ABSTRACT
Cryptococcus deneoformans is a yeast-type fungus that causes fatal meningoencephalitis in immunocompromised patients and evades phagocytic cell elimination through an escape mechanism. Memory T (Tm) cells play a central role in preventing the reactivation of this fungal pathogen. Among these cells, tissue-resident memory T (TRM) cells quickly respond to locally invaded pathogens. This study analyzes the kinetics of effector T (Teff) cells and Tm cells in the lungs after cryptococcal infection. Emphasis is placed on the kinetics and cytokine expression of TRM cells in the early phase of infection. CD4+ Tm cells exhibited a rapid increase by day 3, peaked at day 7, and then either maintained their levels or exhibited a slight decrease until day 56. In contrast, CD8+ Tm cells reached their peak on day 3 and thereafter decreased up to day 56 post-infection. These Tm cells were predominantly composed of CD69+ TRM cells and CD69+ CD103+ TRM cells. Disruption of the CARD9 gene resulted in reduced accumulation of these TRM cells and diminished interferon (IFN) -γ expression in TRM cells. TRM cells were derived from T cells with T cell receptors non-specific to ovalbumin in OT-II mice during cryptococcal infection. In addition, TRM cells exhibited varied behavior in different tissues. These results underscore the importance of T cells, which produce IFN-γ in the lungs during the early stage of infection, in providing early protection against cryptococcal infection through CARD9 signaling.
KEYWORDS: Cryptococcus deneoformans, CLR, tissue-resident memory T cells, MPT cells, Th1 response
INTRODUCTION
The two sister species, Cryptococcus neoformans (formerly C. neoformans var. grubii, serotype A) and C. deneoformans (formerly C. neoformans var. neoformans, serotype D), are yeast-type fungal pathogens characterized by thick capsules composed of polysaccharides such as glucuronoxylomannan and galactoxylomannan (1). These fungi grow in pigeon droppings and enter the lungs via an airborne route. While most healthy individuals experience asymptomatic infection, marked by granulomatous lesions in the lungs caused by these fungi, immunocompromised hosts with severely impaired cellular immunity, such as those with AIDS, frequently suffer from severe lung lesions and disseminated infections that extend to the central nervous system (2).
Host defense against cryptococcal infection is critically regulated by the balance between Th1 and Th2 cytokine responses (2–7). Th1-related cytokines, such as IFN-γ, support host defense by inducing the production of nitric oxide, which enhances macrophages’ ability to eliminate Cryptococcus and facilitates granuloma formation at infection sites, impeding the fungi from expanding in the infected lungs (6, 8–10). In contrast, Th2 immune responses, characterized by IL-4 and IL-13 production, counteract Th1-mediated responses by suppressing granulomatous responses and hindering the host’s ability to fend off the fungal pathogen (6, 8, 11). A recent study conducted by our team demonstrated that IL-17A, one of the Th17-related cytokines, regulates Th1-mediated host defense against cryptococcal infection (12). Other studies have also reported the diverse functions of Th17-related cytokines in this context (13–17). Thus, the commitment of Th phenotypes critically influences host susceptibility to cryptococcal infection.
Innate immune cells, such as macrophages and dendritic cells, highly express pattern recognition receptors (PRRs). These PRRs play a crucial role in recognizing pathogen-associated molecular patterns composed of microbial components and danger-associated molecular patterns released from damaged cells (18). C-type lectin receptors (CLRs) are well-known as representative PRRs and act as sensor molecules for fungal pathogen cell wall polysaccharides (19, 20). Numerous studies have demonstrated the important roles of CLRs in recognizing various fungi and in host defense against these microorganisms, including Candida albicans, Pneumocystis carinii, Aspergillus fumigatus, and C. deneoformans (21–25). However, the roles of CLRs in host defense against cryptococcal infection remain incompletely understood, despite investigations into the impact of deficiencies in Dectin-1, Dectin-2, Dectin-3 (also known as MCL, Clec4D, and Clecsf8), and Mincle on the clinical course of the infection (7, 26–32).
In our previous study using a mouse model, we observed that deficiency of caspase recruitment domain-containing protein 9 (CARD9), a common adaptor molecule for CLRs-mediated signaling, rendered mice highly susceptible to pulmonary infection with C. deneoformans mediated by Th1 immune responses (25). In addition, the early-phase production of IFN-γ from memory phenotype T (MPT) cells after cryptococcal infection was significantly affected in CARD9-deficient mice. MPT cells represent a novel T cell subset characterized by a memory T cell phenotype, even in the absence of exposure to foreign antigens (33, 34). Several reports revealed that IFN-γ production from MPT cells plays an important role in infections with Listeria monocytogenes and Toxoplasma gondii, not limited to C. deneoformans (35–37). On the other hand, tissue-resident memory T (TRM) cells, another novel memory T cell subset, are known for their unique ability to migrate to the tissue and reside there for an extended time without recirculation (38). TRM cells exist in various tissues, such as the gastrointestinal tract, lungs, skin, and genital tract, playing a key role in the acute phase of infection control against invading microbes (39–41). While the role of TRM cells is well understood in viral infection (41–43), it remains unclear how TRM cells contribute to infections with other microorganisms and the relationship between TRM cells and MPT cells.
In the present study, we aim to investigate the details of the accumulated MPT and TRM cells in the lungs after cryptococcal infection, shedding light on the involvement of CLRs in these cells.
MATERIALS AND METHODS
Mice
C57BL/6 mice, purchased from CLEA (Tokyo, Japan), were used as controls [wild-type (WT)]. CARD9 gene-disrupted [knockout (KO)], Dectin-2KO, and MincleKO mice were generated and established as described previously (24, 44, 45) and backcrossed to C57BL/6 mice for more than eight generations. OT-II transgenic mice (OT-II mice) (46), which express T cell receptor (TCR) α and β chains that recognize the major histocompatibility complex (MHC) class II Ib–restricted ovalbumin (OVA) peptide (residues 323–339) in a C57BL/6 background, were kindly provided by Dr. N. Ishii (Tohoku University, Sendai, Japan). Male or female mice at 6–8 weeks of age and 16–24 g of weight were used in the experiments. Mice were allocated to each experimental group randomly. All mice were kept under specific pathogen-free conditions at the Institute for Animal Experimentation, Tohoku University Graduate School of Medicine. The conditions of the breeding room were as follows: room temperature, 20°C–26°C; humidity, 40%–60%; light/dark cycle, 12 h; and ad libitum availability of water and food. Microbial monitoring of mice was regularly carried out by the Central Institute for Experimental Animals. We took utmost care to minimize any pain and suffering experienced by the mice. Mice were sacrificed by cervical dislocation prior to analysis.
Inoculation with Cryptococcus deneoformans
A serotype D strain of C. deneoformans, designated B3501 (a kind gift from Kwong Chung, National Institutes of Health, Bethesda, MD, USA), was used. The yeast cells were cultured on potato dextrose agar (Eiken, Tokyo, Japan) plates for 2–3 days before use. Mice were anesthetized by an intramuscular injection of 0.3 mg/kg of midazolam (Fuji Pharma, Tokyo, Japan) and 0.02 mg/kg of medetomidine hydrochloride (Nippon Zenyaku Kogyo, Fukushima, Japan) and an intraperitoneal injection of 15 mg/kg of pentobarbital (Abbott Laboratory, North Chicago, IL, USA). Live C. deneoformans (1 × 106 cells) was inoculated in a 50 µL volume into the trachea of each mouse using a 24-gauge catheter (Terumo, Tokyo, Japan).
Treatment with FTY720
FTY720 was purchased from Cayman Chemical (Ann Arbor, MI, USA). Mice were orally administered FTY720 by ad libitum availability of water containing 10.5 µg/mL FTY720 every day from 2 days before infection with C. deneoformans.
Preparation of lung leucocytes
Pulmonary intraparenchymal leukocytes were prepared as described previously (12, 25, 27, 32, 47). Briefly, the chest of the mouse was opened, and the lung vascular bed was flushed by injecting 3 mL of chilled physiological saline into the right ventricle. The lungs were then excised and washed in physiological saline. The lungs, teased with stainless mesh, were incubated in RPMI 1640 (Sigma-Aldrich, St. Louis, MO, USA) with 10% fetal calf serum (FCS; Biowest, Nuaillé, France), 100 U/mL penicillin G, 100 µg/mL streptomycin, 10 mM HEPES (4–2-hydroxyethyl-1-piperazineethanesulfoni acid), 50 µM 2-mercaptoethanol, 20 U/mL collagenase, and 1 µg/mL DNase I (Sigma-Aldrich, St. Louis, MO, USA). After incubation for 60 min at 37°C with vigorous shaking, the tissue fragments and most dead cells were removed by passing through the 40 µm cell strainer (BD Biosciences, Franklin Lakes, NJ, USA). In the intracellular staining experiments, to prevent the decrease of intracellular IFN-γ by extracellular excretion during the preparation of lung leukocytes, the lungs were incubated with 2 nM monensin from the time of collection until the intrapulmonary leukocytes were separated by Percoll density gradient centrifugation. After centrifugation, the cell pellet was resuspended in 4 mL of 40% (vol/vol) Percoll (Pharmacia, Uppsala, Sweden) and layered onto 4 mL of 80% (vol/vol) Percoll. After centrifugation at 600 × g for 20 min at 20°C, the cells at the interface were collected, washed three times, and counted with a hemocytometer.
