Abstract
Kidney stone disease affects ~10% of the global population and the incidence continues to rise owing to the associated global increase in the incidence of medical conditions associated with kidney stone disease including, for example, those comprising the metabolic syndrome. Considering that the intestinal microbiome has a substantial influence on host metabolism, that evidence has suggested that the intestinal microbiome might have a role in maintaining oxalate homeostasis and kidney stone disease is unsurprising. In addition, the discovery that urine is not sterile but, like other sites of the human body, harbours commensal bacterial species that collectively form a urinary microbiome, is an additional factor that might influence the induction of crystal formation and stone growth directly in the kidney. Collectively, the microbiomes of the host could influence kidney stone disease at multiple levels, including intestinal oxalate absorption and direct crystal formation in the kidneys.
The human microbiome comprises all of the microorganisms that live within as well as on the human host1,2. One of the most frequently studied microbiome sites in the human host is that of the intestine3, which acts as an effective metabolic organ4,5 and has been shown to have important roles in the maintenance of overall human health by not only being critical for the digestion of food and extraction of nutrients but also by regulating the immune response of the host6, preventing overgrowth of pathogens7, regulating cell proliferation and vascularization of the host8,9, and regulating endocrine functions of the intestine10, as well as via neurological signalling11, regulation of energy12, and by regulating the overall metabolism of the host13,14. Disruption in the composition of the microbiome — termed ‘dysbiosis’ — contributes to several disease states within and distant from the intestines15 including inflammatory bowel disease (Crohn’s disease and ulcerative colitis)16-18, asthma19, obesity20,21 and type 2 diabetes22,23, as well as behavioural disorders including anxiety and depression via the gut–brain axis24-26. In general, dysbiosis can be driven either by the loss of health-protective bacteria, such as members of the Lactobacillus, Faecalibacterium, Coprococcus and Ruminococcus species, or by the acquisition and/or overgrowth of disease-causing bacteria such as Clostridium difficile, which are termed loss-of-function and gain-of-function dysbiosis, respectively27.
Intestinal dysbiosis and oxalate homeostasis.
Urinary oxalate, which is derived from both exogenous and endogenous sources, contributes to the formation of 70–80% of kidney stones, and the urine of stone formers is frequently more supersaturated with oxalate than that of healthy subjects28,29. Oxalate-metabolizing bacterial species (OMBS) in the gut have been speculated to have an active role in sustaining oxalate homeostasis by reducing the amount of dietary oxalate absorbed. This mechanism is useful in the scenario of high dietary oxalate intake (the primary focus of this Review) and potentially also by sequestering circulating oxalate into the digestive tract, which could be particularly useful in individuals with high endogenous oxalate production30-33.
Oxalate-degrading microorganisms can be categorized into two groups: obligate oxalate consumers, such as Oxalobacter formigenes, that require oxalate as a carbon and energy source30,34; and facultative oxalate consumers, which include species from the Lactobacillus, Bifidobacterium, Enterococcus, Clostridium, Eggerthella, Providencia, Streptococcus and Leuconostoc genera35, which degrade oxalate when present but often exhibit growth inhibition with oxalate exposure36-38. Attempts to introduce oxalate-degrading microorganisms into the digestive tracts of humans or rodents have typically resulted in an ephemeral decrease in urinary oxalate excretion, with values returning to baseline in as little as 5 days39. In fact, of 14 clinical studies to date that used either O. formigenes or various Lactobacilli as probiotics, only 8 showed varying significant effects of treatment on urinary oxalate levels33,36,40-51. Of the three studies that quantified persistence of the oxalate-degrading function, values of persistence ranged from <2 weeks to >4 months33,42,49. In the three randomized, double-blind, placebo-controlled clinical studies using O. formigenes, the probiotics showed no effect on urinary oxalate excretion40,41,43. However, these clinical trials were notably conducted only on patients with primary hyperoxaluria, meaning that probiotic supplementation of O. formigenes might still be effective in patients with enteric hyperoxaluria. That said, based on the data available to date, our overall understanding of the oxalate-degrading properties of the gut microbiome remains incomplete.
The inconsistency of the results from clinical studies that sought to reduce urinary oxalate through the introduction of specific OMBS into the gut might be partly explained by the heterogeneous aetiology of hyperoxaluria. Individuals whose hyperoxaluria is caused by primary hyperoxaluria, for example, would be unlikely to benefit from enhanced gut microbial oxalate degradation because the source of oxalate in these patients is the liver52. However, regarding patients whose hyperoxaluria arises largely from dietary oxalate absorption and who should, therefore, benefit from increased levels of OMBS, results from metagenomic and animal studies shed light on potential reasons for inconsistent findings. Several metagenomic studies published since 2016 have used comparative 16S rRNA metagenomic sequencing to identify differences in the gut microbiome between patients with an active episode of kidney stone disease and those with no history of stones53-59. Although differences in gut microbiome composition were apparent between groups, particularly with regard to the Bacteroides, Ruminococcus, Coprococcus, Prevotella, and Oscillospira genera, none of the studies reported a difference in O. formigenes. However, one shotgun metagenomic study reported significantly lower diversity in oxalate-degrading genes in the population of patients with a history of kidney stones (P = 0.002)57, whereas another study showed that this population exhibited lower levels of bacterial taxa that are stimulated by dietary oxalate58. Together, these metagenomic studies suggest that some level of metabolic redundancy and/or cooperation within the microbiome help to maintain oxalate homeostasis, rather than a single or a few specific OMBS, and that the loss of these microbial consortia might increase the risk of the development of stones. This hypothesis is supported by animal studies in which faecal transplants from wild animals resulted in persistently lower urinary oxalate levels, the effect of which was greater than from oral probiotics of mixed oxalate-degrading bacteria; however, this effect was eliminated by antibiotics use or a high-fat, high-sugar diet, suggesting that the fine-tuned microbial consortia that convey maximal oxalate degradation can easily be disrupted by various external factors, resulting in increased oxalate absorption and a corresponding increased risk of stone development60-62.
Beyond oxalate, one other animal study in rats found that the gut microbiome broadly affected numerous kidney stone risk factors63. Specifically, urinary calcium was decreased by 55% following faecal transplant (P < 0.001), whereas urinary oxalate levels were 24% lower than baseline levels (P < 0.01). Furthermore, pH increased by 0.6 units from the baseline level of 5.85 (P < 0.001), and a 29% increase in gastrointestinal alkali absorption (P < 0.001) was observed as well as 90% increased expression of Slc26a6 (anion transporter of chloride, oxalate, sulfate and bicarbonate) in the caecum following transplant.
Similarly, faecal transplant into germ-free mice led to decreased urinary calcium and oxalate and increased urinary pH63. All together, these findings suggest that intestinal microbiome composition might have an important effect on metabolism and urinary chemistries relevant to kidney stone disease risk. However, besides these studies, the role of the gut microbiome in risk factors beyond oxalate has been poorly explored to date.
Effect of urinary microbiota on kidney stone disease risk factors
In 2012, Wolfe and colleagues reported the discovery that the urinary tract is not sterile, even in the absence of any symptoms of a urinary tract infection64, opening up a new avenue for research into the influence of the human microbiome on kidney stone disease.
Only two case–control studies have been published investigating the association between the urinary tract microbiome and urinary stone disease, both of which report significant assocations59,65. In a 2020 study, Xie et al.65. studied the urinary microbiome composition of men with calcium-based kidney stones and compared its composition with that of non-stone-forming men. The majority of participants were first-time stone formers (20 of 22, 90.9%) and only 2 were recurrent stone formers. Interestingly, the authors used urine samples taken from both the bladder and renal pelvis of participants and noted no significant differences in the urinary microbiome composition between the two sites in either patients or controls, suggesting that studying bladder urine (which is easier to collect than urine from the pelvis) is representative of the composition in the kidneys where stones form.
The 2019 study by Zampini et al. used metagenomic and metabolomic comparative analysis of the gut, urinary tract and stone microbiomes, and reported that the composition of the urinary tract microbiome could differentiate stone formers and non-stone formers more accurately than the gut microbiome59. Specifically, differential abundance analysis showed that only 1.9% of the operational taxonomic units identified in stool samples were significantly different in stone-forming patients and controls, whereas 8.8% of operational taxonomic units in the urinary microbiome were representative. In particular, Zampini et al. found the taxum Lactobacillus to be most abundant in the urine of healthy individuals, whereas members of the taxum Enterobacteriaceae were most abundant in the stone patient cohort. Thus, they concluded that the urinary microbiome might have an important role in the onset of kidney stone disease, and that the Lactobacillus genus and Enterobacteriaceae family could be important for the protection or promotion of the disease, respectively.
A number of studies have reported the presence of a consistent urinary microbiome among patients who formed specific types of stones59,66-69, which suggests that bacteria might have a direct role in lithogenesis at the site of stone formation. Thus, investigators have employed different in vitro and in vivo study systems to understand the direct mechanistic role of urinary tract bacteria in the formation of stones. One study used a two-stage bioreactor that mimicked the kidney, ureter and bladder to investigate the role of Proteus mirabilis biofilm on struvite stone formation70. The developed model most closely mimics multiple factors affecting stone formation in vivo, including the natural migration of bacteria from a contaminated bladder vessel (that is, the site of bacterial inoculation) against urine flow (from the kidney to the ureter) and subsequent stone formation in the kidney. The researchers concluded that P. mirabilis-induced struvite stones were driven by microbial migration between the bladder and kidneys, bacterial attachment and biofilm formation, and increased urinary pH driven by ureolysis, all of which were followed by microcrystalline growth and aggregation, leading to stone formation70.
