Abstract
Purpose
Neoadjuvant targeted therapy provides a brief, preoperative window of opportunity that can be exploited to individualize cancer care based on treatment response. We investigated whether response to neoadjuvant therapy during the preoperative window confers survival benefit in patients with operable head and neck squamous cell carcinoma (HNSCC).
Experimental Design
A pooled analysis of treatment-naïve patients with operable HNSCC enrolled in one of three clinical trials from 2009–2020 (NCT00779389, NCT01218048, NCT02473731). Neoadjuvant regimens consisted of EGFR inhibitors (n=83) or anti-ErbB3 antibody therapy (n=9) within 28 days of surgery. Clinical to pathologic stage migration was compared to disease-free survival (DFS) and overall survival (OS) while adjusting for confounding factors using multivariable Cox regression. Circulating tumor markers validated in other solid tumor models were analyzed.
Results
92 of 118 patients were analyzed; all patients underwent surgery following neoadjuvant therapy. Clinical to pathologic downstaging was more frequent in patients undergoing neoadjuvant targeted therapy compared to control cohort (P=0.048). Patients with pathologic downstage migration had the highest OS (89.5%, 95% CI 75.7–100) compared to those with no stage change (58%, 95% CI 46.2–69.8) or upstage (40%, 95% CI 9.6–70.4, P=0.003). Downstage migration remained a positive prognostic factor for OS (hazard ratio 0.22, 95% CI 0.05–0.90) while adjusting for measured confounders. Downstage migration correlated with decreased circulating tumor markers, SOX17 and TAC1 (P=0.0078).
Conclusions
Brief neoadjuvant therapy achieved pathologic downstaging in a subset of patients and was associated with significantly better DFS and OS as well as decreased circulating methylated SOX17 and TAC1.
Translational relevance
Window of opportunity trials allow for pharmacodynamic assessment of investigational drugs during the window of preoperative planning prior to definitive treatment. Traditional endpoints for window studies are often aimed at assessing biochemical activity of the drug to confirm target engagement and guide selection of patients in future clinical trials. Here, we demonstrate that pathologic downstaging in response to neoadjuvant targeted therapy in three pooled window trials is associated with a survival benefit in head and neck cancer and may serve as a basis for individualized treatment selection. We further show that circulating tumor markers (circulating methylated SOX17 and TAC1 promoter DNA) may function as non-invasive measures of pathologic response to targeted therapy. Taken together, our findings may form the basis for a novel bioselection approach that optimizes survival and minimizes toxicity with neoadjuvant targeted therapy followed by definitive surgery.
INTRODUCTION
Over the last decade, incremental advances in the treatment of mucosal head and neck squamous cell carcinoma (HNSCC) have occurred. Surgery and chemoradiotherapy endure as the pillars of treatment in this population. However, the overall survival (OS) in patients with operable HNSCC remains modest, with poorer outcomes in patients with nodal involvement and HPV-negative disease (1). Often, such patients develop significant treatment-related toxicity for cure to be achieved (2–4). Consequently, patient selection for curative-intent treatment is challenging as up to 40% of patients develop major adverse events following cancer treatment (2–4). Neoadjuvant systemic therapy followed by extirpative surgery is a novel treatment strategy to reduce tumor burden, while awaiting preoperative surgical planning and medical optimization when necessary, which may be able to mitigate treatment toxicity related to standard regimens similar to breast and prostate treatment protocols. Neoadjuvant trials, for example TAX 324, have typically treated patients with 3 cycles of cytotoxic chemotherapy (5). The current role of neoadjuvant systemic therapy in patients with curable disease remains controversial (5–10). Previous studies on induction chemotherapy with platinum and taxol-based agents in HNSCC proved inconclusive, with a limited number of patients responding and significant treatment-related toxicity (5–10). Additionally, larger phase III trials (DECIDE & PARADIGM) did not yield positive results (9,10). Consequently, neoadjuvant therapy is infrequently used in patients with HNSCC.
In contrast to typical neoadjuvant chemotherapy protocols, window of opportunity trials provide an option to treat patients within a shorter therapeutic interval (typically 6 weeks or less). Window of opportunity trials, where a systemic agent is administered in the neoadjuvant setting followed by definitive surgery, allows for observation of tumor response and analysis of the resected specimen. This short treatment interval further provides an occasion to complete preoperative planning and optimization if the regimen is well tolerated (8,11,12). That being said, in most window of opportunity trials the primary endpoint is typically biochemical since these trials are intended to evaluate the biologic activity of drugs under investigation. In the present study, a pooled analysis of three window of opportunity clinical trials using neoadjuvant EGFR or HER3 targeted therapy for patients with HNSCC prior to definitive surgery was performed. The aim of this study was to ascertain the effect of pathologic stage migration, change from clinical to pathological stage, on (OS) and disease-free survival (DFS) in this population. A secondary outcome was to evaluate circulating tumor DNA markers as a surrogate for pathologic response to targeted therapy. We hypothesize that neoadjuvant targeted therapy can exploit the preoperative window and bioselect patients with improved prognosis allowing for an individualization of cancer care.
MATERIALS AND METHODS
Study Design & Population
We performed an ad-hoc analysis of three window of opportunity trials using neoadjuvant targeted therapy for treatment-naïve patients with HNSCC from 2009–2020 at the University of Pittsburgh Medical Center and Oregon Health & Science University (11–14).
