SUMMARY
In 2023, the World Health Organization designated eumycetoma causative agents as high-priority pathogens on its list of fungal priority pathogens. Despite this recognition, a comprehensive understanding of these causative agents is lacking, and potential variations in clinical manifestations or therapeutic responses remain unclear. In this review, 12,379 eumycetoma cases were reviewed. In total, 69 different fungal species were identified as causative agents. However, some were only identified once, and there was no supporting evidence that they were indeed present in the grain. Madurella mycetomatis was by far the most commonly reported fungal causative agent. In most studies, identification of the fungus at the species level was based on culture or histology, which was prone to misidentifications. The newly used molecular identification tools identified new causative agents. Clinically, no differences were reported in the appearance of the lesion, but variations in mycetoma grain formation and antifungal susceptibility were observed. Although attempts were made to explore the differences in clinical outcomes based on antifungal susceptibility, the lack of large clinical trials and the inclusion of surgery as standard treatment posed challenges in drawing definitive conclusions. Limited case series suggested that eumycetoma cases caused by Fusarium species were less responsive to treatment than those caused by Madurella mycetomatis. However, further research is imperative for a comprehensive understanding.
KEYWORDS: mycetoma, diagnosis, susceptibility, itraconazole, biofilm, grain, neglected tropical disease, molecular diagnostics
INTRODUCTION
Mycetoma was recognized as a neglected tropical disease in 2016 by the World Health Organization (1). It is a disease with a high morbidity, and it is characterized by large tumorous lesions in the subcutaneous tissue (2). Inside the subcutaneous tissue, the causative agent is embedded in a granule called a grain. Mycetoma can be caused by more than 80 different microorganisms, either of a bacterial or fungal nature (3). Bacterial mycetoma is often named actinomycetoma, while fungal mycetoma is named eumycetoma. In 2023, the fungal pathogens able to cause mycetoma were ranked as high-priority pathogens on the WHO fungal priority pathogens list to guide the research, development, and public health action (4). However, it was not clearly stated which fungal species are considered as eumycetoma causative agents. Furthermore, it is currently also not known what are the similarities and differences between these species in terms of pathogenesis and treatment response. Therefore, in this review, we will describe which fungal species are able to cause mycetoma, what the clinical and mycological differences are between these species, and if there are differences in treatment response associated with these pathogens. For this, we have searched the literature using PubMed and included papers from 1945 to 2023.
EUMYCETOMA CAUSATIVE AGENTS
To assess how many eumycetoma causative agents there actually are, we updated the data from references (3, 5, 6) (Table 1). We did this by searching additional papers in the electronic database PubMed from January 2013 to December 2023 on mycetoma. Only case series or case reports presenting novel causative agents were included. The cases presented in these papers were then added to the database. From each paper, the sampling period, the region of sampling, the sex distribution, age distribution, and species isolated were recorded. To determine which fungal species were most prevalent, the total number of cases belonging to a certain species was divided by the total number of fungal causative agents reported and then multiplied by 100 to get the percentage. By adding these cases, the updated database now contained 27,114 mycetoma cases, and for 24,500 cases, it was specified if it was caused by a bacterium or a fungus. The identification of the causative agent differed per study. In some studies, the species were identified based on culture morphology, in others, it was based on histology. Only in a few studies species identification was based on molecular identification. Of those 24,500 mycetoma cases, 12,379 (50.5%) cases were caused by fungi (Table 1). In total, 69 different fungal species were reported to cause eumycetoma. In 86.0% of all eumycetoma cases, the fungus Madurella mycetomatis was the causative agent. The second most common causative agent was Falciformispora senegalensis. This causative agent was encountered in 3.5% of all eumycetoma causative agents (Table 1). We considered a causative agent a common causative agent if it was encountered in ≥10% of independent eumycetoma cases, an occasional causative agent when it was encountered in ≥0.5% of independent eumycetoma cases, and rare if it was encountered in <0.5% of independent eumycetoma cases.
TABLE 1.
Eumycetoma causative species
| Species | No. of isolates | % | Prevalencea | Evidenceb | Color of grains | Size of grains (mm) | Genome sequence | References |
|---|---|---|---|---|---|---|---|---|
| Madurella mycetomatis | 10,556 | 85.3 | C | A | Black | Up to 5.0 or more | GCA_001275765.2 GCA_022530565.1 |
(6–98) |
| Falciformispora senegalensis (Syn. Leptosphaeria senegalensis) | 435 | 3.5 | O | A | Black | 0.5–2.0 | (6, 25, 29, 31, 34, 35, 38, 40, 42, 43, 46, 55, 56, 71, 73, 76, 95–102) | |
| Trematosphaeria grisea (Syn. Madurella grisea) | 173 | 1.4 | O | B | Black | 0.3–0.6 | (28, 48, 65, 68, 69, 73–76, 79–82, 85, 88–90, 95–98, 103, 104) | |
| Scedosporium boydii | 120 | 1.0 | O | A | White/white yellow | 0.2–2.0 | GCA_002221725.1 | (6–8, 22, 23, 28, 35, 38, 42, 47–51, 61, 62, 65, 67, 70, 73, 75–77, 79, 80, 84, 85, 87–91, 100, 104) |
| Medicopsis romeroi (Syn. Pyrenochaeta romeroi) | 78 | 0.6 | O/R | B | Black | 0.5–1.5 | (6, 23, 25, 35, 47, 65, 68, 71, 73, 88, 90, 95–97, 105) | |
| Acremonium blochii | 1 | <0.1 | R | C | White | <1.5 | (67) | |
| Acremonium sclerotigenum | 1 | <0.1 | R | C | White | <1.5 | (26) | |
| Amesia atrobrunnea (Syn. Chaetomium atrobrunneum) | 1 | <0.1 | R | C | Black | (99) | ||
| Aspergillus candidus | 1 | <0.1 | R | C | White | 1.0–2.0 | GCA_002847045.1 | (8, 106) |
| Aspergillus flavus | 14 | 0.1 | R | B | Green/white | GCA_014117465.1 | (61–63, 66, 67, 107–109) | |
| Aspergillus fumigatus | 4 | <0.1 | R | C | White | GCA_000002655.1 | (6, 8, 61, 62) | |
| Aspergillus nidulans | 12 | <0.1 | R | C | White yellow | <0.6–2 | GCA_000011425.1 | (6, 67, 74, 105, 110–114) |
| Aspergillus niger | 3 | <0.1 | R | C | Black | GCA_000002855.2 | (26, 63) | |
| Aspergillus sydowii | 1 | <0.1 | R | C | Black | GCA_001890705.1 | (115) | |
| Aspergillus terreus | 2 | <0.1 | R | C | White | GCA_000149615.1 | (62, 67) | |
| Aspergillus ustus | 1 | <0.1 | R | C | GCA_000812125.1 | (84) | ||
| Cladophialophora bantiana | 4 | <0.1 | R | C | Black | 0.5 | GCA_000835475.1 | (90, 116, 117) |
| Cladophialophora mycetomatis | 1 | <0.1 | R | C | Black | 0.5 | (90) | |
| Cladosporium ramotellenum | 1 | <0.1 | R | C | (26) | |||
| Corynespora cassiicola | 1 | <0.1 | R | C | Black | <1.0 | GCA_022059125.1 | (118) |
| Curvularia geniculata | 4 | <0.1 | R | C | Black | 0.5–1.0 | GCA_016162275.1 | (119–121) |
| Curvularia lunata | 8 | <0.1 | R | C | Black | 0.5–1.0 | GCA_005212705.1 | (6, 35, 67, 97, 122, 123) |
| Diaporthe phaseolorum (Syn. Phomopsis phaseoli) | 1 | <0.1 | R | C | Yellow | (124) | ||
| Emarellia grisea | 6 | <0.1 | R | C | Black | 0.3–0.6 | (95, 96) | |
| Emarellia paragrisea | 1 | <0.1 | R | C | Black | 0.3–0.6 | (95) | |
| Exophiala dermatitidis | 1 | <0.1 | R | C | Black | 0.5–1.0 | (84) | |
| Exophiala jeanselmei | 25 | 0.2 | R | A | Black | 0.5–1.0 | (6, 61, 65, 67, 72, 75, 77, 79, 97, 100, 125–134) | |
| Exophiala oligosperma | 1 | <0.1 | R | C | Black | 0.5–1.0 | GCA_000835515.1 | (84) |
| Exserohilum rostratum | 1 | <0.1 | R | C | GCA_026367775.1 | (135) | ||
| Falciformispora lignatilis | 1 | <0.1 | R | C | Black | (136) | ||
| Falciformispora tompkinsii (Syn. Leptosphaeria tompkinsii) | 11 | <0.1 | R | C | Black | 0.5–1.0 | (60, 95–99, 137–139) | |
| Fusarium falciforme (Syn. Neocosmospora falciformis) | 8 | <0.1 | R | A | White | 0.2–0.5 | GCA_026873545.1 | (6, 8, 20, 69, 78, 99, 140) |
| Fusarium verticillioides (Syn. Fusarium monoliforme) | 1 | <0.1 | R | C | White | 0.5 | GCA_000149555.1 | (141) |
| Fusarium oxysporum | 1 | <0.1 | R | C | White | GCA_013085055.1 | (142) | |
| Fusarium solani (Syn Neocosmospora solani) | 18 | 0.1 | R | C | White | <1.5 | GCA_020744495.1 | (6, 42, 62, 67, 125, 143–148) |
| Fusarium chlamydosporum | 1 | <0.1 | R | C | Zwart | <1.5 | GCA_014898915.1 | (149) |
| Fusarium incarnatum | 1 | <0.1 | R | C | White | GCA_004367075.1 | (67) | |
| Fusarium subglutinans | 1 | <0.1 | R | C | GCA_013396075.1 | (150) | ||
| Fusarium thapsinum | 2 | <0.1 | R | C | White | GCA_013186935.1 | (99) | |
| Geotrichum candidum | 1 | <0.1 | R | C | GCA_013365045.1 | (8) | ||
| Ilyonectria destructans (Syn. Cylindrocarpon destructans) | 1 | <0.1 | R | C | White | 0.5 | GCA_020740775.1 | (151) |
| Macroventuria anomochaeta | 1 | <0.1 | R | C | GCA_010093625.1 | (26) | ||
| Madurella fahalii | 9 | <0.1 | R | C | Black | GCA_029876055.1 | (26, 96, 99, 152–154) | |
| Madurella pseudomycetomatis | 13 | 0.1 | R | C | Black | (13, (84, 91, 99, 154–156) | ||
| Madurella tropicana | 3 | <0.1 | R | C | Black | (13, (99, 154) | ||
| Microascus gracilis | 1 | <0.1 | R | C | White | (157) | ||
| Microsporum audouini | 6 | <0.1 | R | C | White | (6, 22, 31) | ||
| Microsporum canis | 4 | <0.1 | R | C | White | GCA_000151145.1 | (158–161) | |
| Neocosomospora cyanescens (Syn. Cylindrocarpon cyanescens) | 1 | <0.1 | R | C | White | (162) | ||
| Neoscytalidium dimidiatum (Syn. Scytalidium dimidiatum) | 1 | <0.1 | R | C | Brown | 4.0 | GCA_900092665.1 | (66) |
| Neotestudina rosatii | 14 | 0.1 | R | C | White | <0.5–1.5 | (6, 23, 77, 99, 105, 163) | |
| Nigrograna mackinnonii (syn. Pyrenochaeta mackinnonii) | 21 | 0.2 | R | C | Black | 0.3–1.0 | GCA_001007845.1 | (91, 95–98, 164, 165) |
| Paecilomyces variotii | 1 | <0.1 | R | C | White | GCA_004022145.1 | (166) | |
| Penicillium thomii | 1 | <0.1 | R | C | (26) | |||
| Phaeoacremonium krajdenii | 1 | <0.1 | R | C | White | (167) | ||
| Phaeoacremonium parasiticum | 1 | <0.1 | R | C | White | (168) | ||
| Phialophora verrucosa | 1 | <0.1 | R | C | Black | GCA_002099365.1 | (169) | |
| Phomopsis longicola | 1 | <0.1 | R | C | GCA_000800745.1 | (104) | ||
| Pleurostoma ochraceum (Syn. Pleurostomophora ochracea) | 1 | <0.1 | R | C | Yellow | (170) | ||
| Pseudochaetosphaeronema larense | 2 | <0.1 | R | C | Black | (96) | ||
| Rhinocladiella atrovirens | 2 | <0.1 | R | C | (38, 100) | |||
| Rhytidhysteron rufulum | 5 | <0.1 | R | C | Black | GCA_000467735.1 | (95–97) | |
| Sarocladium kiliense (Syn. Acremonium kiliense) | 7 | <0.1 | R | C | White yellow | <1.5 | GCA_030734395.1 | (67, 80) |
| Subramaniula obscura | 1 | <0.1 | R | C | (26) | |||
| Subramaniula thielavioides | 1 | <0.1 | R | C | (26) | |||
| Trichophyton interdigitale | 1 | <0.1 | R | C | White | GCA_019359935.1 | (26) | |
| Trichophyton rubrum | 2 | <0.1 | R | C | White | GCA_000151425.1 | (54, 171) | |
| Xenoacremonium recifei (Syn. Acremonium recifei) | 2 | <0.1 | R | C | White yellow | <1.5 | GCA_012184525.1 | (172) |
| Fungi without species identification | 772 | 6.2 |
C, common (>5% of the reported cases worldwide were caused by this species); O, occasional (caused 1%–5%); and R, rare (caused <1%).
A, strong evidence (microorganism was encountered in more than 50 individual cases, and its ability to form a mycetoma grain was confirmed in in vivo animal models); B, moderate evidence (microorganism was encountered in more than 10 individual cases, and its presence within the grain was confirmed by either immunohistochemistry or in situ hybridization); C, marginal evidence (microorganism was encountered in less than 10 individual cases, and there was no additional evidence that could demonstrate that the fungus that grew out of the grain was also present in the grain in the tissue)
As many of the eumycetoma causative agents are also considered fungi that live freely in the environment or are known to live on the skin, there has been a debate over whether all these can be considered true eumycetoma causative agents. Especially, for those species that can be found in large quantities in the environment, there has been a debate about whether these would be true eumycetoma causative agents or contaminants. Most of the eumycetoma causative agents have long culture times, while Aspergillus and Fusarium species, in general, grow rapidly under laboratory conditions. Therefore, for this review, we also created an evidence-based score, ranging from strong (A) to moderate (B) to marginal (C). Strong evidence was considered when Koch’s postulates were adhered to, meaning that a microorganism was encountered in more than 50 individual cases, and its ability to form a mycetoma grain was confirmed in in vivo animal models. An example of a species that belonged to this category was M. mycetomatis, as its grain-forming potential was confirmed in several animal models (173–176). Moderate evidence was considered when a microorganism was encountered in more than 10 individual cases, and its presence within the grain was confirmed by either immunohistochemistry or in situ hybridization. An example of a species that belonged to this category was Aspergillus flavus because in one case study with Aspergillus-specific antibodies, its presence was demonstrated in the eumycetoma grain in tissue (107). Marginal evidence was considered when a microorganism was encountered in less than 10 individual cases, and there was no additional evidence that could demonstrate that the fungus that grew out of the grain was also present in the grain in the tissue. As can be seen in Table 1, for M. mycetomatis, F. senegalensis, and Scedosporium bodyii, the evidence was strong. For most of the rare causative agents, the evidence was less strong, and the color of the grain was not even known.
Since in most of the studies, identification was based on either culture or histology, misidentifications could have occurred. The ranking presented is likely to shift when molecular identification of eumycetoma causative agents becomes the main identification technique. The study of Borman et al. demonstrated that 28 out of the 31 Trematosphaeria grisea isolates from the British National Collection of Pathogenic Fungi and French Institut Pasteur Culture Collection were in fact other black grain mycetoma causative agents, namely Nigrograna mackinnonii (n = 8), Medicopsis romeroi (n = 10), Rhytidhysteron rufulum (n = 4), Emarellia grisea (n = 5), and Emarellia paragrisea (n = 1) when they were re-identified using sequencing (95). The same was noted in Mexico, where, again, after sequencing, three isolates previously identified as T. grisea appeared to be N. mackinnonii and two isolates previously identified as M. mycetomatis were, in fact, Madurella pseudomycetomatis (164, 177). Therefore, etiologies might differ from what was reported before, and it could be that when molecular identification becomes the standard identification technique, differences in the ranking will be noted. However, as seen in a study in the Mycetoma Research Centre, in some regions, when molecular identification is introduced, the diversity in causative agents might enhance, but M. mycetomatis will remain by far the most common causative agent of eumycetoma (178).
GEOGRAPHICAL DISTRIBUTION OF THE CAUSATIVE AGENTS
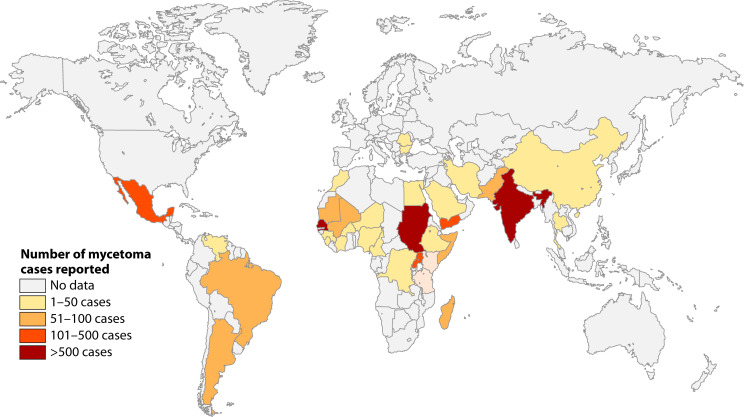
Eumycetoma has been reported from 102 countries, but there are large regional differences in the number of cases reported (179). If we look at eumycetoma alone, the highest number of cases was reported from Sudan (3, 5, 179), Senegal, and India (3, 5, 6, 179) (Fig. 1).
Fig 1.
Number of eumycetoma cases reported based on the metadata used for publications (3, 5) using references (6–94).
Due to the lack of surveillance programs, the prevalence of eumycetoma is largely unknown. In a previous report, the prevalence was calculated based on the number of reported cases divided by the population of the country in a particular year. In that way, a prevalence of 1.8 per 100,000 was obtained for Sudan (5). However, in surveys performed in highly endemic villages in Sudan, a prevalence of 1–5.2 eumycetoma cases per 1,000 inhabitants was found in the 1960s (180) and 6.2–35 cases per 1,000 inhabitants in the 2010s (180, 181), indicating that the prevalence of eumycetoma is much higher than reported.
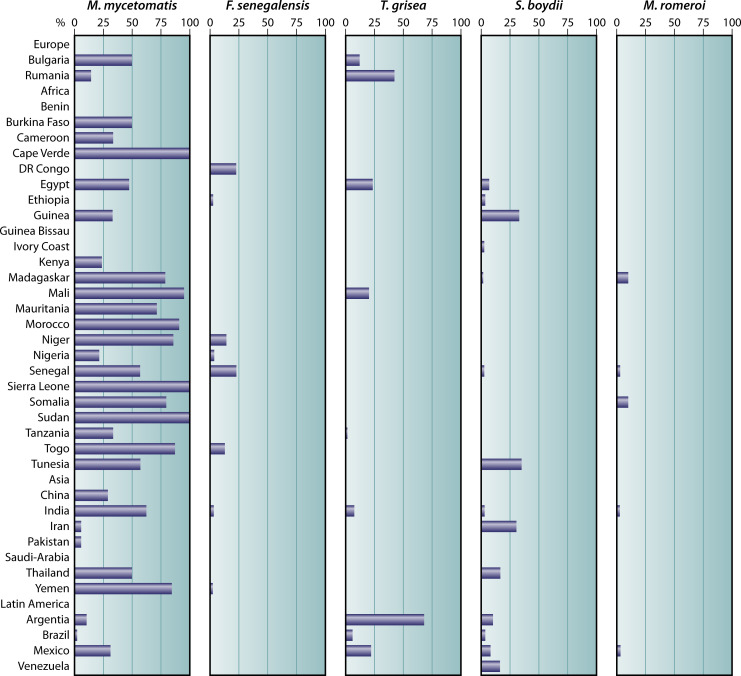
In the villages surveyed in Sudan, the most prevalent causative agent of eumycetoma was M. mycetomatis. In general, M. mycetomatis is the most common causative agent of eumycetoma in most regions in the world. However, the prevalence of F. senegalensis, T. grisea, Scedosporium boydii, and M. romeroi varies per region (Fig. 2).