Preparation of splenocytes
Mouse spleens were collected in RPMI 1640 with 10% FCS, 100 U/mL penicillin G, and 100 µg/mL streptomycin and teased apart between two ground glass slides. After centrifugation at 320 × g for 5 min at 4°C, the cell pellet was hemolyzed using a red blood cell lysis solution (155 mmol/L NH4Cl and 17 mmol/L Tris, pH 7.2) and washed twice, and the tissue fragments and most dead cells were removed by passing through a 40 µm cell strainer.
Preparation of peripheral blood leukocytes
A total of 500 µL of peripheral blood was collected from mice using heparin (AY Pharmaceuticals, Tokyo). The peripheral blood was hemolyzed using a red blood cell lysis solution and washed twice. This hemolysis process was repeated four times to isolate peripheral blood leukocytes.
Flow cytometry
Lung leukocytes, splenocytes, and peripheral blood leukocytes were pre-incubated with anti-FcγRII/III mAb, which was prepared from the culture supernatants of hybridoma cells (clone 2.4G2) on ice for 15 min in phosphate-buffered saline (PBS) containing 1% FCS and 0.1% sodium azide. The cells were stained with PE-conjugated anti-CD3 mAb (clone 145–2C11; Biolegend, San Diego, CA, USA), APC/Cy7-conjugated anti-CD4 mAb (clone GK1.5; Biolegend) or anti-CD8 mAb (clone 53–6.7; Biolegend), Biotin-conjugated anti-CD127 mAb (clone A7R34; Biolegend), Pacific Blue–conjugated anti-CD44 mAb (clone IM7; Biolegend), APC-conjugated anti-CD69 mAb (clone H1.2F3; Biolegend), FITC-conjugated anti-CD103 mAb (clone 2E-7; Biolegend), PE-conjugated anti-CD4 mAb, APC/Cy7-conjugated anti-TCR Vα2 mAb (clone B20.1; Biolegend), APC-conjugated anti-TCR Vβ5.1/5.2 mAb (clone MR9-4; Biolegend), Biotin-conjugated anti-CD127 mAb, Pacific Blue–conjugated anti-CD44 mAb, PE/Cy7-conjugated anti-CD69 mAb (clone H1.2F3; Biolegend), and FITC-conjugated anti-CD103 mAb. After washing twice, the cells were stained with PerCP/Cy5.5-conjugated streptavidin (Biolegend). After washing twice, the cells were incubated in the presence of Cytofix/Cytoperm (BD Biosciences), washed twice in BD Perm/Wash solution (BD Biosciences), and stained with PE/Cy7-conjugated anti-IFN-γ (clone XMG1.2; Biolegend). Isotype-matched IgG was used for control staining. The stained cells were analyzed using a BD FACS Canto II flow cytometer (BD Bioscience, Franklin Lakes, NJ, USA). The gating strategy is shown in Fig. S1.
Statistical analysis
Data were analyzed using JMP Pro 11.2.0 software (SAS Institute Japan, Tokyo, Japan). Data are expressed as the mean ± SD. Differences between groups were examined for statistical significance using Welch’s t test. A P value of less than 0.05 was considered significant.
RESULTS
Kinetics in the accumulation of effector T and memory T cells in the lungs after cryptococcal infection
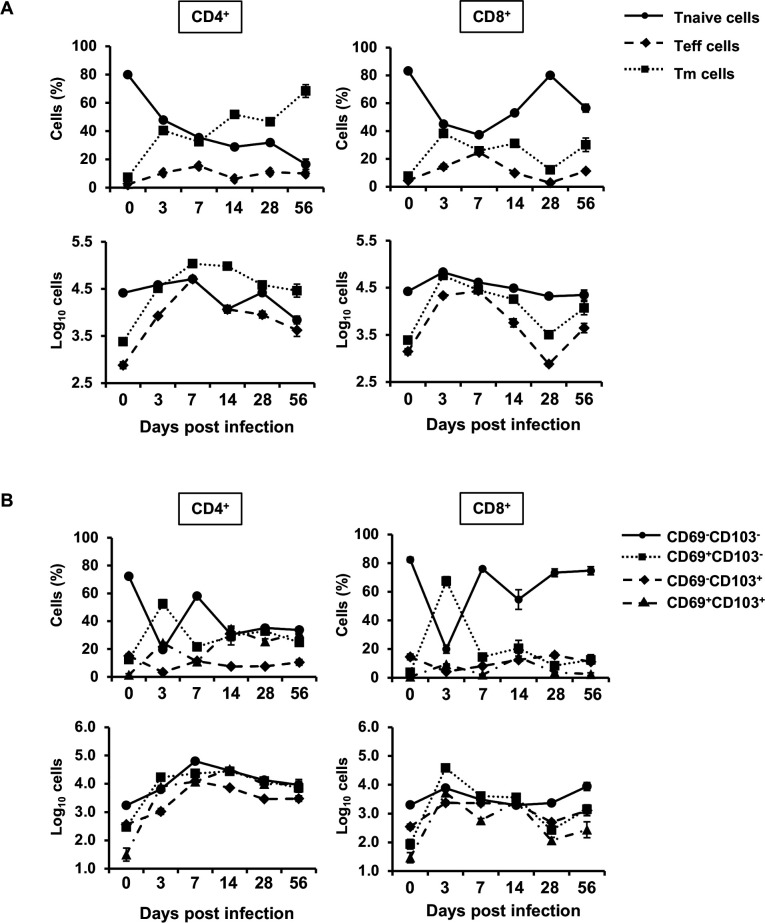
First, we analyzed CD4+ or CD8+ memory T (Tm) cells in the lungs after cryptococcal infection. WT mice were infected intratracheally with C. deneoformans, and then naïve T (Tnaïve) cells, effector T (Teff), and Tm cells were identified on days 0, 3, 7, 14, 28, and 56 after infection. As depicted in Fig. 1A, the number of CD4+ Tm cells drastically increased as early as day 3. CD4+ Tm cells reached their peak on days 7 and 14 and then gradually declined by day 56 post-infection. Similarly, CD4+ Teff cells markedly increased on day 3, peaked on day 7, and then gradually decreased until day 56 (Fig. 1A). In contrast, the number of CD8+ Tm cells peaked on day 3, decreased to basal levels by day 28, and then increased again on day 56 post-infection. Similarly, the number of CD8+ Teff cells reached its peak on days 3 and 7, decreased to basal levels by day 28, and then increased again on day 56. These results suggest that both CD4+ and CD8+ Teff and Tm cells may contribute to host defense in the early phase, such as on day 3 after infection with C. deneoformans.
Fig 1.
Kinetics of pulmonary T cells after C. deneoformans infection WT mice were infected intratracheally with C. deneoformans. The number of naïve T (T naïve: CD44- CD127+), effector T (Teff: CD44bright+ CD127-), and memory T (Tm: CD44bright+ CD127+) cells (A) and CD69 and/or CD103-expressing Tm cells (B) in pulmonary CD4+ or CD8+ T cells were analyzed using flow cytometry on day 0 (uninfected; n = 6), 3 (n = 4), 7 (n = 3), 14 (n = 3), 28 (n = 5), and 56 (n = 5) post-infection. Each column represents the mean ± SD. Representative data demonstrating similar results from independent experiments are presented. Experiments were conducted once for day 0, three times for day 3, twice for days 7 and 14, once for day 28, and once for day 58.
Tm cells, traditionally associated with the acquired immunity phase, exhibited a significant increase during the innate immunity phase. Therefore, we investigated whether these Tm cells were related to TRM cells, which are known for their role in innate immune responses. TRM cells are defined as expressing either or both CD69 and CD103 in CD44bright+ CD127+ Tm cells (48–50). To explore this, WT mice were infected with C. deneoformans, and the various TRM cell subsets in the lungs were analyzed on days 0, 3, 7, 14, 28, and 56 post-infection. CD69+ CD103- and CD69+ CD103+ TRM (double-positive TRM: DP TRM) cells in CD4+ and CD8+ Tm cells significantly increased on day 3 after infection (Fig. 1B). CD69+ CD103- and DP TRM cells in CD8+ TRM cells were increased with a peak level on day 3 and subsequently decreased over the course of infection. However, CD69+ CD103- and DP TRM cells in CD4+ TRM cells were increased to the same level as the peak, and these cells did not exhibit a significant decrease over the course of infection. CD69- CD103+ TRM and CD69- CD103- Tm (double-negative Tm: DN Tm) cells reached their peak on day 7 after infection. These findings suggest that CD69+ CD103- and DP TRM cells, which predominantly compose the increased Tm cells, are involved in host defense against cryptococcal infection in the early phase of infection.
