Abstract
Spinal cord injury (SCI) is a complex tissue injury that results in a wide range of physical deficits, including permanent or progressive disabilities of sensory, motor and autonomic functions. To date, limitations in current clinical treatment options can leave SCI patients with lifelong disabilities. There is an urgent need to develop new therapies for reconstructing the damaged spinal cord neuron-glia network and restoring connectivity with the supraspinal pathways. Neural stem cells (NSCs) possess the ability to self-renew and differentiate into neurons and neuroglia, including oligodendrocytes, which are cells responsible for the formation and maintenance of the myelin sheath and the regeneration of demyelinated axons. For these properties, NSCs are considered to be a promising cell source for rebuilding damaged neural circuits and promoting myelin regeneration. Over the past decade, transplantation of NSCs has been extensively tested in a variety of preclinical models of SCI. This review aims to highlight the pathophysiology of SCI and promote the understanding of the role of NSCs in SCI repair therapy and the current advances in pathological mechanism, pre-clinical studies, as well as clinical trials of SCI via NSC transplantation therapeutic strategy. Understanding and mastering these frontier updates will pave the way for establishing novel therapeutic strategies to improve the quality of recovery from SCI.
Keywords: Spinal cord injury, Neural stem cells, Oligodendrocytes, Myelin regeneration, SCI repair therapy, NSC transplantation
Introduction of the current status of spinal cord injury (SCI)
Spinal cord injury (SCI) is a destructive neurological and pathological condition that leads to a loss of motor, sensory and autonomic functions below the injury site. The permanent or progressive disabilities it brings can have a devastating impact on individuals and a significant burden on the society [1–3]. Most common causes for traumatic SCI are traffic accidents, falls, sports injuries and violence. For the past 30 years (from 1990 to 2019), the incidence of SCI was 0.9 million cases with an estimated 6.2 million cases lived with disability. SCI rates increased substantially for global prevalence (from 74.2 to 87.1%), incidence (from 30.3 to 69.8%) and cases lived with disability (from 56.3 to 76.0%), basing on the data from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019 [4]. Despite considerable strides in trauma management and medical care, available treatments can only provide supportive relief for patients with lifelong disabilities since the lack of effective treatment options for this devastating disease [5–7].
Pathophysiology of SCI
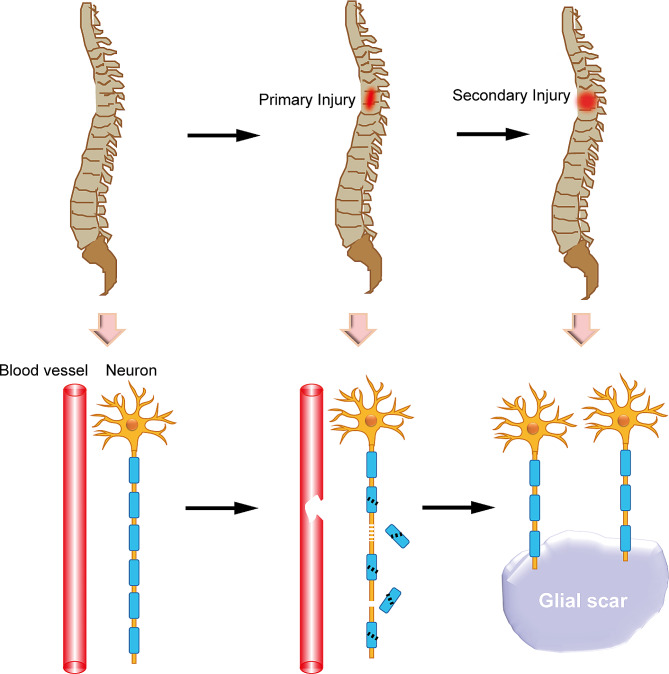
SCI can be divided into primary injury and secondary injury according to the injury time and pathophysiologic changes [8, 9]. The initial stage is known as primary injury, which commonly occurs immediately after the injury caused by a sudden trauma, and is accompanied with bone fragments and tear of spinal ligaments. The secondary injury triggered by the primary injury, which produces further chemical and mechanical damage to spinal tissues, involving gliosis, glial scar, inflammation, demyelination and other reversible pathologic changes (Fig. 1) [2, 10].
Fig. 1.
Primary and secondary injuries following the spinal cord injury. Primary injury results in disruption of the myelin sheath in the spinal cord, bone fragments and tear of spinal ligaments; secondary injury activates the inflammatory cascade, forms glial scars, and inhibits injury repair
The SCI pathophysiology of secondary injury comprises a complex cascade of interrelated events at the molecular, cellular and systemic levels [11, 12]. Injuries caused by trauma or other factors disrupt the structure of the spinal cord, leading to a breakdown of the blood-spinal cord barrier, and initiating an inflammatory response [13].
At the molecular level, the release of inflammatory cytokines, including interleukin (IL)-1a, IL-1b, IL-6, and tumor necrosis factor (TNF), recruits immune cells to the injury site and initiates the inflammatory cascade. This inflammatory response leads to further damage and cell death within the spinal cord [14, 15].
At the cellular level, disruption of blood vessels causes bleeding in spinal cord tissues, followed by invasion of neutrophils, monocytes, macrophages, T and B lymphocytic cells [14, 16]. In addition, glial cells involved in gliosis or the formation of glial scars undergo morphological and functional changes following SCI. Gliosis refers to the reactive response of astrocytes, microglia and other glial cells, to central nervous system (CNS) injury, which can include SCI, trauma, stroke, or neurodegenerative diseases [17, 18]. This response is characterized by the proliferation and hypertrophy of these cells, along with altered gene expression, which can lead to the formation of a glial scar. The glial scar is a complex structure composed of various cell types, including reactive astrocytes, NG2 glia, fibroblasts, microglia, etc. [19, 20]. The glial scar has both beneficial and detrimental effects on CNS repair. On the one hand, it can act as a physical barrier to wall off the injury site, preventing the spread of toxic molecules and curtailing inflammation, which is crucial for the initial stages of wound healing. Reactive astrocytes within the glial scar also secrete growth factors and cytokines that can support survival and regeneration of certain neurons [21]. On the other hand, the glial scar poses significant challenges to axonal regeneration and can contribute to demyelination. Key factors that limit regeneration include physical barrier, chemical inhibition, inflammatory environment, and oligodendrocyte dysfunction [22–25]. Specifically, the dense network of glial cells and extracellular matrix (ECM) proteins, such as chondroitin sulfate proteoglycans (CSPGs), can physically impede the growth of axons, which is critical for restoring neural connections after injury [21, 22]. CSPGs and other molecules within the ECM can release inhibitory signals that actively suppress axonal growth and neurite extension. These molecules can interact with neuronal cell surface receptors, such as the Nogo receptor, to suppress growth signals [23]. Prolonged activation of microglia and the release of pro-inflammatory cytokines can create a hostile environment for axonal growth and contribute to further demyelination and neuronal damage [24]. Additionally, glial scar formation can adversely affect the function of oligodendrocyte precursor cells (OPCs), which are responsible for myelination. This disruption can result in demyelination, which in turn can compromise axonal conduction and further deteriorate neuronal function [20, 25]. Moreover, neurons undergo Wallerian degeneration, characterized by axonal swelling and cytoskeletal rearrangements, leading to the breakdown and degeneration of axons and their innervation structures [26, 27].
At the systems level, SCI results in the disconnection of surviving neural elements, dysfunction of the neural circuits and loss of motor, sensory and autonomic function. The poor axon growth ability and inhibitory factors of scar axon regeneration leads to the failure in regeneration of spinal cord and reconstruction of neural circuits [28, 29]. Therefore, therapies primarily focus on how to promote axon regeneration, axon myelination and neural circuits reconstructing.
The basic characteristics of neural stem cells (NSCs)
Neural stem cells (NSCs) are present in all major subdivisions of the CNS in adult mammals, including the spinal cord [30–32]. NSCs are essential for the maintenance of CNS functions during development and the regeneration of all CNS cell populations [33, 34]. They have indefinite self-renewal capacity and the ability to give rise to neural progenitor cells (NPCs). Additionally, they have the potential to continue developing into mature neural lineages, such as neurons, astrocytes and oligodendrocytes [35–37].
NSCs reside along the axis of CNS. The two major active brain regions for regenerating NSCs are the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the hippocampal dentate gyrus [38, 39]. In addition, the fourth ventricle [40] and the central canal ependyma of the spinal cord also harbors endogenous populations of NSCs, which have the potential to generate neural lineages [41].
Mature oligodendrocytes from NSCs are essential for myelination
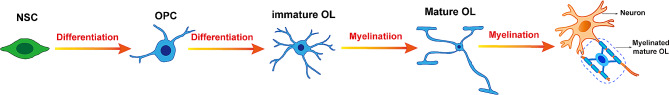
Oligodendrocytes are one of the major glial cell types in the CNS besides astrocytes. During neural development, oligodendrocytes originate from NSCs and undergo a series of differentiation process to achieve a mature phenotype [42–45]. The maturation of oligodendrocytes is a prerequisite for the production and maintenance of the myelin sheath, which is a lipid-rich substance that wraps around axons to provide electrical insulation and support, thus highlighting the importance of oligodendrocyte lineage development for myelination (Fig. 2) [46–49]. Myelin is essential for the rapid transmission of electrical signals along axons and also provides trophic support to neurons [50–52]. Oligodendrocytes are distributed throughout the CNS, including the brain and spinal cord. They are critical for the normal functioning of the nervous system [53, 54]. The loss or damage of oligodendrocytes can lead to demyelination and neurodegeneration, causing various CNS diseases such as multiple sclerosis, acute disseminated encephalomyelitis, and other demyelinating diseases [55–57].