Fig 2.
Distribution of the most common causative eumycetoma agents per country. For each country, the percentage of M. mycetomatis, F. senegalensis, T. grisea, S. boydii, and M. romeroi were calculated by the following formula (number of cases per selected species/total number of eumycetoma cases reported in that country × 100) and displayed in the corresponding panels.
F. senegalensis is mainly found in Western Africa and T. grisea in Europe and Latin America. S. boydii is also more prevalent in temperate regions in Europe and North America.
CLINICAL PRESENTATION
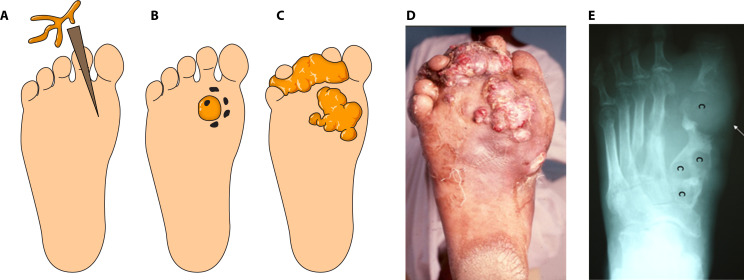
Since there are so many different causative agents able to cause mycetoma, one wonders if there are also differences in the clinical presentation per causative agent. Eumycetoma is an implantation mycosis and, therefore, there are several stages in which a eumycetoma lesion develops (Fig. 3).
Fig 3.
Eumycetoma development. (A) The fungal causative agent is introduced into the subcutaneous tissue via a minor trauma such as a thorn prick. (B) Inside the tissue, the fungus will form a grain. (C) Subcutaneous nodules will form, which will further extend into the subcutaneous tissue. (D) Picture of a mycetoma lesion in real life. (E) The eumycetoma causative agents will invade the bone and cavities (c) will form.
Phase 1: implantation into the subcutaneous tissue
As can be noted from Table 1, most of the eumycetoma causative agents are fungal species that can be found everywhere in our surroundings (S. boydii, Aspergillus species, and Fusarium species), and patients could have acquired from everywhere. Furthermore, these fungi have also been implicated in invasive fungal infections, and only a small portion of patients infected with these fungi develop mycetoma. DNA from the more specific eumycetoma causative agents, such as M. mycetomatis, F. senegalensis, and Falciformispora tompkinsii, was found in soil, thorns, spines, dung, and even in the walls of houses in highly endemic regions (135, 182–185). However, in those studies, it was impossible to culture the fungi from these sources too. In earlier studies, M. mycetomatis was cultured from soil and anthills (186, 187), and F. senegalensis and F. tompkinsii were cultured from about 50% of the examined dry thorns of Acacia trees (188, 189). However, their identity was not confirmed by sequencing.
A penetrating trauma is needed to introduce the causative agent into the subcutaneous tissue. This can be a prick from a thorn, splinter, or fish scale, a cut from a knife or farm implement, or insect and snake bites (180). These penetrating traumas often heal quickly, without any discomfort for the patient but they will result in the introduction of the causative agent in the subcutaneous tissue.
Phase 2: grain formation
Once into the subcutaneous tissue, the fungus will organize itself in a grain. The time of grain formation within the human host is unknown, as subclinical cases of eumycetoma have never been described. However, all grains generally originate within a single lesion from a single isolate introduced into the subcutaneous tissue (190).
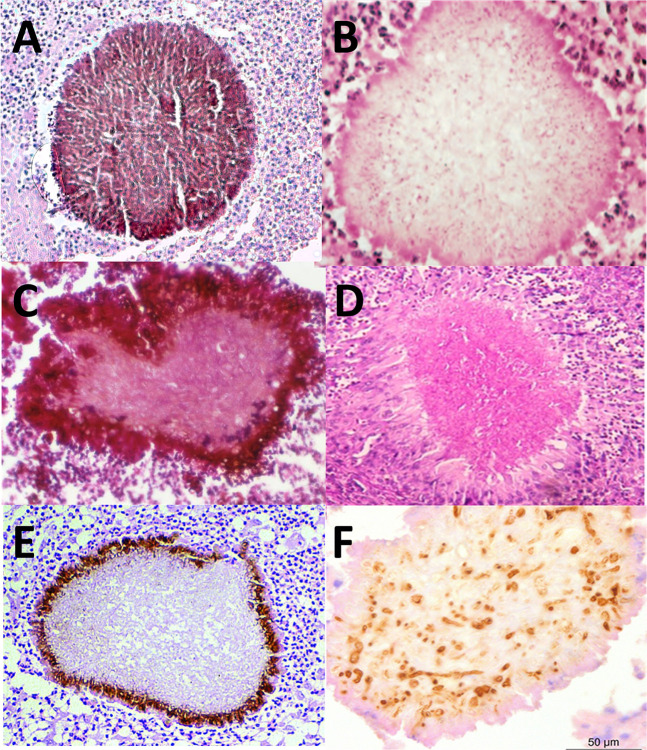
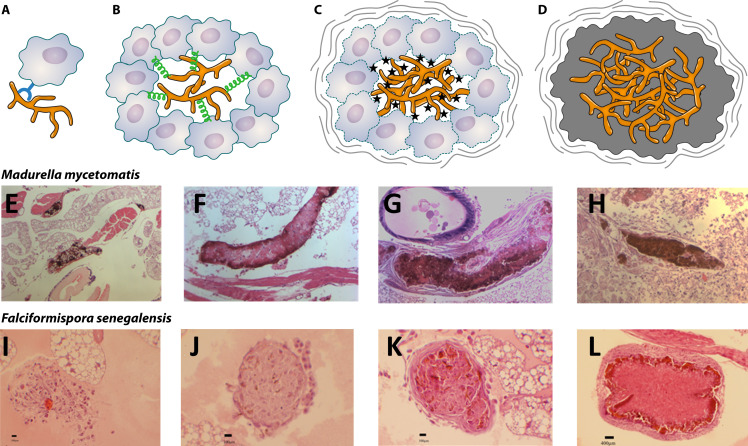
Grains are considered a unique feature of mycetoma, and each species forms its own type of grain (94). The majority of eumycetoma causative agents either produce black or white grains, but the less common causative agents Pleurostoma ochraceum and Aspergillus flavus have been reported to form either yellow or green grains, respectively (Table 1) (170). With histology, clear differences between the grains can be noted.
As shown in Fig. 4, the three black grains all differ and have species-specific features. In all cases, the color of the black grain is caused by melanin, a high molecular weight compound that is anchored to extracellular proteins. M. mycetomatis produces this melanin through the DHN-, DOPA-, and pyomelanin pathways (191, 192). In the M. mycetomatis grain, the hyphae are embedded in a brown matrix material, which is called cement. This cement material comprises areas of amorphous electron-dense material and areas of membrane-bound vesicular inclusions (193). Within this cement material, DNA and proteins of the host and pathogen can be found (194–197). In fact, 99.3% of the DNA and proteins identified in these grains is from humans, only 0.069% from the fungus (194, 198, 199). In M. mycetomatis grains, this cement material is found throughout the grain. Collagen in the grain shows a disintegration of the cross-striations with fiber swelling (200). The collagen appears honeycombed rather than branched (200). Within the hyphae, numerous concentric layers of cell wall material were often noted, indicative of intra-hyphal growth (193).
Fig 4.
Histology of grains. (A) M. mycetomatis; (B) S. boydii picture reprinted from reference (201) with permission of the publisher (McGraw Hill); (C) F. senegalensis, picture reprinted from reference (202); (D) Fusarium, picture reprinted from reference (203) with permission of the publisher (copyright 2016 Blackwell Verlag GmbH); (E) T. grisea, picture reprinted from reference (204) with permission; (F) A. flavus, stained with antibodies against Aspergillus, picture reprinted from reference (107) with permission of the publisher (copyright 2015 Blackwell Verlag GmbH).
The grains of the three Falciformispora species (Fig. 4C) that cause mycetoma are indistinguishable from each other (205) and difficult to distinguish from the grains of T. grisea (Fig. 4E) (193) or M. romeroi (200). At the periphery of these grains, the hyphae are embedded in a black, cement-like substance. In the centrum of the grain, a loose network of hyphae is noted. This portion is non-pigmented (205, 206). The grains of T. grisea resemble those of F. senegalensis and also have a blackish-brown cement-like material at the periphery of the grain. The hyphae in the center of the grain are weakly pigmented (193, 205, 206).
The white grains of Scedosporium and Fusarium species can be histopathologically similar (207). No cement material was noted in the white grains formed by S. boydii and Fusarium species (208). Instead, the central portion of each grain often consists of loosely interwoven hyaline or colorless hyphae (209). Thickening of the fungal cell walls and fusion of the cell walls were noted (208). Widespread cytoplasmic disorganization was noted. With electron microscopy, mitochondria were no longer noted, and increased granularity of the cytoplasm and an increase in the number of intracytoplasmic membranes were noted (208).
The grains described in patients are generally mature, and the early steps of grain formation in humans are unknown. Since grains cannot be formed in vitro, the process of grain formation can only be studied in in vivo grain models developed in the larvae of the greater wax moth Galleria mellonella and mice (173, 174, 210–212). Only for M. mycetomatis, F. senegalensis, S. boydii, Exophiala jeanselmei, and Fusarium falciforme, in vivo grain models have been developed (173, 174, 210–212). For S. boydii, E. jeanselmei, and F. falciforme, only models in mice have been developed, while for M. mycetomatis, grain models exist in mice and in the invertebrate Galleria mellonella, and for F. senegalensis, only a model in G. mellonella has been published (173, 174, 210–212). The grains formed in these animal modes all resembled the grains formed in humans.
In the two G. mellonella models, grain formation was followed in more detail over time. For both species, the process of grain formation can be divided into four stages (Fig. 5). In the first stage, hyphae cluster together and are surrounded by host cells (194). In the second stage, the hyphae are in close contact, and cement material starts to form. Individual host cells are still found between the hyphae, and melanization can also be seen. In the third stage, the cement material is fully formed, and individual host cells are no longer noted between the hyphae. A capsule is found to surround the grain (194). In the fourth stage, a massive influx of host cells is noted, and the granuloma forms, which surrounds the mature grain (194). When comparing the different grain-forming stages between M. mycetomatis and F. senegalensis in the invertebrate G. mellonella, it seems that the same stages are noted, but the timing is different. For instance, cement material already formed within 4 hours in the grain when G. mellonella larvae were infected with M. mycetomatis. When larvae were infected with F. senegalensis this was delayed (Fig. 5) (210).
Fig 5.
Grain formation over time. (A) Fungal cells are recognized by the host via pathogen receptor proteins. (B) Hemocytes will then agglutinate around the fungal hyphae, and host cells will be cross-linked to each other and attach to the fungal cells. The fungal cells will excrete zincophores and siderophores to obtain nutrients. (C) Degranulation of the host cells will occur, and reactive oxygen species will be produced. In order to protect itself, the fungus will produce melanin and trehalose. The host will form a capsule surrounding the granuloma. (D) In the final stage, the fungus will completely disintegrate the host cells and form the cement material. The extracellular matrix will be melanized. Although the steps in grain formation are similar between M. mycetomatis and F. senegalensis, the timing is not. (E) A M. mycetomatis grain in G. mellonella 4 hours after infection. Cement material is forming. (F) A M. mycetomatis grain in G. mellonella 24 hours after infection. Cement material is formed, and only a few host cells are still found within the grain. (G) A mature M. mycetomatis grain in G. mellonella larvae after 3 days. At this time no host cells are present in the cement material. The cement material is melanized, and a capsule surrounds the grain. (H) A massive influx of immune cells toward the M. mycetomatis grains 7 days after infection. (I) No grain is present in G. mellonella larvae infected with F. senegalensis 4 hours after infection. Only loose hyphae are noted. (J) At 24 hours after infection, the first signs of grain formation are noted. There is no cement material yet, and melanization is not noted. (K) F. senegalensis grain at 3 days after infection, cement material is forming, and some melanization of the grain is noted. Also, a capsule is forming. (L) At 7 days after infection, a mature F. senegalensis grain in G. mellonella is noted. Panels A–D are based on reference (194), panels E–H are reprinted from reference (174) (published under a Creative Commons license), and panels I–L are reprinted from reference (213) (published under a Creative Commons license).
In M. mycetomatis, proteomic and transcriptomic analyses have been performed to gain insight into the processes leading to grain formation. Up- and downregulation of genes and proteins at 4, 24, 72 h, and 7 days after infection were mapped (194, 199). The analysis of these responses led to the formation of a theoretical model for M. mycetomatis grain formation in G. mellonella (194) (Fig. 5A through D). In this model, grain formation starts with the recognition of M. mycetomatis by the host via pathogen recognition proteins. In G. mellonella, these receptors are β-glucan-recognizing proteins and hemolin (194). In turn, M. mycetomatis increases vesicle transport to transport building blocks for the extracellular matrix through the cell wall. Adhesion proteins, such as fructose bisphosphate aldolase, glyceraldehyde-3-phosphate dehydrogenase, and enolase, are displayed on the surface allowing M. mycetomatis to attach itself to the host (194). Next, hemocytes will agglutinate around the fungal hyphae, and the production of G. mellonella defense protein Hdd11 is increased, resulting in cross-linking of the hemocytes and attaching to M. mycetomatis. M. mycetomatis will subsequently secrete a zincophore to acquire zinc and siderophores to acquire iron and cross-link the extracellular matrix (194, 199). The cytoplasm of the G. mellonella hemocytes will be discharged, and degranulation occurs, which elevates reactive oxygen species production and the secretion of antimicrobial peptides in the grain (194). Finally, melanin will be produced by the fungus, and a capsule is formed surrounding the grain. In that stage, no hemocytes are found anymore within the grain; they are all lysed, and the extracellular matrix is completely melanized (194, 199). The melanin is most likely both intracellular entrapped in the fungal cell wall and extracellular entrapped into the extracellular matrix. Which of these processes are unique for the M. mycetomatis grain formation or shared within the grain formation of other fungal species is currently unknown; however, it is expected that melanization will be only found in black grain eumycetoma grain formation.
Phase 3: formation of the subcutaneous swelling
Despite the large number of causative agents and the differences in grain formation, the clinical presentation of eumycetoma is almost indistinguishable, and there are no clear differences noted between the different causative agents. The disease usually starts with the formation of a nodule. They are typically painless but painful in 18%–20% of patients, caused by secondary bacterial infection in most of them (214). The swelling is usually firm and rounded, but it may be soft, lobulated, and rarely cystic (214). It is often mobile. As the swelling increases, the skin becomes stretched and attached to the swelling. Localized areas of hyperhidrosis and sweating are occasionally seen in the affected skin (215). After some time, the swelling can suppurate and drain through sinus tracts (214). Prior to discharge, pustules may be visible (214). Secondary nodules evolve. Sinuses can close after discharge during the active phase of the disease, and new adjacent sinuses may open while some of the old ones may heal completely (214). These sinuses are numerous and interconnected with each other. They discharge serous, sero-purulent fluid and can be pussy with secondary bacterial infection. During the active phase of the disease, the sinuses discharge grains (214).
Although the foot is the most commonly affected region of the body, eumycetoma can also develop at other body sites, and no part is exempted. The foot (68.7%) and hand (4.0%) are affected most (5). Extrapedal involvement in the chest, abdominal walls, perineum, eye, gluteal and perineal regions, head, and neck occurs but is relatively rare (5). Mycetoma at these rare sites has high morbidity, is difficult to treat, and can be fatal (216). Furthermore, spreading from the primary site can occur via the blood and the lymphatics (2).
Phase 4: invasion of the bone
When the lesion progresses, the bone may be infected. Initially, the bone is displaced, bowed, or compressed from one or both sites (217). Then, an irritation reaction at the bone surface is noted (217). This can be noted on an X-ray by a periosteal reaction or diffuse reactive sclerosis (217). In the next stage, the fungus penetrates the periosteum and cortex and forms cavities in the bone. Cavitation is initially limited to a solitary bone and then spreads either longitudinally in the bone or horizontally toward other bones (217). Whether there are characteristic differences in bone affection or the appearance of the cavities between fungal species is currently unknown.
DIAGNOSIS AND IDENTIFICATION OF THE FUNGAL PATHOGENS
In order to identify the fungal pathogen, a surgical biopsy or a fine needle aspiration is used to obtain tissue specimens in which grains are present. The biopsy should be checked visually for the presence of grains before it is sent to the laboratory. In the laboratory, tissue materials are divided and submitted for microbiology, histopathology, and when available molecular examination. Tissue biopsies without visible grains usually result in negative cultures (193).
Histology
As mentioned earlier, eumycetoma grains are often either black or white, and there are some characteristic features that can be used to differentiate the causative agents (Fig. 4). The first is, of course, the presence of melanin; this would already differentiate the black (Fig. 4A, C, and E ) from the white grains (Fig. 4B and D). The second is the presence of cement material. The cement material is found throughout the grain in M. mycetomatis (Fig. 4A), but in the black grains of F. senegalensis (Fig. 4C) and T. grisea (Fig. 4E), the center is often non-pigmented, and cement material is absent (193). Of the 750 grains identified as M. mycetomatis by culture, 714 were also identified by histopathology, resulting in a sensitivity of 95.2% and a specificity of 95.4% (218). However, when molecular identification was used as a comparator, the sensitivity still remained at 93.8%, but the specificity dropped to 42.8%, simply because many of the black grains were wrongfully identified as M. mycetomatis in histology while they were in fact other black grain causative agents (178). The average time to identification was 8.5 (range: 2–15) days (178).
Culture
In order to culture the causative agents, grains are usually washed several times in sterile saline, crushed with a sterile glass rod, and plated onto Sabouraud agar plates supplemented with antibiotics. Commonly used antibiotics are gentamicin sulfate (400 mg/mL), penicillin G (20 U/mL), streptomycin (40 mg/mL), or chloramphenicol (50 mg/mL) (193). Plates are often inoculated in duplicate to be able to incubate them at 25°C and 37°C, as not all causative agents are able to grow at 37°C on culture medium. The average time to identification was 21.2 days (range: 2.5–60 days) (178). Identification is mainly based on the colony morphology and the microscopic morphology of the fruiting bodies, if present (Table 2)(193). For species such as M. mycetomatis, it can be difficult to identify the causative agent at the species level, as colony morphologies can be quite diverse, and sporulation is usually not observed. Also, differentiation between T. grisea and M. romeroi is difficult as their colony morphologies are quite similar. In most cases, T. grisea is sterile; however, some isolates are known to produce abortive flask-shaped fruiting bodies bearing conidia like those formed by M. romeroi (193).
TABLE 2.
Growth characteristics of the common and occasional causative agents of eumycetomaa
| Colony morphology | Ascospores | Conidia | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Organism | Color (obverse) | Color (reverse) | Secreted pigment | Texture | Growth at 37°C | Absent | Conidiophore | Shape | Size |
| M. mycetomatis | Yellowish brown to gray | Yellowish brown to gray | Yes | Skin like | Yes | Absent | Phialides with minute conidia | Globose to rounded | 2 × 3 µm |
| F. senegalensis | Gray-black | Black | No | Velvet | Yes | Subhyaline, obovoidal to ellipsoidal 23–36 × 8.0–13.5 µm, 4-septate | Absent | Absent | |
| Trematosphaeria grisea | Gray, becoming faint toward the margin | Dark gray | No | Velvet | No | Absent | Ampulliform phialides | Ellipsoidal to bacilliform | 2.5–4.0 × 1.3–1.5 µm |
| M. romeroi | Gray, becoming faint toward the margin | Dark gray | No | Velvet | No | Absent | Ampulliform phialides | Ellipsoidal to bacilliform | 2.5–4.0 × 1.3–1.5 µm |
| Scedosporium boydii | White gray, becoming darker gray or brown | Dark brown or gray, almost black | No | Downy to cottony | Yes | Absent | On branched cylindrical conidiophores or solitary | Globose to subglobose, thick-walled | 4–9 × 6–10 µm |
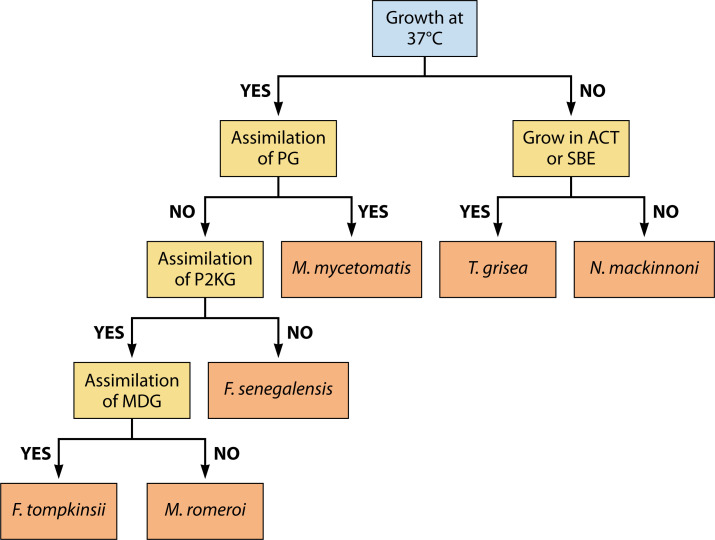
To differentiate the most common black-grain causative agents, one can make use of an identification scheme based on assimilation patterns (Fig. 6)
Fig 6.