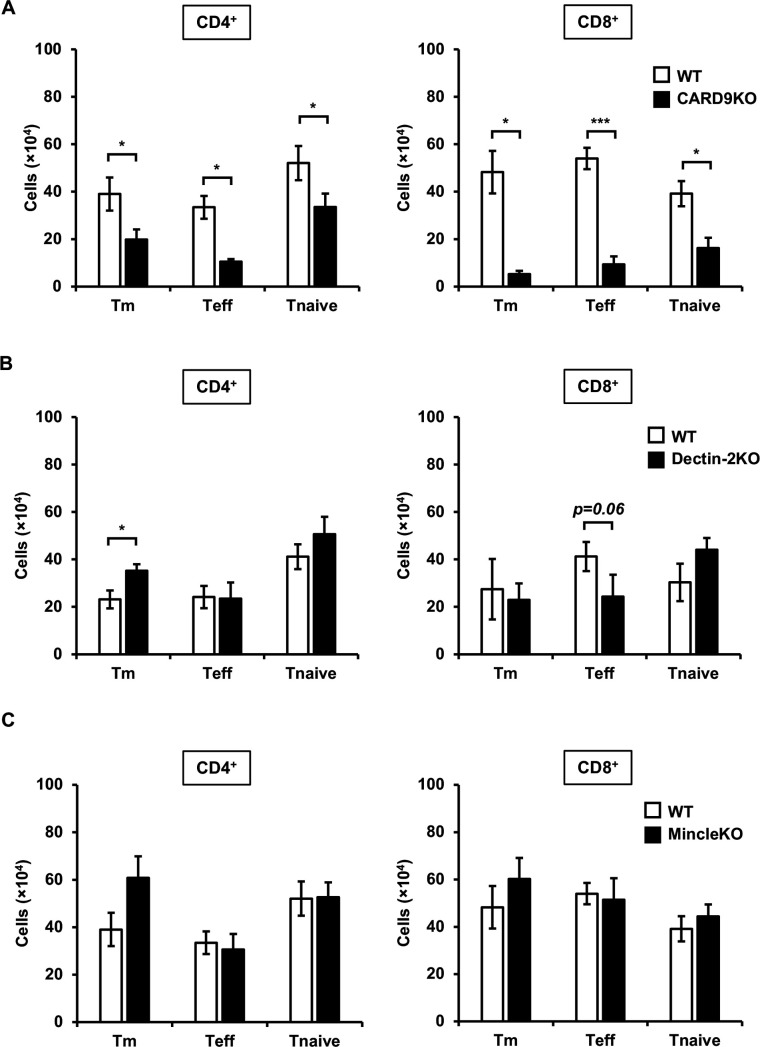
Effect of CARD9, Dectin-2, or Mincle deficiency on Teff and Tm cells in the lungs after cryptococcal infection
In a previous study, we demonstrated that IFN-γ-producing MPT cells were induced via CARD9 signaling in the early phase of cryptococcal infection (25). Consequently, we analyzed the effect of CARD9 deficiency on the accumulation of Teff and Tm cells in the early phase of cryptococcal infection. Three days post-infection, both Teff and Tm cells in CD4+ and CD8+ T cells were significantly lower in the lungs of CARD9KO mice compared to WT mice (Fig. 2A). Therefore, we next investigated which CLRs upstream of CARD9 were involved in the accumulation of these T cells. We previously reported that Dectin-2 and Mincle recognized cryptococcal chitin deacetylase 2 (Cda2) and glucosylceramide (GlcCer), respectively (7, 27–29, 32). However, the accumulation of Teff and Tm cells was not affected by Dectin2 and Mincle deficiency (Fig. 2B and C).
Fig 2.
Effects of CLR signaling on T cell accumulation in the lungs after C. deneoformans infection WT (n = 3), CARD9KO (A, n = 3), Dectin-2KO (B, n = 5), and MincleKO (C, n = 4) mice were infected intratracheally with C. deneoformans. The number of each T cell subset was analyzed using flow cytometry on day 3 post-infection. Each column represents the mean ± SD. *, P < 0.05. ***, P < 0.005. Representative data demonstrating similar results from independent experiments are shown. Experiments were conducted three times for A and B and twice for C. The results for A and C represent experiments conducted simultaneously, and the outcomes for WT mice utilize the same materials.
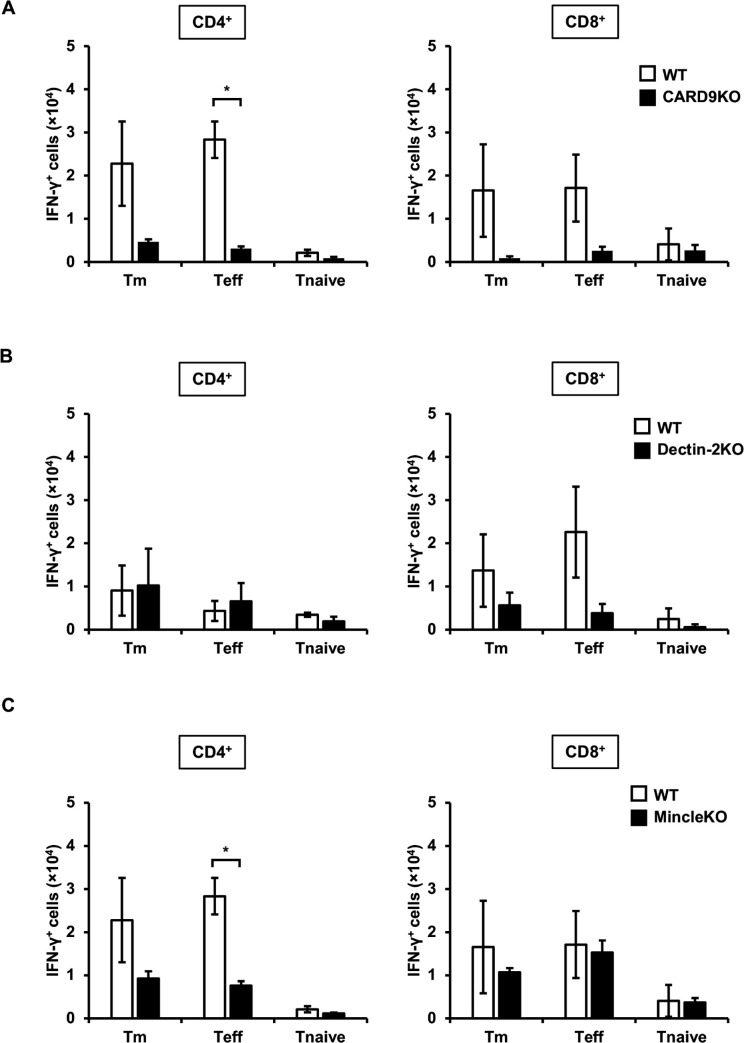
We next examined the effect of CLRs on IFN-γ expression in these T cells in the early phase of cryptococcal infection. Similar to our previous study, 3 days after infection, IFN-γ expression in both Teff and Tm cells in CD4+ and CD8+ T cells was lower in the lungs of CARD9KO mice compared to WT mice (Fig. 3A). IFN-γ-expressing CD8+ Teff cells and CD4+ Teff cells decreased in Dectin-2KO and MincleKO mice, respectively, compared to WT mice, although not to the same extent as observed in CARD9KO mice (Fig. 3B and C).
Fig 3.
Effects of CLR signaling on IFN-γ expression in pulmonary T cells after C. deneoformans infection WT (n = 3), CARD9KO (A, n = 3), Dectin-2KO (B, n = 5), and MincleKO (C, n = 4) mice were infected intratracheally with C. deneoformans. The number of each IFN-γ-expressing T cell subset was analyzed using flow cytometry on day 3 post-infection. Each column represents the mean ± SD. *, P < 0.05. Representative data demonstrating similar results from independent experiments are shown. Experiments were conducted three times for A and B and twice for C. The results for A and C represent experiments conducted simultaneously, and the outcomes for WT mice utilize the same materials.
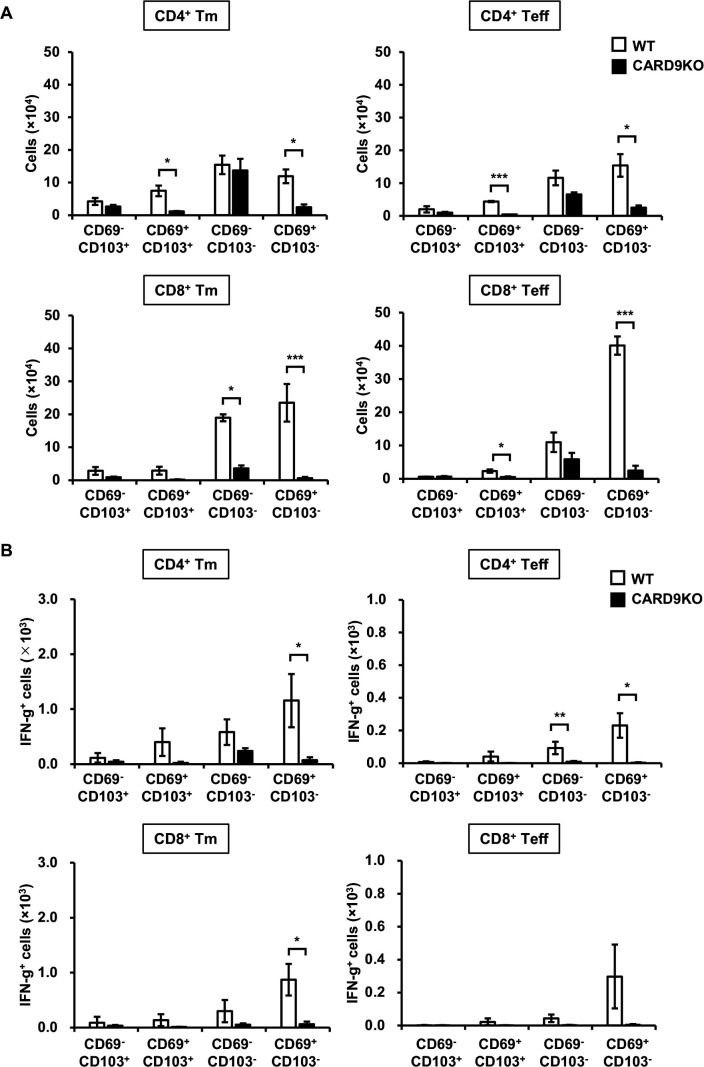
Effect of CARD9 deficiency on the synthesis of IFN-γ by Tm and Teff cells expressing CD69 or CD103 in the lungs after cryptococcal infection
Tm and Teff cells were induced in the lungs 3 days after cryptococcal infection, which precedes the establishment of adaptive immunity, and they produced IFN-γ through CARD9 signaling (Fig. 1to 3A). To confirm the tissue residency of these T cells and assess the effect of CARD9 deficiency on it, we analyzed the expression of CD69 and CD103 on these T cells in WT and CARD9KO mice. While many Tm and Teff cells expressed CD69, CD103-expressing cells were a small proportion in WT mice (Fig. 4A). These CD69-expressing Tm and Teff cells were significantly decreased in CARD9KO mice compared to WT mice (Fig. 4A). In addition, CD69+ Tm and Teff cells, which predominantly expressed IFN-γ in WT mice, were abolished by CARD9 deficiency (Fig. 4B).
