Abstract
Background:
Vitamin D supplementation is supposed to have an important role in the management of several endometriosis-related aspects, offering potential relief to affected individuals. Herein, the authors aim to evaluate the impact of vitamin D on pregnancy rates and clinical symptoms in women with endometriosis.
Methods:
The authors extensively searched PubMed, Cochrane Library, EMBASE, Ovid MEDLINE, and CINAHL from their inception to 20 July 2023.
Results:
Three randomized controlled trials involving 167 patients were included in this meta-analysis. The findings demonstrated that vitamin D supplementation exhibits efficacy in alleviating dysmenorrhea associated with endometriosis, as evidenced by a meta-analysis showing a significant reduction in dysmenorrhea (mean difference −1.41, 95% CI −2.61 to −0.22, P = 0.02). However, the impact on dyspareunia was inconclusive, with a non-significant mean difference of –0.2 (95% CI −1.62 to 1.22, P = 0.78). In contrast, dyschezia significantly decreased with vitamin D supplementation (mean difference −1.10, 95% CI −2.22 to 0.02, P = 0.05 However, the meta-analysis did not show a significant effect of vitamin D on chronic pelvic pain associated with endometriosis.
Conclusion:
While antioxidant vitamin D supplementation demonstrates general effectiveness in alleviating endometriosis symptoms, such as dysmenorrhea, dyspareunia, and dyschezia, the existing literature lacks direct investigations into the specific impact of vitamin D on enhancing pregnancy rates among endometriosis patients. This observation prompts various hypotheses, suggesting that the positive effects of vitamin D supplementation on endometriosis-related symptoms may indirectly contribute to improved pregnancy outcomes and enhanced fertility.
Keywords: endometriosis, endometriosis-related symptoms, pregnancy rate, vitamin D
Introduction
Highlights
Vitamin D supplementation significantly alleviates endometriosis-related dysmenorrhea, offering potential relief to affected individuals.
While chronic pelvic pain associated with endometriosis does not show improvement with vitamin D supplementation, indicating a nuanced effect on different pain aspects.
Notable improvement in catastrophic thinking is observed with vitamin D supplementation, implying a potentially positive impact on mental well-being.
Urgent need for large randomized trials to conclusively determine the relationship between vitamin D supplementation and improved fertility outcomes in individuals with endometriosis.
Endometriosis is a common estrogen-dependent chronic inflammatory condition, and it is characterized by lesions resembling the endometrium found outside the uterus1–3. It predominantly affects women in their reproductive years, with an estimated prevalence ranging from 5 to 10%2. Common symptoms include painful menstruation (dysmenorrhea), pain during intercourse (dyspareunia), chronic pelvic pain, and difficulties with fertility2,3. The exact cause of endometriosis is still not fully understood, and its diverse subtypes (peritoneal, ovarian, deep infiltrating) contribute to variations in clinical presentations and the intricate underlying mechanisms3–5. Nonetheless, genetics, environmental factors, immunity, and persistent inflammation are believed to play roles in its development1–3. Numerous research studies have documented elevated levels of inflammatory markers such as cytokines, neutrophils, macrophages, and tumor necrosis factor-alpha in peritoneal fluid1,6–8. Differences in the mechanisms underlying inflammation may account for the variations in pain and fertility outcomes observed among the main subtypes of endometriosis1. Vitamin D, among the factors that can influence or modulate inflammation, has been the subject of investigation in numerous studies related to endometriosis1,9. Adequate vitamin D levels are defined as circulating concentrations exceeding 30–40 ng/ml, while insufficiency is noted when concentrations range between 20 and 30 ng/ml, and deficiency is characterized by levels below 20 ng/ml. The question of whether the concentration of vitamin D (25-hydroxyvitamin-D3) is linked to the disease and its severity remains a topic of ongoing discussion, with studies reporting both positive and negative associations10–13.
Endometriosis has a profound impact on the quality of life of affected individuals, significantly affecting their daily activities, sexual function, and personal relationships. Furthermore, this condition is associated with depression, fatigue, and a reduction in work productivity, leading to a substantial economic burden4. Research indicates that endometriosis imposes a yearly cost of ~$78 billion in the United States, encompassing both direct healthcare expenses and indirect costs related to healthcare resource utilization and lost productivity14. Despite its widespread prevalence, there is currently no known cure for endometriosis, and diagnosis is often delayed by a span of 4–11 years from the onset of symptoms, even in developed countries15.
While the precise mechanisms underlying the development of endometriosis remain incompletely understood, Sampson’s theory of retrograde menstruation is widely acknowledged as a leading explanation16. However, what distinguishes women with retrograde menstruation who develop endometriosis from those who do not is still not well-defined. Recent research suggests that immunity and persistent inflammation may play a role in its pathogenesis17.
Examinations of peritoneal fluid have revealed heightened levels of inflammatory cytokines, neutrophils, macrophages, and tumor necrosis factor-alpha8,18. Notably, endometriosis is primarily linked to chronic pelvic pain, which results from the activation of macrophages and mast cells, contributing to a continuous cycle of inflammation, oxidative stress, and pain19.
Oxidative stress, which is marked by an inequity between reactive oxygen species (ROS) and natural biological antioxidants, is considered a pivotal element in the pathophysiology of endometriosis20. Cells possess an antioxidant system designed to counteract the repercussions of ROS and uphold a harmonious equilibrium between the defense mechanisms provided by antioxidants and the generation of ROS, ultimately averting cellular harm attributed to ROS and facilitating the repair process21. Consequently, elevating antioxidant levels may have the potential to mitigate the pathological effects of endometriosis resulting from oxidative damage22.
As a result, the effectiveness of antioxidant therapy in the treatment of endometriosis has garnered increasing attention in recent years. A study conducted by Jennifer and colleagues demonstrated that women with endometriosis experienced enhancements in their peripheral antioxidant markers upon adopting a high-antioxidant diet23. Additional research has suggested that garlic extract may alleviate pelvic and back pain, dysmenorrhea, and dyspareunia in women with endometriosis by reducing oxidative stress, curtailing prostaglandin production, restraining endometrial cell proliferation, and enhancing estrogen elimination24,25. Moreover, a higher consumption of fruits, particularly citrus fruits, has been linked to a reduced risk of developing endometriosis26. Vitamin supplementation vitamins has also displayed promise in diminishing pelvic pain associated with endometriosis and enhancing the body’s response to oxidative stress20,27–29. Growing evidence underscores the significant role of oxidative stress in the development of endometriosis, often aligning disease severity with the levels of oxidative stress markers20,30.
While vitamin D’s primary role is to regulate calcium and maintain skeletal homeostasis, research has unveiled its involvement in modulating the immune system31. The vitamin D metabolic pathway is illustrated in Figure 1 32. The majority of vitamin D’s biological effects are mediated through a high-affinity receptor that functions as a transcription factor. The gene responsible for encoding the vitamin D receptor (VDR) is located on chromosome 1233.
Figure 1.
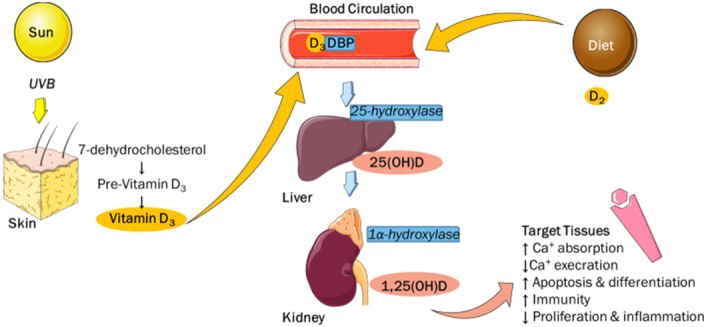
Shows the metabolic pathway of vitamin D and targeted tissues. The images in the figure are adopted from smart.servier.com Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License. DBP, vitamin D binding protein; UVB, ultraviolet B rays.
The presence of VDR protein in healthy and endometriotic endometrium was detected34. While VDR expression is observed in endometriosis, its role as an initiator or consequence of inflammation remains unclear35. Recent research shows VDR expression in peritoneal endometriotic lesions, suggesting it may play a significant role in the condition’s pathophysiology36–38. The authors propose that VDR signaling could be a potential target for innovative therapeutic approaches in endometriosis, including the consideration of vitamin D as an anti-endometriosis agent38.
