Abstract
Chikungunya fever (CF) is caused by an arbovirus whose manifestations are extremely diverse, and it has evolved with significant severity in recent years. The clinical signs triggered by the Chikungunya virus are similar to those of other arboviruses. Generally, fever starts abruptly and reaches high levels, followed by severe polyarthralgia and myalgia, as well as an erythematous or petechial maculopapular rash, varying in severity and extent. Around 40% to 60% of affected individuals report persistent arthralgia, which can last from months to years. The symptoms of CF mainly represent the tissue tropism of the virus rather than the immunopathogenesis triggered by the host's immune system. The main mechanisms associated with arthralgia have been linked to an increase in T helper type 17 cells and a consequent increase in receptor activator of nuclear factor kappa-Β ligand and bone resorption. This review suggests that persistent arthralgia results from the presence of viral antigens post-infection and the constant activation of signaling lymphocytic activation molecule family member 7 in synovial macrophages, leading to local infiltration of CD4+ T cells, which sustains the inflammatory process in the joints through the secretion of pro-inflammatory cytokines. The term "long chikungunya" was used in this review to refer to persistent arthralgia since, due to its manifestation over long periods after the end of the viral infection, this clinical condition seems to be characterized more as a sequel than as a symptom, given that there is no active infection involved.
Keywords: Key ords: Chikungunya, Immunopathology, Inflammation process, Persistent arthralgia, Signaling lymphocytic activation molecule family member 7
Core ip: This review of Chikungunya fever focuses on one of the most prevalent and important symptoms of the disease-arthralgia. The authors propose an approach to explain the persistence of arthralgia for a long time after the resolution of the infection, based on the sustained inflammatory response, mainly by macrophages and T helper type 17 cells. Additionally, it is suggested that, given the context, persistent arthralgia is a sequel of CF and could therefore be termed "long Chikun-gunya".
INTRODUCTION
Chikungunya fever (CF) is a disease caused by the Chikungunya virus (CHIKV), an arbovirus of the Togaviridae family, genus Alphavirus, which has positive single-stranded RNA genetic material[1,2]. CF is transmitted primarily by mosquitoes of the Aedes genus, including Aedes aegypti, Aedes furcifer, Aedes africanus, and Aedes albopictus[2,3]. Transmission can occur through both urban and sylvatic cycles[4,5]. In the urban cycle, transmission occurs between humans and mosquito vectors, while the sylvatic cycle involves non-human primates[5,6]. In addition to transmission through mosquito bites, transmission through blood transfusions is also possible in the urban cycle[7]. The possibility of vertical transmission from an infected mother to fetus and sexual transmission has also been proposed. Sexual transmission has not yet been confirmed, and viruses have only been found in semen[4,6,8].
Since its first recorded appearance in 1952 in Tanzania, Africa, CHIKV has garnered attention for its recurrence, with outbreaks occurring every 7-20 years. In 1958, CHIKV began to be reported in central and southern regions of Africa (Uganda and the sub-Saharan region), followed by outbreaks in Asia between 1958 and 1973, and Kenya in 2004. From Kenya, it spread to the Indian Ocean, India, and Southeast Asia, resulting in more than 6 million cases. Recent outbreaks have been notable for the high number of infected individuals. In addition to the outbreak in India, Comoros recorded 215000 cases of the disease in 2005, with a further 255000 cases reported on Reunion Island, east of Madagascar, between 2005 and 2006[2,9]. Since 2007, health agencies have increased their focus on CF and its causative agent due to its spread to regions of the world previously unaffected[2,10].
In addition to the significant number of cases and its ability to spread, the occurrence of fatal cases contrasts with CF's typical status as a self-limiting and mild disease. Reports of new modes of CF transmission, such as vertical transmission, have also contributed to increased awareness of the disease, which has regained attention recently due to the exponential advancement of global warming. This partly explains the presence of CHIKV in regions previously less affected, such as Europe and the Americas[8,11,12].
In Brazil, especially following the 2014 outbreak in the Northeast region, an association was observed between CF and other arboviruses-Zika and Dengue virus. This association indicates not exacerbation in CF cases, but rather complications in dengue cases leading to hospitalizations[13].
Much of the impact caused by CHIKV and CF stems from the virus's ability to easily adapt to new locations, owing to its capacity to attract new species of anthropophilic vectors[12].
The repercussions of CF extend beyond the realm of health, affecting the economy, social welfare, and other areas. A qualitative study conducted in Curaçao revealed that the social impacts of CF varied depending on the manifestation, duration, and severity of the disease. Patients reported social isolation, inability to engage in physical and daily activities due to physical limitations. Regarding emotional impact, there were reports of stress, anxiety, shame, frustration, despair, feelings of social exclusion, and even personality dissociation. No significant financial impacts were observed, as Curaçao has a public health system that mitigated the damage[14].
CF has an incubation period of 3 to 7 d[2,3,15]. There are three clinical stages defined in CF: The acute stage from the 1st to the 21st d of infection; post-acute stage from the 21st d to the 3rd month, and chronic stage from the 3rd month onwards[16]. Occasionally, infections can be asymptomatic, but these represent a minimal proportion of infected individuals[2,3].
Serological data can indicate leukopenia, thrombocytopenia, and elevated levels of liver transaminases[17]. Some possible findings in individuals experiencing arthralgia include joint effusion, bone erosion, spinal cord edema/erosion, synovial thickening, tendonitis, and tenosynovitis, which can be detected by nuclear magnetic resonance imaging[18].
Clinical manifestations can be acute or chronic. During the acute phase, symptoms may include arthralgia, with or without fever exceeding 38.9 °C, low back pain, headache, fatigue, oligoarthralgia, or polyarthralgia (typically bilateral, predominantly affecting large and peripheral joints such as knees, ankles, wrists, shoulders, and phalanges), ocular hyperemia, oral ulcers[2,3,19], macular or maculopapular skin lesions that are swollen or pruritic, typically affecting the palms of the hands, soles of the feet, torso, and face.
Gastrointestinal symptoms such as nausea, vomiting, and diarrhea may also be present during the acute phase, along with erythema, asthenia, conjunctival effusion, persistent conjunctivitis, and cervical lymphadenopathy, though the latter is less common. The severity and presence of these symptoms are associated with viral load, considered high when it ranges from 105 to 109 copies of viral RNA per milliliter[8], as well as age and biological sex[2,19].
Other atypical manifestations associated with CF have also been reported, such as Guillain-Barré syndrome, partial or total alopecia (predominantly in women), uveitis, and retinitis. Fever, loss of appetite, apnea, skin manifestations, distal and cerebral edema, encephalitis, hemorrhage, cardiac symptoms (myocarditis)[2,20], respiratory issues (Acute respiratory distress syndrome), renal complications (rhabdomyolysis, acute interstitial nephritis, thrombotic microangiopathy, and kidney damage)[20], and gastrointestinal symptoms have been reported in vertically infected neonates.
