Abstract
No meta-analysis is available which has analysed the role of aromatase inhibitors (AIs) in hypogonadism in adult males related to obesity and aging. This meta-analysis intended to address this knowledge gap. Electronic databases were searched for studies involving adult males with hypogonadism. The primary outcomes were changes in total testosterone (TT). Secondary outcomes were alterations in oestradiol, luteinizing hormone (LH), and side-effect profile. From initially screened 177 articles, data from three randomised controlled trials(RCTs) (118 patients) and three uncontrolled studies(52 patients) were analysed. AIs were associated with significantly greater improvement in TT after three months [mean difference (MD) 7.08 nmol/L (95% Confidence Interval (CI): 5.92–8.24); P < 0.01; I2 = 0%], six months [MD 6.61 nmol/L (95% CI: 5.30–7.93); P < 0.01] and 12 months [MD 5.20 nmol/L (95% CI: 3.78–6.62); P < 0.01] therapy. AIs were associated with greater reduction in oestradiol after three months [MD -3.07 pmol/L (95% CI: -5.27– -0.87); P < 0.01; I2 = 40%], six months [MD -5.39 pmol/L (95% CI: -7.18– -3.60); P < 0.01] and 12 months [MD -8.3 pmol/L (95% CI: -15.97– -0.63); P = 0.03] therapy. AIs were associated with greater increase in LH after three months [MD 1.79 IU/L (95% CI: 0.77–2.81); P < 0.01; I2 = 0%], six months [MD 2.20 IU/L (95% CI: 0.29 – 4.11); P = 0.02] and 12 months [MD 1.70 IU/L (95% CI: 0.28–3.12); P = 0.02] therapy. Occurrence of treatment-emergent adverse events[Risk ratio (RR) 1.48 (95% CI: 0.47–4.66); P = 0.45; I2 = 0%] and severe adverse events[RR 2.48 (95% CI: 0.42–14.66); P = 0.32; I2 = 0%] were similar among AIs and controls. Following six-month treatment, AIs were associated with significantly lower bone mineral density (BMD) at lumbar-spine [MD -0.04 gm/cm2 (95% CI: -0.08– -0.01); P = 0.03], but not total hip [MD 0.01 gm/cm2 (95% CI: -0.02–0.04); P = 0.55] and femoral neck [MD 0.02 gm/cm2 (95% CI: -0.01–0.05); P = 0.12] compared to controls. This meta-analysis highlights the good efficacy of AIs in improving TT over 3–12 months of use. Adverse impact on spine bone density remains a concern in obese ageing males and warrants further evaluation.
Keywords: Aging, anastrozole, aromatase inhibitors, letrozole, male hypogonadism, meta-analysis, obesity, safety
INTRODUCTION
The prevalence of obesity has increased exponentially globally in the last half-century. Obesity has been linked with increased occurrence of hypogonadism in adult males, with prevalence ranging from 28–79%.[1] An inverse link has been observed between the severity of obesity and the circulating testosterone levels, viz. greater the obesity, the more severe is the hypogonadism.[2] An increase in age is also associated with lower circulating levels of testosterone and hence increased occurrence of hypogonadism. Apart from decreased testosterone production, ageing has been associated with increased body fat with a relatively greater loss in muscle mass, a phenotype which is also seen in younger men with obesity.[3]
Testosterone replacement has been tried both in obese men with hypogonadism as well as ageing men with hypogonadism.[3] However, use of testosterone remains controversial in these scenarios due to the associated efficacy and safety concerns. Exogenous testosterone administration is associated with further reduction in endogenous testosterone production, reduction in sperm count, and reduced fertility, which becomes a major issue in young obese males with hypogonadism.[3] A well-recognized side effect of exogenous testosterone is the use of gynaecomastia across all age groups. The adverse impact on prostate function and lower urinary tract symptoms (LUTS) may be a major issue in ageing males with hypogonadism.
Increased expression of the aromatase enzyme in the adipose tissue is a common etiologic factor for both obesity and ageing-related male hypogonadism.[4] This increased aromatase activity leads to greater conversion of androgens to oestrogen, resulting in a relative hyperestrogenemia, which exerts negative feedback on the hypothalamic-pituitary-gonadal axis, resulting in lower luteinizing hormone (LH) and follicular stimulating hormone (FSH) production, which ultimately leads to lower testosterone production from testes, causing “functional hypogonadotropic hypogonadism”.[3,4] Hence theoretically, aromatase inhibitors (AIs) like anastrozole and letrozole appear ideally suited to reverse this pathophysiologic change in ageing and obesity-related hypogonadism. AIs have been documented to have a beneficial impact on endogenous testosterone production, improve sperm count and fertility, and reduce hypogonadism-related gynaecomastia.[5] Safety concerns with the use of AIs include reduced bone density, which has primarily been documented in adult cancer survivors.[6]
To date, several randomised controlled trials (RCTs) have been published, evaluating the role of AIs in managing hypogonadism related to ageing and obesity.[7,8] However, to date, no meta-analysis is available which has analysed and summarized the clinical efficacy and safety of AIs in managing hypogonadism related to obesity and aging. Hence the aim of this systematic review and meta-analysis was to evaluate the efficacy and safety of AIs in managing hypogonadism related to obesity and ageing.
METHODS
Methodology
The meta-analysis was carried out according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions.[9] The predefined protocol has been registered in International Prospective Register of Systematic Reviews (PROSPERO), having the Registration number CRD12022296489. All RCTs published between January 1950 and November 2021 were considered for this meta-analysis. This meta-analysis has been reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), the filled checklist of which can be found at the end of the manuscript.[9] Since ethical approval already exists for the individual studies included in the meta-analysis, no separate approval was required for this study.
