Abstract
The interaction between the viral protein linked to the genome (VPg) of turnip mosaic potyvirus (TuMV) and the translation eukaryotic initiation factor eIF(iso)4E of Arabidopsis thaliana has previously been reported. eIF(iso)4E binds the cap structure (m7GpppN, where N is any nucleotide) of mRNAs and has an important role in the regulation in the initiation of translation. In the present study, it was shown that not only did VPg bind eIF(iso)4E but it also interacted with the eIF4E isomer of A. thaliana as well as with eIF(iso)4E of Triticum aestivum (wheat). The interaction domain on VPg was mapped to a stretch of 35 amino acids, and substitution of an aspartic acid residue found within this region completely abolished the interaction. The cap analogue m7GTP, but not GTP, inhibited VPg-eIF(iso)4E complex formation, suggesting that VPg and cellular mRNAs compete for eIF(iso)4E binding. The biological significance of this interaction was investigated. Brassica perviridis plants were infected with a TuMV infectious cDNA (p35Tunos) and p35TuD77N, a mutant which contained the aspartic acid substitution in the VPg domain that abolished the interaction with eIF(iso)4E. After 20 days, plants bombarded with p35Tunos showed viral symptoms, while plants bombarded with p35TuD77N remained symptomless. These results suggest that VPg-eIF(iso)4E interaction is a critical element for virus production.
Potyviruses belong to the supergroup of “picorna-like” viruses. The viral genome is a single RNA molecule of positive polarity of close to 10,000 nucleotides with a poly(A) tract at its 3′ end. It codes for one large polyprotein which is processed into at least 10 mature proteins by three viral proteinases (Pro) (47). The 5′ end of the viral RNA does not have a cap structure (m7GpppN, where N is any nucleotide) but is covalently linked to a virus-encoded protein termed VPg via a tyrosine residue (39, 40). VPg has several suggested roles in the virus life cycle. Interactions of VPg with the viral RNA polymerase in yeast (25, 33) and in vitro (15) support a role in viral RNA synthesis. Additionally, VPg has been implicated in overcoming resistance in plants (27, 35, 41, 42, 55). VPg also performs a yet-to-be-defined function in the nucleus. Indeed, NIa protein of tobacco etch potyvirus, a precursor form of VPg, has been found in the nucleus (10, 23, 46), and mutations in the VPg domain resulting in the inhibition of nuclear transport debilitated viral genome amplification (54). Recently, an interaction was shown to take place between the VPg of turnip mosaic potyvirus (TuMV) and the translation eukaryotic initiation factor (eIF) iso 4E of Arabidopsis thaliana (65). eIF4E is a component of the eIF4F complex and binds the cap structure of cellular mRNAs (6, 36, 38, 43). The cap mediates attachment of mRNAs to small ribosomal subunits, and the association is mediated by eIF4F (through binding to eIF4E) and eIF3 (38, 43). The interaction between VPg and eIF(iso)4E suggests the participation of the viral protein in the initiation of translation of the viral RNA.
Initiation is the rate-limiting step of translation in eukaryotes, and eIF4E has a regulatory role in this cellular event (38, 43, 60). In mammals, eIF4E is the least abundant of the initiation factors (13), although this assertion has been challenged (45). Its cap-binding activity is modulated by phosphorylation (62, 64). It is also regulated by eIF4E-binding proteins (4E-BPs) (31) which, by binding eIF4E, prevent the formation of the eIF4F complex (21, 34, 44). As a consequence, eIF4E plays an important role in the control of cell growth (58). In Saccharomyces cerevisiae, disruption of the gene coding for eIF4E is lethal, and mutants with altered mRNA cap-binding affinity reprogram mRNA selection by ribosomes (2). In mammals, overexpression of eIF4E has been shown to transform cells in tissue culture (11, 32). Elevated eIF4E expression results in the selective increase of a few proteins whose mRNAs are normally translationally repressed, such as ornithine decarboxylase and cyclin (49, 50). Just as elevated levels of eIF4E contribute to the development of a transformed cellular state, the reduction of eIF4E levels, using antisense RNA, has been shown to lengthen cell division times (12). The results of these in vitro studies, which emphasize the importance of eIF4E in the regulation of the cell division cycle, have been extended to clinical observations: eIF4E amounts have been found to be elevated in some human carcinomas (16, 27).
eIF4F is targeted by several animal viruses in their attempt to control host translation for preferential viral mRNA translation. For instance, adenoviruses and influenza viruses affect the phosphorylation state of eIF4E (14, 66). Encephalomyocarditis virus inactivates the initiation factor by enhancing 4E-BP1 binding (18). Finally, picornaviruses induce the cleavage eIF4G, with the consequence that cellular mRNAs linked to eIF4E cannot interact with 40S ribosome complexes (22, 59).
Although most of these observations relating to the role of eIF4E have been made in mammalian cells, the similarities in translation initiation in mammals, plants, and yeasts and the sequence homologies of different translation initiation factors (6, 17) suggest that the plant eIF4E plays as important a role as its mammalian homologue in the regulation of cellular processes. In this study, we investigated the interaction between the VPg of TuMV and eIF(iso)4E and its consequences for viral infection. We found that the cap analogue m7GTP competed with VPg for eIF(iso)4E binding. Furthermore, TuMV whose VPg was mutated at a single residue which abolished in vitro interaction with eIF(iso)4E was debilitated for viral infection in whole plants.
MATERIALS AND METHODS
Microorganisms and media.
Manipulations of bacterial as well as yeast strains and of nucleic acids and proteins were done by standard methods (19, 52). Escherichia coli XL1-Blue was used for subcloning, and E. coli BL21(DE3) (Novagen) was used for protein expression. S. cerevisiae EGY48 (MATa trp1 his3 ura3 8op-Leu2) (19) was used for the interaction study.
Yeast two-hybrid system.
Plasmids employed for the interaction study were as described by Golemis et al. (19). pEG202 was used for the fusion of VPg and its derivatives to the DNA-binding domain of LexA. pJG4-5 was used to express eIF(iso)4E of A. thaliana (pSW56) (65) as a translation fusion to a cassette consisting of the simian virus 40 nuclear localization sequence, the acid blob B42, and the hemagglutinin epitope tag; expression was under the control of the GAL1 inducible promoter. The lacZ reporter plasmid was pSH18-34 containing eight lexA operators. Strength of the interaction was quantified using the β-galactosidase liquid assay (19). β-Galactosidase units were calculated using the following equation: units = 1,000 × (optical density at 420 nm [OD420] − 1.75 × OD550)/(T × V × OD660), where T is time in minutes and V is the volume of culture used in milliliters.
