Abstract
The hippocampus maintains a capacity for neurogenesis throughout life, a capacity that is reduced in models of adult onset hypothyroidism. The effects of developmental thyroid hormone (TH) insufficiency on neurogenesis in the adult hippocampus, however, has not been examined. Graded degrees of TH insufficiency were induced in pregnant rat dams by administration of 0, 3, 10 ppm of 6-propylthiouracil (PTU) in drinking water from gestational day (GD) 6 until weaning. Body, brain, and hippocampal weight were reduced on postnatal day (PN) 14, 21, 78 and hippocampal volume was smaller at the 10 but not 3 ppm dose level. A second experiment examined adult hippocampal neurogenesis following developmental or adult onset hypothyroidism. Two male offspring from 0 and 3 ppm exposed dams were either maintained on control water or exposed to 3 ppm PTU to create 4 distinct treatment conditions (Control-Control; Control-PTU, PTU-Control, PTU-PTU) based on developmental and adult exposures. Beginning on the 29th day of PTU exposure, bromodeoxyuridine (BrdU, 50mg/kg, ip) was administered twice daily for 5 days, and one male from each treatment was sacrificed 24 h and 28 days after the last BrdU dose and brains processed for immunohistochemistry. Although no volume changes were seen in the hippocampus of the neonate at 3 ppm, thinning of the granule cell layer emerged in adulthood. Developmental TH insufficiency produced a reduction in newly born cells, reducing BrdU+ve cells at 1 with no further reduction at 28-days post-BrdU. Similar findings were obtained using the proliferative cell marker Ki67. Neuronal differentiations was also altered with fewer doublecortin (Dcx) expressing cells and a higher proportion of immature Dcx phenotypes seen after developmental but not adult TH insufficiency. An impaired capacity for neurogenesis may contribute to impairments in synaptic plasticity and cognitive deficits previously reported in our laboratory and others following moderate degrees of developmental TH insufficiency induced by this PTU model.
Keywords: hypothyroidism, hippocampus, neurogenesis, brain development, dentate gyrus, developmental neurotoxicity, thyroid hormone
1.1. INTRODUCTION
Thyroid hormone (TH) is critical for brain organization during early development and for normal brain function throughout life (Bernal, 2002, Williams, 2008). In the developing brain, TH is required for optimal neurogenesis, synaptogenesis, neuronal migration, plasticity, and myelination (Auso et al., 2004, Morreale de Escobar et al., 2004, Berbel et al., 2010, Mohan et al., 2012). While severe restrictions of TH undermine these developmental processes producing smaller animals with smaller brains, with particular sensitivity in the hippocampus (Rami et al., 1986a, Rami et al., 1986b, Madeira et al., 1988, Rami and Rabie, 1990, Madeira et al., 1991, Madeira et al., 1992, Hasegawa et al., 2010, Powell et al., 2012), the dose-response relationship of more modest degrees of TH deprivation have not been widely reported. The dentate gyrus (DG) of the hippocampus also represents a brain region where neurogenesis, a process typically relegated to the immature brain, continues throughout life. A significant fraction of these newly born granule cells integrate into the existing DG circuitry to support learning and memory, modulate affect, and respond to injury (Jacobs et al., 2000, Kempermann et al., 2000, Shors et al., 2001, Santarelli et al., 2003, Snyder et al., 2005, Laplagne et al., 2006, Saxe et al., 2006, Samuels and Hen, 2011, Christie and Turnley, 2012, Turnley et al., 2014).
Data from several laboratories have implicated TH in the proliferation of neural stem/progenitor cells (NPC) in the adult rodent brain (Montero-Pedrazuela et al., 2006, Lopez-Juarez et al., 2012, Shiraki et al., 2012b), while others have reported that adult onset hypothyroidism reduces the survival of newly born neurons (Ambrogini et al., 2005, Desouza et al., 2005, Zhang et al., 2009, Kapoor et al., 2011, Kapoor, 2012). These observations derive from studying neurogenesis in adult animals with adult onset hypothyroidism, and under conditions of very severe reductions in TH. It is unclear if milder degrees of TH insult, closer to those that would accompany exposure to environmental contaminants, sub-clinical hypothyroidism, or hypothyroxinemia, are sufficient to impair adult neurogenesis. Neither is it known if transient TH deficiencies initiated in utero but from which animals recover could lead to a persistent impairment adult neurogenesis.
In the present study, we examined the dose-dependency of TH insufficiency induced alterations of common anatomical metrics including brain and hippocampal weight, and hippocampal volume. Despite a wealth of data demonstrating the devastating effects on neuronal development accompanying severe hypothyroidism, relatively few reports of low level, dose-dependent, quantitative assessments are available at relatively modest degrees of developmental hypothyroidism useful for computational modeling. A second study assessed hippocampal neurogenesis in adult offspring of dams experiencing moderate degrees of TH disruption induced by propylthiouracil (PTU). One objective was to examine the potential lasting effects of transient developmental hypothyroidism on neurogenesis in the adult following return to euthyroid status. An additional question focused on the exposure to a similar dose in the adult. Finally, a third objective was to determine if developmental hypothyroidism increased the vulnerability to subsequent hypothyroid insult in adulthood. We report that moderate levels of TH insufficiency limited to adulthood did not affect adult neurogenesis, but developmental hypothyroidism resulted in a significant reduction in cell proliferation/early survival phase of the neurogenesis process. This impairment was accompanied by a reduction in volume of the DG granule cell layer (GCL), an effect that was not detected until adulthood. Additional thyroid insult imposed on the adult after perinatal TH compromise did not produce further decrements in neurogenesis. Deficiencies in adult neurogenesis that persist in response to developmental hormone insufficiency may contribute to impairments in synaptic transmission, long-term potentiation, and hippocampally-based learning and memory previously reported by our laboratory with this low dose PTU model (Gilbert and Sui, 2006, Gilbert, 2011, Gilbert et al., 2016).
2.1. EXPERIMENTAL PROCEDURES
2.1.1. Animals and Treatment:
Pregnant Long-Evans (LE) rats were obtained from Charles River (Raleigh, NC) on gestational day (GD) 2 and housed individually in standard plastic hanging cages in an AAALAC-approved animal facility. All animal treatments were in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animal rooms were maintained on a 12:12 light:dark schedule, and animals were permitted free access to food (Purina 5008 rat chow) and filtered tap water. In Experiment 1 (Figure 1A), pregnant dams were exposed to 0, 3 (0.0003%) or 10 (0.001%) ppm of PTU (Sigma, St. Louis, MO) in the drinking water from GD6 to postnatal day (PN) 30 when all pups were weaned to control drinking water. The lower dose was designed to emulate maternal hypothyroxinemia (reductions in circulating levels of thyroxine, T4), the high dose to induce hypothyroidism (significant reductions in both T4 and triiodothyronine, T3) as previously determined in our laboratory (Gilbert and Sui, 2006, Gilbert, 2011, Gilbert et al., 2016). All litters were culled to 10 pups (keeping the maximum number of males) on PN4. At weaning, pups were transferred to plastic hanging cages (2/cage with same sex littermate), and were permitted free access to food (Purina 5001) and filtered tap water. Pups were weaned on PN30 in this study to increase survivability of offspring in the high dose group. One male pup from each litter was sacrificed by decapitation on PN4 (at cull), PN14, PN21 and PN78, the brain removed and weighed. The hippocampus was dissected and weighed from animals on PN14, 21 and 78. Hippocampal weights were not taken in the PN4 group. On PN23, one male pup per litter was perfused intracardially and prepared for histological assessment.
Figure 1.
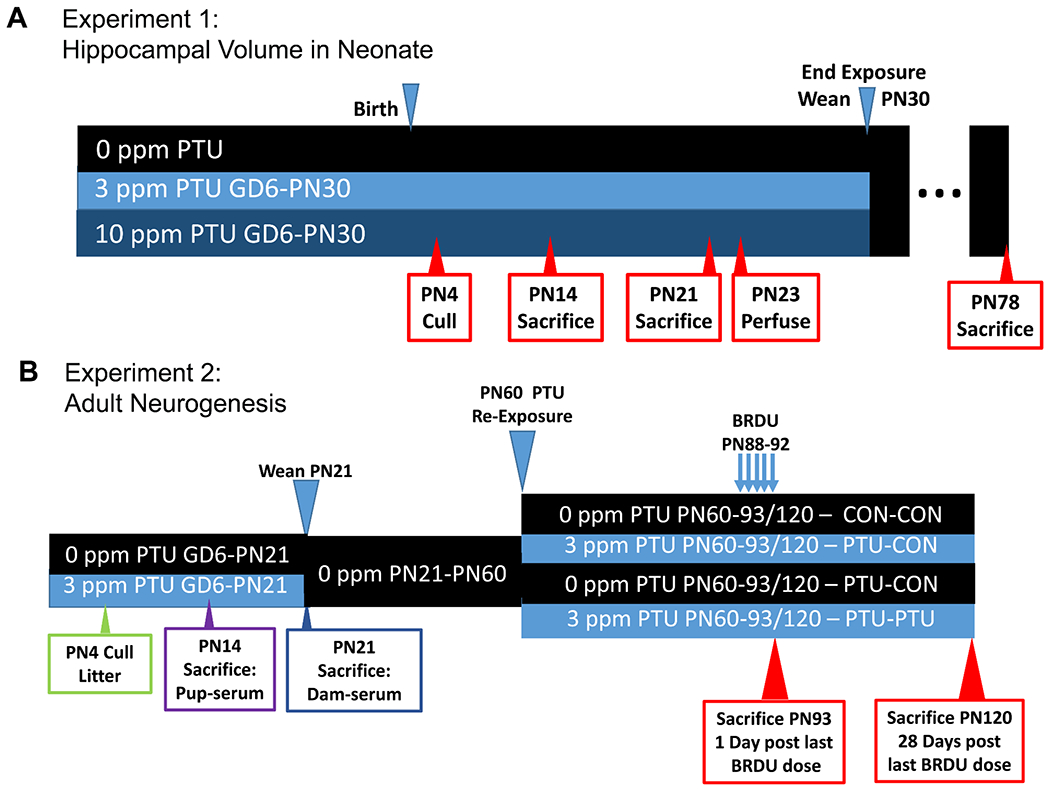
Schematics for exposure and tissue collection for neonatal body and brain weights and hippocampal size of Experiment 1 (A) and adult neurogenesis of Experiment 2 (B).
