Abstract
Superoxide dismutase (SOD) is a common antioxidant enzyme found majorly in living cells. The main physiological role of SOD is detoxification and maintain the redox balance, acts as a first line of defence against Reactive nitrogen species (RNS), Reactive oxygen species (ROS), and other such potentially hazardous molecules. SOD catalyses the conversion of superoxide anion free radicals (O 2 -.) into molecular oxygen (O 2) and hydrogen peroxide (H 2O 2) in the cells. Superoxide dismutases (SODs) are expressed in neurons and glial cells throughout the CNS both intracellularly and extracellularly. Endogenous oxidative stress (OS) linked with enlarged production of reactive oxygen metabolites (ROMs), inflammation, deregulation of redox balance, mitochondrial dysfunction and bioenergetic crisis are found to be prerequisite for neuronal loss in neurological diseases. Clinical and genetic studies indicate a direct correlation between mutations in SOD gene and neurodegenerative diseases, like Amyotrophic Lateral Sclerosis (ALS), Huntington’s disease (HD), Parkinson’s Disease (PD) and Alzheimer’s Disease (AD). Therefore, inhibitors of OS are considered as an optimistic approach to prevent neuronal loss. SOD mimetics like Metalloporphyrin Mn (II)-cyclic polyamines, Nitroxides and Mn (III)- Salen complexes are designed and used as therapeutic extensively in the treatment of neurological disorders. SODs and SOD mimetics are promising future therapeutics in the field of various diseases with OS-mediated pathology.
Keywords: Superoxide anions, Reactive oxygen species, Superoxide dismutase, Neurological disorders, Neurodegenerative diseases, And oxidative stress
1. Introduction
Superoxide dismutase (SOD) is an indigenous antioxidant enzyme, belongs to a class of oxidoreductase which has metal cofactor at their catalytic core (Ighodaro and Akinloye, 2018). SODs are found in the cellular components of almost all living cells, and acts as the first enzymatic antioxidant against oxidative stress (OS) (Islam et al., 2022). SOD catalyses the dismutation of potentially hazardous 2 molecules of superoxide anion (O2) into less harmful oxygen molecules (O2) as well as Hydrogen peroxide (H2O2), which needs a metal cofactor for its antioxidant activity (van der Vliet, 2015). Manganese (Mn), Zinc (Zn), Iron (Fe), and Copper (Cu) are the metal ions that normally bound to SOD in the active site. Based on the kind of metal ions required as a cofactor, superoxide dismutase (SODs) are categorized into three different forms (van der Vliet, 2015). They are (i) Fe-SOD - normally found in chloroplast of number of plants and prokaryotes. (ii) Mn-SOD - found in mitochondria, peroxisome of eukaryotes and prokaryotes. (iii) Cu/Zn-SOD- found in eukaryotes and mostly dispersed essentially in cytosol, and also found in peroxisomes and chloroplasts (Gill and Tuteja, 2010, Karuppanapandian et al., 2011). These various forms of SODs have distinct subcellular localizations. Mainly, intracellular SODs are located in the cytosol, mitochondria, glycosome, and endoplasmic reticulum to remove the toxic actions of superoxide anion radicals (O2•-) and the SODs located in the extracellular matrix and fluids protect the cells from superoxide released by pathogens, and thus SOD is often marked as a strong free radical scavenger (Schatzman et al., 2020, Tamayo et al., 2016). Cu/Zn-containing SOD (SOD1) predominantly found in the cytosol, Mn-containing SOD (SOD2) mainly found in mitochondria and Cu-Zn-containing SOD (SOD3) found in extracellular tissues and fluids (Azadmanesh and Borgstahl, 2018).
1.1. Superoxide anions radical
Superoxide anions radical (O2•-) and its derivatives are generated as a by-product of electron “leak” from respiratory transport chain in the mitochondria (Hayyan et al., 2016, Maier and Chan, 2002). In addition, superoxide radicals are also formed by mitochondrial enzymes, nicotinamide adenine dinucleotide phosphate oxidase, xanthine oxidase, and lipoxygenase, nitric oxide (NO) synthase. During catalytic actions, ribonucleotide reductase uses a free radical at its active site, while activated phagocytes create O2•- during bactericidal actions. Autooxidation of thiols (e.g., glutathione, cysteine), ascorbic acid, flavin coenzymes, and adrenaline also generate superoxide radicals (Cheeseman and Slater, 1993, Wong et al., 2017). In moderate levels, ROS contribute to differentiation and proliferation of healthy neural stem cells, the modulation of redox-sensitive proteins, and promote long-term potentiation (Garbarino et al., 2015). Recently, two-photon imaging techniques have shown the presence of endoplasmic reticulum specific O2•- in the lives cells (Zuo et al., 2020).
Increased production of superoxide radicals indiscriminately induce oxidative damage via several mechanisms such as initiation of lipid peroxidation, inactivation of antioxidant enzymes, attacks phospholipids, proteins and DNA, and damages protein sulfhydryl oxidation leading to neuronal loss and reactive gliosis resulting in impairments in neuronal function and cognition, even cell death (Peluffo et al., 2005, Zhang et al., 2022).
1.2. Physiological role of SODs
SOD protects the cells from oxidative stress by neutralising the superoxide radicals. It converts the O2•- into H2O2 and O2 (I, 1986, McCord and Fridovich, 1988). Then, H2O2 gets converted to hydroxyl radical (OH-), or associated metal related reactive species, in the existence of increased transition metal (Fe2+, Cu+)·H2O2 traverse cell membranes easily and gets converted into H2O by catalase (CAT) (the inner mitochondrial membrane and peroxisomes possess CAT, which is a heme protein) or by (selenium-containing) glutathione peroxidase (GPX; belong to family of cytosolic selenoenzymes) (Benzi and Moretti, 1995). Although, O2•-. is not extremely dangerous on its own, its reaction with NO (formed during the translation of L-arginine to L-citrulline) produces the powerful oxidant, peroxynitrite (ONOO-), which contributes to neuropathological changes by nitrating tyrosine (Peluffo et al., 2004). Peroxynitrite prevents several important physiological functions of NO- such as neurotransmission, vasorelaxation, immune responses, regulation of cell proliferation, gut peristalsis, penile erection and olfactory memory (Groves and Wang, 2000, Huang, 1999, Springall, 1995). SODs protect nitric oxide from oxidation by superoxide anion. In view of these neutralising effects, SOD is considered as the front line of enzymatic defence against toxic superoxide anion radicals by regulating O2•-, H2O2, and obtainable NO (Fig. 1). SODs also act as strong anti-inflammatory agent and prevent the growth of cancerous cells (Yasui and Baba, 2006), thus considered as a potential therapeutic target. These reports show that SODs are considered as key enzymes involved in the detoxification of superoxide radicals and act as an important antioxidant defence against OS in the body..
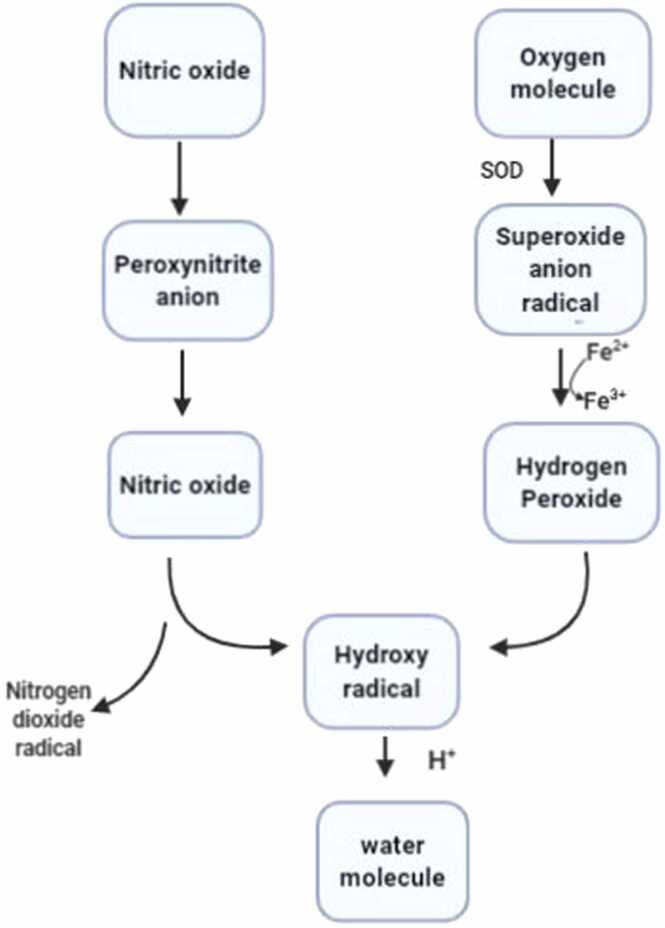
Fig. 1.
Generation of free radicals from an oxygen molecule. Oxygen molecule is converted into Superoxide anion radical by Superoxide Dismutase. This is followed by conversion into hydrogen peroxide through reduction of Ferrous ion to Ferric ion. Simultaneously Nitric oxide is converted into peroxynitrite anion and then to nitric oxide. Both Hydrogen peroxide and Nitric oxide are converted into hydroxy radical and results in formation of a water molecule aby means of hydrogen ion.
2. Classification of SODs (Table 1)
Table 1.
List of SODs and their location and functions.
| Isoform | Characteristics | Metal cofactor | Function | Location | Reference |
|---|---|---|---|---|---|
| SOD1 (Cu/ ZnSOD) |
32 kDa, homodimer | Cu2 + (catalytic) Zn2 + (stability |
Zn is responsible for the correct protein folding and stability | Cytoplasm, mitochondrial IMS. and others (nucleus, lysosomes, peroxisomes) |
(Chang et al., 1988) |
| SOD2 (MnSOD) |
96 kDa, homotetramer | Mn3+ (catalytic) | At the active site of SOD2, Mn acts to catalyse the disproportionation of O2-to oxygen and hydrogen per oxide | Mitochondria matrix | |
| SOD3 Extracellular SOD (ecSOD) |
135 kDa, homotetrameric secretory glycoprotein |
Cu2 + (catalytic) Zn2 + (stability) |
It binds to heparin sulfate proteoglycan (HSPGs), collagen, and fibulin-5 eventually directed to the extracellular matrix and endothelial cell surface | Extracellular matrix, cell surface, extracellular fluids |
2.1. SOD1 (Cu/Zn-SOD)
Cu/Zn-SOD, a key intracellular enzyme present as a 32 kDa homodimer consisting of 153 amino acids, and 21q22.1 region of chromosome 21 of the human genome code for SOD1 gene (Levanon et al., 1985). It is found throughout the cytoplasm, while relatively in traces in the mitochondrial intermembrane space (Crapo et al., 1992, Okado-Matsumoto and Fridovich, 2001, Sturtz et al., 2001). Immunocytochemical analysis have revealed the presence of SOD1 in the nuclei, lysosomes, and peroxisomes of mammalian cells (Chang et al., 1988). In contrast to SOD2, SOD1 is comparatively resistant to cyanide. The enzymatic activity of SOD1 is contributed by the metal ions such as Zn and Cu. Both the placement and quantity of Zn and Cu ions are important for SOD1 activity, as Zn is responsible for the correct protein folding and stability, while the antioxidant activity of SOD1 and remetallated derivatives of SOD is directly relative to the quantity of Cu at its natural metal binding site Cobalt and Cu may replace Zn, and is generally not required for performing enzymatic activity at a lower pH, whereas Cu is irreplaceable with any metals (Babu and Ramanathan, 2011a, Jl et al., 1996, Valentine et al., 1979). The actions of SOD1 are varied such as modifying cellular respiration, scavenging excessive O2•-, posttranslational modifications, and energy metabolism (Saccon et al., 2013).
2.2. SOD2 (Mn-SOD)
SOD2 is a Mn-containing isoenzyme consisting of 96 kDa homotetramer located in the mitochondrial matrix of prokaryotes and eukaryotes. The continuous production of ROS by mitochondrial respiratory chain justifies the presence of SOD2 in the mitochondria to efficiently eliminate superoxide generated from molecular oxygen (van der Vliet, 2015, Weisiger and Fridovich, 1973). Active site of SOD2 is not similar to SOD1, but shows homology to Fe-SOD, which is generally not present in eukaryotes (Borgstahl et al., 1992). SOD2 present in CNS is found to be altered under neuroinflammatory conditions. In active site of SOD2, Mn acts to catalyse the disproportionation of O2•- to oxygen and H2O2, which is found to be similar to SOD1 and SOD3 (Cu/Zn SODs) (Jl et al., 1996). SOD2 is formed in the cytoplasm and transferred to mitochondria by a signal peptide, and then dismutates O2•- produced by respiratory chain enzymes.
2.3. SOD3 (extracellular Cu/Zn-SOD and ecSOD)
SOD3 an extracellular form of Cu/Zn-containing SOD found in eukaryotes. SOD3 is present chiefly in the extracellular matrix of tissues (about 90–99%) and on cell surfaces with less amount in the extracellular fluids and plasma. SOD3 is the main member of SOD family and also known as secretory extracellular Cu/Zn-containing SOD (ecSOD). In a large group of species, SOD3 is present as 135 kDa homotetramer containing two disulphide-linked dimers with a high affinity for heparin (Marklund, 1984a, Marklund, 1984b). Although the tissue distribution of SOD3 varies in different species, in general, it is produced in higher concentrations in tissues like lung, uterus, kidneys, blood vessels, and to a smaller fraction, in the heart (Folz and Crapo, 1994, Strålin et al., 1995, T et al., 1998). Comparable to SOD1, SOD3 enzyme is also sensitive to cyanide. It is mainly produced by fibroblasts in the vascular tissue and vascular smooth muscle cells. SOD3 is also found in atherosclerosis, injured tissue and inflammatory cells (Fukai et al., 1998, Luoma et al., 1998, Tan et al., 2006). SOD3 possess a heparin-binding domain and has high affinity to heparin sulfate proteoglycan, fibulin-5 on the cell surface, collagen, and binds to these molecules directed to the extracellular matrix and endothelial cell surface (Fukai et al., 2002, Nguyen et al., 2004, Petersen et al., 2004). SOD3 is a regulator of NO bioavailability (Pineda et al., 2001), therefore play a crucial role in cerebral vascular and vasomotor functions (Nakane et al., 2001).
2.4. Iron SOD (Fe SOD)
Fe SODs consist of ancient group of SOD, and they are present in both prokaryotes and eukaryotes. It is a tetradentate protein that consists of 3 histidine and 1 aspartic acid donor groups stabilizing the iron ion that is bound in the active site of the enzyme (Stephenie et al., 2020). Fe SOD consist of two distinct groups, first is a homodimer formed with two similar 20 kDa subunit protein containing 1–2 g atom of iron in active site. Second group is present in plants and is a tetramer with four equal subunits of 80–90kDA molecular weight and 2–4 g atom of iron in active centre (Alscher et al., 2002).
2.5. SOD -nanozyme
Nanozymes are nanomaterials that possess activity similar to enzymes, they are recently explored as they have many advantages in comparison to natural enzymes (Zhang et al., 2020). Since SOD possess several limitations, nanozymes that has SOD like activity has been explored. The commonly available nanozymes having SOD like activity are cerium oxide nanoparticles, fullerenes (carbon materials) etc. SOD nanozymes has been used in disease as in possess anti-oxidant property (Zhao et al., 2021). Instillation of both native SOD1 and its nanozyme revealed that nanozyme showed more effective anti-inflammatory effect than native SOD1 in an in-vivo rabbit model of immunogenic uveitis (Kost et al., 2016).
Further, there are several other applications of SOD nanozymes and still it has to be explored well. The conversion of superoxide anion (O2•-) to O2 and H2O2 is catalysed by SOD nanozymes thereby eliminating reactive nitrogen species (RNS) and reactive oxygen species (ROS). Also, they play a vital role in preventing oxidative stress and protects the cells, inhibit inflammatory response and prevent aging (Yang et al., 2022). A cerium doped zeolite-based nanomaterial (Ce/Zeo-NMs) was designed by Huang et al. to protect against ischemic stroke by improving blood brain barrier (BBB) integrity and inhibition of astrocytes and microglia in a rat model of middle cerebral artery occlusion-reperfusion (MCAO/R) (Huang et al., 2022). Boehmite amino nanozyme (BNP) possess SOD- like activity, thereby it scavenges mitochondrial reactive oxygen species (mito ROS) and Cu2+ which disaggregates mutant HTT deposit in the cells. This implies that boehmite nanozymes can be used for treating Huntington disease (HD) (Martínez-Camarena et al., 2022). Prussian blue nanozyme (PBzyme) improves motor function, reduces mitochondrial membrane potential damage, and recovers dopaminergic neurons. In an MPTP-induced Parkinson's disease animal model, intracerebroventricular injection of PBzyme decreases dopaminergic degradation and inhibits neuroinflammation by downregulating the activation of microglial nucleotide-binding domain and leucine-rich repeat family pyrin domain containing 3 (NLRP3) inflammasomes and caspase-1 by scavenging reactive oxygen species which ultimately leads to microglia pyroptosis (Ma et al., 2022).
These data suggest that nanozymes can be used as a potential therapeutic strategy for neurodegenerative diseases as they regulate ROS levels and thereby supressing misfolded protein aggregates (Zhang et al., 2023).
3. SOD and central nervous system
Brain cells are more susceptible to oxidative insult due to their high metabolic rate, increased oxygen consumption, and weak antioxidants defence (Garbarino et al., 2015, Hulbert et al., 2007). Increased levels of omega-3- polyunsaturated fatty acids in brain tissue is responsible for the mass-specific metabolic rate (Pifferi et al., 2021). Moreover, presence of high levels of phospholipids as well as redox-active iron and copper further enhances its susceptibility to peroxidation (Fig. 2). It is very difficult to restore the terminally differentiated neurons and glial cells present in the brain from oxidative damage (Hulbert et al., 2007). In fact central nervous system (CNS) of both rodents and humans have revealed the distribution of SODs in the neurons and reactive glial cells such as astrocytes present in brain and spinal cord (Furuta et al., 1995, Kim et al., 2000, Liu et al., 1994, Moreno et al., 1997, Pardo et al., 1995, Peluffo et al., 2005, Thaete et al., 1986). Supportively, brain-specific SODs are reported to play a chief part in regulating the redox balance and protecting the brain from oxidative injury (Fukai and Ushio-Fukai, 2011).
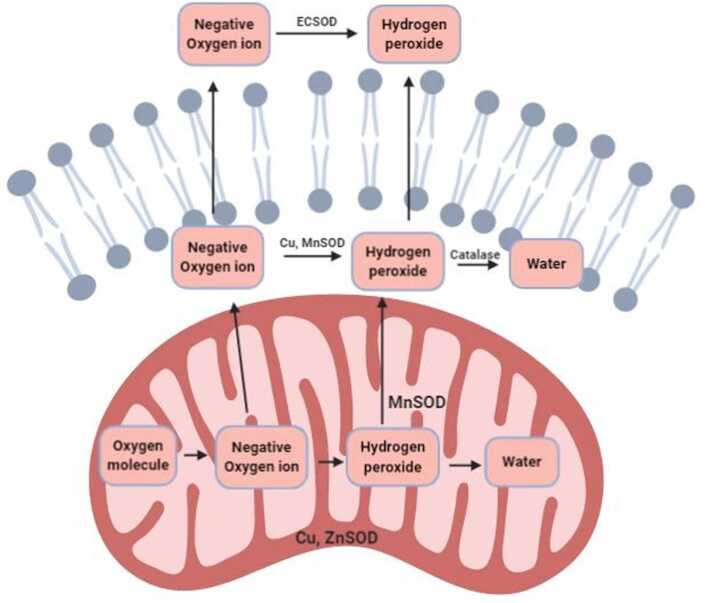
Fig. 2.
Conversion of water from oxygen radical in a healthy mitochondrion. Outside the mitochondria and inside the lipid bilayer membrane, negative oxygen ion is converted into a water molecule by means of Cu and Mn Superoxide dismutase and catalase. Outside the lipid bilayer, negatively charged oxygen ions are converted into hydrogen peroxide by means of extracellular superoxide dismutase.
3.1. Expression of SODs in neurons
In the adult CNS, SOD1 are found to be expressed in neurons of substantia nigra, pyramidal neurons of hippocampal and cortical regions, granule neurons of dentate gyrus, and at significantly higher levels in motor neurons of spinal cord. In the immature CNS, SOD1 expression is found to be higher in neurons of the cortical layers II, III and V, and in neurons of pyriform cortex, hippocampus, and hypothalamus. Following excitotoxic injury, SOD1 in neurons was found to be down-regulated preceding neuronal degeneration, while SOD1 in astrocytes was found to be up-regulated (Peluffo et al., 2005). Instead, SOD2 is detected principally in neurons present all over the brain and spinal cord. SOD2 is observed in relatively lesser quantity in astrocytes than in neurons, whilst following any insult or OS, SOD2 expression increases in astrocytes (Maier and Chan, 2002). A transgenic murine model of brain-specific SOD2-deficient mice (B-Sod2 −/−) exhibited perinatal death along with reduced body weight and smaller brain. Brain slices of B-Sod2 −/− mice revealed spongiform encephalopathy with increased astrocyte activation in distinct brain areas such as cerebral cortex, brain stem, and hippocampus. In addition, selective loss of mitochondrial complex II enzymatic activity with increased lipid peroxidation (marked by increased level of malondialdehyde) indicate the accumulation of OS-mediated injury in the brain lysates of B-Sod2 −/− mice.
3.2. Expression of SODs in glial cells
Earlier studies have reported that the expression of SOD1 and SOD2 are relatively low in oligodendrocytes, endothelial cells, and microglial cells under normal physiological conditions (Furuta et al., 1995, Lindenau et al., 2000, Moreno et al., 1997, Noack et al., 1998). Pardo et al., 1995 reported that SOD1 is not found in the microglial cells and oligodendrocytes. Furthermore, studies investigating expression of SOD1 in astrocytes have produced controversial results. Some studies have revealed the intense expression of SOD1 in astrocytes (Lindenau et al., 2000, Noack et al., 1998), while others have failed to identify their expression (Moreno et al., 1997, Thaete et al., 1986) and few studies have partially identified it in the form of scattering in some astrocytes (Furuta et al., 1995, Pardo et al., 1995). In case of brain injuries such as kainate and quinolinic acid intoxication, transient cerebral ischemia, or Alzheimer's disease or Down's Syndrome, the expression of Cu/Zn SOD are found to be intense in reactive astrocytes (Furuta et al., 1995, Kim et al., 2000, Liu et al., 1994, Noack et al., 1998). An in vitro study (Ishihara et al., 2015) shows that LPS stimulation increased the mRNA and protein levels of SOD2 in rat primary microglia, but not in the astrocytes, or primary neurons signifying the specific activation of microglia under inflammatory conditions. Increased SOD2 conferred high tolerance to OS on rat primary microglia, whilst SOD2 knockdown promoted vulnerability to OS. Increased SOD2 scavenges ROS and reduces the expression of inflammatory cytokines (TNF-α and IL-1β) by attenuating the NF-κB subsequently activating the termination of microglial activation. These data indicate that SOD2 regulates the immune system in the CNS by regulating microglial cells (Ishihara et al., 2015).