In infants, bullous lesions have been reported on the second day following the febrile state. There have also been reports of deaths due to CF in neonates, immunocompromised individuals, and the elderly, possibly linked to neurological disorders[21], such as encephalitis, encephalopathy, cognitive disorders, mood swings, depression, confusion, and memory loss[8,20].
During the chronic phase of CF, the main symptom is persistent arthralgia, which, when it appears after the acute phase of the disease (7-10 d)[2], can last from weeks to years, depending on the affected population, age, and the presence of comorbidities, affecting both peripheral and large synovial joints[2,8]. Additionally, alopecia, depression, mood swings, sleep disturbances, blurred vision, and memory loss have also been associated with the chronic phase of CF[22].
Initially, CHIKV remains present in the blood and lymph, characterizing the acute phase of CF. As the disease progresses, other organs and cell types become infected due to the hematogenous distribution route. Cell types include natural killer (NK) cells, T and B cells, dendritic cells, macrophages, synovial fibroblasts, endothelial cells, and myocytes.
The chronic condition of CF can mimic arthritis, particularly rheumatoid arthritis, with symptoms such as joint effusion, bone erosions, medullary edema, synovial thickening, tendonitis, and tenosynovitis, which are present in around 55% to 65% of cases. Among these cases, 90% reported bilateral joint involvement, 63% reported joint edema, and 39% experienced chronic myalgia. Middle-aged individuals and women are predominantly affected[23].
Regarding CF, studies indicate that the persistence of chronic symptoms is sustained by a prolonged inflammatory response[24]. In rheumatoid arthritis, high levels of interleukin (IL) 12 are found, which is responsible for bone degradation and, consequently, arthralgia. Similarly, CHIKV induces the proliferation of osteoclasts by proliferating in synoviocytes[21,25].
Despite being a serious disease in many respects, there is still no vaccine, and pharmacological treatment is neither specific nor effective, as it currently relies on symptom management using non-steroidal anti-inflammatory drugs in conjunction with corticosteroids and antipyretics[3,7,8,26-28].
Patients with polyarthralgia may present with other associated manifestations, characteristic of the continuation of an inflammatory process, such as swelling, and may therefore require corticosteroid therapy[26]. Due to the clinical similarity between the arthralgia experienced by patients infected with CHIKV and rheumatoid arthritis, treatment typically involves the use of disease-modifying anti-rheumatic drugs, such as methotrexate, sulfasalazine, leflunomide, and hydroxychloroquine[3,28]. In cases where there is an inadequate response to methotrexate, satisfactory therapeutic outcomes have been reported with the administration of tumor necrosis factor (TNF) blockers, such as etanercept[28]. One challenge in developing drugs against CHIKV is the virus's intrinsic ability to mutate, potentially leading to resistance to antivirals[29]. In fact, the exact immunological mechanisms involved in CF symptoms need to be better understood so that targeted management and treatment can be devised in search of more effective results.
CHIKV INFECTION CHRONICITY
CHIKV can be classified as an arthritogenic virus, similar to other clinically relevant alphaviruses, as it typically induces debilitating musculoskeletal diseases characterized by myalgia, arthralgia, and arthritis[30]. It is estimated that 30% to 60% of infected individuals develop long-term sequelae, with symptoms persisting for several years[3,31-33]. Over a third of patients report persistent or recurrent polyarthralgia, with approximately half of them experiencing chronic rheumatic manifestations[34,35].
In preclinical infection trials, it was observed that CF symptoms primarily reflect the tissue tropism of the virus rather than the immunopathogenesis triggered by the host's immune system. These trials demonstrated that the severity of the infection is directly linked to the inefficiency of type I-interferon (I-IFN) signaling[36]. Another study revealed the presence of type I-IFN in the synovial tissue of patients with chronic CHIKV infection[37].
Fibroblasts are the primary targets of CHIKV, and as these cells are found in tissues such as joint capsules, fascia, and muscle insertions, they account for the pronounced intensity of myalgia reported by patients, as these structures contain numerous nociceptive nerve endings[36]. Additionally, there is viral tropism for blood monocytes and joint macrophages, directly contributing to the initiation of an inflammatory process during acute infection[5,37].
In studies conducted on non-human primates, the CHIKV genome was identified in splenic macrophages approximately 3 months post-infection, suggesting the significance of macrophages in viral persistence. Furthermore, the prolonged presence of viral antigens in lymph nodes, liver, and muscles supports the notion that macrophages act as crucial viral reservoirs[38]. Additionally, viral tropism towards muscle satellite cells has been observed in ex vivo and in vitro studies[39].
Viral persistence has been associated with the inefficiency of the host's immune system and the effectiveness of viral evasion mechanisms[37]. In a human study, the severity of the infection was found to be correlated with elevated serum levels of pro-inflammatory cytokines, such as IL-6 and IL-1β, along with a decrease in regulated on activation, normal T cell expressed and secreted (RANTES) levels, also referred to as C-C motif ligand (CCL) 5. During the acute phase, an inflammatory pattern predominates, driven by a Th1 immune response, with circulating cytokine levels even more pronounced in individuals with high viral loads. However, as the infection progresses to symptomatic stages, there is a shift towards Th2 response markers, such as IL-7, IL-10, and IL-15[40,41].
The identification of IL-7 and IL-15 in the tissue and synovial fluids of patients with rheumatoid arthritis suggests that these cytokines may be implicated in the development of arthralgia associated with CHIKV infection[40,42,43]. IL-15 produced by synoviocyte fibroblasts induces the expression of IL-17, which has been linked to the pathogenesis of rheumatoid arthritis and the chronic phase of CHIKV infection[41,43]. Osteoblast infection is also observed in patients with chronic arthritis, leading to detrimental effects on bone mineralization due to the inhibition of osteoprotegerin by IL-6 present in infected joints[3,5,31].
Persistent arthralgia typically emerges after the resolution of the acute CF phase, and despite its chronic nature, little is known about the mechanisms and factors contributing to its progression[3,5,18], although various studies suggest different risk factors. Patients with chronic arthralgia exhibit fibroblast hyperplasia, angiogenesis, tissue damage due to elevated levels of metalloproteinase-2, cell death, and infection of perivascular macrophages in synovial tissue[37].