The mechanisms by which urinary tract bacteria promote calcium oxalate (CaOx) stones has also been investigated. Studies indicate that E. coli are commonly found in CaOx stones and are present both in the centre and the periphery of the stones, suggesting that these microorganisms are not randomly entrapped into the stones but are actually associated with disease pathogenesis59,68,69. Although the exact mechanism has not been elucidated, these findings suggest that bacteria from the urinary tract could colonize or simply become trapped on the surface of the stone as it forms, acting as a nidus for further crystal growth by providing additional attachment points for crystals. An in vitro study confirmed that intact and viable uropathogens, E. coli, Klebsiella pneumoniae, Staphylococcus aureus and Streptococcus pneumoniae all enhance CaOx crystal growth and aggregation71. Furthermore, a follow-up study by the same group found that the effects of intact viable E. coli to promote CaOx crystallization and aggregation were via the flagella72, which potentially facilitate bacteria-assisted CaOx crystal growth and aggregation both indirectly, by promotion of bacterial biofilm formation, and directly by binding CaOx crystals via the dynamic charge of the flagella proteins.
Other components from the bacterial capsule, such as the lipopolysaccharide and the outer membrane vesicles, were found to promote crystal growth and aggregation but were not as potent as the flagella72. These in vitro studies are supported by data from Barr-Beare and colleagues68 who showed that uropathogenic E. coli colonization and biofilm formation can induce substantial renal CaOx deposition in mice when uropathogenic E. coli was administered transurethrally at the same time as intraperitoneal glyoxalate (a precursor to oxalate)68. These data suggest that bacterial colonization and biofilm formation could have an important role in promoting CaOx crystal deposition and growth into larger stones.
The hypothesis that the urinary tract microbiome actively promotes lithogenesis is also supported by an interdisciplinary geobiology study that analysed thin sections of CaOx stones using a combination of optical techniques73. The images produced using this approach indicated that CaOx stones grow in a complex environment of biomass-rich nanolayers through a process of repeated dissolution, crystallization and remodelling, which the authors termed ‘diagenetic phase transitions’. A number of different biomolecules have a role in driving these events and plausibly include biomolecules derived from a resident microbial community66. In fact, stone cultures have shown that nearly one-third of CaOx stones contained bacteria and up to 3% contained fungi74. The authors also observed that CaOx nanolayers are considerably smaller than the microbial layer that directly influences layering in other geological deposits, such as corals, caves and travertine marble on aqueducts. Thus, kidney stone biomineralization might be controlled, at least in part, by microbiome-derived biomolecules.
Similarly, a subsequent study analysed bulk-entombed DNA-sequenced fragments of CaOx, brushite and struvite stones from 11 recurrent kidney stone formers75. The analysis confirmed the presence of an entombed low-diversity community of bacteria and fungi including Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria and Aspergillus niger. Furthermore, in addition to the DNA sequence-based evidence, bacterial cells were also optically observed entombed and well preserved in amorphous hydroxyapatite spherules and fans of needle-like crystals of brushite and struvite75. Collectively, these data indicate that a ‘microbiome’ is incorporated into the CaOx, brushite and struvite stone formation processes. Although bacteria-associated urease activity is known to be the metabolic function driving infection stone formation76, the mechanisms of how microbiome-associated metabolic functions drive lithogenesis of CaOx and brushite stones remains to be elucidated. One possibility is that CaOx and brushite stone-associated microbiomes might be merely bystanders in the urinary microbiome that become incorporated into the stone matrix as the stone forms. However, given the symbiotic nature of bacterial microbiomes, this simple explanation is unlikely to represent the whole story.
The role of microbial metabolites in stone formation
Metabolites are the end product of a cascade of metabolic processes induced by the interaction between an organism and external factors that starts with the genome, and progresses through translation, protein synthesis and finally metabolism.
Thus, as the main communicators produced by the intestinal microbiome, metabolites are often at the intersection between host–microorganism and microorganism–microorganism interactions, such as those that promote kidney stone formation as they interact with and activate various host and microbial pathways involved in the maintenance of overall health77,78. Short-chain fatty acids (SCFAs), for example, are the main metabolites produced by the intestinal microbiome79, and have been shown to have important effects on the metabolism of the host and determinants of health and disease80. Of particular relevance to kidney stone disease, SCFAs such as butyrate substantially affect gut health characteristics, including tight junction expression, transepithelial resistance and the expression of transporters that affect the absorption of compounds and/or ions relevant to kidney stone formation, including sodium, calcium and oxalate81-85.
Previous metabolomic evaluations of the urine of stone formers have shown that metabolites involved in the processes of cell injury, such as lactate, are elevated in individuals with kidney stone disease86. Among the metabolites identified, some bacteria-derived biomolecules are associated with the promotion or inhibition of stone growth59. Among these, hippurate, a metabolic marker of microbiome diversity87, was elevated in healthy individuals compared with those with stones86 and also correlated negatively with kidney stone disease in a metabolomic analysis of urine samples from patients with and without kidney stones88. However, the mechanisms associated with these compounds in inhibiting stone formation require further study.
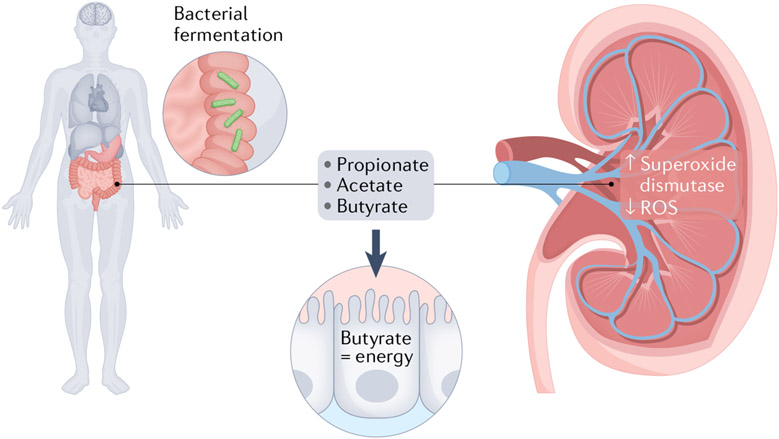
SCFAs, including acetate, propionate and butyrate, are the main metabolites produced by gut microbiota in the colon as a result of fermentation of dietary fibre and digestion-resistant starch79, and are involved in the maintenance of intestinal epithelial health and function (FIG. 1). SCFAs in the lumen of the intestine are absorbed quickly by the duodenal mucosa through the electrogenic high-affinity transporter sodium-coupled monocarboxylate transporter 1 (SMCT1) and slowly by low-affinity SMCT2 in the jejunum or ileum89. Once absorbed, SCFAs are converted into either acetyl-CoA or propionyl-CoA, both of which are used to generate energy for intestinal epithelial cells via the tricarboxylic acid cycle90. As each butyrate molecule generates more ATP molecules than the other SCFAs (27 for butyrate versus 18 for propionate and 10 for acetate), butyrate is the main energy source for colonocytes, providing at least 60–70% of the energy required by these cells91 (FIG. 1).
Fig. 1 ∣. The effect of short-chain fatty acids on mechanisms that might affect oxalate absorption and mechanisms of stone formation.
Short-chain fatty acids (SFCAs) are products of intestinal microbial fermentation of indigestible foods and include propionate, acetate and butyrate. These SCFAs are the main energy source of colonocytes, making them a key determinant of intestinal epithelial layer health. In addition, SCFAs in the gut have distant effects on the kidney, including the production of reactive oxygen species (ROS), which have been implicated in renal changes that trigger the initiation of crystal formation.
Although a role for these important bacterial metabolites has been established in conditions involved in metabolic syndrome, including obesity, high lipid levels, and diabetes12,92-96, early evidence suggests that similar roles exist in the gut–kidney axis. For instance, some SCFAs have been shown to be able to regulate renal dysfunction in acute and chronic kidney disease via activation of anti-inflammatory responses81,97-102. For instance, SCFAs decreased oxidative stress, the maturation of dendritic cells and their ability to induce CD4+ and CD8+ T cell proliferation, all of which are hallmarks of the inflammatory process leading to acute kidney injury97. Furthermore, sodium butyrate attenuated kidney dysfunction and tubular damage, probably because of the reduced expression of ILN6 and NF-κB, both key cytokines involved in the inflammatory process leading to acute kidney injury98. Lastly, both acetate and butyrate have been shown to protect against kidney injury by regulating reactive oxygen species (ROS) production by enhancing superoxide dismutase and catalase activities, which are responsible for breaking down ROSs103. Overall, these data suggest a direct effect of SCFAs produced in the intestine on the kidney via activation of G-protein-coupled receptors after intestinal absorption into the circulation104. Given that a role for ROS and inflammation in the kidneys has been suggested in the initiation of kidney stones105,106, SCFA concentrations and intestinal absorption might affect specific inflammatory events in the kidney that could influence kidney stone formation. This area warrants further investigation.
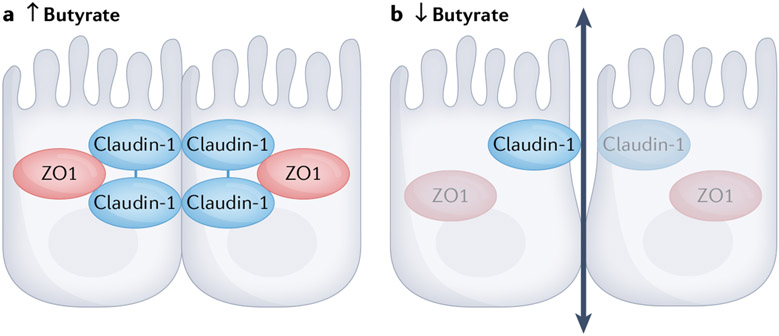
Given the association between CaOx stone disease and intestinal oxalate absorption, the availability and absorption of SCFAs as the main determinants of overall intestinal epithelial health and function might have a direct role in the development of hyperoxaluria via direct effects on intestinal ion transport. For instance, SCFAs have a role in restoring and maintaining normal intestinal barrier function by affecting the expression of tight junction proteins to regulate paracellular permeability and solute transport via channels between cells (FIG. 2). Of all SCFAs, butyrate is the most important regulator of tight junctions by altering the expression of genes encoding tight junction proteins such as claudin-1 and zonula occludens 1 (ZO1) and by regulating the distribution of occludins107,108. In the context of kidney stone disease, decreased tight junction protein expression results in a more permeable gut barrier that enables increased absorption of relevant ions and/or compounds such as oxalate.
