Abstract
Whether the long-term treatment of patients with proton pump inhibitors (PPIs) with different diseases [GERD, Zollinger–Ellison syndrome (ZES), etc.] can result in vitamin B12 (VB12) deficiency is controversial. In this study, in 175 patients undergoing long-term ZES treatment with anti-acid therapies, drug-induced control acid secretory rates were correlated with the presence/absence of VB12 deficiency, determined by assessing serum VB12 levels, measurements of VB12 body stores (blood methylmalonic acid (MMA) and total homocysteine[tHYC]), and other features of ZES. After a mean of 10.2 yrs. of any acid treatment (5.6 yrs. with PPIs), 21% had VB12 deficiency with significantly lower serum and body VB12 levels (p < 0.0001). The presence of VB12 deficiency did not correlate with any feature of ZES but was associated with a 12-fold lower acid control rate, a 2-fold higher acid control pH (6.4 vs. 3.7), and acid control secretory rates below those required for the activation of pepsin (pH > 3.5). Over a 5-yr period, the patients with VB12 deficiency had a higher rate of achlorhydria (73% vs. 24%) and a lower rate of normal acid secretion (0% vs. 49%). In conclusion, in ZES patients, chronic long-term PPI treatment results in marked acid hyposecretion, resulting in decreased serum VB12 levels and decreased VB12-body stores, which can result in VB12 deficiency.
Keywords: Zollinger–Ellison syndrome, PPI, vitamin B12 deficiency, acid hypersecretion, neuroendocrine tumor, gastrinomas, total homocysteine, methylmalonic acid
1. Introduction
The pharmacological control of gastric acid secretion by increasingly potent classes of gastric acid antisecretory drugs has been one of the most successful pharmaceutical accomplishments over the last 50 years. This progression started with the development of histamine H2-receptor antagonists in the 1970s (metiamide, followed by cimetidine, ranitidine, famotidine, nizatidine, etc.), followed by the introduction of inhibitors of the gastric H+K+ ATPase (proton pump inhibitors) (PPIs) in the 1980s (omeprazole, followed by lansoprazole, esomeprazole, pantoprazole, and rabeprazole) [1,2,3] and, very recently, by the introduction of gastric potassium-competitive acid blockers such as vonoprazan [4,5,6,7]. PPIs have been one of the best-selling drugs in the US as well as worldwide for several years [1,2,3], and in 2019, it was the eighth most commonly prescribed medication in the US, with >52 million prescriptions [8], resulting in 7–15% of patients using these drugs at some point [9,10,11]. With the recent availability of PPIs without prescriptions, because of their availability as over-the-counter drugs, the use of PPIs is increasing even further [12]. PPIs have overwhelmingly proven to be safe and effective drugs and are the mainstay for the treatment of gastroesophageal reflux disease (GERD) and peptic ulcer disease, for which they have been approved [1,2,13]. However, PPIs are increasingly being used with less clear indications [12,14,15] and, in fact, in a recent study [16], in nearly two-thirds of the patients, they were used with no clear indications.
This increased use of PPIs, coupled with the fact there is increased long-term use and even potential lifetime treatment with PPIs [17], especially for patients with chronic, advanced GERD, because the symptoms return quickly when the PPI is stopped, has led to increasing concerns about the long-term safety of these drugs [9,10,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. These safety concerns involve not only potential side effects of the PPI’s action but also the development of long-term hypergastrinemia, which invariably develops with their continued use [10,33,34,35,36,37]. These safety concerns have also been heightened by numerous reports, primarily from epidemiological or observational studies of potential serious side effects linked to PPIs [9,10,19,22,23,24,25,26,27,31,38], including bone fractures [18,30,39,40,41,42,43,44,45], chronic renal disease [46,47,48,49,50,51], malabsorption of nutrients [18,52,53,54,55,56], infections [57,58,59,60,61], increased cancer risk [33,34,36,62,63], increased mortality [64,65,66], increased drug–drug interactions with other therapeutic agents [38,67,68], dementia [69,70,71,72], and an increase in other CNS effects [69,73,74,75,76]. Although causality has not been proven by these studies and randomized control studies do not show an increased incidence of side effects [9,38], these concerns persist.
One of the safety issues that remains contentious and unresolved is in regard to the effect of chronic long-term treatment with PPIs on the absorption of the essential nutrient vitamin B12 (VB12) and whether it lowers serum vitamin B12 levels/body stores to the extent that vitamin B12 deficiency can develop, and if it this does occur, through which mechanism do PPIs cause this [18,52,53,54,77,78,79]? This controversy exists not due to a lack of research on this topic but because of the differences in the results of the studies that have been performed. Many of these studies have been performed in patients chronically taking PPIs for GERD, some of which support the conclusion that there is a long-term treatment effect of PPIs, which can result in a decrease in serum VB12 levels/body stores and, thus, the development of VB12 deficiency [54,78,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94]. However, numerous other studies do not support this conclusion and show no effect of PPIs on VB12 stores or the development of VB12 due to PPI usage [79,95,96,97,98,99]. One of the problems with these studies is that except for two studies on patients with Zollinger–Ellison syndrome (ZES) [54,79], none of the other studies found a correlation between the effect of the PPI on acid secretory rate and its effect on VB12 body stores; so, there was no clear relationship between the direct effect of PPI reduction on acid secretion and the effect on VB12 body stores, and thus, they did not provide any insight into the mechanism(s) involved regarding the observed effects of PPIs. The two studies on VB12 status with chronic PPI treatment in ZES patients [54,79] were performed to address this question. They were performed in ZES patients because of their need for life-long anti-acid treatment, which was shown by the fact that prior to the availability of potent acid antisecretory drugs, these patients died from their massive acid hypersecretion unless successful surgical treatment was performed (vagotomies, gastric resection, and total gastrectomies). Moreover, because only 25% of these patients are cured surgically [100,101,102], 75% require life-long treatment with PPIs with regular measurements of drug-induced acid secretory rates to adjust the drug dose [103,104,105] because of the continued acid hypersecretion secondary to the chronic hypergastrinemia caused by the continued ectopic release of gastrin from the gastrinomas [100,101,102]. In both of these ZES studies [54,79], it was found that there was a decrease in serum VB12 with long-term PPI treatment, but in one case [54], it was shown that the change in VB12 levels was due to the PPI-induced hypo-/achlorhydria; however, no study was performed on VB12 body stores to determine whether VB12 deficiency actually developed or examine the relationship between any changes in VB12 levels and the continued PPI treatment or the possible mechanisms involved. In the second study [79], it was concluded that the decrease induced by PPIs in the acid secretion of the ZES patients could not account for the change in serum VB12 or VB12 deficiency, which developed in some patients. Despite these unclear results in these two ZES studies, there are several reasons that the potential study of ZES patients could still provide one of the best opportunities to resolve the issue of whether prolonged PPI use in humans affects VB12 stores, resulting in VB12 deficiency, and to provide insights into the possible mechanisms involved. ZES patients are among the very few patient groups that have regular acid control assessments at all yearly admissions [103,104,105,106,107,108,109,110,111], when they are required to undergo an assessment of the adequacy of the acid secretory control and adjustment of the PPI dose to have secretory control to acceptable levels [103,104,105,106,107,108,109,110,111]. The result of this regular monitoring of acid secretion control allows the acid secretory status induced by the PPI to be continuously determined, which can then be correlated with other factors such as changes in VB12 body stores. This can lead to the potential assessment of effects of PPI-induced acid suppression on VB12 body stores or other PPI-induced effects. Therefore, in the current study, we investigated in detail the relationships of the PPI-induced acid secretory status in ZES patients with changes in VB12 body stores, including effects on both VB12 serum levels and VB12 body status by assessing blood methyl malonic acid levels and total homocysteine levels, which are well-established markers of body VB12 stores and VB12 deficiency [112,113,114,115,116,117,118,119,120,121,122]. In addition to defining the mechanism(s) of any changes in VB12 stores, we also investigated in detail any other factors that might contribute to these changes in VB12 levels, including clinical, tumoral, or laboratory features of ZES. By comparing these changes in patients treated with PPIs to the changes in acid/VB12 levels seen in patients on H2Rs, as well as the presence or absence of VB12 deficiency, we were able to provide evidence that the longstanding, chronic PPI treatment in our patients results in a decrease in serum VB12 levels and VB12 body stores, which can result in VB12 deficiency; this is mediated by the effect of PPI-induced hypo-/achlorhydria, which can cause VB12 mal-digestion/malabsorption, supporting the conclusion that chronic PPI treatment can result in VB12 deficiency under the conditions of this study.
2. Results
2.1. Patient Characteristics [Clinical, Laboratory, Tumoral, Acid Treatment Duration, Drug Type/Dosage, and Result]
One hundred and seventy-five consecutive patients with ZES were included in this study (Table 1). The clinical, laboratory, and tumoral features of the 175 patients are shown in Table 1 and Table 2. The patient’s clinical characteristics were similar to those in most other large series of ZES patients, which were predominantly male and Caucasian and mainly had the sporadic form (74%) of ZES [100,123,124,125,126,127,128,129,130,131,132]. Similarly, the patients resembled those in other series, with a 6-year delay in diagnosis, and at the time of this study, they were middle-aged (age 54 yrs.), with an average of 4 years after their initial evaluation at the NIH, and presented with clinical features usually described in other large series, most prominently including pain, diarrhea, and gastroesophageal reflux disease (GERD) [100,123,124,126,131,133,134,135,136,137,138]. The patients also had a long follow-up at the NIH, almost 20 years since ZES onset and over 10 years since their first admission at the NIH (Table 1). Similarly, the laboratory features with marked hyperchlorhydria/hypergastrinemia characteristic of ZES patients were present with both basal and maximal acid outputs that were markedly increased both in patients with and without previous gastric acid-reducing surgery and marked fasting hypergastrinemia occurred with >6-fold increase in gastrin, similar to that observed in previous series (Table 2) [131,135,137,139,140,141,142,143,144,145,146,147,148,149,150]. The tumoral features (Table 2), which were defined by findings on detailed imaging modalities, as well as surgical exploration in some patients, were also characteristic of the most recent ZES series [103,151,152,153], with most patients having localized disease at presentation, with either primary or secondary lymph node metastases. In addition, the primary tumor was located in the duodenum in the majority of patients and was less common in the pancreas (Table 2), as was originally proposed [123,154,155].
Table 1.
Patient clinical characteristics and disease course.
| Characteristic | Number (%) |
|---|---|
| Patient number | 175 (100%) |
| MEN 1 present (a) | 45 (26%) |
| Gender | |
| Male | 91 (52%) |
| Female | 84 (48%) |
| Race | |
| White | 137 (78%) |
| Black | 29 (17%) |
| Hispanic | 6 (4%) |
| Asian | 3 (1%) |
| Age at ZES onset (yrs.) (b) | |
| Mean ± SEM | 39.9 ± 0.9 |
| (Range) | (12.9–65.0) |
| Age at ZES diagnosis (yrs.) (c) | |
| Mean ± SEM | 45.8 ± 0.9 |
| (Range) | (14.2–70.6) |
| Age at 1st NIH visit | |
| Mean ± SEM | 47.8 ± 0.9 |
| (Range) | (13.1–71.0) |
| Age at the time of present study | |
| Mean ± SEM | 53.8 ± 0.9 |
| (Range) | (21.3–81.8) |
| Presenting clinical symptoms/features (d) | |
| Pain | 135 (77%) |
| Ulcer history | 121 (63%) |
| Diarrhea | 140 (80%) |
| GERD (any) | 88 (50%) |
| GERD(severe) | 24 (14%) |
| GERD/PUD Complication (e) | 31 (18%) |
| Bleeding | 46 (26%) |
| Duration (yrs.) | |
| From ZES onset to the present study | |
| Mean ± SEM | 13.7 ± 0.6 |
| (Range) | (0.5–42.1) |
| From ZES onset to last follow-up/death (f) | |
| Mean ± SEM | 19.4 ± 1.3 |
| (Range) | (2.4–40.7) |
| From 1st NIH visit to last follow-up/death (f) | |
| Mean ± SEM | 11.4 ± 0.9 |
| (Range) | (1.2–23.7) |
Abbreviations: ZES—Zollinger–Ellison syndrome; MEN1—Multiple Endocrine Neoplasia type 1; yrs.—years; NIH—National Institutes of Health; GERD—gastroesophageal reflux disease; PUD—peptic ulcer disease; and PMD—patient’s private medical doctor. (a) MEN1 was diagnosed using family history, serum calcium, prolactin, and PTH assays as described previously [100,156,157,158,159,160,161]. (b) Onset is defined as the onset of recurrent and/or persistent symptoms compatible with ZES as defined previously [106,162]. (c) Criteria for diagnosis of ZES required assessment of BAO/fasting gastrin/secretin test as described previously [104,150,163]. (d) Clinical symptoms were determined as described previously [164,165]. (e) Other GERD/PUD complications include nonbleeding complications (stricture [esophageal, duodenal, and small intestine], perforation, penetration, obstruction, and advanced Barrett’s esophagus) as defined previously [103,164,166]. (f) All patients were followed in the long term (≥5 years) at NIH and treated with gastric antisecretory drugs, except for patients who had an early death, surgical cure with low acid secretion [162,167,168,169], or returned to their PMD after diagnosis stabilization and evaluation [106]. During follow-up, 25 patients died, with 8 patients having a ZES-related death, and in 17 patients, the death was not ZES-related [101,170].
Table 2.
Patient laboratory/tumor characteristics.
| Characteristic | Number (%) |
|---|---|
| I. LABORATORY RESULTS | |
| BAO (mEq/h) (no gastric surgery) (a) | |
| Mean ± SEM | 43.6 ± 2.0 |
| (Range) | (1.8–159) |
| BAO (mEq/h) (previous gastric surgery) (a) | 19.3 ± 2.6 |
| Mean ± SEM | (8.2–33.1) |
| (Range) | |
| MAO (mEq/h) (no gastric surgery) (b) | |
| Mean ± SEM | 66.7 ± 2.5 |
| (Range) | (13–159) |
| MAO (mEq/h) (previous gastric surgery) (b) | |
| Mean ± SEM | 28.4 ± 3.7 |
| (Range) | (11.0–44.0) |
| Fasting serum gastrin (FSG) (pg/mL) | |
| Mean ± SEM | 2717 ± 863 |
| (Range) | (52–110,000) |
| Median | 618 |
| II. TUMORAL FEATURES | |
| Tumor extent (c) | |
| Overall tumor localization | |
| Localized disease | 40 (23%) |
| Not localized | 135 (77%) |
| Specific tumor extent (c) | |
| Primary only | 68 (39%) |
| Primary and lymph node metastases | 59 (34%) |
| Primary and liver metastases | 39 (22%) |
| Primary tumor location (d) | |
| Duodenum | 87 (50%) |
| Pancreas | 30 (17%) |
| Lymph node primary (e) | 22 (13%) |
| Other (f) | 12 (12%) |
| Unknown (g) | 36 (21%) |
Abbreviations: BAO—Basal acid output; MAO—Maximal acid output; FSG—serum fasting gastrin. (a) 163 patients had a preoperative BAO (152 with no gastric surgery and 11 with previous gastric acid-reducing surgery) determined as described previously [104,150]. (b) A total of 143 patients had an MAO (133 with no gastric surgery and 10 with previous gastric acid-reducing surgery) determined as described previously [104,150]. (c) General tumor extent is determined by imaging and surgery in all patients as described previously [103,171,172,173]. Specific localization in 9 patients with regional disease could not be determined because no surgery was performed. Localized disease refers to patients with regional disease without distant metastases to liver/bone/or other sites. (d) The primary tumor site was established during surgery or endoscopy or by imaging as described previously [167,174,175,176,177,178]. (e) Primary lymph node gastrinomas were identified as described previously [179]. (f) Non-pancreatic-duodenal/lymph node primary sites occurred as described previously in the hepato-biliary tract [102,180], ovary, jejunum, mesentery, heart, lung cancer, and gastric antrum [103,181,182,183]. (g) Patients with diffuse liver metastases, with MEN1/ZES, or severe co-morbidities did not undergo routine surgical exploration as described previously [151,159,178,184], and the primary location, if not clearly identified on the imaging/endoscopy, was listed as an unknown primary site.
All the ZES patients enrolled in the present study had been treated prior to this study using long-term (mean of 10.2 yrs.) chronic gastric acid antisecretory drugs, and at the time of this study, they were all still being treated with chronic gastric acid antisecretory drugs (Table 3 and Table 4). This result is consistent with other reports on the long-term treatment of ZES patients [79,100,106,109,110,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209] due to the following reasons. The majority (80%) of ZES patients cannot be surgically cured, with 20–30% having MEN1/ZES and multiple microscopic duodenal gastrinomas, frequently with lymph node metastases [151,178,210,211]. Thus, the MEN1/ZES patients cannot be cured without a Whipple resection, which is not recommended in most guidelines [103,212]. Furthermore, 30–35% of patients have distant metastases at presentation, which are unresectable, and thus, over all, only 30–40% of the patients with sporadic ZES (75% of all the patients) can be surgically cured [103]. In addition, even in those cured surgically, a high proportion continues to have marked gastric hypersecretion, requiring chronic long-term antisecretory drug treatment [169], which necessitates the constant monitoring of gastric output to appropriately establish the required drug dose, which can change with time [106,213,214]. This makes ZES an excellent model to study the long-term possible side effects of chronic drug-induced gastric acid inhibition, including its possible effect on nutrient absorption such as with VB12 (which requires gastric acid secretion for the absorption of food-bound VB12), which is an essential vitamin that needs to be absorbed from food [18,52,215]. Prior to this study, 75% of patients had undergone long-term H2R treatment at some point (mean of 5.8 yrs.), and 96% received long-term PPI treatment more recently (mean of 5.6 yrs.) (Table 3 and Table 4) because the antisecretory treatment had been frequently started at the onset of symptoms (mean of 3.6 yrs. prior to the study), even before the diagnose of ZES was established (Table 3). The primary H2R that the patients had been previously treated with was ranitidine (62%), with 25% treated with cimetidine at some point, whereas of the 96% receiving PPIs (26% without previous H2R treatment), the most frequent PPI used was omeprazole (96%), with 15% receiving lansoprazole (Table 4). During the study period (1997–2001), 166 (95%) of patients were treated with PPIs (100% omeprazole), and 5% were treated with H2Rs (Table 4). Both with the initial H2R dosing (n = 9), as well as the H2R dose at the time of this study, high daily doses of H2Rs were required to control acid secretion (981 ± 86 mg and 1083 ± 391 mg of ranitidine-equivalent daily dose), which is similar to the findings of other studies on ZES patients [79,106,197,198]. Similarly, higher doses of PPIs than are characteristically used in the treatment of patients with idiopathic peptic ulcer disease or GERD [203,216,217,218,219,220] were also initially required to control the acid hypersecretion, as was the case in the present study as well (n = 166 pts) (71.4 ± 3.0 and 61.7 ± 3.0 mg/day of omeprazole-equivalent dose) (Table 4), which is a similar result to that reported in numerous other studies on ZES patients [203,216,217,218,219,220]. Before the availability of PPIs, gastric acid-reducing surgery was often performed in addition to the use of H2Rs alone [103,146,221,222], which required frequent high dosing to control acid hypersecretion. In older studies, gastric resections were reported to also affect VB12 absorption [18,223,224]; so, this also has to be noted in the subsequent analyses. In our study, 11 patients (6.3%) had vagotomies ± Billroth resections prior to being referred to the NIH, and another 22 (12.5%) patients had parietal cell vagotomies at the NIH (Table 3).
Table 3.
Acid treatment data: surgical and medical (duration).
| Characteristic | Number (% Total) |
|---|---|
| A. Gastric acid-reducing surgery | |
| I. Prior to the initial visit to the NIH | |
| No | 11 |
| Yes | 164 |
| II. Type of prior surgical treatment of acid hypersecretion | |
| Vagotomy-pyloroplasty/selective vagotomy | 11 (4.6%) |
| Billroth I resection | 4 (2.0%) |
| Billroth 2 resection | 5 (3%) |
| III. Highly selective vagotomy at NIH (a) | 22 (12.5%) |
| B. Medical treatment of acid hypersecretion prior to the present study (duration) (yrs.) | |
| I. Any medical acid treatment (yrs.) (n = 175) (b) | |
| Mean ± SEM | 10.2 ± 0.5 |
| Range | (0.1–30.1) |
| Any Tx > 10 yrs. | 20 (11%) |
| II. Any treatment with H2R (yrs.) (n = 130) (b) | |
| Mean ± SEM | 5.8 ± 0.4 |
| Range | (0.2–20) |
| III. Any treatment with PPI (yrs.) (n = 169) (b) | |
| Mean ± SEM | 5.6 ± 0.3 |
| Range | (0–14.9) |
| PPI Tx > 10 yrs. | 20 (11%) |
| IV. Time until medical acid treatment started (yrs.) (c) | |
| From ZES onset (yrs.) (c) | |
| Mean ± SEM | 3.6 ± 0.4 |
| (Range) | (0.0–26.0) |
| Prior to ZES diagnosis (yrs.) (n = 122) (c) | |
| Mean ± SEM | 3.7 ± 0.4 |
| (Range) | (0.01–26.2) |
| After ZES diagnosis (yrs.) (n = 51) (c) | |
| Mean ± SEM | 1.5 ± 0.5 |
| (Range) | (0.01–18.1) |
Abbreviations: H2R—histamine H2-receptor antagonists; NIH—National Institutes of Health; PPI—proton pump inhibitors; ZES—Zollinger–Ellison syndrome; and others—see legends in Table 1 and Table 2. (a) Prior to the availability of PPIs, selective patients with high BAOs and antisecretory drug requirements, which can persist even after tumor resections, had a highly selective vagotomy performed at the time of any surgical exploration, as recommended from 1980 to 1983 [169,219,225]. (b) In the NIH perspective trials [106,214], H2Rs were the first effective acid antisecretory medical therapy, with cimetidine being first used in 1978, ranitidine in 1982, and famotidine in 1983 [198,226,227]. PPIs were first used in 1983 with omeprazole and with lansoprazole in 1989 [106,197,220,228]; so, all patients initially enrolled in this study were first treated with H2Rs (cimetidine, ranitidine, and famotidine) and later, most of them switched to PPIs (omeprazole and lansoprazole) while new patients generally started treatment with PPIs [106,229]. (c) The times of ZES onset and diagnosis were determined as described in the Methods section and Table 1 footnote. In 51 patients, the diagnosis of ZES was after or at the time of the earliest medical therapy, whereas in 122 patients, some initial medical therapy preceded the diagnosis of ZES, as defined in the Methods section.
Table 4.
Acid treatment: drug schedule, and dose.
| Characteristic | Number (% Total) |
|---|---|
| I. Treatment schedule | |
| I.A. First acid medical drug treatment (n = 175) (a) | |
| H2R | 130 (74%) |
| PPI | 45 (26%) |
| I.B. Total acid treatment: drug (n = 175) (a) | |
| H2R-related | |
| H2R antagonist (cimetidine, ranitidine, famotidine, and nizatidine) at any time | 130 (74%) |
| Only H2R antagonists without any PPI treatment at any time | 6 (3.4%) |
| H2R with an anticholinergic agent at any time (b) | 16 (9.1%) |
| H2R without an anticholinergic agent at any time | 158 (90%) |
| H2R followed by PPI (c) | 124 (71%) |
| PPI-related | |
| PPI (omeprazole, lansoprazole, and pantoprazole) at any time | 169 (96.5%) |
| PPI only | 45 (26%) |
| PPI followed by H2R (d) | 3 (1.7%) |
| II. Antisecretory drugs used at any time (a and e) | |
| II.A. H2R (a and e) | |
| Cimetidine | 44 (25%) |
| Ranitidine | 108 (62%) |
| Famotidine | 18 (10%) |
| Nizatidine | 3 (1.7%) |
| II.B. PPI (a and e) | |
| Omeprazole | 168 (96%) |
| Lansoprazole | 26 (15%) |
| Pantoprazole/esomeprazole | 4 (2.2%) |
| III. Treatment: acid secretory drug dose | |
| III.A. Initial antisecretory treatment | |
| H2R (f) | |
| # of patients | 128 |
| Initial dose (mg/day) | |
| Mean ± SEM | 918 ± 86 |
| (Range) | (100–4800) |
| PPI | |
| # of patients | 170 |
| Initial dose (mg/day) (g) | |
| Mean ± SEM | 71.4 ± 3.0 |
| (Range) | (20–240) |
| III.B. Current study: antisecretory drug dose | |
| H2R | |
| # of patients | 9 |
| current dose (mg/day) (f) | |
| Mean ± SEM | 1083 ± 391 |
| (Range) | (300–3600) |
| PPI | |
| # of patients | 166 |
| Current dose (mg/day) (g) | |
| Mean ± SEM | 61.7 ± 3.0 |
| (Range) | (20–240) |
Abbreviations; H2R—Histamine H2-receptor antagonists; PPI—Proton pump inhibitors; ZES—Zollinger–Ellison syndrome. (a) In the NIH perspective trials [106,214], H2Rs were the first effective acid antisecretory medical therapy, with cimetidine first used in 1978, ranitidine in 1982, and famotidine in 1983 [198,226,227]. PPIs were first used in 1983 with omeprazole and then with lansoprazole in 1989 [106,197,220,228]; so, all patients initially enrolled in this study were first treated with H2Rs (cimetidine, ranitidine, and famotidine) and later, most of them switched to PPIs (omeprazole and lansoprazole). New patients generally started treatment with PPIs [106,229]. (b) When only H2R antagonists were available, many patients required high and frequent dosing to control the acid hypersecretion [177,226,227]. The addition of an anticholinergic drug such as isopropamide or probanthine potentiated the H2R inhibitory effect and was thus frequently added [106,230]. (c) Patients with active disease were initially treated with H2Rs and then switched to PPIs as described previously [106]. (d) Patients with active disease were initially treated with PPIs and then switched to H2Rs, particularly after curative resections [169,225]. (e) The total number of patients treated with a given PPI or H2R in total was greater than the number of patients initially treated with PPIs/H2Rs because many of them received more than one antisecretory drug over time. (f) The daily H2R dosage is listed as a ranitidine-equivalent dose calculated as described previously [106,227] using their relative potencies of famotidine/ranitidine/cimetidine of 1:9:32 based on a previous study on ZES patients [227]. (g) The PPI dose listed is listed as a daily omeprazole-equivalent dose as described previously from data demonstrating that omeprazole (20 mg) was equivalent to 40 mg of esomeprazole, 30 mg of lansoprazole, 40 mg of pantoprazole, and 20 mg of rabeprazole [231].