Persistent arthralgia resulting from CHIKV infection also demonstrates elevated serum concentrations of IL-1Ra, IL-1β, IL-6, IL-7, IL-8, IL-12, IL-15, and IFN-α, including IL-17, a cytokine prominent in rheumatoid arthritis; this indicates that the persistent arthralgia of CF physiologically resembles rheumatoid arthritis. However, unlike rheumatoid arthritis, which exhibits serum levels of anti-cyclic citrullinated peptide) and anti-rheumatoid factor antibodies, along with an increase in the CCL5/RANTES chemokine ratio, the persistent arthralgia of CF is characterized by the presence of anti-CHIKV IgM or IgG, a decrease in the CCL5/RANTES ratio, and an increase in granulocyte macrophage-colony stimulating factor (GM-CSF) and TNF-α[23]. An in vivo study comparing serum levels of TNF-α, IL-13, IL-2, and IL-4 during acute infection in patients who developed chronic arthralgia and those without persistent manifestations over a 20-month period post-infection observed that an intense cytokine response during the acute phase led to a reduced incidence of chronic arthralgia, whereas low cytokine levels were associated with pronounced chronic joint pain[27].
ARTHRALGIA TRIGGERED BY CHIKV
The immunopathogenic mechanisms of CF are akin to those responsible for the immune response against CHIKV. Acute CHIKV infection is characterized by elevated serum levels of pro-inflammatory chemokines, such as CCL2, CCL4, C-X-C motif chemokine ligand (CXCL) 9, and CXCL10, as well as cytokines IFN type 1, IFN-γ, IL-6, IL-8, IL-17, growth factors, and GM-CSF[31]. This inflammatory microenvironment triggers a robust migration of phagocytic cells such as macrophages and activation of CD8+ T cells and NK cells to eradicate the viral agent[44,45]. Although macrophages are pivotal for the protective response, their presence in the joints contributes to the inflammatory process[46].
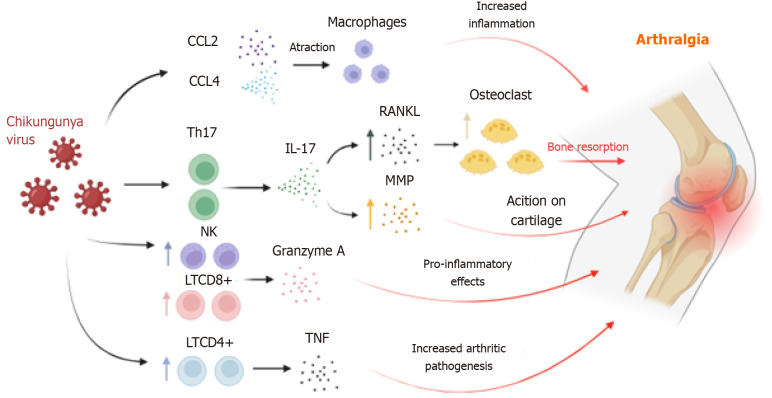
The number of effector lymphocytes increases during the pathogenic process of CF, and this augmentation correlates with the onset of arthralgia[47]. There is a differentiation of T cells into the Th17 subtype, observed in animal models of rheumatoid arthritis and associated with pain manifestation in clinical settings[23]. Thus, Th17 polarization leads to elevated circulating concentrations of IL-17, linked to bone matrix destruction and stimulation of other cytokines, pro-inflammatory chemokines, and matrix metalloproteinases (MMPs), promoting cartilage degradation. Indeed, MMP2 was found in high levels in the synovial fluid of patients with chronic arthralgia[48,49].
Therefore, IL-17 is crucial for arthralgia development, playing a role in bone resorption and weakening through receptor activator of nuclear factor kappa-Β ligand (RANKL) expression, which binds to RANK and regulates osteoclast differentiation, thereby increasing bone resorption and exacerbating joint pain[50]. It is hypothesized that the increase in CD4+ T cells in the joint inflammatory microenvironment may worsen the condition due to intense TNF-α release, as this cytokine contributes to the pathogenesis of psoriatic arthritis and rheumatoid arthritis[51-53].
Studies suggest that NK cells are also involved in acute arthralgia caused by CHIKV in murine models[54-56], as the increased presence of these cells in the synovial region correlates with the arthralgic mechanism in rheumatoid arthritis through the action of TNF-α and IFN-γ, although this mechanism is not fully understood[55,57]. Granzyme A, released by CD8+ T cells and NK cells, also plays a significant role in CHIKV-induced arthralgia[58]. Granzyme A promotes the degradation of type IV collagen and lymphocyte migration to the synovial joint[59-61]. This association is underscored by higher serum levels of granzyme A in CHIKV-infected individuals compared to uninfected individuals, along with its proteolytic and pro-inflammatory activity[55,60,62-64] (Figure 1).
Figure 1.
Supposed mechanisms involved in the pathogenesis of arthritis caused by Chikungunya virus. The elevation in levels of pro-inflammatory chemokines induces T helper type 17 response polarization, leading to interleukin-17 expression and subsequent increase in receptor activator of nuclear factor kappa-Β ligand. Granzyme A released by natural killer cells and CD8+ T cells targets type IV collagen, contributing to progressive joint damage. Th17: T helper type 17; CCL4: C-C motif ligand 4; NK: Natural killer; LTCD8: CD8 T lymphocytes; LTCD4: CD4 T lymphocytes; RANKL: Receptor activator of nuclear factor kappa-Β ligand; MMP: Matrix metalloproteinases; IL: Interleukin; TNF: Tumor necrosis factor.
IMMUNOPATHOLOGY OF PERSISTENT ARTHRALGIA
Some proposals attempt to explain the chronic pathogenesis of CHIKV in the joints. The establishment of the virus, through tissue tropism, in fibroblasts, satellite cells, and myoblasts[36,39,65] makes these cells important reservoirs for the persistence of viral antigens, such as viral RNA, in the affected organism, even after the acute infection has resolved[37,38,66]. The presence of these antigens in the joints may be one of the causes of persistent arthralgia, although the mechanisms are not fully understood. In addition to antigenic persistence, the presence of T cells in the joints, in a chronic state, can be correlated with an increase in local IL-17 levels and other pro-inflammatory cytokines, which exacerbate joint pain[67]. The actions of IL-17, along with RANKL, are related to the development of arthralgia, as previously mentioned[23,41,68-70].