Fig. 2.
Schematic of oligodendroglial lineage depicting different developmental stages from neural stem cells (NSCs) to myelinate mature oligodendrocytes (OLs). Oligodendrocyte progenitor cells (OPCs) are capable to differentiate into immature oligodendrocytes (immature OLs), and then myelinate into mature OLs. The newly formed myelin wraps around axons, thus creating a new myelin sheath
Myelin regeneration process
SCI often results in damage to the myelin sheath, the insulating layer that surrounds nerve fibers and effectively transmits electrical signals [58]. Therefore, myelin regeneration is significant for the recovery process following SCI. Remyelination is the myelin regenerative response that follows demyelination, which restores saltatory conduction and function, maintains axon health, and is essential for the recovery from demyelinating diseases and injuries [59, 60]. NSC-derived oligodendrocytes have been shown to integrate into existing neural networks and to remyelinate damaged nerve fibers [61]. It is a complex process that involves the recruitment of NSC-derived oligodendrocyte precursor cells (OPCs) to the site of demyelination, their differentiation into mature oligodendrocytes and the formation of new myelin sheaths around axons [62, 63]. This process is controlled by a network of signaling molecules [64], transcription factors [65, 66] and microRNAs [67, 68].
Endogenous NSCs neurogenesis after SCI
NSCs play a crucial role in remyelination after SCI. Given the right conditions, endogenous NSC niche in the SCI can be activated and start to proliferate rapidly in response to injuries. The inflammatory response following the injury releases signaling molecules and growth factors, which contribute to the activation and multiplication of NSCs [13, 69]. Subsequently, NSCs, guided by chemokines, growth factors and extracellular matrix, migrate from the NSC niche towards the injury site and the area requiring remyelination [70]. Once reaching the injury site, NSCs differentiate into oligodendrocytes, regulated by several signaling molecules such as Olig1/2 [71, 72], MBP [73, 74], PLP [75, 76] and MAG [77, 78]. The newly formed oligodendrocytes then produce myelin and wrap it around axons, thus creating a new myelin sheath [63]. While the neurogenesis of endogenous NSCs is a complicated and difficult process, considerable advancements are making strides towards leveraging this process for regenerative medicine treatments in SCI [79, 80].
Potential sources of NSCs for transplantation strategies
Transplantation of NSCs has emerged as a potential therapeutic approach for SCI, aiming to replace lost cells, promote tissue repair and restore neural function. However, the identification of suitable sources for NSCs remains a critical issue. There are several potential sources of NSCs that can be used for transplantation strategies in SCI, including adult NSCs, embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs) and fetal neural progenitor cells (FNPCs) [81, 82]. They have the ability to self-renew and differentiate into neurons, astrocytes and oligodendrocytes.
Adult NSCs
Transplantation of adult NSCs has shown beneficial effects in terms of availability, potency, immune rejection, ethical considerations and risk of tumorigenesis [83]. Originally, Reynolds and Weiss succeeded in isolating a population of cells from the striatum of adult mice that had the ability to proliferate and differentiate into neurons and glial cells in vitro, and formally proposed the concept of NSCs [84]. Later, Zhao and colleagues revealed that adult NSCs transplantation improved locomotor function in spinal cord transection rats, which associated with nerve regeneration and IGF-1R expression [85]. Recently, Fauser and colleagues successfully transplanted adult NSCs from midbrain periventricular regions into the hippocampal neurogenic niche [86]. Lv and colleagues evaluated the safety and efficacy of adult NSCs transplantation for cerebral palsy [87].
Embryonic stem cells (ESCs)
ESCs are pluripotent stem cells derived from the inner cell mass of the blastocyst. They have the potential to self-renew and differentiate into any cell type in the body [88–90]. To prepare region-specific NPCs from ESCs for regenerative medicine researches, various in vitro directed methods have been established based on developmental stages that NSCs undergo during neural induction and differentiation in the CNS [81]. Initially, Bain and colleagues performed neural induction of mouse ESCs using embryoid body formation assay, and ESC-derived NSCs could differentiate into neurons capable of generating action potentials after RA treatment [91]. Subsequently, Chambers and colleagues proposed a novel method for generation of NPCs from ESCs by dual SMAD inhibition, which provided a more efficient approach for preparing NPCs from ESCs by remarkedly improving the conversion efficiency of ESCs to neural rosette and reducing the duration for neural induction [92]. Additionally, researchers described a straightforward approach for efficiently generating NSCs from ESCs using simplified medium formulations and procedures, indicating that dynamic changes of cell-substrate matrix interactions through short suspension culture period facilitated ectodermal lineage specification [93].
The differentiation of ESCs into NSCs offers a potential source of NSCs for transplantation strategies in SCI and other CNS disorders. However, ethical concerns and potential immunological rejection limit the use of ESCs in transplantation [94].
Induced pluripotent stem cells (iPSCs)
iPSCs are artificially generated from reprogrammed somatic cells via the induction of pluripotent genes, and share the same developmental pluripotency as ESCs [95]. As such, they resolve the ethical controversy of ESCs, realize autologous cell transplantation and prevent immune rejection [96, 97].
In 2006, Yamanaka’s research team developed the reprograming technology to generate iPSCs from accessible somatic cells, thus ushering in a new era in translational and regenerative medicine [98]. Since similar properties of ESCs and iPSCs, iPSCs can share a similar approach with ESCs to the induction of NSCs. In 2014, D’Aiuto and colleagues reported a scalable protocol that allows robust and cost-effective generation of NSCs from iPSCs [99]. For the past decade, researchers have made great progress in stem cell therapies of iPSC-derived NSCs. Transplanting iPSC-derived NSCs improved CNS functional recovery after SCI in mice [100–105] and rats [106–108], by inhibiting demyelination and promoting synapse formation. In addition, Strnadel and colleagues reported the survival of iPSC-derived NSCs after spinal grafting in minipigs [109].
Fetal neural progenitor cells (FNPCs)
FNPCs are neural progenitor cells derived from fetal tissue that have the ability to self-renew and differentiate into neural lineages. FNPCs have been used in transplantation strategies for SCI with promising results. Studies have shown their positive role in promoting tissue repair and functional recovery of the injured spinal cord [110, 111]. However, there are also potential risks that limit the use of FNPCs in clinical applications, such as immune rejection and ethical concerns surrounding the use of fetal tissue.
Comparative evaluation of various sources of NSCs
In pre-clinical studies, several factors need to be considered when selecting the above sources of NSCs, including differentiation potential, integration and survival abilities, immunogenicity, ethical considerations, availability and scalability, safety and tumorigenicity, and functional outcomes.
Specifically, as for differentiation potential, ESCs and iPSCs are unrivaled, holding the theoretical capacity to differentiate into any cell type within the body, encompassing the full spectrum of neural lineages. Adult NSCs, while more limited in their plasticity, retain the ability to differentiate into a variety of neural cells, such as neurons, astrocytes, and oligodendrocytes. FNPCs exhibit multipotency as well, yet their differentiation potential is more constrained relative to ESCs and iPSCs [81, 111]. Regarding integration and survival abilities, adult NSCs and FNPCs, due to their closer developmental alignment with the host tissue in SCI, tend to integrate more effectively into the neural tissue. In contrast, ESCs and iPSCs necessitate meticulous differentiation to ensure proper integration and to prevent the risk of forming teratomas or other tumors [112]. In terms of immunogenicity, ESCs derived from non-autologous sources pose a higher risk of provoking an immune response, potentially leading to rejection. Autologous iPSCs, generated from an individual’s own cells, significantly mitigate this risk [113, 114]. Similarly, adult NSCs and FNPCs, particularly when autologous, present a reduced likelihood of immune rejection [111, 115]. From the ethical considerations, ESCs are derived from embryos, raising considerable ethical dilemmas [94]. Remarkably, iPSCs avoid these concerns by being generated from somatic cells and reprogrammed to a pluripotent state [96, 97]. Both adult NSCs and FNPCs are generally considered more ethically acceptable; however, it should be noted that FNPCs, derived from fetal tissue, may also engender ethical issues [112]. Concerning availability and scalability, iPSCs present the advantage of being scalable and the ability to be generated from any patient, which is highly promising for personalized medicine [114]. Adult NSCs, while more limited, can be isolated from various regions of the adult brain and expanded in vitro. Conversely, FNPCs face constraints due to the scarcity of available fetal tissue [116]. With respect to safety and tumorigenicity, adult NSCs are generally considered less likely to induce substantial gliosis, ascribed to their closer developmental congruence with the host tissue, while ESCs and iPSCs possess high differentiation potential and thus bear the inherent risk of gliosis if their differentiation process being misguided. ESCs and iPSCs carry a heightened risk of tumor formation and require meticulous differentiation approaches to mitigate this risk [94]. Adult NSCs and FNPCs are generally considered to have a reduced tumorigenic risk, favoring their use in certain applications [115]. With regard to functional outcomes, pre-clinical studies have shown that all of these mentioned cell types can contribute to a certain degree of functional improvement in animal models of SCI, with the magnitude of recovery being variable [85, 100, 110, 117, 118]. Factors such as the specific cell type, the timing and technique of transplantation, and the SCI model used are recognized to impact the outcomes.