The pLex-VPg plasmids were constructed as follows. The region coding for VPg in plasmid pETPro/24 (30) was amplified by PCR using the 5′ and 3′ primer pairs listed in Table 1. The amplified fragment was digested with BamHI and XhoI, ligated with similarly restricted pEG202, and introduced into E. coli XL1 and ultimately into S. cerevisiae EGY48. pEGVPgΔ59–93 was produced by amplification of pETPro/24 with a first set of primers (Table 1); the amplified fragment was digested with EcoRI and XhoI and ligated into similarly digested pKS pBluescript I (Stratagene) to produce pKS-VPg3′. Plasmid pETPro/24 was also amplified with a second set of primers, and the amplified fragment was digested with BamHI and EcoRI and ligated in similarly digested pKS-VPg3′. This plasmid was digested with BamHI and XhoI, and the VPg-containing fragment was ligated into BamHI- and XhoI-digested pEG202.
TABLE 1.
List of oligonucleotides used in this study for plasmid construction and site-directed mutagenesis
| Plasmid construct | 5′ Oligonucleotide sequence (5′→3′) | 3′ Oligonucleotide sequence (5′→3′) |
|---|---|---|
| pEGVPg7–191 | AAAGGCAGGATCCAAAGACAG | AGTTACTCTCGAGGTCCACT |
| pEGVPg94–191 | CCATTCACGGAATTCACCCTTGTA | AGTTACTCTCGAGGTCCACT |
| pEGVPg62–191 | GAACAGGAGGATCCTTAACA | AGTTACTCTCGAGGTCCACT |
| pEGVPg7–63 | AAAGGCAGGATCCAAAGACAG | GATCAAACTCGAGCATGTTA |
| pEGVPgΔ59–93 | AAAGGCAGGATCCAAAGACAGa | CATGTTAATGAATTCCCTGTTCTT |
| CCATTCACGGAATTCACCCTTGTA | AGTTACTCTCGAGGTCCACT | |
| pETeIF4EAt | TAATTTAGGGAATTCGGAGAAACA | GCAAAGATTCTCGAGGTTTCAAGC |
| pEGVPgF59A | CATGAAGCGGAATTCAAGAGGCAA | CTGACTGTTCTCGAGTGGCATTAT |
| AACAGGGCAATCAACATGTAT | ATACATGTTGATTGCCTTCCTGTT | |
| pEGVPgY63A | CATGAAGCGGAATTCAAGAGGCAA | CTGACTGTTCTCGAGTGGCATTAT |
| TTCATCAACATGGCCGGCTTTGAT | ATCAAAGCGGGCCATGTTGATGAA | |
| pEGVPgD77A | CATGAAGCGGAATTCAAGAGGCAA | CTGACTGTTCTCGAGTGGCATTAT |
| CGTTTCGTGGCGCCACTCACAGGA | TGTGAGTGGCGCCACGAAACG | |
| pEGVPgD77E | CATGAAGCGGAATTCAAGAGGCAA | CTGACTGTTCTCGAGTGGCATTAT |
| CGTTTCGTGGAGCCACTCACAGGA | TCCTGTGAGTGGCTCCACGAAACG | |
| pEGVPgD77N | CATGAAGCGGAATTCAAGAGGCAA | CTGACTGTTCTCGAGTGGCATTAT |
| CGTTTCGTGAACCCACTCACAGGA | TCCTGTGAGTGGGTTCACGAAACG |
Oligonucleotides on the same line were used in pairs, and amplified fragments were assembled as described in Materials and Methods.
Recombinant protein expression in E. coli and purification.
Plasmid pETtag(iso)4EAt codes for eIF(iso)4E of A. thaliana and was produced by digestion of plasmid pSW56 with EcoRI and XhoI and ligation of the 0.7-kb insert with similarly restricted pET21a (Novagen). The resulting eIF(iso)4E is fused at its N-terminal end to the 11-amino-acid N-terminal peptide of the T7 gene 10 protein (T7 tag), which is recognized by the anti-T7 tag monoclonal antibody (Novagen). Plasmid pETtag(iso)4ETa codes for eIF(iso)4E of Triticum aestivum (wheat) and was produced by digestion of plasmid pGAG424/eIF(iso)4E (a generous gift from K. S. Browning, University of Texas) with EcoRI and SalI and ligation with EcoRI- and XhoI-restricted pET21a. The resulting protein is fused at its N-terminal end with the T7 tag. Plasmid pETtag4EAt codes for eIF4E of A. thaliana and was produced by amplification of plasmid pET14b/eIF4E (kindly provided by C. Robaglia, Centre d'Énergie Atomique) with the primers listed in Table 1; the amplified fragment was digested with EcoRI and XhoI and ligated with EcoRI- and XhoI-restricted pET21a. The resulting recombinant protein is fused at its N-terminal end with the T7 tag. Plasmids were introduced into E. coli BL21(DE3). Recombinant proteins were purified as described earlier (65).
VPgPro was purified as previously described (37). VPgΔPro was produced as follows. pETPro/24 and pEGVPgΔ59–93 were digested with NcoI and StuI. The 5.5- and 0.4-kb fragments from pETPro/24 and pEGVPgΔ59–93, respectively, were purified and ligated. The ligation product was introduced into E. coli XL1-Blue and ultimately into BL21(DE3). The recombinant protein was expressed and purified as described above for VPgPro.
ELISA-based binding assay.
Purified VPgPro was adsorbed to the wells of an enzyme-linked immunosorbent assay (ELISA) plate (1.0 μg/well) by overnight incubation at 4°C, and the wells were blocked with 5% Blotto in phosphate-buffered saline (PBS). Purified initiation factor was diluted in 1% Blotto in PBS with 0.2% Tween and was incubated for 1 h at 4°C with the previously coated wells. Detection of bound initiation factor was achieved as in the ELISA assays with the anti-T7 tag antibody and peroxidase-labeled goat anti-mouse immunoglobulin G (KPL). Wells were washed three times with 0.05% Tween between incubations.
Site-directed mutagenesis.
PCR site-directed mutagenesis by the overlap extension method was done as described previously (24). Primers used for mutagenesis are listed in Table 1, and plasmid p35Tunos (53) was used as a template. Amplification was performed with the Pwo DNA polymerase (Roche).
Particle bombardment.
Plasmid p35TuD77N was constructed by digesting p35Tunos (53) with ClaI and ligating the 3.8-kb fragment with similarly digested pKS pBluescript I (Stratagene), resulting in the recombinant plasmid pKS-Tunos/Cla. Plasmid pEGVPgD77N was digested with PmlI and SpeI, and the corresponding fragment was inserted into pKS-Tunos/Cla linearized with SpeI and partially digested with PmlI. This last construction was digested with ClaI, and the fragment was ligated back into p35Tunos. Proper assembly was verified by nucleic acid sequencing. Particle bombardment was done in the Biolistic PDS-1000/He instrument (Bio-Rad). Then, 7 μg of DNA was mixed with 3 mg of gold particles in 2.5 M CaCl2 and 0.1 M spermidine. This mixture was diluted 1:5 in ethanol, and 5 μl was placed in the center of a 900-lb/in2 rupture disk. Brassica perviridis plants at the two-leaf stage were used.
RESULTS
Interaction of VPg with eIF4E of A. thaliana and eIF(iso)4E of T. aestivum.