Patients with operable and non-metastatic HNSCC with an Eastern Cooperative Oncology Group score of 0 or 1, adequate bone marrow function (hemoglobin ≥10 g/dL, absolute neutrophil count ≥1500/mm3, platelet count ≥ 100,000 /mm3) and serum creatinine ≤ 1.5g/dL were included in the clinical trials (11–14). All pediatric patients, pregnant women, those currently on immunosuppression, patients with previous cancer therapy within 2 years of enrollment or those with prior treatment for HNSCC were excluded from the study. Additionally, patients in a placebo arm (i.e. clinical trial NCT00779389) were excluded from the data analysis. All patients provided written informed consent and studies were conducted in accordance with the U.S. Common Rule and with approval of the Institutional Review Board of the University of Pittsburgh Medical Center.
Study trial NCT00779389 was a phase I trial of erlotinib/dasatinib taken daily for 14–21 days prior to definitive surgery (12,13). The second study, NCT01218048, was a phase II study evaluating cetuximab 2 weeks prior to surgery (11). In NCT02473731, the authors administered KTN3379, an anti-ErbB3 antibody, preoperatively in a phase I clinical trial (14).
Measurement of Exposure & Outcome
The exposure of interest was stage migration, defined as a change from clinical to pathological stage (downstage, no change or upstage) following definitive surgery. This was compared to a cohort of similar patients with head and neck cancer undergoing upfront surgery at the same institution (15). Two control groups were included in the study—a historical cohort of 49 patients with HNSCC in the University of Pittsburgh Medical Center network registry undergoing primary surgery (15) and a cohort of 100 consecutive patients with HNSCC enrolled between October 2020 and November 2021 in the University of Pittsburgh Medical Center network registry undergoing primary surgery. TNM staging was evaluated using both the American Joint Committee on Cancer (AJCC) 7th and 8th editions. Sociodemographic covariates including age, sex, alcohol consumption, smoking history and medical comorbidities were recorded. Relevant pathologic features, namely positive margin status, extranodal extension, perineural invasion, tumor differentiation, p16 status and number of involved lymph nodes were evaluated. Common Terminology Criteria for Adverse Events (CTCAE) for neoadjuvant systemic therapy, type and extent of surgery, and need for adjuvant therapy were noted. Criteria for adjuvant therapy included close (<5 mm) or positive margins, nodal disease, extranodal extension, perineural invasion or T3-T4 tumors. The primary outcome was overall survival (OS) defined from time of diagnosis to time of last follow-up or death. Locoregional recurrence and distant metastasis were recorded. Disease-free survival (DFS), defined as time after definitive treatment with no evidence of disease, was evaluated as a secondary outcome. As all patients were enrolled in a clinical trial, missing data and loss to follow-up were minimized.
Plasma DNA isolation, bisulfite conversion, and DNA methylation analysis
To determine whether noninvasive cancer-specific biomarkers correlate with pathologic stage migration, we evaluated a panel of 4 methylated promoter genes (CDO1, HOXA7, SOX17, TAC1) validated in other solid tumors and with available panels at our institution (16). Peripheral whole blood collected in EDTA-coated tubes (BD, Franklin Lakes, NJ) was spun at 1000 g to obtain plasma. Plasma was obtained at baseline and following a brief course of neoadjuvant targeted therapy. DNA extraction from plasma using methylation-on-beads (MOB), bisulfite conversion, and methylation analyses have been previously described (16). Briefly, plasma, proteinase K, and buffer AL (Qiagen, Germantown, MD) were incubated at 55°C for 1 hour. Following digestion, silica superparamagnetic beads and isopropanol were added. Lysate was incubated for 10 minutes prior to carrier RNA addition. For bisulfite conversion, lightning conversion reagent, M-binding buffer, M-desulphonation buffer, and M-wash buffer (ThermoFisher Scientific, Waltham, MA) were utilized. DNA was eluted from magnetic beads using M-elution buffer (ThermoFisher Scientific, Waltham, MA). Analysis was done using real-time methylation specific PCR. All primer and probe sequences have been previously reported (16).
Statistical Analysis
Descriptive statistics using the Mann-Whitney U tests (for continuous variables) and Chi-square tests (for categorical variables) were performed to compare covariates and pathologic response. Kaplan-Meier survival curves and Cox Proportional Hazards regression were used to compare OS and DFS. Multivariable analysis was subsequently performed with inclusion of clinically relevant covariates to adjust for measured confounding including age, sex, smoking history, p16 status, tumor location, adverse pathologic features and stage. Multivariable modelling with stage migration using both AJCC 7th and 8th editions was performed to reduce co-liner variables (e.g. extranodal extension). Wilcoxon signed-rank test was used for comparison of plasma methylated DNA levels before and after therapy. All data was analyzed with R software (R Foundation for Statistical Computing, Vienna, Austria) with packages “survminer” and “ggplot2” or with GraphPad Prism 9 software (La Jolla, CA). Adjustment for pathologic stage migration based on previous institutional data in patients not receiving adjuvant therapy was performed (15).
Data Availability Statement
The data generated in this study are available upon request from the corresponding author.
RESULTS
Patient characteristics
A total of 92 of the 118 patients enrolled in one of three neoadjuvant window of opportunity clinical trials were included in the pooled analysis (Supplemental Figure 1, Supplemental Table 4, NCT00779389, NCT01218048 and NCT02473731). 26 patients were excluded as they either belonged to the placebo arm of trial NCT00779389 (n=15) or had no available clinical information (n=11). There were no CTCAE grade 3 or greater adverse events following neoadjuvant therapy and all patients underwent curative-intent surgery following targeted therapy. Median age of patients was 58 (range 31–93) years old with a median follow-up of 56.5 months. 76 patients had clinical stage III/IV disease with 50% (46/92) oral cavity, 24% (22/92) larynx/hypopharynx and 26% (24/92) oropharynx primaries (Table 1). Seventy (76%) patients received adjuvant therapy with 31 (34%) patients requiring chemoradiation.