2.2. Patient Serum VB12 and MMA Levels, Plasma tHCY Levels, and Identification of VB12-Deficient Patients
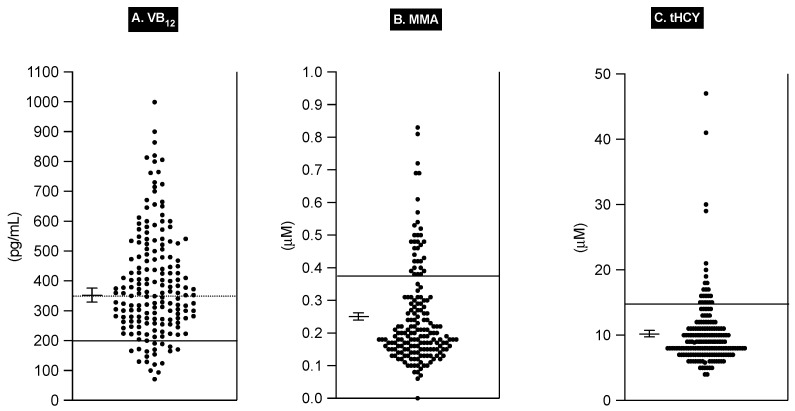
The serum VB12 levels varied markedly in the 175 patients, with a mean ± SEM value of 394 ± 14 pg/mL and a range of 71 to 999 pg/mL (Figure 1 and Table 5). In various studies, several serum VB12 levels have been widely proposed as useful cut-offs for the lower level of normal to identify VB12-deficient subjects [118,232]. These include serum VB12 levels < 200 pg/mL (148 pmoles/L) [112,113,114,115,116,118,120,233,234] as the most frequently recommended level (sensitivity = 38–39%) [116,232]), as well as other values of 250/270/280 pg/mL [113,115,118], 337/348/350 pg/mL [113,115,116,118], and 1001 pg/mL [115], which have increasing specificity but rapidly decreasing sensitivity [116,118,232].
Figure 1.
Scatter diagram of serum vitamin B12 (VB12), serum methyl malonic acid (MMA), and plasma total homocysteine (tHCY) values of 175 ZES patients. Each solid dot represents the value for one of the three parameters for a given patient. The mean ± SEM for all patients is shown for each serum parameter. For VB12, the solid line at <200 pg/mL represents a commonly used value proposed for identifying patients with VB12 deficiency [112,113,114,115,118,120,233,235]. The VB12 dotted line represents the upper limit of the 200–350 pg/mL VB12 range, referring to patients with low normal levels of VB12, a proportion of whom can be VB12-deficient, which can be identified by accompanying blood MMA and/or tHCY levels [115,116,118,232]. In the serum MMA and tHCY panels, the solid line represents the upper limit of the normal level for the assays of 0.37 uM and 0.15 uM for MMA and tHCY, respectively.
Table 5.
Mean VB12/MMA and plasma tHCY levels and % VB12-deficient patients according to different criteria.
| Characteristic | Number (% Total) |
|---|---|
| A. Single serum measurements [VB12, MMA, and tHCY] | |
| I. VB12 levels | |
| Ia. VB12 level (pg/mL) | |
| Mean ± SEM | 394 ± 14 |
| (range) | (71–999) |
| Ib. Proposed VB12 deficiency | |
| # with VB12 level < 200 pg/mL (a) | 18 (10%) |
| # with VB12 level 200–350 pg/mL (b) | 67 (38%) |
| II. MMA levels | |
| IIa. MMA value (uM) | |
| Mean ± SEM | 0.25 ± 0.01 |
| (range) | (0.06–0.83) |
| IIb. Proposed VB12 deficiency < 200 pg/mL (c) | |
| # with MMA level > 0.26 (uM) (a) | 53 (30%) |
| # with MMA level > 0.37 (uM) (b) | 32 (18%) |
| IIII. Plasma tHCY levels | |
| IIIa. tHCY value (uM) | |
| Mean ± SEM | 10.20 ± 0.41 |
| (range) | (4.0–47) |
| IIIb. Proposed VB12 deficiency (d) | |
| # with tHCY level > 13 uM (a) | 22 (13%) |
| # with tHCY level > 15 uM (a) | 14 (8%) |
| B. Combination blood measurements [VB12, MMA, and tHCY] | |
| # with VB12 level < 200 pg./mL + MMA > 0.37 uM (e) | 14 (8%) |
| # with VB12 level < 200 pg./mL + tHCY > 15 uM (e) | 8 (4.6%) |
| # with MMA level > 0.37 uM or tHCY > 15 uM and response to VB12 administration [normal folate] (f) | 37 (21%) |
Abbreviations: MMA—serum methylmalonic acid level; tHCY—plasma total homocysteine level; and VB12 level: serum vitamin B12 concentration. (a) Numerous studies have proposed a serum VB12 level of <200 pg/mL for diagnosing VB12-deficient patients [112,113,114,115,118,120,233,235]. (b) Serum VB12 levels over the range of 200–350 pg/mL have been reported to represent a low-level range that could suggest VB12 deficiency [115,116,118]. (c) Many studies propose that an MMA level > 0.37 uM should be generally used to identify VB12-deficient patients [115,116,117,118] and a few recommend using an MMA level of >0.26 uM [116,235,236,237]. These recommendations are only for patients with normal renal function [113,116,236,238]. (d) Various studies have proposed either a tHCY level of >13 uM [113,115,234] or >15 uM [112,114,115,235] as the upper limit of normal. (e) Numerous combinations of serum VB12 levels with either MMA or tHCY levels have been reported to be more sensitive than either one alone [112,113,114,115,119,120]. (f) The response of blood MMA/tHCY with normal serum folate levels and renal function to the administration of crystalline VB12 either given orally or parenterally is considered by many to be one of the single best measurements of VB12 deficiency [112,114,117,236,237].
In addition, serum VB12 levels of 200–300 pg/mL [116,233,234,237,239] and 200–350/470 pg/mL [116,215] have been reported to be marginally low levels of serum VB12. In our study, 18 patients (10%) had a serum VB12 level <200 pg/mL, and another 67 patients (38%) had VB12 levels between 200 and 350 pg/mL (Table 5), suggesting that a significant percentage of our patients could be VB12-deficient using these criteria.
Unfortunately, in general, it is now generally recognized that no single level of serum VB12 alone can identify almost all patients with VB12 deficiency [113,114,116,118,120,215,232]. To increase the sensitivity and specificity of the diagnosis of VB12 deficiency, two different blood determinations (i.e., the assessment of total homocysteine (tHYC) and methylmalonic acid (MMA) levels) have been developed and are now widely used, either alone or in combination with serum VB12 levels, and the combined use of tHYC and MMA is now the recommended approach to diagnose VB12 deficiency [84,112,113,114,118,120,215,232]. Both these tests measure the functional effect of VB12 (cobalamin) deficiency on metabolic enzymes; the tHCY assay takes into account that methylcobalamin and folate are essential coenzymes for the biosynthesis of methionine from homocysteine, catalyzed by the cytosolic enzyme methionine synthetase, which is essential for de novo nucleic acid biosynthesis, and thus, with a deficiency in either of these coenzymes, there is an accumulation of HCY in the blood [112,113,114,115,116,117,118,120,215,232]. The assessment of blood MMA is even more specific for VB12 deficiency because adenosyl cobalamin is essential for the conversion of methyl malonyl-Co-A to succinyl Co-A, catalyzed by methyl malonyl-CoA synthase, which is needed for the proper function of the Krebs cycle and heme biosynthesis, such that VB12 deficiency, rather than folate deficiency, results in a buildup of methyl malonyl-CoA, which enters the circulation as free MMA [112,113,114,115,116,117,118,120,215,232]. In the 175 ZES patients, the serum MMA level varied widely, from 0.06 to 0.83 uM (Figure 1 and Table 5). Several upper limits of normal cut-off values for serum MMA have been proposed, including both 0.26 uM [116,118,236,237] and 0.37 uM [115,116,117,118], which are the most commonly used values [115,116,117,118]. In our study, 53 patients (30%) had a serum MMA value > 0.26 uM, and 32 patients (18%) had a serum MMA value > 0.37 (Figure 1 and Table 5). In the 175 patients, the plasma tHCY level also varied widely, from 4.0 to 47 uM (Figure 1 and Table 5). Similar to MMA levels, several upper limits of normal levels for plasma tHCY have also been proposed, with the most frequent being >15 uM [112,114,115,235,240], but a limit >13 uM has also been proposed in several studies [113,115,234]. In our study, 22 patients (13%) had a tHYC level > 13 uM, and 14 patients (8%) had a value > 15 uM (Table 5). In total, 39 patients (22%) had an elevated serum level of MMA > 0.37 or an elevated level of tHYC > 15 uM, with 7 patients (4%) having an elevated level of both. Of the 32 patients having a serum MMA > 15 uM, 7 had a plasma tHYC >15 uM, whereas of the 14 patients with tHCY >15 uM, 7 patients had a serum MMA >15 uM. In numerous studies, it has been reported that both the serum MMA levels and the plasma tHYC levels are very sensitive to alterations in renal function [116,117,238] and that the tHCY levels can also be affected by folate deficiency [113,240]. Neither of these variables was a contributing factor to the serum MMA or plasma tHCY elevations in our patients as all the patients had multiple assessments of serum creatinine as well as serum folate levels, and in all patients, they were within the normal range.
In numerous studies, various combinations of serum VB12/MMA and plasma tHYC values have been proposed to better identify patients with VB12 deficiencies compared to serum VB12/MMA or plasma tHYC alone [112,113,114,115,119,120]. We investigated the results using two of these commonly used criteria in our patients [117,240], which involved identifying patients with a decreased serum VB12 level to <200 pg/mL combined with either an elevated serum level of serum MMA (i.e., >0.37 uM) or an elevated level of plasma tHYC (i.e., >15 uM) (Table 5). The result of the combination of VB12 and MMA was observed in 14 patients (8%), and the result of the combination of VB12 and tHYC was observed in 8 patients (4.6%) (Table 5). Of the 175 patients, one or the other of these two combination criteria was found to be positive in 17 patients (10%), with only the VB12/tHYC combination criterion observed in 3 patients, only the criterion of the VB12/MMA combination observed in 9 patients, and both combinations of the VB12/MMA and VB12/tHCY criteria observed in 5 patients.
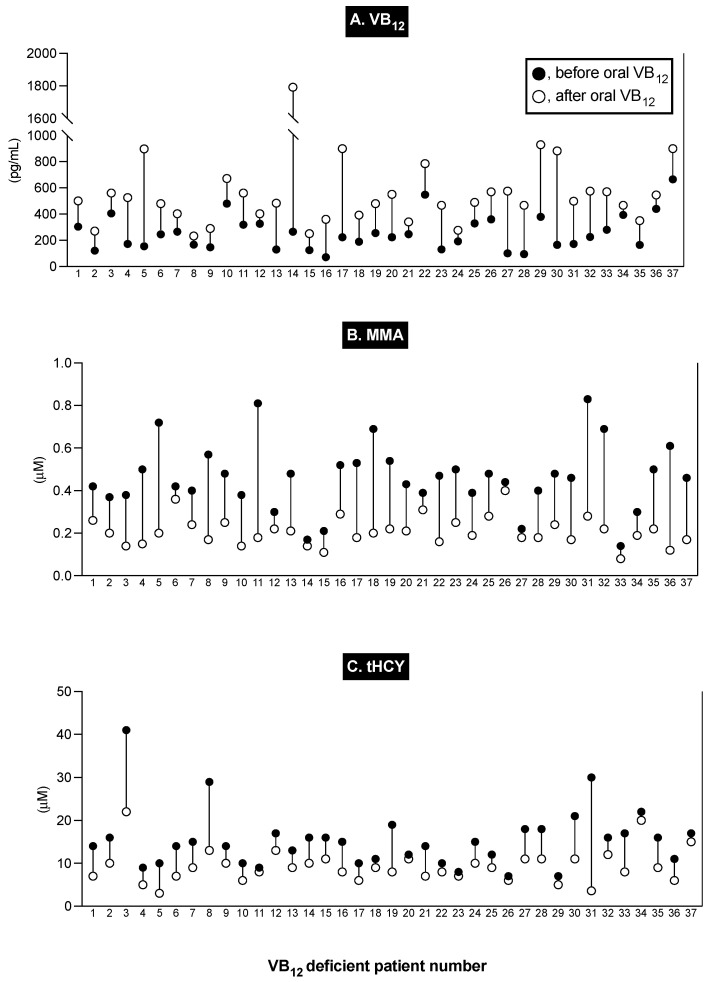
Numerous studies show that to identify all patients with VB12 deficiency, the inclusion of a criterion of serum VB12 < 200 pg/mL, either alone or in combination with MMA/tHCY levels, has a low sensitivity [113,116,118] because a significant proportion of VB12-deficient patients are now known to have serum VB12 levels in the range of 200–350 pg/mL or even higher [115,116,118,241]. This is particularly true of patients with subclinical cobalamin deficiency, in which the blood MMA or tHYC identifies VB12 deficiency, but the patients are asymptomatic and do not have hematological changes [118,232,240]. One additional criterion frequently used to diagnose VB12 deficiency is the assessment of changes in blood MMA/tHYC after the administration of VB12, which, in several studies, has been stated to be the single best method of detecting VB12 deficiency [112,114,117,236,237,242]. Therefore, we assessed the response to the administration of VB12 levels in all the patients with elevated levels of blood MMA or tHCY. Figure 2 shows the results for the 37 patients who met the criteria of having both an elevated blood MMA or tHCY level and who showed an increase in serum VB12 level and a decrease in MMA and/or tHCY levels after the administration of VB12. For the 37 patients, the mean serum VB12 level increased 2.2-fold from 256 ± 22 to 559 ± 47 (p < 0.0001), while the mean serum MMA level showed a decrease of 56% from 0.462 ± 0.025 to 0.208 ± 0.011 (p < 0.0001), and the mean plasma tHCY showed a decrease of 40% from 15.4 ± 1.11 to 9.30 ± 0.64 (p < 0.0001) (Figure 2). This latter combination criterion using two different markers, both widely used to identify VB12 deficiency due to an elevated MMA level > 0.37 uM or an elevated tHYC level > 15 uM (with normal renal function and normal folate levels), combined with the appropriate response to the administration of VB12 (increased serum VB12, decreased serum MMA, or plasma tHCY), was therefore used to identify the 37 ZES patients (21%) with VB12 deficiency in our study (Table 5).
Figure 2.
Serum VB12, serum MMA, and plasma tHCY results from 37 VB12-deficient patients after taking crystalline VB12. Patient numbers are shown on the X axis, and the change in serum VB12, MMA, and tHYC are shown on the Y axis. The solid and the open circles represent the serum values before and after taking VB12, respectively.
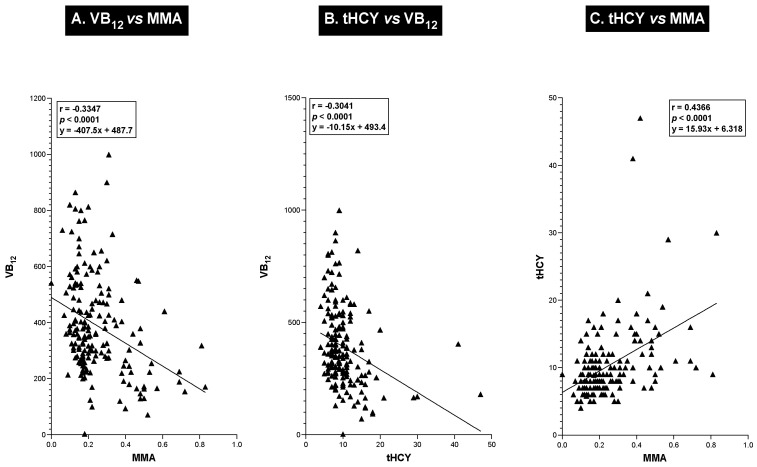
A correlation analysis of the relationship between serum VB12 levels and serum MMA/plasma tHCY levels in a given patient supports the above results that these have a reciprocal relation, with an increase in both blood MMA and tHCY levels as the VB12 levels decrease (Figure 3A,B). As seen in Figure 3A,B, there was a highly significant negative correlation (r = −0.304 and r = −0.335) (p < 0.0001) between the serum VB12 level and the serum MMA level (Figure 3A) or between the serum VB12 level and the plasma tHCY level (Figure 3B) in a given patient, and the serum MMA level directly (r = 0.437) and significantly (p < 0.0001) correlated with the plasma tHCY level in a given patient (Figure 3C).
Figure 3.
Correlations between patient values for serum MMA and plasma tHYC with serum vitamin B12 levels. Each solid circle or triangle is a value from one patient determined from the same NIH admission. The correlation coefficient, its significance, and the best-fitted regression line equation (using least squares regression analysis) for each correlation are shown.
2.3. Comparison of Vitamin B12 Markers (i.e., Blood VB12, MMA, and tHCY) and Clinical, Laboratory, and Tumoral Characteristics of ZES Patients with or without VB12 Deficiency
Our patients with VB12 deficiency had a significantly lower serum VB12 level, with more than 40% having a level below 200 pg/mL (p < 0.0001) compared to patients without VB12 deficiency (Table 6). Furthermore, the VB12-deficient patients had a 2.4-fold increase in serum MMA levels (p < 0.0001), with 81% exceeding an MMA level > 0.37 uM, all significantly higher than in patients without VB12 deficiency (Table 6). Similarly, the plasma tHCY level was increased by almost 2-fold, with almost 40% having a level > 15 uM, which were all markedly increased (p < 0.0001) compared to patients without VB12 deficiency (Table 6).
Table 6.
Comparison of serum VB12/MMA and plasma tHCY levels in patients with or without VB12 deficiency.
| Number (% Total Group) | |||
|---|---|---|---|
| VB12 Deficiency | |||
| Yes | No | p-Value | |
| Characteristic | (n = 37) | (n = 138) | |
| Serum VB12 levels (pg/ML) | |||
| Mean ± SEM | 254 ± 22 | 433 ± 15 | <0.0001 |
| (Range) | (71–665) | (150–999) | |
| # with VB12 level < 200 pg/mL (a) | 16 (43%) | 2 (1.4%) | <0.0001 |
| # with VB12 level 200–350 pg/mL (b) | 14 (38%) | 53 (38%) | 0.99 |
| Serum MMA levels (uM) | |||
| Mean ± SEM | 0.46 ± 0.03 | 0.19 ± 0.01 | <0.0001 |
| (Range) | (0.14–0.83) | (0.06–0.42) | |
| # with MMA level > 0.26 (uM) (c) | 31 (84%) | 22(16%) | <0.0001 |
| # with MMA level > 0.37 (uM) (c) | 30 (81%) | 2 (1.4%) | <0.0001 |
| Plasma tHCY levels (uM) | |||
| Mean ± SEM | 15.3 ± 1.1 | 8.8 ± 0.3 | <0.0001 |
| (Range) | (7.0–41.0) | (4.0–47.0) | |
| # with tHCY level > 13 uM (d) | 18 (49%) | 4 (2.9%) | <0.0001 |
| # with tHCY level > 15 uM (d) | 14 (39%) | 0 (0%) | <0.0001 |
Abbreviations: See legends in Table 1, Table 2, Table 3, Table 4 and Table 5. (a) Numerous studies have proposed a serum VB12 level of <200 pg/mL for diagnosing VB12-deficient patients [112,113,114,115,118,120,233,235]. (b) Serum VB12 levels over the range of 200–350 pg/mL have been reported to represent a low-level range that could suggest VB12 deficiency [115,116,118]. (c) Several studies propose classifying those with serum MMA > 0.37 uM as VB12-deficient patients [115,116,117,118] and a few recommend using a serum MMA level of >0.26 uM [116,235,236,237]. These recommendations are only for patients with normal renal function [113,116,236,238]. (d) Various studies have proposed either a plasma tHCY level of >13 uM [113,115,234] or >15 uM [112,114,115,235] as the upper limit of normal.
The 37 ZES patients with VB12 deficiency in our study had no clinical symptoms that indicated the presence of this disorder, and their hematological profile did not differ from the 138 ZES patients without VB12 deficiency (Table 7 and Table 8). No patient had an increase in mean corpuscular volume (i.e., MCV > 100 fL), megaloblastic anemia, or reported hypersegmented neutrophils [240]. Therefore, all our VB12-deficient patients could be classified as having subclinical cobalamin deficiency, which is commonly observed in older patients, primarily due to food-bound cobalamin malabsorption occurring in up to 40% of patients [113,118,240]. Furthermore, in regard to the clinical/demographic features of ZES, they did not differ between patients with or without VB12 deficiency in age, gender, race, presence or absence of ZES symptoms, presence or absence of MEN1, or duration from ZES onset to the time of this study or age at ZES onset (Table 8). Furthermore, the VB12-deficient and non-VB12-deficient patients did not differ in the magnitude of their original gastric acid hypersecretion (either BAO or MAO), the magnitude of their hypergastrinemia, or the occurrence of previous gastric acid-reducing surgery, including partial gastrectomy (Table 8). In terms of the tumoral features of the gastrinomas, there were no differences between patients with or without VB12 deficiency in terms of tumor extent or primary tumor locations (Table 8).
Table 7.
Comparison of hematological/serum folate values in patients with or without VB12 deficiency.
| VB12 Deficiency | |||
|---|---|---|---|
| Yes | No | p-Value | |
| Characteristic | (n = 37) | (n = 138) | |
| Hematological value (a) | |||
| Hematocrit (%) | 41.5 ± 0.6 | 40.9 ± 0.6 | 0.61 |
| Mean corpuscular volume (fL) | 90.8 ± 0.9 | 89.3 ± 0.9 | 0.70 |
| Leukocytes (×103/mm3) | 6.1 ± 0.3 | 6.4 ± 0.1 | 0.32 |
| Red blood cell count (×103/mm3) | 4.6 ± 0.9 | 4.7 ± 0.4 | 0.67 |
| Serum folate levels (ng/mL) | |||
| Mean ± SEM | 11.6 ± 0.77 | 11.4 ± 0.43 | 0.70 |
| Median (range) | 10.60 (4.4–24.5) | 10.50 (3.8–30.0) | |
Table 8.
Comparison of ZES Clinical/lab/tumoral features in patients with or without VB12 deficiency.
| Number (% Total Group) | |||
|---|---|---|---|
| VB12 Deficiency | |||
| Yes | No | p-Value | |
| Characteristic | (n = 37) | (n = 138) | |
| I. Clinical features/disease course | |||
| Age at study (yrs.) (Mean ± SEM) (a) | 54.1 ± 2.0 | 53.7 ± 1.0 | 0.93 |
| Male gender | 24 (65%) | 67 (49%) | 0.096 |
| Race | |||
| White | 30 (81%) | 107 (78%) | 0.82 |
| Nonwhite | 7 (19%) | 31 (23%) | |
| Age at ZES onset (yrs.) (Mean ± SEM) (a) | 40.3 ± 2.0 | 39.8 ± 1.0 | 0.71 |
| Presenting symptom (a) | |||
| Pain | 29 (78%) | 106 (77%) | 0.99 |
| GERD | 15 (40%) | 72 (52%) | 0.27 |
| Diarrhea | 32(86%) | 108 (78%) | 0.36 |
| MEN-1 present (a) | 9 (24%) | 36 (26%) | 0.84 |
| Duration (yrs.) (mean ± SEM) | |||
| Time ZES onset to study | 13.8 ± 1.3 | 13.7 ± 0.7 | 0.93 |
| II. LABORATORY RESULTS | |||
| BAO (mEq/h) (Mean ± SEM) (b) | 41.7 ± 3.8 | 42.2 ± 2.2 | 0.82 |
| MAO (mEq/h) (Mean ± SEM) (c) | 57.3 ± 4.8 | 66.2 ± 2.9 | 0.10 |
| # Previous gastric acid-reducing surgery) (a) | 2 (5.4%) | 9 (6.5%) | 0.99 |
| Fasting serum gastrin (FSG) (pg/mL)) | |||
| Mean ± SEM | 1953 ± 401 | 2921 ± 1090 | 0.84 |
| (Range) | (172–8900) | (52–110,000) | |
| Median | 742 | 597 | |
| III. TUMORAL FEATURES | |||
| Tumor extent | |||
| Overall tumor localization (d and e) | |||
| Localized disease | 29 (78%) | 106 (77%) | 0.99 |
| Not localized | 8 (22%) | 32 (23%) | |
| Specific tumor extent (e and f) | |||
| Primary only | 10 (27%) | 58 (42%) | 0.13 |
| Primary and lymph node metastases | 17 (46%) | 42 (30%) | 0.082 |
| Primary and liver metastases | 8 (22%) | 31 (22%) | 0.99 |
| Not established: no surgery | 2 (5.4%) | 7 (5.1%) | 0.99 |
| Primary tumor location (f) | |||
| Duodenum | 21 (55%) | 66 (48%) | 0.47 |
| Pancreas | 8 (22%) | 22 (16%) | 0.46 |
| Lymph node primary (g) | 5 (14%) | 17 (12%) | 0.78 |
| Other (h) | 2 (5%) | 10 (7%) | 0.99 |
| Unknown (i) | 6 (16%) | 30 22%) | 0.65 |
Abbreviations: BAO-Basal acid output; MAO- Maximal acid output; FSG-serum fasting gastrin. (a) See legends in Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6 for an explanation of variables. (b) A total of 163 patients had a preoperative BAO (152 with no gastric surgery and 11 with previous gastric acid-reducing surgery) determined as described previously [104,150]. (c) A total of 143 patients had an MAO (133 with no gastric surgery and 10 with previous gastric acid-reducing surgery) determined as described previously [104,150]. (d) Localized disease included patients with regional disease without distant metastases as defined previously [171]. (e) General tumor extent determined by imaging and surgery in all patients as described previously [103,171,172]. Specific localization in 9 patients with regional disease could not be determined because no surgery was performed. (f) The primary tumor site was established during surgery or endoscopy or by imaging as described previously [167,174,175,177]. (g) Primary lymph node gastrinomas were identified as described previously [103]. (h) Non-pancreatic-duodenal/lymph node primary sites occurred, as described previously, in the hepato-biliary tract [102,180], ovary, jejunum, mesentery, heart, lung cancer, and gastric antrum [103,181,182,183]. (i) Patients with diffuse liver metastases, with MEN1/ZES, or severe co-morbidities did not undergo routine surgical exploration, as described previously [151,159,178], and the primary location, if not clearly identified on the imaging/endoscopy, was listed as the primary site unknown.