In Experiment 2, two groups of pregnant dams (10-11 dams/dose) were exposed to 0 or 3 ppm PTU beginning on GD6 and ending on PN21. Animals were weaned on PN21 and pair housed with littermates. On PN60, four males from each litter were selected. Two of these male pups were exposed to 3 ppm PTU, the other two remained on control water. This created four treatment conditions, offspring from control (Con) litters that were administered PTU as adults (Con-PTU), offspring from PTU litters that were either re-exposed to PTU as adults (PTU-PTU) or remained on control drinking water (PTU-Con), and offspring only exposed to control water (Con-Con). Beginning on the 28th day of adult exposure to 0 or 3ppm PTU (PN60-PN88) all animals received an ip injection of BrdU (50mg/kg; 25 mg/ml solution; Sigma) twice daily at 8-hr intervals (8:00 am and 4:00 pm) for 5 consecutive days for incorporation into mitotic cells in the S-phase of the cell cycle (Kee et al., 2002). Twenty-four hours after the last injection, half of the animals were perfused intracardially and prepared for histological analysis. The remaining animals continued on control or PTU conditions and were sacrificed 28 days after the last injection. The short animal survival time of 24-hours after the last BrdU injection was used to investigate the early ‘proliferative phase’ of NPC. The longer survival time of 28 days was used to monitor long-term cell survival and integration into the granule cell layer (GCL). It is recognized that the early timepoint assessed after 5 consecutive days of dosing with BrdU represents a mix of proliferating cells and newly born cells in the early phase of survival, and will be referred to as ‘proliferation/early survival phase’. In contrast, the 28-day survival time will be referred to as ‘long-term survival phase’. This dosing regime was chosen as it used relatively low doses of BrdU, circumventing cytotoxicity that accompanies higher doses, while still labeling a sufficient number of cells for analysis. It is based on paradigms previously described in the literature to identify changes due to long-lasting treatment manipulations as is hypothyroidism (e.g., aging, exercise, chronic ethanol exposure, environmental housing, epilepsy, viral infection) (Kempermann et al., 1998, Scharfman et al., 2000, Cameron and McKay, 2001, Sharma et al., 2002, Herrera et al., 2003, Gilbert et al., 2005). The PTU dosing regimen and schedule of BrdU treatment and sacrifice are summarized in Figure 1B.
Intracardiac Perfusions.
Animals were sacrificed by administering an overdose of Euthasol® (100 mg/kg, ip). In Experiment 1, serum TH was assessed from trunk blood collected in littermates of animals sacrificed on PN21, while in Experiment 2, blood was collected from the heart at the time of perfusion. Animals were perfused through the heart with phosphate buffered saline (PBS; pH 7.2) followed by a 4% solution of paraformaldehyde. Following perfusion, brains were allowed to post-fix in the cranium overnight in 4% paraformaldehyde then excised from the cranium and placed in 4% paraformaldehyde/PBS for 3-5 days before transfer to an antigen preserve cryoprotectant solution (de Olmos, 1977) and storage at 4°C until histological processing.
Histology.
Brains from neonates (PN23) in Experiment 1 were vibratome-sectioned at 50μM in the coronal plane throughout the dorsal hippocampus. Sections were collected in a 1-in-5 series with 250 μM between each section in the series. One set of sections from each animals was mounted on slides for Nissl staining. In Experiment 2, 7-9 animals/treatment condition from each of 4 treatment conditions and 2 post-BrdU survival times (1 and 28 days) were embedded in a 4 16-brain gelatin matrix using MultiBrain® Technology (NeuroScience Associates, Knoxville, TN). Only 1 animal/litter is represented for any given treatment and survival time. The MultiBrain® blocks were sectioned coronally on a freezing microtome at 50μm, every 8th section collected for staining, and stereological analysis of volume and cell number. Free floating sections were incubated overnight at room temperature with a primary BrdU-antibody primary (Novus, NB 500-235, 1:40,000) followed by application of a biotinylated secondary antibody (Vector, BA-6000, 1:238), avidin-biotin-HRP complex (Vectastain Elite ABC, Vector, Burlingame, CA), and nickel diaminobenzidine tetrahydrochloride (Ni-DAB) as the chromogen. Sections were rinsed, mounted on gelatin coated glass slides, air dried, and counterstained with a Hematoxylin and coverslipped. Two additional sets of sections 1 of 8 series were similarly processed for Ki67 (VP-RM04; Vector Laboratories, Burlingame, CA, USA; 1:1000) to identify proliferating cells (Kee et al., 2002), and doublecortin (Dcx, SC-8066; Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1: 200) to identify immature dentate granule neurons (Ramos et al., 2013).
Hippocampal Subregion Volume and Morphometrics in the Neonate.
One set of stereologically collected Nissl-stained sections from each animal was examined using an Olympus BH-51 microscope under a 4X objective. A minimum of 5 sections was evaluated from each brain collected on PN23. Random sampling began on sections where both blades of the DG were present and attached at the medial aspect (~Plate 20 of Paxinos and Watson (1989)), and extended to that depicted in Plate 23. Using this stereological approach, area measurements were collected on 5 hippocampal subregions ; Cornu Ammonis (CA)1 pyramidal cell layer; CA1 – Stratum radiatum (apical dendritic zone); CA3 – pyramidal cell layer, DG granule cell layer (GCL); DG molecular layer and the DG hilus, for each section from every animal using the Cavalieri estimator (StereoInvestigator™ software, MicroBrightfield, Williston, Vermont; Figure 3A). Regional volumes were calculated by summing the areas within the hippocampus of right hemisphere for each brain, multiplying by section thickness (i.e., 50μM) and section interval (i.e., 5). Linear estimates of these same subregions were collected from the same hemisphere of each section in the series according to the scheme depicted in Figure 3B. Mean length/region was calculated across all 5 sections from for each animal and these means compared across dose groups.
Figure 3.
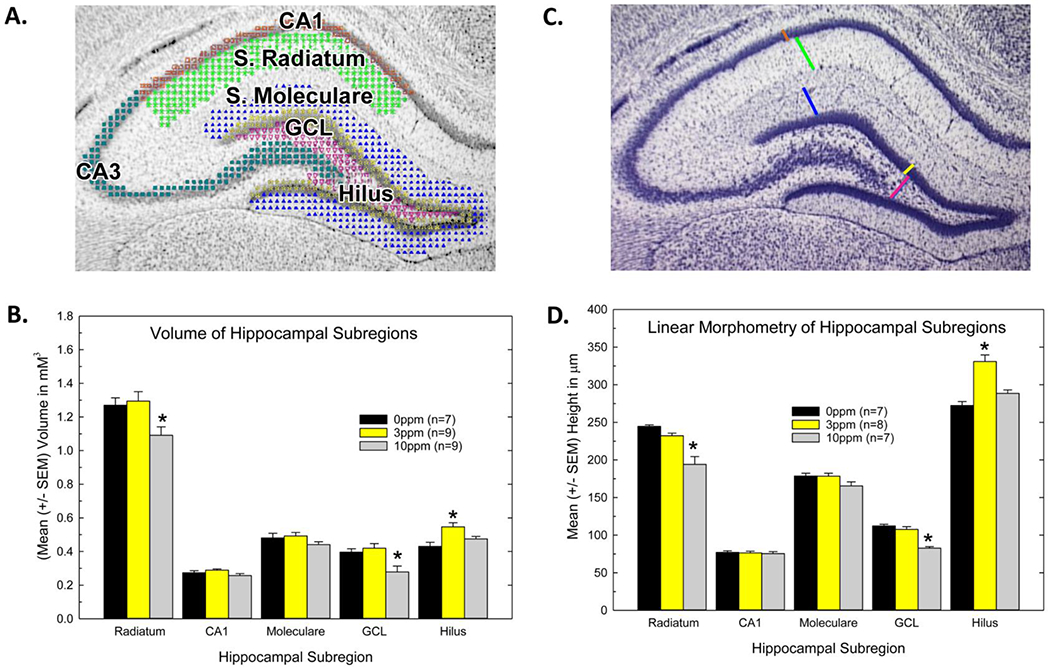
Hippocampal structure in neonatal offspring of PTU-treated dams. A) Volumetric analysis was performed in five subregions of the hippocampus as depicted across 5 sections sampled at 250 μm intervals throughout the dorsal hippocampus. B) Reductions in the volume of the CA1 dendritic field stratum radiatum and granule cell layer (GCL) were observed and restricted to the high dose group. Increases in volume and were seen in the hilar region at the low dose, with no change at the high dose. C) Linear estimates of the hippocampus were assessed in the same sections as shown B by estimating the length of the line as depicted. A mean for each region was calculated across the section series for each animal. D) A similar pattern detected with volume was evident using simple morphometry techniques. Mean contrast tests after significant ANOVA confirmed reductions in volume and length were evident in the high dose group. Dunnett’s T-test, * p<0.05
Dentate Gyrus Volume and Adult Neurogenesis.
Digital photomicrographs of the DG in adult brains were captured using an Olympus BH-51 microscope. The GCL in every section for which BrdU cells were to be counted was traced at 10X using Nikon NIS (ver. 3.22) software. The mean area of the GCL for each animal was calculated by summing the individual GCL areas and dividing by the number of sections examined. The volume of GCL for each animal was calculated similarly to that described above in the neonate, but in this case GCL areas for both hemispheres were summed. Both hemispheres were examined to allow a more reliable estimate for calculation of BrdU-positive cell number and density. As above, volume was calculated using Cavalieri’s principle (Gundersen and Jensen, 1987) by summing total GCL areas across all sections, multiplied by section thickness (i.e., 50μm) and section interval (i.e., 8).
Cell Counts.
Cells expressing BrdU, Ki67, and Dcx were exhaustively counted in every 8th section throughout the dorsal hippocampus. Both superior and inferior blades of the DG were counted by an experimenter blinded to treatment condition. Sections were examined using a VANOX microscope at a magnification level of 40X. The first section in the series chosen for each animals was one in which both blades of the DG were present and attached at the medial aspect, corresponding to ~Plate 20 of Paxinos and Watson (1989). Counting of each 1-in-8 sections continued until the DG extended ventrally and merged with the ventral hippocampus (~Plate 26), typically 6-8 sections/animal. Cell number was expressed in two ways. First, a simple mean of cells/hemisphere of a single section was estimated by summing the total number of BrdU-positive cells across all sections evaluated and dividing by the number of hemispheres examined (i.e., typically 12-14). Total number of cells within the entire hippocampus was also estimated stereologically by summing the cells in GCL of each section examined, multiplying by section thickness (50μM) and section interval (8). Cell density within the GCL of the whole hippocampus was then calculated by dividing the total cell count across all sections by GCL volume (i.e., number of BrdU-positive cells/mM3 of GCL).
Ki67-positive cells were counted in a parallel series of sections. Dcx-positive cells were more numerous so counting was restricted to one hemisphere in each brain section. Similar to procedures described for BrdU, mean cell count/section, total cell number/hippocampus, and cell density as a function of GCL volume were also calculated for Ki67 and Dcx.
Granule Cell Maturation.
