Abstract
Objective
We aimed to analyze the relationship between non-alcoholic fatty liver and progressive fibrosis and serum 25-hydroxy vitamin D (25(OH)D) in patients with type 2 diabetes mellitus.
Methods
A total of 184 patients with T2DM who were hospitalized in the Department of Endocrinology of the ShiDong Clinical Hospital between January 2023 and June 2023 were selected. We compared review of anthropometric, biochemical, and inflammatory parameters and non-invasive scores between groups defined by ultrasound NAFLD severity grades.We determine the correlation between 25(OH)D and FLI and FIB-4 scores, respectively.
Results
Statistically significant differences were seen between BMI, WC, C-peptide levels, FPG, ALT, serum 25(OH)D, TC, HDL, lumbar spine bone density, FLI, and FIB-4 in different degrees of NAFLD. Multivariate logistic regression analysis showed that 25(OH)D (OR = 1.26, p = 0.001), age (OR = 0.93, P < 0.001) and BMI (OR = 1.04, p = 0.007) were independent predictors of NAFLD in patients with T2DM.
Conclusions
This study revealed the correlation between serum 25(OH)D levels and NAFLD in patients with T2DM. We also demonstrated that serum 25(OH)D levels were negatively correlated with FLI/FIB-4 levels in patients with T2DM with NAFLD, suggesting that vitamin D deficiency may promote hepatic fibrosis progression in T2DM with NAFLD.
Keywords: Type 2 diabetes mellitus, Non-alcoholic fatty liver disease, Progressive liver fibrosis, 25-hydroxyvitamin D
Introduction
Nonalcoholic Fatty Liver Disease (NAFLD) is a liver disease characterized by an excessive accumulation of fat in the liver but not associated with excessive alcohol consumption [1]. Non-alcoholic steatohepatitis (NASH) is a severe type of NAFLD characterized pathologically by hepatic steatosis, balloon-like degeneration of hepatocytes, and lobular inflammation [2]. They are related to abnormal liver enzyme levels, insulin resistance, type 2 diabetes mellitus (T2DM), and increased cardiovascular risk [3]. The global prevalence of NAFLD and NASH is estimated to be around 25% and 5%, respectively, and is increasing [4]. NASH is indeed a serious health problem, and its progression can lead to a variety of serious complications. As NASH continues to develop, the damage to the liver becomes progressively worse, eventually leading to the development of cirrhosis. Liver failure is also one of the serious complications that may occur in the later stages of NASH development [5].
Steatosis and fibrosis are common pathologic processes in liver diseases, and their early detection and monitoring of their course are crucial for patient treatment and management. Traditional diagnostic methods for liver diseases, such as liver biopsy, although highly accurate, are invasive and carry certain risks. Therefore, in recent years, noninvasive scoring methods have received widespread attention and application. Non-invasive scoring methods are mainly based on biochemical parameters, epidemiological data, and anthropometric indicators, combined with non-invasive imaging techniques such as ultrasound and magnetic resonance to assess the degree of hepatic steatosis and fibrosis. These methods can help in the early detection of liver lesions, monitoring of disease progression, and assessment of treatment efficacy [6–8]. The European Association for the Study of Diabetes (EASD) has approved the use of the Fatty Liver Index (FLI) and the Fibrosis-4 Index (FIB-4) to assess liver fibrosis [9].
Approximately 60% of T2DM patients suffer from NAFLD [10]. Several studies have emphasized the strong interaction between NAFLD and T2DM and described a complex bidirectional relationship [11].
Studies have found that people with fatty liver often have abnormal levels of vitamins. Vitamins play a role in the development of NAFLD [12], but the roles and mechanisms of many vitamins in NAFLD are not clear at present.
Vitamin D is a steroid hormone that plays an important role in the regulation of bone and calcium homeostasis [13]. In addition to its classical role in bone health, vitamin D has recently been shown to have a variety of biological roles in different cell and tissue types, regulating cell proliferation, differentiation and immune regulation [14]. Indeed, many studies have shown that low circulating levels of vitamin D are associated with the development of liver fibrosis in patients with various chronic liver diseases (CLDs) [15, 16]. Vitamin D also play an important role in modifying the risk of T2DM, mainly in terms of its mediation of beta-cell function, insulin sensitivity, and systemic inflammatory responses [17].
