Summary
Background
Furmonertinib showed superior efficacy compared with gefitinib as first-line therapy in patients with epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC) in the FURLONG study. Here we present prespecified secondary endpoints of patient-reported outcomes (PRO).
Methods
In this multicentre, double-blind, double-dummy, randomised phase 3 study, patients were 1:1 randomly assigned to receive furmonertinib 80 mg once daily or gefitinib 250 mg once daily. PROs assessed by the European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire Core 30 and Quality-of-Life Questionnaire Lung Cancer 13 were analysed using a mixed model for repeated measures and time-to-event analyses. A difference in score of 10 points or more was deemed clinically relevant.
Findings
Three hundred and fifty-seven patients (furmonertinib group, n = 178; gefitinib group, n = 179) received at least one dose of the study drug, all of whom completed at least one PRO assessment. Statistically significant difference of overall score changes from baseline favoured furmonertinib in physical functioning (between-group difference 2.14 [95% CI 0.25–4.04], p = 0.027), nausea/vomiting (−1.56 [95% CI −2.62 to −0.49], p = 0.004), appetite loss (−2.24 [95% CI −4.26 to −0.23], p = 0.029), diarrhoea (−3.36 [95% CI −5.19 to −1.54], p < 0.001), alopecia (−2.62 [95% CI −4.54 to −0.71], p = 0.007), and pain in other parts (−4.55 [95% CI −7.37 to −1.74], p = 0.002), but not reached clinical relevance. Time to deterioration in physical functioning (hazard ratio 0.63 [95% CI 0.42–0.94], p = 0.021), cognitive functioning (0.73 [95% CI 0.54–0.98], p = 0.034), nausea/vomiting (0.64 [95% CI 0.41–0.99], p = 0.042), appetite loss (0.63 [95% CI 0.43–0.92], p = 0.016), diarrhoea (0.63 [95% CI 0.46–0.85], p = 0.002), dyspnoea (0.72 [95% CI 0.53–0.98], p = 0.034), cough (0.67 [95% CI 0.44–1.00], p = 0.049), dysphagia (0.54 [95% CI 0.35–0.83], p = 0.004), and alopecia (0.62 [95% CI 0.42–0.90], p = 0.012) was longer with furmonertinib versus gefitinib.
Interpretation
In patients with locally advanced or metastatic EGFR mutation-positive NSCLC, furmonertinib showed improved scores and delayed deterioration in several functioning and symptoms compared to gefitinib.
Funding
Shanghai Allist Pharmaceutical Technology Co., Ltd and the National Science and Technology Major Project for Key New Drug Development (2017ZX09304015).
Keywords: Non-small cell lung cancer, Epidermal growth factor receptor, Furmonertinib, AST2818, Patient-reported outcomes
Research in context.
Evidence before this study
We searched PubMed for research articles published in English from database inception to April 10, 2024, with the search terms “advanced non-small-cell lung cancer (NSCLC)” and “patient-reported outcomes (PRO)” or “health-related quality of life”. Evidence regarding the effect of new lung cancer therapy on PROs in patients with advanced or metastatic NSCLC is emerging. However, open-label trials had increasingly published separate PRO articles to provide a comprehensive and systematic presentation of PRO analysis. On the other hand, double-blind studies had seldom published PRO articles as standalone publications. To the best of our knowledge, osimertinib is the only tyrosine-kinase inhibitor systematically reported PRO data of advanced NSCLC patients in blinded randomised clinical trials. A statistically significant difference favouring osimertinib for chest pain was not clinically relevant, and no difference in time-to-symptom deterioration was observed comparing with gefitinib.
Added value of this study
We analysed the prespecified secondary patient-reported outcomes in FURLONG, a multicentre, double-blind, randomised, placebo-controlled, phase 3 study done in 55 hospitals across mainland China. In this study, furmonertinib provided improvements compared with gefitinib in PRO profile, with statistically better mean score changes from baseline up to, including, end of treatment, as well as the significantly delayed time to deterioration in several functioning and symptoms.
Implications of all the available evidence
These favourable PRO findings complement the superior efficacy and manageable safety profile demonstrated with furmonertinib over gefitinib in the FURLONG study, and further support use of furmonertinib as a first-line therapy in Chinese patients with locally advanced or metastatic EGFR mutation-positive NSCLC.
Introduction
Lung cancer is the leading cause of cancer-related death worldwide.1 Epidermal growth factor receptor (EGFR) mutation is one of the most frequent driven mutations detected in non-small cell lung cancer (NSCLC), especially in Asian patients with lung adenocarcinoma, which can be as high as 51.4%.2 Introduction of targeted treatment with EGFR tyrosine kinase inhibitors (TKIs) has significantly improved progression-free survival (PFS) versus chemotherapy and has changed the treatment landscape of EGFR mutated advanced NSCLC.3, 4, 5, 6, 7, 8 Third-generation EGFR TKIs, primarily developed to overcome the secondary resistant EGFR T790M mutation post first- or second-generation EGFR TKIs treatment, which selectively inhibited both EGFR sensitizing and EGFR T790M resistance mutations, with lower activity against wild-type EGFR, were found to have superior efficacy compared with first-generation EGFR TKIs in first-line settings on the basis of the results of well-designed randomised controlled trials and markedly prolonged median PFS to around 20 months. These third-generation EGFR TKIs are considered as new standard of care for treatment naïve patients with EGFR mutated advanced NSCLC.9, 10, 11
Patient-reported outcomes (PRO) data now play an increasing role in anti-cancer treatment selection and in drug evaluation by payers and health technology assessment agencies.12,13 Assessments of PROs capture patient perspectives on disease, treatment burden and their impact on health-related quality of life (HRQoL) and can provide a more comprehensive evaluation of the impact of cancer therapy on patient experience and quality of life, since the management of symptoms and potential adverse events is of paramount importance in NSCLC patients.14,15
Furmonertinib (AST2818) is a potent, orally bioavailable, highly brain penetrant, third-generation EGFR TKI with a unique trifluoroethoxypyridine-based molecule structure designed to improve potency and specificity for EGFR sensitizing and resistant mutations while sparing wildtype EGFR.16 In the multicentre, double-blind, double-dummy, randomised phase 3 FURLONG study conducted in 55 hospitals across the People's Republic of China (Appendix 1 pp 1–3), furmonertinib significantly improved the median PFS compared with gefitinib (20.8 versus 11.1 months, hazard ratio [HR] 0.44, 95% confidence interval [CI] 0.34–0.58, p < 0.0001). Central nervous system (CNS) analysis showed that furmonertinib also significantly improved the median CNS PFS (20.8 versus 9.8 months, HR 0.40, 95% CI 0.23–0.71, p = 0.0011), and the CNS objective response rate (ORR) was 91% with furmonertinib and 65% with gefitinib (odds ratio [OR] 6.82, 95% CI 1.23–37.67, p = 0.0277). The rate of treatment-related adverse events (TRAEs) of grade 3 or higher was lower with furmonertinib than with gefitinib (11% versus 18%).11,17 These data formed the basis for the subsequent approvals of furmonertinib as a first-line treatment for patients with advanced EGFR mutation-positive NSCLC by the People's Republic of China National Medical Products Administration.