Identification of causative agents by growth characteristics. Assimilation of or growth on potassium gluconate (PG), potassium 2-keto-gluconate (P2KG), methyl-D-glucopyranoside (MDG), actidione (ACT), or L-sorbose can help in differentiating the most common causative agents of black grain mycetoma. Adapted from references (94, 220).
Identification by matrix-assisted laser desorption ionization time-of-flight mass spectrometry
Identification of isolates can also occur via matrix-assisted laser desorption ionization time-of-flight mass spectrometry. For this, isolates are cultured on Sabouraud agar and then proteins are extracted by sequential ethanol, 70% formic acid, and acetonitrile precipitation and then applied to the target plate and overlaid with α-cyano-4-hydroxycinnamic acid matrix and measured (96). In the current Version MALDI Biotyper database of Brüker, 222 fungal species are included, including the rare eumycetoma causative agents Acremonium sclerotigenum, Aspergillus fumigatus, Aspergillus nidulans, Aspergillus niger, Aspergillus sydowii, Aspergillus terreus, Aspergillus ustus, Exophiala dermatitidis, Fusarium chlamydosporum, Fusarium oxysporum, Fusarium solani, Fusarium verticillioides, Microsascus gracilis, Neoscytalidum dimidiatum, and Sarocladium kiliense (221). For some eumycetoma causative agents, the Büker database only identifies to a species complex such as Aspergillus flavus/oryzae group, the Microsporium audouinii/canis group, and the Trichophyton rubrum group, or the genus level, such as Scedosporium and Curvularia species (221). For the more common eumycetoma causative agents, several labs have built their own databases so that they could identify the more common eumycetoma causative agents, such as Madurella mycetomatis, Falciformispora senegalensis, Trematosphaeria grisea, Scedosporium boydii, and Medicospsis romeroi to the species level (96, 222, 223). Furthermore, these databases also included samples of Curvularia lunata, Emarellia grisea, Emarellia paragrisea, Exophiala jeanselmei, Exserohilum rostratum, Madurella fahalii, Nigrograna mackinnonii, and Rhytidhysteron rufulum (96, 222).
Molecular identification
When DNA is extracted directly from grains, the time to identification can be reduced to 2.8 (range: 2.4–3.1) hours (178). In order to isolate DNA directly from grains, bead beating with metal beads is performed, after which a standard DNA isolation kit can be used. To identify the common and occasional causative agents and several rare eumycetoma causative agents, some specific amplification techniques have been developed. For the common and occasional causative agents, the amplification techniques are listed in Table 3.
TABLE 3.
Primers and probes for the identification of the common and occasional causative agents of eumycetoma
| Species | Primer/probe name | Sequence | Reference |
|---|---|---|---|
| Madurella mycetomatis | 26.1a | 5′-AATGAGTTGGGCTTTAACGG-3′ | (224) |
| 28.3a | 5′-TCCCGGTAGTGTAGTGTCCCT-3′ | (224) | |
| mm-fw | 5′-TCTCCTGTCCTACGACATCTGTGG-3′ | (225) | |
| mm-rv | 5′-TTCCTCACCTCCCAGCCCTTT-3′ | (225) | |
| PF2-specific-Myc | 5’-TGACCGTCGGCGTCTCTT-3’ | (226) | |
| R2-specific-Myc | 5′-TAGGCTGTCAGAAAACACATCG-3′ | (226) | |
| PF3-Myc | 5'-CTCCCGGTAGTGTAGTGT-3’ | (226) | |
| R3-Myc | 5'-CAGAAGACTCAGAGAGGCC-3’ | (226) | |
| M.m_forward | 5′-CCTCCCGGTAGTGTAGTGTCC-3′ | (227) | |
| M.m_reverse | 5′-GAGAGGCCGTACAGAGCAAAT-3′ | (227) | |
| M.m_probe | 5′-FAM-GGCGTCCGCCGGAGGATTATACAAC-BHQ1-3′ | (227) | |
| Falciformispora senegalensis | F.s_forward | 5′-GTTCCTACGCCGGCAAC-3′ | (227) |
| F.s_reverse | 5′-AGACAGGTATACTGCTTTTGCTGC-3′ | (227) | |
| F.s_probe | 5′-HEX- GCCGCTGGGTCTCCACC-BHQ1-3′ | (227) | |
| Scedosporium boydii | Forward | 5′-TGGCGAGCACGGTCTTG-3′ | (228) |
| Reverse | 5′-ACATTCACGGCAGACACTGATT-3′ | (228) | |
| Apioboy_P | 5'-FAM-TAGCAACGGAGTGTACGGAACCACCC-BBQ-3’ | (228) |
For the other causative agents, amplification of the internally transcribed spacer region (ITS) with either primers V9G (5′-TTACGTCCCTGCCCTTTGTA-3′) and LS266 (5′-GCATTCCCAAACAACTCGACTC-3′), ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) and ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG-3′), or ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) and ITS3 (5′GCATCGATGAAGAACGCAGC-3′) is used, followed by sanger sequencing or rolling circle amplification (RCA) with species-specific probes (229, 230) (Table 4). For most causative agents, each of the three primer sets for the ITS regions can be used; however, in the study of Desnos-Ollivier (97), four M. mycetomatis strains could not be amplified with the V9D and LS266 or the ITS4 and ITS5 primers. In the studies where amplification was performed directly on grains, in general, the pan-fungal primers ITS4 and ITS5 or the M. mycetomatis-specific primers 26.1A and 28.3A were used (13, 99).
TABLE 4.
Rolling circle amplification for the identification of eumycetoma causative agentsa
| Species | Primer/probe name | Sequence |
|---|---|---|
| RCA1 | 5′-ATGGGCACCGAAGAAGCA-3′ | |
| RCA2 | 5′-CGCGCAGACACGATA-3′ | |
| M. mycetomatis | MYC | 5′p-ACTACACTACCGGGAGGCCCgatcaTGCTTCTTCGGTGCCCATtaccggtgcggatagctac CGCGCAGACACGATAgtctaAGGGGGCCGAGGGAC-3′ |
| F. senegalensis | FSEN | 5′p-ACATAGACAAGGGTGTTGCCGGCgatcaTGCTTCTTCGGTGCCCATtaccggtgcggatagctac CGCGCAGACACGATAgtctaCAACGTACGGTAC-3′ |
| T. grisea | TGRIS | 5′p-ACCCGTAGGTCCTCCCAAAAGCGgatcaTGCTTCTTCGGTGCCCATtaccggtgcggatagctac CGCGCAGACACGATAgtctaTGGACGCCAGTCC-3′ |
| S. boydii | BoyRCA | 5′p-GGGTCGCGAAGACTCGCCGTAgatcaTGCTTCTTCGGTGCCCATtaccggtgcggatagctac CGCGCAGACACGATAgtctaTTTCAGGGCCTACGGA |
| S. apiospermum | ApioRCA | 5′p-CATCGTCCTCTTYTCAGAGGGGgatcaTGCTTCTTCGGTGCCCATtaccggtgcggatagctac CGCGCAGACACGATAgtctaCCGGCGGGAGGGG |
| M. romeroi | MRO | 5′p-AAGGCGAGTCCACGCACTCTGGgatcaTGCTTCTTCGGTGCCCATtaccggtgcggatagctac CGCGCAGACACGATAgtctaCTGCCAATGACTTT-3′ |
TREATMENT AND IN VITRO SUSCEPTIBILITY OF THE PATHOGENS
The standard treatment of eumycetoma is combination therapy with either an azole or terbinafine and surgery (231). In a survey by the WHO held in 2022, it appeared that 85% of the respondents use itraconazole to treat eumycetoma (125, 232, 233). This was followed by terbinafine (48%), voriconazole (41%), and posaconazole (33%). Voriconazole and posaconazole were used mainly in high-income countries (125, 232, 233). There is only one double-blind clinical trial performed for eumycetoma. That is, trial NCT03086226, Proof-of-Concept Superiority Trial of Fosravuconazole Versus Itraconazole for Eumycetoma in Sudan (234). In that trial, only cases of M. mycetomatis mycetoma were included, which were either treated with itraconazole or fosravuconazole, the prodrug of ravuconazole. Therefore, no comparison can be made if eumycetoma cases where other fungi were the causative agent will respond similarly to the treatment given. Therefore, due to the lack of clinical trials, we can only review in vitro susceptibility data, animal models, and case series and estimate if there are likely differences in treatment response between different causative agents. Future clinical trials should be performed to be able to answer this question properly.
In vitro susceptibility data
The in vitro susceptibility data for the different eumycetoma causative agents were all generated with the protocols from the Clinical and Laboratory Standards Institute (CLSI) or the European Committee on Antimicrobial Susceptibility Testing (235). These were either in-house assays (236–242) or using the commercial YeastOne testing panel (242–244). For some of the non-sporulating causative agents, such as Madurella species, a small adaptation to these protocols was needed to perform in vitro susceptibility testing with hyphal fragments instead of conidia (235). These adaptations were recently reviewed and will not be discussed here (235).
In Table 5, the published in vitro susceptibilities toward the antifungal agents commonly used to treat mycetoma are listed, as well as those for ravuconazole. When we focus on the common and occasional causative agents of eumycetoma, we can already see some clear differences. For itraconazole, the concentration that will inhibit 90% of all tested isolates in growth (MIC90) is 0.25 μg/ml for M. mycetomatis and 1 μg/ml F. senegalensis, which are lower than the serum levels that are usually attained with this drug. For T. grisea, S. boydii, and M. romeroi, the MIC90 is above these serum levels, indicating that these three species most likely will not be inhibited in growth in a lesion (245, 235). Also, for some of the more rare causative agents, such as the Fusarium species and M. fahalii, higher MIC90s for itraconazole are noted. The high MIC found in M. fahalli was most likely due to the insertion of glutamic acid at position 149 and a shift from isoleucine to valine at position 153 of the CYP51 protein (152). For the newer azoles, voriconazole and posaconazole, T. grisea and M. romeroi seem to be more susceptible as their MIC90s were within range of the serum levels. However, for most Fusarium species, also for voriconazole, posaconazole, and terbinafine, high MIC90s were obtained (245).
TABLE 5.
In vitro susceptibilities of eumycetoma causative agents as determined by the CLSI methodology
| Itraconazole | Ravuconazole | Posaconazole | Voriconazole | Terbinafine | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Occurrence | N | MIC90 (μg/ml)a | Range (μg/ml) | Ref | N | MIC90 (μg/ml)a | Range (μg/ml) | Ref | N | MIC90 (μg/ml)a | Range | Ref | N | MIC90 (μg/ml)a | Range (μg/ml) | Ref | N | MIC90 (μg/ml)a | Range (μg/ml) | Ref |
| Madurella mycetomatis | C | 131 | 0.25 | <0.008–1 | (246) | 131 | 0.032 | <0.002–0.125 | (246) | 34 | 0.064 | <0.03–0.125 | (240) | 34 | 0.25 | <0.016–0.5 | (242) | 34 | 16 | 2–> 16 | (240) |
| Falciformispora senegalensis | O | 6 | 1 | 0.03–1 | (235) | 6 | 0.25 | 0.064–0.25 | (247) | 4 | 0.125 | 0.03–1 | (235) | 4 | 0.25 | 0.25 | (235) | 3 | 0.25 | 0.125–0.25 | (235) |
| Trematosphaeria grisea | O | 11 | 4 | 0.125–4 | (235) | 4 | 8 | 0.125–8 | (247) | 3 | 0.25 | 0.016–0.25 | (235) | 3 | 0.25 | 0.125–0.25 | (235) | ||||
| Scedosporium boydii | O | 39 | >4 | >4 | (248) | 30 | 4 | 0.5–16 | (249) | 48 | 2 | 0.125–16 | (250) | 39 | 1 | 0.125–16 | (248) | 30 | >16 | >16 | (249) |
| Medicopsis romeroi | O/R | 23 | >8 | 1- > 8 | (95, 235) | 6 | 1 | 0.25–1 | (247) | 5 | 1 | 0.25–1 | 23 | 0.5 | 0.125–0.5 | (95, 235) | |||||
| Acremonium blochii | R | ||||||||||||||||||||
| Acremonium sclerotigenum | R | ||||||||||||||||||||
| Amesia atrobrunnea | R | 4 | 0.07 | 0.04–0.07 | (251) | 7 | 0.25 | 0.06–0.25 | (251) | 7 | 0.5 | 0.125–0.5 | (251) | ||||||||
| Aspergillus candidus | R | 3 | 0.25 | 0.03–0.25 | (252) | 3 | 1 | 0.05–1 | (252) | ||||||||||||
| Aspergillus flavus | R | 175 | 1 | 0.125–1 | (250) | 13 | 1 | 0.125–1 | (253) | 175 | 0.5 | 0.06–1 | (250) | 175 | 1 | 0.125–2 | (250) | ||||
| Aspergillus fumigatus | R | 1,263 | 1 | 0.125- > 8 | (250) | 114 | 0.5 | 0.25–4 | (253) | 1,263 | 0.5 | 0.03–4 | (250) | 1,263 | 0.5 | 0.06- > 8 | (250) | 82 | 2 | 0.05–4 | (254) |
| Aspergillus nidulans | R | 38 | 1 | 0.125–1 | (250) | 38 | 0.5 | 0.125–0.5 | (250) | 38 | 0.25 | 0.03–0.5 | (250) | ||||||||
| Aspergillus niger | R | 174 | 2 | 0.125–4 | (250) | 22 | 2 | 0.5–4 | (253) | 174 | 1 | 0.06–1 | (250) | 174 | 1 | 0.03–2 | (250) | ||||
| Aspergillus sydowii | R | 23 | 2 | 0.5–2 | (250) | 9 | 2 | 0.125–2 | (253) | 23 | 1 | 0.25–1 | (250) | 23 | 1 | 0.06–2 | (250) | ||||
| Aspergillus terreus | R | 75 | 1 | 0.125–1 | (250) | 8 | 0.5 | 0.25–0.5 | (253) | 75 | 0.5 | 0.125–1 | (250) | 75 | 0.5 | 0.125–2 | (250) | ||||
| Aspergillus ustus | R | 11 | 4 | 2–4 | (255) | 3 | 1 | 0.25–1 | (256) | 3 | 16 | 4–16 | (256) | 11 | 8 | 0.25–8 | (255) | 11 | 0.5 | 0.06–5 | (255) |
| Cladophialophora bantiana | R | 37 | 0.125 | <0.016–0.25 | (257) | 37 | 0.125 | <0.016–0.125 | (257) | 37 | 2 | 0.125–4 | (257) | ||||||||
| Cladophialophora mycetomatis | R | ||||||||||||||||||||
| Cladosporium ramotellenum | R | ||||||||||||||||||||
| Corynespora cassiicola | R | 1 | 0.5 | 0.5 | 1 | 2 | 2 | 1 | 0.25 | 0.25 | (258) | ||||||||||
| Curvularia geniculata | R | 14 | 0.25 | 0.06–1 | (259) | 14 | 0.25 | <0.03–0.5 | (259) | 14 | 1 | 0.125–4 | (259) | ||||||||
| Curvularia lunata | R | 10 | 0.25 | 0.125– > 16 | (259) | 10 | 0.25 | <0.03–0.5 | (259) | 10 | 1 | 0.25–1 | (259) | ||||||||
| Diaporthe phaseolorum | R | ||||||||||||||||||||
| Emarellia grisea | R | 5 | 0.5 | 0.06–0.5 | (95) | 5 | 0.5 | 0.125–0.5 | (95) | ||||||||||||
| Emarellia paragrisea | R | 1 | 0.125 | 0.125 | (95) | 1 | 0.125 | 0.125 | (95) | ||||||||||||
| Exophiala dermatitidis | R | 51 | 2 | <0.015–2 | (260) | 51 | 2 | <0.03–2 | (260) | 51 | 2 | 0.06–2 | (260) | 51 | 4 | <0.03–4 | (260) | ||||
| Exophiala jeanselmei | R | 17 | 0.25 | 0.016–0.25 | (235) | 17 | 0.06 | 0.016–0.06 | (235) | 17 | 0.5 | 0.125–2 | (235) | ||||||||
| Exophiala oligosperma | R | 4 | 8 | 0.06–8 | (261) | 2 | 0.06 | <0.03–0.06 | (261) | 5 | 8 | 0.125–8 | (261) | ||||||||
| Exserohilum rostratum | R | 34 | 0.03 | <0.03–0.125 | (262) | 34 | 0.03 | <0.03–0.125 | (262) | 34 | 0.25 | <0.03–1 | (262) | 34 | 0.03 | <0.03–0.03 | (262) | ||||
| Falciformispora lignatilis | R | ||||||||||||||||||||
| Falciformispora tompkinsii | R | 3 | 0.25 | 0.25 | (235) | 3 | 0.25 | 0.25 | (235) | 3 | 0.5 | 0.5 | (235) | ||||||||
| Fusarium falciforme | R | ||||||||||||||||||||
| Fusarium verticillioides | R | 13 | 16 | 1–16 | (245) | 13 | 16 | 2–16 | (245) | 13 | 16 | 0.25–16 | (245) | 13 | 16 | 1–16 | (245) | 13 | 32 | 1–32 | (245) |
| Fusarium oxysporum | R | 14 | 16 | 1–16 | (245) | 14 | 16 | 1–16 | (245) | 14 | 16 | 0.06–16 | (245) | 14 | 16 | 0.5–16 | (245) | 14 | 32 | 0.5–32 | (245) |
| Fusarium solani | R | 22 | 16 | 16 | (245) | 22 | 16 | 4–16 | (245) | 22 | 16 | 16 | (245) | 22 | 16 | 4–16 | (245) | 22 | 32 | 16–32 | (245) |
| Fusarium chlamydosporum | R | 4 | 50 | 12.5–50 | (263) | ||||||||||||||||
| Fusarium incarnatum | R | 1 | >8 | >8 | (264) | 1 | 8 | 8 | (264) | ||||||||||||
| Fusarium subglutinans | R | 1 | >16 | >16 | (150) | 1 | >16 | >16 | (150) | 1 | 2 | 2 | (150) | ||||||||
| Fusarium thapsinum | R | 5 | >16 | >16 | (265) | 5 | >16 | 8–>16 | (265) | 5 | >16 | >16 | (265) | 5 | 4 | 2–4 | (265) | 5 | 0.5 | 0.25–0.5 | (265) |
| Geotrichum candidum | R | ||||||||||||||||||||
| Ilyonectria destructans | R | ||||||||||||||||||||
| Macroventuria anomochaeta | R | ||||||||||||||||||||
| Madurella fahalii | R | 1 | >16 | >16 | (235) | 2 | 0.016 | 0.004–0.016 | (247) | 1 | 1 | 1 | (235) | 1 | 1 | 1 | (235) | ||||
| Madurella pseudomycetomatis | R | 7 | 0.06 | 0.016–0.06 | (235) | 6 | 0.032 | 0.008–0.032 | (247) | 7 | 0.06 | 0.008–0.06 | (235) | 7 | 0.25 | 0.008–0.25 | (235) | ||||
| Madurella tropicana | R | 1 | 0.01 | 0.01 | (235) | 3 | 0.064 | 0.016–0.064 | (247) | 1 | 0.03 | 0.03 | (235) | 1 | 0.03 | 0.03 | (235) | ||||
| Microascus gracilis | R | ||||||||||||||||||||
| Microsporum audouini | R | ||||||||||||||||||||
| Microsporum canis | R | 100 | 4 | 0.03–8 | (266) | 42 | 0.06 | <0.03–0.25 | (267) | 100 | 1 | 0.08–2 | (266) | 100 | 0.06 | 0.08–0.5 | (266) | 100 | 0.25 | 0.08–0.5 | (266) |
| Neocosomospora cyanescens | R | ||||||||||||||||||||
| Neoscytalidium dimidiatum | R | 17 | >16 | <0.03- > 16 | (268) | 17 | 2 | 0.125–2 | (268) | 17 | 0.25 | <0.03–0.5 | (268) | 17 | 0.5 | 0.125–0.5 | (268) | ||||
| Neotestudina rosatii | R | ||||||||||||||||||||
| Nigrograna mackinnonii | R | 10 | 2 | (0.25–2) | (95) | 10 | 1 | 0.125–1 | (95) | ||||||||||||
| Paecilomyces variotii | R | 26 | 0.25 | 0.016–0.5 | (269) | 26 | 16 | 0.016–16 | (269) | 26 | 0.125 | 0.016–0.5 | (269) | 26 | 16 | 0.03–16 | (269) | 26 | 4 | 0.125–32 | (269) |
| Penicillium thomii | R | ||||||||||||||||||||
| Phaeoacremonium krajdenii | R | 11 | 16 | 16–>16 | (270) | 11 | 0.5 | 0.125–0.5 | (270) | 11 | 0.5 | 0.125–0.5 | (270) | ||||||||
| Phaeoacremonium parasiticum | R | 16 | >16 | >16 | (270) | 16 | 1 | 0.25–1 | (270) | 16 | 1 | 0.25–1 | (270) | ||||||||
| Phialophora verrucosa | R | 46 | 2 | 0.25–4 | (271) | 46 | 0.5 | 0.03–1 | (271) | 46 | 1 | 0.06–4 | (271) | 46 | 0.25 | 0.002–1 | (271) | ||||
| Phomopsis longicola | R | ||||||||||||||||||||
| Pleurostoma ochraceum | R | 1 | 0.25 | 0.25 | (170) | 1 | 1 | 1 | (170) | 1 | 0.5 | 0.5 | (170) | ||||||||
| Pseudochaetosphaeronema larense | R | ||||||||||||||||||||
| Rhinocladiella atrovirens | R | ||||||||||||||||||||
| Rhytidhysteron rufulum | R | 2 | 1 | 0.5–1 | (95) | 2 | 0.25 | 0.125–0.25 | (95) | ||||||||||||
| Sarocladium kiliense | R | 4 | >16 | >16 | (272) | 4 | >16 | 4–>16 | (272) | 4 | 1 | 0.5–1 | (272) | 4 | 0.5 | 0.5 | (272) | ||||
| Subramaniula obscura | R | ||||||||||||||||||||
| Subramaniula thielavioides | R | ||||||||||||||||||||
| Trichophyton interdigitale | R | ||||||||||||||||||||
| Trichophyton rubrum | R | 111 | 2 | 0.03–16 | (273) | 16 | 0.125 | 0.03–0.125 | (274) | 111 | 0.5 | 0.016–1 | (273) | 111 | 0.125 | 0.03–16 | (273) | 111 | 0.06 | 0.008–0.06 | (273) |
| Xenoacremonium recifei | R | ||||||||||||||||||||
| Serum level | >1–2 | >0.5–1.5 | >1–6 | 2.8 | |||||||||||||||||
The MIC90 values above the therapeutic goal according to reference (275) are bold and underlined, the MIC90 values equal to the upper boundary of the therapeutic goal according to reference (275) are in bold, and the MIC90 values lower than the upper boundary of the therapeutic goal according to reference (275) are in normal font.