Dcx is expressed during the initial steps of neuronal differentiation and is maintained as the cells develop before switching to a fully mature neuronal phenotype that expresses NeuN and not Dcx. Initially Dcx-expressing cells can be seen as nonpolarized cells with short blunt processes running parallel to the granule cell layer. Over time, highly polarized cells emerge that extend their processes into the molecular layer and exhibit rich dendritic branching. The maturity of Dcx-expressing cells was determined based on developmental stages previously described by Plumpe et al. (2006) and Workman et al. (2015). A subset of the Dcx –stained sections described above (condition=4/treatment condition) was examined using the optical fractionator probe (StereoInvestigator™ software, MicroBrightfield, Williston, Vermont). This group of animals was taken from the 28-day survival timepoint such that the adult exposure to PTU was 9 weeks. In a minimum of 5 sections, the GCL in both hemispheres was outlined at a magnification of 10X. The software randomly placed a series of counting frames (15μM X 15μM), throughout the contour and the analysis of cellular morphology was performed at a magnification of 40X. Only those cell bodies falling within counting frame for which the presence or absence of a clearly defined dendrite could be determined assigned to one of three developmental stages as described below. Counting frames that did not contain any cells or contained cells whose dendrite could not be resolved were skipped. Using this method, the mean number of Dcx-labeled cells evaluated for each of 4 animals in each treatment condition ranged from 230-300 cells. Cells were classified as being in one of three developmental stages based on morphological attributes: 1) immature, no processes or short, stunted processes parallel to the GCL 2) intermediate, one thin or medium process extending towards the molecular layer; 3) mature, at least one long branching dendrite reaching the molecular layer.
Serum Hormones.
Serum hormones from trunk blood collected from dams and pups at the time of weaning for both experiments were previously reported (Gilbert and Sui, 2006, Lasley and Gilbert, 2011). Similar radioimmunoassay procedures were performed on blood collected at the time of perfusion from all animals treated with BrdU for neurogenesis studies.
Statistical Analyses.
Standard general linear models analysis of variance (ANOVA) were performed using SAS (version 9.2, SAS Institute, Cary, NC). Body weights of dams and pups, serum TH, and GCL volumes were evaluated using one-way ANOVAs. Hippocampal morphometrics were evaluated using a 2-factor ANOVA (PTU dose and hippocampal subregion). Step-down ANOVAs by region were conducted in the presence of a significant interaction in the overall ANOVA. Mean BrdU cell count and BrdU-positive cell densities were assessed using a two-factor ANOVA for Treatment, Survival Time (1 or 28 days), and Treatment X Survival Time interaction. Ki67 and Dcx mean cell counts and densities were evaluated using one-way ANOVAs to assess the effect of Treatment. Mean contrast tests for Day and Treatment conditions utilized Dunnett’s or Duncan’s t-test where appropriate. For all analyses, the alpha level was set at 0.05.
RESULTS
Serum Thyroid Hormones.
On PN21, serum T4 was reduced by 75% at 3 ppm and 90% at 10 ppm dose levels in animals comprising the morphological data reported in Experiment 1 (hormone data previously reported in (Gilbert and Sui, 2006) but is included here for completeness). Similar reductions in serum T4 were observed at weaning for pups of Experiment 2 who as adults were examined for hippocampal neurogenesis. Hormone profiles in dams and neonates of this cohort of animals were previously reported in Lasley and Gilbert (2011). As adults, exposure to 3 ppm of PTU significantly reduced serum T4 in both PTU-groups exposed at the time of sacrifice (Con-PTU and PTU-PTU), with no differences between Con-Con and PTU-Con groups (Table 1). Interestingly, animals with a previous history of developmental exposure to PTU exhibited greater declines in serum T4 than their naïve counterparts (compare with PTU-PTU with Con-PTU).
Table 1.
Serum T4 (Mean±SEM) in adults at time of sacrifice for neurogenesis was reduced in animals exposed at the time of sacrifice. Recovery to control levels was seen in animals exposed until weaning. T4: [F(3,82)=57.44, p<0.0001] ; T3: [F(3,82)=7.76, p<0.0001].
| Treatment | n | T4 ng/ml | T3 ng/dl |
|---|---|---|---|
| Con-Con | 20 | 42.4±2.3 | 59.8±3.4 |
| Con-PTU | 22 | 18.7±2.4* | 54.2±2.3 |
| PTU-Con | 22 | 36.6±1.2 | 50.3±1.9 |
| PTU-PTU | 22 | 10.8±1.4*# | 44.1±1.6* |
Duncan’s Multiple Range Test, p<0.05 different from Con-Con,
different from Con-PTU. Adult exposure to PTU appeared to have a greater effect on serum TH in animals previously experiencing thyroid disruption during development.
Brain Weight and Hippocampal Morphometrics in the Neonate.
Pup body weight was similar across dose groups on PN4, but lower weights were evident on PN14 [F(2,30)=4.49, p<0.02] and PN21 [F(2,29)=17.45, p<0.0001] and persisted to PN78 [F(2,25)=9.24, p<0.001] despite removal of PTU and recovery of serum hormones. Body weight deficits were largely restricted to the high dose group (10 ppm) although a s slight and transient body weight deficit was seen in the low dose group (3 ppm) on PN21 (Figure 2A). Whole brain and hippocampus weights were also reduced, but these changes in brain did not emerge until PN21 [F(2,29)=6.44, p<0.0048] and in hippocampus not until adulthood [F(2,25)=4.4, p<0.03] and in all cases were limited to the high dose group (Figure 2B and 2C).
Figure 2.
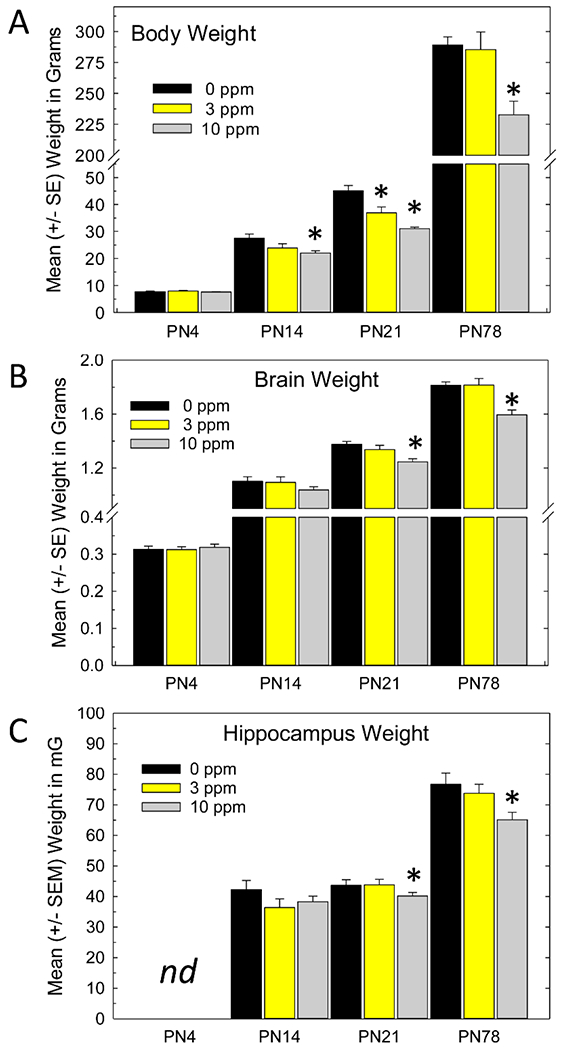
Body, brain and hippocampus weights on postnatal (PN) days 4, 14, 21 and PN78. A) Reduced body weights were detected on PN21 that persisted to adulthood after termination of exposure and return of thyroid hormone status. B) Brain weight reductions were not seen on PN4 or PN14, but were revealed on (PN21) and persisted to PN78, but were restricted to the high dose group. C) Similarly, hippocampal weights were not evident on PN14 but were present on PN21 and PN78. Hippocampal weights were not taken in the PN4 neonate. Mean contrast test after significant effect of Treatment, Dunnett’s T-test, * p<0.05.
The areas of interest identified for regional volume and morphometric assessments of the hippocampus are depicted in Figures 3A and 3C. Reductions in volume were evident in the stratum radiatum of CA1 and the GCL of the DG and limited to the high dose group (Figure 3B). An overall ANOVA on volume estimates by Region and Dose revealed significant main effects (p<0.003) and a significant Dose X Region interaction (p<0.0006). Stepdown ANOVAs by region showed reductions in volume of stratum radiatum at the high dose of PTU (10 ppm) [F(2,22)=4.84, p<0.02] with no change in CA1 (p>0.10). In the high dose group this was accompanied by a volume reduction of the GCL [F(2,22)=6.87, p<0.005], with no change in the volume of the stratum moleculare (p>0.20) , the dendritic field of the GCL. At the mid-dose (3 ppm) level, the hilus was significantly increased in volume with no differences observed at the high dose (10 ppm) level [F(2,22)=7.22, p<0.004]. Simple linear estimates of hippocampal regions showed similar effects with a significant decrease in height measurements of the stratum radiatum and the GCL in the high dose group and an increase in height measurements of the hilus in the mid-dose (3 ppm) group (Figure 3D).
Granule Cell Layer Area and Volume Estimates in the Adult:
Area measurements of the GCL were collected in animals prepared for evaluation of neurogenesis. A significant main effect of Treatment [F(7,56)=4.24, p<0.0008] was observed, with no effect of Survival Time [F(1,56)=0.02, p>0.89] or Treatment X Survival Time interaction [F(3,56)=2.12, p>0.10]. The lack of effect of survival time simply indicates that the GCL area was not different between animals sacrificed 1-day after the last BrdU dose or 28 days later. Therefore, the area data were collapsed across day and presented in a single plot (Figure 4A) depicting the mean GCL area (mean across GCL on every section/animal). Animals exposed to PTU during development had smaller mean GCL relative to controls or animals exposed only as adults. Figure 4B depicts the mean volume of GCL. Results and statistical analysis for volume paralleled that observed for individual area measurement (i.e., significant main effect of Treatment [F(3,56)=7.75, p<0.0002] but no effect of Survival Time [F(1,56)=0.06, p>0.80] or Treatment X Survival Time interaction [F(3,56)=2.12, p>0.11]. Both mean GCL area and total GCL volume calculations reveal reductions, relative to controls (Con-Con), of similar magnitude, in treatments that included developmental exposure (PTU-Con and PTU-PTU), but not in exposure that began in adulthood (Con-PTU).
Figure 4.