To further assess the benefit of furmonertinib treatment, PROs were evaluated as a prespecified secondary objective in the FURLONG study to determine whether furmonertinib could reduce disease/treatment burden, improve HRQoL and delay time to deterioration compared with gefitinib.
Methods
Study design and participants
Detailed information about study design, patient eligibility criteria, study protocol and primary efficacy results of the phase 3 FURLONG study (NCT03787992) have been previously published.11 Patients aged 18 years or older who had histologically confirmed locally advanced or metastatic, stage ⅢB, ⅢC, or Ⅳ unresectable NSCLC with tissue biopsy-confirmed EGFR mutation (exon 19 deletions or exon 21 L858R) by a central laboratory were screened.
This study was conducted in accordance with the Good Clinical Practice guidelines (as defined by the International Conference on Harmonization) and the Declaration of Helsinki. All the patients provided written informed consent before any study-specific procedures were performed. The protocol was approved by the institutional review board of the National Cancer Centre/National Clinical Research Centre for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, the People's Republic of China (approval number 18-206/1784), along with all participating centres.
Randomisation and masking
Patients were stratified according to EGFR mutations (exon 19 deletions or exon 21 L858R) and CNS metastases (with or without) and were randomly assigned (1:1) to receive either furmonertinib plus dummy gefitinib placebo or gefitinib plus dummy furmonertinib placebo orally once daily in 21-day cycles until disease progression, occurrence of intolerable toxicities, withdrawal of consent, or other discontinuation reasons judged by the investigators. Randomisation was performed by an independent team from Aikeman (Nanjing, the People's Republic of China) using a central interactive web response system provided by Medidata (New York, NY, USA). Investigators, participants, independent review centre (IRC), and the sponsor were all blinded to the patient's allocation. Unblinding would be done after the primary analysis but could be done beforehand if urgent interventions were required, the EGFR T790M mutation was confirmed after progression, or next-line treatment could be affected by blinding status when the patient did not have the EGFR T790M mutation after progression. In cases of urgent unblinding, treatment information would not be provided to the sponsor.
Procedures
Patients received 80 mg of oral furmonertinib (two tablets taken once daily) plus gefitinib-matching oral placebo once daily or 250 mg of oral gefitinib (one tablet) plus furmonertinib-matching oral placebo once daily in 21-day cycles until disease progression, the occurrence of intolerable toxicities, withdrawal of consent, or other discontinuation reasons judged by the investigators. The doses of furmonertinib or furmonertinib-matching placebo could be reduced by half but dose reduction of gefitinib or gefitinib-matching placebo was not allowed according to the prescribing information of gefitinib. After dose reduction, dose escalation was not permitted.
A valid, reliable simplified Chinese version of the multidimensional, self-administered European Organization for Research and Treatment of Cancer (EORTC) Quality-of-Life Questionnaire Core 30 (QLQ-C30) version 3 and its lung cancer-specific module Quality-of-Life Questionnaire Lung Cancer 13 (QLQ-LC13) were used to evaluate patient-reported symptoms, functioning, and HRQoL of patients.18, 19, 20 PROs were assessed with paper-based questionnaires at randomisation, every first day in cycle 2–6, and every two cycles in cycle 7–18, then every four cycles until treatment discontinuation. The follow-up PRO assessment for patients who discontinued treatment was taken at day 7 after the final dose of the study drug. Patients completed the questionnaires prior to any clinical assessment and disease status discussions with the medical staff.
Outcomes
The primary endpoint of the FURLONG study, which have been published previously,11 was PFS centrally assessed by an IRC in the full analysis set (FAS) population, key secondary endpoints included PFS assessed by investigators; overall survival; objective response rate, disease control rate, duration of response, depth of response, and time to progression as assessed by investigators and the IRC; the type, frequency, severity, and degree of treatment emergent adverse events, according to the Common Terminology Criteria for Adverse Events version 5.0, and their causal relationship with study drugs, as assessed by investigators; and patient-reported outcomes on EORTC QLQ-C30 and EORTC QLQ-LC13.
Statistical analysis
The statistical methods for primary analyses have been described previously.11 The analysis of data on PROs including patients in the FAS population was a prespecified secondary analysis. No power calculation was done for PROs; p values for these analyses all were two-sided and were provided to aid interpretation, but must be interpreted conservatively, given the multiple scales, time points, and hypotheses.
Patients were considered to have completed at least one PRO assessment if they had completed at least one item in a PRO questionnaire. Compliance with the PRO assessments was defined as the proportion of patients who completed at least one item among those expected to complete the questionnaires (i.e., those who remained on treatment and had a scheduled study visit; PRO assessments were no longer expected from patients who had died).21
The EORTC QLQ-C30 and QLQ-LC13 scores were standardized to a scale ranging from 0 to 100 by linear transformation. Higher scores on symptom scales and items represent more/worse symptoms, and higher scores on HRQoL and functioning scales indicate better health status or function. A difference in score of 10 points or more was deemed clinically relevant, corresponding to at least a moderate change in quality of life as reported by patients.22
A longitudinal, mixed model for repeated measure (MMRM) was used to evaluate EORTC QLQ-C30 and QLQ-LC13 score changes from baseline up to, including, end of treatment. Patient was fitted as a random effect, with fixed categorical effects of treatment, visit, and treatment-by-visit interaction as explanatory variables, then baseline score and baseline score-by-visit interaction as continuous fixed covariates. The missing scores were not imputed. The least-squares (LS) means, which were the group mean changes from baseline to a given timepoint after adjusting for covariates, for each scale/item were presented with corresponding 95% CI, and the between-group mean LS differences were estimated and tested.