4. SODs in neurological disorders
OS plays a crucial role in the pathogenesis of numerous neurological disorders including neurodegenerative diseases, neuropsychiatric disorders, and cerebrovascular disorders (Babu and Ramanathan, 2011b, Maier and Chan, 2002). Neurons and glial cells are prone to oxidative damage because of their moderate anti-oxidant defence system, reduced level of antioxidants (especially enzymatic antioxidants), along with increased membrane polyunsaturated fatty acids (PUFAs) and release of iron easily from injured cells. For example, in neurodegenerative diseases like Alzheimer’s disease (AD), a positive correlation between OS and neuronal damage, characterized by the reduced levels of antioxidant enzymes such as CAT, glutathione peroxidase, and SOD in the affected brain regions (Pappolla et al., 1992, Younus, 2018, Zemlan et al., 1989). In mouse model of AD, experimental overexpression of SOD2 prevented the memory deficits by reducing hippocampal superoxide (Massaad et al., 2009). In Parkinson’s disease (PD), oxidative damage is associated predominantly in substantia nigra region (Kondo, 1996, Pall et al., 1987). Several research findings suggest that oxidative damage resulting from disproportion between reactive oxygen metabolites (ROMs) production and antioxidant activity generates deleterious effects on signal transduction, mitochondrial complex (I-IV) activities, structural plasticity, oxidation of proteins, and lipid peroxidation in membranes and genes leading to neuronal death (Takuma et al., 2004). Dysregulation of mitochondrial functions and immune response further intensifies the ROS production and oxidative damage (Serrano and Klann, 2004). Therefore, we have summarized the role of SODs in different neurodegenerative diseases. We used major search engines such as PubMed, SCOPUS, MDPI and Google Scholar to collect the scientific literature.
4.1. SOD and Alzheimer’s disease
AD is one of the major neurodegenerative diseases pathologically characterized by accumulation of intracellular neurofibrillary tangles and extracellular amyloid-β peptide (Aβ) plaques in the brain leading to atrophy and neurodegeneration (Dubois et al., 2007). The major clinical symptoms include dementia, apathy, social withdrawal, mood swings, depression, disturbed sleep, and delusions. These pathological aggregates of insoluble protein primarily consist of hyper-phosphorylated tau and misfolded amyloid-β isoforms in conjunction with metals, which may result in vascular dysregulation (due to injury to the endothelial lining of the vascular rings), and OS, which triggers the progression of AD (Perry et al., 2002). Progression of AD marked by the loss of neurons has been reported to be connected with reduced mitochondrial complex IV activity and increased ROS production (Misrani et al., 2021, Wang et al., 2020, Wong-Riley et al., 1997). Several transgenic AD mouse models have clearly shown the link between SOD gene and manifestation of AD-like symptoms. For instance, deletion of one SOD allele in transgenic mice over-expressing β-APP accelerated the onset of behavioural changes and Aβ plaques in brain parenchyma, but increased the development of cerebrovascular amyloidosis, gliosis, and neuritic dystrophy. Similarly, transgenic human APP mice also show altered behaviour such as reduced anxiety and disinhibition (Resende et al., 2008). Inactivation of one SOD allele in transgenic (Tg2576) mouse model of AD has shown to bolster the accumulation of Aβ plaques (Murakami et al., 2011) while overexpression of SOD in mice has shown to reduce the plaque formation and lower cognitive deficits (Massaad et al., 2009). Supplementation with SOD has shown to reverse AD-like pathological features and delayed the onset of cognitive decline by encountering OS (Fleming et al., 2012, Niedzwiecki et al., 2013) in AD mouse models. OS-mediated upregulation of BACE1 (a β-site AβPP cleavage enzyme 1) was significantly reduced by SOD treatment in Tg19959 mice, which was reflected by lower brain levels of Aβ (Hampel et al., 2021). Moreover, post-mortem brain samples of AD patients have revealed elevated markers of OS including protein and lipid peroxidation (Butterfield and Lauderback, 2002, Sultana et al., 2006). AD patients have shown upregulation of antioxidant enzymes like SOD1, SOD2 and CAT early in the disease progression (De Leo et al., 1998). Other contributing factors to increased OS include “vicious loop” of mitochondrial dysfunction leading to Aβ plaque formation, which intensifies mitochondrial aberrations (Wallace, 2010). Generally, chronic treatment with SOD or SOD mimetics have evidently shown neuroprotective effects in ageing mice such as reduction in lipid peroxidation, nucleic oxidation and ROS concentrations, and improve age related-cognitive decline, mainly by neutralizing the age-dependent OS increase in hippocampus and amygdala (Clausen et al., 2010). Especially, supplementation with SOD/CAT mimetic (EUK-207) have shown to inhibit the onset of amyloid tau pathology as well as cognitive decline in an aggressive mouse model of AD (Clausen et al., 2012, Persichilli et al., 2015). These results suggests that mutation of SOD gene leads to Aβ formation, and treatment with SOD /SOD mimetics decline the pathological symptoms by regulating oxidative stress.
4.2. SOD and Parkinson’s disease
PD is the second leading neurodegenerative disease characterized by clinical symptoms such as bradykinesia, resting tremor, rigidity, postural instability, and sleep disorders (Jankovic, 2008, Jankovic and Tan, 2020). PD is associated with progressive degeneration of dopaminergic neurons in the substantial nigra pars compacta, reduced dopamine secretion, loss of glutathione and appearance of Lewy bodies (Helley et al., 2017, Jiang and Dickson, 2018)(Dickson, 2018). Specific genetic mutations resulting in the Parkinsonian phenotype are found to be associated with the loss of mitochondrial complex I activity within the electron transport chain, which accounts for approximately 10% of PD cases (i.e., familial type of PD) (Helley et al., 2017, Larsen et al., 2018) The pathophysiology of PD includes selective degeneration of midbrain dopaminergic neurons which leads to dopamine deficiency. The molecular mechanisms underlying neuronal death include chronic inflammatory response and OS (Gaki and Papavassiliou, 2014, Stojkovska et al., 2015), which is characterised by the presence of microglial cell activation, reactive astrogliosis (Marino et al., 2020). The production of ROMs is increased significantly in PD due to mitochondrial energy crisis, aging, neuroinflammation, high levels of iron or Ca2+, and GSH depletion (Meiser et al., 2013). Other causes of neurodegeneration include exogenous and endogenous neurotoxins, altered antioxidant defence system and enhanced dopamine turnover (Kocatürk et al., 2000, Olanow and Arendash, 1994).
Transplantation of substantia nigra grafts from transgenic mice overexpressing SOD into Parkinsonian model rats (induced by 6-hydroxydopamine toxin) enhanced their survival and functional recovery, by regulating the expression of SOD and protecting tyrosine hydroxylase-immunoreactive cells (Nakao et al., 1995). Another in vitro study showed that dopaminergic neurons present in SOD1 transgenic mice show better survival than those from non-transgenic littermates against dopaminergic neurotoxin (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)), which is reflected by reduced dopamine depletion (Przedborski et al., 1992). Microglial cells transfected with human SOD1 release SOD3 into the medium under proper stimulation, which in turn protects neurons against 6-hydroxydopamine -induced cell damage (Polazzi et al., 2013). A preclinical study demonstrates that Drosophila melanogaster treated with M40403 compound showed decreased loss of endogenous SOD enzymes, acting both at a cytosolic and mitochondrial level, and prevents oxidative damage induced by paraquat (Filograna et al., 2016).
Clinical studies have shown enhanced levels of circulating inflammatory cytokine profiles (IL6, IL10, IL-2, and IL-1β and TNF-α,) and OS markers (C-reactive protein (CRP)), and reduced levels of antioxidants in PD patients, specifically in blood (Medeiros et al., 2016, Qin et al., 2016, Yuan et al., 2016). Post-mortem brain samples of PD patients show increased iron and decreased copper in substantia nigra (Ayton et al., 2013) and basal ganglia (Dexter et al., 1989, Riederer et al., 1989). A clinical study reports significant reduction in plasma levels of SOD, cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol, and an increase in the plasma level of high-sensitivity CRP in PD patients when compared to healthy controls (Yang et al., 2020). In PD patients, plasma SOD level negatively correlates with hs-CRP and positively with total cholesterol, HDL-C, and LDL-C. Reduced plasma SOD level correlate with PD disease severity. In organotypic midbrain-striatum co-cultures increase in midbrain SOD activity increases the resistance of dopaminergic neurons to NO cytotoxicity via suppressing the formation of peroxynitrite (Katsuki et al., 2001)(Tabrizi et al., 2020). These reports indicate that SOD plays a crucial role in Parkinson’s disease, by preventing OS, and dopaminergic neurotoxicity.
4.3. SOD and Huntington’s disease
HD is a genetic disorder linked with progressive degeneration of striatal spiny neurons, and present as a triad of motor, cognitive, and psychiatric symptoms. Motor dysfunction include rigidity, writhing movements, abnormal posture, involuntary and uncontrollable movements called as chorea, cognitive decline can lead to dementia, and neuropsychiatric symptoms involve anxiety, depression, aggression and compulsive behaviour. HD is caused by an unstable expansion of cytosine-adenine-guanine repeats within the exon1 of the huntingtin (HTT) gene resulting in a mutant HTT protein (McColgan and Tabrizi, 2018). Mutant HTT directly attacks the mitochondrial membrane and alters calcium homeostasis (Suzuki et al., 2012), and induces mitochondrial dysfunction by damaging mitochondrial DNA (Yang et al., 2008) mitochondrial fission/fusion mechanisms, and mitochondrial energy metabolism (Song et al., 2011). Specifically, mutant HTT aggregates in the striatal spiny neurons directly inhibit the activities of mitochondrial complex II (succinate dehydrogenase) causing increased production of ROS and decreased production of ATP (Bossy-Wetzel et al., 2008), which further results in apoptosis and cell death. Other studies have shown that direct suppression of PGC-1α (a transcriptional co-regulator of mitochondrial biogenesis) transcription by mutant HTT increase ROS production in HD (Chaturvedi et al., 2012, Johri et al., 2013). Reduced PGC-1α activity in turn reduces the expression level of mitochondrial uncoupling proteins as well as SOD1, SOD2, and GPX (St-Pierre et al., 2006), which contributes to mitochondrial dysfunction in HD pathology. Indeed, overexpression of PGC-1α prevented HD-induced neurodegeneration moderately by decreasing OS (Tsunemi et al., 2012). Transgenic mutant mice with PGC-1α deletion showed mitochondrial dysfunction, striatal degeneration, and HD symptoms (Lin et al., 2004). Mutant HTT interacts with Drp1, a transcriptional co-regulator of mitochondrial dynamics, and disrupts the mitochondrial fission–fusion processes (Shirendeb et al., 2011, Shirendeb et al., 2012, Song et al., 2011). An in vitro study shows that mutant striatal cells expressing mutant huntingtin with 111 glutamines (STHdhQ111/Q111) exhibit higher mitochondrial ROS and reduced NADPH oxidase and xanthine oxidase activities, indicating reduction in superoxide cytosolic generation as well as increase in SODs and components of glutathione redox cycle·H2O2 and staurosporine exposure increased the ROS production in mutant cells and primarily enhanced xanthine oxidase activity. Staurosporine bolster the mitochondrial ROS production and caspase-3 activity·H2O2 and staurosporine increased SOD1 activity slightly and reduced glutathione reductase (Ribeiro et al., 2013). These results indicate that both dysfunctional mitochondrial and increased ROS act synergistically to promote OS, resulting in neuronal loss in the striatum and cortex (Gil-Mohapel et al., 2014, Zuccato et al., 2010). Injection of mitochondrial toxin (3-nitropropionic acid (3-NP)) in transgenic mice with partial deficiency of SOD2 (heterozygous SOD2-knockout) induced significantly larger striatal lesions, proving the fact that SOD play an imminent role in neurotoxicity studies of HD (Andreassen et al., 2001, Chidambaram et al., 2017).
Mutant HTT proteins tend to aggregate throughout the brain of HD patients and disrupt the protein quality control and transcription process which causes aberrant motor and cognitive deficits. HD patients have shown reduced oxygen consumption in the cortex and basal ganglia along with increased lactate accumulation in the occipital cortex (Bowling and Beal, 1995). Another study found increased levels of antioxidant defence proteins such as peroxiredoxins 1, 2 and 6 and GPX-1 and 6 in post mortem human brain samples of HD patients (Sorolla et al., 2008). The level of SOD1 was found to be significantly accelerated in HD patients compared to controls, representing a compensatory response to increased oxidative levels in HD (Ciancarelli et al., 2015). Therefore, these literatures suggest that SOD play’s role in HD by increasing mitochondrial ROS and oxidative levels.
4.4. SOD and ischemic stroke
Ischemic stroke is a brain lesion caused by inadequate supply of blood and oxygen to the affected region due to blockage of an artery, resulting in neuronal death. The most common symptoms include muscular weakness, paralysis, and loss or abnormal sensation on one side of the body, dizziness, trouble in speaking, vision loss or double vision, confusion, and loss of balance or coordination (Hui et al., 2022). Following focal and global ischemia, ROS including O2•-. and its reaction product peroxynitrite have shown to play a prominent role in the pathogenesis of cerebral infarction. Patients following an ischemic stroke showed reduced capacity for antioxidant activity (Ciancarelli et al., 2012, Ullegaddi et al., 2005). A clinical study reported significantly elevated OS (marked by increased levels of CRP, malondialdehyde (MDA) and uric acid along with reduced activity of antioxidants (marked by lower plasma levels of GSH, CAT and GPX along with lower levels of SOD in plasma and red blood cells) in patients with acute ischemic stroke, suggesting a possible therapeutic intervention with antioxidant agents (Milanlioglu et al., 2016). A biochemical study reported lower serum levels of vitamin E, vitamin C, total antioxidant capacity (TAC) and SOD in ischemic stroke patients compared to those of healthy controls, whilst serum uric acid levels was significantly higher in ischemic stroke cases than controls (Srikrishna and Suresh, 2009). Stroke patients with large vessel or cortical and subcortical infarcts showed significantly reduced serum levels of vitamin E, vitamin C and SOD, as well as significantly increased serum levels of uric acid than those of stroke patients with small vessel or lacunar infarcts. TAC showed a strong positive correlation with serum uric acid levels, but negative correlation with vitamin C, vitamin E, and SOD. A case-control study (Zheng et al., 2020) showed that patients with acute cerebral infarction or cerebral haemorrhage had higher complement C3 level, and lower complement C4, SOD and TAC levels than the healthy controls. Additionally, severity of neurologic injury correlated directly with increased complement C3 level, and reduced SOD and TAC levels.
To ameliorate ischemia-induced brain damage, the main approaches used to delirious effects of ROS reduce include up-regulation of endogenous ROS-scavenging mechanisms, administration of ROS-scavenging drugs, and inhibition of leukocyte infiltration into affected brain tissue (Nilupul Perera et al., 2006, Popa-Wagner et al., 2007). Transgenic mice overexpressing SOD1 show reduced ischemic/reperfusion (I/R) injury following focal cerebral ischemia (Yang et al., 1994). Mutant mice with SOD2 deficiency (established via targeted deletion of SOD2) deteriorates the outcome of both temporary and permanent middle cerebral artery occlusions (Kim et al., 2002, Murakami et al., 1998). Inhibition of NADPH oxidase by apocynin protected the brain by alleviating OS and reducing the inflammation process following cerebral focal I/R injury in mice. Cerebral I/R injury induced upregulation of the NADPH oxidase subunit gp91phox expression, which was further enhance by genetic ablation of SOD1, but reduced in SOD1-overexpressing mice, indicating that post-ischemic gp91phox overexpression was regulated by endogenous SOD1 level (Chen et al., 2009).
SOD mimetic removes the products of ROMs that aids in the prevention of tissue damage and cellular energetic failure related with ischemia and perfusion (Salvemini and Cuzzocrea, 2002). For example, pre-treatment with a novel SOD2 mimic, MnTm4PyP, promoted the survival of primary cultured cortical neurons by efficiently restoring the redox balance following injury by H2O2. MnTm4PyP pre-treatment significantly reduced the infarct size and neurological deficits by alleviating OS and neuronal apoptosis in mice subjected to acute ischemic stroke (Huang et al., 2012). Several in vitro and in vivo therapeutic studies have shown that SOD2 lipophilic mimic, Mn (III) meso-tetrakis (N-n-hexylpyridinium-2-yl) porphyrin, (H2O) MnTnHex-2-PyP5 + (MnHex) can cross the BBB and accumulate in mitochondria than cytosol (Leu D et al., 2017; Spasoievic I et al., 2011). An in vivo study showed that MnHex reduce the infarct volume and neuroinflammation, and improve the neurological functions in a rat ischemic stroke model (induced by middle cerebral artery occlusion) (Sheng et al., 2011). Another SOD2 mimic, Fe analog, (OH)FeTnHex-2-PyP4 + (FeHex), has shown higher neuroprotective efficacy than MnHex in an SOD-deficient E. coli model (JI132) of OS (Leu et al., 2017, p. 5; Li et al., 2020; Spasojevic et al., 2011; Tovmasyan et al., 2013). Generally, clinical and basic studies in stroke patients show that reducing oxidative damage may be important part in addition to restoring blood flow in order to reduce inflammation, and promote the best outcome in the patient (Nilupul Perera et al., 2006). Thus, reduction in oxidative stress, neuroinflammation, and alleviation of neurological functions can be achieved by administering SOD or SOD mimetics.
4.5. SOD and depression
Depression is the most common psychological disorder affecting more than 300 million of lives every year (Santomauro et al., 2021). Depression is characterised by long-lasting anhedonia, fatigue, negative thinking, mood swings, lower self-esteem, insomnia or hypersomnia, memory deficits, disturbed appetite, social isolation and crying spells for no apparent reason. Suicidal thoughts are more frequent in patients with severe depression (Cao et al., 2020). The fatality rate of depression accounts for approximately 800,000 deaths per annum (Cheung et al., 2019). The pathophysiology of major depressive disorder (MDD) includes chronic inflammatory response, psychological stress, and autoimmune tissue damage leading to increased OS. Lower levels of antioxidants along with higher levels of MDA results in OS. Both adolescent and adult depressive patients showed increased markers of OS (such as lipid hydroperoxides, 8-isoprostane and protein carbonyls) and reduced levels of antioxidant enzymes (such as SOD1 and GPX) (Black et al., 2015, Siwek et al., 2013) (Black et al., 2017). A meta-analysis by Liu and others (Liu et al., 2015) showed that the serum TAC, antioxidant levels and paraoxonase were significantly reduced, while the serum oxidative stress markers such as malondialdehyde, nitrate/nitrite, 8-iso-PGF2α were increased in depressed patients than healthy controls.
Proton magnetic resonance spectroscopy revealed lower GSH levels in brains of adolescents with depressive disorders (Freed et al., 2017). Young adults with the early stages of bipolar disorder had increased protein carbonyl levels compared to the controls (Magalhães et al., 2012). Indeed, Scola et al. reported that the levels of lipoperoxides correlate negatively with the severity of depression and positively with the severity of manic symptoms (Scola et al., 2016). These results reveal the pathophysiological relationships between OS and depression. A cross-sectional study reported higher SOD activities and SOD/GPX + CAT ratio in maniac and depressive patients compared to than both euthymics and controls (Andreazza et al., 2007). A clinical study showed increased serum concentrations of SOD and CAT and increased protein carbonyl content in MDD patients than those of healthy subjects, which may be an early adaptive phenomenon (Tsai and Huang, 2016). Another clinical study shows increased MDA levels in MDD subjects when compared to healthy controls (Bajpai et al., 2014). Serum levels of nitrites, ascorbic acid as well as SOD were found to be significantly low than that of healthy controls. A recent study showed that the levels of 8-isoprostane, advanced oxidation protein products and nitrotyrosine were elevated in paediatric and adolescent patients with depressive disorder compared to healthy controls. A supplementation with omega-3 fatty acid, but not with omega-6 fatty acid, reduced 8-isoprostane, advanced oxidation protein products and nitrotyrosine levels, and enhanced Trolox equivalent antioxidant capacity and SOD activity (Katrenčíková et al., 2021). Another clinical study showed that MDD patients had elevated levels of MDA, and reduced levels of SOD and nuclear factor erythroid 2-related factor 2 compared to non-depressed subjects (Hamed, 2020). These data indicate that SOD plays a prominent role in depression pathology. Although in majority of the studies the depressive subjects are observed to suffer with decreased anti-oxidants levels; however, the contradictory reports may be speculated to the difference in disease duration or severity.
4.6. SOD and schizophrenia
Schizophrenia is a serious debilitating neurodevelopmental disorder characterized predominantly by hallucinations, delusions, disordered thinking and abnormal behaviour. Patients with schizophrenia exhibit three types of symptoms such as positive symptoms (hallucinations, delusions and active manifestation of anomalous behaviour), negative symptoms (social withdrawal, anhedonia, and deficits in affect) and disorganized symptoms (disordered speech, disorganized thinking, extremely disorganized or abnormal motor behaviour (termed as tardive dyskinesia), and cognitive impairments)(Jauhar et al., 2022). Brain imaging studies of schizophrenic patients showed increased activity in the substantia nigra and basal ganglia, but diminished activity in prefrontal cortex, and reduced functional connectivity between these regions and prefrontal cortex (Yoon et al., 2013). Increased production of free radicals attacks the cellular constituents, which raises the lipid peroxidation in membranes, leading to reformed membrane structure and function as well as increased levels of lipid peroxidation products in red blood cells, cerebrospinal fluid, plasma and post-mortem brain tissues of schizophrenic patients (Flatow et al., 2013, Wu et al., 2013). Numerous clinical studies reported that SOD activity have been consistently elevated in adult patients with chronic schizophrenia (Kunz et al., 2008, Wu et al., 2012) and in schizophrenia patients treated with haloperidol or clozapine (Gama et al., 2006). Another study had shown significantly increased levels of OS in schizophrenic patients, which is indicated by higher levels of pro-oxidants NO and MDA, and declined levels of antioxidants GSH, SOD, GPX and neurotrophins (NT)− 4/5 (Dietrich-Muszalska and Kwiatkowska, 2014). Additionally, increased ROS levels were found in the periphery of schizophrenia patients (Miyaoka et al., 2015, Sarandol et al., 2015).