Currently, there are few studies on the relationship between 25(OH)D levels and the degree of liver fibrosis in patients with T2DM combined with NAFLD. We hypothesized that serum 25(OH)D levels may play a role in the pathogenesis of NAFLD and its progression to fibrosis in T2DM patients.
Materials and methods
Patients and study design
184 patients with T2DM who were hospitalized in the Department of Endocrinology of the ShiDong Clinical Hospital between January 2023 and June 2023 were selected, and the diagnosis of T2DM was based on the 1999 WHO Diagnostic and Classification Criteria.
Inclusion Criteria: 1)Age ≥ 18 years. 2) T2DM conformed to 1999 WHO Diagnostic Criteria. 3) Ultrasound Diagnosis Grading Criteria: assessed according to the degree of echo weakening in the liver by color ultrasound examination. Liver ultrasound was performed by the same ultrasonographer experienced in liver disease ultrasonography aiming to obtain uniform NAFLD stratification and to avoid interobserver variations in findings. On each study participant, liver ultrasound was performed by a Toshiba Xario SSA-660 A (Toshiba, Japan) device, equipped with a 5 MHz convex probe. The NAFLD grade description is based on the ultrasonographic finding. There are three grades of NAFLD based on visual analysis. Grade 1 NAFLD presents with increased liver echogenicity, grade 2 NAFLD with the echogenic liver obscuring the echogenic walls of the portal venous branches, and grade 3 NAFLD diaphragmatic out line is obscured.The levels of 25(OH)D in the patients were divided into three grades: <20 ng/mL, 20–30 ng/mL and > 30 ng/mL.
Exclusion Criteria: For patients who drink a lot of alcohol, the amount of alcohol consumed should equate to the amount of alcohol consumed, which is more than 140 g/week for men and more than 70 g/week for women; use of medications that affect vitamin D levels; T1DM, special type diabetes mellitus, acute complications of diabetes mellitus; pregnant and lactating women; ethanol-induced or drug-induced liver disease, autoimmune disease, viral hepatitis, cholestasis, and metabolic liver disease, hepatomegaly; total parenteral nutrition; autoimmune liver disease; thyroid disease; rheumatoid immune disease.
Data collection
General clinical information [gender, age, waist circumference (WC), height, weight, blood pressure was collected from the medical history. Blood measurements included fasting plasma glucose(FPG), fasting C-peptide, fasting insulin, glycosylated hemoglobin (HbA1c), 25-hydroxy vitamin D[25(OH)D], total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), low-density lipoprotein cholesterol (LDL-C), alanine aminotransferase (ALT), aspartate aminotransferase (AST), uric acid (UA), creatinine (Cr), urea nitrogen (BUN), and other biochemicals. Body mass index (BMI) is determined by the formula weight (kg)/height (m)2.
All patients were grouped according to their diagnosis after a color ultrasound of the liver. Lumbar spine bone mineral density was measured using dual-energy X-bone absorptiometry in the enrolled patients.
Statistical analysis
The statistical analysis of this study was conducted using IBM SPSS statistics software Version 26. Measurement data were subjected to K-S normality test, and the data conforming to normal distribution were expressed as mean ± standard deviation (x ± s).Comparisons between groups were analyzed by one-way ANOVA.We used the parametric Pearson test and the nonparametric Spearman test to determine correlation. Multivariate logistic regression modeling were performed with the effect of several predictors on ultrasound grading results in nonalcoholic fatty liver disease.
Results
A total of 184 T2DM patients with NAFLD were enrolled in the study. 89 (48%) of the 184 patients with NAFLD had mild fatty liver, 55 (30%) had moderate fatty liver, and 40 (22%) had severe fatty liver. Table 1 shows the differences in anthropometric, metabolic profile and non-invasive scores of steatosis between the groups. Statistically significant differences were seen between BMI, WC, C-peptide levels, FPG, ALT, serum 25(OH)D, TC, HDL, lumbar spine bone density, FLI, and FIB-4 in different degrees of NAFLD.