This systematic review and meta-analysis aim to provide a comprehensive synthesis of existing evidence on the impact of vitamin D treatment on the pregnancy rate among women diagnosed with endometriosis, either through surgical confirmation or based on clinical symptoms. The study also seeks to explore the effects of vitamin D supplementation on infertility and other endometriosis-related symptoms. Shedding the light on its role as an adjunctive therapy for endometriosis in improving fertility and alleviating associated symptoms. The findings from this study may have implications for clinical practice and inform future research directions in the management of endometriosis.
Material and methods
This systematic review adhered to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA-P Statement, Supplemental Digital Content 4, http://links.lww.com/MS9/A477)39 and was duly registered with PROSPERO under ID number of CRD42023444184 (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=444184). This study has been reported following AMSTAR 2 guidelines40, Supplemental Digital Content 3, http://links.lww.com/MS9/A476.
Data sources and search strategy
For conducting this study, a comprehensive search strategy was employed to locate relevant published studies. Electronic databases such as PubMed, Cochrane Library, EMBASE, MEDLINE, and Cumulative Index to Nursing & Allied Health (CINAHL) were searched from inception up to 20 July 2023. Additionally, the reference lists of relevant studies were also meticulously examined to minimize any potential omissions. No limitations regarding the type of publication or language were included in this research. The details of the search strategy can be found in Supplementary Data File S1, Supplemental Digital Content 1, http://links.lww.com/MS9/A474.
Study selection and eligibility criteria
The criteria for inclusion and exclusion were determined using the PICOS (Population/patients, Intervention/exposure, Control/comparison, Outcome, and Study type) approach, which is detailed in Table 1. Studies published solely as abstracts, conference proceedings, books, or editorials will be excluded, as they may lack the depth of data and rigorous peer-review required for this systematic review. In addition, studies focusing on menopausal women with chronic pelvic pain will not be considered, as endometriosis predominantly affects individuals of reproductive age. Case reports will be excluded from this review, as they typically do not provide the comprehensive data necessary for systematic analysis. Studies centered on asymptomatic patients will not be included, as the primary focus of this review is on women diagnosed with endometriosis and its associated symptoms. These exclusion criteria have been defined to ensure that the review encompasses pertinent and scientifically rigorous research aligning with the study’s objectives.
Table 1.
Formulated question of the study based on PICOS
| Inclusion criteria | |
| P (Participants) | Women of reproductive age who have been diagnosed with endometriosis either through surgical confirmation or based on clinical symptoms. |
| I (Intervention) | Vitamin D at any dosage and via any route of administration. |
| C (Control) | No supplementation or placebo |
| O (Outcomes) | Primary outcome: The impact of vitamin D on pregnancy rates and infertility among individuals with endometriosis Secondary outcomes: (I) chronic pelvic pain, (II) mood disorders, (III) urinary problems, (IV) endometriosis regression, (V) secondary dysmenorrhea, (VI) dyspareunia and dyschezia, and (VII) the effectiveness of vitamin D supplementation in recurrent and/or refractory endometriosis. |
| S (Study type) | Experimental studies like RCTs, Observational studies including Cohort studies, Case series with any language |
RCT, randomized controlled trial.
Data extraction, quality assessment, and risk of bias
All the search results have been uploaded into COVIDENCE to facilitate the process of screening. The duplicate studies were removed. Consequently, two independent authors screened the titles and abstracts and then proceeded toward the full-text review, applying the inclusion and exclusion criteria. One author gathered the data, while the other reviewed it, resolving any inconsistencies through discussion and consensus. The extracted data encompassed details such as authors, publication year, study location, sample size, study type, participant age, and specifics of vitamin D supplementation, including dose, duration, and administration route. Methodological quality assessment was conducted by two reviewers using the Cochrane risk of bias assessment tool for rigorous evaluation41. The GRADE analysis of quality has been supplied as a supplementary material in S2 table, Supplemental Digital Content 2, http://links.lww.com/MS9/A475.
Statistical methods
Meta-analysis was conducted using ReVMan software to calculate pooled effect estimates, the mean differences, for continuous data and corresponding 95% CIs. Fixed-effects models were chosen based on the level of heterogeneity observed. Forest plots were generated to visually represent individual study effects.
Results
Study selection
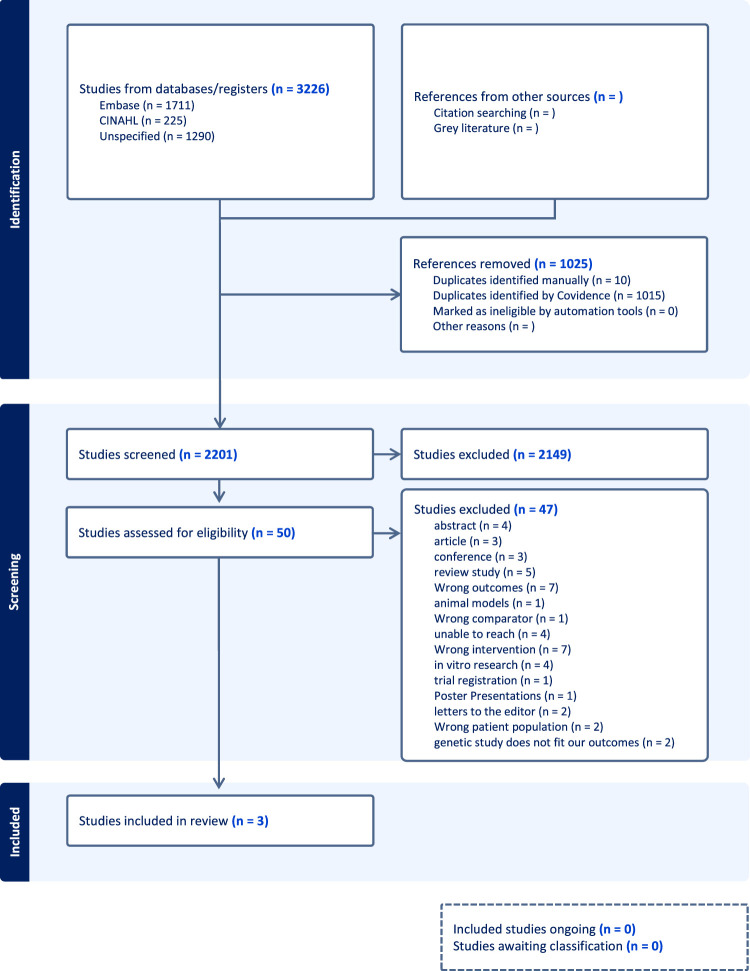
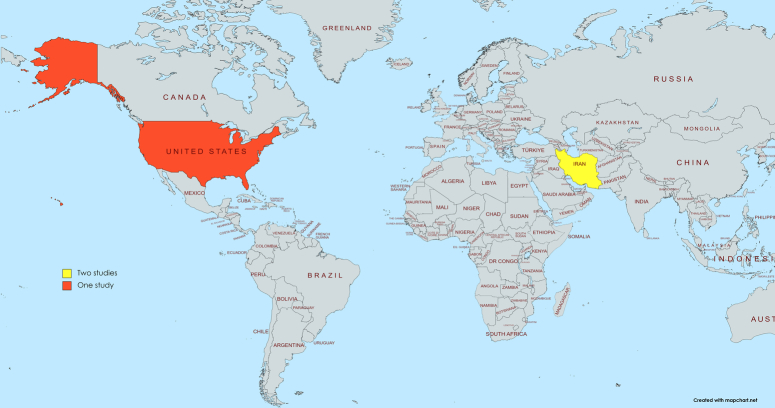
The electronic database search initially produced 3226 articles, out of which 1024 were duplicates. After screening based on titles and abstracts, 2149 articles were excluded, resulting in 3 studies that met the eligibility criteria for inclusion, as illustrated in the PRISMA flowchart shown in Fig. 2. These studies originated from Iran (2) and the USA (1) (Fig. 3).
Figure 2.
PRISMA flowchart. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Figure 3.
Geographic distribution of the countries of the included studies: both of Almassinokiani 2016 and Mehdizadehkashi 2021 in Iran while Nodler 2020 in USA.
Study characteristics
The goal of our systematic review is to synthesize the available evidence from several separate trials regarding the effectiveness of vitamin D in managing endometriosis and increasing pregnancy rates as a primary outcome. Additionally, we assessed the impact of vitamin D on endometriosis-related symptoms or complications, including chronic pelvic pain, mood disorders, urinary problems, endometriosis regression, secondary dysmenorrhea, dyspareunia, and the effectiveness of vitamin D supplementation in recurrent and/or refractory endometriosis, as secondary outcomes.