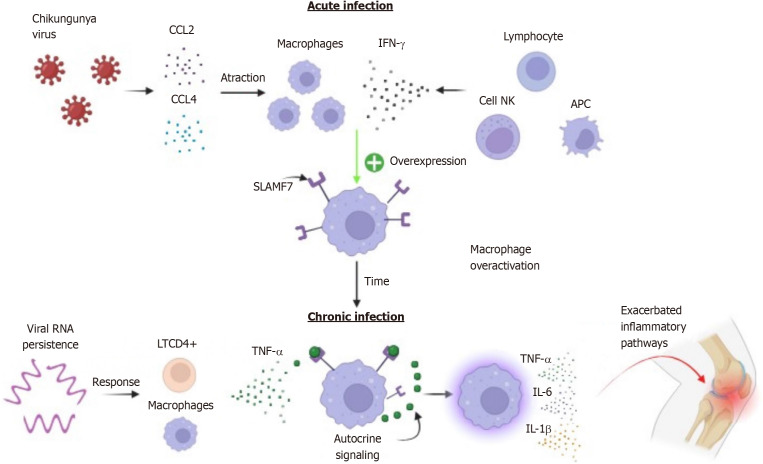
While macrophages are essential phagocytes for controlling numerous microbial infections, they can contribute to an increase in acute inflammation, directing it towards a chronic state and causing tissue damage[71,72]. Dysfunctional macrophages are commonly found in autoimmune diseases such as rheumatoid arthritis[73,74]. Macrophage overactivation occurs through receptors and signaling molecules in the infectious microenvironment, and some studies have shown that the signaling lymphocytic activation molecule family member 7 (SLAMF7) receptor or CD139 plays a crucial role in transforming these phagocytic cells into an explosive, highly inflammatory, and potentially pathogenic state[75,76].
In an unstimulated state, the SLAMF7 receptor may be expressed in plasma cells, NK cells, B and T cells, albeit at low levels in macrophages[77,78]. In vitro treatment of macrophages with IFN-γ has been shown to increase SLAMF7 expression, as well as IFN-β and TNF-α[76]. IFN-γ has been identified as a key regulator of SLAMF7; activation of the SLAMF7 receptor by r-SLAMF7 protein led to up-regulation of TNF-α, IL-1β, IL-6, CCL3, CCL4, CXCL1, CXCL2, and CXCL8, suggesting an intrinsic up-regulation feedback loop. Additionally, an increase in IL-6 and TNF-α levels was observed after stimulation[76].
The initial SLAMF7 activation sequence involves IFN-γ expression. IFN-γ potentiates and increases the number of SLAMF7 receptors on the surface of macrophages, and after the initial stimulus, receptor engagement triggers a highly potent activation of the inflammatory state in these cells[76]. Furthermore, after SLAMF7 induction by IFN-γ, TNF-α from the microenvironment can recruit molecules from autocrine amplification pathways. This suggests that TNF-α participates in an additional step in the maintenance of the inflammatory process[76].
During the acute viral infection phase, the host immune system responds to the infection by releasing IFN-γ by NK cells, antigen-presenting cells, and B cells; in addition to acting in an autocrine and paracrine manner in Th1 cell differentiation, intervening in viral replication[79-81]. IFN-γ release, along with macrophage/monocyte chemotaxis by the chemokines CCL2 and CCL4, may lead to increased SLAMF7 receptor expression in phagocytic cells that have migrated to the inflammatory site[46,76,82].
Thus, in the long term, the activation of SLAMF7 receptors in synovial macrophages associated with the persistence of viral antigens promotes the constant presence of activated CD4+ T cells in the joints; together with macrophages, T cells lead to an increase and continuous release of TNF-α, which in turn acts in an autocrine manner to amplify inflammatory signaling pathways[47,76,83-86]. This condition contributes to a state of constant inflammatory activation, perpetuating the release of cytokines that allow the configuration of an inflammatory joint microenvironment, resulting in greater tissue damage and clinical worsening (Figure 2).
Figure 2.
Hypothesis for persistent arthralgia caused by Chikungunya virus infection. During the initial viremia phase, interferon gamma stimulates increased expression of the signaling lymphocytic activation molecule family member 7 receptor in macrophages, which persists into the chronic phase due to continuous stimulation by Tumor necrosis factor-alpha. Activation of the signaling lymphocytic activation molecule family member 7 receptor and persistence of viral antigens (RNA) enable macrophages to sustain a state of chronic inflammatory over-activation, perpetuating the release of cytokines that facilitate the formation of an inflammatory microenvironment, tissue damage, and clinical deterioration. SLAMF7: Signaling lymphocytic activation molecule family member 7; LTCD4: CD4 T lymphocytes; CCL: C-C motif ligand; NK: Natural killer; TNF-α: Tumor necrosis factor-alpha; IL: Interleukin; APC: Antigen presenting cells; IFN-γ: Interferon gamma.
CONCLUSION
The arthralgia is one of the most prevalent and relevant symptoms of CF in both the acute and chronic phases of the disease. The fact that arthralgia is reported by individual months and even years after the infection has resolved characterizes this manifestation as persistent. Although arthralgia was triggered by the viral infection, persistent arthralgia is not supported by the presence of the infection. In this context, persistent arthralgia, in the absence of infection, classifies this clinical condition as a sequel rather than a symptom. Therefore, in comparison to similar cases involving viral agents, this post-infection condition could be determined, for the first time, as "long chikungunya".
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Virology
Country/Territory of origin: Brazil
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade C
Scientific Significance: Grade C
P-Reviewer: Chen K, China S-Editor: Liu H L-Editor: A P-Editor: Bu BZ
Contributor Information
Jayme Euclydes Picasky Silveira-Freitas, Campus Toledo, Universidade Federal do Paraná, Toledo, Paraná 85919-899, Brazil.
Maria Luiza Campagnolo, Campus Toledo, Universidade Federal do Paraná, Toledo, Paraná 85919-899, Brazil.
Mariana dos Santos Cortez, Campus Toledo, Universidade Federal do Paraná, Toledo, Paraná 85919-899, Brazil.
Fabrício Freire de Melo, Instituto Multidisciplinar em Saúde, Universidade Federal da Bahia, Campus Anísio Teixeira, Vitória da Conquista, Bahia 45029-094, Brazil.
Ana Carla Zarpelon-Schutz, Campus Toledo, Universidade Federal do Paraná, Toledo, Paraná 85919-899, Brazil; Programa de Pós-graduação em Biotecnologia, Palotina, Universidade Federal do Paraná-Setor Palotina, Paraná 85950-000, Brazil.
Kádima Nayara Teixeira, Campus Toledo, Universidade Federal do Paraná, Toledo, Paraná 85919-899, Brazil; Programa Multicêntrico de Pós-graduação em Bioquímica e Biologia Molecular, Palotina, Universidade Federal do Paraná-Setor Palotina, Paraná 85950-000, Brazil. kadimateixeira@ufpr.br.
