Abstract
Problem
The NHS is perceived to have a poor record of learning from incidents. Despite efforts of the Medical Devices Agency, which issues safety warnings, adverse incidents with medical devices continue to occur, some of which result in serious injury or death through device failures, user errors, and organisational problems.
Design
Introduction of feedback notes on a supportive investigation that seeks to determine latent factors, immediate triggers, causes, and positive actions taken by staff that minimised adverse consequences.
Background and setting
Medical physics department providing equipment management services in a major NHS teaching trust.
Key measures for improvement
Reduction in repetitions of adverse incidents and improved staff competency in using devices.
Strategy for change
A feedback note was developed to describe the incident and generic details of the equipment, summarise the investigation (focusing on latent causes and immediate triggers), and describe lessons to be learnt and positive actions by staff.
Effects of change
Feedback notes have been used in teaching sessions and given to ward link nurses. Despite being new, the positive supportive approach has encouraged an open reporting culture.
Lessons learnt
Adverse incidents are typically caused by alignment of different factors, but good practice can prevent errors becoming incidents. Careful analysis of incidents reveals both the multifactorial causes and the good practices that can help minimise repetitions.
Context
Errors in medicine and the inevitability of human error are now widely recognised,1–8 and the health service is perceived as having a poor record of learning from previous incidents.4 Adverse incidents are typically caused by several factors, particularly organisational and structural defects as well as unpredictable human errors.1–5 The NHS has been urged to change from a “passive” to an “active” learning organisation, establishing a culture of self reflection and appraisal to minimise adverse incidents.7
Outline of problem
Medical devices have been termed “members” of the healthcare team,3 reflecting their importance in healthcare delivery, but using them has risks as well as benefits. Risks may stem from design, manufacture, maintenance, storage, housekeeping, or a lack of user competence.
Each year about 400 people are killed or seriously injured in adverse incidents involving medical devices.4,9 The Medical Device Agency helps ensure safe use and operates an incident reporting system that encourages reporting of latent defects as well as incidents with adverse consequences.10,11 Although the adverse incidents are often ascribed to human factors, including users' inexperience,12–16 they are typically multifactorial in origin, with latent factors, faults, errors, and mistakes aligning together (the Swiss cheese model).5
Analyses of incidents should explore the background conditions as well as the triggering factors and unsafe actions.2,17 Factors that minimise adverse consequences should also be investigated,1 as understanding them can help prevent repetitions and contribute to an open supportive environment.
The key problem is that lessons from previous incidents are not always learnt, so safety warnings are sometimes not heeded.18 We describe an extension of Reason's incident analysis model to help in learning from adverse incidents.5 The incident is investigated and then a feedback note, which includes a description and analysis of the incident with recommendations on how to avoid repetitions, is compiled. The note recognises and highlights positive actions of staff to minimise adverse consequences.
Key measures for improvement
The feedback note is designed to be an educational tool that supports staff and encourages them to think of the wider issues that promote safe practice. This in turn should help prevent repetitions of previously reported problems with medical devices. The key measure of improvement is no repetition of previously reported adverse incidents. Feedback should also help improve staff understanding of the characteristics and limitations of medical devices and the factors that can lead to incidents. The note is also designed to encourage a supportive environment in which staff feel free to discuss problems that they experience; this involves a change of staff attitudes.
Process of gathering information
The process starts when a clinical department reports an incident involving a medical device or technical staff report a defect. A clinical area requests repair of a broken medical device by submitting a servicing request form to the workshop, accompanied by a copy of the hospital's incident report form. Not all failures of medical devices constitute adverse incidents, however, and supervisors of the servicing workshop must exercise judgment about which failures to report. The Medical Devices Agency advises that device failures should be reported not only if they caused injury but also if they have the potential to injure patients, staff, or visitors.10 Supervisors should be told about devices that are intrinsically unsafe, either mechanically or functionally, and histories or patterns of failures. Incident reports involving medical devices should be submitted to the Medical Devices Agency (or Scottish Healthcare Services in Scotland).
