Abstract
Interstitial cytomegalovirus (CMV) pneumonia is a clinically relevant complication in recipients of bone marrow transplantation (BMT). Recent data for a model of experimental syngeneic BMT and concomitant infection of BALB/c mice with murine CMV (mCMV) have documented the persistence of tissue-resident CD8 T cells after clearance of productive infection of the lungs (J. Podlech, R. Holtappels, M.-F. Pahl-Seibert, H.-P. Steffens, and M. J. Reddehase, J. Virol. 74:7496–7507, 2000). It was proposed that these cells represent antiviral “standby” memory cells whose functional role might be to help prevent reactivation of latent virus. The pool of pulmonary CD8 T cells was composed of two subsets defined by the T-cell activation marker L-selectin (CD62L): a CD62Lhi subset of quiescent memory cells, and a CD62Llo subset of recently resensitized memory-effector cells. In this study, we have continued this line of investigation by quantitating CD8 T cells specific for the three currently published antigenic peptides of mCMV: peptide YPHFMPTNL processed from the immediate-early protein IE1 (pp89), and peptides YGPSLYRRF and AYAGLFTPL, derived from the early proteins m04 (gp34) and M84 (p65), respectively. IE1-specific CD8 T cells dominated in acute-phase pulmonary infiltrates and were selectively enriched in latently infected lungs. Notably, most IE1-specific CD8 T cells were found to belong to the CD62Llo subset representing memory-effector cells. This finding is in accordance with the interpretation that IE1-specific CD8 T cells are frequently resensitized during latent infection of the lungs and may thus be involved in the maintenance of mCMV latency.
In human cytomegalovirus (hCMV) infection after bone marrow transplantation (BMT), recovery from CMV disease correlates with efficient reconstitution of CD8 T cells (50). Preemptive cytoimmunotherapy by adoptive transfer of hCMV-specific CD8 T-cell clones was found to be beneficial in that it reduced the incidence of CMV disease in BMT recipients (51, 56). Proof of principle for the protective effect of antiviral CD8 T cells was provided by the model of murine CMV (mCMV) infection of BALB/c mice subjected to hematoablative treatment. Early experiments performed in the absence of BMT documented an antiviral and protective function of adoptively transferred mCMV-specific CD8 T cells in the lungs as well as in other target organs of the disease (44, 46, 48; for a review, see reference 23). More recently, the course of mCMV infection was analyzed in the specific context of hematolymphopoietic reconstitution after either syngeneic BMT (18, 38, 39) or BMT performed across a single major histocompatibility complex (MHC) class I antigen disparity (1). Prevention of a disseminated and fulminant interstitial CMV pneumonia by the antiviral function of endogenously reconstituted CD8 T cells was inferred from the following observations: (i) CD8 T cells rather than CD4 T cells were recruited to infected lungs much more efficiently than to uninfected lungs (18); (ii) lung-infiltrating, blastoid CD62Llo CD8 T cells were not randomly distributed in lung tissue but were found to colocalize with infected lung cells in inflammatory foci, thereby secluding the infected cells from health tissue (18, 38); (iii) when isolated from the infiltrates, these activated CD8 T cells exerted ex vivo cytolytic activity against infected target cells (18) and secreted gamma interferon (IFN-γ) upon polyclonal triggering via CD3ɛ (38); (iv) the kinetics of infiltration correlated with resolution of the productive infection of the lungs (1, 18, 38); (v) selective in vivo depletion of reconstituting CD8 T cells, but not of CD4 T cells, resulted in a fulminant lung infection associated with severe histopathology (38, 39); and (vi) pulmonary CD8 T cells, but not CD4 T cells, protected against lethal infection of indicator recipients upon cell transfer (1, 38).
In a recent report we operationally defined two phases of lung histopathology during a nonlethal, controlled mCMV infection of the lungs after syngeneic BMT: phase 1, characterized by focal pulmonary infiltrates confining productive infection; and phase 2, characterized by persistence of interstitial T cells after resolution of productive infection (38). These phase 2 pulmonary T cells were no longer organized in foci but were found to be distributed evenly in lung tissue. Unlike the blastoid phase 1 T cells, most phase 2 T cells were resting according to morphological criteria. However, expression of the T-cell activation marker CD62L, a member of the selectin family that is rapidly shed from the cell surface upon cell activation (for a review, see reference 55), revealed the presence of CD62Lhi and CD62Llo subsets of tissue-resident CD8 T cells in phase 2 lungs (38), supposed to represent quiescent memory cells and sensitized memory-effector cells, respectively (3, 34).
Resolution of productive infection of the lungs is not accompanied by clearance of the viral genome. The lungs are a site at which mCMV latency is established with a particularly high tissue load of the latent viral genome and an accordingly high risk of viral transcriptional reactivation and virus recurrence after secondary immunoablative treatment (6, 43, 53). A role for CD8 T cells in the prevention of recurrent infection was inferred from the finding that their selective depletion increases the incidence of recurrence (40). Several previous reports have dealt with pulmonary mCMV latency, reactivation, and recurrence in the specific model of syngeneic experimental BMT (27–29, 53). In accordance with the focal character of acute pulmonary infection (18, 38), reactivation and recurrence were found to be focal too (27, 28). Notably, the latent viral genome was not transcriptionally silent. While ie3 transcripts specifying immediate-early (IE)-phase protein IE3, the essential transactivator of early (E) gene expression (2, 32), were absent in latently infected lungs, generation of ie1 transcripts occurred randomly, with a frequency of ca. 10 events per lung at any moment during latency (27, 28). Provided that these IE1 transcripts are translated into the IE1 protein pp89, one may speculate that transient but iterative presentation of the MHC class I Ld-presented immunodominant IE1 peptide 168YPHFMPTNL176 (18, 47; for a review, see reference 42) could lead to frequent pulses of memory T-cell stimulation and account for phase 2 CD8 T cells with a CD62Llo activation phenotype.
This study documents a pronounced and preferential enrichment of IE1 peptide-specific CD8 T cells in the CD62Llo memory-effector subset of tissue-resident CD8 T cells during mCMV latency in the lungs. This finding supports the hypothesis of immune surveillance of viral latency by “standby” memory-effector CD8 T cells.
MATERIALS AND METHODS
BMT and concurrent mCMV infection.
Animal experiments were approved by the Ethics Commission, permission no. 177-07/991-35, according to German federal law. Donors and recipients of a syngeneic experimental BMT were 8-week-old, female BALB/c (haplotype H-2d) mice. Hematoablation was performed by total-body γ irradiation of the recipients with a single dose of 6.5 Gy delivered by a 137Cs γ-ray source. About 6 h after irradiation, hematopoietic reconstitution was accomplished by intravenous infusion of 5 × 106 tibial and femoral donor bone marrow cells, isolated and depleted of contaminating intravascular CD8 T cells as described previously (1). About 2 h after BMT, recipients were infected subcutaneously in the left hind footpad with 105 PFU of purified, cell culture-propagated mCMV, strain Smith ATCC VR-194/1981 (29). A control group of recipients was left uninfected. Under the specific conditions chosen for BMT, almost all uninfected as well as infected recipients survived owing to an efficient endogenous reconstitution of hematopoietic cell lineages, including CD8 T cells, which resolve the acute infection (18, 38, 39).
Isolation and immunomagnetic enrichment of pulmonary CD8 T cells.
Mononuclear leukocytes were isolated from lung tissue as described previously (17, 18) by collagenase-DNase digestion of lung parenchyma followed by Ficoll density gradient centrifugation. Analyses were performed with cells pooled from 10 to 30 mice per group, depending on cell yield from the lungs and cell need for subsequent purification procedures and assays. Specifically, the relative numbers of pulmonary T cells were ca. 1:10:25 in uninfected BMT controls and in phase 2 (3 months) and phase 1 (4 weeks) infected groups, respectively (38). Cells were used with no further physical purification for cytofluorometric analyses of cell surface marker expression (see below). In the case of enzyme-linked immunospot (ELISPOT) assays (see below), cells were subjected to positive immunomagnetic (magnetically activated cell sorter [MACS]) sorting (MidiMACS separation unit; Miltenyi Biotec Systems, Bergisch-Gladbach, Germany) for purification of CD8 T cells (anti-CD8a MicroBeads; rat immunoglobulin G2a [IgG2a], clone 53-6.7; catalog no. 494-01; Miltenyi Biotec) as described in more detail previously (1) and essentially as suggested by the supplier. The purity of the population was found to be >95% when determined by cytofluorometric reanalysis with phycoerythrin (PE)-Cy5-conjugated anti-CD8a monoclonal antibody (MAb) (clone 53-6.7; catalog no. 01048A; PharMingen, San Diego, Calif.).