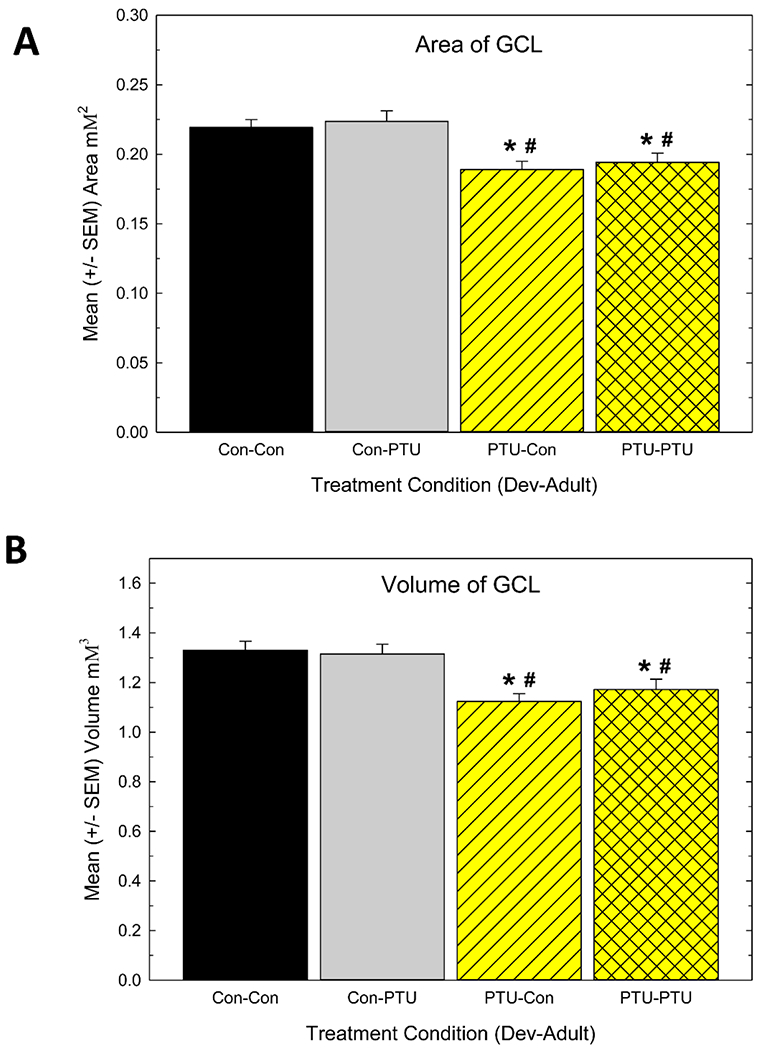
A) Mean granule cell layer (GCL) area/section and B) mean GCL volume/hippocampus in controls (Con-Con), adult only exposure (Con-PTU), developmental exposure (PTU-Con) and developmental and adult exposure (PTU-PTU) conditions. Animals experiencing developmental TH insufficiency were distinct from the control and the adult only hypothyroid groups. Significant effects in ANOVA were followed by Duncan’s Multiple Range Test for between group comparisons, * different from Con-Con, # different from Con-PTU, p<0.05.
BrdU Cell Number and Cell Density:
Figure 5 is a composite of a representative section from each Treatment condition at the 1-day survival time. BrdU-positive cells can be seen lining the inner edge of the SGZ of both blades of the DG. Fewer cells were present in developmentally hypothyroid animals (Figure 5C, D) relative to controls (Figure 5A) or those exposed as adults (Figure 5B). Cell counts expressed as the mean number of BrdU-positive cells/section and density of cells which corrects for changes in GCL volume are summarized in Figure 6. In animals sacrificed 1-day after the last BrdU injection (Figure 6A and 6C), mean cell number/section and the density based on GCL volume were reduced in animals exposed to PTU during development (PTU-Con) relative to controls (Con-Con), but not in those exposed only as adults (Con-PTU). No greater reduction in cell number was revealed in animals exposed to PTU as neonates and again as adults (PTU-PTU). As expected, fewer BrdU-positive cells were detected in controls at the longer survival time, reflecting ~ a 20% loss over the 4-week survival period (total cell count mean in Con-Con=1397±100.6 and 1127±89.3 for 1 and 28 days, respectively), however, the relative pattern of the effect of PTU treatment was similar to that observed at the 1-day timepoint (Figure 6B and 6D). These findings are supported by the results of ANOVA which revealed significant main effects of Treatment [F(3,56)=8.57, p<0.0001] and Survival Time [F(1,56)=7.10, p<0.01], but no significant Treatment X Survival Time interaction [F(3,56)=0.68, p>0.56]. At both time points, mean contrast tests confirmed fewer cells were present in animals exposed during development (PTU-Con and PTU-PTU) relative to those exposed as adults (Con-PTU) or not at all (Con-Con). That no differential effect on BrdU-positive cell number is seen across Survival Time is clear when data are expressed as a percent of control (Figure 6E and 6F). BrdU cell number when expressed as a percent of control (Con-Con) at the 1-day (Figure 6E) and 28- day (Figure 6F) was reduced ~40-50% in both developmentally exposed groups (PTU-Con and PTU-PTU), and this decline was similar in magnitude at both the 1-day and 28-day survival times. ANOVA revealed a significant main effect of Treatment [F(3,56)=6.96, p<0.0001] in the absence of an effect of Survival Time or Treatment X Survival Time interaction (both p’s>.64). The findings are consistent with a preferential effect in animals exposed during development on the proliferation/early survival phase of neurogenesis, with no additional effect on long-term cell survival.
Figure 5.
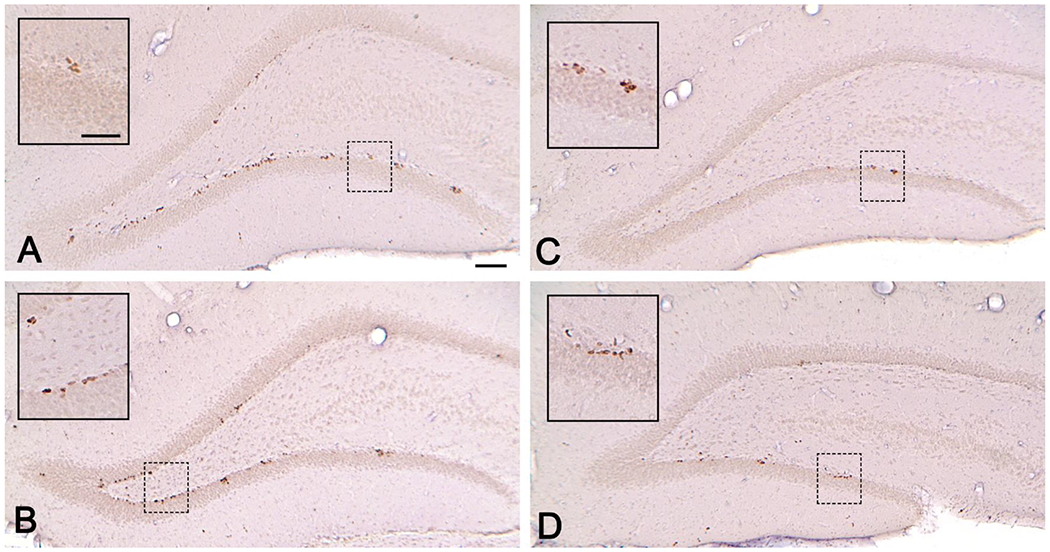
BrdU-positive in the granule cell layer (GCL) of a (A) Con-Con (B) Con-PTU (C) PTU-Con and (D) PTU-PTU animal at the 1-day survival time. BrdU+ cells line the zone of both blades. Inset shows higher magnification. All BrdU+ cells within the GCL and subgranular zone (SGZ) of the dentate gyrus were counted at a magnification of 40X in a 1-in-8 series of sections throughout the dorsal hippocampus. Scale bars = (A) 100 μM and (B) inset 200 μM.
Figure 6.
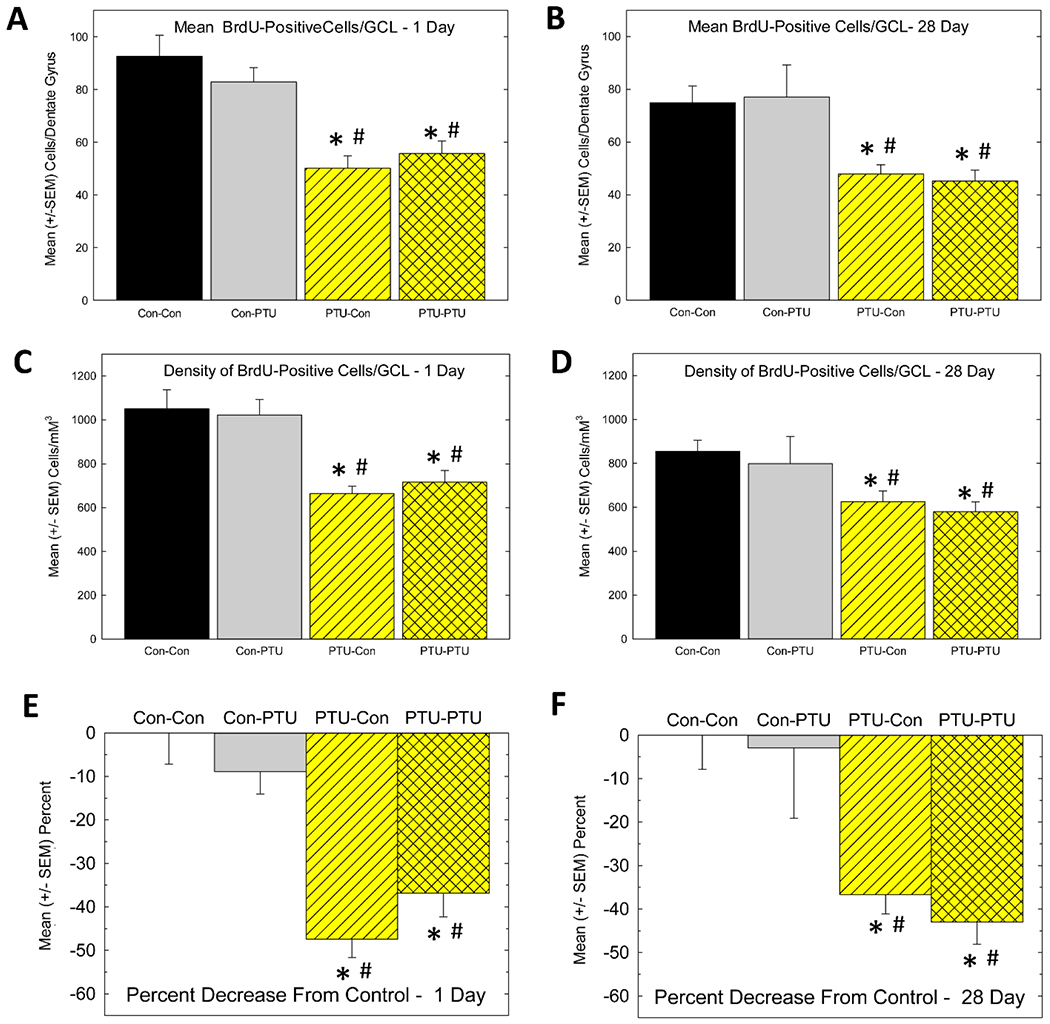
Cell Proliferation and Cell Survival. (A and B) Mean (± SEM) BrdU-positive cells at the 1-day (A) and 28-day (B) survival time. This estimate reflects the average number of cells counted in the GCL of one hemisphere of one section calculated from the mean of all the sections (6-8) across both hemispheres assessed for each animal. (C and D) Mean (± SEM) BrdU-positive cell density at the 1-day (C) and 28-day (D) survival time. This estimate is the calculated total number of BrdU-positive cells within the hippocampus using stereological methods and normalized to the volume of the GCL (see text). E) Percent decrease from control in each treatment condition at 1-day (E) and 28-day (F) survival time. CC, Control-Control; CP, Control-PTU, PC PTU-Control, PP, PTU-PTU. * significantly different from control (Con-Con, CC), # different from Con-PTU, Duncan’s Multiple Range Test, p<0.05 following significant ANOVA.