Table 1.
A comparative review of biochemical and inflammatory parameters and non-invasive scores between groups defined by ultrasound NAFLD severity grades
| Characteristics | Grade 1 of liver steatosis (n = 89) | Grade 2 of liver steatosis (n = 55) | Grade 3 of liver steatosis (n = 40) | F | P |
|---|---|---|---|---|---|
| BMI (kg/m2 ) | 25.61 ± 2.13 | 26.50 ± 1.83 | 27.67 ± 1.62ab | 6.049 | 0.003 |
| WC (cm) | 90.43 ± 7.75 | 102.12 ± 7.66 | 102.21 ± 8.01ab | 94.200 | 0.000 |
| HbA1c (%) | 7.55 ± 0.80 | 7.47 ± 0.53 | 7.47 ± 0.53 | 0.225 | 0.799 |
| C-peptide (ng/ml) | 2.43 ± 0.58 | 4.13 ± 1.20 | 4.99 ± 0.61ab | 48.9 | 0.000 |
| FPG (mmol/l) | 7.67 ± 1.23 | 6.41 ± 1.00 | 7.40 ± 0.63ab | 4.49 | 0.009 |
| Platelet count(*109 /L) | 132 ± 43 | 144 ± 29 | 138 ± 38 | 7.431 | 0.099 |
| ALT (U/L) | 24.70 ± 10.36 | 32.33 ± 11.82 | 54.55 ± 4.42ab | 18.05 | 0.000 |
| AST (U/L) | 20.91 ± 3.61 | 19.05 ± 2.90 | 31.20 ± 3.64 | 0.834 | 0.438 |
| γGT (U/L) | 22 ± 13 | 69 ± 20 | 122 ± 31 | 5.833 | 0.001 |
| 25(OH)D (ng/ml) | 35.69 ± 6.10 | 26.70 ± 3.10a | 14.53 ± 6.26ab | 7.17 | 0.001 |
| PTH (pg/ml) | 37.67 ± 11.23 | 36.41 ± 14.00 | 37.40 ± 10.63 | 4.99 | 0.339 |
| TC (mmol/l) | 6.63 ± 1.39 | 7.67 ± 1.23 | 7.67 ± 1.23ab | 4.208 | 0.018 |
| TG (mmol/l) | 2.61 ± 0.71 | 2.48 ± 0.69 | 2.54 ± 0.62 | 0.411 | 0.664 |
| HDL (mmol/l) | 1.38 ± 0.28 | 1.72 ± 0.39 | 1.66 ± 0.38ab | 9.800 | 0.000 |
| LDL (mmol/l) | 3.84 ± 0.80 | 3.59 ± 0.69 | 3.73 ± 0.99 | 1.077 | 0.345 |
| FLI | 57.74 ± 17.30 | 75.89 ± 9.65 | 89.86 ± 9.56ab | 5.286 | 0.007 |
| FIB−4 | 1.67 ± 0.44 | 1.95 ± 0.99 | 2.46 ± 0.23ab | 4.206 | 0.018 |
| lumbar spine bone density (g/cm2) | 0.99 ± 0.44 | 0.87 ± 0.61 | 0.75 ± 0.59ab | 3.734 | 0.006 |
BMI body mass index, WC waist circumference, HbA1c glycated hemoglobin, 25(OH)D 25-hydroxy vitamin D, FPG fasting plasma glucose, ALT alanine transaminase, AST aspartate aminotransferase, γGT gamma-glutamyl transferase, PTH Parathyroid Hormone, TC total cholesterol, TG triglycerides, HDL high density lipoprotein, LDL low density lipoprotein, FLI Fatty liver index score, FIB-4- Fibrosis-4 score
a indicates P<0.05, compare with hepatic steatosis grade 1; b indicates P<0.05, compare with hepatic steatosis grade 2。
Multivariate logistic regression analysis was performed with NAFLD as the dependent variable and anthropometric, metabolic profile and non-invasive scores of steatosis as independent variables. The results showed that 25(OH)D (OR = 1.26, p = 0.001), age (OR = 0.93, P < 0.001) and BMI (OR = 1.04, p = 0.007) were independent predictors of NAFLD in patients with T2DM (Table 2).