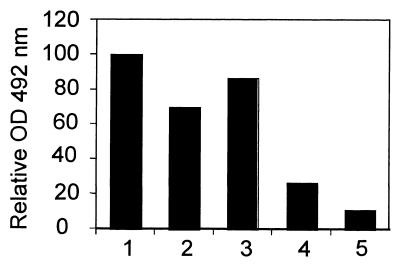
Plants have two isomers of the cap-binding initiation factor, namely, eIF(iso)4E and eIF4E (7, 8). A third factor was recently identified (51), but its involvement in translation initiation relative to the eIF4E isomers is unclear. Since both isomers of eIF4E participate in translation initiation, it was speculated that VPg would bind to both forms and to eIF(iso)4E from a monocotyledenous species such as T. aestivum (wheat). A. thaliana is a dicotyledenous plant and is infected by TuMV, whereas wheat is not a host of the virus. Interactions between the viral protein and these initiation factors were investigated using an ELISA-based binding assay. The initiation factors were produced in E. coli as recombinant proteins fused at their N-terminal end to the 11-amino-acid N-terminal peptide of the T7 gene 10 protein (T7 tag), which is recognized by an anti-T7 tag monoclonal antibody. The proteins were purified by m7GTP-Sepharose chromatography. ELISA plate wells were coated with 1.0 μg of recombinant VPgPro (see protein purity in Fig. 2A, lane 1) and incubated with 2.0 μg of the different initiation factors. VPgPro, a precursor form of VPg, was used because it is purified more easily than VPg in E. coli and because it had been shown that the Pro domain does not participate in eIF(iso)4E binding (65). Complex formation was detected using anti-T7 tag antibodies. Figure 1 shows that VPgPro interacted most effectively with eIF(iso)4E of A. thaliana, and the level of that interaction was given as a relative value of 100 (lane 1). The interaction was specific for the viral protein since the initiation factor was not retained when wells were not coated with VPgPro (lane 5), nor was it retained with an E. coli lysate not containing VPgPro (65). Figure 1 also shows that eIF4E from A. thaliana (lane 2) and eIF(iso)4E from wheat (lane 3) interacted with VPgPro. Once the OD values were corrected for background noise (i.e., the OD value obtained in the absence of initiation factors [lane 4]), the binding values of VPgPro to eIF4E from A. thaliana and eIF(iso)4E from wheat were 60 and 80%, respectively, of the binding to eIF(iso)4E from A. thaliana. This experiment indicated that the VPg of TuMV interacted with several initiation factor species, with similar binding affinities.
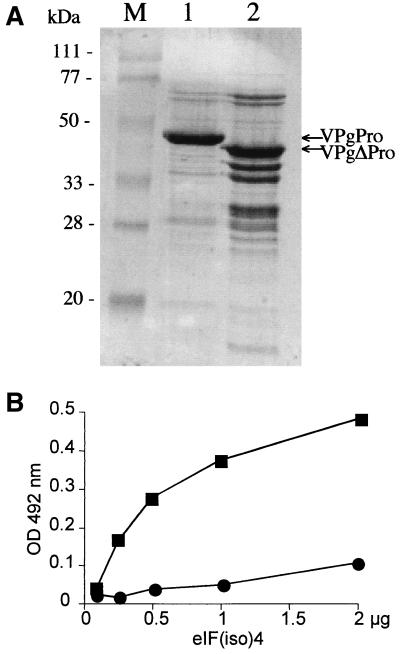
FIG. 2.
VPgPro and VPgΔPro interaction with eIF(iso)4E of A. thaliana as demonstrated by ELISA-based binding assay. (A) Purification of VPgPro and VPgΔPro. Expression and purification were as described in Materials and Methods. Samples were loaded on a sodium dodecyl sulfate-polyacrylamide gel and stained with Coomassie blue. Lane 1, VPgPro (5 μg); lane 2, VPgΔPro (20 μg); lane M, molecular mass standards. (B) ELISA-based binding assay. Wells were coated with 1.0 μg of VPgPro (■) or 4.0 μg of VPgΔPro (●) and then incubated with increasing concentrations of eIF(iso)4E from A. thaliana. Complexes were detected using anti-T7 tag antibodies. Values are averages of two replicates from a typical experiment.
FIG. 1.
VPg interaction with eIF4E isomers as demonstrated by ELISA-based binding assay. Wells precoated with 1.0 μg of VPgPro were incubated with 2.0 μg of eIF(iso)4E (lane 1) and eIF4E (lane 2) from A. thaliana, eIF(iso)4E from T. aestivum (lane 3), or no added initiation factor (lane 4). In lane 5, wells were coated with Blotto only and incubated with 2.0 μg of eIF(iso)4E from A. thaliana. Complexes were detected using anti-T7 tag antibodies. Values are averages of two replicates from a typical experiment.
Mapping of the VPg interaction domain.
Since VPg interacted with the different isomers of the initiation factor and since the interaction is likely to be important for all potyviruses, it was hypothesized that the VPg domain responsible for the eIF(iso)4E interaction would be conserved among different potyviral VPgs. The VPg domain involved in the interaction with eIF(iso)4E was mapped using the yeast two-hybrid system. Deletions in the VPg gene were made by PCR and were fused to the gene coding for the DNA-binding domain of LexA in pEG202. These recombinant plasmids were introduced into the yeast EGY48 strain, which contained either pJG4-5 (carrying the activation domain without insert) or pSW56 which codes for eIF(iso)4E of A. thaliana fused to the activation domain of pJG4-5. The lacZ reporter plasmid pSH18-34 was also present in the yeast cells. Interaction between the different deleted VPg domains and eIF(iso)4E was measured by β-galactosidase assay. The near-full-length VPg comprising amino acids 7 to 191 (VPg7–191) strongly interacted with eIF(iso)4E, providing on average 659 U of β-galactosidase activity (Table 2). No activity was measured when the initiation factor was omitted. VPg fragments comprising amino acids 7 to 63 (VPg7–63) or amino acids 94 to 191 (VPg94–191) failed to interact with the initiation factor. However, the VPg fragment comprising amino acids 62 to 191 (VPg62–191) strongly interacted with eIF(iso)4E. This suggests that the region comprising amino acids 62 to 93 was involved in the interaction. This was confirmed by the deletion of amino acids 59 to 93 from VPg; this deletion mutant (VPgΔ59–93) exhibited extremely low levels of interaction (17 U of β-galactosidase).
TABLE 2.
β-Galactosidase activity displayed by various VPg deletions in yeast expressing eIF(iso)4E from A. thaliana fused to the B42 activation domain
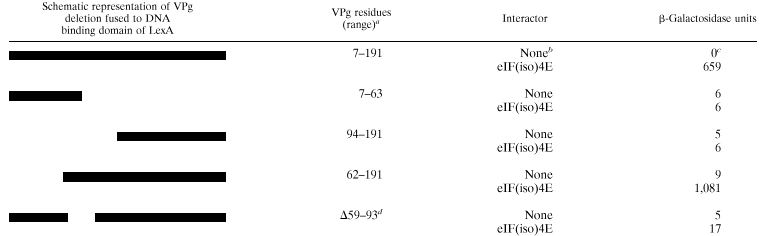 |
Numbers represent first and last residues of VPg fused to DNA binding domain of LexA.