Time to deterioration (TTD) defined as the time from randomisation until the date of the first clinically relevant deterioration (≥10-point increase for symptom scales; ≥10-point decrease for function scales and HRQoL) or death from any cause, patients had not deteriorated without death were censored at the time when they last completed a PRO assessment. We assessed between-group differences in TTD using a stratified log-rank test, with the HR and 95% CI determined using the COX proportional hazards model stratified by randomisation factors with treatment as a covariate. The Kaplan–Meier method was used to determine the median values and the corresponding two-sided 95% CI.
Statistical analyses were done by use of SAS version 9.2 (SAS Institute, Inc., Cary, NC, USA). This study was registered with ClinicalTrials. gov, NCT03787992.
Role of the funding source
This study was funded by Shanghai Allist Pharmaceuticals Co., Ltd and the National Science and Technology Major Project for Key New Drug Development (2017ZX09304015). The FURLONG study was designed by the sponsor and the principal investigator (Prof Yuankai Shi). The sponsor provided funding and organisational support and had a role in data collection, data analysis, data interpretation, and medical writing. All the authors had access to the raw data, reviewed this report and approved the final submission. The corresponding author (Prof Yuankai Shi) had the final responsibility for decisions related to the submission of the results for publication.
Results
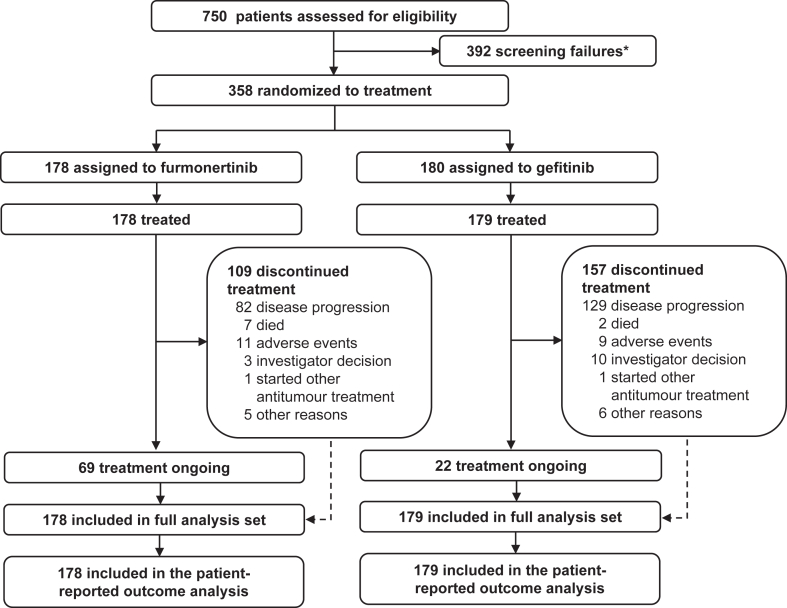
Of 750 patients screened between May 30, 2019, and December 5, 2019, 358 were enrolled; 178 were randomly assigned to the furmonertinib group and 180 to the gefitinib group, and one patient who was randomly assigned to the gefitinib group did not receive the study drug treatment and was excluded from the FAS (Fig. 1). By the data cutoff date of September 15, 2021, median follow-up was 21.0 months (interquartile range [IQR] 18.0–23.5) in the furmonertinib group and 21.0 months (IQR 18.0–23.5) in the gefitinib group. All the patients in the FAS, 178 (100%) and 179 (100%) who received furmonertinib and gefitinib, respectively, had a baseline assessment available for QLQ-C30 and QLQ-LC13 data and were included in the PRO analysis. As previously reported, the baseline demographic and clinical characteristics were well balanced between the groups (Appendix 1 pp 4). Patient-reported HRQoL, functioning, and symptom scores were generally comparable between the two groups at baseline; the most severe symptom at baseline was cough (QLQ-LC13, 33.9 in the furmonertinib group and 30.9 in the gefitinib group) (Table 1).
Fig. 1.
Study profile. ∗Ten patients had two reasons for screening failure.
Table 1.
EORTC QLQ-C30 and QLQ-LC13 scores at baseline.
| Baseline score | Furmonertinib (n = 178) |
Gefitinib (n = 179) |
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| EORTC QLQ-C30 | ||||
| HRQoL | 64.4 | 21.0 | 63.7 | 20.9 |
| Physical functioning | 87.9 | 16.3 | 85.8 | 15.2 |
| Role functioning | 90.2 | 20.2 | 88.6 | 19.0 |
| Emotional functioning | 88.4 | 13.1 | 87.7 | 14.0 |
| Cognitive functioning | 91.4 | 12.6 | 91.7 | 13.5 |
| Social functioning | 83.9 | 22.5 | 82.0 | 21.7 |
| Fatigue | 19.7 | 21.7 | 22.4 | 16.9 |
| Nausea/vomiting | 4.8 | 12.3 | 3.9 | 10.5 |
| Pain | 17.4 | 20.2 | 22.1 | 20.6 |
| Dyspnoea | 17.0 | 24.1 | 17.3 | 19.8 |
| Insomnia | 16.7 | 24.9 | 16.9 | 22.7 |
| Appetite loss | 12.2 | 21.4 | 11.2 | 18.3 |
| Constipation | 6.4 | 15.3 | 7.3 | 15.9 |
| Diarrhoea | 2.1 | 8.0 | 2.6 | 9.6 |
| Financial difficulties | 34.1 | 31.7 | 35.0 | 32.8 |
| EORTC QLQ-LC13 | ||||
| Dyspnoea | 19.8 | 19.5 | 20.7 | 19.7 |
| Cough | 33.9 | 24.9 | 30.9 | 29.6 |
| Haemoptysis | 3.7 | 11.1 | 3.4 | 12.3 |
| Sore mouth | 3.6 | 13.5 | 3.2 | 11.0 |
| Dysphagia | 4.5 | 16.0 | 3.7 | 12.7 |
| Peripheral neuropathy | 7.9 | 17.0 | 11.9 | 21.4 |
| Alopecia | 5.2 | 12.7 | 4.7 | 12.6 |
| Chest pain | 20.2 | 25.9 | 19.0 | 23.2 |
| Pain in arm or shoulder | 15.0 | 22.7 | 19.6 | 26.1 |
| Pain in other parts | 16.7 | 23.4 | 22.7 | 25.8 |
Each scale/item score range is 0–100, where higher scores indicated better functioning or HRQoL in those scales and more/worse symptoms in the symptom scale.
EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire Core 30; EORTC QLQ-LC13, European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire Lung Cancer 13; SD, standard deviation; HRQoL, health-related quality of life.
Compliance with the PRO assessments was at least 95.0% at all on-treatment assessments. High compliance rates were also observed during the follow-up PRO assessments (83.9%–84.3%), which occurred seven days after the final dose of randomised treatment. Patients expected to complete the questionnaires declined faster in the gefitinib group than in the furmonertinib group, aligning with the differences in PFS rates between the two groups (Appendix 1 pp 5).
As previously reported,11 the HRQoL score improved from baseline to randomised treatment discontinuation by 3.80 (95% CI 1.76–5.85) in the furmonertinib group, was similar with 2.60 (95% CI 0.51–4.69) in the gefitinib group, with an adjusted between-group LS mean difference of 1.21 (95% CI −1.71 to 4.13, p = 0.417). Score changes significantly favoured furmonertinib over gefitinib for physical functioning (adjusted between-group mean difference 2.14 [95% CI 0.25–4.04], p = 0.027), nausea/vomiting (−1.56 [95% CI −2.62 to −0.49], p = 0.004), appetite loss (−2.24 [95% CI −4.26 to −0.23], p = 0.029), diarrhoea (−3.36 [95% CI −5.19 to −1.54], p < 0.001, all QLQ-C30), alopecia (−2.62 [95% CI −4.54 to −0.71], p = 0.007), and pain in other parts (any pain other than chest, arm, or shoulder pain; −4.55 [95% CI −7.37 to −1.74], p = 0.002, QLQ-LC13). However, no clinically relevant difference (≥10 points) was found between the furmonertinib and gefitinib group. Clinically relevant overall changes from baseline were observed in cough (LS mean −15.08 [95% CI −17.21 to −12.96] with furmonertinib and −16.28 [95% CI −18.47 to −14.09] with gefitinib) and chest pain (−10.39 [95% CI −12.20 to −8.57] with furmonertinib only), all from QLQ-LC13 (Appendix 1 pp 6 and 7).
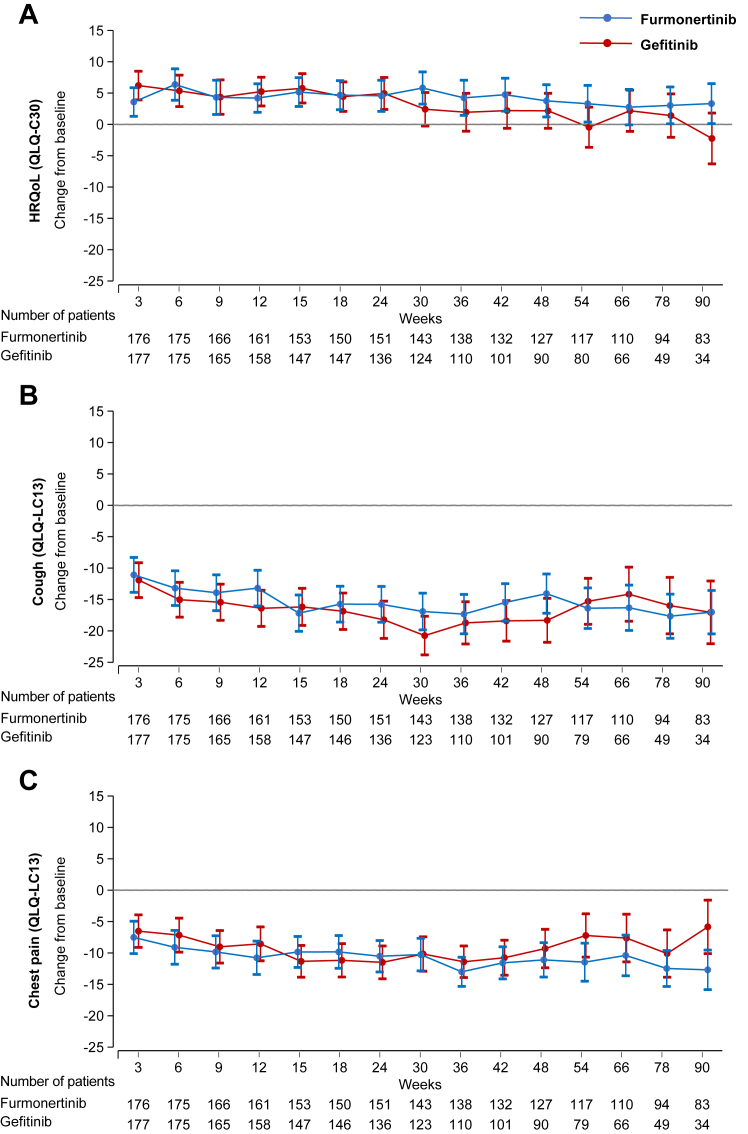
Among the patients who remained on treatment and for whom PRO data were available, the mean QLQ-C30 HRQoL scores improved from baseline to week 24 in both treatment groups (Fig. 2A). Although the scores subsequently declined in both groups, those in the furmonertinib group remained above baseline at week 90 (3.32 [95% CI 0.12–6.51]), whereas those in the gefitinib group fell below baseline (−2.23 [95% CI −6.27 to 1.81]). The difference in adjusted mean changes favoured furmonertinib treatment (5.55 [95% CI 0.41–10.68, p = 0.035]). Clinically relevant improvements in cough were seen as early as week 3 in the two groups (−11.08 [95% CI −13.86 to −8.30] in furmonertinib group and −11.82 [95% CI −14.58 to −9.06] in gefitinib group) and remained stable up to week 90 (−17.02 [95% CI −20.48 to −13.56] and −16.92 [95% CI −21.89 to −11.95]) (Fig. 2B). Both groups reported clinically relevant improvement in chest pain from 12 weeks in the furmonertinib group (−10.77 [95% CI −13.43 to −8.11]) and from 15 weeks in the gefitinib group (−11.26 [95% CI −13.77 to −8.75]), but patients treated in the furmonertinib group improved more over time, and between-group difference was observed at week 90 (−6.89 [95% CI −12.19 to −1.59, p = 0.011]) (Fig. 2C). The change scores of other on-treatment PROs were presented in Appendix 1 (pp 10–21). Clinically relevant improvements were seen in the furmonertinib group for the pain in other parts (week 66 –10.53 [95% CI −13.65 to −7.42]), and financial difficulties (week 78 –11.12 [95% CI −15.21 to −7.03] and week 90 –11.91 [95% CI −16.63 to −7.18]), but not in the gefitinib group.