Table 2.
Multivariate logistic regression analysis of factors associated with NAFLD in T2DM patients
| Covariate | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age (years) | 1.02 | 0.99–1.07 | < 0.001 | 0.93 | 0.89–0.95 | < 0.001 |
| Sex | ||||||
| Male | ||||||
| Female | 0.68 | 0.55–0.87 | 0.465 | |||
| BMI (kg/m2 ) | 1.12 | 1.02–1.14 | 0.004 | 1.04 | 1.00-1.07 | 0.007 |
| WC (cm) | 1.13 | 1.07–1.15 | 0.038 | 0.83 | 0.79–0.85 | 0.344 |
| HbA1C(%) | 0.93 | 0.88–0.97 | 0.067 | |||
| C-peptide (ng/ml) | 0.73 | 0.69–0.78 | 0.694 | |||
| FPG(mmol/l) | 0.67 | 0.61–0.73 | 0.072 | |||
| ALT(U/L) | 0.66 | 0.62–0.70 | 0.029 | |||
| AST(U/L) | 0.73 | 0.68–0.76 | 0.018 | |||
| 25(OH)D(ng/ml) | 1.17 | 1.14–1.23 | 0.003 | 1.26 | 1.22–1.29 | 0.001 |
| PTH(pg/ml) | 0.13 | 0.07–0.16 | 0.562 | |||
| TC(mmol/l) | 0.46 | 0.42–0.49 | 0.849 | |||
| TG(mmol/l) | 0.69 | 0.63–0.73 | 0.712 | |||
| HDL-C(mmol/l) | 0.34 | 0.30–0.37 | 0.088 | |||
| LDL-C(mmol/l) | 1.28 | 0.24–0.33 | 0.672 | |||
| FLI | 1.18 | 1.14–1.22 | 0.012 | 0.74 | 0.71–0.77 | 0.027 |
| FIB-4 | 1.23 | 1.19–1.27 | 0.025 | 1.09 | 1.06–1.12 | 0.011 |
BMI body mass index, WC waist circumference, HbA1c glycated hemoglobin, 25(OH)D 25-hydroxy vitamin D, FPG fasting plasma glucose, ALT alanine transaminase, AST aspartate aminotransferase, γGT gamma-glutamyl transferase, PTH Parathyroid Hormone, TC total cholesterol TG triglycerides, HDL high density lipoprotein, LDL low density lipoprotein, FLI Fatty liver index score, FIB-4 Fibrosis-4 score
a indicates P<0.05, compare with hepatic steatosis grade 1; b indicates P<0.05, compare with hepatic steatosis grade 2
Correlation analysis revealed that there occurs a negative association between 25(OH)D and FLI (r = -0.45, P < 0.001) (Fig. 1A). As shown in Fig. 1B, there was negative association between 25(OH)D and FIB-4 (r = -0.67, P < 0.001).
Fig. 1.