The PICOS criterion was used to screen and select the studies for this meta-analysis, with patients (P) being adult males with hypogonadism; intervention (I) being the use of AIs (anastrozole or letrozole) for managing hypogonadism; control (C) being patients receiving placebo or any other approved medication for managing hypogonadism like human chorionic gonadotropin (hCG) and selective oestrogen receptor modulators (SERMs); outcomes (O) being evaluated were impact on serum testosterone, oestradiol, LH, FSH, other hormone parameters, metabolic parameters and any adverse effects noted; and (S) being studies included which were RCTs. Only adults with obesity or ageing-related hypogonadism were considered for this meta-analysis. Adults with other established aetiologies of hypogonadism like a primary testicular failure (due to trauma, infection, Klinefelter syndrome) or secondary hypogonadism (isolated, multiple pituitary hormone deficiencies, post brain surgery, trauma), history of radiation or chemotherapy exposure were excluded. Patients with chronic disorders which may interfere with serum testosterone levels like chronic liver disease, chronic kidney disease, cardiac disease, vasculitis, autoimmune disorders, malignancy, severe LUTS, and history of metabolic/bariatric surgery were excluded. Patients with a history of androgen replacement or substance abuse were excluded. Only those studies were included in this meta-analysis that had at least two treatment groups of adults with hypogonadism, with one of the groups receiving AIs and the other group receiving either placebo or any other medication in place of AIs.
The primary outcomes were to evaluate the changes in total testosterone levels. The secondary outcomes were to evaluate alterations in LH, FSH, oestradiol, other hormone parameters, metabolic parameters, bone mineral and body composition changes, and any side effects reported.
Search method for identification of studies
A detailed search was done of electronic databases of Medline (Via PubMed), Embase (via Ovid SP), Cochrane central register of controlled trials (CENTRAL) (for trials only), ctri.nic.in, clinicaltrials.gov, global health, and Google scholar using a Boolean search strategy: [(aromatase inhibitors) OR (anastrozole) OR (letrozole)] AND [(hypogonadism)].
Data extraction and study selection
Data extraction was carried out independently by two authors using standard data extraction forms. In cases where more than one publication of a single study group were found, results were grouped and relevant data from each report were used in the analyses. Data on the primary and secondary outcomes, as stated above, was extracted. Patient characteristics (including demographic information and comorbidities) from the different RCTs included in the meta-analysis were noted in a tabular form [Table 1]. All disagreements were resolved by the third and fourth authors. Data from uncontrolled studies have been elaborated in Table 2.
Table 1.
Characteristics of patients in the different randomized controlled trials evaluated in this meta-analysis on the use of aromatase inhibitors in hypogonadism related to obesity and ageing
| Study details | Number and nature of patients in the study | AI studied; study duration | Baseline hormone parameters | End of study hormone parameters |
|---|---|---|---|---|
| Burnett-Bowie et al. 2009[7] | Anz group: 34 men; age 66 4 years; BMI 30 ± 5 kg/m2 Placebo group: 35 men; age 65 4 years; BMI 32 ± 5 kg/m2 | Anz 1 mg/day; 1 year Matched placebo in control group for 1 year | Anz group: TT: 11.2 ± 3.3 nmol/l E2: 55.8 ± 15.4 pmol/l LH: 4 ± 3 U/l Placebo group: TT: 11.7 ± 3.3 nmol/l E2: 68.6 ± 18.7 pmol/l LH: 3.8 ± 2.56 U/l |
Anz group: TT: 16.5 ± 5.1 nmol/l E2: 45.5 ± 15.4 pmol/l LH: 6 ± 4 U/l Placebo group: TT: 11.9 ± 2.9 nmol/l E2: 67.1 ± 19.8 pmol/l LH: 3.8 ± 2.2 U/l |
| Colleluori et al. 2020[8] | Anz group: 12 men; age 52 6 years; BMI 39.6 ± 5.1 kg/m2 Placebo group: 11 men; age 51 ± 6 years; BMI 41.5 ± 6.9 kg/m2 | Anz 1 mg/day; 6 months Matched placebo in control group for 6 months | Anz group: TT: 9.941 ± 3.8 nmol/l E2: 104.9 ± 27.9 pmol/l LH: 4.6 ± 2.4 U/l Placebo group: TT: 9.48 ± 2.65 nmol/l E2: 107.9 ± 43.3 pmol/l LH: 5.2 ± 2.2 U/l |
Anz group: TT: 18.39 ± 4.5 nmol/l E2: 51.33 ± 38.91 pmol/l LH: 6.2 ± 4.1 U/l Placebo group: TT: 9.76 ± 3.3 nmol/l E2: 106.82 ± 29 pmol/l LH: 4.8 ± 2.9 U/l |
| Leder et al. 2004[10] & Leder 2005[11] | Anz group: 12 men; age 67 3 years; BMI 29 ± 5 kg/m2 Placebo group: 14 men; age 67 ± 4 years; BMI 28 ± 5 kg/m2 | Anz 1 mg/day; 12 weeks months Matched placebo in control group for 12 weeks | Anz group: TT: 10.0 ± 1.28 nmol/l E2: 95.4 ± 26.5 pmol/l LH: 5.1 ± 4.8 U/l Placebo group: TT: 10.1 ± 1.76 nmol/l E2: 103.2 ± 41.3 pmol/l LH: 6.1 ± 4.5 U/l |
Anz group: TT: 19.85 ± 4.82 nmol/l E2: 62.4 ± 29.43 pmol/l LH: 7.9 ± 6.5 U/l Placebo group: TT: 10.0 ± 2.12 nmol/l E2: 106.82 ± 29 pmol/l LH: 6.2 ± 4.4 U/l |
AI: aromatase inhibitor; Anz: Anastrozole; BMI: body mass index; conc: concentration; mill: million; Lz: letrozole; TT: total testosterone; E2: estradiol; LH: luteinizing hormone
Table 2.
