Abstract
Background
Neurofibromatosis type 1, NF2-related schwannomatosis and non-NF2-related schwannomatosis (grouped under the abbreviation “NF”) are rare hereditary tumor predisposition syndromes. Due to the low prevalence, variability in the range, and severity of manifestations, as well as limited treatment options, these conditions require innovative trial designs to accelerate the development of new treatments.
Methods
Within European Patient-Centric Clinical Trial Platforms (EU-PEARL), we designed 2 platform-basket trials in NF. The trials were designed by a team of multidisciplinary NF experts and trial methodology experts.
Results
The trial will consist of an observational and a treatment period. The observational period will serve as a longitudinal natural history study. The platform trial design and randomization to a sequence of available interventions allow for the addition of interventions during the trial. If a drug does not meet the predetermined efficacy endpoint or reveals unacceptable toxicities, participants may stop treatment on that arm and re-enter the observational period, where they can be re-randomized to a different treatment arm if eligible. Intervention-specific eligibility criteria and endpoints are listed in intervention-specific-appendices, allowing the flexibility and adaptability needed for highly variable and rare conditions like NF.
Conclusions
These innovative platform-basket trials for NF may serve as a model for other rare diseases, as they will enhance the chance of identifying beneficial treatments through optimal learning from a small number of patients. The goal of these trials is to identify beneficial treatments for NF more rapidly and at a lower cost than traditional, single-agent clinical trials.
Keywords: neurofibromatosis, schwannomatosis, clinical trial, platform trial, rare diseases
Neurofibromatosis type 1 (NF1), NF2-related schwannomatosis and non-NF2-related schwannomatosis (grouped under the abbreviation “NF”) are rare tumor predisposition syndromes, with respective incidences of approximately 1 in 2000,1 1 in 27 9561,2 and 1 in 68 956.3 The hallmark of these conditions is the development of benign nerve sheath tumors. NF1 displays a wide variety of other disease manifestations in almost all organ systems, with a pronounced variability in clinical expression.2,4,5 Most of the NF-associated tumors are low-grade with a small risk of malignant transformation. Still, they can cause significant morbidity due to their size and/or location.
The current treatment options for NF-associated disease manifestations are limited. Studies of the underlying signaling pathways have revealed potential targets for drug treatments,6,7 but the translation into clinical trials has been slow. From 2010 to 2021, the results of just 42 clinical trials in NF were published.8 Indeed, conducting a clinical trial for NF comes with an array of challenges. The timing and associated morbidity of manifestations vary substantially between individuals,9 which results in variable treatment needs. Limited patient numbers are another restriction, given the low prevalence of the disorders. The phenotypic variability adds an additional layer of complexity, that is the number of patients with a specific manifestation that requires treatment is even lower. This complicates the collection of adequate safety and efficacy data, especially when using standard trial designs.10 Moreover, the natural history of most of the manifestations of NF has not been fully described. Data on the natural history of NF-associated tumors and other manifestations may provide insight into possible predictors of progression and disease burden, help define the best time point for initiating treatment, and help define benchmarks for treatment success.10,11
For patients with rare diseases like NF, the time from drug discovery to approval is lengthy. Novel approaches are needed to accelerate the development of effective therapies for rare diseases. The European Patient-CEntric ClinicAl TRial PLatforms (EU-PEARL), a project funded under the Innovative Medicines Initiative (IMI), aims to establish the use of platform trials for the efficient clinical evaluation of therapies for patients in areas of unmet medical need.12 EU-PEARL chose 4 contrasting disease areas as exemplars to create a framework for platform trials in Europe: major depressive disorder, tuberculosis, nonalcoholic steatohepatitis (NASH), and NF. Under EU-PEARL we developed 2 platform-basket trials, 1 for NF1, and 1 for NF2-related schwannomatosis and non-NF2-related schwannomatosis, which share the same principles and structure. The design of these platform-basket trials provides an opportunity to gain insight into the potential benefits of adaptive clinical trials for rare diseases, and how they could advance the development of effective therapies for these patients. In this article, we present the principles of platform trials, and subsequently the platform-basket trial design we developed for NF.
Platform Trials
Platform trials are defined by the adaptive platform trials coalition as “the study of multiple targeted therapies to a single disease in a perpetual manner, with therapies allowed to enter or leave the platform on the basis of a predefined decision algorithm.”13 If more than one disease or disease subgroup is included in a platform trial, it is called a platform-basket trial.