Schizophrenic patients also showed elevated levels of plasma thiobarbituric acid reactive substances (TBARS) (Kunz et al., 2008, Ranjekar et al., 2003), reduced levels of essential polyunsaturated fatty acids (PUFAs), altered immune function and altered levels of phospholipids in both brain and peripheral membranes (Yao et al., 2003, Yao et al., 1994), indicating the link between OS-mediated neuronal damage, which is directly reflected as impaired social cognition and neurocognition. A post-mortem study (Karry et al., 2004) have reported that both protein levels and mRNA of 24-kDa and 51-kDa subunits in mitochondrial complex I were substantially reduced in prefrontal cortex, whilst elevated in ventral parietooccipital cortices of schizophrenia subjects associated with control individuals, supporting the hypothesis of mitochondrial dysfunction leading to impaired cerebral circuitry in schizophrenia. The plasma SOD2 activity was found to be decreased significantly in subjects with schizophrenia compared to that of normal control subjects, and the association between higher SOD2 activity and cognitive impairment in schizophrenia is dependent on the Ala-9Val polymorphism of SOD2 gene (Zhang et al., 2014). In parallel to previous reports in adult schizophrenia (Gonzalez-Liencres et al., 2014, Zhang et al., 2013). A recent study also revealed that in patients with late-life schizophrenia, peripheric SOD levels were independently linked with cognition, language, and general intellectual functions (Huo et al., 2021). Furthermore, increased SOD activity correlated positively with negative symptoms in schizophrenia patients as they aged (Cohen et al., 2015). In chronic schizophrenia patients, SOD activity correlated positively with both positive and negative symptoms, as well as general psychopathology (Bai et al., 2018, Zhang et al., 2009). In contrast, a study showed that SOD activity was associated inversely with positive symptoms (Wu et al., 2012). Another study showed that SOD-associated clinical symptoms vary between men and women, as increased SOD activity correlated with negative symptoms in men, while increased SOD activity correlated with positive symptoms in women. Further, the evidences from this study reveal that SOD activity plays role in the pathology of schizophrenia but it may vary among gender, age and lifestyle.
4.7. SOD and Down Syndrome
Down syndrome (DS) is a chromosomal disorder primarily triggered by an extra copy of chromosome 21, therefore also known as trisomy 21, and characterised by short stature, facial dysmorphology, larger tongue, low muscle tone, and intellectual disability (Karmiloff-Smith et al., 2016). Triplication of chromosome 21, on which 21q22.1 gene encoding for SOD-1 is localized, leads to an increased SOD protein expression, and an imbalance in the ratio of SOD1 to CAT and GPX, which results the accumulation of H2O2 and consequently damage to DS brain (Zana et al., 2007). DS patients contain approximately 50% higher SOD1 levels in various DS cells and tissues, including erythrocytes, brain, fibroblasts, B and T lymphocytes, which leads to chronic OS and manifests as a wide array of neurological symptoms (Turrens, 2001; Barone E et al., 2018). Excessive glial proliferation in the DS brain is the main cause for increased SOD1, as a response to OS (Gulesserian et al., 2001). Both DS mouse models and DS patients have shown that several genes encoded on chromosome 21 such as SOD1, APP, BACH1 (BTB Domain and CNC Homolog 1), Et2 (ETS Protooncogene 2, Transcription Factor), and S100B (S100 Calcium-Binding Protein, Beta) are associated with increased OS (Barone et al., 2018, Perluigi and Butterfield, 2012). Elevated expression of SOD1 and APP were found to be associated with mitochondrial aberrations, increased oxidative damage on neurons, and early onset of senile plaques accumulation (Fig. 3). Overexpression of Ets-2, BACH1, and S100β were associated with increased neuronal apoptosis, neurodegeneration and increased ROS production, respectively. Higher SOD1 levels alters the activity and response of T cells (Ferrari and Stagi, 2021). An increase in levels of total protein carbonyls, TBARS and advanced glycation end products (AGEs) were observed in the cortex of DS foetal brains compared with controls (Odetti et al., 1998), while a marked accumulation of nitro tyrosine, 8-hydroxy-2-deoxyguanosine, and oxidized proteins was reported in the cerebral neuron cytoplasm in DS (Nunomura et al., 2000). Increased ROS production, elevated levels of lipid peroxidation and mitochondrial dysfunction are observed in cells of DS patients from their embryonic life (Busciglio and Yankner, 1995) which subsequently leads to neurodegeneration. Cultures of astrocytes, neurons, and foetal fibroblasts from DS patients have evidently shown several mitochondrial DNA mutations; reduced mitochondrial membrane potential; altered cristae morphology, and macroscopic alteration of mitochondrial morphology (Helguera et al., 2013, Izzo et al., 2018, Piccoli et al., 2013).
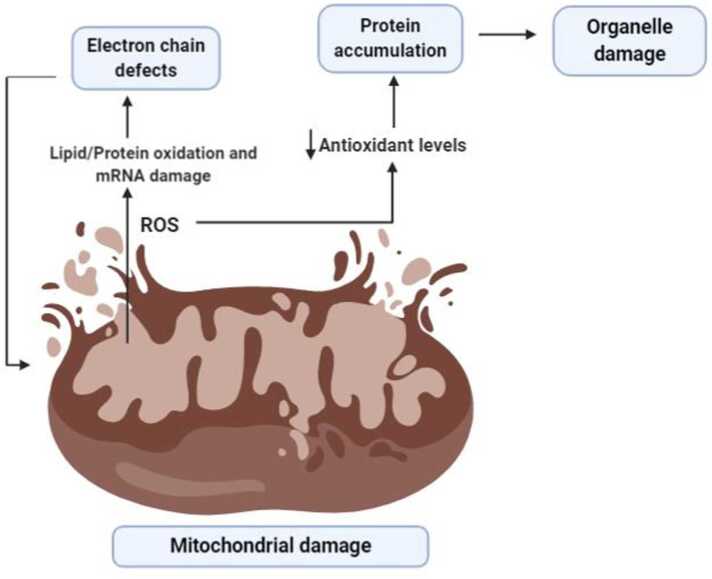
Fig. 3.
Role of reactive oxygen species in organ damage. A damaged mitochondria gives rise to reactive oxygen species leading to lipid/ protein oxidation as well as mRNA damage followed by electron chain defects. These further increases the release of ROS. Another mechanism of ROS activity is by decreasing anti-oxidant levels and resulting in protein accumulation and organelle damage.
Transgenic mice carrying SOD1 overexpression have shown to develop increased lipid peroxidation and oxidative damage (Ceballos-Picot et al., 1992). Further, using a transgenic mouse overexpressing wild-type human SOD1, Shin et al. (2004) showed that abnormal neuronal and mitochondrial proteins induce swelling, vacuolization, and increased ROS levels intracellularly, and manifests as learning and memory deficits (Shin et al., 2004, p. 1). Thus, these data suggest that in Down syndrome there is increased expression of SOD which further progress the disease by increased ROS that leads to damage of neurons, cognitive decline etc.
4.8. SOD in Amyotrophic Lateral Sclerosis
ALS is a progressive neurodegenerative disease caused by the death of huge motor neurons in motor cortex, brain stem and spinal cord, and characterized by progressive loss of muscle control resulting in paralysis (Taylor et al., 2016). The main pathological mechanisms of ALS include peroxidation and excitotoxicity, ROS-mediated injury to vital cellular proteins. A breakthrough study by Rosen and others (Rosen et al., 1993) recognized that the SOD1 gene-associated mutations encode for the 2% of ALS and 20% of familial amyotrophic lateral sclerosis (FALS) cases (Gamez et al., 2006). More than 70 different types of SOD1 mutations result in an autosomal dominant phenotype of ALS. Point mutations in the SOD1 gene which are linked with loss of motor neurons have been detected in patients with FALS (Choi et al., 2005, Cleveland and Rothstein, 2001).
Although the exact molecular mechanisms behind the death of motor neurons are still under investigation, results of some studies have shown the abnormal accumulation of SOD1 protein, increased nitrosylation of essential proteins by mutant SOD1, increased affinity for toxic Cu in the active site of mutant SOD1, enhanced disorganization of neurofilaments, mitochondrial dysfunctions, dysregulation of intracellular calcium, and glutamate-mediated excitotoxicity (Riva et al., 2016, Rowland and Shneider, 2001). Mutant SOD1 may accumulate in the intermembrane space (IMS), overriding the physiological retention controlled by the copper chaperone for superoxide dismutase (CCS), and that misfolded SOD1 may deposit on the outer mitochondrial membrane (OMM), preventing the transport across mitochondrial membranes and inducing mitochondrial-dependent cell apoptosis (Lee et al., 2016, Li et al., 2011).
Transgenic mice overexpressing human SOD1 with a glycine to alanine mutation in position 93 (mutant-SOD1 mice) have shown to develop selective motor neuron degeneration resembling FALS-like phenotype, which is linked to SOD1 mutations in 15–25% of cases (Gurney et al., 1994). The spinal cord, frontal cortex and striatum of mutant-SOD1 mice and sporadic ALS patients showed increased levels of 8-hydroxy-2′-deoxyguanosine (an indicator of oxidative DNA damage), which correlate positively with disease progression and severity (Aguirre et al., 2005, Bogdanov et al., 2000, Fitzmaurice et al., 1996). In contrast, SOD1 knockout mice do not develop ALS-like phenotype, despite the presence of excess superoxide radicals (Shefner et al., 1999). A recent Polish prospective study reported identification of 15 SOD1 mutations in 21.1% of FALS cases and 2.3% of sporadic ALS cases (Berdyński et al., 2022). The most frequent SOD1 mutations among Polish ALS patients were found to be L144S and K3E. ALS patients with SOD1 mutations of conserved residues were found to have shorter life span as well as shorter time between the disease onset and respiratory failure. ALS patients carrying L144S and G41S mutation had the longest and shortest overall survival, respectively. Substantial disturbance of SOD1 structure were found to be associated with clinically relevant endpoints such as loss of motor functions, bulbar involvement, respiratory insufficiency and overall survival (Berdyński et al., 2022). Therefore, these evidences reveal that mutation in SOD gene which progress the disease.
4.9. SOD in epilepsy
Epilepsy is a chronic neurological disorder caused by abnormal brain activity resulting in recurrent unprovoked seizures (2 or more epileptic seizure episodes occurring more than 24 h apart). The main symptom of epilepsy is recurrent seizures. Accumulating evidence suggests that neuronal hyperexcitability and oxidative injury play in the pathogenesis and progression of epilepsy (Xiang and Jiang, 2014). Both the autopsy of patients with temporal lobe epilepsy and experimental rodent models of partial complex seizures have shown increased production of ROS/RNS and proinflammatory cytokines (IL-6, IL-1β, TNFα, and interferons), neurodegeneration, axonal sprouting within excitatory pathways along with reactive astrogliosis, death of inhibitory interneurons and microgliosis, damage to BBB integrity, and abnormal neuronal cytoarchitecture (Kalozoumi et al., 2018, Sharma et al., 2018). These changes results in higher brain excitability which ultimately leads to enhanced epileptiform spiking, neuronal hyper-synchronization and spontaneous recurrent seizures (Waldbaum and Patel, 2010). Several studies have confirmed defective oxidative status in epileptic patients reflected by increased MDA levels and markedly decreased serum TAC (çevik, 2012, Pandey et al., 2012, Yiş et al., 2009). A prospective study showed decreased SOD1 levels in epileptic patients when compared to healthy controls (Al-Muhammadi et al., 2015). Another clinical study showed increased lipid peroxidation in patients with epilepsy when compared to controls. Serum MDA levels were significantly higher, while SOD in red blood cells and serum vitamin E levels were significantly reduced in epilepsy patient when compared with controls (Nemade, n.d.). A case-control study showed that patients with genetic generalised epilepsy had markedly high levels of serum NO, MDA and low levels of plasma TAC compared to healthy controls, while erythrocyte SOD, CAT and GPX activities were significantly lower in epileptic patients compared to controls (Prasad et al., 2017). In addition, a significant reduction in the activities of SOD, CAT and TAC, and increase in the level of NO and MDA in patients treated with anti-epileptic drugs compared to untreated patients suggest the induction of additional OS, utilization of antioxidant enzymes and reduction of the antioxidant capacity, which implicate as seizure recurrence and drug unresponsiveness. In contrast, some studies have shown increased SOD levels or no changes in epileptic patients (Hung-Ming et al., 2002). A recent meta-analysis showed that lower levels of SOD and GPX in erythrocytes, serum and plasma in patients with epilepsy compared to that of healthy controls, indicating the oxidative damage in epilepsy.
Preclinical studies in rodents with pilocarpine- and kainate-induced temporal lobe epilepsy showed high levels of ROS/RNS, lipid peroxidation with consequent hippocampal apoptosis, damage of membranes and organelles, protein synthesis in survived neurons and altered gene expression, and GSH action was reduced extensively (Atanasova et al., 2013, Pearson et al., 2015). These evidences reveal that there are reduced levels of SOD in patients with epilepsy which produces increased ROS and further damage of organelles etc.
5. SOD mimetics
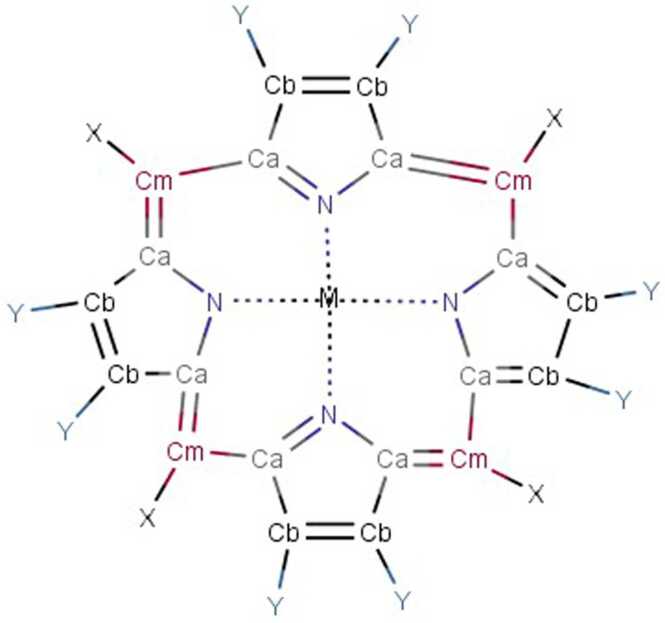
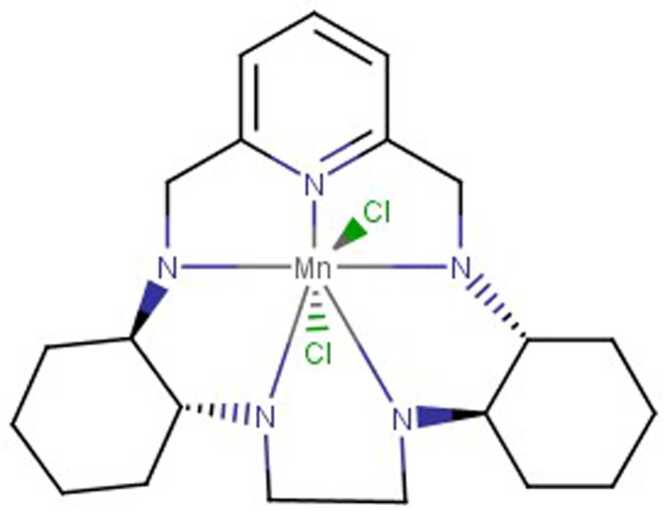
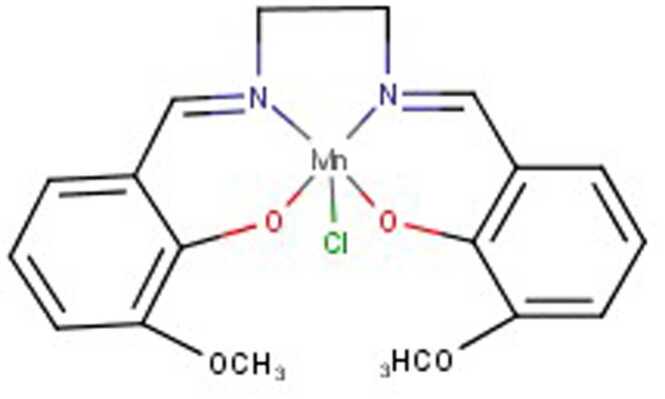
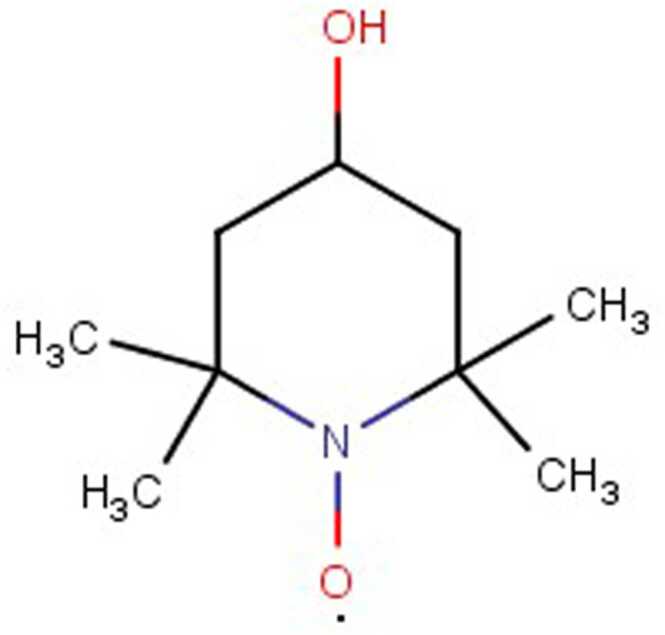
There are many chemical synthetic compounds which are designed conceivably to mimic the antioxidant actions of SOD enzyme. These small molecular catalytic antioxidants exerts beneficial effects against OS-mediated injuries (Mansuroğlu et al., 2015, Younus, 2018). SOD mimetics efficiently convert O2•- into H2O2, which is further converted into water by CAT, and their design is based on the subsequent production of Mn (II) and Fe (III) complexes (Salvemini et al., 2002a). The stability, as well as high SOD value, has been determined by means of computer-aided modelling and they have also paved the way for design and development of newer categories of highly stable and active SOD catalysts(Rosa et al., 2021; Singh et al., 2017). These synthesized SOD mimetics are illustrated by the prototype complexes, M40401 and M40403 resulting from the 15-membered macrocyclic ligand 1,4,7,10,13-pentaazacyclopentadecane, consisting of bis (cyclohexyl pyridine) functional groups (Salvemini et al., 1999). A vital feature of these SOD mimetics is their ability to selectively remove superoxide anion (O2•-) by catalysation without reacting with other reactive species like hydrogen peroxide, peroxynitrite, peroxyl radical, nitric oxide, hypochlorite or molecular oxygen (De Lazzari et al., 2018, Salvemini et al., 2001, p. 40403) and supress cytokines production (Fig. 4). They also possess catalytic activity as the original enzyme as well as benefit by being much smaller in size. (e.g., MW483-M40403 vs. MW30000 for the mimetic and native enzyme respectively). Different classes of SOD mimetics have been identified and their specific antioxidant effects have also been illustrated (Jan Olof G. Karlsson et al., 2012; Miriyala et al., 2012; Rosa et al., 2021). The different classes of SOD mimetics include (Fig. 5A - 5D): Fig. 6, Fig. 7, Fig. 8
-
▪
Porphyrin-based SOD mimetics (Mn-metalloporphyrin’s)
-
▪
Mn (II)-cyclic polyamines-based SOD mimetics
-
▪
Salen–Mn complexes
-
▪
Mn-PLED derivatives
-
▪
Nitroxides
-
▪
Metal based compounds (E.g. [Fe (HPCINOL)Cl2]NO3, RM191A)
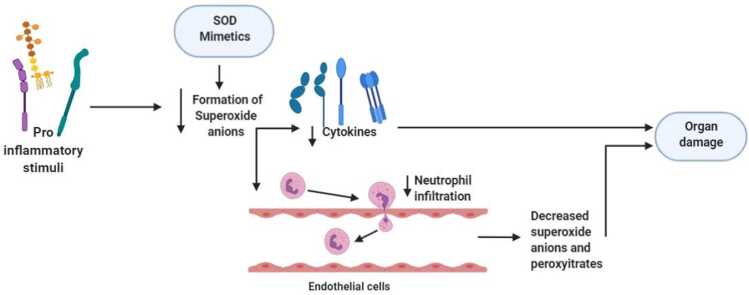
Fig. 4.
. SOD mimeticsMechanism of action of SOD mimetic alleviated pro-inflammatory stimuli triggered productions of superoxide anions and cytokines followed by the decrease in neutrophil infiltration and eventually protects the organ damage.
Fig. 5.
Structures of SOD mimetics. Basic structure of poryphyrin based SOD mimetics.
Fig. 6.
: Structure of M40403 SOD mimetics.
Fig. 7.
: Structure of Salen Manganese complex- EUK 134.
Fig. 8.
: Chemical structure of Nitric Oxide based - tempol.
The major characteristic features of SODs mimics:
-
▪
Low molecular weight with longer half-life in blood
-
▪
Non-peptide, non-immunogenic, and non-antigenic
-
▪
Ability to penetrate cellular and subcellular membranes
-
▪
Shows catalytic activity that facilitate free radicals scavenging
-
▪
Selectively acts on superoxide
-
▪
Stability in in-vivo, promising integrity of the metal site under biologic conditions
-
▪
Possess limitless possibilities of altering the porphyrin core structure
-
▪
Possess protective actions in different models of shock, acute and chronic inflammation, and also reperfusion injury.
5.1. Therapeutic implications of SOD mimetics
SOD mimetics are found to be effective against OS-mediated injuries in several experimental models and a few of them are already in the market (Table2). In metabolic diseases, reduction of SOD2 activity is associated with systemic OS and dysfunctional mitochondria. Hence, the catalytic SOD2 mimetic alleviates liver steatosis and insulin sensitivity as well as decreases inflammation related with obesity-induced type 2 diabetes (Coudriet et al., 2017). An in-vitro study demonstrates that overexpression of SOD2 in transgenic mouse embryonic fibroblasts devoid of functional Sirtuin 3 gene (a mitochondrial fidelity protein that protects redox homeostasis) protected these cells from mitochondrial OS and altered metabolism on exposure to 3,3'-dichlorobiphenyl metabolite (3,3’-dichlorobiphenyl-4-ol) (Alam et al., 2018). In vivo studies have proven the beneficial effects of SOD mimetics as protectors against ischemia reperfusion injury, radiation injuries, diabetes, or and enhancing anti-cancer effects (Coudriet et al., 2017, El-Mahdy et al., 2020, Moreton et al., 2022). These findings reveal the potential therapeutic effects of SOD mimetics in the treatment and management of OS-mediated injuries that underlie mitochondrial dysfunctional, neurodegenerative, and inflammation.
