Abstract
Rice dwarf virus (RDV) is a double-shelled particle that contains a major capsid protein (P8), a major core protein (P3), several minor core proteins, and viral genomic double-stranded RNA. Coexpression of P8 and P3 in transgenic rice plants resulted in formation of double-shelled, virus-like particles (VLPs) similar to the authentic RDV particles. The VLPs were not detected in transgenic rice plant cells expressing P8 alone. This in vivo result suggests that P8 interacted with P3 and that these two proteins provide the structural integrity required for the formation of VLPs in rice cells independently of other structural proteins, nonstructural proteins, or viral genomic double-stranded RNAs.
Rice dwarf virus (RDV), which is the pathogen causing rice dwarf disease, belongs to the family Reoviridae and the genus Phytoreovirus. RDV can replicate both in the rice plant and in its leafhopper vectors (Nephotettix cincticeps or Resilia dorsalis) (11). It is an icosahedral double-shelled particle approximately 70 nm in diameter (5, 7, 9, 23). The genome of RDV is composed of 12-segment, double-stranded RNAs (dsRNAs) (S1 to S12), which encode at least six structural proteins, P1, P2, P3, P5, P7, and P8, and other nonstructural proteins (17, 20). The outer shell of the RDV capsid is composed of two proteins (P2 and P8), and the inner core consists of four structural proteins (P1, P3, P5, and P7) and the 12 segments of dsRNAs (6, 9, 10). P8 (46 kDa) is the main component of the outer shell, and P3 (114 kDa) is the major core protein of the inner core shell (5, 10, 12, 20, 23; S. Ueda and I. Uyeda, Molecular Plant Pathology On-Line [http://www.bspp.org.uk/mppol/]). These two proteins account for approximately 52 and 29% of the total proteins of an intact viral particle, respectively (10).
The three-dimensional structure of the RDV capsid was determined by electron cryomicroscopy, and computer reconstruction revealed a T=13 outer icosahedral shell composed of trimeric clusters of P8 and a T=1 inner icosahedral shell of P3 dimers, indicating that the two RDV shells have mismatched lattice symmetries (5). The lack of matched lattice symmetries suggests that the other minor proteins (e.g., P2 or P7) or the structural variations in the floor domains of P8 may be involved in accommodating the mismatched lattice symmetries of the outer and inner shells in assembly of the RDV particle (5; Molecular Plant Pathology On-Line). Indeed, it was found that the appearances of viral particles with or without P2 present were indistinguishable (19). Thus, the involvement of P2 or P7 in capsid formation is unclear. Takahashi et al. (13) demonstrated the reassembly of RDV and rice gall dwarf virus (RGDV) core particles with homologous and heterologous capsids extracted from purified virions in vitro. Ueda et al. (14; Molecular Plant Pathology On-Line) suggested that P7 may play an important role as a “hinge” for the assembly of viral structural proteins and genomic RNA, based on in vitro interactions between P7 and other viral structural proteins or viral dsRNAs using far-Western blotting and Northwestern blotting. However, the requirement of P7 for viral particle assembly has not been determined. So far, little is known about interactions among structural proteins and the in vivo assembly process of RDV.
In this study, we coexpressed P8 and P3 in transgenic rice plants, the host plants of RDV, to investigate whether P8 and P3 interact with each other to form a virus-like structure (e.g., virus-like particle [VLP]). Results from this work will help to determine the viral proteins necessary to form a double-shelled, viral structure.
The coding regions of S8 and S3, which encode P8 and P3 of RDV, respectively, were cloned from a Fujian isolate of RDV (20, 22). The S8 and S3 genes were then cloned into plant expression vectors under the control of the CaMV 35S promoter. These vectors contained either neomycin phosphotransferase or hygromycin phosphotransferase selective markers. Plasmid constructs harboring the S8 or S3 gene were cotransformed into rice embryogenic callus by using a particle bombardment technique as described previously (22). After selection using 100 mg of G418/liter and 50 mg of hygromycin/liter, putative transgenic rice plants were regenerated (22). The regenerated transgenic rice plants were maintained in the tissue culture vessels and analyzed for gene expression.
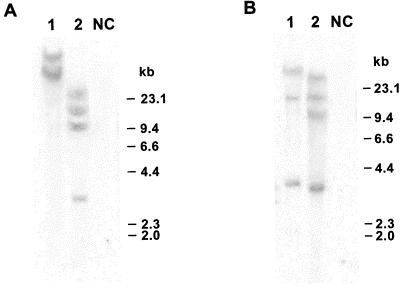
To confirm the integration of the S8 and S3 genes in the rice genome, total DNA was extracted from leaf tissue of transformed and untransformed rice plants as previously described (8) and subjected to Southern blot analysis (Fig. 1). A 32P-labeled, 1.4-kb cDNA probe specific for the S8 sequence was used to probe the XhoI-digested plant DNA, and a 32P-labeled 3.2-kb cDNA probe specific for the S3 gene was used to probe the SalI-digested plant DNA. Results of this work show that both S8 and S3 genes were integrated into the genome of the independent transgenic rice plant lines 1 and 2 (Fig. 1).