Advancements in NSC transplantation therapies for SCI treatment
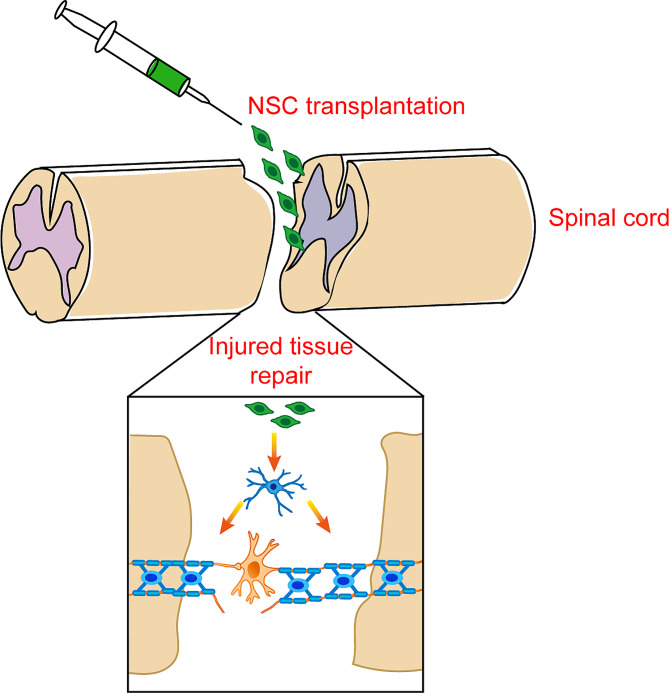
NSC transplantation is a prospective therapeutic strategy for SCI. This approach aims to improve functional recovery by replacing damaged cells, restoring lost neural circuits, modifying SCI environment and promoting endogenous repair (Fig. 3) [119, 120]. With the development of regenerative medicine based on stem cell research, advanced novel strategies and technologies have been implemented in pre-clinical studies, some of which have already been applied in clinical trials [121, 122]. Nowadays, considerable progress has been made in pathological mechanism research, pre-clinical studies and clinical trials.
Fig. 3.
The transplantation of NSCs in the repair and recovery of injured spinal cord. After injecting into the injury site, NSCs rapidly proliferate and differentiate into oligodendrocytes. NSC-derived oligodendrocytes integrate into the destroyed neural networks and remyelinate damaged nerve fibers
Studies on pathological mechanism
In the past few decades, researchers have been attempting to fully elucidate the pathological mechanism of SCI, and to seek for effective strategies to promote axon regeneration and neural circuit remodeling; however, they have not made satisfactory achievements. Recently, single-cell sequencing technology and multi-omics analyses have been widely used in SCI research, providing a broader vision to elucidate pathological mechanisms of SCI [123–126].
A relatively early studies has shown that transplantation of NSCs together with administration of valproic acid dramatically enhanced the restoration of hind limb function, thus raising the possibility that epigenetic status in transplanted NSCs could be manipulated for providing effective treatment for SCI [127]. Another study revealed that NSC transplantation could modulate SCI-induced inflammatory responses and enhance neurological function after SCI via reducing M1 macrophage activation and infiltrating neutrophils [128].
Lately, researchers put forward that LncRNA-GAS5 promoted spinal cord repair and inhibited neuronal apoptosis through the transplantation of 3D-printed scaffold loaded with iPSC-derived NPCs [129]. STAT3 was identified as a target for NSCs to promote neuronal differentiation and functional recovery in rats with SCI [130]. Transplantation of Wnt4-modified NSCs improved inflammatory micro-environment of SCI by mediating M2 polarization [131]. Transplantation of NSC which preconditioned with hypoxia promoted SCI in rats by affecting transmembrane immunoglobulin domain-containing [132].
Pre-clinical studies
Pre-clinical studies have shown that NSC transplantation can effectively promote spinal cord repair [133, 134]. In various animal models, NSCs have been successfully transplanted into injured spinal cords, and the therapeutic potential, safety and technical challenges of NSCs transplantation have been tested under multiple conditions [135–137]. These cells integrate with the host tissue, forming functional synapses and improving motor and sensory function. Pre-clinical studies have also evaluated different sources of NSCs, such as fetal spinal cord, adult brain and iPSCs. The use of iPSCs offers an attractive alternative since they can be derived from patients’ own cells, avoiding ethical issues and immune rejection [137].
In 1999, scientists first demonstrated the therapeutic potential of NSC transplantation in the context of SCI. The grafts, which derived from transplanting NSCs into a rat spinal cord, were observed to have the capacity to survive and differentiate into neurons, oligodendrocytes and astrocytes [138]. Since then, plenty of studies have shown that NSCs can be successfully transplanted into the injured spinal cord, and are beneficial to the functional recovery. Takano and colleagues showed that the neurotrophic factor HGF plays a key role in the enhanced functional recovery after NSC transplantation observed in aged mice with SCI [139]. Zhang and colleagues treated transplanted NSC with LiCl, and promoted the functional recovery in SCI rat [140]. Xue and colleagues found that transplantation of NSCs which preconditioning with high‑mobility group box 1 facilitated functional recovery after SCI in rats [141]. Kong and colleagues showed the effective recovery of acute SCI in mice via transplanting hiPSC-derived NSCs [103]. Ko and colleagues induced cellular differentiation of transplanted NSCs into neurons, and found that transplantation of neuron-inducing grafts positively charged gold nanoparticles for the treatment of SCI [142]. The pre-clinical studies on NSC transplantation therapy in SCI animal models are summarized in Table 1.
Table 1.
Summary of pre-clinical studies on NSC transplantation therapy in SCI Animal models
| Donor cells | Quantity of transplanted cells | Method of cell delivery | Time of transplantation | Outcomes | References |
|---|---|---|---|---|---|
| Human iPSC-derived-NSCs | 1 × 105 | Injected into mouse spinal cord after laminectomy | about 1 month | Promoted functional recovery in mice by replacing missing neurons and attenuating fibrosis, glial scar formation, and inflammation | [103] |
| Fetal rat spinal cord-derived NSCs | 1–4 × 105 | Transplanted into rat spinal cord at 9 days after contusion injury | 5 weeks | Graft neurons extended processes into host tissue and formed synaptic formation with host neurons | [111] |
| Mouse ESCs- derived NSCs | Not mentioned | Transplanted into rat spinal cord 9 days after traumatic injury | 2–5 weeks | Showed hindlimb weight support and partial hindlimb coordination | [138] |
| Mouse striata- derived NSCs | 5 × 105 | Transplanted into mouse spinal cord after laminectomy | 7 weeks | Showed the importance of neurotrophic factor HGF in the enhanced functional recovery | [139] |
| Rat spinal cord-derived NSCs | 1 × 105 | Transplanted into rat spinal cord after injury | 4 weeks | Showed that Lithium promoted survival and neuronal generation of grafted NSCs | [140] |
| Rat neocortices-derived NSCs | 2 × 105 | Injected into rat spinal cord after injury | about 1 month | Showed that preconditioning with high‑mobility group box 1 facilitated functional recovery | [141] |
| Rat spinal cord-derived NSCs | 4 × 105 | Injected into rat spinal cord after contusion injures | 6 weeks | Suggested that neuron-inducing grafts embedding positively charged gold nanoparticles for the treatment of SCI | [142] |
| Fetal mouse spinal cord-derived NSCs | Not mentioned | Injected into mouse spinal cord immediately after dorsal column lesion | 1–3 months | Graft neurons receive synaptic inputs from all host spinal cord tracts | [143] |
| Human embryonic spinal cord-derived NSCs | 2 × 107 | Transplanted into monkey cervical after injury | 2 weeks | Axon regeneration with synapse formation | [144] |
| Rat enteric nervous system-derived NSCs | 1 × 106 | Injected into rat thoracic after drop weight contusion injury | 3 days | Gastrointestinal tract could be a viable option for cell source | [145] |
Clinical trials
Numerous clinical trials on the stem cell treatment of SCI have been conducted worldwide [146–148], of which, several clinical trials are based on NSCs transplantation [149–151]. Due to the limitations of small sample sizes, variable trial designs and short follow-up durations in most studies, longer-term follow-up data are needed to evaluate the long-term safety and efficacy of NSC transplantation in the treatment of SCI [152].
Clinical trials have shown promising results, with some patients experiencing significant improvements in motor function and sensory perception following NSC transplantation. Research published in 2018 reported a first-in-human, phase I study of NSCs transplantation for chronic SCI. Unfortunately, this study is insufficient and debatable due to the lack of a control group and a small number of subjects, yet it paves the way for future research [153].
Several clinical trials have been conducted to evaluate the safety and efficacy of NSC transplantation in patients with SCI. A study published in 2019 showed clinical outcomes from a multi-center study of human NSC transplantation in chronic cervical SCI, aiming at assessing the safety and feasibility of NSC transplants for the treated participants [154]. Another study conducted in 2020 showed that the long-term results of the NSC transplants in spinal cords of 12 participants were reassuring. A six-year follow-up clinical assessment, containing sensory thresholds and neuroimaging augmenting, revealed short- and long-term surgical and medical safety for NSC transplantation therapy [155]. In 2021, researchers conducted a first-in-human clinical trial of transplantation of iPSC-derived NSCs in subacute complete SCI, with assessment of the safety of iPSC-derived NSC transplantation in patients and its impact on neurological function and quality-of-life outcomes [156]. Initial studies have demonstrated the safety of this approach, with no significant adverse events reported, however, results regarding efficacy have been ambiguous. Some studies have reported improvements in motor function and sensation, while others have not observed significant changes. The differences in outcomes may be attributed to factors such as source of transplanted cells, quantity of cells, and the time post-transplantation after injury [122, 157, 158]. The clinical trials on NSC transplantation therapy in SCI treatment are summarized in Table 2.