The structure of a platform trial generally consists of a master protocol and intervention-specific appendices (ISAs).13 Each intervention tested in the platform trial has its own ISA. In the NF platform trials, manifestation-specific sub-sections are also incorporated in this structure. The master protocol contains the overarching design of the platform trial, including all elements that are common across the different interventions or therapies that fall under the master protocol.14 Examples of such elements include the study rationale, the primary objective of the trial, a description of the target patient population and the corresponding trial eligibility criteria, the study duration, and the statistical analysis plan. The ISAs contain further information on a specific intervention, describing features that can vary from intervention to intervention. Examples of elements included in the ISA are the rationale for testing this intervention in the described patient population, benefit/risk information, specific eligibility criteria that apply to the intervention (eg, age restrictions), dosing specifications, and specific measurements for each intervention (eg, related to safety and tolerability, pharmacokinetics, and biomarkers). ISAs function as plug-ins to the master protocol and are the mechanism through which new interventions are added to the platform. As such, an ISA has the same structure as the master protocol and complements the latter. The manifestation-specific sub-sections, or disease subgroup sub-sections, can be used to provide further information on elements that only apply to a certain subgroup of patients. In the NF platform trials, each included disease manifestation has its own sub-sections. These sub-sections provide information on elements that may be different across disease manifestations, for example, eligibility criteria, the assessment schedule, outcome measures and endpoints, the response assessment, and treatment success/futility criteria.
Platform trials allow for the incorporation of interim analyses and response-adaptive randomization. Interim analyses of outcome data can be used for early withdrawal of interventions from the trial, either due to proof-of-concept, futility or unacceptable levels of toxicity. By using response-adaptive randomization, allocation ratios to the different interventions may change during the course of the trial to increase the likelihood for participants to be randomized to interventions with better efficacy. The functionality of a platform trial can improve the outcomes for individual trial participants, and shorten the time that is required to evaluate the performance of an intervention.15
Platform trials have already been successfully utilized in the field of oncology,16,17 and are becoming more common in other fields, including neurology18,19 and COVID-19.20 Compared to trials for a single intervention in one disease, platform trials provide numerous benefits. Platform trials are hypothesized to be more efficient at finding effective treatments by reducing start-up times and using fewer resources.21 This increased efficiency has also been demonstrated in economic evaluations and real-world scenarios.22–24 In addition, platform trials utilize an already operational trial infrastructure and clinical networks to investigate new medications.25 Also, platform trials can reduce trial costs and increase statistical efficiency through the sharing of resources (like control group data) between multiple sponsors and investigational medicinal product (IMP) owners.
Methods
The EU-PEARL trials for NF were developed by a team of clinicians with expertise in NF, methodology and trial design experts, experts in trials for NF, and patient representatives (Annex I). The general trial design was inspired by the “Innovative Trial for Understanding the Impact of Targeted Therapies in NF2” (INTUITT-NF2), a pioneering platform trial in NF2-related schwannomatosis.26 The master protocols were based on the master protocol template as provided by TransCelerate (version 8, copyright TransCelerate Biopharma Inc. 2019 – 2021) and a modified version of this template, published as a deliverable by Work Package 2 of EU-PEARL.27 Approval of an institutional review board or ethics committee was not applicable to this study. The patient representatives that were involved in the design of the trial participated as advisors, not as study subjects.
The first key task was to select the manifestations of NF that were to be included in the platform-basket trials. In a separate effort, the team prioritized manifestations for inclusion in the trial through a modified Delphi procedure, asking for the opinions of both health professionals and patient representatives.28 The NF1 manifestations that were selected for inclusion as a result of the Delphi procedure were plexiform neurofibroma (PN), cutaneous neurofibroma (cNF), optic pathway glioma (OPG), and nonoptic pathway low-grade glioma (LGG). The selected manifestations of NF2-related schwannomatosis and non-NF2-related schwannomatosis include: (1) vestibular schwannoma in NF2-related schwannomatosis, (2) meningioma in NF2-related schwannomatosis, (3) ependymoma in NF2-related schwannomatosis, (4) radiologic progressive nonvestibular schwannoma, and (5) static and pain causing nonvestibular schwannoma in non-NF2-related schwannomatosis.
Results
Two separate master protocols were developed: one for NF1, and one that included both NF2-related and non-NF2-related schwannomatosis manifestations. Both master protocols follow the same structure and apply the same flowchart as described below. The corresponding trials are open-label, Phase I/Phase II proof-of-concept, platform-basket studies. The aim of the trials is to screen the effectiveness of investigational agents in different manifestations of NF1 and NF2-related and non-NF2-related schwannomatosis. Each of 2 platform-basket studies can be viewed as a collection of single-arm proof-of-concept studies with a binary (response/nonresponse) endpoint. Each combination of an investigational agent and a manifestation is treated as a separate proof-of-concept study because recruitment times and endpoints vary greatly across the included manifestations.
Pillars of the Study Flow: Decision Points
To streamline the development of the manifestation-specific sub-sections of the master protocols, the team reviewed a participant’s journey through the platform trial. By describing and harmonizing the different decisions that need to be made for individual participants across all manifestations, we limited these decisions to just 4 key decision points.
These 4 different decision points correspond to 4 questions:
Does the patient meet the master protocol eligibility criteria?
Does the patient qualify to receive an investigational intervention under the master protocol (treatment eligibility criteria)?
Does the patient meet the intervention-specific eligibility criteria?
Does the patient meet the criteria for treatment discontinuation?
The criteria to answer these 4 questions are specific to the different manifestations. The key decision points allowed us to harmonize the manifestation-specific sub-sections of the master protocols. We assigned sub-teams of experts to each manifestation to develop these sub-sections. There was 1 sub-team for NF2-related schwannomatosis and non-NF2-related schwannomatosis, and 4 for NF1, each dedicated to a specific manifestation of NF1. To ensure future data comparability, meetings with experts on trials in NF from the USA and paediatric neuro-oncologists were arranged, to align the protocols to current or upcoming trials for NF-associated tumors as best as possible.