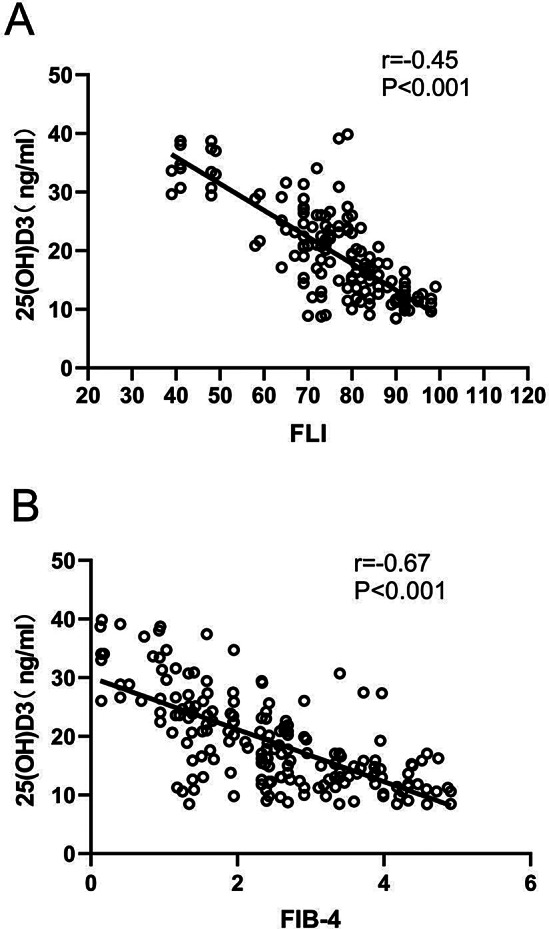
Showed the correlation between (A) 25(OH)D and Fibrosis- 4 (FIB-4) score and (B) 25(OH)D and fatty liver index (FLI) score
Discussion
The direct mechanism between 25(OH)D deficiency and liver fibrosis has not yet been clearly revealed by scientific research. However, we can explore the relationship between the two in terms of their respective effects and possible interactions. In the ANOVA, there was a significant difference in 25(OH)D levels between the different groups. In correlation analysis, we have found a significant negative correlation between 25(OH)D and FIB-4 and FLI. Serum 25(OH)D was an influential factor for NAFLD in multivariate logistic regression analysis. 25(OH)D activation exerts anti-inflammatory and anti-fibrotic effects in the liver by modulating the immune system, inhibiting the expression of pro-fibrotic inflammatory mediators such as platelet-derived growth factor (PDGF) and transforming growth factor β (TGF-β), and promoting the expression of collagen, α-smooth muscle actin, and tissue inhibitor of metalloproteinase-1 [18, 19]. However, once cirrhosis develops, vitamin D loses its antifibrotic protective effect [20].
Some studies suggest that 25(OH)D levels may decline as the severity of NAFLD increases [21]. This decline may be related to impaired liver function, as the liver is an important organ for 25(OH)D synthesis and metabolism [22].Liangpunsakul S et al. found that serum vitamin D levels were significantly lower in those with unexplained elevations in ALT than in controls. In this article, we observed significant differences in ALT between subgroups with different degrees of NAFLD, besides, in a multiple logistic regression analysis, ALT was also found to have a significant effect on the severity of NAFLD [23]. However, in a meta-analysis based on six cross-sectional biopsy studies, Saberi et al. did not find a significant difference between 25(OH)D levels and the degree of liver fibrosis in patients with nonalcoholic fatty liver disease (NAFLD) [24].
In this study, FLI and FIB-4 were statistically different between groups determined by ultrasound NAFLD severity. Therefore, we concluded that FLI and FIB-4 scores were significantly associated with ultrasound NAFLD stratification. We used both of these indicators to score the degree of hepatic fibrosis in T2DM patients. We found that 25(OH)D was negatively correlated with FIB4 and FLI, all of which suggest that 25(OH)D deficiency may promote the progression of hepatic fibrosis in T2DM patients with NAFLD [25].
Our study showed that the was lumbar spine bone density significantly associated with ultrasound NAFLD stratification. The results of this study suggest a correlation between NAFLD and osteoporosis. However, our findings do not support a causal role of osteoporosis on NAFLD. This finding suggests that there may be bone-liver interactions, which are worthy of further study [26].
There was a statistically significant difference in BMI between groups. BMI showed a significant association with NAFLD in both univariate and multivariate analyses. The higher the BMI, the greater the risk of NAFLD (OR 1.12 in univariate analysis and 1.04 in multivariate analysis). This suggests that obesity is a significant risk factor for NAFLD. The increased prevalence of NAFLD is associated with a rising trend in obesity, especially among morbidly obese patients [27–29].
Among the glycemic-related indices, the levels of C-peptide and fasting glucose (FPG) varied with increasing grades of NAFLD. C-peptide levels were significantly higher in grades 2 and 3, whereas FPG levels were relatively high in grades 1 and 3. These changes may be related to insulin resistance due to hepatic steatosis. Alfadda et al. investigated the prevalence of NAFLD in patients with T2DM using transient elastography and found that 80.8% of T2DM patients had steatosis, of which 82.3% had severe steatosis and 17.6% had mild to moderate steatosis [30]. 25(OH)D deficiency promotes the development and progression of NAFLD by causing insulin resistance, increasing hepatic resistin gene expression, and upregulating hepatic steatosis and oxidative stress gene expression [31].