References
- 1.Vairo F, Haider N, Kock R, Ntoumi F, Ippolito G, Zumla A. Chikungunya: Epidemiology, Pathogenesis, Clinical Features, Management, and Prevention. Infect Dis Clin North Am. 2019;33:1003–1025. doi: 10.1016/j.idc.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Burt FJ, Chen W, Miner JJ, Lenschow DJ, Merits A, Schnettler E, Kohl A, Rudd PA, Taylor A, Herrero LJ, Zaid A, Ng LFP, Mahalingam S. Chikungunya virus: an update on the biology and pathogenesis of this emerging pathogen. Lancet Infect Dis. 2017;17:e107–e117. doi: 10.1016/S1473-3099(16)30385-1. [DOI] [PubMed] [Google Scholar]
- 3.de Lima Cavalcanti TYV, Pereira MR, de Paula SO, Franca RFO. A Review on Chikungunya Virus Epidemiology, Pathogenesis and Current Vaccine Development. Viruses. 2022;14 doi: 10.3390/v14050969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganesan VK, Duan B, Reid SP. Chikungunya Virus: Pathophysiology, Mechanism, and Modeling. Viruses. 2017;9 doi: 10.3390/v9120368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartholomeeusen K, Daniel M, LaBeaud DA, Gasque P, Peeling RW, Stephenson KE, Ng LFP, Ariën KK. Chikungunya fever. Nat Rev Dis Primers. 2023;9:17. doi: 10.1038/s41572-023-00429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sreekanth R, Venugopal L, Arunkrishnan B, Chaturvedi S, Sundaram S. Neonatal chikungunya encephalitis. Trop Doct. 2022;52:199–201. doi: 10.1177/00494755211063268. [DOI] [PubMed] [Google Scholar]
- 7.Moizéis RNC, Fernandes TAAM, Guedes PMDM, Pereira HWB, Lanza DCF, Azevedo JWV, Galvão JMA, Fernandes JV. Chikungunya fever: a threat to global public health. Pathog Glob Health. 2018;112:182–194. doi: 10.1080/20477724.2018.1478777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva JVJ Jr, Ludwig-Begall LF, Oliveira-Filho EF, Oliveira RAS, Durães-Carvalho R, Lopes TRR, Silva DEA, Gil LHVG. A scoping review of Chikungunya virus infection: epidemiology, clinical characteristics, viral co-circulation complications, and control. Acta Trop. 2018;188:213–224. doi: 10.1016/j.actatropica.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramful D, Carbonnier M, Pasquet M, Bouhmani B, Ghazouani J, Noormahomed T, Beullier G, Attali T, Samperiz S, Fourmaintraux A, Alessandri JL. Mother-to-child transmission of Chikungunya virus infection. Pediatr Infect Dis J. 2007;26:811–815. doi: 10.1097/INF.0b013e3180616d4f. [DOI] [PubMed] [Google Scholar]
- 10.Powers AM. How Chikungunya Virus Virology Affects Its Epidemiology and Transmission: Implications for Influencing Public Health. J Infect Dis. 2016;214:S449–S452. doi: 10.1093/infdis/jiw382. [DOI] [PubMed] [Google Scholar]
- 11.Madariaga M, Ticona E, Resurrecion C. Chikungunya: bending over the Americas and the rest of the world. Braz J Infect Dis. 2016;20:91–98. doi: 10.1016/j.bjid.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chala B, Hamde F. Emerging and Re-emerging Vector-Borne Infectious Diseases and the Challenges for Control: A Review. Front Public Health. 2021;9:715759. doi: 10.3389/fpubh.2021.715759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pescarini JM, Rodrigues M, Paixão ES, Cardim L, Brito CAA, Costa MDCN, Santos AC, Smeeth L, Teixeira MDG, Souza APF, Barreto ML, Brickley EB. Dengue, Zika, and Chikungunya viral circulation and hospitalization rates in Brazil from 2014 to 2019: An ecological study. PLoS Negl Trop Dis. 2022;16:e0010602. doi: 10.1371/journal.pntd.0010602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elsinga J, Grobusch MP, Tami A, Gerstenbluth I, Bailey A. Health-related impact on quality of life and coping strategies for chikungunya: A qualitative study in Curaçao. PLoS Negl Trop Dis. 2017;11:e0005987. doi: 10.1371/journal.pntd.0005987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuri-Morales PA, Guzmán-Morales E, De La Paz-Nicolau E, Salas-Fernández A. [Emerging and reemerging diseases] Gac Med Mex. 2015;151:674–680. [PubMed] [Google Scholar]
- 16.Simon F, Javelle E, Cabie A, Bouquillard E, Troisgros O, Gentile G, Leparc-Goffart I, Hoen B, Gandjbakhch F, Rene-Corail P, Franco JM, Caumes E, Combe B, Poiraudeau S, Gane-Troplent F, Djossou F, Schaerverbeke T, Criquet-Hayot A, Carrere P, Malvy D, Gaillard P, Wendling D Société de pathologie infectieuse de langue francaise. French guidelines for the management of chikungunya (acute and persistent presentations). November 2014. Med Mal Infect. 2015;45:243–263. doi: 10.1016/j.medmal.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Marques MA, Adami de Sá FP, Lupi O, Brasil P, von Ristow A. Trombose venosa profunda e vírus chicungunha. J Vasc Bras. 2017;16:60–62. doi: 10.1590/1677-5449.009616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manimunda SP, Vijayachari P, Uppoor R, Sugunan AP, Singh SS, Rai SK, Sudeep AB, Muruganandam N, Chaitanya IK, Guruprasad DR. Clinical progression of chikungunya fever during acute and chronic arthritic stages and the changes in joint morphology as revealed by imaging. Trans R Soc Trop Med Hyg. 2010;104:392–399. doi: 10.1016/j.trstmh.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Hossain MS, Hasan MM, Islam MS, Islam S, Mozaffor M, Khan MAS, Ahmed N, Akhtar W, Chowdhury S, Arafat SMY, Khaleque MA, Khan ZJ, Dipta TF, Asna SMZH, Hossain MA, Aziz KS, Mosabbir AA, Raheem E. Chikungunya outbreak (2017) in Bangladesh: Clinical profile, economic impact and quality of life during the acute phase of the disease. PLoS Negl Trop Dis. 2018;12:e0006561. doi: 10.1371/journal.pntd.0006561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Traverse EM, Millsapps EM, Underwood EC, Hopkins HK, Young M, Barr KL. Chikungunya Immunopathology as It Presents in Different Organ Systems. Viruses. 2022;14 doi: 10.3390/v14081786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinheiro TJ, Guimarães LF, Silva MT, Soares CN. Neurological manifestations of Chikungunya and Zika infections. Arq Neuropsiquiatr. 2016;74:937–943. doi: 10.1590/0004-282X20160138. [DOI] [PubMed] [Google Scholar]