Outcomes of uncontrolled studies evaluating role of aromatase inhibitors in hypogonadism related to obesity and ageing
| Study details | Number and nature of patients in the study | AI studied; study duration | Baseline hormone parameters | End of study hormone parameters |
|---|---|---|---|---|
| De Boer et al.[12] | 10 obese men; age 48.2 ± 2.3 years; BMI 42.1 ± 2.6 kg/m2 | Lz (7.5 to 17.5 mg per week); 6 weeks | TT: 7.5 ± 1.0 nmol/l E2: 120 ± 20 pmol/l LH: 4.5 ± 0.8 U/l |
TT: 23.8 ± 3.0 nmol/l E2: 70 ± 9 pmol/l LH: 14.8 ± 2.3 U/l |
| Loves et al.[13] | 12 obese men; age 48.4 ± 3.3 years; BMI 45.7 ± 3.0 kg/m2 | Lz 2.5 mg weekly; 6 months | TT: 5.9 ± 0.5 nmol/l E2: 117.47 ± 15 pmol/l LH: 4.4 ± 0.6 U/l |
TT: 19.6 ± 1.4 nmol/l E2: 58.3 ± 9.1 pmol/l LH: 11.1 ± 1.5 U/l |
| Shah et al.[14] | 30 overweight men; age 34 ± 3.1 years; BMI: 28.7 ± 3.5 kg/m2 | Anz 1 mg daily; 5 months | TT: 9.39 ± 2.3 nmol/l E2: 123 ± 11 pmol/l LH: 3.4 ± 1.1 U/l Sperm conc.:7.8 mill/ml |
TT: 14.29 ± 3.92 nmol/l E2: 58 ± 7 pmol/l LH: 5.4 ± 2.1 U/l Sperm conc.:14.2 mill/mL |
AI: aromatase inhibitor; Anz: Anastrozole; BMI: body mass index; conc: concentration; mill: million; Lz: letrozole; TT: total testosterone; E2: estradiol; LH: luteinizing hormone
Assessment of risk of bias in included studies
Three authors independently assessed the risk of bias using the risk of the bias assessment tool in Review Manager (Revman) Version 5.3 (The Cochrane Collaboration, Oxford, UK 2014) software.[15] The different types of bias looked for have already been elaborated on in a previous publication from our group[16]
Measures of treatment effect
For continuous variables, the outcomes were expressed as mean differences (MD). SI (International System) units were used for analysis, and all studies reporting results in conventional units were converted to SI units for analysis. For dichotomous outcomes (treatment success), results were expressed as risk ratios (RR) with 95% confidence intervals (CI). For adverse events, results were expressed as post-treatment absolute risk differences. RevMan 5.3 was used for comparing MD of the different primary and secondary outcomes between AIs and the control groups.
Assessment of heterogeneity
Heterogeneity was initially assessed by studying the forest plot generated for the primary and secondary outcomes of this study. Subsequently, heterogeneity was analysed using a Chi-square test on N-1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test.[15] Details of interpretation of heterogeneity have been elaborated elsewhere.[16]
Grading of the results
An overall grading of the evidence (certainty of the evidence) related to each of the primary and secondary outcomes of the meta-analysis was done using the GRADE (Grades of Recommendation, Assessment, Development, and Evaluation) approach.[17] The details of how the GRADE pro-Guideline Development Tool (GDT) software (McMaster University and Evidence Prime Inc, 2015) was used to create the Summary of Findings (SoF) table has been elaborated elsewhere.[16]
Publication bias was assessed by plotting the Funnel Plot, which specifically targets small study bias, in which small studies tend to show larger estimates of effects and greater variability than larger studies.[9] The presence of one or more of the smaller studies outside the inverted funnel plot was taken as evidence of the presence of significant publication bias.[18]
Data synthesis
Data was pooled as a random effect model for the analysis of primary and secondary outcomes. The outcomes were expressed as 95% confidence intervals (95% CI). Forrest plots were plotted with the left side of the graph favoring AIs and the right side of the graph favoring control using RevMan 5.3 software. P < 0.05 was considered statistically significant.
RESULTS
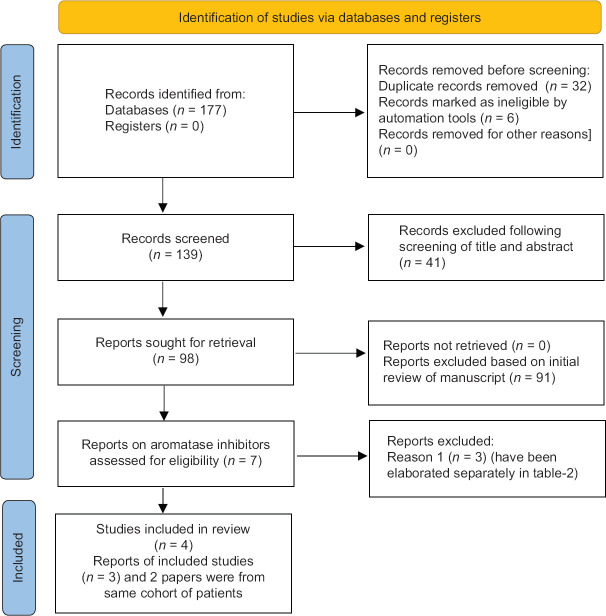
A total of 177 articles were found after the initial search [Figure 1]. Following the screening of the titles and abstracts, the search came down to 139 articles. Thirty-two duplicates were removed. Ninety-eight articles were reviewed in detail, from which four RCTs that fulfilled all inclusion and exclusion criteria were included in the meta-analysis [Figure 1].[7,8,10,18] The RCT by Leder (2005) et al.[11] evaluating the bone health outcomes with the use of anastrozole was a part of the original RCT published by Leder (2004) et al.[10] Hence to prevent duplication of patients, in our meta-analysis, the data of the above two studies have been pooled together and analysed by Leder (2004) et al.[10]
Figure 1.