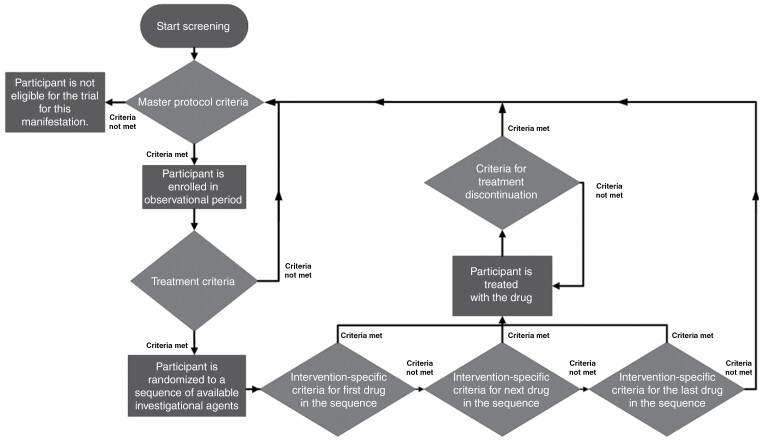
The diagram in Figure 1 shows a participant’s journey through the NF platform-basket trials. The master protocol eligibility criteria define whether a participant can be enrolled in the observational period for a particular manifestation (first diamond in Figure 1). The eligibility criteria for the master protocol are specific to each manifestation. Eligible participants will remain in the observational period until there is an investigational intervention available, which could be the case immediately upon admission to the observational period.
Figure 1.
Participant flow through the EU-PEARL-NF platform-basket trials. The 4 main decision points are represented by the diamonds.
Once there are one or more investigational interventions available under the master protocol for the participant’s manifestation, it will be checked whether the participant meets the criteria to receive an investigational treatment, which is defined separately for each manifestation (second diamond in Figure 1). If yes, the participant will be assigned to a treatment arm. Participants not eligible for treatment will remain on the observational period.
If only one investigational intervention is available, the participant will be assigned to the corresponding treatment arm. The participant will receive the intervention if they meet the intervention-specific eligibility criteria as defined in the corresponding ISA. If several interventions are available, the assignment will be based on randomization. Participants will be randomized to a sequence of available interventions. Participants will receive the first intervention in the sequence for which they fulfil the intervention-specific eligibility criteria (bottom diamonds in Figure 1). If they do not meet the criteria for any of the interventions, participants will remain in the observational period until a new intervention becomes available.
Randomizing to a sequence of interventions is primarily done for operational simplicity. It does not mean that participants will receive this sequence of treatments, rather, they will receive the first treatment in the sequence for which they meet the ISA-specific eligibility criteria. After completing a treatment arm or treatment discontinuation, participants will be re-randomized to a sequence of treatments, which would include all treatments that are currently available. This sequence could include an intervention which the participant has already received before. Each ISA will include an exclusion criterion that precludes the participants from receiving the same treatment twice.
The participant will remain on a treatment arm until they complete it as per protocol, or until they meet the criteria for treatment discontinuation (eg, adverse events and disease progression) (central diamond in Figure 1). Regardless of the reason for treatment discontinuation, the participant may be re-admitted to the observational period, given that they still meet the master protocol eligibility criteria. From here, the participant journey as described above may begin again. If participants complete the treatment arm as per protocol, follow-up visits and data collection will take place as specified in the corresponding ISA, and eligible participants may re-enroll in the platform-basket trial if needed. Participants may also receive an investigational intervention for a different manifestation from the one for which they were originally admitted to the master protocol, if they meet the corresponding eligibility criteria. As such, participants may receive multiple investigational interventions over time, possibly for different manifestations.
Characteristics of the Observational Period of the Trials
The observational period will serve as a longitudinal natural history study (LNHS). Participants that are enrolled on this observational period will receive the current standard of care according to local practice. During this observational period, minimal data will be collected. Sites can collect additional data according to their standard of practice. The required data includes administrative and operational data, demographics, data on primary endpoints specific to each manifestation (such as MRI for PN and LGG, and visual acuity for OPG), information on tumor-directed therapies (surgery, chemotherapy, etc.), as well as patient reported outcome measures (PROMs), clinical data, and functional assessments. The assessment schedule for the observational period is aligned with regular clinical care and therefore less frequent as compared to the treatment arms. As most centers see the patient population for the EU-PEARL-NF trials at least once per year, participants on the observational period are to be seen minimally once every 12 months. The data collected during the observational period will be used to understand the long-term natural history of the disease. The collected data will be used to update the target responder rates (“P0” and “P1” in the statistical section below) for evaluation of the investigational interventions.