Overall, further studies are needed to clarify the apparent differences in 25(OH)D levels between different degrees of NAFLD at this time. Future studies could conduct larger surveys and clinical trials in patients with different degrees of NAFLD to more accurately assess the relationship between 25(OH)D levels and NALD severity. There is also a need to further explore the mechanism of 25(OH)D’s role in the pathogenesis and treatment of NAFLD and how to improve the condition of patients with NAFLD by adjusting 25(OH)D levels. This will help provide new ideas and methods for the prevention and treatment of NAFLD.
Conclusion
This study revealed the correlation between serum 25(OH)D levels and NAFLD in patients with T2DM. We also demonstrated that serum 25(OH)D levels were negatively correlated with FLI/FIB-4 levels in patients with T2DM with NAFLD, suggesting that vitamin D deficiency may promote hepatic fibrosis progression in T2DM with NAFLD.
Acknowledgements
We acknowledge Dr QingSu for guidance in statistical analysis for English editing.
Author contributions
JF conceived the study and wrote the manuscript. YH, JM and HZ collected clinical data, analyzed and interpreted the data. XH, YH, FH, QG and SW made a revised version. All authors read and agreed to the final version of the manuscript.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not profit sector.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information.
Declarations
Ethics approval and consent to participate
Research was in accordance with Declaration of Helsinki. All participants signed an informed consent, and the Institutional Review Boards of all participating institutions approved the study. The study protocol was approved by the Ethics Committee of Shanghai Yangpu District Shidong Hospital.
Human and animal rights
No animals were used for the study. All human procedures were followed in accordance with the Helsinki Declaration of 1975 as revised in 2013.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jing-Xian Fang and Yu Han shared first authorship.
Contributor Information
Qing Gu, Email: drguqingsd@163.com.
Sui-Jun Wang, Email: drwangsuijun@163.com.
References
- 1.Petroni ML et al. Moderate Alcohol Intake in non-alcoholic fatty liver disease: to drink or not to drink? Nutrients, 2019. 11(12). [DOI] [PMC free article] [PubMed]
- 2.Huby T, Gautier EL. Immune cell-mediated features of non-alcoholic steatohepatitis. Nat Rev Immunol. 2022;22(7):429–43. doi: 10.1038/s41577-021-00639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hassen G, et al. Nonalcoholic fatty liver disease: an emerging modern-day risk factor for Cardiovascular Disease. Cureus. 2022;14(5):e25495. doi: 10.7759/cureus.25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younossi ZM, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Schuster S, et al. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol. 2018;15(6):349–64. doi: 10.1038/s41575-018-0009-6. [DOI] [PubMed] [Google Scholar]
- 6.Wieckowska A, Feldstein AE. Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Semin Liver Dis. 2008;28(4):386–95. doi: 10.1055/s-0028-1091983. [DOI] [PubMed] [Google Scholar]
- 7.Loomba R, Adams LA. Advances in non-invasive assessment of hepatic fibrosis. Gut. 2020;69(7):1343–52. doi: 10.1136/gutjnl-2018-317593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bedogni G, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Mauro S et al. Clinical and molecular biomarkers for diagnosis and staging of NAFLD. Int J Mol Sci, 2021. 22(21). [DOI] [PMC free article] [PubMed]
- 10.Arteh J, Narra S, Nair S. Prevalence of vitamin D deficiency in chronic liver disease. Dig Dis Sci. 2010;55(9):2624–8. doi: 10.1007/s10620-009-1069-9. [DOI] [PubMed] [Google Scholar]
- 11.Tanase DM et al. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD) J Diabetes Res, 2020. 2020: p. 3920196. [DOI] [PMC free article] [PubMed]