Table 1 –
Patient characteristics of pooled cohort
| Patient Characteristic | Downstage (n=18) |
Upstage (n=9) | No change (n=65) |
P-Value |
|---|---|---|---|---|
| Age at NST start, mean (SD) | 56 (±14) | 58 (±9.7) | 59 (±10) | P=0.725 |
| Sex, male (%) | 14 (78) | 8 (89) | 43 (66) | P=0.120 |
| Smoking history >10 PYs, (%) | 14 (78) | 6 (67) | 38 (58) | P=0.728 |
| Primary site (%) | ||||
| Oral Cavity | 11 (61) | 4 (44) | 31 (48) | P=0.200 |
| Oropharynx | 6 (33) | 1 (11) | 17 (26) | |
| Larynx/Hypopharynx | 1 (6) | 4 (44) | 17 (26) | |
| p16 status (for oropharynx primary) | 3 (50) | 1 (100) | 12 (71) | P=0.389 |
| cT classification * | ||||
| cT1/T2 | 8 (44) | 6 (67) | 29 (45) | P=0.414 |
| cT3/T4 | 10 (64) | 3 (33) | 36 (55) | |
| cN classification * | ||||
| cN0 | 6 (33) | 5 (56) | 25 (38) | P=0.509 |
| cN+ | 12 (67) | 4 (44) | 40 (62) | |
| Clinical stage * | ||||
| Stage I/II | 3 (17) | 4 (44) | 9 (14) | P=0.067 |
| Stage III/IV | 15 (83) | 5 (55) | 56 (86) | |
| Neoadjuvant systemic therapy | ||||
| Erlotinib/Dasatinib (NCT00779389) | 8 (43) | 8 (80) | 27 (42) | P=0.350 |
| Cetuximab (NCT01218048) | 9 (47) | 1 (10) | 30 (46) | |
| KTN3379-Anti ErbB3 (NCT02473731) | 1 (5) | 0 | 8 (12) | |
| Margin Status, positive (%) | 0 | 1 (11) | 7 (11) | P=0.354 |
| Extranodal Extension | 1 (6) | 3 (33) | 18 (28) | P=0.101 |
| Adjuvant therapy | ||||
| None | 7 (39) | 1 (11) | 16 (25) | P=0.060 |
| Radiotherapy | 10 (56) | 3 (33) | 25 (38) | |
| Chemoradiotherapy | 1 (6) | 5 (56) | 24 (37) |
NST: Neoadjuvant systemic treatment.
AJCC 7th Edition. cN+: clinical lymph node positive. PY: Pack-years
Clinical to pathological stage migration
Clinical to pathologic downstaging occurred in 20% (18/92) of patients, with six patients no longer meeting criteria for adjuvant treatment and were subsequently observed. In all 18 patients who had clinical to pathologic downstaging, there was evidence of pathological treatment effect. Nine patients showed pathological upstaging. Of the nine patients with pathologic upstaging, 6 patients had recurrent cancer after completion of primary treatment and eventually succumbed to the disease within 2 years of treatment. Clinical to pathologic downstaging in HNSCC was more frequent in patients undergoing neoadjuvant targeted therapy (18/92) compared to a historic cohort from the University of Pittsburgh Medical Center without neoadjuvant therapy (4/49, Chi-squared test P=0.048, Supplemental Table 1) (15) and a recent cohort of 100 consecutive patients with HNSCC without neoadjuvant therapy undergoing upfront surgery (3/100, Chi-squared test P=0.0002, Supplemental Table 2 and 3). Comparison of pathologic upstage migration between the current pooled cohort (8/92) and a historic one showed no statistical difference (7/49, Chi-squared test P=0.20). A significant difference in clinical to pathologic upstaging was noted in patients undergoing upfront surgery compared to those in neoadjuvant targeted therapy trial (32/100 vs 9/92, P=0.0002, respectively).
Prognostic factors and overall survival
The 5-year OS for all patients undergoing neoadjuvant targeted therapy was 53.1% (95% CI of 39.9–66.3, Figure 1A). Patients with advanced age, clinical stage III/IV, non-oropharynx site or extranodal extension had poorer OS on univariate analysis (Table 2). Patients with a pathologic downstage migration (89.5%, 95% CI 75.7–100) had the highest OS compared to those with no change (58%, 95% CI 46.2–69.8) or upstage (40%, 95% CI 9.6–70.4, P=0.003). On multivariable analysis, downstage migration remained a positive prognostic variable with a hazard ratio of 0.22 (95% CI 0.05–0.90) while adjusting for measured confounders (age, clinical stage, primary tumor site, p16 status and extranodal extension, Table 2). All six patients with pathologic downstaging who were subsequently observed (i.e. did not receive adjuvant therapy) were alive and disease free 5 years from diagnosis of HNSCC.