Ki-67 and Dcx Cell Number and Density.
Cell counts for the proliferation marker Ki-67 (Figure 7) were also reduced in PTU-treated animals [F(3, 28)=9.35, p<0.0002]. As shown in Figure 7E, the reduction in Ki-67 was limited to animals experiencing TH insufficiency during development. Consistent with BrdU counts at the 24-hour sacrifice time, adult exposure to PTU or additional exposure in adulthood following developmental exposure did not further alter the number of Ki-67-positive cells. When Ki-67 cells were expressed as a density measure, however, reductions in cell number remained [Figure 7F, F(3,28)=3.76, p<0.02], but significant declines were restricted to the PTU-PTU treatment condition relative to control (p<0.05).
Figure 7.
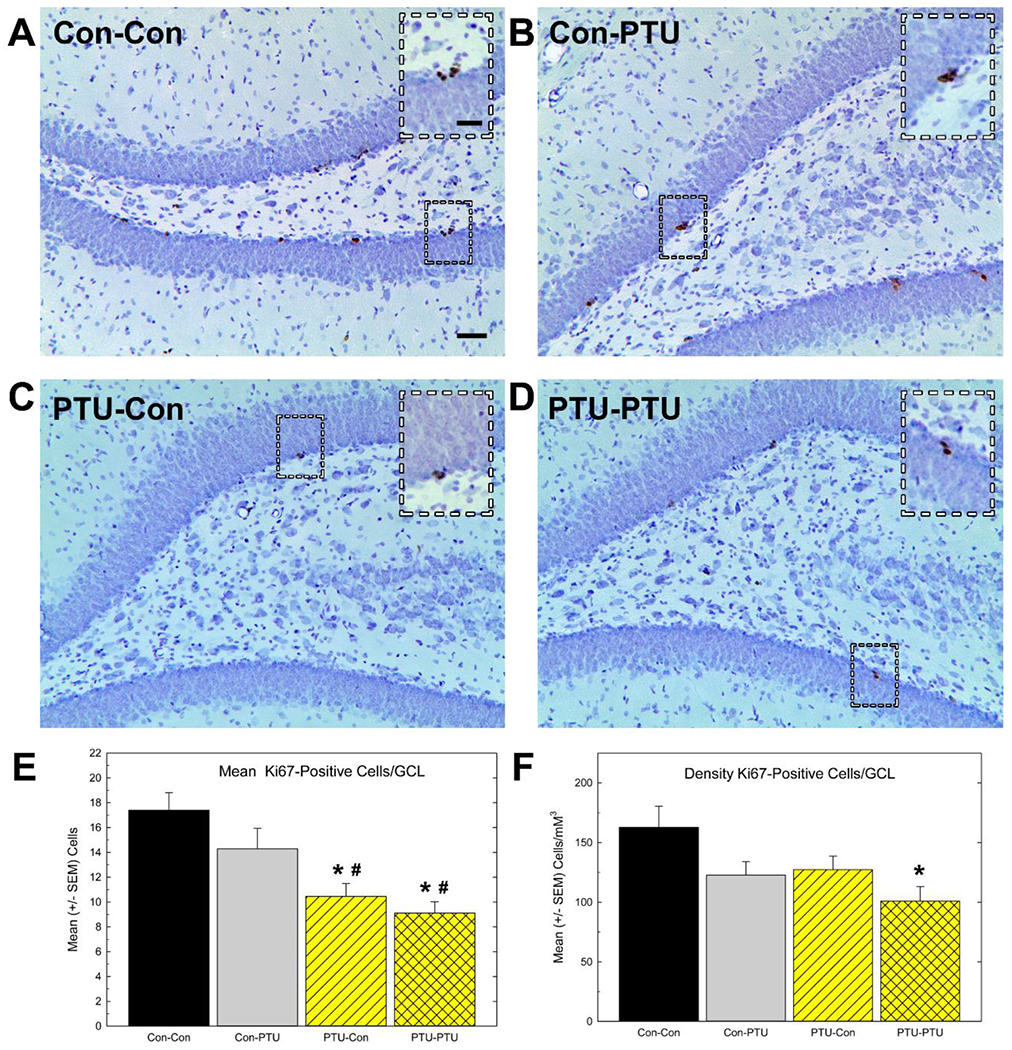
Immunostaining of cell proliferation marker Ki67. (A-D) Low and high (inset) magnification images of Ki-67 positive cells in the granule cell layer (GCL) of adult hippocampus. (E) Mean (±SEM) Ki-67-positive cell number/section. This estimate reflects the average number of cells counted in the GCL of one hemisphere of one section calculated from the mean of all the sections (6-8) across both hemispheres assessed for each animal. (F) Mean (± SEM) Ki-67-positive cell density. This estimate is the calculated total number of Ki-67-positive cells within the hippocampus using stereological methods and normalized to the volume of the GCL (see text).
Significant effects in ANOVA were followed by Duncan’s Multiple Range Test for between group comparisons, * different from Con-Con, # different from Con-PTU at p<0.05. Calibration bars 25 and 50 μM.
Immunohistochemistry for Dcx was examined to assess the number of cells within the GCL that exhibited a phenotype characteristic of immature neurons (Figure 8). Dcx-positive cell number was reduced by PTU-exposure [Figure 8E; F(3,28)=13.4, p<0.0001]. As with BrdU and Ki-67, these reductions were limited to the animals experiencing TH insufficiency during development and were not altered by adult onset hypothyroidism. When expressed as a function of GCL volume, however, a significant effect of Treatment for Dcx density was revealed by ANOVA [F(3,28)=4.75, p<0.009], but mean contrast tests indicated no treatment groups were significantly different from controls (Figure 8F).
Figure 8.
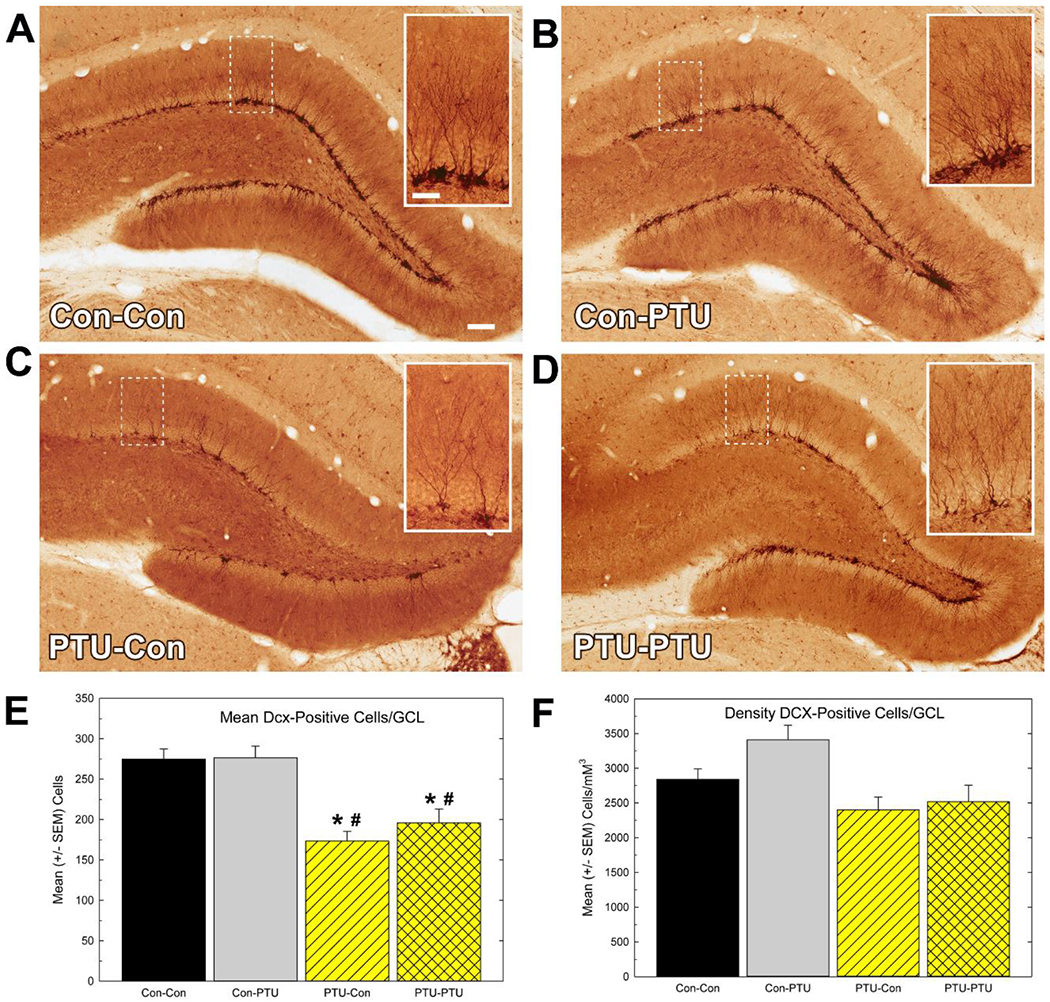
Immunostaining of neuronal differentiation marker doublecortin (Dcx). (A-D) Low and (B) high magnification (inset) images of Dcx-positive cells in granule cell layer (GCL) of adult hippocampus. (E) Mean (±SEM) Dcx-positive cell number/section. This estimate reflects the average number of cells counted in the GCL of one hemisphere in one section calculated from the mean of all the sections (6-8) assessed for each animal. (F) Mean (± SEM) Dcx-positive cell density. This estimate is the calculated total number of Dcx-positive cells within the hippocampus using stereological methods and normalized to the volume of the GCL (see text). Significant effects in ANOVA were followed by Duncan’s Multiple Range Test for between group comparisons, * different from Con-Con, # different from Con-PTU at p<0.05. Calibration bars 35 and 140 μM.