Fig. 2.
Changes in HRQoL, cough, and chest pain from baseline to week 90. For HRQoL, higher scores denote improved functioning; for symptom scales, higher scores denote worse symptoms. Data are mean change in mixed model for repeated measures-adjusted scores, error bars indicate 95% CI around the mean. Data were (A) HRQoL, (B) cough and (C) chest pain based on relevant items in the QLQ-LC13 and QLQ-C30. HRQoL, health-related quality of life; CI, confidence interval; QLQ-C30, European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire Core 30; QLQ-LC13, European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire Lung cancer 13.
Analyses of changes from baseline to follow-up PRO assessment (seven days after final dose) showed that in patients who discontinued treatment, mean changes from baseline in the EORTC QLQ-C30 and QLQ-LC13 scores were small, whereas the improvements for cough in both treatment groups (−10.08 [95% CI −14.34 to −5.81] with furmonertinib and −13.64 [95% CI −17.31 to −9.97] with gefitinib, from QLQ-LC13), were still clinically relevant. Statistically significant differences favouring furmonertinib for dyspnoea (adjusted between-group mean difference −5.17 [95% CI −10.30 to −0.05], p = 0.048), diarrhoea (−3.65 [95% CI −7.16 to −0.13], p = 0.042, both QLQ-C30), and alopecia (−5.02 [95% CI −8.69 to −1.35], p = 0.008, QLQ-LC13) were not clinically relevant (Appendix 1 pp 8 and 9).
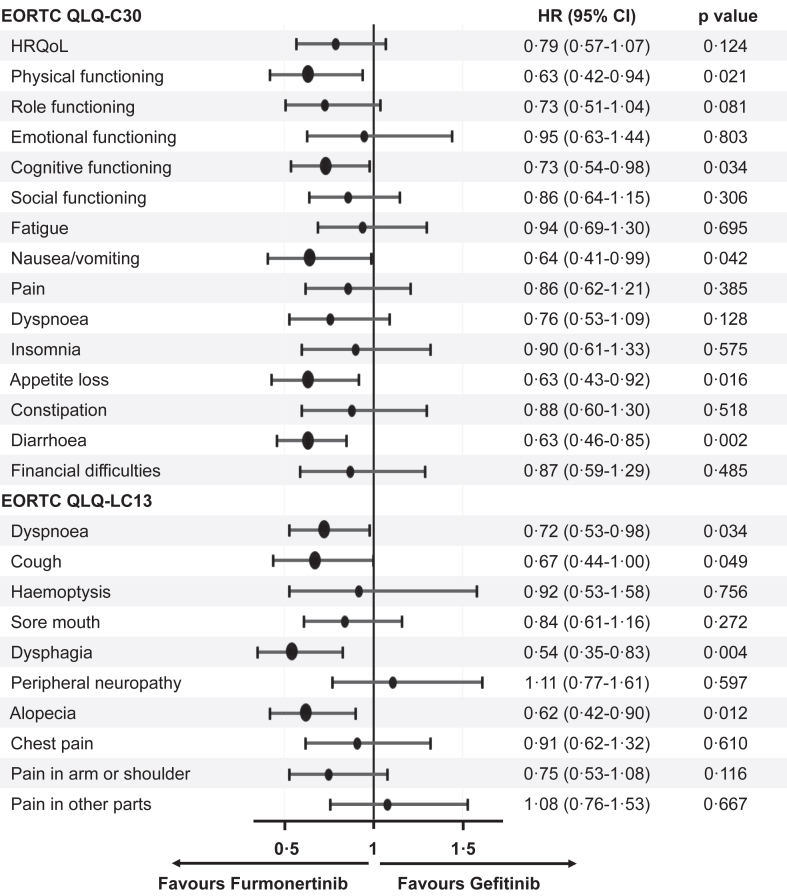
Median time to deterioration of HRQoL was 23.6 months (95% CI 15.0 months to not available [NA]) in the furmonertinib group versus 12.4 months (95% CI 8.4–22.5) in the gefitinib group (HR 0.79 [95% CI 0.57–1.07], p = 0.124), was not significantly different. However, analyses of the time to deterioration in some symptoms and functioning terms showed clinically important between-group differences (Fig. 3). Furmonertinib significantly delayed TTD of physical functioning (median TTD not reached [NR] versus NR, HR 0.63 [95% CI 0.42–0.94], p = 0.021), cognitive functioning (21.6 versus 9.6 months, HR 0.73 [95% CI 0.54–0.98], p = 0.034), nausea/vomiting (NR versus NR, HR 0.64 [95% CI 0.41–0.99], p = 0.042), appetite loss (NR versus 20.9 months, HR 0.63 [95% CI 0.43–0.92], p = 0.016), diarrhoea (NR versus 6.9 months, HR 0.63 [95% CI 0.46–0.85], p = 0.002), dyspnoea (23.6 versus 11.0 months, HR 0.72 [95% CI 0.53–0.98], p = 0.034), cough (NR versus NR, HR 0.67 [95% CI 0.44–1.00], p = 0.049), dysphagia (NR versus NR, HR 0.54 [95% CI 0.35–0.83], p = 0.004), alopecia (NR versus NR, HR 0.62 [95% CI 0.42–0.90], p = 0.012) versus that of gefitinib. Details of TTD were presented in Appendix 1 (pp 22–35).
Fig. 3.