Figure 1 – Kaplan-Meier Curves by Pathologic Stage Migration:
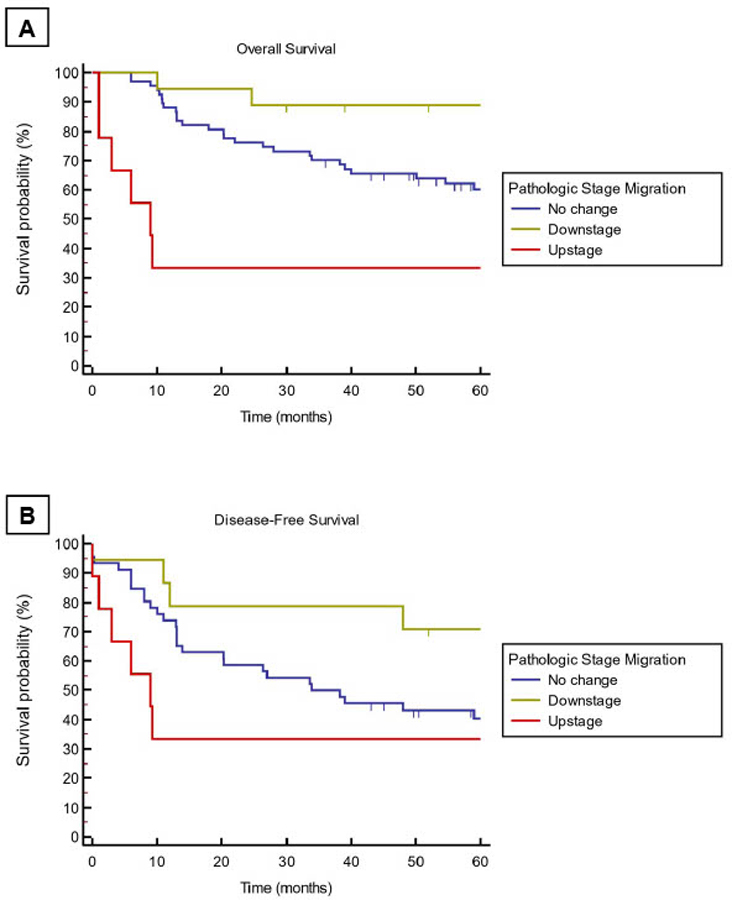
Unadjusted Kaplan-Meier curves for overall survival (A) and disease-free survival (B) stratified by pathologic stage migration. Logrank tests for Kaplan-Meier curves A is P=0.0013 and B is P=0.035.
Table 2 –
Association of prognostic factors and overall survival
| Risk Factor | Univariable HR | Multivariable HR |
|---|---|---|
| Age | 1.04 (1.01–1.08) | 1.05 (1.02–1.09) |
| Sex, male | 0.89 (0.46–1.71) | - |
| Smoking history >10 PY | 0.71 (0.34–1.45) | - |
| Clinical stage* | ||
| Stage I/II | REF | REF |
| Stage III/IV | 1.80 (0.70–4.59) | 3.39 (0.62–18.3) |
| P16 status | 0.21 (0.05–0.89) | 0.20 (0.03–1.17) |
| Stage Migration | ||
| No change | REF | REF |
| Downstage | 0.18 (0.04–0.75) | 0.25 (0.06–0.98) |
| Upstage | 2.19 (0.92–5.24) | 1.03 (0.23–4.70) |
| Site | ||
| Oral Cavity | REF | REF |
| Oropharynx | 0.37 (0.14–0.98) | 1.05 (0.30–3.72) |
| Larynx/Hypopharynx | 1.29 (0.66–2.51) | 1.11 (0.50–2.48) |
| Extranodal extension | 1.94 (0.97–3.92) | 2.08 (0.96–4.50) |
| Margin status, positive | 1.65 (0.58–4.72) | - |
REF: Reference. HR: Hazard Ratio.
AJCC 7th Edition. PY: Pack-year
Clinical factors and disease-free survival
The five-year DFS in the pooled cohort was 48.0% (95% CI 35.7–60.3). When stratified by pathologic stage migration, patients who experienced pathologic downstaging, had a DFS of 84.4% (95% CI 64.0–100) compared with 40.0% (95% CI 9.6–70.4) for upstaged patients and 41.4% (95% CI 26.5–56.3) for patients with no stage change (P=0.04, Figure 1B). When adjusted for age, clinical stage, primary site p16 status and extranodal extension, downstage migration showed a hazard ratio of 0.12 (0.01–0.95) for DFS (Table 3). In multivariate analysis, pathological upstaging did not impact survival in a statistically significant fashion. Adjusted OS and DFS survival curves are illustrated in Figure 2A, B, respectively. There was no statistical association between individual clinical trials and OS or DFS. Testing of Schoenfeld residuals of the Cox models did not show statistically significant differences globally as well as for covariates confirming the proportional hazard assumptions. No significant difference in OS and DFS amongst patients treated with the various clinical trials.
Table 3 –
Association of clinical factors and disease-free survival
| Risk Factor | Univariable HR | Multivariable HR |
|---|---|---|
| Age | 1.04 (1.01–1.07) | 1.03 (0.99–1.08) |
| Sex, male | 0.96 (0.51–1.80) | - |
| Smoking history >10 PY | 0.99 (0.50–1.96) | - |
| Clinical stage* | ||
| Stage I/II | REF | REF |
| Stage III/IV | 1.21 (0.42–3.60) | 1.68 (0.53–5.38) |
| P16 status | 0.28 (0.07–1.14) | 0.15 (0.03–0.64) |
| Stage Migration | ||
| No change | REF | REF |
| Downstage | 0.39 (0.15–0.99) | 0.12 (0.01–0.95) |
| Upstage | 1.20 (0.50–2.85) | 3.25 (0.05–199) |
| Site | ||
| Oral Cavity | REF | - |
| Oropharynx | 0.52 (0.21–1.27) | - |
| Larynx/Hypopharynx | 1.19 (0.62–2.26) | - |
| Extranodal extension | 1.53 (0.78–3.02) | 1.82 (0.79–4.22) |
| Margin status, positive | 1.64 (0.58–4.66) | - |
REF: Reference. HR: Hazard Ratio.