Statistical Considerations
For the statistical analysis, each investigational agent, when assessed for treatment of a specific manifestation of NF1 or NF2-related or non-NF2-related schwannomatosis, is regarded as a stand-alone single-arm proof-of-concept study. If an investigational agent is tested for 2 different indications, there are correspondingly 2 proof-of-concept studies with that agent. Regardless of the manifestation, the primary endpoint is a binary response/nonresponse endpoint. Each proof-of-concept study will be analyzed separately. Inference is about the unknown responder probability P. The primary analysis is based on a confidence distribution,29 which is a frequentist analogue of a Bayesian posterior distribution. The confidence distribution is obtained from the data of the proof-of-concept study and summarizes our (data-driven) knowledge about the response probabilities at the end of the trial. To conduct the analysis, a range of ineffective response probabilities (from 0 to P0, say) and a range of desired (or clinically relevant) response probabilities (from P1 to 1, say) will be defined (with P0 < P1). Values of P0 and P1 will initially be based on clinical consensus, but over time will be updated based on the observed response rates in the observational period. The confidence distribution assigns probabilities to these ranges, and proof of concept will be declared if the probability assigned to the range of ineffective probabilities is small (smaller than a predefined value α), and if the probability assigned to the range of desired response probabilities is large (larger than a predefined value 1 − β). Details on the statistical analysis approach and its operating characteristics will be published separately.
For each manifestation, the default sample sizes have been set based on estimated patient availability. For PN and cNF, the default sample size is currently set to 40 participants in each intervention arm. For OPG and LGG, the default sample size is set to 10 participants in each intervention arm. The small sample size for OPG and LGG reflects that patients will be difficult to recruit due to the rarity of the manifestation (LGG) and/or the defined eligibility criteria for that manifestation. The default sample size is 20 participants in study arms corresponding to vestibular schwannoma in NF2-related schwannomatosis, meningioma in NF2-related schwannomatosis, and radiologic progressive nonvestibular schwannoma. For ependymoma in NF2-related schwannomatosis and the static, pain causing nonvestibular schwannoma in non-NF2-related schwannomatosis, the default sample size is 10 participants. The default sample size may be changed depending upon the needs related to the different investigational agents in the different manifestations.
Discussion
Following the principles of the INTUITT-NF2 trial and platform trials in general, we developed 2 platform-basket trials: 1 for NF1 and 1 for NF2-related and non-NF2-related schwannomatosis. The trials follow the same structure and principles and consist of an observational and a treatment period. The observational period will provide longitudinal natural history data, which aims to generate reference data for the treatment arms. Different layers of eligibility criteria for both the observational and the treatment period are defined separately for each manifestation, making the trial design flexible and adaptable to highly variable disorders like NF. Randomization to a sequence of available interventions allows for the addition of interventions during the trial. If a drug does not meet the predetermined efficacy endpoint or reveals unacceptable toxicities, participants may re-enter the observational phase or be re-randomized to a different treatment arm, if eligible.
A key success factor for the design of these platform-basket studies was the use of the 4 decision points. This simplification of decision-making for individual patients allowed us to write the manifestation-specific sub-sections in an efficient manner. By keeping a common structure across all manifestations, we maximized consistency, which also allows investigators to readily add new manifestations and treatment indications to the protocol.
Several core assumptions and pre-established limitations shaped the design of the EU-PEARL-NF trials. The trials had to be designed in a drug-agnostic fashion. As no investigational agents had been selected at the time of protocol development, the trial design had to be adaptable to all types of systemic and topical investigational agents. Additionally, given the varying treatment needs throughout the lifetime of a patient, the study had to allow participants to enrol sequentially for multiple manifestations into the trial. Another key premise of the EU-PEARL-NF trials is to neither include a placebo nor an active control group. This is in line with other successful trials in NF, such as the SPRINT-trial,30 the NF10431 and NF10532 trials, and the INTUITT-NF2 platform trial.26,33 Since the patients that are eligible for the designed trials suffer from more severe forms of the included disease manifestations, it is less preferable to randomize them to a placebo group. The use of an active control group, such as a standard of care in combination with a placebo, is also not applicable to this trial. The current treatment options for several NF-associated tumors tend to be limited, and the standard of care varies significantly between participating countries and centers. In addition, there is variable data available on the effect of standard of care, a gap that may be partly addressed by the data from the observational period of the presented trial. Due to the lack of an active control group and the associated risk of bias, the results of trials with the presented design will be considered less robust compared to the results of randomized controlled trials. Since there will be a danger of overestimating the efficacy of investigational agents,34 mitigation measures must be taken, such as the choice of objective study endpoints, the inclusion of a larger sample size where possible, and applying a more stringent significance level. To avoid erroneous conclusions regarding the efficacy of new treatments, we stress the importance of phase III confirmation for the investigational agents that show promise in the EU-PEARL-NF proof-of-concept trials.