Table 2.
Superoxide Dismutase mimetics: Chemical name and Commercial name.
| Class | Chemical name | Product |
|---|---|---|
| Mn-based porphyrin | MnIIImeso-tetrakis(N-n-butoxyethylpyridinium-2-yl) porphyrin | MnBuOE |
| Mn cyclic polyamines | N,N′-bis{(1 R,2 R)-[2-(amino)] cyclohexyl}− 1,2-diaminoethanetetra hydrochloride | M40403 |
| Mn-salen | N,N′-bis(salicylidene) ethylenediamine chloride complex | EUK-8 |
| Mn-PLED | MnDPDP | Mangafodipir |
| Nitroxide | 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl | TEMPOL |
5.1.1. Porphyrin-based SOD mimetics
Porphyrin-based mimetics comprise more than 50 effective water-soluble molecules with lower toxicities and more catalytic property as well as different physicochemical and functional properties (Salvemini et al., 2001, p. 40403). Mn-centred porphyrins (MnPs) were found to be O2·−/ONOO− scavengers and modulate the redox-sensitive cellular transcriptional activity (Batinić-Haberle et al., 2010). MnPs dose-dependently catalysed the oxidation of hydrogen sulfide (H2S) to longer-chain polysulfides and hydrogen persulfide (H2S2) (Olson et al., 2019). The Mn (III) meso-tetrakis (N-ethylpyridinium-2-yl) porphyrin, MnIIITE-2-PyP5+ (AEOL-10113) has shown to be useful in treating OS-induced conditions such as radiation damage, CNS trauma, cancer, and diabetes (Spasojević et al., 2007). The ability of MnIIITE-2-PyP5+ to dismutate O2•−. parallels its capability to scavenge ONOO-. and CO3-. (Ferrer-Sueta et al., 2003). AEOL-11114 is found to be an effective Mn-centred porphyrin against MPTP mouse model of PD. AEOL-11114 increased absorption in gastrointestinal tract, longer plasma half-life, blood brain barrier penetration and attainment of active concentration in brain (Liang et al., 2017). Together, these results suggest that MnPs are effective cytoprotectors by acting as sulfide oxidation catalysts generating per/polysulfides.
5.1.2. Mn (II)-cyclic polyamines-based SOD mimetics
Mn (II)-cyclic polyamines-based SOD mimetics are newer class which were found to be effective against inflammation as well as OS-related conditions. The centre of these SOD mimetics contains a Mn (II) which is responsible for its selectivity property. It undergoes oxidation into Mn (III) by protonated superoxide which is reactive, but when it reduces to its Mn form in cases of diffusion-controlled rates, the complex is relatively inert against reducing agents. Oxidants containing one electron have not shown oxidative properties against these associated complexes (including NO and oxygen) as they are relatively difficult to oxidize. M40403 and other components of this class of SOD mimetics behave as selective probes by taking up the role of superoxide anion present in the biological system, where other applicable biological oxidants may be existing (Salvemini et al., 2002a).
5.1.2.1. M40403
M40403 is prototypical complex of the family, as it contains aromatic rings synchronized with Mn centre. M40403 has less molecular weight molecule, which is much smaller than natural SODs and are more lipid soluble, thus it can pass through the cellular membrane and BBB, (Salvemini et al., 1999) and were found to be more stable than other mimetics (Baudry et al., 1993). M40403 exhibit resistance to oxidative degradation and catalyse superoxide radicals and other species such as NO, hypochlorite, hydrogen peroxide, and peroxynitrite. Both M40403 and M40401 complexes produce valuable effects in models of ischemia, intestinal and acute inflammation (Cuzzocrea et al., 2001b; Aston et al., 2001). Clinical studies have shown the analgesic effects, for post-operative pain relief from dental operations and bunionectomy when combining M40403 with other approaches (Salvemini et al., 2002b). Hence, newer derivatives with greater stability are being designed and developed.
5.1.3. Salen–manganese complexes
Synthetic salen-Mn complexes represent a class of SOD and CAT mimetics whose hydrogen peroxide scavenging and cellular protective effects can be significantly manipulated. Derivatives of salen-Mn complexes have low molecular weight, better catalytic activity, and higher cell-permeability. Therefore, salen-Mn complexes are found to have more therapeutic potential in several diseases linked to OS and nitrosative stress (Salvemini et al., 2002b, Watanabe et al., 2007). EUK compounds (given via injection or infusion) are found to effective against OS in epileptic rat model with seizure-induced hippocampal injury, mouse model of ALS, rat stroke model and PD, and a human prion disease mouse model (Brazier et al., 2008, Melov et al., 2001, Peng et al., 2005). Salen-Mn complexes were found to extend survival and reduce oxidative pathologies in mice with inactivated SOD2 (sod2 nullizygous mice).
5.1.3.1. EUK-113
EUK-113, a demonstrative of the Mn-salen complexes, shows two-fold SOD activity. SOD activity of EUK-113 is observed in both Hepes and phosphate buffer (Friedel et al., 2012). The corresponding manganese ion present in oxidation state in N,N′-bis(salicylidene) ethylenediamine chloride (EUK-8) complex governs whether Mn-salen complex acts as an SOD or catalase mimetic (Sharpe et al., 2002).
5.1.3.2. EUK-8
EUK-8 is more active than other salen-Mn complex derivatives and the most water-soluble derivative exhibiting both CAT and SOD activities (Doctrow et al., 1997, Gonzalez et al., 1995a) indicating that salen-Mn complexes can scavenge hydrogen peroxide in addition to superoxide. In an in vitro study, EUK-8 has shown to protect organotypic hippocampal cultures from Aβ toxicity (Bruce et al., 1996). In vivo studies have demonstrated that repeated injection of EUK-8 inhibited the degeneration of dopaminergic neurons in murine model (Doctrow et al., 1997) and also suppressed the progress of paralysis in mouse experimental autoimmune encephalomyelitis (a human model for multiple sclerosis) (Malfroy et al., 1997). Intravenously infusion of EUK-8 ameliorated acute lung injury in a highly stringent porcine endotoxin-induced model for the adult respiratory distress syndrome(Gonzalez et al., 1995b).
5.1.3.3. EUK-134
EUK-134 is a 3,3′- methoxy salen ring-disubstituted EUK-8 analogue with a low molecular weight and equivalent SOD activity, but increased CAT activity. EUK-134 is found to be potent neuroprotective than EUK-8 in a rat model of stroke (Baker et al., 1998). EUK-134 prevented OS injury in hippocampal neurons and attenuated numerous indicators of neuropathology in kainic acid-induced seizure model in rats (Rong et al., 1999). A study has shown that EUK-8 and EUK-134 reduced indicators of spinal cord OS and prolonged the survival in a mouse model of FALS (Jung et al., 2001). Previous reports have shown that both EUK-134 and EUK-8 extend the lifetime of Caenorhabditis elegans, a nematode worm (Melov et al., 2000).
5.1.4. Mn-PLED derivatives
MnPLED-derivatives are another important group of complexes showing SOD-activity. Mainly, Mangafodipir (Manganese dipyridoxyl diphosphate (MnDPDP)) prevent the destruction of endothelium-derived NO by superoxide in bovine mesenteric arteries (Asplund et al., 1994). Both in-vitro and in-vivo studies confirmed the benefits of MnPLED derivatives in cancer treatment, ischemia-reperfusion injury in mouse models of liver toxicity and acute myocardial infarction (Asplund et al., 1994; Jan Olof G. Karlsson et al., 2012). In an ex-vivo study, MnDPDP improved contractile function and reduced the release of lactate dehydrogenase in rat hearts exposed to hypoxia-reoxygenation (Brurok et al., 1999). Animal experimental studies have shown that MnDPDP protects against paracetamol intoxication (Bedda et al., 2003) and paclitaxel myelosuppression (Alexandre et al., 2006). MnDPDP treatment can be given to cancer patients with pre-existing oxaliplatin (a chemotherapy drug)-associated peripheral sensory neurotoxicity (Brurok et al., 1999). Replacement of 80% of Mn2+ in mangafodipir with Ca2+ results in the formation of another MnPLED-derivative, calmangafodipir. Calmangafodipir binds readily with considerable amount of plasma Zn2+ resulting in significant retention of Mn2+ by reducing its release from the brain. At corresponding Mn2+ doses, calmangafodipir was found to be substantially more efficient than MnDPDP to protect BALB/c mice against myelosuppressive effects of oxaliplatin (Jan Olof G Karlsson et al., 2012, p. 1).
5.1.5. Nitroxides
Nitroxides constitute a class of weaker SOD mimetics. Under in vivo conditions, reduction of nitroxides to hydroxylamine functions as an antioxidant (Trnka et al., 2009). TEMPOL (4-hydroxy-2,2,6,6-tetramethylpiperidine-Noxyl) is an aqueous soluble as well as redox cycling nitroxide SOD mimetic agent. It neutralises ROS and RNS, and due to its less molecular weight passes through the biological membrane. Tempol exhibits protective effects against metabolic syndrome, shock, and radiation as well as kidney, heart and CNS protection (Bernardy et al., 2017, Krishna et al., 1992). In-vitro models have proven the protective effects of TEMPOL against hypoxanthine/xanthine oxidase or hydrogen peroxide exposure (Samuni et al., 1991). In established model of multiple sclerosis, oral intake of TEMPOL modulates the immune response and limits the autoimmune disease and damages in the CNS (Neil et al., 2017). TEMPOL prevents thermal hyperalgesia, neuropathic pain induced by chronic constriction injury, superoxide anion-induced neuronal firing, chemotherapy in rats and oedema induced by carrageenan (Khattab, 2006, Kim et al., 2017, Zhao et al., 2016). TEMPOL inhibits neutrophils recruitment in mice and TNF-induced mechanical hyperalgesia (Abouzied et al., 2016). Treatment with TEMPOL also removes free radicals and prevents lipid peroxidation in vitro and in vivo (Abouzied et al., 2016, Ali et al., 2016).
5.2. Clinical trials
Since there is no sufficient documentation of clinical trials data of SOD mimetics, the information generated in the form of a table depicts the studied relating to anti-oxidants and neurological disorders and their specific interventions (Table 3).
Table 3.
List of antioxidants used in neurological disorders clinical trials.
| S. No |
Study title (Trial number) |
Conditions | Interventions | Location | Status | Outcomes | References |
| 1 | Anti-Oxidant Treatment of Alzheimer's Disease (NCT00117403) |
Alzheimer’s Disease | Drug: Vitamin E, Vitamin C, and Alpha-lipoic Acid Drug: Coenzyme Q Drug: Placebo capsules Drug: Placebo wafers |
University of Arizona Tucson, Arizona, United States University of California- Irvine Irvine, California, United States University of California, San Diego La Jolla, California, United States |
Completed | Anti-oxidant administration in AD subjected decreased CSF biomarkers related to oxidative damage, varied a beta42 and a beta40 concentrations. | (National Institute on Aging (NIA), 2009) |
| 2 | Glutathione and Health with Post-Polio Syndrome (NCT01402570) |
Depression | Glutathione | University of Michigan Ann Arbor, Michigan, United States |
Completed | Glutathione intervention increased ability to carry out physical activities from self-care to complex activities. Increased sleep efficiency, and decreased fatigue | (Kalpakjian, 2016) |
| 3 | Mitochondrial Dysfunction in the Pathophysiology and Treatment of Bipolar Disorder (NCT00327756) |
Depression | Drug: Coenzyme Q10 | National Institutes of Health Clinical Centre, 9000 Rockville Pike Bethesda, Maryland, United States |
Completed | No results posted | (National Institute of Mental Health (NIMH), 2011) |
| 4 | Clinical Trial of High Dose CoQ10 in ALS (NCT00243932) |
Amyotrophic Lateral Sclerosis | Drug: coenzyme Q10 Drug: Placebo |
University of Arkansas for Medical Sciences, Department of Neurology Little Rock, Arkansas, United States California Pacific Medical Centre San Francisco, California, United States University of California at San Francisco San Francisco, California, United States |
Completed | CoQ10 improved scores on ALS functional rating scale, changes in forced vital capacity, fatigue severity scale, and decreased 8OH2Dg in ALS patients | (Ferrante et al., 2005) |
| 5 | Expanded Controlled Study of Safety and Efficacy of MCI-186 in Patients with Amyotrophic Lateral Sclerosis (ALS) (NCT00424463) |
Amyotrophic Lateral Sclerosis | Drug: MCI-186 Drug: Placebo of MCI-186 |
National Hospital Organization Miyagi National Hospital Watari-gun, Miyagi-ken, Japan |
Completed | MCI-186 improved ALSFRS score and changes in forced vital capacity in ALS subjects | (Abe et al., 2014) |
| 6 | Phase IIb Study of Intranasal Glutathione in Parkinson's Disease (NCT02424708) |
Parkinson’s Disease | Drug: Reduced Glutathione 100 mg Drug: Reduced Glutathione 200 mg Drug: Placebo |
Bastyr University Kenmore, Washington, United States University of Washington Seattle, Washington, United State |
Completed | Glutathione improved total and motor scores in PD subjects | (Mischley et al., 2017) |
| 7 | CNS Uptake of Intranasal Glutathione (NCT02324426) |
Parkinson’s Disease | Drug: Reduced Glutathione | University of Washington Seattle, Washington, United States |
Completed | Intranasal administration of GSH elevated the brain GSH levels in patients with Parkinson’s disease. | (Mischley, 2015) |
| 8 | Role of antioxidants (Vitamin C and E) in the Management of Schizophrenia (NCT04078048) |
Schizophrenia | Dietary Supplement: antioxidants (Vitamin C, E) | SaimaKouse Lahore, Punjab, Pakistan |
Completed | No results posted. | (Zahid, 2019) |
| 9 | Treatment of Schizophrenia with an Omega-3 Fatty Acid (EPA) and antioxidant (NCT00419146) |
Schizophrenia | Drug: Ethyl-eicosapentaenoic acid (EPA) Drug: Vitamins E + C Other: Etyl EPA (placebo) Other: Vitamins E + C (placebo) |
Aker University Hospital Oslo, Norway |
Completed | No results posted | (National Center for Complementary and Integrative Health (NCCIH), 2006) |
| 10 | Effects of EGCG (Epigallocatechin Gallate) in Huntington’s disease (ETON-Study) (NCT01357681) |
Huntington’s disease | Drug: (2)-epigallocatechin-3-gallate (EGCG) Drug: Placebo |
Department of Neuropsychiatry Berlin, Germany NeurologischeKlinik der Ruhr-Universität Bochum Bochum, Germany, Universitätsklinikum Ulm, KlinikfürNeurologie Ulm, Germany |
Completed | No results posted | (Priller, 2015) |
| 11 | Natural anti-oxidants in the Treatment of multiple sclerosis (NCT00010842) |
Multiple Sclerosis | Drug: Ginkgo biloba Drug: Alpha-lipoic acid Drug: Vitamin E/Selenium Drug: Essential fatty acids |
Oregon Health Sciences University Portland, Oregon, United States |
Completed | No results posted | (National Center for Complementary and Integrative Health (NCCIH), 2006) |
| 12 | Defining the Anti-inflammatory Role of Lipoic Acid in multiple sclerosis (NCT00997438) |
Multiple Sclerosis | Dietary Supplement: Lipoic Acid | Oregon Health & Science University Portland, Oregon, United States Portland VA Medical Center Portland, Oregon, United States |
Completed | Lipoic acid supplementation stimulated cAMP production reduced disease severity. Decreased brain atrophy was also seen among the MS patients | (Carr, 2016) |
| 13 | Cognitive Impact of Pomegranate Polyphenols Following Ischemic Stroke (NCT02442804) |
Stroke | Dietary Supplement: POMx Other: Placebo |
Loma Linda University East Campus Hospital Loma Linda, California, United States |
Completed | POMs improved neuropsychological functions, functional independence score and decreased scores on Trial-making Test. | (Bellone, 2017) |
| 14 | Safety and Pharmacokinetics of MCI-186 in Subjects with Acute Ischemic Stroke (NCT00821821) |
Acute Ischemic Stroke (AIS) | Drug: MCI-186 Drug: Placebo |
Helsinki University Central Hospital Helsinki, Finland Erasmus Medical Centre Rotterdam, Netherlands Newcastle upon Tyne Hospitals NHS Foundation Trust Newcastle, United Kingdom |
Completed | MCI-186 was well tolerated at the tested doses in AIS patients. Adverse events also seen among 109 patients | (Mitsubishi Tanabe Pharma Corporation, 2014) |
| 15 | Study of Glutathione, Vitamin C and Cysteine in Children with Autism and Severe Behaviour Problems | Autism Severe Behaviour Disorder |
Drug: placebo Drug: glutathione Drug: glutathione, vit C and NAC |
KCPCRU Louisville, Kentucky, United States |
Completed | No results posted | |
| 16 | Treating Oxidative Stress and the Metabolic Pathology of Autism (NCT00572741) |
Autistic Disorder | Dietary Supplement: B12 Dietary Supplement: Placebo Dietary Supplement: folate |
Arkansas Children's Hospital Research Institute Little Rock, Arkansas, United States |
Completed | No results posted | (Arkansas Children’s Hospital Research Institute, 2016) |
| 17 | Vitamins and Minerals for Children with Down’s Syndrome (NCT00378456) |
Trisiomy 21 Down’s Syndrome | Drug: Anti-oxidants (Vitamins A, C, E Selenium and Zinc) Drug: Folinic acid Drug: Vitamins A, C, E Selenium and Zinc and Folinic acid. Drug: Placebo |
Institute of Child Health Birmingham, United Kingdom Peninsular Medical School Exeter, United Kingdom Institute of Child Health, London London, United Kingdom |
Completed | Anti-oxidant supplementation didn’t show any improvement in children with Down’s syndrome. | (Blair et al., 2008) |
| 18 | Normalization of dyrk1A and APP Function as an Approach to Improve Cognitive Performance and Decelerate AD Progression in DS Subjects: Epigallocatechin Gallate as Therapeutic Tool (NCT01699711) |
Down’s Syndrome | Dietary Supplement: Epigallocatechin-3-gallate (EGCG) | IMIM (Institut Hospital del Mar d'InvestigacionsMèdiques) Barcelona, Spain |
Completed | EGCG and cognitive training for 12 months was found to be effective than placebo | (Torre, 2016) |
| 19 | Double-Blind, Placebo-Controlled Trial of Vitamin E as Add-on Therapy for Children with Epilepsy (NCT00004637) |
Epilepsy | Drug: Vitamin E | The Children's Hospital, Neurology B155 Denver, Colorad o, United States |
Completed | Children received Vit-E has increased vitamin-E levels | (Gupta et al., 2004) |
| 20 | Turmeric as Treatment in Epilepsy (NCT03254680) |
Epilepsy | Dietary Supplement: Turmeric | New York University School of Medicine New York, New York, United States |
Completed | No results posted. | (NYU Langone Health, 2018) |
6. Limitations of SOD mimetics
In clinical applications, the use of naïve SODs has been limited due to instability, immunogenicity, low half-life, and increased renal excretion. These problems may be overcome by developing novel drug deliveries using liposomes. Several conjugates of SOD are being developed to overcome these limitations and many of these have shown promising therapeutic effects in-vivo (Younus, 2018). Another general limitation of these agents is they react with a wide variety of ROS and RNS, without specificity. From a pharmacological perspective, SOD enzymes are expected to remove only superoxide anions without reacting with other reactive species, but non-selectivity of SOD mimetics clouds the interpretation of the results. Thus, great caution must be taken before using SOD mimetics by clearly defining the class of mimetic and type of ROS involved in the disease pathology, without making general assumptions (Muscoli et al., 2003). For example, MnSOD mimetics conjugated with lipophilic cations for mitochondria specificity might have cytotoxic effect as they interfere with adenosine triphosphate production (ATP) (Liu et al., 2022).
6.1. Future implications of the strategy
SOD mimetics gains more attention in chronic illness such as cancer, stroke, allergy, neurodegenerative diseases, and neuropsychiatric disorders. They can be used in combination with conventional treatment, thereby synergising the effectiveness of such treatments and attenuating drug side-effects (Bonetta, 2018). Successful development of SOD mimetics with specific selectivity towards particular OS species will enhance the safety of the therapeutic approaches. Extensive preclinical and clinical research are needed to design selective SOD mimics designed for the development of chronic neurological disorders.
7. Conclusion
Superoxide dismutase play crucial role in physiological and diseased states. This review provides a comprehensive function of SOD in neurological disorders such as Alzheimer’s disease, Parkinson’s disease, stroke, epilepsy, and neuropsychiatric disorders. The pathophysiological basis of these disorders indicates the overproduction of superoxide anions, mitochondrial dysfunction, and altered signalling transduction that with damages DNA, proteins, or lipids resulting in inflammation and cell death. This provides a unique window to reduce OS-mediated injuries by removing superoxide anions using antioxidants. Exogenous application of SOD enzymes has been used as a pharmaceutical in OS-related diseases, but imposes therapeutic challenges due to its high molecular weight, reduced cellular permeability and stability, and shorter half-life. Recent development of synthetic small molecular conjugates of SOD with redox modulating properties have demonstrated improved performance by overcoming the limitations of the naïve SOD enzymes. This review also highlights the beneficial effects of SOD mimetics and thus may probably be the future of OS targeted therapies in neurological disorders. In summary, this review hints the use of SOD mimetics in the treatment of neurological disorders, and they can be considered as adjunct therapies, at least partly.
Ethics approval and consent to participate
Not Applicable.
Author contributions
Conceptualization, design, methodology, writing & original draft preparation, and formal analysis: SBC; writing, review and editing: Nikilesh, SRV,SR, CV, AS & AMM; MME: participated in manuscript drafting, revising and editing.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgments
The authors thank their respective institutions for providing the facilities during the manuscript preparation.
Contributor Information
Saravana Babu Chidambaram, Email: babupublications@gmail.com.
Nikhilesh Anand, Email: nanand@auamed.net.
Musthafa Mohamed Essa, Email: drmdessa@gmail.com.
Data Availability
Data that support the findings of this study are available in standard research databases such as PubMed, Science Direct, or Google Scholar, and/or on public domains that can be searched with either key words or DOI numbers.