Three randomized controlled trials involved 167 women with endometriosis at different stages (76 women in the vitamin D groups and 91 women in the placebo groups).Among the included studies, two evaluated the effects of vitamin D supplementation on clinical symptoms associated with endometriosis42,43, and one focused on the physical and mental quality of life among endometriosis patients44. Endometriosis was surgically diagnosed in two of the studies, while it was not mentioned in the other. Vitamin D treatment was administered orally in all of them with different doses and durations. Detailed information about each individual study, including their characteristics and outcomes, can be found in Tables 2–4.
Table 2.
Clinical Trials examined the impact of vitamin D supplementation in women with endometriosis
| References | Study title | Country | Study design | Registration | Ethical consideration | Final results |
|---|---|---|---|---|---|---|
| Almassinokiani et al.42 | Effects of Vitamin D on Endometriosis-Related Pain: A Double-Blind Clinical Trial | Iran | Double-blind clinical trial | IRCT | Authorization by the university Ethics Committee | Vitamin D treatment did not have a significant effect in reducing dysmenorrhea and/or pelvic pain. |
| Mehdizadehkashi et al.43 | The effect of vitamin D supplementation on clinical symptoms and metabolic profiles in patients with endometriosis | Iran | Double-blind clinical trial | E Iranian website for registration of clinical trials | Ethics committee of Iran University of Medical Sciences | Vitamin D intake in patients with endometriosis resulted in a significant improvement of pelvic pain, total-/HDL-cholesterol ratio, hs-CRP, and TAC levels, but did not affect other clinical symptoms and metabolic profiles. |
| Nodler et al.44 | Supplementation with vitamin D or ω-3 fatty acids in adolescent girls and young women with endometriosis (SAGE): a double-blind, randomized, placebo-controlled trial | USA | Double-blind clinical trial | clinicaltrials.gov | The BCH Institutional Review Board approved this trial | Supplementation with vitamin D led to statistically significant improvements in pelvic pain and catastrophic thinking; however, they did not differ in magnitude from the effect observed among those who received placebo. |
IRCT, Iranian Registry of Clinical Trials; CRP: C-Reactive Protein, HDL; High Density Lipoprotein, TAC; Total Antioxidant Capacity.
Table 4.
Population characteristics of vitamin D group and placebo group
| Mean Age | Height | Weight | BMI | Marital status | Race | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| References | Vitamin D | Placebo | Vitamin D | Placebo | Vitamin D | Placebo | Vitamin D | Placebo | Vitamin D | Placebo | Vitamin D | Placebo |
| Almassinokiani et al.42 | 30.84 | 28.95 | Not stated | Not stated | Not stated | Not stated | 22.46 | 23 | Not married 5 (26.32%) Married 14 (73.68%) | Not married 9 (47.37%) Married 10 (52.63%) | Iranian | Iranian |
| Mehdizadehkashi et al.43 | 34.8 ± 7.1 | 35.6 ± 7.0 | 162.8 ± 5.9 | 162.1 ± 5.5 | 63.4 ± 11.1 | 67.4 ± 10.4 | 23.8 ± 3.6 | 25.6 ± 3.4 | Not stated | Not stated | Not stated | Not stated |
| Nodler et al.44 | 20.0 ± 2.7 | 20.1 ± 3.5 | Not stated | Not stated | Under- to normal weight 15 (55.8) Overweight 3 (11.1) Obese 9 (33.3) | Under- to normal weight 13 (59.1) Overweight 3 (13.6) Obese 6 (27.3) | 26.2 ± 5.7 | 25.6 ± 5.1 | Not stated | Not stated | White 24 (88.9) Other 3 (11.1) | White 21 (95.5) Other 1 (4.5) |
Table 3.
Shows the criteria of inclusion and exclusion of involved studies, the vitamin D regimen and diagnosis of endometriosis
| References | Total sample size | Inclusion criteria | Exclusion criteria | Vitamin D dose | Duration of vit D treatment | Method of diagnosis | Endometriosis stage |
|---|---|---|---|---|---|---|---|
| Almassinokiani et al.42 | 38 | Women aged 15–40 years with proven endometriosis by laparoscopy and a visual analog scale (VAS) test score of 3 or more for dysmenorrhea and/or pelvic pain at second menses after operative laparoscopy. | 1. Patients with vitamin D treatment in the last 6 months prior to surgery; 2. Patients with known systemic diseases (e.g. hypertension, diabetes, coronary, renal, and hepatic diseases); 3. Patients with known malignancy; 4. Menopausal women; 5. Patients with hormonal treatment, including oral contraceptive pills, in the last 6 months. | 50 000 IU/weekly | 12 weeks | laparoscopy | Minimal 0 (0.00%) Mild 1 (5.26%) Moderate 10 (52.63%) Sever 8 (42.11%) |
| Mehdizadehkashi et al.43 | 60 | Women diagnosed with endometriosis, BMI (<25, 25 or more kg/m2) And age (<30, 30 or more years) | Patients with diabetes mellitus, thyroid disorders, hypertension, hyperprolactinemia, and Cushing syndrome were excluded | 50 000 IU vitamin D supplements each 2 weeks | 12 weeks | Not mentioned | Not mentioned |
| Nodler et al. 44 | 69 | Non-pregnant females aged 12–25 years with a surgical diagnosis of endometriosis with a pelvic pain of visual analog scale (VAS) score ≥ 3 out of 10 for their worst pain in the month preceding study enrollment. | Patients with a history of kidney stones or concurrent chronic illnesses known to affect gastrointestinal absorption of nutrients were ineligible | 2000 IU Vitamin D3 | 6 months | surgically | Endometriosis rASRM stage I 23 (85.2) II 3 (11.1) III 0 (0.0) IV 1 (3.7) |
Risk of bias of included studies
The quality of the trials was evaluated independently by two reviewers, and any disparities were resolved through consensus. The assessment of the risk of bias in randomized controlled trials (RCTs) adhered to the guidelines outlined in the Cochrane Handbook for the Development of Systematic Reviews of Intervention (Version 5.4.1). This assessment utilized the Cochrane Risk of Bias Tool (Figs. 4, 5), which encompasses six domains: selection bias, performance bias, attrition bias, detection bias, reporting bias, and other potential sources of bias. Each element within these domains was categorized as presenting either a low, high, or unclear risk of bias45.
Figure 4.
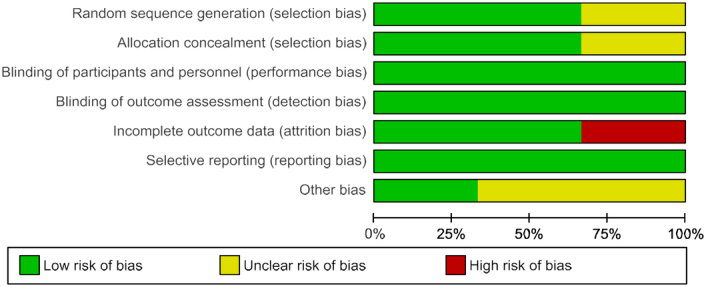
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
Figure 5.
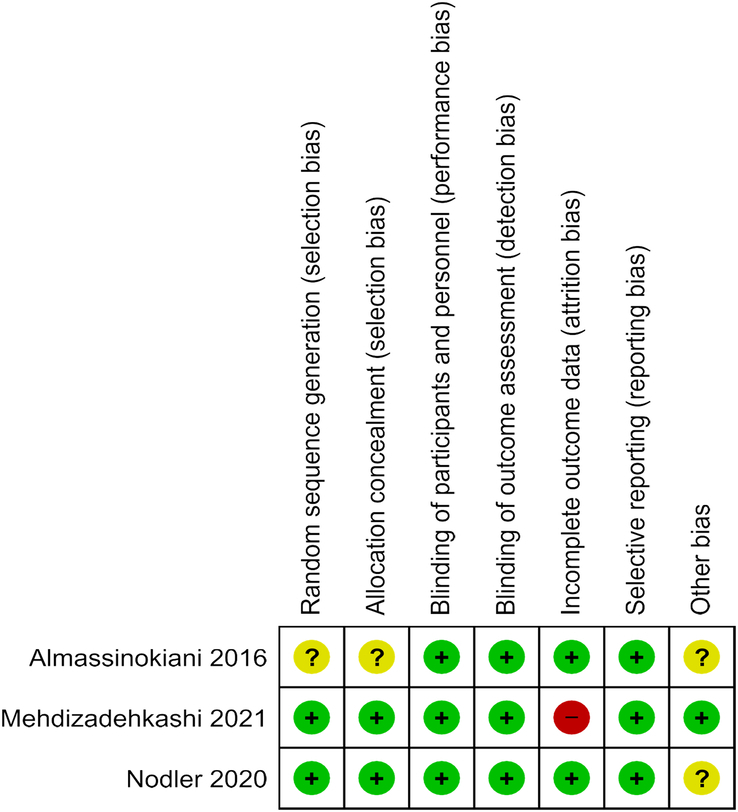
Shows risk of bias summery.