Table 2.
Summary of clinical trials on NSC transplantation therapy in SCI treatment
| Donor cells | Quantity of transplanted cells | Method of cell delivery | Outcomes | References |
|---|---|---|---|---|
| Human CNS derived-NSCs | 2 × 107 | Transplanted into 29 patients with chronic traumatic SCI | Perilesional intramedullary injections after thoracic and cervical SCI respectively proved safe and feasible | [151] |
| Human spinal cord derived-NSCs | 1.2 × 106 | Injected into chronic spinal trauma patients | NSCs transplanted in the spinal injury site of patients can be performed safely | [153] |
| Human NSCs released by StemCells Inc. | 1.5/3/4 × 107 | Transplanted into three cohorts with chronic SCI | Assessed the safety and feasibility of NSC transplants for the treated participants | [154] |
| Human CNS derived-NSCs | 2 × 107 | Transplanted into phase I/IIa SCI patients | Revealed short- and long-term surgical and medical safety for NSC transplantation therapy | [155] |
| Human iPSC-derived NSCs/NPCs | 2 × 106 | Transplanted into the injured spinal cord parenchyma 14–28 days post-injury of 4 patients | Assessed the safety of iPSC-derived NSC transplantation in patients and its impact on neurological function | [156] |
| Human CNS derived-NSCs | 4 × 107 | Injected into 5 chronic cervical SCI patients > 4 months post-injury | Observed improvements in overall mean functional outcomes measures by 12-month clinical follow-up | [159] |
Despite the promising results of NSC transplantation in SCI treatment, there are still several challenges that need to be addressed. Future clinical trials should focus on optimizing cell sources, transplant techniques, and outcome measures to further enhance the therapeutic potential of NSC transplantation. The identification of appropriate patient populations and the development of combinatorial approaches that combine NSC transplantation with other regenerative strategies may also bring more effective treatment outcomes.
Concluding remarks and future perspectives
In summary, SCI is a complex condition that results in significant functional and anatomical changes within the spinal cord. A comprehensive understanding of the molecular, cellular and systems-level changes following SCI is essential for the development of effective therapeutic strategies to promote neural repair and functional recovery. Despite significant efforts in the field of regenerative medicine, effective treatment options for SCI remain limited. NSCs represent a promising regenerative strategy for SCI treatment due to their ability to differentiate into neurons and glial cells, of which, oligodendrocytes play a crucial role in myelin regeneration, demyelination repair and neural circuit reconstruction. In addition, the trophic factors secreted in these processes can promote tissue repair and functional recovery. Over the past decades, many preclinical studies have shown the improved motor and neural function following successful NSC transplantation in animal models of SCI. And several clinical trials tested the efficacy and safety of NSC transplantation in patients, with delightful results.
For future perspectives, although NSC transplantation holds promise for treating SCI, there are still several challenges that need to be addressed before it can be translated into clinical practice. Challenges include improving cell survival and differentiation rates, ensuring safe and effective delivery methods and uncovering the mechanisms underlying the therapeutic effects of NSC transplantation [160]. Continued studies should focus on extending cell sources, optimizing transplantation strategies and matching pre-clinical animal models with human SCI. In addition to cellular transplantation alone, combinatorial strategies based on NSCs such as co-transplantation therapies, cell delivery methods, enrichment of microenvironment, pharmacotherapeutics, biomaterial scaffolds and neurorehabilitation may have the potential to enhance injury repair following SCI [161–163]. We are confident that in the near future, NSCs have the potential to make remarkable breakthroughs in both the study of pathological mechanisms and clinical treatments for SCI.
Acknowledgements
Not applicable.
Abbreviations
- SCI
Spinal cord injury
- NSCs
Neural stem cells
- IL-1a
Interleukin-1a
- IL-1b
Interleukin-1b
- IL-6
Interleukin-6
- TNF
Tumor necrosis factor
- CNS
Central nervous system
- NPCs
Neural progenitor cells
- SVZ
Subventricular zone
- SGZ
Subgranular zone
- OLs
Oligodendrocytes
- OPCs
Oligodendrocyte precursor cells
- ESCs
Embryonic stem cells
- iPSCs
Induced pluripotent stem cells
- FNPCs
Fetal neural progenitor cells
Author contributions
All authors contributed to the conception of this work. CL drafted the main text, tables, and figures. YPL provided scientific suggestions to refine the manuscript. SGL was responsible for supervision, funding acquisition, and manuscript revising. All authors have read and agreed the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 31830111, 82171387) and Key Research and Innovation Program of Shanghai Municipal Education Commission (2019-01-07-00-07-E00040).
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Badhiwala JH, Wilson JR, Fehlings MG. Global burden of traumatic brain and spinal cord injury. Lancet Neurol. 2019;18(1):24–5. doi: 10.1016/S1474-4422(18)30444-7. [DOI] [PubMed] [Google Scholar]
- 2.Anjum A, Yazid MD, Fauzi Daud M, Idris J, Ng AMH, Selvi Naicker A, et al. Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci. 2020;21(20):7533. doi: 10.3390/ijms21207533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crispo JAG, Kuramoto LK, Cragg JJ. Global burden of spinal cord injury: future directions. Lancet Neurol. 2023;22(11):976–8. doi: 10.1016/S1474-4422(23)00366-6. [DOI] [PubMed] [Google Scholar]
- 4.GBD Spinal Cord Injuries Collaborators Global, regional, and national burden of spinal cord injury, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet Neurol. 2023;22(11):1026–47. doi: 10.1016/S1474-4422(23)00287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan B, Wei Z, Yao X, Shi G, Cheng X, Zhou X, et al. Microenvironment Imbalance of spinal cord injury. Cell Transpl. 2018;27(6):853–66. doi: 10.1177/0963689718755778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russo GS, Mangan JJ, Galetta MS, Boody B, Bronson W, Segar A, et al. Update on spinal cord injury management. Clin Spine Surg. 2020;33(7):258–64. doi: 10.1097/BSD.0000000000000956. [DOI] [PubMed] [Google Scholar]
- 7.Grijalva-Otero I, Doncel-Pérez E. Traumatic human spinal cord injury: are single treatments enough to solve the problem? Arch Med Res. 2024;55(1):102935. doi: 10.1016/j.arcmed.2023.102935. [DOI] [PubMed] [Google Scholar]
- 8.Eftekharpour E, Karimi-Abdolrezaee S, Fehlings MG. Current status of experimental cell replacement approaches to spinal cord injury. Neurosurg Focus. 2008;24(3–4):E19. doi: 10.3171/FOC/2008/24/3-4/E18. [DOI] [PubMed] [Google Scholar]
- 9.Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, Fehlings MG. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:17018. doi: 10.1038/nrdp.2017.18. [DOI] [PubMed] [Google Scholar]
- 10.Katoh H, Yokota K, Fehlings MG. Regeneration of spinal cord connectivity through stem cell transplantation and biomaterial scaffolds. Front Cell Neurosci. 2019;13:248. doi: 10.3389/fncel.2019.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp (Wars) 2011;71(2):281–99. doi: 10.55782/ane-2011-1848. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Zhang Y, Wang Y, Qian T. Inflammatory response to spinal cord injury and its treatment. World Neurosurg. 2021;155:19–31. doi: 10.1016/j.wneu.2021.07.148. [DOI] [PubMed] [Google Scholar]
- 13.Hellenbrand DJ, Quinn CM, Piper ZJ, Morehouse CN, Fixel JA, Hanna AS. Inflammation after spinal cord injury: a review of the critical timeline of signaling cues and cellular infiltration. J Neuroinflammation. 2021;18(1):284. doi: 10.1186/s12974-021-02337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turtle JD, Henwood MK, Strain MM, Huang YJ, Miranda RC, Grau JW. Engaging pain fibers after a spinal cord injury fosters hemorrhage and expands the area of secondary injury. Exp Neurol. 2019;311:115–24. doi: 10.1016/j.expneurol.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He B, Niu L, Li S, Li H, Hou Y, Li A, et al. Sustainable inflammatory activation following spinal cord injury is driven by thrombin-mediated dynamic expression of astrocytic chemokines. Brain Behav Immun. 2024;116:85–100. doi: 10.1016/j.bbi.2023.11.035. [DOI] [PubMed] [Google Scholar]
- 16.Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol. 2019;10:282. doi: 10.3389/fneur.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hara M, Kobayakawa K, Ohkawa Y, Kumamaru H, Yokota K, Saito T, et al. Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat Med. 2017;23(7):818–28. doi: 10.1038/nm.4354. [DOI] [PubMed] [Google Scholar]