Consecutive two-marker immunomagnetic cell sorting.
Pulmonary infiltrate cells retrieved from the Ficoll interphase were incubated at 4°C for 5 min in blocking solution (1) to saturate unspecific binding sites and were then labeled with fluorescein (FITC) [fluorescein isothiocyanate]-conjugated anti-CD8a MAb (clone 53-6.7; catalog no. 01044A; Becton Dickinson). After two wash steps with MACS buffer (FACS buffer with no NaN3; see reference 1), ca. 107 cells were resuspended in 90 μl of MACS buffer and 10 μl of MultiSort anti-FITC MicroBeads (colloidal superparamagnetic beads conjugated to MAb anti-FITC isomer 1, mouse IgG1; anti-FITC MultiSort kit, order no. 587-01; Miltenyi Biotec) and incubated in the dark for 15 min at 6 to 12°C. After washing with MACS buffer, cells were resuspended in 500 μl of MACS buffer and loaded on an LS+ column equipped with Flow-Resistor 26g (Miltenyi Biotec) under the influence of a magnetic field. Unbound cells were eluted from the column with MACS buffer and, if necessary, residual CD8-positive cells were recovered on an MS+ column (Miltenyi Biotec). CD8-negative cells in the final eluate were discarded. CD8-positive cells were eluted likewise after disconnection of the magnetic field. The beads were released from the cells by using MultiSort release reagent for 10 min at 6 to 10°C (20 μl per ml of cell suspension). Remaining magnetically labeled cells were removed by passage through an MS+ column under the influence of a magnetic field. Released CD8-positive cells (<107 cells) were resuspended in 50 μl of MACS buffer and 30 μl of MultiSort stop reagent. After washing with MACS buffer, cells (<107 cells) were resuspended in 93 μl of MACS buffer and 7 μl of CD62L (L-selectin) MicroBeads (colloidal superparamagnetic beads conjugated to MAb anti-CD62L, clone MEL-14, rat IgG2aκ; order no. 497-01; Miltenyi Biotec) and incubated for 15 min at 4°C. After washing with MACS buffer, separation on an LS+ column was performed as outlined above except that both the CD62Llo/neg (eluate under magnetic field) and the CD62Lhi fraction (eluate after disconnection of magnetic field) were recovered. All steps in the purification and separation were controlled by two-color cytofluorometric analysis of a cell aliquot (see below).
Multicolor cytofluorometric analyses.
Cytofluorometric analyses were performed with a FACSort (Becton Dickinson, San Jose, Calif.) by using CellQuest software (Becton Dickinson) for data processing. All procedures were performed essentially as described previously (1, 18). Thresholds were set in the forward-versus-side scatter (FSC-vs-SSC) plot to exclude particles of the size of erythrocytes or smaller during data acquisition. For calculations, a lymphocyte gate was set in the FSC-vs-SSC plot to largely exclude macrophages and residual granulocytes from the analysis. Throughout, fluorescence channel 1 (FL-1) represents the fluorochrome fluorescein, FL-2 represents the fluorochrome PE, and FL-3 represents either the tandem fluorochrome PE-Cy5 (also known as Cy-Chrome) or the tandem fluorochrome PE-Texas red (also known as RED613 or duochrome). Quadrants in the two-dimensional dot plots were defined by labeling with appropriately conjugated isotype control antibodies.
(i) Quantitation of α/β T cells in pulmonary infiltrates.
Pulmonary infiltrate cells retrieved from the Ficoll interphase were labeled with PE-conjugated anti-T-cell receptor (TCR) α/β MAb (clone H57-597, hamster IgG; catalog no. 01305A; PharMingen) and FITC-conjugated anti-CD3ɛ MAb (clone 145-2C11, hamster IgG; catalog no. 01084A; PharMingen). Absolute numbers of α/β T cells in the lungs were estimated by immunohistological quantitation of CD3ɛ-positive cells (38) in combination with cytofluorometric determination of the proportion of TCR α/β-expressing T cells among the CD3ɛ-expressing cells, which include also γ/δ T cells (17).
(ii) Determination of CD8/CD4 subset ratios among α/β T cells.
Pulmonary infiltrate cells retrieved from the Ficoll interphase were labeled with FITC-conjugated anti-CD8 MAb (see above), PE-conjugated anti-TCR α/β MAb (see above), and RED613-conjugated MAb anti-CD4 (clone H129.19, rat IgG2a; catalog no. 19862-028; Gibco BRL, Eggenstein, Germany). The analysis was restricted to α/β T cells by setting an electronic gate on signals with positive FL-2.
(iii) CD62L activation phenotyping of CD8 T cells.
For reanalysis after two-marker immunomagnetic cell sorting (see above), CD8 T cells (still carrying FITC-conjugated anti-CD8a) were additionally labeled with PE-conjugated MAb anti-CD62L (clone MEL-14; catalog no. 01265B; PharMingen).
IFN-γ-based ELISPOT assays.
Principles of the ELISPOT assay and all details of the procedure have been described previously (33, 54), with modifications described in reference 19. Effector cells were either BALB/c-derived CD8-positive cytolytic T cells (CTL) from a long-term IE1 (peptide YPHFMPTNL)-specific line (18, 19) or immunomagnetically sorted CD8 T cells derived from lung tissue. Effector cells were tested in graded numbers. Unless stated otherwise, effector cell titration was performed in log10 steps beginning with 104 cells. Target cells were P815 mastocytoma cells (H-2d haplotype) stably transfected with human B7-1 cDNA (4), referred to as P815-B7 cells (used with the kind permission of L. L. Lanier, DNAX, Palo Alto, Calif.). The number of target cells was kept constant and was 105 per assay culture. Since the highest number of effector cells was 104 per culture, the effector-to-target cell ratio never exceeded 0.1, so that sterical access to target cells was not a limiting factor in the stimulation of the effector cells.
(i) Peptide-specific ELISPOT assay.
The assay was used to quantitate CD8 T cells specific for antigenic peptides presented by MHC class I molecules and functionally capable of responding with the synthesis of IFN-γ. Peptide-presenting target cells were P815-B7 pulsed for 2 h at 37°C with a saturating concentration of synthetic peptides, which was 10−8 M in the case of the mCMV nonapeptides IE1 (amino acids [aa] 168 to 176, presented by Ld), m04 (aa 243 to 251, presented by Dd), M83 (presented by Ld), and M84 (aa 297 to 305, presented by Kd) and 10−7 M in the case of nonapeptide NP (aa 118 to 126, presented by Ld), which is derived from the nucleoprotein (NP) of lymphocytic choriomeningitis virus (LCMV) (52, 57). It should be noted that use of higher peptide concentrations did not change the results. Excess peptide was washed out before use of the pulsed P815-B7 cells as target cells in the assay. Custom peptide synthesis in 1-mg scale and a purity of >75% was performed by JERINI Bio Tools GmbH (Berlin, Germany). Peptides were dissolved in 30% (vol/vol) acetonitrile in phosphate-buffered saline at a concentration of 10−3 M. Further dilutions were performed in culture medium.
(ii) CD3ɛ-redirected ELISPOT assay.
A modification of the ELISPOT assay was used to quantitate the overall number of effector cells functionally capable of responding with the synthesis of IFN-γ after engagement of the TCR-CD3 complex by anti-CD3ɛ MAb bound to P815-B7 cells via Fc receptor. Specifically, 5 × 105 P815-B7 cells were incubated for 20 min at 20°C in 0.1 ml of culture medium containing 3 to 4 μg of anti-murine CD3ɛ MAb (clone 145-2C11; catalog no. 530-14; Dianova, Hamburg, Germany), depending on the batch of antibody. Excess antibody was washed out before use of the CD3ɛ-armed P815-B7 cells as target cells in the ELISPOT assays. It is important to note that higher doses of anti-CD3ɛ MAb during labeling of the P815-B7 cells did not result in higher numbers of responding effector cells in the assay. Spots, representing individual IFN-γ-secreting cells, were counted under a zoom stereomicroscope (model SZX12; Olympus), and photodocumentation of the ELISPOT microwell membranes was made with a 3- by 12-bit CV12 digital camera using SIS analySIS 3.0 Doku Soft software (Soft Imaging System GmbH, Münster, Germany) and Adobe Photoshop 4.0.
RESULTS
Establishment of a CD3ɛ-redirected ELISPOT assay for quantitation of IFN-γ-secreting CD8 effector T cells.