Fig. 2 ∣. The role of butyrate in regulating intestinal epithelial cell tight junctions.
Butyrate regulates the expression of genes encoding tight junction proteins, including claudin-1 and zonula occludens 1 (ZO1), and regulates the distribution of occludins. In the presence of high levels of butyrate, the expression of tight junction proteins and distribution of occludins is such that tight junctions are well formed. In an environment where butyrate levels are low, decreased expression of key proteins that form tight junctions causes the junctions to loosen and increases absorption.
Butyrate has also been found to have a direct effect on transporters involved in transcellular transport of oxalate across the intestinal epithelium. Butyrate was shown to stimulate SLC26A3 expression and promoter activity via regulation of specific transcription factors, including yin yang 1 and GATA109. Lactobacillus acidophilus was also found to stimulate the expression of SLC26A3 via a transcriptional mechanism, which is interesting as this suggests a direct effect of bacteria on transporter expression110. These data suggest that butyrate producers and butyrate itself could have direct effects on transporters involved in oxalate transport across the intestinal epithelium. In this context, the levels of butyrate producers such as L. acidophilus and butyrate itself in the intestines of recurrent kidney stone formers might affect the expression and activity of transporters directly involved in intestinal oxalate absorption leading to a higher propensity to develop stones.
Butyrate was also found to have an important role in increasing transepithelial electrical resistance (TEER) via the activation of AMP-activated protein kinase (AMPK)91,111, which further supports its effect on both paracellular and transcellular ion transport. TEER is the measure of the net movement of all ions across the intestinal epithelium and reflects the resistance that the epithelium exerts against the movement of ions112-114. Increased permeability and overall ‘leakiness’ of the epithelium results in reduction of TEER and easier passage of ions. Na+ and Cl− are the most common physiological ions and drive the current that determines TEER; given that butyrate enhances colonic NaCl absorption via NHE2 and NHE3 transporters112-114, that butyrate levels affect TEER is not surprising115.
Evidence supporting a role for intestinal barrier function in recurrent CaOx kidney stone disease comes from the fact that dietary oxalate is absorbed via both paracellular and transcellular mechanisms, as the intestinal barrier is a determinant of the absorption of compounds and ions across the intestinal epithelium84,116. Studies involving non-stone-forming individuals have shown that ~5–10% of soluble oxalate is absorbed at the intestinal level (inter-individual and intra-individual variability 1–20%)117. However, intestinal oxalate absorption was found to be higher in stone-forming individuals, suggesting that intestinal integrity and function has an important role in increased oxalate absorption in stone formers. Given its role in maintaining overall intestinal epithelial health and function, butyrate produced by fermentation of dietary constituents by the gut microbiome might, therefore, also be involved in the overall maintenance of oxalate homeostasis and, therefore, CaOx stone risk.
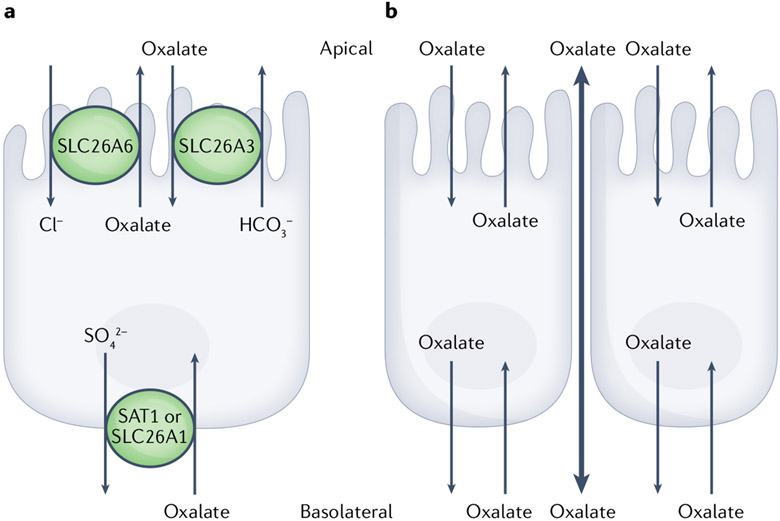
Transcellular transport of oxalate is thought to occur via the activity of specific ion channels, mainly members of the SLC26 family of transporters (FIG. 3). SLC26A6 (also known as PAT1) and SLC26A3 (also known as DRA) have been shown to have key roles in regulating apical oxalate transport116. The corresponding oxalate transporter at the basolateral membrane remains unknown; however, SLC26A1 (also known as SAT1) is believed to be a candidate, based on studies in SAT1-KO mice that showed decreased intestinal oxalate secretion resulting in increased oxalate absorption82.
Fig. 3 ∣. Oxalate transport across intestinal epithelium.
Oxalate absorption and secretion across the intestinal epithelium occur via transcellular (through cells) and paracellular (between cells) mechanisms. Transcellular oxalate absorption and secretion involve transporters found on the apical (lumen of intestine) and basolateral (circulation) sides of the intestinal epithelial layer. For absorption, dietary oxalate enters intestinal epithelial cells on the apical side via SLC26A3 and exits into the circulation via a transport mechanism that remains to be elucidated, but might include SLC26A3. Secretion of oxalate from the circulation back into the lumen of the intestine occurs via SLC26A1 on the basolateral side and SLC26A6 on the apical side of the membrane. Paracellular transport of oxalate is passive and occurs through the tight junctions of the cells on the basolateral side via SLC26A3 and SLC26A6.
In contrast to transcellular transport, paracellular transport involves the movement of oxalate between cells in response to transepithelial electrical and concentration gradients acting on oxalate, as well as membrane tight junction characteristics118. Interestingly, oxalate absorption (movement from the lumen of the intestine into the blood) occurs via both paracellular and transcellular pathways, whereas oxalate secretion (movement from the blood into the intestinal lumen) is believed to be mainly via the transcellular route. As a result, the overall net direction of oxalate movement across epithelia of the intestines is dependent on the relative contribution of each of the unidirectional fluxes and pathways118.
Evidence has suggested that SCFAs might have a role in recurrent kidney stone disease, in particular in CaOx stones119. In a 2020 study, disruption of the intestinal microbiome of rats using antibiotics was shown to increase ethylene-glycol-induced crystal formation in the kidneys of the animals, which could be reduced by the administration of acetate, propionate and butyrate. Furthermore, studies in patients with recurrent CaOx stones showed that the intestinal microbiomes of patients were characterized by lower levels of SCFA-producing bacterial species and a lower abundance of genes involved in metabolic pathways associated with SCFA production compared with individuals without stone disease. Collectively, these data suggest that bacteria-induced SCFAs might have important roles in CaOx kidney stone disease.
Further evidence of a potential role for SCFAs in recurrent kidney stone disease comes from a case–control study of 88 children aged 4–18 years, including 44 patients with stones that contained at least 50% CaOx and 44 controls matched for age, sex and race120. Shotgun metagenomic sequencing revealed 31 bacterial taxa to be less abundant in the gut microbiomes of stone formers, which was reflected in the decreased abundance of the gene encoding butyryl-CoA-dehydrogenase (bcd), a key enzyme in butyrate metabolism. Overall, the study indicated that the loss of gut bacteria involved in butyrate production was associated with perturbations of the metabolome, suggesting that decreased butyrate production might have an important role in early-onset CaOx kidney stone disease.
Diet, microbiome composition and recurrent kidney stones
The human gut microbiome is largely — although not solely — influenced by diet. Nutrients are consumed in relatively large amounts multiple times a day, as they are required for hundreds of bodily functions including as substrates for the synthesis of proteins and other biologically active substances required for function and growth, co-factors for enzymes and structural components for bone, teeth and cell membranes121. Nutrients also regulate fluid and acid–base balance122. In addition to nutrients, food also provides non-nutrient substances that are absorbed and have systemic biological effects; these substances include phytochemicals, non-essential amino acids and fatty acids123. Plant-based foods, in particular, provide edible but indigestible carbohydrate, lignan and lignin components, which are collectively referred to as fibre. At various points along the gastrointestinal tract, different types of fibre (broadly grouped into soluble and insoluble) regulate bowel function, lipid metabolism and blood glucose levels124. As fibre is indigestible, it also reaches the lower intestinal tract to provide sustenance for gut microorganisms, the preponderance of which reside there.
Although fibre was traditionally the major dietary constituent thought to affect the gut microbiome, microbiome-modulating effects of other nutrients (including vitamins, minerals, protein and fats) and of non-nutrient compounds and chemicals such as polyphenols are now recognized85,125. Overall, all aspects of the human diet — not only fibre — seem to influence the gut microbiome in some way or another. As the effects of the gut microbiome on human health and disease are now known to reach far beyond the gastrointestinal tract, probably resulting from symbiotic interplay developed during human evolution126, the optimal diet should, therefore, include foods that deliver sufficient energy and nutrients to maintain the host’s homeostasis and also to optimize the function of the gut microbiome.
Role of diet in recurrent kidney stone disease.