The immediate causes of the incident as well as the background latent conditions should be investigated. The medical device should be examined to ascertain if and how it failed and how any failure contributed to the incident. The maintenance history of the device and the storage environment should be reviewed. Background factors that could have contributed to device malfunction should be explored as well as the normal operating conditions. The investigation should proceed in a supportive manner, avoiding knee jerk conclusions of “user error” or “device error” which close the mind to the wider picture. Good practice that minimised adverse consequences should be highlighted. Thus incident investigations should not simply concentrate on what went wrong, but should also focus on good practices that minimised adverse consequences. This can reassure staff and provide lessons to minimise the risk of recurrences and help change individuals' perceptions of how errors are dealt with by the organisation.
Analysis and interpretation
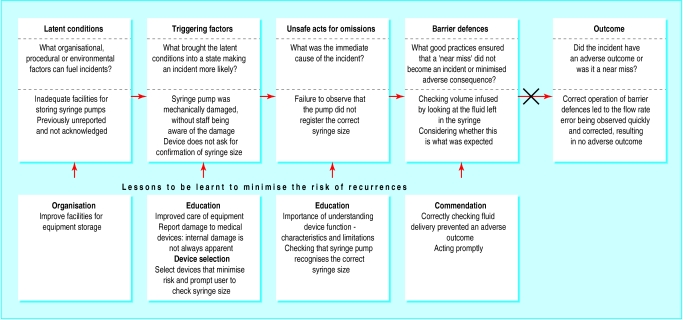
The feedback method is illustrated by the example of a reported overinfusion with a syringe pump (figure). Examination in the workshop confirmed the 50% overinfusion and found that the immediate cause was the incorrect recognition by the pump of the 50 ml syringe as a 20 ml syringe. On internal examination the syringe size detector was found to be damaged.
It is not uncommon for such faults to be corrected and the functional device returned to the ward, a routine breakdown with no adverse consequences. This failure was reported to the workshop together with an incident report, and the failure was reported to the appropriate notification agency and the manufacturer. However, this does not always occur.
The immediate cause of the incident was the failure to notice that the pump indicated an incorrect syringe size. Good practice requires that staff ensure that the pump correctly detects the size before starting an infusion. However, when only 50 ml syringes are used and when the pump “always” registers a 50 ml syringe it is very easy to “see” what we anticipate. Moreover, it is relatively easy to miss information on a crowded display.14 Furthermore, staff may not understand why syringe pumps register the syringe size.
A faulty device whose damage was not apparent to the user caused the overinfusion. To prevent repetitions, the cause of the damage should be examined. Repair records showed that levels of mechanical damage to devices from the ward were higher than normal. Investigation pointed to poor storage and handling.
Good practices that help minimise the consequences should be explored. In this case diligent charting of fluid delivery and checking of the volume remaining in the syringe led to the overinfusion being recognised early. Highlighting good practice supports the staff involved, provides useful lessons in encouraging staff to adopt safer practice, and helps individuals to believe that a “no blame culture” exists.
This incident contains lessons that can be used to improve practice, both in the ward and elsewhere in the hospital, particularly as infusion devices are involved in many incidents.16 Furthermore, servicing supervisors must be alert to device failures that should be reported to the Medical Devices Agency (or other appropriate notification agency). The manufacturer should also be informed, and the feedback note developed can be used for this.
Strategy for change
To learn lessons staff must be informed, in a supportive manner, why the incident occurred. The incident and the sequence of events that caused it should be described. Good clinical practice that helped minimise adverse consequences should be highlighted. We developed an anonymous feedback note to help staff to learn from the incident. It had six sections:
Brief description—an anonymous summary
Device detail (only if pertinent is the specific make and model mentioned)—keeping the device details generic widens the instruction value to staff using other makes
Investigation—details of the investigation with illustrations if appropriate
Possible causes—latent and active causes of the incident are explored
Lessons to be learnt and remedial action to be taken—consideration of the wider lessons to be learnt
Positive actions taken by staff—highlight positive actions taken to minimise adverse consequences. This helps engender a positive supportive culture and emphasises good practice that can prevent errors leading to adverse consequences.