- 22.van Aalst M, Nelen CM, Goorhuis A, Stijnis C, Grobusch MP. Long-term sequelae of chikungunya virus disease: A systematic review. Travel Med Infect Dis. 2017;15:8–22. doi: 10.1016/j.tmaid.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Amaral JK, Bilsborrow JB, Schoen RT. Chronic Chikungunya Arthritis and Rheumatoid Arthritis: What They Have in Common. Am J Med. 2020;133:e91–e97. doi: 10.1016/j.amjmed.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar R, Ahmed S, Parray HA, Das S. Chikungunya and arthritis: An overview. Travel Med Infect Dis. 2021;44:102168. doi: 10.1016/j.tmaid.2021.102168. [DOI] [PubMed] [Google Scholar]
- 25.Bautista-Reyes E, Núñez-Avellaneda D, Alonso-Palomares LA, Salazar MI. Chikungunya: Molecular Aspects, Clinical Outcomes and Pathogenesis. Rev Invest Clin. 2017;69:299–307. doi: 10.24875/RIC.17002029. [DOI] [PubMed] [Google Scholar]
- 26.de Castro APC, Lima RA, Nascimento JS. Chikungunya: a visão do clínico de dor. Rev Dor. 2016;17:299–302. [Google Scholar]
- 27.Tritsch SR, Encinales L, Pacheco N, Cadena A, Cure C, McMahon E, Watson H, Porras Ramirez A, Mendoza AR, Li G, Khurana K, Jaller-Raad JJ, Castillo SM, Barrios Taborda O, Jaller-Char A, Echavez LA, Jiménez D, Gonzalez Coba A, Alarcon Gomez M, Ariza Orozco D, Bravo E, Martinez V, Guerra B, Simon G, Firestein GS, Chang AY. Chronic Joint Pain 3 Years after Chikungunya Virus Infection Largely Characterized by Relapsing-remitting Symptoms. J Rheumatol. 2020;47:1267–1274. doi: 10.3899/jrheum.190162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouquillard E, Combe B. A report of 21 cases of rheumatoid arthritis following Chikungunya fever. A mean follow-up of two years. Joint Bone Spine. 2009;76:654–657. doi: 10.1016/j.jbspin.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Battisti V, Urban E, Langer T. Antivirals against the chikungunya virus. Viruses. 2021;13:1307. doi: 10.3390/v13071307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toivanen A. Alphaviruses: an emerging cause of arthritis? Curr Opin Rheumatol. 2008;20:486–490. doi: 10.1097/BOR.0b013e328303220b. [DOI] [PubMed] [Google Scholar]
- 31.Zaid A, Gérardin P, Taylor A, Mostafavi H, Malvy D, Mahalingam S. Chikungunya Arthritis: Implications of Acute and Chronic Inflammation Mechanisms on Disease Management. Arthritis Rheumatol. 2018;70:484–495. doi: 10.1002/art.40403. [DOI] [PubMed] [Google Scholar]
- 32.Sissoko D, Malvy D, Ezzedine K, Renault P, Moscetti F, Ledrans M, Pierre V. Post-epidemic Chikungunya disease on Reunion Island: course of rheumatic manifestations and associated factors over a 15-month period. PLoS Negl Trop Dis. 2009;3:e389. doi: 10.1371/journal.pntd.0000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heath CJ, Lowther J, Noël TP, Mark-George I, Boothroyd DB, Mitchell G, MacPherson C, Desiree LaBeaud A. The Identification of Risk Factors for Chronic Chikungunya Arthralgia in Grenada, West Indies: A Cross-Sectional Cohort Study. Open Forum Infect Dis. 2018;5:ofx234. doi: 10.1093/ofid/ofx234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weaver SC, Charlier C, Vasilakis N, Lecuit M. Zika, Chikungunya, and Other Emerging Vector-Borne Viral Diseases. Annu Rev Med. 2018;69:395–408. doi: 10.1146/annurev-med-050715-105122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodríguez-Morales AJ, Cardona-Ospina JA, Fernanda Urbano-Garzón S, Sebastian Hurtado-Zapata J. Prevalence of Post-Chikungunya Infection Chronic Inflammatory Arthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res (Hoboken) 2016;68:1849–1858. doi: 10.1002/acr.22900. [DOI] [PubMed] [Google Scholar]
- 36.Couderc T, Chrétien F, Schilte C, Disson O, Brigitte M, Guivel-Benhassine F, Touret Y, Barau G, Cayet N, Schuffenecker I, Desprès P, Arenzana-Seisdedos F, Michault A, Albert ML, Lecuit M. A mouse model for Chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 2008;4:e29. doi: 10.1371/journal.ppat.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoarau JJ, Jaffar Bandjee MC, Krejbich Trotot P, Das T, Li-Pat-Yuen G, Dassa B, Denizot M, Guichard E, Ribera A, Henni T, Tallet F, Moiton MP, Gauzère BA, Bruniquet S, Jaffar Bandjee Z, Morbidelli P, Martigny G, Jolivet M, Gay F, Grandadam M, Tolou H, Vieillard V, Debré P, Autran B, Gasque P. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol. 2010;184:5914–5927. doi: 10.4049/jimmunol.0900255. [DOI] [PubMed] [Google Scholar]
- 38.Labadie K, Larcher T, Joubert C, Mannioui A, Delache B, Brochard P, Guigand L, Dubreil L, Lebon P, Verrier B, de Lamballerie X, Suhrbier A, Cherel Y, Le Grand R, Roques P. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J Clin Invest. 2010;120:894–906. doi: 10.1172/JCI40104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozden S, Huerre M, Riviere JP, Coffey LL, Afonso PV, Mouly V, de Monredon J, Roger JC, El Amrani M, Yvin JL, Jaffar MC, Frenkiel MP, Sourisseau M, Schwartz O, Butler-Browne G, Desprès P, Gessain A, Ceccaldi PE. Human muscle satellite cells as targets of Chikungunya virus infection. PLoS One. 2007;2:e527. doi: 10.1371/journal.pone.0000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ng LF, Chow A, Sun YJ, Kwek DJ, Lim PL, Dimatatac F, Ng LC, Ooi EE, Choo KH, Her Z, Kourilsky P, Leo YS. IL-1beta, IL-6, and RANTES as biomarkers of Chikungunya severity. PLoS One. 2009;4:e4261. doi: 10.1371/journal.pone.0004261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chow A, Her Z, Ong EK, Chen JM, Dimatatac F, Kwek DJ, Barkham T, Yang H, Rénia L, Leo YS, Ng LF. Persistent arthralgia induced by Chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J Infect Dis. 2011;203:149–157. doi: 10.1093/infdis/jiq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Churchman SM, Ponchel F. Interleukin-7 in rheumatoid arthritis. Rheumatology (Oxford) 2008;47:753–759. doi: 10.1093/rheumatology/ken053. [DOI] [PubMed] [Google Scholar]