Benefits of the Observational Period
The embedded observational period and natural history study of the presented trial design come with various advantages. Most importantly, it will provide data on the natural course of the included disease manifestations. The limited and less frequent data collection will limit the utility of the observational period to a certain degree, but the data may be used to decide upon the desired response rates in the treatment arms. Moreover, as this design anticipates on changing treatment needs over time, patients will be allowed to enter the observational period for multiple manifestations as they develop. This method will yield more data from a single patient, enabling a more efficient assessment of the effectiveness of investigational agents in various manifestations of NF. In addition, the observational period could provide opportunities to develop and validate outcome measures in NF. The tumor-related symptoms in patients with NF vary strongly between individuals, and a decrease in tumor size does not always lead to clinical improvement. At this time there is a lack of endpoints specifically designed for NF, and the few existing NF-specific PROMs, for example, the INF1-QOL questionnaire for NF1,35 are often not validated in multiple languages. This restricts the use of NF-PROMs in international trials and was a significant impediment when selecting PROMs for the EU-PEARL-NF trial. Through standardized data collection, the integrated LNHS of the EU-PEARL-NF trials could provide a platform to collect data on new outcome measures in multiple European countries, facilitating the validation process.
Benefits of this Trial Design to the Main Stakeholders
The presented platform trial design will provide numerous benefits to its main stakeholders. For investigators and IMP owners, a considerable benefit is the reduced start-up times for new investigational agents. Since new drugs are added as an extension to the protocol, and do not require the setup of a whole new trial, the time from drug discovery to clinical trial is shortened significantly.14,23–25 As data can be collected for multiple manifestations in the observational period, this may provide additional insight into drug effects across manifestations. Investigational products are assessed for efficacy in a more efficient manner and may progress to the next stage of drug development more rapidly.24,36
Through its innovative design and the use of the decision types and manifestation-specific sub-sections, it will be relatively easy to add new manifestations and treatment indications to the protocol as new investigational agents become available. This is essential for a variable condition like NF, which presents a wide variety of disease manifestations. Another benefit is that investigators, sponsors, and IMP owners will have access to an already existing and operating trial infrastructure. This advantage could be especially relevant for trials in NF and other rare diseases. For rare diseases, it is peculiarly difficult and time-consuming to set up the infrastructure for a multi-center trial, especially when it comes to the identification of sites and investigators. In platform trials, an existing network of experienced sites and investigators can be utilized to roll out new treatment arms. For IMP owners and sponsors, the platform trial framework offers an opportunity to reduce the operational costs and time of evaluating investigational agents.24,36
Platform trials also offer improved screening processes when compared to traditional clinical trials. Since all patients are regularly screened in a standardized manner (both for eligibility for multiple manifestations, as well as eligibility for more than one treatment arm), the screening and enrollment process will be more streamlined. This will likely result in a higher quality screening with less screening failures and shorter times to enrolment.25 In addition, the observational arm will include a pool of patients who need treatment. Patients who require treatment, but who were not eligible for previous investigational agents, can be re-screened as soon as new investigational agents become available. This approach should help to further speed up the recruitment for the different treatment arms. Lastly, the standardized screening in the observational period will provide a reliable estimate of the number of possibly eligible patients. This information could be especially valuable to IMP owners when they are considering the addition of a new treatment arm.
Patients with NF will also benefit from participating in the EU-PEARL-NF trials. Beyond getting access to an investigational treatment like in a standard clinical study, participants are offered the option to re-enrol in a different treatment arm after completing a previous investigational intervention. In addition, participants will be able to sequentially receive treatment for more than one manifestation in the same trial. This provides participants with the opportunity to receive investigational agents more rapidly than they would in individual studies for separate manifestations. Interim analyses allow for the early stopping of treatment arms due to futility, followed by a re-direction of the assignment of participants to more promising interventions. This could increase the likelihood for participants to receive a potentially effective treatment. The screening process will also be favourable to patients. Because patients are screened for all available investigational agents simultaneously, there is no possibility to skip a screening opportunity, as could be the case when conducting independent trials for each agent, resulting in more opportunities to be enrolled.25
Limitations of This Trial Design
Along with the many benefits of platform and platform-basket trials, considerable drawbacks to these study designs must also be considered. Primarily, these study types require a large up-front effort and investment to build the platform and infrastructure for the trial.14 It may be difficult to reach a consensus regarding trial design, operations, and governance with the large number of parties involved. More and larger sources of financial support are needed to start these projects and to ensure the sustainability of the trial. To develop and maintain a network of clinical sites to run the trial also requires considerable effort, involving problems with, for example, the eligibility of sites, logistics, sponsorship, the coordination of various research boards, regulatory approval, and available research infrastructure.36,37 As the initiation and implementation of the trial will be more complex than standard clinical trials, centers might be hesitant to join the platform. To patients, the EU-PEARL-NF trials can appear complex and intimidating, due to their complicated design. The observational period can discourage patients from participating, because they may believe that they will not receive an investigational agent in the trial in a timely manner. Although results from performed patient and public engagement activities indicated that the EU-PEARL-NF trial design was appealing and clear to patients,38 special attention must be given to fully inform patients about the setup of innovative trial designs. Lastly, if well-designed, platform trials may run for long periods of time, and this could mean that changes in the standard of care may require modifications to the general trial design and the master protocol.25
Despite the described challenges, there is ample proof that platform trials work and that they are attractive to participating partners.14 This highlights the need to implement this trial design in rare diseases, where it’s essential to develop innovative trial designs that accelerate the ability to efficiently assess investigational agents.