Developmental TH Insufficiency Reduced Proportion of Mature Dcx-Expressing Cells.
Dcx expression is restricted the neuronal lineage (Plumpe et al., 2006). Semi-quantitative characterization of the developmental status of Dcx-expressing cells (Figure 9A, 9B) revealed a larger proportion of immature cells and a smaller proportion mature cells in DG of animals experiencing developmental hypothyroidism (Figure 9C). No difference among treatment conditions was seen in the proportion of the intermediate cell type category, representing cells transitioning from immature to mature status. Adult onset hypothyroidism (Con-PTU) did not alter the relative proportions of these phenotypes compared to Controls (Con-Con). Adult onset hypothyroidism in addition to developmental thyroid insult did not differ from developmental hypothyroidism alone (PTU-Con was similar to PTU-PTU) These findings are confirmed by a significant main effect of Cell Type [F(2,24)=360, p<0.0001] and a Treatment X Cell Type interaction [F(6,24)=15.5, p<0.0001] and mean contrast tests, p<0.05 (Figure 9C)
Figure 9.
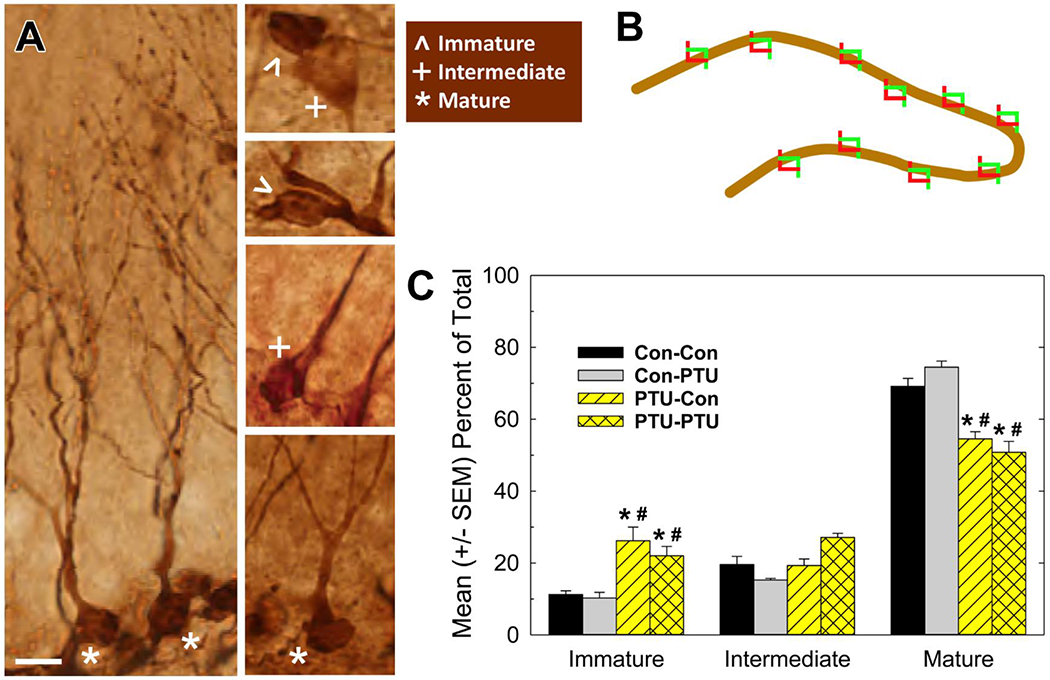
Categorization of Dendritic Morphology in Doublecortin (Dcx) Labelled Neurons. (A) Representative phenotypes of Dcx-positive cells ranging from immature, intermediate, mature. (B) Schematic of granule cell layer (GCL) with software generated counting frames (not to scale) for selection of cells for analysis. The profile of Dcx-positive cells falling within counting frame were categorized as one of the phenotypes depicted in A. The GCL of both hemispheres in 5 sections of each of 4 animals per treatment condition were analyzed resulting in assessment of ~200-300 cells/animal (see text). (C) The mean (+/− SEM) proportion of total number of cell analyzed falling within each category in the different treatment conditions reflect a shift in the distribution to fewer Dcx-positive neurons with mature dendritic morphology in developmentally hypothyroid groups. Significant effects in ANOVA for Cell Type and Treatment X Cell Type interaction were followed by Duncan’s Multiple Range Test for between group comparisons. Within each category, * denote significant differences from control (Con-Con), # different from Con-PTU, p<0.05. Calibration bar 10 μM.
DISCUSSION
Production of new neurons occurs throughout life in the hippocampus of all mammals and in the mature brain supports learning and memory, modulates affect, and provides the possibility for compensation and repair in response to brain injury (Bond et al., 2015). Hippocampal synaptic transmission and plasticity are impaired in the DG following moderate degrees of developmental thyroid hormone insufficiency, and these impairments are accompanied by deficits in behavioral tasks dependent on the hippocampus (radial arm maze learning, Morris water maze, trace-fear conditioning) (Gilbert and Sui, 2006, Gilbert et al., 2007, Axelstad et al., 2008, Opazo et al., 2008, Gilbert, 2011, Gilbert et al., 2016). Reports of alterations in hippocampal anatomy, however, have been largely restricted to very severe hypothyroid models and very little quantitative information is available. The present study documents mild reductions in brain weight and hippocampal volume in the hypothyroid neonatal brain at the highest dose tested. Importantly, transient TH insufficiency incurred during early development impaired adult neurogenesis at a dose that was without effect on these metrics in the neonate. This decrement was seen in the offspring of dams exhibiting hypothyroxinemia, reductions in circulating levels of T4 with no change in serum T3. A similar level of thyroid disruption induced in adulthood, however, did not impair hippocampal neurogenesis and stands in contrast to previous reports with more severe hypothyroid models. Neither did a second hypothyroid insult delivered in adulthood to developmentally exposed offspring exacerbate the effects on neurogenesis beyond those observed with developmental exposure alone. Newly born neurons in the adult DG are proposed to facilitate learning in the (Deng et al., 2010, Stuchlik, 2014, Ortega-Martinez, 2015), deficits in the process may contribute to impairments in synaptic function and behavior previously reported by our laboratory with this this model of moderate TH disruption (Gilbert and Sui, 2006, Gilbert, 2011, Gilbert et al., 2016).
Neurogenesis in the mammalian brain is a complex multifaceted process, the toxicological significance of which are just beginning to be appreciated. The present study interrogated both early and late phases of adult neurogenesis following repeated doses of BrdU over several days. As such, BrdU cell counts obtained at the 24-hour survival time represents net sum of cells actively dividing over the course of the 5 BrdU-dosing days, minus those newly GCL labelled cells that died within this timeframe. However, the consistency in the pattern of BrdU expression with the proliferative marker Ki67 supports the conclusion that the proliferative early phase of neurogenesis is susceptible to interference by thyroid hormone insufficiency. The majority of newly born cells in GCL (>80%) differentiate into neurons (Kempermann et al., 2000, Plumpe et al., 2006). BrdU double-labeling experiments to determine the number of newly born cells that went on to develop a neuronal phenotype were not conducted. However, the similarity in the patterns of cell counts for BrdU and the neuronal marker Dcx suggests that fewer cells were born, and fewer cells differentiated into neurons in animals suffering from developmental but not adult onset hypothyroidism.
A number of reports have demonstrated TH-induced deficits in adult neurogenesis with adult onset hypothyroid models. Some of these reports are consistent with a decrease in NPC proliferation (Montero-Pedrazuela et al., 2006, Kapoor et al., 2010, Kapoor et al., 2011, Lopez-Juarez et al., 2012, Shiraki et al., 2012a), whereas others support a role for TH in the survival of newborn neurons (Ambrogini et al., 2005, Desouza et al., 2005, Zhang et al., 2009). Previous reports have been restricted to very severe degrees of TH disruption. The present results indicate that cell proliferation, neuronal differentiation, and cell survival phases of neurogenesis in the DG do not appear to be affected by more moderate degrees of adult TH compromise. It should be noted that the effects of adult onset hypothyroidism on the proliferation/early survival phase of neurogenesis was assessed after 32 days of PTU exposure (24-hour post BrdU) whereas animals assessed for long term survival endured an additional 28 days of PTU exposure prior to sacrifice at the 28-days timepoint. Despite this extended period of exposure, serum hormones were similar between these groups (data not shown) and no differences in the number of BrdU-positive cells were seen relative to respective controls. These data suggest that neither proliferation or survival of newly born cells was altered when hypothyroidism was induced in adulthood at these concentrations of PTU for 4-8 weeks.
In the developing nervous system, severe hypothyroidism permanently reduces hippocampal size and granule cell number (Rami et al., 1986a, Madeira et al., 1988, Rami and Rabie, 1990, Zhang et al., 2009, Shiraki et al., 2012a). We report smaller hippocampal subregions in the neonate exposed to the 10 ppm dose of PTU but no differences were observed at the lower dosage of 3 ppm. However, at the moderate levels of TH disruption induced by the 3 ppm, reduced GCL volumes emerged in adulthood. Exposure initiated in adulthood was without effect on GCL volume. A reduced neurogenic capacity in the adult following developmental hypothyroidism observed in the present study may contribute to lower GCL volumes. However, it is unclear why smaller GCL volumes were not also evident in the neonate during the period of ongoing TH insufficiency. Less compaction of the maturing GCL may have obfuscated subtle differences in GCL volumes in the neonate at the lower dose level. It is also possible that with modest levels of TH disruption, proliferation, differentiation and survival of cells emanating from a number of distinct proliferating cell pools in the developing DG (Altman and Bayer, 1990) renders the neonate more resilient to TH insult during this robust period of neurogenesis. In the less permissive neurogenic environment of the adult nervous system, where only a single progenitor pool is present, a reduced capacity for neurogenesis becomes more evident and may well be reflected in smaller GCL volumes. Consistent with this hypothesis, Shiraki et al. (2012a) reported fewer Pax6-positive cells, a marker for proliferating NPC, in the adult offspring at a dose of a related goitrogen, methimazole, that did not affect this marker in the neonate.