- 43.Asquith DL, McInnes IB. Emerging cytokine targets in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:246–251. doi: 10.1097/BOR.0b013e3280eec78c. [DOI] [PubMed] [Google Scholar]
- 44.Her Z, Malleret B, Chan M, Ong EK, Wong SC, Kwek DJ, Tolou H, Lin RT, Tambyah PA, Rénia L, Ng LF. Active infection of human blood monocytes by Chikungunya virus triggers an innate immune response. J Immunol. 2010;184:5903–5913. doi: 10.4049/jimmunol.0904181. [DOI] [PubMed] [Google Scholar]
- 45.Wauquier N, Becquart P, Nkoghe D, Padilla C, Ndjoyi-Mbiguino A, Leroy EM. The acute phase of Chikungunya virus infection in humans is associated with strong innate immunity and T CD8 cell activation. J Infect Dis. 2011;204:115–123. doi: 10.1093/infdis/jiq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poo YS, Nakaya H, Gardner J, Larcher T, Schroder WA, Le TT, Major LD, Suhrbier A. CCR2 deficiency promotes exacerbated chronic erosive neutrophil-dominated chikungunya virus arthritis. J Virol. 2014;88:6862–6872. doi: 10.1128/JVI.03364-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miner JJ, Aw-Yeang HX, Fox JM, Taffner S, Malkova ON, Oh ST, Kim AHJ, Diamond MS, Lenschow DJ, Yokoyama WM. Chikungunya viral arthritis in the United States: a mimic of seronegative rheumatoid arthritis. Arthritis Rheumatol. 2015;67:1214–1220. doi: 10.1002/art.39027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 49.Fraser JR, Cunningham AL, Clarris BJ, Aaskov JG, Leach R. Cytology of synovial effusions in epidemic polyarthritis. Aust N Z J Med. 1981;11:168–173. doi: 10.1111/j.1445-5994.1981.tb04226.x. [DOI] [PubMed] [Google Scholar]
- 50.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 51.Wade SM, Canavan M, McGarry T, Low C, Wade SC, Mullan RH, Veale DJ, Fearon U. Association of synovial tissue polyfunctional T-cells with DAPSA in psoriatic arthritis. Ann Rheum Dis. 2019;78:350–354. doi: 10.1136/annrheumdis-2018-214138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rudwaleit M, Yin Z, Siegert S, Grolms M, Radbruch A, Braun J, Sieper J. Response to methotrexate in early rheumatoid arthritis is associated with a decrease of T cell derived tumour necrosis factor alpha, increase of interleukin 10, and predicted by the initial concentration of interleukin 4. Ann Rheum Dis. 2000;59:311–314. doi: 10.1136/ard.59.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haroon N, Srivastava R, Misra R, Aggarwal A. A novel predictor of clinical response to methotrexate in patients with rheumatoid arthritis: a pilot study of in vitro T cell cytokine suppression. J Rheumatol. 2008;35:975–978. [PubMed] [Google Scholar]
- 54.Teo TH, Her Z, Tan JJ, Lum FM, Lee WW, Chan YH, Ong RY, Kam YW, Leparc-Goffart I, Gallian P, Rénia L, de Lamballerie X, Ng LF. Caribbean and La Réunion Chikungunya Virus Isolates Differ in Their Capacity To Induce Proinflammatory Th1 and NK Cell Responses and Acute Joint Pathology. J Virol. 2015;89:7955–7969. doi: 10.1128/JVI.00909-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson JA, Prow NA, Schroder WA, Ellis JJ, Cumming HE, Gearing LJ, Poo YS, Taylor A, Hertzog PJ, Di Giallonardo F, Hueston L, Le Grand R, Tang B, Le TT, Gardner J, Mahalingam S, Roques P, Bird PI, Suhrbier A. RNA-Seq analysis of chikungunya virus infection and identification of granzyme A as a major promoter of arthritic inflammation. PLoS Pathog. 2017;13:e1006155. doi: 10.1371/journal.ppat.1006155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poo YS, Rudd PA, Gardner J, Wilson JA, Larcher T, Colle MA, Le TT, Nakaya HI, Warrilow D, Allcock R, Bielefeldt-Ohmann H, Schroder WA, Khromykh AA, Lopez JA, Suhrbier A. Multiple immune factors are involved in controlling acute and chronic chikungunya virus infection. PLoS Negl Trop Dis. 2014;8:e3354. doi: 10.1371/journal.pntd.0003354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamin R, Berhani O, Peleg H, Aamar S, Stein N, Gamliel M, Hindi I, Scheiman-Elazary A, Gur C. High percentages and activity of synovial fluid NK cells present in patients with advanced stage active Rheumatoid Arthritis. Sci Rep. 2019;9:1351. doi: 10.1038/s41598-018-37448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Long KM, Heise MT. Protective and Pathogenic Responses to Chikungunya Virus Infection. Curr Trop Med Rep. 2015;2:13–21. doi: 10.1007/s40475-015-0037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simon MM, Kramer MD, Prester M, Gay S. Mouse T-cell associated serine proteinase 1 degrades collagen type IV: a structural basis for the migration of lymphocytes through vascular basement membranes. Immunology. 1991;73:117–119. [PMC free article] [PubMed] [Google Scholar]
- 60.Voskoboinik I, Whisstock JC, Trapani JA. Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol. 2015;15:388–400. doi: 10.1038/nri3839. [DOI] [PubMed] [Google Scholar]
- 61.Suhrbier A, Fernan A, Burrows SR, Saul A, Moss DJ. BLT esterase activity as an alternative to chromium release in cytotoxic T cell assays. J Immunol Methods. 1991;145:43–53. doi: 10.1016/0022-1759(91)90309-4. [DOI] [PubMed] [Google Scholar]
- 62.Wensink AC, Hack CE, Bovenschen N. Granzymes regulate proinflammatory cytokine responses. J Immunol. 2015;194:491–497. doi: 10.4049/jimmunol.1401214. [DOI] [PubMed] [Google Scholar]
- 63.Froelich CJ, Pardo J, Simon MM. Granule-associated serine proteases: granzymes might not just be killer proteases. Trends Immunol. 2009;30:117–123. doi: 10.1016/j.it.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 64.Sower LE, Froelich CJ, Allegretto N, Rose PM, Hanna WD, Klimpel GR. Extracellular activities of human granzyme A. Monocyte activation by granzyme A versus alpha-thrombin. J Immunol. 1996;156:2585–2590. [PubMed] [Google Scholar]
- 65.Lohachanakul J, Phuklia W, Thannagith M, Thongsakulprasert T, Smith DR, Ubol S. Differences in response of primary human myoblasts to infection with recent epidemic strains of Chikungunya virus isolated from patients with and without myalgia. J Med Virol. 2015;87:733–739. doi: 10.1002/jmv.24081. [DOI] [PubMed] [Google Scholar]