Flowchart elaborating on study retrieval and inclusion in the meta-analysis. Reason-1: lack of a valid control group; RCT: randomised controlled trial
Anastrozole 1 mg daily for the duration of the study was the AI used in the studies by Burnett-Bowie et al.,[7] Colleluori et al.,[8] and Leder (2004) et al.[10] and (2005) et al.[11] The duration of the study was 12 months, six months, and three months in the RCTs by Burnett-Bowie et al.,[7] Colleluori et al,[8] and Leder (2004 and 2005) et al.,[10,11] respectively. The study by Leder (2004) et al.[10] had another arm in which the participants received anastrozole twice weekly. However, this arm was excluded from the analysis as our study wanted to compare the outcomes regarding the daily use of aromatase inhibitors. Letrozole 2.5 mg daily was used for the duration of study in the study by Loves et al.[13] and De Boer et al.[12] Anastrozole 1 mg for the duration of the study was used by Shah et al.[14] However, these three studies were excluded from the analysis as they did not have a valid control group. The details of the RCTs included in this meta-analysis have been elaborated in Table 1. The outcomes of the uncontrolled studies have been summarized in Table 2 as a part of the systematic review.
In the study by Burnett-Bowie et al.[7] and Leder (2004) et al.[10] and (2005) et al.[11] radio-immunoassay (RIA) was used to measure serum total testosterone, oestradiol, and dihydrotestosterone (DHT). Gonadotropins were measured using a chemiluminescent immunoassay.[7] In the study by Colleluori et al.,[8] chemiluminescent immunoassay was used for the estimation of total testosterone, oestradiol, and gonadotropins. Bioavailable testosterone was measured by radioimmunoassay after ammonium sulphate precipitation in the studies by Burnett-Bowie et al.[7] and Leder et al.[10,11]
Risk of bias in the included studies
The summaries of the risk of bias of the studies included in the meta-analysis have been elaborated in supplementary Figure 1a (80.2KB, tif) and b (80.2KB, tif) . Random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, selective reporting, and other biases were judged to be at low risk of bias in all the studies (100%). Source of funding, especially pharmaceutical, authors from the pharmaceutical organizations, and conflict of interests were looked into the “other bias” section. Attrition bias was judged to be at a low risk in two out of three studies (66.7%) [Supplementary Table 1].
Supplementary Table 1.
Risk of bias assessment table
| Burnett-Bowie 2009 | Risk Of Bias | Author Judgement |
|---|---|---|
| Random Sequence Generation (Selection Bias) | Low Risk | Randomised, double-blind, placebo controlled study |
| Allocation Concealment (Selection Bias) | Low Risk | Subjects were randomized by computer-generated assignment in a blinded 1 : 1 ratio |
| Blinding Of Participants & Personal (Performance Bias) | Low Risk | Yes, double blinded RCT |
| Blinding Of Outcome Assessment (Detection Bias) | Low Risk | Yes, double blinded RCT |
| Incomplete Outcome Data (Attrition Bias) | High Risk | 69 out of 88 patients completed the study (78.4%) Hence attrition rate was high (>20%) |
| Selective Reporting (Reporting Bias) | Low Risk | All pre-specified outcomes were reported |
| Other Biases | Low Risk | This work was supported by National Institute of Health grants K23-RR-161310 (to BZL), R01-AG-025099-03 (to BZL), M01-RR-01066 (to the Mallinckrodt GCRC) and AstraZeneca Pharmaceuticals. |
|
| ||
| Colleluori 2020 | Risk Of Bias | Author Judgement |
|
| ||
| Random Sequence Generation (Selection Bias) | Low Risk | Randomised, double-blind, placebo controlled study |
| Allocation Concealment (Selection Bias) | Low Risk | Participants were randomized by the MEDVAMC pharmacy from a list generated by a research biostatistician to one of two treatment groups |
| Blinding Of Participants & Personel (Performance Bias) | Low Risk | Yes, double blinded RCT |
| Blinding Of Outcome Assessment (Detection Bias) | Low Risk | Yes, double blinded RCT |
| Incomplete Outcome Data (Attrition Bias) | High Risk | 17 out of 23 patients completed the study (73.91%). Hence attrition rate was high (>20%) |
| Selective Reporting (Reporting Bias) | Low Risk | All Pre-Specified Outcomes Were Reported |
| Other Biases | Low Risk | This research did not receive any specific grant from any funding agency in the public, commercial, or not-for profit sector. |
|
| ||
| Leder 2004 | Risk Of Bias | Author Judgement |
|
| ||
| Random Sequence Generation (Selection Bias) | Low Risk | Randomised, double-blind, placebo controlled study |
| Allocation Concealment (Selection Bias) | Low Risk | Subjects were randomized by computer-generated assignment |
| Blinding Of Participants & Personal (Performance Bias) | Low Risk | Yes, double blinded RCT |
| Blinding Of Outcome Assessment (Detection Bias) | Low Risk | Yes, double blinded RCT |
| Incomplete Outcome Data (Attrition Bias) | Low Risk | All patient outcomes reported. NO drop-outs |
| Selective Reporting (Reporting Bias) | Low Risk | All Pre-Specified Outcomes Were Reported |
| Other Biases | Low Risk | This work was supported by National Institutes of Health Grant K23-RR16310 (to B.Z.L.), the Massachusetts General Hospital Clinical Research Center grant (RR-1066), and AstraZeneca Pharmaceuticals. |
|
| ||
| Leder 2005 | Risk Of Bias | Author Judgement |
|
| ||
| Random Sequence Generation (Selection Bias) | Low Risk | Randomised, double-blind, placebo controlled study |
| Allocation Concealment (Selection Bias) | Low Risk | Subjects were randomized by computer-generated assignment |
| Blinding Of Participants & Personal (Performance Bias) | Low Risk | Yes, double blinded RCT |
| Blinding Of Outcome Assessment (Detection Bias) | Low Risk | Yes, double blinded RCT |
| Incomplete Outcome Data (Attrition Bias) | Low Risk | All patient outcomes reported. NO drop-outs |
| Selective Reporting (Reporting Bias) | Low Risk | All Pre-Specified Outcomes Were Reported |
| Other Biases | Low Risk | This work was supported by National Institutes of Health Grant K23-RR16310 (to B.Z.L.), the Massachusetts General Hospital Clinical Research Center grant (RR-1066), and AstraZeneca Pharmaceuticals. |
Effect of aromatase inhibitors on primary outcomes
Total testosterone
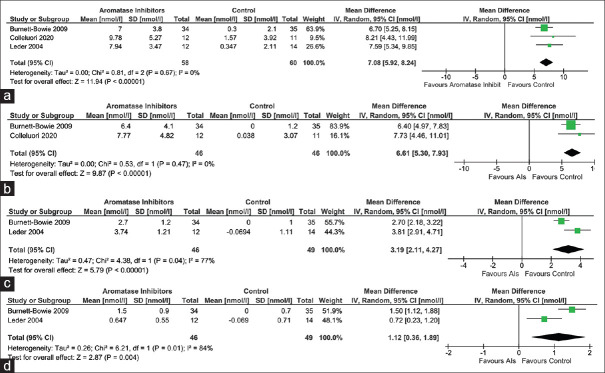
Data from three studies (118 participants), two studies (92 participants), and one study (69 participants; Burnett-Bowie et al.[7]) were analysed to find out the impact of AIs on serum total testosterone after three months, six months, and 12 months therapy, respectively, as compared to those receiving placebo. Individuals receiving AIs had a significantly greater improvement in total testosterone after three months [mean difference (MD) 7.08 nmol/L (95% CI: 5.92–8.24); P < 0.01; I2 = 0% (low heterogeneity); Figure 2a], six months [MD 6.61 nmol/L (95% CI: 5.30–7.93); P < 0.01; I2 = 0% (low heterogeneity); Figure 2b] and 12 months [MD 5.20 nmol/L (95% CI: 3.78–6.62); P < 0.01] therapy.