Although in vitro susceptibility assays can determine if a drug is active against fungal hyphae, this does not always predict if the drug is also active in a more complex structure such as a grain. Only in one study, the MIC of hyphae was compared to the MIC obtained from grains. In this study, the grains were obtained from experimentally infected mice, not humans (276). The MIC obtained with M. mycetomatis hyphae was 1 µg/mL, while grains were not inhibited by concentrations up to 100 µg/mL (276). The same phenomenon was also noted for Exophiala dermatitidis biofilms (277). E. dermatitidis planktonic cells were susceptible to itraconazole, voriconazole, and posaconazole, while the E. dermatitidis biofilm was completely resistant (277, 278). This demonstrates that the cement material present in biofilms and grains can protect the hyphae inside these structures against antifungal agents (235, 277). As previously mentioned, this cement material consists of melanin, proteins, chitin, and polysaccharides. M. mycetomatis melanin was able to bind ketoconazole and itraconazole, and when 250 µg/mL M. mycetomatis melanin was added to an in vitro susceptibility assay, a strong rise in MIC was noted for ketoconazole and itraconazole (191). The polysaccharide β-1,3-D-glucan and extracellular DNA are also known to bind antifungal agents (235).
So, based on the in vitro data generated so far, one can conclude that the different causative agents have different susceptibilities toward the standard drugs used to treat eumycetoma patients. Furthermore, the cement material also seems to protect the fungus inside against these drugs.
Evidence from animal models
Although animal models were developed for M. mycetomatis, F. senegalensis, S. boydii, E. jeanselmei, and F. falciforme (173, 174, 210–212), only in the models for M. mycetomatis and F. senegalensis, antifungal treatments were evaluated. If we look at the two G. mellonella models for M. mycetomatis and F. senegalensis, we see a difference in clinical response toward antifungal agents. In these models, larvae are infected with either M. mycetomatis or F. senegalensis and then treated at 4, 28, and 52 h after infection with either 1 mg amphotericin B/kg of body weight/day, 5.7 mg itraconazole/kg of body weight/day, or 7.14 mg terbinafine/kg of body weight/day (213, 279). Prolonged larval survival was noted when M. mycetomatis-infected larvae were treated with either amphotericin B or terbinafine, not when they were treated with itraconazole (279). For F. senegalensis-infected larvae, prolonged survival was only noted when they were treated with amphotericin B, not when treated with terbinafine or itraconazole (213). A similar comparison could not be made for the studies performed in mouse models, as only in the M. mycetomatis mouse model treatment responses were measured, not in the other mouse models. The treatment response measured in mice infected with M. mycetomatis was similar to that in G. mellonella larvae infected with M. mycetomatis, although the read-out was different. No grains were observed at day 21 in mice infected with M. mycetomatis and treated with 1 mg amphotericin B/kg of body weight/day for 14 days, while grains were still present at day 21 when mice were treated with 40 mg itraconazole/kg of body weight/day for 14 days (280). So, based on the scarce data from animal models, it looks like there is a difference in treatment response between the different species; however, much more data are needed.
Evidence from case series
The most extensive case series of eumycetoma patients treated with either ketoconazole or itraconazole are from the Mycetoma Research Centre (MRC) in Khartoum, Sudan (281, 282), followed by the Université Cheikh Anta Diop de Dakar, Senegal (42) (Table 6). In the Sudanese studies, more than 1,500 eumycetoma cases were followed (281, 282). In all patients, the causative agent was identified as M. mycetomatis, and adjusted cure rates of 56.2% and 67.6% were noted for ketoconazole and itraconazole (Table 6). Furthermore, a complete cure was significantly associated with surgery, smaller lesion sizes, and shorter disease duration (281, 282). In the Senegalese study, the black grain causative agents were not always identified to the species level; however, a better treatment outcome was noted when itraconazole was combined with surgery (adjusted cure rate of 100%) and a comparable treatment outcome when terbinafine was combined with surgery (adjusted cure rate of 55.6%). Also, this study noted an association with better treatment outcomes when medical treatment was combined with surgery (42). Unfortunately, no large cohort studies were performed for the other causative agents, and only a few case series and case reports have been described. For the other occasional eumycetoma causative agents Falciformispora species (136–138, 233), T. grisea (103, 283–285), and S. boydii (286), as well as for the rare Fusarium causative agents (140), corrected treatment responses toward the azoles ranged from 20% to 38.5% and again antifungal treatment combined with surgery had a more favorable outcome. However, the number of cases was small. Strikingly, the corrected treatment responses of in vitro azole-resistant Fusarium species, T. grisea, and S. boydii were much lower than those found in the larger studies of M. mycetomatis. But clearly, more data are needed to indicate whether there is a correlation between the species and treatment outcome.
TABLE 6.
Treatment response as described in case series
| Species | N | Treatment | Surgery (including amputation) | Treatment response | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cured | Still on treatment | Amputation | Not cured or recurrenceb | Lost to follow up | Corrected cure ratea (%) | |||||
| Madurella mycetomatis | 13 | Ketoconazole (100–400 mg/day) | Unknown | 4 (30.8%) | 9 (69.2%) | 30.8 | (287) | |||
| Madurella mycetomatis | 1,242 | Ketoconazole (400–800 mg/day) | 1,242 | 321 (25.8%) | 35 (2.8%) | 215 (17.3) | 671 (54.0%) | 56.2 | (281) | |
| Madurella mycetomatis | 11 | Itraconazole (400 mg/day) | 0 | 1 (9.1%) | 10 (90.9%) | 9.1 | (288) | |||
| Madurella mycetomatis | 377 | Itraconazole (400 mg/day) | 164 | 48 (12.7%) | 306 (81.2) | 23 (6.1) | 13 | 67.6 | (282) | |
| Madurella mycetomatis or Falciformispora senegalensis | 23 | Itraconazole | 23 | 23 (100%) | 100.0 | (42) | ||||
| Madurella mycetomatis or Falciformispora senegalensis | 68 | Terbinafine (500 mg BID) | 49 | 20 (29.4) | 32 | 18 | 2 | 14 | 55.6 | (42) |
| Falciformispora species | 4 | Azoles | 3 (75%) | 1 (25%) | 1 (25%) | 2 (50%) | 25 | (136–138, 233) | ||
| Trematosphaeria grisea | 5 | Azoles | 1 | 1 (20%) | 4 (80%) | 20 | (103, 283–285) | |||
| Scedosporium species | 3 | Azoles | 1 (33.3%) | 1 (33.3%) | 1 (33.3%) | 1 (33.3) | (286) | |||
| Fusarium species | 19 | Azoles | 6 (31.6%) | 5 (26.3%) | 1 (5.3%) | 7 (36.8%) |
6 (31.6%) | 38.5 | (140) | |
For the corrected cure rate, only those patients who had completed their treatment and were not lost to follow-up were included. For that, we used the following calculation: (number of cured patients)/[(total number of patients) − (number of patients under treatment) − (number of patients lost to follow-up)] × 100.
Not cured also includes patients who improved on treatment as they were still not cured.
PREVENTION OF EUMYCETOMA
There are currently no thorough studies performed on the prevention of eumycetoma. However, in the past decade, several modeling studies showed that, in Sudan, the strongest predictors of mycetoma occurrence were aridity, proximity to water, low soil calcium and sodium concentrations, and the distribution of various thorny trees species such as Acacia trees (181, 289–291). Also, most patients seem to live on light clay soil (181, 291). Next to that, a correlation between eumycetoma and close contact with stock animals, such as goats and cows, was noted (292–294). This correlation is most likely due to the environmental conditions associated with cattle. Animal enclosures are often found to be made of thorns, and they often stand in mud, dirt, and dung (294). Also, ticks and other insects are found on these animals, and in one of these ticks M. mycetomatis was detected by PCR (295). Although there are no large case-cohort studies performed yet, it seems clear that improving the living and hygienic standards in mycetoma-endemic regions and villages could help to reduce the risk of contracting eumycetoma (291). Also, wearing shoes might lower the risk of contracting eumycetoma; however, so far, no studies have been performed to prove this assumption.
WAY FORWARD
So, from the in vitro and in vivo comparisons, there is some evidence that not all causative agents respond the same to therapy, but the numbers are small. To enhance the current knowledge, gathering information from individual cases and comparing their treatment response are important. To be able to do that, a few things are needed. First, a proper diagnosis and species identification are needed. This means that more reliable methods need to be used for species identification. So far, the most reliable method was molecular identification; however, species-specific molecular identification tools have been developed for only a few causative agents. The development of more identification tools, which will help diagnose and update the epidemiologic data, is on its way. Second, more data are needed to assess and compare the treatment responses of the different causative agents to standard treatment to develop proper treatment guidelines. In the open-source drug discovery project MycetOS (https://github.com/OpenSourceMycetoma/General-Start-Here), in vitro responses against new drugs are already determined against multiple causative agents so that at least the differences in in vitro susceptibility can be assessed. To assess the differences in treatment responses in patients, the new initiative to collect case reports from implantation mycoses in the openly accessible, publicly-funded CURE ID platform (https://cure.ncats.io) is another step forward (232). These collected case reports can then be analyzed to gain insights into the treatment responses of the different causative agents, which in turn can be further developed into treatment guidelines as a resource for treating physicians.
CONCLUSION
This review established that M. mycetomatis was the most common causative agent of eumycetoma, followed by F. senegalensis, T. grisea, S. boydii, and M. romeroi. Clinically, there were no differences reported on the form of the lesions or the bone involvement between the different causative agents, but the differences in grain morphology and formation were noted. Only for M. mycetomatis and S. boydii species-specific molecular diagnostic tools were available, but for the other causative agents, they are currently being developed. Differences in in vitro susceptibility and treatment responses in animal models and patient series were noted, but the numbers were small. Therefore, there is a large need to gather more case series with proper species identification in order to develop treatment guidelines that also take the causative agent of eumycetoma into account. This is desperately needed to improve the therapeutic outcome in this difficult-to-treat neglected tropical disease.
Biographies

Wendy van de Sande is an associate professor in medical mycology at ErasmusMC, the Netherlands. She has been studying mycetoma since 2001 and obtained her PhD from Erasmus University in Rotterdam in 2007 cumlaude. She published 128 peer-reviewed papers on mycetoma. She and her team developed several diagnostic tools to identify the causative agents at the species level. These tools are currently used worldwide. She also leads the biological screening in the Mycetoma Open Source drug discovery program MycetOS. She is a founding member of the global mycetoma working group, convener of the eumycetoma working group at the International Society of Human and Animal Mycoses and vice chair of the skinNTD subgroup of the Diagnostic Technical Advisory Group of the World Health Organization.

Prof. Ahmed Fahal, a distinguished surgeon and professor, has significantly contributed to medical research, education, and the management of mycetoma. Trained at the University of Khartoum and in the UK, he attained the position of consultant surgeon at Soba University Hospital and a professorship at the University of Khartoum. He excels in mycetoma and tropical surgery, founding the globally recognized Mycetoma Research Centre at the University of Khartoum, WHO Collaborating Center on Mycetoma and Skin NTDs. His extensive research, with over 300 peer-reviewed articles, positions him among the top 2% of global scientists. Playing fundamental roles in educational development and scientific research agencies, he currently serves as the Vice President of the Arab Scientific Research Councils Federation. Prof. Fahal's enduring legacy encompasses groundbreaking clinical trials, the establishment of mycetoma satellite centers, and vocational training for mycetoma patients, reflecting his dedication to comprehensive healthcare. His numerous awards underscore his outstanding achievements in scientific innovation and research.
Contributor Information
Wendy W. J. van de Sande, Email: w.vandesande@erasmusmc.nl.
Graeme N. Forrest, Rush University, Chicago, Illinois, USA
Rod Hay, The International Foundation for Dermatology, London, United Kingdom.
Ferry Hagen, Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands.