2.4. Comparison of Gastric Antisecretory Treatment Characteristics in ZES Patients with or without VB12 Deficiency
In our patients with or without VB12 deficiency, there were no significant differences in the frequency of previous gastric acid-reducing surgeries, including partial gastrectomies, the frequency of the of use H2R or PPI at the time of this study, or the duration of use in the last 10 yrs., although patients with VB12 deficiency generally had longer PPI treatment in the last 10 years (p = 0.063) (68% vs. 49%) (Table 9, Part I). In patients with or without VB12 deficiency, there also was no difference in the ages at which the patients first started gastric acid antisecretory medical treatment or the ages that initial treatment with H2Rs or PPIs (Table 9, Part II). While the time of treatment with only an H2R prior to the present study was significantly longer in patients without VB12 deficiency (p = 0.0022), no other antisecretory drug treatment’s duration differed between patients with or without VB12 deficiency (Table 9, part III).
Table 9.
Comparison of antisecretory treatment results in patients with or without VB12 deficiency.
| Number (% Total Group) | |||
|---|---|---|---|
| VB12 Deficiency | p-Value | ||
| Yes | No | ||
| Characteristic | (n = 37) | (n = 138) | |
| I. Type antisecretory treatment | |||
| # Previous gastric acid-reducing surgery (a) | 2 (5.4%) | 9 (6.5%) | 0.99 |
| Medical treatment: | 37 (100%) | 138 (100%) | |
| At the time of the present study | |||
| H2R (n = 9) | 0 (0%) | 9 (7%) | 0.21 |
| PPI (n = 166) | 37 (100%) | 129 (93%) | |
| During 10 yrs. prior to the present study | |||
| H2R only (n = 6) | 0 (0%) | 6 (4.3%) | 0.34 |
| PPI only (n = 150) | 34 (92%) | 116 (84%) | 0.72 |
| Any PPI (n = 166) | 37 (100% | 129 (93%) | 0.21 |
| H2R 1st then PPI (n = 17) (b) | 3(8.1%) | 15 (11%) | 0.99 |
| PPI > 5 yrs. (n = 93) | 25 (68%) | 68 (49%) | 0.063 |
| II. General medical/surgical acid treatment features | |||
| Age at 1st medical treatment (yrs.) | 43.9 ± 2.0 | 43.6 ± 1.0 | 0.88 |
| Age at H2R initial treatment (yrs.) | 41.7 ± 2.6 | 43.2 ± 1.0 | 0.54 |
| Age at PPI initial treatment (yrs.) | 48.0 ± 2.0 | 47.6 ± 1.0 | 0.88 |
| III. Duration of antisecretory Tx (yrs.) (mean ± SEM) | |||
| ZES onset to any acid Tx (n = 175) (a) | 3.8 ± 0.9 | 3.7 ± 0.4 | 0.92 |
| ZES onset to PPI started (n = 167) (a) | 7.6 ± 1.2 | 7.9 ± 0.6 | 0.76 |
| Initial acid treatment to the present study (n = 175) | 10.2 ± 1.0 | 10.1 ± 0.6 | 0.76 |
| Initial H2R treatment to PPI Tx (Tx PPI/H2R) (n = 124) | 6.0 ± 1.1 | 5.9 ± 0.5 | 0.96 |
| PPI Tx (+/− with H2R) prior to present study (all PPI) (n = 169) | 6.2 ± 0.6 | 5.5 ± 0.3 | 0.22 |
| Time treated only with H2R prior to present study (n = 6) | 0 | 15.0 ± 1.6 | 0.0022 |
| Time treated only with PPI prior to the present study (n = 45) | 3.2 ± 0.6 | 3.4 ± 0.5 | 0.73 |
| IV. Gastric acid control and VB12 status | |||
| IV.A. Correlations with present study for single admission results (n = 175) | |||
| Control acid output (mEq/h) (c) | |||
| mean ± SEM | 0.14 ± 0.04 | 1.71 ± 0.17 | <0.0001 |
| (range) | (0–1.10) | (0–9.5) | |
| Control acid pH | |||
| mean ± SEM | 6.4 ± 0.0.2 | 3.7 ± 0.2 | <0.0001 |
| (range) | (4.0–7.6) | (1.2–7.5) | |
| Number with control acid with pH < 3.5 (d) | 0 (0%) | 78 (56%) | <0.0001 |
| Number with control acid with pH ≥ 7 | 18(49%) | 12 (8.5%) | <0.0001 |
| IV.B. Correlation with acid control results for all admissions over previous 5 yrs. (n = 873) | |||
| With sustained achlorhydria (>50% Adm acid = 0) (e) | 27 (73%) | 33 (24%) | <0.0001 |
| With sustained hypochlorhydria (0.1 to <1 mEq/h (>50%) (e) | 10(27%) | 37(27%) | 0.99 |
| With >50% acid controls ≥1 mEq/h (e) | 0 (0%) | 68 (49%) | 0.0005 |
Abbreviations: Adm—admission; BAO—Basal acid output; MAO—Maximal acid output; FSG—serum fasting gastrin; and Tx—treatment. For other abbreviations, see legends in Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7 and Table 8. (a) See legends in Table 1 and Table 2 for an explanation of each of these characteristics. (b) In the NIH perspective trials [106,214], H2Rs were the first effective acid antisecretory medical therapy, with cimetidine first used in 1978, ranitidine in 1982, and famotidine in 1983 [198,226,227]. PPIs were first used in 1983 with omeprazole and then with lansoprazole in 1989 [106,197,220,228]; so, all patients initially enrolled in this study were first treated with H2Rs (cimetidine, ranitidine, and famotidine) and later, most of them switched to PPIs (omeprazole and lansoprazole), while new patients generally started treatment with PPIs [106,229]. (c) All antisecretory drug doses were determined as described previously based on the results of the acid control secretory rate [104,106,164,227]. This was determined by assessing the drug acid control secretory rate for the hour prior to the next antisecretory dose, and the antisecretory dose was adjusted to control the acid hypersecretion to <10 mEq/h in the majority of patients [104,106,164,227] or to below <5 mEq/h in patients with moderate/severe GERD [103,166] or previous Billroth resections depending on UGI endoscopic findings and symptom control [166,222]. These levels have been shown to result in the healing of mucosal lesions and, if maintained, prevent the development of additional peptic mucosal damage [164,177,222,226]. (d) The frequency of gastric acid > pH 3.5 was included because studies reported that pepsin activation, which cleaves food-bound VB12 during digestion and is essential for VB12 absorption, is inhibited at pH levels > 3.5 [79,243]. (e) Using the 873 gastric acid drug control analysis performed over the 5 years of this study (19967–2001), the patients were divided into one of three acid control categories: the presence of sustained achlorhydria (>50% admission acid control = 0), sustained hypochlorhydria (acid control levels from 0.1 to <1 mEq/h > 50%), and >50% acid controls ≥1 mEq/h. This categorization was determined as described in the Methods section and previous studies [54,106,244].
2.5. Comparison of the Effect of Levels of Control of the Acid Hypersecretion by Gastric Antisecretory Treatment Drugs in ZES Patients with or without VB12 Deficiency or on the Biomarkers Used to Determine the Presence of VB12 Deficiency (i.e., VB12, MMA, and tHCY)
A comparison of the levels of acid control for both the single NIH admission analyzed in detail in the present study (Table 9, Part IV.A) as well as an evaluation of the effect of acid control for all NIH admissions (n = 873) over the full five years of this study were performed in our patients with or without VB12 deficiency (Table 9, Part IV.B).
For the single admission analyzed in detail, there was a highly significant difference in the two patient groups, with patients with VB12 deficiency having a 12-fold lower mean control acid output level (0.14 vs. 1.71 mEq/h) (p < 0.0001) (Table 9, Part IV.A), as well as a highly significant difference in the average acid control pH between VB12-deficient and non-VB12-deficient patients (6.4 vs. 3.7) (p < 0.0001) (Table 9, Part IV.A). Furthermore, there was a very large difference in the percentage of patients with a pH value < 3.5 in the VB12-deficient/non-VB12-deficient groups (0% vs. 56%) (p < 0.0001) (Table 9, Part IV.A), which is the pH value that is required to activate pepsin in the stomach [243]; pepsin is essential for the cleavage of food-bound cobalamin to free cobalamin to allow cobalamin (VB12) conjugation to R-factor proteins in the stomach, which allows its subsequent absorption [240,245,246,247]. In addition, the reverse pattern for pH > 7 was seen in these acid control values for the single admission analyzed in detail, occurring much more frequently in the patients with VB12 deficiency than those without deficiency (49% vs. 8.5%) (p < 0.0001) (Table 9, Part IV.A).
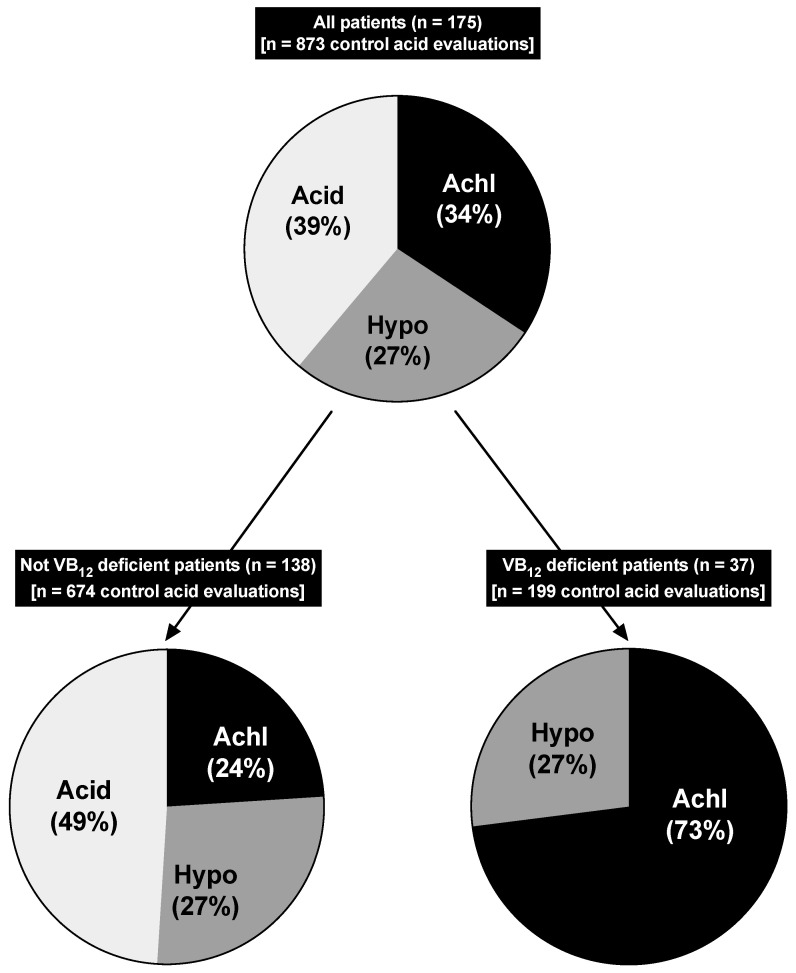
A similar prominent correlation between the lack of acidity and the development of VB12 deficiency was seen in the analysis of the 873 total acid assessments during all admissions for the patients over the full 5-year period (1997–2001) (Table 9, Part IV.B, Figure 4). Similar to previous studies [106,244], this analysis was performed by assigning each patient to one of three acid control categories, which best classify the overall degree of drug-induced acid suppression for each patient for all admissions over this period of time (675 admissions in the non-VB12-deficient patients and 199 admissions in the VB12-deficient patients). The three overall acid control categories that the patients were assigned to were as follows: sustained achlorhydria, defined as having >50% of all acid control values with an acid output of zero; sustained hypochlorhydria, with >50% of admission with acid output controls of 0.1 to <1 mEq/h; and full acid secretion, with >50% of all admissions having an acid output >1 mEq/h (Table 9, Part IV.B, Figure 4). The presence of sustained achlorhydria was 3 times more frequent in the VB12-deficient patients than those without VB12 deficiency (73% vs. 24%) (p < 0.0001), whereas the opposite trend was seen with the full acid category (>50% admissions >1 mEq/h), which occurred in 49% of all patients with no VB12 deficiency but not in any patients with VB12 deficiency (p < 0.0001) (Table 9, Part IV.B, Figure 4). In contrast to the results in these two acid control categories, there was no difference in the frequency of patients in the sustained hypochlorhydria category with or without VB12 deficiency (27% vs. 27%) (Table 9 (Part IV.B) and Figure 4).
Figure 4.
Pie diagram showing mean gastric acid control value acid output category from all patient admissions [n = 873] over a 5-year period (1997–2001) stratified by presence or absence of VB12 deficiency. As outlined in the Methods section and reported previously [54,244], using the mean acid outputs from gastric acid control analysis from past admissions, all patients were assigned to one of three categories: the presence of sustained achlorhydria (>50% admission acid control = 0) [Achl], sustained hypochlorhydria (acid control levels from 0.1 to <1 mEq/h ->50%) [Hypo], and normal gastric acid in >50% acid controls ≥1 mEq/h [Acid]. The percentages represent the percent of patients in each VB12 group [i.e., all pts (n = 175); VB12-deficient patients (n = 37), and non-VB12-deficient patients (n = 138)], which were in each of the mean acid control categories. For all patients (n = 175), VB12-deficient (n = 37) groups, and non-VB12-deficient groups (n = 138), there were 60, 27, and 33 patients in the achlorhydric category, 47, 10, and 37 patients in the sustained hypochlorhydria category, and 68, 0, and 68 patients in the acid category, respectively. The presence of achlorhydria was significantly higher in the vitamin B12-deficient category of patients (73% vs. 24%) (p < 0.001) but not in the hypochlorhydria category (p = 0.99), and the presence of normal acid secretion was significantly higher in the non-VB12-deficient than VB12-deficient patients (49% vs. 0%) (p < 0.001).
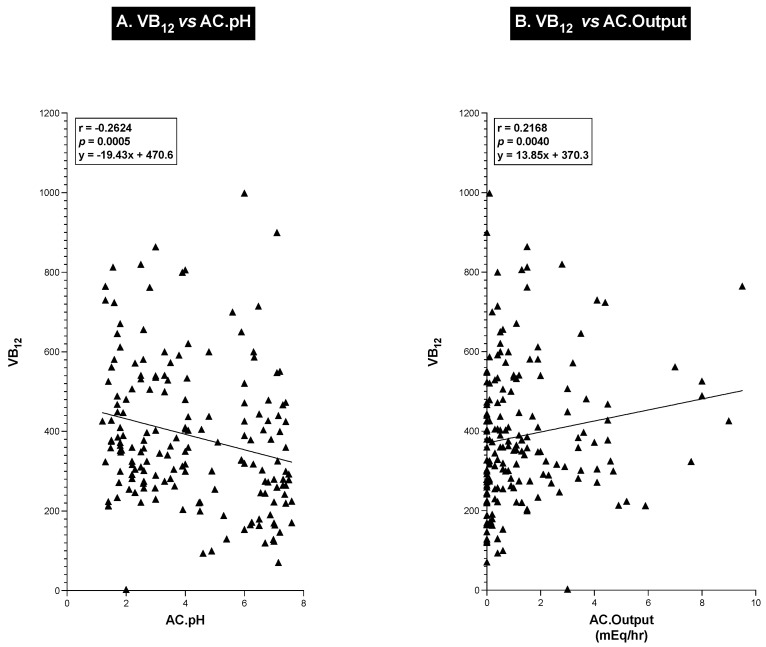
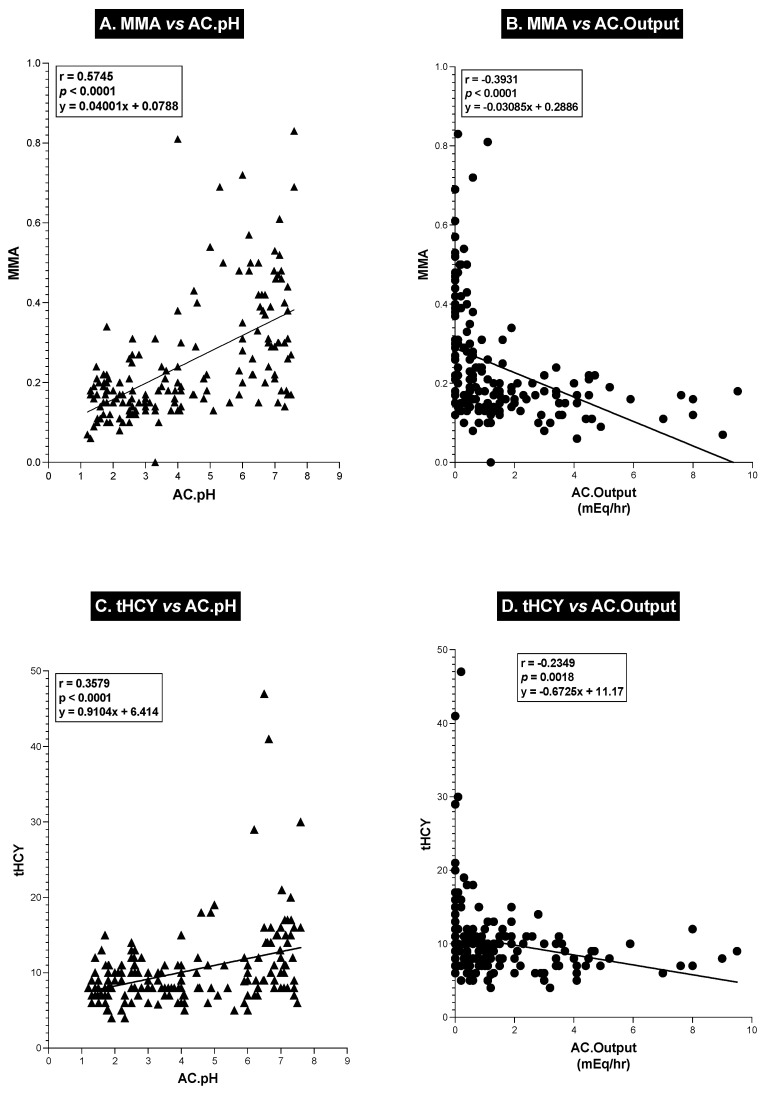
To further explore the relationship between the antisecretory drug acid control levels [acid output per hour for the hour before the next drug dose and the sample’s acid concentration (pH)] and serum VB12 levels, we performed a correlation analysis of these different variables (Figure 5) as well as the relationship between these acid control parameters and serum MMA and tHCY levels in each patient (Figure 6). As shown in Figure 5, the patients’ serum VB12 levels showed a highly significant (p = 0.0005) negative correlation (r = −0.262) with pH, with decreasing levels of acidity (increasing pH) in the control sample associated with a decrease in serum VB12 levels. Conversely, with acid output, there was a significant direct positive correlation between acid output and serum VB12 levels. These results both show that as acid output decreases or with decreasing acidity (pH) of the gastric control fluid, there is a similar proportional decrease in serum VB12 levels. A similar analysis with serum MMA (Figure 6A,B) and plasma tHCY (Figure 6C,D) also showed highly significant correlations (p < 0.0001) between changes in control acid output (mEq/h) as well as acid concentration (pH). There was a highly significant (p < 0.0001) direct correlation (r = 0.574 and r = 0.358) (Figure 6A,C) between both the serum MMA and plasma tHYC levels and the control acid concentration (pH), demonstrating that as the acidity of the sample decreased and the pH of the patients’ acid control samples increased, there was a proportional increase in serum MMA and tHCY levels, which was the opposite pattern seen with changes in serum VB12 levels (compare Figure 6,A,C and Figure 5A). Conversely, both serum MMA and plasma tHYC levels (Figure 6B,D) demonstrated a highly significant negative correlation (r = −0.393 and r = −0.235) (p < 0.0001, p = 0.0018) with changes in control hourly acid output (mEq/h), with increasing hourly acid output (decreasing acid control) associated with decreased blood concentrations of MMA and tHCY, which correlated with the higher serum VB12 levels (compare Figure 6B,D and Figure 5B).
Figure 5.
Correlations between patient values for serum vitamin B12 levels and gastric acid control values after antisecretory drug intake (gastric acid hourly output after drug intake and pH of hourly gastric control sample). Each solid circle or triangle is a value from one patient determined from the same NIH admission. The correlation coefficient, its significance, and the best-fitted regression line equation (using least squares regression analysis) for each correlation are shown.
Figure 6.
Correlations between patient values for serum MMA and plasma tHYC and gastric acid control (A,C) values after antisecretory drug intake (pH and acid hourly output after drug intake). Each solid circle or triangle is a value from one patient determined from the same NIH admission. During this admission, the post-drug acid output was determined for the hour prior to the next drug dose, and both the pH (A,C) and the acid output (B,D) were in mEq/h were determined and used in the correlations. The correlation coefficient, its significance, and the best-fitted regression line equation for each correlation are shown. The results show highly significant correlations with the increasing serum MMA or plasma tHCY, correlating directly with increased pH (decreasing acidity) of the gastric acid control value (A,C) and inversely with the acid output value in mEq/h (B,D).
2.6. Effect of Multivitamin Consumption on Serum VB12, Serum MMA, and Plasma tHCY
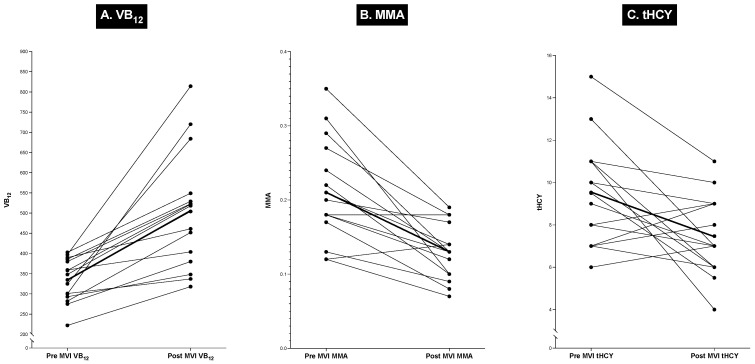
During this study, the patients did not take multivitamin tablets and were questioned about this in detail during each admission. At the end of our study, 15 patients, none of whom were found to be vitamin B12-deficient, started taking standard multivitamin (MVI)/mineral supplements daily on their own. During their subsequent evaluation at the NIH, they were all found to have higher serum vitamin B12 levels compared to the previous admission, when they were not taking supplements (Figure 7) [the tables above do not contain information about this or other post-MVI admissions of these patients]. In these 15 patients, after MVI, the mean serum VB12 level increased by 34%, from a pre-MVI level of 334 ± 15 to 504 ± 40 pg/mL, which was a significant change (p = 0.0003). Similarly, there was a decrease of 38% in the mean serum MMA level, from 0.21 ± 0.02 uM to 0.13 ± 0.01, with 13/15 patients (87%) showing a decrease (Figure 7), which was a highly significant change (p = 0.0009). There was more variation in plasma tHCY levels, but for all the patients, there was a 22% decrease, from 9.50 ± 0.69 uM to 7.43 ± 0.52 uM (Figure 7), which also was a significant change (p = 0.0176). The results from these patients are included here because they illustrate the importance of studying possible vitamin B12 deficiency and the need to carefully evaluate the use of supplemental multivitamin preparations, which, in numerous studies, have been found to be widely used in the US, with an overall frequency of 47–52% [248,249] but up to 80% in some groups, such as patients with cancer [248]. However, their use cannot always be easily established. The importance of this issue will be discussed in more detail in the Discussion section, particularly in relation to the possible contribution to the marked variation in the results of the possible effect of acid suppression drugs on VB12 status, as reported in various studies.
Figure 7.
Serum VB12, serum MMA, and plasma tHCY results from 15 non-VB12-deficient patients after taking multivitamin preparations. The results from 15 patients who had started on multivitamins on their own before their final evaluation are shown. The changes in blood VB12, MMA, and tHYC between their last two NIH evaluations are shown on the Y axis, with one being prior to starting any multivitamin and the other after taking a daily multivitamin preparation, all of which contained crystalline VB12. Each line shows the effects before or after the multivitamin ingestion. The serum VB12, MMA, and tHCY levels for a given patient, shown by the dark lines, represent the mean effect. The mean changes in serum VB12 (334 ± 15 to 504 ± 40 pg/mL (p = 0.0003)), MMA (0.21 ± 0.02 uM to 0.13 ± 0.01, (p = 0.0009)), and tHCY (9.50 ± 0.69 uM to 7.43 ± 0.52 uM (p = 0.0176)) are shown.
3. Discussion
This study investigated the effect of long-term gastric acid antisecretory treatment (primarily PPIs) on body VB12 status for several reasons. First, there is a continuous increase in the usage of gastric acid antisecretory drugs (especially PPIs) [9,10], both used for established indications such as chronic GERD or peptic ulcer disease and for nonspecific, unapproved indications [9,10,11,14,15], such that they are used by 7–15% of the population, according to various studies, and are among the most prescribed medications [9,10]. In addition, an even larger number of patients are using nonprescribed members of this drug class [12]; so, there is increased concern about the long-term possible risks of this drug class due to its widespread use [9,10,18,19,20,22,23,24,27,28,29,31,32,69]. Second, not only is there increased use of these drugs in the short term but an increasing number of patients are taking them in the long term, especially patients with chronic GERD, such that life-long treatment is becoming increasingly frequent [17]. Third, although these drugs have been reported to be remarkably safe and effective [9,10], there is increased concern about their long-term safety due to their possible risks, especially those observed in numerous epidemiological studies as well as other emerging research [9,10,18,19,22,23,24,25,26,27,28,29,30,31,32,43,44,46,62,63,64,65,67,69,70,73,250,251], in addition to increasing concerns about the effect of chronic hypergastrinemia in humans, which is almost invariably induced by the chronic, long-term use of PPIs [18,36,37,252,253,254]. Fifth, one of these proposed side effects, which has remained contentious, is whether long-term PPI treatment causes various nutrient deficiencies, such as interfering with VB12 absorption, and whether its long-term usage can reduce body VB12 stores and cause VB12 deficiency [18,52,53,54,77,78,79]. This issue is controversial because many studies show that there is likely a long-term treatment effect of PPIs on serum VB12 levels and/or causing deficiency [5,54,78,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,255,256], while several others refute this [79,95,96,97,98,99]. Therefore, the resolution of this issue has important implications for long-term treatment with PPIs in a large group of patients. The fact that the possible effect, if any, of the long-term treatment with potent antisecretory agents such as PPIs on VB12 status is still contentious is somewhat surprising because in contrast to several other reported potential side effects of chronic PPI treatment that are receiving considerable attention, the pathogenesis of the possible effect of PPIs on VB12 absorption, leading to possible VB12 deficiency, if it were to occur, seems clear from prior studies. In contrast, it is unclear which possible mechanism(s) are involved in the PPI-induced increase in the development of dementia, as reported in several studies on chronic PPI use [24,69,257,258,259,260] or whether PPIs result in an increased incidence of bone fractures, particularly of the spine [27,39,44,257,261,262], an increased occurrence of declining renal function and/or increased renal disease [24,27,50,257,263,264], an increased incidence of various infections [250,260,265] or overall mortality [27,65,266,267,268], and their possible pathogenesis [257]. The possible mechanism of PPI-induced VB12 deficiency fits well with the results of studies that show that the absorption of VB12 from food can be inhibited by any process that markedly inhibits acid secretion [232,247,269,270,271], which is the main basis for PPIs’ clinical effect in acid/peptic disorders [243,247]. This possible side effect is especially a concern with PPIs because they inhibit the gastric parietal cell H+K+ ATPase, one of the most important distal steps in the acid secretory process, and they have a much longer duration of action than histamine H2-receptor antagonists such that in most patients, not only are they much more potent at acid inhibition than H2Rs but the duration of acid inhibition is markedly longer [197,228,243,272]. VB12 is an essential vitamin for humans and needs to be absorbed from animal food sources, in which it is bound to protein [215,240,247,273,274]. To be absorbed, the protein-bound VB12 needs to be freed from the protein, which occurs in the stomach by the action of pepsin, a protease [215,247], requiring a pH of <3.5 to be active [243], and then, the free VB12 binds to R-protein (haptocorrin) and after the digestion of this complex by pancreatic proteases in the duodenum, the free VB12 binds to an intrinsic factor and is absorbed in the terminal ileum [215,269,275,276]. This proposed mechanism is well supported by experimental/clinical results that show that acid-reducing drugs, including PPIs, inhibit VB12 absorption in humans [99,215,247,274,277,278,279]. Patients with disease-induced achlorhydria (pernicious anemia and severe atrophic gastritis) develop VB12 deficiency [215,240,280], as do patients after total gastrectomy [215,281], total vegans with a strict vegetarian diet [215,282], and patients with inherited disorders disrupting acid production, such as those with inherited disorders of the gastric acid pump due to a defect in gastric H+ K+ ATPase [283].