Diet influences urinary tract calculus formation and growth at multiple points along the lithogenic continuum83. This influence is highly variable between individuals owing, in part, to variable intestinal tract digestive and absorptive function, underlying physiological and metabolic processes, food–drug interactions, and many other factors that potentially include variable gene–nutrient interactions and the expression of various diet-related diseases, such as type 2 diabetes mellitus83. Thus, the role of diet in kidney stone disease is complex, with a plethora of primary dietary factors implicated in the most common types of kidney stones (TABLE 1). In susceptible individuals, dietary risk factors contribute to physiological and/or biochemical mechanisms that cause derangements in renal handling, leading to urinary stone risk factors and the potential to form kidney stones. Notably, kidney stones do not form in all people with dietary risk factors, suggesting that other factors combine with diet to contribute to stone disease. Given that the microbial composition of the human intestinal tract varies widely between individuals, the gut microbiome could be one of the factors that accounts for the variable incidence of stone disease among otherwise similar individuals.
Table 1 ∣.
Common dietary risk factors for calcium and uric acid stones
| Dietary risk factor | Mechanism | Urinary aberrationa | Specific stone risk |
|---|---|---|---|
| Fluid intake insufficient to maintain suitably low urine supersaturation | Concentrated urine | Increased urine supersaturation for crystal formation | All types of stones |
| Intake of bicarbonate precursors insufficient to compensate for acidogenic foods in diet | High dietary acid load inducing bone resorption and increased renal citrate reabsorption | Hypercalciuria Hypocitraturia Overly acidic urine |
CaOx, CaPhos, uric acid |
| Excessive intake of salt (as NaCl) | Expansion of extracellular volume and decreased renal calcium reabsorption in nephron | Hypercalciuria | CaOx, CaPhos |
| Excessive calcium supplementation (above the DRI)b | Increased intestinal calcium absorption | Hypercalciuria | CaOx, CaPhos |
| Excessive vitamin D supplementation (above the DRI)c | Increased intestinal calcium absorption | Hypercalciuria | CaOx, CaPhos |
| Vitamin D intake insufficient to maintain normal limits | Secondary hyperparathyroidism and bone resorption | Hypercalciuria | CaOx, CaPhos |
| Calcium intake insufficient to meet needs for bone, especially in the context of increased dietary protein intake | Rise in calcitriol production | Hypercalciuria | CaOx, CaPhos |
| Calcium intake insufficient to compensate for oxalate load in the diet, especially in the context of malabsorption | Increased intestinal absorption of dietary oxalate | Hyperoxaluria | CaOx |
| Excessive vitamin C supplementation (>2,000 mg/day) | Increased biosynthesis of oxalate | ||
| Excessive intake of animal-derived purines | Increased uric acid biosynthesis | Hyperuricosuria | Uric acid |
CaOx, calcium oxalate; CaPhos, calcium phosphate; DRI, dietary reference intake. aAssessed using 24-h urine collection. bDRI for calcium is 1,200 mg/day for adults >19 years of age. cDRI for vitamin D is 600 International Units (15 mcg)/day for adults.
Diet, microbiome, hyperoxaluria and CaOx stones.
Until ~2017, hyperoxaluria and CaOx kidney stones were the best known links between stone disease and the gut microbiome, owing to data showing that O. formigenes, an obligate oxalotroph common to mammals, was lacking in the faecal microbiomes of people who formed CaOx stones30. The lack of O. formigenes was thought to severely compromise bacterial oxalate degradation such that more dietary oxalate was absorbed and available to be excreted in urine. However, subsequent studies support the existence of oxalate-degrading networks comprising many different microorganisms, even in the absence of O. formigenes, which are responsible for most oxalate degradation127,129. This finding explains why not all individuals with absent or low O. formigenes form CaOx stones. The fact that, even among CaOx stone formers, hyperoxaluria is identified as causative in only 20–30% of cases128; means that other factors — such as hypercalciuria, hypocitraturia, hypomagnesiuria and/or low urine volume — must be involved.
Broader effects of diet on the human microbiome and kidney stones.
Assuming that hyperoxaluria caused by a lack of oxalate degradation in the human gut microbiome is only one of several influences on kidney stones exerted by gut microorganisms, this raises the question of additional pathways that might also be involved. This question is a focus of considerable research efforts and several groups are engaged in promising inquiries. Based on what is known about the effects of diet on the gut microbiome, coupled with known effects of the microbiome on human physiology, some inferences to kidney stones can be made.
First, given that diverse networks of microorganisms seem to be most responsible for oxalate degradation in the human intestinal tract127,129, dietary factors associated with reductions or depletion in microorganisms known to inhabit these networks can be identified. For example, >100 bacterial taxa have been identified as being involved in oxalate degradation128 and dietary factors that affect all of these microorganisms are not yet known. However, Lactobacillus and Bifidobacterium, two species with major roles in stone biology are both less abundant based on metagenomic studies in individuals who report increased salt (sodium chloride) intake129. Second, a reduction in oxalate intake, which is frequently recommended to patients with CaOx stones, promotes the depletion of OMBS, including non-oxalotrophs (bacterial species capable of breaking down and using oxalate as a main energy source)128. Although these changes might not be problematic for individuals who consistently follow a low-oxalate diet, transient consumption of oxalate could result in concomitant spikes in oxalate absorption and urinary excretion, potentially stimulating CaOx crystal formation130. Moreover, the consequences of oxalate-degrading bacterial network depletion for other aspects of human health are unknown. Based on known effects of diet on the gut microbiome, and considering the physiological effects of changes in gut microbiota, several other speculative links between diet, the gut microbiome and kidney stone disease can be made (TABLE 2). For instance, the increased intake of saturated fats promotes colonization with species associated with decreased inulin sensitivity131, resulting in a deficit in ammonia production and lower urinary ammonium excretion132. The resultant increase in urine pH leads to an increased risk of uric acid stone formation.
Table 2 ∣.
Dietary factors influencing the gut microbiome, altering the risk of kidney stone disease
| Increased dietary factor |
Effects on gut microbiome | Potential implications for urolithiasis |
Effect on risk of specific stone types |
|
|---|---|---|---|---|
| Fibre | Expands microbial diversity | Increases oxalate degradation capacity of microbial networks; enhances microbial production of short-chain fatty acids | Reduced dietary oxalate absorption and urinary excretiona | ↓CaOx stones |
| Oxalate | Selects for oxalate-degrading bacteria | Increases oxalate degradation and promotes oxalate secretion into the digestive tract160 | Reduced dietary oxalate absorption and urinary excretiona | |
| Legumes | Promote colonization of bacteria that produce short-chain fatty acids | Increases gut barrier161 and reduces inflammation | Reduced dietary oxalate absorption and urinary excretiona | |
| Inulin-type fructans | Improves colonic mucosa absorptive capacity | Increased dietary magnesium absorption162 and urinary excretion | ||
| Fructo-oligosaccharides | Increase bacterial production of butyrate | Promotes skeletal resistance to parathyroid hormone163 | Reduced urinary calcium excretion | ↓CaOx stones ↓CaPhos stones |
| Various polysaccharides and disaccharides (e.g. inulin, lactulose) | Promote colonization of Bifidobacterium longum and other Bifidobacteria164 | Reduces bone resorption, potentially by downregulating osteoclast activity164 | Reduced urinary calcium excretion | |
| Phytoestrogens | Provide substrate for microbial production of equol | Equol binds to human oestrogen receptor to decrease bone resorption165 | Reduced urinary calcium excretion | |
| Animal meat (including fish and seafood) | Selects for sulfidogenic bacteria | Increases bacterial production of sulphuric acid, leading to increased intestinal permeability166 | Increased dietary oxalate absorption and urinary excretion | ↑CaOx stones |
| Salt (sodium chloride) | Suppresses colonization of Bifidobacteria and Lactobacillus129 | Reduces oxalate degradation capacity of microbiome | Increased dietary oxalate absorption and urinary excretion | |
| Saturated fats | Increase abundance of lipopolysaccharide-bearing bacterial species | Activates Toll-like receptors on immune cells and increased intestinal permeability and inflammation167 | Increased dietary oxalate absorption and urinary excretion | |
| Promote colonization of species associated with decreased insulin sensitivity131 | Promotes defect in ammonia genesis leading to lower urinary ammonium excretion132 | Overly acidic urine | ↑Uric acid stones |
The listed effects do not account for synergy between dietary factors; the impact of any one dietary factor is likely to be less important for the gut microbiome than the dietary pattern as a whole. CaOx, calcium oxalate; CaPhos, calcium phosphate. aAssumes that higher oxalate consumption promotes concomitant increases in oxalate-degrading bacteria.
Although the role of diet in the gut microbiome is reasonably well understood, the effects of diet-induced gut microbiome profiles on kidney stone risk are relatively unknown. Dietary habits among stone formers have been investigated129,133, but no studies have tested whether gut microbial modulation with diet affects stone risk or the incidence or recurrence of stone events. The effect of diet on the gut microbiome and its concomitant influence on kidney stone disease is, therefore, an emerging area of interest.
Antibiotics, dysbiosis and recurrent stones
In addition to diet, antibiotics use is also thought to be linked to an increased risk of kidney stone development. The discovery and administration of antibiotics is one of the most revolutionary advances in the history of medicine, enabling the treatment of bacterial infections and enhancing the safety of modern medical procedures. Thus, antibiotics are administered worldwide on a massive scale: in 2018, ~250 million courses of antibiotics were prescribed in the USA, equivalent to 763 antibiotics prescriptions per 1,000 persons134. In a given year, 30% of people are prescribed at least one antibiotic in the UK135. However, such widespread and frequent antibiotics use is not without risk: in addition to the well-established occurrence of antibiotics resistance, antibiotics use has been shown to be associated with alterations in microbiome composition.