FIG. 1.
Genomic DNA Southern blot analysis. Genomic DNAs isolated from the independent transgenic rice plant lines 1 and 2 and from nontransformed control plants (NC) were digested with restriction enzyme XhoI (A) or SalI (B) and hybridized with a radiolabeled 1.4-kb fragment of the RDV S8 gene (A) or 3.2-kb fragment of the RDV S3 coding region (B). The molecular size markers (in kilobases) are on the right.
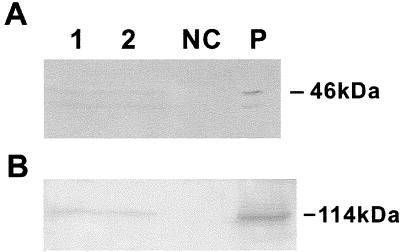
To detect the expressions of P8 and P3, total protein was extracted from leaves of transgenic rice plants and subjected to Western blot assay using polyclonal antiserum against RDV P8 or RDV P3, as previously described (20, 22). The S8- and S3-encoded products were detected in plant lines 1 and 2 (Fig. 2). Together with the 46-kDa band, the major product of S8-encoded protein, a 42-kDa protein band was also observed (Fig. 2A). This lower-molecular-mass band has been identified previously as a posttranslational cleavage product of the 46-kDa protein (6).
FIG. 2.
Western blot analysis showing the coexpression of RDV P8 and P3 in transgenic rice plants. Total proteins extracted from independent transgenic rice plant lines 1 and 2, from nontransformed control plants (NC), and from RDV-infected rice plants (P) were detected with an antiserum against RDV P8 (A) or P3 (B).
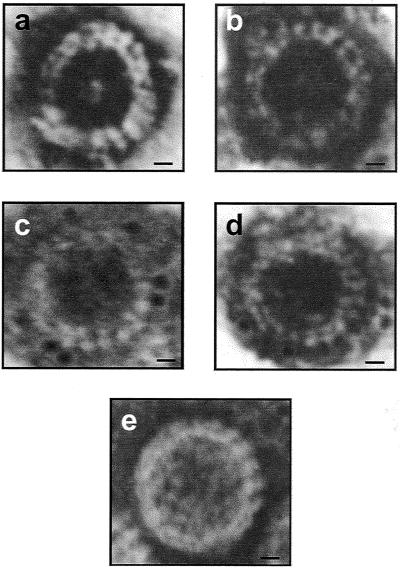
To identify VLPs in leaf extracts by electron microscopy (EM), the tissues from the transgenic rice leaves (100 mg) were homogenized in 200 μl of 0.1 M phosphate buffer (pH 7.0) and clarified using an equal volume of chloroform. For antibody capture, carbon-coated 300-mesh grids were incubated on a 1:200 (vol/vol) dilution of RDV P8 antiserum in phosphate-buffered saline (PBS) for 1 h following three washes in PBS. The antibody-coated grids were then incubated on 10 μl of leaf extracts overnight at 4°C. The grids were washed five or six times in water, stained with 2% uranyl acetate, and examined under an electron microscope (model JEM101; JEOL, Tokyo, Japan). Results of the experiment show that typical double-shelled VLPs were present in the transgenic rice plants harboring both RDV S8 and S3 genes (Fig. 3a and b). The VLPs were similar in size and shape to native double-shelled RDV particles (Fig. 3e). Such VLPs were not found in the extracts from nontransgenic rice plants or from transgenic rice expressing the S8 gene alone. This finding suggests that P8 alone did not form VLPs. A similar result was obtained with purified P8 from virions (23). To further confirm the identity of VLPs, purified antibody against RDV P8 was labeled with 5-nm-diameter colloidal gold as previously described (3, 18). EM grids coated with extracts from transgenic rice plants harboring S3 and S8 were placed onto drops of immunogold solution (1:20 [vol/vol] in 50 mM PBS containing 0.15 M NaCl, 0.02% polyethylene glycol 20,000, and 0.15% NaN3) containing immunogold-conjugated P8 antibody overnight before the negative staining. EM micrographs showed specific immunogold labeling of VLPs (Fig. 3c and d). This result demonstrated clearly that the VLPs contained RDV P8 on the outer capsid shell.
FIG. 3.