- 18.Chen F, Wang D, Jiang Y, Ma H, Li X, Wang H. Dexmedetomidine postconditioning alleviates spinal cord ischemia-reperfusion injury in rats via inhibiting neutrophil infiltration, microglia activation, reactive gliosis and CXCL13/CXCR5 axis activation. Int J Neurosci. 2023;133(1):1–12. doi: 10.1080/00207454.2021.1881089. [DOI] [PubMed] [Google Scholar]
- 19.Rolls A, Shechter R, Schwartz M. The bright side of the glial scar in CNS repair. Nat Rev Neurosci. 2009;10(3):235–41. doi: 10.1038/nrn2591. [DOI] [PubMed] [Google Scholar]
- 20.Clifford T, Finkel Z, Rodriguez B, Joseph A, Cai L. Current advancements in spinal cord injury research-glial scar formation and neural regeneration. Cells. 2023;12(6):853. doi: 10.3390/cells12060853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams KL, Gallo V. The diversity and disparity of the glial scar. Nat Neurosci. 2018;21(1):9–15. doi: 10.1038/s41593-017-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soderblom C, Luo X, Blumenthal E, Bray E, Lyapichev K, Ramos J, et al. Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J Neurosci. 2013;33(34):13882–7. doi: 10.1523/JNEUROSCI.2524-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukherjee N, Nandi S, Garg S, Ghosh S, Ghosh S, Samat R, et al. Targeting chondroitin sulfate proteoglycans: an emerging therapeutic strategy to treat CNS injury. ACS Chem Neurosci. 2020;11(3):231–2. doi: 10.1021/acschemneuro.0c00004. [DOI] [PubMed] [Google Scholar]
- 24.Gaire BP. Microglia as the critical regulators of neuroprotection and functional recovery in cerebral ischemia. Cell Mol Neurobiol. 2022;42(8):2505–25. doi: 10.1007/s10571-021-01145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang HF, Liu XK, Li R, Zhang P, Chu Z, Wang CL, et al. Effect of glial cells on remyelination after spinal cord injury. Neural Regen Res. 2017;12(10):1724–32. doi: 10.4103/1673-5374.217354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Namjoo Z, Moradi F, Aryanpour R, Piryaei A, Joghataei MT, Abbasi Y, et al. Combined effects of rat Schwann cells and 17β-estradiol in a spinal cord injury model. Metab Brain Dis. 2018;33(4):1229–42. doi: 10.1007/s11011-018-0220-8. [DOI] [PubMed] [Google Scholar]
- 27.Lee HY, Moon SH, Kang D, Choi E, Yang GH, Kim KN, et al. A multi-channel collagen conduit with aligned Schwann cells and endothelial cells for enhanced neuronal regeneration in spinal cord injury. Biomater Sci. 2023;11(24):7884–96. doi: 10.1039/D3BM01152F. [DOI] [PubMed] [Google Scholar]
- 28.Yang B, Zhang F, Cheng F, Ying L, Wang C, Shi K, et al. Strategies and prospects of effective neural circuits reconstruction after spinal cord injury. Cell Death Dis. 2020;11(6):439. doi: 10.1038/s41419-020-2620-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruschel J, Hellal F, Flynn KC, Dupraz S, Elliott DA, Tedeschi A, et al. Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science. 2015;348(6232):347–52. doi: 10.1126/science.aaa2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16(23):7599–609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabelström H, Stenudd M, Frisén J. Neural stem cells in the adult spinal cord. Exp Neurol. 2014;260:44–9. doi: 10.1016/j.expneurol.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 32.Mauffrey P, Tchitchek N, Barroca V, Bemelmans AP, Firlej V, Allory Y, et al. Progenitors from the central nervous system drive neurogenesis in cancer. Nature. 2019;569(7758):672–8. doi: 10.1038/s41586-019-1219-y. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi M, Seki T, Imayoshi I, Tamamaki N, Hayashi Y, Tatebayashi Y, et al. Neural stem cells and neuro/gliogenesis in the central nervous system: understanding the structural and functional plasticity of the developing, mature, and diseased brain. J Physiol Sci. 2016;66(3):197–206. doi: 10.1007/s12576-015-0421-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moyon S, Holloman M, Salzer JL. Neural stem cells and oligodendrocyte progenitor cells compete for remyelination in the corpus callosum. Front Cell Neurosci. 2023;17:1114781. doi: 10.3389/fncel.2023.1114781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Homem CC, Repic M, Knoblich JA. Proliferation control in neural stem and progenitor cells. Nat Rev Neurosci. 2015;16(11):647–59. doi: 10.1038/nrn4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu C, Xia K, Gong Z, Ying L, Shu J, Zhang F, et al. The application of neural stem/progenitor cells for regenerative therapy of spinal cord injury. Curr Stem Cell Res Ther. 2019;14(6):495–503. doi: 10.2174/1574888X14666190329095638. [DOI] [PubMed] [Google Scholar]
- 37.Ye D, Wang Q, Yang Y, Chen B, Zhang F, Wang Z, Luan Z. Identifying genes that affect differentiation of human neural stem cells and myelination of mature oligodendrocytes. Cell Mol Neurobiol. 2023;43(5):2337–58. doi: 10.1007/s10571-022-01313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer I, Dulin JN, Lane MA. Transplanting neural progenitor cells to restore connectivity after spinal cord injury. Nat Rev Neurosci. 2020;21(7):366–83. doi: 10.1038/s41583-020-0314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahuja CS, Mothe A, Khazaei M, Badhiwala JH, Gilbert EA, van der Kooy D, et al. The leading edge: emerging neuroprotective and neuroregenerative cell-based therapies for spinal cord injury. Stem Cells Transl Med. 2020;9(12):1509–30. doi: 10.1002/sctm.19-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo Y, Coskun V, Liang A, Yu J, Cheng L, Ge W, et al. Single-cell transcriptome analyses reveal signals to activate dormant neural stem cells. Cell. 2015;161(5):1175–86. doi: 10.1016/j.cell.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stenudd M, Sabelström H, Frisén J. Role of endogenous neural stem cells in spinal cord injury and repair. JAMA Neurol. 2015;72(2):235–7. doi: 10.1001/jamaneurol.2014.2927. [DOI] [PubMed] [Google Scholar]
- 42.Rogister B, Ben-Hur T, Dubois-Dalcq M. From neural stem cells to myelinating oligodendrocytes. Mol Cell Neurosci. 1999;14(4–5):287–300. doi: 10.1006/mcne.1999.0790. [DOI] [PubMed] [Google Scholar]
- 43.Chandran S, Compston A. Neural stem cells as a potential source of oligodendrocytes for myelin repair. J Neurol Sci. 2005;233(1–2):179–81. doi: 10.1016/j.jns.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 44.Grade S, Bernardino L, Malva JO. Oligodendrogenesis from neural stem cells: perspectives for remyelinating strategies. Int J Dev Neurosci. 2013;31(7):692–700. doi: 10.1016/j.ijdevneu.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Langhnoja J, Buch L, Pillai P. Potential role of NGF, BDNF, and their receptors in oligodendrocytes differentiation from neural stem cell: an in vitro study. Cell Biol Int. 2021;45(2):432–46. doi: 10.1002/cbin.11500. [DOI] [PubMed] [Google Scholar]
- 46.Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344(6183):1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cristobal CD, Lee HK. Development of myelinating glia: an overview. Glia. 2022;70(12):2237–59. doi: 10.1002/glia.24238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan L, Trimarco A, Zhang AJ, Fujimori K, Urade Y, Sun LO, et al. Oligodendrocyte-lineage cell exocytosis and L-type prostaglandin D synthase promote oligodendrocyte development and myelination. Elife. 2023;12:e77441. doi: 10.7554/eLife.77441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu Q, Guan T, Guo Y, Kong J. The initial myelination in the central nervous system. ASN Neuro. 2023;15:17590914231163039. doi: 10.1177/17590914231163039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snaidero N, Möbius W, Czopka T, Hekking LH, Mathisen C, Verkleij D, et al. Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell. 2014;156(1–2):277–90. doi: 10.1016/j.cell.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuhn S, Gritti L, Crooks D, Dombrowski Y. Oligodendrocytes in development, myelin generation and beyond. Cells. 2019;8(11):1424. doi: 10.3390/cells8111424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pajevic S, Plenz D, Basser PJ, Fields RD. Oligodendrocyte-mediated myelin plasticity and its role in neural synchronization. Elife. 2023;12:e81982. doi: 10.7554/eLife.81982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487(7408):443–8. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen JF, Wang F, Huang NX, Xiao L, Mei F. Oligodendrocytes and myelin: active players in neurodegenerative brains? Dev Neurobiol. 2022;82(2):160–74. doi: 10.1002/dneu.22867. [DOI] [PubMed] [Google Scholar]
- 55.Kondo Y, Duncan ID. Transplantation of oligodendrocyte progenitor cells in animal models of leukodystrophies. Methods Mol Biol. 2009;549:175–85. doi: 10.1007/978-1-60327-931-4_12. [DOI] [PubMed] [Google Scholar]
- 56.Assinck P, Duncan GJ, Plemel JR, Lee MJ, Stratton JA, Manesh SB, et al. Myelinogenic plasticity of oligodendrocyte precursor cells following spinal cord contusion injury. J Neurosci. 2017;37(36):8635–54. doi: 10.1523/JNEUROSCI.2409-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.López-Muguruza E, Matute C. Alterations of oligodendrocyte and myelin energy metabolism in multiple sclerosis. Int J Mol Sci. 2023;24(16):12912. doi: 10.3390/ijms241612912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Venkatesh K, Ghosh SK, Mullick M, Manivasagam G, Sen D. Spinal cord injury: pathophysiology, treatment strategies, associated challenges, and future implications. Cell Tissue Res. 2019;377(2):125–51. doi: 10.1007/s00441-019-03039-1. [DOI] [PubMed] [Google Scholar]