Yeast containing pJG4-5.
Average value of two replicates from a typical experiment.
Symbol and numbers represent deleted residues on VPg7–191.
The lack of interaction with eIF(iso)4E by VPgΔ59–93 could, however, have been the result of degradation of the fusion protein or lack of nuclear transport in the yeast. To test that this was not the case, in vitro binding assays with purified proteins were performed. The deletion mutant gene was subcloned in the plasmid pET21a and expressed as a Pro fusion (VPgΔPro) in E. coli. The protein was purified using the same procedure as for VPgPro. While VPgPro was purified as a 49-kDa species (Fig. 2A, lane 1), multiple forms of VPgΔPro, with a main band at 46 kDa, were observed (lane 2). This degradation of VPgΔPro suggests that deletion of the amino acids caused the protein to be more susceptible to degradation than the complete VPgPro in E. coli. Once purified, VPgΔPro was not susceptible to further degradation. Conditions for the binding assay were adjusted so that similar concentrations of VPgPro and nondegraded VPgΔPro were used. ELISA plate wells were coated with either 1.0 μg of VPgPro or 4.0 μg of VPgΔPro and then incubated with increasing concentrations of eIF(iso)4E. Compared with wild-type VPgPro, VPgΔPro bound approximately fivefold less initiation factor (Fig. 2B). This experiment suggests that amino acids 59 to 93 of VPg are largely responsible for the binding of eIF(iso)4E.
The 35 amino acids identified above are listed in Fig. 3 and were compared with the corresponding region of eight potyviruses. The comparison indicates that the region is highly conserved among the different potyviruses: of the 35 amino acids, 8 residues are identical for all listed viruses, 13 are identical for most of the listed viruses, and 7 residues belong to the same class.
FIG. 3.
Amino acid sequence of the eIF(iso)4E-binding domain of VPg and comparison with corresponding region from other potyviruses. Amino acid sequences were aligned using BLAST software with the BLOSUM62 matrix provided on the NCBI World Wide Web server. The numbers for TuMV represent the first and last residue positions of VPg; for the other viruses, the numbers represent the first and last residue positions of the polyprotein. Dashes indicate amino acids identical to that of the TuMV VPg. PPV, plum pox potyvirus (accession number S47508); LMV, lettuce mosaic potyvirus (P89876); TVMV, tobacco vein mottling potyvirus (P09814); PVY, potato mosaic potyvirus (1906388); TEV, tobacco etch potyvirus (P04517); BCMV, bean common mosaic potyvirus (Q65399); PRSV, papaya ringspot potyvirus (Q01901); ZYMV, zucchini yellow mosaic potyvirus (Q89330).
Site-directed mutagenesis of the phenylalanine at position 59, the tyrosine at position 63, and the aspartic acid at position 77 of the VPg was undertaken to determine their importance for eIF(iso)4E binding. Phe59 and Asp77 are conserved in all listed potyviruses and are adjacent to other highly conserved residues; Tyr63 is the residue which is covalently linked to the viral RNA (39, 40, 47). The VPg from an infectious TuMV cDNA clone (p35Tunos) derived from the UK1 strain (53) was used for these mutagenesis experiments since introduced mutations could be transferred back into infectious cDNA plasmids without introducing changes elsewhere in the viral genome. The VPg sequence of the Quebec and UK1 strains differ at several nucleic acid positions (mainly at position 3 of the codons) but differ by only four amino acid residues clustered in the middle of the protein. However, these residues are outside of the eIF(iso)4E binding region mapped above. The affinity of VPg from both strains for eIF(iso)4E of A. thaliana was similar, as determined with the yeast two-hybrid system (data not shown).
PCR site-directed mutagenesis by overlap extension was used to introduce substitutions, and the interaction of the VPg mutants with eIF(iso)4E was measured using the yeast two-hybrid system. Here, a portion of Pro was introduced along with VPg in pEG202 for subsequent subcloning into p35Tunos. Mutants VPgF59A and VPgY63A, which introduced alanine residues at positions 59 and 63, respectively, produced β-galactosidase activity levels similar to that of the wild-type VPg, indicating that their modification did not affect VPg interaction with the initiation factor (Table 3). Mutants VPgD77A, VPgD77E, and VPgD77N, which introduced either an alanine, a glutamic acid, or an asparagine, respectively, at position 77, failed, however, to interact with the translation factor. The importance of the aspartic acid in the interaction is stressed by the fact that replacement with related amino acids such as glutamic acid and asparagine abolished binding.
TABLE 3.
β-Galactosidase activity displayed by mutants of VPg in yeast expressing eIF(iso)4E from A. thaliana fused to the activation domain B42
| VPg | Interactor | β-Galactosidase units |
|---|---|---|
| Wild type | Nonea | 0b |
| eIF(iso)4E | 178 | |
| F59Ac | None | 0 |
| eIF(iso)4E | 125 | |
| Y63A | None | 0 |
| eIF(iso)4E | 198 | |
| D77A | None | 0 |
| eIF(iso)4E | 0 | |
| D77E | None | 0 |
| eIF(iso)4E | 0 | |
| D77N | None | 0 |
| eIF(iso)4E | 0 |
Yeast containing pJG4-5.
Average value of three replicates from a typical experiment.
First and second letters represent original and modified residues, respectively; the number is the residue position on VPg.
Effect of m7GTP on the formation of VPg-eIF(iso)4E complex.