Conclusion and Future Directions
This platform-basket trial design solves some of the challenges that are encountered in clinical trials for NF. It allows for optimal learning from a small number of patients with variable disease manifestations. The goal is to identify beneficial treatments for NF more rapidly and at a lower cost than traditional, single-agent clinical trials by optimizing patient inclusion and invigorating international collaborations. This trial design offers benefits to various stakeholders, including patients and IMP owners. The next steps will be the identification of promising investigational agents and the implementation of the trial and the associated trial framework.
Supplementary Material
Acknowledgments
The authors would like to thank the EU-PEARL consortium and the NF community, including the people living with NF1, NF2-related schwannomatosis or non-NF2-related schwannomatosis, the families and caregivers of patients, NF clinicians, representatives of pharmaceutical companies, and NF trial experts for their unwavering support during this project. Special thanks go to the patient representatives that participated in this project and the modified Delphi procedure.
Contributor Information
Britt A E Dhaenens, Department of General Paediatrics, Erasmus MC-Sophia Children’s Hospital, Rotterdam, The Netherlands; ENCORE Expertise Centre for Neurodevelopmental Disorders, Erasmus MC, Rotterdam, The Netherlands.
Günter Heimann, Biostatistics & Pharmacometrics, Novartis Pharma AG, Basel, Switzerland.
Annette Bakker, Children’s Tumor Foundation, New York, New York, USA.
Marco Nievo, Children’s Tumor Foundation, New York, New York, USA.
Rosalie E Ferner, Neurofibromatosis Service, Department of Neurology, Guy’s and St. Thomas’ NHS Foundation Trust London, Great Maze Pond, London, UK.
D Gareth Evans, Centre for Genomic Medicine, Division of Evolution, Infection and Genomic Sciences, University of Manchester, St Mary’s Hospital, Manchester, UK.
Pierre Wolkenstein, Department of Dermatology, Henri-Mondor Hospital, Créteil, France.
Jonas Leubner, Department of Pediatric Neurology, Charité Universitätsmedizin Berlin—Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt Universität zu Berlin, Berlin, Germany.
Cornelia Potratz, Department of Pediatric Neurology, Charité Universitätsmedizin Berlin—Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt Universität zu Berlin, Berlin, Germany.
Charlotte Carton, Department of Human Genetics, KU Leuven, Leuven, Belgium.
Uchenna Iloeje, Medical Affairs, SpringWorks Therapeutics, Stamford, Connecticut, USA.
George Kirk, AstraZeneca Oncology R&D, Cambridge, UK.
Jaishri O Blakeley, Department of Neurology, Neuro-Oncology, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Scott Plotkin, Cancer Center and Department of Neurology, Massachusetts General Hospital, Boston, Massachusetts, USA.
Michael J Fisher, Division of Oncology, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania, USA.
AeRang Kim, Division of Oncology, Children’s National Hospital, Washington DC, District of Columbia, USA.
Pablo Hernáiz Driever, Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt Universität zu Berlin, Berlin, Germany.
Amedeo A Azizi, Division of Neonatology, Pediatric Intensive Care and Neuropediatrics, Department of Pediatrics and Adolescent Medicine, Medical University of Vienna, Wien, Austria.
Brigitte C Widemann, Pediatric Oncology Branch, Center for Cancer Research, National Cancer Institute, Bethesda, Maryland, USA.
Andrea Gross, Pediatric Oncology Branch, Center for Cancer Research, National Cancer Institute, Bethesda, Maryland, USA.
Tom Parke, Berry Consultants, Abingdon, UK.
Eric Legius, Department of Human Genetics, KU Leuven, Leuven, Belgium; Department of Clinical Genetics, UZ Leuven, Leuven, Belgium; Full Member of the European Reference Network on Genetic Tumour Risk Syndromes (ERN GENTURIS), Nijmegen, The Netherlands (E.L., R.O.).
Rianne Oostenbrink, Department of General Paediatrics, Erasmus MC-Sophia Children’s Hospital, Rotterdam, The Netherlands; ENCORE Expertise Centre for Neurodevelopmental Disorders, Erasmus MC, Rotterdam, The Netherlands; Full Member of the European Reference Network on Genetic Tumour Risk Syndromes (ERN GENTURIS), Nijmegen, The Netherlands.
Conflict of interest statement
AAA participates in the advisory board of Alexion/AstraZeneca, and has received scientific support and speaker honoraria from the same company. AB declares no conflict of interest. JOB participates in the advisory board of SpringWorks Therapeutics. CC and BD report grants from EU-PEARL, the innovative Medicines Initiative 2 Joint Undertaking under grant agreement no 853966. PHD participates in the advisory board of Alexion/AstraZeneca and is the ICI of the SPRINKLE study sponsored by Alexion. AG reports no conflict of interest. DGE reports personal fees from AstraZeneca and personal fees from SpringWorks Therapeutics. MJF participates in advisory boards of Alexion/AstraZeneca, SpringWorks Therapeutics and DayOne, and has received research support from Alexion/AstraZeneca, Array Biopharma/Pfizer and Exelixis. REF reports grants and personal fees from AstraZeneca. GH is an employee of Novartis Pharma AG. UI is an employee of SpringWorks Therapeutics. GK is an employee of AstraZeneca. AK reports no conflict of interest. EL reports personal fees from AstraZeneca, personal fees from SpringWorks Therapeutics, and grants from EU-PEARL, the innovative Medicines Initiative 2 Joint Undertaking under grant agreement no 853966. JL declares no conflict of interest. MN declares no conflict of interest. RO reports grants from EU-PEARL, the innovative Medicines Initiative 2 Joint Undertaking under grant agreement no 853966 and has performed unpaid advisory work for Alexion. CP reports no conflict of interest. SP reports being a cofounder of NFlection Therapeutics and NF2 Therapeutics and served as a consultant for Akouos. TP reports no conflict of interest. BCW reports no conflict of interest. PW reports no conflict of interest.
