-resourced communities: Delphi consensus. S Afr J Physiother 2021;77:1531. 10.4102/sajp.v77i1.1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barton CJ, De Oliveira Silva D, Morton S, et al. REPORT-PFP: a consensus from the International Patellofemoral research network to improve reporting of quantitative patellofemoral pain studies. Br J Sports Med 2021;55:bjsports–2020. 10.1136/bjsports-2020-103700 [DOI] [PubMed] [Google Scholar]
- 35. Vicenzino BT, Rathleff MS, Holden S, et al. Developing clinical and research priorities for pain and psychological features in people with patellofemoral pain: an international consensus process with health care professionals. J Orthop Sports Phys Ther 2022;52:29–39. 10.2519/jospt.2022.10647 [DOI] [PubMed] [Google Scholar]
- 36. van Middelkoop M, Bennell KL, Callaghan MJ, et al. International patellofemoral osteoarthritis consortium: consensus statement on the diagnosis, burden, outcome measures, prognosis, risk factors and treatment. Semin Arthritis Rheum 2018;47:666–75. 10.1016/j.semarthrit.2017.09.009 [DOI] [PubMed] [Google Scholar]
- 37. Collins NJ, Barton CJ, van Middelkoop M, et al. 2018 Consensus statement on exercise therapy and physical interventions (orthoses, taping and manual therapy) to treat patellofemoral pain: recommendations from the 5th international patellofemoral pain research retreat, Gold Coast, Australia, 2017. Br J Sports Med 2018;52:1170–8. 10.1136/bjsports-2018-099397 [DOI] [PubMed] [Google Scholar]
- 38. Kolasinski SL, Neogi T, Hochberg MC, et al. American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis & Rheumatology 2019;72:220–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Crossley KM, van Middelkoop M, Callaghan MJ, et al. Patellofemoral pain consensus statement from the 4TH international patellofemoral pain research retreat, Manchester. part 2: recommended physical interventions (exercise, taping, bracing, foot orthoses and combined interventions). Br J Sports Med 2016;50:844–52. 10.1136/bjsports-2016-096268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Helmer-Hirschberg O. Analysis of the Future: The Delphi Method. RAND Corporation, 1967. [Google Scholar]
- 41. Dalkey NC. Delphi. RAND Corporation; 1967. [Google Scholar]
- 42. Delbecq A, Ven A, Gustafson D. Group techniques for program planning: Scott, Foresman and company; 1975 01/01.
- 43. Delbecq AL, Van de Ven AH. A group process model for problem identification and program planning. J Appl Behav Sci 1971;7:466–92:92. 10.1177/002188637100700404 [DOI] [Google Scholar]
- 44. Fitch K, Bernstein SJ, Aguilar MD, et al. The RAND/UCLA Appropriateness Method User’s Manual. RAND Corporation, 2001. [Google Scholar]
- 45. BJSM . BJSM author guidelines for consensus statements. 2022. Available: https://bjsm.bmj.com/bjsm/wp-content/uploads/sites/11/2022/05/BJSM-Author-Guidelines-and-Considerations-for-Consensus-Statements-31-AUG-2021.pdf
- 46. Logullo P, van Zuuren EJ, Winchester C, et al. ACCORD e&e - accurate consensus reporting document (ACCORD) explanation and elaboration: guidance and examples to support reporting consensus methods. Open Science Framework [Preprint] 2024. 10.31219/osf.io/kvjuh [DOI] [PMC free article] [PubMed]
- 47. Hussler C, Muller P, Rondé P. Is diversity in Delphi panelist groups useful? Evidence from a French forecasting exercise on the future of nuclear energy. Technol Forecast Soc Change 2011;78:1642–53. 10.1016/j.techfore.2011.07.008 [DOI] [Google Scholar]
- 48. Kurvers RHJM, Herzog SM, Hertwig R, et al. Boosting medical diagnostics by pooling independent judgments. Proc Natl Acad Sci USA 2016;113:8777–82. 10.1073/pnas.1601827113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Luan S, Katsikopoulos KV, Reimer T. When does diversity trump ability (and vice versa) in group decision making? A simulation study. PLOS ONE 2012;7:e31043. 10.1371/journal.pone.0031043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Díaz-García C, González-Moreno A, Jose Sáez-Martínez F. Gender diversity within R&Amp;D teams: its impact on radicalness of innovation. Innovation 2013;15:149–60. 10.5172/impp.2013.15.2.149 [DOI] [Google Scholar]