In general, antibiotics-induced dysbiosis manifests as a reduction in the level of overall microbial diversity and as declines and expansions in the relative abundances of certain microbial taxa136. Antibiotics treatment affects a specific set of bacteria137, most commonly resulting in increased abundance of Proteobacteria and higher ratios of Firmicutes to Bacteroidetes138. However, the specific pattern of microbiota alteration depends on the characteristics of both the antibiotic and the host. Antibiotics-related factors include antibiotics class, dosage, duration and administration route, and host-related factors include host age, lifestyle and microbiome composition139. Microbial alterations typically persist weeks to months after antibiotics exposure138, but alterations lasting years have also been reported, particularly after administration of antibiotics with strong and broad activity against anaerobes. This effect is mainly because the natural re-introduction of these species into the intestinal environment after antibiotics treatment is limited owing to their high sensitivity to even low concentrations of oxygen136.
Antibiotics exposure has been associated with several diseases, including asthma140 and inflammatory bowel disease141, and these associations are believed to be mediated by perturbation of the microbiome. The rapid shift in the epidemiology of kidney stone disease suggests that environmental exposures, including antibiotics, could also be driving forces for the development of this disease. Research has, therefore, focused on identifying the relationship between antibiotics use and risk of kidney stone disease. For example, antibiotics use for ≥2 months was shown to be associated with a higher risk of incident kidney stones for women in early (age 20–39 years) and middle adulthood (age 40–49 years), compared with no antibiotics use142. However, the mechanism for this effect remains unclear, as only marginally lower urine pH and citrate values were observed among women with varying durations of antibiotics exposure142.
Similarly, a separate study reported that adults with an active episode of kidney stone disease were more likely to have taken oral antibiotics in the year before diagnosis, compared with individuals who had no history of the disease59. The investigators also reported that antibiotics use had a considerable effect on the beta diversity (differences in bacterial species present in a given sample) of the urinary tract microbiome, but not the gut microbiome in this population. Finally, exposure to sulfonylureas, cephalosporins, fluroquinolones, nitrofurantoin/methenamine and broad-spectrum penicillins has been shown to be associated with an increased risk of kidney stone disease143. The magnitude of risk was greatest for exposure 3–6 months before diagnosis and for individuals exposed to antibiotics at younger ages. Although these associations were robust even when excluding individuals with previous UTI, the possibility of unmeasured confounding due to antibiotics prescribed for UTI symptoms without an associated diagnosis of UTI remains. Taken together, the findings of these studies do suggest that antibiotics use is a risk factor for kidney stone disease.
Nonetheless, the mechanisms by which antibiotics affect the gut microbiome in individuals with kidney stone disease is a critical knowledge gap. That antibiotics could alter the composition of the microbiome and metabolism of macronutrients is a plausible explanation — studies have shown that the use of certain antibiotics including chloramphenicol, colistin, doxycycline, erythromycin, polymyxin B, rifampin and tetracycline — markedly decreases the prevalence of colonization by O. formigenes, a major oxalate degrader in the gut144. Indeed, O. formigenes is susceptible to some of the same antibiotics that are associated with an increased risk of kidney stone disease, such as cephalosporins, nitrofurantoin and broad-spectrum penicillins145. Thus, persistent elimination of Oxalobacter after antibiotics exposure might be a risk factor and a mechanism resulting in CaOx kidney stone formation. However, given the complexity of the gut microbiota, decreased colonization by Oxalobacter alone is very unlikely to be responsible for incident kidney stone disease. Instead, an increased risk of stones might arise from dysfunction of bacterial networks.
For example, oxalate homeostasis is known to be maintained by a multispecies bacterial network that includes Ruminococcaceae and Oscillospira129. The bacteria in this network are believed to rely on the by-products of oxalate metabolism — CO2 and formate — which can then be used in several metabolic pathways. Formate is used in most tissues in the synthesis of nucleotides and methyl groups146. In animal studies, exposure to antibiotics disrupts these symbiotic relationships that are critical for oxalate homeostasis and results in increased urinary oxalate levels and risk of CaOx stone formation60.
Another possible mechanism is that antibiotics can select for multidrug-resistant bacteria, which might promote the growth of kidney stones via incorporation into the stone matrix as a component that facilitates crystal attachment and growth. This potential mechanism is supported by a study reporting that 70% of bacteria isolated from CaOx stones were resistant to multiple antibiotics69.
Although antibiotic exposure might be an important link between dysbiosis and kidney stone disease, further studies are required to identify the specific perturbations of the gut and urinary microbiome caused by antibiotics. Potential research areas include identification of the way in which specific classes of antibiotics perturb the gut microbiome and which subgroups of patients are at an increased risk of kidney stones after antibiotics exposure. This information would have important implications for understanding the dramatic increase in the prevalence of kidney stone disease, revealing novel therapeutic pathways, and reducing inappropriate antibiotics use.
Effects of water intake on the intestinal microbiome
A strong correlation exists between low fluid intake and kidney stone formation147. As a result, all individuals, but especially stone formers, are encouraged to increase their fluid intake to ensure adequate hydration and normalization of urine parameters to within non-stone-forming ranges147.
A 2020 systemic review studying the role of fluid intake in kidney stone prevention analysed the effect of six types of water and ten different types of juices on stone formation147 and indicated that fluids low in calcium, as well as grapefruit, apple and orange juice, reduced urine CaOx saturations and subsequent stone formation.
The intestinal microbiome is thought to affect the way in which fluid intake influences overall health. For instance, a 2020 study used a novel approach to assess these effects, by comparing solute concentration measurements of domestic tap water with intestinal microbiome composition and health data from 85 monozygotic twins with existing faecal microbiota profiles148. The use of identical twins is particularly powerful in this study, as this approach controlled for genetic and early life factors, increasing the ability of the study to detect environmental effects. Overall, significant associations were observed between average daily doses of sodium in tap water and microbiota diversity. Furthermore, the overall microbial community composition differed with average daily doses of sulphate and chloride, and the abundance of taxa was associated with the chemical water composition in a number of participants.
A separate study similarly explored associations between source (n = 3,413 subjects) and intake (n = 3,794 subjects) of plain drinking water and gut microbiota composition. Overall, the source of drinking water — including bottled, tap, filtered or well water — was associated with significant variation in alpha and beta diversities. For example, the greatest differences in both alpha and beta diversity were observed between people consuming well water and individuals consuming bottled water. Interestingly, this study also noted differences in the relative abundance of specific bacterial taxa when comparing the intestinal microbiome composition of individuals who consumed low (<1 l/day) versus high amounts (>1 l/day) of water, whereby those consuming <1 l/day tended to have a lower abundance of bacterial taxa, including Gammaproteobacteria, Lactococcus, Streptophyta, Veillonella, Lactobacillales, Clostridium and Erysipelotrichaceae, than those drinking >1 l/day.
Although these data are interesting, direct causality between decreased water intake and changes in gut microbiome composition has not been established. However, stool consistency — which is believed to be partly dependent on the degree of water intake — has been shown to be strongly associated with faecal microbial composition148-150. Of particular relevance to stone disease, constipation has been associated with a relative decrease in obligate bacteria, including Lactobacillus, Bifidobacterium and Bacteroides species, potentially influencing intestinal motility and secretory functions151-154. These particular bacterial species have the ability to degrade oxalate, which, in addition to regulating urinary saturation, might be another mechanism by which water regulates the risk of stone disease. These data emphasize the importance of incorporating water intake into studies on the role of the intestinal microbiome in kidney stone disease.
Future directions
Although research has begun to make clear links between the microbiome and the onset of kidney stone disease, more work is critically needed.
First, although microbial oxalate metabolism in the gut is clearly important in helping to prevent the formation of kidney stones, our understanding of the interactions between oxalate and the gut microbiota is limited. Second, the way in which gut microbiota contribute to other urinary abnormalities — such as imbalances in pH, uric acid, sodium, calcium, magnesium and other urinary risk factors — has received little attention, with the exception of studies that have found increased saturated fat intake to promote the abundance of bacterial species with decreased inulin sensitivity, hence decreasing ammonia genesis, leading to an overly acidic urine and increased risk of uric acid stone formation131,132. Finally, our understanding of the microbially derived proteins and metabolites that contribute to stone formation — either directly by promoting mineral crystallization and aggregation, or indirectly by inducing inflammation, oxidative stress or epithelial barrier function — is also very limited.
These gaps in knowledge can be addressed by performing long-term in vivo studies focused on assessing how changes in microbiome composition alter urine composition in patients and how varying oxalate levels affect the overall symbiotic nature of the urinary microbiome and associated overall metabolic function. That said, studies on how changes in intestinal and/or urinary microbiome compositions affect mineral crystallization and aggregation are more difficult to conduct, as current models of natural and spontaneous stone formation155 include companion animals (dogs and cats), and various captive and wild species, including otters, dolphins and ferrets, that are limited by high costs, given the large number of animals needed to obtain reliable data, and ethical barriers preventing the use of such animals in research.
Future therapeutic potential.
Members of the intestinal microbiome regulate key physiological processes including protective, metabolic, trophic and immune functions156-159. The main function of bacteria in the colon is the fermentation of non-digestible carbohydrates to form SCFAs, which have a key role in regulating gut epithelial integrity and overall health. Given their direct role in the maintenance of processes that are determinants of overall health, the manipulation of the gut microbiome composition has considerable therapeutic potential as a ‘natural’ intervention to treat human diseases. Potential therapeutic approaches might include the introduction of individual bacterial species and/or networks whose loss is associated with a given disease, or even targeted eradication of bacterial species and/or networks identified as directly causing or exacerbating a given disease state.
Although these types of therapeutic approaches sound intriguing, several factors need to be taken into account before they can be implemented. For instance, although the re-introduction of bacteria seems relatively simple in theory, it can be very complex given that one is trying to re-introduce bacteria into an environment in which they did not survive to begin with and, therefore, a favourable overall environment might need to be created that will undoubtedly affect the composition of the pre-existing microbiome. Similarly, although targeted approaches to eradicate ‘unwanted’ bacterial species and/or networks sounds simple, the impact on innocent bystanders must also be taken into consideration. Additional research including these areas must, therefore, be conducted to better understand the potential overall effect of a given intervention before it can be translated into and implemented in clinical practice.