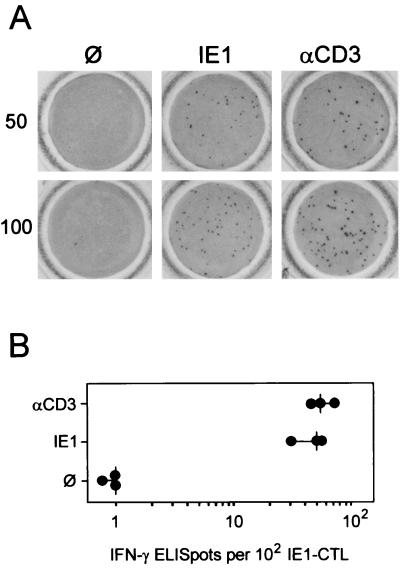
To evaluate the quantitative significance of a particular MHC class I-presented antigenic peptide in the CD8 T-cell response, it is useful to relate the number of peptide-specific CD8 effector T cells to the total number of CD8 effector T cells in a polyclonal and polyspecific population. For cytolytic effector function, the CD3ɛ-redirected cytolysis assay can be used to estimate the overall cytolytic potential of an effector cell population independent of the peptide specificities of the TCRs by polyclonal signaling via the CD3ɛ molecule of the TCR-CD3 complex (24). This is accomplished by anti-CD3ɛ antibodies bound via Fc to Fc receptor-expressing target cells, such as P815 (haplotype H-2d) mastocytoma cells. For a monospecific CTL line (CTLL) specific for the MHC class I Ld-presented IE1 peptide 168YPHFMPTNL176 of mCMV (IE1-CTLL), we have previously shown that peptide-specific cytolytic activity equals the CD3ɛ-redirected cytolytic activity, whereas there was a great difference between the two assays in case of polyspecific CD8 T cells (18). Quantitation of effector cells in ex vivo lymphocyte populations is difficult to achieve on the basis of target cell lysis. Here we have used the principle of CD3ɛ-mediated triggering of effector cell function to establish an ELISPOT assay for the single-cell visualization and quantitation of effector T cells capable of IFN-γ secretion irrespective of TCR specificity. The validity of the assay was evaluated with IE1-CTLL (Fig. 1). In case of a monospecific CTLL, presentation of the respective peptide by MHC class I on the target cells and triggering of the TCR-CD3 complex by anti-CD3ɛ should ideally give the same effector cell numbers. Representative filters are shown in Fig. 1A for 50 and 100 IE1-CTL seeded in the ELISPOT cultures, and data for three independent cultures are compiled in Fig. 1B. Absence of background indicates that IE1-CTL did not spontaneously secrete IFN-γ. Spots generated after stimulation with Ld-presented IE1 peptide 168YPHFMPTNL176 were smaller than those generated by stimulation with anti-CD3ɛ, which reflects a quantitative difference in the amount of released IFN-γ. While the seeded cells were all found to be viable, the response rate was reproducibly lower than 100% (ca. 50% in the example shown [Fig. 1B]). Most importantly, the number of spots and thus the number of functionally competent effector cells were identical in the two types of assay. We infer from this result that even in a CTLL growing in cell culture, individual cells are not permanently in a permissive state for triggering of their effector function. In conclusion, all functionally competent cells of the line, and not just the 50% that responded in the assays, were actually specific for the IE1 peptide. This conclusion entails a very important consequence: frequencies of peptide-specific effector cells measured in ELISPOT assays usually relate to the total number of CD8 T cells present in the tested population instead of to the number of functionally competent CD8 T cells. This means of quantitation is prone to lead to an underestimation of the prevalence of effector cells specific for a particular peptide.
FIG. 1.
Comparison between peptide-specific and CD3ɛ-redirected ELISPOT assays. Assays were performed with an IE1 (YPHFMPTNL) peptide-specific CTLL as effector cells and P815-B7 cells as the stimulating target cells. (A) Photodocumentation of ELISPOT microwell membranes for 50 and 100 IE1-CTL seeded. Shown is one of triplicate assay membranes after staining of bound IFN-γ. ∅, P815-B7 cells with no peptide added; IE1, P815-B7 cells pulsed with a saturating (10−8 M) dose of IE1 peptide; αCD3, P815-B7 cells loaded via their Fc receptors with anti-CD3ɛ MAb. (B) ELISPOT plot showing the number of spots counted. Each dot represents the result for one set of the triplicate assay cultures. The vertical dash indicates the median value.
The assays documented here for an IE1-specific CTLL were also performed with essentially the same results (not shown) for the previously described CTLL specific for the Dd-restricted peptide 243YGPSLYRRF251, which is derived from the E-phase protein m04 (gp34) of mCMV (19). This finding ensured that differences in effector cell frequencies determined for pulmonary CD8 T-cell populations (see below) were not skewed by the assay conditions.
Subset composition of interstitial T cells in different phases of mCMV pneumonia.
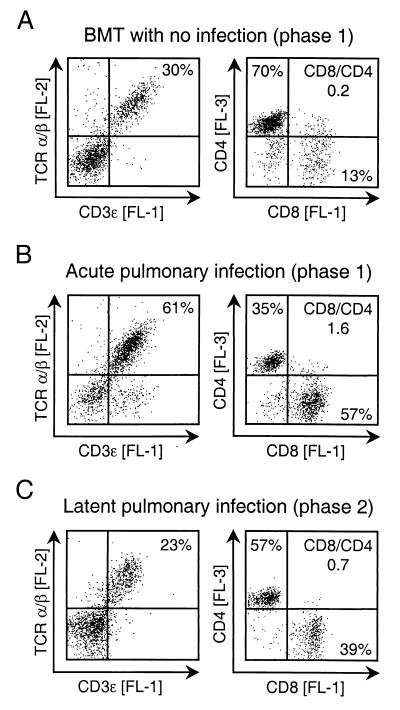
In a preceding report, we defined two phases in the murine model of CMV pneumonia after syngeneic BMT (38). Phase 1 represents the peak of T-cell infiltration during acute, productive infection of the lungs and is histologically characterized by inflammatory foci consisting of infected lung cells and blastoid CD8 T cells which confine and finally resolve the productive infection. Phase 2 represents the situation after resolution of the productive infection and is histologically characterized by the persistence of disseminated interstitial T cells in absence of viral replication. From earlier work in the very same experimental model, it is known that phase 2 lungs remain latently infected (27, 29) and that immunoablative treatment induces reactivation of the productive cycle and virus recurrence (28). The influence of mCMV infection on the composition of the pulmonary T-cell pool is shown for phase 1 by cytofluorometric phenotyping of lung infiltrate cells from uninfected and infected BMT recipients (Fig. 2A and B), and the difference between acute and latent infection of the lungs is shown by phenotyping of phase 1 and phase 2 infiltrates (Fig. 2B and C). In accordance with previous work (1, 18, 38), mCMV infection caused a fulminant recruitment of CD8 T cells to the lungs. Most CD3ɛ-expressing cells in the phase 1 infiltrates in infected lungs (Fig. 2B, left) were TCR α/β T cells, but a notable subset did not express TCR α/β. These cells were previously identified as TCR γ/δ T cells (17), but their role in mCMV infection is still undetermined. After resolution of the acute infection, the proportion of T cells among the infiltrate cells declined, but the proportion of CD8 T cells among the α/β T cells remained elevated. We have previously shown by quantitative immunohistology that T-cell infiltrates persist in the lungs for the life span of the infected BMT recipients (38). In conclusion, CD8 T cells are preferentially recruited to acutely infected lungs, and elevated numbers of CD8 T cells persist in lung tissue during latent infection.
FIG. 2.
Quantitative analysis of T-cell subsets in pulmonary infiltrates. The proportion of T cells among pulmonary infiltrate lymphocytes was determined by setting a lymphocyte gate in the FSC-vs-SSC plot (not shown) followed by two-color cytofluorometric analysis of the cell surface expression of CD3ɛ [FL-1] and TCR α/β [FL-2]. The proportion of CD8 T cells and CD4 T cells was determined by three-color cytofluorometric analysis of the expression of CD8 [FL-1], TCR α/β [FL-2], and CD4 [FL-3]. An electronic gate was set on positive FL-2 to restrict the analysis of CD8 and CD4 expression to α/β T lymphocytes. Shown are two-dimensional dot plots for 10,000 gated cells with 2,500 dots displayed. Quadrants were defined by isotype controls. Percentages of populations of interest are indicated in the appropriate quadrants. CD8/CD4, ratio of CD8 T cells to CD4 T cells. (A) Analysis of pulmonary leukocytes isolated at 4 weeks (phase 1) after BMT performed with no infection. (B) Analysis of pulmonary leukocytes isolated during viral replication in the lungs at 4 weeks (phase 1) after BMT and simultaneous infection with mCMV. (C) Analysis of pulmonary leukocytes isolated after resolution of productive infection at 3 months (phase 2), i.e., during viral latency in the lungs.
Frequency and peptide specificity of interstitial pulmonary CD8 effector T cells.