REFERENCES
- 1. WHO . 2016. WHA69.21: assessing the burden of mycetoma. WHO, Geneva. [Google Scholar]
- 2. Zijlstra EE, van de Sande WWJ, Welsh O, Mahgoub ES, Goodfellow M, Fahal AH. 2016. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis 16:100–112. doi: 10.1016/S1473-3099(15)00359-X [DOI] [PubMed] [Google Scholar]
- 3. van de Sande W, Fahal A, Ahmed SA, Serrano JA, Bonifaz A, Zijlstra E, eumycetoma working group . 2018. Closing the mycetoma knowledge gap. Med Mycol 56:153–164. doi: 10.1093/mmy/myx061 [DOI] [PubMed] [Google Scholar]
- 4. WHO . 2023. WHO fungal priority pathogens list to guide research, development and public health action
- 5. van de Sande WWJ. 2013. Global burden of human mycetoma: a systematic review and meta-analysis. PLoS Negl Trop Dis 7:e2550. doi: 10.1371/journal.pntd.0002550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oladele RO, Ly F, Sow D, Akinkugbe AO, Ocansey BK, Fahal AH, van de Sande WWJ. 2021. Mycetoma in West Africa. Trans R Soc Trop Med Hyg 115:328–336. doi: 10.1093/trstmh/trab032 [DOI] [PubMed] [Google Scholar]
- 7. Balabanoff VA. 1980. Mycetomas originated from South-East Bulgaria (author's transl). Ann Parasitol Hum Comp 55:605–613. [PubMed] [Google Scholar]
- 8. Avram A. 1966. A study of mycetomas of rumania. Mycopathol Mycol Appl 28:1–15. doi: 10.1007/BF02276016 [DOI] [PubMed] [Google Scholar]
- 9. Abbott P. 1956. Mycetoma in the Sudan. Trans R Soc Trop Med Hyg 50:11–24. doi: 10.1016/0035-9203(56)90004-9 [DOI] [PubMed] [Google Scholar]
- 10. Lynch JB. 1964. Mycetoma in the Sudan. Ann R Coll Surg Engl 35:319–340. [PMC free article] [PubMed] [Google Scholar]
- 11. Fahal AH, Sabaa AHA. 2010. Mycetoma in children in Sudan. Trans R Soc Trop Med Hyg 104:117–121. doi: 10.1016/j.trstmh.2009.07.016 [DOI] [PubMed] [Google Scholar]
- 12. Fahal A, Mahgoub ES, El Hassan AM, Jacoub AO, Hassan D. 2015. Head and neck mycetoma: the mycetoma research centre experience. PLoS Negl Trop Dis 9:e0003587. doi: 10.1371/journal.pntd.0003587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ahmed EA, Nour BYM, Abakar AD, Hamid S, Mohamadani AA, Daffalla M, Mahmoud M, Altayb HN, Desnos-Ollivier M, de Hoog S, Ahmed SA. 2020. The genus Madurella: molecular identification and epidemiology in Sudan. PLoS Negl Trop Dis 14:e0008420. doi: 10.1371/journal.pntd.0008420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mohamed ESW, Bakhiet SM, El Nour M, Suliman SH, El Amin HM, Fahal AH. 2021. Surgery in mycetoma-endemic villages: unique experience. Trans R Soc Trop Med Hyg 115:320–323. doi: 10.1093/trstmh/traa194 [DOI] [PubMed] [Google Scholar]
- 15. Hassan R, Deribe K, Fahal AH, Newport M, Bakhiet S. 2021. Clinical epidemiological characteristics of mycetoma in eastern Sennar locality, Sennar state, Sudan. PLoS Negl Trop Dis 15:e0009847. doi: 10.1371/journal.pntd.0009847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siddig EE, Ahmed A, Hassan OB, Bakhiet SM, Verbon A, Fahal AH, van de Sande WWJ. 2023. Using a Madurella mycetomatis-specific PCR on grains obtained via non-invasive fine-needle aspirated material is more accurate than cytology. Mycoses 66:477–482. doi: 10.1111/myc.13572 [DOI] [PubMed] [Google Scholar]
- 17. Abate DA, Ayele MH, Mohammed AB. 2021. Subcutaneous mycoses in Ethiopia: a retrospective study in a single dermatology center. Trans R Soc Trop Med Hyg 115:1468–1470. doi: 10.1093/trstmh/trab080 [DOI] [PubMed] [Google Scholar]
- 18. Tilahun Zewdu F, Getahun Abdela S, Takarinda KC, Kamau EM, Van Griensven J, Van Henten S. 2022. Mycetoma patients in Ethiopia: case series from Boru Meda hospital. J Infect Dev Ctries 16:41S–44S. doi: 10.3855/jidc.16047 [DOI] [PubMed] [Google Scholar]
- 19. Enbiale W, Bekele A, Manaye N, Seife F, Kebede Z, Gebremeskel F, van Griensven J. 2023. Subcutaneous mycoses: endemic but neglected among the neglected tropical diseases in Ethiopia. PLoS Negl Trop Dis 17:e0011363. doi: 10.1371/journal.pntd.0011363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilson AM. 1965. The aetiology of mycetoma in Uganda compared with other African countries. East Afr Med J 42:182–190. [PubMed] [Google Scholar]
- 21. Kwizera R, Bongomin F, Meya DB, Denning DW, Fahal AH, Lukande R. 2020. Mycetoma in Uganda: a neglected tropical disease. PLoS Negl Trop Dis 14:e0008240. doi: 10.1371/journal.pntd.0008240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vanbreuseghem R. 1958. Epidemiologie et therapeutique des pieds de Madura au Congo belge. Bull Soc Pathol Exot 51:759–814. [Google Scholar]
- 23. Destombes P, Mariat F, Rosati L, Segretain G. 1977. Mycetoma in Somalia - results of a survey done from 1959 to 1964. Acta Trop 34:355–373. [PubMed] [Google Scholar]
- 24. Destombes P, Du Tour J, Marquet J, Mariat F, Segretain G. 1958. Les mycétomes en Côte Française des Somalis. Bull Soc Pathol Exot 51:575–581. [Google Scholar]
- 25. Orio J, Destombes P, Mariat F, Segretain G. 1963. Mycetoma in the French Somali coast. Review of 50 cases observed between 1954 and 1962. Bull Soc Pathol Exot Filiales 56:161–173. [PubMed] [Google Scholar]
- 26. Colom MF, Ferrer C, Ekai JL, Ferrández D, Ramírez L, Gómez-Sánchez N, Leting S, Hernández C. 2023. First report on mycetoma in Turkana County-North-Western Kenya. PLoS Negl Trop Dis 17:e0011327. doi: 10.1371/journal.pntd.0011327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mavura D, Chapa P, Sabushimike D, Kini L, Hay R. 2021. Mycetoma in Moshi, Tanzania. Trans R Soc Trop Med Hyg 115:340–342. doi: 10.1093/trstmh/traa190 [DOI] [PubMed] [Google Scholar]
- 28. Agarwal SC, Mathur DR. 1985. Mycetoma in northern Nigeria. Trop Geogr Med 37:133–135. [PubMed] [Google Scholar]
- 29. Mahe A, Develoux M, Lienhardt C, Keita S, Bobin P. 1996. Mycetomas in Mali: causative agents and geographic distribution. Am J Trop Med Hyg 54:77–79. doi: 10.4269/ajtmh.1996.54.77 [DOI] [PubMed] [Google Scholar]
- 30. Traore T, Toure L, Diassana M, Niang M, Ballo E, S Coulibaly B, Hans-Moevi A. 2021. Prise en charge medico-chirurgicale des mycetomes a l'hopital Somine Dolo de Mopti (Mali). Med Trop Sante Int 1:mtsi.2021.170. doi: 10.48327/mtsi.2021.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Philippon M, Larroque D, Ravisse P. 1992. Mycetoma in Mauritania: species found, epidemiologic characteristics and country distribution. Bull Soc Pathol Exot 85:107–114. [PubMed] [Google Scholar]
- 32. Kébé M, Ba O, Mohamed Abderahmane MA, Mohamed Baba ND, Ball M, Fahal A. 2021. A study of 87 mycetoma patients seen at three health facilities in Nouakchott, Mauritania. Trans R Soc Trop Med Hyg 115:315–319. doi: 10.1093/trstmh/traa197 [DOI] [PubMed] [Google Scholar]
- 33. Bourrel P, Cerutti J, Disy P, Olivier R. 1974. Les mycetomas propos de 64 observations. Méd Trop 34:221–247. [Google Scholar]
- 34. Klueken N, Camain R, Baylet M, Basset A. 1965. Epidemiology, clinical aspects and treatment of mycetoma in Western Africa. Hautarzt 16:1–6. [PubMed] [Google Scholar]
- 35. Rey M, Baylet R, Camain R. 1962. Donnees actuelles sur les mycetomes. A propos de 214 cas africains. Ann Derm Syph 89:511–527. [PubMed] [Google Scholar]
- 36. Ndiaye B, Develoux M, Langlade MA, Kane A. 1994. Actinomycotic mycetoma. Apropos of 27 cases in Dakar; medical treatment with cotrimoxazole. Ann Dermatol Venereol 121:161–165. [PubMed] [Google Scholar]
- 37. Dieng MT, Niang SO, Diop B, Ndiaye B. 2005. Actinomycetomas in Senegal: study of 90 cases. Bull Soc Pathol Exot 98:18–20. [PubMed] [Google Scholar]
- 38. Dieng MT, Sy MH, Diop BM, Niang SO, Ndiaye B. 2003. Mycetoma: 130 cases. Ann Dermatol Venereol 130:16–19. [PubMed] [Google Scholar]
- 39. Sarr L, Niane MM, Diémé CB, Diatta BA, Coulibaly NF, Dembélé B, Diouf AB, Kinkpé CAV, Sané AD, Ndiaye A, Seye SIL. 2016. Chirurgie des mycetomes fongiques a grain noir. A propos de 44 patients pris en charge a l'Hopital Aristide le Dantec de Dakar (Senegal) de decembre 2008 a mars 2013. Bull Soc Pathol Exot 109:8–12. doi: 10.1007/s13149-015-0463-4 [DOI] [PubMed] [Google Scholar]
- 40. Diadie S, Ndiaye M, Diop K, Diongue K, Diouf J, Sarr M, Sarr L, Ly F, Dieng MT, Niang SO. 2022. Extrapodal mycetomas in Senegal: epidemiologica, clinical and etiological study of 82 cases diagnoses from 2000 to 2020. Med Trop Sante Int 2:1–8. doi: 10.48327/mtsi.v2i1.2022.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Badiane AS, Ndiaye M, Diongue K, Diallo MA, Seck MC, Ndiaye D. 2020. Geographical distribution of mycetoma cases in Senegal over a period of 18 years. Mycoses 63:250–256. doi: 10.1111/myc.13037 [DOI] [PubMed] [Google Scholar]
- 42. Sow D, Ndiaye M, Sarr L, Kanté MD, Ly F, Dioussé P, Faye BT, Gaye AM, Sokhna C, Ranque S, Faye B. 2020. Mycetoma epidemiology, diagnosis management, and outcome in three hospital centres in Senegal from 2008 to 2018. PLoS One 15:e0231871. doi: 10.1371/journal.pone.0231871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Develoux M, Audoin J, Treguer J, Vetter JM, Warter A, Cenac A. 1988. Mycetoma in the Republic of Niger: clinical features and epidemiology. Am J Trop Med Hyg 38:386–390. doi: 10.4269/ajtmh.1988.38.386 [DOI] [PubMed] [Google Scholar]
- 44. Gamet A, Brottes H, Essomba R. 1964. New cases of mycetoma detected in Cameroun. Bull Soc Pathol Exot Filiales 57:1191–1195. [PubMed] [Google Scholar]
- 45. Pitche P, Napo-Koura G, Kpodzro K, Tchangaï-Wallam K. 1999. Les mycetomes au Togo: aspects épidémiologiques et étiologiques de cas histologiquement diagnostiqués. Med Afr Noire 46:322–325. [Google Scholar]
- 46. Darré T, Saka B, Mouhari-Toure A, Tchaou M, Dorkenoo AM, Doh K, Walla A, Amégbor K, Pitché VP, Napo-Koura G. 2018. Mycetoma in the togolese: an update from a single-center experience. Mycopathologia 183:961–965. doi: 10.1007/s11046-018-0260-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Coulanges P, Vicens R, Rakotonirina-Randriambeloma PJ. 1987. Mycetomas in Madagascar (apropos of 142 cases seen in the laboratory of anatomical pathology of the Pasteur Institute of Madagascar from 1954 to 1984. Arch Inst Pasteur Madagascar 53:35–42. [PubMed] [Google Scholar]
- 48. Ahmed SA, El-Sobky TA, de Hoog S, Zaki SM, Taha M. 2023. A scoping review of mycetoma profile in Egypt: revisiting the global Endemicity map. Trans R Soc Trop Med Hyg 117:1–11. doi: 10.1093/trstmh/trac085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Daoud M, Ezzine Sebai N, Badri T, Mokhtar I, Fazza B, Kamoun MR. 2005. Mycetoma: retrospective study of 13 cases in Tunisia. Acta Dermatovenerol Alp Panonica Adriat 14:153–156. [PubMed] [Google Scholar]
- 50. Elgallali N, El Euch D, Cheikhrouhou R, Belhadj S, Chelly I, Chaker E, Ben Osman A. 2010. Mycetoma in Tunisia: a 15-case series. Med Trop (Mars) 70:269–273. [PubMed] [Google Scholar]
- 51. Aounallah A, Boussofara L, Ben Saïd Z, Ghariani N, Saidi W, Denguezli M, Belajouza C, Nouira R. 2013. Analyse d'une serie tunisienne de 18 cas de mycetomes a l'hopital de Sousse (1974-2010). Bull Soc Pathol Exot 106:5–8. doi: 10.1007/s13149-012-0233-1 [DOI] [PubMed] [Google Scholar]
- 52. Marc S, Meziane M, Hamada S, Hassam B, Benzekri L. 2011. Clinical and epidemiological features of mycetoma in Morocco. Med Mal Infect 41:163–164. doi: 10.1016/j.medmal.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 53. Messoudi A, Fnini S, El Andaloussi Y, Charafeddine A, Marouane S, Ouarab M, Largab A. 2013. Madura foot: a rare pathology in Morocco (about 15 cases). Bull Soc Pathol Exot 106:9–12. doi: 10.1007/s13149-012-0269-2 [DOI] [PubMed] [Google Scholar]
- 54. Tazi S, Konzi KM-E, Chachi EM, Lyagoubi M, Aoufi S. 2019. Epidemiological profile of eumycetomas in Rabat, Morocco, over 40 years. J Mycol Med 29:356–360. doi: 10.1016/j.mycmed.2019.100902 [DOI] [PubMed] [Google Scholar]
- 55. Khatri ML, Al Kubati SAS, Gaffer IA, Majeed SMA. 2022. Mycetoma in North-Western Yemen: clinico-epidemiological and histopathological study. Indian J Dermatol Venereol Leprol 88:615–622. doi: 10.25259/IJDVL_500_2021 [DOI] [PubMed] [Google Scholar]
- 56. Khatri ML, Al-Halali HM, Fouad Khalid M, Saif SA, Vyas MCR. 2002. Mycetoma in Yemen: clinicoepidemiologic and histopathologic study. Int J Dermatol 41:586–593. doi: 10.1046/j.1365-4362.2002.01383.x [DOI] [PubMed] [Google Scholar]
- 57. Yu AM, Zhao S, Nie LY. 1993. Mycetomas in northern Yemen: identification of causative organisms and epidemiologic considerations. Am J Trop Med Hyg 48:812–817. doi: 10.4269/ajtmh.1993.48.812 [DOI] [PubMed] [Google Scholar]
- 58. Bendl BJ, Mackey D, Al-Saati F, Sheth KV, Ofole SN, Bailey TM. 1987. Mycetoma in Saudi Arabia. J Trop Med Hyg 90:51–59. [PubMed] [Google Scholar]
- 59. Mufti ST, Aljhdali H. 2015. Mycetoma at a tertiary care hospital in Saudi Arabia: correlation of histopathological and clinical findings. Asian Pac J Trop Biomed 5:331–336. doi: 10.1016/S2221-1691(15)30353-1 [DOI] [Google Scholar]
- 60. Venugopal PV, Venugopal TV. 1990. Leptosphaeria tompkinsii mycetoma. Int J Dermatol 29:432–433. doi: 10.1111/j.1365-4362.1990.tb03831.x [DOI] [PubMed] [Google Scholar]
- 61. Hashemi SJ, Nasrollahi A, Guerami M, Daei R, Pakshir K, Zibafar A. 2008. Mycetoma in Iran: study of 62 cases. Asian J Epidemiol 1:77–81. doi: 10.3923/aje.2008.77.81 [DOI] [Google Scholar]
- 62. Bassiri-Jahromi S. 2014. Mycetoma in Iran: causative agents and geographic distribution. Indian J Dermatol 59:529. doi: 10.4103/0019-5154.139889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mallick YA, Yaqoob N. 2021. Clinical and epidemiological profile of mycetoma patients from a tertiary care center in Karachi, Pakistan. An Bras Dermatol 96:617–619. doi: 10.1016/j.abd.2020.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zeeshan M, Fatima S, Farooqi J, Jabeen K, Ahmed A, Haq A, Arif MO, Zafar A. 2022. Reporting of mycetoma cases from skin and soft tissue biopsies over a period of ten years: a single center report and literature review from Pakistan. PLoS Negl Trop Dis 16:e0010607. doi: 10.1371/journal.pntd.0010607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Maiti PK, Ray A, Bandyopadhyay S. 2002. Epidemiological aspects of mycetoma from a retrospective study of 264 cases in West Bengal. Trop Med Int Health 7:788–792. doi: 10.1046/j.1365-3156.2002.00915.x [DOI] [PubMed] [Google Scholar]
- 66. Padhi S, Uppin SG, Uppin MS, Umabala P, Challa S, Laxmi V, Prasad VBN. 2010. Mycetoma in South India: retrospective analysis of 13 cases and description of two cases caused by unusual pathogens: Neoscytalidium dimidiatum and Aspergillus flavus. Int J Dermatol 49:1289–1296. doi: 10.1111/j.1365-4632.2010.04610.x [DOI] [PubMed] [Google Scholar]
- 67. Dubey N, Capoor MR, Hasan AS, Gupta A, Ramesh V, Sharma S, Singh A, Rudramurthy SM, Chakrabarti A. 2019. Epidemiological profile and spectrum of neglected tropical disease eumycetoma from Dehli, North India. Epidemiol Infect 147:e294. doi: 10.1017/S0950268819001766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chakraborti A, Singh K. 1998. Mycetoma in Chandigarh and surrounding areas. Indian J Med Microbiol 16:64–65. [Google Scholar]
- 69. Chouhan SS, Agarwal S. 1961. Histological diagnosis of mycetoma – a clinical study of 24 cases. Indian J Med Res 57:71–77. [PubMed] [Google Scholar]
- 70. Venugopal TV, Venugopal PV, Paramasivan CN, Shetty BMV, Subramanian S. 1977. Mycetomas in Madras. Med Mycol 15:17–22. doi: 10.1080/00362177785190041 [DOI] [PubMed] [Google Scholar]
- 71. Reddy C, Samdareshwar B, Rao AP, Reddy SS. 1972. Mycetoma – histological diagnosis of causal agents in 50 cases. Indian J Med Sci 26:733–736. [PubMed] [Google Scholar]
- 72. Talwar P, Sehgal SC. 1979. Mycetomas in North India. Sabouraudia 17:287–291. doi: 10.1080/00362177985380421 [DOI] [PubMed] [Google Scholar]
- 73. Klokke AH, Swamidasan G, Anguli R, Verghese A. 1968. The causal agents of mycetoma in South India. Trans R Soc Trop Med Hyg 62:509–516. doi: 10.1016/0035-9203(68)90135-1 [DOI] [PubMed] [Google Scholar]
- 74. Bakshi R, Mathur DR. 2008. Incidence and changing pattern of mycetoma in Western Rajasthan. Indian J Pathol Microbiol 51:154–155. doi: 10.4103/0377-4929.40433 [DOI] [PubMed] [Google Scholar]
- 75. Mathur DR, Joshi KR, Mathur A. 1979. An etiological and pathological study of mycetoma in Western Rajasthan. Curr Med Pract 23:151–161. [Google Scholar]
- 76. Joshi KR, Sanghvi A, Vyas MC, Sharma JC. 1987. Etiology & distribution of mycetoma in Rajasthan, India. Indian J Med Res 85:694–698. [PubMed] [Google Scholar]
- 77. Kotrajaras R. 1981. Mycetoma, a review of seventeen cases seen at the institute of dermatology. J Dermatol 8:133–137. doi: 10.1111/j.1346-8138.1981.tb02579.x [DOI] [PubMed] [Google Scholar]
- 78. Wang R, Yao X, Li R. 2019. Mycetoma in China: a case report and review of the literature. Mycopathologia 184:327–334. doi: 10.1007/s11046-019-00324-z [DOI] [PubMed] [Google Scholar]
- 79. Negroni R. 1974. Contribucion al estudio de los micetomas en. la Republica Argentina. Med Cut ILA 2:353–362. [PubMed] [Google Scholar]
- 80. Negroni R, López Daneri G, Arechavala A, Bianchi MH, Robles AM. 2006. Clinical and microbiological study of mycetomas at the Muniz hospital of Buenos Aires between 1989 and 2004. Rev Argent Microbiol 38:13–18. [PubMed] [Google Scholar]
- 81. Biagini RE, Museu A, Martinez T, Salvador M. 1977. Micetomas en la provincia de Salta. Archivos Argentinos Dermat 4:217–226. [Google Scholar]
- 82. Biagini RE, Martínez TE, Museli A, Sarmiento Villa H. 1983. Mycetoma in northern Argentina. Med Cutan Ibero Lat Am 11:431–436. [PubMed] [Google Scholar]
- 83. Niño FL, Freire RS. 1966. Maduromycotic mycetoma in the province of Chaco (Argentina). Mycopathol Mycol Appl 28:95–96. doi: 10.1007/BF02276034 [DOI] [PubMed] [Google Scholar]
- 84. Rojas OC, León-Cachón RBR, Moreno-Treviño M, González GM. 2017. Molecular identification of unusual mycetoma agents isolated from patients in Venezuela. Mycoses 60:129–135. doi: 10.1111/myc.12579 [DOI] [PubMed] [Google Scholar]
- 85. Castro LG, Belda Júnior W, Salebian A, Cucé LC. 1993. Mycetoma: a retrospective study of 41 cases seen in Sao Paulo, Brazil, from 1978 to 1989. Mycoses 36:89–95. doi: 10.1111/j.1439-0507.1993.tb00694.x [DOI] [PubMed] [Google Scholar]
- 86. Lacaz CS. 1981. Distribuicao geografica dos micetomas no Brasil. / Geographic distribution of mycetoma in Brazil. An Bras Dermatol 56:167–172. [Google Scholar]