Fig 4.
Effects of CARD9 deficiency on tissue-resident T cells in the lungs after C. deneoformans infection WT (n = 3) and CARD9KO (n = 3) mice were infected intratracheally with C. deneoformans. The number of CD69 and/or CD103-expressing T cells (A) and each IFN-γ-expressing T cell subset (B) in the lungs was analyzed using flow cytometry on day 3 post-infection. Each column represents the mean ± SD. *, P < 0.05. **, P < 0.01. ***, P < 0.005. Representative data from three independent experiments with similar results are shown.
Effect of FTY720 treatment on Tm and Teff cells in the lungs after cryptococcal infection
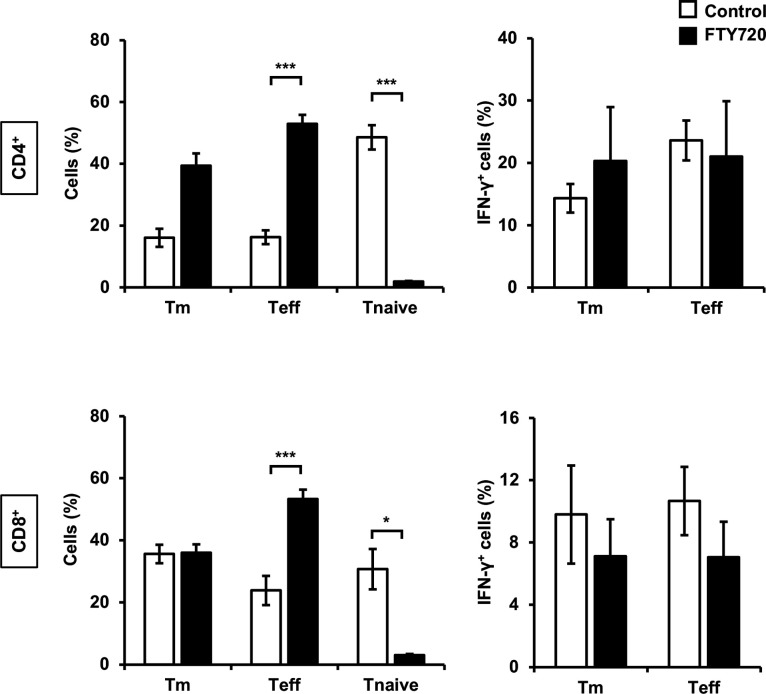
To investigate whether Tm and Teff cells, which increased in the lungs of WT mice in the early phase of cryptococcal infection, migrated to the lungs after proliferating in response to antigen presentation in the lymph nodes, we analyzed these T cells through FTY720 treatment, which suppressed the transmigration of T cells from lymphoid tissues (51). Although CD4+ and CD8+ T naïve cells were significantly decreased by FTY720 treatment compared to the control, the populations of Tm, Teff, and IFN-expressing T cells did not decrease (Fig. 5).
Fig 5.
Effects of FTY720 on T cells in the lungs after C. deneoformans infection WT mice were orally administered distilled water (control, n = 3) or FTY720 (n = 6) each day starting from 2 days before infection, followed by intratracheal infection with C. deneoformans. The number of each T cell subset (A) and each IFN-γ-expressing T cell subset (B) in the lungs was analyzed using flow cytometry on day 3 post-infection. Each column represents the mean ± SD. *, P < 0.05. ***, P < 0.005. Representative data from two independent experiments with similar results are shown.
Origin of TRM cells after cryptococcal infection
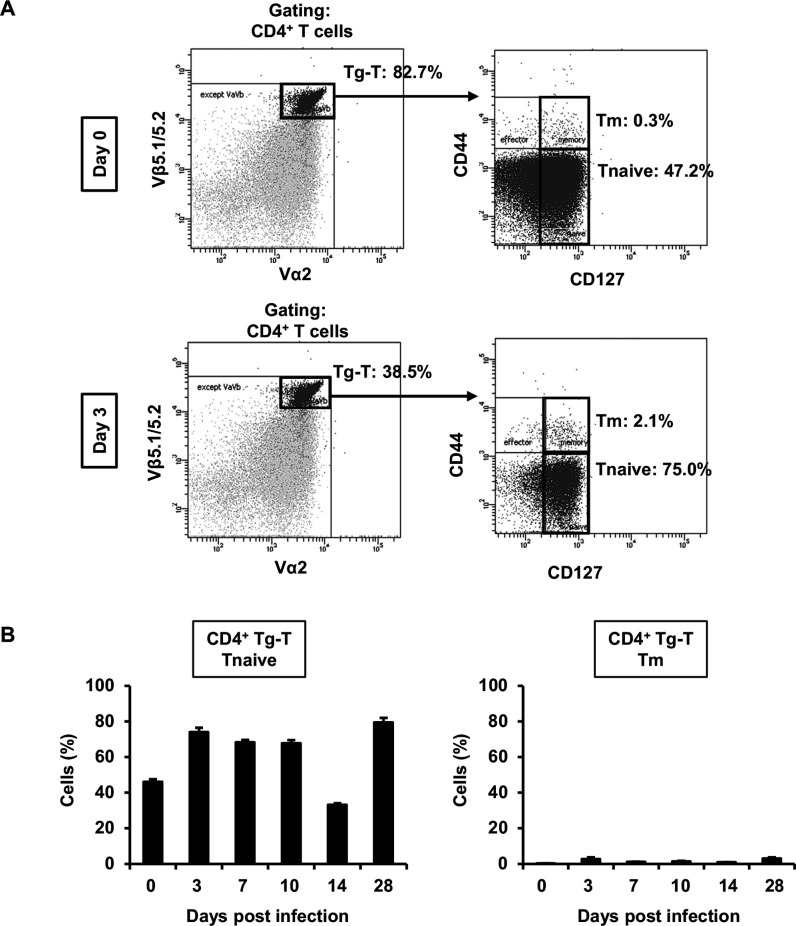
To investigate the origin of TRM cells, which rapidly increased in the early phase of infection and might be involved in IFN-γ production, we examined TRM cells in the lungs of OT-II mice after cryptococcal infection. In OT-II mice, most T cells express high levels of TCRs specific to OVA, and these cells are considered nearly naïve T cells that have not encountered antigens. CD4+ Tm cells in transgenic T (Tg-T) cells expressing TCR specific to OVA did not exhibit a marked change on day 3 post-infection (2.1%) compared to uninfected mice (0.3%; Fig. 6A). In addition, almost all Tg-T cells were composed of T naïve cells, and Tm cells did not exhibit a significant increased at any time point after infection compared to uninfected mice (Fig. 6B). These findings suggest that CD4+ Tm cells did not differentiate from Tg-T cells, which retained their T naïve cells after infection.
Fig 6.
Analysis of TRM and T naïve cells in the lungs of OT-II mice after C. deneoformans infection OT-II mice were infected intratracheally with C. deneoformans. (A) Pulmonary T naïve, Teff, and Tm cells in CD4+ Tg-T cells expressing OVA-specific TCR were analyzed using flow cytometry on days 0 (uninfected, n = 3) and 3 (n = 3) post-infections. Representative dot plots are shown. (B) The number of pulmonary T naïve and Tm cells in CD4+ Tg-T cells were analyzed using flow cytometry on days 0 (uninfected; n = 3), 3 (n = 3), 7 (n = 5), 14 (n = 4), and 28 (n = 6) post-infection. Each column represents the mean ± SD. Representative data demonstrating similar results from independent experiments are shown. Experiments were conducted twice for day 0, four times for day 3, twice for days 7 and 14, once for day 28, and once for day 58. The results for Fig. 6 and Fig. 7 represent experiments conducted simultaneously.