Figure 2.
Forest plot highlighting the impact of aromatase inhibitors (AIs) on (a) total testosterone at three months; (b) total testosterone at six months; (c) bioavailable testosterone at three months; (d) dihydro-testosterone at three months
Bioavailable testosterone
Data from two studies (95 participants) were analysed to find out the impact of AIs on serum bioavailable testosterone after three months of therapy. Individuals receiving AIs had a significantly greater improvement in bioavailable testosterone after three months of therapy [MD 3.19 nmol/L (95% CI: 2.11–4.27); P < 0.01; I2 = 77% (moderate heterogeneity); Figure 2c].
Dihydrotestosterone
Data from two studies (95 participants) and one study (69 participants; Burnett-Bowie et al.[7]) were analysed to find out the impact of AIs on serum dihydro-testosterone (DHT) after three and six months of therapy, respectively. Individuals receiving AIs had a significantly greater improvement in DHT after three months [MD 1.12 nmol/L (95% CI: 0.36–1.89); P < 0.01; I2 = 84% (moderate heterogeneity); Figure 2d] and six months [MD 1.00 nmol/L (95% CI: 0.45–1.55); P < 0.01] of therapy.
Oestradiol
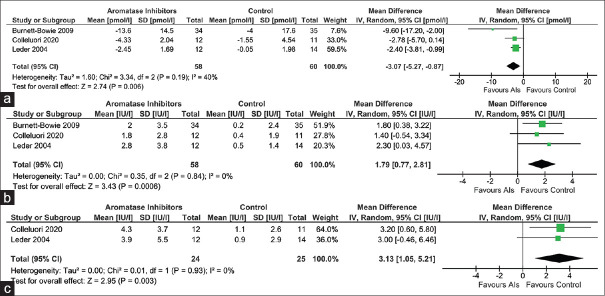
Data from three studies (118 participants), one study (23 participants; Colleluori et al.[8]) and one study (69 participants; Burnett-Bowie et al.[7]) were analysed to find out the impact of AIs on serum oestradiol after three months, six months and 12 months therapy, respectively, as compared to those receiving placebo. Individuals receiving AIs had a significantly greater reduction in serum oestradiol after three months [MD 3.07 pmol/L (95% CI: -5.27– -0.87); P < 0.01; I2 = 40% (low heterogeneity); Figure 3a], six months [MD 5.39 pmol/L (95% CI: -7.18– -3.60); P < 0.01] and 12 months [MD 8.3 pmol/L (95% CI: -15.97– -0.63); P = 0.03] therapy.
Figure 3.
Forest plot highlighting the impact of aromatase inhibitors on (a) estradiol at three months; (b) Luteinizing hormone at three months; (c) Follicle-stimulating hormone at three months
Gonadotropins
Data from three studies (118 participants), one study (23 participants; Colleluori et al.[8]) and one study (69 participants; Burnett-Bowie et al.[7]) were analysed to find out the impact of AIs on serum LH after three months, six months and 12 months therapy, respectively, as compared to those receiving placebo. Individuals receiving AIs had a significantly greater increase in serum LH after three months [MD 1.79 IU/L (95% CI: 0.77–2.81); P < 0.01; I2 = 0% (low heterogeneity); Figure 3b], six months [MD 2.20 IU/L (95% CI: 0.29–4.11); P = 0.02] and 12 months [MD 1.70 IU/L (95% CI: 0.28–3.12); P = 0.02] therapy.
Data from two studies (49 participants) and one study (23 participants; Colleluori et al.[8]) were analysed to find out the impact of AIs on serum FSH after three months and six months therapy, respectively, as compared to those receiving placebo. Individuals receiving AIs had a significantly greater increase in serum FSH after three months [MD 3.13 IU/L (95% CI: 1.05–5.21); P < 0.01; I2 = 0% (low heterogeneity); Figure 3c] but not at six months [MD 2.10 IU/L (95% CI: -1.42–5.62); P = 0.24] of therapy.