Time to deterioration of HRQoL, functioning, symptoms, and financial difficulty. Data are time to deterioration based on relevant items in the QLQ-LC13 and QLQ-C30, hazard ratios and 95% CI were calculated by use of the COX proportional hazards model, p values were calculated with a stratified log-rank test. HRQoL, health-related quality of life; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire Core 30; HR, hazard ratio; CI, confidence interval; EORTC QLQ-LC13, European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire Lung Cancer 13.
Discussion
In the multicentre, double-blind, double-dummy, randomised phase 3 FURLONG study, a prespecified secondary endpoint analysis showed that, furmonertinib provided improvements compared with gefitinib in the PRO profile, with statistically better mean score changes from baseline up to, including, end of treatment, as well as the significantly delayed time to deterioration of several functioning and symptoms.
Evaluated by HRQoL, functioning and lung cancer symptoms, patient-reported disease burden in both furmonertinib and gefitinib groups were low at baseline compared to available reference values from a normative sample of 1262 unselected patients with NSCLC (1046 patients with stage Ⅲ–Ⅳ disease) from the EORTC Quality of Life Group's Cross-Cultural Analysis Project,23, 24, 25 but were mostly comparable with the reported reference baseline data of treatment-naïve advanced NSCLC from the FLAURA, CROWN, KEYNOTE-024, and KEYNOTE-189 studies.26, 27, 28, 29 Owing to the low baseline disease burden in these patients receiving first-line treatment for NSCLC, there were technical challenges in the measurement of improvements of both between groups and comparing with baseline to obtain a difference in score of 10 points or more which deemed clinically relevant.26, 27, 28, 29 Our findings for furmonertinib relative to gefitinib under first-line NSCLC setting in overall score changes for HRQoL, functioning and lung cancer symptoms were in line with those obtained for osimertinib from the FLAURA study as differences that were both statistically significant and clinically relevant were not observed in favour of either treatment group.26 However, in a previous study, the median TTD was similar between osimertinib and first-generation TKI, with overlapping 95% CIs for medians in lung cancer symptoms including cough, dyspnoea, chest pain, fatigue and appetite loss.26 Whereas in FURLONG study, with median follow-up of 21.0 months at data cutoff, furmonertinib significantly delayed median TTD of lung cancer symptoms including cough (NR [95% CI NA to NA] versus NR [21.0 to NA], HR 0.67 [95% CI 0.44–1.00], p = 0.049), dyspnoea (23.6 [95% CI 18.1 to NA] versus 11.0 months [95% CI 3.5 to NA], HR 0.72 [95% CI 0.53–0.98], p = 0.034), appetite loss (NR [95% CI 23.7 to NA] versus 20.9 months [95% CI 17.7 to NA], HR 0.63 [95% CI 0.43–0.92], p = 0.016) compared with gefitinib, as well as of physical functioning (NR [95% CI 23.8 to NA] versus NR [95% CI 20.5 to NA], HR 0.63 [95% CI 0.42–0.94], p = 0.021) and cognitive functioning (21.6 [95% CI 8.5 to NA] versus 9.6 months [95% CI 5.6–17.8], HR 0.73 [95% CI 0.54–0.98], p = 0.034). These findings underscored the efficacy benefits previously observed with furmonertinib in this double-blind, randomised study.
Further analyses of on-treatment PRO assessments including time points up to 90 weeks revealed that improvements from baseline (in which 95% CI did not cross zero) in HRQoL, functioning and lung cancer symptoms were seen as early as week 3 in both groups, and reached the maximum effect interval around week 12. Although the score changes showed variability at individual time points, most improved scores tended to fallback after 30–48 weeks with gefitinib, whereas the improvements persisted in the furmonertinib group through 90 weeks, and treatment differences favouring furmonertinib tended to increase over time. In this study, treatment was often discontinued at the time of disease progression. Analyses evaluating data from patients who discontinued randomised treatment were done to estimate the disease burden of progressed patients and showed that there was no clinically relevant deterioration over baseline in either group. Meanwhile, differences between the two groups observed in dyspnoea, diarrhoea and alopecia still favoured furmonertinib.
A published primary analysis of FURLONG demonstrated that 11% and 18% of patients experienced TRAEs of grade 3 or higher with furmonertinib and gefitinib, respectively, but a relatively low prevalence of treatment-related elevated alanine/aspartate aminotransferase, rash and diarrhoea was observed in the furmonertinib group.11 Here, we reported that furmonertinib resulted in statistically significant better overall score changes from baseline compared with gefitinib in several treatment-related symptoms for multiple gastrointestinal scales, including diarrhoea and nausea/vomiting. Unsurprisingly, the TTD in the furmonertinib group was significantly longer for these gastrointestinal scales. Meanwhile, we found that patients in the furmonertinib group reported less trouble with alopecia as well as a significantly longer TTD. However, there was no question focused on rash or pruritus in the EORTC QLQ-C30 and QLQ LC-13 and further cutaneous relevant analyses could not be conducted. These findings of PROs captured patient perspectives on treatment burden and further substantiated the clinical meaningfulness of the superiority in the safety of first-line furmonertinib in patients with advanced EGFR mutation-positive NSCLC.
FLAURA2, a recent first-line therapy study for patients with locally advanced or metastatic EGFR mutation-positive NSCLC presented PRO data at the European Lung Cancer Congress 2024, showed that there were non-clinically meaningful improvements in HRQoL, physical functioning, fatigue, appetite loss from QLQ-C30, and chest pain, dyspnoea from QLQ-LC13, in both the osimertinib-chemotherapy group and osimertinib group. Meanwhile, the median TTD of HRQoL, cough and dyspnoea was similar between the two groups.30 Despite the notable differences in safety, particularly in the rate of adverse events of grade 3 or higher (64% in the osimertinib-chemotherapy group and in 27% in the osimertinib group), the patient-reported outcomes suggest that the combination of osimertinib and chemotherapy did not significantly impact the patients' quality of life, these finding challenges what we learned in AURA3 study to some extent.31 Notably, open label trials like FLAURA2 and AURA3 had increasingly published separate PRO articles to provide a comprehensive and systematic presentation of PRO analysis. On the other hand, double-blind studies had seldom published PRO articles as standalone publications. However, potentially overly optimistic reports of PROs among patients in the investigational arm and overly pessimistic reports for patients in the control arm might resulting larger treatment effects for PRO outcomes in open-label studies.32,33
Strengths of this study included the high-quality, high-compliance PRO data collected in both treatment groups throughout the study including end of treatment in a blinded fashion. Several measures were taken in the FURLONG study to ensure the validity and robustness of PRO results. Patients were requested to complete the questionnaires independently at the study sites prior to any clinical assessment or disease status discussion with medical staff as the first step of each follow-up visit to avoid bias. PRO assessments were scheduled throughout the study treatment, including a PRO assessment for patients who discontinued treatment, seven days after the final dose. High compliance rates for questionnaire completion minimized the occurrence of missing data, with at least 95% of the patients in both treatment groups completing the questionnaires at most time points.