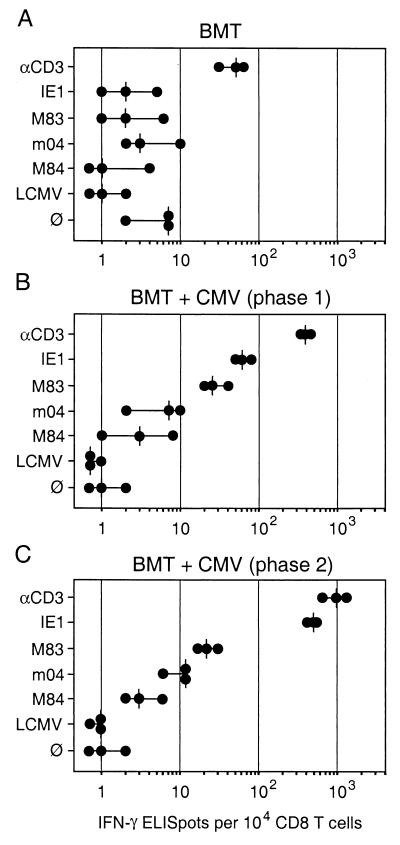
Antiviral effector function of immunomagnetically purified phase 1 and phase 2 pulmonary CD8 T cells was documented recently by means of preemptive adoptive cell transfer in lethally infected, hematoablated indicator recipients (38). Notably, the protective antiviral efficacy was found to be somewhat higher with phase 2 CD8 T cells, suggesting that the frequency of virus-specific effector cells might be higher in a phase 2-derived cell pool. Here we have focused on effector cells defined by target cell-induced secretion of IFN-γ. The CD3ɛ-redirected ELISPOT assay was used to measure the overall frequency of IFN-γ-secreting effector cells in immunomagnetically purified pools of polyclonal CD8 T cells derived directly from pulmonary infiltrates. This number was related to the numbers of peptide-specific CD8 effector T cells measured by the conventional IFN-γ-based ELISPOT assay (Fig. 3). To date, three antigenic peptides are published for mCMV. Peptide 168YPHFMPTNL176 is processed from the IE-phase protein IE1 (gene m123-exon 4; pp89) and is presented by the MHC class I molecule Ld (47). This peptide was the first antigenic peptide identified for a CMV and was considered an immunodominant peptide in the H-2d haplotype (for reviews, see references 22 and 42). Peptide 243YGPSLYRRF251 is processed from the E-phase protein m04 (gene m04; gp34) and is presented by Dd (19). Particular interest in this protein resulted from its binding to MHC class I molecules and its proposed role in silencing natural killer cells (21). Peptide 297AYAGLFTPL305 is processed from the E-phase protein M84 (gene M84; p65) and is presented by Kd (20). Interest in M84-p65 resulted from the findings that it protects against mCMV (36) and possesses significant amino acid homology to hCMV UL83-encoded pp65 (9, 35), which is considered immunodominant in hCMV (7, 31, 59; for a review, see reference 42). In addition, we used an unpublished nonapeptide derived from mCMV protein M83 (gene M83; pp105) and presented by Ld (M. J. Reddehase and R. Holtappels, presented at the 25th International Herpesvirus Workshop, Portland, Oreg., 20 July to 4 August 2000). Gene M83 of mCMV is the positional homolog of hCMV UL83, and its expression product M83-pp105 shows amino acid homology to hCMV UL83-pp65. It resembles its hCMV homolog by virtue of its late expression kinetics, phosphorylation, and virion association (9, 35). For verification of mCMV specificity of responses, we used peptide 118RPQASGVYM126, which is derived from the NP of LCMV and is presented by Ld (52, 57). Finally, the assay baseline defined by the number of cells that spontaneously secrete IFN-γ was determined by omission of stimulating peptide.
FIG. 3.
Frequencies of IFN-γ-secreting effector T cells in pulmonary infiltrates. Effector cells in the ELISPOT assays were immunomagnetically purified CD8 T cells derived from pulmonary infiltrates. (A) Infiltrates isolated at 4 weeks (phase 1) after BMT with no infection. (B) Infiltrates isolated at 4 weeks (phase 1) during acute infection of the lungs. (C) Infiltrates isolated at 3 months during latent infection of the lungs. The CD3ɛ-redirected ELISPOT assay (Fig. 1) was used to determine the frequency of effector cells irrespective of their antigen specificity. Negative controls included the presentation of an unrelated peptide (LCMV NP aa 118 to 126) by the Ld molecule of P815-B7 cells as well as omission of peptide (∅). Specific peptides of mCMV included IE1 (aa 168 to 176) presented by Ld, M83 presented by Ld, m04 (aa 243 to 251) presented by Dd, and M84 (aa 297 to 305) presented by Kd. Dots represent results from individual assay cultures. Vertical dashes indicate median values.
(i) Frequency of pulmonary effector cells after BMT in absence of mCMV infection.
CD8 T cells reconstituted after BMT may potentially be primed by numerous antigens intrinsic to the recipient and unrelated to mCMV. These effector cells were quantitated in phase 1 (that is, 4 weeks after BMT) in uninfected BMT recipients (Fig. 3A). There were indeed effector cells detected by the antigen-independent CD3ɛ-redirected ELISPOT assay in a frequency of 30 to 60 per 104 CD8 T cells, that is, 0.3 to 0.6% (range with a median of 0.5%) of all CD8 T cells in the pulmonary infiltrates. To evaluate the biological significance of this background activity, we wished to obtain an estimate of the absolute numbers (Table 1). We have documented previously first by histological image (18) and later by quantitative immunohistology (38) that infiltration of the lungs is minute in uninfected BMT recipients. The absolute number of immunohistologically detected CD3ɛ-expressing T cells was ca. 3.2 × 105 per lung at 4 weeks after BMT in absence of infection (not shown). Based on the proportion of CD8 T cells (Fig. 2A) and the frequency determined in the CD3ɛ-redirected ELISPOT assay (see above), the absolute number of IFN-γ-defined effector cells was only ca. 200 cells per lung. As revealed by the ELISPOT control cultures with target cells that did not present a defined peptide or that presented a peptide unrelated to mCMV (here LCMV NP aa 118 to 126), the baseline of the ELISPOT assay was found to be in the range of zero to seven spots per 104 CD8 T cells. Operationally, we would define 10 spots, which corresponds to a frequency of 0.1%, as the significance limit. As one would predict for uninfected recipients, frequencies of effector cells specific for any of the four tested mCMV-derived peptides did not exceed the significance limit.
TABLE 1.
Absolute numbers of ELISPOT-reactive effector cells in lungs
| Group (time [wk] after BMT) | Cell no.a
|
IE1/CD3ɛ | |||
|---|---|---|---|---|---|
| CD8 T cellsb | Effector cellsc
|
||||
| CD3ɛ | IE1 | M83 | |||
| No infection (4) | 40,000 | 200 | <DL | <DL | 0 |
| Acute infection (4) | 4,600,000 | 180,000 | 30,000 | 12,000 | 1/6 |
| Latent infection (12) | 1,200,000 | 120,000 | 60,000 | 3,000 | 1/2 |
Determined from pools of lungs and given as absolute number per lung.
Calculated from the absolute numbers of CD3ɛ-positive T cells determined by quantitative immunohistology (not shown) and the percentage of CD8 T cells determined by cytofluorometry (Fig. 2). The minor population of γ/δ T cells contained in the CD3ɛ-positive T-cell population is negligible.
Calculated from the absolute numbers of CD8 T cells (see footnote b) and the percentage of CD8 T cells (median values) that were reactive in the IFN-γ-based CD3ɛ-redirected ELISPOT assay or in the IFN-γ-based IE1 or M83 peptide-specific ELISPOT assay (Fig. 3), as indicated. DL, detection limit of the ELISPOT assay, defined by a frequency of <0.1%.
(ii) Frequency and specificity of pulmonary effector cells during acute infection of the lungs.
In phase 1 infiltrates of infected BMT recipients, the relative frequency of pulmonary effector cells defined by the CD3ɛ-redirected ELISPOT assay was ca. 10-fold higher than in uninfected BMT recipients (Fig. 3B compared with 3A), that is, ca. 400 cells (median) per 104 CD8 T cells or 4% of all CD8 T cells in the infiltrates. Again, it is instructive to consider absolute numbers (Table 1). As determined by quantitative immunohistology using the method described in our previous report (38), the number of CD3ɛ-expressing T cells was ca. 8 × 106 per lung at 4 weeks after BMT in presence of infection. Based on the proportion of CD8 T cells (Fig. 2B) and the relative frequency determined in the CD3ɛ-redirected ELISPOT assay (see above), the absolute number of IFN-γ-defined effector cells was ca. 1.8 × 105 cells per lung, or about 900-fold more than at the same time in the kinetics after BMT with no infection. Effector cells specific for the IE1 peptide were present in phase 1 infiltrates in a lower but clearly significant frequency, 50 to 80 (range, with a median of 60) per 104 CD8 T cells, that is, ca. 0.6% (median) or in absolute terms ca. 30,000 IE1-specific effector cells per lung. Notably, while the two E-phase peptides m04 and M84 were not recognized by a significant number of effector cells, the M83 virion protein-derived peptide was recognized by 20 to 40 (range, with a median of 24) cells per 104 CD8 T cells, which is ca. 12,000 M83-specific effector cells per lung (Table 1).