Safety
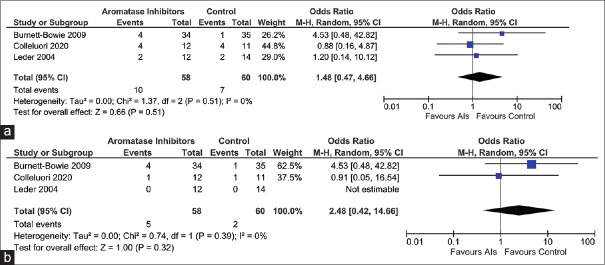
Data from three studies (118 participants) were analysed to evaluate the impact of AIs on the occurrence of adverse events [(treatment emergent adverse events (TAEs) and severe adverse events (SAEs)]. The occurrence of TAEs [Risk ratio (RR) 1.48 (95% CI: 0.47–4.66); P = 0.45; I2 = 0% (low heterogeneity); Figure 4a] and SAEs [RR 2.48 (95% CI: 0.42–14.66); P = 0.32; I2 = 0% (low heterogeneity); Figure 4b; High Certainty of Evidence (HCE)] was not statistically different in participants receiving AIs as compared to controls. Drop-out rates were not different among patients receiving AIs as compared to controls (Burnett-Bowie et al.[7]) [Odd Ratio (OR) 1.61 (95% CI: 0.56–4.69); P = 0.38].
Figure 4.
Forest plot highlighting the impact of aromatase inhibitors on (a) Treatment-emergent Adverse Events (TAEs); (b): Severe Adverse Events (SAEs)
Data from two studies (95 participants) were analysed to evaluate the impact of AIs on serum prostate specific antigen (PSA). No difference in PSA levels with use of AIs as compared to controls [MD 0.14 ng/ml (95% CI: -0.03–0.30); P = 0.10; I2 = 0% (low heterogeneity)].
In the study by Burnett-Bowie et al.,[7] during the course of the study, 11 participants withdrew from the AI group and eight participants from the control group. A rise in serum PSA >2.5 ng/ml was the cause for withdrawal of three participants in the AI group and four participants in the placebo group.[7] The five SAEs responsible for withdrawal in the study by Burnett-Bowie et al.[7] were prostate carcinoma [three months follow-up (FU), (placebo group)], pancreatic carcinoma (five months FU; AI group), hepatitis-A (five months FU, AI group), pulmonary embolism (six month FU, AI group) and embolic stroke (eight months FU, AI group). The two SAEs in the study by Colleluori et al.[8] were suicidal ideation and low back pain. No SAEs were reported in the study by Leder et al.[10,11]
Effect of aromatase inhibitors on bone mineral health and body composition parameters
Leder (2004) et al.[10] showed that the use of AIs was not associated with any significant changes in the bone turn over markers like N-telopeptide [MD 0.40 nmol/L (95% CI: -0.41–1.21); P = 0.33], urine deoxypyridinoline [MD 0.20 nmol/mmol (95% CI: -0.97–0.57); P = 0.61], type-1 procollagen (P1NP) [MD 2.00 ng/ml (95% CI: -5.08–1.08); P = 0.20], osteocalcin [MD 0.60 ng/ml (95% CI: -2.33–1.13); P = 0.50] and osteoprotegerin [MD 0.40 pmol/L (95% CI: -1.52–0.72); P = 0.48], as compared to controls.
Following six month treatment with AIs, no significant difference in bone mineral density (BMD) at total hip [MD 0.01 gm/cm2 (95% CI: -0.02–0.04); P = 0.55] and femoral neck [MD 0.02 gm/cm2 (95% CI: -0.01–0.05); P = 0.12], but lumbar spine BMD was found to be significantly lower [MD -0.04 gm/cm2 (95% CI: -0.08– -0.01); P = 0.03], as compared to controls, was documented by Colleluori et al.[8]
Trabecular bone score (TBS) is a relatively newer tool for evaluating osteoporosis. It is an indirect indicator of bone microarchitecture. It is a textural index that evaluates pixel gray-level variations in the lumbar spine Dual-Energy X-ray Absorptiometry (DEXA) image. In the study by Colleluori et al.,[8] TBS was not significantly different in the AI group as compared to controls [MD 0.07 (95% CI: -0.01–0.15); P = 0.10].
Data from two studies (Burnett-Bowie et al.[7] and Collelluori et al.[8]; 92 participants) were analysed to evaluate the impact of AIs on body composition parameters (total fat mass and total lean mass). Use of AIs was not associated with any significant change in total fat mass [MD 1.53 kg (95% CI: -5.38–2.33); P = 0.44; I2 = 90% (considerable heterogeneity)], and total lean mass [MD 0.45 kg (95% CI: -1.27–0.38); P = 0.29; I2 = 0% (low heterogeneity)].
Effect of aromatase inhibitors on quality of life, muscle strength and lower urinary symptoms
No significant change was noted with six month therapy of AIs by Colleluori et al.[8] on clinical symptoms of androgen deficiency (androgen deficiency in adult male (ADAM) score) [MD 0.30 (95% CI: -4.99–5.59); P = 0.91], lower urinary tract symptoms (IIEF-5 score) [MD 0.80 (95% CI: -3.28–4.88); P = 0.70] and quality of life scores (IWQOL score) [MD 2.0 (95% CI: -19.34–15.34); P = 0.82], as compared to controls. Muscle strength as assessed using 60 degree knee extension peak torque [MD 9.10% (95% CI: -20.752.55); P = 0.13] and 60 degree knee flexion peak torque [MD 3.70% (95% CI: -18.23–10.83); P = 0.62] were not different in patients receiving AIs as compared to controls (Coleluori et al.).[8]
Publication bias for the key outcomes of this meta-analysis was found to be low and has been elaborated on in Supplmentary Figure 2 (66.2KB, tif) . The summary of findings of the key outcomes of this meta-analysis has been elaborated in Table 3.
Table 3.