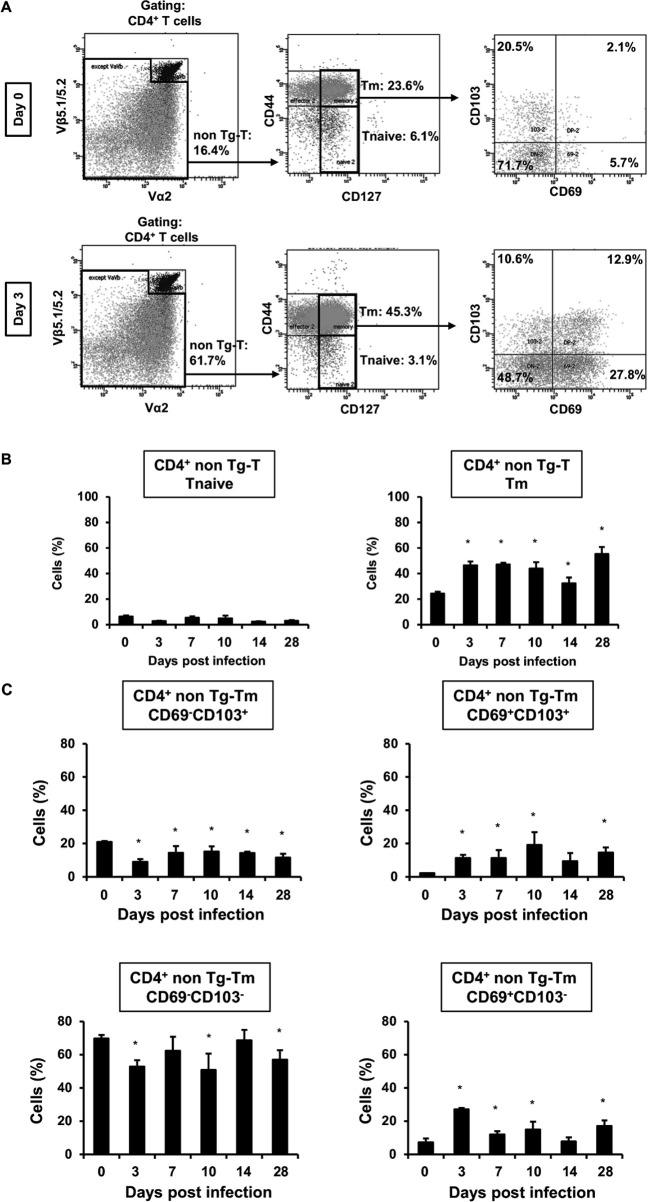
Next, we examined whether CD4+ TRM cells originated from non-transgenic T (nonTg-T) cells expressing OVA-non-specific TCR. Tm cells in nonTg-T cells significantly increased on day 3 post-infection (45.3%) compared to uninfected mice (23.6%; Fig. 7A). In addition, we investigated T naïve and Tm cells in nonTg-T cells on days 0, 3, 7, 14, and 28 after infection. Tm cells in nonTg-T cells were significantly increased at all time points after infection compared to uninfected mice (Fig. 7B). In CD4+ Tm cells in nonTg-T cells, CD69+ CD103- and DP TRM cells exhibited a significant increase, whereas CD69- CD103+ TRM cells were significantly decreased at almost every time point after infection compared to uninfected mice (Fig. 7C). These findings suggest that CD4+ Tm cells, especially CD69+ CD103- and DP TRM cells, were differentiated from nonTg-T cells due to cryptococcal infection in the early phase of infection.
Fig 7.
Analysis of TRM and nonTg-T cells in the lungs after C. deneoformans infection OT-II mice were infected intratracheally with C. deneoformans. (A) Pulmonary TRM cells in CD4+ nonTg-T cells expressing OVA non-specific TCR were analyzed using flow cytometry on days 0 (uninfected, n = 3) and 3 (n = 3) post-infection. Representative dot plots are shown. The number of pulmonary T naïve and Tm cells (B) and CD69- and/or CD103-expressing Tm cells (C) in CD4+ nonTg-T cells were analyzed using flow cytometry on days 0 (uninfected; n = 3), 3 (n = 3), 7 (n = 5), 14 (n = 4), and 28 (n = 6) post-infection. Each column represents the mean ± SD. *, P < 0.05 (vs day 0 post-infection). Representative data demonstrating similar results from independent experiments are shown. Experiments were conducted twice for day 0, four times for day 3, twice for days 7 and 14, once for day 28, and once for day 58. The results for Fig. 6 and 7 represent experiments conducted simultaneously.
Variation of TRM cells in each tissue
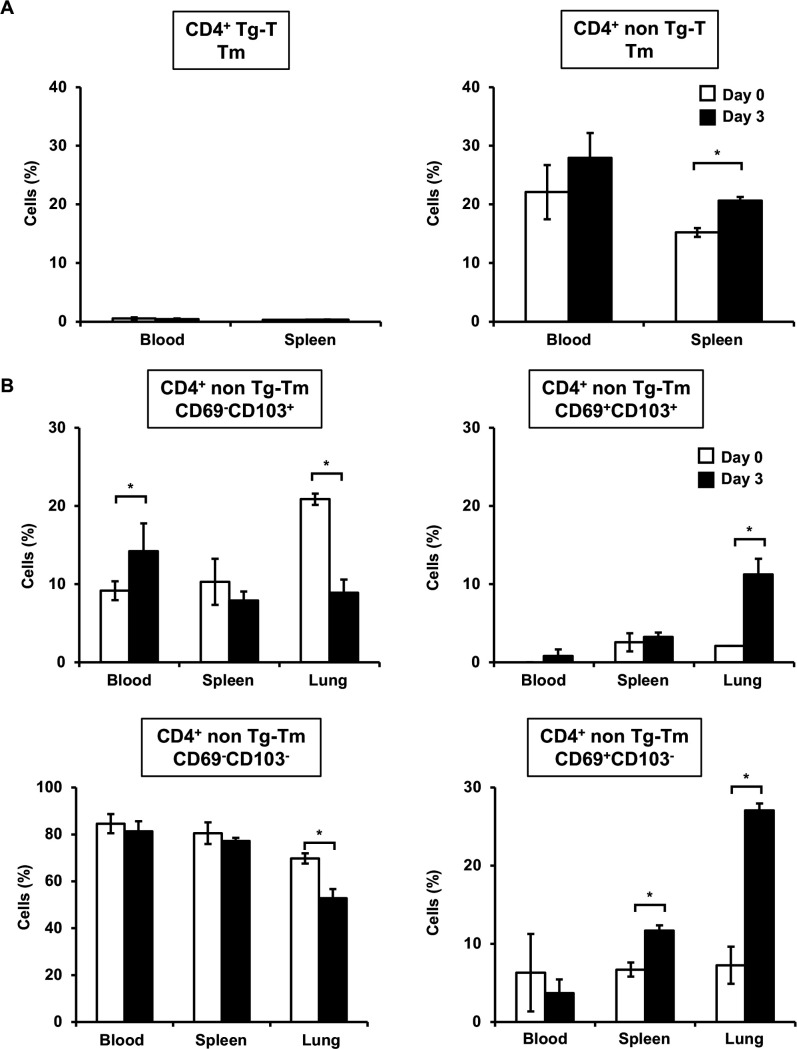
TRM cells are considered not to exist in the peripheral blood and secondary lymphoid tissues as they are primarily tissue-resident memory T cells (39, 40, 52). Therefore, we examined CD4+ TRM cells in peripheral blood lymphocytes and splenocytes after cryptococcal infection. Tm cells in nonTg-T cells, not Tg-T cells, increased in both peripheral blood lymphocytes and splenocytes on day 3 post-infection compared to uninfected mice (Fig. 8A). Similar to the lungs, CD69+ CD103- TRM cells in nonTg-T cells were significantly increased in splenocytes, not peripheral blood lymphocytes, on day 3 after infection compared to uninfected mice (Fig. 8B). On the contrary, DP TRM cells were significantly increased only in the lungs on day 3 after infection compared to uninfected mice. In contrast to the lungs, CD69- CD103+ TRM cells were significantly increased in peripheral blood lymphocytes on day 3 after infection compared to uninfected mice. DN TRM cells were significantly decreased only in the lungs on day 3 after infection compared to uninfected mice.
Fig 8.
Analysis of TRM cells in the peripheral blood and spleen after C. deneoformans infection OT-II mice were infected intratracheally with C. deneoformans. (A) Tm cells in CD4+ Tg-T and nonTg-T cells in the peripheral blood and spleen were analyzed using flow cytometry on days 0 (uninfected, n = 3) and 3 (n = 3) post-infection. (B) TRM cells in CD4+ nonTg-T cells in the peripheral blood, spleen, and lungs were analyzed using flow cytometry on days 0 (uninfected, n = 3) and 3 (n = 3) post-infection. Each column represents the mean ± SD. *, P < 0.05. Representative data demonstrating similar results from independent experiments are shown. Experiments were conducted once for day 0 and twice for day 3. The results for each tissue represent results from the same experiment.
DISCUSSION
In this study, we analyzed the kinetics of TRM cells, whose role in infection is not well understood, and MPT cells, previously reported in our research. We also assessed the production of IFN-γ from these cells using various mouse strains during infection with C. deneoformans. While memory T cells are generally defined as CD44+CD127+ cells, it is known that some MPT cells, effector, and central memory T cells exhibit low expression of CD127 (53, 54). Therefore, there is a possibility that these cells may be misidentified as effector T cells in this study.
TRM cells are defined as expressing either or both CD69, an early activation marker regulating lymphocyte migration, and CD103, which binds to epithelial cells, in CD44bright+ CD127+ Tm cells (48–50). CD69+ and DP TRM cells significantly increased in the lungs on day 3 after cryptococcal infection, suggesting that the increased Tm cells in the early phase were predominantly composed of CD69+ and DP TRM cells. On the contrary, Tm and Teff cells in CD4+ and CD8+ T cells exhibited no increase in the lungs of CARD9KO mice after cryptococcal infection. Moreover, CD69+ and DP TRM cells were significantly decreased in CARD9KO mice compared to WT mice. In addition, IFN-γ expression was observed in CD4+ and CD8+ Tm cells and CD4+ and CD8+ Teff cells in the lungs of WT mice on day 3 after cryptococcal infection, and these cells were decreased in CARD9KO mice compared to WT mice. These findings are consistent with our previous report indicating a decrease in IFN-γ production in the early phase of cryptococcal infection in CARD9KO mice, suggesting that the Tm and Teff cells detected in this study are the same as the MPT cells reported in our previous studies (25). These Tm cells and Teff cells were detected in the lungs during the innate immune phase of cryptococcal infection under specific pathogen free (SPF) conditions, suggesting that these cells were responsive to various antigens encountered thus far, rather than being C. deneoformans-specific T cells. It has been reported that MPT cells are not only found in SPF mice but also in germ-free mice, suggesting that their production is influenced by self-antigens or dietary antigens (37).