- 59.Franklin RJM, Simons M. CNS remyelination and inflammation: from basic mechanisms to therapeutic opportunities. Neuron. 2022;110(21):3549–65. doi: 10.1016/j.neuron.2022.09.023. [DOI] [PubMed] [Google Scholar]
- 60.Chapman TW, Olveda GE, Bame X, Pereira E, Hill RA. Oligodendrocyte death initiates synchronous remyelination to restore cortical myelin patterns in mice. Nat Neurosci. 2023;26(4):555–69. doi: 10.1038/s41593-023-01271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Llorens-Bobadilla E, Chell JM, Le Merre P, Wu Y, Zamboni M, Bergenstråhle J, et al. A latent lineage potential in resident neural stem cells enables spinal cord repair. Science. 2020;370(6512):eabb8795. doi: 10.1126/science.abb8795. [DOI] [PubMed] [Google Scholar]
- 62.Marisca R, Hoche T, Agirre E, Hoodless LJ, Barkey W, Auer F, et al. Functionally distinct subgroups of oligodendrocyte precursor cells integrate neural activity and execute myelin formation. Nat Neurosci. 2020;23(3):363–74. doi: 10.1038/s41593-019-0581-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mezydlo A, Treiber N, Ullrich Gavilanes EM, Eichenseer K, Ancău M, Wens A, et al. Remyelination by surviving oligodendrocytes is inefficient in the inflamed mammalian cortex. Neuron. 2023;111(11):1748–e598. doi: 10.1016/j.neuron.2023.03.031. [DOI] [PubMed] [Google Scholar]
- 64.Fang M, Tang T, Qiu M, Xu X. Hedgehog signaling in CNS remyelination. Cells. 2022;11(14):2260. doi: 10.3390/cells11142260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Caprariello AV, Adams DJ. The landscape of targets and lead molecules for remyelination. Nat Chem Biol. 2022;18(9):925–33. doi: 10.1038/s41589-022-01115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hou J, Zhou Y, Cai Z, Terekhova M, Swain A, Andhey PS, et al. Transcriptomic atlas and interaction networks of brain cells in mouse CNS demyelination and remyelination. Cell Rep. 2023;42(4):112293. doi: 10.1016/j.celrep.2023.112293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nazari B, Namjoo Z, Moradi F, Kazemi M, Ebrahimi-Barough S, Sadroddiny E, et al. miR-219 overexpressing oligodendrocyte progenitor cells for treating compression spinal cord injury. Metab Brain Dis. 2021;36(5):1069–77. doi: 10.1007/s11011-021-00701-y. [DOI] [PubMed] [Google Scholar]
- 68.Ngo C, Kothary R. MicroRNAs in oligodendrocyte development and remyelination. J Neurochem. 2022;162(4):310–21. doi: 10.1111/jnc.15618. [DOI] [PubMed] [Google Scholar]
- 69.Freyermuth-Trujillo X, Segura-Uribe JJ, Salgado-Ceballos H, Orozco-Barrios CE, Coyoy-Salgado A. Inflammation: a target for treatment in spinal cord injury. Cells. 2022;11(17):2692. doi: 10.3390/cells11172692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Samanta J, Grund EM, Silva HM, Lafaille JJ, Fishell G, Salzer JL. Inhibition of Gli1 mobilizes endogenous neural stem cells for remyelination. Nature. 2015;526(7573):448–52. doi: 10.1038/nature14957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wegener A, Deboux C, Bachelin C, Frah M, Kerninon C, Seilhean D, et al. Gain of Olig2 function in oligodendrocyte progenitors promotes remyelination. Brain. 2015;138(Pt 1):120–35. doi: 10.1093/brain/awu375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109(1):61–73. doi: 10.1016/S0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- 73.Brunner C, Lassmann H, Waehneldt TV, Matthieu JM, Linington C. Differential ultrastructural localization of myelin basic protein, myelin/oligodendroglial glycoprotein, and 2’,3’-cyclic nucleotide 3’-phosphodiesterase in the CNS of adult rats. J Neurochem. 1989;52(1):296–304. doi: 10.1111/j.1471-4159.1989.tb10930.x. [DOI] [PubMed] [Google Scholar]
- 74.Huang HT, Ho CH, Sung HY, Lee LY, Chen WP, Chen YW, et al. Hericium erinaceus mycelium and its small bioactive compounds promote oligodendrocyte maturation with an increase in myelin basic protein. Sci Rep. 2021;11(1):6551. doi: 10.1038/s41598-021-85972-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Le Bras B, Chatzopoulou E, Heydon K, Martínez S, Ikenaka K, Prestoz L, et al. Oligodendrocyte development in the embryonic brain: the contribution of the plp lineage. Int J Dev Biol. 2005;49(2–3):209–20. doi: 10.1387/ijdb.041963bl. [DOI] [PubMed] [Google Scholar]
- 76.Michalski JP, Anderson C, Beauvais A, De Repentigny Y, Kothary R. The proteolipid protein promoter drives expression outside of the oligodendrocyte lineage during embryonic and early postnatal development. PLoS ONE. 2011;6(5):e19772. doi: 10.1371/journal.pone.0019772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trapp BD. Myelin-associated glycoprotein location and potential functions. Ann N Y Acad Sci. 1990;605:29–43. doi: 10.1111/j.1749-6632.1990.tb42378.x. [DOI] [PubMed] [Google Scholar]
- 78.Chen L, Yu Z, Xie L, He X, Mu X, Chen C, et al. ANGPTL2 binds MAG to efficiently enhance oligodendrocyte differentiation. Cell Biosci. 2023;13(1):42. doi: 10.1186/s13578-023-00970-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu D, Wu D, Qin M, Nih LR, Liu C, Cao Z, et al. Efficient delivery of nerve growth factors to the central nervous system for neural regeneration. Adv Mater. 2019;31(33):e1900727. doi: 10.1002/adma.201900727. [DOI] [PubMed] [Google Scholar]
- 80.Shu M, Xue X, Nie H, Wu X, Sun M, Qiao L, et al. Single-cell RNA sequencing reveals Nestin(+) active neural stem cells outside the central canal after spinal cord injury. Sci China Life Sci. 2022;65(2):295–308. doi: 10.1007/s11427-020-1930-0. [DOI] [PubMed] [Google Scholar]
- 81.Tang Y, Yu P, Cheng L. Current progress in the derivation and therapeutic application of neural stem cells. Cell Death Dis. 2017;8(10):e3108. doi: 10.1038/cddis.2017.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu B, Yin M, Yang Y, Zou Y, Liu W, Qiao L, et al. Transplantation of neural stem progenitor cells from different sources for severe spinal cord injury repair in rat. Bioact Mater. 2023;23:300–13. doi: 10.1016/j.bioactmat.2022.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Genchi A, Brambilla E, Sangalli F, Radaelli M, Bacigaluppi M, Furlan R, et al. Neural stem cell transplantation in patients with progressive multiple sclerosis: an open-label, phase 1 study. Nat Med. 2023;29(1):75–85. doi: 10.1038/s41591-022-02097-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 85.Zhao XM, He XY, Liu J, Xu Y, Xu FF, Tan YX, et al. Neural stem cell transplantation improves locomotor function in spinal cord transection rats associated with nerve regeneration and IGF-1 R expression. Cell Transpl. 2019;28(9–10):1197–211. doi: 10.1177/0963689719860128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fauser M, Loewenbrück KF, Rangnick J, Brandt MD, Hermann A, Storch A. Adult neural stem cells from midbrain periventricular regions show limited neurogenic potential after transplantation into the hippocampal neurogenic niche. Cells. 2021;10(11):3021. doi: 10.3390/cells10113021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lv Z, Li Y, Wang Y, Cong F, Li X, Cui W, et al. Safety and efficacy outcomes after intranasal administration of neural stem cells in cerebral palsy: a randomized phase 1/2 controlled trial. Stem Cell Res Ther. 2023;14(1):23. doi: 10.1186/s13287-022-03234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 89.Young RA. Control of the embryonic stem cell state. Cell. 2011;144(6):940–54. doi: 10.1016/j.cell.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hackett CH, Fortier LA. Embryonic stem cells and iPS cells: sources and characteristics. Vet Clin North Am Equine Pract. 2011;27(2):233–42. doi: 10.1016/j.cveq.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev Biol. 1995;168(2):342–57. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- 92.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27(3):275–80. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wen Y, Jin S. Production of neural stem cells from human pluripotent stem cells. J Biotechnol. 2014;188:122–9. doi: 10.1016/j.jbiotec.2014.07.453. [DOI] [PubMed] [Google Scholar]
- 94.Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, et al. Ethical and safety issues of stem cell-based therapy. Int J Med Sci. 2018;15(1):36–45. doi: 10.7150/ijms.21666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rezanejad H, Matin MM. Induced pluripotent stem cells: progress and future perspectives in the stem cell world. Cell Reprogram. 2012;14(6):459–70. doi: 10.1089/cell.2012.0039. [DOI] [PubMed] [Google Scholar]