Summary of findings table on the role of aromatase inhibitors in managing hypogonadism in adult males related to obesity and aging: A systematic review and meta-analysis
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
|---|---|---|---|---|---|
|
| |||||
| Risk with Control | Risk with Aromatase Inhibitors | ||||
| Total testosterone (3 months) | The mean total testosterone (3 months) was 10.43 nmol/l | MD 7.08 nmol/l higher (5.92 higher to 8.24 higher) | - | 118 (3 RCTs) | ⨁⨁⨁⨁High |
| Estradiol (3 months) | The mean estradiol (3 months) was 93.73 pmol/l | MD 3.07 pmol/l lower (5.27 lower to 0.87 lower) | - | 118 (3 RCTs) | ⨁⨁⨁⨁High |
| Luteinizing hormone (3 months) | The mean luteinizing hormone (3 months) was 5.03 U/l | MD 1.79 U/l higher (0.77 higher to 2.81 higher) | - | 118 (3 RCTs) | ⨁⨁⨁⨁High |
| Severe adverse events (SAEs) | 33 per 1,000 | 79 per 1,000 (14 to 336) | OR 2.48 (0.42 to 14.66) | 118 (3 RCTs) | ⨁⨁⨁⨁High |
| Treatment emergent adverse events (TAEs) | 117 per 1,000 | 164 per 1,000 (58 to 381) | OR 1.48 (0.47 to 4.66) | 118 (3 RCTs) | ⨁⨁⨁⨁High |
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI); CI: confidence interval; MD: mean difference; OR: odds ratio. GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect
Key outcomes from uncontrolled studies
The key outcomes from the three uncontrolled studies evaluated in this systematic review have been elaborated in Table 2. A robust improvement in serum total testosterone and LH levels with a decline in oestradiol levels was documented with the use of anastrozole or letrozole over a period of six weeks to six months. Shah et al.,[14] in addition documented improvement in sperm count and fertility.
DISCUSSION
This is the first meta-analysis to highlight the efficacy and safety of AIs in managing functional hypogonadism related to obesity and ageing in adult males. An important observation from this meta-analysis is the significant and sustained improvement in serum testosterone over 3–12 months of therapy with adult males. This improvement in serum testosterone was associated with an increase in circulating gonadotropin levels and a significant decline in oestradiol levels. In a recently published systematic review, Raheem et al.[19] documented the beneficial impact of non-testosterone-based treatments (SERMs, hCG, and AIs) in improving serum testosterone levels and ADAM scores in men with hypogonadism. For SERMs (n = 512) and AIs (n = 375), the authors documented the exactly same pre-treatment (5.82 ± 7.03 nmol/L) and post-treatment (12.72 ± 1.12 nmol/L) testosterone values.[19] The corresponding pre-treatment and post-hCG treatment (n = 196) testosterone levels were 9.87 ± 0.47 nmol/L and 19.63 ± 1.38 nmol/L, respectively. Analysing data primarily from uncontrolled studies and analysing all the different aetiologies of hypogonadism together were a limitation of this review.[19]
TAEs and SAEs were not increased with the use of AIs in this meta-analysis. AIs were well-tolerated in the different studies, with no increased drop-outs noted. Bone turnover markers were not affected with short to intermediate-term use of AIs. No significant change in total fat mass and lean mass was documented with the use of AIs. No change in femoral BMD was noted after six months of use of AI. However, a small but statistically significant decline in lumbar spine BMD was noted following six months of use of AI in adult males. Weight has a direct trophic impact on BMD.[20] Weight loss has been linked with lower BMD.[20] In the study by Colleluori et al.,[8] weight loss was significantly higher in the AI group as compared to controls [MD -5.1 kg (95% CI: -9.90– -0.30); P = 0.04]. This significantly higher weight loss in the AI group over six months may have contributed to the lower lumbar spine BMD. It must be remembered that the lumbar spine has predominantly cancellous bone, where changes in BMD occur faster. The femur has predominantly cortical bone, where both increment as well as a decrease in BMD is a much slower process. This may explain the lower BMD noted at the lumbar spine but not the hip. Testosterone is aromatised to oestrogen in the body, which has a direct impact on increased bone formation.[21] Oestrogens have a trophic effect on bone health and BMD. There is data to suggest that androgens increase periosteal bone formation and cortical thickness, thereby increasing BMD and strength.[22] Hence, further studies with longer follow-up years are needed to settle the issue of the use of AIs on long-term bone mineral outcomes.
We have previously documented the beneficial impact of letrozole in improving testosterone levels and pubertal outcomes in children with constitutional delay in growth and puberty (CDGP).[23] No adverse impact on BMD or bone health was noted in children for up to 18–24 months of the use of AIs in them.[22] Higher levels of insulin-like growth factor-1 (IGF-1) and puberty in young children may explain the lack of adverse impact on bone health with the use of AIs, unlike adults and ageing males.
Limitations of this meta-analysis include the small number of patients evaluated in the different RCTs and uncontrolled studies. This meta-analysis highlights the lack of availability of long-term efficacy and safety data of AIs in managing hypogonadism in males due to either ageing or obesity for more than one year. This issue is of vital importance as a decline in BMD in the elderly with ageing-related hypogonadism is undesirable as it would increase the risks of fractures related to falls/trauma. Hence, there remains an urgent need to evaluate long-term bone health data with the use of AIs in the elderly. Without that, long-term use of AIs cannot be recommended in obese and elderly men with hypogonadism.
To conclude, this first meta-analysis on the efficacy and safety of AIs in managing hypogonadism related to obesity and ageing highlights the good efficacy of AIs in improving serum testosterone levels over 3–12 months of clinical use. This meta-analysis raises the important safety issue of adverse impact on bone health, specifically spine BMD with 3–12 months of AIs use. There is an urgent need for clinical trials evaluating the long-term safety and efficacy of AIs in hypogonadism related to obesity and ageing.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
(a) Risk of bias graph: review authors‘ judgements about each risk of bias item presented as percentages across all included studies; (b) Risk of bias summary: review authors‘ judgements about each risk of bias item for each included study.