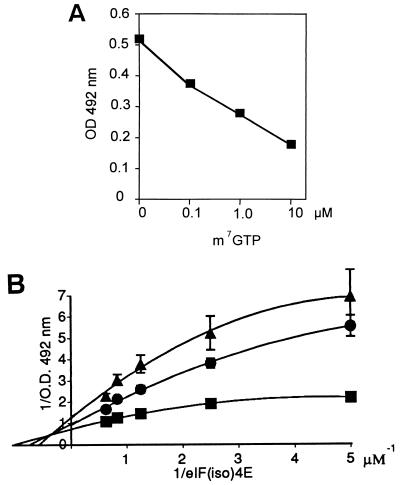
eIF(iso)4E's role in the cell is to initiate assembly of the translation apparatus by binding to the 5′ m7G residue of the mRNAs. In order to test whether the VPg and mRNAs would compete for eIF(iso)4E interaction, the influence of the cap analogue m7GTP on the formation of the VPg-eIF(iso)4E complex was tested. ELISA plate wells were coated with 1.0 μg of recombinant VPgPro and incubated with 2.0 μg of eIF(iso)4E and various concentrations of m7GTP. Complex formation was detected with anti-T7 tag antibodies. Figure 4A shows that increasing concentrations of the analogue progressively prevented the formation of the VPg-eIF(iso)4E complex. These concentrations appear to be physiologically relevant since m7GTP at 4 μM greatly inhibited in vitro translation of RNAs in rabbit reticulocyte lysates (9). The cap analogue m7GTP used at a concentration of 10 μM inhibited complex formation by 60%, while GTP used at the same concentration had no effect on the formation of the complex. To determine what type of ligand relationship (i.e., competitive or noncompetitive) existed between VPg and m7GTP, ELISA plate wells were coated with 1.0 μg of recombinant VPgPro and incubated with increasing concentrations of eIF(iso)4E in the absence or in the presence of 0.5 and 1.0 μM m7GTP. Binding data were treated as enzyme kinetic data and were represented as a Lineweaver-Burk plot [i.e., 1/OD492 versus 1/eIF(iso)4E] (Fig. 4B). The experimental points were not expected to fall on a straight line since VPg and eIF(iso)4E are in the same concentration range, while in enzyme kinetics the substrate concentrations are much higher than the enzyme concentrations. Curves were fitted across the experimental points using least-square analysis, assuming a binomial equation of the following type: y = ax − bx2 + c. The three lines crossed at a single point left of the y axis. Such a pattern is indicative of mixed-type noncompetitive ligand binding, meaning that VPg and m7GTP can simultaneously bind eIF(iso)4E, but the binding of one ligand decreases the binding affinity for the second ligand (56). This binding relationship is depicted in Fig. 5, where K1 and K2 are the dissociation constants for the respective complexes, and “a” is the factor by which the constants increase when the other ligand is already bound. Data of the type shown in Fig. 4B may be used to extract the dissociation constants (Kd) for the VPg-eIF(iso)4E and the m7GTP-eIF(iso)4E complexes (56). When 1/[eIF(iso)4E] approaches zero (i.e., [eIF(iso)4E] > [VPgPro]), the bx2 term becomes negligible and the equation is now y = ax + c and has the same form as the Lineweaver-Burk equation, 1/v = Kapp/(Vmax[S]) + 1/Vmax. Using the values estimated for the constants a and c for each curve, the calculated Kd for the VPg-eIF(iso)4E complex is 0.9 μM, and the Kd for m7GTP is 0.4 μM. The dissociation constant for m7GTP measured here is slightly lower when compared with the Kd of 2 to 9 μM previously obtained for the dissociation of m7GTP with wheat eIF(iso)4E (57, 63) and can be explained by the different experimental procedures used to determine the constant value. Furthermore, the factor by which the Kd of one ligand increases when the other ligand occupies its binding site is estimated to be 4.3.
FIG. 4.
Inhibition by m7GTP of VPg-eIF(iso)4E complex formation as determined by ELISA-based binding assay. (A) Wells were coated with 1.0 μg of VPgPro and incubated with 2.0 μg of eIF(iso)4E from A. thaliana with increasing concentration of m7GTP. Values are averages of two replicates from a typical experiment. (B) Lineweaver-Burk reciprocal representation of binding data. Wells were coated with 1 μg of VPgPro and incubated with increasing concentrations of eIF(iso)4E from A. thaliana in the absence (■) or presence, at 0.5 μM (●) or 1.0 μM (▴), of m7GTP. Values are averages of two replicates from a typical experiment. Solid lines present the best fit of the data to equation y = ax − bx2 + c.
FIG. 5.
Binding of VPg and m7GTP to eIF(iso)4E.
Infection of whole plants.
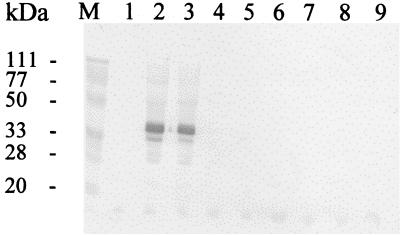
To determine if there is a correlation between the lack of in vitro interaction between VPg and eIF(iso)4E and debilitation of viral production, B. perviridis plants were infected with p35Tunos and p35TuD77N by particle bombardment. p35Tunos is an infectious cDNA clone of TuMV (53), and p35TuD77N is a p35Tunos derivative which contained the D77N mutation in the VPg domain that abolished the interaction with eIF(iso)4E. After bombardment, the plants were kept under an 18-h light regime at 22°C. After 8 days, plants bombarded with the wild-type infectious plasmid showed initial vein clearing followed by systemic mosaic symptoms. After 20 days, 14 of the 15 plants thus bombarded showed full symptoms of TuMV infection. On the other hand, plants bombarded with p35TuD77N remained symptomless. The presence or absence of viral proteins was confirmed by immunoblot analysis using a rabbit anti-TuMV capsid serum. No immunoreactive signal was found in mock-bombarded plants (Fig. 6, lane 1), while a strong signal of the expected molecular weight for the capsid protein was observed in plants bombarded with p35Tunos (lanes 2 and 3). No immunoreactive species were found in those plants bombarded with p35TuD77N (lanes 4 to 9).
FIG. 6.
Immunoblot analysis of B. perviridis plants bombarded with TuMV plasmid cDNA. After bombardment, plants were placed in a growth chamber for 10 days. Proteins were extracted from the new leaf emerging above the one bombarded, separated on a sodium dodecyl sulfate-polyacrylamide gel, transferred on a nitrocellulose membrane, and incubated with a rabbit anti-TuMV capsid serum. Lane 1, plant bombarded with gold particles not coated with DNA; lanes 2 and 3, plants bombarded with p35Tunos; lanes 4 to 9, plants bombarded with p35TuD77N; lane M, molecular mass standards.
DISCUSSION
Viruses use the cellular machinery for their replication, and this implies that viral proteins interact with proteins from the host. In this study, experiments were undertaken to investigate the biological importance of the interaction between the VPg of TuMV and eIF(iso)4E of A. thaliana. In A. thaliana, eIF4E and eIF(iso)4E share 70% identity in their amino acid sequence, and the identity between eIF(iso)4E from A. thaliana and from wheat is equally high at 70% (48). This high sequence homology is found in other plant species as well (6). The two factors are mechanistically equivalent for the translation process but exhibit differences in their ability to bind m7GTP and other cap analogues (8), as well as in their expression in different organs (48). Because of this homology in sequence and function, VPg binding to eIF4E and eIF(iso)4E from A. thaliana as well as to eIF(iso)4E from wheat was expected and indicated that it can take place in many cell types and plant species, both monocotyledenous and dicotyledenous. In addition, the identification of the VPg domain interacting with eIF(iso)4E in a conserved region among potyviruses suggests that this interaction exists with other potyviruses as well. Preliminary experiments with the VPg of tobacco vein mottling potyvirus and plum pox potyvirus showed that indeed they can interact with eIF(iso)4E of A. thaliana (M. G. Fortin et al., unpublished results). Interaction with various initiation factor isomers and the identification of the binding domain in a highly conserved region of the VPg are indications that the interaction plays an important role in the viral life cycle. This presumed important role is supported by the fact that a mutation in VPg which abolished the interaction with the translation factor in vitro debilitated viral infection in whole plants.