Conclusions
The current literature suggests an intriguing association between antibiotics-induced and probably also diet-induced dysbiosis of both the intestinal and urinary microbiomes and recurrent kidney stone disease. Ongoing work is focused on determining the exact mechanisms by which either of these microbiomes affects overall oxalate homeostasis. Although much focus has been on a direct role of members of the intestinal and urinary microbiome on oxalate breakdown, findings that SCFAs such as butyrate might have a role suggest the existence of oxalate-independent mechanisms, for example, via the regulation of immune responses in the kidney that are associated with kidney stone formation, gene and/or protein expression of relevant intestinal and kidney ion transporters, and/or the effect on actual stone growth via incorporation into the stone matrix.
The data collected to date suggest a multi-mechanistic role of both microbiomes in recurrent kidney stone disease and open up an exciting new area of research with the potential to lead to novel treatment options that can target multiple mechanisms of stone formation simultaneously, which is not possible with current treatment options that target a single mechanism.
Key points.
Composition of the intestinal microbiome influences host metabolism and overall health.
Patients with recurrent kidney stone disease have a disrupted intestinal microbiome composition, including reduced overall abundance of butyrate-producing species.
Butyrate is a key short-chain fatty acid responsible for overall intestinal epithelial integrity and health, a major determinant affecting the absorption of oxalate and other ions relevant to kidney stone disease.
Long-term history of antibiotic use is associated with an antibiotics-driven shift in intestinal microbiome composition and increased risk of kidney stone disease.
Members of the microbiome have a role in different processes of stone formation, including intestinal oxalate absorption and crystal formation.
Urine also has a native urinary microbiome, the composition of which is better able to differentiate between stone and non-stone formers than that of the intestine.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Cho I & Blaser MJ The human microbiome: at the interface of health and disease. Nat. Rev. Genet 13, 260–270 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malla MA et al. Exploring the human microbiome: the potential future role of next-generation sequencing in disease diagnosis and treatment. Front. Immunol 9, 2868 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NIH Human Microbiome Portfolio Analysis Team. A review of 10 years of human microbiome research activities at the US National Institutes of Health, fiscal years 2007–2016. Microbiome 7, 31 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke G. et al. Minireview: gut microbiota: the neglected endocrine organ. Mol. Endocrinol 28, 1221–1238 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliphant K & Allen-Vercoe E Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome 7, 91 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fulde M & Hornef MW Maturation of the enteric mucosal innate immune system during the postnatal period. Immunol. Rev 260, 21–34 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Kamada N, Chen GY, Inohara N & Nunez G Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol 14, 685–690 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ijssennagger N. et al. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon. Proc. Natl Acad. Sci. USA 112, 10038–10043 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reinhardt C. et al. Tissue factor and PAR1 promote microbiota-induced intestinal vascular remodelling. Nature 483, 627–631 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuman H, Debelius JW, Knight R & Koren O Microbial endocrinology: the interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev 39, 509–521 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Yano JM et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canfora EE, Jocken JW & Blaak EE Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol 11, 577–591 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Tuddenham S & Sears CL The intestinal microbiome and health. Curr. Opin. Infect. Dis 28, 464–470 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch SV & Pedersen O The human intestinal microbiome in health and disease. N. Engl. J. Med 375, 2369–2379 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Durack J & Lynch SV The gut microbiome: relationships with disease and opportunities for therapy. J. Exp. Med 216, 20–40 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sokol H. et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl Acad. Sci. USA 105, 16731–16736 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schirmer M. et al. Dynamics of metatranscription in the inflammatory bowel disease gut microbiome. Nat. Microbiol 3, 337–346 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gevers D. et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 15, 382–392 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujimura KE & Lynch SV Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe 17, 592–602 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turnbaugh PJ et al. A core gut microbiome in obese and lean twins. Nature 457, 480–484 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turnbaugh PJ et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Larsen N. et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 5, e9085 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin J. et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Marin IA et al. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci. Rep 7, 43859 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang DW et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 5, 10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clapp M. et al. Gut microbiota’s effect on mental health: the gut-brain axis. Clin. Pract 7, 987 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilkins LJ, Monga M & Miller AW Defining dysbiosis for a cluster of chronic diseases. Sci. Rep 9, 1–10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moe OW Kidney stones: pathophysiology and medical management. Lancet 367, 333–344 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Robertson WG, Peacock M, Marshall RW, Marshall DH & Nordin BC Saturation-inhibition index as a measure of the risk of calcium oxalate stone formation in the urinary tract. N. Engl. J. Med 294, 249–252 (1976). [DOI] [PubMed] [Google Scholar]
- 30.Kaufman DW et al. Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J. Am. Soc. Nephrol 19, 1197–1203 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sidhu H. et al. Absence of Oxalobacter formigenes in cystic fibrosis patients: a risk factor for hyperoxaluria. Lancet 352, 1026–1029 (1998). [DOI] [PubMed] [Google Scholar]
- 32.Kumar R. et al. Role of Oxalobacter formigenes in calcium oxalate stone disease: a study from North India. Eur. Urol 41, 318–322 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Duncan SH et al. Oxalobacter formigenes and its potential role in human health. Appl. Environ. Microbiol 68, 3841–3847 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allison MJ, Dawson KA, Mayberry WR & Foss JG Oxalobacter formigenes gen. nov., sp. nov.: oxalate-degrading anaerobes that inhabit the gastrointestinal tract. Arch. Microbiol 141, 1–7 (1985). [DOI] [PubMed] [Google Scholar]
- 35.Miller AW & Dearing D The metabolic and ecological interactions of oxalate-degrading bacteria in the mammalian gut. Pathogens 2, 636–652 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campieri C. et al. Reduction of oxaluria after an oral course of lactic acid bacteria at high concentration. Kidney Int. 60, 1097–1105 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Turroni S. et al. Oxalate consumption by lactobacilli: evaluation of oxalyl-CoA decarboxylase and formyl-CoA transferase activity in Lactobacillus acidophilus. J. Appl. Microbiol 103, 1600–1609 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Turroni S. et al. Oxalate-degrading activity in Bifidobacterium animalis subsp. lactis: impact of acidic conditions on the transcriptional levels of the oxalyl coenzyme A (CoA) decarboxylase and formyl-CoA transferase genes. Appl. Environ. Microbiol 76, 5609–5620 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Batagello CA, Monga M & Miller AW Calcium oxalate urolithiasis: a case of missing microbes? J. Endourol 32, 995–1005 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Hoppe B. et al. A randomised phase I/II trial to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Pediatr. Nephrol 32, 781–790 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Hoppe B. et al. Efficacy and safety of Oxalobacter formigenes to reduce urinary oxalate in primary hyperoxaluria. Nephrol. Dial. Transplant 26, 3609–3615 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Hoppe B. et al. Oxalobacter formigenes: a potential tool for the treatment of primary hyperoxaluria type 1. Kidney Int. 70, 1305–1311 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Milliner D, Hoppe B & Groothoff J A randomised phase II/III study to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Urolithiasis 46, 313–323 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jairath A. et al. Oxalobacter formigenes: opening the door to probiotic therapy for the treatment of hyperoxaluria. Scand. J. Urol 49, 334–337 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Lieske JC, Goldfarb DS, De Simone C & Regnier C Use of a probiotic to decrease enteric hyperoxaluria. Kidney Int. 68, 1244–1249 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Lieske JC et al. Diet, but not oral probiotics, effectively reduces urinary oxalate excretion and calcium oxalate supersaturation. Kidney Int. 78, 1178–1185 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferraz RRN et al. Effects of Lactobacillus casei and Bifidobacterium breve on urinary oxalate excretion in nephrolithiasis patients. Urol. Res 37, 95–100 (2009). [DOI] [PubMed] [Google Scholar]
- 48.Okombo J & Liebman M Probiotic-induced reduction of gastrointestinal oxalate absorption in healthy subjects. Urol. Res 38, 169–178 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Al-Wahsh I, Wu Y & Liebman M Acute probiotic ingestion reduces gastrointestinal oxalate absorption in healthy subjects. Urol. Res 40, 191–196 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Siener R. et al. The role of Oxalobacter formigenes colonization in calcium oxalate stone disease. Kidney Int. 83, 1144–1149 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Goldfarb DS, Modersitzki F & Asplin JR A randomized, controlled trial of lactic acid bacteria for idiopathic hyperoxaluria. Clin. J. Am. Soc. Nephrol 2, 745–749 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Hoppe B & Martin-Higueras C Improving treatment options for primary hyperoxaluria. Drugs 10.1007/s40265-022-01735-x (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stern JM et al. Evidence for a distinct gut microbiome in kidney stone formers compared to non-stone formers. Urolithiasis 44, 399–407 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang R. et al. 16S rRNA gene sequencing reveals altered composition of gut microbiota in individuals with kidney stones. Urolithiasis 10.1007/s00240-018-1037-y (2018). [DOI] [PubMed] [Google Scholar]