In addition to GCL volume deficits, developmental hypothyroidism preferentially reduced NPC number/early survival in the adult DG while leaving long-term survival intact. Some authors report a reduced NPC population in the DG of the hypothyroid animal, but when volume reductions are considered, the overall density of NPC is the same as controls (Zhang et al., 2009). Others maintain that volume, numerical density, and total number of GC are permanently reduced through TH-mediated disruption of NPC proliferation in the developing nervous system, and that these same TH-mediated actions are operative in the adult DG (Madeira et al., 1991). Our data indicate that with milder TH insult, reductions in BrdU cell number remained on correction for slight reductions in GCL volume. Decreases in cell number and cell density of 40-50% were observed in the two treatment groups experiencing developmental TH insufficiency. This pattern was also evident in counts of cells in the GCL expressing the proliferative cell marker, Ki-67. In the long term survival groups, BrdU cell number dropped ~20% in the controls and adult onset hypothyroid group and this drop was not exacerbated by developmental hypothyroidism. The developmental exposure group (PTU-Con) even appeared to have a more limited loss than controls at 28-days relative to counts in this group on Day 1, but this was not recapitulated in the PTU-PTU group. Nonetheless, these observations further support the conclusion that NPC proliferation/early survival and not long-term cell survival appears to be preferentially targeted by developmental TH insufficiencies.
The more subtle perturbation on brain development invoked by the lower levels of TH insufficiency in the present study revealed the lasting effects on NPC proliferation/early survival in the adult offspring that may have been obscured in more severe model systems. In contrast to BrdU and Ki-67, however, the apparent reduction in the immature neuronal marker, Dcx, prominent in our results on cell number, was not evident when values were adjusted for reductions in GCL volume. This suggests a lack of effect of TH insufficiency on the process of neuronal differentiation, an observation consistent with results of Zhang et al. (2009) for this marker of immature neurons. However, an assessment of dendritic morphology of Dcx-expressing neurons revealed a change in the relative proportion of neurons displaying a mature vs an immature phenotype stemming from developmental but not adult hypothyroidism. Under control conditions, previous reports estimate ~70% of all Dcx-expressing granule cells are of the mature phenotype, characterized by dendrites extending to the molecular layer with many exhibiting multiple branching and a delicate dendritic arbor. The remaining 30% of Dcx-expressing cells are of the ‘immature’ or ‘intermediate’ phenotype, either completely denuded or with short stunted processes typically extending parallel to the GCL (Plumpe et al., 2006). Our observations in control animals (Con-Con) and adult onset hypothyroidism (Con-PTU) are consistent with these proportions, whereas developmental TH insufficiency caused a shift to fewer mature neurons and a higher proportion of immature neuronal phenotypes. The incorporation of newly born GC into the neuronal networks is believed to play a significant role in learning and memory (Deng et al., 2010, Aimone et al., 2014, Stuchlik, 2014). A reduction in dendritic development in the adult GCL following developmental hypothyroidism could delay or impair the formation of synaptic connections and contribute to plasticity and memory impairments previously reported by our laboratory and others (Gilbert and Sui, 2006, Axelstad et al., 2008, Opazo et al., 2008, Gilbert, 2011, Gilbert et al., 2016). Collectively these data indicate that moderate degrees of developmental TH insufficiency imparts persistent decrements in the proliferative capacity/early survival of NPCs within the SGZ in addition to altering the dynamics of dendritic development in differentiated neurons.
The mechanisms whereby transient developmental TH insufficiencies reduce the capacity for neurogenesis adult DG are not known. Developmental TH deprivation may have a direct effect on adult neurogenesis by permanently reducing the size of the NPC pool in the hippocampus (Madeira et al., 1991). Mohan et al. (2012) have demonstrated a TH-dependent selective loss of a specific population of NPC cells that regulates cortical thickness in fetal cortex. Reduced rates of cortical neurogenesis of this pool of NPC derived from a TH-dependent disruption of cell cycle kinetics and decreased cell cycle length. A similar TH-dependent action on the NPC population of the DG may underlie the selective effect on proliferative/early survival of NPC and contribute to the observed GCL thinning in the adult hippocampus.
Alternatively developmental TH insufficiency may negatively impact neurogenesis indirectly by its effects on neural activity. Neurogenesis is regulated by synaptic activity - it is enhanced by learning, long-term potentiation, exercise, environmental enrichment, and seizure activity (Laplagne et al., 2006, Bruel-Jungerman et al., 2007, Bruel-Jungerman et al., 2009, Scobie et al., 2009, Kameda et al., 2012, Marlatt et al., 2012). Moderate degrees of developmental TH insufficiency comparable to those used here, reduce excitatory synaptic transmission (Gilbert and Sui, 2006, Gilbert, 2011), long-term potentiation, and hippocampal-dependent learning in adult offspring (Akaike, 1991, Gilbert and Sui, 2006, Axelstad et al., 2008, Gilbert, 2011, Gilbert et al., 2016). Moreover, neurotrophins, including nerve growth factor and brain-derived nerve growth factor, essential for the maintenance of neurogenesis and activity-dependent plasticity (Messaoudi et al., 2002, van Praag et al., 2002, Laplagne et al., 2006, Bruel-Jungerman et al., 2007, Frielingsdorf et al., 2007, Kameda et al., 2012, Marlatt et al., 2012, Ortega-Martinez, 2015), are reduced in expression and in activation in the hippocampus of offspring following moderate developmental TH insufficiency (Royland et al., 2008, Lasley and Gilbert, 2011, Bastian et al., 2012, Bastian et al., 2014, Gilbert et al., 2016) . It is possible that the suppressive effects of developmental TH insufficiency on neural activity evident in the mature brain contributes to reduction in NPC proliferation/early survival to impede neurogenesis in the adult DG. Alterations in dendritic maturation of newly born neurons in the adult DG in developmentally hypothyroid animals may serve to perpetuate the observed deficits in neural transmission, synaptic plasticity, and learning.
In conclusion, we have demonstrated impaired neurogenesis in the adult hippocampus following developmental TH insufficiency. Learning deficits associated with modest degrees of TH insufficiency repeatedly shown with this model and at this and lower levels of TH insufficiency may in part be the result of a disruption of this type of structural neuroplasticity. As plasticity mechanisms play important roles during development in the formation of functional neural networks and in the mature brain during learning and memory processes, their impairment in the absence of sufficient TH may underlie some of the more subtle neurological deficiencies associated with moderate degrees of maternal TH insufficiency. Moreover, our data show that these processes can be impaired in the absence of change in other common metrics of developmental neurotoxicity (i.e., body weight, brain weight, brain volume) used to identify TH-disrupting chemicals. Better characterization of the consequences of moderate degrees of developmental TH insufficiency will not only provide insight into the role of TH in brain development, but serve to identify molecular substrates of TH action that are critical for the development of sensitive assays and endpoints for detection of TH-mediated dysfunction.
Acknowledgements-
The technical assistance of Willard Anderson, Richard (Luke) Ford, Susan Thomas, Ian Gitata, Alan Tennant, John Nance is gratefully acknowledged. The assistance and comments of Dr. Robert Switzer in study design, immunohistochemistry and image analysis, and Dr. Karl Jensen, Keith Tarpley and Katherine O’Shaughnessy in preparation of photomicrographs is very much appreciated. The authors also thank Dr. Jean Harry for very helpful discussions during the conduct of these experiments and for comments on an earlier version of this manuscript.
Footnotes
This document has been subjected to review by the National Health and Environmental Effects Research Laboratory and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
REFERENCES
- Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH (2014) Regulation and function of adult neurogenesis: from genes to cognition. Physiol Rev 94:991–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike M, Kato N, Ohno H, Kobayashi T (1991) Hyperactivity and spatial maze learning impairment of adult rats with temporary neonatal hypothyroidism. Neurotoxicol Teratol 13:317–322. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA (1990) Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol 301:365–381. [DOI] [PubMed] [Google Scholar]
- Ambrogini P, Cuppini R, Ferri P, Mancini C, Ciaroni S, Voci A, Gerdoni E, Gallo G (2005) Thyroid hormones affect neurogenesis in the dentate gyrus of adult rat. Neuroendocrinology 81:244–253. [DOI] [PubMed] [Google Scholar]
- Auso E, Lavado-Autric R, Cuevas E, Del Rey FE, Morreale De Escobar G, Berbel P (2004) A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology 145:4037–4047. [DOI] [PubMed] [Google Scholar]
- Axelstad M, Hansen PR, Boberg J, Bonnichsen M, Nellemann C, Lund SP, Hougaard KS, U H (2008) Developmental neurotoxicity of Propylthiouracil (PTU) in rats: relationship between transient hypothyroxinemia during development and long-lasting behavioural and functional changes. Toxicol Appl Pharmacol 232:1–13. [DOI] [PubMed] [Google Scholar]
- Bastian TW, Anderson JA, Fretham SJ, Prohaska JR, Georgieff MK, Anderson GW (2012) Fetal and neonatal iron deficiency reduces thyroid hormone-responsive gene mRNA levels in the neonatal rat hippocampus and cerebral cortex. Endocrinology 153:5668–5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian TW, Prohaska JR, Georgieff MK, Anderson GW (2014) Fetal and neonatal iron deficiency exacerbates mild thyroid hormone insufficiency effects on male thyroid hormone levels and brain thyroid hormone-responsive gene expression. Endocrinology 155:1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbel P, Navarro D, Auso E, Varea E, Rodriguez AE, Ballesta JJ, Salinas M, Flores E, Faura CC, de Escobar GM (2010) Role of late maternal thyroid hormones in cerebral cortex development: an experimental model for human prematurity. Cereb Cortex 20:1462–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal J (2002) Action of thyroid hormone in brain. J Endocrinol Invest 25:268–288. [DOI] [PubMed] [Google Scholar]
- Bond AM, Ming GL, Song H (2015) Adult Mammalian Neural Stem Cells and Neurogenesis: Five Decades Later. Cell Stem Cell 17:385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Davis S, Laroche S (2007) Brain plasticity mechanisms and memory: a party of four. Neuroscientist 13:492–505. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Veyrac A, Dufour F, Horwood J, Laroche S, Davis S (2009) Inhibition of PI3K-Akt signaling blocks exercise-mediated enhancement of adult neurogenesis and synaptic plasticity in the dentate gyrus. PLoS One 4:e7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McKay RD (2001) Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol 435:406–417. [DOI] [PubMed] [Google Scholar]