Synthesis of results
Our primary outcome is to assess the effect of vitamin D treatment on the pregnancy rate in endometriosis, defined as the number of viable intrauterine pregnancies confirmed by ultrasound. Regrettably, none of the included studies directly provided data on the impact of vitamin D supplementation on pregnancy outcomes in women with endometriosis.
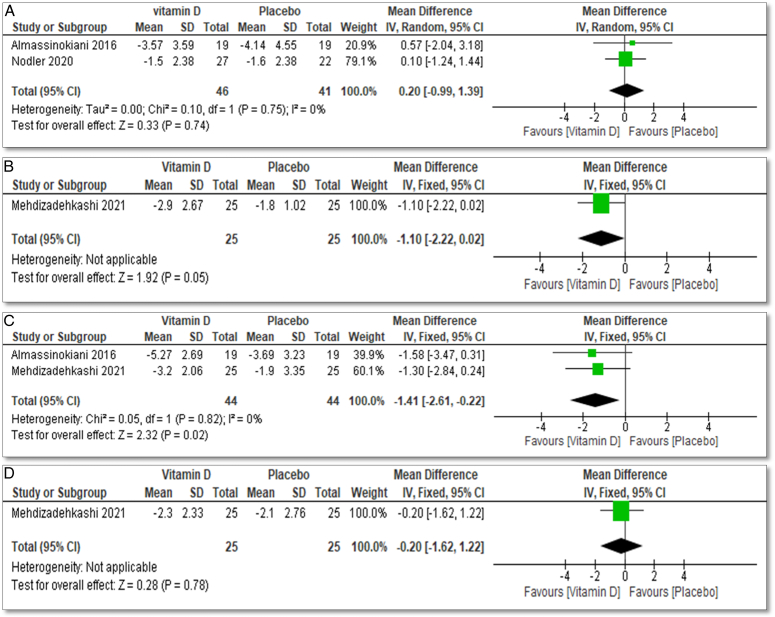
Regarding secondary outcomes, two studies investigated the effect of vitamin D treatment on endometriosis-related pelvic pain measured using a Visual Analog Scale (VAS)46: Almassinokiani 2016 with 38 participants42 and Nodler 2020 with 69 participants44. Meta-analysis showed that vitamin D treatment has no effect on chronic pelvic pain, with a mean difference of 0.2, 95% CI (−0.99 to 1.39), and an insignificant P value of 0.72 (Fig. 6A).
Figure 6.
(A) Forest plot of comparison: vitamin D vs. placebo, outcome: pelvic pain, (B) forest plot of comparison: 1 vitamin D vs. placebo, outcome: dyschezia, (C) forest plot of comparison: 1 vitamin D vs. placebo, outcome: dysmenorrhea, and (D) forest plot of comparison: 1 vitamin D vs placebo, outcome: dyspareunia.
Mehdizadehkashi 202143 included 60 participants to examine the effect of vitamin D supplementation in the treatment of dyschezia among endometriosis patients, which revealed a significant decrease in dyschezia with a mean difference of −1.10, 95% CI (−2.22 to 0.02), and a significant P value of 0.05 (Fig. 6B).
Both Almassinokiani 2016 with 38 participants42 and Mehdizadehkashi 2021 with 60 participants43 highlighted the role of vitamin D in the treatment of dysmenorrhea in the setting of endometriosis. Meta-analysis showed that vitamin D treatment decreased dysmenorrhea with a mean difference of −1.41 with CI 95% (−2.61 to v0.22) and a significant P value 0.02 (Fig. 6C).
For dyspareunia, only Mehdizadehkashi 202143, showed the effect of vitamin D as treatment of endometriosis that it decreases it with a mean difference −0.2 with CI 95% (−1.62 to 122) and insignificant P value 0.78 (Fig. 6D).
In examining the impact on both psychological and physical well-being, a study investigated the influence of vitamin D supplementation on the quality of life among women with endometriosis44. The evaluation employed the validated Short Form 12 (SF12) questionnaire47, which assesses physical and mental components, each rated on a scale of 0–100, where 0 signifies the lowest quality of life. Catastrophic thinking, a validated measure gauging pain sensitivity46, was appraised on a scale of 0–52, with higher scores indicating heightened catastrophizing tendencies, such as envisioning that the pain will never improve.
Across the study groups, no consistent patterns were observed in alterations to physical or mental quality of life, and there was no discernible significant difference in reported changes when comparing the impact of vitamin D supplementation with placebo over the 6-month intervention period.
For the SF-physical components, the intervention group demonstrated a mean change from baseline to the end of the study of 3.1 (95% CI: −4.3, 10.4), while the placebo group exhibited a mean change of 2.3 (95% CI: −2.4, 7.5).
Regarding the SF-mental components, the intervention group displayed a mean change from baseline to the end of the study of −0.9 (95% CI: −9.8, 7.1), whereas the placebo group showed a mean change of 0.6 (95% CI: −9.3, 10.4).
Importantly, participants across all study arms exhibited improvements in catastrophic thinking scores, with a statistically significant mean score enhancement observed only in the arm where vitamin D supplementation was administered (25.3–20.8, P = 0.04).
Specifically for catastrophic thinking, in the intervention group, the mean change from baseline to the end of the study was −4.5 (95% CI: −12.0, 3.0), while in the placebo group, the mean change was −3.5 (95% CI: −7.7, 0.8).
No studies reported the role of vitamin D in treatment of endometriosis on urinary symptoms, endometriosis regression, and refractoriness.
Ethical consideration
No ethical approval was required for this systematic review with meta-analysis, as all data were already published in peer-reviewed journals. No patients were involved in the design, conduct, or interpretation of our study.
Discussion
Endometriosis: definition, clinical presentation, risk factors, pathophysiology and treatment
Endometriosis is a gynecological disease that affects adolescents and women of reproductive age. It is defined as the presence of endometrial tissue outside the uterine cavity48. The clinical presentation of endometriosis varies according to the location and extent of the disease. Patients may present with pelvic pain, dysmenorrhea, dyspareunia, dyschezia, dysuria, abnormal menstrual bleeding, gastrointestinal symptoms, infertility, back pain, fatigue and malaise47. There are several risk factors for endometriosis, which include a family history of endometriosis, especially first-degree relatives, early onset of menstruation or experiencing prolonged heavy menstrual periods, null parity, exposure to high levels of estrogen, lifestyle and diet factors, such as high consumption of red meat and low intake of fruits and vegetables, uterine abnormalities and previous surgeries49.
Several hypotheses explain the pathophysiology of endometriosis, the main one involves retrograde menstruation in which menstrual blood containing endometrial cells flows backwards into the pelvic cavity48. Other theories proposed metaplasia in which extrauterine cells transform into endometrial cells, hormonal influence as endometriotic lesions are sensitive to estrogen which promotes their growth and proliferation, genetic expression and signaling pathways, oxidative stress and inflammation where reactive oxygen species cause DNA damage in endometrial cells, Immune dysfunction as autoimmune diseases activate macrophages and decreased cellular immunity and this inflammatory response will prevent elimination of menstrual cells and promote growth of endometrial cells outside the uterine cavity and angiogenesis to supply blood and nutrients of endometriotic tissue50.
Because the exact mechanisms behind endometriosis are unclear, no definitive curable treatment has been identified. Current treatment options primarily involve medical or surgical options include hormonal medications such as contraceptive pills, medroxyprogesterone, danazol, gastronome, GnRH analogs, or levonorgestrel IUD, or surgical interventions through laparotomy or laparoscopy. Hysterectomy with double adnexectomy is one of the surgical interventions capable of eradicating the disease entirely, but it is usually avoided for young patients or those desiring future pregnancies51. Also avoiding hysterectomy with double adnexectomy to prevent the potential complications associated with early menopause, such as decreased bone density leading to an increased risk of fractures, accelerated progression of cardiovascular disease, psychological impacts like depression, anxiety, and reduced perceived psychosocial support, potential cognitive decline, and the onset of dry eye syndrome52.