AJCC 7th Edition. PY: Pack-year
Figure 2 – Adjusted Cox Proportional Hazards Multivariate Model of Survival by Pathologic Stage Migration:
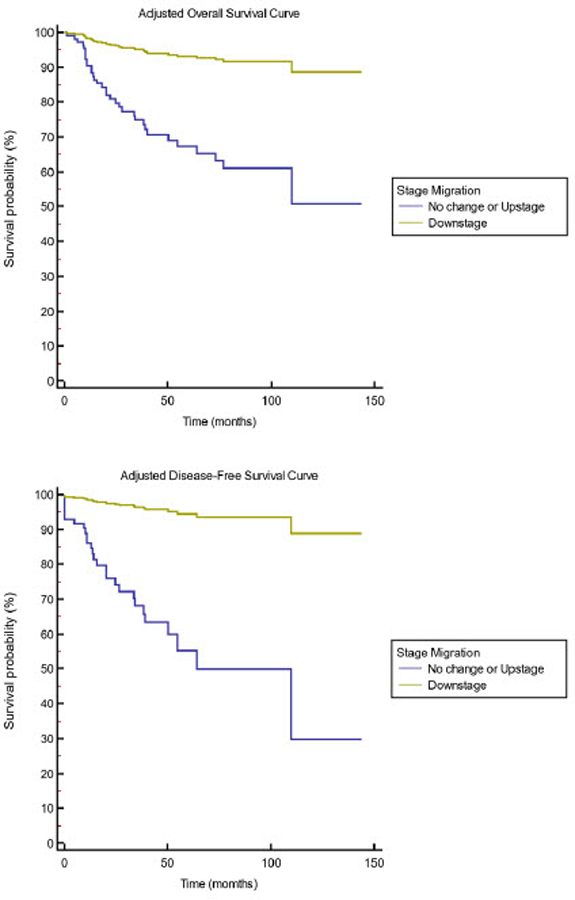
Adjusted overall survival (A) and disease-free survival (B) stratified by pathologic stage migration in the pooled cohort. Multivariable Cox regression survival curves A is P=0.005 and B is P=0.038. Adjustment for age, clinical stage, p16 status, extranodal extension and tumor location.
Pathologic stage migration correlates with circulating methylated DNA
We detected CDO1, SOX17, TAC1, but not HOXA7 in plasma obtained from HNSCC patients (Supplemental Figure 2A, B). Plasma SOX17 and TAC1, but not CDO1, appeared differentially expressed between nonresponders (patients with no stage change or pathologic upstage migration) and responders (patients with pathologic downstage migration) (Supplemental Figure 2C–E) and were selected as biomarkers to monitor response to neoadjuvant targeted therapy. Though not statistically significant, there was a trend towards elevated SOX17 (baseline 0.0036±0.002 vs pre-surgery 0.005±0.003) and TAC1 (baseline 0.037±0.02 vs pre-surgery 0.087±0.07) levels in plasma of nonresponders (P=0.5, Figure 3A). In contrast, we observed marked reduction in plasma SOX17 (baseline 0.0026±0.003 vs pre-surgery 3.14e-021±1.3e-021) and TAC1 (baseline 0.027±0.016 vs pre-surgery 0.01±0.016) levels in responders (P=0.0078, Figure 3B). Collectively, these findings indicate that pathologic response to neoadjuvant targeted therapy correlates with circulating levels of methylated SOX17 and TAC1.
Figure 3 – Pathologic stage migration correlates with circulating methylated DNA:
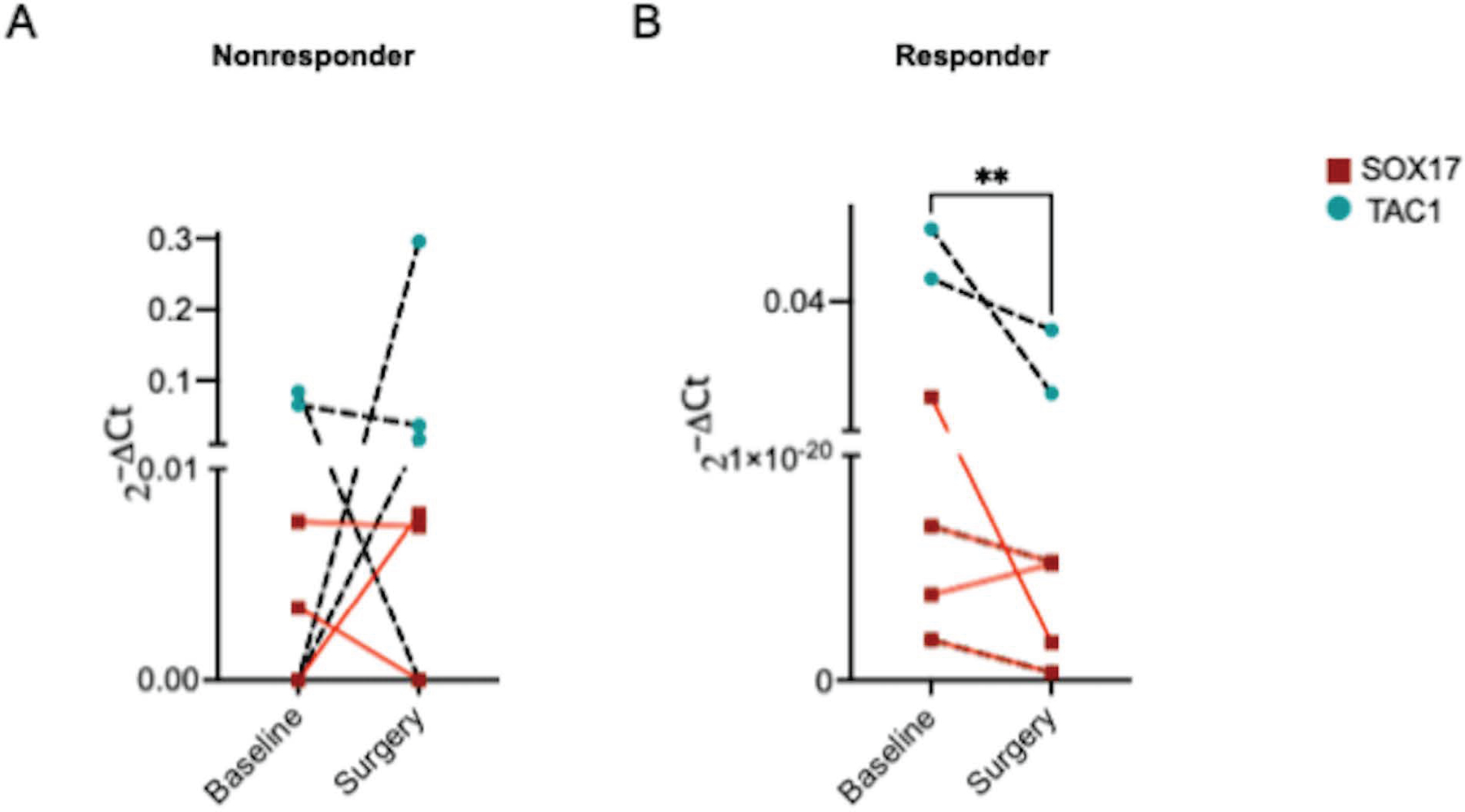
Methylated SOX17 and TAC1 in plasma obtained pre-intervention (baseline) and post-neoadjuvant therapy immediately prior to surgery. n = 4 nonresponders, 4 responders. P < 0.01 by Wilcoxon signed-rank test.
DISCUSSION
In this pooled analysis of three window of opportunity clinical trials evaluating a brief course of neoadjuvant targeted therapy for treatment-naïve patients with operable HNSCC, all patients were able to undergo curative-intent surgery within the timeframe prescribed by the respective trial. There were no CTCAE grade 3 or greater treatment-related toxicities following neoadjuvant targeted therapy and 20% of patients had pathological downstaging following extirpative surgery. Pathologic downstage migration was significantly associated with improved overall and disease-free survival in the multivariate analysis. Patient with pathologic upstaging tended to have worse OS and DFS; however, this did not reach statistical significance. While there can be significant discordance between clinical and pathologic stage categorization, the median survival for clinical and pathologic staging has been shown to be comparable with overlapping confidence intervals and either clinical or pathologic stage can be interchangeably used as tools for prognostication (17).