References
- Abe K., Itoyama Y., Sobue G., Tsuji S., Aoki M., Doyu M., Hamada C., Kondo K., Yoneoka T., Akimoto M., Yoshino H. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph. Lateral Scler. Front. Degener. 2014;15:610–617. doi: 10.3109/21678421.2014.959024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abouzied M.M., Eltahir H.M., Taye A., Abdelrahman M.S. Experimental evidence for the therapeutic potential of tempol in the treatment of acute liver injury. Mol. Cell. Biochem. 2016;411:107–115. doi: 10.1007/s11010-015-2572-2. [DOI] [PubMed] [Google Scholar]
- Aguirre N., Beal M.F., Matson W.R., Bogdanov M.B. Increased oxidative damage to DNA in an animal model of amyotrophic lateral sclerosis. Free Radic. Res. 2005;39:383–388. doi: 10.1080/10715760400027979. [DOI] [PubMed] [Google Scholar]
- Alam S., Carter G.S., Krager K.J., Li X., Lehmler H.-J., Aykin-Burns N. PCB11 Metabolite, 3,3’-Dichlorobiphenyl-4-ol, Exposure Alters the Expression of Genes Governing Fatty Acid Metabolism in the Absence of Functional Sirtuin 3: Examining the Contribution of MnSOD. Antioxid. Basel Switz. 2018;7 doi: 10.3390/antiox7090121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre J., Nicco C., Chéreau C., Laurent A., Weill B., Goldwasser F., Batteux F. Improvement of the therapeutic index of anticancer drugs by the superoxide dismutase mimic mangafodipir. J. Natl. Cancer Inst. 2006;98:236–244. doi: 10.1093/jnci/djj049. [DOI] [PubMed] [Google Scholar]
- Ali M.R.A.-A., Abo-Youssef A.M.H., Messiha B.A.S., Khattab M.M. Tempol and perindopril protect against lipopolysaccharide-induced cognition impairment and amyloidogenesis by modulating brain-derived neurotropic factor, neuroinflammation and oxido-nitrosative stress. Naunyn. Schmiede Arch. Pharmacol. 2016;389:637–656. doi: 10.1007/s00210-016-1234-6. [DOI] [PubMed] [Google Scholar]
- Al-Muhammadi, M., Al-Tameemi, K., Kadhim, H., 2015. Role of Superoxide Dismutase Enzyme in Patients with Epilepsy 12, 1015–1019.
- Alscher R.G., Erturk N., Heath L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002;53:1331–1341. doi: 10.1093/jexbot/53.372.1331. [DOI] [PubMed] [Google Scholar]
- Andreassen O.A., Ferrante R.J., Dedeoglu A., Albers D.W., Klivenyi P., Carlson E.J., Epstein C.J., Beal M.F. Mice with a partial deficiency of manganese superoxide dismutase show increased vulnerability to the mitochondrial toxins malonate, 3-nitropropionic acid, and MPTP. Exp. Neurol. 2001;167:189–195. doi: 10.1006/exnr.2000.7525. [DOI] [PubMed] [Google Scholar]
- Andreazza A.C., Cassini C., Rosa A.R., Leite M.C., de Almeida L.M.V., Nardin P., Cunha A.B.N., Ceresér K.M., Santin A., Gottfried C., Salvador M., Kapczinski F., Gonçalves C.A. Serum S100B and antioxidant enzymes in bipolar patients. J. Psychiatr. Res. 2007;41:523–529. doi: 10.1016/j.jpsychires.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Arkansas Children’s Hospital Research Institute, 2016. Treating Oxidative Stress and the Metabolic Pathology of Autism (Clinical trial registration No. NCT00572741). clinicaltrials.gov.
- Asplund A., Grant D., Karlsson J.O. Mangafodipir (MnDPDP)-and MnCl2-induced endothelium-dependent relaxation in bovine mesenteric arteries. J. Pharmacol. Exp. Ther. 1994;271:609–614. [PubMed] [Google Scholar]
- Atanasova M., Petkova Z., Pechlivanova D., Dragomirova P., Blazhev A., Tchekalarova J. Strain-dependent effects of long-term treatment with melatonin on kainic acid-induced status epilepticus, oxidative stress and the expression of heat shock proteins. Pharmacol. Biochem. Behav. 2013;111:44–50. doi: 10.1016/j.pbb.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Ayton S., Lei P., Duce J.A., Wong B.X.W., Sedjahtera A., Adlard P.A., Bush A.I., Finkelstein D.I. Ceruloplasmin dysfunction and therapeutic potential for Parkinson disease. Ann. Neurol. 2013;73:554–559. doi: 10.1002/ana.23817. [DOI] [PubMed] [Google Scholar]
- Azadmanesh J., Borgstahl G. A Review of the Catalytic Mechanism of Human Manganese Superoxide Dismutase. Antioxidants. 2018;7:25. doi: 10.3390/antiox7020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu C.S., Ramanathan M. Post‐ischemic administration of nimodipine following focal cerebral ischemic‐reperfusion injury in rats alleviated excitotoxicity, neurobehavioural alterations and partially the bioenergetics. Int. J. Dev. Neurosci. 2011;29:93–105. doi: 10.1016/j.ijdevneu.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Babu C.S., Ramanathan M. Post‐ischemic administration of nimodipine following focal cerebral ischemic‐reperfusion injury in rats alleviated excitotoxicity, neurobehavioural alterations and partially the bioenergetics. Int. J. Dev. Neurosci. 2011;29:93–105. doi: 10.1016/j.ijdevneu.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Bai Z.-L., Li X.-S., Chen G.-Y., Du Y., Wei Z.-X., Chen X., Zheng G.-E., Deng W., Cheng Y. Serum Oxidative Stress Marker Levels in Unmedicated and Medicated Patients with Schizophrenia. J. Mol. Neurosci. MN. 2018;66:428–436. doi: 10.1007/s12031-018-1165-4. [DOI] [PubMed] [Google Scholar]
- Bajpai A., Verma A.K., Srivastava M., Srivastava R. Oxidative Stress and Major Depression. J. Clin. Diagn. Res. JCDR. 2014;8:CC04–CC07. doi: 10.7860/JCDR/2014/10258.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K., Marcus C.B., Huffman K., Kruk H., Malfroy B., Doctrow S.R. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: a key role for reactive oxygen species in ischemic brain injury. J. Pharmacol. Exp. Ther. 1998;284:215–221. [PubMed] [Google Scholar]
- Barone E., Arena A., Head E., Butterfield D.A., Perluigi M. Disturbance of redox homeostasis in Down Syndrome: role of iron dysmetabolism. Free Radic. Biol. Med. 2018;114:84. doi: 10.1016/j.freeradbiomed.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batinić-Haberle I., Rebouças J.S., Spasojević I. Superoxide Dismutase Mimics: Chemistry, Pharmacology, and Therapeutic Potential. Antioxid. Redox Signal. 2010;13:877–918. doi: 10.1089/ars.2009.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry M., Etienne S., Bruce A., Palucki M., Jacobsen E., Malfroy B. Salen-manganese complexes are superoxide dismutase-mimics. Biochem. Biophys. Res. Commun. 1993;192:964–968. doi: 10.1006/bbrc.1993.1509. [DOI] [PubMed] [Google Scholar]
- Bedda S., Laurent A., Conti F., Chéreau C., Tran A., Tran-Van Nhieu J., Jaffray P., Soubrane O., Goulvestre C., Calmus Y., Weill B., Batteux F. Mangafodipir prevents liver injury induced by acetaminophen in the mouse. J. Hepatol. 2003;39:765–772. doi: 10.1016/s0168-8278(03)00325-8. [DOI] [PubMed] [Google Scholar]
- Bellone, J., 2017. The Effects of Pomegranate Polyphenols on Neuropsychological Functioning Following Ischemic Stroke (Clinical trial registration No. NCT02442804). clinicaltrials.gov.
- Benzi G., Moretti A. Are reactive oxygen species involved in Alzheimer’s disease? Neurobiol. Aging. 1995;16:661–674. doi: 10.1016/0197-4580(95)00066-n. [DOI] [PubMed] [Google Scholar]
- Berdyński M., Miszta P., Safranow K., Andersen P.M., Morita M., Filipek S., Żekanowski C., Kuźma-Kozakiewicz M. SOD1 mutations associated with amyotrophic lateral sclerosis analysis of variant severity. Sci. Rep. 2022;12 doi: 10.1038/s41598-021-03891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardy C.C.F., Zarpelon A.C., Pinho-Ribeiro F.A., Calixto-Campos C., Carvalho T.T., Fattori V., Borghi S.M., Casagrande R., Verri W.A. Tempol, a Superoxide Dismutase Mimetic Agent, Inhibits Superoxide Anion-Induced Inflammatory Pain in Mice. BioMed. Res. Int. 2017;2017 doi: 10.1155/2017/9584819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C.N., Bot M., Scheffer P.G., Cuijpers P., Penninx B.W.J.H. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology. 2015;51:164–175. doi: 10.1016/j.psyneuen.2014.09.025. [DOI] [PubMed] [Google Scholar]
- Black C.N., Bot M., Scheffer P.G., Penninx B.W.J.H. Oxidative stress in major depressive and anxiety disorders, and the association with antidepressant use; results from a large adult cohort. Psychol. Med. 2017;47:936–948. doi: 10.1017/S0033291716002828. [DOI] [PubMed] [Google Scholar]
- Blair C.K., Roesler M., Xie Y., Gamis A.S., Olshan A.F., Heerema N.A., Robison L.L., Ross J.A. Vitamin supplement use among children with Down syndrome and risk of leukemia: A Children’s Oncology Group (COG) Study. Paediatr. Perinat. Epidemiol. 2008;22:288–295. doi: 10.1111/j.1365-3016.2008.00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov M., Brown R.H., Matson W., Smart R., Hayden D., O’Donnell H., Flint Beal M., Cudkowicz M. Increased oxidative damage to DNA in ALS patients. Free Radic. Biol. Med. 2000;29:652–658. doi: 10.1016/s0891-5849(00)00349-x. [DOI] [PubMed] [Google Scholar]
- Bonetta R. Potential Therapeutic Applications of MnSODs and SOD-Mimetics. Chem. Weinh. Bergstr. Ger. 2018;24:5032–5041. doi: 10.1002/chem.201704561. [DOI] [PubMed] [Google Scholar]
- Borgstahl G.E., Parge H.E., Hickey M.J., Beyer W.F., Hallewell R.A., Tainer J.A. The structure of human mitochondrial manganese superoxide dismutase reveals a novel tetrameric interface of two 4-helix bundles. Cell. 1992;71:107–118. doi: 10.1016/0092-8674(92)90270-m. [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E., Petrilli A., Knott A.B. Mutant huntingtin and mitochondrial dysfunction. Trends Neurosci. 2008;31:609–616. doi: 10.1016/j.tins.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling A.C., Beal M.F. Bioenergetic and oxidative stress in neurodegenerative diseases. Life Sci. 1995;56:1151–1171. doi: 10.1016/0024-3205(95)00055-b. [DOI] [PubMed] [Google Scholar]
- Brazier M.W., Doctrow S.R., Masters C.L., Collins S.J. A manganese-superoxide dismutase/catalase mimetic extends survival in a mouse model of human prion disease. Free Radic. Biol. Med. 2008;45:184–192. doi: 10.1016/j.freeradbiomed.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Bruce A.J., Malfroy B., Baudry M. beta-Amyloid toxicity in organotypic hippocampal cultures: protection by EUK-8, a synthetic catalytic free radical scavenger. Proc. Natl. Acad. Sci. U. S. A. 1996;93:2312–2316. doi: 10.1073/pnas.93.6.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brurok H., Ardenkjaer-Larsen J.H., Hansson G., Skarra S., Berg K., Karlsson J.O., Laursen I., Jynge P. Manganese dipyridoxyl diphosphate: MRI contrast agent with antioxidative and cardioprotective properties? Biochem. Biophys. Res. Commun. 1999;254:768–772. doi: 10.1006/bbrc.1998.0131. [DOI] [PubMed] [Google Scholar]
- Busciglio J., Yankner B.A. Apoptosis and increased generation of reactive oxygen species in Down’s syndrome neurons in vitro. Nature. 1995;378:776–779. doi: 10.1038/378776a0. [DOI] [PubMed] [Google Scholar]
- Butterfield D.A., Lauderback C.M. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic. Biol. Med. 2002;32:1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- Cao C., Liu M., Qu S., Huang R., Qi M., Zhu Z., Zheng J., Chen Z., Wang Z., Han Z., Zhu Y., Huang F., Duan J.-A. Chinese medicine formula Kai-Xin-San ameliorates depression-like behaviours in chronic unpredictable mild stressed mice by regulating gut microbiota-inflammation-stress system. J. Ethnopharmacol. 2020;261 doi: 10.1016/j.jep.2020.113055. [DOI] [PubMed] [Google Scholar]
- Carr, D., 2016. Defining the Anti-inflammatory Role of Lipoic Acid in Multiple Sclerosis (Clinical trial registration No. NCT00997438). clinicaltrials.gov.
- Ceballos-Picot I., Nicole A., Clément M., Bourre J.M., Sinet P.M. Age-related changes in antioxidant enzymes and lipid peroxidation in brains of control and transgenic mice overexpressing copper-zinc superoxide dismutase. Mutat. Res. 1992;275:281–293. doi: 10.1016/0921-8734(92)90032-k. [DOI] [PubMed] [Google Scholar]
- çevik mehmet uğur. Serum paraoxonase-1 activities and malondialdehyde levels in patients with epilepsy. Dicle Med. J. Dicle Tip. Derg. 2012;39:557–560. doi: 10.5798/diclemedj.0921.2012.04.0200. [DOI] [Google Scholar]
- Chang L.Y., Slot J.W., Geuze H.J., Crapo J.D. Molecular immunocytochemistry of the CuZn superoxide dismutase in rat hepatocytes. J. Cell Biol. 1988;107:2169–2179. doi: 10.1083/jcb.107.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi R.K., Hennessey T., Johri A., Tiwari S.K., Mishra D., Agarwal S., Kim Y.S., Beal M.F. Transducer of regulated CREB-binding proteins (TORCs) transcription and function is impaired in Huntington’s disease. Hum. Mol. Genet. 2012;21:3474–3488. doi: 10.1093/hmg/dds178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman K.H., Slater T.F. An introduction to free radical biochemistry. Br. Med. Bull. 1993;49:481–493. doi: 10.1093/oxfordjournals.bmb.a072625. [DOI] [PubMed] [Google Scholar]
- Chen H., Song Y.S., Chan P.H. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J. Cereb. Blood Flow. Metab. . J. Int. Soc. Cereb. Blood Flow. Metab. 2009;29:1262–1272. doi: 10.1038/jcbfm.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung S.G., Goldenthal A.R., Uhlemann A.-C., Mann J.J., Miller J.M., Sublette M.E. Systematic Review of Gut Microbiota and Major Depression. Front. Psychiatry. 2019;10 doi: 10.3389/fpsyt.2019.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidambaram S.B., Vijayan R., Sekar S., Mani S., Rajamani B., Ganapathy R. Simultaneous blockade of NMDA receptors and PARP-1 activity synergistically alleviate immunoexcitotoxicity and bioenergetics in 3-nitropropionic acid intoxicated mice: Evidences from memantine and 3-aminobenzamide interventions. Eur. J. Pharmacol. 2017;803:148–158. doi: 10.1016/j.ejphar.2017.03.023. [DOI] [PubMed] [Google Scholar]
- Choi J., Rees H.D., Weintraub S.T., Levey A.I., Chin L.-S., Li L. Oxidative modifications and aggregation of Cu,Zn-superoxide dismutase associated with Alzheimer and Parkinson diseases. J. Biol. Chem. 2005;280:11648–11655. doi: 10.1074/jbc.M414327200. [DOI] [PubMed] [Google Scholar]
- Ciancarelli I., De Amicis D., Di Massimo C., Sandrini G., Pistarini C., Carolei A., Tozzi Ciancarelli M.G. Influence of intensive multifunctional neurorehabilitation on neuronal oxidative damage in patients with Huntington’s disease. Funct. Neurol. 2015;30:47–52. [PMC free article] [PubMed] [Google Scholar]
- Ciancarelli I., Di Massimo C., De Amicis D., Carolei A., Tozzi Ciancarelli M.G. Evidence of redox unbalance in post-acute ischemic stroke patients. Curr. Neurovasc. Res. 2012;9:85–90. doi: 10.2174/156720212800410885. [DOI] [PubMed] [Google Scholar]
- Clausen A., Doctrow S., Baudry M. Prevention of cognitive deficits and brain oxidative stress with superoxide dismutase/catalase mimetics in aged mice. Neurobiol. Aging. 2010;31:425–433. doi: 10.1016/j.neurobiolaging.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen A., Xu X., Bi X., Baudry M. Effects of the superoxide dismutase/catalase mimetic EUK-207 in a mouse model of Alzheimer’s disease: protection against and interruption of progression of amyloid and tau pathology and cognitive decline. J. Alzheimers Dis. JAD. 2012;30:183–208. doi: 10.3233/JAD-2012-111298. [DOI] [PubMed] [Google Scholar]
- Cleveland D.W., Rothstein J.D. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat. Rev. Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- Cohen C.I., Meesters P.D., Zhao J. New perspectives on schizophrenia in later life: implications for treatment, policy, and research. Lancet Psychiatry. 2015;2:340–350. doi: 10.1016/S2215-0366(15)00003-6. [DOI] [PubMed] [Google Scholar]
- Coudriet G.M., Delmastro-Greenwood M.M., Previte D.M., Marré M.L., O’Connor E.C., Novak E.A., Vincent G., Mollen K.P., Lee S., Dong H.H., Piganelli J.D. Treatment with a Catalytic Superoxide Dismutase (SOD) Mimetic Improves Liver Steatosis, Insulin Sensitivity, and Inflammation in Obesity-Induced Type 2 Diabetes. Antioxidants. 2017;6:85. doi: 10.3390/antiox6040085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo J.D., Oury T., Rabouille C., Slot J.W., Chang L.Y. Copper,zinc superoxide dismutase is primarily a cytosolic protein in human cells. Proc. Natl. Acad. Sci. U. S. A. 1992;89:10405–10409. doi: 10.1073/pnas.89.21.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lazzari F., Bubacco L., Whitworth A.J., Bisaglia M. Superoxide Radical Dismutation as New Therapeutic Strategy in Parkinson’s Disease. Aging Dis. 2018;9:716–728. doi: 10.14336/AD.2017.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leo M.E., Borrello S., Passantino M., Palazzotti B., Mordente A., Daniele A., Filippini V., Galeotti T., Masullo C. Oxidative stress and overexpression of manganese superoxide dismutase in patients with Alzheimer’s disease. Neurosci. Lett. 1998;250:173–176. doi: 10.1016/s0304-3940(98)00469-8. [DOI] [PubMed] [Google Scholar]
- Dexter D.T., Wells F.R., Lees A.J., Agid F., Agid Y., Jenner P., Marsden C.D. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. J. Neurochem. 1989;52:1830–1836. doi: 10.1111/j.1471-4159.1989.tb07264.x. [DOI] [PubMed] [Google Scholar]
- Dietrich-Muszalska A., Kwiatkowska A. Generation of superoxide anion radicals and platelet glutathione peroxidase activity in patients with schizophrenia. Neuropsychiatr. Dis. Treat. 2014;10:703–709. doi: 10.2147/NDT.S60034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doctrow S.R., Huffman K., Marcus C.B., Musleh W., Bruce A., Baudry M., Malfroy B. . Salen-manganese complexes: combined superoxide dismutase/catalase mimics with broad pharmacological efficacy. Adv. Pharmacol. San. Diego Calif. 1997;38:247–269. doi: 10.1016/s1054-3589(08)60987-4. [DOI] [PubMed] [Google Scholar]
- Dubois B., Feldman H.H., Jacova C., Dekosky S.T., Barberger-Gateau P., Cummings J., Delacourte A., Galasko D., Gauthier S., Jicha G., Meguro K., O’brien J., Pasquier F., Robert P., Rossor M., Salloway S., Stern Y., Visser P.J., Scheltens P. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- El-Mahdy M.A., Alzarie Y.A., Hemann C., Badary O.A., Nofal S., Zweier J.L. The novel SOD mimetic GC4419 increases cancer cell killing with sensitization to ionizing radiation while protecting normal cells. Free Radic. Biol. Med. 2020;160:630–642. doi: 10.1016/j.freeradbiomed.2020.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante K.L., Shefner J., Zhang H., Betensky R., O’Brien M., Yu H., Fantasia M., Taft J., Beal M.F., Traynor B., Newhall K., Donofrio P., Caress J., Ashburn C., Freiberg B., O’Neill C., Paladenech C., Walker T., Pestronk A., Abrams B., Florence J., Renna R., Schierbecker J., Malkus B., Cudkowicz M. Tolerance of high-dose (3,000 mg/day) coenzyme Q10 in ALS. Neurology. 2005;65:1834–1836. doi: 10.1212/01.wnl.0000187070.35365.d7. [DOI] [PubMed] [Google Scholar]
- Ferrari M., Stagi S. Oxidative Stress in Down and Williams-Beuren Syndromes: An Overview. Mol. Basel Switz. 2021;26:3139. doi: 10.3390/molecules26113139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Sueta G., Vitturi D., Batinić-Haberle I., Fridovich I., Goldstein S., Czapski G., Radi R. Reactions of Manganese Porphyrins with Peroxynitrite and Carbonate Radical Anion. J. Biol. Chem. 2003;278:27432–27438. doi: 10.1074/jbc.M213302200. [DOI] [PubMed] [Google Scholar]
- Filograna R., Godena V.K., Sanchez-Martinez A., Ferrari E., Casella L., Beltramini M., Bubacco L., Whitworth A.J., Bisaglia M. Superoxide Dismutase (SOD)-mimetic M40403 Is Protective in Cell and Fly Models of Paraquat Toxicity: IMPLICATIONS FOR PARKINSON DISEASE. J. Biol. Chem. 2016;291:9257–9267. doi: 10.1074/jbc.M115.708057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice P.S., Shaw I.C., Kleiner H.E., Miller R.T., Monks T.J., Lau S.S., Mitchell J.D., Lynch P.G. Evidence for DNA damage in amyotrophic lateral sclerosis. Muscle Nerve. 1996;19:797–798. [PubMed] [Google Scholar]
- Flatow J., Buckley P., Miller B.J. Meta-Analysis of Oxidative Stress in Schizophrenia. Biol. Psychiatry. 2013;74:400–409. doi: 10.1016/j.biopsych.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J.L., Phiel C.J., Toland A.E. The role for oxidative stress in aberrant DNA methylation in Alzheimer’s disease. Curr. Alzheimer Res. 2012;9:1077–1096. doi: 10.2174/156720512803569000. [DOI] [PubMed] [Google Scholar]
- Folz R.J., Crapo J.D. Extracellular superoxide dismutase (SOD3): tissue-specific expression, genomic characterization, and computer-assisted sequence analysis of the human EC SOD gene. Genomics. 1994;22:162–171. doi: 10.1006/geno.1994.1357. [DOI] [PubMed] [Google Scholar]
- Freed R.D., Hollenhorst C.N., Weiduschat N., Mao X., Kang G., Shungu D.C., Gabbay V. A pilot study of cortical glutathione in youth with depression. Psychiatry Res. Neuroimaging. 2017;270:54–60. doi: 10.1016/j.pscychresns.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedel F.C., Lieb D., Ivanović-Burmazović I. Comparative studies on manganese-based SOD mimetics, including the phosphate effect, by using global spectral analysis. J. Inorg. Biochem. 2012;109:26–32. doi: 10.1016/j.jinorgbio.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Fukai T., Folz R.J., Landmesser U., Harrison D.G. Extracellular superoxide dismutase and cardiovascular disease. Cardiovasc. Res. 2002;55:239–249. doi: 10.1016/s0008-6363(02)00328-0. [DOI] [PubMed] [Google Scholar]
- Fukai T., Galis Z.S., Meng X.P., Parthasarathy S., Harrison D.G. Vascular expression of extracellular superoxide dismutase in atherosclerosis. J. Clin. Invest. 1998;101:2101–2111. doi: 10.1172/JCI2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai T., Ushio-Fukai M. Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Diseases. Antioxid. Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta A., Price D.L., Pardo C.A., Troncoso J.C., Xu Z.S., Taniguchi N., Martin L.J. Localization of superoxide dismutases in Alzheimer’s disease and Down’s syndrome neocortex and hippocampus. Am. J. Pathol. 1995;146:357–367. [PMC free article] [PubMed] [Google Scholar]
- Gaki G.S., Papavassiliou A.G. Oxidative stress-induced signaling pathways implicated in the pathogenesis of Parkinson’s disease. Neuromolecular Med. 2014;16:217–230. doi: 10.1007/s12017-014-8294-x. [DOI] [PubMed] [Google Scholar]