Endometriosis management lacks consensus as of yet. Since endometriosis primarily affects individuals of reproductive age, some authors suggest removing the ovaries as a potential treatment51. However, such treatment, including ovary removal, can induce early menopause and associated health problems53.
Endometriosis and Vitamin D: a potential relationship
Vitamin D is a fat-soluble vitamin that has crucial functions in the human body. It aids in bone growth and remodeling through calcium and phosphorus regulation, supports the immune system to regulate immune cell functions and reduces inflammation54. Moreover, adequate levels of vitamin D significantly reduce the risk of cardiovascular diseases and cancer prevention55. In addition, it affects hormones such as adrenaline, noradrenaline and dopamine production in the brain and helps to prevent serotonin depletion, so evidence linked vitamin D levels to mood and mental well-being56.
As vitamin D has immune-modulatory and anti-inflammatory properties, it plays a crucial role in mitigating the symptoms and progression of endometriosis57. Its ability to regulate immune cell functions and reduce inflammation suggests a potential impact on the immune dysfunction observed in endometriosis. Moreover, by influencing hormonal levels and aiding in tissue regulation, vitamin D could potentially contribute to hormonal balance, which is pivotal in managing endometriosis-associated symptoms58. It is also associated with the reduction of pelvic pain and discomfort for women with endometriosis59. This meta-analysis evaluated the effect of vitamin D intervention on pelvic pain, the mean difference was 0.2 (95% CI: −0.99 to 1.39, P = 0.75). The negligible mean difference suggests a minimal impact of vitamin D on pelvic pain compared to the control group. The wide confidence interval, [−0.99 to 1.39], indicates substantial uncertainty regarding the true effect size. Importantly, the non-significant p-value of 0.75 suggests that the observed difference is not statistically significant, emphasizing the lack of evidence supporting a meaningful association between vitamin D intervention and pelvic pain reduction. Based on the available studies, these findings suggest that vitamin D supplementation may not have a significant impact on alleviating pelvic pain. one of the included studies confirmed that supplementation of vitamin D can significantly improve endometriosis-related dysmenorrhea and dyschezia as well as the possible impact of vitamin D supplementation on dyspareunia42,43.
Endometriosis and pregnancy rate: impact and correlations
The growth of endometrial tissue outside the uterus can profoundly affect a woman’s fertility. It can obstruct or distort reproductive organs, thus decreeing the chance for sperm to reach the egg or for a fertilized egg to implant in the uterus60. Furthermore, endometriosis cause disruption of the normal ovulation process, which is essential for successful conception, so this will lead to irregular menstrual cycles and difficulty in predicting ovulation accurately. Additionally, the inflammation and scarring associated with endometrial implants in the pelvic region can interfere with the normal functioning of reproductive organs, further impacting on fertility61. Also, the presence of ovarian endometriomas significantly compromises the ovarian reserve, confounding the prospects of fertilization and pregnancy62. The possibility of miscarriage is slightly higher in women with endometriosis is possibly due to inflammation and hormonal imbalances associated with the condition63.
The correlation between endometriosis and infertility depends on several factors. It includes the severity of the disease, severe cases with extensive tissue growth and significant scarring may have a more pronounced effect on fertility compared to mild or moderate cases59. Age is another critical factor. As a woman with endometriosis ages, the combination of the natural age-related decline in fertility and the progression of the condition can make conceiving more challenging. In addition, the location of endometrial implants can affect fertility. Implants on or near the ovaries, fallopian tubes, or uterus can have a more direct impact on fertility by disrupting ovulation, fertilization, or implantation64.
Adequate vitamin D levels have a potential impact on improving pregnancy rates. It improves fertility and reproductive outcomes because vitamin D receptors are present in the reproductive tract, and adequate vitamin D enhances fertility by affecting hormone levels, egg quality, and the uterine environment65. Moreover, it supports regular ovulatory function, which is crucial for achieving pregnancy. Research suggests that vitamin D deficiency can be linked to ovulatory disorders, and correcting this deficiency could enhance ovulation and increase the chances of conception66. In addition, adequate vitamin D level contribute to successful implantation and early pregnancy by fostering a favorable uterine environment while potentially mitigating pregnancy complications like gestational diabetes and pre-eclampsia67. Regrettably, none of the included studies directly provided data on the impact of vitamin D supplementation on pregnancy rates in women with endometriosis. However, as vitamin D has the potential to alleviate symptoms associated with endometriosis, including dysmenorrhea, dyschezia, dyspareunia, and mood disorders. This in turn suggests that addressing vitamin D deficiency could possibly contribute to an improvement in these symptoms. Notably, exploring the potential amelioration of these challenges holds promise for enhancing overall reproductive health and potentially increasing pregnancy rates. It is hypothesized that incorporating adequate vitamin D levels into women’s health strategies might offer a positive impact on both symptom management and fertility outcomes.
It is strongly advised to conduct further research in this field, given the ongoing study whose results have not yet been published. The outcome of this study holds the potential to significantly impact the findings of any meta-analysis within the subject matter. Pending study results could significantly shape the meta-analytical landscape in this field.
Endometriosis and urinary symptoms
Endometrial implants most commonly affect the pelvic region, but they can extend to various body organs including the urinary tract. This can result in a variety of urinary symptoms68. The urinary symptoms associated with endometriosis can include dysuria, hematuria, frequent urination as implants can irritate the bladder, especially during menstrual cycles when endometrial tissue is more active and urinary urgency that disrupts daily activity and quality of life69. In addition, the presence of endometrial tissue near the urinary tract can cause pelvic pressure and prompt a sudden and compelling urge to urinate due to bladder irritation70.
Those urinary symptoms can significantly impact the woman’s ability to conceive and affect the pregnancy rate. Frequent urination and urinary urgency can disrupt the timing and frequency of sexual intercourse. Moreover, chronic urinary symptoms cause emotional stress and anxiety. This will negatively impact hormonal regulation and menstrual cycles which will decrease the pregnancy rate71. Unfortunately, no previous studies reported the possible role of vitamin D supplementation in the treatment of urinary symptoms associated with endometriosis.
Endometriosis and mental health
Endometriosis can impact mood due to its complex interplay with hormones and persistent pain. Chronic pelvic pain can cause emotional distress, which will affect the overall quality of life. Additionally, it exacerbates mood disorders such as anxiety and depression72. Hormonal fluctuation associated with endometriosis can contribute to mood disorders since estrogen plays an important role in regulating neurotransmitters in the brain, which affect mood73. Furthermore, fatigue and sleep disorders associated with endometriosis have an important impact on concentration, mood and overall mental well-being74.
These mood disorders have a negative effect on the pregnancy rate in women with endometriosis. It can lead to a hormonal imbalance that potentially affects ovulation and fertility75. It can also lead to decreased libido and disrupted sexual function. This can reduce the frequency of sexual intercourse, which is essential for conception. In addition, mood disorders decrease adherence to fertility treatment, which will decrease the chance of successful conception76.
Studies have reported varying findings regarding the relationship between endometriosis and mental health (MH) issues. Furthermore, the assessment of chronic pelvic pain (CPP) or pain disorders such as dyspareunia, their reporting, and their impact on MH symptoms can be challenging to evaluate, although some evidence suggests they may contribute to the development of mood disorders77,78. Laganà et al.77. demonstrated a connection between psychiatric disorders, including depression, somatization, and anxiety, among patients with endometriosis. Given the presentation of endometriosis, it is highly likely to have an adverse impact on women’s mental health. However, theories about the underlying mechanisms and pathophysiology remain relatively underexplored and unclear79.
A cross-sectional study has demonstrated that endometriosis can lead to heightened mental health distress, resulting in issues related to body image, loss, hopelessness, alexithymia, and feelings of worthlessness. Aerts et al.80 have summarized the adverse psychological effects on women with endometriosis, highlighting increased levels of pain-related experiences and distress.
In this review, one study specifically examined the impact of vitamin D supplementation on the mental health and quality of life for women with endometriosis. In assessing both physical and mental well-being, participants in the intervention group demonstrated positive changes in SF-physical components compared to the placebo group. While the mean change from baseline to the end of the study was 3.1 for the intervention group and 2.3 for the placebo group, the confidence intervals indicated overlapping ranges, suggesting no clear distinction between the two groups.
Similarly, for SF-mental components, the intervention group showed a slight decrease (−0.9) from baseline to the end of the study, while the placebo group exhibited a small increase (0.6). Again, the confidence intervals overlapped, indicating no significant difference in the observed changes.