- 87. Lavalle P. 1966. New data on the etiology of mycetoma in Mexico and on its pathogenesis. Gac Med Mex 96:545–574. [PubMed] [Google Scholar]
- 88. López Martínez R, Méndez Tovar LJ, Lavalle P, Welsh O, Saúl A, Macotela Ruíz E. 1992. Epidemiology of mycetoma in Mexico: study of 2105 cases. Gac Med Mex 128:477–481. [PubMed] [Google Scholar]
- 89. Buot G, Lavalle P, Mariat F, Suchil P. 1987. Epidemiologic study of mycetomas in Mexico. Apropos of 502 cases. Bull Soc Pathol Exot Filiales 80:329–339. [PubMed] [Google Scholar]
- 90. Lopez-Martinez R, Mendez-Tovar LJ, Bonifaz A, Arenas R, Mayorga J, Welsh O, Vera-Cabrera L, Padilla-Desgarennes MC, Contreras Perez C, Chavez G, Estrada R, Hernandez-Hernandez F, Manzano-Gayosso P. 2013. Actualizacion de la epidemiologia del micetoma en Mexico. Revision de 3,933 casos. Gac Med Mex 149:586–592. [PubMed] [Google Scholar]
- 91. Bonifaz A, Tirado-Sánchez A, Araiza J, Treviño-Rangel R, González GM. 2021. Deep mycoses and pseudomycoses of the foot: a single-center retrospective study of 160 cases, in a tertiary-care center in Mexico. The Foot 46:101770. doi: 10.1016/j.foot.2020.101770 [DOI] [PubMed] [Google Scholar]
- 92. Roberto E, Guadalupe CL, Guadalupe EC, Hay R. 2021. Mycetoma and the community dermatology program, Mexico. Trans R Soc Trop Med Hyg 115:383–386. doi: 10.1093/trstmh/traa199 [DOI] [PubMed] [Google Scholar]
- 93. Cárdenas-de la Garza JA, Welsh O, Cuéllar-Barboza A, Suarez-Sánchez KP, De la Cruz-Valadez E, Cruz-Gómez LG, Gallardo-Rocha A, Ocampo-Candiani J, Vera-Cabrera L. 2020. Clinical characteristics and treatment of actinomycetoma in northeast Mexico: a case series. PLoS Negl Trop Dis 14:e0008123. doi: 10.1371/journal.pntd.0008123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ahmed SA, De Hoog GS, Van de Sande WWJ. 2019. Fungi causing eumycotic mycetoma, p 2261–2277. In Carroll KC, Pfaller MA, Landry ML, McAdam AJ, Patel R, Richter SS, Warnock DW (ed), Manual of clinical microbiology, 12th ed. Vol. 2. ASM Press, Washington, DC. [Google Scholar]
- 95. Borman AM, Desnos-Ollivier M, Campbell CK, Bridge PD, Dannaoui E, Johnson EM. 2016. Novel taxa associated with human fungal black-grain mycetomas: Emarellia grisea gen. nov., sp. nov., and Emarellia paragrisea sp. nov. J Clin Microbiol 54:1738–1745. doi: 10.1128/JCM.00477-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Fraser M, Borman AM, Johnson EM. 2017. Rapid and robust identification of the agents of black-grain mycetoma by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 55:2521–2528. doi: 10.1128/JCM.00417-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Desnos-Ollivier M, Bretagne S, Dromer F, Lortholary O, Dannaoui E. 2006. Molecular identification of black-grain mycetoma agents. J Clin Microbiol 44:3517–3523. doi: 10.1128/JCM.00862-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Borman AM, Desnos-Ollivier M, Miles S-J, Linton CJ, Campbell CK, Bridge PD, Dannaoui E, Johnson EM. 2009. Molecular characterisation of the Madurella grisea complex reveals at least three new taxa associated with human mycetomas. abstr ISHAM 2009, Tokyo. [Google Scholar]
- 99. Siddig EE, Nyuykonge B, Mhmoud NA, Abdallah OB, Bahar MEN, Ahmed ES, Nyaoke B, Zijlstra EE, Verbon A, Bakhiet SM, Fahal AH, van de Sande WWJ. 2023. Comparing the performance of the common used eumycetoma diagnostic tests. Mycoses 66:420–429. doi: 10.1111/myc.13561 [DOI] [PubMed] [Google Scholar]
- 100. N’Diaye B, Develoux M, Dieng MT, Ndiaye PD, Ndir O. 1995. Les mycétomes à la Clinique Dermatologique de Dakar (Sénégal) : aspects épidémiologiques: à propos de 111 cas. Med Afr Noire 42:207–212. [Google Scholar]
- 101. N’diaye B, Dieng MT, Perez A, Stockmeyer M, Bakshi R. 2006. Clinical efficacy and safety of oral terbinafine in fungal mycetoma. Int J Dermatol 45:154–157. doi: 10.1111/j.1365-4632.2004.02392.x [DOI] [PubMed] [Google Scholar]
- 102. Ndiaye D, Ndiaye M, Sène PD, Diouf MN, Diallo M, Faye B, Sakho MG, Ndiaye JL, Tine R, Kane A, Ndir O. 2011. Mycétomes diagnostiqués au Sénégal de 2008 à 2010. J Mycol Med 21:173–181. doi: 10.1016/j.mycmed.2011.07.003 [DOI] [PubMed] [Google Scholar]
- 103. Venucopal PV, Venugopal TV, Laing WN, Humaidan YAl, Namnyak SS, Jama AAAl, Elbashier AM. 1990. Black grain mycetoma caused by Madurella grisea in Saudi Arabia . Int J Dermatol 29:434–435. doi: 10.1111/j.1365-4362.1990.tb03832.x [DOI] [PubMed] [Google Scholar]
- 104. Estrada-Castañón R, Estrada-Chávez G, Chávez-López M de G. 2019. Diagnosis and management of fungal neglected tropical diseases in community settings-mycetoma and sporotrichosis. TropicalMed 4:81. doi: 10.3390/tropicalmed4020081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Baylet R, Camain R, Chabal J, Izarn R. 1968. Nouvelle contribution a l'etude des mycetomes au Senegal. Neotestudina rosatii. Pyrenochaeta romeroi. Aspergillus nidulans. Bull Soc Med Afr Noire Lang Fr 13:311–313. [PubMed] [Google Scholar]
- 106. Kaur M, Singla N, Bhalla M, Kundu R, Gulati N, Chander J. 2021. Aspergillus candidus eumycetoma with review of literature. J Med Mycol 31:101135. doi: 10.1016/j.mycmed.2021.101135 [DOI] [PubMed] [Google Scholar]
- 107. Ahmed SA, Abbas MA, Jouvion G, Al-Hatmi AMS, de Hoog GS, Kolecka A, Mahgoub ES. 2015. Seventeen years of subcutaneous infection by Aspergillus flavus; eumycetoma confirmed by immunohistochemistry. Mycoses 58:728–734. doi: 10.1111/myc.12422 [DOI] [PubMed] [Google Scholar]
- 108. Kamali Sarvestani H, Ahmadi B, Gerami Shoar M, Getso M, Rafat Z, Mahmoudi S, Khansari M, Salahshour F, Fatahi L, Salehi M, Ansari S. 2022. Mycetoma due to Aspergillus flavus in a diabetic patient: case report and literature review. IDCases 29:e01585. doi: 10.1016/j.idcr.2022.e01585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Witzig RS, Greer DL, Hyslop NE. 1996. Aspergillus flavus mycetoma and epidural abscess successfully treated with itraconazole. J Med Vet Mycol 34:133–137. doi: 10.1080/02681219680000201 [DOI] [PubMed] [Google Scholar]
- 110. Veraldi S, Grancini A, Venegoni L, Merlo V, Guanziroli E, Menicanit C, Nazzaro G, Tortorano A. 2016. Mycetoma caused by Aspergillus nidulans. Acta Derm Venereol 96:118–119. doi: 10.2340/00015555-2144 [DOI] [PubMed] [Google Scholar]
- 111. Prasanna S, Grover N, Bhatt P, Sahni AK. 2016. A case of Aspergillus nidulans causing white granule mycetoma. Med J Armed Forces India 72:88–90. doi: 10.1016/j.mjafi.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Joshi KR, Mathur DR, Sharma JC, Vyas MC, Sanghvi A. 1985. Mycetoma caused by Aspergillus nidulans in India. J Trop Med Hyg 88:41–44. [PubMed] [Google Scholar]
- 113. Verma R, Vasudevan B, Sahni AK, Vijendran P, Neema S, Kharayat V. 2015. First reported case of Aspergillus nidulans eumycetoma in a sporotrichoid distribution. Int J Dermatol 54:74–77. doi: 10.1111/ijd.12571 [DOI] [PubMed] [Google Scholar]
- 114. Baylet R, Basset A. 1968. Aspergillus nidulans, agent of mycetoma. Bull Soc Pathol Exot Filiales 61:359–365. [PubMed] [Google Scholar]
- 115. Vera-Cabrera L, Cardenas-de la Garza JA, Cuellar-Barboza A, Gallardo-Rocha A, Molina-Torres CA, Escalante-Fuentes W, Ocampo-Candiani J. 2021. Case report: Coral reef pathogen Aspergillus sydowii causing black grain mycetoma: a review. Am J Trop Med Hyg 104:871–873. doi: 10.4269/ajtmh.20-1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Werlinger KD, Yen Moore A. 2005. Eumycotic mycetoma caused by Cladophialophora bantiana in a patient with systemic lupus erythematosus. J Am Acad Dermatol 52:S114–S117. doi: 10.1016/j.jaad.2004.12.023 [DOI] [PubMed] [Google Scholar]
- 117. Bonifaz A, De Hoog S, McGinnis MR, Saúl A, Rodríguez-Cortés O, Araiza J, Cruz M, Mercadillo P. 2009. Eumycetoma caused by Cladophialophora bantiana successfully treated with itraconazole. Med Mycol 47:111–114. doi: 10.1080/13693780802430639 [DOI] [PubMed] [Google Scholar]
- 118. Mahgoub E. 1969. Corynespora cassiicola, a new agent of maduromycetoma. J Trop Med Hyg 72:218–221. [PubMed] [Google Scholar]
- 119. Bridges CH. 1957. Maduromycotic mycetomas in animals; Curvularia geniculata as an etiologic agent. Am J Pathol 33:411–427. [PMC free article] [PubMed] [Google Scholar]
- 120. Boomker J, Coetzer JA, Scott DB. 1977. Black grain mycetoma (maduromycosis) in horses. Onderstepoort J Vet Res 44:249–251. [PubMed] [Google Scholar]
- 121. Clark FD, Jones LP, Panigrahy B. 1986. Mycetoma in a grand Eclectus (Eclectus roratus roratus) parrot. Avian Dis 30:441–443. [PubMed] [Google Scholar]
- 122. Gunathilake R, Perera P, Sirimanna G. 2014. Curvularia lunata: a rare cause of black-grain eumycetoma. J Mycol Med 24:158–160. doi: 10.1016/j.mycmed.2013.09.005 [DOI] [PubMed] [Google Scholar]
- 123. Garg A, Sujatha S, Garg J, Parija SC, Thappa DM. 2008. Eumycetoma due to Curvularia lunata. Indian J Dermatol Venereol Leprol 74:515–516. doi: 10.4103/0378-6323.44327 [DOI] [PubMed] [Google Scholar]
- 124. Iriart X, Binois R, Fior A, Blanchet D, Berry A, Cassaing S, Amazan E, Papot E, Carme B, Aznar C, Couppié P. 2011. Eumycetoma caused by Diaporthe phaseolorum (Phomopsis phaseoli): a case report and a mini-review of Diaporthe/Phomopsis spp invasive infections in humans. Clin Microbiol Infect 17:1492–1494. doi: 10.1111/j.1469-0691.2011.03568.x [DOI] [PubMed] [Google Scholar]
- 125. Crabol Y, Poiree S, Bougnoux M-E, Maunoury C, Barete S, Zeller V, Arvieux C, Pineau S, Amazzough K, Lecuit M, Lanternier F, Lortholary O, French Mycosis Study Group . 2014. Last generation triazoles for imported eumycetoma in eleven consecutive adults. PLoS Negl Trop Dis 8:e3232. doi: 10.1371/journal.pntd.0003232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Severo LC, Vetoratto G, Oliveira F de M, Londero AT. 1999. Eumycetoma by Madurella grisea. Report of the first case observed in the Southern Brazilian region. Rev Inst Med Trop S Paulo 41:139–142. doi: 10.1590/S0036-46651999000200013 [DOI] [PubMed] [Google Scholar]
- 127. Neumeister B, Zollner TM, Krieger D, Sterry W, Marre R. 1995. Mycetoma due to Exophiala jeanselmei and Mycobacterium chelonae in a 73-year-old man with idiopathic CD4+ T lymphocytopenia. Mycoses 38:271–276. doi: 10.1111/j.1439-0507.1995.tb00406.x [DOI] [PubMed] [Google Scholar]
- 128. Hemashettar BM, Patil CS, Nagalotimath SJ, Thammayya A. 1986. Mycetoma due to Exophiala jeanselmei (a case report with description of the fungus). Indian J Pathol Microbiol 29:75–78. [PubMed] [Google Scholar]
- 129. Thammayya A, Sanyal M. 1980. Exophiala jeanselmei causing mycetoma pedis in India. Med Mycol 18:91–95. doi: 10.1080/00362178085380161 [DOI] [PubMed] [Google Scholar]
- 130. Badali H, Najafzadeh MJ, van Esbroeck M, van den Enden E, Tarazooie B, Meis JFGM, de Hoog GS. 2010. The clinical spectrum of Exophiala jeanselmei, with a case report and in vitro antifungal susceptibility of the species. Med Mycol 48:318–327. doi: 10.1080/13693780903148353 [DOI] [PubMed] [Google Scholar]
- 131. Desoubeaux G, Millon A, Freychet B, de Muret A, Garcia-Hermoso D, Bailly E, Rosset P, Chandenier J, Bernard L. 2013. Eumycetoma of the foot caused by Exophiala jeanselmei in a Guinean woman. J Mycol Med 23:168–175. doi: 10.1016/j.mycmed.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 132. Capoor MR, Khanna G, Nair D, Hasan A, Deb M, Aggarwal P. 2007. Eumycetoma pedis due to Exophiala jeanselmei. Indian J Med Microbiol 25:155–157. doi: 10.4103/0255-0857.32726 [DOI] [PubMed] [Google Scholar]
- 133. Al-Tawfiq JA, Amr SS. 2009. Madura leg due to Exophiala jeanselmei successfully treated with surgery and itraconazole therapy. Med Mycol 47:648–652. doi: 10.1080/13693780802669194 [DOI] [PubMed] [Google Scholar]
- 134. Pattanaprichakul P, Bunyaratavej S, Leeyaphan C, Sitthinamsuwan P, Sudhadham M, Muanprasart C, Feng P, Badali H, de Hoog GS. 2013. An unusual case of eumycetoma caused by Exophiala jeanselmei after a sea urchin injury. Mycoses 56:491–494. doi: 10.1111/myc.12050 [DOI] [PubMed] [Google Scholar]
- 135. Santona A, Mhmoud NA, Siddig EE, Deligios M, Fiamma M, Bakhiet SM, Barac A, Paglietti B, Rubino S, Fahal AH. 2021. Metagenomics of black grains: new highlights in the understanding of eumycetoma. Trans R Soc Trop Med Hyg 115:307–314. doi: 10.1093/trstmh/traa177 [DOI] [PubMed] [Google Scholar]
- 136. Olenski M, Halliday C, Gullifer J, Martinez E, Crowe A, Sheorey H, Darby J. 2021. A case of trauma-induced Falciformispora lignatilis eumycetoma in a renal transplant recipient. TropicalMed 6:144. doi: 10.3390/tropicalmed6030144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Cartwright KE, Clark TW, Hussain AM, Wiselka M, Borman A, Johnson EM. 2011. Eumycetoma of the hand caused by Leptosphaeria tompkinsii and refractory to medical therapy with voriconazole. Mycopathologia 172:311–315. doi: 10.1007/s11046-011-9432-8 [DOI] [PubMed] [Google Scholar]
- 138. Machmachi H, Godineau N, Develoux M, Bretagne S, Bazeli A, Amsellem D, Desnos-Ollivier M, Poirat JB. 2011. Black grain mycetoma caused by Leptosphaeria tompkinsii. Med Mycol 49:186–189. doi: 10.3109/13693786.2010.524945 [DOI] [PubMed] [Google Scholar]
- 139. Welfringer A, Parache RM. 1978. The role of histopathology in the diagnosis of mycetomas. Two cases of maduromycosis. Actinomadura pelletieri and Leptosphaeria tompkinsii (author’s transl). Arch Anat Cytol Pathol 26:50–58. [PubMed] [Google Scholar]
- 140. Siddig EE, Ahmed A, Eltigani HF, Bakhiet SM, van de Sande WWJ, Fahal AH. 2023. The first case of Fusarium falciforme eumycetoma in Sudan and an extensive literature review about treatment worldwide. J Fungi 9:730. doi: 10.3390/jof9070730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Bonifaz A, Saldaña M, Araiza J, Mercadillo P, Tirado-Sánchez A. 2017. Two simultaneous mycetomas caused by Fusarium verticillioides and Madurella mycetomatis. Rev Inst Med Trop Sao Paulo 59:e55. doi: 10.1590/S1678-9946201759055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Martínez López D, Pérez Blasco A, García Ferrer L, Camarena JJ, González R, Rodrigo Perez JL. 2022. Eumicetomas por Fusarium oxysporum y Madurella mycetomatis. Descripcion de dos casos y revision de la bibliografia. Rev Esp Quimioter 35:566–569. doi: 10.37201/req/027.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Correia C, Ferreira J, Soares-de-Almeida L, Filipe P. 2022. An unusual cause of eumycetoma - Fusarium solani keratoplasticum. Actas Dermo-Sifiliográficas 113:899. doi: 10.1016/j.ad.2021.04.013 [DOI] [PubMed] [Google Scholar]
- 144. Das L, Dahiya D, Gupta K, Prakash M, Malhotra B, Rastogi A, Choudhary H, Rudramurthy SM, Dutta P. 2021. Eumycetoma of the foot due to Fusarium solani in a person with diabetes mellitus: report of a case and review of literature. Mycopathologia 186:277–288. doi: 10.1007/s11046-020-00524-y [DOI] [PubMed] [Google Scholar]
- 145. Prinja A, Roberts C, Doherty T, Oddy MJ. 2014. An unusual cause of an ankle mass. BMJ Case Rep 2014:bcr2014204253. doi: 10.1136/bcr-2014-204253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Yera H, Bougnoux ME, Jeanrot C, Baixench MT, De Pinieux G, Dupouy-Camet J. 2003. Mycetoma of the foot caused by Fusarium solani: identification of the etiologic agent by DNA sequencing . J Clin Microbiol 41:1805–1808. doi: 10.1128/JCM.41.4.1805-1808.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Salas-Coronas J, Cabezas-Fernández T, Martínez-Lage MJV, Villarejo-Ordóñez A. 2011. Mycetoma caused by Fusarium solani. Rev Clin Esp 211:e16–e18. doi: 10.1016/j.rce.2010.06.019 [DOI] [PubMed] [Google Scholar]
- 148. Tomimori-Yamashita J, Ogawa MM, Hirata SH, Fischman O, Michalany NS, Yamashita HK, Alchorne M. 2002. Mycetoma caused by Fusarium solani with osteolytic lesions on the hand: case report. Mycopathologia 153:11–14. doi: 10.1023/a:1015294117574 [DOI] [PubMed] [Google Scholar]
- 149. Malisiewicz B, Uhrlaß S, Nenoff P, Schöfer H. 2019. Eumyzetom durch Fusarium chlamydosporum: Fallbericht und Literaturubersicht. Hautarzt 70:612–617. doi: 10.1007/s00105-019-4430-8 [DOI] [PubMed] [Google Scholar]
- 150. Campos-Macías P, Arenas-Guzmán R, Hernández-Hernández F. 2013. Fusarium subglutinans: a new eumycetoma agent. Med Mycol Case Rep 2:128–131. doi: 10.1016/j.mmcr.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zoutman DE, Sigler L. 1991. Mycetoma of the foot caused by cylindrocarpon destructans. J Clin Microbiol 29:1855–1859. doi: 10.1128/jcm.29.9.1855-1859.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Konings M, Gerrits van den Ende B, Raats MWJ, Fahal AH, van de Sande WWJ, Hagen F. 2024. Complete genome sequence of the itraconazole decreased susceptible Madurella fahalii type-strain CBS 129176. Mycopathologia 189:6. doi: 10.1007/s11046-023-00807-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Hallur V, Sirka CS, Sable M, Sethy M, Bag N, Sahu S, Sahoo M, Shivaprakash MR, Das M. 2024. A novel report of mycetoma with spinal spread due to Madurella fahalli from India. Indian J Med Microbiol 48:100523. doi: 10.1016/j.ijmmb.2023.100523 [DOI] [PubMed] [Google Scholar]
- 154. de Hoog GS, van Diepeningen AD, Mahgoub E-S, van de Sande WWJ. 2012. New species of Madurella, causative agents of black-grain mycetoma. J Clin Microbiol 50:988–994. doi: 10.1128/JCM.05477-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Borjian Boroujeni Z, Hashemi SJ, Daie Ghazvini R, Khodavaisy S, Zareei M, Hosseinpour L, Ardi P, Shokri M. 2019. Recurrent eumycetoma caused by novel species Madurella pseudomycetomatis: a case report. Med Mycol Case Rep 26:13–15. doi: 10.1016/j.mmcr.2019.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Yan J, Deng J, Zhou CJ, Zhong BY, Hao F. 2010. Phenotypic and molecular characterization of Madurella pseudomycetomatis sp. nov., a novel opportunistic fungus possibly causing black-grain mycetoma . J Clin Microbiol 48:251–257. doi: 10.1128/JCM.00018-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Mhmoud NA, Siddig EE, Nyuykonge B, Bakhiet SM, van de Sande WWJ, Fahal AH. 2021. Mycetoma caused by Microascus gracilis: a novel agent of human eumycetoma in Sudan . Trans R Soc Trop Med Hyg 115:426–430. doi: 10.1093/trstmh/trab010 [DOI] [PubMed] [Google Scholar]
- 158. Teo TSP, Crawford LC, Pilch WT, Carney B, Solanki N, Kidd SE, Warner MS. 2021. Mycetoma caused by Microsporum canis in a patient with renal transplant: a case report and review of the literature . Transplant Infectious Dis 23:e13516. doi: 10.1111/tid.13516 [DOI] [PubMed] [Google Scholar]