- Gama C., Salvador M., Andreazza A., Kapczinski F., Belmonte-de-Abreu P. Elevated serum superoxide dismutase and thiobarbituric acid reactive substances in schizophrenia: A study of patients treated with haloperidol or clozapine. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:512–515. doi: 10.1016/j.pnpbp.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Gamez J., Corbera-Bellalta M., Nogales G., Raguer N., García-Arumí E., Badia-Canto M., Lladó-Carbó E., Alvarez-Sabín J. Mutational analysis of the Cu/Zn superoxide dismutase gene in a Catalan ALS population: should all sporadic ALS cases also be screened for SOD1? J. Neurol. Sci. 2006;247:21–28. doi: 10.1016/j.jns.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Garbarino V.R., Orr M.E., Rodriguez K.A., Buffenstein R. Mechanisms of oxidative stress resistance in the brain: Lessons learned from hypoxia tolerant extremophilic vertebrates. Arch. Biochem. Biophys. 2015;576:8–16. doi: 10.1016/j.abb.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S.S., Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. PPB. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Gil-Mohapel J., Brocardo P.S., Christie B.R. The role of oxidative stress in Huntington’s disease: are antioxidants good therapeutic candidates? Curr. Drug Targets. 2014;15:454–468. doi: 10.2174/1389450115666140115113734. [DOI] [PubMed] [Google Scholar]
- Gonzalez P.K., Zhuang J., Doctrow S.R., Malfroy B., Benson P.F., Menconi M.J., Fink M.P. EUK-8, a synthetic superoxide dismutase and catalase mimetic, ameliorates acute lung injury in endotoxemic swine. J. Pharmacol. Exp. Ther. 1995;275:798–806. [PubMed] [Google Scholar]
- Gonzalez P.K., Zhuang J., Doctrow S.R., Malfroy B., Benson P.F., Menconi M.J., Fink M.P. EUK-8, a synthetic superoxide dismutase and catalase mimetic, ameliorates acute lung injury in endotoxemic swine. J. Pharmacol. Exp. Ther. 1995;275:798–806. [PubMed] [Google Scholar]
- Gonzalez-Liencres C., Tas C., Brown E.C., Erdin S., Onur E., Cubukcoglu Z., Aydemir O., Esen-Danaci A., Brüne M. Oxidative stress in schizophrenia: a case-control study on the effects on social cognition and neurocognition. BMC Psychiatry. 2014;14 doi: 10.1186/s12888-014-0268-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves J.T., Wang C.C. Nitric oxide synthase: models and mechanisms. Curr. Opin. Chem. Biol. 2000;4:687–695. doi: 10.1016/s1367-5931(00)00146-0. [DOI] [PubMed] [Google Scholar]
- Gulesserian T., Seidl R., Hardmeier R., Cairns N., Lubec G. Superoxide dismutase SOD1, encoded on chromosome 21, but not SOD2 is overexpressed in brains of patients with Down syndrome. J. Investig. Med. . Publ. Am. Fed. Clin. Res. 2001;49:41–46. doi: 10.2310/6650.2001.34089. [DOI] [PubMed] [Google Scholar]
- Gupta M., Gupta Y.K., Agarwal S., Aneja S., Kohli K. A randomized, double-blind, placebo controlled trial of melatonin add-on therapy in epileptic children on valproate monotherapy: effect on glutathione peroxidase and glutathione reductase enzymes. Br. J. Clin. Pharmacol. 2004;58:542–547. doi: 10.1111/j.1365-2125.2004.02210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney M.E., Pu H., Chiu A.Y., Dal Canto M.C., Polchow C.Y., Alexander D.D., Caliendo J., Hentati A., Kwon Y.W., Deng H.X. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Hamed R.A. Biomarkers of Oxidative Stress in Major Depressive Disorder |. Open Access Maced. J. Med. Sci. 2020 [Google Scholar]
- Hampel H., Vassar R., De Strooper B., Hardy J., Willem M., Singh N., Zhou J., Yan R., Vanmechelen E., De Vos A., Nisticò R., Corbo M., Imbimbo B.P., Streffer J., Voytyuk I., Timmers M., Tahami Monfared A.A., Irizarry M., Albala B., Koyama A., Watanabe N., Kimura T., Yarenis L., Lista S., Kramer L., Vergallo A. The β-Secretase BACE1 in Alzheimer’s Disease. Biol. Psychiatry. 2021;89:745–756. doi: 10.1016/j.biopsych.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayyan M., Hashim M.A., AlNashef I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016;116:3029–3085. doi: 10.1021/acs.chemrev.5b00407. [DOI] [PubMed] [Google Scholar]
- Helguera P., Seiglie J., Rodriguez J., Hanna M., Helguera G., Busciglio J. Adaptive Downregulation of Mitochondrial Function in Down Syndrome. Cell Metab. 2013;17:132–140. doi: 10.1016/j.cmet.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helley M.P., Pinnell J., Sportelli C., Tieu K. Mitochondria: A Common Target for Genetic Mutations and Environmental Toxicants in Parkinson’s Disease. Front. Genet. 2017;8 doi: 10.3389/fgene.2017.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E.P. Nitric oxide. Curr. Biol. CB. 1999;9:R42. doi: 10.1016/s0960-9822(99)80004-9. [DOI] [PubMed] [Google Scholar]
- Huang H.-F., Guo F., Cao Y.-Z., Shi W., Xia Q. Neuroprotection by manganese superoxide dismutase (MnSOD) mimics: antioxidant effect and oxidative stress regulation in acute experimental stroke. CNS Neurosci. Ther. 2012;18:811–818. doi: 10.1111/j.1755-5949.2012.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Qian K., Chen J., Qi Y., E Y., Liang J., Zhao L. A biomimetic zeolite-based nanoenzyme contributes to neuroprotection in the neurovascular unit after ischaemic stroke via efficient removal of zinc and ROS. Acta Biomater. 2022;144:142–156. doi: 10.1016/j.actbio.2022.03.018. [DOI] [PubMed] [Google Scholar]
- Hui, C., Tadi, P., Patti, L., 2022. Ischemic Stroke, in: StatPearls. StatPearls Publishing, Treasure Island (FL). [PubMed]
- Hulbert A.J., Pamplona R., Buffenstein R., Buttemer W.A. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol. Rev. 2007;87:1175–1213. doi: 10.1152/physrev.00047.2006. [DOI] [PubMed] [Google Scholar]
- Hung-Ming W., Liu C.-S., Tsai J.-J., Ko L.-Y., Wei Y.-H. Antioxidant and anticonvulsant effect of a modified formula of chaihu-longu-muli-tang. Am. J. Chin. Med. 2002;30:339–346. doi: 10.1142/S0192415X02000235. [DOI] [PubMed] [Google Scholar]
- Huo L., Lu X., Wu F., Chang C., Ning Y., Zhang X.Y. Elevated activity of superoxide dismutase in male late-life schizophrenia and its correlation with clinical symptoms and cognitive deficits. BMC Psychiatry. 2021;21 doi: 10.1186/s12888-021-03604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- I F. Biological effects of the superoxide radical. Arch. Biochem. Biophys. 1986;247 doi: 10.1016/0003-9861(86)90526-6. [DOI] [PubMed] [Google Scholar]
- Ighodaro O.M., Akinloye O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018;54:287–293. doi: 10.1016/j.ajme.2017.09.001. [DOI] [Google Scholar]
- Ishihara Y., Takemoto T., Itoh K., Ishida A., Yamazaki T. Dual Role of Superoxide Dismutase 2 Induced in Activated Microglia. J. Biol. Chem. 2015;290:22805–22817. doi: 10.1074/jbc.M115.659151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M.N., Rauf A., Fahad F.I., Emran T.B., Mitra S., Olatunde A., Shariati M.A., Rebezov M., Rengasamy K.R.R., Mubarak M.S. Superoxide dismutase: an updated review on its health benefits and industrial applications. Crit. Rev. Food Sci. Nutr. 2022;62:7282–7300. doi: 10.1080/10408398.2021.1913400. [DOI] [PubMed] [Google Scholar]
- Izzo A., Mollo N., Nitti M., Paladino S., Calì G., Genesio R., Bonfiglio F., Cicatiello R., Barbato M., Sarnataro V., Conti A., Nitsch L. Mitochondrial dysfunction in down syndrome: molecular mechanisms and therapeutic targets. Mol. Med. Camb. Mass. 2018;24 doi: 10.1186/s10020-018-0004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J. Parkinson’s disease: clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Jankovic J., Tan E.K. Parkinson’s disease: etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry. 2020;91:795–808. doi: 10.1136/jnnp-2019-322338. [DOI] [PubMed] [Google Scholar]
- Jauhar S., Johnstone M., McKenna P.J. Schizophrenia. Lancet. 2022;399:473–486. doi: 10.1016/S0140-6736(21)01730-X. [DOI] [PubMed] [Google Scholar]
- Jiang P., Dickson D.W. Parkinson’s disease: experimental models and reality. Acta Neuropathol. (Berl. ) 2018;135:13–32. doi: 10.1007/s00401-017-1788-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jl H., Y H., C T., D O., Hs N., Dn S. Catalytic properties of human manganese superoxide dismutase. J. Biol. Chem. 1996;271 doi: 10.1074/jbc.271.30.17687. [DOI] [PubMed] [Google Scholar]
- Johri A., Chandra A., Flint Beal M. PGC-1α, mitochondrial dysfunction, and Huntington’s disease. Free Radic. Biol. Med. 2013;62:37–46. doi: 10.1016/j.freeradbiomed.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C., Rong Y., Doctrow S., Baudry M., Malfroy B., Xu Z. Synthetic superoxide dismutase/catalase mimetics reduce oxidative stress and prolong survival in a mouse amyotrophic lateral sclerosis model. Neurosci. Lett. 2001;304:157–160. doi: 10.1016/s0304-3940(01)01784-0. [DOI] [PubMed] [Google Scholar]
- Kalozoumi G., Kel-Margoulis O., Vafiadaki E., Greenberg D., Bernard H., Soreq H., Depaulis A., Sanoudou D. Glial responses during epileptogenesis in Mus musculus point to potential therapeutic targets. PLOS ONE. 2018;13 doi: 10.1371/journal.pone.0201742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalpakjian, C.Z., 2016. Oral Glutathione and Health Outcomes Among Persons With Post-Polio Syndrome (Clinical trial registration No. NCT01402570). clinicaltrials.gov.
- Karlsson Jan Olof G., Adolfsson K., Thelin B., Jynge P., Andersson R.G., Falkmer U.G. First clinical experience with the magnetic resonance imaging contrast agent and superoxide dismutase mimetic mangafodipir as an adjunct in cancer chemotherapy-a translational study. Transl. Oncol. 2012;5:32–38. doi: 10.1593/tlo.11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmiloff-Smith, A., Al-Janabi, T., D’Souza, H., Groet, J., Massand, E., Mok, K., Startin, C., Fisher, E., Hardy, J., Nizetic, D., Tybulewicz, V., Strydom, A., 2016. The importance of understanding individual differences in Down syndrome. F1000Research 5, F1000 Faculty Rev-389. https://doi.org/10.12688/f1000research.7506.1. [DOI] [PMC free article] [PubMed]
- Karry R., Klein E., Ben Shachar D. Mitochondrial complex I subunits expression is altered in schizophrenia: a postmortem study. Biol. Psychiatry. 2004;55:676–684. doi: 10.1016/j.biopsych.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Karuppanapandian T., Moon J.-C., Kim C., Manoharan K., Kim W. Reactive oxygen species in plants: Their generation, signal transduction, and scavenging mechanisms. Aust. J. Crop Sci. 2011;5:709–725. [Google Scholar]
- Katrenčíková B., Vaváková M., Paduchová Z., Nagyová Z., Garaiova I., Muchová J., Ďuračková Z., Trebatická J. Oxidative Stress Markers and Antioxidant Enzymes in Children and Adolescents with Depressive Disorder and Impact of Omega-3 Fatty Acids in Randomised Clinical Trial. Antioxidants. 2021;10:1256. doi: 10.3390/antiox10081256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki H., Tomita M., Takenaka C., Shirakawa H., Shimazu S., Ibi M., Kume T., Kaneko S., Akaike A. Superoxide dismutase activity in organotypic midbrain-striatum co-cultures is associated with resistance of dopaminergic neurons to excitotoxicity. J. Neurochem. 2001;76:1336–1345. doi: 10.1046/j.1471-4159.2001.00136.x. [DOI] [PubMed] [Google Scholar]
- Khattab M.M. TEMPOL, a membrane-permeable radical scavenger, attenuates peroxynitrite- and superoxide anion-enhanced carrageenan-induced paw edema and hyperalgesia: a key role for superoxide anion. Eur. J. Pharmacol. 2006;548:167–173. doi: 10.1016/j.ejphar.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Kim G.W., Kondo T., Noshita N., Chan P.H. Manganese Superoxide Dismutase Deficiency Exacerbates Cerebral Infarction After Focal Cerebral Ischemia/Reperfusion in Mice. Stroke. 2002;33:809–815. doi: 10.1161/hs0302.103745. [DOI] [PubMed] [Google Scholar]
- Kim H., Bing G., Jhoo W., Ko K.H., Kim W.K., Suh J.H., Kim S.J., Kato K., Hong J.S. Changes of hippocampal Cu/Zn-superoxide dismutase after kainate treatment in the rat. Brain Res. 2000;853:215–226. doi: 10.1016/s0006-8993(99)02254-4. [DOI] [PubMed] [Google Scholar]
- Kim H.K., Hwang S.-H., Abdi S. Tempol Ameliorates and Prevents Mechanical Hyperalgesia in a Rat Model of Chemotherapy-Induced Neuropathic Pain. Front. Pharm. 2017;7:532. doi: 10.3389/fphar.2016.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocatürk null, Akbostanci null, Tan null, Kavas null. Superoxide dismutase activity and zinc and copper concentrations in Parkinson’s disease. Pathophysiol. . J. Int. Soc. Pathophysiol. 2000;7:63–67. doi: 10.1016/s0928-4680(00)00030-4. [DOI] [PubMed] [Google Scholar]
- Kondo T. Parkinson’s disease and free radicals. Mechanism of neurodegeneration and neuroprotection. Ann. N. Y. Acad. Sci. 1996;786:206–216. doi: 10.1111/j.1749-6632.1996.tb39063.x. [DOI] [PubMed] [Google Scholar]
- Kost O.A., Beznos O.V., Davydova N.G., Manickam D.S., Nikolskaya I.I., Guller A.E., Binevski P.V., Chesnokova N.B., Shekhter A.B., Klyachko N.L., Kabanov A.V. Superoxide Dismutase 1 Nanozyme for Treatment of Eye Inflammation. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/5194239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna M.C., Grahame D.A., Samuni A., Mitchell J.B., Russo A. Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of superoxide. Proc. Natl. Acad. Sci. U. S. A. 1992;89:5537–5541. doi: 10.1073/pnas.89.12.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz M., Gama C.S., Andreazza A.C., Salvador M., Ceresér K.M., Gomes F.A., Belmonte-de-Abreu P.S., Berk M., Kapczinski F. Elevated serum superoxide dismutase and thiobarbituric acid reactive substances in different phases of bipolar disorder and in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:1677–1681. doi: 10.1016/j.pnpbp.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Larsen S.B., Hanss Z., Krüger R. The genetic architecture of mitochondrial dysfunction in Parkinson’s disease. Cell Tissue Res. 2018;373:21–37. doi: 10.1007/s00441-017-2768-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Hyeon S.J., Im H., Ryu Hyun, Kim Y., Ryu Hoon. Astrocytes and Microglia as Non-cell Autonomous Players in the Pathogenesis of ALS. Exp. Neurobiol. 2016;25:233–240. doi: 10.5607/en.2016.25.5.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu D., Spasojevic I., Nguyen H., Deng B., Tovmasyan A., Weitner T., Sampaio R.S., Batinic-Haberle I., Huang T.-T. CNS bioavailability and radiation protection of normal hippocampal neurogenesis by a lipophilic Mn porphyrin-based superoxide dismutase mimic, MnTnBuOE-2-PyP5. Redox Biol. 2017;12:864–871. doi: 10.1016/j.redox.2017.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levanon D., Lieman-Hurwitz J., Dafni N., Wigderson M., Sherman L., Bernstein Y., Laver-Rudich Z., Danciger E., Stein O., Groner Y. Architecture and anatomy of the chromosomal locus in human chromosome 21 encoding the Cu/Zn superoxide dismutase. EMBO J. 1985;4:77–84. doi: 10.1002/j.1460-2075.1985.tb02320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Tovmasyan A., Sheng H., Xu B., Sampaio R.S., Reboucas J.S., Warner D.S., Batinic-Haberle I., Spasojevic I. Fe Porphyrin-Based SOD Mimic and Redox-Active Compound, (OH)FeTnHex-2-PyP4+, in a Rodent Ischemic Stroke (MCAO) Model: Efficacy and Pharmacokinetics as Compared to Its Mn Analogue, (H2O)MnTnHex-2-PyP5+ Antioxid. Basel Switz. 2020;9 doi: 10.3390/antiox9060467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Spencer N.Y., Pantazis N.J., Engelhardt J.F. Alsin and SOD1(G93A) proteins regulate endosomal reactive oxygen species production by glial cells and proinflammatory pathways responsible for neurotoxicity. J. Biol. Chem. 2011;286:40151–40162. doi: 10.1074/jbc.M111.279711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L.-P., Huang J., Fulton R., Pearson-Smith J.N., Day B.J., Patel M. Pre-clinical therapeutic development of a series of metalloporphyrins for Parkinson’s disease. Toxicol. Appl. Pharmacol. 2017;326:34–42. doi: 10.1016/j.taap.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Wu P.-H., Tarr P.T., Lindenberg K.S., St-Pierre J., Zhang C.-Y., Mootha V.K., Jäger S., Vianna C.R., Reznick R.M., Cui L., Manieri M., Donovan M.X., Wu Z., Cooper M.P., Fan M.C., Rohas L.M., Zavacki A.M., Cinti S., Shulman G.I., Lowell B.B., Krainc D., Spiegelman B.M. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Lindenau J., Noack H., Possel H., Asayama K., Wolf G. Cellular distribution of superoxide dismutases in the rat CNS. Glia. 2000;29:25–34. doi: 10.1002/(sici)1098-1136(20000101)29:1<25::aid-glia3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Liu T., Zhong S., Liao X., Chen J., He T., Lai S., Jia Y. A Meta-Analysis of Oxidative Stress Markers in Depression. PloS One. 2015;10 doi: 10.1371/journal.pone.0138904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.H., Kato H., Araki T., Itoyama Y., Kato K., Kogure K. An immunohistochemical study of copper/zinc superoxide dismutase and manganese superoxide dismutase following focal cerebral ischemia in the rat. Brain Res. 1994;644:257–266. doi: 10.1016/0006-8993(94)91688-8. [DOI] [PubMed] [Google Scholar]
- Luoma J.S., Strålin P., Marklund S.L., Hiltunen T.P., Särkioja T., Ylä-Herttuala S. Expression of extracellular SOD and iNOS in macrophages and smooth muscle cells in human and rabbit atherosclerotic lesions: colocalization with epitopes characteristic of oxidized LDL and peroxynitrite-modified proteins. Arterioscler. Thromb. Vasc. Biol. 1998;18:157–167. doi: 10.1161/01.atv.18.2.157. [DOI] [PubMed] [Google Scholar]
- Ma X., Hao J., Wu J., Li Y., Cai X., Zheng Y. Prussian Blue Nanozyme as a Pyroptosis Inhibitor Alleviates Neurodegeneration. Adv. Mater. Deerfield Beach Fla. 2022;34 doi: 10.1002/adma.202106723. [DOI] [PubMed] [Google Scholar]
- Magalhães P.V.S., Jansen K., Pinheiro R.T., Colpo G.D., da Motta L.L., Klamt F., da Silva R.A., Kapczinski F. Peripheral oxidative damage in early-stage mood disorders: a nested population-based case-control study. Int. J. Neuropsychopharmacol. 2012;15:1043–1050. doi: 10.1017/S1461145711001532. [DOI] [PubMed] [Google Scholar]
- Maier C.M., Chan P.H. Role of superoxide dismutases in oxidative damage and neurodegenerative disorders. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry. 2002;8:323–334. doi: 10.1177/107385840200800408. [DOI] [PubMed] [Google Scholar]
- Malfroy B., Doctrow S.R., Orr P.L., Tocco G., Fedoseyeva E.V., Benichou G. Prevention and suppression of autoimmune encephalomyelitis by EUK-8, a synthetic catalytic scavenger of oxygen-reactive metabolites. Cell. Immunol. 1997 doi: 10.1006/cimm.1997.1091. [DOI] [PubMed] [Google Scholar]
- Mansuroğlu B., Derman S., Yaba A., Kızılbey K. Protective effect of chemically modified SOD on lipid peroxidation and antioxidant status in diabetic rats. Int. J. Biol. Macromol. 2015;72:79–87. doi: 10.1016/j.ijbiomac.2014.07.039. [DOI] [PubMed] [Google Scholar]
- Marino B.L.B., de Souza L.R., Sousa K.P.A., Ferreira J.V., Padilha E.C., da Silva C.H.T.P., Taft C.A., Hage-Melim L.I.S. Parkinson’s Disease: A Review from Pathophysiology to Treatment. Mini Rev. Med. Chem. 2020;20:754–767. doi: 10.2174/1389557519666191104110908. [DOI] [PubMed] [Google Scholar]
- Marklund S.L. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem. J. 1984;222:649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S.L. Extracellular superoxide dismutase in human tissues and human cell lines. J. Clin. Invest. 1984;74:1398. doi: 10.1172/JCI111550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaad C.A., Washington T.M., Pautler R.G., Klann E. Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13576–13581. doi: 10.1073/pnas.0902714106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColgan P., Tabrizi S.J. Huntington’s disease: a clinical review. Eur. J. Neurol. 2018;25:24–34. doi: 10.1111/ene.13413. [DOI] [PubMed] [Google Scholar]
- McCord J.M., Fridovich I. Superoxide dismutase: the first twenty years (1968-1988) Free Radic. Biol. Med. 1988;5:363–369. doi: 10.1016/0891-5849(88)90109-8. [DOI] [PubMed] [Google Scholar]
- Medeiros M.S., Schumacher-Schuh A., Cardoso A.M., Bochi G.V., Baldissarelli J., Kegler A., Santana D., Chaves C.M.M.B.S., Schetinger M.R.C., Moresco R.N., Rieder C.R.M., Fighera M.R. Iron and Oxidative Stress in Parkinson’s Disease: An Observational Study of Injury Biomarkers. PloS One. 2016;11 doi: 10.1371/journal.pone.0146129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiser J., Weindl D., Hiller K. Complexity of dopamine metabolism. Cell Commun. Signal. 2013;11 doi: 10.1186/1478-811X-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melov S., Doctrow S.R., Schneider J.A., Haberson J., Patel M., Coskun P.E., Huffman K., Wallace D.C., Malfroy B. Lifespan Extension and Rescue of Spongiform Encephalopathy in Superoxide Dismutase 2 Nullizygous Mice Treated with Superoxide Dismutase–Catalase Mimetics. J. Neurosci. 2001;21:8348–8353. doi: 10.1523/JNEUROSCI.21-21-08348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melov S., Ravenscroft J., Malik S., Gill M.S., Walker D.W., Clayton P.E., Wallace D.C., Malfroy B., Doctrow S.R., Lithgow G.J. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567–1569. doi: 10.1126/science.289.5484.1567. [DOI] [PubMed] [Google Scholar]
- Milanlioglu A., Aslan M., Ozkol H., Çilingir V., Nuri Aydın M., Karadas S. Serum antioxidant enzymes activities and oxidative stress levels in patients with acute ischemic stroke: influence on neurological status and outcome. Wien. Klin. Wochenschr. 2016;128:169–174. doi: 10.1007/s00508-015-0742-6. [DOI] [PubMed] [Google Scholar]
- Miriyala S., Spasojevic I., Tovmasyan A., Salvemini D., Vujaskovic Z., St Clair D., Batinic-Haberle I. Manganese superoxide dismutase, MnSOD and its mimics. Biochim. Biophys. Acta. 2012;1822:794–814. doi: 10.1016/j.bbadis.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischley, L., 2015. Central Nervous System Uptake of Intranasal Glutathione in Parkinson’s Disease (Clinical trial registration No. NCT02324426). clinicaltrials.gov. [DOI] [PMC free article] [PubMed]
- Mischley L.K., Lau R.C., Shankland E.G., Wilbur T.K., Padowski J.M. Phase IIb Study of Intranasal Glutathione in Parkinson’s Disease. J. Park. Dis. 2017;7:289–299. doi: 10.3233/JPD-161040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misrani A., Tabassum S., Yang L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021;13 doi: 10.3389/fnagi.2021.617588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsubishi Tanabe Pharma Corporation, 2014. A Phase IIa, Multi-centre, Randomised, Double-blind, Placebo Controlled, Clinical Study Investigating the Safety, Tolerability and Pharmacokinetics of MCI-186 in Subjects With Acute Ischemic Stroke (Clinical trial registration No. NCT00821821). clinicaltrials.gov.