We next investigated which CLRs upstream of CARD9 were involved in the accumulation of these T cells. Compared to WT, Dectin-2KO mice exhibited a decrease in IFN-γ-producing CD8+ Teff cells, and MincleKO mice showed a decrease in IFN-γ-producing CD4+ Teff cells. However, the effects observed were not as pronounced as those seen in CARD9KO mice, suggesting that other CLRs might contribute to the induction of these cells upon recognition of C. deneoformans. It has been reported that individual CLRs do not play major roles in phagocytosis and innate defense (55). In addition, since FcRγ and DAP12, which are not part of CLRs, require CARD9 signals (56), further research is needed to investigate the involvement of other CLRs, such as the mannose receptor, DC-SIGN, as well as FcRγ and DAP12.
CD69+ and DP TRM cells, along with IFN-γ expressing cells, were reduced in CARD9KO mice compared to WT mice, suggesting that these T cells were activated within the lungs at the site of infection rather than in secondary lymphoid tissues through CARD9 signaling. CD69 is known to be an initial activating antigen expressed in T cells, B cells, and NK cells activated by anti-CD3/TCR or anti-CD2 antibodies, phorbol 12-myristate 13-acetate that activates protein kinase C (PKC), and phytohemagglutinin known as a T cells’ mitogen (57, 58). In addition, it has been reported that CD69 expression is enhanced by the inflammatory cytokine tumor necrosis factor-α (TNF-α) (59), which is induced by CARD9 signaling (60). These findings suggest that the decreased antigen presentation and/or TNF-α production may lead to decreased CD69 expression in CARD9KO mice. Indeed, some reports have demonstrated that macrophages and dendritic cells derived from CARD9KO mice exhibit reduced phagocytosis of L. monocytogenes and C. deneoformans (29, 61).
To analyze the tissue residency of Tm cells and Teff cells, mice were administered FTY720. The binding of the phospholipid mediator S1P to its receptor triggers the migration of mature T cells from the thymus and secondary lymphoid tissues (48). FTY720, acting as an agonist for the S1P receptor, inhibits the migration of mature T cells from secondary lymphoid tissues by inducing the internalization of the S1P receptor from the cell surface (62). While CD4+ and CD8+ naïve T cells significantly decreased with FTY720 treatment compared to the control, the populations of Tm, Teff, and IFN-expressing T cells did not exhibit a decrease. The decrease in naïve T cells is likely due to the suppression of T cell migration from secondary lymphoid tissues and the decrease in the number of circulating T cells. IFN-γ-expressing Tm and Teff cells were not decreased by FTY720 treatment, suggesting that these cells were present in the lungs before infection and did not migrate to the lungs after activation and proliferation in the regional lymph nodes. Tm and Teff cells detected in the early phase of cryptococcal infection are considered to be T cells resident in the lungs, similar to TRM cells. However, TRM cells are involved in the rapid initial immune response at the infected site during reinfection, and it remains unclear whether these cells respond non-specifically during the initial infection. In addition, as Teff cells, not only Tm cells, were also found to be tissue resident, further investigation is needed to determine if these cells are different from TRM cells.
Next, we attempted to analyze the origin of CD69+ and DP TRM cells using OT-II mice which highly express TCRs that are specific to OVA. T cells in OT-II mice are considered almost naïve T cells that have not encountered antigens. Tm cells were not detected in Tg-T cells before and after infection but were observed in nonTg-T cells. In addition, Tm cells and TRM cells in nonTg-T cells were already present before infection, and their numbers significantly increased after 3 days of infection. CD69+, CD103+, and DP TRM cells exhibited fluctuations over the course of the infection. Therefore, Tm cells and TRM cells were already produced through encounters with various antigens, and these cells were thought to be activated in an antigen-non-specific manner upon infection with C. deneoformans.
Recently, a novel class of lymphocytes, distinct from T cells or B cells, has been identified as innate lymphoid cells (ILCs), involved in innate immunity. ILCs are recognized as important contributors to mucosal immunity, tissue homeostasis, and immune adjustment, categorized into three types by cytokine production profiles (63, 64). ILC1 produces Th1 cytokines such as IFN-γ, ILC2 produces Th2 cytokines such as IL-5 and IL-13, and ILC3 produces IL-17 and IL-22 (64). ILC1 produces IFN-γ and TNF-α and expresses the transcription factor T-bet upon stimulation with IL-12. It is distinguished from CD127- NK cells, CD103+ ILC1, and CD127+ ILC1 (63–65). These ILCs exist as either tissue-resident cells in lymphoid tissues or not, and they fulfill their function by changing the external environment or multiplying (63, 66). In this study, we consider Tm cells expressing CD69 as one subtype of TRM cells, but their function is similar to ILC1, suggesting that these cells might be TRM-like cells that could be classified as a new subtype of ILC. Understanding the processes by which Tm cells and TRM cells, which are present during the non-infection phase, transition from antigen-specific T cells to effector T cells, memory T cells, and ultimately become TRM cells, as well as identifying the cytokines or transcription factors participating in the expression of CD69 and CD103, are potential future research topics.
MPT cells and TRM cells both exhibited a rapid increase on day 3 after infection, indicating their involvement in the innate phase of infection. They were stimulated by antigen non-specifically and produced IFN-γ. However, MPT cells were mostly of the CD8+ type and were found in the peripheral blood or secondary lymphoid tissues. In contrast, TRM cells migrate to tissues after infection with pathogenic microorganisms and reside within the tissues (38). While MPT cells and TRM cells are similar in that they both play an important role against initial infection, considering their nature and cell surface markers, it is assumed that both cells are activated by different mechanisms and perform different functions. In this study, the actual conditions and relevance of these cells were not clarified.
In this study, we demonstrated that (i) CD69+ Tm cells and CD69+ Teff cells in the lungs increased as early as 3 days after cryptococcal infection, (ii) these cells produced IFN-γ through non-specific signaling, and (iii) these cells were resident in tissues. In addition, it was revealed that CARD9 signaling was involved in the induction of these cells and IFN-γ production. The production of IFN-γ from these cells is considered non-specific signaling, as it occurs during the innate immune phase. However, whether specific responses exist during this phase has not been investigated in this study. These results suggest that T cells producing IFN-γ in the lungs during the early stage of infection are important for early protection against cryptococcal infection. It is essential for future research to investigate whether these cells are also detected during infection with other pathogens and whether they are related to antigen-specific Tm cells and Teff cells that are formed during acquired immunity.
ACKNOWLEDGMENTS
We thank the Biomedical Research Unit of Tohoku University Hospital and the Biomedical Research Core, Animal Pathology Platform of Tohoku University Graduate School of Medicine for providing technical support.
This work was supported in part by a Grant-in-Aid for Scientific Research (B; 18H02851 and 21H02965) and Early-Career Scientists (19K17920 and 21K16314) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; by the Strategic International Collaborative Research Program (SICORP), AMED (JP19jm0210073, JP20jm0210073, and JP21jm0210073); by the MSD Life Science Foundation, Public Interest Incorporated Foundation (ID-014); and by the Joint Usage/Research Program of the Medical Mycology Research Center, Chiba University (20-02, 21-04).
Conceived and designed the experiments: A.M., A.U., K.S., and Kazuyoshi K. Performed the experiments: A.M., A.U., K.S., T.M., I.M., T.Z., T.K., A.O., Kotone K., R.Y., and K.Y. Analyzed the data: A.M., A.U., K.S., H.Y., T.M., D.T., X.Y., E.K., H.T., K.I., and Kazuyoshi K. Contributed reagents/materials/analysis tools: H.H., S.Y., S.S., and Y.I. Contributed to the writing of the manuscript: A.M., A.U., K.S., and Kazuyoshi K. All authors reviewed the manuscript.
Contributor Information
Ko Sato, Email: ko-sato@med.tohoku.ac.jp.
Mairi C. Noverr, Tulane University, New Orleans, Louisiana, USA
ETHICS APPROVAL
This study was performed in strict accordance with the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology in Japan, 2006. All experimental procedures involving animals followed the Regulations for Animal Experiments and Related Activities at Tohoku University, Sendai, Japan, and were approved by the Institutional Animal Care and Use Committee at Tohoku University (approval numbers: 2013 IDOU-257, 2013 IDOU-500, 2015 IDOU-061, 2016 IDOU-032, 2016 IDOU-070, 2018 IDOU-031, 2018 IDOU-056, 2019 IDOU-109, 2013 IKUMIKAE-125, 2013 IKUMIKAE-074, 2016 IKUMIKAE-141, 2018 IKUMIKAE-109, and 2018 IKUMIKAE-030). All experiments were performed under anesthesia, and all efforts were made to minimize the suffering of the animals.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/iai.00024-24.