(iii) Increased frequency of memory-effector cells during latent infection of the lungs.
In phase 2 infiltrates during latent mCMV infection, the frequency of pulmonary effector cells defined by the CD3ɛ-redirected ELISPOT assay was ca. 2.5-fold higher than in phase 1 infiltrates (Fig. 3C compared with 3B), that is, ca. 1,000 cells (median) per 104 CD8 T cells or 10% of all tissue-resident, interstitial CD8 T cells. Absolute numbers demonstrate that this frequency reflects only a relative increase (Table 1). In phase 2, specifically after 3 months, the number of immunohistologically detected CD3ɛ-expressing T cells had declined to ca. 3 × 106 per lung. Taking further into account the decreased proportion of CD8 T cells (recall Fig. 2C), the absolute number of IFN-γ-defined effector cells was ca. 1.2 × 105 cells per lung, as opposed to ca. 1.8 × 105 in phase 1 (see above). Notably, the increase in relative frequency was far more pronounced for IE1-specific memory-effector cells: to 500 cells (median) per 104 CD8 T cells, that is, from 0.6 to 5% of all CD8 T cells. In absolute terms, this is 60,000 IE1-specific memory-effector cells per lung, as opposed to only 30,000 in phase 1 (Table 1). The frequency of CD8 T cells specific for the E-phase peptides m04 and M84 remained very low and was barely significant. Most importantly, the relative frequency of M83-specific effector cells did not increase between acute and latent infection, and in absolute terms the number of these cells even decreased to ca. 3,000 (Table 1). This finding documents that the enrichment observed for effector cells in total, and for IE1-specific effector cells in particular, does not apply to every mCMV specificity. In conclusion, of the specificities that were available for testing, only IE1-specific memory-effector cells were found to be enriched in phase 2 pulmonary infiltrates in terms of relative frequency as well as in terms of absolute numbers.
IE1-specific effector cells are enriched in a CD62Llo subset of phase 2 CD8 T cells.
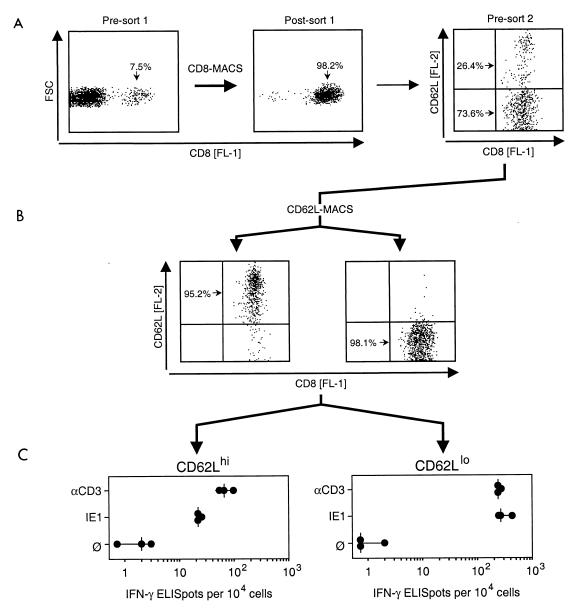
CD62L (also known as L-selectin, Ly-22, and MEL-14 antigen) is a member of the selectin family and contributes to the recruitment of leukocytes into areas of inflammation (for a review, see reference 55). Upon stimulation of lymphocytes via the TCR-CD3 complex, CD62L is rapidly shed from the cell surface by proteolytic cleavage. Resting memory cells among tissue-resident pulmonary CD8 T cells should therefore express CD62L, whereas restimulated memory-effector cells should accumulate in a CD62Llo subset of the CD8 T cells (34). We have recently shown that phase 2 pulmonary CD8 T cells are subdivided into CD62Lhi and CD62Llo subsets (38). After stimulation with soluble anti-CD3ɛ antibodies, cells positive for intracellular IFN-γ were phenotyped as CD62Llo cells (38). However, this approach cannot be used to localize in vivo-activated memory-effector cells to either subset, because in vitro stimulation downregulates CD62L during the assay period (38). It was therefore crucial to sort the cells into CD62Lhi and CD62Llo subsets before entering the assay. Results of such an assay are shown in Fig. 4.
FIG. 4.
Localization of IFN-γ-secreting effector cells in CD8 T-cell subsets defined by expression of the activation marker CD62L. Interstitial leukocytes were isolated from latently infected lungs at 3 months after BMT and primary mCMV infection. (A) CD8 T cells were enriched to almost purity by positive immunomagnetic cell sorting (CD8-MACS, sort 1) and analyzed for cell surface expression of CD8 [FL-1] and L-selectin CD62L [FL-2]. FSC, forward scatter representing cell size. The two-dimensional presort 2 dot plot shows 2,500 cells. (B) Cytofluorometric reanalysis of CD8 T cells after immunomagnetic sorting into CD62Lhi and CD62Llo subsets (CD62L-MACS, sort 2). Analyses were performed for 2,500 sorted cells, with all cells displayed as dots. (C) Frequencies of IFN-γ-secreting effector cells determined by CD3ɛ-redirected and IE1 (YPHFMPTNL) peptide-specific ELISPOT assays. ∅, P815-B7 target cells with no peptide added. Dots represent results from individual assay cultures. Vertical dashes indicate median values.
CD8 T cells present in phase 2 (3 months) pulmonary infiltrates during latent mCMV infection of the lungs were enriched from 7.5% to almost purity in a first step of immunomagnetic sorting. Reanalysis of the sorted cells revealed subpopulations of CD62Lhi and CD62Llo cells (Fig. 4A). The population was then subjected to immunomagnetic sorting with anti-CD62L-conjugated microbeads, and successful separation of the two subsets was verified by cytofluorometric reanalysis (Fig. 4B). The purified CD62Lhi and CD62Llo CD8 T cells were then analyzed for effector function in CD3ɛ-redirected and IE1 peptide-specific IFN-γ-based ELISPOT assays (Fig. 4C). It should be noted that the difference in procedures does not allow a direct frequency comparison between cells that have undergone single sorting (as in Fig. 3) and those that have undergone dual sorting. CD8 effector T cells were clearly found to be enriched in the CD62Llo subset, which implies that their activation resulted from contact with antigen in vivo. Notably, while CD62Lhi cells revealed a significant difference in response to polyclonal and IE1-specific stimulation, which indicates the existence of several specificities in that subpopulation, CD62Llo cells were predominantly IE1 specific.
DISCUSSION
After resolution of productive infection of the lungs in a murine model of CMV pneumonia following syngeneic BMT, mCMV genome is maintained in lung tissue in a latent state for the life span of the recipients (27, 29, 53). The establishment of latency is accompanied by persistence of elevated numbers of tissue-resident T cells (18, 38). Notably, a significant proportion of interstitial CD8 T cells were found to display the phenotype of activated cells, as evidenced from the absence of cell surface L-selectin CD62L (38). A recent report by Harrington et al. noted a characteristic change in the expression of surface O-glycans that differentiates between memory and effector CD8 T cells (14) and will likely help to further characterize the activation state of the persisting pulmonary CD8 T cells. Previous views have proposed that lung infiltrates mediate CMV-associated immunopathology (12, 13), but the demonstration of a protective antiviral function of the persisting pulmonary CD8 T cells upon adoptive transfer in lethally infected indicator recipients led us to infer an antiviral immune surveillance function of these cells in the lungs of the latently infected donors. We proposed a concept of standby memory cells, which might serve to prevent reactivation of latent virus (38). Indirect evidence in support of this hypothesis was provided by the demonstration of recurrent infection in the lungs after secondary immunoablative treatment performed either by γ-irradiation (28, 53) or by selective depletion of CD8 T cells (40).