- 51. Phillips KW, Liljenquist KA, Neale MA. Is the pain worth the gain? The advantages and liabilities of agreeing with socially distinct newcomers. Pers Soc Psychol Bull 2009;35:336–50. 10.1177/0146167208328062 [DOI] [PubMed] [Google Scholar]
- 52. Sommers SR. On racial diversity and group decision making: identifying multiple effects of racial composition on jury deliberations. J Pers Soc Psychol 2006;90:597–612. 10.1037/0022-3514.90.4.597 [DOI] [PubMed] [Google Scholar]
- 53. Belton J, Hoens A, Scott A, et al. Patients as partners in research: it’s the right thing to do. J Orthop Sports Phys Ther 2019;49:623–6. 10.2519/jospt.2019.0106 [DOI] [PubMed] [Google Scholar]
- 54. Concannon TW, Grant S, Welch V, et al. Practical guidance for involving stakeholders in health research. J Gen Intern Med 2019;34:458–63. 10.1007/s11606-018-4738-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Concannon TW, Meissner P, Grunbaum JA, et al. A new taxonomy for stakeholder engagement in patient-centered outcomes research. J Gen Intern Med 2012;27:985–91. 10.1007/s11606-012-2037-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Freeman RE, Kujala J, Sachs S, et al. Stakeholder engagement: practicing the ideas of Stakeholder theory. In: Freeman REKujala J, Sachs S, eds. Stakeholder Engagement: Clinical Research Cases. Cham: Springer International Publishing, 2017: 1–12. [Google Scholar]
- 57. Mitchell RK, Agle BR, Wood DJ. Toward a theory of stakeholder identification and salience: defining the principle of who and what really counts. Acad Manag Rev 1997;22:853. 10.2307/259247 [DOI] [Google Scholar]
- 58. Petkovic J, Riddle A, Akl EA, et al. Protocol for the development of guidance for stakeholder engagement in health and healthcare guideline development and implementation. Syst Rev 2020;9:21. 10.1186/s13643-020-1272-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hofseth LJ. Getting rigorous with scientific rigor. Carcinogenesis 2018;39:21–5. 10.1093/carcin/bgx085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Black N, Murphy M, Lamping D, et al. Consensus development methods: a review of best practice in creating clinical guidelines. J Health Serv Res Policy 1999;4:236–48. 10.1177/135581969900400410 [DOI] [PubMed] [Google Scholar]
- 61. Waggoner J, Carline JD, Durning SJ. Is there a consensus on consensus methodology? Descriptions and recommendations for future consensus research. Acad Med 2016;91:663–8. 10.1097/ACM.0000000000001092 [DOI] [PubMed] [Google Scholar]
- 62. Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol 2014;67:401–9. 10.1016/j.jclinepi.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 63. Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation; 2023. [Google Scholar]
- 64. Manera K, Hanson CS, Gutman T, et al. Consensus methods: nominal group technique. In: Liamputtong P, ed. Handbook of Research Methods in Health Social Sciences. Singapore: Springer Singapore, 2019: 737–50. [Google Scholar]
- 65. Skrabanek P. Nonsensus consensus. The Lancet 1990;335:1446–7. 10.1016/0140-6736(90)91460-R [DOI] [PubMed] [Google Scholar]
- 66. Murphy MK, Black NA, Lamping DL, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess 1998;2:i–iv, . [PubMed] [Google Scholar]
- 67. Nair R, Aggarwal R, Khanna D. Methods of formal consensus in classification/diagnostic criteria and guideline development. Semin Arthritis Rheum 2011;41:95–105. 10.1016/j.semarthrit.2010.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lunny C, Ramasubbu C, Puil L, et al. Over half of clinical practice guidelines use non-systematic methods to inform recommendations: a methods study. PLOS ONE 2021;16:e0250356. 10.1371/journal.pone.0250356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nyanchoka L, Tudur-Smith C, Thu VN, et al. A scoping review describes methods used to identify, prioritize and display gaps in health research. J Clin Epidemiol 2019;109:99–110. 10.1016/j.jclinepi.2019.01.005 [DOI] [PubMed] [Google Scholar]
- 70. Humphrey-Murto S, Varpio L, Gonsalves C, et al. Using consensus group methods such as Delphi and nominal group in medical education research Med Teach 2017;39:14–9. 10.1080/0142159X.2017.1245856 [DOI] [PubMed] [Google Scholar]