Notably, participants across all study arms experienced improvements in catastrophic thinking scores. However, a statistically significant enhancement was only observed in the arm where vitamin D supplementation was administered (25.3–20.8, P = 0.04). Specifically for catastrophic thinking, the intervention group showed a mean change of −4.5, while the placebo group had a mean change of −3.5. The overlapping confidence intervals suggest caution in attributing this difference solely to the intervention, emphasizing the need for further exploration in future studies.
Vitamin D and endometrial regression
Four published studies investigating the role of vitamin D in the treatment of endometriosis in animal models provide valuable insights into the disease’s mechanisms, complications, and potential treatments81–84. However, it is important to note that the lack of specific strategies mirroring those employed in humans may contribute to the observed inconsistencies between findings from animal studies and results obtained from human research in the context of endometriosis. Three studies utilized mouse models by surgically inducing endometriosis through the transplantation of endometrial tissue into the peritoneum81–83, while another study achieved endometriosis in mice by injecting endometrial tissue extracted from donor mice84. Two studies employed intraperitoneal administration of vitamin D (1,2), one study utilized oral administration84, and another study opted for intramuscular administration83.
Despite the absence of published data on endometriosis regression in humans, one study among the four published in animal models reported a reduction in both endometriosis development and peritoneal inflammation following the administration of elocalcitol, a synthetic derivative of vitamin D84. This observed reduction aligns with findings from two additional studies, collectively demonstrating regression of endometriosis following vitamin D treatment82,83. However, in contrast, another study within this set did not observe any significant difference in the regression of endometriosis implants after the administration of vitamin D81.
In the context of endometriosis, animal models have demonstrated a noteworthy reduction in the size of lesions82–84, a phenomenon that may be attributed to diminished cellular motility, proliferation, and invasion85,86. This observed reduction aligns with findings from studies investigating the role of vitamin D in endometriosis regression, where the potential impact on cellular processes may contribute to the observed therapeutic effects. The variability in outcomes across studies underscores the complexity of understanding the potential role of vitamin D in endometriosis regression. This emphasizes the imperative for further research and clinical investigations to elucidate the efficacy of vitamin D in human cases, forming a basis for comprehensive insights into its therapeutic potential.
Possible complications of vitamin D supplementation
Vitamin D toxicity is extremely rare, either from sun exposure or from an exogenous source, because the skin has the ability to destroy the excess of vitamin D, which has a wide therapeutic index. Although it may occur when taking high doses for a long period, resulting in concentrations over 150 ng/ml and hypercalcemia. Symptoms of vitamin D toxicity mimic other conditions that cause high calcium levels, affecting many body systems. Neuropsychiatric effects such as diminished focus, depression, psychosis, or even coma in severe cases. Gastrointestinal effects include vomiting, abdominal discomfort, excessive thirst, loss of appetite, gastric ulcers, and pancreatitis. Cardiovascular implications involve elevated blood pressure, ECG changes such as a shortened QT interval, ST-segment elevation, and bradyarrhythmias with first-degree heart block. Renal manifestations encompass early signs such as excessive calcium in urine, excessive urination, thirst, dehydration, kidney calcification, and ultimately, renal malfunction. Additional VDT symptoms arising from heightened calcium levels include corneal calcification, hearing impairment, and painful calcification around joints87.
Principle findings
The main findings of this systematic review and meta-analysis are as follows: (1) Vitamin D supplementation was found to be effective in alleviating specific symptoms associated with endometriosis, including dysmenorrhea, dyspareunia, and dyschezia; (2) Contrary to the positive effects on specific symptoms, the meta-analysis indicated that vitamin D supplementation did not have a positive impact on chronic pelvic pain associated with endometriosis; (3) There was no consistent pattern of improvement in the mental or physical aspects of the quality of life among individuals with endometriosis who received vitamin D supplementation; (4) While significant improvement in catastrophic thinking was observed with vitamin D supplementation over a 6-month period, there was no significant difference between the vitamin D and placebo groups; (5) Eventually, positive effects of vitamin D supplementation on endometriosis-related symptoms may indirectly contribute to improved pregnancy outcomes and enhanced fertility.
Comparison with existing literature
When compared with existing literature, our review aligns with previous studies indicating the beneficial effects of vitamin D on endometriosis-related symptoms including dysmenorrhea, dyspareunia and dyschezia. Conversely, meta-analysis showed that supplementation of vitamin D has no positive effect on chronic pelvic pain associated with endometriosis There was no consistent pattern of improvement in the mental or physical aspects of the quality of life. However, our findings diverge in certain aspects, such as the lack of an obvious impact on the pregnancy rate. The discussion provides a comprehensive overview of how our results contribute to the existing body of knowledge on vitamin D therapeutic potential in managing endometriosis-related problems.
Strengths and limitations
To the best of our knowledge, this systematic review represents the first comprehensive assessment of the influence of vitamin D on pregnancy rates in women with endometriosis. It rigorously evaluates the findings from available studies. Our study’s strength lies in its extensive search across multiple databases, resulting in a broader and more comprehensive set of results compared to previous reviews. Furthermore, nearly all of the studies included in our analysis were high-quality randomized clinical trials with a low to medium risk of bias.
However, several limitations should be acknowledged: Firstly, the number of studies available for our analysis is limited, and the sample sizes in these trials are relatively small, which could potentially impact the reliability of the results. Secondly, variations in the doses and regimens of vitamin D supplementation used in the included studies may have influenced the outcomes of the meta-analysis. Additionally, confounding factors such as individual differences in sun exposure habits and dietary patterns were not taken into account, which could have had an impact on the analysis results. Lastly, due to insufficient data, we were unable to assess the effects of vitamin D supplementation on pregnancy outcomes.
Conclusion
Our study highlights that the utilization of antioxidant vitamin D supplementation generally proves effective in alleviating symptoms associated with endometriosis. Consequently, this therapy can be considered a viable alternative for managing endometriosis-related symptoms, either as a standalone treatment or in conjunction with other approaches.
It is essential to note that our systematic review is the first to specifically examine the correlation between vitamin D supplementation and pregnancy rates among women of reproductive age with endometriosis. Nevertheless, due to the scarcity of studies investigating the link between vitamin D and pregnancy outcomes, along with the limited sample sizes and study quality, further research is imperative to gain a more comprehensive understanding of the role of vitamin D supplementation in enhancing pregnancy prospects for women with endometriosis. In light of this, it is noteworthy to consider that our secondary outcomes, though not directly addressed in existing studies, may still exert an indirect influence on our primary objective. These secondary outcomes could potentially offer valuable insights into the broader context of factors contributing to endometriosis-related pregnancy challenges.
We also propose conducting future meta-analyses as additional studies with similar objectives are added to the scientific literature. This will help us refine our understanding of the relationship between vitamin D supplementation and pregnancy outcomes in women with endometriosis.
Ethical approval
Ethics approval was not required for this systematic review/meta-analysis.
Consent
Informed consent was not required for this systematic review/meta-analysis
Sources of funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contribution
This work was carried out in collaboration among all authors. O.N.S., H.A.S., Y.S.A., and R.R.S. contributed to the conception of the review and interpreted the literatures based on the level of evidence and revised the manuscript. O.N.S., H.A.S., Y.S.A., and R.R.S. participate in reviewing preparation of the manuscript. O.N.S., H.A.S., Y.S.A., R.R.S., and M.N. participate in preparation and critical review of the manuscripts. In addition, all authors read and approved the manuscript.
Conflicts of interest disclosure
The authors declare no conflict of interest.
Research registration unique identifying number (UIN)
Name of the registry: PROSPERO
Unique Identifying number or registration ID: CRD42023444184.
Hyperlink to your specific registration (must be publicly accessible and will be checked). https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=444184.
Guarantor
Oadi N. Shrateh.
Data availability statement
Dataset is available upon reasonable request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Acknowledgements
The authors are very grateful to Elham Kateeb, Associate professor of Dental Public Health, Deanship of scientific Research, Faculty of Dentistry, Al-Quds University, Palestine for her help and facilitating the process of databases accessibility.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/annals-of-medicine-and-surgery.
Published online 15 May 2024
Contributor Information
Oadi N. Shrateh, Email: oadi.shrateh@students.alquds.edu.
Haneen A. Siam, Email: haneens709@gmail.com.
Yasmeen S. Ashhab, Email: yasmeen.ashhab@students.alquds.edu.
Raneen R. Sweity, Email: raneensweety1999@gmail.com.
Mashhour Naasan, Email: mashhournaasan@yahoo.co.uk.