- Miyaoka T., Ieda M., Hashioka S., Wake R., Furuya M., Liaury K., Hayashida M., Tsuchie K., Arauchi R., Araki T., Shioji I., Ezoe S., Inoue K., Yamaguchi T., Horiguchi J. Analysis of oxidative stress expressed by urinary level of biopyrrins and 8-hydroxydeoxyguanosine in patients with chronic schizophrenia. Psychiatry Clin. Neurosci. 2015;69:693–698. doi: 10.1111/pcn.12319. [DOI] [PubMed] [Google Scholar]
- Moreno S., Nardacci R., Cerù M.P. Regional and ultrastructural immunolocalization of copper-zinc superoxide dismutase in rat central nervous system. J. Histochem. Cytochem. . J. Histochem. Soc. 1997;45:1611–1622. doi: 10.1177/002215549704501204. [DOI] [PubMed] [Google Scholar]
- Moreton N., Puzio M., O’Connor J.J. The effects of the superoxide dismutase mimetic, MnTMPyP, post hypoxia and oxygen glucose deprivation in isolated rat hippocampal slices. Brain Res. Bull. 2022;190:105–115. doi: 10.1016/j.brainresbull.2022.09.021. [DOI] [PubMed] [Google Scholar]
- Murakami K., Kondo T., Kawase M., Li Y., Sato S., Chen S.F., Chan P.H. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J. Neurosci. . J. Soc. Neurosci. 1998;18:205–213. doi: 10.1523/JNEUROSCI.18-01-00205.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K., Shimizu T., Irie K. Formation of the 42-mer Amyloid β Radical and the Therapeutic Role of Superoxide Dismutase in Alzheimer’s Disease. J. Amino Acids. 2011;2011 doi: 10.4061/2011/654207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscoli C., Cuzzocrea S., Riley D.P., Zweier J.L., Thiemermann C., Wang Z.-Q., Salvemini D. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br. J. Pharmacol. 2003;140:445–460. doi: 10.1038/sj.bjp.0705430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane H., Chu Y., Faraci F.M., Oberley L.W., Heistad D.D. Gene transfer of extracellular superoxide dismutase increases superoxide dismutase activity in cerebrospinal fluid. Stroke. 2001;32:184–189. doi: 10.1161/01.str.32.1.184. [DOI] [PubMed] [Google Scholar]
- Nakao N., Frodl E.M., Widner H., Carlson E., Eggerding F.A., Epstein C.J., Brundin P. Overexpressing Cu/Zn superoxide dismutase enhances survival of transplanted neurons in a rat model of Parkinson’s disease. Nat. Med. 1995;1:226–231. doi: 10.1038/nm0395-226. [DOI] [PubMed] [Google Scholar]
- NCCIH), 2006. Natural Antioxidants in the Treatment of Multiple Sclerosis (Clinical trial registration No. NCT00010842). clinicaltrials.gov.
- NIMH), 2011. An Investigation Examining the Evidence for Mitochondrial Dysfunction in the Pathophysiology and Treatment of Bipolar Disorder (Clinical trial registration No. NCT00327756). clinicaltrials.gov.
- NIA), 2009. Evaluation of the Safety, Tolerability and Impact on Biomarkers of Anti-Oxidant Treatment of Mild to Moderate Alzheimer’s Disease (Clinical trial registration No. NCT00117403). clinicaltrials.gov.
- Neil S., Huh J., Baronas V., Li X., McFarland H.F., Cherukuri M., Mitchell J.B., Quandt J.A. Oral administration of the nitroxide radical TEMPOL exhibits immunomodulatory and therapeutic properties in multiple sclerosis models. Brain. Behav. Immun. 2017;62:332–343. doi: 10.1016/j.bbi.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A.D., Itoh S., Jeney V., Yanagisawa H., Fujimoto M., Ushio-Fukai M., Fukai T. Fibulin-5 is a novel binding protein for extracellular superoxide dismutase. Circ. Res. 2004;95:1067–1074. doi: 10.1161/01.RES.0000149568.85071.FB. [DOI] [PubMed] [Google Scholar]
- Niedzwiecki M.M., Hall M.N., Liu X., Oka J., Harper K.N., Slavkovich V., Ilievski V., Levy D., van Geen A., Mey J.L., Alam S., Siddique A.B., Parvez F., Graziano J.H., Gamble M.V. Blood glutathione redox status and global methylation of peripheral blood mononuclear cell DNA in Bangladeshi adults. Epigenetics. 2013;8:730–738. doi: 10.4161/epi.25012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilupul Perera M., Ma H.K., Arakawa S., Howells D.W., Markus R., Rowe C.C., Donnan G.A. Inflammation following stroke. J. Clin. Neurosci. . J. Neurosurg. Soc. Austral. 2006;13:1–8. doi: 10.1016/j.jocn.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Noack H., Lindenau J., Rothe F., Asayama K., Wolf G. Differential expression of superoxide dismutase isoforms in neuronal and glial compartments in the course of excitotoxically mediated neurodegeneration: relation to oxidative and nitrergic stress. Glia. 1998;23:285–297. doi: 10.1002/(sici)1098-1136(199808)23:4<285::aid-glia1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Nunomura A., Perry G., Pappolla M.A., Friedland R.P., Hirai K., Chiba S., Smith M.A. Neuronal oxidative stress precedes amyloid-beta deposition in Down syndrome. J. Neuropathol. Exp. Neurol. 2000;59:1011–1017. doi: 10.1093/jnen/59.11.1011. [DOI] [PubMed] [Google Scholar]
- NYU Langone Health, 2018. Turmeric as Treatment in Epilepsy (Clinical trial registration No. NCT03254680). clinicaltrials.gov.
- Odetti P., Angelini G., Dapino D., Zaccheo D., Garibaldi S., Dagna-Bricarelli F., Piombo G., Perry G., Smith M., Traverso N., Tabaton M. Early glycoxidation damage in brains from Down’s syndrome. Biochem. Biophys. Res. Commun. 1998;243:849–851. doi: 10.1006/bbrc.1998.8186. [DOI] [PubMed] [Google Scholar]
- Okado-Matsumoto A., Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J. Biol. Chem. 2001;276:38388–38393. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- Olanow C.W., Arendash G.W. Metals and free radicals in neurodegeneration. Curr. Opin. Neurol. 1994;7:548–558. doi: 10.1097/00019052-199412000-00013. [DOI] [PubMed] [Google Scholar]
- Olson K.R., Gao Y., Arif F., Patel S., Yuan X., Mannam V., Howard S., Batinic-Haberle I., Fukuto J., Minnion M., Feelisch M., Straub K.D. Manganese Porphyrin-Based SOD Mimetics Produce Polysulfides from Hydrogen Sulfide. Antioxidants. 2019;8:639. doi: 10.3390/antiox8120639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pall H.S., Williams A.C., Blake D.R., Lunec J., Gutteridge J.M., Hall M., Taylor A. Raised cerebrospinal-fluid copper concentration in Parkinson’s disease. Lancet Lond. Engl. 2. 1987:238–241. doi: 10.1016/s0140-6736(87)90827-0. [DOI] [PubMed] [Google Scholar]
- Pandey M.K., Mittra P., Maheshwari P.K. The lipid peroxidation product as a marker of oxidative stress in epilepsy. J. Clin. Diagn. Res. 2012;6:590–592. [Google Scholar]
- Pappolla M.A., Omar R.A., Kim K.S., Robakis N.K. Immunohistochemical evidence of oxidative [corrected] stress in Alzheimer’s disease. Am. J. Pathol. 1992;140:621–628. [PMC free article] [PubMed] [Google Scholar]
- Pardo C.A., Xu Z., Borchelt D.R., Price D.L., Sisodia S.S., Cleveland D.W. Superoxide dismutase is an abundant component in cell bodies, dendrites, and axons of motor neurons and in a subset of other neurons. Proc. Natl. Acad. Sci. U. S. A. 1995;92:954–958. doi: 10.1073/pnas.92.4.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J.N., Rowley S., Liang L.-P., White A.M., Day B.J., Patel M. Reactive oxygen species mediate cognitive deficits in experimental temporal lobe epilepsy. Neurobiol. Dis. 2015;82:289–297. doi: 10.1016/j.nbd.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluffo H., Acarin L., Faiz M., Castellano B., Gonzalez B. Cu/Zn superoxide dismutase expression in the postnatal rat brain following an excitotoxic injury. J. Neuroinflamm. 2005;2 doi: 10.1186/1742-2094-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluffo H., Shacka J.J., Ricart K., Bisig C.G., Martìnez-Palma L., Pritsch O., Kamaid A., Eiserich J.P., Crow J.P., Barbeito L., Estèvez A.G. Induction of motor neuron apoptosis by free 3-nitro-L-tyrosine. J. Neurochem. 2004;89:602–612. doi: 10.1046/j.1471-4159.2004.02363.x. [DOI] [PubMed] [Google Scholar]
- Peng J., Stevenson F.F., Doctrow S.R., Andersen J.K. Superoxide dismutase/catalase mimetics are neuroprotective against selective paraquat-mediated dopaminergic neuron death in the substantial nigra: implications for Parkinson disease. J. Biol. Chem. 2005;280:29194–29198. doi: 10.1074/jbc.M500984200. [DOI] [PubMed] [Google Scholar]
- Perluigi M., Butterfield D.A. Oxidative Stress and Down Syndrome: A Route toward Alzheimer-Like Dementia. Curr. Gerontol. Geriatr. Res. 2012;2012 doi: 10.1155/2012/724904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G., Nunomura A., Hirai K., Zhu X., Pérez M., Avila J., Castellani R.J., Atwood C.S., Aliev G., Sayre L.M., Takeda A., Smith M.A. Is oxidative damage the fundamental pathogenic mechanism of Alzheimer’s and other neurodegenerative diseases? Free Radic. Biol. Med. 2002;33:1475–1479. doi: 10.1016/s0891-5849(02)01113-9. [DOI] [PubMed] [Google Scholar]
- Persichilli S., Gervasoni J., Di Napoli A., Fuso A., Nicolia V., Giardina B., Scarpa S., Desiderio C., Cavallaro R.A. Plasma thiols levels in Alzheimer’s disease mice under diet-induced hyperhomocysteinemia: effect of S-adenosylmethionine and superoxide-dismutase supplementation. J. Alzheimers Dis. JAD. 2015;44:1323–1331. doi: 10.3233/JAD-142391. [DOI] [PubMed] [Google Scholar]
- Petersen S.V., Oury T.D., Ostergaard L., Valnickova Z., Wegrzyn J., Thøgersen I.B., Jacobsen C., Bowler R.P., Fattman C.L., Crapo J.D., Enghild J.J. Extracellular superoxide dismutase (EC-SOD) binds to type i collagen and protects against oxidative fragmentation. J. Biol. Chem. 2004;279:13705–13710. doi: 10.1074/jbc.M310217200. [DOI] [PubMed] [Google Scholar]
- Piccoli C., Izzo A., Scrima R., Bonfiglio F., Manco R., Negri R., Quarato G., Cela O., Ripoli M., Prisco M., Gentile F., Calì G., Pinton P., Conti A., Nitsch L., Capitanio N. Chronic pro-oxidative state and mitochondrial dysfunctions are more pronounced in fibroblasts from Down syndrome foeti with congenital heart defects. Hum. Mol. Genet. 2013;22:1218–1232. doi: 10.1093/hmg/dds529. [DOI] [PubMed] [Google Scholar]
- Pifferi F., Laurent B., Plourde M. Lipid Transport and Metabolism at the Blood-Brain Interface: Implications in Health and Disease. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.645646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda J.A., Aono M., Sheng H., Lynch J., Wellons J.C., Laskowitz D.T., Pearlstein R.D., Bowler R., Crapo J., Warner D.S. Extracellular superoxide dismutase overexpression improves behavioral outcome from closed head injury in the mouse. J. Neurotrauma. 2001;18:625–634. doi: 10.1089/089771501750291864. [DOI] [PubMed] [Google Scholar]
- Polazzi E., Mengoni I., Caprini M., Peña-Altamira E., Kurtys E., Monti B. Copper-zinc superoxide dismutase (SOD1) is released by microglial cells and confers neuroprotection against 6-OHDA neurotoxicity. Neurosignals. 2013;21:112–128. doi: 10.1159/000337115. [DOI] [PubMed] [Google Scholar]
- Popa-Wagner A., Carmichael S.T., Kokaia Z., Kessler C., Walker L.C. The response of the aged brain to stroke: too much, too soon? Curr. Neurovasc. Res. 2007;4:216–227. doi: 10.2174/156720207781387213. [DOI] [PubMed] [Google Scholar]
- Prasad D.K.V., Satyanarayana U., Shaheen U., Prabha T.S., Munshi A. Oxidative Stress in the Development of Genetic Generalised Epilepsy: An Observational Study in Southern Indian Population. J. Clin. Diagn. Res. JCDR. 2017;11 doi: 10.7860/JCDR/2017/29133.10604. BC05–BC08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priller, J., 2015. Effects of EGCG (Epigallocatechin Gallate) in Huntington’s Disease The ETON-Study - A Randomized, Double-blind, Stratified, Placebo-controlled Prospective Investigator Initiated Multicenter Trial - (Clinical trial registration No. NCT01357681). clinicaltrials.gov.