Electron micrographs of uranyl acetate-stained RDV particles. Shown are double-shelled VLPs assembled in cells of transgenic rice lines 1 (a) and 2 (b) coexpressing the P3 and P8 structural proteins. Also shown is immunogold probe detection of P8 in VLPs from transgenic rice plant lines 1 (c) and 2 (d). (e) RDV particle purified from RDV-infected rice plants. Bars = 10 nm.
Our study has demonstrated that coexpression of RDV P8 and P3, but not expression of P8 alone, in rice plants resulted in formation of double-shelled VLPs. These VLPs were similar to authentic RDV particles. This in vivo result suggests that P8 interacts with P3 and that these two proteins provide the structural integrity of the double-shelled particle. Consequently, the minor structural proteins (P1, P2, P5, and P7), nonstructural proteins, and viral dsRNAs are not required for the assembly of these VLPs.
To our knowledge, no VLP formation has been reported for any plant reoviruses in vivo. Expression of coat protein genes of several RNA plant viruses in bacteria or plants resulted in formation of VLPs in different plant virus taxonomic groups (1, 4, 14, 21). For reovirus, it has been showed that the viral cores from purified virions could be recoated with baculovirus-expressed outer capsid proteins μ1 and ς3 in vitro (2).
Our results have provided further information on RDV assembly. VLPs were produced in transgenic rice without the presence of other minor structural proteins, such as P2 and P7, although the previous reports had indicated that P7 might be necessary for protein-RNA interactions and that it also interacts with P3 and P8 in far-Western blotting analysis (14). We conclude that P7 is not necessary for the formation of VLPs. This result does not support the previous hypothesis of Ueda and Uyeda (Molecular Plant Pathology On-Line). Our data also suggested that P8-P3 interaction was necessary for the formation of a structure closely resembling RDV particles. The interaction between P3 and P8 was also demonstrated in far-Western blotting assay by Ueda and Uyeda (Molecular Plant Pathology On-Line). A recent report on the structures of double-shelled RDV and of its single-shelled core determined by cryoelectron microscopy and image reconstruction implied that part of the P8 trimers, anchored by paired P3-P8 interaction at the positions of the threefold axes, would form the seeding center for assembling the symmetry-mismatched outer shell and inner shell (16).
These in vivo results may have significant impact on understanding reovirus assembly and replication.
Acknowledgments
We thank R. S. Nelson and X. S. Ding for helpful suggestions and comments, T. Omura and N. Suzuki for their generous gift of the antiserum against P3, and S. Q. Liu for assistance.
The work was funded by National Key Basic Research (973 contract no. 62000016204), High-Tech (863-103-13-02-05), Natural Sciences Foundation of China (39870027), and Rockefeller Foundation Grant Award RF93022, Allocation 236.
REFERENCES
- 1.Bragard C, Duncan G H, Wesley S V, Naidu R A, Mayo M A. Virus-like particles assemble in plants and bacteria expressing the coat protein gene of Indian peanut clump virus. J Gen Virol. 2000;81:267–272. doi: 10.1099/0022-1317-81-1-267. [DOI] [PubMed] [Google Scholar]
- 2.Chandran K, Walker S B, Chen Y, Contreras C M, Schiff L A, Baker T S, Nibert M L. In vitro recoating of reovirus cores with baculovirus-expressed outer-capsid proteins μ1 and ς3. J Virol. 1999;73:3941–3950. doi: 10.1128/jvi.73.5.3941-3950.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding X, Shintaku M H, Carter S A, Nelson R S. Invasion of minor veins of tobacco leaves inoculated with tobacco mosaic virus mutants defective in phloem-dependent movement. Proc Natl Acad Sci USA. 1996;93:11155–11160. doi: 10.1073/pnas.93.20.11155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hwang D J, Roberts I M, Wilson T M A. Expression of tobacco mosaic virus coat protein and assembly of pseudovirus particles in Escherichia coli. Proc Natl Acad Sci USA. 1994;91:9067–9071. doi: 10.1073/pnas.91.19.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu G, Zhou Z H, Baker M L, Jakana J, Cai D, Wei X, Gu X, Chiu W. Structure of double-shelled rice dwarf virus. J Virol. 1998;72:8541–8549. doi: 10.1128/jvi.72.11.8541-8549.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao Z J, Li Y, Xu H, Zheng H H, Schiemann J, Casper R, Chen Z L. The 42K protein of rice dwarf virus is a post-translational cleavage product of the 46K outer capsid protein. Arch Virol. 1998;143:1831–1838. doi: 10.1007/s007050050421. [DOI] [PubMed] [Google Scholar]