- 96.Engle SJ, Blaha L, Kleiman RJ. Best practices for translational disease modeling using human iPSC-derived neurons. Neuron. 2018;100(4):783–97. doi: 10.1016/j.neuron.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 97.Liu G, David BT, Trawczynski M, Fessler RG. Advances in pluripotent stem cells: history, mechanisms, technologies, and applications. Stem Cell Rev Rep. 2020;16(1):3–32. doi: 10.1007/s12015-019-09935-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 99.D’Aiuto L, Zhi Y, Kumar Das D, Wilcox MR, Johnson JW, McClain L, et al. Large-scale generation of human iPSC-derived neural stem cells/early neural progenitor cells and their neuronal differentiation. Organogenesis. 2014;10(4):365–77. doi: 10.1080/15476278.2015.1011921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Salewski RP, Mitchell RA, Li L, Shen C, Milekovskaia M, Nagy A, et al. Transplantation of induced pluripotent stem cell-derived neural stem cells mediate functional recovery following thoracic spinal cord injury through remyelination of axons. Stem Cells Transl Med. 2015;4(7):743–54. doi: 10.5966/sctm.2014-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kawabata S, Takano M, Numasawa-Kuroiwa Y, Itakura G, Kobayashi Y, Nishiyama Y, et al. Grafted human iPS cell-derived oligodendrocyte precursor cells contribute to robust remyelination of demyelinated axons after spinal cord injury. Stem Cell Rep. 2016;6(1):1–8. doi: 10.1016/j.stemcr.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Luciani M, Garsia C, Mangiameli E, Meneghini V, Gritti A. Intracerebroventricular transplantation of human iPSC-derived neural stem cells (hiPSC-NSCs) into neonatal mice. Methods Cell Biol. 2022;171:127–47. doi: 10.1016/bs.mcb.2022.04.007. [DOI] [PubMed] [Google Scholar]
- 103.Kong D, Feng B, Amponsah AE, He J, Guo R, Liu B, et al. hiPSC-derived NSCs effectively promote the functional recovery of acute spinal cord injury in mice. Stem Cell Res Ther. 2021;12(1):172. doi: 10.1186/s13287-021-02217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fan L, Liu C, Chen X, Zou Y, Zhou Z, Lin C, et al. Directing induced pluripotent stem cell derived neural stem cell fate with a three-dimensional biomimetic hydrogel for spinal cord injury repair. ACS Appl Mater Interfaces. 2018;10(21):17742–55. doi: 10.1021/acsami.8b05293. [DOI] [PubMed] [Google Scholar]
- 105.Kajikawa K, Imaizumi K, Shinozaki M, Shibata S, Shindo T, Kitagawa T, et al. Cell therapy for spinal cord injury by using human iPSC-derived region-specific neural progenitor cells. Mol Brain. 2020;13(1):120. doi: 10.1186/s13041-020-00662-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu Q, Zhang L, Zhang J. Induced pluripotent stem cell-derived neural progenitor cell transplantation promotes regeneration and functional recovery after post-traumatic stress disorder in rats. Biomed Pharmacother. 2021;133:110981. doi: 10.1016/j.biopha.2020.110981. [DOI] [PubMed] [Google Scholar]
- 107.Suematsu Y, Nagoshi N, Shinozaki M, Kase Y, Saijo Y, Hashimoto S, et al. Hepatocyte growth factor pretreatment boosts functional recovery after spinal cord injury through human iPSC-derived neural stem/progenitor cell transplantation. Inflamm Regen. 2023;43(1):50. doi: 10.1186/s41232-023-00298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Amemori T, Ruzicka J, Romanyuk N, Jhanwar-Uniyal M, Sykova E, Jendelova P. Comparison of intraspinal and intrathecal implantation of induced pluripotent stem cell-derived neural precursors for the treatment of spinal cord injury in rats. Stem Cell Res Ther. 2015;6:257. doi: 10.1186/s13287-015-0255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Strnadel J, Carromeu C, Bardy C, Navarro M, Platoshyn O, Glud AN, et al. Survival of syngeneic and allogeneic iPSC-derived neural precursors after spinal grafting in minipigs. Sci Transl Med. 2018;10(440):eaam6651. doi: 10.1126/scitranslmed.aam6651. [DOI] [PubMed] [Google Scholar]
- 110.Watanabe K, Nakamura M, Iwanami A, Fujita Y, Kanemura Y, Toyama Y, et al. Comparison between fetal spinal-cord- and forebrain-derived neural stem/progenitor cells as a source of transplantation for spinal cord injury. Dev Neurosci. 2004;26(2–4):275–87. doi: 10.1159/000082144. [DOI] [PubMed] [Google Scholar]
- 111.Ogawa Y, Sawamoto K, Miyata T, Miyao S, Watanabe M, Nakamura M, et al. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J Neurosci Res. 2002;69(6):925–33. doi: 10.1002/jnr.10341. [DOI] [PubMed] [Google Scholar]
- 112.Li X, Sundström E. Stem cell therapies for central nervous system trauma: the 4 Ws-what, when, where, and why. Stem Cells Transl Med. 2022;11(1):14–25. doi: 10.1093/stcltm/szab006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gao M, Yao H, Dong Q, Zhang H, Yang Z, Yang Y, et al. Tumourigenicity and immunogenicity of induced neural stem cell grafts versus induced pluripotent stem cell grafts in syngeneic mouse brain. Sci Rep. 2016;6:29955. doi: 10.1038/srep29955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jiang Z, Han Y, Cao X. Induced pluripotent stem cell (iPSCs) and their application in immunotherapy. Cell Mol Immunol. 2014;11(1):17–24. doi: 10.1038/cmi.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Borhani-Haghighi M, Mohamadi Y. The protective effects of neural stem cells and neural stem cells-conditioned medium against inflammation-induced prenatal brain injury. J Neuroimmunol. 2021;360:577707. doi: 10.1016/j.jneuroim.2021.577707. [DOI] [PubMed] [Google Scholar]
- 116.Fan Y, Marcy G, Lee ES, Rozen S, Mattar CN, Waddington SN, et al. Regionally-specified second trimester fetal neural stem cells reveals differential neurogenic programming. PLoS ONE. 2014;9(9):e105985. doi: 10.1371/journal.pone.0105985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hwang I, Hahm SC, Choi KA, Park SH, Jeong H, Yea JH, et al. Intrathecal transplantation of embryonic stem cell-derived spinal GABAergic neural precursor cells attenuates neuropathic pain in a spinal cord injury rat model. Cell Transpl. 2016;25(3):593–607. doi: 10.3727/096368915X689460. [DOI] [PubMed] [Google Scholar]
- 118.Salewski RP, Mitchell RA, Shen C, Fehlings MG. Transplantation of neural stem cells clonally derived from embryonic stem cells promotes recovery after murine spinal cord injury. Stem Cells Dev. 2015;24(1):36–50. doi: 10.1089/scd.2014.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W. Cell transplantation therapy for spinal cord injury. Nat Neurosci. 2017;20(5):637–47. doi: 10.1038/nn.4541. [DOI] [PubMed] [Google Scholar]
- 120.Pereira IM, Marote A, Salgado AJ, Silva NA. Filling the gap: neural stem cells as a promising therapy for spinal cord injury. Pharmaceuticals (Basel) 2019;12(2):65. doi: 10.3390/ph12020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kawai M, Imaizumi K, Ishikawa M, Shibata S, Shinozaki M, Shibata T, et al. Long-term selective stimulation of transplanted neural stem/progenitor cells for spinal cord injury improves locomotor function. Cell Rep. 2021;37(8):110019. doi: 10.1016/j.celrep.2021.110019. [DOI] [PubMed] [Google Scholar]
- 122.Hu X, Xu W, Ren Y, Wang Z, He X, Huang R, et al. Spinal cord injury: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2023;8(1):245. doi: 10.1038/s41392-023-01477-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Duan H, Ge W, Zhang A, Xi Y, Chen Z, Luo D, et al. Transcriptome analyses reveal molecular mechanisms underlying functional recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2015;112(43):13360–5. doi: 10.1073/pnas.1510176112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Luo D, Ge W, Hu X, Li C, Lee CM, Zhou L, et al. Unbiased transcriptomic analyses reveal distinct effects of immune deficiency in CNS function with and without injury. Protein Cell. 2019;10(8):566–82. doi: 10.1007/s13238-018-0559-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Milich LM, Choi JS, Ryan C, Cerqueira SR, Benavides S, Yahn SL, et al. Single-cell analysis of the cellular heterogeneity and interactions in the injured mouse spinal cord. J Exp Med. 2021;218(8):e20210040. doi: 10.1084/jem.20210040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li C, Wu Z, Zhou L, Shao J, Hu X, Xu W, et al. Temporal and spatial cellular and molecular pathological alterations with single-cell resolution in the adult spinal cord after injury. Signal Transduct Target Ther. 2022;7(1):65. doi: 10.1038/s41392-022-00885-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Abematsu M, Tsujimura K, Yamano M, Saito M, Kohno K, Kohyama J, et al. Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J Clin Invest. 2010;120(9):3255–66. doi: 10.1172/JCI42957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cheng Z, Zhu W, Cao K, Wu F, Li J, Wang G, et al. Anti-inflammatory mechanism of neural stem cell transplantation in spinal cord injury. Int J Mol Sci. 2016;17(9):1380. doi: 10.3390/ijms17091380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shao R, Li C, Chen Y, Zhang L, Yang H, Zhang Z, et al. LncRNA-GAS5 promotes spinal cord repair and the inhibition of neuronal apoptosis via the transplantation of 3D printed scaffold loaded with induced pluripotent stem cell-derived neural stem cells. Ann Transl Med. 2021;9(11):931. doi: 10.21037/atm-21-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li T, Zhao X, Duan J, Cui S, Zhu K, Wan Y, et al. Targeted inhibition of STAT3 in neural stem cells promotes neuronal differentiation and functional recovery in rats with spinal cord injury. Exp Ther Med. 2021;22(1):711. doi: 10.3892/etm.2021.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pan B, Wu X, Zeng X, Chen J, Zhang W, Cheng X, et al. Transplantation of Wnt4-modified neural stem cells mediate M2 polarization to improve inflammatory micro-environment of spinal cord injury. Cell Prolif. 2023;56(8):e13415. doi: 10.1111/cpr.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fan X, Wei H, Du J, Lu X, Wang L. Hypoxic preconditioning neural stem cell transplantation promotes spinal cord injury in rats by affecting transmembrane immunoglobulin domain-containing. Hum Exp Toxicol. 2022;41:9603271211066587. doi: 10.1177/09603271211066587. [DOI] [PubMed] [Google Scholar]