- Przedborski S., Kostic V., Jackson-Lewis V., Naini A.B., Simonetti S., Fahn S., Carlson E., Epstein C.J., Cadet J.L. Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J. Neurosci. . J. Soc. Neurosci. 1992;12:1658–1667. doi: 10.1523/JNEUROSCI.12-05-01658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X.-Y., Zhang S.-P., Cao C., Loh Y.P., Cheng Y. Aberrations in Peripheral Inflammatory Cytokine Levels in Parkinson Disease: A Systematic Review and Meta-analysis. JAMA Neurol. 2016;73:1316–1324. doi: 10.1001/jamaneurol.2016.2742. [DOI] [PubMed] [Google Scholar]
- Ranjekar P.K., Hinge A., Hegde M.V., Ghate M., Kale A., Sitasawad S., Wagh U.V., Debsikdar V.B., Mahadik S.P. Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res. 2003;121:109–122. doi: 10.1016/s0165-1781(03)00220-8. [DOI] [PubMed] [Google Scholar]
- Resende R., Moreira P.I., Proença T., Deshpande A., Busciglio J., Pereira C., Oliveira C.R. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radic. Biol. Med. 2008;44:2051–2057. doi: 10.1016/j.freeradbiomed.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Ribeiro M., Silva A.C., Rodrigues J., Naia L., Rego A.C. Oxidizing effects of exogenous stressors in Huntington’s disease knock-in striatal cells--protective effect of cystamine and creatine. Toxicol. Sci. . J. Soc. Toxicol. 2013;136:487–499. doi: 10.1093/toxsci/kft199. [DOI] [PubMed] [Google Scholar]
- Riederer P., Sofic E., Rausch W.D., Schmidt B., Reynolds G.P., Jellinger K., Youdim M.B. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J. Neurochem. 1989;52:515–520. doi: 10.1111/j.1471-4159.1989.tb09150.x. [DOI] [PubMed] [Google Scholar]
- Riva N., Agosta F., Lunetta C., Filippi M., Quattrini A. Recent advances in amyotrophic lateral sclerosis. J. Neurol. 2016;263:1241–1254. doi: 10.1007/s00415-016-8091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Y., Doctrow S.R., Tocco G., Baudry M. EUK-134, a synthetic superoxide dismutase and catalase mimetic, prevents oxidative stress and attenuates kainate-induced neuropathology. Proc. Natl. Acad. Sci. U. S. A. 1999;96:9897–9902. doi: 10.1073/pnas.96.17.9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa A.C., Corsi D., Cavi N., Bruni N., Dosio F. Superoxide Dismutase Administration: A Review of Proposed Human Uses. Molecules. 2021;26:1844. doi: 10.3390/molecules26071844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A., Donaldson D., Goto J., O’Regan J.P., Deng H.X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rowland L.P., Shneider N.A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- Saccon R.A., Bunton-Stasyshyn R.K.A., Fisher E.M.C., Fratta P. Is SOD1 loss of function involved in amyotrophic lateral sclerosis? Brain. 2013;136:2342–2358. doi: 10.1093/brain/awt097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini D., Cuzzocrea S. Superoxide, superoxide dismutase and ischemic injury. Curr. Opin. Investig. Drugs Lond. Engl. 2002;2000(3):886–895. [PubMed] [Google Scholar]
- Salvemini D., Mazzon E., Dugo L., Riley D.P., Serraino I., Caputi A.P., Cuzzocrea S. Pharmacological manipulation of the inflammatory cascade by the superoxide dismutase mimetic, M40403. Br. J. Pharmacol. 2001;132:815–827. doi: 10.1038/sj.bjp.0703841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini D., Muscoli C., Riley D.P., Cuzzocrea S. Superoxide Dismutase Mimetics. Pulm. Pharmacol. Ther. 2002;15:439–447. doi: 10.1006/pupt.2002.0374. [DOI] [PubMed] [Google Scholar]
- Salvemini D., Riley D.P., Cuzzocrea S. Sod mimetics are coming of age. Nat. Rev. Drug Discov. 2002;1:367–374. doi: 10.1038/nrd796. [DOI] [PubMed] [Google Scholar]
- Salvemini D., Wang Z.Q., Zweier J.L., Samouilov A., Macarthur H., Misko T.P., Currie M.G., Cuzzocrea S., Sikorski J.A., Riley D.P. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- Samuni A., Mitchell J.B., DeGraff W., Krishna C.M., Samuni U., Russo A. Nitroxide SOD-mimics: modes of action. Free Radic. Res. Commun. 1991;12-13(Pt 1):187–194. doi: 10.3109/10715769109145785. [DOI] [PubMed] [Google Scholar]
- Santomauro D.F., Herrera A.M.M., Shadid J., Zheng P., Ashbaugh C., Pigott D.M., Abbafati C., Adolph C., Amlag J.O., Aravkin A.Y., Bang-Jensen B.L., Bertolacci G.J., Bloom S.S., Castellano R., Castro E., Chakrabarti S., Chattopadhyay J., Cogen R.M., Collins J.K., Dai X., Dangel W.J., Dapper C., Deen A., Erickson M., Ewald S.B., Flaxman A.D., Frostad J.J., Fullman N., Giles J.R., Giref A.Z., Guo G., He J., Helak M., Hulland E.N., Idrisov B., Lindstrom A., Linebarger E., Lotufo P.A., Lozano R., Magistro B., Malta D.C., Månsson J.C., Marinho F., Mokdad A.H., Monasta L., Naik P., Nomura S., O’Halloran J.K., Ostroff S.M., Pasovic M., Penberthy L., Jr R.C.R., Reinke G., Ribeiro A.L.P., Sholokhov A., Sorensen R.J.D., Varavikova E., Vo A.T., Walcott R., Watson S., Wiysonge C.S., Zigler B., Hay S.I., Vos T., Murray C.J.L., Whiteford H.A., Ferrari A.J. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398:1700–1712. doi: 10.1016/S0140-6736(21)02143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarandol A., Sarandol E., Acikgoz H.E., Eker S.S., Akkaya C., Dirican M. First-episode psychosis is associated with oxidative stress: Effects of short-term antipsychotic treatment. Psychiatry Clin. Neurosci. 2015;69:699–707. doi: 10.1111/pcn.12333. [DOI] [PubMed] [Google Scholar]
- Schatzman S.S., Peterson R.L., Teka M., He B., Cabelli D.E., Cormack B.P., Culotta V.C. Copper-only superoxide dismutase enzymes and iron starvation stress in Candida fungal pathogens. J. Biol. Chem. 2020;295:570–583. doi: 10.1074/jbc.RA119.011084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scola G., McNamara R.K., Croarkin P.E., Leffler J.M., Cullen K.R., Geske J.R., Biernacka J.M., Frye M.A., DelBello M.P., Andreazza A.C. Lipid peroxidation biomarkers in adolescents with or at high-risk for bipolar disorder. J. Affect. Disord. 2016;192:176–183. doi: 10.1016/j.jad.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano F., Klann E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res. Rev. 2004;3:431–443. doi: 10.1016/j.arr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Sharma S., Carlson S., Puttachary S., Sarkar S., Showman L., Putra M., Kanthasamy A.G., Thippeswamy T. Role of the Fyn-PKCδ signaling in SE-induced neuroinflammation and epileptogenesis in experimental models of temporal lobe epilepsy. Neurobiol. Dis. 2018;110:102–121. doi: 10.1016/j.nbd.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe M.A., Ollosson R., Stewart V.C., Clark J.B. Oxidation of nitric oxide by oxomanganese-salen complexes: a new mechanism for cellular protection by superoxide dismutase/catalase mimetics. Biochem. J. 2002;366:97–107. doi: 10.1042/BJ20020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefner J.M., Reaume A.G., Flood D.G., Scott R.W., Kowall N.W., Ferrante R.J., Siwek D.F., Upton-Rice M., Brown R.H. Mice lacking cytosolic copper/zinc superoxide dismutase display a distinctive motor axonopathy. Neurology. 1999;53:1239–1246. doi: 10.1212/wnl.53.6.1239. [DOI] [PubMed] [Google Scholar]
- Sheng H., Spasojevic I., Tse H.M., Jung J.Y., Hong J., Zhang Z., Piganelli J.D., Batinic-Haberle I., Warner D.S. Neuroprotective efficacy from a lipophilic redox-modulating Mn(III) N-Hexylpyridylporphyrin, MnTnHex-2-PyP: rodent models of ischemic stroke and subarachnoid hemorrhage. J. Pharmacol. Exp. Ther. 2011;338:906–916. doi: 10.1124/jpet.110.176701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J.-H., London J., Le Pecheur M., Höger H., Pollak D., Lubec G. Aberrant neuronal and mitochondrial proteins in hippocampus of transgenic mice overexpressing human Cu/Zn superoxide dismutase 1. Free Radic. Biol. Med. 2004;37:643–653. doi: 10.1016/j.freeradbiomed.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Shirendeb U., Reddy A.P., Manczak M., Calkins M.J., Mao P., Tagle D.A., Reddy P.H. Abnormal mitochondrial dynamics, mitochondrial loss and mutant huntingtin oligomers in Huntington’s disease: implications for selective neuronal damage. Hum. Mol. Genet. 2011;20:1438–1455. doi: 10.1093/hmg/ddr024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirendeb U.P., Calkins M.J., Manczak M., Anekonda V., Dufour B., McBride J.L., Mao P., Reddy P.H. Mutant huntingtin’s interaction with mitochondrial protein Drp1 impairs mitochondrial biogenesis and causes defective axonal transport and synaptic degeneration in Huntington’s disease. Hum. Mol. Genet. 2012;21:406–420. doi: 10.1093/hmg/ddr475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh O., Tyagi N., Olmstead M.M., Ghosh K. The design of synthetic superoxide dismutase mimetics: seven-coordinate water soluble manganese( ii) and iron( ii) complexes and their superoxide dismutase-like activity studies. Dalton Trans. 2017;46:14186–14191. doi: 10.1039/C7DT03278A. [DOI] [PubMed] [Google Scholar]
- Siwek M., Sowa-Kućma M., Dudek D., Styczeń K., Szewczyk B., Kotarska K., Misztakk P., Pilc A., Wolak M., Nowak G. Oxidative stress markers in affective disorders. Pharmacol. Rep. PR. 2013;65:1558–1571. doi: 10.1016/s1734-1140(13)71517-2. [DOI] [PubMed] [Google Scholar]
- Song W., Chen J., Petrilli A., Liot G., Klinglmayr E., Zhou Y., Poquiz P., Tjong J., Pouladi M.A., Hayden M.R., Masliah E., Ellisman M., Rouiller I., Schwarzenbacher R., Bossy B., Perkins G., Bossy-Wetzel E. Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat. Med. 2011;17:377–382. doi: 10.1038/nm.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorolla M.A., Reverter-Branchat G., Tamarit J., Ferrer I., Ros J., Cabiscol E. Proteomic and oxidative stress analysis in human brain samples of Huntington disease. Free Radic. Biol. Med. 2008;45:667–678. doi: 10.1016/j.freeradbiomed.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Spasojević I., Chen Y., Noel T.J., Yu Y., Cole M.P., Zhang L., Zhao Y., St Clair D.K., Batinić-Haberle I. Mn porphyrin-based superoxide dismutase (SOD) mimic, MnIIITE-2-PyP5+, targets mouse heart mitochondria. Free Radic. Biol. Med. 2007;42:1193–1200. doi: 10.1016/j.freeradbiomed.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasojevic I., Miriyala S., Tovmasyan A., Salvemini D., Fan P., Vujaskovic Z., Batinic-Haberle I., Clair D. Lipophilicity of Mn(III) N-Alkylpyridylporphyrins Dominates Their Accumulation Within Mitochondria and Therefore in Vivo Efficacy: a Mouse Study. FREE Radic. Biol. Med. - FREE Radic. Biol. Med. 2011;51 doi: 10.1016/j.freeradbiomed.2011.10.473. [DOI] [Google Scholar]
- Springall D.R. Nitric oxide--friend and foe. J. Pathol. 1995;175:165–166. doi: 10.1002/path.1711750202. [DOI] [PubMed] [Google Scholar]
- Srikrishna, R., Suresh, D.R., 2009. Biochemical study of antioxidant profile in acute ischemic stroke.
- Stephenie S., Chang Y.P., Gnanasekaran A., Esa N.M., Gnanaraj C. An insight on superoxide dismutase (SOD) from plants for mammalian health enhancement. J. Funct. Foods. 2020;68 doi: 10.1016/j.jff.2020.103917. [DOI] [Google Scholar]
- Stojkovska I., Wagner B.M., Morrison B.E. Parkinson’s disease and enhanced inflammatory response. Exp. Biol. Med. 2015;240:1387–1395. doi: 10.1177/1535370215576313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J., Drori S., Uldry M., Silvaggi J.M., Rhee J., Jäger S., Handschin C., Zheng K., Lin J., Yang W., Simon D.K., Bachoo R., Spiegelman B.M. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Strålin P., Karlsson K., Johansson B.O., Marklund S.L. The interstitium of the human arterial wall contains very large amounts of extracellular superoxide dismutase. Arterioscler. Thromb. Vasc. Biol. 1995;15:2032–2036. doi: 10.1161/01.atv.15.11.2032. [DOI] [PubMed] [Google Scholar]
- Sturtz L.A., Diekert K., Jensen L.T., Lill R., Culotta V.C. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J. Biol. Chem. 2001;276:38084–38089. doi: 10.1074/jbc.M105296200. [DOI] [PubMed] [Google Scholar]
- Sultana R., Perluigi M., Butterfield D.A. Protein oxidation and lipid peroxidation in brain of subjects with Alzheimer’s disease: insights into mechanism of neurodegeneration from redox proteomics. Antioxid. Redox Signal. 2006;8:2021–2037. doi: 10.1089/ars.2006.8.2021. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Nagai Y., Wada K., Koike T. Calcium leak through ryanodine receptor is involved in neuronal death induced by mutant huntingtin. Biochem. Biophys. Res. Commun. 2012;429:18–23. doi: 10.1016/j.bbrc.2012.10.107. [DOI] [PubMed] [Google Scholar]
- T O., N I., O M., T K., S O.-I., C N., Y S., H O. Tissue distribution of immunoreactive mouse extracellular superoxide dismutase. Am. J. Physiol. 1998;275 doi: 10.1152/ajpcell.1998.275.3.C840. [DOI] [PubMed] [Google Scholar]
- Takuma K., Baba A., Matsuda T. Astrocyte apoptosis: implications for neuroprotection. Prog. Neurobiol. 2004;72:111–127. doi: 10.1016/j.pneurobio.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Tamayo D., Muñoz J.F., Lopez Á., Urán M., Herrera J., Borges C.L., Restrepo Á., Soares C.M., Taborda C.P., Almeida A.J., McEwen J.G., Hernández O. Identification and Analysis of the Role of Superoxide Dismutases Isoforms in the Pathogenesis of Paracoccidioides spp. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan R.J., Lee J.S., Manni M.L., Fattman C.L., Tobolewski J.M., Zheng M., Kolls J.K., Martin T.R., Oury T.D. Inflammatory Cells as a Source of Airspace Extracellular Superoxide Dismutase after Pulmonary Injury. Am. J. Respir. Cell Mol. Biol. 2006;34:226–232. doi: 10.1165/rcmb.2005-0212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J.P., Brown R.H., Cleveland D.W. Decoding ALS: from genes to mechanism. Nature. 2016;539:197–206. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaete L.G., Crouch R.K., Nakagawa F., Spicer S.S. The immunocytochemical demonstration of copper-zinc superoxide dismutase in the brain. J. Neurocytol. 1986;15:337–343. doi: 10.1007/BF01611436. [DOI] [PubMed] [Google Scholar]
- Torre, R. de la, 2016. Normalization of dyrk1A and APP Function as an Approach to Improve Cognitive Performance and Decelerate AD Progression in DS Subjects: Epigallocatechin Gallate as Therapeutic Tool (Clinical trial registration No. NCT01699711). clinicaltrials.gov.
- Tovmasyan A., Weitner T., Sheng H., Lu M., Rajic Z., Warner D.S., Spasojevic I., Reboucas J.S., Benov L., Batinic-Haberle I. Differential Coordination Demands in Fe versus Mn Water-Soluble Cationic Metalloporphyrins Translate into Remarkably Different Aqueous Redox Chemistry and Biology. Inorg. Chem. 2013;52:5677–5691. doi: 10.1021/ic3012519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trnka J., Blaikie F.H., Logan A., Smith R.A.J., Murphy M.P. Antioxidant properties of MitoTEMPOL and its hydroxylamine. Free Radic. Res. 2009;43:4–12. doi: 10.1080/10715760802582183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai M.-C., Huang T.-L. Increased activities of both superoxide dismutase and catalase were indicators of acute depressive episodes in patients with major depressive disorder. Psychiatry Res. 2016;235:38–42. doi: 10.1016/j.psychres.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Tsunemi T., Ashe T.D., Morrison B.E., Soriano K.R., Au J., Roque R.A.V., Lazarowski E.R., Damian V.A., Masliah E., La Spada A.R. PGC-1α rescues Huntington’s disease proteotoxicity by preventing oxidative stress and promoting TFEB function. Sci. Transl. Med. 2012;4:142ra97. doi: 10.1126/scitranslmed.3003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens J.F. Increased superoxide dismutase and Down’s syndrome. Med. Hypotheses. 2001;56:617–619. doi: 10.1054/mehy.2001.1327. [DOI] [PubMed] [Google Scholar]
- Ullegaddi R., Powers H.J., Gariballa S.E. Antioxidant supplementation enhances antioxidant capacity and mitigates oxidative damage following acute ischaemic stroke. Eur. J. Clin. Nutr. 2005;59:1367–1373. doi: 10.1038/sj.ejcn.1602248. [DOI] [PubMed] [Google Scholar]
- Valentine J.S., Pantoliano M.W., McDonnell P.J., Burger A.R., Lippard S.J. pH-dependent migration of copper(II) to the vacant zinc-binding site of zinc-free bovine erythrocyte superoxide dismutase. Proc. Natl. Acad. Sci. U. S. A. 1979;76:4245–4249. doi: 10.1073/pnas.76.9.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vliet A. In: Comparative Biology of the Normal Lung. Second edition., Parent R.A., editor. Academic Press; San Diego: 2015. Chapter 25 - Antioxidant Defenses in the Lung; pp. 489–507. [DOI] [Google Scholar]
- Waldbaum S., Patel M. Mitochondrial dysfunction and oxidative stress: a contributing link to acquired epilepsy? J. Bioenerg. Biomembr. 2010;42:449–455. doi: 10.1007/s10863-010-9320-9sss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D.C. Mitochondrial DNA mutations in disease and aging. Environ. Mol. Mutagen. 2010;51:440–450. doi: 10.1002/em.20586. [DOI] [PubMed] [Google Scholar]
- Wang W., Zhao F., Ma X., Perry G., Zhu X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: recent advances. Mol. Neurodegener. 2020;15 doi: 10.1186/s13024-020-00376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Owada S., Kobayashi H.P., Kawakami H., Nagaoka S., Murakami E., Ishiuchi A., Enomoto T., Jinnouchi Y., Sakurai J., Tobe N., Koizumi S., Shimamura T., Asakura T., Nakano H., Otsubo T. Protective effects of MnM2Py4P and Mn-salen against small bowel ischemia/reperfusion injury in rats using an in vivo and an ex vivo electron paramagnetic resonance technique with a spin probe. Transplant. Proc. 2007;39:3002–3006. doi: 10.1016/j.transproceed.2007.08.091. [DOI] [PubMed] [Google Scholar]
- Weisiger R.A., Fridovich I. Superoxide dismutase. Organelle specificity. J. Biol. Chem. 1973;248:3582–3592. [PubMed] [Google Scholar]
- Wong H.-S., Dighe P.A., Mezera V., Monternier P.-A., Brand M.D. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J. Biol. Chem. 2017;292:16804–16809. doi: 10.1074/jbc.R117.789271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley M., Antuono P., Ho K.-C., Egan R., Hevner R., Liebl W., Huang Z., Rachel R., Jones J. Cytochrome oxidase in Alzheimer’s disease: Biochemical, histochemical, and immunohistochemical analyses of the visual and other systems. Vis. Res. 1997;37:3593–3608. doi: 10.1016/S0042-6989(96)00210-6. [DOI] [PubMed] [Google Scholar]
- Wu J.Q., Kosten T.R., Zhang X.Y. Free radicals, antioxidant defense systems, and schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;46:200–206. doi: 10.1016/j.pnpbp.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Wu Z., Zhang X.Y., Wang H., Tang W., Xia Y., Zhang F., Liu J., Fu Y., Hu J., Chen Y., Liu L., Chen D.C., Xiu M.H., Kosten T.R., He J. Elevated plasma superoxide dismutase in first-episode and drug naive patients with schizophrenia: inverse association with positive symptoms. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;36:34–38. doi: 10.1016/j.pnpbp.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Xiang J., Jiang Y. Regulation of Cu-Zn superoxide dismutase on SCN2A in SH-SY5Y cells as a potential therapy for temporal lobe epilepsy. Mol. Med. Rep. 2014;9:16–22. doi: 10.3892/mmr.2013.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Chan P.H., Chen J., Carlson E., Chen S.F., Weinstein P., Epstein C.J., Kamii H. Human copper-zinc superoxide dismutase transgenic mice are highly resistant to reperfusion injury after focal cerebral ischemia. Stroke. 1994;25:165–170. doi: 10.1161/01.str.25.1.165. [DOI] [PubMed] [Google Scholar]
- Yang J., Zhang R., Zhao H., Qi H., Li J., Li J.-F., Zhou X., Wang A., Fan K., Yan X., Zhang T. Bioinspired copper single-atom nanozyme as a superoxide dismutase-like antioxidant for sepsis treatment. Exploration. 2022;2 doi: 10.1002/EXP.20210267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.-L., Weissman L., Bohr V., Mattson M.P. Mitochondrial DNA Damage and Repair in Neurodegenerative Disorders. DNA Repair. 2008;7:1110–1120. doi: 10.1016/j.dnarep.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Chang Z., Que R., Weng G., Deng B., Wang T., Huang Z., Xie F., Wei X., Yang Q., Li M., Ma K., Zhou F., Tang B., Mok V.C.T., Zhu S., Wang Q. Contra-Directional Expression of Plasma Superoxide Dismutase with Lipoprotein Cholesterol and High-Sensitivity C-reactive Protein as Important Markers of Parkinson’s Disease Severity. Front. Aging Neurosci. 2020;12 doi: 10.3389/fnagi.2020.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J.K., Sistilli C.G., van Kammen D.P. Membrane polyunsaturated fatty acids and CSF cytokines in patients with schizophrenia. Prostaglandins Leukot. Essent. Fat. Acids. 2003;69:429–436. doi: 10.1016/j.plefa.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Yao J.K., van Kammen D.P., Gurklis J. Red blood cell membrane dynamics in schizophrenia. III. Correlation of fatty acid abnormalities with clinical measures. Schizophr. Res. 1994;13:227–232. doi: 10.1016/0920-9964(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Yasui K., Baba A. Therapeutic potential of superoxide dismutase (SOD) for resolution of inflammation. Inflamm. Res. . J. Eur. Histamine Res. Soc. Al. 2006;55:359–363. doi: 10.1007/s00011-006-5195-y. [DOI] [PubMed] [Google Scholar]
- Yiş U., Seçkin E., Kurul S.H., Kuralay F., Dirik E. Effects of epilepsy and valproic acid on oxidant status in children with idiopathic epilepsy. Epilepsy Res. 2009;84:232–237. doi: 10.1016/j.eplepsyres.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Yoon J.H., Minzenberg M.J., Raouf S., D’Esposito M., Carter C.S. Impaired prefrontal-basal ganglia functional connectivity and substantia nigra hyperactivity in schizophrenia. Biol. Psychiatry. 2013;74:122–129. doi: 10.1016/j.biopsych.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younus H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018;12:88–93. [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Tong Q., Zhang L., Jiang S., Zhou H., Zhang R., Zhang S., Xu Q., Li D., Zhou X., Ding J., Zhang K. Plasma antioxidant status and motor features in de novo Chinese Parkinson’s disease patients. Int. J. Neurosci. 2016;126:641–646. doi: 10.3109/00207454.2015.1054031. [DOI] [PubMed] [Google Scholar]
- Zahid, S., 2019. Role of Selected Antioxidants (Vitamin C and E) in the Management of Schizophrenia (Clinical trial registration No. NCT04078048). clinicaltrials.gov.
- Zana M., Janka Z., Kálmán J. Oxidative stress: a bridge between Down’s syndrome and Alzheimer’s disease. Neurobiol. Aging. 2007;28:648–676. doi: 10.1016/j.neurobiolaging.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Zemlan F.P., Thienhaus O.J., Bosmann H.B. Superoxide dismutase activity in Alzheimer’s disease: possible mechanism for paired helical filament formation. Brain Res. 1989;476:160–162. doi: 10.1016/0006-8993(89)91550-3. [DOI] [PubMed] [Google Scholar]
- Zhang M.-S., Liang J.-H., Yang M.-J., Ren Y.-R., Cheng D.-H., Wu Q.-H., He Y., Yin J. Low Serum Superoxide Dismutase Is Associated With a High Risk of Cognitive Impairment After Mild Acute Ischemic Stroke. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.834114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.Y., Chen D.C., Xiu M.H., Tan Y.L., Yang F.D., Zhang Laurence Y., Zhang Laura Y., Haile C.N., Kosten T.R. Clinical symptoms and cognitive impairment associated with male schizophrenia relate to plasma manganese superoxide dismutase activity: a case-control study. J. Psychiatr. Res. 2013;47:1049–1053. doi: 10.1016/j.jpsychires.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Zhang X.Y., Chen D.C., Xiu M.H., Yang F.D., Tan Y., Luo X., Zuo L., Kosten T.A., Kosten T.R. Cognitive function, plasma MnSOD activity, and MnSOD Ala-9Val polymorphism in patients with schizophrenia and normal controls. Schizophr. Bull. 2014;40:592–601. doi: 10.1093/schbul/sbt045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.Y., Zhou D.F., Qi L.Y., Chen S., Cao L.Y., Chen D.C., Xiu M.H., Wang F., Wu G.Y., Lu L., Kosten T.A., Kosten T.R. Superoxide dismutase and cytokines in chronic patients with schizophrenia: association with psychopathology and response to antipsychotics. Psychopharmacol. (Berl. ) 2009;204:177–184. doi: 10.1007/s00213-008-1447-6. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Jin Y., Cui H., Yan X., Fan K. Nanozyme-based catalytic theranostics. RSC Adv. 2020;10:10–20. doi: 10.1039/C9RA09021E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhang L., Wang M., Li P. The applications of nanozymes in neurological diseases: From mechanism to design. Theranostics. 2023;13:2492–2514. doi: 10.7150/thno.83370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Pan Y., Wang Z., Tan Y., Song X. Intrathecal Administration of Tempol Reduces Chronic Constriction Injury-Induced Neuropathic Pain in Rats by Increasing SOD Activity and Inhibiting NGF Expression. Cell. Mol. Neurobiol. 2016;36:893–906. doi: 10.1007/s10571-015-0274-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Zhang R., Yan X., Fan K. Superoxide dismutase nanozymes: an emerging star for anti-oxidation. J. Mater. Chem. B. 2021;9:6939–6957. doi: 10.1039/D1TB00720C. [DOI] [PubMed] [Google Scholar]
- Zheng M., Wang X., Yang J., Ma S., Wei Y., Liu S. Changes of complement and oxidative stress parameters in patients with acute cerebral infarction or cerebral hemorrhage and the clinical significance. Exp. Ther. Med. 2020;19:703–709. doi: 10.3892/etm.2019.8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C., Valenza M., Cattaneo E. Molecular mechanisms and potential therapeutical targets in Huntington’s disease. Physiol. Rev. 2010;90:905–981. doi: 10.1152/physrev.00041.2009. [DOI] [PubMed] [Google Scholar]
- Zuo Q., Wu Q., Lv Y., Gong X., Cheng D. Imaging of endoplasmic reticulum superoxide anion fluctuation in a liver injury model by a selective two-photon fluorescent probe. N. J. Chem. 2020;44:5457–5462. doi: 10.1039/D0NJ00487A. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data that support the findings of this study are available in standard research databases such as PubMed, Science Direct, or Google Scholar, and/or on public domains that can be searched with either key words or DOI numbers.