Effects of change
In our example, the feedback note was used to alert staff in the department where the incident occurred to the causes of the overinfusion and to the necessity of checking the syringe size before starting the infusion. The need to store medical devices safely was raised. There have been no repetitions of this incident in the department concerned.
The feedback notes have been used in teaching sessions and have been given to departmental link nurses. Good practices by staff that minimised adverse consequences (or, in the case of a near miss, prevented a series of conditions leading to an incident) are included in the investigation process; this helps encourage an open reporting culture.
The feedback note encapsulates the events leading to the incident, highlights good practice, and points out where lessons can be learnt. It recognises that human error is inevitable and should be anticipated.19 It extends the scope of the Medical Device Agency's safety warnings, seeking to share experiences, pointing out lessons and good practice that can be developed and implemented.
The feedback note encourages the investigation to look beyond the immediate causes of the incident to background factors and positive actions taken by staff. Latent factors include arrangements for equipment storage, cleaning technique, and incorrect operative procedures and techniques. It also recognises that damage to medical devices may not always be apparent.
Design features of medical devices can improve safety. For example, in the incident described, staff would more likely check the syringe size if prompted by the device. Conversely, information not clearly displayed may not be noticed, particularly if the display invariably contains the same information.14
A positive approach to incident investigation is inherent in the design of the feedback note. Simply blaming an individual for incorrect use of a medical device with the label “user error” does not solve the underlying problem and promotes distrust from users. Problems identified in one area may occur in others and thus have lessons for other clinical users. Similarly, simply labelling the cause as equipment failure, without seeking to understand what caused it, may not prevent repetitions. Though it is not uncommon for medical devices to be sent for repair and for the maintenance workshop to find no fault, detailed investigation may uncover underlying problems. The cause may be an obscure or intermittent fault, environmental factors in the ward (high temperatures, for example), or a lack of user competence. It has been gratifying to observe staff volunteering information on incidents involving medical devices, rather than simply trying to hide the event.
Key learning points
Adverse incidents with medical devices are caused by a variety of factors not simply either device or user errors
Lessons from previous incidents involving medical devices are not always learnt
A feedback note that includes a description of the incident and what caused it, as well as outlining the lessons to be learnt and a summary of good practices that helped minimise adverse consequences, will help staff learn lessons from incidents
Highlighting positive actions taken by staff that helped minimise adverse consequences develops a culture that supports learning and promotes good practice
Risk is inherent in the practice of medicine, whether it is associated with pharmacological or surgical interventions or with diagnosis. Safe practice requires that risks be minimised and managed, but it is probably not possible or desirable to entirely eliminate all risks. Indeed, creative and innovative medicine may not be possible without accepting the inevitability of risk, an essential ingredient that has been termed vitamin R.20 Clinical risk management seeks to understand the causes of adverse incidents, to learn from previous incidents, and to put into place procedures to minimise the adverse consequences.
Next steps
The success of the feedback note depends on disseminating the information not only to the department where the incident occurred but also more widely throughout the organisation via the annual nurse clinical update session and the hospital intranet, for example. An audit of nursing training programmes and further development of a link nurse system are helping us to improve the distribution of information.
The trust's new approach to incident reporting takes into account the advice in An Organisation with a Memory and Building a Safer NHS for Patients.4,8 The new investigation form explicitly seeks the broader factors that cause incidents. This will support the gathering of information to develop supportive feedback notes. The feedback note can in turn help to ensure that lessons are learnt, an integral part of the new process.
Supplementary Material
Figure.
A near miss involving a medical device, and lessons learnt (adapted from Reason5)
Acknowledgments
We acknowledge the help and support of the servicing workshops in investigating incidents involving medical devices. Discussions with various staff over the years have provided insights into incident investigation.
Footnotes
Funding: None.
Competing interests: None declared.