What is the signal that causes memory T cells to persist at an extralymphoid tissue site, and what is the stimulus responsible for the perpetuation of an activated memory-effector phenotype in the absence of productive viral replication? The currently accepted view is that the maintenance of long-term memory does not depend on persistent antigenic stimulation. Specifically, memory CD8 T cells were shown to persist indefinitely in the absence of priming antigen, retain a CD44hi memory phenotype (30), and express an increased level of Bcl-2, which is supposed to prolong survival by preventing apoptosis (11). Most convincingly, Murali-Krishna et al. (37) have documented maintenance of memory CD8 T cells in MHC class I-deficient mice, which are unable to present antigenic peptides to CD8 T cells. Notably, memory cells in these mutant mice evolved an MHC class I-independent behavior in that they were capable of maintaining their numbers by homeostatic proliferation that does not involve signaling by MHC-TCR interaction. Nonetheless, these memory cells retained the ability to secrete cytokines upon reencountering the priming antigen. These findings are relevant to the interpretation of our data in that the presence of memory CD8 T cells is per se not an indication for the presentation of antigenic viral peptides during latency, and even the relative enrichment of CD3ɛ-reactive CD8 T cells in latently infected lungs could be explained by homeostatic proliferation, in particular since the absolute numbers had actually declined (Table 1).
A slightly different view of T-cell memory was presented by Zinkernagel and colleagues (5, 25; for a review, see reference 26), who emphasized the different requirements for the maintenance of elevated numbers of quiescent memory CD8 T cells homing in lymphoid tissues and the infiltration of peripheral nonlymphoid organs. While central memory in lymphoid tissues is independent of restimulation by antigen, a certain state of activation by persisting antigen is said to be needed by memory CD8 T cells to operate in nonlymphoid tissues. According to this view, the maintenance of an interstitial CD8 T-cell pool during mCMV latency in the lungs should require the presentation of antigenic peptides, in particular since a significant proportion of these cells displayed the CD62Llo phenotype of recently sensitized cells. It should be noted that our previous immunohistological analysis clearly documented that the persisting pulmonary CD8 T cells are localized in the lung parenchyma (38), that is, at an extralymphoid site.
It is instructive to compare our model of pneumonia caused by a virus that establishes latent infection in the lungs with a model of pneumonia caused by a virus that is cleared after acute infection of the lungs. Notably, like in our phase 1 scenario of acute pulmonary infection with mCMV, Flynn et al. (10) described infiltration of the lungs by antiviral CD8 T cells during primary influenza virus pneumonia. However, after clearance of the primary infection, memory was established in the mediastinal lymph nodes, and it took the memory CD8 T cells 4 to 5 days to relocalize to the respiratory tract after a secondary influenza virus infection. We therefore conclude that the persistence of activated (CD62lo) tissue-resident CD8 T cells during latent mCMV infection indicates an iterative restimulation by viral peptides presented during latency.
A selecting force exerted by restimulation with viral peptides presented during mCMV latency is indicated by the finding that IE1 peptide-specific memory-effector cells were enriched in latently infected lungs to a higher degree than the memory-effector cells defined by the CD3ɛ-redirected ELISPOT assay representing all specificities involved in the polyclonal response. Such a preferential enrichment predicted the existence of specificities that do not take part in the enrichment process. The M83 peptide represents an example of this category of mCMV antigens. Altogether, the CD62Llo phenotype of IE1-specific interstitial CD8 T cells along with their relative and absolute enrichment during latency indicates presentation of the IE1 peptide during latency in the lungs.
Viral transcription and protein synthesis are required for the presentation of antigenic peptides. It was therefore intriguing to find that correctly spliced transcripts of gene ie1, which codes for the IE1 peptide, can be detected by reverse transcriptase PCR in latently infected lungs (27) in the absence of further transcripts of the viral cycle, such as transcripts from genes ie3 and gB, specifying the essential transactivator protein IE3 (2, 32) and the virion envelope glycoprotein gB (41), respectively. However, despite a painstaking approach of screening hundreds of tissue sections from latently infected lungs, we have been unable to detect the intranuclear IE1 protein pp89 by sensitive immunohistology, and nor have we been able to detect naturally processed IE1 peptide with the IE1-CTLL in extracts from latently infected lungs (not shown). A likely reason for these failures is the very low frequency of transcriptional events during latency. Specifically, most viral genomes are transcriptionally silent during latency, and the ie1 transcription is focal and ephemeral, with a frequency of 10 foci, probably just 10 individual cells among ca. 60 million lung cells, at any point of time during latency (27, 28). However, if such an inapparent presentation of the IE1 peptide were to occur during latency and iteratively restimulate the standby CD8 T cells over several months, the CD8-T-cell pool should build up apparent IE1-specific memory reflected by an increased frequency of IE1-specific memory-effector cells. The data presented here support this hypothesis.
It must be pointed out that latency-associated transcription is not necessarily restricted to the ie1 gene but may occur also from other parts of the viral genome, as suggested by representational difference analysis comparing transcription in latently infected lung tissue with that in normal lung tissue (H. W. Virgin IV, plenary lecture at the 7th International Cytomegalovirus Workshop, Brighton, England, 1999). If these transcripts account for the presentation of antigenic peptide(s), memory cells with the corresponding specificity(ies) should coenrich with IE1-specific memory cells during latency. Our data show that there is sufficient space left for unidentified further antigen specificities represented in the pulmonary memory T-cell pool. Accordingly, the decline in the number of M83-specific CD8 T cells predicts that gene M83 is not expressed during latency. Experiments to test this prediction are under way.
The life-long maintenance of the latent viral genome is an obvious problem of the immune surveillance hypothesis. Why does persistent control by CD8 T cells not eventually result in the elimination of latently infected cells and, consequently, in clearance of the viral genome from the lungs? Previous work in this particular model of mCMV latency has estimated a load of ca. 5,000 viral genomes per 106 lung cells (53), or ca. 3 × 105 viral genomes per lung. Even if we assume that the mechanism of the surveillance is the elimination of latently infected lung cells by the cytolytic function of CD8 T cells, there is currently no way to calculate the kinetics of the proposed clearance because the viral DNA copy number per cell and the rate of cell killing are not known. In addition, we must consider the possibility of replenishment by cell division and sterile viral DNA replication. Finally, control may be exerted via cytokines instead of by cytolysis. In short, this question remains unresolved.
One argument against immune surveillance of latency is that the observed enrichment of IE1-specific memory-effector cells may simply reflect a latency-associated presentation of the IE1 peptide, with no causal involvement of CD8 T cells in the maintenance of molecular latency. We have indeed shown previously that only few foci of reactivation after immunoablative treatment proceed to productive infection, whereas most are arrested at earlier stages of viral gene expression (28). On the other hand, the interstitial T cells are clearly not anergic, as can be inferred from their protective antiviral function upon adoptive cell transfer (38) and from their effector function in the cytolytic assay (38) and the IFN-γ-based ELISPOT assay (this report). Clearly, intrinsic molecular mechanisms and T-cell control may cooperate in the maintenance of latency.
Besides the key finding, our data have further implications. It had been a reasonable speculation that the prevalence of IE1-specific CD8 T cells in immunity to mCMV as well as hCMV may result from IE1 peptide presentation during latency (for a review, see reference 42) when presentation is not subverted by the immune evasion functions of E-phase proteins (for reviews, see references 15 and 58). However, since IE1-specific CD8 T cells were relatively immunodominant in phase 1 infiltrates during acute infection of the lungs (Fig. 3B), presentation of the IE1 peptide during latency cannot be the only explanation for the immunodominance of IE1. It should be recalled that the original finding of the immunogenicity of the mCMV IE1 protein was made for CD8 T lymphocytes derived during acute intraplantar infection from the draining popliteal lymph node (45). Thus, events during acute infection must already favor IE1 peptide-specific priming. Recent work by Hengel et al. (16) has suggested the importance of infected macrophages for efficient priming of the IE1 peptide-specific CD8 T-cell response. Notably, unlike the situation in fibroblasts, immunosubversive functions of the m152 and m06 E-gene products gp40 (60) and gp48 (49), respectively, were found not to be effective in productively infected macrophages. Accordingly, the IE1 peptide is presented in infected macrophages throughout the viral replication cycle. However, while this mechanism explains effective IE1-specific priming in vivo, it does not explain the immunodominance of IE1 because presentation of E-phase peptides should benefit too. Yet, as shown herein, the two currently known E-phase peptides m04 243YGPSLYRRF251 (19) and M84 297AYAGLFTPL305 (20) of mCMV were not significantly involved in the pulmonary CD8 T-cell response, neither during acute infection nor during latency. However, we caution against the conclusion that E-phase proteins are generally not relevant for the immune control of mCMV. We have shown previously for phase 1 pulmonary infiltrates that the IE1 peptide accounts for a minor part of the overall cytolytic CD8 T-cell activity and that infected fibroblasts are well lysed in the E phase (18), even though the immunosubversive functions should be effective in fibroblasts during the E phase. Obviously, the relevant E-phase peptides remain to be identified. Work on immunity to influenza virus has revealed that immunodominance in class I-restricted CD8 T-cell responses can have multiple causes (8). This is likely to apply also to mCMV.