- 159. Chiapello LS, Dib MD, Nuncira CT, Nardelli L, Vullo C, Collino C, Abiega C, Cortes PR, Spesso MF, Masih DT. 2011. Mycetoma of the scalp due to Microsporum canis: hystopathologic, mycologic, and immunogenetic features in a 6-year-old girl. Diagn Microbiol Infect Dis 70:145–149. doi: 10.1016/j.diagmicrobio.2011.02.003 [DOI] [PubMed] [Google Scholar]
- 160. Kramer SC, Ryan M, Bourbeau P, Tyler WB, Elston DM. 2006. Fontana-positive grains in mycetoma caused by Microsporum canis. Pediatr Dermatol 23:473–475. doi: 10.1111/j.1525-1470.2006.00286.x [DOI] [PubMed] [Google Scholar]
- 161. Vezon G, Desbois N, Boisseau-Garsaud AM, Helenon R, Jouannelle A, Saint-Cyr I, Cales-Quist D. 2000. Microsporum canis mycetoma of the scalp. Ann Dermatol Venereol 127:729–731. [PubMed] [Google Scholar]
- 162. de Vries GA, de Hoog GS, de Bruyn HP. 1984. Phialophora cyanescens sp. nov. with phaeosclera-like synanamorph, causing white-grain mycetoma in man. Antonie van Leeuwenhoek 50:149–153. doi: 10.1007/BF00400175 [DOI] [PubMed] [Google Scholar]
- 163. Segretain G, Destombes P. 1961. Description of a new agent for maduromycosis, Neotestudina rosatii, n. gen., n. sp., isolated in Africa. C R Hebd Seances Acad Sci 253:2577–2579. [PubMed] [Google Scholar]
- 164. Ahmed SA, González GM, Tirado-Sánchez A, Moreno-López LM, de Hoog S, Bonifaz A. 2018. Nigrograna mackinnonii, not Trematosphaeria grisea (syn., Madurella grisea), is the main agent of black grain eumycetoma in Latin America. J Clin Microbiol 56:e01723-17. doi: 10.1128/JCM.01723-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Zimmer F, Kellner N, Nenoff P, Lübbert C. 2023. Eumycetoma caused by Biatriospora mackinnonii in a young pregnant woman from Somalia. Int J Infect Dis 128:1–2. doi: 10.1016/j.ijid.2022.12.021 [DOI] [PubMed] [Google Scholar]
- 166. Criado PR, Belda Junior W, da Matta VLR. 2021. First report of cutaneous mycetoma by Paecilomyces variotii and the successful treatment with combined itraconazole and terbinafine along with resection surgeries. Australas J Dermatol 62:e397–e399. doi: 10.1111/ajd.13592 [DOI] [PubMed] [Google Scholar]
- 167. Hemashettar BM, Siddaramappa B, Munjunathaswamy BS, Pangi AS, Pattan J, Andrade AT, Padhye AA, Mostert L, Summerbell RC. 2006. Phaeoacremonium krajdenii, a cause of white grain eumycetoma. J Clin Microbiol 44:4619–4622. doi: 10.1128/JCM.01019-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Aguilar-Donis A, Torres-Guerrero E, Arenas-Guzmán R, Hernández-Hernández F, López-García L, Criales-Vera S, Teliz-Meneses MA. 2011. Mycetoma caused by Phaeoacremonium parasiticum--a case confirmed with B-tubulin sequence analysis. Mycoses 54:e615–8. doi: 10.1111/j.1439-0507.2010.01929.x [DOI] [PubMed] [Google Scholar]
- 169. Turiansky GW, Benson PM, Sperling LC, Sau P, Salkin IF, McGinnis MR, James WD. 1995. Phialophora verrucosa: a new cause of mycetoma. J Am Acad Dermatol 32:311–315. doi: 10.1016/0190-9622(95)90393-3 [DOI] [PubMed] [Google Scholar]
- 170. Mhmoud NA, Ahmed SA, Fahal AH, de Hoog GS, Gerrits van den Ende AHG, van de Sande WWJ. 2012. Pleurostomophora ochracea, a novel agent of human eumycetoma with yellow grains. J Clin Microbiol 50:2987–2994. doi: 10.1128/JCM.01470-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Namba HD, Umemura H, Asagoe K. 2017. Eumycetoma caused by Trichophyton species successfully treated with terbinafine and surgery. J Dermatol 44:e97–e98. doi: 10.1111/1346-8138.13669 [DOI] [PubMed] [Google Scholar]
- 172. Koshi G, Padhye AA, Ajello L, Chandler FW. 1979. Acremonium recifei as an agent of mycetoma in India. Am J Trop Med Hyg 28:692–696. [PubMed] [Google Scholar]
- 173. Ahmed AO, van Vianen W, ten Kate MT, van de Sande WWJ, van Belkum A, Fahal AH, Verbrugh HA, Bakker-Woudenberg IAJM. 2003. A murine model of Madurella mycetomatis eumycetoma. FEMS Immunol Med Microbiol 37:29–36. doi: 10.1016/S0928-8244(03)00096-8 [DOI] [PubMed] [Google Scholar]
- 174. Kloezen W, van Helvert-van Poppel M, Fahal AH, van de Sande WWJ. 2015. A Madurella mycetomatis grain model in Galleria mellonella larvae. PLoS Negl Trop Dis 9:e0003926. doi: 10.1371/journal.pntd.0003926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Gumaa SA, Abu-Samra MT. 1981. Experimental mycetoma infection in the goat. J Comp Pathol 91:341–346. doi: 10.1016/0021-9975(81)90003-7 [DOI] [PubMed] [Google Scholar]
- 176. Mahgoub ES. 1978. Experimental infection of athymic nude New Zealand mice, nu nu strain with mycetoma agents. Sabouraudia 16:211–216. doi: 10.1080/00362177885380281 [DOI] [PubMed] [Google Scholar]
- 177. Nyuykonge B, Klaassen CHW, Zandijk WHA, de Hoog GS, Ahmed SA, Desnos-Ollivier M, Verbon A, Bonifaz A, van de Sande WWJ. 2020. Diagnostic implications of mycetoma derived from Madurella pseudomycetomatis isolates from Mexico. J Eur Acad Dermatol Venereol 34:1828–1834. doi: 10.1111/jdv.16402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Siddig EE, Nyuykonge B, Mhmoud NA, Abdallah OB, Bahar MEN, Ahmed ES, Nyaoke B, Zijlstra EE, Verbon A, Bakhiet SM, Fahal AH, van de Sande WWJ. 2023. Comparing the performance of the common used eumycetoma diagnostic tests. Mycoses 66:420–429. doi: 10.1111/myc.13561 [DOI] [PubMed] [Google Scholar]
- 179. Emery D, Denning DW. 2020. The global distribution of actinomycetoma and eumycetoma. PLoS Negl Trop Dis 14:e0008397. doi: 10.1371/journal.pntd.0008397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. van de Sande WWJ, Maghoub ES, Fahal AH, Goodfellow M, Welsh O, Zijlstra E. 2014. The mycetoma knowledge gap: identification of research priorities. PLoS Negl Trop Dis 8:e2667. doi: 10.1371/journal.pntd.0002667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Ganawa ETS, Bushara MA, Musa AEA, Bakhiet SM, Fahal AH. 2021. Mycetoma spatial geographical distribution in the Eastern Sennar locality, Sennar state, Sudan. Trans R Soc Trop Med Hyg 115:375–382. doi: 10.1093/trstmh/trab029 [DOI] [PubMed] [Google Scholar]
- 182. Ajello L. 1962. Epidemiology of human fungus infections, p 69–83. In Dalldorf G (ed), Fungi and fungous diseases. Charles C Thomas Springfield, Illinois. [Google Scholar]
- 183. Baylet R, Camain R, Rey M. 1961. Champignons de mycétomes isolés des épineux au Sénégal. Bull Soc Med Afr Noire Lang Fr 6:317–319. [Google Scholar]
- 184. Emmons CW. 1962. Soil reservoirs of pathogenic fungi. J Wash Acad Sci 52:3–9. [Google Scholar]
- 185. Mackinnon JE, Conti-Diaz IA, Gezuele E, Civila E. 1971. Datos sobre ecologia de Allescheria boydii, Shear. Shear. Rev Urug Pathol Clin Microbiol 9:37–43. [Google Scholar]
- 186. Segretain G. 1972. Recherches sur l’écologie de Madurella mycetomi au Senegal. Bull Soc Fr Mycol Med 14:121–124. [Google Scholar]
- 187. Thirumalachar MJ, Padhye AA. 1968. Isolation of Madurella mycetomi from soil in India. Hindustan Antibiot Bull 10:314–318. [PubMed] [Google Scholar]
- 188. Segretain G, Destombes P. 1961. Description d’un nouvel agent de maduromycose Neotestudina rosatii, n. gen., n. sp. isolé en Afrique. C R Hebd Seances Acad Sci 253:2577–2579. [PubMed] [Google Scholar]
- 189. Segretain G, Mariat F. 1968. echerches sur la presence d’agents de mycetomes dans le sol et sur les épineux du Senegal et de la Mauritanie. Bull Soc Pathol Exot Filiales 61:194–202. [PubMed] [Google Scholar]
- 190. Nyuykonge B, Siddig EE, Konings M, Bakhiet S, Verbon A, Klaassen CHW, Fahal AH, van de Sande WWJ. 2022. Madurella mycetomatis grains within a eumycetoma lesion are clonal. Med Mycol 60:myac051. doi: 10.1093/mmy/myac051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. van de Sande WWJ, de Kat J, Coppens J, Ahmed AOA, Fahal A, Verbrugh H, van Belkum A. 2007. Melanin biosynthesis in Madurella mycetomatis and its effect on susceptibility to itraconazole and ketoconazole. Microbes Infect 9:1114–1123. doi: 10.1016/j.micinf.2007.05.015 [DOI] [PubMed] [Google Scholar]
- 192. Lim W, Konings M, Parel F, Eadie K, Strepis N, Fahal A, Verbon A, van de Sande WWJ. 2022. Inhibiting DHN- and DOPA-melanin biosynthesis pathway increased the therapeutic value of itraconazole in Madurella mycetomatis infected Galleria mellonella. Med Mycol 60:myac003. doi: 10.1093/mmy/myac003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. van de Sande WWJ, Fahal AH, Goodfellow M, Mahgoub ES, Welsh O, Zijlstra EE. 2014. Merits and pitfalls of the currently used diagnostic tools in mycetoma. PLoS Negl Trop Dis 8:e2918. doi: 10.1371/journal.pntd.0002918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Sheehan G, Konings M, Lim W, Fahal A, Kavanagh K, van de Sande WWJ. 2020. Proteomic analysis of the processes leading to Madurella mycetomatis grain formation in Galleria mellonella larvae. PLoS Negl Trop Dis 14:e0008190. doi: 10.1371/journal.pntd.0008190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Geneugelijk K, Kloezen W, Fahal AH, van de Sande WWJ. 2014. Active matrix metalloprotease-9 is associated with the collagen capsule surrounding the Madurella mycetomatis grain in mycetoma. PLoS Negl Trop Dis 8:e2754. doi: 10.1371/journal.pntd.0002754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. de Klerk N, de Vogel C, Fahal A, van Belkum A, van de Sande WWJ. 2012. Fructose-bisphosphate aldolase and pyruvate kinase, two novel immunogens in Madurella mycetomatis. Med Mycol 50:143–151. doi: 10.3109/13693786.2011.593005 [DOI] [PubMed] [Google Scholar]
- 197. van de Sande WWJ, Janse D-J, Hira V, Goedhart H, van der Zee R, Ahmed AOA, Ott A, Verbrugh H, van Belkum A. 2006. Translationally controlled tumor protein from Madurella mycetomatis, a marker for tumorous mycetoma progression. J Immunol 177:1997–2005. doi: 10.4049/jimmunol.177.3.1997 [DOI] [PubMed] [Google Scholar]
- 198. Litvintseva AP, Bakhiet S, Gade L, Wagner DD, Bagal UR, Batra D, Norris E, Rishishwar L, Beer KD, Siddig EE, Mhmoud NA, Chow NA, Fahal A. 2022. Genomics and metagenomics of Madurella mycetomatis, a causative agent of black grain mycetoma in Sudan. PLoS Negl Trop Dis 16:e0010787. doi: 10.1371/journal.pntd.0010787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Abugessaisa I, Konings M, Manabe R-I, Kawashima T, Hasegawa A, Takahashi C, Tagami M, Okazaki Y, Lim W, Verbon A, Fahal AH, Kasukawa T, van de Sande WWJ. 2023. Iron regulatory pathways differentially expressed during Madurella mycetomatis grain development in Galleria mellonella. bioRxiv. doi: 10.1101/2022.12.20.520897 [DOI]
- 200. Segretain G, Destombes P. 1969. Recherches sur mycetomes à Madurella grisea et Pyrenochaeta romeroi. Sabouraudia 7:51–61. [PubMed] [Google Scholar]
- 201. Chandler FW, Ajello L. 1997. Mycetoma, chapter 113, p 1035–1044. In Connor DH, Schwartz DA, Lack EE, Manz H, Lack E, Chandler FW (ed), Pathology of infectious diseases. Appleton & Lange, Stamford, CT. [Google Scholar]
- 202. CDC . 2022. Public health imagge library record 21194, on centers for disease control and prevention. Available from: https://phil.cdc.gov/Details.aspx?pid=21194. Retrieved 10 Feb 2023.
- 203. Al‐Hatmi AMS, Bonifaz A, Tirado‐Sánchez A, Meis JF, de Hoog GS, Ahmed SA. 2017. Fusarium species causing eumycetoma: report of two cases and comprehensive review of the literature . Mycoses 60:204–212. doi: 10.1111/myc.12590 [DOI] [PubMed] [Google Scholar]
- 204. Ellis D. Trematosphaeria grisea. University of Adelaide. Available from: https://www.adelaide.edu.au/mycology/fungal-descriptions-and-antifungal-susceptibility/hyphomycetes-conidial-moulds/trematosphaeria . Accessed 8 December 2023 [Google Scholar]
- 205. Kwon-Chung KJ, Bennet JE. 1992. Medical mycology. Lea and Febiger, Philadelphia. [Google Scholar]
- 206. Verghese A, Klokke AH. 1966. Histologic diagnosis of species of fungus causing mycetoma. Indian J Med Res 54:524–530. [PubMed] [Google Scholar]
- 207. Destombes P. 1964. Histologic structure of mycetomas. Ann Soc Belges Med Trop Parasitol Mycol 44:897–908. [PubMed] [Google Scholar]
- 208. Hay RJ, Collins MJ. 1983. An ultrastructural study of pale eumycetoma grains. Sabouraudia 21:261–269. doi: 10.1080/00362178385380421 [DOI] [PubMed] [Google Scholar]
- 209. Hay RJ, Mackenzie DWR. 1982. The histopathological features of pale grain eumycetoma. Trans R Soc Trop Med Hyg 76:839–844. doi: 10.1016/0035-9203(82)90121-3 [DOI] [PubMed] [Google Scholar]
- 210. Ma J, Konings M, Verbon A, van de Sande WWJ. 2023. A Falciformispora senegalensis grain model in Galleria mellonella larvae. Med Mycol 61:myad070. doi: 10.1093/mmy/myad070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Rao VK, Prasad CR. 1973. Experimental maduromycosis. I. Infection of mice with Phialophora jeanselmei. Mycopathol Mycol Appl 51:119–126. doi: 10.1007/BF02141294 [DOI] [PubMed] [Google Scholar]
- 212. Avram A. 1967. Experimental induction of grains with Cephalosporium falciforme. Med Mycol 5:89–91. doi: 10.1080/00362176785190161 [DOI] [PubMed] [Google Scholar]
- 213. Ma J, Konings M, Verbon A, van de Sande WWJ. 2023. A Falciformispora senegalensis grain model in Galleria mellonella larvae. Med Mycol 61:myad070. doi: 10.1093/mmy/myad070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Fahal AH, Suliman SH, Hay R. 2018. Mycetoma: the spectrum of clinical presentation. Trop Med Infect Dis 3:97. doi: 10.3390/tropicalmed3030097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Fahal A, Mahgoub ES, El Hassan AM, Abdel-Rahman ME. 2015. Mycetoma in the Sudan: an update from the mycetoma research centre. PLoS Negl Trop Dis 9:e0003679. doi: 10.1371/journal.pntd.0003679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Fahal AH, Suliman SH, Gadir AF, el Hag IA, el Amin FI, Gumaa SA, Mahgoub ES. 1994. Abdominal wall mycetoma: an unusual presentation. Trans R Soc Trop Med Hyg 88:78–80. doi: 10.1016/0035-9203(94)90509-6 [DOI] [PubMed] [Google Scholar]
- 217. Abd El Bagi ME. 2003. New radiographic classification of bone involvement in pedal mycetoma. AJR Am J Roentgenol 180:665–668. doi: 10.2214/ajr.180.3.1800665 [DOI] [PubMed] [Google Scholar]
- 218. Siddig EE, Mhmoud NA, Bakhiet SM, Abdallah OB, Mekki SO, El Dawi NI, Van de Sande W, Fahal AH. 2019. The accuracy of histopathological and cytopathological techniques in the identification of the mycetoma causative agents. PLoS Negl Trop Dis 13:e0007056. doi: 10.1371/journal.pntd.0007056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Chen SC-A, Halliday CL, Hoenigl M, Cornely OA, Meyer W. 2021. Scedosporium and Lomentospora infections: contemporary microbiological tools for the diagnosis of invasive disease. J Fungi 7:23. doi: 10.3390/jof7010023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Ahmed SA, van de Sande WWJ, Stevens DA, Fahal A, van Diepeningen AD, Menken SBJ, de Hoog GS. 2014. Revision of agents of black-grain eumycetoma in the order Pleosporales. Persoonia 33:141–154. doi: 10.3767/003158514X684744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Bruker Daltonics GmbH . 2024. Maldi biotyper - MBT HT filamentous fungi IVD module. Bruker. Available from: https://www.bruker.com/en/products-and-solutions/microbiology-and-diagnostics/microbial-identification-for-clinical-laboratories-ivd-ce/mbt-ht-filamentous-fungi-ivd-module.html [Google Scholar]
- 222. Singh A, Singh PK, Kumar A, Chander J, Khanna G, Roy P, Meis JF, Chowdhary A. 2017. Molecular and matrix-assisted laser desorption ionization-time of flight mass spectrometry-based characterization of clinically significant melanized fungi in India. J Clin Microbiol 55:1090–1103. doi: 10.1128/JCM.02413-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Coulibaly O, Marinach-Patrice C, Cassagne C, Piarroux R, Mazier D, Ranque S. 2011. Pseudallescheria/Scedosporium complex species identification by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Med Mycol 49:621–626. doi: 10.3109/13693786.2011.555424 [DOI] [PubMed] [Google Scholar]
- 224. Ahmed A, Desplaces N, de Hoog S, Verbrugh H, van Belkum A. 2003. Eumycetoma caused by Madurella mycetomatis identified by PCR and sequencing: a report of two cases. J Clin Microbiol 41:1813–1816. doi: 10.1128/JCM.41.12.5813-5816.2003 [DOI] [Google Scholar]
- 225. Lim W, Siddig E, Eadie K, Nyuykonge B, Ahmed S, Fahal A, Verbon A, Smit S, van de Sande WW. 2020. The development of a novel diagnostic PCR for Madurella mycetomatis using a comparative genome approach. PLoS Negl Trop Dis 14:e0008897. doi: 10.1371/journal.pntd.0008897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Arastehfar A, Lim W, Daneshnia F, van de Sande WWJ, Fahal AH, Desnos-Ollivier M, de Hoog GS, Boekhout T, Ahmed SA. 2020. Madurella real-time PCR, a novel approach for eumycetoma diagnosis. PLoS Negl Trop Dis 14:e0007845. doi: 10.1371/journal.pntd.0007845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Hashizume H, Taga S, Sakata MK, Hussein M, Siddig EE, Minamoto T, Fahal AH, Kaneko S. 2023. Environmental detection of eumycetoma pathogens using multiplex real-time PCR for soil DNA in Sennar state, Sudan. Trop Med Health 51:71. doi: 10.1186/s41182-023-00563-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Lu Q, Gerrits van den Ende AHG, Bakkers JMJE, Sun J, Lackner M, Najafzadeh MJ, Melchers WJG, Li R, de Hoog GS. 2011. Identification of Pseudallescheria and Scedosporium species by three molecular methods. J Clin Microbiol 49:960–967. doi: 10.1128/JCM.01813-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Ahmed SA, van den Ende BHGG, Fahal AH, van de Sande WWJ, de Hoog GS. 2014. Rapid identification of black grain eumycetoma causative agents using rolling circle amplification. PLoS Negl Trop Dis 8:e3368. doi: 10.1371/journal.pntd.0003368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Lackner M, Najafzadeh MJ, Sun J, Lu Q, Hoog GS de. 2012. Rapid identification of Pseudallescheria and Scedosporium strains by using rolling circle amplification. Appl Environ Microbiol 78:126–133. doi: 10.1128/AEM.05280-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Welsh O, Al-Abdely HM, Salinas-Carmona MC, Fahal AH. 2014. Mycetoma medical therapy. PLoS Negl Trop Dis 8:e3218. doi: 10.1371/journal.pntd.0003218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Milani B, Dagne DA, Choi HL, Schito M, Stone HA. 2023. Diagnostic capacities and treatment practices on implantation mycoses: results from the 2022 WHO global online survey. PLoS Negl Trop Dis 17:e0011443. doi: 10.1371/journal.pntd.0011443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Mattioni S, Develoux M, Brun S, Martin A, Jaureguy F, Naggara N, Bouchaud O. 2013. Management of mycetomas in France. Med Mal Infect 43:286–294. doi: 10.1016/j.medmal.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 234. Anonymous . 2017. Proof-of-concept superiority trial of fosravuconazole versus itraconazole for eumycetoma in Sudan. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT03086226. Retrieved 8 Dec 2023. [Google Scholar]