Funding
Some of the authors are members of the EU Patient-centric clinical trial platform (EU-PEARL). EU-PEARL has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No [853966]. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation program and EFPIA and Children’s Tumor Foundation, Global Alliance for TB Drug Development non-profit organization, SpringWorks Therapeutics Inc. This publication reflects the authors’ views. Neither IMI nor the European Union, EFPIA, or any Associated Partners are responsible for any use that may be made of the information contained herein.
Author contributions
Authors B.D. and G.H. wrote the first draft of the manuscript. All authors contributed significantly to the design of the presented trial. All authors have provided critical revisions of all versions and have read and approved the final version of the manuscript.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. Uusitalo E, Leppävirta J, Koffert A, et al. Incidence and mortality of neurofibromatosis: a total population study in Finland. J Invest Dermatol. 2015;135(3):904–906. [DOI] [PubMed] [Google Scholar]
- 2. Evans DG, Howard E, Giblin C, et al. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A. 2010;152A(2):327–332. [DOI] [PubMed] [Google Scholar]
- 3. Evans DG, Bowers NL, Tobi S, et al. Schwannomatosis: a genetic and epidemiological study. J Neurol Neurosurg Psychiatry. 2018;89(11):1215–1219. [DOI] [PubMed] [Google Scholar]
- 4. Ferner RE. The neurofibromatoses. Pract Neurol. 2010;10(2):82–93. [DOI] [PubMed] [Google Scholar]
- 5. Korf BR. Neurofibromatosis. Handb Clin Neurol 2013;111:333–340. [DOI] [PubMed] [Google Scholar]
- 6. Walker JA, Upadhyaya M.. Emerging therapeutic targets for neurofibromatosis type 1. Expert Opin Ther Targets. 2018;22(5):419–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tamura R. Current understanding of neurofibromatosis type 1, 2, and schwannomatosis. Int J Mol Sci. 2021;22(11):5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dhaenens BAE, Ferner RE, Evans DG, et al. Lessons learned from drug trials in neurofibromatosis: a systematic review. Eur J Med Genet. 2021;64(9):104281. [DOI] [PubMed] [Google Scholar]
- 9. Blakeley JO, Plotkin SR.. Therapeutic advances for the tumors associated with neurofibromatosis type 1, type 2, and schwannomatosis. Neuro Oncol. 2016;18(5):624–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kempf L, Goldsmith JC, Temple R.. Challenges of developing and conducting clinical trials in rare disorders. Am J Med Genet A. 2018;176(4):773–783. [DOI] [PubMed] [Google Scholar]
- 11. U.S. Department of Health and Human Services, Center for Biologics Evaluation and Research (CBER), Office of Orphan Products Development (OOPD) Rare Diseases: Natural History Studies for Drug Development Guidance for Industry—Draft Guidance. 2019. [Google Scholar]
- 12. EU Patient-Centric Clinical Trial Platforms (EU-PEARL). https://eu-pearl.eu/. Accessed 23 January 2023. [DOI] [PubMed]
- 13. Adaptive Platform Trials C. Adaptive platform trials: definition, design, conduct and reporting considerations. Nat Rev Drug Discov. 2019;18(10):797–807. [DOI] [PubMed] [Google Scholar]
- 14. Paganoni S, Berry JD, Quintana M, et al. ; Healey ALS Platform Trial Study Group. Adaptive platform trials to transform amyotrophic lateral sclerosis therapy development. Ann Neurol. 2022;91(2):165–175. [DOI] [PubMed] [Google Scholar]
- 15. Meurer WJ, Lewis RJ, Berry DA.. Adaptive clinical trials: a partial remedy for the therapeutic misconception? JAMA. 2012;307(22):2377–2378. [DOI] [PubMed] [Google Scholar]
- 16. Alexander BM, Ba S, Berger MS, et al. ; GBM AGILE Network. Adaptive global innovative learning environment for glioblastoma: GBM AGILE. Clin Cancer Res. 2018;24(4):737–743. [DOI] [PubMed] [Google Scholar]
- 17. Simon R. Critical review of umbrella, basket, and platform designs for oncology clinical trials. Clin Pharmacol Ther. 2017;102(6):934–941. [DOI] [PubMed] [Google Scholar]
- 18. Connick P, De Angelis F, Parker RA, et al. ; UK Multiple Sclerosis Society Clinical Trials Network. Multiple Sclerosis-Secondary Progressive Multi-Arm Randomisation Trial (MS-SMART): a multiarm phase IIb randomised, double-blind, placebo-controlled clinical trial comparing the efficacy of three neuroprotective drugs in secondary progressive multiple sclerosis. BMJ Open. 2018;8(8):e021944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bateman RJ, Benzinger TL, Berry S, et al. ; DIAN-TU Pharma Consortium for the Dominantly Inherited Alzheimer Network. The DIAN-TU next generation Alzheimer’s prevention trial: adaptive design and disease progression model. Alzheimers Dement. Jan 2017;13(1):8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vanderbeek AM, Bliss JM, Yin Z, Yap C.. Implementation of platform trials in the COVID-19 pandemic: a rapid review. Contemp Clin Trials. 2022;112:106625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saville BR, Berry SM.. Efficiencies of platform clinical trials: a vision of the future. Clin Trials. 2016;13(3):358–366. [DOI] [PubMed] [Google Scholar]