Conclusion.
An immune surveillance function of IE-specific CD8 T cells in CMV latency had been proposed when the immunogenicity of IE-phase proteins was discovered in 1984 for the example of mCMV (45). The data presented here provide initial experimental evidence in support of this hypothesis.
ACKNOWLEDGMENTS
Hans-Peter Steffens (now at Miltenyi Biotec GmbH, Bergisch-Gladbach, Germany) contributed cytofluorometric data in earlier stages of the project. We appreciated his ongoing advice regarding immunomagnetic cell sorting. Jürgen Podlech helped with the photodocumentation and contributed data on immunohistological T-cell quantitation.
Support was provided by a grant to M.J.R. from the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 490, individual project B1, “Immune Control of Latent Cytomegalovirus Infection.”
REFERENCES
- 1.Alterio de Goss M, Holtappels R, Steffens H-P, Podlech J, Angele P, Dreher L, Thomas D, Reddehase M J. Control of cytomegalovirus in bone marrow transplantation chimeras lacking the prevailing antigen-presenting molecule in recipient tissues rests primarily on recipient-derived CD8 T cells. J Virol. 1998;72:7733–7744. doi: 10.1128/jvi.72.10.7733-7744.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angulo A, Ghazal P, Messerle M. The major immediate-early gene ie3 of mouse cytomegalovirus is essential for viral growth. J Virol. 2000;74:11129–11136. doi: 10.1128/jvi.74.23.11129-11136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashton-Rickardt P G, Opferman J T. Memory T lymphocytes. Cell Mol Life Sci. 1999;56:69–77. doi: 10.1007/s000180050007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azuma M, Cayabyab M, Buck D, Philipps J H, Lanier L L. CD28 interaction with B7 costimulates primary allogeneic proliferative responses and cytotoxicity mediated by small, resting T lymphocytes. J Exp Med. 1992;175:353–360. doi: 10.1084/jem.175.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachmann M F, Kundig T M, Hengartner H, Zinkernagel R M. Protection against immunopathological consequences of a viral infection by activated but not resting cytotoxic T cells: T cell memory without “memory T cells”? Proc Natl Acad Sci USA. 1997;94:640–645. doi: 10.1073/pnas.94.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balthesen M, Messerle M, Reddehase M J. Lungs are a major organ site of cytomegalovirus latency and recurrence. J Virol. 1993;67:5360–5366. doi: 10.1128/jvi.67.9.5360-5366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boppana S B, Britt W J. Recognition of human cytomegalovirus (HCMV) gene products by HCMV-specific cytotoxic T cells. Virology. 1996;222:293–296. doi: 10.1006/viro.1996.0424. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Anton L C, Bennink J R, Yewdell J W. Dissecting the multifactorial causes of immunodominance in class I-restricted T cell responses to viruses. Immunity. 2000;12:83–93. doi: 10.1016/s1074-7613(00)80161-2. [DOI] [PubMed] [Google Scholar]
- 9.Cranmer L D, Clark C L, Morello C S, Farrell H E, Rawlinson W D, Spector D H. Identification, analysis, and evolutionary relationships of the putative murine cytomegalovirus homologs of the human cytomegalovirus UL82 (pp71) and UL83 (pp65) matrix phosphoproteins. J Virol. 1996;70:7929–7939. doi: 10.1128/jvi.70.11.7929-7939.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn K J, Belz G T, Altman J D, Ahmed R, Woodland D L, Doherty P C. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 11.Grayson J M, Zajac A J, Altman J D, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J Immunol. 2000;164:3950–3954. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 12.Grundy J E. Virologic and pathogenetic aspects of cytomegalovirus infection. Rev Infect Dis. 1990;12(Suppl. 7):S711–S719. doi: 10.1093/clinids/12.supplement_7.s711. [DOI] [PubMed] [Google Scholar]
- 13.Grundy J E, Shanley J D, Griffiths P D. Is cytomegalovirus interstitial pneumonitis in transplant recipients an immunopathological condition? Lancet. 1987;ii:996–999. doi: 10.1016/s0140-6736(87)92560-8. [DOI] [PubMed] [Google Scholar]
- 14.Harrington L E, Galvan M, Baum L G, Altman J D, Ahmed R. Differentiating between memory and effector CD8 T cells by altered expression of cell surface O-glycans. J Exp Med. 2000;191:1241–1246. doi: 10.1084/jem.191.7.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hengel H, Brune W, Koszinowski U H. Immune evasion by cytomegalovirus-survival strategies of a highly adapted opportunist. Trends Microbiol. 1998;6:190–197. doi: 10.1016/s0966-842x(98)01255-4. [DOI] [PubMed] [Google Scholar]
- 16.Hengel H, Reusch U, Geginat G, Holtappels R, Ruppert T, Hellebrand E, Koszinowski U H. Macrophages escape inhibition of major histocompatibility complex class I-dependent antigen presentation by cytomegalovirus. J Virol. 2000;74:7861–7868. doi: 10.1128/jvi.74.17.7861-7868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holtappels R. Peptide recognition by cytolytic T lymphocytes derived from pulmonary infiltrates after bone marrow transplantation and concurrent cytomegalovirus infection in a murine model. Ph.D. thesis. Mainz, Germany: Johannes Gutenberg University; 1996. [Google Scholar]
- 18.Holtappels R, Podlech J, Geginat G, Steffens H-P, Thomas D, Reddehase M J. Control of murine cytomegalovirus in the lungs: relative but not absolute immunodominance of the immediate-early 1 nonapeptide during the antiviral cytolytic T-lymphocyte response in pulmonary infiltrates. J Virol. 1998;72:7201–7212. doi: 10.1128/jvi.72.9.7201-7212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holtappels R, Thomas D, Podlech J, Geginat G, Steffens H-P, Reddehase M J. The putative natural killer decoy early gene m04 (gp34) of murine cytomegalovirus encodes an antigenic peptide recognized by protective antiviral CD8 T cells. J Virol. 2000;74:1871–1884. doi: 10.1128/jvi.74.4.1871-1884.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holtappels, R., D. Thomas, and M. J. Reddehase. Identification of a Kd-restricted antigenic peptide encoded by murine cytomegalovirus early gene M84. J. Gen. Virol., in press. [DOI] [PubMed]
- 21.Kleijnen M F, Huppa J B, Lucin P, Mukherjee S, Farrell H, Campbell A E, Koszinowski U H, Hill A B, Ploegh H L. A mouse cytomegalovirus glycoprotein, gp34, forms a complex with folded class I MHC molecules in the ER which is not retained but is transported to the cell surface. EMBO J. 1997;16:685–694. doi: 10.1093/emboj/16.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koszinowski U H, Reddehase M J, del Val M. Principles of cytomegalovirus antigen presentation in vitro and in vivo. Semin Immunol. 1992;4:71–79. [PubMed] [Google Scholar]
- 23.Koszinowski U H, Reddehase M J, Jonjic S. The role of T-lymphocyte subsets in the control of cytomegalovirus infection. In: Thomas D B, editor. Viruses and the cellular immune response. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 429–445. [Google Scholar]
- 24.Kranz D M, Tonegawa S, Eisen H N. Attachment of an anti-receptor antibody to non-target cells renders them susceptible to lysis by a clone of cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 1984;81:7922–7926. doi: 10.1073/pnas.81.24.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kundig T M, Bachmann M F, Oehen S, Hoffmann U W, Simard J J, Kalberer C P, Pircher H, Ohashi P S, Hengartner H, Zinkernagel R M. On the role of antigen in maintaining cytotoxic T-cell memory. Proc Natl Acad Sci USA. 1996;93:9716–9723. doi: 10.1073/pnas.93.18.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kundig T M, Bachmann M F, Ohashi P S, Pircher H, Hengartner H, Zinkernagel R M. On T cell memory: arguments for antigen dependence. Immunol Rev. 1996;150:63–90. doi: 10.1111/j.1600-065x.1996.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 27.Kurz S K, Rapp M, Steffens H-P, Grzimek N K A, Schmalz S, Reddehase M J. Focal transcriptional activity of murine cytomegalovirus during latency in the lungs. J Virol. 1999;73:482–494. doi: 10.1128/jvi.73.1.482-494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurz S K, Reddehase M J. Patchwork pattern of transcriptional reactivation in the lungs indicates sequential checkpoints in the transition from murine cytomegalovirus latency to recurrence. J Virol. 1999;73:8612–8622. doi: 10.1128/jvi.73.10.8612-8622.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurz S K, Steffens H-P, Mayer A, Harris J R, Reddehase M J. Latency versus persistence or intermittent recurrences: evidence for a latent state of murine cytomegalovirus in the lungs. J Virol. 1997;71:2980–2987. doi: 10.1128/jvi.71.4.2980-2987.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau L L, Jamieson B D, Somasundaram T, Ahmed R. Cytotoxic T cell memory without antigen. Nature. 1994;369:648–652. [PubMed] [Google Scholar]