This study was performed in patients with Zollinger–Ellison syndrome (ZES) for several reasons. These patients have neuroendocrine tumors (gastrinomas) [103], which ectopically secrete gastrin [103,163], resulting in marked basal acid hypersecretion (mean 4-fold increase but can be up to a 15-fold increase) [150] as well as maximal acid secretion [150] because of the trophic effects of gastrin on the gastric mucosa cells (gastric enterochromaffin-like cells and parietal cells) [36,161,189]. Because <25% of all ZES patients are cured by surgical resection (i.e., due to diffuse metastatic disease, multiple tumors as in MEN1/ZES, and recurrent disease after surgery) [100,101,102], the majority of these patients require life-long treatment with gastric acid antisecretory drugs (now, >95% are treated with PPIs) [103,104,105]. In the NIH prospective studies of ZES patients, all the antisecretory drug doses were titrated to reduce the basal acid hypersecretion to <10 mEq/h prior to the next drug dose [104,164]. In ZES patients with advanced GERD [166], previous gastric resections, or with continual symptomatic patients, acid output levels were reduced even further to control symptoms [103,104]. The result of this approach is that all ZES patients treated in this manner at the NIH or other specialty centers [103,105,106] can be used to generate a unique database such that the level of acid secretion on PPIs or other antisecretory drugs is known over a long period of time [103,105,106]. Such an acid secretory database is not available for other diseases, but in ZES patients it’s availability means that acid secretory suppression can be correlated with the possible side effects of PPIs, which might be a result of their profound inhibitory effect on acid secretion, such as its effect on the absorption of nutrients, such as VB12. In fact, three previous studies [54,79,244] have used this unique feature of PPI treatment in ZES patients to create an acid secretory database to investigate the effect of PPI acid suppression on patient nutrient body stores (two studies for VB12 [54,79] and one for iron absorption [244]). In one study [54], 131 consecutive ZES patients were treated with omeprazole for a mean of 4.5 yrs. (range of 0.2–12 yrs.) and with H2Rs for an additional 5 yrs. (range of 0.2–18 yrs.); decreasing serum VB12 levels were found with increasing duration of PPI treatment, the magnitude of the decrease correlated with the degree of acid suppression, and eight patients (6%) were found to have a serum VB12 level below 200 pg/mL, a level that is common used to suggest the presence of VB12 deficiency [112,113,114,115,118,120,233,235]. However, the exact percentage of patients with VB12 deficiency was not confirmed because neither blood MMA nor tHYC levels were assessed. In addition, no patient showed clinical symptoms/signs consistent with VB12 deficiency, and macrocytosis was seen in only one patient. This study concluded that long-term PPI treatment caused a decrease in serum VB12 levels, which, in some of the patients, was similar to levels seen in VB12-deficient patients, and that it was directly due to the effect of PPI-induced hypo-/achlorhydria. In the second study [79], 46 ZES patients were studied during treatment with the PPI, i.e., lansoprazole or previously with omeprazole, for a total PPI duration of 11.6 yrs. After 8 yrs., the serum VB12 levels started to decrease, without symptoms of VB12 deficiency or hematological changes, and by assessing the response of the serum MMA/tHCY level to the administration of VB12, 31% of the patients were reported to have developed VB12 deficiency. However, in contrast to the first study [54], it was concluded that these changes in serum VB12 levels/stores were not due to PPI-induced changes in acid secretion because the changes in acid secretion were not prolonged or profound enough to explain those seen serum/body VB12 changes. Therefore, these two previous studies in ZES patients [54,79] did not explain the effect of chronic PPI treatment on serum/body VB12 levels/stores or the possible role of PPIs’ effect on acid secretion for any observed changes and gave mixed results, similar to those obtained in the large number of studies reviewed above in non-ZES patients, which attempted to determine the effect of PPIs on VB12 levels/stores and the role of PPI-induced acid suppression regarding any observed changes, resulting in equally divided conclusions.
The present study was designed to attempt to resolve the issue of whether long-term, chronic PPI use could affect serum VB12 levels in ZES patients, which could lead to VB12 deficiency, and whether this was mediated by the effect of PPIs on gastric acid suppression. To carry out this study, we included a larger number of ZES patients (n = 175) than the previous studies reviewed above [54,79] because of our experience with previous ZES studies [103,169,184,198]. Previous studies have demonstrated that there could be a wide variation in PPI-induced acid suppression not only between patients but also in a given patient over time, which could be influenced by adjustments in PPI dosage/and or frequency, as well as aspects of the ZES itself (tumor growth, level of gastrin ectopic release, hormonal aspects such as serum calcium levels in MEN1/ZES patients, tumor load changes after surgical resection, treatments against advanced disease, etc.) [103,169,184,198]. We extended this study over a 5-year period (1997–2001), with yearly admissions and assessments of tumor activity by detailed imaging studies [102]. Furthermore, during each assessment, we conducted a careful review of whether any oral VB12 supplements were being used and determined serum VB12, serum MMA, and plasma tHCY levels at all admissions with appropriate controls (assessment of renal function and serum folate levels). In addition, other studies were regularly performed, including hematologic studies and an assessment of gastric acid secretory control at that admission. Lastly, to clearly identify patients with proven VB12 deficiency, we measured the blood level of either MMA or tHCY, which are established markers of body VB12 deficiency [112,113,114,115,116,117,118,119,120], as well as a response toward an elevated MMA/tHCY level after the administration of oral VB12 supplements, which is considered by many to be the most sensitive measurement of VB12 deficiency [112,114,117,236,237]. The increased number of patients and patient admissions/assessments over time allowed us to collect sufficient data to systematically assess all clinical, laboratory, and tumoral factors, PPI treatment, and acid secretory controls that could affect VB12 blood and/or VB12 body stores in the patients and to perform appropriate correlative analyses to gain insights into any effects found on VB12 levels/stores in our patients.
Our results strongly support the conclusion that in our patients, the chronic, long-term use of antisecretory drugs, particularly PPIs, has a prominent effect on the serum VB12 levels as well the VB12 body stores, with the development of VB12 deficiency in 20% of the patients. First, in our study, the mean serum VB12 level was 394 ± 14 pg/mL, with a mean value of 390 ± 14 in those taking PPIs, which is significantly lower (p = 0.03) than that in the patients taking only H2Rs in the present study (502 ± 44); it was also significantly lower (p < 0.001) than the value reported in 20 ZES patients undergoing long-term treatment with only ranitidine in our previous study using the same VB12 assay (582 ± 63 pg/mL) [54]. These results are consistent with two previous studies on ZES patients [54,79], which reported that chronic antisecretory treatment with PPIs decreased serum VB12, with a decrease of 53% in one of the studies [54] over a 10-year period. Second,18% of our patients had a serum VB12 < 200 pg/mL, which is commonly used in VB12 studies to indicate that a given patient likely has VB12 deficiency [112,113,114,115,118,120,233,235], and 38% of our patients had a serum VB12 level of 200–350 pg/mL, which was considered a low level of serum VB12 in numerous studies [115,116,118]. Third, 18% of our patients had an increased serum MMA level of >0.37 uM, which is a frequently used criterion to identify the presence of VB12 deficiency [115,116,117,118] because its elevation shows a metabolic insufficiency of VB12 body stores to carry out a critical VB12-dependent metabolic pathway [115,116,117,118,235,236,237]. Similarly, 8% of all patients had an elevated total homocysteine (tHCY) plasma level (i.e., >15 uM), which, in the presence of normal renal function and a normal serum folate level, is commonly used as another criterion for the diagnosis of VB12 deficiency [112,114,115,235] because its elevation also shows a metabolic insufficiency of VB12 body stores to carry out a critical VB12-dependent metabolic pathway [112,113,114,115,234]. Fourth, with the administration of oral crystalline VB12 supplements, each of the 37 patients (21%) with either an elevated blood MMA or tHCY level showed a concomitant increase in serum VB12 levels combined with a decrease toward normal levels of both the blood MMA and tHCY levels, which are considered by many to represent the most sensitive measurements of VB12 deficiency [112,114,117,236,237]. Fifth, we observed that the decreasing serum VB12 levels are in fact closely coupled with the increases in blood MMA and tHCY levels, shown by a highly significant (p < 0.0001) inversed correlation of serum VB12 levels with both blood MMA and tHCY levels. Sixth, the claim that the development of VB12 deficiency is due to the prolonged chronic use of PPIs is supported by our finding that none of the patients undergoing long-term treatment with only H2Rs developed VB12 deficiency, as shown by significantly higher serum VB12 levels in the latter group of patients and by our previous study [54], which showed significantly higher serum VB12 levels in ZES patients chronically treated with H2R than those treated with PPIs. Furthermore, in our previous study [54], a significantly higher proportion (p < 0.001) of patients who had received long-term treatment with PPIs compared to those receiving long-term treatment with H2Rs had serum VB12 levels < 200 pg/mL, which is a criterion widely used to identify a patient with VB12 deficiency [112,113,114,115,118,120,233,235]. These results strongly support the conclusion that ZES patients who received long-term treatment with PPIs developed a decreased serum VB12 level, which increased with the duration of treatment [54,79], potentially resulting in VB12 deficiency. Furthermore, it directly establishes the mechanism by which the PPI causes decreased body stores of VB12 in these patients.
One could propose several different mechanisms through which PPIs could interfere with VB12 absorption, including that PPIs inhibit acid secretion to such an extent that it interferes with its essential role for VB12 absorption from the typical diet, which has been extensively shown in studies on other acid-reducing disorders [54,85,247]; that PPIs interfere with the secretion of the intrinsic factor (IF), which per se is needed for the efficient absorption of VB12 after VB12 is released from R-factors by pancreatic protease digestion and then complexes with IF, which is essential for the specific receptor-mediated absorption of VB12 in the ileum [247,284]; that bacterial overgrowth develops or results in VB12 deficiency [247,285,286], which could occur due to bowel dysfunction due to the frequent GI surgeries these patients had or due to the presence of hypo-/achlorhydria [247,285,286]; due to previous gastric resections or other gastric acid-reducing surgeries that many of these patients frequently had prior to the availability of potent medical acid secretory drugs [113,224,247]; due to a direct effect of PPI-induced hypochlorhydria on the absorption of VB12; due to the development of atrophic gastritis or pernicious anemia, each of which is frequently associated with VB12 deficiency, perhaps due to H. pylori infections, which are common found in elderly patients with severe VB12 deficiency [233,247,287]; due to a specific ZES tumor variable such as an effect of hypergastrinemia and tumor-induced cachexia; due to anti-tumor treatments in patients with advanced disease; or due to the tumor’s location, which induces intestinal dysfunction, with effects on VB12 absorption. Many of these possibilities can be excluded based on the known actions of PPIs, whereas many of the others can be excluded using the detailed analyses of these variables, which were performed in this study or other studies. Previous studies demonstrated that PPIs do not alter IF secretion [288,289], that gastrinomas do not alter IF secretion by inducing hypergastrinemia, and that in fact, ZES patients are hypersecretors of IF [275]. No cases of blind loop syndrome with bacterial overgrowth in ZES patients have been reported in the literature even though these patients received abdominal surgeries very frequently, especially in the past, and furthermore, in our series of more than 400 ZES patients, we did not have any cases with this diagnosis. Also, there is no evidence in the literature that hypergastrinemia per se affects vitamin B12 absorption or that hypochlorhydria per se affects free VB12 ‘s absorption. Although hypo-/achlorhydria has been shown to have a marked effect on the absorption of food-bound VB12, it had no effect on the absorption of crystalline VB12 in several ZES patients [54,290,291,292]. However, in one study [293], when the duodenal pH was very acidic in a ZES patient, VB12 absorption was impaired; however, when the pH was raised to neutral (pH 7), in the same patient, the absorption of VB12 was normalized. While there are occasional case reports of the development of atrophic gastritis in ZES patients [290,294,295,296], in some cases, after acute gastrointestinal infections [296,297,298], this is a rare occurrence, and in our series of 400 patients, we did not see any cases of this. Many of the proposed possible explanations for the PPI-associated VB12 malabsorption that are specifically related to features of ZES can be excluded by the analyses performed in the present study, especially when combined with the results of other studies. In particular, no clinical feature (age, gender, duration of disease, presence of ZES symptoms, or presence of MEN1) or laboratory feature (level of BAO, MAO, or fasting gastrin) was correlated with the presence or absence of VB12 deficiency (Table 8). A particularly important finding is that the presence or absence of a history of any surgical gastric acid surgical procedure [223] had no effect on the development of VB12 deficiency in our study (Table 8), even though in numerous studies that included a follow-up of non-ZES patients who underwent such surgical procedures, especially after gastric resection, there was an increased occurrence of VB12 deficiency [113,224,247]. Similarly, there was no specific tumoral feature, including the location of the primary gastrinoma, or extent of the disease, including patients with extensive liver metastases, many of whom underwent anti-tumor therapies. These results agree with our previous study [54], which showed a correlation between the serum VB12 levels in ZES patients and chronic long-term treatment with PPIs and/or H2R gastric antisecretory therapy, with similar clinical, laboratory, and tumoral variables to those assessed in the present study, both showing that the presence or absence these ZES variables in any of these areas did not correlate with the effect of antisecretory therapy on serum VB12 levels either positively or negatively. The above analysis provides evidence to exclude all of the above possibilities, except the first mechanism proposed above, which is that PPIs inhibit acid secretion to such an extent that it interferes with its essential role for VB12 absorption from the typical diet, which has been extensively shown in the literature for other acid-reducing disorders [54,85,247].
Many results of the analyses of the relationships between their drug gastric acid secretory control rates and their various body VB12 measurements, including their VB12 serum levels, VB12 body stores, and the presence or absence of VB12 deficiency in the individual patients, provide direct evidence in support of the proposed conclusion that PPI-induced sustained hypo-/achlorhydria directly results in decreased VB12 body stores in our patients, which led to the development of VB12 deficiency in some of our patients, according to each of the criteria used (MMA, tHCY, and response to VB12 supplementation). First, at the time of the admission, a highly significant (p < 0.0001) direct correlation was found between the individual patients’ serum VB12 level and the control acid output on the drug for the last hour prior to the next drug dose, whereas with the acid control pH, the opposite trend was seen, with a highly significant (p < 0.0001) inverse relationship between the acid fluid pH and the serum VB12 levels. This relationship strongly supports the conclusion that in these patients, as the gastric acid output decreased due to a greater inhibitory effect of the PPI, coupled with the decreased acidity, resulting in an increased gastric pH, there was a decrease in the level of the serum VB12 over time. We conclude that this was due to the PPI based on the finding that the serum VB12 levels were significantly higher in the patients being treated with only H2Rs and that none of the patients treated with only H2R developed VB12 deficiency, whereas the lower levels of serum VB12 in the chronic PPI-treated patients resulted in 22% of these patients developing VB12 deficiency. This conclusion is also supported by our previous study [54], which demonstrated that drug acid control rates were 3-fold higher in patients with chronic long-term treatment with only an H2R compared to those treated with PPIs; the percentage with stringent acid control (<1 mEq/h) was significantly less, and the VB12 levels were significantly higher. Second, not only were the changes in serum VB12 levels closely correlated with the changes in the gastric acid control levels but there was also a highly significant (p < 0.0001) and close correlation between the levels of blood MMA and tHYC levels and both the control acid output levels as well as the acidity (i.e., pH) of the sample, with the correlations showing an opposite trend compared to the changes in the serum VB12 levels. This occurs because as the gastric output is reduced, coupled with reduced acidity (increasing pH), lower VB12 levels and, over time, VB12 deficiency develop, resulting in increasing MMA/tHCY levels, which change in the opposite direction compared to VB12 levels because they directly monitor the adequacy of body VB12 given that the enzyme’s activity responsible for the cascade of metabolism involving these substances directly depends on the availability of VB12. Thus, as VB12 stores decrease, both blood MMA and tHCY levels increase [115,116,117,120,232,233,240]. Third, the mean drug control acid output for the hour prior to the next dose of antisecretory drug during the admission was 12-fold lower in patients with VB12 deficiency compared to those without VB12 deficiency, with the result that the gastric pH was 2-fold higher and none of the VB12-deficient patients had a gastric acid control pH < 3.5, which is the level required for the activation of gastric pepsin [243]. This result supports the conclusion that the mechanism of gastric acid hyposecretion/achlorhydria causes a decrease in serum VB12 levels, which resulted in the development of VB12 deficiency in our patients. VB12 deficiency occurred because free VB12 could not be released from the food-bound form in the stomach by the action of gastric proteases, which require this degree of acidic pH, and as a result, free VB12 could not be generated to allow its subsequent binding to gastric R-factor proteins [215,245,246,247] and, later, the intrinsic factor, which are all required for its efficient absorption [215,247,269,275]. Fourth, the conclusion for the central role of the profound inhibition of gastric acid secretion mediating the VB12 changes in our patients is also supported by the analysis of all the gastric acid controls from the patients’ yearly admissions (n = 873) over the 5 years of this study. This showed that a 3-fold higher percentage of patients in the VB12-deficient group of patients had sustained achlorhydria than the patients without VB12-deficient (75% vs. 24%), whereas the reverse was true in the non-VB12-deficient patients, with half showing high acid controls, whereas none of the VB12-deficient patients had high acid controls. Therefore, the results of our study provide strong support for the conclusion that chronic, long-term treatment with PPIs can result in decreasing serum VB12 levels, which, in time, can further result in VB12 deficiency, mediated by PPI-induced acid hyposecretion, resulting in chronic food-mediated VB12 malabsorption.
In conclusion, one could raise the question of whether the findings in this study on ZES patients that received prolonged treatment with PPIs can have sufficient hyposecretion of gastric acid secretion to cause VB12 deficiency and whether it can provide any insights into the much larger and still unanswered and controversial question of this possible long-term effect of chronic PPI treatment in patients with idiopathic GERD. One could argue the answer is clearly no because the patient populations and treatment strategies are very different in several ways. First, although there is a more recent attempt to lower the PPI daily dose in ZES patients [106], our ZES patients’ mean daily dose of PPIs was higher (60 mg/day omeprazole-equivalent dose) than that generally used in most patients with idiopathic GERD [3,299]. Second, 71% of our patients required more than a daily dose of PPIs (i.e., BID or TID), which is more frequent than that of patients in the series with idiopathic GERD, in which this percentage is usually less than 30% [300,301]. This is an important difference because numerous studies have shown that increasing a single daily PPI dose results in less acid suppression and symptom control than splitting the same dose not only in ZES patients [106,228,302] but also in patients with idiopathic GERD [301,303]. Third, many patients with ZES have a prolonged history of the disease before the diagnosis is made (4–7 yrs.), during which time their gastric acid hypersecretion is often inadequately controlled, which can lead not only to persistent UGI acid/peptic symptoms but also to malabsorption, including for VB12 [293], which, when combined with a less-than-optimum diet due to general ill health, can lead to lower body VB12 body stores, at the time that adequate acid control measurements are started. Therefore, this could lead to a shortened time to develop even lower levels of body VB12 if the acid hypersecretion is over-controlled due to PPI-induced achlorhydria. Fourth, a similar logic to that in the third point described above is found in the subsequent natural history of patients with ZES after diagnosis, which separates their probability of developing low VB12 body stores and/or VB12 deficiency from patients with idiopathic GERD, who are usually healthier. This occurs because, after diagnosis, many of these patients undergo various procedures/treatments (surgeries, treatment of advanced disease, and surgeries/treatments for those with MEN1/ZES) [103] that have extended periods of poor nutrition. However, the present study demonstrates these results in humans for the first time, with correlative acid measurements showing that in at least one group of patients, i.e., those with ZES, PPI-induced acid hyposecretion can result in low body VB12 stores and VB12 deficiency, using three different widely used criteria of VB12 deficiency, and thus resolves the controversy of whether PPI suppression of acid secretion is responsible for the decrease in serum VB12 levels in this group of patients, as previously reported in these patients in two studies [54,79]. Whether similar findings to those in our patients might be found in patients chronically taking PPIs for other diseases, such as GERD, can be determined by a similar protocol to ours in the other groups of patients. However, it is unlikely that this will ever be investigated because gastric acid measurements have largely disappeared from all but a few centers; therefore, the type of correlations we performed in this study will be very difficult to complete. On the other hand, approximately half of the studies on patients with chronic, long-term PPI treatment in other diseases (primarily GERD) support the conclusion that a similar process may occur with PPI treatment in these patients [54,78,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94], while the other half of the studies do not support this conclusion [79,95,96,97,98,99]. The explanation for these different results is not currently clear, although our studies provide one insight that may have been overlooked in these studies and that could contribute to the differences in the results. This insight was that at the end of our study, in 15 patients, the blood VB12 levels began to rise, with a concomitant decrease in blood MMA and tHCY levels, although none of these patients had reached levels where they were defined as being VB12-deficient. In each case, this turned out to be due to the use of multivitamins obtained by the patient themselves without any doctor’s prescription but instead obtained over the counter. Even though, whenever possible, we asked the patients and their spouses detailed questions about the use of multivitamins or vitamin supplements upon every admission, which our patients denied taking throughout this study, we still did not find any group of patients that had started taking multivitamins on their own; the patients stated that they did not take multivitamins when questioned. Multivitamins are now used in 47–52% of the population [248,249] and up to 80% in patients with cancer [248], and as can be seen in our study, their use can sometimes be difficult to identify. Our result shows that in these 15 patients, a moderate amount of crystalline VB12 in the standard multivitamin (7–55 ug/tablet) that our patients were taking was enough to reverse the changes in VB12, blood MMA, and tHCY levels induced by chronic PPI use. This result is comparable to the reports of the effect of standard multivitamin use in various patient groups, including patients with diabetes, which resulted in an increase in serum VB12 levels and a correction of the presence of VB12 deficiency manifested by reversing the elevated blood levels of MMA and tHCY [241,304,305,306,307]. It is not clear whether this contributes to the differences in results regarding PPI-induced effects in other diseases studied, which reported differing effects of chronic PPI use on VB12 levels/body stores. However, as seen in our study, any intermittent or even regular use of multivitamin preparations can be difficult to detect or exclude. This possibility is supported by studies that show sensitivity for the detection of the use of dietary supplements by self-reporting, which was found to be 66%, 69%, and 75% in three studies, thus suggesting that it can frequently be underestimated [276,308,309]. Furthermore, at least one study [87] examining the effect of PPIs on serum VB12 levels in elderly patients concluded that the failure to detect a PPI effect might be because 41% of the patients were taking multivitamin supplements. Considering that in the US, patients can easily buy MVIs without prescriptions, it is very difficult to completely exclude periodic MVI use in any study.
4. Materials and Methods
The patients in this study are part of an ongoing NIH prospective study on various aspects of Zollinger–Ellison syndrome (ZES) that started in 1974, as approved by the clinical research committee of the National Institute of Diabetes, Digestive and Kidney Diseases of the National Institutes of Health (NIH), the characteristics of which have been described previously [100,150,163,310]. All subjects gave their informed consent for inclusion before they participated in this study. This study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the clinical research committee of the National Institute of Diabetes, Digestive and Kidney Diseases of the National Institutes of Health (NIH). This cohort includes all patients with established ZES who had acid antisecretory treatment with either H2Rs or PPIs and who had their antisecretory doses set according to the results of gastric acid testing as described previously and reviewed briefly below [166,227,228].
All patients who were diagnosed with Zollinger–Ellison syndrome (ZES) without a total gastrectomy at the National Institutes of Health from 1997 to 2001 or who were taking gastric antisecretory drugs because they were not surgically cured were eligible [106,168,225]. The diagnostic criteria for ZES were as previously described, which involved demonstrating inappropriate hypersecretion of gastrin by demonstrating hypergastrinemia in the presence of acid hypersecretion, positive provocative testing with either secretin or calcium, a positive histologic diagnosis of gastrinoma, or a combination of these as described previously [103,150,310,311]. To accomplish the diagnosis, all patients underwent nasogastric aspiration, a basal acid output (BAO), and pentagastrin-stimulated maximal acid output (MAO) when initially assessed, which were performed as described previously [104,150]. Also, they all underwent gastrin provocative testing (all had secretin testing and, if equivocal, calcium provocative testing) as previously described [163,310] as well as multiple fasting gastrin tests, some of which were performed without the administration of any antisecretory drugs, as described previously [104,163]. Serum gastrin analysis was performed by Bioscience Laboratories (New York, NY, USA), and all samples were diluted into the normal range for accurate determination [213]. The possible presence of MEN1 was investigated in all patients using the assessment of family history, in addition to serum/plasma parathormone, prolactin, and ionized calcium assays as described previously [100,101,156,161].
All patients were initially assessed for tumor location and tumor extent using multiple tumor localization approaches, including imaging studies performed as previously described, including computed tomography scan with contrast (CT scan) [172,312], magnetic resonance imaging with contrast (MRI) [313], somatostatin receptor imaging [314,315], and transabdominal ultrasonography. In patients with equivocal results, a proportion also underwent selective celiac angiography with contrast, with or without secretin-stimulated gastrin sampling or portal venous gastrin sampling [177,316,317].