- Christie KJ, Turnley AM (2012) Regulation of endogenous neural stem/progenitor cells for neural repair-factors that promote neurogenesis and gliogenesis in the normal and damaged brain. Front Cell Neurosci 6:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Olmos JS (1977) An improved HRP method for the study of central nervous connections. Exp Brain Res 29:541–551. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH (2010) New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 11:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desouza LA, Ladiwala U, Daniel SM, Agashe S, Vaidya RA, Vaidya VA (2005) Thyroid hormone regulates hippocampal neurogenesis in the adult rat brain. Mol Cell Neurosci 29:414–426. [DOI] [PubMed] [Google Scholar]
- Frielingsdorf H, Simpson DR, Thal LJ, Pizzo DP (2007) Nerve growth factor promotes survival of new neurons in the adult hippocampus. Neurobiol Dis 26:47–55. [DOI] [PubMed] [Google Scholar]
- Gilbert ME (2011) Impact of low-level thyroid hormone disruption induced by propylthiouracil on brain development and function. Toxicol Sci 124:432–445. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Kelly ME, Samsam TE, Goodman JH (2005) Chronic developmental lead exposure reduces neurogenesis in adult rat hippocampus but does not impair spatial learning. Toxicol Sci 86:365–374. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Sanchez-Huerta K, Wood C (2016) Mild Thyroid Hormone Insufficiency During Development Compromises Activity-Dependent Neuroplasticity in the Hippocampus of Adult Male Rats. Endocrinology 157:774–787. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Sui L (2006) Dose-dependent reductions in spatial learning and synaptic function in the dentate gyrus of adult rats following developmental thyroid hormone insufficiency. Brain Res 1069:10–22. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Sui L, Walker MJ, Anderson W, Thomas S, Smoller SN, Schon JP, Phani S, Goodman JH (2007) Thyroid hormone insufficiency during brain development reduces parvalbumin immunoreactivity and inhibitory function in the hippocampus. Endocrinology 148:92–102. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB (1987) The efficiency of systematic sampling in stereology and its prediction. J Microsc 147:229–263. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Kida I, Wada H (2010) A volumetric analysis of the brain and hippocampus of rats rendered perinatal hypothyroid. Neurosci Lett 479:240–244. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, Romero FJ, Garcia-Verdugo JM (2003) Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proc Natl Acad Sci U S A 100:7919–7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, van Praag H, Gage FH (2000) Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatry 5:262–269. [DOI] [PubMed] [Google Scholar]
- Kameda M, Taylor CJ, Walker TL, Black DM, Abraham WC, Bartlett PF (2012) Activation of latent precursors in the hippocampus is dependent on long-term potentiation. Transl Psychiatry 2:e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor R, Ghosh H, Nordstrom K, Vennstrom B, Vaidya VA (2011) Loss of thyroid hormone receptor beta is associated with increased progenitor proliferation and NeuroD positive cell number in the adult hippocampus. Neurosci Lett 487:199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor R, van Hogerlinden M, Wallis K, Ghosh H, Nordstrom K, Vennstrom B, Vaidya VA (2010) Unliganded thyroid hormone receptor alpha1 impairs adult hippocampal neurogenesis. FASEB J 24:4793–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor RD, L. A; Nanavaty IN; Kernie SG; Vaidya VA; (2012) Thyroid hormone accelerates the differentiation of adult hippocampal progenitors. J Neuroendocrinol 24:1259–1271. [DOI] [PubMed] [Google Scholar]
- Kee N, Sivalingam S, Boonstra R, Wojtowicz JM (2002) The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods 115:97–105. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH (1998) Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci 18:3206–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, van Praag H, Gage FH (2000) Activity-dependent regulation of neuronal plasticity and self repair. Prog Brain Res 127:35–48. [DOI] [PubMed] [Google Scholar]
- Laplagne DA, Esposito MS, Piatti VC, Morgenstern NA, Zhao C, van Praag H, Gage FH, Schinder AF (2006) Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol 4:e409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasley SM, Gilbert ME (2011) Developmental thyroid hormone insufficiency reduces expression of brain-derived neurotrophic factor (BDNF) in adults but not in neonates. Neurotoxicol Teratol 33:464–472. [DOI] [PubMed] [Google Scholar]
- Lopez-Juarez A, Remaud S, Hassani Z, Jolivet P, Pierre Simons J, Sontag T, Yoshikawa K, Price J, Morvan-Dubois G, Demeneix BA (2012) Thyroid hormone signaling acts as a neurogenic switch by repressing Sox2 in the adult neural stem cell niche. Cell Stem Cell 10:531–543. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Cadete-Leite A, Andrade JP, Paula-Barbosa MM (1991) Effects of hypothyroidism upon the granular layer of the dentate gyrus in male and female adult rats: a morphometric study. J Comp Neurol 314:171–186. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Paula-Barbosa M, Cadete-Leite A, Tavares MA (1988) Unbiased estimate of hippocampal granule cell numbers in hypothyroid and in sex-age-matched control rats. J Hirnforsch 29:643–650. [PubMed] [Google Scholar]
- Madeira MD, Sousa N, Lima-Andrade MT, Calheiros F, Cadete-Leite A, Paula-Barbosa MM (1992) Selective vulnerability of the hippocampal pyramidal neurons to hypothyroidism in male and female rats. J Comp Neurol 322:501–518. [DOI] [PubMed] [Google Scholar]
- Marlatt MW, Potter MC, Lucassen PJ, van Praag H (2012) Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Dev Neurobiol 72:943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi E, Ying SW, Kanhema T, Croll SD, Bramham CR (2002) Brain-derived neurotrophic factor triggers transcription-dependent, late phase long-term potentiation in vivo. J Neurosci 22:7453–7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan V, Sinha RA, Pathak A, Rastogi L, Kumar P, Pal A, Godbole MM (2012) Maternal thyroid hormone deficiency affects the fetal neocorticogenesis by reducing the proliferating pool, rate of neurogenesis and indirect neurogenesis. Exp Neurol 237:477–488. [DOI] [PubMed] [Google Scholar]
- Montero-Pedrazuela A, Venero C, Lavado-Autric R, Fernandez-Lamo I, Garcia-Verdugo JM, Bernal J, Guadano-Ferraz A (2006) Modulation of adult hippocampal neurogenesis by thyroid hormones: implications in depressive-like behavior. Mol Psychiatry 11:361–371. [DOI] [PubMed] [Google Scholar]
- Morreale de Escobar G, Obregon MJ, Escobar del Rey F (2004) Role of thyroid hormone during early brain development. Eur J Endocrinol 151 Suppl 3:U25–37. [DOI] [PubMed] [Google Scholar]
- Opazo M, Gianini A PF, Azkcona G, Alarcón L, Lizana R, Noches V, Gonzalez PAPM, Mora S, Rosenthal D, Eugenin E, Naranjo D, Bueno SM, Kalergis AMRC, (2008) Maternal hypothyroxinemia impairs spatial learning and synaptic nature and function in the offspring. Endocrinology 149:5097–5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Martinez S (2015) A new perspective on the role of the CREB family of transcription factors in memory consolidation via adult hippocampal neurogenesis. Front Mol Neurosci 8:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1989) The rat brain in stereotaxic coordinates. 2nd Edition New York: Academic Press. [Google Scholar]
- Plumpe T, Ehninger D, Steiner B, Klempin F, Jessberger S, Brandt M, Romer B, Rodriguez GR, Kronenberg G, Kempermann G (2006) Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci 7:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell MH, Nguyen HV, Gilbert M, Parekh M, Colon-Perez LM, Mareci TH, Montie E (2012) Magnetic resonance imaging and volumetric analysis: novel tools to study the effects of thyroid hormone disruption on white matter development. Neurotoxicology 33:1322–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rami A, Patel AJ, Rabie A (1986a) Thyroid hormone and development of the rat hippocampus: morphological alterations in granule and pyramidal cells. Neuroscience 19:1217–1226. [DOI] [PubMed] [Google Scholar]
- Rami A, Rabie A (1990) Delayed synaptogenesis in the dentate gyrus of the thyroid-deficient developing rat. Dev Neurosci 12:398–405. [DOI] [PubMed] [Google Scholar]
- Rami A, Rabie A, Patel AJ (1986b) Thyroid hormone and development of the rat hippocampus: cell acquisition in the dentate gyrus. Neuroscience 19:1207–1216. [DOI] [PubMed] [Google Scholar]
- Ramos RL, Van Dine SE, George E, Patel D, Hoplight BJ, Leheste JR, Richfield EK, Torres G (2013) Molecular layer heterotopia of the cerebellar vermis in mutant and transgenic mouse models on a C57BL/6 background. Brain Res Bull 97:63–68. [DOI] [PubMed] [Google Scholar]
- Royland JE, Parker JS, Gilbert ME (2008) A genomic analysis of subclinical hypothyroidism in hippocampus and neocortex of the developing rat brain. J Neuroendocrinol 20:1319–1338. [DOI] [PubMed] [Google Scholar]
- Samuels BA, Hen R (2011) Neurogenesis and affective disorders. Eur J Neurosci 33:1152–1159. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR (2006) Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A 103:17501–17506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL (2000) Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci 20:6144–6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scobie KN, Hall BJ, Wilke SA, Klemenhagen KC, Fujii-Kuriyama Y, Ghosh A, Hen R, Sahay A (2009) Kruppel-like factor 9 is necessary for late-phase neuronal maturation in the developing dentate gyrus and during adult hippocampal neurogenesis. J Neurosci 29:9875–9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Valadi N, Miller AH, Pearce BD (2002) Neonatal viral infection decreases neuronal progenitors and impairs adult neurogenesis in the hippocampus. Neurobiol Dis 11:246–256. [DOI] [PubMed] [Google Scholar]
- Shiraki A, Akane H, Ohishi T, Wang L, Morita R, Suzuki K, Mitsumori K, Shibutani M (2012a) Similar distribution changes of GABAergic interneuron subpopulations in contrast to the different impact on neurogenesis between developmental and adult-stage hypothyroidism in the hippocampal dentate gyrus in rats. Arch Toxicol 86:1559–1569. [DOI] [PubMed] [Google Scholar]
- Shiraki A, Alame J, Ohishi T, Wang L, Moria R, Suzuki K, Mitsumori K, Shibutani M (2012b) Similar distribution changes of GABergic interneuron subpopulations in contrast to the different impact on neurogeneisis between developmetnal and adult-stage hypothyroidism in the hippocampal dentate gyrus in rats. Archives of Toxicology 86:1559–1569. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E (2001) Neurogenesis in the adult is involved in the formation of trace memories. Nature 410:372–376. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM (2005) A role for adult neurogenesis in spatial long-term memory. Neuroscience 130:843–852. [DOI] [PubMed] [Google Scholar]
- Stuchlik A (2014) Dynamic learning and memory, synaptic plasticity and neurogenesis: an update. Front Behav Neurosci 8:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnley AM, Basrai HS, Christie KJ (2014) Is integration and survival of newborn neurons the bottleneck for effective neural repair by endogenous neural precursor cells? Front Neurosci 8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH (2002) Functional neurogenesis in the adult hippocampus. Nature 415:1030–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GR (2008) Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol 20:784–794. [DOI] [PubMed] [Google Scholar]
- Workman JL, Raineki C, Weinberg J, Galea LA (2015) Alcohol and pregnancy: Effects on maternal care, HPA axis function, and hippocampal neurogenesis in adult females. Psychoneuroendocrinology 57:37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Blomgren K, Kuhn HG, Cooper-Kuhn CM (2009) Effects of postnatal thyroid hormone deficiency on neurogenesis in the juvenile and adult rat. Neurobiol Dis 34:366–374. [DOI] [PubMed] [Google Scholar]