The ELISA-based binding experiments indicated that the initiation factor can simultaneously make a complex with VPg and m7GTP. Ligand binding showed negative cooperativity (i.e., one ligand decreases the affinity of the initiation factor for the other ligand). Lower ligand affinity can result from the binding of the first ligand physically hindering the binding of the second ligand. It can also be the consequence of eIF(iso)4E undergoing a conformational change, which is known to take place when eIF(iso)4E binds m7GTP (57). This binding cooperativity seems to be a feature of eIF4E to regulate its activity. For instance, binding of mammalian eIF4G to eIF4E increased the affinity of the latter for the cap analogue (22), and the wheat germ poly(A) binding protein enhanced the binding affinity of eIF4E isomers for the cap analogue (63). A consequence of negative binding cooperativity would be that the interaction of VPg with eIF(iso)4E can lower the affinity of the initiation factor for the cap structure of mRNAs in planta, which may lead to a decrease in host protein synthesis.
Interaction of plant viruses with the host translation machinery and its consequence on protein synthesis has not been intensively investigated (4). Recently, inhibition of host gene expression has been associated with potyvirus replication. By examining the front of virus invasion in immature pea embryos infected with pea seed-borne mosaic potyvirus, decreased levels of host transcripts were observed (61), but not for all transcripts (5). Although no experimental explanation was provided, reduced transcript levels can result from an inhibition of transcription and/or from hydrolysis of mRNAs. However, transcript hydrolysis may be the consequence of inhibition of translation since there is a relationship between translatability and mRNA stability (1); it has been proposed that factors that stimulate translation initiation minimize the rate of entry of mRNA into decay pathways (26). For instance, a cis-acting mRNA stability determinant is the m7Gppp cap. If less eIF(iso)4E is available for cap binding, the cap structure of mRNAs may become more susceptible to hydrolysis by decapping enzyme(s) (29), which then leads to degradation by 5′→3′ exonuclease(s) (1, 26). It remains to be seen if the interaction between VPg and eIF4E has any part to play in the observed inhibition of host gene expression during potyvirus infection.
This study showed that the ability of VPg to make a complex with eIF(iso)4E in vitro correlated with viral infection in planta. We are now attempting to elucidate the precise role of the VPg-eIF(iso)4E interaction in virus replication, i.e., whether VPg, when linked to viral RNA, can still bind the initiation factor and provide for the viral RNA a competitive edge over cellular mRNAs in translation initiation.
ACKNOWLEDGMENTS
We are grateful to Christophe Robaglia and Karen S. Browning and particularly to Fernando Ponz for plasmids pET14-4E, pGAG424-eIF(iso)4E, and p35Tunos, respectively. We thank Armand Séguin and Denis Lachance for their help in particle bombardment. We thank Nahum Sonenberg for critically reading the manuscript.
This work was supported by the Natural Sciences and Engineering Research Council of Canada and Le Fonds pour la Formation des Chercheurs et l'Aide à la Recherche du Québec.
REFERENCES
- 1.Abler M L, Green P J. Control of mRNA stability in higher plants. Plant Mol Biol. 1996;32:63–78. doi: 10.1007/BF00039377. [DOI] [PubMed] [Google Scholar]
- 2.Altmann M, Trachsel H. Altered cap recognition activity of initiation factor 4E in the yeast cell cycle division mutant cdc33. Nucleic Acids Res. 1989;17:5923–5931. doi: 10.1093/nar/17.15.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altmann M, Schmitz N, Berset C, Trachsel H. A novel inhibitor of cap-dependent translation initiation in yeast: p20 competes with eIF4G for binding to eIF4E. EMBO J. 1997;16:1114–1121. doi: 10.1093/emboj/16.5.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aranda M, Maule A. Virus-induced host gene shutoff in animals and plants. Virology. 1998;243:261–267. doi: 10.1006/viro.1998.9032. [DOI] [PubMed] [Google Scholar]
- 5.Aranda M A, Escaler M, Wang D, Maule A J. Induction of HSP70 and polyubiquitin expression associated with plant virus replication. Proc Natl Acad Sci USA. 1996;93:15289–15293. doi: 10.1073/pnas.93.26.15289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browning K S. The plant translational apparatus. Plant Mol Biol. 1996;32:107–144. doi: 10.1007/BF00039380. [DOI] [PubMed] [Google Scholar]
- 7.Browning K S, Lax S R, Ravel J M. Identification of two messenger RNA cap binding proteins in wheat germ. Evidence that the 28-kDa subunit of eIF-4B and the 26-kDa subunit of eIF-4F are antigenically distinct polypeptides. J Biol Chem. 1987;262:11228–11232. [PubMed] [Google Scholar]
- 8.Browning K S, Webster C, Roberts J K M, Ravel J M. Identification of an isozyme form of protein synthesis initiation factor 4F in plants. J Biol Chem. 1992;267:10096–10100. [PubMed] [Google Scholar]
- 9.Carrington J C, Freed D D. Cap-independent enhancement of translation by a plant potyvirus 5′ nontranslated region. J Virol. 1990;64:1590–1597. doi: 10.1128/jvi.64.4.1590-1597.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrington J C, Freed D D, Leinicke A J. Bipartite signal sequence mediates nuclear translocation of the plant potyviral NIa protein. Plant Cell. 1991;3:953–962. doi: 10.1105/tpc.3.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Benedetti A, Rhoads R E. Overexpression of eukaryotic protein synthesis initiation factor 4E in HeLa cells results in aberrant growth and morphology. Proc Natl Acad Sci USA. 1990;87:8212–8216. doi: 10.1073/pnas.87.21.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Benedetti A, Joshi-Barve S, Rinker-Schaeffer C, Rhoads R E. Expression of antisense RNA against initiation factor eIF-4E mRNA in HeLa cells results in lengthened cell division times, diminished translation rates, and reduced levels of both eIF-4E and the p220 component of eIF-4F. Mol Cell Biol. 1991;11:5435–5445. doi: 10.1128/mcb.11.11.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duncan R, Milburn S C, Hershey J W B. Regulated phosphorylation and low abundance of HeLa cell initiation factor eIF-4F suggest a role in translational control. Heat shock effects on eIF-4F. J Biol Chem. 1987;262:380–388. [PubMed] [Google Scholar]