Funnel plot of all the included studies in the meta-analysis (assessing the publication bias) of the main outcomes assessed (a) total testosterone three months; (b) estradiol three months; (c) luteinizing hormone three months; (d): treatment-emergent adverse events; (e): Severe adverse events
REFERENCES
- 1.Pellitero S, Olaizola I, Alastrue A, Martinez E, Granada ML, Balibrea JM, et al. Hypogonadotropic hypogonadism in morbidly obese males is reversed after bariatric surgery. Obes Surg. 2012;22:1835–42. doi: 10.1007/s11695-012-0734-9. [DOI] [PubMed] [Google Scholar]
- 2.Aguirre LE, Colleluori G, Dorin R, Robbins D, Chen R, Jiang B, et al. Hypogonadal men with higher body mass index have higher bone density and better bone quality but reduced muscle density. Calcif Tissue Int. 2017;101:602–11. doi: 10.1007/s00223-017-0316-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, et al. Testosterone therapy in adult men with androgen deficiency syndromes:An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995–2010. doi: 10.1210/jc.2005-2847. [DOI] [PubMed] [Google Scholar]
- 4.Cohen PG. The hypogonadal-obesity cycle:Role of aromatase in modulating the testosterone-estradiol shunt–A major factor in the genesis of morbid obesity. Med Hypotheses. 1999;52:49–51. doi: 10.1054/mehy.1997.0624. [DOI] [PubMed] [Google Scholar]
- 5.La Vignera S, Izzo G, Emerenziani GP, Cannarella R, Condorelli RA, Calogero AE, et al. Male hypogonadism:Therapeutic choices and pharmacological management. Minerva Endocrinol. 2020;45:189–203. doi: 10.23736/S0391-1977.20.03195-8. [DOI] [PubMed] [Google Scholar]
- 6.Monteverdi S, Pedersini R, Gallo F, Maffezzoni F, Dalla Volta A, Di Mauro P, et al. The interaction of lean body mass with fat body mass is associated with vertebral fracture prevalence in women with early breast cancer undergoing aromatase inhibitor therapy. JBMR Plus. 2020;5:e10440. doi: 10.1002/jbm4.10440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnett-Bowie SA, Roupenian KC, Dere ME, Lee H, Leder BZ. Effects of aromatase inhibition in hypogonadal older men:A randomized, double-blind, placebo-controlled trial. Clin Endocrinol (Oxf) 2009;70:116–23. doi: 10.1111/j.1365-2265.2008.03327.x. [DOI] [PubMed] [Google Scholar]
- 8.Colleluori G, Chen R, Turin CG, Vigevano F, Qualls C, Johnson B, et al. Aromatase inhibitors plus weight loss improves the hormonal profile of obese hypogonadal men without causing major side effects. Front Endocrinol (Lausanne) 2020;11:277. doi: 10.3389/fendo.2020.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JP, Altman DG, Gotzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leder BZ, Rohrer JL, Rubin SD, Gallo J, Longcope C. Effects of aromatase inhibition in elderly men with low or borderline-low serum testosterone levels. J Clin Endocrinol Metab. 2004;89:1174–80. doi: 10.1210/jc.2003-031467. [DOI] [PubMed] [Google Scholar]
- 11.Leder BZ, Finkelstein JS. Effect of aromatase inhibition on bone metabolism in elderly hypogonadal men. Osteoporos Int. 2005;16:1487–94. doi: 10.1007/s00198-005-1890-8. [DOI] [PubMed] [Google Scholar]
- 12.de Boer H, Verschoor L, Ruinemans-Koerts J, Jansen M. Letrozole normalizes serum testosterone in severely obese men with hypogonadotropic hypogonadism. Diabetes Obes Metab. 2005;7:211–5. doi: 10.1111/j.1463-1326.2004.00397.x. [DOI] [PubMed] [Google Scholar]
- 13.Loves S, Ruinemans-Koerts J, de Boer H. Letrozole once a week normalizes serum testosterone in obesity-related male hypogonadism. Eur J Endocrinol. 2008;158:741–7. doi: 10.1530/EJE-07-0663. [DOI] [PubMed] [Google Scholar]
- 14.Shah T, Nyirenda T, Shin D. Efficacy of anastrozole in the treatment of hypogonadal, subfertile men with body mass index≥25 kg/m2. Transl Androl Urol. 2021;10:1222–8. doi: 10.21037/tau-20-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and metaanalyses of studies that evaluate healthcare interventions:Explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dutta D, Jindal R, Kumar M, Mehta D, Dhall A, Sharma M. Efficacy and safety of once weekly thyroxine as compared to daily thyroxine in managing primary hypothyroidism:A systematic review and meta-analysis. Indian J Endocrinol Metab. 2021;25:76–85. doi: 10.4103/ijem.IJEM_789_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE:An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ. Publication and related biases. Health Technol Assess. 2000;4:1–115. [PubMed] [Google Scholar]
- 19.Raheem OA, Chen T, Akula KP, Greenberg J, Le TV, Chernobylsky D, et al. Efficacy of non-testosterone-based treatment in hypogonadal men:A review. Sex Med Rev. 2021;9:381–92. doi: 10.1016/j.sxmr.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Maisnam I, Dutta D, Mukhopadhyay S, Chowdhury S. Lean mass is the strongest predictor of bone mineral content in type-2 diabetes and normal individuals:An eastern India perspective. J Diabetes Metab Disord. 2014;13:90. doi: 10.1186/s40200-014-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar M, Mukhopadhyay S, Dutta D. Challenges and controversies in diagnosis and management of gonadotropin dependent precocious puberty:An Indian perspective. Indian J Endocrinol Metab. 2015;19:228–35. doi: 10.4103/2230-8210.149316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seeman E. Periosteal bone formation—A neglected determinant of bone strength. N Engl J Med. 2003;349:320–3. doi: 10.1056/NEJMp038101. [DOI] [PubMed] [Google Scholar]
- 23.Dutta D, Singla R, Surana V, Sharma M. Efficacy and safety of letrozole in the management of constitutional delay in growth and puberty:A systematic review and meta-analysis. J Clin Res Pediatr Endocrinol. 2021 doi: 10.4274/jcrpe.galenos.2021.2021.0169. doi:10.4274/jcrpe.galenos.2021.2021.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) Risk of bias graph: review authors‘ judgements about each risk of bias item presented as percentages across all included studies; (b) Risk of bias summary: review authors‘ judgements about each risk of bias item for each included study.
Funnel plot of all the included studies in the meta-analysis (assessing the publication bias) of the main outcomes assessed (a) total testosterone three months; (b) estradiol three months; (c) luteinizing hormone three months; (d): treatment-emergent adverse events; (e): Severe adverse events