Furthermore, we demonstrate that pathologic stage migration can be monitored with circulating tumor DNA markers, SOX17 and TAC1. SOX17 promoter methylation in plasma DNA has been shown to have diagnostic or prognostic utility in breast cancer (18), gastric cancer (19), and lung cancer (16). To our knowledge, this is the first study demonstrating its potential utility as a biomarker in HNSCC. Though not as robustly studied, TAC1 hypermethylation has been detected in plasma DNA obtained from patients with esophageal cancer (20) and lung cancer (16). Here, we show that circulating methylated TAC1 is detected in HNSCC and plasma TAC1 levels correlate with therapeutic response.
This is the first study to report bioselection using a brief course of neoadjuvant targeted therapy prior to extirpative surgery for HNSCC in a large cohort of patients. Several studies have shown significant pathologic response to neoadjuvant cisplatin, fluorouracil and taxotere in patients with HPV-associated oropharynx cancer, up to 50% pathologic response following two-to-three cycles of chemotherapy in certain studies (5–7). In comparison, neoadjuvant pembrolizumab showed a pathologic response rate of 34–44% (21,22); however, this was after a 6–8 week window (21,22). Importantly, patients with a pathologic response to immunotherapy also had improved 1-year DFS (21,22). Furthermore, when compared to chemotherapy, neoadjuvant targeted therapy is better tolerated with fewer significant adverse events (11–14). This may enhance completion of prescribed treatment regimens with fewer delays associated with treatment toxicity. That said, any pathologic response to neoadjuvant systemic therapy may be associated with improved outcomes in patients with HNSCC. The underlying commonality by which neoadjuvant systemic agents produce a pathologic response and improve survival may be a mechanism of identifying tumors with biologically less aggressive features. The hiatus between HNSCC diagnosis and definitive surgery can be exploited in a short trial of systemic therapy. This allows the treatment team to prepare patients for surgical therapy through nutritional supplementation, prehabilitation, surgical planning and medical optimization during the window (23–29).