The feedback note appears on bmj.com
References
- 1.Runciman WB, Sellen A, Webb RK, Williamson JA, Currie M, Morgan C, et al. Errors, incidents and accidents in anaesthetic practice. Anaesth Intensive Care, 1993;21:506–519. doi: 10.1177/0310057X9302100506. [DOI] [PubMed] [Google Scholar]
- 2.Vincent C, Taylor-Adams S, Stanhope N. Framework for analysing risk and safety in medicine. BMJ. 1998;316:1154–1157. doi: 10.1136/bmj.316.7138.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohn LT, Corrigan JM, Donaldson MM, editors. To err is human. Building a safer health system. Washington, DC: National Academy Press; 1999. [PubMed] [Google Scholar]
- 4.Department of Health. An organisation with a memory: report of an expert group on learning from adverse events in the NHS chaired by the Chief Medical Officer. London: Stationery Office; 2000. [Google Scholar]
- 5.Reason J. Human error: models and management. BMJ. 2000;320:768–770. doi: 10.1136/bmj.320.7237.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leape LL, Berwick DM. Safe health care: are we up to it? BMJ. 2000;320:725–726. doi: 10.1136/bmj.320.7237.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barach P, Small SD. How the NHS can improve safety and learning. BMJ. 2000;320:1683–1684. doi: 10.1136/bmj.320.7251.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Department of Health. Building a safer NHS for patients. London: Stationery Office; 2001. [Google Scholar]
- 9.Webb RK, Russel WJ, Klepper I, Runciman WB. Equipment failure: an analysis of 2000 incident reports. Anaesth Intensive Care, 1993;21:673–677. doi: 10.1177/0310057X9302100533. [DOI] [PubMed] [Google Scholar]
- 10.Department of Health. Medical device and equipment management for hospital and community-based organisations. London: Medical Device Agency; 1998. . (Bulletin MDA DB 9801.) [Google Scholar]
- 11.Scottish Office. Reporting of adverse incidents and defective equipment. Edinburgh: Scottish Office; 1995. . (Management Executive Letter MEL (1995)74.) [Google Scholar]
- 12.Cooper JB, Newbower RS, Kitz RJ. An analysis of major errors and equipment failures in anesthesia management: considerations for prevention and detection. Anesthesiology. 1984;60:34–42. doi: 10.1097/00000542-198401000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Wright D, Mackenzie SJ, Buchan I, Cairns CS, Price LE. Critical incidents in the intensive therapy unit. Lancet. 1991;338:676–678. doi: 10.1016/0140-6736(91)91243-n. [DOI] [PubMed] [Google Scholar]
- 14.Arnstein F. Catalogue of human error. Br J Anaesth. 1997;79:645–656. doi: 10.1093/bja/79.5.645. [DOI] [PubMed] [Google Scholar]
- 15.Department of Health. Equipped to care: the safe use of medical devices in the 21st century. London: Medical Devices Agency; 2000. [Google Scholar]
- 16.Williams C, Lefever J. Reducing the risk of user error with infusion pumps. Prof Nurse. 2000;15:382–384. [PubMed] [Google Scholar]
- 17.Vincent C, Taylor-Adams S, Chapman EJ, Hewett D, Prior S, Strange P, et al. How to investigate and analyse clinical incidents: clinical risk unit and association of litigation and risk management protocol. BMJ. 2000;320:777–781. doi: 10.1136/bmj.320.7237.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scottish Office. Syringe pumps: uncontrolled infusion. Edinburgh: Scottish Healthcare Supplies, Incident Reporting and Investigation Centre; 1988. , 1993, 1994. (Hazard Bulletin HC(Hazard)(88)9, 1988; Safety Action Bulletins SAB(93)7, 1993 and SAB(94)26, June 1994.) [Google Scholar]
- 19.Cohen MR, Senders J, Davis NM. Failure mode and effects analysis: A novel approach to avoiding dangerous medication errors and accidents. Hosp Pharm. 1994;29:319–324. ,326-8,330. [PubMed] [Google Scholar]
- 20. Wolff HSW. Millennium homes: technology as a catalyst for community involvement. Proceedings of the Institute of Physics and Engineering in Medicine 6th annual conference, Southampton, 2000.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.