References
- 1.Kalaitzopoulos DR, Lempesis IG, Athanasaki F, et al. Association between vitamin D and endometriosis: a systematic review. Hormones 2020;19:109–121. [DOI] [PubMed] [Google Scholar]
- 2.Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med 2020;382:1244–1256. [DOI] [PubMed] [Google Scholar]
- 3.Kalaitzopoulos DR, Lempesis IG, Samartzis N, et al. Leptin concentrations in endometriosis: a systematic review and meta-analysis. J Reprod Immunol 2021;146:103338. [DOI] [PubMed] [Google Scholar]
- 4.Chapron C, Marcellin L, Borghese B, et al. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol 2019;15:666–682. [DOI] [PubMed] [Google Scholar]
- 5.Kalaitzopoulos DR, Samartzis N, Kolovos GN, et al. Treatment of endometriosis: a review with comparison of 8 guidelines. BMC Women’s Health 2021;21:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nirgianakis K, Bersinger NA, McKinnon B, et al. Regression of the inflammatory microenvironment of the peritoneal cavity in women with endometriosis by GnRHa treatment. Eur J Obstetr Gynecol Reprod Biol 2013;170:550–554. [DOI] [PubMed] [Google Scholar]
- 7.Nirgianakis K, Grandi G, McKinnon B, et al. Dienogest mediates midkine suppression in endometriosis. Hum Reprod 2016;31:1981–1986. [DOI] [PubMed] [Google Scholar]
- 8.Nirgianakis K, McKinnon B, Ma L, et al. Peritoneal fluid biomarkers in patients with endometriosis: a cross-sectional study. Horm Mol Biol Clin Investig 2020;42:113–122. [DOI] [PubMed] [Google Scholar]
- 9.Nirgianakis K, Egger K, Kalaitzopoulos DR, et al. Effectiveness of dietary interventions in the treatment of endometriosis: a systematic review. Reprod Sci 2022;29:26–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Somigliana E, Panina-Bordignon P, Murone S, et al. Vitamin D reserve is higher in women with endometriosis. Hum Reprod 2007;22:2273–2278. [DOI] [PubMed] [Google Scholar]
- 11.Anastasi E, Fuggetta E, De Vito C, et al. Low levels of 25-OH vitamin D in women with endometriosis and associated pelvic pain. Clin Chem Lab Med (CCLM) 2017;55:e282–e284. [DOI] [PubMed] [Google Scholar]
- 12.Buggio L, Somigliana E, Pizzi MN, et al. 25-Hydroxyvitamin D serum levels and endometriosis: results of a case–control study. Reprod Sci 2019;26:172–177. [DOI] [PubMed] [Google Scholar]
- 13.Miyashita M, Koga K, Izumi G, et al. Effects of 1, 25-dihydroxy vitamin D3 on endometriosis. J Clin Endocrinol Metab 2016;101:2371–2379. [DOI] [PubMed] [Google Scholar]
- 14.Evans S, Fernandez S, Olive L, et al. Psychological and mind-body interventions for endometriosis: a systematic review. J Psychosom Res 2019;124:109756. [DOI] [PubMed] [Google Scholar]
- 15.Sukan B, Akdevelioğlu Y, Sukan VN. Effect of antioxidant supplementation on endometriosis-related pain: a systematic review. Curr Nutr Rep 2022;11:753–764. [DOI] [PubMed] [Google Scholar]
- 16.Ye L, et al. Easily missed: endometriosis. Br Med J (BMJ) 2022;379:068950. [Google Scholar]
- 17.Zhou F, Li C, Zhang S-Y. NLRP3 inflammasome: a new therapeutic target for high-risk reproductive disorders? Chin Med J 2021;134:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ansariniya H, Yavari A, Javaheri A, et al. Oxidative stress‐related effects on various aspects of endometriosis. Am J Reprod Immunol 2022;88:e13593. [DOI] [PubMed] [Google Scholar]
- 19.Cacciottola L, Donnez J, Dolmans M-M. Can endometriosis-related oxidative stress pave the way for new treatment targets? Int J Mol Sci 2021;22:7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clower L, Fleshman T, Geldenhuys WJ, et al. Targeting oxidative stress involved in endometriosis and its pain. Biomolecules 2022;12:1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mittler R, Zandalinas SI, Fichman Y, et al. Reactive oxygen species signalling in plant stress responses. Nat Rev Mol Cell Biol 2022;23:663–679. [DOI] [PubMed] [Google Scholar]
- 22.Assaf L, Eid AA, Nassif J. Role of AMPK/mTOR, mitochondria, and ROS in the pathogenesis of endometriosis. Life Sci 2022;306:120805. [DOI] [PubMed] [Google Scholar]
- 23.Mier-Cabrera J, Aburto-Soto T, Burrola-Méndez S, et al. Women with endometriosis improved their peripheral antioxidant markers after the application of a high antioxidant diet. Reprod Biol Endocrinol 2009;7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redwood E, Lam V, Takechi R, et al. Aged garlic extract as a potential prophylactic to reduce the progression of endometriosis and associated pain burden.. Front Pain Res 2022;3:1057830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amirsalari S, Behboodi Moghadam Z, Taghizadeh Z, et al. The effect of garlic tablets on the endometriosis-related pains: a randomized placebo-controlled clinical trial. Evid Based Complement Alternat Med 2021;2021:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris HR, Eke AC, Chavarro JE, et al. Fruit and vegetable consumption and risk of endometriosis. Hum Reprod 2018;33:715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ibrahim Abd El-Fadil Sehsah F, Taha Abd El-Fattah A, Mohammed Saeed A. The role of antioxidant supplementation in reducing the endometriosis related chronic pelvic pain in women. Al-Azhar Med J 2022;51:121–134. [Google Scholar]
- 28.Alnaggar MA, Abdelfattah AT, Saeed IM. Role of antioxidants (vitamin E and vitamin C) supplementation for management of chronic pelvic pain related to endometriosis.. Zagazig Univ Med J 2022;28:1269–1273. [Google Scholar]
- 29.Sinha A. The role of antioxidant supplementation in endometriosis therapy. J Gynecol Women’s Health 2017;3:555601. [Google Scholar]
- 30.Liu Y, Wang J, Zhang X. An update on the multifaceted role of NF-kappaB in endometriosis. Int J Biol Sci 2022;18:4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aranow C. Vitamin D and the immune system. J Investig Med 2011;59:881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Froicu M, Weaver V, Wynn TA, et al. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol 2003;17:2386–2392. [DOI] [PubMed] [Google Scholar]
- 33.Whitfield GK, Hsieh JC, Jurutka PW, et al. Genomic actions of 1, 25-dihydroxyvitamin D3. J Nutr 1995;125:1690S–1694S. [DOI] [PubMed] [Google Scholar]
- 34.Vienonen A, Miettinen S, Bläuer M, et al. Expression of nuclear receptors and cofactors in human endometrium and myometrium. J Soc Gynecol Investig JSGI 2004;11:104–112. [DOI] [PubMed] [Google Scholar]
- 35.Viganò P, Lattuada D, Mangioni S, et al. Cycling and early pregnant endometrium as a site of regulated expression of the vitamin D system. J Mol Endocrinol 2006;36:415–424. [DOI] [PubMed] [Google Scholar]
- 36.Agic A, Xu H, Altgassen C, et al. Relative expression of 1, 25-dihydroxyvitamin D3 receptor, vitamin D 1α-hydroxylase, vitamin D 24-hydroxylase, and vitamin D 25-hydroxylase in endometriosis and gynecologic cancers. Reprod Sci 2007;14:486–497. [DOI] [PubMed] [Google Scholar]
- 37.Lopez A, Cruz ML, Chompre G, et al. Influence of stress on the vitamin D-vitamin D receptor system, macrophages, and the local inflammatory milieu in endometriosis. Reprod Sci 2020;27:2175–2186. [DOI] [PubMed] [Google Scholar]
- 38.Cermisoni G, Alteri A, Corti L, et al. Vitamin D and endometrium: a systematic review of a neglected area of research. Int J Mol Sci 2018;19:2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 40.Shea BJ, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.RoB 2:. A revised Cochrane risk-of-bias tool for randomized trials. 2023. Accessed 29 November 2023. https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials
- 42.Almassinokiani F, Khodaverdi S, Solaymani-dodaran M, et al. Effects of vitamin D on endometriosis-related pain: a double-blind clinical trial. Med Sci Monitor 2016;22:4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehdizadehkashi A, Rokhgireh S, Tahermanesh K, et al. The effect of vitamin D supplementation on clinical symptoms and metabolic profiles in patients with endometriosis. Gynecol Endocrinol 2021;37:640–645. [DOI] [PubMed] [Google Scholar]
- 44.Nodler JL, DiVasta AD, Vitonis AF, et al. Supplementation with vitamin D or ω-3 fatty acids in adolescent girls and young women with endometriosis (SAGE): a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 2020;112:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess 1995;7:524. [Google Scholar]
- 47.Galle PC. Clinical presentation and diagnosis of endometriosis. Obstet Gynecol Clin North Am 1989;16:29–42. [PubMed] [Google Scholar]
- 48.https://my.clevelandclinic.org/health/diseases/24432-retrograde-menstruation professional, C.C.m. Retrograde Menstruation. 2022. Accessed 14 October 2023.