All patients were assessed for possible surgical cure, except patients with MEN1/ZES with imaged tumors < 1.5–2 cm [102,167] or those with advanced unresectable disease, who were instead assessed for the treatment of metastatic disease (chemotherapy, interferon, or somatostatin) as described previously [103,176,184].
Study Design
The primary purpose of this study was to assess ZES patients who require life-long gastric acid antisecretory agents for possible vitamin B12 deficiency by assessing serum methylmalonic acid (MMA), total homocysteine (tHCY), and VB12 levels and to provide insights into its possible causative factors, especially the role of the long-term suppression of gastric acid hypersecretion. Possible causality was assessed by correlating the patient’s VB12 status to various possible contributing clinical, laboratory, tumoral, and treatment features of the ZES patients. After having their gastric acid hypersecretion controlled for at least 6 months, consecutive patients with ZES were enrolled in this study. Prior to this study, all patients entering this study, who had their gastric acid hypersecretion initially controlled long-term before 1983, were treated with a histamine H2-receptor antagonist (H2R) (cimetidine or ranitidine), either alone or with an anticholinergic agent [106,198,214,225,226]. After 1983, in almost all cases, patients were initially treated with a more potent H2R antagonist (famotidine) [214,227], switched to a PPI, or initially started on a PPI (omeprazole or lansoprazole) [197,214,228].
The maintenance dose of antisecretory drugs was established in all cases by drug dose titration based on gastric acid antisecretory measurements for the hour prior to the next drug dose, determined as described previously [104,106,164,214]. The primary criterion for the effective control of acid hypersecretion was to reduce the acid output to <10 mEq/h prior to the next dose [104,164,214], which has been shown to allow the healing of mucosal lesions, as well as prevent additional acid-induced lesions in ZES patients [104,164,214]. However, in patients with moderate to severe GERD [166] or a previous partial gastrectomy, acid reduction to lower levels (<5 mEq/h or less) is frequently required to completely control all symptoms and result in complete mucosal healing. A similar approach was used with both H2Rs and PPIs with stepwise dose adjustment after starting with 150/600 mg every 6–8 h for cimetidine, 150/300 mg with ranitidine, 20/40 mg of famotidine, or a once-daily dose of omeprazole/lansoprazole of 60 mg [104,106,164,214]. The initial daily dose of PPIs, which were the most frequent antisecretory drug used during this study period (1997–2001), was increased by 20 mg/day (15 mg for lansoprazole) until acid output was controlled; if not, the daily dose was increased to 120 mg/day, and the dosage was split to 60 BID and increased further as needed [106,214]. A prior study established that the relative potencies for famotidine/ranitidine/cimetidine (i.e., H2Rs) in ZES patients are 1:9:32 [227], and for PPIs, these values are as follows: omeprazole (20 mg) = 40 mg esomeprazole, 30 mg lansoprazole, 40 mg pantoprazole, and 20 mg of rabeprazole [231]. Using these equivalent doses, a ranitidine-equivalent dose or omeprazole-equivalent dose was calculated for each patient to allow the comparison of drug dosing in different patients on different drugs, as described previously [106].
Upon entering the present study and during each subsequent yearly evaluation at the NIH (1997–2001), a complete medical history and a complete physical examination were performed to assess for any symptoms/signs that might indicate VB12 deficiency. In addition, a complete blood count, a complete clinical chemistry profile (creatinine, BUN, liver function studies, and serum electrolytes), and the levels of fasting serum gastrin, serum folate, serum vitamin B12, serum MMA, and serum tHCY were determined by the NIH Clinical Center Hematological and Clinical Chemistry laboratories. During all visits, patients were questioned about the use of any multivitamin preparations to ensure that none were being taken. Serum vitamin B12 and serum folate levels were measured by radio-immunoassays (Ciba-Corning Co., Medford, MA, USA). The inter-assay variation for the serum vitamin B12 and serum folate levels for four different standards was as follows: 140 pg/mL (8%), 387 pg/mL (6%), 681 pg/mL (8%), and 964 pg/mL (10%) for vitamin B12 and 1.5 ug/mL (11%), 3 ug/mL (9.8%), 5 ug/mL (6.4%), and 11 ug/mL (7.5%) for serum folate levels. In the patients who were found to have low vitamin B12 serum levels, anti-parietal and anti-intrinsic factor antibodies were obtained, which were negative in all patients tested. All hematology measurements were obtained with the National Institutes of Health Clinical Chemistry/Hematology Laboratories. Serum MMA levels were measured through the National Institutes of Health Clinical Chemistry laboratory by gas chromatography/mass spectrometry, with the normal level being <0.37 uM, and plasma tHCY levels were measured using the Abbott IMx immunoassay and assessed using a normal level < 15 uM. Patients were classified as VB12-deficient if they had an elevated serum MMA level or tHYC level in the presence of normal serum folate levels (>3.0 ug/L) and a normal serum creatinine level; and the serum MMA and tHYC levels decreased toward normal levels after taking oral crystalline VB12 (50 ug BID). Patients were classified as having subclinical cobalamin deficiency (SCCD), as defined previously [113,232,234], if they had compromised cobalamin metabolism, as defined by abnormal MMA and/or tHCY levels without folate deficiency/altered renal function, and were asymptomatic and nonanemic. Patients diagnosed with SCCD were treated with crystalline V12 supplements (primarily oral 50 ug BID crystalline cobalamin) and reassessed to confirm the normalization of MMA and tHCY levels with elevated VB12 levels.
Gastric acid secretory drug control rates were reviewed for all admissions over the 5-year study period. Based on their acid secretory rate over all the evaluations during the study period, patients were classified into different acid control categories, similar to other studies on ZES patients [54,106,244]. Three different acid control categories were identified: sustained achlorhydria (>50% admission acid control = 0), sustained hypochlorhydria (acid control levels from 0.1 to <1 mEq/h (>50%), and normal secretion with >50% acid controls ≥1 mEq/h.
The results of the single admission over the 5-year study period, which showed the most advanced MMA/tHCY changes, were used to classify the patient as VB12-deficient or non-VB12-deficient, and MMA/tHCY and VB12 levels as well as the acid secretory data during this admission were used for correlative and comparative analyses.
The patient variables used in comparative analyses to identify possible contributing factors to the development of VB12 deficiency, with correlations with VB12, MMA, and tHCY levels, including those for clinical characteristics and disease course, tumoral characteristics, and laboratory characteristics, including acid secretion, duration dosage, and the type of previous acid secretory treatments, are defined in the tables above.
Statistical analysis was performed using the Mann–Whitney–Wilcoxon test and the Fisher’s exact test. Least squared regression analysis was used to calculate correlation coefficients, with p-values < 0.05 considered significant. All the continuous variables are reported as mean ± SEM.
Author Contributions
Detailed review of the literature—T.I., R.T.J. and I.R.-A.; Validation T.I., I.R.-A. and R.T.J.; Writing: original draft preparation—T.I. and R.T.J.; Writing: review and editing—T.I., I.R.-A. and R.T.J.; Supervision: R.T.J.; and Funding: R.T.J. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This research has been approved by the clinical research committee of the National Institute of Diabetes, Digestive and Kidney Diseases of the National Institutes of Health: the protocol for the use of histamine H2-receptor antagonists was approved on 8/20/1978 (89-DK-15), and the protocol for the PPI omeprazole was approved on 1/15/83.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Funding Statement
This research was partially supported by the NIDDK intramural program (NIDDK #DK053200-26) of the NIH.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Strand D.S., Kim D., Peura D.A. 25 Years of Proton Pump Inhibitors: A Comprehensive Review. Gut Liver. 2017;11:27–37. doi: 10.5009/gnl15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinoshita Y., Ishimura N., Ishihara S. Advantages and Disadvantages of Long-term Proton Pump Inhibitor Use. J. Neurogastroenterol. Motil. 2018;24:182–196. doi: 10.5056/jnm18001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plehhova K., Haering M., Wray J., Coyle C., Ibanez E., Kostev K. Prescribing Patterns of Proton Pump Inhibitors in Germany: A Retrospective Study Including 472,146 Patients. J. Prim. Care Community Health. 2023;14:21501319231221002. doi: 10.1177/21501319231221002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang K.Z., Weber H.C. Potassium-competitive acid blockers and acid-related disorders. Curr. Opin. Endocrinol. Diabetes Obes. 2024;31:107–114. doi: 10.1097/MED.0000000000000858. [DOI] [PubMed] [Google Scholar]
- 5.Bandyopadhyay S., Verma P., Samajdar S.S., Das S. Vonoprazan causes symptomatic improvement in non-erosive gastroesophageal reflux disease: A systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2024;48:102373. doi: 10.1016/j.clinre.2024.102373. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y., Gao Z., Hou X. Potassium-competitive acid blockers and proton-pump inhibitors for healing of erosive esophagitis: A systematic review and network meta-analysis. Therap. Adv. Gastroenterol. 2024;17:17562848241251567. doi: 10.1177/17562848241251567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L., Shi H., Shi Y., Wang A., Guo N., Li F., Nahata M.C. Vonoprazan-based therapies versus PPI-based therapies in patients with H. pylori infection: Systematic review and meta-analyses of randomized controlled trials. Helicobacter. 2024;29:e13094. doi: 10.1111/hel.13094. [DOI] [PubMed] [Google Scholar]
- 8.Shanika L.G.T., Reynolds A., Pattison S., Braund R. Proton pump inhibitor use: Systematic review of global trends and practices. Eur. J. Clin. Pharmacol. 2023;79:1159–1172. doi: 10.1007/s00228-023-03534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Targownik L.E., Fisher D.A., Saini S.D. AGA Clinical Practice Update on De-Prescribing of Proton Pump Inhibitors: Expert Review. Gastroenterology. 2022;162:1334–1342. doi: 10.1053/j.gastro.2021.12.247. [DOI] [PubMed] [Google Scholar]
- 10.Freedberg D.E., Kim L.S., Yang Y.X. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology. 2017;152:706–715. doi: 10.1053/j.gastro.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 11.Lassalle M., Tri T.L., Biour M., Kirchgesner J., Rouby F., Dumaecet N., Zureik M., Dray-Spira R. Use of proton pump inhibitors in adults in France: A nationwide drug utilization study. Eur. J. Clin. Pharmacol. 2020;76:449–457. doi: 10.1007/s00228-019-02810-1. [DOI] [PubMed] [Google Scholar]
- 12.Bustillos H., Leer K., Kitten A., Reveles K.R. A cross-sectional study of national outpatient gastric acid suppressant prescribing in the United States between 2009 and 2015. PLoS ONE. 2018;13:e0208461. doi: 10.1371/journal.pone.0208461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pottegard A., Broe A., Hallas J., de Muckadell O.B., Lassen A.T., Lodrup A.B. Use of proton-pump inhibitors among adults: A Danish nationwide drug utilization study. Therap. Adv. Gastroenterol. 2016;9:671–678. doi: 10.1177/1756283X16650156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutta A.K., Sharma V., Jain A., Elhence A., Panigrahi M.K., Mohta S., Kirubakaran R., Philip M., Goenka M., Bhatia S., et al. Inappropriate use of proton pump inhibitors in clinical practice globally: A systematic review and meta-analysis. Gut. 2024 doi: 10.1136/gutjnl-2024-332154. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Eltriki M., Chhabra M., Cassels A., Wright J.M. Inappropriate Use of Proton Pump Inhibitor Among Elderly Patients in British Columbia: What are the Long-term Adverse Events? Curr. Drug Saf. 2024;19:244–247. doi: 10.2174/1574886318666230726124540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rotman S.R., Bishop T.F. Proton pump inhibitor use in the U.S. ambulatory setting, 2002–2009. PLoS ONE. 2013;8:e56060. doi: 10.1371/journal.pone.0056060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Othman F., Card T.R., Crooks C.J. Proton pump inhibitor prescribing patterns in the UK: A primary care database study. Pharmacoepidemiol. Drug Saf. 2016;25:1079–1087. doi: 10.1002/pds.4043. [DOI] [PubMed] [Google Scholar]
- 18.Ito T., Jensen R.T. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin b(12), iron, and magnesium. Curr. Gastroenterol. Rep. 2010;12:448–457. doi: 10.1007/s11894-010-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elias E., Targownik L.E. The Clinician’s Guide to Proton Pump Inhibitor Related Adverse Events. Drugs. 2019;79:715–731. doi: 10.1007/s40265-019-01110-3. [DOI] [PubMed] [Google Scholar]
- 20.Scarpignato C., Gatta L., Zullo A., Blandizzi C. Effective and safe proton pump inhibitor therapy in acid-related diseases—A position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016;14:179. doi: 10.1186/s12916-016-0718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nehra A.K., Alexander J.A., Loftus C.G., Nehra V. Proton Pump Inhibitors: Review of Emerging Concerns. Mayo Clin. Proc. 2018;93:240–246. doi: 10.1016/j.mayocp.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 22.Vaezi M.F., Yang Y.X., Howden C.W. Complications of Proton Pump Inhibitor Therapy. Gastroenterology. 2017;153:35–48. doi: 10.1053/j.gastro.2017.04.047. [DOI] [PubMed] [Google Scholar]
- 23.Jaynes M., Kumar A.B. The risks of long-term use of proton pump inhibitors: A critical review. Ther. Adv. Drug Saf. 2018;10:2042098618809927. doi: 10.1177/2042098618809927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatnagar M.S., Choudhari S., Pawar D., Sharma A. Long-Term Use of Proton-Pump Inhibitors: Unravelling the Safety Puzzle. Cureus. 2024;16:e52773. doi: 10.7759/cureus.52773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yibirin M., De Oliveira D., Valera R., Plitt A.E., Lutgen S. Adverse Effects Associated with Proton Pump Inhibitor Use. Cureus. 2021;13:e12759. doi: 10.7759/cureus.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chinzon D., Domingues G., Tosetto N., Perrotti M. Safety of long-term proton pump inhibitors: Facts and myths. Arq. Gastroenterol. 2022;59:219–225. doi: 10.1590/s0004-2803.202202000-40. [DOI] [PubMed] [Google Scholar]
- 27.Maes M.L., Fixen D.R., Linnebur S.A. Adverse effects of proton-pump inhibitor use in older adults: A review of the evidence. Ther. Adv. Drug Saf. 2017;8:273–297. doi: 10.1177/2042098617715381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alla D., Shah D.J., Seepana M., Salian R.B., Alla S.S.M., Mohanan M.K., Saburoglu M., Vegesna M.S.S., Singh A., Gupta S., et al. Safety of Proton Pump Inhibitors in Pediatric Population: A Systematic Review. Glob. Pediatr. Health. 2024;11:2333794X241248967. doi: 10.1177/2333794X241248967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savarino V., Marabotto E., Furnari M., Zingone F., Zentilin P., Savarino E. Latest insights into the hot question of proton pump inhibitor safety—A narrative review. Dig. Liver Dis. 2020;52:842–852. doi: 10.1016/j.dld.2020.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Alanazi A.S., Almutairi H., Gupta J.K., Mohanty D., Rath D., AlOdan A.A., Mahal A., Khatib M.N., Gaidhane S., Zahiruddin Q.S., et al. Osseous implications of proton pump inhibitor therapy: An umbrella review. Bone Rep. 2024;20:101741. doi: 10.1016/j.bonr.2024.101741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thurber K.M., Otto A.O., Stricker S.L. Proton pump inhibitors: Understanding the associated risks and benefits of long-term use. Am. J. Health Syst. Pharm. 2023;80:487–494. doi: 10.1093/ajhp/zxad009. [DOI] [PubMed] [Google Scholar]
- 32.Veettil S.K., Sadoyu S., Bald E.M., Chandran V.P., Khuu S.A.T., Pitak P., Lee Y.Y., Nair A.B., Antony P.T., Ford A.C., et al. Association of proton-pump inhibitor use with adverse health outcomes: A systematic umbrella review of meta-analyses of cohort studies and randomised controlled trials. Br. J. Clin. Pharmacol. 2022;88:1551–1566. doi: 10.1111/bcp.15103. [DOI] [PubMed] [Google Scholar]
- 33.Waldum H.L., Hauso O., Brenna E., Qvigstad G., Fossmark R. Does long-term profound inhibition of gastric acid secretion increase the risk of ECL cell-derived tumors in man? Scand. J. Gastroenterol. 2016;51:767–773. doi: 10.3109/00365521.2016.1143527. [DOI] [PubMed] [Google Scholar]
- 34.Waldum H.L., Sagatun L., Mjones P. Gastrin and Gastric Cancer. Front. Endocrinol. 2017;8:1. doi: 10.3389/fendo.2017.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waldum H.L., Hauso O., Fossmark R. The regulation of gastric acid secretion—Clinical perspectives. Acta Physiol. 2014;210:239–256. doi: 10.1111/apha.12208. [DOI] [PubMed] [Google Scholar]
- 36.Lee L., Ramos-Alvarez I., Ito T., Jensen R.T. Insights into Effects/Risks of Chronic Hypergastrinemia and Lifelong PPI Treatment in Man Based on Studies of Patients with Zollinger-Ellison Syndrome. Int. J. Mol. Sci. 2019;20:5128. doi: 10.3390/ijms20205128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waldum H., Mjones P. The central role of gastrin in gastric cancer. Front. Oncol. 2023;13:1176673. doi: 10.3389/fonc.2023.1176673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moayyedi P., Eikelboom J.W., Bosch J., Connolly S.J., Dyal L., Shestakovska O., Leong D., Anand S.S., Stork S., Branch K.R.H., et al. Safety of Proton Pump Inhibitors Based on a Large, Multi-Year, Randomized Trial of Patients Receiving Rivaroxaban or Aspirin. Gastroenterology. 2019;157:682–691. doi: 10.1053/j.gastro.2019.05.056. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y.X., Lewis J.D., Epstein S., Metz D.C. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947–2953. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 40.Andersen B.N., Johansen P.B., Abrahamsen B. Proton pump inhibitors and osteoporosis. Curr. Opin. Rheumatol. 2016;28:420–425. doi: 10.1097/BOR.0000000000000291. [DOI] [PubMed] [Google Scholar]
- 41.Yu E.W., Bauer S.R., Bain P.A., Bauer D.C. Proton pump inhibitors and risk of fractures: A meta-analysis of 11 international studies. Am. J. Med. 2011;124:519–526. doi: 10.1016/j.amjmed.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poly T.N., Islam M.M., Yang H.C., Wu C.C., Li Y.J. Proton pump inhibitors and risk of hip fracture: A meta-analysis of observational studies. Osteoporos. Int. 2019;30:103–114. doi: 10.1007/s00198-018-4788-y. [DOI] [PubMed] [Google Scholar]
- 43.Palmowski A., Schmajuk G., Yazdany J., Katz P., Li J., Stovall R., Kersey E., Nielsen S.M., Christensen R., Bliddal H., et al. Proton Pump Inhibitor Use and Bone Health in Patients with Rheumatic Diseases: A Cross-Sectional Study. Mayo Clin. Proc. 2024 doi: 10.1016/j.mayocp.2023.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Philippoteaux C., Paccou J., Chazard E., Cortet B. Proton pump inhibitors, bone and phosphocalcic metabolism. Joint Bone Spine. 2024;91:105714. doi: 10.1016/j.jbspin.2024.105714. [DOI] [PubMed] [Google Scholar]
- 45.Thong B.K.S., Ima-Nirwana S., Chin K.Y. Proton Pump Inhibitors and Fracture Risk: A Review of Current Evidence and Mechanisms Involved. Int. J. Environ. Res. Public Health. 2019;16:1571. doi: 10.3390/ijerph16091571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parmar M.P., Kaleem S., Samuganathan P., Ishfaq L., Anne T., Patel Y., Bollu S., Vempati R. Impact of Proton Pump Inhibitors on Kidney Function and Chronic Kidney Disease Progression: A Systematic Review. Cureus. 2023;15:e49883. doi: 10.7759/cureus.49883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edinoff A.N., Wu N.W., Parker K., Dudossat E., Linquest L., Flanagan C.J., Dharani A., Patel H., Willett O., Cornett E.M., et al. Proton Pump Inhibitors, Kidney Damage, and Mortality: An Updated Narrative Review. Adv. Ther. 2023;40:2693–2709. doi: 10.1007/s12325-023-02476-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hart E., Dunn T.E., Feuerstein S., Jacobs D.M. Proton Pump Inhibitors and Risk of Acute and Chronic Kidney Disease: A Retrospective Cohort Study. Pharmacotherapy. 2019;39:443–453. doi: 10.1002/phar.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jain D., Sharma G., Kumar A. Adverse effects of proton pump inhibitors (PPIs) on the renal system using data mining algorithms (DMAs) Expert. Opin. Drug Saf. 2023;22:741–752. doi: 10.1080/14740338.2023.2189698. [DOI] [PubMed] [Google Scholar]
- 50.Wu C.C., Liao M.H., Kung W.M., Wang Y.C. Proton Pump Inhibitors and Risk of Chronic Kidney Disease: Evidence from Observational Studies. J. Clin. Med. 2023;12:2262. doi: 10.3390/jcm12062262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Munch P.V., Norgaard M., Heide-Jorgensen U., Jensen S.K., Birn H., Christiansen C.F. Proton pump inhibitors and the risk of acute kidney injury in cancer patients receiving immune checkpoint inhibitors: A Danish population-based cohort study. Int. J. Cancer. 2024;154:1164–1173. doi: 10.1002/ijc.34788. [DOI] [PubMed] [Google Scholar]
- 52.Heidelbaugh J.J. Proton pump inhibitors and risk of vitamin and mineral deficiency: Evidence and clinical implications. Ther. Adv. Drug Saf. 2013;4:125–133. doi: 10.1177/2042098613482484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McColl K.E. Effect of proton pump inhibitors on vitamins and iron. Am. J. Gastroenterol. 2009;104((Suppl. S2)):S5–S9. doi: 10.1038/ajg.2009.45. [DOI] [PubMed] [Google Scholar]
- 54.Termanini B., Gibril F., Sutliff V.E., III, Yu F., Venzon D.J., Jensen R.T. Effect of long-term gastric acid suppressive therapy on serum vitamin B12 levels in patients with Zollinger-Ellison syndrome. Am. J. Med. 1998;104:422–430. doi: 10.1016/S0002-9343(98)00087-4. [DOI] [PubMed] [Google Scholar]
- 55.Cheungpasitporn W., Thongprayoon C., Kittanamongkolchai W., Srivali N., Edmonds P.J., Ungprasert P., O’Corragain O.A., Korpaisarn S., Erickson S.B. Proton pump inhibitors linked to hypomagnesemia: A systematic review and meta-analysis of observational studies. Ren. Fail. 2015;37:1237–1241. doi: 10.3109/0886022X.2015.1057800. [DOI] [PubMed] [Google Scholar]
- 56.William J.H., Danziger J. Proton-pump inhibitor-induced hypomagnesemia: Current research and proposed mechanisms. World J. Nephrol. 2016;5:152–157. doi: 10.5527/wjn.v5.i2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dahabra L., Kreidieh M., Abureesh M., Abou Y.A., Deeb L. Proton Pump Inhibitors Use and Increased Risk of Spontaneous Bacterial Peritonitis in Cirrhotic Patients: A Retrospective Cohort Analysis. Gastroenterol. Res. 2022;15:180–187. doi: 10.14740/gr1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zerr B., Vazquez A., Erstad B.L. Infection risk and management strategies for patients with cirrhosis taking proton pump inhibitors. Am. J. Health Syst. Pharm. 2023;80:967–973. doi: 10.1093/ajhp/zxad089. [DOI] [PubMed] [Google Scholar]
- 59.Low E.X.S., Wang Y.P., Lu C.L. Risks of Proton Pump Inhibitors in Patients with Cirrhosis: Please Peruse the Indications. Dig. Dis. Sci. 2024;69:7–9. doi: 10.1007/s10620-023-08153-3. [DOI] [PubMed] [Google Scholar]