- 12.Abe RAM, et al. The role of vitamins in non-alcoholic fatty liver disease: a systematic review. Cureus. 2021;13(8):e16855. doi: 10.7759/cureus.16855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson PH, Turner AG, Morris HA. Vitamin D actions to regulate calcium and skeletal homeostasis. Clin Biochem. 2012;45(12):880–6. doi: 10.1016/j.clinbiochem.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 14.Hossein-nezhad A, Holick MF. Vitamin D for health: a global perspective. Mayo Clin Proc. 2013;88(7):720–55. doi: 10.1016/j.mayocp.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petta S, et al. Low vitamin D serum level is related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis C. Hepatology. 2010;51(4):1158–67. doi: 10.1002/hep.23489. [DOI] [PubMed] [Google Scholar]
- 16.Terrier B, et al. Low 25-OH vitamin D serum levels correlate with severe fibrosis in HIV-HCV co-infected patients with chronic hepatitis. J Hepatol. 2011;55(4):756–61. doi: 10.1016/j.jhep.2011.01.041. [DOI] [PubMed] [Google Scholar]
- 17.Mitri J, Pittas AG. Vitamin D and diabetes. Endocrinol Metab Clin North Am. 2014;43(1):205–32. doi: 10.1016/j.ecl.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timms PM, et al. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM. 2002;95(12):787–96. doi: 10.1093/qjmed/95.12.787. [DOI] [PubMed] [Google Scholar]
- 19.Targher G, et al. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2007;17(7):517–24. doi: 10.1016/j.numecd.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Yuan S, Larsson SC. Inverse Association between serum 25-Hydroxyvitamin D and nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2023;21(2):398–e4054. doi: 10.1016/j.cgh.2022.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Ciardullo S, et al. Low 25 (OH) vitamin D levels are associated with increased prevalence of nonalcoholic fatty liver disease and significant liver fibrosis. Diabetes Metab Res Rev. 2023;39(5):e3628. doi: 10.1002/dmrr.3628. [DOI] [PubMed] [Google Scholar]
- 22.Cai J, et al. Correlation between serum 25-OH vitamin D expression and non-alcoholic fatty liver disease. Exp Ther Med. 2020;19(3):1681–6. doi: 10.3892/etm.2020.8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liangpunsakul S, Chalasani N. Serum vitamin D concentrations and unexplained elevation in ALT among US adults. Dig Dis Sci. 2011;56(7):2124–9. doi: 10.1007/s10620-011-1707-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saberi B, et al. Vitamin D levels do not predict the stage of hepatic fibrosis in patients with non-alcoholic fatty liver disease: a PRISMA compliant systematic review and meta-analysis of pooled data. World J Hepatol. 2018;10(1):142–54. doi: 10.4254/wjh.v10.i1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taban L et al. Vitamin D status and Steatohepatitis in obese Diabetic and non-diabetic patients. J Clin Med, 2022. 11(18). [DOI] [PMC free article] [PubMed]
- 26.Cui A, et al. Causal association of NAFLD with osteoporosis, fracture and falling risk: a bidirectional mendelian randomization study. Front Endocrinol (Lausanne) 2023;14:1215790. doi: 10.3389/fendo.2023.1215790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quek J, et al. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2023;8(1):20–30. doi: 10.1016/S2468-1253(22)00317-X. [DOI] [PubMed] [Google Scholar]
- 28.Barranco-Fragoso B, et al. Identification of hepatic dendritic cells in liver biopsies showing steatosis in patients with metabolic dysfunction-Associated fatty liver Disease (MAFLD) Associated with obesity. Med Sci Monit. 2022;28:e937528. doi: 10.12659/MSM.937528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, et al. Associations between abdominal obesity indices and nonalcoholic fatty liver disease: Chinese visceral Adiposity Index. Front Endocrinol (Lausanne) 2022;13:831960. doi: 10.3389/fendo.2022.831960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alfadda AA, et al. Transient elastography for the prevalence of non-alcoholic fatty liver disease in patients with type 2 diabetes: evidence from the CORDIAL cohort study. Saudi J Gastroenterol. 2022;28(6):426–33. doi: 10.4103/sjg.sjg_73_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Contreras-Bolívar V et al. Mechanisms involved in the relationship between Vitamin D and insulin resistance: impact on clinical practice. Nutrients, 2021. 13(10). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data supporting the findings of this study are available within the paper and its Supplementary Information.