- 7.Mizuno H, Kano H. Crystallization and preliminary X-ray study of a double-shelled spherical virus, rice dwarf virus. J Mol Biol. 1991;219:665–669. doi: 10.1016/0022-2836(91)90663-q. [DOI] [PubMed] [Google Scholar]
- 8.Murray M G, Thompson W F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naitow H, Morimoto Y, Mizuno H, Kano H, Omura T, Koizumi M, Tsukihara T. A low-resolution structure of rice dwarf virus determined by ab initio phasing. Acta Crystallogr Sect D. 1999;55:77–84. doi: 10.1107/S0907444998007677. [DOI] [PubMed] [Google Scholar]
- 10.Omura T, Ishikawa K, Hirano H, Ugaki M, Minobe Y, Tsuchizaki T, Kato H. The outer capsid protein of rice dwarf virus is encoded by genome segment S8. J Gen Virol. 1989;70:2759–2764. doi: 10.1099/0022-1317-70-10-2759. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki N, Sugamara M, Kusano T, Mori H, Matsuura Y. Immunodetection of rice dwarf phytoreoviral proteins in both insect and plant hosts. Virology. 1994;202:41–48. doi: 10.1006/viro.1994.1320. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki N, Watenabe U, Kusano T, Kitagawa Y. Sequence analysis of the rice dwarf phytoreovirus segment S3 transcript encoding for major structural core protein of 114 kDa. Virology. 1990;179:455–459. doi: 10.1016/0042-6822(90)90314-h. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi Y, Tomiyama M, Hibino H, Omura T. Conserved primary structures in core capsid proteins and reassembly of core particles and outer capsids between rice gall dwarf and rice dwarf phytoreoviruses. J Gen Virol. 1994;75:269–275. doi: 10.1099/0022-1317-75-2-269. [DOI] [PubMed] [Google Scholar]
- 14.Ueda S, Masuta C, Uyeda I. Hypothesis on particle structure and assembly of rice dwarf phytoreovirus: interactions among multiple structural proteins. J Gen Virol. 1997;78:3135–3140. doi: 10.1099/0022-1317-78-12-3135. [DOI] [PubMed] [Google Scholar]
- 15.Wellink J, Verver J, Van Lent L, Van Kammen A. Capsid proteins of cowpea mosaic virus transiently expressed in protoplasts form virus-like particles. Virology. 1996;224:352–355. doi: 10.1006/viro.1996.0541. [DOI] [PubMed] [Google Scholar]
- 16.Wu S, Hammar L, Xing L, Markarian S, Yan J, Iwasaki K, Fujiyoshi Y, Omura T, Cheng R H. Phytoreovirus T=1 core plays critical roles in organizing the outer capsid of T=13 quasi-equivalence. Virology. 2000;271:18–25. doi: 10.1006/viro.2000.0300. [DOI] [PubMed] [Google Scholar]
- 17.Xu H, Li Y, Mao Z J, Li Y, Wu Z, Qu L, An C, Ming X, Schiemann J, Casper R, Chen Z L. Rice dwarf phytovirus segment s11 encodes a nucleic acid binding protein. Virology. 1998;240:267–272. doi: 10.1006/viro.1997.8945. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Hu D W, Fang Y X. Preparation of immuno-gold probes for plant virus identification. J Zhejiang Univ. 1998;24(s):27–30. [Google Scholar]
- 19.Yan J, Tomaru M, Takahashi A, Kimura I, Hibino H, Omura T. P2 protein encoded by genome segment S3 of rice dwarf phytoreovirus is essential for virus infection. Virology. 1996;224:539–541. doi: 10.1006/viro.1996.0560. [DOI] [PubMed] [Google Scholar]
- 20.Zhang F, Li Y, Liu Y F, An C C, Chen Z L. Molecular cloning, sequencing, and functional analysis and expression in E. coli of major core protein gene (S3) of rice dwarf virus Chinese isolate. Acta Virol. 1997;141:161–168. [PubMed] [Google Scholar]
- 21.Zhao X, Fox J M, Olson N H, Baker T S, Young M J. In vitro assembly of cowpea chlorotic mottle virus from coat protein expressed in E. coli and in vitro transcribed viral cDNA. Virology. 1995;207:486–494. doi: 10.1006/viro.1995.1108. [DOI] [PubMed] [Google Scholar]
- 22.Zheng H H, Li Y, Yu Z H, Li W, Chen M Y, Ming X T, Casper R, Chen Z L. Recovery of transgenic rice plants expressing the rice dwarf virus outer coat protein gene (S8) Theor Appl Genet. 1997;94:522–527. [Google Scholar]
- 23.Zhu Y F, Hemmings A M, Iwasaki K, Fujiyoshi Y, Zhong B X, Yan J, Isogai M, Omura T. Details of the arrangement of the core capsid of rice dwarf phytoreovirus, as visualized by two-dimensional crystallography. J Virol. 1997;71:8899–8901. doi: 10.1128/jvi.71.11.8899-8901.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]