- 235. van de Sande WWJ. 2021. In vitro susceptibility testing for black grain eumycetoma causative agents. Trans R Soc Trop Med Hyg 115:343–354. doi: 10.1093/trstmh/traa184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Ahmed AOA, van de Sande WWJ, van Vianen W, van Belkum A, Fahal AH, Verbrugh HA, Bakker-Woudenberg IAJM. 2004. In vitro susceptibilities of Madurella mycetomatis to itraconazole and amphotericin B assessed by a modified NCCLS method and a viability-based 2,3-bis(2-methoxy-4-nitro-5- sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) assay. Antimicrob Agents Chemother 48:2742–2746. doi: 10.1128/AAC.48.7.2742-2746.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Ahmed SA, Kloezen W, Duncanson F, Zijlstra EE, de Hoog GS, Fahal AH, van de Sande WWJ. 2014. Madurella mycetomatis is highly susceptible to ravuconazole. PLoS Negl Trop Dis 8:e2942. doi: 10.1371/journal.pntd.0002942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Kloezen W, Meis JF, Curfs-Breuker I, Fahal AH, van de Sande WWJ. 2012. In vitro antifungal activity of isavuconazole to Madurella mycetomatis. Antimicrob Agents Chemother 56:6054–6056. doi: 10.1128/AAC.01170-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Lim W, Eadie K, Konings M, Rijnders B, Fahal AH, Oliver JD, Birch M, Verbon A, van de Sande W. 2020. Madurella mycetomatis, the main causative agent of eumycetoma, is highly susceptible to olorofim. J Antimicrob Chemother 75:936–941. doi: 10.1093/jac/dkz529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. van Belkum A, Fahal AH, van de Sande WWJ. 2011. In vitro susceptibility of Madurella mycetomatis to posaconazole and terbinafine. Antimicrob Agents Chemother 55:1771–1773. doi: 10.1128/AAC.01045-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. van de Sande WWJ, Fahal AH, Bakker-Woudenberg IAJM, van Belkum A. 2010. Madurella mycetomatis is not susceptible to the echinocandin class of antifungal agents. Antimicrob Agents Chemother 54:2738–2740. doi: 10.1128/AAC.01546-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. van de Sande WWJ, Luijendijk A, Ahmed AOA, Bakker-Woudenberg IAJM, van Belkum A. 2005. Testing of the in vitro susceptibilities of Madurella mycetomatis to six antifungal agents by using the sensititre system in comparison with a viability-based 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5 [(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) assay and a modified NCCLS method. Antimicrob Agents Chemother 49:1364–1368. doi: 10.1128/AAC.49.4.1364-1368.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Ahmed SA, de Hoog GS, Stevens DA, Fahal AH, van de Sande WWJ. 2015. In vitro antifungal susceptibility of coelomycete agents of black grain eumycetoma to eight antifungals. Med Mycol 53:295–301. doi: 10.1093/mmy/myu098 [DOI] [PubMed] [Google Scholar]
- 244. Nyuykonge B, Croughs PD, Fahal AH, Verbon A, van de Sande WWJ. 2019. Pyomelanin secretion in Madurella mycetomatis interferes with spectrophotometric endpoint reading using the sensititre YeastOne alamarBlue assay but not with visual endpoint reading. Antimicrob Agents Chemother 64:e01532-19. doi: 10.1128/AAC.01532-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Alastruey-Izquierdo A, Cuenca-Estrella M, Monzón A, Mellado E, Rodríguez-Tudela JL. 2008. Antifungal susceptibility profile of clinical Fusarium spp. isolates identified by molecular methods. J Antimicrob Chemother 61:805–809. doi: 10.1093/jac/dkn022 [DOI] [PubMed] [Google Scholar]
- 246. Nyuykonge B, Siddig EE, Mhmoud NA, Nyaoke BA, Zijlstra EE, Verbon A, Bakhiet S, Fahal AH, van de Sande WWJ. 2022. Epidemiological cut-off values for itraconazole and ravuconazole for Madurella mycetomatis, the most common causative agent of mycetoma. Mycoses 65:1170–1178. doi: 10.1111/myc.13509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Nyuykonge B, Lim W, van Amelsvoort L, Bonifaz A, Fahal A, Badali H, Abastabar M, Verbon A, van de Sande W. 2022. Eumycetoma causative agents are inhibited in vitro by luliconazole, lanoconazole and ravuconazole. Mycoses 65:650–655. doi: 10.1111/myc.13442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Pfaller MA, Carvalhaes CG, Castanheira M. 2023. Susceptibility patterns of amphotericin B, itraconazole, posaconazole, voriconazole and caspofungin for isolates causing invasive mould infections from the SENTRY antifungal surveillance program (2018-2021) and application of single-site epidemiological cutoff values to evaluate amphotericin B activity. Mycoses 66:854–868. doi: 10.1111/myc.13620 [DOI] [PubMed] [Google Scholar]
- 249. Gilgado F, Serena C, Cano J, Gené J, Guarro J. 2006. Antifungal susceptibilities of the species of the Pseudallescheria boydii complex. Antimicrob Agents Chemother 50:4211–4213. doi: 10.1128/AAC.00981-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Pfaller MA, Carvalhaes CG, Messer SA, Rhomberg PR, Castanheira M. 2021. In vitro activity of posaconazole and comparators versus opportunistic filamentous fungal pathogens globally collected during 8 years. Diagn Microbiol Infect Dis 101:115473. doi: 10.1016/j.diagmicrobio.2021.115473 [DOI] [PubMed] [Google Scholar]
- 251. Guarro J, Soler L, Rinaldi MG. 1995. Pathogenicity and antifungal susceptibility of Chaetomium species. Eur J Clin Microbiol Infect Dis 14:613–618. doi: 10.1007/BF01690737 [DOI] [PubMed] [Google Scholar]
- 252. Wildfeuer A, Seidl HP, Paule I, Haberreiter A. 1998. In vitro evaluation of voriconazole against clinical isolates of yeasts, moulds and dermatophytes in comparison with itraconazole, ketoconazole, amphotericin B and griseofulvin. Mycoses 41:309–319. doi: 10.1111/j.1439-0507.1998.tb00344.x [DOI] [PubMed] [Google Scholar]
- 253. Pfaller MA, Messer SA, Hollis RJ, Jones RN, Group SP. 2002. Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: report from SENTRY antimicrobial surveillance program, 2000. Antimicrob Agents Chemother 46:1032–1037. doi: 10.1128/AAC.46.4.1032-1037.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Ryder NS. 1999. Activity of terbinafine against serious fungal pathogens. Mycoses 42 Suppl 2:115–119. doi: 10.1111/j.1439-0507.1999.tb00026.x [DOI] [PubMed] [Google Scholar]
- 255. Verweij PE, van den Bergh MF, Rath PM, de Pauw BE, Voss A, Meis JF. 1999. Invasive aspergillosis caused by Aspergillus ustus: case report and review. J Clin Microbiol 37:1606–1609. doi: 10.1128/JCM.37.5.1606-1609.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Alastruey-Izquierdo A, Cuesta I, Houbraken J, Cuenca-Estrella M, Monzón A, Rodriguez-Tudela JL. 2010. In vitro activity of nine antifungal agents against clinical isolates of Aspergillus calidoustus. Med Mycol 48:97–102. doi: 10.3109/13693780902803040 [DOI] [PubMed] [Google Scholar]
- 257. Badali H, de Hoog GS, Curfs-Breuker I, Klaassen CHW, Meis JF. 2010. Use of amplified fragment length polymorphism to identify 42 Cladophialophora strains related to cerebral phaeohyphomycosis with in vitro antifungal susceptibility. J Clin Microbiol 48:2350–2356. doi: 10.1128/JCM.00653-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Wang Q, Wang L, Lian L, Pu X, Tang L, Li Y, Liu Y. 2023. Case report: a case of ocular infection caused by Corynespora cassiicola. Front Cell Infect Microbiol 13:1160831. doi: 10.3389/fcimb.2023.1160831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. da Cunha Keith C, Sutton DA, Fothergill AW, Gené J, Cano J, Madrid H, Hoog S de, Crous PW, Guarro J. 2013. In vitro antifungal susceptibility and molecular identity of 99 clinical isolates of the opportunistic fungal genus Curvularia. Diagn Microbiol Infect Dis 76:168–174. doi: 10.1016/j.diagmicrobio.2013.02.034 [DOI] [PubMed] [Google Scholar]
- 260. Gülmez D, Doğan Ö, Boral B, Döğen A, İlkit M, de Hoog GS, Arikan-Akdagli S. 2018. In vitro activities of antifungal drugs against environmental Exophiala isolates and review of the literature. Mycoses 61:561–569. doi: 10.1111/myc.12779 [DOI] [PubMed] [Google Scholar]
- 261. Ng CY, de Hoog S, Li H-E, Lee Y-Y, Chen C-B, Sun P-L. 2017. Cutaneous Exophiala oligosperma infection in a patient with bullous pemphigoid with a review of the literature. Mycopathologia 182:539–547. doi: 10.1007/s11046-016-0104-6 [DOI] [PubMed] [Google Scholar]
- 262. da Cunha KC, Sutton DA, Gené J, Capilla J, Cano J, Guarro J. 2012. Molecular identification and in vitro response to antifungal drugs of clinical isolates of Exserohilum. Antimicrob Agents Chemother 56:4951–4954. doi: 10.1128/AAC.00488-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Sekhon AS, Padhye AA, Garg AK, Ahmad H, Moledina N. 1994. In vitro sensitivity of medically significant Fusarium species to various antimycotics. Chemotherapy 40:239–244. doi: 10.1159/000239199 [DOI] [PubMed] [Google Scholar]
- 264. Kawakami H, Inuzuka H, Hori N, Takahashi N, Ishida K, Mochizuki K, Ohkusu K, Muraosa Y, Watanabe A, Kamei K. 2015. Inhibitory effects of antimicrobial agents against Fusarium species. Med Mycol 53:603–611. doi: 10.1093/mmy/myv016 [DOI] [PubMed] [Google Scholar]
- 265. Azor M, Gené J, Cano J, Sutton DA, Fothergill AW, Rinaldi MG, Guarro J. 2008. In vitro antifungal susceptibility and molecular characterization of clinical isolates of Fusarium verticillioides (F. moniliforme) and Fusarium thapsinum. Antimicrob Agents Chemother 52:2228–2231. doi: 10.1128/AAC.00176-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Aneke CI, Rhimi W, Hubka V, Otranto D, Cafarchia C. 2021. Virulence and antifungal susceptibility of Microsporum canis strains from animals and humans. Antibiotics (Basel) 10:296. doi: 10.3390/antibiotics10030296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Kano R, Watanabe M, Tsuchihashi H, Ogawa T, Ogawa Y, Komiyama E, Hirasawa Y, Hiruma M, Ikeda S. 2023. Antifungal susceptibility testing for Microsporum canis from cats in Japan. Med Mycol J 64:19–22. doi: 10.3314/mmj.22-00014 [DOI] [PubMed] [Google Scholar]
- 268. Lacroix C, de Chauvin MF. 2008. In vitro activity of amphotericin B, itraconazole, voriconazole, posaconazole, caspofungin and terbinafine against Scytalidium dimidiatum and Scytalidium hyalinum clinical isolates. J Antimicrob Chemother 61:835–837. doi: 10.1093/jac/dkn011 [DOI] [PubMed] [Google Scholar]
- 269. Castelli MV, Alastruey-Izquierdo A, Cuesta I, Monzon A, Mellado E, Rodriguez-Tudela JL, Cuenca-Estrella M. 2008. Susceptibility testing and molecular classification of Paecilomyces spp. Antimicrob Agents Chemother 52:2926–2928. doi: 10.1128/AAC.00538-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Badali H, Khodavaisy S, Fakhim H, de Hoog GS, Meis JF, Chowdhary A. 2015. In vitro susceptibility profiles of eight antifungal drugs against clinical and environmental strains of Phaeoacremonium. Antimicrob Agents Chemother 59:7818–7822. doi: 10.1128/AAC.01733-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Li Y, Wan Z, Li R. 2014. In vitro activities of nine antifungal drugs and their combinations against Phialophora verrucosa. Antimicrob Agents Chemother 58:5609–5612. doi: 10.1128/AAC.02875-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Yao L, Wang H, Wan Z, Li R, Yu J. 2019. The high diversity and variable susceptibility of clinically relevant Acremonium-like species in China. Mycopathologia 184:759–773. doi: 10.1007/s11046-019-00399-8 [DOI] [PubMed] [Google Scholar]
- 273. Deng S, Zhang C, Seyedmousavi S, Zhu S, Tan X, Wen Y, Huang X, Lei W, Zhou Z, Fang W, Shen S, Deng D, Pan W, Liao W. 2015. Comparison of the in vitro activities of newer triazoles and established antifungal agents against Trichophyton rubrum. Antimicrob Agents Chemother 59:4312–4314. doi: 10.1128/AAC.00244-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Singh J, Zaman M, Gupta AK. 2007. Evaluation of microdilution and disk diffusion methods for antifungal susceptibility testing of dermatophytes. Med Mycol 45:595–602. doi: 10.1080/13693780701549364 [DOI] [PubMed] [Google Scholar]
- 275. Andes D, Pascual A, Marchetti O. 2009. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob Agents Chemother 53:24–34. doi: 10.1128/AAC.00705-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Murray IG, Colichon H. 1962. Chemotherapy of maduromycosis. I. Preliminary experiments with diamidinodi-phenylamine dihydrochloride and amphotericin B in vivo and in vitro. Trans R Soc Trop Med Hyg 56:156–165. doi: 10.1016/0035-9203(62)90143-8 [DOI] [PubMed] [Google Scholar]
- 277. Gao L, Jiang S, Sun Y, Deng M, Wu Q, Li M, Zeng T. 2016. Evaluation of the effects of photodynamic therapy alone and combined with standard antifungal therapy on planktonic cells and biofilms of Fusarium spp. and Exophiala spp. Front Microbiol 7:617. doi: 10.3389/fmicb.2016.00617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Sun Y, Gao L, He C, Li M, Zeng T. 2018. In vitro interactions between IAP antagonist AT406 and azoles against planktonic cells and biofilms of pathogenic fungi Candida albicans and Exophiala dermatitidis. Med Mycol 56:1045–1049. doi: 10.1093/mmy/myx150 [DOI] [PubMed] [Google Scholar]
- 279. Kloezen W, Parel F, Brüggemann R, Asouit K, Helvert-van Poppel M, Fahal A, Mouton J, van de Sande W. 2018. Amphotericin B and terbinafine but not the azoles prolong survival in Galleria mellonella larvae infected with Madurella mycetomatis. Med Mycol 56:469–478. doi: 10.1093/mmy/myx064 [DOI] [PubMed] [Google Scholar]
- 280. van de Sande WWJ, van Vianen W, ten Kate M, Fahal A, Bakker-Woudenberg I. 2015. Amphotericin B but not itraconazole is able to prevent grain formation in experimental Madurella mycetomatis mycetoma in mice. Br J Dermatol 173:1561–1562. doi: 10.1111/bjd.14025 [DOI] [PubMed] [Google Scholar]
- 281. Zein HAM, Fahal AH, Mahgoub ES, El Hassan TA, Abdel-Rahman ME. 2012. Predictors of cure, amputation and follow-up dropout among patients with mycetoma seen at the mycetoma research centre, University of Khartoum, Sudan. Trans R Soc Trop Med Hyg 106:639–644. doi: 10.1016/j.trstmh.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 282. Musa EA, Abdoon IH, Bakhiet SM, Osman B, Abdalla SA, Fahal AH. 2023. Mycetoma management and clinical outcomes: the mycetoma research center experience. Trans R Soc Trop Med Hyg 117:12–21. doi: 10.1093/trstmh/trac069 [DOI] [PubMed] [Google Scholar]
- 283. Vilela R, Duarte OMV, Rosa CA, Castro JGF, Lyon S, Motta RL, Moura ACL. 2004. A case of eumycetoma due to Madurella grisea in northern Brazil. Mycopathologia 158:415–418. doi: 10.1007/s11046-004-2844-y [DOI] [PubMed] [Google Scholar]
- 284. Machado LA, Rivitti MC, Cucé LC, Salebian A, Lacaz C da S, Heins-Vaccari EM, Belda Júnior W, de Melo NT. 1992. Black-grain eumycetoma due to Madurella grisea. A report of 2 cases. Rev Inst Med Trop Sao Paulo 34:569–580. [PubMed] [Google Scholar]
- 285. Belda Junior W, Cuce LC, Dias MC, Lacaz Cda S. 1989. Eumicetoma de graos pretos por Madurella grisea. Rev Inst Med Trop Sao Paulo 31:195–199. doi: 10.1590/S0036-46651989000300010 [DOI] [PubMed] [Google Scholar]
- 286. Sampaio FMS, Wanke B, Freitas DFS, Coelho JMC de O, Galhardo MCG, Lyra MR, Lourenço MC da S, Paes R de A, do Valle ACF. 2017. Review of 21 cases of mycetoma from 1991 to 2014 in Rio de Janeiro, Brazil. PLoS Negl Trop Dis 11:e0005301. doi: 10.1371/journal.pntd.0005301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Mahgoub ES, Gumaa SA. 1984. Ketoconazole in the treatment of eumycetoma due to Madurella mycetomii. Trans R Soc Trop Med Hyg 78:376–379. doi: 10.1016/0035-9203(84)90126-3 [DOI] [PubMed] [Google Scholar]
- 288. Fahal AH, Rahman IA, El-Hassan AM, Rahman MEAEL, Zijlstra EE. 2011. The safety and efficacy of Itraconazole for the treatment of patients with eumycetoma due to Madurella mycetomatis. Trans R Soc Trop Med Hyg 105:127–132. doi: 10.1016/j.trstmh.2010.11.008 [DOI] [PubMed] [Google Scholar]
- 289. Samy AM, van de Sande WWJ, Fahal AH, Peterson AT. 2014. Mapping the potential risk of mycetoma infection in Sudan and South Sudan using ecological niche modeling. PLoS Negl Trop Dis 8:e3250. doi: 10.1371/journal.pntd.0003250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Hassan R, Simpson H, Cano J, Bakhiet S, Ganawa E, Argaw D, Newport MJ, Deribe K, Fahal AH. 2021. Modelling the spatial distribution of mycetoma in Sudan. Trans R Soc Trop Med Hyg 115:1144–1152. doi: 10.1093/trstmh/trab076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Fahal AH, Bakhiet SM. 2023. Mycetoma and the environment. PLoS Negl Trop Dis 17:e0011736. doi: 10.1371/journal.pntd.0011736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Hounsome N, Hassan R, Bakhiet SM, Deribe K, Bremner S, Fahal AH, Newport MJ. 2022. Role of socioeconomic factors in developing mycetoma: results from a household survey in Sennar state. PLoS Negl Trop Dis 16:e0010817. doi: 10.1371/journal.pntd.0010817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Hassan R, Deribe K, Simpson H, Bremner S, Elhadi O, Alnour M, Fahal AH, Newport M, Bakhiet S. 2022. Individual risk factors of mycetoma occurrence in Eastern Sennar locality, Sennar state, Sudan: a case-control study. TropicalMed 7:174. doi: 10.3390/tropicalmed7080174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Bakhiet SM, Fahal AH, Musa AM, Mohamed ESW, Omer RF, Ahmed ES, El Nour M, Mustafa ERM, Sheikh A Rahman ME, Suliman SH, El Mamoun MAG, El Amin HM. 2018. A holistic approach to the mycetoma management. PLoS Negl Trop Dis 12:e0006391. doi: 10.1371/journal.pntd.0006391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Azrag RS, Bakhiet SM, Mhmoud NA, Almalik AM, Mohamed AH, Fahal AH. 2021. A possible role for ticks in the transmission of Madurella mycetomatis in a mycetoma-endemic village in Sudan . Trans R Soc Trop Med Hyg 115:364–374. doi: 10.1093/trstmh/trab030 [DOI] [PubMed] [Google Scholar]