- 66.Hawman DW, Stoermer KA, Montgomery SA, Pal P, Oko L, Diamond MS, Morrison TE. Chronic joint disease caused by persistent Chikungunya virus infection is controlled by the adaptive immune response. J Virol. 2013;87:13878–13888. doi: 10.1128/JVI.02666-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mostafavi H, Tharmarajah K, Vider J, West NP, Freitas JR, Cameron B, Foster PS, Hueston LP, Lloyd AR, Mahalingam S, Zaid A. Interleukin-17 contributes to Ross River virus-induced arthritis and myositis. PLoS Pathog. 2022;18:e1010185. doi: 10.1371/journal.ppat.1010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Phuklia W, Kasisith J, Modhiran N, Rodpai E, Thannagith M, Thongsakulprasert T, Smith DR, Ubol S. Osteoclastogenesis induced by CHIKV-infected fibroblast-like synoviocytes: a possible interplay between synoviocytes and monocytes/macrophages in CHIKV-induced arthralgia/arthritis. Virus Res. 2013;177:179–188. doi: 10.1016/j.virusres.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 69.Herrero LJ, Nelson M, Srikiatkhachorn A, Gu R, Anantapreecha S, Fingerle-Rowson G, Bucala R, Morand E, Santos LL, Mahalingam S. Critical role for macrophage migration inhibitory factor (MIF) in Ross River virus-induced arthritis and myositis. Proc Natl Acad Sci U S A. 2011;108:12048–12053. doi: 10.1073/pnas.1101089108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen W, Foo SS, Rulli NE, Taylor A, Sheng KC, Herrero LJ, Herring BL, Lidbury BA, Li RW, Walsh NC, Sims NA, Smith PN, Mahalingam S. Arthritogenic alphaviral infection perturbs osteoblast function and triggers pathologic bone loss. Proc Natl Acad Sci U S A. 2014;111:6040–6045. doi: 10.1073/pnas.1318859111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 73.Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996;39:115–124. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- 74.Smiljanovic B, Grützkau A, Sörensen T, Grün JR, Vogl T, Bonin M, Schendel P, Stuhlmüller B, Claussnitzer A, Hermann S, Ohrndorf S, Aupperle K, Backhaus M, Radbruch A, Burmester GR, Häupl T. Synovial tissue transcriptomes of long-standing rheumatoid arthritis are dominated by activated macrophages that reflect microbial stimulation. Sci Rep. 2020;10:7907. doi: 10.1038/s41598-020-64431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, De Nardo D, Gohel TD, Emde M, Schmidleithner L, Ganesan H, Nino-Castro A, Mallmann MR, Labzin L, Theis H, Kraut M, Beyer M, Latz E, Freeman TC, Ulas T, Schultze JL. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40:274–288. doi: 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simmons DP, Nguyen HN, Gomez-Rivas E, Jeong Y, Jonsson AH, Chen AF, Lange JK, Dyer GS, Blazar P, Earp BE, Coblyn JS, Massarotti EM, Sparks JA, Todd DJ Accelerating Medicines Partnership (AMP) RA/SLE Network, Rao DA, Kim EY, Brenner MB. SLAMF7 engagement superactivates macrophages in acute and chronic inflammation. Sci Immunol. 2022;7:eabf2846. doi: 10.1126/sciimmunol.abf2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karampetsou MP, Comte D, Kis-Toth K, Kyttaris VC, Tsokos GC. Expression patterns of signaling lymphocytic activation molecule family members in peripheral blood mononuclear cell subsets in patients with systemic lupus erythematosus. PLoS One. 2017;12:e0186073. doi: 10.1371/journal.pone.0186073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Veillette A. SLAM-family receptors: immune regulators with or without SAP-family adaptors. Cold Spring Harb Perspect Biol. 2010;2:a002469. doi: 10.1101/cshperspect.a002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ge Y, Huang M, Yao YM. Autophagy and proinflammatory cytokines: Interactions and clinical implications. Cytokine Growth Factor Rev. 2018;43:38–46. doi: 10.1016/j.cytogfr.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 80.Laurent X, Bertin B, Renault N, Farce A, Speca S, Milhomme O, Millet R, Desreumaux P, Hénon E, Chavatte P. Switching invariant natural killer T (iNKT) cell response from anticancerous to anti-inflammatory effect: molecular bases. J Med Chem. 2014;57:5489–5508. doi: 10.1021/jm4010863. [DOI] [PubMed] [Google Scholar]
- 81.Dörries R. The role of T-cell-mediated mechanisms in virus infections of the nervous system. Curr Top Microbiol Immunol. 2001;253:219–245. doi: 10.1007/978-3-662-10356-2_11. [DOI] [PubMed] [Google Scholar]
- 82.Rulli NE, Rolph MS, Srikiatkhachorn A, Anantapreecha S, Guglielmotti A, Mahalingam S. Protection from arthritis and myositis in a mouse model of acute chikungunya virus disease by bindarit, an inhibitor of monocyte chemotactic protein-1 synthesis. J Infect Dis. 2011;204:1026–1030. doi: 10.1093/infdis/jir470. [DOI] [PubMed] [Google Scholar]
- 83.Nakaya HI, Gardner J, Poo YS, Major L, Pulendran B, Suhrbier A. Gene profiling of Chikungunya virus arthritis in a mouse model reveals significant overlap with rheumatoid arthritis. Arthritis Rheum. 2012;64:3553–3563. doi: 10.1002/art.34631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gasque P, Jaffar-Bandjee MC. Blunting CHIKV infection by keeping T cells in check. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aam6567. [DOI] [PubMed] [Google Scholar]
- 85.Lentscher AJ, McCarthy MK, May NA, Davenport BJ, Montgomery SA, Raghunathan K, McAllister N, Silva LA, Morrison TE, Dermody TS. Chikungunya virus replication in skeletal muscle cells is required for disease development. J Clin Invest. 2020;130:1466–1478. doi: 10.1172/JCI129893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nair S, Poddar S, Shimak RM, Diamond MS. Interferon Regulatory Factor 1 Protects against Chikungunya Virus-Induced Immunopathology by Restricting Infection in Muscle Cells. J Virol. 2017;91 doi: 10.1128/JVI.01419-17. [DOI] [PMC free article] [PubMed] [Google Scholar]