Gating strategy of T cells.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Hagen F, Khayhan K, Theelen B, Kolecka A, Polacheck I, Sionov E, Falk R, Parnmen S, Lumbsch HT, Boekhout T. 2015. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet Biol 78:16–48. doi: 10.1016/j.fgb.2015.02.009 [DOI] [PubMed] [Google Scholar]
- 2. Perfect JR, Casadevall A. 2002. Cryptococcosis. Infect Dis Clin North Am 16:837–874. doi: 10.1016/s0891-5520(02)00036-3 [DOI] [PubMed] [Google Scholar]
- 3. Gilbert AS, Wheeler RT, May RC. 2014. Fungal pathogens: survival and replication within macrophages. Cold Spring Harb Perspect Med 5:a019661. doi: 10.1101/cshperspect.a019661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feldmesser M, Tucker S, Casadevall A. 2001. Intracellular parasitism of macrophages by Cryptococcus neoformans. Trends Microbiol 9:273–278. doi: 10.1016/s0966-842x(01)02035-2 [DOI] [PubMed] [Google Scholar]
- 5. Lim TS, Murphy JW. 1980. Transfer of immunity to cryptococcosis by T-enriched splenic lymphocytes from Cryptococcus neoformans-sensitized mice. Infect Immun 30:5–11. doi: 10.1128/iai.30.1.5-11.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koguchi Y, Kawakami K. 2002. Cryptococcal infection and Th1-Th2 cytokine balance. Int Rev Immunol 21:423–438. doi: 10.1080/08830180213274 [DOI] [PubMed] [Google Scholar]
- 7. Sato K, Kawakami K. 2022. Pamps and host immune response in cryptococcal infection. Med Mycol J 63:133–138. doi: 10.3314/mmj.22.005 [DOI] [PubMed] [Google Scholar]
- 8. Arora S, Olszewski MA, Tsang TM, McDonald RA, Toews GB, Huffnagle GB. 2011. Effect of cytokine interplay on macrophage polarization during chronic pulmonary infection with Cryptococcus neoformans. Infect Immun 79:1915–1926. doi: 10.1128/IAI.01270-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tohyama M, Kawakami K, Futenma M, Saito A. 1996. Enhancing effect of oxygen radical Scavengers on murine macrophage anticryptococcal activity through production of nitric oxide. Clin Exp Immunol 103:436–441. doi: 10.1111/j.1365-2249.1996.tb08299.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hardison SE, Ravi S, Wozniak KL, Young ML, Olszewski MA, Wormley FL. 2010. Pulmonary infection with an interferon-gamma-producing Cryptococcus neoformans strain results in classical macrophage activation and protection. Am J Pathol 176:774–785. doi: 10.2353/ajpath.2010.090634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Müller U, Stenzel W, Köhler G, Werner C, Polte T, Hansen G, Schütze N, Straubinger RK, Blessing M, McKenzie ANJ, Brombacher F, Alber G. 2007. IL-13 induces disease-promoting type 2 Cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J Immunol 179:5367–5377. doi: 10.4049/jimmunol.179.8.5367 [DOI] [PubMed] [Google Scholar]
- 12. Sato K, Yamamoto H, Nomura T, Kasamatsu J, Miyasaka T, Tanno D, Matsumoto I, Kagesawa T, Miyahara A, Zong T, Oniyama A, Kawamura K, Yokoyama R, Kitai Y, Ishizuka S, Kanno E, Tanno H, Suda H, Morita M, Yamamoto M, Iwakura Y, Ishii K, Kawakami K. 2020. Production of IL-17A at innate immune phase leads to decreased Th1 immune response and attenuated host defense against infection with Cryptococcus deneoformans. J Immunol 205:686–698. doi: 10.4049/jimmunol.1901238 [DOI] [PubMed] [Google Scholar]
- 13. Zhang Y, Wang F, Tompkins KC, McNamara A, Jain AV, Moore BB, Toews GB, Huffnagle GB, Olszewski MA. 2009. Robust Th1 and Th17 immunity supports pulmonary clearance but cannot prevent systemic dissemination of highly virulent Cryptococcus neoformans H99. Am J Pathol 175:2489–2500. doi: 10.2353/ajpath.2009.090530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murdock BJ, Huffnagle GB, Olszewski MA, Osterholzer JJ. 2014. Interleukin-17A enhances host defense against cryptococcal lung infection through effects mediated by leukocyte recruitment, activation, and gamma interferon production. Infect Immun 82:937–948. doi: 10.1128/IAI.01477-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Szymczak WA, Sellers RS, Pirofski L. 2012. IL-23 Dampens the allergic response to Cryptococcus neoformans through IL-17-independent and -dependent mechanisms. Am J Pathol 180:1547–1559. doi: 10.1016/j.ajpath.2011.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hardison SE, Wozniak KL, Kolls JK, Wormley FL. 2010. Interleukin-17 is not required for classical macrophage activation in a pulmonary mouse model of Cryptococcus neoformans infection. Infect Immun 78:5341–5351. doi: 10.1128/IAI.00845-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wozniak KL, Hole CR, Yano J, Fidel PL, Wormley FL. 2014. Characterization of IL-22 and antimicrobial peptide production in mice protected against pulmonary Cryptococcus Neoformans infection. Microbiology (Reading) 160:1440–1452. doi: 10.1099/mic.0.073445-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takeuchi O, Akira S. 2010. Pattern recognition receptors and inflammation. Cell 140:805–820. doi: 10.1016/j.cell.2010.01.022 [DOI] [PubMed] [Google Scholar]
- 19. Figdor CG, van Kooyk Y, Adema GJ. 2002. C-type lectin receptors on dendritic cells and langerhans cells. Nat Rev Immunol 2:77–84. doi: 10.1038/nri723 [DOI] [PubMed] [Google Scholar]
- 20. Willment JA, Brown GD. 2008. C-type lectin receptors in antifungal immunity. Trends Microbiol 16:27–32. doi: 10.1016/j.tim.2007.10.012 [DOI] [PubMed] [Google Scholar]
- 21. Yang H, He H, Dong Y. 2011. Card9 Syk-dependent and Raf-1 Syk-independent signaling pathways in target recognition of Candida albicans by Dectin-1. Eur J Clin Microbiol Infect Dis 30:303–305. doi: 10.1007/s10096-010-1103-z [DOI] [PubMed] [Google Scholar]
- 22. Hernández-Santos N, Gaffen SL. 2012. Th17 cells in immunity to Candida albicans. Cell Host Microbe 11:425–435. doi: 10.1016/j.chom.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, Brown GD. 2007. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol 8:31–38. doi: 10.1038/ni1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung S, Komatsu R, Miura N, Adachi Y, Ohno N, Shibuya K, Yamamoto N, Kawakami K, Yamasaki S, Saito T, Akira S, Iwakura Y. 2010. Dectin-2 recognition of alpha-Mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32:681–691. doi: 10.1016/j.immuni.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 25. Yamamoto H, Nakamura Y, Sato K, Takahashi Y, Nomura T, Miyasaka T, Ishii K, Hara H, Yamamoto N, Kanno E, Iwakura Y, Kawakami K. 2014. Defect of CARD9 leads to impaired accumulation of gamma interferon-producing memory phenotype T cells in lungs and increased susceptibility to pulmonary infection with Cryptococcus neoformans. Infect Immun 82:1606–1615. doi: 10.1128/IAI.01089-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakamura K, Kinjo T, Saijo S, Miyazato A, Adachi Y, Ohno N, Fujita J, Kaku M, Iwakura Y, Kawakami K. 2007. Dectin-1 is not required for the host defense to Cryptococcus neoformans. Microbiol Immunol 51:1115–1119. doi: 10.1111/j.1348-0421.2007.tb04007.x [DOI] [PubMed] [Google Scholar]
- 27. Nakamura Y, Sato K, Yamamoto H, Matsumura K, Matsumoto I, Nomura T, Miyasaka T, Ishii K, Kanno E, Tachi M, Yamasaki S, Saijo S, Iwakura Y, Kawakami K. 2015. Dectin-2 deficiency promotes Th2 response and mucin production in the lungs after pulmonary infection with Cryptococcus neoformans. Infect Immun 83:671–681. doi: 10.1128/IAI.02835-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tanno D, Yokoyama R, Kawamura K, Kitai Y, Yuan X, Ishii K, De Jesus M, Yamamoto H, Sato K, Miyasaka T, Shimura H, Shibata N, Adachi Y, Ohno N, Yamasaki S, Kawakami K. 2019. Dectin-2-mediated signaling triggered by the cell wall polysaccharides of Cryptococcus neoformans. Microbiol Immunol 63:500–512. doi: 10.1111/1348-0421.12746 [DOI] [PubMed] [Google Scholar]
- 29. Kitai Y, Sato K, Tanno D, Yuan X, Umeki A, Kasamatsu J, Kanno E, Tanno H, Hara H, Yamasaki S, Saijo S, Iwakura Y, Ishii K, Kawakami K. 2021. Role of Dectin-2 in the phagocytosis of Cryptococcus neoformans by Dendritic cells. Infect Immun 89:e0033021. doi: 10.1128/IAI.00330-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Campuzano A, Castro-Lopez N, Wozniak KL, Leopold Wager CM, Wormley FL. 2017. Dectin-3 is not required for protection against Cryptococcus neoformans infection. PLoS One 12:e0169347. doi: 10.1371/journal.pone.0169347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang H-R, Li F, Han H, Xu X, Li N, Wang S, Xu J-F, Jia X-M. 2018. Dectin-3 recognizes glucuronoxylomannan of Cryptococcus Neoformans Serotype AD and Cryptococcus gattii serotype B to initiate host defense against cryptococcosis. Front Immunol 9:1781. doi: 10.3389/fimmu.2018.01781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sato Y, Sato K, Yamamoto H, Kasamatsu J, Miyasaka T, Tanno D, Miyahara A, Kagesawa T, Oniyama A, Kawamura K, Yokoyama R, Kitai Y, Umeki A, Ishizuka S, Takano K, Shiroma R, Nakahata N, Kawakami K, Kanno E, Tanno H, Yamasaki S, Hara H, Ishii K, Kawakami K. 2020. Limited role of mincle in the host defense against infection with Cryptococcus deneoformans. Infect Immun 88:e00400–20. doi: 10.1128/IAI.00400-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sprent J, Surh CD. 2011. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol 12:478–484. doi: 10.1038/ni.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hu J, August A. 2008. Naive and innate memory phenotype CD4+ T cells have different requirements for active Itk for their development. J Immunol 180:6544–6552. doi: 10.4049/jimmunol.180.10.6544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yajima T, Nishimura H, Ishimitsu R, Yamamura K, Watase T, Busch DH, Pamer EG, Kuwano H, Yoshikai Y. 2001. Memory phenotype Cd8(+) T cells in IL-15 transgenic mice are involved in early protection against a primary infection with Listeria monocytogenes . Eur J Immunol 31:757–766. doi: [DOI] [PubMed] [Google Scholar]