- 49.Peterson CM, Johnstone EB, Hammoud AO, et al. Risk factors associated with endometriosis: importance of study population for characterizing disease in the ENDO Study. Am J Obstet Gynecol 2013;208:451.e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med 2014;2014:179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Acién P, Velasco I. Endometriosis: a disease that remains enigmatic. ISRN Obstet Gynecol 2013;2013:242149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vincent AJ, Laven JS. Early menopause/premature ovarian insufficiency. Semin Reprod Med 2020;38:235–236. [DOI] [PubMed] [Google Scholar]
- 53.Hegazy AA. Is there any mean to postpone the menopausal ovarian senescence? Int J FertilSteril 2020;13:346–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nair R, Maseeh A. Vitamin D: the “sunshine” vitamin. J Pharmacol Pharmacother 2012;3:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCullough ML, Zoltick ES, Weinstein SJ, et al. Circulating vitamin D and colorectal cancer risk: an international pooling project of 17 cohorts. JNCI: J Natl Cancer Inst 2019;111:158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shulman Dorothy, MD. Endocrine-related Organs and Hormones. 2022. Accessed 14 October 2023. https://www.endocrine.org/patient-engagement/endocrine-library/hormones-and-endocrine-function/endocrine-related-organs-and-hormones#:~:text=Researchers%20have%20found%20that%20vitamin,individual’s%20risk%20of%20depression%20significantly
- 57.Kalaitzopoulos DR, Samartzis N, Daniilidis A, et al. Effects of vitamin D supplementation in endometriosis: a systematic review. Reprod Biol Endocrinol 2022;20:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang L, Zhang X. Research progress on roles of vitamin D in endometriosis. Zhejiang Da Xue Xue Bao Yi Xue Ban 2018;47:413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Organization, WH Endometriosis. 2023. Accessed 14 October 2023. https://www.who.int/news-room/fact-sheets/detail/endometriosis/?gclid=EAIaIQobChMI0LjQouTwgQMVSQAGAB2liwCgEAAYASAAEgLx7vD_BwE
- 60.Bulletti C, Coccia ME, Battistoni S, et al. Endometriosis and infertility. J Assist Reprod Genet 2010;27:441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Filip L, Duică F, Prădatu A, et al. Endometriosis associated infertility: a critical review and analysis on etiopathogenesis and therapeutic approaches. Medicina (Kaunas) 2020;56:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zeng C, Lu R, Li X, et al. The presence of ovarian endometrioma adversely affect ovarian reserve and response to stimulation but not oocyte quality or IVF/ICSI outcomes: a retrospective cohort study. J Ovarian Res 2022;15:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frincu F, Carp-Veliscu A, Petca A, et al. Maternal-fetal outcomes in women with endometriosis and shared pathogenic mechanisms. Medicina (Kaunas) 2021;57:1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Casey FE. Overview of Contraception. 2023. Accessed 14 October 2023. https://www.msdmanuals.com/professional/gynecology-and-obstetrics/family-planning/oral-contraceptives
- 65.Várbíró S, Takács I, Tűű L, et al. Effects of vitamin D on fertility, pregnancy and polycystic ovary syndrome-a review. Nutrients 2022;14:1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Butts SF, Seifer DB, Koelper N, et al. Vitamin D deficiency is associated with poor ovarian stimulation outcome in PCOS but Not unexplained infertility. J Clin Endocrinol Metab 2019;104:369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Palacios C, Kostiuk LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev 2019;7:Cd008873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smolarz B, Szyłło K, Romanowicz H. Endometriosis: epidemiology, classification, pathogenesis, treatment and genetics (review of literature). Int J Mol Sci 2021;22:10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leonardi M, Espada M, Kho RM, et al. Endometriosis and the urinary tract: from diagnosis to surgical treatment. Diagnostics (Basel) 2020;10:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.MedicalNewsToday. Can endometriosis cause bladder pain? 2023. Accessed 14 October 2023. https://www.medicalnewstoday.com/articles/321439
- 71.Uzelpasaci E, Çinar GN, Baran E, et al. Trimester-based changes in urogenital symptoms and their impact on the quality of life in pregnant women: a preliminary report. Curr Urol 2021;15:167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Estes SJ, Huisingh CE, Chiuve SE, et al. Depression, anxiety, and self-directed violence in women with endometriosis: a retrospective matched-cohort study. Am J Epidemiol 2021;190:843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chantalat E, Valera MC, Vaysse C, et al. Estrogen receptors and endometriosis. Int J Mol Sci 2020;21:2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Facchin F, Buggio L, Roncella E, et al. Sleep disturbances, fatigue and psychological health in women with endometriosis: a matched pair case-control study. Reprod Biomed Online 2021;43:1027–1034. [DOI] [PubMed] [Google Scholar]
- 75.Szypłowska M, Tarkowski R, Kułak K. The impact of endometriosis on depressive and anxiety symptoms and quality of life: a systematic review. Front Public Health 2023;11:1230303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bonavina G, Taylor HS. Endometriosis-associated infertility: from pathophysiology to tailored treatment. Front Endocrinol (Lausanne) 2022;13:1020827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laganà AS, La Rosa V, Petrosino B, et al. Comment on “Risk of developing major depression and anxiety disorders among women with endometriosis: a longitudinal follow-up study. J Affect Disord 2017;208:672–673. [DOI] [PubMed] [Google Scholar]
- 78.Cavaggioni G, Lia C, Resta S, et al. Are mood and anxiety disorders and alexithymia associated with endometriosis? A preliminary study. Biomed Res Int 2014;2014:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rolla E. Endometriosis: advances and controversies in classification, pathogenesis, diagnosis, and treatment. F1000Res 2019;8:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aerts L, Grangier L, Streuli I, et al. Psychosocial impact of endometriosis: from co-morbidity to intervention. Best Pract Res Clin Obstetr Gynaecol 2018;50:2–10. [DOI] [PubMed] [Google Scholar]
- 81.Akyol A, Şimşek M, İlhan R, et al. Efficacies of vitamin D and omega-3 polyunsaturated fatty acids on experimental endometriosis. Taiwan J Obstet Gynecol 2016;55:835–839. [DOI] [PubMed] [Google Scholar]
- 82.Abbas MA, Taha MO, Disi AM, et al. Regression of endometrial implants treated with vitamin D3 in a rat model of endometriosis. Eur J Pharmacol 2013;715:72–75. [DOI] [PubMed] [Google Scholar]
- 83.Yildirim B, Guler T, Akbulut M, et al. 1-alpha,25-dihydroxyvitamin D3 regresses endometriotic implants in rats by inhibiting neovascularization and altering regulation of matrix metalloproteinase. Postgrad Med 2014;126:104–110. [DOI] [PubMed] [Google Scholar]
- 84.Mariani M, Vigano P, Gentilini D, et al. The selective vitamin D receptor agonist, elocalcitol, reduces endometriosis development in a mouse model by inhibiting peritoneal inflammation. Hum Reprod 2012;27:2010–2019. [DOI] [PubMed] [Google Scholar]
- 85.Delbandi AA, Mahmoudi M, Shervin A, et al. 1,25-dihydroxy vitamin D3 modulates endometriosis-related features of human endometriotic stromal cells. Am J Reprod Immunol 2016;75:461–473. [DOI] [PubMed] [Google Scholar]
- 86.Ingles SA, Wu L, Liu BT, et al. Differential gene expression by 1,25(OH)(2)D(3) in an endometriosis stromal cell line. J Steroid Biochem Mol Biol 2017;173:223–227. [DOI] [PubMed] [Google Scholar]
- 87.Marcinowska-Suchowierska E, Kupisz-Urbańska M, Łukaszkiewicz J, et al. Vitamin D toxicity—a clinical perspective. Front Endocrinol (Lausanne) 2018;9:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Dataset is available upon reasonable request.