- 14.Feigenblum D, Schneider R J. Modification of eukaryotic initiation factor 4F during infection by influenza virus. J Virol. 1993;67:3027–3035. doi: 10.1128/jvi.67.6.3027-3035.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fellers J, Wan J, Hong Y, Collins G B, Hunt A G. In vitro interactions between a potyvirus-encoded, genome-linked protein and RNA-dependent RNA polymerase. J Gen Virol. 1998;79:2043–2049. doi: 10.1099/0022-1317-79-8-2043. [DOI] [PubMed] [Google Scholar]
- 16.Flynn A, Proud C G. The role of eIF4 in cell proliferation. Cancer Surv. 1996;27:293–310. [PubMed] [Google Scholar]
- 17.Gallie D R. Translational control of cellular and viral mRNAs. Plant Mol Biol. 1996;32:145–158. doi: 10.1007/BF00039381. [DOI] [PubMed] [Google Scholar]
- 18.Gingras A-C, Svitkin Y, Belsham G J, Pause A, Sonenberg N. Activation of the translational suppressor 4E-BP1 following infection with encephalomyocarditis virus and poliovirus. Proc Natl Acad Sci USA. 1996;93:5578–5583. doi: 10.1073/pnas.93.11.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golemis E A, Gyuris H, Brent R. Interaction trap/two-hybrid system to identify interacting proteins. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seldman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1996. pp. 20.1.1–20.1.28. [Google Scholar]
- 20.Haghighat A, Sonenberg N. eIF4G dramatically enhances the binding of eIF4E to the mRNA 5′-cap structure. J Biol Chem. 1997;272:21677–21680. doi: 10.1074/jbc.272.35.21677. [DOI] [PubMed] [Google Scholar]
- 21.Haghighat A, Mader S, Pause A, Sonenberg N. Repression of cap-dependent translation by 4E-binding protein 1: competition with p220 for binding to eukaryotic initiation factor-4E. EMBO J. 1995;14:5701–5709. doi: 10.1002/j.1460-2075.1995.tb00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haghighat A, Svitkin Y, Novoa I, Kuechler E, Skern T, Sonenberg N. The eIF4G-eIF4E complex is the target for direct cleavage by the rhinovirus 2A proteinase. J Virol. 1996;70:8444–8450. doi: 10.1128/jvi.70.12.8444-8450.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajimorad M R, Ding X S, Flasinski S, Mahajan S, Graff E, Haldman-Cahill R, Carrington J C, Cassidy B G. NIa and NIb of peanut stripe potyvirus are present in the nucleus of infected cells, but do not form inclusions. Virology. 1996;224:368–379. doi: 10.1006/viro.1996.0544. [DOI] [PubMed] [Google Scholar]
- 24.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 25.Hong Y, Levay K, Murphy J F, Klein P G, Shaw J G, Hunt A G. A potyvirus polymerase interacts with the viral coat protein and VPg in yeast cells. Virology. 1995;214:159–166. doi: 10.1006/viro.1995.9944. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson A, Peltz S W. Interrelationships of the pathways of mRNA decay and translation in eukaryotic cells. Annu Rev Biochem. 1996;65:693–739. doi: 10.1146/annurev.bi.65.070196.003401. [DOI] [PubMed] [Google Scholar]
- 27.Keller K E, Johansen I E, Martin R R, Hampton R O. Potyvirus genome-linked protein (VPg) determines pea seed-borne mosaic virus pathotype virulence in Pisum sativum. Mol Plant-Microbe Interact. 1998;11:124–130. doi: 10.1094/MPMI.1998.11.2.124. [DOI] [PubMed] [Google Scholar]
- 28.Kerekatte V, Smiley K, Hu B, Smith A, Gelder F, De Benedetti A. The proto-oncogene/translation factor eIF4E: a survey of its expression in breast carcinomas. Int J Cancer. 1995;64:27–31. doi: 10.1002/ijc.2910640107. [DOI] [PubMed] [Google Scholar]
- 29.LaGrandeur T E, Parker R. Isolation and characterization of Dcp1p, the yeast mRNA decapping enzyme. EMBO J. 1998;17:1487–1496. doi: 10.1093/emboj/17.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laliberté J-F, Nicolas O, Chatel H, Lazure C, Morosoli R. Release of a 22-kDa protein derived from the amino-terminal domain of the 49-kDa NIa of turnip mosaic potyvirus in Escherichia coli. Virology. 1992;190:510–514. doi: 10.1016/0042-6822(92)91244-o. [DOI] [PubMed] [Google Scholar]
- 31.Lawrence J C, Abraham R T. PHAS/4E-BPs as regulators of mRNA translation and cell proliferation. Trends Biochem Sci. 1997;22:345–349. doi: 10.1016/s0968-0004(97)01101-8. [DOI] [PubMed] [Google Scholar]
- 32.Lazaris-Karatzas A, Montine K S, Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5′ cap. Nature. 1990;345:544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]
- 33.Li X H, Valdez P, Olvera R E, Carrington J C. Function of the tobacco etch virus RNA polymerase (NIb): subcellular transport and protein-protein interaction with VPg/proteinase (NIa) J Virol. 1997;71:1598–1607. doi: 10.1128/jvi.71.2.1598-1607.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mader S, Lee H, Pause A, Sonenberg N. The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-4 gamma and the translational repressors 4E-binding proteins. Mol Cell Biol. 1995;15:4990–4997. doi: 10.1128/mcb.15.9.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masuta C, Nishimura M, Morishita H, Hataya T. A single amino acid change in viral genome-associated protein of potato virus Y correlates with resistance breaking in “virgin A mutant” tobacco. Phytopathology. 1999;89:118–123. doi: 10.1094/PHYTO.1999.89.2.118. [DOI] [PubMed] [Google Scholar]
- 36.McKendrick L, Pain V P, Morley S J. Translation initiation factor 4E. Int J Biochem Cell Biol. 1999;31:31–35. doi: 10.1016/s1357-2725(98)00129-0. [DOI] [PubMed] [Google Scholar]
- 37.Ménard R, Chatel H, Dupras R, Plouffe C, Laliberté J-F. Purification of turnip mosaic potyvirus viral protein genome-linked proteinase expressed in Escherichia coli and development of a quantitative assay for proteolytic activity. Eur J Biochem. 1995;229:107–112. doi: 10.1111/j.1432-1033.1995.tb20444.x. [DOI] [PubMed] [Google Scholar]
- 38.Merrick W C. Mechanisms and regulation of eukaryotic protein synthesis. Microbiol Rev. 1992;56:291–315. doi: 10.1128/mr.56.2.291-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy J F, Klein P G, Hunt A G, Shaw J G. Replacement of the tyrosine residue that links a potyviral VPg to the viral RNA is lethal. Virology. 1996;220:535–538. doi: 10.1006/viro.1996.0344. [DOI] [PubMed] [Google Scholar]
- 40.Murphy J F, Rychlik W, Rhoads R E, Hunt A G, Shaw J G. A tyrosine residue in the small nuclear inclusion protein of tobacco vein mottling virus links the VPg to the viral RNA. J Virol. 1991;65:511–513. doi: 10.1128/jvi.65.1.511-513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicolas O, Dunnington S W, Gotow L F, Pirone T P, Hellmann G M. Variations in the VPg protein allow a potyvirus to overcome va gene resistance in tobacco. Virology. 1997;237:452–459. doi: 10.1006/viro.1997.8780. [DOI] [PubMed] [Google Scholar]