This study demonstrates the utility of a neoadjuvant targeted therapy followed by surgery in patients with HNSCC. With just a small window of targeted therapy, we observed pathologic response and subsequent improvement in survival, indicating that pathologic downstage migration following neoadjuvant systemic therapy can serve as a surrogate marker for treatment response. Neoadjuvant bioselection, using pathologic response and circulating tumor DNA biomarkers, can allow for individualization of cancer treatment plans. In the current study, six patients were observed after response to neoadjuvant therapy and extirpative surgery based on multidisciplinary team consensus. These six patients were disease-free at 5 years following treatment. One concern about window of opportunity trials is the potential for progression from a delay of definitive surgery while on systemic therapy. However, we found that pathologic upstaging occurred less frequently in operable HNSCC with neoadjuvant therapy than in previous institutional data (15). Moreover, the therapeutic period for neoadjuvant therapy administered in window trials is often much shorter than traditional neoadjuvant protocols. Another important difference compared to neoadjuvant immunotherapy trials is that given the short window for administration which is enough to show bioselection, there are essentially no delays to definitive surgery. Further investigations are necessary to evaluate survival outcomes in patients who respond to systemic therapy as seen in neoadjuvant trials in operable melanoma (30).
A pooled analysis of patients receiving neoadjuvant systemic therapy has limitations attributed to clinical trial heterogeneity and inclusion of patients in the trial. Our study was also limited by a small sample size in evaluating circulating tumor DNA markers. Given the ad-hoc analysis, external validation is required. Measurable confounding variables including age, sex, smoking history, clinical stage, adverse pathologic features and p16 status were adjusted for in multivariable Cox regression models. It is intuitively reasonable to hypothesize that patients with pathologic downstage migration following neoadjuvant therapy and extirpative surgery have improved OS and DFS. Moreover, patients enrolled in a window trial showed an almost tenfold reduction in clinical to pathologic upstaging compared to patients undergoing upfront surgery. It is more challenging to comment on the underlying mechanisms at work in patients with pathologic upstaging as they portend a poorer prognosis. It is possible this represents more aggressive tumor biology rather than tumor progression during the 28-day delay of definitive therapy. Consequently, these patients may ultimately benefit from treatment escalation. Validation of pathological stage migration in patients undergoing neoadjuvant targeted therapy with an external cohort is necessary to confirm the study results.
Neoadjuvant targeted therapy in patients with operable HNSCC exploits the preoperative window and can allow for individualization of cancer care based on treatment response. Even with a short treatment window, pathologic downstaging was achieved and was independently associated with significantly improved overall and disease-free survival as well as decreased circulating tumor DNA markers SOX17 and TAC1. Notwithstanding the need for external validation, it is conceivable that a novel standard-of-care paradigm utilizing neoadjuvant targeted therapy be a viable treatment option in patients with HNSCC.
Supplementary Material
Funding:
US Department of Veterans Affairs, National Institute of Dental and Craniofacial Research (NIDCR)
Footnotes
Conflict of interest: The authors declare no potential conflicts of interest.
References
- 1.Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suh JD, Sercarz JA, Abemayor E, Calcaterra TC, Rawnsley JD, Alam D, et al. Analysis of outcome and complications in 400 cases of microvascular head and neck reconstruction. Arch Otolaryngol Head Neck Surg 2004;130:962–6. [DOI] [PubMed] [Google Scholar]
- 3.L’Esperance HE, Kallogjeri D, Yousaf S, Piccirillo JF, Rich JT. Prediction of mortality and morbidity in head and neck cancer patients 80 years of age and older undergoing surgery. Laryngoscope. 2018;128:871–7. [DOI] [PubMed] [Google Scholar]
- 4.Adams P, Ghanem T, Stachler R, Hall F, Velanovich V, Rubinfeld I. Frailty as a predictor of morbidity and mortality in inpatient head and neck surgery. JAMA Otolaryngol Head Neck Surg 2013;139:783–9. [DOI] [PubMed] [Google Scholar]
- 5.Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 2007;357:1705–15. [DOI] [PubMed] [Google Scholar]
- 6.Mirghani H, Amen F, Blanchard P, Moreau F, Guigay J, Hartl DM, et al. Treatment de-escalation in HPV-positive oropharyngeal carcinoma: ongoing trials, critical issues and perspectives. Int J Cancer. 2015;136:1494–503. [DOI] [PubMed] [Google Scholar]
- 7.Masterson L, Moualed D, Liu ZW, Howard JEF, Dwivedi RC, Tysome JR, et al. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma: a systematic review and meta-analysis of current clinical trials. Eur J Cancer. 2014;50:2636–48. [DOI] [PubMed] [Google Scholar]
- 8.Zorat PL, Paccagnella A, Cavaniglia G, Loreggian L, Gava A, Mione CA, et al. Randomized phase III trial of neoadjuvant chemotherapy in head and neck cancer: 10-year follow-up. J Natl Cancer Inst 2004;96:1714–7. [DOI] [PubMed] [Google Scholar]