- 60.Liu Y.B., Chen M.K. The impact of proton pump inhibitors in liver diseases and the effects on the liver. J. Dig. Dis. 2022;23:196–208. doi: 10.1111/1751-2980.13093. [DOI] [PubMed] [Google Scholar]
- 61.D’Silva K.M., Mehta R., Mitchell M., Lee T.C., Singhal V., Wilson M.G., McDonald E.G. Proton pump inhibitor use and risk for recurrent Clostridioides difficile infection: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2021;27:697–703. doi: 10.1016/j.cmi.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 62.Sawaid I.O., Samson A.O. Proton Pump Inhibitors and Cancer Risk: A Comprehensive Review of Epidemiological and Mechanistic Evidence. J. Clin. Med. 2024;13:3052. doi: 10.3390/jcm13071970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo H., Zhang R., Zhang P., Chen Z., Hua Y., Huang X., Li X. Association of proton pump inhibitors with gastric and colorectal cancer risk: A systematic review and meta-analysis. Front. Pharmacol. 2023;14:1129948. doi: 10.3389/fphar.2023.1129948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ben-Eltriki M., Green C.J., Maclure M., Musini V., Bassett K.L., Wright J.M. Do proton pump inhibitors increase mortality? A systematic review and in-depth analysis of the evidence. Pharmacol. Res. Perspect. 2020;8:e00651. doi: 10.1002/prp2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song H.J., Seo H.J., Jiang X., Jeon N., Lee Y.J., Ha I.H. Proton pump inhibitors associated with an increased risk of mortality in elderly: A systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2024;80:367–382. doi: 10.1007/s00228-023-03606-0. [DOI] [PubMed] [Google Scholar]
- 66.Tran T.H., Myung S.K., Trinh T.T.K. Proton pump inhibitors and risk of gastrointestinal cancer: A meta-analysis of cohort studies. Oncol. Lett. 2024;27:28. doi: 10.3892/ol.2023.14161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ben Ghezala I., Luu M., Bardou M. An update on drug-drug interactions associated with proton pump inhibitors. Expert. Opin. Drug Metab. Toxicol. 2022;18:337–346. doi: 10.1080/17425255.2022.2098107. [DOI] [PubMed] [Google Scholar]
- 68.Farhat N., Fortin Y., Haddad N., Birkett N., Mattison D.R., Momoli F., Wu W.S., Krewski D. Systematic review and meta-analysis of adverse cardiovascular events associated with proton pump inhibitors used alone or in combination with antiplatelet agents. Crit. Rev. Toxicol. 2019;49:215–261. doi: 10.1080/10408444.2019.1583167. [DOI] [PubMed] [Google Scholar]
- 69.Khan Z., Mehan S., Saifi M.A., Gupta G.D., Narula A.S., Kalfin R. Proton Pump Inhibitors and Cognitive Health: Review on Unraveling the Dementia Connection and Co-morbid Risks. Curr. Alzheimer Res. 2024;20:739–757. doi: 10.2174/0115672050289946240223050737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahn N., Nolde M., Krause E., Guntner F., Guntner A., Tauscher M., Gerlach R., Meisinger C., Linseisen J., Baumeister S.E., et al. Do proton pump inhibitors increase the risk of dementia? A systematic review, meta-analysis and bias analysis. Br. J. Clin. Pharmacol. 2023;89:602–616. doi: 10.1111/bcp.15583. [DOI] [PubMed] [Google Scholar]
- 71.Patel D., Bertz R., Ren S., Boulton D.W., Nagard M. A Systematic Review of Gastric Acid-Reducing Agent-Mediated Drug-Drug Interactions with Orally Administered Medications. Clin. Pharmacokinet. 2020;59:447–462. doi: 10.1007/s40262-019-00844-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raoul J.L., Moreau-Bachelard C., Gilabert M., Edeline J., Frenel J.S. Drug-drug interactions with proton pump inhibitors in cancer patients: An underrecognized cause of treatment failure. ESMO Open. 2023;8:100880. doi: 10.1016/j.esmoop.2023.100880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Slavin M., Frankenfeld C.L., Guirguis A.B., Seng E.K. Use of Acid-Suppression Therapy and Odds of Migraine and Severe Headache in the National Health and Nutrition Examination Survey. Neurol. Clin. Pract. 2024;14:e200302. doi: 10.1212/CPJ.0000000000200302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fong P., Chan S.T., Lei P.N., Cheong H.I., Cheong I.M., Hoe W.L. Association of suicidal ideation and depression with the use of proton pump inhibitors in adults: A cross-sectional study. Sci. Rep. 2022;12:19539. doi: 10.1038/s41598-022-24244-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin J., Liao P., Qian J., Qin Y., Xu D., He W.Q., Liang X., Qin X. Association between proton pump inhibitor use and neurological or psychiatric disorders: A systematic review protocol. JBI Evid. Synth. 2023;21:2239–2246. doi: 10.11124/JBIES-22-00447. [DOI] [PubMed] [Google Scholar]
- 76.Wang Y.H., Wintzell V., Ludvigsson J.F., Svanstrom H., Pasternak B. Proton pump inhibitor use and risk of depression and anxiety in children: Nationwide cohort study. Clin. Transl. Sci. 2022;15:1112–1122. doi: 10.1111/cts.13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson D.A. Nutritional consequences of long-term acid suppression; are they clinically important? Curr. Opin. Gastroenterol. 2016;32:136–140. doi: 10.1097/MOG.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 78.Lam J.R., Schneider J.L., Zhao W., Corley D.A. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310:2435–2442. doi: 10.1001/jama.2013.280490. [DOI] [PubMed] [Google Scholar]
- 79.Hirschowitz B.I., Worthington J., Mohnen J. Vitamin B12 deficiency in hypersecretors during long-term acid suppression with proton pump inhibitors. Aliment. Pharmacol. Ther. 2008;27:1110–1121. doi: 10.1111/j.1365-2036.2008.03658.x. [DOI] [PubMed] [Google Scholar]
- 80.Choudhury A., Jena A., Jearth V., Dutta A.K., Makharia G., Dutta U., Goenka M., Kochhar R., Sharma V. Vitamin B12 deficiency and use of proton pump inhibitors: A systematic review and meta-analysis. Expert. Rev. Gastroenterol. Hepatol. 2023;17:479–487. doi: 10.1080/17474124.2023.2204229. [DOI] [PubMed] [Google Scholar]
- 81.Jung S.B., Nagaraja V., Kapur A., Eslick G.D. Association between vitamin B12 deficiency and long-term use of acid-lowering agents: A systematic review and meta-analysis. Intern. Med. J. 2015;45:409–416. doi: 10.1111/imj.12697. [DOI] [PubMed] [Google Scholar]
- 82.Mitchell S.L., Rockwood K. The association between antiulcer medication and initiation of cobalamin replacement in older persons. J. Clin. Epidemiol. 2001;54:531–534. doi: 10.1016/S0895-4356(00)00340-1. [DOI] [PubMed] [Google Scholar]
- 83.Force R.W., Meeker A.D., Cady P.S., Culbertson V.L., Force W.S., Kelley C.M. Ambulatory care increased vitamin B12 requirement associated with chronic acid suppression therapy. Ann. Pharmacother. 2003;37:490–493. doi: 10.1345/aph.1C037. [DOI] [PubMed] [Google Scholar]
- 84.Damodharan S., Raj G.M., Sakthibalan M., Dakshinamoorthy K., Muraliswaran P. Effect of long-term acid suppression therapy with proton pump inhibitors or H(2) receptor blockers on serum vitamin B(12) levels in elderly population. Ir. J. Med. Sci. 2021;190:1213–1217. doi: 10.1007/s11845-020-02399-w. [DOI] [PubMed] [Google Scholar]
- 85.Mumtaz H., Ghafoor B., Saghir H., Tariq M., Dahar K., Ali S.H., Waheed S.T., Syed A.A. Association of Vitamin B12 deficiency with long-term PPIs use: A cohort study. Ann. Med. Surg. 2022;82:104762. doi: 10.1016/j.amsu.2022.104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pipingas A., Camfield D.A., Stough C., Scholey A.B., Cox K.H., White D., Sarris J., Sali A., Macpherson H. Effects of multivitamin, mineral and herbal supplement on cognition in younger adults and the contribution of B group vitamins. Hum. Psychopharmacol. 2014;29:73–82. doi: 10.1002/hup.2372. [DOI] [PubMed] [Google Scholar]
- 87.Hartman B., Donnelly-VanderLoo M., Watson T., O’Connor C., Madill J. Proton-pump inhibitor therapy and vitamin B(12) status in an inpatient hospital setting. Appl. Physiol. Nutr. Metab. 2016;41:1071–1076. doi: 10.1139/apnm-2016-0020. [DOI] [PubMed] [Google Scholar]
- 88.Qorraj-Bytyqi H., Hoxha R., Sadiku S., Bajraktari I.H., Sopjani M., Thaci K., Thaci S., Bahtiri E. Proton Pump Inhibitors Intake and Iron and Vitamin B12 Status: A Prospective Comparative Study with a Follow up of 12 Months. Open Access Maced. J. Med. Sci. 2018;6:442–446. doi: 10.3889/oamjms.2018.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Presse N., Perreault S., Kergoat M.J. Vitamin B12 Deficiency Induced by the Use of Gastric Acid Inhibitors: Calcium Supplements as a Potential Effect Modifier. J. Nutr. Health Aging. 2016;20:569–573. doi: 10.1007/s12603-015-0605-x. [DOI] [PubMed] [Google Scholar]
- 90.Valuck R.J., Ruscin J.M. A case-control study on adverse effects: H2 blocker or proton pump inhibitor use and risk of vitamin B12 deficiency in older adults. J. Clin. Epidemiol. 2004;57:422–428. doi: 10.1016/j.jclinepi.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 91.Swarnakari K.M., Bai M., Manoharan M.P., Raja R., Jamil A., Csendes D., Gutlapalli S.D., Prakash K., Desai D.M., Desai A., et al. The Effects of Proton Pump Inhibitors in Acid Hypersecretion-Induced Vitamin B12 Deficiency: A Systematic Review (2022) Cureus. 2022;14:e31672. doi: 10.7759/cureus.31672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rozgony N.R., Fang C., Kuczmarski M.F., Bob H. Vitamin B(12) deficiency is linked with long-term use of proton pump inhibitors in institutionalized older adults: Could a cyanocobalamin nasal spray be beneficial? J. Nutr. Elder. 2010;29:87–99. doi: 10.1080/01639360903574734. [DOI] [PubMed] [Google Scholar]
- 93.Dharmarajan T.S., Kanagala M.R., Murakonda P., Lebelt A.S., Norkus E.P. Do acid-lowering agents affect vitamin B12 status in older adults? J. Am. Med. Dir. Assoc. 2008;9:162–167. doi: 10.1016/j.jamda.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 94.Lewis J.R., Barre D., Zhu K., Ivey K.L., Lim E.M., Hughes J., Prince R.L. Long-term proton pump inhibitor therapy and falls and fractures in elderly women: A prospective cohort study. J. Bone Miner. Res. 2014;29:2489–2497. doi: 10.1002/jbmr.2279. [DOI] [PubMed] [Google Scholar]
- 95.Attwood S.E., Ell C., Galmiche J.P., Fiocca R., Hatlebakk J.G., Hasselgren B., Langstrom G., Jahreskog M., Eklund S., Lind T., et al. Long-term safety of proton pump inhibitor therapy assessed under controlled, randomised clinical trial conditions: Data from the SOPRAN and LOTUS studies. Aliment. Pharmacol. Ther. 2015;41:1162–1174. doi: 10.1111/apt.13194. [DOI] [PubMed] [Google Scholar]
- 96.Chappell L., Brown S.A., Wensel T.M. Evaluation of Vitamin B12 Monitoring in Patients on Concomitant Metformin and Proton Pump Inhibitors. Innov. Pharm. 2020;11 doi: 10.24926/iip.v11i4.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.den Elzen W.P., Groeneveld Y., de Ruijter W., Souverijn J.H., le Cessie S., Assendelft W.J., Gussekloo J. Long-term use of proton pump inhibitors and vitamin B12 status in elderly individuals. Aliment. Pharmacol. Ther. 2008;27:491–497. doi: 10.1111/j.1365-2036.2008.03601.x. [DOI] [PubMed] [Google Scholar]
- 98.Lerman T.T., Cohen E., Sochat T., Goldberg E., Goldberg I., Krause I. Proton pump inhibitor use and its effect on vitamin B12 and homocysteine levels among men and women: A large cross-sectional study. Am. J. Med. Sci. 2022;364:746–751. doi: 10.1016/j.amjms.2022.07.006. [DOI] [PubMed] [Google Scholar]
- 99.Schenk B.E., Festen H.P., Kuipers E.J., Klinkenberg-Knol E.C., Meuwissen S.G. Effect of short- and long-term treatment with omeprazole on the absorption and serum levels of cobalamin. Aliment. Pharmacol. Ther. 1996;10:541–545. doi: 10.1046/j.1365-2036.1996.27169000.x. [DOI] [PubMed] [Google Scholar]
- 100.Gibril F., Schumann M., Pace A., Jensen R.T. Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. A prospective study of 107 cases and comparison with 1009 patients from the literature. Medicine. 2004;83:43–83. doi: 10.1097/01.md.0000112297.72510.32. [DOI] [PubMed] [Google Scholar]
- 101.Ito T., Igarashi H., Uehara H., Berna M.J., Jensen R.T. Causes of Death and Prognostic Factors in Multiple Endocrine Neoplasia Type 1: A Prospective Study: Comparison of 106 MEN1/Zollinger-Ellison Syndrome Patients with 1613 Literature MEN1 Patients with or without Pancreatic Endocrine Tumors. Medicine. 2013;92:135–181. doi: 10.1097/MD.0b013e3182954af1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Norton J.A., Foster D.S., Blumgart L.H., Poultsides G.A., Visser B.C., Fraker D.L., Alexander H.R., Jensen R.T. Incidence and Prognosis of Primary Gastrinomas in the Hepatobiliary Tract. JAMA Surg. 2018;153:e175083. doi: 10.1001/jamasurg.2017.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jensen R.T., Ito T. Gastrinoma. Endotext. 2023. [(accessed on 27 September 2023)]. Available online: www.endotext.org.
- 104.Metz D.C., Pisegna J.R., Fishbeyn V.A., Benya R.V., Jensen R.T. Control of gastric acid hypersecretion in the management of patients with Zollinger-Ellison syndrome. World J. Surg. 1993;17:468–480. doi: 10.1007/BF01655106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hirschowitz B.I., Mohnen J., Shaw S. Long-term treatment with lansoprazole for patients with Zollinger-Ellison syndrome. Aliment. Pharmacol. Ther. 1996;10:507–522. doi: 10.1046/j.1365-2036.1996.10152000.x. [DOI] [PubMed] [Google Scholar]
- 106.Ito T., Ramos-Alvarez I., Jensen R.T. Successful lifetime/lifelong medical treatment of acid in Zollinger-Ellison syndrome (ZES): Myth or fact? Insights from an analysis of results of NIH-long-term prospective studies of ZES. Cancers. 2023;15:1377. doi: 10.3390/cancers15051377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hirschowitz B.I., Simmons J.L., Johnson L.F., Mohnen J. Risk factors for esophagitis in extreme acid hypersecretors with and without Zollinger-Ellison syndrome. Clin. Gastroenterol. Hepatol. 2004;2:220–229. doi: 10.1016/S1542-3565(04)00009-6. [DOI] [PubMed] [Google Scholar]
- 108.Hirschowitz B.I., Simmons J., Mohnen J. Minor effects of Helicobacter pylori on gastric secretion and dose of lansoprazole during long-term treatment in ZE and non-ZE acid hypersecretors. Aliment. Pharmacol. Ther. 2002;16:303–313. doi: 10.1046/j.1365-2036.2002.01175.x. [DOI] [PubMed] [Google Scholar]
- 109.Mignon M., Hochlaf S., Forestier S., Ruszniewski P., Vatier J., Joubert-Collin M. [Dose-response effect of lansoprazole in patients with Zollinger-Ellison syndrome] Gastroenterol. Clin. Biol. 1994;18:13–16. [PubMed] [Google Scholar]
- 110.Ramdani A., Mignon M., Samoyeau R. Effect of pantoprazole versus other proton pump inhibitors on 24-hour intragastric pH and basal acid output in Zollinger-Ellison syndrome. Gastroenterol. Clin. Biol. 2002;26:355–359. [PubMed] [Google Scholar]
- 111.Vezzadini P., Bonora G., Tomassetti P., Pazzaglia M., Labo G. Medical treatment of Zollinger-Ellison syndrome with ranitidine. Int. J. Tiss. Reac. 1983;4:339–343. [PubMed] [Google Scholar]
- 112.Clarke R., Refsum H., Birks J., Evans J.G., Johnston C., Sherliker P., Ueland P.M., Schneede J., McPartlin J., Nexo E., et al. Screening for vitamin B-12 and folate deficiency in older persons. Am. J. Clin. Nutr. 2003;77:1241–1247. doi: 10.1093/ajcn/77.5.1241. [DOI] [PubMed] [Google Scholar]
- 113.Hannibal L., Lysne V., Bjorke-Monsen A.L., Behringer S., Grunert S.C., Spiekerkoetter U., Jacobsen D.W., Blom H.J. Biomarkers and Algorithms for the Diagnosis of Vitamin B12 Deficiency. Front. Mol. Biosci. 2016;3:27. doi: 10.3389/fmolb.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Devalia V., Hamilton M.S., Molloy A.M. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br. J. Haematol. 2014;166:496–513. doi: 10.1111/bjh.12959. [DOI] [PubMed] [Google Scholar]
- 115.Harrington D.J. Laboratory assessment of vitamin B12 status. J. Clin. Pathol. 2017;70:168–173. doi: 10.1136/jclinpath-2015-203502. [DOI] [PubMed] [Google Scholar]
- 116.Carmel R. Biomarkers of cobalamin (vitamin B-12) status in the epidemiologic setting: A critical overview of context, applications, and performance characteristics of cobalamin, methylmalonic acid, and holotranscobalamin II. Am. J. Clin. Nutr. 2011;94:348S–358S. doi: 10.3945/ajcn.111.013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lindenbaum J., Savage D.G., Stabler S.P., Allen R.H. Diagnosis of cobalamin deficiency: II. Relative sensitivities of serum cobalamin, methylmalonic acid, and total homocysteine concentrations. Am. J. Hematol. 1990;34:99–107. doi: 10.1002/ajh.2830340205. [DOI] [PubMed] [Google Scholar]
- 118.Carmel R. Subclinical cobalamin deficiency. Curr. Opin. Gastroenterol. 2012;28:151–158. doi: 10.1097/MOG.0b013e3283505852. [DOI] [PubMed] [Google Scholar]
- 119.Stabler S.P., Allen R.H., Fried L.P., Pahor M., Kittner S.J., Penninx B.W., Guralnik J.M. Racial differences in prevalence of cobalamin and folate deficiencies in disabled elderly women. Am. J. Clin. Nutr. 1999;70:911–919. doi: 10.1093/ajcn/70.5.911. [DOI] [PubMed] [Google Scholar]
- 120.Allen R.H., Stabler S.P., Savage D.G., Lindenbaum J. Diagnosis of cobalamin deficiency I: Usefulness of serum methylmalonic acid and total homocysteine concentrations. Am. J. Hematol. 1990;34:90–98. doi: 10.1002/ajh.2830340204. [DOI] [PubMed] [Google Scholar]
- 121.Obeid R., Andres E., Ceska R., Hooshmand B., Gueant-Rodriguez R.M., Prada G.I., Slawek J., Traykov L., Ta Van B., Varkonyi T., et al. Diagnosis, Treatment and Long-Term Management of Vitamin B12 Deficiency in Adults: A Delphi Expert Consensus. J. Clin. Med. 2024;13:2176. doi: 10.3390/jcm13082176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sands T., Jawed A., Stevenson E., Smith M., Jawaid I. Vitamin B(12) deficiency: NICE guideline summary. BMJ. 2024;385:q1019. doi: 10.1136/bmj.q1019. [DOI] [PubMed] [Google Scholar]
- 123.Ellison E.H., Wilson S.D. The Zollinger-Ellison syndrome: Re-appraisal and evaluation of 260 registered cases. Ann. Surg. 1964;160:512–530. doi: 10.1097/00000658-196409000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Soga J., Yakuwa Y. The gastrinoma/Zollinger-Ellison syndrome: Statistical evaluation of a Japanese series of 359 cases. J. Hep. Bil. Pancr. Surg. 1998;5:77–85. doi: 10.1007/PL00009955. [DOI] [PubMed] [Google Scholar]
- 125.Zollinger R.M., Ellison E.C., Fabri P.J., Johnson J., Sparks J., Carey L.C. Primary peptic ulcerations of the jejunum associated with islet cell tumors. Twenty-five-year appraisal. Ann. Surg. 1980;192:422–430. doi: 10.1097/00000658-198009000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Farley D.R., Van Heerden J.A., Grant C.S., Miller L.J., Ilstrup D.M. The Zollinger-Ellison syndrome. A collective surgical experience. Ann. Surg. 1992;215:561–569. doi: 10.1097/00000658-199206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Doherty G.M., Olson J.A., Frisella M.M., Lairmore T.C., Wells S.A., Jr., Norton J.A. Lethality of multiple endocrine neoplasia Type I. World J. Surg. 1998;22:581–587. doi: 10.1007/s002689900438. [DOI] [PubMed] [Google Scholar]
- 128.Mignon M., Cadiot G., Rigaud D., Ruszniewski P., Jais P., Lehy T., Lewin M.J.M. Management of islet cell tumors in patients with multiple endocrine neoplasia type 1. In: Mignon M., Jensen R.T., editors. Endocrine Tumors of the Pancreas: Recent Advances in Research and Management. Series: Frontiers in Gastrointestinal Research. Volume 23. S. Karger; Basel, Switzerland: 1995. pp. 342–359. [Google Scholar]
- 129.Ruszniewski P., Podevin P., Cadiot G., Marmuse J.P., Mignon M., Vissuzaine C., Bonfils S., Lehy T. Clinical, anatomical, and evolutive features of patients with the Zollinger-Ellison syndrome combined with type I multiple endocrine neoplasia. Pancreas. 1993;8:295–304. doi: 10.1097/00006676-199305000-00003. [DOI] [PubMed] [Google Scholar]
- 130.Mignon M., Ruszniewski P., Haffar S., Rignaud D., Rene E., Bonfils S. Current approach to the management of tumoral process in patients with gastrinoma. World J. Surg. 1986;10:703–710. doi: 10.1007/BF01655562. [DOI] [PubMed] [Google Scholar]
- 131.Stage J.G., Stadil F. The clinical diagnosis of the Zollinger-Ellison syndrome. Scand. J. Gastroenterol. Suppl. 1979;53:79–91. [PubMed] [Google Scholar]
- 132.Kaplan E.L., Horvath K., Udekwu A., Straus F., Schark C., Ferguson D.J., Skinner D.B. Gastrinomas: A 42-year experience. World J. Surg. 1990;14:365–375. doi: 10.1007/BF01658530. [DOI] [PubMed] [Google Scholar]
- 133.Mignon M., Ruszniewski P., Podevin P., Sabbagh L., Cadiot G., Rigaud D., Bonfils S. Current approach to the management of gastrinoma and insulinoma in adults with multiple endocrine neoplasia type 1. World J. Surg. 1993;17:489–497. doi: 10.1007/BF01655108. [DOI] [PubMed] [Google Scholar]
- 134.Christlieb A.R., Schuster M.M. Zollinger-Ellison syndrome. Arch. Intern. Med. 1964;114:381–388. doi: 10.1001/archinte.1964.03860090115012. [DOI] [PubMed] [Google Scholar]
- 135.Way L., Goldman L., Dunphy J.E. Zollinger-Ellison syndrome. An analysis of twenty-five cases. Am. J. Surg. 1968;116:293–304. doi: 10.1016/0002-9610(68)90507-2. [DOI] [PubMed] [Google Scholar]
- 136.Straus E., Yalow R.S. Differential diagnosis of hypergastrinemia. In: Thompson J.C., editor. Gastrointestinal Hormones. University of Texas Press; Austin, TX, USA: 1975. pp. 99–113. [Google Scholar]
- 137.Thompson J.C., Reeder D.D., Villar H.V., Fender H.R. Natural history and experience with diagnosis and treatment of the Zollinger-Ellison syndrome. Surg. Gynecol. Obstet. 1975;140:721–739. [PubMed] [Google Scholar]
- 138.Regan P.T., Malagelada J.R. A reappraisal of clinical, roentgenographic, and endoscopic features of the Zollinger-Ellison syndrome. Mayo Clin. Proc. 1978;53:19–23. [PubMed] [Google Scholar]
- 139.Aoyagi T., Summerskill W.H. Gastric secretion with ulcerogenic islet cell tumor. Importance of basal acid output. Arch. Intern. Med. 1966;117:667–672. doi: 10.1001/archinte.1966.03870110059012. [DOI] [PubMed] [Google Scholar]
- 140.Winship D.H. Problems in the diagnosis of Zollinger-Ellison-syndrome by analysis of gastric secretion. In: Demling I., Ottenjann R., editors. Non-Insulin-Producing Tumors of the Pancreas. Modern Aspects on Zollinger-Ellison-Syndrome and Gastrin. Georg Thieme Verlagh; Erlangen, Germany: 1968. pp. 129–140. [Google Scholar]
- 141.Sanchez R.E., Longmire W.P., Jr., Passaro E., Jr. Acid secretion and serum gastrin levels in the Zollinger-Ellison syndrome. Calif. Med. 1972;116:1–7. [PMC free article] [PubMed] [Google Scholar]
- 142.Cameron A.J., Hoffman H.N. Zollinger-Ellison syndrome; Clinical features and long-term follow-up. Mayo Clin. Proc. 1974;49:44–51. [PubMed] [Google Scholar]
- 143.Malagelada J.R., Davis C.S., O’Fallon W.M., Go V.L. Laboratory diagnosis of gastrinoma. I. A prospective evaluation of gastric analysis and fasting serum gastrin levels. Mayo Clin. Proc. 1982;57:211–218. [PubMed] [Google Scholar]
- 144.Mee A.S., Ismail S., Bornman P.C., Marks I.N. Changing concepts in the presentation, diagnosis and management of the Zollinger-Ellison syndrome. Q. J. Med. 1983;52:256–267. [PubMed] [Google Scholar]
- 145.Vezzadini P., Tomassetti P., Toni R., Bonora G., Labo G. Omeprazole in the medical treatment of Zollinger-Ellison syndrome. Curr. Ther. Res. 1984;35:772–776. [Google Scholar]
- 146.Richardson C.T., Peters M.N., Feldman M., McClelland R.N., Walsh J.H., Cooper K.A., Willeford G., Dickerman R.M., Fordtran J.S. Treatment of Zollinger-Ellison syndrome with exploratory laparotomy, proximal gastric vagotomy, and H2-receptor antagonists. A prospective study. Gastroenterology. 1985;89:357–367. doi: 10.1016/0016-5085(85)90337-3. [DOI] [PubMed] [Google Scholar]