- 42.Nicolas O, Pirone T P, Hellmann G M. Construction and analysis of infectious transcripts from a resistance-breaking strain of tobacco vein mottling potyvirus. Arch Virol. 1996;141:1535–1552. doi: 10.1007/BF01718253. [DOI] [PubMed] [Google Scholar]
- 43.Pain V M. Initiation of protein synthesis in eukaryotic cells. Eur J Biochem. 1996;236:747–771. doi: 10.1111/j.1432-1033.1996.00747.x. [DOI] [PubMed] [Google Scholar]
- 44.Poulin F, Gingras A-C, Olsen H, Chevalier S, Sonenberg N. 4E-BP3, a new member of the eukaryotic initiation factor 4E-binding protein family. J Biol Chem. 1998;273:14002–14007. doi: 10.1074/jbc.273.22.14002. [DOI] [PubMed] [Google Scholar]
- 45.Rau M, Ohlmann T, Morley S J, Pain V M. A reevaluation of the cap-binding protein, eIF4E, as a rate-limiting factor for the initiation of translation in reticulocyte lysate. J Biol Chem. 1996;271:8983–8990. doi: 10.1074/jbc.271.15.8983. [DOI] [PubMed] [Google Scholar]
- 46.Restrepo M A, Freed D D, Carrington J C. Nuclear transport of plant potyviral proteins. Plant Cell. 1990;2:987–998. doi: 10.1105/tpc.2.10.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riechmann J L, Lain S, Garcia J A. Highlights and prospects of potyvirus molecular biology. J Gen Virol. 1992;73:1–16. doi: 10.1099/0022-1317-73-1-1. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez C M, Freire M A, Camilleri C, Robaglia C. The Arabidopsis thaliana cDNAs coding for eIF4E and eIF(iso)4E are not functionally equivalent for yeast complementation and differentially expressed during plant development. Plant J. 1998;13:465–473. doi: 10.1046/j.1365-313x.1998.00047.x. [DOI] [PubMed] [Google Scholar]
- 49.Rosenwald E B, Lazaris-Karatzas A, Sonenberg N, Schmidt E V. Elevated levels of cyclin D1 protein in response to increased expression of eukaryotic initiation factor 4E. Mol Cell Biol. 1993;13:7358–7363. doi: 10.1128/mcb.13.12.7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rousseau D, Kaspar R, Rosenwald I, Gehrke L, Sonenberg N. Translation initiation of ornithine decarboxylase and nucleocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. Proc Natl Acad Sci USA. 1996;93:1065–1070. doi: 10.1073/pnas.93.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruud K A, Kuhlow C, Goss D J, Browning K S. Identification and characterization of a novel cap-binding protein from Arabidopsis thaliana. J Biol Chem. 1998;273:10325–10330. doi: 10.1074/jbc.273.17.10325. [DOI] [PubMed] [Google Scholar]
- 52.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 53.Sanchez F, Martinez-Herrera D, Aguilar I, Ponz F. Infectivity of turnip mosaic potyvirus cDNA clones and transcripts on the systemic host Arabidopsis thaliana and local lesion hosts. Virus Res. 1998;55:207–219. doi: 10.1016/s0168-1702(98)00049-5. [DOI] [PubMed] [Google Scholar]
- 54.Schaad M C, Haldeman-Cahill R, Cronin S, Carrington J C. Analysis of the VPg-proteinase (NIa) encoded by tobacco etch potyvirus: effects of mutations on subcellular transport, proteolytic processing, and genome amplification. J Virol. 1996;70:7039–7048. doi: 10.1128/jvi.70.10.7039-7048.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schaad M C, Lellis A D, Carrington J C. VPg of tobacco etch potyvirus is a host genotype-specific determinant for long-distance movement. J Virol. 1997;71:8624–8631. doi: 10.1128/jvi.71.11.8624-8631.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Segel I H. Enzyme kinetics: behavior and analysis of rapid equilibrium and steady-state enzyme systems. New York, N.Y: John Wiley & Sons, Inc.; 1975. [Google Scholar]
- 57.Sha M, Wang Y, Xiang T, van Heerden A, Browning K S, Goss D J. Interaction of wheat germ protein synthesis initiation factor eIF(iso)4F and its subunits p28 and p86 with m7GTP and mRNA analogues. J Biol Chem. 1995;270:29904–29909. doi: 10.1074/jbc.270.50.29904. [DOI] [PubMed] [Google Scholar]
- 58.Sonenberg N, Gingras A-C. The mRNA 5′ cap-binding protein eIF4E and control of cell growth. Curr Opin Cell Biol. 1998;10:268–275. doi: 10.1016/s0955-0674(98)80150-6. [DOI] [PubMed] [Google Scholar]
- 59.Svitkin Y V, Gradi A, Imataka H, Morino S, Sonenberg N. Eukaryotic initiation factor 4GII (eIF4GII), but not eIF4GI, cleavage correlates with inhibition of host cell protein synthesis after human rhinovirus infection. J Virol. 1999;73:3467–3472. doi: 10.1128/jvi.73.4.3467-3472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thach R E. Cap recap: the involvement of eIF-4F in regulating gene expression. Cell. 1992;68:177–180. doi: 10.1016/0092-8674(92)90461-k. [DOI] [PubMed] [Google Scholar]
- 61.Wang D, Maule A J. Inhibition of host gene expression associated with plant virus replication. Science. 1995;267:229–231. doi: 10.1126/science.267.5195.229. [DOI] [PubMed] [Google Scholar]
- 62.Wang X, Flynn A, Waskiewicz A J, Webb B L J, Vries R G, Baines I A, Cooper J A, Proud C G. The phosphorylation of eukaryotic initiation factor eIF4E in response to phorbol esters, cell stresses, and cytokines is mediated by distinct MAP kinase pathways. J Biol Chem. 1998;273:9373–9377. doi: 10.1074/jbc.273.16.9373. [DOI] [PubMed] [Google Scholar]
- 63.Wei C-C, Balasta M L, Ren J, Goss D J. Wheat germ poly(A) binding protein enhances the binding affinity of eukaryotic initiation factor 4F and (iso)4F for cap analogues. Biochemistry. 1998;37:1910–1916. doi: 10.1021/bi9724570. [DOI] [PubMed] [Google Scholar]
- 64.Whalen S G, Gingras A-C, Amankwa L, Mader S, Branton P E, Aebersold R, Sonenberg N. Phosphorylation of eIF-4E on serine 209 by protein kinase C is inhibited by the translational repressors, 4E-binding proteins. J Biol Chem. 1996;271:11831–11837. doi: 10.1074/jbc.271.20.11831. [DOI] [PubMed] [Google Scholar]
- 65.Wittmann S, Chatel H, Fortin M G, Laliberté J-F. Interaction of the viral protein genome linked of turnip mosaic potyvirus with the translational eukaryotic initiation factor (iso)4E of Arabidopsis thaliana using the yeast two-hybrid system. Virology. 1997;234:84–92. doi: 10.1006/viro.1997.8634. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Feigenblum D, Schneider R J. A late adenovirus factor induces eIF-4E dephosphorylation and inhibition of cell protein synthesis. J Virol. 1994;68:7040–7050. doi: 10.1128/jvi.68.11.7040-7050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]