- 133.Zhu Y, Uezono N, Yasui T, Nakashima K. Neural stem cell therapy aiming at better functional recovery after spinal cord injury. Dev Dyn. 2018;247(1):75–84. doi: 10.1002/dvdy.24558. [DOI] [PubMed] [Google Scholar]
- 134.Lee S, Nam H, Joo KM, Lee SH. Advances in neural stem cell therapy for spinal cord injury: safety, efficacy, and future perspectives. Neurospine. 2022;19(4):946–60. doi: 10.14245/ns.2244658.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gao L, Peng Y, Xu W, He P, Li T, Lu X, et al. Progress in stem cell therapy for spinal cord injury. Stem Cells Int. 2020;2020:2853650. doi: 10.1155/2020/2853650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Farid MF, Rizk YSA. Stem cell treatment trials of spinal cord injuries in animals. Auton Neurosci. 2021;238:102932. doi: 10.1016/j.autneu.2021.102932. [DOI] [PubMed] [Google Scholar]
- 137.Du X, Amponsah AE, Kong D, He J, Ma Z, Ma J, et al. hiPSC-neural stem/progenitor cell transplantation therapy for spinal cord injury. Curr Stem Cell Res Ther. 2023;18(4):487–98. doi: 10.2174/1574888X17666220509222520. [DOI] [PubMed] [Google Scholar]
- 138.McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999;5(12):1410–2. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- 139.Takano M, Kawabata S, Shibata S, Yasuda A, Nori S, Tsuji O, et al. Enhanced functional recovery from spinal cord injury in aged mice after stem cell transplantation through HGF induction. Stem Cell Rep. 2017;8(3):509–18. doi: 10.1016/j.stemcr.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang LQ, Zhang WM, Deng L, Xu ZX, Lan WB, Lin JH. Transplantation of a peripheral nerve with neural stem cells plus Lithium Chloride injection promote the recovery of rat spinal cord injury. Cell Transpl. 2018;27(3):471–84. doi: 10.1177/0963689717752945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xue X, Zhang L, Yin X, Chen XX, Chen ZF, Wang CX, et al. Transplantation of neural stem cells preconditioned with high–mobility group box 1 facilitates functional recovery after spinal cord injury in rats. Mol Med Rep. 2020;22(6):4725–33. doi: 10.3892/mmr.2020.11565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ko WK, Kim SJ, Han GH, Lee D, Jeong D, Lee SJ, et al. Transplantation of neuron-inducing grafts embedding positively charged gold nanoparticles for the treatment of spinal cord injury. Bioeng Transl Med. 2022;7(3):e10326. doi: 10.1002/btm2.10326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Adler AF, Lee-Kubli C, Kumamaru H, Kadoya K, Tuszynski MH. Comprehensive monosynaptic rabies virus mapping of host connectivity with neural progenitor grafts after spinal cord injury. Stem Cell Rep. 2017;8(6):1525–33. doi: 10.1016/j.stemcr.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rosenzweig ES, Brock JH, Lu P, Kumamaru H, Salegio EA, Kadoya K, et al. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat Med. 2018;24(4):484–90. doi: 10.1038/nm.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jevans B, James ND, Burnside E, McCann CJ, Thapar N, Bradbury EJ, et al. Combined treatment with enteric neural stem cells and chondroitinase ABC reduces spinal cord lesion pathology. Stem Cell Res Ther. 2021;12(1):10. doi: 10.1186/s13287-020-02031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yamazaki K, Kawabori M, Seki T, Houkin K. Clinical trials of stem cell treatment for spinal cord injury. Int J Mol Sci. 2020;21(11):3994. doi: 10.3390/ijms21113994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zipser CM, Cragg JJ, Guest JD, Fehlings MG, Jutzeler CR, Anderson AJ, et al. Cell-based and stem-cell-based treatments for spinal cord injury: evidence from clinical trials. Lancet Neurol. 2022;21(7):659–70. doi: 10.1016/S1474-4422(21)00464-6. [DOI] [PubMed] [Google Scholar]
- 148.Szymoniuk M, Litak J, Sakwa L, Dryla A, Zezuliński W, Czyżewski W, et al. Molecular mechanisms and clinical application of multipotent stem cells for spinal cord injury. Cells. 2022;12(1):120. doi: 10.3390/cells12010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hosseini SM, Borys B, Karimi-Abdolrezaee S. Neural stem cell therapies for spinal cord injury repair: an update on recent preclinical and clinical advances. Brain. 2024;147(3):766–93. doi: 10.1093/brain/awad392. [DOI] [PubMed] [Google Scholar]
- 150.Shin JC, Kim KN, Yoo J, Kim IS, Yun S, Lee H, et al. Clinical trial of human fetal brain-derived neural stem/progenitor cell transplantation in patients with traumatic cervical spinal cord injury. Neural Plast. 2015;2015:630932. doi: 10.1155/2015/630932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Levi AD, Okonkwo DO, Park P, Jenkins AL, 3rd, Kurpad SN, Parr AM, et al. Emerging safety of intramedullary transplantation of human neural stem cells in chronic cervical and thoracic spinal cord injury. Neurosurgery. 2018;82(4):562–75. doi: 10.1093/neuros/nyx250. [DOI] [PubMed] [Google Scholar]
- 152.Hu XC, Lu YB, Yang YN, Kang XW, Wang YG, Ma B, et al. Progress in clinical trials of cell transplantation for the treatment of spinal cord injury: how many questions remain unanswered? Neural Regen Res. 2021;16(3):405–13. doi: 10.4103/1673-5374.293130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Curtis E, Martin JR, Gabel B, Sidhu N, Rzesiewicz TK, Mandeville R, et al. A first-in-human, phase I study of neural stem cell transplantation for chronic spinal cord injury. Cell Stem Cell. 2018;22(6):941–e506. doi: 10.1016/j.stem.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 154.Levi AD, Anderson KD, Okonkwo DO, Park P, Bryce TN, Kurpad SN, et al. Clinical outcomes from a multi-center study of human neural stem cell transplantation in chronic cervical spinal cord injury. J Neurotrauma. 2019;36(6):891–902. doi: 10.1089/neu.2018.5843. [DOI] [PubMed] [Google Scholar]
- 155.Curt A, Hsieh J, Schubert M, Hupp M, Friedl S, Freund P, et al. The damaged spinal cord is a suitable target for stem cell transplantation. Neurorehabil Neural Repair. 2020;34(8):758–68. doi: 10.1177/1545968320935815. [DOI] [PubMed] [Google Scholar]
- 156.Sugai K, Sumida M, Shofuda T, Yamaguchi R, Tamura T, Kohzuki T, et al. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: study protocol. Regen Ther. 2021;18:321–33. doi: 10.1016/j.reth.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hejrati N, Wong R, Khazaei M, Fehlings MG. How can clinical safety and efficacy concerns in stem cell therapy for spinal cord injury be overcome? Expert Opin Biol Ther. 2023;23(9):883–99. doi: 10.1080/14712598.2023.2245321. [DOI] [PubMed] [Google Scholar]
- 158.Ribeiro BF, da Cruz BC, de Sousa BM, Correia PD, David N, Rocha C, et al. Cell therapies for spinal cord injury: a review of the clinical trials and cell-type therapeutic potential. Brain. 2023;146(7):2672–93. doi: 10.1093/brain/awad047. [DOI] [PubMed] [Google Scholar]
- 159.Ghobrial GM, Anderson KD, Dididze M, Martinez-Barrizonte J, Sunn GH, Gant KL, et al. Human neural stem cell transplantation in chronic cervical spinal cord injury: functional outcomes at 12 months in a phase II clinical trial. Neurosurgery. 2017;64(CNsuppl1):87–91. doi: 10.1093/neuros/nyx242. [DOI] [PubMed] [Google Scholar]
- 160.Zeng CW. Advancing spinal cord injury treatment through stem cell therapy: a comprehensive review of cell types, challenges, and emerging technologies in regenerative medicine. Int J Mol Sci. 2023;24(18):14349. doi: 10.3390/ijms241814349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu S, Schackel T, Weidner N, Puttagunta R. Biomaterial-supported cell transplantation treatments for spinal cord injury: challenges and perspectives. Front Cell Neurosci. 2017;11:430. doi: 10.3389/fncel.2017.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen X, Wang Y, Zhou G, Hu X, Han S, Gao J. The combination of nanoscaffolds and stem cell transplantation: paving a promising road for spinal cord injury regeneration. Biomed Pharmacother. 2021;143:112233. doi: 10.1016/j.biopha.2021.112233. [DOI] [PubMed] [Google Scholar]
- 163.Pieczonka K, Fehlings MG. Incorporating combinatorial approaches to encourage targeted neural stem/progenitor cell integration following transplantation in spinal cord injury. Stem Cells Transl Med. 2023;12(4):207–14. doi: 10.1093/stcltm/szad008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.