- 147.Larkin C.J., Joy E.S., Ardill J.E., Johnston C.F., Collins J.S., Buchanan K.D. Gastrinomas and the change in their presentation and management in Northern Ireland, UK, from 1970 to 1996. Eur. J. Gastroenterol. Hepatol. 1998;10:947–952. doi: 10.1097/00042737-199811000-00008. [DOI] [PubMed] [Google Scholar]
- 148.Waddell W.R., Leonsins A.J., Zuidema G.D. Gastric secretory and other laboratory studies on two patients with Zollinger-Ellison syndrome. N. Engl. J. Med. 1959;260:56–62. doi: 10.1056/NEJM195901082600202. [DOI] [PubMed] [Google Scholar]
- 149.Mignon M., N’Go Y., Gobet B., Piper A., Duet M., Vatier J. Sensitivity and specificity of cut-off points values for acid output and serum gastrin.in basal state and upon secretin for the diagnosis of Zollinger-Ellison syndrome: A study of 57 postoperative ulcer patients. Gastroenterology. 1990;98:A227. [Google Scholar]
- 150.Roy P.K., Venzon D.J., Feigenbaum K.M., Koviack P.D., Bashir S., Ojeaburu J.V., Gibril F., Jensen R.T. Gastric secretion in Zollinger-Ellison syndrome: Correlation with clinical expression, tumor extent and role in diagnosis—A prospective NIH study of 235 patients and review of the literature in 984 cases. Medicine. 2001;80:189–222. doi: 10.1097/00005792-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 151.MacFarlane M.P., Fraker D.L., Alexander H.R., Norton J.A., Jensen R.T. A prospective study of surgical resection of duodenal and pancreatic gastrinomas in multiple endocrine neoplasia-Type 1. Surgery. 1995;118:973–980. doi: 10.1016/S0039-6060(05)80102-3. [DOI] [PubMed] [Google Scholar]
- 152.Kong W., Albers M.B., Manoharan J., Goebel J.N., Kann P.H., Jesinghaus M., Bartsch D.K. Pancreaticoduodenectomy Is the Best Surgical Procedure for Zollinger-Ellison Syndrome Associated with Multiple Endocrine Neoplasia Type 1. Cancers. 2022;14:1928. doi: 10.3390/cancers14081928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Imamura M., Komoto I., Taki Y. How to treat gastrinomas in patients with multiple endocrine neoplasia type1: Surgery or long-term proton pump inhibitors? Surg. Today. 2023;53:1325–1334. doi: 10.1007/s00595-022-02627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zollinger R.M., Ellison E.H. Primary peptic ulcerations of the jejunum associated with islet cell tumors of the pancreas. Ann. Surg. 1955;142:709–728. doi: 10.1097/00000658-195510000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ellison E.C., Johnson J.A. The Zollinger-Ellison syndrome: A comprehensive review of historical, scientific, and clinical considerations. Curr. Probl. Surg. 2009;46:13–106. doi: 10.1067/j.cpsurg.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 156.Ben Benya R.V., Metz D.C., Venzon D.J., Fishbeyn V.A., Strader D.B., Orbuch M., Jensen R.T. Zollinger-Ellison syndrome can be the initial endocrine manifestation in patients with multiple endocrine neoplasia-type 1. Am. J. Med. 1994;97:436–444. doi: 10.1016/0002-9343(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 157.Asgharian B., Chen Y.J., Patronas N.J., Venzon D.J., Peghini P., Reynolds J.C., Vortmeyer A., Zhang Z.P., Gibril F., Jensen R.T. Meningiomas may be a component tumor of MEN1. Clin. Cancer Res. 2004;10:869–880. doi: 10.1158/1078-0432.CCR-0938-3. [DOI] [PubMed] [Google Scholar]
- 158.Gibril F., Venzon D.J., Ojeaburu J.V., Bashir S., Jensen R.T. Cutaneous tumors in patients with MEN1 and gastrinomas: Prospective study of frequency and development of criteria with high sensitivity and specificity. J. Clin. Endocrinol. Metab. 2004;89:5328–5336. doi: 10.1210/jc.2004-0218. [DOI] [PubMed] [Google Scholar]
- 159.Gibril F., Venzon D.J., Ojeaburu J.V., Bashir S., Jensen R.T. Prospective study of the natural history of gastrinoma in patients with MEN1: Definition of an aggressive and a nonaggressive form. J. Clin. Endocrinol. Metab. 2001;86:5282–5293. doi: 10.1210/jcem.86.11.8011. [DOI] [PubMed] [Google Scholar]
- 160.Goebel S.U., Heppner C., Burns A.D., Marx S.J., Spiegel A.M., Zhuang Z.P., Gibril F., Jensen R.T., Serrano J. Geneotype/phenotype correlations of MEN1 gene mutations in sporadic gastrinoma. J. Clin. Endocrinol. Metab. 2000;85:116–123. doi: 10.1210/jcem.85.1.6260. [DOI] [PubMed] [Google Scholar]
- 161.Norton J.A., Melcher M.L., Gibril F., Jensen R.T. Gastric carcinoid tumors in multiple endocrine neoplasia-1 patients with Zollinger-Ellison syndrome can be symptomatic, demonstrate aggressive growth, and require surgery. Surgery. 2004;136:1267–1274. doi: 10.1016/j.surg.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 162.Jensen R.T. Natural history of digestive endocrine tumors. In: Mignon M., Colombel J.F., editors. Recent Advances in Pathophysiology and Management of Inflammatory Bowel Diseases and Digestive Endocrine Tumors. John Libbey Eurotext Publishing Co.; Paris, France: 1999. pp. 192–219. [Google Scholar]
- 163.Berna M.J., Hoffmann K.M., Long S.H., Serrano J., Gibril F., Jensen R.T. Serum gastrin in Zollinger-Ellison syndrome: II. Prospective study of gastrin provocative testing in 293 patients from the National Institutes of Health and comparison with 537 cases from the literature. evaluation of diagnostic criteria, proposal of new criteria, and correlations with clinical and tumoral features. Medicine. 2006;85:331–364. doi: 10.1097/MD.0b013e31802b518c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Raufman J.P., Collins S.M., Pandol S.J., Korman L.Y., Collen M.J., Cornelius M.J., Feld M.K., McCarthy D.M., Gardner J.D., Jensen R.T. Reliability of symptoms in assessing control of gastric acid secretion in patients with Zollinger-Ellison syndrome. Gastroenterology. 1983;84:108–113. doi: 10.1016/S0016-5085(83)80173-5. [DOI] [PubMed] [Google Scholar]
- 165.Ito T., Lee L., Jensen R.T. Carcinoid-syndrome: Recent advances, current status and controversies. Curr. Opin. Endocrinol. Diabetes Obes. 2018;25:22–35. doi: 10.1097/MED.0000000000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Miller L.S., Vinayek R., Frucht H., Gardner J.D., Jensen R.T., Maton P.N. Reflux esophagitis in patients with Zollinger-Ellison syndrome. Gastroenterology. 1990;98:341–346. doi: 10.1016/0016-5085(90)90823-J. [DOI] [PubMed] [Google Scholar]
- 167.Norton J.A., Alexander H.R., Fraker D.L., Venzon D.J., Jensen R.T. Does the use of routine duodenotomy (DUODX) affect rate of cure, development of liver metastases or survival in patients with Zollinger-Ellison syndrome (ZES)? Ann. Surg. 2004;239:617–626. doi: 10.1097/01.sla.0000124290.05524.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fishbeyn V.A., Norton J.A., Benya R.V., Pisegna J.R., Venzon D.J., Metz D.C., Jensen R.T. Assessment and prediction of long-term cure in patients with Zollinger-Ellison syndrome: The best approach. Ann. Intern. Med. 1993;119:199–206. doi: 10.7326/0003-4819-119-3-199308010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pisegna J.R., Norton J.A., Slimak G.G., Metz D.C., Maton P.N., Jensen R.T. Effects of curative resection on gastric secretory function and antisecretory drug requirement in the Zollinger-Ellison syndrome. Gastroenterology. 1992;102:767–778. doi: 10.1016/0016-5085(92)90157-T. [DOI] [PubMed] [Google Scholar]
- 170.Norton J.A., Harris E.J., Chen Y., Visser B.C., Poultsides G.A., Kunz P.C., Fisher G.A., Jensen R.T. Pancreatic endocrine tumors with major vascular abutment, involvement, or encasement and indication for resection. Arch. Surg. 2011;146:724–732. doi: 10.1001/archsurg.2011.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Krampitz G.W., Norton J.A., Poultsides G.A., Visser B., Sun L., Jensen R.T. Lymph nodes and survival in duodenal and pancreatic neuroendocrine tumors. Arch. Surg. 2012;147:820–827. doi: 10.1001/archsurg.2012.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Krudy A.G., Doppman J.L., Jensen R.T., Norton J.A., Collen M.J., Shawker T.H., Gardner J.D., McArthur K.K., Gorden P. Localization of islet cell tumors by dynamic CT: Comparison with plain CT, arteriography, sonography and venous sampling. Am. J. Roentgenol. 1984;143:585–589. doi: 10.2214/ajr.143.3.585. [DOI] [PubMed] [Google Scholar]
- 173.Sutliff V.E., Doppman J.L., Gibril F., Yu F., Serrano J., Venzon D.J., Jensen R.T. Growth of newly diagnosed, untreated metastatic gastrinomas and predictors of growth patterns. J. Clin. Oncol. 1997;15:2420–2431. doi: 10.1200/JCO.1997.15.6.2420. [DOI] [PubMed] [Google Scholar]
- 174.Frucht H., Norton J.A., London J.F., Vinayek R., Doppman J.L., Gardner J.D., Jensen R.T., Maton P.N. Detection of duodenal gastrinomas by operative endoscopic transillumination: A prospective study. Gastroenterology. 1990;99:1622–1627. doi: 10.1016/0016-5085(90)90466-E. [DOI] [PubMed] [Google Scholar]
- 175.Doppman J.L., Miller D.L., Chang R., Maton P.N., London J.F., Gardner J.D., Jensen R.T., Norton J.A. Gastrinomas: Localization by means of selective intraarterial injection of secretin. Radiology. 1990;174:25–29. doi: 10.1148/radiology.174.1.2294556. [DOI] [PubMed] [Google Scholar]
- 176.Ito T., Igarashi H., Jensen R.T. Therapy of metastatic pancreatic neuroendocrine tumors (pNETs): Recent insights and advances. J. Gastroenterol. 2012;47:941–960. doi: 10.1007/s00535-012-0642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cherner J.A., Doppman J.L., Norton J.A., Miller D.L., Krudy A.G., Raufman J.P., Collen M.J., Maton P.N., Gardner J.D., Jensen R.T. Selective venous sampling for gastrin to localize gastrinomas. A prospective study. Ann. Intern. Med. 1986;105:841–847. doi: 10.7326/0003-4819-105-6-841. [DOI] [PubMed] [Google Scholar]
- 178.Thom A.K., Norton J.A., Axiotis C.A., Jensen R.T. Location, incidence and malignant potential of duodenal gastrinomas. Surgery. 1991;110:1086–1093. [PubMed] [Google Scholar]
- 179.Norton J.A., Alexander H.A., Fraker D.L., Venzon D.J., Gibril F., Jensen R.T. Possible primary lymph node gastrinomas: Occurrence, natural history and predictive factors: A prospective study. Ann. Surg. 2003;237:650–659. doi: 10.1097/01.SLA.0000064375.51939.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Martignoni M.E., Friess H., Lubke D., Uhl W., Maurer C., Muller M., Richard H.P., Reubi J.C., Buchler M.W. Study of a primary gastrinoma in the common hepatic duct—A case report. Digestion. 1998;60:187–190. doi: 10.1159/000007645. [DOI] [PubMed] [Google Scholar]
- 181.Bollen E.C.M., Lamers C.B., Jansen J.B., Larsson L.I., Joosten H.J. Zollinger-Ellison syndrome due to a gastrin-producing ovarian cystadenocarcinoma. Br. J. Surg. 1981;68:776–777. doi: 10.1002/bjs.1800681107. [DOI] [PubMed] [Google Scholar]
- 182.Luiso D., Zuccarino F., Tizon-Marcos H., Ble M. Primary intracardiac gastrinoma causing Zollinger-Ellison syndrome. Eur. Heart J. 2019;41:3376. doi: 10.1093/eurheartj/ehaa512. [DOI] [PubMed] [Google Scholar]
- 183.Rafieian S., Vahedi M., Jahanbin B., Ghasemloee A. Primary thoracic gastrinoma causing Zollinger-Ellison syndrome. Indian. J. Thorac. Cardiovasc. Surg. 2021;37:706–709. doi: 10.1007/s12055-021-01211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.von Schrenck T., Howard J.M., Doppman J.L., Norton J.A., Maton P.N., Smith F.P., Vinayek R., Frucht H., Wank S.A., Gardner J.D., et al. Prospective study of chemotherapy in patients with metastatic gastrinoma. Gastroenterology. 1988;94:1326–1334. doi: 10.1016/0016-5085(88)90670-1. [DOI] [PubMed] [Google Scholar]
- 185.Burkitt M.D., Pritchard D.M. Review article: Pathogenesis and management of gastric carcinoid tumours. Aliment. Pharmacol. Ther. 2006;24:1305–1320. doi: 10.1111/j.1365-2036.2006.03130.x. [DOI] [PubMed] [Google Scholar]
- 186.Rindi G., Bordi C., Rappel S., La Rosa S., Stolte M., Solcia E. Gastric carcinoids and neuroendocrine carcinomas: Pathogenesis, pathology, and behavior. World J. Surg. 1996;20:168–172. doi: 10.1007/s002689900026. [DOI] [PubMed] [Google Scholar]
- 187.Rindi G., Azzoni C., La Rosa S., Klersy C., Paolotti D., Rappel S., Stolte M., Capella C., Bordi C., Solcia E. ECL cell tumor and poorly differentiated endocrine carcinoma of the stomach: Prognostic evaluation by pathological analysis. Gastroenterology. 1999;116:532–542. doi: 10.1016/S0016-5085(99)70174-5. [DOI] [PubMed] [Google Scholar]
- 188.Rindi G., Solcia E. Endocrine hyperplasia and dysplasia in the pathogenesis of gastrointestinal and pancreatic endocrine tumors. Gastroenterol. Clin. N. Am. 2007;36:851–865. doi: 10.1016/j.gtc.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 189.Peghini P.L., Annibale B., Azzoni C., Milione M., Corleto V.D., Gibril F., Venzon D.J., Delle Fave G., Bordi C., Jensen R.T. Effect of chronic hypergastrinemia on human enterochromaffin-like cells: Insights from patients with sporadic gastrinomas. Gastroenterology. 2002;123:68–85. doi: 10.1053/gast.2002.34231. [DOI] [PubMed] [Google Scholar]
- 190.Oates J.A., Sjoerdsma A. A unique syndrome associated with secretion of 5-hydroxytryptophan by metastatic gastric carcinoids. Am. J. Med. 1962;32:333–342. doi: 10.1016/0002-9343(62)90124-9. [DOI] [PubMed] [Google Scholar]
- 191.Gladdy R.A., Strong V.E., Coit D., Allen P.J., Gerdes H., Shia J., Klimstra D.S., Brennan M.F., Tang L.H. Defining Surgical Indications for Type I Gastric Carcinoid Tumor. Ann. Surg. Oncol. 2009;16:3154–3160. doi: 10.1245/s10434-009-0687-y. [DOI] [PubMed] [Google Scholar]
- 192.Hopper A.D., Bourke M.J., Hourigan L.F., Tran K., Moss A., Swan M.P. En-bloc resection of multiple type 1 gastric carcinoid tumors by endoscopic multi-band mucosectomy. J. Gastroenterol. Hepatol. 2009;24:1516–1521. doi: 10.1111/j.1440-1746.2009.05909.x. [DOI] [PubMed] [Google Scholar]
- 193.Delle Fave G., Capurso G., Milione M., Panzuto F. Endocrine tumours of the stomach. Best. Pract. Res. Clin. Gastroenterol. 2005;19:659–673. doi: 10.1016/j.bpg.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 194.Grozinsky-Glasberg S., Kaltsas G., Gur C., Gal E., Thomas D., Fichman S., Alexandraki K., Barak D., Glaser B., Shimon I., et al. Long-acting somatostatin analogues are an effective treatment for type 1 gastric carcinoid tumours. Eur. J. Endocrinol. 2008;159:475–482. doi: 10.1530/EJE-08-0420. [DOI] [PubMed] [Google Scholar]
- 195.Richards M.L., Gauger P., Thompson N.W., Giordano T.J. Regression of type II gastric carcinoids in multiple endocrine neoplasia type 1 patients with Zollinger-Ellison syndrome after surgical excision of all gastrinomas. World J. Surg. 2004;28:652–658. doi: 10.1007/s00268-004-7345-0. [DOI] [PubMed] [Google Scholar]
- 196.Tomassetti P., Migliori M., Caletti G.C., Fusaroli P., Corinaldesi R., Gullo L. Treatment of type II gastric carcinoid tumors with somatostatin analogues. N. Engl. J. Med. 2000;343:551–554. doi: 10.1056/NEJM200008243430805. [DOI] [PubMed] [Google Scholar]
- 197.Jensen R.T., Metz D.C., Koviack P.D., Feigenbaum K.M. Prospective study of the long-term efficacy and safety of lansoprazole in patients with Zollinger-Ellison syndrome. Aliment. Pharmacol. Ther. 1993;7((Suppl. S1)):41–50. doi: 10.1111/j.1365-2036.1993.tb00588.x. [DOI] [PubMed] [Google Scholar]
- 198.Jensen R.T., Collen M.J., McArthur K.E., Howard J.M., Maton P.N., Cherner J.A., Gardner J.D. Comparison of the effectiveness of ranitidine and cimetidine in inhibiting acid secretion in patients with gastric hypersecretory states. In: Misiewicz J.J., Wood J.R., editors. Ranitidine: Therapeutic Advances. Excerpta Medica; Amsterdam, The Netherlands: 1984. pp. 168–198. [PubMed] [Google Scholar]
- 199.Pospai D., Cadiot G., Forestier S., Ruszniewski P., Coste T., Escourrou J., Mignon M. Efficacite et tolerance due lansoprazole dans le traitement du syndrome de Zollinger-Ellison. Gastroenterol. Clin. Biol. 1998;22:801–808. [PubMed] [Google Scholar]
- 200.Lloyd-Davies K.A., Rutgersson K., Solvell L. Omeprazole in the treatment of Zollinger-Ellison syndrome: A 4-year international study. Aliment. Pharmacol. Ther. 1988;2:13–32. doi: 10.1111/j.1365-2036.1988.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 201.Wilcox C.M., Seay T., Arcury J.T., Mohnen J., Hirschowitz B.I. Zollinger-Ellison syndrome: Presentation, response to therapy, and outcome. Dig. Liver Dis. 2011;43:439–443. doi: 10.1016/j.dld.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 202.Phan J., Benhammou J.N., Pisegna J.R. Gastric Hypersecretory States: Investigation and Management. Curr. Treat. Options. Gastroenterol. 2015;13:386–397. doi: 10.1007/s11938-015-0065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Morocutti A., Merrouche M., Bjaaland T., Humphries T., Mignon M. An open-label study of rabeprazole in patients with Zollinger-Ellison syndrome or idiopathic gastric acid hypersecretion. Aliment. Pharmacol. Ther. 2006;24:1439–1444. doi: 10.1111/j.1365-2036.2006.03137.x. [DOI] [PubMed] [Google Scholar]
- 204.Metz D.C., Forsmark C., Lew E.A., Starr J.A., Soffer E.F., Bochenek W., Pisegna J.R. Replacement of oral proton pump inhibitors with intravenous pantoprazole to effectively control gastric acid hypersecretion in patients with Zollinger-Ellison syndrome. Am. J. Gastroenterol. 2001;96:3274–3280. doi: 10.1111/j.1572-0241.2001.05325.x. [DOI] [PubMed] [Google Scholar]
- 205.Riff B.P., Leiman D.A., Bennett B., Fraker D.L., Metz D.C. Weight Gain in Zollinger-Ellison Syndrome After Acid Suppression. Pancreas. 2016;45:193–197. doi: 10.1097/MPA.0000000000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bardram L., Stadil F. Omeprazole in the Zollinger-Ellison syndrome. Scand. J. Gastroenterol. 1986;21:374–378. doi: 10.3109/00365528609003090. [DOI] [PubMed] [Google Scholar]
- 207.Delchier J.C., Soule J.C., Mignon M., Goldfain D., Cortot A., Travers B., Isal J.P., Bader J.P. of omeprazole in seven patients with Zollinger- Ellison syndrome resistant to histamine H2-receptor antagonists. Dig. Dis. Sci. 1986;31:693–699. doi: 10.1007/BF01296445. [DOI] [PubMed] [Google Scholar]
- 208.Raddatz D., Horstmann O., Basenau D., Becker H., Ramadori G. Cushing’s syndrome due to ectopic adrenocorticotropic hormone production by a non-metastatic gastrinoma after lonterm conservative treatment of Zollinger-Ellison syndrome. Ital. J. Gastroenterol. Hepatol. 1998;30:636–640. [PubMed] [Google Scholar]
- 209.Imamura M., Komoto I., Wada M., Doi R., Adachi Y., Yabana T., Kabumoto T., Miyamoto S., Hirota T., Aiko T. Clinical evaluation of rabeprazole in patients with Zollinger-Ellison syndrome. Therpeutic Res. 2005;26:1287–1308. [Google Scholar]
- 210.Maton P.N., Gardner J.D., Jensen R.T. Cushing’s syndrome in patients with Zollinger-Ellison syndrome. N. Engl. J. Med. 1986;315:1–5. doi: 10.1056/NEJM198607033150101. [DOI] [PubMed] [Google Scholar]
- 211.Thompson N.W., Vinik A.I., Eckhauser F.E. Microgastrinomas of the duodenum. A cause of failed operations for the Zollinger-Ellison syndrome. Ann. Surg. 1989;209:396–404. doi: 10.1097/00000658-198904000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hofland J., Falconi M., Christ E., Castano J.P., Faggiano A., Lamarca A., Perren A., Petrucci S., Prasad V., Ruszniewski P., et al. European Neuroendocrine Tumor Society 2023 guidance paper for functioning pancreatic neuroendocrine tumour syndromes. J. Neuroendocrinol. 2023;35:e13318. doi: 10.1111/jne.13318. [DOI] [PubMed] [Google Scholar]
- 213.Ito T., Jensen R.T. Perspectives on the Current Pharmacotherapeutic Strategies for Management of Functional Neuroendocrine Tumor Syndromes. Expert. Opin. Pharmacother. 2021;22:685–693. doi: 10.1080/14656566.2020.1845651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ito T., Igarashi H., Uehara H., Jensen R.T. Pharmacotherapy of Zollinger-Ellison syndrome. Expert. Opin. Pharmacotherapy. 2013;14:307–321. doi: 10.1517/14656566.2013.767332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Herrmann W., Obeid R. Cobalamin deficiency. Subcell. Biochem. 2012;56:301–322. doi: 10.1007/978-94-007-2199-9_16. [DOI] [PubMed] [Google Scholar]
- 216.Metz D.C., Comer G.M., Soffer E., Forsmark C.E., Cryer B., Chey W., Pisegna J.R. Three-year oral pantoprazole administration is effective for patients with Zollinger-Ellison syndrome and other hypersecretory conditions. Aliment. Pharmacol. Ther. 2006;23:437–444. doi: 10.1111/j.1365-2036.2006.02762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Metz D.C., Soffer E., Forsmark C.E., Cryer B., Chey W., Bochenek W., Pisegna J.R. Maintenance oral pantoprazole therapy is effective for patients with Zollinger-Ellison syndrome and idiopathic hypersecretion. Am. J. Gastroenterol. 2003;98:301–307. doi: 10.1111/j.1572-0241.2003.07262.x. [DOI] [PubMed] [Google Scholar]
- 218.Metz D.C., Sostek M.B., Ruszniewski P., Forsmark C.E., Monyak J., Pisegna J.R. Effects of esomeprazole on Acid output in patients with zollinger-ellison syndrome or idiopathic gastric Acid hypersecretion. Am. J. Gastroenterol. 2007;102:2648–2654. doi: 10.1111/j.1572-0241.2007.01509.x. [DOI] [PubMed] [Google Scholar]
- 219.McArthur K.E., Richardson C.T., Barnett C.C., Eshaghi N., Smerud M.J., McClelland R.N., Feldman M. Laparotomy and proximal gastric vagotomy in Zollinger-Ellison syndrome: Results of a 16-year prospective study. Am. J. Gastroenterol. 1996;91:1104–1111. [PubMed] [Google Scholar]
- 220.Lamers C.B.H.W., Lind T., Moberg S., Jansen J.B.M.J., Olbe L. Omeprazole in Zollinger-Ellison syndrome: Effects of a single dose and of long term treatment in patients resistant to histamine H2-receptor antagonists. N. Engl. J. Med. 1984;310:758–761. doi: 10.1056/NEJM198403223101205. [DOI] [PubMed] [Google Scholar]
- 221.Richardson C.T., Feldman M., McClelland R.N., Dickerman R.M., Kumpuris D., Fordtran J.S. Effect of vagotomy in Zollinger-Ellison syndrome. Gastroenterology. 1979;77:682–686. doi: 10.1016/0016-5085(79)90221-X. [DOI] [PubMed] [Google Scholar]
- 222.Maton P.N., Frucht H., Vinayek R., Wank S.A., Gardner J.D., Jensen R.T. Medical management of patients with Zollinger-Ellison syndrome who have had previous gastric surgery: A prospective study. Gastroenterology. 1988;94:294–299. doi: 10.1016/0016-5085(88)90415-5. [DOI] [PubMed] [Google Scholar]
- 223.Sumner A.E., Chin M.M., Abrahm J.L., Berry G.T., Gracely E.J., Allen R.H., Stabler S.P. Elevated methylmalonic acid and total homocysteine levels show high prevalence of vitamin B12 deficiency after gastric surgery. Ann. Intern. Med. 1996;124:469–476. doi: 10.7326/0003-4819-124-5-199603010-00002. [DOI] [PubMed] [Google Scholar]
- 224.Rygvold O. Hypovitaminosis B12 following partial gastrectomy by the Billroth II method. Scand. J. Gastroenterol. Suppl. 1974;29:57–64. [PubMed] [Google Scholar]
- 225.Metz D.C., Benya R.V., Fishbeyn V.A., Pisegna J.R., Orbuch M., Strader D.B., Norton J.A., Jensen R.T. Prospective study of the need for long-term antisecretory therapy in patients with Zollinger-Ellison syndrome following successful curative gastrinoma resection. Aliment. Pharmacol. Ther. 1993;7:247–257. doi: 10.1111/j.1365-2036.1993.tb00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Collen M.J., Howard J.M., McArthur K.E., Raufman J.P., Cornelius M.J., Ciarleglio C.A., Gardner J.D., Jensen R.T. Comparison of ranitidine and cimetidine in the treatment of gastric hypersecretion. Ann. Intern. Med. 1984;100:52–58. doi: 10.7326/0003-4819-100-1-52. [DOI] [PubMed] [Google Scholar]
- 227.Howard J.M., Chremos A.N., Collen M.J., McArthur K.E., Cherner J.A., Maton P.N., Ciarleglio C.A., Cornelius M.J., Gardner J.D., Jensen R.T. Famotidine, a new, potent, long-acting histamine H2-receptor antagonist: Comparison with cimetidine and ranitidine in the treatment of Zollinger-Ellison syndrome. Gastroenterology. 1985;88:1026–1033. doi: 10.1016/S0016-5085(85)80024-X. [DOI] [PubMed] [Google Scholar]