This study had some limitations. PRO assessments beyond seven days following the final dose of randomised treatment were not collected, and a very small amount of missing data was still unavoidable despite careful planning and collection strategies. Therefore, the results must be interpreted as PROs while patients were on randomised treatment and up until the point of randomised treatment discontinuation. In this study, treatments were mostly discontinued at the time of disease progression, meaning that symptom deterioration beyond disease progression was not taken into account by the analysis. Nevertheless, the collection of PRO data after disease progression might have had an effect on data interpretation because of variations in post disease progression treatments. Moreover, the PROs were secondary endpoint measures with no power calculation lacked formal statistical testing, and we presented a very large number of analyses based on multiple PRO sub-scales or multiple assessment points of a PRO scale. Therefore, these PRO results should be interpreted with caution and there was a substantial multiple comparisons problem. Finally, the commonly used cancer-targeted PRO instruments including the EORTC QLQ-C30 and QLQ-LC13 were developed in a prior therapeutic era dominated by cytotoxic chemotherapy. The questions were fixed, irrespective of the disease stage or therapies being studied. Some targeted therapy associated symptoms (pruritus and rash) were not considered by these instruments. The lack of flexibility, especially with respect to symptoms toxicities, could be problematic in an era of novel therapies with multiple mechanistic classes and unique symptomatic adverse events, and it is important to note that the criterion of 10-point change in score commonly accepted as the minimal clinically important difference in phase 3 advanced NSCLC trials was derived from a study conducted in 1990s.22,26, 27, 28, 29, 30, 31 The development of a new PRO instrument, as well as a study on the interpretation of changes in HRQoL based on new treatment scenarios and patient perspectives might be warranted.15
In conclusion, furmonertinib demonstrated statistically significant improvements in physical functioning, nausea/vomiting, appetite loss, diarrhoea, alopecia, and pain in other parts, as well as delayed deterioration in physical functioning, cognitive functioning, nausea/vomiting, appetite loss, diarrhoea, dyspnoea, cough, dysphagia, and alopecia compared to gefitinib. Together with the previously published superior PFS and manageable safety profile, furmonertinib had a significantly superior benefit compared to gefitinib as first-line therapy in Chinese patients with locally advanced or metastatic EGFR mutation-positive NSCLC.
Contributors
Yuankai Shi was the leading principal investigator and designed the study with the sponsor. Yuankai Shi, Gongyan Chen, Xiang Wang, Yunpeng Liu, Lin Wu, Yanrong Hao, Chunling Liu, Shuyang Zhu, Xiaodong Zhang, Yuping Li, Jiwei Liu, Lejie Cao, Ying Cheng, Hui Zhao, Shucai Zhang, Aimin Zang, Jiuwei Cui, Jian Feng, and Nong Yang contributed to patient recruitment and data acquisition. Yuankai Shi, Fei Liu, Yong Jiang, and Nan Ge accessed and verified the data. Jie Hu, Fei Liu, Yong Jiang, and Nan Ge were involved in data analysis and data interpretation. Nan Ge did the manuscript writing. Yuankai Shi revised and editing the manuscript. All authors reviewed and approved the final version of the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing statement
The data generated and analysed in this study are on file with Shanghai Allist Pharmaceuticals Co., Ltd and are not publicly available according to company policy.
Declaration of interests
JH, FL, YJ and NG are employees and shareholders of Shanghai Allist Pharmaceuticals Co., Ltd. All other authors declare no competing interests.
Acknowledgements
This study was funded by Shanghai Allist Pharmaceuticals Co., Ltd and the National Science and Technology Major Project for Key New Drug Development (2017ZX09304015). The authors thank all the hospitals that participated and contributed to patient recruitment, the patients who participated in this study and their families, and participating study teams. Medical writing assistance for this manuscript was provided by Maggie Li from YY Science funded by Shanghai Allist Pharmaceuticals Co., Ltd. The authors also thank Dr. Zucheng Xie and Dr. Haohua Zhu (National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College) for the editing assistance of this manuscript.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2024.101122.