- 55.Suryavanshi MV et al. Hyperoxaluria leads to dysbiosis and drives selective enrichment of oxalate metabolizing bacterial species in recurrent kidney stone endures. Sci. Rep 6, 34712 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suryavanshi MV, Bhute SS, Gune RP & Shouche YS Functional eubacteria species along with trans-domain gut inhabitants favour dysgenic diversity in oxalate stone disease. Sci. Rep 8, 1–11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ticinesi A. et al. Understanding the gut–kidney axis in nephrolithiasis: an analysis of the gut microbiota composition and functionality of stone formers. Gut 67, 2097–2106 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Miller AW, Choy D, Penniston KL & Lange D Inhibition of urinary stone disease by a multi-species bacterial network ensures healthy oxalate homeostasis. Kidney Int. 96, 180–188 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zampini A, Nguyen AH, Rose E, Monga M & Miller AW Defining dysbiosis in patients with urolithiasis. Sci. Rep 9, 5425 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller AW, Orr T, Dearing D & Monga M Loss of function dysbiosis associated with antibiotics and high fat, high sugar diet. ISME J. 13, 1379–1390 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller AW, Oakeson KF, Dale C & Dearing MD Microbial community transplant results in increased and long-term oxalate degradation. Microb. Ecol 72, 470–478 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller AW, Dale C & Dearing MD The induction of oxalate metabolism in vivo is more effective with functional microbial communities than with functional microbial species. mSystems 2, e00088–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stern JM et al. Fecal transplant modifies urine chemistry risk factors for urinary stone disease. Physiol. Rep 7, e14012 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolfe AJ et al. Evidence of uncultivated bacteria in the adult female bladder. J. Clin. Microbiol 50, 1376–1383 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie J. et al. Profiling the urinary microbiome in men with calcium-based kidney stones. BMC Microbiol. 20, 41 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwaderer AL & Wolfe AJ The association between bacteria and urinary stones. Ann. Transl. Med 5, 32 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dornbier RA et al. The microbiome of calcium-based urinary stones. Urolithiasis 48, 191–199 (2019). [DOI] [PubMed] [Google Scholar]
- 68.Barr-Beare E. et al. The interaction between Enterobacteriaceae and calcium oxalate deposits. PLoS ONE 10, e0139575 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tavichakorntrakool R. et al. Extensive characterizations of bacteria isolated from catheterized urine and stone matrices in patients with nephrolithiasis. Nephrol. Dial. Transplant 27, 4125–4130 (2012). [DOI] [PubMed] [Google Scholar]
- 70.Hobbs T, Schultz LN, Lauchnor EG, Gerlach R & Lange D Evaluation of biofilm induced urinary infection stone formation in a novel laboratory model system. J. Urol 199, 178–185 (2018). [DOI] [PubMed] [Google Scholar]
- 71.Chutipongtanate S, Sutthimethakorn S, Chiangjong W & Thongboonkerd V Bacteria can promote calcium oxalate crystal growth and aggregation. J. Biol. Inorg. Chem 18, 299–308 (2013). [DOI] [PubMed] [Google Scholar]
- 72.Kanlaya R, Naruepantawart O & Thongboonkerd V Flagellum is responsible for promoting effects of viable Escherichia coli on calcium oxalate crystallization, crystal growth, and crystal aggregation. Front. Microbiol 10, 2507 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sivaguru M. et al. Geobiology reveals how human kidney stones dissolve in vivo. Sci. Rep 8, 1–9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sivaguru M. et al. Human kidney stones: a natural record of universal biomineralization. Nat. Rev. Urol 18, 404–432 (2021). [DOI] [PubMed] [Google Scholar]
- 75.Saw J. et al. In vivo entombment of bacteria and fungi during calcium oxalate, brushite, and struvite urolithiasis. Kidney 360 2, 298–311 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Flannigan R, Choy WH, Chew B & Lange D Renal struvite stones-pathogenesis, microbiology, and management strategies. Nat. Rev. Urol 11, 333–341 (2014). [DOI] [PubMed] [Google Scholar]
- 77.Jansma J & El Aidy S Understanding the host-microbe interactions using metabolic modeling. Microbiome 9, 16 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Donia MS & Fischbach MA Human microbiota. Small molecules from the human microbiota. Science 349, 1254766 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Silva YP, Bernardi A & Frozza RL The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol 11, 25 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van der Hee B & Wells JM Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 29, 700–712 (2021). [DOI] [PubMed] [Google Scholar]
- 81.Al-Harbi NO et al. Short chain fatty acid, acetate ameliorates sepsis-induced acute kidney injury by inhibition of NADPH oxidase signaling in T cells. Int. Immunopharmacol 58, 24–31 (2018). [DOI] [PubMed] [Google Scholar]
- 82.Dawson PA et al. Urolithiasis and hepatotoxicity are linked to the anion transporter Sat1 in mice. J. Clin. Invest 120, 706–712 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heilberg IP & Goldfarb DS Optimum nutrition for kidney stone disease. Adv. Chronic Kidney Dis 20, 165–174 (2013). [DOI] [PubMed] [Google Scholar]
- 84.Mitchell T. et al. Dietary oxalate and kidney stone formation. Am. J. Physiol. Renal Physiol 316, F409–F413 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rowland I. et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr 57, 1–24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Duan X. et al. 1 H NMR-based metabolomic study of metabolic profiling for the urine of kidney stone patients. Urolithiasis 48, 27–35 (2020). [DOI] [PubMed] [Google Scholar]
- 87.Pallister T. et al. Hippurate as a metabolomic marker of gut microbiome diversity: modulation by diet and relationship to metabolic syndrome. Sci. Rep 7, 1–9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang X. et al. Identification of urine biomarkers for calcium-oxalate urolithiasis in adults based on UPLC-Q-TOF/MS. J. Chromatogr. B 1124, 290–297 (2019). [DOI] [PubMed] [Google Scholar]
- 89.Iwanaga T & Kishimoto A Cellular distributions of monocarboxylate transporters: a review. Biomed. Res 36, 279–301 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Schonfeld P & Wojtczak L Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J. Lipid Res 57, 943–954 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suzuki T, Yoshida S & Hara H Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br. J. Nutr 100, 297–305 (2008). [DOI] [PubMed] [Google Scholar]
- 92.Macfarlane GT & Macfarlane S Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int 95, 50–60 (2012). [DOI] [PubMed] [Google Scholar]
- 93.Matey-Hernandez ML et al. Genetic and microbiome influence on lipid metabolism and dyslipidemia. Physiol. Genomics 50, 117–126 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.den Besten G. et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res 54, 2325–2340 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Druart C. et al. Role of the lower and upper intestine in the production and absorption of gut microbiota-derived PUFA metabolites. PLoS ONE 9, e87560 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lehnen TE, da Silva MR, Camacho A, Marcadenti A & Lehnen AM A review on effects of conjugated linoleic fatty acid (CLA) upon body composition and energetic metabolism. J. Int. Soc. Sports Nutr 12, 36 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Andrade-Oliveira V. et al. Gut bacteria products prevent AKI induced by ischemia-reperfusion. J. Am. Soc. Nephrol 26, 1877–1888 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Machado RA et al. Sodium butyrate decreases the activation of NF-κB reducing inflammation and oxidative damage in the kidney of rats subjected to contrast-induced nephropathy. Nephrol. Dial. Transpl 27, 3136–3140 (2012). [DOI] [PubMed] [Google Scholar]
- 99.Marzocco S. et al. Supplementation of short-chain fatty acid, sodium propionate, in patients on maintenance hemodialysis: beneficial effects on inflammatory parameters and gut-derived uremic toxins, a pilot study (PLAN Study). J. Clin. Med 10.3390/jcm7100315 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang J. et al. Effects of prebiotic fiber xylooligosaccharide in adenine-induced nephropathy in mice. Mol. Nutr. Food Res 10.1002/mnfr.201800014 (2018). [DOI] [PubMed] [Google Scholar]
- 101.Vaziri ND et al. High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS ONE 9, e114881 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wong J. et al. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol 39, 230–237 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang W, Zhou L, Guo H, Xu Y & Xu Y The role of short-chain fatty acids in kidney injury induced by gut-derived inflammatory response. Metabolism 68, 20–30 (2017). [DOI] [PubMed] [Google Scholar]
- 104.Li LZ, Tao SB, Ma L & Fu P Roles of short-chain fatty acids in kidney diseases. Chin. Med. J 132, 1228–1232 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Khan SR Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J. Urol 189, 803–811 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Khan SR, Canales BK & Dominguez-Gutierrez PR Randall’s plaque and calcium oxalate stone formation: role for immunity and inflammation. Nat. Rev. Nephrol 17, 417–433 (2021). [DOI] [PubMed] [Google Scholar]
- 107.Feng W. et al. Sodium butyrate attenuates diarrhea in weaned piglets and promotes tight junction protein expression in colon in a GPR109A-dependent manner. Cell Physiol. Biochem 47, 1617–1629 (2018). [DOI] [PubMed] [Google Scholar]
- 108.Wang HB, Wang PY, Wang X, Wan YL & Liu YC Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig. Dis. Sci 57, 3126–3135 (2012). [DOI] [PubMed] [Google Scholar]
- 109.Alrefai WA et al. Molecular cloning and promoter analysis of downregulated in adenoma (DRA). Am. J. Physiol. Gastrointest. Liver Physiol 293, G923–G934 (2007). [DOI] [PubMed] [Google Scholar]