- 228.Metz D.C., Pisegna J.R., Ringham G.L., Feigenbaum K.M., Koviack P.D., Maton P.N., Gardner J.D., Jensen R.T. Prospective study of efficacy and safety of lansoprazole in Zollinger-Ellison syndrome. Dig. Dis. Sci. 1993;38:245–256. doi: 10.1007/BF01307541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hirschowitz B.I., Simmons J., Mohnen J. Long-term lansoprazole control of gastric acid and pepsin secretion in ZE and non-ZE hypersecretors: A prospective 10-year study. Aliment. Pharmacol. Ther. 2001;15:1795–1806. doi: 10.1046/j.1365-2036.2001.01097.x. [DOI] [PubMed] [Google Scholar]
- 230.McCarthy D.M., Hyman P.E. Effect of isopropamide on response to oral cimetidine in patients with Zollinger-Ellison syndrome. Dig. Dis. Sci. 1982;27:353–359. doi: 10.1007/BF01296756. [DOI] [PubMed] [Google Scholar]
- 231.Graham D.Y., Tansel A. Interchangeable Use of Proton Pump Inhibitors Based on Relative Potency. Clin. Gastroenterol. Hepatol. 2018;16:800–808. doi: 10.1016/j.cgh.2017.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Carmel R. Diagnosis and management of clinical and subclinical cobalamin deficiencies: Why controversies persist in the age of sensitive metabolic testing. Biochimie. 2013;95:1047–1055. doi: 10.1016/j.biochi.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 233.Allen L.H. How common is vitamin B-12 deficiency? Am. J. Clin. Nutr. 2009;89:693S–696S. doi: 10.3945/ajcn.2008.26947A. [DOI] [PubMed] [Google Scholar]
- 234.Campbell A.K., Miller J.W., Green R., Haan M.N., Allen L.H. Plasma vitamin B-12 concentrations in an elderly latino population are predicted by serum gastrin concentrations and crystalline vitamin B-12 intake. J. Nutr. 2003;133:2770–2776. doi: 10.1093/jn/133.9.2770. [DOI] [PubMed] [Google Scholar]
- 235.Refsum H., Yajnik C.S., Gadkari M., Schneede J., Vollset S.E., Orning L., Guttormsen A.B., Joglekar A., Sayyad M.G., Ulvik A., et al. Hyperhomocysteinemia and elevated methylmalonic acid indicate a high prevalence of cobalamin deficiency in Asian Indians. Am. J. Clin. Nutr. 2001;74:233–241. doi: 10.1093/ajcn/74.2.233. [DOI] [PubMed] [Google Scholar]
- 236.Dierkes J., Domrose U., Ambrosch A., Schneede J., Guttormsen A.B., Neumann K.H., Luley C. Supplementation with vitamin B12 decreases homocysteine and methylmalonic acid but also serum folate in patients with end-stage renal disease. Metabolism. 1999;48:631–635. doi: 10.1016/S0026-0495(99)90062-8. [DOI] [PubMed] [Google Scholar]
- 237.Solomon L.R. Cobalamin-responsive disorders in the ambulatory care setting: Unreliability of cobalamin, methylmalonic acid, and homocysteine testing. Blood. 2005;105:978–985. doi: 10.1182/blood-2004-04-1641. [DOI] [PubMed] [Google Scholar]
- 238.Lewerin C., Ljungman S., Nilsson-Ehle H. Glomerular filtration rate as measured by serum cystatin C is an important determinant of plasma homocysteine and serum methylmalonic acid in the elderly. J. Intern. Med. 2007;261:65–73. doi: 10.1111/j.1365-2796.2006.01732.x. [DOI] [PubMed] [Google Scholar]
- 239.Green R. Indicators for assessing folate and vitamin B12 status and for monitoring the efficacy of intervention strategies. Food Nutr. Bull. 2008;29:S52–S63. doi: 10.1177/15648265080292S108. [DOI] [PubMed] [Google Scholar]
- 240.Green R., Allen L.H., Bjorke-Monsen A.L., Brito A., Gueant J.L., Miller J.W., Molloy A.M., Nexo E., Stabler S., Toh B.H., et al. Vitamin B(12) deficiency. Nat. Rev. Dis. Primers. 2017;3:17040. doi: 10.1038/nrdp.2017.40. [DOI] [PubMed] [Google Scholar]
- 241.Garcia A., Paris-Pombo A., Evans L., Day A., Freedman M. Is low-dose oral cobalamin enough to normalize cobalamin function in older people? J. Am. Geriatr. Soc. 2002;50:1401–1404. doi: 10.1046/j.1532-5415.2002.50362.x. [DOI] [PubMed] [Google Scholar]
- 242.Matchar D.B., McCrory D.C., Millington D.S., Feussner J.R. Performance of the serum cobalamin assay for diagnosis of cobalamin deficiency. Am. J. Med. Sci. 1994;308:276–283. doi: 10.1097/00000441-199411000-00004. [DOI] [PubMed] [Google Scholar]
- 243.Hirschowitz B.I., Keeling D., Lewin M., Okabe S., Parsons M., Sewing K., Wallmark B., Sachs G. Pharmacological aspects of acid secretion. Dig. Dis. Sci. 1995;40:3S–23S. doi: 10.1007/BF02214869. [DOI] [PubMed] [Google Scholar]
- 244.Stewart C.A., Termanini B., Sutliff V.E., Serrano J., Yu F., Gibril F., Jensen R.T. Assessment of the risk of iron malabsorption in patients with Zollinger-Ellison syndrome treated with long-term gastric acid antisecretory therapy. Aliment. Pharmacol. Ther. 1998;12:83–98. doi: 10.1046/j.1365-2036.1998.00274.x. [DOI] [PubMed] [Google Scholar]
- 245.Stenman U.H. Characterization of R-type vitamin B12-binding proteins by isoelectric focusing. II. Comparison of cobalophilin (r proteins) from different sources. Scand. J. Clin. Lab. Investig. 1975;35:147–155. doi: 10.1080/00365517509087218. [DOI] [PubMed] [Google Scholar]
- 246.Stenman U.H. Vitamin B12-binding proteins of r-type, cobalophilin. Scand. J. Haematol. 1975;14:91–107. doi: 10.1111/j.1600-0609.1975.tb00301.x. [DOI] [PubMed] [Google Scholar]
- 247.Gueant J.L., Gueant-Rodriguez R.M., Alpers D.H. Vitamin B12 absorption and malabsorption. Vitam. Horm. 2022;119:241–274. doi: 10.1016/bs.vh.2022.01.016. [DOI] [PubMed] [Google Scholar]
- 248.Rock C.L. Multivitamin-multimineral supplements: Who uses them? Am. J. Clin. Nutr. 2007;85:277S–279S. doi: 10.1093/ajcn/85.1.277S. [DOI] [PubMed] [Google Scholar]
- 249.Goodwin J.C., Wang Q., Lin P.H., Shrubsole M.J., Epplein M. Supplement use and gastric cancer risk in the Southern Community Cohort Study. Cancer Causes Control. 2023;34:897–907. doi: 10.1007/s10552-023-01734-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Salvo E.M., Ferko N.C., Cash S.B., Gonzalez A., Kahrilas P.J. Umbrella review of 42 systematic reviews with meta-analyses: The safety of proton pump inhibitors. Aliment. Pharmacol. Ther. 2021;54:129–143. doi: 10.1111/apt.16407. [DOI] [PubMed] [Google Scholar]
- 251.Arufe M.C., Beckett G.J., Duran R., Alfonso M. Effect of okadaic acid and calyculin-A, two protein phosphatase inhibitors, on thyrotropin-stimulated triiodothyronine secretion in cultured sheep thyroid cells. Endocrine. 1999;11:235–240. doi: 10.1385/ENDO:11:3:235. [DOI] [PubMed] [Google Scholar]
- 252.Waldum H.L., Fossmark R., Bakke I., Martinsen T.C., Qvigstad G. Hypergastrinemia in animals and man: Causes and consequences. Scand. J. Gastroenterol. 2004;39:505–509. doi: 10.1080/00365520410005072. [DOI] [PubMed] [Google Scholar]
- 253.Waldum H.L., Sordal O., Fossmark R. Proton pump inhibitors (PPIs) may cause gastric cancer—Clinical consequences. Scand. J. Gastroenterol. 2018;53:639–642. doi: 10.1080/00365521.2018.1450442. [DOI] [PubMed] [Google Scholar]
- 254.Wormsley K.G. Is chronic long-term inhibition of gastric secretion really dangerous. Scand. J. Gastroenterol. Suppl. 1988;146:166–174. doi: 10.3109/00365528809099143. [DOI] [PubMed] [Google Scholar]
- 255.Dinesh D., Lee J.S., Scott T.M., Tucker K.L., Palacios N. Association between Acid-Lowering Agents, Metformin, and Vitamin B12 among Boston-Area Puerto Ricans. J. Nutr. 2023;153:2380–2388. doi: 10.1016/j.tjnut.2023.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Long A.N., Atwell C.L., Yoo W., Solomon S.S. Vitamin B(12) deficiency associated with concomitant metformin and proton pump inhibitor use. Diabetes Care. 2012;35:e84. doi: 10.2337/dc12-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Corsonello A., Lattanzio F., Bustacchini S., Garasto S., Cozza A., Schepisi R., Lenci F., Luciani F., Maggio M.G., Ticinesi A., et al. Adverse events of proton pump inhibitors: Potential mechanisms. Curr. Drug Metab. 2017;19:142–154. doi: 10.2174/1389200219666171207125351. [DOI] [PubMed] [Google Scholar]
- 258.Haenisch B., von Holt K., Wiese B., Prokein J., Lange C., Ernst A., Brettschneider C., Konig H.H., Werle J., Weyerer S., et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur. Arch. Psychiatry Clin. Neurosci. 2015;265:419–428. doi: 10.1007/s00406-014-0554-0. [DOI] [PubMed] [Google Scholar]
- 259.Batchelor R., Gilmartin J.F., Kemp W., Hopper I., Liew D. Dementia, cognitive impairment and proton pump inhibitor therapy: A systematic review. J. Gastroenterol. Hepatol. 2017;32:1426–1435. doi: 10.1111/jgh.13750. [DOI] [PubMed] [Google Scholar]
- 260.Koyyada A. Long-term use of proton pump inhibitors as a risk factor for various adverse manifestations. Therapie. 2021;76:13–21. doi: 10.1016/j.therap.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 261.Paudel Y., Najam B., Desai H.N., Illango J., Seffah K.D., Kumar M., Naveen N., Pachchipulusu V.K., Penumetcha S.S. Use of Proton Pump Inhibitors and Risk of Fracture in Adults: A Review of Literature. Cureus. 2023;15:e49872. doi: 10.7759/cureus.49872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Targownik L.E., Goertzen A.L., Luo Y., Leslie W.D. Long-Term Proton Pump Inhibitor Use Is Not Associated with Changes in Bone Strength and Structure. Am. J. Gastroenterol. 2017;112:95–101. doi: 10.1038/ajg.2016.481. [DOI] [PubMed] [Google Scholar]
- 263.Nochaiwong S., Ruengorn C., Awiphan R., Koyratkoson K., Chaisai C., Noppakun K., Chongruksut W., Thavorn K. The association between proton pump inhibitor use and the risk of adverse kidney outcomes: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2018;33:331–342. doi: 10.1093/ndt/gfw470. [DOI] [PubMed] [Google Scholar]
- 264.Han C.T., Islam M.M., Poly T.N., Lu Y.C., Lin M.C. A Meta-Analysis of Proton Pump Inhibitor Use and the Risk of Acute Kidney Injury: Geographical Differences and Associated Factors. J. Clin. Med. 2023;12:2467. doi: 10.3390/jcm12072467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Maideen N.M.P. Adverse Effects Associated with Long-Term Use of Proton Pump Inhibitors. Chonnam. Med. J. 2023;59:115–127. doi: 10.4068/cmj.2023.59.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Xie Y., Bowe B., Li T., Xian H., Yan Y., Al-Aly Z. Risk of death among users of Proton Pump Inhibitors: A longitudinal observational cohort study of United States veterans. BMJ Open. 2017;7:e015735. doi: 10.1136/bmjopen-2016-015735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Xie Y., Bowe B., Yan Y., Xian H., Li T., Al-Aly Z. Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: Cohort study. BMJ. 2019;365:l1580. doi: 10.1136/bmj.l1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Brown J.P., Tazare J.R., Williamson E., Mansfield K.E., Evans S.J., Tomlinson L.A., Bhaskaran K., Smeeth L., Wing K., Douglas I.J. Proton pump inhibitors and risk of all-cause and cause-specific mortality: A cohort study. Br. J. Clin. Pharmacol. 2021;87:3150–3161. doi: 10.1111/bcp.14728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Seetharam B. Gastrointestinal absorption and transport of cobalamin (vitamin B12) In: Johnson L.R., Alpers D.H., Christensen J., Jacobson E.D., Walsh J.H., editors. Physiology of the Gastrointestinal Tract. 3rd ed. Volume 2. Raven Press; New York, NY, USA: 1994. pp. 1997–2026. [Google Scholar]
- 270.Dutta S.K. Editorial: Vitamin B12 malabsorption and omeprazole therapy. J. Am. Coll. Nutr. 1994;13:544–545. doi: 10.1080/07315724.1994.10718444. [DOI] [PubMed] [Google Scholar]
- 271.Koop H. Review article: Metabolic consequences of long-term inhibition of acid secretion by omeprazole. Aliment. Pharmacol. Ther. 1992;6:399–406. doi: 10.1111/j.1365-2036.1992.tb00553.x. [DOI] [PubMed] [Google Scholar]
- 272.Shin J.M., Munson K., Vagin O., Sachs G. The gastric HK-ATPase: Structure, function, and inhibition. Pflug. Arch. 2009;457:609–622. doi: 10.1007/s00424-008-0495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Koop H., Bachem M.G. Serum iron, ferritin, and vitamin B12 during prolonged omeprazole therapy. J. Clin. Gastroenterol. 1992;14:288–292. doi: 10.1097/00004836-199206000-00005. [DOI] [PubMed] [Google Scholar]
- 274.Saltzman J.R., Kemp J.A., Golner B.B., Pedrosa M.C., Dallal G.E., Russell R. Effect of hypochlorhydria due to omeprazole treatment or atrophic gastritis on protein-bound vitamin B12 absorption. J. Am. Coll. Nutr. 1994;13:584–591. doi: 10.1080/07315724.1994.10718452. [DOI] [PubMed] [Google Scholar]
- 275.Festen H.P. Intrinsic factor secretion and cobalamin absorption. Physiology and pathophysiology in the gastrointestinal tract. Scand. J. Gastroenterol. 1991;26:1–7. doi: 10.3109/00365529109111222. [DOI] [PubMed] [Google Scholar]
- 276.Messerer M., Wolk A. Sensitivity and specificity of self-reported use of dietary supplements. Eur. J. Clin. Nutr. 2004;58:1669–1671. doi: 10.1038/sj.ejcn.1602021. [DOI] [PubMed] [Google Scholar]
- 277.Marcuard S.P., Albernaz L., Khazanie P.G. Omeprazole therapy causes malabsorption of cyanocobalamin (Vitamin B12) Ann. Intern. Med. 1994;120:211–215. doi: 10.7326/0003-4819-120-3-199402010-00006. [DOI] [PubMed] [Google Scholar]
- 278.Festen H., Tertoolen J. Malabsorption of protein-bound but not unbound cobalamin during treatment with omeprazole. Gastroenterology. 1988;94:A125. [Google Scholar]
- 279.Steinberg W.M., King C.E., Toskes P.P. Malabsorption of protein-bound cobalamin but not unbound cobalamin during cimetidine administration. Dig. Dis. Sci. 1980;25:188–191. doi: 10.1007/BF01308137. [DOI] [PubMed] [Google Scholar]
- 280.Porter K.M., Hoey L., Hughes C.F., Ward M., Clements M., Strain J., Cunningham C., Casey M.C., Tracey F., O’Kane M., et al. Associations of atrophic gastritis and proton-pump inhibitor drug use with vitamin B-12 status, and the impact of fortified foods, in older adults. Am. J. Clin. Nutr. 2021;114:1286–1294. doi: 10.1093/ajcn/nqab193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Bahardoust M., Mousavi S., Ziafati H., Alipour H., Haghmoradi M., Olamaeian F., Tayebi A., Tizmaghz A. Vitamin B12 deficiency after total gastrectomy for gastric cancer, prevalence, and symptoms: A systematic review and meta-analysis. Eur. J. Cancer Prev. 2024;33:208–216. doi: 10.1097/CEJ.0000000000000838. [DOI] [PubMed] [Google Scholar]
- 282.Herrmann W., Schorr H., Obeid R., Geisel J. Vitamin B-12 status, particularly holotranscobalamin II and methylmalonic acid concentrations, and hyperhomocysteinemia in vegetarians. Am. J. Clin. Nutr. 2003;78:131–136. doi: 10.1093/ajcn/78.1.131. [DOI] [PubMed] [Google Scholar]
- 283.Calvete O., Reyes J., Zuniga S., Paumard-Hernandez B., Fernandez V., Bujanda L., Rodriguez-Pinilla M.S., Palacios J., Heine-Suner D., Banka S., et al. Exome sequencing identifies ATP4A gene as responsible of an atypical familial type I gastric neuroendocrine tumour. Hum. Mol. Genet. 2015;24:2914–2922. doi: 10.1093/hmg/ddv054. [DOI] [PubMed] [Google Scholar]
- 284.Alpers D.H., Russell-Jones G. Gastric intrinsic factor: The gastric and small intestinal stages of cobalamin absorption. a personal journey. Biochimie. 2013;95:989–994. doi: 10.1016/j.biochi.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 285.Losurdo G., Caccavo N.L.B., Indellicati G., Celiberto F., Ierardi E., Barone M., Di Leo A. Effect of Long-Term Proton Pump Inhibitor Use on Blood Vitamins and Minerals: A Primary Care Setting Study. J. Clin. Med. 2023;12:2910. doi: 10.3390/jcm12082910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Singh A., Cresci G.A., Kirby D.F. Proton Pump Inhibitors: Risks and Rewards and Emerging Consequences to the Gut Microbiome. Nutr. Clin. Pract. 2018;33:614–624. doi: 10.1002/ncp.10181. [DOI] [PubMed] [Google Scholar]
- 287.Carmel R., Aurangzeb I., Qian D. Associations of food-cobalamin malabsorption with ethnic origin, age, Helicobacter pylori infection, and serum markers of gastritis. Am. J. Gastroenterol. 2001;96:63–70. doi: 10.1111/j.1572-0241.2001.03453.x. [DOI] [PubMed] [Google Scholar]
- 288.Kittang E., Aadland E., Schjonsby H., Rohss K. The effect of omeprazole on gastric acidity and the absorption of liver cobalamins. Scand. J. Gastroenterol. 1987;22:156–160. doi: 10.3109/00365528708991873. [DOI] [PubMed] [Google Scholar]
- 289.Kittang E., Aadlang E., Schonsby H. Effect of omeprazole on the secretion of intrinsic factor, gastric acid and pepsin in man. Gut. 1985;26:594–598. doi: 10.1136/gut.26.6.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Griffith J.L., Cummings O.W., Hirschowitz B.I. Development of sustained achlorhydria in a patient with the Zollinger-Ellison syndrome treated with omeprazole. Gastroenterology. 1991;101:242–246. doi: 10.1016/0016-5085(91)90484-3. [DOI] [PubMed] [Google Scholar]
- 291.King C.E., Toskes P.P. Nutrient malabsorption in the Zollinger-Ellison Syndrome. Arch. Intern. Med. 1983;143:349–351. doi: 10.1001/archinte.1983.00350020175033. [DOI] [PubMed] [Google Scholar]
- 292.Shum H.Y., O’Neill B.J., Streeter A.M. Vitamin-B 12 absorption and the Zollinger-Ellison syndrome. Lancet. 1971;1:1303. doi: 10.1016/S0140-6736(71)91824-1. [DOI] [PubMed] [Google Scholar]
- 293.Shimoda S.S., Saunders D.R., Rubin C.E. The Zollinger-Ellison syndrome with steatorrhea. II. The mechanism of fat and vitamin B 12 malabsorption. Gastroenterology. 1968;55:705–723. doi: 10.1016/S0016-5085(19)33989-7. [DOI] [PubMed] [Google Scholar]
- 294.Desai H.G., Antia F.P. Spontaneous achlorhydria with atrophic gastritis in the Zollinger-Ellison syndrome. Gut. 1969;10:935–939. doi: 10.1136/gut.10.11.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Lawrie R., Hunt J.N., Williamson A.W.R. Treament of the Zollinger-Ellison syndrome. Lancet. 1966;1:203. doi: 10.1016/S0140-6736(66)90726-4. [DOI] [PubMed] [Google Scholar]
- 296.Melnyk C.S., Krippaehne W.W., Benson J.A., Dunphy J.E. Spontaneous remission in Zollinger-Ellison syndrome. Arch. Intern. Med. 1965;115:42–47. doi: 10.1001/archinte.1965.03860130044007. [DOI] [PubMed] [Google Scholar]
- 297.Ramsey E.J., Carey K.V., Peterson W.L., Jackson J.J., Murphy F.K., Read N.W., Taylor K.B., Trier J.S., Fordtran J.S. Epidemic gastritis with hypochlorhydria. Gastroenterology. 1979;76:1449–1457. doi: 10.1016/0016-5085(79)90415-3. [DOI] [PubMed] [Google Scholar]
- 298.Wiersinga W.M., Tytgat G.N. Clinical recovery owing to target parietal cell failure in a patient with Zollinger-Ellison syndrome. Gastroenterology. 1977;73:1413–1417. doi: 10.1016/S0016-5085(19)31525-2. [DOI] [PubMed] [Google Scholar]
- 299.Maret-Ouda J., Markar S.R., Lagergren J. Gastroesophageal Reflux Disease: A Review. JAMA. 2020;324:2536–2547. doi: 10.1001/jama.2020.21360. [DOI] [PubMed] [Google Scholar]
- 300.Zhang H., Yang Z., Ni Z., Shi Y. A Meta-Analysis and Systematic Review of the Efficacy of Twice Daily PPIs versus Once Daily for Treatment of Gastroesophageal Reflux Disease. Gastroenterol. Res. Pract. 2017;2017:9865963. doi: 10.1155/2017/9865963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Fass R., Shapiro M., Dekel R., Sewell J. Systematic review: Proton-pump inhibitor failure in gastro-oesophageal reflux disease--where next? Aliment. Pharmacol. Ther. 2005;22:79–94. doi: 10.1111/j.1365-2036.2005.02531.x. [DOI] [PubMed] [Google Scholar]
- 302.Meijer J.L., Jansen J.B., Lamers C.B. Omeprazole in the treatment of Zollinger-Ellison syndrome and histamine H2-antagonist refractory ulcers. Digestion. 1989;44((Suppl. S1)):31–39. doi: 10.1159/000200102. [DOI] [PubMed] [Google Scholar]
- 303.Kinoshita Y., Hongo M. Efficacy of twice-daily rabeprazole for reflux esophagitis patients refractory to standard once-daily administration of PPI: The Japan-based TWICE study. Am. J. Gastroenterol. 2012;107:522–530. doi: 10.1038/ajg.2012.19. [DOI] [PubMed] [Google Scholar]
- 304.Fuchs H.E., O’Connell K., Du M., Navarro S.L., Brasky T.M., Kantor E.D. Vitamin B(12) Supplementation and Vitamin B(12) Blood Serum Levels: Evaluation of Effect Modification by Gender and Smoking Status. Nutr. Cancer. 2022;74:2373–2383. doi: 10.1080/01635581.2021.2007271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Kancherla V., Garn J.V., Zakai N.A., Williamson R.S., Cashion W.T., Odewole O., Judd S.E., Oakley G.P., Jr. Multivitamin Use and Serum Vitamin B12 Concentrations in Older-Adult Metformin Users in REGARDS, 2003–2007. PLoS ONE. 2016;11:e0160802. doi: 10.1371/journal.pone.0160802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Pflipsen M.C., Oh R.C., Saguil A., Seehusen D.A., Seaquist D., Topolski R. The prevalence of vitamin B(12) deficiency in patients with type 2 diabetes: A cross-sectional study. J. Am. Board. Fam. Med. 2009;22:528–534. doi: 10.3122/jabfm.2009.05.090044. [DOI] [PubMed] [Google Scholar]
- 307.Evatt M.L., Terry P.D., Ziegler T.R., Oakley G.P. Association between vitamin B12-containing supplement consumption and prevalence of biochemically defined B12 deficiency in adults in NHANES III (third national health and nutrition examination survey) Public. Health Nutr. 2010;13:25–31. doi: 10.1017/S1368980009990279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Dorant E., van den Brandt P.A., Goldbohm R.A., Hermus R.J., Sturmans F. Agreement between interview data and a self-administered questionnaire on dietary supplement use. Eur. J. Clin. Nutr. 1994;48:180–188. [PubMed] [Google Scholar]
- 309.Ishihara J., Sobue T., Yamamoto S., Sasaki S., Akabane M., Tsugane S. Validity and reproducibility of a self-administered questionnaire to determine dietary supplement users among Japanese. Eur. J. Clin. Nutr. 2001;55:360–365. doi: 10.1038/sj.ejcn.1601164. [DOI] [PubMed] [Google Scholar]
- 310.Berna M.J., Hoffmann K.M., Serrano J., Gibril F., Jensen R.T. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine. 2006;85:295–330. doi: 10.1097/01.md.0000236956.74128.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Ito T., Igarashi H., Jensen R.T. Zollinger-Ellison syndrome: Recent advances and controversies. Curr. Opin. Gastroenterol. 2013;29:650–661. doi: 10.1097/MOG.0b013e328365efb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Wank S.A., Doppman J.L., Miller D.L., Collen M.J., Maton P.N., Vinayek R., Slaff J.I., Norton J.A., Gardner J.D., Jensen R.T. Prospective study of the ability of computerized axial tomography to localize gastrinomas in patients with Zollinger-Ellison syndrome. Gastroenterology. 1987;92:905–912. doi: 10.1016/0016-5085(87)90963-2. [DOI] [PubMed] [Google Scholar]
- 313.Frucht H., Doppman J.L., Norton J.A., Miller D.L., Dwyer A.J., Frank J.A., Vinayek R., Maton P.N., Jensen R.T. Gastrinomas: Comparison of MR Imaging with CT, angiography and US. Radiology. 1989;171:713–717. doi: 10.1148/radiology.171.3.2655004. [DOI] [PubMed] [Google Scholar]
- 314.Gibril F., Reynolds J.C., Doppman J.L., Chen C.C., Venzon D.J., Termanini B., Weber H.C., Stewart C.A., Jensen R.T. Somatostatin receptor scintigraphy: Its sensitivity compared with that of other imaging methods in detecting primary and metastatic gastrinomas: A prospective study. Ann. Intern. Med. 1996;125:26–34. doi: 10.7326/0003-4819-125-1-199607010-00005. [DOI] [PubMed] [Google Scholar]
- 315.Gibril F., Doppman J.L., Reynolds J.C., Chen C.C., Sutliff V.E., Yu F., Serrano J., Venzon D.J., Jensen R.T. Bone metastases in patients with gastrinomas: A prospective study of bone scanning, somatostatin receptor scanning, and MRI in their detection, their frequency, location and effect of their detection on management. J. Clin. Oncol. 1998;16:1040–1053. doi: 10.1200/JCO.1998.16.3.1040. [DOI] [PubMed] [Google Scholar]
- 316.Maton P.N., Miller D.L., Doppman J.L., Collen M.J., Norton J.A., Vinayek R., Slaff J.I., Wank S.A., Gardner J.D., Jensen R.T. Role of selective angiography in the management of Zollinger- Ellison syndrome. Gastroenterology. 1987;92:913–918. doi: 10.1016/0016-5085(87)90964-4. [DOI] [PubMed] [Google Scholar]
- 317.Thom A.K., Norton J.A., Doppman J.L., Chang R., Miller D.L., Jensen R.T. Prospective study of the use of intraarterial secretin injection and portal venous sampling to localize duodenal gastrinomas. Surgery. 1992;112:1002–1008. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.