- 9.Cohen EEW, Karrison TG, Kocherginsky M, Mueller J, Egan R, Huang CH, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol 2014;32:2735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haddad R, O’Neill A, Rabinowits G, Tishler R, Khuri F, Adkins D, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol 2013;14:257–64. [DOI] [PubMed] [Google Scholar]
- 11.Duvvuri U, George J, Kim S, Alvarado D, Neumeister VM, Chenna A, et al. Molecular and Clinical Activity of CDX-3379, an Anti-ErbB3 Monoclonal Antibody, in Head and Neck Squamous Cell Carcinoma Patients. Clin Cancer Res 2019;25:5752–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauman JE, Duvvuri U, Gooding WE, Rath TJ, Gross ND, Song J, et al. Randomized, placebo-controlled window trial of EGFR, Src, or combined blockade in head and neck cancer. JCI Insight 2017;2:e90449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross ND, Bauman JE, Gooding WE, Denq W, Thomas SM, Wang L, et al. Erlotinib, erlotinib-sulindac versus placebo: a randomized, double-blind, placebo-controlled window trial in operable head and neck cancer. Clin Cancer Res 2014;20:3289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferris RL, Kim S, Trivedi S, Srivastava RM, Concha-Benavente F, Heron DE, et al. Correlation of anti-tumor adaptive immunity with clinical response in a phase II “Window” trial of neoadjuvant cetuximab in patients with resectable stage III-IV head and neck squamous carcinoma (HNSCC). JCO 2016;34:6060–6060. [Google Scholar]
- 15.Walvekar RR, Li RJ, Gooding WE, Gibson MK, Heron D, Johnson JT, et al. Role of surgery in limited (T1–2, N0–1) cancers of the oropharynx. Laryngoscope. 2008;118:2129–34. [DOI] [PubMed] [Google Scholar]
- 16.Hulbert A, Jusue-Torres I, Stark A, Chen C, Rodgers K, Lee B, et al. Early detection of lung cancer using DNA promoter hypermethylation in plasma and sputum. Clin Cancer Res 2017;23:1998–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch WM, Ridge JA, Forastiere A, Manola J. Comparison of clinical and pathological staging in head and neck squamous cell carcinoma: results from Intergroup Study ECOG 4393/RTOG 9614. Arch Otolaryngol Head Neck Surg 2009;135:851–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chimonidou M, Strati A, Malamos N, Georgoulias V, Lianidou ES. SOX17 promoter methylation in circulating tumor cells and matched cell-free DNA isolated from plasma of patients with breast cancer. Clin Chem 2013;59:270–9. [DOI] [PubMed] [Google Scholar]
- 19.Balgkouranidou I, Karayiannakis A, Matthaios D, Bolanaki H, Tripsianis G, Tentes AA, et al. Assessment of SOX17 DNA methylation in cell free DNA from patients with operable gastric cancer. Association with prognostic variables and survival. Clin Chem Lab Med 2013;51:1505–10. [DOI] [PubMed] [Google Scholar]
- 20.Jin Z, Olaru A, Yang J, Sato F, Cheng Y, Kan T, et al. Hypermethylation of tachykinin-1 is a potential biomarker in human esophageal cancer. Clin Cancer Res 2007;13:6293–300. [DOI] [PubMed] [Google Scholar]
- 21.Uppaluri R, Chernock R, Mansour M, Jackson R, Rich J, Pipkorn P, et al. Enhanced pathologic tumor response with two cycles of neoadjuvant pembrolizumab in surgically resectable, locally advanced HPV-negative head and neck squamous cell carcinoma (HNSCC). JCO 2021;39:6008–6008. [Google Scholar]
- 22.Wise-Draper TM, Takiar V, Mierzwa ML, Casper K, Palackdharry S, Worden FP, et al. Association of pathological response to neoadjuvant pembrolizumab with tumor PD-L1 expression and high disease-free survival (DFS) in patients with resectable, local-regionally advanced, head and neck squamous cell carcinoma (HNSCC). JCO 2021;39:6006–6006. [Google Scholar]
- 23.Menzies AM, Amaria RN, Rozeman EA, Huang AC, Tetzlaff MT, van de Wiel BA, et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat Med 2021;27:301–9. [DOI] [PubMed] [Google Scholar]
- 24.Cramer JD, Patel UA, Samant S, Smith SS. Postoperative complications in elderly patients undergoing head and neck surgery: opportunities for quality improvement. Otolaryngol Head Neck Surg 2016;154:518–26. [DOI] [PubMed] [Google Scholar]
- 25.Carli F, Charlebois P, Stein B, Feldman L, Zavorsky G, Kim DJ, et al. Randomized clinical trial of prehabilitation in colorectal surgery. Br J Surg 2010;97:1187–97. [DOI] [PubMed] [Google Scholar]
- 26.Twomey R, Culos-Reed SN, Dort JC. Exercise Prehabilitation-Supporting Recovery From Major Head and Neck Cancer Surgery. JAMA Otolaryngol Head Neck Surg 2020;146:689–90. [DOI] [PubMed] [Google Scholar]
- 27.Loewen I, Jeffery CC, Rieger J, Constantinescu G. Prehabilitation in head and neck cancer patients: a literature review. J Otolaryngol Head Neck Surg 2021;50:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paccagnella A, Morello M, Da Mosto MC, Baruffi C, Marcon ML, Gava A, et al. Early nutritional intervention improves treatment tolerance and outcomes in head and neck cancer patients undergoing concurrent chemoradiotherapy. Support Care Cancer. 2010;18:837–45. [DOI] [PubMed] [Google Scholar]
- 29.Swartz JE, Pothen AJ, Wegner I, Smid EJ, Swart KMA, de Bree R, et al. Feasibility of using head and neck CT imaging to assess skeletal muscle mass in head and neck cancer patients. Oral Oncol 2016;62:28–33. [DOI] [PubMed] [Google Scholar]
- 30.Dort JC, Farwell DG, Findlay M, Huber GF, Kerr P, Shea-Budgell MA, et al. Optimal perioperative care in major head and neck cancer surgery with free flap reconstruction: A consensus review and recommendations from the enhanced recovery after surgery society. JAMA Otolaryngol Head Neck Surg 2017;143:292–303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available upon request from the corresponding author.