- 110.Raheja G. et al. Lactobacillus acidophilus stimulates the expression of SLC26A3 via a transcriptional mechanism. Am. J. Physiol. Gastrointest. Liver Physiol 298, G395–G401 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peng L, Li ZR, Green RS, Holzman IR & Lin J Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr 139, 1619–1625 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Krishnan S, Rajendran VM & Binder HJ Apical NHE isoforms differentially regulate butyrate-stimulated Na absorption in rat distal colon. Am. J. Physiol. Cell Physiol 285, C1246–C1254 (2003). [DOI] [PubMed] [Google Scholar]
- 113.Musch MW, Bookstein C, Xie Y, Sellin JH & Chang EB SCFA increase intestinal Na absorption by induction of NHE3 in rat colon and human intestinal C2/bbe cells. Am. J. Physiol. Gastrointest. Liver Physiol 280, G687–G693 (2001). [DOI] [PubMed] [Google Scholar]
- 114.Kiela PR, Hines ER, Collins JF & Ghishan FK Regulation of the rat NHE3 gene promoter by sodium butyrate. Am. J. Physiol. Gastrointest. Liver Physiol 281, G947–G956 (2001). [DOI] [PubMed] [Google Scholar]
- 115.Farre R, Fiorani M, Abdu Rahiman S & Matteoli G Intestinal permeability, inflammation and the role of nutrients. Nutrients 10.3390/nu12041185 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Freel RW, Whittamore JM & Hatch M Transcellular oxalate and Cl-absorption in mouse intestine is mediated by the DRA anion exchanger Slc26a3, and DRA deletion decreases urinary oxalate. Am. J. Physiol. Gastrointest. Liver Physiol 305, G520–G527 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hesse A, Schneeberger W, Engfeld S, Von Unruh GE & Sauerbruch T Intestinal hyperabsorption of oxalate in calcium oxalate stone formers: application of a new test with [13C2]oxalate. J. Am. Soc. Nephrol 10, S329–S333 (1999). [PubMed] [Google Scholar]
- 118.Whittamore JM & Hatch M The role of intestinal oxalate transport in hyperoxaluria and the formation of kidney stones in animals and man. Urolithiasis 45, 89–108 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu Y. et al. The relationship between gut microbiota and short chain fatty acids in the renal calcium oxalate stones disease. FASEB J. 34, 11200–11214 (2020). [DOI] [PubMed] [Google Scholar]
- 120.Denburg MR et al. Perturbations of the gut microbiome and metabolome in children with calcium oxalate kidney stone disease. J. Am. Soc. Nephrol 31, 1358–1369 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen Y, Michalak M & Agellon LB Importance of nutrients and nutrient metabolism on human health. Yale J. Biol. Med 91, 95–103 (2018). [PMC free article] [PubMed] [Google Scholar]
- 122.Remer T. Influence of nutrition on acid-base balance-metabolic aspects. Eur. J. Nutr 40, 214–220 (2001). [DOI] [PubMed] [Google Scholar]
- 123.Orzechowski A, Ostaszewski P, Jank M & Berwid SJ Bioactive substances of plant origin in food-impact on genomics. Reprod. Nutr. Dev 42, 461–477 (2002). [DOI] [PubMed] [Google Scholar]
- 124.McRorie JW Jr & McKeown NM Understanding the physics of functional fibers in the gastrointestinal tract: an evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J. Acad. Nutr. Diet 117, 251–264 (2017). [DOI] [PubMed] [Google Scholar]
- 125.Schmidt TSB, Raes J & Bork P The human gut microbiome: from association to modulation. Cell 172, 1198–1215 (2018). [DOI] [PubMed] [Google Scholar]
- 126.Schroeder BO & Backhed F Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med 22, 1079–1089 (2016). [DOI] [PubMed] [Google Scholar]
- 127.Suryavanshi MV, Bhute SS, Gune RP & Shouche YS Functional eubacteria species along with trans-domain gut inhabitants favour dysgenic diversity in oxalate stone disease. Sci. Rep 8, 16598 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Miller AW, Dale C & Dearing MD Microbiota diversification and crash induced by dietary oxalate in the mammalian herbivore Neotoma albigula. mSphere 10.1128/mSphere.00428-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Seck EH et al. Salt in stools is associated with obesity, gut halophilic microbiota and Akkermansia muciniphila depletion in humans. Int. J. Obes 43, 862–871 (2019). [DOI] [PubMed] [Google Scholar]
- 130.Bernardino M & Parmar MS Oxalate nephropathy from cashew nut intake. CMAJ 189, E405–E408 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Murphy EA, Velazquez KT & Herbert KM Influence of high-fat diet on gut microbiota: a driving force for chronic disease risk. Curr. Opin. Clin. Nutr. Metab. Care 18, 515–520 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Abate N, Chandalia M, Cabo-Chan AV Jr., Moe OW & Sakhaee K The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int. 65, 386–392 (2004). [DOI] [PubMed] [Google Scholar]
- 133.Ticinesi A. et al. Understanding the gut-kidney axis in nephrolithiasis: an analysis of the gut microbiota composition and functionality of stone formers. Gut 67, 2097–2106 (2018). [DOI] [PubMed] [Google Scholar]
- 134.Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions — United States, 2018. CDC; https://www.cdc.gov/antibiotic-use/community/programs-measurement/state-local-activities/outpatient-antibiotic-prescriptions-US-2018.html (2018). [Google Scholar]
- 135.Shallcross L, Beckley N, Rait G, Hayward A & Petersen I Antibiotic prescribing frequency amongst patients in primary care: a cohort study using electronic health records. J. Antimicrob. Chemother 72, 1818–1824 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Modi SR, Collins JJ & Relman DA Antibiotics and the gut microbiota. J. Clin. Invest 124, 4212–4218 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ferrer M, Méndez-García C, Rojo D, Barbas C & Moya A Antibiotic use and microbiome function. Biochem. Pharmacol 134, 114–126 (2017). [DOI] [PubMed] [Google Scholar]
- 138.Neuman H, Forsythe P, Uzan A, Avni O & Koren O Antibiotics in early life: dysbiosis and the damage done. FEMS Microbiol. Rev 42, 489–499 (2018). [DOI] [PubMed] [Google Scholar]
- 139.Ianiro G, Tilg H & Gasbarrini A Antibiotics as deep modulators of gut microbiota: between good and evil. Gut 65, 1906–1915 (2016). [DOI] [PubMed] [Google Scholar]
- 140.Risnes KR, Belanger K, Murk W & Bracken MB Antibiotic exposure by 6 months and asthma and allergy at 6 years: findings in a cohort of 1,401 US children. Am. J. Epidemiol 173, 310–318 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kronman MP, Zaoutis TE, Haynes K, Feng R & Coffin SE Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics 130, e794–e803 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ferraro PM, Curhan GC, Gambaro G & Taylor EN Antibiotic use and risk of incident kidney stones in female nurses. Am. J. Kidney Dis 74, 736–741 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tasian GE et al. Oral antibiotic exposure and kidney stone disease. J. Am. Soc. Nephrol 29, 1731–1740 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kelly JP, Curhan GC, Cave DR, Anderson TE & Kaufman DW Factors related to colonization with Oxalobacter formigenes in US adults. J. Endourol 25, 673–679 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lange JN et al. Sensitivity of human strains of Oxalobacter formigenes to commonly prescribed antibiotics. Urology 79, 1286–1289 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pietzke M, Meiser J & Vazquez A Formate metabolism in health and disease. Mol. Metab 33, 23–37 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gamage KN et al. The role of fluid intake in the prevention of kidney stone disease: a systematic review over the last two decades. Turk. J. Urol 46, S92–S103 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bowyer RCE et al. Associations between UK tap water and gut microbiota composition suggest the gut microbiome as a potential mediator of health differences linked to water quality. Sci. Total. Environ 739, 139697 (2020). [DOI] [PubMed] [Google Scholar]
- 149.Vandeputte D. et al. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 65, 57–62 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Falony G. et al. Population-level analysis of gut microbiome variation. Science 352, 560–564 (2016). [DOI] [PubMed] [Google Scholar]
- 151.Zhao Y & Yu YB Intestinal microbiota and chronic constipation. Springerplus 5, 1130 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gerritsen J, Smidt H, Rijkers GT & de Vos WM Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 6, 209–240 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kirgizov IV, Sukhorukov AM, Dudarev VA & Istomin AA Hemostasis in children with dysbacteriosis in chronic constipation. Clin. Appl. Thromb. Hemost 7, 335–338 (2001). [DOI] [PubMed] [Google Scholar]
- 154.Khalif IL, Quigley EM, Konovitch EA & Maximova ID Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig. Liver Dis 37, 838–849 (2005). [DOI] [PubMed] [Google Scholar]
- 155.Alford A, Furrow E, Borofsky M & Lulich J Animal models of naturally occurring stone disease. Nat. Rev. Urol 17, 691–705 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ley RE, Turnbaugh PJ, Klein S & Gordon JI Microbial ecology: human gut microbes associated with obesity. Nature 444, 1022–1023 (2006). [DOI] [PubMed] [Google Scholar]
- 157.Sekirov I & Finlay BB The role of the intestinal microbiota in enteric infection. J. Physiol 587, 4159–4167 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sekirov I, Russell SL, Antunes LC & Finlay BB Gut microbiota in health and disease. Physiol. Rev 90, 859–904 (2010). [DOI] [PubMed] [Google Scholar]
- 159.Mukherjee S, Joardar N, Sengupta S & Sinha Babu SP Gut microbes as future therapeutics in treating inflammatory and infectious diseases: lessons from recent findings. J. Nutr. Biochem 61, 111–128 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hatch M. Gut microbiota and oxalate homeostasis. Ann. Transl. Med 5, 36 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sanchez-Tapia M. et al. Consumption of cooked black beans stimulates a cluster of some clostridia class bacteria decreasing inflammatory response and improving insulin sensitivity. Nutrients 10.3390/nu12041182 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Scholz-Ahrens KE & Schrezenmeir J Inulin and oligofructose and mineral metabolism: the evidence from animal trials. J. Nutr 137, 2513S–2523S (2007). [DOI] [PubMed] [Google Scholar]
- 163.Xu X et al. Intestinal microbiota: a potential target for the treatment of postmenopausal osteoporosis. Bone Res. 5, 17046 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kiousi DE et al. Probiotics in extraintestinal diseases: current trends and new directions. Nutrients 10.3390/nu11040788 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Weaver CM & Legette LL Equol, via dietary sources or intestinal production, may ameliorate estrogen deficiency-induced bone loss. J. Nutr 140, 1377S–1379S (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Blaut M & Clavel T Metabolic diversity of the intestinal microbiota: implications for health and disease. J. Nutr 137, 751S–755S (2007). [DOI] [PubMed] [Google Scholar]
- 167.Guo S. et al. Lipopolysaccharide regulation of intestinal tight junction permeability is mediated by TLR4 signal transduction pathway activation of FAK and MyD88. J. Immunol 195, 4999–5010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]