- 22. Park JJH, Sharif B, Harari O, et al. Economic evaluation of cost and time required for a platform trial vs conventional trials. JAMA Netw Open. 2022;5(7):e2221140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sydes MR, Parmar MK, Mason MD, et al. Flexible trial design in practice—stopping arms for lack-of-benefit and adding research arms mid-trial in STAMPEDE: a multi-arm multi-stage randomized controlled trial. Trials. 2012;13:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang H, Yee D.. I-SPY 2: A neoadjuvant adaptive clinical trial designed to improve outcomes in high-risk breast cancer. Curr Breast Cancer Rep. 2019;11(4):303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woodcock J, LaVange LM.. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med. 2017;377(1):62–70. [DOI] [PubMed] [Google Scholar]
- 26. ClinicalTrials.gov. Innovative Trial for Understanding the Impact of Targeted Therapies in NF2 (INTUITT-NF2). 2023; https://clinicaltrials.gov/ct2/show/NCT04374305. Accessed February 9, 2023.
- 27. Work Package 2 of EU-PEARL Provisional Generic Master Protocol Template and Intervention Specific Appendix for IRPs. 2021; https://eu-pearl.eu/wp-content/uploads/2023/03/1.EU-PEARL_D2.3-Provisional-Generic-Master-Protocol-Template-Intervention-Specific-Append.pdf. Accessed May 18, 2023.
- 28. Dhaenens BAE, Ferner RE, Bakker A, et al. Identifying challenges in neurofibromatosis: a modified Delphi procedure. Eur J Hum Genet. 2021;29(11):1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xie M, Singh K.. Confidence distribution, the frequentist distribution estimator of a parameter: A review. Int Stat Rev. 2013;81(1):3–39. [Google Scholar]
- 30. Gross AM, Wolters PL, Dombi E, et al. Selumetinib in children with inoperable plexiform neurofibromas. N Engl J Med. 2020;382(15):1430–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Plotkin SR, Duda DG, Muzikansky A, et al. Multicenter, prospective, phase ii and biomarker study of high-dose bevacizumab as induction therapy in patients with neurofibromatosis type 2 and progressive vestibular schwannoma. J Clin Oncol. 2019;37(35):3446–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fisher MJ, Shih CS, Rhodes SD, et al. ; Neurofibromatosis Clinical Trials Consortium. Cabozantinib for neurofibromatosis type 1-related plexiform neurofibromas: a phase 2 trial. Nat Med. 2021;27(1):165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Plotkin SR, Babovic-Vuksanovic D, Dinh C, et al. CTNI-80 INTUITT-NF2, An adaptive platform-basket trial for NF2-related schwannomatosis patients with progressive schwannomas, meningiomas, and ependymomas: Primary outcome of the brigatinib treatment arm. Neuro Oncol. 2023;25(Supplement_5):96. [Google Scholar]
- 34. Food and Drug Administration (FDA) Guidance for Industry: E10 choice of control group and related issues in clinical trials. 2001; https://www.fda.gov/media/71349/download. Accessed December 11, 2023.
- 35. Ferner RE, Thomas M, Mercer G, et al. Evaluation of quality of life in adults with neurofibromatosis 1 (NF1) using the Impact of NF1 on Quality Of Life (INF1-QOL) questionnaire. Health Qual Life Outcomes. 2017;15(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pericàs JM, Tacke F, Anstee QM, et al. ; EU-PEARL NASH Investigators. Platform trials to overcome major shortcomings of traditional clinical trials in non-alcoholic steatohepatitis? Pros and cons. J Hepatol. Feb 2023;78(2):442–447. [DOI] [PubMed] [Google Scholar]
- 37. Minisman G, Bhanushali M, Conwit R, et al. Implementing clinical trials on an international platform: challenges and perspectives. J Neurol Sci. 2012;313(1-2):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dhaenens BAE, Mahler F, Batchelor H, et al. Optimizing expert and patient input in pediatric trial design: Lessons learned and recommendations from a collaboration between conect4children and European Patient-CEntric ClinicAl TRial PLatforms. Clin Transl Sci. 2023;16(8):1458–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analysed in support of this research.