- 36. Umeda K, Sun X, Guo Y, Yamada H, Shibata K, Yoshikai Y. 2011. Innate memory phenotype CD4+ T cells play a role in early protection against infection by Listeria monocytogenes in a CD30L-dependent manner. Microbiol Immunol 55:645–656. doi: 10.1111/j.1348-0421.2011.00362.x [DOI] [PubMed] [Google Scholar]
- 37. Kawabe T, Jankovic D, Kawabe S, Huang Y, Lee P-H, Yamane H, Zhu J, Sher A, Germain RN, Paul WE. 2017. Memory-phenotype CD4+ T cells spontaneously generated under steady-state conditions exert innate TH1-like effector function. Sci Immunol 2:eaam9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wakim LM, Gebhardt T, Heath WR, Carbone FR. 2008. Cutting edge: local recall responses by memory T cells newly recruited to peripheral nonlymphoid tissues. J Immunol 181:5837–5841. doi: 10.4049/jimmunol.181.9.5837 [DOI] [PubMed] [Google Scholar]
- 39. Turner DL, Gordon CL, Farber DL. 2014. Tissue-resident T cells, in situ immunity and transplantation. Immunol Rev 258:150–166. doi: 10.1111/imr.12149 [DOI] [PubMed] [Google Scholar]
- 40. Shin H, Iwasaki A. 2013. Tissue-resident memory T cells. Immunol Rev 255:165–181. doi: 10.1111/imr.12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McMaster SR, Wilson JJ, Wang H, Kohlmeier JE. 2015. Airway-resident memory CD8 T cells provide antigen-specific protection against respiratory virus challenge through rapid IFN-γ production. J Immunol 195:203–209. doi: 10.4049/jimmunol.1402975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu T, Hu Y, Lee Y-T, Bouchard KR, Benechet A, Khanna K, Cauley LS. 2014. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol 95:215–224. doi: 10.1189/jlb.0313180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Laidlaw BJ, Zhang N, Marshall HD, Staron MM, Guan T, Hu Y, Cauley LS, Craft J, Kaech SM. 2014. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity 41:633–645. doi: 10.1016/j.immuni.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hara H, Ishihara C, Takeuchi A, Imanishi T, Xue L, Morris SW, Inui M, Takai T, Shibuya A, Saijo S, Iwakura Y, Ohno N, Koseki H, Yoshida H, Penninger JM, Saito T. 2007. The Adaptor protein CARD9 is essential for the activation of myeloid cells through ITAM-associated and toll-like receptors. Nat Immunol 8:619–629. doi: 10.1038/ni1466 [DOI] [PubMed] [Google Scholar]
- 45. Yamasaki S, Matsumoto M, Takeuchi O, Matsuzawa T, Ishikawa E, Sakuma M, Tateno H, Uno J, Hirabayashi J, Mikami Y, Takeda K, Akira S, Saito T. 2009. C-type lectin mincle is an activating receptor for pathogenic fungus, Malassezia. Proc Natl Acad Sci USA 106:1897–1902. doi: 10.1073/pnas.0805177106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barnden MJ, Allison J, Heath WR, Carbone FR. 1998. Defective TCR expression in transgenic mice constructed using cDNA- based Α- and Β-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol 76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x [DOI] [PubMed] [Google Scholar]
- 47. Kawakami K, Kohno S, Morikawa N, Kadota J, Saito A, Hara K. 1994. Activation of macrophages and expansion of specific T lymphocytes in the lungs of mice Intratracheally Inoculated with Cryptococcus neoformans. Clin Exp Immunol 96:230–237. doi: 10.1111/j.1365-2249.1994.tb06547.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shiow LR, Rosen DB, Brdicková N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. 2006. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440:540–544. doi: 10.1038/nature04606 [DOI] [PubMed] [Google Scholar]
- 49. Hadley GA, Higgins JMG. 2014. Integrin ΑEβ7: molecular features and functional significance in the immune system. Adv Exp Med Biol 819:97–110. doi: 10.1007/978-94-017-9153-3_7 [DOI] [PubMed] [Google Scholar]
- 50. Mackay LK, Braun A, Macleod BL, Collins N, Tebartz C, Bedoui S, Carbone FR, Gebhardt T. 2015. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J Immunol 194:2059–2063. doi: 10.4049/jimmunol.1402256 [DOI] [PubMed] [Google Scholar]
- 51. Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P. 2010. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. 11. Nat Rev Drug Discov 9:883–897. doi: 10.1038/nrd3248 [DOI] [PubMed] [Google Scholar]
- 52. Mueller SN, Mackay LK. 2016. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol 16:79–89. doi: 10.1038/nri.2015.3 [DOI] [PubMed] [Google Scholar]
- 53. Kawabe T, Ciucci T, Kim KS, Tayama S, Kawajiri A, Suzuki T, Tanaka R, Ishii N, Jankovic D, Zhu J, Sprent J, Bosselut R, Sher A. 2022. Redefining the foreign antigen and self-driven memory CD4+ T-cell compartments via transcriptomic, phenotypic, and functional analyses. Front Immunol 13:870542. doi: 10.3389/fimmu.2022.870542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Paiardini M, Cervasi B, Albrecht H, Muthukumar A, Dunham R, Gordon S, Radziewicz H, Piedimonte G, Magnani M, Montroni M, Kaech SM, Weintrob A, Altman JD, Sodora DL, Feinberg MB, Silvestri G. 2005. Loss of CD127 expression defines an expansion of effector CD8+ T CelLS in HIV-infected individuals. J Immunol 174:2900–2909. doi: 10.4049/jimmunol.174.5.2900 [DOI] [PubMed] [Google Scholar]
- 55. Walsh NM, Wuthrich M, Wang H, Klein B, Hull CM. 2017. Characterization of C-type Lectins reveals an unexpectedly limited interaction between Cryptococcus neoformans spores and Dectin-1. PLOS ONE 12:e0173866. doi: 10.1371/journal.pone.0173866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hara H, Ishihara C, Takeuchi A, Xue L, Morris SW, Penninger JM, Yoshida H, Saito T. 2008. Cell type-specific regulation of ITAM-mediated NF-kappaB activation by the adaptors, CARMA1 and CARD9. J Immunol 181:918–930. doi: 10.4049/jimmunol.181.2.918 [DOI] [PubMed] [Google Scholar]
- 57. Marzio R, Mauël J, Betz-Corradin S. 1999. Cd69 and regulation of the immune function. Immunopharmacol Immunotoxicol 21:565–582. doi: 10.3109/08923979909007126 [DOI] [PubMed] [Google Scholar]
- 58. Lauzurica P, Sancho D, Torres M, Albella B, Marazuela M, Merino T, Bueren JA, Martínez-A C, Sánchez-Madrid F. 2000. Phenotypic and functional characteristics of hematopoietic cell lineages in CD69-deficient mice. Blood 95:2312–2320. [PubMed] [Google Scholar]
- 59. Turner DL, Farber DL. 2014. Mucosal resident memory CD4 T cells in protection and immunopathology. Front Immunol 5:331. doi: 10.3389/fimmu.2014.00331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Strasser D, Neumann K, Bergmann H, Marakalala MJ, Guler R, Rojowska A, Hopfner K-P, Brombacher F, Urlaub H, Baier G, Brown GD, Leitges M, Ruland J. 2012. Syk kinase-coupled C-type lectin receptors engage protein kinase C-δ to elicit CARD9 adaptor-mediated innate immunity. Immunity 36:32–42. doi: 10.1016/j.immuni.2011.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wu W, Hsu Y-M, Bi L, Songyang Z, Lin X. 2009. CARD9 facilitates microbe-elicited production of reactive oxygen species by regulating the LyGDI-Rac1 complex. 11. Nat Immunol 10:1208–1214. doi: 10.1038/ni.1788 [DOI] [PubMed] [Google Scholar]
- 62. Chiba K, Yanagawa Y, Masubuchi Y, Kataoka H, Kawaguchi T, Ohtsuki M, Hoshino Y. 1998. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J Immunol 160:5037–5044. [PubMed] [Google Scholar]
- 63. Lai D-M, Shu Q, Fan J. 2016. The origin and role of innate lymphoid cells in the lung. Mil Med Res 3:25. doi: 10.1186/s40779-016-0093-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, Powrie F, Vivier E. 2013. Innate lymphoid cells — a proposal for uniform nomenclature. 2. Nat Rev Immunol 13:145–149. doi: 10.1038/nri3365 [DOI] [PubMed] [Google Scholar]
- 65. Klose CSN, Flach M, Möhle L, Rogell L, Hoyler T, Ebert K, Fabiunke C, Pfeifer D, Sexl V, Fonseca-Pereira D, Domingues RG, Veiga-Fernandes H, Arnold SJ, Busslinger M, Dunay IR, Tanriver Y, Diefenbach A. 2014. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 157:340–356. doi: 10.1016/j.cell.2014.03.030 [DOI] [PubMed] [Google Scholar]
- 66. Gasteiger G, Fan X, Dikiy S, Lee SY, Rudensky AY. 2015. Tissue Residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 350:981–985. doi: 10.1126/science.aac9593 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gating strategy of T cells.