Appendix A. Supplementary data
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Shi Y., Au J.S.-K., Thongprasert S., et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER) J Thorac Oncol. 2014;9:154–162. doi: 10.1097/JTO.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mok T.S., Wu Y.-L., Thongprasert S., et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 4.Mitsudomi T., Morita S., Yatabe Y., et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 5.Zhou C., Wu Y.-L., Chen G., et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 6.Shi Y.K., Wang L., Han B.H., et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28:2443–2450. doi: 10.1093/annonc/mdx359. [DOI] [PubMed] [Google Scholar]
- 7.Wu Y.-L., Zhou C., Hu C.-P., et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 8.Yang J.C.-H., Wu Y.-L., Schuler M., et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141–151. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 9.Soria J.-C., Ohe Y., Vansteenkiste J., et al. Osimertinib in untreated EGFR -mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 10.Lu S., Dong X., Jian H., et al. AENEAS: a randomized phase III trial of aumolertinib versus gefitinib as first-line therapy for locally advanced or MetastaticNon-small-cell lung cancer with EGFR exon 19 deletion or L858R mutations. J Clin Oncol. 2022;40:3162–3171. doi: 10.1200/JCO.21.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Y., Chen G., Wang X., et al. Furmonertinib (AST2818) versus gefitinib as first-line therapy for Chinese patients with locally advanced or metastatic EGFR mutation-positive non-small-cell lung cancer (FURLONG): a multicentre, double-blind, randomised phase 3 study. Lancet Respir Med. 2022;10:1019–1028. doi: 10.1016/S2213-2600(22)00168-0. [DOI] [PubMed] [Google Scholar]
- 12.Kleijnen S., Leonardo Alves T., Meijboom K., et al. The impact of quality-of-life data in relative effectiveness assessments of new anti-cancer drugs in European countries. Qual Life Res. 2017;26:2479–2488. doi: 10.1007/s11136-017-1574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oderda G., Brixner D., Biskupiak J., et al. Payer perceptions on the use of patient-reported outcomes in oncology decision making. J Manag Care Spec Pharm. 2022;28:188–195. doi: 10.18553/jmcp.2021.21223. [DOI] [PubMed] [Google Scholar]
- 14.Gralla R.J., Hollen P.J., Msaouel P., Davis B.V., Petersen J. An evidence-based determination of issues affecting quality of life and patient-reported outcomes in lung cancer: results of a survey of 660 patients. J Thorac Oncol. 2014;9:1243–1248. doi: 10.1097/JTO.0000000000000244. [DOI] [PubMed] [Google Scholar]
- 15.Kluetz P.G., Slagle A., Papadopoulos E.J., et al. Focusing on core patient-reported outcomes in cancer clinical trials: symptomatic adverse events, physical function, and disease-related symptoms. Clin Cancer Res. 2016;22:1553–1558. doi: 10.1158/1078-0432.CCR-15-2035. [DOI] [PubMed] [Google Scholar]
- 16.Shi Y., Zhang S., Hu X., et al. Safety, clinical activity, and pharmacokinetics of alflutinib (AST2818) in patients with advanced NSCLC with EGFR T790M mutation. J Thorac Oncol. 2020;15:1015–1026. doi: 10.1016/j.jtho.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Shi Y., Chen G., Wang X., et al. Central nervous system efficacy of furmonertinib (AST2818) versus gefitinib as first-line treatment for EGFR-mutated NSCLC: results from the FURLONG study. J Thorac Oncol. 2022;17:1297–1305. doi: 10.1016/j.jtho.2022.07.1143. [DOI] [PubMed] [Google Scholar]
- 18.Aaronson N.K., Ahmedzai S., Bergman B., et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 19.Bergman B., Aaronson N.K., Ahmedzai S., Kaasa S., Sullivan M. The EORTC QLQ-LC13: a modular supplement to the EORTC core quality of life questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC study group on quality of life. Eur J Cancer. 1994;30A:635–642. doi: 10.1016/0959-8049(94)90535-5. [DOI] [PubMed] [Google Scholar]
- 20.Wan C., Zhang C., Tu X., et al. Validation of the simplified Chinese version of the quality of life instrument EORTC QLQ-LC43 for patients with lung cancer. Cancer Invest. 2008;26:504–510. doi: 10.1080/07357900701781788. [DOI] [PubMed] [Google Scholar]
- 21.Coens C., Pe M., Dueck A.C., et al. International standards for the analysis of quality-of-life and patient-reported outcome endpoints in cancer randomised controlled trials: recommendations of the SISAQOL Consortium. Lancet Oncol. 2020;21:e83–e96. doi: 10.1016/S1470-2045(19)30790-9. [DOI] [PubMed] [Google Scholar]
- 22.Osoba D., Rodrigues G., Myles J., Zee B., Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 23.Scott N.W., Fayers P., Aaronson N.K., et al. EORTC quality of life group. 2nd ed. 2008. EORTC QLQ-C30 reference values manual.https://www.eortc.org/app/uploads/sites/2/2018/02/reference_values_manual2008.pdf Available at: [Google Scholar]
- 24.Scott N.W., Fayers P.M., Bottomley A., et al. Comparing translations of the EORTC QLQ-C30 using differential item functioning analyses. Qual Life Res. 2006;15:1103–1115. doi: 10.1007/s11136-006-0040-x. [DOI] [PubMed] [Google Scholar]
- 25.Scott N.W., Fayers P.M., Aaronson N.K., et al. The use of differential item functioning analyses to identify cultural differences in responses to the EORTC QLQ-C30. Qual Life Res. 2007;16:115–129. doi: 10.1007/s11136-006-9120-1. [DOI] [PubMed] [Google Scholar]
- 26.Leighl N.B., Karaseva N., Nakagawa K., et al. Patient-reported outcomes from FLAURA: osimertinib versus erlotinib or gefitinib in patients with EGFR-mutated advanced non-small-cell lung cancer. Eur J Cancer. 2020;125:49–57. doi: 10.1016/j.ejca.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Mazieres J., Iadeluca L., Shaw A.T., et al. Patient-reported outcomes from the randomized phase 3 CROWN study of first-line lorlatinib versus crizotinib in advanced ALK-positive non-small cell lung cancer. Lung Cancer. 2022;174:146–156. doi: 10.1016/j.lungcan.2022.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Brahmer J.R., Rodríguez-Abreu D., Robinson A.G., et al. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): a multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol. 2017;18:1600–1609. doi: 10.1016/S1470-2045(17)30690-3. [DOI] [PubMed] [Google Scholar]
- 29.Garassino M.C., Gadgeel S., Esteban E., et al. Patient-reported outcomes following pembrolizumab or placebo plus pemetrexed and platinum in patients with previously untreated, metastatic, non-squamous non-small-cell lung cancer (KEYNOTE-189): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:387–397. doi: 10.1016/S1470-2045(19)30801-0. [DOI] [PubMed] [Google Scholar]
- 30.Lee C.K., Cheng Y., Laktionov K., et al. 9P First-line (1L) osimertinib (osi) ± platinum-pemetrexed in EGFRm advanced NSCLC: FLAURA2 patient-reported outcomes (PROs) ESMO Open. 2024;9 [Google Scholar]
- 31.Lee C.K., Novello S., Rydén A., Mann H., Mok T. Patient-reported symptoms and impact of treatment with osimertinib versus chemotherapy in advanced non-small-cell lung cancer: the AURA3 trial. J Clin Oncol. 2018;36:1853–1860. doi: 10.1200/JCO.2017.77.2293. [DOI] [PubMed] [Google Scholar]
- 32.Roydhouse J.K., Fiero M.H., Kluetz P.G. Investigating potential bias in patient-reported outcomes in open-label cancer trials. JAMA Oncol. 2019;5:457–458. doi: 10.1001/jamaoncol.2018.6205. [DOI] [PubMed] [Google Scholar]
- 33.Wood L., Egger M., Gluud L.L., et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008;336:601–605. doi: 10.1136/bmj.39465.451748.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.