- 31.McLaughlin-Taylor E, Pande H, Forman S J, Tanamachi B, Li C R, Zaia J A, Greenberg P D, Riddell S R. Identification of the major late human cytomegalovirus matrix protein pp65 as a target antigen for CD8+ virus-specific cytotoxic T lymphocytes. J Med Virol. 1994;43:103–110. doi: 10.1002/jmv.1890430119. [DOI] [PubMed] [Google Scholar]
- 32.Messerle M, Bühler B, Keil G M, Koszinowski U H. Structural organization, expression, and functional characterization of the murine cytomegalovirus immediate-early gene 3. J Virol. 1992;66:27–36. doi: 10.1128/jvi.66.1.27-36.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyahira Y, Murata K, Rodriguez D, Rodriguez J R, Esteban M, Rodrigues M M, Zavala F. Quantification of antigen-specific CD8+ T cells using an ELISPOT assay. J Immunol Methods. 1995;181:45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- 34.Mobley J L, Rigby S M, Dailey M O. Regulation of adhesion molecule expression by CD8 T cells in vivo. II. Expression of L-selectin (CD62L) by memory cytolytic T cells responding to minor histocompatibility antigens. J Immunol. 1994;153:5443–5452. [PubMed] [Google Scholar]
- 35.Morello C S, Cranmer L D, Spector D H. In vivo replication, latency, and immunogenicity of murine cytomegalovirus mutants with deletions in the M83 and M84 genes, the putative homologs of human cytomegalovirus pp65 (UL83) J Virol. 1999;73:7678–7693. doi: 10.1128/jvi.73.9.7678-7693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morello C S, Cranmer L D, Spector D H. Suppression of murine cytomegalovirus (MCMV) replication with a DNA vaccine encoding MCMV M84 (a homolog of human cytomegalovirus pp65) J Virol. 2000;74:3696–3708. doi: 10.1128/jvi.74.8.3696-3708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murali-Krishna K, Lau L L, Sambhara S, Lemonnier F, Altman J, Ahmed R. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 38.Podlech J, Holtappels R, Pahl-Seibert M-F, Steffens H-P, Reddehase M J. Murine model of interstitial cytomegalovirus pneumonia in syngeneic bone marrow transplantation: persistence of protective pulmonary CD8-T-cell infiltrates after clearance of acute infection. J Virol. 2000;74:7496–7507. doi: 10.1128/jvi.74.16.7496-7507.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Podlech J, Holtappels R, Wirtz N, Steffens H-P, Reddehase M J. Reconstitution of CD8 T cells is essential for the prevention of multiple-organ cytomegalovirus histopathology after bone marrow transplantation. J Gen Virol. 1998;79:2099–2104. doi: 10.1099/0022-1317-79-9-2099. [DOI] [PubMed] [Google Scholar]
- 40.Polic B, Hengel H, Krmpotic A, Trgovcich J, Pavic I, Lucin P, Jonjic S, Koszinowski U H. Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J Exp Med. 1998;188:1047–1054. doi: 10.1084/jem.188.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rapp M, Messerle M, Bühler B, Tannheimer M, Keil G M, Koszinowski U H. Identification of the murine cytomegalovirus glycoprotein B gene and its expression by recombinant vaccinia virus. J Virol. 1992;66:4399–4406. doi: 10.1128/jvi.66.7.4399-4406.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reddehase M J. The immunogenicity of human and murine cytomegaloviruses. Curr Opin Immunol. 2000;12:390–396. doi: 10.1016/s0952-7915(00)00106-0. [DOI] [PubMed] [Google Scholar]
- 43.Reddehase M J, Balthesen M, Rapp M, Jonjic S, Pavic I, Koszinowski U H. The conditions of primary infection define the load of latent viral genome in organs and the risk of recurrent cytomegalovirus disease. J Exp Med. 1994;179:185–193. doi: 10.1084/jem.179.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reddehase M J, Jonjic S, Weiland F, Mutter W, Koszinowski U H. Adoptive immunotherapy of murine cytomegalovirus adrenalitis in the immunocompromised host: CD4-helper-independent antiviral function of CD8-positive memory T lymphocytes derived from latently infected donors. J Virol. 1988;62:1061–1065. doi: 10.1128/jvi.62.3.1061-1065.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reddehase M J, Koszinowski U H. Significance of herpesvirus immediate early gene expression in cellular immunity to cytomegalovirus infection. Nature (London) 1984;312:369–371. doi: 10.1038/312369a0. [DOI] [PubMed] [Google Scholar]
- 46.Reddehase M J, Mutter W, Münch K, Bühring H-J, Koszinowski U H. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol. 1987;61:3102–3108. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reddehase M J, Rothbard J B, Koszinowski U H. A pentapeptide as minimal antigenic determinant for MHC class I-restricted T lymphocytes. Nature (London) 1989;337:651–653. doi: 10.1038/337651a0. [DOI] [PubMed] [Google Scholar]
- 48.Reddehase M J, Weiland F, Münch K, Jonjic S, Lüske A, Koszinowski U H. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J Virol. 1985;55:264–273. doi: 10.1128/jvi.55.2.264-273.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reusch U, Muranyi W, Lucin P, Burgert H G, Hengel H, Koszinowski U H. A cytomegalovirus glycoprotein re-routes MHC class I complexes to lysosomes for degradation. EMBO J. 1999;18:1081–1091. doi: 10.1093/emboj/18.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reusser P, Riddell S R, Meyers J D, Greenberg P D. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991;78:1373–1380. [PubMed] [Google Scholar]
- 51.Riddell S R, Watanabe K S, Goodrich J M, Li C R, Agha M E, Greenberg P D. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 52.Schulz M, Aichele P, Schneider R, Hansen T H, Zinkernagel R M, Hengartner H. Major histocompatibility complex binding and T cell recognition of a viral nonapeptide containing a minimal tetrapeptide. Eur J Immunol. 1991;21:1181–1185. doi: 10.1002/eji.1830210513. [DOI] [PubMed] [Google Scholar]
- 53.Steffens H-P, Kurz S, Holtappels R, Reddehase M J. Preemptive CD8-T-cell immunotherapy of acute cytomegalovirus infection prevents lethal disease, limits the burden of latent viral genome, and reduces the risk of virus recurrence. J Virol. 1998;72:1797–1804. doi: 10.1128/jvi.72.3.1797-1804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taguchi T, McGhee J R, Coffman R L, Beagley K W, Eldridge J H, Takatsu K, Kyono H. Detection of individual mouse splenic T cells producing IFN-γ and IL-5 using the enzyme-linked immunospot (ELISPOT) assay. J Immunol Methods. 1990;128:65–73. doi: 10.1016/0022-1759(90)90464-7. [DOI] [PubMed] [Google Scholar]
- 55.Tedder T F, Steeber D A, Chen A, Engel P. The selectins: vascular adhesion molecules. FASEB J. 1995;9:866–873. [PubMed] [Google Scholar]
- 56.Walter E A, Greenberg P D, Gilbert M J, Finch R J, Watanabe K S, Thomas E D, Riddell S R. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 57.Whitton J L, Tishon A, Lewicki H, Gebhard J, Cook T, Salvato M, Joly E, Oldstone M B. Molecular analyses of a five-amino-acid cytotoxic T-lymphocyte (CTL) epitope: an immunodominant region which induces nonreciprocal CTL cross-reactivity. J Virol. 1989;63:4303–4310. doi: 10.1128/jvi.63.10.4303-4310.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiertz E, Hill A, Tortorella D, Ploegh H. Cytomegaloviruses use multiple mechanisms to elude the host immune response. Immunol Lett. 1997;57:213–216. doi: 10.1016/s0165-2478(97)00073-4. [DOI] [PubMed] [Google Scholar]
- 59.Wills M R, Carmichael A J, Mynard K, Jin X, Weekes M P, Plachter B, Sissons J G P. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996;70:7569–7579. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ziegler H, Muranyi W, Burgert H G, Kremmer E, Koszinowski U H. The luminal part of the murine cytomegalovirus glycoprotein gp40 catalyzes the retention of MHC class I molecules. EMBO J. 2000;19:870–881. doi: 10.1093/emboj/19.5.870. [DOI] [PMC free article] [PubMed] [Google Scholar]