- 71. Crossley KM, Stefanik JJ, Selfe J, et al. Patellofemoral pain consensus statement from the 4TH international patellofemoral pain research retreat, manchester. part 1: terminology, definitions, clinical examination, natural history, patellofemoral osteoarthritis and patient-reported outcome measures. Br J Sports Med 2016;50:839–43. 10.1136/bjsports-2016-096384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Boulkedid R, Abdoul H, Loustau M, et al. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLOS ONE 2011;6:e20476. 10.1371/journal.pone.0020476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jandhyala R. Delphi, non-RAND modified Delphi, RAND/UCLA appropriateness method and a novel group awareness and consensus methodology for consensus measurement: a systematic literature review. Curr Med Res Opin 2020;36:1873–87. 10.1080/03007995.2020.1816946 [DOI] [PubMed] [Google Scholar]
- 74. Elsevier . Gender report 2020: the researcher journey through a gender lens. 2020. Available: https://www.elsevier.com/connect/gender-report
- 75. Carlson J, Mitchell R, Bailey A. Refining the principle of who or what really counts: a normative foundation for stakeholder theory. AMPROC 2013;14090. 10.5465/ambpp.2013.14090abstract [DOI] [Google Scholar]
- 76. Mohammed S, Ringseis E. Cognitive diversity and consensus in group decision making: the role of inputs, processes, and outcomes. Organ Behav Hum Decis Process 2001;85:310–35. 10.1006/obhd.2000.2943 [DOI] [PubMed] [Google Scholar]
- 77. Bini SA, Mahajan J. Achieving 90% adoption of clinical practice guidelines using the Delphi consensus method in a large orthopedic group. J Arthroplasty 2016;31:2380–4. 10.1016/j.arth.2015.12.050 [DOI] [PubMed] [Google Scholar]
- 78. Haddaway NR, Page MJ, Pritchard CC, et al. Prisma2020: an R package and shiny App for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and open synthesis. Campbell Syst Rev 2022;18:e1230. 10.1002/cl2.1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Powers CM, Bolgla LA, Callaghan MJ, et al. Patellofemoral pain: proximal, distal, and local factors—second International research retreat, August 31–September 2, 2011, Ghent, Belgium. J Orthop Sports Phys Ther 2012;42:A1–54. 10.2519/jospt.2012.0301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Witvrouw E, Callaghan MJ, Stefanik JJ, et al. Patellofemoral pain: consensus statement from the 3RD International Patellofemoral pain research retreat held in Vancouver, September 2013. Br J Sports Med 2014;48:411–4. 10.1136/bjsports-2014-093450 [DOI] [PubMed] [Google Scholar]
- 81. Powers CM, Witvrouw E, Davis IS, et al. Evidence-based framework for a pathomechanical model of patellofemoral pain: 2017 patellofemoral pain consensus statement from the 4th international patellofemoral pain research retreat, Manchester, UK: part 3. Br J Sports Med 2017;51:1713–23. 10.1136/bjsports-2017-098717 [DOI] [PubMed] [Google Scholar]
- 82. Huang D, Liu Y-Q, Liang L-S, et al. The diagnosis and therapy of degenerative knee joint disease: expert consensus from the Chinese pain medicine panel. Pain Res Manag 2018;2018:2010129. 10.1155/2018/2010129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Guanghua ea. Chinese clinical practice guideline for patellofemoral osteoarthritis (2020 edition). Chin J Orthop 2020;40:1227–34. [Google Scholar]
- 84. Keshmiri A, Dirisamer F, et al. Operative treatment options for patellofemoral arthritis: an expert recommendation of the AGA patellofemoral committee. Orthop J Sports Med 2021;9. 10.1177/2325967121994849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. National Clinical Research Center For Geriatric Disorders Xiangya Hospital CSU, Joint Surgery Branch Of The Chinese Orthopedic Association . [Expert consensus on surgical treatment of patellofemoral osteoarthritis]. Chin J Reparative and Reconstr Surg 2021;35:1–7. 10.7507/1002-1892.202012037 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjsports-2023-107552supp001.pdf (411.7KB, pdf)
bjsports-2023-107552supp002.pdf (117.5KB, pdf)
bjsports-2023-107552supp003.pdf (177.6KB, pdf)
bjsports-2023-107552supp004.pdf (139.9KB, pdf)